Note: Descriptions are shown in the official language in which they were submitted.
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
FORMULATIONS CONTAINING DIACEREIN AND METHODS OF
LOWERING BLOOD LEVELS OF URIC ACID USING THE SAME
CROSS-REFERENCES TO RELATED APPLICATIONS
Not applicable.
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to a diacerein formulation, especially to a
method of
lowering blood levels of uric acid using this formulation.
Descriptions of the Related Art
Chemically, rhein is 9, 10-dihydro-4, 5-dihydroxy-9, 10-dioxo-2-anthracene
carboxylic
acid having a structure of Formula (I), and one of its prodrugs, diacerein, is
4, 5-bis
(acetyloxy) 9, 10-dihydro-4, 5-dihydroxy-9, 10-dioxo-2-anthracenecarboxylic
acid having a
structure of Formula (II). Diacerein is entirely converted into rhein before
reaching the
systemic circulation, and exerts its physiological function in form of rhein
within the body.
Formula (I)
0 4,11
40100
(1)43i=I
Formula (II)
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
0
0110 cool
o oh,
0
Diacerein is an anti-inflammatory agent widely used in the treatment of
osteoarthritis,
which has been demonstrated to inhibit interleukin-1 (IL-1) signaling.
Presently, diacerein
capsules are available in 50 mg strength and are marketed under various trade
names in
different countries, including Art SO , Artrodar , etc. As disclosed in US
patent No.
8,536,152, diacerein can also be used as an adjunctive treatment for type II
diabetes mellitus.
Although diacerein can be administered by oral route, it cannot be completely
absorbed by the
digestive tract, and the oral bioavailability of diacerein has been estimated
to be
approximately 40% to 60%. The incomplete absorption of diacerein may result in
undesirable side effects such as diarrhea or soft stools. In vitro and in vivo
studies have
showed that non-absorbed diacerein is metabolized to rhein in the colon, which
then induces a
laxative effect. Thus, there is still a need in the art for a diacerein
formulation having
reduced adverse side effects and/or higher bioavailability compared to the
current commercial
formulations.
As disclosed in US patent No. 8,865,689, diacerein was found to be effective
in reducing
the blood uric acid levels, and can be used for treating hyperuricemia or a
metabolic disorder
associated with hyperuricemia. However, no diacerein formulations specific for
lowering
the blood uric acid levels have been developed so far.
In view of the above demand, the present invention provides a diacerein
formulation
2
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
having improved properties, as well as its uses in treating diseases
including, but not limited
to, hyperuricemia, a metabolic disorder associated with hyperuricemia,
osteoarthritis and type
2 diabetes mellitus.
SUMMARY OF THE INVENTION
In one embodiment, the invention provides a controlled-release formulation
with reduced
adverse side effects and/or higher bioavailability, comprising an immediate-
release layer and
an extended-release layer.
In another embodiment, this invention provides a method of lowering blood
levels of
uric acid in a subject, comprising administering the above controlled-release
formulation to
the subject in need thereof
In another embodiment, this invention provides a method of lowering blood
levels of
uric acid in a subject, comprising administering to the subject in need
thereof a formulation
containing a therapeutically effective amount of a compound selected from the
group
consisting of diacerein, rhein, monoacetylrhein, a prodrug and a
pharmaceutically acceptable
salt thereof, wherein when the formulation is administered to said subject it
provides at least
one of the following pharmacokinetic parameters: (i) a maximum plasma
concentration C.
of rhein above 5.0 i.tg/m1; (ii) an area under the concentration time curve
AUCo_t or AUC0, of
rhein above 35.0 i.tg=hr/m1; (iii) Tmax of about 3 to 4.5 hours after oral
administration to the
subject under a fed condition; and (iv) a plasma concentration of rhein above
2.8 .tg/m1 for at
least 4 hours.
3
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
In another embodiment, this invention provides a method of lowering blood
levels of
uric acid in a subject, comprising administering to the subject in need
thereof a formulation
containing at least about 75 mg of a compound selected from the group
consisting of
diacerein, rhein, monoacetylrhein, a prodrug and a pharmaceutically acceptable
salt thereof
The detailed technology and preferred embodiments implemented for the subject
invention are described in the following paragraphs accompanying the appended
drawings for
people skilled in this field to well appreciate the features of the claimed
invention.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG 1 shows the dissolution profiles of the controlled-release formulations A
and F of
the present invention, measured by the United States Pharmacopeia (USP)
Apparatus II
(Paddle) at 50 rpm in 900 ml of pH 6.8 PBS at 37 C;
FIG 2 is a statistical bar graph showing inhibition of uric acid uptake by
different doses
of rhein;
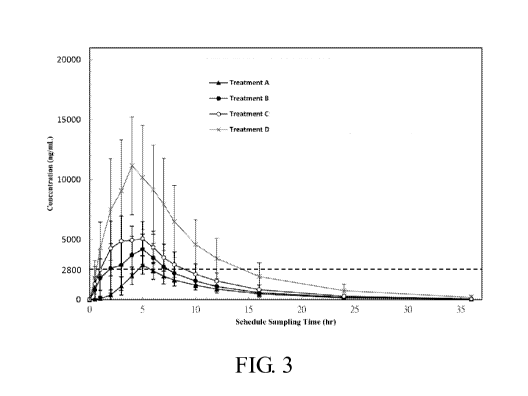
FIG 3 shows the average plasma concentration-time profiles of rhein after
subjects
received treatment with different diacerein formulations; and
FIG 4 is a statistical bar graph showing the serum uric acid concentrations
before and
after the treatment with different diacerein formulations.
DETAILED DESCRIPTION OF THE INVENTION
The term "Immediate-Release" or "IR," as used herein, means that a drug (e.g.,
diacerein)
is released in a conventional or non-modified way.
The term "Controlled-Release" or "CR" and "Extended-Release" or "ER," as used
herein,
4
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
refers to the gradual release of a drug at a predetermined rate other than an
immediate release
manner over a period of time.
The term "therapeutically effective amount," as used herein, refers to an
amount that
alleviates or reduces one or more symptoms of a disease.
The term "Cmax," as used herein, refers to the maximum observed plasma
concentration,
calculated as the mean of the individual maximum blood plasma concentrations.
The term "average plasma concentration," as used herein, refers to the
arithmetic mean
blood plasma concentration.
The term "Tmax," as used herein, refers to the time at which the peak
(maximum)
observed blood plasma drug concentration for each individual participating in
the
bioavailability study.
The term "AUC0" or "AUCõ,f," as used herein, refers to the mean area under the
plasma/serum/blood concentration-time curve extrapolated to infinity. It is
calculated as the
arithmetic mean of the area under the plasma concentration-time curve from
time zero
extrapolated to infinity, calculated for each individual participating in the
bioavailability
study.
The term "AUCo_t," as used herein, refers to the area under the
plasma/serum/blood
concentration-time curve from time zero to time t, where "t" is the last
sampling time point
with measurable concentration for individual formulation.
The term "diacerein or its analogs," as used herein, refers to diacerein,
rhein,
monoacetylrhein, or a pharmaceutically acceptable salt or a prodrug thereof
5
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
Unless otherwise stated herein, the terms "a (an)", "the" or the like used in
this
specification (especially in the Claims hereinafter) shall be understood to
encompass both the
singular form and the plural form.
As stated above, to improve adverse side effects and/or bioavailability of
diacerein, the
present invention provides a controlled-release formulation, comprising an
immediate-release
layer and an extended-release layer.
In one embodiment, the immediate-release layer comprises a therapeutically
effective
amount of a compound selected from the group consisting of diacerein, rhein,
monoacetylrhein, a prodrug and a pharmaceutically acceptable salt thereof
(hereinafter
referred to as "diacerein or its analogs"); a filler; a binder; a
disintegrant; and a lubricant; and
the extended-release layer comprises a therapeutically effective amount of
diacerein or its
analogs, a controlled-release polymer, a filler, and a lubricant; and wherein
the weight ratio of
diacerein or its analogs in the immediate-release layer to that in the
extended-release layer is
about 2:1 to about 1:9.
In one embodiment, the formulation further comprises a cosmetic coating.
Preferably, in the formulation of the present invention, the immediate-release
layer
comprises about 5% to about 60% by weight, preferably about 5% to about 50% by
weight of
diacerein or its analogs; about 30% to about 95% by weight, preferably about
40% to about
85% by weight of a filler; about 0.1% to about 20% by weight, preferably about
1% to about
10% by weight of a binder; about 0.1% to about 20% by weight, preferably about
1% to about
10% by weight of a disintegrant; and about 0.01% to about 5% by weight,
preferably about
6
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
0.1% to about 2.5% by weight of a lubricant, based on the total weight of the
immediate-release layer; and the extended-release layer comprises about 5% to
about 60% by
weight, preferably about 5% to about 50% by weight of diacerein or its
analogs; about 1% to
about 60% by weight, preferably about 10% to about 50% by weight of a
controlled-release
polymer; about 1% to about 70% by weight, preferably about 10% to about 55% by
weight of
a filler; and about 0.01% to about 5% by weight, preferably about 0.1% to
about 2.5% by
weight of a lubricant, based on the total weight of the extended-release
layer.
Examples of fillers include, but are not limited to, lactose monohydrate,
lactose
anhydrous, and starches. Preferably, the filler is lactose monohydrate.
Examples of binders include, but are not limited to, povidone, starch,
gelatin, tragacanth,
methylcellulose, hypromellose, and hydroxypropylcellulose.
Preferably, the binder is
povidone.
Suitable disintegrants include, but are not limited to, sodium
carboxymethylcellulose,
L-hydroxypropylcellulose, cropovidone, corn starch, sodium starch glycolate,
starch,
croscarmellose sodium, and alginic acid or its sodium salt. Preferably, the
disintegrant is
croscarmellose sodium.
Suitable lubricants include, but are not limited to, light anhydrous silicic
acid, talc,
stearic acid and its zinc, magnesium, or calcium salt, and polyethyleneglycol.
Preferably, the
lubricant is magnesium stearate.
Controlled-release polymers that can be used in the present invention may be,
for
instance, hydroxypropyl methylcellulose (HPMC), hydroxypropyl cellulose,
sodium alginate,
7
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
carbomer, sodium carboxymethyl cellulose, xanthan gum, guar gum, locust bean
gum, poly
vinyl acetate, polyvinyl alcohol carboxyvinyl polymers, polyvinyl alcohols,
glucans,
scleroglucans, mannans, xanthans, alginic acid and its derivatives,
polyanhydrides,
polyaminoacids, carboxymethyl cellulose, cross-linked sodium carboxymethyl
cellulose,
polyvinyl pyaolidone, cross-linked polyvinyl pyaolidone, carboxymethylamide,
potassium
methacrylate/divinylbenzene copolymer, starches and their derivatives, P-
cyclodextrin,
dextrin derivatives with linear or branched chains, ethyl cellulose, methyl
cellulose,
methacrylic acid copolymers, and cellulose derivatives. Preferably, the
controlled-release
polymer is hydroxypropyl methylcellulose (HPMC).
Because the formulation of the invention has reduced adverse side effects, it
can deliver
a higher dose of diacerein without increasing the side effects like diarrhea.
Specifically, it
can be administered to patients with a higher dose compared to the commercial
diacerein
drugs (e.g., Artrodar , 50 mg Q.D. or B.I.D, 50 or 100 mg daily in total), and
may contain at
least about 75 mg, preferably about 75 to 200 mg, more preferably about 75 to
100 mg of
diacerein or its analogs, thereby enhancing the treatment effect in a single
dose.
In another aspect, the inventors of the present application found that the
formulation
containing at least about 75 mg of diacerein is more effective in reducing
blood levels of uric
acid than Artrodar (an immediate-release formulation containing 50 mg of
diacerein). Thus,
the formulation of the invention comprises preferably at least about 75 mg,
more preferably
about 75 to 200 mg, most preferably about 75 to 100 mg of diacerein or its
analogs.
In a dissolution test, the controlled-release formulation preferably has an in
vitro
8
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
dissolution rate when measured by the United States Pharmacopeia (USP)
Apparatus II
(Paddle) at 50 rpm in 900 ml of pH 6.8 PBS at 37 C, between about 30% and
about 45%,
preferably about 35% and about 40%, diacerein released after 1 hour; between
about 50% and
about 60% diacerein released after 4 hours; between about 60% and about 75%,
preferably
about 65% and about 75%, diacerein released after 8 hours; and not less than
about 80%
diacerein released after 16 hours, by weight.
In one embodiment, the controlled-release formulation of the invention, when
administered to a subject, may provide at least one of the following
pharmacokinetic
parameters: (i) a maximum plasma concentration Cmax of rhein above 5.0 mg/m1;
(ii) an area
under the concentration time curve AUCo_t or AUC0, of rhein above 35.0
mg=hr/m1; (iii) Tmax
of about 3 to 4.5 hours after oral administration to a subject under a fed
condition; and (iv) a
plasma concentration of rhein above 2.8 mg/m1 for at least 4 hours. A
formulation exhibiting
the above pharmacokinetic parameters shows reduced adverse side effects,
reduced food
effect, higher bioavailability, and/or better effect in reducing the blood
uric acid levels as
compared to the conventional immediate-release formulation.
Preferably, the formulation is a once-daily (i.e., taken once per day)
controlled-release
formulation.
Because the formulation of the invention has the above-mentioned advantages,
it is
beneficial when used for treating all the diseases to which diacerein is
therapeutically
effective. These diseases include, but are not limited to, hyperuricemia, a
metabolic disorder
associated with hyperuricemia, osteoarthritis and type 2 diabetes mellitus.
The metabolic
9
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
disorder associated with hyperuricemia includes, but is not limited to, acute
gout, chronic gout,
gout arthritis, gout flares, uric acid nephrolithiasis, gouty nephropathy,
cardiovascular
diseases (e.g., hypertension and atherosclerosis), obesity, chronic kidney
disease, and insulin
resistance.
The formulation can be used for decreasing inflammatory effects of gout
arthritis and
gout flares induced by hyperuricemia; and/or dissolving kidney stones; and/or
reducing the
recurrence rate of acute inflammatory arthritis induced by hyperuricemia;
and/or slowing
down the progression of urate nephropathy in a subject.
In one embodiment, the formulation may further comprise one or more additional
therapeutic agent, such as an anti-inflammatory agent or a urate-lowering
agent to enhance the
therapeutic effect of diacerein. Examples of the anti-inflammatory agents
include, but are
not limited to, non-steroidal anti-inflammatory drugs (NSAIDs),
corticosteroids, and
colchicines. Examples of the urate-lowering agents include, but are not
limited to, xanthine
oxidase inhibitors, uricosuric agents, urate oxidases, urinary alkalinizers,
and fenofibrate.
The present invention also provides a method of lowering blood levels of uric
acid in a
subject, comprising administering to the subject in need thereof a formulation
containing
diacerein or its analogs.
The formulations that can be used in this method may have the structure,
composition
and other properties as those defined above for the formulation of the present
invention.
Alternatively, the formulations suitable for this method may have different
structure and
composition as long as when administered to a subject they can provide at
least one of the
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
following pharmacokinetic parameters: (i) a maximum plasma concentration Cmax
of rhein
above 5.0 lag/m1; (ii) an area under the concentration time curve AUCo_t or
AUCo, of rhein
above 35.0 lag=hr/m1; (iii) Tmax of about 3 to 4.5 hours after oral
administration to the subject
under a fed condition; and (iv) a plasma concentration of rhein above 2.8
lag/m1 for at least 4
hours.
In another embodiment, the formulation used in the method contains at least
about 75 mg,
preferably about 75 to 200 mg, more preferably about 75 to 100 mg of diacerein
or its
analogs.
Hereinafter, the present invention will be further illustrated with reference
to the
following examples. However, these examples are only provided for illustrate
purpose, but
not to limit the scope of the present invention.
[Preparation Example] Preparation of a controlled-release formulation
containing
diacerein
Ten controlled-release tablet formulations containing 75 or 100 mg of
diacerein were
prepared according to Tables 1(a) and 1(b). The prepared tablets were used in
the following
in vivo study.
11
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
Table 1(a). 75 mg diacerein
Tablet A Tablet B Tablet C Tablet D
Tablet E
Ingredients
mg % mg % mg % mg % mg %
/tab w/w /tab w/w /tab w/w /tab w/w /tab w/w
Diacerein
25 25 25 25 37.5 37.5 7.5 7.5 7.5 7.5
Lactose 63.5 63.5 63.5 63.5 53 53 83 83 85
85
Povidone 5 5 5 5 5 5 5 5 5
5
IR Layer
Croscarmellose Sodium 6 6 6 6 4 4 4 4 2
2
Magnesium Stearate 0.5 0.5 0.5 0.5 0.5 0.5 0.5 0.5
0.5 0.5
Sub Total 100 100 100 100 100 100 100 100
100 100
Diacerein
50 29.7 50 29.7 37.5 22.28 67.5 40.1 67.5 40.1
Hypromellose (HPMC) 33.5 19.9 67.34 40 50.5 30 16.83
10 67.34 40
Lactose 83.83 49.8 49.99 29.7 79.33 47.12 83
49.3 32.49 19.3
ER Layer FD&C Blue Aluminum
0.17 0.1 0.17 0.1 0.17 0.1 0.17 0.1 0.17 0.1
Lake
Magnesium Stearate 0.84 0.5 0.84 0.5 0.84 0.5 0.84 0.5
0.84 0.5
Sub Total
168.34 100 168.34 100 168.34 100 168.34 100 168.34 100
Total Core Tablet Weight 268.34 268.34 268.34 268.34 268.34
Cosmetic
Op
0 adry II Yellow 8.3 3 8.3 3 8.3 3 8.3 3 8.3
3
Coating
Total Coated Tablet Weight
276.64 100 276.64 100 276.64 100 276.64 100 276.64 100
Table 1(b). 100 mg diacerein
Tablet F Tablet G Tablet H Tablet I
Tablet J
Ingredients
mg % mg _____________________________________ % mg % mg % mg %
/tab w/w /tab w/w /tab w/w /tab w/w /tab w/w
Diacerein
33.33 25 33.33 25 50 37.5 10 7.5 10 7.5
Lactose 84.67 63.5
84.66 63.49 70.66 52.99 110.66 82.99 113.32 84.99
Povidone 6.67 5 6.67 5 6.67 5 6.67 5 6.67 5
IR Layer
_______________________________________________________________________
Croscarmellose Sodium 8 6 8 6 5.33 4 5.33 4
2.67 2
Magnesium Stearate 0.67 0.5 0.67 0.5 0.67 0.5
0.67 0.5 0.67 0.5
Sub Total 133.34 100 133.33 100 133.33 100 133.33 100 133.33
100
Diacerein
66.67 29.7 66.67 29.7 50 22.28 90 40.1 90 40.1
Hypromellose (HPMC) 44.67 19.9 89.78 40 67.34 30 22.45 10 89.78
40
ER Layer Lactose
111.77 49.8 66.65 29.7 105.77 47.12 110.66 49.3 43.32 19.3
FD&C Blue Aluminum
0.23 0.1 0.23 0.1 0.23 0.1 0.23 0.1 0.23 0.1
Lake
12
CA 02983394 2017-10-19
WO 2016/171656 PCT/US2015/026678
Tablet F Tablet G Tablet H Tablet I Tablet J
Ingredients
mg % __ mg % mg % mg % mg %
/tab w/w /tab w/w /tab w/w /tab w/w /tab w/w
Magnesium Stearate 1.12 0.5 1.12 0.5 1.12 0.5 1.12
0.5 1.12 0.5
Sub Total
224.45 100 224.45 100 224.45 100 224.45 100 224.45 100
Total Core Tablet Weight 357.79 357.78 357.78 357.78
357.78
Cosmetic
p= 0 adry II Red 11.05 3 11.05 3 11.05 3
11.05 3 11.05 3
Coating
Total Coated Tablet Weight
368.84 100 368.83 100 368.83 100 368.83 100 368.83 100
[EXAMPLE I] Dissolution Assay for Diacerein Controlled-Release Formulations
In this example, dissolution was performed in accordance with the USP
Apparatus II
(Paddle). A solution of pH 6.8 PBS was used as the dissolution medium. Samples
were
taken at suitable time intervals and analyzed for diacerein content by means
of high-pressure
liquid chromatography (HPLC).
Table 2 summarizes the raw data of the dissolution of Tablets A and F of the
present
invention, and FIG. 1 shows the dissolution profiles.
Table 2
Time Tablet A Tablet F
(hrs) (% released) (% released)
0 0 0
0.5 31 33
1 39 40
2 47 46
4 57 53
6 65 60
8 71 65
77 70
12 81 74
14 84 78
16 87 80
18 89 83
13
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
Time Tablet A Tablet F
(hrs) (% released) (% released)
20 91 85
Dissolution method: USP Apparatus II (Paddle), 50
rpm/ 900 mL pH 6.8 PBS, 37 C
[EXAMPLE 2] Human URAT1 Dependent Uric Acid Uptake Assay
Uric acid is mainly eliminated through urinary excretion and up to 90% of
filtered urate
is re-absorbed. A decrease in an excretion rate of urate is considered to
elevate serum uric acid,
resulting in hyperuricemia. URAT1 (urate transporter 1, the SLC22Al2 gene) is
the main
transporter responsible for tubular reabsorption of urate and is thought to be
the major
mechanism for regulating blood urate levels. URAT1 has been genetically
associated with
urate levels, and inhibition of URAT1 may decrease serum uric acid.
In this study, an in vitro method was established to investigate the hURAT1-
mediated
uric acid [814C]
uptake in transiently transfected HEK293T cells, a human embryonic kidney
293 cells containing the URAT1 transporter.
After 24h to 72h incubation of the transfected HEK293T cells, they were
reseeded in a
microplate. At least 12 hours after the cell plated, the culture medium was
removed and the
cells were washed and then incubated in a 100 n1 Cl-free HBSS Buffer for 5 to
10 mins. The
buffer was removed and 50 per well of Cl-free HBSS Buffer containing 50 M
uric acid
[8-14C] (0.13nCi/well) was added with or without rhein (under four doses, 30,
10, 3.3 and 1.1
M) to the cells incubated for 5 mins at 37 C. At the end of the incubation,
uric acid [8-14C]
uptake was stopped. Cells were washed three times and 50 ul per well 100 mM
NaOH was
added to lyse cells, which were then agitated at 600 rpm for at least 20 mins.
The cells
14
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
lysate was collected and 200 ul per well UltimaGold TM XR scintillation was
added, and the
mixture was agitated at 600 rpm for 10 mins. Finally, the microplate was
counted. The
results are shown in FIG 2.
FIG. 2 shows that the uptake of uric acid was inhibited by rhein at 3.3 and 10
uM for
49.4% 22.2%, and at 30 uM for 79.3% 1.5%. ICso of rhein in URAT1 inhibition is
10 uM,
which is about 2.8 ug/ml. Maintenance of plasma rhein concentration exceed 2.8
ug/ml may
perform uric acid lowering effect.
This study shows that diacerein or its analogs can lower serum uric acid by
inhibiting
URAT1, and thus can be used to treat hyperuricemia and a metabolic disorder
associated with
hyperuricemia.
[EXAMPLE 3] Pharmacokinetic Study
A Phase 1, randomized, open-Label, single dose, 4-Treatment, 4-sequence, 4-
period,
crossover, pharmacokinetic study of a diacerein immediate-release formulation
(Artrodar 50
mg Capsule) and three different doses of the diacerein controlled-release
formulations of the
present invention was conducted in healthy male and female volunteers under
fed conditions.
Methodology: A 4-way crossover comparative pharmacokinetic study of Artrodar
50
mg and three different doses of the controlled-release formulations (75, 100
and 200 mg) by
oral administration in healthy male and female volunteers was conducted. There
was a
7-day washout period separating the treatment periods.
Subjects: Healthy volunteers met all the inclusion and none of the exclusion
criteria of
the study.
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
Procedure: Diacerein administered in the different formulations and doses was
compared in randomized fashion with a washout period of 7 days between
periods. The
subjects were randomly assigned to one of the treatment sequences as the
following Table 3.
The study started with a screening visit. Only eligible subjects participated
in the study.
5Table 3. Study Sequence
Period 1 Period 2 Period 3
Period 4
Sequence 1 Treatment A Treatment B Treatment C
Treatment D
Sequence 2 Treatment B 7 days Treatment D 7 days Treatment A 7 days Treatment
C
Sequence 3 Treatment C Treatment A Treatment D
Treatment B
Sequence 4 Treatment D Treatment C Treatment B
Treatment A
= Treatment A: 1 x Artrodar 50 mg Capsule;
= Treatment B: 1 x 75 mg Tablet;
= Treatment C: 1 x 100 mg Tablet;
= Treatment D: 2 x 100 mg Tablet (200 mg).
The comparison between the different formulations and doses is based on a
comparison
within subjects rather than between subjects. The washout period of 7 days was
estimated to
be adequate in avoiding carry-over effects of the preceding treatments.
Statistical method(s) for efficacy/pharmacokinetic evaluations: AUCo_t, AUCoõ,
Cmax,
and Tmax for rhein in plasma of per-protocol (PP) population were determined
and calculated
by non-compartment methods. Analysis of Variance (ANOVA) was used for AUCo_t,
AUCoõ,
Cmax, and Tmax. Tmax was analyzed using an additional non-parametric test
(Wilcoxon test).
Safety assessment was performed for all subjects who had been administered at
least one
dose of the study drug. The investigator obtained and recorded all observed
adverse events
16
CA 02983394 2017-10-19
WO 2016/171656 PCT/US2015/026678
(AEs) on the CRF or those voluntarily reported, including its intensity and
relationship
assessment with the investigational products. For all AEs, the investigator
pursued and
obtained information adequate to determine both the outcome of the AE and
whether it met
any seriousness criterion. All AEs had to be followed up until resolution or
stabilization at a
level acceptable to the investigator.
Pharmacokinetics results are shown in Tables 4 and 5 and FIG. 3. Subjects
(n=23) were
screened and 16 subjects were randomized into the study. There were 13
subjects who
completed the whole study (4 periods) for estimating pharmacokinetics of PP
population.
Only the data obtained from these subjects was reported in the following
tables.
Table 4. Pharmacokinetic parameters of rhein for PP population
Parameter*
) Treatment A Treatment B Treatment C Treatment D
(N=13
AUC0-i 22,541.0 36,166.4 49,661.3 108,367.6
(hrxng/mL) (4675.5) (8983.9) (10258.2) (30657.3)
AUC0_a, 22,853.0 36,569.1 50,216.7 110,025.6
(hrxng/mL) (4794.0) (9266.3) (10595.4) (32067.3)
Cmax 3,018.5 5,360.0 6,483.8 14,590.0
(ng/mL) (663.8) (986.3) (1109.9) (3116.0)
Tmax 5.00 3.64 3.62 4.16
(hr) (0.41) (1.50) (1.50) (1.46)
*data were shown as mean (SD)
Table 5. The ratio of rhein for PP population
Ratio of treatment group ln-trans formednon ln-trans formed
(N=13) AUCo_t AUC0-00 Cmax Tmax
Ratio of B/A (%) 106.8 106.6 114.8 72.7
Ratio of C/A (%) 110.3 110.1 103.8 72.3
Ratio of D/A (%) 116.9 116.9 115.2 83.2
Safety results are shown in Table 6. There were no reported significant
adverse events,
17
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
death, or serious adverse events. During the study, the most commonly reported
adverse
events were diarrhea followed by nausea, vomiting, rash, blood creatine
phosphokinase
increased, dermatitis contact, hypotension and somnolence. Diarrhea events are
almost the
same between the 50 mg capsule and 75 mg and 100 mg Tablets. All adverse
events were
reported to be mild in intensity and were resolved in the end. In conclusion,
50 mg of
Artrodar capsule and 75, 100 and 200 mg of the Tablets are safe to use.
Table 6. Summary of Adverse Events (By event)
Total Treatment A Treatment B Treatment C Treatment
D
Total AEs 32 5 6 4 17
Soft stool 11(34.4%) 1(20.0%) 2(33.3%) 3(75.0%) 5(29.4%)
Diarrhea 8 (25.0%) 1 (20.0%) 2 (33.3%) 1 (25.0%) 4
(23.5%)
Nausea 5 (15.6%) 0 (0.0%) 2 (33.3%) 0 (0.0%) 3 (17.6%)
Vomit 2 (6.3%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 2 (11.8%)
Elevated CK 2 (6.3%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 1 (5.9%)
Skin rash 2 (6.3%) 1 (20.0%) 0 (0.0%) 0 (0.0%) 1 (5.9%)
Contact dermatitis 1(3.1%) 1(20.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%)
Sleepy 1(3.1%) 1(20.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%)
Hypotension 1(3.1%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 1(5.9%)
The above results showed that the controlled-release formulations of the
present
invention exhibited Cmax of rhein above 5.0 ug/ml, AUCo_t or AUC0, of rhein
above 35.0
ug=hr/ml, and Tmax of about 3 to 4.5 hours. In addition, the formulations of
the invention
provided a blood concentration of rhein above 2.8 ug/ml (the treatment
effective
18
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
concentration in Example 2) for at least 4 hours (Treatment B: 4.2 hours;
Treatment C: 7
hours; Treatment D: 12.7 hours) and had greater bioavailability than the
commercial
immediate-release formulation, with about 10% greater dose-normalized AUC and
C.
values. It was found that AUC and Cmax values increased generally proportional
with
increasing doses of diacerein.
The controlled-release formulations at 75 mg and 100 mg had similar
tolerability to the
immediate-release formulation at 50 mg, while the 200 mg dose was associated
with a higher
gastrointestinal AE incidence.
Therefore, compared to Artrodar formulation, the
formulations of the invention demonstrated improved safety in higher dose
strengths at 75 mg
and 100 mg, and provided reduced adverse side effects accordingly. This allows
patients to
be treated with a higher dose at 75 or 100 mg once per day without increasing
the side effects.
Mean blood rhein reached peak concentrations was approximately 3.62 to 4.16
hours
postdose in the tablet group of the present invention and 5.00 hours in
Artrodar capsule
group under fed conditions. It has been demonstrated that fasting Tmax of
diacerein was 2.4
hours after a single oral administration of 50 mg in healthy volunteers and
increased to 5.2
hours with a meal (Petitjean et al., Clinical Pharmacokinetics, November 1998,
Volume 35,
Issue 5, pp 347-359). The formulations of the present invention were absorbed
faster under
fed conditions and showed less food effect when compared to Artrodar capsule.
[EXAMPLE 4] Serum Uric Acid Study
Serum uric acid reduction valuation in the study of diacerein immediate-
release
19
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
formulation (Artrodar 50 mg Capsule) and three different doses of the
diacerein
controlled-release formulations of the present invention in healthy volunteers
under fed
conditions was conducted.
In the pharmacokinetic study of Example 3, the effects of Artrodar 50 mg and
three
different doses of the tablets of the present invention (75, 100 and 200 mg)
on serum uric acid
of healthy volunteers under fed conditions were also post-analyzed for intend-
to-treat (ITT)
population. A total of 15 subjects were analyzed. The serum uric acid
concentrations were
compared by before and after treatments with paired t-test analysis.
The results were revealed in Figure 4. The serum uric acid concentration after
Treatment A (Artrodar 50 mg) was not significantly different from that before
treatment.
However, the serum uric acid concentration was lowered after Treatment B than
that before
treatment. Same outcomes were also demonstrated in Treatment C and Treatment
D. Such
difference in lowering of serum uric acid might be due to the duration that
rhein maintained at
an effective blood concentration above 2.8 ug/ml. As shown in FIG. 3,
Treatment A reached
rhein blood concentration above 2.8 ug/ml for quite a short period, which was
insufficient to
exert urate-lowering effect.
This study revealed that serum uric acid was significantly lowered by the
controlled-release formulations of the present invention at different doses
above 75 mg.
The above disclosure is related to the detailed technical contents and
inventive features
thereof People skilled in this field may proceed with a variety of
modifications and
replacements based on the disclosures and suggestions of the invention as
described without
CA 02983394 2017-10-19
WO 2016/171656
PCT/US2015/026678
departing from the characteristics thereof
21