Note: Descriptions are shown in the official language in which they were submitted.
CA 03005854 2018-05-18
WO 2017/138923 PCT/US2016/017135
DEGRADABLE CASING JOINTS FOR USE IN
SUBTERRANEAN FORMATION OPERATIONS
BACKGROUND
[0001] The present
disclosure relates to subterranean formation
operations and, more particularly, to degradable casing joints for completion
of
lateral wellbores.
[0002] Hydrocarbon producing
wells (e.g., oil producing wells, gas
producing wells, and the like) are created and stimulated using treatment
fluids
introduced into the wells to perform a number of subterranean formation
operations. A treatment fluid in this context refers generally to any fluid
that
may be used in a subterranean application in conjunction with a desired
function
and/or for a desired purpose, but unless otherwise indicated does not imply
any
particular action by the fluid or any component thereof.
[0003] Hydrocarbon producing
wells are first formed by drilling a
parent wellbore into a subterranean formation, involving circulating a
drilling
treatment fluid as the wellbore is bored out using a drill bit. The parent
wellbore
is then completed by positioning a casing string (which generally includes a
plurality of interconnected casing joints) within the wellbore and cementing
the
casing string in position. A casing string is used to line a wellbore, which
may be
cemented in place, and which increases the integrity of the wellbore and
provides a flow path between the casing surface and a selected subterranean
formation. This flow path may be used to introduce treatment fluids into the
surrounding formation to stimulate production, to receive the flow of
hydrocarbons from the formation, and to permit the introduction of fluids for
reservoir management or disposal purposes.
[0004] To make the
production of hydrocarbons more economical,
one or more lateral wellbores may be drilled from a wellbore and similarly
completed. Typically, to form a lateral wellbore, a whipstock is positioned in
the
casing string in the parent wellbore at a desired intersection, and then a
rotating
mill is used to form an access window through the casing sidewall. Thereafter,
a
drill bit is used to bore the lateral wellbore through the window.
1
CA 03005854 2018-05-18
WO 2017/138923 PCT/US2016/017135
BRIEF DESCRIPTION OF THE DRAWINGS
[0005] The following figures
are included to illustrate certain aspects
of the present disclosure and should not be viewed as exclusive examples. The
subject matter disclosed is capable of considerable modifications,
alterations,
combinations, and equivalents in form and function, as will occur to one
having
ordinary skill in the art and the benefit of this disclosure.
[0006] FIG. 1 is a schematic
cross-sectional view of an offshore oil
and gas platform using an exemplary well system subassembly.
[0007] FIG. 2 is a schematic
cross-sectional view of an enlarged
view of a well system subassembly.
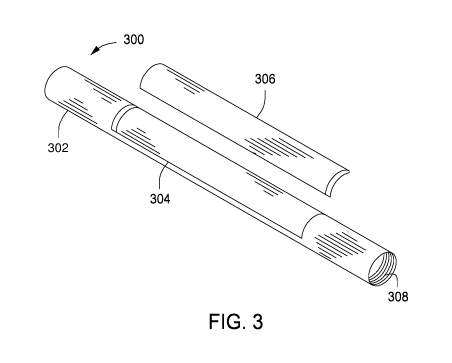
[0008] FIG. 3 is a schematic perspective view of a casing joint.
[0009] FIGS. 4A-4B are
schematic cross-sectional views of a casing
joint.
DETAILED DESCRIPTION
[0010] The present
disclosure relates to subterranean formation
operations and, more particularly, to the use of degradable materials to
temporarily occlude a window (Le., aperture(s)) formed through a sidewall of a
tubular body, (e.g., a casing joint), so that the window can be selectively
exposed (i.e., unoccluded) for lateral entry or exit, such as in the
completion of
lateral wellbores.
[0011] A casing joint may
have a tubular body, a window formed
through a sidewall of the tubular body, and a degradable material substrate
secured to the tubular body to initially occlude the window and form a fluidic
seal
therewith. The fluidic seal assures that no appreciable fluid will leak out
from
the occluded window while the degradable material substrate remains intact. A
typical casing joint may include, for example, a length of casing (e.g., steel
pipe.). Multiple casing joints may be assembled to form a casing string of a
desired length and specification for the wellbore in which it is installed.
Each
casing joint may be assembled, for example, by male to female threading either
between the two joints or with a threaded casing coupling. The window may be
a portion of the casing joint through which access to one or more specified
zones
of a subterranean formation to form a lateral wellbore is made. The window of
the present disclosure is pre-milled such that an opening in the sidewall
tubular
body of the casing joint is made prior to its placement in a wellbore. The
fluidic
2
CA 03005854 2018-05-18
WO 2017/138923
PCT/US2016/017135
seal (which may have grammatical variants below) refers to a seal that
prevents
fluid flow between opposite sides of the seal (e.g., between an inside of the
tubular body of the casing joint and through the occluded window to the
outside
of the tubular body of the casing joint). In the examples below, a sidewall
may
refer to a structural wall (including any surface thereof) of an apparatus
(e.g.,
the tubular wall of a casing joint), which may but does not necessarily extend
between the apparatus' top surface and the apparatus' bottom surface.
[0012] The
window of the casing joint described herein includes a
degradable material secured to the tubular body to occlude the window and form
a fluidic seal. The degradable materials may refer to materials that wholly or
partially degrade in the presence of a reactant in a downhole environment. The
degradable materials discussed in greater detail below, have a degradation
rate
of greater than about 0.1 (e.g., as low as 0.0095) milligrams per square
centimeters (mg/cm2) at 93.3 C (200 F) when exposed to a 15% potassium
chloride (KCI) solution. Accordingly, upon contact with an appropriate
reactant
in a wellbore, the degradable material degrades to expose the window for
lateral
exit or entry, such as for access to forming a lateral wellbore therethrough.
[0013] Thus,
the casing joints of the present disclosure may avoid or
reduce costs and equipment usage associated with milling a window in a casing
string, avoid or reduce costs and equipment usage associated with actuating a
pre-milled slidable sleeve window or use of a specialized milling tool to
remove a
smaller section of a pre-milled window, avoid or reduce operational costs
associated with running tools into and out of a wellbore during the formation
of a
lateral wellbore, and the like. Instead, the casing joints described herein
are
able to be placed downhole using standard equipment and to endure typical
downhole environments (e.g., temperature, pressure, salinity, and the like),
and
thereafter upon contact with a reactant have a window exposed (i.e.,
unoccluded) by degradation of the degradable material. Moreover, despite the
presence of the degradable material, the casing joint of the present
disclosure
behaves as a standard section of casing string as it is placed downhole and
until
degradation is stimulated by contact with a reactant.
Additionally, once
degradation of the degradable material is stimulated, the structural integrity
of
the casing window assembly is not hindered, as only the degradable material
itself is degraded, which may be designed to be merely large enough to
3
CA 03005854 2018-05-18
WO 2017/138923 PCT/US2016/017135
accommodate equipment for drilling and completing the lateral wellbore through
the window (e.g., a drilling assembly, a multilateral junction, and the like).
[0014] Not all features of
an actual implementation are described or
shown in this application for the sake of clarity. It is understood that
numerous
implementation-specific decisions may need to be made to achieve the
developer's goals, such as compliance with system-related, lithology-related,
business-related, government-related, and other constraints, which vary by
implementation and from time to time. While a developer's efforts might be
complex and time-consuming, such efforts would be, nevertheless, a routine
undertaking for those of ordinary skill in the art having benefit of this
disclosure.
[0015] At the very least,
and not as an attempt to limit the
application of the doctrine of equivalents to the scope of the claim, each
numerical parameter herein should at least be construed in light of the number
of reported significant digits and by applying ordinary rounding techniques.
[0016] While compositions and methods are described herein in
terms of "comprising" various components or steps, the compositions and
methods can also "consist essentially of" or "consist of" the various
components
and steps. When "comprising" is used in a claim, it is open-ended.
[0017] As used herein, the
term 'substantially" means largely, but
not necessarily wholly.
[0018] The use of
directional terms such as above, below, upper,
lower, upward, downward, left, right, uphole, downhole and the like are used
as
they are depicted in the figures, and unless otherwise indicated, the upward
direction being toward the top of the corresponding figure and the downward
direction being toward the bottom of the corresponding figure, the uphole
direction being toward the surface of the well and the downhole direction
being
toward the toe of the well.
[0019] As used herein, the
term "degradable," and grammatical
variants thereof (e.g., "degrade," 'degradation," "degrading," "dissolve,"
dissolving," and the like), refers to the dissolution or chemical conversion
of solid
materials such that reduced-mass solid end products result by at least one of
solubilization, hydrolytic degradation, biologically formed entities (e.g.,
bacteria
or enzymes), chemical reactions (including electrochemical and galvanic
reactions), thermal reactions, reactions induced by radiation, or combinations
thereof. The term "degradable," and its grammatical variants, does not imply
4
CA 03005854 2018-05-18
WO 2017/138923
PCT/US2016/017135
complete degradation, although complete degradation may take place without
departing from the scope of the present disclosure. The degradable materials
of
the present disclosure are at least partially degraded or wholly degraded,
where
at least partial degradation refers to a degradation of at least about 8 0%
(e.g.,
as low as 76%) of the volume of the degradable material.
[0020] The conditions for degradation are generally downhole
conditions in a wellbore environment where an external reactant may be used to
initiate or affect the rate of degradation. The external reactant (or simply
"reactant) is introduced into the wellbore (e.g., fluids, chemicals). However,
naturally occurring wellbore environment conditions may additionally influence
either initiation or the rate of degradation of the degradable material (e.g.,
pressure, pH, temperature, and the like), without departing from the scope of
the present disclosure. Accordingly, the term "wellbore environment" includes
both naturally occurring wellbore environments and materials or fluids
introduced into the wellbore.
[0021] Referring to FIG. 1, illustrated is an offshore oil and
gas
platform 100 that is able to use one or more of the exemplary casing joint
assemblies described herein. Even though FIG. 1 depicts an offshore oil and
gas
platform 100, the casing joints disclosed herein may be equally well suited
for
use in or on other types of oil and gas rigs, such as land-based oil and gas
rigs
or rigs located at any other geographical site. The platform 100 may be a semi-
submersible platform 102 centered over a submerged oil and gas formation 104
located below the sea floor 106. A subsea conduit 108 extends from the deck
110 of the platform 102 to a wellhead installation 112 that includes one or
more
blowout preventers 114. The platform 102 has a hoisting apparatus 116 and a
derrick 118 for raising and lowering pipe strings, such as a drill string 120,
within the subsea conduit 108.
[0022] As depicted, a parent wellbore 122 has been drilled
through
the various earth strata, including the formation 104. The term "parent"
wellbore
is used herein to designate a wellbore from which another wellbore is drilled.
It
is to be noted, however, that a parent or parent wellbore does not necessarily
extend directly to the earth's surface, but could instead be a branch of
another
wellbore. A casing string 124 is at least partially cemented within the parent
wellbore 122.
5
CA 03005854 2018-05-18
WO 2017/138923
PCT/US2016/017135
[0023] A
casing joint 126 may be interconnected between elongate
portions or lengths of the casing string 124 and positioned at a desired
location
within the wellbore 122 where a lateral wellbore 128 is to be drilled (already
drilled as shown). The term 'lateral" wellbore is used herein to designate a
wellbore that is drilled outwardly from its intersection with another
wellbore,
such as a parent wellbore. Moreover, a lateral wellbore may have another
lateral wellbore drilled outwardly therefrom. A multilateral assembly 130 may
be positioned within the casing string 124 and/or the casing joint 126. The
multilateral assembly 130 may deflect one or more cutting tools (i.e., drill
bit(s))
through an open window 132 formed in a sidewall of the casing joint 126. The
window 132, as described herein, is occluded by a degradable material that
degrades in a wellbore environment (e.g., upon contact of a reactant) to cause
the window 132 to become exposed (i.e., unoccluded), such that the one or
more cutting tools of the multilateral assembly 130 can drill the lateral
wellbore
128.
[0024] The
multilateral assembly 130 may include a variety of
additional components in addition to the drill bit(s) including, but not
limited to,
a whipstock, a motor, a multilateral junction, one or more screens, a motor, a
deflector, one or more latching mechanisms for anchoring the multilateral
assembly 130 or components thereof in the parent wellbore 122 and/or the
lateral wellbore 128, and the like, without departing from the scope of the
present disclosure.
[0025] While
FIG. 1 depicts a vertical section of the parent wellbore
122, the casing joints described may be equally applicable for use in
wellbores
having other directional configurations including horizontal wellbores,
deviated
wellbores, combinations thereof, and the like. Moreover, while FIG. 1 depicts
a
deviated and horizontal section of the lateral wellbore 128, the lateral
wellbore
128 may extend from the parent wellbore 122 in other directional
configurations
including vertical wellbores depending on the directional configuration of the
parent wellbore 122 and the location of desired reservoirs in the formation
104.
[0026]
Referring now to FIG. 2, with continued reference to FIG. 1,
illustrated is an enlarged view of the junction or intersection between the
main
wellbore 122 and the lateral wellbore 128, as shown in FIG. 1. As illustrated,
the multilateral assembly 130 may be coupled to or otherwise arranged adjacent
to various tools (e.g., a measurement while drilling tool) and/or tubular
lengths
6
CA 03005854 2018-05-18
WO 2017/138923 PCT/US2016/017135
202, and either arranged within or interconnected with a portion of the casing
string 124. Such tools and/or tubular lengths 202 may be used to determine the
appropriate circumferential angle and orientation for the formation of the
lateral
wellbore 128 through the window 132 of the casing joint 126. As illustrated,
the
multilateral assembly 130 may include a deflector surface 204 operable to
direct
a cutting tool through the window 132 of the casing joint 126 to create the
lateral wellbore 128.
[0027] The casing joint 126
may be coupled to and otherwise
interpose separate elongate segments of the casing string 124. Each end of the
casing joint 126 may be threaded to the corresponding elongate lengths of the
casing string 124. Alternatively, the casing joint 126 may be coupled to the
casing string 124 via couplings 206 made of, for example, steel or a steel
alloy
(e.g., low alloy steel). One or more ends of the casing joint 126 may
alternatively be tapered to wedge within corresponding elongate lengths of the
casing string 124, or the corresponding elongate lengths of the casing string
124
may be tapered to wedge into the ends of the casing joint 126.
[0028] Referring now to FIG.
3, illustrated is a casing joint 300,
which may be substantially similar or the same as casing joint 126 in FIGS. 1
and 2. As shown, the casing joint 300 has a tubular body 302 and a window 304
formed in the sidewall of the tubular body 302. The tubular body 302 may be
made from a corrosive-resistant material such as steel (e.g., 13-chromium
steel,
28-chromium steel), or other stainless steel or nickel alloys that are
corrosive-
resistant. One or both ends of the tubular body 302 of the casing joint 300
may
comprise threading 308 (one shown), as previously described, to accept an
adjacent casing string or joint. The threading 308 may be male or female
depending on the configuration of the adjacent casing string or casing joint
the
casing joint 300 is intended to be coupled or connected.
[0029] As shown, the window
304 is a pre-milled window in the
sidewall of the tubular body 302, such that a fluidic opening is formed
through
the window 304. The window 304 can be formed by any mechanism, including,
but not limited to, casting (i.e., the mold is formed with the window 304
opening), etching (which may require several etch passes until the window 304
is formed), cutting, chiseling, and the like, and any combination thereof. The
shape of the window 304 is determined based on the drill bit size and shape
that
is to be used to form the lateral wellbore 128 (FIGS. 1 & 2). As shown, the
7
CA 03005854 2018-05-18
WO 2017/138923
PCT/US2016/017135
window 304 is rectangle-shaped; however, the window 304 may have a different
shape, such as a teardrop-shape, a circle-shape, an oval-shape, or a square-
shaped, without departing from the scope of the present disclosure. For
example, the window 304 may be tear-drop shaped to accommodate the angle
at which a drill bit is defected to drill the lateral wellbore 128 (FIGS. 1 &
2)
through the window 304.
[0030] The
casing joint 300 includes a degradable material 306
secured to the tubular body 302 to occlude the window 304; the degradable
material 306 is shown detached from the tubular body 302 merely for
illustrative
purposes. The degradable material is secured to the tubular body 302 to
occlude the window 304 and form a fluidic seal between the interior of the
casing
joint 300 and the exterior of the casing joint 300. This fluidic seal allows
operations, such as primary cementing operations, to be performed as normal
despite the presence of the degradable material 306. After such operations are
" completed, the degradable material 306 is degraded (e.g., by contact with a
reactant) to remove the degradable material 306 and expose the opened window
304. That is, the casing joint 300 may be cemented within a wellbore (e.g.,
parent wellbore 124 of FIGS. 1 & 2) prior to degrading the degradable material
306. The degradable material 306 occludes and forms the fluidic seal by any
means that is compatible with the material of the tubular body 302, the
material
of the degradable material 306, and the wellbore environment. For example,
the degradable material 306 is secured to the tubular body by one or more of
an
adhesive, an epoxy, an elastomer, a weld, brazing, a mechanical seal (e.g., a
shrink fit, a metal-to-metal seal, a threaded engagement, and the like), and
the
like, and any combination thereof.
[0031] As
previously discussed, the degradable material of the
present disclosure has a degradation rate of greater than 0.0095 mg/crn2 at
93.3 C when exposed to a 15% KCI solution (a 'reactant"). The degradable
material loses typically greater than 0.095% of its total mass per day at 93.3
C
in a 15% KCI solution. Typically, the degradation of the degradable material
for
use in forming the casing joint of the present disclosure degrades in the
range of
2 hours to 120 days, encompassing any value and subset therebetween. Each
of these values depend on a number of factors including, but not limited to,
the
type of degradable material, the type of reactant contacting the degradable
material, the wellbore environment, and the like, and any combination thereof.
8
CA 03005854 2018-05-18
WO 2017/138923
PCT/US2016/017135
The degradation rate of the degradable material 306 described herein allows
performance of various wellbore operations while the casing joint 300 is in a
downhole environment prior to degradation, such as where a fluidic seal is
required (e.g., primary cementing operations). The degradation rate thus
permits the casing joint to 300 function as a typical casing joint (i.e., one
lacking
a degradable window) during the normal course of operations, followed by
subsequent degradation of the degradable material 306 after those operations
are complete, as described herein.
[0032] The
degradable material described herein may degrade by a
number of mechanisms. For example, the degradable material may degrade by
swelling, dissolving, undergoing a chemical change, undergoing an
electrochemical change, undergoing thermal degradation in combination with
any of the foregoing, and any combination thereof. Degradation by swelling
involves the absorption by the degradable material of a fluid in the wellbore
environment such that the mechanical properties of the degradable material
degrade. That is, the degradable material continues to absorb the fluid until
its
mechanical properties are no longer capable of maintaining the integrity of
the
degradable material and it at least partially falls apart.
Degradation by
dissolving involves use of a degradable material that is soluble or otherwise
susceptible to a fluid in the wellbore environment (e.g., an aqueous fluid or
a
hydrocarbon fluid), such that the fluid is not necessarily incorporated into
the
degradable material (as is the case with degradation by swelling), but becomes
soluble upon contact with the fluid. Degradation by undergoing a chemical
change may involve breaking the bonds of the backbone of the degradable
material (e.g., polymer backbone) or causing the bonds of the degradable
substance to crosslink, such that the degradable substance becomes brittle and
breaks into small pieces upon contact with even small forces expected in the
wellbore environment. Degradation by undergoing an electrochemical change
involves corrosion (e.g., galvanic corrosion) by an electrolytic process
(e.g.,
oxidation of the degradable material). Thermal degradation involves a chemical
decomposition due to heat, such as the heat present in a wellbore environment.
Thermal degradation of some degradable substances described herein may occur
at wellbore environment temperatures of greater than 93.3 C (200 F), or
greater than 50 C (122 F). Each degradation method may work in concert with
9
CA 03005854 2018-05-18
WO 2017/138923 PCT/US2016/017135
one or more of the other degradation methods, without departing from the
scope of the present disclosure.
[0033] Although the
degradation rate of the degradable material is
defined in terms of exposure to a 15% KCI solution at 93.3 C, other reactant
= 5
types can be used to initiate or accelerate the degradation of the degradable
material. Reactants can be introduced into a formation (e.g., pumped) to
contact the degradable material and initiate or accelerate degradation
thereof.
As discussed previously, the reactants may be selected to work in concert with
the surrounding wellbore environment to enhance or delay degradation
compared to the combination of the reactant(s) and the degradable material in
the absence of the wellbore environment, without departing from the scope of
the present disclosure. Examples of suitable reactants include, but are not
limited to, an acid, a base, an electrolyte (e.g., a brine), and any
combination
thereof.
[0034] Suitable acid
reactants may also include chemical etchants
that are capable of etching completely through the degradable material to
expose the window (unocclude) of the casing joint described herein. The acid
reactants are selected to have a pKa of less than 1.84. Examples of suitable
acid reactants include, but are not limited to, ferric chloride, hydrochloric
acid,
hydroiodic acid, perchloric acid, nitric acid, sulfuric acid, hydrobromic
acid,
chloric acid, acetic acid, boric acid, carbonic acid, citric acid,
hydrofluoric acid,
oxalic acid, phosphoric acid, picric acid, acetic-picral, p-toluenesulfonic
acid,
methanesulfonic acid, hydronium ion, bromic acid, perbromic acid, iodic acid,
periodic acid, fluoroantimonic acid, triflic acid, fluorosulfuric acid, and
any
combination thereof.
[0035] Suitable base
reactants may include any base suitable for use
in a subterranean formation and capable of degrading the degradable materials
as described herein. Examples of suitable base reactants include, but are not
limited to a hydroxide (e.g., sodium hydroxide, magnesium hydroxide, barium
hydroxide, lithium hydroxide, potassium hydroxide, strontium hydroxide, cesium
hydroxide, rubidium hydroxide, and the like), an oxide (e.g., magnesium oxide,
calcium oxide, barium oxide, silicon dioxide, aluminum oxide, beryllium oxide,
and the like), butyl lithium, lithium diisopropylamide, lithium diethylamide,
sodium hydride, sodium amide, lithium bis(trimethylsilyl)amide, and any
combination thereof.
CA 03005854 2018-05-18
WO 2017/138923 PCT/US2016/017135
[0036] The electrolyte
reactants described herein may be solutions
of salt (e.g., a salt dissolved in water), which provides free ions for
reacting with
the degradable material to initiate or accelerate degradation of the
degradable
material. Common free ions in an electrolyte reactant include, but are not
limited to, sodium (Na) ions, potassium (K+) ions, calcium (Ca2+) ions,
magnesium (Mg2+) ions, chloride (Cl) ions, bromide (B-) ions, hydrogen
phosphate (HP042-) ions, hydrogen carbonate (HCO3-) ions, and any combination
thereof. The electrolyte reactant can be a fluid that is introduced into a
wellbore
to contact the degradable material or a fluid emanating from the wellbore
itself,
such as from a surrounding subterranean formation, without departing from
the
scope of the present disclosure.
[0037] The degradable
material may be a degradable metal material
and the reactant causes degradation thereof by corrosion. Examples of suitable
degradable metal materials include, but are not limited to, gold, a gold-
platinum
alloy, silver, nickel, a nickel-copper alloy, a nickel-chromium alloy, copper,
a
copper alloy (e.g., brass, bronze, and the like), chromium, tin, aluminum, an
aluminum alloy, iron, an iron alloy, magnesium, a magnesium alloy, beryllium,
tungsten, zinc, a zinc alloy, and any combination thereof.
[0038] Suitable magnesium
alloys include alloys having magnesium
at a concentration in the range of from 38% to 99% by weight of the
magnesium alloy, encompassing any value and subset therebetween. Each of
these values may depend on a number of factors including, but not limited to,
the type of magnesium alloy, the desired degradability of the magnesium alloy,
and the like. Magnesium alloys comprise at least one other ingredient besides
the magnesium. The other ingredients can be selected from one or more
metals, one or more non-metals, or a combination thereof.
[0039] Suitable metals that
may be alloyed with magnesium include,
but are not limited to, lithium, sodium, potassium, rubidium, cesium,
beryllium,
calcium, strontium, barium, aluminum, gallium, indium, tin, thallium, lead,
bismuth, scandium, titanium, vanadium, chromium, manganese, iron, cobalt,
nickel, copper, zinc, yttrium, zirconium, niobium, molybdenum, ruthenium,
rhodium, palladium, praseodymium, silver, lanthanum, hafnium, tantalum,
tungsten, terbium, rhenium, osmium, iridium, platinum, gold, neodymium,
gadolinium, erbium, oxides of any of the foregoing, and any combinations
thereof.
11
CA 03005854 2018-05-18
WO 2017/138923 PCT/US2016/017135
[0040] Suitable non-metals
that may be alloyed with magnesium
include, but are not limited to, graphite, carbon, silicon, boron nitride, and
combinations thereof. The carbon can be in the form of carbon particles,
fibers,
nanotubes, fullerenes, and any combination thereof. The graphite can be in the
form of particles, fibers, graphene, and any combination thereof. The
magnesium and its alloyed ingredient(s) may be in a solid solution and not in
a
partial solution or a compound where inter-granular inclusions may be present.
The magnesium and its alloyed ingredient(s) may be uniformly distributed
throughout the magnesium alloy; however, some variations in the distribution
of
particles of the magnesium and its alloyed ingredient(s) can occur. The
magnesium alloy may also or instead be a sintered construction.
[0041] Suitable aluminum
alloys include alloys having aluminum at a
concentration in the range of from 38% to 99% by weight of the aluminum alloy,
encompassing any value and subset therebetween. Each of these values may
depend on a number of factors including, but not limited to, the type of
aluminum alloy, the desired degradability of the aluminum alloy, and the like.
The aluminum alloys may be wrought or cast aluminum alloys and comprise at
least one other ingredient besides the aluminum. The other ingredients can be
selected from one or more any of the metals, non-metals, and combinations
thereof described above with reference to magnesium alloys, with the addition
of
the aluminum alloys additionally being able to comprise magnesium.
[0042] Suitable zinc alloys
include alloys having zinc at a
concentration in the range of from 38% to 99% by weight of the zinc alloy,
encompassing any value and subset therebetween. Each of these values may
depend on a number of factors including, but not limited to, the type of zinc
alloy, the desired degradability of the zinc alloy, and the like. The zinc
alloys
may be wrought or cast zinc alloys and comprise at least one other ingredient
besides the zinc. The other ingredients can be selected from one or more of
any
of the metals, non-metals, and combinations thereof described above with
reference to magnesium alloys, with the addition of the zinc alloys
additionally
being able to comprise magnesium and/or aluminum.
[0043] Alternatively or in
combination with the degradable metal
materials (i.e., a combination of both degradable metal and degradable non-
metal materials), the degradable material may be a non-metal degradable
material that at least partially degrades in a wellbore environment upon
contact
12
CA 03005854 2018-05-18
WO 2017/138923
PCT/US2016/017135
with one or more reactants. Suitable non-metal degradable materials include,
but are not limited to, a polyurethane rubber (e.g., cast polyurethanes,
thermoplastic polyurethanes, polyethane polyurethanes); a polyester-based
polyurethane rubber (e.g., lactone polyester-based thermoplastic
polyurethanes); a polyether-based polyurethane rubber; a thiol-based polymer
(e.g., 1,3,5,-triacryloylhexahydro-1,3,5-triazine); a thiol-epoxy polymer
(e.g.,
having an epoxide functional group, such as bisphenol-A diglycidyl ether,
triglycidylisocyanurate, and/or trimethylolpropane triglycidyl ether); a
hyaluronic
acid rubber; a polyhydroxobutyrate rubber; a polyester elastomer; a polyester
amide elastomer; a starch-based resin (e.g., starch-poly(ethylene-co-vinyl
alcohol), a starch-polyvinyl alcohol, a starch-polylactic acid, starch-
polycaprolactone, starch-poly(butylene succinate), and the like); a
polyethylene
terephthalate polymer; a polyester thermoplastic (e.g., polyether/ester
copolymers, polyester/ester copolymers); a polylactic acid polymer; a
polybutylene succinate polymer; a polyhydroxy alkanoic acid polymer; a
polybutylene terephthalate polymer; a polysaccharide; chitin; chitosan; a
protein; an aliphatic polyester; poly(E-caprolactone); a
poly(hydroxybutyrate);
poly(ethyleneoxide); poly(phenyllactide); a poly(amino
acid); a
poly(orthoester); polyphosphazene; a polylactide; a polyglycolide; a
poly(anhydride) (e.g., poly(adipic anhydride), poly(suberic anhydride),
poly(sebacic anhydride), poly(dodecanedioic anhydride), poly(maleic
anhydride),
and poly(benzoic anhydride), and the like); a polyepichlorohydrin; a copolymer
of ethylene oxide/polyepichlorohydrin; a terpolymer of
epichlorohydrin/ethylene
oxide/allyl glycidyl ether; copolymers thereof; terpolymers thereof; and any
combination thereof.
[0044]
Referring now to FIGS. 4A-46, illustrated are two cross-
sectional views of a casing joint 400. FIGS. 4A and 4B show two configurations
in which the degradable material 406 can be secured to the tubular body 402 to
occlude the window 404. Referring first to FIG. 4A, shoulders 408 are defined
in
the sidewall of the tubular body 402 at the window 404. As shown, the
shoulders 408 are defined at each end of the tubular body 402 at the window
404 along the inner diameter of the tubular body 402, such that the window 404
occupies a greater circumference of the inner radial surface of the tubular
body
402 than the outer radial surface of the tubular body 402. Although the
shoulders 408 are defined along the inner radial surface of the tubular body
402,
13
CA 03005854 2018-05-18
WO 2017/138923
PCT/US2016/017135
such shoulders 408 may alternatively be defined along the outer radial surface
of
the tubular body 402, without departing from the scope of the present
disclosure. Moreover, a single shoulder 408 at only one end of the window 404
may additionally be defined rather than two shoulders 408 at each end, without
departing from the scope of the present disclosure. The degradable material
406 is received by the shoulders 408 and otherwise flush with the radial
surface(s) that does not have a shoulder 408 (e.g., the outer radial surface,
as
shown in FIG. 4A). Referring now to FIG. 4B, the degradable material 406 is
flush with both the outer radial surface and the inner radial surface of the
tubular body 402, such that no shoulders, protrusion, or depression forming
the
ends of the window 404.
[0045] Additionally or alternatively, a shoulder, protrusion,
and/or
depression (e.g., divot) can be located at any position along one or both
edges
of the window 404 and extending from or into one or both edges of the tubular
body 402. Such shoulders, protrusions, and/or depressions, for example, may
be along the inner radial surface of the tubular body 402 (as shown in FIG.
4A),
along the outer radial surface of the tubular body 402, and/or any surface
therebetween. The shoulders, protrusions, and/or depressions can receive the
degradable material 406 and aid in securing the degradable material 406 to the
tubular body 402 to occlude the window 404, and work synergistically with the
one or more mechanisms for forming a fluidic seal with the degradable material
406, as described above.
[0046] While various examples have been shown and described
herein, modifications may be made by one skilled in the art without departing
from the scope of the present disclosure. The examples described here are
exemplary only, and are not intended to be limiting. Many
variations,
combinations, and modifications of the examples disclosed herein are possible
and are within the scope of the disclosure. Accordingly, the scope of
protection
is not limited by the description set out above, but is defined by the claims
which
follow, that scope including all equivalents of the subject matter of the
claims.
[0047] Examples disclosed herein include:
[0048] Example A: A casing joint comprising: a tubular body; a
window formed through a sidewall of the tubular body; and a degradable
material secured to the tubular body to occlude the window, wherein the
degradable material has a degradation rate of greater than 0.0095 milligrams
14
CA 03005854 2018-05-18
WO 2017/138923 PCT/US2016/017135
per square centimeters (mg/cm') at 93.3 C when exposed to a 15% potassium
chloride solution.
[0049]
Example B: A method comprising: lining a wellbore
with casing that includes a casing joint interconnected in the casing, wherein
the
casing joint includes a tubular body, a window formed through a sidewall of
the
tubular body, and a degradable material secured to the tubular body to occlude
1
the window, wherein the degradable material has a degradation rate of greater
than 0.0095 milligrams per square centimeters (mg/cm2) at 93.3 C when
exposed to a 15% potassium chloride solution; and degrading the degradable
material to expose the window.
[0050]
Example C: A system comprising: a wellbore lined
with casing that includes a casing joint interconnected in the casing, wherein
the
casing joint includes a tubular body, a window formed through a sidewall of
the
tubular body, and a degradable material secured to the tubular body to occlude
the window, wherein the degradable material has a degradation rate of greater
than 0.0095 milligrams per square centimeters (mg/cm2) at 93.3 C when
exposed to a 15% potassium chloride solution.
[0051] Examples A, B, and C
may have one or more of the following
additional elements in any combination:
[0052] Element 1: Wherein
the degradable material is a degradable
metal.
[0053] Element 2: Wherein
the degradable material is a degradable
metal selected from the group consisting of gold, a gold-platinum alloy,
silver,
nickel, a nickel-copper alloy, a nickel-chromium alloy, copper, a copper
alloy,
chromium, tin, aluminum, an aluminum alloy, iron, an iron alloy, magnesium, a
magnesium alloy, beryllium, tungsten, zinc, a zinc alloy, and any combination
thereof.
[0054] Element 3: Wherein
the window exhibits a shape selected
from the group consisting of teardrop-shaped, circle-shaped, oval-shaped,
square-shaped, rectangle-shaped, and any combination thereof.
[0055] Element 4: Wherein
the tubular body has a first end and a
second end, and wherein at least one of the first and second ends is threaded
for
coupling to a wellbore casing.
CA 03005854 2018-05-18
WO 2017/138923 PCT/US2016/017135
[0056] Element 5: Wherein the
degradable material fluidically seals
the window using at least one of an adhesive, an epoxy, an elastomer, a weld,
brazing, a mechanical seal, and any combination thereof.
[0057] Element 6: Wherein the
tubular body comprises an inner
radial surface and an outer radial surface, and wherein the degradable
material
is flush with the inner and outer radial surfaces.
[0058] Element 7: Wherein a
shoulder is defined in the sidewall of
the tubular body at the window, and wherein the degradable material is
received
by the shoulder.
[0059] Element 8: Wherein
when the casing joint is interconnected
in casing lined in a wellbore, and further comprising introducing a reactant
into
the wellbore to degrade the degradable material, wherein the reactant is
selected from the group consisting of an acid, a base, an electrolyte, and any
combination thereof; or wherein the wellbore further comprises the one or more
of the reactants contacting the degradable material.
[0060] Element 9: Wherein
when the casing joint is interconnected
in casing lined in a wellbore, and further comprising introducing a reactant
into
the wellbore to degrade the degradable material, wherein reactant is selected
from the group consisting of sodium ions, potassium ions, calcium ions,
magnesium ions, chloride ions, bromide ions, hydrogen phosphate ions,
hydrogen carbonate ions, ferric chloride, hydrochloric acid, hydroiodic acid,
perchloric acid, nitric acid, sulfuric acid, hydrobromic acid, chloric acid,
acetic
acid, boric acid, carbonic acid, citric acid, hydrofluoric acid, oxalic acid,
phosphoric acid, picric acid, acetic-picral, p-toluenesulfonic acid,
methanesulfonic
acid, hydronium ion, bromic acid, perbromic acid, iodic acid, periodic acid,
fluoroantimonic acid, triflic acid, fluorosulfuric acid, a hydroxide, an
oxide, butyl
lithium, lithium diisopropylannide, lithium diethylamide, sodium hydride,
sodium
amide, lithium bis(trimethylsilyl)amide, and any combination thereof, or
wherein
the wellbore further comprises the one or more of the reactants contacting the
degradable material.
[0061] Element 10: Wherein
when the casing joint is interconnected
in casing lined in a wellbore, and further comprising cementing the casing
joint
in the wellbore prior to degrading the degradable material, or wherein the
casing
joint is cemented in the wellbore.
16
CA 03005854 2018-05-18
WO 2017/138923 PCT/US2016/017135
[0062] Element 11: Wherein
when the casing joint is interconnected
in casing lined in a wellbore, and further comprising drilling a lateral
wellbore
through the window after degrading the degradable material, or wherein the
degradable material is degraded and further comprising a lateral wellbore
extending through the window.
[0063] By way of non-
limiting example, exemplary combinations
applicable to A, B, and C include: 1-11; 1, 2, and 10; 3, 4, and 11; 5 and 6;
3,
7, and 9; 8 and 10; 2 and 4; 5, 7, and 8; 10 and 11; and the like.
[0064] Therefore, the
present disclosure is able to attain the ends
and advantages mentioned as well as those that are inherent therein. The
particular Examples disclosed above are illustrative only, as the present
disclosure may be modified and practiced in different but equivalent manners
apparent to those skilled in the art having the benefit of the teachings
herein.
Furthermore, no limitations are intended to the details of construction or
design
herein shown, other than as described in the claims below. It is therefore
evident that the particular illustrative Examples disclosed above may be
altered,
combined, or modified and all such variations are considered within the scope
and spirit of the present disclosure. The disclosure illustratively disclosed
herein
suitably may be practiced in the absence of any element that is not
specifically
disclosed herein and/or any optional element disclosed herein. While
compositions and methods are described in terms of "comprising," 'containing,"
or "including" various components or steps, the compositions and methods can
also "consist essentially of" or 'consist of" the various components and
steps.
All numbers and ranges disclosed above may vary by some amount. Whenever
a numerical range with a lower limit and an upper limit is disclosed, any
number
and any included range falling within the range are specifically disclosed. In
particular, every range of values (of the form, "from a to b," or,
equivalently,
"from approximately a to b," or, equivalently, 'from approximately a-b")
disclosed herein is to be understood to set forth every number and range
encompassed within the broader range of values. Also, the terms in the claims
have their plain, ordinary meaning unless otherwise explicitly and clearly
defined
by the patentee. Moreover, the indefinite articles "a" or "an," as used in the
claims, are defined herein to mean one or more than one of the element that it
introduces.
17