Note: Descriptions are shown in the official language in which they were submitted.
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
IDENTIFICATION OF IMMUNOGENIC MHC CLASS II PEPTIDES FOR
IMMUNE-BASED THERAPY
CROSS-REFERENCE TO RELATED APPLICATIONS
This is a continuation-in-part of 'application Serial No.
PCT/US16/21042 filed March 4, 2016, which in turn is a continuation-in-part of
PCT/US1.5/41034 filed July 17, 2015, which in turn claims priority and benefit
from
U.S. Provisional Application Serial No. 62./076,789 filed November 7,2014. and
U.S.
Provisional Application Serial No. 62/025,681 tiled July 17, 2014, the
contents of
each of which are incorporated by reference herein in their entireties.
SEQUENCE LISTING
The instant application contains a Sequence Listing which has been
submitted electronically in ASCII format and is hereby incorporated by
reference in
its entirety. Said ASCII copy, created on June 14, 2016, is named 319-
003_PCT_CIP2_SL.txt and is 2,666 bytes in size.
BACKGROUND
In 25-30% of breast cancers, amplification and overexpression of the
growth factor receptor gene HER2 (human epidermal growth factor receptor-2,
also
known as nettierbB2) is associated with enhanced tumor aggressiveness and a
high
risk of relapse and death (Slamon. D., et al., 1987, Science 235: i77; Yarden,
Y.,
2001, Oncology 1:1): This oncogene encodes a 185 kilodalton (kDa)
transmembrane
receptor tyrosine kinase ("RTK"). As one of the four members of the human
epidermal growth factor receptor ("EGFR") family, HER2 distinguishes itself in
several ways. First. HER2 is an orphan receptor. No high-affinity ligand has
been
identified. Second. HER2 is a preferred partner for other EGFR family members
(HEM/EGER. HER3, and HER4) for the formation of heterodimers, which show
high ligand affinity and superior signaling activity. Third, full-length HER2
undergoes proteolvtic cleavage, releasing a soluble extracellidar domain
("ECD").
. Shedding of the ECD has been shown to represent an alternative activation
mechanism of' full-length HER2 both in vitro and in vivo, as it leaves a
membrane-
anchored fragment with kinase activity. The central role of HER2 in EGFR
family
signaling correlates with its involvement in the oncogenesis of several types
of
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
cancers, such as breast, ovarian, colon, and gastric cancers, regardless of
its
expression level (Slamon, D., et al., 1989, Science 244:707; Hynes, N., et
al., 1994,
Biochem. Biophys. Acta. 1198:165). HER2 may also render tumor cells resistant
to
certain chemotherapeutics (Pegram. M., et al., 1997, Oncogene 15:537). Given
its
vital role in tumorigenesis, HER2 is an important target for cancer
therapeutics.
The human EGF receptor ("HER") family of RIKs regulates a large
variety of biological processes including cell proliferation, -migration, -
invasion and -
survival. The family consists of four members: EGER (HER1), HER2 (mu or
ErbB2), HER3 (ErbB3) and HER4 (ErhB4). To date, eleven ligands have been
reported including epidermal growth factor ("EGF"), heparin-binding EGF-like
growth factor ("HB-EGF"), transforming growth factor .alpha. (TGF0,),
amphiregulin
(AR), epiregulin, betacellulin and the heregulins. These ligands bind directly
to their =
cognate receptors, which leads to the formation of receptor homo- or
heterodimers
that trigger the activation of multiple signaling pathways. Dysregulation of
members
of the HER-family either by activating mutations, receptor over expression or
aberrant
ligand release leads to the development of a variety of human tumors. HER3 is
over
expressed in breast-, ovarian- and lung cancer and this genetic feature has
been
correlated with poor prognosis. Upon activation by heregulins. HER3 dimerizes
with
HER2 and EGER to form potent oncogenic receptor heterodimers. Within this
complex, HER3 preferentially recruits P13 kinase to its cytoplasmic docking
sites
thereby regulating cell proliferation and -survival. So far it was assumed
that HER3
is kinase-inactive due to apparently aberrant sequence characteristics in its
kinase
domain and that it requires heterodimerization with a kinase-intact member of
the
HER-family in order to initiate signaling events. Consistent with this, it was
shown
that HER2 requires HER3 to drive breast tumor cell proliferation. However,
recent
findings of showed that HER3 is able to phosphorylate Pyk2 which results in
the
activation of the MAPK pathway in human glioma cells. Furthermore, monoclonal
antibodies specific for HER3 can inhibit the proliferation and migration of
cancer cell
lines. Interestingly, it was shown recently that cancer cells escape HER-
family
inhibitor therapy by up-regulation of HER3 signaling and that HER3 inhibition
abrogates HER2-driven ta.moxi fen resistance in breast cancer cells. Moreover,
= resistance to Gefitinib (lressa) therapy; an EGFR small molecule
inhibitor, was shown
to be connected to HER3 signal activation.
2
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
HER3 is a receptor protein that plays an important role in regulating
normal cell growth. HER3 lacks an intrinsic kinase activity and relies on the
presence
of FIER2 to transduce signals across the cell membrane. As initially
transcribed, the
pre-mRNA for HER3 contains 28 exons and 27 introns. The fully spliced HER3
= =5 inRNA from which the introits have been spliced out is composed of
28 exons.
Targeted therapy has emerged as the cornerstone of cancer therapeutics
in the last decade. Members of the EGF receptor family - namely EGFR (or HER!)
and ErbB2 (or HERZ/neu )-have evolved as particularly attractive targets,
since these
R.TKs are deregulated in a multitude of cancers. The oncogenic functions of
another
member of the EGF receptor family - ErbB3 or HER3- have only been recently
scrutinized due its major role in mediating resistance to HER2 and PI3K
pathway-
directed therapies. Activating mutations in and/or overexpression of HER3 has
been
identified in a number of different tumor types, including breast, gastric,
colon,
bladder cancer, and melanoma, and portend a worse overall prognosis in these
tumors.
Despite advances in the field, it is still uncertain whether effective
immune responses can be generated in humans using cell- or protein-based
vaccine
strategies targeting HER3. Accordingly, there is a need in the art to have
additional
immunotherapeutic approaches for treating or preventing breast cancer and
other
= malignancies with which overexpression of the 1-IER3 protein is
associated. The
present embodiments fulfill this need.
BRIEF DESCRIPTION OF THE DRAWINGS
The following detailed description of preferred embodiments of the
invention will be better understood when read in conjunction with the appended
drawings. For the purpose of illustrating the invention, there are shown in
the
drawings embodiments which are presently preferred. It should be understood,
however, that the invention is not limited to the precise arrangements and
instrumentalities of the embodiments shown in the drawings.
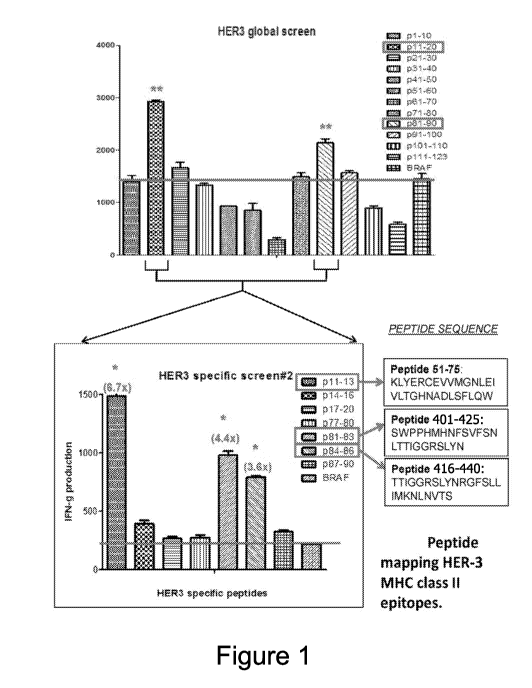
Figure! shows immunogenic peptides from HER3 that exhibit the
ability to activate CD4 T cells across-many patients (SEQ ID NOS 1-3,
respectively,
in order of appearance).
Figure 2 shows a HER3 global screen with groups of 10 peptide
fragments. Figure 2 also shows HER3 screen with single peptides (SEQ ID NOS 4-
7,
respectively, in order of appearance).
3
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
Figure 3 shows a HER3 global screen with groups of 10 peptide
fragments. Figure 3 also shows HER3 screen with single peptides (SEQ ID NOS 4
and 7, respectively, in order of appearance).
Figure 4 shows a HER3 global screen with groups of 10 peptide
fragments. Figure 4 also shows HER3 screen with single peptides (SEQ ID NOS 1-
3,
respectively, in order of appearance).
Figure 5 shows IFN-y production from different HER3 peptides.
Figure 6 shows IFN-y production from different HER3 peptides.
Figure 7 shows IFN-y production from a "REVERSE" screen, starting
with previously identified peptides, sensitizing to peptides and HER3
extracellular
domain.
Figure 8 shows IFN-y production from a "REVERSE" screen, starting
with previously identified peptides sensitizing to peptides and HER3
extracellular
domain,
Figure 9 shows IFN-y production from a "REVERSE" screen in a
patient not previously sensitized with HER extracellular domain, starting with
peptides, sensitizing to peptides and HER3 extracellular domain.
Figure 10 shows IFN-y production from a "REVERSE" screen in a
patient not previously sensitized with HER extracellular domain, starting with
whole
peptide library, sensitizing io peptides and HER3 extracellular domain.
Figure 11 shows IFINI-7 production from a "REVERSE" screen in a
patient not previously sensitized with HER extracellular domain, starting with
whole
peptide librai-y, sensitizing to peptides and HER3 extracellular domain.
Figure 12 shows IFN-y production from a "REVERSE" screen in a
patient not previously sensitized with HER extracellular domain, starting with
peptides, sensitizing to peptides and HER3 extracellular domain.
Figure 13 shows a sequential peptide screen in donor f UPCC 15107-
24.
Figure 14 shows a sequential peptide screen in donor 4 UPCC 15107-
38.
Figure 15 shows "REVERSE" sensitization in donor 4 UPCC 15107-
38 and UPCC 15107-24.
4
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
Figure 16 shows that immunogenic HER3 epitope-pulsed DC]
sensitized CD4+ Thl and overcame anti-1-TER3 immune tolerance in donor # UPCC
15107-30 and UPCC 15107-32 (both patients with known anti-1-TER3 non-
reactivity
to identified HER3 peptides and/or native HER3 ECD)..
Figure 17 shows immunogenic CD4+ HER3 epitopes demonstrate
MHC class IT promiscuity.
Figure 18 shows that when activated HER3 CD4+ cells are placed next
to HER3 expressing cells in a chamber, the HER3 CD4+ cells cause apoptosis or
death of 1-IER3 expressing cells breast cancer cells.
Figure 19 shows methods for identification of immunogenic Class II-
promiscuous HER3 CD4+ peptides using the ECD of HER3 as a tumor antigen in
order to generate anti-HER3 Thl cellular immunity.
Figure 20 shows confirmation of immunogenicity of identified CD4+
HER3 ECD epitopes by "reverse" sensitization. A HER3 ECD screen was performed
with single peptides shown.
Figure 21 shows additional results of confirmation of iminutiogenic4
of identified CD4+ HER3 ECD epitopes by "reverse" sensitization.
Figure 22 shows photographs of immunohistochemistry scoring of
HER staining.
Figures 23A and 23B are histograms showing rate of HER family
overexpression in Barrett's esophagus with low-grade dysplasia (LGD) or high-
grade
dysplasia (HGD) (Figure 23A) , and high-grade dysplastic Barrett's lesions
with
(HGD with carcinoma)) or without associated invasive cancer (HGD) (Figure
23B).
Figures 24A-24C show anti-HER3 C1)4 'Di I cell responses decline
from HDs (healthy donors) to ER IBC/ERP"sIBC (estrogen receptor positive
invasive
breast cancer ("IBC")) and TN IBC (triple negative IBC). The figures show
histograms (left panels) of IFN-y ELISpot analysis of systemic CD4 Thi cell
response. Patient groups studied were: HD; BD (benign breast biopsy): DC/IS
(HER2
positive ("HER2P '") ductal carcinoma in situ); HER2 IBC/HER2"' IBC; ER
IBCIERP" IBC (estrogen receptor positive IBC); and TN IBC (triple negative
IBC).
Corresponding tables to the right of the respective histograms are individual
comparisons by student's t-test between two groups at a time. One-way ANOVA
tests were performed on all groups. Figure 24A shows cumulative anti-HER3 CD4
Tcell response as measured by IFN-y spots per million cells via ELISpot assay
5
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
declined significantly going from HDs to BDs to DCIS to HER2 Pes IBC to ERP's
IBC
and finally to TN IBC (90 versus 80 versus 66 versus 79 versus 48 versus 40, p-
0.01,
respectively). Figure 248 shows repertoire, or the number of HER3 peptides
with a
positive CD4 Thl response, declined significantly going from HDs to BDs to
DCIS to
HER2P"s IBC to ERP" IBC and finally to TN IBC (1.0 versus 0.6 versus 0.8
versus 0.8
versus 0.5 versus 0.3, p=0.003, respectively). Figure 24C shows responsivity,
the
percent of subjects responding to at least I peptide, declined significantly
going from
liDs to BDs to DCIS to HER21"5 IBC to ERP" IBC and finally to TN IBC (76.7%
versus 63.6% versus 53.8% versus 66.7% versus 45.0% versus 33.3%, p-0.02,
respectively).
Figures 25A and 258 show loss of CD4 I cell response is specific to
HER3 as there are no differences in tetatnus or anti-CD3/CD28 stimulation
between
tested patient groups. The figures show histograms (left panels) of IFN-7
ELISpot
analysis of systemic CD4 f Thl cell response. Patient groups studied were: HD;
BD;
DCIS; HER2 IBC/HER21"5 IBC; ER IBC/ERP`" IBC; and TN IBC. Corresponding
tables to the right of the respective histograms are individual comparisons by
student's Hest between two groups at a time. One-way ANOVA tests were
performed on all groups. Figure 25A shows there were no statistically
significant
differences in tetanus response as measured by ITN-7 spots per 200,000 cells
via
ELISpot assay between Ds, BDs, Das, HER2 IIICHER2P05 IBC, ER IBC/ERP"
IBC or TN IBC (37 versus 30 versus 19 versus 34 versus 24 versus 29, p=0.65,
respectively). Figure 25B shows there were no statistically significant
differences in
anti-CD3lanti-CD28 polyclonal stimulation as measured by 1FN-7 spots per
200,000
cells via ELISpot assay between HDs, BDs, DCIS, HER2 IBC/HER2P" IBC, ER
IBC/ER" IBC or TN IBC (688 versus 549 versus 804 versus 699 versus 629 versus
675, p-0.68, respectively).
Figures 26A-26C show anti-HER3 CD4 Tcell responses correlate with
recurrence and response to neo-adjuvant chemotherapy, but not with lymph node
metastasis. Figure 26A has four histograms comparing IBC patients' immune
responses by lymph node status at initial surgely (lymph node positive FIN+"
or
versus lymph node negative ("1_,N-" or "1_,N0e5"))showing there were no
statistically significant differences in cumulative response (top panel) (40
versus 56,
p=0.12, respectively), repertoire (second panel) (0.4 versus 0.6, p-0.08,
respectively),
responsivity (third panel) (35.7% versus 54.8%, p-0.19, respectively) or
tetanus
6
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
response (bottom panel) (22 versus 29, p=-0.35, respectively) between le-NIP '
and 1_,1\1'"g
IBC patients. Figure 2613 has four histograms comparing IBC patients' immune
responses by recurrence versus non-recurrence (disease-free) in patients who
were at
least 1 year our from diagnosis had significantly lower cumulative response
(top
panel) (17 versus 66, p=0.04, respectively), repertoire (second panel) (0.0
versus 0.6,
p<0.05, respectively) and responsivity (third panel) (0% versus 55.6%, p-0.0
respectively). There was no difference in tetanus response between recurrent
and
non-recurrent IBC patients (bottom panel) (27 versus 35, p-0.65,
respectively).
Figure 26C has four histograms comparing II3C patients' immune responses by
response to neo-adjuvant chemotherapy (pathologic complete response ("pCR")
versus residual disease ("<pCR")). Of patients receiving neo-adjuvant
chemotherapy,
those with a pCR, compared to those with <pCR, displayed significantly higher
cumulative response (top panel) (144 versus 32, p-0.004, respectively) and
repertoire
(second panel) (0.8 versus 0.4, p=0.05, respectively). There was no difference
in
responsivity (third panel) (80.0% versus 27.3%, p=0.10, respectively) or
tetanus
response (bottom panel) (17 versus 59, p-0.15, respectively) between pCR and
<pCR
patient immune responses.
Figures 27A-27D show anti-HER3 CD4 T cell responses are
significantly higher in post-menopausal IlDs/BDs but do not differ by age,
race or
pregnancy history. Figure 27A has four histograms comparing HD patients'
immune
responses by age (<50 years old ("yo") versus >50 years old). There were no
statistically significant differences by age in cumulative response (top
panel) (77
versus 103, p=0.25, respectively), repertoire (second panel) (0.8 versus 1.1,
p=0.38,
respectively), responsivity (third panel) (72.0% versus 75.0%, p=1.0,
respectively) or
tetanus response (bottom panel) (39 versus 30, p=0.40, respectively). Figure
27B has
four histograms comparing HD patients' immune responses by race (Caucasian
versus
African American versus Other).. There were no statistically significant
differences
by race in cumulative response (top panel) (87 versus 83 versus 95, p=0.96,
respectively), repertoire (second panel) (0.9 versus 0.7 versus 1.4, p=0.31,
respectively), responsivity (third panel) (69.0% versus 71.4% versus 100%,
p=0.35,
respectively) or tetanus response (bottom panel) (33 versus 51 versus 26,
p=0,30,
respectively). Figure 27C has four histograms comparing HD patients' immune
responses by pregnancy history/parity (0 pregnancies versus 1 or more
pregnancies)
There were no statistically significant differences by pregnancy history in
cumulative
7
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
response (top panel) (82 versus 91, p=0.71, respectively), repertoire (second
panel)
(1.0 versus 0.9, p=0.62, respectively) or responsivity (third panel) (76.5%
versus
70.8%, p=0.74. repectively). Of interest, tetanus response (bottom panel) was
significantly higher in nulliparous females compared to those with at least
one
pregnancy (47 versus 27, p-0.04, respectively). Figure 27D has four histograms
comparing HD patients' immune responses by menopausal status (pre-menopausal
versus post-menopausal). Post-menopausal HDs/BDs, compared to pre-menopausal
IIDs/13Ds, displayed significantly higher cumulative response (top panel) (136
versus
70 spots per million cells, p=0.005, respectively) and repertoire (second
pane]) (1.4
versus 0.8 peptides, p=0.03, respectively). There was no difference between
post- and
pre-menopausal HD/sBDs by responsivity (third panel) (90.9% versus 66.7%,
p=0.23, respectively) or tetanus response (bottom panel) (38 versus 28,
p=0.37,
respectively).
Figure 28 is a graph of ELISpot linearity determination. ELISpot
assays were determined to be linear and precise under the operator who
performed all
assays for this study by serial dilution of a known anti-1-IER3 CD4 T cell
responder
into media. Cumulative response followed a linear regression curve going from
a
dilution of 1.0 to 0.1 to 0.01 to 0.001 (230 to 35 to 12 to 5 spots per
million cells,
p<0.0001, r2= 0.88, respectively).
DETAILED DESCRIPTION
The present embodiments provide isolated peptides of the HER family
of proteins as well as other RTKs. In one embodiment, there are isolated
peptides of
One or more or HER]. HER3, and c-MET protein. in one embodiment, a peptide
represents an epitope of HER!. In one embodiment, the peptide represents an
epitope
of 1-IER3. In one embodiment, the peptide represents an epitope of c-MET.
In some embodiments, the epitope of the corresponding HER family of
proteins as well as other RIKs is immunogenic. The present embodiments
additionally provide compositions that include one or more peptides of the
embodiments. In one embodiment, there is provided a chimeric peptide, wherein
the
chimeric peptide comprises one or more peptides of the embodiements.
One embodiment includes a. composition comprising a multivalent
peptide. The multivalent peptide includes two or more of the peptides of the
invention.
8
= SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
Methods of stimulating an immune response and methods of treating
cancer in a subject are additionally provided. Vaccines are also provided for
therapeutic and prophylactic use. The peptides of the embodiments, either
alone or in
the context of chimeric peptides, as described herein, are capable of invoking
an
immune response. In one embodiment, the immune response is a bumoral response.
In another embodiment, the immune response is a cell-mediated response.
According
to sonic embodiments, the peptides of the invention confer a protective
effect.
In another embodiment HER3 expression can be used as a marker of tumor
progression in premalignant lesions of the gastroesophageal junction.
In another embodiment anti-HER3CD4 Thi loss is determined comprising use
of HER3 MHC Class II immunogenic peptides.
Definitions
Unless defined otherwise, all technical and scientific terms used herein
have the same meaning as commonly understood by one of ordinary skill in the
art to
which this invention belongs. Although any methods and materials similar or
equivalent to those described herein can be used in the practice or testing of
the
present invention, the preferred methods and materials are described.
Generally, the nomenclature used herein and the laboratory procedures
in cell culture, molecular genetics, organic chemistry, and nucleic acid
chemistry and
hybridization are those well-known and commonly employed in the art.
Standard techniques are used for nucleic acid and peptide synthesis.
The techniques and procedures are generally performed according to
conventional
methods in the art and various general references (e.g.. Sambrook and Russell,
2012,
Molecular Cloning, A Laboratory Approach, Cold Spring Harbor Press, Cold
Spring
Harbor, NY, and Ausubel et at., 2012, Current Protocols in Molecular Biology,
John
Wiley & Sons, NY), which are provided throughout this document.
The nomenclature used herein and the laboratory procedures used in
analytical chemistry and organic syntheses described below are those well-
known and
commonly employed in the art. Standard techniques or modifications thereof are
used
for chemical syntheses and chemical analyses.
As used herein, each of the following terms has the meaning associated
with it in this section.
9
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCTATS2016/026542
The articles "a" and "an" are used herein to refer to one or to more
than one e., to at least one) of the grarnmatical object of the article. By
way of
example, "an element" means one element or more than one element,
"About" as used herein when referring to a measurable value such as
an amount, a temporal duration, and the like, is meant to encompass variations
of
:1,20%, or +10%, or .5%, or +1%, or +0.I.% from the specified value, as such
variations are appropriate to perform the disclosed methods.
The term "abnormal" when used in the context of organisms, tissues,
cells or components thereof, refers to those organisms, tissues, cells or
components
thereof that differ in at least one observable or detectable characteristic
(e.g., aae,
treatment, time of day, etc.) from those organisms, tissues, cells or
components
thereof that display the "normal" (expected) respective characteristic.
Characteristics
which are normal or expected for one cell or tissue type, might be abnormal
for a
different cell or tissue type.
"Adjuvant therapy" for breast cancer as used herein refers to any
treatment given after primary therapy (i.e., surgery) to increase the chance
of long-
term survival. "Neoadjuvant or neo-adjuvant therapy" or is treatment given
before
primary therapy.
The term "antigen" or "ag" as used herein is defined as a molecule that
provokes an immune response. This immune response may involve either antibody
production, or the activation of specific immunologically-competent cells, or
both.
The skilled artisan will understand that any macromolecule, including
virtually all
proteins or peptides, can serve as an antigen. Furthermore, antigens can be
derived
from recombinant or genomic DNA. A skilled artisan will understand that any
DNA,
. which comprises a nucleotide sequences or a partial nucleotide sequence
encoding a
protein that elicits an immune response therefore encodes an "antigen" as that
term is
used herein. Furthermore, one skilled in the art will understand that an
antigen need
not be encoded solely by a full length nucleotide sequence of a gene It is
readily
apparent that the present invention includes, but is not limited to, the use
of partial
nucleotide sequences of more than one gene and that these nucleotide sequences
are
arranged in various combinations to elicit the desired immune response.
Moreover, a
skilled artisan will understand that an antigen need not be encoded by a
"gene" al all.
it is readily apparent that an antigen can be generated synthesized or can be
derived
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
from a biological sample. Such a biological sample can include, but is not
limited to a
tissue sample, a tumor sample, a cell or a. biological -fluid.
"An antigen presenting cell" ("APC") is a cell that are capable of
activating T cells, and includes, but is not limited to,
monocytes/macrophages. B cells
and dendritic cells (DCs).
"Antigen-loaded APC" or an "antigen-pulsed APC" includes an APC,
which has been exposed to an antigen and activated by the antigen. For
example, an
APC may become Ag-loaded in vitro, e.g., during culture in the presence of an
antigen. The APC may also be loaded in vivo by exposure to an antigen. An
"antigen-loaded APC" is traditionally prepared in one of two ways: (I) small
peptide
fragments, known as antigenic peptides, are "pulsed" directly onto the outside
of the
APCs; or (2) the APC is incubated with whole proteins or protein particles
which are
then ingested by the APC. These proteins are digested into small peptide
fragments
by the APC and are eventually transported to and presented on the .APC
surface. In
addition, the antigen-loaded APC can also be generated by introducing a
polynucleotide encoding an antigen into the cell.
"Anti-HER3 response," "anti -HER3 CD4 Thl response" "anti-HER3
CD4 T cell response¨ and the like refer to the immune response specifically
against
HER3 protein.
The term "anti-tumor effect" as used herein, refers to a biological
effect which can be manifested by a decrease in tumor volume, a decrease in
the
number of tumor cells, a decrease in the number of metastases, an increase in
life
expectancy, or amelioration of various physiological symptoms associated with
the
cancerous condition. An "anti-tumor effect" can also be manifested by the
ability of
the peptides, polynucleotides, cells and antibodies of the invention in
prevention of
the occ-urrence of tumor in the first place.
The term "autoimmune disease" as used herein is defined as a disorder
that results from an autoimmune response. An autoimmune disease is the result
of an
inappropriate and excessive response to a self-antigen. Examples of autoimmune
diseases include but are not limited to, Addision's disease, alopecia areata,
ankylosing
spondylitis, autoimmune hepatitis, autoimmune parotitis, Crohn's disease,
diabetes
(Type I), dystrophic epidennolysis bullosa, epididymitis, Ell
omerulonephritis, Graves'
disease, Guillain-Barr syndrome, Hashitnoto's disease, hemolytic anemia,
systemic
lupus erythematosus, multiple sclerosis, myasthenia gra.vis, pemphigus
vulgaris,
11
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
psoriasis, rheumatic fever, rheumatoid arthritis, sarcoidosis, scleroderma,
Sjogren's
syndrome, spondyloarthropathies, thyroiditis, vasculitis, vitiligo,
niyxedeina,
pernicious anemia, ulcerative colitis, among others.
As used herein, the term "autologous" is meant to refer to any material
derived from the same individual to which it is later to be re-introduced into
the
The term "B cell" as used herein is defined as a cell derived from the
bone marrow and/or spleen. B cells can develop into plasma cells which produce
antibodies.
The term "cancer" as used herein is defined as a hyperproliferation of
cells whose unique trait--loss of normal control--results in unregulated
growth, lack of
differentiation, local tissue invasion, andior metastasis. Examples include
but are not
limited to, breast cancer, prostate cancer, ovarian cancer, cervical cancer,
skin cancer,
pancreatic cancer, colorectal cancer, renal cancer, liver cancer, brain
cancer,
lymphoma, leukemia, lung cancer, germ-cell tumors, and the like.
"CD4+ Thl "Thl cells," "CD4+ T-helper type teens," "CD4+-
T cells," and the like are defined as a subtype of T-helper cells that express
the
surface protein CD4 and produce high levels of the cytokinelFN-y. See also, "T-
helper cells."
"Cumulative response" means the combined immune response of a
patient group expressed as the total sum of reactive spots (spot-forming cells
"SFC"
per 10' cells from IFN-y ELISpot analysis) from all MHC class 11 binding
peptides
from a given patient group.
"DC vaccination," "DC immunization," "DC1 immunization,' and the
like refer to a strategy using autologous dendritic cells to harness the
immune system
to recognize specific molecules and mount specific responses against them.
The term "dendritic cell" or "DC" is an antigen presenting cell existing
in vivo, in vitro, ex vivo, or in a host or subject, or which can be derived
from a
hematopoietic stem cell or a monocyte. Dendritic cells and their precursors
can be
isolated from a variety of lymphoid organs, e.g,, spleen, lymph nodes, as well
as from
bone marrow and peripheral blood. DCs have a characteristic morphology with
thin
sheets (lamellipodia) extending in multiple directions away from the dendritic
cell
body. Typically, dendritic cells express high levels of MI-IC and
costimulatory (e.g.,
B7-1 and B7-2) molecules. Dendritic cells can induce antigen specific
differentiation
12
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
. WO
2017/014816 PCT/ITS2016/026542
of T cells in vitro, and are able to initiate primary T cell responses in
vitro and in vivo.
In the context of vaccine production, an "activated DC" is a DC that has been
exposed
to a Toll-like receptor agonist such as lipopolysaccharide "ITS." An activated
DC
may or may not be loaded with an antigen.
A "disease" is a state of health of an animal wherein the animal cannot
maintain homeostasis, and wherein if the disease is not ameliorated then the
animal's
health continues to deteriorate,
A "disorder" in an animal is a state of health in which the animal is
able to maintain homeostasis, but in which the animal's state of health is
less
favorable than it would be in the absence of the disorder. Left untreated, a
disorder
does not necessarily cause a further decrease in the animal's state of health.
A disease or disorder is "alleviated" if the severity or frequency of at
least one sign or symptom of the disease or disorder experienced by a patient
is
. reduced.
"Effective amount" or "therapeutically effective amount" are used
interchangeably herein, and refer to an amount of a compound, formulation,
material,
or composition, as described herein effective to achieve a particular
biological result.
Such results may include, but are not limited to, the inhibition of virus
infection as
determined by any means suitable in the art
As used herein "endogenous" refers to any material from or produced
inside an organism, cell, tissue or system.
As used herein, the term "exogenous" refers to any material introduced
from or produced outside an organism, cell, tissue or system.
A "HER receptor" is a receptor protein tyrosine kinase which belongs
to the HER receptor family and includes EGER (,ErbBI, HER1), HER2 tabB2),
HER3 (ErbB3) and HEM (ErbB4) receptors. The HER receptor will generally
comprise an extracellular domain, which may bind an HER ligand and/or dimerize
= with another HER receptor molecule; alipophilic transmembrane domain; a
conserved intracellular tyrosine kinase domain; and a carboxyl-terminal
signaling
domain harboring several tyrosine residues which can be phosphorylated. The
HER
receptor may be a "native sequence" HER receptor or an "amino acid sequence
variant" thereof. Preferably the HER receptor is a native sequence human HER
receptor.
13
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/1JS2016/026542
The "HER pathway" refers to the signaling network mediated by the
HER receptor family.
"HER activation" refers to activation, or phosphorylation, of any one
or more HER receptors. Generally. HER activation results in signal
transduction (e.g.
that caused by an intracellular kinase domain of a HER receptor
phosphorylating
tyrosine residues in the HER receptor or a substrate polypeptide). HER
activation may
be mediated by HER ligand binding to a HER dimer comprising the HER receptor
of
interest. HER ligand binding to a HER dimer may activate a kinase domain of
one or
more of the HER receptors in the dirner and thereby results in phosphorylation
of
tyrosine residues in one or more of the HER receptors and/or phosphorylation
of
tyrosine residues in additional substrate polypeptides(s), such as Akt or MARK
intracellular kinases.
"HER2" is a member of the human epidermal growth factor receptor
("EGFR") family. HER2 is overexpressed in approximately 20-25% of human breast
= cancer and is expressed in many other cancers.
"FIER2P'"' is the classification or molecular subtype of a type of breast
cancer as well as numerous other types of cancer. HER2 positivity is currently
defined by gene amplification by FISH (fluorescent in situ hybridization)
assay and
2+ or 3+ on intensity of pathological staining.
"HER2ln" is defined by lack of gene amplification by FISH, and can
encompass a range of patholonic staining form 0 to 2+ in most cases.
"HER3" and "ErbB3" refer to the receptor polypeptide as disclosed,
for example, in U.S. Pat. Nos. 5,183,884 and 5,480,968 as well as Kraus et at.
PNAS
(US4) 86:9193-9197(1989).
"HER3 extracellular domain" or "HER3ECD" refers to a domain of
HER3 that is outside of a cell, either anchored to a cell membrane, or in
circulation,
including fragments thereof In one embodiment, the extracellular domain of
HER3
may comprise four domains: Domain I. Domain TI, Domain Hi, and Domain IV. In
= one embodiment, the HER3ECD comprises amino acids 1-636 (numbering
including
signal peptide). In one embodiment. HER3 domain III comprises amino acids 328-
532 (numbering including signal peptide).
"HER3 immunogenic peptides," HER3 binding peptides," "HER3 epitopes"
and the like as used herein refer to MFIC Class II peptides derived from or
based on
the sequence of the HER3 protein, specifically HER3ECD, and their equivalents.
14
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
HER3 peptides can activate CD4 T cells across many patients. The peptides can
be
used to pulse dendritic cells and educate T cells to recognize HER3. HER3 is
expressed in triple negative breast cancer and can impart resistance to anti-
estrogen in
ER1'" breast cancers. HER3 is also expressed in other cancers, including
melanoma,
lung, colon, prostate cancer, and metastatic brain tumors. According to a
preferred
embodiment four HER3 immunogenic peptides (epi topes) or binding peptides have
been identified as follows:
P12 (Peptide 56-70): CEVVMGNLEIVLIGH (SEQ ID NO: 4);
p81 (Peptide 401-415): SWPPHMHNESVFSNL (SEQ ID NO: 5):
p84 (Peptide 416-430): TTIGGRSLYNR.GESL (SEQ fD NO: 6); and
p91 (Peptide 451-465): AGRIYISANRQLCYH (SEQ ID NO: 7).
"Homologous" as used herein, refers to the subunit sequence similarity
between two polymeric molecules, e.g., between two nucleic acid molecules,
e.g.. two
DNA molecules or two RNA molecules, or between two polypeptide molecules.
When a subunit position in both of the two molecules is occupied by the same
monomeric subunit, e.g., if a position in each of two DNA molecules is
occupied by
adenine, then they are completely or 100% homologous at that position. The
percent
homology between two sequences is a direct function of the number of matching
or
homologous positions, e.g., if half (e.g., five positions in a polymer ten
subunits in
length) of the positions in two compound sequences are homologous then the two
sequences are 50% identical, if 90% of the positions, e.g., 9 of 10, are
matched or
homologous, the two sequences share 90% homology. By way of example, the DNA
sequences .5`ATIGCC3 and 5iTATGGC3' share 50% homology.
In addition, when the terms "homology" or "identity" are used herein
to refer to the nucleic acids and proteins, it should be construed to be
applied to
homology or identity at both the nucleic acid and the amino acid sequence
levels.
The term "hyperproliferative disease" is defined as a disease that
results from a hyperproliferation of cells. Exemplary hyperproliferative
diseases
include, but are not limited to, cancer or autoimmune diseases. Other
hyperproliferative diseases may include vascular occlusion, restenosis.
atherosclerosis, or inflammatory bowel disease, for example.
As used herein, an "instructional material" includes a publication, a
recording, a diagram, or any other medium of expression which can be used to
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
communicate the usefulness of the compositions and methods of the invention.
The
instructional material of the kit of the invention may, for example, be
affixed to a
container which contains the nucleic acid, peptide, and/or composition of the
invention or be shipped together with a container which contains the nucleic
acid,
peptide, and/or composition. Alternatively, the instructional material may be
shipped
separately from the container with the intention that the instructional
material and the
compound be used cooperatively by the recipient.
"Immune response" as used herein means the activation of a host's
immune system, e.g., that of a. mammal, in response to the introduction of
antigen.
The immune response can be in the fomi of a cellular or humoral response, or
both.
"Isolated" means altered or removed from the natural state. For
example, a nucleic acid or a peptide naturally present in a living animal is
not
"isolated," hut the same nucleic, acid or peptide partially or completely
separated from
the coexisting materials of its natural state is "isolated." An isolated
nucleic acid or
protein can exist in substantially purified form, or can exist in a non-native
environment such as, for example, a host cell.
By the term "modulating," as used herein, is meant mediating a
detectable increase or decrease in the level of a response in a subject
compared with
the level of a response in the subject in the absence of a treatment or
compound,
and/or compared with the level of a response in an otherwise identical but
untreated
subject. The term encompasses perturbing and/or affecting a native signal or
response
thereby mediating a beneficial therapeutic response in a subject, preferably,
a human.
"Metrics" of CD4-1 Thl responses or "metrics of immune responses"
are defined for each subject group analyzed for anti-HER.3 CD4+ Thl immune
response: (a) overall anti-FIER3 responsivity (expressed as percent of
subjects
responding to 1-1 immunogenic peptide); (h) response repertoire (expressed as
mean
number of immunogenic peptides (n) recognized by each subject group); and (c)
cumulative response (expressed as total sum of reactive spots (spot-forming
cells
"SEC" per 10" cells from IEN-y EL1Spot analysis) from 4 MHC Class H HER3
immunogenic peptides from each subject group.
A "peptide," "protein," or "polypeptide" as used herein can mean a
linked sequence of amino acids and can be natural, synthetic, or a
modification or
combination of natural and synthetic.
16
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
As used herein, a "population" includes reference to an isolated culture
comprising a homogenous, a substantially homogenous, or a heterogeneous
culture of
cells. Generally, a "population" may also be regarded as an "isolated" culture
of cells.
Receptor tyrosine kinases ("RTI(s") are the high-affinity cell surface
receptors for many polvTeptide growth factors, cytohines, and hormones. The
human
EGE receptor ("HER") family of RTI(s regulates a large variety of biological
processes including cell proliferation, migration, invasion, and survival. The
family
consists of four members: HERI (ErbB1), HER2 (neu or ErbB2), HER3 (ErbB3), and
HER4 (ErbB4).
As used herein, a "recombinant cell" is a host cell that comprises a
recombinant polynucleotide.
"Responsivity" or "anti-HER3 responsivity" are used interchangeably
herein to mean the percentage of subjects responding to at least 1 of 4 HER3
immunogenic peptides.
"Response repertoire" is defined as the mean number ("n") of HER3
immunogenic peptides recognized by each subject group.
"Sample" or "biological sample" as used herein means a biological
material from a subject, including but is not limited to organ, tissue,
exosome, blood,
plasma, saliva, urine and other body fluid. A sample can be any source of
material
obtained from a. subject.
"Signal 1" as used herein generally refers to the first biochemical
signal passed from an activated DC to a T cell. Signal I is provided by an
antigen
expressed at the surface of the DC and is sensed by the T cell through the I
cell
receptor.
"Signal 2" as used herein generally refers to the second signal provided
by DCs to T cells. Signal 2 is provided by "costimulatory" molecules on the
activated
DC, usually CD80 andlor CDS6 (although there are other co-stimulatory
molecules
known), and is sensed by the I cell through the surface receptor CD28.
"Signal 3" as used herein generally refers to the signal generated from
soluble proteins (usually cytokines) produced by the activated DC. These are
sensed
through receptors on the T lymphocyte. The 3rd signal instructs the I cell as
to which
phenotypical or functional features they should acquire to best deal with the
current
threat.
17
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
By the term "specifically binds," as used herein, is meant a molecule,
such as an antibody, which recognizes and binds to another molecule or
feature, but
does not substantially recognize or bind other molecules or features in a
sample.
The terms "subject," "patient," "individual," and the like are used
interchangeably herein, and refer to any animal, or cells thereof whether in
vitro or in
situ, amenable to the methods described herein. In certain non-limiting
embodiments,
the patient, subject or individual is a human.
The term "targeted therapies" as used herein refers to cancer treatments
that use drugs or other substances that interfere with specific target
molecules
involved in cancer cell growth usually while doing little damage to normal
cells to
achieve an anti-tumor effect. Traditional cytotoxic chemotherapy drugs, by
contrast,
act against all actively dividing cells. In breast cancer treatment monoclonal
antibodies, specifically trastuzumab/HERCEPTIN targets the HER2Ineu receptor.
The terms "T cell" or T-cell" as used herein is defined as a thymus-
derived cell that participates in a variety of cell-mediated immune reactions.
The term "T-helper" as used herein with reference to cells indicates a
sub-group of lymphocytes (a type of white blood cell or leukocyte) including
different
cell types identifiable by a skilled person. In particular, T-helper cell
according to the
present disclosure include effector Th cells (such as Thl, Th2 and Th17).
These Th
cells secrete cytokines, proteins or peptides that stimulate or interact with
other
leukocytes.
The terms "T-helper cells," "helper T cells," "Th cells," and the like
are used herein with reference to cells indicates a sub-group of lymphocytes
(a type of
white blood cell or leukocyte) including different cell types identifiable by
a skilled
person in the art. In particular, 1'-helper cells are effector T cells whose
primary
function is to promote the activation and functions of other B and T
lymphocytes
andlor macrophages. Helper T cells differentiate into two major subtypes of
cells
known as "Th]" or "Type I" and "Th2" or -type 2" phenotypes. These Th cells
secrete cytokines, proteins, or peptides that stimulate or interact with other
leukocytes.
"Thl. cell," "CD4+ Thl cell," "CD4-1- T-helper typel cell," ¶CD4+ T
cell" and the like as used herein refer to a mature T-cell that has expressed
the surface
glycoprotein CD4. CD4+ I-helper cells become activated when they are presented
with peptide antigens by MHC class II molecules which are expressed on the
surface
of antigen-presenting peptides ("APCs") such as dendritic cells. Upon
activation of a
18
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
C D4+ T helper cell by the MHC-antigen complex, it secretes high levels of
cytokines
such as interferon-7 ("IFN-7"). Such cells are thought to be highly effective
against
certain disease-causing microbes that live inside host cells, and are critical
in
antitumor response in human cancer against certain disease-causing microbes
that live
inside host cells, and cancer as well.
"Th17 T cell" as used herein refers to a T cell that produces high levels
of the cytokines 1L-17 and 1L-22 and is thought to be highly effective against
disease-
causing microbes that live on inucousal surfaces.
"Therapeutically effective amount" is an amount of a. compound of the
invention, that when administered to a patient, ameliorates a symptom of the
disease.
The amount of a compound of the invention which constitutes a "therapeutically
. effective amount" will vary depending on the compound, the disease state and
its
severity, the age of the patient to be treated, and the like. The
therapeutically
effective amount can be determined routinely by one of ordinary skill in the
art having
regard to his own knowledge and to this disclosure.
The terms "treat," "treating," and "treatment," refer to therapeutic or
preventative measures described herein. The methods of "treatment" employ
administration to a subject, in need of such treatment, a composition of the
present
invention, for example, a subject afflicted a disease or disorder, or a
subject who
ultimately may acquire such a disease or disorder, in order to prevent, cure,
delay,
reduce the severity of, or ameliorate one or more symptoms of the disorder or
recurring disorder, or in order to prolong the survival of a subject beyond
that
expected in the absence of such treatment.
"Triple negative" and "TN" breast cancer refer to any breast cancer
cells that test negative for estrogen receptor ("ER"), progesterone receptor
("PR") and
HER2.
The term "vaccine" as used herein is defined as a material used to
provoke an immune response after administration of the material to an animal,
preferably a mammal. and more preferably a human. Upon introduction into a
subject, the vaccine is able to provoke an imintaie response including, but
not limited
to, the production of antibodies, cytokines and/or other cellular responses.
"Variant" with respect to a peptide or polypeptide that differs in amino
acid sequence by the insertion, deletion, or conservative substitution of
amino acids.
but retain at least one biological activity. 'Variant can also mean a protein
with an
19
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
amino acid sequence that is substantially identical to a referenced protein
with an
amino acid sequence that retains at least one biological activity. A
conservative
substitution of an amino acid, i.e., replacing an amino acid with a different
amino acid
of similar properties (e.g., hydrophilicity, degree and distribution of
charged regions)
is recognized in the art as typically involving a minor change. These minor
changes
can be identified, in part, by considering the hydropathic index of amino
acids, as
understood in the art. Kyle et at.. J. Mol. Biol. 157:105-132 (1982). The
hydropathic
index of an amino acid is based on a consideration of its hydrophobicity and
charge.
It is known in the art that amino acids of similar hydropathic indexes can be
substituted and still retain protein function. In one aspect, amino acids
having
hydropathic indexes of 12 are substituted. The hydrophilicity of amino acids
can also
be used to reveal substitutions that would result in proteins retaining
biological
= function. A consideration of the hydrophilicity of amino acids in the
context of a.
peptide permits calculation of the greatest local average hydrophil icily of
that peptide,
a useful measure that has been reported to correlate well with antigenicity
and
immunogenicity. U.S. Patent No. 4,554,101, incorporated fully herein by
reference.
Substitution of amino acids having similar hydrophilicity values can result in
peptides
retaining biological activity, for example immunogenicity, as is understood in
the art.
Substitutions can be performed with amino acids having hydrophilicity values
within
12 of each other. Both the hyrophobicity index and the hydrophilicity value of
amino
acids are influenced by the particular side chain of that amino acid.
Consistent with
that observation, amino acid substitutions that are compatible with biological
function
are understood to depend on the relative similarity of the amino acids, and
particularly
the side chains of those amino acids, as revealed by the hydrophobicity,
hydrophilicity, charge, size, and other properties.
Ranges: throughout this disclosure, various aspects of the invention
can be presented in a range format. It should be understood that the
description in
range format is merely for convenience and brevity and should not be construed
as an
inflexible limitation on the scope of the invention. Accordingly, the
description of a
range should be considered to have specifically disclosed all the possible
subranges as
well as individual numerical values within that range. For example,
description of a
range such as from Ito 6 should be considered to have specifically disclosed
subranges such as from 1 to 3, from 1 to 4, from I to 5, from 2 to 4, from 2
to 6, from
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
3 to 6 etc., as well as individual numbers within that range for example. I,
2. 2.7, 3,
4, 5, 5.3, and 6. This applies regardless of the breadth of the range.
Description
The embodiments provide an immunological composition comprising
a peptide of a HER family of proteins as well as other RTKs. In one
embodiment,
there are provided isolated peptides of one or more of HERI. HER3, and c-MET
protein. In one embodiment, the peptides are useful in eliciting an immune
response.
A composition comprising a peptide of the embodiments is useful as a
prophylactic
therapeutic agent for initial protection as well as useful as a therapeutic
agent for
treatment of an ongoing condition.
The present invention also provides methods for treating or preventing
cancer. Such methods involve the step of administering to a subject in need
thereof a
peptide or combinations of peptides of the invention. Administration of such
peptide(s) results in the induction of anti-tumor immunity. Thus, the present
invention provides methods for inducing anti-tumor immunity in a subject, such
methods involving the step of administering to the subject the peptide or
combination
of peptides of the invention, as well as pharmaceutical compositions and
cellular
compositions derived thereof
The invention encompasses a method for inducing a T cell response to
in a mammal. The method comprises administering an antigen presenting cell
(APC)
that specifically induces proliferation of a T cell. In one embodiment, method
comprises administering a dendritic cell vaccine pulsed with a peptide of the
invention to thereby specifically induce proliferation of a. T cell against
the antigen
corresponding to the peptide.
In one embodiment, APCs pulsed with the peptide of the invention can
be used to culture expand T cells. Once sufficient numbers of antigen-specific
T cells
are obtained using the APC to expand the T cell, the antigen-specific T cells
so
obtained are administered to the mammal, thereby inducing an antigen specific
T cell
response in the mammal.
The invention includes a preparation of activated DCs. In one
embodiment, the DC preparations are greater than 90% pure. In another
embodiment,
the DC preparations are fully activated. For example, the DCs are activated
with a
DC activation regimen comprising contacting the DC with a MR. agonist (e.g..
I.PS).
21
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/1JS2016/026542
In another embodiment, the DCs are activated with a calcium mobilizing
treatment in
conjunction with other DC activation regimens (e.g., activating agents) that
enhance
different 3rd signal cytokines.
The present invention includes mature, antigen loaded DCs activated
by any DC activation regimen. The DCs of the present invention produce
desirable
levels of cytokines and chemokines. In one embodiment, the invention provides
a
method to pulse and activate cells, whereby the cells maintain the active
state
following cryopreservation. A benefit of the DC preparation of the invention
is that
- the cells are efficiently cryopreserved from a single letikapheresis
(patient collection)
into an initial vaccine plus multiple "booster" doses (e.g., 10 or more) that
can be
thawed as needed at remote treatment locations without any specialized cell
processing facilities or further required quality control testing.
The present invention also relates to the cryopreservation of these
activated DCs in a manner that retains their potency and functionality in
presenting
antigen as well as their production of various cytokines and chemokines after
thawing, such that the cryopreserved and subsequently thawed activated DCs are
as
clinically effective as freshly harvested and activated DCs.
As contemplated herein, the present invention provides a method for
generating and cryopreserving DCs with superior functionality in producing
stronger
signals to T cells, and thus resulting in a more potent DC-based vaccine. By
effectively cryopreserving such cells, samples can be stored and thawed for
later use,
thereby reducing the need for repeated pheresis and elutriation processes
during
- vaccine production. Being able to freeze DCs and then thaw them out later is
an
advantage because it means that a single round of vaccine production can be
divided
into small parts, frozen away, and then administered one at a time to a
patient over the
course of weeks, months, or years to give "booster- vaccinations that
strengthen
immunity. .
The present embodiments also include use of HER3 expression as a
marker of tumor progression in premalignant lesions of the gastroeophageal
junction.
also known as Barrett's esophagus. The marker has prognostic and therapeutic
uses
in invasive esophagogastric carcinoma.
Compositions
22
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCTA1S2016/026542
The present invention provides isolated peptides of the HER family of
proteins as well as other RTKs. In one embodiment, the invention provides
isolated
.
peptides of one or more of HER1. HER3, and c-MET protein. in one embodiment,
the peptides of the invention represent epitopes of the corresponding HER or c-
MET
protein. In some embodiments, the epitopes of the corresponding HER or c-MET
protein are immunogenic.
The present invention provides compositions that include one or more
peptides of the invention. The present invention also provides compositions
that
include one or more chimeric peptides. In one embodiment, the chimeric
peptides
include one more of the epitopes of the corresponding HER or c-MET protein.
Additionally, compositions having one or more multivalent peptides
are provided. These multivalent peptides include two or more of the epitopes
of the
invention.
Methods of stimulating an immune response and methods of treating
cancer in a subject using the compositions of the invention are included in
the
invention. Vaccines are also provided for therapeutic and prophylactic use.
The
epitopes of the invention, either alone or in the context of chimeric
peptides, as
described herein, is capable of invoking an immune response. In one
embodiment,
the immune response is a humoral response. In another embodiment, the immune
response is a cell mediated response. According to some embodiments, the
epitopes
or peptides of the invention confer a protective effect.
In one embodiment, the HER3 epitopes or otherwise peptides of the
invention include:
p11-13 (Peptide 51-75): KLYERCEVVMGNLEIVETGIENADESFLQW (SEQ ID
NO: 1);
p81-83 (Peptide 401-425): SWPPHMI-INFSVESNETTIGGRSLYN (SEQ ID NO: 2);
p84-86 (Peptide 416-440): TTIGGRSLYNRCEFSELIMKNENVTS (SEQ ID NO: 31;
pi 2 (Peptide 56-70): CEVVVIGNERVETGH (SEQ ID NO: 4):
p81 (Peptide 401-415): SWPPHIVIHNFSV.ESNE (SEQ ID NO: 5);
p84 (Peptide 416-430): TTIGGRSLYNRGESE (SEQ ID NO: 6);
p91 (Peptide 451-465): AGRIVISANRQUAH (SEQ ID NO: 7);
The HER3 peptides or any peptide of the invention may be cyclized or
linear. When cyclized, the epitopes may be cyclized in any suitable manner.
For
23
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
example, disulfide bonds may be formed between selected cysteine (Cys) pairs
in
order to provide a desired confirmation. It is believed that the formation of
cyclized
epitopes may provide conformations that improve the humoral response, thus
improving the protective effect.
The HER3 epitope identified by SEQ ID NO: 4 represents positions
56-70 of the HER3 protein. The HER3 epitope identified by SEQ ID NO: 5
represents
positions 401-415 of the HER3 protein. The HER3 epitope identified by SEQ ID
NO:
6 represents positions 416-430 oldie HER3 protein. The HER3 epitope identified
by
SEQ ID NO: 7 represents positions 451-465 of the HER3 protein.
As described herein, the HER3 epitopes of the invention also
encompass peptides that are functional equivalents of the peptides identified
by SEQ
ID NOs. Such functional equivalents have an altered sequence in which one or
more
of the amino acids in the corresponding HER3 epitope sequence is substituted
or in
which one or more amino acids are deleted from or added to the corresponding
reference sequence. For example 1 to 3 amino acids may be added to the amino
terminus, ca.rboxy terminus; or both. In some examples, the HER3 epitopes are
glycosylated.
In other examples, the HER3 epitopes may be the retro-inverso
isomers of the HER3epitopes. The retro-inverso modification comprises the
reversal
of all amide bonds within the peptide backbone. This reversal may be achieved
by
reversing the direction of the sequence and inverting the chirality of each
amino acid
residue by using D-amino acids instead of the L-amino acids. This retro-
inverso
isomer form may retain planarity and conformation restriction of at least some
of the
peptide bonds.
Non-conservative amino acid substitutions and/or conservative
substitutions may be made. Substitutions are conservative amino acid
substitutions
when the substituted amino acid has similar structural or chemical properties
with the
corresponding amino acid in the reference sequence. By way of example,
conservative amino acid substitutions involve substitution of one aliphatic or
hydrophobic amino acids, e,g., alanine, valine. Ieucine and isoleucine, with
another;
substitution of one hydroxyl-containing amino acid, e.g., serine and
threonine, with
another: substitution of one acidic residue, e.g., glutamic acid or aspartic
acid, with
another; replacement of one amide,-containing residue, e.g., asparagine and
glutamine,
with another; replacement of one aromatic residue, e.g., phenylalt-mine and
tyrosine,
24
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/1JS2016/026542
with another; replacement of one basic residue, e.g., lysine, arginine and
histidine,
with another; and replacement of one small amino acid, e.g., alanine, scrim,
threonine, methionine. and glycine, with another.
In some examples, the deletions and additions are located at the amino
= terminus, the carboxy terminus, or both, of one of the sequences of the
peptides of the
invention. For example, the HER3 epi tope equivalent has an amino acid
sequence
which is at least 70% identical, at least 80% identical, at least 85%
identical, at least
90% identical, at least 91%, at least 92%, at least 93%, at least 94%, at
least 95%, at
least 96%, at least 97%, at least 98%, or at least 99% identical to the
corresponding
HER3 epitope sequences. Sequences which are at least 90% identical have no
more
than I alteration, i.e., any combination of deletions. additions or
substitutions, per 10
amino acids of the reference sequence. Percent identity is determined by
comparing
the amino acid sequence of the variant with the reference sequence using known
or to
be developed programs in the art.
For functional equivalents that are longer than a corresponding HER3
epitope sequence, the functional equivalent may have a sequence which is at
least
90% identical to the HER3 epitope sequence and the sequences which flank the
HER3
epitope sequences in the wild-type HER3 protein.
Functional equivalents of the HER3 epitopes may be identified by
modifying the sequence of the epitope and then assaying the resulting
polypeptide for
the ability to stimulate an immune response, e.g., production of antibodies.
Such
antibodies may be found in a variety of body fluids including sera and
ascites.
Briefly, a body fluid sample is isolated from a warm-blooded animal, such as a
human. for whom it is desired to determine whether antibodies specific for
HER3
polypeptide are present. The body fluid is incubated with HER3 polypeptide
under
conditions and for a time sufficient to permit immunocomplexes to form between
the
polypeptide and antibodies specific for the protein and then assayed,
preferably using
an RBA technique.
In accordance with other embodiments of the present invention,
chimeric peptides and compositions comprising one or more chimeric peptides
are
provided. According to various embodiments, the chimeric peptides comprise a
HER3 epitope, another epitope, and a linker joining the HER3 epitope to the
other
epitope. In one embodiment, the other epitope can include hut is not limited
to another
HER3 epitope, a HER1 epitope, a HER2 epitope, and a c-Met epitope. It will be
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
further understood that any suitable linker may be used. For example,
depending upon
the epitope used, the HER3 epitope may be linked to either the amino or the
catboxy
terminus of the other epitope. The location and selection of the other epitope
depends
on the structural characteristics of the HER3 epitope, whether alpha helical
or beta-
turn or strand.
In one embodiment, the linker may be a peptide of from about 2 to
about 15 amino acids, about 2 to about 10 amino acids, or from about 2 to
about 6
amino acids in length. The chimeric peptides may be linear or cyclized.
Additionally,
the HER3 epitopes, the other epitopes, and/or the linker may be in retro-
inverso form.
Thus the HER3 epitope along could be in retro inverso form. Alternatively, the
HER3
epitope and the other epitope could be in retro inverso form. In another
example, the
HER3 epitope, the other epitope, and the linker could be in retro inverso
form.
In another embodiment, the peptides of the invention can be in a
mixture together instead of being in a form of a chimeric peptide. In any
event, the
compositions of the invention comprising the peptides may be useful agents to
pulse
antigen presenting cells (e.g., dendritic cells) for the generation of
cellular vaccines.
In another embodiment, the compositions of the invention comprising the
peptides
may be useful immunonens for inducing production of antibodies. The
compositions
of the invention may also be used to immunize a subject and retard or prevent
tumor
development. The compositions of the invention may he used in vaccines to
provide
a protective effect.
In accordance with additional embodiments of the present invention,
compositions comprising a mixture of two or more of the peptides or chimeric
peptides of the invention are provided. In some examples, the HER3 epitope of
each
of the two or more chimeric peptides are different. In other examples, one of
the
HER3 epitopes is selected from SEQ ID NOs: 1-7.
Peptides, including chimeric peptides, of the present invention can be
prepared using well known techniques. For example, the peptides can be
prepared
synthetically, using either recombinant DNA technology or chemical synthesis.
Peptides of the present invention may be synthesized individually or as longer
polypeptides composed of two or more peptides. 'The peptides of the present
invention are preferably isolated, i.e., substantially free of other naturally
occurring
host cell proteins and fragments thereof.
26
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
The peptide and chimeric peptides of the invention may be synthesized
using commercially available peptide synthesizers. For example, the chemical
methods described in K.aumaya et al., "De Novo" Engineering of Peptide
Immunogenic and Antigenic Determinants as Potential Vaccines, in Peptides.
Design,
Synthesis and Biological Activity (1994), pp 133-164, which is specifically
incorporated herein by reference, may be used. For example, IIER3 epitopes may
be
synthesized co-linearly with the other epitope to form a chimeric peptide.
Peptide
synthesis may be performed using Fmoc/t-But chemistry. The peptides and
chimeric
peptides may be cycli.zed in any suitable manner. For example, disulfide bonds
may
be achieved using differentially protected cysteine residues, iodine
oxidation, the
addition of water to boost removal of Acrn group and the concomitant formation
of a
disulfide bond, andlor the silyl chloride-sulfoxide method.
The peptides and chimeric peptides may also be produced using cell-
free translation systems and RNA molecules derived from DNA constructs that
encode the epitope or peptide. Alternatively, the epitopes or chimeric
peptides are
made by transfecting host cells with expression vectors that comprise a DNA
sequence that encodes the respective epitope or chimeric peptide and -then
inducing
expression of the polypeptide in the host cells. For recombinant production,
recombinant constructs comprising one or more of the sequences which encode
the
epitope, chimeric peptide, or a variant thereof are introduced into host cells
by
conventional methods such as calcium phosphate transfection, DEAF-dextran
mediated transfection, microinjection, cationic lipid-mediated transfection,
electroporation, transduction, scrape lading, ballistic introduction or
infection.
The peptides of the present invention may contain modifications, such
as glycosylation, side chain oxidation, or phosphorylation; so long as the
modifications do not destroy the biological activity of the peptides. Other
modifications include incorporation of D-amino acids or other amino acid
nnineties
that can be used, for example, to increase the serum half-life of the
peptides,
The peptides of the invention can be prepared as a combination_ which
includes two or more of peptides of the invention, for use as a vaccine for a
disease,
e.g. cancers. The peptides may be in a cocktail or may be conjugated to each
other
using standard techniques. For example, the peptides can be expressed as a
single
polypeptide sequence. The peptides in the combination may be the same or
different.
27
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
The present invention should also be construed to encompass
"mutants," "derivatives," and "variants' of the peptides of the invention (or
of the
DNA encoding the same) which mutants, derivatives and variants are peptides
which
are altered in one or more amino acids (or, when referring to the nucleotide
sequence
encoding the same, are altered in one or more base pairs) such that the
resulting
peptide for DNA) is not identical to the sequences recited herein, but has the
same
biological property as the peptides disclosed herein.
The invention also provides a polynucleotide encoding at least one
peptide selected from a peptide having the sequence of any one or more of SEQ
ID
NOs 1-7. The nucleic acid sequences include both the DNA sequence that is
transcribed into RNA and the RNA sequence that is translated into a peptide.
According to other embodiments, the polynucleotides of the invention are
inferred
from the amino acid sequence of the peptides of the invention. As is known in
the art
several alternative polynucleotides are possible due to redundant codons,
while
retaining the biological activity of the translated peptides.
Further, the invention encompasses an isolated nucleic acid encoding a
peptide having substantial homology to the peptides disclosed herein.
Preferably, the
nucleotide sequence of an isolated nucleic acid encoding a peptide of the
invention is
"substantially homologous", that is, is about 60% homologous, more preferably
about
70% homologous, even more preferably about 80% homologous, more preferably
about 90% homologous, even more preferably, about 95% homologous, and even
more preferably about 99% homologous to a nucleotide sequence of an isolated
nucleic acid encoding a peptide of the invention.
It is to be understood explicitly that the scope of the present invention
encompasses homologs, analogs, variants, derivatives and salts, including
shorter and
longer peptides and polynucleotides, as well as peptide and polvnueleotide
analogs
with one or more amino acid or nucleic acid substitution, as well as amino
acid or
nucleic acid derivatives, non-natural amino or nucleic acids and synthetic
amino or
nucleic acids as are known in the art, with the stipulation that these
modifications
must preserve the biological activity of the original molecule. Specifically
any active
fragments of the active peptides as well as extensions, conjugates and
mixtures are
disclosed according to the principles of the present invention.
The invention should be construed to include any and all isolated
nucleic acids which are homologous to the nucleic acids described and
referenced
28
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
herein, provided these homologous DNAs have the biological activity of the
peptides
disclosed herein.
The skilled artisan would understand that the nucleic acids of the
invention encompass an RNA or a DNA sequence encoding a peptide of the
invention, and any modified forms thereof, including chemical modifications of
the
DNA or RNA which render the micleonde sequence more stable when it is cell
free or
when it is associated with a cell. Chemical modifications of nucleotides may
also be
used to enhance the efficiency with which a nucleotide sequence is taken up by
a cell
or the efficiency with which it is expressed in a cell. Any and all
combinations of
If) modifications of the nucleotide sequences are contemplated in the
present invention.
further, any number of procedures may be used for the generation of
mutant, derivative or variant forms of a protein of the invention using
recombinant
DNA methodology well known in the art such as, for example, that described in
Sambrook and Russell, supra_ and Ausubel et al., supra. Procedures for the
introduction of amino acid changes in a peptide or polypeptide by altering the
DNA
sequence encoding the polypeptide are well known in the art and are also
described in
these, and other, treatises.
The nucleic acids encoding the peptides of the invention can be
incorporated into suitable vectors e.g. retroviral vectors. These vectors are
well known
in the art. The nucleic acids or the vectors containing them usefully can be
transferred
into a desired cell, which cell is preferably from a patient. Advantageously,
the
invention provides an off-the-shelf composition allowing rapid modification of
a
patient's own cells (or those of another mammal) to rapidly and easily produce
modified cells having excellent cancer cell killing properties.
Vectors
In other related aspects, the invention includes an isolated nucleic acid
encoding one or more of peptides having a sequence selected from the group
consisting of SEQ ID NOs: 1-7.
In one embodiment, the invention includes a nucleic acid sequence
encoding one or more Peptides of the invention operably linked to a. nucleic
acid
comprising a. promoter/regulatory sequence such that the nucleic acid is
preferably
capable of directing expression of the protein encoded by the nucleic acid.
Thus, the
invention encompasses expression vectors and methods for the introduction of
29
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
exogenous DNA into cells with concomitant expression of the exogenous DNA in
the
cells such as those described, for example; in Sambrook et al. (2012,
Molecular
Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, New York), and in
Ausubel et al. (1997, Current Protocols in Molecular Biology, John Wiley &
Sons,
New York). The incorporation of a desired polynucleotide into a vector and the
choice
of vectors is well-known in the art as described in; for example, Sambrook et.
al.,
= supra, and .Ausubel et al., supra.
The polynucleotide can be cloned into a number of types of vectors.
However, the present invention should not be construed to be limited to any
particular
vector. Instead, the present invention should be construed to encompass a wide
plethora of vectors which are readily available and/or well-known in the art.
For
example, the polynucleotide of the invention can be cloned into a vector
including,
but not limited to a plasmid, a phagemid, a phage derivative, an animal virus,
and a
cosmid. Vectors of particular interest include expression vectors, replication
vectors,
probe generation vectors, and sequencing vectors.
In specific embodiments, the expression vector is selected from the
group consisting of a viral vector, a bacterial vector and a mammalian cell
vector.
Numerous expression vector systems exist that comprise at least a part or all
of the
compositions discussed above. Prokaryote- and/or eukaiyote-vector based
systems
can be employed for use with the present invention to produce polynucleotides.
or
= their cognate polypeptides. Many such systems are commercially and widely
available.
Further, the expression vector may be provided to a cell in the form of
a viral vector. Viral vector technology is well known in the art and is
described, for
example, in Sambrook et al. (2012), and in Ausubel et at. (1997), and in other
virology and molecular biology manuals. Viruses, which are useful as vectors
include,
but are not limited to, retroviruses, adenoviruses, adeno-associated viruses,
herpes
viruses, and lenti viruses. In general, a suitable vector contains an origin
of replication
functional in at least one organism, a promoter sequence, convenient
restriction
endonuclease sites, and one or more selectable markers. (See, e.g., WO
01/96584;
WO 01/29058; and U.S. Pat, No. 6,326,193,
For expression of the desired nucleotide sequences of the invention, at
least one module in each promoter functions to position the start site for RNA
synthesis. The best known example of this is the TATA box, but in some
promoters
=
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
lacking a TATA box, such as the promoter for the mammalian terminal
deoxynucleatidyl transferase gene and the promoter for the SV40 genes, a
discrete
element overlying the start site itself helps to fix the place of initiation.
Additional promoter elements, i.e., enhancers, regulate the frequency
of transcriptional initiation. Typically, these are located in the region 30-
110 bp
upstream of the start site, although a number of promoters have recently been
shown
to contain functional elements downstream of the start site as well. The
spacing
between promoter elements frequently is flexible, so that promoter Function is
preserved when elements are inverted or moved relative to one another. In the
thymidine kinase (tk) promoter, the spacing between promoter elements can be
increased to 50 bp apart before activity begins to decline. Depending on the
promoter.
it appears that individual elements can function either co-operatively or
independently
to activate transcription.
A promoter may be one naturally associated with a gene or
polynucleotide sequence, as may be obtained by isolating the 5' non-coding
sequences
located upstream of the coding segment and/or eX011. Such a promoter can be
referred.
to as "endogenous." Similarly, an enhancer may be one naturally associated
with a
poly-nucleotide sequence, located either downstream or upstream of that
sequence.
Alternatively, certain advantages will be gained by positioning the coding
polynucleotide segment under the control of a recombinant or heterologous
promoter,
which refers to a promoter that is not normally associated with a
polynucleotide
sequence in its natural environment. A recombinant or heterologous enhancer
refers
also to an enhancer not normally associated with a polynucleotide sequence in
its
natural environment. Such promoters or enhancers may include promoters or
enhancers of other genes, and promoters or enhancers isolated from any other
prokaiyotic, viral, or eukaryotic cell, and promoters or enhancers not
"naturally
occurring," i.e., containing different elements of different transcriptional
regulatory
regions, and/or mutations that alter expression. In addition to producing
nucleic acid
sequences of promoters and enhancers synthetically, sequences may be produced
using recombinant cloning and/or nucleic acid amplification technology.
including
PCRThr, in connection with the compositions disclosed herein (U.S. Patent
4,683,202,
U.S. Patent 5,928,906). Furthermore, it is contemplated the control sequences
that
31
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
direct transcription and/or expression of sequences within non-mid ear
organelles such
as mitochondria, chloroplasts, and the like, can be employed as well.
Naturally, it will be important to employ a promoter and/or enhancer
that effectively directs the expression of the DNA segment in the cell type,
organelle,
and organism chosen for expression. Those of skill in the art of molecular
biology.
generally know how to use promoters, enhancers, and cell type combinations for
protein expression, for example, see Sambrook et al. (2012). The promoters
employed
may be constitutive, tissue-specific, inducible, and/or useful under the
appropriate
conditions to direct high level expression of the introduced DNA segment, such
as is
advantageous in the large-scale production of recombinant proteins and/or
peptides.
The promoter may be heterologous or endogenous.
A promoter sequence exemplified in the experimental examples
presented herein is the immediate early cytomegalovirus (CMV) promoter
sequence.
This promoter sequence is a strong constitutive promoter sequence capable of
driving
high levels of expression of any polynucleotide sequence operatively linked
thereto.
However, other constitutive promoter sequences may also be used, including,
but not
limited to the simian virus 40 (SV40) early promoter, mouse mammary tumor
virus
(MMTV), human immunodeficiency virus (HIV) long terminal repeat (UR)
. promoter, Moloney virus promoter, the avian leukemia virus promoter, Epstein-
Barr
virus immediate early promoter. Rous sarcoma virus promoter, as well as human
gene
promoters such as, but not limited to, the actin promoter, the myosin
promoter, the
hemoglobin promoter, and the muscle creatine prothoter. Further, the invention
should not be limited to the use of constitutive promoters. Inducible
promoters are
also contemplated as part of the invention. The use of an inducible promoter
in the
invention provides a molecular switch capable of turning on expression of the
polynudeotide sequence which it is operatively linked when such expression is
desired, or turning off the expression when expression is not desired.
Examples of
inducible promoters include, but are not limited to a metallothionine
promoter, a
glucocorticoid promoter, a progesterone promoter, and a tetracycline promoter.
Further, the invention includes the use of a tissue specific promoter, which
promoter
is active only in a desired tissue.
In order to assess the expression of the nucleotide sequences encoding
the peptides of the invention, the expression vector to be introduced into a
cell can
also contain either a selectable marker gene or a reporter gene or both to
facilitate
32
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
identification and selection of expressing cells from the population of cells
sought to
be transfected or infected through viral vectors. In other embodiments, the
selectable
marker may be carried on a separate piece of DNA and used in a co-transfection
procedure. Both selectable markers and reporter genes may be flanked with
appropriate regulatory sequences to enable expression in the host cells.
Useful
selectable markers are known in the art and include, for example, antibiotic-
resistance
genes, such as neo and the like.
Reporter genes are used for identifying potentially transfected cells and
for evaluating the functionality of regulatory sequences. Reporter genes that
encode
for easily assayable proteins are well known in the art. In general, a
reporter gene is a
gene that is not present in or expressed by the recipient organism or tissue
and that
encodes a protein whose expression is manifested by some easily detectable
property,
e.g., enzymatic activity. Expression of the reporter gene is assayed at a
suitable time
after the DNA has been introduced into the recipient cells.
Suitable reporter genes may include genes encoding luciferase, beta-
galactosidse, chloramplienicol acetyl transferase, secreted alkaline
phosphatase, or
the green fluorescent protein gene (see, e.g., Ui-Tei et al., 2000 FEBS Lett.
4179:79-
82). Suitable expression systems are well known and may be prepared using well
known techniques or obtained commercially. Internal deletion constructs may be
generated using unique internal restriction sites or by partial digestion of
non-unique
restriction sites. Constructs may then be transfected into cells that display
high levels
of siRNA polynucleotide andlor polypeptide expression. In general, the
construct with
the minimal 5' flanking region showing the highest level of expression of
reporter
gene is identified as the promoter. Such promoter regions may be linked to a
reporter
gene and used to evaluate agents for the ability to modulate promoter-driven
transcription.
Vaccine
.....
In one embodiment, the present invention is directed to a vaccine
comprising a peptide of the invention. The vaccine of the invention can
provide any
combination of particular peptides for the particular prevention or treatment
of the
cancer of a subject that is in need of treatment.
The vaccine of the invention can induce antigen-specific T cell aniior
high titer antibody responses, thereby inducing or eliciting an immune
response that is
33
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
directed to or reactive against the cancer or tumor expressing the antigen. In
some
embodiments, the induced or elicited immune response can be a cellular,
humoral, or
both cellular and Immoral immune responses. In some embodiments, the induced
or
elicited cellular immune response can include induction or secretion of
interferon-
gamma (IFN-y) andlor tumor necrosis factor alpha (TNF-00.
In one embodiment, the present invention is directed to an anti-cancer
vaccine. The vaccine can comprise one or more cancer antigens. The vaccine can
prevent tumor growth. The vaccine can reduce tumor growth. The vaccine can
prevent metastasis of tumor cells. Depending upon the cancer antigen. the
vaccine
can be targeted to treat breast cancer, liver cancer, prostate cancer,
melanomas, blood
cancers, head and neck cancer, glioblastoma, recurrent respiratory
papillomatosis,
anal cancer, cervical cancer, brain cancer, and the like.
In a particular embodiment, the vaccine can mediate clearance or
prevent growth of tumor cells by inducing (1) humoral immunity via B cell
responses
to generate desirable antibodies; (2) increase cytotoxic T lymphocyte such as
CD8'
(CTL) to attack and kill tumor cells: (3) increase T helper cell responses;
(4) and
increase inflammatory responses via IFN-y and TFN-o. or preferably all of the
aforementioned. The vaccine can increase tumor free survival by 30%, 31%, 32%,
33%, 34%, 35%, 36%, 37%, 38%, 39%,40%, 41%, 42%, 43%, 44%, and 45%. The
vaccine can reduce tumor mass by 30%, 31%, 32%, 33%, 34%, 35%, 36%. 37%,
38%, 39%, 40%. 41%, 42%, 43%, 44%, 45%, 46%, 47%, 48%, 49%, 50%, 51%,
52%, 53%, 54%, 55%, 56%, 57%õ 58%, 59%, and 60% after immunization.
The vaccine can increase a cellular immune response in a subject
administered the vaccine by about 50-fold to about 6000-fold, about 50-fold to
about
5500-fold, about 50-fold to about 5000-fold, about 50-fold to about 4500-fold,
about
100-fold to about 6000-fold, about 150-fold to about 6000-fold, about 200-fold
to
about 6000-fold, about 250-fold to about 6000-fold, or about 300-fold to about
6000-
fold as compared to a cellular immune response in a subject not administered
the
vaccine. In some embodiments the vaccine can increase the cellular immune
response
in the subject administered the vaccine by about 50-fold, 100-fold, 150-fold,
200-fold,
250-fold, 300-fold, 350-fold, 400-fold, 450-fold, 500-fold, 550-fold, 600-
fold, 650-
fold, 700-fold. 750-fold, 800-fold, 850-fold, 900-fold, 950-fold, 1000-fold.
1100-fold,
1200-fold, 1300-fold, 1400-fold, 1500-fold, 1600-fold, 1700-fold, 1800-fold,
1900-
fold, 2000-fold, 2100-fold, 2200-fold, 2300-fold, 2400-fold, 2500-fold, 2600-
fold,
34
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
2700-fold, 2800-fold, 2900-fold, 3000-fold, 3100-fold, 3200-fold, 3300-fold,
3400-
fold, 3500-fold, 3600-fold, 3700-fold, 3800-fold, 3900-fold, 4000-fold, 4100-
fold,
4200-fold, 4300-fold, 4400-fold, 4500-fold, 4600-fold, 4700-fold, 4800-fold,
4900-
fold, 5000-fold, 5100-fold, 5200-fold, 5300-fold, 5400-fold, 5500-fold, 5600-
fold,
5700-fold, 5800-fold, 5900-fold, or 6000-fold as compared to the cellular
immune
response in the subject not administered the vaccine.
The vaccine can increase interferon gamma (1FN-y) levels in a subject
administered the vaccine by about 50-fold to about 6000-fold, about 50-fold to
about
5500-fold, about 50-fold to about 5000-fold, about 50-fold to about 4500-fold,
about
100-fold to about 6000-fold, about 150-fold to about 6000-fold, about 200-fold
to
about 6000-fold, about 250-fold to about 6000-fold, or about 300-fold to about
6000-
fold as compared to IFN-y levels in a subject not administered the vaccine. In
some
embodiments the vaccine can increase IFN-7 levels in the subject administered
the
vaccine by about 50-fold, 100-fold, 150-fold, 200-fold, 250-fold, 300-fold,
350-fold,
400-fold, 450-fold, 500-fold, 550-fold, 600-fold, 650-fold, 700-fold, 750-
fold, 800-
fold, 850-fold, 900-fold, 950-fold, 1000-fold, 1100-fold, 1200-fold, 1300-
fold, 1400-
fold, 1500-fold, 1600-fold. 1700-fold, 1800-fold, 1900-fold, 2000-fold, 2100-
fold,
2200-fold, 2300-fold, 2400-fold, 2500-fold, 2600-fold, 2700-fold, 2800-fold,
2900-
fold, 3000-fold, 3100-fold, 3200-fold, 3300-fold, 3400-fold, 3500-fold, 3600-
fold,
3700-fold, 3800-fold. 3900-fold, 4000-161d, 4100-fold, 4200-fold, 4300-fold,
4400-
fold, 4500-fold, 4600-fold, 4700-fold, 4800-fold, 4900-fold, 5000-fold. 5100-
fold,
5200-fold, 5300-fold, 5400-fold, 5500-fold, 5600-fold, 5700-fold, 5800-fold,
5900-
fold, or 6000-fold as compared to IFN-y levels in the subject not administered
the
vaccine.
The vaccine of the present invention can have features required of
effective vaccines such as being safe so that the vaccine itself does not
cause illness or
death; being protective against illness; inducing neutralizing antibody;
inducing
protective T cell responses; and providing ease of administration, few side
effects,
biological stability, and low cost per dose. The vaccine can accomplish some
or all of
these features by containing the cancer antigen as discussed below,
Generation of a loaded (pulsed) immune cell
The present invention includes a cell that has been exposed or
otherwise "pulsed" with an antigen or otherwise a peptide of the invention.
For
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/1JS2016/026542
example, an APC, such as a DC, may become Ag-loaded in vitro, e.g., by culture
ex
vi vu in the presence of an antigen, or in vivo by exposure to an antigen.
A person skilled in the art would also readily understand that an APC
can be "pulsed" in a manner that exposes the APC to an antigen for a time
sufficient
to promote presentation of that antigen on the surface of the APC. For
example, an
APC can be exposed to an antigen in the form of small peptide fragments, known
as
antigenic peptides, which are "pulsed" directly onto the outside of the APCs
(Mehta-
Damani et at., 1994); or APCs can be incubated with whole proteins or protein
particles which are then ingested by the APCs. These whole proteins are
digested into
small peptide fragments by the APC and eventually carried to and presented on
the
APC surface (Cohen et at., 1994). Antigen in peptide form may be exposed to
the cell
by standard "pulsing" techniques described herein.
Without wishing to be bound by any particular theory, the antigen in
the form of a foreign or an autoantigen is processed by the APC of the
invention in
order to retain the immunogenic form of the antigen. The immunogenic form of
the
antigen implies processing of the antigen through fragmentation to produce a
form of
the antigen that can be recognized by and stimulate immune cells, for example
T cells.
Preferably, such a foreign or an autoantigen is a protein which is processed
into a
peptide by the APC. The relevant peptide which is produced by the APC may be
extracted and purified for use as an immunogenic composition. Peptides
processed by
the APC may also be used to induce tolerance to the proteins processed by the
.APC.
The antigen-loaded APC, otherwise known as a "pulsed APC" of the
invention, is produced by exposure of the APC to an antigen either in vitro or
in vivo.
In the case where the APC is pulsed in vitro, the APC can be plated on a
culture dish
and exposed to an antigen in a sufficient amount and for a sufficient period
of time to
allow the antigen to bind to the APC. The amount and time necessary- to
achieve
binding of the antigen to the APC may be determined by using methods known in
the
art or otherwise disclosed herein. Other methods known to those of skill in
the art. thr
example immunoassays or binding assays, may be used to detect the presence of
antigen on the APC following exposure to the antigen.
In a further embodiment of the invention, the APC may be transfected
with a vector which allows for the expression of a specific protein by the
APC. The
protein which is expressed by the APC may' then be processed and presented on
the
36
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
cell surface. The transfected APC may then be used as an immunogenic
composition
to produce an immune response to the protein encoded by the vector.
As discussed elsewhere herein, vectors may be prepared to include a.
specific polynucleotide which encodes and expresses a protein to which an
immunogenic response is desired. Preferably, retroviral vectors are used to
infect the
cells. More preferably, a.denoviral vectors are used to infect the cells.
In another embodiment, a vector may be targeted to an APC by
modifying the viral vector to encode a protein or portions thereof that is
recognized by
. a receptor on the APC, whereby occupation. of the APC receptor by the vector
will
initiate endocytosis of the vector, allowing for processing and presentation
of the
antigen encoded by the nucleic acid of the viral vector. The nucleic acid
which is
delivered by the virus may be native to the virus, which when expressed on the
APC
encodes viral proteins which are then processed and presented on the WIC
receptor
of the APC.
As contemplated herein, various methods can be used for transfecting a
polynucleotide into a host cell. The methods include, but are not limited to,
calcium
phosphate precipitation, lipofection, particle bombardment, microinjection,
electroporation, colloidal dispersion systems (i.e. macromolecule complexes,
nanocapsules, microspheres, beads, and lipid-based systems including oil-in-
water
emulsions, micelles, mixed micelles, and liposomes). These methods are
understood
in the art and are described in published literature so as to enable one
skilled in the art
to perform these methods.
In another embodiment, a polynucleotide encoding an antigen can be
cloned into an expression vector and the vector can be introduced into an APC
to
otherwise generate a loaded APC. Various types of vectors and methods of
introducing nucleic acids into a cell are discussed in the available published
literature.
For example, the expression vector can be transferred into a host cell by
physical,
chemical or biological means. See, for example. Sambrook et al. (2012,
Molecular
Cloning: A Laboratory Manual, Cold Spring Harbor Laboratoiy, New York), and in
Ausubel et al. (1997, Current Protocols in Molecular Biology, John Wiley &
Sons,
New York). It is readily understood that the introduction of the expression
vector
comprising a polynucleotide encoding an antigen yields a pulsed cell.
The present invention includes various methods for pulsing APCs
including, but not limited to, loading APCs with whole antigen in the form of
a
37
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
protein, cONA or mRNA. However, the invention should not be construed to be
limited to the specific form of the antigen used for pulsing the APC. Rather,
the
= invention encompasses other methods known in the art for generating an
antigen
loaded APC. Preferably, the APC is tranfected with mRNA encoding a defined
antigen. mRNA corresponding to a gene product whose sequence is known can be
rapidly generated in vitro using appropriate primers and reverse transcriptase-
polymerase chain reaction (RT-PCR) coupled with transcription reactions.
Transfection of an APC with an mRNA provides an advantage over other antigen-
loading techniques for generating a pulsed APC. For example, the ability to
amplify
R.NA from a microscopic amount of tissue, i.e. tumor tissue, extends the use
of the
APC for vaccination to a large number of patients.
For an antigenic composition to be useful as a vaccine, the antigenic
composition must induce an immune response to the antigen in a cell, tissue or
mammal (e.g., a human). As used herein, an "immunological composition" may
comprise an antigen (e.g., a peptide or polypeptide), a nucleic acid encoding
an
antigen (e.g., an antigen expression vector), or a cell expressing or
presenting an
= antigen or cellular component. In particular embodiments the antigenic
composition
comprises or encodes all or part of any antigen described herein, or an
immunologically functional equivalent thereof. In other embodiments, the
antigenic
composition is in a mixture that comprises an additional imrnunostimulatory
agent or
nucleic acids encoding such an agent. Immunostimulatory agents include but are
not
limited to an additional antigen, an immunomodulator, an antigen presenting
cell or
an adjuvant. In other embodiments, one or more of the additional agent(s) is
covalently bonded to the antigen or an immunostimulatory agent, in any
combination.
In certain embodiments, the antigenic composition is conjugated to or
comprises an
HLA anchor motif amino acids.
A vaccine, as contemplated herein, may vary in its composition of
nucleic acid and/or cellular components. In a non-limiting example, a nucleic
encoding an antigen might also be formulated with an adjuvant. Of course, it.
will be
understood that various compositions described herein may further comprise
= additional components. For example, one or more vaccine components may be
comprised in a lipid or liposome. In another non-limiting example, a vaccine
may
comprise one or more adjuvants. A vaccine of the present invention, and its
various
38
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
components, may be prepared andior administered by any method disclosed herein
or
as would be known to one of ordinary skill in the art, in light of the present
disclosure,
It is understood that an antigenic composition of the present invention
may be made by a method that is well known in the art, including but not
limited to
chemical synthesis by solid phase synthesis and purification away from the
other
products of the chemical reactions by FIPLC, or production by the expression
ola
nucleic acid sequence (e.g., a DNA sequence) encoding a peptide or polypeptide
comprising an antigen of the present invention in an in vitro translation
system or in a
living cell. In addition, an antigenic composition can comprise a cellular
component
isolated from a biological sample. The antigenic composition isolated and
extensively
dialyzed to remove one or more undesired small molecular weight molecules
and/or
lyophilized for more ready formulation into a desired vehicle. It is further
understood
that additional amino acids, mutations, chemical modification and such like,
if any,
that are made in a vaccine component will preferably not substantially
interfere with
the antibody recognition of the epitopic sequence.
Antigen Presenting Cell Therapy
The invention encompasses a method of producing a population of
APCs (e.g., dendritic cells; DCs) that present the peptides of the invention
on their
surface that may be subsequently used in therapy. Such a method may be carried
out
ex vivo on a sample of cells that have been obtained from a patient The APCs
produced in this way therefore form a pharmaceutical agent that can be used in
the
treatment or prevention of cancer. The cells should be accepted by the immune
system
of the individual because they derive from that individual. Delivery of cells
that have
been produced in this way to the individual from whom they were originally
obtained,
thus forms a therapeutic embodiment of the invention.
DCs are derived from pluripotent monocytes that serve as antigen-
presenting cells (APCs). DCs are ubiquitous in peripheral tissues, where they
are
prepared to capture antigens. Upon antigen capture. DCs process the antigen
into
small peptides and move towards secondary lymphoid organs. It is within the
. lymphoid organs that DCs present antigen peptides to naive T cells, thereby
initiating
a cascade of signals that polarizes T cell differentiation. Upon exposure, DCs
present
antigen molecules bound to either IVIFIC class I or class II binding peptides
and
activate CD8 or CD4' T cells, respectively (Steinman, 1991, Annu. Rev.
Immunol.
39
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
9:271-296; Banchereau et al.. 1998, Nature392,245-252; Steinman, et at., 2007.
Nature 449:419-426; Ginhoux et at., 2007, J. Exp. ivied. 204:3133-3146;
Banerjee ci
at.. 2006, Blood 108:2655-2661; Sallusto et at., 1999, J. Exp. Med. 189:611-
614;
Reid et al., 2000, Curr. Opin. Immuno1.12:114-121; Bykovskaia et al., 1999, J.
Leukoc. Biol. 66:659-666; Clark et al., 2000, Microbes Infect. 2:257-272).
Des are responsible for the induction, coordination and regulation of
the adaptive immune response and also serve to orchestrate communication
between
effectors of the innate arm and the adaptive arm of the immune system. These
features
have made Des strong candidates for immunotherapy. Des have a unique capacity
to
sample the environment through macropinocytosis and receptor-mediated
endocytosis
(Gemer et al., 2008, J. Immuno1.181:155-164; Stoitzner et al., 2008, Cancer
Immunol. Immunother 57:1665-1673; Lanzevecchia A., 1996, Cuff. Opin.
Imrnuno1.8:348-354; Delamarre et al., 2005, Science, 307(5715):1630-1634).
Des also require maturation signals to enhance their antigen-
presenting capacity. Des upregulate the expression of surface molecules, such
as
CD80 and CD86 (also known as second signal molecules) by providing additional
maturation signals, such as TNT-a. CD4OE or calcium signaling agents
(Czemiecki ci
1997,. j. Immuno1.159:3823-3837; Bedrosian etal. 2000, J. Immunother. 23:311-
' 320; Mailliard et al., 2004, Cancer Res.64,5934-5937; Brossart et al., 1998.
Blood
92:4238-4247; Jin et al., 2004, Rum. Immunol. 65:93-103). It has been
established
that a mixture of cytokines, including TNE-u., IL-1 i. 11.-6 and prostaglandin
E2
(PGE2), have the ability to mature DC (Jonuleit, et al., 2000, Arch. Derm.
Res.
292:325-332). Des can also be matured with calcium ionophore prior to being
pulsed
with antigen.
In addition to pathogen-recognition receptors, such as PKR and MDA-
5 (Kalali et al., 2008, J. Immunol. 181:2694-2704; Nallagatla et al., 2008.
RNA Biol.
5(3):140-144), Des also contain a series of receptors, known as Toll-like
receptors
(TERs), that are also capable of sensing danger from pathogens. When these
TERs are
triggered, a series of a.ctivational changes are induced in Des, which lead to
maturation and signaling of T cells (Boullart et al. 2008, Cancer Immunol.
Immunother. 57(11):158-1597; Kaisho et al., 2003, Cum Mol. Med. 3(4):373-385;
Pulendran et al., 2001, Science 293(5528):253--256; Napolitani et al., 2005,
Nat.
Itnmunol. 6(8):769-776). DCs can activate and extend the various arms of the
cell-
mediated response, such as natural killer 7-6 T and a-13 T cells and, once
activated,
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/1JS2016/026542
DCs retain their immunizing capacity (Steinman, 1991, Annu. Rev. Inummol.
296; Banchereau et al., 1998, Nature 392:245-252; Reid et al., 2000, Curr.
Opin.
Immunol. 12:114-121; Bykovskaia et ale 1999, J. Letikoc. Bio1.66:659-666;
Clark et
al., 2000, Microbes Infect. 2:257-2721
The present invention also provides methods of inducing antigen
presenting cells (APCs) using one or more peptides of the invention. The APCs
can
be induced by inducing dendri.tic cells from the peripheral blood monocytes
and then
contacting (stimulating) them with one or more peptides of this invention in
vitro, ex
vivo or in vivo, When peptides of the present invention are administered to
the
mammal in need thereof, APCs that have the peptides of this invention
immobilized
to them are induced in the body of the mammal. Alternatively, after
immobilizing the
peptides of this invention to the APCs, the cells can be administered to the
subject as
a vaccine. For example, the ex vivo administration may include the steps of:
collecting
APCs from a mammal, and contacting the APCs with a peptide of the present
invention.
The present invention also provides APCs presenting complexes
formed between 1-ILA antigens and one or more peptides of this invention. 'The
APCs,
obtained through contact with the peptides of this invention or the
nucleotides
encoding such peptides, are preferably derived from subjects who are the
target of
treatment and/or prevention, and can be administered as vaccines, alone or in
combination with other drugs, including the peptides, exosomes, or T cells of
the
present invention.
The present invention provides compositions and methods for
stimulating APC, preferably DCs, in the context of immunotherapy to stimulate
the
immune response in a mammal. DCs can be manipulated by stimulating them with a
peptide or combination of peptides of the invention and causing the DCs to
mature so
that they stimulate anti-tumor immunity in a mammal in need thereof.
In one embodiment, the invention includes a method for inducing al
cell response in a mammal. The method comprising administering an APC, such as
a
DC, wherein the APC has been activated by contacting the APC with a peptide or
combination of peptides of the invention thereby generating a peptide-loaded
APC.
In one embodiment, the invention relates to novel APCs produced and
methods for their use to, inter alio, expand a desired T cell, to activate T
cells, to
expand specific T cell, as well as numerous therapeutic uses relating to
expansion and
41
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
stimulation of T cells using the peptide-load APC and peptides of the
invention. In
some instances, the OCT4 stimulated DCs can be used to expand peptide-specific
T
cells.
The present invention relates to the discovery that a DC contacted with
a peptide or combination of peptides of the invention can be used to induce
expansion
ofpeptide-specilic T cells. A skilled artisan would recognize that the DCs
contacted
with the peptides of the invention are considered primed or otherwise peptide-
loaded.
The peptide-loaded DCs of the invention are useful for eliciting an immune
response
against a desired antigen, for example HER3. Accordingly, the peptide-load DCs
of
the invention can be used to treat a disease associated with unregulated
expression of
HERI
Methods for Treating a Disease
The present invention also encompasses methods of treatment and/or
prevention of a disease caused by pathogenic microorganisms, autoimmune
disorder
and/or a hyperproliferative disease.
Diseases that may be treated or prevented by use of the present
invention include diseases caused by viruses, bacteria, yeast, parasites,
protozoa,
cancer cells and the like. The pharmaceutical composition of the present
invention
may be used as a generalized immune enhancer (DC activating composition or
system) and as such has utility in treating diseases. Exemplary diseases that
can be
treated and/or prevented utilizing the pharmaceutical composition of the
present
invention include, but are not limited to infections of viral etiology such as
HIV,
influenza, Herpes, viral hepatitis, Epstein Bar, polio, viral encephalitis,
measles,
chicken pox, Papilloma virus etc.; or infections of bacterial etiology such as
pneumonia, tuberculosis, syphilis, etc.; or infections of parasitic etiology
such as
malaria, trypanosoiniasis,leishmaniasis, trichomoniasiS, aMOebiasis, etc.
Preneoplastic or hyperplastic states that may be treated or prevented
using the pharmaceutical composition of the present invention (transduced DCs,
expression vector, expression construct, etc.) of the present invention
include but are
not limited to preneoplastic or hyperplastic states such as colon polyps.
Crohn's
disease, ulcerative colitis, breast lesions and the like.
Cancers that may be treated using the composition of the present
invention of the present invention include, but are not limited to primary or
metastatic
42
SUBSTITLTTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
melanoma, adenocarcinoma, squamous cell carcinoma, adenosquamous cell
carcinoma thymoma., lymphoma., sarcoma, lung cancer, liver cancer, non-
Hodgkin's
lymphoma, Hodgkin's lymphoma, leukemias, uterine cancer, breast cancer,
prostate
cancer, ovarian cancer, pancreatic cancer, colon cancer, multiple myeloma,
neuroblastoma, gastrointestinal cancer, brain cancer, bladder cancer, cervical
cancer
and the like.
Other hyperproliferative diseases that may be treated using DC
activation system of the present invention include, but are not limited to
rheumatoid
arthritis, inflammatory bowel disease, osteoarthritis, lei.ornyomas. adenomas,
lipomas,
hemangiomas. fibromas, vascular occlusion, restenosis, atherosclerosis, pre-
neoplastic
lesions (such as adenomatous hyperplasia and prostatic intraepithelial
neoplasia),
carcinoma in situ, oral hairy leukoplakia, or psoriasis.
Autoirmnune disorders that may be treated using the composition of
the present invention include, but are not limited to, AIDS, Addison's
disease, adult
respiratory distress syndrome, allergies, anemia, asthma, atherosclerosis,
bronchitis,
cholecystitis, Crohn's disease, ulcerative colitis, atopic dermatitis,
dermatomyositis,
diabetes mellitus, emphysema, erythema nodosum, atrophic gastritis,
glomerulonephritis, gout, Graves' disease, hypereosinophilia, irritable bowel
syndrome, lupus erythematosus, multiple sclerosis, myasthenia gravis,
myocardial or
pericardial inflammation, osteoarthritis, osteoporosis, pancreatitis,
polymyositis,
rheumatoid arthritis, scleroderma. Sjogren's syndrome. and autoimmilile
thyroiditis:
complications of cancer, hemodialysis, and extracorporeal circulation; viral,
bacterial,
fungal, parasitic, protozoal, and helminthic infections; and trauma.
In the method of treatment, the administration of the composition of
the invention may be for either "prophylactic" or "therapeutic" purpose. When
provided prophylactically, the composition of the present invention is
provided in
advance of any symptom, although in particular embodiments the vaccine is
provided.
following the onset of one or more symptoms to prevent further symptoms from
developing or to prevent present symptoms from becoming worse. The
prophylactic,
administration of composition serves to prevent or ameliorate any subsequent
infection or disease. When provided therapeutically, the pharmaceutical
composition
is provided at or after the onset of a symptom of infection or disease. Thus,
the
present invention may be provided either prior to the anticipated exposure to
a
disease-causing agent or disease state or after the initiation of the
infection or disease.
43
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
An effective amount of the composition would be the amount that
achieves this selected result of enhancing the immune response, and such an
amount
could be determined as a matter of routine by a person skilled in the art. For
example,
an effective amount of for treating an immune system deficiency against cancer
or
pathogen could be that amount necessary to cause activation of the immune
system,
resulting in the development am antigen specific immune response upon exposure
to antigen. The tennis also synonymous with "sufficient amount."
The effective amount for any particular application can vary depending
on such factors as the disease or condition being treated, the particular
composition
being administered, the size of the subject, and/or the severity of the
disease or
condition. One of ordinary skill in the art can empirically determine the
effective
amount of a particular composition of the present invention without
necessitating
undue experimentation.
Vaccine Formulations
The present invention further includes vaccine formulations suitable
for use in immunotherapy. In certain embodiments, vaccine formulations are
used for
the prevention and/or treatment of a disease, such as cancer and infectious
diseases. In
one embodiment, the administration to a patient of a vaccine in accordance
with the
present invention for the prevention and/or treatment or cancer can take place
before
or after a surgical procedure to remove the cancer, before or after a
chemotherapeutic
. procedure for the treatment of cancer, and before or after radiation therapy
for the
treatment of cancer and any combination thereof. In other embodiments, the
vaccine
formulations may be administrated to a patient in conjunction or combination
with
another composition or pharmaceutical product. It should be appreciated that
the
present invention can also be used to prevent cancer in individuals without
cancer, but
who might be at risk of developing cancer.
The administration of a cancer vaccine prepared in accordance with the
present invention, is broadly applicable to the prevention or treatment of
cancer,
determined in part by the selection of antigens forming part of the cancer
vaccine.
Cancers that can be suitably treated in accordance with the practices of the
present
invention include, without limitation, cancers of the lung, breast, ovary,
cervix, colon,
head and neck, pancreas, prostate, stomach, bladder, kidney, bone, liver,
esophagus,
brain, testicle, uterus and the various leukemias and lymphomas.
44
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
In one embodiment, vaccines in accordance with this invention can be
derived from the tumor or cancer cells to be treated. For example, in the
treatment of
lung cancer, the lung cancer cells would be treated as described hereinabove
to
produce a lung cancer vaccine. Similarly, breast cancer vaccine, colon cancer
vaccine,
pancreas cancer vaccine, stomach cancer vaccine, bladder cancer vaccine,
kidney
cancer vaccine and the like, would be produced and employed as
immunotherapeutic
agents in accordance with the practices for the prevention and/or treatment of
the
tumor or cancer cell from which the vaccine was produced.
In another embodiment, vaccines in accordance with the present
invention could, as stated, also be prepared to treat various infectious
diseases which
affect mammals, by collecting the relevant antigens shed into a culture medium
by the
pathogen. As there is heterogenecity in the type of immunogenic and protective
antigens expressed by different varieties of organisms causing the same
disease,
polyvalent vaccines can be prepared by preparing the vaccine from a pool of
organisms expressing the different antigens of importance.
In another embodiment of the present invention, the vaccine can be
administered by intranodal injection into groin nodes. Alternatively, and
depending on
the vaccine target, the vaccine can be intrademially or subcutaneously
administered to
the extremities, arms and legs, of the patients being treated. Although this
approach is
generally satisfactory for melanoma and other cancers, including the
prevention or
treatment of infectious diseases, other routes of administration, such as
intramuscularly or into the blood stream may also be used.
Additionally, the vaccine can be given together with adjuvants and/or
immuno-modulators to boost the activity of the vaccine and the patient's
response.
Such adjuvants and/or immuno-modulators are understood by those skilled in the
art,
and are readily described in available published literature.
As contemplated herein, arid depending on the type of vaccine being
generated, the production of vaccine can, if desired, be scaled up by
culturing cells in
bioreactors or fennentors or other such vessels or devices suitable for the
growing of
cells in bulk. In such apparatus, the culture medium would be collected
regularly,
frequently or continuously to recover therefrom any materials or antigens
before such
materials or antigens are degraded in the culture medium.
If desired, devices or compositions containing the vaccine or antigens
produced and recovered, in accordance with the present invention, and suitable
for
4
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
sustained or intermittent release could be, in effect, implanted in the body
or topically
applied thereto for a relatively slow or timed release of such materials into
the body.
Other steps in vaccine preparation can be individualized to satisfy the
requirements of particular vaccines. Such additional steps will be understood
by those
skilled in the art. For example, certain collected antigenic materials may be
= concentrated and in some cases treated with detergent and
ultracentrifuged to remove
transplantation alloantigens.
HER3 Expression as a Biomarker for Diagnosis and Treatment of Disease
In another embodiment HER3 expression can serve as a biomarker for
occult invasive disease in patients with Barrett's esophagus and high-grade
dysplasia
(HGD). Additionally contemplated herein are therapeutics for targeting HER3 or
CMET that may afford secondary prevention of gastroesophageal carcinoma in
some
patients.
These methods described herein are by no means all-inclusive, and
further methods to suit the specific application will be apparent to the
ordinary skilled
artisan. Moreover, the effective amount of the compositions can be further
approximated through analogy to compounds known to exert the desired effect.
= EXPERIMENTAL EXAMPLES
The invention is further described in detail by reference to the
following experimental examples. These examples are provided for purposes of
illustration only, and are not intended to be limiting unless otherwise
specified. Thus,
the invention should in no way be construed as being limited to the following
examples, but rather, should be construed to encompass any and all variations
which
become evident as a result of the teaching provided herein.
Without further description, it is believed that one of ordinary skill in
the art can, using the preceding description and the following illustrative
examples,
make and utilize the present invention and practice the claimed methods. The
following working examples therefore, specifically point out the preferred
embodiments of the present invention, and are not to be constmed as limiting
in any
way the remainder of the disclosure.
46
SUBSTITUTE SHEET '(RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCMTS2016/026542
EXAMPLE I: Creating Peptide Vaccines Against Other Receptor Tyrosine Kinases
("RTKs") That Cause Breast Cancer and Other Solid Cancers
Experiments were designed to develop alternative therapies against
patients designated as BRCA mutation carriers. That is, there is an unmet need
for
younger patients genetically at risk for breast cancer who are seeking
alternatives to
bilateral mastectomy.
Women with the breast cancer gene mutations BRCA1/BRCA2 have a
70% lifetime risk of developing breast cancer, and BRCA I mutation carriers
often
develop triple negative breast cancer. Experiments were designed to develop
vaccines
for this group and evaluate their safety in an immune-inducing trial, which is
the first
attempt ever at vaccination for primary prevention of breast cancer. BRCA2
mutation
carriers will also be included to see if estrogen receptor-P"iu" breast cancer
can be
prevented using the multivalent vaccine of the invention.
Experiments were designed to study RIK expression in breast cancers
and DCIS from BRCA mutation carriers. It was observed that tumors from the
BRCA
mutation carriers frequently over-expressed the c-MET oncogene and HER3 early
on
while the tumors from non-mutation or sporadic patients expressed HER2 and
HER3.
This is important because targets for tumor immunotherapy that can be used to
develop vaccines for sporadic and BRCA mutation carriers it is now known based
on
the disclosure presented herein. This is the first distinguishing feature that
can be
= targeted using immune response for prevention. Accordingly, the invention
includes
compositions and methods for developing vaccines and uses thereof for
prevention as
an alternative to bilateral mastectomies.
Creating peptide vaccines
The HER family consists of four related signaling molecules¨HERI,
HER2. HER3, and HER4¨that are involved in a variety of cancers. It is known
that
over-expression of HER2 is found in 20% to 30% of breast cancers. The results
presented herein demonstrate that other HER family members are involved in
both
early and invasive breast cancer, as well as other cancers. For example, HERI
is
expressed on a small number of breast cancers, generally those that are triple
negative, c-MET is a growth factor receptor involved in recurrence of many
cancers
that activates HER3. HER3 is over-expressed in colon, prostate, breast and
melanoma. HER3 is expressed in a large number of DCIS lesions and breast
cancers.
47
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
HER3 can be detected in the residual XIS at the time of surgely in some
patients
who received a HER2 vaccine. As a result of these findings, the potential to
target
these molecules in addition to HER2 in breast cancer is believed to be
beneficial.
Immunogenic peptides from HER3 have been identified (Figures I and
2) as follows:
pl 1-13 (Peptide 51-75): KLYERCEVVMGNLEIVLTGHNADLSFLQW (SEQ ID
NO: 1);
p81.-83 (Peptide 401-425): SWPPIIMFINFSVESNETTIGGRSLYN (SEQ ID NO: 2);
p84-86 (Peptide 416-440): TTIGGRSINNRGFSELIMKNENVTS (SEQ ID NO: 3);
p12 (Peptide 56-70): CEVVMONLEIVETGH (SEQ ID NO: 4);
p81 (Peptide 401-415): SWPPHMHNFSVFSNE (SEQ ID NO: 5);
p84 (Peptide 416-430): TTIGGRSLYNRGFSE (SEQ ID NO: 6); and
p91 (Peptide 451-465): AGRIYISANR.QLCYFI (SEQ ID NO: 7).
The results presented herein demonstrate that these peptides can
activate CD4 T cells across many patients. The peptides can be used to pulse
dendritic cells and educate T cells to recognize HER3. HER3 is expressed in
triple-
negative breast cancer and can impart resistance to anti-estrogen in ER"P'smve
breast
cancers. HER3 is also expressed in other cancers, including melanoma, lung,
colon,
prostate cancer, and metastatic brain tumors. Without wishing to be bound by
any
particular theory, peptides from the intracellular part of the molecule may
also be
advantageous.
Based on the disclosure presented herein, immunogenic peptides for
HER1 and the c-MET RTK molecules can be screened and identified based on the
procedure that identified immunogenic peptides for HER3. The immunogenic
peptides of the invention can be used to prepare a multivalent preventive
vaccine for
breast cancer as well as other cancers.
The results presented herein show the identification of the role of
HER2's sister proteins in breast cancer. These sister proteins can be
effectively
targeted and vaccines for other solid tumors can be developed. Peptides that
can be
used to target HERI and HER3 have been developed. In DCIS specifically,
specific
anti-HER], HER2, and HER3 responses in patients before and after vaccination
have
been identified, which provides support for the development of a multivalent
vaccine
that can be used to prevent early cancer or treat women who have DCIS. The
compositions of the invention is useful to treat other cancers including but
not limited
48
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
to colon cancer, melanoma, brain tumors, lung cancer, ovarian cancer, and
other
tumors.
Melanoma
Melanoma is an aggressive skin cancer that can be deadly if not caught
early. Experiments were conducted in mice using a standard dendritic cell
vaccine
wherein the dendritic cell was engineered to exhibit a mutated protein (BR.AF)
that
causes about 70% of melanomas. Vaccination with these dendritic cells
protected the
mice from challenge with melanoma cells, demonstrating that it is possible to
develop
vaccines for melanoma. Without wishing to be bound by any particular theory,
combinations of BIW.and HER3 targeting may be useful for treating melanomas as
well as other cancers including but not limited to solid cancers, such as
colon,
pancreatic, and lung cancers, and other gastrointestinal tumors.
In addition, it has been shown that melanoma tumors use B cells to
escape immune surveillance, and therefore it is believed that eliminating
certain B
cells can improve therapy. Experiments can be designed to assess whether
altering the
tumor microenvironment to a Thl-type response can help to prevent escape.
In some instances, the vaccine of the invention can be used to treat
melanoma that has spread. In some instances, the invention provides therapies
to
eliminate remaining cells that often become resistant to drug therapy.
EXAMPLE 2: Novel Strategy to Identify MHC Class II-Promiscuous CD4 Peptides
from Tumor Antigens for Utilization in Vaccination
Although cytotoxic CD8+ 1' lymphocytes (CIE.) were historically
considered primary effectors of antitumor immunity, solely boosting CU
responses
with CD8+ vaccines in various tumor types has yielded unpredictable clinical
results,
possibly because CTEs function suboptimally without adequate CD4+ T-Iymphocyte
help. CD4+ T-helper type I (Th I) cells secrete INF-'r/TNT-a, inducing tumor
senescence and apoptosis. As such, successful incorporation of CD4+ epitopes
into
cancer vaccine construction and generation of durable antigen-specific CD4+
immunity remains a challenge. Using the extracellular domain (ECD) of HER3 as
a
candidate "oncodriver" tumor antigen, experiments were performed to identify
immunogenic HER3 CD4+ peptides that demonstrate Class II promiscuity and
generate anti-HER3 CD4-1 immunity for inclusion in a vaccine construct.
49
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
The materials and methods employed in these experiments are now
described.
MATERIALS AND METHODS
Experiments were designed to identify immunogenic Class
promiscuous HER3 CD4-i- peptides using the ECD of HER3 as a tumor antigen in
order to generate anti-HER3 Thl cellular immunity.
Protocol Overview
Alibraiy of 15-iner long peptides that overlap by 5 amino acids was created
from the HER3 ECD. These peptides were pulsed onto monocyte-derived DCs from
donors and were matured to type 1-polarized (DC1; IL-12-secreting) phenotype.
The
DC 1s were harvested and co-cultured with purified CD4+ T cells from subjects
who
had known anti-HER3 Thl responses from our DCIS vaccine study. Large pools of
10 peptides were used and the identification process was progressively
narrowed
down to single reactive epitopes as measured by interferon gamma (IFN-y)
secretion
of the CD4+ T cells. Upon screening 5-6 subjects, 4 peptides were identified
that
seemed to react across most donors i.e., HER:356-70 (SEQ ID NO: 4), HE,R341-
415(SEQ
ID NO: 5), HER34 16-430 (SEQ ID NO: 6), and HER3451.465(SEQ ID NO: 7).
Subjects
with no evidence of reactivity to CD4+ T cell recognition of HER3
extracellular
domain were identified and their DC1s were pulsed with the four HER3 peptides
and
the pulsed DC1s were cultured with CD4 T cells for a week and then tested for
reactivity against HEIL' peptide and reaction to extracellular HER3 protein.
In all
cases, at least I peptide led to recognition of both the peptide pulsed on
monocytes
and the whole HER3 protein suggesting that primary sensitization had taken
place ex
vivo. It was also shown that healthy donors can react to these peptides and in
triple
negative breast cancer patients where there is a loss of anti-HER3 Thl
responses.
See, also, Gala, K., et al., Clin. Cancer Res 2014; 20: 1410-1416 and Datta,
J., et al.,
'Progressive Loss of Anti-HER2 CD4+ T-helper Type 1 Response in Breast
Tumorigenesis and the Potential for Immune Restoration". Oncohnmunology (in
press).
Protocol Highlights as further illustrated in Figure 19:
= A library comprising 123 overlapping 15 amino acid-long peptide fragments
that overlapped by 5 amino acids was generated from the HER3 extracellular
domain (ECD).
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
* Autologous monocyte-derived dendritic cells (DC) from donors were rapidly
matured to a type 1-polarized (DC1 IL-12 secreting) phenotype via GM-
CST. IFN-y and LPS, and pulsed with relevant peptides (e.g., HER3 ECD or
HER3 Cal peptides, where indicated). DC" polarize Thl responses via
elaboration of 1L-12.
= Harvested DC1s were allosensitized with purified CD4 T-cells in 8-10
day
co-cultures.
= Sensitized CD4- T-cells (a large fraction of which are expected to become
antigen-specific) were restimulated against immature DCs (iDC) that were
pulsed with a specific CD4+ peptide of interest (e.g.. HER3 library peptide
clusters') or irrelevant class H peptide control.
= The supernatant from these co-cultures were then harvested. Thi
responses,
measured by IFN-y ELISA, were considered antigen-specific if IFN-y
production was at least twice that of irrelevant control.
1 * ITLA-DR, DP, DQ typing was performed on donors by the Clinical
Immunology laboratory at the Hospital of the University of Pennsylvania in
order to assess WIC class TI promiscuity of CD4+ Thi responses.
A library comprising 123 overlapping 15 amino acid-long peptide
fragments was generated from the HER3-ECD. Autologous monocyte-derived DCs
from donors were matured to DC Is, and pulsed with HER3-ECD, Harvested DC Is
were co-cultured with purified CD4 T cells. After 10 days, sensitized CD4 T
cells
were restimulated against immature DCs (iDC) that were pulsed with IIER3
library
peptide clusters or irrelevant CD4 control peptide'. Thl responses, measured
by PFN-y
ELISA, were considered antigen-specific if IFN-y production was at least twice
that
of irrelevant control.
Experiments were performed in a 3-step process: 1) breast cancer
patients with known anti-HER3 LCD reactivity following HER2-pulsed DC.1
vaccine
were obtained in order to identify immunogenic CD4+ peptides; 2) the
immunogenicity of these peptides were confirmed in the same patients by a
process of
"reverse" sensitization; 3) patients with knovin anti-HER3 ECD non-reactivity
following vaccination were obtained and used to identify CD44- peptides to see
if the
51
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
cells were sensitized to the native HER3 ECD, thus overcoming/abrogating self-
antigen (i.e.. HER3) tolerance.
The results of the experiments are now described.
Sequential screening of HER3 ECD peptide library to ident0 immunogenic
epitopes
recognized by HER3 ECD-sensitized CD4+ Thl cells
Thl sensitization was initially performed in 5 breast cancer patients
with known ami-HER3 ECD reactivity in order to identify single immunogenic
HER3
CD4+ epitopes. To achieve this, HER3 ECD-sensitized CD4+ Thl were sequentially
restirnulated against 1.0-peptide clusters (1-10, 11-20, ... etc.), narrowed
to 3-peptide
clusters (1-3, 3-6, 7-10, ... etc.), and ultimately to single immunogenic HER3
peptides. Representative screens are shown in Figures 2, 13 and 14. Four
immunogenic peptides - HER3(56-70) (SEQ ID NO: 4), HER3(401-415) (SEQ ID
NO: 5), HER3(41.6-430) (SEQ ID NO: 6), and HF,R3(451-465) (SEQ ID NO: 7) -
were reproducibly identified and promiscuous across HLA-DR. DP, and DQ
-15 subtypes. When Thl cells from 4 non-HER3 reactive donors were
sensitized using
DC 1s pulsed with the four identified HER3 peptides, and subsequently
challenged to
recognize HER3 ECD-pulsed iDCs, all donors demonstrated successful
sensitization
not only to individual immunogenic HER3 peptides, but also recognized native
HER3-ECD.
The results presented herein demonstrate that DCI pulsed with an
overlapping tumor antigen-derived peptide library can identify promiscuous
class II
peptides for CD4 T cell vaccine development. In this study, immunogenic HER3
CD4 peptides effectively overcome immune tolerance to self-tumor antigens.
Utilization of these HER3 CD4 peptides in vaccine construction can be applied
to
patients harboring HER3-overexpressing cancers. Additionally, these results
represent a novel strategy to rapidly and reproducibly identify class II-
promiscuous
immunogenic CD4 epitopes from any tumor antigen for cancer immunotherapy using
a DC1-Th I platform. Table 1 below shows initial identification of immunogenic
CD4+ HER3 ECD peptides in patients with known anti-HER3 reactivity. Table 2
shows the amino acid sequences of the four immunogenic HER3 CD4+ epitopes
identified by the sequential screening.
Table 1 - Four immunogenic peptides ¨ HER356-70 (SEQ ID NO: 4), 11ER340I-
415 (SEQ ID NO: 5), HER3416-430 (SEQ ID NO: 6), 11ER3451-465 (SEQ ID
52
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816 PCT/US2016/026542
NO: 7)¨ were reproducibly identified across 5 donors previously sensitized to
HER3 ECD
' Donor # HER356-7o HER3401-4is 11ER3416-430
HER3451-465
15107-38
15107-24
26113-03 '-
15107-31
Table 2 Amino acid sequences of immunogenic HER3 C04+ epitopes
_ __________________________________________________
HE R356-70 CEVVMGNLEIVLTGH (SEQ ID NO: 4)
HER34o1-415 SWPPIIMIINFSVFSNL (SEQ ID NO: 5)
HER34 16-430 TTIGGRSLYNRGFSL (SEQ ID NO: 6)
HER3451_465 AGRIY ISANRQLCYH (SEQ ID NO: 7)
Confirmation of immunogenicity of identified CD4-l- HER3 ECD enitopes by
"reverse" sensitization ¨ i.e. ability of individual epi tope-sensitized CD4+
Thi to
recP.grlit..11?t! .... ....................
Figure 15 shows that in the donors with known HER3 ECD reactivity,
CD4+ T-cells were sensitized with respective donor-specific immunogenic HER3
epitope-pulsed DC is, and restimulated against iDCs pulsed with respective
HER3
epitope and native HER3 ECD.
Figures 20 and 21show additional results of "reverse" sensitization
CD4+ 'Th I sensitized with immunogenic HER3 epitopuised DC1 appears to
abrogate anti-HER3 immune self-tolerance
As seen in Figure 16, when CD4+ Thl cells from four HER3 ECD
nonreactive donors were sensitized using DC1s pulsed with the four identified
HER3
s3
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/1JS2016/026542
peptides, and subsequently challenged to recognize -HER3 ECD-pulsed iDCs, all
donors demonstrated successful sensitization not only to individual HER3 epi
topes,
but also recognized native HER3 ECD.
CD4+ HER3 epitopes demonstrate WIC class II promiscuity
Using the extracellular domain (ECD) of HER3 as a candidate
4oncodriver" tumor antigen, experiments were performed -to identify
immunogenic
HER3 CD4+ peptides that demonstrate Class 11 promiscuity and generate anti-
HER3
CD4+ immunity that can be used in a vaccine construct as seen in Figure 17.
Peptides From Tumor Antigens
The results presented herein demonstrate that:
= DC1 pulsed with an overlapping tumor antigen-derived peptide library can
identify promiscuous MHC class H peptides for CD4+ I-cell vaccine
development.
4* Immunogenic HER3 CD4+ peptides effectively overcome immune tolerance
to self-tumor antigens.
= These results represent a novel strategy to rapidly and reproducibly
identify
class II-promiscuous immunogenic CD4+ epitopes from any tumor antigen for
cancer immunotherapy using a DC1-CD4+ Thl platform.
= Utilization of these HER3 CD4i, peptides in vaccine construction warrants
investigation in patients harboring HER3-overexpressing cancers.
EXAMPLE 3: HER3 Expression is a Marker of Tumor Progression in Premalignant
Lesions of the Gastroesophaizeal Junction
Over-expression of RTKs including members of the HER family, has
prognostic and therapeutic significance in invasive esophag,oga.stric
carcinoma. RTK
expression in premalignant gastroesophageal lesions has not been extensively
explored previously.
Barrettes esophagus, or the presence of metaplastic columnar epithelium in the
distal esophagus, predisposes to the development of esophageal adenocarcinoma.
(Cameron, A.J., et al., Gastroenterology 109(5):1541-6 (1995).1 While the
histologic
transition from dyspl aS ia. to invasive malignancy is well characterized,
carcinogenesis
54
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
in metaplastic cells involves genetic alterations that are incompletely
understood.
Several recent reports have identified human epidermal growth factor receptor
2
(HER2) expression in a subset of Barrett's esophagus lesions with dysplasia.
(Almhanna. K., et al., App/. Inuntoolustochein. Mol.Morphol. July 16 2015)
(Almhanna, et al.); Fassan; M., et al., Hi.slopathology 61(5): 769-76 (2012)
(Fassan, et
al.); and Rossi, E., et al.õ /. Celt 11,101. "VW 13(9B):3826-33 (2009) (Rossi,
et al).)
Furthermore, the rate of HER2 expression correlates with degree of dysplasia,
implicating related pathways in tumorigenesis.
Oyer-expression of RTK molecules, including members of the HER family
(HER1, HER2, and HER3) and (MET, the meserichymal-epithelial transition
factor.
have been demonstrated in many of the more common malignancies, including
breast,
lung, and gastrointestinal cancers (Yokata, J., et al., Lancet 1:765-767
(1986) as well
as in esophagogastric carcinomas. The identification or HER2 overexpression in
a
subset of breast carcinomas, the association of HER2 overexpression with more
aggressive biology and effective targeting of HER2 with a monoclonal antibody
were
pivotal events in the evolution of targeted therapies for the treatment of
solid tumors.
(Joensuu. H., et al., N Eng. J. Med. 354(8):809-20 (2006).) This experience
has
provided a foundation for further efforts to target RTK molecules in the
treatment of
other malignancies.
HER2 overexpression has been demonstrated in a minority of gastric cancers
and has been targeted with trastuzumab in the metastatic setting with a modest
impact
on outcome. (Bang, 'VI, et al.. Lancet 376(9742) 687-97 (2010) (Bang, et
al.).)
HER2 overexpression is more frequently expressed in proximal gastric and
gastroesophageal junction compared to more distal gastric adenocarcinomas
(Rajagopal, 1., et al.õ /. Clin. Diagn. Res. 9(3)1E06-10 (2015)) and has been
targeted
with trastuzumab in the metastatic setting with a modest impact on outcome.
(Bang,
et at. and Fichter. C.D., et al., Int J. Cancer 135(7):1517-30 (2014)
(Fichter, et al.))
HER.1 and HER3 expression in gastric carcinomas have been associated with poor
prognosis in most studies. (Kande'. C., et at.. J 67(4):307-12 (2014)
and Hayashi, M., et al., Chn. Cancer Res. 14(23)7843-9 (2008).1 Overexpression
of
cMET is associated with poor prognosis in esophageal adenocarcinoma and
inhibition
of cMET-dependent signaling regulates the activity of HER1 and HER3. (Liu, X.,
et
al., Gin. Cancer Res. 17(22):7127-38 (2011).) These data provide rationale for
further efforts to characterize RTK expression in esophagogastric cancers and
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
precursor lesions. The studies described below are aimed to characterize RTK
expression in dysplastic lesions of the gastroesophag,eal :junction in efforts
to identify
potential targets for treatment and primary prevention.
METHODS
Following approval by the Institutional Review Board of the University of
Pennsylvania; the clinical records and histologic specimens from 73 patients
with
Barrett's esophagus with dysplasia (low-grade dysplasia (LGD), n=32, or high-
grade
dysplasia(HCiD), n=59) were retrospectively reviewed. Formalin-fixed paraffin-
embedded tissue blocks from stored endoscopic biopsy and mucosa' resection
specimens from 2003-2012 were sectioned at 5um on plus slides (Fisher
Scientific,
Waltham, MA) and subsequently deparaffinized and rehydrated. All biopsy
materials
were immunostained for HER1 (clone H11; 1:50; DAKO), HER2 (HercepTest,
DAKO. Carpinteria,CA) and HER3 (clone RT,1.2; 1:30; Santa Cruz Biotechnology,
Dallas, TX) (Leica Bond-Ill instrument) and evaluated under the
microscope(Leica
Bond-III) by a single pathologist. Membrane 3+ -1'1ER staining was considered
positive, as was membrane 2+ HER2 staining in >10% of tumor cells as seen in
Figure 22. cMET immunohisiochemistry was performed in 42 cases when sufficient
tissue was available; moderate or strong membranous staining in ','-!.50% of
tumor cells
was considered positive. RTK overexpression was correlated with clinical data
to
evaluate for associations with invasive carcinoma, either paired dyspl ia-
adenocarcinoma biopsy specimens or the diagnosis of adenocarcinoma on
subsequent
biopsy specimens.
Statistical 4nalvsLs
Two tailed tests were used for all analyses. Descriptive statistics are
presented as frequencies for categorical variables and median (interquartile
range
(1QR) for continuous variables. Pearson's x2 or Fisher's
exact tests and Wilcoxon rank-sum test were used to analyze categorical and
continuous variables, respectively. P-values :Ø05 were considered
statistically
significant; all tests were two-sided. Analyses were carried out using SPSS
v22.0
(IBM, Armonk, NY).
RESULTS
56
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
A total of 73 patients with Barrett's esophagus with low-grade dysplasia
(n=32) or high-grade dysplasia (n=59) were identified and analyzed for HER!.
HER2,
HER:3 and cMET expression by immunohistochemistry. Median age of the cohort
was 65 years (IQR 60-73 years); 81.9% were male and 87.5% were Caucasian. The
rate of alcohol use in the cohort was 14.3% and the rate of active cigarette
use was
6.3%, yet 55.6% were former smokers, 26.4% had a family history of malignancy.
There were no significant differences between the LOD and HOD cohorts in the
measured clinical and demographic variables as seen below in Table 3.
57
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
Table 3 Demographic and Clinical Characteristics of Cohort with Dysplastic
Barrett's Esophagus, and Univariate Comparison of Low-grade and High-grade
ILGD HGD p-value
Median (IQR) or Median (IQR) or
no. of patients (%) no. of patients (%)
Age, years I 64.0 (60.0-77.5) 66.0 (63.0-710)
0.621
Sex, male 19(73.!) 41 (87.2) 0,130
Caucasian race , 23 (95.8) 41 (93.2) 0,755
Cigarette use I Current 2(9.1) 2 (4.8) 0,681
1 Former 13 (59.1) 23 (54.8)
Alcohol use 2 (9.1) 7 (16.7) 0.683
Positive family history 6 (28.6) 13 (32.5) 0.753
Dysplastic Patients
High-grade dysplasia (HGD) was associated with overexpression of HER1
(22.4% vs. 3.1%, p=0.016), HER2 (5.3% vs. 0.0%, p=0.187) and HER3 (45.6% vs.
9.4%, p<0.001) compared to low-grade dysplasia (1_,GD).
Foci of invasive esophageal adenocarcinoma were associated with dysplastic
lesions in 6 cases, all of which arose in association with HGD (HGD: 10.2% vs.
LGD:
0.0%, p<0.001). An additional 9 patients were diagnosed with invasive
esophageal
adenocarcinoma on subsequent biopsy specimens (HGD: 17.0% vs. LGD: 0.0%,
p=0.017). There was a significant association of HER3, but not HER! or HER2
(increase in HER1 (26.7% vs. 20.5%, p-0.616) and HER2 (14.3% vs. 2.3%,
p-0.077), overexpression in HGD lesions compares with those without foci of
invasive carcinoma (71.4% vs. 38.6%, p=0.032) as seen in Figures 23A and 23B.
Overexpression of cMET was observed in 18 of 42 (42.9%) evaluated
specimens and was increasingly observed in HGD compared toL,GD specimens
(58.3% vs. 36.7%, p=0.200) and was most often co-expressed with HER3 (62.5% of
HER3-positive specimens vs. 38.2% of HER3-negative specimens (p=0.212)).
Similar trends were not observed in HER1-positive (p-0.729) or HER2-positive
(p-NA) specimens. One of the 42 (5.6%) patients had invasive carcinoma
identified,
cMET was overexpressed in this patient (p=0.243).
DISCUSSION:
This analysis of RTK expression in dysplastic lesions of the gastroesophageal
junction confirms that (1) HER family proteins are upregulated in Barrett's
esophagus
with dysplasia; (2) the frequency of HER family and cMET overexpression is
positively correlated with the degree of dysplasia; and (3) HER protein
upregulation,
58
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
particularly in dysplastic lesions, is associated with an increased incidence
of
associated invasive cancer.
HER3 may therefore serve as a biomarker for occult invasive disease in
patients with Barrett's esophagus and HGD. Additionally, therapeutics
targeting
HER3 or MET may afford secondary prevention of gastroesophageal carcinoma in
subsets of patients.
Previous evaluations of HER expression in _Barrett's esophagus have been
limited to an assessment of FIER2, which is overexpressed in a minority of
cases.
See, Al mhanna, et al.. FaSSari, et al., and Rossi, et al. HER2 overexpression
in this
study was present in 3.3% of biopsy specimens, lower than the rate of HER] or
HER3
overexpression. This pattern is consistent with HER family protein expression
in
invasive gastroesophageal junction cancers. where 1-IER3 is overexpressed more
commonly than HERZ. Fichter, et al. Increasing HER3 protein overexpression
with
progression fromEGD to HGD and frequent overexpression of HER3 in particular,
represent novel, though not unanticipated findings. Homo- and hetero-
dimerization of
HER receptors drive signal activation; clustered overexpression of multiple
members
of the HER family have been observed in other tumor types. In conjunction,
activated
c-MET positively regulates the activity of HER1 and HER311. Indeed, the
interplay
between these receptors have provided rationale for multivalent therapeutic
approaches targeting multiple RTKs. (Baselga, J., et al., N. Eng. 1 Med.
366(2): I 09-
19 (2012); Waddell, T., et al., lancet Oneol. 14(6):481-489 (2013))
The present data also suggest an opportunity for targeted secondary prevention
of gastroesophageal carcinoma that has not yet been explored. Previously
targeted
HER2 expression in ductal carcinoma in situ (XIS) of the breast has been
targeted
with promising results. See, U.S. Published Application US 2015/0323547 Al;
U.S.
Ser. No. 14/985,303 filed December 30, 201., Dan, J., etal., Oncohninunology
48
e1022301 (2015) DOI:10. 1080/2162402X.2015, 1022301; Datta, J., et al.. Breast
cancer Res. 17(1):71 (2015). Such an approach remains a more distant goal for
gastrointestinal malignancies. Notwithstanding. current treatment options for
Barrett's esophagus, including endoscopic resection and ablative modalities
and
radical surgery all have significant limitations. Alternative strategies that
spare
morbidity and mitigate the risk of invasive carcinoma are needed. This
analysis of
RTK expression in dysplastic lesions of the gastroesophageal junction confimis
that
(I) HER family proteins are upregulated in Barrett's esophagus with dysplasia,
(2) the
4;9
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US 2016/026542
frequency of HER family and cMET overexpression is positively correlated with
the
degree of dysplasia; and (3) HER protein upregulationõ particularly in
dysplastic
lesions, is associated with an increased incidence of associated invasive
cancer.
HER3 may therefore serve as a biomarker for occult invasive disease in
patients with Barrett's esophagus and HGD. Additionally, therapeutics
targeting
HER3 or CMET may afford secondary prevention of gastroesophageal carcinoma in
subsets of patients.
In summary, the present data indicate a relationship between frequent
overexpression of HER3 in high-grade dysphistic lesions of the
gastroesophageal
junction, especially those with occult invasive carcinoma and malignant
transformation. These findings may justify a more aggressive management
approach
for HER3-expressing dysplastic lesions and provide rationale for the future
application of HER3-targeted therapeutics in an early disease setting as will
be readily
appreciated by those skilled in the art.
We have previously shown a progressive loss in the native anti-HER-2 CD4
Thl during HER-2P" breast tumorigenesis. This loss of response was associated
with
lack of pathologic complete response ("pCR") to neoadjuvant treatment, and
correlated with elevated risk of breast cancer recurrence and could be
restored with
vaccination. Example 4 below explores whether there is a similar loss in anti-
HER3
Th I response during breast tumorigenesis.
EXAMPLE 4: Loss of Anti-HER3 CD4 Thl Occurs in Breast Tumorigenesis and is
Neg,atively Associated with Outcomes
Overall Example 4 Summary
We have previously shown a progressive loss in the native anti-HER2 CD4
Thl during HER2P's breast tumorigenests. This loss of response was associated
\kith
lack of pathologic complete response ("pCR") to neoadjuvant treatment, and
correlated with elevated risk abreast cancer recurrence and could be restored
with
vaccination. This Example explores whether there is a similar loss in anti-
HER3 Thl
response during breast tumorigenesis.
Peripheral blood from 131 subjects, including healthy donors ("HDs"), benign
breast disease ("BD"), ductal carcinoma in situ ("DCIS") and invasive breast
cancer
(IBC") patients was collected. Immune responses to four different HER3
immunogenic peptides identified in Examples I and 2 above were tested via.
enzyme
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
-finked immunosorbent (ELISpot) assay and all metrics of immune response were
analyzed.
There was a significant decline in the anti-HER3 response going from HiDs to
IBC. Triple negative ("TN") IBC had the lowest response across all three
immune
parameters. FIDs had significantly higher immune responses than both FRP"' IBC
and
TN IBC patients across all three immune parameters. Interestingly, HER2N5 II3C
displayed immune responses similar to that of HDs and BDs. Patients with
recurrent
breast cancer and lack of pCR to neoadjuvant therapy had significantly lower
anti-
HER.3 CD4 Thl responses than patients with no subsequent. recurrences or those
having a pCR to neoadjuvant therapy.
Thus, it was found that CD4 Thl anti-HER3 are lost during breast
tumorigenesis, most notably in TN IBC, a group with limited treatment options
and
markedly worse prognosis with HER3 overexpression. These findings have
implication for attempting to restore this response to prevent recurrence.
Background
Nearly one in eight women will develop breast cancer in their lifetime. Of
those, cancers over-expressing HER2 are associated with a higher rate of
distant
metastases and overall worse prognosis. The introduction of trastuzumab, a
monoclonal antibody against HER2, has dramatically enhanced progression free
survival and overall survival in patients with HER2 positive cancers, pointing
to
HER2's key role in modulating breast cancer progression. Giordano S.H., et
al., J.
Once/. (2014): JC0-2013 [Published online before print May 5, 2014,
doi:10,1200X0.2013.54.09481.
The immune system plays a key role in modulating HER2 expressing tumors.
It has been previously shown there is a step-wise decline in native anti-HER2
CD4 T
cell response going from healthy subjects to HER2P'5 DCIS to HERD" IBC, but
not
HER25eg IBC. Further, lower anti-1-JER2 immune responses correlated with
subsequent breast cancer recurrence while higher anti-HER2 immune responses
correlated with pathologic complete response to neoadjuvarn chemotherapy,
implicating the immune system's role in HER2P's tumorigenesis, See, Dana, J.,
et al.,
Oncolmmunology 4(10): el 027474. 1)01:10.1080/2162402X.2015. 1022301 (2015)
and U.S. Published Application US 2015/0323547 Al (collectively hereinafter,
"Datta, et al."). Our croup has developed a HER2 pulsed dendritic cell vaccine
that
restores anti-HER2 CD4 and CDS T cell responses in both DOS and IBC patients.
61
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
Sharma, A., et al., Cancer 118(17)4354-4362 (2012); Koski, G.K., et al., J.
Immunother. 35(1):54-65 (2102); and U.S. Published Application US 2015/0323547
Al.
HER2 is a member of the EGFR family, a group of RTKs that also include
HER1 and HER3. While it is well known that FIER2 self-dimerizes, the role of
HER3
in signaling is less clear and it may act to dimerize both with itself and
HER2. HER3
dimerization with HER2 has been proposed as an escape mechanism in breast
cancer
patients treated with trastuzumab. Czopek, J., et al., Contemp. Oncol.
17(5):446-9
(2013) ("Czopek, et al.") and Bae. S.Y., et al., Breast Cancer Res. Treat.
139(3):741-
50 (2013) ("Bae, et al."). Pertuzumab, a recent addition to the market and the
first
oncology drug to receive accelerated FDA approval as neoadjuvant treatment,
inhibits
HER2/HER3 dimerization and has been shown to have an overall survival benefit
when used in combination with trastuzuma.b for breast cancer patients.
;Thayer', K., et
alai, Nati. Compr. Canc. Nem,. 12(4):591-8 (2014) and Harbeck, N., et al.,
Breast
Care 8(0:49-55 (2013).
HER3 expression is less clearly delineated among the sub-types of breast
cancer although there is overexpression seen in some ER-positive, FIER2-
positive and
triple negative ("TN") subtypes. Moeder, C., et al.. Cancer 115(10:2400-9
(2009). It
is of interest that while HER3 overexpression may be more conunon in HER21"s
IBC,
its prognostic value is more significant in TN IBC. While HER3 expression in
ERP"/HER2P IBC did not impact disease-free survival ("DES") or overall
survival
("OS"), 1-IER3 expression in TN IBC was correlated with both a worse 5-year
DES
and 10-year OS. Bae, et al. and Czopek, et al. Notably, the sizeable subset of
TN
IBC patients with HER3 overexpression, a group that by definition does not
have any
of the classical treatment options, may benefit from a recognizable new
target. It is
unknown whether the anti-HER3 CD4 Thl response exists in healthy donors and
whether this response changes during breast twriorigenesis. The present stud
seeks
answers to these questions.
METHODS
Subject enrollment
A total of 131 subjects met study criteria and were consecutively
enrolled at the University of Pennsylvania with informed consent. This study
was
approved by the institutional Review Board of the University of Pennsylvania
and the
62
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
Abramson Cancer Center prior to subject enrollment. Of 131 subjects, nine had
insufficient peripheral blood monocytes to perform assays, leaving 122
subjects with
immune response data for review. CD4 T cell responses to four different HER3
immunogenic peptides were compared between healthy donors (HD, n=30), patients
with benign breast disease (BD, rr- II), DCIS (n-13), HERD' IBC (n-21), ERP"'
invasive breast cancer (ERP" IBC, n-20) and triple negative IBC (TN IBC,
n=27).
Peripheral blood mono CV te collection
Peripheral blood was collected by venipuncture. Blood was diluted in
Hank's buffer or PBS at a 1:1 ratio and lymphocyte separation media was
layered
below diluted blood in conical tubes. Blood was then separated by density
centrifugation at 1200 rpm for 30 minutes. Monocyte layers were collected and
washed twice in Hank's buffer or PBS. Cells were counted and resuspended at 10
million cells per milliliter and frozen at minus 80 C for 24-48 hours before
being
transferred to minus 200 C, where cells remained stored until experimental
assay.
Measuring anti-HER3 CD4 Thl response
Anti-HER3 CD4 Thl cell response were measured by ELISpot assay,
according to the manufacturer's protocol. Briefly, 96 well PVDF membrane
plates
were activated with 70% ethanol, washed with PBS then coated with anti-ITN-
gamma
(anti-IFN-y) antibody and incubated overnight at 4 C. 24 hours later, plates
were
again washed with PBS then blocked with Iscove's media with 10% human scrum
for
1 hour. Peripheral blood monocytes were thawed at 37 C, washed in PBS or
Hank's
buffer, counted and resuspended at I million cells per milliliter then plated
at 200,000
cells per well with one of four immunogenic HER3 peptides (p12 (Peptide 56-
70):
CEVNINIGNLEIVETGH (SEQ ID NO: 4); p81 (Peptide 401-415):
SWPPHMHNESVESNL (SEQ ID NO: 5); p84 (Peptide 416-430):
TTIGGRSLYNRUSE (SEQ ID NO: 6), and p91 (Peptide 451-465):
AGRWISANROLCYH (SEQ ID NC): 7), anti-CD3/CD28 (polycloncal stimulus:
positive control) tetanus toxoid (Santa Cruz Biotechnology, Dallas, TX) or
nothing
(unstimulated control). Assays were performed in triplicate. Plates were
incubated at
38 C for 48 hours. After 48 hours, plates were washed with PBS, biotinylated
anti-
IFN- y antibody was then added and plates were incubated at 38 C for two
hours.
=
63
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
Plates were again washed with PBS, streptavidin-FIRP was added and plates were
incubated at 38 C for one hour. Finally, plates were washed with PBS then TMB
substrate solution was added. Color development was stopped after five minutes
by
washing extensively with tap water. Plates were dried overnight at 4 C.
Immune response analysis
Spots were counted via irnmunospot software. Three parameters or
metrics were quanitifed to determine immune response: (1) cumulative response,
or
the summed response to all four 1-IER3 immunogenic peptides in spots per
million
cells, (2) repertoire, or the number of peptides per subject with 20 or more
spots, and
(3) responsivity, or the percent of subjects responding to at least one
peptide (defined
as a threshold of 20 or more spots). Tetanus response in spots per 200,000
cells and
anti-CD3/CD28 response in spots per 200,000 cells were also quantified as
controls.
All immune response metrics were analyzed in graphpad prism software.
RESULTS
Study Subject Characteristics
A total of 131 subjects met study criteria and were consecutively enrolled
with
informed consent at the Hospital of the University of Pennsylvania. Nine
subjects had
insufficient cells for analysis, leaving 122 subjects. Of these, the mean age
was 50,
ranging from 25 to 83. 72.1% were Caucasian, 18.0% African American and 9.8%
another race. Subjects fell into one of live groups: FIDs (n=30), .BDs (n=11),
DOS
(n=13), FIER2P' IBC (n=21), ER" IBC (n=20) or rs1 IBC (n=27). Of the 68 IBC
subjects, 35 (51.5%) were Stage 1, 22 (32.4%) were Stage 11, 9 (13.2%) were
Stage III
and 2 (2.9%) were Stage IV. 52 (76.5%) had undergone chemotherapy and/or
herceptin and/or ta.moxilen treatment, while 16 (23.5%) were treatment-naïve.
Three
DCIS patients and three HER2P" IBC patients received HER2 pulsed dendritic
cell
vaccination. Other characteristics are reported in Table 4 below.
64
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816 PCT/US2016/026542
Table 4 Characteristics of study subjects
ti
7
itSV-20, . SP. *mar 1.118cateaaffie m2ttestilt &filthy COMM at** &Via 0,R00
1
Olnats &sawn* fa 1 km:5m Immo I brawl:mew *nosy* &most
gay I Camp' 1 61.11SV
R. X) 11 IS I 22 2t) 27
. .. I=gr la ;43-fta Se PP/3) S424242:22 I 2222-iki) !
sr,142432 slis4,22
t
k c=
._... i == ::- =
tas.taSaµt Pitt t 411874 4 t?2.7S1 4 Dift.rat ! la in.13...t
SS NW Ig iS4.17gt
t-
, - 1 St,116) 1 (3g.i331 4 (3$:.01 I 1
(343%) 48f0IS 8123t OS
atmoicats i =
i , e
i.,titiq rti i A :AN) 10 mi DiN1 3 (14=3$62 1i%
................. 1_ _
O.:ego:a:strata*
--- ___ ________________________
===='''"'=== = = *=::.:=,:::H,::: l'.'==;:':=.::'=:,:::::,:===H:':.=-==17,-
:=...:::=::::::ti:t.:=i=:'==:i:::.::g?:==.:i::=.,::?:==.:
ilAvitesoc.itts 13i10X1
xi PapanArt i 1530:4
t............... -....i.................................. , -..
============= == = = == .::: -::== === = = = ¨
Alsattosussi St3143
. . ''''''''' 1:.:t:=-
.3:.::.=,:i..::::.=:':::=:t:i;t:::.:=:==.:=:::3::,===::t:=.-
.T:==::..:it:'...:=:..:=.::=::=:.
$.0 ir. 22212.2222 *:?4="1
:'.,:i..:......:.:1.1=4.....1::.!1.:..i.'51....,...:,:... ,::.......
,:::.,:..::,: ::, .....õ: :,.,:,:.,:: '
::..:..:õ:i,,,:::...::::...:....::,::.:...:::.:,.
*92*. . JJ ',111-7941 IT:?333(
;',::: '....:::::==:==::::::.::::==:=:t.-.:::,,=,=,:==,.,=:t=,::=,:
:::.:=:.3tt:':: ::::=:::*=:::.:::.,=::',. =.:.=: '..::::*=.=:,.:';:=::::.'..
'..: ===::.:=:::.:.,t
Mactoparticl
Itecaptots.
' t .
'-'-'=-''''"'= = T...::::=-..*:..*...!sz...::.:?.:.,..:.:.-....:...: ' ....,..
ai .0 ov i Is :,1.4,0 if: ilõ.,,,,
' "'
.', ' = : . . ::= . .
.......,,,,.,,,= __ t __
lint.). i.'-i.:::...=:.:.i:.::::.].....:.::i '..
::::'::::.,::'::'...,:,i.,::::,.::::.: a 022.)2i2 1 11f1Mi 01 %)
1 0 tirki
..:.====:..'::'.::::µ:::..:,,,.i::.,::: :::::r.,;.i=i::,:..:::
1"''''"=,',.- '' '.'...". ...7.7%"''' ........1
10-1 ::: :.;:......::
. ... .
....-
Ste;2
_______________________________________________ ¨ ¨
_.-
: tt '-,.H ,.....:.'..,:., =.,:.=-,"=.=
:,:'=::i:.,=:=::.:,::,,::::H:==:=,, =:.'i ,:i;:.i:',.::..,===.=::=,:g:IZI--
1,1t.16.,,x,.., -=- s2.10,4 Ti-j---..,s1 ,rxi
Stal4t 1,=,=:';':=,.....:',-....-
r=:=======:==:1=1':=,===:=:=:=:=:=:=:.=.:=:::::':=;:'....::::':*:=:',::=:'...',
===':: :::=:=::=,t,...:=:...............:....:,..,<..
=<=,=,',.= ' ' ' = ' =,=:=:=:=:,:i:=:===::.:=:'====':;,=:-
..'.====:.=,=::==:===.=: :'.'...,...=..::i=ti=i..,'.::::::,]':.i: $
(23.9131 a t.201i.1 i 13 Y61%
:=:" '': =!:::=:: '' = '' = ==:==== = = = : :=:
=:::.===,::=:=..:,=:,,,.- ..
==== . = : . ===== = ===== ====== = .. ,== =-= = =... ....
'--------- ' ...'. i ::: ' . : ... . . = ::::' : :- .= ' ::::: '-=I:
= ; :::',=:====,'=:::::.=:=:='H===: ... 3 P.3õ3 3!13%1 I I V..4
IN
'
t ..-µ..'"µ=µµ's="'-µµ..µ'''''''7-.r.µ.:7.:µ,7'.:"7....:7,..r.:r.:...7:7.r...t
a tax) 1 2 (s*2 3
2 0. )x)
22..1,. sv !:.::::.:::::!::!::::!:,.. ..::.j,:.:... ...,
.,:.:,::....:::..:,,..:=,.j..:,:.: :=.:', .:j.....,.....:.,...;:..::::.,.,.,1
_ ....L. ......i....................._,
. - ..............
- = = .22.: Yin,,rt at Mt=tr. F.wy
i'=.: :======= '...:', =:.:.:,...:,......::,...:::,,:::::::::.,, . ..
1,...,04, I 4 i,,s,,,
ii P.3K1 ) ilS.0%)
i
ChipepothvagSY 3.:::::..:: . : . :,:,::.:;:. . : . . f = ,
c.....4,...;;;;;..........".......,;,_
Se,a-.14..vrtt. i==,=:::====.:,:if.:== :....:=,=;.=::
::.:':::::::=?,=.:;:i::.:=:::.=,:::: 0234 ?cm 3222 .2 fswa
8;248331
Ctratothent:1 ,...::.ii.E.':i:.'.=:1I''j': '.::=,'. :','.:.=.=:. :.:.:.
t'....:'...:.::';;.:;::::.:==.:::=..:' .....ss,..
ss.õ...............,,,,,,,4.......,...........ss,...,,,ss.....ss......µs,µ,,,,,
,,,...4
------ it....r..r-'-r..r'.777777**7
t,361
- i 10 ia7148 I 14(10M L44
Acti3saca t:,:,',=,F,,,...::,.,:.:,..i.=:.: ,..:::: ,:.-. = . .::::=:-
.=,.::.:,..,... .3
,=3111:3KNAC tt ,:l.'::=-=:=...;::..::''.;:.='-:::==;t:f::.'=:', .'-
=':;;:....::: . = . i . .:t=:.'::'''.::::l..:,
1
1,7111,113Xvi ,:==:=.,'.'=:.i=i=======:Ht.:='-.::: t,:.::.
:.:=.,=:=:.*:=:.t:'.,':'..::.:==:::' .
___________________________________________________________ ..--.
., t=:':.='il=:===::.:. ==..:===,:.:.tH:t..tt...t=õ.:,: Al
3 r.143
a1 ;,1.,4 :at Stte.Cto. = . . .... .. ............ ....
..... ............... SS Fiffi Li P.0%;
J 3...
LINO:
'IX.) S. at fattle Soasat=
-.17 _ õ
õ...õ,õ.õ............õ....,õ,õ.._....õ,..õ . ...............
af.,lith.. :.,=::.,:='.::.=j. .. '.::::777TH:i; .:.........:::::::-
.. - .tt i ;:g.K) ii41....,..:::, i 12 !,s6ii 2.4242 .424
YMIAMANMAM1
081* 1 g c4034 la i..(1.9%;
Pialft 1.;....:;.:.:....'..:: .....,.i,... . .H.,:',..:,:,:.:: r::. ,
I .
r=-==== .... .....-
================================.....ss
f:...77.7:'.7.7.7.7 4 cm.ili , (a sv , saw
II y4S.334
" Wt.'
1:'.':=:.'.....'.1:.*:'.::1'....==:....:...:====: :::::::::.'...:::';','...'.
!
_________________________________________ - i
'''------"-t.'"...'.....17.77.=: : '.:..:...: .: :::;::.:::',]-::,,.::::,.i
,('L$; a 07.1%) I ts(?3%1 IS (SCL4222
- ..... ¨.. ===
snuixtught220,422 4,2,2,=:4 f hrto al Ytta= itl:t. I.......... ... . .
. ......... . , . ..... , . ..
NtS'ibaNt t :, . :. . . :. .:.: ,, . .. . ...:::::: . : , ,.: :::
= tti ;313N 13 t'.4.11S) 13 ($11.3'31
vat* 1 t::. : :: = 1: -:' ' : , : = ,,=.',. . :., ,:: :,
. , ::
teettsmtytt t ::,!: = : = : = ' .= ' = = = =:, ' ' ' = =
: .. .
. __ ... __
ttatatolt i,.::',,,= = õ= = ,= .. : ., i
,...,i:....t.=:::::;:::::::::, 2 (16./N3 293.314 3f1k.itg
tirn..s3C tlxri 1.:.,..:.:.:,.:.: = :
........1,....,....:,..:===:=:=.,=:=......:=;=.::===...,...:
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
There is a Decline in CD4 Thl Cell Anti-HER3 Immune Responses from Healthy
Donors to Subsets of Invasive Breast Cancer
Comparing HDs, BDs, DCIS, HERD"' IBC, ERP" IBC, and TN IBC,
there was a decline in all three immune parameters, reaching the lowest point
in TN
IBC: cumulative response (90 versus 80 versus 66 versus 79 versus 48 versus
40,
p=0.0l, respectively, as shown in Figure 24A), repertoire (1.0 versus 0.6
versus 0.8
versus 0,8 versus 0,5 versus 0.3, p=0.003, respectively, as shown in Figure
24B) and
. responsivity (76.7% versus 63.6% versus 53.8% versus 66.7% versus 45.0%
versus
33,3%, p=0,02, respectively, as shown in Figure 24C). Notably, these
differences
were not only statistically significantly higher, but also more than double,
in HDs
compared to TN IBC patients across all three immune parameters: cumulative
response (90 versus 40, p=0.002), repertoire (1.0 versus 0.3, p-0.0004) and
responsivity (76.7% versus 33.3%, p-0.001). Compared to TN IBC patients, BDs
had significantly higher cumulative response (40 versus 80, p=0.007,
respectively).
DCIS patients had significantly higher repertoire (0.8 versus 0.3, p=0.04,
respectively) and HER2P' IBC had significantly higher repertoire (0.3 versus
0.8,
p=0.01) and responsivity (33.3% versus 66.7%, p=0.04. respectively). FR'' IBC
patients had the second lowest anti-HER3 CD4 T cell responses and displayed
statistically significantly lower responses compared to HDs across all three
immune
parameters: cumulative response (48 versus 90, p-0.03, respectively),
repertoire (0.5
versus 1.0, p=0.008. respectively) and responsivity (45.0% versus 76.7%,
p=0.03,
. respectively). Of note is the fact that, HER2P" IBC anti-HER3 responses did
not vary
significantly from IlDs, BDs or DCIS subjects.
Lower CD4 Thl Cell Anti-HER3 immune Responses in Invasive Breast Cancer
Patients are Specific to 1'IER3 and are Not Attributable to a Broad Deficiency
in the
Immune Response
Tetanus responses and polyclonal stimulation with anti-CD3/CD28 responses
were analyzed to compare and control for overall immune responsiveness. There
was
no difference in CD4 Thl cell anti-tetanus response, as measured via ELISpot
assay
in spots per 200,000 cells, between HDs, BDs, DCIS, HER2P" IBC, ERP" IBC or TN
IBC patients (37 versus 30 versus 19 versus 34 versus 24 versus 29, p=0.65,
respectively, as shown in Figure 25A). Importantly, anti-tetanus responses
between
HDs and TN IBC patients, the groups with the most divergent anti-HER3 CD4 Thl
66
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
cell responses, were similar (37 versus 29, p=0.37). Likewise, there was no
difference
in polyclonal stimulation with anti-CD3/CD28õ with the majority of subjects in
each
group having robust spot development that was too numerous to count. Of those
that
were countable, there was no statistically significant difference between HIN,
BDs,
DCIS, HER2P"'IBC, ERP"s IBC or TN IBC patients (688 versus 549 versus 804
versus 699 versus 629 versus 675, p=0.68, respectively, as shown in Figure
25B).
Invasive Breast Cancer Patients' Anti-HER3 CD4 Thl Cell Responses Correlate
with
Prognosis and Characteristics of Tumor Aggression
To determine whether anti-HER3 CD4 T cell responses correlated with
characteristics of tumor aggression, IBC patients' immune responses were
compared
by lymph node status at initial surgery (lymph node positive ("LNP"') versus
lymph
node negative ("LN"g ')), recurrence versus non-recurrence in patients who
were at
least 1 year out from diagnosis and response to neoadjuyant chemotherapy
(pathologic complete response ("pCR") versus residual disease ("<pCR")). While
LNP's IBC patients (n=28) had overall lower immune responses compared to LNncg
patients (n=31) across all three parameters, none were statistically
significant:
cumulative response (40 versus 56, p=0.12, respectively), repertoire (0.4
versus 0.6,
p=0.08, respectively) and responsivity (35.7% versus 54.8%, p=0.19,
respectively) as
shown in Figure 26A. Of note, LNP" subjects included those with lymph node
metastasis after neoadj uv ant chemotherapy. Subjects who were LN"g post-
neoadjuvant chemotherapy were excluded from analysis, given it was unknown
whether they may have had positive nodes prior to treatment. Of patients who
were at
least a year out from diagnosis, those with recurrent breast cancer (either
local or
distant metastasis, n=7) had significantly lower anti-HER3 responses across
all three
immune parameters compared to those who remained disease-free (n=36):
cumulative
response (17 versus 66,p-0.04, respectively), repertoire (0.0 versus 0.6,
p<0.05,
respectively) and responsivity (0% versus 55.6%, p-0.01, respectively) as
shown in
Figure 26B. Lastly, of patients receiving neoadjuvant chemotherapy (a-1 (A pCR
(n=5) compared to <pCR (n=11) had significantly higher cumulative response
(144
versus 32, p-0.004, respectively) and repertoire (0.8 versus 0.4, p0.05,
respectively)
as shown in Figure 26C, There was no statistically significant difference in
responsivity between pCR and <pCR (80.0% versus 27.3%, p-=0.10, respectively).
It
67
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
is note, there were no differences in tetanus response between LNP's and LN"cg
patients (22 versus 29, p-0.35, respectively), recurrent versus non-recurrent
patients
(27 versus 35, p=0.65, respectively) and pCR versus <pCR (17 versus 59.
p=0.15,
respectively). Thus, lower CD4 Thl cell responses in 113C patients with
characteristics of more aggressive tumors is specific to HER3.
Anti-HER3 CD4 Thi Cell Responses by Healthy Donor Characteristics
Anti-HER3 CD4 .Thi responses were compared in HDs and BDs by age (<50
years (n=25) or ?.50 years (n=16)), race (Caucasian (n=29), African American
(n=12)
or other (n=5)), pregnancy status (0 (n=17) or 1 or more pregnancies (n=24))
and
menopausal status (pre-menopausal (n-30) or post-menopausal (n--11)). There
were
no differences in cumulative peptide response, repertoire or responsivity by
age
(Figure 27A), race (Figure 278) or history of prior pregnancy (Figure 27C).
However, as seen in Figure 27D, post-menopausal women, compared to pre-
menopausal women, had significantly higher cumulative response (136 versus 70
spots per million cells, p-0.005, respectively) (top panel) and repertoire
(1.4 versus
0.8 peptides, p=0.03, respectively) (second panel). There was no statistically
significant difference in responsivity (90.9% versus 66.7%, p-0.23,
respectively)
(third panel). There was also no statistically significant difference in
tetanus response
between pre- and post-menopausal women (bottom panel), indicating the
difference in
immune response by menopausal status was specific to HER3.
EL1Suot Assays are Precise as Demonstrated by a Linearity Precision Assay
EL1Spot assays have been previously validated in our laboratory. To confirm
the precision of this assay under the operator who conducted all experiments
for this
study, a linearity precision assay was performed with serial dilutions from a
known
high anti-HER3 CD4 T cell responder. Peripheral blood monocytes were serially
diluted into media from a concentration of 1,0 to 0.1 to 0,01 to 0.001 and
cumulative
anti-HER3 immune response was measured in spots per million cells. Figure 28
shows there was a linear decline in spots going from a cumulative value or 230
to 35
to 12 to 5, respectively (p<0,0001, r=0,88),
68
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
DISCUSSION
Knowledge of the immune system's role in cancer development, progression
and prognosis is rapidly expanding. It is well established that
immunodeficient states
increase risk of cancer development, not only from tumors of viral origin but
also
from tumors of non-viral origin. Boshoff, C., et al., Nature Rev. Cancer 2:373-
82
(2002); Shed, A.G., Wor/a'J. Surg. 10:389-96 (1986); Penn, 1., Transplantation
61:274-78 (1996); and Penn, 1., Transplantation 60:1485-91 (1995). It is also
known
that certain immune phenotypes are associated with breast cancer; circulating
inflammatory cytokines TNF-a and IL-6 are higher in breast cancer patients and
low
CD4P"/CD8P's T cell ratios are linked to more aggressive breast cancer
phenotypes
while tumor infiltrating lymphocytes are associated with a better prognosis in
some
breast cancers. Alokail. M.S., et al.,114ed Oncol. 31(438 (2014) doi:
10.1007/s12032-014-0038-0, Jai, Y., et al., Med. Oncol. 31:981 (2014); and
Matsumoto. H., et al.,!. Clin. Pothol. doi:10.1136,Jcli path-2015-202944.
However,
evidence linking loss of immune recognition to specific molecular oncodrivers
in
otherwise immunocompetent hosts is relatively newer. Only recently has there
been
shown to be a decline in native anti-HER2 CD4 Thl cell responses going from
healthy donors to HER2P`" DC1S to HER2P" IBC, one of the first studies showing
a
lost immune response to this specific oncodriver in breast tumorigenesis.
Those
having ordinary skill in the art will readily appreciate that the
identification and
understanding of such specific losses will have much potential for specific
immuno-
targeting therapy.
This study showed (1) there is a decline in the anti-HER3 CD4 Thlcell
response going from IIDs to ERP" and TN IBC, (2) the anti-HER3 response
correlates
with prognosis, specifically lower responses are associated with recurrence
while
higher responses are associated with pCR to neo-adjuvant chemotherapy, and (3)
post-menopausal fiDs have significantly higher anti-HER3 immune responses. All
of these findings will have diagnostic and clinical uses for anti-HET3 CD4 Thi
cell
response.
Anti-HER3 CD4 Thlcell responses were highest in 1-IDs and lowest in TN
IBC, a group whose prognosis is more severely impacted by HER3 overexpression
than other types of IBC. Bae, et al. and Czopek, J.. et al. While HER3
expression is
unknown in the presently sutudied cohort of IBC patients, it may be that the
TN IBC
69
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
and FRP" IBC groups have higher levels of HER3 expression compared to the
HER2P" IBC cohort, which displayed responses similar to that of 'Ms. Indeed,
our
prior study showed the anti-HER2 CD4 Thl cell response correlated directly
with
HER2 expression: there was a significant decline in HER2" IBC but not in
HER2"g
= IBC. Not only does HER3 have a greater impact on prognosis of TN IBC
compared
to receptor-expressing breast cancers, but even in TN IBC it may have a
greater
impact on prognosis of HER2 (0) compared to HER2 (1+) tumors. Schmidt, G., et
ale
Arch. Gynceol. 0/islet. 290:1221-29 (2(14) This may in part explain the
similarity in
immune response between HER2P'" IBC and 1-IDs. -lithe tumor is already
propogating due to HER2 overexpression, there is no drive for tumor evolution
to
evade immunosurveillance. if. however, the immune system is already
recognizing
and targeting HER2, the tumor may adapt via HER3 overexpression, where immune
evasion becomes evolutionary advantageous to tumor cell survival.
Interestingly, ERP"s IBC displayed ant-HER3 CD4 [cell responses similar to
that of TN IBC and significantly lower than HDs or HER2P" IBC. While HER3
expression is less prognostically significant in ER"' IBC compared to TN IBC,
evidence indicates HER3 mRNA expression is positively correlated with ER
expression. Fujiwara. S., et al., Breast Cancer 21;472-81 (2014) This may
explain
= the lower immune response seen in this subgroup of IBC.
Breast cancer patients with recurrent disease had lower anti-HER3 CD4 Thl
responses compared to patients who remained disease-free, indicating
immunosurveillance may be an important mechanism for long-term therapeutic
success. It is also possible recurrent patients were more likely to have high
HER3
expressing tumors, which itself correlates with higher risk of recurrence,
metastasis
and worse overall survival. Li. Q., et al.; Oncoloxv Reports 30:2563-70
(2013);
Smirnoya, T.; et al.. Oncogene 31:706-15 (2012); and Deana; A., et al., JAI.
C./.
105(4):266-73 (2013). Moreover, immuno-editing has been proposed as an escape
mechanism whereby neoplastic cells are selectively eliminated by the immune
system
until they evolve to express molecular oncodrivers, such as HER3, that evade
immune
recognition. Dunn, G.P., et al., .Nature immunology 3(11):991-8 (2008). Thus,
HER3
expression may be possible due to lack of immunosurveillance, which then
enhances
risk of recurrence. Interestingly, recent evidence suggests recurrent tumors
may not
= be pathologically identical to primary tumors. Discordant rates between
primary and
secondary tumors are particularly high for progesterone receptor and
discordance of
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
any type points to worse prognosis of recurrent tumors. klinsinghe, P.K.A., et
al.,
Am. ,I. Pathol. 133:416-29 (2010); Broom, R.I, et al., Anticancer
Research
29: 557-62 (2009); and Liedtke, C.. et al., Annals of Oncology 20:1953-58
(2009). It
is believed no studies to date have compared HER3 expression between primary
and
secondary tumors; it is unknown whether HER3 expression is discordant between
primary and recurrent tumors and whether HER3 expression may represent an
escape
mechanism for recurrence to occur. If so, targeting patients with lov,r anti-
HER3 CD4
T cell responses may boost immunosurveillance and help prevent long-term
recurrence.
Also implicating the immune system's role in prognosis, patients with pCR to
neoadjuvant chemotherapy had significantly higher anti-HER3 CD4 T cell
responses
than patients with <pCR. HER3 signalling has been shown to mediate acquired
resistance to targeted therapies. Serg,ina, N.V., et al.. Nature 445:437-41
(2007); and
frogne, T., et al., Breast Cancer Res. 1}cat. 114:263-75 (2009). Here, it is
implicated
not in acquired resistance but in initial resistance, making the anti-HER3
immune
response a potential prognostic marker of patients that would most benefit
from neo-
adjuvant treatment. Further studies should elucidate whether the anti-HER3
immune
response is not only prognostic but can also be intervened upon to boost
response to
treatment.
In the present study a subset of }Os, specifically post-menopausal women,
demonstrated higher anti-HER3 responses. Unlike prior findings with anti-HER2
responses, however, there was no difference based on pregnancy history. While
biologically this higher anti-HER2 response could be attributed to breast
involution
and the subsequent exposure of cellular proteins to immune surveillance with
pregnancy, such changes in the breast parenchyma are less likely in menopause.
Press. M.F.; et al., Oncogene 5:953-62(1990). Changes in breast density with
hormonal changes (as in menopause) have been observed on imaging, which may
mimic breast involution in pregnancy and likewise expose cellular proteins
that are
normally expressed in breast tissue to the immune system. Clendenen. TV., et
al.,
Magnetic Resonance Imaging 31:1-9 (2013). An alternate explanation may
attribute
the higher anti-HER3 immune response in post-menopausal women to a difference
in
risk. Expression of various breast cancer oncodrivers is clearly age
dependent:
HER2P"s IBC becomes less likely with age while ERP's IBC becomes more likely.
Clark, G.A4., et al., J. Clin. Oncol. 2:1102-09 (1984); Eppenberger-Castori,
S., et al.,
71
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
Int. J. Biochem. and Celt Riot. 34:1318-30 (2002). Further, these two
receptors are
not independent of each other as ER/PR expression with age is 1IER2 dependent
and
vice versa, Neyen, P., et al., Breast Cancer Res. Treat. 110:153-59 (2008). TN
IBC
patients, a group with the lowest anti-HER3 immune response and most sensitive
prognostically to HER3 overexpression, occurs more frequently in pre-
menopausal
women. Bae, et al. and Howlander. N., et al., JNX1. 106(5):1-8(2014). Thus,
the
= subset of pre-menopausal HDs represent a group at higher risk of
developing TN IBC
while the post-menopausal FIDs represent a group that has already surpassed
this
higher risk period and actually represent a group at lower risk of haying both
HER2
and HER3 overexpressing breast cancer. It is also notable that while TN IBC is
more
common in younger women, its occurrence in an older population portends a
better
prognosis for unknown reasons. Aapro, M., et al., annals of Oncology
23(6):vi52-55
(2012).1f the higher anti-HER3 immune response is indeed due to a biological
mechanism that occurs with menopause rather than a risk averse group, this may
partially explain the better prognosis of TN IBC in post-menopausal women.
CONCLUSION
This example demonstrated a decline in the anti-HER3 CD4 'fedi
response going from HDs, BDs and DCIS to ERI's and TN IBC. Furthermore, lower
anti-1-IER3 responses correlated with recurrence and <pCR to neoadjuyant
treatment,
indicating this immune response may also play a prognostic role in invasive
breast
cancer. Most importantly, these results min-or those of the prior study
showing a
decline in the native anti-HER2 immune response going from HDs to HER2P's DCIS
to HER2P's IBC. Such similar results are promising in not only confirming
prior
findings but also pointing to a larger role of the immune system in patrolling
molecular oncodrivers. Interestingly, post-menopausal HDs had significantly
higher
immune responses than pre-menopausal 1-IDs, a group that is generally at
higher risk
of developing HER.3 overexpressing breast cancer and potentially pointing to
mechanism that mediates this risk. It will be important to determine whether
HER3
pulsed DCI vaccination can have a therapeutic and/or risk-modifying effect on
the
development or FIER3 overexpressing breast cancer. It will likewise be
important to
continue to examine the immune system's role in other oncodriver-specific
cancers.
72
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
Front of paper Conclusions: CD4 Thl cell anti-HER-3 immune responses are
lost from healthy donors to invasive breast cancer, most notably in TN IBC, a
group
with limited treatment options and markedly worse prognosis with HER-3
overexpression. Anti-HER3 immune responses also mitigate response to treatment
and prognosis, pointing to a potential immunotherapy target. Addition of HER3
immunogenic peptides to DC1 vaccine may increase the population of IBC
patients
that could benefit from vaccination. Most importantly, these results mirror
prior
findings and point to a larger role of the immune system in patrolling
molecular
oncodrivers.
The findings in this Example, namely, that there is a significant loss of
anti-HER3 CD4+ Thl in breast tutnorigenesis going from HDs to IBC can be
appreciated by those of ordinary skill in the art to be useful in the
diagnosis and
treatment of ITER3-expressing cancers, in particular breast cancers and in
particular
triple negative IBC. It is contemplated that blood tests can be developed to
detect the
circulating anti-cancer CD4+ Thl response in subjects to take advantage of
these
findings. Preferably such blood tests will employ HER3 immunogenic peptides
such
as the 4 enumerated HER3 immunogenic peptides employed herein or any other
MFIC
class II immunogenic peptides based on the type of cancer the patient is
afflicted with
and which are capable of inducing an immune response in the patient. As a non-
limiting example, patients with recurrent breast cancer and lack of pCR to
neoadjuvant therapy can be monitored with such blood tests to determine their
anti-
HER3 CD4 Thl response and treated accordingly.
Low anti-HER3 response detected by a patient blood test or other
means can be countered by restoration methods such as, for example, vaccines,
and
preferably vaccines based on a patient's monocyte-derived dendritic cells that
are
pulsed/incubated with HER3 immunogenic peptides, such as, for example, the 4
HER3 immunogenic peptides used in the herein Example. Those of ordinary skill
in
the art will readily appreciate there are other ways to restore patient immune
response.
In particular, for TN IBC patients, a group that inherently has limited
treatment
options, methods of measuring HER3 response, and if needed, methods to restore
such response via a DC1 vaccine, may prove invaluable. Anti-HER3 immune
response can also be used as a potential prognostic biomarker of patients
needing
neoadjuvant treatment. A HER3-pulsed DCI vaccine or other suitable vaccine
might
have a. therapeutic and/or risk-modifying effect on the development of HER3-
73
SUBSTITUTE SHEET (RULE 26)
CA 02992937 2018-01-17
WO 2017/014816
PCT/US2016/026542
overexpressing breast cancers as well as other HER3-expressing cancers. The
finding
herein can be appreciated to be useful for the development of an array blood
tests and
assays as contemplated herein for diagnosis and/or therapy.
The disclosures of each and every patent, patent application, and
publication cited herein are hereby incorporated herein by reference in their
entirety.
While this invention has been disclosed with reference to specific
embodiments, it is
apparent that other embodiments and variations of this invention may be
devised by
others skilled in the art without departing from the true spirit and scope of
the
invention. The appended claims are intended to be construed to include all
such
embodiments and equivalent variations.
74
SUBSTITUTE SHEET (RULE 26)