Note: Descriptions are shown in the official language in which they were submitted.
CA 02983626 2017-10-23
WO 2016/169679
PCT/EP2016/054180
1
Device and process for automated extraction of nucleic acids
[0001] The subject matter of the invention is a device and a process with
which nucleic acids
can be isolated and purified rapidly and highly efficiently as well as
quantitatively in an
automated process.
[0002] Under traditional conditions, DNA is isolated from cells and tissues by
digesting the
starting materials containing nucleic acids under strongly denaturing and
reducing conditions,
sometimes also with use of protein-degrading enzymes, purifying the resulting
nucleic acid
fractions via phenol/chloroform extraction steps and obtaining the nucleic
acids from the
aqueous phase by means of dialysis or precipitation with ethanol (Sambrook,
J., Fritsch, E.F.
and Maniatis, T., 1989, CSH, "Molecular Cloning"). These "traditional methods"
for isolation
of nucleic acids from cells and especially from tissues are very time-
consuming (sometimes
longer than 48 hours), require highly complex apparatus and beyond that are
also not feasible
under field conditions. Moreover, such methods are hazardous to health to a
not inconsiderable
degree because of the chemicals used, such as phenol and chloroform.
[0003] The next generation of methods for isolation of nucleic acids is based
on a method for
preparative and analytical purification of DNA fragments from agarose gels,
developed and
described for the first time by Vogelstein und Gillespie (Proc. Natl. Acad.
Sci. USA, 1979, 76,
615 - 619). The method combines the dissolution of the agarose containing the
DNA bands to
be isolated in a saturated solution of a chaotropic salt (NaI), with binding
of the DNA on glass
particles. The DNA fixed on the glass particles is then washed with a washing
solution (20 mM
Tris HC1 [pH 7.2]; 200 mM NaCl; 2 mM EDTA; 50% v/v ethanol) and then detached
from the
carrier particles. Heretofore this method has undergone a series of
modifications and at present
is applied for different methods of extraction and purification of nucleic
acids from different
sources, ultimately becoming the basis for almost all commercially available
kits for manual
and also automated isolation of nucleic acids. Furthermore, numerous patents
and publications
are now known that relate to the basic principle of isolation of nucleic acids
published for the
first time by Vogelstein and Gillespie, some of them containing further
advantages. These
variants concern both the use of different mineral carrier materials and the
type of buffers used
for binding the nucleic acids. Examples include the binding of nucleic acids
on mineral carriers
CA 02983626 2017-10-23
WO 2016/169679
PCT/EP2016/054180
2
in the presence of solutions of different chaotropic salts, in which finely
ground glass powder
(B10 101, La Jolla, CA), diatomaceous earths (Sigma Co.) or even silica gels
or silica
suspensions or glass-fiber filters or mineral ores (DE 41 39 664 Al; US
5,234,809; WO-A
95/34569 DE 4321904; DE 20207793) are used as carrier materials. All of these
patents are
based on the binding of nucleic acids on a mineral carrier material on the
basis of glass or
silicon in the presence of chaotropic salt solutions. In more recent patent
specifications, it is
disclosed that so-called anti-chaotropic salts as components of lysing/binding
buffer systems
can likewise be used very efficiently and successfully for adsorption of
nucleic acids on the
mineral materials known to and used by the person skilled in the art (EP
1135479). In summary,
the prior art may therefore be described to the effect that nucleic acids bind
to mineral materials
in the presence of buffers that contain chaotropic or anti-chaotropic salts or
even in the presence
of buffers that contain mixtures of chaotropic and anti-chaotropic salts, and
in this way can then
also be isolated. In this connection, preferred variants are also known in
which aliphatic
alcohols are additionally used for mediation of binding. It is also known to
the person skilled in
the art that all common commercial products for isolation and purification of
nucleic acids are
based on this principle. The mineral carriers used for this purpose have the
form of loose bulk
materials, the form of filter membranes or even the form of suspensions.
Paramagnetic or
magnetic particles are often used to perform automated extraction processes.
Examples of these
are silicate materials with a magnetic or paramagnetic core, or else iron
oxide particles, the
surface of which has been modified such that they have the functionalities
necessary for binding
nucleic acids. Modified pipette tips have been used, especially so that
automated extractions can
be performed more easily. These are characterized in that they already contain
the carrier
materials (porous mineral carrier materials or porous anion exchangers, etc.)
necessary for
binding nucleic acids. Thus patent specification DE3717211 describes a pipette
tip with a
porous chromatography material for isolation of nucleic acids. Patent
specification EP1951904
discloses a pipette tip consisting of an upper and lower part, between which a
porous
chromatographic carrier material is likewise disposed and which is intended
for use in the
automated isolation of nucleic acids. A modified pipette tip for extraction of
nucleic acids is
also disclosed in patent specification US2013/0078619. This pipette tip also
contains a porous
mineral carrier material (porous glass) for direct binding of nucleic acids.
It is common to all of
CA 02983626 2017-10-23
WO 2016/169679
PCT/EP2016/054180
3
these modified pipette tips that they contain a porous chromatographic
material (loose bulk
material or solid porous bodies). These carrier materials are always disposed
horizontally inside
the pipette tips. The liquids to be processed flow through the porous material
being used. The
extraction process is based on the fact that, after lysis of the sample and
adjustment of necessary
binding conditions for adsorption of the nucleic acids on the carrier
material, this mixture is
drawn by means of a pipetting process through the porous carrier material. The
nucleic acids
bind on the carrier material. Thereupon washing buffers are pipetted through
the carrier
material. Then a drying step is performed (by frequently filling and emptying
the pipette or by
applying vacuum). Finally, the eluent is pipetted through the carrier
material. In the process, the
bound nucleic acid is detached from the carrier material. The use of pipette
tips containing
carrier material is intended to greatly simplify the extraction of nucleic
acids (especially) by an
automated process. Although these ideas are already relatively old in some
cases (patent
specification DE3717211 dates back to 22 May 1987), such a method has not
become widely
accepted. The reason for this lies in some fundamental problems:
1) The pipetting of highly viscous lysates containing nucleic acids
functions to
only a limited extent or leads to complete clogging of the chromatographic
material. Thus extraction is not possible.
2) The pipetting of lysates through a porous material causes foaming. This is
intensified with the increasing number of pipetting steps and it can likewise
make the extraction process impossible.
3) The removal of alcoholic components from a porous material is difficult
and in many cases is not satisfactorily solved.
[0004] The object underlying the invention was therefore to solve the known
problems and thus
to make it possible to perform the automated extraction of nucleic acids much
more easily and
rapidly than heretofore. A further goal of the invention is to make it
possible to use existing
liquid-handling instrument platforms for automated extraction of nucleic
acids. It is intended
that this will be universally possible with simple means.
[0005] The object has been achieved according to the features of the claims.
Claim 1 describes a
device for automated extraction of nucleic acids, comprising a body that can
be immersed partly
CA 02983626 2017-10-23
WO 2016/169679
PCT/EP2016/054180
4
or completely in a reaction cavity, wherein at least the part immersed in the
reaction cavity has a
rough or structured surface. It is preferable to use a corresponding pipette
tip, which either has
been roughened or onto which a rough or structured object has been slipped.
The nucleic acids
are precipitated onto this object when the polarity of the solution is
lowered. This takes place
preferably either by addition of an organic solvent (alcohols) or by a binding
buffer known in
the prior art. It has been found that binding of the nucleic acids on this
object does not take
place if the object has a smooth surface. Claims 2 to 6 relate to preferred
embodiments of the
invention.
[0006] Subject matter of the invention is also an instrument according to the
walk-away
principle, with which the inventive device is equipped, e.g. an automated
pipetting system or an
automated extraction system.
[0007] The basis of the invention is the observation that nucleic acids are
adsorbed on the
surface of structured or rough materials (e.g. on polymer materials). For this
purpose, it is
merely necessary to lyse a biological sample containing a nucleic acid, in
order to liberate the
nucleic acid. This can be accomplished with buffers known to the person
skilled in the art. After
lysis, a substance that lowers the polarity of the aqueous solution,
preferably organic solvents
such as alcohols or ketones, is mixed with the sample. This mixture is now
brought into contact
with a material characterized by a non-smooth surface. Under these conditions,
nucleic acids are
adsorbed on the surface of the material being used. Thereupon washing steps
with known
alcoholic washing solutions may be carried out. After drying, the adsorbed
nucleic acid is
detached from the material by addition of water or a buffer of low salt
concentration (e.g. 10
mM Tris HCl), whereupon it can be used for downstream applications. The
inventive device
and the inventive method use this capability for a simple and automated
extraction process. This
device consists of a body that can be immersed partly in a reaction cavity,
wherein the part
immersed in this reaction cavity has a non-smooth surface. It is preferable to
use a hollow body,
which can receive and release liquids. A pipette tip is used particularly
preferably. The pipette
tip is constructed such that a structured or rough material is disposed on its
outer surface in the
last bottom third. This may be achieved, for example, by slipping on a fitting
ring (e.g. such a
ring can be slipped onto common pipette tips, thus emphasizing the
universality). However, the
hollow body itself may likewise have such a structural feature (roughness) and
thus consist of
CA 02983626 2017-10-23
WO 2016/169679 PCT/EP2016/054180
one part and be produced in an injection-molding die. An example of the
inventive device for a
modified pipette tip is sketched in Fig. 1.
[0008] The invention also relates to a process for automated extraction of
nucleic acids,
characterized by the following steps:
a) A lysed biological sample is introduced into a reaction cavity and at
least one substance
that lowers the polarity of the aqueous solution or a means for binding
nucleic acids on a
solid phase is mixed therewith
b) A device according to one of claims 1 to 5 is immersed in this cavity,
whereupon the
nucleic acids bind to the rough or structured part of this device
c) If necessary, the device is transferred into at least one further cavity
for washing the bound
nucleic acids
d) If necessary, the bound nucleic acids are dried
e) The dried, bound nucleic acids are transferred into a further cavity for
elution of the nucleic
acids
100091 The term "rough surface" is to be understood as a surface that is
obviously not smooth to
the touch or to the eye. However, it may also be a surface that has a
structure (e.g. grooves).
Because of this structure, the smoothness of the surface is eliminated, even
if the structure, i.e.
the grooves, may itself be smooth. According to the invention, such surfaces
are referred to as
"structured surfaces". If it is not obvious to the eye or to the touch whether
a surface is smooth
or rough, a test in which a laser beam is directed onto this surface may be
performed. If the
surface is smooth, the laser will be reflected only in the primary direction
at the surface. In the
case of rough surfaces, scattering takes place in all spatial directions. Such
a test has been
described.
100101 The inventive device is used with the inventive process for automated
extraction of
nucleic acids as follows: Preferably a traditional walk-away principle is
applied, i.e. the
solutions needed for extraction are introduced beforehand and successively
involved in the
Date Recue/Date Received 2021-07-16
CA 02983626 2017-10-23
WO 2016/169679
PCT/EP2016/054180
6
extraction process. Corresponding to the stated goal of the present invention,
even commercially
available automated extraction systems or automated pipetting systems may be
used for the
automated extraction process, provided they meet the necessary technical
criteria. The sample is
introduced into a reaction cavity, then lysis buffer and if necessary
proteolytic enzymes are
mixed therewith. Thereupon sample lysis takes place. After lysis of the sample
with known
lysing buffers, an organic component is added to the lysate. The inventive
device is immersed in
this solution and is moved vertically up and down several times in the
solution. Now the nucleic
acids are disposed on this device. Thereupon the device is removed from the
solution and made
to move in a new reaction cavity. This contains an alcoholic washing buffer
(known washing
buffers may also be used for this purpose) or only an alcohol. The inventive
means is immersed
in this solution and is moved vertically up and down several times in the
solution. The washing
steps may be repeated several times. After the last washing step, the device
is removed from the
solution and dried briefly outside the cavity, so that the remaining alcohol
is removed. In the
last step, the inventive device is immersed in a further cavity, in which
water or another buffer
of low salt concentration is disposed. The inventive device is also immersed
in this cavity and is
moved vertically up and down several times in the solution. This leads to
detachment of the
bound nucleic acids. From this general process protocol, it is obvious how
simple the automated
extraction is. It is no longer necessary to separate magnetic particles, as
would otherwise be the
case during automated extraction by means of magnetic particles. The method
does not need
any vacuum-filtration steps, as are required when filter plates are used. It
requires only the
inventive device as well as a pipetting platform. In this connection, the
universality and
simplicity of the inventive device are naturally advantageous, since
commercially available
standard pipette tips can be used. These pipette tips are modified in such a
way by slipping on a
ring, for example, with the specific surface property needed for isolation of
nucleic acids, that
they become the inventive device and thus any appropriate pipetting platforms
may be used for
the isolation of nucleic acids.
[0011J Furthermore, the reagents needed for extraction may already be
introduced beforehand
into appropriate reaction cavities, so that the extraction process can take
place according to the
walk-away principle. A further particular advantage is disclosed in that the
inventive means
permits not only binding of the nucleic acid but furthermore is also still
able to move liquids in
CA 02983626 2017-10-23
WO 2016/169679
PCT/EP2016/054180
7
separate ways. In this combined function, the extraction process can be still
further optimized.
Thus lysis of the sample can already be achieved on an automated system. The
continuous
movement of the sample needed for lysis is achieved by the pipetting function
of the inventive
device. Furthermore, after the final elution step, the eluate can also be
removed from the elution
cavity by means of the pipetting function and transferred into a storage
vessel. Thus the degree
of automation can be flexibly enhanced by this easy-to-implement double
function of the
inventive means.
100121 The invention will be explained in more detail hereinafter on the basis
of exemplary
embodiments. These exemplary embodiments do not represent any limitation of
the invention.
Exemplary embodiment
Automated extraction of nucleic acid from NIH 3T3 cells by means of the
inventive process and
using a modified pipette tip as well as using a commercially available
automated extraction
system
Variant A: semiautomated extraction_process (sample lysis takes place
separately)
100131 The InnuPure C16 (Analytik Jena AG) was used as an example of a
standard automated
extraction system. This system is a magnetic-particle-based extraction system,
which was used
outside its normal purpose to perform the inventive method. At the lower end
of the pipette tips
used for the InnuPure C16 automated system, a ring was slipped on externally
in such a way
that the pipetting function was not impaired. This externally slipped-on ring
consists of a
polymer and has a structured surface. The combination of hollow body and ring
fastened
thereon forms the means for performing the inventive method.
100141 Different quantities of NIH 3T3 cells were used for the extraction of
nucleic acids. The
extraction chemistry used for isolation of the nucleic acids was obtained in
part from the
commercial extraction kit known as innuPREP Blood DNA Kit/IPC 16X (Analytik
Jena AG).
CA 02983626 2017-10-23
WO 2016/169679
PCT/EP2016/054180
8
Using a lysis buffer (Lysis Solution CBV) as well as Proteinase K, the cells
were lysed at 60 C
for 15 minutes in a 2.0-mL reaction vessel. This lysis was not performed in
the automated
extraction system. Subsequently, the automated method of the Innupure C16 was
used for
purification of the nucleic acids. The solutions needed for extraction were
present in a prefilled
deep-well plate. The lysates described hereinabove were introduced into
cavities filled with 400
!AL isopropanol. The pipette tips equipped with the ring (the inventive means)
were subjected to
80 cycles of vertical immersion movement in these cavities, each including a
waiting period of
2 s at the bottom of the cavity for incubation. Then the pipette tips modified
with the ring were
successively immersed 10 times each in three further cavities, which contained
the alcoholic
washing buffer (Washing Solution LS, 80% ethanol, 80% ethanol).
[0015] Following the last washing step, the ring on the hollow body was dried
for 10 minutes
outside the cavity, and in this way the remaining ethanol was removed. The
nucleic acids were
eluted by 30 repetitions of immersion in and removal from 200 L Elution
Buffer, which had
been previously adjusted to a temperature of 50 C by the instrument. In the
same cavity, a
mixing step then took place by means of 80 cycles of pipetting of 100 uL at 40
C. The
inventive double function of the inventive means was used for this purpose.
[0016] The method is extremely easy to perform and thereby is extremely fast.
Compared with
the standard method of nucleic acid extraction with the InnuPure C16 and the
use of magnetic
particles for binding the nucleic acids, the time savings is greater than 50%.
[0017] The isolated nucleic acid was detected by means of spectrophotometric
measurement
combined with gel-electrophoretic visualization in an agarose gel.
100181 Results of the spectrophotometric measurement:
Sample Concentration Yield Ratio Ratio
(ng/p1) (Fig) A260 A280 A260 A230
1 5 x 105 NIH 3T3 cells 72.52 14.5 1.79 1.53
2 5 x i05 NIH 3T3 cells 64.11 12.8 1.96 1.58
3 2.5 x 105 NIH 3T3 cells 45.19 9.0 1.74 1.41
CA 02983626 2017-10-23
WO 2016/169679
PCT/EP2016/054180
9
4 2.5 x 105 NIH 3T3 cells 32.88 6.8 1.91 1.29
1.25 x 105 NIH 3T3 cells 19.4 3.9 1.8 1.1
6 1.25 x 105 NIH 3T3 cells 10.47 2.1 1.76 1.05
7 0.62x 105 NIH 3T3 cells 5.65 1.1 1.34 0.76
8 0.62 x 105 NIH 3T3 cells 5.84 1.2 1.9 0.7
100191 As the results show, it is possible with the inventive means, solely by
using standard
extraction chemistry and commercially available extraction platforms, to bind
and to isolate
nucleic acids. It is evident that the yields are extremely high and that
graduations can be
observed in the yields depending on the cell quantities used.
100201 Fig. 2 shows the separation of the isolated nucleic acids by gel
electrophoresis. It
illustrates the nucleic acid isolated by means of the inventive method and
separated
electrophoretically in an 0.8% agarose gel. The samples were applied from left
to right,
beginning with sample I. The applied volume was 5 L.
Variant B: fully automated extraction process (sample lysis takes place in the
instrument)
100211 In a further embodiment, lysis of the sample likewise takes place in an
automated
process. Thus only the sample and the Proteinase K must be added by the user,
while the further
preparation takes place by a completely automated process using the technique
of the InnuPure
C16. For lysis of the sample, it is heated by the Innupure C16 to 50 C, and
lysis is further
intensified by 250 cycles of filling and emptying of the pipette. Thereafter
400 L isopropanol
from a prefilled cavity is introduced into the cavity containing the lysate by
the pipetting
function of the hollow body. All further steps took place as described
hereinabove.
Sample Concentration Yield Ratio Ratio
(ngint) (11g) A260 : A280 A260: A230
1 2.5 x 105 NIH 3T3 cells 33.07 6.6 1.72 1.14
2 2.5 x 105 NIH 3T3 cells 34.02 6.8 1.62 1.13
CA 02983626 2017-10-23
WO 2016/169679
PCT/EP2016/054180
[0022] Fig. 3 shows the separation of the isolated nucleic acids by gel
electrophoresis. It
illustrates the nucleic acid isolated by means of the inventive method and
internal lysis then
separated eleetrophoretically in an 0.8% agarose gel. The samples were applied
from left to
right, beginning with sample 1. The applied volume was 5 L.
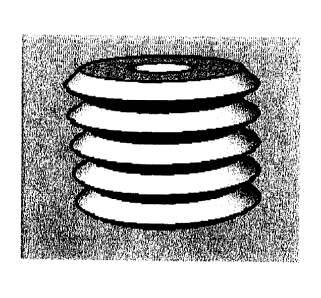
[0023] Fig. 1 is an exemplary embodiment of the ring to be slipped onto a
hollow body. The
illustration is highly enlarged.
[0024] It illustrates an exemplary embodiment of the ring just as it can be
used for nucleic acid
extraction according to the inventive method. This shaped body with non-smooth
surface can be
slipped onto any appropriate commercially available pipette tips, in such a
way that it is then
disposed in the last bottom third of the tip.