Note: Descriptions are shown in the official language in which they were submitted.
TITLE OF THE INVENTION
DELIVERING OSMOLYTES BY NASAL CANNULA
BACKGROUND_OF.THE_INVENTION
= Field ent"o
The present invention relates to the aerosolized delivery of hypertonic saline
(HS) and other esmelytes to provide overnight nasal hydration to patients with
all
forms of chronic obstructive pahrionary disease (COM)) over along period of
time,
The present invention also relates to a device and apparatus with a sufficient
reservoir
to accomplish the same,
sae_tpliz_ef_the Backstround
The mucosal surfaces at the interface between the environment and the body
have evolved a number of "innate defenses", i.e., protective mechanisms. A
principal
form of such innate defense is to cleanse these surfaces with liquid.
Typically, the
quantity of the liquid layer on a mucosal surfaoe reflects the balance between
epithelial liquid secretion, often reflecting active anion (C1- and/or HCO3`)
secretion
coupled with water (and a cation counter-ion), and epithelial liquid
absorption, often
reflecting ,active Na4- absorption, coupled with water and counter anion (Cl"
and/or
HCO3'). Many diseases of mucosal surfaces are caused by too little protective
liquid.
on those mucosal surfaces created by an imbalance between secretion (too
little) and
absorption (relatively too much), The defective salt transport processes that
characterize these mucosal dysfuttctions reside itt the epithelial layer of
the mucosa!
surface.
One approach to replenish the protective liquid layer on mucosal surfaces is
to
"re-balance" the system by blOckingNa+ channel and liquid absorption. The
, epithelial protein that mediates the tate=limiting step of Nil' and
liquid absorption is
the epithelial Na' channel (ENaC). ENaC is positioned on the apical surface of
the
1
CA 3003780 2018-05-03
epithelium, i.e. the mucosal surface-environmental interface. Therefore, to
inhibit
ENaC mediated Na 4 and liquid absorption, an ENaC blocker of the arniloride
class
(which blocks from the extraceilular domain of ENaC) must be delivered to the
mucosal surface and, importantly, be maintained at this site, to achieve
therapeutic
utility, The present invention describes diseases characterized by too little
liquid on
mucosal surfaces and "topical" sodium channel blockers designed to exhibit the
increased potency, reduced mucosal absorbtion, and slow dissociation
("unbinding"
or detachment) from ENaC required for therapy of these diseases.
Chronic obstructive pulmonary diseases arc characterized by dehydration of
airway surfaces and the retention of mucous secretions in the lungs, Examples
of
such diseases include cystic fibrosis, chronic bronchitis, and primary or
secondary
ciliary dyskinesia. Such diseases affect approximately 1$ million patients in
the
United States, and are the sixth leading cause of death. Other airway or
pulmonary
diseases characterized by the accumulation of retained mucous secretions
include
sinusitis can inflammation of the para.nasal sinuses associated with upper
respiratory
infection) and pneumonia.
U.S. patent No, 5,817,028 to Anderson describes a method for the provocation
of air passage narrowing (for evaluating susceptibility to asthma) and/or the
induction
of sputum in subjects via the inhalation of mannitol. It is suggested that the
same
technique can be used to induce sputum and promote mucociliary clearance.
Substances suggested include osmolytes such as sodium chloride, potassium
chloride,
mannitol and dextrose.
Chronic bronchitis (CD), including the most common lethal genetic form of
chronic bronchitis, cystic fibrosis (CF), a disease that reflects the body's
failure to
clear mucus normally from the lungs, which ultimately produces chronic airways
infection. In the normal lung, the primary defense against chronic
intrapulmonary
airways infection (chronic bronchitis) is mediated by the continuous clearance
of
mucus from bronchial airway surfaces, This function in health effectively
removes
from the lung potentially noxious toxins and pathogens. Recent data indicate
that the
initiating problem, i.e., the "basic defect," in both CB and CF is the failure
to clear
mucus from airway surfaces, The failure to clear mucus reflects dehydration of
airway
surfaces that reflects an imbalance between the amount of liquid and mucirt on
airway
surfaces. This "airway surface liquid" (ASL) is primarily composed of salt and
water
in proportions similar to plasma (i.e., isotonic). Mucin macromolecules
organize into
2
CA 3003780 2018-05-03
a well defined "mucus layer" which nortnally traps inhaled bacteria and is
transported
out of the lung via the actions of cilia which beat in a watery, low viscosity
solution
termed the "poriciliary liquid" (PCL). In the disease state, there is an
imbalance in thc
quantities of mucins (too much) and ASL (too little) on airway surfaces that
produces
airway surface dehydration. This dehydration leads to mucus concentration,
reduction
in the lubricant activity of the PCL, and a failure to clear mucus via ciliary
activity to
the mouth, The reduction in mechanical clearance of mucus from the lung leads
to
chronic airways inflammation and bacterial colonization of mucus adherent to
airway
surfaces. It is the chronic retention of bacteria, the failure of local
antimicrobial
substances to kill mucus-entrapped bacteria on a chronic basis, and the
consequent
chronic inflammatory responses of the body to this type of surface infection,
that lead
to the destruction of the lung in CB and CF.
The current afflicted population in the U.S. is 12,000,000 patients with the
acquired (primarily from cigarette smoke exposure) form of chronic bronchitis
and
approximately 30,000 patients with the genetic form, cystic fibrosis.
Approximately
equal numbers of both populations are present in Rurope, In Asia, there is
little CF
but the incidence of CB is high and, like the rest of the world, is
increasing.
There is currently a large, unmet medical need for products that specifically
treat CB and CF at the level of the basic defect that cause these diseases.
The current
therapies for chronic bronchitis and cystic fibrosis focus on treating the
symptoms
and/or the late effects of these diseases. Thus, for chronic bronchitis,
g=agonists,
inhaled steroids, anti-cholinergic agents, and oral theophyllines and
phosphodiesterase
inhibitors are all in development. However, none of these drugs treat
effectively the
fundamental problem of the failure to clear mucus from the lung. Similarly, in
cystic
fibrosis, the same spectrum of pharmacologic agents is used, These strategies
have
been complemented by more recent strategies designed to clear the CF lung of
the
DNA ("Pulmozyme"; Genentech) that has been deposited in the lung by
neutrophils
that have futilely attempted to kill the bacteria that grow In adherent mucus
masses
and through the use of inhaled antibiotics ("T0131") designed to augment the
lungs'
own killing mechanisms to rid the adherent mucus plaques of bacteria, A
general
principle of the body is that if the initiating lesion is not treated, in this
case mucus
retention/obstruction, bacterial infections became chronic And increasingly
refractory
to antimicrobial therapy. Thus, a major unmet therapeutic need for both CB and
CF
lung diseases is an effective means of re-hydrating airway mucus (i.e,,
3
CA 3003780 2018-05-03
restoring/expanding the volume of the ASL) and promoting its clearancz, with
bacteria, from the lung.
The inhalation of osmolytes/osmolyte solutions, such as hypertonic saline (3.
12% preferred embodiment 7%) has been demonstrated to be a safe and effective
treatment for individuals with cystic fibrosis. Inhaled hypertonic saline
improves
mucus hydration and clearance, and is assoeiatedwith improvements in lung
function,
as well as, a reduction in the number of infectious exacerbations over one
year
(Donaldson et al. N. Engl. J. Ivied, 354, 3, January 19, 2006, pp, 241-250)
and Elkins
et. al. (N. Engl. J. Med, 354, 3, January 19,2006, pp, 229-240). -
A limitation of inhaled osmolytes to increase mucosal hydration is tho
durability of the therapeutic effect of the osmolytes, In cell based assays,
the ability
of the mucosa] epithelium to efficiently absorb fluid results in the reversal
of
osmolyte-induced surface hydration. The relatively short therapeutic benefit
of
inhaled osmolytes can be overcome by increasing the number of treatments per
day.
For example, Donaldson at at. (N. Engl. J Med. 354, 3, January 19, 2006, pp.
241-
250) showed inhaling 7% HS four times daily increased FEV1 by two fold grcater
than observed by Elkins et al. (A( Engl. J, Med, 354,3, Sanuary 19, 2006, pp,
229-
240) in CF patients inhaling 7% HS twice daily. However, increasing the dosing
frequency of hypertonic saline or other osmolytes is inconvenient for subjects
in need
thereof, requiring hours of time taking medications during the day,
Clearly, what are needed are treatments that are more effective at restoring
the
clearance of mucus from the lungs of patients with CB/CF. The value of these
new
therapies will be reflected in improvements in the quality and duration of
life for both
the CF and the CB populations.
In U.S. patent publication no. 2008090841, R,C, Boucher and M.R.
Johnson describe a method to extend the duration of osmolyte therapy by co-
administering a potent sodium channel blockers. The inhibition of epithelial
sodium
transport prevents the reabsorption of HS osmolytes, and thereby, slows
mucosa!, fluid
absorption and extends the duration of mucosal hydration. The present
invention
describes an alternative approach to improving both the therapeutic benefit
and
convenience to the of inhaled osmolyte. treatements,
4
CA 3003780 2018-05-03
SinsiMaySE INVENTIQN
The present invention is designed to improve the dosing of an osmolyte (e,g.,
}IS) delivered to the lungs of subjects in need of airway surface rchydration
by
delivering the osmelyte to the lung via nasal cannulae. The present invention
will
permit subjects to be treated for long periods of time (e.g., hours) while
sleeping or
performing daily fiCtiVitICL
Thus, an object of the present invention is a method of treating chronic
obstructive pulmonary disease by administering an effective amount clan
aerosolized
osololYte to a subject in need thereof with a nebulizer connected to a nasal
cannula.
Another object of the present invention is a nasal cannula system for
delivering an esmolyte, comprising:
a nebulizer and
tubing, whore one end of the tubing is connected to the nebulizer and another
end of the tubing is tapered to fit in the nostril of a. subject.
A more complete appreciation of the invention and many of the attendant
advantages thereof will be readily obtained as the same becomes better
understood by
reference to the following figure and detailed description,
Ligluja.SWIDA, 12_,Mlatgla
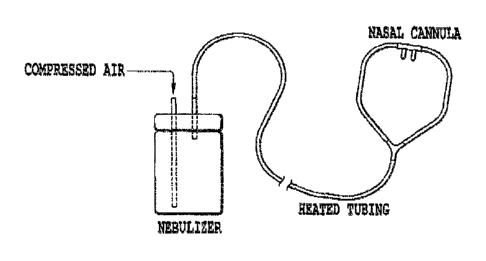
Figure 1: Example of a nebulizer device capable of delivering osmolytes for
extended periods of time. The diagram shows a standard large volume nebulizer
(with >100 ml capacity) connected to a nasal cannula with heated tubing.
CA 3003780 2018-05-03
MAU DESCRIPTION OP Tligjravik,Nnoti
Osmolytes are well-known therapeutics in the field of respiratory
therapeutics.
These agents are molecules or compounds that are osmotically active are
"osmolytes"). "Osmotically active" compounds of the present invention are
rnembrane-impenneable (i.e,, essentially non-absorbablc) on the airway or
pulmonary
epithelial surface, The terms "airway strike" and "pulmonary surface," as used
.
herein, include pulmonary airway surfaces such as the bronchi and bronchioles,
alveolar surfaces, and nasal and sinus surfaces, Active compounds of the
present
invention may be ionic osmolytes OA, salts), or may be non-ionic osmolytcs
(i.e.,
sugars, sugar alcohols, and organic osmolytes). It is specifically intended
that both
raterriic forms of the active compounds that are racerrtic in nature are
included in the
group of active compounds that are usefµ.11 in the present invention. It is to
be noted
that all racematess enantiomers, diastereomers, tautomers, polymorphs and
psoudopolymorphs and racemic mixtures of the osmotically active compounds are
embraced by the present invention.
Active osmolytes uaeful in the present invention that are ionic osmolytes
include any salt of a pharmaceutically acceptable anion and a pharmaceutically
acceptable cation. Preferably, either (or both) of the anion and cation are
non-
absorbable (i.e., osmotically active and not subject to rapid active
transport) in
relation to the airway surfaces to which they are administered. Such compounds
include but are not limited to anions and cations that are contained in FDA
approved
commercially marketed salts, see, e.g., Remington; The Science and Practice of
Pharmacy,V al. II, pg. 1457 (l9' Ed, 1995), incorporated herein by reference,
and
can be used in any combination including their conventional combinations.
Phamiaceutically acceptable osmotically active anions that can be used to
carry out the present invention include, but are not limited to, acetate,
benzonesulfonate, benzoate, bicarbonate, bitartrate, bromide, calcium edetate,
camsylate (camphorsulfonate), carbonate, chloride, citrate, dihydrochloride,
edetate,
edisylate (1,2-ethanedisulfonate), estolate (lauryl sulfate), esylate (1,2-
ethanedisutfonate), fumarate, glueeptate, glueonate, glutamate,
glycollylarsanilate (p-
glycollarnidophenylarsonate), hexylresorcinate, hydrabamine (N,N'-
Di(dehydtoitb(etyl)ethylenedimtint), hydrobromide, hydrochloride,
hydroxynaphthoate, iodide, isethionate, lactate, lactobionate, malate,
maleate,
6
CA 3003780 2018-05-03
mandelate, mesylate, methylbromide, methylnitratc, methylsulfate, rnucate,
napsylate,
nitrate, nitrte, pamoate (embonate), pantothenate, phosphate or diphosphate,
potygalacturonate, salieylate, stcarate, subacetate, suceinate, sulfate,
tannate, tartrate,
tecielate (8-chlorotheophyllinate), triethiodide, bicarbonate, etc.
Particularly preferred
anions include chloride sulfate, nitrate, gluconate, iodide, bicarbonate,
bromide, and
phosphate.
Pharmaceutically acceptable cations that can be used to carry out the present
invention include, but are not limited to, organic catione such as benzathine
(NIAdibenzyethylenediaminc), chloroprocaine, cholinc, diethanolamine,
ethylenediamine,
meglumine (N-methyl D-glucamine), procaine, D-lysine, L-lysine, D-arginine,
arginine, triethylammonium, N.methyl D-glycerol, and the like. Particularly
preferred organic cations are 3-carbon, 4-carbon1 5-carbon and 6-carbon
organic
cations, Metallic cations useful in the practice of the present invention
include but are
not limited to aluminum, calcium, lithium, magnesium, potassium, sodium, zinc,
iron,
ammonium, and the like Particularly preferred cations include sodium,
potassium,
choline, lithium, meglurnine, D-lysine, ammonium, magnesium, and calcium.
Specific examples of osmotically active salts that may be used with the
sodium channel blockers described herein to carry out the present invention
include,
but are not limited to, sodium chloride, potassium chloride, Moline chloride,
eholine
iodide, lithium chloride, meglumine chloride, L-lysine chloride, D-lysine
chloride,
ammonium chloride, potassium sulfate, potassium nitrate, potassium gluconate,
potassium iodide, ferric chloride, ferrous chloride, potassium bromide, eta.
Either a
single salt or a combination of diffbrent osmotically active salts may be used
to carry
out the present invention. Combinations of different salts are preferred, When
different salts are used, one of the anion or cation may be the same among the
differing salts,
Osmotically active compounds of the present invention also include non-ionic
osmolytes such 25 sugars, sugar-alcohols, and organic osmolytes. Sugars and
sugar-
alcohols useful in the practice of the present invention include but are not
limited to
3-carbon sugars (e.g., glycerol, dihydroxyacctone); 4-carbon sugars (e.g.,
both the 13
and L forms of erythrose, threose, and erythrulose); 5-carbon sugars (e.g.,
both the
and I., forms of ribose, arabirtose, xylose, lyxose, psicose, fructose,
eorbose, and
tagatose); and 6-carbon sugars (e.g., both the D. and I.-forms of allose,
Mose,
7
CA 3003780 2018-05-03
glucose, mannose, gulose, idose, galactose, and tatose, and the D- and L-forms
of
allo-heptulose, allo-hepulose, gluco-heptulose, marmo-heptilose, gulo-
heptulose, ido-
heptulose, galacto-heptulose, talo-heptalose). Additional sugars useful in the
practice
of the present invention include raffinose, raffinose series oligosaccharides,
and
stachyose, Both the 1)- and L-forms of the reduced form of each sugar/sugar
alcohol
= Useful in the present invention are also active compounds within the
scope of the
invention, For example, glucose, when reduced, becomes sorbitol; within the
scope
of the invention, sorbitol and other reduced forms of sugar/sugar alcohols
(e.g.,
marmitol, duicitol, arabitoI) are accordingly active compounds of the present
invention,
Osmotically active compounds of the present invention additionally include
the family of non-lank osmolytes termed "organic osmolytes." The term "organic
osmolytes" is generally used to refer to molecules used to control
intracellular
osmolality in the kidney. See e.g., J. S. Handler et al., Camp. Boehm.
Physiol,117,
301-306 (1997); M. Burg, Am. .1 Physiol. 268, F983,7996 (1995).
Although the inventor does not wish to be bound to any
particular theory of the invention, it appears that these organic osmolytes
are useful in
controlling extracellular volume on the airway/pulmonary surface, Organic
osmolytes
useful as active compounds in the present invention include but are not
limited to
three major classes of compounds; polls (polyhydric alcohols), methylatnines,
and
amino acids. The polyol organic osmolytes considered useful in the practice of
this
invention include, but are not limited to, inositol, myo-inositol, and
sorbitol. The
tnethylarnine organic osmolytes uaeful in the practice of the invention
include, but are
not limited to, choline, betaine, carnitine (L-, D- and DL.forms),
phosphorylcholine,
lyso-phosphorylcholine, glycerophosphorylcholine, creatine, and creatine
phosphate.
The amino acid organic osmolytes of the invention include, but are not limited
to, the
D- and L-forms of garine, alanine, glutamine, glutamate, aspartate, proline
and
taurine. Additional osmolytes useful in the practice of the invention include
tihulose
and sarcosine, Mammalian organic osmolytes are preferred, with human organic
'osmolytes being most preferred. However, certain organic osmolytes are of
bacterial,
yeast, and marine animal origin, and these compounds are also useful active
compounds within the scope of the present invention.
Under certain circumstances, an osmolyte precursor may be administered to
the subject. Accordingly, these compounds are also useful in the practice of
the
8
CA 3003780 2018-05-03
invention. The term "osmolyte precursor" as used herein refers to a compound
which
is converted into an osmolyte by a metabolic step, either catabolic or
anabolic. The
osmolyte precursors of this invention include, but are not limited to,
glucose, glucose
polymers, glycerol, choline, phosphatidylcholine, lyso-phosphatidyleholine and
inorganic phosphates, which are precursors of polyols and methylamines.
Precursors
of amino acid osmolytes within the scope of this invention include proteins,
peptides,
and. polyamino acids, which are hydrolyzed to yield osmolyte amino acids, and
metabolic precursors which can be converted into osmolyte amino acids by a
metabolio step such as transamination. For example, a precursor of the amino
acid
glutamine is poly-L.glutamine, and a precursor of glutamate is poly-L,glutamic
acid.
Also included within the scope of this invention are chemically modified
ostnolytes or osmolyte precursors. Such chemical modifications involve linking
to
the osmolyte (or precursor) an additional chemical group which alters or
enhances the
effect of the osmolyte or osmolyte precursor (e,g,, inhibits degradation of
the .
osmolyte molecule). Such chemical modifications have been utilized with drugs
or
prodrugs and are known in the art. (See, for exantple, U.S. Pat. Nos,
4,479,932 and
4,540,564; Slick, E. et al,, Med. Chem. 19:113-117 (1976); Bodor, N. et al.,
J.
Pharm, Sci, 67:1045-1050 (1978); Bodin, N. et al., 3 Med. Chem. 26:313-318
(1983);
Bodor, N. et alõ J. Pharrn, Sci, 75:29-35 (1986).
lin general, osmotically active compounds of the present invention (both ionic
and non-ionic) that do not promote, armn fact deter or retard bacterial
growth, are
preferred.
It is an object of the present invention to provide a nebulizer connected to a
nasal cannula to deliver aerosolized osmolytes (e.g., HS) to subjects over
long time
intervals. The nebulizer will have the capacity for a large volume of osmolyte
solution (up to 2 liters) and will produce aerosol particles in the respirable
range(1 -
microns MIvID) at a rate that will produce good lung deposition and will be
continuous, i.e. will not require refilling over long time periods (8-24 hrs),
An
example of such a nebulizer is the Westmed Heart High Output Nebulizer, A
nasal
cannula/tubing will be connected to the nebulizerby a. tapered fitting, The
dimensions of the tubing will be ¨3-5 min with an inner diameter with a length
of 2-
,
4 meters. The end of the tubing may end in one or two tapered ends that fit
into the
nostrils, although face masks are alternatives.
9
CA 3003780 2018-05-03
Both nebulizers and nasal cannulas are well-known in the field of respiratory
treatment, See Critical Care Medicine (Michael lames Murray, American Society
of
Critical Care Anesthesiologists, Douglas B. Coursin, Ronald G. Pearl, Donald
S.
Prough), pp. 431 and 439-445. However, commercial nebulizers are generally
designed to rapidly delivery therapeutic agents via the mouth or mask. Nasal
cannulas are generally used to delivery oxygen (gasses) to the lungs through
the nose.
Nasal cannulas are preferred for the delivery of gasses as they are
comfortable to wear
for long periods of time, The adaptation of a nasal cannula on a nebulizer
provides a
novel meant to deliver inhaled osmolytes that offers the following advantages.
(I)
The nasal cannulainebulizer device is comfortable and can be worn for extended
periods of time. (2) Thc device can deliver osmolytes for long periods of
time, thus,
increasing the therapeutic benefit of these treatments,
Due to the narrow diameter of oxygen tubing and nasal cannulas, the output
from a nebulizer will lead to the deposition of aerosol on the inner surface
of the
tubing, leading to the "condensation" and accumulation of fluid droplets.
Fluid inside
the tubing can occlude the flow of aerosol inside the tubing, as well as,
result droplets
blowing out the nasal cannula that would "drown" the subject with boluses of
liquid.
Several modifications improve the performance of the nasal cannula/nebulizer
device to prevent fluid condensation on the inner surface of the tubing and
nasal
cannula. It is an object of the present invention to heat all the fittings,
tubing, and/or
the nasal cannula of the device to retard condensation in the tubing. Thus the
heated, inner surface coated cannula will ensure that the aerosol generated
will be
delivered to the nostril as a respirable particle. It is another object of the
present
invention that the tubing will contain a coating on its inner surface so as to
prevent
condensation of solution in the lumen, It is anticipated that the subject will
use the
heated cannulae to receive HS for periods of minutes to daily.
gxamoles
The nebulizer system shown in Figure 1 was run for SO minutes with 7%
hypertonic saline. The build-up of fluid within the oxygen tubing was observed
with
and without heating the oxygen tubing in a water bath, For this system, the
tubing
became occluded with water droplets within 23 minutes of continuous nebulizer
operation. Externally heating the tubing to 60 C allow the nebulizer system to
run fbr
the full 80 minutes without occlusion from water droplets.
CA 3003780 2018-05-03
Table]. The effect of heating on fluid condensation within the oxygen tubing.
External Tubing Time to
Nebulizer/Cornpressor Tubing Temperature Condensation
laari-LC Star with ProNeb Oxygen Tubing with Ambient 23
min'
Compressor Adult nasal cannula
¨Pari-LC Star with ProNeb Oxygen Tubing with 60'C No significant
__________ Compressor , Adult qua] cannula condensation
Obviously, numerous modifications and variations of the present invention are
possible in light of the above teachings. It is therefore to be understood
that within
the scope of the appended claims, the invention may be practiced otherwise
than as
specifically described herein.
11
CA 3003780 2018-05-03