Note: Descriptions are shown in the official language in which they were submitted.
METHOD AND SYSTEM FOR
MODIFYING EYE TISSUE AND INTRAOCULAR LENSES
[0001]
BACKGROUND OF THE INVENTION
100021 Cataract extraction is one of the most commonly performed surgical
procedures in the
world. A cataract is the pacification of the crystalline lens or its envelope
- the lens capsule - of
the eye. It varies in degree from slight to complete opacity that obstructs
the passage of light.
Early in the development of age-related cataract the power of the lens may be
increased, causing
near-sightedness (myopia), and the gradual yellowing and opacification of the
lens may reduce
the perception of blue colors as those wavelengths are absorbed and scattered
within the
crystalline lens. Cataract typically progresses slowly to cause vision loss
and are potentially
blinding if untreated.
[0003] Treatment is performed by removing the opaque crystalline lens and
replacing it with
an artificial intraocular lens (TOL). An estimated 3 million cases are
presently performed
annually in the United States and 15 million worldwide. This market is
composed of various
segments including intraocular lenses for implantation, viscoelastic polymers
to facilitate
1
Date Recue/Date Received 2020-07-15
CA 02994799 2018-02-05
WO 2017/023296 PCMJS2015/043504
surgical maneuvers, disposable instrumentation including ultrasonic
phacoemulsification tips,
tubing, and various knives and forceps.
[0004] Modern cataract surgery is typically performed using a technique termed
phacoemulsification in which an ultrasonic tip with associated irrigation and
aspiration ports is
used to sculpt the relatively hard nucleus of the lens to facilitate it
removal through an opening
made in the anterior lens capsule termed anterior capsulotomy or more recently
continuous
curvilinear capsulorhexis (CCC). Finally, a synthetic foldable intraocular
lens is inserted into the
remaining lens capsule of the eye through a small incision.
[0005] One of the most technically challenging and critical steps in the
procedure is making
the capsulorhexis. This step evolved from an earlier technique termed can-
opener capsulotomy
in which a sharp needle was used to perforate the anterior lens capsule in a
circular fashion
followed by the removal of a circular fragment of lens capsule typically in
the range of 5-8 mm
in diameter. This facilitated the next step of nuclear sculpting by
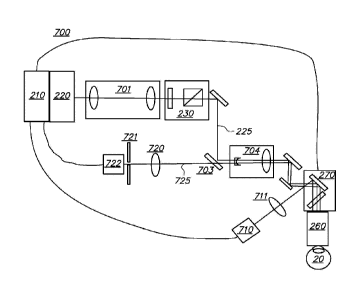
phacoemulsification. Due to a
variety of complications associated with the initial can-opener technique,
attempts were made by
leading experts in the field to develop a better technique for removal of the
anterior lens capsule
preceding the emulsification step.
[0006] The concept of the continuous curvilinear capsulorhexis is to provide a
smooth
continuous circular opening through which not only the phacoemulsification of
the nucleus can
be performed safely and easily, but also for easy insertion of the intraocular
lens. It provides
both a clear central access for insertion, a permanent aperture for
transmission of the image to
the retina by the patient, and also a support of the IOL inside the remaining
capsule that would
limit the potential for dislocation.
[0007] Problems may develop related to inability of the surgeon to adequately
visualize the
capsule due to lack of red reflex, to grasp it with sufficient security, to
tear a smooth circular
opening of the appropriate size and in the correct location without creating
radial rips and
extensions. Also present are technical difficulties related to maintenance of
the anterior chamber
depth after initial opening, small size of the pupil, or the absence of a red
reflex due to the lens
opacity. Some of the problems with visualization have been minimized through
the use of dyes
such as methylene blue or indocyanine green. Additional complications arise in
patients with
2
weak zonules (typically older patients) and very young children that have very
soft and elastic
capsules, which are very difficult to controllably and reliably rupture and
tear.
[0008] Many cataract patients have astigmatic visual errors. Astigmatism can
occur when the
corneal curvature is unequal in all directions. Nowadays, IOLs are used to
correct for
astigmatism but require precise rotational and central placement.
Additionally, IOLs are not
used for correction beyond 5D of astigmatism, even though many patients have
more severe
aberrations. Higher correction beyond 5D is required to reshape the cornea to
become more
spherical. There have been numerous approaches, including Corneaplasty,
Astigmatic
Keratotomy, Corneal Relaxing incision (CRI) and Limbal Relaxing Incision
(LRI). Except the
Corneaplasty, all procedures are done by placing corneal incisions in a well
defined manner and
depth to allow the cornea to change shape to become more spherical. Nowadays,
these delicate
cuts are placed manually with its implication on its limited precision.
100091 But, not only cuts are desired for ophthalmic therapies. There is also
the need for more
gentle modifications of the eye tissue which result in weakening of the
tissues mechanical
properties and or changes of the optical properties of the treated tissue. In
this case, the effect
should be gentle enough to allow structural modifications of the eye tissue
without mechanical
disruption. Ding et al., Intratissue Refractive Index Shaping (IRIS) of the
Cornea and Lens Using
a Low-Pulse-Energy Femtosecond Laser Oscillator (Investigative Ophthalmology &
Visual
Science, 2008 (49), 12, pp 5532-5539) showed modification of corneal tissue
with sub-rupture
femtosecond laser pulses and could demonstrate changes in the refractive index
by about 1% by
applying diffraction patterns into the corneal tissue. The practical
application of Ding's
technique is although limited by the need to apply 100,000,000 laser pulses
per cubic micrometer
of treated tissue.
[0010] Vogel et al. ( US 2010/0163540 Al) describes a method for machining and
cutting of
transparent material with temporal smooth laser beams to generate a low
density plasma without
the formation of plasma luminescence. In the teaching, they describe that
linear absorption of
the exposed material is especially to be avoided as it leads to the random
generation of seeding
electrons which in turn generates a stochastic variation in the plasma
threshold. Additionally,
they describe that the low density plasma formation is always associated with
the formation of
cavitation bubbles.
3
Date Recue/Date Received 2020-07-15
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
[0011] This is in strong contrast to the present invention in which two
working regimens are
described. It was discovered that using a laser wavelength that has some
linear absorption in the
target tissue enables to create extremely low threshold effect. Additionally,
a temporal smooth
pulse shape is not required in the current invention. Also, the formation of a
cavitation bubble is
not desired in one embodiment of the invention as the effect is induced by
linear absorption
enhanced photodecomposition. Also, Vogel' s data show that there is still more
than one order
difference in achieving plasma formation when comparing IR femtosecond lasers
and 355 sub-ns
laser. In our embodiment, due to the use of the linear absorption of tissue
intrinsic chromophores
(or via the addition of exogenous chromophores) the energy threshold for the
355nm sub-
nanosecond laser is even slightly lower when compared to femtosecond laser
pulses using the
same numerical aperture optics.
[0012] Braun et al. (DE 198 55 623 Cl) describes a method for precise
machining inside of
glass using a laser with wavelength outside the transmission plateau of the
glass. This laser is
then used to specifically create material defects inside the glass without
comprising the surface.
This method allows them to place material defects closer to the surface
without damaging the
surface itself. No surface effects are described. It also does not create any
cavitation event as its
used only on glass in which no cavitation bubble is formed.
[0013] Koenig et al. (WO 2007/057174) claims a system for the surgical
intervention of the
eye by using femtosecond laser pulses in the IJV spectral range. In his
teaching, he describes the
use of higher numerical apertures of 0.8 for his invention which lowers the
threshold
significantly into the nanoJoule regimen. But, he makes the transfer of this
system into a useable
product so difficult as it is optically difficult to have these numerical
apertures combined with a
wide scan ranges of 6 to 10 mm typically used for ophthalmic applications.
Also, the generation
of femtosecond UV laser pulses is technically challenging.
[0014] Therefore, methods, techniques, and an apparatus to advance the
standard of care of the
ophthalmic patient are needed.
4
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
SUMMARY OF THE INVENTION
[0015] Accordingly, this disclosure provides systems and methods for use in
suitable
ophthalmic laser surgery systems so as to obviate one or more problems due to
limitations and
disadvantages of the related art. One embodiment is directed to a system for
ophthalmic surgery,
comprising a laser source configured to deliver a laser beam comprising a
plurality of laser
pulses having a wavelength between about 320 nanometers and about 430
nanometers and a
pulse duration between about 1 picosecond and about 100 nanoseconds; and an
optical system
operatively coupled to the laser source and configured to focus and direct the
laser beam in a
pattern into one or more intraocular targets within an eye of a patient, such
that interaction
between the one or more targets and the laser pulses is characterized by
linear absorption
enhanced photodecomposition without formation of a plasma or associated
cavitation event. The
wavelength may be about 355nm. The pulse duration may be between about 400
picoseconds
and about 700 picoseconds. The pulses may have a pulse energy between about
0.01
microJoules and about 500 microJoules. The pulses may have a pulse energy of
between about
0.5 microJoules and about 10 microJoules. The plurality of laser pulses may
have a repetition
rate of between about 500 Hertz and about 500 kiloHertz. The optical system
may be
configured to focus the laser beam to create a beam diameter of between about
0.5 microns and
about 10 microns within the one or more intraocular targets. At least one of
the one or more
intraocular targets may be selected from the group consisting of a cornea, a
limbus, a sclera, a
lens capsule, a crystalline lens, and a synthetic intraocular lens implant.
The pattern may be
configured to create one or more physical modifications, such as cuts
(incisions) and refractive
index changes, in the intraocular target in a configuration selected from the
group consisting of
corneal relaxing incisions, limbal relaxing incisions, astigmatic
keratotomies, and capsulotomies.
The optical system and laser source may be configured to structurally alter at
least one of the one
or more intraocular targets such that an index of refraction of the altered
tissue structure target is
changed.
[0016] Another embodiment is directed to a system for ophthalmic surgery,
comprising a
laser source configured to deliver a laser beam comprising a plurality of
laser pulses having a
wavelength between about 320 nanometers and about 430 nanometers and a pulse
duration
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
between about 1 picosecond and about 100 nanoseconds; and an optical system
operatively
coupled to the laser source and configured to focus and direct the laser beam
in a pattern into one
or more tissue structure targets within an eye of a patient, such that
interaction between the one
or more targets and the laser pulses is characterized by localized formation
of a plasma that is
facilitated by linear absorption. The wavelength may be about 355nm. The pulse
duration may
be between about 400 picoseconds and about 700 picoseconds. The pulses may
have a pulse
energy between about 0.01 microJoules and about 500 microJ oules. The pulses
may have a
pulse energy of between about 0.5 microJoules and about 10 microJ oules. The
plurality of laser
pulses may have a repetition rate of between about 500 Hertz and about 500
kiloHertz. The
optical system may be configured to focus the laser beam to create a beam
diameter of between
about 0.5 microns and about 10 microns within the one or more tissue structure
targets. At least
one of the one or more tissue structure targets may be selected from the group
consisting of a
cornea, a limbus, a sclera, a lens capsule, a crystalline lens, and a
synthetic intraocular lens
implant. The pattern may be configured to create one or more cuts in the
intraocular target that
is tissue structure target in a configuration selected from the group
consisting of corneal relaxing
incisions, limbal relaxing incisions, astigmatic keratotomies, and
capsulotomies.
[0017] Another embodiment is directed to a system for ophthalmic surgery,
comprising a
laser source configured to deliver a laser beam comprising a plurality of
laser pulses having a
wavelength between about 320 nanometers and about 430 nanometers and a pulse
duration
between about 1 picosecond and about 100 nanoseconds; and an optical system
operatively
coupled to the laser source and configured to focus and direct the laser beam
in a pattern into one
or more targets within an eye of a patient, such that interaction between the
one or more targets
and the laser pulses is characterized by linear absorption enhanced
photodecomposition without
formation of a plasma or associated cavitation event. The pattern may be
configured such that
the operation of the optical system and laser source causes physical
alteration of the one or more
targets. The physical alteration may be manifested as a change in refractive
index of the one or
more targets or one or more incisions. At least one of the one or more targets
may be a cornea or
an artificial intraocular lens. The physical alteration may be configured to
change the refractive
profile of the target.
6
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
[0018] Another embodiment is directed to a system for ophthalmic surgery,
comprising a
laser source configured to deliver a laser beam comprising a plurality of
laser pulses having a
wavelength between about 320 nanometers and about 430 nanometers and a pulse
duration
between about 1 picosecond and about 100 nanoseconds; an optical system
operatively coupled
to the laser source and configured to focus and direct the laser beam in a
pattern into one or more
tissue structure targets within an eye of a patient, such that interaction
between the one or more
targets and the laser pulses is characterized by linear absorption enhanced
photodecomposition
without formation of a plasma or associated cavitation event; and an
integrated imaging
subsystem that captures in a confocal arrangement back-reflected light from a
sample provided
by the laser source. The laser pulses may induce fluorescence that is
collected by the imaging
subsystem. The system may be configured to provide interleaved lower energy
pulses for
imaging and higher energy pulses for treatment. The imaging subsystem may
comprise an
optical coherence tomography system, a Purkinje imaging system, and/or a
Scheimpflug imaging
system. The system may further comprise a controller configured to determine
the locations &
shapes of ocular structures, to determine pattern placement and/or laser
parameters, and position
the patterns within the defined targets.
[0019] Another embodiment is directed to a system for ophthalmic surgery,
comprising a
laser source configured to deliver a laser beam comprising a plurality of
laser pulses having a
wavelength between about 320 nanometers and about 430 nanometers and a pulse
duration
between about 1 picosecond and about 100 nanoseconds; an optical system
operatively coupled
to the laser source and configured to focus and direct the laser beam in a
pattern into one or more
tissue structure targets within an eye of a patient, such that interaction
between the one or more
targets and the laser pulses is characterized by linear absorption enhanced
photodecomposition
without formation of a plasma or associated cavitation event; and an exogenous
chromophore
introduced to the target structure to create/enhance linear absorption. The
exogenous
chromophore may be trypan blue.
[0020] Another embodiment is directed to a system for ophthalmic surgery,
comprising a laser
source configured to deliver a laser beam comprising a plurality of laser
pulses having a
wavelength between about 320 nanometers and about 430 nanometers and a pulse
duration
7
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
between about 1 picosecond and about 100 nanoseconds; and an optical system
operatively
coupled to the laser source and configured to focus and direct the laser beam
in a pattern into one
or more intraocular targets within an eye of a patient, such that interaction
between the one or
more targets and the laser pulses is characterized by linear absorption
enhanced
photodecomposition without formation of a plasma or associated cavitation
event; with the
addition of a second laser source configured to fragment the lens utilizing a
wavelength between
about 800nm and about 1100nm. The second laser may be a pulsed infrared laser.
The second
laser may have a pulse duration between about 1 picosecond and about 100
nanoseconds. The
second laser may be a Q-switched Nd:YAG laser.
[0021] Another embodiment is directed to a system for ophthalmic surgery of
an eye of a
patient, which comprises: a laser source configured to deliver an ultraviolet
laser beam
comprising a plurality of ultraviolet laser pulses having a wavelength between
320 nanometers
and 370 nanometers to photodecompose one more intraocular targets within the
eye with
chromophore absorbance, a pulse duration between 1 picosecond and 100
nanoseconds, and a
pulse energy between 0.01 microJoules and 500 microJoules; and an optical
system operatively
coupled to the laser source and configured to focus the ultraviolet laser beam
to a focal spot and
direct the focal spot in a pattern into the one or more intraocular targets
selected from the group
consisting of a cornea, a limbus, a sclera, a lens capsule, a crystalline
lens, and a synthetic
intraocular lens implant; the pulse energy, the pulse duration, and the focal
spot being configured
such that an irradiance of the ultraviolet laser beam at the focal spot is
sufficient to
photodecompose the one or more intraocular targets with chromophore absorbance
without
exceeding a threshold of formation of a plasma and an associated cavitation
event, wherein the
ultraviolet laser beam is focused by the optical system at the one or more
intraocular targets at a
numerical aperture that provides for the focal spot of the laser beam to be
scanned over a scan
range of 6 mm to 10 mm in a direction lateral to a Z-axis that is aligned with
the laser beam. The
numerical aperture of the system is less than 0. 6, preferably between 0.05 to
0.4.
[0022] Another embodiment is directed to a system for ophthalmic surgery of
an eye of a
patient, which comprises: a laser source configured to deliver an ultraviolet
laser beam
comprising a plurality of ultraviolet laser pulses having a wavelength, a
pulse duration, and a
8
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
pulse energy, wherein the plurality of ultraviolet laser pulses has a
wavelength between 320 and
370 nanometers to photodecompose one or more intraocular targets within the
eye with
chromophore absorbance; and an optical system operatively coupled to the laser
source and
configured to focus the ultraviolet laser beam to a focal spot and direct the
focal spot in a pattern
into the one or more intraocular targets selected from the group consisting of
a cornea, a limbus,
a sclera, a lens capsule, a crystalline lens, and a synthetic intraocular lens
implant; the pulse
energy, the pulse duration, and the focal spot being configured such that an
irradiance of the
ultraviolet laser beam at the focal spot is sufficient to photodecompose the
one or more
intraocular targets with chromophore absorbance without exceeding a threshold
of formation of a
plasma and an associated cavitation event, and wherein the ultraviolet laser
beam is focused by
the optical system at the one or more intraocular targets at a numerical
aperture less than 0.6.
The numerical aperture of the system is preferably 0.05 to 0.4.
[0023] This summary and the following detailed description are merely
exemplary, illustrative,
and explanatory, and are not intended to limit, but to provide further
explanation of the invention
as claimed. Additional aspects, features, objectives and advantages of the
invention will be set
forth in the descriptions that follow, and in part will become apparent from
the written
description, taken in conjunction with the accompanying drawings, illustrating
by way of
example the principles of the invention, or may he learned by practice of the
invention.
BRIEF DESCRIPTION OF THE DRAWINGS
[0024] The novel features of the invention are set forth with particularity
in the appended
claims. A better understanding of the features and advantages will be
facilitated by referring to
the following detailed description that sets forth illustrative embodiments
using principles of the
invention, as well as to the accompanying drawings, in which like numerals
refer to like parts
throughout the different views. Like parts, however, do not always have like
reference numerals.
Further, the drawings are not drawn to scale, and emphasis has instead been
placed on
illustrating the principles of the invention. All illustrations are intended
to convey concepts,
9
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
where relative sizes, shapes, and other detailed attributes may be illustrated
schematically rather
than depicted literally or precisely.
[0025] Figure 1 illustrates a high-level flowchart in accordance with an
embodiment of the
present invention.
[0026] FIGS. 2A & B are illustrations of system embodiments.
[0027] FIG. 3 shows a flowchart of a method in accordance with an alternate
embodiment.
[0028] FIG. 4 is an illustration of the line pattern applied across the lens
for depth ranging
measurement (OCT, confocal reflection, confocal autofluorescence, ultrasound)
of the axial
profile of the anterior chamber of the eye.
[0029] FIG. 5 is a top view diagram of a rotationally asymmetric capsulorhexis
incision.
[0030] FIG. 6 is a top view diagram of a complementary rotationally asymmetric
IOL.
[0031] FIG. 7 is a top view of the IOL of Figure 6 positioned in the lens
capsule of FIG. 5.
[0032] FIGS. 8 and 9 are side views of the rotationally asymmetric IOL of FIG.
6.
[0033] FIG. 10 illustrates fragmentation patterns of an ocular lens produced
by one
embodiment of the present invention.
[0034] FIG. 11 illustrates a line pattern 501 applied across the cornea 504
and lens for depth
ranging measurement (OCT, confocal reflection, confocal autofluorescence,
ultrasound) of the
axial profile of the anterior chamber of the eye. It goes over the iris 502
and the lens 402 (not
shown)
[0035] FIG 12 illustrates a measured scan pattern across the cornea and lens
which can be used
for depth ranging by OCT
[0036] FIG 13 illustrates a measured scan pattern across the lens which can be
used for depth
ranging by confocal autofluorescence using a pulsed 320NM TO 430NM laser.
[0037] FIG 14 is another illustration of a system in accordance with an
embodiment of this
invention.
[0038] FIG 15 shows a histological cross section of a corneal cut produced by
one embodiment
of the present invention in which no cavitation bubbles were formed but the
tissue was modified.
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
[0039] FIG 16 shows a histological cross section of a opened corneal cut which
was produced
by one embodiment of the present invention in which no cavitation bubbles were
formed as
shown in FIG 15. The cut opened up effortless along the modified tissue
structure.
[0040] FIG 17 shows a histological cross section of a corneal cut produced by
one embodiment
of the present invention in which cavitation bubbles were formed.
[0041] FIG 18 shows an illustration of the refractive index changes 822
locally induced to the
corneal tissue 504 by said invention. As seen in FIG 15 in this case no
cavitation bubbles will be
created. This effect will induce a change of the refractive index profile of
the corneal tissue.
[0042] FIG 19 shows a high resolution SEM image of the excised human lens
capsule
processed with the current invention. Compared to FIG 20 this sample has a
much smoother
edge quality and does not show any effect of cavitation bubbles.
[0043] FIG 20 shows a high resolution SEM image of an excised human lens
capsule
processed with a femtosecond laser. The effect of each single laser shot with
spacing of 5
micrometer is visible as the mechanical effect of cavitation causes the
rupture of the capsular
tissue.
[0044] FIG. 21 is a graph of average power (W) of the laser as a function of
NA with 355 nm
laser light at repetition rates of 70 kHz and 100 kHz, respectively. The time
required to modify
tissue, i.e., to complete a cut, is also a function of the system NA.
[0045] FIG. 22 is a graph of the time required to modify tissue, i.e., "cut
time" per mm2, as a
function of NA with 355 nm light at repetition rates of 70 kIIz and 100 kIIz,
respectively.
[0046] Figure 23 is a graph of a relative exposure ratio as a function of NA
as a function of NA
with 355 nm light at repetition rates of 70 kHz and 100 kHz, respectively.
[0047] FIG. 24 is a combination that combines the considerations of cut time
and iris exposure.
DETAILED DESCRIPTION OF ILLUSTRATED EMBODIMENTS
[0048] The present invention relates to method and systems for making an
incision in eye
tissue to alter its mechanical or optical properties. The following
description is presented to
enable one of ordinary skill in the art to make and use the invention and is
provided in the
context of a patent application and its requirements. Various modifications to
the embodiments
11
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
and the generic principles and features described herein will be readily
apparent to those skilled
in the art. Thus, the present invention is not intended to be limited to the
embodiment shown but
is to be accorded the widest scope consistent with the principles and features
described herein.
[0049] As shown in the drawings for purposes of illustration, a method and
system for making
an incision in eye tissue or alter its mechanical or optical properties are
disclosed. In varying
embodiments, the method and system disclosed herein provide many advantages
over the current
standard of care. Specifically, rapid and precise openings in the lens capsule
are enabled using a
320nm to 430nm laser to facilitate the placement and stability of intraocular
lenses.
[0050] Other procedures enabled by the techniques described herein include the
treatment of
astigmatism. Intraocular lens (IOLs) are typically used for correcting
astigmatism but require
precise placement, orientation and stability. Complete and long lasting
correction using IOLs is
difficult. It often involves further surgical intervention to make the corneal
shape more
spherical, or at least less radially asymmetrical. This can be accomplished by
making Corneal or
Limbal Relaxing Incisions. Other procedures include the creation of corneal
flaps for LASIK
procedure and the creation of matching corneal transplant shapes of the donor
and recipient
cornea. The present invention may be employed to perform these delicate
incisions.
[0051] FIG. 1 is a flowchart of a method in accordance with an embodiment. A
first step 101
involves generating a beam of light from a 320nm to 430nm laser system having
at least a first
pulse of light. A next step 102 involves passing the beam of light through an
optical element so
that the beam of light is focused at a predetermined depth in the eye tissue.
By implementing
this method, rapid and precise openings in the lens capsule are enabled
thereby facilitating the
placement and stability of intraocular lenses.
[0052] The present invention can be implemented by a system 200 that projects
or scans an
optical beam into a patient's eye 20, such as the system shown in FIG. 2A. The
system 200
includes control electronics 210, a light source 220, an attenuator 230, a
beam expander 240,
focusing lens' 250, 260 and reflection means 270. Control electronics 210 may
be a computer,
microcontroller, etc. Scanning may be achieved by using one or more moveable
optical elements
(e.g. lenses 250, 260, reflection means 270) which also may be controlled by
control electronics
12
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
210, via input and output devices (not shown). Another means of scanning might
be enabled by
an electro optical deflector device (single axis or dual axis) in the optical
path.
[0053] During operation, the light source 220 generates an optical beam 225
whereby
reflection means 270 may be tilted to deviate the optical beam 225 and direct
beam 225 towards
the patient's eye 20. Focusing lens' 250, 260 can be used to focus the optical
beam 225 into the
patient's eye 20. The positioning and character of optical beam 225 and/or the
scan pattern it
forms on the eye 20 may be further controlled by use of an input device such
as a joystick, or any
other appropriate user input device.
[0054] The present invention alternatively can be implemented by a system 700
that
additionally does a range finding of patient's eye 20, such as the system
shown in FIG. 14. The
system 700 includes control electronics 210, a light source 220, an attenuator
230, a beam
expander 701, an optical variable beam attenuator 230, an separate focus lens
combination 704
and a beam reflection and scanning means 270. The light beam 225 of light
source 220 is
focused through focusing lens 260 to its target location 20. This will be
controlled by electronics
210 which is connected to deflection unit 270. Additionally the auto
fluorescence light 725 of the
target structure 20 is de-scanned by the similar optical path shared with
laser light 225 by
preferred means of a dichroic beam splitter 703 and focused by a lens 720. An
aperture pinhole
721 is placed in the focal spot of formed beam 725 as a conjugate of the laser
beam (225) focus
in target structure 20. The intensity of the transmitted auto fluorescence
light through beam
aperture 721 is detected and converted to an electrical signal which can be
read by the control
unit 210. Also an image of the treated area is imaged by lens 711 on an image
capture device 710
which can be a CCD or a CMOS camera. Also this signal is transmitted to
control unit 210.
[0055] In another variation of system 700 the detection combination unit 703,
720, 721, 722 is
used to confocally detect the back reflected light of beam 225 from sample 20.
[0056] The underlying mechanism of varying embodiment employs a 320nm to 430nm
laser
source. The ultraviolet optical spectrum is technically subdivided into three
major spectral
regions which are: UVA (400nm-315nm), UVB (315nm ¨ 280nm), UVC (280nm ¨
100nm).
Due to their high single photon energy, UVB and UVC light is commonly
associated with
carcinogenic effects due to their ability to directly modify DNA. While water
is still transparent
13
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
down to 200nm the absorption of proteins strongly increases around 240nm. This
strong protein
absorption in the UVC spectral region, which is also the leading absorption in
corneal tissue, is
clinically used nowadays in Laser-Assisted in situ Keratomileusis (LASIK)
procedures to
precisely ablate the corneal tissue.
[0057] UVC lasers have been used to ablate biological tissue through
photodissociation, the
absorption of a high energy photon to break bonds within an organic molecule.
A list of such
common bonds is given in the table below along with their dissociation
energies listed in terms
of wavelength. The shorter the wavelength, the stronger the bond.
Bond Energy (nm)
C-H, sp3 292
C-H, sp 239
C=C 199
[0058] From this table it is obvious that highly energetic photons are
required for the
photodissociation of biological materials, such as is discussed in U.S. Pat.
No. 4,784,135 by
Blum, et al. This effect is the basis of numerous photo-medical systems,
especially in
ophthalmology where 193nm excimer lasers are routinely used for corneal
modification.
Embodiments of the present invention utilize an altogether different physical
phenomenon and
different spectral region (UVA to green) to modify and or ablate biological
tissue that is neither
present nor considered in the prior art.
[0059] In an embodiment, the light source 220 is a 320nm to 430nm laser source
such as an
Nd:YAG laser source operating at the 3'd harmonic wavelength, 355nm. The
transmission of the
cornea at 355nm is about 85% and starts to strongly drop off at 320nm (50%
transmission) to
300nm with about 2% transmission whereas the lens absorption is ¨99%. Also,
for older people,
14
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
light scattering of the cornea is minimal while light scattering of the lens
has considerably
increased (cataract).
[0060] The effect of light scattering is sensitive to wavelength. In case of
scatter centers
smaller than the used wavelength, the scattering coefficient scales as 2J4.
For larger scatterers
with a size range within the size of the wavelength, the Mie approximation is
well suited for
describing the scattering function. For particles with sizes between 350 and
700nm in size, the
scattering coefficient scales as 2L-1. The aged lens itself absorbs all
wavelengths shorter than
420nm and is a strong scatterer. This implies that shorter wavelengths can be
used for the laser
cutting of the anterior part of the aged lens, especially the lens capsule,
while serving to protect
the retina by effectively attenuating the light ultimately disposed there.
[0061] Q-switched infrared lasers with energies of several milliJoule and in
the IR spectral
range (1064nm) are routinely employed to treat posterior cataract
opacification. They do so by
providing a reliable plasma formation directly behind the posterior lens
capsule. These pulses
create cavitation bubbles of several millimeters in size and peak pressures in
the kilobar range.
Mechanical effects of the cavitation bubbles with their sizes in the
millimeter range are the
limiting factor for highly precise cutting in a liquid environment. In order
to reduce the bubble
size and commensurate mechanical side-effects that yield incisions with poor
edge quality and
therefore poor mechanical strength, laser pulse energy must he significantly
reduced. Such an
interaction would, however, be well suited for the application of lens
conditioning.
[0062] Q-switched green lasers with energies of several milliJoule and several
nanoseconds
pulse duration are routinely employed to treat open angle glaucoma of the eye.
This therapy
named Selective Laser Trabecuplasty (SLT) utilizes the specific targeting of
the melanin
chromophore naturally present in the trabecular meshwork. The laser itself
uses a relatively large
200 micrometer spot size to cover most of the target issue area. The laser
produces also a
cavitation bubble around the melanin absorber but this effect is due to linear
heating than plasma
formation as used in the posterior cataract treatment with Q-switched IR laser
pulses.
[0063] In an embodiment of the invention the use of UV wavelengths,
significantly reduces
the threshold for plasma formation and associated formation of cavitation
bubbles but also
decreases the threshold energy required for linear absorption enhanced
photodecomposition
without the formation of cavitation bubbles for a few reasons. First, the
focused spot diameter
scales linearly with wavelength which squares the peak radiant exposure within
the focal plane.
Second, the linear absorption of the material itself allows an even lower
threshold for plasma
formation or low density photodecomposition as initially more laser energy is
absorbed in the
target structure. Third, the use of UV laser pulses in the nanosecond and sub-
nanosecond regime
enables linear absorption enhanced photodecomposition and chromophore guided
ionization.
[0064] Furthermore, this chromophore guided ionization strongly lowers the
threshold for
ionization in case of plasma formation as well lowers the threshold for low
density
photodecomposition for material modification or alteration without cavitation
even under very
weak absorption. Due to the high fluence densities even minimal linear
absorption strongly
lowers the threshold for an effect. It has been shown (Colombelli et al.,
Ultraviolet diffraction
limited nanosurgery of live biological tissues, Rev. Sci. Instrum. 2004, Vol
75, pp. 472-478) that
the threshold for plasma formation and the generation of cavitation bubbles
can be lowered by an
order of magnitude if one only changes from high purity water to water with a
physiologic
NADH concentration of 38mMol. The linear absorption also allows for the
specific treatment of
topical lens structures (e.g. the lens capsule) as the optical penetration
depth of the laser beam is
limited by the linear absorption of the lens. This is especially true for aged
lenses which
absorption in the UV-blue spectral region increases strongly compared to young
lenses.
100651 Additionally in another embodiment of this invention the linear
absorption effect on the
target structures can be even enhanced by applying exogenouse chromophors. One
such useful
chromophore is trypan blue which is commonly used in surgery to stain the lens
capsule in case
of the absence of the fundus red reflex. Trypan blue also has an increased
linear absorption at
wavelengths shorter than 370 nm. This linear absorption further reduces the
energy required to
create disclosed effect on the lens capsular surface.
[0066] This method can also be used for the alteration of the overall
refractive power of the
human eye by:
Create cuts (incisions) within the cornea to change its shape to alter
its refractive power
16
Date Recue/Date Received 2020-07-15
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
Modify the refractive index of the corneal tissue to induce a change
of its effective refractive power.
Modify the refractive index of an implanted synthetic JUL by
writing Fresnel lenses or such other similar into the JUL material to change
its effective
refractive power
iv. Any combination of i, ii, &
[0067] The present inventive system enables surgical techniques that include
utilizing a pulsed
320nm to 430nm laser to perform highly precise physical modifications of
ocular targets,
including tissues (such as lens, lens capsule, cornea, etc.) and synthetic
intraocular lens implants.
This can be done in two different operating regimes; with or without
cavitation bubble
formation. The sub-cavitation regime can also be used to modify the refractive
index of ocular
targets. Although the wavelengths used in the present invention are shorter or
in the range than
those associated with retinal blue light toxicity, the absorption of the 320nm
to 400nm laser light
within the aged lens further minimizes the risk of retinal damage, as this
light will be absorbed
by the lens volume. Furthermore, the risk of damaging the corneal endothelium
or other corneal
structures is also minimized. The threshold pulse energy will be Eth=41)*d2/4,
where 01:0 is the
threshold radiant exposure and d is the focal spot diameter. Here, the focal
spot diameter, d, is
d.U/Db where is the wavelength, F is the focal length of the last focusing
element and Db is
the beam diameter of the last lens. For stable and reproducible operation,
pulse energy should
exceed the threshold by at least a factor of 2, however, the energy level can
be adjusted to avoid
damage to the corneal endothelium.
[0068] The incident light of the laser used for the modification of the eye
tissue generally has a
wavelength of between 320 nm and 430 nm, preferably between 320 and 400 nm,
preferably
between 320 to 370 nm, and more preferably between 340nm and 360 nm. In many
embodiments, the laser light has a wavelength of 355 nm.
[0069] The pulse energy of laser pulses is generally between 0.010 and 5000.
In many
embodiments, the pulse energy will be between 0.1 .1 and 100 J, or more
precisely, between
0.1 J and 40 J, or between 0.1 ILO and 10 [.t.T, or between 0.5 J and 8 J.
17
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
[0070] A pulse repetition rate of the laser pulses is generally between 500Hz
and 500kHz. In
many embodiments, the pulse repetition rate is between lkHz to 200 kHz, or
between 1 KHz to
100 KHz.
[0071] Spot sizes of the laser pulses are generally smaller than 10 pm. In
many embodiments,
the spot size is preferably smaller than 5 pm, typically 0.5pm to 3p m. In
some embodiments,
the spot size is in the range of 1 pm to 2 p.m.
[0072] A pulse duration of the laser pulses is generally between 1ps and
100ns. In many
embodiments, the pulse duration is between 100 ps to 10 ns, or between 100 ps
and 1 ns. In a
preferred embodiment, the pulse duration is between 300 ps and 700 ps,
preferably 400 ps to 700
PS.
[0073] In some embodiments, the beam quality, also referred to as M2
factor, is between
1 and 1.3. The M2 factor is a common measure of the beam quality of a laser
beam. In brief, the
M2 factor is defined as the ratio of a beam's actual divergence to the
divergence of an ideal,
diffraction limited, Gaussian TEM00 beam having the same waist size and
location as is
described in ISO Standard 11146.
[0074] A peak power density (irradiance), obtained by dividing the peak
power of the
laser pulse by the area of the focused spot, is generally expressed in units
of GW/cm2. In
general, the peak power density (irradiance) of the laser pulses should he
sufficiently high to
modify the ocular tissue to be treated. As would be understood by those
ordinarily skilled in the
art, the peak power density (irradiance) depends upon a number of factors,
including the pulse
energy, pulse duration, and focused spot size. Note that the wavelength
indirectly affects the
irradiance since the minimum focused spot size for any given convergence angle
is proportional
to the wavelength. The practical effect of this is that smaller focused spots
can be easier to obtain
with a shorter wavelength. In some embodiments, a peak power density generally
in the range of
20 GW/cm2 to 2000 GW/cm2 will be used to cut ocular tissue with 355 nm light.
Note that the
"peak" power density (irradiance = power per unit area) in a Gaussian beam is
typically
calculated using the beam diameter specified at the "1/e of peak intensity"
width. In this case the
average pulse power is calculated from the pulse energy divided by the pulse
duration at the full
width half maximum point. Then, the average irradiance in time, at the
geometric peak of the
1 g
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
intensity profile (center of the beam) is the power divided by the "1/e" beam
diameter. This is
the value represented in the ranges 20 GW/cm2 to 2000 GW/cm2. The true peak
instantaneous
irradiance and the center of the beam is actually higher due to the "Gaussian"
like temporal
shape of the pulse power.
[0075] The scan range of the laser surgical system is preferably in the
range of 6 to 10
mm.
[0076] In many embodiments for the modification of ocular tissue, spot
spacing between
adjacent laser pulses is typically in the range of about 0.20 lam to 10 lam,
preferably 0.2 lam to 6
m.
[0077] A numerical aperture should be selected that preferably provides for
the focal spot
of the laser beam to be scanned over a scan range of 6 mm to 10 mm in a
direction lateral to a Z-
axis that is aligned with the laser beam. The NA of the system should be less
than 0.6, preferably
less than 0.5 and more preferably in a range of 0.05 to 0.4, typically between
0.1 and 0.3. In
some specific embodiments, the NA is 0.15. For each selected NA, there are
suitable ranges of
pulse energy and beam quality (measured as an M2 value) necessary to achieve a
peak power
density (irradiance) in the range required to cut the ocular tissue. Further
considerations when
choosing the NA include available laser power and pulse rate, and the time
needed to make a cut.
Further, in selection of an appropriate NA, it is preferable to ensure that
there is a safe incidental
exposure of the iris, and other ocular tissues, that are not targeted for
cuts.
[0078] FIG. 21 is a graph of average power (W) of the laser as a function
of NA with 355
nm laser light at repetition rates of 70 kHz and 100 kHz, respectively. Laser
power required to
modify tissue, as a function of NA, increases as the NA decreases. As such,
smaller NA values
generally lead to a potentially undesirable need for a larger (higher average
power) laser. As
shown in Fig. 21, average power is preferably less than about 4 W.
[0079] The time required to modify tissue, i.e., to complete a cut, is also
a function of the
system NA. FIG. 22 is a graph of the time required to modify tissue, i.e.,
"cut time" per mm2, as
a function of NA with 355 nm light at repetition rates of 70 kHz and 100 kHz,
respectively. The
time needed for a cut of unit area (1 mm2) increases with increasing NA due to
lower threshold
19
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
energies, and the consequent need for increased number of pulses. As shown in
FIG. 22,
increased NA tends to lead to longer cut times, favoring lower NA systems from
this perspective.
[0080] Further, these so-called "cut times" affect the exposure of non-
target tissue that is
incidentally exposed while making laser cuts in ocular tissue. For instance,
the limit of safe
exposure of the iris while treating the cornea may be expressed according to
the following
formula:
L (J/ cm2) = C x
wherein L is a safe limit of safe exposure, C is a constant and T is the total
exposure time for
modifying tissue. Figure 23 is a graph of the relative exposure ratio as a
function of NA as a
function of NA with 355 nm light at repetition rates of 70 kHz and 100 kHz,
respectively. In
FIG. 23, the relative exposure ratio is defined as a ratio of the actual
delivered exposure divided
by the safe limit of exposure, L. In the relative exposure ratios of FIG. 23,
values of C are
normalized to match the exposure at 0.15 NA in order to illustrate the effects
of varying NA on
the relative exposure. As shown in Fig. 22, the relative exposure ratio
increases with
decreasing NA.
FIG. 24 is a graph combining FIGS. 22 and 23, i.e., FIG. 24 combines the
considerations of cut
time and iris exposure. From FIG. 24, it can be seen that there is an optimum
at an intermediate
NA in the range of 0.05 to 0.40, and preferably 0.1 to 0.3.
[0081] Table I and Table 2, below, show typical representative laser beam
parameters in
accordance with many embodiments of the present invention.
CA 02994799 2018-02-05
WO 2017/023296 PCT/1JS2015/043504
[0082] TABLE 1:
wavelength (nrn) 355 355 355 355 355 355
energy (u.1) 1 4 2.25 9 0.36 1.44
pulse rate (kHz) 70 100 70 100 70000 100
Pulse length (s) 6.00E-10 6.00E-10 6.00E-10 6.00E-10
6.00E-10 6.00E-10
NA (1/e^2) 0.15 0.15 0.1 0.1 0.25 0.25
MA2 (1/e^2) 1.3 1 1.3 1 1.3 1
spot spacing (pm) 1 2 1.5 3 0.6 1.2
theta (rad, 1/e^2) 0.3 0.3 0.2 0.2 0.5 0.5
BP (p.m, 1/e^2) 0.588 0.452 0.588 0.452 0.587
0.452
SS (pm, 1/e^2) 1.95 1.5 2.94 2.26 1.18
0.904
area (mm^2, 1/e^2) 3.01E-06 1.78E-06 6.77E-06 4.01E-06
1.08E-06 6.42E-07
area (cm^2, 1/e^2) 3.01E-08 1.78E-08 6.78E-08 4.01E-08
1.08E-08 6.42E-09
peak energy density 66.4 449 66.4 449 66.34 449
(J/cm^2)
peak power density 1.E+11 7.E+11 1.E+11 7.E+11 1.E+11
7.E+11
(W/cm^2)
peak power density 111 748 111 748 111 748
(GW/cm^2)
ratio to NS 100% 100% 100% 100% 100% 100%
average power (W) 0.07 0.4 0.158 0.9 0.0252
0.144
spots per mmA2 1,000,000 250,000 444,000 111,000
2,778,000 694,000
time per pattern mmA2 (s) 14.3 2.500 6.35 1.11 39.7
6.94
average pattern energy 100 100 100 100 100 100
density (J/cm^2)
relative possible iris safety 353 95.4 192 51.9 758
205
limit (8*6TA.75 (Pcm^2))
ratio energy density 0.284 1.05 0.521 1.93 0.132
0.487
delivered/safety
21
CA 02994799 2018-02-05
WO 2017/023296
PCT/US2015/043504
[0083] TABLE 2:
wavelength (nm) 355 355 355 355
energy (uJ) 9 36 0.141 0.562
pulse rate (Hz) 70000 100000 70000 100000
Pulse length (s) 6.00E-10 6.00E-10 6.00E-10
6.00E-10
NA (1/02) 0.05 0.05 0.4 0.4
M''2 (1/e"2) 1.3 1 1.3 1
spot spacing (um) 3 6 0.375 0.75
theta (rad, 1/02) 0.1 0.1 0.8 0.8
BP (um, 1/02) 0.588 0.452 0.0588 0.452
SS (um, 1/02) 5.88 4.52 0.735 0.565
area (mna^2, 1/02) 2.71E-05 1.61E-05 4.24E-07
2.51E-07
area (cm^2, 1/02) 2.71E-07 1.61E-07 4.24E-09
2.51E-09
peak energy density (J/cm^2) 66.4 449 66.4 449
peak power density (W/cm^2) 1.E+11 7.E+11 1.E+11 7.E+11
peak power density (GW/cm^2) 111 748 111 748
ratio to NS 100.00% 100.00% 100.00%
100.00%
average power (W) 0.63 3.6 0.00984 0.0563
spots per mmA2 111,000 27,800
7,111,000 1,778,000
time per pattern mmA2 (s) 1.59 0.278 102 17.8
average pattern energy density 100.000 100.000 100.000
100.000
(J/cm^2)
relative possible iris safety limit 67.9 18.4 154 416
(8*6TA.75 (J/cm^2))
ratio energy density delivered/safety 1.47 5.45 0.065
0.241
22
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
[0084] In Tables 1 and 2, theta is the divergence half-angle, BP is the beam
parameter
product, SS is the spot size, and the area is the area of the laser spot.
Here, the 1/e2 width is
equal to the distance between the two points on the marginal distribution that
are 1/e2 = 0.135
times the maximum value.
[0085] An example of the results of such a system on an actual human
crystalline lens is
shown in FIG. 10. A beam of 40, 400 ps pulses delivered at a pulse repetition
rate of 0.5kIIz
from a laser operating at a wavelength of 355nm was focused at NA=0.15, using
an irradiance of
about 120 gigaWatts per square centimeter. This produced the capsulotomy
patterns in the
human lens shown in FIG. 10. In this case no cavitation bubbles were formed to
induce the cuts.
This was confirmed visually under the microscope but also by using a
hydrophone for the
detection of the acoustic sound wave emitted by cavitation bubbles. For laser
cataract surgery,
the only high precision cut on the lens itself is the capsulotomy. For the
softening or
fragmentation of the lens nucleus, the patterns don't need a high spatial
confinement. So for this
application even if there is a longer pulse, a higher fluence and/or
irradiance threshold is
acceptable.
[0086] FIG. 3 shows a flowchart of a method in accordance with an alternate
embodiment. A
first step 301 involves generating a beam of light from a 320nm to 430nm laser
system. A next
step 302 involves translating the focused beam of light within the eye tissue
in a controlled
fashion thereby forming an incision. In an embodiment, the incision is formed
in the anterior
lens capsule of the eye tissue in the performance of a capsulorhexis.
Alternately, the incision
may be in the cornea for the purposes of astigmatic correct or creating
surgical access. For
example, clear corneal cataract instrumentation and paracentesis incisions
maybe used to provide
surgical access.
[0087] The control electronics 210 and the lights source 220 can be set to
target the surfaces of
the targeted structures in the eye 20 and ensure that the beam 225 will be
focused where
appropriate and not unintentionally damage non-targeted tissue. Imaging
modalities and
techniques described herein, such as for example, Optical Coherence Tomography
(OCT),
Purkinje imaging, Scheimpflug imaging, autofluorescence imaging, confocal
autofluorescence,
confocal reflectance imaging or ultrasound may be used to determine the
location and measure
23
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
the thickness of the lens and lens capsule to provide greater precision to the
laser focusing
methods, including 2D and 3D patterning. Laser focusing may also be
accomplished using one
or more methods including direct observation of an aiming beam, OCT, Purkinje
imaging,
Scheimpflug imaging, structured light illumination, ultrasound, or other known
ophthalmic or
medical imaging modalities and/or combinations thereof. It should be noted
that the imaging
depth need only include the anterior most portion of the intraocular target,
and not necessarily
the entire eye or even the anterior chamber.
[0088] Additionally confocal reflectometry can be used for the adjustment of
delivered laser
energy during treatment as it will be able to detect if a cavitation bubble is
formed after a laser
pulse and adjust the energy of subsequent laser pulses or monitor the laser
induced change of the
refractive index of said tissue.
[0089] Accordingly, a three dimensional application of laser energy can be
applied across the
capsule along the pattern produced by the laser-induced effect in a number of
ways. For
example, the laser can be employed to produce several circular or other
pattern scans
consecutively at different depths with a step equal to the axial length of the
effect zone. Thus, the
depth of the focal point (waist) in the tissue is stepped up or down with each
consecutive scan.
The laser pulses are sequentially applied to the same lateral pattern at
different depths of tissue
using, for example, axial scanning of the focusing elements or adjusting the
optical power of the
focusing element while, optionally, simultaneously or sequentially scanning
the lateral pattern.
[0090] The adverse result of laser beam scattering on bubbles, cracks and/or
tissue fragments
prior to reaching the focal point can be avoided by first producing the
pattern/focusing on the
maximal required depth in tissue and then, in later passes, focusing on more
shallow tissue
spaces. Not only does this "bottom up" treatment technique reduce unwanted
beam attenuation in
tissue above the target tissue layer, but it also helps protect tissue
underneath the target tissue
layer. By scattering the laser radiation transmitted beyond the focal point on
gas bubbles, cracks
and/or tissue fragments which were produced by the previous scans, these
defects help protect
the underlying retina. Similarly, when segmenting a lens, the laser can be
focused on the most
posterior portion of the lens and then moved more anteriorly as the procedure
continues.
24
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
[0091] The present invention can be implemented by a system that projects or
scans an optical
beam into a patient's eye 68, such as system 2 shown in Figure 2B which
includes a
TREATMENT light source 4 (e.g. a short pulsed 355nm laser). Using this system,
a beam may
be scanned in a patient's eye in three dimensions: X, Y, Z. Safety limits with
regard to
unintended damage to non-targeted tissue bound the upper limit with regard to
repetition rate and
pulse energy; while threshold energy, time to complete the procedure and
stability bound the
lower limit for pulse energy and repetition rate.
[0092] The laser 4 is controlled by control electronics 300, via an input and
output device 302,
to create optical beam 6. Control electronics 300 may be a computer,
microcontroller, etc. In
this example, the entire system is controlled by the controller 300, and data
moved through
input/output device JO 302. A graphical user interface GUI 304 may be used to
set system
operating parameters, process user input (UI) 306 on the GUI 304, and display
gathered
information such as images of ocular structures.
[0093] The generated TREATMENT light beam 6 proceeds towards the patient eye
68 passing
through half-wave plate, 8, and linear polarizer, 10. The polarization state
of the beam can be
adjusted so that the desired amount of light passes through half-wave plate 8
and linear polarizer
10, which together act as a variable attenuator for the TREATMENT beam 6.
Additionally, the
orientation of linear polarizer 10 determines the incident polarization state
incident upon
beamcombiner 34, thereby optimizing beamcombiner throughput.
[0094] The TREATMENT beam proceeds through a shutter 12, aperture 14, and a
pickoff
device 16. The system controlled shutter 12 ensures on/off control of the
laser for procedural
and safety reasons. The aperture sets an outer useful diameter for the laser
beam and the pickoff
monitors the output of the useful beam. The pickoff device 16 includes of a
partially reflecting
mirror 20 and a detector 18. Pulse energy, average power, or a combination may
be measured
using detector 18. The information can be used for feedback to the half-wave
plate 8 for
attenuation and to verify whether the shutter 12 is open or closed. In
addition, the shutter 12 may
have position sensors to provide a redundant state detection.
[0095] The beam passes through a beam conditioning stage 22, in which beam
parameters such
as beam diameter, divergence, circularity, and astigmatism can be modified. In
this illustrative
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
example, the beam conditioning stage 22 includes a 2 element beam expanding
telescope
comprised of spherical optics 24 and 26 in order to achieve the intended beam
size and
collimation. Although not illustrated here, an anamorphic or other optical
system can be used to
achieve the desired beam parameters. The factors used to determine these beam
parameters
include the output beam parameters of the laser, the overall magnification of
the system, and the
desired numerical aperture (NA) at the treatment location. In addition, the
optical system 22 can
be used to image aperture 14 to a desired location (e.g. the center location
between the 2-axis
scanning device 50 described below), ln this way, the amount of light that
makes it through the
aperture 14 is assured to make it through the scanning system. Pickoff device
16 is then a
reliable measure of the usable light.
[0096] After exiting conditioning stage 22, beam 6 reflects off of fold
mirrors 28, 30, & 32.
These mirrors can be adjustable for alignment purposes. The beam 6 is then
incident upon beam
combiner 34. Beamcombiner 34 reflects the TREATMENT beam 6 (and transmits both
the OCT
114 and aim 202 beams described below). For efficient beamcombiner operation,
the angle of
incidence is preferably kept below 45 degrees and the polarization where
possible of the beams
is fixed. For the TREATMENT beam 6, the orientation of linear polarizer 10
provides fixed
polarization.
[0097] Following the beam combiner 34, the beam 6 continues onto the z-adjust
or Z scan
device 40. In this illustrative example the z-adjust includes a Galilean
telescope with two lens
groups 42 and 44 (each lens group includes one or more lenses). Lens group 42
moves along the
z-axis about the collimation position of the telescope. In this way, the focus
position of the spot
in the patient's eye 68 moves along the z-axis as indicated. In general there
is a fixed linear
relationship between the motion of lens 42 and the motion of the focus. In
this case, the z-adjust
telescope has an approximate 2x beam expansion ratio and a 1:1 relationship of
the movement of
lens 42 to the movement of the focus. Alternatively, lens group 44 could be
moved along the z-
axis to actuate the z-adjust, and scan. The z-adjust is the z-scan device for
treatment in the eye
68. It can be controlled automatically and dynamically by the system and
selected to be
independent or to interplay with the X-Y scan device described next. Mirrors
36 and 38 can be
used for aligning the optical axis with the axis of z-adjust device 40.
26
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
[0098] After passing through the z-adjust device 40, the beam 6 is directed to
the x-y scan
device by mirrors 46 & 48. Mirrors 46 & 48 can be adjustable for alignment
purposes. X-Y
scanning is achieved by the scanning device 50 preferably using two mirrors 52
& 54 under the
control of control electronics 300, which rotate in orthogonal directions
using motors,
galvanometers, or any other well known optic moving device. Mirrors 52 & 54
are located near
the telecentric position of the objective lens 58 and contact lens 66
combination described below.
Tilting these mirrors 52/54 causes them to deflect beam 6, causing lateral
displacements in the
plane of TREATMENT focus located in the patient's eye 68. Objective lens 58
may be a
complex multi-element lens element, as shown, and represented by lenses 60,
62, and 64. The
complexity of the lens 58 will be dictated by the scan field size, the focused
spot size, the
available working distance on both the proximal and distal sides of objective
58, as well as the
amount of aberration control. An objective lens 58 of focal length 60mm,
operating over a field
of 7mm, with an input beam size of 20mm diameter is an example. Alternatively,
X-Y scanning
by scanner 50 may be achieved by using one or more moveable optical elements
(e.g. lenses,
gratings) which also may be controlled by control electronics 300, via input
and output device
302.
[0099] The aiming and treatment scan patterns can be automatically generated
by the scanner
50 under the control of controller 300. Such patterns may be comprised of a
single spot of light,
multiple spots of light, a continuous pattern of light, multiple continuous
patterns of light, and/or
any combination of these. In addition, the aiming pattern (using aim beam 202
described below)
need not be identical to the treatment pattern (using light beam 6), but
preferably at least defines
its boundaries in order to assure that the treatment light is delivered only
within the desired target
area for patient safety. This may be done, for example, by having the aiming
pattern provide an
outline of the intended treatment pattern. This way the spatial extent of the
treatment pattern
may be made known to the user, if not the exact locations of the individual
spots themselves, and
the scanning thus optimized for speed, efficiency and accuracy. The aiming
pattern may also be
made to be perceived as blinking in order to further enhance its visibility to
the user.
[00100] An optional contact lens 66, which can be any suitable ophthalmic
lens, can be used to
help further focus the optical beam 6 into the patient's eye 68 while helping
to stabilize eye
27
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
position. The positioning and character of optical beam 6 and/or the scan
pattern the beam 6
forms on the eye 68 may be further controlled by use of an input device such
as a joystick, or any
other appropriate user input device (e.g. GUI 304) to position the patient
and/or the optical
system.
[00101] The TREATMENT laser 4 and controller 300 can be set to target the
surfaces of the
targeted structures in the eye 68 and ensure that the beam 6 will be focused
where appropriate
and not unintentionally damage non-targeted tissue. Imaging modalities and
techniques
described herein, such as for example, Optical Coherence Tomography (OCT),
Purkinje
imaging, Scheimpflug imaging, structured light illumination, confocal back
reflectance imaging,
fluorescence imaging, or ultrasound may be used to determine the location and
measure the
thickness of the lens and lens capsule to provide greater precision to the
laser focusimg methods,
including 2D and 3D patterning, or other known ophthalmic or medical imaging
modalities
and/or combinations thereof. In the embodiment of Figure 2A, an OCT device 100
is described,
although other modalities are within the scope of the present invention. An
OCT scan of the eye
will provide information about the axial location of the anterior and
posterior lens capsule, the
boundaries of the cataract nucleus, as well as the depth of the anterior
chamber. This
information is then be loaded into the control electronics 300, and used to
program and control
the subsequent laser-assisted surgical procedure. The information may also he
used to determine
a wide variety of parameters related to the procedure such as, for example,
the upper and lower
axial limits of the focal planes used for modifying the lens capsule, cornea,
and synthetic
intraocular lens implant, among others.
[00102] The OCT device 100 in Figure 2A includes a broadband or a swept light
source 102
that is split by a fiber coupler 104 into a reference arm 106 and a sample arm
110. The reference
arm 106 includes a module 108 containing a reference reflection along with
suitable dispersion
and path length compensation. The sample arm 110 of the OCT device 100 has an
output
connector 112 that serves as an interface to the rest of the TREATMENT laser
system. The
return signals from both the reference and sample arms 106, 110 are then
directed by coupler 104
to a detection device 128, which employs one of the following; time domain,
frequency domain,
28
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
or single point detection techniques. In Figure 2A, a frequency domain
technique is used with an
OCT wavelength of 920nm and bandwidth of 100nm.
[00103] Exiting connector 112, the OCT beam 114 is collimated using lens 116.
The size of the
collimated beam 114 is determined by the focal length of lens 116. The size of
the beam 114 is
dictated by the desired NA at the focus in the eye and the magnification of
the beam train leading
to the eye 68. Generally, OCT beam 114 does not require as high an NA as the
TREATMENT
beam 6 in the focal plane and therefore the OCT beam 114 is smaller in
diameter than the
TREATMENT beam 6 at the beamcombiner 34 location. Following collimating lens
116 is
aperture 118 which further modifies the resultant NA of the OCT beam 114 at
the eye. The
diameter of aperture 118 is chosen to optimize OCT light incident on the
target tissue and the
strength of the return signal. Polarization control element 120, which may be
active or dynamic,
is used to compensate for polarization state changes which may be induced by
individual
differences in corneal birefringence, for example. Mirrors 122 & 124 are then
used to direct the
OCT beam 114 towards beamcombiners 126 & 34. Mirrors 122 & 124 may be
adjustable for
alignment purposes and in particular for overlaying of OCT beam 114 to
TREATMENT beam 6
subsequent to beamcombiner 34. Similarly, beamcombiner 126 is used to combine
the OCT
beam 114 with the aim beam 202 described below.
[00104] Once combined with the TREATMENT beam 6 subsequent to beamcombiner 34,
OCT
beam 114 follows the same path as TREATMENT beam 6 through the rest of the
system. In this
way, OCT beam 114 is indicative of the location of TREATMENT beam 6. OCT beam
114
passes through the z-scan 40 and x-y scan 50 devices then the objective lens
58 , contact lens 66
and on into the eye 68. Reflections and scatter off of structures within the
eye provide return
beams that retrace back through the optical system, into connector 112,
through coupler 104, and
to OCT detector 128. These return back reflections provide the OCT signals
that are in turn
interpreted by the system as to the location in X, Y Z of TREATMENT beam 6
focal location.
[00105] OCT device 100 works on the principle of measuring differences in
optical path length
between its reference and sample arms. Therefore, passing the OCT through z-
adjust 40 does
not extend the z-range of OCT system 100 because the optical path length does
not change as a
function of movement of 42. OCT system 100 has an inherent z-range that is
related to the
29
CA 02994799 2018-02-05
WO 2017/023296
PCT/US2015/043504
detection scheme, and in the case of frequency domain detection it is
specifically related to the
spectrometer and the location of the reference arm 106. In the case of OCT
system 100 used in
Figure 2A, the z-range is approximately 1-2mm in an aqueous environment.
Extending this
range to at least 4mm involves the adjustment of the path length of the
reference arm within
OCT system 100. Passing the OCT beam 114 in the sample arm through the z-scan
of z-adjust
40 allows for optimization of the OCT signal strength. This is accomplished by
focusing the
OCT beam 114 onto the targeted structure while accommodating the extended
optical path
length by commensurately increasing the path within the reference arm 106 of
OCT system 100.
[00106] Because of the fundamental differences in the OCT measurement with
respect to the
TREATMENT focus device due to influences such as immersion index, refraction,
and
aberration, both chromatic and monochromatic, care must be taken in analyzing
the OCT signal
with respect to the TREATMENT beam focal location. A calibration or
registration procedure
as a function of X, Y Z should be conducted in order to match the OCT signal
information to the
TREATMENT focus location and also to the relate to absolute dimensional
quantities.
[00107] Observation of an aim beam may also be used to assist the user to
directing the
TREATMENT laser focus. Additionally, an aim beam visible to the unaided eye in
lieu of the
infrared OCT and TREATMENT beams can be helpful with alignment provided the
aim beam
accurately represents the infrared beam parameters. An aim subsystem 200 is
employed in the
configuration shown in Figure 2A. The aim beam 202 is generated by an aim beam
light source
201, such as a helium-neon laser operating at a wavelength of 633nm.
Alternatively a laser
diode in the 630-650nm range could be used. The advantage of using the helium
neon 633nm
beam is its long coherence length, which would enable the use of the aim path
as a laser unequal
path interferometer (LUPI) to measure the optical quality of the beam train,
for example.
[00108] It should be also noted that TREATMENT beam may also be attenuated to
the
nanoJoule level and used instead of the OCT system described above. Such a
configuration
provides for the most direct correlation between the position of the focal
locations for imaging
and treatment ¨ they are the same beam.
[00109] In this embodiment, the same laser assembly is used both for
treatment (i.e.
modification) and imaging of the target tissue. For instance, the target
tissue may be imaged by
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
raster scanning the pulsed laser beam along the target tissue to provide for a
plurality of data
points, each data point having a location and intensity associated with it for
imaging of the target
tissue. In some embodiments, the raster scan is selected to deliver a sparse
pattern in order to
limit the patient's exposure, while still discerning a reasonable map of the
intraocular targets. In
these embodiments, the spacing between at least two adjacent laser spots
during an imaging
raster scan of a target tissue is greater than a spot spacing of the adjacent
laser spots in a
treatment scan of the same target tissue. In order to image the target tissue,
the treatment laser
beam (i.e. the laser beam having the parameters suitably chosen as described
above for the
modification of tissue) is preferably attenuated to the nanoJoule level for
imaging of the
structures to be treated. When used for imaging, the attenuated laser beam may
be referred to as
an imaging beam. In many embodiments, the treatment beam and the imaging beam
may be the
same except that the pulse energy of the laser source is lower than the
treatment beam when the
laser beam is used for imaging. In many embodiments, the pulse energy of the
laser beam when
used for imaging is preferably from about 0.1 nJ to 10 nJ, preferably less
than 2 nJ and more
preferably less than 1.8 nJ. The use of the same laser beam for both treatment
and imaging
provides for the most direct correlation between the position of the focal
locations for imaging
and treatment ¨ they are the same beam. This attenuated imaging beam can be
used directly in a
back reflectance measuring configuration, hut, alternatively, may he used
indirectly in a
fluorescence detection scheme. Since increases in both backscatter and
fluorescence within
tissue structures will be evident, both approaches have merit.
[00110] In a preferred embodiment, imaging of a first target area to be
modified is
performed sequentially with the modification of the tissue in the first target
area before moving
on to a second, different, target area, i.e. imaging is performed sequentially
with treatment in a
predetermined target area. Thus, for instance imaging of the lens capsule is
preferably followed
by treatment of the lens capsule before imaging is carried out on other either
structures, such as
the cornea or iris. In another embodiment, imaging of a first target area
where a first incision to
be place is performed sequentially with the scanning the treatment beam to
perform the incision
in the first target area before moving on to a second target area for
performing a second incision,
31
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
i.e. imaging of the area to be incised is performed sequentially with scanning
the treatment beam
to perform in the predetermined target area.
[00111] In another embodiment, a cataract procedure comprises a capsulotomy
incision, and at
least one of a cataract incision and a timbal relaxing incision. In one
embodiment, imaging of
the target tissue where the capsulotomy is to be performed is followed by
scanning of the
treatment to perform the capsulotomy, and then the treatment beam is scanned
to perform the
capsulotomy. Subsequently, imaging of the target tissue where the at least one
of the cataract
incisions (Cl) and the limbal relaxing incision (LRI) is carried out and then
the treatment beam is
scanned to perform the at least one of the LRI and the CI. When an LRI is
selected, this
minimizes the chance for the patient to move between imaging and treatment for
the LRIs which
are the most critical / sensitive to eye movements between image and
treatment. Furthermore,
since the requisite precision and inclusion size are much more relaxed for
lens conditioning as
compared to the incision of cornea and lens capsule, the present invention
contemplates the
addition of a short pulsed IR laser source to the above described system for
lens treatments, as
was mentioned above in the discussion of the use of milliJoule pulse from Q-
switched Nd:YAG
lasers for the treatment of posterior pacification. Such pulse energies will
cause larger
inclusions, which unsuitable for capsular and corneal incisions could provide
for robust
separation of a cataractous lens. The NIR wavelength is not strongly absorbed
or scattered by
the lens, as opposed to shorter wavelengths. This second treatment source may
have its beam
combined with that of the first treatment beam by means of another beam
splitter. The large
difference in wavelength makes this a fairly straightforward design. However,
that same spectral
difference will require a different registration to the imaging and/or ranging
modality, as was
discussed above with respect to Figure 2B.
[00112] FIG. 4 is an illustration of the line pattern applied across the lens
for OCT measurement
of the axial profile of the anterior chamber of the eye 20. OCT imaging of the
anterior chamber
of the eye 20 can be performed along a simple linear scan across the lens
using the same laser
and/or the same scanner used to produce the patterns for cutting. This scan
will provide
information about the axial location of the anterior and posterior lens
capsule, the boundaries of
the cataract nucleus, as well as the depth of the anterior chamber. This
information may then be
32
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
loaded into the laser scanning system, and used to program and control the
subsequent laser
assisted surgical procedure. The information may be used to determine a wide
variety of
parameters related to the procedure such as, for example, the upper and lower
axial limits of the
focal planes for cutting the lens capsule and segmentation of the lens cortex
and nucleus, the
thickness of the lens capsule among others.
[00113] FIGS. 5 through 9 illustrate different aspects of an embodiment of the
present
invention, which can be implemented using the system 200 described above. As
shown in FIG.
5, a capsulorhexis incision 400 (which may be created using system 200) is
tailored for
astigmatism-correcting intraocular lenses (IOLs). Such astigmatism-correcting
IOLs need to be
placed not only at the correct location within the capsule 402 of the eye 20,
but also oriented at
the correct rotational/clocking angle. Thus, they have inherent rotational
asymmetries, unlike
spherical IOLs. The incision 400 shown in this example is elliptical; however,
other shapes are
also useful. Incision 400 may be made continuously, or piecewise to largely
maintain the
structural integrity of the lens-capsule apparatus of the patient's eye 20.
[00114] Such incomplete incisions 400 may be thought of as perforated
incisions, and may be
made to be removed gently in order to minimize their potential to
inadvertently extend the
capsulorhexis. Either way, incision 400 is an enclosed incision, which for the
purposes of this
disclosure means that it starts and ends at the same location and encircles a
certain amount of
tissue therein. The simplest example of an enclosed incision is a circular
incision, where a round
piece of tissue is encircled by the incision. It follows therefore that an
enclosed treatment pattern
(i.e. generated by system 200 for forming an enclosed incision) is one that
also starts and ends at
the same location and defines a space encircled thereby.
[00115] One key feature of the enclosed incision 400 is that it includes a
registration feature to
orient the IOL that will be placed inside it. For the illustrated elliptical
incision 400, it elliptical
shape is it's registration feature, which allows for the accurate placement of
an IOL by virtue of
its inherent rotational asymmetry, unlike the desired circular outcome of a
manual CCC. The
elliptical major axis 404 and minor axis 406 of incision 400 are shown. Major
axis 404 and
minor axis 406 are not equal. Incision 400 may be made at any rotational angle
relative to the
eye 20 of a patient, although it is shown in this example to be in the plane
of the iris with its
33
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
major axis 404 lying along the horizontal. Incision 400 is intended to mate
with one or more
complementary registration features on an IOL. The system 200 may be used to
precisely define
the surface of the capsule 402 to be incised. This may serve to isolate the
laser pulses nominally
to the vicinity of the targeted capsule 402 itself, thus minimizing the energy
required and the
treatment time and commensurately increasing patient safety and overall
efficiency.
[00116] As shown in FIG. 6, an JUL 408 includes an optic portion 410 used to
focus light and a
haptic 416 used to position the IOL 408. Optic 410 is a rotationally
asymmetric lens (about its
optical axis) that include an elliptically shaped peripheral sidewall or edge
412, the
complementary registration feature that mates with elliptically shaped
incision 400. In this
example, the elliptically shaped edge 412 includes a major axis 418 and minor
axis 420. Major
axis 418 and minor axis 420 are not equal. IOL 408 further contains surface
414 that serves to
hold haptics element 416 and provide a resting place for capsule 402 to secure
optic 410 of
intraocular lens 408 in the proper orientation and position within the capsule
402 of a patient's
eye 20. Surface 414 is shown as elliptical, but need not be.
[00117] Haptics 416 provide stability and may serve to seat edge 412 of
intraocular lens 408 in
incision 400 by applying retaining force towards the anterior portion of
capsule 402. Haptics
416 may be deployed in any orientation. The orientation of the cylindrical
correction of optic
410 of intraocular lens 408 may he made to coincide with either its major axis
418 or its minor
axis 420. In this way, intraocular lenses JUL 408 and optic 410 may be
manufactured in a
standardized manner and the rotational orientation of incision 400 and the
spherical and
cylindrical optical powers of optic 410 may be made to vary to suit the
individual optical
prescription of the eye 20 of a patient.
[00118] FIG. 7 shows the proper immediate disposition of intraocular lens 408
once installed
into capsule 402 with mating registration features edge 412 and incision 400
engaged, and
resting upon surface 414. Major axis 404 and major axis 418 are not of equal
length. Minor axis
406 and minor axis 420 are not the same length, either. This is done to
accommodate the fact the
capsule 402 may contract somewhat subsequent to capsulorhexis incision. The
difference
between the lengths of these axes is intended to allow the capsule 402 to
contract and still better
seat intraocular lens 408 into capsule 402 via incision 400. These differences
should be limited
34
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
to allow for reasonable contraction, but not so much as to allow for
significant rotation of
intraocular lens 408. Typical values for these length differences may range
from 100 i_.tm to 500
um, for example.
[00119] FIG. 8 shows a side view on the same intraocular lens 408 depicted in
FIGS. 6 and 7.
In this schematic representation, edge 412 is shown on the same side of optic
410 as surface 424
of intraocular lens 408. The surface 422 on intraocular lens 408 serves to
maintain the integrity
of fit between edge 412 and incision 400. Edge 412 is seen as the projection
of surface 422 in
the alternate view depicted in FIGS. 6 and 7. Optical axis 411 of optic 410 is
shown. Haptics
416 lie along the line of sight in this view.
[00120] FIG. 9 is a side view of the lens configuration of FIG. 8, but rotated
90 degrees to show
that displaying surface 426 is not curved in both directions (i.e. shaped as a
cylindrical lens).
This cylindrical or tonic optical system of optic 410 provides cylindrical
correction for the
astigmatism of a patient. Haptics 416 lie perpendicular to the line of sight
in this view.
[00121] As shown in Figure 15 the system can also be used to alter the
structure of for example
corneal tissue without generating a cavitation bubble as shown in Figure 16.
These alterations of
the corneal tissue can be used to shape the refractive index profile of the
cornea 504 itself as
illustrated in Figure 18. A multitude of small localized modifications 822 can
be induced within
the cornea which will change the refractive profile by altering the refractive
index itself hut also
the mechanical strength of corneal tissue. So not only a change of index but
also a change of
corneal topography can be used. This is achieved by tightly controlling the
lateral spacing of the
laser effects utilizing beam deflection units 270 and focus shifting unit 704
through focusing unit
260.
[00122] As shown in the drawings for purposes of illustration, a method and
system for making
physical modifications (structural alterations) or incisions in eye tissue has
been disclosed. In
varying embodiments, the method and system disclosed herein provide many
advantages over
the current standard of care. Specifically, rapid and precise openings in the
lens capsule are
enabled using a 320nm to 430nm laser to facilitate the placement and stability
of intraocular
lenses. But also the alteration of the refractive power of the corneal tissue
by locally altering the
refractive index and reshaping the corneal topography.
1001231 Without further analysis, the foregoing so fully reveals the gist of
the present inventive
concepts that others can, by applying current knowledge, readily adapt it for
various applications
without omitting features that, from the standpoint of prior art, fairly
constitute essential
characteristics of the generic or specific aspects of this invention.
Therefore, such applications
should and are intended to be comprehended within the meaning and range of
equivalents of the
following claims. Although this invention has been described in terms of
certain embodiments,
other embodiments that are apparent to those of ordinary skill in the art are
also within the scope
of this invention.
1001241 The use of the terms "a" and "an" and "the" and similar referents in
the context of
describing the invention (especially in the context of the following claims)
are to be construed to
cover both the singular and the plural, unless otherwise indicated herein or
clearly contradicted
by context. The terms "comprising," "having," "including," and "containing"
are to be construed
as open-ended terms (i.e., meaning "including, but not limited to,") unless
otherwise noted. The
term "connected" is to be construed as partly or wholly contained within,
attached to, or joined
together, even if there is something intervening. Recitation of ranges of
values herein are merely
intended to serve as a shorthand method of referring individually to each
separate value falling
within the range, unless otherwise indicated herein, and each separate value
is incorporated into
the specification as if it were individually recited herein. All methods
described herein can be
performed in any suitable order unless otherwise indicated herein or otherwise
clearly
contradicted by context. The use of any and all examples, or exemplary
language (e.g., "such
as") provided herein, is intended merely to better illuminate embodiments of
the invention and
does not pose a limitation on the scope of the invention unless otherwise
claimed. No language
in the specification should be construed as indicating any non-claimed element
as essential to the
practice of the invention.
1001251 While certain illustrated embodiments of this disclosure have been
shown and
described in an exemplary form with a certain degree of particularity, those
skilled in the art will
understand that the embodiments are provided by way of example only, and that
various
36
Date Recue/Date Received 2020-07-15
CA 02994799 2018-02-05
WO 2017/023296 PCT/US2015/043504
variations can be made without departing from the spirit or scope of the
invention. Thus, it is
intended that this disclosure cover all modifications, alternative
constructions, changes,
substitutions, variations, as well as the combinations and arrangements of
parts, structures, and
steps that come within the spirit and scope of the invention as generally
expressed by the
following claims and their equivalents.
37