Note: Descriptions are shown in the official language in which they were submitted.
PROCESS FOR TREATMENT OF MINE IMPACTED WATER
TECHNICAL FIELD
[0001] The present application relates to the treatment of mine impacted
water.
BACKGROUND
[0002] Mine impacted water (MIW) is water that has been impacted by a
mining operation. Such water may include run-off water that has contacted
waste rock dumps generated during mining of coal, copper, or zinc. Over time
chemical and biological processes can become established within waste rock
dumps, gradually releasing constituents of interest from the waste rock to the
MIW.
[0003] Substantial removal of constituents of interest present in MIW can
be done using physical-chemical or biological water treatment processes or a
combination thereof depending on the constituents of interest to be removed.
[0004] For the purposes of the present application, MIW includes influent
water to or effluent water from the aforementioned physical-chemical or
biological water treatment processes, which can be active, semi-passive (e.g.
saturated rock fills), or passive (e.g. wetlands) water treatment facilities
in which
key constituents of interest are substantially removed.
[0005] Selenium, a key constituents of interest, may be present in MIW
and removal of selenium from MIW is desirable because of the possible effect
that selenium, above certain concentrations, may have on aquatic life and
organisms.
- 1 -
CA 2988187 2017-12-07
[0006] Many active, passive, and semi-passive water treatment processes
have been identified to remove selenium species from water. Processes using
biological treatment are currently utilized to remove selenium species from
MIW.
The final effluent from biological treatment processes may contain selenium in
reduced oxidation states in the form of organic and inorganic compounds.
[0007] Soluble selenium species exist in any of the (+6), (+4), (+2), (-
1)
and (-2) oxidation states.
[0008] The least bioavailable form of soluble selenium in receiving
environments is the selenate oxyanion in which selenium exists in the (+6)
oxidation state. Thus, if selenium is present in MIW, the (+6) form of
selenium is
desirable because this form of soluble selenium is the least bioavailable.
[0009] PCT patent application publication WO 2013/041898 describes a
method for removing trace organic and volatile selenium species present in
drinking water. Some of these species were considered contributors to bad
odour
in the water. The described method includes adsorption of reduced selenium
species on media containing oxides of either iron, aluminium, or titanium.
Reduced selenium species were largely adsorbed on the media and then partially
removed from the media by passing ozonated water through the media.
Selenium accumulation in the media was significant and in some cases less than
one percent of the selenium present in the media was removed by ozonated
water.
[0010] PCT patent application publication WO 2009/005834 describes the
introduction of ozone to the soil of a contaminated site followed by periodic
addition of hydrogen peroxide to inhibit formation of hexavalent chromium
within
the soil.
- 2 -
CA 2988187 2017-12-07
[0011] PCT Patent application publication WO 2009117141 discloses a
modular water treatment apparatus that utilizes side stream injection of
ozone.
The system utilizes ozone, with or without hydrogen peroxide, for disinfection
or
decontamination of the wastewater. The disinfected or decontaminated water is
suitable for reuse.
[0012] PCT Patent application publication WO 2017070347 describes a
method and system for decreasing the concentration of selenium species in
water, particularly water containing difficult-to-remove selenium species.
Water
containing selenium is first treated with potassium permanganate then treated
in
a two-step system comprising (a) a reactive solid containing zero-valent iron
and
iron oxide minerals in contact therewith and (b) ferrous iron. Thus, a
chemical
oxidant is utilized to oxidize the reduced selenium species (mostly selenite)
to
selenate as a pre-treatment to a physical-chemical treatment process (in this
case a zero-valent iron process). For the water tested, potassium permanganate
is the preferred oxidant. Hypochlorite is also utilized.
[0013] None of the above-noted references address the treatment of
various selenium species present in MIW using an advanced oxidation process
(AOP) system.
SUMMARY
[0014] According to a first aspect, a process for treating MIW including
one
or more selenium species is provided. The process includes subjecting the MIW
to an advanced oxidation process (AOP). The process includes subjecting the
MIW to an AOP including ultraviolet light and ozone or ozone and hydrogen
peroxide to oxidize the one or more selenium species to selenate and thereby
- 3 -
CA 2988187 2017-12-07
provide an AOP treated water. Residual oxidants are then removed from the
AOP treated water.
[0015] The primary treatment objective of AOP is to oxidize selenium
compounds present in MIW to selenate, thereby producing an AOP-treated water
that decreases selenium bioavailability of the treated water. Residual
oxidants
left after AOP treatment (mainly ozone and hydrogen peroxide) are removed
utilizing either chemical or physical-chemical means.
[0016] Other aspects and features of the present application will become
apparent to those ordinarily skilled in the art upon review of the following
description of specific embodiments of the application in conjunction with the
accompanying figures.
BRIEF DESCRIPTION OF THE DRAWINGS
[0017] Embodiments of the present application will now be described, by
way of example only, with reference to the attached figures, in which:
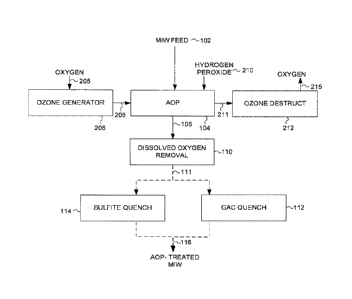
[0018] FIG. 1 is a simplified process flow diagram illustrating a process
for
treating MIW in accordance with an embodiment;
[0019] FIG. 2 is a simplified process flow diagram illustrating a process
for
treating MIW in accordance with another embodiment.
DETAILED DESCRIPTION
[0020] For simplicity and clarity of illustration, reference numerals may
be
repeated among the figures to indicate corresponding or analogous elements.
Numerous details are set forth to provide an understanding of the examples
described herein. The examples may be practiced without these details. In
other
- 4 -
CA 2988187 2017-12-07
instances, well-known methods, procedures, and components are not described
in detail to avoid obscuring the examples described. The description is not to
be
considered as limited to the scope of the examples described herein.
[0021] Generally, the present application discloses a process for
treating
MIW that contains one or more selenium species. The process includes
subjecting the MIW to an advanced oxidation process (AOP) including
ultraviolet
light and ozone or ozone and hydrogen peroxide to oxidize the one or more
selenium species to selenate and thereby provide an AOP treated water.
Residual oxidants are then removed from the AOP treated water.
[0022] As utilized herein, MIW refers to water that has been impacted by
a
mining operation such as a coal, zinc, or copper mining operation.
[0023] Water soluble reduced selenium species present in MIW are those
species in which selenium has an oxidation state less than (+6), including
selenite ion, selenocyanate and selenosulfate ions, low-molecular weight semi-
volatile species (e.g., dimethylselenide and dimethyldiselenide), and
organoselenium species such as dimethylselenoxide, methylseleninic acid and
seleno-amino acids such as selenomethionine and selenocysteine.
[0024] Because selenium in the (+6) oxidation state (selenate) is the
least
bioavailable form of dissolved selenium, it is desirable to oxidize reduced
selenium species to selenate. This is particularly important when MIW is
treated
to remove dissolved selenium by a water treatment facility (WTF) utilizing
biological treatment processes. Although, the dissolved selenium concentration
in
MIW after a biological WTF may be lower than 20 pg/L, some of this selenium
may be present in reduced oxidation states, often mostly in the (+4) oxidation
state as selenite ion (Se032-), but with material amounts of organoselenium
species.
- 5 -
CA 2988187 2017-12-07
[0025] Reduced selenium species present in MIW may be oxidized by an
AOP. The feed to AOP may be MIW at any state, that is as untreated water from
waste rock dumps, or water after full or partial treatment to remove selenium
in
a WTF. Treating MIW at the end of a WTF process train with an AOP is desirable
because the WTF process also decreases the total selenium concentration in the
water discharged to the receiving environment.
[0026] Referring now to FIG. 1, a process for treating MIW is shown. As
illustrated in FIG. 1, MIW 102 is subjected to an AOP treatment at 104. The
AOP
104 is carried out in presence of ozone 206 generated in a commercially
available ozone generator 208 that produces ozone gas from oxygen 205, at
concentrations of 2% to 20% weight (the remainder is principally oxygen
carrier
gas). The targeted transfer of ozone to water ranges from 3 mg/L to 25 mg/L.
Ozone transfer is achieved by injecting the oxygen-ozone gas mixture 206 into
the MIW 102 utilizing a suitable commercially-available ozone injection
system.
Ozone transfer efficiency (defined as the difference between feed gas and off-
gas
ozone concentrations divided by ozone concentration in feed gas) in commercial
systems may be over 90%, and may be higher than 95%.
[0027] Prior to or shortly after ozone addition to the MIW 102, hydrogen
peroxide 210 is added to the MIW to achieve a hydrogen peroxide concentration
in the range from 1 to 25 mg/L. Hydrogen peroxide is added to a target initial
hydrogen peroxide to ozone transferred mass ratio of 0.2 to 2, preferably 0.4
to
0.7. The AOP reactions then progress in single- or multi-stage commercially-
available contactors to achieve a desired gas-liquid contact time.
[0028] The total contact time of the MIW 102 with ozone 206 and hydrogen
peroxide 210 in the AOP treatment 104 may be 1 to 10 minutes and preferably 3
to 6 minutes. The oxidation reaction of selenium species may be completed in
less than 1 minute, and the remainder of the residence time in the contactors
is
- 6 -
CA 2988187 2017-12-07
utilized to separate and remove the excess gases (namely ozone and oxygen
mixture) from water.
[0029] The released off-gases 211 after AOP treatment are captured and
sent to an ozone destruct system 212 where ozone is converted to oxygen 215,
which is then vented to atmosphere.
[0030] A fraction of the excess dissolved oxygen present in the AOP
treated
water (produced from decomposition of the injected ozone) is removed utilizing
a
suitable dissolved oxygen removal process 110, such as a water cascade system
or a deaeration tank. The dissolved oxygen concentration present in the AOP
treated water may still be above its saturation level (up to 10 mg/L), after
subjecting the water to the dissolved oxygen removal process 110. The
presence of this excess dissolved oxygen does not appear to interfere with the
subsequent quenching step 112 or 114.
[0031] After the dissolved oxygen removal step, stream 111 is treated in
a
quenching step 112 or 114. Quenching is utilized to remove residual ozone and
hydrogen peroxide in the water to values below 10 pg/L and 0.5 mg/L,
respectively, because both of these oxidants may be toxic to aquatic life at
above
these concentrations. Removal of residual oxidants by quenching to these
target
values is effective despite still having excess dissolved oxygen in the water.
[0032] Residual ozone in the water is not stable and may decay within
tens
of minutes. Hydrogen peroxide also decays over time but at much slower rates
(tens of hours). Also, if residual hydrogen peroxide is present in stream 105,
residual ozone may be less than 10 pg/L. In absence of hydrogen peroxide in
steam 105, residual ozone concentrations may be higher than 10 pg/L.
[0033] Both residual ozone and hydrogen peroxide may be sufficiently
removed in a quenching step utilizing a variety of methods, for example
utilizing
- 7 -
CA 2988187 2017-12-07
reducing agents such as sodium or potassium sulfite or thiosulfate salts, or
utilizing granular activated carbon bed filters, or sand bed filters.
[0034] Residual oxidants may be removed by passing stream 111 through
a granular activated carbon column 112 having an empty bed contact time
(EBCT) of 2 to 10 minutes. Alternatively, residual oxidants may be removed by
sodium sulfite 114 utilizing, for example, 10 to 80 mg sodium sulfite per L of
water. Sodium sulfite dosage of 20 mg/L may be sufficient if residual hydrogen
peroxide in stream 111 is less than 1 mg/L and residual ozone is less than
pg/L.
[0035] After quenching step 112 or 114, at least 90% of the dissolved
selenium present in the MIW treated by the AOP system, stream 116, is present
as selenate ions. A fraction of the remaining dissolved selenium species
present
in stream 116 are selenite ions. This is a significant improvement from MIW
feed
102 in which, for some streams (e.g., after a WTF) less than 30% of the
dissolved selenium can be present as selenate ions. Thus, depending on the
total
concentration of reduced selenium species in the feed MIW 102 to be treated,
selenite concentration in the resulting AOP treated MIW 116 may be less than
2 pg/L, and may be less than 0.5 pg/L. Other residual reduced selenium species
may be present in MIW 116, at values close to or below analytical detection
limits.
[0036] The AOP treated MIW 116 contains low concentrations of selenite
ions and reduced selenium species. After equilibration with air (that brings
the
dissolved oxygen concentration to near its saturation value), MIW treated by
the
AOP system 116 may be further processed or discharged to the receiving
environment.
- 8 -
CA 2988187 2017-12-07
[0037] Referring now to FIG. 2, another process for treating MIW is
shown.
As illustrated in FIG. 2, the MIW 102 may be subjected to AOP treatment at 104
in presence of ultraviolet light (UV) 103 with continuous addition of ozone
206.
[0038] UV dosage may range from 1.4 to 2.4 Wh/L, with continuous
addition of ozone resulting in applied ozone dosages of from 47 to 57 mg/L.
[0039] The remaining processes indicated in Fig. 2 may be similar to
those
shown in FIG. 1 and described above. In the present example, utilizing UV, no
residual hydrogen peroxide is expected in stream 111 (since none was added).
However, some residual ozone may be present in this stream. The residual ozone
may be sufficiently removed to values below 10 pg/L using either the sodium
sulfite quench 114 or the GAC quench 112 as described above.
[0040] The process described herein is applicable to the treatment of MIW
under varying operating conditions (at various UV and ozone dosages, various
ozone and hydrogen peroxide dosages, and contact times). These operating
conditions may be adjusted depending on the MIW chemistry (e.g., total
selenium concentrations, oxidation states of selenium species, alkalinity,
total
suspended solids, total dissolved solids, pH, water temperature, total and
dissolved organic carbon, sulphate ion concentration, and concentrations of
other
organic and inorganic compounds). These variations may affect the oxidation of
selenium species, but the proposed process is still suitable under varied
conditions.
[0041] Advantageously, the present processes may be added at any stage
of a WTF to oxidize reduced selenium species to selenate. In addition, the
reagents used do not add deleterious compounds to the water being released to
receiving environment, i.e., ozone and hydrogen peroxide decompose to oxygen
and water and sodium sulfite is converted to sodium sulfate that already
exists in
the water. The process may also oxidize residual nitrite to nitrate in MIW
from a
- 9 -
CA 2988187 2017-12-07
biological WTF. Other advantages of the processes include short contact times,
relatively low ozone and hydrogen peroxide additions, or low UV light exposure
dosage in an advanced oxidation process to oxidize reduced selenium compounds
to selenate.
EXAMPLES
[0042] The following examples are submitted to illustrate embodiments of
the present invention. These examples are intended to be illustrative only and
not intended to limit the scope of the present invention. Tests were carried
out
using MIW that had been biologically treated in a WTF containing various
concentrations of selenium species and other constituents of interest.
[0043] Bench-scale tests were carried out to study the use of a chemical
to
oxidize or remove reduced selenium species in MIW (Method A) as a comparison
to the processes illustrated in FIG. 1 and FIG. 2. The tests were carried out
utilizing beakers holding 1-L to 2.5-L water volumes and using reagent grade
chemicals. The chemicals included potassium permanganate, potassium
persulfate, and hydrogen peroxide as oxidants and granular activated carbon as
adsorbent. Agitation was carried out utilizing radial impellers at 150 RPM.
The
tests were carried out at room temperature (17 C to 20 C) unless otherwise
specified.
[0044] Bench-scale batch AOP tests (Method B) were carried out to
evaluate ozone and hydrogen peroxide dosages applicable to the process shown
in FIG. 1, utilizing a glass reactor containing up to 2.5 L of MIW. Agitation
was
carried out using a magnetic stirrer. A mixture of oxygen and ozone gas was
continuously sparged at the bottom of the reactor using a ceramic frit
sparger.
Ozone was generated by passing 99% Vol. oxygen gas through a bench-top
corona discharge ozone generator (HG-1500 from Enaly). Hydrogen peroxide
doses were added at preset times during the experiments. The ozone generation
- 10 -
CA 2988187 2017-12-07
rate was approximated from equipment manufacturer data based on the oxygen
gas flow rate and electrical power setting. Ozone generation rate using 0.5
L/min
(STP) of 99% Vol oxygen flow rate was constant at about 10 to 20 mg of ozone
per minute. The produced ozone concentration was 2% to 5% Wt. and remaining
was oxygen. Treatment by AOP 104 (FIG.1) lasted from 5 min to 60 min. After
treatment, samples were aerated for 10 to 30 minutes to remove oxygen super
saturation. Residual hydrogen peroxide and ozone were removed with sodium
sulfite as quenching reagent. Chemical assays and spectrophotometry analytical
techniques were utilized to measure the residual ozone and hydrogen peroxide
in
AOP treated MIW.
[0045] Bench-scale AOP tests (Method C) were carried out to evaluate UV
light wavelength and dosages and ozone dosages applicable to the process
illustrated in Fig. 2. A bench-top UV unit (from ozone solutions UV-LAB-DE03)
was used with either a 254 nm lamp (part GL287/4, with "L" glass) or a 185 nm
lamp (part G8-9150, with "VH" glass). The lamps had a nominal output power of
14 W at 380 mA current, and a diameter of 15 mm and a length of 287 mm. The
UV lamp was installed inside a cylindrical chamber with a working volume of
water of 0.7 L. In some tests, two UV units were used in series to increase
contact time. In some examples, the feed MIW was recirculated through one UV
unit using a pump at a flow rate of 0.2 L/min (2-L total water treated) for a
contact time of 12 min, corresponding to a UV dose of 1.4 Wh/L (Method C.1).
In other examples two UV units were connected in series, treating water at a
flow rate of 0.2 L/min in a single pass treatment, corresponding to a UV dose
of
2.4 Wh/L (Method C.2). In both cases, ozone was generated by passing 99% Vol.
oxygen gas through a bench-top corona discharge ozone generator (HG-1500
from Enaly) as described above. The ozone gas was continuously injected in-
line
to the water fed to the UV units. In the UV-ozone tests, the ozone generation
- 11 -
CA 2988187 2017-12-07
rate based on equipment manufacturer data using 0.5 L/min (STP) 99% Vol
oxygen flow rate was constant at about 10 to 20 mg of ozone per minute. The
produced ozone concentration was 2% to 5% Wt. and the remaining was
oxygen. After treatment, samples were aerated for 10 to 30 minutes to remove
oxygen super saturation. Residual ozone was removed with sodium sulfite as
quenching reagent.
[0046] Pilot-scale testing (Method D) was also carried out for ozone-
hydrogen peroxide AOP system, as shown in FIG. 1, utilizing a commercially-
available AOP pilot unit for AOP 104 coupled to other equipment to carry out
the
dissolved oxygen removal 110, the sulfite quench 114, and the granular
activated carbon quench 112. The feed to the AOP pilot plant was MIW that was
previously partially treated in a full-scale biological WTF. The pilot plant
feed
water treatment rate was as high as 38 L/min. Ozone-oxygen gas mixture was
injected to the MIW using a venturi (side stream) system. Hydrogen peroxide
was added to the water prior to or shortly after injection of ozone. The water
was
then passed through an in-line mixer to mix fully the ozone and hydrogen
peroxide, and then sent to an array of in-series contactors, each with a
residence
time of about 1 min for a total of 4 min contact time. Ozone was generated by
passing 88% Vol. oxygen through a pilot-scale corona discharge ozone generator
at oxygen flow rate of 1.7-10.8 L/min (standard temperature and pressure),
producing 0.1 to 1 g/min ozone. The oxygen gas was produced from a pilot-scale
pressure swing adsorption air separator unit. Produced ozone concentration in
the gas phase was in the range 3-12 wt%, with the balance principally oxygen.
Transferred ozone dosage was calculated from ozone concentrations in the inlet
gas (containing the ozone-oxygen gas mixture) and in the off-gas. The off-gas
streams from the contactors were collected and were treated using a catalytic
ozone destruct unit to remove residual ozone before releasing the off-gas to
- 12 -
CA 2988187 2017-12-07
atmosphere. Oxygen super saturation from the A0P-treated MIW was removed in
a cascade system within the vessel for dissolved oxygen removal 110 in FIG. 1.
In the quenching step, sodium sulfite l0wt /0 solution was added to remove
residual ozone and hydrogen peroxide to values below 10 pg/L and 0.5 mg/L,
respectively. In parallel, two in-series granular activated carbon columns for
a
total empty bed contact time (EBCT) of 10 min were also tested to remove
residual ozone and hydrogen peroxide.
[0047]
Specialized analytical assay procedures were utilized to analyze the
water for selenium species. These assay procedures were carried out in a
commercially-certified analytical lab utilizing state-of-the-art techniques
and
analytical instruments:
1) Total and dissolved selenium assays were carried out by digestion of the
analytes in a closed vessel (bomb) with nitric and hydrochloric acids. The
dissolved selenium assay sample was filtered through a 0.45 pm filter prior to
digestion. The digested samples were analyzed for selenium content using
inductively coupled plasma-dynamic reaction-cell mass spectrometry (ICP-DRC-
MS).
2) Selenium speciation assays were carried out by chromatographically
separating the various selenium species in an ion exchange column and then
quantifying them using inductively coupled plasma collision reaction cell mass
spectrometry (ICP-CRC-MS).
As a reference, representative analytical detection limits (also referred to
as
Method Detection Limits) for the various selenium species are provided in
Tables 2 and 3. Selenium assays presented in Table 4 had the analytical
detection limits provided in Table 2. Selenium assays presented in Tables 5
and 6
had the analytical detection limits provided in Table 3.
- 13 -
CA 2988187 2017-12-07
Additional AOP tests were conducted at the commercial analytical lab site
adjacent to the ICP-CRC-MS such that the samples were processed immediately
to develop the sample preparation methods and to ensure that samples
remained stable during transportation to an external analytical lab.
EXPERIMENTAL
[0048] Table 1 shows approximate MIW chemistry that may be treated
utilizing the present method. The assays indicated in the average column
approximate the water chemistry utilized to carry out some of the experiments
presented in the examples. In the pilot plant campaign carried out as part of
this
work, MIW with the range of water composition indicated by the minimum and
maximum values Table 1 was tested.
- 14 -
CA 2988187 2017-12-07
[0049] In some experiments, feed MIW was spiked with reduced selenium
species to test the performance of the AOP at higher selenium concentrations.
Table 1: Approximate composition ranges of MIW from mining of coal
constituents of interest Unit Minimum Maximum Average
pH s.u. 6.5 8.0 7.3
Total suspended solids, TSS mg/L <1 40 1.5
Total dissolved solids, TDS mg/L 1160 2080 1671
Alkalinity as CaCO3 mg/L 295 576 341
Selenium-total g/L 11 325 19
Selenium, Dissolved p.g/L 10 333 17
Selenium+6 g/L 0.1 330 1.5
Reduced Selenium species [ig/L 3 94 12.7
Sulphate mg/L 534 907 821
Chloride mg/L 4 182 74
Nitrate-N mg-NIL <0.005 18.3 0.014
Nitrite-N mg-NIL <0.001 1.9 - 0.002
Ammonia-N mg-NIL <0.005 1.6 0.007
Magnesium mg/L 108 183 161
Calcium mg/L 175 277 248
Total Organic Carbon (TOC) mg/L 0.8 16.4 1.5
Total Inorganic Carbon (TIC) mg/L 40 171
67
UV transmittance % 93.5 98.6 96.3
[0050] In the following examples:
Se+4 is selenite ion;
Se+6 is selenate ion;
SeMet is selenomethionine;
MeSe is methylseleninic acid;
DMetSe0 is dimethyl selenoxide,
SeCN is selenocyanate ion;
SeS03 is selenosulphate ion;
- 15 -
CA 2988187 2017-12-07
UnK represents the total concentration of any unidentified selenium-containing
species detected by the speciation analytical technique;
SeD is the concentration of selenium in all dissolved selenium species in the
water (samples were filtered using a 0.45 pm filter prior to analysis);
SeT is the total concentration selenium in the water;
SeTd is the sum of the concentrations of all the selenium species assayed by
the
ICP-CRC-MS speciation analytical technique. Note SeD can be different than
SeTd
as some selenium species may not be detected using selenium speciation
analytical technique and because there are measurement uncertainties in both
the speciation and total dissolved selenium assays causing analytical errors;
Se+6R is the Selenate Ratio expressed as the ratio of the concentration of
selenium in the (+6) oxidation state to the sum of the concentrations of
selenium
species (SeTd). The higher this value, higher the relative proportion of
selenate
in the water;
ORP, mV, is the oxidation reduction potential of the solution at the end of
the
test (vs. Ag/AgCI);
HCI is hydrochloric acid;
03 is ozone; and
H202 is hydrogen peroxide
In the examples below the UV lamps and the ozone generator were sufficiently
run ("warmed up" for at least 15 min) prior to use to ensure stable operating
conditions.
Example 1
[0051] The following example illustrates the effect of various chemicals
as
oxidants and an adsorbent to oxidize or remove reduced selenium species from a
MIW treated in abiological WTF with water chemistry similar to that provided
by
- 16 -
CA 2988187 2017-12-07
the average assays in Table 1. The lab scale tests were carried out using
Method
A, referred to above, at room temperature (17 to 20 C).
[0052] Reagents were added to the MIW and after 240 min of mixing,
samples were collected and analyzed for the selenium species. Results are
provided in Table 2. Concentrations that were below the analytical detection
limit
(shown in the first row of the Table) are shown as zero.
TABLE 2
Selenate
Selenium Speciation,
ratio (%)
Reagent
6
Reagent dosage
ORP
mV' Se4 + Se+ Semet MeSe SeCN UnK SeS03 SeTd Se+6R
(mg/L)
Analytical Detection limit, g/L 0.15 0.3 0.15 0.15 0.25
0.30 0.3 -
Feed MIW 220 4.8 0.6 0 0 0 3.7 0 9.1 7
H202 1000 214 0.0 5.7 0.0 2.3 0.0 3.2 0.0
11.2 51
KMn04 500 605 8.6 6.9 0.0 8.4 0.0 4.2 0.0
28.1 25
K2S208 100 279 4.8 0.5 0.0 1.2 0.0 3.0 0.0
9.5 5
Activated
5000 165 6.1 4.1 0.0 0.3 0.0 0.0 0.0 10.5 39
Carbon
Conclusion
[0053] Hydrogen peroxide provided the highest selenate ratio (51%). The
other treatments were less effective. None of the treatments achieved the
target
selenate ratio of greater than 90%.
Example 2
[0054] The following example illustrates the use of AOP to treat samples
of
MIW that were partially treated in a WTF that utilizes a biological process to
remove nitrate and selenium.
- 17 -
CA 2988187 2017-12-07
[0055] Various water samples were taken every few days from three
different WTF process streams and were treated utilizing Method B and Method
C.2 (using 254 nm UV lamps).
[0056] Both treatments were carried out at a water temperature ranging
between 8 C and 12 C. The results from 16 different tests are shown in Table
3.
(N/A means not available and selenium assay values shown as averages for the
number of tests indicated in the brackets).
[0057] For AOP treatment utilizing Method B, the applied ozone rate was
constant at 9.5 mg/min. Ozone transfer efficiency was not measured as no off-
gas ozone monitor was available. Ozone sparging lasted for 15 min, for a total
applied ozone dosage of 57 mg/L treating 2.5 L of water. Hydrogen peroxide was
dosed at 10 mg/L every 5 min starting one minute after continuous ozone
sparging addition started (total hydrogen peroxide added was 30 mg/L). After
15
min of treatment utilizing ozone and hydrogen peroxide, MIW was aerated for 10
to 30 min by sparging air in the water and then samples were taken and
prepared for analysis.
[0058] For AOP treatment utilizing Method C.2, ozone was injected to the
feed MIW at a rate of 9.5 mg/min prior to passing it through to the UV system.
After 28 minutes of continuous treatment (to ensure steady state conditions),
ozone addition and UV exposure were stopped. Treated water samples were
collected over the last 7 min of treatment. Total applied ozone during the 7
min
of treatment was 47.5 mg/L and applied UV dose was 2.4 Wh/L. The treated
water were aerated for up to 30 minutes and then samples were taken and
prepared for analysis.
- 18 -
CA 2988187 2017-12-07
TABLE 3
Total and
Selenium Speciation, ug/L Dissolved
Selenate
Se, ug/L ratio, %
Se-'4 Se+6 SeMet MeSe SeCN DmSe UnK SeS03 SeTd SeD SeT Se+6R
Analytical
Detection limit, 0.015 0.030 0.005 0.005 0.025 0.005 0.060
0.030 - 0.7 0.7
ug/L
Feed MIW-1 4.7 0.7 0 0.13 0.18 1.4 0 0.019 7.1
10.9 14.1 10
Treatment (No. of
repeats)
03+H202 (5) 0.04 14.6 0 0.01 0.01 0.02 0.07 0 14.8
N/A N/A 99
UV+03 (2) 0.05 14.1 0 0 0 0.02 0.07 0 14.2
N/A N/A 99
Feed MIW-2 4.8 0.24 0 0.09 0.21 0.63 0 0 6.0
10.7 22.3 4
03+H202 (3) 0.06 16.4 0 0 0 0 0.17 0 16.6 N/A
N/A 99
UV+03 (2) 1.1 15.6 0 0.01 0 0 0.08 0 16.8
N/A N/A 93
Feed MIW-3 0.76 0.08 0 0.35 0.81 0.05 0.03 0.05
2.1. 7.2 23.7 4
03+H202 (3) 0.08 17.2 0 0 0 0.01 0.4 0 17.7
N/A N/A 97
UV+03 (1) 4.4 8.9 0 1 0 0.5 0.5 0 15.3
N/A N/A 58
Conclusion
[0059] The difference between SeTd and SeD assays for the feed MIW
waters revealed the presence of unaccounted reduced selenium species which
were not detected by the speciation analytical procedure. The higher SeT
values
versus SeD indicated the presence of particulate selenium.
[0060] The AOP treatment using ozone and hydrogen peroxide and
treatment using UV and ozone were both effective at increasing the proportion
of
selenate ion in the treated MIW as indicated by the increase in selenate
ratio.
[0061] The highest
selenate ratios (>96%) were consistently observed
when MIW was treated with ozone and hydrogen peroxide.
- 19 -
CA 2 9 8 8 1 8 7 2 0 1 7-1 2-0 7
[0062] For both MIW-2 and MIW-3, treatment using ozone and hydrogen
peroxide was more effective than treatment using UV and ozone, with up to
4.4 pg/L of selenite still present after treating MIW-3 with ozone and UV.
[0063] Treatment of MIW-3 using UV and ozone, resulted in only 58%
selenate ratio, compared to 97% using ozone and hydrogen peroxide.
[0064] Both feed MIW-2 and MIW-3 had more reduced selenium species
than feed MIW-1, as indicated by the lower selenate ratio water values in feed
MIW-2 and MIW-3 versus that of feed MIW-1, and the UV-ozone treatment may
be less effective as a result.
Example 3
[0065] The following example illustrates the use of ozone at different pH
to
oxidize reduced selenium species present in MIW with and without addition of
hydrogen peroxide.
[0066] The initial pH of the MIW was adjusted using hydrochloric acid,
HCI.
The range of pH tested was 4.6 to 8.4.
[0067] Also, feed MIW-4 and MIW-5 were spiked with reduced selenium
species to assess the robustness of selenium oxidation using AOP. The assay
results for the spiked feed MIW samples are shown in Table 4.
[0068] Tests were carried out under similar conditions as those described
in
Example 2 using ozone and hydrogen peroxide (Method B). In these tests, ozone
was added to the water at an applied rate of 9.5 mg of ozone per minute. Feed
MIW volume was 1.5 L and temperature was 10 to 13 C.
[0069] Ozone addition continued for either 15 minutes or 60 minutes, as
indicated in Table 4. Hydrogen peroxide, if added, was dosed at 10 mg/L every
5
or 10 minutes after commencing the addition of ozone, as indicated in Table 4.
- 20 -
CA 2988187 2017-12-07
[0070]
After the indicated total experiment time (15 min or 60 min), ozone
addition was stopped and water was aerated for 10 to 30 minutes and then
samples were taken for analysis.
Table 4
Dissolved
H202:
Selenium Speciation, ug/L Se, p.g/L Selenate
TOC
ratio, %
mg/L
Reagent pH added t,
Se+4 Se+6 SeMet MeSe SeCN UnK SeS03 SeTd SeD nigiL Se+6R
at min
Feed
MIW-4 8.2 12.2 1.3
0 2.2 2.2 0.1 0.02 18.0 26.9 3.2 7
(spiked)
03 8.4 60 0.1
29.7 0 0.06 0 1.7 0 31.6 32.8 5.5 94
03 6.1 60 0.2 21 0 0.03 0 5.7 0 26.9 27.1
4.9 78
every
03+H202 8.6 10 60 0.04 27.8 0 0 0 0.05 0 27.9 28 3.3 100
min
every
03+H202 4.6 10 60 0 25.1 0 0 0 0.3 0 25.4 28.1 2.6 99
min
Feed
MIW-5 8 10 1.1 1.6 2.5 1.8 2.3 0 19.3
27.1 1.4 6
(spiked)
03 7.9 60 0.1 19.1 0 0.1 0 2.6 0 21.9
26.7 2.2 87
03 8 15 0.1
18.6 0 0.1 0 2.5 0 21.3 25.7 1.7 87
every
03+H202 8 10 60 0 20.2 0 0 0 0.04 0 20.2 28.5 1.7 100
min
03+H202 8.1 every15 0 20.3 0 0 0 0.05 0 20.4 26.5 1.5 100
5 min
Conclusion
[0071] The difference between SeTd and SeD assays for the feed MIW
waters tested revealed the presence of unaccounted reduced selenium species in
the feed MIWs which were not detected by the speciation analytical procedure.
The higher SeT values versus SeD indicated the presence of particulate
selenium.
After AOP treatment, the SeTd and SeD assays were within measurement
- 21 -
CA 2988187 2017-12-07
uncertainty; however, indicating that most of the unaccounted selenium species
were oxidized to species that were detectable by the analytical procedure.
[0072] The results shown in Table 4 demonstrate that the reduced
selenium species (including selenomethionine and selenocyanate) were
significantly oxidized to selenate by AOP using ozone and hydrogen peroxide,
achieving a selenate ratio greater than 99%.
[0073] Ozone alone did not consistently achieve high selenate ratio
values
and oxidation target.
[0074] Good results were obtained using ozone and hydrogen peroxide
combination, even at pH as low as 4.6.
[0075] Total organic carbon (TOC) concentrations did not decrease in
these
tests, indicating that mineralization of TOC (i.e., conversion or organics to
carbon
dioxide) was not significant.
Example 4
[0076] The following example illustrates the use of UV light with ozone
to
treat MIW spiked with various selenium species.
[0077] Tests were carried out at different pH (natural pH and pH-adjusted
with HCl). A single UV unit was utilized with a lamp at a wavelength of either
185
nm or 254 nm. Water was recirculated through a single UV unit at a rate of 0.7
L/min from a beaker with 2 L total water volume into which ozone was
continuously sparged (Method C.1). Water temperature was kept at 12 to 15 C.
[0078] Ozone was added at a rate of 9.5 mg/min to a total of 57 mg/L over
12 minutes of. The total UV dose was 1.4 Wh/L. After 12 minutes of treatment,
ozone addition and UV exposure were stopped. The water was aerated for up to
30 minutes and then samples were taken for analysis.
- 22 -
CA 2988187 2017-12-07
TABLE 5
Dissolved
Selenium Speciation, ug/L Se assay, TOC
Se+6R
ug/L
pH Se+4 Se+6 Semet MeSe SeCN UnK SeS03 SeTd SeD mg/L
Feed MIW-6
7.9 16.6 1.2 0.02 4.4 0.7 0.6 0.08 23.4 29.7 1.8 5
(spiked)
185 nm 8 0.2 26 0 0.03 0 0.52 0 26.1 28.9
2.7 98
185 nm 6.1 7 16 0 0.03 0 1.1 0 24.5 29.2
2.6 67
Feed MIW-7
7.9 20.3 1.3 0 9.4 0.05 0.7 0.02
31.7 33.5 1.8 4
(spiked)
254 nm 8 0.2 28 0 0.04 0 0.8 0 28.3 31.1
2.6 97
254 nm 6.2 12.8 14 0 0.2 0 1.7 0 28.8 32.3
3.9 50
Conclusion
[0079] Sufficient oxidization of reduced selenium species was achieved
(selenate ratio higher than 97%) utilizing ozone and UV light, at both tested
wavelengths, at pH 8. Oxidation of selenium species was not sufficient at pH
6.1-
6.2 (selenate ratio 67% and 50%).
[0080] Total organic carbon (TOC) concentration did not decrease
indicating that mineralization of TOC did not occur.
Example 5
[0081] Examples 1 to 4 indicate that AOP treatments with either ozone and
hydrogen peroxide or UV and ozone can successfully oxidize reduced selenium
species. However, these examples were conducted at a small bench scale and
mostly batch mode in which important process parameters, including ozone
transferred to the water, were not measured.
[0082] Example 5 illustrates the treatment of MIW from a biological WTF
using ozone and hydrogen peroxide AOP and ozone only. A pilot-scale AOP
system was utilized to validate the success of AOP treatment at larger scale
and
- 23 -
CA 2988187 2017-12-07
continuous operating mode and to verify full-scale ozone transfer, contact
time,
and identify other process parameters.
[0083] Tests were carried out according to Method D and the process
shown in FIG. 1 to test the ozone and hydrogen peroxide AOP at different
operating conditions. Tests ran for several months at a temperature of 9 C to
11 C, at a water treatment rate of 38 L/min, a pH of 7.3 to 7.6, and a contact
time of 4 minutes. The pilot plant feed MIW composition was consistent with
values listed in the "Average" column of Table 1.
[0084] The AOP system was operated at constant transferred ozone
dosage. The hydrogen peroxide dosing rate was adjusted to obtain 0.5-1 mg/L
residual hydrogen peroxide in stream 105 of FIG. 1 after the AOP step 104. The
residual ozone concentration in stream 105 after the AOP step 104 was usually
less than 10 pg/L, but was as high as 2 mg/L. The measured dissolved oxygen in
this stream was generally 30-40 mg/L.
[0085] Transferred ozone dosage was calculated from the ozone gas
concentrations measured in the feed gas stream and in the off-gas streams and
water flow rate.
[0086] After the AOP, oxygen super saturation was normally removed by a
cascade system as described above as Method D. Residual oxidants (ozone and
hydrogen peroxide) were removed to values below 10 pg/L and 0.5 mg/L,
respectively, utilizing a sodium sulfite quench system or a granular activated
carbon bed with 10 min EBCT as referred to above as Method D.
[0087] A sodium sulfite dosage of 20 mg/L was found to be sufficient to
achieve the target for the residual oxidants. Samples of stream 116 were taken
after sulfite quenching for selenium speciation. The selenium speciation
assays
are shown in Table 6.
- 24 -
CA 2988187 2017-12-07
Table 6
Total and
Selenium Speciation, ttg/L Dissolved
Selenate
Se, lig/L
ratio %
Ozone
H202
transferr
Treatmen adde Se. Se. sem MeS SeC DMetSe Un SeS SeT Se
ed 4 6 SeT
Se+6R
t d et e N 0 K 03 d D
Dosage,
mg/L mg/L
Feed MIW- 10. 14.
- 3.8 0.5 0 0.1 0.1 1.3 0 0 5.8 8
8 4 3
03 o 4.7 0.1 9.8 0 0.1 0.0 1.5 5.2 0 59
16. 14. 15.
7 6 7
Feed MIW- 10.
- 2.9 0.7 0.0 0.1 0.1 1.4 0.0 0 5.2 13 13
9 9
0.0 13. 14. 13. 13.
03+ H202 1.2 5.1 0 0.00 0 0.1 0.9 0
93
2 3 6 8
0.1 13. 14. 13. 14.
03+ H202 2.0 5.1 0 0.03 0 0.1 1.1 0
90
5 1 5 4 1
Feed MIW- 10.
- 2.8 0.7 0.0 0.13 0.2 1.6 0.0 0 5.4 12 14
2
0.0 12. 13. 13. 13.
03+ H202 1.6 7.9 0 0.02 0 0.2 1.1 0
91
5 6 9 5 8
0.1 13. 14. 13. 14.
03+ H202 3.0 7.7 o o o 0.1 0.4 0
96
6 4 0 6 8
Conclusion
[0088] As in previous Examples, SeTd was less that SeD assays for the
feed MIW waters tested, indicating the presence of unaccounted reduced
selenium species, which were not detected by the speciation analytical
procedure. However, after AOP treatment, the SeTd and SeD assays were within
measurement uncertainty, indicating that most of the unaccounted selenium
species were oxidized to species that were detectable by the analytical
procedure.
[0089] Ozone alone was not effective at oxidizing all reduced selenium
species to selenate.
- 25 -
CA 2988187 2017-12-07
[0090] Ozone and hydrogen peroxide AOP achieved the target selenate
ratio (greater than 90%) at a relatively short contact time of four minutes in
the
AOP step.
[0091] Integration of oxygen removal, ozone destruction, and quenching as
part of an AOP system were also demonstrated in a pilot plant operating in a
continuous mode.
[0092] AOP oxidized some of the insoluble selenium, indicated by smaller
diffidence between SeT and SeD and the increase of the SeD after AOP than
before.
[0093] The total and dissolved selenium assays after AOP treatment were
close to one other, unlike in the feed MIW before AOP treatment.
[0094] In the feed MIW, a fraction of the selenium species could not be
accounted for by the speciation technique, but after AOP treatment, most of
the
soluble selenium species could be accounted for in the analytical technique
used.
[0095] MIW partially treated in a WTF was used in these tests. The MIW
had a significant level of background components (e.g., sulfate, carbonate,
chloride ions and total suspended solids) that did not impair the AOP
performance.
Example 6
[0096] Example 6 illustrates treatment of MIW from a biological WTF thaw
was also spiked with additional constituents of interest using ozone and
hydrogen
peroxide AOP. The pilot-scale AOP system of Example 5 was utilized to validate
the success of AOP treatment at larger scale and continuous operating mode and
to verify full-scale ozone transfer, contact time, and identify other process
parameters.
- 26 -
CA 2988187 2017-12-07
[0097] Tests were carried out according to Method D and process shown in
Fig.1 to test the ozone and hydrogen peroxide AOP at higher concentrations
constituents of interest. Tests ran for several days at a temperature of 9 C
to
C, at a water treatment rate of 38 L/min, a pH of 7.5 to 8.0, and a contact
time of 4 minutes.
[0098] These tests illustrate the performance of the AOP system at higher
alkalinity (440-508 mg/L), chloride (157-179 mg/L), TDS (1974-2015 mg/L), TIC
(123-135 mg/L using sodium bicarbonate), pH (7.6-7.7), and dissolved selenium
(56-66 pg/L) than for the tests of Example 5. The feed to the pilot AOP system
was otherwise similar to that in Example 5.
[0099] The AOP system was evaluated at three transferred ozone dosages.
The hydrogen peroxide dosing rate was adjusted to obtain 0.5-1 mg/L residual
hydrogen peroxide in stream 105 of FIG. 1 after the AOP step 104. The residual
ozone concentration in stream 105 after the AOP step 104 was usually less than
10 pg/L, but was as high as 2 mg/L. The measured dissolved oxygen in this
stream was in the range 34-50 mg/L.
[00100] Transferred ozone dosage was calculated from the ozone gas
concentrations measured in the feed gas stream and in the off-gas streams and
water flow rate.
[00101] After the AOP, oxygen super saturation was normally removed by a
cascade system as described above as Method D. Residual oxidants (ozone and
hydrogen peroxide) were removed to values below 10 pg/L and 0.5 mg/L,
respectively, utilizing a sodium sulfite quench system or a granular activated
carbon bed with 10 min EBCT as referred to above as Method D.
- 27 -
CA 2988187 2017-12-07
[00102] A sodium sulfite dosage of 20 mg/L was found to be sufficient to
achieve the target for the residual oxidants. Samples of stream 116 were taken
after sulfite quenching for selenium speciation.
[00103] The selenium speciation assays after the sulfite quench step are
shown in Table 7. The bracketed numbers indicates the number of samples used
to calculate the average values shown.
Table 7
Total and
Selenat
Selenium Speciation, g/L Dissolved
e ratio
Se, g/L %
Ozone
H202
transferre
Treatmen adde Se+ +6 SeMe MeS SeC DMetSe Un SeS0 Se
d 4 Se SeTd SeT
Se+6R
t d Dosage t e N 0 K D
3
,
mg/L
_____________ mg/L
Feed MIW- 44.
(1) 63. 0.9 0 0.2 0.1 1.3 0 0 46.7
61.7 2
11 (spiked) 2 5
59. 03+ H202(2) 3.7 4.9 0.9 o o o 0.01 0.3 0
60.8 65.57.0 98
8 5
Feed MIW- 38. 0.1 56.2
(8) 1.0 0 0.10 0.09 1.8 0.0 41.4 55.3 2.3
12 (spiked) 4 0
0.00 0.1
03+ H202(8) 6.9 11.0 0.3 56. 0.0 0.0 0.003
0.007 57.0 61.57.9 99
9 4 3 5 8
Feed MIW- 43. 0.1
(2) 1.1 0 0 0 2.20 0
47.0 62. 57.3 2
13 (spiked) 4 0 1
58. 0.1 . 7
03+ H202(2) 10.9 11.3 0.3 0 0 0 0.00 0 58.5
63 62.1 99
0 9
Conclusion
[00104] As in previous Examples, SeTd was less that SeD assays for the
feed MIW waters tested, indicating the presence of unaccounted reduced
selenium species, which were not detected by the speciation analytical
procedure. However, after AOP treatment, the SeTd and SeD assays were closer,
indicating some unaccounted selenium species were oxidized to species that
were detectable by the analytical procedure.
- 28 -
CA 2988187 2017-12-07
[00105] Ozone and hydrogen peroxide AOP achieved above the target
selenate ratio (greater than 90%) at a relatively short contact time of four
minutes.
[00106] Integration of quenching as part of an AOP system was also
demonstrated in a pilot plant operating in a continuous mode.
[00107] AOP oxidized some of the insoluble selenium, indicated by the
increase of the SeD after AOP than before.
[00108] In the feed MIW, a fraction of the selenium species could not be
accounted for by the speciation analytical technique, but after AOP treatment,
most of the soluble selenium species could be accounted for.
[00109] MIW partially treated in a WTF and spiked with alkalinity,
selenite,
and chloride was used in these tests. The higher concentrations of these
constituents of interest than in Example 5 did not impair the AOP performance.
[00110] The above-described embodiments of the application and the
examples are examples only. Alterations, modifications, and variations can be
applied to the particular embodiments by those skilled in the art without
departing from the scope of the application, which is defined solely by the
claims
appended hereto.
- 29 -
CA 2988187 2017-12-07