Note: Descriptions are shown in the official language in which they were submitted.
COMPOSMONS AND METHODS TO PROMOTE BONE FORMATION
BACKGROUND ART
[0003] Bone formation and degradation are tightly regulated by growth factor
signaling
between osteoblasts that are responsible for bone formation and osteoctasts
that are
responsible for bone re-absorption. Coupling bone formation by osteoblasts
with degradation
by osteoclasts has recently become a topic of intense study; with the list of
growth factors
identified as coupling factors expanding. Coupling bone formation with bone ri
-absorption
requires the recruitment of osteoblasts and osteoclasts in parallel with the
recruitment of
their respective progenitor cells. Osteoblasts derive from mesenchymal stem
cell (MSC)
while osteoclasts derive from monocytes that are a part of the myeloid-
lineage; however, it
remains unknown how MSC or monocytes migrate from their niche in the bone
marrow to
sites of new bone formation. The current understanding of the spatial and
temporal
regulation of osleogenesis proposes that MSC migrate from their bone marrow
niche to the
endosteal surface; where the MSC ,differentiate into osteoblasts that produce
new bone. In
parallel, monocytes also migrate from their bone marrow niche to the endosteal
surface;
where they subsequently differentiate into osteoclasts that re-absorb bone.
Growth factors
known to regulate bone formation include TGF13-, SNP- and the canonical Wnt-
ligands.
Osteoclast formation from monocyte precursors and bone re-absorption are
regulated
through the expression of MSCF, OPG and RANK-ligand. In parallel, osteoclast
activity is
also regulated by the expression of the TGFp-, SMP- and the non-canonical Wnt-
ligands.
However, many developmental growth factors involved in tissue patterning,
including TG93,-.
BMP- and the Wnt-ligands, promote bone formation and re-absorption. The
maintenance of
1
Date Recue/Date Received 2021-09-23
CA 02984889 2017-11-02
WO 2015/184059 PCT/US2015/032820
healthy bone requires constant remodeling, in which bone is made and destroyed
continuously.
[0004] The introduction of an implant into bone results in a biochemical
cascade that drives
the pro-inflammatory response that is partially mediated by macrophage
activity, which are
derived from the myeloid lineage and can contribute to the degradation of bone
or an implant
material. Currently implants and implant materials are chosen to minimize the
macrophage
response while being optimally osteo-conductive and promoting maximum bone-
implant
integration. Alternatively, the introduction of autograft with an implant or
the use of
devitalized bone tissue graft (autograft) has been employed in concert with
the material
properties of an implant as a means of increasing osteo-integration; however,
these
approaches have often been problematic. Ideally, materials could be designed
to be both
self-organizing and self-assembling.
[0005] Generating bone as an adjuvant therapeutic approach employed during
orthopedic
trauma procedures or during routine spine fusion procedures represents a
continuing
challenge in orthopedic surgery. Specifically, these adjuvant bone-generating
therapies seek
to increase the growth of healthy bone at the site of surgical intervention in
parallel with
decreasing the healing time for bone. In the last several decades a number of
attempts have
been made to use various growth factors with osteogenic potential, including
Bone
Morphogenci Protein (BMP). Unfortunately, BMP based therapies intended to
generate bone
also carry a risk for tumorigenesis in patients, particularly those who may be
undergoing X-
radiation therapy or possess nascent undetected tumor. Further, BMP based
therapies
cannot be used in patients with active tumor, which is particularly
unfortunate since these
patients would benefit significantly from therapies that increase bone
formation during
surgical intervention.
[0006] Impaired fracture healing continues to present a significant challenge
in orthopedic
surgery and bone healing. Fracture non-union rates as high as 5-20% have been
reported.
The morbidity and cost associated with the treatment of patients developing
non-unions can
be substantial. Approximately 10% of the 6.2-million fractures encountered
each year have
difficulty healing. Various options exist to help accelerate bone healing,
with unproven
efficacy. Iliac crest bone graft is still considered to be the gold standard
but has significant
issues related to harvest site co-morbidity. Growth factor based therapies
that include
platelet-derived growth factor (PDGF), fibroblast growth factor (FGF) and
parathyroid
hormone (PTH) has shown initial success in cell culture studies; however,
their efficacy
2
CA 02984889 2017-11-02
WO 2015/184059 PCT/US2015/032820
remains unproven in clinical application. An additional option, such as bone
morphogenic
protein-2 (BMP2) and BMP7, has been shown to have success in accelerating
fracture
healing with diaphyseal fractures. However, there are risks associated with
the use of BMP
that include increased infection, increased risk of tumor growth, and an
increased risk of
local osteolysis. Many of the risks associated with treatments that include
BMP also preclude
the use of BMP for patients with other pathologies.
[0007] The therapeutic ability to increase bone formation, as an adjuvant
during orthopedic
surgery, while not increasing the potential for tumor growth is currently a
limitation of
commercially available biologics, when treating complex orthopedic problems
such as spine
fusion, fracture healing and the management of fracture non-unions.
[0008] In the field of orthopedic trauma, particularly with open fractures
with large defects
and non-unions; autogenous/ allogenic bone grafts are the primary treatment
options.
However, autogenous harvested bone graft, used as the gold standard to achieve
bone
formation, has risks of infection and donor site pain. Other allogenic bone
graft substitutes
have shown poor healing when used singularly. The same limitations exist for
spine
surgeries when these graft options are used to achieve fusions.
[0009] Cortical and cancellous bone derived from cadaveric sources serves to
fill space and
is primarily osteo-conductive without significant osteo-inductive potential.
Hence, biologics
such as PDGF, VEGF and BMP are used to increase rates of healing or spine
fusion, and
their application adds to the cost of treatment. However, these biologic
therapies stimulate
proliferation during development in a range of cell phenotypes, which presents
an inherent
and unacceptable tumor risk.
[00010] De-mineralized bone matrix and calcium phosphate substitutes have not
shown
high efficacy at accelerated bone healing and also have significant cost
associated with
them due to production costs.
[00011] Recombinant BMP2 (rhBMP2) is an implant commercially developed by
Medtronic
known as INFUSE that is distributed in small (4.2-mg of BMP2 with 2x collagen
sponges for
a 15-mg/ cm3 implant), medium (8.4-mg of BMP2 with 4x collagen sponges for a
15-mg/ cm'
implant), large (12-mg of BMP2 with 6x collagen sponges for a 15-mg/ cm'
implant) and
large-II (12-mg of BMP2 with lx collagen sponge for a 15-mg/ cm3 implant). All
sizes of the
INFUSE implant are approved for spine and maxillofacial applications while
only the large-II
3
CA 02984889 2017-11-02
WO 2015/184059
PCT/US2015/032820
implant is approved for fracture. An INFUSE implant is administered by
reconstituting the
powdered BMP2 with sterile saline and then adding the BMP2-saline solution to
the collagen
sponge; after which the implant is delivered locally during surgical
intervention.
[00012] Recombinant BMP7 (rhBMP7 or OP1) is an implant commercially developed
by
Stryker and now owned by Olympus known as OP1. OP1 implants are distributed as
OP1-
putty (20-mL vial containing powdered bovine cartilage and 3.3-mg of BMP7) or
OP1-implant
(1-g of powdered bovine cartilage and 3.3-mg of BMP7). The OP1-putty is
approved for
spine fusion surgeries while the OP1-implant is approved for treating
fractures and fracture
non-union surgery. The OP1-putty or the OP1-implant is administered by,
reconstituting the
powdered BMP7 with sterile saline first, and then adding the BMP7-saline
solution to the
collagen implant; after which the implant is delivered locally during surgical
intervention.
[00013] The opioid growth factor-receptor (OGFR or c-opioid receptor) is a non-
canonical,
pen-nuclear opioid-receptor that does not share structural homology with the
canonical K-
and 5-opioid-receptors (OPRM, OPRK and OPRD, respectively) and binds the
native opioid-
ligands less efficiently than the canonical opioid receptors. The opioid
growth factor (OGF or
met-5 enkephalin: met5) is the native ligand for the OGFR. Met5 is derived
from the pro-
hormone pro-enkephalin (PENK) and to a lesser extent pro-opiomelanocortin
(POMC),
which are first reduced by prohormone convertase (PCSK1 and PCSK2) and then
carboxypeptidase E or D (CPE or CPD; enkephalin convertase) to form five
copies of met5-
enkephalin. Previous work identified met5 expression in osteoblasts and
osteoprogenitors
(Rosen et al., Proc Natl Acad Sci 88(9):3705-9, 1991; Rosen et al., J Bone
Miner Res.
13(10):1515-20, 1998; Elhassan et al., J Bone and Miner Res., 13(1): 88-95,
1998: Cheng et
al., Mol Biol Cell. 20(1):319-27, 2009). Additionally, Kuis et al. identified
met5 in monocytes
of the peripheral blood and spleen (Kuis et al., J Olin Invest. 88(3):817-24,
1991).
Nevertheless, these investigators failed to identify a functional significance
for OGFR-
signaling in mesenchymal of myeloid lineages. Elhassan et al. (J Bone and
Miner Res,
13(1): 88-95, 1998) discloses the presence of met5 in bone and joint tissues.
However,
there is no demonstrable link between met5 and bone formation.
SUMMARY OF THE DISCLOSURE
[00014] It has been identified herein that inhibition of opioid growth factor
signaling,
promotes bone formation and/or reduces bone destruction. Without intending to
be bound by
any particular theory, it is believed that inhibition of the opioid growth
factor signaling through
the opioid growth factor receptor (OGFR) is effective to promote bone
formation and/or
4
CA 02984889 2017-11-02
W02015/184059 PCT/US2015/032820
reduce bone destruction. It has been demonstrated herein that inhibition of
opioid growth
factor signaling promotes bone formation in an animal by locally administering
an inhibitor of
the opioid growth factor (OGFR) signaling pathway directly to the site where
bone formation
is desired. It has also been demonstrated that administration of an inhibitor
of the opioid
growth factor signaling pathway directly to the site where bone formation is
desired is
required to promote the differentiation of MSC to become osteoblasts and
prevent the
differentiation of monocytes into osteoclasts, which leads to the increase in
mineralization,
and the increase in bone formation at a site of bone injury or surgery.
[00015] Accordingly, the present invention is directed to a method for
promoting bone
formation or reducing bone destruction, by administering to an animal in need
thereof, an
amount of an antagonist of the opioid growth factor receptor effective to
promote bone
formation and/or reduce bone destruction directly to a site where bone
formation is desired.
[00016] In one aspect, the OGFR antagonist employed in the present method
blocks the
binding of opioid growth factor to the OGFR.
[00017] In some embodiments, the OGFR antagonist that blocks the binding of
opioid
growth factor (met5) to OGFR is naloxone or a functional derivative thereof,
naltrexone or a
functional derivative thereof, or a combination thereof.
[00018] In other embodiments, OGFR antagonists are derived from oxymorphone
and bind
to the OGFR, which include: naloxone, naltrexone, nalorphine, naloxonazine,
levallorphan,
nalmefene, cyprodime, cyclorphan, cyclazocine, oxilorphan, LY113878, MR2266,
diprenorphine, WIN 44,441-3, naltindole, or norbinaltorphimine.
[00019] In still other embodiments, OGFR antagonists are derived from trans-
3,4-dimethy1-
4-phenylpiperidine and bind to the OGFR, which include: LY99335, LY25506,
LY117413, or
LY255582.
[00020] In some embodiments, OGFR antagonists are derived from the met5-
enkephalin or
leu-enkephalin peptides, bind to the OGFR, and minimally include the following
amino acid
sequences as a means of targeting the OGFR: Tyr--Gly¨Gly¨Phe¨Met (SEQ ID NO:
1) for
those derived from met5-enkephalin or Tyr¨Gly¨Gly¨Phe¨Leu (SEQ ID NO: 2) for
those
derived from the leu-enkephalin.
[00021] In still other embodiments, OGFR antagonists are derived from the
peptide
antagonist 1C1174864 (N,N-diallyl-Tyr-Aib-Aib-Phe-Leu-OH, SEQ ID NO: 3;
CA 02984889 2017-11-02
WO 2015/184959 PCT/US2015/032820
Aib=aminoisobutytic acid) or somatostatin analog CTP (D-Phe-Cys-Tyr-D-Trp-Lys-
Thr-Pen-
Thr-NH2, SEQ ID NO: 4).
[00022] In another aspect, the OGFR antagonist employed in the present methods
is a
molecule that disrupts the nuclear localization sequence found within OGFR:
251
QSALDYFMFAVRCRHQRRQLVHFAWEHFRPRCKFVWGPQDKLRRFKPSSL (SEQ ID NO:
5).
[00023] In still another aspect, the OGFR antagonist employed in the present
methods is a
small-hairpin (sh)-RNA or a small-interfering (si)-RNA directed against the
OGFR gene and
effective in disrupting OGFR gene expression.
[00024] In another aspect, this disclosure provides a method for promoting the
recruitment
of mesenchymal stem cells (MSC) to a local site of injury or surgical
intervention in bone to
promote healing while inhibiting osteoclast driven bone degradation (or re-
absorption). The
method is based on administration of an amount of an OGFR antagonist to
promote bone
formation while inhibiting bone degradation. The injury can be, e.g., bone
fracture or a
surgical intervention. In some embodiments, the OGFR antagonist is
administered locally to
the site of injury or site of surgical intervention.
DESCRIPTION OF THE DRAWINGS
[00025] Figures 1A-1I: (A) The addition of naloxone between 1-11M and 1-mM
with
osteoinduction media substantially increased mineral accumulation (red
staining) in MSC
cultures induced to become osteoblasts. The addition of naltrexone between 1-
m and 1-
mm with osteoinduction media also increased mineral, but to a lesser extent
than naloxone.
(B) The delta opioid receptor (OPRD) gene expression decreased substantially
in
osteoblasts (* = p<0.001) and osteoclasts (" = p<0.0084) while opioid growth
factor receptor
(OGFR) gene expression (C) increased significantly in osteoblasts (* = p<0.02)
and
osteoclasts (" = p<0.0001). (D) The OGFR-ligand, met5-enkephalin (met5), is
derived from a
larger precursor protein known as pro-enkaphalin (PENK). The addition of 5- or
50-4M of
met5 had no effect on mineral formation in culture. (E) Importantly, adding 5-
jiM met5
(PENK) to osteoinduction media had no effect on mineral accumulation while 50-
pm met5
decreased mineral accumulation slightly. However, when 1-mM of naloxone was
added with
5- M or 50-RM met5 and osteoinduction media, naloxone treatment was able to
abrogate
the anti-osteogenic effects of met5. (F) The addition of osteoinduction media
resulted in
mineral formation relative to control cultures. BMP2 increased mineral
formation relative to
6
CA 02984889 2017-11-02
WO 2015/184059 PCTJUS2015/032820
controls while a single treatment (1x dose) with naloxone (1-mM) and two
treatments (2x-
dose) with naloxone increased mineral formation (red staining). Treatment with
naloxone at
each media change (continuous dose) suppressed mineral formation. (G) The
addition of
naloxone to MSC cultures decreased cell number at 72- and 120-hours (" =
p<0.017). (H)
The addition of naloxone to monocyte cultures also reduced cell number
significantly (" =
p<0.0001). (I) Monocytes cultured to become osteoclasts were unaffected by
treatment with
met5 relative to control osteoclast cultures (TRAP staining is purple). The
addition of 1- M of
naloxone or 1-1iM naltrexone did not significantly reduce osteoclast number
while the
addition of 1-mM of naloxone or 1-mM naltrexone reduced osteoclast number
substantially.
[00026] Figures 2A-2E: (A) MSC transfected with OGFR shRNA expressed
significantly
less OGFR gene expression ("` = p<0.0085), which was corroborated (B) in
decreased
OGFR in nuclear and cytoplasmic protein lysates. (C) In addition, in OGFR
deficient MSC
SMAD1 gene expression was significantly greater than in control MSC and GFP
transfected
control cultures (* = p<0.0008). (D) ID1 gene expression was also increased in
the OGFR
deficient MSC relative to the control MSC and GFP transfected control cultures
(" =
p<0.0217). (E) The osteoblast specific protein osteocalcin (OCN) was also
significantly
increased in the OGFR deficient MSC induced to become osteoblasts relative to
control
cultures and GFP transfected control (* = p<0.0215).
[00027] Figures 3A-3G: (A) The addition of 1-mM of naloxone treatment
increased bone
mass (By/Tv) 1.53-fold (" =134.001) in the unicortical defects relative to
control PBS or met5
treated defects. (B) CT images of the control, naloxone, or met5 treated
group defects. (C)
Elevated bone mass (By/Tv) paralleled a 1.2-fold increase in trabecular number
(TbN) (" =
p<0.047). (D) The surgical administration of a unicortical defect in the
surgical control group
(Sx Control) increased the By/Tv (bone volume corrected by total volume)
relative to the
control non-surgical group, which corresponds to bone mass that has
accumulated within the
defect (* = p<0.034). Defects treated with a bovine collagen implant and PBS
or met5 were
not different from surgical controls. However, both the PBS (" = p<0.0021) and
the met5 (" =
p<0.0009) treatment groups were significantly increased relative to the non-
surgical control
group. Defects treated with BMP2, naltrexone or naloxone were all increased
relative to non-
surgical controls (* = p<0.0001). The BMP2, naltrexone, and naloxone treatment
groups
were significantly increased when compared to the surgical control group (X =
p<0.0005),
the PBS treatment group (# p<0.0124) and the met5 treatment group (-4- =
p<0.011). The
BMP2 treatment group By/Tv was not different from the naltrexone treatment
group while the
naloxone treatment group By/Tv was significantly increased (0 = p<0.035). (E)
Trabecular
7
CA 02984889 2017-11-02
WO 2015/184059 PCT/US2015/032820
thickness (TbTh) was increased in the surgical control (Sx Control) group, the
met5
treatment group, the BMP2 treatment group, the naltrexone treatment group and
the
naloxone treatment group (* = p<0.04). Only the TbTh in the naloxone treatment
group was
greater than the BMP2 treatment group or the naltrexone treatment group (** =
p<0.0002).
(F) The collagen implant increased bone formation in the lumbar spine relative
to the SHAM
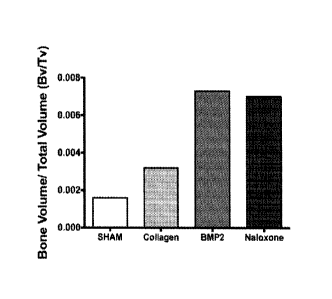
surgery controls. However, the BMP2 + collagen or the naloxone + collagen
implant
increased bone formation over the collagen implants alone. (G) uCT images of
the lumbar
bone fusion mass for the collagen implant and the naloxone + collagen implant.
[00028] Figures 4A-4D: (A) OGFR gene expression was observed in osteoblasts (*
¨
p<0.018), RDES Ewing's sarcoma of bone tumor cells (* = p<0.0014), Hs822t
Ewing's
sarcoma of bone tumor cells (* = p<0.0001), Hs863t Ewing's sarcoma of bone
tumor cells (*
= p<0.039) and Sa0S2 osteosarcoma tumor cells (" = p<0.05). (B) Seventy-two
hours after
the addition of either 1-mM of naloxone or 1-mM of naltrexone, SaOS2
osteosarcoma cell
number decreased significantly relative to the control cultures (*= p<0.0001).
The OGFR
ligand, met5, had not effect on cell number. (C) The Hs822t Ewing's sarcoma of
bone tumor
cell line are adherent in culture. Seventy-two hours after the addition of a 1-
mM dose of
naltrexone, Hs822t Ewing's sarcoma of bone tumor cell number decreased
relative to the
control cultures (" = p<0.0025). Naloxone had no effect of Hs822t tumor cell
number. In
contrast, the addtion of 50-mM of met5 resulted in a significant increase in
the number of
Hs822t tumor cells (X = p<0.03). (D) The RDES Ewing's sarcoma of bone tumor
cells are
loosely adherent in culture. Seventy-two hours after the addition of either 1-
mM of naloxone
or 1-mM of naltrexone dose, RDES Ewing's sarcoma of bone tumor cell number
decreased
significantly relative to the control cultures (* = p<0.0005). The addition of
met5 had no effect
on RDES tumor cell number.
DETAILED DESCRIPTION
[00029] It has been demonstrated herein that naloxone or naltrexone increases
bone
formation while decreasing osteoclast number. Increased bone formation is
supported by
mineral formation observed in culture and bone formation measured using micro-
CT
following a surgically induced unicortical defect with or without a bovine
collagen carrier, or
following fusion the posterolateral vertebral processes using a bovine
collagen carrier. The
surgical model resulted in an injury containing abundant albumin: which can
sequester
naloxone and naltrexone thereby supporting an extended availability of these
OGFR
antagonists.
8
CA 02984889 2017-11-02
WO 2015/184059 PCT/US2015/032820
[00030] In one aspect, the invention provides a method for promoting bone
formation and/
or reducing bone degradation. The method includes administering an amount of
an OGFR
antagonist to a local site of injury or surgical intervention in bone.
[00031] As used herein, a "surgical intervention" includes a surgical
procedure to repair a
fracture, a surgical procedure used to fuse vertebral bones (e.g. spine
fusion), or a surgical
procedure that includes, for example, integration of an implant during total
joint arthroplasty,
bone screws used during fracture repair, bone screws used to anchor tendons or
ligaments,
or any orthopedic hardware designed to mechanically stabilize the orthopedic
surgical site.
OGFR Antagonist
[00032] By "OGFR antagonist" is meant any molecule that inhibits, suppresses
or causes
the cessation of at least one OGFR -mediated biological activity.
[00033] In some embodiments, an OGFR antagonist is an OGFR binding antagonist,
namely, a molecule that, interferes with, blocks or otherwise prevents the
interaction or
binding of the met5-ligand (OGF) to the OGFR. An OGFR binding antagonist can
function in
two ways: First, the OGFR antagonist can compete with the met5-ligand for
binding to the
OGFR on the surface of the nuclear membrane, thereby interfering with,
blocking or
otherwise preventing the binding of the me15-ligand to the OGFR, without
triggering the
downstream signaling that would otherwise be induced by the binding of the
met5-ligand to
the OGFR. Alternatively, an OGFR binding antagonist can bind to or sequester
PENK or the
met5-ligand with sufficient affinity and specificity to substantially
interfere with, block or
otherwise prevent binding of met5-ligand to the OGFR, thereby inhibiting,
suppressing or
causing the cessation of at least one OGFR-mediated biological activity.
Generally speaking,
OGFR binding antagonists can be large molecules (e.g., antibodies) or small
molecules
(e.g., compounds of a molecular weight of less than 15-kD, 12-kD, 10-kD or
even 8-kD), and
can be a polypeptide, nucleic acid, or a synthetic small molecule compound.
OGFR binding
antagonists can be identified with any in vitro assay readily selected by one
of skill in the art.
For example, OGFR antagonists can be identified using the methods described in
U.S.
Patent No. 5,882,944, U.S. Patent No. 6,007,986, or U.S. Patent No. 6,270,979.
[00034] in one embodiment, the OGFR binding antagonist is naloxone or a
functional
derivative thereof, naltrexone or a functional derivative thereof, or a
combination thereof.
[00035] As used herein, a "functional derivative" refers to a derivative or
analog that is
structurally and functionally analogous to the originating molecule (e.g.,
maintains the
9
CA 02984889 2017-11-02
WO 2015/184059 PCT/US2015/032820
function of naftrexone or naloxone as an OGFR antagonist). Nafoxone and
naltrexone
analogs can be synthesized using standard synthetic procedures such as those
described in
March J., Advanced Organic Chemistry, 3rd Ed. (1985). Examples of naltrexone
and
naloxone functional derivatives include salt forms, e.g., naloxone
hydrochloride dihydrate or
naltrexone hydrochloride. Additional examples of naltrexone and naloxone
functional
derivatives suitable for use in the present methods include naltrexone and
naloxone analogs
disclosed in U.S. Patent Application Publication No. 2007/0197573 Al, U.S.
Patent No.
6,713,488, for example.
[00036] In another embodiment, an OGFR binding antagonist is derived from
oxymorphone
and binds to the OGFR, which includes naloxone, naltrexone, nalorphine,
naloxonazine,
levallorphan, nalmefene, cyprodime, cyclorphan, cyclazocine, oxilorphan,
LY113878,
MR2266, diprenorphine, WIN 44,441-3, nattindole, or norbinaltorphimine.
[00037] In still another embodiment, an OGFR binding antagonist is derived
from trans-3,4-
dimethy1-4-phenylpiperidine and binds to the OGFR, which includes LY99335,
LY25506,
LY117413, or LY255582.
[00038] In another embodiment, an OGFR binding antagonist is derived from the
met5-
enkephalin or leu-enkephalin peptides, binds to the OGFR, and minimally
includes the
following amino acid sequences as a means of targeting the OGFR:
Tyr¨Gly¨Gly¨Phe¨Met
(SEQ ID NO: 1) for those derived from met5-enkephalin or Tyr-Gly-Gly-Phe-Leu
(SEQ ID
NO:2) for those derived from the leu-enkephalin.
[00039] In still another embodiment, an OGFR binding antagonist is derived
from the
peptide antagonist IC1174864 (N,N-diallyl-Tyr-Aib-Aib-Phe-Leu-OH, SEQ ID NO:
3;
Aib=aminoisobutytic acid) or somatostatin analog CTP (D-Phe-Cys-Tyr-D-Trp-Lys-
Thr-Pen-
Thr-NH2. SEQ ID NO: 4).
[00040] In other embodiments, the OGFR antagonist, instead of being an OGFR
binding
antagonist, is a molecule that disrupts the nuclear localization sequence
found within OGFR:
251 QSALDYFMFAVRCRHORROLVHFAWEHFRPRCKFWVGPODKLRRFKPSSL (SEQ ID
NO: 5).
[00041] In still other embodiments, the OGFR antagonist employed in the
present methods
is a small-hairpin (sh)-RNA or a small-interfering (si)-RNA directed against
the OGFR gene
and effective in disrupting OGFR gene expression.
CA 02984889 2017-11-02
WO 2015/184059
PCT/US2015/032820
[00042] The OGFR antagonists described herein can be administered individually
or in
combination. Suitable combinations include, for example, naloxone and
naltrexone;
naloxone and/or naltrexone, in combination with another OGFR binding
antagonist or
another OGFR antagonist.
Combination of OGFR Antagonists with Other Active Agents
[00043] An OGFR antagonist described herein can be administered in combination
with one
or more other active agents that promote bone formation or growth via SMAD
signaling,
which can be synergistic to an OGFR antagonist. Examples of such active agents
include,
but are not limited to, a BMP molecule (e.g., BMP-2, BMP-4, BMP6 and BMP-7),
which can
regulate SMAD1/5/8 signaling. A TGFp molecule that can expand the MSC pool
that can
then be regulated by an OGFR antagonist. TGFp inhibitors, that include
LY2109761, that
reduce TGFp signaling decrease inhibitor SMAD (SMAD6 and SMAD7) signaling and
then
antagonize BMP signaling and reduce bone formation. For example, a collagen
implant can
be infused with a desirable BMP molecule, a TGFP molecule or a TGFp inhibitor
molecule
and an OGFR antagonist for use in the present methods.
[00044] An OGFR antagonist described herein can be administered in combination
with one
or more other active agents that promote bone formation or growth. Examples of
such
active agents include, but are not limited to one or more growth factors, such
as EGF,
VEGF, PDGF, IGF, FGF, TGFa, and cytokines; cells such as mesenchymal stem
cells,
chondrocytes, and bone marrow cells. For example, a collagen implant can be
infused with
both a desirable growth factor such as a VEGF molecule and an OGFR antagonist
for use in
the present methods. An OGFR antagonist can also be administered in
combination with a
chemotherapeutic agent in treating a cancer patient to promote bone formation
or growth in
that patient.
Delivery Systems/Carriers
[00045] An OGFR antagonist described herein can be administered with or
without a carrier
locally to accelerate fracture repair or promote fusion of vertebral bone.
In some
embodiments, an OGFR antagonist is combined with or encapsulated within a
carrier for
administration.
[00046] Suitable carriers can be in bead, microsphere or nanoparticle form,
and can be
made of natural and/or synthetic biocompatible polymers. Examples of
suitable
11
CA 02984889 2017-11-02
W02015/184059 PCT/US2015/032820
biocompatible polymers include hyaluronic acid, collagen, tricalcium
phosphate, chondroitin
sulfate, polybutyrate, polylactide, polyglycolide, and lactide/glycolide
copolymers, and
mixtures or copolymers thereof. Suitable carriers also include non-polymer
systems such as
carboxylic acids, fatty acids, phospholipids, amino acids, lipids such as
sterols, hydrogel
release system; silastic system; peptide-based system; implants and the like.
[00047] In one embodiment, the carrier is a hygroscopic collagen based carrier
(e.g., a
collagen sponge, a collagen scaffold, a powdered collagen, or a collagen based
gelatin
hydrogel).
[00048] In another embodiment, the carrier is a hydrophilic hydrogel based
carrier (e.g.,
poly lactic acid, poly glycolic acid), which allows an OGFR antagonist (e.g.,
naloxone or
naltrexone or a functional derivative thereof) infused therein to be released
over a period of
time.
[00049] In still another embodiment, the carrier is albumin, a derivative or
fragment of
albumin that maintains the naloxone/ morphine binding site located at the
interface between
the IA and I IA domains, and/ or maintains the naloxone binding site around
tryptophan (Trp)-
214, that binds an OGFR antagonist such as naloxone or naltrexone or a
functional
derivative thereof and allows for a slow release of the OGFR antagonist.
[00050] In still another embodiment, methyl cellulose, and an insert gel, for
example, that
binds an OGFR antagonist such as naloxone or naltrexone or a functional
derivative thereof
and allows for a slow release of the OGFR antagonist.
[00051] In a further embodiment, the carrier is a bovine collagen implant. An
OGFR
antagonist, e.g., naloxone or naltrexone or a functional derivative thereof,
can be combined
with a bovine collagen implant, in a manner similar to either INFUSE (BMP2) or
OP1-putty or
OP1-implant, that is supplied with a bovine collagen sponge, powdered bovine
collagen, or
collagen based gelatin construct. Administration of naloxone, naltrexone or a
functional
derivative thereof can be achieved by, e.g., reconstituting the powdered
naloxone or
naltrexone or a functional derivative thereof with sterile saline and then
adding the OGFR
antagonist-saline solution to the collagen implant; after which the implant
can be delivered
locally to the site of surgical intervention.
12
CA 02984889 2017-11-02
WO 2015/184059 PCT/US2015/032820
[00052] In still another embodiment, the carrier is a surgical implant, or a
surgical implant
delivery system selected from e.g., cages, screws, rods, plates, expandable
cages, anchors
(metal based, synthetic or biodegradable anchors).
[00053] In a further embodiment, the carrier is composed of beta tricalcium
phosphate in the
form of chips or powers, cement (polymethylmethacrylate or "PMMA"), or a
demineralized
bone matrix scaffold in the forms of e.g., putty, paste, boats, or injectable
formulations.
[00054] In another embodiment, the carrier is a carrier composed of PGA (poly
glycolic
acid)-PLGA (polylactic glycolic acid) spheres, which can encapsulate an OGFR
antagonist to
provide for immediate, delayed or sustained release.
[00055] In another embodiment, the carrier is an allograft such as
corticocancellous
allograpft, cortical chips and structural allograft.
[00056] In another aspect, this disclosure provides a method for promoting the
recruitment
of mesenchymal stem cells (MSC) to a local site of injury or surgical
intervention in bone to
promote healing while inhibiting osteoclast driven bone degradation (or re-
absorption). The
method is based on administration of an amount of an OGFR antagonist described
herein
(e.g., naloxone or naltrexone or a functional derivative thereof) to promote
bone formation
while inhibiting bone degradation.
[00057] In still another aspect, this disclosure provides a method of treating
osteonecrosis
or osteoradionecrosis based on administration of an OGFR antagonist described
herein
(e.g., naloxone or naltrexone or a functional derivative thereof).
[00058] In some embodiments, this disclosure provides a suitable dose range,
through
which naloxone (400-rig! mL (1- M) to 400-jig/ mL (1-mM)) and/ or naltrexone
(378-ng/ mL
(1-p,M) to 378-pg/ mL (1-mM)) is administered at doses that range between 1-
jiM and 1-mM
and are effective to increase bone formation and/or decrease bone degradation
through
decreased osteoclast formation. Specific dose amounts of naloxone can be, for
example, 400-ng/ mL, 800-ng/ mL, Hug/ mL, 10-jig! mL, 50- g/ mL, 100-11g/ mL,
400-jig/ mL
or an amount between any of the listed doses so long as these doses range
between 1- M
and 1-mM. These dosage amounts can be delivered in a single administration or
multiple
administrations. The precise, total amount of naloxone that is effective will
depend on the
extent of the injury or surgical application and the carrier to be used. For
example, 400-jig/
mL naloxone-saline solution, or any equivalent dose, that is equal to 400- g/
cm' naloxone-
13
saline solution, would be recommended for every collagen implant with
dimensions 2-cm x
2-cm x 0.25-cm or 1-cm-1 of naloxone (e.g., collagen infused with 1-mlel of a
naloxone-saline
solution) or a functional derivative thereof, which can be administered to an
injury or local
surgical site that results in substantial bone formation and inhibition of
osteoclast numbers
within the surgical area. Specific dose amounts of naltrexone can be, for
example, 378-ngi
mL, 756-ngi mL, leig( mL, 10-pgi mL, 5014 mL, 10014 mL, 378-jig/ mL or an
amount
between any of the listed doses, The precise, total amount of naltrexone that
is effective will
depend on the extent of the injury or surgical application and the carrier to
be used. For
example, 378-egt mL naltrexone-saline solution, or any equivalent dose, that
is equal to 378-
pee cm/ naltrexone-saline solution, would be required for every collagen
implant with
dimensions 2-cm x 2-cm x 0.25-cm or 1-cm/ of naltrexone (e.g,, collagen
infused with 1-mM
of a naltrexone-saline solution) or a functional derivative thereof, which can
be administered
to an injury or local surgical site that results in substantial bone formation
and inhibition of
osteoclast numbers within the surgical area.
i00059] The present description is further illustrated by the following
examples, which
should not be construed as limiting in any way.
(C0060) Example 1: Opioid Antagonists Regulate Bone Formation and Re-
absorption.
(000611 Methods: Human bone marrow was collected from consenting adult
patients
undergoing either an elective primary proximal femoral total hip arthroplasty
or elective
primary distal femoral total knee arthroplasty (ne6. mean age 65) as a part of
an IRE
approved study. Human MSC were derived from the adherent fraction of cells
derived from
each whole bone marrow aspirate collected, while the monocyte population was
collected
from the non-adherent fraction of the bone marrow. The monocyte fraction was
enriched
through sub-culture with 100-ngi mi. recombinant human macrophage colony-
stimulating
factor (MCSF: Wyett). In parallel experiments described below, the femurs from
3-week
(ne10) and 16-week (re=20) old male mice were collected and then the bone
marrow was
flushed from the femur according to the following: A 21-gauge needle was
inserted into the
femoral intramedular canal after the removal of the proximal and distal ends
of the femur.
Media was then carefully passed through the proximal end of the femur, which
forced the
bone marrow to pass out of the bone_ Finally, the bone marrow pellet was
mechanically
disassociated using an 18-gauge needle and then passed through a 70-pm mesh
filter,.
14
Date Recue/Date Received 2021-09-23
CA 02984889 2017-11-02
WO 2015/184059
PCT/US2015/032820 -
These whole bone marrow aspirates were used to generate osteoclasts. Cells
were
maintained in Dulbecco's Modification of Eagle's Media (DMEM) containing 10%
fetal calf
serum (v/v) and 1% penicillin-streptomycin-glutamine (PSG; Cellgro,
Mediatech).
Recombinant human netrin-ligands (NTN1 and NTN4) were diluted in PBS (R&D
Systems).
The responsible IACUC committee approved all of the animal studies described
in this work.
[00062] Gene Expression Analysis: MSC, osteoblasts and adipocytes derived from
human bone marrow were assayed for changes in gene expression. In parallel,
osteoclasts
derived from human monocytes were also assayed for changes in myeloid gene
expression.
Gene data were derived from two independently generated samples collected from
at least
three patients. mRNA was purified using RNeasy Plus Mini columns (Qiagen) and
cDNA
was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad). Gene
expression was
analyzed using quantitative PCR (qPCR) using 100-ng of cDNA mixed with Fast
Plus
EvaGreen Master Mix (Biotium). In each experiment GAPDH served as a control,
negative
controls contained no-template and a standard curve was generated using serial
dilutions of
a chemically synthesized sequence for GAPDH (0, 1, 10 and 100 femtograms;
Integrated
DNA Technologies). Gene expression was evaluated using Pfaffl's method, in
which the
efficiency of each primer (E) and the starting gene product concentration (R,)
are calculated
from the linear region of the fluorescence-crossing threshold curve using the
software
LinRegPCR (v2013.0). Experiments were considered valid when the control gene
GAPDH
fell within the standard curve and the primer efficiencies (E) were calculated
to be E>=1.8.
The presence of a single gene product was confirmed using a melt-curve
analysis and
product size was confirmed using gene product gel-electrophoresis.
[00063] Protein Expression through Western Blot Analysis: Human MSC,
osteoblasts
and osteoclasts were lysed with cold RIPA buffer (Pierce Thermo Scientific)
containing 2-
mM iodoacetamide, 2-mM benzamidine hydrochloride, 0.1-mM ethylmaleimide, 1%
PMSF
and the Halt Protease Inhibitor Cocktail (Pierce Thermo Scientific). Protein
lysates were
analyzed from at least two replicates generated from three patient samples.
Total protein
was assayed using the BCA Protein Assay Kit (Thermo) following the
manufacturers
instructions. Samples were loaded (20-pg/ well) onto a 10-20% Mini-Protean
Tris-Tricine
Precast Gel (Bio-Rad) with the Page Ruler Pre-stained MR Protein Ladder (Bio-
Rad) and
transferred to a nitrocellulose membrane (Bio-Rad). OGFR was identified on
membranes
blocked using 5% non-fat milk with a OGFR primary antibody (Santa Cruz
Biotechnologies).
Actin (1:500) and 8-tubulin served as loading controls. Antibodies were
detected using an
HRP-conjugated micro-polymer conjugated secondary antibody (ImmPress kit,
Vector Labs)
CA 02984889 2017-11-02
WO 2015/184059 PCT/US2015/032820
in conjunction with the Clarity Western ECL substrate (Bio-Rad). Mouse brain
protein lysates
(mB) were used as positive-expression controls.
[00064] Osteogenesis: Osteogenic potential in MSC was assayed by chemically
inducing
mineral formation. MSC from at least three human patients were seeded at 5x103
cells per
well and allowed to become confluent and woven prior to the addition of osteo-
induction
media. Induction media consisted of DMEM containing 20% FCS (v/v) and 1% PSG
supplemented with 25- g/ mL of acscorbic-2-phosphate (Sigma), 100-nM
dexamethasone
(Sigma) and the following dosing regimen of I3-glycerophosphate (BOP; Sigma):
lx media
change with 5-mM BOP, lx media change with 10-mM BOP and lx media change with
20-
mM of BGP. Met5-ligand (0-, [5- M] 2.87-, or [50-pM] 28.7-jig; Sigma),
naloxone (0-, 400-fg,
400-pg, 400-ng, 400- g; Sigma) or naltrexone (0-, 378-fg, 378-pg, 378-ng, 378-
jig; Sigma)
was added as follows: 1) lx, with the first addition of osteo-induction media,
2) 2x, with the
first and second addition of osteo-induction media and 3) with each post-
induction media
change. Positive control wells were treated with 25-ng of the recombinant
human
BMP2/BMP7-ligand (R&D Systems) with the first addition of induction media.
After the
appearance of mineral nodules, cells were fixed with 70% ice-cold Et0H (Sigma)
and then
stained using 40-mM alizarin red-S (pH 4.2, Sigma). Osteogenesis experiments
were
repeated at least twice for each patient.
[00065] Assay of Cell Number: Following the addition of 1-mM naloxone or 1-mM
naltrexone, viable cell number was determined with the MIT assay. After 72-
hours and 120-
hours, MIT (5 mg/ ml (w/v), Sigma) was added to each well, incubated for 2-
hours, after
which the cells lysed with 500-pl of DMSO (Sigma). MTT was measured at 570-nm
and the
effects of therapy on cell proliferation were determined by normalizing
treated wells relative
to mean values from non-treated wells: Fold change in cell number =
100"[treated cells
optical density/ mean control optical density].
[00066] TRAP Staining and the Assay of Osteoclast Number: Osteoclasts were
derived
from either an enriched population of human monocytes or from mouse non-
enriched whole
bone marrow aspirates. Three human patient bone marrow samples were assayed in
parallel with samples collected from 3-week (n=10) mouse bone marrow. The
monocyte
fraction was stimulated to become osteoclasts by culturing 1x106 cells with 25-
ng/ mL of
MCSF and 25-ng/ mL of recombinant human or mouse RANK-ligand (R&D Systems) in
the
presence of the met5-ligand ([5- M] 2.87-, or [50-pM] 28.7-jig; Sigma),
naloxone (400-ng or
16
CA 02984889 2017-11-02
WO 2015/184059 PCT/US2015/032820
400-gg; Sigma) or naltrexone (378-ng or 378-pig; Sigma). Osteoclasts were
stained with
tartrate resistant acid phosphatase (TRAP; Sigma Leukocyte Acid Phosphatase
Kit 387-A)
and counted when cells stained TRAP-positive and had at least three nuclei.
Estimates of
osteoclast number were obtained by Cavalieri sampling and a modification of
the fractionator
technique.
[00067] shRNA Knock-down of the OGF-receptor: OGF-receptor activity was
inhibited by
transfecting MSC using a commercially available neogenin shRNA-lentivirus, or
with a GFP
lentivirus as a control (Santa Cruz Biotechnologies). MSC were then induced to
become
osteoblasts and subsequently assayed for BMP-target genes (I01, ID2, SMAD1,
SMAD2,
SMAD3, SMAD4, SMAD5, SMAD6, SMAD7, SMA08/9 and osteocalcin (OCN)).
[00068] Unicortical Defect Model: Male 3-week old C57BL/6 mice (n=5 per
treatment
group) were injected with met5, naloxone or naltrexone following the creation
of a unicortical
defect. Briefly, a small incision (approximately 3-mm) was made just below the
knee joint,
located on the medial side of the tibia just below the tibial tuberosity on
the tibial crest. In
young animals the physeal plate is clearly visible and the drill bit was
placed approximately
1-mm below this point. The drill-bit produces a unicortical defect with
dimensions 300-gm
diameter x 1-mm depth. A Hamilton Neuros RN 10-gL syringe with a 33-gauge
blunt tip
needle was used to inject 2-pt of met5 (28.7-gg), naloxone (400-gg) or
naltrexone (378-jig)
resuspended in saline directly into the unicortical defect at a rate no faster
than
approximately 0.1-4 per second. The left-limb tibias served as contra-lateral
surgical
controls, in which animals received a unicortical defect and 2-4 of saline was
injected. Mice
were euthanized 5-days after surgery, hind limbs were collected and tibias
were fixed for
immunofluorescense, TRAP staining and OTC associated bone growth.
[00069] Implantation of Opioid Antagonist Containing Collagen Implant into a
Unicortical Defect Model: Male 5-week old C57BL/6 mice (n=5 per treatment
group for a
total of 25 total animals) were used in this study. Treatment groups consisted
of the
following: 1) PBS + collagen sponge; 2) met5 + collagen sponge; 3) BMP2 +
collagen
sponge; 4) naltrexone + collagen sponge; 5) naloxone + collagen sponge.
Unicortical defects
were surgically administered through a small incision (approximately 3-mm)
made just below
the knee joint, located on the medial side of the tibia just below the tibial
tuberosity on the
tibial crest. In 5-weeks old male mice, the physeal plate is clearly visible
and the drill bit was
placed approximately 1-mm below this point. The drill-bit produces a
unicortical defect with
17
CA 02984889 2017-11-02
WO 20151184959 PCT/US2015/032820
dimensions 300- m diameter x 1-mm depth. Bovine collagen sponge implants
(Duraform)
were prepared (approximately 1-mm x 1-mm) and then soaked in PBS, 50- M (28.7-
g in
10-4), met-5 enkephalin (met5), 25-ng BMP2, 1-mM naltrexone (378- g in 10-4)
or
naloxone 1-mM (400- g in 10-4) (n=5 for each group, 25 total animals). A
unicortical defect
was administered to a separate group of mice (n=5; Surgical Controls for
cortical defect) that
did not receive a collagen sponge implant. In parallel, a separate group of
animals served as
non-surgical controls. Mice were euthanized 7-days after surgery, hind limbs
were collected
and tibias were prepared for micro-CT (jACT) analysis.
[00070] Rat Posterolateral Spinal Fusion of L5-L6 Vertebrae: 20 male, 1-month
old
skeletally mature Sprague-Dawley rats underwent bilateral lumbar
posterolateral spinal
fusion at the L5¨L6 vertebrae. Treatment groups will consist of the following:
1) sham
surgery control; 2) collagen sponge implant; 3) BMP2 + collagen sponge
implant; 4)
naloxone + collagen sponge implant. Under sterile conditions a 2-cm long
posterior midline
incision centered at L5¨L6 vertebrae was made. A muscle-splitting approach was
used,
lateral to the facet joints, to expose the transverse processes of a
particular vertebra. A high-
speed 1-mm burr was used to decorticate the transverse processes of the L5-L6
vertebrae.
Implants were prepared and implanted between the transverse processes
bilaterally in the
paraspinal muscle bed. Bovine collagen sponge implants will be prepared
(approximately 1-
cm x 1-cm) and then soaked in PBS, 25-ng BMP2, 1-mM naloxone (400- g in 10-
L).
Animals were euthanized 2-months (n=5 rats per time point per treatment group
for a total of
n=20 rats) spines were collected and prepared for micro-CT ( CT) analysis.
[00071] MicroCT Analysis of Unicortical Defects: High-resolution images of the
tibia
were acquired with a CT imaging system ( CT40; Scanco Medical). Tibias were
scanned at
45-keV with an isotropic voxel size of 12-pm. An analysis region was selected
from axial
sections to include the entire unicortical defect bounded by the endosteal
cortical wall. The
volume corrected bone volume (bone volume/ total volume; By/Tv), trabecular
number (TbN)
and trabecular thickness (TbTh) were calculated using the Scanco software.
[00072] Statistical Analyses: Prism statistical software (Graphpad) was used
to analyze
data. Means and standard deviations were calculated. Data were analyzed by 1-
way
ANOVA using the Holm-Sidak post-hoc correction for multiple comparisons with
significance
set at p<0.05.
18
CA 02984889 2017-11-02
WO 2015/184059 PCT/U52015/032820
[00073] Results:
[00074] Naloxone and naltrexone increased MSC differentiation into osteoblasts
and
mineral formation while decreasing osteoclast number: The addition of naloxone
between 1-1.1M and 1-mM with osteoinduction media substantially increased
mineral
accumulation (red staining) in MSC cultures induced to become osteoblasts
(Figure 1A). The
addition of naltrexone between 1- rn and 1-mm with osteoinduction media also
increased
mineral, but to a lesser extent than naloxone (Figure 1A). The delta opioid
receptor (OPRD)
gene expression was greatest in the MSC and significantly decreased in
osteoblasts
(p<0.0074) and osteoclasts (p<0.0084) (Figure 1B). The OGFR gene expression
was
increased in osteoblasts (p<0.011) and osteoclasts (p<0.0001) relative to MSC
cultures
(Figure 1C). Met5 (PENK) gene expression was not different between MSC and
osteoblasts;
however, PENK gene expression was significantly decreased in osteoclasts
(p<0.0018)
(Figure 1D). Adding 5-KM met5 (PENK) to osteoinduction media had no effect on
mineral
accumulation while 50- m met5 decreased mineral accumulation slightly (Figure
1E).
However, when 1-mM of naloxone was added with 5-IJM or 50-11M met5 and
osteoinduction
media, naloxone treatment was able to abrogate the anti-osteogenic effects of
met5 (Figure
1E). The wopioid receptor, the K-opioid receptor, the met5 precursor POMC and
the CPA1
enzyme gene expression were never observed. PCSK1, PCSK2, CPD and CPE gene
expression were observed in MSC, osteoblasts and osteoclasts, but were not
significantly
different from one another. The addition of a single, 'pulse' dose of naloxone
(1-mM) or a
double, 'pulse' dose of naloxone (1-mM) 72-hours after the first 'pulse' dose
resulted in a
substantial increase in mineral accumulation while continuous naloxone dosing
(e.g. with
every media change) was slightly depressed (Figure 1F). In addition, we found
that the
addition of 1-mM naloxone suppressed, but did not stop, MSC proliferation at
72-hours
(p<0.0177) and 120-hours (p<0.0001) (Figure 1G). Naloxone similarly decreased
proliferation in monocyte cultures (p<0.0001) (Figure 1H). Monocytes cultured
to become
osteoclasts were unaffected by treatment with met5 relative to control
osteoclast cultures
(TRAP staining is purple) (Figure 11). The addition of 1-1.tM of naloxone or 1-
1iM naltrexone
did not significantly reduce osteoclast number while the addition of 1-mM of
naloxone or 1-
mM naltrexone reduced osteoclast number substantially (Figure ll).
[00075] The loss of OGFR expression lead to increased expression of osteogenic
transcriptional regulators in parallel with increased osteocalcin expression.
MSC
transfected with OGFR shRNA showed significantly reduced OGFR gene expression
(p<0.0085) (Figure 2A), which was corroborated in decreased OGFR in nuclear
and
19
CA 02984889 2017-11-02
WO 2015/184059 PCT/US2015/032820
cytoplasmic protein lysates (Figure 2B). In addition, in OGFR deficient MSC,
SMAD1 gene
expression was significantly greater than in control MSC and GFP transfected
control
cultures (p<0.0008) (Figure 2C). IDl gene expression was also increased in the
OGFR
deficient MSC relative to the control MSC and OFF transfected control cultures
(p<0.0217)
(Figure 2D). The osteoblast specific protein osteocalcin (OCN) was also
significantly
increased in the OGFR deficient MSC induced to become osteoblasts relative to
control
cultures and OFF transfected control (p<0.0215) (Figure 2E).
[00076] Treatment with naloxone or naltrexone increased bone formation in
surgical
defect in an animal model. Unicortical defects were surgically administered to
mouse tibias
that were then treated with naloxone (1-mM) or met5 (50- M). Naloxone
increased bone
healing while met5 had no effect (Figure 3A and 3B). Naloxone treatment
increased bone
mass (By/Tv) 1.53-fold (p<0.001) (Figure 3A). The elevated bone mass that we
measured
was driven by a 1.2-fold increase in trabecular number (TbN) (p<0.047) (Figure
3C). Seven
days after the surgical administration, fracture healing of a unicortical
defect as measured by
By/Tv, was increased 25.6% in the surgical control group (Sx Control) relative
to the non-
surgical controls (p<0.034) (Figure 3D). Defects treated with PBS or met5 in
combination
with a collagen implant were not significantly different from the surgical
control group, with
respect to fracture healing. However, the PBS or met5 treatment coupled with
the collagen
implant resulted in approximately 37% greater than the non-surgical control
group,
consistent with the difference observed between the surgical control and the
non-surgical
control groups (p<0.003) (Figure 3D). Treating the defect with BMP2 and the
collagen
implant resulted in increased By/Tv 32.4% relative to the PBS treated group
and 55.6%
relative to the surgical control group (p<0.0124 and p<0.005, respectively)
(Figure 3D).
There was no significant difference between the BMP2 treated group and the
naltrexone
treated group. The defects treated with naltrexone and a collagen implant
increased By/Tv
39.2% relative to the PBS treatment group and 63.5% relative to the surgical
control group
(p<0.0043 and p<0.0001, respectively) (Figure 3D). Finally, the defects
treated with
naloxone and the collagen implant had an increased By/Tv of 56.5% relative to
the PBS
treated defects and 83.8% relative to the surgical control group (p<0.0001)
(Figure 3D).
Further, naloxone treatment increased By/Tv in the defect 18.2% relative to
BMP2 treatment
group (p<0.035) (Figure 3D). Trabecular thickness is a structural parameter
that relates to
By/Tv, which relates to bone mass within the field of interest. The trabecular
thickness was
increased within the defect significantly in the surgical control (p<0.032),
the met5 (p<0.039),
the BMP2 (p<0.025), the naltrexone (p<0.025) and the naloxone (p<0.001) groups
relative to
the control non-surgical group (Figure 3E). Treating the defect with naloxone
increased
CA 02984889 2017-11-02
WO 2015/184059 PCT/US2015/032820
trabecular thickness approximately 37% relative to all other treatment groups
(p<0.001)
(Figure 3E). Also, the increase in By/Tv observed with naloxone treatment of
the defects
appears to be driven mostly through increased trabecular thickness while
treatment with
BMP2 or naltrexone increased By/Tv through both trabecular number and
trabecular
thickness. The addition of naloxone infused collagen implant to posterolateral
lumbar spine
resulted in a substantial increase in By/Tv relative to spines implanted with
collagen or the
control sham surgery group (Figures 3F and 3G).
[00077] Example 2: The OGFR antagonists do not stimulate sarcoma tumor
proliferation despite OGFR gene expression in sarcoma cells.
[00078] Methods: Human bone marrow was collected from consenting adult
patients
undergoing either an elective primary proximal femoral total hip arthroplasty
or elective
primary distal femoral total knee arthroplasty (n=6, mean age 65) as a part of
an IRB
approved study. Human MSC were derived from the adherent fraction of whole
bone marrow
aspirates. Ewing's sarcoma tumor cells (RDES, Hs822 and Hs863) and Sa0S2
osteosarcoma tumor cells were obtained from ATCC. Cells were maintained in
Dulbecco's
Modification of Eagle's Media (DMEM) containing 10% fetal calf serum (v/v) and
1%
penicillin-streptomycin-glutamine (PSG; Cellgro, Mediatech).
[00079] Gene Expression Analysis: MSC, osteoblasts and adipocytes derived from
human bone marrow were assayed for changes in gene expression. In parallel,
osteoclasts
derived from human monocytes were also assayed for changes in myeloid gene
expression.
Gene data were derived from two independently generated samples collected from
at least
three patients. mRNA was purified using RNeasy Plus Mini columns (0iagen) and
cDNA
was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad). Gene
expression was
analyzed using quantitative PCR (qPCR) using 100-ng of cDNA mixed with Fast
Plus
EvaGreen Master Mix (Biotium). In each experiment GAPDH served as a control,
negative
controls contained no-template and a standard curve was generated using serial
dilutions of
a chemically synthesized sequence for GAPDH (0, 1, 10 and 100 femtograms;
Integrated
DNA Technologies). Gene expression was evaluated using Pfaffl's method, in
which the
efficiency of each primer (E) and the starting gene product concentration (No)
are calculated
from the linear region of the fluorescence-crossing threshold curve using the
software
LinRegPCR (v2013.0). Experiments were considered valid when the control gene
GAPDH
fell within the standard curve and the primer efficiencies (E) were calculated
to be E>=1.8.
21
CA 02984889 2017-11-02
WO 2015/184059 PCT/US2015/032820
The presence of a single gene product was confirmed using a melt-curve
analysis and
product size was confirmed using gene product gel-electrophoresis.
[00080] Assay of Cell Number: Following the addition of 1-mM naloxone or 1-mM
naltrexone, viable cell number was determined with the MTT assay. After 72-
hours and 120-
hours, MTT (5 mg/ ml (w/v), Sigma) was added to each well, incubated for 2-
hours, after
which the cells lysed with 500-pl of DMSO (Sigma). MTT was measured at 570-nm
and the
effects of therapy on cell proliferation were determined by normalizing
treated wells relative
to mean values from non-treated wells: Fold change in cell number =
1001treated cells
optical density/ mean control optical density].
[00081] Statistical Analyses: Prism statistical software (Graphpad) was used
to analyze
data. Means and standard deviations were calculated. Data were analyzed by 1-
way or 2-
way ANOVA using the Holm-Sidak post-hoc correction for multiple comparisons
with
significance set at p<0.05.
[00082] Results:
[00083] Osteosarcoma and Ewing's sarcoma tumors express the OGFR and naloxone
and naltrexone inhibit tumor proliferation. OGFR gene expression was observed
in
osteoblasts (p<0.018), RDES Ewing's sarcoma of bone tumor cells (p<0.0014),
Hs822t
Ewing's sarcoma of bone tumor cells (p<0.0001), Hs863t Ewing's sarcoma of bone
tumor
cells (p<0.039) and Sa0S2 osteosarcoma tumor cells (p<0.05) (Figure 4A).
Seventy-two
hours after the addition of either 1-mM of naloxone or 1-mM of naltrexone,
Sa0S2
osteosarcoma cell number decreased significantly relative to the control
cultures (p<0.0001).
The OGFR ligand, met5, had not effect on cell number (Figure 4B). The Hs822t
Ewing's
sarcoma of bone tumor cell line are adherent in culture. Seventy-two hours
after the addition
of a 1-mM dose of naltrexone, Hs822t Ewing's sarcoma of bone tumor cell number
decreased relative to the control cultures (p<0.0025). Naloxone had no effect
of Hs822t
tumor cell number. In contrast, the addition of 50-1..LM of met5 resulted in a
significant
increase in the number of Hs822t tumor cells (p<0.03) (Figure 4C). The RDES
Ewing's
sarcoma of bone tumor cells are loosely adherent in culture. Seventy-two hours
after the
addition of either 1-mM of naloxone or 1-mM of naltrexone dose, RDES Ewing's
sarcoma of
bone tumor cell number decreased significantly relative to the control
cultures (p<0.0005).
The addition of met5 had no effect on RDES tumor cell number (Figure 4D).
22
CA 02984889 2017-11-02
WO 2015/184059 PCT/US2015/032820
[00084] Summary of Results from Examples 1 and 2:
[00085] Naloxone and naltrexone increase bone formation and reduce bone re-
absorption
(destruction) through a reduction in osteoclast number.
[00086] Naloxone or naltrexone infused collagen implants increased bone
formation in a
unicortical defect or fused lumbar vertebral bones.
[00087] Despite the presence of the OGFR, naloxone or naltrexone did not
increase
sarcoma tumor cell proliferation.
23