Note: Descriptions are shown in the official language in which they were submitted.
WO 2016/168719 PCT/US2016/027917
PROPPANT HAVING NON-UNIFORM ELECTRICALLY CONDUCTIVE
COATINGS AND METHODS FOR MAKING AND USING SAME
Cross-Reference to Related Patent Applications
[0001] This application claims priority to U.S. Patent Application No.
14/826,965 filed on
August 14, 2015, which is a continuation in part of U.S. Patent Application
No. 14/572,486
filed on December 16, 2014. This application claims priority to U.S. Patent
Application No.
14/942,304 filed on November 16, 2015, which is a continuation-in-part of U.S.
Patent
Application No. 14/629,004 filed on February 23, 2015, which is a continuation-
in-part of U.S.
Patent Application No. 14/593,447 filed on January 9, 2015, which is a
continuation of U.S.
Patent Application No. 14/147,372, now U.S. Patent No. 8,931,553, filed on
January 3,2014
and International Patent Application No. PCT/U52014/010228 filed January 3,
2014. U.S.
Patent Application No. 14/942,304, U.S. Patent Application No. 14/629,004,
U.S. Patent
Application No. 14/593,447, U.S. Patent Application No. 14/147,372, and
International Patent
Application No. PCT/U52014/010228 each claims the benefit of U.S. Provisional
Patent
Application No. 61/749,093 filed January 4, 2013. This application also claims
priority to U.S.
Provisional Patent Application No. 62/148,422 filed April 16, 2015.
Field
[0002] Embodiments of the present invention relate generally to hydraulic
fracturing of
geological formations, and more particularly to electrically conductive
proppants used in the
hydraulic fracture stimulation of gas, oil, or geothermal reservoirs.
Embodiments of the
present invention relate to compositions and methods for the founation of the
electrically
conductive proppants for use in the electromagnetic methods for detecting,
locating and
characterizing such proppants.
Background
[0003] In order to stimulate and more effectively produce hydrocarbons from
downhole
formations, especially formations with low porosity and/or low permeability,
induced
fracturing (called "frac operations", "hydraulic fracturing", or simply
"fracing") of the
Date Recue/Date Received 2021-04-15
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
hydrocarbon-bearing formations has been a commonly used technique. In a
typical frac
operation, fluids are pumped downhole under high pressure, causing the
formations to
fracture around the borehole, creating high permeability conduits that promote
the flow of the
hydrocarbons into the borehole. These frac operations can be conducted in
horizontal and
deviated, as well as vertical, boreholes, and in either intervals of uncased
wells, or in cased
wells through perforations.
[0004] In cased boreholes in vertical wells, for example, the high pressure
fluids exit the
borehole via perforations through the casing and surrounding cement, and cause
the
formations to fracture, usually in thin, generally vertical sheet-like
fractures in the deeper
formations in which oil and gas are commonly found. These induced fractures
generally
extend laterally a considerable distance out from the wellbore into the
surrounding
formations, and extend vertically until the fracture reaches a formation that
is not easily
fractured above and/or below the desired frac interval. The directions of
maximum and
minimum horizontal stress within the formation determine the azimuthal
orientation of the
induced fractures. Normally, if the fluid, sometimes called slurry, pumped
downhole does not
contain solids that remain lodged in the fracture when the fluid pressure is
relaxed, then the
fracture re-closes, and most of the permeability conduit gain is lost.
[0005] These solids, called proppants, are generally composed of sand
grains or ceramic
particles, and the fluid used to pump these solids downhole is usually
designed to be
sufficiently viscous such that the proppant particles remain entrained in the
fluid as it moves
downhole and out into the induced fractures. Prior to producing the fractured
formations,
materials called "breakers", which are also pumped downhole in the frac fluid
slurry, reduce
the viscosity of the frac fluid after a desired time delay, enabling these
fluids to be easily
removed from the fractures during production, leaving the proppant particles
in place in the
induced fractures to keep them from closing and thereby substantially
precluding production
fluid flow there through.
[0006] The proppants may also be placed in the induced fractures with a low
viscosity
fluid in fracturing operations referred to as "water fracs" or "slick water
fracs". The
fracturing fluid in water fracs is water with little or no polymer or other
additives. Water
fracs are advantageous because of the lower cost of the fluid used. Also when
using cross-
linked polymers, it is essential that the breakers be effective or the fluid
cannot be recovered
from the fracture, effectively restricting flow of formation fluids. Water
fracs, because the
fluid is not cross-linked, do not rely on the effectiveness of breakers.
2
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
[0007] Commonly used proppants include naturally occurring sands, resin
coated sands,
and ceramic proppants. Ceramic proppants are typically manufactured from
naturally
occurring materials such as kaolin and bauxitic clays, and offer a number of
advantages
compared to sands or resin coated sands principally resulting from the
compressive strength
of the manufactured ceramics and their highly spherical particle shape.
[0008] Although induced fracturing has been a highly effective tool in the
production of
hydrocarbon reservoirs, the amount of stimulation provided by this process
depends to a large
extent upon the ability to generate new fractures, or to create or extend
existing fractures, as
well as the ability to maintain connection to the fractures through
appropriate placement of
the proppant. Without appropriate placement of the proppant, fractures
generated during the
hydraulic fracturing may tend to close, thereby diminishing the benefits of
the hydraulic
fracturing treatment. However, reliable methods for detecting, locating and
characterizing
the placement of proppant within fractures at relatively far distances from
the wellbore and
thus confirming whether or not such placement has been appropriate are not
available.
[0009] Current state of the art proppant identification techniques are
limited to relatively
short distances (12 inches to 18 inches maximum) from the wellbore.
Radioactive and
non-radioactive tracers and proppants are currently used to infer the presence
of proppant in
the near well bore region. A better understanding of proppant placement in the
far field
regions of a hydraulic fracture is needed.
[0010] Previous work for massive hydraulic fracture mapping is summarized
in Bartel, L.
C., McCann, R. P., and Keck, L. J., Use of potential gradients in massive
hydraulic fracture
mapping and characterization, prepared for the 51st Annual Fall Technical
Conference and
Exhibition of Society of Petroleum Engineers, New Orleans, Oct 3-6, 1976 paper
SPE 6090
In this previous work, the electric potential differences were measured
between two
concentric circles of voltage electrodes around a vertical fracture well at
the earth's surface.
The well was electrically energized at the top of the well casing or at the
depth of the
fracture. The electrical ground was established at a well located at a
distance of
approximately one mile from the fracture well. At that time, the fact that the
grounding wire
acted as a transmitting antenna was not taken into account. The water used for
the fracture
process contained potassium chloride (KCl) to enhance its electrical
conductivity and the
fracture was propped using non-conducting sand. A 1 Hz repetition rate square
wave input
current waveform was used and only the voltage difference amplitudes were
measured.
Voltages using an elementary theory based on current leakage from the well
casing and the
3
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
fracture into a homogeneous earth were used to produce expected responses.
Comparing the
field data to results from the elementary model showed that a fracture
orientation could be
inferred, however, since the model did not account for the details of the
fracture, other
fracture properties could not be determined using the elementary model.
[0011] Electrically conductive proppant has been used to determine a
proppant pack
location within one or more fracture(s). For example, U.S. Patent No.
8,931,553 discloses
providing proppant particles having a substantially uniform coating of
electrically conductive
material of at least 500 nm in thickness for detection, location and
characterization of the
proppant particles in one or more fractures via electromagnetic (EM) methods.
However,
coating proppant particles with a substantially uniform coating of
electrically conductive
material can be cost prohibitive.
[0012] There is a need, therefore, for a method of detecting, locating and
characterizing
the location of the proppant as placed in a hydraulic fracture at distances of
more than several
inches from the cased wellbore in a manner that is not cost prohibitive.
Brief Description of the Drawings
[0013] The invention may best be understood by referring to the following
description
and accompanying drawings that are used to illustrate embodiments of the
invention. In the
drawings:
[0014] FIG. 1 depicts a perspective view an illustrative proppant particle
have a non-
uniform coating of electrically conductive material.
[0015] FIG. 2 depicts a perspective view of an illustrative electrically
conductive
proppant pack 200 containing the proppant particle of FIG. 1 in contact with
two other
proppant particles having non-uniform coatings of electrically conductive
material.
[0016] FIG. 3 is a flow chart showing steps of an electroless coating
method for
electrically conductive material onto a proppant substrate to provide the
proppant particle of
FIG. 1.
[0017] FIG. 4 is another flow chart showing alternative steps of an
electroless coating
method for electrically conductive material onto a proppant substrate to
provide the proppant
particle of FIG. 1.
[0018] FIG. 5 is a diagram of the geometric layout of a vertical or
deviated well in which
layers of the earth having varying electrical and mechanical properties are
depicted.
[0019] FIG. 6 is a schematic of an installed horizontal wellbore casing
string traversing a
4
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
hydrocarbon bearing zone with proppant filled fractures in which layers of the
earth having
varying electrical and mechanical properties are depicted.
[0020] FIG. 7 is a schematic cross-sectional illustration of a hydraulic
fracture mapping
system which depicts two embodiments for introducing electric current into a
wellbore,
namely energizing the wellbore at the surface and energizing via a wireline
with a sinker bar
near perforations in the wellbore
[0021] FIG. 8 is a schematic plan illustration of a hydraulic fracture
mapping system.
[0022] FIG. 9 is a schematic perspective illustration of a hydraulic
fracture mapping
system
[0023] FIG 10A is a schematic illustration of an electrically insulated
casing joint
[0024] FIG. 10B is a schematic illustration of an electrically insulated
casing collar.
[0025] FIG. 11 is schematic illustration of a test system for measuring
proppant electrical
resistance.
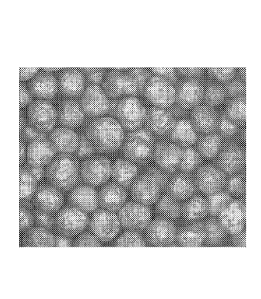
[0026] FIG. 12 shows a sample of 20/40 Mesh CARBOECONOPROP non-uniformly
coated with a nickel alloy coating having an average thickness of about 192
nm.
[0027] FIG. 13 shows a sample of 20/40 Mesh CARBOECONOPROP non-uniformly
coated with a nickel alloy coating having an average thickness of about 390
nm.
[0028] FIG. 14 shows a sample of 20/40 Mesh CARBOECONOPROP non-uniformly
coated with a nickel alloy coating having an average thickness of about 670
nm.
[0029] FIG. 15 shows a sample of 20/40 Mesh CARBOECONOPROP uniformly coated
with a nickel alloy coating having an average thickness of about 780 nm.
[0030] FIG. 16 shows a sample of 20/40 Mesh CARBOECONOPROP uniformly coated
with a nickel alloy coating having an average thickness of about 1,080 nm.
[0031] FIG 17 shows a sample at 7x magnification of 25 Mesh KRYPTOSPHERE
HD
non-uniformly coated with a nickel alloy coating having an average thickness
of about 200
nm.
[0032] FIG. 18 shows a sample at 20x magnification of 25 Mesh KRYPTOSPHERE
HD
non-uniformly coated with a nickel alloy coating having an average thickness
of about 200
nm.
[0033] FIG. 19 shows a sample at 7x magnification of 25 Mesh KRYPTOSPHERE
HD
uniformly coated with a nickel alloy coating having an average thickness of
about 500 nm.
[0034] FIG. 20 shows a sample at 20x magnification of 25 Mesh KRYPTOSPHERE
HD
uniformly coated with a nickel alloy coating having an average thickness of
about 500 nm.
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
Detailed Description
[0035] In the
following description, numerous specific details are set forth. However, it
is understood that embodiments of the invention may be practiced without these
specific
details. In other instances, well-known structures and techniques have not
been shown in
detail in order not to obscure the understanding of this description.
[0036]
Described herein are electrically conductive sintered, substantially round and
spherical particles and methods for preparing such electrically conductive
sintered,
substantially round and spherical particles, referred to hereinafter as
"electrically conductive
proppant," from a slurry of an alumina containing raw material for use as
proppants
detectable by electromagnetic (EM) methods. In particular, electrically
conductive proppant
having irregular, or non-uniform, coatings of electrically conductive material
on its outer
surfaces are described herein. Also described herein are electrically
conductive proppant
packs having an electrically conductive portion and a non-electrically
conductive portion.
The electrically conductive portion can include electrically conductive
proppant having
uniform or non-uniform coatings of electrically conductive material on its
outer surfaces.
The term "substantially round and spherical" and related forms, as used
herein, is defined to
mean an average ratio of minimum diameter to maximum diameter of about 0.8 or
greater, or
having an average sphericity value of about 0.8 or greater compared to a
Krumbein and Sloss
chart. Also described herein are electromagnetic methods for detecting,
locating, and
characterizing the electrically conductive proppants used in the hydraulic
fracture stimulation
of gas, oil, or geothermal reservoirs.
[0037] The
electrically conductive proppant can include an irregular or non-uniform
coating of electrically conductive material. FIG. 1
depicts an illustrative electrically
conductive proppant particle 100 having a non-uniform coating of an
electrically conductive
material 106. As shown in FIG. 1, a proppant particle 102 such as sand or
ceramic proppant
can be partially coated with the electrically conductive material 106 to
provide the electrically
conductive proppant particle 100. The coating of electrically conductive
material 106 can
cover or coat any suitable portion of the surface 104 of the proppant particle
102. In one or
more exemplary embodiments, the coating of electrically conductive material
106 can cover at
least about 10%, at least about 15%, at least about 20%, at least about 30%,
at least about 40%,
or at least about 50% of the surface 104 of the electrically conductive
proppant particle 100. In
one or more exemplary embodiments, the coating of electrically conductive
material 106 can
cover less than 100%, less than 99%, less than 95%, less than 90%, less than
85%, less than
6
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
800/o, less than 75%, less than 65%, less than 50%, less than 40%, or less
than 35% of the
surface 104 of the electrically conductive proppant particle 100. In one or
more exemplary
embodiments, about 25%, about 30%, about 35%, or about 45% to about 55%, about
65%,
about 75%, about 85%, about 900/0, about 95%, or about 99% or more of the
surface 104 of the
electrically conductive proppant particle 100 can be covered by the
electrically conductive
material 106. For example, the coating of electrically conductive material 106
can cover from
about 10% to about 99%, from about 15% to about 95%, from about 20% to about
75%, from
about 25% to about 65%, from about 30% to about 45%, from about 35% to about
75%, from
about 45% to about 90%, or from about 40% to about 95% of the surface 104 of
the electrically
conductive proppant particle 100.
[0038] The coating of electrically conductive material 106 can have any
suitable thickness.
In one or more exemplary embodiments, the coating of electrically conductive
material 106 can
have an average thickness ranging from about 5 nm, about 10 nm, about 25 nm,
about 50 nm,
about 100 nm, or about 200 nm to about 300 nm, about 400 nm, about 500 nm,
about 750 nm,
about 1,000 about 1,500 nm, about 2,000 nm, or about 5,000 nm. For example,
the average
thickness of the coating of electrically conductive material 106 can be from
about 400 nm to
about 1,000 nm, from about 200 nm to about 600 nm, or from about 100 nm to
about 400 nm.
The coating of electrically conductive material 106 can also have any suitable
variation in
thickness. In one or more exemplary embodiments, the thickness of the coating
of electrically
conductive material 106 can vary from about 10 nm to about 1,000 nm, from
about 50 nm to
about 500 nm, from about 100 nm to about 400 nm, or from about 400 nm to about
1,000 nm.
[0039] Two or more electrically conductive proppant each having the
irregular or non-
uniform coating of electrically conductive material can be in contact with one
another to form
an electrically conductive proppant pack FIG. 2 depicts a perspective view of
an illustrative
electrically conductive proppant pack 200 containing the electrically
conductive proppant
particle 100, or first electrically conductive proppant particle 100, in
contact with second and
third electrically conductive proppant particles 202, 300 having second and
third non-uniform
coatings of electrically conductive material 208, 306, respectively. Similar
to the first
electrically conductive proppant particle 100, the second and third
electrically conductive
proppant particles 202, 300 can include second and third proppant particles
204, 302 such as
sand or ceramic proppant partially coated with the second and third
electrically conductive
materials 208, 306, respectively. The coatings of the second and third
electrically conductive
materials 208, 306 can cover or coat any suitable portion of the second and
third surfaces 206,
7
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
304 of the proppant particles 204, 302, respectively. In one or more exemplary
embodiments,
the coatings of electrically conductive materials 208, 306 can cover at least
1%, at least 5%, at
least 10%, at least 15%, at least 20%, at least 30%, at least 40%, or at least
50% of the
respective surfaces 206, 304 of the second and third electrically conductive
proppant particles
202, 300. In one or more exemplary embodiments, the coatings of electrically
conductive
material 208, 306 can cover less than 100%, less than 99%, less than 95%, less
than 90%, less
than 85%, less than 80%, less than 75%, less than 65%, less than 50%, less
than 40%, less than
25%, less than 20%, or less than 10% of the surfaces 206, 304 of the
electrically conductive
proppant particles 202, 300. In one or more exemplary embodiments, about I %,
about 4%,
about 8%, about 12%, about 18%, about 25%, about 35%, or about 45% to about
55%, about
65%, about 75%, about 85%, about 90%, about 95%, or about 99% or more of the
surfaces
206, 304 of the electrically conductive proppant particles 202, 300 can be
covered by the
electrically conductive materials 208, 306. For example, the coatings of
electrically conductive
materials 208, 306 can cover from about 1% to about 99%, from about 5% to
about 95%, from
about 10% to about 75%, from about 15% to about 65%, from about 25% to about
45%, from
about 35% to about 75%, from about 45% to about 90%, or from about 40% to
about 95% of
the surfaces 206, 304 of the electrically conductive proppant particles 202,
300.
[0040] The coatings of second and third electrically conductive materials
208, 306 can
have any suitable thickness. In one or more exemplary embodiments, the
coatings of second
and third electrically conductive materials 208, 306 can have an average
thickness ranging from
about 5 nm, about 10 nm, about 25 nm, about 50 nm, about 100 nm, or about 200
nm to about
300 nm, about 400 nm, about 500 nm, about 750 nm, about 1,000 about 1,500 nm,
about 2,000
nm, or about 5,000 nm. For example, the average thickness of the coatings of
second and third
electrically conductive materials 208, 306 can be from about 400 nm to about
1,000 nm, from
about 200 nm to about 600 nm, or from about 100 nm to about 400 nm. The
coatings of second
and third electrically conductive materials 208, 306 can also have any
suitable variation in
thickness. In one or more exemplary embodiments, the thickness of the coatings
of second and
third electrically conductive materials 208, 306 can vary from about 10 nm to
about 1,000 nm,
from about 50 nm to about 500 nm, from about 100 nm to about 400 nm, or from
about 400 nm
to about 1,000 nm.
[0041] As shown in FIG. 2, the coating of electrically conductive material
106 of particle
100 can be in direct contact with coating of electrically conductive material
208 of the
adjacently disposed particle 202. The direct contact of the electrically
conductive material
8
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
106 with the electrically conductive material 208 can form a conductive bridge
or link so that
the electrically conductive material 106 is electrically coupled to the
electrically conductive
material 208. This conductive bridge can be imperfect, meaning there can be
some contact
resistance in the conductive bridge that can lead to charge build up at the
interfaces of the
particles. When the energizing field is turned off, these charges will flow
back to their
equilibrium state, which does not occur instantaneously. This charge
redistribution leads to
the induced polarization effect discussed below in greater detail. The
conductive bridge can
allow an electric and/or magnetic field or charge to move across the
electrically conductive
proppant pack 200, from the first electrically conductive proppant particle
100 to the second
electrically conductive proppant particle 202.
[0042] Also shown in FIG. 2 is a gap D that can be present between the
second
electrically conductive material 208 and the third electrically conductive
material 306 in the
proppant pack 200. The gap D can have any length suitable to permit the
electric and/or
magnetic field or charge to move from the second electrically conductive
proppant particle
202 to the third electrically conductive proppant particle 300 via the gap D.
Even though the
second electrically conductive material 208 is not in direct physical contact
with the third
electrically conductive material 306, the charges on materials 106 and 208
will induce
charges onto the material 306. In other words, even though the metallic
coating 306 is not
directly contacting the metallic coatings 106 and/or 208, an energizing
electric field will
cause charge separation in the 306 coating thereby influencing flow in
coatings 106 and 208.
For a time-dependent source field, coating 306 will capacitively couple to
coatings 106 and
208 resulting in a displacement current that can flow between particles that
are not in direct
physical contact with each other, such as coatings 306 and 106 and/or 208. In
one or more
exemplary embodiments, the gap D can be from about 1 micron, about 5 microns,
about 25
microns, about 50 microns, about 100 microns, or about 250 microns to about
300 microns,
about 400 microns, about 500 microns, about 750 microns, or about 1,000
microns or more.
[0043] The electrically conductive proppant pack 200 can have any suitable
electrical
conductivity. In one or more exemplary embodiments, the electrically
conductive proppant
pack 200 can have an electrical conductivity of at least about 1 Siemen-meter
(Siemen-m), at
least about 5 Siemen-m, at least about 15 Siemen-m, at least about 50 Siemen-
m, at least
about 100 Siemen-m, at least about 250 Siemen-m, at least about 500 Siemen-m,
at least
about 750 Siemen-m, at least about 1,000 Siemen-m, at least about 1,500 Siemen-
m, or at
least about 2,000 Siemen-m. The electrical conductivity of the pack 200 can
also be from
9
WO 2016/168719 PCT/US2016/027917
about 10 Siemen-m, about 50 Siemen-m, about 100 Siemen-m, about 500 Siemen-m,
about
1,000 Siemen-m, or about 1,500 Siemen-m to about 2,000 Siemen-m, about 3,00
Siemen-m,
about 4,000 Siemen-m, about 5,000 Siemen-m, or about 6,000 Siemen-m. The
electrically
conductive proppant pack 200 can have any suitable resistivity. In one or more
exemplary
embodiments, the pack 200 can have a resistivity of less than 100 Ohm-cm, less
than 80
Ohm-cm, less than 50 Ohm-cm, less than 25 Ohm-cm, less than 15 Ohm-cm, less
than 5
Ohm-cm, less than 2 Ohm-cm, less than 1 Ohm-cm, less than 0.5 Ohm-cm, or less
than 0.1
Ohm-cm.
[0044] In one or more exemplary embodiments, increasing a load or pressure
onto the
pack of the electrically conductive proppant pack 200 by a factor of 2, a
factor of 5, or a
factor of 10 can increase the electrical conductivity of the pack of the
electrically conductive
proppant pack 200 by at least about 50%, at least about 75%, at least about
100%, at least
about 150%, or at least about 200%. In one or more exemplary embodiments,
increasing a
load or pressure onto the pack of the electrically conductive proppant pack
200 by a factor of
'") a factor of 5, or a factor of 10 can decrease the resistivity of the pack
of the electrically
conductive proppant pack 200 by from about 1%, about 2%, or about 5% to about
10%, about
15%, or about 25%.
[0045] According to embodiments of the present invention, the electrically
conductive
proppant can be made from a conventional proppant such as a ceramic proppant,
sand, plastic
beads and glass beads. Such conventional proppants can be manufactured
according to any
suitable process including, but not limited to continuous spray atomization,
spray fluidization,
spray drying, or compression. Suitable conventional proppants and methods for
their
manufacture are disclosed in U.S. Patent Nos. 4,068,718, 4,427,068, 4,440,866,
5,188,175, and
7,036,591.
[0046] Ceramic proppants vary in properties such as apparent specific
gravity by virtue of
the starting raw material and the manufacturing process. The term "apparent
specific
gravity" as used herein is the weight per unit volume (grams per cubic
centimeter) of the
particles, including the internal porosity. Low density proppants generally
have an apparent
specific gravity of less than 3.0 g/cm3 and are typically made from kaolin
clay and other
alumina, oxide, or silicate ceramics. Intermediate density proppants generally
have an
apparent specific gravity of about 3.1 to 3.4 g/cm3 and are typically made
from bauxitic clay.
High strength proppants are generally made from bauxitic clays with alumina
and have an
apparent specific gravity above 3.4 g/cm3.
Date Recue/Date Received 2021-04-15
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
[0047] As described herein, sintered, substantially round and spherical
particles, or
proppants, are prepared from a slurry of alumina-containing raw material. In
certain
embodiments, the particles have an alumina content of from about 40% to about
55% by
weight. In certain other embodiments, the sintered, substantially round and
spherical
particles have an alumina content of from about 41.5% to about 49% by weight.
[0048] In certain embodiments, the proppants have a bulk density of from about
1.35 g/cm3
to about 1.55 g/cm3. The term "bulk density", as used herein, refers to the
weight per unit
volume, including in the volume considered, the void spaces between the
particles. In certain
other embodiments, the proppants have a bulk density of from about 1.40 g/cm3
to about 1.50
g/cm3.
[0049] According to several exemplary embodiments, the proppants have any
suitable
permeability and fluid conductivity in accordance with ISO 13503-5:
"Procedures for
Measuring the Long-term Conductivity of Proppants," and expressed in terms of
Darcy units,
or Darcies (D). The proppants can have a long term permeability at 7,500 psi
of at least
about 1 D, at least about 2 D, at least about 5 D, at least about 10 D, at
least about 20 D, at
least about 40 D, at least about 80 D, at least about 120 D, or at least about
150 D. The
proppants can have a long term permeability at 12,000 psi of at least about 1
D, at least about
2 D, at least about 3 D, at least about 4 D, at least about 5 D, at least
about 10 D, at least
about 25 D, or at least about 50 D. The proppants can have a long term
conductivity at 7,500
psi of at least about 100 millidarcy-feet (mD-ft), at least about 200 mD-ft,
at least about 300
mD-ft, at least about 500 mD-ft, at least about 1,000 mD-ft, at least about
1,500 mD-ft, at
least about 2,000 mD-ft, or at least about 2,500 mD-ft. For example, the
proppants can have
a long term conductivity at 12,000 psi of at least about 50 mD-ft, at least
about 100 mD-ft, at
least about 200 mD-ft, at least about 300 mD-ft, at least about 500 mD-ft, at
least about 1,000
mD-ft, or at least about 1,500 mD-ft.
[0050] In certain embodiments, the proppants have a crush strength at 10,000
psi of from
about 5% to about 8.5%, and a long term fluid conductivity at 10,000 psi of
from about 2500
mD-ft to about 3000 mD-ft. In certain other embodiments, the proppants have a
crush
strength at 10,000 psi of from about 5% to about 7.5%.
[0051] The proppants can have any suitable apparent specific gravity. In
one or more
exemplary embodiments, the proppants have an apparent specific gravity of less
than 5, less
than 4.5, less than 4.2, less than 4, less than 3.8, less than 3.5, or less
than 3.2. In still other
embodiments, the proppants have an apparent specific gravity of from about
2.50 to about
11
WO 2016/168719 PCT/US2016/027917
3.00, about 2.75 to about 3.25, about 2.8 to about 3.4, about 3.0 to about
3.5, or about 3.2 to
about 3.8. In one or more exemplary embodiments, the proppants can have a
specific gravity
of about 5 or less, about 4.5 or less, about 4.2 or less, about 4 or less, or
about 3.8 or less.
The term "apparent specific gravity," (ASG) as used herein, refers to a number
without units
that is defined to be numerically equal to the weight in grams per cubic
centimeter of volume,
including void space or open porosity in determining the volume.
[0052] In one or more exemplary embodiments, the ceramic proppant can be
manufactured in a manner that creates porosity in the proppant grain. A
process to
manufacture a suitable porous ceramic proppant is described in U.S. Patent No.
7,036,591.
In this case the electrically
conductive material can be impregnated into the pores of the proppant grains
to a
concentration of about 0.01 wt%, about 0.05 wt%, about 0.1 wt%, about 0.5 wt%,
about 1
wt%, about 2 wt%, or about 5 wt% to about 6 wt%, about 8 wt%, about 10 wt%,
about 12
wt%, about 15 wt?/o, or about 20 wt% based on the weight of the electrically
conductive
proppant. Water soluble coatings such as polylactic acid can be used to coat
these particles to
allow for delayed/timed release of conductive particles.
[0053] The ceramic proppants can have any suitable porosity. The ceramic
proppants can
include an internal interconnected porosity from about 1%, about 2%, about 4%,
about 6%,
about 8%, about 10%, about 12%, or about 14% to about 18%, about 20%, about
22%, about
24%, about 26%, about 28%, about 30%, about 34%, about 38%, or about 45% or
more. In
several exemplary embodiments, the internal interconnected porosity of the
ceramic
proppants is from about 5 to about 35%, about 5 to about 15%, or about 15 to
about 35%.
According to several exemplary embodiments, the ceramic proppants have any
suitable
average pore size. For example, the ceramic proppant can have an average pore
size from
about 2 nm, about 10 nm, about 15 nm, about 55 nm, about 110 nm, about 520 nm,
or about
1,100 nm to about 2,200 nm, about 5,500 nm, about 11,000 nm, about 17,000 nm,
or about
25,000 nm or more in its largest dimension. For example, the ceramic proppant
can have an
average pore size from about 3 nm to about 30,000 nm, about 30 nm to about
18,000 nm,
about 200 nm to about 9,000 nm, about 350 nm to about 4,500 nm, or about 850
nm to about
1,800 nm in its largest dimension.
[0054] Suitable sintered, substantially round and spherical particles can
also include
proppants manufactured according to vibration-induced dripping methods, herein
called "drip
casting." Suitable drip casting methods and proppants made therefrom are
disclosed in U.S.
12
Date Recue/Date Received 2021-04-15
WO 2016/168719 PCT/US2016/027917
Patent Nos. 8,865,631, 8,883,693 and 9,175,210, U.S. Patent Application
Publication Nos. US
2015/0166880 and US 2016/0017214, and U.S. Patent Application No. 15/066,936.
Proppants produced from the drip
cast methods can have a specific gravity of at least about 2.5, at least about
2.7, at least about
3, at least about 3.3, or at least about 3.5. Proppants produced from the drip
cast methods can
have a specific gravity of about 5 or less, about 4.5 or less, or about 4 or
less. The drip cast
proppants can also have a surface roughness of less than 5 um, less than 4 um,
less than 3
um, less than 2.5 um, less than 2 pm, less than 1.5 gm, or less than 1 um. In
one or more
exemplary embodiments, the drip cast proppants have an average largest pore
size of less
than about 25 um, less than about 20 um, less than about 18 um, less than
about 16 um, less
than about 14 um, or less than about 12 um and/or a standard deviation in pore
size of less
than 6 um, less than 4 um, less than 3 um, less than 2.5 um, less than 2 um,
less than 1.5 um,
or less than 1 um. In one or more exemplary embodiments, the drip cast
proppants have less
than 5,000, less than 4,500, less than 4,000, less than 3,500, less than
3,000, less than 2,500,
or less than 2,200 visible pores at a magnification of 500x per square
millimeter of proppant
particle.
[0055] The proppants, produced by the drip casting methods or the
conventional methods,
can have any suitable composition. The proppants can be or include silica
and/or alumina in
any suitable amounts. According to one or more embodiments, the proppants
include less
than 80 wt%, less than 60 wt%, less than 40 wt%, less than 30 wt%, less than
20 wt%, less
than 10 wt%, or less than 5 wt% silica based on the total weight of the
proppants. According
to one or more embodiments, the proppants include from about 0.1 wt% to about
70 wt%
silica, from about 1. wt% to about 60 wt% silica, from about 2.5 wt% to about
50 wt% silica,
from about 5 wt% to about 40 wt% silica, or from about 10 wt% to about 30 wt%
silica.
According to one or more embodiments, the proppants include at least about 30
wt?/o, at least
about 50 wt%, at least about 60 wt%, at least about 70 wt%, at least about 80
wt%, at least
about 90 wt%, or at least about 95 wt% alumina based on the total weight of
the proppants.
According to one or more embodiments, the proppants include from about 30 wt%
to about
99.9 wt% alumina, from about 40 wt% to about 99 wt% alumina, from about 50 wt%
to about
97 wt% alumina, from about 60 wt% to about 95 wt% alumina, or from about 70
wt% to
about 90 wt% alumina. In one or more embodiments, the proppants produced by
the
processes disclosed herein can include alumina, bauxite, or kaolin, or any
mixture thereof.
For example, the proppants can be composed entirely of or composed essentially
of alumina,
13
Date Recue/Date Received 2021-04-15
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
bauxite, or kaolin, or any mixture thereof. The term "kaolin" is well known in
the art and can
include a raw material having an alumina content of at least about 40 wt% on a
calcined basis
and a silica content of at least about 40 wt% on a calcined basis. The term
"bauxite" is well
known in the art and can be or include a raw material having an alumina
content of at least
about 55 wt% on a calcined basis.
[0056] The
proppants can also have any suitable size. According to one or more
exemplary embodiments, the proppants can have a size of at least about 100
mesh, at least
about 80 mesh, at least about 60 mesh, at least about 50 mesh, or at least
about 40 mesh. For
example, the proppants can have a size from about 115 mesh to about 2 mesh,
about 100
mesh to about 3 mesh, about 80 mesh to about 5 mesh, about 80 mesh to about 10
mesh,
about 60 mesh to about 12 mesh, about 50 mesh to about 14 mesh, about 40 mesh
to about 16
mesh, or about 35 mesh to about 18 mesh. In a particular embodiment, the
proppants have a
size of from about 20 to about 40 U.S. Mesh.
[0057]
According to certain embodiments described herein, the proppants are made in a
continuous process, while in other embodiments, the proppants are made in a
batch process.
[0058] An
electrically conductive material such as a metal, a conductive polymer, or a
conductive particle may be added at any suitable stage in the manufacturing
process of any
one of these proppants to result in an electrically conductive proppant
suitable for use
according to certain embodiments of the present invention. The electrically
conductive
material can also be added to any one of these proppants after manufacturing
of the
proppants. In one or more exemplary embodiments, the proppant can be a porous
proppant,
such that the electrically conductive material can be impregnated or infused
into the pores of
the proppant to provide the electrically conductive proppant. The porous
proppant can be
impregnated or infused with the electrically conductive material in any
suitable amounts,
such as from about 1% to 15% by weight. Water soluble coatings such as
polylactic acid can
be used to coat these particles to allow for delayed/timed release of
conducting particles.
[0059] The
electrically conductive material can be or include any suitable electrically
conductive metal. For example, the metal can be or include iron, silver, gold,
copper,
aluminum, calcium, tungsten, zinc, nickel, lithium, platinum, palladium,
rhodium, tin, carbon
steel, or any combination or oxide thereof. In one or more exemplary
embodiments, the
electrically conductive material can be selected from one or more of aluminum,
copper,
nickel, and phosphorus and any alloy or mixture thereof. The electrically
conductive
proppant can have an electrically conductive metal concentration of about 0.01
wt%, about
14
WO 2016/168719 PCT/US2016/027917
0.05 wt?/o, about 0.1 wt?/o, about 0.5 wt%, about 1 wt?/o, about 2 wt%, or
about 5 wt% to
about 6 wt%. about 8 wt%, about 10 wt%, about 12 wt%, or about 14 wt%. In one
or more
exemplary embodiments, the metals can include aluminum, copper and nickel and
can be
added to result in a proppant having a metal content of from about 5% to about
10% by
weight.
[0060] The
electrically conductive material can be or include any suitable electrically
conductive polymer. Suitable
conductive polymers include poly(3,4-
ethylenedioxythiophene) poly(styrenesulfonate) (PEDOT:PSS), polyanilines
(PANI), and
polypyrroles (PPY) and can be added to result in a proppant having any
suitable conductive
polymer content, such as from about 0.1% to about 10% by weight. In one or
more
exemplary embodiments, the electrically conductive proppant can have a
conductive polymer
concentration of about 0.01 wt%, about 0.05 wt%, about 0.1 wt%, about 0.5 wt%,
about 1
wt%, about 2 wt%, or about 5 wt% to about 6 wt%, about 8 wt%, about 10 wt%,
about 12
wt%, or about 14 wt%. Suitable PEDOT:PSS, PANI and PYY conductive polymers are
commercially available from Sigma-Aldrich.
[0061] In one
or more exemplary embodiments, the electrically conductive material can
be added at any stage in a method of manufacture of a conventional ceramic
proppant. The
method of manufacture of a conventional ceramic proppant can be or include a
method
similar in configuration and operation to that described in U.S. Patent No.
4,440,866.
In one or more exemplary
embodiments, the electrically conductive material can be added at any stage in
a method of
manufacture of drip cast proppant. Suitable drip casting methods and proppants
made
therefrom are disclosed in U.S. Patent Nos 8,865,631 and 8,883,693, U.S.
Patent Application
Publication No. 2012/0227968, and U.S. Patent Application No. 14/502,483.
[0062]
According to certain embodiments of the present invention, the electrically
conductive material is coated onto the proppants to provide the electrically
conductive
proppant. The coating may be accomplished by any coating technique well known
to those
of ordinary skill in the art such as spraying, sputtering, vacuum deposition,
dip coating,
extrusion, calendaring, powder coating, electroplating, transfer coating, air
knife coating,
roller coating and brush coating. In one or more exemplary embodiments, the
electrically
conductive material is coated onto the proppants with an electroless plating
or coating
method.
Date Recue/Date Received 2021-04-15
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
[0063] Referring now to FIG. 3, a flow chart is depicted showing steps of a
process 300
for electroless coating of the electrically conductive material onto proppant
utilizing a
conditioning step. In the electroless coating process 300, a supply of
proppant via line 302
can be introduced to one or more washing units 304 where the proppant via line
302 can be
contacted with a first washing solution to remove dust and/or fines to provide
a clean
proppant via line 306. The washing unit 304 can be or include one or more
tanks, one or
more vessels, one or more conveyance systems, one or more conduits, or the
like. The first
washing solution can be or include an aqueous solution containing an acid or
base, such as
water containing dilute acid, or an organic phase solution, such as a liquid
hydrocarbon, this
washing can also be conducted at an elevated temperature Clean proppant via
line 306 can
be withdrawn from the washing unit 304 and introduced to one or more
pretreatment units
308 where the clean proppant via line 306 can be contacted with a conditioning
solution. The
pretreatment unit 308 can be or include one or more tanks, one or more
vessels, one or more
conveyance systems, one or more conduits, or the like. The conditioning
solution can be or
include an alkaline solution to adjust the pH of the surface of the proppant
to alkaline levels
(pH >7). The alkaline solution can include one or more of a hydroxide,
ammonia, or a
carbonate.
[0064] The conditioning in the pretreatment unit 308 can be further
enhanced by
combining or mixing a suitable surfactant with the conditioning solutions.
Suitable
surfactants can include, but are not limited to, anionic, cationic, nonionic,
and amphoteric
surfactants, or combinations thereof. According to several exemplary
embodiments, suitable
surfactants include but are not limited to saturated or unsaturated long-chain
fatty acids or
acid salts, long-chain alcohols, polyalcohols, polysorbates,
dimethylpolysiloxane and
poly ethyl hydrosiloxane. According to several exemplary embodiments, suitable
surfactants
include but are not limited to linear and branched carboxylic acids and acid
salts having from
about 4 to about 30 carbon atoms, linear and branched alkyl sulfonic acids and
acid salts
having from about 4 to about 30 carbon atoms, linear alkyl benzene sulfonate
wherein the
linear alkyl chain includes from about 4 to about 30 carbon atoms,
sulfosuccinates,
phosphates, phosphonates, phospholipids, ethoxylated compounds, carboxylates,
sulfonates
and sulfates, polyglycol ethers, amines, salts of acrylic acid, pyrophosphate
and mixtures
thereof. In one or more exemplary embodiments, the surfactant is a
polysorbate, such as
Tweenfm 20 (PEG(20) sorbitan monolaurate).
[0065] The clean proppant via line 306 can contact the conditioning
solution in the
16
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
pretreatment unit 308 under any suitable conditions to provide a conditioned
proppant via
line 310. Suitable conditions can include a temperature of about 10 'C, about
25 C, about 30
C, about 35 C, about 40 C, about 45 C to about 47 C, about 50 C, about 55 C ,
about
60 C, about 75 C, or about 100 C under a residence time of about 1 second
(s), about 5 s,
about 15 s, about 25 s, about 45 s, or about 55 s to about 65 s, about 75 s,
about 100 s, about 2
minutes (min), about 5 min, or about 10 min. The conditioning solution can
have a pH of at
least about 7.2, at least about 8, at least about 8.5, at least about 9, at
least about 10, at least
about 11, at least about 12, at least about 12.5, or at least about 13.
[0066] The conditioned proppant via line 310 can be withdrawn from the
pretreatment
unit 308 and introduced to one or more turbidity reduction units 312 where the
conditioned
proppant via line 310 can be contacted with a second washing solution to
further remove dust
and/or fines to provide a washed proppant via line 314 having a reduced
turbidity compared
to the conditioned proppant via line 310. The turbidity reduction unit 312 can
be or include
one or more tanks, one or more vessels, one or more conveyance systems, one or
more
conduits, or the like. The second washing solution can be the same as or
similar to the first
washing solution and can include an aqueous solution, such as water, or an
organic phase
solution, such as a liquid hydrocarbon. The second washing solution can also
have a
sensitizer which aids the activator in the subsequent step. The sensitizer can
be any agent
that reduces the activator, such as tin chloride, sodium borohydride or sodium
hypophosphite
or any other known reducing agent. In one or more exemplary embodiments, the
second
washing solution does not contain the sensitizer. The sensitizer step would be
followed by
another rinse step, but in some embodiments may be omitted.
[0067] Washed proppant via line 314 can be withdrawn from the turbidity
reduction unit
312 and introduced to one or more catalyst reduction units 316 where the
washed proppant
via line 314 can be contacted with an activation solution. The activation
solution can activate
the proppant by attaching catalytically active material, such as palladium or
silver, to the
proppant surface. The activation solution can be or include one or more
palladium salts, such
as palladium chloride or palladium ammonium chloride, and/or silver nitrate.
The activation
solution can be an aqueous phase solution or an organic phase solution. The
activation
solution can have a palladium salt concentration of about 0.1 milligrams of
Pd2+ per liter
(mg/1), about 0.5 mg/1, about 1 mg/1, about 5 mg/1, about 10 mg/1, or about 20
mg/1 to about
30 mg/1, about 35 mg/1, about 40 mg/1, about 50 mg/1, or about 100 mg/l. The
activation
solution can also contain a reducing agent, or sensitizer. The reducing agent
can be or
17
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
include a tin salt, such as stannous chloride. In one or more exemplary
embodiments, the
activation solution does not contain the reducing agent.
[0068] The
washed proppant via line 314 can contact the activation solution in the
catalyst reduction unit 316 under any suitable conditions to provide an
activated proppant via
line 318. Suitable conditions can include a temperature of about 20 C, about
35 C, about 50
C, about 65 C, about 75 C, about 78 C to about 82 C , about 85 'C, about 90
'C, about 95
'C, about 100 'C, or about 105 C under a residence time of about 1 min, about
2 min, about
3 min, about 4 min, about 5 min, or about 7 min to about 8 min, about 9 min,
about 10 min,
about 12 min, about 15 min, or about 20 min or more and/or until the bath is
substantially
exhausted. The activation solution can have a pH of about 7.1, about 7.2,
about 7.4, about
7.6, or about 7.8 to about 8, about 8.5, about 9, about 9.5, about 10, about
11, about 12, or
about 13 or more.
[0069] The
activated proppant via line 318 can be withdrawn from the activation unit 316
and introduced to one or more rinse units 320 where the activated proppant via
line 318 can
be contacted with a third washing solution to remove excess activation
solution from the
activated proppant. The rinse unit 320 can be or include one or more tanks,
one or more
vessels, one or more conveyance systems, one or more conduits, or the like.
The third
washing solution can include an aqueous solution, such as tap water or de-
ionized water.
[0070] Rinsed
proppant via line 322 can be withdrawn from the rinse unit 320 and
introduced to one more metallization units 324 where the rinsed proppant via
line 322 can be
subjected to metal plating. In the metallization unit 324, the rinsed proppant
via line 322 can
be immersed in a plating bath solution having a temperature of about 20 'C,
about 35 'C,
about 50 'C, about 60 'C, or about 70 'C to about 75 about 80
'C, about 90 'C, about 95
C, about 100 C, about 110 C, or about 120 C or more under a residence time of
about 1
min, about 2 min, about 4 min, about 8 min, about 12 min, or about 14 min to
about 16 min,
about 20 min, about 25 min, about 30 min, about 45 min, or about 60 min or
more and/or
until the bath is substantially exhausted. After immersion, a film of
electrically conductive
material ranging from about 10 nanometers (nm), about 50 nm, about 100 nm,
about 250 nm,
or about 400 nm to about 500 nm, about 600 nm, about 700 nm, about 800 nm,
about 900 nm,
about 1,000 nm, or about 1,200 nm or more can be non-uniformly coated onto the
rinsed
proppant to provide the electrically conductive proppant such as the
electrically conductive
proppant 100 depicted in FIG. 1.
[0071] The
plating bath solution can be an aqueous solution containing water or an
18
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
organic phase solution containing one or more hydrocarbons. The plating bath
solution can
be basic or acidic and can include a metal salt, a complexing agent, a
reducing agent, and a
buffer. For example, the plating bath solution can include a salt of nickel
such as nickel
sulfate, nickel sulphate hexahydrate, and nickel chloride. The complexing
agent can include
acetate, succinate, aminoacetate, malonate, pyrophosphate, malate, or citrate
or any
combination thereof. The reducing agent can include sodium borohydride,
dimethylamine
borane, or hydrazine or any combination thereof. The buffer can include acetic
acid,
propionic acid, glutaric acid, succinic acid, or adipic acid or any
combination thereof. Those
of ordinary skill in the art will understand that any conventional electroless
nickel, copper,
silver or gold plating bath solution can also be utilized such as those that
are commercially
available from suppliers such as Uyemura, Transene, Caswell, and Metal-Chem.
[0072] Additional and/or alternative steps can be employed in the
electroless plating
process. Referring now to Figure 4, a flow chart is depicted showing steps of
a process 400
for electroless coating of the electrically conductive material onto proppant
in which
alternative activation and metal deposition steps are depicted. Proppant
particles can be
subjected to alkaline conditioning 401, which can be the same as or similar to
the alkaline
conditioning in the pretreatment unit 308, to provide conditioned proppant
particles.
[0073] After being subjected to the alkaline conditioning step 401, the
conditioned
proppant particles can be subjected to an activation step 402 prior to
electroless metal
deposition 403. The conditioned particles can be sensitized using a sensitizer
solution of
tin(II) 404 to produce sensitized particles. After subsequent exposure to
palladium(II)
activation solution 405, palladium(II) is reduced to palladium metal (Pd2+ ->
Pd ) on the
surface of the sensitized particles and tin(II) is oxidized to tin(IV) (Sn2+ -
> Sn4+). An
accelerator solution 406 can be used to remove oxidized tin(IV) after exposure
to
palladium(II) activation solution 405 and prior to electroless metal
deposition 403
Alternative embodiments involve a combined tin(IV) and palladium(II) activator
and
sensitizer colloidal suspension 407 which can be followed by the accelerator
solution 406.
The accelerator solution 406 can be an aqueous solution and can include one or
more
accelerator agents including, but not limited to, one or more organic sulfide
compounds, such
as bis(sodium-sulfopropyl)disulfide, 3-mercapto-1-propanesulfonic acid sodium
salt, N,N-
dimethyl-dithiocarbamyl propylsulfonic acid sodium salt or 3-S-isothiuronium
propyl
sulfonate, and mixtures thereof. Other suitable accelerator agents can
include, but are not
limited to, thiourea, allylthiourea, acetylthiourea, and pyridine and the
like.
19
WO 2016/168719 PCT/US2016/027917
[0074] In certain embodiments, specific to proppant particle surfaces, the
alkaline
conditioning can enable activation using only the Pd activator as shown in
step 408. The
conditioned particles are activated using a solution of any suitable palladium
salt, such as
palladium chloride or palladium ammonium chloride, in a concentration of from
about 0.1,
about 0.5, about 1, about 5, about 10, about 15 or about 20 to about 25, about
30, about 35,
about 40, or about 50 or more milligrams Pd2+ per liter, where the pH of the
solution can be
adjusted between 7 and 14 using any suitable bases such as, for example,
sodium hydroxide.
[0075] In one or more exemplary embodiments, intrinsic surface activation
409 can be
accomplished prior to electroless metal deposition 403. In this embodiment,
iron or any other
suitable metal ion incorporated into the proppant particles during firing or
sintering that are
expressed at the surface of the proppant, can serve to directly activate the
particles. In one or
more exemplary embodiments, the surface of the particles is activated by
soaking the
particles in a reducing agent solution, such as sodium borohydride, sodium
hypophosphite or
sodium cyanoborohydride, where this solution can be transferred directly to
the electroless
plating bath with the particles still wet from the solution, or dried onto the
particles prior to
electroless metal plating 403, or rinsed completely from the particles.
[0076] Ceramic proppant particles can contain a significant amount of
oxidized iron. In
one or more exemplary embodiments of intrinsic surface activation 409, these
iron moieties
can be reduced to elemental iron, or other reduced foun [iron (II)] which is
catalytically
active to copper, nickel and other noble metal electroless plating solutions.
By utilizing the
native iron content intrinsic to the particle, it is possible to plate onto
the particles without Pd
activators. The reduction of surface iron ions to atomic iron can occur within
a sintering
device, or subsequent to sintering, by maintaining a reducing environment in
the kiln, which
is characterized by the presence of carbon monoxide or other products of
partial combustion.
Iron on the surface of the proppant particles can also be reduced after
manufacturing by
exposing the surfaces of the proppant particles to carbon monoxide or hydrogen
at any
suitable temperatures such as, for example, about 200 C, about 300 C, about
400 C, about
500 C, or about 600 C to about 750 C, about 900 C, about 1,100 C, or about
1,500 C.
[0077] After particle activation 402, activated proppant 410 can be
converted into
electrically conductive proppant 411 by electroless metal deposition 403.
Processes for
electrolytic and electroless coating are well-known to those of ordinary skill
in the art. See,
for example, U.S. Patent No. 3,556,839.
According to several exemplary embodiments, and in accordance with
Date Recue/Date Received 2021-04-15
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
conventional autocatalytic or electroless plating methods, the activated
proppant sample can
be coated with metal and metal alloys by various methods.
[0078] After
activation 402, the substrate can be immersed in, submerged in, or otherwise
contacted with a plating bath of the electroless metal deposition 403 to
provide the
electrically conductive proppant 411. The plating bath can be heated to a
temperature of
from about 35 C, about 45 C, about 55 C, about 65 C, or about 75 C to
about 85 , about
95 C, about 105 C, or about 120 C or more. In one or more embodiments, the
plating bath
can be or include an acidic, nickel-containing bath with a high phosphorous
content (about 5
wt% to about 12 wt% phosphorous by weight of the resulting nickel-phosphorous
alloy film)
412. The high phosphorous content bath can include, for example, an aqueous
solution
containing a salt of nickel and a phosphorous-containing reducing agent such
as sodium
hypophosphite in the presence of salts such as sodium citrate and sodium
acetate. The pH of
the high phosphorous content bath solution can be from about 2, about 3, about
3.5, about 4,
or about 4.5 to about 5, about 5.5, about 6, or about 6.5.
[0079] In one
or more embodiments, the plating bath can be an alkaline, nickel-
containing bath 413 with a low phosphorous content (about > 1 wt% to about 4.9
wt%
phosphorous by weight of the resulting nickel-phosphorous alloy film). The pH
of the
alkaline plating bath 313 with a low phosphorous content can be from about 7,
about 7.5,
about 8, about 8.5, or about 9 to about 10, about 10.5, about 11, about 12, or
about 13 or
more. The alkaline plating bath 413 can chelate free nickel ions to prevent
solution reactivity
with Pd, as can occur with Pd solution drag out, and therefore offer a
preferred reaction
environment for high surface area materials such as ceramic proppant. Those of
ordinary
skill in the art will understand that any conventional electroless nickel,
copper, silver or gold
plating bath solution may be utilized with any range of pH such as those that
are
commercially available from suppliers such as Metal-Chem, Enthone, Uyemura,
Transene or
Caswell. In one or more exemplary embodiments, the plating bath can be or
include alkaline
electroless copper 414 containing formaldehyde as a reducing agent. In one or
more
exemplary embodiments, the plating bath can include electroless noble metals
415, such as
silver, gold, and platinum. For example, the plating bath can be or include a
silver nitrate
solution.
[0080] The
electrically conductive material can also be incorporated into a resin
material.
Ceramic proppant or natural sands can be coated with the resin material
containing the
electrically conductive material such as metal clusters, metal flake, metal
shot, metal powder,
21
WO 2016/168719 PCT/US2016/027917
metalloids, metal nanoparticles, quantum dots, carbon nanotubes,
buckminsterfullerenes, and
other suitable electrically conductive materials to provide electrically
conductive material-
containing proppant that can be detected by electromagnetic means. Processes
for resin
coating proppants and natural sands are well known to those of ordinary skill
in the art. For
example, a suitable solvent coating process is described in U.S. Patent No.
3,929,191, to
Graham et al. Another suitable process such as that described in U.S. Patent
No. 3,492,147
to Young et al., involves the coating of a particulate substrate with a
liquid, uncatalyzed
resin composition characterized by its ability to extract a catalyst or curing
agent from a
non-aqueous solution. Also, a suitable hot melt coating procedure for
utilizing
phenol-formaldehyde novolac resins is described in U.S. Patent No. 4,585,064,
to Graham et
al. Those of ordinary skill in the art will be familiar with still other
suitable methods for
resin coating proppants and natural sands.
[0081]
According to certain embodiments of the present invention, the electrically
conductive material is incorporated into a resin material and ceramic proppant
or natural
sands are coated with the resin material containing the electrically
conductive material.
Processes for resin coating proppants and natural sands are well known to
those of ordinary
skill in the art. For example, a suitable solvent coating process is described
in U.S. Patent
No. 3,929,191, to Graham et al. Another suitable process such as that
described
in U.S. Patent No. 3,492,147 to Young et al., involves the coating of a
particle
substrate with a liquid, uncatalyzed resin composition characterized by its
ability to extract a
catalyst or curing agent from a non-aqueous solution. Also a suitable hot melt
coating
procedure for utilizing phenol-formaldehyde novolac resins is described in
U.S. Patent No.
4,585,064, to Graham et al. Those of ordinary skill in the art will be
familiar with still other
suitable methods for resin coating proppants and natural sands.
[0082]
According to several exemplary embodiments, the proppants disclosed herein are
coated with a resin material to provide resin coated proppant particulates.
According to
several exemplary embodiments, the electrically conductive material can be
mixed with the
resin material and coated onto the proppants to provide the resin coated
proppant particulates.
According to several exemplary embodiments, at least a portion of the surface
area of each of
22
Date Recue/Date Received 2021-04-15
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
the resin coated proppant particulates is covered with the resin material.
According to several
exemplary embodiments, at least about 10%, at least about 25%, at least about
50%, at least
about 75%, less than 90%, less than 95%, or less than 99% of the surface area
of the resin
coated proppant particulates is covered with the resin material. According to
several
exemplary embodiments, about 40% to about 90%, about 25% to about 80%, or
about 10% to
about 50% of the surface area of the resin coated proppant particulates is
covered with the
resin material. According to several exemplary embodiments, the entire surface
area of the
resin coated proppant particulates is covered with the resin material. For
example, the resin
coated proppant particulates can be encapsulated with the resin material.
[0083] According to several exemplary embodiments, the resin material is
present on the
resin coated proppant particulates in any suitable amount. According to
several exemplary
embodiments, the resin coated proppant particulates contain at least about 0.1
wt% resin, at
least about 0.5 wt% resin, at least about 1 wt% resin, at least about 2 wt%
resin, at least about
4 wt% resin, at least about 6 wt% resin, at least about 10 wt% resin, or at
least about 20 wt%
resin, based on the total weight of the resin coated proppant particulates.
According to
several exemplary embodiments, the resin coated proppant particulates contain
about 0.01
wt%, about 0.2 wt%, about 0.8 wt%, about 1.5 wt%, about 2.5 wt%, about 3.5
wt%, or about
wt% to about 8 wt%, about 15 wt%, about 30 wt%, about 50 wt%, or about 80 wt%
resin,
based on the total weight of the resin coated proppant particulates.
[0084] According to several exemplary embodiments, the resin material
includes any
suitable resin. For example, the resin material can include a phenolic resin,
such as a phenol-
formaldehyde resin. According to several exemplary embodiments, the phenol-
formaldehyde
resin has a molar ratio of formaldehyde to phenol (F:P) from a low of about
0.6:1, about
0.9:1, or about 1.2:1 to a high of about 1.9:1, about 2.1:1, about 2.3:1, or
about 2.8:1. For
example, the phenol-formaldehyde resin can have a molar ratio of formaldehyde
to phenol of
about 0.7:1 to about 2.7:1, about 0.8:1 to about 2.5:1, about 1:1 to about
2.4:1, about 1.1:1 to
about 2.6:1, or about 1.3:1 to about 2:1. The phenol-formaldehyde resin can
also have a
molar ratio of formaldehyde to phenol of about 0.8:1 to about 0.9:1, about
0.9:1 to about 1:1,
about 1:1 to about 1.1:1, about 1.1:1 to about 1.2:1, about 1.2:1 to about
1.3:1, or about 1.3:1
to about 1.4:1.
[0085] According to several exemplary embodiments, the phenol-formaldehyde
resin has
a molar ratio of less than 1:1, less than 0.9:1, less than 0.8:1, less than
0.7:1, less than 0.6:1,
or less than 0.5:1. For example, the phenol-formaldehyde resin can be or
include a phenolic
23
WO 2016/168719 PCT/US2016/027917
novolac resin. Phenolic novolac resins are well known to those of ordinary
skill in the art, for
instance see U.S. Patent No. 2,675,335 to Rankin, U.S. Patent No. 4,179,429 to
Hanauye,
U.S. Patent No. 5,218,038 to Johnson, and U.S. Patent No. 8,399,597 to
Pullichola . Suitable
examples of commercially available novolac resins include novolac resins
available from
PlencoTM, Durite resins available from Momentive, and novolac resins
available from S.I. Group.
[0086] The electrically conductive material can also be in the form of
particles and/or
nanoparticles that are separate and distinct from the proppant particles prior
to injection into
the formation. The electrically conductive particles can be or include
pyrolytic carbon,
carbon black, graphite, coke breeze, carbon fiber, or carbon nanotubes or any
mixture or
combination thereof. The electrically conductive particles can be or include
any suitable
magnetic material. In one or more exemplary embodiments, the electrically
conductive
particles can be or include any suitable metallic and/or non-metallic
material. The
electrically conductive particles can be or include any metal selected from
Groups 3-12 of the
Periodic Table or any oxides thereof. For example, the electrically conductive
particles can
be or include iron, cobalt, nickel, gadolinium, or oxides thereof, or any
combination or
mixture thereof. The electrically conductive particles can also be or include
ferromagnetic
particles. The electrically conductive particles can be or include aluminum,
boron, or carbon
or any combination or mixture thereof
[0087] The electrically conductive particles can survive or remain stable
under any
suitable downhole conditions. According to several exemplary embodiments, the
electrically
conductive particles are survivable under downhole conditions. According to
several
exemplary embodiments, the electrically conductive particles are survivable
under
temperatures of at least about 100 C, at least about 125 C, at least about 150
C, or at least
about 300 C. In one or more embodiments, the electrically conductive particles
are
survivable at temperatures of about 80 C, about 120 C, about 160 C, or about
200 C to about
250 C, about 300 C, about 350 C, or about 400 C. According to several
exemplary
embodiments, the electrically conductive particles do not degrade due to being
under
temperatures of at least about 100 C, at least about 125 C, at least about 150
C, or at least
about 300 C In one or more embodiments, the electrically conductive particles
do not
degrade due to being at temperatures of about 80 C, about 120 C, about 160 C,
or about
200 C to about 250 C, about 300 C, about 350 C, or about 400 C.
[0088] The electrically conductive particles can have any suitable size.
The electrically
24
Date Recue/Date Received 2021-04-15
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
conductive particles can have a size from about 1 nanometers (nm), about 5 nm,
about 10 nm,
about 50 nm, about 100 nm, or about 500 nm in their largest dimension. For
example, the
electrically conductive particles can be from about 2 nm to about 500 nm,
about 25 nm to
about 450 nm, about 150 nm to about 400, about 250 nm to about 350 nm, or
about 275 nm
to about 325 nm in their largest dimension. The electrically conductive
particles can be or
include nanoparticles. According to several exemplary embodiments, the
electrically
conductive particles are nanoparticles. In one or more exemplary embodiments,
the
nanoparticles are nanowires.
[0089] The electrically conductive particles can have a size of at least
about 100 mesh, at
least about 80 mesh, at least about 60 mesh, at least about 50 mesh, or at
least about 40 mesh
For example, the electrically conductive particles can have a size from about
115 mesh to
about 2 mesh, about 100 mesh to about 3 mesh, about 80 mesh to about 5 mesh,
about 80
mesh to about 10 mesh, about 60 mesh to about 12 mesh, about 50 mesh to about
14 mesh,
about 40 mesh to about 16 mesh, or about 35 mesh to about 18 mesh. In a
particular
embodiment, the electrically conductive particles have a size of from about 20
to about 40
U.S. Mesh.
[0090] The electrically conductive particles can be present in any suitable
amounts. For
example, the electrically conductive particles can be present in an
electrically conductive
particle to proppant weight ratio of about 1:1,000, about 1:1,000, about
1:500, about 1:200,
about 1:100, or about 1:50 to about 1:25, about 1:20, about 1:15, about 1:10,
about 1:8, about
1:4, about 1:3, about 1:2, or about 1:1 or more.
[0091] In one or more exemplary embodiments, the electrically conductive
particles are
treated and/or coated with one or more chemicals or ligands to impart surface
functionality to
the electrically conductive particles. These coatings can be selected from
organic compound
containing materials and/or organic compounds of varying chain lengths, each
having
functional groups on the terminus of their respective chains to modify or
tailor the affinity of
the electrically conductive particles with surface functionality of the
proppant. Many
commercially available surfactants can be used for this purpose. Ligands that
are multi-
functional can also be used as a coating, with one end of the ligand molecule
binding to at
least a portion of the electrically conductive particle and the other end of
the ligand molecule
affecting the dispersibility of the functionalized nanoparticle in the desired
organic or
aqueous solvent or carrier fluid so that the electrically conductive particle
are not completely
"soluble" in the carrier fluid or production fluid to cause the electrically
conductive particles
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
to have a tendency to settle and remain on proppant surfaces and not be mixed
with and thus
carried away with any produced fluids. These multi-functional ligands can be
modified by
traditional organic synthetic methods and principles to decrease solubility of
the electrically
conductive particles in organic and/or aqueous reservoir fluids, which can
affect the settling
rate of the electrically conductive particles. The property of the terminus
groups on the
multi-functional ligand molecule can also affect the solubility of the
electrically conductive
particle in the fluid. Examples of the types of functional groups that can be
used are
carboxylates, amines, thiols, polysiloxanes, silanes, alcohols, and other
species capable of
binding to the electrically conductive particle and providing binding affinity
to the proppant
surface. In one or more exemplary embodiments, the electrically conductive
particles can
adhere to and/or coat the proppant particles after the electrically conductive
particles
encounter the proppant in a downhole environment, for example a subterranean
fracture. In
one or more exemplary embodiments, the particles can self-accumulate in the
downhole
environment or subterranean fracture. For example, the electrically conductive
particles can
have a modified surface functionalization that encourages or causes the
electrically
conductive particles to adhere or bind to one another in groups or clusters in
a downhole or
subterranean environment. In one or more exemplary embodiments, the
electrically
conductive particles can bind to one another to form clusters of electrically
conductive
particles having a size that can be the same as or similar to the size of the
proppant.
[0092] The electromagnetic methods described herein can include
electrically energizing
the earth at or near a fracture at depth and measuring the electric and
magnetic responses at
the earth's surface or in adjacent wells/boreholes. The electromagnetic
methods described
herein can include energizing the earth in the fractured well/borehole or in a
well/borehole
adjacent to the fractured well/borehole. The electromagnetic methods described
herein are
can be used in connection with a cased wellbore, such as well 20 shown in FIG.
5, or in an
uncased wellbore (not shown). As shown in FIG. 5, casing 22 extends within
well 20 and
well 20 extends through geological strata 24a-24i in a manner that has three
dimensional
components.
[0093] Referring now to FIG. 6, a partial cutaway view is shown with
production well 20
extending vertically downward through one or more geological layers 24a-24i
and
horizontally in layer 24i. While wells are conventionally vertical, the
electromagnetic
methods described herein are not limited to use with vertical wells. Thus, the
terms
"vertical" and "horizontal" are used in a general sense in their reference to
wells of various
26
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
orientations.
[0094] The preparation of production well 20 for hydraulic fracturing can
include drilling
a bore 26 to a desired depth and then in some cases extending the bore 26
horizontally so that
the bore 26 has any desired degree of vertical and horizontal components. A
casing 22 can be
cemented 28 into well 20 to seal the bore 26 from the geological layers 24a-
24i in FIG. 6.
The casing 22 can have a plurality of perforations 30 and/or sliding sleeves
(not shown). The
perforations 30 are shown in FIG. 6 as being located in a horizontal portion
of well 20 but
those of ordinary skill in the art will recognize that the perforations can be
located at any
desired depth or horizontal distance along the bore 26, but are typically at
the location of a
hydrocarbon bearing zone in the geological layers 24, which may be within one
or more of
the geological layers 24a-24j. Those of ordinary skill in the art will also
recognize that the
well 20 can include no casing, such as in the case of an open-hole well. The
hydrocarbon
bearing zone may contain oil and/or gas, as well as other fluids and materials
that have fluid-
like properties. The hydrocarbon bearing zone in geological layers 24a-24j is
hydraulically
fractured by pumping a fluid into casing 22 and through perforations 30 at
sufficient rates and
pressures to create fractures 32 and then incorporating into the fluid an
electrically
conductive proppant which will prop open the created fractures 32 when the
hydraulic
pressure used to create the fractures 32 is released.
[0095] The hydraulic fractures 32 shown in FIG. 6 are oriented radially
away from the
metallic well casing 22. This orientation is exemplary in nature. In practice,
hydraulically-
induced fractures 32 may be oriented radially as in FIG. 6, laterally or
intermediate between
the two. Various orientations are exemplary and not intended to restrict or
limit the
electromagnetic methods described herein in any way.
[0096] The electrically conductive proppant can be introduced into one or
more
subterranean fractures during any suitable hydraulic fracturing operation to
provide an
electrically conductive proppant pack. The electrically conductive proppant
pack can include
the electrically conductive proppant pack 200 depicted in FIG. 2. In one or
more exemplary
hydraulic fracturing operations, any combination of the electrically
conductive proppant and
a non-electrically conductive proppant can be introduced into one or more
fractures to
provide an electrically conductive proppant pack. The electrically conductive
proppant of the
electrically conductive proppant pack can include a non-uniform coating of
electrically
conductive material as disclosed herein and/or a substantially uniform coating
of electrically
conductive material.
27
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
[0097] The
substantially uniform coating of electrically conductive material can have any
suitable thickness. In one or more exemplary embodiments, the substantially
uniform coating
of electrically conductive material can have a thickness of about 5 nm, about
10 nm, about 25
nm, about 50 nm, about 100 nm, or about 200 nm to about 300 nm, about 400 nm,
about 500
nm, about 750 nm, about 1,000 about 1,500 nm, about 2,000 nm, or about 5,000
nm. For
example, the thickness of the substantially uniform coating of electrically
conductive material
can be from about 400 nm to about 1,000 nm, from about 200 nm to about 600 nm,
or from
about 100 nm to about 400 nm.
[0098] The
electrically conductive proppant pack can include non-electrically conductive
proppant in any suitable amounts. The non-electrically conductive proppant can
have any
suitable resistivity. For example, the non-electrically conductive proppant
can have a
resistivity of at least about 1 x 105 Ohm-cm, at least about 1 x 108 Ohm-cm,
at least about 1 x
1010 Ohm-cm, at least about 1 x 1011 Ohm-cm, or at least about 1 x 1012 Ohm-
cm. The
electrically conductive proppant pack can include any suitable amount of non-
electrically
conductive proppant. In one or more exemplary embodiments, the electrically
conductive
proppant pack can include at least about 1 wt%, at least about 5 wt%, at least
about 10 wt%,
at least about 20 wt%, at least about 40 wt%, at least about 50 wt%, at least
about 60 wt%, at
least about 70 wto, at least about 80 wt%, at least about 90 wt%, or at least
about 95 wt%
non-electrically conductive proppant. In one or more exemplary embodiments,
the
electrically conductive proppant pack can include at least about 1 wt%, at
least about 5 wt%,
at least about 10 wt%, at least about 20 wt%, at least about 40 wt%, at least
about 50 wt%, at
least about 60 wt%, at least about 70 wt%, at least about 80 wt%, at least
about 90 wt%, or at
least about 95 wt% electrically conductive proppant In one
or more exemplary
embodiments, the electrically conductive proppant pack can have an
electrically conductive
proppant concentration of about 2 wt%, about 4 wt?/o, about 8 wt%, about 12
wt%, about 25
wt%, about 35 wt%, or about 45 wt% to about 55 wt%, about 65 wt%, about 75
wt%, about
85 wt%, or about 95 wt% based on the total weight of the proppant pack. In one
or more
exemplary embodiments, the electrically conductive proppant pack can include
from about 1
wt% to about 10 wt%, from about 10 wt% to about 25 wt%, about 25 wt% to about
50 wt%,
from about 50 wt% to about 75 wt%, or from about 75 wt% to about 99 wt% non-
electrically
conductive proppant.
[0099] The non-
electrically conductive proppant can be dispersed throughout the
electrically conductive proppant pack in any suitable manner. For example, the
non-
28
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
electrically conductive proppant can be substantially evenly dispersed
throughout the
electrically conductive proppant pack. In one or more exemplary embodiments,
the proppant
pack has a minimum threshold concentration of electrically conductive proppant
needed to
make the proppant pack electrically conductive when the non-electrically
conductive
proppant are substantially evenly dispersed throughout the proppant pack. The
minimum
threshold concentration of electrically conductive proppant can be least about
20%, at least
about 25%, at least about 300/, or at least about 35%, based on the total
number of proppant
particles in the proppant pack. In one or more exemplary embodiments, the
minimum
threshold concentration of electrically conductive proppant can be from about
20% to about
40%, from about 25% to about 35%, from about 28% to about 32%, or from about
30% to
about 33%, based on the total number of proppant particles in the proppant
pack.
[00100] The electrically conductive proppant pack containing the non-
conductive proppant
can have any suitable resistivity. In one or more exemplary embodiments, the
electrically
conductive proppant pack containing at least about 20 wt%, at least about 40
wt%, at least
about 50 wt?/o, or at least about 60 wt% non-conductive proppant can have a
resistivity of less
than 1,000 Ohm-cm, less than 500 Ohm-cm, less than 200 Ohm-cm, less than 100
Ohm-cm,
less than 80 Ohm-cm, less than 50 Ohm-cm, less than 25 Ohm-cm, less than 15
Ohm-cm, less
than 5 Ohm-cm, less than 2 Ohm-cm, less than 1 Ohm-cm, less than 0.5 Ohm-cm,
or less
than 0.1 Ohm-cm. The electrically conductive proppant pack containing the non-
conductive
proppant can have any suitable electrical conductivity. In one or more
exemplary
embodiments, the electrically conductive proppant pack containing at least
about 20 wt?/o, at
least about 40 wt%, at least about 50 wt%, or at least about 60 wt% non-
conductive proppant
can have an electrical conductivity of at least about 0.1 Siemen-m, at least
about 0,5 Siemen-
m, at least about 1 Siemen-m, at least about 5 Siemen-m, at least about 15
Siemen-m, at least
about 50 Siemen-m, at least about 100 Siemen-m, at least about 250 Siemen-m,
at least about
500 Siemen-m, at least about 750 Siemen-m, at least about 1,000 Siemen-m, at
least about
1,500 Siemen-m, or at least about 2,000 Siemen-m.
[00101] According to certain embodiments of the electromagnetic method of the
present
invention and as shown schematically in FIG. 7, electric current is carried
down wellbore 20
to an energizing point which will generally be located within 10 meters or
more (above or
below) of perforations 30 in casing 22 via a seven strand wire line insulated
cable 34, such as
those which are well known to those of ordinary skill in the art and are
widely commercially
available from Camesa Wire, Rochester Wire and Cable, Inc., WireLine Works,
Novametal
29
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
Group, and Quality Wireline & Cable Inc. In other exemplary embodiments, the
wire line
insulated cable 34 can contain 1 to 6 strands or 8 or more strands. A sinker
bar 36 connected
to the wire line cable 34 contacts or is in close proximity to the well casing
22 whereupon the
well casing 22 becomes a current line source that produces subsurface electric
and magnetic
fields. In other exemplary embodiments, the wire line cable 34 can be
connected to or
otherwise attached to a centralizer and/or any other suitable downhole tool in
addition to or in
lieu of the sinker bar 26. These fields interact with the fracture 32
containing electrically
conductive proppant to produce secondary electric and magnetic fields that
will be used to
detect, locate, and characterize the proppant-filled fracture 32.
[00102] According to certain embodiments of the electromagnetic method of the
present
invention and as shown schematically in FIG. 7, a power control box 40 is
connected to
casing 22 by a cable 42 so that electric current is injected into the fracture
well 20 by directly
energizing the casing 22 at the well head or any other suitable surface
location. In one
embodiment, the power control box 40 is connected wirelessly by a
receiver/transmitter 43 to
a receiver/transmitter 39 on equipment truck 41. Those of ordinary skill in
the art will
recognize that other suitable means of carrying the current to the energizing
point may also
be employed.
[00103] As shown schematically in FIGS. 7-9, a plurality of electric and
magnetic field
sensors 38 will be located on the earth's surface in a rectangular or other
suitable array
covering the area around the fracture well 20 and above the anticipated
fracture 32. In one
embodiment, the sensors 38 are connected wirelessly to a receiver/transmitter
39 on
equipment truck 41. The maximum dimension of the array (aperture) in general
should be at
least 80 percent of the depth to the fracture zone The sensors 38 will measure
the x, y and z
component responses of the electric and magnetic fields. It is these responses
that will be
used to infer location and characterization of the electrically conductive
proppant through
comparison to numerical simulations and/or inversion of the measured data to
determine the
source of the responses. The responses of the electric and magnetic field
components will
depend upon: the orientation of the fracture well 20, the orientation of the
fracture 32, the
electrical conductivity, magnetic permeability, and electric permittivity of
layers 24a-24j, the
electrical conductivity, magnetic permeability, and electric permittivity of
the proppant filled
fracture 32, and the volume of the proppant filled fracture 32. Moreover, the
electrical
conductivity, magnetic permeability and electric permittivity of the
geological layers residing
between the surface and the target formation layers 24a-24j influence the
recorded responses.
WO 2016/168719 PCT/US2016/027917
From the field-recorded responses, details of the proppant filled fracture 32
can be
determined.
[00104] In another embodiment, electric and/or magnetic sensors may be located
in
adjacent well/boreholes. The adjacent well/boreholes can be in one or more new
or pre-
existing hydrocarbon production wells, water production wells, and/or water
injection wells.
The new or pre-existing wells can be off-set from the fracture(s) by any
suitable distance.
For example, the wells can be off-set from the fracture(s) by about 0.5 meter
(m), about 1 m,
about 2 m, about 5 m to about 7 m, about 10 m, about 15 m, about 20 m, or
about 50 m or
more. In one or more exemplary embodiment the adjacent well/borehole can
bisect, intersect,
abut or otherwise be disposed proximate to a fracture. In one or more
exemplary
embodiments, the adjacent well/borehole can be drilled and/or placed before,
during or after
the fracture has been formed. The adjacent well/borehole can be a test well
that is drilled to
bisect, intersect, abut or otherwise be disposed proximate to a fracture.
[00105] Depending upon the conductivity of the earth surrounding the well
casing 22, the
current may or may not be uniform as the current flows back to the surface
along the well
casing 22. According to both embodiments shown in FIG. 7, current leakage
occurs along
wellbore 20 such as along path 50 or 52 and returns to the electrical ground
54 which is
established at the well head. As described in U.S. Patent Application No.
13/206,041 filed
August 9, 2011 and entitled "Simulating Current Flow Through a Well Casing and
an
Induced Fracture," the well
casing is represented as a leaky transmission line in data analysis and
numerical modeling.
Numerical simulations have shown that for a conducting earth (conductivity
greater than
approximately 0.05 siemens per meter (S/m)), the current will leak out into
the formation,
while if the conductivity is less than approximately 0.05 S/m the current will
be more-or-less
uniform along the well casing 22. As shown in FIGS. 10A and 10B, to localize
the current in
the well casing 22, electrically insulating pipe joints or pipe collars may be
installed.
According to the embodiment shown in FIG. 10A, an insulating joint may be
installed by
coating the mating surfaces 60 and 62 of the joint with a material 64 having a
high dielectric
strength, such as any one of the well-known and commercially available plastic
or resin
materials which have a high dielectric strength and which are of a tough and
flexible
character adapted to adhere to the joint surfaces so as to remain in place
between the joint
surfaces. As described in U.S. Patent No. 2,940,787,
such plastic or resin materials include epoxies, phenolics,
31
Date Recue/Date Received 2021-04-15
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
rubber compositions, and alkyds, and various combinations thereof. Additional
materials
include polyetherimide and modified polyphenylene oxide. According to the
embodiment
shown in FIG. 10B, the mating ends 70 and 72 of the joint are engaged with an
electrically
insulated casing collar 74. The transmission line representation is able to
handle various well
casing scenarios, such as vertical only, slant wells, vertical and horizontal
sections of casing,
and, single or multiple insulating gaps.
[00106] The detection, location, and characterization of the electrically
conductive
proppant in a fracture will depend upon several factors, including but not
limited to the net
electrical conductivity of the fracture, fracture volume, the electrical
conductivity, magnetic
permeability, and electric permittivity of the earth surrounding the fracture
and between the
fracture and surface mounted sensors. The net electrical conductivity of the
fracture means
the combination of the electrical conductivity of the fracture, the proppant
and the fluids
when all are placed in the earth minus the electrical conductivity of the
earth formation when
the fracture, proppant and fluids were not present. Also, the total electrical
conductivity of
the proppant filled fracture is the combination of the electrical conductivity
created by
making a fracture, plus the electrical conductivity of the new/modified
proppant plus the
electrical conductivity of the fluids, plus the electro-kinetic effects of
moving fluids through a
porous body such as a proppant pack. The volume of an overly simplified
fracture with the
geometric form of a plane may be determined by multiplying the height, length,
and width
(i.e. gap) of the fracture. A three dimensional (3D) finite-difference
electromagnetic
algorithm that solves Maxwell's equations of electromagnetism may be used for
numerical
simulations. In order for the electromagnetic response of a proppant filled
fracture at depth to
be detectable at the Earth's surface, the net fracture conductivity multiplied
by the fracture
volume within one computational cell of the finite difference (FD) grid must
be larger than
approximately 100 Sm2 for a Barnett shale-like model where the total fracture
volume is
approximately 38 m3. For the Barnett shale model, the depth of the fracture is
2000 m.
These requirements for the numerical simulations can be translated to
properties in a field
application for formations other than the Barnett shale.
[00107] The propagation and/or diffusion of electromagnetic (EM) wavefields
through
three-dimensional (3D) geological media are governed by Maxwell's equations of
electromagnetism.
[00108] According to one embodiment of the present invention, the measured
three
dimensional components of the electric and/or magnetic field responses may be
analyzed
32
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
with imaging methods such as an inversion algorithm based on Maxwell's
equations and
electromagnetic migration and/or holography to determine proppant pack
location. Inversion
of acquired data to determine proppant pack location involves adjusting the
earth model
parameters, including but not limited to the proppant location within a
fracture or fractures
and the net electrical conductivity of the fracture, to obtain the best fit to
forward model
calculations of responses for an assumed earth model. As described in Bartel,
L. C., Integral
wave-migration method applied to electromagnetic data, SEG Technical Program
Expanded
Abstracts, 1994, 361-364, the electromagnetic integral wave migration method
utilizes
Gauss's theorem where the data obtained over an aperture is projected into the
subsurface to
form an image of the proppant pack. Also, as described in Bartel, L. C.,
Application of EM
Holographic Methods to Borehole Vertical Electric Source Data to Map a Fuel
Oil Spill, SEG
Technical Program Expanded Abstracts, 1987, 49-51, the electromagnetic
holographic
method is based on the seismic holographic method and relies on constructive
and destructive
interferences where the data and the source wave form are projected into an
earth volume to
form an image of the proppant pack. Due to the long wave lengths of the low
frequency
electromagnetic responses for the migration and holographic methods, it may be
necessary to
transform the data into another domain where the wave lengths are shorter. As
described in
Lee, K.H., et al., A new approach to modeling the electromagnetic response of
conductive
media, Geophysics, Vol. 54, No. 9 (1989), this domain is referred to as the q-
domain.
Further, as described in Lee, K.H., et al., Tomographic Imaging of Electrical
Conductivity
Using Low-Frequency Electromagnetic Fields, Lawrence Berkeley Lab, 1992, the
wave
length changes when the transformation is applied.
[00109] Also, combining Maxwell's equations of electromagnetism with
constitutive
relations appropriate for time-independent isotropic media yields a system of
six coupled
first-order partial differential equations referred to as the "EH" system. The
name derives
from the dependent variables contained therein, namely the electric vector E
and the magnetic
vector H. Coefficients in the EH system are the three material properties,
namely electrical
current conductivity, magnetic permeability, and electric permittivity. All
of these
parameters may vary with 3D spatial position. The inhomogeneous terms in the
EH system
represent various body sources of electromagnetic waves, and include
conduction current
sources, magnetic induction sources, and displacement current sources.
Conduction current
sources, representing current flow in wires, cables, and borehole casings, are
the most
commonly-used sources in field electromagnetic data acquisition experiments.
33
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
[001101 In one or more exemplary embodiments, an explicit, time-domain, finite-
difference (TDFD) numerical method is used to solve the EH system for the
three
components of the electric vector E and the three components of the magnetic
vector H, as
functions of position and time. A three-dimensional gridded representation
of the
electromagnetic medium parameters, referred to as the "earth model" is
required, and may be
constructed from available geophysical logs and geological information. A
magnitude,
direction, and waveform for the current source are also input to the
algorithm. The waveform
may have a pulse-like shape (as in a Gaussian pulse), or may be a repeating
square wave
containing both positive and negative polarity portions, but is not limited to
these two
particular options. Execution of the numerical algorithm generates
electromagnetic responses
in the form of time series recorded at receiver locations distributed on or
within the gridded
earth model. These responses represent the three components of the E or H
vector, or their
time-derivatives.
[00111] Repeated execution of the finite-difference numerical algorithm
enables a
quantitative estimate of the magnitude and frequency-content of
electromagnetic responses
(measured on the earth's surface or in nearby boreholes) to be made as
important modeling
parameters are varied. For example, the depth of current source may be changed
from
shallow to deep. The current source may be localized at a point, or may be a
spatially-
extended transmission line, as with an electrically charged borehole casing.
The source
waveform may be broad-band or narrow-band in spectral content. Finally,
changes to the
electromagnetic earth model can be made, perhaps to assess the shielding
effect of shallow
conductive layers. The goal of such a modeling campaign is to assess the
sensitivity of
recorded electromagnetic data to variations in pertinent parameters. In turn,
this information
is used to design optimal field data acquisition geometries that have enhanced
potential for
imaging a proppant-filled fracture at depth.
[00112] The electric and magnetic responses are scalable with the input
current magnitude.
In order to obtain responses above the background electromagnetic noise, a
large current on
the order of 10 to 100 amps may be required. The impedance of the electric
cable to the
current contact point and the earth contact resistance will determine the
voltage that is
required to obtain a desired current. The contact resistance is expected to be
small and will
not dominate the required voltage. In addition, it may be necessary to sum
many repetitions
of the measured data to obtain a measurable signal level over the noise level.
In the field
application and modeling scenarios, a time-domain current source waveform may
be used. A
34
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
typical time-domain waveform consists of an on time of positive current
followed by an off
time followed by an on time of negative current. In other words, + current,
then off, then ¨
current, then off again. The repetition rate to be used would be determined by
how long the
current has to be on until a steady-state is reached or alternatively how long
the energizing
current has to be off until the fields have died to nearly zero. In this
exemplary method, the
measured responses would be analyzed using both the steady-state values and
the decaying
fields following the current shut-off. The advantage of analyzing the data
when the
energizing current is zero (decaying fields) is that the primary field
contribution (response
from the transmitting conductor; i.e., the well casing) has been eliminated
and only the earth
responses are measured. In addition, the off period of the time domain input
signal allows
analysis of the direct current electrical fields that may arise from electro-
kinetic effects,
including but not limited to, flowing fluids and proppant during the
fracturing process.
Fracture properties (orientation, length, volume, height and asymmetry will be
determined
through inversion of the measured data and/or a form of holographic
reconstruction of that
portion of the earth (fracture) that yielded the measured electrical responses
or secondary
fields. According to certain embodiments, a pre-fracture survey will be
prepared to isolate
the secondary fields due to the fracture. Those of ordinary skill in the art
will recognize that
other techniques for analyzing the recorded electromagnetic data, such as use
of a pulse-like
current source waveform and full waveform inversion of observed
electromagnetic data may
also be used.
[00113] In one or more exemplary embodiments, a frequency domain finite-
difference
(FDFD) numerical method is used to solve the EH system for the three
components of the
electric vector E and the three components of the magnetic vector H. The earth
model,
magnitude, direction, and waveform for the current source can be inputted to
the algorithm.
Similar to that of the TDFD numerical method, the waveform may have a pulse-
like shape (as
in a Gaussian pulse), or may be a repeating square wave containing both
positive and
negative polarity portions, but is not limited to these two particular
options. Execution of the
numerical algorithm generates electromagnetic responses in the form of
frequency series
recorded at receiver locations distributed on or within the gridded earth
model. These
responses represent the three components of the E or H vector, or their
frequency-derivatives.
[00114] In one or more exemplary embodiments, an induced polarization (IP)
effect is
used to determine a location of the proppant. The IP effect is present in the
time domain
where the effect is measured flowing the cessation of the driving electric
field. The IP effect
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
is also present in the frequency domain wherein the effect is explained in
terms of complex
impedance. For time domain measurements the received voltage decay as a
function of time
is made when the input current is off. The frequency domain measures the phase
delay from
the input current and the effects of frequency on the received voltage.
[00115] The IP effect arises from various causes and different dependencies on
the
frequency of an impressed electric field. Central to some of the theories is
fluid flow in
porous media. In a porous medium the earth material is generally slightly
negatively
charged, thereby attracting positive charged ions in the fluid that makes up
the electric double
layer (EDL). This leaves the fluid in the pore space somewhat rich in negative
charges that
now conduct current in a porous medium The ionic current is the difference in
the
concentrations of positive and negative ions. The flow of ions takes place due
to an
impressed electric field, pressure gradient, and/or diffusion where the pore
space available for
transport is restricted by the EDL. In addition, there are other restrictions
for flow (pore
throats, other material in the pore space) that can cause charge build up. A
metallic ore,
which is an electronic conductor, also affects the flow of the ions. Once the
forcing electric
field is switched off, the charge distribution "wants" to seek a lower energy
state, which is the
equilibrium condition. Diffusion of charges plays a major role in the quest to
obtain
equilibrium. In other words, when a surface is immersed or created in an
aqueous solution, a
discontinuity is formed at the interface where such physicochemical variables
as electric
potential and electrolyte concentration change significantly from the aqueous
phase to
another phase. Because of the different chemical potentials between the two
phases, charge
separation often occurs at the interfacial region. This interfacial region,
together with the
charged surface, is usually known as the EDL. This EDL, or layer, which can
extend as far
as 100 nm in a very dilute solution to only a few angstroms in a concentrated
solution, plays
an important role in electrochemistry, colloid science, and surface chemistry
(Devasenathipathy and Santiago, 2003; Kirby and Hasselbrink, 2004; Yang et
al., 2004).
[00116] Once the conducting proppant has been placed into the fracture(s) and
an electric
current is supplied to the well casing, the component of the electric field
perpendicular to the
direction of the fracture will generally be larger than the component parallel
to the fracture.
The component of the electric field parallel to the fracture will induce ionic
conductivity in
the fracture fluid that will be impeded due to the ion mobility in the
presence of the EDL and
the charges induced on the conductive proppant. In addition, there will be
electronic current
flow via electrically conductive proppant that are in contact with each other.
The current
36
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
flow perpendicular to the fracture will not depend appreciably on the ionic
flow but more on
electronic conduction via the metallic coated proppant particles. The
electronic conduction
of electrical current will depend on the volume of the metal present and will
rely on proppant
particles to be in contact with each other.
[00117] If the energizing current is on for a sufficient amount of time so
that the
movement of charges has reached a steady state in the presence of the applied
electric field,
then when the current is terminated and the applied electric field goes to
zero the charges
must redistribute themselves to come to an equilibrium charge distribution.
This
redistribution does not occur instantaneously, but involves several decay
mechanisms.
Membrane IP effects can occur along with the electrode polarization effect The
conductive
coatings present at or on the proppant surface can produce a significant IP
response through
the chargeability that is related to the surface impedance term. The surface
impedance term
will have some time (or frequency) dependent decay characteristic. This IP
response from
the conductive proppant particles will depend upon the total surface coated
area of these
proppant particles. For example, for a 1 micron thick metallic coating on a
proppant particle
substrate having a diameter 700 microns, the volume of metallic coating is
approximately
15x1013- m-3 and the surface are per proppant particle is 1.54x10-6m2. A 75%
packing
factor, for example, would mean 4.14 x 109 proppant particles per unit volume,
where the
total volume of metal is 0.0062 m3 per cubic meter while the total surface
area is 6380 m2 per
cubic meter. This calculation shows that the IP effect due to the metallic
coated proppant
particles has the potential to be greater than the enhanced conductivity
effect of the metallic
coated proppant particles.
[00118] Another EM response that impacts 1P measurements is the inductive
response of
the earth. The inductive response arises from the Faraday/Lentz law which
produces eddy
currents in conductive media. The response is based upon the time-rate-of-
change of the
magnetic field; if the magnetic field is increasing, eddy currents are
generated in the
conductor (earth) to create a magnetic field opposite to the increasing
magnetic field, and if
the magnetic field is decreasing eddy currents are generated in the conductor
to create a
magnetic field opposite that of the decreasing magnetic field. The result of
this is to produce
a response much like the IP response; i.e., after a turn on of a primary
magnetic field (turning
on the current), the response takes time to achieve saturation and following
the turn off of the
primary magnetic field (turning off the current) the response slowly decays to
zero. Along
37
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
with the surrounding conducting earth, the conducting fracture (fluid and
proppant) will
generate an inductive response in addition to the IP response discussed above.
Due to the
coupling of electric and magnetic field through Maxwell's equations, the
magnetic induction
manifests itself in the electric field as well The inductive and IP effects
are additive. These
two responses can be separated in the magnetic field due to their different
frequency
responses.
[00119] Also, the finite-difference solutions to Maxwell's equations, FDEM,
includes the
inductive responses, but not the IP responses. In one or more exemplary
embodiments, the IP
effects can be included into the FDEM algorithm by treating the IP effect as a
time dependent
source term If the IP effect is treated as a time dependent source term, then
the IP effect can
be much larger than the pure conductive response.
[00120] A field data acquisition experiment was conducted to test the
transmission line
representation of a well casing current source. The calculated electric field
and the measured
electric field are in good agreement. This test demonstrates that the
transmission line current
source implementation in the 3D finite-difference electromagnetic code gives
accurate
results. The agreement, of course, depends upon an accurate model describing
the
electromagnetic properties of the earth. In this field data acquisition
experiment, common
electrical logs were used to characterize the electrical properties of the
earth surrounding the
test well bore and to construct the earth model.
[00121] The following examples are included to demonstrate illustrative
embodiments of
the present invention. It will be appreciated by those of ordinary skill in
the art that the
techniques disclosed in these examples are merely illustrative and are not
limiting. Indeed,
those of ordinary skill in the art should, in light of the present disclosure,
appreciate that
many changes can be made in the specific embodiments that are disclosed, and
still obtain a
like or similar result without departing from the spirit and scope of the
invention
Example 1
[00122] The electrical conductivity of various proppant samples was measured
using the
test device shown in FIG. 11. As shown in FIG. 11, the test system 1000
included an
insulating boron nitride die 1002, having an inside diameter of 0.5 inches and
an outside
diameter of 1.0 inches, disposed in a bore 1004 in a steel die 1006 in which
the bore 1004 had
an inside diameter of 1.0 inches. Upper and lower steel plungers 1008 and 1010
having an
outside diameter of 0.5 inches were inserted in the upper and lower ends 1012,
1014,
38
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
respectively, of the insulating boron nitride die 1002 such that a chamber
1016 is formed
between the leading end 1018 of the upper plunger 208, the leading end 1020 of
the lower
plunger 1010 and the inner wall 1022 of the boron nitride sleeve 1002. Upper
plunger 1008
was removed from the insulating boron nitride die 1002 and proppant was loaded
into the
chamber 1016 until the proppant bed 1024 reached a height of about 1 to 2 cm
above the
leading end 1020 of the lower plunger 1010. The upper plunger 1008 was then
reinstalled in
the insulating boron nitride die 1002 until the leading end 1018 of the upper
plunger 1008
engaged the proppant 1024. A copper wire 1026 was connected to the upper
plunger 1008
and one pole of each of a current source 1028 and a voltmeter 1030. A second
copper wire
was connected to the lower plunger 1010 and the other pole of each of the
current source
1028 and the voltmeter 1030. The current source may be any suitable DC current
source well
known to those of ordinary skill in the art such as a Keithley 237 High
Voltage Source
Measurement Unit in the DC current source mode and the voltmeter may be any
suitable
voltmeter well known to those of ordinary skill in the art such as a Fluke 175
True RMS
Multimeter which may be used in the DC mV mode for certain samples and in the
ohmmeter
mode for higher resistance samples.
[00123] The current source was powered on and the resistance of the test
system 1000 with
the proppant bed 1024 in the chamber 1016 was then determined. The resistance
of the
proppant 1024 was then measured with the Multimeter as a function of pressure
using the
upper plunger 1008 and lower plunger 1010 both as electrodes and to apply
pressure to the
proppant bed 1024. Specifically, R = ¨ the resistance of the system with
the plungers
touching is subtracted from the values measured with the proppant bed 1024 in
the chamber
1016 and the resistivity, p = R*A/t where A is the area occupied by the
proppant bed 1024
and t is the thickness of the proppant bed 1024 between the upper plunger 1008
and the lower
plunger 1010.
[00124] The results were as follows:
[00125] Electrical measurements of base proppants without the addition of any
conductive
material were conducted at 100 V DC on samples that were 50 volume % proppant
in wax
that were pressed into discs nominally 1 inch in diameter and approximately 2
mm thick.
Using these values to calculate the resistivity and using the measured
resistivity for pure wax,
the values below were extrapolated by plotting log(resistivity) vs. volume
fraction proppant
and extrapolating to a volume fraction of one:
CARBOPROP 40/70: 2 x 1012 Ohm-cm
39
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
CARBOPROP 20/40: 0.6 x 1012 Ohm-cm
CARBOHYDROPROP: 1.8 x 1012 Ohm-cm
CARBOECONOPROP: 9 x 1012 Ohm-cm
[00126] It should be noted that the resistivities of the samples measured
above are very
high and not suitable for detection in the present invention.
Example 2
[00127] Three batches of 20/40 mesh size conventional ceramic proppant
(EconoProp),
commercially available as CARBOECONOPROP from CARBO Ceramics Inc., were
separately non-uniformly coated with a nickel-phosphorous alloy to provide non-
uniformly
coated coventional proppant with different coating thicknesses of 192 nm, 390
nm, and 670
nm as shown in FIGS. 12-14, respectively. Two more batches of EconoProp were
separately
uniformly coated with the nickel-phosphorous alloy to provide uniformly coated
proppant
with different coating thicknesses of 780 nm and 1,080 nm as shown in FIGS. 15
and 16,
respectively. Each of the batches shown in FIGS. 12-16 were coated by placing
the uncoated
EconoProp into an electroless plating bath solution comprising a nickel and
phosphorous
solution.
[00128] A batch of 25 mesh size drip cast proppant (KS-H 25), commercially
available as
KRYPTOSPHEREco HD from CARBO Ceramics Inc., was non-uniformly coated with the
nickel-phosphorous alloy to provide non-uniformly coated drip cast proppant
shown in FIGS.
17 and 18 at 7x and 20x magnification, respectively. The batch shown in FIGS.
17-18 was
coated by placing the KS-H 25 into an electroless plating bath solution
comprising a nickel
and phosphorous solution for 25 minutes to provide an approximate coating
thickness of 200
nm. Another batch of KS-H 25 was uniformly coated with the nickel-phosphorous
alloy to
provide uniformly coated proppant shown in FIGS. 19 and 20 at 7x and 20x
magnification,
respectively. The batch shown in FIGS. 19-20 was coated by placing the KS-H 25
into an
electroless plating bath solution comprising a nickel and phosphorous solution
for 45 minutes
to provide an approximate coating thickness of 500 nm.
[00129] The electrical conductivity of each batch was independently tested
using the test
device shown in FIG. 11. The results and respective coating thicknesses for
each batch are
shown in Table 1 below.
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
Table 1
Surface Approximate Nickel Approximate Ni Coating
Resistivity
Ceramic Substrate Area (m2/g) Thickness (nn) Coverage (%)
(Ohms)
20/40 EconoProp .0238 192 45 5
20/40 EconoProp .0238 390 75 1.9
20/40 EconoProp .0238 670 90 0.9
20/40 EconoProp .0238 780 100 0.8
20/40 EconoProp .0238 1,080 100 0.6
KS-H 25 .0156 200 50 1.1
KS-H 25 .0156 500 100 0.3
[00130] For successful EM detection it can be desirable for the proppant to
have a
resistivity of about 1 ohm or less. Surprisingly, the incomplete coverage of
the non-uniform
coatings was able to provide the target 1 ohm resistivity with both the
EconoProp and the KS-
H. Also surprising, was the lower required coating thickness for the drip cast
KS-H batches
when compared to the conventional EconoProp batches. These results suggest
that the
features of the drip cast proppant, such as smoothness and shape, allows for
low resistivity
with non-uniform coatings having low coating thicknesses.
Example 3
[00131] For this example, a first batch of 40/70 mesh size EconoProp coated
with nickel
and having a resitivity of 0.3 Ohm, a second batch of 40/70 mesh size
EconoProp coated with
nickel and having a resitivity of 0.7 Ohm, and a third batch of 40/70 mesh
size EconoProp
coated with nickel and having a resitivity of 0.3 Ohm admixed with 40/70 bare
sand on a 1:1
weight ratio were separately tested for conductivity at varied pressures.
[00132] The electrical conductivity of each batch 1-3 was independently tested
using the
test device shown in FIG. 11. The results and respective coating thicknesses
for each batch
are shown in Table 1 below.
Table 1
Batch Compressive Stress (psi) Conductivity (S/m)
0.3 Ohm 40/70 CEP (Batch 1)
509.42 2596.71
636.78 2702.82
764.14 2815.47
891.49 2935.27
1018.85 3062.92
1273.56 3199.23
1528.27 3421.94
41
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
1782.99 3584.17
2037.7 3669.87
2292.41 3758.87
2547.12 3851.36
0.7 Ohm 40/70 CEP (Batch 2)
509.42 1069
636.78 1156.38
764.14 1251.63
891.49 1334.24
1018.85 1377.9
1273.56 1519.48
1528.27 1596.91
1782.99 1707.99
2037.7 1767.24
2292.41 1829.18
2547.12 1894
1:1 mixture of 40/70 sand and
0.3 Ohm 40/70 CEP (Batch 3)
509.42 38.95
636.78 43.02
764.14 47.12
891.49 51.26
1018.85 55.43
1273.56 63.88
1528.27 72.46
1782.99 76.81
2037.7 81.2
2292.41 90.08
2547.12 99.12
[00133] For successful EM detection it can be desirable for the proppant to
have an
electrical conductivity of at least 10 S/m. The 1:1 mixture of electrically
conductive proppant
(0.3 Ohm 40/70 CEP) and non-electrically conductive bare sand was able to
provide and
surpass the target 10 S/m conductivity, suggesting that a mixture of
electrically conductive
and non-electrically conductive proppant can form a proppant pack having an
electrical
conductivity suitable for detection using the methods disclosed herein.
[00134] When used as a proppant, the particles described herein may be handled
in the
same manner as ordinary proppants. For example, the particles may be delivered
to the well
42
CA 02982453 2017-10-11
WO 2016/168719 PCT/US2016/027917
site in bags or in bulk form along with the other materials used in fracturing
treatment.
Conventional equipment and techniques may be used to place the particles in
the formation as
a proppant. For example, the particles are mixed with a fracture fluid, which
is then injected
into a fracture in the formation.
[00135] In an exemplary method of fracturing a subterranean formation, a
hydraulic fluid
is injected into the formation at a rate and pressure sufficient to open a
fracture therein, and a
fluid containing sintered, substantially round and spherical particles
prepared from a slurry as
described herein and having one or more of the properties as described herein
is injected into
the fracture to prop the fracture in an open condition.
[00136] The foregoing description and embodiments are intended to illustrate
the
invention without limiting it thereby. It will be understood that various
modifications can be
made in the invention without departing from the spirit or scope thereof.
43