Note: Descriptions are shown in the official language in which they were submitted.
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
MULTILAYER FILMS AND METHODS THEREOF
TECHNICAL FIELD
Embodiments of the present disclosure generally relate to multilayer films,
and more
particularly, to multilayer films having a high cling force and are
substantially free of
polyisobutylene (FIB).
BACKGROUND
Multilayer films are often used in packaging, and may package diverse items,
such as, bulk
farm materials like grass and hay to small grocery store items like meats and
vegetables.
For all of these items it is usually desirable to have a strong, stretchy film
that has a
sufficient level of tack or cling such that the film can releasably adhere to
itself and/or an
article that is wrapped with the film.
To achieve the desired level of cling, additives, such as FIB, may be
incorporated into a
cling layer to improve the tack of the cling layer. However, films that
include such
additives can have one or more drawbacks such as 1) being excessively noisy
when
unwound from a film-roll when utilized on a high speed wrapping machine, 2)
having to be
aged for a period of time so that the additive migrates to the surface of the
film (i.e.,
blooms) during the aging period, 3) contaminating process equipment, and 4)
causing two-
sided cling when one-sided cling is desired. In addition, such additives can
cause undue
handling issues when they are in liquid form and drip to an undue degree from
process
equipment.
The multilayer films may also incorporate high levels of ethylene/alpha-olefin
elastomers to
achieve a higher level of tack or cling; however, ethylene/alpha-olefin
elastomers can make
the multilayer films very expensive. In addition, the films can be difficult
to process using
blown film techniques when ethylene/alpha-olefin elastomers are used at high
levels (e.g.,
greater than 90% by weight in a cling layer) because of their tackiness.
Accordingly, alternative multilayer films may be desired having improved
properties, such
as, high cling, while also being cost-effective and/or relatively easy to
fabricate using blown
film techniques.
- 1 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
SUMMARY
Disclosed in embodiments herein are multilayer films. The multilayer films
have a cling
layer and a release layer. The cling layer comprises (i) an ethylene/alpha-
olefin elastomer
having a density in the range of 0.855 to 0.890 grams/cm3 and a melt index
(12) in the range
of 0.1 to 30 grams/10 minutes; and (ii) a polyethylene polymer selected from
ultra-low
density polyethylene, a very low density polyethylene, or combinations
thereof, wherein the
polyethylene polymer has a density in the range 0.885 to 0.915 grams/cm3, a
melt index (12)
in the range of 0.1 to 30 grams/10 minutes, and a purge fraction greater than
20 percent as
determined by the Crystallization Elution Fractionation (CEF) test method. The
release
layer comprises a polyethylene composition which comprises the reaction
product of
ethylene and, optionally, one or more alpha olefin comonomers, wherein the
polyethylene
composition is characterized by one or more of the following properties: (a) a
melt index, 12,
of from 0.1 to 2 g/10 min, (b) a density of from 0.910 to 0.930 g/cc, (c) a
melt flow ratio,
110/12, of from 6 to 7.5, and (d) a molecular weight distribution, (Mw/Mn) of
from 2.5 to 3.9.
Also disclosed in embodiments herein are methods of making multilayer films.
The
methods comprise coextruding a cling layer composition with a release layer
composition in
an extruder to form a tube having a cling layer and a release layer, and
cooling the tube to
form a multilayer film. The cling layer compositions comprise (i) an
ethylene/alpha-olefin
elastomer having a density in the range of 0.855 to 0.890 grams/cm3 and a melt
index(I2) in
the range of 0.1 to 30 grams/10 minutes; and (ii) a polyethylene polymer
selected from
ultra-low density polyethylene, a very low density polyethylene, or
combinations thereof,
wherein the polyethylene polymer has a density in the range 0.885 to 0.915
grams/cm3, a
melt index(I2) in the range of 0.1 to 30 grams/10 minutes, and a purge
fraction greater than
20 percent as determined by the Crystallization Elution Fractionation (CEF)
test method.
The release layer composition comprises a polyethylene composition which
comprises the
reaction product of ethylene and, optionally, one or more alpha olefin
comonomers, wherein
the polyethylene composition is characterized by one or more of the following
properties:
(a) a melt index, 12, of from 0.1 to 2 g/10 min, (b) a density of from 0.910
to 0.930 g/cc, (c)
a melt flow ratio, 110/12, of from 6 to 7.5, and (d) a molecular weight
distribution, (Mw/Mn)
of from 2.5 to 3.9.
Additional features and advantages of the embodiments will be set forth in the
detailed
description which follows, and in part will be readily apparent to those
skilled in the art
from that description or recognized by practicing the embodiments described
herein,
- 2 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
including the detailed description which follows, the claims, as well as the
appended
drawing.
It is to be understood that both the foregoing and the following description
describe various
embodiments and are intended to provide an overview or framework for
understanding the
nature and character of the claimed subject matter. The accompanying drawing
is included
to provide a further understanding of the various embodiments, and is
incorporated into and
constitutes a part of this specification. The drawing illustrates the various
embodiments
described herein, and together with the description serves to explain the
principles and
operations of the claimed subject matter.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 graphically depicts the cling force for several inventive films
according to one or
more embodiments described herein in comparison to several comparative films.
DETAILED DESCRIPTION
Reference will now be made in detail to embodiments of multilayer films and
materials
used to make such films. The multilayer films may be used in stretch-cling
applications. It
is noted, however, that this is merely an illustrative implementation of the
embodiments
disclosed herein. The embodiments are applicable to other technologies that
are susceptible
to similar problems as those discussed above. For example, the multilayer
films described
herein may be used as surface protection films, agricultural films, such as
silage wrap, or in
other flexible packaging applications, such as, shrink films, heavy duty
shipping sacks,
liners, sacks, stand-up pouches, detergent pouches, sachets, etc., all of
which are within the
purview of the present embodiments.
In embodiments described herein, the multilayer films comprise a cling layer
and a release
layer. Optionally, one or more core layers may be positioned between the cling
layer and
the release layer. The cling layer is an outer layer of the multilayer film
that has a sufficient
level of adhesive tack such that the cling layer of the multilayer film may
form a releasable
bond when brought into contact with a surface, such as, the surface of an
article or the
surface of the release layer. The release layer is an outer layer of the
multilayer film that
exhibits low adhesion to the cling layer. The release layer can allow for
separation to occur
between the cling layer/release layer interface on a roll such that the
multilayer film may be
unrolled from a spool without undue force or without the film tearing.
- 3 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
The thickness of the cling and release layers can vary over a wide range. In
some
embodiments, the cling layer may have a thickness that is from 5-50 percent of
the overall
thickness of the film, from 5-30 percent of the overall thickness of the film,
or even from
10-30 percent of the overall thickness of the film. The release layer may have
a thickness
that is from 5-50 percent of the overall thickness of the film, from 5-30
percent of the
overall thickness of the film, or even from 10-30 percent of the overall
thickness of the film.
In some embodiments, where one or more core layers are present, the one or
more core
layers may have a thickness that is from 0-90 percent of the overall thickness
of the film,
10-90 percent of the overall thickness of the film, 20-90 percent of the
overall thickness of
the film, 30-90 percent of the overall thickness of the film, 40-90 percent of
the overall
thickness of the film, or 40-80 percent of the overall thickness of the film.
The ratio of the
thicknesses among a cling layer, a release layer, and any optional core layers
can be any
ratio that provides desirable properties such as cling, release, and the like.
In some
embodiments, a multilayer film can have a cling layer thickness, a core layer
thickness, and
a release layer thickness in a ratio in the range of 1:8:1 to 3:4:3.
Cling Layer
The cling layer may comprise an ethylene/alpha-olefin elastomer and a
polyethylene
polymer selected from ultra-low density polyethylene, a very low density
polyethylene, or
combinations thereof. In some embodiments, the cling layer comprises an
ethylene/alpha-
olefin elastomer and an ultra-low density polyethylene. In other embodiments,
the cling
layer comprises an ethylene/alpha-olefin elastomer and a very low density
polyethylene. In
further embodiments, the cling layer comprises an ethylene/alpha-olefin
elastomer, an ultra-
low density polyethylene, and a very low density polyethylene.
In embodiments described herein, the ethylene/alpha-olefin elastomers may
comprise
greater than 50%, by weight, of the units derived from ethylene. All
individual values and
subranges of greater than 50%, by weight, are included and disclosed herein.
For example,
the ethylene/alpha-olefin elastomer may comprise at least 60%, at least 70%,
at least 80%,
at least 90%, at least 92%, at least 95%, at least 97%, at least 98%, at least
99%, at least
99.5%, from greater than 50% to 99%, from greater than 50% to 97%, from
greater than
50% to 94%, from greater than 50% to 90%, from 70% to 99.5%, from 70% to 99%,
from
70% to 97%, from 70% to 94%, from 80% to 99.5%, from 80% to 99%, from 80% to
97%,
from 80% to 94%, from 80% to 90%, from 85% to 99.5%, from 85% to 99%, from 85%
to
97%, from 88% to 99.9%, 88% to 99.7%, from 88% to 99.5%, from 88% to 99%, from
88%
- 4 -
84217324
to 98%, from 88% to 97%, from 88% to 95%, from 88% to 94%, from 90% to 99.9%,
from 90%
to 99.5%, from 90% to 99%, from 90% to 97%, from 90% to 95%, from 93% to
99.9%, from 93%
to 99.5%, from 93% to 99%, or from 93% to 97%, by weight, of the units derived
from ethylene.
The ethylene/alpha-olefin elastomer may comprise less than 50%, by weight, of
units derived from
one or more alpha-olefin comonomers. All individual values and subranges of
less than 50%, by
weight, are included herein and disclosed herein. For example, the
ethylene/alpha-olefin elastomer
may comprise less than 45%, less than 40%, less than 35%, less than 30%, less
than 25%, less than
20%, less than 18%, less than 15%, less than 12%, less than 10%, less than 8%,
less than 5%, less
than 4%, less than 3%, from 0.2 to 15 %, 0.2 to 12%, 0.2 to 10%, 0.2 to 8%,
0.2 to 5%, 0.2 to 3%,
.. 0.2 to 2%, 0.5 to 12%, 0.5 to 10%, 0.5 to 8%, 0.5 to 5%, 0.5 to 3%, 0.5 to
2.5%, 1 to 10%, 1 to
8%, 1 to 5%, 1 to 3%, 2 to 10%, 2 to 8%, 2 to 5%, 3.5 to 12%, 3.5 to 10%, 3.5
to 8%, 3.5% to 7%,
or 4 to 12%, 4 to 10%, 4 to 8%, or 4 to 7%, by weight, of units derived from
one or more alpha-
olefin comonomers. The comonomer content may be measured using any suitable
technique, such
as techniques based on nuclear magnetic resonance ("NMR") spectroscopy, and,
for example, by
13C NMR analysis as described in U.S. Patent 7,498,282.
Suitable alpha-olefin comonomers include those containing from 3 to 20 carbon
atoms (C3-C20).
For example, the alpha-olefin may be a C4-C20 alpha-olefin, a C4-C12 alpha-
olefin, a C3-C10
alpha-olefin, a C3-C8 alpha-olefin, a C4-C8 alpha-olefin, or a C6-C8 alpha-
olefin. In some
embodiments, the alpha-olefin is selected from the group consisting of
propylene, 1-butene, 1-
pentene, 1-hexene, 4-methyl- 1-pentene, 1-heptene, 1-octene, 1-nonene and 1-
decene. In other
embodiments, the alpha-olefin is selected from the group consisting of
propylene, 1-butene, 1-
hexene, and 1-octene. In further embodiments, the alpha-olefin is selected
from the group
consisting of 1-hexene and 1-octene.
Exemplary ethylene/alpha-olefin elastomers for use in a cling layer are
commercially available
under the trade names AFFINITYTm from the Dow Chemical Company, ENGAGETM from
the
Dow Chemical Company, INFUSETM from the Dow Chemical Company, EXACT from
ExxonMobil Chemical, and TAFMERTm from Mitsui Chemicals, Inc. Suitable
ethylene/alpha-
olefin elastomers are further described in U.S. Pat. No. 5,272,236 (Lai et
al.), U.S. Pat. No.
6,486,284 (Karande et al.), and U.S. Pat. No. 6,100,341 (Friedman).
Ethylene/alpha-olefin elastomers may be produced using single-site catalysts.
Methods for
producing olefin polymers using single site catalysts are described in U.S.
Pat. No. 5,272,236 (Lai
- 5 -
Date Regue/Date Received 2022-09-16
84217324
et al.) and U.S. Pat. No. 6,486,284 (Karande et al.). Single-site catalyst
systems may include
metallocene catalysts and post-metallocene catalysts. In
exemplary embodiments, the
ethylene/alpha-olefin elastomer may be produced by a metallocene catalyst or a
post-metallocene
catalyst.
In some embodiments, the ethylene/alpha-olefin elastomer can include one or
more olefin block
copolymers. Olefin block copolymers are polymers comprising two or more
chemically distinct
regions or segments (referred to as "blocks") that may be joined in a linear
manner, that is, a
polymer comprising chemically differentiated units, which are joined end-to-
end with respect to
polymerized ethylenic functionality, rather than in pendent or grafted
fashion. The blocks may
differ in the amount or type of incorporated comonomer, density, amount of
crystallinity,
crystallite size attributable to a polymer of such composition, type or degree
of tacticity (isotactic
or syndiotactic), regio-regularity or regio-irregularity, amount of branching
(including long chain
branching or hyper-branching), homogeneity or any other chemical or physical
property. Suitable
olefin block copolymers are further described in U.S. Pat. No. 7,608,668.
In embodiments described herein, the ethylene/alpha-olefin elastomers have a
density in the range
of 0.855 to 0.890 grams/cc. All individual values and subranges of from 0.855
g/cc to 0.890 g/cc
are included and disclosed herein. For example, in some embodiments, the
ethylene/alpha-olefin
elastomers may have a density of from 0.860 g/cc to 0.890 g/cc. In other
embodiments, the
ethylene/alpha-olefin elastomers may have a density of from 0.865 g/cc to
0.890 g/cc. Density
may be measured according to ASTM D792.
In embodiments described herein, the ethylene/alpha-olefin elastomers have a
melt index (12) in
the range of 0.1 to 30 grams/10 minutes. All individual values and subranges
of from of 0.1 to 30
grams/10 minutes are included and disclosed herein. For example, in some
embodiments, the
ethylene/alpha-olefin elastomers may have a melt index (12) in the range of
0.1 to 20 gams/10
minutes. In other embodiments, the ethylene/alpha-olefin elastomers may have a
melt index (12)
in the range of 0.1 to 15 grams/10 minutes. In further embodiments, the
ethylene/alpha-olefin
elastomers may have a melt index (12) in the range
- 6 -
Date Regue/Date Received 2022-09-16
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
of 0.1 to 10 grams/10 minutes. Melt index (I2) may be measured according to
ASTM
D1238, condition 190 C/2.16 kg.
The ethylene/alpha-olefin elastomer can be incorporated into a cling layer
formulation in an
amount based on a variety of factors, such as, amounts of other polymers
(e.g., ULDPE or
ultra-low density polyethylene and VLDPE or very low density polyethylene),
desired
tack/cling; cost; tack stability during manufacturing, transportation,
storage, and/or use
conditions. In some embodiments, the ethylene/alpha-olefin elastomer is
present in the
cling layer in an amount in the range of 10 to 90 percent by weight of the
cling layer, in the
range of 15 to 90 percent by weight of the cling layer, in the range of 30 to
90 percent by
.. weight of the cling layer, or even in the range of 40 to 85 percent by
weight of the cling
layer. Of course, all individual values and subranges of 10 to 90 percent by
weight of the
cling layer are included and disclosed herein.
The cling layer also comprises a polyethylene polymer selected from ULDPE,
VLDPE, and
combinations thereof. ULDPE and/or VLDPE can be incorporated into cling layer
formulations in an amount based on a variety of factors, such as, the amounts
of other
ingredients (e.g., ethylene/alpha-olefin elastomer) present in the cling
layer, desired
tack/cling properties in the film; cost; tack stability during manufacturing,
transportation,
storage, and/or use conditions. In some embodiments, ULDPE and/or VLDPE is
present in
the cling layer in an amount in the range of 10 to 90 percent by weight of the
cling layer, in
the range of 20 to 85 percent by weight of the cling layer, in the range of 30
to 70 percent
by weight of the cling layer, or even in the range of 35 to 70 percent by
weight of the cling
layer.
ULDPE or VLDPE comprises, in polymerized form, a majority weight percent of
units
derived from ethylene, based on the total weight of the ULDPE or VLDPE. The
ULDPE or
.. VLDPE may be an interpolymer of ethylene and at least one ethylenically
unsaturated
comonomer. In some embodiments, the comonomer is a C3-C20 alpha-olefin. In
other
embodiments, the comonomer is a C3-C8 alpha-olefin. In further embodiments,
the C3-C8
alpha-olefin is selected from propylene, 1-butene, 1-hexene, or 1-octene. In
even further
embodiments, the ULDPE or VLDPE may be an ethylene/propylene copolymer,
ethylene/butene copolymer, ethylene/hexene copolymer, or ethylene/octene
copolymer.
ULDPE or VLDPE can be made using Ziegler-Natta catalyst techniques to provide
a
desired level of purge fraction. Ziegler-Natta catalysts are described in U.S.
Publication
- 7 -
84217324
Numbers 2008/0038571 (Klitzmiller et al.) and 2008/0176981 (Biscoglio et al.).
In some
embodiments, Ziegler¨Natta catalyzed ULDPE or VLDPE includes a copolymer of
ethylene and
3.5 to 10.5 mol percent of at least one comonomer selected from the group
consisting of C3-C20 a-
olefins, dienes, and cycloalkenes. "ULDPE" and "VLDPE" can be used
interchangeably. See,
e.g., U.S. Publication Number 2008/0038571(Klitzmiller et al.). In some
embodiments, VLDPE
refers to ULDPEs or VLDPEs that are manufactured by gas phase reaction
techniques and ULDPE
refers to ULDPEs or VLDPEs that are manufactured by liquid phase (solution)
reaction
techniques. Suitable ULDPEs include ATTANETm 4404 available from The Dow
Chemical
Company. Suitable VLDPEs include DFDB-9042 NT VLDPE, available from The Dow
Chemical
Company.
In embodiments described herein, the polyethylene polymer has a density of
0.885 to 0.915 g/cc.
All individual values and subranges of from 0.885 to 0.915 g/cc are included
and disclosed herein.
For example, in some embodiments, the polyethylene polymer has a density of
0.885 to 0.910 g/cc.
In other embodiments, the polyethylene polymer has a density of 0.890 to 0.915
g/cc. In further
.. embodiments, the polyethylene polymer has a density of 0.890 to 0.912 g/cc.
In even further
embodiments, the polyethylene polymer has a density of 0.895 to 0.905 g/cc. In
even further
embodiments, the polyethylene polymer has a density of 0.899 to 0.905 g/cc.
Density may be
measured according to ASTM D792.
In embodiments described herein, the polyethylene polymer has a melt index
(12) in the range of
0.1 to 30 grams/10 minutes. All individual values and subranges of from 0.1 to
30 grams/10
minutes are included and disclosed herein. For example, in some embodiments,
the polyethylene
polymer has a melt index (12) in the range of 0.1 to 25 g/10 minutes. In other
embodiments, the
polyethylene polymer has a melt index (12) in the range of 0.1 to 20 g/10
minutes. In further
embodiments, the polyethylene polymer has a melt index (12) in the range of
0.1 to 15 g/10 minutes.
.. In even further embodiments, the polyethylene polymer has a melt index (12)
in the range of 0.1 to
10 g/10 minutes. In even further embodiments, the polyethylene polymer has a
melt index (12) in
the range of 0.5 to 10 grams/10 minutes. Melt index (12) may be measured
according to ASTM
D1238, condition 190 C/2.16 kg.
In embodiments described herein, the polyethylene polymer may have a molecular
weight
distribution (Mw/Mn) of from 3.0 to 6Ø Molecular weight distribution can be
described as
- 8 -
Date Regue/Date Received 2022-09-16
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
the ratio of weight average molecular weight (Mw) to number average molecular
weight
(M0) (i.e., Mw /Mn), and can be measured by gel permeation chromatography
techniques.
In embodiments described herein, the polyethylene polymer has a purge fraction
of greater
than 20 percent as determined by the Crystallization Elution Fractionation
(CEF) test
method. The purge fraction can qualitatively refer to branched (e.g., highly-
branched) and
non-crystallizable polyolefin copolymers that can be generated during a
polymerization
process via a Ziegler¨Natta catalyst ("Z-N" catalyst), and become part of the
final
polyethylene product. Without being bound by theory, it is believed that a
polyethylene
polymer having a purge fraction of at least 20 wt.% as determined by the CEF
test method
can be blended with ethylene/alpha-olefin elastomer to provide a cling layer
with desirable
cling properties. In some embodiments, the polyethylene polymer has a purge
fraction of
greater than 22 percent, or greater than 25 percent. In other embodiments, the
polyethylene
polymer may have a purge fraction of less than 45 percent, or less than 40
percent. Of
course, it should be understood that polyethylene polymers having higher purge
fraction
amounts may be utilized.
Without being bound by theory, it is believed that the combination of (i) an
ethylene/alpha-
olefin elastomer and (ii) a polyethylene polymer having a purge fraction
greater than 20
percent, can provide similar or enhanced cling in the cling layer as compared
to a cling
layer having a higher level of PE (polyethylene) elastomer and no polyethylene
polymer
having a purge fraction greater than 20 percent. Specifically, it is believed
that
ethylene/alpha-olefin elastomers can give the cling layer a smooth surface
(i.e., better
surface conformability) while the polyethylene polymer having a purge fraction
greater than
20 percent can enable a diffusion mechanism across the polymer interface to
form
entanglement within the polymer matrix. Reducing the amount of PE elastomer in
a cling
layer to provide desired cling properties can be advantageous as PE elastomer
can be
relatively expensive and/or can be difficult to process with blown film
techniques when
used at relatively high levels (e.g., greater than 90% by weight of a layer)
because of its
tackiness. Further, the cling layer can have desired cling properties without
including
polyisobutylene (PIB) (i.e., PIB-free). Eliminating the need for FIB additives
can be
advantageous as the additives are sometimes subjected to a time consuming
aging period to
migrate the additive to the surface of the film (i.e., bloom). In addition,
the additives can be
in liquid form, and therefore, drip to an undue degree from process equipment.
Further, the
- 9 -
84217324
additives may contaminate process equipment and/or cause two-sided cling where
it is not desired.
The polyethylene polymer (ULDPE and/or VLDPE) may be incorporated into the
cling layer at a
sufficient level to permit a lower amount of ethylene/alpha-olefin elastomer
present in the cling
layer, while still providing desired cling properties. This can be
advantageous as ethylene/alpha-
olefin elastomers can be relatively more expensive than the polyethylene
polymer (ULDPE and/or
VLDPE). In addition, the ethylene/alpha-olefin elastomer can be difficult to
process using blown
film techniques, particularly, when the ethylene/alpha-olefin elastomer is
present at relatively high
levels (e.g., greater than 90 wt.%) in the cling layer due to its tackiness.
In some embodiments,
the cling layer may comprise 30 wt.% to 70 wt.% of the polyethylene polymer
(ULDPE and/or
VLDPE) and 70 wt.% to 30 wt.% of the ethylene/alpha-olefin elastomer.
Optionally, the cling layer can include one or more additives and/or
additional polymers. For
example, in some embodiments, the cling layer can optionally include low
density polyethylene
(LDPE) and/or linear low density polyethylene (LLDPE) as desired. Low density
polyethylene
can have a density in the range in the range of 0.915 to 0.935 grams/cm3 and a
melt index in the
range of 0.1 to 30 grams/10 minutes. Linear low density polyethylene can have
a density in the
range of 0.912 to 0.940 grams/cm3 and a melt index in the range of 0.5 to 30
grams/10 minutes.
The cling layer can include LDPE in an amount from 0 to 30 percent by weight
of the cling layer.
The cling layer can include LLDPE in an amount from 0 to 30 percent by weight
of the cling layer.
In some embodiments, the cling layer can include LDPE in an amount from 0 to
30 percent by
.. weight of the cling layer and LLDPE in an amount from 0 to 30 percent by
weight of the cling
layer.
The ethylene/alpha-olefin elastomer can be dry blended with the polyethylene
polymer to form a
cling layer blend. Methods of dry blending resins can be found in U. S. Pat.
No. 3,318,538
(Needham). The ethylene/alpha-olefin elastomer can also be melt-blended with
the polyethylene
polymer to form a cling layer blend. Methods of melt blending resins can be
found in U. S. Pat.
No. 6,111,019 (Arjunan et al.). The cling layer blend can be used in an
extrusion process to form
a cling layer via, for e.g., blown film techniques.
Release Layer
The release layer comprises a polyethylene composition that comprises the
reaction product of
ethylene and, optionally, one or more alpha olefin comonomers. The
polyethylene composition
- 10 -
Date Regue/Date Received 2022-09-16
84217324
comprises greater than 50 wt.% of the units derived from ethylene and less
than 30 wt.% of the
units derived from one or more alpha-olefin comonomers. In some embodiments,
the polyethylene
composition comprises (a) greater than or equal to 55%, for example, greater
than or equal to 60%,
greater than or equal to 65%, greater than or equal to 70%, greater than or
equal to 75%, greater
than or equal to 80%, greater than or equal to 85%, greater than or equal to
90%, greater than or
equal to 92%, greater than or equal to 95%, greater than or equal to 97%,
greater than or equal to
98%, greater than or equal to 99%, greater than or equal to 99.5%, from
greater than 50% to 99%,
from greater than 50% to 97%, from greater than 50% to 94%, from greater than
50% to 90%,
from 70% to 99.5%, from 70% to 99%, from 70% to 97% from 70% to 94%, from 80%
to 99.5%,
from 80% to 99%, from 80% to 97%, from 80% to 94%, from 80% to 90%, from 85%
to 99.5%,
from 85% to 99%, from 85% to 97%, from 88% to 99.9%, 88% to 99.7%, from 88% to
99.5%,
from 88% to 99%, from 88% to 98%, from 88% to 97%, from 88% to 95%, from 88%
to 94%,
from 90% to 99.9%, from 90% to 99.5% from 90% to 99%, from 90% to 97%, from
90% to 95%,
from 93% to 99.9%, from 93% to 99.5% from 93% to 99%, or from 93% to 97%, by
weight, of
the units derived from ethylene; and (b) optionally, less than 30 percent, for
example, less than 25
percent, or less than 20 percent, less than 18%, less than 15%, less than 12%,
less than 10%, less
than 8%, less than 5%, less than 4%, less than 3%, less than 2%, less than 1%,
from 0.1 to 20 %,
from 0.1 to 15 %, 0.1 to 12%, 0.1 to 10%, 0.1 to 8%, 0.1 to 5%, 0.1 to 3%, 0.1
to 2%, 0.5 to 12%,
0.5 to 10%, 0.5 to 8%, 0.5 to 5%, 0.5 to 3%, 0.5 to 2.5%, 1 to 10%, 1 to 8%, 1
to 5%, 1 to 3%, 2
to 10%, 2 to 8%, 2 to 5%, 3.5 to 12%, 3.5 to 10%, 3.5 to 8%, 3.5% to 7%, or 4
to 12%, 4 to 10%,
4 to 8%, or 4 to 7%, by weight, of units derived from one or more a-olefin
comonomers. The
comonomer content may be measured using any suitable technique, such as
techniques based on
nuclear magnetic resonance ("NMR") spectroscopy, and, for example, by 13C NMR
analysis as
described in U.S. Patent 7,498,282.
Suitable comonomers may include alpha-olefin comonomers, typically having no
more than 20
carbon atoms. The one or more alpha-olefins may be selected from the group
consisting of
C3-C20 acetylenically unsaturated monomers and C4-C18 diolefins. Those skilled
in the art will
understand that the selected monomers are desirably those that do not destroy
- 11 -
Date Regue/Date Received 2022-09-16
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
conventional Ziegler-Natta catalysts. For example, the alpha-olefin comonomers
may have
3 to 10 carbon atoms, or 3 to 8 carbon atoms. Exemplary alpha-olefin
comonomers include,
but are not limited to, propylene, 1-butene, 1-pentene, 1-hexene, 1-heptene, 1-
octene, 1-
nonene, 1-decene, and 4-methyl-1 -pentene. The one or more alpha-olefin
comonomers
may, for example, be selected from the group consisting of propylene, 1-
butene, 1-hexene,
and 1-octene; or in the alternative, from the group consisting of 1-butene, 1-
hexene and 1-
octene. In some embodiments, the polyethylene composition comprises greater
than 0 wt.%
and less than 30 wt.% of units derived from one or more of octene, hexene, or
butene
comonomers.
In some embodiments, the polyethylene composition of the release layer is
formed in the
presence of a catalyst composition comprising a multi-metallic procatalyst via
solution
polymerization. The multi-metallic procatalyst used in producing the reaction
product is at
least trimetallic, but may also include more than three transition metals, and
thus may in one
embodiment be defined more comprehensively as multi-metallic. These three, or
more,
transition metals are selected prior to production of the catalyst. In a
particular
embodiment, the multi-metal catalyst comprises titanium as one element.
The catalyst compositions may be prepared beginning first with preparation of
a
conditioned magnesium halide based support. Preparation of a conditioned
magnesium
halide based support begins with selecting an organomagnesium compound or a
complex
including an organomagnesium compound. Such compound or complex is desirably
soluble in an inert hydrocarbon diluent. The concentrations of components are
preferably
such that when the active halide, such as a metallic or non-metallic halide,
and the
magnesium complex are combined, the resultant slurry is from about 0.005 to
about 0.25
molar (moles/liter) with respect to magnesium. Examples of suitable inert
organic diluents
include liquefied ethane, propane, isobutane, n-butane, n-hexane, the various
isomeric
hexanes, isooctane, paraffinic mixtures of alkanes having from 5 to 10 carbon
atoms,
cyclohexane, methylcyclopentane, dimethylcyclohexane, dodecane, industrial
solvents
composed of saturated or aromatic hydrocarbons such as kerosene, naphthas, and
combinations thereof, especially when freed of any olefin compounds and other
impurities,
and especially those having boiling points in the range from about -50 C to
about 200 C.
Also included as suitable inert diluents are ethylbenzene, cumene, decalin and
combinations
thereof.
- 12 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
Suitable organomagnesium compounds and complexes may include, for example,
magnesium C2-C8 alkyls and aryls, magnesium alkoxides and aryloxides,
carboxylated
magnesium alkoxides, and carboxylated magnesium aryloxides. Preferred sources
of
magnesium moieties may include the magnesium C2-C8 alkyls and C1-C4 alkoxides.
Such
organomagnesium compound or complex may be reacted with a metallic or non-
metallic
halide source, such as a chloride, bromide, iodide, or fluoride, in order to
make a
magnesium halide compound under suitable conditions. Such conditions may
include a
temperature ranging from -25 C to 100 C, alternatively, 0 C to 50 C; a
time ranging from
1 to 12 hours, alternatively, from 4 to 6 hours; or both. The result is a
magnesium halide
based support.
The magnesium halide support is then reacted with a selected conditioning
compound
containing an element selected from the group consisting of boron, aluminum,
gallium,
indium and tellurium, under conditions suitable to form a conditioned
magnesium halide
support. This compound and the magnesium halide support are then brought into
contact
under conditions sufficient to result in a conditioned magnesium halide
support. Such
conditions may include a temperature ranging from 0 C to 50 C, or
alternatively, from 25
C to 35 C; a time ranging from 4 to 24 hours, or alternatively, from 6 to 12
hours; or both.
The conditioning compound has a molar ratio constitution that is specific and
which is
believed to be an important feature in ensuring the desirable catalyst
performance.
Specifically, the procatalyst desirably exhibits a molar ratio of the
magnesium to the
conditioning compound that ranges from 3:1 to 6:1. Without wishing to be bound
by any
theory of mechanism, it is suggested that this aging serves to facilitate or
enhance
adsorption of additional metals onto the support.
Once the conditioned support is prepared and suitably aged, it is brought into
contact with a
titanium compound which may be added individually or as a mixture with the
"second
metal". In certain preferred embodiments titanium halides or alkoxides, or
combinations
thereof, may be selected. Conditions may include a temperature within the
range from 0 C
to 50 C, alternatively from 25 C to 35 C; a time from 3 hours to 24 hours,
alternatively
from 6 hours to 12 hours; or both. The result of this step is adsorption of at
least a portion
of the titanium compound onto the conditioned magnesium halide support.
Finally, one or two additional metals, referred to herein as "the second
metal" and "the third
metal" for convenience, will also be adsorbed onto the magnesium-based
support, The
"second metal" and the "third metal" are independently selected from zirconium
(Zr),
- 13 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
hafnium (Hp, vanadium (V), niobium (Nb), tantalum (Ta), chromium (Cr),
molybdenum
(Mo), and tungsten (W). These metals may be incorporated in any of a variety
of ways
known to those skilled in the art, but generally contact between the
conditioned magnesium
based halide support including titanium and the selected second and third
metals, in, e.g.,
liquid phase such as an appropriate hydrocarbon solvent, will be suitable to
ensure
deposition of the additional metals to form what may now be referred to as the
"procatalyst," which is a multi-metallic procatalyst.
The multi-metallic procatalyst has a molar ratio constitution that is specific
and which is
believed to be an important feature in ensuring the desirable polymer
properties that may be
attributed to the catalyst made from the procatalyst. Specifically, the
procatalyst desirably
exhibits a molar ratio of the magnesium to a combination of the titanium and
the second and
third metals that ranges from 30:1 to 5:1; under conditions sufficient to form
a multi-
metallic procatalyst. Thus, the overall molar ratio of magnesium to titanium
ranges from
8:1 to 80:1.
Once the procatalyst has been formed, it may be used to form a final catalyst
by combining
it with a cocatalyst consisting of at least one organometallic compound such
as an alkyl or
haloalkyl of aluminum, an alkylaluminum halide, a Grignard reagent, an alkali
metal
aluminum hydride, an alkali metal borohydride, an alkali metal hydride, an
alkaline earth
metal hydride, or the like. The formation of the final catalyst from the
reaction of the
procatalyst and the organometallic cocatalyst may be carried out in situ, or
just prior to
entering the polymerization reactor. Thus, the combination of the cocatalyst
and the
procatalyst may occur under a wide variety of conditions. Such conditions may
include, for
example, contacting them under an inert atmosphere such as nitrogen, argon or
other inert
gas at temperatures in the range from 0 C to 250 C, preferably from 15 C to
200 C. In
the preparation of the catalytic reaction product, it is not necessary to
separate hydrocarbon
soluble components from hydrocarbon insoluble components. Time for contact
between the
procatalyst and cocatalyst may desirably range, for example, from 0 to 240
seconds,
preferably from 5 to 120 seconds. Various combinations of these conditions may
be
employed.
In embodiments described herein, the polyethylene composition may have a metal
catalyst
residual of greater than or equal to 1 parts by combined weight of at least
three metal
residues per one million parts of polyethylene polymer, wherein the at least
three metal
residues are selected from the group consisting of titanium, zirconium,
hafnium, vanadium,
- 14 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
niobium, tantalum, chromium, molybdenum, tungsten, and combinations thereof,
and
wherein each of the at least three metal residues is present at greater than
or equal to 0.2
ppm, for example, in the range of from 0.2 to 5 ppm. All individual values and
subranges
from greater than or equal to 0.2 ppm are included herein and disclosed
herein; for example,
the polyethylene composition may further comprise greater than or equal to 2
parts by
combined weight of at least three metal residues remaining from the multi-
metallic
polymerization catalyst per one million parts of the polyethylene composition.
In some embodiments, the polyethylene composition comprises at least 0.75 ppm
of V
(Vanadium). All individual values and subranges from at least 0.75 ppm of V
are included
and disclosed herein; for example the lower limit of the V in the polyethylene
composition
may be 0.75, 1, 1.1, 1.2, 1.3 or 1.4 ppm to an upper limit of the V in the
polyethylene
composition may be 5, 4, 3, 2, 1.9, 1.8, 1.7, 1.6, 1.5, or 1 ppm. The vanadium
catalyst metal
residual concentration for the polyethylene composition can be measured using
the Neutron
Activation Method for Metals described below.
In some embodiments, the polyethylene composition comprises at least 0.3 ppm
of Zr
(Zirconium). All individual values and subranges of at least 0.3 ppm of Zr are
included and
disclosed herein; for example the lower limit of the Zr in the polyethylene
composition may
be 0.3, 0.4, 0.5, 0.6 or 0.7 ppm. In yet another embodiment, the upper limit
of the Zr in the
polyethylene composition may be 5, 4, 3, 2, 1, 0.9, 0.8 or 0.7 ppm. The
zirconium catalyst
.. metal residual concentration for the polyethylene composition can be
measured using the
Neutron Activation Method for Metals described below.
In embodiments described herein, the polyethylene composition may have a
density of
0.910 g/cc to 0.930 g/cc. All individual values and subranges of at least
0.910 g/cc to 0.930
g/cc are included and disclosed herein. For example, in some embodiments, the
polyethylene has a density of 0.910 to 0.927 g/cc, 0.910 to 0.925 g/cc, 0.915
to 0.930 g/cc,
0.915 to 0.925 g/cc, or 0.916 to 0.922 g/cc. Density may be measured in
accordance with
ASTM D792.
In embodiments described herein, the polyethylene composition may have a melt
index, 12,
of 0.1 g/10 min to 2.0 g/10 min. All individual values and subranges of at
least 0.1 g/10
.. min to 2.0 g/10 min are included and disclosed herein. For example, in some
embodiments,
the polyethylene composition may have a melt index, 12, of 0.1 g/10 min to 1.8
g/10 min,
0.1 g/10 min to 1.6 g/10 min, or 0.1 g/10 min to 1.5 g/10 min. In other
embodiments, the
- 15 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
polyethylene composition may have a melt index, 12, 0.4 g/10 min to 2.0 g/10
min, 0.4 g/10
min to 1.8 g/10 min, or 0.4 g/10 min to 1.5 g/10 min. In further embodiments,
the
polyethylene composition may have a melt index, 12, 0.5 g/10 min to 1.5 g/10
minõ 0.5
g/10 min to 1.0 g/10 mm, or 0.7 g/10 min to 1.0 g/10 min. Melt index, 12, may
be measured
in accordance with ASTM D1238 (190 C. and 2.16 kg).
In embodiments described herein, the polyethylene composition may have a melt
flow ratio,
110/12, of less than 7.6. All individual values and subranges of less than 7.6
are included
and disclosed herein. For example, in some embodiments, the polyethylene
composition
may have a melt flow ratio, I10/12, of less than 7.5, 7.4, 7.3, 7.2, 7.1 or
7Ø In other
embodiments, the polyethylene composition may have a melt flow ratio, 110/12,
of from 6.0
to 7.5, 6.2 to 7.5, 6.5 to 7.5, 6.5 to 7.4, or, 6.5 to 7.3. In further
embodiments, the
polyethylene composition may have a melt flow ratio, 110/12, of from 6.2 to
7.5, 6.3 to 7.4,
6.4 to 7.3, or 6.5 to 7.2. Melt index, 110, may be measured in accordance with
ASTM
D1238 (190 C. and 10.0 kg).
In embodiments described herein, the polyethylene composition may have a
molecular
weight distribution (Mw/Mn) of from 2.5 to 4Ø All individual values and
subranges of
from 2.5 to 4.0 are included and disclosed herein. For example, the
polyethylene
composition may have an Mw/Mn ratio from a lower limit of 2.5, 2.6, 2.7, or
2.8 to an
upper limit of 4.0, 3.9, 3.8, or 3.7. In some embodiments, the polyethylene
composition
may have an Mw/Mn ratio of from 2.7 to 3.9, 2.8 to 3.9, or 2.8 to 3.7. In
other
embodiments, the polyethylene composition may have an Mw/Mn ratio of from 3.0
to 4.0,
3.1 to 3.9, 3.2 to 3.9, 3.3 to 3.8, or 3.4 to 3.7. Molecular weight
distribution can be
described as the ratio of weight average molecular weight (M,) to number
average
molecular weight (Mr,) (i.e., Mw /M), and can be measured by gel permeation
chromatography techniques.
In embodiments described herein, the polyethylene composition may have a
number
average molecular weight, Mn (g/mol), of from 30,000 to 50,000 g/mol. All
individual
values and subranges of from 30,000 to 50,000 g/mol are included and disclosed
herein.
For example, the polyethylene composition may have a Mn from 30,000 to 45,000
g/mol,
30,000 to 40,000 g/mol, 32,000 to 38,000 g/mol, 34,000 to 37,000 g/mol, or
35,000 to
36,000 g/mol.
- 16 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
In embodiments described herein, the polyethylene composition may have a
weight average
molecular weight, Mw (g/mol), of from 110,000 to 140,000 g/mol. All individual
values
and subranges of from 110,000 to 140,000 g/mol are included and disclosed
herein. For
example, the polyethylene composition may have an Mw from 115,000 to 135,000
g/mol,
117,000 to 133,000 g/mol, or 119,000 to 131,000 g/mol.
In embodiments described herein, the polyethylene composition may have a z
average
molecular weight, Mz (g/mol), of from 300,000 to 425,000 g/mol. All individual
values
and subranges of from 300,000 to 425,000 g/mol are included and disclosed
herein. For
example, the polyethylene composition may have an Mz from 325,000 to 425,000
g/mol,
330,000 to 425,000 g/mol, or 360,000 to 411,000 g/mol.
In embodiments described herein, the polyethylene composition may have a melt
strength
of from 2 ¨ 7 cN at 190 C. All individual values and subranges of from 2 to 7
cN are
included and disclosed herein. For example, the polyethylene composition may
have a melt
strength from 2.5 to 6 cN, 2.75 to 5.5 cN, or 2.5 to 5.5 cN at 190 C.
In embodiments described herein, the polyethylene composition may have a wt%
of Zone 1
or a purge fraction, as determined by CEF, of 3% to 6%. All individual values
and
subranges of from 3% to 6% are included and disclosed herein. For example, in
some
embodiments, the polyethylene composition may have a wt% of Zone 1 or a purge
fraction,
as determined by CEF, of from 3.3% to 5.5%, or 3.6% to 5.0%. Details of the
CEF method
are described below.
In embodiments described herein, the polyethylene composition may have a
copolymer
fraction, as determined by CEF, of 60% - 80%. All individual values and
subranges of from
60% to 80% are included and disclosed herein. For example, the polyethylene
composition
may have a copolymer fraction, as determined by CEF, of from 65% to 80%, 65%
to 75%,
68% to 76%, or 68% to 72%.
In embodiments described herein, the polyethylene composition may have a high
density
fraction, as determined by CEF, of 15% - 30%. All individual values and
subranges of from
15% to 30% are included and disclosed herein. For example, the polyethylene
composition
may have a high density fraction, as determined by CEF, of from 17% to 29%, or
18% to
28%.
- 17 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
In embodiments described herein, the polyethylene composition may have a
viscosity ratio
(viscosity at 0.1 rad/s / viscosity at 100 rad/s, both measured at 190 C) of 3
to 6. All
individual values and subranges of from 3 to 6 are included and disclosed
herein. For
example, the polyethylene composition may have a viscosity ratio of from 4 to
6, or 4.5 to
5.5.
In embodiments described herein, the polyethylene composition may have a tan
delta at 0.1
rad/s measured at 190 C of 5 to 25. All individual values and subranges of
from 5 to 25 are
included and disclosed herein. For example, the polyethylene composition may
have a tan
delta at 0.1 rad/s measured at 190 C of from 5 to 20, 5 to 15, or 10 to 13.
In embodiments described herein, the polyethylene composition may have a
composition
distribution breadth index, CDBI, of less than 60%. All individual values and
subranges of
less than 60% are included and disclosed herein. For example, in some
embodiments, the
polyethylene composition may have a CDBI of less than 58%, 55%, 53%, 51%,
50.5%, or
50.0%. In other embodiments, the CDBI may be from 30% to 60%, 35% to 50%, or
from
.. 40% to 48%.
The CDBI may be defined as the weight percent of the polymer molecules having
a
comonomer content within 50 percent of the median total molar comonomer
content. The
CDBI of linear polyethylene, which does not contain a comonomer, is defined to
be 100%.
The CDBI of a copolymer is readily calculated from data obtained from
crystallization
elution fractionation ("CEF") as described below. Unless otherwise indicated,
terms such
as "comonomer content", "average comonomer content" and the like refer to the
bulk
comonomer content of the indicated interpolymer blend, blend component, or
fraction on a
molar basis.
In embodiments described herein, the polyethylene composition may be further
characterized by one or more of the following properties: melt index (I2),
melt flow ratio
(110/12), density, Mw/Mn, or CDBI, as previously described herein. In some
embodiments,
the polyethylene composition is further characterized by one or more of the
following
properties: melt index (12), melt flow ratio (I10/12), or density. In other
embodiments, the
polyethylene composition is further characterized by one or more of the
following
properties: (a) a melt index, 12, measured according to ASTM D 1238 (2.16 kg
@190 C), of
from 0.1 to 2 g/10 min; (b) a density (measured according to ASTM D792) from
0.910 to
0.930 g/cm3; or (c) a melt flow ratio, 110/12, of from 6 to 7.6.
- 18 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
In embodiments herein, the release layer comprises from 20 wt.% to 80 wt.% of
the
polyethylene composition. All individual values and subranges of from 20 wt.%
to 80 wt.%
are included and disclosed herein. For example, in some embodiments, the
release layer
comprises from 25 wt.% to 70 wt.%, 30 wt.% to 65 wt.%, or 30 wt.% to 60 wt.%,
by weight
of the release layer, of the polyethylene composition.
In embodiments described herein, the release layer may further comprise one or
more of
low density polyethylene (LDPE), linear low density polyethylene (LLDPE),
polypropylene, and/or ethylene vinyl acetate (EVA). In some embodiments, the
release
layer may further comprise an LDPE. In other embodiments, the release layer
may further
comprise an LDPE present in an amount ranging from 1 wt.% to 100 wt.%, 20 wt.%
to 80
wt.%, 20 wt.% to 70 wt.%, 30 wt.% to 70 wt.%, or 40 wt.% to 70 wt.%, by weight
of the
release layer. Also, in some embodiments, the release layer may further
comprise an
LLDPE. In other embodiments, the release layer may further comprise an LLDPE
in an
amount ranging from 1 wt.% to 100 wt.%, 1 wt.% to 50 wt.%, 1 wt.% to 25 wt.%,
5 wt.% to
25 wt.%õ or 5 wt.% to 20 wt.%, by weight of the release layer. The LDPE may
have a
density in the range of 0.915 to 0.935 grams/cm3 and a melt index in the range
of 0.1 to 30
grams/10 minutes. The LLDPE may have a density in the range in the range of
0.912 to
0.940 grams/cm3 and a melt index in the range of 0.5 to 30 grams/10 minutes.
Core Layer
Optionally, a multilayer film described herein can include one or more core
layers
positioned between the cling layer and the release layer. In some embodiments,
the
multilayer film comprises a core layer positioned between the cling layer and
the release
layer. In other embodiments, the multilayer film comprises a single core layer
positioned
between and contacting at least a portion of the cling layer and the release
layer.
The core layer can include one or more of LLDPE, LDPE, ethylene/alpha-olefin
elastomer,
polypropylene elastomer, and/or ethylene vinyl acetate (EVA). In some
embodiments, the
core layer comprises LLDPE in an amount from 0 to 100 percent, 25 to 100
percent, 30 to
100 percent, 40 to 100 percent, 50 to 100 percent, 60 to 100 percent, 65 to
100 percent, 70
to 100 percent, 75 to 100 percent, by weight of the core layer. In other
embodiments, the
core layer comprises LLDPE and one or more of ethylene/alpha-olefin elastomer,
polypropylene elastomer, or ethylene vinyl acetate. The one or more of
ethylene/alpha-
olefin elastomer, polypropylene elastomer, or ethylene vinyl acetate may be
present in
- 19 -
84217324
amounts ranging from 1 to 30 percent, 1 to 25 percent, 1 to 20 percent, or 1
to 15 percent, by weight,
of the core layer. In further embodiments, the core layer may comprise LLDPE
and LDPE. The LDPE
may be present in amounts ranging from 1 to 50 percent, 1 to 35 percent, 1 to
25 percent, or 1 to 20
percent, by weight, of the core layer. Exemplary LLDPE for use in the core
layer of a multilayer film
is commercially available under the trade names ELITETm, TUFLINTm, and
DOWLEXTM from the
Dow Chemical Company.
The multilayer films described herein can be made by a variety of techniques,
such as, blown film
techniques. Methods of making multilayer blown films are described in U.S.
Patent No. 6,521,338
(Maka). For example, in some embodiments, a multilayer blown film can be made
by co-extruding a
cling layer composition with the release layer composition (and, optionally, a
core layer composition)
in an extruder to form a tube having a cling layer and a release layer, and
cooling the tube to form a
multilayer blown stretch film.
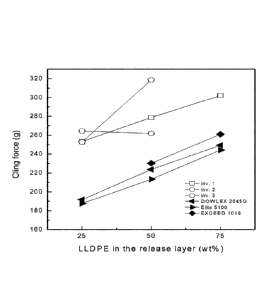
In embodiments described herein, the multilayer films may exhibit a cling
force according to the
following equation:
Cling Force (g) = (0.97 x wt.% of Polyethylene Composition in the Release
Layer) + 204
Embodiments of the multilayer films will now be further described in the
following illustrative
examples.
TEST METHODS
Density
.. Density can be measured in accordance with ASTM D-792.
Melt Index
Melt index (I2) can be measured in accordance with ASTM D-1238, Procedure B
(condition
190 C/2.16 kg). Melt index (ho) can be measured in accordance with ASTM D-
1238, Procedure B
(condition 190 C/10.0 kg).
Gel Permeation Chromatography (GPC)
The chromatographic system consisted of a PolymerChar GPC-IR (Valencia, Spain)
high temperature
GPC chromatograph equipped with an internal IRS detector. The autosampler
- 20 -
Date Regue/Date Received 2022-09-16
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
oven compartment was set at 160 Celsius and the column compartment was set at
150
Celsius. The columns used were 3 Agilent "Mixed B" 30cm 10-micron linear mixed-
bed
columns and a 10-um pre-column. The chromatographic solvent used was 1,2,4
trichlorobenzene and contained 200 ppm of butylated hydroxytoluene (BHT). The
solvent
source was nitrogen sparged. The injection volume used was 200 microliters and
the flow
rate was 1.0 milliliters/minute.
Calibration of the GPC column set was performed with 21 narrow molecular
weight
distribution polystyrene standards with molecular weights ranging from 580 to
8,400,000
and were arranged in 6 "cocktail" mixtures with at least a decade of
separation between
individual molecular weights. The standards were purchased from Agilent
Technologies.
The polystyrene standards were prepared at 0.025 grams in 50 milliliters of
solvent for
molecular weights equal to or greater than 1,000,000, and 0.05 grams in 50
milliliters of
solvent for molecular weights less than 1,000,000. The polystyrene standards
were
dissolved at 80 degrees Celsius with gentle agitation for 30 minutes. The
polystyrene
standard peak molecular weights were converted to polyethylene molecular
weights using
Equation 1 (as described in Williams and Ward, J. Polym. Sci., Polym. Let., 6,
621 (1968)).:
Mpolyethyiene = A x (11/1
\--polystyrene)B (EQ1)
where M is the molecular weight, A has a value of 0.4315 and B is equal to
1Ø
A fifth order polynomial was used to fit the respective polyethylene-
equivalent calibration
.. points. A small adjustment to A (from approximately 0.415 to 0.44) was made
to correct
for column resolution and band-broadening effects such that NIST standard NBS
1475 is
obtained at 52,000 Mw.
The total plate count of the GPC column set was performed with Eicosane
(prepared at 0.04
g in 50 milliliters of TCB and dissolved for 20 minutes with gentle
agitation.) The plate
.. count (Equation 2) and symmetry (Equation 3) were measured on a 200
microliter injection
according to the following equations:
Plate Count = 5.54 * RVPeak Max (EQ2)
Peak Width at -height)
where RV is the retention volume in milliliters, the peak width is in
milliliters, the peak max
is the maximum height of the peak, and 1/2 height is 1/2 height of the peak
maximum.
- 21 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
(Rear Peak RVone tenth height¨ RV Peak max)
Symmetry (EQ3)
(RV Peak max¨Front Peak RV one tenth height)
where RV is the retention volume in milliliters and the peak width is in
milliliters, Peak
max is the maximum position of the peak, one tenth height is 1/10 height of
the peak
maximum, rear peak refers to the peak tail at later retention volumes than the
peak max, and
front peak refers to the peak front at earlier retention volumes than the peak
max. The plate
count for the chromatographic system should be greater than 24,000 and
symmetry should
be between 0.98 and 1.22.
Samples were prepared in a semi-automatic manner with the PolymerChar
"Instrument
Control" Software, wherein the samples were weight-targeted at 2 mg/ml, and
the solvent
(contained 200ppm BHT) was added to a pre nitrogen-sparged septa-capped vial,
via the
PolymerChar high temperature autosampler. The samples were dissolved for 2
hours at
160 Celsius under "low speed" shaking.
The calculations of Mn, Mw, and Mz were based on GPC results using the
internal IR5
detector (measurement channel) of the PolymerChar GPC-IR chromatograph
according to
Equations 4-6, using PolymerChar GPCOneTM software, the baseline-subtracted IR
chromatogram at each equally-spaced data collection point (i), and the
polyethylene
equivalent molecular weight obtained from the narrow standard calibration
curve for the
point (i) from Equation 1.
I IR,
Mn = _____________________
i / (EQ 4)
M polyethylene i
i
Ri * M polyethylene i)
Mw = ______________________
IR (EQ 5)
- 22 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
IR 2
* M polyethylene
Mz = _______________________
(EQ 6)
E* M polyethylthei)
In order to monitor the deviations over time, a flowrate marker (decane) was
introduced into
each sample via a micropump controlled with the PolymerChar GPC-IR system.
This
flowrate marker was used to linearly correct the flowrate for each sample by
alignment of
the respective decane peak within the sample to that of the decane peak within
the narrow
standards calibration. Any changes in the time of the decane marker peak are
then assumed
to be related to a linear shift in both flowrate and chromatographic slope. To
facilitate the
highest accuracy of a RV measurement of the flow marker peak, a least-squares
fitting
routine is used to fit the peak of the flow marker concentration chromatogram
to a quadratic
equation. The first derivative of the quadratic equation is then used to solve
for the true
peak position. After calibrating the system based on a flow marker peak, the
effective
flowrate (as a measurement of the calibration slope) is calculated as Equation
7. Processing
of the flow marker peak was done via the PolymerChar GPCOneTM Software.
FlowMarkerCalibration
Flowrateef fective = FlOWratenominat x ______________ (EQ7)
Ftowmarkerobserved
Neutron Activation Method for Metals
Two sets of duplicate samples were prepared by transferring approximately 3.5
grams of the
pellets into pre-cleaned 2 dram polyethylene vials. Standards were prepared
for each metal
tested from their NIST traceable standard solutions (Certi. pure from SPEX)
into 2-dram
polyethylene vials. They were diluted using milli-Q pure water to 6m1 and the
vials were
heat-sealed. The samples and standards were then analyzed for these elements,
using a
Mark I TRIGA nuclear reactor. The reactions and experimental conditions used
for these
elements are summarized in the table below. The samples were transferred to un-
irradiated
vials before doing the gamma-spectroscopy. The elemental concentrations were
calculated
using CANBERRA software and standard comparative technique. Table 1 provides
measurement parameters for metals determination.
- 23 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
Table 1: Reactions and experimental conditions used for elements during
neutron
activation.
Reactor
Elements Nuclear reaction Isotope Half life
Power
Al 27A1(n, y)28A1 28A1 2.24 m 250 kW
Cl 37C1(11,Y)38C1 38C1 37.2 m 250 kW
Cr 50Cr(n,y)51Cr 51Cr 27.7 d 250 kW
Hf 180111(n,y)181Hf , isiiif 42.4 d 250 kW
Mg 26mg(n,y)27mg 27A wiA-g __
9.46 m 250 kW
.
Mo 98M0(11,y)99M0 99Mo 66.0 h 250 kW
Nb 93Nb(n,y)94'1\1b 94mNb 6.26 m 250 kW
Ta 181Ta(n,y)182Ta i82Ta 114.4 d 250 kW
Ti soTi(n,y)siTi 51Ti 5.76 m 250 kW
W 186w(n,y)187w 187w 23.7 h 250 kW
V 51 V(I1,y)52V 52V 3.75 m 250 kW
Zr 96Zi(n,y)97Zr 97Zr 16.91 h 250 kW
Table 1 Continued
Elements Irradiation Time Waiting Time Counting Time Gamma Energy, keV
Al 2m 4m 4.5 min 1778.5
Cl 2m 4m 4.5 min 1642.5, 2166.5
Cr 90m 5h 1.6h 320
Hf 90m 5h 1.6h 133,482
Mg 2m 4m 4.5 min 843.8, 1014
Mo 90 na 5h 1.6h 181,739.7, 141
Nb 2m 4m 4.5 min 871
Ta 90m 5h 1.6h 1121, 1222
,
Ti 2m 4m 4.5 min 320
W 90m 5h 1.6h 135,481
V 2m 4m 4.5 min 1434
Zr 90m 5h 1.6h 743.4
Differential Scanning Calorimetry (DSC)
DSC was used to measure the melting and crystallization behavior of a polymer
over a wide
range of temperatures. For example, the TA Instruments Q1000 DSC, equipped
with an
RCS (refrigerated cooling system) and an autosampler was used to perform this
analysis.
During testing, a nitrogen purge gas flow of 50 ml/min was used. Each sample
was melt
pressed into a thin film at about 175 C; the melted sample was then air-cooled
to room
- 24 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
temperature (approx. 25 C). The film sample was formed by pressing a "0.1 to
0.2 gram"
sample at 175 C at 1,500 psi, and 30 seconds, to form a "0.1 to 0.2 mil thick"
film. A 3-10
mg, 6 mm diameter specimen was extracted from the cooled polymer, weighed,
placed in a
light aluminum pan (ca 50 mg), and crimped shut. Analysis was then performed
to
determine its thermal properties.
The thermal behavior of the sample was determined by ramping the sample
temperature up
and down to create a heat flow versus temperature profile. First, the sample
was rapidly
heated to 180 C, and held isothermal for five minutes, in order to remove its
thermal
history. Next, the sample was cooled to -40 C, at a 10 C/minute cooling rate,
and held
isothermal at -40 C for five minutes. The sample was then heated to 150 C
(this is the
"second heat" ramp) at a 10 C/minute heating rate. The cooling and second
heating curves
were recorded. The cool curve was analyzed by setting baseline endpoints from
the
beginning of crystallization to -20 C. The heat curve was analyzed by setting
baseline
endpoints from -20 C to the end of melt. The values determined were peak
melting
temperature (Tm), peak crystallization temperature (TA heat of fusion (Hf) (in
Joules per
gram), and the calculated % crystallinity for polyethylene samples using: %
Crystallinity =
((Hf)/(292 J/g)) x 100. The heat of fusion (Hf) and the peak melting
temperature were
reported from the second heat curve. Peak crystallization temperature is
determined from
the cooling curve.
Tml is the highest temperature peak melting temperature, Tm2 is the second
highest peak
melting temperature, and Tm3 is the third highest peak melting temperature.
Tcl is the
highest temperature peak crystallization temperature, Tc2 is the second
highest peak
crystallization temperature, and Tc3 is the third highest peak crystallization
temperature.
Melt Strength
Melt strength was measured at 190 C using a Goettfert Rheotens 71.97
(Goettfert Inc.;
Rock Hill, SC), melt fed with a Goettfert Rheotester 2000 capillary rheometer
equipped
with a flat entrance angle (180 degrees) of length of 30 mm and diameter of
2.0 mm. The
pellets (20-30 gram pellets) were fed into the barrel (length = 300 mm,
diameter = 12 mm),
compressed and allowed to melt for 10 minutes before being extruded at a
constant piston
speed of 0.265 mm/s, which corresponds to a wall shear rate of 38.2 s-1 at the
given die
diameter. The extrudate passed through the wheels of the Rheotens located 100
mm below
the die exit and was pulled by the wheels downward at an acceleration rate of
2.4 mm/s2.
- 25 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
The force (in cN) exerted on the wheels was recorded as a function of the
velocity of the
wheels (in mm/s). Melt strength is reported as the plateau force (cN) before
the strand
broke.
Dynamic Mechanical Spectroscopy (DMS)
Resins were compression-molded into "3 mm thick x 1 inch" circular plaques at
350 F, for
five minutes, under 1500 psi pressure, in air. The sample was then taken out
of the press,
and placed on a counter to cool.
A constant temperature frequency sweep was performed using a TA Instruments
"Advanced
Rheometric Expansion System (ARES)," equipped with 25 mm (diameter) parallel
plates,
under a nitrogen purge. The sample was placed on the plate, and allowed to
melt for five
minutes at 190 C. The plates were then closed to a gap of "2 mm," the sample
trimmed
(extra sample that extends beyond the circumference of the "25 mm diameter"
plate was
removed), and then the test was started. The method had an additional five
minute delay
built in, to allow for temperature equilibrium. The experiments were performed
at 190 C
over a frequency range of 0.1 to 100 rad/s. The strain amplitude was constant
at 10%. The
complex viscosity Ti*, tan (6) or tan delta, viscosity at 0.1 rad/s (V0.1),
the viscosity at 100
rad/s (V100), and the viscosity ratio (VO.1/V100) were calculated from these
data.
Crystallization Elution Fractionation (CEF) Method
The Crystallization Elution Fractionation (CEF) technology is conducted
according to
Monrabal et al, Macromol. Symp. 257, 71-79 (2007). The CEF instrument is
equipped with
an IR-4 or IR-5 detector (such as that sold commercially from PolymerChar,
Spain) and a
two angle light scattering detector Model 2040 (such as those sold
commercially from
Precision Detectors). A 10 micron guard column of 50 mm x 4.6 mm (such as that
sold
commercially from PolymerLabs) is installed before the IR-4 or IR-5 detector
in the
detector oven. Ortho-dichlorobenzene (ODCB, 99% anhydrous grade) and 2,5-di-
tert-
buty1-4-methylphenol (BHT) (such as commercially available from Sigma-Aldrich)
are
obtained. Silica gel 40 (particle size 0.2-0.5 mm) (such as commercially
available from
EMD Chemicals) is also obtained. The silica gel is dried in a vacuum oven at
160 C for at
least two hours before use. ODCB is sparged with dried nitrogen (N2) for one
hour before
use. Dried nitrogen is obtained by passing nitrogen at <90 psig over CaCO3 and
5A
molecular sieves. ODCB is further dried by adding five grams of the dried
silica to two
- 26 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
liters of ODCB or by pumping through a column or columns packed with dried
silica
between 0.1m1/min to 1.0m1/min. Eight hundred milligrams of BHT are added to
two liters
of ODCB if no inert gas such as N2 is used in purging the sample vial. Dried
ODCB with or
without BHT is hereinafter referred to as "ODCB-m." A sample solution is
prepared by,
using the autosampler, dissolving a polymer sample in ODCB-m at 4 mg/ml under
shaking
at 160 C for 2 hours. 300 I, of the sample solution is injected into the
column. The
temperature profile of CEF is: crystallization at 3 C/min from 110 C to 30 C,
thermal
equilibrium at 30 C for 5 minutes (including Soluble Fraction Elution Time
being set as 2
minutes), and elution at 3 C/min from 30 C to 140 C. The flow rate during
crystallization
is 0.052 ml/min. The flow rate during elution is 0.50 ml/min. The IR-4 or IR-5
signal data
is collected at one data point/second.
The CEF column is packed with glass beads at 125 pm 6% (such as those
commercially
available with acid wash from MO-SCI Specialty Products) with 1/8 inch
stainless tubing
according to U.S. 8,372,931. The internal liquid volume of the CEF column is
between 2.1
ml and 2.3 ml. Temperature calibration is performed by using a mixture of NIST
Standard
Reference Material linear polyethylene 1475a (1.0 mg/m1) and Eicosane (2
mg/ml) in
ODCB-m. The calibration consists of four steps: (1) calculating the delay
volume defined
as the temperature offset between the measured peak elution temperature of
Eicosane minus
30.00 C; (2) subtracting the temperature offset of the elution temperature
from the CEF raw
temperature data. It is noted that this temperature offset is a function of
experimental
conditions, such as elution temperature, elution flow rate, etc.; (3) creating
a linear
calibration line transforming the elution temperature across a range of 30.00
C and
140.00 C such that NIST linear polyethylene 1475a has a peak temperature at
101.00 C,
and Eicosane has a peak temperature of 30.00 C, (4) for the soluble fraction
measured
.. isothermally at 30 C, the elution temperature is extrapolated linearly by
using the elution
heating rate of 3 C/min. The reported elution peak temperatures are obtained
such that the
observed comonomer content calibration curve agrees with those previously
reported in
USP 8,372,931.
The CEF chromatogram is divided into three zones, the elution temperature
range of each
zone is specified in Table 8. The wt% of the lowest temperature zone is
generally called the
wt% of Zone 1 or the wt% of the purge fraction. The wt% of the intermediate
temperature
zone is generally called the wt% of Zone 2 or the wt% of the copolymer
fraction. The wt%
- 27 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
of the highest temperature zone is generally called the wt% of Zone 3 or the
wt% of the
high density fraction.
Comonomer Distribution Breadth Index (CDBI)
The CDBI is calculated using the methodology described in WO/93/03093 from
data
obtained from CEF. CDBI is defined as the weight percent of the polymer
molecules
having a comonomer content within 50 percent of the median total molar
comonomer
content. It represents a comparison of the comonomer distribution in the
polymer to the
comonomer distribution expected for a Bernoullian distribution.
CEF is used to measure the short chain branching distribution (SCBD) of the
polyolefin. A
CEF molar comonomer content calibration is performed using 24 reference
materials (e.g.,
polyethylene octene random copolymer and ethylene butene copolymer) with a
narrow
SCBD having a comonomer mole fraction ranging from 0 to 0.108 and a Mw from
28,400
to 174,000 g/mole. The In (mole fraction of ethylene), which is the In
(comonomer mole
fraction), versus 1/T (K) is obtained, where T is the elution temperature in
Kelvin of each
reference material. The comonomer distribution of the reference materials is
determined
using 13C NMR analysis in accordance with techniques described, for example,
in U.S.
Patent No. 5,292,845 (Kawasaki, et al.) and by J. C. Randall in Rev.
Macrornol. Chem.
Phys., C29, 201-317.
Cling
On-pallet stretch cling (for stretch cling performance) can be measured by
Lantech SHS test
equipment. The test consists of stretching the film at 200% at a constant
force F2 of 8 lbs.
for 6 wraps with the turntable running at a rate of 10 rpm. The end of the
film is then
attached to a load cell which measures the amount of force, in grams, needed
to pull the
film off the drum.
EXAMPLES
Cling & Core Layers
The resins used in the cling and core layers are shown in Table 2. The resins
in Table 2 are
available from the Dow Chemical Company.
- 28 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
Table 2: Resins used in the cling and core layers
Purge fraction,
Melt index
Density Product (MI or 12) (g/10 as
determined by
(g/cm3) CEF
min)
(wt.%)
Resin for cling layer
SAMPLE 1 ULDPE 0.900 5.0 32
AFBINITYTm EG 8100G PE
0.870 1.0 Not applicable
Elastomer
Resin for core layer
DOWLEXTM 2045G LLDPE 0.920 1.0
The core layer consists of 100 wt.% of DOWLEXTM 2045G LLDPE. The cling layer
consists of 65 wt.% of AFFIN1TYTm EG 8100G PE Elastomer and 35 wt.% of SAMPLE
1
ULDPE.
Preparation of the Ziegler-Natta (Z-N) Catalyst to make Sample 1 ULDPE
The Z-N catalyst was prepared according to the following procedure.
Ethylaluminium
dichloride (EADC) solution (15 wt.% EADC dissolved in Isopar E (available from
ExxonMobil Chemical Co., Houston, Tex.)) was transferred into the stirred
vessel
containing magnesium chloride (MgCl2) slurry (0.2M in Isopar E) and aged while
stirring
for 6 hours prior to use. Titanium tetraisopropoxide (Ti(OiPr)4) was
transferred to the
MgCl2/EADC slurry vessel, followed by at least 8 hours of aging to obtain the
procatalyst.
The ratio of MgC12:EADC:Ti(OiPr)4 was such that the metal ratio (Mg:Al:Ti) in
the
procatalyst was 40:12.5:3.
Preparation of Sample 1 ULDPE
A solution polymerization reactor system was used. A hydrocarbon solvent and
monomer
(ethylene) were injected into the reactor as a liquid. Comonomer (1-octene)
was mixed
with the liquid solvent. This feed stream was cooled to less than 20 C before
injection into
the reactor system. The reactor system was operated at polymer concentrations
in excess of
10 wt.%. The adiabatic temperature rise of the solution accounts for the heat
removal from
the polymerization reactions.
The solvent used in the solution polyethylene process was a high purity iso-
paraffinic
fraction of C6-C8 hydrocarbons. Fresh 1-octene was purified and mixed with the
recycle
- 29 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
solvent stream (contained solvent, ethylene, 1-octene, and hydrogen). After
mixing with the
recycle stream the combined liquid stream was further purified before using a
600-1000
psig pressure feed pump to pump the contents to the reactor. Fresh ethylene
was purified
and compressed to 600-1000 psig. Hydrogen (a telogen used to reduce molecular
weight)
and ethylene were flow controlled into the recycle solvent stream and the
total feed stream
was cooled to the appropriate feed temperature, which can be <40 C. The
process used the
Ziegler-Natta catalyst described above to catalyze the polymerization
reactions. The reactor
was operated at pressures > 400 psig and temperatures in excess of 70 C. The
ethylene
conversion was maintained in the reactor by controlling the catalyst injection
rate. The
residence time was relatively short (less than 30 minutes). The ethylene
conversion per
reaction pass was greater than 80 wt.% ethylene.
Upon exiting the reactor, water and antioxidant additives were injected in the
polymer
solution. The water hydrolyzed the catalyst, terminating the polymerization
reaction. Some
of the additives such as antioxidants remained with the polymer and function
as stabilizers
to prevent polymer degradation. The post reactor solution was superheated from
reactor
temperature (> 70 Deg C) to 210 ¨ 260 Deg C in preparation for a two-stage
devolatization
to recover the solvent and unreacted monomers. Residual volatiles in the
polymer were less
than 2,000 ppm by weight. The polymer melt was pumped to a die for underwater
pellet
cutting.
Release Layer
The release layer consists of a blend of a low density polyethylene (LDPE) and
a
polyethylene composition as further outlined in Table 12 below. The low
density
polyethylene has a 0.922 g/cc density and a melt index, 12, of 1.9 g/10 mm,
and is produced
in a high pressure, free radical process (LDPE 5011, available from the Dow
Chemical
Company, Midland, MI). The comparative polyethylene compositions used in the
release
layer, ethylene/alpha-olefin resins and additional details are shown in Table
3 below.
EXCEEDTM 1018 is available from the ExxonMobil Corporation. DOWLEXTM 2045G,
TUFLINTm 7046, and ELITETm 5100 are available from The Dow Chemical Company.
The inventive resins (Inv. 1, Inv. 2, Inv. 3) and comparative resin E
(comparative
polyethylene) were prepared as described below. The inventive and comparative
resins
underwent characterization testing as shown below in Tables 5-10.
- 30 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
Table 3: Comparative polyethylene compositions used in the release layer
Target Density Target MI
Label Product
(g/cm) (g / 10 min)
Comp.
DOWLEXTM 2045G 0.920 1.0
A
Comp.
ELITETm 5100 0.920 0.85
Comp.
EXCEEDTM 1018 0.918 1.0
Comp.
TUFLINTm 7046 0.919 1.0
Comp.
Comp. PE 0.917 3.0
A multi-metal catalyst is prepared (Catalyst 1). Catalyst 1 is then used to
prepare the
inventive polyethylene compositions and comparative resin E in a solution
polymerization.
Catalyst 1 Preparation
To approximately 6,718 kg of 0.20 M MgCl2 slurry was added 219 kg of EADC
solution
(15 wt% in heptanes), followed by agitation for 8 hours. A mixture of
TiC14/VOC13 (239
Kg and 155 Kg, respectively) at 6% was then added, followed by 275 Kg of a 6%
solution
of Zr(TMHD)4 in Isopar E. These two additions were performed sequentially
within 3
hours of each other. The resulting catalyst premix was aged with agitation for
an additional
8 hours prior to use.
Each of the catalysts prepared hereinabove is then used to prepare
Polyethylene
Compositions as described below.
Production of Inventive & Comparative Polyethylene Composition Examples
The inventive resins and comparative resin E were made according to the
following
procedures: A heterogeneously branched ethylene/a-olefin copolymer is prepared
using a
multi-constituent catalyst system, as described hereinabove, suitable for
(co)polymerizing
ethylene and one or more a-olefin comonomers, e.g. 1-octene or 1-hexene, in an
adiabatic
continuously stirred tank reactor, CSTR, under a solution phase polymerization
condition.
More specifically for this example the reactor consists of two adiabatic
reactors linked
together in series, operating under a solution phase polymerization condition.
All feed
- 31 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
streams are introduced into the first reactor which is a mechanically agitated
adiabatic
CSTR.
The solvent, e.g. Petrosol D, ethylene monomer, and 1-octene or 1-hexene
comonomer
reactor feed streams are purified using molecular sieves prior to introduction
in the reaction
environment. The solvent, ethylene monomer, and 1-octene or 1-hexene comonomer
are
combined into a single feed stream prior to introduction into the reaction
environment and
are temperature controlled. The hydrogen is also added to the combined single
feed stream
prior to introduction into the reaction environment.
The catalyst system is fed to the reaction environment separately from the
single feed
stream. The catalyst-premix is combined in line to the reactor with a dilute
stream of tri-
ethyl aluminum, TEA. The TEA flow is controlled to achieve a specified molar
ratio of Al
to Ti with the catalyst premix. The catalyst-premix is flow controlled to
control the extent
of reaction in the reaction environment.
The first reactor temperature and the overall ethylene conversion are
controlled by adjusting
the catalyst-premix flow and the total solvent flow introduced into the
reaction
environment. The melt index of the overall polymer is controlled by adjusting
the hydrogen
feed to the reaction environment. The density of the overall polymer is
controlled by
adjusting the comonomer feed to the reaction environment. Values for the
measured
parameters are contained in data Table 4.
After leaving the reaction environment, the reaction is stopped by the
addition of and
reaction of the active catalyst with a fluid especially designed for that
purpose, typically
water. The polymer is separated from the solvent and any unreacted monomer,
comonomer(s), and hydrogen; the isolated polymer melt is then pelletized and
packaged.
The separated stream containing solvent, monomer, comonomer(s), and hydrogen
is
recycled after removal of a purge stream.
- 32 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
Table 4: Reactor Data
Description Units . Inv. 1 Inv. 2 Inv. 3 Comp E
Reactor Feed (Solvent Mass
Flow / Ethylene Mass Flow) g / g 4.79 4.84 4.90 4.81
Reactor Feed (Comonomer
Mass Flow / Ethylene Mass
Flow) g/g 0.52 0.48 0.49 0.56
Reactor Feed (Fresh
Hydrogen Mass Flow /
Ethylene Mass Flow) g / g 1.81E-05 9.76E-06 9.63E-06 7.32E-
05
Reactor Feed Temperature C 15.5 14.7 14.5 14.0
Reactor 1 Temperature C 186.0 185.1 , 182.4 185.1
Reactor 2 Temperature C 212.8 211.4 209.1 213.0
Overall Ethylene Conversion wt% 92.3 92.3 92.5 92.0
mole /
Al : Ti molar ratio mole . 10.0 10.5 10.4 10.5
gPoly
Ti Catalyst Efficiency / g Ti 1,100,000 1,132,000 1,248,000
1,015,000
Reactor 1 viscosity cP 417 447 490 186
Comonomer type _ 1-octene 1-hexene 1-hexene 1-
hexene
Table 5: Measured Melt Index and Density Data
Density
Type 12, g/10 min In/I2
(g/cc)
Inv. 1 0.98 7.12 0.9204
Inv. 2 0.91 6.52 0.9180
Inv. 3 0.72 7.33 0.9170
Comp. A 1.04 8.13 0.9206
Comp. B 0.91 8.37 0.9193
Comp. C 0.98 5.79 0.9200
Comp. D 1.00 7.84 0.9188
Comp. E 2.67 6.86 0.9160
- 33 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
Table 6: Conventional GPC Data
Mn Mw Mz
Type Mw/Mn
(g/mol) (g/mol) (g/mol)
Inv. 1 34,901 119,909 361,775 3A4
Inv. 2 35,127 122,811 376,444 - 3.50
Inv. 3 35,635 130,416 410,843 3.66
Comp. A 29,853 114,087 337,994 3.82
Comp. B 29,660 110,671 269,439 3.73
Comp. C 51,294 112,087 196,331 2.19
Comp. D 29,511 120,359 365,314 4.08
Comp. E 25,993 87,775 241,586 3.38
Table 7: Melt Strength Data
Type Velocity at Break (mm/s) Melt Strength (cN)
Inv. I 296 3.0
Inv. 2 265 3.9
Inv. 3 258 5.0
Comp. A 259 3.3
Comp. B 217 3.8
Comp. C 343 2.9
Comp. D Not measured Not measured
Comp. E 404 0.91
- 34 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
Table 8: CEF and CDBI Data
Peak Temp and Temperature Range (in
Wt% of Each zone
parenthesis) of Each Zone ( C)
Type Zone 1 Zone 2 Zone 3 Zone
1Zone 2Zone 3CDBI
28.4 86.4 98.8
Inv. 1 3.8 70.3 26.0
40.6
(25.04-34.45) (34.55-93.76) (93.85-119.99)
28.5 83.9 98.1
Inv. 2 4.4 75.6 20.1
47.1
(26.54-34.48) (34.58-92.93) (93.06-114.99)
28.2 84.6 97.9
Inv. 3 4.7 74.8 20.5
46.9
(25.01-34.46) (34.56-92.76) (92.84-119.97)
28.4 83.3 98.9
Comp. A 4.2 66.0
29.7 35.4
(25.18-32.00) (32.05-92.97) (93.06-119.99)
28.5 68.2 98.3
Comp. B 2.4 56.7
40.8 28.7
(26.34-31.97) (32.02-85.97) (86.06-119.98)
29.4 86.0 87.2
Comp. C 0.6 52.0
47.1 61.1
(25.04-31.98) (32.03-85.97) (86.07-119.95)
28.1 86.2 99.2
Comp. D 15.2 47.5
37.3 21.5
(26.03-31.98) (32.03-92.46) (92.52-107.97)
28.5 82.9 97.9
Comp. E 7.0 76.2
16.9 66.3
(25.02-34.48) (34.56-92.68) (92.79-119.97)
- 35 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
Table 9: DMS Rheology Data (at 190 C)
Viscosity (Pa-s at 190 C) Viscosity Ratio
0.1 1 10 100 (Viscosity O. 1 rad/s) Tan Delta
Type
rad/s rad/s rad/s rad/s
(Viscosity 100 rad/s) 0.1 rad/s
Inv. 1 7,724 6,531 4,275 1,824 4.24 12.77
Inv. 2 9,214 7,571 4,758 1,956 4.71 10.54
Inv. 3 10,218 8,310 5,114 2,047 4.99 10.08
Comp. A 8,161 6,563 3,986 1,603 5.09 9.82
Comp. B 10,597 6,882 3,799 1,510 7.02 4.24
Comp. C 6,780 6,314 4,927 2,358 2.88 30.64
Comp. D 8,220 6,594 3,974 1,588 5.18 9.51
Comp. E 2,854 2,633 2,025 1,070 2.67 28.61
Table 10: DSC Data
Heat of
Tmi Tm2 Tm3 % Tel Ira
Tc3
Type ( C) ( C) ( C) Fusion
(J/g) Crystallinity ( C) ( C) ( C)
Inv. 1 122.7 120.4 111.2 138.4 47.4 107.9 65.4 ND
Inv. 2 121.2 118.3 107.8 147.2 50.4 106.0 63.6 ND
Inv. 3 121.3 118.2 107.6 150.2 51.4 106.1 63.6 ND
Comp. A 123.7 120.9 ND 148.5 50.9 106.8 64.3 ND
Comp. B 123.9 121.0 ND 141.5 48.5 107.2 88.7 62.3
Comp. C 117.8 109.6 ND 143.9 49.3 106.1 68.1 ND
Comp. D 123.9 ND ND 138.5 47.4 111.2 ND ND
Comp. E 122.0 117.4 106.0 137.0 46.9 104.8 61.4 ND
ND refers to not detectable.
- 36 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
Table 11: Neutron Activation Data*
Type Al, ppm Mg, ppm Ti, ppm V, ppm Hf, ppb Zr, ppm Cl, ppm
Inv. 1 9.2 0.4 16 2 0.67 0.03
1.6 0.1 0.26 0.03 0.88 0.08 58 2
Inv. 2 9.9 0.4 18 2 1.02 0.06
1.7 0.1 0.23 0.03 0.96 0.09 61 2
Inv. 3 9.0 0.4 15 2 0.77 0.03
1.5 0.1 0.26 0.03 0.83 0.09 55 2
* Niobium (Nb) (5 ppm), tantalum (Ta) (50 ppb), chromium (Cr) (0.5 ppm),
molybdenum
(Mo) (50 ppb), and tungsten (W) (5ppm) were not detected in any of the
examples at their
respective detection limits, as indicated in the parentheses following each
element.
Films
Three layer blown films were made using a Hosokawa Alpine 7-layer blown film
line. The
cling layer (outside of the bubble) with layer ratio of 15% is produced from
extruder 1. The
core layer with layer ratio of 70% is produced from extruder 2, 3, 4, 5 and 6.
The release
layer (inside of the bubble) with layer ratio of 15% is produced from extruder
7. All
extruders are groove-feed and L/D ratio is 30 with diameter of 50 mm. Melt
temperature of
extrusion for all extruders is ranged from 450 to 480 F and die temperature is
450 F. Die
gap is 78.7 mil. Blow up ratio is 2.5 and film gauge is 1 mil. Output rate is
300 lbs/hr. The
film structures are further outlined in Table 12 below.
Table 12: Blown Film Structures
Cling Layer Core Layer Release Layer
(15% of Overall Film) (70% of Overall Film) (15% of Overall
Film)
Inv. Film 1 65 wt.% 100 wt.% 25 wt.% Inv.1
AFFINITYTm 8100G DOWLEXTm 2045G 75 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Inv. Film 2 65 wt.% 100 wt.% 50 wt.% Inv. 1
AFFINITYTm 8100G DOWLEXTM 2045G 50 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Inv. Film 3 65 wt.% 100 wt.% 75 wt.% Inv. 1
ANFINITYTm 8100G DOWLEXTM 2045G 25 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
- 37 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
Inv. Film 4 65 wt.% 100 wt.% 25 wt.% Inv.2
AH-INITYTm 8100G DOWLEXTM 2045G 75 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Inv. Film 5 65 wt.% 100 wt.% 50 wt.% Inv. 2
All-INITYTm 8100G DOWLEXTM 2045G 50 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Inv. Film 6 65 wt.% 100 wt.% 25 wt.% Inv.3
ANPINITYTm 8100G DOWLEXTM 2045G 75 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Inv. Film 7 65 wt.% 100 wt.% 50 wt.% Inv. 3
A141-4NITYTm 8100G DOWLEXTM 2045G 50 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Comp. Film 1 65 wt.% 100 wt.% 25 wt.% Comp. A
AFFINITYTm 8100G DOWLEXTM 2045G 75 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Comp. Film 2 65 wt.% 100 wt.% 50 wt.% Comp. A
AFFINITYTm 8100G DOWLEXTM 2045G 50 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Comp. Film 3 65 wt.% 100 wt.% 75 wt.% Comp. A
APPINITYTm 8100G DOWLEXTM 2045G 25 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Comp. Film 4 65 wt.% 100 wt.% 25 wt.% Comp. B
All-INITYTm 8100G DOWLEXTM 2045G 75 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Comp. Film 5 65 wt.% 100 wt.% 50 wt.% Comp. B
ANFINITYTm 8100G DOWLEXTM 2045G 50 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Comp. Film 6 65 wt.% 100 wt.% 75 wt.% Comp. B
AFFINITYTm 8100G DOWLEXTM 2045G 25 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
- 38 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
Comp. Film 7 65 wt.% 100 wt.% 50 wt.% Comp. C
AH-INITYTm 8100G DOWLEXTM 2045G 50 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Comp. Film 8 65 wt.% 100 wt.% 75 wt.% Comp. C
APHNITYTm 8100G DOWLEXTM 2045G 25 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Comp. Film 9 65 wt.% 100 wt.% 25 wt.% Comp. D
ANPINITYTm 8100G DOWLEXTM 2045G 75 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Comp. Film 10 65 wt.% 100 wt.% 50 wt.% Comp. D
A141-4NITYTm 8100G DOWLEXTM 2045G 50 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Comp. Film 11 65 wt.% 100 wt.% 75 wt.% Comp. D
AFFINITYTm 8100G DOWLEXTM 2045G 25 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Comp. Film 12 65 wt.% 100 wt.% 25 wt.% Comp. E
AFFINITYTm 8100G DOWLEXTM 2045G 75 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Comp. Film 13 65 wt.% 100 wt.% 50 wt.% Comp. E
A1411NITYTm 8100G DOWLEXTM 2045G 50 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
Comp. Film 14 65 wt.% 100 wt.% 75 wt.% Comp. E
A1-1-INITYTm 8100G DOWLEXTM 2045G 25 wt.% LDPE 5011
35 wt.%
Sample 1 ULDPE
- 39 -
CA 02996337 2018-02-21
WO 2017/039987
PCT/US2016/046252
TABLE 13 ¨ The Effect of Release Layer Formulation on Stretch Cling
Performance
Film # Cling Force (g) per 20 inch film width
Inv. Film 1 253
Inv. Film 2 278
Inv. Film 3 302
Inv. Film 4 264
Inv. Film 5 261
Inv. Film 6 252
Inv. Film 7 318
Comp. Film 1 191
Comp. Film 2 223
Comp. Film 3 249
Comp. Film 4 187
Comp. Film 5 213
Comp. Film 6 244
Comp. Film 7 230
Comp. Film 8 261
Comp. Film 9 Unmeasurable ¨ Too difficult to unwind the film
(i.e., the film blocked)
Comp. Film 10 Unmeasurable ¨ Too difficult to unwind the film
(i.e., the film blocked)
Comp. Film 11 Unmeasurable ¨ Too difficult to unwind the film
(i.e., the film blocked)
Comp. Film 12 Unmeasurable ¨ Too difficult to unwind the film
(i.e., the film blocked)
Comp. Film 13 Unmeasurable ¨ Too difficult to unwind the film
(i.e., the film blocked)
Comp. Film 14 Unmeasurable ¨ Too difficult to unwind the film
(i.e., the film blocked)
- 40 -
84217324
Further shown in FIG. 1 is a graph of cling force performance as the amount of
polyethylene
composition in the release layer increases. As depicted, the inventive films
have significantly
higher cling force.
The dimensions and values disclosed herein are not to be understood as being
strictly limited to
.. the exact numerical values recited. Instead, unless otherwise specified,
each such dimension is
intended to mean both the recited value and a functionally equivalent range
surrounding that value.
For example, a dimension disclosed as "40 mm" is intended to mean "about 40
mm."
The citation of any document is not an admission that it is prior art with
respect to any invention
disclosed or claimed herein or that it alone, or in any combination with any
other reference or
references, teaches, suggests or discloses any such invention. Further, to the
extent that any
meaning or definition of a term in this document conflicts with any meaning or
definition of the
same twit in a document referenced herein, the meaning or definition assigned
to that term in this
document shall govern.
While particular embodiments of the present invention have been illustrated
and described, it
.. would be obvious to those skilled in the art that various other changes and
modifications can be
made without departing from the spirit and scope of the invention. It is
therefore intended to cover
in the appended claims all such changes and modifications that are within the
scope of this
invention.
- 41 -
Date Regue/Date Received 2022-09-16