Note: Descriptions are shown in the official language in which they were submitted.
Title: Electrolytic reactor
Description
In process engineering, electrolytic reactors comprising one cathode and one
anode are
often used. When the reactor is operating, an electrical voltage is applied
between the
cathode and the anode such that the anode is consumed (sacrificial anode). For
example,
DE 10 2010 050 691 B3 and DE 10 2010 050 692 B3 describe a method and a
reactor for
recovering phosphate salts from a liquid whereby the sacrificial electrodes
consist of a
magnesium-containing material.
In particular, in the state of the art, many documents already disclose how in
the case of
sacrificial anodes, the cathode is designed to be movable such that the
distance between
the cathode and the anode can be kept constant. The object of the present
invention is to
provide an electrolytic reactor comprising two electrodes of different
polarity whereby at
least one of the two electrodes is a sacrificial electrode consisting of a
magnesium-
containing material and whereby the electrodes can be provided as cost-
effectively as
possible.
According to the invention, this object is achieved with an electrolytic
reactor, in particular
for separating phosphate from phosphate-containing liquids and recovering
phosphate
salts, comprising a housing, an inlet and an outlet for the liquid, and two
electrodes of
different polarity which enclose a reaction chamber between them, whereby at
least one of
the two electrodes is a sacrificial electrode made of a magnesium-containing
material
whereby the sacrificial electrode is constructed of trapezoidal bars having a
first and a
second upper surface where the first upper surface is smaller than the second
upper
surface, and where four lateral surfaces are provided which connect the first
upper surface
with the second upper surface.
1
CA 2986748 2017-11-27
Preferably, the sacrificial electrode consists of crude magnesium. In the
manufacturing
process, magnesium is cast in the form of trapezoidal bars as semi-finished
products. By
using the bars without another processing step, the cost of the magnesium that
is to be
used in a reactor can be kept relatively low. Alternatively the bars are
called ingots. The
electrolytic recovery of phosphorus as a crystallized magnesium ammonium
phosphate
(MAP or Struvite), with magnesium deficiency in the initial substrate,
proceeds according to
the following formula:
Mg2+ + NH4 + + P043- + 6H20¨> MgNH4 PO4 -6 H20
by liberating magnesium ions on the surface of a sacrificial anode of
magnesium. Then the
crystallized MAP can be removed from the liquid.
The bars are designed such that at least two of the edge lengths of the first
upper surface
are parallel, but that preferably all four edge lengths are shorter than those
of the second
upper surface. Hereafter, the latter will be called all-round trapezoidal
bars.
It is especially preferred when the bars - to form a continuous surface - are
arranged with
the first and second upper surfaces alternately facing the reaction chamber
and
complementing each other in form. The bars are arranged such that they abut
each other
with their preferably long lateral surfaces in case of rectangular basic
shapes such that the
one slanted lateral surface extends from the larger to the smaller surface of
a second bar
and is complemented such that an upper surface is plane when the electrode is
seen in top
view.
A plane or substantially plane surface is understood as such that a constant
continuous or
homogeneous surface is formed as seen in top view, and only smaller than 5%,
in
particular smaller than 3% of the entire electrode surface does not
participate in the surface
lying in one plane. Due to the diagonal lateral surfaces between the first and
second upper
surface, the plane surface of the electrode is somewhat smaller than the
surface actually
filled with the electrodes. This effect in the edge region, which with bars
arranged in two
2
CA 2986748 2017-11-27
rows can also occur between the rows, does not compromise the electrodes and
is
neglected in the definition of the plane surface if the portion is smaller
than 5% and
preferably smaller than 2%. The plane surface is to be maintained throughout
the entire
consumption of the electrode.
A particularly preferred arrangement can be that the bars have a longitudinal
direction and
that the longitudinal direction is transverse to the flow direction of the
reactor, i.e. in terms
of their first and second upper surfaces, the bars have a longer lateral edge
and a shorter
lateral edge, whereby the direction of the longer lateral edge is transverse
to the flow
direction of the reactor.
Alternatively, designs are also conceivable where the bars are arranged
lengthwise in flow
direction of the reactor. In principle it can be provided that the bars abut
each other with
their lateral surfaces which run in longitudinal direction. However, it is
also feasible in
principle that alternatively or additionally other bars are connected to the
shorter lateral
surface.
It is particularly preferred when both electrodes are sacrificial electrodes
and both are made
of a magnesium-containing material, and it is also preferred when both
electrodes consist
of trapezoidal magnesium-containing bars. Preferably, the bars can be made of
crude
magnesium. In that way, it can be achieved that by reversing the polarity, the
upper as well
as the lower electrode can be consumed since they are alternating between
serving as
anode and as cathode. Furthermore, by reversing the polarity, it can be
achieved that there
are no deposits forming on the electrode that serves as a cathode; otherwise
such deposits
must be removed by means of repeated rinsing. When the polarity is reversed
from one
electrode to the other, only minor deposits may result which are removed again
when the
electrode is subsequently used as an anode. This makes it possible to conduct
a
particularly good and continuous process.
In addition, it is particularly advantageous when an electrode can be moved
relative to the
other electrode. In that way, regardless of the consumption of the electrodes,
the distance
3
CA 2986748 2017-11-27
between the two electrodes can always remain constant. Therefore the
electrical field
between the electrodes is always even, and optimal conversion rates are
achieved in the
reactor while energy consumption is low.
It is generally advantageous when one of the electrodes is connected with a
housing base
and another electrode with an upper housing part. It is particularly
advantageous and
especially easy to accomplish when the electrode for the upper housing part is
moved
relative to the other electrode. If that electrode is not designed to be a
sacrificial electrode,
it can be made of stainless steel or other non-corrosive electrically
conductive material. It is
of special importance for the process that a constant distance is maintained
between the
surfaces of the electrodes regardless of any consumption. These surfaces
border a
channel through which the liquid to be treated is flowing. When the geometry
of the channel
and the electrical field between the electrodes (anode and cathode) are
constant, very
defined and very good conversion rates are achieved with a minimum of energy
consumption.
In an advantageous embodiment of the invention, the desired distance can be
achieved
when between the surfaces of the electrodes bordering the channel at least one
electrically
non-conductive spacer is provided that can be made of plastic or other
material. To prevent
the tilting of the electrodes against each other, it is usually an advantage
to provide at least
two spacers. The spacers are dimensioned such that the distance between the
electrodes
is maintained regardless of whether the electrodes are being consumed. The
channel
between the electrodes is regarded as a reaction chamber.
.. The distance can be held constant by means of gravity, by one or more
springs, or by one
or more actuators. When gravity is used, for example to track the upper
electrode to the
lower electrode - when the reactor is being operated - as a rule the electrode
provided in
vertical direction above the other electrode is tracked to the lower
electrode. If the
described spacer is provided between the electrodes, the distance between the
surfaces
bordering the reaction chamber is always held constant in a very simple and
reliable way
regardless of whether the electrodes are being consumed or not.
4
CA 2986748 2017-11-27
When actuators are used to track one electrode to the other, the spacing
between the
electrodes can be regulated or controlled with sensors which detect the
consumption, i.e.
the remaining thickness of the electrodes as part of a control circuit. All
commercially
available types of sensors can be used for this.
In principle, although not preferred, it is also possible with this invention
to move both
electrodes to keep the spacing between the electrodes constant.
It is easiest to keep the reaction room or the channel between the bordering
upper surfaces
of the electrodes constant, when the surfaces of both electrodes bordering the
reaction
chamber are plane.
Furthermore it is especially preferred when the surfaces of the electrodes
which border the
channel are rectangular, which means that essentially a cubic shape is
provided for the two
electrodes in spite of their thickness, whereby it applies what was said
above, that only the
edge region of the electrodes should deviate from the cubic form, where a
deviation of 5%,
but in particular of 2% related to the total surface of the bars is regarded
as a cubic form.
It is also possible to provide the inventive reactor with means to detect the
position of the
electrodes, to gauge the reactor operation, the process and the consumption of
the at least
one sacrificial electrode. For example, these means to detect the position of
at least one
electrode can consist of a position sensor of any design. Preferably this
position sensor is
fastened to the electrode that is movably attached to the housing of the
reactor if such an
electrode is provided. This is how the consumption of the sacrificial
electrodes can be
monitored in a simple and very reliable manner.
Finally, means are also provided to detect the electrical current flowing
between the
electrodes and/or the voltage applied between the electrodes. With this, the
process taking
place in the reactor can be monitored simply and reliably. Potential
malfunctions of the
5
CA 2986748 2017-11-27
process can lead to a change in the electrical current and/or voltage and can
thus be
simply detected.
It is particularly advantageous when the reaction chamber has a rectangular
cross-section
in flow direction and has a constant flow cross-section throughout the entire
reaction
chamber. This is how a particularly even and good reaction process can be
achieved, and
the conversion can be optimal.
The at least one electrode, namely the sacrificial electrode, consists of a
magnesium-
containing material. It is especially advantageous when pure magnesium is used
for this. It
is particularly preferred when both electrodes are designed as sacrificial
electrodes made
of magnesium-containing material, especially of pure magnesium.
To allow for a particularly simple way of contact for the electrodes,
especially when a
movable electrode is planned, it can be provided that the electrode is
contacted via a
flexible contact strip or a flexible contact chain that is in contact with
every one of the bars.
That way it can be assured that each of the electrodes can be securely
contacted. In
particular in case of bar-shaped elements, problems may occur since due to the
casting
process, the bars may show certain differences in height.
As a rule, the bars, in particular magnesium bars, are not cast in large-scale
processes but
manually or in small quantities, which means that fill fluctuations can be
expected which
may have the result that not all bars in the electrode may come in contact
with a contact
plate in the same way. Also, a certain shrinkage takes place during cooling,
which means
that the upper surfaces of the bars may not be completely plane but may show
some
retraction.
Since an oxide layer may form on the bars, it can happen that the potentials
of an electrode
do not equalize between the bars. It is therefore advantageous when contacting
is the
same via all the bars. By means of a contact chain or a flexible contact strip
it can be
6
CA 2986748 2017-11-27
achieved that the contact strip or the chain comes to lie on all the bars and
that therefore all
the bars are electrically contacted.
Other advantages and characteristics of the invention are shown in the
following drawings
where
Fig. 1 shows a longitudinal section through a magnesium bar in view (a)
and a cross-
section thereof in view (b),
Fig. 2 shows a first electrode design,
Fig. 3 shows an alternative electrode design, and
Fig. 4 shows an arrangement of the upper and lower electrode,
Fig. 5 shows an electrode in top view, and
Fig. 6 shows a sectional view through the bars of the upper electrode
with intended
contacting,
Fig. 7 shows two views of inventive reactors.
Fig. 1 shows a longitudinal section through a commercially available magnesium
bar with a
smaller upper side 1 and a larger upper side 2, whereby the two lateral
surfaces 4 and 5
between the upper surfaces 1 and 2 are shown in Fig. (a) and (b). The bar is
all-round
trapezoid.
Fig. 2 illustrates the alternate laying of the bars where the upper surface 1
and the upper
surface 2 are alternating depending on the assembly situation. Due to the
alternate laying
of the bars whose side surfaces 5 have the same slant, a plane upper surface
10 results
which serves to delimit the reaction chamber and as the upper surface of an
electrode. The
7
CA 2986748 2017-11-27
slanted parts of the lateral surfaces 4 and the slant of the outermost lateral
surfaces 5 (here
shown as 5a) have the effect that the plane surface in the edge regions cannot
be
completely maintained. However, the portion of this edge region is less than
5% of the total
surface and can therefore be neglected, such that the electrode surface can be
called a
plane surface 10. Apart from that it is also possible to connect further rows
of bars, as
shown in Fig. 3, with the result being an electrode 12 with a plane surface.
In this case,
depending on the arrangement, there can also be regions between the rows of
all-round
trapezoid bars which deviate from the plane surface. These regions are shown
here as 4a.
However, according to the invention, the total of all these regions is smaller
than 5% of the
total surface 10 of electrode 12.
The flow direction of a medium is indicated by reference number 14.
Such magnesium electrodes are used in particular to separate phosphate from
phosphate-
containing waste water where they serve as sacrificial electrodes in the
reactor.
Fig. 4 shows the arrangement of two electrodes 12 and 16, whereby electrode
16, which is
the upper electrode in the drawing, is movable in the direction of arrow 18
such that the
reaction chamber 20 between the electrodes always remains of the same size and
is thus
able to provide flow rates and conversion rates as constant as possible. The
polarity of
electrodes 12, 16 is alternated at intervals to reduce deposits forming on
electrodes 12, 16.
Both electrodes 12, 16 serve as sacrificial electrodes as required, whereby
deposits usually
form on the cathode. After the polarity is changed, the deposits can be
removed by the
liquid stream. In this case, too, the flow direction is indicated by reference
number 14.
Fig. 5 shows a top view of the lower electrode 12 where the upper surfaces 1
and 2, but
also the lateral surfaces 4 and 5 can be seen. The tapered lateral regions
ought to be no
more than 2% of the plane electrode surface.
Fig. 6 shows the upper electrode 16 in a cut-out view which indicates that due
to
differences in casting, the upper surfaces 1 and 2 on the side opposite
surface 10 can have
8
CA 2986748 2017-11-27
different height levels when surface 10 is plane, such that it is difficult to
establish contact
via a plate. Therefore, it is preferable according to the invention to make
contact for
alternating the polarity via a flexible contact strip 22, for example in the
form of a link chain,
such that every single bar comes in contact with contact strip 22.
In this manner, the secure contacting of all bars can be achieved.
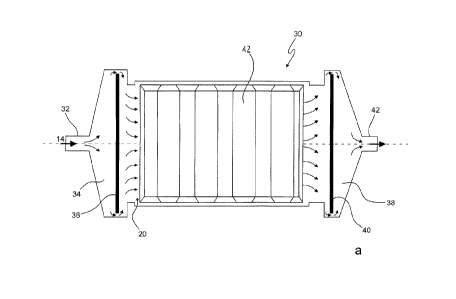
In views (a) and (b), Fig. 7 shows inventive reactors 30, whereby Fig. 7a
shows a section
through a reactor 20 with a view of electrode 12 according to Fig. 5. Here,
the liquid flows
via inlet 32 into a pre-chamber 34 and around a bulkhead 36 provided such that
the whole
stream is directed around bulkhead 36 which serves to equalize the flow. Then
the liquid
enters reaction chamber 20 between the electrodes 12 and 16. In reaction room
20, the
electrodes 12 and 16 are provided in the form of bars laid in alternation.
After flowing
through reaction chamber 20, the liquid flows through an after-chamber 38, and
there again
around a bulkhead 40 and through an outlet 42.
Fig. 7b shows a reactor 30' in another sectional plane, without any pre-
chamber or after-
chamber. Electrodes 12 und 16 constructed of the bars are accommodated in
housing 44
which comprises an upper part 48 and a base 46.
9
CA 2986748 2017-11-27