Note: Descriptions are shown in the official language in which they were submitted.
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
THERAPEUTIC USES OF BERBERINE FORMULATIONS
BACKGROUND OF THE INVENTION
[001] Red face related skin disorders, which share symptomatic similarities
and probably
pathological causes, include rosacea, acne vulgaris, seborrheic dermatitis,
photodermatitis and
contact dermatitis. These red face related conditions may range from feelings
of heat and
sensitivity to flushing or burning with intense sensitivity. Patients with red
face related skin
disorders often exhibit extreme sensitivity to environmental and topical
factors. Steroid-induced
rosacea-like dermatitis (or steroid rosacea) is papular or pustular lesions
with erythematous and
edematous base with or without telangiectasia, which is caused by prolonged
application of
topical steroids to the face or as a rebound condition after discontinuation
of topical steroids.
[002] Dermatologic toxicities are known cutaneous adverse events associated
with targeted
therapies or immunotherapy and share similar symptoms and probable pathologic
causes of the
red face-related skin disorders. Targeted therapies such as epidermal growth
factor receptor
(EGFR) inhibitors, multityrosine kinase (MTK) inhibitors, MEK inhibitors,
phosphoinositide
3-kinase (PI3K) inhibitors, protein kinase B (AKT) inhibitors, BRAF
inhibitors, HER2 inhibitor,
multikinase angiogenesis inhibitors, mTOR inhibitors, ALK/c-met inhibitors,
multikinase Abl
inhibitors, BTK inhibitors, HDAC inhibitors, proteasome inhibitors, and
retinoid X receptor (RXR)
agonists; immunotherapies such as cancer vaccines, cytokine agents (e.g.,
granulocyte-macrophage colony-stimulating factor (GM-CSF), interferons, and
interleukin-2
(IL-2)), cell therapies (e.g., tumor-infiltrating lymphocytes (TILs), T-cell
receptor
(TCR)-engineered peripheral blood lymphocytes (PBL), and chimeric antigen
receptor
(CAR)-engineered PBL), immune checkpoint protein inhibitors (e.g., PD-1, PD-
L1, CTLA-4,
TIM-3, LAG-3, BTLA, VISTA, and TIG1T), and immune checkpoint protein
stimulators (e.g.,
1
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
0028, ICOS, 4-1BB, 0X40, BITR, CD27, TWEAKR, HVEM, TIM-1, and CD-40); or a
combination of any of the above therapies could induce toxicities including
papulopustular rash,
maculopapular rash, erythema, telangiectasias flushing, paronychia and
fissure, hair changes,
xerosis, mucositis, pruritus, and hand-foot skin reaction, which may occur in
more than 90% of
patients and may also superinfected with bacteria, such as staphylococcus
aureus (Wollenberg,
Kroth et al., Cutaneous side effects of EGFR inhibitors--appearance and
management, Dtsch
Med Wochenschr 2010; Lacouture, Maitland et al., A proposed EGFR inhibitor
dermatologic
adverse event-specific grading scale from the MASCO skin toxicity study group,
Support Care
Cancer, 2010; Curry, Torres-Cabala et al., Dermatologic toxicities to targeted
cancer therapy:
shared clinical and histologic adverse skin reactions, International Journal
of Dermatology, 2014;
Jeffrey S. Weber et. al., Toxicities of Immunotherapy for the Practitioner,
Journal of Clinical
Oncology, Vol. 33, 2015; Grace K. Dy and Alex A. Adjei, Understanding,
Recognizing, and
Managing Toxicities of Targeted Anticancer Therapies, CA Cancer J Olin, Vol.
63, 2013; Ahmad
Tarhini, Immune-Mediated Adverse Events Associated with lpilimumab CTLA-4
Blockade
Therapy: The Underlying Mechanisms and Clinical Management, Scientifica, 2013;
J Larkin et al.,
Combined Nivolumab and lpilimumab or Monotherapy in Untreated Melanoma, N.
Engl, J. Med.,
Vol. 373, 2015). Histopathologic findings of such skin toxicities showed that
inflammation is
frequently involved and leads to acneiform skin rash. A papulopustular rash
was more frequently
reported on EGFR inhibitors like cetuximab (83% of patients) and afatinib (90%
of patients), and
MEK inhibitors like selumetinib (93% of patients) and trametinib (80% of
patients) therapy. A
maculopapular rash was more commonly described with PI3K inhibitors like 8KM-
120 (37% of
patients), MK2206 (52% of patients) therapy, immune checkpoint protein
inhibitors like
anti-CTLA-4 inhibitor ipilimumab (33% of patients), anti-PD-1 inhibitor
nivolumab (26% of
patients), or combination of ipilimumab and nivolimumab (more than 40% of
patients).
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
[003] Berberine (Natural Yellow 18, 5,6-dihydro-9,10-dimethoxybenzo(g)-1,3-
benzodioxolo
(5,6-a) quinolizinium) is an isoquinoline alkaloid present in herb plants,
such as coptis (Coptidis
rhizome), phellodenron, Scutellaria baicalensis, Mahonia aquifolium and
berberis. Berberine and
its derivatives have been found to have antimicrobial and antimalarial
activities. It can act against
various kinds of pathogens such as fungi, saccharomycete, parasite, bacterium
and virus.
[004] Berberine also has anti-inflammatory function, yet the exact
mechanism is unknown.
[005] U.S. Pat. No. 6,440,465 pertains to topical skin formulations of
glucosamine in an
emollient base which contains berberine for the treatment of psoriasis. U.S.
Patent Publication
No. 2005/0158404 pertains to a nutritional product, dietary supplement or
pharmaceutical
composition which contains vitamin A, vitamin E, selenium. vitamin B6, zinc,
chromium, and an
herbal source of berberine for the treatment of acne in oral administration.
U.S. Pat. No.
6,974,799 relates to topical compositions comprising a tripeptide (N-palmitoyl-
Gly-His-Lys) and a
tetrapeptide (N-palmitoyl-Gly-Gln-Pro-Arg) for the treatment of visible signs
of aging including
wrinkles, stretch marks, dark circles. The formulation may contain additional
ingredients,
including berberine. In these inventions, berberine is included as one of the
many ingredients
and its concentration is not specified.
[006] U.S. Patent Publication 2004/0146539 relates to topical nutraceutical
compositions
with body slimming and tone-firming anti-aging benefits that may be used to
treat skin aging, skin
wrinkle, skin exfoliating, acne, rosacea and other skin problems. The
composition of this
invention includes antimicrobial agents selected from several agents including
berberine. In
these nutraceutical compositions, berberine is included as one of the many
ingredients and its
concentration is not specified. There has been a 10% Mahonia aquifolium cream
(RelievaTM,
Apollo Pharmaceutical Canada Inc) containing 0.1% berberine for the treatment
of psoriasis.
3
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
[007] U.S. Patent Publication 2012/0165357 discloses the use of berberine
to treat various
red face related skin disorders but it does not disclose any specific
formulations of berberine that
would be found to be effective for the treatment of specific conditions,
[008] Therefore, there is still a need to develop new effective methods for
the treatment of
various red face related skin disorders as well as dermatologic toxicities
induced by targeted
therapy and/or immunotherapy.
SUMMARY OF THE INVENTION
[009] The present invention provides pharmaceutical compositions for the
treatment and/or
prevention of red face related skin disorders and dermatologic toxicities
induced by targeted
therapy and/or immunotherapy. The provided formulations are either cream-based
(i.e., cream)
formulations or gel-based formulations.
[010] In particular, the invention provides a pharmaceutical composition
comprising
berberine, wherein said composition is a cream formulation comprising a water
phase and an oil
phase.
[011] In one embodiment, the concentration of berberine in the provided
cream formulations
is between 0.01% and 10%, preferably between 0.01% and 0.3% w/w, more
preferably between
0.1% and 0.2% w/w, even more preferably between 0.1% and 0.15% w/w, and most
preferably
about 0.12% w/w.
[012] Unless explicitly stated otherwise, whenever the application
describes amounts or
concentrations in the w/w format, the weight of each ingredient is by the
total weight of the
formulation.
4
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
[013] The pharmaceutical compositions of the invention may further comprise
a penetration
enhancer.
[014] In one embodiment, the penetration enhancer is an anionic penetration
enhancer.
[015] In another embodiment, the penetration enhancer comprises Tween 60
and glycerin.
[016] In one embodiment, berberine is the only pharmaceutically active
component in the
provided formulations.
[017] In one embodiment, the pharmaceutical compositions of the invention
have a pH of
between about 4 and about 7, and more preferably of about 5.5.
[018] In a preferred embodiment, the invention provides a pharmaceutical
composition
comprising berberine as the only pharmaceutically active component, wherein
said berberine is
at a concentration of between 0.1% and 0.2% w/w, wherein said composition is a
cream
formulation comprising a water phase and an oil phase, wherein said
composition comprises a
penetration enhancer, a preservative, and a stabilizer, and wherein said
composition has a pH of
between about 4 and about 7.
[019] In an even more preferred embodiment, the invention provides a
pharmaceutical
composition comprising berberine as the only pharmaceutically active
component, wherein said
berberine is at a concentration of about 0.12% w/w, wherein said composition
is a cream
formulation comprising a water phase and an oil phase, wherein said
composition comprises a
penetration enhancer, a preservative, and a stabilizer, and wherein said
composition has a pH of
about 5.5.
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
[020] In another embodiment, the invention provides a pharmaceutical
composition
comprising berberine, wherein said composition is a gel-based formulation,
wherein said
composition comprises an anionic penetration enhancer.
[021] In a preferred embodiment, the anionic penetration enhancer comprises
sodium
dodecyl sulfate (SDS).
[022] In one embodiment, in the gel-based pharmaceutical compositions
provided by the
invention, about 90% of an average particle size of the berberine is less than
10 pm.
[023] In another embodiment, in the gel-based pharmaceutical compositions
provided by the
invention, about 50% of an average particle size of the berberine is less than
4 pm.
[024] In one embodiment, the concentration of berberine in the provided gel-
based
formulations is between 0.01% and 0.3% w/w, more preferably between 0.1% and
0.2% w/w,
even more preferably between 0.1% and 0.15% w/w, and most preferably about
0.12% w/w.
[025] The invention also provides methods of treating a red face related
skin disorder
comprising administering to a patient in need thereof a pharmaceutically
effective amount of the
pharmaceutical composition of the invention.
[026] In one embodiment, red face related skin disorder is selected from
the group consisting
of rosacea. acne vulgaris, seborrheic dermatitis, photodermatitis, contact
dermatitis,
steroid-induced rosacea-like dermatitis, and epidermal growth factor receptor
(EGFR)
inhibitor-induced skin disorder.
[027] The invention further provides methods of treating and/or preventing
dermatologic
toxicities induced by targeted therapy and/or immunotherapy comprising
administering to a
6
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
patient in need thereof a pharmaceutically effective amount of berberine
and/or a biologically
equivalent analogue thereof.
[028] In a preferred embodiment, the targeted therapy comprises therapies
by EGFR
inhibitors; MTK inhibitors, MEK inhibitors, PI3K inhibitors, AKT inhibitors,
BRAF inhibitors, HER2
inhibitor, multikinase angiogenesis inhibitors, mTOR inhibitors, ALK/c-met
inhibitors, multikinase
Abl inhibitors, BTK inhibitors, HDAC inhibitors, proteasome inhibitors, and
RXR agonists.
[029] In a preferred embodiment, the immunotherapy comprises therapies by
cancer
vaccines, cytokine agents (e.g., granulocyte-macrophage colony-stimulating
factor (GM-CSF),
interferons, and interleukin-2 (1L-2)), cell therapies (e.g., tumor-
infiltrating lymphocytes (TILs),
T-cell receptor (TCR)-engineered peripheral blood lymphocytes (PBL), and
chimeric antigen
receptor (CAR)-engineered PBL), immune checkpoint protein inhibitors (e.g., PD-
1, PD-L1,
CTLA-4, TIM-3, LAG-3, BTLA, VISTA, and TIGIT), and immuno checkpoint protein
stimulators
(e.g., CD28, 1COS, 4-1BB, 0X40, BITR, CD27, TWEAKR, HVEM, TIM-1, and CD-40).
[030] In a preferred embodiment, berberine and/or the biologically
equivalent analogue
thereof is administered to the patient in form of a topical pharmaceutical
composition.
BRIEF DESCRIPTION OF THE DRAWINGS
[031] The patent or application file contains at least one drawing executed
in color. Copies of
this patent or patent application publication with color drawing(s) will be
provided by the Office
upon request and payment of the necessary fee.
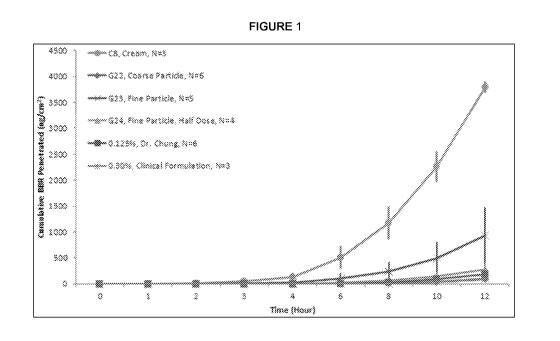
[032] Figure 1 is a plot of cumulative berberine penetrated (ng/cm2) vs
time for six tested
formulations of berberine.
7
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
[033] Figure 2 is a plot of cumulative berberine penetrated (ng/cm2) vs
time for three gel
suspension formulations of berberine (G22, G23 and G24).
[034] Figure 3 is a picture showing hematoxylin and eosin (H&E) staining of
bilateral skin
biopsies from nasolabial folds of a patient receiving afatinib and topically
administered with a gel
formulation (G23) on one side of his face.
[035] Figure 4 is a picture showing H&E staining of bilateral skin biopsies
from nasolabial
folds of the patient receiving the EGFR inhibitor afatinib and topically
administered with a vehicle
gel (G23 without berberine) on the other side of his face.
[036] Figure 5 is a plot of papule counts vs time for a patient receiving
afatinib and topically
administered with a gel formulation (G23) on one side of his face and a
vehicle gel (G23 without
berberine) on the other side of his face (* denotes P< 0.05 by Wilcoxon Signed
Rank test).
[037] Figure 6 is a plot of pustule counts vs time for the patient
receiving afatinib and
topically administered with the gel formulation (G23) on one side of his face
and the vehicle gel
(G23 without berberine) on the other side of his face (* denotes P< 0.05 by
Wilcoxon Signed
Rank test).
[038] Figure 7 is a plot of total counts (papule plus pustule) vs time for
the patient receiving
afatinib and topically administered with the gel formulation (G23) on one side
of his face and the
vehicle gel (G23 without berberine) on the other side of his face (* denotes
P< 0.05 by Wilcoxon
Signed Rank test).
DETAILED DESCRIPTION OF THE INVENTION
[039] Depending on a particular disorder being treated, it is important
that berberine can
efficiently penetrate the skin. Berberine is a hydrophilic compound (a
partition coefficient of 1.07
8
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
in an octanol-water system) which makes it hard for berberine to penetrate
through the stratum
corneum (SC) to reach the target site, e.g. dermis or epidermis, where red
face related skin
disorders or targeted therapy-induced dermatologic toxicities may occur.
Further, berberine is
rather soluble (solubility of 1.57 mg/ml) and will therefore be quickly
released into the target cells,
leading to a temporary effect.
[040] The present invention thus provides pharmaceutical compositions
having an improved
penetration rate of berberine for the treatment and/or prevention of red face
related skin
disorders and dermatological toxicities induced by targeted therapy and/or
immunotherapy. The
provided formulations are either cream-based (i.e., cream) formulations or gel-
based
formulations.
[041] In particular, the invention provides a pharmaceutical composition
comprising
berberine, wherein said composition is a cream formulation comprising a water
phase and an oil
phase.
[042] Because the cream formulations of the invention may promote the
penetration of
berberine into the skin, a relatively small amount of berberine is sufficient
to achieve desired
treating effects. In one embodiment, the concentration of berberine in the
provided cream
formulations is between 0.01% and 10% w/w, preferably 0.01% and 0.3% w/w, more
preferably
between 0.1% and 0.2% w/w, even more preferably between 0.1% and 0.15% w/w,
and most
preferably about 0.12% w/w, on the basis of the total weight of the
formulation.
[043] The pharmaceutical compositions of the invention may further comprise
a penetration
enhancer.
[044] In one embodiment, the penetration enhancer is an anionic penetration
enhancer.
For example, the anionic penetration enhancer may comprise sodium dodecyl
sulfate (SDS).
9
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
[045] In another embodiment, the penetration enhancer comprises Tween0 60
and glycerin.
The cream formulations of the invention preferably include Tween 60 and
glycerin as
penetration enhancers. When the same penetration enhancers are used in non-
cream
formulations, they do not result in an improved penetration rate, suggesting
that there is
something unique about the cream-based formulations.
[046] In one embodiment, berberine is the only pharmaceutically active
component in the
provided formulations. Even if an ingredient of the provided formulations may
be an active
component in prior art formulations for purposes other than treatment of
dermatologic toxicities
induced by targeted therapy or immunotherapy, it is still considered a
pharmaceutical excipient
for the purposes of the provided formulations as long as this ingredient is
not present at an
amount sufficient to effectively treat dermatologic toxicities induced by
targeted therapy or
immunotherapy.
[047] In one embodiment, the pharmaceutical compositions of the invention
have a pH of
between about 4 and about 7, and more preferably of about 5.5.
[048] In a preferred embodiment, the invention provides a pharmaceutical
composition
comprising berberine as the only pharmaceutically active component, wherein
said berberine is
at a concentration of between 0.1% and 0.2% w/w, wherein said composition is a
cream
formulation comprising a water phase and an oil phase, wherein said
composition comprises a
penetration enhancer, a preservative, and a stabilizer, and wherein said
composition has a pH of
between about 4 and about 7.
[049] In an even more preferred embodiment, the invention provides a
pharmaceutical
composition comprising berberine as the only pharmaceutically active
component, wherein said
berberine is at a concentration of about 0.12% w/w, wherein said composition
is a cream
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
formulation comprising a water phase and an oil phase, wherein said
composition comprises a
penetration enhancer, a preservative, and a stabilizer, and wherein said
composition has a pH of
about 5.5.
[050] It was very surprisingly and unexpectedly found that the cream
formulations of the
invention have a superior penetration rate compared to non-cream berberine
formulations.
[051] In another embodiment, the invention provides a pharmaceutical
composition
comprising berberine, wherein said composition is a gel-based formulation,
wherein said
composition comprises an anionic penetration enhancer.
[052] In a preferred embodiment, the anionic penetration enhancer comprises
sodium
dodecyl sulfate (SDS). Including SDS as an anionic penetration enhancer
results in the provided
gel-based formulations being hydrophobic (a partition coefficient of 50.1 in
an octanol-water
system) and having a dramatically lower solubility of about 0.011 mg/ml,
allowing for a slow
release of berberine into the target cells, resulting in an extended release
profile.
[053] It was found in the present invention that, in a pH of between 4 and
7, berberine
solubility in the presence of SDS ranges from 0.01 to 0.06 mg/mL, i.e., 25 to
150 times lower
than aqueous berberine solubility (1.57 mg/mL), and is relatively low at pH
5.5.
[054] It was surprisingly found that out of all tested penetration
enhancers (SDS, glycerol,
propylene glycol, PEG 400, ethanol, and Tween ), the addition of SDS in the
gel-based
formulations resulted in the most enhanced penetration rate and increased
local concentration of
berberine in epidermis and dermis.
[055] In one embodiment, in the gel-based pharmaceutical compositions
provided by the
invention, about 90% of an average particle size of the berberine is less than
10 pm.
11
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
[056] In another embodiment, in the gel-based pharmaceutical compositions
provided by the
invention, about 50% of an average particle size of the berberine is less than
4 pm.
[057] It was also surprisingly found that in the gel-based formulations
there was a positive
correlation between the amount of SDS and the penetration rate, and negative
correlation
between the size of berberine and the penetration rate.
[058] Because the gel-based formulations of the invention may promote the
penetration of
berberine into the skin, a relatively small amount of berberine is sufficient
to achieve desired
treating effects. In one embodiment, the concentration of berberine in the
provided gel-based
formulations is between 0.01% and 0.3% w/w, more preferably between 0.1% and
0.2% w/w,
even more preferably between 0.1% and 0.15% w/w, and most preferably about
0.12% w/w, on
the basis of the total weight of the formulation.
[059] The invention also provides methods of treating a red face related
skin disorder
comprising administering to a patient in need thereof a pharmaceutically
effective amount of the
pharmaceutical composition of the invention.
[060] In one embodiment, red face related skin disorder is selected from
the group consisting
of rosacea, acne vulgaris, seborrheic dermatitis, photodermatitis, contact
dermatitis,
steroid-induced rosacea-like dermatitis, and epidermal growth factor receptor
(EGFR)
inhibitor--induced skin disorder.
[061] The invention further provides methods of treating and/or preventing
dermatologic
toxicities induced by targeted therapy and/or immunotherapy comprising
administering to a
patient in need thereof a pharmaceutically effective amount of the
pharmaceutical composition of
the invention.
12
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
[062] In one embodiment, said targeted therapy is selected from the group
consisting of
EGFR, multityrosine kinase (MTK). MEK, phosphoinositide 3-kinase (PI3K),
protein kinase B
(AKT), BRAF inhibitors. HER2 inhibitor, multikinase angiogenesis inhibitors,
mTOR inhibitors,
ALK/c-met inhibitors, multikinase Abl inhibitors, BTK inhibitors, HDAC
inhibitors, proteasorne
inhibitors, and RXR agonists; said immunotherapy is selected from the group
consisting of
cancer vaccines, cytokine agents (e.g., granulocyte-macrophage colony-
stimulating factor
(GM-CSF), interferons, and interleukin-2 (1-2)), cell therapies (e.g., tumor-
infiltrating
lymphocytes (TILs), T-cell receptor (TCR)-engineered peripheral blood
lymphocytes (PBL), and
chimeric antigen receptor (CAR)-engineered PBL), immune checkpoint protein
inhibitors (e.g.,
PD-1, PD-L1, CTLA-4, TIM-3, LAG-3, BTLA, VISTA, and TIGIT), and immune
checkpoint protein
stimulators (e.g., CD28, ICOS, 4-1BB, 0X40, BITR, CD27, TWEAKR, HVEM, TIM-1,
and
CD-40); and said dermatologic toxicity induced by targeted therapy and/or
immunotherapy is
selected from the group consisting of papulopustular rash, maculopapular rash,
erythema,
telangiectasias flushing, paronychia and fissure, hair changes, xerosis,
mucositis, pruritus, and
hand-foot skin reaction.
[063] The concentrations of berberine in epidermis, dermis, and receiver
(which refers to a
container filled with PBS that in contact with the skin) are measured by the
following approach.
Franz diffusion cell setup is essentially a piece of skin clamped between two
clamps. The drug is
applied on one side of the skin (top) and drug concentration is measured in
the received portion
(bottom) of the setup.
[064] As used herein, the term "penetration rate" refers to an amount of
berberine that
presents in per gram of epidermis or dermis tissue, or an amount of berberine
per cm2 of skin
13
CA 02990237 2017-12-19
WO 2016/210230
PCT/US2016/039180
that presents in the receiver, after a certain time period from the
application of a formulation to
the skin.
[065] Amount of the drug measured in the receiver indicates the total
amount that penetrated
through SC, epidermis and dermis region of the skin. The pharmaceutical
composition of the
present invention has improved penetration rate, and the preferred range of
the penetration rate
is as follows:
Epidermis: 0.4 to 4000 pg of berberine per gram of tissue
Dermis: 0.003 to 30 pg of berberine per gram of tissue
Receiver: 0.0001 to 1 pg of berberine per 1X1 cm2 of skin.
[066] The following Table 1 lists various ingredients that may be used in
the compositions of
the invention. This list, however, is only provided for illustration purpose,
but not to limit the scope
of the present invention. Further, different ingredients/excipients can act in
more than one way,
e.g. can function as a penetration enhancer, an emulsifying agent, a wetting
agent, etc.
TABLE 1
Concentration
Excipient Function Replacements/Analogs
range ( /0)
Anionic
Sodium LaurylSulfate, Sulfonate, Phosphate, Oleate,
penetration 0.5-2.5
Sulfate monostearate and Carboxylates
enhancer
Carbopol
Carbopol 940, Carbopol 941, Carbopol
Gelling agent 0.3-3
934P 971, Carbopol 974, Carbopol 980,
1
Carbopol 981, Carbopol 5984EP,
Carbopol ETD 2020,
Hydroxyethyl Hydroxyethylmethyl cellulose,
Gelling agent 0.5-8
Cellulose
Hydroxypropyl cellulose, Hydroxypropyl
cellulose (Low-substituted), Methyl
cellulose, Methylhydroxypropyl cellulose
14
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
Concentration
Excipient Function Replacements/Analogs
range (%)
Quaternary ammonium compounds,
Methylparaben 0.02-0.3
Amino aryl acid esters, Alkyl/Aryl
alcohols, Alkyl/Aryl acids, Alkyl/Aryl
Preservatives
amides, Organomercurials,
Propylparaben 0.01-0.8
Formaldehyde donators, Biguanides,
Phenols
Dipotassium edetate, Disodium edetate,
EDTA Antioxidant 0.005-0.1 Edetate calcium
disodium, Sodium
edetate, Trisodium edetate
Glycerin Humectant
0.01-30 Propylene glycol, Polyethylene glycol
Tween 80 Wetting agent 0.1-3
Polyethylene glycol, Sorbitan esters
Anhydrous citric acid, Fumaric acid,
Citric acid
0.1-2
Malic acid, Sodium citrate dehydrate,
monohydrate
Buffering agent Tartaric acid
Sodium citrate
Anhydrous sodium citrate; citric acid
0.3-2
dihydrate monohydrate
Water Water
Calcium stearate, Magnesium stearate,
Polyoxyethylene stearates, Purified
Stearic acid Oil base 1-20
stearic acid, Zinc stearate, Lauric acid,
Myristic acid, Palmitic acid, Oleic acid
Mineral oil, Almond oil, Cocoa oil, Corn
Castor oil Oil base 5-12.5
oil, Coconut oil, Cotton seed oil, Linseed
oil, Olive oil, Soybean oil
Yellow petrolatum, Liquid petrolatum,
White
Oil base 4-56 Paraffin, Ceresin,
Microcrystalline wax,
petrolatum
Plastibase
Polyoxyethylene sorbitan fatty acid
SPAN 60 Emulsifying agent 1-15
esters,
Tween 60 Emulsifying agent 1-15 Polyethylene glycol, Sorbitan
esters i
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
[067] As used herein, the term
"berberine" refers to
5,6-dihydro-9,10-dirnethoxybenzo(g)-1,3-benzodioxolo (5,6-a) quinolizinium.
The invention also
contemplates the use of analogues of berberine which include but are not
limited to jatrorrhizine,
palmatine, coptisine, 9-demethylberberine, 9-demethylpalmatine, 13-
hydroxyberberine,
berberrubine, palmatrubine, 9-0-ethylberberrubine,
9-0-ethyl-13-ethylberberrubine,
13-methyldihydroberberine N-methyl salt, tetrahydroprotoberberines and N-
methyl salts thereof,
9-lauroylberberrubine chloride, and pharmaceutically acceptable salts of all
these compounds.
[068] As used herein, the term "pharmaceutically acceptable salts" includes
salts of acidic or
basic groups. Examples of pharmaceutically acceptable salts include those
derived from
inorganic acids, such as hydrochloric, hydrobromic, nitric, carbonic,
monohydrogencarbonic,
phosphoric, monohydrogenphosphoric, dihydrogenphosphoric, sulfuric,
monohydrogensulfuric,
hydriodic, or phosphorous acids and the like, as well as the salts derived
from relatively nontoxic
organic acids, such as acetic; propionic; isobutyric; maleic; malonic;
benzoic; succinic; suberic;
fumaric; mandelic; phthalic; benzenesulfonic; toluenesulfonic, including p-
toluenesulfonic,
m-toluenesulfonic, and o-toluenesulfonic; citric; tartaric; methanesulfonic;
and the like. Also
included are salts of amino acids such as arginate and the like, and salts of
organic acids, such
as glucuronic or galacturonic acids and the like.
[069] As used herein, the terms "treatment" and "treating" include
inhibiting the disease or
condition, causing a reduction in severity and/or frequency of symptoms,
elimination of
symptoms and/or underlying cause, prevention of the occurrence of symptoms
and/or their
underlying cause, ameliorating and/or improving a patient's condition. Thus,
"treating" a patient
with said compositions of the invention includes prevention of a particular
disorder in a
susceptible individual, as well as management of a clinically symptomatic
individual to inhibit or
16
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
cause regression of a disorder or disease, and maintenance of the current
state and/or
prevention of a progression of a disorder or disease. Treatment can include
prophylaxis, therapy,
or cure
[070] As used herein, the term "pharmaceutically effective amount" of the
compounds and/or
pharmaceutical compositions of the invention refers to a sufficient amount of
the compound
and/or composition to treat, inhibit, ameliorate or prevent various red face
related skin disorders,
including but not limited to, targeted therapy-induced dermatologic
toxicities, at a reasonable
benefit/risk ratio applicable to any medical treatment. It will be understood,
however, that the
total daily usage of the compounds and/or compositions of the present
invention will be decided
by the attending physician within the scope of sound medical judgment. The
specific effective
dose level for any particular patient will depend upon a variety of factors,
including the disorder
being treated and the severity of the disorder; activity of the specific
compound employed; the
specific composition employed; the age, body weight, general health, sex and
diet of the patient;
the time of administration, route of administration, and rate of excretion of
the specific compound
employed; the duration of the treatment; drugs used in combination or
coincidental with the
specific compound employed; and like factors well known in the medical arts.
For example, it is
well within the skill of the art to start doses of the composition at levels
lower than required to
achieve the desired therapeutic effect and to gradually increase the dosage
until the desired
effect is achieved.
[071] The pharmaceutical composition can further include a pharmaceutically
acceptable
carrier, and can be in solid or liquid form, including but not limited to,
tablets, powders, capsules,
pellets, solutions, suspensions, elixirs, emulsions, gels, creams, patch, or
suppositories,
including rectal and urethral suppositories.
17
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
[072] As used herein, the term "pharmaceutically acceptable carrier" refers
to a
pharmaceutically acceptable material, composition or vehicle, such as a liquid
or solid filler,
diluent, excipient, solvent or encapsulating material. A pharmaceutically
acceptable carrier is
compatible with the other ingredients of the composition, with the mode of
administration, and
not injurious to the patient. A pharmaceutically acceptable carrier may be
either aqueous or
non-aqueous. Pharmaceutically acceptable carriers include gums, starches,
sugars, cellulosic
materials, and mixtures thereof. Some examples of materials which can serve as
pharmaceutically-acceptable carriers include, but are not limited to: (a)
sugars, such as lactose,
glucose and sucrose; (b) starches, such as corn starch and potato starch; (c)
cellulose, and its
derivatives, such as sodium carboxymethyl cellulose, ethyl cellulose and
cellulose acetate; (d)
powdered tragacanth; (e) malt; (f) gelatin; (g) talc; (h) excipients, such as
cocoa butter and
suppository waxes; (i) oils, such as peanut oil, cottonseed oil, safflower
oil, sesame oil, olive oil,
corn oil and soybean oil; (j) glycols, such as propylene glycol; (k) polyols,
such as glycerin,
sorbitol, mannitol and polyethylene glycol; (I) esters, such as ethyl oleate
and ethyl laurate; (m)
agar; (n) buffering agents, such as magnesium hydroxide, aluminum hydroxide,
boric acid and
sodium borate, and phosphate buffers; (o) alginic acid; (p) pyrogen-free
water; (q) isotonic
saline; (r) Ringer's solution; (s) ethyl alcohol; (t) phosphate buffer
solutions; and (u) other
non-toxic compatible substances suitable for use in pharmaceutical
compositions.
[073] The compositions of the invention may be administered using any means
known in the
art, including but not limited to oral, nasal, parenteral, topical,
transdermal, or rectal routes of
administration. Preferably, the compositions are adapted for oral or topical
administration. For
example, the active ingredient of the composition can be formulated with
suitable excipients for
the preparation of tablets, capsules, pellets, troches, lozenges, solutions,
powders or granules,
suspensions, hard or soft capsules, patches and any other suitable forms.
18
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
[074] The invention also provides a method of treating and/or preventing
dermatologic
toxicities induced by targeted therapy and/or immunotherapy comprising
administering to a
patient in need thereof a pharmaceutically effective amount of berberine or a
biologically
equivalent analogue thereof.
[075] The invention further provides a method of treating and/or preventing
dermatologic
toxicities induced by targeted therapy and/or immunotherapy comprising
topically applying to
affected skin a pharmaceutically effective amount of a topical pharmaceutical
composition
comprising berberine or a biologically equivalent analogue thereof.
[076] In one embodiment, the topical pharmaceutical composition is in the
form of a lotion,
cream, ointment, paste, gel, spray, suspension, emulsion, foam, patch, powder
and liniment.
[077] In one embodiment, the topical pharmaceutical composition comprises
at least 0.02%
w/w, preferably about 0.1% to about 2% w/w of berberine or a biologically
equivalent analogue
thereof, wherein the amounts are by the total weight of the composition.
[078] In one embodiment, berberine or the biologically equivalent analogue
of berberine is
the primary pharmaceutically acceptable active component.
[079] In another embodiment, berberine or the biologically equivalent
analogue of berberine
is the only pharmaceutically acceptable active component.
[080] The following Examples demonstrate some aspects of the invention. The
Examples
are not meant to limit the invention in any way.
Example 1
Mouse Skin Penetration Study of Berberine Formulations
[081] The following six berberine formulations were compared: 08, 0.125%,
0.3%, G22, G23
and G24.
19
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
Formulations
[082] The formulations were as follows:
C8 (a cream-based formulation):
Water Phase
berberine (0.12%), Tween0 60 (1%), Glycerin (3%), methylparaben (0.1%),
propylparaben (0.02%), NaOH (to adjust pH to 5.5), and EDTA (0.02%).
Oil Phase
stearic acid (7.5%), castor oil (8%), white petrolatum (6%), and SPAN 60 (2%).
0.125% (gel-based formulation):
berberine (0.125%), ethanol (2.5%), glycerol (10%), phenoxyethanol (0.3%),
carbomer.
0.3% (gel-based formulation):
berberine (0.3%), propylene glycol (9.25%), PEG 400 (5.03%), methylparaben
(0.1%), propylparaben (0.02%), NaOH (0.4%), EDTA (0.02%), Carbomer 934P
(1%).
G22 (gel-based formulation):
berberine (0.1%), SDS (0.086%), glycerol (10%), Tween8 80 (0.5%),
methylparaben (0.1%), propylparaben (0.02%), citric acid (0.033%), sodium
citrate dihydrate (0.115%), NaOH, EDTA (0.02%), Carbomer 934P (0.3%), HEC
250 HHX (1.2%). Particle Size Distribution: 3.83/11.34/27.24 (in the format
D10/D50/D90, where each value refers to the respective percentage of particles
below the stated size, i.e. 10% of the particles are less than 3.83, and so
on).
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
G23 (gel-based formulation):
berberine (0.1%), SDS (0.086%), glycerol (10%), Tween 80 (0.5%),
methylparaben (0.1%), propylparaben (0.02%), citric acid (0.033%), sodium
citrate dihydrate (0.115%), NaOH, EDTA (0.02%), Carbomer 934P (0.3%), HEC
250 HHX (1.2%). Particle Size Distribution: 1.45/2.85/9.30
G24 (gel-based formulation):
berberine (0.1%), SDS (0.043%), glycerol (10%), Tween 80 (0.5%),
methylparaben (0.1%), propylparaben (0.02%), citric acid (0.033%), sodium
citrate dihydrate (0.115%), NaOH, EDTA (0.02%), Carbomer 934P (0.3%), HEC
250 HHX (1.2%). Particle Size Distribution: 1.55/2.86/5.44
[083] The particle size of G22, G23 and G24 formulations were determined as
follows.
[084] Purified water was prepared, then berberine chloride, Tween 80 and
sodium lauryl
sulfate (SDS) were added. After well dispersed, the mixture was micronized.
After that, the
particle size was measured by a diffraction analyzer.
Experimental Conditions
(085] Mice were sacrificed by cervical dislocation. The full-thickness
flank skin was removed
and placed on the diffusion cell in contact with receptor phase, which was
0.01M PBS (pH 7.4 at
37 C). Buffers were pumped through the receiver compartment at a flow rate of
3 - 4 mL/h. 3000
of formulations were added onto the skin surface in the donor compartment.
Receiver solutions
were collected at hour 0, 1, 2, 3, 4, 6, 8, 10, and 12 for HPLC analysis. Skin
flux was calculated
from slope of the linear part of the cumulative amount berberine chloride
penetrated versus time
curve.
21
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
Results
[086] Figure 1 demonstrates a plot of cumulative berberine penetrated
(ng/cm2) vs time for
all 6 tested formulations. As one can see, C8 (cream formulation) and G23 (gel-
based
formulation) penetrated the best as compared to the other formulations. This
was unexpected
because theoretically, all six formulations should penetrate with a similar
rate due to physical
properties of berberine in water phase.
[087] Figure 2 demonstrates a plot of cumulative berberine penetrated
(ng/cm2) vs time for
three gel suspension formulations (G22, G23 and G24). As one can see,
penetration rate is
positively correlated to the penetration enhancer (SDS) but is negatively
correlated to berberine
size. G23 and G24 with berberine size of D90 less than 10 pm have higher
penetration rate than
G22 with D90 higher than 10 pm.
Example 2
Mini-pig Penetration Study of Berberine Formulations
[088] The following berberine formulations were compared: 1) C8, G22, and
G23; and 2)
0.125%, 0.30%, and G23.
[089] Skin: Mini-pig ( Lanyu pig or Lee sung pig) skin dermatomed to 700
.Lni with electrical
resistance > 10 kO (Millicell-ERS, Millipore).
Penetration experiments:
[090] Pig skin was placed on the diffusion cell with dermal side in contact
with receptor
phase, which was filled with PBS (pH 7.4 at 37 C). 20 I of formulations were
added onto the skin
surface in the donor compartment. After 8 hours, the residual formulation on
the skin surface was
removed using three dry cotton swabs. At the end of 12 and 24 hours of
treatment with
formulations, skin was dismounted from the diffusion cell, again skin surface
was cleaned
22
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
carefully with three water-soaked cotton swabs. 10 tape-strippings were
employed to remove
stratum corneum. The skin was then placed on glass disc and heat-separated
into epidermis and
dermis at 60 C water bath for 90 seconds. Both the separated epidermis and
dermis were
weighed and minced, and extracted with 0.5 ml diluent (1%H3PO4:CH3OH (1:1)).
The skin
'extracts were centrifuged at 14,500 rpm for 20 min. Berberine chloride
concentrations in the
receiver solutions and supernatants from skin extracts were determined by
HPLC. Recovery of
berberine chloride from skin was determined by spiking known amounts of the
drug into skin
tissues and processed as described above.
Results
[091] Table 2 summarizes the results of this experiment.
Table 2
Run 1 (Comparing C8, G22, G23)
12hr Epidermis (pg/g) Dermis (pg/g) Receiver (pg/cm2)
08 6.15 0.22 0.069
G22 7.55 0.14 0.0625
G23 11.99 0.09 0.0025
24hr Epidermis (pg/g) Dermis (pg/g) Receiver (pg/cm2)
08 14.675 0.2 0.029
G22 30.95 0.43 0.1905
G23 100.315 0.64 0.0285
Run 2 (Comparing 0.125%, 0.30%, G23)
12hr Epidermis (pg/g) Dermis (pg/g) Receiver (pg/cm2)
0.125% 11.67 0.14 0
0.30% 22.09 0.41 0
G23 16.68 0.1 0
23
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
24hr Epidermis (pg/g) Dermis (pg/g) Receiver (pg/cm2)
0.125% 18.74 0.19 0.01
0.30% 39.83 0.15 0
G23 35.75 0.27 0.009
[092] The mini-pig skin penetration results indicate that: a) C8 (cream
formulation)
penetrates surprisingly well; b) formulations containing berberine particles
continuously released
over the 24 hours (G22, G23 vs 08, 0.125% and 0.3%); c) G22 & G23
(formulations containing
berberine particles) retained more berberine in the epidermis and dermis after
24 hours as
compared to 08 (cream formulation); d) formulations containing berberine
particles penetrated
better than formulations with berberine in solution (G23 vs 0.3%); e) G23
retained approximately
the same amount of berberine in the epidermis and more berberine in the dermis
after 24 hours
as compared to the 0.3% formulation even though G23 contained only 0.1%
berberine; and f)
compared with other penetration enhancers (ethanol and glycerol in the 0.125%
formulation and
propylene glycol and PEG 400 in the 0.3% formulation), the addition of SDS in
G23 resulted in
enhanced penetration rate and increased local concentration of berberine in
epidermis and
dermis.
Example 3
syin. Biopsy Results from a Patient Treated by Topical Formulation Comprising
Berberine
[093] The subject tested was a 56 year old male who received afatinib, an
EGFR inhibitor,
for treatment of non-small cell lung cancer (NSCLC). Upon receiving afatinib,
the subject started
applying topical gel of G23 formulation on one side of his face and vehicle
gel (G23 with no
berberine) on the other side once daily.
24
CA 02990237 2017-12-19
WO 2016/210230 PCT/US2016/039180
[094] Bilateral skin biopsies from nasolabial folds (both sides of the
nose) were collected
from the subject completing two-week topical treatment. Skin specimens were
obtained by
incisional biopsy measuring 1.0cm x 0.5cm, then histologically processed using
hematoxylin and
eosin (H&E) staining. Evaluation was performed by a trained
dermatopathologist.
[095] The H&E staining results (Figures 3 & 4) show that follicular
structure remains intact
and there was no inflammatory cell infiltrate for skin area treated with G23
(Figure 3) while there
was destruction of the follicular structure, profuse infiltration of
inflammatory cells at the
perifollicular region vacuolar change of the dermal-epidermal junction of
follicular epithelium for
skin treated with vehicle gel (Figure 4), indicating potential anti-
inflammatory effect of berberine
(G23) for treating EGFR inhibitor-associated skin toxicity.
Example 4
Facial Lesion Results from a Patient Treated by Topical
Formulation Comprising Borberine
[096] Subjects initiating afatinib inhibitors (EGFRI therapy) were enrolled
to receive half face
for G23 and the other half for vehicle gel (G23 with no berberine) during the
4-week treatment
period. Subjects were assigned at a 1:1 ratio to determine the side of face
for application of
study medications. Subjects initiated study medications within 1 day before or
after initiation of
EGFRI therapy. Study medication was administered once daily (QD) at bedtime
(HS) to the
designated half of the face.
[097] Number of facial lesions (papules and pustules) were evaluated at
weekly visits for four
weeks. As shown in Figures 5 to 7, a clear trend was found that the number of
lesions on the half
face treated with G23 is significantly lower than that on the other half,
indicating potential
therapeutic effect of berberine for treating EGFR inhibitor- or other targeted
therapy-induced or
immunotherapy-induced dermatologic toxicities.