Note: Descriptions are shown in the official language in which they were submitted.
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
PEPTIDES AND METHODS OF
TREATING ENDOMETRIOSIS USING THE SAME
FIELD
The present disclosure generally relates to compositions and methods for the
diagnosis and treatment of endotnetriosis. The disclosure also relates to pre-
implantation
factor (PIF) mutants and methods of treatment such as treating endometriosis,
BACKGROUND
Endogenous Pre-Implantation Factor (PIF) is a 15 amino acid peptide
(MVRIKPGSANKPSDD, SEQ ID NO:1) expressed by the embryo/fetus and placenta and
is
present in circulation of viable mammals throughout pregnancy starting post
fertilization,
playing a critical determining role to create and maternal tolerance without
immune
suppression. PIF exerts broad neurotrophic and neuroprotective effects. PIF
regulates
immunity, inflammation and transplant acceptance. By creating a favorable
maternal milieu
PIF specifically reduces neural damage while it promotes neural development,
protecting
against maternal adverse environment. PIF precisely targets proteins in the
embryo to reduce
oxidative stress and protein misfolding. In vivo PIF reduces spontaneous and
LPS induced
pregnancy loss by decreasing the pro-inflammatory response in the placenta.
It has been observed that PIF and synthetic PIF analogs (sPIF) have immune
modulatory properties and such peptides are useful in the prevention and/or
treatment of
various immune-mediated diseases, including, but not limited to,
endometriosis, a chronic
inflammatory condition that affects reproductive age women. Compositions and
methods for
diagnosing, treating and/or preventing endometriosis are provided herein.
SUMMARY
The present disclosure relates to a method of treating or preventing
endometriosis in a
subject in need thereof, the method comprising administering to the subject at
least one pre-
implantation factor (PIF) peptide, a mimetic thereof, an analog thereof, or a
pharmaceutically
acceptable salt thereof
In some embodiments, the step of administering to the subject at least one PIF
peptide, a mimetic thereof, an analog thereof, or a pharmaceutically
acceptable salt thereof
comprises administering a therapeutically effective dose of the at least one
PIF molecule, an
-1-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
analog thereof, or a pharmaceutically acceptable salt thereof
In some embodiments, the step of administering to the subject at least one PIF
peptide, a mimetic thereof, an analog thereof, or a pharmaceutically
acceptable salt thereof
comprises administering a therapeutically effective dose of the PIF peptide,
an analog
thereof, or pharmaceutically acceptable salt thereof from about 0.001 mg/kg to
about 200
mg/kg.
In some embodiments, the step of administering to the subject at least one PIF
peptide, a mimetic thereof, an analog thereof, or a pharmaceutically
acceptable salt thereof
comprises administering a therapeutically effective dose of the PIF peptide,
an analog
thereof, or pharmaceutically acceptable salt thereof from about 0.5 mg/kg to
about 5 mg/kg.
In some embodiments, the at least the PIF peptide, a mimetic thereof, an
analog
thereof, or pharmaceutically acceptable salt thereof comprises a chemical
targeting moiety
and/or a radioactive moiety.
In some embodiments, the at least one inhibitor of nuclear translocation of
beta-
catenin or pharmaceutically acceptable salt thereof comprises at least one
radioactive moiety
comprising at least one or a combination of the following isotopes: 2H, 3H,
13c, 14c, 15N, 160,
170, 31F, 32F, 35s, 18F, and 36c1.
In some embodiments, the method further comprises administering at least one
analgesic and/or one anti-inflammatory compound.
In some embodiments, the method further comprises administering at least one
analgesic and or one anti-inflammatory compound before, after, or
simultaneously with the
administration of a therapeutically effective dose of at least one PIF
peptide, an analog
thereof or pharmaceutically acceptable salt thereof
In some embodiments, the therapeutically effective dose is from about 1.0
mg/kg to
about 5.5 mg/kg, wherein kg is kilograms of the subject and mg is milligrams
of the
therapeutically effective dose. In some embodiments, the therapeutically
effective dose is
from about 1.0 mg/kg to about 5.0 mg/kg, wherein kg is kilograms of the
subject and mg is
milligrams of the therapeutically effective dose. In some embodiments, the
therapeutically
effective dose is from about 1.0 mg/kg to about 4.5 mg/kg, wherein kg is
kilograms of the
subject and mg is milligrams of the therapeutically effective dose. In some
embodiments, the
therapeutically effective dose is from about 1.0 mg/kg to about 4.0 mg/kg,
wherein kg is
kilograms of the subject and mg is milligrams of the therapeutically effective
dose. In some
embodiments, the therapeutically effective dose is from about 1.0 mg/kg to
about 3.5 mg/kg,
wherein kg is kilograms of the subject and mg is milligrams of the
therapeutically effective
-2-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
dose. In some embodiments, the therapeutically effective dose is administered
subcutaneously, intravenously, intraperitoneally, topically, orally,
sublingually, intranasally,
or intramuscularly. In some embodiments, the therapeutically effective dose is
administered
once a week, twice a week, three times a week, four times a week, or five days
per week, and
optionally, wherein the dose is administered once in two, three, four or five
days in
succession. In some embodiments, the therapeutically effective dose is
administered once
every two weeks, once every three weeks, once every four weeks, once every
five weeks,
once every six weeks, once every seven weeks, once every eight weeks, once
every nine
weeks, once every ten weeks, once every eleven weeks, or once every twelve
weeks. In some
embodiments, the therapeutically effective dose is administered once a month,
twice a month,
three times a month, four times a month, or five times a month. In some
embodiments, the
therapeutically effective dose is administered once a year, twice a year,
three times a year,
four times a year, five times a year, six times a year, seven times a year,
eight times a year,
nine times a year, ten times per year, or eleven times per year.
In some embodiments, the PIF peptide comprises SEQ ID NO:1, SEQ ID NO:2,
and/or SEQ ID NO:3. In some embodiments, the PIF peptide comprises SEQ ID NO:4
or a
pharmaceutically acceptable salt thereof In some embodiments, the PIF peptide
comprises
SEQ ID NO:5 or a pharmaceutically acceptable salt thereof In some embodiments,
the PIF
peptide comprises SEQ ID NO:6 or a pharmaceutically acceptable salt thereof In
some
embodiments, the PIF peptide comprises SEQ ID NO:7 or a pharmaceutically
acceptable salt
thereof In some embodiments, the PIF peptide comprises SEQ ID NO:8 or a
pharmaceutically acceptable salt thereof In some embodiments, the PIF peptide
comprises
SEQ ID NO:9 or a pharmaceutically acceptable salt thereof In some embodiments,
the PIF
peptide comprises SEQ ID NO:10 or a pharmaceutically acceptable salt thereof
In some
embodiments, the PIF peptide comprises SEQ ID NO:11 or a pharmaceutically
acceptable
salt thereof In some embodiments, the PIF peptide comprises SEQ ID NO:12 or a
pharmaceutically acceptable salt thereof In some embodiments, the PIF peptide
comprises
SEQ ID NO:13 or a pharmaceutically acceptable salt thereof In some
embodiments, the PIF
peptide comprises SEQ ID NO:14 or a pharmaceutically acceptable salt thereof
In some
embodiments, the PIF peptide comprises SEQ ID NO:15 or a pharmaceutically
acceptable
salt thereof In some embodiments, the PIF peptide comprises SEQ ID NO:16 or a
pharmaceutically acceptable salt thereof In some embodiments, the PIF peptide
comprises
SEQ ID NO:17 or a pharmaceutically acceptable salt thereof In some
embodiments, the PIF
peptide comprises SEQ ID NO:18 or a pharmaceutically acceptable salt thereof
In some
-3-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
embodiments, the PIF peptide comprises SEQ ID NO:19 or a pharmaceutically
acceptable
salt thereof In some embodiments, the PIF peptide comprises SEQ ID NO:20 or a
pharmaceutically acceptable salt thereof In some embodiments, the PIF peptide
comprises
SEQ ID NO:21 or a pharmaceutically acceptable salt thereof In some
embodiments, the PIF
peptide comprises SEQ ID NO:22 or a pharmaceutically acceptable salt thereof
In some
embodiments, the PIF peptide comprises SEQ ID NO:23 or a pharmaceutically
acceptable
salt thereof In some embodiments, the PIF peptide comprises SEQ ID NO:24 or a
pharmaceutically acceptable salt thereof In some embodiments, the PIF peptide
comprises
SEQ ID NO:25 or a pharmaceutically acceptable salt thereof In some
embodiments, the PIF
peptide comprises SEQ ID NO:26 or a pharmaceutically acceptable salt thereof
In some
embodiments, the PIF peptide comprises SEQ ID NO:27 or a pharmaceutically
acceptable
salt thereof In some embodiments, the PIF peptide comprises SEQ ID NO:28 or a
pharmaceutically acceptable salt thereof In some embodiments, the PIF peptide
comprises
SEQ ID NO:29 or a pharmaceutically acceptable salt thereof
In a further embodiment, a compound of the formula R1-R2-R3-R4 (SEQ ID NO:31)
is
provided, wherein R1 is Pro or a mimetic of Pro, R2 is GIy or a mimetic of
GIy, R3 is Ser or a
mimetic of Ser, and R4 is Ala or a mimetic of Ala. In alternative embodiments,
the compound
may comprise one or more of up to 11 additional amino acid residues.
Embodiments include
those peptides derived from pre-implantation embryos that induces TH2 type
cytokines like
IL-10 synthesis or secretion from lymphocytes or other white blood cells and
pharmacophores that binds specifically to PIF receptors (such but not limited
to PGSA
(A)VRIKPGSANKPSDD or (Q)VRIKPGSANKPSDD) or by substituting with D amino
acids or by adding PEG. Preferably such peptides are from pre-implantation
embryos and
increases TH2/TH1 ratio through increased number of lymphocytes containing the
desired
cytokines and or by preferential secretion or TH2 over THI cytokines into the
media. Such
pre-implantation embryo-derived peptide may be used to cause a shift from pro-
inflammatory to anti- inflammatory activities in lymphocytes. In a further
embodiment, the
pharmaceutical composition comprises a compound of the formula MVRIK (SEQ ID
NO:
32).
In some embodiments, the pharmaceutically acceptable carrier is sterile and
pyrogen-
free water or aqueous buffer, such as saline or Lactated Ringer's solution.
In some embodiments, the therapeutically effective dose is about 1.0 mg/kg,
wherein
kg is kilograms of the subject and mg is milligrams of the therapeutically
effective dose. In
some embodiments, the therapeutically effective dose is about 2.0 mg/kg,
wherein kg is
-4-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
kilograms of the subject and mg is milligrams of the therapeutically effective
dose. In some
embodiments, the therapeutically effective dose is about 3.0 mg/kg, wherein kg
is kilograms
of the subject and mg is milligrams of the therapeutically effective dose. In
some
embodiments, the therapeutically effective dose is about 4.0 mg/kg, wherein kg
is kilograms
of the subject and mg is milligrams of the therapeutically effective dose. In
some
embodiments, the therapeutically effective dose is about 0.2 mg/kg, wherein kg
is kilograms
of the subject and mg is milligrams of the therapeutically effective dose. In
some
embodiments, the therapeutically effective dose is about 0.3 mg/kg, wherein kg
is kilograms
of the subject and mg is milligrams of the therapeutically effective dose. In
some
embodiments, the therapeutically effective dose is about 0.4 mg/kg, wherein kg
is kilograms
of the subject and mg is milligrams of the therapeutically effective dose. In
some
embodiments, the therapeutically effective dose is about 0.5 mg/kg, wherein kg
is kilograms
of the subject and mg is milligrams of the therapeutically effective dose. In
some
embodiments, the therapeutically effective dose is about 0.6 mg/kg, wherein kg
is kilograms
of the subject and mg is milligrams of the therapeutically effective dose. In
some
embodiments, the therapeutically effective dose is about 0.7 mg/kg, wherein kg
is kilograms
of the subject and mg is milligrams of the therapeutically effective dose. In
some
embodiments, the therapeutically effective dose is about 0.8 mg/kg, wherein kg
is kilograms
of the subject and mg is milligrams of the therapeutically effective dose.
The present disclosure also relates to a pharmaceutical composition comprising
(i) a
therapeutically effective dose of one or a combination of PIF peptide or
analogs thereof or
pharmaceutically acceptable salts thereof; and (ii) a pharmaceutically
acceptable carrier.
In some embodiments, the composition further comprises a therapeutically
effective
dose of one or a plurality of active agents.
In some embodiments, the one or plurality of active agents is one or a
combination of
compounds chosen from: an anti-inflammatory compound, alpha-adrenergic
agonist,
antiarrhythmic compound, analgesic compound, and an anesthetic compound, or a
hormone
therapy.
In some embodiments, the therapeutically effective dose of one or a
combination of
PIF peptide or mimetics thereof or thereof analogs thereof or pharmaceutically
acceptable
salts thereof is about 1.0 mg/kg, wherein kg is kilograms of the subject and
mg is milligrams
of the therapeutically effective dose.
In some embodiments, the therapeutically effective dose of one or a
combination of
PIF peptide or mimetics thereof or analogs thereof or pharmaceutically
acceptable salts
-5-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
thereof is about 2.0 mg/kg, wherein kg is kilograms of the subject and mg is
milligrams of the
therapeutically effective dose.
In some embodiments, the therapeutically effective dose of one or a
combination of
PIF peptide or analogs thereof or pharmaceutically acceptable salts thereof is
about 3.0
mg/kg, wherein kg is kilograms of the subject and mg is milligrams of the
therapeutically
effective dose.
In some embodiments, the therapeutically effective dose of one or a
combination of
PIF peptide or analogs thereof or pharmaceutically acceptable salts thereof is
about 4.0
mg/kg, wherein kg is kilograms of the subject and mg is milligrams of the
therapeutically
effective dose.
In some embodiments, wherein the therapeutically effective dose of one or a
combination of PIF peptide or analogs thereof or pharmaceutically acceptable
salts thereof is
about 0.2 mg/kg, wherein kg is kilograms of the subject and mg is milligrams
of the
therapeutically effective dose.
In some embodiments, the therapeutically effective dose of one or a
combination of
PIF peptide or analogs thereof or pharmaceutically acceptable salts thereof is
about 0.3
mg/kg, wherein kg is kilograms of the subject and mg is milligrams of the
therapeutically
effective dose.
In some embodiments, the therapeutically effective dose of one or a
combination of
PIF peptide or analogs thereof or pharmaceutically acceptable salts thereof is
about 0.4
mg/kg, wherein kg is kilograms of the subject and mg is milligrams of the
therapeutically
effective dose.
In some embodiments, the therapeutically effective dose of one or a
combination of
PIF peptide or analogs thereof or pharmaceutically acceptable salts thereof is
about 0.5
mg/kg, wherein kg is kilograms of the subject and mg is milligrams of the
therapeutically
effective dose.
In some embodiments, the therapeutically effective dose of one or a
combination of
PIF peptide or analogs thereof or pharmaceutically acceptable salts thereof is
about 0.6
mg/kg, wherein kg is kilograms of the subject and mg is milligrams of the
therapeutically
effective dose.
In some embodiments, the therapeutically effective dose of one or a
combination of
PIF peptide or analogs thereof or pharmaceutically acceptable salts thereof is
about 0.7
mg/kg, wherein kg is kilograms of the subject and mg is milligrams of the
therapeutically
effective dose.
-6-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
In some embodiments, wherein the therapeutically effective dose of one or a
combination of PIF peptide or analogs thereof or pharmaceutically acceptable
salts thereof is
about 0.8 mg/kg, wherein kg is kilograms of the subject and mg is milligrams
of the
therapeutically effective dose.
In some embodiments, the therapeutically effective dose of one or a
combination of
PIF peptide or analogs thereof or pharmaceutically acceptable salts thereof is
about 0.9
mg/kg, wherein kg is kilograms of the subject and mg is milligrams of the
therapeutically
effective dose.
In some embodiments, the composition further comprises one or a plurality of
stem
cells. In some embodiments, the stem cell is an autologous stem cell.
In some embodiments, the pharmaceutical composition is administered via
parenteral
injection, subcutaneous injection, intravenous injection, intramuscular
injection,
intraperitoneal injection, transdermally, orally, buccally, ocular routes,
intravaginally, by
inhalation, by depot injections, or by implants.
In some embodiments, the compositions further comprise one or a combination of
active agents chosen from: an anti-inflammatory compound, alpha-adrenergic
agonist,
antiarrhythmic compound, analgesic compound, and an anesthetic compound.
In some embodiments, the one or combination of active agents is selected from
Table
Y.
The present disclosure also relates to a method of improving the clinical
outcome in a
subject suffering with, diagnosed with or suspected of having endometriosis
comprising
administering to the subject at least one pharmaceutical composition
comprising: pre-
implantation factor (PIF) peptide, an analog thereof, or a pharmaceutically
acceptable salt
thereof; and a pharmaceutically acceptable carrier.
In some embodiments, methods of diagnosing endometriosis in a human subject
are
provided. In some embodiments, the methods comprises measuring pre-
implantation factor
(PIF) protein or mRNA expression levels from an endometrial tissue sample from
the subject;
and comparing the PIF protein expression levels from the endometrial sample to
the PIF
expression levels in a control normal sample; wherein the patient is diagnosed
with
endometriosis if the PIF expression levels from the endometrial sample are
greater than the
PIF expression levels from the control normal sample.
In some embodiments, methods of treating endometriosis in a human subject are
provided. In some embodiments, the methods comprise measuring pre-implantation
factor
(PIF) protein or mRNA expression levels from a endometrial sample from the
subject;
-7-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
comparing the PIF protein or mRNA expression levels from the endometrial
sample to the
PIF expression levels in a control normal sample, wherein the patient is
diagnosed with
endometriosis if the PIF expression levels from the endometrial sample are
greater than the
PIF expression levels from the control normal sample; and administering to the
subject at
least one pharmaceutical composition comprising a therapeutically effective
amount of a PIF
peptide, mimetics thereof, analogs thereof, or a pharmaceutically acceptable
salt thereof and a
pharmaceutically acceptable carrier.
BRIEF DESCRIPTION OF THE DRAWINGS
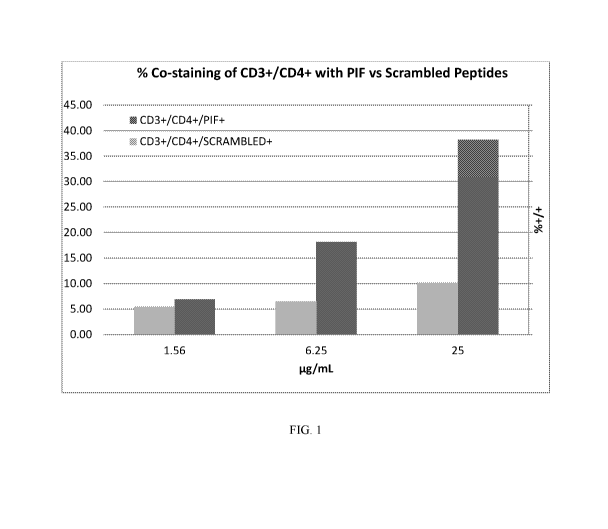
FIG. 1 depicts a graph showing the dose dependent increase in the binding of
PIF with
CD4+/CD25+ cells, in contract to a control scrambled-FITC-PIF.
FIG. 2 depicts a graph showing the dose dependent increase in the binding of
PIF with
CD4+/CD25+/FoxP3+ cells.
FIGs. 3A ¨ 3D depict results showing that PIF imparts epithelial ectopic
endometria.
FIG. 3A depicts PIF staining in epithelial and stromal cells. FIG. 3B depicts
graphs showing
the semi-quantitative evaluation of the PIF positive staining from FIG. 3A.
FIG. 3C depicts a
graph showing the cell viability with increasing sPIF concentration in
epithelial cells
compared to controls. FIG. 3D depicts a graph showing the cell viability with
increasing sPIF
concentration in stromal cells.
FIG. 4A depicts results of a global gene array from sPIF treated epithelial
ectopic
cells lines. FIG. 4B depicts a heat map analysis of T-cell receptor genes
identified in FIG. 4A.
FIGs. 5A ¨ 5E depict results showing that PIF interacts with FoxP3 positive
cells.
FIG. 5A depicts FoxP3 and PIF staining in eutopic and ectopic tissues. FIG. 5B
depicts
graphs showing the semi-quantitative evaluation of the PIF positive staining
from FIG. 5A.
FIG. 5C depicts the dose dependent increase in the binding of FITC-PIF to
CD4+/CD25+/FoxP3+ cells using flow cytometry. FIG. 5D depicts a graph showing
the cell
viability with increasing sPIF concentration in epithelial cells with TNFa
pretreatment. FIG.
5E depicts a cartoon showing the hypothesis that PIF re-expresses in the
epithelial
compartment of ectopic endometria resulting in the recruitment of FoxP3+ cells
into the
stromal compartment creating a positive feed-back loop leading to cellular
survival.
DETAILED DESCRIPTION
Before the present compositions and methods are described, it is to be
understood that
this disclosure is not limited to the particular molecules, compositions,
methodologies or
-8-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
protocols described, as these may vary. It is also to be understood that the
terminology used
in the description is for the purpose of describing the particular versions or
embodiments
only, and is not intended to limit the scope of the present disclosure which
will be limited
only by the appended claims. It is understood that these embodiments are not
limited to the
particular methodology, protocols, cell lines, vectors, and reagents
described, as these may
vary. It also is to be understood that the terminology used herein is for the
purpose of
describing particular embodiments only, and is not intended to limit the scope
of the present
embodiments or claims. The compositions described herein may include D amino
acids, L
amino acids, a racemic backbone of D and L amino acids, or any mixture thereof
at each
residue. That is, at each position, the residue may be a D amino acid residue
or a L-amino
acid residue and each position can be independently D or L of each other
position, unless
context dictates otherwise.
Unless defined otherwise, all technical and scientific terms used herein have
the same
meanings as commonly understood by one of ordinary skill in the art. Although
any methods
and materials similar or equivalent to those described herein can be used in
the practice or
testing of embodiments of the present disclosure, the preferred methods,
devices, and
materials are now described. All publications mentioned herein are
incorporated by reference.
Nothing herein is to be construed as an admission that the disclosure is not
entitled to
antedate such disclosure by virtue of prior disclosure.
As used herein, the phrase "in need thereof means that the animal or mammal
has
been identified or suspected as having a need for the particular method or
treatment. In some
embodiments, the identification can be by any means of diagnosis or
observation. In any of
the methods and treatments described herein, the animal or mammal can be in
need thereof
As used herein, the term "subject," "individual" or "patient," used
interchangeably.
As used herein, the terms "a" or "an" means that "at least one" or "one or
more" unless
the context clearly indicates otherwise. It must also be noted that as used
herein and in the
appended claims, the singular forms "a", "an", and "the" include plural
reference unless the
context clearly dictates otherwise. Thus, for example, reference to "a cell"
is a reference to
one or more cells and equivalents thereof known to those skilled in the art,
and so forth.
As used herein, the term "about" means that the numerical value is approximate
and
small variations would not significantly affect the practice of the disclosed
embodiments.
Where a numerical limitation is used, unless indicated otherwise by the
context, "about"
means the numerical value can vary by 10% and remain within the scope of the
disclosed
-9-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
embodiments. Where a numerical value is used with the term "about" the
numerical value
without the term "about" is also disclosed and can be used without the term
"about."
As used herein, the terms "comprising" (and any form of comprising, such as
"comprise", "comprises", and "comprised"), "having" (and any form of having,
such as
"have" and "has"), "including" (and any form of including, such as "includes"
and "include"),
or "containing" (and any form of containing, such as "contains" and
"contain"), are inclusive
or open-ended and do not exclude additional, unrecited elements or method
steps.
As used herein, the phrase "integer from X to Y" means any integer that
includes the
endpoints. That is, where a range is disclosed, each integer in the range
including the
endpoints is disclosed. For example, the phrase "integer from X to Y"
discloses about 1, 2, 3,
4, or 5 as well as the range from about 1 to about 5.
As used herein, the phrase "therapeutically effective amount" means the amount
of
active compound or pharmaceutical agent that elicits the biological or
medicinal response that
is being sought in a tissue, system, animal, individual or human by a
researcher, veterinarian,
medical doctor or other clinician. The therapeutic effect is dependent upon
the disorder being
treated or the biological effect desired. As such, the therapeutic effect can
be a decrease in the
severity of symptoms associated with the disorder and/or inhibition (partial
or complete) of
progression of the disorder, or improved treatment, healing, prevention or
elimination of a
disorder, or side-effects. The amount needed to elicit the therapeutic
response can be
determined based on the age, health, size and sex of the subject. Optimal
amounts can also be
determined based on monitoring of the subject's response to treatment.
As used herein, the terms "treat," "treated," or "treating" can refer to
therapeutic
treatment and/or prophylactic or preventative measures wherein the object is
to slow down
(lessen) an undesired physiological condition, disorder or disease, or obtain
beneficial or
desired clinical results. For purposes of the embodiments described herein,
beneficial or
desired clinical results include, but are not limited to, alleviation of
symptoms; diminishment
of extent of condition, disorder or disease; stabilized (i.e., not worsening)
state of condition,
disorder or disease; delay in onset or slowing of condition, disorder or
disease progression;
amelioration of the condition, disorder or disease state or remission (whether
partial or total),
whether detectable or undetectable; an amelioration of at least one measurable
physical
parameter, not necessarily discernible by the patient; or enhancement or
improvement of
condition, disorder or disease. Treatment can also include eliciting a
clinically significant
response without excessive levels of side effects. Treatment also includes
prolonging survival
as compared to expected survival if not receiving treatment. Thus, "treatment
of
-10-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
endometriosis " means an activity that prevents, alleviates or ameliorates any
of the primary
phenomena or secondary symptoms associated with the endometriosis.
This application describes compounds and methods of administering those
compounds to a subject in need thereof In some embodiments, "preimplantation
factor" or
"PIF" may also refer to synthetic PIF-1, which replicates the native peptide's
effect and exerts
potent immune modulatory effects on activated peripheral blood mononuclear
cell (PBMC)
proliferation and cytokine secretion, acting through novel sites on PBMCs and
having an
effect which is distinct from known immunosuppressive drugs. In some
embodiments,
"preimplantation factor" or "PIF" or "PIF analog" refers to an amino acid
selected from SEQ
ID NO:1, SEQ ID NO:2, SEQ ID NO:3, SEQ ID NO:4, SEQ ID NO:5, SEQ ID NO:13,
SEQ ID NO:14, SEQ ID NO:15, SEQ ID NO:16, SEQ ID NO:20, SEQ ID NO:21, SEQ ID
NO:22, SEQ ID NO:23, SEQ ID NO:24, SEQ ID NO:25, SEQ ID NO:26, SEQ ID NO:27,
SEQ ID NO:28, SEQ ID NO:29, SEQ ID NO: 31, SEQ ID NO:32, or peptidomimetics or
mutants thereof, and combinations thereof that are about 75, 80, 81, 82, 83,
84 85, 86, 87, 88,
89, 90, 91, 92, 93, 94, 95, 96, 97, 98, or 99% homologous to any such amino
acid. In some
embodiments, the terms "PIF," "PIF peptide," or "PIF analog" refers to an
amino acid
sequence of Table 1 or any peptidomimetics, mutant or analog thereof, that is
from about
75% to about 100% homologous to any sequence of Table 1, optionally fused or
unfused to
one or more other amino acid sequences at its carboxy and/or its amino
terminal ends.
Pharmaceutical compositions of the present disclosure relate to any or all of
the compounds
or PIF peptides disclosed herein or their respective pharmaceutically
effective salts or
polymorphs.
Without being bound by any particular theory, the compounds described herein
may
act as agonists of PIF-mediated signal transduction via the receptor or
receptors of PIF. Thus,
these compounds modulate signaling pathways that provide significant
therapeutic benefit in
the treatment of, but not limited to, endometriosis. The compounds of the
present disclosure
may exist in unsolvated forms as well as solvated forms, including hydrated
forms. The
compounds of the present disclosure also are capable of forming both
pharmaceutically
acceptable salts, including but not limited to acid addition and/or base
addition salts.
Furthermore, compounds of the present disclosure may exist in various solid
states including
an amorphous form (non-crystalline form), and in the form of clathrates,
prodrugs,
polymorphs, bio-hydrolyzable esters, racemic mixtures, non-racemic mixtures,
or as purified
stereoisomers including, but not limited to, optically pure enantiomers and
diastereomers. In
general, all of these forms can be used as an alternative form to the free
base or free acid
-11-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
forms of the compounds, as described above and are intended to be encompassed
within the
scope of the present disclosure.
A "polymorph" refers to solid crystalline forms of a compound. Different
polymorphs
of the same compound can exhibit different physical, chemical and/or
spectroscopic
properties. Different physical properties include, but are not limited to
stability (e.g., to heat
or light), compressibility and density (important in formulation and product
manufacturing),
and dissolution rates (which can affect bioavailability). Different physical
properties of
polymorphs can affect their processing. The disclosure relates to a polymorph
of any of the
disclosed PIF peptides.
As noted above, the compounds of the present disclosure can be administered,
inter
alia, as pharmaceutically acceptable salts, esters, amides or prodrugs. The
term "salts" refers
to inorganic and organic salts of compounds of the present disclosure. The
salts can be
prepared in situ during the final isolation and purification of a compound, or
by separately
reacting a purified compound in its free base or acid form with a suitable
organic or inorganic
base or acid and isolating the salt thus formed. Representative salts include
the
hydrobromide, hydrochloride, sulfate, bisulfate, nitrate, acetate, oxalate,
palmitiate, stearate,
laurate, borate, benzoate, lactate, phosphate, tosylate, citrate, maleate,
fumarate, succinate,
tartrate, naphthylate, mesylate, glucoheptonate, lactobionate, and
laurylsulphonate salts, and
the like. The salts may include cations based on the alkali and alkaline earth
metals, such as
sodium, lithium, potassium, calcium, magnesium, and the like, as well as non-
toxic
ammonium, quaternary ammonium, and amine cations including, but not limited
to,
ammonium, tetramethylammonium, tetraethylammonium, methylamine, dimethylamine,
trimethylamine, triethylamine, ethylamine, and the like. See, for example, S.
M. Berge, et al.,
"Pharmaceutical Salts," J Pharm Sci, 66: 1-19 (1977). The term "salt" refers
to acidic salts
formed with inorganic and/or organic acids, as well as basic salts formed with
inorganic
and/or organic bases. Examples of these acids and bases are well known to
those of ordinary
skill in the art. Such acid addition salts will normally be pharmaceutically
acceptable
although salts of non-pharmaceutically acceptable acids may be of utility in
the preparation
and purification of the compound in question. Salts include those formed from
hydrochloric,
hydrobromic, sulphuric, phosphoric, citric, tartaric, lactic, pyruvic, acetic,
succinic, fumaric,
maleic, methanesulphonic and benzenesulphonic acids.
In some embodiments, salts of the compositions comprising either a PIF or PIF
analog or PIF mutant may be formed by reacting the free base, or a salt,
enantiomer or
racemate thereof, with one or more equivalents of the appropriate acid. In
some
-12-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
embodiments, pharmaceutical acceptable salts of the present disclosure refer
to analogs
having at least one basic group or at least one basic radical. In some
embodiments,
pharmaceutical acceptable salts of the present disclosure comprise a free
amino group, a free
guanidino group, a pyrazinyl radical, or a pyridyl radical that forms acid
addition salts. In
some embodiments, the pharmaceutical acceptable salts of the present
disclosure refer to
analogs that are acid addition salts of the subject compounds with (for
example) inorganic
acids, such as hydrochloric acid, sulfuric acid or a phosphoric acid, or with
suitable organic
carboxylic or sulfonic acids, for example aliphatic mono- or di-carboxylic
acids, such as
trifluoroacetic acid, acetic acid, propionic acid, glycolic acid, succinic
acid, maleic acid,
fumaric acid, hydroxymaleic acid, malic acid, tartaric acid, citric acid or
oxalic acid, or amino
acids such as arginine or lysine, aromatic carboxylic acids, such as benzoic
acid, 2-phenoxy-
benzoic acid, 2-acetoxybenzoic acid, salicylic acid, 4-aminosalicylic acid,
aromatic-aliphatic
carboxylic acids, such as mandelic acid or cinnamic acid, heteroaromatic
carboxylic acids,
such as nicotinic acid or isonicotinic acid, aliphatic sulfonic acids, such as
methane-, ethane-
or 2-hydroxyethane-sulfonic acid, or aromatic sulfonic acids, for example
benzene-, p-
toluene- or naphthalene-2-sulfonic acid. When several basic groups are present
mono- or
poly-acid addition salts may be formed. The reaction may be carried out in a
solvent or
medium in which the salt is insoluble or in a solvent in which the salt is
soluble, for example,
water, dioxane, ethanol, tetrahydrofuran or diethyl ether, or a mixture of
solvents, which may
be removed in vacuo or by freeze drying. The reaction may also be a
metathetical process or
it may be carried out on an ion exchange resin. In some embodiments, the salts
may be those
that are physiologically tolerated by a patient. Salts according to the
present disclosure may
be found in their anhydrous form or as in hydrated crystalline form (i.e.,
complexed or
crystallized with one or more molecules of water).
Examples of pharmaceutically acceptable esters of the compounds of the present
disclosure include C1-C8 alkyl esters. Acceptable esters also include C5-C7
cycloalkyl esters,
as well as arylalkyl esters such as benzyl. Ci-C4 alkyl esters are commonly
used. Esters of
compounds of the present disclosure may be prepared according to methods that
are well
known in the art. Examples of pharmaceutically acceptable amides of the
compounds of the
present disclosure include amides derived from ammonia, primary C1-C8 alkyl
amines, and
secondary C1-C8 dialkyl amines. In the case of secondary amines, the amine may
also be in
the form of a 5 or 6 membered heterocycloalkyl group containing at least one
nitrogen atom.
Amides derived from ammonia, C1-C3 primary alkyl amines and C1-C2 dialkyl
secondary
amines are commonly used. Amides of the compounds of the present disclosure
may be
-13-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
prepared according to methods well known to those skilled in the art.
"Administering" when used in conjunction with a therapeutic means to
administer a
therapeutic directly into or onto a target tissue or to administer a
therapeutic to a patient
whereby the therapeutic positively impacts the tissue to which it is targeted.
Thus, as used
herein, the term "administering", when used in conjunction with PIF, can
include, but is not
limited to, providing PIF peptide into or onto the target tissue; providing
PIF peptide
systemically to a patient by, e.g., intravenous injection whereby the
therapeutic reaches the
target; providing PIF peptide in the form of the encoding sequence thereof to
the target (e.g.,
by so-called gene-therapy techniques). "Administering" a composition may be
accomplished
by parenteral, oral or topical administration.
As used herein, the terms "pharmaceutically acceptable", "physiologically
tolerable"
and grammatical variations thereof, as they refer to compositions, carriers,
diluents and
reagents, are used interchangeably and represent that the materials are
capable of
administration upon a mammal without the production of undesirable
physiological effects
such as nausea, dizziness, rash, or gastric upset. In a preferred embodiment,
the therapeutic
composition is not immunogenic when administered to a subject for therapeutic
purposes.
As used herein, the terms "pharmaceutically acceptable", "physiologically
tolerable"
and grammatical variations thereof, as they refer to compositions, carriers,
diluents and
reagents, are used interchangeably and represent that the materials are
capable of
administration upon a mammal without the production of undesirable
physiological effects
such as nausea, dizziness, rash, or gastric upset. In a preferred embodiment,
the therapeutic
composition is not immunogenic when administered to a subject for therapeutic
purposes.
As used herein, the term "therapeutic" means an agent utilized to treat,
combat,
ameliorate, prevent or improve a subject with endometriosis or at risk of
developing
endometriosis. Examples of such therapeutics, include, but are not limited to,
the PIF
peptides described herein.
A "therapeutically effective amount" or "effective amount" or "physiologically
relevant amount" of a composition is an amount calculated to achieve a desired
effect, i.e., to
effectively inhibit or reduce symptoms and/or complications associated with
endometriosis.
Effective amounts of compounds of the present disclosure can objectively or
subjectively
reduce or decrease the severity or frequency of symptoms associated with
endometriosis. The
specific dose of a compound administered according to this disclosure to
obtain therapeutic
and/or prophylactic effects will, of course, be determined by the particular
circumstances
surrounding the case, including, for example, the compound administered, the
route of
-14-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
administration, and the condition being treated. The compounds are effective
over a wide
dosage range and, for example, dosages per day will normally fall within the
range of from
about 0.01 mg/kg to about 100 mg/kg, more preferably about 0.1 mg/kg to about
1 mg/kg. In
some embodiments, the therapeutically effective dose of PIF or PIF analog or
peptide is
about 0.1mg/kg, 0.2mg/kg, 0.3mg/kg, 0.4mg/kg, 0.5mg/kg, 0.6mg/kg, 0.7mg/kg,
0.8mg/kg,
0.9mg/kg, and lmg/kg, where "mg" is milligram of PIF analog or peptide "kg" is
kilogram of
the subject.
In some embodiments, the pharmaceutical compositions comprise a
therapeutically
effective amount of PIF peptide or analog but the composition is free of SEQ
ID NO:1 or a
pharmaceutically acceptable salt thereof
It will be understood that the effective amount administered will be
determined by the
physician in the light of the relevant circumstances including the condition
to be treated, the
choice of compound to be administered, and the chosen route of administration,
and therefore
the above dosage ranges are not intended to limit the scope of the disclosure
in any way. A
therapeutically effective amount of compound of this disclosure is typically
an amount such
that when it is administered in a physiologically tolerable excipient
composition, it is
sufficient to achieve an effective systemic concentration or local
concentration in the tissue.
In some embodiments, the term "therapeutically effective amount" as used
herein, refers to
that amount of active compound or pharmaceutical agent that elicits the
biological or
medicinal response in a tissue system, animal or human that is being sought by
a researcher,
veterinarian, medical doctor or other clinician, which includes alleviation of
the symptoms of
the disease or disorder being treated. In one aspect, the therapeutically
effective amount is
that which may treat or alleviate the disease or symptoms of the disease at a
reasonable
benefit/risk ratio applicable to any medical treatment. However, it is to be
understood that the
total daily usage of the compounds and compositions described herein may be
decided by the
attending physician within the scope of sound medical judgment. The specific
therapeutically-effective dose level for any particular patient will depend
upon a variety of
factors, including the disorder being treated and the severity of the
disorder; activity of the
specific compound employed; the specific composition employed; the age, body
weight,
general health, gender and diet of the patient: the time of administration,
route of
administration, and rate of excretion of the specific compound employed; the
duration of the
treatment; drugs used in combination or coincidentally with the specific
compound
employed; and like factors well known to the researcher, veterinarian, medical
doctor or other
clinician of ordinary skill.
-15-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
It is also appreciated that the therapeutically effective amount, whether
referring to
monotherapy or combination therapy, is advantageously selected with reference
to any
toxicity, or other undesirable side effect, that might occur during
administration of one or
more of the compounds described herein. Further, it is appreciated that the co-
therapies
described herein may allow for the administration of lower doses of compounds
that show
such toxicity, or other undesirable side effect, where those lower doses are
below thresholds
of toxicity or lower in the therapeutic window than would otherwise be
administered in the
absence of a co-therapy.
As used herein, the term "composition" generally refers to any product
comprising the
specified ingredients in the specified amounts, as well as any product which
results, directly
or indirectly, from combinations of the specified ingredients in the specified
amounts. It is to
be understood that the compositions described herein may be prepared from
isolated
compounds described herein or from salts, solutions, hydrates, solvates, and
other forms of
the compounds described herein. It is also to be understood that the
compositions may be
prepared from various amorphous, non-amorphous, partially crystalline,
crystalline, and/or
other morphological forms of the compounds described herein. It is also to be
understood that
the compositions may be prepared from various hydrates and/or solvates of the
compounds
described herein. Accordingly, such pharmaceutical compositions that recite
compounds
described herein are to be understood to include each of, or any combination
of, the various
morphological forms and/or solvate or hydrate forms of the compounds described
herein.
Illustratively, compositions may include one or more carriers, diluents,
and/or
excipients. The compounds described herein, or compositions containing them,
may be
formulated in a therapeutically effective amount in any conventional dosage
forms
appropriate for the methods described herein. The compounds described herein,
or
compositions containing them, including such formulations, may be administered
by a wide
variety of conventional routes for the methods described herein, and in a wide
variety of
dosage formats, utilizing known procedures (see generally, Remington: The
Science and
Practice of Pharmacy, (21st ed., 2005)).
The terms "treat," "treated," or "treating" as used herein refers to both
therapeutic
treatment and prophylactic or preventative measures, wherein the object is to
prevent or slow
down (lessen) an undesired physiological condition, disorder or disease, or to
obtain
beneficial or desired clinical results. For the purposes of this disclosure,
beneficial or desired
clinical results include, but are not limited to, alleviation of symptoms;
diminishment of the
extent of the condition, disorder or disease; stabilization (i.e., not
worsening) of the state of
-16-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
the condition, disorder or disease; delay in onset or slowing of the
progression of the
condition, disorder or disease; amelioration of the condition, disorder or
disease state; and
remission (whether partial or total), whether detectable or undetectable, or
enhancement or
improvement of the condition, disorder or disease. Treatment includes
eliciting a clinically
significant response without excessive levels of side effects. Treatment also
includes
prolonging survival as compared to expected survival if not receiving
treatment.
"Immune-modulating" refers to the ability of a compound of the present
disclosure to
alter (modulate) one or more aspects of the immune system. The immune system
functions to
protect the organism from infection and from foreign antigens by cellular and
humoral
mechanisms involving lymphocytes, macrophages, and other antigen-presenting
cells that
regulate each other by means of multiple cell-cell interactions and by
elaborating soluble
factors, including lymphokines and antibodies, that have autocrine, paracrine,
and endocrine
effects on immune cells.
"Auto-immune disease" refers to various diseases that arise from an abnormal
immune response of the body against substances and tissues normally present in
the body.
This may be restricted to certain organs or involve a particular tissue in
different places. A
large number of auto-immune diseases are recognized, including, but not
limited to,
Hashimoto's thyroiditis, pernicious anemia, Addison's disease, type I (insulin
dependent)
diabetes, rheumatoid arthritis, systemic lupus erythematosus, dermatomyositis,
Sjogren's
syndrome, lupus erythematosus, multiple sclerosis, myasthenia gravis, Reiter's
syndrome, and
Grave's disease, alopecia greata, anklosing spondylitis, antiphospholipid
syndrome, auto-
immune hemolytic anemia, auto-immune hepatitis, auto-immune inner ear disease,
auto-
immune lymphoproliferative syndrome (ALPS), auto-immune thrombocytopenic
purpura
(ATP), Behcet's disease, bullous pemphigoid, cardiomyopathy, celiac sprue-
dermatitis,
chronic fatigue syndrome immune deficiency syndrome (CFIDS), chronic
inflammatory
demyelinating polyneuropathy, cicatricial pemphigoid, cold agglutinin disease,
CREST
syndrome, Crohn's disease, Dego's disease, dermatomyositis, dermatomyositis,
discoid lupus,
essential mixed cryoglobulinemia, fibromyalgia-fibromyositis, Guillain-Barre
syndrome,
idiopathic pulmonary fibrosis, idiopathic thrombocytopenia purpura (ITP), IgA
nephropathy,
juvenile arthritis, Meniere's disease, mixed connective tissue disease,
pemphigus vulgaris,
polyarteritis nodosa, polychondritis, polyglancular syndromes, polymyalgia
rheumatica,
polymyositis, primary agammaglobulinemia, primary biliary cirrhosis,
psoriasis, Raynaud's
phenomenon, rheumatic fever, sarcoidosis, scleroderma, stiff-man syndrome,
Takayasu
-17-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
arteritis, temporal arteritis/giant cell arteritis, ulcerative colitis,
uveitis, vasculitis, vitiligo, and
Wegener's granulomatosis.
"Inflammatory response" or "inflammation" is a general term for the local
accumulation of fluid, plasma proteins, and white blood cells initiated by
physical injury,
infection, or a local immune response. Inflammation is an aspect of many
diseases and
disorders, including but not limited to diseases related to immune disorders,
viral infection,
arthritis, autoimmune diseases, collagen diseases, allergy, asthma,
pollinosis, and atopy.
Inflammation is characterized by rubor (redness), dolor (pain), calor (heat)
and tumor
(swelling), reflecting changes in local blood vessels leading to increased
local blood flow
which causes heat and redness, migration of leukocytes into surrounding
tissues
(extravasation), and the exit of fluid and proteins from the blood and their
local accumulation
in the inflamed tissue, which results in swelling and pain, as well as the
accumulation of
plasma proteins that aid in host defense. These changes are initiated by
cytokines produced
by activated macrophages. Inflammation is often accompanied by loss of
function due to
replacement of parenchymal tissue with damaged tissue (e.g., in damaged
myocardium),
reflexive disuse due to pain, and mechanical constraints on function, e.g.,
when a joint swells
during acute inflammation, or when scar tissue bridging an inflamed joint
contracts as it
matures into a chronic inflammatory lesion.
"Anti-inflammatory" refers to the ability of a compound to prevent or reduce
the
inflammatory response, or to soothe inflammation by reducing the symptoms of
inflammation
such as redness, pain, heat, or swelling. Inflammatory responses can be
triggered by injury,
for example injury to skin, muscle, tendons, or nerves. Inflammatory responses
can also be
triggered as part of an immune response. Inflammatory responses can also be
triggered by
infection, where pathogen recognition and tissue damage can initiate an
inflammatory
response at the site of infection. Generally, infectious agents induce
inflammatory responses
by activating innate immunity. Inflammation combats infection by delivering
additional
effector molecules and cells to augment the killing of invading microorganisms
by the front-
line macrophages, by providing a physical barrier preventing the spread of
infection, and by
promoting repair of injured tissue. "Inflammatory disorder" is sometimes used
to refer to
chronic inflammation due to any cause.
It is understood that the terms "immune disorder" and "inflammatory response"
are
not exclusive. It is understood that many immune disorders include acute
(short term) or
chronic (long term) inflammation. It is also understood that inflammation can
have immune
aspects and non-immune aspects. The role(s) of immune and nonimmune cells in a
particular
-18-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
inflammatory response may vary with the type of inflammatory response, and may
vary
during the course of an inflammatory response. Immune aspects of inflammation
and diseases
related to inflammation can involve both innate and adaptive immunity. Certain
diseases
related to inflammation represent an interplay of immune and nonimmune cell
interactions,
for example intestinal inflammation (Fiocchi et al., 1997 Am J Physiol
Gastrointest Liver
Physiol 273: G769-G775), pneumonia (lung inflammation), or glomerulonephritis.
As used herein, "conservative" amino acid substitutions may be defined as set
out in
Tables A, B, or C below. The PIF compounds of the disclosure include those
wherein
conservative substitutions (from either nucleic acid or amino acid sequences)
have been
introduced by modification of polynucleotides encoding polypeptides of the
disclosure.
Amino acids can be classified according to physical properties and
contribution to secondary
and tertiary protein structure. A conservative substitution is recognized in
the art as a
substitution of one amino acid for another amino acid that has similar
properties. In some
embodiments, the conservative substitution is recognized in the art as a
substitution of one
nucleic acid for another nucleic acid that has similar properties, or, when
encoded, has similar
binding affinities. Exemplary conservative substitutions are set out in Table
A.
Table A -- Conservative Substitutions I
Side Chain Characteristics Amino Acid
Aliphatic
Non-polar GAPILVF
Polar-uncharged CSTMNQ
Polar-charged DEKR
Aromatic HFWY
Other NQDE
Alternately, conservative amino acids can be grouped as described in
Lehninger,
(Biochemistry, Second Edition; Worth Publishers, Inc. NY, N.Y. (1975), pp. 71-
77) as set
forth in Table B.
Table B -- Conservative Substitutions II
Side Chain Characteristic Amino Acid
Non-polar (hydrophobic)
Aliphatic: ALIVP.
Aromatic: F W Y
-19-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
Sulfur-containing:
Borderline: G Y
Uncharged-polar
Hydroxyl: STY
Amides: NQ
Sulfhydryl:
Borderline: G Y
Positively Charged (Basic): K R H
Negatively Charged (Acidic): D E
Alternately, exemplary conservative substitutions are set out in Table C.
Table C -- Conservative Substitutions III
Original Residue Exemplary Substitution
Ala (A) Val Leu Ile Met
Arg (R) Lys His
Asn (N) Gln
Asp (D) Glu
Cys (C) Ser Thr
Gln (Q) Asn
Glu (E) Asp
Gly (G) Ala Val Leu Pro
His (H) Lys Arg
Ile (I) Leu Val Met Ala Phe
Leu (L) Ile Val Met Ala Phe
Lys (K) Arg His
Met (M) Leu Ile Val Ala
Phe (F) Trp Tyr Ile
Pro (P) Gly Ala Val Leu Ile
Ser (S) Thr
Thr (T) Ser
Trp (W) Tyr Phe Ile
Tyr (Y) Trp Phe Thr Ser
Val (V) Ile Leu Met Ala
-20-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
It should be understood that the inhibitors described herein are intended to
include
nucleic acids and, where the inhibitors include polypeptide, polypeptides
bearing one or more
insertions, deletions, or substitutions, or any combination thereof, of amino
acid residues as
well as modifications other than insertions, deletions, or substitutions of
amino acid residues.
As used herein, the terms "peptide," "polypeptide" and "protein" are used
interchangeably and refer to two or more amino acids covalently linked by an
amide bond or
non-amide equivalent. The peptides of the disclosure can be of any length. For
example, the
peptides can have from about two to about 100 or more residues, such as, about
4 to about 15,
about 12 to about 15, about 8 to about 18, about 18 to about 25, about 15 to
about 50,about 50
to about 75, or about 75 to about 100 or more amino acids in length.
Preferably, peptides are
from about 4 to about 18 residues in length. The peptides of the disclosure
also include 1- and
d-isomers, and combinations of 1- and d-isomers. The peptides can include
modifications
typically associated with posttranslational processing of proteins, for
example, cyclization
(e.g., disulfide or amide bond), phosphorylation, glycosylation,
carboxylation, ubiquitination,
myristylation, or lipidation. In some embodiments, the compositions or
pharmaceutical
compositions of the disclosure relate to analogs of any PIF sequence set forth
in Table 1 that
share no less than about 70%, about 75%, about 79%, about 80%, about 85%,
about 86%,
about 87%, about 90%, about 93%, about 94% about 95%, about 96%, about 97%,
about
98%, about 99% homology with any one or combination of PIF sequences set forth
in Table
1. In some embodiments, PIF or PIF peptide may refer to an amino acid sequence
selected
from SEQ ID NOs: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 ,14 ,15, 16 ,17,
18, 19, 20, 21, 22,
23, 24, 25, 26, 27, 28, 29 or a functional fragment thereof that is about 70%,
75%, 80%, 85%,
90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% homologous to any such
amino
acid sequence. In some embodiments, PIF may refer to an amino acid sequence
comprising,
consisting essentially of, or consisting of a sequence that is at least 70%,
75%, 80%, 85%,
86%, 87%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% homologous to
SEQ
ID. NO: 20. In some embodiments, PIF may refer to an amino acid sequence
comprising,
consisting essentially of, or consisting of a sequence that is at least 70%,
75%, 80%, 85%,
86%, 87%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% homologous to
SEQ
ID. NO: 21. In some embodiments, PIF may refer to an amino acid sequence
comprising,
consisting essentially of, or consisting of a sequence that is at least 70%,
75%, 80%, 85%,
86%, 87%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% homologous to
SEQ
ID. NO: 22. In some embodiments, PIF may refer to an amino acid sequence
comprising,
consisting essentially of, or consisting of a sequence that is at least 70%,
75%, 80%, 85%,
-21-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
860o, 870o, 900o, 910o, 920o, 930o, 940o, 950o, 960o, 970o, 980o, or 990o
homologous to SEQ
ID. NO: 23. In some embodiments. PIF may refer to an amino acid sequence
comprising,
consisting essentially of, or consisting of a sequence that is at least 700o,
750o, 800o, 850o,
860o, 870o, 900o, 910o, 920o, 930o, 940o, 950o, 960o, 970o, 980o, or 990o
homologous to SEQ
ID. NO: 24. In some embodiments, PIF may refer to an amino acid sequence
comprising,
consisting essentially of, or consisting of a sequence that is at least 700o,
750o, 800o, 850o,
860o, 870o, 900o, 910o, 920o, 930o, 940o, 950o, 960o, 970o, 980o, or 990o
homologous to SEQ
ID. NO: 25. In some embodiments, PIF may refer to an amino acid sequence
comprising,
consisting essentially of, or consisting of a sequence that is at least 700o,
750o, 800o, 850o,
860o, 870o, 900o, 910o, 920o, 930o, 940o, 950o, 960o, 970o, 980o, or 990o
homologous to SEQ
ID. NO: 26. In some embodiments, PIF may refer to an amino acid sequence
comprising,
consisting essentially of, or consisting of a sequence that is at least 7000,
75%, 800o, 85%,
860o, 87%, 90%, 91%, 92%, 930o, 940o, 950o, 96%, 970o, 98%, or 99% homologous
to SEQ
ID. NO: 27. In some embodiments, PIF may refer to an amino acid sequence
comprising,
consisting essentially of, or consisting of a sequence that is at least 70%,
750o, 800o, 850o,
86%, 87%, 90%, 91%, 92%, 930o, 940o, 950o, 96%, 970o, 98%, or 99% homologous
to SEQ
ID. NO: 28. In some embodiments, PIF may refer to an amino acid sequence
comprising,
consisting essentially of, or consisting of a sequence that is at least 70%,
7500, 80%, 85%,
86%, 87%, 90%, 91%, 92%, 930o, 940o, 950o, 96%, 970o, 98%, or 99% homologous
to SEQ
ID. NO: 29. In some embodiments, the PIF mutant comprises a sequence selected
from:
XVZIKPGSANKPSD, XVZIKPGSANKPS XVZIKPGSANKP XVZIKPGSANK
XVZIKPGSAN, XVZIKPGSA, XVZIKPGS, XVZIKPG, XVZIKP, XVZIK, XVZI, XVZ
wherein X is a non-natural amino acid or a naturally occurring amino acid. In
some
embodiments, the PIF mutant comprises a sequence selected from:
XVZIKPGSANKPSD,
XVZIKPGSANKPS XVZIKPGSANKP XVZIKPGSANK XVZIKPGSAN, XVZIKPGSA,
XVZIKPGS, XVZIKPG, XVZIKP, XVZIK, XVZI, XVZ wherein X is a non-natural amino
acid or a naturally occurring amino acid except that X is not methionine if Z
is arginine, and
Z is not arginine if X is methionine. In some embodiments, the PIF analog or
mutant is
synthetic or synthetically made.
Peptides disclosed herein further include compounds having amino acid
structural and
functional analogs, for example, peptidomimetics having synthetic or non-
natural amino
acids (such as a norleucine) or amino acid analogues or non-natural side
chains, so long as
the mimetic shares one or more functions or activities of compounds of the
disclosure. The
compounds of the disclosure therefore include "mimetic" and "peptidomimetic"
forms. As
-22-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
used herein, a "non-natural side chain" is a modified or synthetic chain of
atoms joined by
covalent bond to the a-carbon atom, 3-carbon atom, or y-carbon atom which does
not make
up the backbone of the polypeptide chain of amino acids. The peptide analogs
may comprise
one or a combination of non-natural amino-acids chosen from: norvaline, tert-
butylglycine,
phenylglycine, He, 7-azatryptophan, 4-fluorophenylalanine, N-methyl-
methionine, N-methyl-
valine, N-methyl-alanine, sarcosine, N-methyl-tert-butylglycine, N-methyl-
leucine, N-
methyl-phenylglycine, N-methyl-isoleucine, N-methyl-tryptophan, N-methy1-7-
azatryptophan, N-methyl-phenylalanine, N-methyl-4-fluorophenylalanine, N-
methyl-
threonine, N-methyl-tyrosine, N-methyl-valine, N-methyl-lysine, homocysteine,
and Tyr;
Xaa2 is absent, or an amino acid selected from the group consisting of Ala, D-
Ala, N-methyl-
alanine, Glu, N-methyl-glutamate, D-Glu, Gly, sarcosine, norleucine, Lys, D-
Lys, Asn, D-
Asn, D-Glu, Arg, D-Arg, Phe, D-Phe, N-methyl-phenylalanine, Gin, D-Gln, Asp, D-
Asp, Ser,
D-Ser, N-methyl-serine, Thr, D-Thr, N-methyl-threonine, D-Pro D-Leu, N-methyl-
leucine,
D-Ile, N-methyl-isoleucine, D-Val, N-methyl-valine, tert-butylglycine, D-tert-
butylglycine,
N-methyl-tert-butylglycine, Trp, D-Trp, N-methyl-tryptophan, D-Tyr, N-methyl-
tyrosine, 1-
aminocyclopropanecarboxylic acid, 1-aminocyclobutanecarboxylic acid, 1-
aminocyclopentanecarboxylic acid, 1-aminocyclohexanecarboxylic acid, 4-
aminotetrahydro-
2H-pyran-4-carboxylic acid, aminoisobutyric acid, (5)-2-amino-3-0H-tetrazol-5-
y0propanoic
acid, Glu, Gly, N-methyl-glutamate, 2-amino pentanoic acid, 2-amino hexanoic
acid, 2-amino
heptanoic acid, 2-amino octanoic acid, 2-amino nonanoic acid, 2-amino decanoic
acid, 2-
amino undecanoic acid, 2-amino dodecanoic acid, octylglycine, tranexamic acid,
aminovaleric acid, and 2-(2-aminoethoxy)acetic acid. The natural side chain,
or R group, of
an alanine is a methyl group. In some embodiments, the non-natural side chain
of the
composition is a methyl group in which one or more of the hydrogen atoms is
replaced by a
deuterium atom. Non-natural side chains are disclosed in the art in the
following publications:
WO/2013/172954, W02013123267, WO/2014/071241, WO/2014/138429,
WO/2013/050615, WO/2013/050616, WO/2012/166559, US Application No.
20150094457,
Ma, Z., and Hartman, M.C. (2012). In Vitro Selection of Unnatural Cyclic
Peptide Libraries
via mRNA Display. In J.A. Douthwaite & R.H. Jackson (Eds.), Ribosome Display
and
Related Technologies: Methods and Protocols (pp. 367-390). Springer New York.,
all of
which are incorporated by reference in their entireties.
The terms "mimetic," "peptide mimetic" and "peptidomimetic" are used
interchangeably herein, and generally refer to a peptide, partial peptide or
non-peptide
-23-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
molecule that mimics the tertiary binding structure or activity of a selected
native peptide or
protein functional domain (e.g., binding motif or active site). These peptide
mimetics include
recombinantly or chemically modified peptides, as well as non-peptide agents
such as small
molecule drug mimetics, as further described below. The term "analog" refers
to any
polypeptide comprising at least one a-amino acid and at least one non-native
amino acid
residue, wherein the polypeptide is structurally similar to a naturally
occurring full-length PIF
protein and shares the biochemical or biological activity of the naturally
occurring full-length
protein upon which the analog is based. In some embodiments, the compositions,
pharmaceutical compositions and kits comprise a peptide or peptidomimeic
sharing share no
less than about 70%, about 75%, about 79%, about 80%, about 85%, about 86%,
about 87%,
about 90%, about 93%, about 94% about 95%, about 96%, about 97%, about 98%,
about
99% homology with any one or combination of PIF sequences set forth in Table
1; and
wherein one or a plurality of amino acid residues is a non-natural amino acid
residue or an
amino acid residue with a non-natural sidechain. In some embodiments, peptide
or peptide
mimetics are provided, wherein a loop is formed between two cysteine residues.
In some
embodiments, the peptidomimetic may have many similarities to natural
peptides, such as:
amino acid side chains that are not found among the known 20 proteinogenic
amino acids,
non-peptide-based linkers used to effect cyclization between the ends or
internal portions of
the molecule, substitutions of the amide bond hydrogen moiety by methyl groups
(N-
methylation) or other alkyl groups, replacement of a peptide bond with a
chemical group or
bond that is resistant to chemical or enzymatic treatments, N- and C-terminal
modifications,
and conjugation with a non-peptidic extension (such as polyethylene glycol,
lipids,
carbohydrates, nucleosides, nucleotides, nucleoside bases, various small
molecules, or
phosphate or sulfate groups). As used herein, the term "cyclic peptide
mimetic" or "cyclic
polypeptide mimetic" refers to a peptide mimetic that has as part of its
structure one or more
cyclic features such as a loop, bridging moiety, and/or an internal linkage.
As used herein, the
term "bridging moiety" refers to one or a series of bonded atoms that
covalently link one or a
plurality of amino acid side chains to one another within an amino acid
sequence.
In some embodiments, peptide or peptide mimetics are provided, wherein the
loop
comprises a bridging moiety selected from the group consisting of:
-24-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
xs,),
s
x, ======y-
,x
= x ====
-
X"x:X' x'x'x
s IL 111.
1
'11 `Y x- - ¨ x-xty=C
x,x,x
IV. V. VI.
ti*ti 7 tkrli
VU. VIII,
õ..14
IX.
X.
'rtg , = I-IL =
= = ==,;, a,
Cr ;'
XVI
e2A =
=
wherein each X is independently N or CH, such that no ring contains more than
2 N; each
Z is independently a bond, NR, 0, S, CH2, C(0)NR, NRC(0), S(0)vNR, NRS(0)v;
each
m is independently selected from 0, 1, 2, and 3; each vis independently
selected from 1
and 2; each R is independently selected from Hand Ci-C6; and each bridging
moiety is
connected to the peptide by independently selected Co-C6 spacers.
In some embodiments, the PIF peptides of the disclosure are modified to
produce
peptide mimetics by replacement of one or more naturally occurring side chains
of the 20
genetically encoded amino acids (or D amino acids) with other side chains, for
instance with
groups such as alkyl, lower alkyl, cyclic 4-, 5-, 6-, to 7 membered alkyl,
amide, amide lower
alkyl, amide di (lower alkyl), lower alkoxy, hydroxy, carboxy and the lower
ester derivatives
thereof, and with 4-, 5-, 6-, to 7 membered heterocyclics. For example,
proline analogs can be
made in which the ring size of the proline residue is changed from 5 members
to 4, 6, or 7
members. Cyclic groups can be saturated or unsaturated, and if unsaturated,
can be aromatic
or nonaromatic. Heterocyclic groups can contain one or more nitrogen, oxygen,
and/or
sulphur heteroatoms. Examples of such groups include the furazanyl,furyl,
imidazolidinyl,
imidazolyl, imidazolinyl, isothiazolyl, isoxazolyl, morpholinyl (e.g.
morpholino ), oxazolyl,
piperazinyl (e.g. 1-piperazinyl), piperidyl (e.g. 1-piperidyl, piperidino ),
pyranyl, pyrazinyl,
pyrazolidinyl, pyrazolinyl, pyrazolyl, pyridazinyl, pyridyl, pyrimidinyl,
pyrrolidinyl (e.g. 1-
-25-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
pyrrolidinyl), pyrrolinyl, pyrrolyl, thiadiazolyl, thiazolyl, thienyl,
thiomorpholinyl (e.g.
thiomorpholino ), and triazolyl. These heterocyclic groups can be substituted
or
unsubstituted. Where a group is substituted, the substituent can be alkyl,
alkoxy, halogen,
oxygen, or substituted or unsubstituted phenyl. Peptidomimetics may also have
amino acid
residues that have been chemically modified by phosphorylation, sulfonation,
biotinylation,
or the addition or removal of other moieties.
In a further embodiment a compound of the formula R1-R2-R3-R4-R5-R6-R7-R8- R9-
R10-R11-R12-R13-R14-R15, wherein R1 is Met or a mimetic of Met, R2 is Val or a
mimetic of
Val, R3 is Arg or a mimetic of Arg, or any amino acid, R4 is Ile or a mimetic
of Ile, R5 is Lys
or a mimetic of Lys, R6 is Pro or a mimetic of Pro, R7 is Gly or a mimetic of
Gly, R8 is Ser or
a mimetic of Ser, R9 is Ala or a mimetic of Ala, R10 is Asn or a mimetic of
Asn, R11 is Lys or
a mimetic of Lys, R12 is Pro or a mimetic of Pro, R13 is Ser or a mimetic of
Ser, R14 is Asp or
a mimetic of Asp and R15 is Asp or a mimetic of Asp is provided. In a further
embodiment, a
compound comprising the formula R1-R2-R3-R4-R5-R6-R7-R8- R9-R10-R11-R12-R13-
R14-R15,
wherein R1 is a mimetic of the naturally occurring residue at position 1 of
SEQ ID NO:20,
SEQ ID NO:21 SEQ ID SEQ ID NO:23 SEQ ID NO:2* SEQ ID NO:25 SEQ ID
NO:26 SEQ ID NO:27, SEQ ID NO:28, or SEQ ID NO:29 or the residue at that
position of
such sequences; wherein R2 is a mimetic of the naturally occurring residue at
position 2 of
SEQ ID NO:20, SEQ ID NO:21 SEQ ID SEQ
ID NO:23 SEQ ID NO:2* SEQ ID
NO:25 SEQ ID NO:26 SEQ ID NO:27, SEQ ID NO:28, or SEQ ID NO:29 or the residue
at
that position of such sequences; wherein R3 is a mimetic of the naturally
occurring residue at
position 3 of SEQ ID NO:20, SEQ ID NO:21 SEQ ID SEQ ID NO:23 SEQ ID
NO:2* SEQ ID NO:25 SEQ ID NO:26 SEQ ID NO:27, SEQ ID NO:28, or SEQ ID NO:29
or the residue at that position of such sequences; wherein R4 is a mimetic of
the naturally
occurring residue at position 4 of SEQ ID NO:20, SEQ ID NO:21 SEQ ID SEQ ID
NO:23 SEQ ID NO:24L SEQ ID NO:25 SEQ ID NO:26 SEQ ID NO:27, SEQ ID NO:28,
or SEQ ID NO:29 or the residue at that position of such sequences; wherein R5
is a mimetic
of the naturally occurring residue at position 5 of SEQ ID NO:20, SEQ ID NO:21
SEQ ID
SEQ ID NO:23 SEQ ID NO:2* SEQ ID NO:25 SEQ ID NO:26 SEQ ID NO:27,
SEQ ID NO:28, or SEQ ID NO:29 or the residue at that position of such
sequences; wherein
R6 is a mimetic of the naturally occurring residue at position 6 of SEQ ID
NO:20, SEQ ID
NO:21 SEQ ID SEQ
ID NO:23 SEQ ID NO:24L SEQ ID NO:25 SEQ ID NO:26
SEQ ID NO:27, SEQ ID NO:28, or SEQ ID NO:29 or the residue at that position of
such
sequences; wherein R7 is a mimetic of the naturally occurring residue at
position 7 of SEQ ID
-26-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
NO:20, SEQ ID NO:21 SEQ ID SEQ
ID NO:23 SEQ ID NO:24L SEQ ID NO:25
SEQ ID NO:26 SEQ ID NO:27, SEQ ID NO:28, or SEQ ID NO:29 or the residue at
that
position of such sequences; wherein R8 is a mimetic of the naturally occurring
residue at
position 5 of SEQ ID NO:20, SEQ ID NO:21 SEQ ID SEQ ID NO:23 SEQ ID
NO:2* SEQ ID NO:25 SEQ ID NO:26 SEQ ID NO:27, SEQ ID NO:28, or SEQ ID NO:29
or the residue at that position of such sequences; wherein R9 is a mimetic of
the naturally
occurring residue at position 9 of SEQ ID NO:20, SEQ ID NO:21 SEQ ID SEQ
ID
NO:23 SEQ ID NO:24L SEQ ID NO:25 SEQ ID NO:26 SEQ ID NO:27, SEQ ID NO:28,
or SEQ ID NO:29 or the residue at that position of such sequences; wherein R10
is a mimetic
of the naturally occurring residue at position 10 of SEQ ID NO:20, SEQ ID
NO:21 SEQ ID
SEQ ID NO:23 SEQ ID NO:2* SEQ ID NO:25 SEQ ID NO:26 SEQ ID NO:27,
SEQ ID NO:28, or SEQ ID NO:29 or the residue at that position of such
sequences; wherein
R11 is a mimetic of the naturally occurring residue at position 11 of SEQ ID
NO:20, SEQ ID
NO:21 SEQ ID SEQ
ID NO:23 SEQ ID NO:24L SEQ ID NO:25 SEQ ID NO:26
SEQ ID NO:27, SEQ ID NO:28, or SEQ ID NO:29 or the residue at that position of
such
sequences; wherein R12 is a mimetic of the naturally occurring residue at
position 12 of SEQ
ID NO:20, SEQ ID NO:21 SEQ ID SEQ ID NO:23 SEQ ID NO:2* SEQ ID
NO:25 SEQ ID NO:26 SEQ ID NO:27, SEQ ID NO:28, or SEQ ID NO:29 or the residue
at
that position of such sequences; wherein R13 is a mimetic of the naturally
occurring residue at
position 13 of SEQ ID NO:20, SEQ ID NO:21 SEQ ID SEQ ID NO:23 SEQ ID
NO:2* SEQ ID NO:25 SEQ ID NO:26 SEQ ID NO:27, SEQ ID NO:28, or SEQ ID NO:29
or the residue at that position of such sequences; wherein R14 is a mimetic of
the naturally
occurring residue at position 14 of SEQ ID NO:20, SEQ ID NO:21 SEQ ID SEQ
ID
NO:23 SEQ ID NO:24L SEQ ID NO:25 SEQ ID NO:26 SEQ ID NO:27, SEQ ID NO:28,
or SEQ ID NO:29 or the residue at that position of such sequences; wherein R15
is a mimetic
of the naturally occurring residue at position 15 of SEQ ID NO:20, SEQ ID
NO:21 SEQ ID
SEQ ID NO:23 SEQ ID NO:2* SEQ ID NO:25 SEQ ID NO:26 SEQ ID NO:27,
SEQ ID NO:28, or SEQ ID NO:29 or the residue at that position of such
sequences.
In some embodiments, the pharmaceutical composition comprising the formula R1-
R2-R3-R4-R5-R6-R7-R8- R9-R10-R11-R12-R13-R14-R15-R16-R17-R18, wherein R1 is
Ser or a
mimetic of Ser, R2 is Gly or a mimetic of Gly, R3 is Ile or a mimetic of Ile,
R4 is Val or a
mimetic of Val, R5 is Ile or a mimetic of Ile, R6 is Tyr or a mimetic of Tyr,
R7 is Gln or a
mimetic of Gln, R8 is Tyr or a mimetic of Tyr, R9 is Met or a mimetic of Met,
R10 is Asp or a
mimetic of Asp, R11 is Asp or a mimetic of Asp, R12 is Arg or a mimetic of
Arg, R13 is Tyr or
-27-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
a mimetic of Tyr, R14 is Val or a mimetic of Val, R15 is Gly or a mimetic of
Gly, R16 is Ser or
a mimetic of Ser, R17 is Asp or a mimetic of Asp and R18 is Leu or a mimetic
of Leu; and a
compound comprising the formula R1-R2-R3-R4-R5-R6-R7-R8- R9, wherein R1 is Val
or a
mimetic of Val, R2 is Ile or a mimetic of Ile, R3 is Ile or a mimetic of Ile,
R4 is Ile or a
mimetic of Ile, R5 is Ala or a mimetic of Ala, R6 is Gln or a mimetic of Gln,
R7 is Tyr or a
mimetic of Tyr, R8 is Met or a mimetic of Met, and R9 is Asp or a mimetic of
Asp is
provided. In some embodiments, R3 is not Arg or a mimetic of Arg.
A variety of techniques are available for constructing peptide mimetics with
the same
or similar desired biological activity as the corresponding native but with
more favorable
activity than the peptide with respect to solubility, stability, and/or
susceptibility to hydrolysis
or proteolysis (see, e.g., Morgan & Gainor, Ann. Rep. Med. Chem. 24,243-
252,1989).
Certain peptidomimetic compounds are based upon the amino acid sequence of the
peptides
of the disclosure. Often, peptidomimetic compounds are synthetic compounds
having a three
dimensional structure (i.e. a "peptide motif') based upon the three-
dimensional structure of a
selected peptide. The peptide motif provides the peptidomimetic compound with
the desired
biological activity, i.e., binding to PIF receptors, wherein the binding
activity of the mimetic
compound is not substantially reduced, and is often the same as or greater
than the activity of
the native peptide on which the mimetic is modeled. Peptidomimetic compounds
can have
additional characteristics that enhance their therapeutic application, such as
increased cell
permeability, greater affinity and/or avidity and prolonged biological half-
life.
Peptidomimetic design strategies are readily available in the art (see, e.g.,
Ripka &
Rich, Curr. Op. Chem. Bioi. 2,441-452,1998; Hruby et al., Curr. Op.Chem. Bioi.
1,114-
119,1997; Hruby & Baise, Curr.Med. Chem. 9,945-970,2000). One class of
peptidomimetics
a backbone that is partially or completely non-peptide, but mimics the peptide
backbone
atom-for atom and comprises side groups that likewise mimic the functionality
of the side
groups of the native amino acid residues. Several types of chemical bonds,
e.g., ester,
thioester, thioamide, retroamide, reduced carbonyl, dimethylene and
ketomethylene bonds,
are known in the art to be generally useful substitutes for peptide bonds in
the construction of
protease-resistant peptidomimetics. Another class of peptidomimetics comprises
a small non-
peptide molecule that binds to another peptide or protein, but which is not
necessarily a
structural mimetic of the native peptide. Yet another class of peptidomimetics
has arisen from
combinatorial chemistry and the generation of massive chemical libraries.
These generally
comprise novel templates which, though structurally unrelated to the native
peptide, possess
-28-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
necessary functional groups positioned on a nonpeptide scaffold to serve as
"topographical"
mimetics of the original peptide (Ripka & Rich, 1998, supra).
A list of PIF peptides are provided below in Table 1. Antibodies to various
PIF
peptides and scrambled PIF peptides are also provided.
Table 1. PIF Peptides
(SEQ ID NO) Peptide Amino Acid Sequence
SEQ ID NO:1 nPIF-115 MVRIKPGSANKPSDD
isolated native, matches region of
Circumsporozoite protein (Malaria)
SEQ ID NO:2 nPIF -1 (15-a1ter) MVRIKYGSYNNKPSD
isolated native, matches region of
Circumsporozoite protein (Malaria)
SEQ ID NO:3 nPIF-1 (13) MVRIKPGSANKPS
isolated native, matches region of
Circumsporozoite protein (Malaria)
SEQ ID NO:4 nPIF-1 (9) MVRIKPGSA
isolated native, matches region of
Circumsporozoite protein (Malaria)
SEQ ID NO:5 scrPIF-115 GRVDPSNKSMPKDIA
synthetic, scrambled amino acid sequence
from region of Circumsporozoite protein
Malaria
SEQ ID NO:6 nPIF-2(lo) SQAVQEHAST
isolated native, matches region of human
retinoid and thyroid hormone receptor-
SMRT
SEQ ID NO:7 nPIF-2(13) SQAVQEHASTNMG
isolated native, matches region of human
retinoid and thyroid hormone receptor
(SMRT)
SEQ ID NO:8 scrPIF-2(13) EVAQHSQASTMNG
synthetic, scrambled amino acid sequence
from region of human retinoid and thyroid
hormone receptor SMRT
SEQ ID NO:9 scrPIF-2(14) GQASSAQMNSTGVH
SEQ ID NO:10 nPIF-3(18) SGIVIYQYMDDRYVGSDL
isolated native, matches region of Rev Trans
SEQ ID NO:11 Neg control GMRELQRSANK
synthetic, scrambled amino acid sequence for negPIF-
from region of Circumsporozoite protein 1(15)
Malaria
SEQ ID NO:12 nPIF-4(9) VIIIAQYMD
isolated native, matches region of Rev Trans
antibody of native isolated nPIF-115 AbPIF-105)
(SEQ ID NO:13) 5PIF-1(15) MVRIKPGSANKPSDD
-29-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
synthetic, amino acid sequence from region
of Circumsporozoite protein Malaria
(SEQ ID NO:14) 5PIF-2(13) SQAVQEHASTNMG
synthetic, amino acid sequence from of
human retinoid and thyroid hormone
receptor SMRT
(SEQ ID NO:15) 5PIF-3(18) SGIVIYQYMDDRYVGSDL
synthetic, amino acid sequence from region
of Circumsporozoite protein Malaria
(SEQ ID NO: 16) 5PIF-1 (9) MVRIKPGSA
synthetic, amino acid sequence from region
of Circumsporozoite protein Malaria
antibody of native isolated nPIF-2(13) AbPIF-2(13)
antibody of native isolated nPIF -3(18) AbPIF-3(18)
(SEQ ID NO: 17) 5PIF-4(9) VIIIAQYMD
Synthetic
SEQ ID NO: 18 5PIF-1 (5) MVRIK
Synthetic
SEQ ID NO: 19 5PIF-1 (4) PGSA
Synthetic
SEQ ID NO: 20 PIF (-3) MVXIKPGSANKPSDD
SEQ ID NO: 21 PIF (-1) XVRIKPGSANKPSDD
SEQ ID NO: 22 PIF (-1, -3) XVXIKPGSANKPSDD
SEQ ID NO: 23 PIF (-6) MVRIKXGSANKPSDD
SEQ ID NO: 24 PIF (-4) MVRXKPGSANKPSDD
SEQ ID NO: 25 PIF (-2) MXRIKP GS ANKPSDD
SEQ ID NO: 26 mutl MVRIKEGSANKPSDD
SEQ ID NO: 27 mut3 MVRGKPGSANKPSDD
SEQ ID NO: 28 mut4 MERIKPGSANKPSDD
SEQ ID NO: 29 mut5 AVRIKPGSANKPSDD
n=native, s= synthetic, scr =scrambled, same AA, ( )= number of AA,
Ab=antibody, X = any
amino acid, except arginine
In some embodiments of the present disclosure, a PIF peptide (or analog) is
provided.
In some embodiments, the PIF analog binds or associates with human insulin
degrading
enzyme (IDE ¨ SEQ ID NO:30) at least 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, or
10% or
higher than native or wild-type PIF sequences. In some embodiments, the PIF
analog may
have a binding affinity for insulin degrading enzyme (IDE) that has at least
1%, 2%, 3%, 4%,
5%, 6%, 7%, 8%, 9%, or 10% or higher than native or wild-type PIF sequences.
In some
embodiments, the PIF analog may have a binding affinity for insulin degrading
enzyme that
has from about 1% to about 30% or higher than the affinity native or wild-type
PIF sequences
have for IDE. In some embodiments, the PIF analog may have a binding affinity
for insulin
degrading enzyme that has from about 1% to about 10% or higher than the
affinity native or
-30-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
wild-type PIF sequences have for IDEIn some embodiments, the PIF analog may
have a
binding affinity for insulin degrading enzyme that has from about 1% to about
20% or higher
than the affinity native or wild-type PIF sequences have for IDE. In some
embodiments, the
PIF analog may have a binding affinity for insulin degrading enzyme that has
from about
10% to about 20% or higher than the affinity native or wild-type PIF sequences
have for IDE.
Such PIF peptides in therapeutically effective amounts may be useful for
treating any of the
diseases or disorder disclosed herein.
IDE sequence:
MRYRLAWLLHPALPSTFRSVLGARLPPPERLCGFQKKTYSKMNNPAIKRIGNHITKSP
EDKREYRGLELANGIKVLLISDPTTDKSSAALDVHIGSLSDPPNIAGLSHFCEHMLFLG
TKKYPKENEYSQFLSEHAGSSNAFTSGEHTNYYFDVSHEHLEGALDRFAQFFLCPLF
DESCKDREVNAVDSEHEKNVMNDAWRLFQLEKATGNPKHPFSKFGTGNKYTLETR
PNQEGIDVRQELLKFHSAYYSSNLMAVCVLGRESLDDLTNLVVKLF SEVENKNVPLP
EFPEHPFQEEHLKQLYKIVPIKDIRNLYVTFPIPDLQKYYKSNPGHYLGHLIGHEGPGS
LLSELKSKGWVNTLVGGQKEGARGFMFFIINVDLTEEGLLHVEDIILHMFQYIQKLRA
EGPQEWVFQECKDLNAVAFRFKDKERPRGYTSKIAGILHYYPLEEVLTAEYLLEEFR
PDLIEMVLDKLRPENVRVAIVSKSFEGKTDRTEEWYGTQYKQEAIPDEVIKKWQNAD
LNGKFKLPTKNEFIPTNFEILPLEKEATPYPALIKDTAMSKLWFKQDDKFFLPKACLN
FEFFSPFAYVDPLHCNMAYLYLELLKDSLNEYAYAAELAGLSYDLQNTIYGMYLSV
KGYNDKQPILLKKIIEKMATFEIDEKRFEIIKEAYMRSLNNFRAEQPHQHAMYYLRLL
MTEVAWTKDELKEALDDVTLPRLKAFIPQLLSRLHIEALLHGNITKQAALGIMQMVE
DTLIEHAHTKPLLPSQLVRYREVQLPDRGWFVYQQRNEVHNNCGIEIYYQTDMQSTS
ENMFLELFCQIISEPCFNTLRTKEQLGYIVFSGPRRANGIQGLRFIIQSEKPPHYLESRV
EAFLITMEKSIEDMTEEAFQKHIQALAIRRLDKPKKLSAECAKYWGEIISQQYNFDRD
NTEVAYLKTLTKEDIIKFYKEMLAVDAPRRHKVSVHVLAREMDSCPVVGEFPCQNDI
NLSQAPALPQPEVIQNMTEFKRGLPLFPLVKPHINFMAAKL (SEQ ID NO: 30).
In another embodiment, a pharmaceutical composition comprising a PIF peptide
is
provided. In preferred embodiments, the pharmaceutical composition comprises a
therapeutically effective amount of a PIF peptide or a pharmaceutically
acceptable salt
thereof
In another embodiment, a method of treating endometriosis is provided. In a
preferred
embodiment, the method comprises administering an effective amount of a PIF
peptide to a
subject in need thereof In a further embodiment, a method for treating
endometriosis
comprising administering an effective amount of a PIF peptide in combination
with one or
-31-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
more immunotherapeutic, anti-epileptic, diuretic, or blood pressure
controlling drugs or
compounds to a subject in need thereof is provided. Such a combination may
enhance the
effectiveness of the treatment of either component alone, or may provide less
side effects
and/or enable a lower dose of either component.
In some embodiments, a PIF peptide is provided. Such PIF peptides may be
useful for
treating or ameliorating immune-mediated disorders, such as autoimmune
diseases, such as
endometriosis. The PIF peptide can be, for example, as described herein.
In some embodiments, a pharmaceutical composition comprising a PIF peptide or
a
pharmaceutically acceptable salt thereof is provided. In preferred
embodiments, the
pharmaceutical composition comprises a therapeutically effective amount of a
PIF peptide or
a pharmaceutically acceptable salt thereof
For therapeutic treatment of the specified indications, a PIF peptide may be
administered as such, or can be compounded and formulated into pharmaceutical
compositions in unit dosage form for parenteral, transdermal, rectal, nasal,
local intravenous
administration, or, preferably, oral administration. Such pharmaceutical
compositions are
prepared in a manner well known in the art and comprise at least one active
PIF peptide
associated with a pharmaceutically carrier. The term "active compound", as
used throughout
this specification, refers to at least one compound selected from compounds of
the formulas
or pharmaceutically acceptable salts thereof
In such a composition, the active compound is known as "active ingredient." In
making the compositions, the active ingredient will usually be mixed with a
carrier, or diluted
by a carrier, or enclosed within a carrier that may be in the form of a
capsule, sachet, paper or
other container. When the carrier serves as a diluent, it may be a solid,
semisolid, or liquid
material that acts as a vehicle, excipient of medium for the active
ingredient. Thus, the
composition can be in the form of tablets, pills, powders, lozenges, sachets,
cachets, elixirs,
emulsion, solutions, syrups, suspensions, soft and hard gelatin capsules,
sterile injectable
solutions, and sterile packaged powders.
The terms "pharmaceutical preparation" or "pharmaceutical composition"
includes
preparations suitable for administration to mammals, e.g., humans. When the
compounds of
the present disclosure are administered as pharmaceuticals to mammals, e.g.,
humans, they
can be given per se or as a pharmaceutical composition containing, for
example, from about
0.1 to about 99.5% of active ingredient in combination with a pharmaceutically
acceptable
carrier.
The phrase "pharmaceutically acceptable carrier" is art recognized and
includes a
-32-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
pharmaceutically acceptable material, composition or vehicle, suitable for
administering
compounds of the present disclosure to mammals. The carriers include, for
example, liquid or
solid filler, diluent, excipient, solvent or encapsulating material, involved
in carrying or
transporting the subject agent from one organ, or portion of the body, to
another organ, or
portion of the body. Each carrier must be "acceptable" in the sense of being
compatible with
the other ingredients of the formulation. Some examples of materials which can
serve as
pharmaceutically acceptable carriers include: sugars, such as lactose, glucose
and sucrose;
starches, such as corn starch and potato starch; cellulose, and its
derivatives, such as sodium
carboxymethyl cellulose, ethyl cellulose and cellulose acetate; powdered
tragacanth; malt;
gelatin; talc; excipients, such as cocoa butter and suppository waxes; oils,
such as peanut oil,
cottonseed oil, safflower oil, sesame oil, olive oil, corn oil and soybean
oil; glycols, such as
propylene glycol; polyols, such as glycerin, sorbitol, mannitol and
polyethylene glycol;
esters, such as ethyl oleate and ethyl laurate; agar; buffering agents, such
as magnesium
hydroxide and aluminum hydroxide; alginic acid; pyrogen-free water; isotonic
saline;
Ringer's solution; ethyl alcohol; phosphate buffer solutions; and other non-
toxic compatible
substances employed in pharmaceutical formulations. Suitable pharmaceutical
carriers are
described in "Remington's Pharmaceutical Sciences" by E. W. Martin, which is
incorporated
herein by reference in its entirety. In some embodiments, the pharmaceutically
acceptable
carrier is sterile and pyrogen-free water. In some embodiments, the
pharmaceutically
acceptable carrier is Ringer's Lactate, sometimes known as lactated Ringer's
solution.
Wetting agents, emulsifiers and lubricants, such as sodium lauryl sulfate and
magnesium stearate, as well as coloring agents, release agents, coating
agents, sweetening,
flavoring and perfuming agents, preservatives and antioxidants can also be
present in the
compositions.
Examples of pharmaceutically acceptable antioxidants include: water soluble
antioxidants, such as ascorbic acid, cysteine hydrochloride, sodium bisulfate,
sodium
metabisulfite, sodium sulfite and the like; oil-soluble antioxidants, such as
ascorbyl palmitate,
butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), lecithin,
propyl gallate,
.alpha.-tocopherol, and the like; and metal chelating agents, such as citric
acid,
ethylenediamine tetraacetic acid (EDTA), sorbitol, tartaric acid, phosphoric
acid, and the like.
Formulations of the present disclosureinclude those suitable for oral, nasal,
topical,
buccal, sublingual, rectal, vaginal and/or parenteral administration. The
formulations may
conveniently be presented in unit dosage form and may be prepared by any
methods well
known in the art of pharmacy. The amount of active ingredient that can be
combined with a
-33-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
carrier material to produce a single dosage form will generally be that amount
of the
compound that produces a therapeutic effect. Generally, out of one hundred
percent, this
amount will range from about 1 percent to about ninety-nine percent of active
ingredient,
preferably from about 5 percent to about 70 percent, most preferably from
about 10 percent to
about 30 percent.
Some examples of suitable carriers, excipients, and diluents include lactose,
dextrose,
sucrose, sorbitol, mannitol, starches, gum acacia, calcium phosphate
alginates, calcium
salicate, microcrystalline cellulose, polyvinylpyrrolidone, cellulose,
tragacanth, gelatin,
syrup, methyl cellulose, methyl- and propylhydroxybenzoates, tale, magnesium
stearate,
water, and mineral oil. The formulations can additionally include lubricating
agents, wetting
agents, emulsifying and suspending agents, preserving agents, sweetening
agents or flavoring
agents. The compositions may be formulated so as to provide quick, sustained,
or delayed
release of the active ingredient after administration to the patient by
employing procedures
well known in the art.
For oral administration, a compound can be admixed with carriers and diluents,
molded into tablets, or enclosed in gelatin capsules. The mixtures can
alternatively be
dissolved in liquids such as 10% aqueous glucose solution, isotonic saline,
sterile water, or
the like, and administered intravenously or by injection.
The local delivery of inhibitory amounts of active compound for the treatment
of
immune disorders can be by a variety of techniques that administer the
compound at or near
the targeted site. Examples of local delivery techniques are not intended to
be limiting but to
be illustrative of the techniques available. Examples include local delivery
catheters, site
specific carriers, implants, direct injection, or direct applications, such as
topical application.
Local delivery by an implant describes the surgical placement of a matrix that
contains the pharmaceutical agent into the affected site. The implanted matrix
releases the
pharmaceutical agent by diffusion, chemical reaction, or solvent activators.
For example, in some aspects, the disclosure is directed to a pharmaceutical
composition comprising a PIF peptide, and a pharmaceutically acceptable
carrier or diluent,
or an effective amount of pharmaceutical composition comprising a PIF peptide.
The compounds of the present disclosure can be administered in the
conventional
manner by any route where they are active. Administration can be systemic,
topical, or oral.
For example, administration can be, but is not limited to, parenteral,
subcutaneous,
intravenous, intramuscular, intraperitoneal, transdermal, oral, buccal, ocular
routes,
intravaginally, by inhalation, by depot injections, or by implants. Thus,
modes of
-34-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
administration for the compounds of the present disclosure(either alone or in
combination
with other pharmaceuticals) can be, but are not limited to, subligual,
injectable (including
short-acting, depot, implant and pellet forms injected subcutaneously or
intramuscularly), or
by use of vaginal creams, suppositories, pessaries, vaginal rings, rectal
suppositories,
intrauterine devices, and transdermal forms such as patches and creams.
Specific modes of administration will depend on the indication. The selection
of the
specific route of administration and the dose regimen is to be adjusted or
titrated by the
clinician according to methods known to the clinician in order to obtain the
optimal clinical
response. The amount of compound to be administered is that amount which is
therapeutically effective. The dosage to be administered will depend on the
characteristics of
the subject being treated, e.g., the particular mammal or human treated, age,
weight, health,
types of concurrent treatment, if any, and frequency of treatments, and can be
easily
determined by one of skill in the art (e.g., by the clinician).
Pharmaceutical formulations containing the compounds of the present disclosure
and
a suitable carrier can be solid dosage forms which include, but are not
limited to, tablets,
capsules, cachets, pellets, pills, powders and granules; topical dosage forms
which include,
but are not limned to, solutions, powders, fluid emulsions, fluid suspensions,
semi-solids,
ointments, pastes, creams, gels and jellies, and foams; and parenteral dosage
forms which
include, but are not limited to, solutions, suspensions, emulsions, and dry
powder; comprising
an effective amount of a polymer or copolymer of the present disclosure. It is
also known in
the art that the active ingredients can be contained in such formulations with
pharmaceutically acceptable diluents, fillers, disintegrants, binders,
lubricants, surfactants,
hydrophobic vehicles, water soluble vehicles, emulsifiers, buffers,
humectants, moisturizers,
solubilizers, preservatives and the like. The means and methods for
administration are known
in the art and an artisan can refer to various pharmacologic references for
guidance. For
example, Modern Pharmaceutics, Banker & Rhodes, Marcel Dekker, Inc. (1979);
and
Goodman & Gilman's The Pharmaceutical Basis of Therapeutics, 6th Edition,
MacMillan
Publishing Co., New York (1980) can be consulted.
The compounds of the present disclosure can be formulated for parenteral
administration by injection, e.g., by bolus injection or continuous infusion.
The compounds
can be administered by continuous infusion subcutaneously over a predetermined
period of
time. Formulations for injection can be presented in unit dosage form, e.g.,
in ampoules or in
multi-dose containers, with an added preservative. The compositions can take
such forms as
-35-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
suspensions, solutions or emulsions in oily or aqueous vehicles, and can
contain formulatory
agents such as suspending, stabilizing and/or dispersing agents.
For oral administration, the compounds can be formulated readily by combining
these
compounds with pharmaceutically acceptable carriers well known in the art.
Such carriers
enable the compounds of the disclosure to be formulated as tablets, pills,
dragees, capsules,
liquids, gels, syrups, slurries, suspensions and the like, for oral ingestion
by a patient to be
treated. Pharmaceutical preparations for oral use can be obtained by adding a
solid excipient,
optionally grinding the resulting mixture, and processing the mixture of
granules, alter adding
suitable auxiliaries, if desired, to obtain tablets or dragee cores. Suitable
excipients include,
but are not limited to, fillers such as sugars, including, but not limited to,
lactose, sucrose,
mannitol, and sorbitol; cellulose preparations such as, but not limited to,
maize starch, wheat
starch, rice starch, potato starch, gelatin, gum tragecanth, methyl cellulose,
hydroxypropylmethyl-celllose, sodium carboxymethylcellulose, and
polyvinylpyrrolidone
(PVP). If desired, disintegrating agents can be added, such as, but not
limited to, the cross-
linked polyvinyl pyrrolidone, agar, or alginic acid or a salt thereof such as
sodium alginate.
Dragee cores can be provided with suitable coatings. For this purpose,
concentrated
sugar solutions can be used, which can optionally contain gum arabic, talc,
polyvinyl
pyrrolidone, carbopol gel, polyethylene glycol, and/or titanium dioxide,
lacquer solutions,
and suitable organic solvents or solvent mixtures. Dyestuffs or pigments can
be added to the
tablets or dragee coatings for identification or to characterize different
combinations of active
compound doses.
Pharmaceutical preparations which can be used orally include, but are not
limited to,
push-fit capsules made of gelatin, as well as soft, scaled capsules made of
gelatin and a
plasticizer, such as glycerol or sorbitol. The push-fit capsules can contain
the active
ingredients in admixture with filler such as, e.g., lactose, binders such as,
e.g., starches,
and/or lubricants such as, e.g., talc or magnesium stearate and, optionally,
stabilizers. In soft
capsules, the active compounds can be dissolved or suspended in suitable
liquids, such as
fatty oils, liquid paraffin, or liquid polyethylene glycols. In addition,
stabilizers can be added.
All formulations for oral administration should be in dosages suitable for
such administration.
For buccal administration, the compositions can take the form of, e.g.,
tablets or
lozenges formulated in a conventional manner.
For administration by inhalation, the compounds for use according to the
present
disclosure are conveniently delivered in the form of an aerosol spray
presentation from
pressurized packs or a nebulizer, with the use of a suitable propellant, e.g.,
-36-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
dichlorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane,
carbon dioxide
or other suitable gas. In the case of a pressurized aerosol the dosage unit
can be determined
by providing a valve to deliver a metered amount. Capsules and cartridges of,
e.g., gelatin for
use in an inhaler or insufflator can be formulated containing a powder mix of
the compound
and a suitable powder base such as lactose or starch.
The compounds of the present disclosure can also be formulated in rectal
compositions such as suppositories or retention enemas, e.g., containing
conventional
suppository bases such as cocoa butter or other glycerides.
In addition to the formulations described previously, the compounds of the
present
disclosure can also be formulated as a depot preparation. Such long acting
formulations can
be administered by implantation (for example subcutaneously or
intramuscularly) or by
intramuscular injection.
Depot injections can be administered at about 1 to about 6 months or longer
intervals.
Thus, for example, the compounds can be formulated with suitable polymeric or
hydrophobic
materials (for example as an emulsion in an acceptable oil) or ion exchange
resins, or as
sparingly soluble derivatives, for example, as a sparingly soluble salt.
In transdermal administration, the compounds of the present disclosure, for
example,
can be applied to a plaster, or can be applied by transdermal, therapeutic
systems that are
consequently supplied to the organism.
Pharmaceutical compositions of the compounds also can comprise suitable solid
or
gel phase carriers or excipients. Examples of such carriers or excipients
include but are not
limited to calcium carbonate, calcium phosphate, various sugars, starches,
cellulose derivates,
gelatin, and polymers such as, e.g., polyethylene glycols.
For parenteral administration, analog can be, for example, formulated as a
solution,
suspension, emulsion or lyophilized powder in association with a
pharmaceutically
acceptable parenteral vehicle. Examples of such vehicles are water, saline,
Ringer's solution,
dextrose solution, and 5% human serum albumin. Liposomes and nonaqueous
vehicles such
as fixed oils may also be used. The vehicle or lyophilized powder may contain
additives that
maintain isotonicity (e.g., sodium chloride, mannitol) and chemical stability
(e.g., buffers and
preservatives). The formulation is sterilized by commonly used techniques. For
example, a
parenteral composition suitable for administration by injection is prepared by
dissolving 1.5%
by weight of analog in 0.9% sodium chloride solution.
The present embodiments also relate to routes of administration include
intramuscular, sublingual, intravenous, intraperitoneal, intrathecal,
intravaginal, intraurethral,
-37-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
intradermal, intrabuccal, via inhalation, via nebulizer and via subcutaneous
injection.
Alternatively, the pharmaceutical composition may be introduced by various
means into cells
that are removed from the individual. Such means include, for example,
microprojectile
bombardment and liposome or other nanoparticle device.
Solid dosage forms for oral administration include capsules, tablets, pills,
powders
and granules. In solid dosage forms, the analogs are generally admixed with at
least one inert
pharmaceutically acceptable carrier such as sucrose, lactose, starch, or other
generally
regarded as safe (GRAS) additives. Such dosage forms can also comprise, as is
normal
practice, an additional substance other than an inert diluent, e.g.,
lubricating agent such as
magnesium state. With capsules, tablets, and pills, the dosage forms may also
comprise a
buffering agent. Tablets and pills can additionally be prepared with enteric
coatings, or in a
controlled release form, using techniques know in the art.
Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, solutions, suspensions and syrups, with the elixirs containing an
inert diluent
commonly used in the art, such as water. These compositions can also include
one or more
adjuvants, such as wetting agent, an emulsifying agent, a suspending agent, a
sweetening
agent, a flavoring agent or a perfuming agent.
In some embodiments, the compounds and compositions described are used to
treat
a patient suffering from, or susceptible to endometriosis and an autoimmune
disease.
One of skill in the art will recognize that the appropriate dosage of the
compositions
and pharmaceutical compositions may vary depending on the individual being
treated and the
purpose. For example, the age, body weight, and medical history of the
individual patient
may affect the therapeutic efficacy of the therapy. Further, a lower dosage of
the composition
may be needed to produce a transient cessation of symptoms, while a larger
dose may be
needed to produce a complete cessation of symptoms associated with the
disease, disorder, or
indication. A competent physician can consider these factors and adjust the
dosing regimen to
ensure the dose is achieving the desired therapeutic outcome without undue
experimentation.
It is also noted that the clinician and/or treating physician will know how
and when to
interrupt, adjust, and/or terminate therapy in conjunction with individual
patient response.
Dosages may also depend on the strength of the particular analog chosen for
the
pharmaceutical composition.
The dose of the composition or pharmaceutical compositions may vary. The dose
of
the composition may be once per day. In some embodiments, multiple doses may
be
administered to the subject per day. In some embodiments, the total dosage is
administered in
-38-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
at least two application periods. In some embodiments, the period can be an
hour, a day, a
month, a year, a week, or a two-week period. In an additional embodiment of
the invention,
the total dosage is administered in two or more separate application periods,
or separate doses
over the course of about an hour, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours,
7 hours, 8 hours,
9 hours, 10 hours, 11 hours, 12 or more hours. a day, a month, a year, a week,
or a two-week
period.
In some embodiments, subjects can be administered the composition in which the
composition is provided in a daily dose range of about 0.0001 mg/kg to about
5000 mg/kg of
the weight of the subject. The dose administered to the subject can also be
measured in terms
of total amount of PIF peptide or PIF analog or pharmaceutically acceptable
salt thereof
administered per day. In some embodiments, a subject is administered from
about 0.001 to
about 3000 milligrams of PIF peptide or PIF analog or pharmaceutically
acceptable salt
thereof per day. In some embodiments, a subject is administered up to about
2000 milligrams
of PIF peptide or PIF analog or pharmaceutically acceptable salt thereof per
day. In some
embodiments, a subject is administered up to about 1800 milligrams of PIF
peptide or PIF
analog or pharmaceutically acceptable salt thereof per day. In some
embodiments, a subject is
administered up to about 1600 milligrams of PIF peptide or PIF analog or
pharmaceutically
acceptable salt thereof per day. In some embodiments, a subject is
administered up to about
1400 milligrams of PIF peptide or PIF analog or pharmaceutically acceptable
salt thereof per
day. In some embodiments, a subject is administered up to about 1200
milligrams of PIF
peptide or PIF analog or pharmaceutically acceptable salt thereof per day. In
some
embodiments, a subject is administered up to about 1000 milligrams of PIF
peptide or PIF
analog or pharmaceutically acceptable salt thereof per day. In some
embodiments, a subject is
administered up to about 800 milligrams of PIF peptide or PIF analog or
pharmaceutically
acceptable salt thereof per day. In some embodiments, a subject is
administered from about
0.001 milligrams to about 700 milligrams of PIF peptide or PIF analog or
pharmaceutically
acceptable salt thereof per dose. In some embodiments, a subject is
administered up to about
700 milligrams of PIF peptide or PIF analog per dose. In some embodiments, a
subject is
administered up to about 600 milligrams of PIF peptide or PIF analog or
pharmaceutically
acceptable salt thereof per dose. In some embodiments, a subject is
administered up to about
500 milligrams of PIF peptide or PIF analog or pharmaceutically acceptable
salt thereof per
dose. In some embodiments, a subject is administered up to about 400
milligrams of PIF
peptide or PIF analog or pharmaceutically acceptable salt thereof per dose. In
some
embodiments, a subject is administered up to about 300 milligrams of PIF
peptide or PIF
-39-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
analog or pharmaceutically acceptable salt thereof per dose. In some
embodiments, a subject
is administered up to about 200 milligrams of PIF peptide or PIF analog or
pharmaceutically
acceptable salt thereof per dose. In some embodiments, a subject is
administered up to about
100 milligrams of PIF peptide or PIF analog or pharmaceutically acceptable
salt thereof per
dose. In some embodiments, a subject is administered up to about 50 milligrams
of PIF
peptide or PIF analog or pharmaceutically acceptable salt thereof per dose.
In some embodiments, subjects can be administered the composition in which the
composition comprising a PIF peptide or PIF analog or pharmaceutically
acceptable salt
thereof is administered in a daily dose range of about 0.0001 mg/kg to about
5000 mg/kg of
the weight of the subject. In some embodiments, the composition comprising a
PIF analog or
pharmaceutically acceptable salt thereof is administered in a daily dosage of
up to about 450
mg/kg of the weight of the subject. In some embodiments, the composition
comprising a PIF
peptide or PIF analog or pharmaceutically acceptable salt thereof is
administered in a daily
dosage of up to about 400 mg/kg of the weight of the subject. In some
embodiments, the
composition comprising a PIF peptide or PIF analog or pharmaceutically
acceptable salt
thereof is administered in a daily dosage of up to about 350 mg/kg of the
weight of the
subject. In some embodiments, the composition comprising a PIF peptide or PIF
analog or
pharmaceutically acceptable salt thereof is administered in a daily dosage of
up to about 300
mg/kg of the weight of the subject. In some embodiments, the composition
comprising a PIF
peptide or PIF analog or pharmaceutically acceptable salt thereof is
administered in a daily
dosage of up to about 250 mg/kg of the weight of the subject. In some
embodiments, the
composition comprising PIF peptide or a PIF analog or pharmaceutically
acceptable salt
thereof is administered in a daily dosage of up to about 200 mg/kg of the
weight of the
subject. In some embodiments, the composition comprising PIF peptide or a PIF
analog or
pharmaceutically acceptable salt thereof is administered in a daily dosage of
up to about 150
mg/kg of the weight of the subject. In some embodiments, the composition
comprising a PIF
peptide or a PIF analog or pharmaceutically acceptable salt thereof is
administered in a daily
dosage of up to about 100 mg/kg of the weight of the subject. In some
embodiments, the
composition comprising a PIF peptide or a PIF analog or pharmaceutically
acceptable salt
thereof is administered in a daily dosage of up to about 50 mg/kg of the
weight of the subject.
In some embodiments, the composition comprising PIF peptide or a PIF analog or
pharmaceutically acceptable salt thereof is administered in a daily dosage of
up to about 25
mg/kg of the weight of the subject.
In some embodiments, the composition comprising a PIF peptide or a PIF analog
or
-40-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
pharmaceutically acceptable salt thereof is administered in a daily dosage of
up to about 10
mg/kg of the weight of the subject. In some embodiments, the composition
comprising PIF
peptide or a PIF analog or pharmaceutically acceptable salt thereof is
administered in a daily
dosage of up to about 5 mg/kg of the weight of the subject. In some
embodiments, the
composition comprising a PIF peptide or a PIF analog or pharmaceutically
acceptable salt
thereof is administered in a daily dosage of up to about 2.0 mg/kg of the
weight of the
subject. In some embodiments, the composition comprising a PIF peptide or a
PIF analog or
pharmaceutically acceptable salt thereof is administered in a daily dosage of
up to about 3.0
mg/kg of the weight of the subject. In some embodiments, the composition
comprising PIF
peptide or a PIF analog or pharmaceutically acceptable salt thereof is
administered in a daily
dosage of up to about 1 mg/kg of the weight of the subject. In some
embodiments, the
composition comprising a PIF peptide or a PIF analog or pharmaceutically
acceptable salt
thereof is administered in a daily dosage of up to about 0.1 mg/kg of the
weight of the
subject. In some embodiments, the composition comprising a PIF analog or
pharmaceutically
acceptable salt thereof is administered in a daily dosage of up to about 0.01
mg/kg of the
weight of the subject. In some embodiments, the composition comprising a PIF
analog or
pharmaceutically acceptable salt thereof is administered in a daily dosage of
up to about
0.001 mg/kg of the weight of the subject. The dose administered to the subject
can also be
measured in terms of total amount of a PIF peptide or PIF analog administered
per day.
In some embodiments, a subject in need thereof is administered from about 1 ng
to
about 500 pg of analog or pharmaceutically salt thereof per day. In some
embodiments, a
subject in need thereof is administered from about 1 ng to about 10 ng of
analog or
pharmaceutically salt thereof per day. In some embodiments, a subject in need
thereof is
administered from about 10 ng to about 20 ng of analog or pharmaceutically
salt thereof per
day. In some embodiments, a subject in need thereof is administered from about
10 ng to
about 100 ng of analog or pharmaceutically salt thereof per day. In some
embodiments, a
subject in need thereof is administered from about 100 ng to about 200 ng of
analog or
pharmaceutically salt thereof per day. In some embodiments, a subject in need
thereof is
administered from about 200 ng to about 300 ng of analog or pharmaceutically
salt thereof
per day. In some embodiments, a subject in need thereof is administered from
about 300 ng to
about 400 ng of analog or pharmaceutically salt thereof per day. In some
embodiments, a
subject in need thereof is administered from about 400 ng to about 500 ng of
analog or
pharmaceutically salt thereof per day. In some embodiments, a subject in need
thereof is
administered from about 500 ng to about 600 ng of analog or pharmaceutically
salt thereof
-41-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
per day. In some embodiments, a subject in need thereof is administered from
about 600 ng to
about 700 ng of analog or pharmaceutically salt thereof per day. In some
embodiments, a
subject in need thereof is administered from about 800 ng to about 900 ng of
analog or
pharmaceutically salt thereof per day. In some embodiments, a subject in need
thereof is
administered from about 900 ng to about 1 pg of analog or pharmaceutically
salt thereof per
day. In some embodiments, a subject in need thereof is administered from about
1 pg to
about 100 pg of analog or pharmaceutically salt thereof per day. In some
embodiments, a
subject in need thereof is administered from about 100 pg to about 200 pg of
analog or
pharmaceutically salt thereof per day. In some embodiments, a subject in need
thereof is
administered from about 200 pg to about 300 pg of analog or pharmaceutically
salt thereof
per day. In some embodiments, a subject in need thereof is administered from
about 300 pg
to about 400 pg of analog or pharmaceutically salt thereof per day. In some
embodiments, a
subject in need thereof is administered from about 400 pg to about 500 pg of
analog or
pharmaceutically salt thereof per day. In some embodiments, a subject in need
thereof is
administered from about 500 pg to about 600 pg of analog or pharmaceutically
salt thereof
per day. In some embodiments, a subject in need thereof is administered from
about 600 pg
to about 700 pg of analog or pharmaceutically salt thereof per day. In some
embodiments, a
subject in need thereof is administered from about 800 pg to about 900 pg of
analog or
pharmaceutically salt thereof per day. In some embodiments, a subject in need
thereof is
administered from about 900 pg to about 1 mg of analog or pharmaceutically
salt thereof per
day.
In some embodiments, a subject in need thereof is administered from about
.0001 to
about 3000 milligrams of a PIF peptide or PIF analog or pharmaceutically salt
thereof per
day. In some embodiments, a subject is administered up to about 2000
milligrams of a PIF
peptide or PIF analog or pharmaceutically salt thereof day. In some
embodiments, a subject is
administered up to about 1800 milligrams of a PIF peptide or PIF analog or
pharmaceutically
salt thereof per day. In some embodiments, a subject is administered up to
about 1600
milligrams of a PIF peptide or PIF analog or pharmaceutically salt thereof per
day. In some
embodiments, a subject is administered up to about 1400 milligrams of a PIF
peptide or PIF
analog or pharmaceutically salt thereof per day. In some embodiments, a
subject is
administered up to about 1200 milligrams of a PIF peptide or PIF analog or
pharmaceutically
salt thereof per day. In some embodiments, a subject is administered up to
about 1000
milligrams of a PIF peptide or PIF analog or pharmaceutically salt thereof per
day. In some
embodiments, a subject is administered up to about 800 milligrams of a PIF
peptide or PIF
-42-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
analog or pharmaceutically salt thereof per day. In some embodiments, a
subject is
administered from about 0.0001 milligrams to about 700 milligrams of a PIF
peptide or PIF
analog or pharmaceutically salt thereof per dose. In some embodiments, a
subject is
administered up to about 700 milligrams of a PIF peptide or PIF analog or
pharmaceutically
salt thereof per dose. In some embodiments, a subject is administered up to
about 600
milligrams of a PIF peptide or PIF analog or pharmaceutically salt thereof per
dose. In some
embodiments, a subject is administered up to about 500 milligrams of a PIF
peptide or PIF
analog or pharmaceutically salt thereof per dose. In some embodiments, a
subject is
administered up to about 400 milligrams of a PIF peptide or PIF analog or
pharmaceutically
salt thereof per dose. In some embodiments, a subject is administered up to
about 300
milligrams of a PIF peptide or PIF analog or pharmaceutically salt thereof per
dose. In some
embodiments, a subject is administered up to about 200 milligrams of a PIF
peptide or PIF
analog or pharmaceutically salt thereof per dose. In some embodiments, a
subject is
administered up to about 100 milligrams of a PIF peptide or PIF analog or
pharmaceutically
salt thereof per dose. In some embodiments, a subject is administered up to
about 50
milligrams of a PIF peptide or PIF analog or pharmaceutically salt thereof per
dose. In some
embodiments, a subject is administered up to about 25 milligrams of a PIF
peptide or PIF
analog or pharmaceutically salt thereof per dose. In some embodiments, a
subject is
administered up to about 15 milligrams of a PIF peptide or PIF analog or
pharmaceutically
salt thereof per dose. In some embodiments, a subject is administered up to
about 50, 60, 70,
80, 90, or 100 milligrams of a PIF peptide or PIF analog or pharmaceutically
salt thereof per
dose.
In some embodiments, a subject is administered up to about 10 milligrams of a
PIF
peptide or PIF analog or pharmaceutically salt thereof per dose. In some
embodiments, a
subject is administered up to about 5 milligrams of a PIF peptide or PIF
analog or
pharmaceutically salt thereof per dose. In some embodiments, a subject is
administered up to
about 1 milligram of a PIF peptide or PIF analog or pharmaceutically salt
thereof per dose. In
some embodiments, a subject is administered up to about 0.1 milligrams of a
PIF peptide or
PIF analog or pharmaceutically salt thereof per dose. In some embodiments, a
subject is
administered up to about 0.001 milligrams of a PIF peptide or PIF analog or
pharmaceutically
salt thereof per dose.
The dose administered to the subject can also be measured in terms of total
amount of
a PIF peptide or PIF analog or pharmaceutically salt thereof administered per
ounce of liquid
prepared. In some embodiments, the PIF peptide or PIF analog or
pharmaceutically salt
-43-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
thereof is at a concentration of about 2.5 grams per ounce of solution. In
some embodiments,
the PIF peptide or PIF analog or pharmaceutically salt thereof is at a
concentration of about
2.25 grams per ounce of solution. In some embodiments, the PIF peptide or PIF
analog or
pharmaceutically salt thereof is at a concentration of about 2.25 grams per
ounce of solution.
In some embodiments, the PIF peptide or PIF analog or pharmaceutically salt
thereof is at a
concentration of about 2.0 grams per ounce of solution. In some embodiments,
the PIF
peptide or PIF analog or pharmaceutically salt thereof is at a concentration
of about 1.9 grams
per ounce of solution. In some embodiments, the PIF peptide or PIF analog or
pharmaceutically salt thereof is at a concentration of about 1.8 grams per
ounce of solution.
In some embodiments, the PIF analog or pharmaceutically salt thereof is at a
concentration of
about 1.7 grams per ounce of solution. In some embodiments, the PIF peptide or
PIF analog
or pharmaceutically salt thereof is at a concentration of about 1.6 grams per
ounce of
solution. In some embodiments, the PIF peptide or PIF analog or
pharmaceutically salt
thereof is at a concentration of about 1.5 grams per ounce of solution. In
some embodiments,
the PIF peptide or PIF analog or pharmaceutically salt thereof is at a
concentration of about
1.4 grams per ounce of solution. In some embodiments, the PIF peptide or PIF
analog or
pharmaceutically salt thereof is at a concentration of about 1.3 grams per
ounce of solution.
In some embodiments, the PIF peptide or PIF analog or pharmaceutically salt
thereof is at a
concentration of about 1.2 grams per ounce of solution. In some embodiments,
the PIF
peptide or PIF analog or pharmaceutically salt thereof is at a concentration
of about 1.1 grams
per ounce of solution. In some embodiments, the PIF peptide or PIF analog or
pharmaceutically salt thereof is at a concentration of about 1.0 grams per
ounce of solution.
In some embodiments, the PIF peptide or PIF analog or pharmaceutically salt
thereof
is at a concentration of about 0.9 grams per ounce of solution. In some
embodiments, the PIF
peptide or PIF analog or pharmaceutically salt thereof is at a concentration
of about 0.8 grams
per ounce of solution. In some embodiments, the PIF peptide or PIF analog or
pharmaceutically salt thereof is at a concentration of about 0.7 grams per
ounce of solution.
In some embodiments, the PIF peptide or PIF analog or pharmaceutically salt
thereof is at a
concentration of about 0.6 grams per ounce of solution. In some embodiments,
the PIF
peptide or PIF analog or pharmaceutically salt thereof is at a concentration
of about 0.5 grams
per ounce of solution. In some embodiments, the PIF peptide or PIF analog or
pharmaceutically salt thereof is at a concentration of about 0.4 grams per
ounce of solution.
In some embodiments, the PIF peptide or PIF analog or pharmaceutically salt
thereof is at a
concentration of about 0.3 grams per ounce of solution. In some embodiments,
the PIF
-44-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
peptide or PIF analog or pharmaceutically salt thereof is at a concentration
of about 0.2 grams
per ounce of solution. In some embodiments, the PIF peptide or PIF analog or
pharmaceutically salt thereof is at a concentration of about 0.1 grams per
ounce of solution.
In some embodiments, the PIF peptide or PIF analog or pharmaceutically salt
thereof is at a
concentration of about 0.01 grams per ounce of solution. In some embodiments,
the PIF
peptide or PIF analog or pharmaceutically salt thereof is at a concentration
of about 0.001
grams per ounce of solution prepared. In some embodiments, the PIF peptide or
PIF analog
or pharmaceutically salt thereof is at a concentration of about 0.0001 grams
per ounce of
solution prepared. In some embodiments, the PIF peptide or PIF analog or
pharmaceutically
salt thereof is at a concentration of about 0.00001 grams per ounce of
solution prepared. In
some embodiments, the PIF peptide or PIF analog or pharmaceutically salt
thereof is at a
concentration of about 0.000001 grams per ounce of solution prepared.
Dosage may be measured in terms of mass amount of analog per liter of liquid
formulation prepared. One skilled in the art can increase or decrease the
concentration of the
analog in the dose depending upon the strength of biological activity desired
to treat or
prevent any above-mentioned disorders associated with the treatment of
subjects in need
thereof For instance, some embodiments of the invention can include up to
0.00001 grams of
analog per 5 mL of liquid formulation and up to about 10 grams of analog per 5
mL of liquid
formulation. In some embodiments, the pharmaceutical compositions comprising a
PIF
analog or any of such compositions in any of the disclosed methods are free of
SEQ ID NO:l.
In some embodiments, the pharmaceutical compositions comprising a PIF analog
or any of
such compositions in any of the disclosed methods are free of SEQ ID NO:2. In
some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:3. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:4. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:5. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:6. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:7. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:8. In some
-45-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:9. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:10. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:11. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:12. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:13. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:14. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:15. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:16. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:17. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:18. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:19. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:20. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:21. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:22. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:23. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:24. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:25. In some
-46-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:26. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:27. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:28. In some
embodiments, the pharmaceutical compositions comprising a PIF analog or any of
such
compositions in any of the disclosed methods are free of SEQ ID NO:29.
In some embodiments the pharmaceutical compositions of the claimed invention
comprises at least one or a plurality of active agents other than the PIF
peptide, analog or
pharmaceutically acceptable salt thereof In some embodiments the active agent
is covalently
linked to the PIF peptide or PIF analog disclosed herein optionally by a
protease cleavable
linker (including by not limited to Pro-Pro or Cituline-Valine di-a-amino acid
linkers). In
some embodiments, the one or plurality of active agents is one or a
combination of
compounds chosen from: an anti-inflammatory compound, alpha-adrenergic
agonist,
antiarrhythmic compound, analgesic compound, and an anesthetic compound.
Table Y
Examples of anti-inflammatory compounds include:
aspirin
celecoxib
diclofenac
diflunisal
etodolac
ibuprofen
indomethacin
ketoprofen
ketorolac nabumetone
naproxen
oxaprozin
piroxicam
salsalate
sulindac
tolmetin
Examples of alpha-adrenergic agonists include:
Methoxamine
Methylnorepinephrine
Midodrine
-47-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
Oxymetazoline
Metaraminol
Phenylephrine
Clonidine (mixed alpha2-adrenergic and imidazoline-Il receptor agonist)
Guanfacine, (preference for alpha2A-subtype of adrenoceptor)
Guanabenz (most selective agonist for alpha2-adrenergic as opposed to
imidazoline-I1)
Guanoxabenz (metabolite of guanabenz)
Guanethidine (peripheral alpha2-receptor agonist)
Xylazine,
Tizanidine
Medetomidine
Methyldopa
Fadolmidine
Dexmedetomidine
Examples of antiarrhythmic compound include:
Amiodarone (Cordarone, Pacerone)
Bepridil Hydrochloride (Vascor)
Disopyramide (Norpace)
Dofetilide (Tikosyn)
Dronedarone (Multaq)
Flecainide (Tambocor)
Ibutilide (Corvert)
Lidocaine (Xylocaine)
Procainamide (Procan, Procanbid)
Propafenone (Rythmol)
Propranolol (Inderal)
Quinidine (many trade names)
Sotalol (Betapace)
Tocainide (Tonocarid)
Examples of analgesic compound include:
codeine
hydrocodone (Zohydro ER),
oxycodone (OxyContin, Roxicodone),
methadone
hydromorphone (Dilaudid, Exalgo),
morphine (Avinza, Kadian, MSIR, MS Contin), and
fentanyl (Actiq, Duragesic)
Examples of anesthetic compounds include:
Desflurane
Isoflurane
Nitrous oxide
Sevoflurane
Xenon
-48-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
The compounds of the present disclosure can also be administered in
combination
with other active ingredients, such as, for example, adjuvants, or other
compatible drugs or
compounds where such combination is seen to be desirable or advantageous in
achieving the
desired effects of the methods described herein.
The methods disclosed herein can be used with any of the compounds,
compositions,
preparations, and kits disclosed herein.
In some embodiments, methods of diagnosing endometriosis in a human subject
are
provided. In some embodiments, the method comprises measuring pre-implantation
factor
(PIF) protein or mRNA expression levels from an endometrial tissue sample from
the subject;
and comparing the PIF protein expression levels from the endometrial sample to
the PIF
expression levels in a control normal sample; wherein the patient is diagnosed
with
endometriosis if the PIF expression levels from the endometrial sample are
greater than the
PIF expression levels from the control normal sample. In some embodiments, the
endometrial sample is an endometrial epithelial tissue sample.
In some embodiments, the sample is a biopsy sample. That is the endometrial
sample
can, for example, be obtained by a biopsy.
Methods of measuring PIF protein expression levels can be performed by any
method.
For example, in some embodiments, the measuring of PIF protein expression
levels
comprises contacting the sample with an anti-PIF antibody and detecting bound
anti-PIF
antibody to PIF in the sample. The detection can by staining methods or other
type methods
using modified antibodies to visualize and/or quantitate the amount of PIF in
the sample. The
amount of the bound antibody can be converted into the amount of PIF present
in the sample
by comparing to controls, which is routine in the art. In some embodiments,
the amount of
bound antibody indicates the PIF protein expression levels. The sample can
also be stained
for PIF protein expression levels using available protocols. As a non-limiting
example, the
method can comprise staining the sample with an anti-PIF antibody that binds
to PIF in the
sample. In some embodiments, instead of measuring protein levels, the mRNA
levels
encoding PIF are measured. Accordingly, in some embodiments, the measuring of
PIF
mRNA expression levels comprises contacting the sample with a probe that binds
to PIF
mRNA and detecting the bound probe in the sample. The probe can be detected by
performing amplification methods, such as RT-PCR, PCR, and the like. In some
embodiments, the probe is linked to fluorescent or radioactive moiety and the
probe is
detected by measuring the fluorescence or radioactive signal to determine the
presence or
absence of PIF in the sample.
-49-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
In some embodiments, a method of treating endometriosis is combined with a
method
of diagnosing endometriosis. In some embodiments, the method comprises
measuring pre-
implantation factor (PIF) protein or mRNA expression levels from a endometrial
sample from
the subject; comparing the PIF protein or mRNA expression levels from the
endometrial
sample to the PIF expression levels in a control normal sample, wherein the
patient is
diagnosed with endometriosis if the PIF expression levels from the endometrial
sample are
greater than the PIF expression levels from the control normal sample; and
administering to
the subject at least one pharmaceutical composition comprising a
therapeutically effective
amount of a PIF peptide, mimetics thereof, analogs thereof, or a
pharmaceutically acceptable
salt thereof and a pharmaceutically acceptable carrier. In some embodiments,
the endometrial
sample is an endometrial epithelial tissue sample . In some embodiments, the
endometrial
tissue sample is a biopsy sample. The PIF protein expression levels and the
PIF mRNA
expression levels can be measured, for example, according to the methods
described herein.
In some embodiments, if the PIF expression levels in the endometrial
epithelial tissue
sample are detectable or greater than as compared to the control, the patient
is diagnosed with
endometriosis. In some embodiments, the PIF expression levels in the
endometrial epithelial
tissue sample are at least 1% greater than the PIF expression levels in the
control sample, the
patient is diagnosed with endometriosis. In some embodiments, the PIF
expression levels in
the endometrial epithelial tissue sample are at least 5% greater than the PIF
expression levels
in the control sample, the patient is diagnosed with endometriosis. In some
embodiments, the
PIF expression levels in the endometrial epithelial tissue sample are at least
10% greater than
the PIF expression levels in the control sample, the patient is diagnosed with
endometriosis.
In some embodiments, the PIF expression levels in the endometrial epithelial
tissue sample
are at least 15% greater than the PIF expression levels in the control sample,
the patient is
diagnosed with endometriosis. In some embodiments, the PIF expression levels
in the
endometrial epithelial tissue sample are at least 20% greater than the PIF
expression levels in
the control sample, the patient is diagnosed with endometriosis. In some
embodiments, the
PIF expression levels in the endometrial epithelial tissue sample are at least
25% greater than
the PIF expression levels in the control sample, the patient is diagnosed with
endometriosis.
In some embodiments, the PIF expression levels in the endometrial epithelial
tissue sample
are at least 30% greater than the PIF expression levels in the control sample,
the patient is
diagnosed with endometriosis. In some embodiments, the PIF expression levels
in the
endometrial epithelial tissue sample are at least 50% greater than the PIF
expression levels in
the control sample, the patient is diagnosed with endometriosis. In some
embodiments, the
-50-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
PIF expression levels in the endometrial epithelial tissue sample are at least
100% greater
than the PIF expression levels in the control sample, the patient is diagnosed
with
endometriosis. In some embodiments, the PIF expression levels in the
endometrial epithelial
tissue sample are at least 200% greater than the PIF expression levels in the
control sample,
the patient is diagnosed with endometriosis. In some embodiments, the PIF
expression levels
in the endometrial epithelial tissue sample are at least 300% greater than the
PIF expression
levels in the control sample, the patient is diagnosed with endometriosis. In
some
embodiments, the PIF expression levels in the endometrial epithelial tissue
sample are at least
400% greater than the PIF expression levels in the control sample, the patient
is diagnosed
with endometriosis. In some embodiments, the PIF expression levels in the
endometrial
epithelial tissue sample are at least 500% greater than the PIF expression
levels in the control
sample, the patient is diagnosed with endometriosis. In some embodiments, the
PIF
expression levels in the endometrial epithelial tissue sample are at least
1000% greater than
the PIF expression levels in the control sample, the patient is diagnosed with
endometriosis.
In some embodiments, the patient is diagnosed with endometriosis if the PIF
expression
levels from the endometrial epithelial sample are at least 10% greater than
the PIF expression
levels from the control normal sample. In some embodiments, the patient is
diagnosed with
endometriosis if the PIF expression levels from the endometrial epithelial
sample are at least
50% greater than the PIF expression levels from the control normal sample. In
some
embodiments, the patient is diagnosed with endometriosis if the PIF expression
levels from
the endometrial epithelial sample are at least 100% greater than the PIF
expression levels
from the control normal sample. In some embodiments, the patient is with
endometriosis if
the PIF expression levels from the endometrial epithelial sample are at least
200% greater
than the PIF expression levels from the control normal sample. For the
avoidance of doubt,
a control sample is a control normal sample unless otherwise indicated by
context.
The disclosure also relates to methods for treating endometriosis comprising
administering an effective amount of the compositions described herein to a
subject in need
thereof
In an embodiment, the composition is administered once a day to a subject in
need
thereof In another embodiment, the composition is administered every other
day, every third
day or once a week. In another embodiment, the composition is administered
twice a day. In
still another embodiment, the composition is administered three times a day or
four times a
day. In a further embodiment, the composition is administered at least once a
day for at least
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12 weeks. In still a further embodiment,
the composition is
-51-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
administered at least once a day for a longer term such as at least 4, 6, 8,
10, 12 or 24 months.
Administration in some embodiments includes but is not limited to a dosage of
10-50 mg of
composition at a frequency of minimum 1, 2, 3 or 4 times per day. Optionally,
administration
continues until all symptoms are resolved and cleared by medical personnel via
standardized
testing such as SCAT 2.
In some embodiments, the composition is administered at least once a day until
the
condition has ameliorated to where further treatment is not necessary. In
another
embodiment, the composition is administered until all symptoms of
endometriosis are
resolved. In further embodiments, the composition is administered for at least
1, 2, 3, 6, 8, 10
or 12 or 24 months after the subject is asymptomatic.
The compositions of the present disclosure are useful and effective when
administered
to treat endometriosis. The amount of each component present in the
composition will be the
amount that is therapeutically effective, i.e., an amount that will result in
the effective
treatment of the condition (e.g., endometriosis) when administered. The
therapeutically
effective amount will vary depending on the subject and the severity and
nature of the injury
and can be determined routinely by one of ordinary skill in the art.
In some embodiments, the disclosure relates to a method of treating or
preventing any
of the indications set forth in US Pat. Nos. 8,222,211, 7,723,289, 7,723,290,
8,454,967,
9,097,725, (each of which are incorporated by reference in their entireties)
comprising
administering compositions or pharmaceutical compositions comprising any one
or plurality
of PIF peptides, analogs, or pharmaceutically acceptable salts thereof
disclosed herein.
In some methods, the disclosure relates to a method of stimulating the
differentiation
and/or proliferation of stem cells in a subject in need thereof comprising
administering
compositions or pharmaceutical compositions comprising any one or plurality of
PIF
peptides, analogs, or pharmaceutically acceptable salts thereof disclosed
herein.
In some embodiments, the disclosure relates to any of the methods disclosed in
US
Pat. Nos. 7,273,708, 7,695,977, 7,670,852, 7,670,851, 7,678,582, 7,670,850,
8,012,700 (each
of which are incorporated by reference in their entireties) comprising
administering
compositions or pharmaceutical compositions comprising any one or plurality of
PIF
peptides, analogs, or pharmaceutically acceptable salts thereof disclosed
herein.
This disclosure also incorporates by reference in their entireties US Pat.
Nos.
7,789,289, 7,723,290, 8,222,211, and 8,454,967.
-52-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
In some embodiments, the disclosure relates to a method of treating
endometriosis by
administering at least one or a plurality of compositions disclosed herein
comprising PIF
peptide, an analog thereof, or a pharmaceutically acceptable salt thereof
In some embodiments, the disclosure relates to a method of endometriosis by
administering a therapeutically effective amount or dose of one or a plurality
of compositions
disclosed herein comprising at least one PIF peptide, an analog thereof, or a
pharmaceutically
acceptable salt thereof
In some embodiments, the disclosure relates to a method of treating
endometriosis by
administration of a pharmaceutical composition comprising a therapeutically
effective
amount or dose of at least one PIF peptide, an analog thereof, or a
pharmaceutically
acceptable salt thereof, and a pharmaceutically acceptable carrier.
In some embodiments, the disclosure relates to a pharmaceutical composition
comprising a therapeutically effective amount or dose of at least one PIF
peptide, an analog
thereof, or a pharmaceutically acceptable salt thereof, and a pharmaceutically
acceptable
carrier for the treatment of endometriosis.
In some embodiments, the disclosure relates to the use of a therapeutically
effective
amount or dose of any one or plurality of compositions disclosed herein
comprising at least
one PIF peptide, an analog thereof, or a pharmaceutically acceptable salt
thereof, and a
pharmaceutically acceptable carrier in the manufacture of a medicament for the
treatment of
endometriosis.
In some embodiments, the disclosure relates to the use of a pharmaceutical
composition comprising a therapeutically effective amount or dose at least one
PIF peptide,
an analog thereof, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically
acceptable carrier in the manufacture of a medicament for the endometriosis.
In some embodiments, the disclosure relates to a method of inducing an
immunomodulation effect in a subject in need thereof, when subject has been or
is suspect of
having endometriosis.
Kits
According to some embodiments of the invention, the formulation may be
supplied as
part of a kit. In some embodiments, the kit comprises comprising a PIF peptide
and/or a PIF
analog or pharmaceutically acceptable salt thereof, the PIF peptide and/or a
PIF analog or
pharmaceutically acceptable salt thereof comprises a non-natural amino acid or
is at least
70% homologous to SEQ ID NO: 1. In some embodiments, the kit comprises
comprising a
PIF peptide and/or a PIF analog or pharmaceutically acceptable salt thereof,
the PIF peptide
-53-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
and/or a PIF analog or pharmaceutically acceptable salt thereof comprises a
non-natural
amino acid or is at least 70% homologous to SEQ ID NO:2. In some embodiments,
the kit
comprises comprising a PIF peptide and/or a PIF analog or pharmaceutically
acceptable salt
thereof, the PIF peptide and/or a PIF analog or pharmaceutically acceptable
salt thereof
comprises a non-natural amino acid or is at least 70% homologous to SEQ ID
NO:3. In some
embodiments, the kit comprises comprising a PIF peptide and/or a PIF analog or
pharmaceutically acceptable salt thereof, the PIF peptide and/or a PIF analog
or
pharmaceutically acceptable salt thereof comprises a non-natural amino acid or
is at least
70% homologous to SEQ ID NO:4. In some embodiments, the kit comprises
comprising a
PIF peptide and/or a PIF analog or pharmaceutically acceptable salt thereof,
the PIF peptide
and/or a PIF analog or pharmaceutically acceptable salt thereof comprises a
non-natural
amino acid or is at least 70% homologous to SEQ ID NO:5. In some embodiments,
the kit
comprises comprising a PIF peptide and/or a PIF analog or pharmaceutically
acceptable salt
thereof, the PIF peptide and/or a PIF analog or pharmaceutically acceptable
salt thereof
comprises a non-natural amino acid or is at least 70% homologous to SEQ ID
NO:6. In some
embodiments, the kit comprises comprising a PIF peptide and/or a PIF analog or
pharmaceutically acceptable salt thereof, the PIF peptide and/or a PIF analog
or
pharmaceutically acceptable salt thereof comprises a non-natural amino acid or
is at least
70% homologous to SEQ ID NO:7. In some embodiments, the kit comprises
comprising a
PIF peptide and/or a PIF analog or pharmaceutically acceptable salt thereof,
the PIF peptide
and/or a PIF analog or pharmaceutically acceptable salt thereof comprises a
non-natural
amino acid or is at least 70% homologous to SEQ ID NO:8. In some embodiments,
the kit
comprises comprising a PIF peptide and/or a PIF analog or pharmaceutically
acceptable salt
thereof, the PIF peptide and/or a PIF analog or pharmaceutically acceptable
salt thereof
comprises a non-natural amino acid or is at least 70% homologous to SEQ ID
NO:9. In some
embodiments, the kit comprises comprising a PIF peptide and/or a PIF analog or
pharmaceutically acceptable salt thereof, the PIF peptide and/or a PIF analog
or
pharmaceutically acceptable salt thereof comprises a non-natural amino acid or
is at least
70% homologous to SEQ ID NO:10. In some embodiments, the kit comprises
comprising a
PIF peptide and/or a PIF analog or pharmaceutically acceptable salt thereof,
the PIF peptide
and/or a PIF analog or pharmaceutically acceptable salt thereof comprises a
non-natural
amino acid or is at least 70% homologous to SEQ ID NO:11. In some embodiments,
the kit
comprises comprising a PIF peptide and/or a PIF analog or pharmaceutically
acceptable salt
thereof, the PIF peptide and/or a PIF analog or pharmaceutically acceptable
salt thereof
-54-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
comprises a non-natural amino acid or is at least 70% homologous to SEQ ID
NO:12. In
some embodiments, the kit comprises comprising a PIF peptide and/or a PIF
analog or
pharmaceutically acceptable salt thereof, the PIF peptide and/or a PIF analog
or
pharmaceutically acceptable salt thereof comprises a non-natural amino acid or
is at least
70% homologous to SEQ ID NO:13. In some embodiments, the kit comprises
comprising a
PIF peptide and/or a PIF analog or pharmaceutically acceptable salt thereof,
the PIF peptide
and/or a PIF analog or pharmaceutically acceptable salt thereof comprises a
non-natural
amino acid or is at least 70% homologous to SEQ ID NO:14. In some embodiments,
the kit
comprises comprising a PIF peptide and/or a PIF analog or pharmaceutically
acceptable salt
thereof, the PIF peptide and/or a PIF analog or pharmaceutically acceptable
salt thereof
comprises a non-natural amino acid or is at least 70% homologous to SEQ ID
NO:15. In
some embodiments, the kit comprises comprising a PIF peptide and/or a PIF
analog or
pharmaceutically acceptable salt thereof, the PIF peptide and/or a PIF analog
or
pharmaceutically acceptable salt thereof comprises a non-natural amino acid or
is at least
70% homologous to SEQ ID NO:16. In some embodiments, the kit comprises
comprising a
PIF peptide and/or a PIF analog or pharmaceutically acceptable salt thereof,
the PIF peptide
and/or a PIF analog or pharmaceutically acceptable salt thereof comprises a
non-natural
amino acid or is at least 70% homologous to SEQ ID NO:17. In some embodiments,
the kit
comprises comprising a PIF peptide and/or a PIF analog or pharmaceutically
acceptable salt
thereof, the PIF peptide and/or a PIF analog or pharmaceutically acceptable
salt thereof
comprises a non-natural amino acid or is at least 70% homologous to SEQ ID
NO:18. In
some embodiments, the kit comprises comprising a PIF peptide and/or a PIF
analog or
pharmaceutically acceptable salt thereof, the PIF peptide and/or a PIF analog
or
pharmaceutically acceptable salt thereof comprises a non-natural amino acid or
is at least
70% homologous to SEQ ID NO:19. In some embodiments, the kit comprises
comprising a
PIF peptide and/or a PIF analog or pharmaceutically acceptable salt thereof,
the PIF peptide
and/or a PIF analog or pharmaceutically acceptable salt thereof comprises a
non-natural
amino acid or is at least 70% homologous to SEQ ID NO:20. In some embodiments,
the kit
comprises comprising a PIF peptide and/or a PIF analog or pharmaceutically
acceptable salt
thereof, the PIF peptide and/or a PIF analog or pharmaceutically acceptable
salt thereof
comprises a non-natural amino acid or is at least 70% homologous to SEQ ID
NO:21. In
some embodiments, the kit comprises comprising a PIF peptide and/or a PIF
analog or
pharmaceutically acceptable salt thereof, the PIF peptide and/or a PIF analog
or
pharmaceutically acceptable salt thereof comprises a non-natural amino acid or
is at least
-55-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
70% homologous to SEQ ID NO:22. In some embodiments, the kit comprises
comprising a
PIF peptide and/or a PIF analog or pharmaceutically acceptable salt thereof,
the PIF peptide
and/or a PIF analog or pharmaceutically acceptable salt thereof comprises a
non-natural
amino acid or is at least 70% homologous to SEQ ID NO:23. In some embodiments,
the kit
comprises comprising a PIF peptide and/or a PIF analog or pharmaceutically
acceptable salt
thereof, the PIF peptide and/or a PIF analog or pharmaceutically acceptable
salt thereof
comprises a non-natural amino acid or is at least 70% homologous to SEQ ID
NO:24. In
some embodiments, the kit comprises comprising a PIF peptide and/or a PIF
analog or
pharmaceutically acceptable salt thereof, the PIF peptide and/or a PIF analog
or
pharmaceutically acceptable salt thereof comprises a non-natural amino acid or
is at least
70% homologous to SEQ ID NO:25. In some embodiments, the kit comprises
comprising a
PIF peptide and/or a PIF analog or pharmaceutically acceptable salt thereof,
the PIF peptide
and/or a PIF analog or pharmaceutically acceptable salt thereof comprises a
non-natural
amino acid or is at least 70% homologous to SEQ ID NO:26. In some embodiments,
the kit
comprises comprising a PIF peptide and/or a PIF analog or pharmaceutically
acceptable salt
thereof, the PIF peptide and/or a PIF analog or pharmaceutically acceptable
salt thereof
comprises a non-natural amino acid or is at least 70% homologous to SEQ ID
NO:27. In
some embodiments, the kit comprises comprising a PIF peptide and/or a PIF
analog or
pharmaceutically acceptable salt thereof, the PIF peptide and/or a PIF analog
or
pharmaceutically acceptable salt thereof comprises a non-natural amino acid or
is at least
70% homologous to SEQ ID NO:28. In some embodiments, the kit comprises
comprising a
PIF peptide and/or a PIF analog or pharmaceutically acceptable salt thereof,
the PIF peptide
and/or a PIF analog or pharmaceutically acceptable salt thereof comprises a
non-natural
amino acid or is at least 70% homologous to SEQ ID NO:29. In another
embodiment, the kit
comprises a pharmaceutically acceptable salt of an analog with a rehydration
mixture. In
another embodiment, the pharmaceutically acceptable salt of an analog are in
one container
while the rehydration mixture is in a second container. The rehydration
mixture may be
supplied in dry form, to which water or other liquid solvent may be added to
form a
suspension or solution prior to administration. Rehydration mixtures are
mixtures designed to
solubilize a lyophilized, insoluble salt of the invention prior to
administration of the
composition to a subject takes at least one dose of a purgative. In another
embodiment, the kit
comprises a pharmaceutically acceptable salt in orally available pill form.
The kit may contain two or more containers, packs, or dispensers together with
instructions for preparation and administration. In some embodiments, the kit
comprises at
-56-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
least one container comprising the pharmaceutical composition or compositions
described
herein and a second container comprising a means for delivery of the
compositions such as a
syringe. In some embodiments, the kit comprises a composition comprising an
analog in
solution or lyophilized or dried and accompanied by a rehydration mixture. In
some
embodiments, the analog and rehydration mixture may be in one or more
additional
containers.
The compositions included in the kit may be supplied in containers of any sort
such
that the shelf-life of the different components are preserved, and are not
adsorbed or altered
by the materials of the container. For example, suitable containers include
simple bottles that
may be fabricated from glass, organic polymers, such as polycarbonate,
polystyrene,
polypropylene, polyethylene, ceramic, metal or any other material typically
employed to hold
reagents or food; envelopes, that may consist of foil-lined interiors, such as
aluminum or an
alloy. Other containers include test tubes, vials, flasks, and syringes. The
containers may have
two compartments that are separated by a readily removable membrane that upon
removal
permits the components of the compositions to mix. Removable membranes may be
glass,
plastic, rubber, or other inert material.
Kits may also be supplied with instructional materials. Instructions may be
printed on
paper or other substrates, and/or may be supplied as an electronic-readable
medium, such as a
floppy disc, CD-ROM, DVD-ROM, zip disc, videotape, audio tape, or other
readable
memory storage device. Detailed instructions may not be physically associated
with the kit;
instead, a user may be directed to an intern& web site specified by the
manufacturer or
distributor of the kit, or supplied as electronic mail.
In another embodiment, a packaged kit is provided that contains the
pharmaceutical
formulation to be administered, i.e., a pharmaceutical formulation containing
PIF peptide
and/or a PIF analog or pharmaceutically acceptable salt thereof, a container
(e.g., a vial, a
bottle, a pouch, an envelope, a can, a tube, an atomizer, an aerosol can,
etc.), optionally
sealed, for housing the formulation during storage and prior to use, and
instructions for
carrying out drug administration in a manner effective to treat any one or
more of the
indications disclosed herein. The instructions will typically be written
instructions on a
package insert, a label, and/or on other components of the kit.
Depending on the type of formulation and the intended mode of administration,
the kit
may also include a device for administering the formulation (e.g., a
transdermal delivery
device). The administration device may be a dropper, a swab, a stick, or the
nozzle or outlet
of an atomizer or aerosol can. The formulation may be any suitable formulation
as described
-57-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
herein. For example, the formulation may be an oral dosage form containing a
unit dosage of
the active agent, or a gel or ointment contained within a tube. The kit may
contain multiple
formulations of different dosages of the same agent. The kit may also contain
multiple
formulations of different active agents. The kit may contain a number of
pharmaceutically
effective dosages in separate containers or syringes necessary to treat one or
more symptoms
of endometriosis. in some embodiments, the kit contains about 1, 2, 3, 4, or 5
or more
dosages in 1, 2, 3, 4, or five containers (such as a syringe), that enable
administration of any
of the dosages into the subject in need thereof
The present kits will also typically include means for packaging the
individual kit
components, i.e., the pharmaceutical dosage forms, the administration device
(if included),
and the written instructions for use. Such packaging means may take the form
of a cardboard
or paper box, a plastic or foil pouch, etc.
According to some embodiments, the kits are provided that can be used for
diagnosing subjects with endometriosis. The kits can, for example, comprises
reagents that
are used to detect PIF in the endometrial sample. These reagents can be
buffers, antibodies to
PIF, secondary antibodies, stains, probes to PIF mRNA and the like. The kit
can also
comprise instructions for performing methods described herein. In some
embodiments, the
kit also comprises reagents for processing the endometrial sample before
detecting PIF
expression.
This disclosure and embodiments illustrating the method and materials used may
be
further understood by reference to the following non-limiting examples.
Example 1: PIF expression in endometriosis
Endometriosis is a chronic benign disease characterized by the presence of
endometrial tissue, both stromal and epithelial components, outside the
uterine cavity,
affecting women in their reproductive age (Giudice LC 2010). Endometriosis
lesions can be
found mostly in the pelvis, where the most common sites are the ovaries,
anterior and
posterior cul-de-sac, broad and utero-sacral ligaments, uterus, fallopian
tubes, sigmoid colon
and appendix (Olive et al 1993). At the moment the most accepted hypothesis
regarding its
pathogenessis is the retrograde menstruation, even though stem cell origin as
well as its
genetic cause are gaining attention (Macer et al 2012).
Several evidences have shown the presence of putative stem cells in the human
endometrium as well as in other mammals (Taylor 2004, Du et al 2007, Kao et al
2011,
Hafnagel et al 2015), and it has been showed that stem cells for the
endometrium may been
-58-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
involved in endometriosis pathogenesis: apart from their of endometrial origin
they may be
mesenchymal stem cells derived from bone marrow.
Pre-Implantation Factor or PIF is a fifteen amino acid linear peptide secreted
by
viable human, bovine and murine embryos (Stamatkin et al 2011, Stamatkin et al
2011b,
Barnea et al 2012, Ramu et al 2013). In singly cultured bovine and murine
embryos increased
levels of PIF in the media correlate with embryos development whereas PIF is
absent in non-
viable embryos (Stamatkin et al 2011). PIF plays an essential role in human
pregnancy, as it
primes the endometrium for implantation, promotes trophoblast invasion and
regulates
systemic immune response (Barnea et al 2012, Paidas et al 2010, Barnea et al
2012b, Duzyj
et al 2010). Relevant to PIF's immune regulatory features, translational
aspects to treatment
of non-pregnant autoimmune and transplantation models were documented (Weiss
et al 2011,
Weiss et al 2012, Azar et al 2013). PIF also promotes trophoblast invasion,
and orchestrates
maternal systemic immune response (Barnea et al 2012, Duzyj et al, Roussev et
al 2013).
Pathway analysis in autoimmunity and transplantation models demonstrate that
sPIF,
administered as a single agent to non-pregnant mice, acts by reducing
oxidative stress and
protein misfolding (Weiss et al 2011, Weiss et al 2012, Paidas et al 2012,
Azer et al 2013,
Shainer et al 2013).
Tregs are derived from the CD4 lineage of T cells and are produced naturally
in the
thymus, express IL-10 receptor (CD25+) and the forkhaed box P3 transcription
factor
(Foxp3+). CD4+ T cells become Tregs by the Foxp3 expression induced by the
cytokines
increase in the microenvironment. Activated Tregs suppress the response of
effector T cell
indirectly by inhibiting the dendritic cells or the other antigen presenting
cells (APC) from
triggering effector T cell proliferation (Tang et al 2006). Tregs induce
immune tolerance
through the production of IL-10 and Transforming Growth Factor-0, anti-
inflammatory
cytokines or Th2, which inhibit T helper cell activation. (von Boemer et al
2006). The
absence or depletion of Tregs leads to multi-systemic autoimmunity in mice and
humans
(Bruckner et al 2010). It has been reported that Tregs are critical for tumor
growth, since they
may provide an immunologically protected micro environment (Bergman et al
2007, Strauss
et al 2007,Bergmann et al 20011, Wang et al 2012). The presence of Tregs in
eutopic and
ectopic endometrium of women affected by endometriosis has been reported
(Budiu et al
2009, Berbic et al 2010).
The aim of the study was to evaluate the possible expression of PIF and FOX-P3
in
the ectopic endometrium in order to evaluate the expression of these antigens
in the ectopic
-59-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
endometrium, and identify cells that are able to modulate immune competent
cells response
in endometriotic lesions.
Material and Methods
Synthetic PIF15 (MVRIKPGSANKPSDD) and a scrambled peptide same amino acid
sequence but in random order (GRVDPSNKSMPKDIA) were synthesized by solid-phase
peptide synthesis (Peptide Synthesizer, Applied Biosystems) employing Fmoc (9-
fluorenylmethoxycarbonyl) chemistry at Bio-Synthesis, Inc. (Lewisville, TX).
Final
purification was carried out by reversed-phase HPLC and identity was verified
by matrix-
assisted laser desorption/ionization time-of-flight mass spectrometry and
amino acid analysis
at >95% purity. Fluorescein labeled FITC-PIF and scrambled PIF (FITC- PIFscr)
were also
generated as previously reported (Barnea AJOG 2012). Anti-PIF monoclonal
antibody
against MVRIKPGSANKPSDD was generated in (Genway, SanDiego, CA). et al 2012).
Patient tissue material: Tissue specimens were obtained from 25 women who
underwent laparoscopic surgery for severe endometriosis according to the
revised criteria of
the American Society Reproductive Medicine. The surgical procedures were
carried out in
CERM-Hungaria Institute, Rome, Italy, from September 2014 through April 2015.
The
project had the approval of the Hospital's Ethical Committee. Samples were
obtained from
the ectopic endometrium, ovarian endometriomas and peritoneal implants. A
total of 25
eutopic endometria, 25 ovarian endometriomas and 10 peritoneal implants were
collected
from patients. Furthermore, the endometria of 10 healthy women in different
phases of the
menstrual cycle were used as controls. Biopsy samples were fixed in 4% neutral
buffered
formalin overnight and were subsequently paraffin embedded.
Before performing immunohistochemistry sections of the tissues were stained
with
eosin and hematoxylin to select tissues with ectopic epthelial cells. Serial
sections of the
same selected samples 51.tm thick were used for immunohistochemistry.
Commercially
available monoclonal antibodies were used for the detection of PIF (Bioincept,
LLC) and
FOXP3 (Santa Cruz, Santa Cruz, CA, USA). Immunohistochemistry was performed
according to Hsu et al 1981. Briefly, tissue sections were dewaxed and
rehydratated
conventionally, after quenching the endogenous peroxidase by incubation with
0.3%
hydrogen peroxide in methanol for 30 minutes at room temperature sections were
exposed to
a non-immune block with normal horse serum for 30 minutes at room temperature.
Incubation with the first antibody was carried out at 4 C overnight with a
dilution of 1 to 100
for the monoclonal mouse anti-human PIF and with a dilution of 1 to 50 for the
monoclonal
-60-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
mouse anti-human FOXP3. Thereafter tissue sections were labeled with the
avidin-biotin-
peroxidase detection system Vectastain (Vector Laboratories, Burlingthon VT,
USA). Each
step was followed by meticulous washing with PBS. Finally, 3,3'-
diaminobenzidine (DAB)
and/or 3-amino-9-ethylcarbazole (AEC) were used as chromogens for single or
double
staining. Counterstaining was performed with hematoxylin. The positive
controls were PIF
and FOXP3 tissues. Negative controls were performed by replacing the primary
antibody
with mouse immunoglobulin at the same concentration as the primary antibody.
In order to
perform the statistical analysis of immunostaining for each protein, a semi-
quantitative
analysis of specific staining was performed using an HSCORE system according
to Mc Carty
et al. 1985. The HSCORE was calculated using the following equation:
HSCORE=IPi(i+1),
where i is the intensity of staining with a value of 1, 2, or 3 (weak, strong
or very strong
respectively) and Pi is the percentage of stained cells for each intensity,
varying from 0% to
100%. For all samples, ten microscopic fields were counted by two of the
authors
independently in each slide.
The intra-observer and inter-observer coefficient of variation were 3.4% and
4.2%
respectively. Three slides of each sample were observed for both antigens
tested under the
microscope and each observer was blind to which sample it was. The slides were
numbered
progressively by a technician, who reported on a separate worksheet the number
of the slide
and the corresponding name of the patient or control. Only after the slide was
analyzed by the
two different observers and scored for the HSCORE each value was reported on
the
worksheet against the corresponding name of the patient for each slide number.
The
HSCORE analysis was performed separately for the component of endometrium,
glandular
and stromal cells, and for the ectopic tissue (each observer performed 4
different HSCOREs
for each slide).
All data are reported as a mean + standard deviation. Statistical analysis was
performed by SPSS statistical package (Chicago, IL USA), using the Mann-
Whitney Sum
Rank test as appropriate.
FITC-PIF Flow Cytometry Studies: Non-pregnant infertile and first trimester
pregnant
patients at Millenova Immunology Laboratories who were undergoing fertility
treatments
signed a standard informed consent (CART, Institute, Chicago) as we have
previously
reported 17,18. All experiments were performed in accordance with the
guidelines and
regulations of CART, Institute, Chicago and with the approval from the
Institutional Review
Board of the University of Illinois at Chicago in March 2006, Dr. R. Roussev,
PI. The blood
was drawn as part of their work-up process with the use of excess specimen
without
-61-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
identifiers. We reported on FITC-PIF binding to CD14+ and CD3+, cells in both
pregnant
and non-pregnant patients.17 In addition binding to activated CD4+ cells were
documented.
Whether PIF targets regulatory T-cells was examined by using specific anti-
CD4+, anti-
CD25+ and anti-FoxP3+ antibodies. Binding was compared with scrambled-FITC-PIF
used
as a negative control. Following separation using Ficoll-Hypaque, PBMC were
isolated.
Binding to Isotype control served as negative controls. Two- three color
staining was done
using conventional techniques. Fluorescence measurements (20,000-50,000 gated
events per
sample) were performed in a Coulter Epics XLTM Flow Cytometer using System
II
software for data acquisition and analysis (Beckman Coulter, Inc., Miami, FL).
Results
In all eutopic endometria of patients with endometriosis and in healthy
controls
neither epithelial and stromal cells were negative for PIF specific staining.
The specific
staining for FOX-P3 was observed with low intensity in epithelial cells of
eutopic
endometrium, and in few stromal cells (putative Treg cells), regardless
whether patients or
controls. The percentage of cells expressing this antigen was 9.2+2.1% of
stromal cells and
32.8+0.9% in the glands, with an HSCORE of 78+7 in epithelial cells and 26+4
in stromal
cells. No differences were observed among the endometria of women with and
without
endometriosis
In the ectopic tissue of endometriotic lesions, 25 ovarian cyst walls and 10
peritoneal
implants, we observed the expression of specific staining for PIF mostly in
the epithelial
cells, even though not in all cells and glands, but with a segmental pattern,
in almost samples
examined. The stromal cells did not showed specific staining for PIF. The
specific staining
for PIF was observed in 65.9+9.4% of epithelial cells with an HSCORE of
138+49, whereas
in the stromal cells the specific staining for PIF was not observed.
The specific staining for FOX-P3 was observed with low intensity in epithelial
cells
of ectopic endometrium, and in few stromal cells (putative Treg cells), even
though in larger
amount than in eutopic samples. The percentage of stromal cells expressing
this antigens was
(17.2+ 3.2%) with a HSCORE of 114+35. The percentage of ectopic epithelial
cells showing
specific staining for FOX-P3 was (12.4+5.6%) with a HSCORE of (67+11). All
data set are
reported in Table 2. The cells showing specific staining for FoxP3 observed in
the stroma
(putative Treg cells) were mostly localized in the stoma surrounding the
ectopic glandular
cells expressing PIF such as shown by the double-staining immune
histochemistry.
-62-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
Table 2: Immunohistochemical findings for PIF and FOXP3 in eutopic and ectopic
endometrial samples
% of cells Positivity HSCORE
PIF
Eutopic endometrium
Epithelia cells 0
Stromal cells 0
Ectopic endometrium
Epithelia cells 65.9 9.4 ++ 138 49
Stromal cells 0
FOXP3
Eutopic endometrium
Epithelia cells 3.8 0.9 78 7
Stromal cells 13.2 2.1 26 4
Ectopic endometrium
Epithelia cells 12.4 1.5 67 11
Stromal cells 17.2 3.2 ++ 114 35
Co-expression of PIF and Tregs FoxP3+ cells was found in ectopic endometrium.
Therefore whether PIF also interacts with these subset of cells in PBMC was
examined. Flow
cytometry data showed that in non-pregnant women there is a dose dependent
increase in the
binding to CD4+/CD25+ cells, which is in contrast to scrambled-FITC-PIF used
as control
which binding was minimal (FIG. 1). Further experiments examined the specific
interaction
of FITC-PIF with FoxP3+ cells. Data showed that FITC-PIF targets those
specific systemic
immune cells in a dose dependent manner (FIG. 2). This reflects possible PIF
induced Tregs
regulation.
Discussion
In this study we showed for the first time that ectopic endometrial epithelial
cells
express PIF, which in contrast it was not found in the epithelial layer of
eutopic
endometrium,. Furthermore, PIF expression, evident only in the epithelial
layer, was co-
expressed with FoxP3+, an antigen expressed by regulatory T cells, which
expression was not
specific in the ectopic endometrium since it was found also in eutopic
tissues. Noteworthy,
-63-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
stromal cells in ectopic endometrium expressing FoxP3 (putative Treg cells)
were
preferentially observed in proximity of ectopic epithelial cells expressing
PIF, in a way that
suggest as PIF may target systemic FoxP3+ immune cells to engraft
endometriotic lesions to
play their role locally, downregulating the inflammatory process and immune
response to
ectopic endometrium. Our flow cytometry data indicated that FITC-PIF targets
CD4+/CD25+/FoXP3+ systemic immune cells confirming partially a functional link
between
PIF and regulation of Treg cells in establishing a local immune privilege in
endometriosis
lesions.
The presence of Tregs in eutopic and ectopic endometrium of women affected by
endometriosis has been reported (Budiu et al 2009, Berbic et al 2010). The
mRNA of FoxP3,
specific of Tregs, seems to be increased in ectopic endothelial tissue (Budiu
et al 2009).
Furthermore, it has been reported that the percentage of Tregs cells was
significantly
decreased in the peripheral blood in women with endometriosis as compared to
health
controls, whereas Treg cells percentage was increased in the peritoneal fluid
of these patients
with respect to controls. (Olkonska-Truchanowicz et al 2013). Our study
evidenced for the
first time the presence of Treg cells in the endometriotic lesion and their
number increase in
relation with PIF expression by epithelial cell lineage, suggesting a role for
these cells in
determining an immune privilege for endometriosis, such as similarly we
previously reported
for Fas-FasLigand system (Sbracia et al 2015). The role of these cells in
several diseases has
been highlighted, and in particular autoimmune disorders might be due to a
dysregulation of
T regulatory cells function, in particular of CD4+-CD25+-FoxP3+ regulatory
cells, which
play a key role in the suppression of many types of effector cells such as
macrophages, NK
cells, dendritic cells, cytotoxic T cells (Sathaguchi et el 2008, Berbic &
Fraser 2011).
The observation that PIF is highly and specifically expressed in the ectopic
endometrial epithelial cells is reported for the first time. We confirmed that
expression of PIF
is not expressed by normal eutopic endometrium but only in the ectopic tissue.
Such reflects
that PIF-like molecule re-expression is conditioned by the abnormal cells
likely multipotent
stems cells which also in certain cases lead to development of this diseases
as well as the
endometroid cancer. The lack of expression in stroma while the high expression
being in the
epithelial layer supports interaction mostly with rapidly proliferating cells
that may have even
an oncogenic potential. Under close observation of the cells expressing PIF it
shows the
presence of several large and even abnormal looking nuclei reflecting their
possible
hyperplastic features. As cells become more hyperplastic they tend to resemble
stem cells
which we showed that PIF regulates. Testing effect of PIF on singly cultured
bovine embryo
-64-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
was shown to promote their development when added in culture. In addition the
dependence
of murine embryos on endogenously secreted PIF was demonstrated since addition
of an anti-
PIF antibody severely blocked cultured embryos development. Thus PIF and stem
cells
appear to have a symbiotic relationship. Whether in the case of endometriosis
PIF re-
emergence to regulate the non-pregnant stems cells is plausible. This stems of
the fact that
PIF was shown effective in promoting both semi and allogenic stems cells
transplantation in a
murine graft vs host disease model. Thus confirming also in vivo the close
link and the direct
effect of PIF on healthy stem cells engraftment irrespective whether they are
matched or not.
Importantly in this study the beneficial graft vs leukemia was preserved
strongly indicating
the protective effect of PIF against tumors development. Thus the ensemble of
the data
support the view that PIF expression in the ectopic endometrium may reflect a
protect effect
against the localized inflammation.
The expression of FoxP3+ by epithelial cells was analyzed showing a
heterogenous
expression both in normal and ectopic endometrium with no differences in
intensity of
expression between ectopic and eutopic epithelial cells. This is suggests that
in contrast to
PIF, FoxP3, which is a transcription factor, is more likely to have a role in
regulate epithelial
transition and differentiation instead that reflecting a reaction to the
inflammatory process.
Whether PIF interacts with the immune suppressive Treg cells was examined.
This stems
from the fact that these cells expression appears to increase close to
implantation therefore
they are associated with development of immune tolerance for the embryo. The
observation
that PIF specifically targets those cells combined their co-localization
supports a possible
regulatory role systemically as shown both in vitro and in vivo setting in
CD4+ cells. It also
raises the possibility that similar action also takes place locally on the
site of endometriosis.
Indeed a number of preclinical models have documented that PIF has both a
local and
systemic integrated protective effect.
Our findings seem depict a possible biological scenario where endometriotic
stem
cells, which in turn may originate the disease, with the onset of inflammatory
reaction to
endometriotic implants by the host tissues, such as ovary or peritoneum, to
protect
themselves express and produce PIF to recall cells like Treg in order to
induce an immune
privileged environment. These findings may suggest a potential role for PIF
not only in the
pathogenesis of endometriosis, but also a potential target for a biological
treatment of this
diseases.
Example 2: PIF in endometriosis:a potential role in in inducing immune
privilege
-65-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
Endometriosis is a chronic inflammatory condition characterised by the growth
of
endometrial epithelial and stromal cells outside the uterine cavity. Besides
Sampson's theory
of retrograde menstruation, endometriosis pathogenesis is related to the
privileged
inflammatory microenvironment in these lesions. One of the pivotal factors are
T regulatory
FoxP3+ expressing T cells (Tregs). PreImplantation factor (PIF) is a peptide
essential for
pregnancy recognition and development. Besides immune modulatory function the
synthetic
PIF analog (sPIF) was successfully tested in multiple animal models. We report
that PIF re-
expresses in the epithelial ectopic cells in close proximity to FoxP3+ stromal
cells. We
provide evidence that PIF interacts with FoxP3+ cells and modulates cell
viability diversely
depending on cell source and presence of inflammatory mediators. Our finding
represent a
novel PIF-based mechanism in endometriosis, thus holding potential of novel
therapeutics.
Material and methods
Production and labelling of synthetic PIE Synthetic PIF15(MVRIKPGSANKPSDD)
and a scrambled peptide sequence with the same amino acids in random order
(GRVDPSNKSMPKDIA) were synthesized by solid-phase peptide synthesis (Peptide
Synthesizer, Applied Biosystems) employing Fmoc (9-fluorenylmethoxycarbonyl)
chemistry
at Bio-Synthesis, Inc. (Lewisville, TX, USA). Final purification was carried
out by reversed-
phase HPLC and identity was verified by matrix-assisted laser
desorption/ionization time-of-
flight mass spectrometry and amino acid analysis at >95% purity. Fluorescein
labeled FITC-
PIF and scrambled PIF (FITC- PIFscr) were also generated as previously
reported (Barnea et
al, 2012a; Barnea et al, 2012c). Anti-PIF monoclonal antibody against
MVRIKPGSANKPSDD was generated in (Genway, SanDiego, CA, USA).
Immunohistochemistry. Tissue specimens were obtained from 25 women who
underwent laparoscopic surgery for severe endometriosis according to the
revised criteria of
the American Society Reproductive Medicine. The surgical procedures were
carried out in
CERM-Hungaria Institute, Rome, Italy, from September 2014 through April 2015.
The
project had the approval of the Hospital's Ethical Committee. Samples were
obtained from
the ectopic endometrium, ovarian endometriomas and peritoneal implants. A
total of 25
eutopic endometria, 25 ovarian endometriomas and 10 peritoneal implants were
collected
from patients. Furthermore, the endometria of 10 healthy were used as
controls. Biopsy
samples were fixed in 4% neutral buffered formalin overnight and were
subsequently paraffin
embedded.
Before performing immunohistochemistry (IHC) tissue sections were stained with
-66-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
eosin and hematoxylin to select tissue with ectopic epithelial cells. Serial
sections of 51.tm
thick were used for IHC. Commercially available monoclonal antibodies were
used for the
detection of PIF (BioIncept, LLC, NJ, USA) and Foxp3 (number: sc-53876, Santa
Cruz, CA,
USA). IHC was performed according to manuals instructions. Briefly, tissue
sections were
dewaxed and re-hydrated and endogenous peroxidase activity quenched by
incubation with
0.3% hydrogen peroxide in methanol for 30 minutes at room temperature.
Sections were
exposed to a non-immune block with normal horse serum for 30 minutes at room
temperature. Incubation with the first antibody was carried out at 4 C
overnight with a
dilution of 1 to 100 for the monoclonal mouse anti-human PIF and with a
dilution of 1 to 50
for the monoclonal mouse anti-human Foxp3. Thereafter tissue sections were
labelled with
the avidin-biotin-peroxidase detection system Vectastain (Vector Laboratories,
Burlingthon
VT, USA). Each step was followed by meticulous washing with PBS. Finally, 3,3'-
diaminobenzidine (DAB) and/or 3-amino-9-ethylcarbazole (AEC) were used as
chromogens
for single or double staining. Counterstaining was performed with hematoxylin.
The positive
controls were PIF and Foxp3 tissues. Negative controls were performed by
replacing the
primary antibody with mouse immunoglobulin at the same concentration as the
primary
antibody.
A semi-quantitative statistical analysis of specific staining was performed
using an
HSCORE system (McCarty et al, 1985). The HSCORE was calculated using the
following
equation: HSCORE=OPi(i+1), where i is the intensity of staining with a value
of 1, 2, or 3
(weak, strong or very strong respectively) and Pi is the percentage of stained
cells for each
intensity, varying from 0% to 100%. For all samples, ten microscopic fields
were counted by
two observers blinded to the different groups. Three slides of each sample
were analysed. The
HSCORE analysis was performed separately for the component of endometrium,
glandular
and stromal cells, and for the ectopic tissue (each observer performed 4
different HSCOREs
for each slide). The intra-observer and inter-observer coefficient of
variation were 3.4% and
4.2% respectively.
FITC-PIF Flow Cytometry. Non-pregnant infertile and first trimester pregnant
patients at Millenova Immunology Laboratories who were undergoing fertility
treatments
signed a standard informed consent (CART, Institute, Chicago, usa). All
experiments were
performed in accordance with the guidelines and regulations of CART,
Institute, Chicago and
with the approval from the Institutional Review Board of the University of
Illinois at Chicago
in March 2006, Dr. R. Roussev, PI. The blood was drawn as part of their work-
up process
with the use of excess specimen without identifiers. We reported on FITC-PIF
binding to
-67-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
CD14+ and CD3+, cells in both pregnant and non-pregnant patients (Barnea et
al, 2012b). In
addition binding to activated CD4+ cells were documented. Whether PIF targets
regulatory T-
cells was examined by using specific anti-CD4+, anti-CD25+ and anti-FoxP3+
antibodies (BD,
Pharmingen, USA). Binding was compared with scrambled-FITC-PIF used as a
negative
control. Following separation using Ficoll-Hypaque, peripheral blood
mononuclear cells
(PBMC) were isolated. Binding to Isotype control served as negative controls.
Two- three
color staining was done using conventional techniques. Fluorescence
measurements (20,000-
50,000 gated events per sample) were performed in a Coulter Epics XLTM Flow
Cytometer
using System II software for data acquisition and analysis (Beckman Coulter,
Inc., Miami,
FL, USA).
Isolation and culture of in vitro cell models. After ethical approval and
informed
consent was obtained endometrial biopsies were collected via soft curette
(Pipelle de Cornier,
Laboratorie CCD, France) and stored in RNAlater at -80 C from women undergoing
laparoscopic surgery at the University Hospital Bern, Switzerland as described
previously
(Santi et al, 2011). The pelvic cavity was examined and any endometriotic
lesions were
removed and the presence of endometriosis confirmed via histological
investigation.
Endometriosis biopsies were collected from women both with (n = 4) and without
(n = 5)
endometriosis. All surgeries were performed during the proliferative phase of
the menstrual
cycle. Primary endometrial stromal cells (ESC) from women with and without
endometriosis
were prepared via a collagenase digestion and size exclusion protocol as
described previously
(McKinnon et al, 2013).
Isolated ESC were maintained in Iscoves's modified Eagle medium (IMEM)
(Invitrogen) supplemented with 10% fetal calf serum (fcs) (Invitrogen) and 1%
antibiotic/antimycotic (Invitrogen). Immortalized epithelial cell lines were
kindly provided by
Professor Kyo, Kanazawa, Japan and were isolated from eutopic endometrium, EM
E6/E7
(Kyo et al, 2003) and an ectopic endometriomas, EM'osis, (Bono et al, 2012).
Epithelial cells
were cultured in Dulbecco's modified Eagles medium (DMEM) (invitrogen) with
10% fcs
and 1% antibiotic/antimycotic.
Analysis of sPIF influence on cell viability. For the MTS assay cell were
plated into
96 well plates at a density of approximately 6 x 103/well. Sixteen hours prior
to treatments
cells were changed into a reduced serum media (0.5% FCS). sPIF was prepared by
diluting
into phosphate buffered saline (PBS) at a final concentration of 300nM.
Subsequent 1:3 serial
dilutions (111nM, 37nM, 12.3nM and 4.12nM) were prepared for a dose-response
assay.
Treatment was performed for a total of 48 hours with the treatment media
replaced after the
-68-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
first 24 hours. Cell viability was measured with the CellTiter 96 Aqueous
Solution Cell
Proliferation Assay (Promega, Madison, Wisconsin, USA). A control (without
sPIF) was
included for each experiment and all subsequent values expressed as a percent
of control. For
assay including TNFa recombinant human TNFa (R&D, Cat No; 210-TA, Minneapolis,
MN,
USA) was diluted into PBS at 10Ong/m1 and included in treatment media.
Whole transcriptome expression array. For whole transcriptome expression array
analysis EM'osis cells were plated into 6 well plates at approximately 2x
105/well and grown
until approximately 80% confluent. Sixteen hours prior to treatment cells were
transferred to
0.5 FCS media to synchronize cell cycles. Treatment with 100nM PIF was
performed for
48 hours with the treatment media replenished after 24 hours. After treatment
period cells
were lysed in Qiazol lysis buffer (Qiagen) and RNA isolated using the RNAeasy
mini kit
(Qiagen), as per the manufacturer's instructions. RNA quantity was measured
via the
Nanodrop 2000 (Witec) and quality via the Bioanalyser 2000 (Agilent). RNA was
considered
of sufficient quality if RNA integrity number (RIN) was above 9.8. A final
concentration for
all samples was approximately 200ng/ml. 6 RNA samples were analysed using the
Affymetrix platform according to the manual' s instructions (GeneChip Human
Transcriptome Array 2.0 and miRNA Array, Affymetrix).
Statistical Analysis. IHC data are reported as a mean standard deviation.
Statistical
analysis was performed by SPSS statistical package (Chicago, IL USA), using
the Mann-
Whitney Sum Rank test as appropriate. Analysis of cell viability in the in
vitro cell models in
response to PIF treatment was performed with a two-way analysis of variance
(ANOVA) test
with a post-hoc Sidak's multiple comparison test to determine if cell
viability was increased
by treatment conditions from control. Significance was considered a value for
p < 0.05 and
analysis performed with Graphpad Prism 6. The raw microarray data was
background-
corrected, normalized using the RMA method as implemented in the
R/Bioconductor package
affy (Gautier et al, 2004). Probe sets where redefined using the alternative
chip definition file
mogene2Ostmmentrezgcdf (Dai et al, 2005). Differential gene expression was
calculated
using the moderated t-test as described previously and implemented in the
R/Bioconductor
package limma (Ritchie et al, 2015).
The Pathway analysis: The output of limma was used to perform gene set
enrichment
analysis (GSEA) using the SetRank method. The key principle of this algorithm
is that it
discards gene sets that have initially been flagged as significant, if their
significance is only
due to the overlap with another gene set. It calculates the p-value of a gene
set using the
ranking of its genes in the ordered list of p-values as calculated by limma.
The following
-69-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
databases were searched for significant gene sets: BIOCYC, Gene Ontology,
KEGG,
Pathway Interaction Database, REACTOME, and WikiPathways.
Results
PIF imparts epithelial ectopic endometria. To determine the role of PIF in
endometriosis, tissue samples were obtained from women with or without
endometriosis
including the ectopic endometrium, ovarian endometriomas and peritoneal
implants during
the proliferative and secretory phases. We could not detect PIF positive cells
in both
epithelial and stromal cells of healthy controls (FIGs. 3A and 3B). However,
we detected PIF
positive cells in the epithelial compartment of ectopic endometria documenting
for the first
time re-expression of PIF outside of pregnancy. We hypothesized that PIF re-
expression may
be induced as a protective mechanism so we tested the effect of sPIF using an
in-vitro system
next.
To begin to elucidate the potential role of PIF in endometriosis we used both
epithelial cell lines isolated from eutopic endometrium (EM E6/E7) (Kyo et al,
2003) and
ectopic endometriomas (EM'osis) (Bono et al, 2012) and primary endometrial
stromal cells
(ESC) from women with and without endometriosis. We tested sPIF treatment in
ascending
dose in epithelial cells first. Indeed, sPIF treatment resulted in a
significantly decreased cell
viability compared to control (FIG. 3C solid line). Furthermore and supporting
PIF s role
during embryo implantation, sPIF increased the viability of eutopic
endometrium cells as
well (FIG. 3D dashed line) (Barnea et al, 2015). We tested sPIF effects in
stromal cells next.
In line with our previous reports and observation in human tissues (FIG. 3B),
sPIF treatment
did not alter stromal cell viability (Barnea et al, 2012a). Together, both PIF
re-expression and
sPIF treatment impart epithelial but not stromal endometriotic cells
significantly suggesting a
novel pathogenesis of endometriosis. To further dissect the underlying
mechanisms, we
performed a global gene array from sPIF treated ectopic endometrial epithelial
cells next.
sPIF modulates T-cell receptor signalling. Having a screening approach in
mind, we
performed a global gene array from sPIF s treated epithelial ectopic cell
lines as sPIF imparts
those cells (FIG. 4A). In line with previous reports (Paidas et al, 2010),
sPIF treatment
resulted in modulation of multiple signaling pathways. We detected significant
changes in
channels activity pathways with cation and potassium channels activity being
crucial. It
seems that these modifications are central to sPIF functions especially as one
of previously
identified sPIF targets is the potassium channel (Chen et al, 2016). Another
interesting
observation are the changes in multiple pathways involved in neuronal
development,
-70-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
plasticity, and protection. Again, this is in line with previous reports of
sPIF neuroprotective
effects (Mueller et al, 2015; Mueller et al, 2014; Weiss et al, 2012b).
Interestingly, we
detected changes in T-cell receptor signalling as well. This is of potential
interest as
involvement of T cell signalling in the pathogenesis of endometriosis was
reported previously
(Berbic & Fraser, 2011; Sakaguchi et al, 2008). Therefore, we performed a
detailed heat map
analysis and detected multiple genes being modulated by sPIF treatment (FIG.
4B). Of
special interest is the FoxP3 gene as FoxP3+ Tregs fail to decline during the
secretory phase
in endometriotic lesions which may lead to their survival and subsequent
establishment
(Berbic & Fraser, 2011). Given the importance of FoxP3 in the pathogenesis of
endometriosis
(Berbic & Fraser, 2011; Berbic et al, 2010), we aimed to confirm PIF
interactions with FoxP3
signalling next.
PIF interacts with FoxP3 positive cells. We stained FoxP3 and PIF in human
samples
and detected FoxP3+ cells in both eutopic and ectopic tissues (FIGs. 5A and
5B).
Interestingly, we detected increased number of FoxP3+ cells in the ectopic
endometria which
was especially evident during the secretory phase (Berbic et al, 2010; Budiu
et al, 2009). As
hypothesized, FoxP3+ cells in the stromal compartment of the ectopic
endometria was in
close proximity to the positive PIF cells in the epithelial compartment (FIG.
5B). This
observation suggests that the increased FoxP3 expression may be due to the re-
expression of
PIF in these lesions. To support the PIF and FoxP3+ interactions, we tested
PTV s effects in
well-defined system of peripheral non-pregnant blood mononuclear cells
(PBMCs). Using
flow cytometry, we documented a dose dependent increase in the binding of FITC-
PIF to
CD4+/CD25+/FoxP3+ cells (Fig.5C) confirming this specific interaction.
Finally, we aimed to detect sPIF effects in the inflammatory endometriotic
environment. Notably, multiple chemokines affect FoxP3+ cells function and
FoxP3 cells
regulate the cellular survival of ectopic endometria (Li et al, 2014). We
decided to test sPIF
effect on cell viability in the presence of TNFa as TNFa was identified as one
of the pivotal
chemokines involved in endometriosis and cell death (Han et al, 2015). Indeed,
in the
presence of TNFa sPIF s specific effect on cell viability in epithelial
ectopic cells was
abolished suggesting the pivotal involvement of TNFa (FIG. 5D) in sPIF' s
mediated effects.
This observation supports the notion of a disturbed balance between pro-
inflammatory and
anti-inflammatory factors in endometriosis affecting the cellular survival
(Tagashira et al,
2009).
Together, we hypothesize that PIF re-expresses in the epithelial compartment
of
ectopic endometria resulting in the recruitment of FoxP3+ cells into the
stromal
-71-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
compartment. The additional influx of chemokines and pro-inflammatory factors
such as
TNFa result in divergent sPIF effect on these cells creating a positive feed-
back loop. It leads
to cellular survival which again contribute to a significant control of the
immune privilege in
the endometriotic microenvironment (FIG. 5E). Thus, PIF may be a crucial
factor
contributing to the pathogenesis of endometriosis on the local level. However,
the immune
modulatory effect of sPIF applied peripherally as a therapeutic option may
lead to a
decreased inflammatory response on the global level, leading to the recovery
of proper
immune balance on the local level and finally cell death of ectopic
endometria. Studies
investigating this hypothesis are currently underway.
Discussion
Our study showed for the first time the presence of FoxP3+ cells in the
endometriotic
lesion in close proximity to PIF expressing epithelial cells. The presence of
Tregs in eutopic
and ectopic endometrium of women with endometriosis has been reported
previously (Berbic
et al, 2010; Budiu et al, 2009). FoxP3 mRNA is increased in ectopic
endothelial tissue and
the percentage of Tregs is significantly decreased in the peripheral blood of
women with
endometriosis, compared to healthy controls. However in the peritoneal fluid
the Treg
percentage is increased (Olkowska-Truchanowicz et al, 2013). This discrepancy
suggests a
differential immune modulatory system response on the local and global level.
Given that
sPIF imparts ectopic endometrial cells and interacts with FoxP3+ cells, we
hypothesize that
PIF re-expression may determine an immune privilege for endometriotic lesions.
A similar
role was reported for Fas-FasLigand system previously (Sbracia et al, 2016).
Notably, in
autoimmune disorders CD4+/CD25+/FoxP3+ dependent suppression of effector cells
(macrophages, natural killer, dendritic, and cytotoxic T cells) was reported
(Berbic & Fraser,
2011; Sakaguchi et al, 2008). As FoxP3+ cells were present in ectopic and
eutopic epithelial
cells, we hypothesize that FoxP3 may regulate epithelial transition and
differentiation as a
transcription factor instead of reflecting a reaction to the inflammatory
process as in case of
TNFa (Han et al, 2015).
Recent evidence suggest that apoptotic epithelial cells control Treg survival
and
abundance (Nakahashi-Oda et al, 2016). Such a role for PIF would be plausible
as cellular
expression increases close to implantation and thus it is associated with the
development of
immune tolerance for the embryo (Barnea et al, 2012a; Duzyj et al, 2010). The
observation
that PIF specifically targets those cells combined with their co-localization
support a potential
regulatory function, as shown in both in vitro and in vivo setting in CD4+
cells. It also raises
-72-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
the possibility that similar action takes place locally at the site of
endometriosis. Indeed a
number of preclinical models have documented that PIF has both a local and
global
integrated protective effects (Barnea et al, 2015). Furthermore, a potential
for a bi-directional
communication between PIF expressing epithelial cells and Treg cells exists.
PIF increased
cell viability of epithelial cells derived from the eutopic endometrium and
decreased cell
viability of cells derived from ectopic tissue (FIGs. 3C and 3D). These
results indicate that
PIF may have local paracrine effects on the epithelial cells within the
endometriotic lesion.
Furthermore, this effect may be modulated by the inflammatory microenvironment
as in case
of TNFa (FIG. 5E). The whole genome transcriptome analysis showed a
significant influence
on the expression of T cell receptor signalling pathways raising the
possibility that not only
does the paracrine effects of PIF influence the survival of ectopic epithelial
cells, but that it
also influences gene expression and the ability of these epithelial cells to
respond to the
immune regulating effects of infiltrating Tregs.
The variation in response to PIF between the eutopic derived and ectopic
derived
epithelial cells is also of interest. Although the pathogenesis of
endometriosis is still not
resolved, the notion that ectopic endometriotic cells have an inherent
characteristic that leads
to implantation is intriguing (Macer & Taylor, 2012). Such as a pathological
alteration may
be due to the ectopic environment or may have the origin in stem cells (Macer
& Taylor,
2012). Since embryo development largely dependent on PIF and sPIF promotes
cultured
embryos and stem cells development support such a premise (Shainer et al,
2016; Stamatkin
et al, 2011a; Stamatkin et al, 2011b). Although hypothetical the idea that a
peptide such as
PIF may be the missing link between the role of stems cells and immune
responses in the
pathogenesis of endometriosis is intriguing (Berbic & Fraser, 2011; Han et al,
2015; Macer &
Taylor, 2012). Lastly, endometriotic derived PIF may influence the
symptomology of
endometriosis as well. sPIF is a well described neuroprotective compound
(Barnea et al,
2015). Recent evidence suggest that the pain experienced by endometriosis
sufferers occurs
through an interaction with endometriotic associated nerve fibers detected in
close proximity
to the lesions (McKinnon et al, 2012; McKinnon et al, 2015). The
neuroprotective effects of
epithelia derived PIF may stimulate an enhanced nerve presence and influence
the
interpretation of pain in endometriosis. On the other hand peripheral sPIF and
the ensuing
reduced neuroinflammation may limit such neurotropic pain as well (Mueller et
al, 2014;
Weiss et al, 2012b).
In summary, our report suggest a possible biological scenario where
endometriotic
cells, as a possible lesion source, combined with the stimulation of the
inflammatory reaction
-73-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
to endometriotic implants, express and produce PIF to recall Treg cells in
order to induce an
immune privileged environment (to protect themselves). The presence of both
PIF and Treg
cells in the microenvironment may modulate the endometriotic lesions
regulating survival
and development, especially in reaction to inflammatory agents such as TNFa.
Our findings
suggest a potential role for PIF not only in the pathogenesis of
endometriosis, but also sPIF as
a potential compound for a biological treatment of endometriosis in the
future.
Example 3: sPIF has similar binding to lymphocytes compared to standard PIF.
The sPIF sequences provided herein (SEQ ID NOs: 20-29) are non-naturally
occurring mutants of PIF. In order to determine if they sPIF sequences
provided herein have
similar properties to PIF, FITC-PIF assays was performed to examine sPIF
binding to
monocytes. SEQ ID NO: 27 and SEQ ID NO: 29 were compared to natural PIF in
binding to
CD11 b (macrophage) and CD19 (B cell) monocytes.
Table 3: PIF v sPIF binding
PIF type CD11b % CD19 %
SEQ ID NO: 27 35.48% 5.60%
control PIF 27.94% 3.23%
SEQ ID NO: 29 33.54% 5.90%
control PIF 27.11% 4.34%
The sPIF sequences SEQ ID NOs: 27 and 29 showed higher binding affinity to
both
CD11 b and CD19 monocytes. This result lends support to the idea that the sPIF
sequences
provided here (SEQ ID NOs: 20-29) may bind to PIF receptors at least as well
as, if not better
than, native PIF.
Example 4: sPIF used as therapeutic in endometriosis animal model
To test whether sPIF may be effective for human treatment, an animal model
described in Greaves et al., Am J Pathol. 2014 Jul;184(7):1930-9, will be used
prior to
primate experimentation. That reference is incorporated by reference in its
entirety, however,
pertinent components of the experiment are disclosed below to describe how
induction of an
endometriotic condition in mice can be studied to test a potential treatment
for human use.
Animals
-74-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
Mature (approximately 8-week-old) female C57BL/6 mice will purchased from an
animal supplier and will be allowed to acclimate for 1 week before surgery.
Some
experiments may be performed using transgenic Cfslr-EGFP mice (MacGreen) that
were
originally generated as described in detail elsewhere. Two heterozygous
enhanced GFP+
males and two heterozygous enhanced GFP+ females were cross-bred with wild-
type (WT)
C57BL/6 mice to form a breeding colony and were bred under standard
conditions. Offspring
will be genotyped and classified as either MacGreen or WT. Mice will be
maintained on
standard chow and water available ad libitum and will be housed in
environmentally
controlled facilities illuminated between 7:00 am and 7:00 pm. All the animal
procedures will
be performed in accordance with legal requirements related to animal care and
under licensed
approval from the appropriate ethical authorities.
Mouse Model of Endometriosis
Menstruation will be induced in adult donor mice (approximately 8 weeks of
age)
using a protocol developed in-house set forth in PLoS One. 2014;9:e86378. In
brief,
ovariectomized mice (day 1) will be primed with s.c. injections of 100 ng of
estradio1-170
(days 7 to 9), treated with progesterone (P4; Sigma-Aldrich, Dorset, UK)
delivered via a
SILASTIC implant (Dow Corning Corp, Midland, MI) from days 13 to 19, and
injected with
5 ng of estradio1-170 in sesame oil on days 13, 14, and 15. Decidualization
will be induced in
one uterine horn using 20 pL of oil (day 15), and endometrial tissue in the
process of being
shed from the decidualized horn will be recovered from mice culled on day 19,
4 hours after
P4 withdrawal (removal of pellet) by opening the horn longitudinally in a
petri dish and
scraping the tissue away from the myometrial layer using a scalpel. The tissue
mass will be
suspended in 0.2 mL of PBS and passed through a 19-gauge needle before being
injected i.p.
into anesthetized recipient mice (approximately 8 weeks of age) that had been
previously
ovariectomized and implanted with an estradio1-170 (Sigma-Aldrich) SILASTIC
implant.
Tissue from one decidualized donor horn will be used to inoculate each
recipient mouse
(approximately 40 mg tissue/0.2 mL PBS per mouse). Three weeks after i.p.
injection,
recipient mice will be culled (photographs of the body cavity taken and were
taken the
lesions carefully dissected from surrounding tissue) and tissues will be
either fixed in 4%
normal buffered formaldehyde for histologic analysis or placed in RNA Save
(Geneflow Ltd,
Lichfield, UK) for RNA extraction. In total, endometriosis will be induced in
about 18 mice.
Of these, 10 mice will be used in the MacGreen reciprocal transfers: MacGreen
donor and
WT recipient (n = 6) and WT donor and MacGreen recipient (n = 4).
-75-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
Endometiral tissues will be fixed to slides and stained using known
immunostaining
procedures. Briefly, after fixation, mouse lesions will be stained using
H&Eosin; only lesions
containing identifiable stromal and epithelial compartments will be used for
further analysis.
Single-color IHC analysis will be performed according to standard
protocols24,26 with
citrate antigen retrieval followed by blocking endogenous peroxidase with 3%
H202 in
methanol. A streptavidin-biotin block (Vector Laboratories, Peterborough, UK)
will be
performed, and any nonspecific staining will be reduced using species-specific
blocking
solution (1:4 serum in Tris-buffered saline + 5% bovine serum albumin).
Incubation with the
appropriate dilution of primary antibody will be performed overnight at 4 C in
blocking
solution (Table 2). Biotinylated secondary antibodies (dilution 1:500) will be
diluted in
blocking solution and incubated at room temperature for 30 minutes. A
streptavidin¨
horseradish peroxidase conjugate (dilution 1:1000; Sigma-Aldrich) will be
diluted in Tris-
buffered saline and incubated at room temperature for 1 hour, followed by
visualization using
ImmPACT diaminobenzidine peroxidase substrate (Vector Laboratories). For anti-
CD31 IHC
analysis, nonspecific epitopes will be blocked with Bloxall blocking solution
(Dako UK Ltd,
Ely, UK), and the secondary antibody will be detected using a
streptavidin¨alkaline
phosphatase conjugate and visualized using PermaRed (Dako UK Ltd). Images will
be
captured using AxioVision software version 4.8.2.0 (Carl Zeiss Ltd, Cambridge,
UK) or a
similar system and a Provis microscope (Olympus America Inc., Center Valley,
PA). Dual
immunofluorescence was performed as previously herein with a secondary F (ab)
polyclonal
antibody to IgG (horseradish peroxidase), and antibody detection will be
performed using a
TSA system kit (PerkinElmer, Waltham, MA) labeled with either Cy3 (red) or
fluorescein
(green). For detection of the second antigen, sections will be microwaved for
2.5 minutes in
boiling citrate buffer, and the second primary antibody was applied overnight
at 4 C.
Secondary antibody will be detected as before with an appropriate TSA system,
and sections
will be counterstained with DAPI (dilution 1:500). Slides will be mounted in
PermaFluor
medium (Thermo Fisher Scientific, Waltham, MA). Sections will be evaluated
using an LSM
710 confocal microscope and ZEN 2009 software (Carl Zeiss Ltd) or another
microscope of a
similar likeness.
List of Antibodies Used in IHC Analysis
Antibody specificity (supplier)
Raised in Used on Dilution
ERP [AbD Serotec (#MCA19745)1 Mouse Human 1:50
-76-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
ERr3 [Santa Cruz Biotechnology (#SC-8974)1 Rabbit Mouse 1:500
ERa (Vector Laboratories) Mouse Mouse 1:50
CD31 (Dako) Rabbit Mouse 1:800
Cytokeratin [Sigma-Aldrich (#C2562)] Mouse Mouse 1:2000
Vimentin [Cell Signaling Technology Inc. (#5741)] Rabbit Mouse 1:600
GFP [Invitrogen Molecular Probes (#A11122) Rabbit Mouse 1:250
Biotinylated anti-mouse (Vector Laboratories) Goat 1:500
Biotinylated anti-rabbit (Vector Laboratories) Goat 1:500
Horseradish peroxidase¨conjugated streptavidin (Dako) NA 1:200
Variable Mouse
Sample size (No.) about 18
Recovery rate (%)
Number of lesions (mean SD)
Lesions with glands and stroma (%)
Mice will be divided into two sets: those left treated with PIF and those left
untreated.
Immunohistochemistry will reveal the experimental factors disclosed in the
table to the left.
In mice that are treated with one or more PIF peptides, we anticipate that
recovery rate of the
animals will improve, lesion size will decrease, and the number of lesions
will decrease as
compared to mice untreated with PIF peptide. By using this study we can
confirm that
induction of a menses-like event in mice was associated with the presence of
macrophages in
the uterus and that lesions formed in mice contained tissue-resident
macrophages and a
proinflammatory microenvironment. We expect that therapeutically effective
levels of PIF
peptide or their pharmaceutically acceptable salts will be able to lessen the
severity of the
endometriotic state induced in the mice.
Example 5: sPIF used as therapeutic for treatment of human endometriosis.
To test whether one or more PIF peptides will be effective to treat
endometriosis, sPIF
will be administered to healthy female patients and/or female patients
diagnosed with
-77-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
endometriosis. The purpose of this study is to examine the safety and
tolerability of synthetic
PreImplantation Factor (sPIF) in female patients with endometriosis. PIF
apparently initiates
both maternal tolerance preventing the loss/rejection of the fetus. Synthetic
PIF (sPIF)
successfully translates PIF endogenous properties to treatment of pregnant and
non-pregnant
immune disorders. sPIF was found to be effective in preclinical models of
autoimmunity and
transplantation (published). In FDA mandated safety studies in humans (Phase I
clinical
trial), sPIF administration to subjects diagnosed with autoimmune hepatitis
followed by
observation period demonstrated that at doses of sPIF ranging from 0.1 mg/kg
and 1.0 mg/kg
were tolerated safely. This study using sPIF a dose dependent design will
evaluate the safety,
tolerability and pain assessment of patients with endometriosis. The primary
endpoint will be
the total pain burden (dysmenorrhoea, deep dyspareunia and non-menstrual pain)
rated on a
daily basis by the patient using standard pain scales and analgesic intake as
the outcome
measures. Secondary endpoints will include the volume of the endometriotic
nodule assessed
clinically and on transvaginal ultrasound, the amount of pelvic tenderness on
clinical
examination and the presence of other endometriotic lesions at laparoscopy.
Arms Assigned Interventions
Active Comparator: SAD Normal sPIF Drug: sPIF
Within each cohort (at least 3 patients/cohort; at least 9 Other Name:
synthetic
subjects in total), patients with normal uterine function tests
PreImplantation Factor in
will be randomized in a 2:1 ratio (active drug: placebo) to Ringer's
lactate
receive a single dose of sPIF or placebo as follows:
Cohort 1: single dose 0.1 mg/kg sPIF Day 1 given
subcutaneously (SQ)
Cohort 2: single dose 0.5 mg/kg sPIF Day 1 given SQ
Cohort 3: single dose 1.0 mg/kg sPIF Day 1 given SQ
Placebo Comparator: SAD Normal Placebo Drug: Placebo
Within each cohort (at least 3 patients/cohort; at least 9 Other Name:
Ringer's lactate
subjects in total), patients with normal uterine function will to mimic
sPIF solution for
be randomized in a 2:1 ratio (active drug: placebo) to injection
receive a single dose of sPIF or placebo as follows:
Cohort 1: single dose placebo Day 1 given SQ
Cohort 2: single dose placebo Day 1 given SQ
Cohort 3: single dose placebo Day 1 given SQ
-78-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
Active Comparator: SAD Abormal sPIF Drug: sPIF
Within each cohort (at least 3 patients/cohort; at least 9 Other Name:
synthetic
subjects in total), subjects with abnormal uterine function PreImplantation
Factor
will be randomized in a 2:1 ratio (active drug: placebo) to
receive a single dose of sPIF or placebo as follows:
Cohort 1: single dose 0.1 mg/kg sPIF Day 1-5 given SQ
Cohort 2: single dose 0.5 mg/kg sPIF Day 1-5 given SQ
Cohort 3: single dose 1.0 mg/kg sPIF Day 1-5 given SQ
Placebo Comparator: single ascending dose (SAD) Drug: Placebo
Abnormal LFTs Placebo
Other Name: Ringer's lactate
Within each cohort (at least 3 patients/cohort; at least 9 to mimic sPIF
solution for
subjects in total), subjects with abnormal uterine function injection
tests will be randomized in a 2:1 ratio (active drug : placebo)
to receive a single dose of sPIF or placebo as follows:
Cohort 1: single dose placebo Day 1-5 given SQ
Cohort 2: single dose placebo Day 1-5 given SQ
Cohort 3: single dose placebo Day 1-5 given SQ
Active Comparator: multiple ascending dose (MAD) Normal Drug: sPIF
sPIF
Other Name: synthetic
Within each cohort ( at least 3 patients/cohort), subjects with
PreImplantation Factor
normal uterine function tests will be randomized in a 2:1
ratio (active drug: placebo) to multiple doses of sPIF
administered subcutaneously once a day for 5 consecutive
days (Days 1 to 5):
Cohort 1: 0.1 mg/kg sPIF Days 1-5 given SQ
Cohort 2: 0.5 mg/kg sPIF Days 1-5 given SQ
Cohort 3: 1.0 mg/kg sPIF Days 1-5 given SQ
Placebo Comparator: MAD Normal Placebo Drug: Placebo
Within each cohort (at least 3 patients/cohort), subjects with Other Name:
Ringer's lactate
normal liver function tests will be randomized in a 2:1 ratio to mimic sPIF
solution for
(active drug: placebo) to multiple doses of placebo injection
administered subcutaneously once a day for 5 consecutive
days (Days 1 to 5):
Cohort 1: placebo Days 1-5 given SQ
Cohort 2: placebo Days 1-5 given SQ
Cohort 3: placebo Days 1-5 given SQ
-79-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
Active Comparator: MAD Abnormal sPIF Drug: sPIF
Within each cohort (at least 3 patients/cohort), subjects with Other Name:
synthetic
abnormal uterine function tests will be randomized in a 2:1 PreImplantation
Factor
ratio (active drug: placebo) to multiple doses of sPIF
administered subcutaneously once a day for 5 consecutive
days (Days 1 to 5):
Cohort 1: 0.1 mg/kg sPIF Days 1-5 given SQ
Cohort 2: 0.5 mg/kg sPIF Days 1-5 given SQ
Cohort 3: 1.0 mg/kg sPIF Days 1-5 given SQ
Placebo Comparator: MAD Abnormal Placebo Drug: Placebo
Within each cohort (at least 3 patients/cohort), subjects with Other Name:
Ringer's lactate
abnormal uterine function tests will be randomized in a 2:1 to mimic sPIF
solution for
ratio (active drug: placebo) to multiple doses of placebo injection
administered subcutaneously once a day for 5 consecutive
days (Days 1 to 5):
Cohort 1: placebo Days 1-5 given SQ
Cohort 2: placebo Days 1-5 given SQ
Cohort 3: placebo Days 1-5 given SQ
After a sufficient observation period, the patients will be evaluated for
changes in
volume of the endometriotic nodule assessed clinically and/or on transvaginal
ultrasound,
degree of pelvic tenderness on clinical examination and the presence of other
endometriotic
lesions at laparoscopy. We expect that sPIF-treated cohorts will experience
less pain than
untreated patients presenting with abnormal uterine dysfunction. We also
expect that sPIF-
treated cohorts will experience reduced volume of endometriotic nodules, less
tenderness and
fewer lesions or lesions with decreased size as compared non treated subjects.
Although the present invention has been described in considerable detail with
reference to certain preferred embodiments thereof, other versions are
possible. Therefore the
spirit and scope of the appended claims should not be limited to the
description and the
preferred versions contain within this specification. Any patent applications
or other journal
articles disclosed herein are incorporated by reference in their entireties.
-80-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
References
1. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010; 362:
2389-98.
2. Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993 Jun
17;328(24):1759-69.
3. Macer ML, Taylor HS. Endometriosis and infertility: a review of the
pathogenesis and
treatment of endometriosis-associated infertility. Obstet Gynecol Clin North
Am.
2012 Dec;39(4):535-49.
4. Taylor HS. Endometrial cells derived from donor stem cells in bone
marrow
transplant recipients. JAMA. 2004; 292: 81-5.
5. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to
endometrium
and endometriosis. Stem Cells. 2007; 25: 2082-6.
6. Kao AP, Wang KH, Chang CC, Lee JN, Long CY, Chen HS, Tsai CF, Hsieh TH,
Tsai
EM. Comparative study of human eutopic and ectopic endometrial mesenchymal
stem
cells and the development of an in vivo endometriotic invasion model. Fertil
Steril.
2011; 95:1308-15.
7. Hufnagel D, Li F, Cosar E, Krikun G, Taylor HS. The Role of Stem Cells
in the
Etiology and Pathophysiology of Endometriosis. Semin Reprod Med. 2015
Sep;33(5):333-40.
8. Stamatkin CW, Roussev RG, Stout M, Absalon-Medina V, Ramu S, Goodman C,
Coulam CB, Gilbert RO, Godke RA, Barnea ER. PreImplantation Factor (PIF)
correlates with early mammalian embryo development-bovine and murine models.
Reprod Biol Endocrinol. 2011 May 15;9:63.
9. Stamatkin CW, Roussev RG, Stout M, Coulam CB, Triche E, Godke RA, Barnea
ER.
Preimplantation factor negates embryo toxicity and promotes embryo development
in
culture. Reprod Biomed Online. 2011b Oct;23(4):517-24.
10. Ramu S, Stamatkin C, Timms L, Ruble M, Roussev RG, Barnea ER.
PreImplantation
factor (PIF) detection in maternal circulation in early pregnancy correlates
with live
birth (bovine model). Reprod Biol Endocrinol. 2013 Nov 15;11:105
11. Barnea ER, Kirk D, Ramu S, Rivnay B, Roussev R, Paidas MJ.
PreImplantation
Factor (PIF) orchestrates systemic antiinflammatory response by immune cells:
effect
on peripheral blood mononuclear cells. Am J Obstet Gynecol. 2012,
Oct;207(4):313.e1-11.
12. Barnea ER, Kirk D, Paidas MJ. Preimplantation factor (PIF) promoting
role in
embryo implantation: increases endometrial integrin-a2r33, amphiregulin and
-81-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
epiregulin while reducing betacellulin expression via MAPK in decidua. Reprod
Biol
Endocrinol. 2012b Jul 12;10:50.
13. Paidas MJ, Krikun G, Huang SJ, Jones R, Romano M, Annunziato J, Barnea
ER. A
genomic and proteomic investigation of the impact of preimplantation factor on
human decidual cells. Am J Obstet Gynecol. 2010 May;202(5):459.
14. Duzyj CM, Barnea ER, Li M, Huang SJ, Krikun G, Paidas MJ.
Preimplantation factor
promotes first trimester trophoblast invasion. Am J Obstet Gynecol. 2010
Oct;203(4):402.
15. Weiss L, Bernstein S, Jones R, Amunugama R, Krizman D, Jebailey L,
Almogi-
Hazan 0, Yekhtin Z, Shiner R, Reibstein I. et al. Preimplantation factor (PIF)
analog
prevents type I diabetes mellitus (TIDM) development by preserving pancreatic
function in NOD mice. Endocrine. 2011;40:41-54.
16. Weiss L, Or R, Jones RC, Amunugama R, JeBailey L, Ramu S, Bernstein SA,
Yekhtin Z, Almogi-Hazan 0, Shainer R. et al. Preimplantation factor (PIF*)
reverses
neuroinflammation while promoting neural repair in EAE model. J Neurol Sci.
2012;312:146-157.
17. Azar Y, Shainer R, Almogi-Hazan 0, Bringer R, Compton SR, Paidas MJ,
Barnea
ER, Or R. Preimplantation factor reduces graft-versus-host disease by
regulating
immune response and lowering oxidative stress (murine model). Biol Blood
Marrow
Transplant. 2013 Apr;19(4):519-28
18. Roussev RG, Dons'koi By, Stamatkin C, Ramu S, Chernyshov VP, Coulam CB,
Barnea ER. Preimplantation factor inhibits circulating natural killer cell
cytotoxicity
and reduces CD69 expression: implications for recurrent pregnancy loss
therapy.
Reprod Biomed Online. 2013 Jan;26(1):79-87.
19. Paidas MJ, Annunziato J, Romano M, Weiss L, Or R, Barnea ER. Pregnancy
and
multiple sclerosis (MS): a beneficial association. Possible therapeutic
application of
embryo-specific pre-implantation factor (PIF*). Am J Reprod Immunol. 2012
Dec;68(6):456-64.
20. Shainer R, Azar Y, Almogi-Hazan 0, Bringer R, Compton SR, et al. (2013)
Immune
Regulation and Oxidative Stress Reduction by Preimplantation Factor following
Syngeneic or Allogeneic Bone Marrow Transplantation. Conference Papers in
Medicine 2013: 1-8
21. Tang Q, Bluestone JA. Regulatory T-cell physiology and application to
treat
autoimmunity. Immunol Rev. 2006 Aug;212:217-37.
-82-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
22. von Boehmer H. Can studies of tolerance ever lead to therapy? Ann Rheum
Dis. 2006
Nov;65 Suppl 3:41-3
23. Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+)
regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849-
859.
24. Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The
frequency and
suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of
patients
with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007 Nov
1;13(21):6301-11.
25. Bergmann C, Wild CA, Narwan M, Lotfi R, Lang S, Brandau S. Human tumor-
induced and naturally occurring Treg cells differentially affect NK cells
activated by
either IL-2 or target cells. Eur J Immunol. 2011 Dec;41(12):3564-73.
26. Wang W, Hodkinson P, McLaren F, MacKinnon A, Wallace W, Howie S, Sethi
T.
Small cell lung cancer tumour cells induce regulatory T lymphocytes, and
patient
survival correlates negatively with FOXP3+ cells in tumour infiltrate. Int J
Cancer.
2012 Sep 15;131(6):E928-37.
27. Budiu RA, Diaconu I, Chrissluis R, Dricu A, Edwards RP, Vlad AM. A
conditional
mouse model for human MUC1-positive endometriosis shows the presence of anti-
MUC1 antibodies and Foxp3+ regulatory T cells. Dis Model Mech. 2009 Nov-
Dec;2(11-12):593-603.
28. Berbic M, Hey-Cunningham AJ, Ng C, Tokushige N, Ganewatta S, Markham R,
Russell P, Fraser IS. The role of Foxp3+ regulatory T-cells in endometriosis:
a
potential controlling mechanism for a complex, chronic immunological
condition.
Hum Reprod. 2010 Apr;25(4):900-7.
29. Hsu SM, Raine L, Fanger H: Use of avidin-biotin-peroxidase complex
(ABC) in
immunoperoxidase techniques: a comparison between ABC and unlabeled antibody
(PAP) procedures. J Histochem Cytochem. 1981, 29, 577-80.
30. McCarty KS, Miller LS, Cox EB: Estrogen receptor analysis, correlation
of
biochemical and immunohistochemical methods using monoclonal anti-receptor
antibodies. Arch Pathol Lab Med 1985, 109, 716-21.
31. Olkowska-Truchanowicz J, Bocian K, Maksym RB, Bialoszewska A,
Wlodarczyk D,
Baranowski W, Zabek J, Korczak-Kowalska G, Malejczyk J. CD4+ CD25+ FOXP3+
regulatory T cells in peripheral blood and peritoneal fluid of patients with
endometriosis. Hum Reprod. 2013 Jan;28(1):119-24.
-83-
CA 03004055 2018-05-02
WO 2017/079430
PCT/US2016/060319
32. Sbracia M, Valeri C, Antonini G, Biagiotti G, Pacchiarotti A,
Pacchiarotti A. Fas and
Fas-Ligand in Eutopic and Ectopic Endometrium of Women With Endometriosis: The
Possible Immune Privilege of Ectopic Endometrium. Reprod Sci. 2016
Jan;23(1):81-
6.
33. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and
immune
tolerance. Cell. 2008 May 30;133(5):775-87.
34. Berbic M, Fraser IS. Regulatory T cells and other leukocytes in the
pathogenesis of
endometriosis. J Reprod Immunol. 2011 Mar;88(2):149-55.
-84-