Note: Descriptions are shown in the official language in which they were submitted.
1
Device for covering and/or reconstructing a bone defect site; method for
produc-
ing a cap of a covering device for a bone defect site
Prior art
Bone defect sites in the form of recesses or hollowed-out areas in the body's
own
bone tissue are often filled with bone augmentation material in bone surgery,
for ex-
ample in the reconstruction of bone in orthopaedic, neurosurgical, or plastic
surgery,
or in maxillofacial surgery operations. In general, the bone augmentation
material
consists of a mixture of synthetic bone replacement material (for example
hydroxyl
apatite granulate) and natural bone particles. In order for osseous growth
through
the bone augmentation material to take place essentially exclusively from the
bone
side, the recess is closed off with a covering membrane, as described in the
patent
document DE 43 02 708 C2. The covering membrane is attached using attachment
nails to the body's own healthy bone adjacent to the bone defect site, wherein
the
attachment process requires a maximum degree of technical skill of the
surgeon,
because the covering membrane consists of a flexible material.
In order to overcome this disadvantage of a lack of support function of the
covering
membrane, a covering membrane is described in the patent document US 4 816
339, which consists of multiple layers, wherein these layers do not consist of
ab-
sorbable membrane material. Here, it might be required that after the bone
defect
has healed, a second operation is necessary to remove material that is foreign
to the
body.
In the patent document DE 10 2005 039 382 54, a biodegradable hollow body that
particularly has the shape of a hollow cylinder or conical cylinder is
proposed. The
hollow body has a plurality of openings in its walls, through which take-in of
blood
and therefore the growth of the body's own bone is possible. It is
disadvantageous,
in this case, that in order to insert the hollow body, a cylindrical bore must
be intro-
duced into the existing bone, by means of a drill.
Date Recue/Date Received 2020-11-20
2
In DE 10 2006 047 054 Al, an implant socket is proposed, which is
characterised by
high fit accuracy and stability, so that the treating physician can handle and
implant it
in simple manner. The implant socket, which is made from hydroxyl apatite, and
has
a thin membrane, particularly one consisting of absorbable material, for
protection of
the mucous membrane from mechanical effects and for protection of the implant
socket from tissue growing into it from the mucous membrane, on the side
facing the
mucous membrane, is produced using an augmenting method of manufacture, so
that the material composition forms a "gradient structure" in the sense of a
decreas-
ing density, particularly toward the inside. Here, a method of construction
with a par-
ticularly porous structure is provided on the side facing the bone, and a
compact
method of construction is provided on the outside of the implant socket, on
which a
structure for holding a tooth implant and/or a dental prosthesis is situated.
Furthermore, devices for a bone defect site are described in the laid-open
(unexamined)
patent applications DE 198 30 992 Al, DE 10 2005 060 761 Al, DE 42 26 465 Al,
WO
01/91818 Al, DE 10 2005 041 412 Al, DE 10 2006 047 054 Al, US 2011/0151400 Al,
WO 00/59409 Al, WO 96/12446 Al, EP 2 737 871 A2 and WO 2006/051401 A2 and in
patent document US 7 172 422 BI, wherein all of these solutions have the
disadvantage
that they affect the healthy bone present next to the bone defect site.
In order to avoid this disadvantage, a covering device with an accurate fit is
proposed in
the laid-open (unexamined) patent application DE 10 2011 011 191 Al, which
covering
device, however, has the disadvantage that the positioning of said device at
the bone
defect site is hindered by the accuracy of fit itself.
The invention and its advantages
The invention proceeds from a device for covering and/or reconstructing a bone
defect site, a device for covering, reconstructing, or covering and
reconstructing a
bone defect site, with a cap, which has an edge and a wall directed away from
the
bone defect and a wall directed towards the bone defect, wherein the cap
consists of
a dimensionally stable material and the wall directed towards the bone defect
of the
Date Recue/Date Received 2021-02-18
3
cap directed towards the bone defect or the wall directed away from the bone
defect
of the cap directed away from the bone defect corresponds to the shape of the
regenerated bone, characterized in that at least one opening-free positioning
means
is arranged at the edge of the cap, which positioning means has a wall which
is
directed towards a healthy bone adjacent to the bone defect, or in that the
device
has at least one fixing means for fixing the cap to a bone within the bone
defect site,
and at least one positioning means, which has a wall which is directed towards
a
healthy bone adjacent to the bone defect, is arranged at the edge of the cap,
and a
method for producing a cap of a covering device for a bone defect site, a
method for
producing a cap of a covering device for a bone defect site, consisting of the
following method steps: recording a first data record, which represents the
affected
bone defect site in its actual state, comparing the first data record with a
second data
record, which represents the target state of a bone regenerated at the bone
defect
site, wherein the second data record is produced by calculation or was
recorded at a
moment in time at which the bone at the position now to be regenerated was
still a
healthy bone, using the first data record and the second data record to plan
the cap,
which has an edge and a wall directed away from the bone defect and a wall
directed towards the bone defect, whereby the cap can be arranged exclusively
in
the region of the bone defect site, and which can be fixed to the bone with at
least
one fixing means, as appropriate, implementing the planning of the cap in the
form of
a planning data record, and providing the planning data record to a
manufacturing
method, in particular to a computer-controlled manufacturing method, in which
the
cap is formed from a dimensionally stable material and the wall thereof
directed
towards the bone defect or the wall thereof directed away from the bone defect
corresponds to the shape of the regenerated bone in the target state, wherein,
during the manufacture of the cap, at least one positioning means is arranged
at the
edge of the cap, which positioning means is used to position the cap on a
healthy
bone adjacent to the bone defect site, wherein a positioning means has a wall
directed away from the healthy bone and a wall directed towards the healthy
bone
and corresponding at least in part thereto.
Date Recue/Date Received 2021-02-18
4
The device according to the invention for covering and/or reconstructing a
bone de-
fect site, wherein the term "bone defect site" refers to a site of a bone (for
example
hip, spine, head, jaw, or the like) of a human or animal (said bone being
diseased,
deformed, injured, modified by ageing processes, modified by degeneration (for
ex-
ample following tooth extraction, tumour, etc.) and/or modified in volume),
that devi-
ates from the shape and/or volume of a healthy bone, having the features of a
device
for a bone defect site, the device comprising: a cap comprising a rim and a
first side
configured to face away from the bone defect and a second side configured to
face
toward the bone defect, wherein the cap comprises a dimensionally stable
material,
and the second side of the cap or the first side of the cap is configured to
correspond
to the shape of a regenerated bone, wherein the device has at least one
positioning
portion having a continuous wall configured to face a healthy bone that
borders on
the bone defect, the at least one positioning portion being disposed at the
rim of the
cap, or the device has at least one connector for fixation of the cap within
the region
of the bone defect, and the device has at least one positioning portion having
a wall
configured to face a healthy bone that borders on the bone defect, the at
least one
positioning portion being disposed at the rim of the cap, and the method
according to
the invention for producing a cap of a covering device for a bone defect site,
having
the features of a method for production of a cap of a covering device for a
bone de-
fect site, comprising the following method steps: recording of a first data
set that
represents the affected bone defect site in the actual state, comparison of
the first
data set with a second data set, which represents the reference state of a
bone re-
generated at the bone defect site, wherein the second data set is produced via
cal-
culation or was recorded at a time when the bone at the site now to be
regenerated
was still a healthy bone, use of the first data set and of the second data set
for plan-
ning of the cap, which has a rim and a first side configured to face away from
the
bone defect and a second side configured to face the bone defect, thereby
making it
possible for the cap to be disposed exclusively in the region of the bone
defect site,
and to be fixed in place on a bone, conversion of the planning of the cap to a
plan-
ning data set, and provision of the planning data set to a manufacturing
method, in
which the cap is formed from a dimensionally stable material, and the first
side or the
second side corresponds to the shape of the regenerated bone in the reference
state, wherein during manufacturing of the cap, at least one positioning
portion is
Date Recue/Date Received 2020-11-20
5
disposed at the rim of the cap, and serves for positioning of the cap on a
healthy
bone that borders on the bone defect site, wherein the at least one
positioning por-
tion has a positioning device first wall that is configured to face away from
the
healthy bone and a positioning device second wall that is configured to face
the
healthy bone and to correspond to the healthy bone, at least in part, by
contrast have
the advantage that the device for covering and/or reconstructing a bone defect
site
consists of a cap (for example moulded shell, rigid shell, shaped body) having
an
edge, which cap can be formed in one or more layers, and, as appropriate, of
at
least one fixing means, which can be arranged within the bone defect site, for
fixing
the cap on a bone, wherein, at the edge of the cap, which is characterised by
having
a dimensionally stable (rigid) nature and being in contact with the bone at
least in
part (at the edge) in the boundary region between the bone defect site and the
adja-
cent healthy bone, there is arranged at least one positioning means, wherein a
wall
(in the sense of a surface or side) of the cap directed towards the bone
defect or a
wall (in the sense of a surface or side) of the cap directed away from the
bone defect
corresponds to the shape of the bone regenerated within the bone defect site,
said
bone regaining the shape of a healthy bone on account of its regeneration. Due
to
the at least one positioning means, it is possible to position the cap at a
healthy bone
adjacent to the bone defect site, since the positioning means has a wall (in
the sense
of a surface or side) directed away from the healthy bone and a wall (in the
sense of
a surface or side) directed towards the healthy bone and corresponding at
least in
part thereto. The cap is arranged and/or fixed exclusively in the region of
the bone
defect site covered fully or at least in part by the cap, so that the cap does
not affect
the healthy bone adjacent to the bone defect site, where no regeneration of
the bone
takes place anyway on account of the healthy nature of said healthy bone.
Instead of
the merely partial covering of the bone defect site by the cap, the cap is
thus
adapted to the bone defect site, preferably with an accurate fit, and
preferably ends
flush with the healthy bone. The bone defect site is hereby fully covered by
the cap,
which does not protrude beyond the bone defect site.
According to an advantageous embodiment of the device according to the
invention,
the cap and/or at least one fixing means and/or at least one positioning means
con-
sist at least in part of a biocompatible material.
Date Recue/Date Received 2020-11-20
6
According to an additional advantageous embodiment of the device according to
the
invention, the material is of organic and/or inorganic origin. The material
can also be
an autogenic, syngenic, allogenic, xenogenic, synthetic, or alloplastic
material.
According to an additional advantageous embodiment of the device according to
the
invention, the cap and/or at least one fixing means and/or at least one
positioning
means consist at least in part of a biodegradable material.
According to an additional advantageous embodiment of the device according to
the
invention, the cap and/or at least one fixing means and/or at least one
positioning
means consist at least in part of an absorbable material. Advantageously, the
ab-
sorption time of the rigid shell can be controlled by means of its absorption
gradients
and/or the absorption time can also amount to less than six months, so that
the im-
plant can be placed in a timely manner. Absorbable metals or alloys, in
particular
magnesium or magnesium alloys, are preferably used. The 3D models (for example
the cap and/or the fixing means) are preferably constructed by means of a
laser
melting method under vacuum, wherein a 3D printer is preferably used.
According to an additional advantageous embodiment of the device according to
the
invention, the cap and/or at least one fixing means and/or at least one
positioning
means consist at least in part of a polymer or a polymer compound.
According to an additional advantageous embodiment of the device according to
the
invention, the cap and/or at least one fixing means and/or at least one
positioning
means consist at least in part of polylactide. Polylactides are composed of
many lac-
tic acid molecules chemically bound to one another, and belong to the
polymers. The
advantage of polylactide plastics, which are also referred to as polylactic
acids
(PLAs), is that they are plastics that are deformable by supplying heat, and
are bio-
com patible.
Date Recue/Date Received 2020-11-20
7
According to an additional advantageous embodiment of the device according to
the
invention, the cap and/or at least one positioning means has a constant or
varying
wall thickness.
According to an embodiment of the device according to the invention that is
advan-
tageous in this regard, the wall thickness should amount to at least 0.2 mm,
prefera-
bly 0.5 mm, but at least to such an amount that the moulded shell or a
positioning
means is dimensionally stable.
According to an additional advantageous embodiment of the device according to
the
invention, the fixing means is a pin, a screw, a nail and/or a bone adhesive.
In order
to protect the healthy bone, the fixing means is/are preferably arranged in
the region
of the bone defect site.
According to an additional advantageous embodiment of the device according to
the
invention, the cap has at least one milled region (bore for the fixing means).
According to an embodiment of the device according to the invention that is
advan-
tageous in this regard, the milled region corresponds to the fixing means.
According to an additional advantageous embodiment of the device according to
the
invention, the wall directed towards the bone defect has a surface
conditioning.
According to an embodiment of the device according to the invention that is
advan-
tageous in this regard, the surface can have a microstructuring, pores,
osteoblast
attraction substances, means for promoting bone growth, and/or bone
replacement
material that contains bone morphogenetic protein (BMP).
According to an additional advantageous embodiment of the device according to
the
invention, the cap has at least one opening. This means that the cap does not
have
to have a closed wall. The cap can have a mesh structure, at least at
individual
points, on account of a multiplicity of openings, wherein the wall of the mesh
struc-
Date Recue/Date Received 2020-11-20
8
ture directed away from the bone defect or the wall of the mesh structure
directed
towards the bone defect corresponds to the shape of the regenerated bone.
According to an additional advantageous embodiment of the device according to
the
invention, the cap has at least one predetermined breaking point. The
predetermined
breaking point brings the advantage that, provided the cap is to be removed
follow-
ing successful bone regeneration, this removal can be performed in a minimally
in-
vasive manner, without "having to open everything up", since the cap can be
divided
into at least two parts on account of the predetermined breaking point. The
cap can
therefore be removed (for example following bone regeneration) very easily.
Fur-
thermore, the predetermined breaking point can be used to separate parts of
the cap
not required from the rest of the cap.
In accordance with an additional advantageous embodiment of the device
according
to the invention, the cap has at least one fastening device (for example a
recess) for
at least one implant to be fitted.
According to an embodiment of the device according to the invention that is
advan-
tageous in this regard, at least one fastening device (for example a recess)
is cov-
ered at least in part by a part of the cap which is connected by means of at
least one
predetermined breaking point to the rest of the cap.
According to an embodiment of the device according to the invention that is
advan-
tageous in this regard, at least one predetermined breaking point is arranged
be-
tween the cap and a positioning means. A positioning means resting on the
healthy
bone and thus possibly causing discomfort can thus be removed from the part of
the
cap possibly remaining, for example once the cap has been fixed or following
the
regeneration of the bone at the bone defect site.
According to an advantageous embodiment of the method according to the
invention
for producing a cap of a covering device for a bone defect site, in which the
comput-
er-assisted design (CAD) of the cap is combined with computer-assisted
manufactur-
ing (CAM), as CAD/CAM, so that a design model of the cap developed on the com-
Date Recue/Date Received 2020-11-20
9
puter is transmitted directly electronically to the manufacture, consisting of
the follow-
ing method steps:
- recording a data record, which represents the affected bone defect site
in its
three-dimensionality, by means of tomography or similar imaging methods
- using the data record for planning of the cap, which has a wall directed
away
from the bone defect and a wall directed towards the bone defect, and can be
fixed in place on a bone with at least one fixing means
- implementing the planning of the cap in a planning data record, and
- providing the planning data record to a computer-controlled manufacturing
method,
wherein the cap is formed from a dimensionally stable material and its wall
(in the
sense of a surface or side) directed towards the bone defect or its wall (in
the sense
of a surface or side) directed away from the bone defect corresponds to the
shape of
the regenerated bone, and the data record representing the affected bone
defect site
in its three-dimensionality is recorded by means of tomography, computed
tomogra-
phy, digital volume tomography, sonography, or the like, wherein at least one
posi-
tioning means is arranged at the edge of the cap during and/or after the
manufacture
of the cap, which positioning means is used to position the cap at a healthy
bone
adjacent to the bone defect site and has a wall (in the sense of a surface or
side)
directed away from the healthy bone and a wall (in the sense of a surface or
side)
directed towards the healthy bone and corresponding at least in part thereto,
the re-
cording of the first data record represents the affected bone defect site in
its three-
dimensionality and/or the recording of the second data record represents the
shape
of the still healthy bone in its three-dimensionality.
According to an additional advantageous embodiment of the method according to
the invention for producing a cap of a covering device for a bone defect site,
the first
data record, which represents the actual state, and/or the second data record,
which
represents the target state, are/is recorded by at least one imaging method.
According to an additional advantageous embodiment of the method according to
the invention for producing a cap of a covering device for a bone defect site,
the first
data record and/or the second data record are/is recorded by means of at least
one
Date Recue/Date Received 2020-11-20
10
method which enables a three-dimensional representation of a bone. In
particular,
the first data record and/or the second data record are/is recorded by means
of to-
mography, computer tomography, digital volume tomography, sonography, or the
like.
According to an additional advantageous embodiment of the method according to
the invention for producing a cap of a covering device for a bone defect site,
the data
record of the healthy bone is recorded once the healthy bone is mature. it is
thus
possible that the ideal state (target state) of the bone might be documented,
so that it
is known how a bone potentially requiring regeneration later should look. In
humans,
the data record of the healthy bone should preferably be recorded between the
ages
of 18 and 25 years. Of course, it is also conceivable for a plurality of
healthy bones
or the entire skeleton of a human or animal to be recorded, documented and/or
stored once a mature state of the bones has been reached. It is also
conceivable to
manufacture a cap at least in part already at the time at which the healthy
bone is
recorded.
According to an embodiment of the method according to the invention for
producing
a cap of a covering device for a bone defect site that is advantageous in this
regard,
the data record of the healthy bone is stored (preserved) for its later use,
on a stor-
age medium.
According to an additional advantageous embodiment of the method according to
the invention for producing a cap of a covering device for a bone defect site,
the cap
is formed by means of milling, in the manufacturing process.
According to an additional advantageous embodiment of the method according to
the invention for producing a cap of a covering device for a bone defect site,
at least
one fastening device for at least one implant to be fitted is arranged on the
cap dur-
ing the manufacture of the cap. The fastening device, for example, can be
formed as
a recess.
Date Recue/Date Received 2020-11-20
11
According to an embodiment of the method according to the invention for
producing
a cap of a covering device for a bone defect site that is advantageous in this
regard,
at least one fastening device (for example recess) is exposed by removing part
of
the cap connected by means of at least one predetermined breaking point to the
rest
of the cap prior to the removal. The moment in time at which the fastening
device is
exposed can be before or after the moment in time at which the covering device
is
arranged at the bone defect site.
According to an additional advantageous embodiment of the method according to
the invention for producing a cap of a covering device for a bone defect site,
at least
one predetermined breaking point is arranged on the cap during the manufacture
of
the cap.
According to an additional advantageous embodiment of the method according to
the invention for producing a cap of a covering device for a bone defect site,
at least
one predetermined breaking point is arranged between the cap and a positioning
means.
According to an additional advantageous embodiment of the method according to
the invention for producing a cap of a covering device for a bone defect site,
a clean-
ing and/or sterilisation process is performed during the manufacture of the
cap.
According to an additional advantageous embodiment of the method according to
the invention for producing a cap of a covering device for a bone defect site,
the cap
can be used in a device for covering and/or reconstructing a bone defect site
accord-
ing to the presently claimed invention. In this way, a device for covering
and/or re-
constructing a bone defect site can be created, the cap and/or fixing means of
which,
for example made of an artificial material and/or of a material of autogenic,
syngenic,
allogenic or xenogenic origin from human and/or animal bone or the human,
animal
or a synthetic matrix, has a shape by means of which the region situated
between
the bone and the desired shape of the regenerated bone is completely or almost
completely filled. To this end, a bone block is removed from the donor, for
example,
and subsequently modelled by means of CAD/CAM, as appropriate.
Date Recue/Date Received 2020-11-20
12
By means of the method according to the invention, a device according to the
inven-
tion for covering and/or reconstructing a bone defect site can be created, the
cap
and/or fixing means of which for example originates from a material of organic
and/or
inorganic origin. This can also be a synthetic material and/or a material of
autogenic,
syngenic, allogenic or xenogeneic, alloplastic, human and/or animal origin.
Here, the
human, animal or synthetic matrix can also have a shape by means of which the
re-
gion situated between the bone and the desired shape of the regenerated bone
is
completely or almost completely filled. For this purpose, a bone block is
removed
from the donor (same patient or third-party donor), for example, and
subsequently
modelled by means of CAD/CAM, as appropriate.
Further features and advantageous embodiments of the invention can be derived
from the following description and drawings.
Drawing
Exemplary embodiments of the subject matter of the invention are shown in the
drawing
and will be explained in greater detail in the following. The figures show:
Fig. 1 a representation of a device according to the invention for covering
and/or
reconstructing a bone defect site,
Fig. 2 a representation of a differently shaped device according to the
invention
for covering and/or reconstructing a bone defect site,
Fig. 3 a representation of a differently shaped device according to the
invention
for covering and/or reconstructing a bone defect point,
Fig. 4 a representation of a differently shaped device according to the
invention
for covering and/or reconstructing a bone defect site,
Fig. 5 a detail of a cap,
Date Recue/Date Received 2020-11-20
13
Fig. 6 to 8 various representations of a cap with positioning means, and
Fig. 9 a cap arranged at a bone defect site.
Description of the exemplary embodiments
Fig. 1 shows a representation of a device 1 according to the invention, for
covering
and/or reconstructing a bone defect site 2 (bone defect) of a bone,
particularly of a
jaw bone 3. The device 1 consists of a cap 4, which is formed in a single
layer, and
of a fixing means 5, which is shown as a pin in Fig. 1 and which is arranged
in the
bone defect site 2 so as not to injure the healthy bone adjacent to the bone
defect
site 2. Of course, it is also conceivable for a plurality of fixing means 5 to
be used to
fix the cap 4, wherein these fixing means would likewise be arranged in the
bone
defect site 2. The cap 4 is made of a dimensionally stable material, so that
it sup-
ports itself and no additional support is required. For fixing of the cap 4
(moulded
shell, rigid shell), the fixing means 5 is pushed through a bore 6, into the
cap 4, and
subsequently introduced into the bore 7 arranged in the jaw bone 3. The cap 4
is
subsequently fixed preferably by means of ultrasonic welding. During
ultrasonic
welding, an ultrasound generator preferably generates a precisely defined
frequency
that is bundled by way of a sonotrode. Once the absorbable fixing means 5
(pin) has
been fitted onto a bore hole (bore 7) pre-drilled in the bone, a vibration
that is pro-
duced ensures liquefaction of the pin surface at its edges, thereby bringing
about the
result that the pin slides into the bore hole. By means of a change in the
state of ag-
gregation, the pin also penetrates into the osseous cavities that cannot be
reached
by a conventional bone screw, so that great initial strength is achieved.
Furthermore,
the pin head connects to the cap 4 and ensures a stable three-dimensional
construct
with an interlocking mechanism. During ultrasonic welding, the fixing means 5
is
therefore softened, so that it connects to the jaw bone 3 and the cap 4. By
means of
the cap 4, which has been fixed in place, a sealed interior space 8 is formed
be-
tween the jaw bone 3 and the cap 4, which space is filled by means of the
regenera-
tion of the bone and/or by means of the introduction of a material of organic
and/or
Date Recue/Date Received 2020-11-20
14
inorganic origin, which material can also be an autogenic, syngenic,
allogenic, xeno-
genic, synthetic and/or alloplastic material, so that the regenerated bone or
the intro-
duced material corresponds to the shape of the wall 9 (in the sense of a
surface or
side) of the single-layer cap 4 directed towards the bone defect site 2. In
order to
accelerate the regeneration process of the jaw bone 3, the wall 9 of the cap 4
di-
rected towards the bone defect can have a surface conditioning (for example a
mi-
crostructuring, pores, osteoblast attraction substances, means for promoting
bone
growth, and/or bone replacement material that contains BMP).
Fig. 2 shows a representation of a differently shaped device 1 according to
the in-
vention for covering and/or reconstructing a bone defect site 2 (bone defect)
of a
bone, particularly of a jaw bone 3. In this figure, the gum 10 is additionally
shown.
Fig. 3 shows a representation of a differently shaped device 1 according to
the in-
vention for covering and/or reconstructing a bone defect site 2 (bone defect)
of a
bone, in particular a jaw bone 3. In this figure, the cap 4 is formed as a
moulded
body, for example made of human or animal bone, and has a wall 9 (in the sense
of
a surface or side) directed towards the bone defect, which wall is adapted to
the re-
lief of the bone defect site 2, and a wall 11 (in the sense of a surface or
side) di-
rected away from the bone defect, which wall corresponds to the shape of the
re-
generated bone.
Fig. 4 shows a representation of a differently shaped device 1 according to
the in-
vention for covering and/or reconstructing a bone defect site 2 (bone defect)
of a
bone, in particular a jaw bone 3. In this figure, the cap 4 is formed as a
moulded
body, for example made of human or animal bone, and has a wall 9 directed
towards
the bone defect and a wall 11 directed away from the bone defect, which corre-
sponds to the shape of the regenerated bone. An interior space 8 is situated
be-
tween the wall 9 and the bone defect site 2, which space is filled by means of
the
regeneration of the bone and/or by means of the introduction of autogenic,
syngenic,
allogenic, xenogeneic, synthetic and/or alloplastic material.
Date Recue/Date Received 2020-11-20
15
Fig. 5 shows a detail of a cap 4, the wall 9 of which directed towards the
bone defect
has openings 12, thus resulting in a mesh-like structure.
Fig. 6 to 8 show various representations of a cap 4, which has a wall 9
directed to-
wards a bone defect site and a wall 11 directed away from the bone defect
site, with
positioning means 13 arranged at the edge 16 of the cap 4, which positioning
means
have a wall 14 directed towards a healthy bone and a wall 15 directed away
from the
healthy bone. When the cap 4 is arranged correctly at the bone defect site,
this being
assisted by the positioning means 13, the walls 14 directed towards a healthy
bone
contact the healthy bone, whereby the positioning means 13 ensure that the cap
4
sits in a perfect position, even without arrangement of at least one fixing
means (not
shown in Fig. 6 to 8) or at least until at least one fixing means is arranged
in the
bone defect site, as appropriate. So as to be able to easily remove the cap 4
follow-
ing the bone regeneration, said cap has predetermined breaking points 17,
whereby
it can be divided into two parts for its removal once these predetermined
breaking
points have been severed.
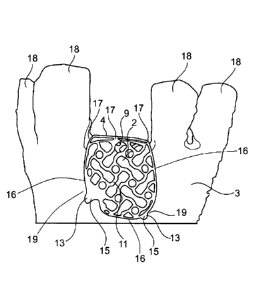
Fig. 9 shows a cap 4 arranged at a bone defect site 2 of a jaw bone 3 (shown
in
part), which has teeth 18. It can thus be seen that the cap 4 is arranged
preferably
only in the region of the bone defect site 2 of the jaw bone 3, so that it
neither spans
nor contacts a healthy bone 19. Thus, only the positioning means 13 arranged
on the
cap 4 are in contact with the healthy bone 19.
In Fig. 6 to 9 a cap 4 is shown, the wall 9 of which directed towards the bone
defect
corresponds to the shape of the regenerated bone. It is also conceivable for
the posi-
tioning means 13 to be arranged on the cap 4 in such a way that the wall 11
thereof
directed away from the bone defect corresponds to the shape of the regenerated
bone. This could also be achieved for example by arranging the positioning
means
13 on the wall 11 of the cap 4 directed away from the bone defect.
All of the features presented here can be essential to the invention
individually and in
any combination with one another.
Date Recue/Date Received 2020-11-20
16
List of reference signs
1 device
2 bone defect site
3 jaw bone
4 cap
fixing means
6 bore
7 bore
8 interior space
9 wall
gum
11 wall
12 opening
13 positioning means
14 wall
wall
16 edge
17 predetermined breaking point
18 tooth
19 healthy bone
Date Recue/Date Received 2020-11-20