Note: Descriptions are shown in the official language in which they were submitted.
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
RATIOMETRIC BIOSENSORS AND
NON-GEOMETRICALLY MODULATED FRET
RELATED APPLICATIONS
This application claims benefit of priority to U.S. Provisional Application
No.
62/257,850, filed November 20, 2015, U.S. Provisional Application No.
62/257,859, filed
November 20, 2015, U.S. Provisional Application No. 62/257,863, filed November
20, 2015,
and U.S. Provisional Application No. 62/257,796, filed November 20, 2015, the
entire
contents of each which are incorporated herein by reference.
INCORPORATION-BY-REFERENCE OF SEQUENCE LISTING
The contents of the text file named "35327-521001WO_Sequence_Listing.txt",
which
was created on November 19, 2016 and is 390 KB in size, is hereby incorporated
by
reference in its entirety.
FIELD OF THE INVENTION
The present invention relates to compositions and methods for detecting
compounds
and determining the concentration thereof.
BACKGROUND
Determination of analyte concentrations using fluorescent probes is a powerful
technique in analytical chemistry. Fluorescent chemosensors have wide-ranging
applications
in cell biology and analytical chemistry.
The majority of fluorescent sensors and biosensors do not undergo changes in
emission spectral shape upon analyte binding and accordingly evince
monochromatic
intensity changes, rather than the dichromatic responses required for
ratiometric sensing.
SUMMARY OF THE INVENTION
The present subject matter provides methods for converting monochromatic
responses
into dichromatic responses that enable ratiometric sensing. If the
fluorescence emission
spectrum changes shape in response to analyte binding such that the ratio of
emission
intensities at two appropriately chosen wavelengths reports on analyte
concentration
(dichromatic response), then ratiometric measurements can be used to monitor
analyte
1
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
concentrations. In embodiments, these methods are based on establishing non-
geometrically
modulated Forster resonance energy transfer (ngmFRET) between a monochromatic,
chemoresponsive fluorophore (a directly responsive partner), and a second
fluorophore that
neither interacts directly with the ligand, nor is sensitive to ligand-
mediated changes in its
environment (an indirectly responsive partner). Biosensors that undergo
ngmFRET (or
altered ngmFRET) upon ligand binding are also provided herein, as well as
compositions and
devices comprising such biosensors.
Methods, compounds, and compositions provided herein overcome challenges
regarding the design of biosensors that produce a ratiometric signal. For
example, a
biosensor that exhibits a monochromatic response (which does not produce a
ratiometric
signal) to ligand binding may be converted into a biosensor that produces a
dichromatic/ratiometric signal. Moreover, the number of fluorophores that may
be utilized in
ratiometric biosensors is dramatically increased by the present subject
matter. For example,
fluorophores that typically do not show a dichromatic response to ligand
binding (such as
fluorescein and derivatives thereof) may be used together with an additional
reporter group
(such as another fluorophore) to produce a ratiometric signal. Also included
are methods,
compounds, and compositions relating to biosensors with multiple reporter
groups that have
improved ratiometric signals compared to other ratiometric biosensors (e.g.,
ratiometric
biosensors having a single reporter group).
Traditional/conventional geometrically-modulated Fluorescence Resonance Energy
Transfer (tgmFRET) is a physical phenomenon that was first described over 50
years ago. In
tgmFRET, the transfer of excited state energy from a donor fluorophore to an
acceptor
fluorophore (i.e. energy transfer) is modulated by a ligand-binding event
through changes in
the distance and/or angle between the donor and acceptor fluorophores. tgmFRET
is
manifested by opposing changes in the fluorescence emission intensities of the
donor and
acceptor fluorophores, respectively, in response to ligand binding. For
instance, a decrease in
distance results in a decrease of the donor fluorescence emission intensity
and an increase in
the acceptor fluorescence intensity, as energy is transferred from the former
to the latter. A
ligand-mediated increase in the distance between the partners has the opposite
effect (the
fluorescence emission intensity of the donor increases, whereas that of the
acceptor
decreases). In tgmFRET, ligand-mediated modulation of fluorescence intensity
arises from
global changes in the entire system, and can occur only if both partners are
present.
2
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
By contrast, in ngmFRET ligand-mediated modulation of fluorescence intensity
arises
from changes that are localized to the photophysics of the directly responsive
fluorophore.
Unlike tgmFRET, ligand-mediated changes in fluorescence therefore occur also
if only the
directly responsive partner is present in isolation by itself. Although the
entire ngmFRET
system comprising two partners is not required for evincing ligand-mediated
changes in
fluorescence emission intensity, the response of such a system is
qualitatively changed or
quantitatively enhanced over the responses of the isolated directly responsive
partner (e.g.
converting a monochromatic into a dichromatic response, thereby enabling
ratiometry).
Furthermore, unlike tgmFRET, the pattern of fluorescence intensity changes
manifested by
ligand binding in ngmFRET systems are not limited to opposing changes only.
Instead, in
ngmFRET almost all combinations of emission intensity changes are possible:
opposing
changes in the two partners, both partners increase, both decrease, one
partner remains
unchanged whereas the other increases or decreases. The majority of these
responses evince
changes that are unequal in magnitude and/or direction (i.e. increase,
decrease), and
accordingly are manifested as ligand-mediated changes in the ratio of the two
fluorescence
emission intensities. This versatility of ngmFRET system response patterns has
great utility
in the field of fluorescent biosensors.
The ligand-mediated alteration of the photophysics of the directly responsive
partner
includes changes to its spectral properties such as the shape of the
excitation or emission
spectra, and the ratio of radiative to non-radiative emission rates. The
fluorescence emission
intensity of the indirectly responsive partner in isolation does not change in
response to
ligand binding; its intensity changes only in the presence of a directly
responsive partner in
the complete ngmFRET system. In the field fluorescence spectroscopy, the term
"quenching" has often been used loosely to refer to a decrease fluorescence
emission
intensity. However, as used herein, the term "quenching" strictly means a
"change in the
ratio of radiative to non-radiative emission rates" of a fluorophore.
Aspects of the present subject matter provide biosensors in which ngmFRET
occurs
between two or more reporter groups (e.g., a donor fluorophore and an acceptor
fluorophore)
of the biosensor. For example, ngmFRET may change (e.g., increase or decrease)
when
ligand is bound to the biosensor and a donor fluorophore is contacted with
radiation within its
excitation wavelength. Effects from tgmFRET and ngmFRET may occur together and
be
combined into an overall ligand-mediated change in fluorescence emission
intensity. In
preferred embodiments, less than half or none of the change in overall ligand-
mediated
3
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
change in fluorescence emission intensity is due to tgmFRET. In embodiments,
most of the
overall ligand-mediated change in fluorescence emission intensity change is
not due to a
change in the distance between the donor and acceptor fluorophore or as a
result of a change
in the orientation between the donor and acceptor fluorophore. In non-limiting
examples,
less than about 25%, 20%, 15%, 10%, 5%, 4%, 3%, 2%, 1%, or 0.5% of the change
in overall
ligand-mediated change in fluorescence emission intensity is due to tgmFRET.
In various
embodiments, at least about 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99%,
99.5%,
99.9%, or 99.99% of the ligand-mediated change in fluorescence emission
intensity is due to
ngmFRET. For example, the change in overall ligand-mediated change in
fluorescence
emission intensity comprises a spectral change (e.g., in the excitation or
emission spectrum)
and/or a change in the ratio of the radiative to non-radiative decay rates of
one of the
fluorophores (by itself and regardless of the presence of any other
fluorophore/partner) upon
ligand binding.
In some embodiments, ligand binding mediates spectral shifts in the absorption
or
emission spectrum of the directly responsive partner. In certain embodiments
such changes
are due at least in part to a switch between different excited states in the
ligand-free and
ligand-bound biosensor. The two excited states are associated with different
transition
dipoles. This class of changes is termed "dipole switching" herein. Non-
limiting examples
of biosensors that show dipole sensing include ttGGBP 17C=Badan-f3Zif Alexa532
and
ttGGBP 182C =Acrylodan-f3Zif Alexa532.
In embodiments, the reporter groups include a directly responsive partner
(which may
be a donor fluorophore or an acceptor fluorophore) and an indirectly
responsive partner
(which may be a donor fluorophore or an acceptor fluorophore). Depending on
context, a
"directly responsive" partner is a fluorophore that responds to (i) ligand-
induced protein
conformational changes upon ligand binding to a ligand-binding protein; or
(ii) ligand
binding to the directly responsive partner itself. In some embodiments, the
directly
responsive partner comprises a fluorophore (i.e., it is a directly responsive
fluorophore). In
various embodiments, the directly responsive fluorophore exhibits a
monochromatic or
dichromatic spectral change, and/or a change in the ratio of radiative to non-
radiative
emission rates, upon ligand binding. In certain embodiments relating to ligand
binding to the
directly responsive partner itself, the directly responsive partner may be a
fluorophore such as
a fluorescent protein or a small molecule fluorescent compound. An "indirectly
responsive"
partner is a fluorophore for which no change in emission spectra, excitation
spectra, or
4
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
change in the ratio of radiative to non-radiative emission rates is caused by
ligand binding in
the absence of a directly responsive partner. In some embodiments, the
indirectly responsive
partner comprises a fluorophore (i.e., it is an indirectly responsive
fluorophore). When paired
with a directly responsive partner with which the indirectly responsive
partner is a ngmFRET
donor or acceptor, the emission fluorescence intensity of the indirectly
responsive partner
changes due to a change in energy flow in the ngmFRET pathway upon ligand
binding. See,
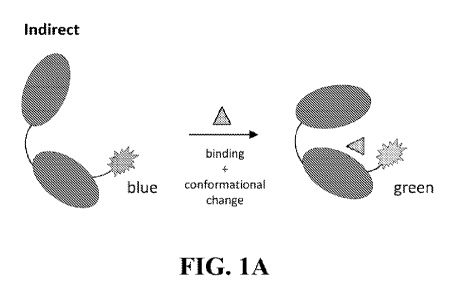
e.g., FIG. 28.
ngmFRET Biosensors
Provided herein are methods, compositions, biosensors, and devices comprising
multiple reporter groups, e.g. a directly responsive fluorophore and an
indirectly responsive
fluorophore, between which ngmFRET occurs.
Aspects include a method of detecting a ligand in a sample, comprising
contacting a
biosensor with a ligand. The biosensor comprises a ligand-binding protein, a
directly
responsive fluorophore and an indirectly responsive fluorophore. The directly
responsive and
the indirectly responsive fluorophores are located at two distinct sites of
the ligand-binding
protein. In some embodiments, the directly responsive fluorophore is a donor
fluorophore
and the indirectly responsive fluorophore is an acceptor fluorophore.
Alternatively, the
directly responsive fluorophore is an acceptor fluorophore and the indirectly
responsive
fluorophore is a donor fluorophore. The method includes contacting the
biosensor with
radiation comprising a wavelength within the excitation spectrum of the donor
fluorophore.
When the biosensor is contacted with such radiation, a fluorescence property
of the directly
responsive fluorophore changes in response to ligand binding. This change in
fluorescent
property is independent of the indirectly responsive fluorophore, and occurs
regardless of
whether the indirectly responsive fluorophore is absent or present. The
fluorescence
properties of the indirectly responsive fluorophore do not change in response
to ligand
binding in the absence of the directly responsive fluorophore. When the
biosensor is
contacted with radiation comprising a wavelength within the excitation
spectrum of the donor
fluorophore, then (i) ngmFRET occurs between the directly responsive
fluorophore and the
indirectly responsive fluorophore; (ii) fluorescent light is emitted from the
biosensor, and the
light emitted from the biosensor comprises a combination of light emitted from
the directly
responsive fluorophore and light emitted from the indirectly responsive
fluorophore; and (iii)
the ratio of the fluorescence emission intensity emitted from the biosensor at
each of two
5
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
distinct wavelengths changes in response to ligand binding. In various
embodiments, the
method further comprises measuring fluorescent light that is emitted from the
directly
responsive fluorophore and the indirectly responsive fluorophore, and
calculating a
ratiometric signal to detect the ligand in the sample.
The ratiometric signal (R1,2) comprises a quotient of two intensities, /xi and
/x2,
measured at two independent wavelengths, Xi and k2 and is calculated according
to the
following equation:
R1,2 = /Ai //A2 =
In various embodiments, the change in the fluorescent property of the directly
responsive fluorophore comprises (i) a bathochromic or hypsochromic shift in
the emission or
excitation spectrum thereof; and/or (ii) a change in the ratio of radiative to
non-radiative
emission rates thereof.
In some embodiments, the directly responsive fluorophore is Badan and emission
intensity is measured at a wavelength or range of wavelengths between about
400 nm and
1000nm (e.g., including a wavelength of about 450, 451, 452, 453, 454, 455,
456, 457, 458,
459, 460, 461, 462, 463, 464, 465, 466, 467, 468, 469, 470, 471, 472, 473,
474, or 475 nm),
and wherein the indirectly responsive fluorophore is 5-
iodoacetamidofluorescein (5-IAF) and
emission intensity is measured at a wavelength or range of wavelengths between
about 400
nm and 1000nm (e.g., including a wavelength of about 510, 511, 512, 513, 514,
515,
516,517, 518, 519, 520, 521, 522, 523, 524, 525, 526, 527, 528, 529, or 530
nm).
In certain embodiments, the directly responsive fluorophore is Badan and
emission
intensity is measured at a wavelength or range of wavelengths between about
400 nm and
1000nm (e.g., including a wavelength of about 450, 451, 452, 453, 454, 455,
456, 457, 458,
459, 460, 461, 462, 463, 464, 465, 466, 467, 468, 469, 470, 471, 472, 473,
474, or 475 nm),
and wherein the indirectly responsive fluorophore is A1exa532 and emission
intensity is
measured at a wavelength or range of wavelengths between about 400 nm and
1000nm (e.g.,
including a wavelength of about 550, 551, 552, 553, 554, 555, 556, 557, 558,
559, 560, 561,
562, 563, 564, 565, 566, 567, 568, 569, or 570 nm).
In various embodiments, the directly responsive fluorophore is Pacific Blue
and
emission intensity is measured at a wavelength or range of wavelengths between
about 400
nm and 1000nm (e.g., including a wavelength of about 445, 446, 447, 448, 449,
450, 451,
452, 453, 454, 455, 456, 457, 458, 459, 460, 461, 462, 463, 464, or 465 nm),
and wherein the
6
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
indirectly responsive fluorophore is 5-IAF and emission intensity is measured
at a
wavelength or range of wavelengths between about 400 nm and 1000nm (e.g.,
including a
wavelength of about 510, 511, 512, 513, 514, 515, 516,517, 518, 519, 520, 521,
522, 523,
524, 525, 526, 527, 528, 529, or 530 nm).
In some embodiments, the directly responsive fluorophore is Acrylodan and
emission
intensity is measured at a wavelength or range of wavelengths between about
400 nm and
1000nm (e.g., including a wavelength of about 450, 451, 452, 453, 454, 455,
456, 457, 458,
459, 460, 461, 462, 463, 464, 465, 466, 467, 468, 469, 470, 471, 472, 473,
474, or 475 nm),
and wherein the indirectly responsive fluorophore is 5-IAF and emission
intensity is
measured at a wavelength or range of wavelengths between about 400 nm and
1000nm (e.g.,
including a wavelength of about 510, 511, 512, 513, 514, 515, 516,517, 518,
519, 520, 521,
522, 523, 524, 525, 526, 527, 528, 529, or 530 nm).
In some embodiments, the directly responsive fluorophore is Acrylodan and
emission
intensity is measured at a wavelength or range of wavelengths between about
400 nm and
1000nm (e.g., including a wavelength of about 470, 471, 472, 473, 474, 475,
476, 477, 478,
479, 480, 481, 482, 483, 484, 485, 486, 487, 488, 489, 490 nm), and wherein
the indirectly
responsive fluorophore is A1exa532 and emission intensity is measured at a
wavelength or
range of wavelengths between about 400 nm and 1000nm (e.g., including a
wavelength of
about 540, 541, 542,543, 544, 545, 546, 547, 548, 549, 550, 551, 552, 553,
554, 555, 556,
557, 558, 559, or 560 nm).
In certain embodiments, the directly responsive fluorophore is 5-IAF and
emission
intensity is measured at a wavelength or range of wavelengths between about
400 nm and
1000nm (e.g., including a wavelength of about 445, 446, 447, 448, 449, 450,
451, 452, 453,
454, 455, 456, 457, 458, 459, 460, 461, 462, 463, 464, or 465 nm), and wherein
the indirectly
responsive fluorophore is Pacific Blue and emission intensity is measured at a
wavelength or
range of wavelengths between about 400 nm and 1000nm (e.g., including a
wavelength of
about 510, 511, 512, 513, 514, 515, 516,517, 518, 519, 520, 521, 522, 523,
524, 525, 526,
527, 528, 529, or 530 nm).
In various embodiments, the directly responsive fluorophore is Oregon Green
and
emission intensity is measured at a wavelength or range of wavelengths between
about 400
nm and 1000nm (e.g., including a wavelength of about 445, 446, 447, 448, 449,
450, 451,
452, 453, 454, 455, 456, 457, 458, 459, 460, 461, 462, 463, 464, or 465 nm),
and wherein the
indirectly responsive fluorophore is Pacific Blue and emission intensity is
measured at a
7
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
wavelength or range of wavelengths between about 400 nm and 1000nm (e.g.,
including a
wavelength of about 510, 511, 512, 513, 514, 515, 516,517, 518, 519, 520, 521,
522, 523,
524, 525, 526, 527, 528, 529, or 530 nm).
In some embodiments, the directly responsive fluorophore is N-
(Iodoacetaminoethyl)-
1-naphthylamine-5-sulfonic acid (IAEDANS) and emission intensity is measured
at a
wavelength or range of wavelengths between about 400 nm and 1000nm (e.g.,
including a
wavelength of about 450, 451, 452, 453, 454, 455, 456, 457, 458, 459, 460,
461, 462, 463,
464, 465, 466, 467, 468, 469, 470, 471, 472, 473, 474, or 475 nm), and wherein
the indirectly
responsive fluorophore is 5-IAF and emission intensity is measured at a
wavelength or range
of wavelengths between about 400 nm and 1000nm (e.g., including a wavelength
of about
510, 511, 512, 513, 514, 515, 516,517, 518, 519, 520, 521, 522, 523, 524, 525,
526, 527, 528,
529, or 530 nm).
In some embodiments, the directly responsive fluorophore is A1exa532 and
emission
intensity is measured at a wavelength or range of wavelengths between about
400 nm and
1000 nm (e.g. including a wavelength of about 530, 531, 532, 534, 534, 535,
536, 537, 538,
539, 540, 541, 542, 543, 544, 545, 546, 547, 548, 549, 550, 551, 552, 553,
554, 555, 556,
557, 558, 559, 560, 561, 562, 563, 564, 565, 566, 567, 568, 569, or 570 nm),
and wherein the
indirectly responsive fluorophore is Acrylodan and emission intensity is
measured at a
wavelength or range of wavelengths between about 400 nm and 1000 nm (e.g.
including 470,
471, 472, 473, 474, 475, 476, 477, 478, 479, 480, 481, 482, 483, 484, 45, 496,
487, 488, 489,
490, 491, 492, 493, 494, 495, 496, 497, 499, 500, 501, 502, 503, 504, 505,
506, 507, 508,
509, or 510 nm).
In various embodiments, the directly responsive fluorophore is a yellow
fluorescent
protein and emission intensity is measured at a wavelength or range of
wavelengths between
about 400 nm and 1000nm (e.g., including a wavelength of about 520, 521, 522,
523, 524,
525, 526, 527, 528, 529, 530, 531, 532, 533, 534, 535, 536, 537, 538, 539, or
540 nm), and
wherein the indirectly responsive fluorophore is Acrylodan and emission
intensity is
measured at a wavelength or range of wavelengths between about 400 nm and
1000nm (e.g.,
including a wavelength of about 490, 491, 492, 493, 494, 495, 496, 497, 498,
499, 500, 501,
502, 503, 504, 505, 506, 507, 508, 509, or 510 nm. In certain embodiments, the
directly
responsive fluorophore is a yellow fluorescent protein and emission intensity
is measured at a
wavelength or range of wavelengths between about 400 nm and 1000nm (e.g.,
including a
wavelength of about 520, 521, 522, 523, 524, 525, 526, 527, 528, 529, 530,
531, 532, 533,
8
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
534, 535, 536, 537, 538, 539, or 540 nm), and wherein the indirectly
responsive fluorophore
is Pacific Blue and emission intensity is measured at a wavelength or range of
wavelengths
between about 400 nm and 1000nm (e.g., including a wavelength of about 445,
446, 447,
448, 449, 450, 451, 452, 453, 454, 455, 456, 457, 458, 459, 460, 461, 462,
463, 464, or 465
nm).
In embodiments, the directly responsive fluorophore comprises a donor
fluorophore
and the indirectly responsive fluorophore comprises an acceptor fluorophore.
In some
embodiments, the emission intensity of the donor fluorophore decreases and the
emission
intensity of the acceptor fluorophore increases upon ligand binding to the
ligand-binding
protein when the donor fluorophore is contacted with radiation within the
excitation spectrum
of the donor fluorophore. In some embodiments, the emission intensity of the
donor
fluorophore increases and the emission intensity of the acceptor fluorophore
decreases upon
ligand binding to the ligand-binding protein when the donor fluorophore is
contacted with
radiation within the excitation spectrum of the donor fluorophore. In some
embodiments, the
emission intensities of the donor fluorophore and the acceptor fluorophore
both decrease
upon ligand binding to the ligand-binding protein when the donor fluorophore
is contacted
with radiation within the excitation spectrum of the donor fluorophore. In
some
embodiments, the emission intensity of the donor fluorophore decreases and the
emission
intensity of the acceptor fluorophore increases, decreases, or remains about
the same upon
ligand binding to the ligand-binding protein when the donor fluorophore is
contacted with
radiation within the excitation spectrum of the donor fluorophore. In some
embodiments, the
emission intensity of the donor fluorophore increases, decreases, or remains
about the same
and the emission intensity of the acceptor fluorophore decreases upon ligand
binding to the
ligand-binding protein when the donor fluorophore is contacted with radiation
within the
excitation spectrum of the donor fluorophore. In some embodiments, the
emission intensities
of the donor fluorophore and the acceptor fluorophore both increase upon
ligand binding to
the ligand-binding protein when the donor fluorophore is contacted with
radiation within the
excitation spectrum of the donor fluorophore. In some embodiments, the
emission intensity
of the donor fluorophore increases, decreases, or remains about the same and
the emission
intensity of the acceptor fluorophore increases upon ligand binding to the
ligand-binding
protein when the donor fluorophore is contacted with radiation within the
excitation spectrum
of the donor fluorophore. In some embodiments, the emission intensity of the
donor
fluorophore increases and the emission intensity of the acceptor fluorophore
increases,
9
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
decreases, or remains about the same upon ligand binding to the ligand-binding
protein when
the donor fluorophore is contacted with radiation within the excitation
spectrum of the donor
fluorophore.
In embodiments the directly responsive fluorophore comprises an acceptor
fluorophore and the indirectly responsive fluorophore comprises a donor
fluorophore. In
some embodiments, the emission intensity of the donor fluorophore decreases
and the
emission intensity of the acceptor fluorophore increases, decreases, or
remains about the
same upon ligand binding to the ligand-binding protein when the donor
fluorophore is
contacted with radiation within the excitation spectrum of the donor
fluorophore. In some
embodiments, the emission intensity of the donor fluorophore increases and the
emission
intensity of the acceptor fluorophore increases, decreases, or remains about
the same upon
ligand binding to the ligand-binding protein when the donor fluorophore is
contacted with
radiation within the excitation spectrum of the donor fluorophore. In some
embodiments, the
emission intensity of the donor fluorophore remains about the same and the
emission
intensity of the acceptor fluorophore decreases upon ligand binding to the
ligand-binding
protein when the donor fluorophore is contacted with radiation within the
excitation spectrum
of the donor fluorophore. In some embodiments, the emission intensity of the
donor
fluorophore decreases and the emission intensity of the acceptor fluorophore
increases,
decreases, or remains about the same upon ligand binding to the ligand-binding
protein when
the donor fluorophore is contacted with radiation within the excitation
spectrum of the donor
fluorophore. In some embodiments, the emission intensity of the donor
fluorophore increases
and the emission intensity of the acceptor fluorophore increases, decreases,
or remains about
the same upon ligand binding to the ligand-binding protein when the donor
fluorophore is
contacted with radiation within the excitation spectrum of the donor
fluorophore. In some
embodiments, the emission intensity of the donor fluorophore remains about the
same and the
emission intensity of the acceptor fluorophore increases upon ligand binding
to the ligand-
binding protein when the donor fluorophore is contacted with radiation within
the excitation
spectrum of the donor fluorophore. In some embodiments, the emission intensity
of the
donor fluorophore decreases and the emission intensity of the acceptor
fluorophore increases
upon ligand binding to the ligand-binding protein when the donor fluorophore
is contacted
with radiation within the excitation spectrum of the donor fluorophore. In
some
embodiments, the emission intensity of the donor fluorophore increases and the
emission
intensity of the acceptor fluorophore remains about the same, increases, or
decreases upon
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
ligand binding to the ligand-binding protein when the donor fluorophore is
contacted with
radiation within the excitation spectrum of the donor fluorophore.
In instances in which an emission intensity increases, the increase may be,
e.g.,
between about 0.1% to 10%, 10% to 50%, or 50% to 100%, or at least about 0.1%,
0.5%, 1%,
2%, 3%, 4%, 5%, 10%, 15%, 20%, 25%, 50%, 75%, 100%, 2-fold, 3-fold, 4-fold, 5-
fold, 6-
fold, 7-fold, 8-fold, 9-fold, or 10-fold. In instances in which an emission
intensity decreases,
the decrease may be, e.g., a decrease of between about at least about 0.1% to
10%, 10% to
50%, or 50% to 100%, or at least about 0.1%, 0.5%, 1%, 2%, 3%, 4%, 5%, 10%,
15%, 20%,
25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, or 90%. In
various embodiments in which both the emission intensity of the donor
fluorophore and the
acceptor fluorophore increases, then the increases are not equal. In certain
embodiments in
which both the emission intensity of the donor fluorophore and the acceptor
fluorophore
decreases, then the decreases are not equal.
In various embodiments, the ligand-binding protein comprises the directly
responsive
fluorophore. For example, the directly responsive fluorophore is formed by an
autocatalytic
cyclization of an oligopeptide within the ligand-binding protein. In some
embodiments, the
oligopeptide is located within an interior a helix. In certain embodiments,
the oligopeptide
comprises 2, 3, 4, 5, 6, 7, 8, 9, or 10 consecutive residues. In embodiments,
the directly
responsive fluorophore is formed by an autocatalytic cyclization of a
tipeptide located in an
interior a helix of the ligand-binding protein. In various embodiments, ligand-
binding
protein comprises a yellow fluorescent protein (YFP), i.e. the YFP binds to
ligand such as a
halide anion.
In some embodiments, ligand binding causes a change in signaling by the
directly
responsive fluorophore.
Also provided is a method of detecting a ligand in a sample, comprising
contacting a
biosensor with a ligand, wherein the biosensor comprises an amino acid or
polypeptide, a
directly responsive fluorophore and an indirectly responsive fluorophore. The
directly
responsive and the indirectly responsive fluorophores are located at two
distinct sites of the
amino acid or polypeptide, and the directly responsive fluorophore is
chemoresponsive. The
method may further comprise contacting the biosensor with radiation comprising
a
wavelength within the excitation spectrum of the donor fluorophore, wherein
(i) a
fluorescence property of the directly responsive fluorophore changes in
response to ligand
binding in the absence or presence of the indirectly responsive fluorophore;
(ii) a
11
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
fluorescence property of the indirectly responsive fluorophore does not change
in response to
ligand binding in the absence of the directly responsive fluorophore; (iii)
ngmFRET occurs
between the directly responsive fluorophore and the indirectly responsive
fluorophore; (iv)
fluorescent light is emitted from the biosensor, wherein the light emitted
from the biosensor
comprises a combination of light emitted from the directly responsive
fluorophore and light
emitted from the indirectly responsive fluorophore; and (v) the ratio of the
fluorescence
emission intensity emitted from the biosensor at each of two distinct
wavelengths changes in
response to ligand binding. The method may also include measuring fluorescent
light that is
emitted from the directly responsive fluorophore and the indirectly responsive
fluorophore
and calculating a ratiometric signal, to detect the ligand in the sample. The
ratiometric signal
(R1,2) comprises a quotient of two intensities, Ai and A2, measured at two
independent
wavelengths, Xi and k2 and is calculated according to the following equation:
R1,2 = '2i/'22 =
As used herein, a "chemoresponsive" fluorophore is a fluorophore to which
ligand
binds, wherein ligand binding causes a change in signaling by the fluorophore.
As used herein, "signaling" refers to the emission of energy (which may be
referred to
as a "signal") by one or more reporter groups. In various implementations, the
signal
comprises electromagnetic radiation such as a light. In some embodiments, the
signal is
detected as a complete emission spectrum (or spectra) or a portion (or
portions) thereof. For
example, a signal may comprise emitted light at a particular wavelength or
wavelengths, or
range(s) of wavelengths. In some embodiments, a change in signaling comprises
a spectral
change (e.g., a spectral shift and/or change in intensity). In some
embodiments, a change in
signaling comprises a dichromatic shift or a monochromatic fluorescence
intensity change.
In some embodiments, the directly responsive fluorophore is a donor
fluorophore and
the indirectly responsive fluorophore is an acceptor fluorophore.
Alternatively, the directly
responsive fluorophore is an acceptor fluorophore and the indirectly
responsive fluorophore
is a donor fluorophore.
In various embodiments, the change in the fluorescent property of the directly
responsive fluorophore comprises (i) a bathochromic or hypsochromic shift in
the emission or
excitation spectrum thereof; and/or (ii) a change in the ratio of radiative to
non-radiative
emission rates thereof.
12
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
In some embodiments, the directly responsive fluorophore is 5-IAF and emission
intensity is measured at a wavelength or range of wavelengths between about
400 nm and
1000nm (e.g., including a wavelength of about 450, 451, 452, 453, 454, 455,
456, 457, 458,
459, 460, 461, 462, 463, 464, 465, 466, 467, 468, 469, or 470 nm), and wherein
the indirectly
responsive fluorophore is Acrylodan and emission intensity is measured at a
wavelength or
range of wavelengths between about 400 nm and 1000nm (e.g., including a
wavelength of
about 510, 511, 512, 513, 514, 515, 516,517, 518, 519, 520, 521, 522, 523,
524, 525, 526,
527, 528, 529, or 530 nm).
In certain embodiments, the directly responsive fluorophore is 5-IAF and
emission
intensity is measured at a wavelength or range of wavelengths between about
400 nm and
1000nm (e.g., including a wavelength of about 510, 511, 512, 513, 514, 515,
516,517, 518,
519, 520, 521, 522, 523, 524, 525, 526, 527, 528, 529, or 530 nm), and wherein
the indirectly
responsive fluorophore is Pacific Blue and emission intensity is measured at a
wavelength or
range of wavelengths between about 400 nm and 1000nm (e.g., including a
wavelength of
about 445, 446, 447,448, 449, 450, 455, 456, 457, 458, 459, 460, 461, 462,
463, 464, 465,
nm).
Any amino acid or polypeptide may be used to link the chemoresponsive directly
responsive fluorophore with the indirectly responsive fluorophore, provided
the two
fluorophores are close enough for ngmFRET to occur. Suitable distances may be
determined
in part by the distance-dependence of the energy transfer between a given
donor-acceptor pair
(see, e.g, J.R. Lakowicz, 2006, Principles of Fluorescence Spectroscopy,
Springer,
incorporated herein by reference). In various embodiments, the amino acid or
polypeptide
comprises 1 amino acid, or a stretch of at least 1, 2, 3, 4, 5, 6, 7, 8, 9,
10, 20, 30, 40, 50, 60,
70, 80, 90, 100, 150, 200, 250, 500, 750, or 1000 amino acids. In some
embodiments, the
amino acid or polypeptide comprises at least 1, 2, or 3 thiol groups; at least
1, 2, or 3
cysteines that each comprise a sulfhydryl group; at least 1, 2, or 3 primary
amine groups; or
at least 1, 2, or 3 lysines that each comprise a primary amine. In certain
embodiments, the
polypeptide comprise two cysteines, and there is no disulfide bond between the
two
cysteines. In some embodiments there is no disulfide bond between any pair of
cysteines
within the amino acid sequence of the polypeptide.
In a non-limiting example, the polypeptide comprises a stretch of at least 50,
60, 70,
80, 90, or 100 amino acids in a sequence that is at least about 85%, 90%, 95%,
or 99%
identical to the amino acid sequence of ecTRX (SEQ ID NO: 151). In some
embodiments,
13
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
the polypeptide comprises a mutant of ecTRX comprising a D3X, K4X, K19X, D27X,
K37X,
K53X, K58X, K70X, R74X, K83X, K91X, K97X, or K101X mutation, or any
combination
thereof, wherein X is any amino acid, and wherein each ecTRX amino acid
position is
numbered as in SEQ ID NO: 151. In certain embodiments, the polypeptide
comprises a
mutant of ecTRX comprising a D3A, K4R, K4Q, K19R, K19Q, D27A, K37R, K53M,
K53R,
K58M, K7OR, R74C, K83R, K91R, K97R, or K101R mutation, or any combination
thereof,
wherein each ecTRX amino acid position is numbered as in SEQ ID NO: 151. In
various
embodiments, the polypeptide comprises a mutant of ecTRX that does not
comprise a lysine.
In certain embodiments, the polypeptide comprises amino acids in the sequence
of any one of
SEQ ID NOS: 69-86 or 151.
In certain embodiments, the polypeptide further comprises a hexahistidine tag.
In some embodiments, the ligand comprises a hydrogen ion. For example, the
biosensor for pH, wherein the directly responsive fluorophore is pH-sensitive.
In various
embodiments, the fully excited emission intensity of the directly responsive
fluorophore is
different at a pH less than about 7.0 (e.g. 6.9, 6.8, 67, 6.6, 6.5, 6.4, 6.3,
6.2, 6.1, or 6.0), or
about 4.0 to 10.0, compared to a pH of about 7.3, 7.4, 7.5, 7.6, or 7.7.
In various embodiments, the directly responsive fluorophore comprises a pH-
sensitive
fluorophore comprising fluorescein or a derivative thereof. In embodiments,
the directly
responsive fluorophore transitions from a monoanion to a dianion at a pH that
is less than 7.0
in an aqueous solution.
In certain embodiments, the indirectly responsive fluorophore is attached to
the
ligand-binding protein via a covalent bond. Various approaches for attaching
reporter groups
such as directly and indirectly responsive fluorophores to an amino acid or a
polypeptide such
as a ligand-binding protein are described herein. In some embodiments, the
covalent bond
comprises a disulfide bond, a thioester bond, a thioether bond, an ester bond,
an amide bond,
or a bond that has been formed by a click reaction.
In some embodiments, the indirectly responsive fluorophore is attached to the
ligand-
binding protein via a non-covalent bond. In certain embodiments, the
indirectly responsive
fluorophore is attached to a cysteine or a lysine of the protein.
In various embodiments, the indirectly responsive fluorophore is attached to
the N-
terminus or the C-terminus of the protein. In some embodiments, the indirectly
responsive
fluorophore is attached to the N-terminus or the C-terminus of the protein via
a fluorophore
attachment motif.
14
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
In some embodiments, fluorophore attachment motif comprises an amino acid or
polypeptide. Various embodiments may be used to link a fluorophore with a
ligand-binding
protein. In some embodiments, the amino acid or polypeptide comprises 1 amino
acid, or a
stretch of at least 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 20, 30, 40, 50, 60, 70, 80,
90, 100, 150, 200, 250,
500, 750, or 1000 amino acids. In a non-limiting example, the polypeptide
comprises amino
acids in the sequence of PZif (SEQ ID NO: 42). In another non-limiting
example, the
polypeptide comprises a stretch of at least 50, 60, 70, 80, 90, or 100 amino
acids in a
sequence that is at least about 85%, 90%, 95%, or 99% identical to the amino
acid sequence
of E. coli thioredoxin (ecTRX; SEQ ID NO: 151).
In some embodiments, the directly responsive fluorophore is attached to the
ligand-
binding protein via a covalent bond. In various embodiments, the covalent bond
comprises a
disulfide bond, a thioester bond, a thioether bond, an ester bond, an amide
bond, or a bond
that has been formed by a click reaction. In directly responsive fluorophore
is attached to a
cysteine or a lysine of the protein.
In various embodiments, if the acceptor fluorophore comprises palladium,
platinum,
ruthenium, or osmium, then the acceptor fluorophore is not attached to the
amino group of
the N-terminus of the ligand-binding protein. In some embodiments, the
acceptor
fluorophore does not comprise [Itu(bpy)3j2"-, iltu(Ph2phen)3i2+,
[Itu(bpy)2(dobpy)]2+, or
[Ru(lopy)2(phen-ITC)]24, where bpy is 2,2!--bipyridine, phen is 1,10-
phenanthroline, debpy is
4,4'-dicarboxy-2,2'-bipyridine, and ITC is isothiocyanate. In certain
embodiments, the
biosensor does not comprise an E. coli glutamine-binding protein with
Acrylodan attached to
179C. In some embodiiments, the biosensor does not comprise E. coli glucose-
binding
protein with Acrylodan attached to 255C.
In some embodiments, an overlap of the emission spectrum of the donor
fluorophore
and the excitation spectrum of the acceptor fluorophore increases upon ligand
binding. In
certain embodiments, the directly responsive fluorophore comprises the donor
fluorophore,
and the increase results from a bathochromic shift in the emission spectrum of
the donor
fluorophore. Alternatively, the directly responsive fluorophore comprises the
acceptor
fluorophore, and the increase results from a hypsochromic shift in the
excitation spectrum of
the acceptor fluorophore.
In various embodiments, an overlap of the emission spectrum of the donor
fluorophore and the excitation spectrum of the acceptor fluorophore decreases
upon ligand
binding. In some embodiments, the directly responsive fluorophore comprises
the donor
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
fluorophore, and the decrease results from a hypsochromic shift in the
emission spectrum of
the donor fluorophore. In certain embodiments, the directly responsive
fluorophore
comprises the acceptor fluorophore, and the decrease results from a
bathochromic shift in the
excitation spectrum of the acceptor fluorophore.
In some embodiments, the directly responsive fluorophore has a monochromatic
spectral change upon ligand binding. Alternatively, the directly responsive
fluorophore has a
dichromatic spectral change upon ligand binding.
In certain embodiments, the emission intensity of the donor fluorophore and/or
the
acceptor fluorophore increases in two phases as ligand concentration
increases.
In various embodiments, the ratio of radiative to non-radiative emission or
intensity of
the directly responsive fluorophore increases by at least about 0.1%, 0.5%,
1%, 2%, 3%, 4%,
5%, 10%, 15%, 20%, 25%, 50%, 75%, 100%, 2-fold, 3-fold, 4-fold, 5-fold, 6-
fold, 7-fold, 8-
fold, 9-fold, or 10-fold upon ligand binding to the ligand-binding protein.
Alternatively, the
ratio of radiative to non-radiative emission or intensity of the directly
responsive fluorophore
decreases by at least about 0.1%, 0.5%, 1%, 2%, 3%, 4%, 5%, 10%, 15%, 20%,
25%, 50%,
75%, 90%, 95%, or 99% upon ligand binding to the ligand-binding protein.
In embodiments, the directly responsive fluorophore and the indirectly
responsive
fluorophore are not a naphthalene derivative. In some embodiments, the
directly responsive
fluorophore and the indirectly responsive fluorophore are not Prodan,
Acrylodan, or Badan.
In certain embodiments, the directly responsive fluorophore is not a
naphthalene derivative.
In some embodiments, the directly responsive fluorophore is not Prodan,
Acrylodan, or
Badan.
In various embodiments, the directly responsive fluorophore comprises
xanthene, a
xanthene derivative, fluorescein, a fluorescein derivative, coumarin, a
coumarin derivative,
cyanine, a cyanine derivative, rhodamine, a rhodamine derivative, phenoxazine,
a
phenoxazine derivative, squaraine, a squaraine derivative, coumarin, a
coumarin derivative,
oxadiazole, an oxadiazole derivative, anthracene, an anthracene derivative, a
boradiazaindacine (BOD1PY) family fluorophore, pyrene, a pyrene derivative,
acridine, an
acridine derivative, arylmethine, an arylmethine derivative, tetrapynole, or a
tetrapynole
derivative. In some embodiments, the directly responsive fluorophore comprises
fluorescein
or a derivative thereof.
In some embodiments, the directly responsive fluorophore and/or the indirectly
responsive fluorophore comprises a fluorescent protein. In various
embodiments, the directly
16
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
responsive fluorophore and/or the indirectly responsive fluorophore comprises
an organic
compound having a molecular weight less than about 2000 Da (e.g., 5-
iodoacetamidofluorescein (5-IAF) or 6-iodoacetamidofluorescein (6-IAF),
rhodamine,
Oregon Green, eosin, Texas Red, indocarbocyanine, oxacarbocyanine,
thiacarbocyanine,
merocyanine, Badan, Acrylodan, IAEDANS, comprising 3-cyano-7-hydroxycoumarin,
7-
hydroxycoumarin-3-carboxylic acid, 6,8-difluoro-7-hydroxy- 4-methylcoumarin,
or 7-amino-
4-methylcoumarin, pyridyloxazole, nitrobenzoxadiazole, benzoxadiazole, DRAQ5,
DRAQ7,
or CyTRAK Orange, cascade blue, Nile red, Nile blue, cresyl violet, oxazine
170, proflavin,
acridine orange, acridine yellow, auramine, crystal violet, malachite green,
porphin,
phthalocyanine, bilirubin, pyrene, N,Nt-dimethyl-N-(iodoacety1)-N'-(7-
nitrobenz-2-ox- a-1,3-
diazol-4-ypethylenediamide (NBD), N-((2-(iodoacetoxy)ethyl)-N-methy- 1)amino-7-
nitrobenz-2-oxa-1,3-diazole (NBDE), JPW4039, JPW4042, JPW4045, Pacific Blue,
CPM,
N,Nt-Dimethyl-N-(Iodoacety1)-N'-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-
y1)Ethylenediamine
(IANBD), 7-diethylamino-3-(4'-maleimidylpheny1)-4-methylcoumarin (CPM), BODIPY
499,
BODIPY 507/545, BODIPY 499/508, Alexa 432, A1exa488, A1exa532, A1exa546, Cy5,
or 1-
(2-maleimidylethyl)-4-(5-(4-methoxyphenypoxazol-2-yppyridinium
methanesulfonate
(PyMPO maleimide) (PyMPO)). Numerous combinations of directly responsive
fluorophores and indirectly responsive fluorophores are possible. For example,
in various
non-limiting examples, (a) the donor fluorophore comprises Pacific Blue and
the acceptor
fluorophore comprises 5-IAF or 6-iodoacetamidofluorescein (6-IAF); (b) the
donor
fluorophore comprises Pacific Blue and the acceptor fluorophore comprises
Oregon Green;
(c) the donor fluorophore comprises IAEDANS and the acceptor fluorophore
comprises 5-
IAF or 6-IAF; (d) the donor fluorophore comprises acrylodan and the acceptor
fluorophore
comprises Alexa532; (e) the donor fluorophore comprises acrylodan and the
acceptor
fluorophore comprises 5-IAF or 6-IAF; (f) the donor fluorophore comprises
acrylodan and
the acceptor fluorophore comprises Pacific Blue or YFP; (g) the donor
fluorophore comprises
5-IAF or 6-IAF and the acceptor fluorophore comprises Pacific Blue; (h) the
donor
fluorophore comprises badan and the acceptor fluorophore comprises 5-IAF or 6-
IAF; or (i)
the donor fluorophore comprises badan and the acceptor fluorophore comprises
Alexa532.
Any of the ligand-binding proteins disclosed herein, as well as others, may be
included in the biosensors and methods that are provided. In some embodiments,
the ligand-
binding protein is selected from the group consisting of a glucose-galactose
binding protein
(GGBP), a glucose-binding protein, a urea-binding protein (UBP), a lactate-
binding protein
17
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
(LacBP), a calcium-binding protein, a calcium-bicarbonate binding protein
(BicarbBP), and
an iron-bicarbonate binding protein (FeBP).
Aspects include a biosensor for a ligand comprising a ligand-binding protein,
a
directly responsive fluorophore and an indirectly responsive fluorophore, the
directly
responsive and the indirectly responsive fluorophores being located at two
distinct sites of the
ligand-binding-protein, wherein (i) the directly responsive fluorophore is a
donor fluorophore
and the indirectly responsive fluorophore is an acceptor fluorophore; or (ii)
the directly
responsive fluorophore is an acceptor fluorophore and the indirectly
responsive fluorophore
is an donor fluorophore, and wherein if the acceptor fluorophore comprises
ruthenium or
osmium, then the acceptor fluorophore is not attached to the amino group of
the N-terminus
of the ligand-binding protein.
In some embodiments, the ligand-binding protein comprises the directly
responsive
fluorophore. In certain embodiments, the directly responsive fluorophore is
formed by an
autocatalytic cyclization of an oligopeptide within the ligand-binding
protein.
In various embodiments, the ligand-binding protein comprises a Yellow
Fluorescent
Protein (YFP; SEQ ID NO: 149) or a fluorescent mutant thereof, and the ligand
comprises a
halide anion. For example, the halide anion comprises a fluoride (F), chloride
(CF), a
bromide (BO, an iodide (F), an astatide (At-) anion, or an ununseptide (Ts)
anion. In some
embodiments, the mutant comprises a mutation that alters the interaction of
the mutant with a
bound halide anion compared to YFP. In certain embodiments, the mutant
comprises a
mutation that alters the affinity and/or specificity of the mutant for a
halide anion compared
to YFP. In various embodiments, the ligand-binding protein comprises 1, 2, 3,
4, or 5 halide
anion binding sites.
In some embodiments, at least one amino acid of the YFP or the fluorescent
mutant
thereof has been substituted with a cysteine. For example, the cysteine is
within a first J3-
strand (Pi), a second J3-strand (f32), a third J3-strand (f33), a fourth J3-
strand (f34), a fifth J3-strand
(135), a sixth J3-strand (P), a seventh J3-strand (P), an eighth J3-strand
(f38), a ninth J3-strand
(P), a tenth J3-strand (010), or an eleventh J3-strand (01i) of the YFP or the
fluorescent mutant
thereof. In certain embodiments, the ligand-binding protein comprises one or
more of the
following substitutions: E17X, E32X, T43X, F64X, G65X, L68X, Q69X, A72X, H77X,
K79X, R80X, E95X, R109X, R122X, D133X, H148X, N149X, V163X, N164X, D173X,
Y182X, Q183X, Y203X, Q204X, L221X, and H231X, wherein X is any amino acid, a
conservative substitution, or a cysteine, wherein each YFP amino acid position
is numbered
18
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
as in SEQ ID NO: 150. In non-limiting examples, the ligand-binding protein
comprises one
or more of the following substitutions: F64L, G65T, L68V, Q69T, A72S, K79R,
R80Q,
H148Q, H148G, V163A, H231L, H148Q, or Q183A, wherein each YFP amino acid
position
is numbered as in SEQ ID NO: 150. In various embodiments, the ligand-binding
protein
comprises an R at the 96 position, a Y at the 203 position, a S at the 205
position, and an E at
the 222 position, wherein each YFP amino acid position is numbered as in SEQ
ID NO: 150.
In various embodiments, ligand binding causes a change in signaling by the
directly
responsive fluorophore. In embodiments, the ligand-binding protein comprises a
mutation
compared to a naturally occurring protein. For example, at least one amino
acid of the
ligand-binding protein has been substituted with a cysteine. In some
embodiments, the
ligand-binding protein comprises a mutant of a microbial ligand-binding
protein. In certain
embodiments, the ligand-binding protein comprises a mutant of a microbial
periplasmic
ligand-binding protein.
In certain embodiments, the ligand comprises glucose, galactose, lactose,
arabinose,
ribose, maltose, lactate, urea, bicarbonate, phosphate, sulfate, chloride,
fluoride, iodide,
astatide, ununseptide, bromide, calcium, a hydrogen ion, a dipeptide,
histidine, glutamine,
glutamate, aspartate, or iron.
In some embodiments, the ligand-binding protein comprises a GGBP. For example,
the GGBP comprises or comprises a mutant of: an Escherichia sp. GGBP; a
Thermoanaerobacter sp. GGBP; a Clostridium sp. GGBP; a Salmonella sp. GGBP; a
Caldicellulosiruptor sp. GGBP; a Paenibacillus sp. GGBP; a Butyrivibrio sp.
GGBP; a
Roseburia sp. GGBP; a Faecalibacterium sp. GGBP; an Erysipelothrix sp. GGBP;
or an
Eubacterium sp. GGBP.
In some embodiments, the ligand-binding protein comprises a UBP. For example,
the
UBP comprises or comprises a mutant of: an Marinomas sp. UBP; a Marinobacter
sp. UBP;
a Bacillus sp. UBP; a Desulfotomaculum sp. UBP; a Geobacillus sp. UBP; a
Clostridium sp.
UBP; a Caldicellulosiruptor sp. UBP; a Thermocrinis sp. UBP; a Synechoccus sp
UBP; a
Paenibacillus sp. UBP; or a Thermosynechococcus sp UBP.
In some embodiments, the ligand-binding protein comprises a GBP. For example,
the
GBP comprises or comprises a mutant of: an Thermus sp GBP; a Deinococcus sp.
GBP; a
Thermotoga sp. GBP; a Kosmotoga sp. GBP; a Bacillus sp. GBP; a Staphylothermus
sp.
GBP; or an Arthrobacter sp. GBP.
19
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
In some embodiments, the ligand-binding protein comprises a LacBP. For
example,
the LacBP comprises or comprises a mutant of: a Thermus sp. LacBP; a
Thioalkalivibrio sp.
LacBP; a Roseobacter sp. LacBP; a Marinobacter sp. LacBP; a Anaeromyxobacter
sp.
LacBP; a Pseudomonas sp. LacBP; a Rhodobacter sp. LacBP;, a Flexistipes sp.
LacBP; or a
Thermanaerovibrio sp. LacBP.
In some embodiments, the ligand-binding protein comprises a calcium-binding
protein or a BicarbBP. For example, the ligand-binding protein comprises or
comprises a
mutant of: a Synechocystis sp. BicarbBP; a Thermosynechococcus sp. BicarbBP; a
Chroococcidiopsis sp. BicarbBP; a Calothrix sp. BicarbBP; a Anabaena sp.
BicarbBP; or a
Chamaesiphon sp. BicarbBP.
In some embodiments, the ligand-binding protein comprises a FeBP. For example,
the ligand-binding protein comprises or comprises a mutant of: a Mannheimia
sp. FeBP; an
Exiguobacterium sp. FeBP; a Thermosynechococcus sp FeBP; a Candidatus
Nitrospira sp.
FeBP; a Thermus sp. FeBP; a Meiothermus sp. FeBP; a Salinibacter sp. FeBP; or
a
Halorubrum sp. FeBP.
Also provide is a biosensor for a ligand comprising an amino acid or a
polypeptide, a
directly responsive fluorophore and an indirectly responsive fluorophore, the
directly
responsive and the indirectly responsive fluorophores being located at two
distinct sites of the
amino acid or polypeptide, wherein the directly responsive fluorophore is
chemoresponsive,
and wherein (i) the directly responsive fluorophore is a donor fluorophore and
the indirectly
responsive fluorophore is an acceptor fluorophore; or (ii) the directly
responsive fluorophore
is an acceptor fluorophore and the indirectly responsive fluorophore is an
donor fluorophore.
As noted above, any amino acid or polypeptide may be used to link the
chemoresponsive directly responsive fluorophore with the indirectly responsive
fluorophore,
provided the two fluorophores are close enough for ngmFRET to occur. In some
embodiments, the amino acid or polypeptide comprises 1 amino acid, or a
stretch of at least
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 150, 200,
250, 500, 750, or 1000
amino acids.
In some embodiments, the polypeptide comprises a stretch of at least 50, 60,
70, 80,
90, or 100 amino acids in a sequence that is at least about 85%, 90%, 95%, or
99% identical
to the amino acid sequence of ecTRX (SEQ ID NO: 151). In certain embodiments,
the
polypeptide comprises a mutant of ecTRX comprising a D3X, K4X, K1 9X, D27X,
K37X,
K53X, K58X, K70X, R74X, K83X, K91X, K97X, or K101X mutation, or any
combination
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
thereof, wherein X is any amino acid, and wherein each ecTRX amino acid
position is
numbered as in SEQ ID NO: 151. In some embodiments, the polypeptide comprises
a mutant
of ecTRX comprising a D3A, K4R, K4Q, K19R, K19Q, D27A, K37R, K53M, K53R, K58M,
K7OR, R74C, K83R, K91R, K97R, or K101R mutation, or any combination thereof,
wherein
each ecTRX amino acid position is numbered as in SEQ ID NO: 151. In some
embodiments,
the polypeptide comprises a mutant of ecTRX that does not comprise a lysine.
In various
embodiments, the biosensor comprises amino acids in the sequence of any one of
SEQ ID
NOS: 69-86 or 151.
In some embodiments, the polypeptide further comprises a hexahistidine tag.
In certain embodiments, the amino acid or polypeptide comprises at least 1, 2,
or 3
thiol groups; at least 1, 2, or 3 cysteines that each comprise a sulfhydryl
group; at least 1, 2,
or 3 primary amine groups; or at least 1, 2, or 3 lysines that each comprise a
primary amine.
In some embodiments, there is no disulfide bond between cysteines within the
amino acid
sequence of the polypeptide.
In various embodiments, the ligand comprises a hydrogen ion. In some
embodiments,
the biosensor is a biosensor for pH, wherein the directly responsive
fluorophore is pH-
sensitive. In certain embodiments, the fully excited emission intensity of the
directly
responsive fluorophore is different at a pH less than about 7.0 compared to a
pH of 7.5. In
some embodiments, the directly responsive fluorophore comprises a pH-sensitive
fluorophore
comprising fluorescein or a derivative thereof. In some embodiments, the
directly responsive
fluorophore transitions from a monoanion to a dianion at a pH that is less
than 7.0 in an
aqueous solution.
In some embodiments, the directly responsive fluorophore is attached to the
ligand-
binding protein, the amino acid, or the polypeptide via a covalent bond. In
some
embodiments, the covalent bond comprises a disulfide bond, a thioester bond, a
thioether
bond, an ester bond, an amide bond, or a bond that has been formed by a click
reaction. In
certain embodiments, the directly responsive fluorophore is attached to a
cysteine or a lysine
of the protein.
In various embodiments, the indirectly responsive fluorophore is attached to
the N-
terminus or the C-terminus of the protein. In some embodiments, the indirectly
responsive
fluorophore is attached to the N-terminus or the C-terminus of the protein via
a fluorophore
attachment motif. In some embodiments, the fluorophore attachment motif
comprises an
amino acid or a polypeptide. In certain embodiments, the polypeptide comprises
amino acids
21
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
in the sequence of PZif (SEQ ID NO: 42). In various embodiments, polypeptide
comprises a
stretch of at least 50, 60, 70, 80, 90, or 100 amino acids in a sequence that
is at least about
85%, 90%, 95%, or 99% identical to the amino acid sequence of E. coli
thioredoxin (ecTRX;
SEQ ID NO: 151).
In certain embodiments, the indirectly responsive fluorophore is attached to
the
ligand-binding protein via a covalent bond. In some embodiments, the covalent
bond
comprises a disulfide bond, a thioester bond, a thioether bond, an ester bond,
an amide bond,
or a bond that has been formed by a click reaction. In various embodiments,
the indirectly
responsive fluorophore is attached to a cysteine or a lysine of the protein.
Aspects of the present subject matter further provide a method for assaying
the level
of a ligand in a subject, comprising contacting a biosensor with a biological
sample from the
subject. Non-limiting examples of ligands include glucose, galactose, lactose,
arabinose,
ribose, maltose, lactate, urea, bicarbonate, phosphate, sulfate, chloride,
fluoride, iodide,
astatide, ununseptide, bromide, calcium, a hydrogen ion, a dipeptide,
histidine, glutamine,
glutamate, aspartate, and iron.
In some embodiments, the subject has or is suspected of having abnormal kidney
function, abnormal adrenal gland function, diabetes, hypochloremia, bromism,
hypothyroidism, hyperthyroidism, cretinism, depression, fatigue, obesity, a
low basal body
temperature, a goiter, a fibrocystic breast change, lactic acidosis, septic
shock, carbon
monoxide poisoning, asthma, a lung disease, respiratory insufficiency, Chronic
Obstructive
Pulmonary Disease (COPD), regional hypoperfusion, ischemia, severe anemia,
cardiac arrest,
heart failure, a tissue injury, thrombosis, or a metabolic disorder, diarrhea,
shock, ethylene
glycol poisoning, methanol poisoning, diabetic ketoacidosis, hypertension,
Cushing
syndrome, liver failure, cancer, or an infection.
In various embodiments, the biological sample comprises sweat, tear fluid,
blood,
serum, plasma, interstitial fluid, amniotic fluid, sputum, gastric lavage,
skin oil, milk, fecal
matter, emesis, bile, saliva, urine, mucous, semen, lymph, spinal fluid,
synovial fluid, a cell
lysate, venom, hemolymph, or a fluid obtained from a plant.
Also provided is a method for assaying the level of ligand in an environmental
sample, comprising contacting a biosensor with the environmental sample. In
some
embodiments, the environmental sample is from an environmental site that is
suspected of
being polluted. In some embodiments, the environmental sample has been
obtained or
provided from an environmental substance, fluid, or surface. In various
embodiments, the
22
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
environmental substance comprises (a) rock, soil, clay, sand, a meteorite, an
asteroid, dust,
plastic, metal, a mineral, a fossil, a sediment, or wood; (b) the
environmental surface
comprises the surface of a satellite, a bike, a rocket, an automobile, a
truck, a motorcycle, a
yacht, a bus, or a plane, a tank, an armored personnel carrier, a transport
truck, a jeep, a
mobile artillery unit, a mobile antiaircraft unit, a minesweeper, a Mine-
Resistant Ambush
Protected (MRAP) vehicle, a lightweight tactical all-terrain vehicle, a high
mobility
multipurpose wheeled vehicle, a mobile multiple rocket launch system, an
amphibious
landing vehicle, a ship, a hovercraft, a submarine, a transport plane, a
fighter jet, a helicopter,
a rocket, or an Unmanned Arial Vehicle, a drone, a robot, a building,
furniture, or an
organism; or (c) the environmental fluid comprises marine water, well water,
drinking well
water, water at the bottom of well dug for petroleum extraction or
exploration, melted ice
water, pond water, aquarium water, pool water, lake water, mud, stream water,
river water,
brook water, waste water, treated waste water, reservoir water, rain water, or
ground water.
Aspects of the present subject matter further provide a method for monitoring
the
level of a ligand, comprising periodically continuously detecting the level of
the ligand,
wherein detecting the level of the ligand comprises (a) providing or obtaining
a sample; (b)
contacting the sample with a biosensor for the ligand; and (c) detecting a
signal produced by
the biosensor. In some embodiments, the sample is provided or obtained from a
subject or
from a culture of microbial cells.
Additional embodiments and methods for detecting the presence and/or amount of
a
ligand are disclosed herein.
Aspects of the present subject matter also provide a method for constructing a
biosensor, comprising: (a) providing a ligand-binding protein; (b) identifying
at least one
putative allosteric, endosteric, or peristeric site of the ligand-binding
based a structure of the
ligand-binding protein; (c) mutating the ligand-binding protein to substitute
an amino acid at
the at least one putative allosteric, endosteric, or peristeric site of the
second protein with a
cysteine; (d) conjugating a donor fluorophore or an acceptor fluorophore to
the cysteine to
produce single labeled biosensor; (e) detecting whether there is a spectral
shift or change in
emission intensity of the single labeled biosensor upon ligand binding when
the donor
fluorophore or the acceptor fluorophore is fully excited; and (f) if a
spectral shift or change in
emission intensity is detected in (e), attaching a donor fluorophore to the
second protein if an
acceptor fluorophore is attached to the cysteine, and attaching an acceptor
fluorophore to the
second protein if an acceptor fluorophore is attached to the cysteine.
23
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
In various embodiments, the ligand-binding protein has been identified by (i)
selecting a first protein having a known amino acid sequence (seed sequence),
wherein the
first protein is known to bind a ligand; (ii) identifying a second protein
having an amino acid
sequence (hit sequence) with at least 15% sequence identity to the seed
sequence; (iii)
aligning the seed amino acid sequence and the hit sequence, and comparing the
hit sequence
with the seed sequence at positions of the seed sequence that correspond to at
least 5 primary
complementary surface (PCS) amino acids, wherein each of the at least 5 PCS
amino acids
has a hydrogen bond interaction or a van der Waals interaction with ligand
when ligand is
bound to the first protein; and (iv) identifying the second protein to be a
ligand-binding
protein if the hit sequence comprises at least 5 amino acids that are
consistent with the PCS.
In some embodiments, the spectral shift comprises a monochromatic fluorescence
intensity change or a dichromatic spectral shift.
Also provided is a method of converting a biosensor that shows a monochromatic
response upon ligand binding into a biosensor with a dichromatic response upon
ligand
binding, the method comprising (a) selecting a biosensor that exhibits a
monochromatic
response upon ligand binding, wherein the biosensor comprises a ligand-binding
protein and
a first reporter group; and (b) attaching a second reporter group to the
biosensor, wherein the
second reporter group has (i) an excitation spectrum that overlaps with the
emission spectrum
of the first reporter group; or (ii) an emission spectrum that overlaps with
the excitation
spectrum of the first reporter group.
The present subject matter also includes method of converting a biosensor that
shows
a monochromatic response upon ligand binding into a biosensor with a
dichromatic response
upon ligand binding, the method comprising (a) selecting a biosensor that
exhibits a
monochromatic response upon ligand binding, wherein the biosensor comprises a
ligand-
binding fluorescent protein; and (b) attaching an acceptor fluorophore or a
donor fluorophore
to the biosensor, wherein (i) the acceptor fluorophore has an excitation
spectrum that overlaps
with the emission spectrum of the fluorescent protein; or (ii) the donor
fluorophore has an
emission spectrum that overlaps with the excitation spectrum of the
fluorescent protein.
Also provided is a method of increasing a dichromatic response of a biosensor
to
ligand binding, the method comprising (a) selecting a biosensor that exhibits
a dichromatic
response upon ligand binding, wherein the biosensor comprises a ligand-binding
protein and
a first reporter group; and (b) attaching a second reporter group to the
biosensor, wherein the
second reporter group has (i) an excitation spectrum that overlaps with the
emission spectrum
24
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
of the first reporter group; or (ii) an emission spectrum that overlaps with
the excitation
spectrum of the first reporter group.
In some embodiments, the second reporter group is within about 0.1, 0.2, 0.3,
0.4, 0.5,
0.6, 0.7, 0.8, 0.9, 1.0, 1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 2, 4, 6,
8, 10, 11, 12, 13, 14, 15,
16, 17, 18, 19, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90,
95, 100, 125, 150, or
200 angstroms (A) of the first reporter group regardless of whether ligand is
bound to the
biosensor. Suitable distances may be determined in part by the distance-
dependence of the
energy transfer between a given donor-acceptor pair (see, e.g, J.R. Lakowicz,
2006,
Principles of Fluorescence Spectroscopy, Springer, incorporated herein by
reference). In
some embodiments, when the ligand is bound to the biosensor, the average
distance between
the first reporter group and the second reporter group changes by less than
about 5, 4, 3, 2, 1,
0.9, 0.8, 0.7, 0.6, 0.5, 0.4, 0.3, 0.2, 0.1, 0.05, or 0.01 angstroms (A)
compared to when ligand
is not bound to the ligand-binding protein.
The present subject matter further provides a method of converting a biosensor
that
shows a monochromatic response upon ligand binding into a biosensor with a
dichromatic
response upon ligand binding, the method comprising (a) selecting a biosensor
that exhibits a
monochromatic response upon ligand binding, wherein said biosensor comprises
an amino
acid or polypeptide and a first reporter group, wherein the first reporter
group comprises a
chemoresponsive fiuorophore; and (b) attaching a second reporter group to said
biosensor,
wherein said second reporter group has (i) an excitation spectrum that
overlaps with the
emission spectrum of said first reporter group; or (ii) an emission spectrum
that overlaps with
the excitation spectrum of said first reporter group.
Also included is a method of increasing a dichromatic response of a biosensor
to
ligand binding, the method comprising (a) selecting a biosensor that exhibits
a dichromatic
response upon ligand binding, wherein said biosensor comprises an amino acid
or a
polypeptide and a first reporter group, wherein the first reporter group
comprises a
chemoresponsive fiuorophore; and (b) attaching a second reporter group to said
biosensor,
wherein said second reporter group has (i) an excitation spectrum that
overlaps with the
emission spectrum of said first reporter group; or (ii) an emission spectrum
that overlaps with
the excitation spectrum of said first reporter group.
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
tgmFRET Biosensors
While ngmFRET is preferred to tgmFRET, tgmFRET may be used alternatively or in
addition to ngmFRET in certain embodiments.
In various embodiments, the biosensor comprises multiple reporter groups,
including
a first reporter group and a second reporter group. For example, the first
reporter group may
comprise a donor fluorophore and the second reporter group may comprise an
acceptor
fluorophore. In certain embodiments, FRET is detectable by a change in the
fluorescence of
the acceptor fluorophore or by a decrease in donor fluorophore fluorescence.
In various
embodiments, the donor fluorophore, and/or the acceptor fluorophore is
fluorescent. In some
embodiments, both the donor fluorophore and the acceptor fluorophore are
fluorescent.
In various embodiments, the angle and/or distance between the donor
fluorophore and
the acceptor fluorophore changes upon ligand binding. In some embodiments,
neither the
donor fluorophore nor the acceptor fluorophore is directly responsive to
ligand binding. In
some embodiments the donor fluorophore and/or the acceptor fluorophore is
attached to the
N-terminus or the C-terminus of the ligand-binding protein (e.g., directly or
via a fluorophore
attachment motif). In certain embodiments, the donor fluorophore and/or the
acceptor
fluorophore is attached to a fluorophore attachment motif. For example, the
fluorophore
attachment motif may be conjugated to the N-terminus or the C-terminus of the
ligand-
binding protein.
In some embodiments, the donor fluorophore and/or the acceptor fluorophore
comprises a fluorescent protein. In various embodiments, the donor fluorophore
and/or the
acceptor fluorophore comprises an organic compound having a molecular weight
less than
about 2000 Da (e.g., 5-iodoacetamidofluorescein (5-IAF) or 6-
iodoacetamidofluorescein (6-
IAF), rhodamine, Oregon Green, eosin, Texas Red, indocarbocyanine,
oxacarbocyanine,
thiacarbocyanine, merocyanine, Badan, Acrylodan, IAEDANS, comprising 3-cyano-7-
hydroxycoumarin, 7-hydroxycoumarin-3-carboxylic acid, 6,8-difluoro-7-hydroxy-
4-
methylcoumarin, or 7-amino-4-methylcoumarin, pyridyloxazole,
nitrobenzoxadiazole,
benzoxadiazole, DRAQ5, DRAQ7, or CyTRAK Orange, cascade blue, Nile red, Nile
blue,
cresyl violet, oxazine 170, proflavin, acridine orange, acridine yellow,
auramine, crystal
violet, malachite green, porphin, phthalocyanine, bilirubin, pyrene, N,N-
dimethyl-N-
(iodoacety1)-N'-(7-nitrobenz-2-ox- a-1,3-diazol-4-ypethylenediamide (NBD), N-
((2-
(iodoacetoxy)ethyl)-N-methy- 1)amino-7-nitrobenz-2-oxa-1,3-diazole (NBDE),
Acrylodan,
JPW4039, JPW4042, JPW4045, Oregon Green, Pacific Blue, CPM, N,N'-Dimethyl-N-
26
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
(Iodoacety1)-N'-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-y1)Ethylenediamine (IANBD), 7-
diethylamino-3-(4'-maleimidylpheny1)-4-methylcoumarin (CPM), BODIPY 499,
BODIPY
507/545, BODIPY 499/508, Alexa 432, A1exa488, A1exa532, A1exa546, Cy5, or 1-(2-
maleimidylethyl)-4-(5-(4-methoxyphenypoxazol-2- yppyridinium methanesulfonate
(PyMPO maleimide) (PyMPO)). For example, the organic compound is a
fluorophore.
Numerous combinations of donor and acceptor fluorophores are possible.
Reporter Group Attachment
Aspects of the present subject matter provide a biosensor that comprises a one
or
more reporter groups attached to a ligand-binding protein, wherein binding of
a ligand to a
ligand-binding domain of the ligand-binding protein causes a change in
signaling by the
reporter group. In various embodiments, the reporter group is attached to an
endosteric site,
an allosteric site, or a peristeric site of the ligand-binding protein. In
embodiments, the
reporter group is covalently or noncovalently attached to the ligand-binding
protein.
For convenience and depending on context, a reporter group may be referred to
by a
name of an unattached form of the reporter group regardless of whether the
reporter group is
attached to a ligand-binding protein. For example, a compound known as
"Compound A"
when in an unconjugated form may be referred to herein as "Compound A" when in
a form
that is attached to a ligand-binding protein. In a specific example, the term
"Acrylodan" is
used to refer to unreacted/unconjugated Acrylodan, as well as Acrylodan that
is conjugated to
a ligand-binding protein.
In certain embodiments, a biosensor comprises a reporter group that is
conjugated to a
ligand-binding protein, and the reporter group is conjugated to an amino acid
of the protein
that is at least about 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.1,
1.2, 1.3, 1.4, 1.5, 1.6,
1.7, 1.8, 1.9, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19,
20, 25, 30, 35, 40, 45,
50, 55, 60, 65, 70, 75, 80, 85, 90, 95, or 100 angstroms (A) from the ligand
when the ligand is
bound to the protein. In embodiments, the reporter group is conjugated to an
amino acid of
the protein that is about 0.1 A to about 5 A, about 5 A to about 10 A, about
10 A to about 20
A, about 20 A to about 50 A, about 50 A to about 75 A, or about 75 A to about
100 A from
the ligand when the ligand is bound to the protein. In some embodiments, the
reporter group
is conjugated to an amino acid of the protein that is within an a-helix or a
J3-strand. In some
embodiments, the reporter group is conjugated to an amino acid that (i) is not
within an a-
helix or a J3-strand, but is within about 10, 9, 8, 7, 6, 5, 4, 3, 2, or 1
amino acids of an amino
27
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
acid of the protein's amino acid sequence that is within an a-helix or a J3-
strand. In some
embodiments, the reporter group is conjugated to an amino acid that is in an
inter-domain
hinge amino acid region between two domains of a protein. In some embodiments,
the
reporter group is conjugated to an amino acid that is in an inter-domain hinge
amino acid
region between (i) a a-helix and a J3-strand; (ii) two a-helixes; or (iii) two
J3-strands of a
protein. In some embodiments, the reporter group is conjugated to an amino
acid (e.g., a
cysteine such as a cysteine added by substitution compared to a naturally
corresponding
polypeptide) between positions 1-25, 25-50, 50-75, 75-100, 100-125, 125-150,
150-175, 175-
200, 200-225, 225-250, 250-275, 275-350, 275-300, 275-325, 300-325, 300-350,
300-400, or
350-450 (inclusive) of a polypeptide (e.g., not including N-terminal fusion
proteins compared
to the polypeptide's naturally occurring counterpart).
In certain embodiments, the directly or indirectly responsive fluorophore is
conjugated (directly or via a fluorophore attachment motif) to an amino acid
that is no more
than about 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22,
23, 24, 25, 26, 27,
28, 29, 30, 40, 50, 60, 70, 80, 90, 100, 5-15, 5-20, 5-25, 5-100, 10-15, 10-
20, 10-25, 10-50,
10-100, 25-50, 25-75, or 25-100 amino acids from the N-terminus or the C-
terminus of the
ligand-binding protein. In some embodiments, the directly or indirectly
responsive
fluorophore is conjugated (directly or via a fluorophore attachment motif) to
an amino acid
that is at least about 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19,
20, 21, 22, 23, 24, 25,
26, 27, 28, 29, 30, 40, 50, 60, 70, 80, 90, 100, 5-15, 5-20, 5-25, 5-100, 10-
15, 10-20, 10-25,
10-50, 10-100, 25-50, 25-75, or 25-100 amino acids from the N-terminus or the
C-terminus
of the ligand-binding protein. In some embodiments, about 5, 6, 7, 8, 9, 10,
11, 12, 13, 14,
15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 40, 50, 60,
70, 80, 90, 100, 5-15,
5-20, 5-25, 5-100, 10-15, 10-20, 10-25, 10-50, 10-100, 25-50, 25-75, or 25-100
amino acids
(including or not including the signal peptide) have been deleted (e.g. are
absent) from the N-
terminus of the protein compared to its naturally occurring counterpart. In
some
embodiments, less than 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19,
20, 21, 22, 23, 24,
25, 26, 27, 28, 29, 30, 40, 50, 60, 70, 80, 90, 100, 5-15, 5-20, 5-25, 5-100,
10-15, 10-20, 10-
25, 10-50, 10-100, 25-50, 25-75, or 25-100 amino acids (including or not
including the signal
peptide) have been deleted (e.g. are absent) from the N-terminus of the
protein compared to
its naturally occurring counterpart. In some embodiments, about 5, 6, 7, 8, 9,
10, 11, 12, 13,
14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 40, 50,
60, 70, 80, 90, 100, 5-
15, 5-20, 5-25, 5-100, 10-15, 10-20, 10-25, 10-50, 10-100, 25-50, 25-75, or 25-
100 amino
28
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
acids have been deleted (e.g. are absent) from the C-terminus of the protein
compared to its
naturally occurring counterpart. In some embodiments, less than 5, 6, 7, 8, 9,
10, 11, 12, 13,
14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 40, 50,
60, 70, 80, 90, 100, 5-
15, 5-20, 5-25, 5-100, 10-15, 10-20, 10-25, 10-50, 10-100, 25-50, 25-75, or 25-
100 amino
acids have been deleted (e.g. are absent) from the C-terminus of the protein
compared to its
naturally occurring counterpart.
Periplasmic binding proteins are characterized by two lobes connected by a
hinge
region; ligand bind at a location at the interface between the two domains.
Such proteins or
engineered versions thereof (as described herein) can adopt two different
conformations: a
ligand-free open form and a ligand-bound closed form, which interconvert
through a
relatively large bending motion around the hinge (FIG. 1A; Dwyer et al., 2004,
Current
Opinion in Structural Biology 12:495-504).
The remarkable adaptability of this superfamily of ligand-binding proteins is
likely to
have arisen from positioning the location of binding of the ligand at the
interface between the
lobes and from the large ligand-mediated conformational change. In this
arrangement, ligands
are placed within an environment that resembles a protein interior, but the
residues forming
the contact points or contact sites with the ligand are positioned at the
surface of the lobes.
Direct signaling relationships between proteins and reporter groups are
readily
designed by replacing a residue known to form a ligand contact with a cysteine
to which the
fluorophore is attached ("endosteric" attachment site). Other, indirect
signaling relationships
can be established in two ways. The first relies on visual inspection of the
ligand complex
structure, and identifying residues that are located in the vicinity of the
binding site, but do
not interact directly with the ligand, and that are likely to be involved in
conformational
changes. Typically, such "peristeric" sites are located adjacent to the
residues that form
direct contacts with the bound ligand. In the case of the bPBPs, such residues
are located at
the perimeter of the inter-domain cleft that forms the ligand binding site
location. The
environment of these peristeric sites changes significantly upon formation of
the closed state.
These are examples of positions which are proximal to the ligand-binding
pocket/domain.
The second, most general, approach identifies sites in the protein structure
that are located
anywhere in the protein, including locations at some distance away from the
ligand-binding
site (i.e., distal to the ligand-binding pocket/domain), and undergo a local
conformational
change in concert with ligand binding. If the structures of both the open and
closed states are
known, then such "allosteric" sites can be identified using a computational
method that
29
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
analyzes the conformational changes that accompany ligand binding (Marvin et
al., Proc.
Natl. Acad. Sci. USA 94:4366-4371, 1997). Alternatively, once allosteric sites
have been
identified in one bPBP, modeling and structural homology arguments can be
invoked to
identify such sites in other bPBPs in which only one state has been
characterized (Marvin &
Hellinga, J. Am. Chem. Soc. 120:7-11, 1998). This generalized conformational
analysis also
may identify peristeric and endosteric sites, which were identified and
classified by visual
inspection. The domain or region involved in ligand binding is comprised of a
plurality of
residues, e.g., non-contiguous amino acids of the ligand-binding protein,
which are contact
points or sites of contact between the ligand and its cognate ligand-binding
protein.
In non-limiting implementations, the reporter group is attached to the ligand-
binding
protein via a biotin-avidin interaction. The reporter group may be, e.g.,
conjugated to biotin
and the ligand-binding protein is conjugated to avidin. In an example, the
avidin is bound to
four biotin molecules wherein each biotin molecule is individually conjugated
to a reporter
group. Alternatively, the reporter group is conjugated to avidin and the
ligand-binding
protein is conjugated to biotin. For example, the avidin is bound to four
biotin molecules,
wherein each biotin molecule is individually conjugated to a ligand-binding
protein.
As used herein, "conjugated" means covalently attached. One compound may be
directly conjugated to another compound, or indirectly conjugated, e.g., via a
linker.
In some embodiments, the reporter group is directly attached to the ligand-
binding
protein. In various embodiments, the reporter group is attached to an amino
acid of the
ligand-binding protein that is at least about 2, 4, 6, 8, 10, 11, 12, 13, 14,
15, 16, 17, 18, 19,
20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95, or 100
angstroms (A) from the
ligand when the ligand is bound to the ligand-binding protein. In certain
embodiments, the
reporter group is conjugated to an amino acid having a position within
positions 1-25, 25-50,
50-75, 75-100, 100-125, 125-150, 150-175, 175-200, 200-225, 225-250, 250-275,
or 275-300
of the ligand-binding protein, wherein position 1 is the N-terminal amino acid
of the ligand-
binding protein. In non-limiting examples, the reporter group is conjugated to
an amino acid
of the ligand-binding protein that is (a) within an a-helix or a J3-strand of
the ligand-binding
protein; (b) not within an a-helix; (c) not within a J3-strand; (d) within
about 5 or 10 amino
acids of an amino acid that is within an a-helix or J3-strand; (e) within a
stretch of consecutive
amino acids that links two domains of the ligand-binding protein; (f) within a
stretch of
consecutive amino acids that links an a-helix and a J3-strand; (g) within a
stretch of
consecutive amino acids that links two a-helices; or (h) within a stretch of
consecutive amino
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
acids that links two J3-strands. In some embodiments, the reporter group is
directly attached
to the N-terminus or the C-terminus of the ligand-binding protein.
The reporter group may be conjugated to the ligand-binding protein a variety
of
linkers or bonds, including (but not limited to) a disulfide bond, an ester
bond, a thioester
bond, an amide bond, or a bond that has been formed by a click reaction. In
some
embodiments, the click reaction is a reaction between (a) an azide and an
alkyne; (b) an azide
and an alkyne in the presence of Cu(I); (c) an azide and a strained
cyclooctyne; (d) an azide
and a dibenzylcyclooctyne, a difiuorooctyne, or a biarylazacyclooctynone; (e)
a diaryl-
strained-cyclooctyne and a 1,3-nitrone; (f) an azide, a tetrazine, or a
tetrazole and a strained
alkene; (g) an azide, a tetrazine, or a tretrazole and a oxanorbomadiene, a
cyclooctene, or a
trans-cycloalkene; (h) a tetrazole and an alkene; or (i) a tetrazole with an
amino or styryl
group that is activated by ultraviolet light and an alkene. These exemplary
click chemistry
reactions have high specificity, efficient kinetics, and occur in vivo under
physiological
conditions. See, e.g., Baskin et al. Proc. Natl. Acad. Sci. USA
104(2007):16793; Oneto et al.
Acta biomaterilia (2014); Neves et al. Bioconjugate chemistry 24(2013):934;
Koo et al.
Angewandte Chemie 51(2012):11836; Rossin et al. Angewandte Chemie
49(2010):3375, and
U.S. Patent Application Publication No. 20160220686, published August 4, 2016,
the entire
content of each of which is incorporated herein by reference. For a review of
a wide variety
of click chemistry reactions and their methodologies, see e.g., Nwe K and
Brechbiel M W,
2009 Cancer Biotherapy and Radiopharmaceuticals, 24(3): 289-302; Kolb H C et
al., 2001
Angew. Chem. Int. Ed. 40: 2004-2021. The entire contents of each of the
foregoing
references are incorporated herein by reference.
As used herein, the term "linker" refers to a molecule or sequence (such as an
amino
acid sequence), that attaches, as in a bridge, one molecule or sequence to
another molecule or
sequence. "Linked" means attached or bound by covalent bonds, or non-covalent
bonds, or
other bonds, such as van der Waals forces. In some embodiments, a linker
comprises a
chemical structure that has resulted from a reaction used to attach one
molecule to another.
In various implementations of the present subject matter, the reporter group
is
conjugated to a cysteine of the ligand-binding protein. The cysteine may be
present on a
natural counterpart or version of the ligand-binding protein or added to the
ligand-binding
protein by a substitution mutation. In some embodiments, the cysteine is at
the N-terminus or
the C-terminus of the ligand-binding protein.
31
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Non-limiting examples relate to the conjugation of a reporter group to a
primary
amine of the ligand-binding protein. In certain embodiments, the primary amine
is present in
a lysine of the ligand-binding protein. The lysine may be present on a natural
counterpart or
version of the ligand-binding protein or added to the ligand-binding protein
by a substitution
mutation. In various embodiments, the lysine is at the N-terminus or the C-
terminus of the
ligand-binding protein.
Aspects of the present subject matter provide a biosensor in which the
reporter group
is attached to the ligand-binding protein via a linker. In some embodiments,
the linker
comprises an organic compound that is less than about 30, 20, 15, or 10 A
long. Non-
limiting examples of linkers include 0, S, NH, PH, and alkyl linkers.
"Alkyl," as used herein, refers to the radical of saturated or unsaturated
aliphatic
groups, including straight-chain alkyl, alkenyl, or alkynyl groups, branched-
chain alkyl,
alkenyl, or alkynyl groups, cycloalkyl, cycloalkenyl, or cycloalkynyl
(alicyclic) groups, alkyl
substituted cycloalkyl, cycloalkenyl, or cycloalkynyl groups, and cycloalkyl
substituted alkyl,
alkenyl, or alkynyl groups. Unless otherwise indicated, a straight chain or
branched chain
alkyl has 30 or fewer carbon atoms in its backbone (e.g., C1-C30 for straight
chain, C3-C30 for
branched chain), more preferably 20 or fewer carbon atoms, more preferably 12
or fewer
carbon atoms, and most preferably 8 or fewer carbon atoms. Likewise, preferred
cycloalkyls
have from 3-10 carbon atoms in their ring structure, and more preferably have
5, 6 or 7
carbons in the ring structure. The ranges provided above are inclusive of all
values between
the minimum value and the maximum value. The term "alkyl" includes both
"unsubstituted
alkyls" and "substituted alkyls," the latter of which refers to alkyl moieties
having one or
more substituents replacing a hydrogen on one or more carbons of the
hydrocarbon backbone.
Such substituents include, but are not limited to, halogen, hydroxyl, carbonyl
(such as a
carboxyl, alkoxycarbonyl, formyl, or an acyl), thiocarbonyl (such as a
thioester, a thioacetate,
or a thioformate), alkoxyl, phosphoryl, phosphate, phosphonate, a phosphinate,
amino,
amido, amidine, imine, cyano, nitro, azido, sulfhydryl, alkylthio, sulfate,
sulfonate,
sulfamoyl, sulfonamido, sulfonyl, heterocyclyl, aralkyl, or an aromatic or
heteroaromatic
moiety. Unless the number of carbons is otherwise specified, "lower alkyl" as
used herein
means an alkyl group, as defined above, but having from one to ten carbons,
more preferably
from one to six carbon atoms in its backbone structure. Likewise, "lower
alkenyl" and "lower
alkynyl" have similar chain lengths. Preferred alkyl groups are lower alkyls.
The alkyl
groups may also contain one or more heteroatoms within the carbon backbone.
Preferably the
32
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
heteroatoms incorporated into the carbon backbone are oxygen, nitrogen,
sulfur, and
combinations thereof. In certain embodiments, the alkyl group contains between
one and
four heteroatoms.
In some embodiments, the linker comprises a bond formed by a chemical reaction
involving a reactive group such as a maleimide group. Alternatively or in
addition, the linker
comprises a stretch of amino acids. In a non-limiting example, the linker
comprises a
polyglycine linker. In embodiments, the polyglycine linker comprises 2, 3, 4,
5, or more
glycines. Optionally, the polyglycine linker further comprises a serine.
In various implementations, the reporter group is attached to a linker via a
covalent
bond and the linker is attached to a ligand-binding protein via a covalent
bond. In
embodiments, the covalent bond between the linker and the reporter group
and/or the
covalent bond between the linker and the ligand-binding protein is a disulfide
bond, an ester
bond, a thioester bond, an amide bond, a carbamate bond, or a bond that has
been formed by
a click reaction. Non-limiting examples of click reactions include reactions
between an azide
and an alkyne; an azide and an alkyne in the presence of Cu(I); an azide and a
strained
cyclooctyne; an azide and a dibenzylcyclooctyne, a difluorooctyne, or a
biarylazacyclooctynone; a diaryl-strained-cyclooctyne and a 1,3-nitrone; an
azide, a tetrazine,
or a tetrazole and a strained alkene; an azide, a tetrazine, or a tretrazole
and a
oxanorbomadiene, a cyclooctene, or a trans-cycloalkene; a tetrazole and an
alkene; or a
tetrazole with an amino or styryl group that is activated by ultraviolet light
and an alkene.
The present subject matter also includes biosensors having one or more
reporter
groups attached to a ligand-binding protein via a fluorophore attachment
motif.
Fluorophore Attachment Motifs
Aspects of the present subject matter include the use of one or more
fluorophore
attachment motifs to attach one or more reporter groups to a ligand-binding
protein. For
example, a reporter group may be attached to a fluorophore attachment motif
that is attached
to the N-terminus or the C-terminus of the ligand-binding protein.
In various implementations, the fluorophore attachment motif comprises a
polypeptide. In some embodiments, the polypeptide comprises amino acids in the
PZif amino
acid sequence (SEQ ID NO: 42).
In some embodiments, the polypeptide comprises a stretch of at least 50, 60,
70, 80,
90, or 100 amino acids in a sequence that is at least about 85%, 90%, 95%, or
99% identical
33
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
to the amino acid sequence of E. coli thioredoxin (ecTRX; SEQ ID NO: 151). In
some
embodiments, the polypeptide is a mutant of ecTRX comprising a D3X, K4X, K1
9X, D27X,
K37X, K53X, K58X, K70X, R74X, K83X, K91X, K97X, or K101X mutation, or any
combination thereof, wherein X is any amino acid, and wherein each ecTRX amino
acid
position is numbered as in SEQ ID NO: 151. In certain embodiments, the
polypeptide is a
mutant of ecTRX comprising a D3A, K4R, K4Q, K19R, K19Q, D27A, K37R, K53M,
K53R,
K58M, K7OR, R74C, K83R, K91R, K97R, or K101R mutation, or any combination
thereof,
wherein each ecTRX amino acid position is numbered as in SEQ ID NO: 151.
In non-limiting examples, the polypeptide comprises amino acids in the
sequence of
any one of SEQ ID NOS: 69-86 or 151.
In certain embodiments, the polypeptide comprises (a) at least 1, 2, or 3
thiol groups;
(b) at least 1, 2, or 3 cysteines that each comprise a sulfhydryl group; (c)
at least 1, 2, or 3
primary amine groups; and/or (d) at least 1, 2, or 3 lysines that each
comprise a primary
amine. In some embodiments there is no disulfide bond between cysteines within
the amino
acid sequence of the polypeptide.
In some embodiments, the polypeptide comprises a hexahistidine tag. In some
embodiments, the hexahistidine tag is attached to another portion of the
polypeptide via a
GGS linker.
Reporter Groups
Various types of reporter groups may be used in embodiments of the present
subject
matter. For example, the reporter group may comprise a fluorophore that
produces a
fluorescent signal. Biosensors comprising a fluorophore may be referred to
herein as
fluorescently responsive sensors (FRSs).
Preferably, the binding of ligand to an FRS results in a change in ratiometric
AR in
the signal from a reporter group. A ratiometric signal (R1,2) is defined as
the quotient of two
intensities, /xi and Ix2, measured at two independent wavelengths, k1 and k2
and may be
calculated according to the following equation:
R1,2 = -1,11 /-1,12
The two independent wavelengths Xi and k2 may be from a single fluorophore or
from
a combination of two or more fluorophores (e.g., a pair of fluorophores
between which
tgmFRET and/or ngmFRET occurs). In some embodiments, k1 falls within the
emission
34
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
spectrum of a directly responsive fluorophore and k2 falls within the emission
spectrum of an
indirectly responsive fluorophore. In certain embodiments, Xi falls within the
emission
spectrum of an indirectly responsive fluorophore and k2 falls within the
emission spectrum of
a directly responsive fluorophore. In various embodiments, k1 falls within the
emission
spectrum of both a directly responsive fluorophore and an indirectly
responsive fluorophore.
In various embodiments, k2 falls within the emission spectrum of both a
directly responsive
fluorophore and an indirectly responsive fluorophore.
In some embodiments, intensities are, e.g., integrated, filtered, assessed,
detected, or
evaluated over a range of wavelengths. In some embodiments, intensities are
integrated over
a range of wavelengths in a recorded emission spectrum. In some embodiments, a
range of
wavelengths is selected using a filter. In some embodiments, k1 is the
intensity over a 1 nm
to 60 nm interval centered between 400 and 1000 nm, and k2 is the intensity
over a 1 nm to
60 nm interval centered between 400 nm and 1000 nm. In some embodiments,
intensities are
integrated, filtered, assessed, detected, or evaluated over a 1 nm, 2 nm,
lOnm, 15nm, 20nm,
25nm, 30nm, 35nm, 40nm, 45nm, 50nm, 55 nm, 60 nm, 75 nm, 100 nm, 10-40 nm, 10-
50
nm, 20-50 nm, or 10-100 nm regions, centered between 400-1000 nm, e.g. between
420 nm
and 520 nm for k1, and 400-1000nm, e.g. between 500 nm to 600 nm for k2. In
some
embodiments, intensities are recorded through a bandpass filter. A non-
limiting example of a
bandpass filter is a 10 nm, 15 nm, 20 nm, 25 nm, 30 nm, 35 nm, 40 nm, 45 nm,
50 nm, 75
nm, 100 nm, 10-40 nm, 10-50 nm, 20-50 nm, or 10-100 nm bandpass filter,
centered between
400-1000 nm, e.g. at 452 nm forki and at 400-1000nm, e.g. at 528 nm (k2).
Aspects of the present subject matter provide FRSs whose emission spectra
change
(e.g., the shape of the emission spectra change) in response to ligand
binding. In various
embodiments, the ratio of intensities at two chosen wavelengths of an FRS's
emission
spectrum changes upon ligand binding.
In various embodiments, the emission spectra of two or more fluorophores
contributes
to /xi and/or A2. In some embodiments, the emission spectrum of a directly
responsive
fluorophore contributes to Ai and/or A2 and the emission spectrum of an
indirectly responsive
fluorophore contributes to Ai and/or A2. In certain embodiments, a directly
responsive
fluorophore contributes to Ai and the emission spectrum of an indirectly
responsive
fluorophore contributes to A2. In some embodiments, a directly responsive
fluorophore
contributes to A2 and the emission spectrum of an indirectly responsive
fluorophore
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
contributes to Ai. In various embodiments, both the emission spectrum of a
directly
responsive fluorophore and the emission spectrum of an indirectly responsive
fluorophore
contributes to Ai. In some embodiments, both the emission spectrum of a
directly responsive
fluorophore and the emission spectrum of an indirectly responsive fluorophore
contributes to
I.
In some embodiments, the emission wavelength and/or intensity of a fluorophore
(e.g., a single fluorophore in a biosensor comprising one reporter group or a
directly
responsive fluorophore comprising reporter groups between which tgmFRET and/or
ngmFRET occurs) changes when the positions of atoms within the fluorophore
change with
respect to each other (e.g., due to the rotation of bound atoms with respect
to each other or a
change in the angle of a bond). In non-limiting examples, the emission
wavelength and/or
intensity of the fluorophore changes when (i) one portion of the fluorophore
rotates around a
bond axis compared to another portion of the fluorophore and/or (ii) when the
angle of a bond
between two atoms of the fluorophore changes. In a non-limiting example, the
fluorophore is
a prodan-derived fluorophore (e.g., Acrylodan or Badan) and binding of ligand
alters the
orientation of a dimethylamino group, a naphthalene ring, and/or a carbonyl
with respect to
the ligand-binding protein and/or each other. In a non-limiting example, the
degree of
polarization of a dipole on the fluorophore changes in response to ligand
binding. In various
embodiments, the emission wavelength and/or intensity of the fluorophore
changes when an
atom electrostatically interacts with the fluorophore. For example, the
emission wavelength
and/or intensity of the fluorophore changes when the source of a positive or
negative charge
changes its distance with respect to the fluorophore within about 1, 2, 3, 4,
5, or 10 A of the
fluorophore. In some embodiments, the fluorophore exhibits hypsochromicity or
bathochromicity upon ligand binding to the ligand-binding domain of the ligand-
binding
protein. In certain embodiments, the fluorophore has an emission spectrum
comprising
radiation with a wavelength (e.g., a peak emission wavelength) of about 400
nm, 410 nm, 420
nm, 430 nm, 440 nm, 450 nm, 460 nm, 470 nm, 480 nm, 490 nm, 500 nm, 510 nm,
520 nm,
530 nm, 540 nm, 550 nm, 560 nm, 570 nm, 580 nm, 590 nm, 600 nm, 610 nm, 620
nm, 630
nm, 640 nm, 650 nm, 660 nm, 670 nm, 680 nm, 690 nm, 700 nm, 710 nm, 720 nm,
730 nm,
740 nm, 750 nm, 760 nm, 770 nm, 780 nm, 790 nm, 800 nm, 850 nm, 900 nm, 950
nm, or
1000 nm, or about 400 nm to about 450 nm, about 450 nm to about 500 nm, about
500 nm to
about 550 nm, about 550 nm to about 600 nm, about 600 nm to about 650nm, about
650 to
36
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
about 700 nm, about 700 nm to about 750 nm, about 750 nm to about 800 nm, or
about 800
nm to about 1000 nm.
In some embodiments, the signal comprises the emission intensity of the FRS
recorded at a single wavelength or range of wavelengths. The change in signal
may be a shift
in the single wavelength or range of wavelengths. In some embodiments, the
shift in the
wavelength is at least about 1 nm, at least about 2 nm, at least about 3 nm,
at least about 4
nm, at least about 5 nm, at least about 6 nm, at least about 7 nm, at least
about 8 nm, at least
about 9 nm, at least about 10 nm, at least about 11 nm, at least about 12 nm,
at least about 13
nm, at least about 14 nm, at least about 15 nm, at least about 16 nm, at least
about 17 nm, at
least about 18 nm, at least about 19 nm, at least about 20 nm, at least about
25 nm, at least
about 30 nm, at least about 35 nm, at least about 40 nm, at least about 45 nm,
at least about
50 nm, at least about 55 nm, at least about 60 nm, at least about 65 nm, at
least about 70 nm,
at least about 75 nm, at least about 80 nm, at least about 85 nm, at least
about 90 nm, at least
about 95 nm, at least about 100 nm, at least about 105 nm, at least about 110
nm, at least
about 115 nm, at least about 120 nm, at least about 125 nm, or at least about
130 nm. In some
embodiments, the shift in the wavelength is about 1 nm to about 20 nm, about 2
nm to about
nm, about 3 nm to about 20 nm, about 4 nm to about 20 nm, about 5 nm to about
20 nm,
about 1 nm to about 19 nm, about 1 nm to about 18 nm, about 1 nm to about 17
nm, 1 nm to
about 16 nm, about 1 nm to about 15 nm, about 1 nm to about 14 nm, about 1 nm
to about 13
20 nm, about 1 nm to about 12 nm, about 1 nm to about 11 nm, or about 1 nm
to about 10 nm. In
some embodiments, the shift in the wavelength is about 1 nm to about 20 nm. In
some
embodiments, the shift in the wavelength is about 1 nm to about 130 nm.
In certain embodiments, the signal comprises the ratio or quotient of the
emission
intensities recorded at two distinct wavelengths or ranges of wavelengths,
i.e. , a ratiometric
signal. For example, as shown in FIG. 1, ligand binding may be determined by
measuring the
ratio of blue to green emission intensities. The change in signal may be
decreased emission
intensity at one wavelength, and no change in emission intensity at the other
wavelength. The
change in signal may be increased emission intensity at one wavelength, and no
change in
emission intensity at the other wavelength. The change in signal may be
increased emission
intensity at one wavelength, and increased emission intensity at the other
wavelength. The
change in signal may be decreased emission intensity at one wavelength, and
decreased
emission intensity at the other wavelength. The change in signal may be
increased emission
intensity at one wavelength, and decreased emission intensity at the other
wavelength. In
37
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
some embodiments, the change in ratio of the emission intensities recorded at
two distinct
wavelengths or ranges of wavelengths may be at least about 1.1-fold, at least
about 1.2-fold,
at least about 1.4-fold, at least about 1.6-fold, at least about 1.8-fold, at
least about 2.0-fold, at
least about 2.5-fold, at least about 3-fold, at least about 3.5-fold, at least
about 4-fold, at least
about 4.5-fold, at least about 5-fold, at least about 5.5-fold, at least about
6-fold, at least about
6.5-fold, at least about 7-fold, at least about 7.5-fold, at least about 8-
fold, at least about 8.5-
fold, at least about 9-fold, at least about 9.5-fold, at least about 10-fold,
at least about 12-fold,
at least about 14-fold, at least about 16-fold, at least about 18-fold, at
least about 20-fold, at
least about 25-fold, at least about 30-fold, at least about 35-fold, at least
about 40-fold, at
least about 45-fold, at least about 50-fold, at least about 55-fold, at least
about 60-fold, at
least about 65-fold, at least about 70-fold, at least about 75-fold, at least
about 80-fold, at
least about 85-fold, at least about 90-fold, at least about 95-fold, or at
least about 100-fold. In
some embodiments, the change in ratio of the emission intensities recorded at
two distinct
wavelengths or ranges of wavelengths may be a decrease of at least about 5%,
10%, 15%,
20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%,
95%,
96%, 97%, 98%, or 99%, or of 5-25%, 25-50%, 25-75%, 50-75%, 50-90%, or 75-99%
or the
reciprocal thereof.
The change in signal may be a change in the ratio of the two distinct
wavelengths or
ranges of wavelengths. The change in signal may be a shift in the two distinct
wavelengths or
ranges of wavelengths. In some embodiments, one wavelength shifts. In some
embodiments,
both wavelengths shift. In some embodiments, the shift in the wavelength is at
least about 1
nm, at least about 2 nm, at least about 3 nm, at least about 4 nm, at least
about 5 nm, at least
about 6 nm, at least about 7 nm, at least about 8 nm, at least about 9 nm, at
least about 10 nm,
at least about 11 nm, at least about 12 nm, at least about 13 nm, at least
about 14 nm, at least
about 15 nm, at least about 16 nm, at least about 17 nm, at least about 18 nm,
at least about
19 nm, at least about 20 nm, at least about 25 nm, at least about 30 nm, at
least about 35 nm,
at least about 40 nm, at least about 45 nm, at least about 50 nm, at least
about 55 nm, at least
about 60 nm, at least about 65 nm, at least about 70 nm, at least about 75 nm,
at least about
80 nm, at least about 85 nm, at least about 90 nm, at least about 95 nm, at
least about 100 nm,
at least about 105 nm, at least about 110 nm, at least about 115 nm, at least
about 120 nm, at
least about 125 nm, or at least about 130 nm. In some embodiments, the shift
in the
wavelength is about 1 nm to about 20 nm, about 2 nm to about 20 nm, about 3 nm
to about 20
nm, about 4 nm to about 20 nm, about 5 nm to about 20 nm, about 1 nm to about
19 nm,
38
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
about 1 nm to about 18 nm, about 1 nm to about 17 nm, 1 nm to about 16 nm,
about 1 nm to
about 15 nm, about 1 nm to about 14 nm, about 1 nm to about 13 nm, about 1 nm
to about 12
nm, about 1 nm to about 11 nm, or about 1 nm to about 10 nm. In some
embodiments, the
shift in the wavelength is about 1 nm to about 20 nm. In some embodiments, the
shift in the
wavelength is about 1 nm to about 130 nm.
A fluorophore may comprise, e.g., a fluorescent protein or an organic compound
having a molecular weight less than about 2000 Daltons (Da). Non-limiting
examples of
commercially available fluorophores include such as 5-iodoacetamidofluorescein
(5-IAF) or
6-iodoacetamidofluorescein (6-IAF), rhodamine, Oregon Green, eosin, Texas Red,
indocarbocyanine, oxacarbocyanine, thiacarbocyanine, merocyanine, Badan,
Acrylodan,
IAEDANS, comprising 3-cyano-7-hydroxycoumarin, 7-hydroxycoumarin-3-carboxylic
acid,
6,8-difluoro-7-hydroxy- 4-methylcoumarin, or 7-amino-4-methylcoumarin,
ppidyloxazole,
nitrobenzoxadiazole, benzoxadiazole, DRAQ5, DRAQ7, or CyTRAK Orange, cascade
blue,
Nile red, Nile blue, cresyl violet, oxazine 170, proflavin, acridine orange,
acridine yellow,
auramine, crystal violet, malachite green, porphin, phthalocyanine, bilirubin,
pyrene, N,1\11-
dimethyl-N-(iodoacety1)-N'-(7-nitrobenz-2-ox- a-1,3-diazol-4-ypethylenediamide
(NBD), N-
((2-(iodoacetoxy)ethyl)-N-methy- 1)amino-7-nitrobenz-2-oxa-1,3-diazole (NBDE),
Acrylodan, JPW4039, JPW4042, JPW4045, Oregon Green, Pacific Blue, CPM, N,N-
Dimethyl-N-(Iodoacety1)-N-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-y1)Ethylenediamine
(IANBD),
7-diethylamino-3-(4'-maleimidylpheny1)-4-methylcoumarin (CPM), BODIPY 499,
BOD1PY
507/545, BOD1PY 499/508, Alexa 432, A1exa488, A1exa532, A1exa546, Cy5, or 1-(2-
maleimidylethyl)-4-(5-(4-methoxyphenypoxazol-2- yppyridinium methanesulfonate
(PyMPO maleimide) (PyMPO). In various embodiments, the reporter group was
thiol-
reactive prior to being conjugated to a polypeptide disclosed herein. In
embodiments, the
reporter group is linked to a polypeptide disclosed herein via a disulfide
bond. Additional
non-limiting examples of commercially available fluorophores include
fluorescent proteins
such as Blue Fluorescent Protein (BFP), TagBFP, mTagBFP2, Azurite, Enhanced
Blue
Florescent Protein 2 (EBFP2), mKalamal, Sirius, Sapphire, T-Sapphire, Cyan
Fluorescent
Protein (CFP); Enhanced Cyan Fluorescent Protein (ECFP), Cerulean, SCFP3A,
mTurquoise,
mTurquoise2, monomeric Midoriishi-Cyan, TagCFP, mTFP1, AmCyanl, Green
Fluorescent
Protein (GFP), Enhanced Green Fluorescent Protein (EGFP), Emerald, Superfolder
GFP,
AcGFP1, ZsGreenl, Monomeric Azami Green, TagGFP2, mUKG, mWasabi, Clover,
mNeonGreen, Yellow Fluorescent Protein (YFP), Enhanced Yellow Fluorescent
Protein
39
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
(EYFP), Citrine, Venus, Super Yellow Fluorescent Protein 2 (SYFP2), TagYFP,
ZsYellowl,
mBanana, Orange Fluorescent Protein (OFP), Monomeric Kusabira-Orange (mK0),
mKOK,
mK02, mOrange, mOrange2, Red Fluorescent Protein (RFP), DsRed-Express, DsRed-
Express2, DsRed2, AsRed2, mRaspbeny, mCheny, mStrawberry, mTangerine,
tdTomato,
TagRFP, TagRFP-T, mApple, mRuby, mRuby2, mPlum, HcRed-Tandem, mKate2,
mNeptune, HcRedl, E2-Crimson, NirFP, TagRF'P657, 1FP1.4, or iRFP.
In some embodiments, the fluorophore comprises xanthene, a xanthene
derivative,
fluorescein, a fluorescein derivative, coumarin, a coumarin derivative,
cyanine, a cyanine
derivative, rhodamine, a rhodamine derivative, phenoxazine, a phenoxazine
derivative,
squaraine, a squaraine derivative, coumarin, a coumarin derivative,
oxadiazole, an oxadiazole
derivative, anthracene, an anthracene derivative, a boradiazaindacine (BODIPY)
family
fluorophore, pyrene, a pyrene derivative, acridine, an acridine derivative,
arylmethine, an
arylmethine derivative, tetrapynole, or a tetrapynole derivative. Non-limiting
aspects of
fluorophores are discussed in Lavis and Raines (2014) ACS Chem. Biol. 9, 855-
866, the
entire content of which is incorporated herein by reference. For example, the
fluorophore
may comprise a xanthene derivative comprising fluorescein or a fluorescein
derivative,
rhodamine, Oregon Green, eosin, or Texas Red. Non-limiting examples of
fluorescein
derivatives include 5-fluorescein, 6-carboxyfluorescein, 3'6-
carboxyfluorescein, 5(6)-
carboxyfluorescein, 6-hexachlorofluorescein, 6-tetrachlorofluorescein, or
isothiocyanate. In
some embodiments, the fluorophore comprises a cyanine derivative comprising
indocarbocyanine, oxacarbocyanine, thiacarbocyanine, or merocyanine. In
certain
embodiments, the fluorophore comprises a squaraine derivative comprising a
ring-substituted
squaraine. In various embodiments, the fluorophore comprises a naphthalene
derivative
comprising a dansyl or prodan naphthalene derivative. In a non-limiting
example, the
fluorophore comprises prodan or a derivative thereof. In certain embodiments,
the
fluorophore comprises Badan, Acrylodan, or N-(Iodoacetaminoethyl)-1-
naphthylamine-5-
sulfonic acid (IAEDANS). In some embodiments, the fluorophore comprises a
coumarin
derivative such as 3-cyano-7-hydroxycoumarin, 7-hydroxycoumarin-3-carboxylic
acid, 6,8-
difluoro-7-hydroxy- 4-methylcoumarin (DiFMU), or 7-amino-4-methylcoumarin. In
various
embodiments, the fluorophore comprises an oxadiazole derivative such as
pyridyloxazole,
nitrobenzoxadiazole, or benzoxadiazole. In certain embodiments, the
fluorophore comprises
an anthracene derivative comprising an anthraquinone such as DRAQ5, DRAQ7, or
CyTRAK Orange. In various embodiments, the fluorophore comprises a pyrene
derivative
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
comprising cascade blue. In non-limiting examples the fluorophore comprises an
oxazine
derivative such as Nile red, Nile blue, cresyl violet, or oxazine 170. In some
embodiments,
the fluorophore comprises an acridine derivative such as proflavin, acridine
orange, or
acridine yellow. In certain embodiments, the fluorophore comprises an
arylmethine
derivative such as auramine, crystal violet, or malachite green. In various
embodiments, the
fluorophore comprises a tetrapynole derivative comprising porphin,
phthalocyanine, or
bilirubin.
Aspects of the present subject matter relate to the use of fluorophores that
may readily
be attached to a ligand-binding protein disclosed herein, e.g., at a cysteine
residue. For
example, a fluorophore may comprise a sulfhydryl group prior to attachment to
a ligand-
binding protein that is reacted with a moiety of the ligand-binding protein to
attach the
fluorophore to the ligand-binding protein. In some embodiments, the
fluorophore comprised
a thiol group prior to attachment to the ligand-binding protein. For example,
the fluorophore
was thiol reactive prior to attachment to the ligand-binding protein. Non-
limiting examples
of fluorophores that may readily be attached to ligand-binding proteins using
thiol reactions
include fluorescein, pyrene, NBD, NBDE, Acrylodan (6-acryloyl 1-2-
dimethylaminonaphthalene), Badan (6-bromo-acetyl-2-dimethylamino-naphthalene),
JPW4039, JPW4042, or JPW4045.
In certain embodiments, the fluorophore comprises a derivative of a Prodan-
based
fluorophore such as Acrylodan or Badan. The excitation and emission properties
of the
Prodan-based fluorophores Acrylodan and Badan can be altered by manipulating
the
fluorescent ring system, while preserving the dimethylamino donor group, and
the twistable
carbonyl acceptor (Klymchenko 2013 Progress in Molecular Biology and
Translational
Science, 35-58). Replacement of the two-ring naphthalene with a three-ring
anthracene (Lu
2006 J. Org. Chem., 71, 9651-9657), fluorene (Kucherak 2010 J. Phys. Chem.
Lett., 1, 616-
620), pyrene (Niko 2013 Chem. Eur. J, 19, 9760-9765), or styrene (Benedetti
2012 J. Am.
Chem. Soc., 134, 12418-12421) cores significantly red-shift the excitation and
emission
properties, and in the case of the latter two, improve brightness through
improvements in
their excitation peak extinction coefficients. The entire content of each of
the references
cited above (as well as all other references referred to herein including the
contents of nucleic
acid and amino acid sequence accession number references) are incorporated
herein by
reference. Non-limiting examples of prodan analogues include 2-cyano-6-
41
CA 03005883 2018-05-18
WO 2017/087912 PCT/US2016/062958
dihexylaminoanthracene and 2-propiony1-6-dihexylaminoanthracene, as well as
fluorophores
comprising the following structures:
j
N1\1'
4,11
fi \
Itr
1
Q.
or
In some embodiments, the fluorophore comprises a fluorescent protein.
Fluorescent
proteins that emit blue, cyan, green, yellow, orange, red, far-red, or near
infrared radiation
when contacted with excitation radiation are known in the art and commercially
available as
proteins and via the expression of vectors that encode the fluorescent
protein. Non-limiting
examples of fluorescent proteins include Blue Fluorescent Protein (BFP),
TagBFP,
mTagBFP2, Azurite, Enhanced Blue Florescent Protein 2 (EBFP2), mKalamal,
Sirius,
Sapphire, T-Sapphire, Cyan Fluorescent Protein (CFP); Enhanced Cyan
Fluorescent Protein
(ECFP), Cerulean, SCFP3A, mTurquoise, mTurquoise2, monomeric Midoriishi-Cyan,
TagCFP, mTFP1, AmCyanl, Green Fluorescent Protein (GFP), Enhanced Green
Fluorescent
Protein (EGFP), Emerald, Superfolder GFP, AcGFP1, ZsGreenl, Monomeric Azami
Green,
TagGFP2, mUKG, mWasabi, Clover, mNeonGreen, Yellow Fluorescent Protein (YFP),
Enhanced Yellow Fluorescent Protein (EYFP), Citrine, Venus, Super Yellow
Fluorescent
Protein 2 (SYFP2), TagYFP, ZsYellowl, mBanana, Orange Fluorescetn Protein
(OFP),
Monomeric Kusabira-Orange (mK0), mKOK, mK02, mOrange, mOrange2, Red
Fluorescent
Protein (RF'P), DsRed-Express, DsRed-Express2, DsRed2, AsRed2, mRaspberry,
mCherry,
mStrawbeny, mTangerine, tdTomato, TagRF'P, TagRF'P-T, mApple, mRuby, mRuby2,
mPlum, HcRed-Tandem, mKate2, mNeptune, HcRedl, E2-Crimson, NirFP, TagRF'P657,
1FP1.4, or iRFP.
In some embodiments, the fluorophore comprises a quantum dot (Medintz et al.
2005)
(Sapsford, Beni and Medintz 2006 Angew Chem Int Ed Engl, 45, 4562-89; Resch-
Genger et
al. 2008 Nat Methods, 5, 763-75). In some embodiments the emission properties
of the
42
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
conjugated protein are enhanced by immobilization on or near metallic
nanoparticles (Zeng et
al. 2014 Chem Soc Rev, 43, 3426-52; Shen et al. 2015 Nanoscale, 7, 20132-41).
In various embodiments, the peak emission wavelength and/or the emission
intensity
of the biosensor change when the ligand binds to the ligand-binding protein.
In some
embodiments, the biosensor exhibits a dichromatic signaling change when the
ligand binds to
the ligand-binding protein. In various embodiments, the peak emission
wavelength of the
biosensor shifts by at least about 5, 10, 15, 20, 30, 40, 50, or by about 5-50
nm when the
biosensor binds to ligand. In certain embodiments, the emission intensity of
the biosensor
increases by at least about 5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%,
100%,
150%, 200%, or 300% when the biosensor binds to ligand. In various
embodiments, the
signal produced by the reporter group persists for at least 1 nanoseconds
(ns), 5 ns, 10 ns, 25
ns, 50 ns, 75 ns, 100 ns, 200 ns, 300 ns, 400 ns, 500 ns, 600 ns, 700 ns, 800
ns, 900 ns, 0.001
milliseconds (ms), 0.01 ms, 0.1 ms, 1 ms, 5 ms, 10 ms, 20 ms, 25 ms, 50 ms,
100 ms, or 500
ms when the ligand binds to the ligand-binding protein.
Ligand-Binding Proteins
Aspects of the present subject matter provide biosensors comprising a ligand-
binding
protein that binds a ligand of interest. Non-limiting examples of ligands
include sugars (such
as glucose, galactose, lactose, arabinose, ribose, and maltose), lactate,
urea, anions (e.g.,
bicarbonate, phosphate, sulfate, and halide anions such as chloride, fluoride,
iodide, astatide,
ununseptide, and bromide), cations (e.g., calcium, iron, and hydrogen ions),
dipeptides, and
amino acids (such as histidine, glutamine, glutamate, aspartate).
The ligand-binding protein may comprise a naturally occurring protein or a
protein
that is modified compared to a naturally occurring protein. For example, the
ligand-binding
protein may comprise one or more mutations compared to a naturally occurring
protein. In
some embodiments, the naturally occurring protein is a naturally occurring
counterpart of the
ligand-binding protein (e.g., the ligand-binding protein is a mutant of the
naturally occurring
counterpart).
A "naturally occurring counterpart" of a mutant polypeptide is a polypeptide
produced in nature from which the mutant polypeptide has been or may be
derived (e.g., by
one or more mutations). For example, the naturally occurring counterpart is an
endogenous
polypeptide produced by an organism in nature, wherein the endogenous
polypeptide
typically does not have one or more of the mutations present in the mutant
polypeptide. For
43
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
convenience and depending on context, a naturally occurring counterpart may be
referred to
herein for the purpose of comparison and to illustrate the location and/or
presence of one or
more mutations, binding activities, and/or structural features.
As used herein, a "mutation" is a difference between the amino acid sequence
of a
modified polypeptide/protein and a naturally occurring counterpart. A
polypeptide having a
mutation may be referred to as a "mutant." Non-limiting examples of mutations
include
insertions, deletions, and substitutions. However, the term "mutation"
excludes (i) the
addition of amino acids to the N-terminus or C-terminus of a polypeptide, and
(ii) the
omission/deletion/replacement of a polypeptide's signal peptide (e.g.,
replacement with
another signal peptide or with a methionine).
The addition of amino acids to the N-terminus or C-terminus of a protein via a
peptide
bond may be referred to herein as a "fusion" of the amino acids to the
protein. Similarly, an
exogenous protein fused to amino acids (e.g., another protein, a fragment, a
tag, or a
polypeptide moiety) at its N-terminus or C-terminus may be referred to as a
"fusion protein."
The added amino acids may comprise a non-native polypeptide, e.g., a
polypeptide reporter
group such as a fluorescent protein, a moiety that facilitates the isolation
or modification of a
polypeptide, or a moiety that facilitates the attachment of a polypeptide to a
substrate or
surface. As used herein, "non-native" when referring to the added amino acids
(e.g., a
"polypeptide") of a fusion protein indicates that the polypeptide is not
naturally part of the
protein to which it is fused in the fusion protein. For example, the sequence
of a non-native
polypeptide ("added amino acids") that is fused to a protein is encoded by an
organism other
than the organism from which the protein is derived, is not known to be
naturally encoded by
any organism, or is encoded by a gene other than the wild-type gene that
encodes an
endogenous version of the protein.
As used herein the term "signal peptide" refers to a short (e.g., 5-30 or 10-
100 amino
acids long) stretch of amino acids at the N-terminus of a protein that directs
the transport of
the protein. In various embodiments, the signal peptide is cleaved off during
the post-
translational modification of a protein by a cell. Signal peptides may also be
referred to as
"targeting signals," "leader sequences," "signal sequences," "transit
peptides," or
"localization signals." In instances where a signal peptide is not defined for
a ligand-binding
protein discussed herein, the signal peptide may optionally be considered to
be, e.g., the first
5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25,
26, 27, 28, 29, 30,
40, 50, 60, 70, 80, 90, 100, 5-15, 5-20, 5-25, 5-100, 10-15, 10-20, 10-25, 10-
50, 10-100, 25-
44
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
50, 25-75, or 25-100 amino acids from the N-terminus of the translated protein
(compared to
a protein that has not had the signal peptide removed, e.g., compared to a
naturally occurring
protein).
In some embodiments, the ligand-binding protein comprises 1, 2, 3, 4, 5, 6, 7,
8, 9, 10,
15, 20, 25, 30, 40, 50, 60, 70, 80, 90, 100, 1-10, 1-15, 1-20, 5-15, 5-20, 10-
25, 10-50, 20-50,
25-75, 25-100 or more mutations compared to a naturally occurring protein
while retaining at
least about 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%,
75%,
80%, 85%, 90%, 95%, 96%, 97%, 98%, 99%, or 99.5%, or about 100% of the
activity of the
naturally occurring protein. Mutations include but are not limited to
substitutions, insertions,
and deletions. Non-limiting examples of ligand-binding proteins may have 1, 2,
3, 4, 5, 6, 7,
8, 9, 10, 15, 20, 25, 30, 40, 50, 60, 70, 80, 90, 100, 1-10, 1-15, 1-20, 5-15,
5-20, 10-25, 10-50,
20-50, 25-75, 25-100, or more substitution mutations compared to a naturally
occurring
protein while retaining at least about 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%,
50%,
55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99%, or 99.5%, or
about 100% of the activity of the naturally occurring protein. In embodiments,
at least one
amino acid of the ligand-binding protein has been substituted with a cysteine.
Alternatively
or in addition, a ligand-binding protein may include one or more mutations
that remove a
cysteine, e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more substitutions or
deletions of a cysteine
compared to a naturally occurring protein.
Alternatively, the ligand-binding protein is not a mutant. For example, a
reporter
group is fused to the N-terminus or the C-terminus of the ligand-binding
protein.
In various embodiments, a ligand-binding protein may comprise a stretch of
amino
acids (e.g., the entire length of the ligand-binding protein or a portion
comprising at least
about 50, 100, 200, 250, 300, or 350 amino acids) in a sequence that is at
least about 90%,
91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, 99.1%, 99.2%, 99.3%, 99.4%, or
99.5%
identical to an amino acid sequence of a naturally occurring protein.
In some embodiments, the mutations are conservative, and the present subject
matter
includes many ligand-binding proteins in which the only mutations are
substitution
mutations. In non-limiting examples, a ligand-binding protein has no deletions
or insertions
compared to a naturally occurring protein (e.g., a naturally occurring
counterpart).
Alternatively, a ligand-binding protein may have (i) less than about 5, 4, 3,
2, or 1 inserted
amino acids, and/or (ii) less than about 5, 4, 3, 2, or 1 deleted amino acids
compared to a
naturally occurring protein.
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
In various embodiments, a naturally occurring protein to which a ligand-
binding
protein is compared or has been derived (e.g., by mutation, fusion, or other
modification)
from a prokaryotic ligand-binding protein such as a bacterial ligand-binding
protein. For
example, the prokaryotic ligand-binding protein is a mutant, fragment, or
variant of a natural
(i.e., wild-type) bacterial protein. In various embodiments, the bacterial
ligand-binding
protein is from a thermophilic, mesophilic, or cryophilic prokaryotic
microorganism (e.g., a
thermophilic, mesophilic, or cryophilic bacterium).
A microorganism is "thermophilic" if it is capable of surviving, growing, and
reproducing at temperatures between 41 and 140 C (106 and 284 F), inclusive.
In various
embodiments, a thermophilic organism has an optimal growth temperature between
41 and
140 C, or that is at least about 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95,
100, 105, 110, 115,
120, 125, 130, 135, or 140 C. Many thermophiles are archaea. Thermophilic
eubacteria are
suggested to have been among the earliest bacteria. Thermophiles are found in
various
geothermally heated regions of the Earth, such as hot springs and deep sea
hydrothermal
vents, as well as decaying plant matter, such as peat bogs and compost. Unlike
other types of
microorganisms, thermophiles can survive at much hotter temperatures, whereas
other
bacteria would be damaged and sometimes killed if exposed to the same
temperatures.
Thermophiles may be classified into three groups: (1) obligate thermophiles;
(2) facultative
thermophiles; and (3) hyperthermophiles. Obligate thermophiles (also called
extreme
thermophiles) require such high temperatures for growth, whereas facultative
thermophiles
(also called moderate thermophiles) can thrive at high temperatures, but also
at lower
temperatures (e.g. below 50 C). Hyperthermophiles are particularly extreme
thermophiles
for which the optimal temperatures are above 80 C. Some microorganisms can
live at
temperatures higher than 100 C at large depths in the ocean where water does
not boil
because of high pressure. Many hyperthermophiles are also able to withstand
other
environmental extremes such as high acidity or radiation levels. A compound
(e.g., a protein
or biosensor) is "thermotolerant" if it is capable of surviving exposure to
temperatures above
41 C. For example, in some embodiments a thermotolerant biosensor retains its
function and
does not become denatured when exposed to a temperature of about 45, 50, 55,
60, 65, 70,
75, 80, 85, 90, 95, 100, 105, 110, 115, 120, 125, 130, 135, or 140 C for at
least about 1, 2, 3,
4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30 or more minutes. In some embodiments, the
thermotolerant
compound survives exposure to 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95, 100,
105, 110, 115,
120, 125, 130, 135, or 140 C under pressure.
46
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
A microorganism is "mesophilic" if it is capable of surviving, growing, and
reproducing at temperatures between 20 and 40 C (68 and 104 F), inclusive.
"Psychrophiles" or "cryophiles" are microorganisms that are capable of growth
and
reproduction in cold temperatures. In various embodiments, a psychrophile is
capable of
growth and reproduction at a temperature of 10 C or less, e.g., between ¨20 C
and +10 C.
In some embodiments, the microbial protein is produced by a bacterial
microorganism, an archaean microorganism, an algal microorganism, a protozoan
microorganism, or a fungal microorganism. In non-limiting examples, the
microbial protein
is produced by a Gram-positive bacterium or a Gram-negative bacterium. In
various
embodiments, a biosensor comprises a modified (e.g., mutated, fused, and/or
conjugated)
periplasmic binding protein or a cytoplasmic binding protein.
Aspects of the present subject matter provide a ligand-binding protein with a
mutation
that alters the interaction of the ligand-binding protein with a ligand. For
example, the
ligand-binding protein comprises a mutation that alters the interaction of the
ligand-binding
protein with the ligand compared to a naturally occurring counterpart. In some
embodiments,
the ligand-binding protein comprises a mutation that alters the interaction of
an amino acid of
the ligand-binding protein with a water molecule compared to a naturally
occurring
counterpart.
In some embodiments, the ligand-binding protein does not comprise a signal
peptide.
For example, the signal peptide (e.g., that is present in a naturally
occurring counterpart) may
be replaced with a methionine.
Exemplary implementations relate to a ligand such as sugars (such as glucose,
galactose, lactose, arabinose, ribose, and maltose), lactate, urea, anions
(e.g., chloride,
bicarbonate, phosphate, and sulfate), cations (e.g., calcium and iron),
dipeptides, amino acids
(such as histidine, glutamine, glutamate, and aspartate). For example, the
biosensor may
comprise a mutant of, a fragment of, or a fusion protein comprising a
microbial ligand-
binding protein. In embodiments, the ligand-binding protein is not a mutant or
fragment to
which a non-native polypeptide has been attached or added.
The ratiometric reagentless biosensors produce precise measurements over
extended
concentration ranges, e.g. from 0.0001 mM to 100 mM, in sample volumes of less
than about
10, 9, 8, 7, 6, 5, 4, 3, 2, or 1 1.1.1. In some embodiments, the ligand-
binding protein comprises
a mutation that alters (e.g., increases or decreases) the interaction of the
mutant with bound
ligand compared to a naturally occurring protein (e.g., a microbial ligand-
binding protein). In
47
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
non-limiting examples, the ligand-binding protein comprises a mutation that
alters (e.g.,
increases or decreases) the mutant's affinity and/or specificity for ligand
compared to a
unmutated ligand-binding protein (e.g., a microbial ligand-binding protein).
In certain
embodiments, the ligand-binding protein comprises a mutation that alters the
interaction
between the protein and bound ligand, a mutation that alters the equilibrium
between the
open and closed states of the ligand-binding protein, a mutation that alters
the interaction
between the ligand-binding protein and a reporter group (such as a fluorescent
conjugate,
e.g., the interaction with a carbonyl group or a naphthalene ring of a prodan-
derived
fluorophore such as Acrylodan or Badan), and/or a mutation that impacts
indirect interactions
that alter the geometry of the ligand binding site. In various embodiments,
the mutation does
not reduce, or negligibly impacts, the thermostability of the ligand-binding
protein. In some
embodiments, the mutation alters the thermostability of the ligand-binding
protein by less
than about 1, 2, 3, 4, 5, or 10 C.
The present subject matter provides a glucose-galactose binding protein GGBP
that is
or is a mutant of: an Escherichia sp. (e.g., E. albertii, E. coli, E.
fergusonii, E. hermannii, or
E. vulneris) GGBP; a Thermoanaerobacter sp. (e.g., T. acetoethylicus, T.
brockii, T.
ethanolicus, T. italicus, T. kivui, T. mathranii, T. pseudethanolicus, T.
siderophilus, T.
sulfurigignens, T. sulfurophilus, T. thermocopriae, T. thermohydrosulfuricus,
T.
thermosaccharolyticum, T. uzonensis, or T. wiegelii) GGBP; a Clostridium sp.
(e.g., C.
absonum, C. aceticum, C. acetireducens, C. acetobutylicum, C. acidisoli, C.
aciditolerans, C.
acidurici, C. aerotolerans, C. aestuarii, C. akagii, C. aldenense, C.
aldrichii, C. algidicarni,
C. algidixylanolyticum, C. algifaecis, C. algoriphilum, C. alkalicellulosi, C.
aminophilum, C.
aminovalericum, C. amygdalinum, C. amylolyticum, C. arbusti, C. arcticum, C.
argentinense,
C. asparagiforme, C. aurantibutyricum, C. autoethanogenum, C. baratii, C.
barkeri, C.
bartlettii, C. beijerinckii, C. bifermentans, C. bolteae, C. bornimense, C.
botulinum, C.
bowmanii, C. bryantii, C. butyricum, C. cadaveris, C. caenicola, C.
caminithermale, C.
carboxidivorans, C. carnis, C. cavendishii, C. celatum, C. celerecrescens, C.
cellobioparum,
C. cellulofermentans, C. cellulolyticum, C. cellulosi, C. cellulovorans, C.
chartatabidum, C.
chauvoei, C. chromiireducens, C. citroniae, C. clariflavum, C.
clostridioforme, C. coccoides,
C. cochlearium, C. colletant, C. colicanis, C. colinum, C. collagenovorans, C.
cylindrosporum, C. difficile, C. diolis, C. disporicum, C. drakei, C. durum,
C. estertheticum,
C. estertheticum estertheticum, C. estertheticum laramiense, C. fallax, C.
felsineum, C.
fervidum, C. fimetarium, C. formicaceticum, C. frigidicarnis, C. frigoris, C.
ganghwense, C.
48
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
gasigenes, C. ghonii, C. glycolicum, C. glycyrrhizinilyticum, C. grantii, C.
haemolyticum, C.
halophilum, C. hastiforme, C. hathewayi, C. herbivorans, C. hiranonis, C.
histolyticum, C.
homopropionicum, C. huakuii, C. hungatei, C. hydrogeniformans, C.
hydroxybenzoicum, C.
hylemonae, C. jejuense, C. indolis, C. innocuum, C. intestinale, C.
irregulare, C. isatidis, C.
josui, C. kluyveri, C. lactatifermentans, C. lacusfryxellense, C. laramiense,
C. lavalense, C.
lentocellum, C. lentoputrescens, C. leptum, C. limosum, C. litorale, C.
lituseburense, C.
ljungdahlii, C. lortetii, C. lundense, C. magnum, C. malenominatum, C.
mangenotii, C.
mayombei, C. methoxybenzovorans, C. methylpentosum, C. neopropionicum, C.
nexile, C.
nitrophenolicum, C. novyi, C. oceanicum, C. orbiscindens, C. oroticum, C.
oxalicum, C.
papyrosolvens, C. paradoxum, C. paraperfringens, C. paraputrificum, C. pascui,
C.
pasteurianum, C. peptidivorans, C. perenne, C. perfringens, C. pfennigii, C.
phytofermentans, C. piliforme, C. polysaccharolyticum, C. populeti, C.
propionicum, C.
proteoclasticum, C. proteolyticum, C. psychrophilum, C. puniceum, C.
purinilyticum, C.
putrefaciens, C. putrificum, C. quercicolum, C. quinii, C. ramosum, C. rectum,
C. roseum, C.
saccharobutylicum, C. saccharogumia, C. saccharolyticum, C.
saccharoperbutylacetonicum,
C. sardiniense, C. sartagoforme, C. scatologenes, C. schirmacherense, C.
scindens, C.
septicum, C. sordellii, C. sphenoides, C. spiroforme, C. sporogenes, C.
sporosphaeroides, C.
stercorarium, C. stercorarium leptospartum, C. stercorarium stercorarium, C.
stercorarium
thermolacticum, C. sticklandii, C. straminisolvens, C. subterminale, C.
sufflavum, C.
sulfidigenes, C. symbiosum, C. tagluense, C. tepidiprofundi, C. termitidis, C.
tertium, C.
tetani, Clostridium tetanomorphum, C. thermaceticum, C. thermautotrophicum, C.
thermoalcaliphilum, C. thermobutyricum, C. thermocellum, C. thermocopriae, C.
thermohydrosulfuricum, C. thermolacticum, C. thermopalmarium, C.
thermopapyrolyticum,
C. thermosaccharolyticum, C. thermosuccinogenes, C. thermosulfurigenes, C.
thiosulfatireducens, C. tyrobutyricum, C. uliginosum, C. ultunense, C.
villosum, C. vincentii,
C. viride, C. xylanolyticum, or C. xylanovorans) GGBP; a Salmonella sp. [e.g.,
S. bongori, S.
enterica, S. enterica subspecies enterica, S. enterica subspecies salamae, S.
enterica
subspecies arizonae, S. enterica subspecies diarizonae, S. enterica subspecies
houtenae, S.
enterica subspecies indica, or S. enterica subspecies enterica serovar
Typhimurium (S.
typhimurium)] GGBP; a Caldicellulosiruptor sp. (e.g., C. saccharolyticus, C.
acetigenus, C.
bescii, C. changbaiensis, C. hydrothermalis, Caldicellulosiruptor hydrother,
C.
kristjanssonii, C. kronotskyensis, C. lactoaceticus, C. owensensis, or C.
obsidiansis) GGBP; a
Paenibacillus sp. (e.g., P. agarexedens, P. agaridevorans, P. alginolyticus,
P. alkaliterrae, P.
49
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
alvei, P. amylolyticus, P. anaericanus, P. antarcticus, P. assamensis, P.
azoreducens, P.
azotofixans, P. barcinonensis, P. borealis, P. brasilensis, P. brassicae, P.
campinasensis, P.
chinjuensis, P. chitinolyticus, P. chondroitinus, P. cineris, P. cookii, P.
curdlanolyticus, P.
daejeonensis, P. dendritiformis, P. durum, P. ehimensis, P. elgii, P.
favisporus, P.
glucanolyticus, P. glycanilyticus, P. gordonae, P. graminis, P. granivorans,
P. hodogayensis,
P. illinoisensis, P. jamilae, P. kobensis, P. koleovorans, P. koreensis, P.
kribbensis, P. lactis,
P. larvae, P. lautus, P. lentimorbus, P. macerans, P. macquariensis, P.
massiliensis, P.
mendelii, P. motobuensis, P. naphthalenovorans, P. nematophilus, P. odorifer,
P. pabuli, P.
peoriae, P. phoenicis, P. phyllosphaerae, P. polymyxa, P. popilliae, P.
pulvifaciens, P.
rhizosphaerae, P. sanguinis, P. stellifer, P. terrae, P. thiaminolyticus, P.
timonensis, P.
tylopili, P. turicensis, P. validus, P. vortex, P. vulneris, P. wynnii, P.
xylanilyticus) GGBP; a
Butyrivibrio sp. (e.g., B. proteoclasticus, B. crossotus, B. fibrisolvens, or
B. hungatei) GGBP;
a Roseburia sp. (e.g., R. intestinalis, R. faecis, R. hominis, or R.
inulinivorans) GGBP; a
Faecalibacterium sp. (e.g., F. prausnitzii) GGBP; an Erysipelothrix sp. (e.g.,
E.
rhusiopathiae, E. inopinata, or E. tonsillarum) GGBP; or an Eubacterium sp.
(e.g., E. rectale,
E. acidaminophilum, E. nodatum, E. oxidoreducens, or E. foedans) GGBP.
The present subject matter provides a urea-binding protein that is or is a
mutant of: an
Marinomas sp. (e.g., M posidonica ) urea-binding protein; a Marinobacter sp.
(e.g., M
adhaerens, M algicola, M alkaliphilus, M antarcticus, M arcticus,
Maromaticivorans, M
bryozoorum, M daepoensis, M daqiaonensis, M excellens, M. flavimaris, M
gudaonensis,
M guineae, M halophilus, M gudaonensis, M hydrocarbonoclasticus, M koreensis,
M
lacisalsi, M lipolyticus, M litoralis, M lutaoensis, M maritimus, M mobilis, M
nitratireducens, M oulmenensis, M pelagius, M persicus, M psychrophilus, M
nanhaiticus,
M salarius, M salicampi, M salsuginis, M santoriniensis, M sediminum, M
segnicrescens,
M shengliensis, M squalenivorans, M similis, M szutsaonensis, M vinifirmus, M
xestospongiae, M zhanjiangensis, or M zhejiangensis) urea-binding protein; a
Bacillus sp.
(e.g., B. acidiceler, B. acidicola, B. acidiproducens, B. acidocaldarius, B.
acidoterrestris, B.
aeolius, B. aerius, B. aerophilus, B. agaradhaerens, B. agri, B. aidingensis,
B. akibai, B.
alcalophilus, B. algicola, B. alginolyticus, B. alkalidiazotrophicus, B.
alkalinitrilicus, B.
alkalisediminis, B. alkalitelluris, B. altitudinis, B. alveayuensis, B. alvei,
B.
amyloliquefaciens, B. a. subsp. amyloliquefaciens, B. a. subsp. plantarum, B.
amylolyticus, B.
andreesenii, B. aneurinilyticus, B. anthracis, B. aquimaris, B. arenosi, B.
arseniciselenatis,
B. arsenicus, B. aurantiacus, B. arvi, B. aryabhattai, B. asahii, B.
atrophaeus, B.
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
axarquiensis, B. azotofixans, B. azotoformans, B. badius, B. barbaricus, B.
bataviensis, B.
beijingensis, B. benzoevorans, B. beringensis, B. berkeleyi, B. beveridgei, B.
bogoriensis, B.
boroniphilus, B. borstelensis, B. brevis Migula, B. butanolivorans, B.
canaveralius, B.
carboniphilus, B. cecembensis, B. cellulosilyticus, B. centrosporus, B.
cereus, B.
chagannorensis, B. chitinolyticus, B. chondroitinus, B. choshinensis, B.
chungangensis, B.
cibi, B. circulans, B. clarkii, B. clausii, B. coagulans, B. coahuilensis, B.
cohnii, B. composti,
B. curdlanolyticus, B. cycloheptanicus, B. cytotoxicus, B. daliensis, B.
decisifrondis, B.
decolorationis, B. deserti, B. dipsosauri, B. drentensis, B. edaphicus, B.
ehimensis, B.
eiseniae, B. enclensis, B. endophyticus, B. endoradicis, B. farraginis, B.
fastidiosus, B.
fengqiuensis, B. firmus, B. flexus, B. foraminis, B. fordii, B. formosus, B.
fortis, B. fumarioli,
B. funiculus, B. fusiformis, B. galactophilus, B. galactosidilyticus, B.
galliciensis, B. gelatini,
B. gibsonii, B. ginsengi, B. ginsengihumi, B. ginsengisoli, B. globisporus, B.
g. subsp.
globisporus, B. g. subsp. marinus, B. glucanolyticus, B. gordonae, B.
gottheilii, B. graminis,
B. halmapalus, B. haloalkaliphilus, B. halochares, B. halodenitrificans, B.
halodurans, B.
halophilus, B. halosaccharovorans, B. hemicellulosilyticus, B. hemicentroti,
B.
herbersteinensis, B. horikoshii, B. horneckiae, B. horti, B. huizhouensis, B.
humi, B.
hwajinpoensis, B. idriensis, B. indicus, B. infantis, B. infernus, B.
insolitus, B. invictae, B.
iranensis, B. isabeliae, B. isronensis, B. jeotgali, B. kaustophilus, B.
kobensis, B. kochii, B.
kokeshiiformis, B. koreensis, B. korlensis, B. kribbensis, B. kndwichiae, B.
laevolacticus, B.
larvae, B. laterosporus, B. lautus, B. lehensis, B. lentimorbus, B. lentus, B.
licheniformis, B.
ligniniphilus, B. litoralis, B. locisalis, B. luciferensis, B. luteolus, B.
luteus, B. macauensis, B.
macerans, B. macquariensis, B. macyae, B. malacitensis, B. mannanilyticus, B.
marisflavi, B.
marismortui, B. marmarensis, B. massiliensis, B. megaterium, B. mesonae, B.
methanolicus,
B. methylotrophicus, B. migulanus, B. mojavensis, B. mucilaginosus, B.
muralis, B.
murimartini, B. mycoides, B. naganoensis, B. nanhaiensis, B. nanhaiisediminis,
B. nealsonii,
B. neidei, B. neizhouensis, B. niabensis, B. niacini, B. novalis, B.
oceanisediminis, B.
odysseyi, B. okhensis, B. okuhidensis, B. oleronius, B. oryzaecorticis, B.
oshimensis, B.
pabuli, B. pakistanensis, B. pallidus, B. pallidus, B. panacisoli, B.
panaciterrae, B.
pantothenticus, B. parabrevis, B. paraflexus, B. pasteurii, B. patagoniensis,
B. peoriae, B.
persepolensis, B. persicus, B. pervagus, B. plakortidis, B. pocheonensis, B.
polygoni, B.
polymyxa, B. popilliae, B. pseudalcalophilus, B. pseudofirmus, B.
pseudomycoides, B.
psychrodurans, B. psychrophilus, B. psychrosaccharolyticus, B.
psychrotolerans, B.
pulvifaciens, B. pumilus, B. purgationiresistens, B. pycnus, B. qingdaonensis,
B. qingshengii,
51
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
B. reuszeri, B. rhizosphaerae, B. rigui, B. ruris, B. safensis, B. salarius,
B. salexigens, B.
saliphilus, B. schlegelii, B. sediminis, B. selenatarsenatis, B.
selenitireducens, B.
seohaeanensis, B. shacheensis, B. shackletonii, B. siamensis, B. silvestris,
B. simplex, B.
siralis, B. smithii, B. soli, B. solimangrovi, B. solisalsi, B. songklensis,
B. sonorensis, B.
sphaericus, B. sporothermodurans, B. stearothermophilus, B. stratosphericus,
B.
subterraneus, B. subtilis, B. s. subsp. inaquosorum, B. s. subsp. spizizenii,
B. s. subsp.
subtilis, B. taeanensis, B. tequilensis, B. thermantarcticus, B.
thermoaerophilus, B.
thermoamylovorans, B. thermocatenulatus, B. thermocloacae, B. thermocopriae,
B.
thermodenitrificans, B. thermoglucosidasius, B. thermolactis, B.
thermoleovorans, B.
thermophilus, B. thermoruber, B. thermosphaericus, B. thiaminolyticus, B.
thioparans, B.
thuringiensis, B. tianshenii, B. trypoxylicola, B. tusciae, B. validus, B.
vallismortis, B.
vedderi, B. velezensis, B. vietnamensis, B. vireti, B. vulcani, B. wakoensis,
B.
weihenstephanensis, B. xiamenensis, B. xiaoxiensis, or B. zhanjiangensis) urea-
binding
protein; a Desulfotomaculum sp. (e.g., D. ruminis, D. nigrificans, D.
australicum, D.
thermobenzoicum, D. geothermicum, D. thermocisternum, D. aeronauticum, D.
halophilum,
D. kuznetsovii, D. thermoacetoxidans, D. thermosapovorans, D. acetoxidans, D.
reducens, D.
putei, D. luciae, D. gibsoniae, D. sapomandens, D. alkaliphilum, D. sp. FSB6,
D. sp. ASRB-
Zg, D. sp. 175,D. sp. 176,D. sp. 171,D. sp. C40-3,D. sp. TPOSR, D. sp. WW1, D.
sp.
SRB-M, D. sp. Mechichi-2001, D. solfataricum, D. sp. ECP-05, D. sp. MPNegl, D.
sp.
0x39, D. sp. RL50L1, D. alcoholivorax, D. sp. NC402, D. sp. NB401, D. sp.
NA401, D.
salinum, D. carboxydivorans, D. arcticum, D. thermosubterraneum, D. indicum,
D. sp. Lac2,
D. sp. CYP1, D. sp. CYP9, D. sp. IS3205, D. sp. Srb55, D. sp. Iso-W2, D. sp.
2, D.
hydrothermale, D. sp. ADR22, D. sp. Hbr7, D. sp. J 175, D. sp. J 176, D. sp.
DSM 7440,
D. sp. DSM 7474, D. sp. DSM 7475, D. sp. DSM 7476, D. sp. DSM 8775, D. sp. cs1-
2, or D.
sp. MJ1) urea-binding protein; a Geobacillus sp. (e.g., G.
thermoglucosidasius, G.
stearothermophilus, G. jurassicus, G. toebii) urea-binding protein; a
Clostridium sp. (e.g., C.
absonum, C. aceticum, C. acetireducens, C. acetobutylicum, C. acidisoli, C.
aciditolerans, C.
acidurici, C. aerotolerans, C. aestuarii, C. akagii, C. aldenense, C.
aldrichii, C. algidicarni,
C. algidixylanolyticum, C. algifaecis, C. algoriphilum, C. alkalicellulosi, C.
aminophilum, C.
aminovalericum, C. amygdalinum, C. amylolyticum, C. arbusti, C. arcticum, C.
argentinense,
C. asparagiforme, C. aurantibutyricum, C. autoethanogenum, C. baratii, C.
barkeri, C.
bartlettii, C. beijerinckii, C. bifermentans, C. bolteae, C. bornimense, C.
botulinum, C.
bowmanii, C. bryantii, C. butyricum, C. cadaveris, C. caenicola, C.
caminithermale, C.
52
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
carboxidivorans, C. carnis, C. cavendishii, C. celatum, C. celerecrescens, C.
cellobioparum,
C. cellulofermentans, C. cellulolyticum, C. cellulosi, C. cellulovorans, C.
chartatabidum, C.
chauvoei, C. chromiireducens, C. citroniae, C. clariflavum, C.
clostridioforme, C. coccoides,
C. cochlearium, C. colletant, C. colicanis, C. colinum, C. collagenovorans, C.
cylindrosporum, C. difficile, C. diolis, C. disporicum, C. drakei, C. durum,
C. estertheticum,
C. estertheticum estertheticum, C. estertheticum laramiense, C. fallax, C.
felsineum, C.
fervidum, C. fimetarium, C. formicaceticum, C. frigidicarnis, C. frigoris, C.
ganghwense, C.
gasigenes, C. ghonii, C. glycolicum, C. glycyrrhizinilyticum, C. grantii, C.
haemolyticum, C.
halophilum, C. hastiforme, C. hathewayi, C. herbivorans, C. hiranonis, C.
histolyticum, C.
homopropionicum, C. huakuii, C. hungatei, C. hydrogeniformans, C.
hydroxybenzoicum, C.
hylemonae, C. jejuense, C. indolis, C. innocuum, C. intestinale, C.
irregulare, C. isatidis, C.
josui, C. kluyveri, C. lactatifermentans, C. lacusfryxellense, C. laramiense,
C. lavalense, C.
lentocellum, C. lentoputrescens, C. leptum, C. limosum, C. litorale, C.
lituseburense, C.
ljungdahlii, C. lortetii, C. lundense, C. magnum, C. malenominatum, C.
mangenotii, C.
mayombei, C. methoxybenzovorans, C. methylpentosum, C. neopropionicum, C.
nexile, C.
nitrophenolicum, C. novyi, C. oceanicum, C. orbiscindens, C. oroticum, C.
oxalicum, C.
papyrosolvens, C. paradoxum, C. paraperfringens, C. paraputrificum, C. pascui,
C.
pasteurianum, C. peptidivorans, C. perenne, C. perfringens, C. pfennigii, C.
phytofermentans, C. piliforme, C. polysaccharolyticum, C. populeti, C.
propionicum, C.
proteoclasticum, C. proteolyticum, C. psychrophilum, C. puniceum, C.
purinilyticum, C.
putrefaciens, C. putrificum, C. quercicolum, C. quinii, C. ramosum, C. rectum,
C. roseum, C.
saccharobutylicum, C. saccharogumia, C. saccharolyticum, C.
saccharoperbutylacetonicum,
C. sardiniense, C. sartagoforme, C. scatologenes, C. schirmacherense, C.
scindens, C.
septicum, C. sordellii, C. sphenoides, C. spiroforme, C. sporogenes, C.
sporosphaeroides, C.
stercorarium, C. stercorarium leptospartum, C. stercorarium stercorarium, C.
stercorarium
thermolacticum, C. sticklandii, C. straminisolvens, C. subterminale, C.
suffiavum, C.
sulfidigenes, C. symbiosum, C. tagluense, C. tepidiprofundi, C. termitidis, C.
tertium, C.
tetani, Clostridium tetanomorphum, C. thermaceticum, C. thermautotrophicum, C.
thermoalcaliphilum, C. thermobutyricum, C. thermocellum, C. thermocopriae, C.
thermohydrosulfuricum, C. thermolacticum, C. thermopalmarium, C.
thermopapyrolyticum,
C. thermosaccharolyticum, C. thermosuccinogenes, C. thermosulfurigenes, C.
thiosulfatireducens, C. tyrobutyricum, C. uliginosum, C. ultunense, C.
villosum, C. vincentii,
C. viride, C. xylanolyticum, or C. xylanovorans) urea-binding protein; a
Caldicellulosiruptor
53
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
sp. (e.g., C. acetigenus, C. bescii, C. changbaiensis, C. hydrothermalis, C.
kristjanssonii, C.
kronotskyensis, C. lactoaceticus, C. owensensis, or C. saccharolyticus) urea-
binding protein;
a Thermocrinis sp. (e.g., T. ruber, T. albus, or T. minervae) urea-binding
protein; a
Synechoccus sp. (e.g., S. ambiguus, S. arcuatus var. cakicolus, S.
bigranulatus, S.
brunneolus S. caldarius, S. capitatus, S. carcerarius, S. elongatus, S.
endogloeicus, S.
epigloeicus, S. ferrunginosus, S. intermedius, S. koidzumii, S. lividus, S.
marinus, S.
minutissimus, S. mundulus, S. nidulans, S. rayssae, S. rhodobaktron, S. roseo-
persicinus, S.
roseo-purpureus, S. salinarum, S. salinus, S. sciophilus, S. sigmoideus, S.
spongiarum, S.
subsalsus, S. sulphuricus, S. vantieghemii, S. violaceus, S. viridissimus, or
S. vtdcanus) urea-
binding protein; a Paenibacillus sp. (e.g., P. agarexedens, P. agaridevorans,
P. alginolyticus,
P. alkaliterrae, P. alvei, P. amylolyticus, P. anaericanus, P. antarcticus, P.
assamensis, P.
azoreducens, P. azotofixans, P. barcinonensis, P. borealis, P. brasilensis, P.
brassicae, P.
campinasensis, P. chinjuensis, P. chitinolyticus, P. chondroitinus, P.
cineris, P. cookii, P.
curdlanolyticus, P. daejeonensis, P. dendritiformis, P. durum, P. ehimensis,
P. elgii, P.
favisporus, P. glucanolyticus, P. glycanilyticus, P. gordonae, P. graminis, P.
granivorans, P.
hodogayensis, P. illinoisensis, P. jamilae, P. kobensis, P. koleovorans, P.
koreensis, P.
kribbensis, P. lactis, P. larvae, P. lautus, P. lentimorbus, P. macerans, P.
macquariensis, P.
massiliensis, P. mendelii, P. motobuensis, P. naphthalenovorans, P.
nematophilus, P.
odorifer, P. pabuli, P. peoriae, P. phoenicis, P. phyllosphaerae, P. polymyxa,
P. popilliae, P.
pulvifaciens, P. rhizosphaerae, P. sanguinis, P. stellifer, P. terrae, P.
thiaminolyticus, P.
timonensis, P. tylopili, P. turicensis, P. validus, P. vortex, P. vulneris, P.
wynnii, P.
xylanilyticus) urea-binding protein; or a Thermosynechococcus sp. (e.g., T.
elongatus or T.
vulcanus) urea-binding protein.
The present subject matter provides a glucose-binding protein that is or is a
mutant of:
an Thermus sp. (e.g., T. caldophilus, T. eggertssonii, T. kawarayensis, T.
murrieta, T.
nonproteolyticus, T. parvatiensis, T. rehai, T. yunnanensis, T.
amyloliquefaciens, T.
antranikianii, T. aquaticus, T. arciformis, T. brockianus, T. caliditerrae, T.
chliarophilus, T.
composti, T. filiformis, T. igniterrae, T. islandicus, T. oshimai, T.
profundus, T. scotoductus,
T. tengchongensis, or T. thermophilus) glucose-binding protein; a Deinococcus
sp. (e.g., D.
aquivivus, D. puniceus, D. soli, D. xibeiensis, D. aerius, D. aerolatus, D.
aerophilus, D.
aetherius, D. alpinitundrae, D. altitudinis, D. apachensis, D. aquaticus, D.
aquatilis, D.
aquiradiocola, D. caeni, D. cellulosilyticus, D. claudionis, D. daejeonensis,
D.
depolymerans, D. deserti, D. erythromyxa, D. ficus, D. frigens, D.
geothermalis, D.
54
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
gobiensis, D. grandis, D. hohokamensis, D. hopiensis, D. indicus, D.
maricopensis, D.
marmoris, D. metalli, D. misasensis, D. murrayi, D. navajonensis, D.
papagonensis, D.
peraridilitoris, D. pimensis, D. piscis, D. proteolyticus, D. radiodurans, D.
radiomollis, D.
radiophilus, D. radiopugnans, D. reticulitermitis, D. roseus, D. saxicola, D.
sonorensis, D.
wulumuqiensis, D. xibeiensis, D. xinjiangensis, D. yavapaiensis, or D.
yunweiensis) glucose-
binding protein; a Thermotoga sp. (e.g., T. caldifontis, T. elfii, T. hypogea,
T. lettingae, T.
maritima, T. naphthophila, T. neapolitana, T. petrophila, T. profunda, T.
subterranea, or T.
thermarum) glucose-binding protein; a Kosmotoga sp. (e.g., K olearia, K
arenicorallina, K
pacifica, or K shengliensis) glucose-binding protein; a Bacillus sp. (e.g., B.
acidiceler, B.
acidicola, B. acidiproducens, B. acidocaldarius, B. acidoterrestris, B.
aeolius, B. aerius, B.
aerophilus, B. agaradhaerens, B. agri, B. aidingensis, B. akibai, B.
alcalophilus, B. algicola,
B. alginolyticus, B. alkalidiazotrophicus, B. alkalinitrilicus, B.
alkalisediminis, B.
alkalitelluris, B. altitudinis, B. alveayuensis, B. alvei, B.
amyloliquefaciens, B. a. subsp.
amyloliquefaciens, B. a. subsp. plantarum, B. amylolyticus, B. andreesenii, B.
aneurinilyticus, B. anthracis, B. aquimaris, B. arenosi, B. arseniciselenatis,
B. arsenicus, B.
aurantiacus, B. arvi, B. aryabhattai, B. asahii, B. atrophaeus, B.
axarquiensis, B. azotofixans,
B. azotoformans, B. badius, B. barbaricus, B. bataviensis, B. beijingensis, B.
benzoevorans,
B. beringensis, B. berkeleyi, B. beveridgei, B. bogoriensis, B. boroniphilus,
B. borstelensis, B.
brevis Migula, B. butanolivorans, B. canaveralius, B. carboniphilus, B.
cecembensis, B.
cellulosilyticus, B. centrosporus, B. cereus, B. chagannorensis, B.
chitinolyticus, B.
chondroitinus, B. choshinensis, B. chungangensis, B. cibi, B. circulans, B.
clarkii, B. clausii,
B. coagulans, B. coahuilensis, B. cohnii, B. composti, B. curdlanolyticus, B.
cycloheptanicus,
B. cytotoxicus, B. daliensis, B. decisifrondis, B. decolorationis, B. deserti,
B. dipsosauri, B.
drentensis, B. edaphicus, B. ehimensis, B. eiseniae, B. enclensis, B.
endophyticus, B.
endoradicis, B. farraginis, B. fastidiosus, B. fengqiuensis, B. firmus, B.
flexus, B. foraminis,
B. fordii, B. formosus, B. fortis, B. fumarioli, B. funiculus, B. fusiformis,
B. galactophilus, B.
galactosidilyticus, B. galliciensis, B. gelatini, B. gibsonii, B. ginsengi, B.
ginsengihumi, B.
ginsengisoli, B. globisporus, B. g. subsp. globisporus, B. g. subsp. marinus,
B.
glucanolyticus, B. gordonae, B. gottheilii, B. graminis, B. halmapalus, B.
haloalkaliphilus, B.
halochares, B. halodenitrificans, B. halodurans, B. halophilus, B.
halosaccharovorans, B.
hemicellulosilyticus, B. hemicentroti, B. herbersteinensis, B. horikoshii, B.
horneckiae, B.
horti, B. huizhouensis, B. humi, B. hwajinpoensis, B. idriensis, B. indicus,
B. infantis, B.
infernus, B. insolitus, B. invictae, B. iranensis, B. isabeliae, B.
isronensis, B. jeotgali, B.
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
kaustophilus, B. kobensis, B. kochii, B. kokeshiiformis, B. koreensis, B.
korlensis, B.
kribbensis, B. kndwichiae, B. laevolacticus, B. larvae, B. laterosporus, B.
lautus, B. lehensis,
B. lentimorbus, B. lentus, B. licheniformis, B. ligniniphilus, B. litoralis,
B. locisalis, B.
luciferensis, B. luteolus, B. luteus, B. macauensis, B. macerans, B.
macquariensis, B. macyae,
B. malacitensis, B. mannanilyticus, B. marisflavi, B. marismortui, B.
marmarensis, B.
massiliensis, B. megaterium, B. mesonae, B. methanolicus, B. methylotrophicus,
B.
migulanus, B. mojavensis, B. mucilaginosus, B. muralis, B. murimartini, B.
mycoides, B.
naganoensis, B. nanhaiensis, B. nanhaiisediminis, B. nealsonii, B. neidei, B.
neizhouensis, B.
niabensis, B. niacini, B. novalis, B. oceanisediminis, B. odysseyi, B.
okhensis, B. okuhidensis,
B. oleronius, B. oryzaecorticis, B. oshimensis, B. pabuli, B. pakistanensis,
B. pallidus, B.
pallidus, B. panacisoli, B. panaciterrae, B. pantothenticus, B. parabrevis, B.
paraflexus, B.
pasteurii, B. patagoniensis, B. peoriae, B. persepolensis, B. persicus, B.
pervagus, B.
plakortidis, B. pocheonensis, B. polygoni, B. polymyxa, B. popilliae, B.
pseudalcalophilus, B.
pseudofirmus, B. pseudomycoides, B. psychrodurans, B. psychrophilus, B.
psychrosaccharolyticus, B. psychrotolerans, B. pulvifaciens, B. pumilus, B.
purgationiresistens, B. pycnus, B. qingdaonensis, B. qingshengii, B. reuszeri,
B.
rhizosphaerae, B. rigui, B. ruris, B. safensis, B. salarius, B. salexigens, B.
saliphilus, B.
schlegelii, B. sediminis, B. selenatarsenatis, B. selenitireducens, B.
seohaeanensis, B.
shacheensis, B. shackletonii, B. siamensis, B. silvestris, B. simplex, B.
siralis, B. smithii, B.
soli, B. solimangrovi, B. solisalsi, B. songklensis, B. sonorensis, B.
sphaericus, B.
sporothermodurans, B. stearothermophilus, B. stratosphericus, B. subterraneus,
B. subtilis,
B. s. subsp. inaquosorum, B. s. subsp. spizizenii, B. s. subsp. subtilis, B.
taeanensis, B.
tequilensis, B. thermantarcticus, B. thermoaerophilus, B. thermoamylovorans,
B.
thermocatenulatus, B. thermocloacae, B. thermocopriae, B. thermodenitrificans
, B.
thermoglucosidasius, B. thermolactis, B. thermoleovorans, B. thermophilus, B.
thermoruber,
B. thermosphaericus, B. thiaminolyticus, B. thioparans, B. thuringiensis, B.
tianshenii, B.
trypoxylicola, B. tusciae, B. validus, B. vallismortis, B. vedderi, B.
velezensis, B.
vietnamensis, B. vireti, B. vulcani, B. wakoensis, B. weihenstephanensis, B.
xiamenensis, B.
xiaoxiensis, or B. zhanjiangensis) glucose-binding protein; a Staphylothermus
sp. (e.g., S.
hellenicus or S. marinus) glucose-binding protein; or an Arthrobacter sp.
(e.g., A. agilis, A.
alkaliphilus, A. alpinus, A. antarcticus, A. aurescens, A. bambusae, A.
castelli, A.
chlorophenolicus, A. citreus, A. cryoconiti, A. cryotolerans, A.
crystallopoietes, A. cumminsii,
A. cupressi, A. defluvii, A. enclensis, A. flavus, A. gandavensis, A.
globiformis, A.
56
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
gyeryongensis, A. halodurans, A. histidinolovorans, A. humicola, A. koreensis,
A. liuii, A.
livingstonensis, A. luteolus, A. methylotrophus, A. monumenti, A.
nanjingensis, A.
nasiphocae, A. nicotinovorans, A. nitroguajacolicus, A. oryzae, A. parietis,
A. pascens, A.
pigmenti, A. pityocampae, A. psychrochitiniphilus, A. psychrolactophilus, A.
ramosus, A.
rhombi, A. roseus, A. russicus, A. sanguinis, A. soli, A. stackebrandtii, A.
subterraneus, A.
tecti, A. tumbae, A. viscosus, or A. woluwensis) glucose-binding protein.
The present subject matter provides a lactate-binding protein that is or is a
mutant of:
a Thermus sp. (e.g., T. caldophilus, T. eggertssonii, T. kawarayensis, T.
murrieta, T.
nonproteolyticus, T. parvatiensis, T. rehai, T. yunnanensis, T.
amyloliquefaciens, T.
antranikianii, T. aquaticus, T. arciformis, T. brockianus, T. caliditerrae, T.
chliarophilus, T.
composti, T. filiformis, T. igniterrae, T. islandicus, T. oshimai, T.
profundus, T. scotoductus,
T. shimai, T. tengchongensis, or T. thermophilus) lactate-binding protein, a
Thioalkalivibrio
sp. (e.g., T. denitrificans, T. halophilus, T. jannaschii, T. nitratireducens,
T. nitratis, T.
paradoxus, T. sulfidiphilus, T. thiocyanodenitrificans, T. thiocyanoxidans,
and T. versutus)
lactate-binding protein, a Roseobacter sp. (e.g., R. dentrificans, R.
litoralis, R. pelophilus, R.
prionitis, R. sp. 14111/A01/004, R. sp. 1922, R. sp. 27-4, R. sp. 3008, R. sp.
38.98, R. sp.
3X/A02/234, R. sp. 4318-8/1,R. sp. 812,R. sp. 8-1,R. sp. ANT8230, R. sp.
AN'T9082, R. sp.
ANT909, R. sp. ANT9234, R. sp. ANT9240, R. sp. ANT9270, R. sp. ANT9274, R. sp.
ANT9276a, R. sp. AN'T9283, R. sp. ARCTIC-P4, R. sp. ARK9990, R. sp. AzwK-3b,
R. sp.
AzwLept-lc, R. sp. B09, R. sp. B-1039, R. sp. B11, R. sp. Ber2105, R. sp.
Ber2107, R. sp.
BS36, R. sp. BS90, R. sp. C115, R. sp. C23, R. sp. CCS2, R. sp. COL2P, R. sp.
COLSP, R. sp.
DG1132, R. sp. DG869, R. sp. DG889, R. sp. DG942, R. sp. Do-34, R. sp. DSS-1,
R. sp.
DSS-8, R. sp. H264, R. sp. H265, R. sp. H454, R.. HJ105, R. sp. HJ247, R. sp.
HYL-SA-18,
R. sp. HZBC52, R. sp. HZDC27, R. sp. HZDC41, R. sp. HZDC42, R. sp. HZDC43, R.
sp.
HZDC7, R. sp. J2W, R. sp. J356, R. sp. J392, R. sp. J483, R. sp. J486, R. sp.
J504, R. sp. J8W,
R. sp. JL-126, R. sp. JL-129, R. sp. JL-131, R.. JL-132, R. sp. JL-135, R. sp.
JL-137, R. sp.
JL-351, R. sp. JL985, R. sp. JLN-A020, R. sp. JLN-A036, R. sp. JLN-A530, R.
sp. KAT10, R.
sp. KAT3, R. sp. KT0202a, R. sp. KT0917, R. sp. KT1117, R. sp. LA7, R. sp.
LA9, R. sp.
LOB-8, R. sp. LZXC12, R. sp. LZXC14, R. sp. LZXC15, R. sp. LZXC16, R. sp.
LZXC20, R.
sp. LZXC23, R. sp. LZXC26, R. sp. LZXC29, R. sp. LZXC4, R. sp. LZXC7, R. sp.
MBT21,
R. sp. MBT22, R. sp. MED001, R. sp. MED006, R. sp. MED007, R. sp. MED008, R.
sp.
MED193, R. sp. MED24, R. sp. MED26, R. sp. MED61, R. sp. MED6, R. sp. M5I042,
R. sp.
NO51, R. sp. NJ5527, R. sp. NT N37, R. sp. 0C-B2-7, R. sp. 0C-C4-20, R. sp. 00-
A3-7, R.
57
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
sp. 00-C4-10, R. sp. Pht-4, R. sp. PIC-68, R. sp. PRLIST02, R. sp. PRLIST06,
R. sp.
PRLISY01, R. sp. PRLISY03, R. sp. QSSC9-8, R. sp. RED15, R. sp. RED1, R. sp.
RED59, R.
sp. RED68, R. sp. RED85, R. sp. S03, R. sp. SC-B2-2,R. sp. SCB28, R. sp.
SCB31, R. sp.
5CB34, R. sp. 5CB48, R. sp. SDBC1, R. sp. SDBC6, R. sp. SFLA13, R. sp. SIO, R.
sp.
5K209-2-6, R. sp. 5KA26, R. sp. 5KA44, R. sp. 5L25, R. sp. S03, R. sp. 5P0804,
R. sp.
SY0P1, R. sp. SY0P2, R. sp. TM1035, R. sp. TM1038, R. sp. TM1040, R. sp.
TM1042, R.
sp. TP9, R. sp. UAzPsJAC-lb, R. sp. UAzPsK-5, R. sp. WED10.10, R. sp. WED1.1,
R. sp.
WHOI JT-01, R. sp. WHOI JT-08, R. sp. WHOI JT-22, R. sp. WM2, R. sp. Y2, R.
sp. Y3F, R.
sp. YS-57, R. sp. YSCB-1, or R. sp. YSCB-3) lactate-binding protein, a
Marinobacter sp.
(e.g., M adhaerens, M algicola, M alkaliphilus, M antarcticus, M arcticus,
M.aromaticivorans, M bryozoorum, M daepoensis, M daqiaonensis, M excellens, M
flavimaris, M gudaonensis, M guineae, M halophilus, M gudaonensis, M
hydrocarbonoclasticus, M koreensis, M lacisalsi, M lipolyticus, M litoralis, M
lutaoensis,
M maritimus, M mobilis, M nitratireducens, M oulmenensis, M pelagius, M
persicus, M
psychrophilus, M nanhaiticus, M salarius, M salicampi, M salsuginis, M
santoriniensis,
M sediminum, M segnicrescens, M shengliensis, M squalenivorans, M similis, M
szutsaonensis, M vinifirmus, M xestospongiae, M zhanjiangensis, or M
zhejiangensis)
lactate-binding protein, a Anaeromyxobacter sp. (e.g., A. dehalogenans)
lactate-binding
protein, a Polymorphum sp. (e.g., P. gilvum ) lactate-binding protein, a
Pseudomonas sp.
(e.g., P. aeruginosa, P. alcaligenes, P. anguilliseptica, P. argentinensis, P.
borbori, P.
citronellolis, P. flavescens, P. mendocina, P. nitroreducens, P. oleovorans,
P.
pseudoalcaligenes, P. resinovorans, P. straminea, P. chlororaphis, P.
asplenii, P.
aurantiaca, P. aureofaciens, P. chlororaphis, P. corrugata, P. fragi, P.
lundensis, P.
taetrolens, P. antarctica, P. azotoformans, P. blatchfordae, P.
brassicacearum, P. brenneri,
P. cedrina, P. corrugata, P. fluorescens, P. gessardii, P. libanensis, P.
mandelii, P.
marginalis, P. mediterranea, P. meridiana, P. migulae, P. mucidolens, P.
orientalis, P.
panacis, P. protegens, P. proteolytica, P. rhodesiae, P. synxantha, P.
thivervalensis, P.
tolaasii, P. veronii, P. denitrificans, P. pertucinogena, P. cremoricolorata,
P. entomophila,
P. fulva, P. monteilii, P. mosselii, P. oryzihabitans, P. parafulva, P.
plecoglossicida, P.
putida, P. balearica, P. luteola, P. stutzeri, P. amygdali, P. avellanae, P.
caricapapayae, P.
cichorii, P. coronafaciens, P. ficuserectae, P. helianthi, P. meliae, P.
savastanoi, P. syringae,
P. tomato, or P. viridiflava) lactate-binding protein, a Rhodobacter sp.
(e.g., R. aestuarii, R.
azotoformans, R. blasticus, R. capsulatus, R. johrii, R. maris, R.
megalophilus, R. ovatus,
58
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
R. sphaeroides, R. veldkampii, R. vinaykumarii, or R. viridis) lactate-binding
protein, a
Flexistipes sp. (e.g., F. sinusarabici) lactate-binding protein, or a
Thermanaerovibrio sp.
(e.g., T. acidaminovorans or T. velox) lactate-binding protein.
The present subject matter provides a ligand-binding protein that is or is a
mutant of:
a Synechocystis sp. (e.g., S. sp. PCC6803) bicarbonate-binding protein, a
Thermosynechococcus sp. (e.g., T. vulcanus, T. elongatus, or T. elongatus BP-
1) bicarbonate-
binding protein, a Chroococcidiopsis sp. (e.g., C. thermalis, C. gigantea, C.
cubana, or C.
codiicola) bicarbonate-binding protein, a Calothrix sp. (e.g., C. aberrans, C.
adscencens, C.
aeruginea, C. africana, C. allorgei, C. australiensis, C. baileyi, C.
bharadwajae, C. borealis,
C. braunii, C. breviarticulata, C. calida, C. castellii, C. capitularis, C.
cavernarum
Copeland, C. charicola, C. davata, C. clavatoides, C. codicola, C. columbiana,
C. compacta,
C. confervicola, C. contarenii, C. coriacea, C. crustacea, C. cylindrica, C.
desertica, C.
elsteri, C. epiphytica, C. evanescens, C. estonica, C. fasciculata, C.
feldmannii, C. flahaultii,
C. floccosa, C. fritschii, C. fuellebornii, C. fusca, C. fusco-violacea, C.
geilterii, C. geitonos,
C. ghosei, C. gigas, C. gloeocola, C. goetzei, C. hunanica, C. inaequabilis,
C. inserta, C.
javanica, C. karnatakensis, C. kawraiskyi, C. kossinskajae, C. kuntzei, C.
linearis, C. minima,
C. nidulans, C. parasitica, C. parietina, C. parva, C. pilosa, C. prolifera,
C. pulvinata, C.
rectangularis, C. reptans, C. rodriguezii, C. santapaui, C. scopulorum, C.
scytonemicola, C.
simplex, C. simulans, C. stagnalis, C. subantarctica, C. subsimplex, C.
tenella, C. thermalis,
C. twfosa, C. viguieri, C. vivipara, C. violacea, C. weberi, C. wembaerensis,
C. aeruginosa,
C. aestuarii, C. antarctica, C. atricha, C. bossei, C. brevissima, C. clausa,
C. conica, C.
dnieprensis, C. elenkinii, C. fonticola, C. galpinii, C. gelatinosa, C.
gracilis, C. intricata, C.
litoralis, C. marchica, C. nodulosa, C. obtusa, C. rhizosoleniae, or C.
schweickertii)
bicarbonate-binding protein, a Anabaena sp. (e.g., A. aequalis, A. affinis, A.
angstumalis
angstumalis, A. angstumalis marchita, A. aphanizomendoides, A. azollae, A.
bornetiana, A.
catenula, A. cedrorum, A. circinalis , A. confervoides, A. constricta, A.
cyanobacterium, A.
cycadeae, A. cylindrica, A. echinispora, A. felisii, A. flos-aquae flos-aquae,
A. flos-aquae
minor, A. flos-aquae treleasei, A. helicoidea, A. inaequalis, A. lapponica, A.
laza, A.
lemmermannii, A. levanderi, A. limnetica, A. macrospora macrospora, A.
macrospora
robusta, A. monticulosa, A. nostoc, A. oscillarioides, A. planctonica, A.
raciborskii, A.
scheremetievi, A. sphaerica, A. spiroides crassa, A. spiroides spiroides, A.
subcylindrica, A.
torulosa, A. unispora, A. variabilis, A. verrucosa, A. viguieri, A.
wisconsinense, or A.
zierlingii) bicarbonate-binding protein, or a Chamaesiphon sp. (e.g., C.
africanus, C.
59
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
amethystinus, C. britannicus, C. carpaticus, C. confervicola, C. cylindricus,
C.
cylindrosporus, C. halophilus, C. incrustans, C. investiens, C. jaoi, C.
komarekii, C. longus,
C. macer, C. major, C. minimus, C. minutus, C. portoricensis, C. rostafinskii,
C. sideriphilus,
C. tibeticus, C. aggregatus, C. fallax, C. fuscus, C. geitleri, C. mollis, C.
niger, C.
ocobyrsiodes, C. polonicus, C. polymorphus, C. starmachii, C. stratosus, or C.
subglobosus)
bicarbonate-binding protein.
The present subject matter provides a ligand-binding protein that is or is a
mutant of:
a Mannheimia sp. (e.g., M caviae, M glucosida, M granulomatis, M haemolytica,
M
ruminalis, or M varigena) bicarbonate and iron binding protein, an
Exiguobacterium sp.
(e.g., E. acetylicum, E. aestuarii, E. alkaliphilum, E. antarcticum, E.
aquaticum, E. artemiae,
E. aurantiacum, E. enclense, E. indicum, E. marinum, E. mexicanum, E.
oxidotolerans, E.
profundum, E. sibiricum, E. soli, or E. undae) bicarbonate and iron binding
protein, a
Thermosynechococcus sp. (e.g., T. vulcanus, T. elongatus, or T. elongatus BP-
1) bicarbonate
and iron binding protein, a Candidatus Nitrospira sp. (e.g., Candidatus
Nitrospira defluvii,
Candidatus Nitrospira nitrificans, Candidatus Nitrospira nitrosa, Candidatus
Nitrospira
inopinata, Candidatus Magnetobacterium casensis, Candidatus Magnetobacterium
bavaricum, Candidatus Magnetoovum chiemensis) bicarbonate and iron binding
protein, a
Thermus sp. (e.g., T. caldophilus, T. eggertssonii, T. kawarayensis, T.
murrieta, T.
nonproteolyticus, T. parvatiensis, T. rehai, T. yunnanensis, T.
amyloliquefaciens, T.
antranikianii, T. aquaticus, T. arciformis, T. brockianus, T. caliditerrae, T.
chliarophilus, T.
composti, T. filiformis, T. igniterrae, T. islandicus, T. oshimai, T.
profundus, T. scotoductus,
T. tengchongensis, or T. thermophilus) bicarbonate and iron binding protein, a
Meiothermus
sp. (M chiliarophilus, M cerbereus, M granaticius, M rosaceus, M ruber, M
rufus, M
silvanus, M taiwanensis, or M timidus) bicarbonate and iron binding protein, a
Salinibacter
sp. (e.g., S. ruber, S. iranicus, or S. luteus) bicarbonate and iron binding
protein, or a
Halorubrum sp. (e.g., H. aidingense, H. alkaliphilum, H. arcis, H.
californiensis, H. coriense,
H. distributum, H. ejinorense, H. ezzemoulense, H. kocurii, H. lacusprofundi,
H. lipolyticum,
H. litoreum, H. luteum, H. orientalis, H. saccharovorum, H. salsolis, H.
sodomense, H.
tebenquichense, H. terrestre, H. tibetense, H. trapanicum, H. vacuolatum, or
H. xinjiangense)
bicarbonate and iron binding protein.
With regard to a defined polypeptide, % identity figures higher or lower than
those
provided herein will encompass various embodiments. Thus, where applicable, in
light of a
minimum % identity figure, a polypeptide may comprise an amino acid sequence
which is at
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
least 60%, 65%, 70%, 75%, 76%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%,
97%,
98%, 99%, 99.1%, 99.2%, 99.3%, 99.4%, 99.5%, 99.6%, 99.7%, 99.8%, or 99.9%
identical
to the reference SEQ ID NO or to each of the reference SEQ ID NOs. Where
applicable, in
light of a maximum % identity to a reference sequence, a polypeptide may
comprise an
amino acid sequence which is less than 75%, 70%, 65%, 60%, 59%, 58%, 57%, 56%,
55%,
54%, 53%, 52%, 51%, 50%, 49%, 48%, 47%, 46%, 45%, 44%, 43%, 42%, 41%, 40%,
39%,
38%, 37%, 36%, 35%, 34%, 33%, 32%, 31%, 30%, 29%, 28%, 27%, 26%, 25%, 24%,
23%,
22%, 21%, 20%, 19%, 18%, 17%, 16%, or 15% identical to the reference SEQ ID NO
or to
each of the reference SEQ ID NOs. In certain embodiments, a polypeptide
comprises amino
acids in a sequence that is preferably at least about 10%, 11%, 12%, 13%, 14%,
15%, 16%,
17%, 18%, 19%, 20%, 21%, 22%, 23%, 24%, 25%, 26%, 27%, 28%, 29%, or 30% and
less
than about 75%, 70%, 65%, 60%, 55%, 50%, 45%, 44%, 43%, 42%, 41%, 40%, 39%,
38%,
37%, 36%, 35%, 34%, 33%, 32%, 31%, or 30% identical to the reference SEQ ID NO
or to
each of the reference SEQ ID NOs. Non-limiting examples of reference proteins
and amino
acid sequences disclosed herein include:
(i) a glucose-galactose binding protein from Escherichia coli (ecGGBP;
genome,
NC 002695; protein, WP 032329053, SEQ ID NO: 87);
(ii) a glucose-galactose binding protein from Thermoanaerobacterium
thermosaccharolyticum (ttGGBP; genome, NC_014410; protein,
YP 003852930.1, SEQ ID NO: 88);
(iii) a glucose-galactose binding protein from Salmonella typhimurium (stGGBP;
genome, NC_003197; protein, WP_001036943, SEQ ID NO: 89);
(iv) a glucose-galactose binding protein from Caldicellulosiruptor
hydrothermalis
(chyGGBP; genome, NC_014652; protein identifier, YP_003991244.1, SEQ
ID NO: 90);
(v) a glucose-galactose binding protein from Caldicellulosiruptor
obsidiansis
(cobGGBP; genome, NC_014392; protein, YP_003839461.1, SEQ ID NO:
91);
(vi) a glucose-galactose binding protein from Paenibacillus sp. (pspGGBP;
genome, NC_013406; protein, YP_003243743.1, SEQ ID NO: 92);
(vii) a glucose-galactose binding protein from Clostridium saccharolyticum
(csaGGBP; genome, NC_014376; protein, YP_003822565.1, SEQ ID NO:
93);
61
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
(viii) a glucose-galactose binding protein from Butyrivibrio proteoclasticus
(bprGGBP; genome, NC_014387; protein, YP_003830205.1, SEQ ID NO:
94);
(ix) a glucose-galactose binding protein from Roseburia intestinalis
(rinGGBP_A;
genome, NC_021012; protein, YP_007778116.1, SEQ ID NO: 95);
(x) a glucose-galactose binding protein from Faecalibacterium prausnitzii
(fprGGBP; genome, NC_021020; protein, YP_007799070.1, SEQ ID NO:
96);
(xi) a glucose-galactose binding protein from Clostridium ljungdahlii
(cljGGBP;
genome, NC_014328; protein, CLJU_c08950, SEQ ID NO: 97);
(xii) a glucose-galactose binding protein from Clostridium autoethanogenum
(cauGGBP; genome, NC_022592; protein, CAETHG_2989, SEQ ID NO: 98);
(xiii) a glucose-galactose binding protein from Roseburia intestinalis
(rinGGBP_B;
genome, NC_021012; protein, YP_007778124.1, SEQ ID NO: 99);
(xiv) a glucose-galactose binding protein from Erysipelothrix rhusiopathiae
(erhGGBP; genome, NC_015601; protein, YP_004561181.1, SEQ ID NO:
100);
(xv) a glucose-galactose binding protein from Eubacterium rectale (ereGGBP;
genome, NC_012781; protein, YP_002936409.1, SEQ ID NO: 101);
(xvi) a urea-binding protein from Marinomas posidonica (mpUBP; genome,
NC 015559, protein, YP 004483096.1; SEQ ID NO: 102);
(xvii) a urea-binding protein from Marinobacter hydrocarbanoclasticus (mhUBP;
genome, NC_017067, protein, YP_005430828.1; SEQ ID NO: 103);
(xviii) a urea-binding protein from Bacillus sp. (bsUBP; genome, NC_017743,
protein, YP_006233530.1; SEQ ID NO: 104);
(xix) a urea-binding protein from Desulfotomaculum carboxydivorans (dsUBP;
genome, NC_015565, protein, YP_004496535.1; SEQ ID NO: 105);
(xx) a urea-binding protein from Geobacillus thermoglucosidasius (gtUBP;
genome, NC_015660, protein, YP_004588319.1; SEQ ID NO: 106);
(xxi) a urea-binding protein from Clostridium thermocellum (ctUBP; genome,
NC 009012, protein, YP 001038237.1; SEQ ID NO: 107);
(xxii) a urea-binding protein from Caldicellulosiruptor saccharolyticus
(csUBP;
genome, NC_009437, protein, YP_001181243.1; SEQ ID NO: 108);
62
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
(xxiii) a urea-binding protein from Thermocrinis albus (taUBP; genome,
NC 013894, protein, YP 003473480.1; SEQ ID NO: 109);
(xxiv) a urea-binding protein from Geobacillus kaustophilus (gkUBP; genome,
NC 006510, protein, YP 147790.1; SEQ ID NO: 110);
(xxv) a urea-binding protein from Paenibacillus sp. (psUBP; genome, NC_013406,
protein, YP_003241723.1; SEQ ID NO: 111);
(xxvi) a urea-binding protein from Thermosynechococcus elongatus (teUBP;
genome, NC_004113, protein, YP_681910.1; SEQ ID NO: 112);
(xxvii) a glucose-binding protein from Thermus thermophilus (ttGBP; genome,
NC 005835, protein, YP 004303.1 and WP 011172778; SEQ ID NO: 113);
(xxviii)a glucose-binding protein from Thermus scotoductus (tsGBP; genome,
NC 014974, protein, YP 004202647.1; SEQ ID NO: 114);
(xxix) a glucose-binding protein from Deinococcus maricopensis (dmGBP; genome,
NC 014958, protein, YP 004171760.1; SEQ ID NO: 115);
(xxx) a glucose-binding protein from Thermotoga neapolitana (tnGBP; genome,
NC 011978, protein, YP 002534202.1; SEQ ID NO: 116);
(xxxi) a glucose-binding protein from Kosmotoga olearia (koGBP; genome,
NC 012785, protein, YP 0029416871.1; SEQ ID NO: 117);
(xxxii) a glucose-binding protein from Bacillus halodurans (bhGBP; genome,
NC 002570, protein, YP 244712.1; SEQ ID NO: 118);
(xxxiii)a glucose-binding protein from Staphylothermus marinus (smGBP; genome,
NC 009033, protein, YP 001041152.1; SEQ ID NO: 119);
(xxxiv)a glucose-binding protein from Arthrobacter sp. (asGBP; genome,
NC 008541, protein, YP 8313491.1; SEQ ID NO: 120);
(xxxv) a lactate-binding protein from Thermus thermophilus (ttLacBP; genome,
NC 006461, protein, YP 144032.1; SEQ ID NO: 121);
(xxxvi)a lactate-binding protein from Thermus scotoductus (tscLacBP; genome,
NC 014974, protein YP 004202714.1; SEQ ID NO: 122);
(xxxvii) a lactate-binding protein from Thermus oshimai (toLacBP; genome,
NC 019386, protein YP 019386; SEQ ID NO: 123);
(xxxviii) a lactate-binding protein from Thioalkalivibrio sulfidophilus
(tsuLacBP; genome, NC_011901, protein YP_002514099.1; SEQ ID NO:
124);
63
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
(xxxix)a lactate-binding protein from Roseobacter denitrificans (rdLacBP;
genome,
NC 008209, protein YP 683924.1; SEQ ID NO: 125);
(xl) a lactate-binding protein from Marinobacter sp. (msLacBP; genome,
NC 018268, protein YP 006556686.1; SEQ ID NO: 126);
(xli) a lactate-binding protein from Thermus sp. (tspLacBP; genome, NC_017278,
protein YP_005654632.1; SEQ ID NO: 127);
(xlii) a lactate-binding protein from Marinobacter adhaerens (maLacBP; genome,
NC 017506, protein YP 005686720.1; SEQ ID NO: 128);
(xliii) a lactate-binding protein from Anaeromyxobacter dehalogens (adLacBP;
genome, NC_007760, protein YP_466_099.1; SEQ ID NO: 129);
(xliv) a lactate-binding protein from Polymorphum gilvum (pgLacBP; genome,
NC 015259, protein YP 4304976.1; SEQ ID NO: 130);
(xlv) a lactate-binding protein from Pseudomonas stuztzeri (psLacBP; genome,
NC 018177, protein YP 00652676.1; SEQ ID NO: 131);
(xlvi) a lactate-binding protein from Rhodobacter sphaeroides (rsLacBP;
genome,
NC 007494, protein RSP 3372; SEQ ID NO: 132);
(xlvii) a lactate-binding protein from Flexistipes sinusarabici (fsLacBP;
genome,
NC 015672, protein YP 004603455.1; SEQ ID NO: 133);
(xlviii) a lactate-binding protein from Thermanaerovibrio acidaminovorans
(taLacBP;
genome, NC_013522, protein YP_003317968.1; SEQ ID NO: 134);
(xlix) a bicarbonate-binding protein from Synechocystis sp. (synBicarbBP1;
genome,
NC 017052, protein YP 005410477.1; SEQ ID NO: 135);
(1) a bicarbonate-binding protein from Thermosynechococcus
elongatus
(teBicarbBP2; genome, NC_004113, protein NP_682790.1; SEQ ID NO:
136);
(1i) a bicarbonate-binding protein from Chroococcidiopsis thermalis
(ctBicarbBP3; genome, NC_019695, protein YP_007090308.1; SEQ ID NO:
137);
(lii) a bicarbonate-binding protein from Calothrix sp. (calBicarbBP4; genome,
NC 019751, protein YP 007137061.1; SEQ ID NO: 138);
(liii) a bicarbonate-binding protein from Anabaena variabilis (avBicarbBP5;
genome, NC_007413, protein YP_321546.1; SEQ ID NO: 139);
64
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
(liv) a bicarbonate-binding protein from Chamaesiphon minutus (cmBicarbBP6;
genome, NC_019697, protein YP_007099445.1; SEQ ID NO: 140);
(1v) a bicarbonate and iron binding protein from Mannheimia haemolytica
(mhFeBP1; genome, NC_0121082, protein, YP_007884192.1; SEQ ID NO:
141);
(lvi) a bicarbonate and iron binding protein from Exiguobacterium sp.
(exiFeBP2;
genome, NC_012673, protein, YP_002886303.1; SEQ ID NO: 142);
(lvii) a bicarbonate and iron binding protein from Thermosynechoccus elongatus
(teFeBP3; genome, NC_004113, protein, NP_681303.1; SEQ ID NO: 143);
(lviii) a bicarbonate and iron binding protein from Candidatus nitrospira
(cnFeBP4;
genome, NC_014355, protein, YP_003796723.1; SEQ ID NO: 144);
(lix) a bicarbonate and iron binding protein from Thermus thermophilus
(ttFeBP5;
genome, NC_006461, protein, YP_144894.1; SEQ ID NO: 145);
(1x) a bicarbonate and iron binding protein from Meiothermus silvanus
(msFeBP6;
genome, NC_014212, protein, YP_003686074.1; SEQ ID NO: 146);
(lxi) a bicarbonate and iron binding protein from Salinibacter ruber (srFeBP7;
genome, NC_014032, protein, YP_003572493.1; SEQ ID NO: 147); and
(lxii) a bicarbonate and iron binding protein from Halorubrum lacusprofundi
(h1FeBP8; genome, NC_012029, protein, YP_002564837.1; SEQ ID NO:
148).
The ligand-binding proteins disclosed herein may optionally be fused (e.g., at
their N-
terminal and/or C-terminal ends) to a motif comprising a stretch of amino
acids that
facilitates the isolation or other manipulation such as conjugation to a
moiety or
immobilization on a substrate such as a plastic, a cellulose product such as
paper, polymer,
metal, noble metal, semi-conductor, or quantum dot (e.g., a fluorescent
quantum dot) . A
non-limiting example of such a stretch of amino acids has the sequence:
GGSHHHHHH
(SEQ ID NO: 152). This motif is not required for, is not believed to influence
or affect
ligand-binding activity or signal transduction, and may be omitted from any
ligand-binding
protein or biosensor disclosed herein. Additionally, for every sequence
disclosed herein that
includes GGSHHHHHH (SEQ ID NO: 152), a corresponding sequence that is
identical
except that it lacks GGSHHHHHH (SEQ ID NO: 152) is also provided and intended
to be
disclosed. For example, each of SEQ ID NOs: 1-41 (and the non-limiting
examples of other
proteins used in the experiments disclosed herein) comprises this motif (SEQ
ID NO: 152).
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Alternatively or in addition, a ligand-binding protein may be fused to a non-
native
polypeptide or "added amino acids" that facilitates the attachment thereof to
a surface, such
as the surface of a device.
In some embodiments, a polypeptide comprises 1, 2, 3, 4, 5, or more
substitutions or
deletions of a cysteine compared to the naturally occurring counterpart of the
polypeptide
(i.e., 1, 2, 3, 4, 5, or more native cysteines have been removed), e.g., 1, 2,
3, 4, 5, or more
cysteine to alanine substitutions compared to the naturally occurring
counterpart of the
polypeptide. In some embodiments, all of the cysteines of a polypeptide have
been deleted
and/or substituted compared to its natural counterpart. In some embodiments,
one or more
cysteines of a polypeptide have been substituted with an alanine, a serine, or
a threonine.
In embodiments, the amino acid sequence of a protein comprises no more than 1,
2, 3,
4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35, 40, 45, or 50 mutations compared to
its naturally
occurring counterpart. In some embodiments, less than 50, 45, 40, 35, 30, 25,
20, 15, 10, 9,
8, 7, 6, 5, 4, 3, or 2 of the mutations is a deletion or insertion of 1, 2, 3,
4, or 5 or no more
than 1, 2, 3, 4, or 5 amino acids. In some embodiments, 1, 2, 3, 4, 5, 6, 7,
8, 9, 10, 15, 20, 25,
30, 35, 40, 45, 50 or more of the mutations is a substitution mutation. In
certain
embodiments, every mutation to a protein compared to its naturally occurring
counterpart is a
substitution mutation. In various embodiments, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10,
15, 20, 25, 30, 35,
40, 45, 50 or more or all of the mutations to a protein compared to its
naturally occurring
counterpart is a conservative substitution mutation.
In various embodiments, a polypeptide does not have any insertion or deletion
compared to its natural counterpart, other than (optionally) the removal of
the signal peptide
and/or the fusion of compounds such as another polypeptide at the N-terminus
or C-terminus
thereof.
Exemplary Ligand-Binding Proteins
Various biosensors provided herein comprise ligand-binding proteins, such as
proteins
that have altered amino acid sequences compared to their naturally occurring
counterparts. In
embodiments, such proteins are conjugated to reporter groups.
In various embodiments, the Ca, root-mean-square deviation (RMSD) between the
backbone of a ligand-binding protein and its naturally occurring counterpart
is, e.g., between
about 0-3 A, 0-1 A, 0-1.5 A, 0-2 A, 0.1-3 A, 0.5-1 A, 0.5-1.5 A, or 0.5-2 A,
or less than about
0.1 A, 0.2 A, 0.3 A, 0.4 A, 0.5 A, 0.6 A, 0.7 A, 0.8 A, 0.9 A, 1.0 A, 1.5 A,
1.6 A, 1.7 A, 1.8
66
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
A, 1.9 A, 2.0 A, 2.5 A, or 3 A. In some embodiments, the Ca RMSD between the N-
terminal
domain (i.e., the portion of the protein at the N-terminal side of the binding
domain hinge)
backbone of the ligand-binding protein and the corresponding domain of its
naturally
occurring counterpart is, e.g., between about 0-3 A, 0-1 A, 0-1.5 A, 0-2 A,
0.1-3 A, 0.5-1 A,
0.5-1.5 ik, or 0.5-2 ik, or less than about 0.1 ik, 0.2 ik, 0.3 ik, 0.4 ik,
0.5 ik, 0.6 ik, 0.7 ik, 0.8
A, 0.9 A, 1.0 A, 1.5 A, 1.6A, 1.7A, 1.8A, 1.9 A, 2.0 A, 2.5 A, or 3 A. In
certain
embodiments, the Ca RMSD between the C-terminal domain (i.e., the portion of
the protein
at the C-terminal side of the binding domain hinge) backbone of the ligand-
binding
polypeptide and the corresponding domain of its naturally occurring
counterpart is, e.g.,
between about 0-3 A, 0-1 A, 0-1.5 A, 0-2 A, 0.1-3 A, 0.5-1 A, 0.5-1.5 A, or
0.5-2 A, or less
than about 0.1 ik, 0.2 ik, 0.3 ik, 0.4 ik, 0.5 ik, 0.6 ik, 0.7 ik, 0.8 ik, 0.9
ik, 1.0 ik, 1.5 ik, 1.6 ik,
1.7 A, 1.8 A, 1.9 A, 2.0 A, 2.5 A, or 3 A. Non-limiting considerations
relating to the
sequence and structural differences between homologous proteins are discussed
in Chothia
and Lesk (1986) The EMBO Journal, 5(4):823-826, the entire content of which is
incorporated herein by reference.
Non-limiting examples of ligand-binding polypeptides that are useful in
biosensors
provided herein include variants of the naturally occurring proteins disclosed
herein that
comprise cysteine substitutions and/or N-terminal and/or C-terminal fusions
(e.g., to a
fluorophore attachment moiety). In embodiments, a biosensor comprises a
modified ligand-
binding protein having an amino acid substitution compared to its naturally
occurring
counterpart, such that the polypeptide has a cysteine at one or more of
position 1, 2, 3, 4, 5, 6,
7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26,
27, 28, 29, 30, 31, 32,
33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51,
52, 53, 54, 55, 56, 57,
58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76,
77, 78, 79, 80, 81, 82,
83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 101, 102,
103, 104, 105, 106,
107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121,
122, 123, 124,
125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139,
140, 141, 142,
143, 144, 145, 146, 147, 148, 149, 150, 160, 161, 162, 163, 164, 165, 166,
167, 168, 169,
170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184,
185, 186, 187,
188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202,
203, 204, 205,
206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220,
221, 222, 223,
224, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235, 236, 237, 238,
239, 240, 241,
242, 243, 244, 245, 246, 247, 248, 249, 250, 251, 252, 253, 254, 255, 256,
257, 258, 259,
67
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
260, 270, 271, 272, 273, 274, 275, 276, 277, 278, 279, 280, 281, 282, 283,
284, 285, 286,
287, 288, 289, 290, 292, 293, 294, 295, 296, 297, 298, 299, 300, 301, 302,
303, 304, 305,
306, 307, 308, 309, 310, 311, 312, 313, 314, 315, 316, 317, 318, 319, 320,
321, 322, 323,
324, 325, 326, 327, 328, 329, 330, 331, 332, 333, 334, 335, 336, 337, 338,
339, 340, 341,
342, 343, 344, 345, 346, 347, 348, 349, 350, 351, 352, 353, 354, 355, 356,
357, 358, 359,
360, 361, 362, 363, 364, 365, 366, 367, 368, 369, 370, 371, 372, 373, 374,
375, 376, 377,
378, 379, 380, 381, 382, 383, 384, 385, 386, 387, 388, 389, 390, 391, 392,
393, 394, 395,
396, 397, 398, 399, or 400 or any combination of 1, 2, 3, 4, or 5 thereof,
wherein the position
corresponds a SEQ ID NO disclosed herein. In embodiments, the cysteine is
conjugated to a
reporter group.
In various embodiments, the dissociation constant of the mutant ligand-binding
polypeptide differs by at least about 1 1.1,M, 5 1.1,M, 10 ,M, 20 1.1,M, 25
,M, 30 ,M, 35 1.1,M, 40
1.1,M, 45 1.1,M, 50 ,M, 75 ,M, 100 ,M, 200 ,M, 300 1.1,M, 400 1.1,M, 500
1.1,M, 600 ,M, 700 ,M,
800 1.1,M, 900 1.1,M, 1 mM, 2 mM, 3 mM, 4 mM, 5 mM, 6 mM, 7 mM, 8 mM, 9 mM, 10
mM,
20 mM, 30 mM, 40 mM, 50 mM, 60 mM, 70 mM, 80 mM, 90 mM, or 100 mM (increase or
decrease) compared to its naturally occurring counterpart.
The biosensors and ligand-binding proteins provided herein are robust and
useful at a
wide range of physical conditions, e.g., pressure, temperature, salinity,
osmolality, and pH
conditions. For example, biosensors and ligand-binding proteins provided
herein may
survive substantial periods of time after being dried or exposed to high
temperatures. In
some embodiments, the biosensor maintains at least about 75%, 80%, 85%, 90%,
95%, 96%,
97%, 98%, 99%, 99.5%, 99.9%, or more of its signal transduction activity after
exposure to a
temperature of about 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95, 100, 105,
110, 115, 120, or
125, or 40-125 C for about 1, 2, 3, 4, 5, 6, 15, 30, 60, 120, 180, 240, or 360
minutes. In
certain embodiments, the biosensor maintains at least about 75%, 80%, 85%,
90%, 95%,
96%, 97%, 98%, 99%, 99.5%, 99.9%, or more of its signal transduction activity
after 1, 2, 3,
4, or 5 freeze-thaw cycles in an aqueous solution. In various embodiments, the
biosensor
maintains at least about 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99%, 99.5%,
99.9%,
or more of its signal transduction activity after storage at a temperature of
between 20-37 C
for about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 18, 24, or 1-24 months in dry
form. In some
embodiments, the optimal functional temperature of the biosensor is between 41
and 122 C,
between 20 and 40 C, or less than about 10 C (e.g., between -20 and +10 C).
Devices,
compositions, and biosensors provided herein may be stored, e.g., with or
without protection
68
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
from exposure to light. In some embodiments, the devices, compositions, and
biosensors are
stored in the dark, e.g., with protection from light.
Non-limiting examples of glucose-binding proteins include variants of ecGGBP,
ttGGBP, stGGBP, chyGGBP, cobGGBP, pspGGBP, csaGGBP, bprGGBP, rinGGBP_A,
rinGGBP_B, fprGGBP, cljGGBP, cauGGBP, erhGGBP, ereGGBP, and chyGGBP.
In embodiments, a biosensor comprises a modified ecGGBP. In non-limiting
examples, the modified ecGGBP may comprise one or more, or any combination of
the
following substitutions compared to its naturally occurring counterpart: Y10X,
D14X, N15X,
F16X, P7OX, N91X, K92X, S112X, 5115X, E149X, H152X, P153X, D154X, A155X,
R158X, M182X, W183X, N211X, D212X, D236X, L238X, L255X, N256X, D257X, P294X,
and V296X, where X is any amino acid, an amino acid that results in a
conservative
substitution, or a cysteine, and where each position is counted in ecGGBP
without including
the signal peptide (SEQ ID NO: 153). In some embodiments, the modified ecGGBP
comprises 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or 11 of the following substitutions:
YlOA, YlOC,
D14C, D14A, D14Q, D14N, D145, D14T, D14E, D14H, D14L, D14Y, D14F, N15C, F16L,
F16A, F16C, F16Y, N91C, N91A, K92A, K92C, E93C, S112A, S115A, E149C, E149K,
E149Q, E1495, H152C, H152A, H152F, H152Q, H152N, D154C, D154A, D154N, A155C,
A1555, A155H, A155L, A155F, A155Y, A155N, A155K, A155M, A155W, A155Q, R158C,
R158A, R158K, M182C, M182W, W183C, W183A, N211C, N211F, N211W, N211K,
N211Q, N2115, N211H, N211M, N211C, D212C, L238C, D236C, D236A, D236N, L255C,
N256A, N256D, D257C, P294C, and V293C.
In various embodiments, a biosensor comprises a modified ttGGBP. In non-
limiting
examples, the modified ttGGBP may comprise one or more, or any combination of
the
following substitutions compared to its naturally occurring counterpart: YllX,
D15X, T16X,
F17X, G20X, N42X, V67X, R69X, R91X, E92X, Al 11X, Q148X, H151X, Q152X, A154X,
N181X, W182X, D183X, D211X, T237X, T240X, L257X, N258X, D259X, A260X, and
K300X where X is any amino acid, an amino acid that results in a conservative
substitution,
or a cysteine, and where each position is counted in ttGGBP with the signal
peptide replaced
with a methionine (SEQ ID NO: 154). In some embodiments, the modified ttGGBP
comprises 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or 11 of the following substitutions:
Y11C, D15A,
D15E, D15N, DISC, T165, T16N, T16C, F17C, G20A, G20C, N42C, V67C, R69P, R69C,
R91K, E92C, AMC, Q1485, Q148K, Q148E, Q148C, H151Q, H151N, H151F, H151C,
Q152P, Q152C, A1545, A154N, A154M, A154F, A154C, N181C, W182C, D183C, D211A,
69
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
D211C, T237C, T240A, L257C, N258D, N258S, N258A, N258C, D259C, A260N, A260Q,
A260R, A260K, A260W, A260F, A260Y, A260S, A260C, and K300C.
In embodiments, a biosensor comprises a modified stGGBP. In non-limiting
examples, the modified stGGBP may comprise one or more, or any combination of
the
following substitutions compared to its naturally occurring counterpart: Y1
1X, Y13X, D15X,
N16X, F17X, P71X, N92X, K93X, P113X, S116X, E150X, H153X, P154X, D155X,
A156X, R159X, M183X, W184X, N211X, N212X, D213X, A214X, D237X, L239X,
D258X, P295X, and V297X where X is any amino acid, an amino acid that results
in a
conservative substitution, or a cysteine, and where each position is counted
in stGGBP with
the signal peptide replaced with a methionine (SEQ ID NO: 155). In some
embodiments, the
modified stGGBP comprises 1, 2 or 3 of the following mutations: Y13C, F17C,
and W184C.
In embodiments, a biosensor comprises a modified chyGGBP. In non-limiting
examples, the modified chyGGBP may comprise one or more, or any combination of
the
following substitutions compared to its naturally occurring counterpart: F12X,
D14X, T15X,
F16X, R68X, N89X, R90X, All0X, 5113X, E147X, H150X, Q151X, D152X, A153X,
R156X, M180X, W181X, N207X, N208X, D209X, D210X, D237X, T239X, D258X,
V296X, and Y298X where X is any amino acid, an amino acid that results in a
conservative
substitution, or a cysteine, and where each position is counted in chyGGBP
with the signal
peptide replaced with a methionine (SEQ ID NO: 156). In some embodiments, the
modified
chyGGBP comprises 1, 2, or 3 of the following mutations: F12C, F16C, C39A,
W181C, and
C206A.
In embodiments, a biosensor comprises a modified cobGGBP. In non-limiting
examples, the modified cobGGBP may comprise one or more, or any combination of
the
following substitutions compared to its naturally occurring counterpart: F12X,
D14X, T15X,
F16X, C39X, R68X, N89X, R90X, Al 10X, 5113X, E147X, H150X, Q151X, D152X,
A153X, R156X, C173X, M180X, W181X, C206X, N207X, N208X, D209X, D210X,
D237X, T239X, D258X, P297X, and Q299X where X is any amino acid, an amino acid
that
results in a conservative substitution, or a cysteine, and where each position
is counted in
cobGGBP with the signal peptide replaced with a methionine (SEQ ID NO: 157).
In some
embodiments, the modified cobGGBP comprises 1, 2, or 3 of the following
mutations: Fl 2C,
F16C, C39A, C173A, W181C, and C206A.
In embodiments, a biosensor comprises a modified pspGGBP. In non-limiting
examples, the modified pspGGBP may comprise one or more, or any combination of
the
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
following substitutions compared to its naturally occurring counterpart: F9X,
D11X, T12X,
F13X, R65X, N86X, R87X, A107X, 5110X, E144X, H147X, Q148X, D149X, A150X,
R153X, M177X, W178X, N204X, N205X, D206X, D207X, D234X, T236X, 255X, A294X,
and K296X where X is any amino acid, an amino acid that results in a
conservative
substitution, or a cysteine, and where each position is counted in pspGGBP
with the signal
peptide replaced with a methionine (SEQ ID NO: 158). In some embodiments, the
modified
pspGGBP comprises 1, 2, or 3 of the following mutations: F9C, F13C, and W178C.
In embodiments, a biosensor comprises a modified csaGGBP. In non-limiting
examples, the modified csaGGBP may comprise one or more, or any combination of
the
following substitutions compared to its naturally occurring counterpart: Y14X,
D16X, F18X,
C62X, I72X, C82X, N93X, R94X, C113A, S118X, A121X, E152X, N155X, E156X,
D157X, S158X, R161X, N185X, W186X, C211X, D241X, L243X, D262X, D290X, I292X,
I297X, F299X, Q301X, and T302X where X is any amino acid, an amino acid that
results in
a conservative substitution, or a cysteine, and where each position is counted
in csaGGBP
with the signal peptide replaced with a methionine (SEQ ID NO: 159). In some
embodiments, the modified csaGGBP comprises 1, 2, 3, 4, 5, 6, 7, or 8 of the
following
mutations: Y14C, F18C, C62A, C82A, C113A, W186C, and C211A.
In embodiments, a biosensor comprises a modified bprGGBP. In non-limiting
examples, the modified bprGGBP may comprise one or more, or any combination of
the
following substitutions compared to its naturally occurring counterpart: C8X,
K12X, D14X,
N15X, F16X, 572X, N93X, R94X, C112X, C116X, Al 18X, 5121X, A153X, N156X,
I157X,
D158X, A159X, C179X, N186X, W187X, C211X,N212X,N213X, D214X, A215X,
D241X, D243X, K251X, C289X, D290X, and V292X where X is any amino acid, an
amino
acid that results in a conservative substitution, or a cysteine, and where
each position is
counted in bprGGBP with the signal peptide replaced with a methionine (SEQ ID
NO: 160).
In some embodiments, the modified bprGGBP comprises 1, 2, 3, 4, 5, 6, 7, 8, or
9 of the
following mutations: C8A, K12C, F16C, C112A, C116A, C179A, W187C, C211A, and
C289A.
In embodiments, a biosensor comprises a modified rinGGBP_A. In non-limiting
examples, the modified rinGGBP_A may comprise one or more, or any combination
of the
following substitutions compared to its naturally occurring counterpart: C6X,
Fl OX, D12X,
N13X, F14X, 570X, N91X, R92X, C114X, Al 16X, Q118X, D151X, N154X, V155X,
D156X, A157X, R160X, C177X, N184X, W185X, C210X, N211X, N212X, D213X,
71
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
A214X, D240X, L242X, L250X, C288X, D289X, and V291X where X is any amino acid,
an
amino acid that results in a conservative substitution, or a cysteine, and
where each position
is counted in rinGGBP_A with the signal peptide replaced with a methionine
(SEQ ID NO:
161). In some embodiments, the modified rinGGBP_A comprises 1, 2, 3, 4, 5, 6,
7, or 8 of
the following mutations: C6A, FlOC, F14C, C114A, C177A, W185C, C210A, and
C288A.
In embodiments, a biosensor comprises a modified rinGGBP_B. In non-limiting
examples, the modified rinGGBP_B may comprise one or more, or any combination
of the
following substitutions compared to its naturally occurring counterpart: Q13X,
D15X, T16X,
F17X, C66X, C70A, R76X, N97X, R98X, Al 18X, S121X, E155X, H158X, Q159X, D160X,
A161X, R164X, N188X, W189X, N215X,N216X, D217X, D218X, D244X, T246X,
D265X, P301X, A303X, and C306X where X is any amino acid, an amino acid that
results in
a conservative substitution, or a cysteine, and where each position is counted
in rinGGBP_B
with the signal peptide replaced with a methionine (SEQ ID NO: 165). In some
embodiments, the modified rinGGBP_B comprises 1, 2, 3, 4, 5, or 6 of the
following
mutations: Q13C, F17C, C66A, C70A, W189C, and C306A.
In embodiments, a biosensor comprises a modified fprGGBP. In non-limiting
examples, the modified fprGGBP may comprise one or more, or any combination of
the
following substitutions compared to its naturally occurring counterpart: C8A,
Fl 2X, D14X,
N15X, F16X, T69X, N90X, R91X, C105X, C106X, Al 13X, 5116X, C143X, D146X,
N149X, 1150X, D151X, A152X, R155X, N179X, W180X, C205A, N206X, N207X, D208X,
A209X, D235X, L237X, N243X, D284X, and V286X where X is any amino acid, an
amino
acid that results in a conservative substitution, or a cysteine, and where
each position is
counted in fprGGBP with the signal peptide replaced with a methionine (SEQ ID
NO: 162).
In some embodiments, the modified fprGGBP comprises 1, 2, 3, 4, 5, 6, or 7 of
the following
mutations: C8A, F12C, F16C, C105A, C106A, C143A, W180C, and C205A.
In embodiments, a biosensor comprises a modified cljGGBP. In non-limiting
examples, the modified cljGGBP may comprise one or more, or any combination of
the
following substitutions compared to its naturally occurring counterpart: F11X,
N13X, T14X,
W15X, V67X, C77X, N88X, R89X, A109X, S112X, E142X, N145X, Q146X, D147X,
A148X, R151X, M175X, W176X, C198X, N201X, N202X, D203X, D204X, D231X,
T233X, D252X, D291X, and K294X where X is any amino acid, an amino acid that
results in
a conservative substitution, or a cysteine, and where each position is counted
in cljGGBP
with the signal peptide replaced with a methionine (SEQ ID NO: 163). In some
72
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
embodiments, the modified cljGGBP comprises 1, 2, 3, 4, or 5 of the following
mutations:
Fl1C, W15C, C77A, W176C, and C198A.
In embodiments, a biosensor comprises a modified cauGGBP. In non-limiting
examples, the modified cauGGBP may comprise one or more, or any combination of
the
following substitutions compared to its naturally occurring counterpart: F12X,
N14X, T15X,
W16X, V68X, C78X, N89X, R90X, Al 10X, S113X, E143X, N146X, Q147X, D148X,
A149X, R152X, M176X, W177X, C199X, N203X, N204X, D205X, D206X, D233X,
T235X, D254X, D293X, and K295X where X is any amino acid, an amino acid that
results in
a conservative substitution, or a cysteine, and where each position is counted
in cauGGBP
with the signal peptide replaced with a methionine (SEQ ID NO: 164). In some
embodiments, the modified cauGGBP comprises 1, 2, 3, 4, or 5 of the following
mutations:
F12C, W16C, C78A, W177C, and C199A.
In embodiments, a biosensor comprises a modified erhGGBP. In non-limiting
examples, the modified erhGGBP may comprise one or more, or any combination of
the
following substitutions compared to its naturally occurring counterpart: F13X,
D15X, N16X,
F17X, P76X, N97X, R98X, Al 19X, S122X, D153X, N156X, V157X, D158X, A159X,
R162X, N187X, W188X, N214X, N215X, D216X, G217X, D243X, I245X, D264X, E312X,
and V314X where X is any amino acid, an amino acid that results in a
conservative
substitution, or a cysteine, and where each position is counted in erhGGBP
with the signal
peptide replaced with a methionine (SEQ ID NO: 166). In some embodiments, the
modified
erhGGBP comprises 1, 2, or 3 of the following mutations: F13C, F17C, and
W188C.
In embodiments, a biosensor comprises a modified ereGGBP. In non-limiting
examples, the modified ereGGBP may comprise one or more, or any combination of
the
following substitutions compared to its naturally occurring counterpart: Q13X,
D15X, T16X,
F17X, C29X, C65X, C69X, R75X, N96X, R97, Al 17X, 5120X, E154X, H157X, Q158X,
D159X, A160X, R163X, C183X, N187X, W188X,N214X, N215X, D216X, A217X,
D243X, T245X, D264X, P301X, and E303X where X is any amino acid, an amino acid
that
results in a conservative substitution, or a cysteine, and where each position
is counted in
ereGGBP with the signal peptide replaced with a methionine (SEQ ID NO: 167).
In some
embodiments, the modified ereGGBP comprises 1, 2, 3, 4, 5, 6, 7, or 8 of the
following
mutations: ClOA, Q13C, F17C, C29A, C65A, C69A, C183A, and W188C.
73
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Fluorescent Proteins
Various biosensors provided herein comprise fluorescent proteins, such as
fluorescent
proteins that have altered amino acid sequences compared to their naturally
occurring
counterparts. In embodiments, such proteins are conjugated to reporter groups.
In
embodiments, the proteins are not conjugated to a reporter group (i.e., a
biosensor comprising
the fluorescent protein that does not undergo tgmFRET or ngmFRET is provided).
Aspects of the present subject matter provide a biosensor for ligand,
comprising a
ligand-binding protein, wherein the ligand-binding protein is a fluorescent
protein, and
wherein binding of the ligand to a ligand-binding domain of the fluorescent
protein causes a
change in fluorescence by the fluorescent protein. In some embodiments, the
biosensor
further comprises a reporter group, e.g., a fluorophore that acts as a ngmFRET
donor
fluorophore or a ngmFRET acceptor fluorophore with respect to the fluorescent
protein.
Green Fluorescent Protein (GFP) and its derivatives such as Yellow Fluorescent
Protein (YFP) form their internal fluorophore through an autocatalytic,
posttranslational
cyclization of a tipeptide from its own amino acid sequence (M. Zimmer, 2002,
Chem. Rev.
102, 759-781). This process entails three steps: a nucleophilic attack to
create a cyclic
peptide, dehydration, and a final oxidation to introduce conjugation (D.P.
Barondeau et al.,
2003, Proc. Natl. Acad. Sci. USA, 100, 12111-12116). The formation of GFP's or
YFP's
fluorophore is an autocatalytic process that requires no catalyst external to
these proteins.
In some embodiments, the ligand comprises a halide anion such as a fluoride
(F),
chloride (Cr), a bromide (BC), an iodide (n, astatide (At-) anion, or an
ununseptide (Ts-)
anion. In certain embodiments, the fluorescent protein has an affinity (Kd)
for the halide
anion that is within the concentration range of the halide anion in a subject.
Non-limiting
examples of fluorescent proteins that bind halide anions include Yellow
Fluorescent Protein
(YFP; SEQ ID NO: 149) and mutants thereof. In some embodiments, the
fluorescent protein
comprises a mutation that alters the interaction of the mutant with a bound
halide anion
compared to YFP. For example, the mutation that may alter the fluorescent
protein's affinity
and/or specificity for a halide anion compared to YFP. In various embodiments,
the
fluorescent protein comprises 1 halide anion binding site. Alternatively the
fluorescent
protein comprises at least 2, 3, 4, or 5 halide anion binding sites. In some
embodiments, at
least one amino acid of the YFP has been substituted with a cysteine.
YFP is a non-limiting reference protein respect to fluorescence proteins.
In various embodiments, a polypeptide of the present disclosure comprises
74
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
(a) amino acids in a sequence that is preferably (i) at least about 10%, 11%,
12%,
13%, 14%, or 15%, and (ii) less than about 75%, 70%, 65%, 60%, 55%, 50%,
45%, 44%, 43%, 42%, 41%, 40%, 39%, 38%, 37%, 36%, or 35% identical to
YFP;
(b) a stretch of at least 5, 10, or 20 amino acids having at least about 50%,
55%, 60%,
65%, 75%, 80%, 85%, 90%, or 95% identity to a stretch of consecutive amino
acids including position 17, 32, 43, 77, 95, 109, 122, 133, 149, 164, 173,
182, 204,
and/or 221 of YFP;
(c) no more than 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or 15 deleted or inserted
amino acids
compared to YFP, not including added amino acids added to the N-terminus or C-
terminus of the polypeptide compared to its natural counterpart, and including
or
not including the signal peptide of the natural counterpart of the
polypeptide;
(d) at least 9, 10, 11, or 12 f3-strands or exactly 9, 10, 11, or 12 f3-
strands.
In some embodiments, the fluorescent polypeptide comprises a f3-barrel. In
certain
embodiments, the f3-barrel comprises 9, 10, 11, or 12 f3-strands. In some
embodiments, the
fluorescent protein comprises a cysteine within the first f3-strand (f31), the
second f3-strand
(132), the third f3-strand (f33), the fourth f3-strand (f34), the fifth f3-
strand (f35), the sixth f3-strand
(f36), the seventh f3-strand (f37), the eighth f3-strand (f38), the ninth f3-
strand (f39), the tenth f3-
strand (f310), or the eleventh f3-strand (f3n) of a YFP. In some embodiments,
the polypeptide
comprises (i) 1, 2, or 3 amino acid substitutions between f31 and f32; (ii) 1,
2, or 3 amino acid
substitutions between f32 and f33; (iii) 1, 2, or 3 amino acid substitutions
between the f33 and
J34; (iv) 1, 2, or 3 amino acid substitutions between the f34 and f35; (v) 1,
2, or 3 amino acid
substitutions between f35 and f36; (vi) 1, 2, or 3 amino acid substitutions
between f36 and f37;
(vii) 1, 2, or 3 amino acid substitutions between the f37 and f38; (viii) 1,
2, or 3, amino acid
substitutions between f38 and f39; (ix) 1, 2, or 3 amino acid substitutions
between the f39 and
f310; and/or (x) 1, 2, or 3 amino acid substitutions between f310 and f311. In
various
embodiments, the 1 or more substitutions comprise a substitution with
cysteine. In certain
embodiments, the cysteine follows f3i 1 in the amino acid sequence of the YFP.
Alpha-helical and f3-strand segments assignments are calculated from a three-
dimensional protein structure as follows, and as described in C.A.F. Andersen,
B. Rost, 2003,
Structural Bioinformatics, 341-363, P.E. Bourne, ed., Wiley, the entire
content of which is
incorporated herein by reference. First for a given residue, i, the backbone
trace angle, r, is
calculated, defined as the dihedral angle between the four successive Ca, atom
positions of
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
residues in the linear protein sequence i, i+1, i+2, i+3. These values are
calculated for all
residues. Second, the residues that form backbone hydrogen bonds with each
other are
recorded. A hydrogen bond is scored if the distance between the backbone amide
nitrogen
and carbonyl oxygen of two different residues in the protein is calculated to
be 2.5A or less,
and if the calculated angle between the nitrogen, its amide proton, and the
carbonyl is greater
than 120 . A residue is deemed to be in an a-helix, if 35 r 65, and it makes a
backbone
hydrogen bond with its i+4th neighbor in the linear amino acid sequence. It is
deemed to be
in a (3-strand, if the absolute t value falls in the interval 120 k-1 180 and
if it makes at least
one hydrogen bond with another residue with the same r value range. Alpha-
helical
segments comprise at least four residues; (3-strand residues comprise at least
three residues.
In embodiments, a biosensor comprises a modified YFP polypeptide having an
amino
acid substitution compared to its naturally occurring counterpart, such that
the polypeptide
has a cysteine at position 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15,
16, 17, 18, 19, 20, 21,
22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40,
41, 42, 43, 44, 45, 46,
47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65,
66, 67, 68, 69, 70, 71,
72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90,
91, 92, 93, 94, 95, 96,
97, 98, 99, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113,
114, 115, 116,
117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131,
132, 133, 134,
135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149,
150, 160, 161,
162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176,
177, 178, 179,
180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194,
195, 196, 197,
198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212,
213, 214, 215,
216, 217, 218, 219, 220, 221, 222, 223, 224, 225, 226, 227, 228, 229, 230,
231, 232, 233,
234, 235, 236, 237, 238, 239, 240, 241, 242, 243, 244, 245, 246, 247, 248,
249, or 250, or any
combination of 1, 2, 3, 4, or 5 thereof, wherein the position corresponds a
SEQ ID NO
disclosed herein for YFP. In embodiments, the cysteine is conjugated to a
reporter group.
In embodiments, a biosensor comprises a modified YFP. In non-limiting
examples,
the modified YFP may comprise one or more, or any combination of the following
substitutions compared to its naturally occurring counterpart: E17X, E32X,
T43X, F64X,
G65X, L68X, Q69X, A72X, H77X, K79X, R80X, E95X, R109X, R122X, D133X, H148X,
N149X, V163X,N164X, D173X, Y182X, Q183X, Y203X, Q204X, L221X, and H231X,
where X is any amino acid or is an amino acid that results in a conservative
substitution. In
76
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
some embodiments X is cysteine. In some embodiments, the modified YFP
comprises 1, 2,
3, 4, 5, 6, 7, 8, 9, 10, 11, 12, or 13 of the following substitutions: F64L,
G65T, L68V, Q69T,
A72S, K79R, R80Q, H148Q, H148G, V163A, H231L, H148Q, or Q183A, wherein each
YFP
amino acid position is numbered as in SEQ ID NO: 150. In some embodiments, the
fluorescent protein comprises an R at the 96 position, a Y at the 203
position, a S at the 205
position, and an E at the 222 position compared to YFP, wherein each YFP amino
acid
position is numbered as in SEQ ID NO: 150. C1BP1 (also referred to as laYFP)
comprises
L68V, K79R, R80Q, H2131L compared to YFP (as numbered in SEQ ID NO: 150).
Adaptor Proteins
Aspects provide biosensor for a ligand, comprising (a) a polypeptide; (b) a
directly
responsive fluorophore, wherein binding of a ligand to the directly responsive
fluorophore
causes a change in signaling by the directly responsive fluorophore (i.e., the
fluorophore is
chemoresponsive); and (b) an indirectly responsive fluorophore. The directly
responsive
fluorophore may be a donor fluorophore or an acceptor fluorophore. In some
embodiments,
the directly responsive fluorophore is a donor fluorophore and the indirectly
responsive
fluorophore is an acceptor fluorophore. In some embodiments, the directly
responsive
fluorophore is an acceptor fluorophore and the indirectly responsive
fluorophore is a donor
fluorophore. ngmFRET occurs between the donor fluorophore and the acceptor
fluorophore
when the donor fluorophore is contacted with radiation comprising the
excitation wavelength
of the donor fluorophore.
Any polypeptide may be used to link a directly responsive fluorophore (e.g., a
chemoresponsive fluorophore) with an indirectly responsive fluorophore. Such a
polypeptide
is referred to as an adaptor protein herein. In some embodiments, the
polypeptide comprises
a stretch of at least 2, 3, 4, 5, 6, 47, 8, 9, 10, 20, 30, 40, 50, 60, 70, 80,
90, 100, 150, 200, 250,
or 500 amino acids.
In some embodiments, the polypeptide comprises a stretch of at least 50, 60,
70, 80,
90, or 100 amino acids in a sequence that is at least about 85%, 90%, 95%, or
99% identical
to the amino acid sequence of ecTRX (SEQ ID NO: 151). In certain embodiments,
the
polypeptide comprises at least 1, 2, or 3 thiol groups; at least 1, 2, or 3
cysteines that each
comprise a sulfhydryl group; at least 1, 2, or 3 primary amine groups; or at
least 1, 2, or 3
lysines that each comprise a primary amine. In various embodiments, there is
no disulfide
bond between cysteines within the amino acid sequence of the polypeptide.
77
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
In some embodiments, the polypeptide comprises a mutant of ecTRX comprising a
D3X, K4X, K19X, D27X, K37X, K53X, K58X, K70X, R74X, K83X, K91X, K97X, or
K101X mutation, or any combination thereof, wherein X is any amino acid, and
wherein each
ecTRX amino acid position is numbered as in SEQ ID NO: 151.
In various embodiments, the polypeptide comprises a mutant of ecTRX comprising
a
D3A, D3A, K4R, K4Q, K19R, K19Q, D27A, K37R, K53M, K53R, K58M, K7OR, R74C,
K83R, K91R, K97R, or K101R mutation, or any combination thereof, wherein each
ecTRX
amino acid position is numbered as in SEQ ID NO: 151. In certain embodiments,
the
polypeptide comprises a mutant of ecTRX that does not comprise a lysine.
In embodiments, the polypeptide further comprises a hexahistidine tag. In some
embodiments the polypeptide comprises amino acids in the sequence of any one
of SEQ ID
NOS:24-41 or 151.
In various embodiments, the biosensor is a pH biosensor and the ligand
comprises a
hydrogen ion. In embodiments, the directly responsive fluorophore is pH-
sensitive. For
example, the fully excited emission intensity of the directly responsive
fluorophore is
different at a pH less than about 7.0 compared to a pH of 7.5. In certain
embodiments, the
directly responsive fluorophore transitions from a monoanion to a dianion at a
pH that is less
than 7.0 in an aqueous solution. In some embodiments, the directly responsive
fluorophore
comprises a pH-sensitive fluorophore comprising fluorescein or a derivative
thereof.
Exemplary Methods of Using Biosensors Provided Herein
Aspects of the present subject matter provide a method of assaying for a
ligand in a
sample. The method may include contacting the sample with a biosensor
disclosed herein
under conditions such that the ligand-binding protein of the biosensor binds
to the ligand if
ligand is present in the sample. The method also comprises detecting (i)
whether a signal is
produced by a reporter group of the biosensor; and/or (ii) the a signal
produced by a reporter
group of the biosensor. In a non-limiting example, a reporter group of the
biosensor is
fluorescent, and the method further comprises contacting the reporter group
with
electromagnetic radiation having a wavelength that comprises a wavelength
within the band
of excitation wavelengths of the reporter group.
In various embodiments, the method further comprises (i) comparing a signal
produced by a reporter group of the biosensor when the biosensor is contacted
with the
sample with a signal produced by a control sample containing a known quantity
of ligand;
78
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
and (ii) detecting the presence or absence of ligand in the sample based on
this comparison.
Alternatively or in addition, the method further comprises (i) comparing a
signal produced by
a reporter group of the biosensor when the biosensor is contacted with the
sample with
signals produced by a series of control samples containing known quantities of
ligand; and
(ii) determining the quantity of ligand in the sample based on this
comparison. In some
embodiments, the series of control samples comprises at least 2, 3, 4, 5, 6,
7, 8, 9, or 10
control samples, and wherein each control sample comprises a different
quantity of ligand.
Alternatively or in addition, the method further comprises determining the
concentration of a
ligand in a sample, wherein determining the concentration of the ligand in the
sample
comprises comparing the signal to a standard hyperbolic ligand binding curve
to determine
the concentration of the ligand in the test sample, wherein the standard
hyperbolic ligand
binding curve is prepared by measuring the signal produced by the reporter
group of the
biosensor when the biosensor is contacted with control samples containing
known
concentrations of ligand. In various embodiments, the method comprises (i)
measuring a
ratiometric change (AR) and/or an intensity change (AI) of a signal produced
by the reporter
group. In some embodiments, the method includes quantitating the level of
ligand present in
the sample.
Aspects of the present subject matter also provide a method of assaying for
multiple
ligands in a sample, wherein the multiple ligands comprise a first ligand and
a second ligand.
Such a method may include contacting the sample with (i) a first biosensor a
first ligand
provided herein and (ii) a second biosensor for the second ligand, under
conditions such that
the ligand-binding protein of the first biosensor binds to the first ligand,
if the first ligand is
present in the sample, and detecting (i) a signal produced by a reporter group
of the first
biosensor, or (ii) whether a signal is produced by a reporter group of the
first biosensor. In
some embodiments, the second biosensor is also a biosensor provided herein,
and the second
biosensor is contacted with the second ligand under conditions such that the
ligand-binding
protein of the second biosensor binds to the second ligand it is present in
the sample. The
method may further comprise detecting (i) a signal produced by a reporter
group of the
second biosensor, or (ii) whether a signal is produced by a reporter group of
the second
biosensor.
In some embodiments, the signal produced by the reporter group of the first
biosensor
is different than the signal produced by the reporter group of the second
biosensor. In a non-
limiting example, the reporter group of the first biosensor and the reporter
group of the
79
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
second biosensor are each fluorescent, and the peak emission wavelength of the
reporter
group of the first biosensor is at least about 10, 25, 50, 75, or 100 nm
greater or lower than
the peak emission wavelength of the reporter group of the second biosensor.
Non-limiting examples of biosensors include biosensors with ligand-binding
proteins
comprising a GGBP (e.g., an E. coli GGBP) or a derivative or mutant thereof;
(ii) an E. coli
arabinose binding protein (e.g., an E. coli arabinose binding protein) or a
derivative or mutant
thereof; (iii) a dipeptide binding protein (e.g., an E. coli dipeptide binding
protein) or a
derivative or mutant thereof; (iv) a histidine binding protein (e.g., an E.
coli, histidine binding
protein) or a derivative or mutant thereof; (v) a ribose binding protein
(e.g., an E. coli ribose
binding protein) or a derivative or mutant thereof; (vi) a sulfate binding
protein (e.g., an E.
coli sulfate binding protein) or a derivative or mutant thereof; (vii) a
maltose binding protein
(e.g., an E. coli maltose binding protein) or a derivative or mutant thereof;
(viii) a glutamine
binding protein (e.g., an E. coli glutamine binding protein) or a derivative
or mutant thereof;
(ix) a glutamate/aspartate binding protein (e.g., an E. coli
glutamate/aspartate binding
protein) or a derivative or mutant thereof; (x) a phosphate binding protein
(e.g., an E. coli
phosphate binding protein) or a derivative or mutant thereof; or (xi) an iron
binding protein
[e.g., a Haemophilus influenza (H. influenzae) iron binding protein] or a
derivative or mutant
thereof. For example, the second biosensor comprises an E. coli GGBP having a
YlOA,
YlOC, D14C, D14A, D14Q, D14N, D14S, D14T, D14E, D14H, D14L, D14Y, D14F, N15C,
Fl6L, Fl6A, Fl6C, F16Y, N91C, N91A, K92A, K92C, E93C, S112A, S115A, E149C,
E149K, E149Q, E1495, H152C, H152A, H152F, H152Q, H152N, D154C, D154A, D154N,
A155C, A155S, A155H, A155L, A155F, A155Y, A155N, A155K, A155M, A155W, A155Q,
R158C, R158A, R158K, M182C, M182W, W183C, W183A, N211C, N211F, N211W,
N211K, N211Q, N211S, N211H, N211M, N211C, D212C, L238C, D236C, D236A, D236N,
L255C, N256A, N256D, D257C, P294C, and V293C mutation (e.g., comprising 1, 2,
3, 4, 5
or more of these mutations), wherein each amino acid position is numbered as
in (SEQ ID
NO: 153); (ii) an E. coli arabinose binding protein having a D257C, F23C,
K301C, L253C, or
L298C mutation (e.g., comprising 1, 2, 3, 4, or 5 of these mutations) (see,
e.g., U.S. Patent
Application Publication No. 2004/0118681, the entire contents of which are
incorporated
herein by reference) (see, e.g., U.S. Patent Application Publication No.
2004/0118681, the
entire contents of which are incorporated herein by reference); (iii) an E.
coli dipeptide
binding protein having a D450C, K394C, R141C, S111C, T44C, or W315C mutation
(e.g.,
comprising 1, 2, 3, 4, 5 or 6 of these mutations) (see, e.g., U.S. Patent
Application Publication
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
No. 2004/0118681, the entire contents of which are incorporated herein by
reference); (iv) an
E. coli, histidine binding protein having a E167C, K229C, V163C, Y230C, F231C,
Y88C
mutation (e.g., comprising 1, 2, 3, 4, 5 or 6 of these mutations) (see, e.g.,
U.S. Patent
Application Publication No. 2004/0118681, the entire contents of which are
incorporated
herein by reference); (v) an E. coli ribose binding protein having a T135C,
D165C, E192C,
A234C, L236C, or L265C mutation (e.g., comprising 1, 2, 3, 4, 5 or 6 of these
mutations)
(see, e.g., U.S. Patent Application Publication No. 2004/0118681, the entire
contents of
which are incorporated herein by reference); (vi) an E. coli sulfate binding
protein having a
L65C, N70C, Q294C, R134C, W290C, or Y67C mutation (e.g., comprising 1, 2, 3,
4, 5 or 6
of these mutations) (see, e.g., U.S. Patent Application Publication No.
2004/0118681 the
entire content of which is incorporated herein by reference); (vii) an E. coli
maltose binding
protein having a D95C, F92C, E163C, G174C, 1329C, or S233C mutation (e.g.,
comprising
1, 2, 3, 4, 5 or 6 of these mutations) (see, e.g., U.S. Patent Application
Publication No.
2004/0118681 the entire content of which is incorporated herein by reference);
(viii) an E.
coli glutamine binding protein having a N160C, F221C, K219C, L162C, W220C,
Y163C, or
Y86C mutation (e.g., comprising 1, 2, 3, 4, 5 or more of these mutations)
(see, e.g., U.S.
Patent Application Publication No. 2004/0118681 the entire content of which is
incorporated
herein by reference); (ix) an E. coli glutamate/aspartate binding protein
having a A207C,
A210C, El 19C, F126C, F131C, F270C, G211C, K268C, Q123C, or T129C mutation
(e.g.,
comprising 1, 2, 3, 4, 5 or more of these mutations) (see, e.g., U.S. Patent
Application
Publication No. 2004/0118681 the entire content of which is incorporated
herein by
reference); (x) an E. coli phosphate binding protein having a A225C, N223C,
N226C,
5164C, or 539C mutation (e.g., comprising 1, 2, 3, 4, or 5 of these mutations)
(see, e.g., U.S.
Patent Application Publication No. 2004/0118681 the entire content of which is
incorporated
herein by reference); or (xi) a Haemophilus influenza (H. influenzae) iron
binding protein
having a E203C, K202C, K85C, or V287C mutation (e.g., comprising 1, 2, 3, or 4
of these
mutations) (see, e.g., U.S. Patent Application Publication No. 2004/0118681
the entire
content of which is incorporated herein by reference). In various embodiments,
the sample is
suspected of comprising a ligand, such as a ligand disclosed, described, or
otherwise
mentioned herein.
81
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
References and PDBa Iìies for bPBP structures, gents, and ligand binding
Crystal structure
bPBP open form closed form DNA sequence ligand affinity
a rahinose BP Quiocho and Scripture et al., Clark et
11.,
yyas, .1984 'ABE: 1987 198-2; Miller et
al., 1983
clipcptidc BP Nickitcnk-c ct Dunten & Abouhamacl et Guyer ct
al., 1995 1DPE Mowbrav, 1995 al., 1991 1986; Smith et.
1 DPP
ciittiAsp BP Barash Halpern,
1975; Willis
Furlong, 1975
Fe(III) BP Bruns et al., Bruns et al., 1997 Sanders et al.,Nhikri
et al.,
2001 1D9V 1MRP 1994 1995
glucose BP Vyas et al., 1988.; Scholle et al.,
Anraku, 196
Vyas et al., 1994 1987
1.61..Ci-
histidine BP Yao et al., 1994 Joshi & Ames Miller et al.,
HISL 1996 1983
maltose BP Sharif ct al., Spurlino et al., Duplay et al,
Scl-wavy er
1992 lOMP 1991; Quiocho et al., 1984 1976
1997 1ANF
phosphate BP t,edvina et al., Lueeke & Magota et al., Medveczky
1996 101B Quiocho, 1990 1984 Rosenberg, 1969.
1IXE-T
glutamine BP lisiu Lal., Sun el al., 1998 ''.µ,-ohno et
al.,'Ikr'ciner ct
1996 1CiGG 1\VDN 1986 1971
ribose BP Bjorkhian. & Mowbray & Cole, Groarke et al., 'Willis &
Mov, btay, 199S 1992 2DRI 1983 Furlong, 1974
1.URP
sulfate BP Pflug,rath Hellinga & Jacobson 8z.
Quiocho, 1985: Evans., 1985 Quiocho, 198g
ckz Quiocho,
1993 1SBP
aProicin Data Bank (Burman ct 2000)
Abouhamad et al., Molec. Microbiol. 5: 1035-104'7 (1991)
Adhikari et al.. J. Biol. Chem, 270: 25142-25149 (1995).
Anraku, J. Biol. Chem. 2,13: 3116-3122 (1968)
Barash & Halpern, Biochim. Acta 386:168-180 (1975).
82
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Ejorkman & Movvbtay, J. NU. Biol, 279: 651-664 (1998)
Barns et al., Biochemistry 40: 156.31-15637 (20(31)
Bruii et al., Nal.. &met. Biol. 4: 919-924 (1997)
Clark et al.. Biochemistry 21: 2227-2233 (1982)
Dunten & Mowbray, Protein Sci. 4: 2327-2334 (1995)
Duplay et al., J. Biol. Chem. 259: 10606-10613 (1984)
Groarke a al., J. Biol. Chcm. 258: 12.952-12956 (1983';
Guver et al., J. Bacterioh 168: 775-779 (1986)
He Sz Quiocho, Protein Sci. 2: 1643-1(47 ;-1003):
Hellinga Sz Evans, Eur. J. Biochc.ni. 149: 353-373 (1985)
Ijsiao et al., J. Mol. Biol. 262: 225-242 (1996)
Jacobson & Quiocho, J. Mot. Biol. 204: 783-787 (1988)
Joshi & Ames, GenBank Accession Number T.:47027 (1996)
Ledvina et al., Proc. Natl. Acad. Sci. USA 93: 6786-6791 (1990)
Lueeke & Quiocho, Nature 347: 402-1.06 (1990)
Magolõa et al., J. Ba.cteriol. 157: 909-917 (i984)
Medveczky &, Rosenhergõ Biochim. Bic.)phys. Acta 192:: 369.371 (1969)
Miller et al., J. Biol. Chem. 258: 13665-13672 (1983)
Mowbray & Cole, J. Mol. Biol. 225: 155-175 (1992)
Nickitenko et al., Biochemistry 34: 16585-16595 (1995y
Nohno et 21., Malec. Gen. Cienet. 205: 260-269 (1986)
Ptlugrath & Quiocho, Nature 314: 2,57-260 (1985)
Quiocho et al., Structure 5: Çì97-11i (] (1997)
Quiocho & Vyas, Natlife 310: 381-3,-Th (1984)
Sanders et al., Infect. Immun. 62: 4515-4525 (1994)
Scholle et al., Molee. (ìen. Genet. 208: 247-253 (1987)
Scripture et al., J. Mol. Biol. 197: 37-46 (1987)
Schwartz et al., Elf. J. Biochem, 71: 167-170 (1976)
1SharIT et al.. Biochemistry 31: 10657-10663 (1992).
Smith et al., Microbiology 145: 2891-2901 (1999)
Spurlino et al., J. Biol. Chem. 266: 5202-5219 (1991)
Sun et al:, J. Mol. Biol. 278: 219-229 (1998)
Vyas et al., Biochemistry 33: 4762-4768 (1994)
Vyas dt al., Science 242: 1290-1295 (1988)
Weiner et al., Arch. Biochem. Blophys. 142: 7157717 (1971)
NVillis Fut long, i. Biol. Chem. 249: 6926-6929 (197L)
& Furlong, J. Biol. Chem. 250: 2574-2580 (1975)::
Yao et al., Biochemistry 33: 4769-4779 (1994)
83
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Various types of samples may be used in methods provided herein. In non-
limiting
examples, a sample may comprise a reaction product, a buffer, and/or a
solvent. In some
embodiments, the solvent is an aqueous solvent. In some embodiments, the
solvent
comprises a non-polar solvent, a polar aprotic solvent, and/or a polar protic
solvent. For
example, a sample may comprise water, liquid ammonia, liquid sulfur dioxide,
sulfuryl
chloride, sulfuryl chloride fluoride, phosphoryl chloride, dinitrogen
tetroxide, antimony
trichloride, bromine pentafluoride, hydrogen fluoride, dimethyl sulfoxide,
hexane, benzene,
toluene, 1,4-dioxane, chlorogorm, diethyl ether, dichloromethane, N-
methylpynolidone,
tetrahydrofuran, ethyl acetate, acetone, dimethylformamide, acetonitrile,
tormic acid, n-
butanol, isopropanol, nitromethane, ethanol, methanol, and/or acetic acid.
In embodiments, a sample comprises a Newtonian liquid, a shear thickening
liquid, a
shear thinning liquid, a thixotropic liquid, a rheopectic liquid, or a Bingham
plastic. In some
implementations, a sample has a dynamic viscosity of at least about 0.5, 0.6,
0.7, 0.8, 0.9, 1,
1.1, 1.2, 1.3, 1.4, 1.5, or 2 pascal-seconds (Pa.$) or less than about 2, 1.5,
1.4, 1.3, 1.2, 1.1, 1,
0.9, 0.8, 0.7, 0.6, 0.5 Pa=s; and/or a kinematic viscosity of at least about
0.5, 0.6, 0.7, 0.8, 0.9,
1, 1.1, 1.2, 1.3, 1.4, 1.5, or 2 centistokes (cSt) or less than about 2, 1.5,
1.4, 1.3, 1.2, 1.1, 1,
0.9, 0.8, 0.7, 0.6, 0.5 cSt.
In various embodiments, the sample comprises a biological sample. The sample
may
comprise, e.g., a clinical sample (i.e., a sample collected in a clinical or
veterinary setting,
e.g., by or at the request or supervision or direction of a doctor, nurse, aid
worker, or medic)
and/or a physiological sample (a sample collected from an organism, e.g., a
mammal such as
a human). In certain embodiments, the biological sample comprises or has been
provided or
obtained from a skin surface or a mucosal surface. In some embodiments, the
biological
sample comprises a biological fluid. Non-limiting examples of biological
fluids include
sweat, tear fluid, blood, serum, plasma, interstitial fluid, amniotic fluid,
sputum, gastric
lavage, skin oil, milk, fecal matter, emesis, bile, saliva, urine, mucous,
semen, lymph, spinal
fluid, synovial fluid, a cell lysate, venom, hemolymph, and fluid obtained
from plants such as
the fluid transported in xylem cells or phloem sieve tube elements of a plant
(e.g. sap).
The present subject matter also provides biosensors, methods, compositions,
and
devices useful for measuring the level of a ligand within a liquid solution or
suspension or
composition comprising cultured cells or tissue or a supernatant of such a
solution or
suspension, e.g., a sample of conditioned media or a sample of growth media in
which a
population of cells was cultured. In some embodiments, the sample is within a
culture (e.g.,
84
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
inserted into a bioreactor) or provided from a media, culture, or reaction,
e.g., in a bioreactor.
For example, the sample may be within or provided from a fermenter such as a
culture or
culture supernatant from a fermentation reaction (e.g., an ongoing
fermentation). Thus, the
level of a ligand can be assayed at a timepoint of interest or at a series of
timepoints over the
duration of cell culture, e.g. continuously, in or from a reaction or culture.
Bioreactors
include devices or systems that support a biologically active environment. For
example, a
bioreactor may comprise a vessel in which a chemical process is carried out
which involves
organisms or biochemically active substances derived from such organisms. Such
a process
can either be aerobic or anaerobic. Organisms growing in bioreactors may be,
e.g.,
submerged or suspended in liquid medium or may be attached to the surface of a
solid
medium. Submerged cultures may be suspended or immobilized. Suspension
bioreactors can
use a wider variety of organisms, since special attachment surfaces are not
needed, and can
operate at much larger scale than immobilized cultures. However, in a
continuously operated
process the organisms will be removed from the reactor with the effluent.
Immobilization is
a general term describing a wide variety of cell or particle attachment or
entrapment. It can
be applied to basically all types of biocatalysis including enzymes, cellular
organelles, and
cells (e.g., animal cells, plant cells, fungal cells, and bacterial cells).
Immobilization is useful
for continuously operated processes, since the organisms will not be removed
with the reactor
effluent, but is limited in scale because the cells are only present on the
surfaces of the vessel.
A bioreactor may also refer to a device or system meant to grow cells or
tissues in the context
of cell culture. The interrogation and/or monitoring of ligand levels in such
samples permits
the evaluation of the status of growth of the cells or production of secreted
products by the
cells to inform harvest or feeding or other modification of the culture.
Aspects of the present subject matter relate to the use of methods and
biosensors
provided herein to detect contamination.
In some embodiments, the sample comprises an environmental sample. Depending
on context, there are instances in which a biological sample may also be, or
may be within, an
environmental sample. In certain embodiments, an environmental sample
comprises a solute
obtained from a biological composition, such as bone, nail, hair, shell, or
cartilage. In
various embodiments, an environmental sample comprises a solute obtained from
an
environmental substance and/or an environmental surface. For example, the
solute may be
dissolved/obtained from the environmental substance and/or an environmental
surface using
an aqueous or nonaqueous solution. In some embodiments, an aqueous may
optionally
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
comprise a nonaqueous solvent (e.g., mixed with an aqueous solvent). Non-
limiting
examples of environmental substances include rock, soil, clay, sand,
meteorites, asteroids,
dust, plastic, metal, mineral, fossils, sediment, and wood. Non-limiting
examples of
environmental surfaces include the surface of a vehicle such as a civilian
vehicle (e.g., a
satellite, a bike, a rocket, an automobile, a truck, a motorcycle, a yacht, a
bus, or a plane) or a
military vehicle (e.g., a tank, an armored personnel carrier, a transport
truck, a jeep, a mobile
artillery unit, a mobile antiaircraft unit, a minesweeper, a Mine-Resistant
Ambush Protected
(MRAP) vehicle, a lightweight tactical all-terrain vehicle, a high mobility
multipurpose
wheeled vehicle, a mobile multiple rocket launch system, an amphibious landing
vehicle, a
ship, a hovercraft, a submarine, a transport plane, a fighter jet, a
helicopter, a rocket, or an
Unmanned Arial Vehicle), a drone, a robot, a building, furniture, or an
organism other than a
human. In some embodiments, the sample comprises an environmental fluid. Non-
limiting
examples of environmental fluids include marine water, well water, drinking
well water,
water at the bottom of well dug for petroleum extraction or exploration,
melted ice water,
pond water, aquarium water, pool water, lake water, mud, stream water, river
water, brook
water, waste water, treated waste water, reservoir water, rain water, and
ground water. In
some embodiments, waste water comprises sewage water, septic tank water,
agricultural
runoff, water from an area in which chemical or oil spill has or is suspected
of having
occurred (e.g., an oil spill into a marine environment), water from an area
where a radiation
leak has or is suspected of having occurred (e.g., coolant from a nuclear
reactor), water
within the plumbing of a building, water within or exiting a research
facility, and/or water
within or exiting a manufacturing facility such as a factory.
As used herein, "suspected" with respect to an event means that there has been
at least
one test (e.g., a test other than a method or assay provided herein),
occurrence (e.g., that is
likely to or that may cause the event such as an emergency, leak, accident,
flood, earthquake,
storm, fire, malfunction, sunk vessel, or crash), or report (e.g., by a
witness, informant, or
observer) that is consistent with the event having occurred.
In certain embodiments, the sample comprises a food or beverage additive
and/or a
food or beverage composition. In some embodiments, the food or beverage
composition
comprises a fermented composition. In various embodiments, the sample
comprises a fluid
obtained from a food composition. Alternatively or in addition, the sample may
comprise a
solute dissolved from a food composition. In some examples, a solute is or has
been
dissolved from a food composition with an aqueous or nonaqueous solution. In
various
86
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
implementations, an aqueous solution may optionally comprise a nonaqueous
solvent. In
certain embodiments, a sample comprises a food composition in semisolid or
liquid form.
Non-limiting examples of such compositions include yogurt, soup, ice cream, a
broth, a
puree, a shake, a smoothie, a batter, a condiment, a sauce, and any
combination thereof. In
some implementations, a sample is a food engineering process (e.g., obtained
from a food
design, storage, transport, or production process or from equipment intended
to process,
transport, or store food). A food composition may comprise, e.g., a plant or a
composition
isolated from a plant, and/or an animal or a composition isolated from an
animal. In various
embodiments, a sample comprises a beverage composition. Non-limiting examples
of
beverage compositions include soft drinks, fountain beverages, water, coffee,
tea, milk, dairy-
based beverages, soy-based beverages (e.g., soy milk), almond-based beverages
(e.g., almond
milk), vegetable juice, fruit juice, fruit juice-flavored drinks, energy
drinks, sports and fitness
drinks, alcoholic products, and beverages comprising any combination thereof.
Non-limiting
examples of beverage compositions comprising water include purified water
(e.g., filtered
water, distilled water, or water purified by reverse osmosis), flavored water,
mineral water,
spring water, sparkling water, tonic water, and any combination thereof. In
various
embodiments, the sample comprises alcohol. Non-limiting examples of such
samples include
samples comprising or obtained/provided from beer, malt beverages, liqueur,
wine, spirits,
and any combination thereof.
In some embodiments, a sample comprises a nutritional or supplement
composition.
In certain implementations, the nutritional or supplement composition
comprises an omega-3
fatty acid, a vitamin, a mineral, a protein powder, or a meal supplement.
In certain embodiments, a biosensor is implanted in a subject's body. For
example, a
biosensor may be implanted in a subject's blood vessel, vein, eye, natural or
artificial
pancreas, alimentary canal, stomach, intestine, esophagus, or skin (e.g.,
within the skin or
under the skin). In various embodiments, the biosensor is configured within or
on the surface
of a contact lens. In some embodiments, the biosensor is configured to be
implanted in or
under the skin. In non-limiting examples, the biosensor is implanted in a
subject with an
optode and/or a microbead. In certain embodiments, the biosensor generates a
signal
transdermally.
Aspects of the present subject matter provide a method for assaying the level
of ligand
in a subject. The method may comprise contacting a biological sample from the
subject with
a biosensor for ligand under conditions such that the biosensor binds to
ligand present in the
87
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
biological sample. The biosensor comprises reporter group that is attached to
a ligand
binding protein, and binding of ligand to a ligand-binding domain of the
ligand binding
protein causes a change in signaling by the reporter group.
In various embodiments, the subject has or is suspected of having a disease or
disorder, such as abnormal kidney function, abnormal adrenal gland function,
diabetes,
hypochloremia, bromism, hypothyroidism, hyperthyroidism, cretinism,
depression, fatigue,
obesity, a low basal body temperature, a goiter, a fibrocystic breast change,
lactic acidosis,
septic shock, carbon monoxide poisoning, asthma, a lung disease, respiratory
insufficiency,
Chronic Obstructive Pulmonary Disease (COPD), regional hypoperfusion,
ischemia, severe
anemia, cardiac arrest, heart failure, a tissue injury, thrombosis, or a
metabolic disorder,
diarrhea, shock, ethylene glycol poisoning, methanol poisoning, diabetic
ketoacidosis,
hypertension, Cushing syndrome, liver failure, cancer, or an infection.
As used herein, "suspected" with respect to a subject's condition (e.g.,
disease or
injury) means that the subject has at least one symptom or test (e.g., a test
other than an assay
or method provided herein) that is consistent with the condition.
In some embodiments, the biological sample comprises blood, plasma, serum,
sweat,
tear fluid, or urine. In certain embodiments, the biological sample is present
in or on the
surface of the subject. In various implementations, the biosensor is applied
onto or inserted
into the subject. For example, the biosensor may be tattooed into the subject
or is in or on a
device that is implanted into the subject. In some embodiments, the biosensor
may be present
in or on a contact lens that is worn by the subject. Methods for determining
the level of
ligand in a subject who has or is suspected of having a disease that results
in or from (or
otherwise involves) an altered level of a ligand, may be performed without
other testing
related to the disease performed as part of a battery of clinical testing.
The present subject matter includes a method for monitoring the level of a
ligand,
comprising periodically or continuously detecting the level of the ligand,
wherein detecting
the level of the ligand comprises (a) providing or obtaining a sample; (b)
contacting the
sample with a biosensor for the ligand under conditions such that the ligand-
binding protein
of the biosensor binds to the ligand, and (c) detecting a signal produced by
the biosensor.
Aspects of the present subject matter also provide a method for monitoring the
level
of a ligand in a subject, comprising periodically detecting the level of the
ligand in the
subject. Detecting the level of the ligand in the subject may comprise (a)
providing or
obtaining a biological sample from the subject; (b) contacting the biological
sample with a
88
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
biosensor for the ligand provided herein under conditions such that the ligand-
binding protein
of the biosensor binds to the ligand, if the ligand is present in the
biological sample, and (c)
detecting (i) a signal produced by a reporter group of the biosensor, or (ii)
whether a signal is
produced by a reporter group of the biosensor. The level of the ligand may be
detected, e.g.,
at least once every 1, 2, 3, 6, or 12 hours, at least once every 1, 2, 3, or 4
days, at least once
every 1, 2, or three weeks, or at least once every 1, 2, 3, 4, 6, or 12
months.
The present subject matter also provides a method for monitoring the level of
a ligand
in a subject. The method comprises (a) administering a biosensor provided
herein or a device
comprising a biosensor provided herein to the subject, wherein after
administration the
biosensor is in contact with a bodily fluid or surface that typically
comprises the ligand, and
(b) detecting (i) a signal produced by a reporter group of the biosensor
continuously or
repeatedly at intervals less than about 30 minutes (m), 15m, 10m, 5m, lm, 30
seconds (s),
15s, 10s, 5s, ls, 0.1s, 0.001s, 0.0001s, or 0.00001 apart, and/or (ii) whether
a signal is
produced by a reporter group of the biosensor continuously or repeatedly at
intervals less than
about 30m, 15m, 10m, 5m, lm, 30s, 15s, 10s, 5s, ls, 0.1s, 0.001s, 0.0001s, or
0.00001apart.
Non-limiting aspects of continuously monitoring ligand levels are described in
Weidemaier et al. (2011) Biosensors and Bioelectronics 26, 4117-4123 and Judge
et al.
(2011) Diabetes Technology & Therapeutics, 13(3):309-317, the entire contents
of each of
which are hereby incorporated herein by reference.
Also within the invention is a composition comprising a purified thermostable,
ligand-
binding fluorescently-responsive sensor protein and a solid substrate, e.g., a
particle, a bead
such as a magnetic bead, or a planar surface such as a chip or slide, wherein
the sensor
protein is immobilized onto the solid substrate. An exemplary solid substrate
solid substrate
comprises a cyclic olefin copolymer.
A thermostable ligand sensor protein is one in which the activity (ligand
binding) is
retained after exposure to relatively high temperatures. For example, the
ligand sensor
protein comprises a mid-point thermal melt transition greater than 50 C,
greater than 60 C,
greater than 70 C, greater than 80 C, greater than 90 C, or greater than 100
C. In some
embodiments, the sensor protein contains a single cysteine residue. In some
embodiments,
the single cysteine residue is located in a site of the ligand-binding
protein, where it responds
to ligand binding. In some examples, the protein comprises the amino acid
sequence of SEQ
ID NO: 16 (ttGGBP.17C.O.bZif) and 19 (ttGGBP.182C.O.bZif), and in some
examples, the
single cysteine is conjugated to Badan, Acrylodan, or a derivative thereof.
For example, the
89
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
derivative comprises a replacement of the two-ring naphthalene of Acrylodan or
Badan with
a three-ring anthracene, a fluorene, or a styrene. A reporter group is
covalently bound to the
single cysteine. In some situations, the solid substrate comprises a plurality
of sensor
proteins, each of which comprises a different dissociation constant (K ) for
ligand, e.g., for
detecting and quantifying ligand levels across many ranges of concentrations.
The present subject matter also includes a composition comprising purified
ligand
sensor protein with less than 65% identity and greater than 27% identity
(e.g., 44-48%
sequence identity) to any ligand-binding protein disclosed herein, wherein the
sensor protein
comprises a single cysteine residue, such that the sensor protein is
immobilized onto the solid
substrate. As described above, a reporter group is covalently bound to the
single cysteine. In
some example, the
solid substrate comprises a plurality of sensor proteins, each of which
comprises a different
dissociation constant (Kd) for ligand for sensing over a wide range or ranges
of ligand
concentrations.
In some embodiments, a method of detecting the presence of or the quantity of
ligand
in a test sample is carried out using the following steps: contacting the test
sample with the
biosensor or sensor protein/solid support construct to yield a complex of
ligand and the
ligand-binding protein or biosensor protein; contacting the complex with an
excitation light;
measuring an emission intensity of the reporter group from at least two
wavelengths;
computing a ratiometric signal from the two (or more) wavelengths; and
comparing the signal
to a known ligand binding curve of signals to identify the presence of or
calculate the
quantity of ligand in the test sample. The test sample may be obtained from a
variety of
sources. For example, the test sample may be selected from a bodily fluid, a
food, a
beverage, or a bioreactor culture broth. The testing method may be carried out
in vivo, e.g.,
using an implantable device or dermal patch, or ex vivo.
In various embodiments, the subject to be tested is a mammal, e.g., a primate
(such as
a human, a monkey, a chimpanzee, or a gorilla), a fish, a bird, a reptile, an
amphibian, or an
arthropod. In some embodiments, the subject is a fish, a cow, a pig, a camel,
a llama, a horse,
a race horse, a work horse, a goat, a rabbit, a sheep, a hamster, a guinea
pig, a cat, a wolf, a
dog (e.g., a pet dog, a work dog, a police dog, or a military dog), a rat, a
mouse, a seal, a
whale, a manatee, a lizard, a snake, a chicken, a goose, a swan, a duck, or a
penguin.
In some embodiments, the ligand comprises a halide anion and the ligand-
binding
protein comprises a fluorescent protein. Aspects of the present subject matter
provide a
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
method for detecting the level of a halide anion in a sample, comprising
contacting the
sample with a biosensor for a halide anion under conditions such that the
biosensor binds to a
halide anion present in the sample. In various embodiments the biosensor
comprises a halide
anion-binding fluorescent protein, and binding of the halide anion to a halide
anion-binding
domain of the fluorescent protein causes a change in signaling by the
fluorescent protein. In
some embodiments, the sample is an environmental sample. In a non-limiting
example, the
sample comprises treated wastewater or drinking water.
Aspects of the present subject matter further provides a method for assaying
the level
of chloride in a subject, comprising contacting a biological sample from the
subject with a
biosensor for chloride under conditions such that the biosensor binds to
chloride present in
the biological sample. The biosensor may comprise, e.g., a chloride-binding
fluorescent
protein, and binding of chloride to a chloride-binding domain of the
fluorescent protein
causes a change in signaling by the fluorescent protein. In some embodiments,
the subject
has or is suspected of having hypochloremia. Alternatively or in addition, the
subject has or
is suspected of having abnormal kidney or adrenal gland function. In certain
embodiments,
the biological sample comprises blood, plasma, serum, sweat, tear fluid, or
urine. In some
embodiments, the method is performed as part of a battery of clinical testing.
Also provided is a method for assaying the level of iodide in a subject,
comprising
contacting a biological sample from the subject with a biosensor for iodide
under conditions
such that the biosensor binds to iodide present in the biological sample,
wherein the biosensor
comprises an iodide-binding fluorescent protein. Binding of iodide to an
iodide-binding
domain of the fluorescent protein causes a change in signaling by the
fluorescent protein. In
some embodiments, the subject has or is suspected of having hypothyroidism,
hyperthyroidism, cretinism, depression, fatigue, obesity, a low basal body
temperature, a
goiter, or a flbrocystic breast change. In some embodiments, the biological
sample comprises
blood, plasma, serum, sweat, tear fluid, or urine. In some embodiments, the
method is
performed as part of a battery of clinical testing.
The present subject matter further includes a method for assaying the level of
bromide
in a subject, comprising contacting a biological sample from the subject with
a biosensor for
bromide under conditions such that the biosensor binds to bromide present in
the biological
sample, wherein the biosensor comprises a bromide-binding fluorescent protein.
Upon
binding of bromide to a bromide-binding domain of the fluorescent protein, the
signal of the
fluorescent protein changes. In some embodiments, the subject has or is
suspected of having
91
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
bromism. In certain embodiments, the biological sample comprises blood,
plasma, serum,
sweat, tear fluid, or urine. In some embodiments, the method is performed as
part of a battery
of clinical testing.
Exemplary Devices and Compositions Comprising Biosensors
Aspects of the present subject matter provide a device comprising one or more
biosensors provided herein. Such devices may be, e.g., wearable, implantable,
portable, or
fixed.
In some embodiments, the device is a nanoparticle or a microparticle
comprising the
biosensor. Non-limiting examples of devices include devices comprising a test
strip, patch,
plate, bead, or chip comprising a biosensor provided herein. In certain
embodiments, a
device may comprise a desiccated biosensor.
The present subject matter also provides a contact lens or a skin patch
comprising a
biosensor provided herein. In some embodiments, the biosensor is throughout
the contact
lens or skin patch or within a particular region or zone of a contact lens or
skin patch (e.g., in
one or more shapes (e.g., a square, circle, or star), dots, lines, or zones,
located at the
periphery or a portion of the periphery of a contact lens or patch). In some
embodiments, the
skin patch comprises an adhesive that facilitates attachment of the patch to
the surface of
skin.
Devices provided herein may include a variety of structural compositions. For
example, many polymers (including copolymers), and plastics may be used. Non-
limiting
examples of compositions useful in certain devices include glass, polystyrene,
polypropylene,
cyclic olefin copolymers, ethylene-norbomene copolymers, polyethylene,
dextran, nylon,
amylase, paper, a natural cellulose, a modified cellulose, a polyacrylamide,
gabbros, gold,
and magnetite (as well as combinations thereof). In some embodiments, the
device
comprises a hydrogel, a cryogel, or a soluble gel. For example, the biosensor
may be
incorporated into or onto the hydrogel, cryogel, or soluble gel. In various
embodiments, the
device comprises a matrix comprising nanopores, micropores, and/or macropores.
In certain
embodiments, the surface of a device comprises a polymer. In an embodiment,
the surface
comprises the surface of a particle or a bead having a diameter of about 0.001-
1, 0.001-0.1,
0.01-0.1, 0.001-0.01, 0.1-1, 0.1-0.5, or 0.01-0.5 centimeters (cm). For
example, the particle
comprises a nanoparticle or a microparticle.
92
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Non-limiting examples of polymers include cyclic olefin copolymers, ethylene-
norbomene copolymers, polylactic acid, polyglycolic acid, agarose, alginate,
poly(lactide-co-
glycolide), gelatin, collagen, agarose, natural and synthetic polysaccharides,
polyamino acids,
poly(lysine), polyesters, polyhydroxybutyrates, polyanhydrides,
polyphosphazines, polyvinyl
alcohol, polyalkylene oxide, polyethylene oxide, polyallylamines,
polyacrylates, modified
styrene polymers, poly(4-aminomethylstyrene), pluronic polyols, polyoxamers,
polyuronic
acid, polyvinylpynolidone, hydroxyethyl (meth)acrylate, polyolefins,
polyurethane,
polystyrene, ethylene/methacrylic acid copolymers, ethylene/methyl
methacrylate
copolymers, polyester, and polyurethane. In some embodiments, the patch
comprises a
woven fabric, a knitted fabric, or a nonwoven fabric of a synthetic fiber
and/or natural fiber.
Non-limiting examples of temporary tattoo compositions for application to a
subject's
skin are discussed in U.S. Patent Application Publication No. 20090325221,
published
December 31, 2009, and U.S. Patent No. 6,428,797, the entire contents of each
of which are
incorporated herein by reference. Biosensor disclosed herein may be
incorporated into any
temporary tattoo or other composition for application to the skin. For
example, a temporary
tattoo decal for application to a subject's skin and configured to detect the
presence of a
ligand may comprise, e.g., a base paper or plastic; a water-soluble slip layer
applied to the
base paper or plastic; a temporary tattoo applied to the water-soluble release
layer on the base
paper, wherein the temporary tattoo comprises a biosensor disclosed herein; an
adhesive layer
overlying the temporary tattoo; and a protective sheet overlying the adhesive
layer.
In some embodiments, the device comprises a plastic polymer comprising cyclic
olefin copolymer (COC), such as e.g. TOPAS COC. Several types of cyclic
olefin
copolymers are available based on different types of cyclic monomers and
polymerization
methods. Cyclic olefin copolymers are produced by chain copolymerization of
cyclic
monomers such as 8,9,10-trinorbom-2-ene (norbomene) or 1,2,3,4,4a,5,8,8a-
octahydro-
1,4:5,8-dimethanonaphthalene (tetracyclododecene) with ethene (such as TOPAS
Advanced
Polymer's TOPAS, Mitsui Chemical's APEL), or by ring-opening metathesis
polymerization
of various cyclic monomers followed by hydrogenation (Japan Synthetic Rubber's
ARTON,
Zeon Chemical's Zeonex and Zeonor). See, e.g., International Union of Pure and
Applied
Chemistry (2005) Purr. AppL Chem. 77(5):801-814. These later materials using a
single type
of monomer may be referred to as cyclic olefin polymers (COPs). A CAS Registry
number
for COC is 26007-43-2.
93
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
In certain embodiments, the device is attached to a surface of a device or is
not
attached to a surface of the device (e.g., the biosensor is present loosely
within the device as a
component of a solution or powder).
A biosensor may be attached to a device via a variety or means, e.g., via
attachment
motif. In some embodiments, the attachment motif is attached to the N-terminus
or the C-
terminus of the biosensor. In certain embodiments, the biosensor is linked to
an attachment
motif via a covalent bond. In various embodiments, the biosensor is linked to
the attachment
motif via a linker. A non-limiting example of a linker is a polyglycine
comprising 2, 3, 4, 5,
or more glycines and optionally further comprising a serine. In some
embodiments, the
attachment motif comprises a polypeptide. Non-limiting examples of
polypeptides useful in
attachment moieties include hexahistidine peptides, hexalysine peptides, zinc-
finger domains
(ZF-QNKs), and disulfide-containing truncated zinc fingers (f3Zifs). An
example of a
hexalysine peptide comprises amino acids in the sequence of SEQ ID NO: 45, an
example of
a ZF-QNK comprises amino acids in the sequence of SEQ ID NO: 43, and an
example of a
PZif comprises amino acids in the sequence of SEQ ID NO: 42. In some
embodiments, the
attachment motif comprises a polypeptide that binds to plastic or cellulose.
The hexahistidine, hexalysine, PZif and QNK-ZF fusions enable FRSs to be
immobilized onto chemically functionalized surfaces. Non-limiting aspects of
chemically
functionalized surfaces are discussed in Biju, V. (2014) Chem Soc Rev, 43, 744-
64 and
McDonagh (2008) Chem Rev, 108, 400-422, the entire contents of which are
incorporated
herein by reference. Directed evolution methods have been used to develop
peptides that
bind directly to non-functionalized surfaces (Care, Bergquist and Sunna 2015
Trends
Biotechnol, 33, 259-68; Baneyx 2007 Curr. Opin. Biotechnol., 18, 312-317;
Gunay and Klok
2015 Bioconjug Chem, 26, 2002-15), including various plastics (Adey et al.
1995 Gene, 156,
27-31; Serizawa et al. 2005 J Am Chem Soc, 127, 13780-1; Serizawa, Sawada and
Kitayama
2007a Angew Chem Int Ed Engl, 46, 723-6; Serizawa, Sawada and Matsuno 2007b
Langmuir, 23, 11127-33; Serizawa, Techawanitchai and Matsuno 2007c
Chembiochem, 8,
989-93; Matsuno et al. 2008 Langmuir, 24, 6399-403; Chen, Serizawa and
Komiyama 2011 J
Pept Sci, 17, 163-8; Kumada 2010 J. Biosci. and BioEng., 109, 583-587; Date et
al. 2011
ACS Appl Mater Interfaces, 3, 351-9; Kumada 2012, Vodnik, Strukelj and Lunder
2012 J.
Biotech., 160, 222-228; Kumada 2014 Biochem. et Biophys. Acta, 1844, 1960-
1969; Ejima,
Matsuno and Serizawa 2010 Langmuir, 26, 17278-85), inorganic materials(Hnilova
2012 Soft
Matter, 8, 4327-4334; Care et al. 2015 Trends Biotechnol, 33, 259-68),
nanoparticles
94
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
(Avvakumova et al. 2014 Trends Biotechnol, 32, 11-20), and cellulosic paper
(Guo et al.
2013 Biomacromolecules, 14, 1795-805). Such peptides, or natural material-
binding domains
(Oliveira et al. 2015 Biotechnol Adv, 33, 358-69), also can be fused to FRSs
to direct site-
specific, oriented immobilization on their target materials while preserving
FRS function.
For instance, plastic-binding peptides have been developed that direct
immobilization on
polystyrene (Adey et al. 1995 Gene, 156, 27-31; Serizawa et al. 2007c
Chembiochem, 8, 989-
93; Kumada 2010 Biochem. et Biophys. Acta, 1844, 1960-1969; Vodnik et al. 2012
Anal
Biochem, 424, 83-6), polymethyl acrylate (Serizawa et al. 2005 J Am Chem Soc,
127, 13780-
1; Serizawa et al. 2007a Angew Chem Int Ed Engl, 46, 723-6; Serizawa et al.
2007b
Langmuir, 23, 11127-33; Kumada 2014 Biochem. et Biophys. Acta, 1844, 1960-
1969),
polycarbonate (Kumada 2012 J. Biotech., 160, 222-228), polylactide (Matsuno et
al. 2008
Langmuir, 24, 6399-403), and polyphenylene vinylene (Ejima et al. 2010
Langmuir, 26,
17278-85). Cellulose-binding peptides (Guo et al. 2013 Biomacromolecules, 14,
1795-805)
and natural domains (Oliveira et al. 2015 Biotechnol Adv, 33, 358-69;
Shoseyov, Shani and
Levy 2006 Microbiol Mol Biol Rev, 70, 283-95) can be used to immobilize fusion
proteins on
paper. Inorganic material include noble metals (Hnilova 2012 Soft Matter, 8,
4327-4334),
semi-conductors (Care et al. 2015 Trends Biotechnol, 33, 259-68), and
fluorescent quantum
dots(Medintz et al. 2005 Nat Mater, 4, 435-46; Lee et al. 2002 Science, 296,
892-5). The
entire contents of each of the references above (and all other references
herein) is
incorporated herein by reference.
In some embodiments, the attachment motif is attached to a device surface
and/or
within a matrix of the device. In some embodiments, a biosensor is attached to
an attachment
motif via a covalent bond and the attachment motif is attached to a device via
a covalent
bond. Non-limiting examples of covalent bonds include disulfide bonds, ester
bonds,
thioester bonds, amide bonds, and bonds that have been formed by click
reactions. Non-
limiting examples of a click reaction include a reaction between an azide and
an alkyne; an
azide and an alkyne in the presence of Cu(I); an azide and a strained
cyclooctyne; an azide
and a dibenzylcyclooctyne, a difluorooctyne, or a biarylazacyclooctynone; a
diaryl-strained-
cyclooctyne and a 1,3-nitrone; an azide, a tetrazine, or a tetrazole and a
strained alkene; an
azide, a tetrazine, or a tretrazole and a oxanorbomadiene, a cyclooctene, or a
trans-
cycloalkene; a tetrazole and an alkene; or a tetrazole with an amino or styryl
group that is
activated by ultraviolet light and an alkene.
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Alternatively or in addition, a surface of a device may be modified to contain
a moiety
(e.g. a reactive group) what facilitates the attachment of a biosensor and/or
binds to the
biosensor. In some embodiments, the biosensor is attached to a surface via a
biotin-avidin
interaction.
In various implementations, the device comprises a first region for receiving
a sample
and second a region that comprises the biosensor, wherein the first region is
separated from
the second region by a filter. In some examples, the filter is impermeable to
compounds
greater than about 1, 2, 3, 4, 5, 10, 50, 200, or 250 kiloDalton (kDa) in
size. The sample may
comprise, e.g., a tube, such as a tube that is configured for centrifugation.
When sample is
placed into the first region and the device is centrifuged, then a portion of
the sample
comprising a ligand flows through the filter into the second region where the
biosensor is
contacted.
Non-limiting examples of devices provided herein include endoscopy probes and
colonoscopy probes.
In some embodiments, the device comprises an optode. In non-limiting examples,
the
optode comprises an optical fiber and a single biosensor or composite
biosensor. In certain
embodiments, the single biosensor or composite biosensor is immobilized on the
surface or at
an end of the optical fiber. In some embodiments, the optode is configured for
implantation
into a subject. Alternatively or in addition, the optode is configured for
insertion into a
sample.
The devices provided herein may optionally comprise a biosensor panel, a
composite
sensor, a sensor array, and/or a composition comprising a plurality of
biosensors. In various
embodiments, a device comprises multiple ligand biosensors that detect a range
of different
ligand concentrations in a single sample and/or assay run (i.e., each
biosensor has a different
affinity for ligand). Devices may provide spatial localization of multiple
biosensors to
provide the necessary addressability of different elements in a multi-sensor
array comprising
sensors that differ in their engineered affinities for coverage of a wide
range of ligand
concentrations, or sensors that each detects distinct analytes.
Aspects of the present subject matter provide a biosensor panel comprising a
plurality
of biosensors, wherein the plurality of biosensors comprises at least one
biosensor disclosed
herein. In some embodiments, the plurality comprises at least about 2, 3, 4,
5, 10, 20, 30, 40,
50, 60, 70, 80, 90, or 100 biosensors.
96
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
The present subject matter also provides a composite sensor. The composite
sensor
may comprise a sensor element, wherein the sensor element comprises 2 or more
biosensors,
wherein at least 1 of the 2 or more biosensors is a biosensor disclosed
herein. In some
embodiments, the biosensors are not spatially separated in the sensor element,
e.g., the
biosensors are mixed within a solution or on a surface of the sensor element.
In various
embodiments, the composite sensor comprises a plurality of sensor elements,
wherein each
sensor element of the plurality of sensor elements comprises 2 or more
biosensors, wherein at
least 1 of the 2 or more biosensors is a biosensor provided herein. In some
embodiments, the
plurality of sensor elements comprises at least about 2, 3, 4, 5, 10, 20, 30,
40, 50, 60, 70, 80,
90, or 100 sensor elements.
Also included herein is a sensor array comprising a plurality of biosensors of
the
present subject matter. The sensor array may include, e.g., multichannel array
or a
multiplexed array. In some embodiments, the biosensors of the plurality of
biosensors are
spatially separated from each other. In certain embodiments, the biosensors
are arranged
linearly or in a grid on a surface of the array.
The present subject matter provides a composition comprising a plurality of
biosensors including at least one biosensor disclosed herein. Also provided is
a non-human
mammal comprising a biosensor or device disclosed herein.
Exemplary Polypeptides and Polynucleotides
The present subject matter provides polynucleotides encoding any one of the
polypeptides disclosed herein. The polypeptides are also provided. In various
embodiments,
the polynucleotides are codon-optimized for expression in a desired host cell,
such as
bacterial cells (e.g., E. coli), yeast, insect cells, plant cells, algal
cells, or mammalian cells.
The polypeptides provided herein include polypeptides comprising the amino
acid sequence
of any one of SEQ ID NOS: 1-41, 87-151, or 153-167. The polynucleotides
provided herein
include polynucleotides encoding a polypeptide comprising the amino acid
sequence of any
one of SEQ ID NOS: 1-41, 87-151, or 153-167.
The polypeptides and biosensors provided herein may be in a variety of forms,
e.g.,
purified in solution, dried (e.g. lyophilized) such as in the form of a
powder, and in the form
of a crystal (e.g., a crystal suitable for x-ray crystallography). Thus,
aspects of the present
subject matter provide crystal structures and crystalized forms of the ligand-
binding proteins
97
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
and biosensors disclosed herein. Such crystal structures and crystalized
proteins are useful
for designing and optimizing biosensors using principles and methods discussed
herein.
Also provided are expression vectors comprising a polynucleotide of the
present
subject matter and/or encoding a polypeptide disclosed herein. Non-limiting
examples of
expression vectors include viral vectors and plasmid vectors. In some
embodiments, an
expression vector comprises nucleotides in the sequence set forth as any one
of SEQ ID
NOS: 46-86. In various embodiments, a polynucleotide encoding a ligand-binding
protein
and/or biosensor is operably linked to a promoter. The promoter may be
expressed, e.g., in a
prokaryotic and/or a eukaryotic cell.
The subject matter further includes an isolated cell comprising an expression
vector
provided herein. The isolated cell may be, e.g., a bacterial cell, a yeast
cell, an algal cell, a
plant cell, an insect cell, or a mammalian cell. Also included is a non-human
multicellular
organism such as a plant or an animal (e.g., an insect, a mammal, a worm, a
fish, a bird, or a
reptile) comprising an expression vector disclosed herein.
Exemplary Methods for Designing Biosensors
Aspects of the present subject matter provide method of identifying a
candidate
ligand-binding protein for use in a biosensor, comprising: (a) selecting a
first protein having a
known amino acid sequence (seed sequence), wherein the first protein is a
ligand binding
protein; (b) identifying a second protein having an amino acid sequence (hit
sequence) with at
least 15% sequence identity to the seed sequence; (c) aligning the seed amino
acid sequence
and the hit sequence, and comparing the hit sequence with the seed sequence at
positions of
the seed sequence that correspond to at least 5 primary complementary surface
(PCS) amino
acids, wherein each of the at least 5 PCS amino acids has a hydrogen bond
interaction or a
van der Waals interaction with ligand when ligand is bound to the first
protein; and (d)
identifying the second protein to be a candidate ligand-binding protein if the
hit sequence
comprises at least 5 amino acids that are consistent with the PCS.
The present subject matter also includes a method for constructing a candidate
biosensor, comprising: (a) providing a candidate ligand-binding protein; (b)
generating a
structure of the second protein; (c) identifying at least one putative
allosteric, endosteric, or
peristeric site of the second protein based on the structure; (d) mutating the
second protein to
substitute an amino acid at the at least one putative allosteric, endosteric,
or peristeric site of
the second protein with a cysteine; and (e) conjugating a fluorescent compound
to the
98
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
cysteine. In some embodiments, the structure comprises a homology model of the
second
protein generated using a structure of the first protein. In some embodiments,
the structure
comprises a structure experimentally determined by nuclear magnetic resonance
spectroscopy
or X-ray crystallography.
Aspects of the present subject matter further provide a method for
constructing a
biosensor comprising a desired dissociation constant (Kd) for ligand,
comprising: (a)
providing an initial biosensor that does not comprise the desired Kd for
ligand, wherein the
initial biosensor is a biosensor provided herein; (b) mutating the initial
biosensor to (i) alter a
direct interaction in the PCS between the initial biosensor and bound ligand;
(ii) manipulate
the equilibrium between open and closed states of the initial biosensor; (iii)
alter an
interaction between the ligand-binding protein and the reporter group of the
initial biosensor;
or (iv) alter an indirect interaction that alters the geometry of the binding
site of the
biosensor, to produce a modified biosensor; and (c) selecting the modified
biosensor if the
modified biosensor comprises the desired Kd for ligand. In some embodiments,
the reporter
comprises Acrylodan, Badan, or a derivative thereof, and mutating the initial
biosensor in (b)
comprises altering an interaction between the ligand-binding protein and a
carbonyl group of
the Acrylodan, Badan, or derivative thereof. In some embodiments, the reporter
group
comprises Acrylodan, Badan, or a derivative thereof, and mutating the initial
biosensor in (b)
comprises altering an interaction between the ligand-binding protein and a
naphthalene ring
of the Acrylodan, Badan, or derivative thereof. In some embodiments, mutating
the initial
biosensor comprises introducing a substitution mutation into the initial
biosensor. In some
embodiments, the method further comprises immobilizing the affinity-tuned
biosensor on a
substrate.
In some embodiments, the second protein comprises (i) amino acids in the
sequence
of any one of SEQ ID NOS: 1-41, 87-151, or 153-167; (ii) a stretch of amino
acids in a
sequence that is least about 95, 96, 97, 98, or 99% identical to the sequence
of any one of
SEQ ID NOS: 1-41, 87-151, or 153-167; (iii) a stretch of at least about 50,
100, 150, 200,
250, 300, 350, or 400 amino acids in a sequence that is at least about 95, 96,
97, 98, or 99%
identical to a sequence within any one of SEQ ID NOS: 1-41, 87-151, or 153-
167; or (iv) a
stretch of at least about 50, 100, 150, 200, 250, 300, 350, or 400 amino acids
in a sequence
that is identical to a sequence within any one of SEQ ID NOS: 1-41, 87-151, or
153-167. In
various embodiments, attaching the reporter group to the putative allosteric,
endosteric, or
peristeric site of the first protein comprises substituting a cysteine at the
site with a cysteine.
99
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
For example, the reporter group is conjugated to the cysteine. Preferably,
attaching a reporter
group to the corresponding amino acid of the second protein produces a
functional biosensor.
Aspects also provide method for constructing a biosensor, comprising (a)
providing a
ligand-binding protein; (b) identifying at least one putative allosteric,
endosteric, or peristeric
site of the ligand-binding based a structure of the ligand-binding protein;
(c) mutating the
ligand-binding protein to substitute an amino acid at the at least one
putative allosteric,
endosteric, or peristeric site of the second protein with a cysteine; (d)
conjugating a donor
fluorophore or an acceptor fluorophore to the cysteine to produce single
labeled biosensor;
(e) detecting whether there is a spectral shift or change in emission
intensity of the single
labeled biosensor upon ligand binding when the donor fluorophore or the
acceptor
fluorophore is fully excited; and (f) if a spectral shift or change in
emission intensity is
detected in (g), attaching a donor fluorophore to the second protein if an
acceptor fluorophore
is attached to the cysteine, and attaching an acceptor fluorophore to the
second protein if an
acceptor fluorophore is attached to the cysteine.
In some embodiments, the ligand-binding protein has been identified by (i)
selecting
a first protein having a known amino acid sequence (seed sequence), wherein
the first protein
is a ligand-binding protein; (ii) identifying a second protein having an amino
acid sequence
(hit sequence) with at least 15% sequence identity to the seed sequence; (iii)
aligning the seed
amino acid sequence and the hit sequence, and comparing the hit sequence with
the seed
sequence at positions of the seed sequence that correspond to at least 5
primary
complementary surface (PCS) amino acids, wherein each of the at least 5 PCS
amino acids
has a hydrogen bond interaction or a van der Waals interaction with ligand
when ligand is
bound to the first protein; and (iv) identifying the second protein to be a
ligand-binding
protein if the hit sequence comprises at least 5 amino acids that are
consistent with the PCS.
The spectral shift may comprise, e.g., a monochromatic fluorescence intensity
change or a
dichromatic spectral shift.
Also provided is a method of converting a biosensor that shows a monochromatic
response upon ligand binding into a biosensor with a dichromatic response upon
ligand
binding, the method comprising (a) selecting a biosensor that exhibits a
monochromatic
response upon ligand binding, wherein the biosensor comprises a ligand-binding
protein and
a first reporter group; and (b) attaching a second reporter group to the
biosensor, wherein the
second reporter group has (i) an excitation spectrum that overlaps with the
emission spectrum
of the first reporter group; or (ii) an emission spectrum that overlaps with
the excitation
100
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
spectrum of the first reporter group. In certain embodiments, the second
reporter group is
within about 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.1, 1.2, 1.3,
1.4, 1.5, 1.6, 1.7, 1.8,
1.9, 2, 4, 6, 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 25, 30, 35, 40,
45, 50, 55, 60, 65, 70,
75, 80, 85, 90, 95, 100, 125, 150, or 200 angstroms (A) of the first reporter
group regardless
of whether ligand is bound to the biosensor. In some embodiments, when the
ligand is bound
to the biosensor, the average distance between the first reporter group and
the second reporter
group changes by less than about 5, 4, 3, 2, 1, 0.9, 0.8, 0.7, 0.6, 0.5, 0.4,
0.3, 0.2, 0.1, 0.05, or
0.01 angstroms (A) compared to when ligand is not bound to the ligand-binding
protein.
Aspects include a method of converting a biosensor that shows a monochromatic
response upon ligand binding into a biosensor with a dichromatic response upon
ligand
binding, the method comprising (a) selecting a biosensor that exhibits a
monochromatic
response upon ligand binding, wherein the biosensor comprises a ligand-binding
fluorescent
protein; and (b) attaching an acceptor fluorophore or a donor fluorophore to
the biosensor,
wherein (i) the acceptor fluorophore has an excitation spectrum that overlaps
with the
emission spectrum of the fluorescent protein; or (ii) the donor fluorophore
has an emission
spectrum that overlaps with the excitation spectrum of the fluorescent
protein.
Also provided is a method of increasing a dichromatic response of a biosensor
to
ligand binding, the method comprising (a) selecting a biosensor that exhibits
a dichromatic
response upon ligand binding, wherein the biosensor comprises a ligand-binding
protein and
a first reporter group; and (b) attaching a second reporter group to the
biosensor, wherein the
second reporter group has (i) an excitation spectrum that overlaps with the
emission spectrum
of the first reporter group; or (ii) an emission spectrum that overlaps with
the excitation
spectrum of the first reporter group.
The selected first protein (e.g., the amino acid sequence thereof) may be
novel or
known. However, in many instances, the function of the first protein will not
be known. In a
non-limiting example, identifying a protein not previously known to have
ligand binding
activity may comprise a structurally assisted functional evaluation (SAFE)
homolog search
method comprising the following steps:
(1) Collecting a sequence homology set using a BLAST sequence alignment tool
starting with ligand binding protein sequence disclosed herein as a seed.
Permissive settings
are used, such that pairwise hits are required to have a minimum of only,
e.g., 20%, 25%,
30%, 35% or 40% sequence identity with the seed sequence. The lengths of the
hit and seed
101
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
are mutually constrained such that the alignment covers at least, e.g., 60%,
65%, 70%, 85%,
or 90% within each partner.
(2) Structure-based encoding of biological function: A primary complementary
surface (PCS) comprising the protein residues that form hydrogen bonds and van
der Waals
contacts with a bound ligand is defined using computer-assisted, visual
inspection of the
three-dimensional structure of the biosensor-ligand complex. This definition
specifies
residue positions and their permitted amino acid identity. Multiple amino acid
identities are
permitted at each position to encode functionally equivalent residues. This
definition
establishes a search filter for the accurate prediction of ligand-binding
proteins within the
universe of sequence homologs collected in (1).
(3) Accurate sequence alignment: Tools such as ClustalW are used to construct
an
accurate alignment of all the sequence homologs. The ligand-binding protein
seed sequence
is included in the alignment. This multiple sequence alignment establishes the
equivalent
positions of the seed sequence (primary complementary surface) PCS in each
sequence
homolog.
(4) Function evaluation: The ligand-binding properties of each of the aligned
sequence homologs is determined by measuring their compliance with the PCS
sequence
filter. A "Hamming distance", H, is assigned for each homolog, which specifies
the degree
of sequence identity of all the residues at the aligned PCS positions. A value
of H=0
indicates that the identities of all the residues at the aligned PCS positions
match the amino
acid(s) allowed in the PCS search filter; H>0, indicates that one or more
aligned positions
have disallowed residues. Sequences for which H=0 are predicted to encode
ligand-binding
proteins.
(5) Selection of representative SAFE homologs: The sequence homologs are
ordered
by (a) identity with the seed PCS, as measured by the Hamming distance, (b)
fractional
overall sequence identity with the seed sequence. A subset for sequences with
H=0, sampling
the fractional overall sequence identity is selected for experimental
verification.
In a non-limiting example, identifying a protein not previously known to have
ligand
binding activity may comprises the following steps:
(1) performing a computational search of sequence databases to define a broad
group
of simple sequence or structural homologs of any known, ligand binding
protein;
(2) using the list from step (1), deriving a search profile containing common
sequence and/or structural motifs shared by the members of the list [e.g. by
using computer
102
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
programs such as MEME (Multiple Em for Motif Elicitation available at
meme.sdsc.edu/meme/cgi-bin/meme.cgi) or BLAST];
(3) searching sequence/structural databases, using a derived search profile
based on
the common sequence or structural motif from step (2) as query (e.g., using
computer
programs such as BLAST, or MAST (Motif Alignment Search Tool available at
meme.sdsc.edu/meme/cgi-bin/mast.cgi), and identifying a candidate sequence,
wherein a
sequence homology and/or structural similarity to a reference ligand binding
protein is a
predetermined percentage threshold;
(4) compiling a list of candidate sequences to generate a list of candidate
ligand
binding proteins;
(5) expressing the candidate ligand-binding proteins in a host organism; and
(6) testing for ligand binding activity, wherein detection of ligand binding
in the
organism (or the media thereof) indicates that the candidate sequence
comprises a novel
ligand binding protein.
In non-limiting examples, the MEME suite of sequence analysis tools
(meme.sdsc.edu/meme/cgi-bin/meme.cgi) can also be used as an alternative to
BLAST.
Sequence motifs are discovered using the program "MEME". These motifs can then
be used
to search sequence databases using the program "MAST." The BLAST search
algorithm is
well-known.
In various embodiments relating to alignments using a ClustalW aligment
program,
the ClustalW alignment program may be, e.g., ClustalW alignment program
version 2.1.
Each embodiment disclosed herein is contemplated as being applicable to each
of the
other disclosed embodiments. Thus, all combinations of the various elements
described
herein are within the scope of the invention.
Other features and advantages of the invention will be apparent from the
following
description of the preferred embodiments thereof, and from the claims. Unless
otherwise
defined, all technical and scientific terms used herein have the same meaning
as commonly
understood by one of ordinary skill in the art to which this invention
belongs. Although
methods and materials similar or equivalent to those described herein can be
used in the
practice or testing of the present invention, suitable methods and materials
are described
below.
103
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
DESCRIPTION OF THE DRAWINGS
FIGS. lA and B are cartoons showing fluorescent probes. FIG. 1A is a cartoon
relating to indirect fluorescent responses. Fluorescent biosensors can be
constructed by site-
specifically attaching a fluorophore to a protein that undergoes a
conformational change upon
binding ligand (triangle) in a location between the two lobes of the protein
(periplasmic
binding protein or engineered derivative thereof), such that the shape and
intensities of the
fluorescent conjugate emission spectra changes. FIG. 1B is a cartoon relating
to direct
fluorescent responses. Fluorescent chemosensors based on fluorophores that
interact directly
with an analyte.
FIGS. 2A-C are graphs illustrating ratiometry. If the fluorescence emission
spectrum
changes shape in response to binding of an analyte, such as glucose, then the
ratio of
emission intensities at two appropriately chosen wavelengths reports on
analyte
concentration. FIG. 2A: In the absence of ligand, the emitted fluorescence
color is
predominantly blue, whereas the ligand complex fluoresces green. Arrows
indicate the
direction of change upon ligand addition. FIG. 2B: The ligand dependence of
the absolute
blue and green intensities. FIG. 2C: The ratio of the blue and green
intensities reports
enables ligand binding to be determined.
FIGS. 3A-D are graphs and diagrams showing three dominant factors that affect
overall ligand-mediated change in fluorescence emission intensity between
donor and
acceptors in which one partner responds to ligand binding. FIG. 3A: Simplified
Jablonski
diagram illustrating radiative and non-radiative pathways in the donor and
acceptor. The
donor excited state (D*) is formed through illumination by the excitation
source (wavy
arrow) whereas the acceptor excited state (A*) is formed by resonance energy
transfer
(dashed arrow). The fluorescence intensity is determined by the ratio of
radiative decay (gray
arrows) of the excited states (gray lines) to the ground state (black line)
relative to all non-
radiative processes (black arrows), and the resonance energy transfer rate,
kt, from donor to
acceptor. FIG. 3B: Inter-dipole geometry. Top, energy transfer efficiency
( f = Qr I (Q0 ¨ Q,), where the Qr, Qo, Q , are the quantum efficiencies at
distances r, closest
approach, and infinity, respectively) varies as the 6th power of the distance
between two
dipoles. Bottom, energy transfer efficiency varies as the square of the
orientation factor K,
where K = sin D sin Acos x ¨ 2 cos OD cos OA with OD and OA the angles of
the donor (blue)
and acceptor (red) electronic transition dipoles with the line connecting
them, and x the angle
104
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
between the planes within which they lie. FIG. 3C: Spectral overlap (gray
area) between the
donor fluorescence emission (DJ, blue) and acceptor fluorescence excitation
(AA, black)
spectra. This overlap increases with bathochromic or hypsochromic shifts of
the donor
emission (red arrow) and acceptor excitation (dotted blue arrow) spectra,
respectively. Shifts
in the opposite directions decreases spectral overlap.
FIGS. 4A-C are illustrations of the construction of ngmFRET pairs by combining
a
fluorophore that responds directly to ligand binding with a non-interacting,
indirectly
responsive partner. FIG. 4A: A glucose-binding protein in which a directly
responsive
partner is positioned in the vicinity of the glucose-binding site, and the
indirectly responsive
partner is fused as the protein C-terminus. FIG. 4B: The internally positioned
fluorophore of
Yellow Fluorescent Protein responds directly chloride binding by itself and
regardless of the
presence of any other fluorophore/partner. Its monochromatic response can be
converted to a
dichromatic one by positioning a second, indirectly responsive fluorophore on
the surface of
the protein. FIG. 4C: An adaptor protein such as E. coli thioredoxin can be
used to position
an directly responsive chemosensor next to an indirectly responsive partner,
thereby
converting a monochromatic into a dichromatic signal.
FIGS. 5A-E are cartoons of fusion constructs that enable site-specific
labeling of
cysteines at two independently addressable sites with distinct, thiol-reactive
fluorophores.
FIG. 5A: In the first labeling step, an unprotected single thiol (circle)
reacts with a
fluorophore, while thiols at a second site remain protected within a disulfide
bridge. In the
second labeling step, the disulfide is deprotected by reduction, and
fluorophores are coupled
to the second site. FIG. 5B: C-terminal fusion of a PZif domain (slanted
lines) to ttGGBP
(solid gray). FIG. 5C: N-terminal fusion of PZif. FIG. 5D: C-terminal fusion
ecTRX
(horizontal lines). FIG. 5E: N-terminal fusion of ecTRX.
FIG. 6 is a structural depiction of ionization states affecting fluorescence
of the YFP
fluorophore (by itself and regardless of the presence of any other
fluorophore/partner).
FIG. 7 shows the sequence of laYFP and the locations of cysteine mutations for
the
construction of semisynthetic chloride sensors. Gray: the tripeptide that
forms the
fluorophore in the matured protein. Underlined: mutations that tune the YFP
wavelength
relative to GFP. laYFP retains the wild-type GFP residues H148 and V68 which
affect
chloride location and affinity (Wang 2015). Standard numbering is used in
which the start
methionine is 0. Structure taken from Protein Data Bank (PDB) Accession Code:
3sve.
105
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
FIGS. 8A and B are structures showing the locations of the cysteine mutations
on the
surface of laYFP. Arrows indicate (3-barrel; central a helix; fluorophore; and
chloride-
binding site (dark gray sphere). Light gray spheres indicate location of
surface cysteine
mutations (numbered according to Fig. 7). FIG. 8A: Side-view showing that the
cysteine
mutations on the surface of the barrel (3 strands form an annulus that
approximately encircles
the fluorophore in the hydrophobic core. FIG. 8B: Top view showing the
positions of all the
annulus cysteine mutations around the barrel (end mutations omitted for
clarity).
FIGS. 9A and B are graphs showing the chloride-dependent responses of the
emission
intensity spectra of representative Acrylodan and Pacific Blue YFP conjugates.
Left column,
normalized corrected emission spectra (see notes to Table 2): purple line, no
chloride; red
line, high chloride concentration; thin black lines, intermediate
concentrations. YFP emission
intensity peak is centered at 530 nm in both conjugates. Arrows indicate
direction of change
with increased chloride concentrations. Middle column, fit of ratiometric
signal (R12) to a
Langmuir binding isotherm (yields aPPKd; see notes to Table 2). Right column:
fits of
Langmuir binding isotherm to monochromatic intensity (/) signals (yields
mieKd); gray circles,
YFP intensity; black circles, Acrylodan or Pacific Blue intensity. FIG. 9A:
C1BP4=Acrylodan
(k1 = 530 nm, k2= 500 nm; aPPKd = 41 mM; mieKd=129 mM). FIG. 9B:
C1BP10=Pacific Blue
(Xi = 530 nm, k2= 455 nm; aPPKd = 260 mM; frueKd=105 mM).
FIG. 10 is an illustration of a structure showing positions for introducing
cysteine
mutations in ttGGBP to which fluorophores can be covalently coupled for
reagentless
biosensor construction. Positions 17, 91, 151, and 182 are endosteric;
positions 11, 16, 42,
67, 92, 111, 148, 152, 181, and 183 are peristeric; and positions 257, 259,
and 300 are
allosteric
FIGS. 11A-P are illustrations of fluorophore structures. Naphthalene family
(arrows
indicate known or potential internal twists): FIG. 11A shows Acrylodan; FIG.
11B shows
Badan; FIG. 11C shows IAEDANS. Xanthene family: FIG. 11D shows Fluorescein (5-
IAF
and 6-IAF); FIG. 11E shows Oregon Green; FIG. 11F shows Alexa 432; FIG. 11G
shows
Alexa 532; FIG. 11H shows Alexa 546; FIG. 11I shows Texas Red. Coumarin
family: FIG.
11J shows Pacific Blue; FIG. 11K shows CPM. benzoxadiazole family: FIG. 11L
shows
IANBD. Boradiazaindacine (BODIPY) family: FIG. 11M shows BODIPY 499/508; FIG.
11N shows BODIPY 507/545. Cyanine family: FIG. 110 shows Cy5. Miscellaneous:
FIG.
11P shows PyMPO.
106
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
FIGS. 12A and B is a pair of graphs showing donor quenching effects. FIG. 12A:
The normalized fluorescence intensity (1(2); purple: apo-protein; red, high
glucose; thin
black line: intermediate concentrations) of the singly labeled F 1 7C=Pacific
Blue conjugate
increases (blue arrow) in response to glucose binding without significant
shifts in the
wavelength of the intensity maximum. FIG. 12B: In the doubly labeled fusion
protein, the
fluorescence emission intensities (color scheme as in FIG. 12A) of the
Fl7C=Pacific Blue
directly responsive donor and the f3ZiPIAF indirectly responsive acceptor both
increase in
response to glucose binding. In the absence changes in spectral overlap, the
observed
intensity response pattern is consistent with decreases in the non-radiative
decay rate of the
Pacific Blue donor (model crgf, Table 1). The relative changes in donor and
acceptor
emissions are unequal, enabling ratiometric sensing (inset) based on the ratio
(R12) of
intensities at 456 nm (donor) and 520 nm (acceptor) so that the glucose
concentration can be
fit to a single-site Langmuir binding isotherm (equations 22 and 24); constant
baselines for
the apo-protein and ligand complex; aPPKd = 31 mM).
FIG. 13A is a cartoon and FIG. 13B is a structural illustration relating to
ratiometric
sensing using Forster resonance energy transfer between pairs of glucose-
responsive and non-
responsive fluorophores attached site-specifically in a fusion protein that
enables orthogonal
site-specific cysteine labeling. FIG. 13A: Fusion of a single cysteine ttGGBP
mutant (gray
line; two alternative cysteine positions indicated, i.e., F 17C and W182C) and
a disulfide-
containing PZif domain (disulfide indicated), separated by a linker (thin
line), enables
sequential labeling with two different fluorophores (line sizes indicate
relative size of the
fusion domains). FIG. 13B: Model showing the positions of the C-terminal PZif
fusion
relative to the experimentally determined F17C=Badan and W182C=Acrylodan
conjugates of
ttGGBP. This domain is connected to ttGGBP via a flexible linker, and is
therefore likely to
adopt an ensemble of conformations, the approximate extent which is indicated
by the oval.
FIGS. 14A-D are graphs showing acceptor dipole switching and quenching effects
in
fluorescein conjugates. FIG. 14A: W182C=5-IAF (directly responsive acceptor)
PZif=Pacific
Blue (indirectly responsive donor). Normalized emission intensities are
colored according to
glucose concentration range: purple line, apo-protein; dotted gray line,
emission at saturated
glucose level for higher affinity binding site (phase I response, see main
text); red line,
emission intensity at highest glucose concentration measured; solid black,
intermediate
glucose concentrations for phase I; dotted black lines, elevated glucose
concentrations in the
107
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
phase II response. Directions of signal change: bottom arrow, phase I; top
arrow, phase II.
Inset, Langmuir binding isotherm of ratiometic signal R 1 2 at 456 nm and 520
nm, aPPKd = 2.9
mM, constant and linear baselines for apo-protein and ligand complex
respectively. FIG.
14B: Contour plot of FIG. 14A, indicating phase I and phase II responses (see
main text).
FIG. 14C: W182C=Oregon Green (directly responsive acceptor) gif=Pacific Blue
(indirectly
responsive donor) coloring as in FIG. 14A. FIG. 14D: Contour plot of FIG. 14C.
FIGS. 15A and B are graphs showing glucose-dependent emission spectra of
Fl7C=Badan and W182C=Acrylodan conjugates of ttGGBP. Corrected spectra (apo-
protein,
dark red; saturated glucose, purple; intermediate glucose concentrations,
black). Insets, fit of
the ratiometric signal (equation 1 and 2; 20 nm integration bandwidth): gray
circles,
experimentally observed ratios; black line, calculated fit (baselines; apo-
protein, constant;
saturated glucose complex, linear). FIG. 15A: Fl7C=Badan (hypsochromic; ki,
470 nm; k2,
542 nm; aPPKd, 0.18 mM); FIG. 15B: F182C=Acrylodan (bathochromic; Xi, 475 nm;
k2, 545
nm; aPPKd, 2.2 mM).
FIGS. 16A-D are graphs showing glucose dependence of electronic transitions in
the
fluorescence emission intensity spectra of Fl7C=Badan and W182C=Acrylodan
ttGGBP
conjugates. Columns: left, singular value decomposition (SVD) of the glucose-
dependent
corrected spectra; right, Gaussian analysis of electronic transitions in the
fluorescence
emission intensity. FIGS. 16A and B: F17C=Badan; FIGS. 16C and D:
F182C=Acry1odan.
For SVD analysis, frequency transformations of the spectra (equation 30) were
decomposed
into principal components (equation 31). The contribution of the first
component, Ci (black),
is largely invariant with glucose concentration, whereas the second component,
C2 (red),
encode accounts for the glucose-dependent changes in the spectra (inserts:
glucose
dependence of the fractional contribution of each component, equation 32). In
the Gaussian
analysis, the spectral emission intensities can be accounted for to a first
approximation by
two excited state electronic transitions: a low-energy, Si (Acrylodan: 521 9
nm; Badan:
530 14 nm) and a high-energy, S2 (Acrylodan: 477 14 nm; Badan: 477 16 nm)
transition.
Glucose binding shifts the population of these excited states. At each
titration point, the
experimentally observed emission intensities were modeled with Gaussians fits
(equation 33)
for Si and S2 electronic transition. Experimental emission spectra and
Gaussian fits are
shown only for the apo-protein, and saturated glucose complex. Emission
spectra: dashed
black line, apo-protein; solid black line, glucose complex. Gaussians (Si,
lines; 52, lines):
dashed lines, apo-protein; solid lines, glucose complex. Thin black lines:
residuals (equation
108
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
35) at each titration point. Inserts show the population fractions (equation
34) of the Si and
S2 transitions extracted from the spectra at each titration point (black
circles) fit to Langmuir
binding isotherms (solid lines) with aPPKd values constrained to be the same
for both
populations. As a first approximation, the wavelengths of Si or S2 transitions
are the same in
apo-protein and the saturated glucose complex. The residuals indicate that a
more extensive
treatment is required in which the Si and S2 are split into multiple
transitions to fully fit the
spectra. Wavelength shifts occur if there is a significant redistribution of
the two excited state
populations in the apo-protein and the saturated ligand complexes. In the
bathochromic
ratiometric 182C=Acrylodan conjugate, the Si state dominates in the glucose
complex (FIG.
16D); in the hypsochromic conjugate 17C=Badan (FIG. 16B) the apo-protein
comprises a
mixture of the two states, whereas the glucose complex contains almost
exclusively the S2
state.
FIGS. 17A-D are graphs showing glucose dependence of the absorption spectra of
ttGGBP Acrylodan and Badan conjugates that undergo wavelength shifts in their
fluorescence emission intensities in response to ligand binding Columns: left,
singular value
decomposition (SVD) of the glucose-dependent corrected spectra; right,
Gaussian analysis of
electronic transitions in the absorption spectra. FIGS. 17A and B: F17C=Badan;
FIGS. 17C
and D: F182C=Acrylodan. For SVD analysis, frequency transformations of the
spectra
(equation 30) were decomposed into principal components (equation 31). Inserts
show
glucose dependence of the fractional contribution of each component (equation
32). As with
the fluorescence emission spectra (FIG. 16), in the Gaussian analysis, the
spectral emission
intensities can be accounted for to a first approximation by two ground state
electronic
transitions (dashed black line, apo-protein; solid black line, glucose
complex): Gi (386 nm)
and G2 (359 nm; apo-protein, dashed; glucose complex, solid). Inset, glucose
dependence of
population fractions at each glucose titration (black circles) of the Gi and
G2 transitions fit to
Langmuir binding isotherms (solid lines) with aPPKd values constrained to be
the same for
both populations (residuals, thin lines).
FIGS. 18A-F are a set of graphs showing donor dipole switching effects in
Acrylodan
and Badan conjugates. Four doubly labeled conjugates were constructed, in
which Acrylodan
or Badan directly responsive donors were combined with A1exa532 or 5-IAF
indirectly
responsive acceptors. FIG. 18A: F17C=Badan gif Alexa532. 1(2) , normalized
emission
intensity: purple line, apo-protein; red line, saturating glucose
concentration; thin black lines,
intermediate glucose concentrations. Blue arrow, direction of change with
increased glucose
109
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
concentration. Inset, Langmuir binding isotherm of ratio, R12, of the
intensities at 467 nm
and 560 nm (equations 23 and 24; constant baselines for apo-protein and ligand
complex;
aPPKd = 0.16 mM). FIG. 18B: F17C=Badan f3Zif5-IAF (color scheme as for FIG.
18A). Inset,
Langmuir isotherm of ratiometric signal R12 at 467 nm and 520 nm, aPPKd = 0.18
mM,
constant and linear baselines for apo-protein and ligand complex respectively.
FIG. 18C:
W182C=Acrylodan PZifAlexa532. Normalized emission intensities are colored
according to
glucose concentration range: purple line, apo-protein; dotted red line,
emission at saturated
glucose level for higher affinity binding site (phase I response, see main
text); red line,
emission intensity at highest glucose concentration measured; solid black,
intermediate
glucose concentrations for phase I; dotted black lines, elevated glucose
concentrations in the
phase II response. Directions of signal change: blue arrow, phase I; red
arrow, phase II.
Inset, Langmuir binding isotherm of ratiometric signal R12 at 480 nm and 550
nm, aPPKd = 1.7
mM, constant and linear baselines for apo-protein and ligand complex
respectively. FIG.
18D: W182C=Acrylodan f3Zif5-IAF. Coloring according to FIG. 18C. Inset,
Langmuir
binding isotherm of ratiometric signal Ri2 at 465 nm and 520 nm, aPPKd = 1.9
mM, constant
and linear baselines for apo-protein and ligand complex respectively. FIG.
18E:
W182C=Acrylodan PZifAlexa532 contour plot of the glucose dependence of
emission
intensities, indicating phase I and phase II responses (see main text). FIG.
18F:
W182C=Acry1odan f3Zif5-IAF contour plot.
FIGS. 19A and B are graphs providing a comparison of singly-labeled
ttGGBP17C=Badan (A) and the C-terminal doubly labeled ttGGBP17C=Badan-
ecTRX=Alexa532 fusion (B). Note the appearance of the approximately invariant
emission
peak (arrow) of the indirectly responsive A1exa532 acceptor.
FIGS. 20A-D are graphs showing donor dipole switching effects in
W182CIAEDANS. FIG. 20A: Singular value decomposition analysis of the change in
the
emission intensity of singly labeled W182CIAEDANS in response to glucose,
showing the
wavenumber dependence of the invariant (black line) and variant (red line)
spectral
components. Inset shows change in contribution of the two spectral components
with respect
to glucose concentration. FIG. 20B: The glucose response is accounted for
largely by two
electronic transitions (green, 542 nm; blue, 485 nm) which were fits as
Gaussians to the
experimental emission intensities (purple line, in the absence of glucose; red
line, saturating
glucose). Black lines show residuals between observed and calculated spectra.
Inset shows
1o
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
the change in the populations of the blue and green transitions in response to
glucose. FIG.
20C: W182C=IAEDANS f3Zif5-IAF. Normalized emission intensities, I(2), are
colored
according to glucose concentration range: purple line, apo-protein; dotted red
line, emission
at saturated glucose level for higher affinity binding site (phase I response,
see main text); red
line, emission intensity at highest glucose concentration measured; solid
black, intermediate
glucose concentrations for phase I; dotted black lines, elevated glucose
concentrations in the
phase II response. Directions of signal change: blue arrow, phase I; red
arrow, phase II.
Inset, Langmuir binding isotherm of ratiometic signal R12 at 465 nm and 520
nm, aPPKd =
0.09 mM, constant and linear baselines for apo-protein and ligand complex
respectively.
FIG. 20D: Contour plot of the glucose dependence of W182CIAEDANS f3Zif5-IAF
emission intensities, indicating phase I and phase II responses (see main
text).
FIG. 21 is a structural illustration of ionization equilibria of the
fluorescein
carboxylate and phenolic hydroxyl (Martin 1975).
FIG. 22 is an illustration of the structure of E. coli thioredoxin (Kati 1990)
showing
positions of mutations constructed in the adaptor proteins. Disulfide
(C32,C35) is indicated.
The D2A, D26A, and K57M background mutations constructed in all adaptor
proteins are
indicated (D2A removes adventitious N-terminal Cu(II)-binding site; D26A and
K57M
remove charges buried in the hydrophobic core). Large gray spheres: surface
lysines mutated
to arginine in Adaptor2.0a (K4Q and K1 8Q in Adaptor2.0b). Structure from PDB
accession
code 2trx.
FIG. 23 shows amino acid sequences of the engineered adaptor proteins based on
E.
coli thioredoxin. Numbering according the X-ray structure of the mature
protein, lacking the
initial methionine. Wild-type sequence is shown in full, mutations are given
below (blank
indicates wild-type residue).
FIGS. 24A-D are graphs showing the pH dependence of the emission spectra of
Adaptor1.0 conjugates. Left column: corrected fluorescence emission intensity
spectra (see
notes to Table 9) of doubly labeled conjugates (purple, pH 4.0; red, pH 9.5;
thin black lines,
intermediate values at 0.5 pH unit intervals); middle column: ratiometic
response, R12 = 11/12
(black circles), as a function of pH (gray lines, fits to Langmuir binding
isotherm give aPPpKa
values), where /1 and 12 are the intensities integrated over a 20 nm band
centered at k1 and k2;
right column: integrated fluorescence emission intensity changes, I, at the
two individual
wavelengths as a function of pH (gray lines, fits give mlepKa values). In all
cases /1 (gray
circles) corresponds to the Fluorescein integrated intensities at 520 nm (red
increases with
111
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
pH, arrows), and 12 (black circles) to 460 nm and 455 nm for Acrylodan and
Pacific Blue
(PB), respectively. FIG. 24A: Fluorescein attached to C73, and Acrylodan to
the disulfide
(aPPpKa = 6.22, mlepKa = 6.16). FIG. 24B: Fluorescein attached to the
disulfide, and
Acrylodan to C73 (aPPpKa = 6.09, mlepKa = 6.13). FIG. 24C: Fluorescein
attached to C73,
Pacific Blue to the disulfide (aPPpKa = 6.26, tuepKa = 6.22); FIG. 24D:
Fluorescein attached to
the disulfide, Pacific Blue to C73 (aPPpKa = 6.14, thlepKa = 6.17).
FIGS. 25A-D are graphs showing the pH dependence of the absorption spectra of
Adoptor1.0 conjugates. Left column: corrected absorbance, A(20, spectra (see
notes to Table
9) of doubly labeled conjugates (purple, pH 4.0; red, pH 9.5; thin black
lines, intermediate
values at 0.5 pH unit intervals); middle column: ratiometric response, R12 =
Ail A2 (black
circles), as a function of pH (gray lines, fits to Langmuir binding isotherm
give aPPpKa
values), where A1 and A2 are the absorbances integrated over a 20 nm band
centered at Xi and
X2; right column: integrated absorbance changes, A, at the two individual
wavelengths as a
function of pH (gray lines, fits give mlepKa values). In all cases A1 (gray
circles) corresponds
to the Fluorescein integrated absorbances at 495 nm (increases with pH,
arrows), and A2
(black circles) to 390 nm and 410 nm for Acrylodan and Pacific Blue (PB),
respectively
(decreases with pH, arrows). FIG. 25A: Fluorescein attached to C73, and
Acrylodan to the
disulfide (aPPpKa = 6.52, mlepKa = 6.40). FIG. 25B: Fluorescein attached to
the disulfide, and
Acrylodan to C73 (aPPpKa = 6.70, mlepKa = 6.62). FIG. 25C: Fluorescein
attached to C73,
Pacific Blue to the disulfide (aPPpKa = 6.72, tuepKa = 6.77); FIG. 25D:
Fluorescein attached to
the disulfide, Pacific Blue to C73 (aPPpKa = 6.73, thlepKa = 6.66).
FIGS. 26A and B are graphs showing the pH dependence of the fluorescence
emission
and absorption spectra of Adaptor2.0a conjugate labeled with Fluorescein at
the amino
terminus, and Acrylodan at the disulfide. Left column, corrected spectra
(purple, pH 4.0; red,
pH 9.5; thin black lines, intermediate values at 0.5 pH unit intervals).
Middle column:
ratiometric responses (black circles, ratiometric signals; gray lines, fits to
Langmuir binding
isotherm give aPPpKa values). Right column, integrated changes at the two
individual
wavelengths gray circles, k1; black circles, k2) as a function of pH (gray
lines, fits give fruepKa
values). FIG. 26A: pH dependence of fluorescence emission (Xi = 520 nm, k2 =
460 nm;
aPPpKa = 5.83, mlepKa = 5.82). FIG. 26B: pH dependence of absorption
properties (Xi = 498
nm, k2 = 375 nm; aPPpKa = 6.36, mlepKa = 6.08).
112
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
FIGS. 27A-F are graphs showing temperature dependence and pH dependence of
Adaptor1.0 and Adaptor 2.0a fluorescent conjugates measured on a Roche
LightCycler. Left
column: Adaptor1.0 R73C=Pacific Blue, disulfide=Fluorescein, fluorescence
ratio recorded at
488 nm and 510 nm; right column: Adaptor 2.0=Fluorescein, disulfide Pacific
Blue,
fluorescent ratio recorded at 488 nm and 580 nm. FIGS. 27A, B: pH- and
temperature-
dependent landscape of the fluorescence ratio (Z axis): dashed-dotted line,
approximate mid-
point concentrations (pKa) of the response to pH; dashed line, approximate mid-
point
temperatures (Tin) of thermal stability (equation 36). FIGS. 27C, D: Thermal
melt at pH 4.5,
as monitored by R12=/(488 nm)//(510 nm) emission intensity ratio: blue,
experimental data;
green, fit to two-state van 't Hoff thermal denaturation (T. ¨360K). FIGS.
27E, F:
Temperature dependence of pKa values: crosses, measured pKa values; line, fit
to Gibbs-
Helmholtz temperature dependence of the free energy all+ binding (equation
37).
FIG. 28 is a diagram relating to directly responsive partners and indirectly
responsive
partners in ngmFRET pathways.
FIG. 29 shows the sequence of an exemplary chloride-binding protein (YFP) 1
(C1BP1) expression construct (SEQ ID NO: 46).
FIG. 30 shows the sequence of an exemplary C1BP2 expression construct (SEQ ID
NO: 47).
FIG. 31 shows the sequence of an exemplary C1BP3 expression construct (SEQ ID
NO: 48).
FIG. 32 shows the sequence of an exemplary C1BP4 expression construct (SEQ ID
NO: 49).
FIG. 33 shows the sequence of an exemplary C1BP5 expression construct (SEQ ID
NO: 50).
FIG. 34 shows the sequence of an exemplary C1BP6 expression construct (SEQ ID
NO: 51).
FIG. 35 shows the sequence of an exemplary C1BP7 expression construct (SEQ ID
NO: 52).
FIG. 36 shows the sequence of an exemplary C1BP8 expression construct (SEQ ID
NO: 53).
FIG. 37 shows the sequence of an exemplary C1BP9 expression construct (SEQ ID
NO: 54).
113
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
FIG. 38 shows the sequence of an exemplary C1BP10 expression construct (SEQ ID
NO: 55).
FIG. 39 shows the sequence of an exemplary C1BP11 expression construct (SEQ ID
NO: 56).
FIG. 40 shows the sequence of an exemplary C1BP12 expression construct (SEQ ID
NO: 57).
FIG. 41 shows the sequence of an exemplary C1BP13 expression construct (SEQ ID
NO: 58).
FIG. 42 shows the sequence of an exemplary C1BP14 expression construct (SEQ ID
NO: 59).
FIG. 43 shows the sequence of an exemplary ttGGBP.11C.O.bZif expression
construct
(SEQ ID NO: 60).
FIG. 44 shows the sequence of an exemplary ttGGBP.17CØbZif expression
construct
(SEQ ID NO: 61).
FIG. 45 shows the sequence of an exemplary ttGGBP.111C.O.bZif expression
construct (SEQ ID NO: 62).
FIG. 46 shows the sequence of an exemplary ttGGBP.151CØbZif expression
construct (SEQ ID NO: 63).
FIG. 47 shows the sequence of an exemplary ttGGBP.182C.O.bZif expression
construct (SEQ ID NO: 64).
FIG. 48 shows the sequence of an exemplary ttGGBP.17C.3.Trx expression
construct
(SEQ ID NO: 65).
FIG. 49 shows the sequence of an exemplary Trx.ttGGBP.17C.3 expression
construct
(SEQ ID NO: 66).
FIG. 50 shows the sequence of an exemplary ttGGBP.182C.2.Trx expression
construct (SEQ ID NO: 67).
FIG. 51 shows the sequence of an exemplary Trx.ttGGBP.182C.2 expression
construct (SEQ ID NO: 68).
FIG. 52 shows the sequence of an exemplary Adaptor expression construct (SEQ
ID
NO: 69).
FIG. 53 shows the sequence of an exemplary Adaptor1.0 expression construct
(SEQ
ID NO: 70).
114
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
FIG. 54 shows the sequence of an exemplary Adaptor2.0a expression construct
(SEQ
ID NO: 71).
FIG. 55 shows the sequence of an exemplary Adaptor2.0b expression construct
(SEQ
ID NO: 72).
FIG. 56 shows the sequence of an exemplary Adaptor3.0 expression construct
(SEQ
ID NO: 73).
FIG. 57 shows the sequence of an exemplary Adaptor4.0 expression construct
(SEQ
ID NO: 74).
FIG. 58 shows the sequence of an exemplary Adaptor5.0 expression construct
(SEQ
lD NO: 75).
FIG. 59 shows the sequence of an exemplary Adaptor6.0 expression construct
(SEQ
1D NO: 76).
FIG. 60 shows the sequence of an exemplary Adaptor7.0 expression construct
(SEQ
1D NO: 77).
FIG. 61 shows the sequence of an exemplary Adaptor8.0 expression construct
(SEQ
1D NO: 78).
FIG. 62 shows the sequence of an exemplary Adaptor9.0 expression construct
(SEQ
1D NO: 79).
FIG. 63 shows the sequence of an exemplary Adaptorl 0.0 expression construct
(SEQ
lD NO: 80).
FIG. 64 shows the sequence of an exemplary Adaptorl 1.0 expression construct
(SEQ
1D NO: 81).
FIG. 65 shows the sequence of an exemplary Adaptorl 2.0 expression construct
(SEQ
1D NO: 82).
FIG. 66 shows the sequence of an exemplary Adaptor13.0 expression construct
(SEQ
1D NO: 83).
FIG. 67 shows the sequence of an exemplary Adaptor14.0 expression construct
(SEQ
1D NO: 84).
FIG. 68 shows the sequence of an exemplary Adaptorl 5.0 expression construct
(SEQ
lD NO: 85).
FIG. 69 shows the sequence of an exemplary Adaptorl 6.0 expression construct
(SEQ
1D NO: 86).
115
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
DETAILED DESCRIPTION
Biosensors are analytical tools that can be used to measure the presence of a
single
molecular species in a complex mixture by combining the exquisite molecular
recognition
properties of biological macromolecules with signal transduction mechanisms
that couple
ligand binding to readily detectable physical changes (Hall, Biosensors,
Prentice-Hall,
Englewood Cliffs, N.J.; Scheller et al., Curr. Op. Biotech. 12:35-40, 2001).
Ideally, a
biosensor is reagentless and, in contrast to enzyme-based assays or
competitive
immunoassays, does not change composition as a consequence of making the
measurement
(Hellinga & Marvin, Trends Biotech. 16:183-189, 1998). Most biosensors combine
a
naturally occurring macromolecule such as an enzyme or an antibody, with the
identification
of a suitable physical signal particular to the molecule in question, and the
construction of a
detector specific to that system (Meadows, Adv. Drug Deliv. Rev. 21:177-189,
1996).
Recently, molecular engineering techniques have been explored to develop
macromolecules
that combine a wide range of binding specificities and affinities with a
common signal
transduction mechanism, to construct a generic detection system for many
different analytes
(Hellinga & Marvin, Trends Biotech. 16:183-189, 1998).
Escherichia coli periplasmic binding proteins are members of a protein
superfamily
(bacterial periplasmic binding proteins, bPBPs) (Tam & Saier, Microbiol. Rev.
57:320-346,
1993). These proteins comprise two domains linked by a hinge region (Quiocho &
Ledvina,
Molec. Microbiol. 20:17-25, 1996). The ligand-binding site is located at the
interface
between the two domains. The proteins typically adopt two conformations: a
ligand-free
open form, and a ligand-bound closed form, which interconvert via a hinge-
bending
mechanism upon ligand binding. This global, ligand-mediated conformational
change has
been exploited to couple ligand binding to changes in fluorescence intensity
by positioning
single, environmentally sensitive fluorophores in locations that undergo local
conformational
changes in concert with the global change (Brune et al., Biochemistry 33:8262-
8271, 1994;
Gilardi et al., Prot. Eng. 10:479-486, 1997; Gilardi et al., Anal. Chem.
66:3840-3847, 1994;
Marvin et al., Proc. Natl. Acad. Sci. USA 94:4366-4371, 1997, Marvin and
Hellinga, J. Am.
Chem. Soc. 120:7-11, 1998; Tolosa et al., Anal. Biochem. 267:114-120, 1999;
Dattelbaum &
Lakowicz, Anal. Biochem. 291:89-95, 2001; Marvin & Hellinga, Proc. Natl. Acad.
Sci. USA
98:4955-4960, 2001; Salins et al., Anal. Biochem. 294:19-26, 2001).
Provided herein are improved biosensors that rapidly, reliably, and accurately
detect
and quantify ligands with significant advantages over previous systems. The
present
116
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
disclosure provides a biosensor for ligand, comprising a ligand-binding
protein that is
attached to one or more reporter group (e.g., 1, 2, 3, or more reporter
groups). The binding of
a ligand to the ligand-binding domain of the ligand-binding protein causes a
change in
signaling by the biosensor. In various implementations, the biosensor may
produce a signal
when a ligand is bound to the ligand binding domain that is not produced
(and/or that is
different from a signal that is produced) when the ligand is absent from the
ligand binding
domain. These biosensors have widespread utility including in clinical,
industrial, and
environmental settings.
Various biosensors provided herein produce a dichromatic, ratiometric signal,
i.e., the
signal is defined as the quotient of the intensities at two independent
wavelengths. The
advantage of such a signal is that it provides an internally consistent
reference. The self-
calibrating nature of a ratiometric measurement removes the necessity for
carrying out on-
board calibration tests prior to each measurement. The biosensors are
reagentless in that their
monitoring mechanism requires neither an enzyme nor additional substrates for
a signal to
develop, nor measurement of substrate consumption or product generation rates
to determine
ligand concentrations.
Reagentless, fluorescently responsive biosensors present a number of
advantages over
enzyme-based biosensors, including elimination of chemical transformations,
elimination of
substrate requirements, and self-calibration, which together lead to rapid
response times,
continuous monitoring capabilities, simple sample-handling, and lower cost due
to simplified
manufacturing and distribution processes.
ngmFRET for Ratiometric Measurements Using Reagentless Analyte Sensors
Determination of analyte concentrations using fluorescent probes is a powerful
technique in analytical chemistry (FIGS. lA and B). Fluorescent chemosensors
based on
small-molecule fluorophores that interact directly with an analyte (Zhang, Yin
and Yoon
2014, Lavis and Raines 2008, Lavis and Raines 2014) and fluorescent biosensors
based on
engineered proteins that couple analyte-binding events to changes in the
emission properties
of fluorophores (being fluorescent by themselves and regardless of the
presence of any other
fluorophore/partner) (Okumoto 2012) or semi-synthetically (Wang 2009)
incorporated
fluorophores have wide-ranging applications in cell biology and analytical
chemistry(Borisov
and Wolfbeis 2008, Liu 2015, Matzeu 2015, Heo and Takeuchi 2013). If the
fluorescence
emission spectrum changes shape in response to analyte binding such that the
ratio of
117
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
emission intensities at two appropriately chosen wavelengths reports on
analyte concentration
(dichromatic response), then ratiometric measurements can be used to monitor
analyte
concentrations (FIGS. 2A-C). Ratiometry is essential for devices that rely on
quantifying
changes in fluorescence emission intensities, because it provides an
internally consistent
reference (Demchenko 2010, Demchenko 2014). The self-calibrating nature of a
ratiometric
measurement removes the necessity for carrying out on-board calibration tests
prior to each
measurement (Choleau et al. 2002), obviating the need for multiple components
and fluidic
circuitry. Accordingly, reagentless, ratiometric fluorescent sensors have many
uses in
process engineering, environmental or clinical chemistry, including single-use
point-of-care
applications (Kozma et al. 2013, Ahmed et al. 2014, Mohammed 2011, Ispas 2012,
Rogers
and Boutelle 2013, Robinson and Dittrich 2013, Arora et al. 2010, Gubala et
al. 2012),
wearable devices (Badugu, Lakowicz and Geddes 2005), optodes for continuous
monitoring
(Weidemaier et al. 2011, Judge et al. 2011), or implanted "tattoos" that are
interrogated
transdermally (Bandodkar et al. 2015).
The majority of fluorescent chemosensors and biosensors do not undergo changes
in
emission spectral shape upon analyte binding and accordingly evince
monochromatic
intensity changes, rather than the dichromatic responses required for
ratiometric sensing (de
Lorimier et al. 2002). The present subject matter provides methods for
converting
monochromatic responses into dichromatic responses that enable ratiometric
sensing. In
embodiments, these methods are based on establishing non-geometrically
modulated Forster
Resonance Energy Transfer (ngmFRET) between the monochromatic fluorophore
(directly
responsive partner), and a second fluorophore that neither interacts directly
with the ligand,
nor is sensitive to ligand-mediated changes in its environment (indirectly
responsive partner).
Unlike tgmFRET-based chemical sensing systems (Valeur 2012), this arrangement
does not
rely on analyte-mediated geometrical changes (inter-fluorophore distance or
angle) between
the donor and acceptor, but instead exploits effects by analyte binding, which
alter the
photophysics of only the directly responsive partner such as changes in its
spectral properties
and non-radiative decay rates (FIG. 3).
The exemplary and non-limiting studies described herein demonstrate how these
ngmFRET effects were used to convert monochromatic into dichromatic responses
and
thereby improve the ratiometric properties of dichromatic responses, using
three classes of
examples that illustrate the application of this technique both to biosensors
and chemosensors
(FIG. 4):
118
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
1. The analyte recognition element is a protein that undergoes an analyte-
mediated
conformational change that is alters the properties of an environmentally
responsive directly responsive partner (FIG. 4A): a glucose-binding protein in
which a conjugated directly responsive fluorophore respond via a glucose-
induced
protein conformational change that alters its emission properties. This
fluorophore is paired with a second, indirectly responsive partner attached to
a
fusion domain (such as a fluorophore attachment motif attached to, e.g., the
first
or the last amino acid of the ligand-binding protein). The resulting ngmFRET
established between the two partners converts a monochromatic response of the
directly responsive partner into a dichromatic response, or improves its
dichromatic, ratiometric properties.
2. The analyte recognition element is a rigid protein with an analyte-binding
site
located adjacent to an fluorophore (having fluorescence by itself and
regardless of
the presence of any other fluorophore/partner; FIG. 4B): the monochromatic
response of the fluorophore to chloride ion binding in a yellow fluorescent
protein
is converted to a dichromatic response using a indirectly responsive extrinsic
fluorophore site-specifically attached to the protein surface.
3. The analyte recognition element is a synthetic chemoresponsive fluorophore
(FIG.
4C): an adaptor protein is engineered to establish ngmFRET between two, site-
specifically attached extrinsic fluorophores. The monochromatic response of
the
directly responsive partner to proton binding is converted into a dichromatic
signal.
The first example represents a large class of protein-based fluorescent
biosensors
which undergo ligand-mediated conformational changes that alter the local
environment of an
attached fluorophore. Such conformational changes are found in many proteins;
coupling
these to fluorescent responses therefore provides a rich source for
engineering fluorescent
biosensors. For instance, the glucose-binding protein used in this example is
a member of the
bacterial periplasmic-binding protein (PBP) superfamily which combines a large
diversity of
ligand specifities with a common structural mechanism (Bemtsson et al. 2010)
that is well
suited to the construction of fluorescent sensors (de Lorimier et al. 2002,
Grunewald 2014).
However, in these proteins, engineered fluorescent responses are more commonly
monochromatic than dichromatic. This first example (FIG. 4A) therefore
demonstrates how
119
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
ngmFRET can be used to improve the success rate for engineering ratiometric
biosensors that
exploit ligand-mediated protein motions. Non-limiting examples of ligand-
binding proteins
include proteins that bind sugars (such galactose-binding proteins, lactose-
binding proteins,
arabinose-binding proteins, ribose-binding proteins, and maltose-binding
proteins), urea-
binding proteins, bicarbonate-binding proteins, phosphate-binding proteins,
sulfate-binding
proteins, calcium-binding proteins, dipeptide-binding proteins, amino acid-
binding proteins
(such as histidine-binding proteins, glutamine-binding proteins, glutamate-
binding proteins,
and aspartate-binding proteins), and iron-binding proteins.
The second example (FIG. 4B) represents a smaller set of proteins that contain
fluorophores formed by a self-catalyzed cyclization of a peptide within their
sequences such
as Green Fluorescent Protein, its engineered variants and homologs(Tsien 1998,
Zimmer
2002). Some of these fluorophores function as direct natural chemosensors by
interacting
with ligands such as protons and halides (Miesenbock, De Angelis and Rothman
1998,
Grimley et al. 2013), but typically evince only monochromatic responses. The
example
demonstrates how ratiometic sensing mechanisms can be engineered into these
proteins.
The third example (FIG. 4C) illustrates how protein engineering was used to
improve
the properties of synthetic directly responsive chemosensors, by incorporating
them as
extrinsic fluorophores into a protein and combining with a second fluorophore
to introduce
ngmFRET. In this example, the protein therefore functions as an "adaptor"
scaffold/compound that enables facile integration of multiple functionalities.
Although many
small-molecule fluorescent chemosensors have been developed that measure a
wide variety
of ligands, prior to the invention it was challenging to engineer dichromatic
responses. The
use of an adaptor protein provides a facile route to integrate multiple
fluorophores to generate
a dichromatic sensor construct, enabling the power of monochromatic
chemosensors to be
harnessed and optimized.
In the third example, a chemoresponsive (directly responsive) fluorophore may
be
linked to another fluorophore (an indirectly responsive fluorophore) using
virtually any
polypeptide sequence. Though a pH-sensitive chemoresponsive fluorophore is
exemplified
in the examples, any other chemoresponsive fluorophore may be used. The
principles
demonstrated for converting the monochromatic response of fluorescein to
protons into a
dichromatic signal by incorporation into a dually labeled adaptor protein can
be extended to
other chemoresponsive fluorophores for the detection of a wide variety of
analytes. A well-
known thiol- or amine-reactive functional group (imidoesters, NHS esters,
carbodiimides,
120
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
maleides, aziridines, arcyloyls; see, e.g., G.T. Hermanson, 2013, Bionjugate
Techniques,
Academic Press, incorporated herein by reference) may be incorporated into the
chemoresponsive fluorophore such that the chemoresponsive fluorophore can be
coupled to
the adaptor protein (M.S.T. Goncalves, 2009, Chem. Rev., 190-212).
Chemoresponsive
sensors can bind specifically to small molecules such as ions,
monosaccharides, amino acids,
and short peptides, using a variety of molecular recognition units. Such units
are then linked
to a fluorescent group, the properties of which are altered upon binding the
ligand (A. P.
Demchenko, 2015, Introduction to Fluorescence Sensing, Springer). A variety of
schemes
can be used to couple proton-binding groups such as amines to fluorescence
responses (op.
cit.). Fluoresccent chemosensors have been developed for toxic metals such as
lead,
cadmium, and mercury (K.P. Carter et al., 2014, Chem. Rev., 114, 4564-4601).
Chemoresponsive fluorophores have been developed for glucose (X.S. Sun, T.D.
James,
2015, Chem. Rev., 115, 8001-8037), and other organic analytes including
amines, urea, and
guanidinium (T.W. Bell and N.M. Hext, 2004, Chem. Soc. Rev., 33, 589-598).
The fluorescent glucose sensor described in the first example has utility in
glucose
monitoring is essential for the management of diabetes mellitus, a disease
that affects at least
366 million people world-wide(Yoo and Lee 2010, Cash and Clark 2010) and is
increasing
every year. The majority of current glucose-monitoring technologies rely on
enzymes for
which glucose is one of the substrates(Wang 2008, Bergel, Souppe and Comtat
1989).
Glucose concentration measurements therefore are subject to variations in
second substrate
concentrations consumed in the enzyme reaction, such as oxygen in the case of
glucose
oxidase(Tang et al. 2001). Additional complications arise in systems where
reaction rates are
measured for enzymes immobilized on electrodes. In such arrangements, accuracy
is
compromised by factors that alter the rate at which glucose arrives at the
electrode surface
interfere with accuracy, such as hematocrit levels(Karon et al. 2008, Tang et
al. 2000), or
surface "fouling" by deposition of proteins and cells in the foreign body
response(Koschwanez and Reichert 2007, Gifford et al. 2006, Wisniewski and
Reichert
2000). Ratiometic fluorescent glucose sensors obviate these problems, and
accordingly have
been incorporated successfully in optodes for continuous glucose monitoring in
animals and
humans.
Determination of the concentration of chloride, an essential electrolyte, is a
routine
measurement in clinical chemistry carried out using potentiometric or optical
methods(Huber
2001), and has applications to environmental chemistry(Huber 2000) and
corrosion
121
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
control(de Graaf 2015) as well. Development of a fluorescent, ratiometric
chloride
biosensor, such as described in the second example enables chloride sensing to
be
incorporated into point-of-care devices and continuous monitoring systems.
The determination of proton concentrations is necessary for a broad range of
applications, ranging from clinical chemistry to environmental science.
Accordingly, the
development of fluorescent and other optical probes is an active area(Han
2010, Wencel
2014).
Together these examples demonstrate that the ngmFRET mechanism is applicable
to
the engineering of a wide variety of semi-synthetic protein-based fluorescent
biosensors by
combining properties unique to proteins with those of synthetic fluorophores.
Akin to natural
cofactors, the fluorophores endow the proteins with functions that cannot be
encoded in
amino acids, and the proteins modulate the properties of the fluorophores. As
illustrated by
the analytes chosen in the examples, such engineered fluorescent biosensors
have many
potential applications, including medical diagnostics.
Mechanisms for Ligand Sensing using Non-Geometric Modulation of FRET
The subject matter disclosed herein is not limited to or bound by any
particular
scientific theory. However, the discussion below is provided to facilitate the
understanding
of possible mechanisms involved with ngmFRET signaling in various embodiments
described herein. Equations for calculating various values mentioned herein
are also
provided.
The total signal, S, of a fluorescent sensor (either single-wavelength
emission
intensities, IA, or ratios of intensities at two wavelengths, R12) is the sum
of the fluorescence
due to the ligand-free (apo) and ligand-bound states:
S = 4- )+ g 1
where a and ig are the fluorescent baselines in the ligand-free and -bound
states, respectively,
and is the fractional occupancy of the binding sites. For a single binding
site is given by
[L]
y 2
- = r 1
Lid + K d
where [L] is the ligand (analyte) concentration and Kd the dissociation
constant corresponding
to an apparent, aPPKd, or true, "e1Cd, value for fits to R12 and IA
respectively.
122
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Fluorescence quantum yields are the fractions of photons emitted by the
excited state
relative to the total absorbed, and correspond to the ratio of the radiative
decay rate relative to
the sum of the rates of all possible decay pathways (FIGS. 3A-D). For a single
fluorophore:
kr
Q= kr ___ + kn, 3
where kr and km are the radiative and non-radiative decay rates of the excited
state,
respectively. If we define q as the ratio between the radiative and non-
radiative decay rates,
km
q =. ¨ 4
Icr
then the quantum yield can be written as
Q 1
= 5
q+1
Chemical sensors exploit the ligand-mediated shift of a fluorescent system
between
the ligand-free and ligand-bound states which each exhibit distinct quantum
yields:
Qobs = Qapo(1¨ .0+ Qsatii 6
where Qobs, Qapo and Qsat are the quantum yield of the total system, the apo-
protein, and the
ligand-bound complex, respectively. In a system involving energy transfer
between a donor
and acceptor fluorophore, the Qapo and Qsat quantum yields each are
combinations of their
respective donor and acceptor quantum yields:
Qapo=D Qapo+A Qapo and 0
---sat=D Qsat+AQsat 7
where the superscripts D and A indicate donor and acceptor fluorophores
respectively. To
understand ngmFRET-based sensors, we therefore need to examine the factors
that affect
each of these four quantum yields.
The intensity of the light emitted by a donor or its acceptor is determined by
the rate
of photon emission from their respective excited states (FIG. 3A). The excited
state of a
donor is formed by the incident light from the excitation source, and there
are three pathways
by which this state decays: radiative and non-radiative decay and resonance
transfer (by itself
and regardless of the presence of any other fluorophore/parter). By contrast,
the rate of
formation of the acceptor excited state is determined by the resonance
transfer rate from the
donor, and there are only two processes that determine its decay rate: the
radiative and non-
radiative pathways (by itself and regardless of the presence of any other
fluorophore/parter).
In an ngmFRET system, the patterns of ligand-mediated fluorescence intensity
changes
123
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
therefore depend on whether the fluorophore that responds directly to ligand
binding
functions as a donor or acceptor. To understand these relationships, we
analyzed the factors
that determine the rates of formation and decay of the donor and acceptor
excited states.
The rate of resonance energy transfer, kt, along a non-radiative pathway
between
donor and acceptor (FIG. 3A) is a fraction of the donor radiative emission
pathway rate (by
itself and regardless of the presence of any other fluorophore/parter), D kr
(the emission rate in
the absence of an acceptor) multiplied by the energy transfer coupling factor,
0, (Lakowicz
2006, Valeur 2012):
kf=coQDDkr 8
where QD is the donor quantum yield in the absence of an acceptor.
According to the Forster model of weakly coupled oscillators (Lakowicz 2006,
Valeur
2012), the energy transfer coupling factor is dependent on the spectral
overlap, J, of the donor
emission, Dile,õ and acceptor excitation spectrum, A2,,,, and the variation of
the geometry, G,
between the donor and acceptor excited state transition dipoles with distance,
r, and
orientation factor, K:
\ 90001n10
co = GO - ,K)J(D /Ie., A
/ler ) 9
1287r5N An 4
where
K2
GO - ,K)= 10
r
and
J(D2em,A2)=.1.F(D2eni(A2),14d2 11
with n the refractive index of medium, NA Avogrado's number, fi(Dkem) the
normalized
donor emission spectrum, and c(A2,x) the absorption coefficient of the
acceptor excitation
spectrum [this analysis is a re-arrangement of the traditional presentation of
the equations
describing tgmFRET, separating the different contributions (geometry, spectral
overlap,
quenching)]. Ligand-mediated modulation of r, K and J therefore affects kt
(Fig. 3b-d),
leading to changes in donor and acceptor emission intensities (see below).
At steady state, the concentration of the donor excited state, [D*], is given
by the
following rate balance equation (see Fig. 3a):
Nook ¨ [D* IDIcnr+Dkr + Icf) = 0 12
124
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
where No is the population of ground state fluorophores, kex the rate of
excitation photon
absorption, a the effective illumination, kt, the resonance energy transfer
rate, Dknr and Dkr
the radiative and non-radiative decay rates of the donor (by itself and
regardless of the
presence of any other fluorophore/parter) in the absence of acceptor,
respectively.
Substituting Dkr(d +1) for Dkr+Dknr (using equation 4, with dq, the ratio of
non-
radiative to radiative decay rates in the donor), and replacing kt with
equation 8 (with
QD =0+ d), according to equation 5), we obtain
Nook, ¨[Dtkr (1+ d + ___ 9 1+ dj= 0 13
Hence
[D]= N 0akex 14
Dkr(l+d+ 9 j
1+d
The intensity of the emitted donor light, ID, is
I D 4114 N7. = 0ak
ex 15
1+d
The donor quantum yield, QD, is this emission intensity relative to the
intensity of the
excitation, kexallo
1
QD =/ 16
(1+ d + 9 )
1+d
The rate balance equation for the acceptor excited state concentration, [A*],
is given
by
[D*], _ [A * jAkr Akrir ) 17
Consequently, by applying equations 5, 8 and 16, the acceptor quantum yield,
QA, is
QA= 9 18
(1+41+41+d+ 9 j
1+d
where a is the ratio of the radiative and non-radiative pathways in the
acceptor.
The ratio of the acceptor and donor quantum yields therefore is
QA = 9 19
QB (1+ d)(1 a)
125
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
This equation clearly shows that a ligand-mediated change in energy transfer
(0) or a change
in the ratio of radiative to non-radiative emission rates of either the donor
(d) or acceptor (a)
leads to a change in the ratio of donor and acceptor emission intensities,
thereby enabling
ratiometry.
Classical ligand-mediated modulation of tgmFRET (traditional geometric FRET -
) is
concerned only with ligand-mediated changes in the distance between the donor
and acceptor
(Clegg 1995, Cheung 1991), and does not take advantage of effects that alter
the
photophysics of individual fluorophores. By contrast, in ngmFRET systems, the
directly
responsive partner (DRP) responds to ligand binding through ligand mediated
changes that
alter the ratio of its radiative and non-radiative pathways (quenching, d or
a) or its spectral
properties (J), whereas the indirectly responsive partner (LRP) changes only
as a consequence
of the effect that such change have on the resonance energy transfer rate
(kt). It is important
to realize that the DRP can function either as a ngmFRET donor an acceptor,
depending on
how the spectral overlap is set up with the IRP. Regardless of whether the DRP
is a donor or
acceptor, ligand-mediated alteration of its non-radiative to radiative decay
rate ratio
(parameter d for a DRP donor; a for an acceptor; by itself and regardless of
the presence of
any other fluorophore/parter) changes its emission intensity. In DRP donors
quenching also
alters the ngmFRET transfer rate (see equations 8 and 13), thereby changing
the emission
intensities of not only itself but also its IRP. By contrast, in DPR acceptors
quenching does
not alter ngmFRET, and hence do not affect its lRP donor intensity. A DRP
acceptor
therefore can alter intensities of its donor IRP only if ligand binding
changes 0. If the DRP is
a donor, then manipulation of the ngmFRET coupling factor, 0, changes the rate
of excited
state decay; if it is an acceptor, the rate of excited state formation is
altered.
Regardless of whether the DRP is a donor or acceptor, a change in any of the
two
parameters (0 and d or a) alters the ratio of the donor and acceptor quantum
yields (equation
19), thereby enabling ratiometry. Ligand-mediated donor DRP quenching affects
the
quantum yields of both the donor, QD, and acceptor, QA, quantum yields
(equations 16, 18).
Quenching of an acceptor DRP alters only QA (equation 16). Changes in 0 affect
quantum
yields of both fluorophores, regardless whether the DRP functions as the donor
or acceptor
(equations 9-11, 16, 18). For systems in which there is no ligand-mediated
change in the
(average) distance between the two fluorophores, 0 changes only if the DRP
switches
between two different excited state populations ("dipole switching") in
response to ligand
126
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
binding and if the two excited states differ in their spectral properties
(emission for donor
DRPs; absorption for acceptor DRPs). Excited state dipoles usually also differ
in their dipole
orientations, so it is likely that changes in spectral overlap involve (re-
)orientation effects.
They are also likely to differ in the relative rates of their radiative and
non-radiative decay
rates. Dipole switching therefore is likely to involve a combination of
changes in ngmFRET
and quenching effects.
There are eight possible combinations of ligand-mediated changes in quenching
and
ngmFRET parameters, which have different outcomes on the two emission
intensities and
their ratio, depending on whether the DRP is the donor or acceptor. The
qualitative behavior
of the resulting sixteen possibilities in ngmFRET systems are shown in Table
1. Twelve of
these have a predictable outcome on the direction of change in the ratio of
the two emission
intensities. The effect on the direction of change for both donor and acceptor
emission
intensities can be predicted for seven models. For the other models, the
direction of change
of one or both peaks depends on the size of the change in the underlying
parameters. Purely
geometric effects (changes in inter-dipole distance or orientation) always
result in anti-
correlated changes in emission intensity changes (i.e. one increases and the
other decreases,
or vice versa). Correlated (i.e. both intensities increase or decrease) or
uncorrelated (one
changes, the other remains constant) intensity changes therefore are prima
facie evidence for
an ngmFRET effect.
25
127
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Table 1. Qualitative analysis of the patterns of donor and acceptor emission
intensity
changes in ngmFRETa
Directly responsive partner Model QA/QD QD QA
Donor 4+ 1' .1, 1'
d 0- .1, 1' .1,
ctO
d+0+ .1, *
6' 0" sl, * 4,
d 0 T T T
do+ T * ,i,
d 0" "r *
Acceptor a 0+ 1' si, *
a 0" 4, 1' *
a+0 si, 0 si,
a+ 0+ *
a+ si, 1' *
6/0 1' 0 1'
a", 1' .1, 1'
a" 0" 1' *
aThe effects of increasing or decreasing quenching in the directly responsive
ngmFRET
partner (d for donors, a for acceptors) or the energy transfer coupling (0)
between the donor
and acceptor are tabulated. The consequences of using a directly responsive
donor or
acceptor are examined. Changes in quenching and energy transfer coupling
parameters can
occur singly or in combination, leading to 16 possible models. The models
examine the
effects of the direction of change in quenching parameters (no change, d or
a0; increase di- or
a+; decrease, d or d) and the energy transfer coupling factor (no change, 05 ;
increase, 0+;
decrease, 0") on the patterns in the direction of change of the donor, QD
(equation 16) or
acceptor, QA (equation 18) quantum yields, and their ratio, QA/QD (equation
19): 1', increase;
, decrease; 0, no change; *, response is dependent on precise quantitation
rather than
direction of change in the underlying parameter values.
Exemplary Dual Labeling Techniques to Construct Donor-Acceptor Pairs
The directly responsive fluorophore needs to be site-specifically attached to
a site in
the protein where it can respond to the ligand-binding event (the one
exception is the case
where the directly responsive fluorophore is integral to the protein itself,
as is the case for
YFP). Site-specific attachment of the second, passive fluorophore also is
desirable, because
it establishes a unique ngmFRET interaction, whereas random attachment gives
rise to
multiple interactions, only some of which are likely to be result in usable
signals with the
others contributing to background.
128
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Cysteine thiols provide a convenient, chemically unique functionality for site-
specific
attachments at positions defined by the protein sequence. Furthermore,
straightforward,
reversible protection strategies have been developed that enable multiple
thiols to be labeled
orthogonally at independently addressable sites in successive reactions (Smith
et al. 2005).
In this scheme, two labeling sites can be engineered using fusions that
combine two proteins
or domains containing a single thiol and disulfide bridge, respectively (FIG.
5). In such
constructs, labeling proceeds in two steps. First, the unprotected thiol is
modified with the
first fluorophore under conditions in which the disulfide bridge is fully
formed protecting the
second thiols. After this labeling step is complete, unincorporated first
fluorophore is
removed from the reaction. In the second labeling step the thiols in the
disulfide bridge are
deprotected by reduction, enabling attachment of the ngmFRET partner
fluorophore at their
positions.
Typically, the single cysteine is located in a protein that binds the analyte
of interest
(e.g. the periplasmic glucose-binding protein) for coupling of the directly
responsive
fluorophore, and the disulfide is located in a small domain or protein fused
at the N- or C-
terminus for attachment of the indirectly responsive ngmFRET partner. In the
examples
shown here we have used an 17-residue peptide, PZif, derived from a zinc
finger
protein(Smith et al. 2005) as the disulfide-containing domain. The disulfide-
containing
protein thioredoxin from Escherichia co/i(Holmgren 1985, Qi 2005, Katti 1990),
ecTRX, was
also used as a fusion partner. The ecTRX also has been used to construct the
adaptor protein
by installing a single cysteine mutation that introduces an unprotected thiol
on the surface of
the protein (see below).
Thiols are not the only means to establish orthogonal chemistries. For
example,
amines also have a highly selective chemistry. To demonstrate this approach,
an unusual
version of ecTRX was created in which the amino acid alphabet was limited to
19 residues,
eliminating all lysines. This leaves only one reactive primary amine in the
protein: the amino
terminus. In this manner, constructed a doubly labeled protein by adding the
first label to the
amino terminus, and the second to the reduced disulfide bridge. A label may be
conjugated
to ecTRX using a variety of linkers or bonds, including (but not limited to) a
disulfide bond,
an ester bond, a thioester bond, an amide bond, or a bond that has been formed
by a click
reaction.
129
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Biosensors
Biosensors are molecular recognition elements that transduce ligand-binding
events
into physical detectable signals. Biosensors as detailed herein bind at least
one ligand and
emit a signal. A ligand-bound biosensor results in a signal that is different
from the unbound
biosensor. This difference facilitates detection of the at least one ligand
and/or determination
of ligand concentration. The biosensors may be used alone, i.e., without the
presence or
assistance of other reagents.
Described herein are reagentless biosensors engineered to produce a detectable
ratiometric detection signal. These biosensors may have altered ligand-binding
affinities,
tailored ligand-binding specificities, and/or temperature dependencies of
ligand binding or
stability compared to corresponding naturally occurring ligand-binding
proteins. For
example, the herein described engineered ligand biosensors provide high-
accuracy
information related to extended ligand concentration ranges.
Binding of ligand mediates conformational changes in the biosensor, such as
hinge-bending motions of the polypeptide. The conformational changes affect
the
environment of the reporter such that a change in the reporter-generated
signal occurs. That
is, without ligand bound, the biosensor results in signal generated from the
reporter, and
when ligand is bound, the signal generated from the reporter changes. The
ligand-bound
biosensor results in a reporter-generated signal that is different from the
unbound biosensor.
Among the advantages of these fluorophone-containing protein constructs is
their
high durability. The constructs retain their ability to bind ligand, change
shape and thus
detect the ligand (a) even when immobilized (directly or indirectly) onto a
solid surface such
as a bead, plate, or sheet; (b) even after desiccation (and subsequent
reconstitution in a
physiological buffer solution); (c) even when subjected to ambient conditions,
e.g.,
conditions that can be encountered in storage and/or transportation; and (d)
even when
aged/stored for extended periods of time, e.g., weeks, months, or even years.
Thus, the
biosensors do not require refrigeration or a cold chain for distribution,
permitting a wider
range of applicability such as in-the-field use and reducing the cost of the
sensor product.
For clinical applications, microliter volumes, e.g., 10,9, 8, 7, 6, 5, 4, 3,
2, 1, 1.1.1 or less
of a bodily fluid may be used. In some embodiments, the volume of sample that
is applied to
a biosensor or a device comprising a biosensor is less than 0.1, 0.5, 1, 2, 3,
4, 5, 6, 7, 8, 9, 10,
25, 50, 75, 100, 150, 300, 500, or 1000 pl. In some embodiments, the volume is
about 0.1 1.11
to about 1000 Ill, about 0.1 1.11 to about 100 Ill, about 1 1.11 to about 1000
Ill, about 1 p,1 to
130
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
about 10 Ill, about 1 1.11 to about 100 0, about 1 1.11 to about 50 ta, about
10 tato about 50 0,
or about 5 ta to about 50 0. Moreover compared to conventional enzyme-based or
antibody
based assay systems, the results are achieved virtually instantaneously, e.g.,
within 30-60
seconds. A further advantage is that the sensors consistently and reliably
bind to and detect
the analyte in complex fluids such as whole blood. Thus in a clinical setting,
whole blood
need not be processed, thereby reducing time and cost of the diagnostic
procedure.
In non-clinical situations, e.g., industrial of commercial settings such as
analysis of
waste water, food or beverage production, or bioreactor/fermentation
monitoring, the samples
to be analyzed can be used directly upon sampling without further purification
or processing,
similarly reducing time and expense of the test. Moreover, the immobilized
sensors need not
be washed to remove unbound material following contacting the test sample with
the sensors,
because the unbound material ("contaminants") do not materially affect the
production of a
precise, reliable detectable assay signal.
In some embodiments, the methods and compositions include a plurality of a
single type of biosensor. The biosensors may be identical in structure and
function. For
example, the biosensors of a single type may have the same polypeptide, the
same reporter,
and the same ligand affinity.
In other embodiments, the methods and compositions include a plurality of
different types of biosensors. A plurality of these different types of
biosensors may be
arranged or incorporated in a panel. As used herein, a "panel" refers to two
or more
biosensors. The two or more biosensors may be different from each other. The
biosensors
may differ in structure and/or function. Biosensors may differ in polypeptide
sequence,
reporter, ligand affinities, or a combination thereof. Accordingly, there may
be different types
of biosensors. In some embodiments, each biosensor in the panel comprises the
same reporter
group. In some embodiments, each biosensor in the panel comprises a different
reporter
group. The panel may include at least 2, at least 3, at least 4, at least 5,
at least 6, at least 7, at
least 8, at least 9, at least 10, at least 11, at least 12, at least 13, at
least 14, at least 15, at least
16, at least 17, at least 18, at least 19, at least 20, at least 21, at least
22, at least 23, at least
24, at least 25, at least 30, at least 35, at least 40, at least 45, at least
50, at least 55, at least
60, at least 65, at least 70, at least 75, at least 80, at least 85, at least
90, at least 95, or at least
100 biosensors.
The panel of biosensors includes at least one sensor element. "Sensor element"
131
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
refers to a single spot, site, location, or well for the at least one
biosensor, to which a sample
or aliquot thereof may be applied. The panel may be a composite sensor or an
array.
In some embodiments, the panel is a composite sensor. In a composite sensor,
each sensor element includes a mixture of two or more different biosensors. In
some
embodiments, the composite sensor includes one sensor element. In some
embodiments, the
composite sensor includes two or more sensor elements. In some embodiments,
signals are
measured from a composite sensor in which the signals arise from one or more
biosensors in
the sensor element. For example, signals may be measured from a composite
sensor in which
the signals arise from a subset of the total number of biosensors in the
sensor element. For
example, signals may be measured from a composite sensor in which the signals
arise from
two of five biosensors in the sensor element.
In some embodiments, the panel is an array. In an array, each sensor element
includes a single type of biosensor. An array comprises a plurality of
individually and
spatially localized sensor elements. Each sensor element includes a biosensor
that is different
than or the same as the biosensor of a different sensor element. In some
embodiments,
signals are measured from an array in which the signals arise separately from
two or more
selected biosensors in separate sensor elements. An array may comprise a
plurality of sensor
elements of a variety of sizes and configurations. An array may comprise a
plurality of sensor
elements arranged linearly. For example, an array may comprise a plurality of
micrometer-
sized sensor elements arranged in a single row. An array may comprise a
plurality of sensor
elements arranged in a grid. The grid may be two- or three-dimensional. In
some
embodiments, the grid is a spatially addressable grid. In some embodiments,
the biosensors
are incorporated into an array, such as a multichannel or multiplexed array.
The biosensors of the present disclosure can be used in any setting where
ligand detection is required or desired, such a medical setting (e.g.,
determining the level of
blood ligand in a subject), environmental setting (e.g., determining the level
of ligand in an
environmental sample), biological setting (e.g., determining the presence or
amount of ligand
in a reaction), or in process engineering, such as monitoring the amount of
ligand in a
fermentation reaction (e.g., beer/wine production, etc.). Other examples
include, but are not
limited to, uses in the food industry (Suleiman et al, In: Biosensor Design
and Application:
Mathewson and Finley Eds; American Chemical Society, Washington, DC 1992, vol.
511); in
clinical chemistry (Wilkins et al., Med. Eng. Phys. 1996, 18, 273-288; Pickup,
Tr. Biotech.
1993, 11, 285-291; Meyerhoff et al., Endricon 1966, 6, 51-58; Riklin et al.,
Nature 1995, 376,
132
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
672-675); Willner et al., J. Am. Chem. Soc. 1996, 118, 10321-10322); as the
basis for the
construction of a fluorescent flow cell containing immobilized ligand binding
protein-FAST
conjugates (see, e.g., Wilkins et al., Med. Eng. Phys. 1966, 18, 273-288;
Pickup, Tr. Biotech.
1993, 11, 285-291; Meyerhoff et al., Endricon. 1966, 6, 51; Group, New Engl.
J. Med. 1993,
329, 977-986; Gough et al., Diabetes 1995, 44, 1005-1009); and in an
implantable devices.
The biosensors as detailed herein may be administered in a variety of ways
known by
those of skill in the art, as appropriate for each application. Biosensors may
be provided in a
solution. The solution may be buffered. Biosensors may be provided in a
solution and mixed
directly with a sample. In some embodiments, a biosensor is immobilized onto a
surface.
Biosensors may be immobilized within a disposable cartridge into which a
sample may be
introduced or applied. Biosensors may be implanted or incorporated in a
wearable device.
The biosensor may be provided as an optode.
The biosensor may be attached to or incorporated in a wearable device.
Wearable
devices may include, for example, adhesive strips, patches, and contact
lenses. The biosensor
may be configured for placement in contact with a subject's skin or mucosal
surface. In some
embodiments, the biosensor is configured as an adhesive strip. In some
embodiments, the
biosensor is configured within or on the surface of a contact lens. In some
embodiments, the
contact lens is formed from a transparent substrate shaped to be worn directly
over a subject's
eye, as described in, for example, U.S. Patent No. 8,608,310.
The biosensor may be implanted. The biosensor may be implanted in a subject's
body. The biosensor may be implanted in a subject's blood vessel, vein, eye,
natural or
artificial pancreas, skin, or anywhere in the alimentary canal including the
stomach, intestine
and esophagus. The biosensor may be implanted in a subject with a microbead.
In some
embodiments, the biosensor is configured to be implanted in the skin. The
biosensor may be
implanted in a subject sub-dermally. The biosensor may generate the signal
trans-dermally.
In some embodiments, the biosensor may be implanted in a subject with
transdermal
microbeads, wherein the optical signals can be transmitted remotely between
the biosensor
and detecting device.
In some embodiments, the biosensor is administered as an optode. As used
herein, "optode" refers to an optical fiber with a single biosensor, or a
composite biosensor,
immobilized at the surface or at the end. An "optode" may also be referred to
as an
"optrode." In some embodiments, the biosensor is implanted in a subject as an
optode. The
optode may be incorporated with or into a needle. The optode may be
incorporated with a
133
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
probe such as endoscopy or colonoscopy probes. The optode may be used in a
tumor, near a
tumor, or at the periphery of a tumor. In some embodiments, the biosensor may
be implanted
in a subject as an optode, wherein the optical signals can be transmitted
between the
biosensor and detecting device using physical links. In some embodiments, the
biosensor is
administered as an optode to a sample or reaction. The optode may be contacted
with a
sample or reaction. In some embodiments, an optode is used to continuously or
episodically
monitor a ligand in a sample or reaction.
Methods Of Detecting The Presence Of A Ligand
Provided herein is a method of detecting the presence of a ligand in a sample.
The
method may include contacting the biosensor with the sample; measuring a
signal from the
biosensor; and comparing the signal to a ligand-free control. A difference in
signal indicates
the presence of ligand in the sample.
Also provided herein is a method of detecting the presence of ligand in a
sample. The
method may include (a) providing a ligand biosensor disclosed herein in which
the reporter
group is attached the ligand so that a signal transduced by the reporter group
when the ligand
is bound to ligand differs from a signal transduced by the reporter group when
the ligand is
not bound to ligand; (b) contacting the biosensor with the test sample under
conditions such
that the biosensor can bind to ligand present in the test sample; and (c)
comparing the signal
transduced by the reporter group when the biosensor is contacted with the test
sample with
the signal transduced by the reporter group when the biosensor is contacted
with a ligand-free
control sample, wherein a difference in the signal transduced by the reporter
group when the
biosensor is contacted with the test sample, as compared to when the biosensor
is contacted
with the control sample, indicates that the test sample contains ligand.
Methods Of Determining The Concentration Of A Ligand
Provided herein is a method of determining the concentration of a ligand in a
sample.
The method may include contacting the biosensor with the sample; measuring a
signal from
the biosensor; and comparing the signal to a standard hyperbolic ligand
binding curve to
determine the concentration of ligand in the test sample. The standard
hyperbolic ligand
binding curve may be prepared by measuring the signal transduced by the
biosensor when
contacted with control samples containing known concentrations of ligand.
134
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Another aspect of the present disclosure provides a method of determining the
concentration of ligand in a test sample comprising, consisting of, or
consisting essentially of:
(a) providing a ligand biosensor comprising a ligand biosensor as described
herein in which
the reporter group is attached the ligand so that a signal transduced by the
reporter group
when the ligand is bound to ligand differs from a signal transduced by the
reporter group
when the ligand is not bound to ligand; (b) contacting the biosensor with the
test sample
under conditions such that the biosensor can bind to ligand present in the
test sample; and (c)
comparing the signal transduced by the reporter group when the biosensor is
contacted with
the test sample with a standard hyperbolic ligand binding curve prepared by
measuring the
signal transduced by the reporter group when the biosensor is contacted with
control samples
containing known quantities of ligand to determine the concentration of ligand
in the test
sample.
Methods Of Monitoring The Presence Of A Ligand
The present invention is directed to a method of episodically or continuously
monitoring the presence of a ligand in a reaction. In certain embodiments, the
biosensors
may be used in the continuous monitoring of ligand in a reaction. In certain
embodiments,
the ligand sensors may be used in episodic monitoring of sample aliquots.
The method of episodically or continuously monitoring the presence of a ligand
in a
reaction may include contacting the biosensor with the reaction; maintaining
the reaction
under conditions such that the polypeptide is capable of binding ligand
present in the
reaction; and episodically or continuously monitoring the signal from the
biosensor in the
reaction.
The method of episodically or continuously monitoring the presence of a ligand
in a
reaction may include contacting the biosensor with the reaction; maintaining
the reaction
under conditions such that the polypeptide is capable of binding ligand
present in the
reaction; episodically or continuously monitoring the signal from the
biosensor in the
reaction; and comparing the signal to a standard hyperbolic ligand binding
curve to determine
the concentration of ligand in the test sample. The standard hyperbolic ligand
binding curve
may be prepared by measuring the signal transduced by the biosensor when
contacted with
control samples containing known concentrations of ligand.
In some embodiments, the method further includes comparing the signal to a
ligand-
free control, wherein a difference in signal indicates the presence of ligand
in the reaction.
135
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
In some embodiments, the method further includes comparing the signal to a
standard
hyperbolic ligand binding curve to determine the concentration of ligand in
the test sample.
The standard hyperbolic ligand binding curve may be prepared by measuring the
signal
transduced by the biosensor when contacted with control samples containing
known
concentrations of ligand.
Another aspect of the present disclosure provides a method of continuously
monitoring the presence of ligand in a reaction comprising, consisting of, or
consisting
essentially of: (a) providing a ligand biosensor as described herein in which
the reporter
group is attached the ligand-binding protein so that a signal transduced by
the reporter group
when the ligand is bound to ligand-binding protein differs from a signal
transduced by the
reporter group when the ligand is not bound to the ligand-binding protein; (b)
maintaining the
biosensor within the reaction and under conditions such that the biosensor can
bind to ligand
present in the reaction; (c) continuously monitoring the signal transduced by
the reporter
group when the biosensor is contacted with the ligand present in the reaction;
and optionally
(d) comparing the signal transduced by the reporter group when the biosensor
is contacted
with the ligand present in the reaction with the signal transduced by the
reporter group when
the biosensor is contacted with a ligand-free control sample, wherein a
difference in the
signal transduced by the reporter group when the biosensor is contacted with
the ligand
present in the reaction, as compared to when the biosensor is contacted with
the control
sample, indicates ligand is present in the reaction.
Yet another aspect of the present disclosure provides a method of continuously
monitoring the concentration of ligand in a reaction comprising, consisting
of, or consisting
essentially of: (a) providing a ligand biosensor comprising a ligand biosensor
as described
herein in which the reporter group is attached the ligand so that a signal
transduced by the
reporter group when the ligand is bound to ligand differs from a signal
transduced by the
reporter group when the ligand is not bound to ligand; (b) maintaining the
biosensor within
the reaction under conditions such that the biosensor can bind to ligand
present in the
reaction; and (c) continuously monitoring the signal transduced by the
reporter group when
the biosensor is contacted with the ligand present in the reaction; and (d)
comparing the
signal transduced by the reporter group when the biosensor is contacted with
the ligand
present in the reaction with a standard hyperbolic ligand binding curve
prepared by
measuring the signal transduced by the reporter group when the biosensor is
contacted with
136
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
control samples containing known quantities of ligand to determine the
concentration of
ligand in the reaction.
General Definitions
Unless specifically defined otherwise, all technical and scientific terms used
herein
shall be taken to have the same meaning as commonly understood by one of
ordinary skill in
the art (e.g., in cell culture, molecular genetics, and biochemistry).
As used herein, the term "about" in the context of a numerical value or range
means
10% of the numerical value or range recited or claimed, unless the context
requires a more
limited range.
In the descriptions above and in the claims, phrases such as "at least one of'
or "one
or more of' may occur followed by a conjunctive list of elements or features.
The term
"and/or" may also occur in a list of two or more elements or features. Unless
otherwise
implicitly or explicitly contradicted by the context in which it is used, such
a phrase is
intended to mean any of the listed elements or features individually or any of
the recited
elements or features in combination with any of the other recited elements or
features. For
example, the phrases "at least one of A and B;" "one or more of A and B;" and
"A and/or B"
are each intended to mean "A alone, B alone, or A and B together." A similar
interpretation
is also intended for lists including three or more items. For example, the
phrases "at least one
of A, B, and C;" "one or more of A, B, and C;" and "A, B, and/or C" are each
intended to
mean "A alone, B alone, C alone, A and B together, A and C together, B and C
together, or A
and B and C together." In addition, use of the term "based on," above and in
the claims is
intended to mean, "based at least in part on," such that an unrecited feature
or element is also
permissible
It is understood that where a parameter range is provided, all integers within
that
range, and tenths thereof, are also provided by the invention. For example,
"0.2-5 mg" is a
disclosure of 0.2 mg, 0.3 mg, 0.4 mg, 0.5 mg, 0.6 mg etc. up to and including
5.0 mg.
A small molecule is a compound that is less than 2000 daltons in mass. The
molecular mass of the small molecule is preferably less than 1000 daltons,
more preferably
less than 600 daltons, e.g., the compound is less than 500 daltons, 400
daltons, 300 daltons,
200 daltons, or 100 daltons.
137
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
As used herein, an "isolated" or "purified" nucleic acid molecule,
polynucleotide,
polypeptide, or protein, is substantially free of other cellular material, or
culture medium
when produced by recombinant techniques, or chemical precursors or other
chemicals when
chemically synthesized. Purified compounds are at least 60% by weight (dry
weight) the
compound of interest. Preferably, the preparation is at least 75%, more
preferably at least
90%, and most preferably at least 99%, by weight the compound of interest. For
example, a
purified compound is one that is at least 90%, 91%, 92%, 93%, 94%, 95%, 98%,
99%, or
100% (w/w) of the desired compound by weight. Purity is measured by any
appropriate
standard method, for example, by column chromatography, thin layer
chromatography, or
high-performance liquid chromatography (HPLC) analysis. A purified or isolated
polynucleotide (ribonucleic acid (RNA) or deoxyribonucleic acid (DNA)) is free
of the
genes/nucleic acids or sequences/amino acids that flank it in its naturally-
occurring state.
Purified also defines a degree of sterility that is safe for administration to
a human subject,
e.g., lacking infectious or toxic agents.
Similarly, by "substantially pure" is meant a nucleotide or polypeptide that
has been
separated from the components that naturally accompany it. Typically, the
nucleotides and
polypeptides are substantially pure when they are at least 60%, 70%, 80%, 90%,
95%, or
even 99%, by weight, free from the proteins and naturally-occurring organic
molecules with
they are naturally associated.
The transitional term "comprising," which is synonymous with "including,"
"containing," or "characterized by," is inclusive or open-ended and does not
exclude
additional, unrecited elements or method steps. By contrast, the transitional
phrase
"consisting of' excludes any element, step, or ingredient not specified in the
claim. The
transitional phrase "consisting essentially of' limits the scope of a claim to
the specified
materials or steps "and those that do not materially affect the basic and
novel
characteristic(s)" of the claimed invention.
"Subject" as used herein refers to any organism from which a biological sample
is
obtained. For example, the sample is a biological fluid or tissue. For
example, a subject is
one who wants or is in need of detecting ligand or determining the
concentration of ligand
with the herein described biosensors. The subject may be a human or a non-
human animal.
The subject may be a mammal. The mammal may be a primate or a non-primate. The
mammal can be a primate such as a human; a non-primate such as, for example,
dog, cat,
horse, cow, pig, mouse, rat, camel, llama, goat, rabbit, sheep, hamster, and
guinea pig; or
138
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
non-human primate such as, for example, monkey, chimpanzee, gorilla,
orangutan, and
gibbon. The subject may be of any age or stage of development, such as, for
example, an
adult, an adolescent, or an infant.
As used herein, an "expression vector" is a DNA or RNA vector that is capable
of
effecting expression of one or more polynucleotides. Preferably, the
expression vector is also
capable of replicating within the host cell. Expression vectors can be either
prokaryotic or
eukaryotic, and are typically include plasmids. Expression vectors of the
present invention
include any vectors that function (i.e., direct gene expression) in host cells
of the present
invention, including in one of the prokaryotic or eukaryotic cells described
herein, e.g., gram-
positive, gram-negative, pathogenic, non-pathogenic, commensal, cocci,
bacillus, or spiral-
shaped bacterial cells; archaeal cells; or protozoan, algal, fungi, yeast,
plant, animal,
vertebrate, invertebrate, arthropod, mammalian, rodent, primate, or human
cells. Expression
vectors of the present invention contain regulatory sequences such as
transcription control
sequences, translation control sequences, origins of replication, and other
regulatory
sequences that are compatible with the host cell and that control the
expression of a
polynucleotide. In particular, expression vectors of the present invention
include transcription
control sequences. Transcription control sequences are sequences which control
the initiation,
elongation, and termination of transcription. Particularly important
transcription control
sequences are those which control transcription initiation such as promoter,
enhancer,
operator and repressor sequences. Suitable transcription control sequences
include any
transcription control sequence that can function in at least one of the cells
of the present
invention. A variety of such transcription control sequences are known to
those skilled in the
art.
As used herein, the singular forms "a," "an," and "the" include the plural
reference
unless the context clearly dictates otherwise. Thus, for example, a reference
to "a disease,"
"a disease state", or "a nucleic acid" is a reference to one or more such
embodiments, and
includes equivalents thereof known to those skilled in the art and so forth.
As used herein, "pharmaceutically acceptable" carrier or excipient refers to a
carrier
or excipient that is suitable for use with humans and/or animals without undue
adverse side
effects (such as toxicity, irritation, and allergic response) commensurate
with a reasonable
benefit/risk ratio. It can be, e.g., a pharmaceutically acceptable solvent,
suspending agent or
vehicle, for delivering the instant compounds to the subject.
139
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
The term "diagnosis" refers to a determination that a disease is present in
the subject.
Similarly, the term "prognosis" refers to a relative probability that a
certain future outcome
may occur in the subject. For example, in the context of the present
disclosure, prognosis can
refer to the likelihood that an individual will develop a disease, or the
likely severity of the
disease (e.g., severity of symptoms, rate of functional decline, survival,
etc.).
Unless required otherwise by context, the terms "polypeptide" and "protein"
are used
interchangeably.
A polypeptide or class of polypeptides may be defined by the extent of
identity (%
identity) of its amino acid sequence to a reference amino acid sequence, or by
having a
greater % identity to one reference amino acid sequence than to another. A
variant of any of
genes or gene products disclosed herein may have, e.g., 50%, 55%, 60%, 65%,
70%, 75%,
80%, 85%, 90%, 95%, 96%, 97%, 98%, or 99% sequence identity to the nucleic
acid or
amino acid sequences described herein. The term "% identity," in the context
of two or more
nucleic acid or polypeptide sequences, refers to two or more sequences or
subsequences that
are the same or have a specified percentage of amino acid residues or
nucleotides that are the
same, when compared and aligned for maximum correspondence, as measured using
a
sequence comparison algorithm or by visual inspection. For example, % identity
is relative
to the entire length of the coding regions of the sequences being compared, or
the length of a
particular fragment or functional domain thereof. Variants as disclosed herein
also include
homologs, orthologs, or paralogs of the genes or gene products described
herein. In some
embodiments, variants may demonstrate a percentage of homology or identity,
for example,
at least about 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%,
98%, or
99% identity conserved domains important for biological function, e.g., in a
functional
domain, e.g. a ligand-binding or catalytic domain.
For sequence comparison, one sequence acts as a reference sequence, to which
test
sequences are compared. When using a sequence comparison algorithm, test and
reference
sequences are input into a computer, subsequence coordinates are designated,
if necessary,
and sequence algorithm program parameters are designated. The sequence
comparison
algorithm then calculates the percent sequence identity for the test
sequence(s) relative to the
reference sequence, based on the designated program parameters. Percent
identity is
determined using BLAST. For the BLAST searches, the following parameters were
employed: (1) Expect threshold is 10; (2) Gap cost is Existence:11 and
Extension:1; (3) The
Matrix employed is BLOSUM62; (4) The filter for low complexity regions is
"on."
140
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
The present invention also provides for functional fragments of the genes or
gene
products described herein. A fragment of a protein is characterized by a
length (number of
amino acids) that is less than the length of the full length mature form of
the protein. A
fragment, in the case of these sequences and all others provided herein, may
be a part of the
whole that is less than the whole. Moreover, a fragment ranges in size from a
single
nucleotide or amino acid within a polynucleotide or polypeptide sequence to
one fewer
nucleotide or amino acid than the entire polynucleotide or polypeptide
sequence. Finally, a
fragment is defined as any portion of a complete polynucleotide or polypeptide
sequence that
is intermediate between the extremes defined above.
For example, fragments of any of the proteins or enzymes disclosed herein or
encoded
by any of the genes disclosed herein can be 10 to 20 amino acids, 10 to 30
amino acids, 10 to
40 amino acids, 10 to 50 amino acids, 10 to 60 amino acids, 10 to 70 amino
acids, 10 to 80
amino acids, 10 to 90 amino acids, 10 to 100 amino acids, 50 to 100 amino
acids, 75 to 125
amino acids, 100 to 150 amino acids, 150 to 200 amino acids, 200 to 250 amino
acids, 250 to
300 amino acids, or 300 to 350 amino acids. The fragments encompassed in the
present
subject matter comprise fragments that retain functional fragments. As such,
the fragments
preferably retain the binding domains that are required or are important for
functional
activity. Fragments can be determined or generated by using the sequence
information
herein, and the fragments can be tested for functional activity using standard
methods known
in the art. For example, the encoded protein can be expressed by any
recombinant
technology known in the art and the binding activity of the protein can be
determined.
As used herein a "biologically active" fragment is a portion of a polypeptide
which
maintains an activity of a full-length reference polypeptide. Biologically
active fragments as
used herein exclude the full-length polypeptide. Biologically active fragments
can be any
size as long as they maintain the defined activity. Preferably, the
biologically active fragment
maintains at least 10%, at least 50%, at least 75% or at least 90%, of the
activity of the full
length protein.
Amino acid sequence variants/mutants of the polypeptides of the defined herein
can
be prepared by introducing appropriate nucleotide changes into a nucleic acid
defined herein,
or by in vitro synthesis of the desired polypeptide. Such variants/mutants
include, for
example, deletions, insertions or substitutions of residues within the amino
acid sequence. A
combination of deletion, insertion and substitution can be made to arrive at
the final
141
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
construct, provided that the final peptide product possesses the desired
activity and/or
specificity.
Mutant (altered) peptides can be prepared using any technique known in the
art. For
example, a polynucleotide defined herein can be subjected to in vitro
mutagenesis or DNA
shuffling techniques as broadly described by Harayama (1998). Products derived
from
mutated/altered DNA can readily be screened using techniques described herein
to determine
if they possess, for example, ligand binding activity.
In designing amino acid sequence mutants, the location of the mutation site
and the
nature of the mutation will depend on characteristic(s) to be modified. The
sites for mutation
can be modified individually or in series, e.g., by (1) substituting first
with conservative
amino acid choices and then with more radical selections depending upon the
results
achieved, (2) deleting the target residue, or (3) inserting other residues
adjacent to the located
site.
Amino acid sequence deletions generally range from about 1 to 15 residues,
more
preferably about 1 to 10 residues and typically about 1 to 5 contiguous
residues. In some
embodiments, a mutated or modified protein does not comprise any deletions or
insertions.
In various embodiments, a mutated or modified protein has less than about 10,
9, 8, 7, 6, 5, 4,
3, or 2 deleted or inserted amino acids.
Substitution mutants have at least one amino acid residue in the polypeptide
molecule
removed and a different residue inserted in its place. Sites may be
substituted in a relatively
conservative manner in order to maintain activity and/or specificity. Such
conservative
substitutions are shown in the table below under the heading of "exemplary
substitutions."
In certain embodiments, a mutant/variant polypeptide has only, or not more
than, one
or two or three or four conservative amino acid changes when compared to a
naturally
occurring polypeptide. Details of conservative amino acid changes are provided
in the table
below. As the skilled person would be aware, such minor changes can reasonably
be
predicted not to alter the activity of the polypeptide when expressed in a
recombinant cell.
142
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Exemplary Substitutions
Original Residue Exemplary Substitutions
Alanine (Ala) Val; Leu; Ile; Gly
Arginine (Arg) Lys
Asparagine (Asn) Gln; His
Cysteine (Cys) Ser
Glutamine (Gin) Asn; His
Glutamic Acid (Glu) Asp
Glycine (Gly) Pro; Ala
Histidine (His) Asn; Gln
Isoleucine (Ile) Leu; Val; Ala
Leucine (Leu) Ile; Val; Met; Ala; Phe
Lysine (Lys) Arg
Methionine (Met) Leu; Phe
Phenylalanine (Phe) Leu; Val; Ala
Proline (Pro) Gly
Serine (Ser) Thr
Threonine (Thr) Ser
Tryptophan (Trp) Tyr
Tyrosine (Tyr) Trp; Phe
Valine (Val) Ile; Leu; Met; Phe; Ala
Mutations can be introduced into a nucleic acid sequence such that the encoded
amino
acid sequence is altered by standard techniques, such as site-directed
mutagenesis and PCR-
mediated mutagenesis. Preferably, conservative amino acid substitutions are
made at one or
more predicted non-essential amino acid residues. A "conservative amino acid
substitution"
is one in which the amino acid residue is replaced with an amino acid residue
having a
similar side chain. Families of amino acid residues having similar side chains
have been
defined in the art. Certain amino acids have side chains with more than one
classifiable
characteristic. These families include amino acids with basic side chains
(e.g., lysine,
arginine, histidine), acidic side chains (e.g., aspartic acid, glutamic acid),
uncharged polar
side chains (e.g., glycine, asparagine, glutamine, serine, threonine,
tyrosine, tryptophan,
143
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
cysteine), nonpolar side chains (e.g., alanine, valine, leucine, isoleucine,
proline,
phenylalanine, methionine, tyrosine, tryptophan), beta-branched side chains
(e.g., threonine,
valine, isoleucine) and aromatic side chains (e.g., tyrosine, phenylalanine,
tryptophan,
histidine). Thus, a predicted nonessential amino acid residue in a given
polypeptide is
replaced with another amino acid residue from the same side chain family.
Alternatively, in
another embodiment, mutations can be introduced randomly along all or part of
a given
coding sequence, such as by saturation mutagenesis, and the resultant mutants
can be
screened for given polypeptide biological activity to identify mutants that
retain activity.
Conversely, the invention also provides for variants with mutations that
enhance or increase
the endogenous biological activity. Following mutagenesis of the nucleic acid
sequence, the
encoded protein can be expressed by any recombinant technology known in the
art and the
activity/specificity of the protein can be determined. An increase, decrease,
or elimination of
a given biological activity of the variants disclosed herein can be readily
measured by the
ordinary person skilled in the art, i.e., by measuring the capability for
binding a ligand and/or
signal transduction.
In various embodiments, a polypeptide comprises mutations such that 1, 2, 3,
4, 5, 6,
7, 8, 9, or 10, or less than about 10, 9, 8, 7, 6, 5, 4, 3, or 2 amino acids
is substituted with a
cysteine and/or a lysine.
Polypeptides can be produced in a variety of ways, including production and
recovery
of natural polypeptides or recombinant polypeptides according to methods known
in the art.
In one embodiment, a recombinant polypeptide is produced by culturing a cell
capable of
expressing the polypeptide under conditions effective to produce the
polypeptide, such as a
host cell defined herein.
Key to the Sequence Listing
SEQ ID NO Sequence Name
1 C1BP1, also referred to herein as laYFP
2 C1BP2
3 C1BP3
4 C1BP4
5 C1BP5
6 C1BP6
7 C1BP7
8 C1BP8
144
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
9 C1BP9
C1BP10
11 C1BP11
12 C1BP12
13 C1BP13
14 C1BP14
ttGGBP.11C.O.bZif (11C substitution mutant with bZif fusion, signal
peptide replaced with M and a GGSHHHHHH at C-terminus)
16 ttGGBP.17C.O.bZif (17C substitution mutant with bZif fusion,
signal
peptide replaced with M and a GGSHHHHHH at C-terminus)
17 ttGGBP.111C.O.bZif (111C substitution mutant with bZif fusion,
signal
peptide replaced with M and a GGSHHHHHH at C-terminus)
18 ttGGBP.151C.O.bZif (151C substitution mutant with bZif fusion,
signal
peptide replaced with M and a GGSHHHHHH at C-terminus)
19 ttGGBP.182C.O.bZif (182C substitution mutant with bZif fusion,
signal
peptide replaced with M and a GGSHHHHHH at C-terminus)
ttGGBP.17C.3.Trx (17C, 16N, 211A substitution mutant with ecTRX
fusion, signal peptide replaced with M and a GGSHHHHHH at C-
terminus)
Trx.ttGGBP.17C.3 (17C, 16N, 211A substitution mutant with ecTRX
21 fusion, signal peptide replaced with M and a GGSHHHHHH at C-
terminus)
ttGGBP.182C.2.Trx (182C, 69P, 152P substitution mutant with ecTRX
22 fusion, signal peptide replaced with M and a GGSHHHHHH at C-
terminus)
Trx.ttGGBP.182C.2 (182C, 69P, 152P substitution mutant with ecTRX
23 fusion, signal peptide replaced with M and a GGSHHHHHH at C-
terminus)
24 Adaptor
Adaptor1.0
26 Adaptor2.0a
27 Adaptor2.0b
28 Adaptor3.0
29 Adaptor4.0
Adaptor5.0
31 Adaptor6.0
32 Adaptor7.0
33 Adaptor8.0
34 Adaptor9.0
Adaptor10.0
36 Adaptor11.0
37 Adaptor12.0
38 Adaptor13.0
39 Adaptor14.0
Adaptor15.0
41 Adaptor16.0
145
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
42 PZif
43 ZF-QNK
44 Hexahistidine Tag
45 Hexalysine Tag
46 C1BP1 expression construct
47 C1BP2 expression construct
48 C1BP3 expression construct
49 C1BP4 expression construct
50 C1BP5 expression construct
51 C1BP6 expression construct
52 C1BP7 expression construct
53 C1BP8 expression construct
54 C1BP9 expression construct
55 C1BP10 expression construct
56 C1BP11 expression construct
57 C1BP12 expression construct
58 C1BP13 expression construct
59 C1BP14 expression construct
60 ttGGBP.11C.O.bZif expression construct
61 ttGGBP.17C.O.bZif expression construct
62 ttGGBP.111C.O.bZif expression construct
63 ttGGBP.151C.O.bZif expression construct
64 ttGGBP.182C.O.bZif expression construct
65 ttGGBP.17C.3.Trx expression construct
66 Trx.ttGGBP.17C.3 expression construct
67 ttGGBP.182C.2.Trx expression construct
68 Trx.ttGGBP.182C.2 expression construct
69 Adaptor expression construct
70 Adaptor1.0 expression construct
71 Adaptor2.0a expression construct
72 Adaptor2.0b expression construct
73 Adaptor3.0 expression construct
74 Adaptor4.0 expression construct
75 Adaptor5.0 expression construct
76 Adaptor6.0 expression construct
77 Adaptor7.0 expression construct
78 Adaptor8.0 expression construct
79 Adaptor9.0 expression construct
80 Adaptor10.0 expression construct
81 Adaptor11.0 expression construct
82 Adaptor12.0 expression construct
83 Adaptor13.0 expression construct
84 Adaptor14.0 expression construct
146
CA 03005883 2018-05-18
WO 2017/087912 PCT/US2016/062958
85 Adaptor15.0 expression construct
86 Adaptor16.0 expression construct
87 ecGGBP [U.S. National Center for Biotechnology Information (NCBI)
Accession No. WP 032329053]
88 ttGGBP (NCBI Accession Nos. YP_003852930.1 and WP_013298803.1)
89 stGGBP (NCBI Accession No. WP_001036943)
90 chyGGBP (NCBI Accession Nos. WP 013402088.1 and
YP_003991244.1)
91 cobGGBP (NCBI Accession Nos. WP 013289482.1 and
YP_003839461.1)
92 pspGGBP (NCBI Accession Nos. WP 015735911.1 and
YP_003243743.1)
93 csaGGBP (NCBI Accession Nos. WP 013273028.1 and
YP_003822565.1)
94 bprGGBP (NCBI Accession Nos. WP 013280279.1 and
YP_003830205.1)
95 rinGGBP A (NCBI Accession Nos. WP 006855636.1 and
YP_007778116.1)
96 fprGGBP (NCBI Accession Nos. WP 015536639.1 and
YP 007799070.1)
97 cljGGBP (NCBI Accession No. CLJU_c08950)
98 cauGGBP (NCBI Accession No. CAETHG_2989)
99 rinGGBP B (NCBI Accession Nos. WP 006855628.1 and
YP_007778124.1)
100 erhGGBP (NCBI Accession Nos. WP 003775352.1 and
YP_004561181.1)
101 ereGGBP (NCBI Accession Nos. WP 012741392.1 and
YP_002936409.1)
02 mpUBP; [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 004483096.1 and WP 013797647.1]
03 mhUBP; [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 005430828.1 and WP 014422383.1]
04 bsUBP; [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 006233530.1 and WP 014665698.1]
05 dcUBP; [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 004496535.1 and WP 013809819.1]
06 gtUBP; [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 004588319.1 and WP 013877063.1 ]
07 ctUBP; [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 001038237.1 and WP 003515797.1]
08 csUBP; [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 001181243.1 and WP 011917972.1]
09 taUBP; [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 003473480.1 and WP 012991759.1]
glcUBP; [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 147790.1 and WP 011231423.1]
111 psUBP; [U.S. National Center for Biotechnology Information (NCBI)
147
CA 03005883 2018-05-18
WO 2017/087912 PCT/US2016/062958
Accession Nos. YP 003241723.1 and WP 015734090.1]
teUBP; [U.S. National Center for Biotechnology Information (NCBI)
112
Accession No. YP 681910.1 and WP 011567844.1]
13 ttGBP [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 004303.1 and WP 011172778.1]
14 tsGBP [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 004202647.1 and WP 015717367.1]
dmGBP [U.S. National Center for Biotechnology Information (NCBI)
115
Accession Nos. YP 004171760.1 and WP 013557600.1]
6 tnGBP [U.S. National Center for Biotechnology Information (NCBI)
11
Accession Nos. YP 002534202.1 and WP 015919155.1]
17 koGBP [U.S. National Center for Biotechnology Information (NCBI)
1
Accession No. YP 002941687.1 and WP 015869326.1]
8 bhGBP [U.S. National Center for Biotechnology Information (NCBI)
11
Accession Nos. NP 244712.1 and WP 010899970.1]
19 smGBP [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 001041152.1 and WP 011839435.1]
20 asGBP [U.S. National Center for Biotechnology Information (NCBI)
1
Accession No. YP 831349.1 and WP 011691715.1]
21 ttLacBP1 [U.S. National Center for Biotechnology Information (NCBI)
1
Accession No. YP 144032.1]
22 tsLacBP2 [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 004202714.1 and WP 015717434.1 ]
123 toLacBP3 [U.S. National Center for Biotechnology Information (NCBI)
Accession Nos. YP 006972155.1 and WP 016329249.1]
24 tsLacBP4 [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 002514099.1 and WP 012638591.1]
25 rdLacBP5 [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 683924.1 and WP 011569849.1]
26 msLacBP6 [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 006556686.1 and WP 014869652.1]
27 tsLacBP7 [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP_005654632.1 and WP_014515914.1]
128 maLacBP8 [U.S. National Center for Biotechnology Information (NCBI)
Accession Nos. YP 005886720.1 and WP 014578260.1]
29 adLacBP9 [U.S. National Center for Biotechnology Information (NCBI)
1
Accession No. YP_466099.1 and WP_011421944.1]
30 pgLacBP10 [U.S. National Center for Biotechnology Information (NCBI)
1
Accession No. YP_004304976.1 and WP_013653981.1]
31 psLacBP11 [U.S. National Center for Biotechnology Information (NCBI)
1
Accession No. YP_006522676.1 and WP_014851134.1]
32 rsLacBP12 [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. RSP 3372 and YP 354877.1]
33 fsLacBP13 [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP_004603455.1 and WP_013886373.1]
34 taLacBP14 [U.S. National Center for Biotechnology Information (NCBI)
1
Accession No. YP_003317968.1]
135 synBicarbBP1 [U.S. National Center for Biotechnology Information
148
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
(NCBI) Accession Nos. YP_005410477.1 and WP_Ol 0873997.1]
teBicarbBP2 [U.S. National Center for Biotechnology Information
136
(NCBI) Accession No. NP_682790.1]
ctBicarbBP3 [U.S. National Center for Biotechnology Information
137
(NCBI) Accession Nos. YP_007090308.1 and WP_Ol 5152989.1]
calBicarbBP4 [U.S. National Center for Biotechnology Information
138
(NCBI) Accession Nos. YP_007137061.1 and WP_015197735.1]
avBicarbBP5 [U.S. National Center for Biotechnology Information
139
(NCBI) Accession Nos. YP_321546.1 and WP_011317875.1]
140 cmBicarbBP6 [U.S. National Center for Biotechnology Information
(NCBI) Accession Nos. YP 007099445.1 and WP 015162006.1]
41 mhFeBP1 [U.S. National Center for Biotechnology Information (NCBI)
1
Accession Nos. YP 007884192.1 and WP 006253500.1]
142 exiFeBP2 [U.S. National Center for Biotechnology Information (NCBI)
Accession Nos. YP 002886303.1 and WP 015880417.1]
143 teFeBP3 [U.S. National Center for Biotechnology Information (NCBI)
Accession No. NP 681303.1]
144 cnFeBP4 [U.S. National Center for Biotechnology Information (NCBI)
Accession Nos. YP 003796723.1 and WP 013247623.1]
145 ttFeBP5 [U.S. National Center for Biotechnology Information (NCBI)
Accession No. YP 144894.1]
146 msFeBP6 [U.S. National Center for Biotechnology Information (NCBI)
Accession Nos. YP 003686074.1 and WP 013159102.1]
147 srFeBP7 [U.S. National Center for Biotechnology Information (NCBI)
Accession Nos. YP 003572493.1 and WP 013062602.1]
148 h1FeBP8 [U.S. National Center for Biotechnology Information (NCBI)
Accession Nos. YP 002564837.1 and WP 012659409.1]
149 Yellow Fluorescent Protein
150 Yellow Fluorescent Protein without N-terminal Methionine
151 ecTrx
152 GGSHHHHHH Sequence
153 ecGGBP without signal peptide
154 ttGGBP (with signal peptide replaced with M)
155 stGGBP (with signal peptide replaced with M)
156 chyGGBP (with signal peptide replaced with M)
157 cobGGBP (with signal peptide replaced with M)
158 pspGGBP (with signal peptide replaced with M)
159 csaGGBP (with signal peptide replaced with M)
160 bprGGBP (with signal peptide replaced with M)
161 rinGGBP_A (with signal peptide replaced with M)
162 fprGGBP (with signal peptide replaced with M)
163 cljGGBP (with signal peptide replaced with M)
164 cauGGBP (with signal peptide replaced with M)
165 rinGGBP_B (with signal peptide replaced with M)
166 erhGGBP (with signal peptide replaced with M)
167 ereGGBP (with signal peptide replaced with M)
149
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
The terms "bZir and "f3Zif" are used synonymously herein.
Exemplary amino acid sequences are listed below for convenience:
ecTrx (SEQ ID NO: 151)
MSDKIIHLTDDSFDTDVLKADGAILVDFWAEWCGPCKMIAPILDEIADEY
QGKLTVAKLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLA
YFP (SEQ ID NO: 149)
MVSKGEELFTGVVP ILVELDGDVNGHKF SVS GEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGLQCFARYPDHMKRHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITHGMDELYKGGSNDYKDDDD
K
C1BP1 (SEQ ID NO: 1)
MVSKGEELFTGVVP ILVELDGDVNGHKF SVS GEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
C1BP2 (SEQ ID NO: 2)
MVSKGEELFTGVVP ILVELDGDVNGHKF SVS GEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
C1BP3 (SEQ ID NO: 3)
MVSKGEELFTGVVP ILVELDGDVNGHKF SVS GEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
C1BP4 (SEQ ID NO: 4)
MVSKGEELFTGVVP ILVELDGDVNGHKF SVS GEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
C1BP5 (SEQ ID NO: 5)
150
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
MVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
C1BP6 (SEQ ID NO: 6)
MVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
C1BP7 (SEQ ID NO: 7)
MVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
C1BP8 (SEQ ID NO: 8)
MVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
C1BP9 (SEQ ID NO: 9)
MVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
C1BP10 (SEQ ID NO: 10)
MVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
C1BP11 (SEQ ID NO: 11)
MVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICT
151
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
C1BP12 (SEQ ID NO: 12)
MVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
C1BP13 (SEQ ID NO: 13)
MVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
C1BP14(SEQ II) NO: 14)
MVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICT
TGKLPVPWPTLVTTFGYGVQCFARYPDHMRQHDFFKSAMPEGYVQERTIF
FKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHN
VYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNH
YLSYQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGGSNDYKDDDD
KGGSHHHHHH**
Glucose-Galactose Binding Protein Fusions
ttGBP.11CØbZif (SEQ ID NO: 15)
MKQLNIGVAICKFDDTFMTGVRNAMTAEAQGKAKLNMVDSQNSQPTQNDQ
VDLFITKKMNALAINPVDRTAAGTIIDKAKQANIPVVFFNREPLPEDMKK
WDKVYYVGAKAEQSGILQGQIMADYWKAHPEADKNHDGVMQYVMLMGQPG
HQDAILRTQYSIQTVKDAGIKVQELAKDYANWDRVTAHDKMAAWLSSFGD
KIEAVFANNDDMALGAIEALKSAGYFTGNKYIPVVGVDATAPGIQAIKDG
TLLGTVLNDAKNQAKATFNIAYELAQGITPTKDNIGYDITDGKYVWIPYK
KITKDNISDAEQGGSGGSTGEKPYKCPECGKSFSRSGGSHHHHHH**
ttGBP.17CØbZif (SEQ II) NO: 16)
MKQLNIGVAIYKFDDTCMTGVRNAMTAEAQGKAKLNMVDSQNSQPTQNDQ
VDLFITKKMNALAINPVDRTAAGTIIDKAKQANIPVVFFNREPLPEDMKK
WDKVYYVGAKAEQSGILQGQIMADYWKAHPEADKNHDGVMQYVMLMGQPG
HQDAILRTQYSIQTVKDAGIKVQELAKDYANWDRVTAHDKMAAWLSSFGD
KIEAVFANNDDMALGAIEALKSAGYFTGNKYIPVVGVDATAPGIQAIKDG
TLLGTVLNDAKNQAKATFNIAYELAQGITPTKDNIGYDITDGKYVWIPYK
KITKDNISDAEQGGSGGSTGEKPYKCPECGKSFSRSGGSHHHHHH**
152
Egi
BHHHHEISODbaVUSIMIXIDDIAdIMAANDUL
IaApimaxianobviaAvimaivxvbxxvaminipTunax[vtlloavi
VGADAAdIANNWAADVSNIVMVMVIAIWINNVJAVBDICMSS1AWVIAIN
CEEIVIAINIMNIVAGNIFIHbANIDIAINAIbISAbilflIVCOHDdbDIATINAANN St'
AouHKxava=dHvxmAavv\abotrnoSbHVNVDAAAA)DaAVXNIAICEdldMIN
aannamvbxvxlmunvviwanamirwmAiminaianbambiabsmbs
GAIAININVND6VHVBAIVNIIADENDMICHNAIVADINioNSDOVINVGIMI
lbaNgIVDANIVIVAHON)LITITIAIDIRDAMIVIDdNbUININVAEIND6
AHCIVIarlIdVIIAINDaDDAOVAUCINIIVDCIVNIAGICLISCRIEIHEINCESIAI 0.17
( I Z :ON 0:11 bas) C'DL, UdEEDIrckl,
HHHHEIFISDOVINVCaMlbaNgIVDANIVIVA
HoNalr-rualmlloxxavioambau=rDwArniptuaavlaaimvunix
Dappmaymaaniwosavrinaialsaarnilixassoobavasimaxux sc
NAdIMAANDULICIADINGXIALLID6V1HAVINILVNV6NNWIWIAIDTIL
DUX[VbIDdIVIVGADAAdIANNDLIADVSNIVMVMVIAIVCINNVJAVBDI
(MIS SlAWVIAINCEEIVIAIMMNIVAGNIFIHbANIDIAINAIbI sAbilnivabH
pabovvimutnninosaHmxsavaaavxmAsavw[botinosbavxvonAAAxam
muniaa=nammaannamvbxvxlmunvviwanaminvmnixxunan oc
boalloid6SN6SCIAIAININVND6VHVBAIVNUADENDMICLINAIVADINitYNIAI
(CIZ :ON 0:11 bas) X-11.C.DLI.JEDU
**HHHHEIFISDOSIISJSNDDHJDNA=INHDISDDSOD6HVGSINCULDI
NAdIMAANDULKIADINGXIALLID6V1HAVINILVNV6NNWIWIAIDTIL SZ
DCDIPAnDdIVIVGADAAdIANNDLIADVSNIVMVMVIAKKINNVJAVBDI
CMS glAWVIAINCEEIVIAWDNIVAGNIFIHbANIDIAINAIbIsAbilnivabH
pabovvimutnninommxsavaaavxmAsavw[botinosbavxvonAAAxam
muniaa=nammaannamvbxvxlmunvviwanaminvmnixxunan
bambiabsmbsamAthrixvxotwavEnwmunonnaLaamuvnowitYmni oz
(61 :ON sm bas) J!Zcl. DZ 8 I 'clEEDU
**HHHHEIFISDOSIISJSNDDHJDNA=INHDISDDSOD6HVGSINCULDI
NAdIMAANDULKIADINGXIALLID6V1HAVINILVNV6NNWIWIAIDTIL
DUX[VbIDdIVIVGADAAdIANNDLIADVSNIVMVMVIAKKINNVJAVBDI
(MIS SlAWVIAINCEEIVIAIMMNIVAGNIFIHbANIDIAINAIbISAbITIPAItO
pabovvimutnninommixsavaaavxmAsavw[botinosbavxvonAAAxam
muniaa=nammaannamvbxvxlmunvviwanaminvmnixxunan
bambiabsmbsamAthrixvxotwavEnwmunonnaLaamuvnowitYmni
(81 :ON sm bas)PZTODISI'dEEDII 0
**HHHHEIFISDOSIISJSNDDHJDNA=INHDISDDSOD6HVGSINCULDI
NAdIMAANDULKIADINGXLIED6VIHAVINILVNVoNNVCIN1AIDTIL
DUX[VbIDdIVIVGADAAdIANNDLIADVSNIVMVMVIAKKINNVJAVBDI
(MIS SlAWVIAINCEEIVIAIMMNIVAGNIFIHbANIDIAINAIbI SAbilflIVG6H
pabovvimutnninommixsavaaHvxmAsavw[botinosbaDxvonAAAxam
muniaa=nammaannamvbxvxlmunvviwanaminvmnixxunan
bambiabsmbsamAthrnivxotwavEnwmunonnuiaamuvnowitYmni
(LT :om(JJ bas)PZTODI I I'dEEDU
8S6Z90/910ZSI1/134:1 ZI6L80/LIOZ OM
81-50-810Z 885000 VD
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
ttGBP.182C.2.Trx (SEQ ID NO: 22)
MKQLNIGVAIYKFDDTFMTGVRNAMTAEAQGKAKLNMVDSQNSQPTQNDQ
VDLFITKKMNALAINPVDRTAAGTBDKAKQANIPVVFFNKEPLPEDMKK
WDKVYYVGAKAEQSGILQGQIMADYWKAHPEADKNHDGVMQYVMLMGEPG
HQDAILRTQYSIQTVKDAGIKVQELAKDYANCDRVTAHDKMAAWLSSFGD
KIEAVFANNDDMALGAIEALKSAGYFTGNKYIPVVGVDATAPGIQAIKDG
TLLGTVLNDAKNQAKATFNIAYELAQGITPTKDNIGYDITDGKYVWIPYK
KITKDNISDAEQGGSSDKIIHLTDDSFDTDVLKADGAILVDFWAEWCGPC
KMIAPILDEIADEYQGKLTVAKLNIDQNPGTAPKYGIRGIPTLLLFKNGE
VAATKVGALSKGQLKEFLDANLAGGSHHHHHH
Trx.ttGBP.182C.2 (SEQ II) NO: 23)
MSDKIIHLTDDSFDTDVLKADGAILVDFWAEWCGPCKMIAPILDEIADEY
QGKLTVAKLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSKQLNIGVAIYKFDDTFMTGVRNAMTAEAQGKAKLNMVD
SQNSQPTQNDQVDLFITKKMNALAINPVDRTAAGTIIDKAKQANIPVVFF
NKEPLPEDMKKWDKVYYVGAKAEQSGILQGQIMADYWKAHPEADKNHDGV
MQYVMLMGEPGHQDAILRTQYSIQTVKDAGIKVQELAKDYANCDRVTAHD
KMAAWLSSFGDKIEAVFANNDDMALGAIEALKSAGYFTGNKYIPVVGVDA
TAPGIQAIKDGTLLGTVLNDAKNQAKATFNIAYELAQGITPTKDNIGYDI
TDGKYVWIPYKKITKDNISDAEQGGSHHHHHH
Adaptor Proteins
Adaptor0 (SEQ II) NO: 24)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor1.0 (SEQ II) NO: 25)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGICGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor2.0a (SEQ II) NO: 26)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGICGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor2.0b (SEQ II) NO: 27)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGICGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor3.0 (SEQ II) NO: 28)
MSAKIIHLTDCSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor4.0 (SEQ II) NO: 29)
154
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
MSAKIIHLTDDSFDCDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor5.0 (SEQ ID NO: 30)
MSAKIIHLTDDSFDTDVLKACGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor6.0 (SEQ II) NO: 31)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAECCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor7.0 (SEQ II) NO: 32)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKCIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor8.0 (SEQ II) NO: 33)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDCIADEY
QGKLTVAMLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor9.0(SEQ II) NO: 34)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGCLTVAMLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor10.0 (SEQ II) NO: 35)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDCNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor11.0 (SEQ II) NO: 36)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKCGIRGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor12.0 (SEQ II) NO: 37)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGIRGIPTLLLFKNGECAATKVGALSKGQL
KEFLDANLAGGSHHHHHH***
Adaptor13.0 (SEQ II) NO: 38)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKCGALSKGQL
KEFLDANLAGGSHHHHHH***
155
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Adaptor14.0 (SEQ ID NO: 39)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSCGQL
KEFLDANLAGGSHHHHHH***
Adaptor15.0 (SEQ ID NO: 40)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGCL
KEFLDANLAGGSHHHHHH***
Adaptor16.0 (SEQ ID NO: 41)
MSAKIIHLTDDSFDTDVLKADGAILVAFWAEWCGPCKMIAPILDEIADEY
QGKLTVAMLNIDQNPGTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQL
KEFLDCNLAGGSHHHHHH***
EXAMPLES
Examples are provided below to facilitate a more complete understanding of the
invention. The following examples illustrate the exemplary modes of making and
practicing
the invention. However, the scope of the invention is not limited to specific
embodiments
disclosed in these Examples, which are for purposes of illustration only,
since alternative
methods can be utilized to obtain similar results.
EXAMPLE 1: SENSING CHLORIDE USING ngmFRET EFFECTS ENGINEERED
INTO YELLOW FLUORESCENT PROTEIN.
As the first, simplest, example of ngmFRET, we use YFP, a mutant of Green
Fluorescent Protein (GFP) that contains a serendipitous chloride-binding site
located next to
its fluorophore (Wachter 1999, Jayaraman 2000, Kuner 2000). Chloride binding
elicits a
monochromatic response of the fluorophore, quenching its fluorescence with
increasing
chloride concentrations (by itself and regardless of the presence of any other
fluorophore/parter). Binding is not accompanied by a protein conformational
change
(Grimley et al. 2013), therefore clearly ruling out any distance-dependent
effects on energy
transfer, which limits the number of possible photophysical models.
The YFP fluorophore is formed by an autocatalytic cyclization of a tripeptide
located
in an interior a helix (Zimmer 2002). It exists in four possible states
(anionic, cationic,
zwitterionic, and neutral), of which only the anionic fluorophore is
fluorescent(Elsliger 1999)
(FIG. 6). Binding of chloride at a site located adjacent to the fluorophore
destabilizes the
156
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
anionic form, raising the excited state pK: resulting in protonation of the
fluorophore and
quenching of its fluorescence emission intensity(Grimley et al. 2013). The YFP
fluorescence
emission spectrum changes intensity but not shape in response to chloride
binding
(monochromatic response).
We have set up an ngmFRET system in which the fluorophore functions as a
directly
responsive acceptor, with an indirectly responsive donor site-specifically
coupled on the YFP
surface. In this system both donor and acceptor intensities change in response
to chloride
binding. Our analysis has shown that in the absence of inter-fluorophore
distance changes, as
is the case for these YFP conjugates, the emission intensity of the indirectly
responsive donor
can change only if the efficiency of resonance energy transfer from the donor
to the directly
responsive acceptor changes as a consequence of alterations in the degree of
spectral overlap
due to ligand-mediated shifts in the excitation spectrum of the directly
responsive acceptor.
To construct this ngmFRET system, we used the laYFP variant (Grimley et al.
2013
(FIG. 7), which binds chloride at a site ¨14 A from the YFP phenolic hydoxyl
with affinity of
¨160 mM at neutral pH. A parent construct (C1BP0) was designed for
heterologous
expression in Escherichia coli by optimizing the nucleotide sequence of the
open reading
frame (Allert, Cox and Hellinga 2010). Thirteen single cysteine mutants (C1BP
2-14) were
designed for attachment of the extrinsic indirectly responsive donor
fluorophore, located in
an annulus around the main YFP (3-barrel and at both ends of the barrel (FIG.
8).
Acrylodan (Prendergast 1983) and Pacific Blue(Sun 1998) were each attached to
purified mutant proteins. In these conjugates both donor and acceptor
intensities changed in
response to chloride and pH, and the ratio of acceptor to donor intensities
decreases (FIG. 9;
Table 2). This can happen only if the directly responsive acceptor undergoes
dipole
switching such that its absorption spectrum undergoes a bathochromic shift and
alters the
energy transfer coupling factor, thereby decreasing energy transfer efficiency
and increasing
the intensity of the indirectly responsive donor (equation 16). Chloride
binding decreases the
fluorescence of the unlabeled YFP, indicating that increased directly
responsive acceptor
quenching also plays a role in the photophysics of this ngmFRET system. This
pattern
corresponds to model a+ in Table 1.
157
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Table 2. Chloride affinities and pKa values of semisynthetic YFP chloride
sensors'.
Acrylodanb Pacific Blue
Kd(C1)(mM) Kd(C1)(mM)
Name Mutation Location aPPKd fru% pKa aPPKd iraeKd PKa
C1BP2 E17C 01 308 52 5.8 30 60 5.6
C1BP3 E32C 02 19 46 5.8 76 126 5.8
C1BP4 T43C 03 41 129 5.3 96 136 4.4
C1BP5 E95C 134 37 77 5.7 220 340 <4
C1BP6 R109C 135 77 106 5.4 208 545 <4
C1BP7 R122C 06 35 65 5.4 180 360 <4
C1BP8 N149C 137 23 34 5.8 122 106 4.4
C1BP9 N164C 08 26 70 5.3 259 364 <4
C1BP10 Y182C 09 35 88 5.2 105 260 <4
C1BP11 Q204C Om 72 55 5.2 310 242 <4
C1BP12 L221C On 40 140 3.9 167 276 <4
C1BP13 H77C End 20 55 5.6 150 290 <4
C1BP14 D173C End 78 110 5.4 217 212 <4
a Determined by fitting ratiometric signal of the intensities measured at k1
and k2 to
equations 20-25.
bRatiometry: ki=530 nm, k2=500 nm.
cRatiometry: k 1=530 nm, k2=455 nm.
The collection of thirteen YFP conjugates (Table 2) illustrates that the
quantitative
details of their ngmFRET behavior have profound effects on sensor utility,
even if all
constructs evince ratiometric responses to chloride binding. The emission peak
of Acrylodan
is centered at ¨500 nm, whereas Pacific Blue emits at 455 nm. The excitation
maximum of
YFP is centered at ¨515 nm. The spectral overlap with the YFP acceptor
therefore is much
greater for Acrylodan than for Pacific Blue donors. Consequently the donor
emission
intensity is weak in Acrylodan conjugates, as most of their excited state
energy is transferred
to YFP, whereas the corresponding intensities of Pacific Blue donors are much
stronger.
Furthermore, the Pacific Blue peak is more responsive than Acrylodan, as small
shifts in
spectral overlap by changes in the directly responsive YFP acceptor have a
relatively larger
effect on the former than the latter. Even among the Pacific Blue conjugates,
there is
quantitative difference in the degree of overlap, depending on the attachment
position, with
concomitant changes in behavior (Table 2). Effective dichromatic chloride
sensors therefore
are constructed by balancing the degree of spectral overlap such that the
donor intensity
contributes significantly to the signal.
The response of biosensors based on Langmuir binding isotherms is most
sensitive at
analyte concentrations that match the apparent Kd value of the protein (Marvin
et al. 1997).
158
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
The various Acrylodan and Pacific Blue conjugates therefore can be used to
measure chloride
concentrations over two orders of magnitude, in the 10-1000 mM concentration
range. These
concentration ranges are relevant to water quality determination, corrosion,
and clinical
chemistry. The clinical reference range (Burtis 2012) for chloride
concentrations is 90-120
mM (normal serum range 97-107 mM). The Pacific Blue conjugates of C1BP3, 6, 8
and 10
exhibit aPPKd values centered around 100 mM Cl" in solutions at pH 7.4 with
ionic strengths of
¨150 mEq (conditions that match that of serum(Burtis 2012)), and therefore are
good
candidates for use in point of care (POC) technology. The pKa values of these
variants are
less than 4; fluctuations in proton concentrations around pH 7.4 therefore
will not interfere
with the functioning of these biosensors, which is an important consideration
in the
development of chloride detection technologies.
EXAMPLE 2: SENSING GLUCOSE USING ngmFRET EFFECTS ENGINEERED
INTO A THERMOSTABLE GLUCOSE, BINDING PROTEIN.
As a second, more complex example we use a glucose-binding protein in which
the
directly responsive fluorophore is site-specifically coupled to a cysteine
mutant located in the
binding pocket of the protein. The indirectly responsive partner is placed
either on a C-
terminal PZif or N-terminal thioredoxin fusion, using the multiple thiol-
labeling strategy
described above. We provide examples of directly responsive donors and
acceptors.
A thermostable homolog of Escherichia colt glucose-galactose binding protein,
ecGGBP (Vyas, Vyas and Quiocho 1988), was identified in the thermophilic
bacterium
Thermoanaerobacter thermosaccharolyticum. Additional non-limiting examples of
glucose-
galactose binding proteins, as well as other ligand-binding proteins, are
disclosed herein.
Additionally, non-limiting examples of glucose-galactose binding proteins are
described in
PCT International Patent Application No. PCT/US2016/050297, filed September 2,
2016.
This homolog, ttGGBP, is a member of the bacterial periplasmic-binding protein
(PBP)
superfamily, which has provided several ligand-responsive reagentless
fluorescent sensors
(Grunewald 2014). The structure of PBPs comprises two domains connected by a
hinge
(Bemtsson et al. 2010). Ligand binding shifts a conformational equilibrium via
a hinge-
bending motion from an ensemble of predominantly "open" states to a "closed"
state in
which the bound ligand is enveloped within the interface between the two
protein domains.
Using site-specific modification of cysteine mutants, environmentally
sensitive fluorophores
have been placed strategically to detect these ligand-mediated conformational
changes (de
159
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Lorimier et al. 2002). The resulting semi-synthetic, fluorescent PBP
conjugates then function
as reagentless, fluorescent biosensors for their cognate ligands.
We constructed a series of single cysteine mutants in ttGGBP (FIG. 10) and
evaluated
the fluorescent responses of Badan and Acrylodan conjugates at these
positions. Three of
the seventeen positions exhibited dichromatic responses with one or both of
these
fluorophores (Table 3). The best ratiometric responses were seen for Badan and
Acrylodan
attached to Fl7C and W182C, respectively. A series of additional mutations
were introduced
within these two cysteine mutants to tune their glucose affinities (Table 4).
Finally, we tested
the responses of a variety of different fluorophores (FIG. 11) at these two
positions (Table 5).
Most of these fluorophores evinced monochromatic responses, with the exception
of the
W182C=IAEDANS conjugate which exhibited a small change in emission spectrum
shape
upon binding glucose. These singly labeled conjugates form a dataset of
directly responsive
fluorophore from which examples were drawn to construct glucose-sensing
ngmFRET
systems.
Table 3. Glucose response of Acrylodan and Badan conjugates in a cysteine scan
of the
ttGGBP scaffold.
Mutation Class' Shapeb Conjugate' aP 7' Emission Kdd,e
(K) wavelen,gth (nm) (Inn
M A2
aPPICa trueKd
Y11C P m A 351 511 470 0.12 0.16
d B 349 492 528 0.28 0.24
T16C P m A 351 519 460 8.8 12
m B 349 nb nb
F17C e d A 349 482 542 0.08 0.06
d B 346 467 491 0.087 0.26
N42C P A 350 nb nb
B 324- - nb
nb
V67C P A 350- - nb nb
B 322- - nb
nb
R91C e m A 349 491 540 0.18 0.17
B 348 nbd nbd
E92C P A 350- - nb nb
B 346- - nb
nb
AMC P m A 350 515 550 0.19d 0.11d
m/d B 348 523 550 0.64 0.55
Q148C p A 351 nb nb
B 349- - nb
nb
H151C e m A 351 511 489
0.012 0.025
160
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
m B 348 523 550
0.018d 0.027d
Q152C P A 351 - - nbd nbd
B 349 - - nb
nb
N181C P A 350 - - nb nb
B 349 - - nb
nb
W182C e d A 347 479 526 2.3 2.3
m B 347 515 550 27 19
D183C P A 348 - - nbd nbd
B 348 - - nb
nb
L257C a A 352 - - nb nb
B 348 - - nb
nb
D259C a A 349 - - nb nb
B 347 - - nb
nb
K300C a A 349 - - nb nb
B 348 - - nb
nb
aa, allosteric; e, endosteric; p, peristeric.
bm, monochromatic; d, dichromatic (i.e. spectral shape change).
A, Acrylodan; B, Badan.
dnoisy data and or bad fit.
enb; no binding, nd; not determined.
Table 4. Responses of mutant ttGGBP17C and ttGGBP182C conjugates'.
Emission
aP T. wavelength Glucosed Galactosed
Protein Mutation Classb Conjugate' (nm) S
(K) 2,1 X2 aPPKd tniaKd tin%
(n1M) (n1M) (nlivi)
ttGGBP17C B 346 467
519 0.10 0.15 0.19 1.3
99 A 349 487 515 0.08 0.09
3.8 43
ttGGBP17C.1 R91K,Q148E 2 B 463
515 0.6 0.8 0.4 0.46
ttGGBP17C.2 R69P,Q152P 2 B 350 479
523 8.6 10.8 5.2 0.48
99 99 2 A 492 515 15 18 5.0 0.28
ttGGBP17C.3 T16N,D211A 2 B 346
471 531 3.4 4.1 2.5 0.61
99 99 2 A 495 529 1.4 1.5 0.53
0.35
ttGGBP17C.4 H151Q 1 B 347 511 457 16
6.4 14 2.2
99 99 1 A 488 550 50 48
14 0.29
ttGGBP17C.5 D15A 1 B 345 487 530 16
16 4.2 0.26
99 99 1 A 348 483 498 6 6 2.4 0.41
ttGGBP17C.6 DISE 1 B 346 467 525 3.3
3.6 3.6e 1
ttGGBP17C.7 D15N 1 B 347 483 515 0.75
1.0 0.64 0.64
99 99 1 A 348 483 515 0.3 0.3 0.3
0.91
ttGGBP17C.8 T16N 2 A 348
487 529 0.61 0.60 0.26 0.43
ttGGBP17C.9 T16S 2 A 487 520 0.2 0.20
nd
ttGGBP17C.10 G20A 3 A 351 487 520 0.4
0.4 nd
ttGGBP17C.11 T240A 3 A 348 487 500 0.04
0.04 nd
ttGGBP17C.19 N258D 3 B 344 nbe nbe nbe
ttGGBP17C.20 N258S 3 B 344 nbe nbe nbe
ttGGBP17C.21 N258A 3 B 345 532 494 61
69 nb
ttGGBP17C.22 A260N 3 B 343 523 492 12 15 530e
ttGGBP17C.23 A260Q 3 B 344 527 490 15
18 nbc
ttGGBP17C.24 A260R 3 B 344 490 515 1.8
1.7 29 17
ttGGBP17C.25 A260K 3 B 515 493 3.2 3.7
nbe
ttGGBP17C.26 A260W 3 B 346 523 509 0.9
0.9 14 16
161
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
ttGGBP17C.27 A260F 3 B 346 523
490 0.2 0.3 8.8 33
ttGGBP17C.28 A260Y 3 B 346 523
494 0.06 0.07 2.6 37
ttGGBP17C.29 A2605 3 B 343 527
496 2.1 2.2 250 114
ttGGBP182C A 347 472 535 2.2 2.3 3.3
1.4
ttGGBP182C.2.0f R91K Q148E 2,3 A 346 475 545 4.5 6.0 18.5
3.1
ttGGBP182C.2.1g A1545 1 A 328 480 552 3.0 4.1 13.1
3.2
ttGGBP182C.2.3g A154N 1 A 346 470 537 16.3 19.0 207
10.9
ttGGBP182C.2.4g A154M 1 A 346 472 540 0.4 0.4 4.5
11.2
ttGGBP182C.2.5g H151Q 1 A 346 477 542 10.8 13.0 20.3 1.6
ttGGBP182C.2.6g H151N 1 A 347
475 537 19.2 17.9 52.6 2.9
ttGGBP182C.2.7g H151F 1 A 346 475 545 121 124
ttGGBP182C.2.8g D15N 1 A 343 474 542 179 209 372
1.8
ttGGBP182C.2.9g A154F 1 A 345 512 477 0.4 0.3 1.6
4.0
ttGGBP182C.3f R91K 2 A 346 494 459 1.8 0.9 3.1
3.4
ttGGBP182C.4f Q148E 2 A 347 475
542 0.6 0.8 6.5 8.1
ttGGBP182C.5f R69P Q152P 2 A 349 477 535 7.5 8.8 7.4
0.8
ttGGBP182C.6f T16N D211A 2 A 345 472 530 21.6 27.6 81 2.9
ttGGBP182C.7f R91K Q1485 2,3 A 346 478 550 0.3 0.4 1.1
3.7
R91K
ttGGBP182C.8f 2,3 A 346 475 545 28.7 44.2 256 5.8
Q148K
ttGGBP182C.9i D15N 1 A 343 475 540 75.1 76.4 282 3.7
'Measured on the Nanodrop at room temperature. kina,, is the wavelength
corresponding to
the maximum emission intensity. Optimal ratiometry wavelengths are determined
according
to the analysis described in Materials and Methods. The tmeKd is determined
from
monochromatic titration curves; aPPKd from dichromatic ratiometry (equations
20-25).
Average relative error in the trueKd values is 5%, in the aPPKd values, 1%. S
is the selectivity
between glucose and galactose, S=mleKd(galactose)/ mieKd(glucose); S>l,
selective for
glucose.
pl, PCS; 2, inter-domain interaction; 3, contact between protein and
fluorophore
CA, acrylodan; B, badan.
dnb, no bonding; nd, not determined.
eNoisy data or bad fit.
'Additional mutation constructed in ttGGBP182C.
gAdditional mutation constructed in ttGGBP182C.2Ø
Table 5. Responses of fluorophores conjugated to F17C and W182C mutants of
ttGGBP'.
b Xex alxiXmax aPoimax satxmax satimax true-
d
Position Fluorophore
(nm)` (nm) (AU x1000) (nm) (AU x1000) (mNI)
17C Acrylodan 391 487 15.9 487 20.0
0.2
Badan 391 519 12.8 467 52.6 0.1
5-IAF 491 523 64.3 523 70.6 61.3
Oregon green 496 523 101.9 523 91.7 -
CPM 384 471 78.5 467 90.9 17.0
IANBD 478 531 15.3 535 19.8 11.8
IAEDANS 336 467 12.5 467 14.4 -
Pacific Blue 410 451 28.6 455 108.6 60.7
182C Acrylodan 391 479 55.0 515 17.2
6.0
Badan 391 515 16.8 515 11.9 64.9
5-IAF 491 519 255.0 519 453.2 5.0
6-IAF 491 513 50.4 513 63.1 750
162
CA 03005883 2018-05-18
WO 2017/087912 PCT/US2016/062958
Oregon green 496 519 78.2 519 186.2 20.0
CPM 384 479 49.5 483 40.3 6.8
IANBD 478 543 29.1 547 12.5 210
IAEDANS 336 487 3.7 483 7.9 0.2
Pacific Blue 410 455 115.0 455 119.4 -
BODIPY 499 499 519 40.1 515 80.2 30.7
BODIPY 507 507 531 32.9 535 31.4 11.0
Alexa 488 495 519 211.6 519 182.2 -
Alexa 532 532 551 63.0 551 61.0 -
Alexa 546 546 571 152.1 571 150.2 -
Texas Red 595 611 23.3 611 23.5 -
Cy5 646 663 19.2 663 23.3 210
PyMPO 415 555 3.7 559 4.2 20.6
akex, preferred excitation wavelength (from supplier); aP 2k.max, observed
maximum emission
wavelength of the apo-protein; aP /max, observed intensity at ank.max; satA,,
, max, observed
maximum emission wavelength of the glucose complex; sat/max, observed
intensity at satkmax;
true/Cd, affinity determined from fit of equations 20-25 to the monochromatic
emission
intensities. Emission spectra were measured on the Nanodrop3300, using -10 iiM
protein.
The observed absolute emission intensities are a rough guide to the relative
brightness of the
conjugate, because the protein concentration was approximately the same for
each
experiment.
bAbbreviations, chemical names and supplier catalogue numbers as follows:
Acrylodan
(A433); Badan (B6057); 5-IAF (130451); Oregon Green 488 (06034); CPM (D346);
IANBD
(D2004); IAEDANS (114); Pacific Blue (P30506); BODIPY 499 (D20350); BODIPY 507
(D6004); 6-IAF (130452); Alexa 488 (A10254); Alexa 532 (A10255); Alexa 546
(A10258);
Texas Red (T6008); PyMPO (M6026) from Life Technologies and Cy5 (13080) from
Lumiprobe.
eThe Nanodrop3300 fixed wavelength LED that most closely matched kex was used
(see
Materials and Methods).
Conversion of monochromatic to dichromatic responses.
Case 1: Directly responsive donor, indirectly responsive acceptor; pure
quenching
(model cr 09)
The singly labeled Pacific Blue conjugate at F 17C exhibits a strong increase
in
emission intensity without significant shifts in emission wavelength maxima in
response to
glucose (FIG. 12A), suggesting that the changes are due primarily to a
decrease in the non-
radiative decay rate of its excited state in the glucose complex. In the
doubly labeled
Fl7C Pacific Blue f3Zif 5-IAF fusion the emission intensities of both the
directly responsive
donor and indirectly responsive acceptor peaks increase with glucose addition
(FIG. 12B).
This correlated increase of both donor and acceptor emission intensities is
inconsistent with
effects confined to changes in the energy transfer coupling factor, 0. The
lack of observable
163
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
changes in the emission spectrum, indicates that the ngmFRET is modulated only
by
differences in the non-radiative decay rate on the Pacific Blue directly
responsive donor in
the ligand-free and ¨bound protein (model d 0 , Table 1). In model JO,
modulation of the
indirectly responsive acceptor emission intensity are due to changes in the
resonance transfer
rate which increases as a consequence of decreased the donor non-radiative
decay rate (by
itself and regardless of the presence of any other fluorophore/parter).
The PZif fusion domain is located at the back of the ttGGBP hinge region,
close to the
attachment point of the directly responsive fluorophore at position Fl7C or
W182C (FIG.
13), forming an ensemble of indirectly responsive fluorophore conformations.
In this
arrangement, the ligand-mediated protein conformational change that converts
the open to the
closed state is unlikely to change the average distance or orientation between
the single
directly responsive fluorophore conformation and the ensemble of indirectly
responsive
fluorophores. The behavior of the F17C= Pacific Blue f3Zif 5-IAF ngmFRET pair
is
consistent with this interpretation. Distance contributions therefore can be
ruled in the
interpretation of the intensity change pattern.
Singly labeled Acrylodan attached to position 111C also exhibits a
monochromatic
increase in emission intensity in response to glucose binding. When paired as
a directly
responsive donor with f3Zif Alexa532 as the indirectly responsive acceptor,
this
monochromatic signal is converted to a dichromatic one (Table 7). As with the
F17C=Pacific
Blue ¨ f3Zif IAF example described above, donor and acceptor emission
intensities increase
unequally upon binding glucose, enabling ratiometry, and indicating that the
JO model is
operational in this construct as well. By contrast, glucose binding evinces
only a
monochromatic signal in the 111C=Acrylodan - f3Zif IAF system. In this
construct the
wavelengths of the Acrylodan donor and IAF acceptor emission peaks are so
close together
that their changes in either cannot be resolved independently. The same
effects are observed
for the Badan conjugate attached to position 151C both singly labeled and
partnered with
f3Zif Alexa532 and f3Zif IAF (Table 7). These observations illustrate the
importance of
carefully tuning donor-acceptor optical properties in order to obtain
dichromatic signals, even
if ngmFRET between the two partners can be established.
164
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Case 2: Directly responsive donor, indirectly responsive acceptor; pure
quenching
(model d+ 0 )
In contrast to the Badan conjugate described above, at positon 151C the singly
labeled
Acrylodan conjugate exhibits a monochromatic emission intensity decrease. When
partnered
with an indirectly responsive gif Alexa532 acceptor, both donor and acceptor
intensities
decrease (Table 6). This behavior is consistent with the pure quenching
(change in the ratio
of radiative to non-radiative emission rates) model di-0 (Table 1). For the
reasons described
above, the 151C=Acrylodan - f3Zif IAF system evinces a monochromatic response.
Case 3: Indirectly responsive donor, directly responsive acceptor: combined
spectral
shift and quenching (model a- 0).
The fluorescence intensities of W182C conjugates singly labeled with closely
related
members of the fluorescein family, 5-IAF and Oregon Green (FIG. 11D and E),
increase in
response to glucose binding (Table 5). This observation suggests that the
glucose complexes
of these two conjugates exhibit a significant decrease in the relative rates
of non-radiative
decay.
These conjugates were paired as directly responsive acceptors with Pacific
Blue as
their indirectly responsive donor. The indirectly responsive donor emission
intensity
increased in concert with that of the directly responsive acceptor in both
ngmFRET pairs
(FIG. 14). As described for YFP in the first example, such a pattern is
inconsistent with pure
geometrical effects. The "feedback" to the indirectly responsive donor
indicates that
ngmFRET efficiency has diminished by a bathochromic shift in the directly
responsive
acceptor excitation spectrum, resulting in increased donor emission. This can
occur only if
the directly responsive acceptor switches dipoles, consistent with the
presence of multiple
excited states in Fluorescein and Oregon Green (Martin 1975). The increase in
intensity of
the singly labeled conjugates indicates that quenching decreases upon glucose
binding. The
modulation mechanism therefore combines quenching and ngmFRET coupling
effects: model
a4 (Table 1).
In both systems, the fluorescent response pattern changes with increased
glucose
concentrations, shifting from an increase (Phase I) to a decrease (Phase II)
in the QD/QA ratio
(Fig. 14), indicating that there are two types of glucose responses. This
phenomenon strongly
suggests the presence of a second, much weaker affinity glucose-binding site
that influences
165
CA 03005883 2018-05-18
WO 2017/087912 PCT/US2016/062958
the environment of the directly responsive acceptor. The intensity of the
indirectly
responsive donor continues to increase with glucose concentration in Phase II,
indicating a
further decrease in 0 with a concomitant bathochromic shift of the acceptor
absorption
spectrum. The pattern of changes in the QD/QA ratio is quantitatively
dependent on the
change in magnitudes of a and 0 in the ci 0" model (Table 1). Accordingly, the
change in the
direction of the ratiometric signal indicates that the relative sizes of the
change in 0 and a are
different in Phases I and II.
Table 6. Glucose affinities of ngmFRET systems in Yl1C, A111C, and H151C
ttGGBP
conjugates.
Emission
wavelengths (nm) Affinity (mM)a
Conjugate Xi X2 aPPKd iraeKd
11C=Badan gif5-IAF 480 565 0.16 0.16
110Badan gifAlexa532 480 565 0.14 0.14
1110Acry1odan gifAlexa532 495 575 0.015 0.012
1510Acrylodan gifA1exa532 520 575 0.28 0.11
1510Badan gif=A1exa532 515 580 0.01 0.002
'Determined by fitting ratiometric signal of the intensities measured at Xi
and k2 to equations
20-25.
Table 7. Ligand affinities of ngmFRET systems of affinity-tuned mutants within
the Fl 7C
and W182C ttGGBP background.
Emission aPPKd (11.1W
wavelengths
(nm) Glucose Galactose
Conjugate X1 X2
R91,Q148 R91K,Q148E R91,Q148 R91K,Q148E
170Badan gif5-IAF 467 520 0.2 0.2
170Badan gifA1exa532 467 560 0.2 0.2
170Pacific Blue 13ZiF5-1AF 456 520 31 19
1820Acrylodan gif5-IAF 465 520 2.0 4.4 3.7 18.4
1820Acrylodan gifA1exa532 480 549 1.7 3.6
182054AF 13Zif=Pacific Blue 455 520 2.9 9.9 nbb
18200regon Green
13Zif=Pacific Blue 455 520 1.8 11.6 nbb
1820IAEDANS gif5-IAF 465 520 0.1 5.3
'Determined by fitting ratiometric signal of the intensities measured at k1
and k2 to equations
20-25.
bNo binding. Phase II response only.
166
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Improvements of dichromatic responses.
Case 1: enhancing intensities in strong ratiometric signals (models . 1 0- and
d* 0 ).
Single Badan and Acrylodan conjugates attached to the F17C and W182C cysteine
mutations within the glucose-binding site of ttGGBP exhibit strong dichromatic
responses to
glucose (FIG. 15). Remarkably, the emission intensity maxima shift in opposite
directions at
these two positions: glucose binding evinces a bathochromic shift for
182C=Acrylodan,
whereas F17C=Badan exhibits a hypsochromic response. Analysis of the emission
intensity
(FIG. 16) and absorption spectra (FIG. 17) indicated that glucose binding
alters the
populations of two dominant electronic transitions in both the excited (Si and
S2) and ground
(Gi and G2) states. These two conjugates therefore switch dipoles in response
to glucose
binding. In the F 1 7C=Badan hypsochromic response, the dominant excited state
electronic
transition shifts from Si (green) in the apo-protein, to S2 (blue) in the
glucose complex; in the
182C=Acrylodan bathochromic response, the opposite redistribution is observed,
and the
glucose complex is dominated by the Si (green) excited state. Similarly, in
the ground state,
the hypsochromic response shift the electronic transitions in the absorbance
spectra from a
low- (Gi) to a high-energy state (G2); whereas in the bathochromic response
the shift is
G2¨> Gl .
Doubly-labeled conjugates were prepared, using C-terminal PZif fusions, in
which
Fl7C=Badan and W182C=Acrylodan function as directly responsive donors and
f3Zif 5-IAF or
f3Zif Alexa532 as indirectly responsive acceptors (Table 7). These two
acceptors have
different spectral characteristics and degrees of spectral overlap with their
donors.
All four conjugates exhibit significant changes in fluorescence intensities in
response
to glucose (FIG. 18). These responses can be divided into two glucose
concentration phases.
In Phase I which covers the 0-50 mM glucose concentration range, the directly
responsive
donors exhibited large changes in emission intensities (increases for
Fl7C=Badan, decreases
for W182C=Acrylodan conjugates), whereas the indirectly responsive acceptors
showed only
small, but discernable changes. At >50 mM glucose concentrations in Phase II,
the
intensities of both directly responsive donor and indirectly responsive
acceptor peaks increase
in concert.
The singly labeled 17C=Badan and 182C=Acrylodan conjugates are good
ratiometric
sensors. Nevertheless, their incorporation into an ngmFRET system provides
additional
advantages. First, it extends the wavelength range over which intensities are
sufficiently
167
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
bright to be measured precisely. This is particularly clear for the f3Zif
Alexa532 acceptors,
which provide bright signals around 560 nm whereas neither the singly labeled
Badan nor the
Acrylodan constructs are particularly bright at this wavelength (compare FIGS.
15 and 18).
Second, the ngmFRET systems increase the brightness of the sensors over the
entire range.
For instance, the glucose complex of the singly labeled 182C=Acrylodan
conjugate is
approximately 50% less bright compared to the apo-protein (Fig. 15b), whereas
these two
forms differ by only ¨20% when partnered with f3Zif Alexa532.
In Phase I, both the A1exa532 and the 5-IAF indirectly responsive partners
decreased
in emission intensity in response to the F17C=Badan directly responsive
partner, whereas
when partnered with W182C=Acrylodan, they showed an increase in intensity.
This
observation is consistent with the two directly responsive partners undergoing
dipole
switches in the opposite direction: F17C=Badan, green¨*blue (hypsochromic
shift;
dim¨*bright; model cr0", Table 1); 182C=Acrylodan, blue¨*green (bathochromic
shift;
bright¨*dim; model dff, Table 1).
In Phase II, at glucose concentrations in excess of'-50 mM, the intensities of
both
donor and acceptor peaks increase in all four conjugates. This is consistent
with a second,
low-affinity glucose binding site, as observed for the directly responsive 5-
IAF and Oregon
Green acceptor system described above. Although both donor and acceptor peak
intensities
increase, the QA/QD decreases, which is inconsistent with only a decrease in
donor
quenching; a change in ngmFRET is also implicated (model 60.
In addition to using C-terminal PZif fusions, we also attached indirectly
responsive
ngmFRET partners to N- and C-terminal ecTRX fusions (Table 8). For these
experiments,
we used the affinity-tuned 17C.3 and 182C.2 variants (Table 4). In all cases,
we used
A1exa352 as the indirectly responsive ngmFRET partner. Surprisingly, ngmFRET
was not
established in either the N- or C-terminal fusions between 182C.2=Acrylodan
and ecTRX
(Table 8), nor in the N-terminal ecTRX-17C.3=Badan fusion; only the C-terminal
17C.3=Badan-ecTRX exhibited coupling between the directly responsive donor and
indirectly
responsive acceptor fluorophores (FIG. 19). The response of this fusion
exhibited pattern
similar to that observed for its PZif counter-part, indicating that, once
established, the
ngmFRET mechanism operates in a manner consistent with the character of the
directly
responsive fluorophore and the logic of the donor-acceptor arrangement. This
results also
indicates that the nature of the site-specific attachment point of the
indirectly responsive
168
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
partner is critical. For instance, the ecTRX fusions could have placed the
acceptor too far
away, or in the incorrect relative orientation relative to the donor for
efficient ngmFRET.
Table 8. Ligand affinities of ttGGBP-Thioredoxin fusion ngmFRET pairs'.
Glucose (mM)
Emission
wavelengths (nm)
Conjugate 261 k2 appiCd trued
Trx=Alexa532-ttGGBP17C.3=Badan 467 543 3.1 3.1
ttGGBP17C.3=Badan-Trx=Alexa532 467 547 3.6 3.7
Trx=Alexa532- 4.7 3.2
ttGGBP182C.2.0=Acrylodan 475 515
ttGGBP182C.2.0=Acrylodan- 5.8 4.3
Trx=A1exa532 475 519
'Determined by fitting ratiometric signal of the intensities measured at k1
and k2 to equations
20-25.
Case 2: enhancing a weak ratiometric signal (model d-q5).
The W182C=IAEDANS-gif 5-IAF (fluorescein) construct is another example of a
directly responsive donor- indirectly responsive acceptor ngmFRET pair. The
singly labeled
W182C=IAEDANS conjugate exhibits a modest hypsochromic shift in response to
glucose,
which, to a first approximation, involves two electronic transitions (FIG.
20). Gaussian fits
revealed that the population of a green state dominates in the apo-protein and
exchanges for a
shorter wavelength (blue) form in the glucose complex (FIG. 20). This behavior
is
reminiscent to that of the other naphthalene-based fluorophore, Acrylodan,
suggesting that
IAEDANS also undergoes a glucose-mediated dipole switch.
The W182C=IAEDANS-gif 5-IAF ngmFRET pair exhibits a concerted increase in
the directly responsive IAEDANS donor and indirectly responsive 5-IAF acceptor
emission
intensities in response to glucose (FIG. 20C). As with the W182C=Acrylodan
conjugate, this
response also can be divided into two phases, corresponding to high- and low-
affinity glucose
binding sites (FIG. 20D). In both phases, the response pattern is consistent
with donor dipole
switching in which a decrease in spectral overlap due to the hypsochromic
shift is
accompanied by a decrease in non-radiative decay (model chi)", Table 1).
The singly labeled Badan conjugate attached at position 11C undergoes a
green¨*blue
hypsochromic shift in response to glucose binding with a concomitant overall
increase in
intensity (Table 3). Partnering this directly responsive donor with a f3Zif
IAF or
169
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
f3Zif Alexa532 indirectly responsive acceptor results in an ngmFRET system
that exhibits
increases in the emission intensities of both donor and acceptor peaks, and a
decrease in their
ratio (Table 6), consistent with the d 0" model (Table 1). The wavelength
separation of the
donor and acceptor peaks is sufficiently large in the 11C=Badan - f3ZiPIAF to
obtain a
dichromatic signal.
EXAMPLE 3: ENGINEERING RATIOMETRIC SIGNALS USING
MONOCHROMATIC CHEMOSENSORS AND ADAPTOR PROTEINS.
Here we demonstrate that ngmFRET can be extended to convert monochromatic
chemosensors to dichromatic responses, by using an "adaptor" protein to pair
directly and
indirectly responsive fluorophores via site-specific, orthogonal conjugation
chemistries. We
used the pH-dependent response of Fluorescein (Martin 1975) as an example of a
monochromatic chemosensor. Ionization of the phenolic hydroxyl (FIG. 21) has a
pKa value
of 6.7, and is accompanied by a change in emission intensity, with the dianion
having a
higher quantum yield than the monoanion. Ionization is accompanied by a
bathochromic
shift of the absorption and excitation spectra, enabling ratiometric
measurements based on
absorption or fluorescence excitation, but not emission wavelengths(Lin 2000,
Han 2010,
Wencel 2014). Fluorescein was paired as the chemoresponsive acceptor with
Pacific
Blue(Sun 1998) or Acrylodan (Prendergast 1983) as pH-insensitive ngmFRET
donors. The
two fluorophores were attached to adaptor proteins constructed out of mutants
of Escherichia
coli thioredoxin (ecTRX), a member of the thioredoxin protein
superfamily(Holmgren 1985,
Qi 2005, Yoshioka 2015, Amer 2000), which were engineered to enable
orthogonal, site-
specific conjugation chemistries at two independently addressable sites.
Two different orthogonal fluorophore attachment chemistries were engineered.
In the
first (Adaptor1.0 and 3.0-16.0), the existing ecTRX disulfide was combined
with an
engineered surface cysteines (FIG. 22). This arrangement enables site-specific
attachment of
one thiol-reactive fluorophore to the surface, keeping the disulfide oxidized,
and a second to
the disulfide, following reductive deprotection. Adaptor1.0 is remarkably
thermostable, with
a Tin value of at least 100 C at neutral pH. In the second adaptor protein
(Adaptor2.0), we
created a "reduced alphabet" protein that lacks lysines, leaving the N-
terminus as the only
primary amine. This arrangement enables site-specific attachment of an amine-
reactive
fluorophore to the adaptor N-terminus, and a second fluorophore to the reduced
disulfide.
170
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
The thermostability of this protein is more modest at ¨60 C, but sufficient
for use in
instrumentation.
Construction of adaptor proteins.
Wild-type ecTRX is a 108-residue a/f3 protein that contains a single disulfide
linkage
within a surface loop(Katti 1990) (FIG. 22). As a starting point for building
adaptor proteins
(Adaptor0) we introduced several mutations that remove an adventitious Cu(II)-
binding site
at the N-terminus (D2A) and charged residues buried within the hydrophobic
core (D26A,
K27M) that tune the redox potential of the disulfide (Ladbury 1993, Hellinga
1992,
Langsetmo 1991b, Langsetmo 1991a) (FIG. 23). A hexahistidine purification tag
was fused
to the C-terminus. In Adaptor1.0 and 3.0-16.0, we further introduced surface
cysteine
residues. These designs enabled site-specific dual labeling with thiol-
reactive probes, the
first to the surface thiol, and the second to the disulfide following a
reductive deprotection
step.
Adaptor2.0a and 2.0b are highly unusual proteins in which we limited the amino
acid
alphabet to 19 residues, and replaced all remaining nine, surface lysines with
arginine (2.0a)
or a combination of arginine and glutamine (2.0b). In these designs, thiol-
reactive probes are
attached at the reduced disulfide, and amine-reactive probes at the N-
terminus.
The response of Adaptors 1.0 and 3.0-16.0 conjugates to pH.
Four dually labeled Adaptor1.0 conjugates were prepared, attaching the thiol-
reactive
Fluorescein derivative 5-IAF to the surface thiol or reduced disulfide, and
Acrylodan or
Pacific Blue to the corresponding, orthogonally reactive site. All conjugates
were illuminated
at 365 nm in a Nanodrop3300 spectrofluorimeter, and their emission intensity
spectra
recorded as a function of pH. All four conjugates exhibited ngmFRET from the
indirectly
responsive Acrylodan or Pacific Blue donor to the directly responsive
Fluorescein acceptor
(FIG. 24A-D, Table 9). In all cases, to a first approximation, the intensity
of the Fluorescein
acceptor decreased, but not the Acrylodan or Pacific Blue donor changed. This
pattern of
intensity changes in characteristic of a ngmFRET system in which the directly
responsive
acceptor quenching changes (a+0 , Table 1). The increase of the Fluorescein
emission
intensity with pH is consistent with the higher quantum yield of the dianion
compared to the
monoanion.
171
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Table 9. pKa values of conjugates measured by emission and absorbance
intensities'.
Fluorescein plC,
Adaptor Donor attachment Emission Absorbance
1.0 Acrylodanb R73C 6.2 6.4
Disulfide 6.1 6.6
Pacific Bluee R73C 6.2 6.8
Disulfide 6.2 6.7
2.0a Acrylodanb N-terminus 5.8 6.1
2.0b Acrylodanb N-terminus 5.8
'Determined by fitting ratiometric signal of the intensities measured at Xi
and k2 to equations
20-25.
bRatiometry: Absorbance, k1=400 nm and k2=496 nm; Emission, k1=465 nm and
k2=520
nm.
cRatiometry: Absorbance, k1=411 nm and k2=496 nm; Emission, k1=455 nm and
k2=520
nm.
The choice of fluorophore attachment sites affects the ngmFRET efficiencies
between
the donors and fluorescein acceptor, as judged by the relative intensity of
the donor emission.
Labeling of the disulfide rather than the surface R73C thiol with the donor
gives better
ngmFRET for either the Acrylodan or Pacific Blue donors. Since the distances
are
approximately the same in these two arrangements, any differences must be due
to changes in
dipole-dipole orientation (Valeur 2012, Lakowicz 2006, Clegg 1995, Wu 1994,
Cheung
1991). ngmFRET is more in efficient in the Acrylodan than the Pacific Blue
conjugates,
presumably reflecting difference in spectral overlap. Fluorescein is maximally
excited at 490
nm, which is better matched with the maximum emission intensity of planar
Acrylodan at
¨500 nm (Allert 2015), than the 450 nm maximum of Pacific Blue.
The absorption spectra of the dually-labeled conjugates shift in response to
pH (Fig.
25A-B), with a strong peak at ¨ 500 nm appearing with the formation of the
phenolate
dianion at high pH value (Martin 1975).
Adaptors 3.0-16.0 were labeled with Acarylodan/Fluorescein and Pacific
Blue/Fluorescein pairs (Table 10). All conjugates demonstrated ngmFRET
responses to
proton binding.
172
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Table 10. pKa values of Adaptor conjugatesa.
Name Cysteine Fluorophore Fluorophore Emission p/f.
position Cysteine disulfide wavelength (nm)
kl 262 aPPO4 fr1ep/4
Ada3.0 D11C Acrylodan 5-IAF 523 460 6.11 6.11
Ada4.0 T15C Acrylodan 5-IAF 523 460 6.23 6.29
Ada5.0 D21C Acrylodan 5-IAF 523 460 6.32 6.36
Ada6.0 W32C Acrylodan 5-IAF 515 460 5.66 5.64
Ada7.0 M38C Acrylodan 5-IAF 515 460 5.84 5.83
Ada8.0 E45C Acrylodan 5-IAF 515 480 6.1 5.93
Ada9.0 K53C Acrylodan 5-IAF 523 460 6.2 6.14
Ada10.0 Q63C Acrylodan 5-IAF 519 465 5.9 5.98
Ada11.0 Y71C Acrylodan 5-IAF 515 460 5.95 5.92
Ada12.0 V87C Acrylodan 5-IAF 523 485 6.22 6.23
Ada13.0 V92C Acrylodan 5-IAF 523 540 6.78b 7.05b
Ada14.0 K97C Acrylodan 5-IAF 519 465 6.15 6.16
Ada15.0 Q99C Acrylodan 5-IAF 523 540 6.54 6.83
Ada16.0 A106C Acrylodan 5-IAF 523 465 6.26 6.32
Ada3.0 D11C Pacific Blue 5-IAF 455 520 5.68 6.0
Ada4.0 T15C Pacific Blue 5-IAF 455 523 5.85 6.21
Ada5.0 D21C Pacific Blue 5-IAF 455 523 5.88 6.23
Ada6.0 W32C Pacific Blue 5-IAF 455 520 5.92 6.18
Ada7.0 M38C Pacific Blue 5-IAF 455 520 6.0 6.21
Ada8.0 E45C Pacific Blue 5-IAF 455 520 5.89 6.11
Ada9.0 K53C Pacific Blue 5-IAF 455 520 5.73 6.0
Ada10.0 Q63C Pacific Blue 5-IAF 455 523 5.76 6.27
Ada11.0 Y71C Pacific Blue 5-IAF 455 520 5.83 6.13
Ada12.0 V87C Pacific Blue 5-IAF 455 525 5.78 6.17
Ada13.0 V92C Pacific Blue 5-IAF 455 523 5.87 6.23
Ada14.0 K97C Pacific Blue 5-IAF 455 523 5.83 6.23
Ada15.0 Q99C Pacific Blue 5-IAF 455 523 5.89 6.2
Ada16.0 A106C Pacific Blue 5-IAF 455 523 5.8 6.26
aDetermined by fitting ratiometric signal of the intensities measured at Xi
and 2.2 to equations 20-25.
b
Poor fit and/or noisy data.
The response of Adaptor2.0 to pH.
The single primary amine at the amino terminus of each lysine-free Adaptor2.0
proteins was labeled with the amine-reactive Fluorescein derivative FAM.
Acrylodan was
attached to the disulfide in a second labeling reaction. Adaptor2.0a and 2.0b
both exhibited
pH-sensitive ngmFRET between the Acrylodan donor and Fluorescein acceptor
(FIG. 26A-
B). Adaptor2.0a was more soluble than Adaptor2.0b at low pH values, consistent
with their
difference in number of surface charged groups.
The relatively high Acrylodan donor emission intensity indicates that the
ngmFRET
efficiency of Adaptor2.0 is less than that of the Adaptor1.0 constructs. This
is likely to be a
consequence of orientation effects, as the distances between the donors and
acceptors are
173
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
similar in both adaptor proteins. Furthermore, the donor exhibits two emission
peaks,
suggesting that the Acrylodan conjugate is located in multiple environments,
or adopts both
twisted and planar conformations (see above). These results indicate that the
environment of
the disulfide attachment point differs in these two adaptor proteins, which
may be a
consequence of the surface redecoration with arginine residues.
The intensity of the shorter wavelength donor peak (460 nm), which is well-
separated
from the main Fluorescein emission wavelength (520 nm), does not change in
response to
pH, suggesting that the ngmFRET efficiency remains constant, consistent with a
mechanism
based on alteration of the non-radiative decay of the Fluorescein in response
to pH, as was
the case for the Adaptor1.0 conjugates. The intensities of neither the
acceptor emission, nor
its 500 nm absorption band exhibit large dependencies on pH, although the
direction of
change is the same as for Adpator1Ø This suggests that the effective charge
on the
phenolate is reduced in this construct, consistent with formation a hydrogen
bond or salt
bridge. K36 is located near to the ecTRX N-terminus (FIG. 22), and is a
possibly candidate
for forming such an interaction (K57 is adjacent to the N-terminus, but has
been mutated to
methionine in the adaptor proteins).
Adaptor protein thermostability.
The temperature dependence of the pH responses was determined for the
Adaptor1.0
R73C=Pacific Blue, disulfide=Fluorescein conjugate using a Roche LightCycler
(FIG. 27).
The thermostability of the conjugate is high, increasing from ¨87 Cat pH 4.5
to >100 C at
neutral pH and above. The temperature dependence of the pKa value is modest,
with a ACp =
0.09 kcal/mol/K.
The thermostability of the Adaptor2.0=Fluorecein, disulfide=Acrylodan is ¨60
Cat
neutral pH (FIG. 27). Unlike Adaptor1.0, thermostability does not vary with
pH. The
temperature dependence of the pKa also is modest, with a ACp = 0.004
kcal/mol/K.
The effect of the adaptor protein on Fluorescein pKa values.
The pKa values of the excited states, as determined from the emission spectra,
are
consistently lower than the corresponding ground state values obtained from
the absorption
spectra (Table 9). This effect is consistent with a Forster cycle in which the
excited state base
has a longer emission wavelength than its conjugate acid such that excited
state is more
acidic than the ground state (Valeur 2012).
The excited and ground state pKa values of the Adaptor2.0 conjugates are
significantly lower (-0.4 pH units) than those of Adaptor1.0 (Table 9). These
results indicate
174
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
that the phenolate is stabilized by the Adaptor2.0 protein matrix, consistent
with a proposed
direct interaction with a protein side-chain (see above).
EXAMPLE 4. MATERIALS AND METHODS FOR EXAMPLES 1-3
Gene construction. In all constructs used in this study open reading frames
(ORFs)
encoding engineered proteins were placed behind an efficient Shine-Dalgamo
ribosome-
binding site, and flanked by a T7 promoter and terminator at the 5' and 3'
ends respectively,
using the GeneFab program(Cox et al. 2007). All DNA sequences were first
designed in
silico, and then synthesized by oligonucleotide assembly and cloned into pUC57
by
GeneWiz, Inc. (South Plainfield, New Jersey).
The E. coli thioredoxin gene (ecTRX) DNA sequence was taken from the
electronic
genomic sequence NC_012947, protein identifier YP_003038425.1. Publicly
available
genome sequences were obtained from the National Center of Biotechnology
Information
(ftp://ftvp.ncbi.nih.gov/genomes/Bacteria/all.gbk.tar.gz). The T.
thermosaccharolyticum
glucose-galactose-binding protein, ttGGBP amino acid sequence was taken from
genome
NC _ 014410, protein identifier YP_ 003852930.1. The putative leader peptide
that mediates
secrection of ttGGBP into the periplasm was identified by alignment with the
mature form of
the Escherichia coli glucose-galactose binding protein, ecGGBP, and removing
the 49 amino
acids N-terminal to the start of the aligned mature ecGGBP amino acid
sequence. A single
endogenous cysteine residue (C207 in the mature protein) was mutated to
alanine, so that
further cysteine mutants would install a unique thiol for site-specific
labeling with
fluorophores. The 18-residue PZif amino acid sequence is or comprises
TGEKPYKCPECGKSFSRS (SEQ ID NO: 42) (Smith et al. 2005). The Yellow Fluorescent
Protein amino acid sequence is described in (SEQ ID NO: 149) (Grimley et al.
2013).
The wild-type genomic ecTRX DNA sequence was used to designs the adaptor
mutants and the fusion proteins described in this study. The ttGGBP or YFP
amino acid
sequences were back-translated into DNA optimized in context of the expression
construct by
the OrfOpt or program designed to predict highly expressed mRNA sequences in
E. coli. The
OrfOpt simultaneously imposes AU-rich nucleotide composition at the 5' and 3'
ends of the
ORF, low RNA secondary structure content and favorable codon usage (Allert et
al. 2010).
Subsequent single and multiple point mutations were designed by preparing
mutant
sequences of the synthetic ORF' sequences using GfMutagenesis, an in-house
program that
introduces point mutations into an ORF' using the most prevalent codon in E.
coli for an
175
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
amino acid. To design the sequences of the ttGGBP fusions with the PZif
peptide, the
peptide sequence was first placed behind the optimized ttGGBP sequence; the
DNA sequence
of the resulting fusion protein was then re-optimized with OrfOpt without
changing the
optimized ttGGBP portion. The N- and C-terminal fusions of ecTRX and ttGGBP
mutants
were designed by joining the respective wild-type and optimized DNA sequences
without
further manipulation. All designed protein sequences were terminated with a C-
terminal
affinity purification tag comprising hexa-histidine peptide placed behind a
GGS linker to
enable metal-mediated affinity purification(Hengen 1995).
Protein expression, purification, and fluorescent conjugate preparation.
Proteins were
prepared by fermentation in Escherichia coli and purified by immobilized metal
affinity
chromatography (IMAC) as described(Sameiro 2009). In the final purification
step,
unlabeled proteins are immobilized on the IMAC beads and labeled overnight (4
C, rotating
end-over-end) with a thiol-reactive fluorophore (five-fold stoichiometric
excess over protein).
Following two rinses with buffer (100 mM NaC1, 1mM CaC12, 20 mM MOPS, pH 6.9)
to
remove unincorporated label, the proteins were either labeled with a second
fluorophore, or
eluted from the beads. In experiments that required a second labeling step,
the immobilized
protein was first extensively washed (5x10 mL), followed by reduction of the
cysteine
residues in the PZif disulfide-containing by addition of 1 mM TCEP. After one
hour, the
protein was washed (5x10 mL), the second fluorophore was added, and incubated
as
described above. To elute labeled protein from the IMAC beads, 6 mL of elution
buffer (400
mM imidazole, 500 mM NaC1, 1mM CaC12, 20 mM MOPS, pH 7.8) was added, and the
beads removed by centrifugation. Following dialysis of the eluate against
three changes of
assay buffer (20 mM KC1, 1 mM CaC12, 20 mM MOPS, pH 7.4), using 10 kDa semi-
perimeable membrane (Snake Skin), the fluorescent conjugates were concentrated
in a 10
kDa cutoff spin concentrator (Vivaspin, GE Healthcare). Their purity was
assessed by
SDS/PAGE.
Preparation of titration series to measure ligand-binding. 12-, 24-, or 48-
point
logarithmic titration series were prepared on a Tecan Freedom liquid-handling
robot, using an
in-house program, `TitrationPlate', that compiles an abstract description of a
multi-
component titration series into machine instructions for operating the robot.
For glucose
titrations in ttGGBP constructs, concentrations were varied from 0-1.7 M in 20
mM KC1, 20
mM MOPS (pH 7.4) supplemented with either 1 mM EGTA or 1 mM CaC12. For
chloride
titrations in C1BP constructs, concentrations were varied from 0-1.95 M in 35
mM potassium
176
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
gluconate, 20 mM MOPS (pH 7.4). For pH titrations in adaptor constructs, pH
was varied
from 4.0-9.5 in 20 mM KC1 and 20 mM MOPS.
Determination of emission intensity spectra. Ligand- and wavelength-dependent
emission intensities were recorded on a Nanodrop3300 (Thermo Scientific) at
room
temperature. Using the LED closest to the optimal excitation wavelength of the
fluorophore
(UV, 365 nm; blue, 470 nm; 'white', 460-550 nm).
Ratiometric analysis of ligand binding. Isothermal urea titrations were
extracted from
the fluorescent landscape or emission spectra datasets obtained as described
above.
Monochromatic emission intensities Ix (these intensities correspond to a
bandpass intensity,
recorded either with a physical, or by integrating in the interval X-8, k-F8
in the case of an
emission spectrum), were fit to
I2=aP'9)62(1¨ Y trujEsaffi A5, frue 12
where ap0/32 and s are the fluorescence baselines associated with the
ligand-free and
ligand-bound states of the protein, respectively, and yfrue the fractional
saturation of the
protein(Layton and Hellinga 2010). Baseline functions can be constant, linear,
or a second-
order polynomial. For the ligand- and temperature-dependent fluorescence
landscapes, we
use a constant value for aP )51 x , but sat 16 x is described by a linear
dependence on urea
concentration, [L]:
sat fix =ax bx[L] 21
For a single ligand-binding site, the fractional saturation is given by
[L]
Y =r , 22
Lid+ K d
where [L] is the ligand concentration and K d the dissociation constant, "elCd
for frue.
A ratiometric signal at a given point in a titration series, R12(t), is given
by the ratio of
intensities at two wavelengths, 67(21,t), 67(22,0 in the emission spectrum
measured at that
point:
õ obs rt iii,t)
R12(t), "t A' 23
, obs r( a f
'"t '\"29`)
where a t is an attenuation factor that describes the effect of variations in
sample size (i.e. the
amount of observable fluorophore) in the tth sample on the wavelength-
independent intensity
177
CA 03005883 2018-05-18
WO 2017/087912 PCT/US2016/062958
of the entire emission spectrum. This signal removes wavelength-independent
emission
intensity attenuation effects due to variations in conjugate concentration,
photobleaching,
fluctuations in excitation source intensities, and detection
efficiency(Demchenko 2010,
Demchenko 2014). It is a key aspect for high-precision sensing using the
reagentless
fluorescently-responsive sensors described here. The ratiometric signal also
can be fit to a
binding isotherm:
R1,2 =aP/a R(1¨ YR)+satfiRY R 24
where aP fiR and sat/3R are the baselines, and R the apparent fractional
saturation of the
protein (with aPPKd ). In general, irueKd #aPPKd ; if both baselines are
constant, a simple
relationship can be derived relating aPPKd to "e1Cd(Grim1ey et al. 2013):
P I
a n
WK. d d sat i="elµ n 25
where aP A2 and sat/22 are the emission intensities of the monochromatic
signal at wavelength
X2 of the ligand-free and ligand-bound protein, respectively.
Following a fit of the titration series using equations 20-24, at values can
be recovered
by taking the average comparison of the observed and calculated intensities at
the two
wavelengths:
1 ( cak Aili,t) cak 10,2,1
a = ___________ 1 + __ n 26
t 2 obsi(i,t) 0õ702,t)
The at value can then be applied to all wavelengths to obtain an emission
spectrum or
integrated intensity of the tth titration point corrected for variations in
sample size:
corr i(A)= at obs/(2) 27
where "r7(2) and bs/(2) are the wavelength-dependent intensities of the
corrected and
observed emission spectra, respectively.
The fractional error in the chemometric concentration measurement, depends on
the
first derivative of the binding isotherm as follows(Marvin et al. 1997):
A
as ei 2 =-xí'1,2
)
_______________________________________________________________ ¨ = = X
28
S S dS )
Where R1,2 is the ratiometric signal (equation 23), ei,2 its experimental
error, and ciS is the
resulting chemometric error in the concentration. We can then define a
relative precision
function
178
CA 03005883 2018-05-18
WO 2017/087912 PCT/US2016/062958
S 1
29
P
where P(S) is the relative precision at concentration S, which reaches a
maximum value (i.e.
lowest error), Pmax, at the Ka.
For a given isothermal titration, values for aPPKd and "elCd were obtained
using a non-
linear fitting algorithm in which these two parameters were simultaneously fit
to the three
experimental binding isotherms using equations 20 and 24, with the two
monochromatic
isotherms sharing the same "elCd value. Three separate pairs of aP 16 and "tfl
were fit in this
procedure, corresponding to the two monochromatic and the ratiometic signals,
respectively.
Two distinct ratiometric response models can be used: coupled (both
wavelengths respond to
ligand); uncoupled ( the second wavelength is non-responsive; i.e. remains
constant).
Optionally, an attenuation vector, a(t) containing at values for each
titration point (equation
26), can be refined by iterative fit cycles in which the a(t) vector of a
previous cycle is used
to adjust the integrated intensities of the next cycle. The program
`Nanodrop3300' was used
analyze ligand binding.
Analysis of emission spectra components. Wavelength-dependent,/( k),emission
intensities
at were converted to wavenumber-dependent intensities(Valeur 2012, Lakowicz
2006), /(v):
/(v) = 22/(2) 30
Singular value decomposition was used for model-free identification of regions
in the
emission spectra that vary with respect to glucose concentration(Henry 1992).
An A. data
matrix was constructed by recording /(v) values of m frequencies in columns
for n titration
points in rows. This matrix was decomposed as
A. =U .S nnVn: 31
where Unin records n spectral components at m frequencies ranked by the weight
of their
contribution to the reconstruction of the experimental data, Vnn records the
contribution of the
nth component to the nth titration point, Snn records the weight of the nth
component.
Decomposition was carried using the in-house Nanodrop3300 program, written in
Python.
The linalg.svd method in the open-source Python scipy package (www.scipy.org,
version
0.7.2) was used to solve the decomposition. The relative weight of the nth
component in U.,
fn, was calculated from Snn, by normalizing the values in S with its trace:
fn = Snn(n n)
32
tr(Snn)
179
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
The fractional states of n individual electronic transitions in a spectrum
were
determined by fitting n Gaussians(Valeur 2012, Lakowicz 2006) to the emission
intensities of
the corrected spectra (equation 27) transformed into the frequency domain
(equation 30):
Cakii (V)_ E
i=n Ai e 2( 0 - 33
1 -Nlr
where pi is the wavenumber corresponding to the peak intensity of the ith
transition, Ai the
area contributed to the total spectrum by this transition, and a-the spectral
width of all
transitions. The fraction, f;, of the ith transition is given by:
Ai
A =
i=n 34
EA;
j=1
Wavelength dependent residuals are given by:
6.6/V's/H¨cak/(v) 35
Fits were carried out by minimizing the least squares difference between
observed and
calculated spectra, using simplex and conjugate gradient methods implemented
in
Nanodrop3300 (scipy package methods optimizelmin and optimizeleastsq,
respectively).
For titration series with N spectra, collected as a function of titrant
concentration, global fits
were used in which, as a first approximation, gi values were kept identical in
the apo-protein
and saturated glucose complex, and a-was universal for all transitions in all
spectra. Au
values were allowed to vary in each kth spectrum. The variation of the
fraction for each
transition,f,k, was then fit to a binding isotherm (equation 20), constraining
the fit aPPKd
value to be common to all transitions. These analyses revealed the shifts in
transition dipole
populations in response to ligand binding.
Analysis of ligand-binding properties using thermal melts. Protein thermal
stabilities were
determined by measuring the temperature-dependence of the fluorescence signal,
using a
Roche LightCycler (Layton and Hellinga 2010). The total fluorescence
intensity, S, is given
by
S = )0F fF fiu fu 36
wherefF and fu are the fractions of protein in the folded and unfolded states,
respectively, and
F and flu the fluorescence baselines of these two states. To get the fractions
of the two
states, we have
180
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
1
fN = 1+ Ku(T) and fu =1- fN 37
where Ku(T) is the temperature-dependent unfolding equilibrium constant, which
by the van't
Hoff approximation is given by
)IR
Ku = e
TTm38
Where T is the temperature, T,õ the unfolding reaction transition mid-point
temperature, and
AHu the enthalpy of unfolding.
To obtain the temperature dependence of the binding reaction, the Kd values of
all the
individually determined isotherms were fit the Gibbs-Hemholtz equation(Layton
and
Hellinga 2010):
AG: (T) =A ref1-1: + ACp,b ¨ Tr4. )¨ T AS + AC ln 39
p,b 7,
A rff
where AG: (T) is the standard free energy of binding at 1 M ligand at
temperature T,
AG: (T) = ¨RT141 + _____________________________________________________ 40
d k7'
H: and Are f S: the molar enthalpy and entropy of binding, respectively, at
the reference
temperature, Tref, and ACp,b the heat capacity of the binding reaction. This
data analysis was
carried out using `TitrationMeltAnalysis'.
RATIOMETRIC ngmFRET BIOSENSORS AND USES THEREOF
The energy transfer between a donor-acceptor pair can be responsive to ligand-
mediated changes in the photophysics of a single partner, without involving a
change in the
geometry (distance, angle) between the two partners (non-geometrically
modulated FRET,
ngmFRET). Changes in the individual fluorophore properties include alterations
in relative
rates of excited state radiative and non-radiative decay rates (quenching) and
switching
between different excited state dipoles (dipole switching). The latter can
alter both the
emission intensity spectrum, and the absorption spectrum if the ground state
electronic
structure is affected by ligand binding. The analysis that we present
(equations 8-19) shows
that the detailed mechanism by which quenching and dipole switching affects
ngmFRET
depends on whether the ligand-responsive fluorophore functions as the donor or
acceptor in
the system (Table 1). Dipole switching affects ngmFRET by altering the
spectral overlap
181
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
between the two partners. Accordingly, if the directly responsive partner is
the donor, then
effects are seen only if ligand binding modulates the excited state
(emission), whereas if it is
the acceptor, only ground state (absorption) effects are observable. Quenching
(change in the
ratio of radiative to non-radiative emission rates) effects in directly
responsive donors alter
the ngmFRET transfer rate, thereby affecting the emission intensities of both
donor and
acceptor; directly responsive acceptor quenching affects only its own
intensity. The possible
choices in quenching, dipole switching and directly responsive partner
functional role
together combine into sixteen different scenarios by which ngmFRET occurs
(Table 1).
We have constructed over 15 different donor-acceptor pairs in three different
protein-
based, semi-synthetic, fluorescent biosensors, all of which show ratiometric
responses to
binding of their cognate ligands (Table 11). Together these conjugates
represent five of the
sixteen possible ngmFRET scenarios. Pure geometry-based (tgmFRET) effects
alter the
donor and acceptor emission intensities in opposing directions only. Seven
conjugates
exhibit parallel ligand-mediated intensity increases or decreases, which
clearly indicate the
presence of non-geometrical ngmFRET effects. Quantitative differential
differences between
the donor and acceptor changes the shape of the emission spectrum. In three
rigid conjugates,
the intensity of a donor changes when partnered with directly responsive
acceptor. This
effect can occur only if the acceptor ground state absorption spectrum changes
in response to
ligand binding, providing clear evidence for dipole switching effects.
25
182
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Table 11. Summary of observed intensity response patterns and ngmFRET
mechanisms.
Response pattern'
Ligand Donor Acceptor A/D
Construct ratio
Modelb
YFP 17C.Pacific Bluee Chloride 1' si, si, a+
ttGGBP 11C.Badan gif.5-1AF Glucose 1' si, si, d 0"
ttGGBP 11C.Badan gif. Alexa532 1' si, si, ct
ttGGBP 17C.Pacific Blue gif.5-1AF 1' 1' 1' d 0
ttGGBP 17C.Badan f3Zif.5-IAFd 1' 4, 4, ct
ttGGBP 17C.Badan gifA1exa532 d 1' 4, 4, dgY
ttGGBP 17C.Badan C- 1' 4, 4, d 0"
ecTRX.Alexa532
ttGGBP 111C.Acrylodan 1' 1' si, d 0
f3Zif. Alexa532
ttGGBP 151C.Acrylodan si, si, 1' d+0
f3Zif. Alexa532
ttGGBP 151C =Badan f3Zif. Alexa532 1' 1' 1' ctO
ttGGBP 182C.Acrylodan gif.5-1AF si, 1' 1' 6'0+
ttGGBP 182C.Acrylodan si, 1' 1' 6'0+
f3Zif. Alexa532d
ttGGBP 182C.IAEDANS f3Zif.5-IAFd 1' 1' 4, d4
Adaptor 1.0e disulfide.5-IAF 11+ 1' si, si, a+ 0"
C73 Pacific Blue
Adaptor 1.0e disulfide.5-IAF 1' si, si, a+
C73.Acrylodan
'Change in magnitude ( 1', increase; sL, decrease; 0, no change) of the donor
and acceptor
fluorescence intensities and their ratio.
bSee Table 1
eResponse pattern and model are the same for all members of the chloride-
binding protein
series C1BP2-14.
dPhase I of the response to glucose (see main text).
eResponse pattern and model are the same for all members of the Adaptor
family.
Fluorescently responsive sensors take advantage of ligand-mediated changes in
the
photophysics of a fluorophore. Analyte binding shifts the population
distribution of the two
fluorophore states whose photophysical properties are associated with the
ligand-free and ¨
bound forms of the sensor, respectively. Ratiometic measurements of
differential changes at
two distinct wavelengths are optimal for taking advantage of ligand-mediated
changes in
emission intensities, because these remove unwanted fluctuations in absolute
intensity.
Ratiometry is possible only if the shape of the fluorescence emission
intensity spectrum
differs in the ligand-free and ¨bound states (dichromatic changes). Many
sensors are based
on fluorescent protein conjugates or chemoresponsive fluorophores.
Unfortunately, the
183
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
emission intensity spectra of many of these materials change only in intensity
and not shape
(monochromatic responses), precluding their use in ratiometry. The ngmFRET
systems
presented here can readily convert monochromatic into dichromatic responses,
thereby
overcoming the limitations and drawbacks of earlier systems. Furthermore, we
have shown
that ratiometric responses in a single-fluorophore conjugate can be improved
if the emission
of one of the switched dipoles is coupled to a significantly brighter
acceptor. We have also
shown that optimal ratiometry requires careful tuning of the spectral overlap
in an ngmFRET
system such that both donor and acceptor exhibit significant emission
intensities.
The ngmFRET approach can be applied to many important sources of fluorescent
sensors. Non-limiting examples include ligand binding proteins for sugars
(such as glucose,
galactose, lactose, arabinose, ribose, and maltose), lactate, urea, anions
(e.g., bicarbonate,
phosphate, sulfate, and halide anions such as chloride, fluoride, iodide,
astatide, ununseptide,
and bromide), cations (e.g., calcium and hydrogen ions), dipeptides, amino
acids (such as
histidine, glutamine, glutamate, aspartate), and elements (e.g., iron).
REFERENCES
Ahmed, M. U., I. Saaem, P. C. Wu & A. S. Brown (2014) Personalized diagnostics
and
biosensors: a review of the biology and technology needed for personalized
medicine.
Crit Rev Biotechnol, 34, 180-96.
Allert, M., J. C. Cox & H. W. Hellinga (2010) Multifactorial determinants of
protein
expression in prokaryotic open reading frames. J Mol Biol, 402, 905-18.
Allert, M. J., Kumar, S. Miriyala, J., Bergeron, A., Beese, L.S., Hellinga,
H.W. (2015)
Conformational coupling between ligand binding and internal fluorophore
twisting in
reagentless fluorescent glucose biosensors derived from periplasmic binding
proteins.
In preparation.
Amer, E. S. J., Holmgren, A. (2000) Physiological functions of thioredoxin and
thioredoxin
reductase. Eur. J. Biochem., 267, 6102-6109.
Arora, A., G. Simone, G. B. Salieb-Beugelaar, J. T. Kim & A. Manz (2010)
Latest
developments in micro total analysis systems. Anal Chem, 82, 4830-47.
Badugu, R., J. R. Lakowicz & C. D. Geddes (2005) A glucose-sensing contact
lens: from
bench top to patient. Curr Opin Biotechnol, 16, 100-7.
184
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Bandodkar, A. J., W. Jia, C. Yardimci, X. Wang, J. Ramirez & J. Wang (2015)
Tattoo-Based
Noninvasive Glucose Monitoring: A Proof-of-Concept Study. Anal. Chem.
(Washington, DC, U. S.), 87, 394-398.
Bergel, A., J. Souppe & M. Comtat (1989) Enzymatic amplification for
spectrophotometric
and electrochemical assays of NAD+ and NADH. Anal Biochem, 179, 382-8.
Bemtsson, R. P., S. H. Smits, L. Schmitt, D. J. Slotboom & B. Poolman (2010) A
structural
classification of substrate-binding proteins. FEBS Lett, 584, 2606-17.
Borisov, S. M. & O. S. Wolfbeis (2008) Optical biosensors. Chem Rev, 108, 423-
61.
Burtis, C. A., Ashwood, E.R., Bruns, D.E. 2012. Tietz Textbook of Clinical
Chemistry and
Molecular Diagnostics. Elsevier.
Cash, K. J. & H. A. Clark (2010) Nanosensors and nanomaterials for monitoring
glucose in
diabetes. Trends Mol Med, 16, 584-93.
Cheung, H. C. (1991) Resonance energy transfer. Topics in Fluorescence
Spectroscopy, 2,
127-176.
Choleau, C., J. C. Klein, G. Reach, B. Aussedat, V. Demaria-Pesce, G. S.
Wilson, R. Gifford
& W. K. Ward (2002) Calibration of a subcutaneous amperometic glucose sensor.
Part 1. Effect of measurement uncertainties on the determination of sensor
sensitivity
and background current. Biosens Bioelectron, 17 , 641-6.
Clegg, R. M. (1995) Fluorescence resonance energy transfer. Curr. Opin.
Biotechnol., 6, 103-
110.
Cox, J. C., J. Lape, M. A. Sayed & H. W. Hellinga (2007) Protein fabrication
automation.
Protein Sci, 16, 379-90.
de Graaf, D. B., Abbas, Y., Bomer, J.G., Olthuis, W., can den Berg, A. (2015)
Sensor-
actuator system for dynamic chloride determination. Anal. Chim. Acta, 888, 44-
51.
de Lorimier, R. M., J. J. Smith, M. A. Dwyer, L. L. Looger, K. M. Sali, C. D.
Paavola, S. S.
Rizk, S. Sadigov, D. W. Conrad, L. Loew & H. W. Hellinga (2002) Construction
of a
fluorescent biosensor family. Protein Sci, 11, 2655-75.
Demchenko, A. P. (2010) The concept of lambda-ratiometry in fluorescence
sensing and
imaging. J Fluoresc, 20, 1099-128.
Demchenko, A. P. (2014) Practical aspects of wavelength ratiometry in the
studies of
intermolecular interactions. Journal of Molecular Structure, 1077, 51-67.
185
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Elsliger, M. A., Wachter, R.M., Hanson, G.T., Kallio, K., Remington, S.J.
(1999) Structural
and spectral response of green fluorescent protein variants to changes in pH.
Biochemistry, 38, 5296-5301.
Gifford, R., J. J. Kehoe, S. L. Barnes, B. A. Komilayev, M. A. Alterman & G.
S. Wilson
(2006) Protein interactions with subcutaneously implanted biosensors.
Biomaterials,
27, 2587-98.
Grimley, J. S., L. Li, W. Wang, L. Wen, L. S. Beese, H. W. Hellinga & G. J.
Augustine
(2013) Visualization of synaptic inhibition with an optogenetic sensor
developed by
cell-free protein engineering automation. J Neurosci, 33, 16297-309.
Grunewald, F. S. 2014. Periplasmic binding proteins in biosensing
applications. In BIOREV,
205-236. Springer Int., Switzerland.
Gubala, V., L. F. Harris, A. J. Ricco, M. X. Tan & D. E. Williams (2012) Point
of care
diagnostics: status and future. Anal Chem, 84, 487-515.
Han, J., Burgess, K. (2010) Fluorescent indicators for intracellular pH. Chem
Rev, 110, 2709-
2728.
Hellinga, H. W., Wynn, R., Richards, F.M. (1992) The hydrophobic core of
Escherichia coli
thioredoxin shows a high tolerance to nonconservative single amino acid
substitutions. Biochemistry, 31, 11203-11209.
Hengen, P. N. (1995) Purification of His-Tag fusion proteins from Escherichia
coli. Trends
Biochem Sci, 20.
Henry, E. R., Hofrichter, J. (1992) Singular value decomposition: application
to analysis of
experimental data. Methods Enzymol, 210, 129-192.
Heo, Y. J. & S. Takeuchi (2013) Towards smart tattoos: implantable biosensors
for
continuous glucose monitoring. Adv Healthc Mater, 2, 43-56.
Holmgren, A. (1985) Thioreodoxin. Annu Rev. Biochem., 54, 237-271.
Huber, C., Klimant, I., Krause, C., Werner, T., Mayr, T., Wolfbeis, O. (2000)
Optical sensor
for seawater salinity. Fresenius J Anal Chem, 368, 196-202.
Huber, C., Klimant, I., Krause, C., Wolfbeis, O. (2001) Dual lifetime
referencing as applied
to a chloride optical sensor. Anal. Chem., 73, 2097-2103.
Ispas, C. R. C., G.; Andreescu, S. (2012) Review: Recent Developments in
Enzyme-Based
Biosensors for Biomedical Analysis. Anal. Lett., 45, 168-186.
186
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Jayaraman, S., Haggie, P., Wachter, R.M., Remington, S.J., Verkman, A.S.
(2000)
Mechanism and cellular applications of a green fluorescent protein-based
halide
sensor. J. Biol. Chem., 275, 6047-6050.
Judge, K., L. Morrow, A. G. Lastovich, D. Kurisko, S. C. Keith, J. Hartsell,
B. Roberts, E.
McVey, K. Weidemaier, K. Win & M. Hompesch (2011) Continuous glucose
monitoring using a novel glucose/galactose binding protein: results of a 12-
hour
feasibility study with the becton dickinson glucose/galactose binding protein
sensor.
Diabetes Technol Ther, 13, 309-17.
Karon, B. S., L. Griesmann, R. Scott, S. C. Bryant, J. A. Dubois, T. L.
Shirey, S. Presti & P.
J. Santrach (2008) Evaluation of the impact of hematocrit and other
interference on
the accuracy of hospital-based glucose meters. Diabetes Technol Ther, 10, 111-
20.
Katti, S., LeMaster, D.M., Eklund, H. (1990) Crystal structure of thioredoxin
from
Escherichia coli at 1.68A resolution. J. Mol. Biol., 212, 167-184.
Koschwanez, H. E. & W. M. Reichert (2007) In vitro, in vivo and post
explantation testing of
glucose-detecting biosensors: current methods and recommendations.
Biomaterials,
28, 3687-703.
Kozma, P., A. Lehmann, K. Wunderlich, D. Michel, S. Schumacher, E. Ehrentreich-
Forster
& F. F. Bier (2013) A novel handheld fluorescent microarray reader for point-
of-care
diagnostic. Biosens Bioelectron, 47, 415-20.
Kuner, T., Augustine, G.J. (2000) A genetically encoded ratiometric indicator
for chloride:
capturing chloride transients in cultured hippocampal neurons. Neuron, 27, 447-
459.
Ladbury, J. E., Wynn, R., Hellinga, H.W., Sturtevant, J.M. (1993) Stability of
oxidized
Escherichia coli thioredoxin and its dependence on protonation of the aspartic
acid
residue in the 26 position. Biochemistry, 32, 7526-7530.
Lakowicz, J. R. 2006. Principles of fluorescence spectroscopy. Springer, New
York.
Langsetmo, K., Fuchs, J.A., Woodward, C. (1991a) The conserved, buried
aspartic acid in
oxidized Escherichia coli thioredoxin has a pKa of 7.5. Its titration produces
a related
shift in global stability. Biochemistry, 30, 7603-7609.
Langsetmo, K., Fuchs, J.A., Woodward, C., Sharp, K.A. (1991b) Linkage of
thioredoxin
stability to titration of ionizable groups with perturbed pKa. Biochemistry,
30, 7609-
7614.
Lavis, L. D. & R. T. Raines (2008) Bright ideas for chemical biology. ACS Chem
Biol, 3,
142-55.
187
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
--- (2014) Bright building blocks for chemical biology. ACS Chem Biol, 9, 855-
66.
Layton, C. J. & H. W. Hellinga (2010) Thermodynamic analysis of ligand-induced
changes in
protein thermal unfolding applied to high-throughput determination of ligand
affinities with extrinsic fluorescent dyes. Biochemistry, 49, 10831-41.
Lin, J. (2000) Recent development and applications of optical and fiber-optic
pH sensors. Tr.
Anal. Chem., 19, 541-551.
Liu, D., Evans, T., Zhang, F. (2015) Applicatiosn and advances of metabolite
biosensors for
metabolic engineering. Metabolic Engin., 31, 35-43.
Martin, M. M., Lindqvist, L. (1975) The pH dependence of fluorescein
fluorescence. J.
Luminesc., 10,381-390.
Marvin, J. S., E. E. Corcoran, N. A. Hattangadi, J. V. Zhang, S. A. Gere & H.
W. Hellinga
(1997) The rational design of allosteric interactions in a monomeric protein
and its
applications to the construction of biosensors. Proc Natl Acad Sci USA, 94,
4366-71.
Matzeu, G., Florea, L., Diamond, D. (2015) Advances in wearable chemical
sensor design for
monitoring biological fluids. Sens Actuators B Chem, 211, 403-418.
Miesenbock, G., D. A. De Angelis & J. E. Rothman (1998) Visualizing secretion
and
synaptic transmission with pH-sensitive green fluorescent proteins. Nature,
394, 192-
5.
Mohammed, M. D., M.P.Y. (2011) Lab-on-a-chip based immunosensor principles and
technologies for the detection of cardiac biomarkers: a review. Lab. chip.,
11, 569-
595.
Okumoto, S., Jones, A., Frommer, W.B. (2012) Quantitative imaging with
fluorescent
biosensors. Annu. Rev. Plant Biol., 63, 663-706.
Prendergast, F. G., Meyer, M., Carlson, G.L., Iida, S., Potter, J.D. (1983)
Synthesis, spectral
properties, and use of 6-acryloy1-2-dimethylaminonapthalene (acrylodan).
Journal of
Biological Chemistry, 258, 7541-7544.
Qi, Y., Grishin, N.V. (2005) Structural classification of thioredoxin-like
fold proteins.
Proteins, 58, 376-388.
Robinson, T. & P. S. Dittrich (2013) Microfluidic technology for molecular
diagnostics. Adv
Biochem Eng Biotechnol, 133, 89-114.
Rogers, M. L. & M. G. Boutelle (2013) Real-time clinical monitoring of
biomolecules. Annu
Rev Anal Chem (Palo Alto Cali fi, 6, 427-53.
188
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Sameiro, M., and Goncalves, T. (2009) Fluorescent Labeling of Biomolecules
with Organic
Probes. Chem. Rev., 109, 190-212.
Smith, J. J., D. W. Conrad, M. J. Cuneo & H. W. Hellinga (2005) Orthogonal
site-specific
protein modification by engineering reversible thiol protection mechanisms.
Protein
Sci, 14, 64-73.
Sun, W. C., Gee, K.R., Haugland, R.P. (1998) Synthesis of novel fluorinated
coumarins:
excellent UV-light excitable fluorescent dyes. Bioorg Med Chem Lett, 8, 3107-
3110.
Tang, Z., J. H. Lee, R. F. Louie & G. J. Kost (2000) Effects of different
hematocrit levels on
glucose measurements with handheld meters for point-of-care testing. Arch
Pathol
Lab Med, 124, 1135-40.
Tang, Z., R. F. Louie, J. H. Lee, D. M. Lee, E. E. Miller & G. J. Kost (2001)
Oxygen effects
on glucose meter measurements with glucose dehydrogenase- and oxidase-based
test
strips for point-of-care testing. Crit Care Med, 29, 1062-70.
Tsien, R. Y. (1998) The green fluorescent protein. Annu Rev Biochem, 67, 509-
44.
Valeur, B., Berberan-Santos, M.N. 2012. Molecular Fluorescence. Principles and
Applications. Weinheim: Wiley.
Vyas, N. K., M. N. Vyas & F. A. Quiocho (1988) Sugar and signal-transducer
binding sites
of the Escherichia coli galactose chemoreceptor protein. Science, 242, 1290-5.
Wachter, R. M., Remington, S.J. (1999) Sensitivity of the yellow variant of
green fluorescent
protein to halides and nitrate. Current Biology, 9, R628-R629.
Wang, H., Nakata, E., Hamachi, I. (2009) Recent progress in strategies for the
creation of
protein-based fluorescent biosensors. Chembiochem, 10, 2560-2577.
Wang, J. (2008) Electrochemical glucose biosensors. Chem Rev, 108, 814-25.
Wang, W., Grimley, J.S., Augustine, G.J., Beese, L.S., Hellinga, H.W. (2015)
Determination
of engineered chloride-binding site structures in fluorescent proteins reveals
principles of halide recognition. In preparation.
Weidemaier, K., A. Lastovich, S. Keith, J. B. Pitner, M. Sistare, R. Jacobson
& D. Kurisko
(2011) Multi-day pre-clinical demonstration of glucose/galactose binding
protein-
based fiber optic sensor. Biosens Bioelectron, 26, 4117-23.
Wencel, D., Abel, T., McDonagh, C. (2014) Optical chemical pH sensors. Anal.
Chem., 86,
15-29.
Wisniewski, N. & M. Reichert (2000) Methods for reducing biosensor membrane
biofouling.
Colloids Swf B Biointerfaces, 18, 197-219.
189
CA 03005883 2018-05-18
WO 2017/087912
PCT/US2016/062958
Wu, P., Brand, L. (1994) Resonance energy transfer: methods and applications.
Anal.
Biochem., 218, 1-13.
Yoo, E.-H. & S.-Y. Lee (2010) Glucose biosensors: an overview of use in
clinical practice.
Sensors (Basel), 10, 4558-76.
Yoshioka, J. (2015) Thioredoxin superfamily and its effect on cardiac
physiology and
pathology. Comprehensive Physiol., 5, 513-529.
Zhang, X., J. Yin & J. Yoon (2014) Recent advances in development of chiral
fluorescent and
colorimetric sensors. Chem Rev, 114, 4918-59.
Zimmer, M. (2002) Green fluorescent protein (GFP): applications, structure,
and related
photophysical behavior. Chem Rev, 102, 759-81.
OTHER EMBODIMENTS
While the invention has been described in conjunction with the detailed
description
thereof, the foregoing description is intended to illustrate and not limit the
scope of the
invention, which is defined by the scope of the appended claims. Other
aspects, advantages,
and modifications are within the scope of the following claims.
The patent and scientific literature referred to herein establishes the
knowledge that is
available to those with skill in the art. All United States patents and
published or unpublished
United States patent applications cited herein are incorporated by reference.
All published
foreign patents and patent applications cited herein are hereby incorporated
by reference.
Genbank and NCBI submissions indicated by accession number cited herein are
hereby
incorporated by reference. All other published references, documents,
manuscripts and
scientific literature cited herein are hereby incorporated by reference.
While this invention has been particularly shown and described with references
to
preferred embodiments thereof, it will be understood by those skilled in the
art that various
changes in form and details may be made therein without departing from the
scope of the
invention encompassed by the appended claims.
190