Note: Descriptions are shown in the official language in which they were submitted.
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
DUAL-CHAMBER PACK FOR EXTENDED RELEASE SUSPENSION
COMPOSITIONS
Field of the Invention
The present invention relates to a dual-chamber pack comprising a first
chamber
prefilled with a suspension base and a second chamber prefilled with a powder
for
suspension comprising an active ingredient, wherein upon activation of the
dual-chamber
pack, the contents of both the chambers are mixed to form an extended release
suspension
composition which is characterized by having no substantial change in the in-
vitro
dissolution release profile of the active ingredient upon storage for at least
seven days.
Background of the Invention
Extended release solid compositions are preferred dosage forms over immediate
release solid compositions, especially for active ingredients showing
fluctuations in the
plasma concentration and for active ingredients having short half-lives.
Extended release
solid compositions can be in the form of tablets or capsules, wherein the
release of the
active ingredient is controlled by using a reservoir or a matrix system.
However, extended
release solid compositions suffer from certain drawbacks such as difficulty in
swallowing,
particularly for certain groups of patients, e.g., pediatrics and geriatrics,
resulting in poor
patient compliance. Further, high doses of active ingredients lead to large-
sized
compositions which aggravates this problem. Also, there remains a tendency to
divide
extended release solid compositions such as tablets into small pieces in order
to facilitate
administration, which may ultimately lead to inaccurate dosing and/or dose
dumping. In
view of all this, extended release liquid compositions provide the best
alternative over
extended release solid compositions. Extended release liquid compositions are
easy to
administer, thereby leading to enhanced patient compliance. Additionally,
extended
release liquid compositions provide a unique advantage of having a flexible
dosing
regimen.
Extended release liquid compositions are conventionally administered as powder
for suspensions which are to be reconstituted by the end users at the time of
administration
using household pre-boiled and cooled water. Alternatively, the diluent or
purified water is
supplied separately along with the bottle having the extended release powder
for
suspension. These conventional packs lack patient compliance and may lead to
1
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
contamination due to improper quality of water. Further, there remains a
possibility of
dosing errors if the diluent or water is not added to the marked level.
U.S. Patent No. 3,156,369; U.S. Patent No. 3,603,469; U.S. Patent No.
3,840,136;
and U.S. Patent No. 4,982,875 disclose the use of dual-chamber packs for
separately
storing two compositions in two compartments which can be admixed at the time
of use.
The two compartments are separated by a breakable membrane which is ruptured
by the
depression of a plunger so that the one composition gets released into another
and is
mixed. However, there remains a possibility that the membrane fragments may
get
detached and fall into the final product. This may lead to undesirable
contamination and
can pose serious health hazards. Furthermore, the dual-chamber packs disclosed
in the
prior art have a limited capacity for the compartments which may not be
suitable for high-
dose drugs or for drugs which require chronic administration. Also, the liquid
composition
may get permeated into the solid composition across the membrane during
storage which
can lead to the agglomeration of the solid composition. This may result in
poor flow of the
solid composition, thus affecting the content uniformity of the final product.
Also, the
liquid composition on permeation can affect the stability of moisture-
sensitive active
ingredients.
The present invention provides a patient compliant dual-chamber pack with a
significant improvement over the prior art and which fulfills the unmet need
of
incorporating variety of active ingredients. The present dual-chamber pack can
be suitable
for any class of active ingredients including the high-dose active
ingredients, active
ingredients requiring chronic administration, and/or moisture-sensitive active
ingredients.
Further, the plunger used in the pack of the instant invention is designed in
a way such that
the breakable membrane remains adhered to the plug at the time of activation
and
membrane fragments do not fall into the final product. During activation, the
pack ensures
that the final product remains safe for the use of patients. The pack also
ensures that the
solid composition is completely released into the liquid composition thereby
maintaining
the content uniformity of the final product. Further, the pack also ensures
that there is no
permeation of moisture into the chamber having solid composition comprising
the active
ingredient, and the stability of the active ingredient remains unaffected
during storage.
Apart from storage, there remains some of the complexities involved in
formulating such reconstituted extended release powder for suspension
compositions.
Upon reconstitution, the important prerequisite of these compositions is to
provide the
2
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
desired extended release of the active ingredient throughout its shelf life,
as irregular
release may lead to sub-therapeutic or toxic effects. Once reconstituted, the
key hurdle
remains to overcome the leaching of the active ingredient from the coated
cores into a
suspension base during storage. The objective for a scientist remains to
develop a
formulation such that the release of the active ingredient into the suspension
base during
storage is avoided, and only when the suspension enters the gastrointestinal
tract the
release is allowed.
The present invention offers the reconstituted suspension compositions which
provide the desired extended release of the active ingredient throughout the
shelf life of
the compositions. In the present invention, the suspension base prevents the
leaching of
the active ingredient from the coated cores and thus ensures substantially
similar in-vitro
dissolution release profile of the active ingredient throughout the shelf life
of the
compositions. This consistent in-vitro release then ensures a steady plasma
concentration
with no fluctuations throughout the shelf life of the compositions.
The present invention thus provides a novel patient-compliant dual-chamber
pack
prefilled with solid and liquid compositions in two chambers, which upon
mixing forms a
unique composition providing the desired extended release of the active
ingredient
throughout the shelf life of the composition. The compositions prefilled in
the dual-
chamber pack remain stable during the storage.
Summary of the Invention
The present invention relates to a dual-chamber pack comprising a first
chamber
prefilled with a suspension base and a second chamber prefilled with a powder
for
suspension comprising an active ingredient, wherein upon activation of the
dual-chamber
pack, the contents of both the chambers are mixed to form an extended release
suspension
composition which is characterized by having no substantial change in the in-
vitro
dissolution release profile of the active ingredient upon storage for at least
seven days. The
pack allows the end-users ease of dispensing with only a few simple steps
required for
reconstitution. The pack is suitable from low to high dose active ingredients,
active
ingredients required for chronic administration as well as moisture-sensitive
active
ingredients. The pack ensures that the powder for suspension falls completely
into the
suspension base thereby maintaining the content uniformity. The pack also
ensures that
final product remains free of any contamination from the pack components and
is safe to
3
CA 02984725 2017-11-01
WO 2016/178132
PCT/IB2016/052486
the end-users. Further, the pack ensures the stability of the active
ingredient during
storage.
Brief Description of the Drawings
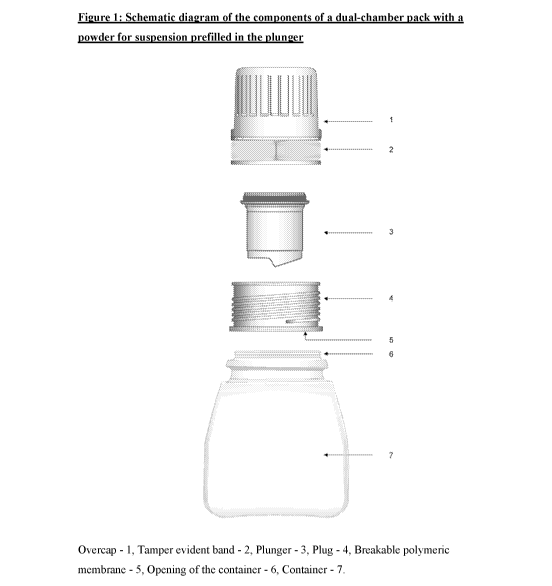
Figure 1: Schematic diagram of the components of a dual-chamber pack with a
powder for suspension prefillcd in the plunger
Figure 2: Schematic diagram of the components of a dual-chamber pack with a
powder for suspension prefilled in the reservoir
Figure 3: Schematic diagram for the biphasic connector ¨ top view and front
view
Figure 4: Schematic diagram representing the assembly of a dual-chamber pack
with a powder for suspension prefilled in the reservoir
Figure 5: Schematic diagram representing the functioning of a dual-chamber
pack
with a powder for suspension prefilled in the reservoir
Detailed Description of the Invention
A first aspect of the invention provides a dual-chamber pack comprising;
(a) a first chamber prefilled with a suspension base; and
(b) a second chamber prefilled with a powder for suspension comprising an
active ingredient;
wherein upon activation of the dual-chamber pack, the contents of both the
chambers are
mixed to form an extended release suspension composition which is
characterized by
having no substantial change in the in-vitro dissolution release profile of
the active
ingredient upon storage for at least seven days.
According to one embodiment of the above aspect, the powder for suspension
prefilled in the second chamber is present in a volume ranging from about 0.5
cc to about
500 cc.
According to another embodiment of the above aspect, the first chamber
comprises
of a container and the second chamber comprises of an overcap, a plunger, and
a plug with
a breakable polymeric membrane. The plunger is prefilled with the powder for
suspension
in a volume ranging from about 0.5 cc to about 30 cc.
4
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
According to another embodiment of the above aspect, the first chamber
comprises
of a container and the second chamber comprises of a reservoir, a biphasic
connector, a
plunger, and a plug with a breakable polymeric membrane. The reservoir is
prefilled with
the powder for suspension in a volume greater than about 30 cc. In particular,
the reservoir
is prefilled with the powder for suspension in a volume ranging from about 30
cc to about
500 cc.
According to another embodiment of the above aspect, the biphasic connector of
the second chamber connects the reservoir to the container of the first
chamber.
According to another embodiment of the above aspect, the plunger ensures the
breakable polymeric membrane remains attached to the plug during activation.
According to another embodiment of the above aspect, the plunger comprise of
one
or more sharp projections with an essential continuous blunt area. In a
preferred
embodiment, the plunger comprise of one sharp projection with an essential
continuous
blunt area. The plunger can further have one or more grooves. The body of the
plunger can
be in the form of a cylinder or a funnel.
According to another embodiment of the above aspect, the plug is made up of a
polymeric material selected from the group comprising polyolefin,
polyethylene,
polypropylene, polyvinyl chloride, cyclic olefin polymer, cyclic olefin co-
polymer,
polyethylene terephthalate, polyethylene terephthalate - G, polypropylene, and
polycarbonate. In a preferred embodiment, the plug is made up of polyethylene.
According to another embodiment of the above aspect, the plug additionally
includes one or more moisture barrier additives.
According to another embodiment of the above aspect, the moisture barrier
additives are selected from the plastic additive group comprising of monomers
and co-
polymers that get activated through polymerization process to form an
effective organic
chemical.
According to another embodiment of the above aspect, the moisture barrier
additives improve the moisture barrier properties by up to 50%. In particular,
the moisture
barrier additives improve the moisture barrier properties by up to 30%.
5
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
According to another embodiment of the above aspect, the plug with the
breakable
polymeric membrane prevents moisture permeation from the first chamber into
the second
chamber.
According to another embodiment of the above aspect, the extended release
suspension composition is a stable composition.
A second aspect of the present invention provides a dual-chamber pack
comprising:
a) a first chamber in the form of a container (7) prefilled with a
suspension
base and provided with an opening (6) at an upper end;
b) a second chamber comprising:
a overcap (1) optionally having a tamper evident band (2) fitted into
a plunger (3);
(ii) the plunger (3) adapted to fit into a plug (4), having a top flat
surface, prefilled with a powder for suspension comprising an active
ingredient;
(iii) the plug (4), with a breakable polymeric membrane (5), adapted to
fit into the opening (6) from a lower end and into the
overcap (1) from the upper end; and
wherein the overcap (1) has a means to exert pressure onto the plunger (3) so
as to
partially rupture the breakable polymeric membrane (5) of the plug and deliver
the powder
for suspension into the suspension base of the container (7); and wherein the
powder for
suspension is mixed with the suspension base to form an extended release
suspension
composition which is characterized by having no substantial change in the in-
vitro
dissolution release profile of the active ingredient upon storage for at least
seven days.
According to one embodiment of the above aspect, the plunger is prefilled with
the
powder for suspension in a volume ranging from about 0.5 cc to about 30 cc.
According to another embodiment of the above aspect, the plunger may be opened
at both the ends. In this case, the plunger is fitted into the overcap first,
and then the
powder for suspension is prefilled into the plunger which is then fitted with
a plug.
6
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
According to another embodiment of the above aspect, the plunger comprise of
one
or more sharp projections with an essential continuous blunt area. In a
preferred
embodiment, the plunger comprise of one sharp projection with an essential
continuous
blunt area.
The overcap exerts pressure onto the plunger when it is screwed during
activation
of the dual-chamber pack.
A third aspect of the present invention provides a dual-chamber pack
comprising:
a) a first chamber in the form of a container (8) prefilled with a
suspension
base provided with an opening (7) at an upper end;
b) a second chamber comprising:
(i) a reservoir (1) adapted to fit into a plunger (2) prefilled with a
powder for suspension comprising an active ingredient; the plunger
(2) is further adapted to fit into a plug (3) having a top flat surface,
(ii) the plug (3), with a breakable polymeric membrane (4), adapted to
fit into the biphasic connector (5) optionally having a tamper
evident band (6) which is further connected from the lower end to
the opening (7) of the container (8);
wherein the reservoir (1) at the top of the second chamber has a means to
exert pressure
onto the plunger (2) so as to partially rupture the breakable polymeric
membrane (4) of the
plug and deliver the powder for suspension into the suspension base of the
container (8);
the second chamber is replaced with a cap (9), and wherein the powder for
suspension is
mixed with the suspension base to form an extended release suspension
composition
which is characterized by having no substantial change in the in-vitro
dissolution release
profile of the active ingredient upon storage for at least seven days.
According to one embodiment of the above aspect, the reservoir is prefilled
with
the powder for suspension in a volume greater than about 30 cc, particularly
in a range
from about 30 cc to about 500 cc.
According to another embodiment of the above aspect, the plunger comprise of
one
or more sharp projections, wherein the plunger essentially has a continuous
blunt area. In a
preferred embodiment, the plunger comprise of one sharp projection with a
continuous
7
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
blunt area. The body of the plunger can be in the form of a cylinder or a
funnel. The funnel
shaped plunger further helps to increase the capacity to incorporate high dose
drugs.
According to another embodiment of the above aspect, the plunger is opened at
both the ends.
According to another embodiment of the above aspect, the cap is a conventional
cap or a child-resistant cap.
According to another embodiment of the above aspect, the biphasic connector
has
a tamper evident band on the side connected to the container of the first
chamber and
grooves on another side for locking with the reservoir of the second chamber.
According to another embodiment of the above aspect, the reservoir exerts
pressure onto the plunger when it is screwed during activation of the dual-
chamber pack.
A fourth aspect of the present invention provides a method of providing an
extended release suspension composition stored in a dual-chamber pack,
comprising the
steps of:
(a) providing a first chamber comprising a container (7), a second chamber
comprising an overcap (1), a plunger (3), a plug (4) with a breakable
polymeric membrane (5);
(b) prefilling the container (7) of the first chamber with a suspension
base;
(c) prefilling the plunger (3) of the second chamber with a powder for
suspension comprising an active ingredient;
(d) fixing the plunger (3) into the plug (4) and mounting the plug on an
opening (6) of the container (7) of the first chamber;
(c) activating the dual-chamber pack by screwing the overcap (1) so
that the
plunger (3) partially ruptures breakable polymeric membrane (5) of the
plug (4); and
(f) shaking the container (7) to allow the mixing of the powder for
suspension
with the suspension base to obtain the extended release suspension
composition which is characterized by having no substantial change in the
in-vitro dissolution release profile of the active ingredient upon storage for
at least seven days.
8
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
According to one embodiment of above aspect, the plunger is prefilled with the
powder for suspension in a volume ranging from about 0.5 cc to about 30 cc.
According to another embodiment of above aspect, the plunger may be open at
both the ends. In this case, the plunger is fitted into the overcap first, and
then the powder
for suspension is prefilled into the plunger which is then fitted with a plug.
Alternatively,
the overcap may be prefitted with the plunger.
The overcap may have a tamper-evident band which is to be removed first to
start
the activation process.
A fifth aspect of the present invention provides a method of providing an
extended
release suspension composition stored in a dual-chamber pack, comprising the
steps of:
(a) providing a first chamber comprising a container (8), a second chamber
comprising a reservoir (1), a plunger (2), a plug (3) with a breakable
polymeric membrane (4), and a biphasic connector (5);
(b) prefilling the container (8) of the first chamber with a suspension
base to
form a first chamber;
(c) prefilling a reservoir (1) of the second chamber with a powder for
suspension comprising an active ingredient;
(d) fixing the biphasic connector (5) into the reservoir (I);
(e) fixing the plunger (2) in the biphasic connector (5);
(0 mounting the plug (3) onto the plunger of the biphasic connector (5) to
form the second chamber;
(g) mounting the second chamber onto the opening (7) of the container (8)
of
the first chamber;
(h) activating the dual-chamber pack by screwing the reservoir (1) of the
second chamber so that the plunger partially ruptures the circumference of
a breakable polymeric membrane; and
removing the second chamber and replacing it with a cap (9);
(i) shaking the container (8) to allow the mixing of the powder for
suspension
with the suspension base to obtain the extended release suspension
composition which is characterized by having no substantial change in the
9
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
in-vitro dissolution release profile of the active ingredient upon storage for
at least seven days.
According to one embodiment of the above aspect, the reservoir is prefilled
with
the powder for suspension in a volume greater than about 30 cc, particularly
in a range
from about 30 cc to about 500 cc.
According to another embodiment of above aspect, the biphasic connector has a
tamper evident band on the side connected to the container of the first
chamber and
grooves on another side for locking with the reservoir of the second chamber.
The tamper
evident band is removed first to start the activation process.
According to another embodiment of the above aspects, the powder for
suspension
comprise of extended release coated cores of an active ingredient, optionally
admixed with
one or more pharmaceutically acceptable excipients. The powder for suspension
may
additionally have one or more osmogents, or one or more suspending agents. The
core
may comprise of a release-controlling agent in the form of a matrix with the
active
ingredient, which can be coated with a coating layer that remain insoluble in
the
suspension base during storage.
According to another embodiment of the above aspects, the extended release
coated cores comprise a core comprising an active ingredient and a coating
layer over said
core comprising one or more release-controlling agents.
According to another embodiment of the above aspects, the core is in the form
of a
bead, a pellet, a granule, a spheroid, or the like.
According to another embodiment of the above aspects, the active ingredient is
layered onto an inert particle to form the core.
Alternatively, the extended release coated cores comprise a core comprising an
active ingredient in a complexed or an ion-exchange resin form and a coating
layer over
said core comprising one or more release-controlling agents.
According to another embodiment of above aspects, the release-controlling
agent is
selected from the group comprising a pH-dependent release-controlling agent, a
pH-
independent release-controlling agent, or mixtures thereof.
According to another embodiment of the above aspects, the extended release
suspension composition is characterized by having an osmolality ratio of at
least about 1.
CA 02984725 2017-11-01
The term "powder for suspension," as used herein, refers to a solid
composition
comprising extended release coated cores of an active ingredient, optionally
admixed with
one or more osmogents, one or more suspending agents, or pharmaceutically
acceptable
excipients. The plunger or reservoir container of the second chamber of the
present
invention is prefilled with the powder for suspension.
The term "suspension base," as used herein, refers to a medium which is used
to
suspend the coated cores of the active ingredient. The suspension base of the
present
invention comprises one or more suspending agents, one or more osmogents, and
a
pharmaceutically acceptable vehicle. It may further comprise one or more
pharmaceutically acceptable excipients. The powder for suspension having
coated cores
of active ingredient may be reconstituted with the suspension base having
suspending
agents, osmogents, pharmaceutically acceptable excipients, and a
pharmaceutically
acceptable vehicle. Alternatively, suspending agents, osmogents, or other
pharmaceutically acceptable excipients may be premixed with the coated cores
which may
be reconstituted with the pharmaceutically acceptable vehicle. The
pharmaceutically
acceptable vehicle may comprise of purified water or a mixture of purified
water with one
or more suitable organic solvents, in particular purified water. The container
of the first
chamber of the present invention is prefilled with a pre-formed suspension
base or a
pharmaceutically acceptable vehicle which forms the suspension base at the
time of
reconstitution. The suspension base generates a hypertonic condition such that
there is no
substantial change in the in-vitro dissolution release profile of the active
ingredient upon
storage of the reconstituted extended release suspension composition for at
least seven
days. The suspension base of the present invention has an osmolality of at
least about 1
osmol/kg of the suspension base.
The term "activation," as used herein means a process which reconstitutes the
powder for suspension with the suspension base. The activation can be done by
the end-
users such as patients, pharmacists, or caregivers. The activation process
starts by either
screwing the overcap or the reservoir.
The term "extended release," as used herein, refers to the release profile of
the
active ingredient over an extended period of time, e.g., over a period of 4,
6, 8, 12, 24
hours, or more.
11
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
The term "hypertonic condition," as used herein, means the suspension base has
higher solute concentration which helps to generate high osmotic pressure such
that there
is no significant leaching of active ingredient from the coated cores into the
suspension
base. In the present invention, the solutes are osmogents i.e.,
pharmaceutically acceptable
inert water-soluble compounds that contribute towards generating hypertonic
conditions in
the suspension base. Alternatively, a saturated solution of the active
ingredient present in
the suspension base or the external phase may prevent the substantial leaching
of the
active ingredient from the extended release coated cores.
The term "osmolality ratio," as used herein, means the ratio of the osmolality
of
the external phase to the osmolality of the internal phase. The external phase
herein means
the suspension base without the multiple extended release coated cores of the
active
ingredient. The internal phase herein means the extended release coated cores
of the active
ingredient. As the direct measurement of the osmolality of the internal phase
i.e., coated
cores is difficult, the osmolality of the internal phase herein, is
represented as the
osmolality of a solution which prevents significant leaching of the active
ingredient from
the coated cores into the solution. The leaching of the active ingredient from
the extended
release coated cores is determined by the difference in the osmolalities
across the coating
layer and the absence of any significant leaching from the extended release
coated cores
directs that the osmolality of the solution has become equal to the osmolality
of the
extended release coated cores. The osmolality ratio of the extended release
suspension
compositions of present invention is at least about 1.
The term "osmolality," as used herein, is expressed as number of moles of any
water-soluble compound per kg of a liquid phase. The liquid phase can be a
suspension
base or a solution. In the present invention, the osmolality may be measured
according to
known methods, such as using a vapor pressure osmometer, a colloid osmometer,
or a
freezing point depression osmometer such as Osmomat 030-D or Osmomat 3000, in
particular by a freezing point depression osmometer.
The term "inert particle," as used herein, refers to a particle made from a
sugar
sphere also known as a non-pareil seed, a microcrystalline cellulose sphere, a
dibasic
calcium phosphate bead, a mannitol bead, a silica bead, a tartaric acid
pellet, a wax based
pellet, and the like.
12
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
The term "substantial," as used herein refers to any value which lies within
the
range as defined by a variation of up to 15 from the average value.
The term "about" as used herein, refers to any value which lies within the
range
defined by a variation of up to 10% of the value.
The term "significant leaching," as used herein means more than 20% of the
active
ingredient is leached out from the extended release coated cores into the
solution.
The term "stable," as used herein, refers to chemical stability, wherein not
more
than 5% w/w of total related substances are formed on storage at 40 C and 75%
relative
humidity (R.H.) or at 25 C and 60% R.H. for a period of at least three months
to the extent
necessary for the sale and use of the composition.
The term "osmogent," as used herein, refers to all pharmaceutically acceptable
inert water-soluble compounds that can imbibe water and/or aqueous biological
fluids.
The osmogent can be present in the suspension base or in the powder for
suspension or
both. Suitable examples of osmogents or pharmaceutically acceptable inert
water-soluble
compounds are selected from the group comprising carbohydrates such as
xylitol,
mannitol, sorbitol, arabinose, ribose, xylose, glucose, fructose, mannose,
galactose,
sucrose, maltose, lactose, dextrose and raffinose; water-soluble salts of
inorganic acids
such as magnesium chloride, magnesium sulfate, potassium sulfate, lithium
chloride,
sodium chloride, potassium chloride, lithium hydrogen phosphate, sodium
hydrogen
phosphate, potassium hydrogen phosphate, lithium dihydrogen phosphate, sodium
dihydrogen phosphate, potassium dihydrogen phosphate, and sodium phosphate
tribasic;
water-soluble salts of organic acids such as sodium acetate, potassium
acetate, magnesium
succinate, sodium benzoate, sodium citrate, and sodium ascorbate; water-
soluble amino
acids such as glycine, leucine, alanine, methionine; urea or its derivatives;
propylene
glycol; glycerin; polyethylene oxide; xanthan gum; hydroxypropylmethyl
cellulose; and
mixtures thereof. Particularly, the osmogcnts used in the present invention
are xylitol,
mannitol, glucose, lactose, sucrose, and sodium chloride.
Suitable suspending agents are selected from the group comprising cellulose
derivatives such as co-processed spray dried forms of microcrystalline
cellulose and
carboxymethyl cellulose sodium, hydroxypropyl cellulose, hydroxyethyl
cellulose,
hydroxypropylmethyl cellulose, methylcellulose, carboxymethyl cellulose and
its
salts/derivatives, and microcrystalline cellulose; carbomers; gums such as
locust bean
13
CA 02984725 2017-11-01
WO 2016/178132
PCT/IB2016/052486
gum, xanthan gum, tragacanth gum, arabinogalactan gum, agar gum, gellan gum,
guar
gum, apricot gum, karaya gum, sterculia gum, acacia gum, gum arabic, and
carrageenan;
pectin; dextran; gelatin; polyethylene glycols; polyvinyl compounds such as
polyvinyl
acetate, polyvinyl alcohol, and polyvinyl pyrrolidone; sugar alcohols such as
xylitol and
mannitol; colloidal silica; and mixtures thereof. Co-processed spray dried
forms of
microcrystalline cellulose and carboxymethyl cellulose sodium have been
marketed under
the trade names Aviccl RC-501, Avicel RC-581, Avicel RC-591, and Avicel CL-
611.
The term "pharmaceutically acceptable excipients," as used herein, refers to
excipients that are routinely used in pharmaceutical compositions. The
pharmaceutically
acceptable excipients may comprise glidants, sweeteners, anti-caking agents,
wetting
agents, preservatives, buffering agents, flavoring agents, anti-oxidants,
chelating agents,
and combinations thereof.
The average diameter of the extended release coated cores ranges from about 10
pm to about 2000 gm, particularly from about 50 gm to about 1000 pm, and more
particularly from about 150 gm to about 500 gm. The finer sizes of the
extended release
coated cores help in avoiding grittiness in the mouth and are therefore more
acceptable.
This dual-chamber pack can be used for active ingredients such as
valacyclovir,
metformin, azithromycin, cloxacillin, clarithromycin, erythromycin,
amoxicillin alone or
in combination with clavulanic acid, cefdinir, cefuroxime axetil, cefixime,
cefadroxil,
cefpodoxime, cefaclor, cefprozil, fluconazole, voriconazole, acarbose,
miglitol, voglibose,
repaglinide, natcglinide, glibenclamide, glimepride, glipizidc, gliclazidc,
chloropropamide,
tolbutamide, phenformin, alogliptin, sitagliptin, linagliptin, saxagliptin,
rosiglitazone,
pioglitazone, troglitazone, faraglitazar, englitazone, darglitazone,
isaglitazone,
zorglitazone, liraglutide, muraglitazar, pcliglitazar, tesaglitazar,
canagliflozin,
dapagliflozin, remogliflozin, sergliflozin, verapamil, albuterol, salmeterol,
acebutolol,
sotalol, penicillamine, norfloxacin, ciprofloxacin, ofloxacin, levofloxacin,
moxifloxacin,
trovafloxacin, gatifloxacin, tetracycline, demeclocycline hydrochloride,
losartan,
irbesartan, eprosartan, valsartan, diltiazem, isosorbide mononitratc,
ranolazine,
propafenone, hydroxyurea, hydrocodone, delavirdine, pentosan polysulfate,
abacavir,
amantadine, acyclovir, ganciclovir, valganciclovir, saquinavir, indinavir,
nelfinavir,
lamivudine, didanosine, zidovudine, nabumetonc, celecoxib, mefenamic acid,
naproxen,
propoxyphene, cimetidine, ranitidine, albendazole, mebendazole, thiobendazole,
pyrazinamide, praziquantel, chlorpromazine, sumatriptan, bupropion,
aminobenzoate,
14
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
pyridostigmine bromide, potassium chloride, niacin, tocainide, quetiapine,
fexofenadine,
sertraline, chlorpheniramine, rifampin, methenamine, nefazodone, modafinil,
metaxalone,
morphine, sevelamer, lithium carbonate, flecainide acetate, simethiconc,
methyldopa,
chlorthiazide, metyrosine, procainamide, entacapone, metoprolol, propanolol
hydrochloride, chlorzoxazone, tolmetin, tramadol, bepridil, phenytoin,
gabapentin,
terbinafine, atorvastatin, doxepine, rifabutin, mesalamine, etidronate,
nitrofurantoin,
choline magnesium trisalicylatc, theophyllinc, nizatidine, methocarbamol,
mycophenolate
mofetil, tolcapone, ticlopidine, capecitabine, orlistat, colsevelam,
meperidine,
hydroxychloroquine, guaifenesin, guanfacine, amiodarone, quinidine,
atomoxetine,
felbamate, pseudoephcdrine, carisoprodol, venlafaxine, etodolac, chondroitin,
lansoprazole, pantoprazole, esomeprazole, dexlansoprazole, dexmethylphenidate,
methylphenidate, sodium oxybate, valproic acid or its salts, divalproex,
topiramate,
carbamazepine, oxcarbazepinc, isotretinoin, oseltamivir, cholestyramine,
nystatin, and a
combination of artemether and lumefantrine.
The suspension base or the powder for suspension of the present invention may
further include an immediate release component of the active ingredient to
have a biphasic
or pulsatile type of release. The immediate release component may be present
in the form
of a powder, a pellet, a bead, a spheroid, or a granule. Alternatively, the
immediate
release component may be present in the form of an immediate release coating
over the
extended release coated cores. The reconstituted extended release suspension
composition
of the present invention may comprise two or more different active ingredients
with
different type of release profiles or incompatible active ingredients.
The release-controlling agents used to form the extended release coating are
selected from a group comprising a pH-dependent release-controlling agent, a
pH-
independent release-controlling agent, or mixtures thereof.
Suitable examples of pH-dependent release-controlling agents are selected from
the group comprising acrylic copolymers such as mcthacrylic acid and methyl
methacrylate copolymers, e.g., Eudragit L 100 and Eudragit S 100,
methacrylic acid and
ethyl acrylate copolymers, e.g., Eudragit L 100-55 and Eudragit L 30 D-55,
dimcthylaminocthyl methacrylate and butyl methacrylate and methyl methacrylate
copolymers e.g., Eudragit E 100, Eudragit E PO, methyl acrylate and
methacrylic acid
and octyl acrylate copolymers, styrene and acrylic acid copolymers, butyl
acrylate and
styrene and acrylic acid copolymers, and ethylacrylate-methacrylic acid
copolymer;
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
cellulose acetate phthalate; cellulose acetate succinates; hydroxyalkyl
cellulose phthalates
such as hydroxypropylmethyl cellulose phthalate; hydroxyalkyl cellulose
acetate
succinates such as hydroxypropylmethyl cellulose acetate succinate; vinyl
acetate
phthalates; vinyl acetate succinate; cellulose acetate trimelliate; polyvinyl
derivatives such
as polyvinyl acetate phthalate, polyvinyl alcohol phthalate, polyvinyl
butylate phthalate,
and polyvinyl acetoacetal phthalate; zein; shellac; and mixtures thereof.
Suitable examples of pH-independent release-controlling agents are selected
from
the group comprising cellulosic polymers such as ethyl cellulose, methyl
cellulose,
hydroxycthyl cellulose, hydroxypropyl cellulose, hydroxyethylmethyl cellulose,
hydroxypropylmethyl cellulose, and carboxy methylcellulose; acrylic copolymers
such as
methacrylic acid copolymers, e.g., Eudragit RS, Eudragit RL, Eudragit NE 30
D;
cellulose acetate; polyethylene derivatives e.g., polyethylene glycol and
polyethylene
= oxide; polyvinyl alcohol; polyvinyl acetate; gums e.g., guar gum, locust
bean gum,
tragacanth, carrageenan, alginic acid, gum acacia, gum arabic, gellan gum, and
xanthan
gum; triglycerides; waxes, e.g., Compritol , Lubritab , and Gelucires ;
lipids; fatty acids
or their salts/derivatives; a mixture of polyvinyl acetate and polyvinyl
pyrrolidonc, e.g.,
Kollidon SR; and mixtures thereof.
Suitable glidants are selected from the group comprising silica, calcium
silicate,
magnesium silicate, colloidal silicon dioxide, cornstarch, talc, stearic acid,
magnesium
stearate, calcium stearate, sodium stearyl fumarate, hydrogenated vegetable
oil, and
mixtures thereof.
Suitable sweeteners are selected from the group comprising saccharine or its
salts
such as sodium, potassium, or calcium, cyclamate or its salt, aspartame,
alitame,
acesulfame or its salt, stevioside, glycyrrhizin or its derivatives,
sucralose, and mixtures
thereof.
Suitable anti-caking agents are selected from the group comprising colloidal
silicon dioxide, tribasic calcium phosphate, powdered cellulose, magnesium
trisilicate,
starch, and mixtures thereof.
Suitable wetting agents are selected from the group comprising anionic,
cationic,
nonionic, or zwitterionic surfactants, or combinations thereof. Suitable
examples of
wetting agents arc sodium lauryl sulphate; cctrimidc; polyethylene glycols;
polyoxyethylene-polyoxypropylene block copolymers such as poloxamers;
polyglycerin
16
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
fatty acid esters such as decaglyceryl monolaurate and decaglyceryl
monomyristate;
sorbitan fatty acid esters such as sorbitan monostearate; polyoxyethylene
sorbitan fatty
acid esters such as polyoxyethylene sorbitan monooleate; polyethylene glycol
fatty acid
esters such as polyoxyethylene monostearate; polyoxyethylene alkyl ethers such
as
polyoxyethylene lauryl ether; polyoxyethylene castor oil; and mixtures thereof
Suitable preservatives are selected from the group comprising parabcns such as
methyl paraben and propyl paraben; sodium benzoate; and mixtures thereof.
Suitable buffering agents are selected from the group comprising citric acid,
sodium citrate, sodium phosphate, potassium citrate, acetate buffer, and
mixtures thereof.
Suitable flavoring agents are selected from the group consisting of
peppermint,
grapefruit, orange, lime, lemon, mandarin, pineapple, strawberry, raspberry,
mango,
passion fruit, kiwi, apple, pear, peach, apricot, cherry, grape, banana,
cranberry, blueberry,
black currant, red currant, gooseberry, lingon berries, cumin, thyme, basil,
camille,
valerian, fennel, parsley, chamomile, tarragon, lavender, dill, bargamot,
salvia, aloe vera
balsam, spearmint, eucalyptus, and combinations thereof.
Suitable anti-oxidants are selected from the group comprising butylatcd
hydroxytoluene (BHT), butylated hydroxyanisole (BHA), sodium metabisulfite,
ascorbic
acid, propyl gallate, thiourea, tocopherols, beta-carotene, and mixtures
thereof.
Suitable chelating agents are selected from the group comprising
ethylencdiamine
tetraacetic acid or derivatives/salts thereof, e.g., disodium edetate;
dihydroxyethyl glycine;
glucaminc; acids, e.g., citric acid, tartaric acid, gluconic acid, and
phosphoric acid; and
mixtures thereof.
The ion-exchange resins such as cation- and anion-exchange matrices are well-
known in the art. Few exemplary resin particles that can be used according to
the
invention include, but are not limited to, Dowex resins and others made by
Dow
Chemical; Amberlite , Amberlyst and other resins made by Rohm and Haas;
Indion
resins made by Ion Exchange, Ltd. (India), Diaion resins by Mitsubishi; Type
AG and
other resins by BioRad; Sephadex and Sepharose made by Amersham; resins by
Lewatit, sold by Fluka; Toyopearl resins by Toyo Soda; IONAC and Whatman
resins
sold by VWR; and BakerBond resins sold by J T Baker; resins having polymer
backbones comprising styrene-divinyl benzene copolymers and having pendant
17
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
ammonium or tetraalkyl ammonium functional groups, available from Rohm and
Haas,
Philadelphia, and sold under the tradename DUOLITErm AP143.
The cores of the present invention comprising the active ingredient can be
prepared
by any method known in the art, e.g, extrusion-spheronoization, wet
granulation, dry
granulation, hot-melt extrusion granulation, spray drying, and spray
congealing.
Alternatively, the active ingredient can be layered onto an inert particle to
form the core.
Further, the active ingredient particles can be directly coated with a release-
controlling
agent to form the microparticles or microcapsules. The microparticles or
microcapsules
can be prepared by a process of homogenization, solvent evaporation,
coacervation phase
separation, spray drying, spray congealing, polymer precipitation, or
supercritical fluid
extraction. The ion-exchange resins comprise loading a plurality of the resin
particles with
the active ingredient to form drug-resin cores. Methods of loading active
ingredients onto
the resin particles are generally known in the art.
The first chamber includes a container which is in the form of a glass or a
plastic or
a metallic bottle. The reservoir of the second chamber can be made of a
plastic, a metal or
a glass; particularly the reservoir is a plastic bottle. The reservoir of the
second chamber
may additionally have a slippery coating or mold polishing. This coating or
polishing will
help to improve the flow characteristics of the powder for suspension
composition during
activation.
In the dual-chamber pack suitable for incorporating powder for suspension in a
volume ranging from about 0.5 cc to about 30 cc, the plunger may be inversely
fitted into
the plug which is subsequently screwed or snuggly fitted on to the opening of
the
container of the first chamber, in particularly it is screwed fitted. The
overcap may be
fitted screwed or snuggly into the plug, in particularly snuggly fitted. The
plunger can be
open at both the ends or closed at one end and open at the other end. In
particular, it is
open at both the ends. The plunger opcncd at both the ends may further
increase the
capacity as well as machine ability. Further, the overcap may be prefitted
with the plunger.
The overcap may have a tamper evident band which is removed first to start the
activation.
In the dual-chamber pack suitable for incorporating for powder for suspension
in a
volume ranging from about 30 cc to about 500 cc, the plunger is opened at both
the ends.
The biphasic connector comprises of cross bridges to give the strength. The
bridges can be
tapered at the edges to avoid any powder deposit. Further, the reservoir can
have serrations
18
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
to have better grip for the end-users. The biphasic connector has a tamper-
evident band on
the side connected to the container of the first chamber which is removed
first to start the
activation process. The biphasic connector is having grooves on other side for
locking
with the reservoir. On this side, there would be instructions for the end-
users regarding
direction of the rotation such as clockwise rotation for activating the pack.
The term "tamper-evident band," as used herein, refers to a band attached co-
axially to the overcap or to the biphasic connector. The band breaks easily on
pulling
apart. The tamper-evident band ensures the overall integrity of the product
until
activation.
The plunger of the instant invention can comprise of one or more sharp
projections
with an essential continuous blunt area. In particular, the plunger comprise
of one sharp
projection with an essential continuous blunt area. Alternatively, the plunger
can have a
single continuous projection with a remaining continuous blunt area which can
be called
as a flute shaped plunger. The plunger can further have one or more grooves.
The body of
the plunger can be in the form of a cylinder or a funnel. The funnel shaped
plunger
provides additional capacity for storing high-dose active ingredients or
active ingredients
required for chronic administration.
The plunger used in the instant invention ensures that the breakable polymeric
membrane remains attached to the plug during activation. The plug and the
plunger may
be made up of a material selected from the group comprising polyolefin,
polyethylene,
polypropylene, polyvinyl chloride, cyclic olefin polymer, cyclic olefin co-
polymer,
polyethylene terephthalate, polyethylene terephthalate - G, polypropylene, and
polycarbonate. Particularly, the plug and the plunger are made up of
polyethylene. More
particularly, the plug and the plunger are made up of linear low density
polyethylene
(LLDPE).
The compositions of the first and second chambers of the container arc
separated
by a polymeric breakable membrane of the plug. The plunger used in the instant
invention
helps to rupture the breakable polymeric membrane upon the application of
pressure by a
screw-based mechanism. When pressure is applied on the overcap or reservoir,
the
breakable polymeric membrane is ruptured by the plunger. The intact polymeric
membrane remains attached to the circumference of the plug. In cases, where a
bottle liner
exists between the first and the second chambers, the plunger would break the
bottle liner
19
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
in the same manner as it ruptures the breakable polymeric membrane. The
unabridged
part of the bottle liner remains attached to the opening of the container. The
plug with the
breakable polymeric membrane prevents moisture permeation from the first
chamber into
the second chamber.
The material used for making the plug may also include moisture barrier
additives
selected from the plastic additive group comprising of monomers and co-
polymers that get
activated through polymerization process to form an effective organic
chemical. The
moisture barrier additives used in the present invention may include any
material that can
prevent moisture permeation. The moisture barrier additives may be present in
the form of
a layer inside the plug. The moisture barrier additives may be present in an
amount of
0.1% to 10% w/w, in particularly, 0.5% to 5% w/w based on total weight of the
material
used for making plug.
The material used for making the reservoir may also include the moisture
barrier
additives. The barrier additives may be present in the form of a layer inside
the reservoir.
The moisture permeation test was carried out on dual chamber packs with
moisture
barrier additives and without moisture barrier additives as per USP (37) ¨ 671
Containers
Performance Testing. The moisture barrier additives used in the present
invention improve
the moisture barrier properties by up to 50%. In particular, the moisture
barrier additives
improves the moisture barrier properties by up to 30%.
The use of moisture barrier additives thus help to prevent the moisture
permeation
from the suspension base into the powder for suspension during storage. The
active
ingredient, particularly moisture-sensitive active ingredient present in the
powder for
suspension thus remains stable during storage. =
The invention may be further illustrated by the following examples, which are
for
illustrative purposes only and should not be construed as limiting the scope
of the
invention in any way.
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
Example 1
Ingredients Quantity (mg/mL)
Core
Metformin hydrochloride 80.00
Microcrystalline cellulose spheres 56.00
Hydroxypropylmcthyl cellulose 4.00
Purified water q.s.
Extended Release Coating
Ethyl cellulose 68.31
Dibutyl sebacate 1.69
Acetone q.s.
Purified water q.s.
Total Weight of Extended Release
210.00 mg
Beads
Suspension base
Metformin hydrochloride 20.00
Xylitol 450.00
Microcrystalline cellulose - sodium
carboxymethyl cellulose (Avicel CL- 20.00
611)
Xanthan gum 1.50
Methyl paraben 1.80
Propyl paraben 0.20
Strawberry flavor 2.00
Sucralose 0.50
Colloidal silicon dioxide 3.50
Purified water 472.00 mg
Procedure:
1. Metformin hydrochloride and hydroxypropylmethyl cellulose were dissolved in
purified water.
2. Microcrystalline cellulose spheres were coated with the solution of step 1.
3. Ethyl cellulose and dibutyl sebacate were dispersed in a mixture of
acetone and
purified water.
4. The beads of step 2 were coated with the coating dispersion of step 3 and
dried to form
a powder for suspension.
5. Purified water was heated to dissolve methyl paraben and propyl paraben.
6. Metformin hydrochloride, xylitol, microcrystalline cellulose - sodium
carboxymethyl
cellulose, xanthan gum, strawberry flavor, sucralose, and colloidal silicon
dioxide
were mixed in the solution of step 5 to form a suspension base.
21
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
7. The powder for suspension of step 4 was prefilled in a plunger of a
second chamber of
a dual-chamber pack.
8. The suspension base of step 6 was prefilled in a container of a first
chamber of a dual-
chamber pack.
9. The two chambers were assembled and the pack was activated to form the
extended
release suspension composition when required.
In-Vitro Studies
The extended release suspension composition prepared as per Example 1 (for a
dose equivalent to 750 mg of metformin hydrochloride) was stored at room
temperature
for 120 days. The in-vitro dissolution was determined at 0, 45, 90, and 120
days using
USP type II apparatus at 100 rpm, in 1000 mL of phosphate buffer with pH 6.8
at 37 C.
The results of the release studies are represented in Table 1.
Table 1: Percentaze ( /0) of the In-Vitro Metformin Release in USP Type!!
Apparatus (Media: Phosphate Buffer, pH 6.8, 1000 mL, and 100 rpm)
Number of 0 45 90 120
Days
Time (hours) Percentage of Metformin Release
0.5 20 21 20 21
1 27 25 27 25
2 55 52 55 52
3 74 72 74 72
4 83 81 83 81
5 85 86 85 86
6 87 90 87 90
8 91 94 91 94
10 93 96 93 96
12 94 97 94 97
From the above in-vitro release data, it is evident that the extended release
suspension composition prepared according to Example 1 provides the
substantially
similar in-vitro metformin release for 120 days.
The dual-chamber pack was kept for 1 month at accelerated conditions i.e.,
40 C/75% R.H. After 1 month, the pack was activated to form an extended
release liquid
composition which was kept for 120 days at room temperature. The in-vitro
dissolution
was determined at 0, 45, 90, and 120 days using USP type II apparatus at 100
rpm, in 1000
22
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
mL of phosphate buffer with pH 6.8 at 37 C. The results of the release studies
are
represented in Table 2.
Table 2: Percentage (/0) of the In-Vitro Metformin Release in USP Type II
Apparatus (Media: Phosphate Buffer, pH 6.8, 1000 mL, and 100 rpm)
Number of 0 45 90 120
Days
Time (hours) Percentage of Metformin
Release
0.5 21 21 21 20
=
1 27 25 26 26
2 56 55 52 54
3 74 74 76 72
4 83 81 82 81
96 96 97 94
5 The dual-chamber pack was kept for 3 months at accelerated conditions
i.e.,
40 C/75% R.H. After 3 months, the pack was activated to form an extended
release liquid
composition which was kept for 45 days at room temperature. The in-vitro
dissolution
was determined at 0 and 45 days using USP type II apparatus at 100 rpm, in
1000 mL of
phosphate buffer with pH 6.8 at 37 C. The results of the release studies are
represented in
10 Table 3.
Table 3: Percentaze (%) of the In-Vitro Metformin Release in USP Type II
Apparatus (Media: Phosphate Buffer, pH 6.8, 1000 mL, and 100 rpm)
Number of Days 0 45
Time (hours) Percentage of Metformin Release
0.5 21 21
1 26 25
2 55 53
3 75 72
4 80 80
10 95 92
From the above data, it is clear that the powder for suspension and suspension
base stored in the dual-chamber pack of the instant invention at accelerated
conditions for
1 month and 3 months, upon activation of the pack forms extended release
suspension
compositions which when stored for 120 days and 45 days respectively at room
temperature provides substantially similar in-vitro metformin release..
23
CA 02984725 2017-11-01
WO 2016/178132
PCT/IB2016/052486
Stability Data
The related substances for the extended release suspension composition
prepared
as per Example I were determined at 0 day and after storage at room
temperature for 45
and 120 days. The powder for suspension and suspension base was stored in the
dual-
chamber pack for one month and for three months at 40 C/75% R.H. After one
month or
three months, the pack was activated to form extended release suspension
compositions
and then related substances were determined at 0 day and after storage at room
temperature for 45 days and 120 days.
The assay of metformin was determined by HPLC method. The results are shown
in Table 4.
Table 4: Stability Data for Metformin
Related Initial 1 month (40 C/75% 3 month
Substances R.H) (40 C/75%
(%w/w) R.H)
0 day 45 120 days 0 day 45 120 0 day 45
days days days days
Cyanoguainidine BLQ 0.001 0.00072 0.001 0.001 0.001 0.001 0.001
Highest unknown 0.05 0.05 0.04 0.05 0.04 0.04 0.05 0.04
impurity
Total impurities 0.05 0.05 0.04 0.05 0.04 0.04 0.09
0.04
*BLQ: Below limit of Quantification
It is evident from the above data that the extended release suspension
composition
prepared as per Example 1 remains stable even after storing at accelerated
conditions for 3
months using the dual-chamber pack.
24
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
Example 2
Ingredients Quantity (mg/mL)
Core
Metformin hydrochloride 80.00
Microcrystalline cellulose spheres 56.00
Hydroxypropylmethyl cellulose 4.00
Purified water q.s.
Extended Release Coating
Ethyl cellulose 61.48
Dibutyl sebacate 1.52
Acetone q.s.
Purified water q.s.
Total Weight of Extended Release Beads 203.00 mg
Metformin hydrochloride 20.00
Xylitol 450.00
Microcrystalline cellulose - sodium
20.00
carboxymethyl cellulose (Avicel CL-611)
Xanthan gum 1.50
Strawberry flavor 2.00
Sucralosc 0.50
Colloidal silicon dioxide 3.50
Vehicle
Purified water q.s. to 1 mL
Procedure:
1. Metformin hydrochloride and hydroxypropylmethyl cellulose were dissolved in
purified water.
2. Microcrystalline cellulose spheres were coated with the solution of step 1.
3. Ethyl cellulose and dibutyl sebacate were dispersed in a mixture of
acetone and
purified water.
4. The beads of step 2 were coated with the coating dispersion of step 3.
5. Metformin hydrochloride, xylitol, microcrystalline cellulose - sodium
carboxymethyl
cellulose, xanthan gum, strawberry flavor, sucralose, and colloidal silicon
dioxide were
mixed.
6. The coated beads of step 4 were mixed with the mixture of step 5 to form a
powder for
suspension.
The powder for suspension may be stored in plunger or reservoir (depending
upon
the volume) of the second chamber and the vehicle (purified water) may be
stored in the
CA 02984725 2017-11-01
WO 2016/178132
PCT/1B2016/052486
container of the first chamber. The two chambers after assembling may be
activated to
form the extended release suspension composition when required.
In-Vitro Studies
The extended release suspension composition prepared as per Example 2 was
stored at room temperature for 30 days. The in-vitro dissolution was
determined at 0 and
30 days using USP type II apparatus at 100 rpm, in 1000 mL of phosphate buffer
with pH
6.8 at 37 C. The results of the release studies are represented in Table 5.
Table 5: Percentage ("/0) of the In-Vitro Metformin Release in USP Type II
Apparatus (Media: Phosphate Buffer, pH 6.8, 1000 mL, and 100 rpm)
Number of Days 0 30
Time (hours) Percentage of Metformin Release
0.5 20 22
1 27 28
2 59 64
3 77 80
4 84 89
5 88 93
6 92 95
8 95 99
10 97 101
12 98 103
From the above in-vitro release data, it is evident that the extended release
suspension composition prepared according to Example 2 provides the
substantially
similar in-vitro metformin release for 30 days.
Osmolalitv Measurement of the Extended Release Suspension
The metformin extended release powder prepared according to the Example 2
(till
step 6) was reconstituted with required amount of purified water. This
suspension was
shaken manually for at least 20 minutes. This suspension was then filtered and
diluted
with purified water and the osmolality was measured using Osmomat 030-D.
The osmolality of the suspension base was found to be 4.112 osmol/kg of the
suspension base on day 0.
The osmolality of the suspension base was found to be 4.328 osmol/kg of the
suspension base on day 7.
26
CA 02984725 2017-11-01
WO 2016/178132 PCTAB2016/052486
It is evident from the above data that the osmolality of the suspension base
of the
extended release suspension composition as per Example 2 remains equivalent
for seven
days.
Osmolality Measurement of the External Phase
The mctformin hydrochloride, xylitol, microcrystalline cellulose - sodium
carboxymethyl cellulose, xanthan gum, strawberry flavor, sucralose, and
colloidal silicon
dioxide were mixed as per step 5 of Example 2. This mixture was reconstituted
with
required amount of purified water. This suspension was then filtered and
diluted with
purified water, and the osmolality was measured using Osmomat 030-D.
The osmolality of the suspension base i.e., external phase was found to be
4.204
osmol/kg of the suspension base.
Osmolality Measurement of the Internal Phase
Various solutions having various concentrations of osmogent (sodium chloride)
were prepared as per Examples 2A-2F. The osmolalities of these solutions were
measured
using Osmomat 030-D.
Ingredient Example Example Example Example Example Example
2A 2B 2C 2D 2E 2F
Sodium Chloride
30.00 60.00 120.00 180.00 240.00 300.00
(mg)
Purified water q.s. to 1 q.s. to 1 q.s. to 1 q.s. to q.s.
to 1 q.s. to 1
mL mL mL 7.5 mL mL mL
Osmolality
0.910 1.787 3.574* 5.361* 7.148* 8.935*
(osmol/kg)
* Extrapolated using values of dilute solutions
The coated beads of step 4 were dispersed in different solutions as per
Examples
2A-2F. These solutions were kept for seven days at room temperature. After
seven days,
each solution was analyzed by HPLC for metformin content. The results are
represented
in following Table 5.
27
CA 02984725 2017-11-01
WO 2016/178132 PCT/1B2016/052486
Table 5: Effect of Osmolalitv on Metformin Leaching
Example Osmolality (osmol/kg) of the solution Metformin Content (%)
2A 0.910 67.3
2B 1.787 30.3
2C 3.574* 2.9
2D 5.361* 1.8
2E 7.148* 1.7
2F 8.935* 1.0
* Extrapolated using values of dilute solutions
From the above data, it is evident that the leaching of metformin from the
coated
beads into the solution was decreasing as the osmolality of the solution was
increasing
from Examples 2A-2F. The leaching is found to be significantly reduced from
Example
2C onwards. The osmolality of Example 2C i.e., 3.574 is considered as
osmolality of the
internal phase.
Osmolalitv Ratio 1.176
28