Note: Descriptions are shown in the official language in which they were submitted.
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1
2
3
4
6
7 Apparatus and Method for Characterization of Acute Otitis
8 Media
9
Field of the Invention
11 The present invention relates to a device for the
12 detection of middle ear effusion with discrimination of
13 fluid type. In particular, the invention relates to the
14 characterization of middle ear effusion behind the tympanic
membrane by stimulating the tympanic membrane using a low
16 frequency excitation such as acoustic and measuring the
17 displacement behavior with a comparatively higher frequency
18 excitation such as ultrasound.
19
Background of the Invention
21 Acute otitis media (ACM) is an inflammatory process in
22 the middle ear and is the most common clinical condition
23 seen by pediatricians in children fifteen years and
1
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 younger. ACM is generally associated with the presence of a
2 middle ear effusion and is considered a middle ear
3 inflammation. Complications of undiagnosed ACM can include
4 hearing loss. Left untreated in children, recurrent ACM can
also lead to delays in the development of speech and
6 language skills.
7 There are two key factors in the diagnosis of ACM:
8 detection of the presence of effusion, and characterization
9 of the type of effusion as either serous, mucoid, purulent
or combinations of these. Decision by the health care
11 provider regarding appropriate treatment relies on
12 confirmation of both the presence of effusion and its type.
13 Health care practitioners use a variety of tests to
14 evaluate a patient suspected of having ACM. The only
definitive tests for ACM are myringotomy and
16 tympanocentisis, procedures which involve direct aspiration
17 of fluid from the middle ear by puncturing the tympanic
18 membrane and drawing fluid, followed by visual and
19 biochemical analysis of the fluid. These are invasive
procedures performed in a surgical setting under
21 anesthesia. Because they are invasive and have significant
22 associated risks of complications, myringotomy and
2
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 tympanocentisis are not used as standard diagnostic methods
2 for ACM except in research settings.
3 Several other non-invasive diagnostic tests are
4 available for evaluating ACM, including acoustic
reflectometry, tympanometry, pneumatic otoscopy, and
6 otoscopy, however, none of these tests achieves the
7 diagnostic accuracy of invasive myringotomy and
8 tympanocentisis; the overall likelihood of obtaining an
9 accurate diagnosis using any of the non-invasive methods is
no better than 50%. More importantly, the various non-
11 invasive methods are useful only in identifying the
12 presence of middle ear effusion; they provide no
13 information regarding the type of effusion. Because of the
14 risks associated with undiagnosed ACM, and the recognized
unreliability of the non-invasive diagnostic tests,
16 patients who are diagnosed with middle ear effusions based
17 on any of these non-invasive tests are often prescribed
18 antibiotics. In many instances, these patients do not have
19 ACM. In addition to the increased cost burden of
unnecessary antibiotic treatment, the patients are exposed
21 to the side effects of antibiotics and the attendant and
22 significant risk of developing antibiotic resistance.
3
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 Acute otitis media is one of the most common causes of
2 childhood health issues, which include for example,
3 bacterial infections, antibiotic overuse, hearing loss, and
4 surgeries. ACM is responsible for more than 12 million
office visits nationwide per year, accounting for over 50
6 percent of all pediatric antibiotic prescriptions and as
7 much as $5 billion in annual costs. The number of operative
8 procedures performed due to unresolved AOM in the United
9 States is estimated at about 600,000 per year.
The majority of children have at least one episode of
11 ACM by the time they are two years of age. ACM is
12 characterized by ear pain, fever, occasional rupture of the
13 ear drum, and findings of middle ear inflammation,
14 including fluid in the middle ear. About 10 percent of
children have recurrent ACM, and these children account for
16 around 40 percent of all ACM episodes. The prevalence of
17 ACM in the United States is increasing. Thus, current
18 diagnostic and treatment methods are not lowering the rate
19 of ACM in the United States.
OM is fundamentally defined by the presence of an
21 effusion in the middle ear. In ACM, the middle ear effusion
22 ("MEE") is induced by infective agents and is often thin or
23 serous with viral infection and thicker and purulent with
4
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 bacterial infection. Acute MEE may persist, even with
2 appropriate antimicrobial treatment. After 30 days, the MEE
3 is termed as chronic, and the condition is referred to most
4 commonly as otitis media with chronic effusion or "OME."
Chronic MEE may be thin and watery, purulent, or, most
6 commonly, thick and mucoid. Mucoid effusion is the hallmark
7 of OME and is often called "glue ear" because of its high
8 viscosity. Because each type of MEE has a different
9 prognosis and treatment, the ability to delineate the type
of the effusion is of great clinical value.
11 In spite of decades of research, optimal management of
12 ON remains controversial. In a recent prospective study,
13 antibiotic treatment of ON accounted for more than 90
14 percent of all antibiotic use during the first two years of
life. It has been estimated that distinguishing AOM from
16 ONE and deferring antibiotics for OME would avoid 6 to 8
17 million courses of unnecessary antibiotic therapy annually.
18 While antibiotics reduce pain symptoms in ACM, their
19 widespread use in ACM has led to an alarming increase in
the prevalence of resistant organisms worldwide without any
21 substantial decrease in complications or sequelae of ACM.
22 Given the high spontaneous resolution rate of ACM, there
23 are serious questions about the need for antibiotics in
5
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 most cases. Thus, physicians and parents are frequently
2 uncertain about proper treatment because there are no
3 clear-cut clinical findings that might reliably predict
4 which cases will resolve spontaneously and which cases
would be better treated with an oral antibiotic. The recent
6 American Academy of Pediatrics 2014 guideline recommended
7 withholding antibiotic when uncertainty exists but did not
8 discuss ways and means to implement the guideline.
9 Many children with fever and a red tympanic membrane
("TM") have no MEE and thus do not have AOM. These children
11 do not benefit from antimicrobial therapy, even though many
12 receive it as a precaution.
13 Similar considerations apply to cases of persistent
14 MEE (OME). Detecting MEE is difficult without expensive
equipment, such as a tympanometer or an audiometer. While
16 screening tympanometers are available, they are not widely
17 used in primary care offices where the majority of cases of
18 AOM/OME are first seen. Acoustic reflectometry was
19 introduced 15 years ago as a method for primary physicians
and parents to indicate MEE presence. Although the
21 sensitivity and specificity of acoustic reflectometry is
22 similar to that of tympanometry, neither device will
23 predict which cases may resolve spontaneously and which
6
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 cases will require treatment. Moreover, neither device is
2 widely used in primary care offices. Chronic MEE is
3 therefore under-diagnosed in primary care practice.
4 ONE may cause hearing loss without other symptoms. The
adverse effects of ONE on hearing and on the development of
6 cognitive, linguistic, additive, and communicative skills
7 are of concern to parents and physicians alike. National
8 guidelines recommend waiting 3 to 6 months before surgical
9 removal of the MRS and insertion of a ventilation tube.
Some effusions cause substantial hearing loss. Typically,
11 middle ears that are impacted with the characteristic
12 viscous effusion (glue ear) are associated with substantial
13 hearing loss that may persist for years. Primary care
14 physicians, unlike ENT specialists, lack a robust clinical
method that can distinguish between a mucoid effusion (glue
16 ear) and one that contains a serous (watery) effusion,
17 which is more likely to resolve spontaneously.
18 One of the major sources of controversy about ON in
19 clinical practice is accuracy of diagnosis. Otoscopy, the
key examination technique, is a visual inspection of the TM
21 by which one may deduce the normal or abnormal middle ear.
22 The equipment and skills for otoscopy are variable.
23 Although with practice, many physicians become proficient
7
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 otoscopists, a monocular examination of the TM of a
2 struggling infant through a tiny speculum remains a
3 difficult and challenging maneuver. Often only a glimpse of
4 the TM is possible. Use of the binocular operating
microscope, which permits a 3D view of the TM, is the most
6 precise method of otoscopy and is widely used by ear, nose,
7 and throat specialists. However, this expensive equipment
8 is rarely found in primary care practices where the
9 majority of ACM diagnoses are made. Accordingly, only 40
percent of primary care pediatricians are confident about
11 their otoscopic findings.
12 The essential elements of otoscopy are a description
13 of: (1) the static characteristics of the TM (color,
14 position, translucency), (2) the contents of the middle ear
(air, ear effusion, other), and (3) the mobility of the TM
16 in response to externally applied air pressure (pneumatic
17 otoscopy). Determining the presence of effusion (liquid) in
18 the middle ear is the critical variable in making a
19 diagnosis of OME. Given that the effusion may vary in
amount and consistency from case to case and may be
21 obscured by the condition of the TM, it is fair to say that
22 even when done under ideal conditions (binocular
23 microscope, pneumatic speculum, and an anesthetized child),
8
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 the otoscopic conclusion regarding the presence or absence
2 of ear effusion may vary from observer to observer. Less
3 than half of pediatricians use pneumatic otoscopy. Similar
4 findings have been found in surveys of practicing
physicians and residents.
6 Tympanometry is an objective measure of the condition
7 of the middle ear. It is widely used in specialty clinics
8 for screening and for diagnostic confirmation. The
9 tympanometer displays the change in the acoustic immittance
of a 226 Hz transducer tone as the pressure in the ear
11 canal is varied in a range within -300 dekapascals (daPa)
12 to +200 daPa. The classic peaked curve indicates an air-
13 containing middle ear while a classic flat curve is
14 associated with middle ear effusion (assuming an intact
TM). Tympanometry is not widely used in primary care
16 offices because of equipment expense and training
17 requirements. The test does require a snug fit between the
18 probe and the ear canal; fitting tightly is not
19 objectionable for older or normal children. However, the
pressurization may cause mild discomfort in the presence of
21 an acute infection.
22 Audiometry often reveals a substantial conductive
23 hearing loss in OME. However, audiometry is expensive and
9
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 not widely used in primary care practice. Infants and
2 children are not difficult to test by experienced
3 audiologists. Audiometry is important in surgical planning
4 but is too nonspecific for evaluation of effusion type.
Acoustic reflectometry (measuring response of the TM
6 to a 1.8 to 4.4 kHz frequency sweep spectrum) was
7 introduced to meet the need for an objective, simple, and
8 safe clinical method for evaluating the condition of the
9 middle ear. While acoustic reflectometry is indeed simple,
safe, and inexpensive, it is too unreliable for making
11 treatment decisions and is used infrequently by physicians.
12 Accordingly, a more reliable, non-invasive method of
13 diagnosing Otitis Media with Effusion (OME) is needed.
14
16
17 Objects of the Invention
18 A first object of the invention is an apparatus and
19 method for detection of acute otitis media (AOM),
specifically inflammatory effusion of the middle ear.
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 A second object of the invention is an apparatus and
2 method for discernment of effusion fluid type in otitis
3 media with effusion (OME) of the middle ear.
4 A third object of the invention is an apparatus for
measurement of fluid viscosity having:
6 a speculum having an extent, the speculum having a
7 smaller outer and inner diameter on a first end of the
8 extent and a comparatively larger inner and outer diameter
9 on an opposite end of the extent;
the speculum having an ultrasound transducer
11 positioned to generate an ultrasound wave directed out of
12 said first end and into an ear canal and also receive
13 reflected ultrasound energy;
14 the speculum coupled to an excitation source for
displacement of a tympanic membrane with a static or
16 dynamic pneumatic excitation;
17 the apparatus actuating the tympanic membrane
18 excitation source and measuring tympanic membrane
19 displacement from a phase shift in ultrasound energy
reflected from a tympanic membrane;
21 thereafter forming an estimate of the viscosity of a
22 fluid which may be present on the far side of the tympanic
23 membrane based on the displacment characteristics of a
11
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 tympanic membrane interacting with the pneumatic
2 excitation.
3 A fourth object of the invention is an ultrasound
4 signal processor for measurement of the viscosity of a
fluid behind a tympanic membrane, the measurement including
6 an excitation resulting in the displacement of the tympanic
7 membrane using the excitation source, the excitation source
8 being sub-audible, audible, or super-audible, the
9 excitation source being either pressure-neutral, pressure-
offset, or periodic, the estimate of fluid viscosity
11 performed by measuring the phase shift of reflected
12 continuous wave (CW) or pulsed ultrasound compared to a
13 transmitted waveform phase.
14
16 Summary of the Invention
17 A speculum tip includes an ultrasound transducer for
18 sending and receiving ultrasound energy through an ear
19 canal and a comparatively low frequency tympanic membrane
excitation source. The tympanic membrane excitation source
21 generates a subtle movement of the tympanic membrane during
22 an interval coincident with an ultrasound transmitter
23 delivering acoustic wave ultrasound energy to the tympanic
12
1 membrane either in CW form or in pulsed form. A receiver
2 for ultrasound reflected from the tympanic membrane
3 measures displacement of the tympanic membrane as a phase
4 change in the received signal when compared to the transmit
frequency, thereby indicating a temporal displacement of
6 the tympanic membrane. An analysis of the temporal
7 displacement of the tympanic membrane, as measured by the
8 phase shifts of the reflected ultrasound in response to the
9 pneumatic excitation coupled to the tympanic membrane, in
combination with comparison to the temporal displacement or
11 from templates or metrics associated with the delay in and
12 amplitude of response between the excitation stimulus to
13 and ultrasound response from the tympanic membrane, is used
14 to determine the viscosity of the fluid behind the tympanic
membrane. Measurement of the viscosity of the fluid behind
16 the tympanic membrane is thereafter used to characterize
17 the type of effusion fluid present in the middle ear as one
18 of: no fluid, serous fluid, or purulent fluid.
19
According to one aspect of the invention there is
provided a device for characterizing a tympanic membrane,
21 the device comprising:
13
Date Recue/Date Received 2021-06-08
1 an excitation generator configured to produce an
2 excitation comprising a pressure modulation in air for
3 application to the tympanic membrane to cause a
4 displacement;
a transducer configured to transmit acoustic waves
6 towards the tympanic membrane during a transmit interval
7 and to receive reflected acoustic waves from the tympanic
8 membrane during a receive interval;
9 an optical source configured to provide a visual
indication of a region of insonification on the tympanic
11 membrane, thereby allowing direction of the acoustic waves
12 from the transducer to a region of interest on the tympanic
13 membrane;
14 a phase and amplitude detector configured to receive a
phase and an amplitude of the reflected acoustic waves,
16 compare a phase of the transmit signal to the phase of the
17 reflected acoustic waves, and generate a phase output; and
18 a response analyzer configured to compare the phase
19 output to the excitation and determine one or more of a
viscosity of a fluid adjacent to the tympanic membrane or a
21 mobility of the tympanic membrane based on the comparison.
13a
Date Recue/Date Received 2021-06-08
1 According to another aspect of the invention
2 there is provided a device for characterizing a tympanic
3 membrane, the device comprising:
4 a speculum tip configured to be inserted into an ear
canal;
6 an excitation generator coupled to the speculum tip
7 and configured to generate an excitation waveform
8 comprising a pressure modulation in an air volume to cause
9 a displacement in the tympanic membrane;
an ultrasound transducer distinct from the excitation
11 generator and positioned in the speculum tip, wherein the
12 ultrasound transducer is configured to transmit ultrasound
13 energy and to receive reflected ultrasound energy from the
14 tympanic membrane;
a transmit/receive switch coupled to the ultrasound
16 transducer;
17 a receiver configured to amplify reflected ultrasound
18 energy received from the ultrasound transducer to form a
19 received signal, wherein the received signal comprises a
phase and amplitude of the reflected ultrasound energy;
13b
Date Recue/Date Received 2021-06-08
1 a detector configured to generate a plurality of phase
2 and amplitude measurements each comprising a difference
3 between the transmitted ultrasound energy and the received
4 signal; and
a signal analyzer configured to compare the plurality
6 of phase and amplitude measurements to the excitation
7 waveform and form an effusion metric.
8 According to a further aspect of the invention
9 there is provided a method for characterizing a tympanic
membrane, the method comprising:
11 applying an excitation to the tympanic membrane, the
12 excitation comprising a pressure modulation;
13 transmitting transmit acoustic waves toward the
14 tympanic membrane during a transmit interval;
receiving reflected acoustic waves from the tympanic
16 membrane during a receive interval;
17 comparing a phase of the transmitted transmit acoustic
18 waves to a phase of the received reflected acoustic waves;
19 generating a phase output in response to the
13c
Date Recue/Date Received 2021-06-08
1 comparison of the phases of the transmitted transmit
2 acoustic waves and the received reflected acoustic waves;
3 comparing the phase output to the excitation; and
4 determining one or more of a displacement or a
mobility of the tympanic membrane in response to the
6 comparison of the phase output to the excitation.
7
8 Brief Description of the Drawings
13d
Date Recue/Date Received 2021-06-08
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
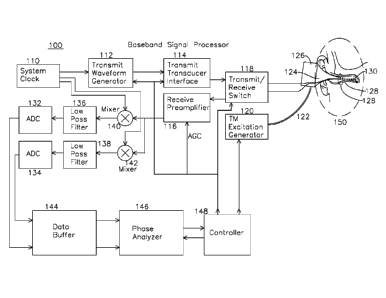
1 Figure 1 is a block diagram of a signal processor
2 system for estimating the characteristics of a fluid behind
3 a tympanic membrane.
4 Figure íA is a detail view of the speculum tip of
figure 1.
6 Figure 1B is a cross section view of figure 1A.
7 Figure 1C shows a view of a tympanic membrane and
8 region of illumination and insonification.
9 Figure 2 is a block diagram as in figure 1 where the
signal processor operates directly on received ultrasound
11 echoes.
12 Figure 3 shows waveforms for the system of figure 1.
13 Figure 4A shows a plot for a sinusoidal excitation
14 applied to an ear canal with a tympanic membrane response
with a phase delay and amplitude level.
16 Figure 4B shows a plot for a step excitation applied
17 to an ear canal with a tympanic membrane response having a
18 phase delay and amplitude level.
19 Figure 40-1 shows a plot of a sinusoidal TM
displacement generating more than +/-1800 of phase shift.
14
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 Figure 4C-2 shows the acquired data with phase wrapped
2 from the large phase shifts of figure 4C-1.
3 Figure 40-3 shows a plot of an unwrapped phase
4 estimate from figure 4C-2.
Figure 5 shows a OW signal processor for continuous
6 interrogation of a tympanic membrane in response to an
7 excitation generator.
8 Figure SA shows a detail view of the transmit
9 transducer and receive transducer of figure 5.
Figure 6 shows the waveforms for the OW system of
11 figure 5.
12 Figure TA is a plot of a sinusoidal excitation source
13 and associated tympanic membrane displacement response.
14 Figure 7B is a plot of a step excitation source and
associated tympanic membrane displacement response.
16
17
18
19 Detailed Description of the Invention
Figure 1 shows a signal processor for an example
21 embodiment of a tympanic membrane characterization system.
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 Region 150 (shown in magnified view figure 1A) includes a
2 cross section view of a middle ear and tympanic membrane
3 130 of a subject being examined. The tympanic membrane
4 130 is interrogated by an ultrasound beam 128 from an
ultrasound transducer 160 (shown in figure 1A) which is
6 optionally mounted on the inner surface of a speculum tip
7 124, and is detachable from an otoscope speculum mounting
8 adapter 126. In one embodiment of the invention, an
9 optical source 161 seen in the figure 1B cross section view
of figure TA, generates a visual indication the region of
11 insonification by the ultrasound by illumination of a
12 target or region of the tympanic membrane within the ear
13 canal, as seen in figure 1C. Figure 1C shows the view of
14 the tympanic membrane as seen through the speculum,
including the tympanic membrane 174, "cone of light" 176,
16 which is a reflective region of the TM which is normal to
17 incident optical illumination and easily located. The
18 optical source 161 may illuminate a small spot 172
19 indicating the center of the region of ultrasonic
insonification 170, or alternatively the spot 172 may be
21 coincident with the ultrasonically insonified region 170.
22 The primary function of the optical source 161 is to
23 provide guidance to a central region 170 of the TM which is
16
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 most likely to provide diagnostic utility in terms of the
2 analysis of TM displacement as a function of the pressure
3 challenge. The optical source 161 may be a visible
4 spectrum semiconductor laser diode, a light emitting diode,
or any other optical emitter which indicates the extent of
6 the region insonified by ultrasound energy and reflecting
7 ultrasound energy for measurement. Preferably, the optical
8 source illuminates a region corresponding to the beam
9 profile of the ultrasonic transducer at the tympanic
membrane. The otoscope mounting adapter 126 and speculum
11 tip 124 have a common interior volume which provides for
12 coupling of dynamic pressures from tympanic membrane
13 excitation generator 120 through hose 122 to the ear canal
14 where the air pressures result in displacement of the
tympanic membrane 130. The excitation generator 120 may
16 generate pressure variations which are coupled into the ear
17 canal through the speculum tip 126. The excitation
18 generator may produce any suitable pressure modulation for
19 displacement of the tympanic membrane, including a sub-
audio frequency below 20Hz, an audio frequency from 20Hz to
21 20Khz, or a super-audio frequency above 20Khz. The nature
22 of the pressure excitation generated by the excitation
23 generator may be an impulsive step or delta (impulse)
17
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 generation, a sinusoidal pressure excitation, a square wave
2 excitation, or any combination of these, and the excitation
3 may be a gated burst or continuous. The pressure
4 excitation may be provided with or without a static
positive or negative pressure bias. Speculum tip 124 also
6 has an associated ultrasound transducer 160 with electrical
7 leads 162 and 164 coupled to transmit receive switch 118.
8 Ultrasound transducer 160 generates ultrasound beam 128
9 which is directed to a central region of the tympanic
membrane 130. A controller 148 generates a variety of
11 control signals which are distributed through the signal
12 processor 100. A system reference clock 110 may be derived
13 from a temporally stable clock source, and the reference
14 clock liU may also be used for demodulation of the received
signal. System reference clock 110 is coupled to a transmit
16 waveform generator 112 which generates a pulse train at or
17 near the center frequency of transducer 160, transmit
18 transducer interface 114 performs voltage level shifting
19 and any required amplification before coupling to the
transmit/receive switch 118, which couples the waveforms
21 from transmit interface 114 to the ultrasonic transducer
22 160 via leads 162 and 164. The ultrasound transducer 160
23 generates and directs the ultrasonic energy in beam 128 to
18
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 the tympanic membrane. Reflected energy from the tympanic
2 membrane is coupled from the transducer 160 back through
3 leads 162 and 164 to the transmit/receive switch 118, where
4 it is directed to the receive preamplifier 116, which
boosts the signal level, and optionally provides automatic
6 gain control through a gain control input from controller
7 148. The output of the receive preamplifier 116 is applied
8 to quadrature mixers 140 and 142, where a quadrature clock
9 from clock generator 110 at the ultrasound transmitting
frequency generates a quadrature output comprising an I
11 (in-phase) baseband channel and Q (quadrature, or 90
12 degrees separated) baseband channel, which are coupled to
13 identical low pass filters 136 and 138, each of which has a
14 respective analog to digital converter 132 and 134, the
output of which is stored in data buffers 144, one for each
16 I and Q channel. The gain control applied to preamplifier
17 116 is set to place the I and Q signals in an optimum
18 converter range for the A/D converters 132 and 134. When
19 the received signal is mixed with the reference clock in
this manner, each transmit pulse generates a single phase
21 value, and over a series of transmit events this sequence
22 of phase differences is used by the phase and amplitude
23 analyzer 146 to estimate the temporal displacement of
19
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 tympanic membrane 130. In one embodiment of the invention,
2 the transmit clock coupled to the transducer during the
3 transmit interval is derived from system clock 110, which
4 is substantially at the center frequency of the transducer.
In an example embodiment where the phase and amplitude
6 analyzer 146 examines primarily the phase of the returned
7 signal, the system clock, at the transmit rate, is also
8 applied to quadrature mixers 140 and 142 during the receive
9 interval to compare the receive signal phase to the system
clock (at the original transmit frequency) to generate a
11 phase difference between the transmitted pulse and the
12 reflected pulse. This phase value may be compared over one
13 or more cycles of the receive signal to establish an
14 average phase value for that particular receive interval,
and then each phase value from each receive interval
16 assembled to provide a continuous estimate of tympanic
17 membrane displacement, based on the wavelength of the
18 acoustic wave and the phase value measured. In another
19 example embodiment, the phase and/or amplitude analyzer 146
may operate on the amplitude of the received signal, which
21 may be analyzed to provide information about the quality of
22 the phase estimate made from the data (such as from signal
23 to noise metrics), or the amplitude of the signal may be
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 analyzed to provide a metric such as db/Mhz-cm falloff, or
2 the amplitude profile may provide an effusion metric which
3 indicates whether fluid is present behind the tympanic
4 membrane based on the strength and characteristic of the
reflection. In general, the effusion metric is any phase
6 or amplitude derived metric from the data presented to the
7 amplitude and phase analyzer 146 which provides a
8 measurement of mobility of the TM, where the mobility is
9 preferentially associated with the presence or absence of
effusion in the middle ear for diagnosis of OM. Controller
11 148 which generates the TM excitation 120 also reads the
12 output of phase and amplitude analyzer 146 over the
13 duration of excitation generator 120 activity, and
14 optionally the amplitude of the reflected signal, to derive
a temporal response of the tympanic membrane to the
16 pneumatic excitation provided through speculum tip 124.
17 The pneumatic excitation may be any sub-audio, audio, or
18 super-audio frequency or pulse as previously described.
19 Figure 2 shows an alternate embodiment of the signal
processor of figure 1, where the signal processor is
21 performing direct sampling of the RF signal from the
22 transducer, rather than using quadrature mixing to baseband
23 of the RF signal. System clock 210 generates the transmit
21
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 clock, which is coupled to transmit waveform generator 112.
2 The operation of transmit waveform generator 112, transmit
3 transducer interface 114, transmit receive switch 118,
4 receive preamplifier 116, tympanic membrane excitation
source 120 and transducer 160 are as previously described
6 for figure 1. The receive preamplifier 116 may be gain
7 controllable, as before, with the gain determined by
8 controller 248 to place the RF signal in optimum A/D
9 converter 232 range. The output of the receive
preamplifier 116 is directed to a band pass filter 236 for
11 reduction of the noise bandwidth applied to the ADC 232,
12 which samples at the Nyquist rate of at least 2X faster
13 than the applied signal. For the case of a 1.5Mhz
14 transducer 160, the Nyquist sampling rate is at least aMhz
plus the skirt falloff associated with the bandwidth of the
16 transducer 160, known in the art of signal sampling as the
17 Nyquist sampling criteria. The single channel output of
18 the ADC 232 is applied to a data buffer 244, and a signal
19 analyzer 246 examines phase shifts in the buffered signal
to determine phase changes of the RF signal to discern
21 movement of the tympanic membrane. The sequence of phase
22 measurements used to form the phase measurement may be a
23 series of measurements which are inverse-time weighted to
22
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 increase the effect of recently acquired measurements, or
2 they may be uniformly weighted over a window of phase
3 samples. The use of a weighting coefficients applied to
4 the stream of measurements over a window may provide
favorable noise rejection characteristics, and weighting
6 may be chosen to favor signals in the excitation source
7 bandwidth to filter and reduce the effect of noise which is
8 outside the excitation source bandwidth.
9 Figure 3 shows example operation of the ultrasound
processor of figure 1. In a pulsed RF mode,
11 transmit/receive events provide an estimate of the tympanic
12 membrane position as a series of phase values during a
13 series of repeated interrogation intervals 340, each of
14 which provides a single phase value. System clock waveform
302 operates continuously, and is furnished by system clock
16 generator 110 of figure 1. The duration of the event
17 interval 340 is determined by the time-of-flight from the
18 transducer 160 to the tympanic membrane 130 and back to the
19 transducer 160 of figure 1. The propagation velocity of
ultrasound in air is 330mt/s (.33mm/us). Accordingly, for
21 a 1.5Mhz transducer, the resultant wavelength of this
22 traveling wave in air is 0.22mm. The total time of flight
23 for an ultrasound signal lOmm each direction is then 60us,
23
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 so duration 340 may be no less than 60 us in this case.
2 This time of flight interval for a transmit pulse to return
3 as a receive signal after reflection is shown as interval
4 343 in figure 3. The time of flight provides an upper limit
to the pulse repetition frequency (PRF) corresponding to
6 the sum of the transmit interval and receive interval. For
7 this example, the transducer with a 1.5Mhz center frequency
8 will have a 220u wavelength traveling in air. A
9 displacement of the TM will result in a shortened path from
the transducer to the TM, and the reflected signal from the
11 TM back to the transducer will return with a phase shift.
12 Accordingly, the phase and amplitude analyzer observing a
13 phase offset of 180 degrees between transmit clock and
14 received signal compared to a datum phase offset will
correspond to a 55u displacement of the TM. A transmit
16 interval 342 for the transmission of a longer pulse train
17 provides improved signal to noise ratio of the receive
18 signal phase and also extends the return time of flight by
19 the duration 342 of the transmit pulse stream, at the
expense of decreased axial resolution, which may be
21 desirable for the case of a discrete moving target such as
22 the tympanic membrane. For a 10 cycle stream at 1.5Mhz,
23 transmit interval 342 is 6.6us, and for the reflected
24
CA 02994244 2018-01-30
W02017/011035
PCT/US2016/019432
1 signal from a previous transmit burst to not interfere with
2 the new transmit burst, the maximum interval 340 is 66.6us,
3 which implies a pulse repetition frequency (PRF) of 15Khz
4 or less. In a limiting case where the TM is 30us one way
time-of-flight distant, and most of the signal energy
6 reflection is at the air/fluid interface of a TM with fluid
7 behind it, and with minimal signal energy reflected from
8 structures beyond the TM, the shortest possible repetition
9 cycle time is 30us (maximum transmit burst length) + 30us
(outgoing time of flight) + 30us (return time of flight).
11 In this idealized scenario, the transducer starts
12 transmitting at t=0 of the repetition cycle. At t=30us,
13 the first cycle of transmit energy reaches the TM at the
14 same time the transducer is finishing sending the last of
the transmit burst. At t=60us, the first reflected cycle
16 is reaching the transducer and the last cycle of the burst
17 is reflecting from the TM, and at t=90us, the last cycle of
18 the burst has reached the transducer. In an actual
19 ultrasound system, the PRF will be much lower to account
for the required attenuation of multi-path reflection
21 energy which will mix with the TM reflections. In a CW
22 system, separate transmit and receive transducers are used
23 and multipath considerations are ignored. It may be
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 preferable for the system to operate in CW mode in some
2 circumstances, and in pulsed mode in others, depending on
3 the nature of the reflected signal energy. For pulsed
4 mode, it is desired to provide many cycles of transmit
energy to improve the phase accuracy of each measurement,
6 particularly where a clear TM reflection boundary is
7 present and most of the signal energy is reflected from the
8 TM. The combined transmit interval and receive interval
9 which determine the PRF may be in the repetition period
range of 50us to lms or more. As multi-path reflections
11 may occur, it may be preferable to reduce the maximum PRF
12 to reduce the effect of ultrasonic reflections from
13 transmit events earlier than the current interval 340, for
14 example. The path length to the TM is also determined by
the offset of the transducer from the end of the speculum
16 tip. Although figure 1A shows transducer 160 positioned
17 near speculum 124 tip, this distance may vary, and the
18 transducer may be offset inside or outside the speculum
19 tip. In one example of the invention, the transducer is
offset substantially 2.5mm to 5mm inside the end of the
21 speculum tip as shown in detail 150 of figure TA. For an
22 ultrasound propagation velocity of .33mm/us, when the
23 separation from the transducer to TM is 15mm, the round
26
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 trip ultrasound path requires -90us, and if the separation
2 distance from transducer to TM is 20mm, the round trip path
3 requires -120us. As an example, for the 20mm separation
4 distance, a transmit burst length of 15 cycles at 1.5Mhz
would add an additional lOus, and adding 20us of settling
6 time for multipath reflections would result in an interval
7 340 of 150us, corresponding to a PRF of -6.67Khz.
8 Transducer waveform 306 shows the transmit waveform 307
9 which includes bias and amplitude corrections during the
transmit interval 342, and a reduced amplitude receive
11 signal 309 from the tympanic membrane. The received signal
12 309 also includes the effects of tympanic membrane
13 displacement in the form of a phase change from the system
14 clock, which must be subtracted from any static phase value
which may be present. Mixer I and Q outputs, after low
16 pass filtering, are shown as waveforms 308 and 310,
17 respectively. Each 66us cycle provides a single phase
18 estimate value, which may be considered in polar
19 coordinates using the I and Q outputs. This may be done
using a range gate select a time of flight interval
21 corresponding to the region containing a reflection from
22 the tympanic membrane to obtain each sample indicating the
23 instantaneous phase of the tympanic membrane for a
27
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 particular sample from a transmit event. Each acquired
2 values within an RX interval 344 is averaged or temporally
3 filtered over the temporal region corresponding to the TM
4 reflected response to reach an average phase estimate shown
as 311 and 313, respectively, for I and Q waveforms 308 and
6 310.] A series of such phase estimates are saved, each of
7 these estimates spanning an extent of the Rx interval 344
8 and which extent corresponds to a reflection from a
9 particular depth. Across multiple data acquisition Ex
intervals 344, the samples of IQ are concatenated to
11 construct a time series describing tympanic membrane
12 motion, since phase change over time is attributed to
13 change in distance from the transducer. A succession of
14 these sampled values are collected and compared against a
tympanic membrane excitation waveform which is used to form
16 a characterization of the tympanic membrane for a
17 particular excitation waveform.
18 Figure 4A shows an example sinusoidal excitation
19 applied to a tympanic membrane, such as a sinusoidal
waveform 321 applied using a voice coil diaphragm
21 displacing a volume sufficient to modulate the ear canal
22 pressure by 100daPa (dekapascals) p-p. Sub-sonic
23 frequencies may require sealing the ear canal, whereas
28
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 audio frequencies and super-audio frequencies may be
2 sufficiently propagated as audio waves without sealing the
3 ear canal. The sinusoidal ear canal pressure excitation
4 321 results in a modulation of the tympanic membrane, which
is shown as phase plot 332, as the modulation in tympanic
6 membrane position corresponds to a change in the phase of
7 the return signal. Each discrete circle of waveform 332
8 represents a sample point such as a polar conversion of
9 average values for I 311 and Q 313. In one example
embodiment of the invention, a series of sinusoidal
11 modulation excitation 321 frequencies are applied, each
12 with a different period 322, and the delay in response 330
13 and peak phase amplitude are used in combination to
14 estimate the viscosity of the fluid behind the ear. Since
each 360 degree phase change of the 1.5Mhz transmit pulse
16 corresponds to lambda / 2 = .11 mm, a phase change of +/-
17 180 degrees total as shown in plot 332 would correspond to
18 .11mm peak to peak displacement of the tympanic membrane.
19 By applying a series of audio and sub-audio tones with
various cycle times 322 and measuring the phase response as
21 shown in plot 332, it is possible to estimate viscosity of
22 the fluid behind the tympanic membrane. For example, an
23 exemplar effusion metric measurement associated with the
29
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 changed density or viscosity of the fluid could be an
2 associated change in tympanic response time. In this
3 manner, a frequency domain response of the tympanic
4 membrane may be made using a series of excitations 321 and
measuring a series of tympanic membrane responses 332.
6 The series of figures 4C-1, 4C-2, and 4C-3 show the
7 effect of reconstructing TM displacements when the received
8 signal phase exceeds X/2 (180 , corresponding to a 2/4 TM
9 displacement). Figure 4C-1 shows a received signal 430
with displacement-associated phase excursions which exceed
11 X/2 (1800). Because phase excursions greater than 180 wrap
12 to -180 , the series of samples of figure 4C-2 wrap and
13 produce the series of samples shown, with samples of
14 individual segments 432, 434, 436, 438, and 440. If a
sufficiently high sample rate is used, it is possible to
16 "unwrap" the samples as shown in figure 4C-3, to provide
17 the original phase information. These techniques are well
18 known in the prior art of Doppler signal reconstruction.
19 Whereas figure 4A shows a sinusoidal excitation which
may be provided in a series of such excitations to generate
21 a phase vs. frequency response plot of the TM displacement
22 from the series of measurements, Figure 4B shows a time
23 domain step response equivalent of figure 4A, where a step
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 pressure excitation 362 of 50 daPa peak is applied to the
2 ear canal, which generates the phase response 372 of the
3 return signal from the tympanic membrane. It is similarly
4 possible to characterize the tympanic membrane response
based on a time delay 374 and amplitude response (shown as
6 180 degrees) for phase response plot 372, corresponding to
7 .11/2 mm displacement. The phase unwrap techniques
8 described in the series of figure 4C-1, 4C-2, 4C-3 may
9 similarly be applied to the samples of waveform 372 of
figure 4B to reconstruct phase shifts in excess of +/-1800.
11 The signal processing of figure 2 operates in a
12 similar manner as was described for figure 3, however the
13 transducer reflection 306 is directly sampled and compared
14 with a reference clock to determine the phase changes
associated with the tympanic membrane movement, for example
16 by multiplying the reference clock with the received signal
17 over a receive signal averaging time, and integrating this
18 value over the duration of the receive signal to estimate a
19 phase value for one receive interval. In a similar manner,
this will result in the generation of response waveform 332
21 from excitation source 321 interacting with the tympanic
22 membrane, as described for figure 4A, or response waveform
31
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 372 from excitation source 362 interacting with the
2 tympanic membrane.
3 Figure 5 shows another embodiment of the invention for
4 OW operation. The signal processor of figure 5 operates as
in figure 1, and with the same block descriptions operative
6 as was present in figure 1, however the transmit interface
7 114 is directly coupled via leads 502/504 to a transmit
8 transducer shown in detail view of figure 5A as 524 and
9 generating transmit beam 526, which is coincident on the
tympanic membrane with the receive beam profile 528 of
11 receive transducer 530, which conveys the receive signal
12 using leads 506/508 to receive amplifier 116, where the
13 signal processing occurs as described previously for figure
14 1, however, the system of figure 5 operates continuously,
with the transmitter continuously transmitting, and the
16 receiver baseband signal being continuously received. This
17 operation is advantageous for detection of signal bandwidth
18 which exceeds the pulsed transmit configuration described
19 in figure 3. Because the CW transmit signal results in a
standing DC offset at the receive mixers 140 and 142, it is
21 desired to provide electronic isolation between transmit
22 element 524 and receive element 530.
32
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 Figure 6 shows waveform plots for the baseband CW
2 system of figure 5. The system clock 110, transmit
3 waveform generator 112, and transmit transducer interface
4 114 generate a biased transducer CW signal waveform 602 of
figure 6, which is applied to the transmit transducer 524
6 of figure 5, and the receive transducer 530 of figure 5
7 generates receive signal 608 of figure 6. The outputs of
8 the I and Q channel low pass filters 136 and 138,
9 respectively, are shown as waveforms 614 and 616. The
phase unwrapping techniques described previously may be
11 applied to these waveforms as well, where the detected
12 phase crosses the +/- 1800 boundary and wraps to the
13 opposite boundary.
14 Figures 7A and 7E show CW output 714 for an excitation
702, and the sample points of 332 and 372 of figures 4A and
16 4B are not present, as the Cw system of figure 5 is not
17 subject to the baseband Nyquist sampling limitations of the
18 pulsed dopper system of figures 2 and 3, provided that the
19 mixer output is sampled at a sufficiently high rate to
satisfy the Nyquist criteria for phase changes at the mixer
21 output.
22 The transducer types for 130 of figures 1 & 2, and 524
23 and 530 of figure aA may be any of capacitive micromachined
33
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 ultrasonic transducer (cMUT) , or piezoelectric transducers,
2 for example, formed with the piezoelectric material PZT.
3 The example embodiments for the signal processors have
4 shown embodiments of a pulsed Doppler system of figures 1
and 2, and a CW Doppler system of figure 5. Each of these
6 systems can be practiced using direct RF sampling, as shown
7 in figure 2, where a bandpass filter is operative to reduce
8 the noise bandwidth of the system to en --V4k1131Z, commonly
9 expressed as nanovolts per root hertz, where
K is the Boltzmann constant 1.38 * 10-23;
11 T is the temperature of the system, assumed to be
12 300 K;
13 B is the bandwidth of the sampled signal (either the
14 bandwidth of the bandpass filter 236 of figure 2, or
bandwidth of the low pass filter 136/138 of figures 1 and
16 5;
17 and R is the resistance generating the Johnson noise,
18 typically 50 ohms.
19 In an ideal system Johnson noise is generated by
transducer 160 and preamplifier 120 of figure 1, and this
21 noise is frequency-limited to reduce its effect on system
22 measurements. The noise floor for a SO ohm system is
34
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 0.9nVIVIlz. It is typically easier to perform narrowband
2 filtering on a baseband signal such as the low pass filters
3 136 and 138 of figure 1 than the bandpass filter 236 of
4 figure 2. For example, a first order band pass filter 236
for a 1.5Mhz system might have a 3db bandwidth of 1 Mhz,
6 whereas the desired signal content is below 1Khz, which is
7 difficult to incorporate into bandpass filter 236, but
8 simple to incorporate into low pass filter 136.
9 Accordingly, the sample noise floor for 1Khz ba3eband
system would 28nV rms whereas the 1Mhz bandwidth direct
11 sampling system would be 30x higher, or 900nV rms with the
12 same signal energy. The noise factor of the system
13 (typically governed by the first few elements in the
14 receive chain) is managed separately, as it would scale the
noise floor by the noise factor, so a 6dB noise factor
16 would approximately double both of the above rms noise
17 floor values.
18 The invention may be practiced many different ways.
19 In one embodiment, the phase and amplitude analyzer
produces an effusion metric which is a characterization of
21 the sequence of phase measurements from the ultrasound
22 reflection from the tympanic membrane in combination with
23 the displacement of the tympanic membrane from the tympanic
CA 02994244 2018-01-30
WO 2017/011035
PCT/US2016/019432
1 membrane excitation source. The effusion metric which is
2 derived from the response of the tympanic membrane may
3 provide an indication of whether the tympanic membrane has
4 an air boundary indicating no effusion, a watery fluid
boundary, or a purulent fluid boundary. When fluid is
6 detected, one effusion metric may be a viscosity estimate,
7 another effusion metric may be a scattering metric.
8 The components of the system are shown in block
9 diagram form for clarity in understanding the invention.
Certain components are indicated as present in a speculum
11 tip, for clarity of understanding the operation of the
12 invention. It should be understood that these components
13 may be located anywhere, including inside or outside the
14 speculum tip, or alternatively the objects of the invention
may be accomplished with the described structures and no
16 speculum tip at all. Alternatively, the speculum tip may be
17 removable with the various structures stationary or
18 removable, including any optical element for viewing of a
19 tympanic membrane, ultrasound transducer, or optical
source. The particular arrangement of the elements with
21 respect to the speculum tip is shown for clarity and to
22 illustrate one example of the invention.
36
1 The excitation generator may be a manual bulb operated by a
2 clinician, an air displacement generator producing
3 alternating pressure, step pressure, or air puffs. The
4 excitation generator output may be sealed to the ear canal
or unsealed and using a puff of gas such as atmospheric air
6 or other suitable gas.
7 The estimate of tympanic membrane deflection may be derived
8 from a velocity, an acceleration, or any other metric
9 associated with deflection over time.
11
12
13
37
Date Recue/Date Received 2021-06-08