Note: Descriptions are shown in the official language in which they were submitted.
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
SHARP AND EROSION RESISTANCE DEGRADABLE MATERIAL
FOR SLIP BUTTONS AND SLIDING SLEEVE BAFFLES
BACKGROUND
[0001] The present disclosure generally relates to a sharp and erosion-
resistant
degradable material used in a component in a downhole tool, and a method of
using said
degradable material. More particularly, the sharp and erosion-resistant
degradable material
includes a dissolvable metal matrix composite wherein the dissolvable metal
matrix composite
includes a dissolvable metal and a dispersed reinforcement material wherein
the dissolvable
metal is capable of dissolving via galvanic corrosion.
[0002] In the drilling, completion and stimulation of hydrocarbon-producing
wells, a
variety of downhole tools are used. For example, it is often desirable to seal
portions of a
wellbore, such as during fracturing operations when various fluids and
slurries are pumped from
the surface into a casing string that lines the wellbore, and forced out into
a surrounding
subterranean formation through the casing string. Sealing the wellbore may
become necessary to
provide zonal isolation at the location of the desired subterranean formation.
Wellbore isolation
devices, such as packers, baffle seats, bridge plugs, and fracturing plugs
(i.e., "frac" plugs), are
designed for these general purposes and are well known in the art of producing
hydrocarbons,
such as oil and gas. Such wellbore isolation devices may be used in direct
contact with the
formation face of the wellbore, with a casing string extended and secured
within the wellbore, or
with a screen or wire mesh.
[0003] After the desired downhole operation is complete, the seal formed by
the
wellbore isolation device must be broken and the tool itself removed from the
wellbore.
Removing the wellbore isolation device may allow hydrocarbon production
operations to
commence without bcing hindered by the presence of the downhole tool. Removing
wellbore
isolation devices, however, is traditionally accomplished by a complex
retrieval operation that
involves milling or drilling out a portion of the wellbore isolation device,
and subsequently
mechanically retrieving its remaining portions. To accomplish this, a tool
string having a mill or
drill bit attached to its distal end is introduced into the wellbore and
conveyed to the wellbore
isolation device to mill or drill out the wellbore isolation device. After
drilling out the wellbore
1
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
isolation device, the remaining portions of the wellbore isolation device may
be grasped onto and
retrieved back to the surface with the tool string for disposal. As can be
appreciated, this
retrieval operation can be a costly and time-consuming process.
[0004] There exists a need for a novel method of removing parts or the entire
wellbore
isolation device in a less expensive and efficient manner with a controlled or
predictable
dissolution rate.
BRIEF DESCRIPTION OF THE DRAWINGS
[0005] The following figures are included to illustrate certain aspects of the
present
disclosure, and should not be viewed as exclusive embodiments. The subject
matter disclosed is
capable of considerable modifications, alterations, combinations, and
equivalents in form and
function, without departing from the scope of this disclosure.
[0006] FIG. 1 is a well system that employs the metal matrix composite in
accordance
with the principles of the present disclosure.
[0007] FIG. 2 is a cross-sectional side view of an exemplary frac plug that
can employ
the metal matrix composite in accordance with the principles of the present
disclosure;
[0008] FIG. 3 is an example of a sliding sleeve that employs the metal matrix
composite
in accordance with the principles of the present disclosure;
[0009] FIG. 4 is an example of a metal matrix composite in accordance with the
principles of the present disclosure.
[0010] FIG. 5 is a micrograph of an example of a metal matrix composite in
accordance
with the principles of the present disclosure.
2
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
DETAILED DESCRIPTION
100111 In the following detailed description of the illustrative embodiments,
reference is
made to the accompanying drawings that form a part hereof. These embodiments
are described
in sufficient detail to enable those skilled in the art to practice the
invention, and it is understood
that other embodiments may be utilized and that logical structural,
mechanical, electrical, and
chemical changes may be made without departing from the spirit or scope of the
invention. To
avoid detail not necessary to enable those skilled in the art to practice the
embodiments described
herein, the description may omit certain information known to those skilled in
the art. The
following detailed description is, therefore, not to be taken in a limiting
sense, and the scope of
the illustrative embodiments is defined only by the appended claims.
100121 Unless otherwise specified, any use of any form of the terms "connect,"
"engage,"
"couple," "attach," or any other term describing an interaction between
elements is not meant to
limit the interaction to direct interaction between the elements and may also
include indirect
interaction between the elements described. In the following discussion and in
the claims, the
terms "including" and "comprising" are used in an open-ended fashion, and thus
should be
interpreted to mean "including, but not limited to". Unless otherwise
indicated, as used
throughout this document, "or" does not require mutual exclusivity.
100131 As used herein, the phrase "consisting essentially of' shall be used as
a
transitional phrase, and will leave the entire phrase including "consisting
essentially of" as being
"open" to include additional elements, but only if those additional elements
do not materially
affect the basic and novel characteristics of the claimed combination
[0014] As used herein, the phrases "hydraulically coupled," "hydraulically
connected,"
"in hydraulic communication," "fluidly coupled," "fluidly connected," and "in
fluid
communication" refer to a form of coupling, connection, or communication
related to fluids, and
the corresponding flows or pressures associated with these fluids. In some
embodiments, a
hydraulic coupling, connection, or communication between two components
describes
components that are associated in such a way that fluid pressure may be
transmitted between or
among the components. Reference to a fluid coupling, connection, or
communication between
two components describes components that are associated in such a way that a
fluid may flow
3
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
between or among the components. Hydraulically coupled, connected, or
communicating
components may include certain arrangements where fluid does not flow between
the
components, but fluid pressure may nonetheless be transmitted such as via a
diaphragm or
piston. The present disclosure generally relates to a sharp and erosion
resistant degradable
material used in a component in a downhole tool and a method of using said
degradable material,
and more particularly, to a dissolvable metal matrix composite.
[00151 As used herein, "about" may mean that the value is within +/-5% of the
measurement.
[00161 As used herein, a "fluid" may include a substance having a continuous
phase that
tends to flow and to conform to the outline of its container when the
substance is tested at a
temperature of 71 F (22 C) and a pressure of one atmosphere "atm" (0.1
megapascals "MPa").
A fluid may be a liquid or gas. A homogenous fluid has only one phase,
whereas, a
heterogeneous fluid has more than one distinct phase. A heterogeneous fluid
may be: a slurry,
which includes a continuous liquid phase and undissolved solid particles as
the dispersed phase;
an emulsion, which includes a continuous liquid phase and at least one
dispersed phase of
immiscible liquid droplets; a foam, which includes a continuous liquid phase
and a gas as the
dispersed phasc; or a mist, which includes a continuous gas phase and a liquid
as the dispersed
phase. A heterogeneous fluid will have only one continuous phase, but may have
more than one
dispersed phase. It is to be understood that any of the phases of a
heterogeneous fluid (e.g., a
continuous or dispersed phase) may contain dissolved or undissolved substances
or compounds.
As used herein, the phrase "base fluid" is the liquid that is in the greatest
concentration in the
wellbore fluid and is the solvent of a solution or the continuous phase of a
heterogeneous fluid.
[00171 A well can include, without limitation, an oil, gas, or water
production well, or an
injection well. As used herein, a "well" includes at least one wellbore. A
wellbore can include
vertical, inclined, and horizontal portions, and it can be straight, curved,
or branched. As used
herein, the term "wellbore" includes any cased, and any uncased, open-hole
portion of the
wellbore. A near-wellbore region is the subterranean material and rock of the
subterranean
formation surrounding the wellbore. As used herein, a "well" also includes the
near-wellbore
region. The near-wellbore region is generally considered to be the region
within approximately
4
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
100 feet radially of the wellbore. As used herein, "into a well" means and
includes into any
portion of the well, including into the wellbore or into the near-wellbore
region via the wellbore.
[0018) A portion of a wellbore may be an open hole or cased hole. In an open-
hole
wellbore portion, a tubing string may be placed into the wellbore. The tubing
string allows fluids
to be introduced into or flowed from a remote portion of the wellbore. In a
cased-hole wellbore
portion, a casing is placed into the wellborc that can also contain a tubing
string. A wellbore can
contain an annulus. Examples of an annulus include, but are not limited to:
the space between
the wellbore and the outside of a tubing string in an open-hole wellbore; the
space between the
wellbore and the outside of a casing in a cased-hole wellbore; and the space
between the inside
of a casing and the outside of a tubing string in a cased-hole wellbore.
[0019] It is not uncommon for a wellbore to extend several hundreds of feet or
several
thousands of feet into a subterranean formation. The subterranean formation
can have different
zones. A zone is an interval of rock differentiated from surrounding rocks on
the basis of its
fossil content or other features, such as faults or fractures. For example,
one zone can have a
higher permeability compared to another zone. It is often desirable to treat
one or more locations
within multiples zones of a formation. One or more zones of the formation can
be isolated
within the wellbore via the use of an isolation device to create multiple
wellbore intervals. At
least one wellbore interval corresponds to a formation zone. The isolation
device can be used for
zonal isolation and functions to block fluid flow within a tubular, such as a
tubing string, or
within an annulus. The blockage of fluid flow prevents the fluid from flowing
across the
isolation device in any direction and isolates the zone of interest. In this
manner, treatment
techniques can be performed within the zone of interest. As used herein, the
term "sealing ball,"
and grammatical variants thereof, refers to a spherical or spheroidal element
designed to seal
perforations of a wellbore isolation device that are accepting fluid, thereby
diverting reservoir
treatments to other portions of a target zone. An example of a sealing ball is
a frac ball in a frac
plug wellbore isolation device. As used herein, the term "packer element"
refers to an
expandable, inflatable, or swellable element that expands against a casing or
wellbore to seal the
wellbore.
[0020] As used herein, the term "wellbore isolation device," and grammatical
variants
thereof, is a device that is set in a wellbore to isolate a portion of the
wellbore thereabove from a
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
portion therebelow so that fluid can be forced into the surrounding
subterranean formation above
the device. Common wellbore isolation devices include, but are not limited to,
a ball and a seat,
a bridge plug, a packer, and a plug. It is to be understood that reference to
a "ball" is not meant
to limit the geometric shape of the ball to spherical, but rather is meant to
include any device that
is capable of engaging with a seat. A "ball" can be spherical in shape, but
can also be a dart, a
bar, or any other shape. Zonal isolation can be accomplished via a ball and
scat by dropping or
flowing the ball from the wellhead onto the seat that is located within the
wellbore. The ball
engages with the seat, and the seal created by this engagement prevents fluid
communication into
other wellbore intervals downstream of the ball and seat. As used herein, the
relative term
"downstream" means at a location further away from a wellhead. In order to
treat more than one
zone using a ball and seat, the wellbore can contain more than one ball seat.
For example, a seat
can be located within each wellbore interval. Generally, the inner diameter
(I.D.) of the ball
seats is different for each zone. For example, the I.D. of the ball seats
sequentially decreases at
each zone, moving from the wellhead to the bottom of the well. In this manner,
a smaller ball is
first dropped into a first wellbore interval that is the farthest downstream;
the corresponding zone
is treated; a slightly larger ball is then dropped into another wellbore
interval that is located
upstream of the first wellbore interval; that corresponding zone is then
treated; and the process
continues in this fashion ¨ moving upstream along the wellbore ¨ until all the
desired zones have
been treated. As used herein, the relative term "upstream" means at a location
closer to the
wellhead.
[0021] A bridge plug is composed primarily of slips, a plug mandrel, and a
rubber
sealing element. A bridge plug can be introduced into a wellbore and the
sealing element can be
caused to block fluid flow into downstream intervals. A packer generally
consists of a sealing
device, a holding or setting device, and an inside passage for fluids. A
packer can be used to
block fluid flow through the annulus located between the outside of a tubular
and the wall of the
wellbore or inside of a casing
[0022] The use of directional terms such as above, below, upper, lower,
upward,
downward, left, right, uphole, downhole and the like are used in relation to
the illustrative
embodiments as they are depicted in the figures, the upward direction being
toward the top of the
corresponding figure and the downward direction being toward the bottom of the
corresponding
6
= CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
figure, the uphole direction being toward the surface of the well and the
downhole direction
being toward the toe of the well.
[0023] As used herein, the term "dissolvable" and all of its grammatical
variants (e.g.,
"degrade," "degradation," "degrading," "dissolve," dissolving," and the like),
refers to the
dissolution or chemical conversion of solid materials such that reduced-mass
solid end products
by at least one of solubilization, hydrolytic degradation, chemical reactions
(including
electrochemical and galvanic reactions), thermal reactions, reactions induced
by radiation, or
combinations thereof.
[0024] As used herein, a "degradable or dissolvable metal" may refer to a
metal that has
a certain rate of dissolution, and the rate of dissolution may correspond to a
rate of material loss
at a particular temperature and within particular wellbore conditions.
[0025] As used herein, an "electrolyte" is any substance containing free ions
(i.e., a
positively or negatively charged atom or group of atoms) that make the
substance electrically
conductive. The electrolyte can be selected from the group consisting of,
solutions of an acid, a
base, a salt, and combinations thereof. A salt can be dissolved in water, for
example, to create a
salt solution. Common free ions in an electrolyte include, but are not limited
to, sodium (Na+),
potassium (le), calcium (Ca2+), magnesium (Mg2-), chloride (Ct), bromide (B)
hydrogen
phosphate (HP042-), hydrogen carbonate (HCO3), and any combination thereof.
Preferably, the
electrolyte contains chloride ions.
[0026] Galvanic corrosion occurs when two different metals or metal alloys are
in
electrical connectivity with each other and both are in contact with an
electrolyte. As used
herein, the phrase "electrical connectivity" means that the two different
metals or metal alloys are
either touching or in close enough proximity to each other such that when the
two different
metals are in contact with an electrolyte, the electrolyte becomes
electrically conductive and ion
migration occurs between one of the metals and the other metal, and is not
meant to require an
actual physical connection between the two different metals, for example, via
a metal wire.
[0027] It is to be understood that as used herein, the term "metal" is meant
to include
pure metals and also metal alloys without the need to continually specify that
the metal can also
be a metal alloy. Moreover, the use of the phrase "metal or metal alloy" in
one sentence or
paragraph does not mean that the mere use of the word "metal" in another
sentence or paragraph
7
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
is meant to exclude a metal alloy. As used herein, the term "metal alloy"
means a mixture of two
or more elements, wherein at least one of the elements is a metal. The other
element(s) can be a
non-metal or a different metal. An example of a metal and non-metal alloy is
steel, comprising
the metal element iron and the non-metal element carbon. An example of a metal
and metal
alloy is bronze, comprising the metallic elements copper and tin.
[0028] In some instances, the degradation of the dissolvable metal matrix
composite or
dissolvable metal may be sufficient for the mechanical properties of the metal
to be reduced to a
point that the metal no longer maintains its integrity and, in essence, falls
apart or sloughs off
into its surroundings. The conditions for degradation are generally wellbore
conditions where an
external stimulus may be used to initiate or effect the rate of degradation,
where the external
stimulus is naturally occurring in the wellbore (e.g., pressure, temperature)
or introduced into the
wellbore (e.g., fluids, chemicals). For example, the pH of the fluid that
interacts with the
material may be changed by introduction of an acid or a base. The term
"wellbore environment"
includes both naturally occurring wellbore environments and materials or
fluids introduced into
the wellbore. The term "at least a portion" with reference to degradation
(e.g., "at least a portion
of the mandrel is degradable" or "at least a portion of the degradable packer
element is
degradable," and variants thereof) refers to degradation of at least about 80%
of the volume of
that part.
[0029] The present disclosure describes embodiments of a component in a
downhole tool
(e.g., wellbore isolation device) that is made of a dissolvable metal matrix
composite. In
particular, the present disclosure describes having a variety of components
including, e.g., a
baffle seats, a shear pin, a slip button, a mandrel, a sealing ball, and an
expandable or inflatable
packer element. The degradable wellbore isolation devices may include e.g.,
frac plugs. Having
a component for wellbore isolation device be made out of a dissolvable metal
matrix composite
would facilitate an easier disposal of the component without an expensive or
labor intensive
procedure to remove said component from the wellbore system.
[0030] The dissolvable metal matrix composite consists essentially of a
dissolvable
metal and a dispersed reinforcement material wherein the dissolvable metal is
capable of
dissolving via galvanic corrosion when the dissolvable metal is in presence of
an electrolyte. The
dispersed reinforcement material may include a ceramic or a hardened metal. In
an alternative
8
= CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
embodiment, the dissolvable metal matrix composite consists essentially of a
dissolvable metal
and a disperse reinforcement material wherein the dissolvable metal is capable
of dissolving via
dissolution when the dissolvable metal is in the presence of water. In another
example, the
dissolvable metal forms a galvanic couple with the dispersed reinforcement
material.
[0031] The dissolvable metal that may be used in accordance with the
embodiments of
the present disclosure includes galvanically-corrodible or degradable metals
and metal alloys.
Such metals and metal alloys may be configured to degrade via an
electrochemical process in
which the galvanically-corrodible metal corrodes in the presence of an
electrolyte (e.g., brine or
other salt-containing fluids present within the wellbore). The electrolyte can
be a fluid that is
introduced into the wellbore or a fluid emanating from the wellbore, such as
from a surrounding
subterranean formation.
[0032] In an embodiment of the present disclosure, the degradability of the
dissolvable
metal matrix composite can be accelerated by creating galvanic couples within
the dissolvable
metal matrix composite. There are two paths for accelerating the corrosion: 1)
alloying the
dissolvable metal with copper, nickel, carbon, or iron, or 2) replacing part
of the ceramic with
cathodic nuggets.
[0033] The dissolvable metal may be alloyed with copper, nickel, or iron as a
solid
solution. The copper, nickel, or iron creates inclusions that have a galvanic
potential that
accelerates the corrosion of the metal.
[0034] A part of the ceramic may be replaced with a cathodic component that
creates a
galvanic potential with the metal matrix. The galvanic coupling may be
generated by embedding
or attaching a cathodic substance or piece of material into an anodic
component. The cathodic
component could be a nugget, a spheroid, a sliver, a fiber, or a weave. In
theory, the cathodic
component could be any material that creates a galvanic potential with the
metal matrix. In a
preferred embodiment of the present disclosure, the cathodic components
include copper, nickel,
steel, or graphite (carbon). Other options may include platinum, silver,
zirconium, titanium, iron,
bronze, chromium, tin, or their alloys. In at least one embodiment of the
present disclosure, the
galvanic coupling may be generated by dissolving aluminum in gallium.
[0035] The metal that is less noble, compared to the other metal, will
dissolve in the
electrolyte. The less noble metal is often referred to as the anode, and the
more noble metal is
9
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
often referred to as the cathode. Galvanic corrosion is an electrochemical
process whereby free
ions in the electrolyte make the electrolyte electrically conductive, thereby
providing a means for
ion migration from the anode to the cathode - resulting in deposition formed
on the cathode.
Metals can be arranged in a galvanic series. The galvanic series lists metals
in order of the most
noble to the least noble. An anodic index lists the electrochemical voltage
(V) that develops
between a metal and a standard reference electrode (gold (Au)) in a given
electrolyte. The actual
electrolyte used can affect where a particular metal or metal alloy appears on
the galvanic series
and can also affect the electrochemical voltage. For example, the dissolved
oxygen content in
the electrolyte can dictate where the metal or metal alloy appears on the
galvanic series and the
metal's electrochemical voltage. The anodic index of gold is -0 V; while the
anodic index of
beryllium is -1.85 V. A metal that has an anodic index greater than another
metal is more noble
than the other metal and will function as the cathode. Conversely, the metal
that has an anodic
index less than another metal is less noble and functions as the anode. In
order to determine the
relative voltage between two different metals, the anodic index of the lesser
noble metal is
subtracted from the other metal's anodic index, resulting in a positive value.
[0036] There are several factors that can affect the rate of galvanic
corrosion. One of the
factors is the distance separating the metals on the galvanic series chart or
the difference between
the anodic indices of the metals. For example, beryllium is one of the last
metals listed at the
least noble end of the galvanic series and platinum is one of the first metals
listed at the most
noble end of the series. By contrast, tin is listed directly above lead on the
galvanic series.
Using the anodic index of metals, the difference between the anodic index of
gold and beryllium
is 1.85 V; whereas, the difference between tin and lead is 0.05 V. This means
that galvanic
corrosion will occur at a much faster rate for magnesium or beryllium and gold
compared to lead
and tin.
[0037] Another factor that can affect the rate of galvanic corrosion is the
temperature
and concentration of the electrolyte. The higher the temperature and
concentration of the
electrolyte, the faster the rate of corrosion In an embodiment of the present
disclosure, the
temperature of the wellbore system may be increased or decreased based on
volume of the
wellbore fluid being pumped into the wellbore system.
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
[0038] Yet another factor that can affect the rate of galvanic corrosion is
the total amount
of surface area of the least noble (anodic metal). The greater the surface
area of the anode that
can come in contact with the electrolyte, the faster the rate of corrosion.
The cross-sectional size
of the anodic metal pieces can be decreased in order to increase the total
amount of surface area
per total volume of the material. The anodic metal or metal alloy can also be
a matrix in which
pieces of cathode material is embedded in the anode matrix.
[0039] Yet another factor that can affect the rate of galvanic corrosion is
the ambient
pressure. Depending on the electrolyte chemistry and the two metals, the
corrosion rate can be
slower at higher pressures than at lower pressures if gaseous components are
generated. Yet
another factor that can affect the rate of galvanic corrosion is the physical
distance between the
two different metal and/or metal alloys of the galvanic system.
[0040] In an embodiment of the present disclosure, the dissolvable metal may
include
gold, gold-platinum alloys, silver, nickel, nickel-copper alloys, nickel-
chromium alloys, copper,
copper alloys (e.g., brass, bronze, etc.), chromium, tin, aluminum, aluminum
alloys, iron, zinc,
magnesium, magnesium alloys, beryllium, any alloy of the aforementioned
materials, and any
combination thereof.
[0041] In another embodiment of the present disclosure, the dissolvable metal
may
include an aluminum alloy that is alloyed with gallium. The gallium acts as a
depassivating agent
and prevents the formation of a protective passivation layer on the surface of
the aluminum.
Indium and tin also act as depassivating agents and help to prevent
passivation on the aluminum.
Examples of aluminum-gallium alloys include 80% aluminum-20% gallium, 80%A1-
10%Ga-
10%In, 75%A1-5%Ga-5%Zn-5%Bi-5%Sn-5%Mg, and 90%A1-2.5%Ga-2.5%Zn-2.5%B
2.5%Sn. Another example is 99.8%A1-0.1%In-0.1%Ga.
[0042] In another embodiment of the present disclosure, the dissolvable metal
may
include an aluminum alloy that is alloyed with copper, with manganese, with
silicon, with
magnesium, with iron, with lithium, carbon, and/or with zinc. Example of
aluminum alloy with
copper is a 2024 aluminum which includes 92%A1-.5%Si-.5%Fe-4.5%Cu-.5%Mn-1.5%Mg-
.1%Cr-.25%Zn-.15%Ti.
[0043] In yet another embodiment of the present disclosure, the dissolvable
metal may
include a magnesium alloy that is alloyed with zinc, aluminum, yttrium,
copper, nickel, cerium,
11
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
and/or iron. Example of a magnesium alloy that is alloyed with aluminum is
AZ91 magnesium
which includes 90.8%Mg-8.25%A1-0.63%Zn-0.035%Si-0.22%Mn. Another example of a
magnesium alloy that is alloyed with zinc is ZK61 which includes 95%Mg-5%Zn-
.3%Zr.
[0044] Magnesium alloys may include at least one other ingredient besides the
magnesium. The other ingredients can be selected from one or more metals, one
or more non-
metals, or a combination thereof. Suitable metals that may be alloyed with
magnesium include,
but are not limited to, lithium, sodium, potassium, rubidium, cesium,
beryllium, calcium,
strontium, barium, aluminum, gallium, indium, tin, thallium, lead, bismuth,
scandium, titanium,
vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc, yttrium,
zirconium, niobium,
molybdenum, ruthenium, rhodium, palladium, praseodymium, silver, lanthanum,
hafnium,
tantalum, tungsten, terbium, rhenium, osmium, iridium, platinum, gold,
neodymium, gadolinium,
erbium, oxides of any of the foregoing, and any combinations thereof.
[0045] Suitable non-metals that may be alloyed with magnesium include, but are
not
limited to, graphite, carbon, silicon, boron nitride, and combinations
thereof. The carbon can be
in the form of carbon particles, fibers, nanotubes, fullerenes, and any
combination thereof. The
graphite can be in the form of particles, fibers, weaves, graphene, and any
combination thereof.
The magnesium and its alloyed ingredient(s) may be in a solid solution and not
in a partial
solution or a compound where inter-granular inclusions may be present. In some
embodiments,
the magnesium and its alloyed ingredient(s) may be uniformly distributed
throughout the
magnesium alloy but, as will be appreciated, some minor variations in the
distribution of
particles of the magnesium and its alloyed ingredient(s) can occur. In other
embodiments, the
magnesium alloy is a sintered construction.
[0046] In some embodiments, the magnesium alloy may have a yield stress in the
range
of from about 10,000 pounds per square inch (psi) to about 50,000 psi,
encompassing any value
and subset therebetween. For example, in some embodiments, the magnesium alloy
may have a
yield stress of about 20,000 psi to about 30,000 psi, or about 30,000 psi to
about 40,000 psi, or
about 40,000 psi to about 50,000 psi, encompassing any value and subset
therebetween.
[0047] Suitable aluminum alloys may include alloys having aluminum at a
concentration
in the range of from about 40% to about 99% by weight of the aluminum alloy,
encompassing
any value and subset therebetween. For example, suitable aluminum alloys may
have aluminum
12
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
concentrations of about 40% to about 50%, or about 50% to about 60%, or about
60% to about
70%, or about 70% to about 80%, or about 80% to about 90%, or about 90% to
about 99% by
weight of the aluminum alloy, encompassing any value and subset therebetween.
[0048] The aluminum alloys may be wrought or cast aluminum alloys and comprise
at
least one other ingredient besides the aluminum. The other ingredients can be
selected from one
or more any of the metals, non-metals, and combinations thereof described
above with reference
to magnesium alloys, with the addition of the aluminum alloys additionally
being able to
comprise magnesium.
[0049] The degradable or dissolvable metal for use in the embodiments
described herein
may also include micro-galvanic metals or materials, such as, for example,
solution-structured
galvanic materials. An example of a solution-structured galvanic material is a
magnesium alloy
containing zinc (Zn), where different domains within the alloy contain
different percentages of
Zn. This leads to a galvanic coupling between these different domains, which
cause micro-
galvanic corrosion and degradation. Micro-galvanically corrodible magnesium
alloys could also
be solution structured with other elements such as zinc, aluminum, manganese,
nickel, cobalt,
calcium, iron, carbon, tin, silver, copper, titanium, rare earth elements,
etc. Examples of
solution-structured micro-galvanically-corrodiblc magnesium alloys include
ZK60, which
includes about 4% to about 7% zinc, about 0% to about 1% zirconium, about 0%
to about 3%
other, and balance magnesium; AZ80, which includes 7% to 10% aluminum, 0% to
1% zinc, 0%
to 1% manganese, 3% other, and balance magnesium; and AZ31, which includes 2%
to 5%
aluminum, 0% to 2% zinc, 0% to 1% manganese, 3% other, and the balance
magnesium. Each
of these examples is % by weight of the metal alloy. In some embodiments,
"other" may include
unknown materials, impurities, additives, any elements on the periodic table,
and any
combination thereof.
00501 The dispersed reinforcement material may include ceramic components or
particles. The ceramic components may be constructed from zirconia (including
zircon), alumina
(including fused alumina, chrome-alumina, and emery), carbide (including
tungsten carbide,
silicon carbide, titanium carbide, and boron carbide), boride (including boron
nitride, osmium
diboride, rhenium boride, titanium boride, and tungsten boride), nitride
(silicon nitride and
aluminum nitride), synthetic diamond, and silica. The ceramic may be an oxide
(like the alumina
13
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
and zirconia) or a non-oxide (like the carbide, nitride, and boride). The
dispersed reinforcement
material may include e.g., a particle, a fiber, a weave, a nugget, and the
like.
[0051] In an alternative embodiment of the present disclosure, the dissolvable
metal
matrix composite may be considered to be a cermet.
[0052] In another alternative embodiment of the present disclosure, a hardened
metal
may be used instead of the ceramic. A medium or high carbon steel with a
carbon content in
excess of 0.25% could be used. A maraging steel, a stainless steel, Inconel,
tool steel, titanium,
nickel, tungsten, chromium, or alloys of any of these materials may also be
used.
[0053] In a preferred embodiment of the present disclosure, the dissolvable
metal matrix
composite includes a dispersed reinforcement material and a dissolvable metal
wherein the
dispersed reinforcement material may include tungsten carbide ceramic with
graphite particles in
a mold, and the dissolvable metal may include aluminum. As the mold is
infiltrated with high
pressure liquid aluminum, the aluminum alloy will be a degradable alloy that
binds together the
tungsten carbide and the graphite particles. Upon exposure to an electrolyte,
the graphite will
galvanically react with the aluminum, and the aluminum will disappear, leaving
behind a
ceramic dust and graphite dust. This degradable or dissolvable metal matrix
composite is best
suited for slip buttons on dissolvable fi-ac plugs as well as for baffle seat
on sliding sleeves. The
dissolvable metal matrix composite of the present disclosure fulfills the need
for the slip buttons
to have the sharpness and the baffle seat to have the erosion resistance while
facilitating easier
and cost efficient degradation of the slip buttons and baffle seat in the
wellbore system.
[0054] In another preferred embodiment of the present disclosure, the
dissolvable metal
matrix composite may include about 20 to about 95 weight percent of the
dispersed
reinforcement material, and may be most typically about 50 to about 70 weight
percent of the
dispersed reinforcement material. The dissolvable metal matrix composite may
include up to
about 95 weight percent of the dissolvable metal.
[0055] In yet another preferred embodiment of the present disclosure, the
dissolvable
metal matrix composite may include a dissolvable metal that exhibits a
degradation rate in an
amount greater than 10 mg/cm2 per hour at a temperature of 200 F (93.3 C)
while exposed to a
15% potassium chloride (KCI) solution.
14
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
[0056] In another embodiment of the present disclosure, the degradation rate
of the
dissolvable metal may be somewhat slower, such that the dissolvable metal
exhibits a
degradation rate in an amount of less than about 10 mg/cm2 per hour at 200 F
(93.3 C) in 15%
KCI solution. In other embodiments, the dissolvable metal exhibits a
degradation rate such that
lower than about 10% but greater than 1% of its total mass is lost per day at
200 F (93.3 C) in
15% KCI solution.
[0057] The degradation of the dissolvable metal may be in the range of from
about 1 day
to about 120 days, encompassing any value or subset therebetween. For example,
the
degradation may be about 5 days to about 10 days, or about 10 days to about 20
days, or about
20 days to about 30 days, or about 30 days to about 120 days, encompassing any
value and
subset therebetween. Each of these values representing the degradable metal
may depend on a
number of factors including, but not limited to, the type of degradable or
dissolvable metal, the
wellbore environment, and the like.
[0058] According to an embodiment of the present disclosure, the dissolvable
metal
matrix composite may include at least one tracer. The tracer(s) can be,
without limitation,
radioactive, chemical, electronic, physical, or acoustic. A tracer can be
useful in determining
real-time information on the rate of dissolution dissolvable metal. For
example, a dissolvable
metal containing a tracer, upon dissolution can be flowed through the wellbore
and towards the
wellhead or into the subterranean formation. By being able to monitor the
presence of the tracer,
workers at the surface can make on-the-fly decisions that can affect the rate
of dissolution of the
remaining dissolvable metal. Such decisions might include to increase or
decrease the
concentration of the electrolyte or to increase or decrease the pH of the
electrolyte.
[0059] Additionally, the dissolution of the dissolvable metal may also be
accelerated by
hydraulically fracturing with an acid or otherwise augmenting the wellbore
fluid with an acid.
For example, all or a portion of the outer surface of a given component of the
wellbore isolation
device may be treated or coated with a substance configured to enhance
degradation of the
dissolvable metal Such a treatment or coating may be configured to remove a
protective coating
or treatment or otherwise accelerate the degradation of the dissolvable metal.
An example is a
galvanically-corroding metal coated with a layer of polyglycolic acid (PGA).
In this example,
= CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
the PGA would undergo hydrolysis and cause the surrounding fluid to become
more acidic,
which would accelerate the degradation of the underlying dissolvable metal.
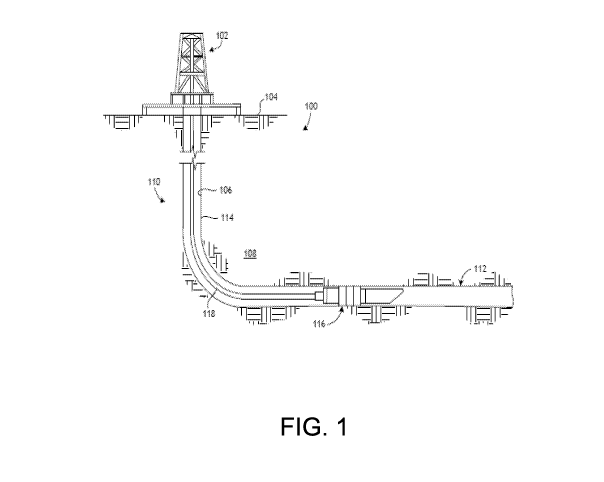
[0060] Referring to FIG. 1, illustrated is a well system 100 that may embody
or
otherwise employ one or more principles of the present disclosure, according
to one or more
embodiments. As illustrated, the well system 100 may include a service rig 102
(also referred to
as a "derrick") that is positioned on the earth's surface 104 and extends over
and around a
wellbore 106 that penetrates a subterranean formation 108. The service rig 102
may be a drilling
rig, a completion rig, a workover rig, or the like. In some embodiments, the
service rig 102 may
be omitted and replaced with a standard surface wellhead completion or
installation, without
departing from the scope of the disclosure. While the well system 100 is
depicted as a land-
based operation, it will be appreciated that the principles of the present
disclosure could equally
be applied in any sea-based or sub-sea application where the service rig 102
may be a floating
platform or sub-surface wellhead installation, as generally known in the art.
[0061] The wellbore 106 may be drilled into the subterranean formation 108
using any
suitable drilling technique and may extend in a substantially vertical
direction away from the
earth's surface 104 over a vertical wellbore portion 110. At some point in the
wellbore 106, the
vertical wellbore portion 110 may deviate from vertical relative to the
earth's surface 104 and
transition into a substantially horizontal wellbore portion 112, although such
deviation is not
required. That is, the wellbore 106 may be vertical, horizontal, or deviated,
without departing
from the scope of the present disclosure. In some embodiments, the wellbore
106 may be
completed by cementing a string of casing 114 within the wellbore 106 along
all or a portion
thereof. As used herein, the term "casing" refers not only to casing as
generally known in the
art, but also to borehole liner, which comprises tubular sections coupled end
to end but not
extending to a surface location. In other embodiments, however, the string of
casing 114 may be
omitted from all or a portion of the wellbore 106 and the principles of the
present disclosure may
equally apply to an "open-hole" environment.
[0062] The well system 100 may further include a wellbore isolation device 116
that
may be conveyed into the wellbore 106 on a conveyance 118 (also referred to as
a "tool string")
that extends from the service rig 102. The wellbore isolation device 116 may
include or
otherwise comprise any type of casing or borehole isolation device known to
those skilled in the
16
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
art including, but not limited to, a frac plug, a deployable baffle, a
wellbore packer, a wiper plug,
a cement plug, or any combination thereof. The conveyance 118 that delivers
the wellbore
isolation device 116 downhole may be, but is not limited to, wireline,
slickline, an electric line,
coiled tubing, drill pipe, production tubing, or the like.
[00631 The wellbore isolation device 116 may be conveyed downhole to a target
location
(not shown) within the wellbore 106. At the target location, the wellbore
isolation device may be
actuated or "set" to seal the wellbore 106 and otherwise provide a point of
fluid isolation within
the wellbore 106. In some embodiments, the wellbore isolation device 116 is
pumped to the
target location using hydraulic pressure applied from the service rig 102 at
the surface 104. In
such embodiments, the conveyance 118 serves to maintain control of the
wellbore isolation
device 116 as it traverses the wellbore 106 and provides the necessary power
to actuate and set
the wellbore isolation device 116 upon reaching the target location. In other
embodiments, the
wellbore isolation device 116 freely falls to the target location under the
force of gravity to
traverse all or part of the wellbore 106.
[0064] It will be appreciated by those skilled in the art that even though
FIG. 1 depicts
the wellbore isolation device 116 as being arranged and operating in the
horizontal portion 112
of thc wellbore 106, the embodiments described herein arc equally applicable
for use in portions
of the wellbore 106 that are vertical, deviated, or otherwise slanted. It
should also be noted that a
plurality of wellbore isolation devices 116 may be placed in the wellbore 106.
In some
embodiments, for example, several (e.g., six or more) wellbore isolation
devices 116 may be
arranged in the wellbore 106 to divide the wellbore 106 into smaller intervals
or "zones" for
hydraulic stimulation.
100651 Referring now to FIG. 2, with continued reference to FIG. 1,
illustrated is a cross-
sectional view of an exemplary wellbore isolation device 200 that may employ
one or more of
the principles of the present disclosure, according to one or more
embodiments. The wellbore
isolation device 200 may be similar to or the same as the wellbore isolation
device 116 of FIG. 1.
Accordingly, the wellbore isolation device 200 may be configured to be
extended into and seal
the wellbore 106 at a target location, and thereby prevent fluid flow past the
wellbore isolation
device 200 for wellbore completion or stimulation operations. In some
embodiments, as
illustrated, the wellbore 106 may be lined with the casing 114 or another type
of wellbore liner or
17
= CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
tubing in which the wellbore isolation device 200 may suitably be set. In
other embodiments,
however, the casing 114 may be omitted and the wellbore isolation device 200
may instead be
set or otherwise deployed in an uncompleted or "open-hole" environment.
[0066] The wellbore isolation device 200 is generally depicted and described
herein as a
hydraulic fracturing plug or "frac" plug. It will be appreciated by those
skilled in the art,
however, that thc principles of this disclosure may equally apply to any of
the other
aforementioned types of casing or borehole isolation devices, without
departing from the scope
of the disclosure. Indeed, the wellbore isolation device 200 may be any of a
frac plug, a bridge
plug, a wellbore packer, a deployable baffle, a ball and seat, a cement plug,
or any combination
thereof in keeping with the principles of the present disclosure.
[0067] As illustrated, the wellbore isolation device 200 may include a ball
cage 204
extending from or otherwise coupled to the upper end of a mandrel 206. A
sealing ball 208 (e.g.,
a frac ball) is disposed in the ball cage 204 and the mandrel 206 defines a
longitudinal central
flow passage 210. The mandrel 206 also defines a ball seat 212 at its upper
end. One or more
spacer rings 214 (one shown) may be secured to the mandrel 206 and otherwise
extend
thereabout. The spacer ring 214 provides an abutment, which axially retains a
set of upper slips
216a that arc also positioned circumferentially about the mandrel 206. As
illustrated, a set of
lower slips 216b may be arranged distally from the upper slips 216a. The lower
slips 216b may
include a lower slip button 215b wherein the slip button 215b may include a
sharp edge 217b
which is configured to bite into the casing 114. The upper slips 216a may
include an upper slip
button 215a wherein the slip button 215a may include a sharp edge 217a which
is configured to
bite into the casing 114. In other embodiments, the sealing ball 208 may be
dropped into the
conveyance 118 (FIG. 1) to land on top of the wellbore isolation device 200
rather than being
carried within the ball cage 204.
[0068] In an embodiment of the present disclosure, the slip buttons 215a and
215b may
be composed of the dissolvable (or degradable) metal matrix composite in
accordance with the
principles of the present disclosure, thereby allowing an easier and cost
efficient disposal of the
slip buttons 215a and 215b in the wellbore.
[0069] One or more slip wedges 218 (shown as upper and lower slip wedges 218a
and
218b, respectively) may also be positioned circumferentially about the mandrel
206, and a
18
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
packer assembly consisting of one or more expandable or inflatable packer
elements 220 may be
disposed between the upper and lower slip wedges 218a,b and otherwise arranged
about the
mandrel 206. It will be appreciated that the particular packer assembly
depicted in FIG. 2 is
merely representative as there are several packer arrangements known and used
within the art.
For instance, while three packer elements 220 are shown in FIG. 2, the
principles of the present
disclosure are equally applicable to wellborc isolation devices that employ
more or less than
three packer elements 220, without departing from the scope of the disclosure.
[0070] A mule shoe 222 may be positioned at or otherwise secured to the
mandrel 206 at
its lower or distal end. As will be appreciated, the lower most portion of the
wellbore isolation
device 200 need not be a mule shoe 222, but could be any type of section that
serves to terminate
the structure of the wellbore isolation device 200, or otherwise serves as a
connector for
connecting the wellbore isolation device 200 to other tools, such as a valve,
tubing, or other
downhole equipment. In some embodiments of the present disclosure, at least a
portion of the
mandrel 206 (such as the interior surface) or at least a portion of the spacer
ring 214 or mule
shoe 222 (such as the exterior surface) may be composed of the dissolvable (or
degradable)
metal matrix composite in accordance with the principles of the present
disclosure, thereby
allowing morc erosion resistance or abrasion resistance of the component.
[0071] In some embodiments, a spring 224 may be arranged within a chamber 226
defined in the mandrel 206 and otherwise positioned coaxially with and fluidly
coupled to the
central flow passage 210. At one end, the spring 224 biases a shoulder 228
defined by the
chamber 226 and at its opposing end the spring 224 engages and otherwise
supports the sealing
ball 208. The ball cage 204 may define a plurality of ports 230 (three shown)
that allow the flow
of fluids therethrough, thereby allowing fluids to flow through the length of
the wellbore
isolation device 200 via the central flow passage 210.
[0072] As the wellbore isolation device 200 is lowered into the wellbore 106,
the spring
224 prevents the sealing ball 208 from engaging the ball seat 212. As a
result, fluids may pass
through the wellbore isolation device 200; i.e., through the ports 230 and the
central flow
passage 210. The ball cage 204 retains the sealing ball 208 such that it is
not lost during
translation into the wellbore 106 to its target location. Once the wellbore
isolation device 200
reaches the target location, a setting tool (not shown) of a type known in the
art can be used to
19
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
move the wellbore isolation device 200 from its unset position (shown in FIG.
2) to a set
position. The setting tool may operate via various mechanisms to anchor the
wellbore isolation
device 200 in the wellbore 106 including, but not limited to, hydraulic
setting, mechanical
setting, setting by swelling, setting by inflation, and the like. In the set
position, the slips 216a,b
and the packer elements 220 expand and engage the inner walls of the casing
114.
[0073] When it is desired to seal the wellbore 106 at the target location with
the wellbore
isolation device 200, fluid is injected into the wellbore 106 and conveyed to
the wellbore
isolation device 200 at a predetermined flow rate that overcomes the spring
force of the spring
224 and forces the sealing ball 208 downwardly until it sealingly engages the
ball seat 212.
When the sealing ball 208 is engaged with the ball seat 212 and the packer
elements 220 are in
their set position, fluid flow past or through the wellbore isolation device
200 in the downhole
direction is effectively prevented. At that point, completion or stimulation
operations may be
undertaken by injecting a treatment or completion fluid into the wellbore 106
and forcing the
treatment/completion fluid out of the wellbore 106 and into a subterranean
formation above the
wellbore isolation device 200.
[0074] Following completion and/or stimulation operations, the wellbore
isolation
device 200 must be removed from the wellbore 106 in order to allow production
operations to
effectively occur without being excessively hindered by the emplacement of the
wellbore
isolation device 200. According to the present disclosure, various components
of the wellbore
isolation device 200 may be made of one or more degrading or dissolving
materials, e.g.,
dissolvable metal matrix composite.
[0075] As at least the mandrel 206 (and, in some embodiments, at least the
sealing ball
208, or any other component) are made of dissolvable metal matrix composite,
it may be
desirable that the wellbore isolation device 200 have a greater flow area or
flow capacity through
and/or around the wellbore isolation device 200. According to the present
disclosure, in some
embodiments the wellbore isolation device 200 may exhibit a large flow area or
flow capacity
through and/or around the wellbore isolation device 200 so that it does not
unreasonably impede,
obstruct, or inhibit production operations while the wellbore isolation device
200 degrades. As a
result, production operations may be undertaken while the wellbore isolation
device 200
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
proceeds to dissolve and/or degrade, and without creating a significant
pressure restriction within
the wellbore 106.
[0076] According to the present disclosure, at least the mandrel 206 (and, in
some
embodiments, at least the sealing ball 208, or any other component) may be
made of or otherwise
include a dissolvable metal matrix composite which includes a dissolvable
metal that is
configured to degrade or dissolve within a wellbore environment. In other
embodiments, othcr
components of the wellbore isolation device 200 may also be made of or
otherwise comprise a
dissolvable metal including, but not limited to, the upper and lower slips
216a,b, the upper and
lower slip wedges 218a,b, and the mule shoe 222.
[0077] In addition to the foregoing, other components of the wellbore
isolation device
200 that may be made of or otherwise comprise a dissolvable metal to include
extrusion limiters
and shear pins associated with the wellbore isolation device 200.
[0078] FIG. 3 shows an example of a sliding sleeve 300 that employs metal
matrix
composite in accordance with the present disclosure. The sliding sleeve 300
may include baffle
portion 310 on top and sliding sleeve portion 315 on bottom. The sliding
sleeve 300 may further
include dissolvable metal matrix composite that degrades in a wellbore fluid.
The dissolvable
metal matrix composite includes dissolvable metal and a dispersed
reinforcement material. The
dispersed reinforcement material provides sharpness and the erosion resistance
needed in a baffle
(or baffle seat) while the metal provides toughness. The erosion resistance
also provides
resistance to the proppant and flow that passes by the baffles. When the metal
dissolves in a
wellbore fluid, all that remains is, e.g., the ceramic dust. The dissolvable
metal matrix composite
enables dissolvable baffle or baffle seats which in turn facilitates easier
removal of the baffle
from the wellbore system.
[0079] The degradable material may be configured to be encapsulated in a
metallic or
polymeric coating. The coating prevents dissolution until the coating is
removed. This prevents
premature degradation. The coating may be removed by erosion during the
hydraulic fracturing
operation. There may be different diameter baffles at different locations in
the wellbore.
[0080] The sliding sleeve 300 may be used to block access to a flow port. The
sliding
sleeve 300 may be held in place at the flow port by a shear pin. When a ball
325 lands on the
21
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
sliding sleeve portion 315, the hydraulic force breaks the shear pin and the
siding sleeve portion
315 shifts towards the right. The flow ports are now open and the frac can
commence.
[0081] As shown in FIG. 3, the baffle 310 where the ball 325 has landed is the
erosion
resistant metal matrix composite. The sliding sleeve 300 may include a
supporting component
320 that supports the baffle 310. The supporting component 320 may include a
degradable
material.
[0082] In alternative embodiments, the baffle 310 and the supporting component
320
may be configured as a single piece and composed entirely of the degradable
metal matrix
composite.
[0083] FIG. 4 shows an example of a metal matrix composite that is constructed
in
accordance with the principles of the present disclosure. The dissolvable or
degradable metal
matrix composite of the present disclosure includes dissolvable metal and a
dispersed
reinforcement material wherein the reinforcement material is a ceramic or a
hardened metal.
[0084] As shown in FIG. 4, a mold may be filled with reinforcement material
and then
infiltrated with dissolvable metal (e.g., aluminum alloy or magnesium alloy).
The dissolvable
metal then glues all the reinforcement material together (as shown in e.g.,
FIG. 5). Any
component or part of the dovvnhole component may include such mold. The
dissolvable metal
may degrade in a wellbore fluid under certain conditions. In one embodiment of
the present
disclosure, the dissolvable metal matrix composite includes connecting
together an element that
is and not degradable (e.g., ceramic or hardened metal) with an element that
is degradable (e.g.,
dissolvable metal) with a degradable metal glue. The rate of degradation of
the dissolvable metal
may depend on a number of factors including, but not limited to, the type of
dissolvable metal
selected and the conditions of the wellbore environment.
[0085] Referring to FIGS. 1-2 and 4 together, the degradable or dissolvable
metal matrix
composite for use in forming components of the wellbore isolation device 200
may degrade, at
least in part, in the presence of an aqueous fluid (e.g., a treatment fluid,
wellbore fluid, acid,
chemical, and the like). The aqueous fluid that may degrade the dissolvable
metal may include,
but is not limited to, fresh water, saltwater (e.g., water containing one or
more salts dissolved
therein), brine (e.g., saturated salt water), seawater, or combinations
thereof. Accordingly, the
aqueous fluid may comprise ionic salts that trigger galvanic corrosion. The
aqueous fluid may
22
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
come from the wellbore 106 itself (i.e., the subterranean formation) or may be
introduced by a
wellbore operator.
[00861 The following clauses represent additional embodiments of the
disclosure:
Clause 1. A component for a downhole tool comprising:
a dissolvable metal matrix composite, wherein the dissolvable metal matrix
composite comprises:
a dissolvable metal that is configured to partially or wholly dissolve when
in contact with the electrolyte; and
a dispersed reinforcement material that is at least one of: a ceramic or a
hardened metal.
Clause 2. The component according to Clause 1, wherein the component is at
least one of
mandrel, a sealing ball, a slip, a slip button, a baffle seat, or a shear pin.
Clause 3. The component according to Clauses 1 or 2, wherein the downhole tool
comprises a
wellbore isolation device that is selected from the group consisting of a frac
plug, a wellbore
packer, a deployable baffle, and any combination thereof
Clause 4. The component according to Clauses 1 or 2, wherein the dissolvable
metal comprises
at least one of aluminum alloy, magnesium alloy, zinc alloy, bismuth alloy,
tin alloy, or any
combination thereof.
Clause 5. The component according to Clause 4, wherein the dissolvable metal
further comprises
the aluminum alloy that is alloyed with indium or gallium wherein the indium
or gallium acts as
a depassivating agent and prevents formation of a protective passivation layer
on a surface of the
aluminum alloy.
Clause 6. The component according to Clause 5, wherein the aluminum and the
gallium is
alloyed together in a ratio that comprises at least one of the following: 80%
A1-20% Ga, 80%A1-
10%Ga-10%In, 75 %A1-5%Ga-5%Zn-5 %B i-5%Sn-5%Mg, 90%A1-2 .5%Ga-2.5%Zn-2 .5%Bi-
2.5%Sn, 99.8%A1-0.1%In-0.1%Ga.
23
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
Clause 7. The component according to Clause 1, wherein the dissolvable metal
further comprises
at least one of the following: the magnesium alloy that is alloyed with zinc,
aluminum,
zirconium, yttrium, copper, nickel, or with iron.
Clause 8. The component according to Clause 7 further comprises at least one
of the following
ratio: about 4% to 7% zinc, about 0% to 1% zirconium, and balance magnesium,
or 7% to 10%
aluminum, 0% to 1% zinc, 0% to 1% manganese, and balance magnesium, or 2% to
5%
aluminum, 0% to 2% zinc, 0% to 1% manganese, and balance magnesium.
Clause 9. The component according to Clause 1, wherein the ceramic comprises
at least one of:
zirconia (including zircon), alumina (including fused alumina, chrome-alumina,
and emery),
carbide (including tungsten carbide, silicon carbide, titanium carbide, and
boron carbide), boride
(including boron nitride, osmium diboride, rhenium boride, titanium boride,
and tungsten
boride), nitride (silicon nitride and aluminum nitride), synthetic diamond,
silica, and any
combination thereof.
Clause 10. The component according to Clause 9, wherein the ceramic comprises
an oxide or a
non-oxide.
Clause 11. The component according to Clauses 9 or 10, wherein the hardened
metal comprises
at least one of: medium or high carbon steel with a carbon content in excess
of 0.25%, a
maraging steel, stainless steel, Inconel, tool steel, titanium, nickel,
tungsten, chromium, or any
combination thereof.
Clause 12. The component according to Clause 1, wherein the dissolvable metal
is alloyed with
at least one of copper, nickel, iron, or any combination thereof, which in
turn creates inclusions
that have a galvanic potential that accelerates dissolution of the dissolvable
metal.
Clause 13. The component according to Clause 1, wherein a portion of the
ceramic is replaced
with a cathodic component which in turn creates a galvanic potential with the
dissolvable metal.
Clause 14. The component according to Clause 13, wherein the cathodic
component comprises at
least one of a nugget, a spheroid, a silver, a fiber, a weave, or any
combination thereof.
Clause 15. A method of removing a component for a wellbore isolation device
comprising:
24
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
contacting or allowing the component to come in contact with an electrolyte,
the
component consists essentially of:
a dissolvable metal and a dispersed reinforcement material, the dissolvable
metal:
(A)is a metal or a metal alloy,
(B) forms a matrix of a portion of the wellbore isolation device, and
(C) partially or wholly dissolves when an electronically conductive path
exists between the dissolvable metal and the dispersed reinforcement
material and at least a portion of the dissolvable metal is in contact
with electrolyte,
and the dispersed reinforcement material comprises at least one of:
(A) a ceramic; or
(B) a hardened metal.
Clause 16. The method according to Clause 15, wherein the wellbore isolation
device is a ball
and a seat, a plug, a bridge plug, a wiper plug, a packer, or a plug for a
base pipe.
Clause 17. The method according to Clauses 15 or 16, wherein the wellbore
isolation device is
capable of restricting or preventing fluid flow between a first wellbore
interval and a second
wellbore interval.
Clause 18. The method according to Clauses 15 or 16, further comprising the
step of placing the
wellbore isolation device into a portion of the wellbore, wherein the step of
placing is performed
prior to the step of contacting or allowing the wellbore isolation device to
come in contact with
the electrolyte.
Clause 19. The method according to Clauses 15 or 16, further comprising the
step of removing
all or a portion of the dissolved dissolvable metal, wherein the step of
removing is performed
after the step of allowing at least the portion of the dissolvable metal to
dissolve.
CA 02998846 2018-03-15
WO 2017/086955 PCT/US2015/061365
Clause 20. A method of removing a component for a downhole tool comprising
introducing the downhole tool into a wellbore, the downhole tool comprising a
wellbore
isolation device that provides a plurality of components including a mandrel,
a packer element,
and a sealing ball, the mandrel defines a central flow passage that allows
fluid flow in at least
one direction through the wellbore isolation device, at least a portion of the
plurality of
components comprises a dissolvable metal matrix component and the dissolvable
metal matrix
component comprises a dissolvable metal, and dispersed reinforcement material;
anchoring the downhole tool within the wellbore at a target location;
performing at least one downhole operation; and
dissolving the dissolvable metal upon exposure to an electrolyte in a wellbore
environment.
26