Note: Descriptions are shown in the official language in which they were submitted.
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
IRRIGATION RESISTANT COMPOSITIONS FOR REGENERATION OF HARD
TISSUES AND METHODS AND KITS OF USING THE SAME
RELATED APPLICATIONS
[0001] The present patent document claims the benefit of the filing date of
U.S.
Patent Application Serial No. 14/695,910, filed April 24, 2015, which is
incorporated
herein by reference in its entirety.
BACKGROUND
[0002] Bone is a composite of collagen, cells, calcium hydroxyapatite
crystals,
and small quantities of other proteins of organic molecules that has unique
proper-
ties of high strength, rigidity, and ability to adapt to varying loads. When
bone inju-
ries occur, it is necessary to fill voids or gaps in the bone as well as to
encourage the
repair and regeneration of bone tissue. There are many materials used today
for the
repair and regeneration of bone defects. For example, one material useful to
en-
courage such repair and regeneration is bioactive glass.
[0003] Bioactive glass was originally developed in 1969 by L. Hench.
Additional-
ly, bioactive glasses were developed as bone replacement materials, with
studies
showing that bioactive glass can induce or aid in osteogenesis (Hench et al.,
J. Bio-
med. Mater. Res. 5:117-141 (1971)). Bioactive glass can form strong and stable
bonds with bone (Piotrowski et al., J. Biomed. Mater. Res. 9:47-61(1975)).
Further,
bioactive glass is not considered toxic to bone or soft tissue from studies of
in vitro
and in vivo models (Wilson et al., J. Biomed. Mater. Res. 805-817 (1981)).
Exempla-
ry bioactive glasses include 45S5, 4555B1, 58S, and 570C30. The original bioac-
tive glass, 45S5, is melt-derived. Sol-gel derived glasses can also be
produced and
include nanopores that allow for increased surface area and bioactivity.
[0004] There are drawbacks to the use of bioactive glass or other materials
in the
form of liquids, pastes, and solids to fill voids or gaps in the bone. A
liquid or a paste
may not remain at the site of the void or gap in the bone. A solid may be
difficult to
apply and may not conform well to the void or gap in the bone. Solids may
migrate or
be displaced from the site through washing or other means.
[0005] These drawbacks may be reduced and/or eliminated by adding materials
to a bone repair composition, such that the composition is rendered irrigation
and
migration resistant.
1
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
BRIEF DESCRIPTION OF THE DRAWINGS
[0006] Figure 1 depicts an exemplary delivery system kit for delivering an
irriga-
tion resistant bone repair composition.
[0007] Figure 2A-B depicts schematic drawings of an adapter (2A) and a
delivery
gun (2B) for the irrigation resistant bone repair composition.
[0008] Figure 3 depicts a schematic drawing of a plunger of the delivery
system.
[0009] Figure 4A depicts exemplary tips for a delivery system.
[0010] Figure 4B depicts exemplary tips for a delivery system.
[0011] Figure 5A is a photograph of the tubes filled with an irrigation
resistant
bone repair composition for use with a delivery system.
[0012] Figure 5B depicts a schematic drawing of a tube for use with a
delivery
system.
[0013] Figure 6A is a photograph of an exemplary delivery system for an
irrigation
resistant bone repair composition.
[0014] Figure 6B is a photograph of an exemplary delivery system for an
irrigation
resistant bone repair composition.
[0015] Figure 7 is a photograph of an exemplary delivery system for an
irrigation
resistant bone repair composition.
[0016] Figure 8 is a photograph of an exemplary delivery system for an
irrigation
resistant bone repair composition.
[0017] Figure 9 is a photograph of an exemplary delivery system for an
irrigation
resistant bone repair composition.
SUMMARY
[0018] Certain embodiments relate to an irrigation resistant bone repair
composi-
tion comprising a biocompatible bone repair material and at least one non-
ionic sur-
factant, wherein the non-ionic surfactant is not a non-random
poly(oxyalkylene) block
copolymer. The non-ionic surfactant is selected from the group consisting of
fatty al-
cohols (e.g., stearyl alcohol), alkoxylated alcohols (e.g., Ecosurf LF 45),
alkoxylated
alkylphenols (e.g., Triton X-100), alkoxylated fatty amides (e.g.,
polyethoxylated tal-
low amine), alkoxylated fatty esters (e.g., PEG 400 monostearate), alkoxylated
fatty
2
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
ethers (e.g., polyethylene glycol lauryl ether (Brij L23), alkoxylated
sorbitan esters
(e.g., Span 85 (sorbitan trioleate)), alkoxylated sorbitan esters (e.g.,
polysorbate 20
and polysorbate 80 also referred to as Tween 20 and Tween 80), fatty acid
esters or
polyol esters (e.g., glycerol monostearate, PEG coconut triglycerides),
polyalkylene
glycols (e.g., PEG 400 and PEG 600), alkoxylated organic acids, hydroxyacids
or
diacids and copolymers therefrom. Preferably, one of the non-ionic surfactants
in the
composition has a melting point above room temperature, and more preferably
above body temperature. The bone repair material can be any number of
materials
that assist in bone repair and production. Such materials include at least
bioactive
glass in the form of a particle, fiber or sphere and calcium salts, i.e., DCP,
alpha
TCP, beta-TCP, hydroxyapatite, calcium sulfates, calcium borates or calcium
sili-
cates, multiphasic calcium phosphates, calcium sulfates, calcium borates or
calcium
silicates along with elemental substitutions within these materials or
coatings applied
to these materials.
[0019] Further embodiments relate to bioactive glass particles including a
coating
comprising at least one non-ionic surfactant, wherein the non-ionic surfactant
is not a
non-random poly(oxyalkylene) block copolymer.
[0020] Another embodiment relates to a putty or paste including bioactive
glass
particles including a coating comprising at least one non-ionic surfactant,
wherein the
non-ionic surfactant is not a non-random poly(oxyalkylene) block copolymer.
[0021] Yet further embodiments relate to methods for treating a bone having
a
bone gap and/or a bone defect with the composition comprising a biocompatible
bone repair material and at least one non-ionic surfactant, wherein the non-
ionic sur-
factant is not a non-random poly(oxyalkylene) block copolymer. The non-ionic
sur-
factant or similar material other than the non-random poly(oxyalkylene) block
copol-
ymer is selected from the group consisting of fatty alcohols (e.g., stearyl
alcohol),
alkoxylated alcohols (e.g., Ecosurf LF 45), alkoxylated alkylphenols (e.g.,
Triton X-
100), alkoxylated fatty amides (e.g., polyethoxylated tallow amine),
alkoxylated fatty
esters (e.g., PEG 400 monostearate), alkoxylated fatty ethers (e.g.,
polyethylene
glycol lauryl ether (Brij L23), alkoxylated sorbitan esters (e.g., Span 85
(sorbitan trio-
leate)), alkoxylated sorbitan esters (e.g., polysorbate 20 and polysorbate
80), fatty
alcohols, fatty acids, fatty acid esters or polyol esters (e.g., glycerol
monostearate,
3
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
PEG coconut triglycerides), polyalkylene glycols (e.g., PEG 400 and PEG 600),
alkoxylated organic acids, hydroxyacids or diacids and copolymers therefrom.
At
least one of the surfactants in the composition has a melting or cloud point
above
room temperature, and more preferably a melting point above body temperature.
[0022] Other embodiments relate to an irrigation resistant bone repair
composi-
tion comprising a biocompatible bone repair material and a mixture of non-
ionic sur-
factants, wherein the non-ionic surfactants are not non-random
poly(oxyalkylene)
block copolymers. The bone repair material can be any number of materials that
as-
sist in bone repair and production. Such materials include at least bioactive
glass,
spherical bioactive glass in a bimodal size distribution, and tricalcium
phosphate, i.e.,
silicated tricalcium phosphate.
[0023] Further embodiments relate to bioactive glass particles including a
coating
comprising two or more non-ionic surfactants, wherein the non-ionic
surfactants are
not non-random poly(oxyalkylene) block copolymers.
[0024] Yet another embodiment relates to a putty or paste including
bioactive
glass particles including a coating comprising two or more non-ionic
surfactants,
wherein the non-ionic surfactants are not non-random poly(oxyalkylene) block
copol-
ymers.
[0025] Yet further embodiments relate to methods for treating a bone having
a
bone gap and/or a bone defect with the composition comprising a biocompatible
bone repair material and a mixture of non-ionic surfactants, wherein the non-
ionic-
surfactants are not non-random poly(oxyalkylene) block copolymers.
DETAILED DESCRIPTION
[0026] Irrigation resistant bone repair compositions comprising (i) a
biocompatible
or bioactive bone repair material and at least one non-ionic surfactant,
wherein the
non-ionic surfactant is not a non-random poly(oxyalkylene) block copolymer, or
(ii) a
mixture of non-ionic surfactants, wherein the non-ionic surfactants are not
non-
random poly(oxyalkylene) block copolymers are provided.
[0027] Specifically, certain embodiments relate to a synthetic bone
grafting com-
position, such as a putty for bone repair that incorporates non-ionic
surfactants or
4
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
other similar materials, other than a non-random poly(oxyalkylene) block
copoly-
mers, having an osteoconductive, osteostimulative and irrigation resistant
properties.
The term "irrigation resistant" in connection with the compositions described
herein
refers to a property of the composition, where the composition can be heavily
irrigat-
ed following placement in a surgical site without being washed away or
displaced
from the surgical site. The composition includes at least one slow dissolving
non-
ionic surfactant(s), other than a non-random poly(oxyalkylene) block
copolymers,
which is mixed with a biocompatible or bioactive bone repair material, such as
bioac-
tive glasses or other osteoconductive salts, glasses or ceramics for use in
methods
for treating a bone having a bone gap and/or a bone defect.
[0028] The irrigation resistant bone repair composition is biocompatible
and or
bioactive and comprised of entirely synthetic materials, which fully
eliminates any
risk of disease transmission that may occur with other products containing
animal or
human derived materials or components to achieve this property.
[0029] The composition promotes osseointegration when introduced into a
bone
gap and/or a bone defect.
[0030] The bone repair composition has a unique physical property of being
irri-
gation resistant. The irrigation resistant characteristics provide a material,
which
maintains position in the surgical site despite the amount of blood, body
fluid or sa-
line to which it is exposed. Irrigation resistance is beneficial to simplify
the application
of the bone graft at the site of defect while preventing migration of the
graft material
during irrigation and after closure of the surgical site. The irrigation
resistance of the
bone repair composition is especially beneficial for its intended use in
orthopedic and
spine processes, as the material will stabilize and maintain placement and
structure
within the body during placement, irrigation and after closure. Specifically,
in certain
embodiments where a non-setting putty material is used, the bone repair
composi-
tion will not be displaced easily during irrigation and closure of the
surgical site.
[0031] An irrigation resistant, fully synthetic and bioactive putty, when
implanted
into the body, will maintain position or placement rather than melt, dissolve
or disin-
tegrate during irrigation or displace upon closure of the surgical site. This
feature
permits the implant to hold in place more easily, and create beneficial
handling prop-
erties. The ability to resist displacement allows the bioactive agent to
remain at the
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
site of implantation to stimulate bone growth for an extended period of time.
The bi-
oactive glass, as the preferred bioactive agent, stimulates the genes
necessary to
differentiate precursor cells into osteoblasts and the subsequent
proliferation of
these cells within the surgical site while undergoing an ionic exchange with
the sur-
rounding body fluid to form microcrystalline hydroxyapatite analogous to
natural
bone mineral. The combination of these properties in one composition is
essential
for bone regeneration and hard tissue repair.
[0032] The composition may be a liquid at room temperature. Alternatively,
the
composition may have the consistency of a solid, gel, putty, paste or any
other non-
liquid substance at room temperature. The composition may also have the form
of a
liquid, solid, gel, putty, paste or any other non-liquid substance at room
temperature.
Additionally the composition may undergo a phase change when warmed from room
temperature to body temperature.
[0033] In some embodiments, the composition is thermoreversible changing
sub-
stantially from a liquid at 5 C and into a solid at 37 C. This effect can
arise from the
type and relative amount of non-ionic surfactants in the composition, which in
turn
determines the viscosity of the composition at room temperature and at body
tem-
perature. For example, as the temperature rises, the composition becomes
substan-
tially more viscous liquid or waxy solid to allow the bone repair material,
for example,
bioactive glass, to more readily remain at the defect site.
[0034] The bone repair composition provides for acceleration in the rate
and an
enhancement in the quality of newly-formed bone. Improved bone healing may oc-
cur in those who may be compromised, such as diabetics, smokers, the obese,
the
elderly, those who have osteoporosis, those who use steroids, and those who
have
infections or other diseases that reduce the rate of healing. The rapid
hardening of
the bone repair composition at the site of the bone defect can serve to
localize the
bone repair material, such as bioactive glass, at the site.
[0035] The bone repair composition may be provided to a site of a bone
defect by
means of a syringe or other injection device. In certain embodiments, the bone
re-
pair composition may be sufficiently liquid so as to be injectable, yet can
harden
suitably at the bone defect site at body temperature. For instance, if the
bone repair
composition is a liquid at room temperature, it may become a thick gel at body
tem-
6
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
perature; in other words, the bone repair composition cures upon application
to a
bone defect at body temperature.
[0036] In certain embodiments, the bone repair composition has the
advantages
of low viscosity, runny liquid composition with regard to the ease of
application to a
bone defect site. Further advantages of the composition include more solid
paste-
like composition characteristics and that it remains positioned at the defect
after be-
ing applied. The solidification of the composition at body temperature
overcomes the
disadvantageous property of other liquid compositions that do not exhibit
irrigation
resistant behavior. At the same time, because the composition is not a solid
at room
temperature, there is greater ease of applying the composition, such as by
means of
a syringe. The composition need not be laboriously painted onto a bone defect
or
applied onto a bone defect by means of pressure.
[0037] Other delivery modes can be used for more viscous bone repair
composi-
tions. These modes include manually placing the gel or paste directly into a
bone
defect or extruding the gel or paste using a syringe, delivery gun or other
means.
[0038] In certain embodiments, if the bone repair composition is a gel at
room
temperature, it may become a paste at body temperature.
[0039] In certain other embodiments, if the bone repair composition is a
thick gel
or paste at room temperature, it may become putty or a solid at body
temperature.
[0040] As noted above, the relative amount of a non-ionic surfactant (other
than a
non-random poly(oxyalkylene) block copolymers), in the composition will
determine
the viscosity at room temperature and at body temperature.
[0041] In certain embodiments, the irrigation resistant composition
includes a bio-
compatible or bioactive bone repair material, and at least one non-ionic
surfactant,
with the proviso that the non-ionic surfactant is not a non-random
poly(oxyalkylene)
block copolymer.
[0042] The non-ionic surfactant or similar material other than the non-
random
poly(oxyalkylene) block copolymer is selected from the group consisting of
fatty ac-
ids (e.g. stearic acid), fatty alcohols (e.g., stearyl alcohol), alkoxylated
alcohols (e.g.,
Ecosurf LF 45), alkoxylated alkylphenols (e.g., Triton X-100), alkoxylated
fatty am-
ides (e.g., polyethoxylated tallow amine), alkoxylated fatty esters (e.g., PEG
400
monostearate), alkoxylated fatty ethers (e.g., polyethylene glycol lauryl
ether (Brij
7
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
L23), polyglycerin fatty acid esters, alkoxylated sorbitan esters (e.g., Span
85 (sorbi-
tan trioleate)), alkoxylated sorbitan esters (e.g., Polysorbate 20 and
Polysorbate 80
also referred to as Tween 20 and Tween 80), fatty acid esters or polyol esters
(e.g.,
glycerol monostearate, PEG coconut triglycerides), polyalkylene glycols (e.g.,
PEG
400 and PEG 600), alkoxylated organic acids, hydroxyacids or diacids and
copoly-
mers therefrom. Specific examples of non-ionic surfactants, other than the non-
random poly(oxyalkylene) block copolymers, include sorbitan tristearate,
polysorbate
20, polysorbate 80, polyoxyethylene 7 coconut, glycerides, poly(ethylene
glycol) 400
monostearate (PEG 400 monostearate), PEG 2000 monomethylether, and PEG 400
distearate.
[0043] Further examples of the non-ionic surfactants suitable for use with
the irri-
gation resistant compositions include polyglycery1-2 isostearate, polyglycery1-
2
di isostearate, polyglycery1-4 isostearate, polyglycery1-6 isostearate,
poly(ethylene
glycol) 8 stearate (MYRJ S8), polyglyceryl-10 isostearate, polyglyceryl-10
diisos-
tearate, poly(ethylene glycol) 25 propylene glycol stearate (MYRJ S25),
poly(ethylene glycol) 400 distearate (PEG 400 distearate), polyglycery1-4
laurate,
polyglycery1-6 laurate, polyglyceryl-10 laurate, polyglyceryl-10 myristate,
polyglycer-
y1-2 oleate, polyglycery1-4 oleate, polyglycery1-6 oleate, polyglyceryl-10
oleate, poly-
glyceryl-10 stearate, and polyglyceryl-10 distearate.
[0044] Yet further examples of the non-ionic surfactants include
polyoxyethylene
7 coconut glyceride (coconut glyceride), polyethylene glycol 2000 monomethyl
ether
(MME), glyceryl monostearate (monostearin), PEG dimethyl ether (dimethyl
polyeth-
ylene glycol), PEG 200 adipate (poly(ethylene glycol) 200 adipate, PEG 6000
dis-
tearate, sorbitan monostearate, cetyl alcohol, ethylene glycol monostearate,
propyl-
ene glycol stearate, polyoxyethylene stearyl ether (Brij 2), polyoxyethylene
stearyl
fatty ether (Brij 10), docosaethylene glycol mono octadecyl ether (Brij 20),
polyeth-
ylene stearyl ether (Brij 100),polyglycerin fatty acid ester (polyglycery1-2
isostearate,
polyglycery1-2 di isostearate, polyglycery1-4 isostearate, polyglycery1-6
isostearate,
polyglyceryl-10 isostearate, polyglyceryl-10 diisostearate, polyglycery1-4
laurate, p01-
yglycery1-6 laurate, polyglyceryl-10 laurate, polyglyceryl-10 myristate,
polyglycery1-2
oleate, polyglycery1-4 oleate, polyglycery1-6 oleate, polyglyceryl-10 oleate,
polyglyc-
ery1-10 stearate, polyglyceryl-10 distearate).
8
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
[0045] In certain embodiments, one of the surfactants in the composition
has a
melting point or cloud point above room temperature, and more preferably above
a
melting point above body temperature.
[0046] In certain other embodiments, at least two non-ionic surfactants,
other
than a non-random poly(oxyalkylene) block copolymers, may be included; alterna-
tively, at least three or more non-ionic surfactants, other than a non-random
poly(oxyalkylene) block copolymers, may be included.
[0047] In some embodiments, the weight ratio of at least one non-ionic
surfactant,
other than poly(oxyalkylene) block copolymer is 1%-99% relative to the weight
of the
bone repair composition. This weight ratio may be from 1-10%, 10-20%, 20-30%,
30%-40%, 40%-50%, 50%-60%, 60%-70%, 70%-80%, 80%-90%, or 90-99%. Alter-
natively, this weight ratio may be about 1%, about 2%, about 3%, about 4%,
about
5%, about 6%, about 7%, about 8%, about 9%, about 10%, about 11%, about 12%,
about 13%, about 14%, about 15%, about 16%, about 17%, about 18%, about 19%,
about 20%, about 21 A, about 22%, about 23%, about 24%, about 25%, about 26%,
about 27%, about 28%, about 29%, about 30%, about 31 A, about 32%, about 33%,
about 34%, about 35%, about 36%, about 37%, about 38%, about 39%, about 40%,
about 41%, about 42%, about 43%, about 44%, about 45%, about 46%, about 47%,
about 48%, about 49%, about 50%, about 51%, about 52%, about 53%, about 54%,
about 55%, about 56%, about 57%, about 58%, about 59%, about 60%, about 61%,
about 62%, about 63%, about 64%, about 65%, about 66%, about 67%, about 68%,
about 69%, about 70%, about 71 A, about 72%, about 73%, about 74%, about 75%,
about 76%, about 77%, about 78%, about 79%, about 80%, about 81 A, about 82%,
about 83%, about 84%, about 85%, about 86%, about 87%, about 88%, about 89%,
about 90%, about 91%, about92%, about 93%, about 94%, about 95%, about 96%,
about 97%, about 98%, or about 99%. The material may have the consistency of a
solid, gel, putty, paste or any other non-liquid substance at room
temperature.
[0048] In some embodiments, the weight ratio of the mixture of at least two
non-
ionic surfactant, other than a non-random poly(oxyalkylene) block copolymers,
is
1%-99% relative to the weight of the bone repair composition. This weight
ratio may
be from 1-10%, 10-20%, 20-30%, 30%-40%, 40%-50%, 50%-60%, 60%-70%, 70%-
80%, 80%-90%, or 90-99%. Alternatively, this weight ratio may be about 1 A,
about
9
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
2%, about 3%, about 4%, about 5%, about 6%, about 7%, about 8%, about 9%,
about 10%, about 11%, about 12%, about 13%, about 14%, about 15%, about 16%,
about 17%, about 18%, about 19%, about 20%, about 21 A, about 22%, about 23%,
about 24%, about 25%, about 26%, about 27%, about 28%, about 29%, about 30%,
about 31%, about 32%, about 33%, about 34%, about 35%, about 36%, about 37%,
about 38%, about 39%, about 40%, about 41%, about 42%, about 43%, about 44%,
about 45%, about 46%, about 47%, about 48%, about 49%, about 50%, about 51%,
about 52%, about 53%, about 54%, about 55%, about 56%, about 57%, about 58%,
about 59%, about 60%, about 61%, about 62%, about 63%, about 64%, about 65%,
about 66%, about 67%, about 68%, about 69%, about 70%, about 71%, about 72%,
about 73%, about 74%, about 75%, about 76%, about 77%, about 78%, about 79%,
about 80%, about 81%, about 82%, about 83%, about 84%, about 85%, about 86%,
about 87%, about 88%, about 89%, about 90%, about 91%, about92%, about 93%,
about 94%, about 95%, about 96%, about 97%, about 98%, or about 99%. The ma-
terial may have the consistency of a solid, gel, putty, paste or any other non-
liquid
substance at room temperature.
[0049] In some embodiments, where the bone repair composition comprises two
non-ionic surfactants, the weight ratio of a first non-ionic surfactant to the
weight ratio
of a second non-ionic surfactant is in the range of from about 1%-99% to about
99%-
1%. Specifically, the weight ratio of a first non-ionic surfactant to the
weight ratio of a
second non-ionic surfactant is about 1 A to 99%; alternatively, the weight
ratio of a
first non-ionic surfactant to the weight ratio of a second non-ionic
surfactant is about
50% to 50%; and alternatively, the weight ratio of a first non-ionic
surfactant to the
weight ratio of a second non-ionic surfactant is about 99% to 1%. The
compositions
may vary in molecular weight and be blended in ratios of 10:1 to 1:10.
[0050] The compositions may further comprise ions and other compounds that
may be dissolved in water. For example, the addition of salts, such as PBS,
can en-
hance solidification and setting properties of non-ionic surfactants. Divalent
salts
may be particularly useful to improve the rheological properties of
compositions con-
taining non-ionic surfactant mixtures and bioactive glass materials as well as
those
of compositions containing non-ionic surfactants and other solid bone repair
materi-
als.
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
[0051] In certain embodiments, the composition may further include at least
one
additive, including but not limited to solvents, linear, straight chain and
branched ali-
phatic hydrocarbons, sugars, polysaccharides, and hydroxyl or alkoxy terminal
poly-
alkylene oxides along with low molecular weight biodegradable polymers
(MW</=10,000).
[0052] Specific examples of additives include sodium hyaluronate,
regenerez,
polypropylene glycol 3000 (poly 3000), seasame oil, candelilla wax, carnauba
wax,
sorbitol (D-Glucitol), polycaprolactone, polycaprolactone diol, coconut oil,
propylene
glycol, polycaprolactone triol, polycaprolactone 10000 mw, mineral oil high
viscosity,
mineral oil low viscosity, polyethylene glycol 400 (PEG-8), butylene glycol,
and
hexylene glycol.
[0053] The biocompatible or bioactive bone repair material may be
osteoinduc-
tive, osteoconductive, or a material that is both osteoinductive and
osteoconductive.
The bone repair material may be xenogeneic, allogeneic, autogeneic, and/or
allo-
plastic.
[0054] The bone repair material can be any number of materials that assist
in
bone repair and production. Such materials include at least bioactive glass in
the
form of a particle, sphere, fiber, mesh, sheet or a combination of these
forms, i.e. fi-
bers within a sphere, and calcium salts, i.e., DCP, alpha TCP, beta-TCP,
hydroxyap-
atite, calcium sulfates, calcium borates or calcium silicates, multiphasic
calcium
phosphates, calcium sulfates, calcium borates or calcium silicates along with
ele-
mental substitutions within these materials or coatings applied to these
materials. In
certain embodiments, the biocompatible or bioactive bone repair material may
also
be any combination of various therapeutic materials. The various types of
bioactive
glass that may be used as bone repair material were previously described In
U.S.
Pub. No. US 2014/0079789, entire content of which is incorporated herein by
refer-
ence.
[0055] In certain embodiments, the composition may be prepared as a
composite
with a biocompatible or bioactive agent, such as a bioactive glass ceramic
which
contains silica or boron. The ceramic releases calcium and silicate or calcium
and
boron ions, which facilitate the differentiation and proliferation of
osteoblasts (defined
as osteostimulation), which in turn increases the rate of regeneration of hard
tissue.
11
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
[0056] In addition, the bioactive glass component undergoes an ion exchange
with the surrounding body fluid to form hydroxyapatite analogous to bone
mineral.
More specifically, dissolution of the bioactive glass ceramics releases the
calcium
and silicate or calcium and boron ions, which stimulate the genes responsible
of the
differentiation and proliferation of osteoblast cells within the bony defect
upon im-
plantation. It is believed that this genetic response is activated through the
introduc-
tion of the genetic cascade responsible for the osteoblast proliferation and
subse-
quently promotes the increased rate of regeneration of hard tissue.
[0057] In certain embodiments, the bone repair material is bioactive glass.
Bioac-
tive glass may be melt-derived or sol-gel derived. Depending on their
composition,
bioactive glasses may bind to soft tissues, hard tissues, or both soft and
hard tis-
sues. The composition of the bioactive glass may be adjusted to modulate the
de-
gree of bioactivity. Furthermore, borate may be added to bioactive glass to
control
the rate of degradation.
[0058] In some embodiments, the bioactive glass contains silica and/or
boron as
well as other ions such as sodium and calcium.
[0059] Certain embodiments relate to an irrigation resistant bone repair
composi-
tion that includes a biocompatible or bioactive bone repair material suspended
in a
mixture of at least two non-ionic surfactant, other than a non-random
poly(oxyalkylene) block copolymer.
[0060] Certain further embodiments relate to an irrigation resistant bone
repair
composition that further includes at least one element selected from the group
con-
sisting of Li, Na, K, Mg, Sr, Ti, Zr, Fe, Co, Cu, Zn, Al, Ga, P, N, S, F, Cl,
Ag, Au, Ce
and I. For example, small amounts of iodine, fluorine or silver can provide
antimi-
crobial properties, while small amount of copper can promote angiogenesis
(i.e., aid
in the formation of blood vessels).
[0061] The preferred embodiment includes non-ionic surfactants, other than
a
non-random poly(oxyalkylene) block copolymers, as carriers for melt and sol-
gel de-
rived bioactive glasses. The composites range from 1 to 99% of a mixture of
non-
ionic surfactants, other than a non-random poly(oxyalkylene) block copolymers,
which is conversely 1-99% bioactive glass.
12
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
[0062] The bioactive glass may be in a form of particles, spheres, fibers,
mesh,
sheets or a combination of these forms i.a fibers within a sphere.
[0063] The compositions may vary in molecular weight and may be blended in
ratios of 10:1 up to 1:10. Compositions of the glass may comprise from 0-90%
silica
or 0-90% boric acid with a plurality of other elements including Li, Na, K,
Mg, Sr, Ti,
Zr, Fe, Co, Cu, Zn, Al, Ga, P, N, S, F, Cl, Ag, Au, Ce and I. The embodiments
take
the consistency of a gel, putty, or waxy solid at room temperature.
[0064] In certain embodiments, bioactive glass is in the form of a
particle. The
composition, porosity and particle sizes of the bioactive glass may vary. In
certain
preferred embodiments, the particles of the glass may range in size from 0.01
pm to
5mm. In certain embodiments, the bioactive glass comprises 0-80% < 100 pm, 0-
80% <500 pm, 0-80% 500-1000 pm, 0-80% 1000-2000 pm, 0-80% 2000-5000 pm,
0-90% 90-710 pm, and 0-90% 32-125 pm bioactive glass. In certain embodiments,
the particles may be porous crystalline. In other embodiments, the particles
may be
porous non-crystalline particles.
[0065] Specifically, the bioactive glass material may have silica, sodium,
calcium,
strontium, phosphorous, and boron present, as well as combinations thereof. In
some embodiments, sodium, boron, strontium, and calcium may each be present in
the compositions in an amount of about 1% to about 99%, based on the weight of
the bioactive glass. In further embodiments, sodium, boron, strontium and
calcium
may each be present in the composition in about 1%, about 2%, about 3%, about
4%, about 5%, about 6%, about 7%, about 8%, about 9%, or about 10%. In certain
embodiments, silica, sodium, boron, and calcium may each be present in the com-
position in about 5 to about 10%, about 10 to about 15%, about 15 to about
20%,
about 20 to about 25%, about 25 to about 30%, about 30 to about 35%, about 35
to
about 40%, about 40 to about 45%, about 45 to about 50%, about 50 to about
55%,
about 55 to about 60%, about 60 to about 65%, about 65 to about 70%, about 70
to
about 75%, about 75 to about 80%, about 80 to about 85%, about 85 to about
90%,
about 90 to about 95%, or about 95 to about 99%. Some embodiments may contain
substantially one or two of sodium, calcium, strontium, and boron with only
traces of
the other(s). The term "about" as it relates to the amount of calcium
phosphate pre-
sent in the composition means 41-0.5%. Thus, about 5% means 5 1-0.5%.
13
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
[0066] The bioactive glass materials may further comprise one or more of a
sili-
cate, borosilicate, borate, strontium, or calcium, including Sr0, CaO, P205,
Si02, and
B203. In certain embodiments, bioactive glass includes about 15-45% CaO, about
30-70% Si02, about 0-25% Na20, about 0-17% P205, about 0-10% MgO and about
0-5% CaF2.
[0067] An exemplary bioactive glass is 45S5, which includes 46.1 mol %
Si02,
26.9 mol % CaO, 24.4 mol % Na20 and 2.5 mol % P205,
[0068] An exemplary borate bioactive glass is 45S5B1, in which the Si02 of
45S5
bioactive glass is replaced by B203.
[0069] Other exemplary bioactive glasses include 58S, which includes 60 mol
%
Si02, 36 mol % CaO and 4 mol % P205, and S70C30, which includes 70 mol % Si02
and 30 mol % CaO.
[0070] In any of these or other bioactive glass materials of the invention,
Sr0 may
be substituted for CaO.
[0071] The following composition provided in Table 1 below, having a weight
% of
each element in oxide form in the range indicated, will provide one of several
bioac-
tive glass compositions that may be used to form a bioactive glass material:
[0072] Table 1:
Si02 0-86
CaO 4-35
Na20 0-35
P205 2-15
CaF2 0-25
B203 0-75
K20 0-8
Md0 0-5
CaF 0-35
[0073] In case of the bioactive glass being in the form of a three-
dimensional
compressible body of loose glass-based fibers, the fibers comprise one or more
glass-formers selected from the group consisting of P205, Si02, and B203. Some
of
the fibers have a diameter between about 100 nm and about 10,000 nm, and a
length:width aspect ratio of at least about 10. The pH of the bioactive glass
can be
adjusted as-needed.
14
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
[0074] The bioactive glass particles, fibers, spheres, meshes or sheets may
fur-
ther comprise any one or more of adhesives, grafted bone tissue, in vitro-
generated
bone tissue, collagen, calcium phosphate, stabilizers, antibiotics,
antibacterial
agents, antimicrobials, drugs, pigments, X-ray contrast media, fillers, and
other ma-
terials that facilitate grafting of bioactive glass to bone.
[0075] The silica and/or calcium ions released by the bioactive glass may
improve
the expression of osteostimulative genes. The silica and/or calcium ions may
also
increase the amount of and efficacy of proteins associated with such
osteostimula-
tive genes. In several embodiments, the bone repair material is
osteostimulative and
can bring about critical ion concentrations for the repair and regeneration of
hard tis-
sue without the necessity of any therapeutic materials or agents.
[0076] In some embodiments, the bone repair material is 45S5 bioactive
glass.
The 45S5 bioactive glass may vary in size from 1 micrometer to 5 millimeters.
The
bioactive glass may be about 1-5 micrometers, about 5-15 micrometers, about 15-
50
micrometers, about 50-200 micrometers, about 200-1,000 micrometers, about 1-2
millimeters, about 2-3 millimeters, about 3-4 millimeters, or about 4-5
millimeters.
[0077] In some embodiments, the bioactive glass particle has a diameter of
be-
tween about 0.01 micrometer and about 5,000 micrometers.
[0078] In some embodiments, the bone repair material is a composition
compris-
ing calcium salt and silica. The silica is in the form of an inorganic
silicate that is ad-
sorbed onto the surface of the calcium salt. The silica is not incorporated
into the
structure of the calcium salt. The composition may be bioactive. These and
other
bone repair materials are described in U.S. Patent Pub. No. US 2013/0330410,
the
entire content of which is herein incorporated by reference.
[0079] In some embodiments, the bone repair material is a composition
compris-
ing suspended autograft bone particles and suspended bioactive glass
particles.
Similar bone repair materials are described in U.S. Pub. No. 2015/0079146, the
en-
tire content of which is incorporated by reference.
[0080] The suspended bioactive glass particle may comprise 5i02.
Alternatively,
the suspended bioactive glass particle may comprise P205, P03. or PO4. The sus-
pended bioactive glass particle may comprise B203 as well. In some
embodiments,
the suspended bioactive glass particle may comprise 40-60% 5i02, 10-20% CaO, 0-
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
4% P205, and 19-30% Na0. The suspended bioactive glass particle may further
comprise a carrier selected from the group consisting of hydroxyapatite and
tricalci-
um phosphate.
[0081] The bioactive glass particles; fibers, meshes or sheets may be
pretreated
in a solution comprising one or more of blood, bone marrow aspirate, bone-
morphogenetic proteins, platelet-rich plasma, and osteogenic proteins.
[0082] In some embodiments, the bone repair material may be bioactive glass
coated with a glycosaminoglycan, in which the glycosaminoglycan is bound to
the
bioactive glass. This and other bone repair materials are described in U.S.
Patent
Pub. No. US 2014/0079789, the entire content of which is incorporated by
reference.
The glycosaminoglycan may be bound to the bioactive glass by means of an ionic
bond or a covalent bond. The glycosaminoglycan may be heparin, heparan
sulfate,
chondroitin sulfate, dermatan sulfate, keratan sulfate, or hyaluronic acid.
[0083] In certain other embodiments, the bone repair material may include
sur-
face immobilized peptides, as previously described in U.S. Pat. Application
No.
14/504,956, filed on October 2, 2014, which is incorporated herein in its
entirety.
[0084] In some further embodiments, the bone repair material is a bimodal
bioac-
tive glass composition comprising large bioactive glass particles and small
bioactive
glass particles. The large bioactive glass particles have a substantially
spherical
shape and a mean diameter of between about 90 micrometers and about 2,000 mi-
crometers. The small bioactive glass particles have a substantially spherical
shape
and a mean diameter of between about 10 micrometers and about 500 micrometers.
[0085] In some embodiments, the bone repair material is a trimodal
bioactive
glass composition comprising large bioactive glass particles, medium bioactive
glass
particles, and small bioactive glass particles. The large bioactive glass
particles
have a substantially spherical shape and a mean diameter of between about 500
mi-
crometers and about 5,000 micrometers. The medium bioactive glass particles
have
a substantially spherical shape and a mean diameter of between about 90
microme-
ters and about 710 micrometers. The small bioactive glass particles have a
substan-
tially spherical shape and a mean diameter of between about 1 micrometers and
about 125 micrometers.
16
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
[0086] In any of the above embodiments, small bioactive glass fibers may be
added to the bone repair material. The small bioactive glass fibers have a
diameter
of less than 2 millimeters. The small bioactive glass fibers may be present in
up to
40% by weight relative to the total weight of the bioactive glass. In various
embodi-
ments, the weight ratio of small bioactive glass fibers to total weight of the
bioactive
glass may be from 0-10%, 0_5%7 5_10%7 5_1,0,/o 7
10-15%, 10-20%, 15-20%, 15-25%,
20-25%, 20-30%, 25-30%, 25-35%, 30-35%, 30-40%, or 35-40%. The weight ratio
of small bioactive glass fibers to total weight of the bioactive glass may be
about 1%,
2%7 3%7 4%7 5%7 6%7 7%7 8%7 9%7 10%7 11%7 12%7 13%7 14%7 15%7 16%7 17%7
18%7 19%7 20%7 21%7 22%, 23%, 24%7 25%, 26% 727%7 28%7 29%7 30%7 31%7
32%7 33%7 34%7 35%7 36%7 37%7 38%7 3,o,/0 7
or 40%.
[0087] In some embodiments, any subset of the bioactive glass present, such
as
bioactive glass particles and/or small bioactive glass fibers, may be coated
with
silane as described in Verne et al. (Verne et al., "Surface functionalization
of bioac-
tive glasses," J. Biomed. Mater. Res. A., 90(4):981-92 (2009)). The silane or
other
functional coatings may then allow for binding of proteins onto the bioactive
glass,
such as BMP-2.
[0088] In some embodiments, any subset of the bioactive glass present, such
as
bioactive glass particles and/or small bioactive glass fibers, may have
additional sili-
cate chains present on them. The additional silicate chains may allow the
bioactive
glass particles and fibers to interact with one another, as well as with
groups of the
non-ionic surfactants. The effect of these interactions may be to reduce the
surface
area of the filler, increase resin demand, and to allow for higher filler
loadings.
[0089] In some embodiments, any subset of the bioactive glass present, such
as
bioactive glass particles and/or small bioactive glass fibers, may have added
hydrox-
yl triethoxysilanes coated onto the glass. Some of these silanes are available
from
Gelest, Inc. For example, the glass may be coated with
hydroxyl(polyethyleneoxy)
propyltriethoxysilane. Additionally, the glass may be coated with other
organic sub-
stituted ethoxy- and methoxy- silanes that are effective to create an
interaction be-
tween the coated glass and the EO/PO carrier.
[0090] In any of the above embodiments, the irrigation resistant bone
repair com-
position may be applied by a syringe at ambient temperature. After application
to the
17
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
bone or other site within the body at 37 C, the bone repair composition will
harden
and have a substantially lower tendency to migrate away from the application
site.
[0091] More viscous bone repair compositions may be applied by painting the
composition onto a site at or near the bone defect. Alternatively, more
viscous bone
repair compositions may be extruded onto the site in the form of a bead.
[0092] Certain embodiments relate to a method for treating hard tissues,
such as
bones using the irrigation resistant bone repair composition.
[0093] Certain other embodiments relate to a method for treating a bone
having a
bone defect comprising contacting the bone at or near the site of the bone
defect
with the irrigation resistant bone repair composition of any of the above-
described
embodiments.
[0094] Any of the above-described materials or methods may be undertaken to
treat any number of bone defects. As such, certain further embodiments relate
to a
method for treating a bone having a bone defect comprising placing an
irrigation re-
sistant bone repair composition of any one of the above-described embodiments
at a
site of a bone gap or a bone defect.
[0095] A bone defect may include bony structural disruptions, in which
repair is
needed or may be a gap in the bone or may arise from lack of adequate bone re-
generation. A bone defect may be a void, which is understood to be a three-
dimension defect that includes a gap, cavity, hole or other substantial
disruption of
the structural integrity of the bone or joint. The bone defects may also be
fractures.
The bone defects may also arise in the context of oral bone defects. The
different
types of bone defects are apparent to those of ordinary skill in the art. Gaps
may be
at least 2.5 cm and are generally in the range of 3-4 cm. This size is large
enough
so that spontaneous repair is not likely to occur and/or be complete.
Exemplary
bone defects include tumor resection, fresh fractures, cranial and facial
abnormali-
ties, spinal fusions, and loss of bone from the pelvis.
[0096] The various embodiments of the invention may be particularly useful
with
respect to orthopedic and spine processes because the material will stabilize
and
hold a better structure as it becomes more solidified when it heats up to body
tem-
perature.
18
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
[0097] Certain further embodiments relate to a method for treating a bone
having
a bone defect comprising placing an irrigation resistant bone repair
composition of
any one of the above-described embodiments at a bone gap or a bone defect.
[0098] In some embodiments, any of the above-described materials or methods
may be combined with autograft bone chips for placement onto or near a bone de-
fect. The materials may be a liquid or a gel at room temperature with the
autograft
bone chips suspended therein. Upon placement at or near the bone defect, the
ma-
terial will solidify around the autograft bone chips and serve to prevent the
autograft
bone chips from migrating away from the surgical sites.
[0099] In some embodiments, any of the above-described materials or methods
may be combined with particles containing allogeneic or xenogeneic bone
mineral
for placement onto or near a bone defect. The materials may be a liquid or a
gel at
room temperature with the particles suspended therein. Upon placement at a
surgi-
cal site, which is at or near the bone defect, the material will solidify
around the parti-
cles and serve to prevent the particles from migrating away from the surgical
site.
[00100] In various embodiments of the invention, the bone repair material
is not a
natural bone material or a synthetic bone material.
[00101] Further embodiments relate to kits that include an irrigation
resistant
bone repair composition including a biocompatible or bioactive bone repair
material,
and at least one surfactant other than the non-random poly(oxyalkylene) block
co-
polymer. The non-ionic surfactant or similar material, other than the non-
random
poly(oxyalkylene) block copolymer, is selected from the group consisting of
fatty ac-
ids (e.g. stearic acid), fatty alcohols (e.g., stearyl alcohol), alkoxylated
alcohols (e.g.,
Ecosurf LF 45), alkoxylated alkylphenols (e.g., Triton X-100), alkoxylated
fatty am-
ides (e.g., polyethoxylated tallow amine), alkoxylated fatty esters (e.g., PEG
400
Monostearate), alkoxylated fatty ethers (e.g., polyethylene glycol lauryl
ether (Brij
L23), polyglycerin fatty acid esters, alkoxylated sorbitan esters (e.g., Span
85 (sorbi-
tan trioleate)), alkoxylated sorbitan esters (e.g., Polysorbate 20 and
Polysorbate 80
also referred to as Tween 20 and Tween 80), fatty acid esters or polyol esters
(e.g.,
glycerol monostearate, PEG coconut triglycerides), polyalkylene glycols (e.g.,
PEG
400 and PEG 600), alkoxylated organic acids, hydroxyacids or diacids and
copoly-
mers therefrom. Specific examples of non-ionic surfactants, other than the non-
19
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
random poly(oxyalkylene) block copolymers, include sorbitan tristearate,
polysorbate
20, polysorbate 80, polyoxyethylene 7 coconut, glycerides, poly(ethylene
glycol) 400
monostearate (PEG 400 monostearate), PEG 2000 monomethylether, and PEG 400
distearate. Further examples of the non-ionic surfactants suitable for use
with the ir-
rigation resistant compositions include polyglycery1-2 isostearate,
polyglycery1-2
di isostearate, polyglycery1-4 isostearate, polyglycery1-6 isostearate,
poly(ethylene
glycol) 8 stearate (MYRJ S8), polyglyceryl-10 isostearate, polyglyceryl-10
diisos-
tearate, poly(ethylene glycol) 25 propylene glycol stearate (MYRJ S25),
poly(ethylene glycol) 400 distearate (PEG 400 distearate), polyglycery1-4
laurate,
polyglycery1-6 laurate, polyglyceryl-10 laurate, polyglyceryl-10 myristate,
polyglycer-
y1-2 oleate, polyglycery1-4 oleate, polyglycery1-6 oleate, polyglyceryl-10
oleate, poly-
glyceryl-10 stearate, and polyglyceryl-10 distearate. Yet further examples of
the non-
ionic surfactants include, polyoxyethylene 7 coconut glyceride (coconut
glyceride),
polyethylene glycol 2000 monomethyl ether (MME), glyceryl monostearate (monos-
tearin), PEG dimethyl ether (dimethyl polyethylene glycol), PEG 200 adipate
(poly(ethylene glycol) 200 adipate, PEG 6000 distearate, sorbitan
monostearate, ce-
tyl alcohol, ethylene glycol monostearate, propylene glycol stearate,
polyoxyethylene
stearyl ether (Brij 2), polyoxyethylene stearyl fatty ether (Brij 10),
docosaethylene
glycol mono octadecyl ether (Brij 20), polyethylene stearyl ether (Brij
100),polyglycerin fatty acid ester (polyglycery1-2 isostearate, polyglycery1-2
diisos-
tearate, polyglycery1-4 isostearate, polyglycery1-6 isostearate, polyglyceryl-
10 isos-
tearate, polyglyceryl-10 diisostearate, polyglycery1-4 laurate, polyglycery1-6
laurate,
polyglyceryl-10 laurate, polyglyceryl-10 myristate, polyglycery1-2 oleate,
polyglyceryl-
4 oleate, polyglycery1-6 oleate, polyglyceryl-10 oleate, polyglyceryl-10
stearate, poly-
glyceryl-10 distearate).
[00102] Further embodiments relate to kits that include an irrigation
resistant
bone repair composition including a biocompatible or bioactive bone repair
material,
and a mixture of at least two non-ionic surfactants or similar materials.
[00103] Exemplary kits for use with bone resistant compositions were
previous-
ly described in U.S. Pub. No. US 2015/030684, which is incorporated herein in
its
entirety.
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
[00104] The kits may further include a dispensing gun, syringe, clam shell,
or
other suitable delivery device and accompanying accessories. Specifically,
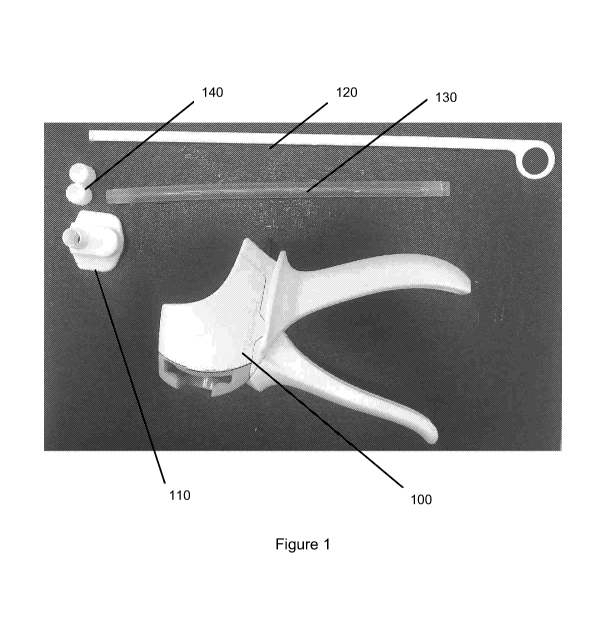
referring
to Figures 1 and 2A-B, the exemplary dispensing gun 100, adapter 110, plunger
120
(see also Figure 3), tube(s) 130 (see also Figures 5A and 5B), caps 140, and
assort-
ed dispensing tips (optional; Figure 4A and Figure 4B) that may be included
with the
kits are shown. The irrigation resistant bone repair composition may be
deposited
into the tube(s) 130 as part of the kit (Figure 5A). An exemplary kit for
delivery of
other materials, such as Bioactive Synthetic Bone Graft Putty is currently
being sold
by NOVABONE (NOVABONE Bioactive Synthetic Bone Graft Putty MIS Cartridge
Delivery System, NovaBone Products, LLC, Alachua, FL).
[00105] Referring to Figures 2A-B, the dispensing gun 100 may include a
cover
150, a latch 160, a lever 170 and a handle 180 (Figure 2B). The adapter 110
(shown
also in Figure 2A) may be inserted into the dispensing gun at an opening 111.
A
plunger (not shown) may be inserted through the front of the gun and pushed
through the opening in the back 190 of the gun.
[00106] Figure 3 depicts an exemplary plunger 120 including gradient
markings
200 facing up.
[00107] Figures 4A-B depict exemplary tips for use with the dispensing gun.
The
tips may be straight (Figure 4A) or at an angle (Figure 4B).
[00108] Figure 5A is a picture of tubes filled with the irrigation
resistant bone re-
pair composition; Figure 5B is a graphical illustration of an exemplary tube
for use
with the kit and specifically with the delivery gun described above. The tubes
have a
substantially constant inner diameter along their entire length such that the
outlets
have substantially the same inner diameters as the rest of the tubes.
[00109] Optionally, a "Y" connector, luer syringe and a tube connector may
be
included to facilitate the simultaneous delivery of biologics and to maintain
position
during shipping (as shown in Figure 9).
[00110] The components of a kit may be packaged and sold as a kit. The com-
ponents of a kit may snap fit into a (inner) tray of a packaging and a
retainer may be
placed over the components of the kit to maintain position of the components
during
shipping. The inner tray may hold up to four tubes that can be prefilled with
the irri-
gation resistant bone repair composition and capped on each end. The inner
tray
21
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
may also contain cavities for the placement of assorted tips, a "Y" connector,
tube
connector, a syringe and aspiration needle.
[00111] The inner tray may be sealed with a lid and placed into an outer
tray also
sealed with a lid. The sealed trays are radiation sterilized for use in
medical applica-
tions. The sealed trays may then be placed in a box.
[00112] Immediately prior to use, the kit may be placed in an operating
room and
the outer tray is opened. The inner tray is removed by a sterile technician
and
placed into the sterile field.
[00113] In the sterile field the inner tray is opened and the dispensing
gun is as-
sembled by inserting the finger grip of the plunger 120 (with the gradient
markings
200 facing up and teeth facing down) through the opening in the front of the
gun 100
and pushing the plunger through the back of the gun until the piston end of
the
plunger is seated completely within the gun (see Figures 6A, 7 and 8). The
adapter
110 is then inserted into the front of the gun 100. Next a prefilled tube is
removed
from the inner tray. One cap is removed from the prefilled tube. The tube is
thread-
ed into the adapter and the other cap is removed from the tube (Figure 6B).
Option-
ally a tip can be placed on the end of the tube to direct the flow of the
graft material.
[00114] The tip of the instrument may be placed into the surgical site.
Upon
pressing the trigger of the gun, the plunger is ratcheted forward to express
the bone
grafting material into the surgical site. The dispensing gun consists of, a
handle, in
which a block is moved forward through pressing the trigger which engages the
teeth
of the plunger moving the piston forward displacing the material from the
tube. The
trigger is manually disengaged by pushing the lever at the back of the
dispensing
gun upward allowing the plunger to be pulled back to a starting position. The
first
tube can be removed from the adapter and additional tubes can be threaded in
place
as needed.
[00115] Another embodiment involves altering the adapter for the attachment
of
two tubes and the plunger modified from a single piston to one have two
pistons
moving simultaneously with each compression of the trigger. Subsequently, the
plungers dispense the material from the two tubes through a static mixer to
facilitate
the addition of a biological or drug material into the non-setting bone
grafting material
during injection into the surgical site. Any of the above-described aspects
and em-
22
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
bodiments of the invention may be in injectable form. Injection may occur by
means
of a syringe, for example. The compositions are particularly useful when
injected in
a gel or liquid form into a bone gap or bone defect. The injected gel or
liquid would
then solidify at body temperature when placed on or near the bone gap or the
bone
defect.
[00116] Example 1
[00117] The purpose of the study was to establish a standard for evaluating
the
handling of irrigation resistant matrix (IRM) samples when immersed in water.
[00118] Materials used in the study were IRM samples as noted in Tables 2-
28,
deionized water, and crystallization dish. Equipment used included hotplate
and an-
alytical balance.
[00119] To preparing for the test deionized water was dispensed into the
crystal-
lization dish so that a 5 ¨ 7.5 g IRM sample was completely immersed. The
crystalli-
zation dish was then set on the hot plate and heated to 37 C
[00120] Next to run the immersed compression test, each IRM sample (5 ¨ 7.5
g)
was molded into a sphere. With the crystallization dish still on the hotplate,
each
sample was pressed flat against the bottom of the dish. The sample was then
picked
up out of the water bath, remolded into a sphere, and the process was
repeated. The
steps were repeated until the sample could no longer be picked up or no longer
molded into a sphere.
[00121] The pass/fail criteria included: each sample that was pressed and
picked up more than once passed the testing; each sample that was only pressed
and picked up once failed the testing. The term "immersed compression" refers
to
the property of stiffness or the ability of the material to be manipulated
under warm
water while the material is still intact.
[00122] The grading used included: 0 and 1 unsatisfactory; 2-4 acceptable;
and >
4 outstanding.
[00123] The results are shown in Tables 2-28.
23
CA 02983486 2017-10-19
WO 2016/172426
PCT/US2016/028764
[00124] Table 2
BG
% Total BG 1- BG 32pm- MYRJ Sodium Polyglycerol
90pm-
Immersed
BG mass 2mm 125pm S25 Hyaluronate sebacate
710pm
Compression
ok 67 30.15 26.80 10.05 13.2 0.3 19.8 0
Kg-01-
08-B-1 67 50 15.075 13.4 5.025 6.6 0.15 9.9 0
[00125] Table 3
PEG PEG
All BG Poly- Glyceryl
Sodium
% Total
bio-
BG 90pm- 32pm_ 400 400 di-
propylene Mono- Hyalu-
Immersed
BG mass 710pm monos- stea-
Compres-
glass 125pm glycol stearate
ronate
tearate rate sion
ok 73 0.00 56.00 13.00 5.7 14.5 5.7 5.7 0.3
Kg-01-
18-A 73 47.6 0 28 6.5 7.25 2.85 0 2.85
0.15 0
Kg-01-
18-B 73 47.6 0 28 6.5 2.85 8.25 0 1.85
0.15 0
Kg-01-
18-C 73 47.6 0 28 6.5 2.85 7.25 0 2.85
0.15 0
Kg-01-
18-D 73 47.6 0 28 6.5 0 7.25 2.85 2.85
0.15 0
Kg-01-
18-E 73 47.6 0 28 6.5 0 7.75 3.35 1.85
0.15 0
[00126] Table 4
BG BG PEG PEG
% Total 400
Poly- Glyceryl
Sodium
Immersed
90pm- 32pm- 400 propylene Mono-
BG mass dis- Hyaluronate Compression
710pm 125pm MONO
tearate glycol stearate
ok 73 56.00 13.00 5.7 14.5 5.7 5.7 0.3
Kg-01-
19-Al 73 95.2 56 13 14.5 5.7 0 5.7 0.3 6
Kg-01-
19-A2 73 95.2 56 13 14.5 0 5.7 5.7 0.3 4
Kg-01-
19-E1 73 95.2 56 13 0 15.5 6.7 3.7 0.3 3
Kg-01-
19-E2 73 95.2 56 13 0 15.5 6.7 3.7 0.3 4
[00127] Table 5
PEG
BG PEG polypro- Cande- Sodium
% Total 90pm- BG 32pm-
400 400 Immersed
pylene Ulla Sorbitol Hyalu-
BG mass 125pm dis-
Compres-
710pm MONO glycol Wax ronate
tearate sion
ok 73 59.00 13.65 14.5 15.2 6.0 6.0 .0 0.3
24
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
PEG
BG PEG polypro- Cande- Sodium
% Total BG 32pm- 400
Immersed
90pm- 400
dis- pylene Ulla Sorbitol Hyalu-
BG mass 125pm
Compres-
710pm MONO tearate glycol Wax
ronate
sion
Kg-01-
22-A 73 49.425 29.5 6.825 7.25 0 2.85 2.85 0 0.15 4
Kg-01-
22-B 73 49.425 29.5 6.825 0 7.75 3.35 1.85 0 0.15 6
Kg-01-
22-C 73 49.425 29.5 6.825 7.25 0 2.85 0 2.85 0.15 0
Kg-01-
22-D 73 49.425 29.5 6.825 0 7.75 3.35 0 1.85 0.15 0
[00128] Table 6
PEG
BG PEG 400 poly-
poly- Immersed
90pm- BG 400 distea poly- pro-
capro- Sodium Com-
pylene
% Total 710p 32pm- MON tea- capro- Cande- 1
col lactone Hyalu- pression
BG mass m 125pm 0 rate lactone !ilia Wax g
Y diol ronate
Kg-01-
23-A 73 98.85 59 13.65 14.5 0 0 5.7 5.7 0 0.3
6
Kg-01-
23-C-1 73 49.425 29.5 6.825 7.25 0 0 0 2.85 2.85
0.15 4
Kg-01-
23-C-2 73 49.425 29.5 6.825 0 7.75 0 0 3.35 1.85
0.15 4
Kg-01-
23-C-3 73 49.425 29.5 6.825 7.25 0 0 0 2.85 2.85
0.15 5
Kg-01-
23-D-1 73 49.425 29.5 6.825 7.25 0 2.85 0 2.85 0 0.15
0
Kg-01-
23-D-2 73 49.425 29.5 6.825 0 7.75 1.85 0 3.35 0 0.15
0
[00129] Table 7
BG
32p
BG m- PEG Glyceryl Poly- Sodium
Immersed
% Total 90pm- 125p 400 Candelilla Mono-
Sesame caprolac- Hyalu- Compres-
BG mass 710pm m MONO Wax stearate Oil tone
diol ronate sion
Kg-01-
25-S-2 73 49.9 29.5 7 7.25 0 3 3 0 0.15 4
Kg-01-
25-S-4 73 49.9 29.5 7 7.25 3 0 3 0 0.15 4
Kg-01-
25-S-5 73 49.9 29.5 7 7.25 0 0 3 3 0.15 2
[00130] Table 8
BG Im-
90pm- BG Glyceryl poly-
Sodium mersed
% Total 710p 32pm- PEG 400 Candelilla Monos-
Coconut caprolac- Hyalu- Com-
BG mass m 125pm MONO Wax tearate Oil
tone diol ronate pression
RK-02-
3-D2 73 49.9 29.5 7 7.25 0 3 3 0 0.15
2
RK-02-
3-D4 73 49.9 29.5 7 7.25 3 0 3 0 0.15
2
RK-02- 73 49.9 29.5 7 7.25 0 0 3 3 0.15
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
3-D5 1
[00131] Table 9
BG BG Im-
90pm- 32pm- Glyceryl
polycapro- Sodium mersed
% Total 710p 125p PEG 400 Candelilla Monos-
Propylene lactone Hyalu- Com-
BG mass m m MONO Wax tearate Glycol
diol ronate pression
RK-02-
3-E2 73 49.9 29.5 7 7.25 o 3 3 o 0.15 2
RK-02-
3-E4 73 49.9 29.5 7 7.25 3 o 3 o 0.15 3
RK-02-
3-E5 73 49.9 29.5 7 7.25 o o 3 3 0.15 2
[00132] Table10
Im-
Bio-
mers
Total Bio- glass PEG Glycer-
Sodi- ed
sam- glass 32pm- 400 Poly- poly- yl Ses-
um Com
% pie
90pm- 125p MON capro- caprolac- polycaprolac- Monos- ame Hyalu- pres-
BG mass 710pm m 0 lactone tone diol tone diol
tearate Oil ronate sion
10k
mw Diol Triol
Kg-01-
27-Al 73 49.9 29.5 7 7.25 0 3 3 o 0 0.15 2
Kg-01-
27-A2 73 49.9 29.5 7 o o 3 10.25 o 0 0.15 2
Kg-01-
27-A3 73 49.9 29.5 7 o o 5 8.25 o 0 0.15 2
Kg-01-
27-B1 73 49.9 29.5 7 7.25 3 o 3 o 0 0.15 0
Kg-01-
27-B2 73 49.9 29.5 7 o 3 o 10.25 o 0 0.15 0
Kg-01-
27-B3 73 49.9 29.5 7 o 5 o 8.25 o 0 0.15 0
[00133] Table 11
BG PEG Poly
Immersed
% Total 90pm- BG 32pm- 400 propylene
Glyceryl Sodium Com-
BG mass 710pm 125pm MONO glycol Monostearate Hyaluronate pression
Kg-01-
28-A 73 47.6 28 6.5 7.25 2.85 2.85 0.15 5
26
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
[00134] Table 12
BG BG PEG Glyceryl poly-
Sodium Immersed
% Total 90pm- 32pm- 400 Candelilla Mono- capro-
Hyalu- Com-
BG mass 710pm 125pm MONO Wax stearate lactone ronate pression
triol diol
RK-02-
4-F2 73 49.9 29.5 7 7.25 o 3 3 0 0.15 4
RK-02-
4-F4 73 49.9 29.5 7 7.25 3 o 3 0 0.15 3
RK-02-
4-F5 73 49.9 29.5 7 7.25 o o 3 3 0.15 3
[00135] Table 13
BG BG Poly pro-
% Total 90pm- 32pm- Bioglass PEG 400
pylene Glyceryl Sodium Immersed
BG mass 710pm 125pm 2-5mm Monostearate glycol Monostearate Hyaluronate
Compression
Kg-01-
29-A 66 47.6 7.75 23.81 7.25 2.85 2.85 0.15 o
[00136] Table 14
BG Poly
BG 32p Mineral ethylene Glycer-
90pm- m- Mineral Oil Oil Low glycol yl
Sodium Immersed
% Total 710p 125p PEG 400 High Viscosi- DiMethyl
PEG Monos- Hyalu- Com-
BG mass m m MONO Viscosity ty Ether -8
tearate ronate pression
Kg-01-
30-E 73 49.9 29.5 7 7.25 3 o o o 3 0.15 4
Kg-01-
30-F 73 49.9 29.5 7 7.25 o 3 o o 3 0.15 3
Kg-01-
30-G 73 49.9 29.5 7 7.25 o o 3 o 3 0.15 2
Kg-01-
30-H 73 49.9 29.5 7 7.25 o o o 3 3 0.15 2
[00137] Table 15
Poly
eth-
ylene
BG BG PEG
Mineral
PEG Sodium
Mineral glycol Im-
Oil
Oil Low DiMe- mersed
% Total 90pm- 32pm- 400 Cande- High
-8 Hyalu- Com-
BG thyl y Com-
BG mass 710pm 125pm MONO Dila Wax Viscosity
ronate
Ether
pression
Kg-01-
30-M 73 49.9 29.5 7 7.25 3 3 o o 0
0.15 3
Kg-01-
30-N 73 49.9 29.5 7 7.25 3 o 3 o 0
0.15 2
Kg-01-
30-0 73 49.9 29.5 7 7.25 3 o o 3 0
0.15 1
Kg-01-
30-P 73 49.9 29.5 7 7.25 3 o o o 3
0.15 3
27
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
[00138] Table 16
BG
32 Im-
pm Mineral
Poly- mersed
BG
1 400 High Vis-
- PEG Mineral Oil Oil Low Poly eth-
capro- Corn-
Total 90pm-
ylene glycol lac- Sodium
pression
DiMethyl PEG- tone Hyalu-
% 5p
mass 710pm MONO Viscosity cosity
BG m Ether 8 diol ronate
Kg-01-
30-Q 73 49.9 29.5 7 7.25 3 0 0 0 3 0.15 1
Kg-01-
30-R 73 49.9 29.5 7 7.25 0 3 0 0 3 0.15 1
Kg-01-
30-S 73 49.9 29.5 7 7.25 0 0 3 0 3 0.15 1
Kg-01-
30-T 73 49.9 29.5 7 7.25 0 0 0 3 3 0.15 3
[00139] Table 17
BG
Poly-
Immersed
% Total 90p BG PEG
Candelilla Propylene Sodium
m- 32pm- 400 caprolactone
Compression
BG mass
Wax Glycol Hyaluronate
710 125pm MONO diol
Pm
RK-02-
7-E4 73 99.8 59 14 14.5 6 6 0 0.3 0
RK-02-
7-E5 73 99.8 59 14 14.5 0 6 6 0.3 0
[00140] Table 18
PEG poly-
Glyceryl PEG Sodium
Total BG BG 400 Candelilla
%BG 90pm- 32pm- capro-
Immersed
Monos- 200 Hyalu-
mass MON Wax lactone Com-
710pm 125pm0 tearate adipate diol
ronate
pression
Kg-01-
31-W 73 49.9 29.5 7 7.25 0 3 3 0 0.15
3
Kg-01-
31-Y 73 49.9 29.5 7 7.25 3 0 3 0 0.15
2
Kg-01-
31-Z 73 49.9 29.5 7 7.25 0 0 3 3 0.15
3
[00141] Table 19
BG
% Total BG 90pm- 32pm- PEG 400
PEG 400 DI Propylene PCL
Sodium
Immersed
BG mass 710pm 125p MONO Glycol diol
Hyalu-
ronate
Compression
m
Kg-01-
34-E51 73 49.9 29.5 7 7.25 3 3 0.15
0
Kg-01-
34-E52 73 49.9 29.5 7 2 5.25 3 3 0.15
2
Kg-01-
34-E53 73 49.85 29.5 7 3.6 3.6 3 3 0.15
2
28
CA 02983486 2017-10-19
WO 2016/172426
PCT/US2016/028764
[00142] Table 20
BG BG
ok Total 90pm- 32 PCL propylene Sodium
pm-
diol glycol Hyaluronate
BG mass
Immersed
710pm 125pm
Compression
Kg-01-35-10 74 49.95 29.5 7 10.3 3 0.15
1
[00143] Table 21
BG BG Name of
% Total Propyleme Variable
Immersed
BG mass 90pm- 32pm-
Glycol Component Variable
Compression
710pm 125pm Component
Ethylene
Glycol
ZT-02-19-4 73 49.7 29.5 7 5.1 8.1
Monostearate 0
Propylene
Glycol
ZT-02-19-5 73 49.7 29.5 7 5.1 8.1 Stearate
0
ZT-02-19-6 73 49.7 29.5 7 5.1 8.1 Brij 2
2
ZT-02-19-7 73 49.7 29.5 7 5.1 8.1 Brij 10
0
ZT-02-19-8 73 49.7 29.5 7 5.1 8.1 Brij 20
0
ZT-02-19-9 73 49.7 29.5 7 5.1 8.1 Brij 100
1
PEG 6000
ZT-02-19-1 73 49.7 29.5 7 5.1 8.1 Distearate
0
Sorbitan
ZT-02-19-2 73 49.7 29.5 7 5.1 8.1
Monostearate 0
ZT-02-19-3 73 49.7 29.5 7 5.1 8.1 Cetyl
Alcohol 0
[00144] Table 22
BG BG Name of
% Total PEG Variable
BG mass 90pm- 32pm-
A Variable Immersed
dipate Component
710pm 125pm Component Compression
PEG 6000
ZT-02-20-A1 73 49.7 29.5 7 5.1 8.1 Distearate 3
ZT-02-20-A6 73 49.7 29.5 7 5.1 8.1 Brij 2 2
ZT-02-20-A7 73 49.7 29.5 7 5.1 8.1 Brij 10 3
ZT-02-20-A8 73 49.7 29.5 7 5.1 8.1 Brij 20 3
ZT-02-20-A9 73 49.7 29.5 7 5.1 8.1 Brij 100 2
Sorbitan
ZT-02-20-A2 73 49.7 29.5 7 5.1 8.1 Monostearate 0
ZT-02-20-A3 73 49.7 29.5 7 5.1 8.1 Cetyl Alcohol 0
ZT-02-20-A4 49.7 29.5 7 5.1 8.1 Ethylene Gly-
29
CA 02983486 2017-10-19
WO 2016/172426
PCT/US2016/028764
BG BG Name of
% Total
90pm- 32pm- PEG Variable
Variable Immersed
BG mass Adipate Component
710pm 125pm Component Compression
col Monos-
73 tearate 0
Propylene
Glycol Stea-
ZT-02-20-A5 73 49.7 29.5 7 5.1 8.1 rate 0
Calcium Stea-
ZT-02-20-A10 73 49.7 29.5 7 5.1 8.1 rate
0
[00145] Table 23
BG BG Name of
% Total Butylene Variable
pp Variable Immersed
90m- 32m-
BG mass Glycol Component
710pm 125pm Component Compression
Ethylene
Glycol
ZT-02-20-B4 73 50.9 29.5 7 6.3 8.1
Monostearate 3
Propylene
Glycol Stea-
ZT-02-20-B5 73 51.7 29.5 7 7.1 8.1 rate
2
Propylene
ZT-02-20- Glycol Stea-
MB5 73 48.8 29.5 7 8.3 4 rate 2
ZT-02-20-B6 73 49.7 29.5 7 5.1 8.1 Brij 2
4
ZT-02-20-B7 73 49.7 29.5 7 5.1 8.1 Brij 10
2
ZT-02-20-B8 73 49.7 29.5 7 5.1 8.1 Brij 20
9
ZT-02-20-B9 73 50.2 29.5 7 5.6 8.1 Brij 100
2
PEG 6000
ZT-02-20-B1 73 49.7 29.5 7 5.1 8.1
Distearate 0
Sorbitan
ZT-02-20-B2 73 49.7 29.5 7 5.1 8.1
Monostearate 0
ZT-02-20-B3 73 49.7 29.5 7 5.1 8.1 Cetyl
Alcohol 0
ZT-02-20- Calcium
B10 73 49.7 29.5 7 5.1 8.1 Stearate 0
[00146] Table 24
BG BG Name of
% Total Hexylene Variable
90pm- 32pm- Variable Immersed
BG mass Glycol Component
710pm 125pm Component Compression
ZT-02-20- Sorbitan
H2 73 50.2 29.5 7 5.6 8.1 Monostearate 0
Ethylene
ZT-02-20- Glycol
H4 73 50.7 29.5 7 6.1 8.1 Monostearate 1
Propylene
ZT-02-20- Glycol Stea-
H5 73 49.7 29.5 7 5.1 8.1 rate 1
ZT-02-20-
H6 73 49.7 29.5 7 5.1 8.1 Brij 2 1
ZT-02-20-
H7 73 49.7 29.5 7 5.1 8.1 Brij 10 1
CA 02983486 2017-10-19
WO 2016/172426
PCT/US2016/028764
BG BG Name of
% Total Hexylene Variable
BG mass 90pm- 32pm-
Glycol Component Variable
Immersed
710pm 125pm Component Compression
ZT-02-20-
H9 73 49.7 29.5 7 5.1 8.1 Brij 100 1
ZT-02-20- PEG 6000
H1 73 49.7 29.5 7 5.1 8.1 Distearate 0
ZT-02-20-
H3 73 49.7 29.5 7 5.1 8.1 Cetyl Alcohol 0
ZT-02-20-
H8 73 49.7 29.5 7 5.1 8.1 Brij 20 1
ZT-02-20- Calcium
H10 73 49.7 29.5 7 5.1 8.1 Stearate 0
[00147] Table 25
BG
BG õ Propylene Ethylene
Immersed
% Total 90pm- --P Hexylene Cetyl PEG PEG
GI col Glycol Compres-
BG mass 710p
12115-p Glycol Alcohol 6E0i0s0 Adipate
Steayrate Mt onots- sion
m m tearate
ZT-02-
23-M1H1 73 49.7 29.5 7 7.2 0 6 0 0 0 1
ZT-02-
23-M2H1 73 49.7 29.5 7 9 0 4.2 0 0 0 0
ZT-02-
23-M1H3 73 49.7 29.5 7 7.2 6 0 0 0 0 0
ZT-02-
23-M2H3 73 49.7 29.5 7 9 4.2 0 0 0 0 0
ZT-02-
23-M1A4 73 49.7 29.5 7 0 0 7.2 0 6 0
ZT-02-
23-M2A4 73 49.7 29.5 7 0 0 9 0 4.2 0
ZT-02-
23-M1A5 73 49.7 29.5 7 0 0 7.2 6 0 0
[00148] Table 26
BG BG
ok Total
BG mass 90pm- 32pm- Brij 20 Brij 100
Propylene Immersed
710pm 125pm Glycol Compression
ZT-02-24-1 73 49.7 29.5 7 0 6 7.2 2
ZT-02-24-2 73 49.7 29.5 7 8.1 0 5.1 9
[00149] Table 27
BG BG
ok Total Propylene
90pm- 32pm- Brij 20 Brij 100 Immersed
BG mass
710pm 125pm Glycol
Compression
Kg-01-37-1 49.7 29.5 7 4 3 6.2
31
CA 02983486 2017-10-19
WO 2016/172426 PCT/US2016/028764
1 73 1 1 1 1 1 1 I 1
I
[00150] Table 28
BG BG
ok Total 90pm- 32pm- Propylene Sodium Immersed
BG mass 710pm 125pm Brij 20 Glycol
Hyaluronate compression
Kg-01-37-6 73 49.85 29.5 7 8.1 5.1 0.15 8
Kg-01-37-7 73 49.75 29.5 7 8.1 5.1 0.05 8
Kg-01-37-8 72 50.85 29.5 7 8.1 6.1 0.15 8
[00151] Overall the irrigation data shows that samples can be manipulated
in
an aqueous environment without migrating, washing away or being displaced from
the site.
[00152] Throughout this specification various indications have been given
as to
preferred and alternative embodiments of the invention. However, the foregoing
de-
tailed description is to be regarded as illustrative rather than limiting and
the inven-
tion is not limited to any one of the provided embodiments. It should be
understood
that it is the appended claims, including all equivalents, are intended to
define the
spirit and scope of this invention.
32