Note: Descriptions are shown in the official language in which they were submitted.
= CA 02993215 2018-01-18
THERAPEUTIC AGENT PREPARATIONS FOR DELIVERY INTO A LUMEN OF THE
INTESTINAL TRACT USING A SWALLOWABLE DRUG DELIVERY DEVICE
CROSS-REFERENCES TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Patent Application No.
62/188,408, entitled
"Therapeutic Agent Preparations For Delivery Into A Lumen Of The Intestinal
Tract Using A
Swallowable Drug Delivery", filed July 2, 2015. This application is also
related to U.S. Patent
No. 8,809,269 and U.S. Patent No. 9,149,617.
BACKGROUND
[0002] Field: Various embodiments of this specification relate to swallowable
drug delivery
devices and more specifically, to swallowable drug delivery devices for
delivering drugs to the
small intestine.
[0003] While there has been an increasing development of new drugs in recent
years for the
treatment of a variety of diseases, many have limited application because they
cannot be given
orally. This is due to a number of reasons including: poor oral toleration
with complications
including gastric irritation and bleeding; breakdown/degradation of the drug
compounds in the
stomach; and poor, slow or erratic absorption of the drug. Conventional
alternative drug delivery
methods such as intravenous and intramuscular delivery have a number of
drawbacks including
pain and risk of infection from a needle stick, requirements for the use of
sterile technique and
the requirement and associated risks of maintaining an IV line in a patient
for an extended period
of time. While other drug delivery approaches have been employed such as
implantable drug
delivery pumps, these approaches require the semi-permanent implantation of a
device and can
still have many of the limitations of IV delivery. Thus, there is a need for
an improved method
for delivery of drugs and other therapeutic agents, including a need for
improved delivery of
insulin and other therapeutic agents for the treatment of diabetes and other
blood glucose
regulation disorders.
1
CA 02993215 2018-01-18
BRIEF SUMMARY
100041 This specification provide devices, systems, kits and methods for
delivering drugs and
other therapeutic agents to various locations in the body. Many embodiments
provide a
swallowable device for delivering drugs and other therapeutic agents within
the Gastrointestinal
(GI) tract. Particular embodiments provide a swallowable device such as a
capsule for delivering
drugs and other therapeutic agents into the wall of the small intestine or
other GI organ wall.
Various embodiments are particularly useful for the delivery of drugs and
other therapeutic
agents which are poorly absorbed, poorly tolerated and/or degraded within the
GI tract. Further,
various embodiments can be used to deliver drugs which were previously only
capable of or
preferably delivered by intravenous or other form of parenteral administration
including various
non-vascular injected forms of administration such as intramuscular or
subcutaneous injection.
100051 In one aspect, this specification provides a therapeutic agent
preparation for delivery
into a wall of the intestinal tract, where the preparation comprises a
therapeutically effective dose
of at least one therapeutic agent. The preparation has a shape and material
consistency to be
contained in a swallowable capsule or other swallowable device and delivered
from the capsule
into the intestinal wall to release the dose of therapeutic agent from within
the intestinal wall.
100061 In another embodiment, this specification provides a therapeutic agent
preparation for
delivery into a wall of the intestinal tract such as the wall of the small
intestine, where the
preparation comprises a therapeutically effective dose of at least one
therapeutic agent. The
preparation is configured to be contained in a swallowable capsule and
operably coupled to an
actuator, expandable balloon or other device having a first configuration and
a second
configuration. The preparation is contained within the capsule in the first
configuration and
advanced out of the capsule and into the intestinal wall in the second
configuration to deliver the
therapeutic agent into the intestinal wall.
100071 In other embodiments, this specification provides a method for
delivering a therapeutic
agent into the wall of the small intestine comprising swallowing a drug
delivery device
comprising a capsule, an actuator and an embodiment of the therapeutic agent
preparation. The
actuator is responsive to a condition in the small intestine such as pH so as
to actuate delivery of
the therapeutic agent preparation into the wall of the small intestine. In
specific embodiments,
2
CA 02993215 2018-01-18
the actuator can comprise a release element or coating on the capsule which is
degraded by a
selected pH in the small intestine. Once degraded, the element or coating
initiates delivery of the
therapeutic agent preparation by one or more delivery means such as the by
expansion of one or
more balloons that are operably coupled to tissue penetrating members that
contain the
therapeutic agent preparation and are configured to penetrate and be advanced
into the intestinal
wall upon expansion of the balloon. Once the tissue penetrating members are in
the intestinal
wall, they degrade to release the therapeutic agent into the bloodstream.
Because the therapeutic
agent preparation is delivered directly into the wall of the small intestine,
the time period
(described herein as Cm) for achieving the maximum concentration of the
therapeutic agent in
the bloodstream or other location in the body is shorter than a corresponding
time period for
achieving such a maximum concentration when the therapeutic agent is non-
vascularly injected
into the body such as by intramuscular or subcutaneous injection. In various
embodiments, the
time period for achieving Cm ax by insertion of the therapeutic preparation
into the intestinal wall
using one or more embodiments of the invention (such as an embodiment of the
swallowable
device) can be 80%, 50%, 30%, 20 or even 10% of the time period for achieving
a Cm ax through
the use of a non-vascular injection of the therapeutic agent. In other
embodiments, the Cmax
achieved by insertion of the therapeutic preparation into the intestinal wall
using one or more
embodiments of the invention, such as an embodiment of the swallowable device,
can be greater
than a Cm ax achieved by taking a convention oral form of the therapeutic
agent (e.g., a pill) where
the therapeutic agent is not inserted into the intestinal wall. In various
embodiments, the Cmax
achieved by insertion of the therapeutic preparation into the intestinal wall
using one or more
embodiments of the invention (such as an embodiment of the swallowable device)
can be 5, 10,
20, 30, 40, 50, 60, 70, 80 or even a 100 times greater than when the
therapeutic agent is delivered
in a pill or other oral form. In other related embodiments, the composition
can be configured to
produce a long-term release of therapeutic agent with a selectable VA, that is
the time period
required for the concentration of the therapeutic agent in the bloodstream or
other location in the
body to reach half its original Cmax value after having reached Cm. For
example, the
selectable VA may be 6, or 9, or 12, or 15 or 18, or 24 hours.
[0008] In another aspect, this specification provides a swallowable device for
delivering a drug
or other therapeutic agent preparation into the wall of the small or large
intestine or other organ
3
CA 02993215 2018-01-18
of the gastro-intestinal tract organ. The devise comprises a capsule sized to
be swallowed and
pass through the gastro-intestinal tract, a deployable aligner positioned
within the capsule for
aligning a longitudinal axis of the capsule with the a longitudinal axis of
the small intestine, a
delivery mechanism for delivering the therapeutic agent into the intestinal
wall and a deployment
member for deploying at least one of the aligner or the delivery mechanism.
The capsule wall is
degradable by contact with liquids in the GI tract but also may include an
outer coating or layer
which only degrades in the higher pH's found in the small intestine, and
serves to protect the
underlying capsule wall from degradation within the stomach before the capsule
reaches the
small intestine at which point the drug delivery is initiated by degradation
of the coating. In use,
such materials allow for the targeted delivery of a therapeutic agent in a
selected portion of the
intestinal tract such as the small intestine. Suitable outer coatings can
include various enteric
coatings such as various co-polymers of Methacrylic Acid and Ethyl Acrylate.
100091 Another embodiment of the capsule includes at least one guide tube, one
or more tissue
penetrating members positioned in the at least one guide tube, a delivery
member and an
actuating mechanism. The tissue penetrating member will typically comprise a
hollow needle or
other like structure and will have a lumen and a tissue penetrating end for
penetrating a
selectable depth into the intestinal wall. In various embodiments, the device
can include a
second and a third tissue penetrating member with additional numbers
contemplated. Each tissue
penetrating member can include the same or a different drug. In preferred
embodiments having
multiple tissue penetrating members, the tissue penetrating members can be
symmetrically
distributed around the perimeter of the capsule so as to anchor the capsule
onto the intestinal wall
during delivery of drug. In some embodiments, all or a portion of the tissue
penetrating member
(e.g., the tissue penetrating end) can be fabricated from the drug preparation
itself. In these and
related embodiments, the drug preparation can have a needle or dart-like
structure (with or
without barbs) configured to penetrate and be retained in the intestinal wall.
[0010] The tissue penetrating member can be fabricated from various
biodegradable materials
(e.g., PGLA, Polyethylene, maltose or other sugar) so as to degrade within the
small intestine
and thus provide a fail-safe mechanism for detaching the tissue penetrating
member from the
intestinal wall should this component become retained in the intestinal wall.
Additionally, in
theses and related embodiments, selectable portions of the capsule can be
fabricated from such
4
CA 02993215 2018-01-18
biodegradable materials so as to allow the entire device to controllably
degrade into smaller
pieces. Such embodiments facilitate passage and excretion of the devices
through GI tract. In
particular embodiments, the capsule can include seams of biodegradable
material which
controllably degrade to break the capsule into pieces of a selectable size and
shape to facilitate
passage through the GI tract. The seams can be pre-stressed, perforated or
otherwise treated to
accelerate degradation. The concept of using biodegradable seams to produce
controlled
degradation of a swallowable device in the GI tract can also be applied to
other swallowable
devices such as swallowable cameras to facilitate passage through the GI tract
and reduce the
likelihood of a device becoming stuck in the GI tract.
100111 The delivery member is configured to advance the drug from the capsule
through the
tissue penetrating member lumen and into the intestinal wall. Typically, at
least a portion of the
delivery member is advanceable within the tissue penetrating member lumen. The
delivery
member can have a piston or like structure sized to fit within the delivery
member lumen. The
distal end of the delivery member (the end which is advanced into tissue) can
have a plunger
element which advances the drug within tissue penetrating member lumen and
also forms a seal
with the lumen. The plunger element can be integral or attached to the
delivery member.
Preferably, the delivery member is configured to travel a fixed distance
within the needle lumen
so as to deliver a fixed or metered dose of drug into the intestinal wall.
This can be achieved by
one or more of the selection of the diameter of the delivery member (e.g., the
diameter can be
distally tapered), the diameter of the tissue penetrating member (which can be
narrowed at its
distal end), use of a stop, and/or the actuating mechanism. For embodiments of
the device
having a tissue penetrating member fabricated from drug (e.g., a drug dart),
the delivery member
is adapted to advance the dart out of the capsule and into tissue.
100121 The delivery member and tissue penetrating member can be configured for
the delivery
of liquid, semi-liquid or solid forms of drug or all three. Solid forms of
drug can include both
powder or pellet. Semi liquid can include a slurry or paste. The drug can be
contained within a
cavity of the capsule, or in the case of the liquid or semi-liquid, within an
enclosed reservoir. In
some embodiments, the capsule can include a first second, or a third drug (or
more). Such drugs
can be contained within the tissue penetrating member lumen (in the case of
solids or powder) or
in separate reservoirs within the capsule body.
CA 02993215 2018-01-18
[0013] The actuating mechanism can be coupled to at least one of the tissue
penetrating
member or the delivery member. The actuating mechanism is configured to
advance the tissue
penetrating member a selectable distance into the intestinal wall as well as
advance the delivery
member to deliver the drug and then withdraw the tissue penetrating member
from the intestinal
wall. In various embodiments, the actuating mechanism can comprise a preloaded
spring
mechanism which is configured to be released by the release element. Suitable
springs can
include both coil (including conical shaped springs) and leaf springs with
other spring structures
also contemplated. In particular embodiments, the spring can be cone shaped to
reduce the
length of the spring in the compressed state even to the point where the
compressed length of the
spring is about the thickness of several coils (e.g., two or three) or only
one coil.
100141 In particular embodiments the actuating mechanism comprises a spring, a
first motion
converter, and a second motion converter and a track member. The release
element is coupled to
the spring to retain the spring in a compressed state such that degradation of
the release element
releases the spring. The first motion converter is configured to convert
motion of the spring to
advance and withdraw the tissue penetrating element in and out of tissue. The
second motion
converter is configured to convert motion of the spring to advance the
delivery member into the
tissue penetrating member lumen. The motion converters are pushed by the
spring and ride
along a rod or other track member which serves to guide the path of the
converters. They engage
the tissue penetrating member and/or delivery member (directly or indirectly)
to produce the
desired motion. They are desirably configured to convert motion of the spring
along its
longitudinal axis into orthogonal motion of the tissue penetrating member
and/or delivery
member though conversion in other directions is also contemplated. The motion
converters can
have a wedge, trapezoidal or curved shape with other shapes also contemplated.
In particular
embodiments, the first motion converter can have a trapezoidal shape and
include a slot which
engages a pin on the tissue penetrating member that rides in the slot. The
slot can have a
trapezoidal shape that mirrors or otherwise corresponds to the overall shape
of the converter and
serves to push the tissue penetrating member during the upslope portion of the
trapezoid and then
pull it back during the down slope portion. In one variation, one or both of
the motion converters
can comprise a cam or cam like device which is turned by the spring and
engages the tissue
penetrating and/or delivery member.
6
CA 02993215 2018-01-18
[0015] In other variations, the actuating mechanism can also comprise an
electro-mechanical
device/mechanism such as a solenoid or a piezoelectric device. In one
embodiment, the
piezoelectric device can comprise a shaped piezoelectric element which has a
non-deployed and
deployed state. This element can be configured to go into the deployed state
upon the
application of a voltage and then return to the non-deployed state upon the
removal of the
voltage. This and related embodiments allow for a reciprocating motion of the
actuating
mechanism so as to both advance the tissue penetrating member and then
withdraw it.
[0016] The release element is coupled to at least one of the actuating
mechanism or a spring
coupled to the actuating mechanism. In particular embodiments, the release
element is coupled
to a spring positioned within the capsule so as to retain the spring in a
compressed state.
Degradation of the release element releases the spring to actuate the
actuation mechanism. In
many embodiments, the release element comprises a material configured to
degrade upon
exposure to chemical conditions in the small or large intestine such as pH.
Typically, the release
element is configured to degrade upon exposure to a selected pH in the small
intestine, e.g., 7.0,
7.1, 7.2, 7.3, 7.4, 8.0 or greater. However, it can also be configured to
degrade in response to
other conditions in the small intestine. In particular embodiments, the
release element can be
configured to degrade in response to particular chemical conditions in the
fluids in the small
intestine such as those which occur after ingestion of a meal (e.g., a meal
high in fats or
proteins).
[0017] Biodegradation of the release element from one or more conditions in
the small
intestine (or other location in the GI tract) can be achieved by selection of
the materials for the
release element, the amount of cross linking of those materials as well as the
thickness and other
dimensions of the release elements. Lesser amounts of cross linking and or
thinner dimensions
can increase the rate of degradation and vice versa. Suitable materials for
the release element
can comprise biodegradable materials such as various enteric materials which
are configured to
degrade upon exposure to the higher pH or other condition in the small
intestine. The enteric
materials can be copolymerized or otherwise mixed with one or more polymers to
obtain a
number of particular material properties in addition to biodegradation. Such
properties can
include without limitation stiffness, strength, flexibility and hardness.
7
CA 02993215 2018-01-18
=
[0018] In particular embodiments, the release element can comprise a film or
plug that fits
over or otherwise blocks the guide tube and retains the tissue penetrating
member inside the
guide tube. In these and related embodiments, the tissue penetrating member is
coupled to a
spring loaded actuating mechanism such that when the release element is
degraded sufficiently, it
releases the tissue penetrating member which then springs out of the guide
tube to penetrate into
the intestinal wall. In other embodiments, the release element can be shaped
to function as a
latch which holds the tissue penetrating element in place. In these and
related embodiments, the
release element can be located on the exterior or the interior of the capsule.
In the interior
embodiments, the capsule and guide tubes are configured to allow for the
ingress of intestinal
fluids into the capsule interior to allow for the degradation of the release
element.
[0019] In some embodiments, the actuating mechanism can be actuated by means
of a sensor,
such as a pH or other chemical sensor which detects the presence of the
capsule in the small
intestine and sends a signal to the actuating mechanism (or to an electronic
controller coupled to
the actuating mechanism to actuate the mechanism). Embodiments of a pH sensor
can comprise
an electrode-based sensor or it can be a mechanically-based sensor such as a
polymer which
shrinks or expands upon exposure to the pH or other chemical conditions in the
small intestine.
In related embodiments, an expandable/contractable sensor can also comprise
the actuating
mechanism itself by using the mechanical motion from the expansion or
contraction of the
sensor.
[0020] According to another embodiment for detecting that the device is in the
small intestine
(or other location in the GI tract), the sensor can comprise a strain gauge or
other pressure/force
sensor for detecting the number of peristaltic contractions that the capsule
is being subject to
within a particular location in the intestinal tract. In these embodiments,
the capsule is desirably
sized to be gripped by the small intestine during a peristaltic contraction.
Different locations
within the GI tract have different number of peristaltic contractions. The
small intestine has
between 12 to 9 contractions per minute with the frequency decreasing down the
length of the
intestine. Thus, according to one or more embodiments detection of the number
of peristaltic
contractions can be used to not only determine if the capsule is in the small
intestine but the
relative location within the intestine as well.
8
CA 02993215 2018-01-18
[0021] As an alternative or supplement to internally activated drug delivery,
in some
embodiments, the user may externally activate the actuating mechanism to
deliver drug by means
of RF, magnetic or other wireless signaling means known in the art. In these
and related
embodiments, the user can use a handheld device (e.g., a hand held RF device)
which not only
includes signaling means, but also means for informing the user when the
device is in the small
intestine or other location in the GI tract. The later embodiment can be
implemented by
including an RF transmitter on the swallowable device to signal to the user
when the device is in
the small intestine or other location (e.g., by signaling an input from the
sensor). The same
handheld device can also be configured to alter the user when the actuating
mechanism has been
activated and the selected drug(s) delivered. In this way, the user is
provided confirmation that
the drug has been delivered. This allows the user to take other appropriate
drugs/therapeutic
agents as well as make other related decisions (e.g., for diabetics to eat a
meal or not and what
foods should be eaten). The handheld device can also be configured to send a
signal to the
swallowable device to over-ride the actuating mechanism and so prevent, delay
or accelerate the
delivery of drug. In use, such embodiments allow the user to intervene to
prevent, delay or
accelerate the delivery of drug based upon other symptoms and/or patient
actions (e.g., eating a
meal, deciding to go to sleep, exercise etc.).
[0022] The user may also externally activate the actuating mechanism at a
selected time period
after swallowing the capsule. The time period can be correlated to a typical
transit time or range
of transit times for food moving through the user's GI tract to a particular
location in the tract
such as the small intestine.
[0023] Another aspect of this specification provides therapeutic agent
preparations for delivery
into the wall of the small intestine (or other wall in the intestinal tract)
using embodiments of the
swallowable device described herein. The preparation comprises a
therapeutically effective dose
of at least one therapeutic agent. It may comprise a solid, liquid or
combination of both and can
include one or more pharmaceutical excipients. The preparation has a shape and
material
consistency to be contained in embodiments of the swallowable capsule,
delivered from the
capsule into the intestinal wall and degrade within the wall to release the
dose of therapeutic
agent. The preparation may also have a selectable surface area to volume ratio
so as enhance or
otherwise control the rate of degradation of the preparation in the wall of
the small intestine or
9
= CA 02993215 2018-01-18
=
other body lumen. In various embodiments, the preparation can be configured to
be coupled to
an actuator such as a release element or actuation mechanism which has a first
configuration in
which the preparation is contained in the capsule and a second configuration
in which the
preparation is advanced out of the capsule and into the wall of the small
intestine. The dose of
the drug or other therapeutic agent in the preparation can be titrated
downward from that which
would be required for conventional oral delivery methods so that potential
side effects from the
drug can be reduced.
[0024] Typically, though not necessarily, the preparation will be shaped and
otherwise
configured to be contained in the lumen of a tissue penetrating member, such
as a hollow needle
which is configured to be advanced out of the capsule and into the wall of the
small intestine.
The preparation itself may comprise a tissue penetrating member configured to
be advanced into
the wall of the small intestine or other lumen in the intestinal tract.
[0025] Another aspect of this specification provides methods for the delivery
of drugs and the
therapeutic agents into the walls of the GI tract using embodiments of the
swallowable drug
delivery devices. Such methods can be used for the delivery of therapeutically
effective amounts
of a variety of drugs and other therapeutic agents. These include a number of
large molecule
peptides and proteins which would otherwise require injection due to chemical
breakdown in the
stomach e.g., growth hormone, parathyroid hormone, insulin, interferons and
other like
compounds. Suitable drugs and other therapeutic agents which can be delivered
by embodiments
of invention include various chemotherapeutic agents (e.g., interferon),
antibiotics, antivirals,
insulin and related compounds, glucagon like peptides (e.g., GLP-1,
exenatide), parathyroid
hormones, growth hormones (e.g., IFG and other growth factors), anti-seizure
agents, immune
suppression agents and anti-parasitic agents such as various anti-malarial
agents. The dosage of
the particular drug can be titrated for the patient's weight, age, condition
or other parameter.
[0026] In various method embodiments, embodiments of the drug swallowable drug
delivery
device can be used to deliver a plurality of drugs for the treatment of
multiple conditions or for
the treatment of a particular condition (e.g., a mixture of protease
inhibitors for treatment HIV
AIDS). In use, such embodiments allow a patient to forgo the necessity of
having to take
multiple medications for a particular condition or conditions. Also, they
provide a means for
facilitating that a regimen of two or more drugs is delivered and absorbed
into the small intestine
= CA 02993215 2018-01-18
and thus, the blood stream at about the same time. Due to differences in
chemical makeup,
molecular weight, etc., drugs can be absorbed through the intestinal wall at
different rates,
resulting in different pharmacokinetic distribution curves. Embodiments of the
invention address
this issue by injecting the desired drug mixtures at about the same time. This
in turn, improves
pharmacoldnetics and thus, the efficacy of the selected mixture of drugs.
[0027] In another aspect, this specification provides therapeutic preparations
comprising a
plurality of glucose regulating compounds, including for example, insulin, GLP-
1 and at least a
third compound. The third compound being selected to produce a synergistic
effect in one or
more of blood glucose reduction, blood glucose regulation, appetite
control/suppression or other
therapeutic effect of the insulin and GLP-1. Such synergistic effects in turn
provide treatment
for various conditions associated with diabetes (or other glucose regulating
disorder) such as
hyperglycemia, insulin resistance or hyperlipidemia. The glucose regulating
compounds may
include those which lower blood glucose, also known as hypoglycemic compounds,
and those
which raise blood glucose. They also include those which affect (e.g., reduce)
glucose directly,
and/or indirectly e.g., by causing the secretion of hormone which subsequently
lower glucose
levels, agonize or enhance the effect of glucose reducing hormones (as is the
case for DDP4
inhibitors with incretins), reduce appetite (as is the case with, peptide YY)
or all of the above.
According to some embodiments, the other glucose regulating compound can be
selected from
the group consisting of glucagon, peptide YY, GIP, metformin, peptide YY,
Dipeptidyl
peptidase-4 (DPP4), DPP4 inhibitors, sodium/glucose co-transporter 2 (SGLT2)
inhibitors along
with their analogues and derivatives. As described herein, the compounds can
be contained in
and/or formed as an embodiment of the tissue penetrating member that is
configured to be
advanced from the swallowable capsule configured to be inserted into the
intestinal wall as is
described herein. Once so inserted into the intestinal wall, the tissue
penetrating member
degrades to release the glucose regulating compounds into the blood stream as
described herein
for other therapeutic agents. In many embodiments, the tissue penetrating
member is configured
to concurrently release the plurality of glucose regulating compounds (e.g.,
by compounding
and/or mixing all of the compounds in the same material used to formulate the
tissue penetrating
member). The concurrent release of the compounds into the wall of the small
intestine and, in
11
CA 02993215 2018-01-18
turn the blood stream, serves to promote and enhance the synergistic effects
between two or
more of the compounds (e.g., increased blood glucose control, appetite
suppression, etc.).
[0028] One embodiment of a therapeutic preparation comprising a plurality of
glucose
regulating compounds includes at least insulin, GLP-1 and a third compound The
third
compound is configured to interact with the insulin or GLP-1 to enhance a
therapeutic effect in a
patient. The preparation is shaped as a solid tissue penetrating member shaped
and configured to
penetrate and be inserted into an intestinal wall after oral ingestion. Upon
insertion, the
preparation concurrently releases the plurality of glucose regulating
compounds into the blood
stream from the intestinal wall. The third compound may be selected from the
group consisting
of glucagon, GIP, Peptide YY, metformin, DPP4, DPP4 inhibitors and SGLT
inhibitors. Also, in
one more embodiments, the tissue penetrating member can be configured such
that upon
insertion the tissue penetrating member releases the glucose regulating
compounds into the blood
stream to achieve a C. in a shorter time period than a time period to achieve
a Cm ax for an
extravascularly injected dose of at least one of the glucose regulating
compounds with
improvements in one or more other pharmacokinetic parameters described herein.
100291 Another aspect of this specification provides a method for delivering a
plurality of
glucose regulating compounds to a patient in need thereof, comprising
providing a solid
therapeutic preparation comprising a plurality of glucose regulating compounds
including at least
insulin, GLP-1 and a third compound, the third compound configured to interact
with the insulin
or GLP-1 to enhance a therapeutic effect in the patient. The preparation is
shaped as a tissue
penetrating member, which is configured to be carried by a swallowable capsule
and penetrate
and be inserted into an intestinal wall, wherein upon ingestion the capsule
advances to the small
intestine of the patient. Then the solid therapeutic preparation is inserted
into the wall of the
small intestine by an application of mechanical force upon a surface of the
tissue penetrating
member from an expandable device operably coupled to the tissue penetrating
member wherein
upon insertion into the intestinal wall. The tissue penetrating member remains
to concurrently
release the plurality of glucose regulating compounds into the blood stream
from the intestinal
wall by degradation of the of the tissue penetrating member. The third
compound interacts with
the insulin or GLP-1 to produce an enhanced therapeutic effect for the patient
in one or more of
improved glucose control, reduction in blood glucose levels, reduction in
period or magnitude of
12
CA 02993215 2018-01-18
hyperglycemia or hypoglycemia, reduction in glycosylated hemoglobin levels,
reduction in
insulin resistance, alfa or beta cell preservation or reduction in
hyperlipidemia. The third
compound can be selected from the group consisting of glucagon, GIP, Peptide
YY, metformin,
DPP4, DPP4 inhibitors and SGLT inhibitors
100301 The invention that is disclosed and claimed herein pertains to a
medicinal preparation
comprising a plurality of glucose regulating compounds including at least
insulin, GLP-1 and a
third compound, the third compound configured to interact with one or both of
the insulin and
the GLP-1 to enhance effect of one or both of the insulin and the GLP-1 in a
patient, the
preparation shaped as a solid tissue penetrating member shaped and configured
for insertion into
an intestinal wall after oral ingestion, and wherein, the preparation is for
concurrent release of
the plurality of glucose regulating compounds into the blood stream from the
intestinal wall.
Also claimed is use of such a preparation for delivery of the glucose
regulating compounds to the
patient. Also claimed is use of such a preparation for treatment of diabetes.
[0031] Further details of these and other embodiments and aspects of the
invention are
described more fully below, with reference to the attached drawing figures.
12a
= CA 02993215 2018-01-18
BRIEF DESCRIPTION OF THE DRAWINGS
[0032] Fig. la is a lateral viewing showing an embodiment of a swallowable
drug delivery
device.
[0033] Fig. lb is a lateral viewing showing an embodiment of a system
including a
swallowable drug delivery device.
[0034] Fig. lc is a lateral viewing showing an embodiment of a kit including a
swallowable
drug delivery device and a set of instructions for use.
[0035] Fig. ld is a lateral viewing showing an embodiment of a swallowable
drug delivery
device including a drug reservoir.
[0036] Fig. 2 is a lateral view illustrating an embodiment of the swallowable
drug delivery
device having a spring loaded actuation mechanism for advancing tissue
penetrating members
into tissue.
[0037] Fig. 3 is a lateral view illustrating an embodiment of the swallowable
drug delivery
device having a spring loaded actuation mechanism having a first motion
converter.
1 2 b
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
[0038] Fig. 4 is a lateral view illustrating an embodiment of the swallowable
drug delivery
device having a spring loaded actuation mechanism having first and a second
motion
converter.
[0039] Fig. 5 is a perspective view illustrating engagement of the first and
second motion
converters with the tissue penetrating member and delivery members.
[0040] Fig. 6 is a cross sectional view illustrating an embodiment of the
swallowable drug
delivery device having a single tissue penetrating member and an actuating
mechanism for
advancing the tissue penetrating member.
[0041] Fig. 7a is a cross sectional view illustrating an embodiment of the
swallowable drug
delivery device having multiple tissue penetrating members and an actuating
mechanism for
advancing the tissue penetrating members.
[0042] Fig. 7b is a cross sectional view illustrating deployment of the tissue
penetrating
members of the embodiment of Fig. 7a to deliver medication to a delivery site
and anchor the
device in the intestinal wall during delivery.
[0043] Figs. 8a-8c are side views illustrating positioning of the drug
delivery device in the
small intestine and deployment of the tissue penetrating members to deliver
drug; Fig. 8a
shows the device in the small intestine prior to deployment of the tissue
penetrating members
with the release element in tact; Fig. 8b shows the device in the small
intestine with the
release element degraded and the tissue penetrating elements deployed; and
Fig. 8c shows the
device in the small intestine with the tissue penetrating elements retracted
and the drug
delivered.
[0044] Fig. 9a shows an embodiment of a swallowable drug delivery device
including a
capsule having bio-degradable seams positioned to produce controlled
degradation of the
capsule in the GI tract.
[0045] Fig. 9b shows the embodiment of Fig. 9a after having been degraded in
the GI tract
into smaller pieces.
[0046] Fig. 10 shows an embodiment of a capsule having biodegradable seams
including
pores and/or perforations to accelerate biodegradation of the capsule.
[0047] Fig. 11 is a lateral viewing illustrating use of an embodiment of a
swallowable drug
delivery device including transit of device in the GI tract and operation of
the device to
deliver drug.
[0048] Figs. 12a and 12b are lateral view illustrating an embodiment of a
capsule for the
swallowable drug delivery device including a cap and a body coated with pH
sensitive
-13-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
biodegradable coatings, Fig. 12a shows the capsule in an unassembled state and
Fig. 12b in
an assembled state
[0049] Figs. 13a and 13b illustrate embodiments of unfolded multi balloon
assemblies
containing a deployment balloon, an aligner balloon, a delivery balloon and
assorted
connecting tubes; Fig. 13a shows an embodiment of the assembly for a single
dome
configuration of the deployment balloon; and Fig. 13b shows an embodiment of
the assembly
for dual dome configuration of the deployment balloon; and.
[0050] Figs. 13c is a perspective views illustrating embodiments of a nested
balloon
configuration which can be used for one or more embodiments of the balloons
described
herein, including the aligner balloon.
[0051] Figs. 14a-14c are lateral views illustrating embodiments of a multi
compartment
deployment balloon; Fig. 14a shows the balloon in a non-inflated state with
the separation
valve closed; Fig. 14b shows the balloon with valve open and mixing of the
chemical
reactants; and Fig. 14c shows the balloon in an inflated state.
[0052] Figs. 15a-15g are lateral views illustrating a method for folding of
the multiple
balloon assembly, the folding configuration in each figure applies to both
single and dual
dome configurations of the deployment balloon, with the exception that Fig.
15c, pertains to a
folding step unique to dual dome configurations; and Fig. 15d, pertains to the
final folding
step unique to dual dome configurations; Fig. 15e, pertains to a folding step
unique to single
dome configurations; and Figs. 15f and 15g are orthogonal views pertaining to
the final
folding step unique to single dome configurations.
[0053] Figs. 16a and 16b are orthogonal views illustrating embodiments of the
final folded
multi balloon assembly with the attached delivery assembly.
[0054] Figs. 17a and 17b are orthogonal transparent views illustrating
embodiments of the
final folded multi balloon assembly inserted into the capsule.
[0055] Fig. 18a is a side view of an embodiment of the tissue penetrating
member.
[0056] Fig. 18b is a bottom view of an embodiment of the tissue penetrating
member
illustrating placement of the tissue retaining features.
[0057] Fig. 18c is a side view of an embodiment of the tissue penetrating
member having a
trocar tip and inverted tapered shaft.
[0058] Fig. 18d is a side view of an embodiment of the tissue penetrating
member having a
separate drug containing section.
-14-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
[0059] Figs. 18e and 8f are side views showing assembly of an embodiment of a
tissue
penetrating member having a shaped drug containing section. Fig. 18e shows the
tissue
penetrating member and shaped drug section prior to assembly; and Fig. 18f
after assembly.
[0060] Fig. 19 provides assorted views of the components and steps used to
assemble an
embodiment of the delivery assembly.
[0061] Figs. 20a-20i provides assorted views illustrating a method of
operation of
swallowabe device to deliver medication to the intestinal wall,
DETAILED DESCRIPTION OF THE INVENTION
[0062] Embodiments of the invention provide devices, systems and methods for
delivering
medications in to various locations in the body. As used herein, the term
"medication" refers
to a medicinal preparation in any form which can include drugs or other
therapeutic agents as
well as one or more pharmaceutical excipients. Many embodiments provide a
swallowable
device for delivering medication within the GI tract. Particular embodiments
provide a
swallowable device such as a capsule for delivering medications to the wall of
the small
intestine or other GI organ. As used herein, "GI tract" refers to the
esophagus, stomach,
small intestine, large intestine and anus, while "Intestinal tract" refers to
the small and large
intestine. Various embodiments of the invention can be configured and arranged
for delivery
of medication into the intestinal tract as well as the entire GI tract.
[0063] Referring now to Figs. 1-11, an embodiment of a device 10 for the
delivery of
medication 100 to a delivery site DS in the intestinal tract such as the wall
of the small
intestine, comprises a capsule 20 including at least one guide tube 30, one or
more tissue
penetrating members 40 positioned or otherwise advanceable in the at least one
guide tube, a
delivery member 50, an actuating mechanism 60 and release element 70.
Medication 100,
also described herein as preparation 100, typically comprises at least one
drug or other
therapeutic agent 101 and may include one or more pharmaceutical excipients
known in the
art. Collectively, one or more of delivery member 50 and mechanism 60 may
comprise a
means for delivery of medication 100 into a wall of the intestinal tract.
Other delivery means
contemplated herein include one or more expandable balloons (e.g., delivery
balloon 172) or
other expandable device/member described herein.
[0064] Device 10 can be configured for the delivery of liquid, semi-liquid or
solid forms of
medication 100 or all three. Solid forms of medication/preparation 100 can
include both
powder or a pellet form. Semi liquid forms can include a slurry or paste.
Whatever the form,
preparation 100 desirably has a shape and material consistency allowing the
medication to be
-15-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
advanced out of the device, into the intestinal wall (or other luminal wall in
the GI tract) and
then degrade in the intestinal wall to release the drug or other therapeutic
agent 101. The
material consistency can include one or more of the hardness, porosity and
solubility of the
preparation (in body fluids). The material consistency can be achieved by one
or more of the
following: i) the compaction force used to make the preparation; ii) the use
of one or more
pharmaceutical di sintegrants known in the art; iii) use of other
pharmaceutical excipients; iv)
the particle size and distribution of the preparation (e.g., micronized
particles); and v) use of
micronizing and other particle formation methods known in the art. Suitable
shapes for
preparation 100 can include cylindrical, cubical, rectangular, conical,
spherical,
hemispherical and combinations thereof. Also, the shape can be selected so as
to define a
particular surface area and volume of preparation 100 and thus, the ratio
between the two.
The ratio of surface area to volume can in turn, be used to achieve a selected
rate of
degradation within the intestinal or other lumen wall within the GI tract.
Larger ratios (e.g.,
larger amounts of surface area per unit volume) can be used to achieve faster
rates of
degradation and vice versa. In particular embodiments, the surface area to
volume ratio can
be in the range of about 1:1 to 100:1, with specific embodiments of 2:1, 5:1,
20:1, 25:1, 50:1
and 75:1. Preparation/medication 100 will typically be pre-packed within a
lumen 44 of
tissue penetrating members 40, but can also be contained at another location
within an
interior 24 of capsule 20, or in the case of a liquid or semi-liquid, within
an enclosed
reservoir 27. The medication can be pre-shaped to fit into the lumen or packed
for example,
in a powder form. Typically, the device 10 will be configured to deliver a
single drug 101 as
part of medication 100. However in some embodiments, the device 10 can be
configured for
delivery of multiple drugs 101 including a first second, or a third drug which
can be
compounded into a single or multiple medications 100. For embodiments having
multiple
medications/drugs, the medications can be contained in separate tissue
penetrating members
40 or within separate compartments or reservoirs 27 within capsule 20. In
another
embodiment, a first dose 102 of medication 100 containing a first drug 101 can
be packed
into the penetrating member(s) 40 and a second dose 103 of medication 100
(containing the
same or a different drug 101) can be coated onto the surface 25 of capsule as
is shown in the
embodiment of Fig. lb. The drugs 101 in the two doses of medication 102 and
103 can be
the same or different. In this way, a bimodal pharmacokinetic release of the
same or different
drugs can be achieved. The second dose 103 of medication 100 can have an
enteric coating
104 to ensure that it is released in the small intestine and achieve a time
release of the
-16-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
medication 100 as well. Enteric coating 104 can include one or more enteric
coatings
described herein or known in the art.
[0065] A system 11 for delivery of medication 100 into the wall of the small
intestine or
other location within the GI tract, may comprise device 10, containing one or
more
medications 100 for the treatment of a selected condition or conditions. In
some
embodiments, the system may include a hand held device 13, described herein
for
communicating with device 10 as is shown in the embodiment of Fig. lb. System
11 may
also be configured as a kit 14 including system 11 and a set of instructions
for use 15 which
are packaged in packaging 12 as is shown in the embodiment of Fig. lc. The
instructions can
indicate to the patient when to take the device 10 relative to one or more
events such as the
ingestion of a meal or a physiological measurement such as blood glucose,
cholesterol, etc.
In such embodiments, kit 14 can include multiple devices 10 containing a
regimen of
medications 100 for a selected period of administration, e.g., a day, week, or
multiple weeks
depending upon the condition to be treated.
[0066] Capsule 20 is sized to be swallowed and pass through the intestinal
tract. The size
can also be adjusted depending upon the amount of drug to be delivered as well
as the
patient's weight and adult vs. pediatric applications. Capsule 20 includes an
interior volume
24 and an outer surface 25 having one or more apertures 26 sized for guide
tubes 30. In
addition to the other components of device 10, (e.g., the actuation mechanism
etc.) the
interior volume can include one or more compartments or reservoirs 27. One or
more
portions of capsule 20 can be fabricated from various biocompatible polymers
known in the
art, including various biodegradable polymers which in a preferred embodiment
can comprise
PGLA (polylactic-co-glycolic acid). Other suitable biodegradable materials
include various
enteric materials described herein as well as lactide, glycolide, lactic acid,
glycolic acid, para-
dioxanone, caprolactone, trimethylene carbonate, caprolactone, blends and
copolymers
thereof. As is described in further detail herein, in various embodiments,
capsule 20 can
include seams 22 of bio-degradable material so as to controllably degrade into
smaller pieces
23 which are more easily passed through the intestinal tract. Additionally, in
various
embodiments, the capsule can include various radio-opaque or echogenic
materials for
location of the device using fluoroscopy, ultrasound or other medical imaging
modality. In
specific embodiments, all or a portion of the capsule can include radio-
opaque/echogenic
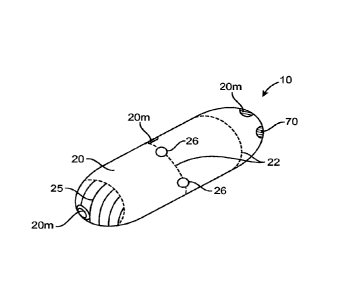
markers 20m as is shown in the embodiment of Figs. la and lb. In use, such
materials not
only allow for the location of device 10 in the GI tract, but also allow for
the determination of
transit times of the device through the GI tract.
-17-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
[0067] In preferred embodiments, tissue penetrating members 40 are positioned
within
guide tubes 30 which serve to guide and support the advancement of members 40
into tissue
such as the wall of the small intestine or other portion of the GI tract. The
tissue penetrating
members 40 will typically comprise a hollow needle or other like structure and
will have a
lumen 44 and a tissue penetrating end 45 for penetrating a selectable depth
into the intestinal
wall IW. Member 40 may also include a pin 41 for engagement with a motion
converter 90
described herein. The depth of penetration can be controlled by the length of
member 40, the
configuration of motion converter 90 described herein as well as the placement
of a stop or
flange 40s on member 40 which can, in an embodiment, correspond to pin 41
described
herein. Medication 100 will typically be delivered into tissue through lumen
44. In many
embodiments, lumen 44 is pre-packed with the desired medication 100 which is
advanced out
of the lumen using delivery member 50 or other advancement means (e.g. by
means of force
applied to a collapsible embodiment of member 40). As an alternative,
medication 100 can
be advanced into lumen 44 from another location/compartment in capsule 20. In
some
embodiments, all or a portion of the tissue penetrating member 40 can be
fabricated from
medication 100 itself. In these and related embodiments, the medication can
have a needle or
dart-like structure (with or without barbs) configured to penetrate and be
retained in the
intestinal wall, such as the wall of the small intestine. The dart can be
sized and shaped
depending upon the medication, dose and desired depth of penetration into the
intestinal wall.
Medication 100 can be formed into darts, pellets or other shapes using various
compression
molding methods known in the pharmaceutical arts.
[0068] In various embodiments, device 10 can include a second 42 and a third
43 tissue
penetrating member 40 as is shown in the embodiments of Figs. 7a and 7b., with
additional
numbers contemplated Each tissue penetrating member 40 can be used to deliver
the same
or a different medication 100. In preferred embodiments, the tissue
penetrating members 40
can be substantially symmetrically distributed around the perimeter 21 of
capsule 20 so as to
anchor the capsule onto the intestinal wall IW during delivery of medications
100.
Anchoring capsule 20 in such a way reduces the likelihood that the capsule
will be displaced
or moved by peristaltic contractions occurring during delivery of the
medication. In specific
embodiments, the amount of anchoring force can be adjusted to the typical
forces applied
during peristaltic contraction of the small intestine. Anchoring can be
further facilitated by
configured some or all of tissue penetrating members 40 to have a curved or
arcuate shape.
[0069] Delivery member 50 is configured to advance medication 100 through the
tissue
penetrating member lumen 44 and into the intestinal wall IW. Accordingly, at
least a portion
-18-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
of the delivery member 50 is advanceable within the tissue penetrating member
lumen 44 and
thus member 50 has a size and shape (e.g., a piston like shape) configured to
fit within the
delivery member lumen 44.
[0070] In some embodiments, the distal end 50d of the delivery member (the end
which is
advanced into tissue) can have a plunger element 51 which advances the
medication within
the tissue penetrating member lumen 44 and also forms a seal with the lumen.
Plunger
element 51 can be integral or attached to delivery member 50. Preferably,
delivery member
50 is configured to travel a fixed distance within the needle lumen 44 so as
to deliver a fixed
or metered dose of drug into the intestinal wall IW. This can be achieved by
one or more of
the selection of the diameter of the delivery member (e.g., the diameter can
be distally
tapered), the diameter of the tissue penetrating member (which can be narrowed
at its distal
end), use of a stop, and/or the actuating mechanism. However in some
embodiments, the
stroke or travel distance of member 50 can be adjusted in situ responsive to
various factors
such as one or more sensed conditions in the GI tract. In situ adjustment can
be achieved
through use of logic resource 29 (including controller 29c) coupled to an el
ectro-mechanical
embodiment of actuating mechanism 60. This allows for a variable dose of
medication
and/or variation of the distance the medication is injected into the
intestinal wall.
[0071] Actuating mechanism 60 can be coupled to at least one of the tissue
penetrating
member 40 or delivery member 50. The actuating mechanism is configured to
advance tissue
penetrating member 40 a selectable distance into the intestinal wall IW as
well as advance the
delivery member to deliver medication 100 and then withdraw the tissue
penetrating member
from the intestinal wall. In various embodiments, actuating mechanism 60 can
comprise a
spring loaded mechanism which is configured to be released by release element
70. Suitable
springs 80 can include both coil (including conical shaped springs) and leaf
springs with
other spring structures also contemplated. In particular embodiments, spring
80 can be
substantially cone-shaped to reduce the length of the spring in the compressed
state even to
the point where the compressed length of the spring is about the thickness of
several coils
(e.g., two or three) or only one coil.
[0072] In particular embodiments actuating mechanism 60 can comprise a spring
80, a first
motion converter 90, and a second motion converter 94 and a track member 98 as
is shown in
the embodiments of Figs. 2, 4 and 8a-8c. The release element 70 is coupled to
spring 80 to
retain the spring in a compressed state such that degradation of the release
element releases
the spring. Spring 80 may be coupled to release element 70 by a latch or other
connecting
element 81. First motion converter 90 is configured to convert motion of
spring 80 to
-19-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
advance and withdraw the tissue penetrating member 40 in and out of the
intestinal wall or
other tissue. The second motion converter 94 is configured to convert motion
of the spring
80 to advance the delivery member 50 into the tissue penetrating member lumen
44. Motion
converters 90 and 94 are pushed by the spring and ride along a rod or other
track member 98
which fits into a track member lumen 99 of converter 90. The track member 98
serves to
guide the path of the converters 90. Converters 90 and 94 engage the tissue
penetrating
member 40 and/or delivery member 50 (directly or indirectly) to produce the
desired motion.
They have a shape and other characteristics configured to convert motion of
the spring 80
along its longitudinal axis into orthogonal motion of the tissue penetrating
member 40 and/or
delivery member 50 though conversion in other directions is also contemplated.
The motion
converters can have a wedge, trapezoidal or curved shape with other shapes
also
contemplated. In particular embodiments, the first motion converter 90 can
have a
trapezoidal shape 90t and include a slot 93 which engages a pin 41 on the
tissue penetrating
member that rides in the slot as is shown in the embodiments of Figs. 2, 3 and
4. Slot 93 can
also have a trapezoidal shape 93t that mirrors or otherwise corresponds to the
overall shape of
converter 90. Slot 93 serves to push the tissue penetrating member 40 during
the upslope
portion 91 of the trapezoid and then pull it back during the down slope
portion 92. In one
variation, one or both of the motion converters 90 and 94 can comprise a cam
or cam like
device (not shown). The cam can be turned by spring 80 so as to engage the
tissue
penetrating and/or delivery members 40 and 50. One or more components of
mechanism 60
(as well as other components of device 10) including motion converters 90 and
94 can be
fabricated using various MEMS-based methods known in the art so as to allow
for selected
amounts of miniaturization to fit within capsule 10. Also as is described
herein, they can be
formed from various biodegradable materials known in the art.
100731 In other variations, the actuating mechanism 60 can also comprise an
electro-
mechanical device/mechanism such as a solenoid or a piezoelectric device. In
one
embodiment, a piezoelectric device used in mechanism 60 can comprise a shaped
piezoelectric element which has a non-deployed and deployed state. This
element can be
configured to go into the deployed state upon the application of a voltage and
then return to
the non-deployed state upon the removal of the voltage or other change in the
voltage. This
and related embodiments allow for a reciprocating motion of the actuating
mechanism 60 so
as to both advance the tissue penetrating member and then withdraw it. The
voltage for the
piezoelectric element can be obtained generated using a battery or a
piezoelectric based
energy converter which generates voltage by mechanical deformation such as
that which
-20-
CA 2993215
occurs from compression of the capsule 20 by a peristaltic contraction of the
small intestine around the
capsule. Further description of piezoelectric based energy converters is found
in U.S. Patent
Application Serial No. 12/556,524. In one embodiment, deployment of tissue
penetrating members 40
can in fact be triggered from a peristaltic contraction of the small intestine
which provides the
mechanical energy for generating voltage for the piezoelectric element.
100741 Release element 70 will typically be coupled to the actuating mechanism
60 and/or a spring
coupled to the actuating mechanism; however, other configurations are also
contemplated. In preferred
embodiments, release element 70 is coupled to a spring 80 positioned within
capsule 20 so as to retain
the spring in a compressed state 85 as shown in the embodiment of Fig. 2.
Degradation of the release
element 70 releases spring 80 to actuate actuation mechanism 60. Accordingly,
release element 70 can
thus function as an actuator 70a (actuator 70 may also include spring 80 and
other elements of
mechanism 60). As is explained further below, release element 70/actuator 70a
has a first configuration
where the therapeutic agent preparation 100 is contained within capsule 20 and
a second configuration
where the therapeutic agent preparation is advanced from the capsule into the
wall of the small intestine
or other luminal wall in the intestinal tract.
[0075] In many embodiments, release element 70 comprises a material configured
to degrade upon
exposure to chemical conditions in the small or large intestine such as pH.
Typically, release element
70 is configured to degrade upon exposure to a selected pH in the small
intestine, e.g., 7.0, 7.1, 7.2, 7.3,
7.4, 7.5, 7.6 8.0 or greater. The release element can also be configured to
degrade within a particular
range of pH such as, e.g., 7.0 to 7.5. In particular embodiments, the pH at
which release element 70
degrades (defined herein as the degradation pH) can be selected for the
particular drug to be delivered
so as to release the drug at a location in small intestine which corresponds
to the selected pH. Further,
for embodiments of device 10 having multiple medications 100, the device can
include a first release
element 70 (coupled to an actuating mechanism for delivering a first drug)
configured to degrade at first
pH and a second release element 70 (coupled to an actuating mechanism for
delivering a second drug)
configured to degrade at a second pH (with additional numbers of release
elements contemplated for
varying number of drugs).
[0076] Release element 70 can also be configured to degrade in response to
other conditions in the
small intestine (or other GI location). In particular embodiments, the release
element 70 can be
configured to degrade in response to particular chemical conditions in the
fluids in the small intestine
such as those which occur after ingestion of a meal (e.g., a meal
- 21 -
Date Regue/Date Received 2023-01-12
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
containing fats, starches or proteins). In this way, the release of medication
100 can be
substantially synchronized or otherwise timed with the digestion of a meal.
[0077] Various approaches are contemplated for biodegradation of release
element 70. In
particular embodiments, biodegradation of release element 70 from one or more
conditions in
the small intestine (or other location in the GI tract) can be achieved by one
or more of the
following approaches: i) selection of the materials for the release element,
ii) the amount of
cross linking of those materials; and iii) the thickness and other dimensions
of the release
element. Lesser amounts of cross linking and or thinner dimensions can
increase the rate of
degradation and vice versa. Suitable materials for the release element can
comprise
biodegradable materials such as various enteric materials which are configured
to degrade
upon exposure to the higher pH in the intestines. Suitable enteric materials
include, but are
not limited to, the following: cellulose acetate phthalate, cellulose acetate
trimellitate,
hydroxypropyl methylcellulose phthalate, polyvinyl acetate phthalate,
carboxymethylethylcellulose, co-polymerized methacrylic acid/methacrylic acid
methyl
esters as well as other enteric materials known in the art. The selected
enteric materials can
be copolymerized or otherwise combined with one or more other polymers to
obtain a
number of other particular material properties in addition to biodegradation.
Such properties
can include without limitation stiffness, strength, flexibility and hardness.
[0078] In alternative embodiments, the release element 70 can comprise a film
or plug 70p
that fits over or otherwise blocks guide tubes 30 and retains the tissue
penetrating member 40
inside the guide tube. In these and related embodiments, tissue penetrating
member 40 is
coupled to a spring loaded actuating mechanism such that when the release
element is
degraded sufficiently, it releases the tissue penetrating member which then
springs out of the
guide tube to penetrate into the intestinal wall. In still other embodiments,
release element 70
can be shaped to function as a latch which holds the tissue penetrating member
40 in place.
In these and related embodiments, the release element can be located on the
exterior or the
interior of capsule 20. In the latter case, capsule 20 and/or guide tubes 30
can be configured
to allow for the ingress of intestinal fluids into the capsule interior to
allow for the
degradation of the release element.
[0079] In some embodiments, actuating mechanism 60 can be actuated by means of
a
sensor 67, such as a pH sensor 68 or other chemical sensor which detects the
presence of the
capsule in the small intestine. Sensor 67 can then send a signal to actuating
mechanism 60 or
to an electronic controller 29c coupled to actuating mechanism 60 to actuate
the mechanism.
Embodiments of a pH sensor 68 can comprise an electrode-based sensor or it can
be a
-22-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
mechanically-based sensor such as a polymer which shrinks or expands upon
exposure to a
selected pH or other chemical conditions in the small intestine. In related
embodiments, an
expandable/contractible sensor 67 can also comprise the actuating mechanism 60
itself by
using the mechanical motion from the expansion or contraction of the sensor.
[0080] According to another embodiment for detecting that the device in the
small intestine
(or other location in the GI tract), sensor 67 can comprise pressure/force
sensor such as strain
gauge for detecting the number of peristaltic contractions that capsule 20 is
being subject to
within a particular location in the intestinal tract (in such embodiments
capsule 20 is
desirably sized to be gripped by the small intestine during a peristaltic
contraction). Different
locations within the GI tract have different number of pedstaltic
contractions. The small
intestine has between 12 to 9 contractions per minute with the frequency
decreasing down the
length of the intestine. Thus, according to one or more embodiments, detection
of the
number of peristaltic contractions can be used to not only determine if
capsule 20 is in the
small intestine, but the relative location within the intestine as well. In
use, these and related
embodiments allow for release of medication 100 at a particular location in
the small
intestine.
[0081] As an alternative or supplement to internally activated drug delivery
(e.g., using a
release element and/or sensor), in some embodiments, the user may externally
activate the
actuating mechanism 60 to deliver medication 100 by means of RF, magnetic or
other
wireless signaling means known in the art. In these and related embodiments,
the user can
use a handheld communication device 13 (e.g., a hand held RF device such as a
cell phone)
as is shown in the embodiment of Fig, lb, to send a receive signals 17 from
device 10. In
such embodiments, swallowable device may include a transmitter 28 such as an
RF
transceiver chip or other like communication device/circuitry. Handheld device
13 may not
only includes signaling means, but also means for informing the user when
device 10 is in the
small intestine or other location in the GI tract. The later embodiment can be
implemented
through the use of logic resources 29 (e.g., a processor 29) coupled to
transmitter 28 to signal
to detect and singe to the user when the device is in the small intestine or
other location (e.g.,
by signaling an input from the sensor). Logic resources 29 may include a
controller 29c
(either in hardware or software) to control one or more aspects of the
process. The same
handheld device can also be configured to alert the user when actuating
mechanism 60 has
been activated and the selected medication 100 delivered (e.g., using
processor 29 and
transmitter 28). In this way, the user is provided confirmation that
medication 100 has been
delivered. This allows the user to take other appropriate drugs/therapeutic
agents as well as
-23-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
make other related decisions (e.g., for diabetics to eat a meal or not and
what foods should be
eaten). The handheld device can also be configured to send a signal to
swallowable device
to over-ride actuating mechanism 60 and so prevent delay or accelerate the
delivery of
medication 100. In use, such embodiments allow the user to intervene to
prevent, delay or
accelerate the delivery of medication, based upon other symptoms and/or
patient actions (e.g.,
eating a meal, deciding to go to sleep, exercise etc.). The user may also
externally activate
actuating mechanism 60 at a selected time period after swallowing the capsule.
The time
period can be correlated to a typical transit time or range of transit times
for food moving
through the user's GI tract to a particular location in the tract such as the
small intestine.
100821 In particular embodiments, the capsule 20 can include seams 22 of
biodegradable
material which controllably degrade to break the capsule into capsule pieces
23 of a
selectable size and shape to facilitate passage through the GI tract as is
shown in the
embodiment of Figs. 10a and 10b. Seams 22 can also include pores or other
openings 22p for
ingress of fluids into the seam to accelerate biodegradation as is shown in
the embodiment of
Fig. 10. Other means for accelerating biodegradation of seams 22 can include
pre-stressing
the seam and/or including perforations 22f in the seam as is also shown in the
embodiment of
Fig. 10. In still other embodiments, seam 22 can be constructed of materials
and/or have a
structure which is readily degraded by absorption of ultrasound energy, e.g.
high frequency
ultrasound (1111FU), allowing the capsule to be degraded into smaller pieces
using externally
or endoscopically (or other minimally invasive method) administered
ultrasound.
100831 Suitable materials for seams 22 can include one or more biodegradable
materials
described herein such as PGLA, glycolic acid etc. Seams 22 can be attached to
capsule body
using various joining methods known in the polymer arts such as molding, hot
melt
junctions, etc. Additionally for embodiments of capsule 20 which are also
fabricated from
biodegradable materials, faster biodegradation of seam 22 can be achieved by
one or more of
the following: i) fabricating the seam from a faster biodegrading material,
ii) pre-stressing
the seam, or iii) perforating the seam. The concept of using biodegradable
seams 22 to
produce controlled degradation of a swallowable device in the GI tract can
also be applied to
other swallowable devices such as swallowable cameras (or other swallowable
imaging
device) to facilitate passage through the GI tract and reduce the likelihood
of such a device
becoming stuck in the GI tract. Accordingly, embodiments of biodegradable seam
22 can be
adapted for swallowable imaging and other swallowable devices.
100841 Another aspect of the invention provides methods for the delivery of
drugs and
other therapeutic agents (in the form of medication 100) into the walls of the
GI tract using
-24-
CA 2993215
one or more embodiments of swallowable drug delivery device 10. An exemplary
embodiment of such
a method will now be described. The described embodiment of drug delivery
occurs in the small
intestine SI. However, it should be appreciated that this is exemplary and
that embodiments of the
invention can be used for delivering drug in a number of locations in the GI
tract including the stomach
and the large intestine. For ease of discussion, the swallowable drug delivery
device 10 will sometimes
be referred to herein as a capsule. As described above, in various
embodiments, device 10 may be
packaged as a kit 14 within sealed packaging 12 that includes device 10 and a
set of instructions for use
15. If the patient is using a handheld device 13, the patient may instructed
to enter data into device 13
either manually or via a bar code 18 (or other identifying indicia 18) located
on the instructions 15 or
packaging 12. If a bar code is used, the patient would scan the bar code using
a bar code reader 19 on
device 13. After opening packaging 12, reading the instructions 15 and
entering any required data, the
patient swallows an embodiment of the swallowable drug delivery device 10.
Depending upon the drug,
the patient may take the device 10 in conjunction with a meal (before, during
or after) or a physiological
measurement. Capsule 20 is sized to pass through the GI tract and travels
through the patient's stomach
S and into the small intestine SI through peristaltic action as is shown in
the embodiment of Fig. 11.
Once in the small intestine, the release element 70 is degraded by the basic
pH in the small intestine (or
other chemical or physical condition unique to the small intestine) so as to
actuate the actuating
mechanism 60 and deliver medication 100 into the wall of the small intestine
SI according to one or
more embodiments of the invention. For embodiments including a hollow needle
or other hollow tissue
penetrating member 40, medication delivery is effectuated by using the
actuating mechanism 60 to
advance the needle 40 a selected distance into the mucosa of the intestinal
wall IS, and then the
medication is injected through the needle lumen 40 by advancement of the
delivery member 50. The
delivery member 50 is withdrawn and the needle 40 is then withdrawn back
within the body of the
capsule (e.g. by recoil of the spring) detaching from the intestinal wall. For
embodiments of device 10
having multiple needles, a second or third needle 42, 43 can also be used to
deliver additional doses of
the same drug or separate drugs 101. Needle advancement can be done
substantially simultaneously or
in sequence. In preferred embodiments that use multiple needles, needle
advancement can be done
substantially simultaneously so as to anchor device 10 in the small intestine
during drug delivery.
100851 After medication delivery, device 10 then passes through the
intestinal tract including the
large intestine LI and is ultimately excreted. For embodiments of the capsule
- 25 -
Date Regue/Date Received 2023-01-12
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
20 having biodegradable seams 22 or other biodegradable portions, the capsule
is degraded in
the intestinal tract into smaller pieces to facilitate passage through and
excretion from the
intestinal tract as is shown in the embodiments of Figs. 9a and 9b. In
particular embodiments
having biodegradable tissue penetrating needles/members 40, should the needle
get stuck in
the intestinal wall, the needle biodegrades releasing the capsule 20 from the
wall.
100861 For embodiments of device 10 including a sensor 67, actuation of
mechanism 60
can be effectuated by the senor sending a signal to actuating mechanism 60
and/or a
processor 29/controller 29c coupled to the actuating mechanism. For
embodiments of device
including external actuation capability, the user may externally activate
actuating
mechanism 60 at a selected time period after swallowing the capsule. The time
period can be
correlated to a typical transit time or range of transit times for food moving
through the user's
GI tract to a particular location in the tract such as the small intestine.
[0087] One or more embodiments of the above methods can be used for the
delivery of
preparations 100 containing therapeutically effective amounts of a variety of
drugs and other
therapeutic agents 101 to -treat a variety of diseases and conditions. These
include a number
of large molecule peptides and proteins which would otherwise require
injection due to
chemical breakdown in the stomach. The dosage of the particular drug can be
titrated for the
patient's weight, age or other parameter. Also the dose of drug 101 to achieve
a desired or
therapeutic effect (e.g., insulin for blood glucose regulation) when delivered
by one or more
embodiments of the invention can be less than the amount required should the
drug have been
delivered by conventional oral delivery (e.g., a swallowable pill that is
digested in the
stomach and absorbed through the wall of the small intestine). This is due to
the fact that
there is no degradation of the drug by acid and other digestive fluids in the
stomach and the
fact that all, as opposed to only a portion of the drug is delivered into the
wall of the small
intestine (or other lumen in the intestinal tract, e.g., large intestine,
stomach, etc.). Depending
upon the drug 101, the dose 102 delivered in preparation 100 can be in the
range from 100 to
5% of a dose delivered by conventional oral delivery (e.g., a pill) to achieve
a desired
therapeutic effect (e.g., blood glucose regulation, seizure regulation, etc.)
with even lower
amounts contemplated. The particular dose reduction can be titrated based upon
the
particular drug, the condition to be treated, and the patient's weight, age
and condition. For
some drugs (with known levels of degradation in the intestinal tract) a
standard dose
reduction can be employed (e.g., 10 to 20%). Larger amounts of dose reduction
can be used
for drugs which are more prone to degradation and poor absorption. In this
way, the potential
toxicity and other side effects (e.g., gastric cramping, irritable bowel,
hemorrhage, etc.) of a
-26-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
particular drug or drugs delivered by device 10 can be reduced because the
ingested dose is
lowered. This in turn, improves patient compliance because the patient has
reduction both in
the severity and incidence of side effects. Additional benefits of embodiments
employing
dose reduction of drug 101 include a reduced likelihood for the patient to
develop a tolerance
to the drug (requiring higher doses) and, in the case of antibiotics, for the
patient to develop
resistant strains of bacteria. Also, other levels of dose reduction can be
achieved for patients
undergoing gastric bypass operations and other procedures in which sections of
the small
intestine have been removed or its working (e.g., digestive) length
effectively shortened.
100881 In addition to delivery of a single drug, embodiments of swallowable
drug delivery
device 10 and methods of their use can be used to deliver a plurality of drugs
for the
treatment of multiple conditions or for the treatment of a particular
condition (e.g., protease
inhibitors for treatment HIV AIDS). In use, such embodiments allow a patient
to forgo the
necessity of having to take multiple medications for a particular condition or
conditions.
Also, they provide a means for facilitating that a regimen of two or more
drugs is delivered
and absorbed into the small intestine and thus, the blood stream, at about the
same time. Due
to difference in chemical makeup, molecular weight, etc., drugs can be
absorbed through the
intestinal wall at different rates, resulting in different pharmacokinetic
distribution curves.
Embodiments of the invention address this issue by injecting the desired drug
mixtures at
substantially the same time. This in turn, improves the pharmacokinetics and
thus the
efficacy of the selected mixture of drugs. Additionally, eliminating the need
to take multiple
drugs is particularly beneficial to patients who have one or more long term
chronic conditions
including those who have impaired cognitive or physical abilities.
100891 In various applications, embodiments of the above methods can be used
to deliver
preparations 100 including drugs and therapeutic agents 101 to provide
treatment for a
number of medical conditions and diseases. The medical conditions and diseases
which can
be treated with embodiments of the invention can include without limitation:
cancer,
hormonal conditions (e.g., hypo/hyper thyroid, growth hormone conditions),
osteoporosis,
high blood pressure, elevated cholesterol and triglyceride, diabetes and other
glucose
regulation disorders, infection (local or septicemia), epilepsy and other
seizure disorders,
osteoporosis, coronary arrhythmia's (both atrial and ventricular), coronary
ischemia anemia
or other like condition. Still other conditions and diseases are also
contemplated.
100901 In many embodiments, the treatment of the particular disease or
condition can be
performed without the need for injecting the drug or other therapeutic agent
(or other non-
oral form of delivery such as suppositories) but instead, relying solely on
the therapeutic
-27-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
agent(s) that is delivered into the wall of the small intestine or other
portion of the GI tract.
Similarly, the patient need not take conventional oral forms of a drug or
other therapeutic
agent, but again rely solely on delivery into the wall of the small intestine
using embodiments
of the swallowable capsule. In other embodiments, the therapeutic agent(s)
delivered into the
wall of the small intestine can be delivered in conjunction with an injected
dose of the
agent(s). For example, the patient may take a daily dose of therapeutic agent
using the
embodiments of the swallowable capsule, but only need take an injected dose
every several
days or when the patient's condition requires it (e.g., hyperglycemia). The
same is true for
therapeutic agents that are traditionally delivered in oral form (e.g., the
patient can take the
swallowable capsule and take the conventional oral form of the agent as
needed). The
dosages delivered in such embodiments (e.g., the swallowed and injected dose)
can be titrated
as needed (e.g., using standard dose response curve and other pharmacokinetic
methods can
be used to determine the appropriate dosages). Also, for embodiments using
therapeutic
agents that can be delivered by conventional oral means, the dose delivered
using
embodiments of the swallowable capsule can be titrated below the dosage
normally given for
oral delivery of the agent since there is little or no degradation of the
agent within the
stomach or other portion of the intestinal tract (herein again standard dose
response curve and
other pharmacokinetic methods can be applied).
[0091] Various groups of embodiments of preparation 100 containing one or more
drugs or
other therapeutic agents 101 for the treatment of various diseases and
conditions will now be
described with references to dosages. It should be appreciated that these
embodiments,
including the particular therapeutic agents and the respective dosages are
exemplary and the
preparation 100 can comprise a number of other therapeutic agents described
herein (as well
as those known in the art) that are configured for delivery into a lumina]
wall in the intestinal
tract (e.g., the small intestinal wall) using various embodiments of device
10. The dosages
can be larger or smaller than those described and can be adjusted using one or
more methods
described herein or known in the art,
Therapeutic Agent Preparations Comprising Insulin
[0092] In one or more groups of embodiments, therapeutic agent preparation 100
can
comprise a therapeutically effective dose of insulin for the treatment of
diabetes and other
glucose regulation disorders. The insulin can be human or synthetically
derived as is known
in the art. In one embodiment, preparation 100 can contain a therapeutically
effective amount
of insulin in the range of about 1-10 units (one unit being the biological
equivalent of about
-28-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
45.5 ug of pure crystalline insulin), with particular ranges of 2-4, 3-9, 4-9,
5-8 or 6-7. Larger
ranges are also contemplated such as 1 to 25 units or 1-50 units. The amount
of insulin in the
preparation can be titrated based upon one or more of the following factors
(herein, "glucose
control titration factors"): i) the patient's condition (e.g., type 1 vs. type
II diabetes; ii) the
patient's previous overall level of glycemic control; iii) the patient's
weight; iv) the patient's
age; v) the frequency of dosage (e.g., once vs. multiple times a day); vi)
time of day (e.g.,
morning vs. evening); vii) particular meal (breakfast vs. dinner); vii)
content/glycemic index
of a particular meal (e.g., high fat/lipid and sugar content (e.g., foods
causing a rapid rise in
blood sugar) vs. low fat and sugar content; and viii) content of the patient's
overall diet (e.g.,
amount of sugars and other carbohydrates, lipids and protein consumed daily).
In use,
various embodiments of the therapeutic preparation 100 comprising insulin or
other
therapeutic agent for the treatment of diabetes or other blood glucose
disorder, to allow for
improved control of blood glucose levels by delivering more precisely
controlled dosages of
insulin without requiring the patient to inject themselves. Also, the patient
can swallow a
device such as swallowable device 10, or 110 (containing insulin and/or other
therapeutic
agent for the treatment of diabetes) at the same time as they take food such
that insulin or
other therapeutic is released into the blood stream from the small intestine
at about the same
time or close to the same time as glucose or other sugar in the food is
released from the small
intestine into the blood stream would raise blood glucose levels. This
concurrent or
otherwise time proximate release allows the insulin to act on various
receptors (e.g., insulin
receptors) to increase the uptake of glucose into muscle and other tissue just
as blood glucose
levels are starting to rise from absorption of sugars into the blood from the
small intestine.
100931 As discussed above, embodiments described herein can include
therapeutic
compositions comprising insulin for the treatment of various disorders such as
diabetes or
other glucose regulation disorder. Such compositions result in the delivery of
insulin with
various desirable pharmacokinetic properties. In this regard, pharmacokinetic
metrics of note
include Cn.õ the peak plasma concentration of insulin after administration;
tn., the time to
reach C11; and tyõ the time required for the plasma concentration of insulin
to reach half its
Cma, value after having reached C. These metrics can be measured using
standard
pharmacokinetic measurement techniques known in the art. In one approach
plasma samples
may be taken at set time intervals (e.g., one minute, five minutes, 1/2 hour,
1 hour, etc.)
beginning and then after administration of the therapeutic composition either
by use of a
swallowable device or by non-vascular injection. The concentration of insulin
in plasma can
then be measured using one or more appropriate analytical methods such as GC-
Mass Spec,
-29-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
LC-Mass Spec, HPLC or various ELISA (Enzyme-linked immunosorbent assays) which
can
be adapted for the particular drug. A concentration vs. time curve (also
herein referred to as a
concentration profile) can then be developed using the measurements from the
plasma
samples. The peak of the concentration curve corresponds to C,m4x. and the
time at which this
occurs corresponds to tmax. The time in the curve where the concentration
reaches half its
maximum value (i.e., C) after having reached Cõ,ax corresponds tot v,this
value is also
known as the elimination half-life of therapeutic agent. The start time for
determination of
can be based on the time at which the injection is made for the case on non-
vascular
injection and the point in time at which embodiments of the swallowable device
advances one
or more tissue penetrating members (containing the drug) into the small
intestine or other
location in the GI tract (e.g., the large intestine). In the latter case, this
time can determined
using one or more means including a remote controlled embodiment of the
swallowable
device which deploys the tissue penetrating members into the intestine wall in
response to an
external control signal (e.g., an RF signal) or for an embodiment of the
swallowable device
which sends an RF or other signal detectable outside the body when the tissue
penetrating
members have been deployed. Other means for detection of tissue penetrating
member
deployment into the small intestine are contemplated such as one more medical
imaging
modalities including for example, ultrasound or fluoroscopy. In any one of
these studies,
appropriate animal models can be used for example, dog, pig, rat etc. in order
to model the
human pharmacokinetic response.
100941 In consideration of the above, one or more embodiments of the invention
provide a
therapeutic composition comprising insulin, the composition adapted for
insertion into an
intestinal wall after oral ingestion, wherein upon insertion, the composition
releases insulin
into the bloodstream from the intestinal wall to achieve a Cmax faster than an
extravascularly
injected dose of insulin. In various embodiments, the therapeutic insulin
composition has a
tmax which is about 80%, or 50%, or 30%, or 200/o, or 10% of a t,õõ for an
extravascularly
injected does of insulin. Such an extravascularly injected dose of insulin can
be, for example,
a subcutaneous injection or an intramuscular injection. In certain embodiments
the Cmax
attained by delivering the therapeutic insulin composition by insertion into
the intestinal wall
is substantially greater, such as 100, or 50, or 10, or 5 times greater, than
the Cm,õ, attained
when the composition is delivered orally without insertion into the intestinal
wall. In some
embodiments, the therapeutic insulin composition is configured to produce a
long-term
release of insulin, such as a long-term release of insulin with a selectable
tiA. For example,
the selectable tv, may be 6, or 9, or 12, or 15 or 18, or 24 hours.
-30-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
[0095] The various embodiments described herein provide a therapeutic agent
composition
(also referred to herein as a preparation or composition) comprising insulin
and/or its
analogues and derivatives. The composition is adapted for insertion into an
intestinal wall
after oral ingestion, wherein upon insertion, the composition releases insulin
into the
bloodstream from the intestinal wall to achieve a C faster than an
extravascularly injected
dose of the therapeutic agent that is to say, achieving a Cmax for the
inserted form of
therapeutic agent in a shorter time period (e.g., a smaller than that for a
dose of the
therapeutic agent that is injected extravascularly Note, that the dose of
therapeutic agent in
the composition delivered into the intestinal wall and the dose delivered by
extravascular
injection, may, but need not, be comparable to achieve these results. In
various
embodiments, the composition is configured to achieve a L. for the insulin
(e.g., by release
of the insulin into the bloodstream from the intestinal wall, e.g., that of
the small intestine)
which is about 80%, or 50%, or 30%, or 20%, or 10% of a tmax for an
extravascularly injected
dose of the insulin. Such an extravascularly injected dose of insulin can be,
for example, a
subcutaneous injection or an intramuscular injection. In certain embodiments,
the Ciõaõ
attained by delivering the therapeutic agent by insertion into the intestinal
wall is
substantially greater, such as 5, 10, 20, 30, 40, 50, 60, 70, 80 or even a 100
times greater, than
the C1 attained when the therapeutic agent is delivered orally without
insertion into the
intestinal wall for example by a pill other convention oral form of the
therapeutic agent or
related compound. Also in various embodiments, the insulin compositions
adapted for
insertion into the wall of the small intestine can be configured to release
the insulin into the
blood stream from the intestinal wall to achieve a ty, that is greater than a
t1/4 for an orally
ingested dose of the therapeutic agent that is not inserted into the
intestinal wall. For
example, the ty, of the dose inserted into the intestinal wall may be 100 or
50 or 10 or 5 times
greater than the dose that is not inserted into the intestinal wall. In some
embodiments, the
insulin compositions adapted for insertion into the wall of the small
intestine may also be
configured to produce a long-term release of insulin with a selectable b,/,.
For example, the
selectable ty, may be 6, or 9, or 12, or 15 or 18, 24 or 48 hours.
[0096] The insulin composition may be in solid form, such as a solid form
composition
configured to degrade in the intestinal wall, and the solid form composition
may have, for
example, a tissue penetrating feature such as a pointed tip. The insulin
composition may
comprise at least one biodegradable material and/or may comprise at least one
pharmaceutical excipient, including a biodegradable polymer such as PGLA or a
sugar such
as maltose.
-31-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
[0097] The insulin composition may be adapted to be orally delivered in a
swallowable
capsule. In certain embodiments such a swallowable capsule may be adapted to
be operably
coupled to a mechanism having a first configuration and a second
configuration, the
therapeutic insulin composition being contained within the capsule in the
first configuration
and advanced out of the capsule and into the intestinal wall in the second
configuration. Such
an operably coupled mechanism may comprise at least one of an expandable
member, an
expandable balloon, a valve, a tissue penetrating member, a valve coupled to
an expandable
balloon, or a tissue penetrating member coupled to an expandable balloon.
100981 In some embodiments, the insulin composition may be configured to be
delivered
within a lumen or other cavity of a tissue penetrating member and/or the
therapeutic
composition may be shaped as a tissue penetrating member advanceable into the
intestinal
wall. The tissue penetrating member may be sized to be completely contained
within the
intestinal wall, and/or it may include a tissue penetrating feature for
penetrating the intestinal
wall, and/or it may include a retaining feature for retaining the tissue
penetrating member
within the intestinal wall. The retaining feature may comprise, for example, a
barb. In
various embodiments, the tissue penetrating member is configured to be
advanced into the
intestinal wall by the application of a force (e.g. a mechanical force) to a
surface of the tissue
penetrating member. According to some embodiments, the force can be applied by
means of
an expandable balloon or other expandable member or structure operatively
coupled to the
tissue penetrating member with the tissue penetrating member configured to
detach or
otherwise disengage from the expandable structure when a direction or
magnitude of force
applied by the expandable structure changes (e.g., when the balloon or other
expandable
member completes its expansion and starts to shrink or otherwise contract). In
preferred
embodiments, the tissue penetrating member has sufficient stiffness and/or
column strength
to be advanced completely into the intestinal wall. In various embodiments,
the column
strength of the tissue penetrating member can range from about 1 to about 20
lbs, 7 to 201bs,
8 to 12 lbs, with individual embodiments of 7, 8, 9, 10 and 11 lbs.
Therapeutic Agent Preparations Comprising Incretins
[0099] In another group of embodiments, therapeutic agent preparation 100 can
comprise a
therapeutically effective dose of one or more incretins for the treatment of
diabetes and other
glucose regulation disorders. Such incretins can include Glucacon like
peptides 1 (e.g., GLP-
1) and their analogues, and Gastric inhibitory peptide (GIP). Suitable GLP-1
analogues
include exenatide, liraglutide, albiglutide and taspoglutide as well as their
analogues,
-32-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
derivatives and other functional equivalents. In one embodiment preparation
100 can contain
a therapeutically effective amount of exenatide in the range of about 1-10 g,
with particular
ranges of 2-4, 4-6, 4-8 and 8-10 jig respectively. In another embodiment,
preparation 100
can contain a therapeutically effective amount of liraglutide in the range of
about 1-2 mg
(milligrams), with particular ranges of 1.0 to 1.4, 1.2 to 1.6 and 1.2 to 1.8
mg respectively.
One or more of the glucose control titration factors can be applied to ti
trate the dose ranges
for exenatide, liraglutide or other GLP-1 analogue or incretin.
Therapeutic Agent Preparations Comprising Combinations of Incretins and
Biguanide
[0100] In yet another group of embodiments, therapeutic agent preparation 100
can
comprise a combination of therapeutic agents for the treatment of diabetes and
other glucose
regulation disorders. Embodiments of such a combination can include for
example,
therapeutically effective doses of incretin and biguanide compounds. An
example biguanide
can include metformin in a dose of 200 to 1000mg, and a preferred does of 200
tO 500 mg.
The incretin can comprise one or more GLP-1 analogues described herein, such
as exenatide
and the biguanide can comprise metformin (e.g., that available under the
Trademark of
GLUCOPHAGO manufactured by Merck Sante S.A.S.) and its analogue, derivatives
and
other functional equivalents. In one embodiment, preparation 100 can comprise
a
combination of a therapeutically effective amount of exenatide in the range of
about 1-10 jig
and a therapeutically effective amount of metformin in a range of about 1 to 3
grams.
Smaller and larger ranges are also contemplated with one or more of the
glucose control
titration factors used to titrate the respective dose of exenatide (or other
incretin) and
metformin or other biguanide. Additionally, the dosages of the exenatide or
other incretin
and metformin or other biguanide can be matched to improved level of glucose
control for
the patient (e.g., maintenance of blood glucose within normal physiological
levels and/or a
reduction in the incidence and severity of instances of hyperglycemia and/or
hypoglycemia)
for extended periods of time ranges from hours (e.g., 12) to a day to multiple
days, with still
longer periods contemplated. Matching of dosages can also be achieved by use
of the
glucose control regulation factors as well as monitoring of the patient's
blood glucose for
extended periods using glycosylated hemoglobin (known as hemoglobin Alc,
HbAlc, Al C,
or Hblc) and other analytes and measurements correlative to long term average
blood
glucose levels.
-33-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
Therapeutic Preparations Comprising Combinations of Glucose Regulating
Compounds
[0101] In another group of embodiments, therapeutic agent preparation 100 can
comprise a
combination of between two to three glucose regulating compounds such as those
compounds
shown in tables 1 and 2 (an "x" in the table indicates that the particular
glucose regulating
compound is included, a "-" indicates that it is not). Such compounds can be
selected to
produce a synergistic effect (e.g. an enhancement) in one or more of blood
glucose reduction,
blood glucose regulation, appetite control/suppression or other therapeutic
effect. Such
synergistic effects in turn provide treatment for various conditions
associated with diabetes
such as hyperglycemia, insulin resistance or hyperlipidemia. According to one
or more
embodiments, the compounds shown in Table 1 can be contained in and/or formed
as an
embodiment of the tissue penetrating member that is configured to be advanced
from the
swallowable capsule configured to be inserted into the intestinal wall as is
described herein.
Once so inserted into the intestinal wall, the tissue penetrating member
degrades to release
the glucose regulating compounds into the blood stream as described herein for
other
therapeutic agents. In many embodiments, the tissue penetrating member is
configured to
concurrently release the plurality of glucose regulating compounds (e.g., by
compounding
and/or mixing all of the compounds in the same material used to formulate the
tissue
penetrating member). The concurrent release of the aforementioned compounds
into the wall
of the small intestine and, in turn the blood stream, serves to promote and
enhance the
synergistic effects between two or more of the compounds (e.g., increased
blood glucose
control, appetite suppression, etc.).
[0102] In additional or alternative embodiments, the compounds shown in Table
2 can be
orally delivered in the form of a dissolvable pill contained in an embodiment
of the
swallowable capsule but in these embodiments are released into the lumen of
the small
intestine where the swallowable capsule dissolves but not into the wall of the
small intestine.
Also it should be noted that the various pharamacokinetic parameters achieved
by delivering
insulin using embodiments of the swallowable capsule (as described above) are
equally
obtainable for each of the respective glucose regulating compounds listed
above in tables 1
and 2, either alone or in combination using embodiments of the swallowable
capsule to
deliver the compounds into the wall of the small intestine or other wall of
the intestinal tract.
-34-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
Table 1 Combination of Compounds Which May Be Delivered by Embodiments of the
Tissue Penetrating Member
GLP-1 insulin Other Compound
X X
X Glucagon
X GIP
X PYY
X X GIP
X X Glucagon
X PYY
X Glucagon
X GIP
X PYY
Table 2 Combination of Compounds Which May Be Delivered in Pill form from
inside the
Swallowable Capsule
GLP-1 Insulin Other
X X metformin
X metformin
X DPP4 Inhibitor
X SG LT2 Inhibitor
101031 In many embodiments, the combination of glucose regulating compounds
may
comprise a plurality of compounds including, for example, insulin, GLP-1 and
at least one
other compound. The glucose regulating compounds may include those which lower
blood
glucose also known as hypoglycemic compounds and those which raise blood
glucose. They
also include those which affect (e.g., reduce) glucose directly, and/or
indirectly e.g., by
causing the secretion of hormone which subsequently lower glucose levels,
agonize or
enhance the effect of glucose reducing hormones (as is the case for DDP4
inhibitors with
incretins), reduce appetite (as is the case with, peptide YY) or all of the
above. According to
some embodiments, the other glucose regulating compound can be selected from
the group
consisting of glucagon, peptide YY, GIP, metformin, peptide YY, Dipeptidyl
peptidase-4
(DPP4), DPP4 inhibitors, sodium/glucose co-transporter 2 (SGLT2) inhibitors
along with
their analogues and derivatives. A brief explanation will now be presented on
several of
these compounds.
Therapeutic Preparations Comprising Peptide YY
[0104] Peptide YY (PYY) also known as peptide tyrosine tyrosine or pancreatic
peptide
YY3-36 is a peptide in the incretin class of molecules that is encoded by the
PPY gene. It is
-35-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
a short (36-amino acid) peptide released by L-cells (being co-released with
GLP-1) in the
ileum and colon in response to feeding (and is co released with GLP-1). In the
blood, gut,
and other elements of periphery of the body, PYY acts to reduce appetite and
when
administered in conjunction with GLP-1, augment the appetite suppressing
effects of that
incretin. According to various embodiments the dose of PPY delivered by
embodiments of
the swallowable capsule can be in a range from 200 to 600 u.g, with a
preferred range of
about 400 to 500 m. Such doses being sufficient to produce a reduction in the
patient's
appetite and/or enhance (e.g. by an amount of 25, 50, 75 or 100%) the appetite
suppressing
effect of GLP-1 when the PPY is co-administered with GLP-1. In use,
embodiments of the
therapeutic preparation comprising insulin, GLP-1 and Peptide YY serve to not
only regulate
glucose homeostasis in the diabetic patient but also to produce weight loss in
the patient from
the synergistic appetite suppressing effect of GLP-1 and the PPY. The latter
effect also
serving to reduce insulin resistance in diabetics thus produce a long term
improvement in
glucose control/homeostasis in the diabetic patient. Further, such
combinations are
particularly useful and novel in that they provide in a single pill an
approach for treating the
different pathophysiologies of diabetes by treating one or more of glucose
regulation
dysfunction, obesity (through appetite suppression) and insulin resistance.
Under
conventional approaches using injected medications, the patient would have to
take three
separate injections all close in time. This is presently not done for type II
diabetes treatment
due to a number of reasons including patient compliance, adverse reactions at
the injections
sites, logistic (three separate liquid doses need to be formulated, stored and
carried by the
patient). Further, the single pill oral delivery approach contemplated by
embodiments of the
invention also provides benefits in that the therapeutics agents are released
in a more
physiologically natural fashion vs injected forms of the drugs. This results
in the
combinations of therapeutic agents which reach the portal circulation first
(vs the heart for
injections resulting the drug concentration subsequently becoming diluted)
which is closer to
a normal physiologic route when such compounds are secreted endogenously.
Therapeutic Preparations Comprising SGLT2 Inhibitors
[0105] The sodium/glucose cotransporter 2 (SGLT2) is a protein that in humans
is encoded
by the SLC5A2 (solute carrier family 5 (sodium/glucose cotransporter)) gene.
SGLT2
inhibitors also known as gliflozins cause a reduction in blood glucose levels.
Specifically,
they block glucose reabsorption in the kidney, thereby, disposing excess
glucose in the urine
and in turn, reducing blood glucose levels. The latter effect serves to
improve insulin
-36-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
resistance in the patient by reducing the blood glucose burden. As such, SGLT2
inhibitors
have potential use for the treatment of type II diabetes to improve glycemic
control. Example
SGLT2 Inhibitors include canagliflozin and dapagliflozin. Canagliflozin has
been shown to
enhance glycemic control as well as reduce body weight and systolic and
diastolic blood
pressure. Specifically clinical trials, canagliflozin have been shown to
produce consistent
dose-dependent decreases in HbAlc levels of 0.77% to 1.16% (from initial
levels of 7.8% to
8.1%) when administered either as monotherapy, in combination with metformin,
in
combination with metformin and a sulfonylurea, in combination with metfoimin
and
pioglitazone, or in combination with insulin. According to various
embodiments, the dose of
canagliflozin delivered by embodiments of the swallowable capsule or other
oral delivery
means described herein can be in a range from about100 to 300 mg, with a
preferred range of
about 400 to 500 ug. Another example of a suitable SGLT2 inhibitor includes
dapagliflozinm. Its dose can be in a range of 5 to 10mg with a preferred range
of 7 to 9mg.
Dapagliflozin inhibits subtype 2 of the sodium-glucose transport proteins
(SGLT2) which are
responsible for at least 90% of the glucose reabsorption in the kidney.
Blocking this
transporter mechanism causes blood glucose to be eliminated through the urine
which lowers
blood glucose. As such when used over extended time periods, dapagliflozin
also produces
reductions in HbAl c levels, in particular cases this can be as much as 0.6
percentage points.
[0106] Embodiments of the invention which deliver one or more of the above
SGLT2
inhibitors in combination with GLP-1 are particularly useful in that these two
compounds
work synergistically to both improve insulin resistance and the efficacy of
the delivered
insulin. (delivering the compounds together in one pill with the ability for
long term release
further enhances these effects). These combined effects in turn not only
greatly improve
glucose homeostasis but also serve to slow, halt or even reverse the
progression of diabetes or
other related glucose control disorder. Also, embodiments which deliver
insulin together
with SGLT2 inhibitors are particularly useful for the treatment of diabetes in
that these two
compounds act synergistically to reduce blood glucose more than each could
alone and thus
in turn reduce HbAl c levels. In specific embodiments the dose of the
particular SGLT2
inhibitor (e.g., canagliflozin, dapagliflozinm, etc.) alone or combination
with the dose of
insulin can titrated in the therapeutic agent combination 100 to produce a
selected decrease in
HbAlc levels e.g., decrease by 0.2 to 1. 2% with a specific range of 0.77% to
1.16% etc.
-37-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
Therapeutic Preparations Comprising DDPP4 Inhibitors
[0107] Dipeptidyl peptidase-4 (DDPP4) Inhibitors, also known as gliptins, are
a class of
oral antidiabetic agents that block the effect of the DPP-4 enzymes which
degrade incretins
such as GLP-1. The net result being the half-life of incretins (both natural
and delivered) in
circulation is increased thereby augmenting anti-diabetic effects of the
incretins. Example
DDPP4 Inhibitors include sitagliptin, with a dose range of about 20 to 100 mg,
and
saxagliptin, with a dose range of about 5 to 10 mg dose. In combination with a
delivered
GLP-1 or GLP-1 mimetic, DDPP4 inhibitors serve to the prolong the duration of
action of the
otherwise short lived incretin peptides ( e.g. natural GLP-1 has a half-life
of about two
minutes). This effect produces a long lived glucose regulating effect of the
GLP-1 eliminate
and/or reduces the need of meal time insulin injection. This effect in turn
reduces the burden
on insulin secreting cells in the pancreas (e.g., pancreatic beta cell) which
in turn, contributes
to slowing the progression of diabetic disease by preserving the function of
pancreatic beta
cells.
Therapeutic Preparations Comprising Gastric Inhibitory Peptides (GIP)
[0108] Gastric inhibitory polypeptide (GIP), also known as the glucose-
dependent
insulinotropic peptide is a member of the secretin family of hormones. GIP is
derived from a
153-amino acid pro-protein encoded by the GIP gene and circulates as a
biologically active
42-amino acid peptide. It is synthesized by K cells, which are found in the
mucosa of the
duodenum and the jejunum of the gastrointestinal tract. GIP reduces blood
glucose levels by
inducing insulin secretion. The dose of GIP delivered by embodiments of the
swallowable
capsule can be in a range from about 50 to about 250 g. Type II diabetics
have lower levels
of GIP secretion after a meal which is believed to adversely effect glucose
disposal by
decreasing insulin production and/or release below typical levels after
ingestion of a meal.
Accordingly, delivering embodiments of therapeutic compositions comprising
GIPs using the
swallowable capsule described herein serves to compensate for the reduction in
endogenous
GIP secretion in diabetic patients and in turn restores the normal
physiological levels of GIP
hormones secreted after a meal, particularly as embodiments of the invention
can deliver
them directly to the small intestine or the wall of the small intestine.
Further, the
administration of embodiments of therapeutic preparations comprising GIP,
insulin and GLP-
1 serve to restore the normal physiological levels of these hormones (these
levels being
comprised in diabetics), thereby restoring normal glucose disposal and, in
turn, glucose
homeostasis. Normal glucose disposal being the ability of the body to prevent
sustained
-38-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
hyperglycemia after a meal by the rapid metabolism and/or storage of
circulating blood
glucose levels.
Therapeutic Preparations Comprising Glucagon
101091 Glucagon is a peptide hormone, produced by alpha cells of the pancreas
and belongs
to a family of hormones known as the seeretin family. It acts to increase the
concentration of
glucose in the bloodstream. Its effect is opposite that of insulin. The
pancreas releases
glucagon when the concentration of glucose in the bloodstream falls too low.
Glucagon
causes the liver to convert stored glycogen into glucose, which is then
released into the
bloodstream raising blood glucose levels. While having opposite effects,
glucagon and
insulin work in concert to maintain glucose levels within normal physiologic
levels (e.g.
euglycemic levels). The diabetic patient has various complication with
glucagon and/or
glucagon metabolism. Specifically, the diabetic patient's tissue/cells have
resistance to one
or both of glucagon and insulin, resulting in the loss of their synergetic
effect in controlling
glucose and in turn, a loss in maintaining glucose homeostasis. Further in
diabetics, glucagon
resistance has been linked to hyperlipidemia and other adverse effects on the
patient's energy
homeostasis. Hyperlipidemia in turn, can result in a number of adverse effects
to the patient
including one or more of increased insulin resistance, cardiovascular disease
and metabolic
syndrome Accordingly, embodiments of therapeutic preparation comprising
insulin, GLP-1
and glucagon can be configured to produce multiple beneficial effects in the
diabetic patient
including: i) restoring insulin resistance by the action of GLp-1; ii) reduce
the burden on the
beta cells of the pancreas by providing basal levels of insulin; iii) reduce
the burden on alfa
cells of the pancreas by providing basal levels of glucagon; iv) prevent or
reduce sustained
excursions in blood glucose levels (e.g., both hyperglycemia and hypoglycemia)
which may
result following a meal and/or an extra-vascularly injected dose of insulin.;
v) reduces
hyperlipidemia.. One or more of the preceding resulting in restoration of
glucose
homeostasis, energy homeostasis (e.g., glucose and fat metabolism) leading to
a reversal in
the course of diabetic disease and/or metabolic syndrome. In various
embodiments the dose
can of glucagon or its analogues delivered by embodiments of the invention can
be in a range
from about 0.5 to about 2mg.
-39-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
Embodiments Contemplating Additional and/or Alternative Drug Delivery Means to
the Tissue Penetrating Member
[0110] Drug delivery compositions and components of known drug delivery
systems may
be employed and/or modified for use in some embodiments of the inventions
described
herein. For example, micro-needles and other microstructures used for delivery
of drugs
through the skin surface with drug patches may be modified and included within
the capsules
described herein and used to instead deliver a drug preparation into a lumen
wall of the
gastrointestinal tract such as the wall of the small intestine. Suitable
polymer micro-needle
structures may be commercially available from Corium of California, such as
the MicroCorTM
micro delivery system technology. Other components of the MicroCorTM patch
delivery
systems, including drug formulations or components, may also be incorporated
into the
capsules described herein. Alternatively, a variety of providers are
commercially available to
formulate combinations of polymers or other drug-delivery matrices with
selected drugs and
other drug preparation components so as to produce desired shapes (such as the
releasable
tissue-penetrating shapes described herein) having desirable drug release
characteristics.
Such providers may, for example, include Corium, SurModics of Minnesota,
BioSensors
International of Singapore, or the like.
Embodiments which Protect Biological Activity of the Therapeutic Agent.
[0111] One advantage and feature of various embodiments of the therapeutic
compositions
described herein is that the biologic drug payload (e.g., the therapeutic
peptide or protein,
e.g., insulin, GLP-1 and a third glucose regulating compound such as PPY, GIP,
Glucagon,
etc.,) is protected from degradation and hydrolysis by the action of
peptidases and proteases
in the gastrointestinal (GI) tract. These enzymes are ubiquitous throughout
living systems.
The GI tract is especially rich in proteases whose function is to break down
the complex
proteins and peptides in one's diet into smaller segments and release amino
acids which are
then absorbed from the intestine. The compositions described herein are
designed to protect
the therapeutic peptide or protein from the actions of these GI proteases and
to deliver the
peptide or protein payload directly into the wall of the intestine. There are
two features in
various embodiments of the compositions described herein which serve to
protect the protein
or peptide payload from the actions of GI proteases. First, in certain
embodiments, the
capsule shell, which contains the deployment engine and machinery, does not
dissolve until it
reaches the duodenal and sub-duodenal intestinal segments, owing to the pH-
sensitive coating
on the outer surface of the capsule which prevents its dissolution in the low
pH of the
-40-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
stomach. Second, in certain embodiments, the tissue penetrating members which
contain the
actual therapeutic peptide or protein; the tissue penetrating members are
designed to penetrate
the intestine muscle as soon as the outer capsule shell dissolves; and the
micro-spears
themselves slowly dissolve in the intestinal muscle wall to release the drug
payload. Thus,
the peptide or protein payload is not exposed to the actions of the GI
proteases and therefore
does not undergo degradation via proteolysis in the GI tract. This feature, in
turn, contributes
to the high % bioavailabilty of the therapeutic peptide or protein.
[0112] Various aspects of the invention also provide other embodiments of a
swallowable
delivery device for the delivery of medication 100 in addition to those
described above.
According to one or more such embodiments, the swallow delivery device can
include one or
more expandable balloons or other expandable devices for use in delivering one
or more
tissue penetrating members including medication 100 into the wall of an
intestine, such as the
small intestine. Referring now to Figs. 12-20, another embodiment of a device
110 for the
delivery of medication 100 to a delivery site DS in the gastro-intestinal (GI)
tract, can
comprise a capsule 120 sized to be swallowed and pass through the intestinal
tract, a
deployment member 130, one or more tissue penetrating members 140 containing
medication
100, a deployable aligner 160 and a delivery mechanism 170. In some
embodiments,
medication 100 (also referred to herein as preparation 100) may itself
comprise tissue
penetrating member 140. The deployable aligner 160 is positioned within the
capsule and
configured to align the capsule with the intestine such as the small
intestine. Typically, this
will entail aligning a longitudinal axis of the capsule with a longitudinal
axis of the intestine;
however, other alignments are also contemplated. The delivery mechanism 170 is
configured
for delivering medication 100 into the intestinal wall and will typically
include a delivery
member 172 such as an expandable member. The deployment member 130 is
configured for
deploying at least one of the aligner 160 or the delivery mechanism 170. As
will be
described further herein, all or a portion of the capsule wall is degradable
by contact with
liquids in the GI tract so as to allow those liquids to trigger the delivery
of medication 100 by
device 110. As used herein, "GI tract" refers to the esophagus, stomach, small
intestine, large
intestine and anus, while "Intestinal tract" refers to the small and large
intestine. Various
embodiments of the invention can be configured and arranged for delivery of
medication 100
into both the intestinal tract as well as the entire GI tract.
[0113] Device 110 including tissue penetrating member 140 can be configured
for the
delivery of liquid, semi-liquid or solid forms of medication 100 or
combinations of all three.
Whatever the form, medication 100 desirably has a material consistency
allowing the
-41-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
medication to be advanced out of device 110, into the intestinal wall (e.g.,
the small or large
intestine) or other luminal wall in the GI tract and then degrade within the
intestinal wall to
release the drug or other therapeutic agent 101. The material consistency of
medication 100
can include one or more of the hardness, porosity and solubility of the
preparation (in body
fluids). The material consistency can be achieved by selection and use of one
or more of the
following: i) the compaction force used to make the preparation; ii) the use
of one or more
pharmaceutical disintegrants known in the art; iii) use of other
pharmaceutical excipients; iv)
the particle size and distribution of the preparation (e.g., micronized
particles); and v) use of
micronizing and other particle formation methods known in the art.
101141 Capsule 120 is sized to be swallowed and pass through the intestinal
tract. The size
can also be adjusted depending upon the amount of drug to be delivered as well
as the
patient's weight and adult vs. pediatric applications. Typically, the capsule
will have a
tubular shape with curved ends similar to a vitamin. In these and related
embodiments,
capsule lengths 120L can be in the range of 0.5 to 2 inches and diameters 120D
in the range
of 0.1 to 0.5 inches with other dimensions contemplated. The capsule 120
includes a capsule
wall 121w, having an exterior surface 125 and an interior surface 124 defining
an interior
space or volume 124v. In some embodiments, the capsule wall 121w can include
one or
more apertures 126 sized for the outward advancement of tissue penetrating
members 140. In
addition to the other components of device 110, (e.g., the expandable member
etc.) the
interior volume can include one or more compartments or reservoirs 127.
101151 Capsule 120 can be fabricated from various biodegradable materials
known in the
pharmaceutical arts such as various gelatins. In various embodiments the
capsule may also
include various enteric coatings 120c, configured to protect the cap from
degradation in the
stomach (due to acids etc.), and then subsequently degrade in the in higher
pH's found in the
small intestine or other area of the intestinal tract. In various embodiments,
the capsule 120
can be formed from multiple portions one or more of which may be
biodegradable. In many
embodiments, capsule 120 can be formed from two portions 120p such as a body
portion
120p" (herein body 120p") and a cap portion 120p' (herein cap 120p), where the
cap fits onto
the body, e.g., by sliding over or under the body (with other arrangements
also
contemplated). One portion such as the cap 120p' can include a first coating
120c'configured
to degrade above a first pH (e.g., pH 5.5) and the second portion such as the
body 120p" can
include a second coating 120c" configured to degrade above a second higher pH
(e.g.6.5).
Both the interior 124 and exterior 125 surfaces of capsule 120 are coated with
coatings 120c'
and 120c" so that that either portion of the capsule will be substantially
preserved until it
-42-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
contacts fluid having the selected pH. For the case of body 120p" this allows
the structural
integrity of the body 120p" to be maintained so as to keep balloon 172 inside
the body
portion and not deployed until balloon 130 has expanded. Coatings 120c' and
120c" can
include various methacrylate and ethyl acrylate based coatings such as those
manufactured by
Evonik Industries under the trade name EUDRAGIT. These and other dual coating
configurations of the capsule 120 allows for mechanisms in one portion of
capsule 120 to be
actuated before those in the other portion of the capsule. This is due to the
fact that intestinal
fluids will first enter those portions where the lower pH coating has degraded
thus actuating
triggers which are responsive to such fluids (e.g., degradable valves). In
use, such dual
coating embodiments for capsule 120 provide for targeted drug delivery to a
particular
location in the small intestine (or other location in the GI tract), as well
as improved
reliability in the delivery process. This is due to the fact that deployment
of a particular
component, such as aligner 160, can be configured to begin in the upper area
of the small
intestine (e.g., the duodenum) allowing the capsule to be aligned within the
intestine for
optimal delivery of the drug (e.g., into the intestinal wall) as well as
providing sufficient time
for deployment/actuation of other components to achieve drug delivery into the
intestinal
wall while the capsule is still in the small intestine or other selected
location.
[0116] As is discussed above, one or more portions of capsule 120 can be
fabricated from
various biocompatible polymers known in the art, including various
biodegradable polymers
which in a preferred embodiment can comprise cellulose, gelatin materials PGLA
(polylactic-
co-glycolic acid). Other suitable biodegradable materials include various
enteric materials
described herein as well as lactide, glycolide, lactic acid, glycolic acid,
para-dioxanone,
caprolactone, trimethylene carbonate, caprolactone, blends and copolymers
thereof.
[0117] In various embodiments, the wall 120w of the capsule is degradable by
contact with
liquids in the GI tract for example liquids in the small intestine. In
preferred embodiments,
the capsule wall is configured to remain intact during passage through the
stomach, but then
to be degraded in the small intestine. In one or more embodiments, this can be
achieved by
the use of an outer coating or layer 120c on the capsule wall 120w, which only
degrades in
the higher pH's found in the small intestine and serves to protect the
underlying capsule wall
from degradation within the stomach before the capsule reaches the small
intestine (at which
point the drug delivery process is initiated by degradation of the coating as
is described
herein). In use, such coatings allow for the targeted delivery of a
therapeutic agent in a
selected portion of the intestinal tract such as the small intestine.
-43-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
[0118] Similar to capsule 20, in various embodiments, capsule 120 can include
various
radio-opaque, echogenic or other materials for location of the device using
one or more
medical imaging modalities such as fluoroscopy, ultrasound, MRI, etc.
[0119] As is discussed further herein, in many embodiments, one or more of the
deployment member 130, delivery member 172 or deployable aligner 160, may
correspond to
an expandable balloon that is shaped and sized to fit within capsule 120.
Accordingly, for
ease of discussion, deployment member 130, delivery member 172 and deployable
aligner
160 will now be referred to as balloon 130, 160 and 172; however, it should be
appreciated
that other devices including various expandable devices are also contemplated
for these
elements and may include for example, various shape memory devices (e.g., an
expandable
basket made from shape memory biodegradable polymer spires), expandable piezo
electric
devices, and/or chemically expandable devices having an expanded shape and
size
corresponding to the interior volume 124v of the capsule 120.
[0120] One or more of balloons 130, 160 and 172 can comprise various polymers
known in
the medical device arts. In preferred embodiments such polymers can comprise
one or more
types of polyethylene (PE) which may correspond to low density PE(LDPE),
linear low
density PE (LLDPE), medium density PE (MDPE) and high density PE (HDPE) and
other
forms of polyethylene known in the art. In one more embodiments using
polyethylene, the
material may be cross-linked using polymer irradiation methods known in the
art so. In
particular embodiments radiation-based cross-linking may be used as to control
the inflated
diameter and shape of the balloon by decreasing the compliance of the balloon
material. The
amount or radiation may be selected to achieve a particular amount of cross
linking to in turn
produce a particular amount of compliance for a given balloon, e.g., increased
irradiation can
be used to produce stiffer less compliant balloon material. Other suitable
polymers can
include PET (polyethylene teraphalate), silicone and polyurethane. In various
embodiments
balloons 130, 160 and 172 may also include various radio-opaque materials
known in the art
such as barium sulfate to allow the physician to ascertain the position and
physical state of
the balloon (e.g., un-inflated, inflated or punctures. Balloons 130, 160 and
172 can be
fabricated using various balloon blowing methods known in the balloon
catheters arts (e.g.,
mold blowing, free blowing, etc.) to have a shape and size which corresponds
approximately
to the interior volume 124v of capsule 120. In various embodiments one or more
of balloons
130, 160 and 172 and various connecting features (e.g., connecting tubes) can
have a unitary
construction being formed from a single mold. Embodiments employing such
unitary
-44-
CA 2993215
construction provide the benefit of improved manufacturability and reliability
since fewer joints must be
made between one or more components of device 110.
[0121] Suitable shapes for balloons 130, 160 and 172 include various
cylindrical shapes having
tapered or curved end portions (an example of such a shape including a hot
dog). In some
embodiments, the inflated size (e.g., diameter) of one or more of balloons
130, 160 and 172, can be
larger than capsule 120 so as to cause the capsule to come apart from the
force of inflation, (e.g., due to
hoop stress). In other related embodiments, the inflated size of one or more
of balloons 130, 160 and
172 can be such that when inflated: i) the capsule 120 has sufficient contact
with the walls of the small
intestine so as to elicit a peristaltic contraction causing contraction of the
small intestine around the
capsule, and/or ii) the folds of the small intestine are effaced to allow.
Both of these results allow for
improved contact between the capsule/balloon surface and the intestinal wall
so as deliver tissue
penetrating members 40 over a selected area of the capsule and/or delivery
balloon 172. Desirably, the
walls of balloons 130, 160 and 172 will be thin and can have a wall thickness
in the range of 0.005 to
0.0001" more preferably, in the range of 0.005 to 0.0001, with specific
embodiments of 0.004, 0.003,
0.002, 0.001, and 0.0005). Additionally in various embodiments, one or more of
balloon 130, 160 or
172 can have a nested balloon configuration having an inflation chamber 160IC
and extended finger
160EF as is shown in the embodiments of Fig. 13c. The connecting tubing 163,
connecting the inflation
chamber 160IC can be narrow to only allow the passage of gas 169, while the
connecting tubing 36
coupling the two halves of balloon 130 can be larger to allow the passage of
water.
[0122] As indicated above, the aligner 160 will typically comprise an
expandable balloon and for
ease of discussion, will now be referred to as aligner balloon 160 or balloon
160. Balloon 160 can be
fabricated using materials and methods described above. It has an unexpanded
and expanded state (also
referred to as a deployed state). In its expanded or deployed state, balloon
160 extends the length of
capsule 120 such that forces exerted by the peristaltic contractions of the
small intestine SI on capsule
120 serve to align the longitudinal axis 120LA of the capsule 120 in a
parallel fashion with the
longitudinal axis LAI of the small intestine SI. This in turn serves to align
the shafts of tissue
penetrating members 140 in a perpendicular fashion with the surface of the
intestinal wall IW to
enhance and optimize the penetration of tissue penetrating members 140 into
the intestinal wall IW. In
addition to serving to align capsule 120 in the small intestine, aligner 160
is also configured to push
delivery mechanism 170 out of capsule 120 prior to inflation of delivery
balloon 172 so that the delivery
balloon and/or mechanism is not encumbered by the capsule. In use, this push
- 45 -
Date Regue/Date Received 2023-01-12
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
out function of aligner 160 improves the reliability for delivery of the
therapeutic agent since
it is not necessary to wait for particular portions of the capsule (e.g.,
those overlying the
delivery mechanism) to be degraded before drug delivery can occur.
[0123] Balloon 160 may be fluidically coupled to one or more components of
device 110
including balloons 130 and 172 by means of polymer tube or other fluidic
couplings 162
which may include a tube 163 for coupling balloons 160 and 130 and a tube 164
for coupling
balloon 160 and balloon 172. Tube 163 is configured to allow balloon 160 to be
expanded/inflated by pressure from balloon 130 (e.g., pressure generated the
mixture of
chemical reactants within balloon 130) and/or otherwise allow the passage of
liquid between
balloons 130 and 160 to initiate a gas generating chemical reaction for
inflation of one or
both of balloons 130 and 160. Tube 164 connects balloon 160 to 172 so as to
allow for the
inflation of balloon 172 by balloon 160. In many embodiments, tube 164
includes or is
coupled to a control valve 155 which is configured to open at a selected
pressure so as to
control the inflation of balloon 172 by balloon 160. Tube 164 may thus
comprise a proximal
portion 164p connecting to the valve and a distal portion 164d leading from
the valve.
Typically, proximal and distal portions 164p and 164d will be connected to a
valve housing
158 as is described below.
[0124] Valve 155 may comprise a triangular or other shaped section 156 of a
material 157
which is placed within a the chamber 158c of a valve housing 158 (alternately,
it may be
placed directly within tubing 164). Section 157 is configured to mechanically
degrade (e.g.,
tears, shears, delaminates, etc.) at a selected pressure so as to allow the
passage of gas
through tube 164 and/or valve chamber 158c. Suitable materials 157 for valve
155 can
include bees wax or other form of wax and various adhesives known in the
medical arts
which have a selectable sealing force/burst pressure. Valve fitting 158 will
typically
comprise a thin cylindrical compartment (made from biodegradable materials) in
which
section 156 of material 157 is placed (as is shown in the embodiment of Fig.
13b) so as to
seal the walls of chamber 158c together or otherwise obstruct passage of fluid
through the
chamber. The release pressure of valve 155 can be controlled through selection
of one or
more of the size and shape of section 156 as well as the selection of material
157 (e.g., for
properties such as adhesive strength, shear strength etc.). In use, control
valve 155 allows for
a sequenced inflation of balloon 160 and 172 such that balloon 160 is fully or
otherwise
substantially inflated before balloon 172 is inflated. This, in turn, allows
balloon 160 to push
balloon 172 along with the rest of delivery mechanism 170 out of capsule 120
(typically from
body portion 120p') before balloon 172 inflates so that deployment of tissue
penetrating
-46-
CA 2993215
members 140 is not obstructed by capsule 120. In use, such an approach
improves the reliability of the
penetration of tissue penetrating members 140 into intestinal wall IW both in
terms of achieving a
desired penetration depth and delivering greater numbers of the penetrating
members 140 contained in
capsule 120 since the advancement of the members into intestinal wall IW is
not obstructed by capsule
wall 120w.
[0125] As is describe above, the inflated length 1601 of the aligner
balloon 160 is sufficient to have
the capsule 120 become aligned with the lateral axis of the small intestine
from peristaltic contractions
of the intestine. Suitable inflated lengths 1601 for aligner 160 can include a
range between about 1/2 to
two times the length 1201 of the capsule 120 before inflation of aligner 160.
Suitable shapes for aligner
balloon 160 can include various elongated shapes such as a hotdog like shape.
In specific embodiments,
balloon 160 can include a first section 160' and a second section 160", where
expansion of first section
160' is configured to advance delivery mechanism 170 out of capsule 120
(typically out of and second
section 160" is used to inflate delivery balloon 172. In these and related
embodiments, first and second
sections 160' and 160" can be configured to have a telescope-style inflation
where first section 160'
inflates first to push mechanism 170 out of the capsule (typically from body
portion 120p') and second
section 160" inflates to inflate delivery member 172. This can be achieved by
configuring first section
160' to have smaller diameter and volume than second section 160" such that
first section 160' inflates
first (because of its smaller volume) and with second section 160" not
inflating until first section 160'
has substantially inflated. In one embodiment, this can be facilitated by use
of a control valve 155
(described above) connecting sections 160' and 160" which does not allow
passage of gas into section
160" until a minimum pressure has been reached in section 160'. In some
embodiments, the aligner
balloon can contain the chemical reactants which react upon mixture with water
or other liquid from the
deploying balloon.
[0126] In many embodiments, the deployment member 130 will comprise an
expandable balloon,
known as the deployment balloon 130. In various embodiments, deployment
balloon 130 is configured
to facilitate deployment/expansion of aligner balloon 160 by use of a gas, for
example, generation of a
gas 169 from a chemical. The gas may be generated by the reaction of solid
chemical reactants 165,
such as an acid 166 (e.g., citric acid) and a base 166 (e.g., potassium
bicarbonate, sodium bicarbonate
and the like) which are then mixed with water or other aqueous liquid 168. The
amount of reactants can
be chosen using stoichiometric methods to produce a selected pressure in one
or more of balloons 130,
160 and 172. The reactants 165 and liquids can be stored separately in balloon
130 and 160 and
- 47 -
Date Regue/Date Received 2023-01-12
CA 2993215
then brought together in response to a trigger event, such as the pH
conditions in the small intestine.
The reactants 165 and liquids 168 can be stored in either balloon, however in
preferred embodiments,
liquid 168 is stored in balloon 130 and reactants 165 in balloon 160. To allow
for passage of the liquid
168 to start the reaction and/or the resulting gas 169, balloon 130 may be
coupled to aligner balloon 160
by means of a connector tube 163 which also typically includes a separation
means 150 such as a
degradable valve 150 described below. For embodiments where balloon 130
contains the liquid, tube
163 has sufficient diameter to allow for the passage of sufficient water from
balloon 130 to balloon 60
to produce the desired amount of gas to inflate balloon 160 as well inflate
balloon 172. Also when
balloon 130 contains the liquid, one or both of balloon 30 and tube 163 are
configured to allow for the
passage of liquid to balloon 160 by one or more of the following: i) the
compressive forced applied to
balloon 130 by peristaltic contractions of the small intestine on the exposed
balloon 130; and ii) wicking
of liquid through tube 163 by capillary action.
101271
Tube 163 will typically include a degradable separation valve or other
separation means 150
which separates the contents of balloon 130, (e.g., water 168) from those of
balloon 160 (e.g., reactants
165) until the valve degrades. Valve 150 can be fabricated from a material
such as maltose, which is
degradable by liquid water so that the valve opens upon exposure to water
along with the various liquids
in the digestive tract. It may also be made from materials that are degradable
responsive to the higher
pH's found in the intestinal fluids such as methacrylate based coatings. The
valve is desirably
positioned at location on tube 163 which protrudes above balloon 130 and/or is
otherwise sufficient
exposed such that when cap 120p' degrades the valve 150 is exposed to the
intestinal liquids which
enter the capsule. In various embodiments, valve 150 can be positioned to lie
on the surface of balloon
130 or even protrude above it (as is shown in the embodiments of Figs. 16a and
16b), so that is has clear
exposure to intestinal fluids once cap 120p' degrades. Various embodiments of
the invention provide a
number of structures for a separation valve 150, for example, a beam like
structure (where the valve
comprises a beam that presses down on tube 163 and/or connecting section 136),
or collar type structure
(where the valve comprise a collar lying over tube 163 and/or connecting
section 136). Still other valve
structures are also contemplated.
101281 Balloon 130 has a deployed and a non-deployed state. In the deployed
state, the deployment
balloon130 can have a dome shape 130d which corresponds to the shape of an end
of the capsule. Other
shapes 130s for the deployed balloon 130 are also contemplated, such as
spherical, tube-shape, etc. The
reactants 165 will typically include at least two reactants 166 and 167, for
example, an acid such as
citric acid and a base such as sodium
- 48 -
Date Regue/Date Received 2023-01-12
CA 2993215
bicarbonate. Other reactants 165 including other acids, e.g., ascetic acid and
bases, e.g., sodium
hydroxide are also contemplated. When the valve or other separation means 150
opens, the reactants
mix in the liquid and produce a gas such as carbon dioxide which expands the
aligner balloon 160 or
other expandable member.
[0129] In an alternative embodiment shown in Fig. 13b, the deployment
balloon 130 can actually
comprise a first and second balloon 130' and 130" connected by a tube 136 or
other connection means
136 (e.g., a connecting section). Connecting tube 136 will typically include a
separation valve 150 that
is degradable by a liquid as described above and/or a liquid having a
particular pH such as basic pH
found in the small intestine (e.g., 5.5 or 6.5). The two balloons 130' and
130" can each have a half
dome shape 130hs allowing them to fit into the end portion of the capsule when
in the expanded state.
One balloon can contain the chemical reactant(s) 165 (e.g., sodium
bicarbonate, citric acid, etc.) the
other the liquid water 168, so that when the valve is degraded the two
components mix to form a gas
which inflates one or both balloons 130' and 130" and in turn, the aligner
balloon 160.
[0130] In yet another alternative embodiment, balloon 130 can comprise a multi-
compartment
balloon 130mc, that is formed or other constructed to have multiple
compartments 130c. Typically,
compartments 130c will include at least a first and a second compartment 134
and 135 which are
separated by a separation valve 150 or other separation means 150 as is shown
in the embodiment of
Fig. 14a. In many embodiments, compartments 134 and 135 will have at least a
small connecting
section 136 between them which is where separation valve 150 will typically be
placed. A liquid 168,
typically water, can be disposed within first compartment 134 and one or more
reactants 165 disposed in
second compartment 135 (which typically are solid though liquid may also be
used) as is shown in the
embodiment of Fig. 14a. When valve 150 opens (e.g., from degradation caused by
fluids within the
small intestine) liquid 168 enters compartment 135 (or vice versa or both),
the reactant(s) 165 mix with
the liquid and produce a gas 169 such as carbon dioxide which expands balloon
130 which in turn can
be used to expand one or more of balloons 160 and 172.
[0131] Reactants 165 will typically include at least a first and a second
reactant, 166 and 167 for
example, an acid such as citric acid and a base such as sodium bi-carbonate or
potassium bi-carbonate.
As discussed herein, in various embodiments they may be placed in one or more
of balloon 130
(including compartments 134 and 135 or halves 130' and 130") and balloon 160.
Additional reactants,
including other combinations of acids and bases which produce an inert gas by
product are also
contemplated. For embodiments using citric acid and sodium or potassium
bicarbonate, the ratio's
between the two reactants (e.g., citric
- 49 -
Date Regue/Date Received 2023-01-12
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
acid to potassium bicarbonate) can be in the range of about 1:1 to about 1:4,
with a specific
ratio of about 1:3. Desirably, solid reactants 165 have little or no absorbed
water.
Accordingly, one or more of the reactants, such as sodium bicarbonate or
potassium
bicarbonate can be pre-dried (e.g., by vacuum drying) before being placed
within balloon
130. Other reactants 165 including other acids, e.g., ascetic acid and bases
are also
contemplated. The amounts of particular reactants 165, including combinations
of reactants
can be selected to produce particular pressures using known stoichiometric
equations for the
particular chemical reactions as well as the inflated volume of the balloon
and the ideal gas
law (e.g., PV=nRT). In particular embodiments, the amounts of reactants can be
selected to
produce a pressure selected one or more of balloons 130, 160 and 172 to: i)
achieve a
particular penetration depth into the intestinal wall; and produce a
particular diameter for one
or more of balloons 130, 160 and 172; and iii) exert a selected amount of
force against
intestinal wall IW. In particular embodiments, the amount and ratios of the
reactants (e.g.,
citric acid and potassium bicarbonate) can be selected to achieve pressures in
one more of the
balloons 130, 160 and 172 in the range of 10 to 15 psi, with smaller and
larger pressures
contemplated. Again the amounts and ratio's of the reactants to achieve these
pressures can
be determined using known stoichiometric equations.
[0132] In various embodiments of the invention using chemical reactants 165 to
generate
gas 169, the chemical reactants alone or in combination with the deployment
balloon 130 can
comprise a deployment engine for 180 deploying one or both of the aligner
balloon 160 and
delivery mechanism 170 including delivery balloon 172. Deployment engine 180
may also
include embodiments using two deployment balloons 130 and 130" (a dual dome
configuration as shown in Fig. 13b), or a multi compartment balloon 130mc as
shown in Fig.
14a. Other forms of a deployment engine 180 are also contemplated by various
embodiments
of the invention such as use of expandable piezo-electric materials (that
expand by
application of a voltage), springs and other shape memory materials and
various thermally
expandable materials.
[0133] One or more of the expandable balloons 130, 160 and 172 will also
typically include
a deflation valve 159 which serves to deflate the balloon after inflation.
Deflation valve 159
can comprise biodegradable materials which are configured to degrade upon
exposure to the
fluids in the small intestine and/or liquid in one of the compartments of the
balloon so as to
create an opening or channel for escape of gas within a particular balloon.
Desirably,
deflation valves 159 are configured to degrade at a slower rate than valve 150
to allow
sufficient time for inflation of balloons, 130, 160 and 172 before the
deflation valve degrades.
-50-
CA 2993215
In various embodiments, of a compartmentalized balloon 130, deflation valve
159 can correspond to a
degradable section 139 positioned on an end portion 131 of the balloon as is
shown in the embodiment
of Fig. 14a. In this and related embodiments, when degradable section 139
degrades from exposure to
the liquid, balloon wall 132 tears or otherwise comes apart providing for a
high assurance of rapid
deflation. Multiple degradable sections 139 can be placed at various locations
within balloon wall 132.
101341 In various embodiments of balloon 172, deflation valve 159 can
correspond to a tube valve
173 attached to the end 172e of the delivery balloon 172 (opposite to the end
which is coupled to the
aligner balloon) as is shown in the embodiment of Fig. 13b. The tube valve 173
comprises a hollow
tube 173t having a lumen that is obstructed at a selected location 1731 with a
material 173m such as
maltose that degrades upon exposure to fluid such as the fluid in the small
intestine. The location 1731
of the obstructing material 173m in tube 173t is selected to provide
sufficient time for the delivery
balloon 172 to inflate and deliver the tissue penetrating members 140 into the
intestinal wall IW before
the obstructing material dissolves to open valve 173. Typically, this will be
close to the end 173e of the
tube 173t, but not quite so as to allow time for liquid to have to wick into
the tube lumen before it
reaches material 173m. According to one or more embodiments, once the
deflation valve 173 opens, it
not only serves to deflate the delivery balloon 172 but also the aligner
balloon 160 and deployment
balloon 130 since in many embodiments, all three are fluidically connected
(aligner balloon being
fluidically connected to delivery balloon 172 and the deployment balloon 130
being fluidically
connected to aligner balloon 160). Opening of the deflation valve 173 can be
facilitated by placing it on
the end 172e of the delivery balloon 172 that is forced out of capsule 120 by
inflation of the aligner
balloon 160 so that the deflation valve has good exposure to liquids in the
small intestine. Similar tube
deflation valves 173 can also be positioned on one or both of aligner balloon
162 and the deployment
balloon 130. In these later two cases, the obstructing material in the tube
valve can be configured to
degrade over a time period to allow sufficient time for inflation of delivery
balloon 172 and
advancement of tissue penetrating members 140 into the intestinal wall.
101351 Additionally, as a further backup for insured deflation, one or more
puncture elements 182
can be attached to the inside surface 124 of the capsule such that when a
balloon (e.g., balloon 130, 160,
172) fully inflates it contacts and is punctured by the puncture element 182.
Puncture elements 182 can
comprise short protrusions from surface 124 having a pointed tip. In another
alternative or additional
embodiment of means for balloon deflation, one or more of the tissue
penetrating members 140 can be
directly coupled to the wall of
- 51 -
Date Regue/Date Received 2023-01-12
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
172w of balloon 172 and configured to tear away from the balloon when they
detach, tearing
the balloon wall in the process.
[0136] A discussion will now be presented of tissue penetrating members 140.
In various
embodiments, tissue penetrating member 140 can be fabricated from various
drugs and other
therapeutic agents 101, one or more pharmaceutical excipients (e.g.,
disintegrants, stabilizers,
etc.) and one or more biodegradable polymers. The later materials chosen to
confer desired
structural and material properties to the penetrating member (for example,
column strength
for insertion into the intestinal wall, or porosity and hydrophilicity for
control the release of
drug). Referring now to Figs. 18a-18f, in many embodiments, the penetrating
member 140
can be formed to have a shaft 144 and a needle tip 145 or other pointed tip
145 so as to
readily penetrate tissue of the intestinal wall as shown in the embodiment of
Fig. 18a. In
preferred embodiments, tip 145 has a trocar shape as is shown in the
embodiment of Fig. 18c.
Tip 145 may comprise various degradable materials (within the body of the tip
or as a
coating), such as sucrose or other sugar which increase the hardness and
tissue penetrating
properties of the tip. Once placed in the intestinal wall, the penetrating
member 140 is
degraded by the interstitial fluids within the wall tissue so that the drug or
other therapeutic
agent 101 dissolves in those fluids and is absorbed into the blood stream. One
or more of the
size, shape and chemical composition of tissue penetrating member 140 can be
selected to
allow for dissolution and absorption of drug 101 in a matter of seconds,
minutes or even
hours. Rates of dissolution can be controlled through the use of various
disintegrants known
in the pharmaceutical arts. Examples of disintegrants include, but are not
limited to, various
starches such as sodium starch glycolate and various cross linked polymers
such as
carboxymethyl cellulose. The choice of disintegrants can be specifically
adjusted for the
environment within the wall of the small intestine.
101371 Tissue penetrating member 140 will also typically include one or more
tissue
retaining features 143 such as a barb or hook to retain the penetrating member
within the
tissue of the intestinal wall IW after advancement. Retaining features 143 can
be arranged in
various patterns 143p to enhance tissue retention such as two or more barbs
symmetrically or
otherwise distributed around and along member shaft 144 as is shown in the
embodiments of
Figs. 18a and 18b. Additionally, in many embodiments, penetrating member will
also
include a recess or other mating feature 146 for attachment to a coupling
component on
delivery mechanism 170.
[0138] Tissue penetrating member 140 is desirably configured to be detachably
coupled to
platform 175 (or other component of delivery mechanism 170), so that after
advancement of
-52-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
the tissue penetrating member 140 into the intestinal wall, the penetrating
member detaches
from the balloon. Detachability can be implemented by a variety of means
including: i) the
snugness or fit between the opening 174 in platform 175 and the member shaft
144); ii) the
configuration and placement of tissue retaining features 143 on penetrating
member 140; and
iii) the depth of penetration of shaft 144 into the intestinal wall. Using one
or more of these
factors, penetrating member 140 be configured to detach as a result of balloon
deflation
(where the retaining features 143 hold the penetrating member 140 in tissue as
the balloon
deflates or otherwise pulls back away from the intestinal wall) and/or the
forces exerted on
capsule 120 by a peristaltic contraction of the small intestine.
101391 In a specific embodiment, the detachability and retention of tissue
penetrating
member 140 in the intestinal wall IW can be enhanced by configuring the tissue
penetrating
member shaft 144 to have an inverse taper 144t as is shown in the embodiment
of Fig.18c.
The taper 144t on the shaft 144 is configured such that the application of
peristaltic
contractile forces from the intestinal wall on the shaft result in the shaft
being forced inward
(e.g., squeezed inward). This is due to the conversion by shaft taper 144t of
the laterally
applied peristaltic force PF to an orthogonal force OF acting to force the
shaft inward into the
intestinal wall. In use, such inverse tapered shaft configurations serve to
retain tissue
penetrating member 140 within the intestinal wall so as to detach from
platform 175 (or other
component of delivery mechanism 170) upon deflation of balloon 172. In
additional
embodiments, tissue penetrating members 140 having an inverse tapered shaft
may also
include one or more retaining features 143 to further enhance the retention of
the tissue
penetrating member within intestinal wall IW once inserted.
101401 As described above, in various embodiments, tissue penetrating member
140 can be
fabricated from a number of drugs and other therapeutic agents 101. Also
according to one
or more embodiments, the tissue penetrating member may be fabricated entirely
from drug
101 or may have other constituent components as well, e.g., various
pharmaceutical
excipients (e.g., binders, preservatives, disintegrants, etc.), polymers
conferring desired
mechanical properties, etc. Further, in various embodiments one or more tissue
penetrating
members 140 can carry the same or a different drug 101 (or other therapeutic
agent) from
other tissue penetrating members. The former configuration allows for the
delivery of greater
amounts of a particular drug 101, while the later, allows two or more
different drugs to be
delivered into the intestinal wall at about the same time to facilitate drug
treatment regimens
requiring substantial concurrent delivery of multiple drugs. In embodiments of
device 110,
having multiple delivery assemblies 178 (e.g., two, one on each face of
balloon 172), a first
-53-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
assembly 178' can carry tissue penetrating members having a first drug 101 and
a second
assembly 178" can carry tissue penetrating members having a second drug 101.
[0141] Typically, the drug or other therapeutic agent 101 carried by the
tissue penetrating
member 140 will be mixed in with a biodegradable material 105 to form tissue
penetrating
member 140. Material 105 may include one or more biodegradable polymers such
as PGLA,
cellulose, as well as sugars such as maltose or other biodegradable material
described herein
or known in the art. In such embodiments, the penetrating member 140 may
comprise a
substantially heterogeneous mixture of drug 101 and biodegradable material
105.
Alternatively, the tissue penetrating member 140 may include a portion 141
formed
substantially from biodegradable material 105 and a separate section 142 that
is formed from
or contains drug 101 as shown in the embodiment of Fig.18d. In one or more
embodiments,
section 142 may correspond to a pellet, slug, cylinder or other shaped section
142s of drug
101. Shaped section 142s may be pre-formed as a separate section which is then
inserted into
a cavity 142c in tissue penetrating member 140 as is shown in the embodiments
of Figs. 18e
and 181 Alternatively, section 142s may be formed by adding of drug
preparation 100 to
cavity 142c. In embodiments, where drug preparation 100 is added to cavity
142c,
preparation may be added in as a powder, liquid, or gel which is poured or
injected into
cavity 142c. Shaped section 142s may be formed of drug 101 by itself or a drug
preparation
containing drug 101 and one or more binders, preservatives, disintegrates and
other
excipients. Suitable binders include polyethylene glycol (PEG) and other
binders known in
the art. In various embodiments, the PEG or other binder may comprise in the
range of about
to 90% weight percent of the section 142s, with a preferred embodiment for
insulin
preparations of about 25-90 weight percent. Other excipients which may be used
for binders
may include, PLA, PLGA, Cyclodextrin, Cellulose, Methyl Cellulose, Maltose,
Dextrin,
Sucrose and PGA. Further information on the weight per cent of excipients in
section 142
may be found in Table 3. For ease of discussion, section 142 is referred to as
a pellet in the
table, but the data in the table is also applicable to other embodiments of
section 142
described herein.
[0142] In various embodiments, the weight of tissue penetrating member 140 can
range
between about 10 to about 15 mg, with larger and smaller weights contemplated.
For
embodiments of tissue penetrating member 140 fabricated from maltose, the
weight can
range from about 11 to 14 mg. In various embodiments, depending upon the drug
101 and
the desired delivered dose, the weight percent of drug in member 140 can range
from about
0.1 to about 15%. In exemplary embodiments these weight per cents correspond
to
-54-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
embodiments of members 140 fabricated from maltose or PGLA, however they are
also
applicable to any of the biodegradable materials 105 used in the fabrication
of members 140.
The weight percent of drug or other therapeutic agent 101 in member 140 can be
adjusted
depending upon the desired dose as well as to provide for structural and
stoichiometric
stability of the drug and also to achieve a desired concentration profile of
the drug in the
blood or other tissue of the body. Various stability tests and models) known
in the art (e.g.,
using the Arrhenius equation and/or known rates of drug chemical degradation
may be used
to make specific adjustments in the weight per cent range. Table 3 lists the
dose and weight
per cent range for insulin and a number of other drugs which may be delivered
by tissue
penetrating member 140. In some cases the tables lists ranges as well a single
value for the
dose, It should be appreciated that these values are exemplary and other
values recited
herein including the claims are also considered. Further, embodiments of the
invention also
consider variations around these values including for example, 1, 5, 10,
25, and even
larger variations. Such variation are considered to fall within the scope of
an embodiment
claiming a particular value or range of values. The table also lists the
weight percentage of
drug in in section 142 for various drugs and other therapeutic agents, where
again for ease of
discussion, section 142 is referred to as a pellet. Again, embodiments of the
invention
consider the variations described above.
Table 3
Drug Dose Via Capsule** % Weight
of Drug in the needle
tnsulm 4-9 units, 5 -30 units, 1-50 Units 2 - 15%
Exenatide 1-10 ug, 1-20 ug, 10 ug <1%, 0.1 -1 %
Liraglutide 0.1-1 mg, 0.5-2 mg, 0.6 mg 3 - 6%
Pramlintide 15 - 120 ug 0.1 - 1 %
Growth Hormone 0.2 - 1 mg, 0.1-4 mg 2 - 10%
Somatostatin and Analogs 50 - 600 ug, 10-100 ug 0.3 - 8%
GnRH and Analogs 0.3 - 1.5 mg, 0.1 -2 mg 2 - 15%
Vasopressin 2- 10 units <1%, 0.1 - 1 %
PTH and Analogues 0.1 to 10 ug, 10-30 ug, 20 ug 1 - 2%
Interferons and analogs
1. For Multiple Sclerosis 0.03 - 0.25 mg
0.1 - 3%
2. For Hep B and HepC 6-20 ug 0.05 - 0.2 %
Adalimumab 1-5 mg,2-4 mg 8 ¨ 12%
-55-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
Infliximab 1-10, 5 mg 8 ¨ 12 %
Etanercept 1-5 mg, 3 mg 8- 12 %
Natalizumab 1-5 mg, 3 mg 8 ¨ 12 %
[0143] Tissue penetrating member 140 can be fabricated using one or more
polymer and
pharmaceutical fabrication techniques known in the art. For example, drug 101
(with or
without biodegradable material 105) can be in solid form and then formed into
the shape of
the tissue penetrating member 140 using molding, compaction or other like
method with one
or more binding agents added. Alternatively, drug 101 and/or drug preparation
100 may be
in solid or liquid form and then added to the biodegradable material 105 in
liquid form with
the mixture then formed into the penetrating member 140 using molding or other
forming
method known in the polymer arts.
[0144] Desirably, embodiments of the tissue penetrating member 140 comprising
a drug or
other therapeutic agent 101 and degradable material 105 are formed at
temperatures which do
not produce any substantial thermal degradation of drug including drugs such
as various
peptides and proteins. This can be achieved through the use of room-
temperature curing
polymers and room temperature molding and solvent evaporation techniques known
in the
art. In particular embodiments, the amount of thermally degraded drug or other
therapeutic
agent within the tissue penetrating member is desirably less than about 10% by
weight and
more preferably, less than 5% and still more preferably less than 1%. The
thermal
degradation temperature(s) for a particular drug are either known or can be
determined using
methods known in the art and then this temperature can be used to select and
adjust the
particular polymer processing methods (e.g., molding, curing, solvent
evaporation methods
etc.) to minimize the temperatures and associated level of drug thermal
degradation.
[0145] A description will be provided of delivery mechanism 170. Typically,
the
mechanism will comprise a delivery assembly 178 (containing tissue penetrating
members
140) that is attached to delivery balloon 172 as is shown in the embodiment of
Figs. 16a and
16b. Inflation of the delivery balloon provides a mechanical force for
engaging delivery
assembly 172 outwards from the capsule and into the intestinal wall IW so as
to insert tissue
penetrating members 140 into the wall. In various embodiments, the delivery
balloon 172
can have an elongated shape with two relatively flat faces 172f connected by
an articulated
accordion-like body 172b. The flat faces 172f can be configured to press
against the
intestinal wall (1W) upon expansion of the balloon 172 so as to insert the
tissue penetrating
members (TPMs) 140 into the intestinal wall. 1PMs 140 (either by themselves or
as part of a
-56-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
delivery assembly 178 described below) can be positioned on one or both faces
172f of
balloon 172 to allow insertion of drug containing TPMs 40 on opposite sides of
the intestinal
wall. The faces 172f of balloon 172 may have sufficient surface area to allow
for placement
of a number of drug containing TPMs 140 on each face.
[0146] Referring now to Fig. 19, a description will now be provided of the
assembly of
delivery assembly 178. In a first step 300, one or more tissue penetrating
members 140 can
be detachably coupled to a biodegradable advancement structure 175 which may
correspond
to a support platform 175 (also known as platform 175). In preferred
embodiments, platform
175 includes one or more openings 174 for insertion of members 140 as shown in
step 300.
Openings 174 are sized to allow for insertion and retention of members 140 in
platform 175
prior to expansion of balloon 172 while allowing for their detachment from the
platform upon
their penetration into the intestinal wall. Support platform175 can then be
positioned within a
carrying structure 176 as shown in step 301. Carrying structure 176 may
correspond to a well
structure 176 having side walls 176s and a bottom wall 176b which define a
cavity or
opening 176c. Platform 175 is desirably attached to inside surface of bottom
wall 176b using
adhesive or other joining methods known in the art. Well structure 176 can
comprise various
polymer materials and may be formed using vacuum forming techniques known in
the
polymer processing arts. In many embodiments, opening 176o can be covered with
a
protective film 177 as shown in step 302. Protective film 177 has properties
selected to
function as a barrier to protect tissue penetrating members 140 from humidity
and oxidation
while still allowing tissue penetrating members 140 to penetrate the film as
is described
below. Film 177 can comprise various water and/or oxygen impermeable polymers
which
are desirably configured to be biodegradable in the small intestine and/or to
pass inertly
through the digestive tract. It may also have a multi-ply construction with
particular layers
selected for impermeability to a given substance, e.g., oxygen, water vapor
etc. In use,
embodiments employing protective film 177 serve to increase the shelf life of
therapeutic
agent 101 in tissue penetrating members 140, and in turn, the shelf life of
device 110.
Collectively, support platform 175 attached tissue penetrating members 140,
well structure
176, and film 177 can comprise a delivery assembly 178. Delivery assemblies
178 having
one or more drugs or therapeutic agents 101 contained within tissue
penetrating member 40
or other drug delivery means can be pre-manufactured, stored and subsequently
used for the
manufacture of device 110 at a later date. The shelf life of assembly 178 can
be further
enhanced by filling cavity 176c of the sealed assembly 178 with an inert gas
such as nitrogen.
-57-
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
[0147] Referring back to Figs. 16a and 16b, assemblies 178 can be positioned
on one or
both faces 172f of balloon 172. In preferred embodiments, assemblies 178 are
positioned on
both faces 172f (as shown in Fig. 16a) so as to provide a substantially equal
distribution of
force to opposite sides of the intestinal wall IW upon expansion of balloon
172. The
assemblies 178 may be attached to faces 172f using adhesives or other joining
methods
known in the polymer arts. Upon expansion of balloon 172, TPMs 140 penetrate
through
film 177, enter the intestinal wall IW and are retained there by retaining
elements 143 and/or
other retaining features of TPM 140 (e.g., an inverse tapered shaft 144t) such
that they detach
from platform 175 upon deflation of balloon 172.
[0148] In various embodiments, one or more of balloons 130, 160 and 172 can be
packed
inside capsule 120 in a folded, furled or other desired configuration to
conserve space within
the interior volume 124v of the capsule. Folding can be done using preformed
creases or
other folding feature or method known in the medical balloon arts. In
particular
embodiments, balloon 130, 160 and 172 can be folded in selected orientations
to achieve one
or more of the following: i) conserve space, ii) produce a desired orientation
of a particular
inflated balloon; and iii) facilitate a desired sequence of balloon
inflations. The embodiments
shown in Figs. 15a-15f illustrate an embodiment of a method of folding and
various folding
arrangements. However, it should be appreciated that this folding arrangement
and the
resulting balloon orientations are exemplary and others may also be used. In
this and related
embodiments, folding can be done manually, by automated machine or a
combination of
both. Also in many embodiments, folding can be facilitated by using a single
multi-balloon
assembly 7 (herein assembly 7) comprising balloons 130, 160, 170; valve
chamber 158 and
assorted connecting tubings 162 as is shown in the embodiments of Figs. 13a
and 13b. Fig.
13a shows an embodiment of assembly 7 having a single dome construction for
balloon 130,
while Fig. 13b shows the embodiment of assembly 7 having dual balloon/dome
configuration
for balloon 130. Assembly 7 can be fabricated using a thin polymer film which
is vacuum-
formed into the desired shape using various vacuum forming and other related
methods
known in the polymer processing arts. Suitable polymer films include
polyethylene films
having a thickness in the range of about 0.003 to about 0.010", with a
specific embodiment of
0.005". In preferred embodiments, the assembly is fabricated to have a unitary
construction
so as to eliminate the need for joining one or more components of the assembly
(e.g.,
balloons 130,160, etc.). However, it is also contemplated for assembly 7 to be
fabricated
from multiple portions (e.g., halves), or components (e.g., balloons) which
are then joined
using various joining methods known in the polymer/medical device arts.
-58-
CA 2993215
[0149]
Referring now to Figs. 15a-15f, 16a-16b and 17a-17b, in a first folding step
210, balloon 160
is folded over onto valve fitting 158 with balloon 172 being flipped over to
the opposite side of valve
fitting 158 in the process (see Fig. 15a). Then in step 211, balloon 172 is
folded at a right angle to the
folded combination of balloon 160 and valve 158 (see Fig. 15b). Then, in step
212 for dual dome
embodiments of balloon 130, the two halves 130' and 130" of balloon 130 are
folded onto each other,
leaving valve 150 exposed (see Fig. 15c, for single dome embodiments of
balloon 130, is folded over
onto itself see Fig. 15e). A final folding step 213 can be done whereby folded
balloon 130 is folded
over 180 to the opposite side of valve fitting 158 and balloon 160 to yield a
final folded assembly 8 for
dual dome configurations shown in the Fig. 15e and a final folded assembly 8'
for single dome
configurations shown in Figs. 15e and 151 One or more delivery assemblies 178
are then be attached to
assembly 8 in step 214 (typically two the faces 172f of balloon 172) to yield
a final assembly 9 (shown
in the embodiments of Figs. 16a and 16b) which is then inserted into capsule
120. After an insertion
step 215, the final assembled version of device 110 with inserted assembly 9
is shown Figs. 17a and
17b.
[0150] Referring now to Figs. 20a-20i, a description will be provided of a
method of using device
110 to deliver medication 101 to a site in the GI tract such as the wall of
the small or large intestine. It
should be appreciated that the steps and their order is exemplary and other
steps and orders also
contemplated. After device 110 enters the small intestine SI, the cap coating
120c' is degraded by the
basic pH in the upper small intestine causing degradation of cap 120p' as
shown in step 400 in Fig. 20b.
Valve 150 is then exposed to fluids in the small intestine causing the valve
to begin degrade as is shown
in step 401 in Fig. 20c. Then, in step 402, balloon 130 expands (due to
generation of gas 169) as shown
in Fig. 20d. Then, in step 403, section 160' of balloon 160 begins to expand
to start to push assembly
178 out of the capsule body as shown in Fig. 20e. Then, in step 404, sections
160' and 160" of balloon
160 become fully inflated to completely push assembly 178 out of the capsule
body extending the
capsule length 1201 so as to serve to align capsule lateral axis 120AL with
the lateral axis of the small
intestine LAI as shown in Fig. 20f. During this time, valve 155 is beginning
to fail from the increased
pressure in balloon 60 (due to the fact that the balloon has fully inflated
and there is no other place for
gas 169 to go). Then, in step 405, valve 155 has completely opened, inflating
balloon 172 which then
pushes the now completely exposed assembly 178 (having been pushed completely
out of body 120p")
radially outward into the intestinal wall IW as shown in Fig. 20g. Then, in
step 406, balloon 172
continues to expand to now advance tissue penetrating members into the
intestinal wall IW as shown in
Fig. 20h. Then, in step
- 59 -
Date Regue/Date Received 2023-01-12
CA 02993215 2018-01-18
WSGR Docket No.: 42197-708602
407, balloon 172, (along with balloons 160 and 130) has deflated pulling back
and leaving
tissue penetrating members retained in the intestinal wall IW. Also, the body
portion 120p"of
the capsule has completely degraded (due to degradation of coating 120c")
along with other
biodegradable portions of device 110. Any portion not degraded is carried
distally through
the small intestine by peristaltic contraction from digestion and is
ultimately excreted.
1015111 The foregoing description of various embodiments of the invention has
been
presented for purposes of illustration and description. It is not intended to
limit the invention
to the precise forms disclosed. Many modifications, variations and refinements
will be
apparent to practitioners skilled in the art. For example, embodiments of the
swallowable
device can be sized and otherwise adapted for various pediatric and neonatal
applications as
well as various veterinary applications. Similarly dosages of therapeutic
agents delivered by
embodiments of the swallowable device can be titrated and/or otherwise
adjusted for
pediatric and neonatal applications. Also those skilled in the art will
recognize, or be able to
ascertain using no more than routine experimentation, numerous equivalents to
the specific
devices and methods described herein. Such equivalents are considered to be
within the
scope of the present invention and are covered by the appended claims below.
101521 Elements, characteristics, or acts from one embodiment can be readily
recombined
or substituted with one or more elements, characteristics or acts from other
embodiments to
form numerous additional embodiments within the scope of the invention.
Moreover,
elements that are shown or described as being combined with other elements,
can, in various
embodiments, exist as standalone elements. Hence, the scope of the present
invention is not
limited to the specifics of the described embodiments, but is instead limited
solely by the
appended claims.
-60-