Note: Descriptions are shown in the official language in which they were submitted.
CA 02986086 2017-11-15
CYCLYC PEPTIDE, AND MEDICINE, EXTERNAL PREPARATION AND
COSMETIC EACH CONTAINING SAID CYCLIC PEPTIDE
[Technical Field]
[0001]
The present invention relates to a cyclic peptide and a medicament, external
preparation and
cosmetic comprising said cyclic peptide.
[Background Arts]
[0002]
BNP (brain natriuretic peptide) is a hormone which is synthesized and secreted
in heart
(predominantly in ventricles). BNP was isolated from and identified in pig
brain in 1988, and it has
been known since that BNP is secreted from ventricular myocardium of human,
etc. An increase in
cardiac stress or development of myocardial hypertrophy induces BNP secretion
and increased in
blood concentration. In general, BNP has activities such as diuretic activity,
vasodilation,
renin-aldosterone secretion suppressing action, sympatholytic activity,
hypertrophy suppressing
action. BNP is considered to a hormone which acts to protect the myocardium
against damages
caused by cardiac stress.
[0003]
Amino acids constituting BNP differ slightly from species to species who
produce BNP.
Nevertheless it been revealed that its structure possesses a cyclic part and a
tail part as a common
structure (Non-Patent Literatures 1-2). For instance, wild-type human BNP has
been known to
consist 32 amino acid residues, and its fragments and derivatives are further
proposed (Patent
Literatures 1-6).
[0004]
In Japan, BNP is currently not used as a therapeutic, but widely used in
clinical practice as a
biochemical marker for cardiac failure. In United States, however, it is used
as a drug for
alleviating symptoms of cardiac failure (trade name: Natrecorg). Recently, it
has been proposed to
utilize an external preparation comprising BNP for treating dermatitis,
rhinitis, alopecia, etc., and
also as a skin-improving agent (Patent Literature7-9).
[Prior Art References]
[Patent Literature]
[0005]
[Patent Literature1] JP A 2007-525213
[Patent Literature2] JP A 2008-509746
=
[Patent Literature3] WO 2008/032450
1
CA 02986086 2017-11-15
[Patent Literature4] US Patent No.6028055
[Patent Literature5J US Patent No.5114923
[Patent Literature6] US Patent No.6818619
[Patent Literaturel WO 2011/010732
[Patent Literature8] WO 2011/024973
[Patent Literature9] WO 2012/099258
[Non-Patent Literature]
[0006]
[Non-Patent Literature1] N. Akizukia, K. Kangawaa, N. Minaminob, H. Matsou,
FEBS Letters 1991
280 (2):357-362.
[Non-Patent Literature2] Takei Y, Fukuzawa A, Itahara Y, Watanabe TX,
Yoshizawa Kumagaye K,
Nakajima K, Yasuda A, Smith MP, Duff DW, Olson KR. FEBS Lett. 1997 Sep 8;414
(2):377-80.
[Summary of the Invention]
[Problems to be Solved by the Invention]
[0007]
As described above, BNP is considered to be a useful substance for the therapy
of various diseases.
However, in order to obtain a better therapeutic effect there is a need for
developing a substance with
a higher drug efficacy and stronger effect and a faster- and longer-acting
substance.
[00081
An improvement in the duration and fast-acting properties of the drug efficacy
and effect is quite
meaningful in light of reducing required number/frequency of treatment,
relieving the sufferings by
the patient/user, and improving the quality of life (Q0L) and the mental,
physical, social or
intellectual satisfaction in dairy living of the user. For example, dermatitis
is often accompanied by
symptoms such as an itch, infiltration or hot flush in the affected site; the
sufferings by the patient
will be reduced if these symptoms can quickly be alleviated. When an alopecia
therapeutic, hair
growing agent or hair growth stimulant is employed, the QOL of the user can be
improved if the
therapeutic effect, hair growth-stimulating or hair growing effect can be
achieved immediately.
[0009]
The inventors focused on these points and tried to search for a novel
substance by reference to the
structure of BNP.
Accordingly, the object of the present invention is to provide a novel peptide
having a strong drug
efficacy and effect, and a medicament or an external preparation comprising
such peptide,
specifically a prophylactic or therapeutic for dermatitis, rhinitis and
alopecia, and a hair growth
stimulant, a hair growing agent, an antipruritic, a cosmetic and a skin-care
product, etc.
2
CA 02986086 2017-11-15
[Means to solve the problems]
[0010]
It was previously considered that the tail part of the wild-type BNP peptide
structure (which is
composed of a cyclic part and tail part) plays at least certain important role
in the binding and
selectivity of BNP to its receptor. However, the inventors focused on the
cyclic part of said structure,
deleted the tail part, and made an intensive research on the resulting cyclic
peptide. Accordingly
we have obtained totally new findings including the effects of such cyclic
peptide, its high drug
efficacy, faster-acting and longer-lasting effect, and further continued the
study and finally
completed the invention.
Accordingly, the present invention relates to the followings:
[0011]
[1] A cyclic peptide having an amino acid sequence expressed by Formula I:
Cys-Phe-X1-X2-X3-X4-Asp-Arg-X5-Xs-X7-X8-X9-X1 -X"-X12-Cys (i)
wherein,
X1 denotes Gly, Val, Ala, Ser or Thr,
X2 denotes Arg, Gln or His,
X3 denotes Lys or Arg,
X4 denotes Met, Leu or Ile,
X5 denotes Ile or Val,
X6 denotes Ser or Gly,
X7 denotes Ser or Ala,
X8 denotes Ser, Gln, Val, Ala, Thr, Leu, Ile or Met,
X0 denotes Ser, Val, Ala or Thr,
X10 denotes Gly or Arg,
X11 denotes Leu, Met, Ile, Val or Ala,
X12 denotes Gly, Ser or Ala, and
the line connecting two Cys denotes a disulfide bond,
and wherein the amino acid sequence does not have a peptide bond that is not
between the amino
acids constituting the amino acid sequence,
or a derivative thereof or a pharmaceutically acceptable salt thereof.
[0012]
[2] The cyclic peptide according to [1], wherein
X2 denotes Arg,
3
CA 02986086 2017-11-15
X4 denotes Met,
X6 denotes Ser,
X7 denotes Ser, and
X10 denotes Gly,
or a derivative thereof or a pharmaceutically acceptable salt thereof.
[3] The cyclic peptide according to [1], wherein the amino acid sequence is
selected from the
amino acid sequences expressed by Formula (Pa) -Formula (I-e):
Cys-Phe-Gly-Arg-Lys-}{et-Asp-Arg-Ile-Ser-Ser-Ser-Ser-Gly-Leu-Gly-Cys (I-a)
Cys-Phe-Gly-Arg-Arg-Leu-Asp-Arg-Ile-Gly-Ser-Leu-Ser-Gly-Leu-Gly-CYs (l-b)
Cys-Phe-Gly-Gln-Lys-Ile-Asp-Arg-Ile-Gly-Ala-Val-Ser-Arg-Leu-Gly-Cys (I-c)
Cys-Phe-Gly-Arg-Arg-Leu-Asp-Arg-Ile-Gly-Ser-Leu-Ser-Gly-Leu-Gly-Cys (I-d)
Cys-Phe-Gly-His-Lys-Ile-Asp-Arg-Ile-Gly-Ser-Val-Ser-Arg-Leu-Gly-CYs (I-e)
wherein, the line connecting two Cys denotes a disulfide bond,
or a derivative thereof or a pharmaceutically acceptable salt thereof.
[0013]
[4] The cyclic peptide according to any one of [1]-[3] or a derivative
thereof or a
pharmaceutically acceptable salt thereof, wherein the derivative is
substituted by a substituent
which is capable of replacing a hydrogen atom, hydroxyl group, carboxy group,
amino group or imino
group in the cyclic peptide.
[5] The cyclic peptide or a derivative thereof or a pharmaceutically
acceptable salt thereof,
which is formed by deleting 1 to 4 amino acids in the cyclic peptide expressed
by any one of the
Formulae (Pa) - (Pe) according to [3], or by replacing them with or adding
them other amino acids,
4
CA 02986086 2017-11-15
and which has an equal function with the cyclic peptide expressed by each of
said formulae.
[6] An external preparation comprising one or more cyclic peptides
according to any one of
[1]-[5] and/or a derivative thereof and/or a pharmaceutically acceptable salt
thereof.
[7] The external preparation according to [6], wherein the external
preparation is an ingredient
for a dermatitis therapeutic, dermatitis prophylactic, antipruritic,
antiphlogistic, epidermis
regeneration accelerating agent, wound epithelialization-accelerating agent or
skin-care product.
[0014]
[8] The external preparation according to [7], wherein the skin-care
product is for moisturizing,
and/or for preventing or improving rough skin, and/or for sebum/acne care,
and/or for irritation
alleviation/anti-inflammation, and/or for skin-lightening, and/or for anti-
aging, and/or for
preventing/alleviating ultraviolet lesion, and/or for slimming, and/or for
skin-cleansing.
[9] The external preparation according to [6], wherein the external
preparation is a bath agent,
a body-cleansing agent or a hair-cleansing agent.
[10] The external preparation according to [6], wherein the external
preparation is an alopecia
therapeutic, an alopecia prophylactic, a hair growing agent and/or a hair
growth stimulant.
[11] The external preparation according to [6], wherein the external
preparation is a rhinitis
therapeutic and/or a rhinitis prophylactic.
[0015]
[12] The external preparation according to [6], wherein the external
preparation is a cosmetic.
[13] The external preparation according to any one of [61112], wherein the
formulation is a solid,
semi-solid, powder, liquid, spray, ointment, cream, emulsion, gel or patch
formulation.
[14] The external preparation according to any one of [611131, wherein the
external preparation
is used as a pharmaceutical product, a quasi-drug or a cosmetic product.
[15] A use of the cyclic peptide according to any one of [1]-[4] and/or a
derivative thereof and/or a
pharmaceutically acceptable salt thereof for preparing the external
preparation.
[0016]
[16] A method of using the external preparation comprising applying the
external preparation
according to any one of [611141 to the skin and/or mucosa of a subject.
[17] The method of using the external preparation according to [16],
wherein the mucosa is labial,
CA 02986086 2017-11-15
oral, nasal, ocular or vaginal mucosa.
[18] The method of using the external preparation according to [16],
wherein the method is a
method of treating and/or preventing dermatitis, a method of alleviating or
resolving itch, a method
of treating eczematous or other erosion or ulcer, or a method for skin-care.
[0017]
[19] The method of using the external preparation according to [16],
wherein the method is a
method of treating and/or preventing alopecia, and/ or a method of stimulating
hair growth, and] or a
method of growing hair.
[20] The method of using the external preparation according to [16],
wherein the method is a
method of treating and/or preventing rhinitis.
[21] A medicament comprising one or more cyclic peptides according to any
one of [1]-[5] or a
derivative thereof or a pharmaceutically acceptable salt thereof.
[22] The medicament according to [21], wherein the medicament is a
therapeutic for
hypertension, unstable angina, acute myocardial infarction, edematous
diseases, renal failure,
cardiac failure, immune diseases, obesity or metabolic syndrome.
The cyclic peptide described herein (hereinafter also referred to as "BNP
cyclic peptide", "B ring" or
"B ring-compound") is, as described above, derived from wild-type BNP. The
wild-type BNP
encompasses BNP from human, as well as BNPs from monkeys, pigs, birds and
rats. Therefore, the
B ring-compound also encompasses the B ring-compound from human, as well as
those from
monkeys, pigs, birds and rats.
[Effect by the Invention]
[0018]
According to the present invention, novel cyclic peptides and compositions
comprising the same can
be provided. Such compositions are applied to an external preparation for
preventing or treating
dermatitis, rhinitis or alopecia, and further applied to a hair growth
stimulant, a hair growing agent
or an antipruritic, each of which are provided as a medicament, a quasi-drug,
a skin-care product, or
a cosmetic product. When an "external preparation" is referred alone in the
present invention, it
means an agent that is applied to skin or mucosa, whose utility is not limited
to a medicament,
quasi-drug, skin-care product and cosmetic product.
[0019]
In general, the external preparation of the present invention has a
significantly higher efficacy
against dermatitis as compared to a conventional steroid external preparation,
as well as an
6
CA 02986086 2017-11-15
excellent immediate effect such that the symptoms start to be improved within
three minutes in
general. Its effect is great and long-lasting, and the amelioration period is
long.
Furthermore, the external preparation of the present invention can, upon being
applied onto the
subject's skin or mucosa suffering a dermatitis, quickly relieve or eliminate
perceptible symptoms
that are or can be caused by dermatitis such as pruritus, soreness (pain), hot
sensation, tautness,
infiltration and erythema, improving the symptoms of the dermatitis.
[0020]
Furthermore, the external preparation of the present invention exerts a
moisturizing effect on the
applied site, while exerting an effect to improve skin texture where the
corneum layer is present at
the applied site. It can thus exerts effects to improve skin texture, improves
dry or rough skin,
softens and moisturizes skin, reduce and diminish wrinkles, and improve
roughened lip.
[0021]
The external preparation of the present invention also has an effect of
stimulating hair growth or
promoting hair growth and at the same time preventing hair loss at the applied
site when being
applied to a decalvant site or a site where hair growth is stimulated. In this
case, stimulated hairs
tend to become terminal hairs and not white hairs. These effects are exhibited
relatively quick as
compared to other active agents that have previously been used in the
therapeutics for alopecia, e.g.,
BNP, and the obtained effects are more significant.
[0022]
Yet the external preparation of the present invention has an immediate, large
and long-lasting effect
effect on rhinitis with a long amelioration period.
[0023]
Besides, since the cyclic peptide of the invention is a peptide sharing a part
of its structure with BNP
which is a hormone inherent in body, it causes little concern about side
effects. It is also considered
to have a small influence on homodynamics as long as used in an appropriate
amount or used
externally onto skin, etc. It therefore can be administered for prolonged
period to a patient in need
thereof, e.g., a patient with chronic dermatitis. Moreover, the external
preparation of the invention
causes no irritation when being taken externally and can be applied safely to
a patient with sensitive
skin, as well as to a child or woman, on face or neck, etc.
[0024]
The reason why the cyclic peptide of the invention has a superior drug
efficacy and effect as
compared with wild-type BNP is not yet clear. However, according to our in
silico analysis, it is
suggested that the cyclic peptide of the invention is more easily and strongly
bound to NPR-A
receptor (GC-A) as compared with wild-type BNP. It is thus predicted that the
cyclic peptide would
7
CA 02986086 2017-11-15
have an excellent drug efficacy and effect, an excellent immediate effect in
particular. As described
above, B ring-compound is derived not only from human, but there also are B
rings from animals of
other species such as monkeys, pigs, birds and rat, and it has been confirmed
that they have similar
structures. From the results of the structural analysis described hereinbelow,
it is strongly
speculated that all these B rings exert similar effects as those of the B ring-
compound from human.
[Brief Description of the Drawings]
[0025]
[Figure 1]
A diagram showing the result of applying BNP cyclic peptide (A) or a gel
formulation (B) on either
left or right side of the face of a subject having large wrinkles on the face.
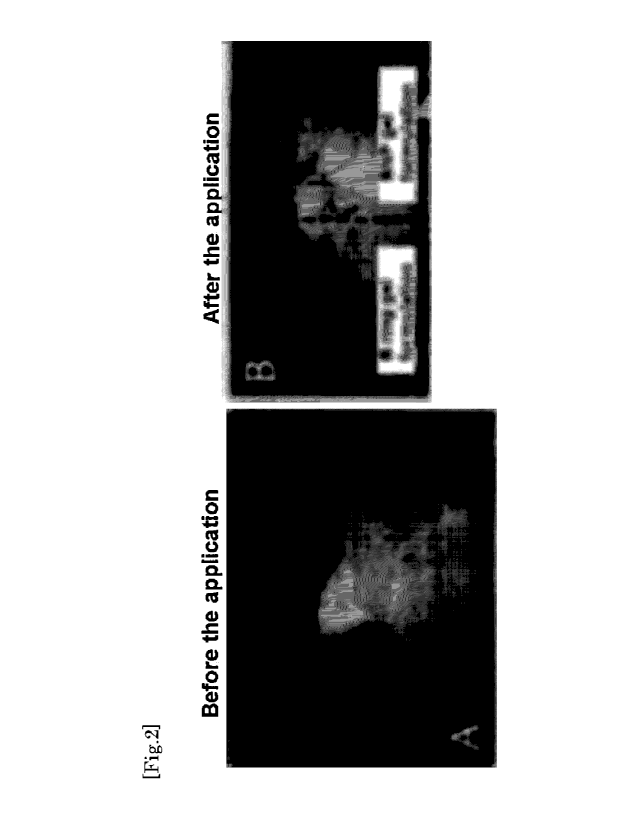
[Figure 2]
A diagram showing the effect before and after the application of either B ring
gel formulation or BNP
gel formulation to a patient with female pattern alopecia and alopecia
pityroides.
[Mode for carrying out the Invention]
[0026]
Hereinafter, the present invention is explained in detail based on its
suitable embodiments.
[0027]
1. The cyclic peptide, a derivative thereof and a pharmaceutically
acceptable salt thereof
Firstly, the cyclic peptide of the invention, a derivative thereof and a
pharmaceutically acceptable
salt thereof are explained.
The cyclic peptide of the present invention has an amino acid sequence
expressed by Formula I:
Cys-Phe-X' -)(2-)(3 -X4-Asp-A rg-X5 -Xs -)(7 -X8 -X' -X'' -X11 -X12 _Cys
(SEQ ID NO: 1)
[0028]
wherein,
Xi denotes Gly, Val, Ala, Ser or Thr,
X2 denotes Arg, Gin or His,
X3 denotes Lys or Arg,
X4 denotes Met, Leu or Ile,
X5 denotes Ile or Val,
X6 denotes Ser or Gly,
8
CA 02986086 2017-11-15
V denotes Ser or Ala,
X8 denotes Ser, Gin, Val, Ala, Thr, Leu, Ile or Met,
V denotes Ser, Val, Ala or Thr,
X10 denotes Gly or Arg,
X11 denotes Leu, Met, Ile, Val or Ala,
X12 denotes Gly, Ser or Ala,
the line connecting two Cys denotes a disulfide bond,
and wherein the amino acid sequence does not have a peptide bond that is not
between the amino
acids constituting the amino acid sequence.
Such cyclic peptide is, similar to previously known BNPs, considered to bind
to a receptor NPR-A
(also known as GC-A) having a guanylate cyclase domain and promote the
production of cyclic
guanosine monophosphate (cGMP), and has activities such as, for example,
diuretic action,
vasodilation, renin-aldosterone secretion suppressing action, sympatholytic
activity and hypertrophy
suppressing action. It has a superior drug efficacy and effect, particularly
an excellent immediate
effect, as compared to BNP.
[0029]
In addition, as described hereinbelow, the above cyclic peptide can be used as
an ingredient of an
external preparation for a dermatitis therapeutic/prophylactic, a rhinitis
therapeutic/prophylactic,
an alopecia therapeutic/prophylactic, a hair growing agent, a hair growth
stimulant, an antipruritic,
etc., or as an ingredient of a skin-care product, a quasi-drug or a cosmetic
product. The above cyclic
peptide can also be used as an alternative for BNP in an medicament utilizing
the activity of BNP as
above, e.g., in an medicament for hypertension, unstable angina, acute
myocardial infarction,
edematous diseases, renal failure, cardiac failure, immune diseases, obesity
or metabolic syndrome.
[0030]
In another embodiment of the present invention, in the cyclic peptide of the
invention, X1-X12 in the
Formula (I) may be defined as one or more selected from the group consisting
of following (1)-(12):
(1) X1 denotes Gly, Val, Ala, Ser or Thr,
(2) X2 denotes Arg, Gin or His,
(3) V denotes Lys or Arg,
(4) V denotes Met, Leu or Ile,
(5) V denotes Ile or Val,
(6) V denotes Ser or Gly,
(7) V denotes Ser or Ala,
(8) X8 denotes Ser, Gin, Val, Ala, Thr, Leu, Ile or Met,
(9) V denotes Ser, Val, Ala or Thr,
(10) X10 denotes Gly or Arg,
9
CA 02986086 2017-11-15
(11) X11 denotes Leu, Met, Ile, Val or Ala, and
(12) X12 denotes Gly, Ser or Ala.
A preferred cyclic peptide of the present invention is a cyclic peptide of
Formula (I), wherein X2
denotes Arg, X4 denotes Met, X6 denotes Ser, X7 denotes Ser, and/or X10
denotes Gly (SEQ ID NO: 2).
[00311
Furthermore, in another embodiment of the invention, in the cyclic peptide of
the invention, the
amino acid sequence expressed by Formula (I) may be selected from SEQ ID NOs:
3-8 and SEQ ID
NOs: 16-75.
[0032]
Furthermore, in the cyclic peptide of the invention, the amino acid sequence
expressed by Formula
(I) is preferably selected from the amino acid sequences expressed by the
Formula (I-a) -Formula
Cys-Phe-G1y-Arg-Lys-Iket-Asp-Arg-1 I e-Ser-Ser-Ser-Ser-Gly-Leu-Gly-Cys ( I-
a)
(SEQ ID NO: 3)
Cys-Phe-Gly-Arg-Arg-Leu-Asp-Arg-1 1 e-Gl y-Ser-Leu-Ser-Gly-Leu-Gly-CYs ( I -
b )
(SEQ ID NO: 4)
Cys-Phe-G ly-G I n-Lys-I 1 e-Asp-Arg-1 1 e-G ly-A 1 a-Val -Ser-Arg-Leu-G 1 y-
CYs I-c)
(SEQ ID NO: 5)
Cys-Phe-G1 y-Arg-Arg-Leu-Asp-Arg-I 1 e-G1y-Ser-Leu-Ser-G1 y-Leu-G ly-Cys (I-
d)
(SEQ ID NO: 6)
CA 02986086 2017-11-15
Cys-Phe-Gly-His-Lys-Ile-Asp-Arg-Ile-Gly-Ser-Val-Ser-Arg-Leu-Gly-Cys (1-0
(SEQ ID NO: 7)
wherein, the line connecting two Cys denotes a disulfide bond.
[00331
Each of the amino acid sequences expressed by these Formulae (Fa) - (Fe) is
the cyclic part of human
BNP (Formula (I-a)), swine BNP (Formula (I-b)), rat BNP (Formula (I-c)),
rabbit BNP (Formula (I-d))
and murine BNP (Formula (I-e)), respectively. Therefore, the cyclic peptide
having such an amino
acid sequence will exhibit the aforementioned effects with more certainty.
Because swine BNP
cyclic peptide is consistent with those of avian (SEQ ID NO: 8,
Cys-Phe-Gly-Arg-Arg-Ile-Asp-Arg-Ile-Gly-Ser-Leu-Ser-Gly-Met-Gly-Cys, wherein,
1st Cys and 17th
Cys form a disulfide bond), bovine (SEQ ID NO: 9,
Cys-Phe-Gly-Arg-Arg-Leu-Asp-Arg-Ile-Gly-Ser-Leu-Ser-Gly-Leu-Gly-Cys, wherein,
1st Cys and 17th
Cys form a disulfide bond), feline (SEQ ID NO: 10,
Cys-Phe-Gly-Arg-Arg-Leu-Asp-Arg-Ile-Gly-Ser-Leu-Ser-Gly-Leu-Gly-Cys, wherein,
1st Cys and 17th
Cys form a disulfide bond), canine (SEQ ID NO: 11,
Cys-Phe-Gly-Arg-Arg-Leu-Asp-Arg-Ile-Gly-Ser-Leu-Ser-Gly-Leu-Gly-Cys, wherein,
1st Cys and 17th
Cys form a disulfide bond), and ovine (SEQ ID NO: 12,
Cys-Phe-Gly-Arg-Arg-Leu-Asp-Arg-Ile-Gly-Ser-Leu-Ser-Gly-Leu-Gly-Cys, wherein,
1st Cys and 17th
Cys form a disulfide bond), BNP cyclic peptides derived from these organisms
also have similar
effects as the cyclic peptide of the present invention. Among those mentioned
above, it is
particularly preferred that the amino acid sequence expressed by Formula (I)
is the amino acid
sequence expressed by Formula (I-a). In another embodiment of the invention,
the cyclic peptide
may be expressed by SEQ ID NOs: 16-75.
[0034]
The cyclic peptide of the present invention encompasses a derivatized form of
said cyclic peptide.
Such a derivative can be used as such, as an active substance, or also used as
a prodrug.
[00351
A derivative of the present invention can be obtained by adding (modifying) a
known substituent to
certain group of an amino acid in the cyclic peptide such as, for example, a
hydrogen atom, hydroxyl
group, carboxy group, amino group or imino group, or replacing it with a known
replaceable
substituent. Modification include, but not limited to, chemical modifications
such as, for example,
glycosylation, acetylation, phosphorylation and lipidation, to an amino acid
within the cyclic peptide.
11
CA 02986086 2017-11-15
[0036]
The addition (modification) or replacement of an amino acid within the cyclic
peptide can occur in
one group, or can occur in more than one group at the same time. Any known
substituents can be
used as long as being capable of replacing above groups and it goes without
saying that such
substituents naturally include, for example, protecting groups such as BOC.
[0037]
The cyclic peptide of the invention further includes a mutated form of said
cyclic peptide. Namely, a
mutant of the present invention can be obtained by deleting an amino acid in
the cyclic peptide,
replacing it with or adding it another amino acid. The number of amino acid to
be deleted, replaced
with other amino acids or added is 4 or less, more preferably 3 or less, even
more preferably 2 or less,
and particularly preferable 1 or less. It also goes without saying that the
deletion, replacement or
addition of amino acids in the cyclic peptide may occur concurrently and
independently to each other.
[0038]
An amino acid that is capable of being replaced with another amino acid can
be, in the case of human
BNP (Formula (I-a)), exemplified as follows, without being limited thereto.
The third amino acid
from left, Gly, may be replaced with either Val, Ala, Ser or Thr. The forth
amino acid from left, Arg,
may be replaced with either Gln or His. The fifth amino acid from left, Lys,
may be replaced with
Arg. The sixth amino acid from left, amino acid, Met, may be replaced with
either Leu or Ile. The
ninth amino acid from left, Ile, may be replaced with Val. The twelfth amino
acid from left, Ser,
may be replaced with either Gln, Val, Ala, Thr, Leu, Ile or Met. The
thirteenth amino acid from left,
Ser, may be replaced with either Val, Ala or Thr. The fourteenth amino acid
from left, Gly, may be
replaced with Arg. The fifteenth amino acid from left, Leu, may be replaced
with either Met, Ile,
Val or Ala. The sixteenth amino acid from left, Gly, may be replaced with
either Ser or Ala. Table
32 summarizes examples of replaceable amino acids in the cyclic peptide of the
invention, though the
invention will not be limited thereto.
[0039]
Cyclic peptides in which one amino acid has been replaced include such as, for
example, SEQ ID
NOs: 16-44, though the invention will not be limited thereto. Cyclic peptides
in which two amino
acids have been replaced include such as, for example, SEQ ID NO: 45-58,
though the invention will
not be limited thereto. Yet cyclic peptides in which three amino acids have
been replaced include
such as, for example, SEQ ID NOs: 59-70, though the invention will not be
limited thereto.
Furthermore, cyclic peptides in which four amino acids have been replaced
include such as, for
example, SEQ ID NOs: 71-75, though the invention will not be limited thereto.
It also goes without
saying that five or more amino acids can be replaced by appropriately
combining the aforementioned
amino acid.
12
CA 02986086 2017-11-15
[0040]
Deletion of one to several amino acids can take place in similar manner to the
replacement of the
aforementioned amino acids with other amino acids.
[0041]
The number of amino acid which are to be deleted or replaced with other amino
acids or added may
be determined such that the cyclic peptide will have 80% homology, preferably
90% homology to the
cyclic peptide of the invention.
[0042]
In addition, the present invention may be a mutant as described above, and a
derivative thereof. As
long as it retains the effect of the invention, any mutant or a derivative
thereof is encompassed in the
cyclic peptide according to the invention. In another embodiment, it has at
least a BNP activity.
For purpose of improving the activity of the cyclic peptide of the invention,
prolonging the effect of
the invention, and/or increasing storage stability of the cyclic peptide of
the invention, the cyclic
peptide of the invention or the amino acids constituting said peptide may be
altered in an
appropriate manner. For example, an amino acid in the cyclic peptide of the
invention may be
chemically modified, some amino acids constituting the cyclic peptide may be
deleted or replaced
with other amino acids, and/or new amino acids may be added.
[0043]
For example, the C-terminal group -COOH of one Cys of the cyclic peptide may
be replaced with
-COOR1, -CONHR1 or -CONR12, and/or the N-terminal group NH2 of the other Cys
of the cyclic
peptide may be replaced with -NHC(0)R1 or -N(C(0)F0)2. Here, each appearance
of R1 is
independently a branched or straight hydrocarbon group or an alkylene glycol
chain or sugar chain
having 1 to 20 carbon atoms. The number of carbon atoms in 10- is preferably 1
to 10, more
preferably 1 to 5, yet more preferably 1 to 2.
[0044]
Pharmaceutically acceptable salts include, without being particularly limited,
as the cyclic peptide of
the invention or a derivative, such as for example, a salt with an inorganic
base, a salt formed with
an organic base, a salt formed with an inorganic acid, a salt formed with an
organic acid, a salt
formed with a basic or acidic amino acid. Examples of suitable salt formed
with inorganic base
include such as, for example, an alkaline metal salt such as a sodium salt and
potassium salt; an
alkaline earth meal salt such as a calcium salt and magnesium salt; and an
aluminum salt and
ammonium salt. Examples of suitable salt formed with organic base include such
as, for example, a
salt with an alkyl amine such as trimethyl amine or triethyl amine; a salt
formed with a heterocyclic
amine such as pyridine and picoline; a salt formed with an alkanol amine such
as ethanol amine,
diethanol amine and triethanol amine; a salt formed with a cycloalkyl amine
such as cyclohexyl
13
CA 02986086 2017-11-15
amine and dicyclohexyl amine; a salt formed with an alkylene diamine
derivative such as
N,N'-dibenzylethylenediamine. Examples of suitable salt formed with inorganic
acid include such
as, for example, a salt formed with hydrochloric acid, hydrobromic acid,
nitric acid, sulfuric acid or
phosphoric acid. Examples of suitable salt formed with organic acid include
such as, for example, a
salt formed with a monocarboxylic acid such as formic acid, acetic acid,
trifluoroacetic acid and
propionic acid; a salt formed with a polyvalent carboxylic acid such as
fumaric acid, oxalic acid and
maleic acid; a salt formed with a oxycarboxylic acid such as tartaric acid,
citric acid, succinic acid and
malic acid; a salt formed with a sulfonic acid such as methane sulfonic acid,
benzezne sulfonic acid
and p-toluene sulfonic acid; and a salt formed with benzoic acid. Examples of
suitable salt formed
with a neutral amino acid include such as, for example, a salt formed with
glycine, valine or leucine;
examples of suitable salt formed with a basic amino acid include such as, for
example, a salt formed
with arginine, lysine or ornithine; and examples of suitable salt formed with
an acidic amino acid
include such as, for example, a salt formed with aspartic acid acid or
glutamic acid.
[0045]
Among those mentioned above, a cyclic peptide composed of the amino acid
sequence expressed by
Formula (I) or a pharmaceutically acceptable salt is preferred. Namely, it is
preferred that the
amino acid sequence expressed by Formula (I) is not replaced.
[0046]
A method for producing the cyclic peptide of the present invention, a
derivative and
pharmaceutically acceptable salt thereof may employ, without being
particularly limited, for
example, any known chemical synthetic or genetic engineering methods.
[0041
When the amino acid sequence as above is chemically synthesized, it may be
synthesized by any
chemical synthetic method, or any known method for peptide synthesis, for
example, or solid-phase
or liquid-phase synthetic method. Moreover, any commercial synthesizer (e.g.,
SHIMADZU
Corporation: PSSM-8) may be used for synthesis.
[0048]
A disulfide bond can be formed in the amino acid sequence, for example,by DMSO
oxidation method
or iodine oxidation method without being particularly limited. In this case,
an intramolecular
disulfide bond can be formed by treating a free sulfhydryl group or a
sulfhydryl group protected by a
protecting group with either DMSO or iodine (I2) to result in said cyclic
peptide.
[00491
A protecting group includes such as, for example, 4-methylbenzyl group (Bzl
(4Me)), trityl group (Trt),
tert-butyl group, N-(acetyl) aminomethyl group (Acm). Deprotection can be
carried out through
14
CA 02986086 2017-11-15
appropriate treatment corresponding to these protecting group, for example,
for 4-methylbenzyl
group by treating with a strong acid, and for N-(acetyl) aminomethyl group by
treating with iodine.
[0050]
Next, if necessary, the cyclic peptide is derivatized to result in a
derivative. Derivatization can be
carried out by known method. Alternatively, a derivative of the cyclic peptide
can be produced at
the time of peptide synthesis by introducing in advance a substituent into the
amino acid
constituting the cyclic peptide.
[00511
Then, if necessary, a pharmaceutically acceptable salt is produced by ion
exchange of the cyclic
peptide or derivative thereof. Ion exchange can be carried out, for example,
by bringing into the
contact the cyclic peptide or derivative thereof with a desired acid or base.
[00521
On the other hand, when the peptide is synthesized by genetic engineering, it
can be synthesized by
any known method such as, for example, methods described in Sambrook J. et
al., Molecular Cloning,
A Laboratory Manual (4th edition) (Cold Spring Harbor Laboratory Press
(2012)). For instance, a
DNA fragment coding for the amino acid sequence expressed by Formula (I) is
prepared at first.
Preparation of the DNA fragment can be performed, for example, if it is a case
in which the DNA
fragment codes for the amino acid sequence expressed by Formula (I-a), by
amplifying the DNA
fragment by PCR using a vector comprising a full-length human BNP gene as
template and primers
designed to synthesize a defined DNA region. Alternatively, a DNA fragment can
be chemically
synthesized.
[0053]
Then, the amplified DNA fragment is ligated into an appropriate vector to
obtain a recombinant
vector for protein expression. Next, the vector for protein expression is
allowed to be taken up by
target cells and the transformed cells are selected. Finally, the protein
produced by the cells (i.e.,
the protein composed of the amino acid sequence expressed by Formula (I)) is
collected.
Formation of disulfide bond, derivatization and salt formation in the
collected protein can be carried
out as described above.
[00541
The identification of the compound of interest such as a cyclic peptide can be
confirmed by known
procedures such as, for example, reverse-phase HPLC or mass spectroscopy.
The presence or absence of BNP activity in the obtained compound can readily
be confirmed by
known means. For instance, it can be confirmed by examining cGMP producing
activity in NPR-A
CA 02986086 2017-11-15
receptor-expressing cells.
[0055]
2. External preparation
Secondly, the external preparation of the present invention is explained.
The external preparation of the present invention comprises one or more cyclic
peptide and/or a
derivative thereof and/or a pharmaceutically acceptable salt thereof as
described above. If two or
more cyclic peptides are used, the number of different cyclic peptide to be
mixed is not limited,
though 2 to 3 are preferred in terms of preparation cost and convenience. It
includes SEQ ID NO:
19, 29, 33, 36, 39 and 43, for example, but the invention is not limited to
these and these cyclic
peptide can appropriately be combined as long as it does not interfere the
effect of the invention.
[0056]
2.1 Application
The external preparation of the present invention can be used for following
uses, for example,
without being particularly limited, and in each case exerts remarkable effect
which had not
previously been achieved. The external preparation of the present invention is
not limited to any
particular use, and can be used as a pharmaceutical product, a quasi-drug
and/or a cosmetic product
depending on its drug efficacy and effect.
[0057]
Hereinbelow, the invention will be described in detail for each of its use.
(1) Therapeutic/prophylactic for dermatitis
The external preparation of the present invention can be a a therapeutic
and/or prophylactic for
dermatitis.
[0058]
The external preparation of the present invention can, upon being applied onto
the subject's skin or
mucosa suffering dermatitis, quickly relieve or eliminate various perceptible
symptoms and
conditions that are or can be caused by dermatitis such as pruritus, soreness
(pain), hot sensation,
tautness, erythema, infiltration, papule, lichenification, crust, exudation or
skin desiccation,
improving the symptoms of dermatitis. In addition, the external preparation of
the present
invention does not cause any irritation at the site of application.
[0059]
Furthermore, such action of the external preparation comprising the cyclic
peptide of the invention is
effected more quickly and for prolonged time period as compared to that of an
external preparation
comprising BNP having the tail part. Although reasons for these advantageous
effects of the cyclic
16
CA 02986086 2017-11-15
peptide over such BNP are not clear, the cyclic peptide of the invention is
considered to have an
advantageous structure for binding to its receptor (NPR-A receptor) over BNP,
thereby enabling
faster binding to the NPR-A receptor in the vicinity of the affected site and
at the same time
prolonging its binding time. Moreover, it is considered that its relatively
quick binding to the
NPR-A receptor near the affected site can prevents the cyclic peptide from
diffusing out of the
affected site via bloodstream, etc., enabling it to stay around the affected
site for relatively long time.
[0060]
The external preparation of the present invention doese not only suppress or
prevent inflammation
in dermatitis but also acts to restore and/or retain the barrier function of
skin. Here, skin's barrier
function works for protecting skin against entry of stimuli and saprophytes
from external
environment or for retaining moisture. The external preparation restores the
barrier function, and
thus able to prevent the progress or exacerbation of inflammation. The barrier
function of skin is
greatly affected by the alignment state of corneocytes in the comeum, i.e.,
state of skin texture and
moisturization. The external preparation of the present invention acts to
improve skin texture and
to moisturize skin. Therefore, its effects of restoring and maintaining skin
barrier function are also
obvious.
[0061]
The external preparation of the present invention is also effective on skin
symptoms commonly
called acne, i.e., such as comedones, red papule, pustula, cysts/tuberosity.
[0062]
Moreover, a quick relief or elimination of perceptible symptoms or conditions
will reduce the burden
of the subject (patient) and improve the QOL of the patient, while preventing
the patient from being
bothered by itch or soreness and from acting to damage the affected site, for
example, touching or
scratching. This effect of the external preparation of the present invention
to relieve or eliminate
perceptible symptoms or conditions is, in general, exhibited within 10
minutes, preferably within 5
minutes, more preferably within 3 minutes after application.
[0063]
In addition, the external preparation of the present invention can treat
dermatitis in which steroid
dermatosis has been developed, or dermatitis that is intractable by other
common drugs for
dermatitis therapy such as steroid and tacrolimus, for example dermatitis for
which a sufficient
therapeutic effect cannot be achieved by these formulations, or dermatitis
which is resistant to these
formulations, or dermatitis for which the use of these formulations is not
suitable or desirable.
Conventional widely-used steroid external preparations have significant
problems upon terminating
application that the severity returns to the pre-application level and that a
rebound phenomenon
may occur and exacerbate the condition. The external preparation of the
present invention has no
17
CA 02986086 2017-11-15
such problems as a rebound phenomenon.
[0064]
Dermatitis is in general a disease which causes inflammation in skin or
mucosa. Dermatitis is
normally accompanied with one or more symptoms selected from acute eczema
symptoms such as
erythema, infiltrative erythema, papule, vesicle, pustula, infiltration,
incrustation and
desquamation; chronic eczema symptoms such as lichenification and
pigmentation; and scales, crust
(scab) attachment, scratching, scratch scar, prurigo nodularis, erosion,
edema, oozing and
squamatization.
[0065]
Dermatitis herein is not particularly limited as long as it is a disease which
is accompanied with
inflammation in skin or mucosa and includes such as, for example, eczema and
atopic dermatitis
such as chronic eczema, dyshidrotic eczema, infantile xerotic eczema; contact
dermatitis such as
allergic contact dermatitis and primary irritant contact dermatitis;
seborrheic dermatitis; asteatotic
dermatitis; autosensitization dermatitis; stasis dermatitis; urticaria such as
allergic urticaria (e.g.,
alimentary urticaria and drug-induced urticaria) and nonallergic urticaria
(e.g., physical urticaria,
solar urticaria and cholinergic urticaria); insect bite; drug eruption;
psoriasis such as plaque
psoriasis, guttate psoriasis, erythrodermic psoriasis, pustular psoriasis and
psoriasis arthropathica;
prurigo such as chronic prurigo, acute prurigo, gestational prurigo and
nodular prurigo; rosacea;
rosacea-like dermatitis; cutaneous vasculitis such as cutaneous allergic
vasculitis; cutaneous
pruritus such as systemic cutaneous pruritus, localized cutaneous pruritus,
senile cutaneous
pruritus and gestational pruritus; solar dermatitis; erythrosis; nummular
dermatitis; localized
scratching dermatitis; perioral dermatitis; pompholyx; keratosis pilaris;
lichen planus, dyshidrotic
eczema, dyshidrosis, miliaria and acne vulgarisacne vulgaris. The external
preparation of the
present invention can be applied to the therapy and prophylaxis of any of
these diseases.
[0066]
The external preparation of the present invention exerts an excellent effect
especially on eczema,
atopic dermatitis, contact dermatitis, seborrheic dermatitis, insect bite,
allergic or nonallergic
urticaria and psoriasis, preferably on eczema, atopic dermatitis, contact
dermatitis and insect bite,
more preferably on eczema and atopic dermatitis. Therefore, the external
preparation of the
present invention can be used for the purpose of the therapy and prophylaxis
of at least one or more
dermatitis selected from above-mentioned group.
[0067]
(2) Ingredients of cosmetic or skin-care product
The external preparation of the present invention may be an ingredient for a
cosmetic or skin-care
product.
18
CA 02986086 2017-11-15
The external preparation of the present invention will, upon being applied to
skin and/or mucosa,
provide moisture to the applied site and exert moisturizing effect, preventing
skin from dryness and
roughness. In cases where corneum is present at the site of application, it
exerts an effect of
improving skin texture there. It also provides and retains moderate elasticity
and flexibility of skin
and mucosa, softens skin, gives skin and mucosa resilience and firmness.
Moreover, it improves
wrinkles (including fine and large wrinkles) and flabbiness at the applied
site, and further
diminishes the appearance of dullness and spots. By diminishing dullness and
spots away, it
consequently provides a skin-lightening effect.
[0068]
As a result, the external preparation of the present invention can be used for
the purpose of
maintaining or improving skin and/or mucosal condition, without being
particularly limited, for
example, for obtaining at least one effects selected from a group consisting
of preventing or
improving dry skin, skin roughness, sensitive skin, roughened lips and
appearance of large and fine
wrinkles, maintaining skin or mucosal condition, anti-aging, and skin-
lightening, or obtaining for at
least one effects selected from the above effects.
Skin roughness herein includes miliaria, chilblain, cracked skin, chapped
skin, acne, diaper rashes,
festering, chafed inner thighs and razor burns.
[0069]
Specific utilities of the external preparation of the present invention as an
ingredient for the
cosmetic or skin-care product includes, without being particularly limited,
for example, cosmetics for
make-up such as face lotion, emulsion, serum, cream, cold cream, gel, mask,
pack, powder, hand soap,
perfume, deodorant, as well as foundation, face powder, eye shadow, eyeliner,
mascara, eyebrow,
blush, makeup base, lip stick, lip cream and nail polish, and furthermore
cosmetics for hair such as
shampoo, rinse, conditioner, hair color, hair tonic, styling agent and perm
chemical, body cleansers
such as face wash, cleansing and body soap, skin-care products such as body
powder, aftershave
lotion and pre-shave lotion, and bath agents.
[0070]
The above effects of the external preparation of the present invention are
quickly exhibited, within
minutes in general, preferably within 5 minutes, more preferably within 3
minutes after
application depending on the type of the effect.
The above effects of the external preparation of the present invention last
for relatively long time.
In general, the effect lasts for 4 hours or more, preferably, 8 hours or more,
more preferably 24 hours
or more after it started to effect depending on the type of the effect.
[0071]
19
CA 02986086 2017-11-15
(3) Antipruritic
The external preparation of the present invention can be an antipruritic.
As mentioned above, the external preparation of the present invention is
suitable for using as
antipruritic because it quickly exerts an excellent antipruritic effect at the
applied site when being
applied to skin or mucosa.
The antipruritic effect of the external preparation of the present invention
as mentioned above is
exhibited within 10 minutes in general, preferably within 5 minutes, more
preferably within 3
minutes after application.
[0072]
(4) Alopecia therapeutic, alopecia prophylactic, hair growing agent and
hair growth stimulant
The external preparation of the present invention can also be an alopecia
therapeutic, alopecia
prophylactic, hair growing agent and/or hair growth stimulant.
When the external preparation of the present invention is applied to a site of
hair loss or a site where
hair growth is to be stimulated, it acts to prevent hair loss and promote hair
growth stimulation and
hair growing at the applied site. It has effects such as nourishing hair,
promoting hair growth, and
improving or preventing hair thinning at the applied site. In this case, hairs
to be stimulated to
grow tend to become terminal hairs or hairs which are not white hairs. These
effects are exhibited
in relatively early stage as compared to other active ingredients previously
used in alopecia
therapeutics e.g., BNP, and the effects obtained are significant.
[0073]
The external preparation of the present invention also has an improving or
preventing effect on
dermatitis as mentioned above. Therefore, it can improve or prevent skin
inflammation associated
with alopecia. Such effects are particularly advantageous when alopecia has
been exacerbated due
to skin condition of the site of application (such as scalp).
[0074]
Moreover, the external preparation of the present invention exerts
moisturizing and skin
texture-improving effects at the applied site when being applied to skin as
mentioned above. It can
remove and suppress dandruff and itching, while giving moisture to hair and
scalp, improving and
preventing dryness and keeping hair and scalp healthy. It also can improve
seborrhea.
[0075]
The external preparation of the present invention also acts to alleviate or
eliminate the perceptible
symptoms or conditions as mentioned above, thereby reducing the burden of the
subject (patient) and
improving QOL of the patient. Moreover, it can prevent the patient from being
bothered by itch or
CA 02986086 2017-11-15
soreness and acting to damage the affected site, for example, touching or
scratching, thereby
preventing exacerbation of skin condition which may induce alopecia.
[0076]
When the external preparation of the present invention is used as alopecia
therapeutic or
prophylactic, applicable alopecia includes such as, for example, alopecia as
listed below, without
being particularly limited. The external preparation can be used for the
purpose of treating or
preventing one ore more of these alopecia.
[0077]
(Acquired alopecia)
(i) Alopecia without accompanying scarring or skin lesion (alopecia areata,
male pattern
alopecia, seborrheic alopecia, alopecia pityroides, female pattern alopecia,
gestational alopecia,
malignant alopecia, senile alopecia, alopecia totalis, alopecia areata
multilocularis, ophiasis,
drug-induced alopecia, cancer chemotherapy-induced alopecia and radiation
exposure-induced
alopecia, traumatic/mechanical alopecia, malnutrition/metabolic disorder
associated alopecia,
endocrine dysfunction associated alopecia and telogen effluvium (post partum
alopecia, alopecia
after high fever)).
[0078]
Alopecia observed on skin lesion or pathologic skin (infection-induced
alopecia,
tumor-induced alopecia, inflammation-induced alopecia)
(iii) Scarring alopecia (skin infection-induced alopecia, alopecia induced
by infiltration of
inflammatory cells)
[0079]
(Congenital alopecia)
Diffuse alopecia, congenital atrichia, alopecia in hereditary syndromes,
localized alopecia,
phakomatosis, aplasia cutis, congenital alopecia triangularis.
[0080]
Among those mentioned above, the external preparation of the present invention
exerts an excellent
effect particularly on acquired alopecia, preferably on alopecia without
accompanying scarring or
skin lesion, more preferably on alopecia areata, male pattern alopecia,
seborrheic alopecia, alopecia
pityroides, female pattern alopecia, gestational alopecia, malignant alopecia,
senile alopecia,
alopecia totalis, alopecia areata multilocularis, ophiasis, drug-induced
alopecia, cancer
chemotherapy-induced alopecia and radiation exposure-induced alopecia,
traumatic/mechanical
alopecia, malnutrition/metabolic disorder associated alopecia, endocrine
dysfunction associated
alopecia and telogen effluvium. Therefore, the external preparation of the
present invention can be
21
CA 02986086 2017-11-15
used for the purpose of treating or preventing at least one or more alopecia
selected from the
above-mentioned group.
[0081]
(5) Rhinitis therapeutic and/or prophylactic
The external preparation of the present invention can also be a rhinitis
therapeutic and/or
prophylactic.
Rhinitis is a disease caused by mucosal inflammation in nasal cavity and/or
paranasal cavity and
induces main symptoms such as nasal obstruction, rhinorrhea, sudden recurrent
sneezing, as well as
symptoms such as pruritus. In the present invention, "rhinitis" does not only
includes rhinitis in a
narrow definition which are accompanied with mucosal inflammation in nasal
cavity, but also
includes sinusitis accompanied with mucosal inflammation in paranasal cavity.
[0082]
The external preparation of the present invention can improve or prevent
various symptoms
associated with rhinitis such as nasal obstruction, rhinorrhea, sneezing and
pruritus, when being
applied to nasal cavity mucosa and/or paranasal cavity mucosa. Especially
these effects are
exhibited quickly and last for a prolonged time as compared to a rhinitis
therapeutic comprising BNP
having the tail part.
[0083]
Such effects of the external preparation of the present invention on rhinitis
are exhibited within 8
minutes in general, preferably within 5 minutes, more preferably within 3
minutes after its
application.
Also the effect of the external preparation of the present invention on
rhinitis as described above
lasts for 4 hours or more in general, preferably for 8 hours or more, more
preferably for 24 hours or
more after it started to effect.
[0084]
When the external preparation of the present invention is used as a rhinitis
therapeutic and/or
prophylactic, applicable rhinitis includes such as, without being particularly
limited, for example,
infectious rhinitis including acute rhinitis and chronic rhinitis;
hypersensitive non-infectious rhinitis
including combined (flower hypersensitivity) rhinitis, rhinitis with
rhinorrhea, congestive rhinitis,
edematous rhinitis and dry-nose type rhinitis; irritant-induced rhinitis
including physical rhinitis,
chemical rhinitis and radiation rhinitis; and atrophic rhinitis and idiopathic
granulomatous rhinitis;
and sinusitis such as acute sinusitis, chronic sinusitis (maxillary empyema),
eosinophilic sinusitis
and paranasal cavity mycosis, The external preparation can be used for the
purpose of treating or
preventing one ore more of the above.
22
CA 02986086 2017-11-15
[0085]
Combined rhinitis includes, for example, allergic rhinitis including perennial
allergic rhinitis and
seasonal allergic rhinitis, and nonallergic rhinitis including vasomotor
(essential) rhinitis and
eosinophilic rhinitis.
Rhinitis with rhinorrhea includes, for example, gustatory rhinitis, cold air-
induced rhinitis and
senile rhinitis.
[0086]
Congestive rhinitis includes, for example, drug-induced rhinitis, psychogenic
rhinitis, gestational
rhinitis, endocrine rhinitis and cold rhinitis.
Edematous rhinitis includes, for example, aspirin-hypersensitive rhinitis.
[00871
Among those mentioned above, the external preparation of the present invention
exerts an excellent
effect particularly on hypersensitive non-infectious rhinitis, irritant-
induced rhinitis and sinusitis,
preferably on hypersensitive non-infectious rhinitis and chronic sinusitis,
more preferably on
combined (flower hypersensitivity) rhinitis, rhinitis with rhinorrhea,
congestive rhinitis, edematous
rhinitis and dry-nose type rhinitis, chronic sinusitis. Therefore, the
external preparation of the
present invention can be used for the purpose of treating or preventing at
least one or more rhinitis
selected from the above-mentioned group.
[00881
In terms of symptoms, since the external preparation of the present invention
acts as described
above, it can be used for any of rhinitis with sneezing/rhinorrhea, rhinitis
with nasal obstruction and
rhinitis with both symptoms.
In addition, the external preparation of the present invention exhibits an
effect of improving rhinitis
which cannot be cured by a rhinitis therapeutic that has conventionally been
used such as steroid
drug.
[00891
(6) Other external preparations
The external preparation of the present invention can also be used for an
application other than
those described above for the effect of the cyclic peptide as described above.
In this case, the
external preparation of the present invention can set an objective that is not
the effects of the cyclic
peptide as described above. In this case, the cyclic peptide of the invention,
a derivative and/or a
pharmaceutically acceptable salt thereof is used for the purpose of assisting
the main effect of the
external preparation or adding another effect to the main effect.
23
CA 02986086 2017-11-15
[00901
Such application include such as, without being particularly limited, for
example, body powder,
deodorant, depilation agent, soap, body shampoo, bath agent, hand soap,
perfume, sunscreen, and
antiinflammatory agent and antifungal agent.
[0091]
2.2 Formulation
The external preparation of the present invention can exert the effect of its
active ingredient, i.e., the
cyclic peptide or a derivative and/or pharmaceutically acceptable salt
thereof, certainly and quickly
in the vicinity of the applied site by being locally applied to the site of
the interest (e.g., affected site)
on skin or mucosa.
Such external preparation can be, without being particularly limited, for
example, an agent for
integument, eye drop, ear drop, nasal drop, buccal agent or suppository. Among
these, when the
external preparation of the present invention is a dermatitis therapeutic, a
dermatitis prophylactic,
an antipruritic, a skin-care product, an alopecia therapeutic, an alopecia
prophylactic, a hair growing
agent or a hair growth stimulant, it is preferably an agent for integument. On
the other hand,
when the external preparation of the present invention is a rhinitis
therapeutic and/or prophylactic,
it is preferably a nasal drop. Furthermore, when it is a
therapeutic/prophylactic of a corneal
disease, it is preferably an eye drop.
[0092]
When the external preparation of the present invention is an agent for
integument, it can be, without
being particularly limited, for example, an external solid formulation, an
external liquid formulation,
a spray formulation, an ointment, an emulsion, a cream, a gel formulation or a
patch.
[0093]
An external solid formulation is a solid formulation for applying or spraying
onto such as skin.
Such an external solid formulation includes, for example, a powder form
external formulation.
An external liquid formulation is a liquid formulation for applying onto such
as skin. Such an
external liquid formulation includes, for example, a lotion and liniment.
[0094]
A spray formulation is a formulation for spraying an active ingredient in a
mist, powder, foam or
paste form onto skin. Such spray formulation includes, for example, an
external aerosol and a
pump spray formulation.
An ointment is a semi-solid formulation which is applied to skin and comprises
an active ingredient
dissolved or dispersed in a base. The ointment can also be a lip cream for
locally applying to lips,
24
CA 02986086 2017-11-15
etc.
A cream is a semi-solid formulation which is applied to skin and emulsified as
either oil-in-water or
water-in-oil type.
[0095]
A gel formulation is a gelled formulation which is applied to skin. The gel
formulation includes, for
example, aqueous gel formulation and oil-based gel formulation.
A patch is a formulation which is attached to skin. The patch includes, for
example, a tape or
plaster.
A nasal drop is a formulation which is administered to nasal cavity or nasal
mucosa. The nasal
drop includes, for example, nasal powder formulation and a nasal drop. Among
these, nasal drop is
preferred.
[0096]
In any formulation described above, the external preparation of the present
invention comprises the
cyclic peptide of the present invention and/or a derivative thereof and/or a
pharmaceutically
acceptable salt thereof as described above.
[0097]
When the formulation is an external liquid formulation, an ointment, a cream
or a gel formulation,
the external preparation of the present invention comprises the cyclic peptide
and/or a derivative
thereof and/or a pharmaceutically acceptable salt thereof at a concentration
of, for example, 0.0001
to 1000000 pg/g, preferably 0.001 to 10000 pg/g, more preferably 0.01 to 1000
pg/g, yet more
preferably 0.1 to 100 pg/g. In another embodiment, it may comprises the cyclic
peptide at a
concentration of 1 to 800 pg/g or 3 to 500 pg/g.
When the formulation is a spray formulation, the external preparation of the
present invention
comprises in the stock solution of the spray formulation the cyclic peptide
and/or a derivative thereof
and/or a pharmaceutically acceptable salt thereof at a concentration of, for
example, 0.0001 to
1000000 pg/mL, preferably 0.001 to 10000 pg/mL, 0.01 to 1000 pg/mL, more
preferably 0.1 to 100
pg/mL, yet more preferably 1 to 100 pg/mL. In another embodiment, it may
comprises the cyclic
peptide at a concentration of 1 to 800 pg/mL or 3 to 500 pg/mL.
[0098]
When the formulation is a patch, the external preparation of the present
invention comprises the
cyclic peptide and/or a derivative thereof and/or a pharmaceutically
acceptable salt thereof at a
concentration of, for example, 0.0001 to 1000000 pg/mL, preferably 0.001 to
10000 pg/mL, more
CA 02986086 2017-11-15
preferably 0.01 to 1000 pg/mL, yet more preferably 0.1 to 100 pg/mL,
particularly preferably 1 to 100
pg/mL. In another embodiment, it may comprises the cyclic peptide at a
concentration of 1 to 800
pg/mL or 3 to 500 pg/mL.
When the formulation is a nasal drop, especially a liquid nasal drop, the
external preparation of the
present invention comprises in the nasal drop solution (nasal drop) the cyclic
peptide and/or a
derivative thereof and/or a pharmaceutically acceptable salt thereof at a
concentration of, for
example, 0.0001 to 1000000 pg/mL, preferably 0.001 to 10000 pg/mL, more
preferably 0.01 to 1000
pg/mL, yet more preferably 0.1 to 100 pg/mL, particularly preferably 1 to 100
pg/mL. In another
embodiment, it may comprises the cyclic peptide at a concentration of 1 to 800
pg/mL or 3 to 500
pg/mL.
[0099]
The external preparation of the present invention can be formulated, for any
formulation as
described above, by using methods and constituent materials known to those
skilled in the art.
Available constituent materials include such as, without being particularly
limited, for example, a
gelator, oily ingredient, higher alcohol, fatty acid, ultraviolet absorbing
agent, ultraviolet scattering
agent, powder, pigment, surfactant, polyhydric alcohol/sugar, polymer,
bioactive ingredient, solvent,
antioxidant, flavor and antiseptic agent.
[0100]
Various organic or inorganic gelator compounds can be used.
An inorganic gelator compound includes such as, for example, hydrous or water-
absorbable silicate,
for example, aluminum silicate (e.g., bentonite), magnesium-aluminum silicate
and colloidal silica.
As an organic gelator compound, a natural, semisynthetic or synthetic polymer
can be used.
natural and semisynthetic polymers include such as, for example,
polysaccharides such as cellulose,
starch, tragacanth, gum arabic, xanthane gum, agar, gelatin, alginic acid and
a salt thereof (e.g.,
sodium alginate and a derivative thereof), lower alkylcellulose (e.g.,
methylcellulose or
ethylcellulose), carboxy- or hydroxy-lower alkylcellulose (e.g.,
carboxymethylcellulose or
hydroxypropylcellulose). A synthetic polymer includes such as, for example,
carboxylvinyl polymer,
sodium polyacrylate, (vinylmethyl ether/ethyl maleate) copolymer,
polymethacrylate, polyvinyl
alcohol, polyvinylpyrrolidone, polyacrylate or polymethacrylate.
Alternatively, as a gel formulation,
commercially available gelators such as, for example, Lubrajel NP, Lubrajel
CG, Lubrajel DV,
Lubrajel MS, Lubrajel OIL, Lubrajel TW, Lubrajel DS (Ashland Inc.) can
also be used.
[01011
As an oily ingredient, for example, various ester, ether, hydrocarbon,
silicone and fluorine oil phase
26
CA 02986086 2017-11-15
ingredient, as well as animal and plant oils and a hardened oil thereof, and
waxes of natural origin.
The ester oil phase ingredients include such as, for example, glyceryl tri(2-
ethyl hexanoate), cetyl
2-ethylhexanoate, isopropyl myristate, butyl myristate, isopropyl palmitate,
ethyl stearate, octyl
palmitate, isocetyl isostearate, butyl stearate, butyl myristate, ethyl
linoleate, isopropyl linoleate,
ethyl oleate, isocetyl myristate, isostearyl myristate, isostearyl palmitate,
octyldocecyl myristate,
isocetyl isostearate, diethyl sebacate, diisopropyl adipate, isoalkyl
neopentanoate, glyceryl
tri(capryl-caprinate), trimethylol propane tri(2-ethylhexanoate), trimethylol
propane triisostearate,
pentaerythritol tetra(2-ethylhexaonate), cetyl caprylate, decyl laurate, hexyl
laurate, decyl myristate,
myristyl myristate, cetyl myristate, stearyl stearate, decyl oleate, cetyl
ricinoleate, isocetyl myristate,
isostearyl myristate, isocetyl palmitate, isostearyl palmitate, octyl
stearate, isocetyl stearate,
isodecyl oleate, octyldodecyl oleate, octyldodecyl linoleate, isopropyl
isostearate, cetostearyl
2-ethylhexanoate, stearyl 2-ethylhexanoate, hexyl isostearate, ethylene glycol
dioctanoate, ethylene
glycol dioleate, propylene glycol dicaprinate, propylene glycol di(capryl-
caprinate), propylene glycol
dicaprylate, neopentyl glycol dicaprinate, neopentyl glycol dioctanoate,
glyceryl tricaprylate, glyceryl
triundecanoate, glyceryl triisopalmitate, glyceryl triisostearate,
octyldodecyl neopentanoate,
isostearyl octanoate, octyl isononanoate, hexyldecyl neodecanoate,
octyldodecyl neodecanoate,
isocetyl isostearate, isostearyl isostearate, octyldecyl isostearate,
polyglycerol oleic acid ester,
polyglycerol isostearic acid ester, dipropyl carbonate, dialkyl (C12-18)
carbonate, triisocetyl citrate,
triisoalkyl citrate, triisooctyl citrate, lauryl lactate, myristyl lactate,
cetyl lactate, octyldecyl lactate,
triethyl citrate, acetyltriethyl citrate, acetyltributyl citrate, trioctyl
citrate, diisostearyl malate,
2-ethylhexyl hydroxystearate, di(2-ethylhexyl) succinate, diisobutyl adipate,
diisopropyl sebacate,
dioctyl sebacate, cholesteryl stearate, cholesteryl isostearate, cholesteryl
hydroxystearate,
cholesteryl oleate, dihydrocholesteryl oleate, phytosteryl isostearate,
phytosteryl oleate, isocetyl
12-stearoylhydroxystearate, stearyl 12-stearoylhydroxystearate and isostearyl
12-stearoylhydroxystearate.
[0102]
Hydrocarbon oil phase ingredients include such as, for example, squalane,
liquid paraffin, a-olefin
oligomer, isoparaffin, ceresin, paraffin, liquid isoparaffin, solid paraffin,
polybutene, microcrystalline
wax and vaseline.
[0103]
Silicone oil phase ingredients include such as, for example,
dimethylpolysiloxane,
methylphenylpolysiloxane, methylcyclopolysiloxane, octamethylpolysiloxane,
dacamethylpolysiloxane, dodecamethylcyclosiloxane, methyl hydrogen
polysiloxane,
polyether-denatured organo(polysiloxane), dimethylsiloxane-
methylcetyloxysiloxane copolymer,
dimethylsiloxane/methylstearoxysiloxane copolymer, alkyl-denatured
organo(polysiloxane),
terminal-denatured organo(polysiloxane), amino-denatured silicone oil, amino-
denatured
27
CA 02986086 2017-11-15
organo(polysiloxane), dimethiconol, silicone gel, acryl silicone, trimethyl
siloxysilicate, silicone RTV
gum.
Fluorine oil phase ingredients include such as, for example,
perfluoropolyether, fluorine-denatured
organo(polysiloxane), pitch fluoride, fluorocarbon, fluoroalcohol and
fluoroalkyl-polyoxyalkylene
co-denatured organo(polysiloxane).
[0104]
Animal and plant oils and a hardened oil thereof, and waxes of natural origin
include such as, for
example, animal and plant oils and the hardened oil thereof such as beef
tallow, hardened beef tallow,
lard, hardened lard, horse oil, mink oil, orange roughy oil, fish oil,
hardened fish oil, egg yolk, jojoba
oil; plant oils and a hardened oil thereof such as avocado oil, almond oil,
olive oil, cacao butter,
apricot kernel oil, kukui nut oil, sesame oil, wheat germ oil, rice germ oil,
rice bran oil, safflower oil,
shea butter, soybean oil, evening primrose oil, camellia oil, corn oil,
rapeseed oil, hardened rapeseed
oil, palm kernel oil, hardened palm kernel oil, palm oil, hardened palm oil,
peanut oil, hardened
peanut oil, castor oil, hardened castor oil, sunflower oil, grape seed oil,
jojoba oil, hardened jojoba oil,
macadamia nut oil, meadowfoam oil, cottonseed oil, hardened cottonseed oil,
palm oil, hardened
palm oil, rose hip oil; waxes such as beeswax, beeswax having high acid value,
lanolin, reduced
lanolin, hardened lanolin, liquid lanolin, carnauba wax and montan wax.
[0105]
Higher alcohols include such as, for example, lauryl alcohol, myristyl
alcohol, cetyl alcohol, stearyl
alcohol, isostearyl alcohol, oleyl alcohol, behenyl alcohol, 2-ethyl hexanol,
hexadecyl alcohol, octyl
dodecanol.
[0106]
Fatty acids include such as, for example, caprylic acid, capric acid,
undecylenic acid, lauric acid,
myristic acid, palmitic acid, palmitoleic acid, stearic acid, isostearic acid,
oleic acid, linoleic acid,
linolenic acid, arachic acid, arachidonic acid, behenic acid, erucic acid, 2-
ethyl hexanoic acid.
[0107]
Ultraviolet absorbing agents include such as, for example, paraminobenzoate,
amyl
paraminobenzoate, ethyl dihydroxypropyl paraminobenzoate, glyceryl
paraminobenzoate, ethyl
paraminobenzoate, octyl paraminobenzoate, octyl dimethyl paraminobenzoate,
ethylene glycol
salicylate, octyl salicylate, triethanol aminesalicylate, phenyl salicylate,
butyl phenyl salicylate,
benzyl salicylate, menthyl salicylate, benzyl cinnamate, octyl paramethoxy
cinnamate, 2-ethylhexyl
paramethoxy cinnamate, glyceryl diparamethoxy cinnamate mono(2-
ethylhexanoate), isopropyl
paramethoxy cinnamate, paramethoxyhydrocinnamate diethanol amine salt,
diisopropyl-disopropyl
cinnamate ester mixture, urocanate, urocanate ethyl, hydroxymethoxy
benzophenone,
28
CA 02986086 2017-11-15
hydroxymethoxy benzophenone sulfonate and a salt thereof,
dihydroxymethoxybenzophenone,
sodium dihydroxymethoxybenzophenone sulfonate, dihydroxybenzophenone,
dihydroxy
dimethoxybenzophenone, hydroxyoctoxybenzophenone, tetrahydroxybenzophenone,
butyl
methoxy-dibenzoyl methan, 2,4,6-trianilino-p-(carbo-2-ethylhexy1-1-oxy)-1,3,5-
triazine,
2-(2-hydroxy-5-methylphenyl) benzotriazole, me thy1-0-aminobenzoate,
2-ethylhexy1-2-cyano-3,3-diphenyl acrylate, phenylbenzimidazole sulfate, 3- (4-
methyl benzylidene)
camphor, isopropyl dibenzoylmethan, 4- (3,4-dimethoxyphenylmethylene)-2,5-
dioxo-1-imidazolidine
propionate 2-ethylhexyl, and polymer derivatives or silane derivative thereof,
titanium oxide and
zinc oxide and dispersion thereof. The zinc oxide and titanium oxide may be
surface-treated.
[0108]
Dermal absorption auxiliary agents include such as, for example, acetic acid,
sodium acetate,
limonene, menthol, salicylic acid, hyaluronic acid, oleic acid, N,N-diethyl-m-
toluamide
(N,N-diethyl-3-methyl benzamide), n-butyl stearate, benzyl alcohol, isopropyl
myristate, isopropyl
palmitate, polypropylene glycol, crotamiton, diethyl sebacate, N-
methylpyrrolidone,
N-ethylpyrrolidone and lauryl alcohol.
[0109]
Powders and pigments include such as, for example, pigments such as Pigment
Red 104, Pigment
Red 201, Pigment Yellow 4, Pigment Blue 1, Pigment Black 401, basic dyes, HC
colors, disperse dyes,
direct colors, lake pigments such as Pigment Yellow 4 AL lake, Pigment Yellow
203 BA lake;
polymers such as nylon powder, silk powder, urethane powder, silicone powder,
methyl
polymethacrylate powder, cellulose powder, starch, silicone elastomer
spherical powder and
polyethylene powder; colored pigments such as yellow iron oxide, red iron
oxide, black iron oxide,
chromic oxide, carbon black, ultramarine, iron blue; white pigments such zinc
oxide, titanium oxide,
cerium oxide; extender pigment such as tare, mica, sericite, kaolin and
tabular barium sulfate; pearl
pigment such as titanium mica; metal salt such as barium sulfate, calcium
carbonate, magnesium
carbonate, aluminum silicate and magnesium silicate; inorganic powder such as
silica, alumina;
metal soap such as aluminum stearate, magnesium stearate, zinc palmitate, zinc
myristate,
magnesium myristate, zinc laurate, zinc undecylenate; bentonite, smectite,
boron nitride, etc. The
shape (spherical, rod-like, needle-like, tabular, amorphous, scaly, spindle-
shaped) and particle
diameter of these powders are not particularly limited.
[0110]
These powders and pigments may be pre-treated by known conventional surface
processing such as,
for example, fluorine compound processing, silicone processing, silicone resin
processing, pendant
processing, processing with silane coupling agent, processing with titanium
coupling agent, oil
solution processing, N-acylated lysine processing, polyacrylic acid
processing, metal soap processing,
amino acid processing, lecithin processing, inorganic compound processing,
plasma processing and
29
CA 02986086 2017-11-15
mechanochemical processing.
[0111]
As the surfactant, any of an anionic surfactant, a cationic surfactant,
ampholytic surfactant and
non-ionic surfactant can be used as appropriate.
Anionic surfactants include such as, for example, fatty acid soap, a-
acylsulfonate, alkylsulfonate,
alkylallylsulfonate, alkylnaphthalenesulfonate, alkylsulfate, POE
alkylethersulfate,
alkylamidesulfate, alkylphosphate, POE alkylphosphate, alkylamidephosphate,
alkyloylalkyl
taurine salt, N-acylamino acid salt, POE alkylether carboxylate,
alkylsulfosuccinate, sodium
a1kylsulfoacetate, acylisethionate, acylated hydrolyzed collagen peptide salt
and perfluoroalkyl
phosphate ester.
[0112]
Cationic surfactants include such as, for example, alkyltrirnethylammonium
chloride,
stearyltrimethylammonium chloride, stearyltrimethylammonium bromide,
cetostearyltrimethylammonium chloride, distearyldimethylammonium chloride,
stearyldimethylbenzylammonium chloride, behenyltrimethylammonium bromide,
benzalkonium
chloride, propyldimethylhydroxypropylammonium behenic amide chloride,
diethylaminoethyl-stearamide, dimethylaminopropyl-stearamide and lanolin
derivative quaternary
ammonium salt. Cationic surfactants also include tertiary amines such as fatty
acid amide dialkyl
amine and salts thereof.
[0113]
Ampholytic surfactants include various ampholytic surfactants, for example,
those of carboxybetain
type, amidebetain type, sulfobetain type, hydroxysulfobetain type,
amidesulfobetain type,
phoshobetain type, aminocarboxylate type, imidazoline derivative type and
amideamine type.
Non-ionic surfactants include such as, for example, propylene glycol fatty
acid ester, glycerin fatty
acid ester, polyglycerin fatty acid ester, sorbitan fatty acid ester, POE
sorbitan fatty acid ester, POE
sorbitol fatty acid ester, POE glycerin fatty acid ester, POE alkyl ether, POE
fatty acid ester, POE
hardened castor oil, POE castor oil, POE = POP copolymer, POE =POP alkyl
ether,
polyether- denatured silicone alkanolamide laurate, alkylamine oxide,
hydrogenated soybean
phospholipid, hydrogenized soybean phospholipid, polymer surfactant and
biosurfactant.
Natual surfactants may also be used, including such as, for example, lecithin,
saponin and
saccharide surfactant.
[0114]
Polyhydric alcohols and sugars include such as, for example, ethylene glycol,
diethylene glycol,
CA 02986086 2017-11-15
polyethylene glycol, propylene glycol, dipropylene glycol, polypropylene
glycol, glycerin, diglycerin ,
polyglycerin , 3-methyl-1,3-butanediol, 1,3-butylene glycol, sorbitol,
mannitol, raffinose, erythritol,
glucose, sucrose, fructose, xylitol, lactose, maltose, maltitol, trehalose,
alkylated trehalose, mixed
isomerized sugar, sulfated trehalose and pullulan. Chemically modificated
forms of these can also
be used.
[01151
Polymers include such as, for example, anionic polymer compounds such as
acrylate
ester/methacrylate ester copolymer, vinyl acetate/crotonate copolymer, vinyl
acetate/crotonate/vinyl
neodecanoate copolymer, methylvinyl ether maleate half ester, t-butyl
acrylate/ethyl
acrylate/methacrylate copolymer, vinylpyrrolidone/vinyl acetate/vinyl
propionate copolymer, vinyl
acetate/crotonatecopolymer (RUBISET CA: BASF), vinyl
acetate/crotonate/vinylpyrrolidone
copolymer, vinylpyrrolidone/acrylate copolymer, acrylate/acrylamide copolymer,
vinyl acetate/butyl
maleate/isoisobornyl acrylate copolymer, carboxyvinyl polymer,
acrylate/methacrylate alkyl
copolymer, and ampholytic acetate of dialkyl aminoethyl methacrylate polymer,
ampholytic polymer
compound such as octyl acrylamide acrylate/hydroxypropyl acrylate/butyl
methacrylateaminoethyl
copolymer, quaternized compound of vinylpyrrolidone/dimethylaminoethyl
methacrylate, cationic
polymer compounds such as methylvinyl imidazolium chloride/vinylpyrrolidone
copolymer, non-ionic
polymer compounds suchs as polyvinylpyrrolidone, vinylpyrrolidone/vinyl
acetate copolymer,
vinylpyrrolidone/dimethylaminoethyl methacrylate copolymer,
vinylcaprolactam/vinylpyrrolidone/dimethylaminoethyl methacrylate copolymer.
Polymer
compounds of natural origin such as cellulose or derivatives thereof, keratin
and collagen or
derivatives thereof, calcium alginate, pullulan, agar, gelatin, tamarind seed
polysaccharides,
xanthan gum, carrageenan, high-methoxyl pectin, low methoxyl pectin, guar gum,
gum arabic,
crystalline cellulose, arabino galactan, can be karaya, gum tragacanth,
alginates, albumin, casein,
curdlan, gellan gum and dextran, and glucooligosaccharide, fucose containing
polysaccharide,
rhamnose containing polysaccharide can also suitably be combined.
[01161
Bioactive ingredients include substances which provide skin with some
bioactivity when being
applied to skin. Bioactive ingredients include those with such as, for
example, skin-lightening,
anti-inflammatory, anti-aging, ultraviolet protection, slimming, firming,
antioxidation, hair growth
stimulating/hair growing, suppressing hair growth, moisturizing, promoting
circulation,
antimicrobial/sterilization, cool/warm feeling, promoting wound cure,
alleviating irritation, analgesic
and cell-activating effects. Bioactive ingredients include such as plant
extracts, seaweed extracts,
vitamins and derivatives thereof, amino acids, various peptides other than the
cyclic peptide,
biopolymers such as sodium hyaluronate and mucopolysaccharide, intercellular
lipid constituents
such as ceramide, phytosphingosine, cholesterol and phytosterol, and analogues
thereof, and
enzymatic ingredients.
31
CA 02986086 2017-11-15
[0117]
Examples of suitable combining ingredient include such as, for example,
Angelica keiskei extract,
avocado extract, sweet Hydrangea leaf extract, althea extract, arnica extract,
aloe extract, apricot
extract, apricot kernel extract, isoflavones, ginkgo extract, fennel extract,
turmeric extract, oolong
tea extract, Rose Fruit extract, echinacea extract, Baikal skullcup extract,
Amur Corktree extract,
Coptis japonica extract, barley extract, Hypericum erectum extract, Lamium
album extract, cress
extract, orange extract, cacao extract, desiccated sea water, seaweed extract,
hydrolyzed elastin,
hydrolyzed wheat powder, hydrolyzed silk, pumpkin seed extract, chamomile
extract, carrot extract,
Artemisia capillaris extract, licorice extract, hibiscus extract, Pyracantha
fortuneana extract, kiwi
extract, cinchona extract, cucumber extract, guanosine, gardenia extract, Sasa
veitchii extract,
Sophora fiavescens extract, cranberry extract, walnut extract, grapefruit
extract, clematis extract,
chlorella extract, mulberry extract, gentian extract, tea extract, yeast
extract, burdock extract, rice
bran fermentation extract, rice germ oil, comfrey extract, collagen, cowberry
extract, Asarum
sieboldii Miq. extract, bupleurum extract, umbilical cord extract, salvia
extract, soapwort extract,
bamboo grass extract, crataegus extract, zanthoxylum extract, shiitake
mushroom extract,
Rehmannia root extract, Lithospermum root extract, Perilla frutescens extract,
nlia japonica (Miq.)
extract, Filipendula multijuga extract, Paeonia lactitlora extract, calamus
extract, white birch
extract, Equisetum arvense extract, Hedera helix extract, Crataegus oxyacantha
extract, European
elder extract, yarrow extract, peppermint extract, sage extract, tree mallow
extract, cnidium extract,
extract of Swertia japonica (Schult.) Makino, soybean extract, jujube extract,
soybean fermentation
extract, thyme extract, tea extract, clove extract, cogon extract, orange peel
extract, Oenothera
tetraptera extract, Centella asiatica extract, Terminalia sericea extract,
dong quai extract, common
marigold extract, peach kernel extract, bitter orange peel extract, extract of
HouttuynM cordata
Thunb., tomato extract, natto extract, ginseng extract, garlic extract, wild
rose extract, hibiscus
extract, Ophiopogon japonicus extract, parsley extract, honey, banana flower
extract, hamamelis
extract, parietaria extract, Isodon japonicus (Burm.) Hara extract, bisabolol,
loquat extract, coltsfoot
extract, butterbur flower extract, Poria Sclerotium extract, butcher's-broom
extract, grape extract,
propolis, luffa extract, safflower extract, peppermint extract, linden
extract, tree peonyextract, hop
extract, pine extract, horse chestnut extract, Lysichiton camtschatcense (L.)
Schott extract, Sapindus
mukurossi Gaertn. extract, Melissa extract, peach extract, bluebottle extract,
eucalyptus extract,
Saxifraga stolonifera extract, Citrus junos extract, coix seed extract,
mugwort extract, lavender
extract, apple extract, lytchee (Litchi) extract, lettuce extract, lemon
extract, Chinese milk vetch
extract, rose extract, rosemary extract, Roman chamomile extract and royal
jelly extract.
[0118]
Examples also include such as biopolymers such as deoxyribonucleic acid,
mucopolysaccharides,
sodium hyaluronate, sodium chondroitin sulfate, collagen, elastin, chitin,
chitosan and hydrolyzed
eggshell membrane; moisturizing ingredients such as amino acids, hydrolyzed
peptides, sodium
32
CA 02986086 2017-11-15
lactate, urea, sodium carbonate pyrrolidone, betain, whey, trimethyl glycine,
polypeptides such as
lysine/arginine condensate etc.; intercellular lipid constituents such as
sphingolipid, ceramide,
phytosphingosine, cholesterol, cholesterol derivative, phytosterol derivative,
phospholipid and
analogues thereof; anti-inflammatory agents such as e-aminoaminocaproic acid,
glycyrrhizic acid,
erglycyrrhetinic acid, lysozyme chloride, guaiazulen, hydrocortisone, tea tree
oil;, vitamins such as
vitamin A and derivatives thereof, vitamin B2 and derivatives thereof, vitamin
B6 and derivatives
thereof, vitamin C and derivatives thereof, vitamin D and derivatives thereof,
vitamin E and
derivatives thereof, calcium pantothenate, biotin and nicotinic acid; active
agents such as allantoin,
diisopropylamine dichloroacetate, 4-aminomethylcyclohexane carbonate;
antioxidants such as
tocopherol, carotenoids, flavonoids, tannin, lignan and saponin; cell
activating agents such as
a-hydroxy acid ande-hydroxy acid; circulation accelerating agents such as y-
oryzanol and vitamin E
derivative; wound curing agents such as retinol and retinol derivative; skin-
lightening agents such
as arbutin, kojic acid, placenta extract, sulfur, ellagic acid, linolenic
acid, tranexamic acid,
glutathione; hair growing agents such as cepharanthine, glycyrrhiza extract,
capsicum tincture,
hinokitiol, garlic extract iodide, pyridoxine hydrochloride, DL-a-tocopherol,
DL-a-tocopherol acetate,
nicotinic ac id, nicotinic acid derivative, calcium pantothenate, D-
pantothenyl alcohol, acetyl
pantothenyl ethyl ether, biotin, allantoin, isopropylmethylphenol, estradiol,
ethinylestradiol,
carpronium chloride, Benzalkonium chloride, diphenhydramine hydrochloride,
Takanal, camphor,
salicylic acid, vanillyl nonylate amide, vanillyl nonanoate amide, piroctone
olamine, glyceryl
pentadecanoate, L-menthol, mononitroguaiacol, resorcin, y-aminobutyric acid,
benzethonium
chloride, mexiletine hydrochloride, auxin, female hormones, cantharides
tincture, Cyclosporine, zinc
pyrithione, hydrocortisone, minoxidil, polyoxyethylene sorbitan monostearate,
peppermint oil,
Sasanishiki extract.
[0119]
Antioxidants include such as, for example, sodium bisulfite, sodium sulfite,
erythorbic acid, sodium
erythorbate, dilauryl thiodipropionate, tocopherol, tolyl biguanide,
nordihydroguaiaretinoic acid,
parahydroxyanisole, butyl hydroxyanisole, dibutyl hydroxytoluene, ascorbyl
stearate, ascorbyl
palmitate, octyl gallate, propyl gallate, carotenoids, flavonoids, tannin,
lignans, saponins and plant
extracts in which the antioxidative effect is recognized such as apple extract
or clove extract.
[0120]
Solvents include such as physiological saline, purified water, ethanol, lower
alcohols, ethers, LPG,
fluorocarbons, N-methylpyrrolidone, fluoroalcohols, volatile straight chain
silicones and
next-generation chlorofluorocarbons.
[0121]
The cyclic peptide of the invention and/or a derivative thereof and/or a
pharmaceutically acceptable
salt thereof can be used for preparation of the external preparation as
described above, and therefore
33
CA 02986086 2017-11-15
the present invention also relates to a use of the cyclic peptide of the
invention and/or a derivative
thereof and/or a pharmaceutically acceptable salt thereof for preparing the
external preparation.
[0122]
3. Method of using the external preparation
Next, methods of using the external preparation of the invention is explained.
A method of using the external preparation of the invention comprises applying
the external
preparation of the present invention as described above to the skin and/or
mucosa of a subject.
[0123]
Subjects include human and vertebrates such as, without being particularly
limited, for example,
birds and mammals. Animals specifically include, for example, experimental
animals including
rodents such as mice, rats, gerbils, hamsters and guinea pigs, domestic
animals such as pigs, goats,
horses, sheep and minks, pet animals such as dogs and cats, primates such as
human, monkeys,
cynomolgus monkeys, rhesus monkeys, marmosets, orangutans and chimpanzees. On
the other
hand, human may be excluded from the subjects.
[0124]
Skin and/or mucosa of a subject to which the external preparation is to be
applied may be skin or
mucosa at any site of the subject, and the external preparation is applicable,
for example, to skin or
mucosa of head (scalp), face, neck, arms, torso, arms, hands or feet, etc.
Specific method for using the external preparation of the present invention is
as follows.
[0125]
(1) Method of treating and preventing dermatitis
When the external preparation of the present invention is used as a
therapeutic/prophylactic of
dermatitis, the external preparation can be applied directly to the aimed site
of skin and/or mucosa
(e.g., the affected site where dermatitis has been developed).
The application frequency is, without being particularly limited, for example,
1 to 10 times a day,
preferably 1 to 5 times a day, yet more preferably 1 to 3 times a day. Because
the external
preparation of the present invention has a long lasting time, it exerts a
sufficient effect even if it is
applied at relatively low frequency, for example, once a day.
In terms of dosage, the total amount of the cyclic peptide of the invention
and a derivative thereof
and a pharmaceutically acceptable salt thereof for one application can be,
without being particularly
limited, for example, 0.0001 to 1000000 pg/mL, preferably 0.001 to 10000
pg/mL, more preferably
0.01 to 1000 pg/mL, yet more preferably 0.1 to 100 pg/mL, particularly
preferably 1 to 100 pg/mL.
34
CA 02986086 2017-11-15
In another embodiment, each dose may comprise the cyclic peptide of the
invention at a
concentration of 1 to 800 pg/mL or 3 to 500 pg/mL.
[0126]
(2) Method of alleviating or resolving itch
When the external preparation of the present invention is used as an
antipruritic, the external
preparation can be applied directly to the aimed site of skin and/or mucosa.
The application frequency is, without being particularly limited, for example,
1 to 10 times a day,
preferably 1 to 5 times a day, yet more preferably 1 to 3 times a day.
In terms of dosage, the total amount of the cyclic peptide of the invention
and a derivative thereof
and a pharmaceutically acceptable salt thereof for one application can be,
without being particularly
limited, for example, 0.0001 to 1000000 pg/mL, preferably 0.001 to 10000
pg/mL, more preferably
0.01 to 1000 pg/mL, yet more preferably 0.1 to 100 pg/mL, particularly
preferably 1 to 100 pg/mL.
In another embodiment, each dose may comprise the cyclic peptide of the
invention at a
concentration of 1 to 80012g/mL or 3 to 500 pg/mL.
[0127]
(3) Method of using as a cosmetic
When the external preparation of the present invention is used as a cosmetic,
the external
preparation can be applied directly to the aimed site of skin and/or mucosa.
The application frequency is, without being particularly limited, for example,
1 to 10 times a day,
preferably 1 to 5 times a day, yet more preferably 1 to 3 times a day.
In terms of dosage, the total amount of the cyclic peptide of the invention
and a derivative thereof
and a pharmaceutically acceptable salt thereof for one application can be,
without being particularly
limited, for example, 0.0001 to 1000000 pg/mL, preferably 0.001 to 10000
pg/mL, more preferably
0.01 to 1000 pg/mL, yet more preferably 0.1 to 100 pg/mL, particularly
preferably 1 to 100 pg/mL,
In another embodiment, each dose may comprise the cyclic peptide of the
invention at a
concentration of 1 to 800 pg/mL or 3 to 500 pg/mL.
[0128]
(4) Method of treating and preventing alopecia, and method of stimulating
hair growth and
method of growing hair
When the external preparation of the present invention is used as a
therapeutic/prophylactic of
alopecia, a hair growth stimulant or a hair growing agent, the external
preparation can be applied
directly to the aimed site (e.g., decalvant site of scalp or skin).
CA 02986086 2017-11-15
The application frequency is, without being particularly limited, for example,
1 to 10 times a day,
preferably 1 to 5 times a day, yet more preferably 1 to 3 times a day.
In terms of dosage, the total amount of the cyclic peptide of the invention
and a derivative thereof
and a pharmaceutically acceptable salt thereof for one application can be,
without being particularly
limited, for example, 0.0001 to 1000000 pg/mL, preferably 0.001 to 10000
pg/mL, more preferably
0.01 to 1000 pg/mL, yet more preferably 0.1 to 100 pg/mL, particularly
preferably 1 to 100 pg/mL.
In another embodiment, each dose may comprise the cyclic peptide of the
invention at a
concentration of 1 to 800 pg/mL or 3 to 500 pg/mL.
[0129]
(5) Method of treating and preventing rhinitis
When the external preparation of the present invention is used as a
therapeutic/prophylactic of
rhinitis, the external preparation can be applied directly to the aimed site
(e.g., nasal cavity mucosa).
The application frequency is, without being particularly limited, for example,
1 to 10 times a day,
preferably I to 5 times a day, yet more preferably 1 to 3 times a day.
In terms of dosage, without being particularly limited, for example, for one
application to each nasal
cavity, the total amount of the cyclic peptide of the invention and a
derivative thereof and a
pharmaceutically acceptable salt thereof can be 0.0001 to 1000000 pg/mL,
preferably 0.001 to 10000
pg/mL, more preferably 0.01 to 1000 pg/mL, yet more preferably 0.1 to 100
pg/mL, particularly
preferably 1 to 100 pg/mL. In another embodiment, each dose may comprise the
cyclic peptide of
the invention at a concentration of 1 to 800 pg/mL or 3 to 500 pg/mL.
[0130]
4. Medicament
Next, the medicament of the present invention is explained.
The medicament of the present invention comprises one or more cyclic peptides
of the invention or
derivatives thereof or pharmaceutically acceptable salts thereof.
[0131]
As mentioned above, similarly to BNP, the cyclic peptide of the present
invention or a derivative
thereof or a pharmaceutically acceptable salt thereof binds to the receptor
NPR-A (also known as
GC-A), which has a guanylate cyclase domain, and promotes the production of
cyclic guanosine
monophosphate (cGMP). It is considered to have effects such as, for example,
diuretic action,
vasodilation, renin-aldosterone secretion suppressing action, sympatholytic
activity and hypertrophy
suppressing action. Also, the compounds of the invention is considered to have
a superior efficacy
and effect, particularly a superior immediate effect, as compared with BNP.
36
CA 02986086 2017-11-15
[01321
Therefore, the medicament of the present invention can be used for a similar
object as a conventional
medicament comprising BNP as an active agent.
Diseases for which the medicament of the present invention is applicable
include such as, without
being particularly limited, for example, the aforementioned various diseases
as well as hypertension,
unstable angina, acute myocardial infarction, edematous diseases, renal
failure, cardiac failure,
immune diseases, obesity and metabolic syndrome.
[0133]
A formulation of the medicament of the present invention can be, without being
particularly limited,
addingly to the external preparations as mentioned above, for example,
formulations for oral
administration such as tablets, capsules, granules, powders, oral solution,
syrup and oral jelly,
formulations for oral application such as buccal tablets, buccal spray
formulation, buccal semi-solid
formulation and mouthwash, formulations for administration via injection such
as an injection and
infusion, dialysis formulation such as dialysis solution, as well as an
inhalant, eye drop, ocular
ointment, ear drop, vaginal tablet and vaginal suppository.
[Working Examples]
[0134]
Hereinbelow, the present invention is further specifically illustrated by
working examples. It
should be noted that the present invention is not limited by these working
examples.
[0135]
1. Preparation of the cyclic peptide
Firstly, a cyclic peptide composed of the amino acid sequence expressed by
Formula I-a is
synthesized.
Specifically, a linear peptide consisting of 17 amino acids was formed by
sequentially binding amino
acids by solid-phase peptide synthesis using a peptide synthesizer.
Subsequently, protecting groups
at Cysl and Cys17 were detached before treating with iodine (12) to form a
cysteine binding between
oxidatively same amino acid residues, thereby forming a cyclic peptide.
A composition comprising the obtained cyclic peptide was purified by reverse-
phase high
performance liquid chromatography (reverse-phase HPLC) and then lyophilized to
yield a purified
cyclic peptide as white powder.
[0136]
Mass spectroscopy was performed on the obtained cyclic peptide.
37
CA 02986086 2017-11-15
Conditions for HPLC are shown below:
Apparatus: Agilent 1100
Flow rate: 1.0 mllmin
Eluent A: 0.1% trifluoroacetic acid/water
Eluent B: 0.1% trifluoroacetic acid/acetonitrile
Gradient: 80% Eluent B, isocratic
Conditions for Mass spectroscopy (MS) are shown below:
Apparatus: Thermo Finnigan LCQ Advantage
Ionization method: electrospray ionization
Analytical method: ion trapping
[0137]
The observed results, m/z=901.83 ([M+2H]2+), m/z=1801.84 ([M+H]+), confirmed
that above peptide is
in agreement with the theoretical values of the molecular weight (1802.07) and
the mass number
(1800.8069) calculated from the composition formula of the cyclic peptide of
interest
(C72H120N24024S3).
[0138]
Furthermore, the purity of the above peptide was measured by HPLC using
following conditions:
Column: Discovery C18, 4.6mmx250mm, particle diameter 5 micron
Column temperature: room temperature
Eluent A: 0.1% trifluoroacetic acid/water
Eluent B: 0.1% trifluoroacetic acid/acetonitrile
Gradient: 10 to 30% Eluent B/20 minutes
Flow rate: 1.2 ml/min
Temperature: room temperature
Injected volume: 20 pl.
Detector: UV detector (detection wavelength: 215nm)
The result of measurement confirmed that the purity of the obtained protein
was 99.2%.
[01391
2. Preparation of formulation
2.1 Preparation of gel formulation
Preparation of a gel-based formulation comprising a cyclic peptide composed of
the amino acid
sequence expressed by Formula I-a (in the working examples below, "B ring" is
referred to mean a
cyclic peptide expressed by such Formula I-a) (B ring gel formulation, Working
Examples) and a
gel-based formulation comprising human BNP (BNP gel formulation, Comparative
Examples) were
carried out as follows.
38
CA 02986086 2017-11-15
0.1 g of methyl-p-hydroxybenzoate (trade name: Mekkins-M, Ueno Fine Chemicals
Industry, Ltd.),
0.2 g of phenoxyethanol and 3.0 g of 1,2-pentanediol were weighed into one
same vessel, heated to 60
to 70 C to make a homogenous solution, and this solution was poured into a
mixer.
Next, added into the mixer 6.0 g of concentrated glycerin, and then the
mixture of 0.44 g of
carboxyvinyl polymer (trade name: Carbopol 940, Lubrizol Advanced Materials
Corporation) and
0.08 g of xanthane gum (trade name: KELTROL T, CP Kelco, Inc.), and the
mixuter was stirred
with a paddle until they dispersed sufficiently.
[0140]
Then 83.95 g of purified water was gradually added with stirring with a
paddle. The mixer was
heated to 70 to 80 C while stirring with a paddle or disper until the
dispersed contents were
dissolved to give a solution. Subsequently, the disper was stopped and the
solution was cooled
immediately after confirming that the contents in the solution were dissolved.
When the
temperature of the mixer reached approximately 40 C, 6.0 g of Lubrajel NP
from Ashland Inc.
(glycerin 2.7 g, carboxyvinyl polymer 0.06 g, sodium polyacrylate 0.018 g,
water 3.222 g) was added
to the solution, mixed uniformly with a paddle. Subsequently, 0.230 g of
potassium hydroxide was
further added to neutralize the solution, then the rotation of the paddle was
stopped when the
temperature of the mixer reached 25 C to prepare a gel base.
[0141]
Next, 20.1 mg of the the cyclic peptide (B ring) composed of the amino acid
sequence expressed by
Formula I-a was dissolved in 144 mL of physiological saline to obtain B ring
solution. 0.131 mL of
this solution was admixed with 10 g of the gel base obtained as described
above, and the mixture was
stirred uniformly to prepare a gel based formulation (B ring gel formulation)
containing B ring at a
concentration of about 1 pM (about 1.8 pg/g). Similarly, gel based
formulations containing B ring at
concentrations of about 0.3 pM (about 0.54 pg/g), about 0.5 pM (about 0.9
pg/g) and about 2.0 pM
(about 3.6 pg/g) were prepared. In the working examples below, as long as it
is specifically
described otherwise, "B ring gel formulation" used contained B ring at a
concentration of about 1 pM.
Next, 20.5mg of human BNP-32 (American Peptide Company) was dissolved in 118mL
of
physiological saline to obtain BNP solution. 0.2mL of this solution was
admixed with 10 g of the gel
base obtained as described above, and the mixure was stirred uniformly to
prepare a gel based
formulation (BNP gel formulation) containing BNP-32 at a concentration of
about 1 pM. Similarly,
gel based formulations containing BNP-32 at concentrations of about 0.5 pM and
2.0 pM were
prepared. In the working examples below, as long as being specifically
described otherwise, "BNP
gel formulation" used contained BNP at a concentration of about 1 pM.
[0142]
39
CA 02986086 2017-11-15
2.2 Preparation of nasal drop
A nasal drop comprising the cyclic peptide (B ring) composed of the amino acid
sequence expressed
by Formula I-a (B ring nasal drop, Working Examples) and a nasal drop
comprising human BNP
(BNP nasal drop, Comparative Examples) were prepared as follows.
[0143]
Firstly, the cyclic peptide (B ring) composed of the amino acid sequence
expressed by Formula I-a
was dissolved in physiological saline, the concentration was adjusted to
prepare B ring nasal drop
containing B ring at a concentration of about 1 pmo1/1 (about 1.8 pg/g).
Next, B ring nasal drop was filled in a quantitative nasal spray container (AS
ONE Corporation) and
the amount ro be sprayed for each administration was adjusted to 100 pl (0.1
ml), resulting B ring
nasal drop.
[0144]
Similarly, a BNP nasal drop comprising human BNP (American Peptide Company) at
a
concentration of 1 pino1/1 was obtained, filled in a quantitative nasal spray
container (AS ONE
Corporation), and the amount ro be sprayed for each administration was
adjusted to 100 pl (0.1 ml),
resulting BNP nasal drop.
[0145]
3. Confirming therapeutic effect on dermatitis
3.1 Confirming the effect of B ring gel formulation
For subjects suffering various dermatitis, B ring gel formulation as described
was applied onto the
affected site, and changes in symptoms before and after the application were
observed. When
possible, as a comparative example, BNP gel formulation as described was
applied onto another
affected site on the same subject where B ring gel formulation had not been
applied, and changes in
symptoms before and after the application were observed. For pruritus, VAS
(Visual Analogue
Scale) was used for evaluating each affected site on 10 stages.
The examination results are shown in Tables 1 to 7 along with age, sexuality,
symptoms of the
subject, and formulation given to the subject.
0
1--,
4=.
(Table 1]
11
Pruritus before
Pre-application symptoms
Case Treatment Disease Post-application
symptoms (severity) and after Notes
(severity)
, application (VAS)
Face: itches removed 2 min after 1
application. Desiccation, scales and
erythema remitted (mild). After 3 days of
application, erythema, scales, infiltration
and desiccation remarkably remitted
2pM (insignificant).
B ring gel formulation Face: presented with
Right forearm: itches stopped to some face 8-.0
1 application erythema, scales and skin
extent after 1.5 min on B ring-applied right arm 7-.0
D1 desiccation (moderate site. No
itching or tingling after 2 mm.
Age: 30's atopic dermatitis disease).
Lichenification/stiffness remitted after 4
Sex: male Limbs: presented with min.
Desiccation improved. Skin
erythema, lichenification, skin moisturized and
soft, with natural
desiccation and scratch scar appearance. No
subjective discomfort P
(severe). (moderate).
0
IV
Face: itches realized after 4 min,
.
00
2pM erythema and scales
more distinguished ..,
0
BNP gel formulation than B ring side.
face 8-.4 00
Left forearm: after 4 min, skin was still
left arm 7-4
1 application
0
4, N i
dry. o improvement in lichenification.
1-
1-,
...3
'
Rough and stiff skin (severe).
1-
1pM Flushing, infiltrating erythema,
Right back: itches stopped after 3 min. 1-
i
02 B ring gel formulation scales, many scratch scars
Flushing erythema, infiltration and scales 8-.0 1-
u,
1
Age: 20's application atopic dermatitis (severe) accompanied
by remitted. Skin became soft
(moderate). .
Sex: male 1pM erythroderma strong itches
causing sleep Left back: still itching after 3 mm. no
BNP gel formulation disruption and desire to
remission of flushing erythema, 8-4
1 application scratch, infiltration and
scales (severe).
_
0.5pM Right back: no
tingling itches 30 sec after
application. Skin felt normal. Erythema,
B ring gel formulation Presented with infiltrating
6-.0
D3 infiltration and
scales remitted after 4
1 application
Age: 10's atopic dermatitis erythema, scales, papules,
min (mild).
y
Sex: male 0.5pM (moderate) accompanied b Left
back: still itching after 4 mm, with no
itches.
BNP gel formulation tinglingremission of erythema,
infiltration and 6-.6
1 application scales (moderate).
_
F
1-,
4=.
--1
[Table 2]
Pruritus before
Pre-application symptoms
Case Treatment Disease Post-application symptoms
(severity) and after Notes
(severity) application (VAS)
Right back: itches relieved just after
Presented with infiltrating application. No
itches after 2 min with
erythema, scales, many remission of
erythema and infiltration
2pM scratch scars with exudation
(moderate).
B ring gel formulation (severe) accompanied by
Forehead: itches reduced after 1.5 min. 10--.0
1 application strong itches that cause sleep After
5 min skin was soft with no
D4 disruption on the back,
lichenification. Erythema and scales
Age: 20's atopic dermatitis lichenification,
erythema, remitted. Wound quickly healed. Scratch
Sex: male scales and scratch scars on scars
epithelized.
forehead (severe). Left back: still
itching after 4 min with no
Back: presented with many remission of
erythema and infiltration
2pM
scratch scars with exudation, (severe).
BNP gel formulation
10 -.3
infiltration, erythema, crusts Forehead: no
improvement in
1 application
(severe). lichenification,
desiccation and scales,
. with remaining itches (severe).
P
Right face: itches, edema and infiltration
0
1.,
remitted/relieved just after application.
'
00
Presented with infiltrating
.
1pM Skin felt no itch
and moisturized after 1 0
erythema,
0
.4.. Bring gel formulation edema and crusts
min (moderate). 9-4 ..,
t\ D5 1 application (severe) accompanied by
No itches at all after 2 min. Edema and
"
0
Age: 20's atopic dermatitis strong tingling itches.
erythema remitted. Right eye became
Sex: male
1-
...3
Back: presented with many
I
1-
scratch scars with exudation, easy to open.
1-
Left face. still itching after 3 min with no
1
1pM infiltration, erythema, crusts
= 1-
.
.
remission of erythema, infiltration and
u,
BNP gel formulation and papules (severe).
9-4
edema. Being ifficult to open left eye
1 application
(severe).
Right back: itches improved/ relieved
after 3 min. Lichenification, prurigo
1pM Presented with lichenification,
nodularis, erythema, scales, scratch
B ring gel formulation prurigo nodularis, erythema,
8.-.0
scars all improved as compared to left.
1 application scales, papules and many
atopic dermatitis Skin was soft and
moisturized
D6 scratch scars (severe)
(moderate).
Age: 10's accompanied by strong itches
prurigo nodularis " Left back: still
itching after 4 min with no
Sex: male Back: presented with many
remission of erythema and infiltration
1pM scratch scars with exudation,
BNP gel formulation infiltration, erythema, crusts
(severe).
8-.3
Forehead: lichenification, desiccation
1 application and papules (severe),
and scales not improved, with itches left
(severe).
F
,-,
.4.
co
[Table 31
Pruritus before
Case Treatment Disease Pre-application symptoms
Post-application symptoms (severity)
and after Notes
(severity)
application (VAS)
Presented with infiltrating No itches after 1
min. Erythema remitted
07 0.3pM erythema, papules, exudation and
exudation stopped after 10 min. 1
intractable
Age: 20's B ring gel formulation atopic dermatitis and many
scratch scars with application/day for 3 days cleared off
10¨.0
steroid-
Sex: male 1 application crust (severe) accompanied
infiltrating erythema, papules, exudation
resistance
by strong itches that cause and scratch scars.
Remarkably improved
sleep disruption. crusts, partly left
mild erythema (mild).
days after application, erythema,
0.5pM Presented with erythema,
infiltration, scales, crusts and wound
intractable
D8 infiltration, scales, crusts,
scratch scars and skin
B ring gel formulation atopic dermatitis quickly cured, with
little scratch scars left 8¨.1 steroid-
Sex: male
1 application, 5 days (mild). Desiccation remarkably remitted,
resistance
desiccation (severe).
Skin moisturized and soft.
_
Left forearm presented with Itches removed in 2
min (VAS 0).
many scratch scars with Erythema and
exudation improved.
09 B ring gel formulation exudation, infiltration,
Severity of rash improved to moderate
P
Age: 30's 2 applications/day atopic dermatitis erythema,
papules, disease level after 5 min. After 1 more 10¨.0 o
Sex: male 1 day accompanied with tingling
application given on the same night,
o
00
strong itches (VAS10) there was no itches
for 3 days thereafter. .
o
(severe). Scratch scars
epithelized. 03
..,
.4- 1p M Right forearm:
itches stopped after 3
cd.., Presented with infiltrating
0
B ring gel formulation min. Erythema and
infiltration improved 8¨.0 1-
010 erythema, scales, papules and
-.3
1 application
i
Age: 50's atopic dermatitis many scratch scars
(severe) (moderate). 1-
1pM Left forearm: still
itching after 4 min with 1-
i
Sex: female BNP gel formulation accompanied by inremediable
no remission of erythema and infiltration
8-4 1-
u,
strong itches -
1 application (severe).
_
Presented with infiltrating Right face:
immediately after application,
1pM erythema, scales and many itches
and skin tautness relieved, with
B ring gel formulation scratch scars (severe)
erythema getting better. No itching after 10¨.0
Dll 1 application accompanied by drastic itches 1 min.
Erythema, infiltration and
Age: 40's atonic dermatitis __________ and tautness. desiccation remitted
(moderate).
Sex: female Back: presented with many
1pM Left face: still itching after 3 min, having
scratch scars with exudation,
BNP gel formulation infiltration, erythema, crusts
erythema, infiltration and edema with 10-4
1 application swollen feeling
(severe).
and papules (severe).
O'
,--,
A
co
[Table 4] -
Pruritus before
Pre-application symptoms
Case Treatment Disease Post-application
symptoms (severity) and after Notes
(severity)
application (VAS)
Right face: itches remitted after 3 mm.
Edema, erythema and infiltration started
to be remitted. After 30 mm, anti-
Strong tingling itches,
104 inflammatory effect observed, exudation
disrupted sleep, infiltrating
13 ring gel formulation stopped, wound
epithelization promoted, 10-0
012 erythema, scales, exudation,
cracks almost epithelized. Edema,
1 application
Age: 40's atopic dermatitis cracks, crusts, scratch
scars;
erythema and infiltration remitted to
Sex: female eyes cannot be opened due to
some extent. Eyelids became openable
exudate sealing eyelids
steadily.
(severe).
1pM Left face: still
having tingling itches after
M
BNP gel formulation 3 m with no
remission of erythema and 10-,6
1 application infiltration. Itches
till left after 30 min. 9
, .
..
Right face: erythema started to be
,a
r.
1phil
8-.0 ,0
remitted and itches removed just after
OD
(immediate after
B ring gel formulation Obvious infiltration, erythema
a,
D13 application.
Erythema and infiltration ,a
1 application and papules all over the face,
application) 0
Age: 20's atonic dermatitis remitted after 4 min
(moderate). m
accompanied with strong
r.
Sex: female 1pM Left face: itching
with hot flush and ,a
itches and hot flash (severe).
8-.0
BNP gel formulation redness just after
application. Itches (after 4 minutes)
--1
1
A
A 1 application _removed after 4 min.
i>
1-,
Right hand: itches removed after 3 min
Steroid- 1
1-'
1 IN
(VASO). Lichenification improved, skin
resistance;
B ring gel formulation Hand eczema of which main
9-40
got soft, erythema and infiltration
intractable
1 application symptom is contact dermatitis
remitted (moderate).
lichenification
014 1pM due to detergent and
which cannot
disinfectant. Presented with
Age: 20's BNP gel formulation
atopic dermatitis be remitted
lichenification, erythema,
Sex: female 1 application Left hand: no
improvement in within this
scales and scratch scars
lichenification and erythema after 10 min
9-.4 short time (3
(severe). Sleep disruption by
(severe),
mm) by
strong itches (VAS9).
conventional
therapy.
F
i¨i
cn
0
(Table 5-1
Pruritus before
Pre-application symptoms
Case Treatment Disease
Post-application symptoms (severity) and after Notes '
(severity)
, application (VAS)
..
Erythema, infiltration, scales and crusts,
wound epithelization improved 3 days
1pM Presented with erythema, after
application. Scratch scars
D15
B ring gel formulation infiltration, scales, crusts,
significantly improved. Only mild
Age: 20's atopic dermatitis
9---.1
scratch scars (severe), with erythema and scales
observed (mild).
Sex: female 1 application/day
3 days strong itches. Desiccation
relieved, improved and
moisturized skin texture. Itches and
scratch scars significantly improved.
Right side: tingling itches removed after
1 min. Itches eliminated after 3 min
1pM Contact dermatitis due to hair
(VASO), felt better than left. Erythema
B ring gel formulation dye with erythema, edema
8¨.0
D16 and infiltration
remitted.
1 application and infiltration along hairline
Age: 60's contact dermatitis Anti-inflammatory
effect observed.
(moderate disease)
Sex: female
accompanied by strong Edema improved
(mild). .
1 pM
tingling itches (VAS8). Left side: symptoms
less improved than P
BNP gel formulation
8¨.4
right (moderate).
0
1 application
IV
tO
Right face: itches stopped just after
0
0
0
application. Erythema remitted. No
0
cn
0
scales and desiccation after 2 mm. Skin
IV
1pM Presented with infiltrating
was moisturized (moderate). 0
F-'
B ring gel formulation erythema, scales, Forehead: itches
controlled after 1.5 M. 10¨.0
D17
...,
'
rn r
1 application lichenification, crusts, papules No
lichenification after 5 min. Skin got r
Age: 40's chronic eczema
,
and skin desiccation (severe) soft. Erythema and
scales remitted, r
Sex: female 01
accompanied by drastic itches scratch scars epithelized and wound
and tautness. quickly healed.
.
1pM Left face: still
itching and taut after 4 min
BNP gel formulation with no remission of
desiccation, scales, 10¨'5
1 application erythema and
infiltration (severe). .
After 15 min, erythema, edema,
1pM
infiltration and scales significantly
B ring gel formulation
8¨.0
D18 Presented with erythema, remitted and skin texture improved
1 application
Age: , eczema edema, infiltration, scales and
(mild).
Sex: female 1pM papules (severe). After 15 min,
still many scales,
BNP gel formulation erythema, edema and
infiltration left. 8¨.4
1 application Skin texture is
rough and still itching.
F
1-,
cn
1-,
¨,
frable 6)
Pruritus before
Pre-application symptoms
Case Treatment Disease Post-application
symptoms (severity) and after Notes
(severity)
application (VAS)
D20 1 tl M Presented with multiple Right lower
limb: itches removed just
wheals erythema and very after application.
Erythema started to be
Age: 20's B ring gel formulation wheal, insect bites
' " 10-.0
Sex: female 1 application strong itches on both lower remitted
after 1.5 min. Wheals
limbs, disappeared in about
1 hour. .
031 1pM Presented with multiple Left lower
limb: itches removed 1 min
Age: 30's B ring gel formulation
wheal, insect bites wheals and very strong itches after application,
wheals remitted after 2 10-.0
Sex: female 1 application on right lower limb.
min. ..
Right back: itches stopped after 30 sec.
Desiccation, scales and erythema
1pM Presented with desiccation,
B ring gel formulation many milary large papules, improved
in few minutes. Skin got
strong pruritus
021 2 applications/day desquamation, , moisturized. After 3
days, wounds 8-.0
childhood xerotic quickly epithelized
leaving one scratch
Age: 10's 3 days and many scratch scars on
eczema scar. Skin is smooth
and moisturized
Sex: female trunk; incrustation, erythema
with no desiccation (insignificant).
and lichenification due to
1pM
P
scratching (severe). Left back:
desiccation, scales and
BNP gel formulation
8--.3 0
erythema left after few minutes.
1 application
.
0
032 1pMPresented with multiple
.
41. parapsoriasis Scales, erythema and
infiltration remitted .
im Age: 40's B ring gel formulation
erythrokeratoderma with N/A 0
..,
guttata after 4 mm.
Sex: female 1 application sticking small scales on back.
_ 0
=
0.5pM Both lower limbs presented
1-
D33 Scales and erythema
started to be -J
,
B ring gel formulation with many red plaques with
1-
Age: 60's psoriasis vulgaris remitted 10 mm after
1st application. N/A 1-
2 applications/day attached small scales having
i
Sex: male After 1 week, no
scales left. 1-
1 week _ clear margin.
ul
- Back psoriasis: thick scales started to be
034 2pM Many red plaques with
remitted in 3 min after application,
attached thick scales having
Age: 30's B ring gel formulation psoriasis vulgaris
clear margin presented on almost disappeared after 30 min. N/A
Sex: male 1 application Neck psoriasis:
erythema and scales
back and neck.
remitted in 3 mm after application.
,
F
i--,
cn
to
[Table 7j
Pruritus before
Pre-application symptoms
Case Treatment Disease Post-application
symptoms (severity) and after Notes
(severity)
application (VAS)
.
2plvt Fingers presented with many Erythema
and bullae remitted in 2 mitt
023
Intractable with
B ring gel formulation dyshidrotic eczema intractable bullae, being
after application. After 1 day, erythema
Age: 30's
N/A steroid
2 applications/day dyshidrosis eczematous with erythema and
scales remitted and bullae
Sex: male
ointment.
1 day and cracks. , disappeared.
024 2pM
Red small papules and flare Pruritus, erythema
and red small
Age: 50's 8 ring gel formulation miliaria
N/A
hyperhidrosis with itches. papules remitted
after 3 nun.
Sex: male 1 application
025 1pM Pruritus removed
just after application.
Many wheals with drastic Wheals started to be
gradually remitted
Age: 50's B ring gel formulation
chronic urticaria 10¨.0
9
itches. just after
application and disappeared
Sex: male 1 application
after? min.
0
Suffering from rosacea for 2
1.)
years. 2 months of facial Tautness and
tingling itches removed Intractable co
0,
0
application of a steroid just after
application. Diffuse flare, after 2 yrs of co
a,
026 1pM
ointment exacerbated the follicular papule
and pustule remitted conventional
1.)
symptoms, developing after 1 min. After 1
week of 2 therapy 0
Age: 30's 8 ring gel formulation
rosacea N/A 1-
-.1
Sex: female 1 application
telangiectasia, diffuse flare, application/day,
anti-inflammatory effect without i
follicular
i-
papule and pustule was observed.
Diffuse flare, follicular satisfactory
I
14 on cheeks. Subjective
papule and pustule remarkably therapeutic
VI
--1 symptoms include tingling
improved. Itches and hot flush removed. effect.
itches and tautness.
2 years of facial application of
Diffuse flare and follicular papule
a steroid ointment resulted in
D35 4pM =
circumoral telan . a remarkably
remitted 2 mm after
Age: 60's B ring gel formulation perioral dermatitis
-aiectass ' application. After 2 application/day for 2 N/A
diffuse flare and follicular
Sex: female 1 application
papule. Subjective symptoms days flare improved and scales
removed.
include heat sensation.
a-,
-
01
o..)
[Table al
-
Pruritus before
Pre-application symptoms
Case Treatment Disease Post-application
symptoms (severity) and after Notes
(severity)
application (VAS)
036 2pM Presented with circumoral Diffuse
flare and follicular papule
Age: 50's B ring gel formulation rosacea telangiectasia,
diffuse flare remarkably remitted 2 min after
Sex: mate 1 application and follicular papule. application
Faster and
Seborrhea remitted 2 min after
stronger
D37 1pM Oily skin accompanied by
application. Anti-inflammatory effect anti-nflammatory
B ring gel formulation acne vulgaris
suppurative inflammation with remitted suppurative inflammation
and effect than
N/A
Age: 20's
Sex: female 1 application/day many pustules and comedos
pustules on lower jaw. Pustules reduced applying/
3 days on lower jaw. and dried 3 days
after application, and administrating
domedos disappeared.
conventional
antibiotics.
,
Anti-inflammatory effect observed 3 min
after application, pustules and papules
Right cheekipM B ring started to be
reduced and flattened, and
P
gel formulation flare started to be
removed. After 1
0
1 application/day week pustules and
papules substantially ND
lt,
2 weeks remitted and flare
further remitted. After 0,
038 Multiple follicular papules and
2 weeks pustules, papules and flare
0
4=- Age: 40's acne vulgaris pustules on both cheek. Oily
almost disappeared.
N/A 0
..,
oo Sex: female skin.
ND
Left cheek: BNP gel
0
single
1-
application, then No change in
symptoms in 3 min after ...3
i
1pM B ring gel application. After
switching to B ring 1-
1-
'
formulation formulation, similar
effects as right 1-
u,
1 application/day cheek were obtained.
2 weeks
.
-Anti-inflammatory effect by B ring
Right cheek: 2pM
application was observed on right cheek
B ring gel formulation Oily skin accompanied by
D39 in 3 min.
Suppurative inflammation
1 application suppurative inflammation with
remitted.
Age: 10's acne vulgaris
Sex: male Left cheek: multiple red papules and
pustules on both cheeks. No anti-inflammatory
effect on
gel formulation
gel-applied left side.
1 application
Comedos faded in 5 mm after
040 4pM Seborrheic skin with multiple
Age: 10's B ring gel formulation acne vulgaris comedos and
follicular application. Oily skin improved to better
texture. Red papules and pustules
Sex: female 1 application papules/pustules on forehead.
reduced.
CA 02986086 2017-11-15
[0154]
As shown in Tables 1 to 8, in all subjects, or in all dermatitis of any
symptoms, emission or
elimination of various symptoms of dermatitis was observedat the site where B
ring gel formulation
was applied.
Specifically, when B ring gel formulation was applied, remission or
elimination of pruritus was
observed immediately after the application. Generally in dermatitis, patients
tend to scratch where
pruritus was felt, potentially causing severer dermatitis. Such a remarkable
remission or
elimination of pruritus in this manner can consequently prevent aggravation of
dermatitis.
In all cases, the effects of B ring gel formulation were superior to those
with BNP gel formulation.
Specifically, B ring gel formulation had faster-acting and longer-lasting
effects as well as more potent
antipruritic effect as compared with BNP gel formulation. It was confirmed
that these effect cannot
be obtained by the gel base.
[0155]
3.2 Comparing B ring gel formulation to gel base by two-sided application
For subjects suffering various dermatitis, either B ring gel formulation or
the gel base was applied to
the affected site, and changes in symptoms before and after the application
were observed (Table
below).
49
[Table 9]
Case Applied site Treatment Disease Pre-application
symptoms Post-application symptoms
El Right B ring atopic dermatitis Face presented
with infiltrating Flare and pigmentation removed 2.5 min after application.
Skin
Age: 20's erythema, scales and elasticity
and desiccation improved. Itches eliminated.
Sex: female __________________________ pigmentation, with intractable
Left gel base No remission of
pigmentation, infiltrating erythema, scales and
itches
desiccation, with itches left.
_
E2 Right B ring chronic eczema Stiffness and
hard lichenification Flare remitted soon after application. After 3 min
skin softened and
Age: 30's on both forearms, with infiltrating
moisturized. Itches eliminated.
Sex: male erythema and desiccation.
Left gel base No improvement in
lichenification, itching and flare after 3 min.
,
_______________________________________________________________________________
___________________
E3 Right B ring atopic dermatitis Presented with
hot flush and Flare started to fade soon after application, and was
remarkably
Age: 10's puffiness, remitted after 3
min.
Sex: female _______________
Left gel base No improvement in
flushing after 3 min.
P
E4 Right B ring chronic eczema Stiffness and
hard lichenification Flare remitted soon after
application, remarkably remitted after 3 min .
Age: 40's on both forearms, with infiltrating
with improved infiltration and hot
sensation, and softened skin. "
Sex: male erythema, scales and hot
..,
Left gel base Infiltration felt
3 min after application. Skin was hard with unimproved 0
sensation.
0
CII flare and hot
sensation.
_
_______________________________________________________________________________
________________________________ N,
.
1-
...]
,
1-
1-
,
1-
0,
CA 02986086 2017-11-15
[0156]
4. Confirming cosmetic effect
Effects of a cosmetic comprising B ring were evaluated from the results
obtained using a gel
formulations, a bath agent and a hair cosmetic product (shampoo or rinse) or
body soap comprising
the B ring to confirm their effects.
4.1.1 Confirming skin-improving effect (comparing B ring gel formulation to
BNP gel
formulation)
The B ring gel formulation or BNP gel formulation was applied to subjects. The
B ring gel
formulation was applied on the right cheek or eye of each subject, the BNP gel
formulation on the left
cheek or eye, and changes in skin conditions were observed and compared (Table
below). The
changes in skin conditions were determined based on physical sensory
evaluation by the subject and
our objective observation as physicians.
51
[Table 10]
Case Applied site: method Pre-application symptoms
Post-application symptoms
Fl Right: B ring gel formulation Presented with wrinkles, cheek
2 min after application, wrinkles on eye corner and forehead were shallowed,
skin was
Age: 69 0.5pmol, 1 application flabbiness, pigmentation
on eye full. After 3 min, cheek flabbiness and skin texture were improved,
and pigmentation on
Sex: female corner, eye corner was less
prominent. After application for 3 days, large wrinkle on cheek was
shallowed, flabbiness was improved, skin was firm, resilient, full and
moisturized.
Left: applied gel base No improvement in wrinkles
after 3 min.
F2 Right: B ring gel formulation Presented with wrinkles, cheek
2 min after application, wrinkles at eye corner due to desiccation was less
prominent, skin
Age: 45 0.5pmol, 1 application flabbiness and
desiccation. texture was improved, moisturized and softened.
Sex: female
Left: applied gel base No improvement in wrinkles
and desiccation after 2 min.
F3 Right: B ring gel formulation Presented with desiccation, light
30 sec after application, skin desiccation was improved, skin was
moisturized and flexible
Age: 21 2pmol, 1 application erythema, tautness and
itch. with no tautness. After 1 min, flare was remitted and itches were
removed. After 4 min,
Sex: female further softness was given
and desiccation was remarkably improved.
Left: applied gel base No improvement observed in
desiccation, itching and flare after 4 min. P
.
IV
lt,
F4 Right: B ring gel formulation Presented with desiccation and
3 min after application, skin
desiccation was improved, skin was moisturized and firm, 0
Age: 24 2pmol, 1 application light erythema.
with improved texture. Skin was
flexible, soft and moisturized. 0
0
c.n t Sex: female Left: applied gel base
No improvement observed in
desiccation and flare after 3 min. "
0
1-
...3
1
1-
F5 Right B ring gel formulation Presented with wrinkles on both
2 min after application, wrinkles on
both corners of eyes were less prominent, cheek 1-
1
=
Age: 28 2pmol, 1 application corners of eye, and
desiccation. dryness was improved, skin was
moisturized and flexible. Perioral desiccation was 1-
u,
Sex: female remitted and skin became
full and flexible.
Left: applied gel base No improvement observed in
desiccation and wrinkles after 2 min at gel-applied site on
the left.
F6 Right: B ring gel formulation Presented with skin desiccation,
1 min after application, itches were remarkably remitted. After 3 min, skin
desiccation and
Age: 30 2pmol, 1 application oily skin and red papules
around flare were improved, and skin was moisturized and flexible with
improved texture. Red
Sex: female nose, and light erythema and papules around
nose almost disappeared, combination state of desiccation and oily skin
rough skin texture. was improved, and skin is
kept healthy.
Left: applied gel base 3 min after application,
desiccation, flare and itches are left, and seborrhea and papules
around nose were not remitted.
c'3'
i2e 0
CD
..2 ,--= CP
00 CD a) ..,1
[Table 11]
I-. =
Case Method of application Pre-application symptoms
Post-application symptoms 0 CD
01 B ring gel formulation 1pmol Presented with cheek flare, open
After Bring application, flare was repressed, skin texture
was improved, skin was P-. Er
Age: 50 1 application pores, rough and hard skin texture, moisturized
and softened.
Sex: female
1-3 cn
0-'
02 B ring gel formulation 2pmol Presented with wrinkles and
3 min after application at B ring-applied site, wrinkles
on forehead and eye corner were 0
Age: 49 1 application desiccation on forehead and eye shallowed and
less prominent, skin desiccation was improved, and skin was moisturized
Sex: female corner.
_ and flexible.
A) P
G3 B ring gel formulation 1pmol Presented with male skin 1 day
after application at ring-applied site, skin desiccation and roughness were
,- =
Age: 40's 1 application desiccation and roughness.
improved, and razor burn was prevented. Cfq N
CD
(l)
Sex: male
07 m
04 B ring gel formulation 2pmol Presented with miliaria and
3 mm after application at B ring-applied site, miliaria
was prevented, flare and itches were 1-= = 0
O X
Age: 50's 1 application pruritus accompanied with flare.
remitted. P
cn
Sex: male
r.
= ts
c) iti
O ci)
O "=
P-=
,--= =
c-t-
P
0
, =
CI)
.-. 0
n,
ed
.
0
ct)
.
a)
,-.' = 0
..,
0-1
ra, crq
co
ct, n,
CD CD
r
...]
I-'
. =
, I
.
. 0
CD P
r
= ul
PI, 0
Cr D'7.
,t-
r= =
C.0 0
r= =
'5' P
Ht co
Ii 4
CD
,=-t-
,_...
O 1-.=
O 0
S:2-= P"
AD
u)
,-- =
,-t-
Sn-= 0-.
CD 0
CO
O ,-/=-
,-5
'6"-' 4
Sa= ...,=`-'
'-<
9)
,-= =
0TA 0
O cm
<
CD
CA 02986086 2017-11-15
[0159]
Accordingly, it was shown that B ring of the present invention has a skin
moisturizing effect and is
capable of improving skin texture, and that it is also effective in improving
skin, for instance in
lightening (spots, dullness) and anti-aging (flabbiness, resilience, large
wrinkles) as well as in
improving mucosal condition such as roughened lips.
[0160]
In all subjects, a remarkable improving effect in skin and its persistence was
observed at the site
where B ring gel formulation had been applied as compared to where BNP gel
formulation had been
applied. Specifically, effects as follows were observed at the site where B
ring gel formulation had
been applied as compared to where BNP gel formulation had been applied with
significance: effects
of improving skin, moisturizing skin, improving skin texture, providing skin
with resilience and
firmness, softening skin, suppressing skin desiccation, fading fine wrinkles,
supplying and keeping
skin with water and oil, shallowing and diminishing crow's feet, eliminating
skin rawness,
eliminating and suppressing itches, relieving skin roughness and maintaining
skin condition
healthy.
[0161]
Particularly, in subjects in their 20's or 30's, remarkable effects such as
improving or eliminating
wrinkles, improving cheek flabbiness, and supplying and keeping skin with
moisture, water and oil,
and the effects such as giving skin firmness and tightness, lifting up cheeks,
eliminating skin
rawness and relieving skin roughness were observed at the site where B ring
gel formulation had
been applied as compared to the site where BNP gel formulation had been
applied, and these effects
were greater as compared to those at BNP gel-applied site.
Moreover, in subjects in their 40's and 50's, remarkable effects such as
improving flabbiness, large
wrinkles and desiccation, giving skin firmness, fading dullness and spots were
exhibited
immediately after the application of B ring gel formulation, and these effects
were greater as
compared to those upon applying BNP gel formulation.
[0162]
The effects exhibited significantly faster in B ring gel formulation as
compared to BNP gel
formulation. Specifically, effects were confirmed in most cases such that
wrinkles were vanished or
started to vanish and faded and skin was made full and resilient. These
effects lasted for at least
several hours thereafter. No irritating symptoms were observed with
application of B ring gel
formulation.
[0163]
Fig. 1 shows a photograph of the right-side face of a subject in her 60's
after the application of B ring
54
CA 02986086 2017-11-15
gel formulation. In this subject, effects were observed at 20 minutes after
the application of B ring
gel formulation such that the skin were moist, provided with moisture,
complexion and softness,
given firmness, improved texture, provided with resilience, and large wrinkles
faded. This result
was compared to BNP gel formulation as a comparative example, indicating that
the cyclic peptide of
the invention is not only effective on fine wrinkles but also is effective on
wrinkles and flabbiness,
and that it is also effective in fading spots and dullness.
[0164]
4.1.2 Confirming skin-improving effect (comparing B ring gel formulation to
gel base)
In order to examine the presence or absence of the contribution by the gel
base to the effects of B ring
gel formulation and BNP gel formulation, three subjects were administered B
ring gel formulation on
their right face and the gel base on the left, and changes in skin conditions
on the subject's faces were
observed (Table below).
o")
cn
[Table 12]
Case Applied site: method Pre-application symptoms
Post-application symptoms
H1 Right: B ring gel formulation Rough, stiff and dry skin
texture. After 2 min skin was felt soft.
Age: 48
Sex: female
Left: applied gel base Skin was tingling. Open
pores and rough skin texture were not improved.
=
H2 Right: B ring Sensitive dry skin with roughness 1 min
after application, skin tingling sensation stopped. After 2 min, B ring-
applied side
Age: 26 and tingling, was felt more moisturized.
Sex: female
Left: applied gel base 1 min after application,
skin was still tingling. After 2 min, the subject felt that moisturizing
effect is weak.
H3 Right: Sensitive skin with tingling.
Age: 30
Sex: female
Left: applied gel base Skin was tingling and
temporally red. Skin desiccation was not improved. White scales
were observed.
01
0
cri
0
CA 02986086 2017-11-15
[0166]
As a result, all subjects felt tingling sensation at the site where the gel
base was applied, and dry
feeling of skin was not improved. Also, in all subjects, no effect (e.g.,
improved skin texture or
closed pores) was observed at the site where the gel base was applied. On the
other hand, in all
subjects, the skin area on which B ring gel formulation was applied has been
moisturized and
softened.
[0167]
4.2 Observation on mucosal condition upon application of B ring gel
formulation.
The B ring gel formulation was applied to normal subjects who had symptoms of
roughened lips, and
changes in mucosal condition were observed (Table below). The changes in
mucosal conditions were
determined based on physical sensory evaluation by the subject and our
objective observation as
physicians.
57
co
[Table 13]
Case Applied site: method Pre-application symptoms
1 Post-application symptoms
Ii B ring 1pmol Cracks on mouth corner with pain. Skin became
moisturized soon after application. After 2 min, cracks on mouth corner
Age: 20's 1 application Desiccation and wrinkles of lips, were
remitted. After 5 min, lips were soft and no longer felt dry or tingling.
Sex: female
12 B ring 0.5pmol Annoying lip desiccation. 3 min after
application, lips became moisturized.
Age: 30's 1 application
Sex: female
14 B ring 2pmol Annoying lip desiccation. After 2 min, lips
were full and soft, and wrinkles were less prominent. Moisturized.
Age: 30's 1 application
Sex: female
13 B ring 2pmol Lips were dry and tingling, with After 2
min, lips were full, soft and moisturized, and wrinkles were less prominent.
Age: 30's 1 application wrinkles and scales. Tingling sensation
stopped.
Sex: female
14 Right: B ring lpmol Cracks on mouth corner with pain. 2 min
after application, cracks on mouth corner were remitted with no more pain, and
Age: 20's 1 application Dry and roughened lips. moisturized.
Sex: male
Left: gel base 2 min after application,
cracks on mouth corner and pain were not improved.
1 application
0
0
co
0
CA 02986086 2017-11-15
[01691
Accordingly, it was confirmed that the cyclic peptide of the invention has an
activity of improving
mucosal condition and it also has an immediate effect in such activity.
[01701
4.3 Observation on scalp/hair condition upon application of B ring gel
formulation.
The B ring gel formulation was applied to normal subjects who had symptoms of
scalp/hair, and
changes in conditions of scalp and hair were observed (Table below). The
changes in scalp/hair were
determined based on physical sensory evaluation by the subject and our
objective observation as
physicians.
59
[Table 14]
Case Applied site: method Pre-application symptoms
Post-application symptoms
J1 B ring 0.5pmol Presented with desiccation and After 2
min, itches were removed, scalp was relieved from roughness and desiccation
Age: 40's 1 application itches of head. and moisturized. After
that scalp was prevented from desiccation and kept moisturized.
Sex: male
J2 B ring lpmol Presented with erythema and itch Hair growth
was stimulated, and an increase in hair volume was observed, and thinning
Age: 50's 1 application/day, 3 weeks of head, and thinning and falling
of of hair was improved. Hair was provided with moisture, flexibility and
radiance.
Sex: female hair.
J3 B ring 1 pmol Presented with thinning and falling A decrease
in falling hair was observed, showing an effect of preventing alopecia. Hair
Age: 40's 1 application/day, 5 days of hair, growth was
stimulated, and an increase in hair volume was observed, and thinning of
Sex: female hair started to be improved. Hair was provided with
moisture, flexibility and radiance,
,
J4 B ring 2pmol Presented with seborrheic dandruff After 2 min,
itches were removed, scalp seborrhea and dandruff started to be improved.
Age: 40's 1 application and itches. After that scalp became
and was kept clean.
Sex: female
--i
J5 B ring 2pmol Presented with desiccation, mild After 1
min, itches were removed. After 2 min, scalp seborrhea and dandruff started to
be
Age: 20's 1 application erythema and itches. improved. After 4 min,
erythema and skin roughness of scalp was improved. After that
Sex: male skin roughness was
prevented. P
J6 B ring 2pmol Presented with scalp seborrhea,
After 2 min, itches were removed,
scalp seborrhea was improved. After that dandruff was 0
Age: 60's 1 application dandruff and itches. removed, scalp became
healthy and clean.
0
Sex: male
.
0
0
..,
m J7 Bring 1pmol From the beginning of 5th month of Upon being
applied to alopecia 5 month post partum, an effect was exhibited on hair loss
o1.,
Age: 30's To decalvant site on head
3rd pregnancy, significant hair loss
in eyebrows and head where hair growth was stimulated, improving thinning of
hair.
1-
...]
Sex: female and brows of subject:
in eyebrows, lashes and scalp i
1-
1 application/day post occurred, loosing almost all hair.
1-
i
partum, continued
1-
u,
CA 02986086 2017-11-15
[0171]
Accordingly, BNP cyclic peptide of the present invention can provide and keep
scalp and hair with
moisture, give them moderate water and oil, improve and prevent desiccation,
and clean scalp and
hair. It is further effective for suppressing itch and dandruff of scalp and
also effective for
preventing thinning or loosing hair, for growing hair and promoting or
stimulating hair growth, and
forcing hair growth, and furthermore effective for an improvement in alopecia
after illness or
postnatal alopecia.
[0172]
4.4 Observation on skin condition upon using bath agent comprising B ring
Subjects were given bath in 37 to 41 C hot water comprising B ring dissolved
at 0.01 pM (100 ml of
20 pM B ring solution dissolved in 200 L hot water) once a day for 14 days,
and skin condition of the
subjects was compared to the case of hot water alone (Table 14). The changes
in skin conditions
were determined based on physical sensory evaluation by the subject and our
objective observation
as physicians.
61
75
,¨,
--1
co
[Table 15)
Pre-application symptoms (miliaria,
Case Treatment Post-application
symptoms Changes in subjective symptoms
cracks, chaps and eczema)
K1 B ring addition Eczema Gradual remission
of erythema and Itches after bath was remitted. Skin was
Age: 30's skin desiccation.
moisturized.
Sex: female
K2 Hot water Eczema Flare and desiccation
exacerbated. Enhanced itches and desiccation after bath.
Age: 30's
Sex: female
K3 BNP addition Eczema No remission of skin
desiccation or No subjective remission of skin desiccation at this
Age: 30's erythema.
concentration.
Sex: female
K4 B ring addition Chaps,
rough skin of hands, itches Remission of desiccation. Remission of itch and
pain. Skin became smooth.
Age: 60's with tingling
Sex: female
K5 Hot water Chaps and rough skin of hands
Exacerbation of desiccation. Exacerbation of itches and tingling after some
Age: 60's time
after bath. P
Sex: female
0
1.,
,
K6 B ring addition Miliaria and itches
Remission of flare and miliaria. Remission of itches and
improvement in skin 0
0
0
Age: 20's
texture. 00
0
cn Sex: male
11ND
Iv
.
0
K7 Hot water Miliaria and itches No improvement in
miliaria. No remission of itches. 1-
...3
1
Age: 20's
1-
1-
Sex: male
1
1-
u,
K8 B ring addition Cracks and pain on sole
Cracks gradually became Relief of pain. Skin was moisturized.
Age: 40's shallower and improved upon
Sex: female application.
K9 Hot water Cracks and pain on sole No
improvement in cracks. No remission of pain.
Age: 40's
Sex: female
CA 02986086 2017-11-15
[0174]
Accordingly, it was confirmed that the bath agent comprising B ring of the
present invention has an
ameliorating effect on miliaria, cracked or chapped skin, and also an
improving effect on eczema.
Furthermore, it was shown to improve desiccation, pain and itch of the skin,
indicating that it is
effective for improvement of skin condition.
[0175]
4.5 Observation on conditions of scalp and hair or skin upon using hair
cosmetics (shampoo,
rinse) or body soap comprising B ring
[0176]
4.5.1 A shampoo comprising B ring was mixed and prepared in composition as
follows.
[Table 16]
Name of ingredient (tradename) Content
(%)
A Sodium cocoylmethyltaurine solution (NIKKOL CMT-30) 10.0
Sodium laureth lactate (NIKKOL SBL-2N-27) 20.0
Lauryl betaine solution (NIKKOL AM-301) 10.0
Cocamide DEA 4.0
Antiseptic q.I.
B Citric acid 0.1
Propylene glycol 2.0
Guar hydroxypropyl trimonium chloride 0.5
Water to fill up 100.0
C Water, B ring (B ring concentration 1pM) 1mL
A and B were dissolved with heat at 70 C. To A, B was added, stirred and
mixed. C was added at
40 to 35 C while being further stirred, and allowed to cool to room
temperature as kept being stirred.
[0177]
4.5.2 A conditioner comprising B ring was mixed and prepared in composition
as follows.
63
CA 02986086 2017-11-15
[Table 17]
Name of ingredient (tradename) Content
( /0)
A Pentylene glycol 1.50
BG 3.50
Glycerin 1.00
Methyl paraben 0.20
Carbomer 0.20
EDTA-2Na 0.10
Water to fill up 100.0
B Composite emulsifier (NIKKOL Nikkomulese LC) 4.00
Methyl heptyl laurate 3.50
Squalane 0.50
Cetearyl alcohol 1.50
Macadamia nut oil 0.50
Avocado oil 0.50
Shea oil 0.50
Propyl paraben 0.10
C Water, B ring (B ring concentration 1pM) 1mL
A and B were dissolved with heat at 80 C. Then A was stirred by a homomixer
while B was
gradually added thereto, and the mixture was emulsified. C was further added
to the mixture, and
the mixture was allowed to cool to 35 C as kept being stirred.
[0178]
4.5.3 A body soap comprising B ring was mixed and prepared in composition
as follows.
64
CA 02986086 2017-11-15
[Table 18]
Name of ingredient (tradename) Content
( /0)
A Cocoyl glutamic acid TEA solution 30.0
Sodium trideceth-4 carboxylate (KIKKOL ECTD-3 NEX) 5.0
Sodium cocoamphoacetate solution (KIKKOL AM-101) 10.0
PEG-50 hydrogenated castor oil (KIKKOL HCO-50) 0.5
1,3-butylene glycol 5.0
Antiseptic q.I.
B EDTA-2Na q.I.
Water to fill up 100.0
C Water, B ring (B ring concentration 1pM) 1 m L
A and B were dissolved with heat at 80 C. Then B was added to A while being
stirred. C was
further added to the mixture at 40 to 35 C while kept being stirred, and the
mixture was allowed to
cool to room temperature as kept being stirred.
[0179]
Subjects used shampoo, rinse or body soap prepared as above once a day for 14
days, and resulted
conditions of scalp and hair or skin of the subjects were assessed (Table
below). Changes in skin
condition was assessed by dandruff of scalp. Changes in erythema was assessed
by visual
observation by the subject. Itches, moisturized feeling, combing, glow,
resilience and elasticity of
hair were assessed by subject's physical sensory evaluation.
[Table 19]
Case Treatment Pre-application symptoms
Post-application Subjective changes
symptoms
L1 shampoo/treatment Presented with erythema and Remission of
scalp Improvement in itches. Hair was
Age: 60's itches on scalp, and thinning erythema and a
decrease moisturized and provided with
Sex: male and falling hair. in hair falling,
resilience, radiance and elasticity.
L2 shampoo Presented with desiccation
Improvement in dandruff Improvement in itches. Hair was
Age: 20's and dandruff on scalp and desiccation of
scalp. moisturized and provided with
Sex: female accompanied with itches
resilience, radiance and elasticity.
Improved. With better combing.
L3 body soap Dry and sensitive skin. Improvement in dry
skin, Improvement in itches after bath.
Age: 60's relief of itching after
bath.
Sex: female
L4 body soap Dry and sensitive skin. Improvement in dry
skin, Improvement in itches after bath.
Age: 60's relief of itching after
bath.
Sex: female
L5 body soap Dry skin. Improvement in dry skin.
Skin was moisturized after bath.
Age: 20's
0-3
cn Sex: male
u,
CA 02986086 2017-11-15
Accordingly, the use of the hair agent comprising B ring of the present
invention had an fast-acting
improving effect on itch, erythema and desiccation of scalp. Moreover, it was
shown to be effective
in improving symptoms of thinning and falling hair when it was used
continuously for at least 2
weeks. Furthermore, it was shown that the use of the body soap comprising B
ring of the present
invention has an improving effect on dry or sensitive skin.
[01801
5. Confirming therapeutic effect on alopecia
Either B ring gel formulation or BNP gel formulation was applied, and their
effects on symptoms
associated to various alopecia were observed (Table below). The changes in
symptoms were
determined based on physical sensory evaluation by the subject and our
objective observation as
physicians.
67
oz
[Table 20]
Case Treatment Disease Post-application symptoms
A21 Right: B ring 1umol Male alopecia 7 weeks
after application, hair became elastic and thick, a drastic decrease in
Sex: male 1 application/day, continued. hair-falling.
Consequently, hair became dense and thinning less prominent. B
Age: 50's Left: BNP gel formulation ring-containing gel
formulation exhibited more remarkable hair-growing effect.
1 application/day, continued.
A5 8 ring gel formulation Male alopecia and
alopecia 2 weeks after starting applying B gel formulation, the existing
soft hair in parietal area
Sex: male 1 application/day onto head pityroides accompanied
with became thicker and longer and turned black.. Hair got resilient and
elastic. Newly grown
Age: 50's decalvant site, continued. dandruff. Previous use of
terminal hairs remarkably decreased thinning area. Dandruff was suppressed
overall,
minoxidil-containing drug caused particularly in parietal
area. Pruritus was eliminated.
strong irritation, itches and
erythema to the subject and its use
was terminated.
A22 B ring gel formulation Seborrheic alopecia
accompanied 2 weeks after application, thinning of hair was improved,
exhibiting no more
Sex: female 2 application/day onto with dandruff. showing-
through of scalp confirming hair-growing stimulation. Dandruff stopped, scalp
Age: 40's parietal decalvant site. seborrhea was improved
and pruritus was removed. Furthermore, scalp and skin
became clean.
0
01
00
0
00
[ND
[Table 21]
A10 B ring gel formulation Alopecia universalis.
Alopecia on 2 weeks after starting application, on both sides of head,
regeneration of pores and
Sex: male 0.5pmol whole head. Previous therapy with growth of
terminal hair were confirmed, which were more remarkable and hair growth
Age: 20's BNP gel formulation carpronium chloride and
steroid area was wider on the B ring gel formulation-applied right side.
Hair growth area kept
0.5pmol pulse first exerted hair increasing to the
whole head. The density, elongation rate and thickness of hair were
growth-stimulating effect, but greater on the B ring gel
formulation-applied right side. Upon increasing the
caused complete hair loss after 1 concentration of
application to lpmol, rate of growth and elongation were increased.
month.
All Right side of head decalvant Ophiasis and multiple alopecia.
Right forehead ophiasis site: 7 days after starting applying B ring gel
formulation, a hair
Sex: female site: B ring gel formulation growth stimulation effect
was observed and hair became thicker terminal hair,
Age: 40's 1 application/day for 1 week. hair-growing area kept
increasing.
1 or 2 applications/week for 1 Left BNP gel formulation-
applied site: few soft hair observed, whose growth was slow
year thereafter. and in obviously smaller
area.
Left side of head decalvant After continuing 1 or 2
applications/week for 1 year:
site: BNP gel formulation Right forehead B ring gel
formulation-applied site: hair-growing area kept increasing and
1 application/day for 1 week. hair elongation rate was
fast (Fig.2).
1 or 2 applications/week for 1 Left BNP gel formulation-
applied site: hair growth was observed though its area was
year thereafter, smaller as compared to
the right side, and majority of hairs was thin and soft (Fig.2).
01
0
01
01
CC)
0
oo
cc
[Table 22]
Al2 Head decalvant site: Alopecia areata 1 week after
application, remarkable stimulation and growth of terminal hair was
Sex: female B ring gel formulation
observed.
Age: 20's 1 application/day, continued.
Al2 Subject's head decalvant Alopecia areata 1 week after
application, remarkable stimulation, growth and elongation of terminal hair
Sex: female site: B ring gel formulation
was observed.
Age: 30's 1 application/day, continued_
A24 Right half of head decalvant Drug-induced alopecia Pre-application:
1 month after termination of anticancer agent therapy. Bilateral severe
Sex: female site: B ring gel formulation
diffuse alopecia on whole head. Thinning of hair on whole head. Hair was short
and thin,
Age: 50's (1pmol), 2 applications/day
mostly white.
for 2 weeks 1 day after application at B ring-
applied site, subjective hair elongation and growth
Left half of head decalvant stimulation were observed, and
grown hairs were predominantly black (non-white).
site: BNP gel formulation After 2 weeks, hair growth was
observed at both sides. Hair were elongated with less
(1pmol), 2 applications/day falling hairs. Hair elongation
rate is faster on B ring-applied site. Hairs are also thicker
for 2 weeks and denser, and contain more non-
white hairs as compared to BNP-applied site. 3
weeks after application, remarkable growth of terminal hairs was observed.
01
0
01
01
CA 02986086 2017-11-15
[01841
5.2 Case summary
In the cases of the subjects having female pattern alopecia, male pattern
alopecia, alopecia
universalis, ophiasis or alopecia areata multilocularis as described above, a
significant decrease in
falling hair was observed, and the area of terminal hair growth was expanded,
and the growth of the
terminal hair was also faster, when B ring gel formulation of the working
example was applied
compared with BNP gel formulation as a comparative control (See, Fig. 2).
Addingly, the existing
hairs obtained resilience and their elasticity was increased. In all cases,
symptoms were
significantly improved as compared to the cases when BNP gel formulation of
the comparative
example was applied. Accordingly, B ring gel formulation had superior effects
on the symptoms
described above as compared to BNP gel formulation.
In addition, the B ring gel formulation could improve seborrhea, clean scalp
and suppress dandruff.
It also prevents hairs from turning white, grows black hairs, and improves
thinning of hair.
Moreover, it exhibits an antipruritic effect within 10 minutes, demonstrating
an excellent immediate
effect. It could suppress ithes within 3 minutes after application in some
cases.
[0185]
6. Confirming therapeutic effect on rhinitis
6.1 Cases
Either ring nasal drop, BNP gel nasal drop was sprayed, and their effects on
rhinitis in the subject
was observed (Table 17). The changes in symptoms were determined based on
physical sensory
evaluation by the subject and our objective observation as physicians.
71
c:.
i-,
oo
cl')
[Table 231 _
Case Treatment Disease Post-application symptoms
(severity)
R1 Right nasal cavity: B ring Perennial chronic rhinitis
and Right nasal cavity (B ring nasal drop-treated side): Subject felt
breath goes through right
Sex: female nasal formulation 0.1m1, 1 rhinorrhea. 20 or more
blow/day nasal cavity soon after application. After 30 sec, air goes
through nasal cavity. After 2
Age: 20's spray mm, nose was clear and the
subject could breathe comfortably. After 3 min, felt fresh
and retained nasal discharge was removed. No discharge by blowing. A day after
application, no discharge from right nasal cavity.
Left nasal cavity (BNP nasal drop-treated side): 3 mm after application, the
discharge
still retained in nasal cavity and drained by blowing. A day after
application, mild nasal
Left nasal cavity: BNP nasal obstruction and rhinorrhea
symptoms in left nasal cavity with small amount of retained
formulation 0.1m1, 1 spray discharge.
R2 Right nasal cavity: B ring Rhinitis with severe nasal
Right nasal cavity (B ring nasal drop-treated side): Subject felt breath
goes through right
Sex: female nasal formulation 0.1m1, 1 obstruction and feeling
heavy, nasal cavity soon after
application. After 30 sec, obstruction was improved, subject's P
Age: 30's spray Right nasal cavity has severer
suffering in breathing was reduced in
right nasal cavity. After 1.5 mm, suffering in 0
1.,
obstruction symptom. The subject breathing was removed in
right nasal cavity and breath went though. A day after
00
feels dry and tingling by steroid application, still no
obstruction or rhinorrhea. The subject felt no irritation upon treatment
..,
0
nasal drops. in right nasal cavity.
00
tso Left nasal cavity (BNP
nasal drop-treated side): 30 sec after application, obstruction of
0
left nasal cavity was slightly improved, though the subject did not feel
breath goes 1-
...3
Left nasal cavity: BNP nasal through as in the right
cavity. A day after application, obstruction or rhinorrhea in left i
1-
formulation 0.1m1, 1 spray nasal cavity were removed.
The subject felt mild dryness in left nasal cavity. 1-
i
1-
R3 Right nasal cavity: B ring Perennial allergic rhinitis
with Right nasal cavity: Subject felt breath goes through nasal cavity 1
min after application.
Sex: female nasal formulation 0.1m1, 1 rhinorrhea and nasal
obstruction. After 3 min nasal obstruction was improved, the subject could
breathe through nose.
Age: 20's spray Right nasal cavity has severer After 5 min
the subject could breathe comfortably through nose, with no nasal discharge
obstruction with retention of nasal by blowing.
discharge.
F
,--
oo
¨I
[Table 24]
R4 Right nasal cavity: B ring Chronic rhinitis with
severe Right nasal cavity (B ring nasal drop-treated side): 1 mm after
treatment, nasal
Sex: female nasal formulation 0.1m1, 1 rhinorrhea and nasal
obstruction, obstruction was improved to some extent. After 2 min,
obstruction was improved, the
Age: 30's spray Application of steroid nasal drops subject
could breathe through nose as usual, and rhinorrhea stopped. After 3 mm, no
Left nasal cavity: BNP nasal could not improve symptoms when nasal discharge
from the right nasal cavity by blowing.
formulation 0.1m1, 1 spray they were severe. Left nasal cavity (BNP
nasal drop-treated side): 1 mm after treatment, nasal discharge
was retained in the left nasal cavity with no improvement in obstruction.
After 2 mm,
obstruction was improved to some extent, though discharge was retained,
showing
rhinorrhea symptom. After 3 min, nasal discharge was not drained by blowing
from left
nasal cavity due to obstruction. After 8 mm, nasal discharge was drained by
blowin..
R5 Right nasal cavity: B ring Allergic rhinitis with
rhinorrhea as Right nasal cavity (B ring nasal drop-treated side): 30 sec
after treatment, obstruction
Sex: female nasal formulation 0.1m1, 1 main symptom and mild
was improved and the subject could breathe through nose. After 2 min, the
subject
Age: 20's spray obstruction. could easily breathe
through nose. After 8 mm, no nasal discharge was drained by
Left nasal cavity: BNP nasal blowing from the right
nasal cavity. A day after treatment (after 26 hours), nasal
formulation 0.1m1, 1 spray discharge was controlled,
and breathing was easier through the right nasal cavity as
compared to the left.
Left nasal cavity (BNP nasal drop-treated side): 2 mm after treatment,
obstruction was
still observed. After 8 min, small amount of discharge was drained by blowing.
A day P
after treatment (after 26 hours), nasal discharge was controlled.
0
R6 Right nasal cavity: B ring
Allergic rhinitis with both
rhinorrhea Right nasal cavity (B ring nasal drop-treated side): nasal
obstruction was slightly "
Sex: female nasal formulation 0.1m1, 1 and nasal obstruction
having improved soon after treatment.
After 1 min, the subject could easily breathe though 00
..,
0
Age: 20's spray constant obstruction and severe
nose. After 4 mm, no nasal discharge
was drained by blowing from the right nasal cavity. 00
--) Left nasal cavity: BNP nasal rhinorrhea. 10-20
blow/day. Nasal obstruction was resolved. After 10 mm, pruritus was
removed. A day after
co
1.,
formulation 0.1m1, 1 spray Regular dose of oral anti-allergic
treatment (after 28 hours), no
obstruction or rhinorrhea was observed. 0
1-
...3
drug did not sufficiently improve Left nasal cavity (BNP
nasal drop-treated side): 1 min after treatment, nasal obstruction ,
nasal obstruction obstruction and rhinorrhea. was not improved. After 3
min, obstruction was still not improved and rhinorrhea was
Steroid nasal nasal spray causes observed. After 3 min,
small amount of discharge was drained by blowing. After 10 mm. .
u,
mucosa pain, obstruction was slightly
improved but not resolved. After 2 hours, obstruction was
resolved and the subject could breathe though nose. A day after treatment
(after 28
hours), no obstruction or rhinorrhea was observed.
oo
co
[Table 25]
R21 Right nasal cavity: B ring Rhinitis with continuous rhinorrhea
Right nasal cavity (B ring nasal drop-treated side): 30 sec after treatment,
the subject
Sex: female nasal formulation 0.1m1, 1 and sneeze. Nasal
discharge was could easily breathe through nose. After 1 min rhinorrhea
stopped. No nasal discharge
Age: 20's spray drained by facing down, by
facing down.
R8 Right nasal cavity: B ring Chronic allergic rhinitis (with
both Right nasal cavity (B ring nasal drop-treated side): 1 min after
treatment, obstruction
Sex: female nasal formulation 0.1m1, 1 rhinorrhea and
obstruction). The was improved and the subject could easily breathe through
nose. The subject felt no
Age: 20's spray +1 spray 20 min later subject regularly
feels nasal irritation by treatment. The effect lasts for 1 week
thereafter, and rhinorrhea and
Left nasal cavity: BNP nasal obstruction. Application of
steroid obstruction were controlled. No nasal discharge by blowing.
formulation 0.1m1, 1 spray +1 nasal formulation causes the Commercial
steroid nasal formulation caused a sharp irritation and recurrence of
spray 20 min later subject strong irritation. symptoms within 2
hours.
Accompanied with rhinorrhea and Left nasal cavity (BNP nasal drop-
treated side):
eye pruritus.
R9 Right nasal cavity: B ring Allergic rhinitis (with
rhinorrhea). Right nasal cavity (B ring nasal drop-treated side): 2 min
after treatment, the subject
Sex: female nasal formulation (1pmol) The subject has
rhinorrhea all day could breathe through nose, with no irritation in the
right nasal cavity. After 4 min the
Age: 30's 0.1m1, 1 spray and usually blows 20 or more times
subject could breathe through nose, with no nasal discharge drained.
Left nasal cavity: BNP nasal a day. Application of steroid
nasal Left nasal cavity (BNP nasal drop-treated side): 2 min after
treatment, obstruction was
formulation (1pmol) 0.1m1, 1 formulation causes the subject
improved and the subject could breathe through nose. After 4 min, in the left
nasal
spray strong dry feeling on mucosae of cavity, the
subject drained small amount of nasal discharge by blowing.
nasal cavity and pharynx. The In the right nasal cavity, the
effect of the nasal treatment lasted for 4 hours. Blowing 0
effect of the steroid nasal caused obstruction in the left
nasal cavity but not in the right.
00
formulation lasts only 3-4 hours, The subject felt that B ring
nasal formulation is much better to the steroid nasal
0
and after the effect ended, formulation regarding the effect
on the symptoms, long-lasting and fast-acting effect,
sneezing and nasal discharge last lower frequency of use required, and absence
of irritating symptom upon use.
0
for several hours and need
frequent blowing.
1¨=
co
[Table 26]
R10 Right nasal cavity: B ring Allergic rhinitis with rhinorrhea
and Right nasal cavity (B ring nasal drop-treated side): 2 min after nasal
treatment,
Sex: male nasal formulation (1pmol) obstruction complicated
with obstruction was improved and the subject could breathe through nose.
The subject
Age: 30's 0.1m1, 1 spray rhinosinusitis. Obstruction in the could
breathe more comfortably in the right nasal cavity as compared to the left.
The
Left nasal cavity: BNP nasal right nasal cavity due to subject felt no
irritation in the right nasal cavity. After 4 min, the subject could very
easily
formulation (1pmol) 0.1m1, 1 rhinosinusitis is severer than the breathe,
with no nasal discharge drained by blowing.
spray left and could not be improved by Left nasal
cavity (BNP nasal drop-treated side): 4 min after nasal treatment, nasal
steroid nasal formulation, making obstruction was not
resolved. Nasal discharge was decreased as compared to
the subject incapable of breathing pre-treatment, though
draining was still observed.
through nose. If left untreated,
nasal discharge is drained from
nostrils spontaneously.
R11 Right nasal cavity: B ring Perennial rhinitis with
rhinorrhea Right nasal cavity (B ring nasal drop-treated side): 2 min after
nasal treatment, the
Sex: male nasal formulation 0.1m1, 1 and obstruction complicated
with subject could breathe through nose. After 5 min, nasal discharge
stopped, enabling the
Age: 20's spray rhinosinusitis, having severe subject to breathe
though nose. The subject felt no obstruction and was able to breathe
Left nasal cavity: BNP nasal obstruction. Very high frequency of through nose
throughout 1 week after nasal treatment.
formulation 0.1m1, 1 spray blowing. These symptoms were not Left nasal
cavity (BNP nasal drop-treated side): 5 min after nasal treatment, obstruction
improved by any previously used was not resolved, with
nasal discharge present in the left nasal cavity. After 10 min,
nasal formulation, obstruction was not
resolved, making the subject incapable of breathing through nose. 0
0
cn
0
CA 02986086 2017-11-15
[0190]
6.2 Case summary
In all cases described above, both rhinorrhea and nasal obstruction were
quickly improved or
eliminated when B ring nasal drop of the working example had been applied as
compared to the case
when BNP nasal drop of comparative example had been applied. Accordingly, B
ring nasal drop had
faster effect than BNP nasal drop. Addingly, the effect of B ring nasal drop
was more than equal to
that of BNP nasal drop, and because the effect lasted for longer time period,
it can suppressing
recurrence of symptoms.
[0191]
The following table summarizes information from the cases described above,
within the range
recognizable at the time when symptoms of nasal obstruction and rhinorrhea
were improved.
[Table 27]
Resolution of rhinorrhea
No
within within within resolution
3 min 5 min 10 min within 10
min
B ring nasal formulation 3 4 1 0
BNP nasal formulation 0 0 0 7
Resolution of nasal obstruction
No
within within within resolution
3 min 5 min 10 min within 10
min
B ring nasal formulation 6 5 0 0
BNP nasal formulation 0 0 0 8
[0192]
7. Analysis of binding state of human BNP and Type A receptor
The superior pharmacological effects of the B ring-compound of the invention
on various diseases
relative to conventional BNP has been explained so far with reference to data
based on respective
clinical cases. Such pharmacological effects is also supported by the result
of the conformation
analysis of the compound performed by the inventors, which is explained by
following experimental
report for reference.
In order to investigate binding state of human BNP (BNP-32) and its receptor
Type A receptor
(NPR-A), an in silico analysis was performed by homology modeling using
conformation. Swiss-Pdb
viewer and SWISS-MODEL were used for modeling.
76
CA 02986086 2017-11-15
[0193]
7.1 Template structure
Firstly, prior to the above analysis, a template structure for investigation
on binding state of human
BNP and Type A receptor was selected. As this template structure, the
conformation of the complex
of rat NPR-A and rat ANP peptide (PDB ID: 1T34) were used. Rat NPR-A is a
homodimer composed
of A and B strands. The conformation of such rat NPR-A has been determined by
X-ray crystalline
structural analysis such that 21 residues from Cys7 to Arg 27 of rat ANP were
bound. The
conformation of rat NPR-A was obtained from the database of protein
conformation, Protein Data
Bank. Amino acid homology between human NPR-A and rat NPR-A is 85%.
[0194]
7.2 BNP peptide model
In the present study, as BNP peptide model, a resion from Cys10 to Arg30 in
human BNP (BNP-32,
SEQ ID NO: 13,
Ser-Pro-Lys-Met-Val-Gln-Gly-Ser-Gly-Cys-Phe-Gly-Arg-Lys-Met-Asp-Arg-Ile-Ser-
Ser-Ser-Ser-Gly-Le
u-Gly-Cys-Lys-Val-Leu-Arg-Arg-His), i.e., an amino acid sequence (SEQ ID NO:
14,
Cys-Phe-Gly-Arg-Lys-Met-Asp-Arg-Ile-Ser-Ser-Ser-Ser-Gly-Leu-Gly-Cys-Lys-Val-
Leu-Arg) was used.
In the peptide model above, the amino acid sequence of B ring of the invention
corresponds to a
resion from Cys10 to Cys26 of human BNP above, i.e.,
Cys-Phe-Gly-Arg-Lys-Met-Asp-Arg-Ile-Ser-Ser-Ser-Ser-Gly-Leu-Gly-Cys (SEQ ID
NO: 15).
[0195]
7.3 Homology modeling
In order to speculate the amino acid residues that are involved in binding of
human NPR-A and
human BNP, a complex model in which BNP peptide is bound to human NPR-A was
constructed by
homology modeling. Specifically, human BNP peptide model was constructed based
on the template
structure of rat ANP peptide, and human NPR-A model structure based on the
template structure of
rat NPR-A.
Next, residues that are involved in the interaction was speculated from the
amino acid residues
detected between human BNP peptide model and human NPR-A.
[0196]
The results showed that BNP peptide model is bound to human NPR-A dimer being
sandwiched
between the A strand and B strand. Amino acids in the amino acid sequence,
e.g., the amino acid
sequence used as the model (SEQ ID NO: 14,
Cys-Phe-Gly-Arg-Lys-Met-Asp-Arg-Ile-Ser-Ser-Ser-Ser-Gly-Leu-Gly-Cys-Lys-Val-
Leu-Arg) are
expressed as "amino acid (number)". Namely, the number in parentheses denotes
the relative
77
CA 02986086 2017-11-15
position counted from the N-terminal of the amino acid sequence, for example,
Phe2 indicates an
amino acid that is the second Phe counted from the N-terminal of the peptide
model.
[0197]
In the constructed complex model, the presence of a hydrophobic bond by Phe2
side chain, a
hydrogen bond by Phe2 main chain, and hydrogen bonds by side chains of Arg4,
Met6, Arg8, Ser10
and Serll were speculated between BNP peptide model and the A strand of NPR-A.
Phe2, Arg4,
Met6, Arg8, Ser10 and Serll in BNP peptide model correspond to Phell, Arg13,
Met15, Arg17, Ser19
and Ser20 in human BNP respectively. Namely, this result suggests that these
amino acid residues
in human BNP is likely to be the residues that contribute to NPR-A activation.
[0198]
7.4 Discussion
Thus, by in silico conformation analysis, it was speculated that, in human
BNP, the amino acid
residues that are present in its cyclic part are likely to contribute to NPR-A
activation, whereas the
amino acid residues present in the tail part are not. It was also suggested
that BNP cyclic structure
is a smaller molecule than BNP-32 and therefore capable of binding easily and
quickly. In fact, a
clinical application of a peptide having BNP cyclic structure confirmed that
BNP cyclic structure
provides faster therapeutic effect than BNP-32. This clinical result is
consistent with that of the in
silico analysis that BNP cyclic structure contributes to NPR-A activation and
can bind to it more
easily and quickly than BNP-32. Thus, BNP cyclic structure has a superior
therapeutic effect than
a general BNP peptide, and therefore is a different substance.
[0199]
On the other hand, an NMR analysis revealed that ANP does not take any
particular conformation in
a solution where its conformation greatly wobbles; this is considered to be
similar for BNP. Namely,
in human BNP, assuming that the amino acid residues in the cyclic part
contribute to its binding to
human NPR-A, it is speculated that the amino acid residues in the tail part
rather prevent human
BNP from entering into the narrow BNP binding site sandwiched between A and B
strands of human
NPR-A due to its large wobbling. Therefore, it is considered that the cyclic
part of human BNP is a
relatively smaller molecule than conventionally known human BNP, enabling
itself to enter into
BNP binding site of NPR-A more easily and quickly. Moreover, it is speculated
that the the cyclic
part of human BNP has a higher affinity to human NPR-A than BNP-32. This
supports the clinical
outcomes described herein that the peptide having only the cyclic part of BNP
exhibits therapeutic
effect faster than BNP-32.
[0200]
In order to speculate the effect of BNP cyclic structure (B ring) from non-
human species on human
Type A receptor, complex models of human NPR-A and BNP rings from pig, bird or
rat were
78
CA 02986086 2017-11-15
generated to surmise interaction. The results suggested that a sufficient
effect of the invention can
be expected by using BNP ring from non-human animal species (e.g., pigs, birds
or rats), as long as it
shows an affinity to human NPR-A.
[0201]
8. Speculating replaceable amino acid residues in BNP cyclic moiety
Using the constructed model structure of the complex, replaceablity of amino
acid residues other
than those considered to be involved in the interaction was investigated.
Specifically, in order to investigate whether the peptide in which amino acid
residue that is not
assumed to be involved in the interaction has been replaced with another amino
acid is capable of
binding to NPR-A, a mutant model of BNP was generated to analyze the
interaction. Swiss-Pdb
viewer was used for modeling.
[0202]
Specifically, amino acid residues G1y12, Lys14, Asp16, I1e18, Ser21, Ser22,
Gly23, Leu24 and G1y25
in human BNP were targeted for the investigation on their replaceability with
other amino acids in
terms of following points:
= No steric hindrance caused by binding to NPR-A. No interatomic collision
observed in the
model structure of NPR-A and BNP.
= No influence on electrostatic potential on surface.
= No large increase in intramolecular energy value (no unnatural angle or
twist caused in
intermolecular binding).
= No non-naturally occurring hydrogen bond formed between the strands of
NPR-A and BNP
and within BNP strand.
= No cavity (cavity, niche) formation.
[0203]
Among those described above, intramolecular energy was calculated by
ComputeEnergy command of
Swiss-Pdb viewer. Intramolecular energy was calculated from the sum of the
length of binding,
bond angle, twist and binding energy, etc. in the unit kilojoule/mol (Kj/mol).
[0204]
The results of analysis indicated the presence of replaceable amino acid
residues in Gly12, Lys14,
Ile18, Ser21, Ser22, Leu24 and G1y25 among the amino acid residues of human
BNP (Table below).
79
CA 02986086 2017-11-15
[Table 28]
human BNP Replaceable amino acid
G1y12 Ala, Val, Ser, Thr
Lys14 Arg
Asp16 none
11e18 Val
Ser21 Thr, Ala, Val, Gln, Leu, Ile, Met
Ser22 Thr, Ala, Val
Gly23 none
Leu24 Ala, Val, Ile, Met
Gly25 Ala, Ser
[02051
Accordingly, even when the replacement of the amino acid corresponding to
those described in the
table above took place in the cyclic part of human BNP, i.e., the peptide
expressed by the Formula I-a,
it was suggested that the peptide replaced in such a way exerts a similar
effect as the peptide
composed of the amino acid sequence expressed by Formula I-a.
CA 02986086 2017-11-15
[02061
9. Confirming therapeutic effect of the cyclic peptide with amino acid
replacement
Next, cyclic peptides with some replaced amino acid were subjected to
following
examination in order to confirm their effect. The methods for preparing the
above
cyclic peptide and confirming amino acid sequence of the prepared peptide were
same as
the method for preparing the cyclic peptide as described above and Mass
spectroscopy
method.
[02071
9.1 Confirming effects on scalp or hair
The obtained cyclic peptide was prepared in purified water at 3, 15, 30, 100
or 500 pg/ml
and applied to the affected site. Each of formulations A to E in the table
indicates the
concentration of cyclic peptide at 3, 15, 30, 100 or 500 pg/ml, respectively.
The absence
of the effect upon the application of purified water confirmed the effect was
not a
placebo effect.
81
=
1-3 o
w tsD
cr o
1¨. co
CD
t=D
CO
SEO ID NO (amino acid
sequence) of the cyclic ID Sex Age Cases Formulation
Treatment Diagnostic impression of hair and scalp
after application 1-.1
peptide used
After 1 day, fluffy hairs started to grow.
Si mute 5o,s multiple alopecia After 3
days, obviously Hair started to grow, restoration and elongation were
C once a day
areata observed.
Hair gained elasticity and resilience, thickened. Hair root regeneration
confirmed.
Idles were remitted immediately after application.
After 3 minutes, seborrhea and erythema remitted.
seborrheic dermatitis
S2 female 40's C once a day
After 1 day, dandruff, redness and seborrhea of scalp improved, falling hairs
male AGA
reduced.
After 7 days, hairs stimulated to grow, restored hair and hair growth
confirmed.
SE) ID NO is S3 female 50's female AGA C once a
day After 1 day, hair gained elasticity, resilience and increased volume.
(CFVRKMDRISSSSGLGC) hair thinning after
After 5 days, hair stimulation and growth confirmed. Hair became dense and
S4 female 50's anticancer agent C
once a day
thick.
P
therapy
o
Iches were improved immediately after application.
n,
After 1 day, fluffy hairs started to grow.
.
multiple alopecia
Ix,
S5 female 20's C once a day
After 3 days, obviously
Hair growth stimulated and restored elongation of hair o,
areata
o
observed, hair gained elasticity and resilience, thickened. Hair root
regeneration 00
CO observed.
o,
CO After 1
day, hair became fuller with increased volume. Hair gained elasticity and
iv
o
S6 female 60's healthy skin (scalp)
C once a day
resilience. After 7 days, grown hairs were predominantly black over white
hairs. r
...1
White hairs tamed black.
1
.
r
multiple alopecia
r
S7 male 40's B once a day
After 3 days, remarkable
terminal hair growth was observed. I
areata
r
.
.
. .
= . in
S8 female 20's multiple alopecia B
once a day After 5 days, newly born hair roots and grown hairs were
observed.
areata
SEO ID NO 17:S9 male 30's male AGA B once a day
After 2 or 3 days, hair gained elasticity and resilience.
(CFGOKMDRISSSSGLGC) After 7
days, hair gained volume, restoration was confirmed.
Hair gained elasticity and resilience, hair volume was increased on the night
of
first application.
S10 female 30's female alopecia C
once a day
After 5 days, appearance of scalp through hair were improved.
The effect lasted for 1 week after stopping application.
1-3 0
5n
IND
C
I¨ C.C)
IND
C.0
S11 male 50's male alopecia B once a
day After 1 week, hair gained thickness and elasticity . hair density was
increased.
LND
After 1 day, hair became fuller with increased volume. Hair gained elasticity
and
SEQ ID NO 18: S12 male 70's healthy skin (scalp) C
once a day resilience. After 7 days, grown hairs were predominantly black
over white hairs.
(CFGHKMDRISSSSGLGC) White hairs
turned black.
S13 female 40's alopecia areata B once
a day After 5 days. hair started to grow, elongation and growth of hair
were observed.
S14 female 50's alopecia areata B once
a day After 5 days, hair started to grow, hair density was increased.
multiple alopecia After 5
days, hair started to grow, elongation and restoration of hair were
515 female 20's B once a day
areata observed.
hair thinning after
S16 female 50's anticancer agent C once
a day After 2 days, hair gained elasticity and resilience. volume of hair
increased.
therapy
SEQ ID NO 19:
(CFGRRMDRISSSSGLGC) S17 male 50's male AGA C once a day
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started
to grow, restoration and elongation were observed.
518 female 40's female alopecia D
once a day After 3 days,
scalp shown through hair due to thinning got obsecure. Reduced P
falling hairs.
0
N)
scalp stlb
)0
sorrfieic
03
S19 female 40's C once a day
After 3 days, seborrhea and
erythema were improved, so was dandff. o,
de
ru o
is
00 SEQ ID NO 20: 520 male 50's male AGA
C once a day After 1
day, hair gained elasticity, resilience and increased volume. is
COiv
(CFGRKLDRISSSSGLGC) multiple alopecia
o
S21 male 40's C once a day
After 3 days, hair density
was increased. Hair started to grow and grew.. 1-)
areata
...]
I
S22 male 40,a amruelatitpale alopecia
C once a day
After 3 days, hair started
to grow at the site of alopecia areata and grew. 1-)
1-)
1
1-)
= =
1 application/day on After 3 days,. hair started to gain
resilience, felt thick. = or
S23 male 40's male alopecia C parietal
male thinning After 7 days, parietal thinning were improved and hair volume
started to increase
area
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started
SEQ ID NO 21: 524 female 40's female AGA C once a
day
to grow, restoration and elongation were observed.
(CFGRKIDRISSSSGLGC)
multiple alopecia After 1
day, hair started to grow.
S25 female 30's areata C once a day
After 2 weeks, obvious lots of hairs started to grow, restoration,
elongation and
growth were observed.
After 2 days, stimulated hair growth was confirmed.
S26 female 10's multiple ophiasis C
once a day After 7 days, obvious stimulated hair growth was observed,
elongated and grown
hair was also observed.
1-3 CD
LND
ZS'
CD
ts.D
02
CD
1 application/day on
$27 male 40's male AGA B forehead thinning
After 2 or 3 days, hair gained elasticity and resilience. After 7 days, hair
gained
CO
volume, restoration was confirmed.
area
$28 male 20's multiple alopecia
C once a day After 3 days,
hair started grow and grew.
areata
S29 female 50's female AGA C once a day
After 1 day, hair gained elasticity, resilience and increased volume.
SEQ ID NO 22:
(CFGRKMDRVSSSSGLGC) S30 female 70's female alopecia C once a day
After 7 days, increased hair volume. Appearance of scalp shown through hair
got
obsecure.
S31 female 20's ophiasis B once a day After
5 days, stimulated hair confirmed along intractable hairline, and elongation
of hair observed.
After 1 day, hair started to grow.
S32 tamale multiple alopecia
C once a day After 6 weeks,
obvious stimulated hair growth, elongation and growth of hair were
areata
observed.
alopecia areata
S33 male 30's C once a day After 3 days,
stimulated and elongated hairs were observed.
universalis
hair thinning after
$34 female 50's anticancer agent C once a day
After 1 day, hair gained elasticity and resilience,
therapy
S35 female 50's female AGA C once a day
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started
to grow, restoration and elongation were observed.
or
00 1 application/day on
or
After 5 days. hair gained elasticity, resilience and increased volume.
Appearance Ia
SEO ID NO 23: S36 female 40's female alopecia
B parietal and forehead 0
(CFGRKMDRIGSSSGLGC) thinning area of scalp
shown through hair got obsecure.
After 1 day, stimulated hair was observed. After 3 days, application was
stoped,
S37 male are arnruelatitpale alopecia
C once a day but stimulation
and elongation of hair contitued. White hairs turned black again,
black hairs started to grow.
S38 female 40's
female alopecia B once a day After 7 days,
hair gained volume, appearance of scalp shown through hair got
seborrheic dermatitis obsecure. Seborrhea
of scalp was improved.
sn female 10's ophiasis B once a day After
4 days, regardless of highly intractable ophiasis, stimulated soft hairs were
observed.
1-3 0
pp
ts.
ts.
c..0
840 female 10's alopecia areata A
once a day After 7 days, stimulated and grown soft hairs were observed.
4
841 female 50's female AGA C once a day After 1 day,
hair gained elasticity, resilience and increased volume.
SEQ ID NO 24:
(CFGRKMDRISASSGLGC) 043 male 30,s multiple alopecia
B once a day After 4
days, stimulation and elongation of hair observed.
areata
543 female 30,s multitple alopecia
After 1 day, hair started to grow. A numbe of stimulated, elongated and grown
C once a day
hairs were observed in 3 weeks,
multiple alopecia
S44 male 30's B once a day After 5 days, hairs started
to grow and grew.
areata
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started
845 male 50's male AGA C once a day
to grow, restoration and elongation were observed.
SEQ ID NO 25: After 5 days,
hair root regeneration and hair growth stimulation were observed.
(CFGRKMDRISSCISGLGC) 546 female 60's multiple alopecia
B once a day
Application continued and a remarkable restoration, elongation and growth of
hair
areata
were observed. Hair elongation rate was fast.
P
After 1 day, hair gained elasticity and resilience. Application continued for
3 days
847 female 40's female alopecia
C once a day 0
and stopped, but hair kept elasticity and resilience. The effect lasted for 1
week. ND
tr,
00
o,
After 3 days, appearance of scalp shown through hair got obsecure. hair gained
0
S48 female 30's female alopecia C once a day
elasticity, resilience and increased volume. Hairs were restored.
00
o,
CO
N)
549
S49 male 30's male alopecia
B once a day After 5 days, volume of hair increased. Hair
gained elasticity and resilience. 0
/
...1
Volume of hair increased and scalp does not show through after 3 days. The
I
S50 female 20's female AGA
C once a day r
effect went on.
r
SEQ ID NO 26:
I
/
After 1 day, hair gained elasticity, resilience and increased volume, Hair
started in
= = (CFGRKMDRISSVSGLGC) S51
female 60's female AGA C - once a day =
to grow, restoration and elongation were observed.
multiple alopecia After 3 days,
stimulated hair was observed.
S52 male 20's C once a day
areata After 7 days,
futther stimulation and growth of hair were confirmed.
553 female 30,s multiple alopecia
C once a day After 1
day, hair started to grow. Terminal hairs started to grow, and restoration,
areata elongation and
growth of hair were observed in 2 weeks,
hair thinning after
S54 female 50's anticancer agent B once a day After 5
days, hair got thick and dense.
therapy .
SEQ ID NO 27: After 1 day,
hair gained elasticity, resilience and increased volume. Hair started
(CFGRKMDRISSLSGLGC) S55 male 50's male AGA C once a day
to grow, restoration and elongation were observed.
556 male 20's
multiple alopecia E once a day After 3
days, hair started to grow. After 7 days, stimulation and growth of hair
areata were
confirmed.
1-3 CD
LND
CD
(.0
S57 female 40's female AGA C once a day
After 1 day, hair gained elasticity, resilience and increased volume.
After 1 day, hair falling was reduced and hair started to grow.
multiple alopecia
C once a day After 7 days,
remarkable stimulation, elongation and growth of hair were
558 male 20's areata
confirmed,
After 1 day, hair started to grow and falling hairs reduced. After 4 days,
multiple alopecia
559 male 20's areata persisted. once a day
application was sloped, but stiumulation, elongation and restoration of
hair
persisted.
SEQ ID NO 28: After 1 day, hair
became fuller with increased volume. Hair gained elasticity and
(CEGRKNIDRISSISGLOC) resilience.
S60 female 50's healthy skin (scalp) C once a
day After 7 days, grown hairs were predominantly black over white hairs.
White hairs
turned black.
S61 female 40's amruelattle alopecia C once a
day After 3 days, hairs started to grow and grew.
After 5 days, stimulated hair growth was confirmed.
S62 female 40's alopecia areata B once a day
After 2 weeks, further growth of terminal hair, elongation and growth of hair
were
confirmed all over alopecia site.
S63 female 40's female alopecia C once a day
After 4 days, hair gained elasticity, resilience and increased volume.
After 1 day, hair volume was Increased. Hair gained elasticity and resilience.
$64 male 40's healthy skin (scalp) C once a
dayor
Hair gained resilience and volume after) days. Application continued for 1
week,
or
CO S65 male 40's male AGA C once a day
and the effect persisted for 1.5 months thereafter.
SEQ ID NO 29:
(CFGRKMDRISSMSGLGC) After 3 days, hair
gained elasticity, resilience and increased volume at thinning
S66 male 30's male alopecia C 2
application/day area on forehead.
for 3 days
After a few days, hair growth stimulation and hair growth were confirmed.
("I .
After 2 days, hair gained elasticity and resilience.
867 female 70's female alopecia C once a day
After 5 days, volume of hair increased. It was confirmed that the stimulated
hairs
are predominantly black over white hair.
After 7 days, stimulated hair growth was confirmed. Even 2 weeks after
stopping
868 female 20's oalpra: areata B once a day
application, hair growth stimulation and restoration persisted, and there were
little
falling hairs.
SEQ ID NO 30:After 2-3 days, hair gained elasticity and resilience.
S69 male 40's male AGA B once a day
(CFGRKMDRISSSVGLOC) After 7 days, hair
gained volume, restoration was confirmed.
S70 male 30's alopecia universalis B once a day
After 3 days, hairs started to grow and grew.
S71 female 30's alopecia C once a day
After 1 day, stimulated hair growth was confirmed.
)-3 0
P ND
we co
CD
¨,
lx.0
CO
ci..
After 7 days, stimulated hair growth was confirmed. Even 2 weeks after
stopping
572 female 20's
oalpozscii: areata
C once a day application,
hair growth stimulation and restoration persisted, and falling hairs
significantly decreased.
SEQ ID NO 31: S73 male 40's healthy skin (scalp) C once
a day After 1 day, hair volume was increased. Hair gained elasticity and
resilience.
(CFGRKMDRISSSSRLGC)
.
S74 male 30's
alopecia areata c 2 application/day
oniversaus for 3 days After 3
days, stimulation and elongation of hair were confirmed.
S75 female 60's multiple alopecia B once a
day After 5 days, hairs stimulated to grow, restored hair and hair growth
were
confirmed. It was confirmed that tair elongation rate is fast.
S76 male 30's male alopecia B once a day
After 7 days, hair gained elasticity. Hair started to grow, and volume of hair
on
parietal forehead was increased.
577 female 50's female AGA C once a day
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started
SEQ ID NO 32: to grow,
restoration and elongation were observed.
(CFGRKMDRISSSSGMGC)P
S78 male 40's alopecia universalis C once a
day After 3 days, hair stimulation and growth were confirmed.
o
hair thinning after
iv
After 2 days, hair gained elasticity, resilience and increased volume.
Appearance up
S79 female 50's anticancer agent
C once a day 03
of scalp shown through hair got obsecure. Hair increase was confirmed,therapy
in
in
multiple alopecia
o,
00 S80 female 50's B once a day After 7
days, stimulated hair growth was confirmed,areata
--.1
iv
After 1 day, hair became fuller with increased volume. Hair gained elasticity
and or
...1
S81 male 50's healthy skin (scalp)
C once a day resilience.
I
SEQ ID NO 33: After 7 days,
grown hairs were predominantly black over white hairs. White hairs r
r .
(CFGRKMDRISSSSGIGC) turned black.
1
multiple alopecia
r
OF
= S82 female 40's ..e.t.
B once a day After 5 days, hair root
regeneration and hair growth stimulation were confirmed. .
S83 male 50's alopecia areata C once a day
After 1 day, apparent hair growth stimulation and restoration were confirmed.
After 4 days, hair elongation were confirmed.
hair thinning after
384 female 50's anticancer agent B once a
day After 5 days, thin and fuzzy hairs along hairline got thicker and
denser.
therapy
S85 male 50's male AGA C once a day
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started
to grow, restoration and elongation were observed.
SEC) ID NO 34:
(CFGRKMDRISSSSGVGC)S86 male 40's male AGA C once a day
Hair gained resilience and volume after 3 days. Application continued for 1
week,
and the effect persisted for 1.5 months thereafter.
.
S87 male 30's male alopecia C once a day
After 3 days, hair gained resilience and volume of hair increased in parietal
thinnig area
multiple alopecia
S88 male 20's areata C once a day
After 1 day, hairs started to grow and grew.
1-3 o
sn
ts.D
cr I¨'
1¨ ,.p.
(1)
i¨.....
l'==
hair thinning after
C.0
589 female 40's anticancer agent B
once a day After 5 days, hair gained elasticity,
resilience, thickness, and increased volume. ---1
,.._,
therapy
590 male 20's multitpale alopecia C once a day
After 3 days, stimulated hair growth was confirmed.
area
After 1 day, hair gained elasticity, resilience and increased volume. Flair
started
SEQ ID NO 35: 591 female 50's female AGA C once a day
to grow, restoration and elongation were observed.
(CFGRKMDRISSSSGAGC)
S92 female 60's amruelatitapie alopecia B once a
day After 5 days, hair root regeneration and hair growth stimulation were
observed.
Then, remarkable restoration, elongation and growth of hair were observed.
arneultitaple alopecia once After 1 day,
stimulated hair was observed. After 2 weeks, there is remarkable
S93 female 30's C on a day elongation of
hair marginal area around large decalvant spot, and new hair
started to grow in center part.
594 male 30's alopecia universalis C once a day
After 4 days, hair stimulation and growth were confirmed.
SEQ ID NO 36: S95 male 40's healthy skin (scalp)
C once a day After 1 day, hair
volume was increased. Hair gained elasticity and resilience. P
(CFGRKMDRISSSSGLSC)0
S96 male 50's seborrheic dermatitis
C once a day After 3 days,
seborrhea and erythema were improved. n,
to
597 male 50's male alopecia B
once a day After 5 days, hair
gained elasticity, resilience and increased volume. 03
o,
o
hair thinning after
a
00 598 female 40's anticancer agent B
once a day After 5 days, hair
gained elasticity, resilience, thickness and increased volume. o,
00 therapy
n,
0
SEQ ID NO 37: 1 applicafion/day on
After 2 or 3 days, hair gained elasticity and resilience. After 7 days, hair
gained r
...1
F"
(CFGRKMDRISSSSGLAC) 899 male 40's male AGA B forehead thinning
volume, restoration was confirmed.
1
area
r
multiple alopecia
1
C once a day
After 5 days, stimulated hair growth was confirmed. r .
S100 male 20's' areata
= , or
S101 female, 20's alopecia areata A ,once a day After 10
days, hair growth stimulation elongation were confirmed.
8102 female 50's female AGA C once a day After 1 day,
hair gained elasticity, resilience and increased volume, Hair started
to grow, restoration and elongation were observed.
SEQ ID NO 38:
Idles stopped in 3 minutes, dandruff was reduced, redness in scalp was
(CFARKMDRISSSSGLGC) S103 female 50's dandruff, itches C once a day
significantly improved.
ectopic multiple
S104 female 10'sC once a day After 3 days, stimulated hair growth was
confirmed in eyebrows.
alopecia
1-3 0
p)
IsD
0- --,
0-- C.Ti
(1)
1,0
S105 male 10's alopecia universalis C once a day
After 3 days. stimulated hair growth was confirmed. CD
do
S106 female 50's dandruff, itches C once ad lches stopped in
1 minute, dandruff was reduced, redness in scalp was
ay
significantly improved.
SEQ ID NO 39: After 1 day, hair
became fuller with increased volume. Hair gained elasticity and
(CFSRKMDRISSSSGLGC) resilience.
$107 female 60's healthy skin (scalp) C once a day
After 7 days, grown hairs were predominantly black over white hairs. White
hairs
turned black.
S108 female 60's
areata
tple alopecia C once a day After 3 days,
stimulated hair growth was confirmed. More black hairs started to
grow than white hairs, and hair elongation rate was fast.
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started
SEQ ID NO 40: S109 male 50's male AGA C once a day to
grow, restoration and elongation were observed.
(CFTRKMDRISSSSGLGC) multiple alopecia
S110 male 20's C once a day After 4 days,
stimulated hair growth was confirmed.
areata
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started
P
SEQ ID NO 41: 5111 female 50's female AGA C once a day
to grow, restoration and elongation were observed.
(CEGRKMDRISSTSGLGC)
o
multiple alopecia
ND
S112 male 20's areata C once a day
After 4 days, stimulated hair growth
was confirmed. vp
.
m
multiple alopecia
o,
$113 male 30's C once a day
After 3 days, stimulated hair growth
was confirmed. o
areata
m
00 SEQ ID NO 42: S114 male 40's healthy skin
(scalp) C once a day After 1 day,
hair volume was increased. Hair gained elasticity and resilience. o,
C.0 (CFGRKMDRISSASGLGC)
ND
0
S115 female 60's After 3 days,
stimulated hair growth was confirmed. Black hairs started to grow r
C once a day
...1
and hair elongation was fast
i
amrttitpale alopecia
r
1 application/day on
r
I
. SEQ ID NO 43: 5116 male 40's male AGA B
forehead thinning After 2-3 days, hair gained elasticity and resilience.
After? days, hair gained volume, restoration was confirmed.
= = r
Or(CFGRKMDRISSSTGLGC) area .
multiple alopecia
S117 male 20's D once a day After 3 days,
stimulated hair growth was confirmed.
areata
SEQ ID NO 44: 5118 female 60's female AGA C once a day
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started
to grow, restoration and elongation were observed.
(CFGRKMDRISSSAGLGC) multiple alopecia
S119 female 20's area, C once a day After 3 days,
stimulated hair growth was confirmed.
1¨ 0
we 0
(D
i---,
t\D
CD
S120 male 40's male AGA C once d
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started
a ay
(SD
SEQ ID NO 45: to grow, restoration
and elongation were observed.
(CFSRRMDRISSSSGLGC) After 1 day, hair
volume was increased. Hair gained elasticity and resilience.
S121 female 40's healthy skin (scalp) C once a day
S122 female 60's female AGA C once a day After 1 day,
hair gained elasticity, resilience and increased volume. Hair started
to grow, restoration and elongation were observed.
SEQ ID NO 46:
(CFSRKMDRISSTSGLGC) After 1 day, hair
became fuller with increased volume. Hair gained elasticity and
S123 female 60's healthy skin (scalp) C once a day resilience.
After 7 days, grown hairs were predominantly black over white hairs. White
hairs
turned black.
After 1 day, hair volume was increased. Hair gained elasticity and resilience.
S124 male 40's healthy skin (scalp) C once a day
SEQ ID NO 47:
(CFSRKMDRISSSTGLGC)
S125 female 50's female AGA C once a day After 1 day,
hair gained elasticity, resilience and increased volume. Hair started
to grow, restoration and elongation were observed.
P
S126 female 40's aTelatitpale alopecia B once a day
After 5 days, hair root regeneration
and hair growth stimulation were confirmed. o
N)
m
a,
After 1 day, hair became fuller with Increased volume. Hair gained elasticity
and o,
SEQ ID NO 48 resilience.
o
S127 female 50's healthy skin (scalp) C once a day
m
C.S) (CFSRKMORISSSSGIGC) After 7 days, grown
hairs were predominantly black over white hairs. White hairs o,
0 turned black.
m
o
S128 female 50's female AGA C once a day
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started r
...1
1
to grow, restoration and elongation were observed.
r
r
I
S129 female 50's female AGA C once a day After 1 day,
hair gained elasticity, resilience and Increased volume.
r
SEQ ID NO 49:
m
=
=
(CFSRKMDRISSSSGLAC) S130 female 50's alopecia areata B once a day '
After 5 days, hair started to grow and hair density was increased.
,
3131 female 40's healthy skin (scalp) C once a day After 1 day,
hair volume was Increased. Hair gained elasticity and resilience.
After 1 day, hair gained elasticity, resilience and Increased volume. Hair
started
SEQ ID NO 50: S132 male 50's male AGA C once a day to
grow, restoration and elongation were observed.
(CFGRRMDRISSTSGLGC)
S133 male 40's healthy skin (scalp) C once a day
After 1 day, hair volume was increased. Hair gained elasticity and
resilience.
c
$1)
tsD
1--.
...A
CD
Is=
1 application/day on
CD
S134 male 40's male AGA B forehead thinning
After 2 or 3 days, hair gamed elasticity and resilience.
i¨k
SEQ ID NO 51: area After 7 days,
hair gained volume, restoration was confirmed.
0
(CFGRRMDRISSSTGLGC) After 1 day,
hair became fuller with increased volume. Hair gained elasticity and
S135 male 50's healthy skin (scalp) C
once a day resilience.
8136 male 60's male AGA C once a day After 1
day, hair gained elasticity, resilience and increased volume. Hair started
to grow, restoration and elongation were observed.
SEQ ID NO 52: 5137 female 40's multiple alopecia
areata B once a day After 5
days, hair root regeneration and hair growth stimulation were confirmed.
(CFGRRMDRISSSSGIGC)
After 1 day, hair became fuller with increased volume. Hair gained elasticity
and
resilience.
S138 female 60's healthy skin (scalp) C once a day After 7
days, grown hairs were predominantly black over white hairs. White hairs
turned black.
8139 female 40's female AGA C once a day After 1
day, hair gained elasticity, resilience and increased volume.
SEQ ID NO 53:
. (CFGRRMDRISSSSGLAC) S140 male 40's healthy skin (scalp)
C once a day After 1 day, hair volume was increased. Hair gained
elasticity and resilience.
P
1 application/day on
0
After 2 or 3 days, hair gained elasticity and resilience. After 7 days, hair
gained n,
S141 male 40's male AGA B
forehead thinning ,.0
volume, restoration was confirmed, mSEQ ID NO 54:
area o,
(CFGRKMORISSTSGIGC) After 1 day,
hair became fuller with Increased volume. Hair gained elasticity and o
S142 female 50's healthy skin (scalp) C once a day
resilience. After 7 days, grown
hairs were predominantly black over wh mite hairs. o, .
C..0 White hairs
turned black. n,
i--,
o
8143 male 50's male AGA C once a day
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started r
...1
.
to grow, restoration and elongation were observed.
I
r
SEQ ID NO 55:
(CFGRKMDRISSTSGLAC)
r
After 1 day, hair became fuller with increased volume. Hair gained elasticity
and 1
r
. resilience.
Lri
.
,
S144 female 60's healthy akin (scalp) C once a day
After 7 days, grown hairs
were predominantly black over white hairs. White hairs .
tamed black.
SEQ ID NO 56 S145 male 40's male AGA C once a day
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started
:
(CFGRKMDRISSSTGIGC) to grow,
restoration and elongation were observed.
S146 male 40's healthy skin (scalp) C
once a day After 1 day, hair volume was increased. Hair gained elasticity
and resilience.
8147 male 50's male AGA C once a day After 1
day, hair gained elasticity, resilience and increased volume. Hair started
SEQ ID NO 57: to grow,
restoration and elongation were observed.
(CFGRKMDRISSSTGLAC)
S148 male 40's healthy skin (scalp) C
once a day After 1 day, hair volume was increased. Hair gained elasticity
and resilience.
=
,---, ,--,
i¨a 0
SID
t=D
tS.
SEQ ID NO 58: 5149 female 40's female AGA C
once a day After 1 day, hair gained elasticity,
resilience and increased volume. CD
i¨,
(CFGRKMDRISSSSGIAC) S150 female 40's healthy skin (scalp) C
once a day After 1 day, hair volume was increased. Hair
gained elasticity and resilience, i--,
SEQ ID NO 59: S151 male 50's male AGA C once a
day After 1 day, hair gained elasticity, resilience and increased volume.
Hair started
(CFSRRMDRISSTSGLGC) to grow, restoration
and elongation were observed.
S152 male 40's healthy skin (scalp) C once a day After 1 day,
hair volume was increased. Hair gained elasticity and resilience,
S153 male 60's male AGA C once a day After 1 day,
hair gained elasticity, resilience and increased volume. Hair started
SEQ ID NO 60: to grow, restoration
and elongation were observed.
(CFSRRMDRISSSTGLGC) After 1 day, hair
volume was increased. Hair gained elasticity and resilience.
S154 male 40's healthy skin (scalp) C once a day
5155 female 60's female AGA C once a day After 1 day,
hair gained elasticity, resilience and increased volume, Hair started
to grow, restoration and elongation were observed.
SEQ ID NO 61: ' After 1 day, hair
became fuller with increased volume. Hair gained elasticity and
(CFSRRMDRISSSSGIGC)
P
S156 male 60's healthy skin (scalp) C once a day resilience.
After 7 days, grown hairs were predominantly black over white hairs. White
hairs
turned black.
IV
0
S157 male 50's male AGA C once a day
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started 00
SEQ ID NO 62: to grow, restoration
and elongation were observed. 00
ai
(CFSRRMDRISSSSGLAC)
o,
CDAfter 1 day, hair volume was increased. Hair gained elasticity and
resilience.
tNQ
$158 male 40's healthy skin (scalp) C once a day
IV
0
I application/clay on
After 2 or 3 days, hair gained elasticity and resilience. After 7 days, hair
gained r
5159 male 60's male AGA B forehead thinning
-J' I
SEQ ID NO 63: volume, restoration
was confirmed. r
fl...
(CFSRKMDRISSTSGIGC)
S160 male 40's healthy skin (scalp) C once a day
After 1 day, hair volume was increased.
Hair gained elasticity and resilience. r 1
r
ut
After 1 day, hair became fuller with increased volume. Hair gained elasticity
and
resilience.
S161 male 60's healthy skin (scalp) C once a day
SEQ ID NO 64: After 7 days, grown
hairs were predominantly black over white hairs. White hairs
(CFSRKMDRISSSTGIGC) turned black,
S162 female 50's female AGA C once a day After 1 day,
hair gained elasticity, resilience and increased volume. Hair started
to grow, restoration and elongation were observed.
i-3 0
Op
lb=D
a)
ts.D
co
1 application/day on
After 2 or 3 days, hair gained elasticity and resilience.
i¨,
SEQ ID NO 65:
5163 male 40's male AGA B forehead thinning
After 7 days, hair gained volume, restoration was confirmed.
(CFSRKMDRISSSTGLAC) area
IsO
5164 male 40's healthy skin (scalp) C once a day After 1 day,
hair volume was increased. Hair gained elasticity and resilience.
SEQ ID NO 66: S165 male 60's male AGA C once a day After
1 day, hair gained elasticity, resilience and increased volume. Hair started
to grow, restoration and elongation were observed.
(CFSRKMDRISSSSGIAC)
5166 male 40's healthy skin (scalp) C once a day After 1 day,
hair volume was increased. Hair gained elasticity and resilience.
1 application/day on
After 2-3 days. hair gained elasticity and resilience.
5167 male 40's male AGA B forehead thinning
After 7 days, hair gained volume, restoration was confirmed.
SEQ ID NO 67: area
(CFGRRMDRISSTSGIGC) After 1 day, hair
became fuller with increased volume. Hair gained elasticity and
8168 female 60's healthy skin (scalp) C once a day resilience.
After 7 days, grown hairs were predominantly black over white hairs. White
hairs
turned black.
SEQ ID NO 68: S169 female 40's female AGA C once a day
After 1 day, hair gained elasticity, resilience and increased volume.
After 1 day, hair volume was increased. Hair gained elasticity and resilience.
P
(CFGRRMDRISSTSGLAC) S170 male 40's healthy skin (scalp) C once a day
o
1 application/day on
$171 male 60's male AGA B forehead thinning
After 2 or 3 days, hair gained elasticity and resilience.
Iv
After 7 days, hair gained volume, restoration was confirmed,
,r,co
o,
area
o
03
multiple alopecia
o,
SEQ ID NO 69: S172 male 30's
areata C once a day After 1 day,
hairs started to grow and grew.
CO (CFGRRMDRISSSTGIGC)
CO After 1 day, hair
became fuller with increased volume. Hair gained elasticity and iv
o
resilience. After 7 days, grown hairs were predominantly black over white
hairs. r
...]
I
S 173 male 60's healthy skin (scalp) C once a day White hairs
turned black.
/
/
i
SEQ ID NO 70: S174 female 50's female AGA C
once a day After 1 day, hair gained
elasticity, resilience and increased volume. r
Or.
After 1 day, hair volume was increased. Hair gained elasticity and resilience.
(CFGRRMDRISSSTGLAC) S175 male 40's healthy skin (scalp) C once a day
S176 male 50's male AGA C once a day After 1 day,
hair gained elasticity, resilience and increased volume. Hair started
=
to grow, restoration and elongation were observed.
SEQ ID NO 71: S177 male 20's rn,u1:t13.le alopecia C once a
day After 1 day. hairs started to grow and grew.
(CFSRRMDRISSTSGIGC) After 1 day, hair
became fuller with increased volume. Hair gained elasticity and
resilience.
S178 male 50's healthy skin (scalp) C once a day After 7 days,
grown hairs were predominantly black over white hairs. White hairs
turned black.
1¨ 0
SID
t=D
0" t\D
cp
LND
1 application/day on 0
After 2 or 3 days. hair gained elasticity and resilience. After 7 days, hair
gained
SEQ ID NO 72: S179 male 40's male AGA B
forehead thinning t--,
volume, restoration was confirmed.
(CFSRRMDRISSTSGLAC) area
CO
5180 male 40's healthy skin (scalp) C once a day
After 1 day, hair volume was increased. Hair gained elasticity and
resilience.
5181 female 40's female AGA C once a day After 1 day,
hair gained elasticity, resilience and increased volume.
S182 female 40's , alopecia areata B once a day After 5 days,
hair started to grow and hair density was increased.
SEQ ID NO 73:
After 1 day, hair became fuller with increased volume. Hair gained elasticity
and
(CFSRRMORISSSTGIGC)
resilience.
S183 female 60's healthy skin (scalp) C once a day After 7 days,
grown hairs were predominantly black over white hairs. White hairs
tamed black.
After 1 day, hair gained elasticity, resilience and increased volume. Hair
started
SEC! ID NO 74: S184 male 60's male AGA C once a day
to grow, restoration and elongation were observed.
(CFSRRMDRISSSTGLAC)
S185 male 40's healthy skin (scalp) C once a day
After 1 day, hair volume was increased. Hair gained elasticity and
resilience.
1 application/day on
After 2 or 3 days. hair gained elasticity and resilience.
S186 male 40's male AGA B forehead thinning
After 7 days, hair gained volume, restoration was confirmed.
P
area
SEQ ID NO 75: S187 female 40's B once a day
After 5 days, hair root regeneration
and hair growth stimulation ware confirrned. o
ND
(CFSRRMORISSSSGIAC) amruelatitpale alopecia
vp
co
After 1 day, hair became fuller with increased volume. Hair gained elasticity
and o,
resilience.
0
S188 female 50's healthy skin (scalp) C once a day
0
After 7 days. grown hairs were predominantly black over white hairs. White
hairs o,
CD
4-
fumed black.
ND
In SEQ ID NOS 16-75, 1st Cys and 17th Cys from left form a disulfide bond.
o
r
...1
I
I"
I"
I
I"
.
.
. =
. Ul
CA 02986086 2017-11-15
[0221]
The result described above demonstrated that the cyclic peptide of the
invention
exhibits a similar effect as the cyclic peptide expressed by Formula I-a even
if some
amino acids of the cyclic peptide have been replaced.
[0222]
Specifically, it has effects of preventing hair loss at the applied site,
stimulating hair
growth or promoting hair growth, giving hair resilience and elasticity,
increasing
volume of hair and dramatically decreasing falling hairs when it was applied
to the site
of alopecia or hair growth stimulation, etc. It also has effects of nourishing
hair,
promoting hair growth stimulation, improving/preventing thinning of hair at
the
applied site. In this case, the stimulated hair tends to become a terminal
(non-white)
hair. Moreover, these effect exhibit significantly faster as compared to other
active
agents conventionally used in alopecia therapeutics such as BNP such that
stimulated
hair growth can be confirmed one day after only one application even in the
case of an
intractable alopecia areata multilocularis or ophiasis. In addition, resulting
effects
persists remarkably even after terminating application and the stimulated hair
keep
growing. In the case of AGA or healthy subject it exert immediate effect of
providing
hair with resilience and elasticity on the following day, increasing the
volume, and
dramatically decreasing falling hairs, which effects last even after
terminating
application. It also has an improving and preventing effect on dermatitis.
Therefore,
it can improve or prevent skin inflammation associated with alopecia. Such
effect is
advantageous in cases where alopecia has been exacerbated due to skin
condition of the
applied site (e.g., scalp). Moreover, the external preparation of the present
invention
exerts a moisturizing and skin texture-improving effects at the applied site
when being
applied to skin as described above. It can remove and suppress dandruff and
itching,
while giving moisture to hair and scalp, improving and preventing dryness and
keeping
hair and scalp healthy. It also can improve seborrhea. On the other hand, when
it is
used as an alopecia therapeutic/prophylactic, it can be used for the purpose
of treating
or preventing one or more of applicable alopecia including, for example, those
described
below, without being particularly limited thereto.
[0223]
(Acquired alopecia)
(i) Alopecia
without accompanying scarring or skin lesion (alopecia areata, male
pattern alopecia, seborrheic alopecia, alopecia pityroides, female pattern
alopecia,
gestational alopecia, malignant alopecia, senile alopecia, alopecia totalis,
alopecia
CA 02986086 2017-11-15
areata multilocularis, ophiasis, drug-induced alopecia, cancer chemotherapy-
induced
alopecia and radiation exposure-induced alopecia, traumatic/mechanical
alopecia,
malnutrition/metabolic disorder associated alopecia, endocrine dysfunction
associated
alopecia and telogen effluvium (post partum alopecia, alopecia after high
fever)).
Alopecia observed in skin lesion or pathologic skin (infection-induced
alopecia,
tumor-induced alopecia, inflammation-induced alopecia).
(iii) Scarring alopecia (skin infection-induced alopecia, alopecia induced
by
infiltration of inflammatory cells).
(Congenital alopecia)
Diffuse alopecia, congenital atrichia, alopecia in hereditary syndromes,
localized
alopecia, phakomatosis, aplasia cutis, congenital alopecia triangularis.
[0224]
It exerts an excellent effect especially on acquired alopecia, preferably on
alopecia
without accompanying scarring or skin lesion, more preferably on alopecia
areata, male
pattern alopecia, seborrheic alopecia, alopecia pityroides, female pattern
alopecia,
gestational alopecia, malignant alopecia, senile alopecia, alopecia totalis,
alopecia
areata multilocularis, ophiasis, drug-induced alopecia, cancer chemotherapy-
induced
alopecia and radiation exposure-induced alopecia, traumatic/mechanical
alopecia,
malnutrition/metabolic disorder associated alopecia, endocrine dysfunction
associated
alopecia and telogen effluvium. Therefore, the external preparation of the
present
invention can be used for the purpose of treating or preventing at least one
or more
alopecia selected from the above-mentioned group.
One simple application onto aforementioned affected site at 3 pg/ml to 500
pg/ml
improved or eliminated itch, dandruff, scalp redness and inflammation
immediately
thereafter (immediate effect). A single application stimulated hair growth,
grew hair,
provided hair with resilience, elasticity and increased volume, and
dramatically
decreased falling hairs, and these effects started on the day after the
application and
persisted for one week or more (long-lasting effect). If application was
carried on for
about one week, these effects are further improved, and even after terminating
application, the effects lasted from 1 to 2 weeks such that stimulation of
hair growth
and restoration of hair continued and there were little or no falling hairs.
[0225]
9.2 Confirming effects on skin
96
CA 02986086 2017-11-15
The obtained cyclic peptide was prepared in purified water at 3, 15, 30, 100
or 500 pg/ml
and applied to affected site. Each of formulations A to E in the table
indicates the
concentration of the cyclic peptide at 3, 15, 30, 100 or 500 pg/ml,
respectively. The
absence of the effect upon the application of purified water without the
peptide
confirmed the effect was not a placebo effect.
97
1-3 0
Po
ts=D
cs=
C...
,--'
C:3)
('D
CO
Amino acid sequence of Diagnosfic
0
cyclic peptide mutant used ID Sex
Age ImpressionFormulation Treatment Diagnostic impression on skin after
application Itches after application
I--,
L.....I
Iches were remitted immediately after
01 female 30's eczema B once a day After 3
minutes, red papulewas reduced and remitted.
application.
Iches were eliminated immediately
Q2 male 20's atopic dermatitis B once a day
After 2 minutes, erythema, edema and infdtrafion was remitted,
after application.
Q once a day, 23 male 60's plaque psoriasis C
After 3 minutes, crusts, scales, erythema and infiltration were remitted,
days
04 male 40's plaque psoriasis C once a day
After 10 minutes, scales, erythema and infiltration were remitted.
SEQ ID NO 16:
(CFVRKMORISSSSGLGC) 05 tamale 5rys eczema C oncea day After 3
minutes. erythema was remitted.
06 male 20's atonic dermatitis B
' 7 Iches were remitted immediately after
Erythema, infiltration and papule were remitted.
application.
After 2 minutes, erythema, infiltration and papule were remitted.
07 female 60's eczema C once a day
After 20 minutes, they were ameliorated.
P
08 female 20's atopic dermatitis C
once a day After 2 minutes,
erythema and infiltration were remitted. Iches were eliminated in 1 minute.
o
5,
or
09 , male 10's atopic dermatitis C
once a day Redness vanished
Immediately after application. Itches were extincted in 1 minute. es
o,
o
010 male 40'0 atopic dermatitis C
once a day After 3 minutes,
erythema and infiltration were remitted. After 3 minutes, iches were
remitted. es
o,
CO
(Xl, SEQ ID NO 17: oil male 10,s eczema nummular
C once a day
After 3 minutes. infiltration,
erythema exudate and scales were remitted. 5,
0
(CFGQKMDRISSSSGLGC)
i-i
-4
After 2 minutes, scales and erythema were remitted.
Iches were eliminated immediately 1
012 male 20's eczema B
once a day i-i
With only 1 application, no recurrence for 7 days thereafter,
after application.
.
1
i-i
013 male 60's miliaria
C once a day After 2 minutes. redness was
remitted. , = = , u'l
SEQ ID NO 18:
(CFGHKMDRISSSSGLGC) After 2 minutes, zits, dryness and rawness on skin
were reduced, skin was
014 female 40's roughened skin A once a day
moisturized and it texture improved. After 7 days, large wrinkles and
nasolabial fold became less deep.
,
1-] 0
a' Ir.D
,--d
....."
CO
0
After 2 minutes, erythema and scales were remitted.
015 male 20's
eczema, atonic a day
After 20 minutes, skin got moisturized. IsD
C once
dermatitis After only 1
application, erythema vanishes and kept in a good condition for
1 week.
' .After 5 mdinutes,
wound was epithefized, erythema and papule were
016 male 20's atonic dermatitis C once a day
After 2 minutes, redness was remitted. Roughness and skin texture were
017 female 40's roughened skin B once a day
SEO 10 NO 19: also improved.
(CFGRRMDRISSSSGLGC)
After 2 minutes, redness was remitted. Roughness and skin texture were
016 female 30's roughened skin B once a day
also improved. Rawness got better. Idles vanishedimmediately after
application!: skin were moisturized and smooth.
019 male 40's plaque psoriasis C once a day
After 3 minutes, erythema, anstsscales and infiltration were remitted.
Itches were extincted immediately
020 male 40's eczema C once a day
Erythema was remitted immediately after application. after
application,
P
021 female 60's eczema C
once a day After 1 minute, red papule was
reduced and extincted. After 3 minutes, lobes were remitted. o
m
io
Itches vanished completely
o)
022 male 20's atonic dermatitis
B once a day After 2
minutes, erythema and scales were remitted. m
immediately after application.
o
Rawness vanished immediately after
0)
023 female 40's eczema C
once a day After 2 minutes,
erythema. scales and papule were remitted. m
0 SEQ ID NO 20:application.
0 (CFGRKLDRISSSSGLGC) roughened skin, After 3 minutes,
zits, ruggedness, dry skin and redness were remitted: skin
lobes were eliminated immediately
m
024 female 30's a B once a day
got full and its texture
improved. Acnes were reduced and redness was 0
after application
r
.
remitted
....1
'
I
025 female 60's wrinkles, dry skin
B once a day After 2
minutes, wrinkles became less deep, dry skin was remitted. r
r
i
. 026 female 60's eczema
C once a day . After 3 minutes, erythema and scales
were remitted . . r
in
027 male 20,s eczema, atopic
C once a day After 3 days, erythema, scales and papule were
improved. Cracks were lobes were eliminated immediately
dermatitis epithelized, skin got
moisturized and improved. after application.
SEO ID NO 21:
After 2 minutes, infiltration, erythema, papule and scratch wound were
(CFGRKIDRISSSSGLGC) 026 female 40's eczema B once a day
After 1 minute, Ickes were eliminated.
remitted.
Alter 3 minutes, Idles were
029 male 40'0 atopic dermatitis
B once a day immediately after application, erythema and scales
were remitted. eliminated,
=
1-3 0
tD
t`O
trO
CD
CO
030 female 30's atopic dermatitis C once arts After 3
minutes, 1 application, erythema and infatrafion were remarkably After 30
seconds, Idles were
y
remitted, dry skin, scales and cracks were also improved. eliminated.
CO
031 female 70's eczema C once a day
immediately after application, erythema and papuleffi Facial rawness
vanished immediatelyremitted. after application.
032 female 30's atopic dermatitis 8 once a day, 7A
remarkable improvement after 2 days, and erythema was eliminated.
days
After 1 minute, erythema was remitted.
After 2 minutes, scratch wound was epithelized.
033 female 40's atopic dermatitis C rime a daY' 2 After 3
minutes, skin texture was improved, lobes were eliminated immediately
ays
d
With only 1-day application, erythema was eliminated, Skin texture was
after application.
improved with no dryness.
SEO ID NO 22:
(CFORKMORVSSSSGLGC)034 male 20's atonic dermatitis C once a day
After 2 minutes, erythema and scales were remitted. Riles were remitted
immediately after
No recurrence for 7 days thereafter. application.
035 female 50's chronic eczema C once a day After 2
appfreations, lichenificated erythema was remitted, lobes were eliminated
immediately
No recurrence for 3 weeks thereafter, after
application.
Skin roughness was cured immediately after application.
036 female 50's roughened skin C once a da After 2
minutes, skin got moisturized, its redness faded. Skin texture was
y 0)
finely improoed . After 7 days, skin tone became bnghter, spots faded and
flabbiness was improved.
037 female 30's roughened skin B once a day After 2
minutes, zits. redness was remitted. Rawness vanished immediately after
After 1 day, skin became normal tone and its texture was improved.
application.
After 2 minutes, scratch wound was epithelized and cured. Erythema was
Q38 female 40's atonic dermatitis C once a day After 1
minute, ides were eliminated.
improved.
039 male 20'0 eczema C once a day After 2 minutes.
erythema, dry skin and scales were improved, and skin got Itches were abated
immediately after
,
moisturized. application.
After 2 minutes, erythema, scales and infiltration were remitted. With 1
Itches were extincted immediately
SEO ID NO 21 040 male 20's atopic dermatitis C once a
day application, no recurmnee for 7 days. after appfication.
(CFGRKMDRIGSSSGIGC)
041 male 40's plaque psoriasis C once a day After 3
Minutes, crustsscalesterythema and infiltration were remitted.
After 2 minutes, roughness, zits and redness were reduced.
042 female 30's roughened skin 8 once a day skin got
After 2 minutes, Itches were extineted.
moisturized and its texture was improved.
After 1 minute, erythema, telangiectasia and acne were remitted.
043 female SO's rosacea. acne C once a day After 2
minutes, papule, infiltration, acne, dry skin and redness was
remitted.
1-3 0
LND
t=
c.0
CO
044 male 10's atopic dermatitis C once a
day After 3 minutes, erythema, scales and infiltration were remitted.
Further improvement after 40 minutes. Scratch wound was epithelized.
045 male 20's atopic dermatitis C
once a day After 3 minutes, erythema and infiltration were remitted.
After 3 minutes, lches were
eliminated.
SEQ ID NO 24:
(CFGRKMDRISASSGLGC)
C146 male 20's eczema C once a After 3 days,
cracks were epithelized. Erythema, scales and infiltration were
day
remitted.
After 2 minutes, wound was epithelized. Eythema, scales and lichenification
iceee were eliminated immediately
047 male 20's atopic dermatitis C once a day
/*emitted.
After 5 minutes, skin got softened. erythema and Infiltration were improved,
after application.
After 2 minutes, erythema. scales and infiltration were remitted. Skin got
lches were remitted immediately after
048 male 20's edeczimema:isaWPic C once a day
moisturized.
application.
SEQ ID NO 25:
(CFGRKMDRISSQSGLGC) After 3 minutes, deep wrinkles
were less deep. dry skin and redness were
049 female 40's roughened skin
improved, skin got moisturized and full. By using for 1 week, large wrinkles
Rawness was eliminated immediately
B dace a day and nasolabial fold
became less deep Skin became brighter. Spots faded, after application.
Flabbiness was improved.
050 male 60's plague psoriasis C once a day
After 2 minutes. scales and infiltration remitted.am
CZ)vu
051 male 30's miliaria C once a day After 2
minutes. remitted.
052 male 20's acne vulgalis C once a day After 6
minutes, acne became dry and was reduced in size.
SEQ ID NO 26:
After 3 minutes, erythema and infiltration were remitted.
(CFGRKMDRISSVSGLGC) 053 female 60's eczema C
once a day = = or
After 4 minutes. ameliorated.
054 female 10's atonic dermatitis C once a day
After 2 minutes, strong itches almost vanished. Erythema and
lichenification were improved, Skin got softened.
055 female 40's roughened skin C once a day
After 2 minutes, zits. redness and roughness were improved. Skin got
moisturized and its texture was improved. Skin was improved.
After 3 minutes, dry skin was improved, skin gat moisturized and its texture
was finely improved. Redness vanished. Inframmation in acne was
Rawness vanished immediately after
056 female 50,e raocunthened skin,
C once a day
remitted, redness faded and dried. By using for 1 week, skin became
application.
SEQ ID NO 27:
=
(CFGRKMDRISSLSGLGC) brighter.
057 male 20's atopic dermatitis C
once a day After 3 minutes, erythema, infiltration and lichenification
were improved. alcftheersoweporiecaterr.mninated immediately
1-3 0
DO ts=
Cr CO
CO
0
058 female 30's eczema C
once a day After 1 minute, erythema and infiltration were
remitted. After 1 minute, Idles were eliminated. i
SEQ ID NO 28: 059 female 20's roughened skin A once a day After
7-day continuous application, skin roughness vanished, wrinkles were
(CFGRKMDRISSISGLGC) less deep, and skin became
brighter.
060 female 20's roughened skin C once a day
After 2 minutes. scales were improved. With 1 application/day, roughness
was improved and skin could be kept moisturized.
SEQ ID NO 29:-
061 female 30's eczema C
once a day After 1 minute, erythema and infiltration were remitted.
Iches were eliminated immediately
(CFGRKMDRISSMSGLGC)
after atunlication.
062 female 20 , once a day, 's atopic
dermatitis 7After 3 days, erythema and infiltration were remitted.
, days
063 male 60's eczema C once a day
After 1 minute, erythema and papule were remitted. lches were eliminated
immediately
After 3 days, they were almost cured,
after application.
064 female 50's dry skin
immediately after application, skin got promptly moisturized and its texture
C aaaa a day
SEQ ID NO 30: was improved. zits, erythema and roughened skin
were improved.
(CFGRKMDRISSSVGLGC)
065 male 20's atopic dermatitis C once a day
After 10 minutes, tichenification was improved. Skin got softened. erythema
Iches were eliminated immediately
and scales were improved,
after application. P
After 1 day with 1 application, skin conditions of roughness. zits and itches
.ND
066 female 40's roughened skin
A once a day were well
improved, and skin texture was finely improved. tff,
o5
The effect lasted for 7 days thereafter.
oi
SEQ ID NO 31:
oPo
t--, (CFGRKMDRISSSSRLGC) 067 male 20's atopic dermatitis
C once a day After 2 minutes,
erythema and papule was remitted. or
0
iv
['0SEQ ID NO 32: 068 male 30's miliaria
C once a day After 2 minutes, red papule was reduced
in size, skin dryness was remitted. 0
/
(CFGRKMDRISSSSGMGC)
..i.]
069 male 10's nat:Pmimaualearrmabas' C OnCe a
day After 3 minutes, exudate stopped. erythema and infiltration were
improved.
/
/
1
/
..
= . =
. . . or
1-3 0
sn
ts.D
cs- co
cr, ¨
co
070 female , 305 contact dermatitis C 2
application After 3 minutes, erythema, infiltration and edema were remitted.
After 1 minute, Iches were eliminated. 0
lobes were eliminated immediately
071 female 30's atonic dermatitis E once a day
immediately alter application, erythema and scales were improved.
SEQ ID NO 33:
after application.
(CFGRKMDRISSSSGIGC) 072 female 70's plaque psoriasis C 2 application
After 5 minutes, erythema. scales and infiltration were remitted.
In 2 minutes after application, skin was improved, Skin texture was finely
073 female 40'5 roughened skin B once a day
improved. Skin got softened.
074 female 30's eczema C 2 application After
3 minutes, erythema, infiltration and edemathemitted. After 1 minute, Iches
were eliminated.
075 male 2Ø eczema, acne
C 2 application After 5 minutes,
acne was dried and reduced in size. Erythema and scales
vulgaris were remitted.
076 female 70's plague psoriasis C 2 application After 5
minutes, erythema, scales and infiltration were remitted.
After 2 minutes, skin texture was improved, skin got full, and redness and
077 female 40's roughened skin C once a day
dryness were improved.
076 female 50's roughened skin 8 once a day After 3
minutes, skin got moisturized and its texture was improved:
SEQ ID NO 34:
Itches were improved immediately
(CEGRKMDRISSSSGVGC) 079 male 20's atopic dermatitis C once a day
After 3 minutes, erythema and scales were improved.
P
after application-
o
After 3 minutes, skin roughness was improved, skin got moisturized and its
nk
texture was finely improved. Skin gained resilience.
II)
080 female 50's roughened skin B
once a day After 7 days, large wrinkles and nasolabial
fold became less deep. By Idles were eliminated immediately 0)
or
o
continuing for 4 weeks, spots faded and skin tone became brighter. Skin
after application. o)
I¨, resilience was improved.
Large wrinkles became less deep. m
0
nk
CO
o
After 3 minutes, skin roughness and redness were removed. Skin texture
r
081 male 30's roughened skin B once a day
was finely improved, By continuing for 7 days, skin resilience was improved.
--I
I
I-I
I-I
After 3 minutes, roughened skin and dry skin were promptly improved, skin
1
C182 female 40's roughened skin B once a day
r
. texture was improved.
kri
.
. .
SEQ ID NO 35:
(CFGRKMDRISSSSGAGC) 083 male 60's plague psoriasis C twice a day
After 30 minutes, erythema, scales and infiltration were impoved.
084 male 40's plaque psoriasis C twice a day After 20
minutes, erythema and scales were irtmoved.
085 female 70's plaque psoriasis C twice a day After 5
minntes. erythema and scales were remitted.
p
ts.
0- co
I¨ t=D
(0
CO
0
After 2 minutes. lichenification was remarkably remitted. erythema, scales.
086 female 10's atopic dermatitis C once a day
infiltration, cracks, scratch wound, etc. all remitted. After 2 minutes,
strong iches wereeliminated.
After 5 minutes, improved with only mild infiltration left.
087 female 20's atopic dermatitis
After 1 minute, erythema, infiltration and papule remitted.
After 2 minutes, iches were
8 once a day
After 4 minutes, infiltration was remited, with only mild erythema left.
eliminated.
SEQ IQ NO 36: 'After 3 minutes. erythema.
scales, infiltration and scratch wound warn After 3 minutes, iches were
(CFGRKMORISSSSGLSC) Q88 mate 10's atopic dermatitis C once a day
remitted, eliminated.
089 female 40's roughened skin 8 once a day immediately
after application, itches and redness were removed.
After 3 minutes. skin got full and resilient, and skin was improved.
090 male 50's seebertietis ic C once a day, 3 immediately
after application, seborrhea was remitted.
days After 3 days, erythema and
seborrhea was remitted. Itches were remitted immediately after
d
application.
091 male 60's plaque psoriasis C once a day After 2 minutes,
erythema and scales were remitted.
092 male 275
atopic dermatitis, After 2 minutes, erythema and
infiltration were remitted. Applied twice. tones were eliminated
immediately
prurigo nodularis C once a day
After 15 minutes, prurigo nodularis on hip was remitted,
after application.
P
SEQ ID NO 37:
(CFGRKMDRISSSSGLAC) 093 female 40's roughened skin 8 once a day After
3 minutes. roughened skin and dry skin were improved promptly, and
skin texture was improved.
0
Na
to
0
094 male 4175 plaque psoriasis C
once a day After 20 minutes, erythema and scales were
remitted. . oi
0
I--I , 095 male 30's atopic dermatitis
C once a day .. After 2 minutes.
scratch wound epithelized. erythema was remitted. 0oi
0
i=P After 3 minutes, cracks was
reduced in size. Erythema, scales and immediately after application,
itcheS 6,
0
095 male 20's atopic dermatitis C once a day
lichenification were remitted,
and rawness were removed, r
--I
I
After 1 minute, dry skin was improved, skin got moisturized and full, and
immediately after application, Itches r
/
1
097 female 40's roughened skin 8 once a day
skin texture was improved,
were removed.
/
. orSEQ ID NO 38: 098 female 20's chronic
eczema C once a day immediately after application, erythema and
scales were remitted.
(CFARKMDRISSSSGLGC)
After 2 minutes, redness was improved.
After 1 minute, iches were eliminated.
099 male 20's atopic dermatitis C once a day After
3 minutes. erythema, infiltration and papule were remitted. After 1
application, no itches for 8
After 1 application, the effect lasted for 8 days with no recurrence.
days thereafter.
0100 female 50's Prudgd dddujads' C once a day eAftpiethrelizmeid
with
minutes, nodes dmesorewereexudflaattrned, scratch wound became dry and
alchfteersa weppriiecoetiiiomninated immediately
1-3 c
p)
C=o
cr. co
co
CB
0101 female SO's derdczd9ema0 daddiada' C once a day After 3 minutes,
nodes were flattened.
00
Altar 2 minutes, an intractable eczema that cannot be remitted by appying
0102 female 10's chronic eczema C once a day strong steroid
ointments was remitted, and erythema, scales and papule
were reduced.
500 10 NO 39, 0103 female 50's eczema
After 2 minutes, erythema, scales and pigmentation were remitted. Skin got
C dada a day
(CFSRKMDRISSSSGLGC) full and soft, with fresh
color.
0104 female 20's atopic dermatitis D once a day Scratch wound was
epithelizecl. Exudate was dried and fichendication was lobes were eliminated
immediately
remitted, erythema and infiltration were remitted,
after application.
After 2 minutes, itches and tingling
0105 male 20's atopic dermatitis B once a
day Alter 3 minutes, erythema and infiltration were remitted. With 1
application, pain were removed.
the effect persisted for 1 week with no recurrence.
The effect persisted for 7 days with no
itches.
Idles were elimi
0106 male 40's atopic dermatitis C once a
day After 2 minutes, erythema, infiltration and lichenificationniremitted.
after applicatinated immediatelyon.
After 1 minute, dry skin was improved, cracks were less deep and
Q107 female 20's roughened lips C 1 application epithelized.
SEQ ID NO 40:
(CFTRKMDRISSSSGLGC) 0108 female 40's roughened skin B once a day
After 3 minutes, redness and roughness were improved.
0
After 3 minutes, dark-reddish skin tone was improved to turn fresh color.
or
0109 female 30's healthy skin C once a day Dryness, tautness,
rawness and hot flash of skin were removed. With 1
application, the effect persisted for 1 week.
or
1-4
C..T1
Na
1-`
1-`
1-`
Ul
1¨a 0
pp
ts.
cr co
(7)
CO
immediately after application, erythema and infiltration of eczeinewere
0
0110 male 10's al Pic
C once a day
dermatitis/acne remitted. The effects were
exhibited remarlably fast.
After 3 minutes, erythema was further remitted. Acne was dried and
lches were eliminated immediately
after application.
C.,0
reduced in size.
immediately after application. erythema and infiltration were remitted.
Iches were eliminated immediately
0111 male 20's atoplc dermatitis C once a day
After 20 minutes, deep scratch wound was epithelized.
after application.
0112 female 40's roughened lips B 1 application After 1 minute, skin
tautness and rawness were improved and skin got
moisturized.
SEQ ID NO 41:
Iches stopped in 30 seconds. Redness and edema were remitted in 1
(CFGRKMDRISSTSGLGC) 0113 female 50's actinic dermatitis B once a day
minute.
After 1 minute, redness was remitted.
0114 female 10's ruZugthaeunuede B once a day
After 3 minutes, skin roughness, zits, rough and stiff skin texture were
After 3 minutes, skin tautness was
improved, skin got full and its texture was finely imprpved. Acne became
removed,
dry and reduced in size.
Rawness vanished in 1 minute. Skin was moisturized. After 2 minutes,
P
0115 female 40's roughened skin B once a day redness, roughness
and stiffness ware remarkably improved. By using for 1
week, skin became brighter. flabbiness was improved.
0
ro
0116 female 40's acne C once a day
After 1 minute, redness was remitted.
In
SEQ ID NO 42:
Iches were eliminated immediately or
0117 male 10's atopic dermatitis B once a day
After 3 minutes, erythema and
infiltration were remitted. o
(CFGRKMDRISSASGLGC)
after application. or
l--1
0118 female 60's eczema C once a day
After 3 minutes, erythema and dry
skin remitted. or
0
After 3 minutes, roughness and Is
a'a
0119 male 40's atopic dermatitis After
2 minutes. no disconfort. felt fresh. Erythema, infiltration and
C once a day
lichenification were remitted
stiffness were removed, and itches 0
r
vanished.
-4
I
I"
0120 female 30's roughened lips C once a day
1 minute after application, dry skin was improved, cracks became less deep
r
I
and epithelized.
r
SEQ ID NO 43:
oil
(CFGRKMDRISSSTGLGC) After 3 minutes, redness and
dry skin were improved. Skin got moisturized
0121 female 40's roughened skin B once a day promptly, and skin
texture was improved. Skin rawness, stiffness and
irritation were eliminated. Pores became less distinguished.
0122 female 40's rosacea/acne G once a day After 7 minutes, acne
was reduced and became dry.
0123 female 30's eczema C once a day
After 1 minute, redness and infiltrationeremitted. Iches were eliminated
immediatety
after application.
1-3 0
CD NO
Cr' CO
CO
Skin got moisturized promptly, wrinkles became less deep, skin texture was
0
improved.
5E0 ID NO 44: 0124 female 60's healthy skin
B once a day By using for 4 weeks,
large wrinkles and nasolabial fold became less deep. 0
i.---i
(CFGRKMDR1SSSAGLGC) Skin resilience
and flabbiness were improved. Spots faded. Skin tone
became brighter.
!
0125 female 60's eczema C once a day
After 1 minute, erythema and infiltration were remitted. dies
were remitted immediately after
application.
After 2 minutes, dryness, tautness and rawness of skin were eliminated.
Skin got full, and wrinkles became less deep. Skin gained elasticity.
SEQ ID NO 45: By using for 5
days, flabbiness was improved. Skin became brighter.
Q126 female 40's roughened skin B once a day
.
(CFSRRMDR1SSSSGLGC) nasolabial fold
became less deep.
By using for 4 weeks, nasolabial fold became less deep. Skin resilience and
flabbiness were improved. Spots faded and skin tone became brighter.
SEC! ID NO 46: After 2
minutes, erythema, scales and infiltration were remitted. Skin got
(CFSRKMDRISSTSGLGC) 0127 female 40's eczema C once a day
softened.
SEQ ID NO 47:
(CFSRKMDRISSSIGLGC) 0128 female 30's chronic eczema C once a day
immediately after application, erythema and scales were remitted.
SEC! ID NO 48:After 2 minutes, erythema, scales and infiltration were
remitted. Skin got P
(CFSRKMDRISSSSGIGC) 0129 male 50's eczema C once a day
moisturized.
SEQ 10 NO 49:0
ND
0130 male 30's chronic eczema C once a day
immediately after applicatioft erythema and scales were remitted.
(CFSRKMDRISSSSGLAC)
0
SEQ ID NO 50:on oo
0131 male 50's eczema C once
a day After 2 minutes, erythema, scales and infiltration were remitted.
(CFGRRMORISSTSGLGC)
0
1--1 SEQ ID NO 51:
0132 female 60's eczema B once a day
After 3 minutes.
erythema and infiltration were remitted. oo
on
0 (CFGRRMORISSSTGLGC)
ND
---,1
0
SEQ ID NO 52: ' After 2 minutes,
erythema, scales and infiltration were remitted. Skin got I"Q133 female
50's eczema C once a day ....1
(CFGRRMDRISSSSGIGC) moisturized.
I
i-i
i-i
5E0 ID NO 53:1
0134 female 20's eczema C once a day After
1 minute, erythema and infiltration were remitted.
=
(CFGRRMDRISSSSGLAC) = . - Or
SEC) ID 140 54 After 2
minutes, dryness, tautness and rawness of skin were eliminated,
0135 female 30's roughened skin B once a day
(CFGRKMDRISSTSGIGC) skin got full,
and wrinkles became less deep. Skin gained elasticity.
SEQ ID NO 55:
(CFGRKMDR1SSTSGUkC) 0136 female 50's eczema C once a day After
2 minutes, erythema, scale and infiltration were remitted.
1-3 0
p)
ts,D
cs- co
(I)
CO
0
SEQ ID NO 56:After 2 minutes, dryness, tautness and rawness of skin were
eliminated, I--,
0137 male 20's roughened skin 8 once a day
(CFGRKMDRISSSTGIGC) skin got full, and
wrinkles became less deep. Skin gained elasticity.
i¨....,
'
SEQ ID NO 57: After 2 minutes,
erythema, scales and infiltration were remitted. scratch
(CFGRKMDRISSSTGLAC) 0138 female 40's eczema C once a day wound
was epithelized and dried.
,
SEQ ID NO 58:
(CFGRKMDRISSSSGIAC) .
0139 female 30's eczema C once a day After
2 minutes, erythema, scales, papule and infiltration were remitted.
SEQ ID NO 59: After 2 minutes,
dryness, tautness and rawness of skin were eliminated,
0140 male 60's roughened skin 8 once a day
(CFSRRMDRISSTSGLGC) = Skin gained
elasticity. wrinkles became less deep
SEQ ID NO 60:
(CFSRRMDRISSSTGLGC) 0141 female 50's chronic eczema C once a day
immediately after application, erythema and scales were remitted.
SEQ ID NO 61:
fCFSRRMORISSSSGIGC) 0142 female 30's chronic eczema C once a day
immediately after application, erythema and scales were remitted.
SEQ ID NO 62:After 2 minutes, dryness, tautness and rawness of skin were
eliminated, P
0143 female 40's roughened skin 8 once a day
o
(CFSRRMDRISSSSGLAC) skin got full, and
wrinkles became less deep. Skin gained elasticity. ND
t.0
.
oo
SEQ ID NO 63:8 once a After 3 minutes, erythema and scales/infiltration
were remitted, and skin got
(CFSRKMDRISSTSGIGC)
en
0144 male 50's erzema
o
day
softened, ot
I--,
o,
0 SEQ ID NO 64:
00 (CFSRKMDRISSSTGIGC) 0145 male 40's chronic eczema
C once a day
immediately after application, erythema and scales were remitted. N)
0
I-'
SEQ ID NO 65: After 1 minute,
skin got moisturized and resilient, skin texture was -4
0146 female 40's roughened skin C once a day
1
(CFSRKMDRISSSTGLAC) improved.
r
r
SEQ ID NO 66:1
0147 male 50's eczema C once a day
After 2 minutes, erythema, scales and infiltration were remitted.
' ' ' r
. (CFSRKMDRISSSSGIAC) ,
Or
SEQ ID NO 67:ed After 2 minutes,
dryness, tautness and rawness of skin were eliminated,
0148 female 70's roughen skin
(CFGRRMDRISSTSGIGC) 8 once a day skin
got full, and wrinkles became less deep. Skin gained elasticity.
SEQ ID NO 68:
(CFGRRMDRISSTSGLAC) 0149 female 30's chronic eczema C once a day
immediately after application, erythema and scales were remitted.
SEQ ID NO 69:
(CFGRRMDRISSSTGIGC) 0150 female 20's eczema C once a day After
2 minutes, erythema, scales, infiltration were improved.
=====3
t=D
0151 female 60's chronic eczema C once a day immediately after
application, erythema and scales were remitted.
CO
SEQ ID NO 70:
(CFGRRMDRISSSTGLAC)
Redness vanished in 30 seconds. After 1 minute, skin texture improved
CO
0152 female 30's roughened skin C once a day
finely_ Pores became less distinguished.
0
SEQ ID NO 71:After 2 minutes, dryness, tautness and rawness of skin were
eliminated,
0153 male 50's roughened skin A once a day
(CFSRRMDRISSTSGIGC) skin got full, and wrinkles became
less deep. Skin gained elasticity.
SEQ ID NO 72:
0154 male 50's chronic eczema C once a day immediately
after application, erythema, scales and papule were remitted.
(CFSRRMDRISSTSGLAC)
SEQ ID NO 73:
0155 male 20's chronic eczema C once a day immediately after
application, erythema and scales were remitted.
(CFSRRMDRISSSTGIGC)
After 1 minute, rawness and hot flash were eliminated, skin got moisturized
0156 female 30's roughened skin C once a day
and its texture improved, wrinkles faded and skin gained elasticity.
SEQ ID NO 74,
(CFSRRMDRISSSTGLAC)
0157 male 60's chronic eczema D once a day Idles were eliminated
immediately after application. Redness was remitted.
SEQ ID NO 75:
0158 female 60's eczema C once a day After 2 minutes. erythema,
scales, papule, infiltration were remitted.
(CFSRRMDRISSSSGIAC)
SEQ ID NO 43:
0
(CFGRKMDRISSSTGLGC)
After 2 minutes, cracks were epithelized with no more pains and itches.
and 0258 male 40's atopic dermatitis C once a day
SEQ ID NO 19: Erythema and scales were also
remitted.
(CFGRRMDRISSSSGLGC)
SEQ ID N043:
(CFGRKMDRISSSTGLGC)After 2 minutes, wound was epithelized quickly, and
erythema, scales and
0356 male 40's eczema C once a day
infiltration were remarkably remitted.
and
SFO ID NO 39:
In SEQ ID NOS 16-75, 1st Cys and 17th Cys from left form a disulfide bond.
CA 02986086 2017-11-15
[0238]
The result described above demonstrated that the cyclic peptide of the
invention
exhibits a similar effect as the cyclic peptide expressed by Formula I-a even
if some
amino acids of the cyclic peptide have been replaced. Moreover, a similar
effect could
be obtained by applying the replaced cyclic peptide in a mixture as long as an
effective
amount of the cyclic peptide is applied.
[0239]
Specifically, in subjects having atopic dermatitis or eczema, effects were
observed
immediately after applying 3 pg/ml to 500 pg/ml to affected site, including
elimination
of itches, epithelialization at infiltrated site, papule or scratch,
termination of exudation,
epithelialization of a crack. Moreover, a single application induced a
persistent effect
for a week or more.
When it was applied to healthy or roughened skin, it exerted a moisturizing
effect
immediately or within a few minutes after application, improving dryness,
providing
and keeping skin and mucosa with moderate elasticity and flexibility,
softening skin,
giving skin or mucosa resilience and firmness. Also, fine wrinkles and
flabbiness are
improved at the applied site, and dullness and spots are further faded. A
single
application induced a persistent effect for a week or more.
[0240]
9.3 Confirming nasal effect
The obtained cyclic peptide was prepared in physiological saline at 3, 15, 30,
100 or 500
pg/ml, and 0.1 ml each was applied to nasal cavity. Each of formulations A to
E in the
table indicates the concentration of the cyclic peptide at 3, 15, 30, 100 or
500 pg/ml,
respectively. The absence of the effect upon the application of physiological
saline
without the peptide confirmed the effect was not a placebo effect.
110
1-3 Ci
CY =
ofli=
CO
1--,
SEQ ID NO (amino acid Diagnostic
Formulatio Diagnostic impression on skin after
i--,
ID Sex Age Treatment
Itches after application i--,
sequence) of cyclic peptides impression n application
,
From immediately after nasal application,
Itches were remitted
once a day, 0.1m1 and
nose got cleared, rhinorrhea stopped.
SEQ ID NO 16:
immediately after nasal
P1 female 10's allergic rhinitis C applied to each
nasal There was no irritationg symptoms, and the
(CFVRKMDRISSSSGLGC) application, and
eliminated
cavity effect
persisted for all day by 1 nasal
after 1 minute.
application.
From immediately after nasal application,
Itches were remitted
SEQ ID NO 17 once a day, 0.1m1 and nose got cleared, rhinorrhea
stopped.
:
P2 male 30's allergic rhinitis
D applied to each nasal There was no irritationg symptoms,
and the =
(CFGQKMDRISSSSGLGC) immediately after nasal
application, and eliminated
cavity effect
persisted for all day by 1 nasal
after 1 minute.
application.
After 3 minutes, and nose got cleared,
once a day, 0.1m1
SEQ ID NO 18:
rhinorrhea stopped. There was no
P3 male 50's allergic rhinitis
C applied to each
nasal P
(CFGHKMDRISSSSGLGC) cavity irritationg symptoms, and the effect
persisted for all day by 1 nasal application.
0
IV
cnronic
0
once a day, 0.1m1 After
1 minute, and nose got cleared. nasal 0
rhinitis,
qi
P4 female 40's perennial 0 Et
applied to each nasal
discharge stopped. There was no irritating 0
i
1--, SEQ ID NO 19:
rhinitic cavity
symptoms. qi
i.--, CFGRRMDRISSSSGLGC
()
iv
i--i once a day, 0.1m1
After 2 minutes, and nose
got cleared, and Itches were remitted 0
P5 female 70's allergic rhinitis B applied to each
nasal no nasal discharge came out by blowing immediately after nasal
...1
cavity nose.
application. i
i-i
,
i-i
SEQ ID NO 20:.
once a day, 0.1m1 After
1 minute, nasal obstruction was I
i-i
= = P6 female 40's allergic rhinitis B applied to each nasal
improved, and nose got cleared. The effect . = izi
(CFGRKLDRISSSSGLGC)
cavity
persisted for all day.
' once a day, 0.1m1
SEQ ID NO 21: After
1 minute' and nose got cleared.
P7 female 20's allergic rhinitis C applied to each
nasal
(CFGRKIDRISSSSGLGC) Nasal
discharge stopped.
, caWty
once a day, 0.1m1 Immediately after nasal
Immediately after nasal application' and
P8 female 50's allergic rhinitis E applied to each
nasal = = application. restless itches and
cavity nose
got cleared.
sneezing stopped.
SEQ ID NO 22:
(CFGRKMDRVSSSSGLGC) chronic
rhinitis, once a day, 0.1m1
After 20 seconds, and nose got cleared.
P9 female 40's C applied to each nasal No nasal discharge after 1
minute. There
perennial
cavity was no
irritating symptoms.
rhinitis
once a day, 0.1m1
SEQ ID NO 23: After
1 minute and nose got cleared'
P10 female 30's allergic rhinitis C applied to each nasal '
(CFGRKMDRIGSSSGLGC) cavity Nasal discharge stopped.
1-3 0
AD
LND
CY i=l=i=
CD 1¨.1
GO
I¨L
C.D
once a day, 0.1m1
SEQ ID NO 24: After 1
minute, and nose got cleared.
P11 female 20's allergic rhinitis C applied to each
nasal
(CFGRKMDRISASSGLGC) Nasal
discharge stopped.
cavity
once a day. 0.1m1
SEQ ID NO 25:
P12 female 20's allergic rhinitis A applied to each
nasal After 2.5 minutes, and nose got cleared.
(CFGRKMDRISSQSGLGC)
cavity
After 1 minute, nose got cleared and
once a day. 0.1m1
SEQ ID NO 26:refreshed.
P13 female 50's allergic rhinitis C applied to each
nasal
(CFGRKMDR1SSVSGLGC) cavity After 2
minutes, no nasal discharge by
brewing.
SEQ ID NO 27:' i once a day, 0 lml
P14 male 20's allergic rhinitis C ii After 1
minute, and nose got cleared.
(CFGRKMDR1SSLSGLGC) applied to each
nasal
once a day, 0.1m1
SEC) ID NO 28: After 1
minute and nose got cleared.
P15 female 40's allergic rhinitis C applied to each
nasal '
(CFGRKMDRISSISGLGC) cavity Nasal
discharge stopped.
once a day, 0.1m1
P
SEQ ID NO 29: After 1
minute, and nose got cleared.
P16 male 50's allergic rhinitis C applied to each
nasal
(CFGRKMDRISSMSGLGC) Nasal
discharge stopped. 0
cavity
ND
VD
SEQ ID NO 30:
once a day, 0.1m1
P17 female 30's allergic rhinitis C applied to each
nasal After 1 minute, rhinorrhea stopped. ci
(CFGRKMDR1SSSVGLGC)
i¨, cavity
m
i¨,
ND
once a day, 0.1m1 After 30
seconds, rhinorrhea stopped. 0
SEQ ID NO 31:
1-,
P18 female 10's allergic rhinitis D applied to each
nasal After 1 minute, no nasal discharge by ...1
(CFGRKMDR1SSSSRLGC)
i
cavity brewing.
.
1-,
. . . once a day, 0.1m1
After 1 minute, nasal obstruction was
ui
P19 male 40's allergic rhinitis C applied to each
nasal improved, and nose got cleared. The effect
cavity
persisted for all day.
SEQ ID NO 32:
(CFGRKMDRISSSSGMGC) chronic
Immediately after nasal application nose
once a day, 0,1m1
rhinitis, got
cleared. The effect was remarkable
P20 female 40's C applied to each
nasal
perennial cavity and
persisted for 7 days after 1 nasal
rhinitis
application.
once a day, 0.1m1 After 30
seconds, rhinorrhea stopped. i
SEQ ID NO 33:
Sneezing and itches n deep
P21 female 40's allergic rhinitis C applied to each
nasal After 1 minute, no nasal discharge by
(CFGRKMDRISSSSGIGC)
inside nose stopped.
cavity browing.
H 0
PD
to
Cr 14-
Ca
SEQ ID NO 34:once a day, 0.1m1
P22 female 40's allergic rhinitis C After 2.5 minutes,
and nose got cleared No irritating symptoms.
(CFGRKMDRISSSSGVGC) applied to each nasal
Ca
once a day, 0.1m1 After 1 minute,
nasal obstruction was
P23 female 30's allergic rhinitis C applied to each nasal
improved, and nose got cleared. The effect
SEQ ID NO 35: cavity persisted for all
day.
(CFGRKMDR1SSSSGAGC) chronic
once a day, 0.1m1
P24 female 40's D applied to each nasal
rhinitis, Immediately after
nasal application, nose
perennial got refreshed and
cleared.
rhinitis cavity
Immediately after nasal
once a day, 0.1m1
SEQ ID NO 36: Immediately after
nasal application, and application, itches were
P25 female 40's allergic rhinitis C applied to each nasal
(CFGRKMDRISSSSGLSC) nose got cleared.
removed. No irritating
cavity
symptoms.
After 30 seconds, rhinorrhea stopped.
Immediate effect to remove
P
Nose got cleard and felt good. Other nose itches.
once a day, 0.1m1 drops causes this
subject to sneeze due to c)
P26 female 40's allergic rhinitis C applied to each nasal some
irritant, but the inventive nose drop iv.
cavity had no irritation.
The effect persisted The .
SEQ ID NO 37 :
03c)
effect persisted for 1 week by 1 nasal
03
(CFGRKMDRISSSSGLAC)
i¨i
application.
1¨,
iv
Co ' chronic
c)
1-
once a day, 0.1m1 ...1
and r
rhinits, cleared
rhinorrhea stopped
P27 female 40's C applied to each nasal Nose got
clear i
perennial in 30 seconds.
1-
cavity
1-
rhinitis
1
.
1-
=
. . u,
SEQ ID NO 38:
once a day, 0.1m1 After 2 minutes,
rhinorrhea stopped. Nasal
P28 male 30's chronic rhinitis C applied to each nasal
obstruction was improved to allow, nasal
(CFARKMDRISSSSGLGC)
cavity breathing.
SEQ ID NO 39:
once a day, 0.1m1 Immediately after
nasal application, nose
P29 male 40's allergic rhinitis C applied to each nasal got
refreshed and cleared.
(CFSRKMDR1SSSSGLGC)
cavity No nasal discharge
after 30 seconds.
SEQ ID NO 40: once a day, 0.1m1
P30 female 30's allergic rhinitis C After 2.5 minutes,
and nose got cleared.
(CFTRKMDRISSSSGLGC) applied to each nasal
SEQ ID NO 41:
once a day, 0.1m1 P31 female 20's allergic rhinitis C applied to each
nasal After 1 minute, rhinorrhea stopped. There
(CFGRKMDRISSTSGLGC) was no irritating
symptoms.
cavity
once a day, 0.1m1
SEQ ID NO 42: Immediately after
nasal application, and
P32 female 10's allergic rhinitis C applied to each nasal
(CFGRKMDR1SSASGLGC)
cavity nose got cleared.
H 0
AD
b=D
Cr' i4
CO
1¨,
SEQ ID NO 43: once a day, 0.1m1
Immediately after nasal application, and i4=.=
(CFGRKMDRISSSTGLGC) P33 male 20's allergic rhinitis C applied to each
nasal nose got cleared. After 1 minute. no nasal
cavity discharge even by
twisting nose
.
.
SEQ ID NO 44 once a day, 0.1m1
: After 1 minute,
rhinontlea stopped. There
P34 mate 40's allergic rhinitis C applied to each nasal
(CFGRKMDRISSSAGLGC)
cavity was no irritating
symptoms.
SEQ ID NO 45:once a day, 0.1m1 After 1 minute, and
nose got cleared.
P35 male 60's allergic rhinitis C
(CFSRRMDRISSSSGLGC) , applied to each nasal
once a day, 0.1m1
SEQ ID NO 47: After 1 minute,
rhinorrhea stopped. There
P36 male 50's allergic rhinitis C applied to each nasal
(CFSRKMDRISSSTGLGC)
cavity was no irritating
symptoms.
once a day, 0.1m1
SEQ ID NO 48: After 1 minute, and
nose got cleared.
P37 female 50's allergic rhinitis C applied to each nasal
(CFSRKMDRISSSSGIGC)
cavity Nasal discharge
stopped.
P
once a day, 0.1m1
SEQ ID NO 49: Immediately after
nasal application, and c,
P38 female 40's allergic rhinitis
C applied to each nasal iv
(CFSRKMDR1SSSSGLAC)
cavity nose got cleared.
0
0
SEQ ID NO 50:
once a day, 0.1m1
0
0
1¨, P39 female 10's allergic rhinitis
C applied to each nasal
Immediately after nasal application, and m
I¨, (CFGRRMDRISSTSGLGC) nose got cleared.
cavity
iv
,I=.=
c,
,0.1m1
1-
SE0 ID NO 51: once a day After 1.5
minutes, nasal obstruction was ...1
P40 male 20's chronic rhinitis
C applied to each nasal 1
(CFGRRMDRISSSTGLGC)
cavity improved, and nose
got cleared. 1-
1-
.
I
once a day, 0.1m1
1-
SEQ ID NO 52: After 1 minute,
rhinorrhea stopped. There . . = u,
P41 male 30's allergic rhinitis D
applied to each nasal
(CFGRRMDR1SSSSGIGC)
cavity was no irritating
symptoms.
once a day, 0,1 ml
SEQ ID NO 53: Immediately after
nasal application, and
P42 male 40's allergic rhinitis C applied to each nasal
(CFGRRMDR1SSSSGLAC)
cavity nose got cleared.
once a day, 0.1m1
SEQ ID NO 54: Immediately after
nasal application, and
P43 female 30's allergic rhinitis C applied to each nasal
(CFGRKMDR1SSTSGIGC)
cavity nose got cleared.
once a day, 0.1m1
SEQ ID NO 55: After 1 minute, and
nose got cleared.
P44 male 50's allergic rhinitis C applied to each nasal
(CFGRKMDRISSTSGLAC)
cavity Nasal discharge
stopped.
SEQ ID NO 56:
once a day, 0.1m1
Immediately after nasal application, and
(CFGRKMDRISSSTGIGC) P45 male 30's allergic rhinitis C applied to each
nasal
cavity nose got cleared.
1-3 0
So NO
Cr 4
CO
once a day, 0.1m1
SEQ ID NO 57: After 1 minute, and
nose got cleared. 61
P46 female 40's allergic rhinitis C applied to each nasal
(CFGRKMDRISSSTGLAC)
cavity Nasal discharge
stopped.
SEQ ID NO 58:
once a day, 0.1m1 After 30 seconds,
rhinorrhea stopped, with Itches were removed in 30
P47 female 40's allergic rhinitis C applied to each nasal no more
sneezing . There was no irritating
seconds.
(CFGRKMDRISSSSG1AC) cavity symptoms.
once a day, 0.1m1
SEQ ID NO 59: Immediately after
nasal application, and
P48 female 20's allergic rhinitis C applied to each nasal
(CFSRRMDRISSTSGLGC)
cavity nose got cleared.
once a day, 0.1m1
SEQ ID NO 60: After 1 minute, and
nose got cleared.
P49 female 40's allergic rhinitis C applied to each nasal
(CFSRRMDRISSSTGLGC)
cavity Nasal discharge
stopped.
once a day, 0.1m1
SEQ ID NO 61: Immediately after
nasal application, and
P50 male 30's allergic rhinitis C applied to each nasal
(CFSRRMDR1SSSSGIGC)
cavity nose got cleared.
P
ip
once a day, 0.1m1 After 1 minute,
rhinorrhea stopped, with no
(CFSRRMDRISSSSGLAC)
IV
SEQ ID NO 62 :
0
P51 female 40's allergic rhinitis
C applied to each nasal more sneezing. There was no irritating 0
cavity symptoms.
0
i--, once a day, 0.1m1
m
1¨, SEQ ID NO 63: . Immediately after
nasal application, and
C)' (CFSRKMDRISSTSGIGC) P52 female 30's
allergic Mir-lifts C applied to each nasal
nose got cleared.
0
0
cavity
1-
...]
I
SEQ ID NO 64:
once a day, 0.1 ml After 1 minute,
rhinorrhea stopped, with no
(CFSRKMDRISSSTGIGC)
1-
1-
P53 male 60's allergic rhinitis
C applied to each nasal more sneezing. There was no irritating i
1-
. . cavity symptoms. .
once a day, 0.1m1
SEQ ID NO 65: After 1 minute, and
nose got cleared.
P54 female 30's allergic rhinitis C applied to each nasal
(CFSRKMDRISSSTGLAC)
cavity Nasal discharge
stopped.
once a day, 0.1m1
SEQ ID NO 66: Immediately after
nasal application, and
P55 female 30's allergic rhinitis C applied to each nasal
(CFSRKMDRISSSSGIAC)
cavity nose got cleared.
_
1-3 0
tap
L=D
Cr 14,-
1¨= (7)
Ca
once a day, 0.1m1
SEQ ID NO 67: After 1 minute and
nose got cleared.ci..,
P56 female 40's allergic rhinitis C applied to each nasal '
(CFGRRMDRISSTSGIGC) Nasal discharge
stopped.
cavity
SEQ ID NO 68:
once a day, 0.1m1 After 1 minute,
rhinorrhea stopped, with no
P57 mate 60's allergic rhinitis C
applied to each nasal more sneezing. There was no irritating
(CFGRRMDRISSTSGLAC)
cavity symptoms.
once a day, 0.1m1
SEQ ID NO 69: Immediately after
nasal application, and
P58 male 20's allergic rhinitis C
applied to each nasal
(CFGRRMDRISSSTGIGC)
cavity nose got cleared.
once a day, 0.1m1
SEQ ID NO 70: Immediately after
nasal application, and
P59 female 40's allergic rhinitis C applied to each nasal
(CFGRRMDRISSSTGLAC)
cavity nose got cleared.
,
SEQ ID NO 71: once a day, 0.1m1 After 1
minute, rhinorrhea stopped, with no
(CFSRRMDRISSTSGIGC) P60 female 30's allergic rhinitis C applied to each
nasal more sneezing. There was no irritating
cavity symptoms. .
SEQ ID NO 72 once a day, 0.1m1 After 1 minute, rhinorrhea stopped,
with no P
:
P61 female 40's allergic rhinitis
C applied to each nasal more sneezing. There was no irritating o
(CFSRRMDRISSTSGLAC)
"
cavity symptoms.
.
or
once a day, 0.1m1
o
SEQ ID NO 73: Immediately after
nasal application, and o,
P62 female 20's allergic rhinitis C applied to each nasal
i-, (CFSRRMDRISSSTGIGC)
, cavity nose got cleared.
o
ni
Cn SEQ ID NO 74:once a day, 0.1m1 After 1 minute,
rhinorrhea stopped, with no
(CFSRRMDRISSSTGLAC)
0
P63 female 40's allergic rhinitis
C applied to each nasal more sneezing. There was no irritating 1-
...1
cavity symptoms.
1
1-
1-
once a day, 0.1m1
1
SEQ ID NO 75: After 1 minute, and
nose got cleared. 1-
(CFSRRMDRISSSSGIAC) P64 = male 20's allergic rhinitis C applied to each
nasal
cavity Nasal discharge
stopped. = . ui
In SEQ ID NOS 16-75, 1st Cys arid 17th Cys from left Rim, a disulfide bond.
CA 02986086 2017-11-15
[0247]
The result described above demonstrated that the cyclic peptide of the
invention
exhibits a similar effect as the cyclic peptide expressed by Formula I-a even
if some
amino acids of the cyclic peptide have been replaced.
[0248]
Specifically, when it was applied to nasal cavity mucosa and/or paranasal
cavity mucosa,
it can improve or prevent various symptoms associated with rhinitis such as
nasal
obstruction, rhinorrhea, sneezing and pruritus. Rhinitis also includes,
without being
particularly limited, for example, infectious rhinitis including acute
rhinitis and chronic
rhinitis; hypersensitive non-infectious rhinitis including combined (flower
hypersensitivity) rhinitis, rhinitis with rhinorrhea, congestive rhinitis,
edematous
rhinitis and dry-nose type rhinitis; irritant-induced rhinitis including
physical rhinitis,
chemical rhinitis and radiation rhinitis; and atrophic rhinitis and idiopathic
granulomatous rhinitis; sinusitis such as acute sinusitis, chronic sinusitis
(maxillary
empyema), eosinophilic sinusitis and paranasal cavity mycosis. The external
preparation can be used for the purpose of treating or preventing one ore more
of the
above.
Combined rhinitis includes, for example, allergic rhinitis including perennial
allergic
rhinitis and seasonal allergic rhinitis, and nonallergic rhinitis including
vasomotor
(essential) rhinitis and eosinophilic rhinitis. Rhinitis with rhinorrhea
includes, for
example, gustatory rhinitis, cold air-induced rhinitis and senile rhinitis.
Congestive rhinitis includes, for example, drug-induced rhinitis, psychogenic
rhinitis,
gestational rhinitis, endocrine rhinitis and cold rhinitis.
Edematous rhinitis includes, for example, aspirin-hypersensitive rhinitis.
[02491
Among those mentioned above, it exerts an excellent effect specifically on
hypersensitive non-infectious rhinitis, irritant-induced rhinitis and
sinusitis, preferably
on hypersensitive non-infectious rhinitis and chronic sinusitis, more
preferably on
combined (flower hypersensitivity) rhinitis, rhinitis with rhinorrhea,
congestive rhinitis,
edematous rhinitis and dry-nose type rhinitis, and chronic sinusitis.
Therefore, the
external preparation of the present invention can be used for the purpose of
treating or
preventing at least one or more rhinitis selected from the above-mentioned
group.
117
CA 02986086 2017-11-15
In terms of its symptoms, for its effects as described above, it can be used
for any of
rhinitis with sneezing/rhinorrhea, rhinitis with nasal obstruction and
rhinitis with both
symptoms.
Immediately after a single application of 0.1 ml of 3 pg/ml to 500 pg/ml
preparation was
applied to the affected site in each nasal cavity, nasal discharge was
stopped, nasal
obstruction was improved, itches in nasal cavity mucosa or sneezing were
stopped
(immediate effect). A single application of a nasal drop induced persistent
effects
lasting for one day, or for a week or more in some cases (long-lasting
effect). In
addition, because it causes no local irritation at all, it can be used safely
even in a
subject who is not desired to use a nasal drop comprising rhinitis therapeutic
that has
conventionally been used such as steroid drug due to its strong local
irritation.
[0250]
Examples of multiple replaceable amino acids in the cyclic peptide of the
invention are
summarized below, based on working examples as described above, without not
being
limited thereto.
118
CA 02906006 201 /-11-16
[0251]
[Table 32]
Candidate multiple replacements of B ring
¨
ifip.sIdui Ammo,,,õ,R=mcILII Arn,no acla Flis.c1.4 '1, ,,
Amh,aaeld --. ' ' 11=111 ExonOio of
replaced am1 no acid sequence
__________________________________________________________ _ -
2 3 A,S 5 R CFSRRMDRISSSSGLGC
. ,
3 A,S 12 0,T .
CFSRKMDRISSTSGLGC
. .
. 3 A,S 13 T CFSRKMDRISSSTGLGC
_._ .
3 A,S 15 M,I,V,A
CFSRKMDRISSSSGIGC
. ._ ... . .. .
. .
: 3 A,S 16 A,S CFSRKMDRISSSSGLAC
R 12 Q,T CFGRRMDRISSTSGLGC
5 R 13 T =
CFGRRMDRISSSTGLGC
. .
5 R 15 M,I,V,A
CFGRRMDRISSSSGIGC
. .
5 R 16 A,S
CFGRRMDRISSSSGLAC
. .
12 Q,T 15 M,I,V,A
CFGRKMDRISSTSGIGC
. _..
12 Q,T 16 A,$
CFGRKMDRISSTSGLAC
, ...
13 T 15 M,I,V,A
CFGRKMDRISSSTGIGC
.. ,
._. .. _
=.. 13 T 16 A,S
CFGRKMDRISSSTGLAC
. _.. .
M,I,V,A 16 A,S . CFGRKMDRISSSSG/AC
3 33,1,S 5 R 12.Q,T CFSRRMDRISSTSGLGC
3-A,S SR 13 T
CFSRRMDRISSSTGLGC
.,..... .. .
3.A,S 5 R 15 M,I,V,A
CFSRAMDRISSSSGIGC
3:A,S 5 R 16.A,S
CFSRRMDRISSSSGLAC
3,A,S 12 Q,T 15,M,I,V,A
CFSRKMDRISSTSGIGC
... . . . .
3;A,S 13 T 15 M,I,V,A
CFSRKMDRISSSTGIGC
3 A,S 13 T 16 A,S
CFSRKMDRISSSTGLAC
.. . .
3 A,S 15 M,I,V,A 16.A,S
CFSRKMDRISSSSGIAC
' 5 R 12 Q,T 15 M,I,V,A CFGRRMDRISSTSGIGC
. ,
5=R 12 0,T 16 A,S
CFGRRMDRISSTSGLAC
5 R 13 T 15.M,I,V,A
CFGRRMDRISSSTGIGC
._,. 5 R 13 T 16 A,S
CFGRRMDRISSSTGLAC
.. ' 5 R 15 M,I,V,A 16 A,S
CFGRAMDRISSSSGIAC
.-
4 3=A,S 5 R 12 Q,T 15 M,I,V,A CFSRRMDRISSTSGIGC
. _.
3 A,S 5 R 12 Q,T 16 A,S
CFSRAMDRISSTSGLAC
3 A,S 5 R 13.T 15 M,I,V,A
CFSRINDRISSSTGIGC
õ.......,¨, . .
= L= '='= 3 A,S 5 R 13.T . 16 A,S
CFSRRMDRISSSTGLAC
,..
5 R 15 .M,I,V,A 16 A,S
CFSRRMDRISSSSGIAC
119