Note: Descriptions are shown in the official language in which they were submitted.
CA 03008040 2018-06-11
WO 2017/100933 PCT/CA2016/051489
RARE EARTH ORE PROCESSING METHODS BY ACID MIXING, SULPHATING AND DECOMPOSING
FIELD OF THE INVENTION
[0001] The present invention relates to processes and methods for the
purification of
rare earth metals and metal oxides from ore deposits.
BACKGROUND OF THE INVENTION
[0002] Several rare earth elements (REEs), such as europium, gadolinium,
dysprosium, terbium, holmium, erbium, thulium, ytterbium, lutetium, and
yttrium or
Eu-Lu+Y, are critical inputs to many clean technologies and generally in short
supply.
REEs are found around the world in varying concentrations in mineral deposits
(for
example, as fluorocarbonates, phosphates, or silicates) and often in
association with
various undesirable metals such as iron, aluminum, zirconium and niobium that
require
complex and uneconomical processing methods. Rare earth minerals can sometimes
be
substantially separated from associated gangue minerals to a degree sufficient
to
produce a mineral concentrate that is suitable for processing by methods well
known by
the industry, such as acid or caustic attacks. With state of the art
processing methods,
however, not all deposits are amenable to sufficient pre-concentration and
separation of
rare earth metals from gangue metals to enable economical extraction of rare
earth
metals. Separation of gangue metals from the rare earths is a major challenge
for many
types of rare earth bearing mineral deposits and is required for economic
viability.
[0003] Indeed it is appreciated in the mining industry that each rare earth
deposit is
unique and consists of different ore bodies, with many different elements in
varying
proportions. Accordingly, processes suitable for separating elements of value
from
gangue elements have been customized to one degree or another for each ore
deposit
based on the initial characterization of the ore deposit with an emphasis on a
few core
elements of interest from an industrial standpoint.
[0004] The deportment of gangue metals to solution following acid or caustic
attack,
especially iron (Fe) and aluminum (Al), is a major driver for the cost and
complexity of
any hydrometallurgical process. If present, removal of Fe and other non-REE
1
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
impurities from pregnant leach solutions (PLS) requires precipitation steps
which
frequently result in significant loss of valuable rare earth metals, or highly
complex
solvent extraction processes to recover the REE product from the impure
solution, and
can render the process uneconomical.
[0005] Most rare earth minerals are insoluble in water, but can be rendered
soluble
for recovery by the addition of sulphuric acid (or alkali) and baking at
temperatures up
to 300 C, or up to 1000 C in the case of alkali cracking. An alkali cracking
stage is
normally followed by mineral acid leaching. A substantial portion of the
gangue
minerals are also converted to soluble sulphates during such state of the art
treatment
processes.
[0006] Acid baking or roasting processes for the decomposition of REE ores to
obtain
REEs have been described wherein concentrated sulphuric acid is mixed with REE
ore
in 1:1 to 2:1 mass ratios and the mixture is heated within ranges of about 150
C to
600 C (sec for example, CN Publication No. 102094116B and CN Publication No.
1173050C). These processes, as described, focus on the extent of the
decomposition of
REE ores following acid roasting, for example, by determining the REE content
in
leachates obtained by an aqueous leaching process applied to acid roasted ore
material
(calcine). These references do not describe or provide the details about the
composition
of the REE ores, nor are further purification steps described for separating
industrially
useable REEs from undesirable non-REE gangue materials, once the ore materials
they
originated from have been decomposed.
[0007] CN Publication No. 102912117B describes a system and provides at a high
level, a continuous acid roasting process for the decomposition of REE ore
powder and
iron powder ore. Given the focus of this reference on describing the
configuration of
the industrial system for decomposing REE ores and recycling heat and
sulphuric acid,
the acid roasting process supported by the system is not disclosed in any
detail and no
information is provided about the composition of the REE ore which may be
processed
by the system and process.
2
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
[0008] As described in CN Publication No. 102912117B, REE ore may be mixed
with sulphuric acid and heated to 300 C until a dried calcine is obtained
comprising
soluble rare earth ore sulphates. This material is then continuously and
gradually heated
to 800 C to complete the roasting decomposition which entails the
decomposition of
sulphuric acid and ferric pyrophosphate, to facilitate removing iron,
phosphorus,
thorium and other impurities (not specifically elaborated on), enhance the
decomposition of rare earth ore and improve the recovery of REEs. Following
the
higher temperature calcination, a dissolution step is applied to the
decomposed roasted
material to facilitate further processing.
100091 In International Patent Application No. PCT/BR2013/000148 a process for
purifying heavy REEs (HREEs) from ore is described wherein sub-stoichiometric
amounts of sulphuric acid are used to obtain sulphates of iron and/or aluminum
from
partially processed ore materials and then submitting the mixture to
temperatures of
between 620 C and 750 C which triggers the sulphation of HREEs at the same
time as
iron and aluminum sulphates are decomposed. The process is demonstrated using
ore
samples containing high levels of iron and very low levels of dysprosium,
europium,
erbium, holmium and lutetium. It is contemplated for the exploitation of low
grade rare
earth ores not amenable to conventional concentrating processes and acid
baking
techniques, due to the requirement for high amounts of acid and the resulting
leachates
containing too many impurities which would require costly removal processes.
100101 In International Patent Application No. PCT/AU2002/00538, a process for
obtaining rutile (titanium oxide) from ilmenite is described using an acid
bake process
with sulphuric acid, wherein the roasting temperature is maintained below 650
C,
followed by magnetic separation of the rutile together with iron. At
temperatures above
640 C (e.g. 700 C), rutile crystallization led to lower yields of rutile in
the magnetic
fraction of leachates. In this process the primary impurity of concern is the
presence of
chromium which can interfere with the use of rutile in titania pigments.
[0011] Processes for the dissolution of elements into leachates following acid
baking
at 300 C (and in one instance at 500 C) are described in International Patent
Application No. PCT/EP2012/050188, with particular emphasis on the
quantitative
3
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
dissolution of niobium and/or tantalum, uranium and cerium, as specifically
disclosed
in the examples of the application, but not other light REEs (LREEs) or HREEs.
Other
elements which can apparently be dissolved into leachates derived from the
acid bake
calcine include iron, aluminum, thorium, manganese, and titanium. The
dissolution
process relies on the presence of iron in a ferric state at concentrations of
at least 50 g/L
and higher, up to about 120 g/L. This concentration of iron allows the Nb and
Ta
sulphates to remain in solution at restricted free acidity which would
otherwise
hydrolyze the sulphates.
[0012] For the acid baking step, mass ratios of sulphuric acid to dry ore
materials are
described as ranging from 100 kg acidit of dry ore material to 3000 kg acid/t
of dry ore
material. Upstream of roasting, the pre-treatment of ore is contemplated to
physically
concentrate or enrich the ore materials subjected to acid baking. For example,
the
process is described as being carried out on material exhibiting a particle
size of less
than 700 microns and advantageously less than 400 microns.
[0013] The effective separation of REEs from one another and from gangue
elements
following acid roasting may be enhanced by using and combining various
purification
steps as described in CN 101012499 (such as ultrasonic extraction in
combination with
electrochemical oxidation and chemical treatments). The purification step
protocols
disclosed in this reference have been described as effective for the
purification of
cerium, lanthanum, praseodymium, neodymium, samarium, europium, gadolinium,
terbium, dysprosium and yttrium oxides, but are numerous and cumbersome from
an
industrial applicability standpoint.
[0014] Processes for the efficient and effective purification of a wide
selection of
REEs from different ore deposit sources using simple and economical processes
continue to be required. More particularly, such processes are required for
high HREE
deposits as demand for HREEs grows for their application in permanent magnets
used
in an array of green, medical imaging and defense technologies.
100151 There also remains a need to improve state of the art acid baking and
leaching
processes for extracting a full range of LREEs and HREEs from different ore
sources
4
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
which are economical, efficient and which can reduce the environmental impact
of
industrial ore processing, by reducing the amount of acid used and recycling
acid lost
due to evaporation, or the generation of sulphur containing off gases arising
as by-
products of acid baking processes.
SUMMARY OF THE INVENTION
[0016] The present invention relates generally to processes and methods for
selectively recovering rare earth metals from ore or mineral concentrates such
as, but
not limited to, those containing rare earth oxides, silicate, carbonate,
fluorcorbonate,
fluoride, or phosphate minerals. It is an object of the present disclosure to
provide
processes and methods suitable for the effective and economic purification of
REE
ores, or mineral concentrates and more particularly from deposits rich in
heavy rare
earth elements (europium, gadolinium, terbium, dysprosium, holmium, erbium,
thulium, ytterbium, lutetium, yttrium), containing one or more of iron,
aluminum,
thorium, and which may also include one or more of scandium, and the light
rare earth
elements (lanthanum, cerium, praseodymium, neodymium, and samarium).
[0017] The processes and methods disclosed herein represent four stages of
processing including a first stage comprising the beneficiation of ore to
prepare a
mineral concentrate (i.e. concentrated and/or enriched in REEs), a second
stage
comprising acid-ore (concentrate) mixing to substantial homogeneity and
heating to
sulphate the REEs and gangue elements in the mineral concentrate, a third
stage
comprising the selective decomposition of gangue element sulphates at high
temperatures into insoluble gangue element compounds; and a fourth stage to
separate
soluble REE compounds from insoluble gangue element compounds, comprising a
leaching step and further downstream processing to extract REE oxides with a
high
recovery rate from the mineral concentrate with substantially reduced gangue
element
impurities.
[0018] According to one aspect, there is provided a process for purifying REEs
from
an ore also comprising gangue elements, comprising the steps of: (i) preparing
a
mineral concentrate from ore material containing REEs and gangue elements; ii)
5
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
combining and mixing concentrated sulphuric acid in a super-stoichiometric
amount to
the mineral concentrate to produce a homogeneous agglomerate or powder
mixture; iii)
heating the homogeneous agglomerate or powder mixture to temperatures between
about 150 C and about 330 C to sulphate the REEs and gangue elements and
produce a
first calcine; iv) heating the first calcine to temperatures between about 400
C to about
800 C to decompose the sulphates of the gangue elements and produce a second
calcine comprising insoluble gangue element compounds, soluble REF sulphates,
and a
gas stream comprising SO3 and/or SO2; and v) subjecting the second calcine to
a
leaching process to obtain a leachate solution of sulphated REEs that is
substantially
free of gangue element sulphates, and a solid residue comprising the insoluble
gangue
element compounds.
[0019] In one embodiment the ore material comprises a mixture of LREEs, HREEs
and one or more gangue elements selected from the group of iron, aluminum,
niobium
and zirconium.
[0020] In another embodiment, the ore material is derived from a silicate,
monazite,
or bastnaesite deposit.
[0021] In yet another embodiment the mineral concentrate is prepared by
beneficiation of the ore material by one or more physical methods selected
from sensor
based sorting, flotation, magnetic separation, and gravity separation.
[0022] In a further embodiment the concentrated sulphuric acid and the mineral
concentrate are combined and mixed in a manner so as to obtain and maintain a
granular mixture consistency throughout mixing
[0023] In still a further embodiment the concentrated sulphuric acid is
combined and
mixed in batches or a stage-wise manner with the mineral concentrate.
[0024] In one embodiment the step of combining and mixing the concentrated
sulphuric acid and the mineral concentrate is done in whole or in part
concurrently with
the step of heating the homogeneous agglomerate or powder mixture to sulphate
REEs
and gangue elements to produce the first calcine.
6
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
[0025] In another embodiment the homogeneous agglomerate or powder mixture is
produced by adding a recycled amount of first calcine during mixing of the
concentrated sulphuric acid and the mineral concentrate.
[0026] In a further embodiment, the step of combining and mixing the
concentrated
sulphuric acid and the mineral concentrate is done in a high sheer mixer, pug
mill,
paddle mixer, ribbon mixer, or rotary drum mixer.
[0027] In a related embodiment, the homogeneous agglomerate or powder mixture
has a substantially uniform pellet diameter or particle size, produced in
whole or in part
by applying a pelletizing mixing action and particle removal method during
mixing of
the concentrated acid and mineral concentrate.
[0028] In a further related embodiment the homogenous agglomerate or powder
mixture has a substantially uniform particle size, produced in whole or in
part by one or
more cycles of grinding and screening.
[0029] In one embodiment the homogenous agglomerate or powder mixture is
heated
to between about 150 C and about 330 C for about 15 to about 240 minutes.
[0030] In a related embodiment, the homogenous agglomerate or powder mixture
is
continuously fed into a first thermal vessel for heating to produce the first
calcine.
[0031] In another embodiment the first calcine is heated to between about 400
C to
about 800 C for about 15 to about 240 minutes.
[0032] In a related embodiment, the first calcine is continuously fed from the
first
thermal vessel into a second thermal vessel for heating to produce the second
calcine.
[0033] In a further embodiment, concentrated sulphuric acid is produced or
recovered
from the gas stream and recycled to produce the homogeneous agglomerate or
powder
mixture.
[0034] In yet another embodiment the leaching of the second calcine is done
with
dilute acid or water.
7
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
100351 In still another embodiment the sulphated REEs in the leachate solution
are
recovered out of solution as REE solids by pH adjustment, precipitation
reactions
and/or solvent extraction methods.
100361 According to another aspect there is provided a process for purifying
REEs
from an ore also comprising gangue elements, comprising the steps of: (i)
preparing a
mineral concentrate from ore material containing REEs and gangue elements; ii)
combining and mixing concentrated sulphuric acid in a super-stoichiometric
amount to
the mineral concentrate in a manner so as to obtain and maintain a granular
mixture
consistency throughout mixing to produce a homogeneous agglomerate or powder
mixture; iii) heating the homogeneous agglomerate or powder mixture to
temperatures
between about 150 C and about 330 C to sulphate the REEs and gangue elements
and
produce a first calcine; iv) heating the first calcine to temperatures between
about
400 C to about 800 C to decompose the sulphates of the gangue elements and
produce
a second calcine comprising insoluble gangue element compounds, soluble REE
sulphates, and a gas stream comprising SO3 and/or SO2; and v) subjecting the
second
calcine to a leaching process to obtain a leachate solution of sulphated REEs
that is
substantially free of gangue element sulphates, and a solid residue comprising
the
insoluble gangue element compounds.
100371 According to yet another aspect there is provided a process for
purifying REEs
from an ore also comprising gangue elements, comprising the steps of: (i)
preparing a
mineral concentrate from ore material containing REEs and gangue elements,
using a
combination of sensor-based sorting and flotation; ii) combining and mixing
concentrated sulphuric acid in a super-stoichiometric amount to the mineral
concentrate
in a manner so as to obtain and maintain a granular mixture consistency
throughout
mixing to produce a homogeneous agglomerate or powder mixture; iii) heating
the
homogeneous agglomerate or powder mixture to temperatures between about 200 C
and about 300 C for one to two hours to sulphate the REEs and gangue elements
and
produce a first calcine; iv) heating the first calcine to temperatures between
about
600 C to about 700 C for one to two hours to decompose the sulphates of the
gangue
elements and produce a second calcine comprising insoluble gangue element
compounds, soluble REE sulphates, and a gas stream comprising SO3 and/or SO2;
and
8
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
v) subjecting the second calcine to a leaching process to obtain a lcachate
solution of
sulphated REEs that is substantially free of gangue element sulphates, and a
solid
residue comprising the insoluble gangue element compounds.
[0038] According to a related aspect there is provided a process for producing
a
homogeneous agglomerate or powder mixture of concentrated sulphuric acid and
mineral concentrate containing REEs and gangue elements, comprising the step
of
combining and mixing concentrated sulphuric acid in a super-stoichiometric
amount to
a mineral concentrate in a manner so as to obtain and maintain a granular
mixture
consistency throughout mixing to produce a homogeneous agglomerate or powder
mixture.
[0039] In one embodiment the concentrated sulphuric acid is combined and mixed
in
batches or a stage-wise manner with the mineral concentrate.
[0040] In another embodiment the concentrated sulphuric acid is combined and
mixed
with the mineral concentrate in dosage amounts each ranging from about 200 kg
to
about 900 kg of acid per tonne of mineral concentrate until the super-
stoichiometric
amount of concentrated sulphuric acid has been combined and mixed with the
mineral
concentrate.
[0041] In a further embodiment the super-stoichiometric amount of acid is
about 800
kg to 1500 kg of acid per tonne of mineral concentrate.
[0042] In yet another embodiment the homogeneous agglomerate or powder mixture
is produced by adding an amount of calcine during mixing of the concentrated
sulphuric acid and the mineral concentrate.
[0043] In yet a further embodiment the mixing of concentrated sulphuric acid
and
mineral concentrate is carried out at temperatures ranging from about 150 C to
about
330 C.
9
SUBSTITUTE SHEET (RULE 26)
BRIEF DESCRIPTION OF THE DRAWINGS
[0044] These and other features of the invention will become more apparent in
the
following detailed description in which reference is made to the appended
drawings.
[0045] Figures 1: is a schematic representation of an X-ray sensor based
sorting
system for the separation of ore material into fractions that are high and low
in REE
content. Sensor systems other than X-ray may be effective on other ores.
[0046] Figures 2: is a graphical representation of the data of Table 1, namely
a
comparison of the extraction of gangue metal elements using a two-step
selective
thermal sulphation process (represented by the circular data points plot)
versus a non-
selective single step acid bake process (represented by the triangular data
points plot).
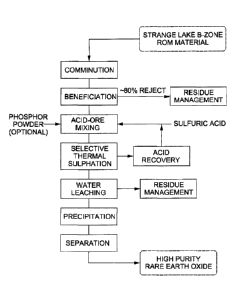
[0047] Figure 3: Block flow diagram of an exemplary process for purifying REEs
from gangue elements staring with ore materials from Strange Lake Deposit
(Zone B)
in Quebec, Canada containing one of the largest rare earth, yttrium and
zirconium
deposits in the world. The Strange Lake Deposit has a HREO:TREO ratio of 50%
in the
enriched zone.
.. [0048] Figure 4: Graphical representation of the effect of ore material
grind size on
flotation recovery of REE in mineral concentrate. The data was generated using
a
FlotinorTM 1682 Collector (and reagent scheme).
[0049] Figure 5: Graphical representation of Mass Pull versus REE recovery
using a
FlotinorTM 1682 reagent scheme, comprising a sodium silicate depressant and
citric/oxalic acid modifiers.
[0050] Figure 6: Effect of acid dosage on the consistency of concentrated
sulphuric
acid/mineral concentrate mixtures. At single stage acid dosages above 625 kg/T
the
consistency of the resulting mixtures becomes increasingly sticky and paste-
like.
[0051] Figure 7: Formation of agglomerates using a multi-stage acid addition
(A)
and recycled calcine (B) methods and low intensity acid mixing. The recycled
calcine
method reduces stickiness and is therefore beneficial to the process.
Date Recue/Date Received 2022-03-16
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
[00521 Figure 8: Effect of mineral concentrate (particle) grind size on
sulphated
element recovery, including REEs (including Y) (diamond data points plot),
iron,
zirconium and niobium (triangular data points plot), after thermal sulphation
under the
following conditions: 300 C, 3 hour bake time, 600kg H2SO4/t ore, and followed
by a
1 h leach at 22 C. The lower plot with square data points depicts the recovery
of iron
and the upper plot of square data points depicts zirconium recovery.
[00531 Figure 9: An exemplary kiln configuration for carrying out a thermal
sulphation protocol according to the disclosure.
[0054] Figure 10: An elemental breakdown of the effect of mineral concentrate
(particle) grind size on sulphated element recovery, following single stage,
low
temperature sulphation under the following conditions: 300 C, 3 hour bake
time, 600kg
H2SO4/t ore, and followed by a 1 h leach at 22 C. The particle sizes in the
legend from
top to bottom correspond to the order of bar groupings from left to right.
[0055] Figure 11: Exemplary Dy recovery following single stage, low
temperature
sulphation at different concentrated sulphuric acid (kg) /mineral concentrate
(T) ratios,
temperatures and heating times (A: 600kg/T; B: 750 kg/T; C: 1200 kg/T; D: 1500
kg/T).
100561 Figure 12: Exemplary HREE recovery following single stage, low
temperature sulphation at different concentrated sulphuric acid (kg) /mineral
concentrate (T) ratios, temperatures and heating times (A: 600kg/T; B: 750
kg/T; C:
1200 kg/T; D: 1500 kg/T).
100571 Figure 13: Exemplary TREE recovery following single stage, low
temperature sulphation at different concentrated sulphuric acid (kg) /mineral
concentrate (T) ratios, temperatures and heating times (A: 600kg/T; B: 750
kg/T; C:
1200 kg/T; D: 1500 kg/T).
[0058] Figure 14: High strength acid recovery at condensation temperatures
between
180 C and 220 C. The results with respect to the bake (BK) samples listed in
the
11
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
legend from top to bottom correspond to the order of bars in each bar grouping
from
left to right.
[0059] Figure 15: An exemplary acid recovery gas condenser train
configuration.
[0060] Figure 16: Schematic representation of an industrial system flow
implementation for a two-step sulphation and selective sulphate decomposition
process
including multi-stage acid/mineral concentrate mixing. Also shown is a two
stage acid
bake with a first stage carried out at 280 C and a second stage carried out at
300 C.
The abbreviations "LT" and "IIT" stand for low temperature and high
temperature,
respectively.
[0061] Figure 17: Schematic representation of an industrial system flow
implementation of a calcine recycling protocol in acid/mineral concentrate
mixing.
DETAILED DESCRIPTION OF THE INVENTION
[0062] The present disclosure relates to processes and methods for separating
REEs
from gangue metals and other materials found in rare earth ores to provide
substantially
pure REEs which can be further processed for industrial application. There is
provided
a selective thermal sulphation process for separating REEs from gangue
minerals and
non-REE metals that can be applied to ore sources rich in REEs, but with
varying
compositions of LREEs, HREEs and gangue-related elements. An overall process
and
method flow diagram is provided at Figure 3. The process can also be applied
for
recovering REEs from secondary sources such as phosphor powder from
fluorescent
lighting devices.
[0063] Unlike state of the art processes, the processes and methods disclosed
have
been demonstrated to facilitate industrially significant recoveries for most
REEs
relative to their original ore source, such as Eu, Gd, Tb, Dy, Ho, Y, Er, Tm,
Yb, Lu and
Y.
[0064] An exemplary source of REEs is the ore, and concentrates derived
therefrom,
of the Strange Lake Deposit, Quebec, Canada. This is one of the largest and
richest
12
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
sources of rare earths (including yttrium) and zirconium in the world. The
Strange Lake
Deposit has a HREO content of 50% of the TREO in the enriched zone and a HREO
content of about 35-38% in the granite domain of the deposit.
Definitions
[0065] Unless defined otherwise, all technical and scientific terms used
herein have
the same meaning as commonly understood by one of ordinary skill in the art to
which
this invention belongs.
[0066] The use of the word "a" or "an" when used herein in conjunction with
the term
"comprising" may mean "one," but it is also consistent with the meaning of
"one or
more," "at least one" and "one or more than one."
[0067] As used herein, the terms "comprising," "having," "including" and
"containing," and grammatical variations thereof, are inclusive or open-ended
and do
not exclude additional, unrecited elements and/or method steps. The term
"consisting
essentially of' when used herein in connection with an apparatus, system, use
or
method, denotes that additional elements and/or method steps may be present,
but that
these additions do not materially affect the manner in which the recited
apparatus,
system, method or use functions. The term "consisting of' when used herein in
connection with an apparatus, system, use or method, excludes the presence of
additional elements and/or method steps. An apparatus, system, use or method
described herein as comprising certain elements and/or steps may also, in
certain
embodiments consist essentially of those elements and/or steps, and in other
embodiments consist of those elements and/or steps, whether or not these
embodiments
are specifically referred to.
[0068] As used herein, the term "about" refers to as much as a +7-10%
variation from
a given value. It is to be understood that such a variation is always included
in any
given value provided herein, whether or not it is specifically referred to.
[0069] The terms, "rare earths," "rare earth element(s)," "rare earth
metal(s)," and
abbreviation "REE(s)" as used herein refer to the industrially relevant
elements of the
periodic table including lanthanum (La), cerium (Ce), praseodymium (Pr),
neodymium
13
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
(Nd), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium
(Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), lutetium (Lu),
as well
as scandium (Sc) and yttrium (Y). Promethium (Pm) is not included as it has no
stable
isotopes and does not exist in nature.
[0070] These elements may be further referred to as "light" and "heavy" REEs
to
further delineate subsets of REEs based on their respective unpaired and
paired electron
configurations, namely (La), cerium (Ce), praseodymium (Pr), neodymium (Nd),
samarium (Sm), europium (Eu), gadolinium (Gd) as light rare earth elements
("LREE(s)") and terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er),
thulium
(Tm), ytterbium (Yb), lutetium (Lu), as heavy rare earth elements ("HREE(s)").
Yttrium is regarded as a HREE due to the similarity of its similar chemical
properties,
whereas scandium's properties are not similar enough to any LREEs or HREEs to
be
classified as such.
[0071] It is understood that reference to REEs, LREEs and HREEs as used
herein,
may be a reference to the element, oxide or other compound forms of these
elements as
they may be found in ores, in the solid product of purification processes, or
in a
solubilized state in solution. To refer to the oxide forms of rare earth
elements, the term
"REO(s)" may be used interchangeably with REE(s) and reference to light and
heavy
REOs may be made as "LREO(s)" and "HREO(s)", and used interchangeably with
LREE(s) and HREE(s), respectively.
[0072[ As used herein, the terms "TREE(s)" and "TREO(s)" may be
interchangeably
used to refer to the total REE/REO present in a given sample, batch or load of
ore
material, mineral concentrate, or recovered amount of solid material from one
or more
ore processing steps.
[0073] The terms "ore(s)", or "ore material(s)" as used herein refer to
(mining output)
material as received from a mine before beneficiation, chemical treatment, or
other
selection and purification procedures applied to change the relative content
of,
composition, or chemical form of REEs in a given ore sample, batch or load
received
from the mine. Ore or mining output material may also include material which
has been
14
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
subjected to physical processing to break it up into smaller pieces for ease
of
management in transportation and for application in beneficiation procedures,
such as,
but not limited to, flotation, and/or (X-ray) sensor based separation
techniques.
[0074] The term "partially processed ore material(s)" refer to ore material(s)
which
have been partially processed, but wherein substantially all or a portion of
REEs are in
their naturally occurring or original mineralized states, whether or not such
mineralized
states include matrix integration or composite structures with gangue and/or
other non-
REE elements. Partially processed ore materials can arise following physical
processing (e.g. crushing or grinding into small particulate matter or
powder),
beneficiation to concentrate and/or enrich the REE content in the ore material
(e.g.
using recycled materials). In other words, partially processed ore materials
have not
undergone any substantial chemical processing to chemically modify,
solubilize,
extract or precipitate REEs. Partially processed ore material may have
undergone
limited chemical treatments as part of flotation methods to chemically alter
ore mineral
surfaces in order to facilitate the physical separation of desirable REEs
minerals from
undesirable sands/silica, silicate gangue or non-REE silicates.
100751 As used herein, the term "beneficiation" refers to processes which can
result in
partially processed ore(s) where the content of REEs is concentrated or
enriched.
Concentration or enrichment can be achieved by the application of one or more
processes or procedures which effect the physical separation of undesirable
materials,
such as sands, which are loosely associated with mineral matrices of REEs or
REE
composite structures in ore materials. The concentration of REE content may be
achieved by magnetic separation, sensor based systems which scan pieces of ore
for
REE content and which can selectively sort and separate low REE content pieces
from
high REE content pieces (e.g. sensor based systems), and the removal of sands
and
non-REE silicates or other gangue minerals (e.g. through flotation
procedures). REE
content in a given sample of partially processed ore material can be further
enriched by
the addition of partially processed ore material from distinct sources, or the
addition of
recycled materials with REE content.
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
[0076] The term "mineral concentrate" refers to partially processed ore
material that
is the product of one or more beneficiation processes that has resulted in the
removal of
gangue material, such as iron, aluminum and silicon compounds. It is
characterized by
having a higher concentration of desirable REEs compared to the ore or mine
output
material it was derived from, and the material which is subjected to chemical
processing, for example, acid baking with concentrated sulphuric acid. An
exemplary
mineral concentrate, derived from ore material of the Strange Lake Deposit,
may
contain a mixture of natural granite and pegmatite minerals containing rare
earth
minerals, zirconium, niobium, iron, calcium, trace beryllium and naturally
occurring
radiological trace elements.
[0077] The term "gangue" as used herein refers to minerals, non-REE metal and
non-
metal elements, found in ore materials, which are targeted for separation from
REEs.
Gangue minerals containing elements such as silicon (Si), iron (Fe), aluminum
(Al),
zirconium (Zr), niobium (Nb), and titanium (Ti) may form matrices in which
desirable
REE minerals are embedded, or otherwise form mineral composites with REEs
which
require chemical or physical processing methods to separate them from REEs.
[0078] The term "calcine" as used herein refers to the product of chemically
processed mineral concentrate which has been subjected to thermally assisted
acid
treatment or thermally assisted sulphate decomposition process.
100791 The terms "acid bake," "acid roast", "acid baking" and "acid roasting"
refer to
a thermal sulphation process consisting of heating a mixture of sulphuric acid
and ore
(or concentrate). This process may be conducted at temperatures ranging
typically
between about 150 C up to about 400 C. By contrast thermal sulphate
decomposition
processes are typically conducted at temperatures above about 500 C to about
800 C.
100801 The terms "leaching" and "leachate" as used herein refer, respectively,
to the
process and product of dissolving desirable (REE) minerals into a liquid
solution by
using a lixiviant reagent of mixture of reagents such as water or a
diluted/mild acid
(typically, sulphuric acid or hydrochloric acid). Leachates contain
solubilized REEs
which have been, or can be separated from solubilized gangue elements and
other
16
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
impurities by way of selective decomposition and precipitation processes, such
as, but
not limited to selective sulphate decomposition. The selective leaching of
soluble REEs
(sulphates) into aqueous solution results in a "pregnant leach solution"
abbreviated as
"PLS" and is a liquor consisting of water soluble salts of desirable REEs.
Pregnant
leach solutions according to the disclosure will typically have low free acid
content and
have an acidic pH of about 2.
[0081] The term "extract," "extracted," or "extraction" is used herein with
reference
to the process of recovering elements in one or more solid forms, such as
metal oxides
from a liquid or solid mixture. The recovered elements may be in solution or
solid
materials that have been separated out from liquid suspensions, precipitated
from liquid
solutions, or mechanically separated from other solids.
[0082] The term "residue" refers to the solid faction of an ore processing or
purification step that contains predominantly undesirable non-REE minerals or
salts
and is not processed in subsequent or downstream steps to obtain purified,
concentrated
or enriched REE materials in solid (e.g. oxide) or liquid form (e.g. sulphated
in
solution).
100831 The term "agglomerate" refers to the association of smaller (primary)
particles
to form larger particles in which the identity of the smaller particles is
still detectable.
The nature of agglomerates is affected by the chemical nature and interaction
between
primary particles, as well as the mixing time, speed and intensity of a given
process or
method to produce them. A homogenous agglomerate as used herein denotes a
mixture
having a consistency amenable for being processed and/or fed continuously
through
different mineral concentrate processing stages. For example, a homogeneous
agglomerate denotes an acid/mineral concentrate mixture that is sufficiently
dry so as
not to be sticky or cause blockages in equipment it is fed through.
[0084] The terms "homogeneous" and "homogeneity" refer to the uniform
composition and distribution of components in a mixture, such as the mixtures
of
concentrated sulphuric acid and mineral concentrate obtained according to the
processes and methods disclosed herein. A mixture of acid and mineral
concentrate can
17
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
be wet and sticky and difficult to handle through process equipment. Under
these
circumstances, recycling calcine can be very beneficial and permit the
relatively
trouble-free processing of what would otherwise be material difficult to
process.
Achieving homogenous mixing of acid and mineral concentrate ensures complete
chemical processing of the mineral concentrate during heat-assisted sulphation
and
selective desulphation processing. Optimal proportions of acid and mineral
concentrate
that have been uniformly mixed to a state of homogeneity according to the
present
disclosure, are evidenced by the formation of sufficiently dry agglomerates or
powders
that can be fed continuously and at a controllable rate into one or more
thermal vessels
without sticking or blockage, and with minimal operational disruptions,
stoppages or
adjustments to maintain the desired feed rate of material through different
processing
stages.
[0085] It is contemplated that any embodiment discussed herein can be
implemented
with respect to any disclosed process, method, use, apparatus or system. For
example,
an apparatus and/or system provided herein can be applied to carry out the
disclosed
processes and methods and said processes and methods can delimit the
operational
parameters and functional characteristics of a disclosed apparatus or system.
Beneficiation of Ore Material
Ore Sorting
[0086] In one embodiment of the disclosed processes and methods, ore materials
are
crushed into smaller pieces (comminution) and sorted to reject coarse-grained
quartz,
feldspar and other pieces of ore material which are relatively low in REE
content.
Sorting may be done using X-ray sensor-based systems (e.g. the Tomra Corn
Tertiary
XRT production scale sorting system) to remove low REE grade rock from higher
grade REE rock. The sorting protocol may include an initial screening to
create more
homogeneously sized fractions of ore material for sorting. In one embodiment
fractions of ore material for sorting comprise rock pieces of about 10-20 mm
or 20-40
mm. In another embodiment, ore materials may be fractionated into pieces of
ore of
about 12 to 20 mm, 10 to 19 mm or 19-38 mm in size. It will be understood by
one
18
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
skilled in the art that such ore fractions may also contain fines of ore
material (i.e.
particles less than 10 mm in size) arising from the crushing process.
[0087] With reference to Figure 1, an exemplary sorting protocol and apparatus
comprises a belt feeding system 1 which facilitates the presentation of non-
uniform
feed, and particle stabilization before scanning with X-ray sensor
transmission
technology that recognizes and separates ore materials based on specific
atomic
density. A broad-band electrical x-ray source 3 is applied to the material to
be sorted as
it is carried on a moving belt. The sensor system including a X-ray camera 2
sits below
the belt in the exemplary configuration shown in Figure 1, and produces a
digital image
of the material being sorted using two different energy bands. Depending on
the
thickness and density of the ore materials scanned, an image transformation of
the
density images of the two bands allows pixel classification relative to a
reference
atomic density. Scanned ore materials are either ejected or accepted as
material streams
A or B in a separation chamber 4, respectively. The material that is ejected
may be
either waste material (barren rock) or ore material targeted for further
processing (e.g.
beneficiation).
[0088] The results of sorting tests using two different ore fraction sizes is
presented
below in Table A:
Summary : fraction 10-20mm +fines
XRT sorter setting 95% 90% 90% IS 85% 80% 75%
mass pull 83.75% 78.11% 76.18% 70.29% 63.96%
56.10%
Total LREE 91.81% 88.13% 87.83% 83.04% 80.38%
72.90%
Total HREE 93.10% 89.08% 88.21% 84.49% 80.80%
76.59%
Total HREE +Y 93.13% 89.28% 89.31% 84.72% 81.75%
76.50%
Total REE +Y 92.52% 88.73% 88.57% 83.90% 81.07%
74.76%
Summary : fraction 19-38 mm +fines
XRT sorter setting 95% 90% 90% IS 85% 80% 75%
mass pull 85.62% 78.93% 76.81% 71.33% 64.49% --
57.26%
Total LREE 92.90% 87.39% 86.22% 82.67% 76.84%
73.83%
Total HREE 92.98% 89.20% 87.34% 85.02% 78.70%
76.17%
Total HREE +Y 93.75% 90.29% 88.61% 85.74% 78.45% --
76.76%
Total REE +Y 93.31% 88.87% 87.42% 84.23% 77.62%
75.29%
19
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
[0089] Such sorting methods allow for the more economical and environmentally
sound processing of lower amounts of ore which contain the majority of REEs of
interest and value. With reference to Table A, it is apparent that at ore
particle sizes of
about 10-20 mm and 19-38 mm respectively, a lower percentage XRT sorter
setting of
75% reduces the mass pull of material, but increases the proportional amount
of REE
content in the material retained. The "mass pull" value indicated in Table A
denotes the
amount of ore material retained following scanning and sorting of an amount of
ore
material fed into the sorting apparatus system and thus comprises the sorter
accept and
the fines that bypassed the sorter and were not sorted, i.e., the -10 mm
material in the
upper portion of the above table and the -19 mm material in the lower portion
of the
table.
100901 It is understood by one skilled in the art that the final amount of
material
retained for further processing may be the result of multiple rounds of
screening and
sorting of ore material fractions. For example, a given input of ore material
may be
separated into fractions categorized as low and high in REE content,
respectively. In
subsequent sorting cycles, the low REE fraction may be further sorted into
fractions
categorized as low and barren/waste (in REE content), and the high REE
fraction may
be further sorted into medium and high REE fractions.
[0091] Depending on the desired ore processing objectives, ore sorting using
such a
system may be sufficient to produce ore material which can be ground to a fine
particulate consistency to produce a mineral concentrate for chemical
treatment (e.g.
acid baking). Accordingly, in one embodiment mineral concentrate is produced
only by
sensor-based sorting.
Flotation
[0092] In another embodiment, a mineral concentrate is produced by applying a
flotation protocol to (pre-sorted) ore materials. This method for separating
desirable
minerals from gangue minerals exploits the hydrophobic properties of different
minerals using surfactants and wetting agents. Undesirable or gangue particles
(e.g.
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
sands and other silicon compounds) are carried by air bubbles to the surface
of a
flotation vessel and removed, while desirable REE minerals are wetted and
remain in
the liquid phase of a mineral/water slurry or vice versa.
[0093] In a further embodiment, prior to subjecting ore material to a
flotation
protocol, ore material is ground into fine particulate matter (micron sized
particles). In
yet another embodiment, ore material is ground to a grind size of 80% passing
("k80")
of about 40 um. Figure 4 demonstrates a relationship between the grind size of
ore
material and the flotation recovery of REE in the resulting mineral
concentrate where
the level of recovery decreases as grind size increases..
[0094] Figure 5 illustrates a relationship between mass pull and REE recovery
following flotation where there is approximately a 10% increase in recovery
for the
indicated REE oxides when the mass pull is approximately doubled. In one
embodiment, a 20% mass pull from flotation achieves 80% REE recovery and is
used
as a source of mineral concentrate in acid baking or a thermal sulphation
protocol. In an
alternative embodiment a mass pull of 20% to 55% from flotation is used as the
source
of mineral concentrate in an acid baking or thermal sulphation protocol.
100951 In another embodiment one or more beneficiation protocols can be
applied to
produce a mineral concentrate, such as sensor-based sorting and flotation.
[0096] The mineral concentrate obtained following ore material beneficiation
may
require further adjustments to meet transport exemption activity levels for U
and Th. In
one embodiment flotation recovery is controlled such that the content of U and
Th in a
mineral (flotation) concentrate is below 10 Bq/g of U and Th combined and
below a
total calculated activity of 110 Bq/g. These levels are set and prescribed in
accordance
with the International Atomic Energy Agency/Canadian Nuclear Safety Commission
(IAEA/CNSC) exempt activity for transportation and dangerous good (DG)
classification purposes. In a related embodiment, the mass pull/recovery can
be
adjusted by changing flotation reagent dosages, and by dilution with lower REE
grade
material. An exemplary activity calculation for Strange Lake Mineral
Concentrate is
provided in Example 3.
21
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
[0097] Prior to processing mineral concentrate to purify REEs, drying will
prevent
dilution of the concentrated sulphuric acid that is added to the mineral
concentrate.
Accordingly, in one embodiment beneficiated ore material is dried, as
required, to
reduce moisture content before being mixed with sulphuric acid. In a related
embodiment the mineral concentrate moisture level after drying is less than
1%.
Sulphation of REEs and Other Metal Elements in Mineral Concentrates
[0098] The step of combining and mixing concentrated sulphuric acid in a super-
stoichiometric amount to mineral concentrate is conducted in such a way so as
to
ensure that there is material (i.e. a mixture of acid and mineral concentrate)
that can be
and is available to be continuously fed into a heated vessel without sticking
or creating
blockages. Combining and mixing acid and concentrate to obtain and maintain a
granular or particulate consistency throughout the mixing process provides for
the
consistent flowability of material through an industrial processing system
(e.g. a plant)
for the purification of REEs from mineral concentrate. The amount of acid used
is
dependent on acid utilization efficiency, while acid/mineral concentrate
mixing is
integrally linked to acid dosage and the total amount of acid used. Using a
super-
stoichiometric amount acid to mineral concentrate (having regard to the amount
of
REEs therein) ensures substantially complete sulphation of REEs, and also
accounts for
acid usage/consumption by gangue and other elements.
[0099] One or more of the following strategies can be used to produce
acid/mineral
concentrate mixing conditions which optimizes acid utilization efficiency:
a) Limitation of sulphuric acid addition (i.e. total acid is delivered in
batches or
dosage amounts for mixing with concentrate) so as to avoid formation of
sticky,
paste-like material;
b) Adjustment of mixing intensity;
c) Pelletization/agglomeration of the acid and mineral mixture;
d) Addition of heat during mixing;
e) Recycling of the heat treated acid and mineral mixture (calcine) to dilute
the
feed to the heated vessel and reduce the acid:solids ratio so as to maintain
the
desired mixture consistency; and/or
22
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
f) Batch addition of acid concurrent with the thermal treatment
process (for
example, portions of the total acid added in advance of multiple and
successive
thermal treatment (sulphation) cycles so that the acid: solid ratio is
maintained at
a low enough level throughout the process to maintain a granular or
particulate
mixture consistency, while allowing sufficient total acid to be added to
achieve
high recovery of REEs).
Acid/Mineral Concentrate Mixing
[00100] The modulation of concentrated sulphuric acid addition to mineral
concentrate
at a rate and/or in dosages that provide for a granular or particulate mixture
consistency
upon mixing can be done in whole or in part by combining acid and concentrate,
and
optionally calcine, at intervals in between cycles of thermal sulphation or
concurrently
with thermal sulphation by controlling the rate of addition of acid to
concentrate in a
heated vessel. Mixture heating can be done in a single or in multiple thermal
vessels (in
kilns or fluidized bed reactors) to apply the strategies for optimizing acid
utilization
efficiencies and for optimizing material flowability during acid/concentrate
mixing and
thermal sulphation of the resulting mixture.
[00101] In one embodiment the dosage of acid combined with concentrate is
selected
from about 225, 250, 275, 300, 325, 350, 375, 400, 425, 450, 475, 500, 525,
550, 575,
600, 625, 650, 675, 700, 725, 750, 775. 800, 825, 850, 875 and 900 kg of acid
per tonne
of mineral concentrate. At higher dosages of acid addition if a slurry of acid
and
mineral concentrate is initially formed, calcine may be added to obtain the
desired
granular or particulate consistency upon mixing
[00102] In one embodiment about 600 to 625 kg/t of concentrated sulphuric acid
to
mineral concentrate is used to produce a homogeneous agglomerate or powder. A
homogeneous, agglomerate or powder has a sufficient level of mechanical
integrity so
that it can be continuously fed into a thermal (sulphation) vessel at a
consistent flow
rate without blockage or sticking. The effect of acid dosage and mixing on the
resulting consistency of an acid/mineral concentrate mixture is illustrated in
Figures 6
and 7.
23
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
1001031 The formation of a homogeneous agglomerate in one embodiment is
prepared
by the stage-wise addition of acid at single dosages ranging between about 220
kg to
about 625 kg of acid per tonne of mineral concentrate, with continuous mixing.
In
another embodiment, acid dosages for stage-wise mixing range between about 200
kg
to about 900 kg of acid per tonne of mineral concentrate, Such protocols
comprise
making successive additions of acid, concurrently with or followed by thermal
processing of acid/concentrate mixture after each stage of acid addition until
a total of
about 800 kg to about 1500 kg of concentrated sulphuric acid per tonne of
mineral
concentrate have been mixed. Using these and other variations of these
protocols as
would be appreciated by one skilled in the art, desirable particulate (e.g.
agglomerate or
powder-like) acid/concentrate mixture properties can be maintained.
[00104] In a further embodiment, the stage-wise mixing of acid and mineral
concentrate is done concurrently with acid baking. In a related embodiment,
stages of
mixing during acid baking include the step of mixture cooling preceding the
addition of
successive doses of acid to partially sulphated mineral concentrate.
[00105] In yet another embodiment the formation of a homogeneous dry
agglomerate
or powder that is easy to handle is facilitated and promoted by recycling a
portion of
sulphated mineral concentrate following acid baking (calcine). Calcine is
added into an
acid/mineral concentrate mixture and remixed. Use of this protocol may require
a larger
vessel to achieve target throughputs.
[00106] In another embodiment concentrated sulphuric acid and mineral
concentrate
are mixed at a low intensity to form a homogeneous agglomerate or powder. In a
further embodiment agglomeration is conducted in a concrete or other kind of
rotary
mixer. In still a further embodiment acid and concentrate are mixed at about
100 rpm.
[00107] In yet another embodiment the grind size of mineral concentrate
particles
mixed with concentrated sulphuric acid is about 40 tim. Figure 8 illustrates
the effect of
mineral concentrate (particle) grind size on sulphated element recovery. A
more
detailed breakdown of element recovery based on grind size is provided in
Figure 10.
24
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
100108] In a further embodiment mixing of acid and mineral concentrate is
carried out
before feeding the mixture into a thermal sulphation vessel. In an alternative
embodiment, mixing of acid and mineral concentrate is carried out in a thermal
sulphation vessel. In yet another alternative embodiment, acid and mineral
concentrate
mixing is carried out before and after feeding the mixture into a thermal
sulphation
vessel. In all of these embodiments temperature may be controlled to optimize
the
sulphation of mineral concentrate elements.
1001091 In a related embodiment, thermal sulphation or acid baking of an
acid/mineral
concentrate mixture is conducted in a rotary kiln, packed bed reactor,
fluidized bed
reactor, moving fixed bed or grate, or a continuous stirred tank. An exemplary
kiln
configuration is provided in Figure 9, including a quartz kiln with lifters
10, operatively
associated with an electric furnace 11, variable-speed kiln drive 12 and air
source 13.
Various temperature sensors and controls (14, 15 and 16) are provided for the
furnace
14, gas inlet 15 and to track burden temperature 16.
Thermal Sulphation
1001101 In one embodiment, the acid and mineral mixture may be heated at a
sustained
temperature of 150 C, 200 C, 250 C, 300 C, or 350 C. Alternatively, in another
embodiment, acid baking may be carried out over a variety of temperature
ranges,
including ranges such as 150 C to less than 400 C, 150 C to 330 C, 200 C to
310 C,
250 C to 330 C, 250 C to 300 C, 270 C to 300 C or 280 C to 300 C. At these
temperatures, rare earth containing minerals react with the sulphuric acid to
form
water-soluble REE sulphates. Metals in gangue minerals, including but not
limited to
iron, aluminum, zirconium and niobium will also form water-soluble sulphates.
These
sulphates complicate and can render uneconomical the downstream processing of
the
leach solution if not converted to insoluble compounds. Examples of reactions
that can
occur when the processes provided and described herein are applied to a
silicate-based
rare earth metal are presented below for sulphation and sulphate decomposition
reactions.
1001111 Examples of sulphation reactions at 300 C:
25
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
Gadolinite Group
Y2Fe2Be2Si2010 + 7H2SO4 Y2(SO4)3 +
2BeSO4 + 1/2Fe2(SO4)3 + 2Si02 + 71-120
1/2S02 (1)
Allanite
2(CaREEAlFe2Ve3+)(SiO4)3(OH) + 15H2SO4 --*2CaSO4 REE2(SO4)3 + Al2(SO4)3 +
SO2 2Fe2(SO4)3 + 16H20 + 6Si02 (2)
Gittinsite
CaZrSi707 + 3H2SO4 ¨> CaSO4 + Zr(SO4)2-xWO + 2Si02 + (3 - x) (3)
Zircon
ZrSiO4 + 2H2SO4 ¨> Zr(SO4)2.xH20 + SiO2 + (2 - x)H20 (4)
Pyrochlore
2CaNaNb206(OH) + 9H2SO4 ¨> Na7SO4 + 2CaSO4 2N13,02(SO4)3 + 10H20 (5)
Hematite
Fe703 + 3H2SO4 ¨4 Fe2(SO4)3 + 3H20 (6)
[00112] The completeness of the sulphation reactions will depend in part on
the length
of time the acid and mineral concentrate mixture is heated. In one embodiment
the acid
and mineral concentrate mixture is continuously fed into a first thermal
vessel wherein
the mixture is heated for a residence time, ranging from 15 to 240 minutes, 30
to 120
minutes or 60 to 90 minutes before the resulting calcine is subjected to a
selective
thermal sulphate decomposition protocol.
1001131 Typical recoveries for desirable elements using different proportions
of acid to
mineral concentrate, expressed as kg/t, over time and with increasing
temperature are
shown in Figures 11 for Dy and Figure 12 for HREEs. Recoveries for other
individual
REEs, TREEs (as shown in Figure 13) and overall recovery value may be
reasonably
extrapolated from such known recoveries using accepted calculation and
statistical
analysis methods known in the art. In this way, one skilled in the art can
reasonably
26
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
predict the efficacy of different mixing and heating parameters/conditions as
illustrated
in Table B, to design appropriate protocols for REE extraction from ore
material. In
Table B below, "A" stands for acid and "C" stands for concentrate:
Max Temperature A/C Time
Value% -C Kg/T min
Dy 98.0 300 1500 120
HREE 96.3 300 1500 120
TREE 95.1 271 1097 120
Recovered 93,7 300 1424 120
Value
[00114] In one embodiment, thermal sulphation of mineral concentrate elements
is
carried out at 300 C, using a total of 1400-1500 kg of concentrated sulphuric
acid per
tonne of mineral concentrate for 120 minutes.
[00115] In another embodiment, thermal sulphation or acid baking of mineral
concentrate elements is carried out at 270 C to less than 300 C, using a total
of about
1000 kg of concentrate sulphuric acid per tonne of mineral concentrate for 120
minutes
to optimize total REE recovery.
[00116] In yet another embodiment thermal sulphation or acid baking is carried
out by
packing agglomerates of acid/mineral concentrate mixtures into a thermal
vessel to
reduce the evaporation of acid during heating (e.g. a packed bed reactor).
[00117] In a further embodiment, thermal sulphation or acid baking is
conducted in a
closed thermal vessel to reduce acid loss during the sulphation of mineral
concentrate
elements. In a related embodiment, the sulphation of mineral concentrate
elements may
be optimized by controlling the feed rate into and amount of acid/mineral
concentrate
agglomerate or powder mixture in the thermal vessel, the pressure in the
vessel and the
atmospheric environment.
27
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
[00118] In another embodiment, the heating rate applied to the acid/mineral
concentrate mixture may be varied to optimize the completeness of mineral
concentrate
element sulphation.
Selective Thermal Decomposition of Gangue (Derived) Element Sulphates
[00119] According to the present disclosure the mixture of rare earth
sulphates and any
gangue-derived, or non-REE metal sulphates present in the calcine product of
an acid
bake are subjected to a higher temperature heating protocol of at least about
4000C but
not more than about 800 C.
1001201 In one embodiment the calcine product resulting from acid baking may
be
heated at a sustained temperature of 400 C, 4500C, 500 C, 550 C, 600 C, 650 C,
700 C
or 750 C. In an alternative embodiment, the calcine product resulting from
acid baking
may be heated over a variety of temperature ranges, including ranges of 400 C
to no
more than 800 C, 550 C to 750 C, 600 C to 750 C, 580 C to 680 C, and 650 C to
700 C. The high temperature provides thermodynamically favourable conditions
for the
preferential decomposition of the gangue metal sulfates, such as those of
iron,
aluminum, niobium and zirconium, while minimizing the decomposition of REEs.
This
thermal sulphate decomposition protocol may be carried out in a separate
thermal
vessel than the lower temperature acid baking protocol.
[00121] Examples of sulphate decomposition reactions:
Fe2(SO4)3 FeO + 3803 (550 C) (7)
Zr(SO4)2 ZrO2 + 2803 (540 C) (8)
REE2(804)3 REE203 + 3803 (700 C - 1300 C) (9)
[00122] A comparison of REE and non-REE element recovery data obtained from a
two-step selective thermal sulphation protocol and one-step non-selective acid
bake
protocols, is provided in Table C below (and plotted in Figure 2):
28
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
Selective Process Unselective Process
Extraction Extraction
Dot Triangle
Elements Extractions, %
La 87 95
Ce 89 95
Pr 91 95
Nd 91 95
Sm 90 93
Eu 89 90
Gd 90 90
Tb 88 87
Dy 86 85
Ho 84 83
86 87
Er 83 82
Tm 81 81
Yb 80 81
Lu 79 81
Th 80 81
78 89
Zr 18 79
Nb 9 93
Fe 10 38
Al 10 11
Si 0 3
Mg 97 96
Ca 24 30
Na 18 11
11 5
Ti 11 69
28 31
Mn 48 48
[00123] The step of decomposing non-REE metal sulphates to insoluble compounds
(for example oxides as in reaction (7) and (8)), while limiting the
decomposition of rare
29
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
earth sulphates, generates a second calcine material that when water leached
will
produce a PLS with improved purity and reduced processing costs when compared
with
state-of-the art standard acid baking methods.
[00124] In one embodiment, the first calcine product of thermal sulphation is
removed
from the thermal sulphation vessel and fed into a selective thermal sulphate
decomposition vessel to produce a second calcine product. In a related
embodiment,
the first calcine product is pre-heated prior to being fed into the selective
thermal
sulphate decomposition vessel.
[00125] Coarser agglomerates containing non-REE sulphates (such as iron,
zirconium,
niobium and aluminum sulphates) exhibit less complete decomposition and have a
grey
colouring when crushed. Finer agglomerates of a similar composition have a
reddish
colour when crushed that is indicative of more complete selective iron
sulphate
decomposition. Accordingly, in another embodiment, the first calcine product
is
mechanically processed prior to or as it is fed into the selective thermal
sulphate
decomposition vessel to optimize agglomerate size and to maximize the
completeness
of selective thermal sulphate decomposition. In a related embodiment the
optimized
agglomerate size is between 3 to 10 mm.
[00126] In yet another embodiment, selective thermal sulphate decomposition is
controlled to achieve maximum Fe sulphate decomposition with less than 5% REE
sulphate decomposition. In a further embodiment, selective thermal sulphate
decomposition is controlled to achieve 80%-90% Fe sulphate decomposition.
Heat and Acid Recovery
[00127] Processing costs may also be reduced by incorporating heat recovery
and acid
recovery methods as part of the processes disclosed herein.
[00128] In one embodiment heat from the selective thermal decomposition of non-
REE
sulphates may be captured and recycled to preheat the calcine product of acid
baking as
it is fed from the acid baking thermal vessel to a another thermal vessel to
carry out the
selective thermal decomposition of gangue element sulphates.
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
[00129] The design of effective acid recovery methods requires consideration
of the
different processing steps in which acid losses occur. Super-stoichiometric
quantities of
sulphuric acid are usually required to achieve economic recoveries of rare
earths in the
typical acid attack process. Excess acid reports to off-gas during
acid/mineral
concentrate mixing, low temperature acid baking and high temperature selective
sulphate decomposition. Residual acid is carried into aqueous solution during
leaching,
and acid is also consumed by gangue metals. Free acid lost to off-gas and
aqueous
solutions or reacted with gangue metals is typically neutralized with an
alkaline
chemical (such as lime or caustic reagents) and replaced with fresh acid. Acid
replacement and neutralization represent significant costs and generates waste
products
that must be disposed of. By offering a method for recycling of excess acid
and
recovering acid consumed by gangue metals, the processes provided herein
reduce acid
requirements and the environmental footprint of rare earth processing.
1001301 Acid Recovery Reactions:
H2SO4 (liquid) ¨3 H2SO4(vapour) (280-338 C) (10)
H2SO4 (vapour) ¨ SOT (gas) + H20 (gas) (>338 C (11)
H2SO4 (vapour) H2SO4 (liquid) (Cooling to 220 C) (12)
SO3 (gas) H20 (gas) ¨3 H2SO4 (liquid) (cooling to 220 C) (13)
[00131] The present disclosure provides a method for the recovery of excess
acid that
is normally lost to the gas phase and PLS and is normally neutralized with
alkaline
chemicals. The majority of acid that is not used in the sulphation reactions,
and SO3
that is liberated according to reactions (7) and (8) is recovered according to
the
reactions (10) to (13). The SO3 recovered can also be used together with
concentrated
sulphuric acid, or on its own as a sulphation agent. Use of SO3 in the
sulphation stage
of the process for REE extraction from mineral concentrate could be carried
out using a
fluidized bed system configuration.
31
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
[001321 In one embodiment high strength acid is recovered at condensation
temperatures of 180 C to 220 C by passing off-gas through a primary condenser
(see
Figure 14). Figure 14 (including a bar graph and table) presents data tracing
the
presence of sulphur and acid in various process fractions, based on baking
acid/concentrate agglomerate mixture to 40 microns, baking and evaporation at
300 C,
drying and a 1 h leach at 22 C . Bar graph groupings A-D represent the amount
of
sulphur to calcine (A), sulphur to calcine titrated as free acid leach (B),
sulphur to first
condenser (C) and acid concentration in first condenser (D). Values shown
above the
bar groupings are average parameter values for 600 and 500 kg acid/t mineral
concentrate (ore). In a related embodiment the strength of the recovered acid
is 93-96%
(i.e. concentrated sulphuric acid). In a further embodiment, low strength acid
is
recovered at temperatures of 40 C to 50 C.
100133] In one embodiment, acid is recovered using equipment including, but
not
limited to, condensers, electrostatic precipitators, or absorption columns. In
another
embodiment an apparatus for the recovery of acid (see Figure 15) is configured
as a gas
train comprising one or more condensers 20, an electrostatic precipitator 21
operatively
associated with a high voltage source, a scrubber 22 and glass fiber mist
filter 23
operatively associated with a vacuum pump 24. One skilled in the art would
appreciate
that there may be other suitable gas train con figurations and that the
apparatus shown in
in Figure 15 is one exemplary apparatus.
Separation of REEs from Gangue (Derived) Metal Elements (Calcine Leaching)
REE Recovery
[00134] The calcine product of selective thermal sulphate decomposition
contains a
mixture of REE sulphates and water insoluble non-REE compounds. To separate
these
two groups of compounds, a water or mild acid leaching protocol may be applied
by
successively adding the calcine to water or mild acidic water according to
known
methods in the art, for example, as described in Example 1 and 2. Further
processing of
the leachate to precipitate out purified REE compounds may also be done
according to
32
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
a number of protocols well known in the art to obtain mixtures of REE
compounds, or
to selectively isolate different REEs from one another.
[00135] In one embodiment, less than 15% of the original Fe content in a
mineral
concentrate is recovered in a leachate.
[00136] In another embodiment, more than 85% of REE content in a mineral
concentrate is recovered in a leachate.
[00137] In still another embodiment the leachate is subjected to a hydromet
(i.e.
hydrometallurgy) protocol that does not require solvent extraction to produce
mixed
REE oxides.
[00138] In a further embodiment, the REE containing leachate solution contains
10%-
20% Fe which is precipitated out of solution using MgCO3.
System Configuration for the Extraction and Purification of REEs from Ore
Materials
1001391 A pilot or industrial scale plant system can be configured to carry
out the
process for purifying REEs from an ore also comprising gangue elements
comprising
the steps of: (i) preparing a mineral concentrate from ore material; ii)
adding
concentrated sulphuric acid in a super-stoichiometric amount to the mineral
concentrate
and mixing to produce a homogeneous agglomerate or powder mixture; iii)
heating the
mixture to temperatures between about 150 C and about 330 C to sulphate the
REEs
and gangue elements and produce a first calcine; iv) heating the first calcine
to
temperatures between about 400 C to about 800 C to decompose the sulphates of
the
gangue elements and produce a second calcine comprising insoluble gangue
element
compounds, soluble REE sulphates, and a gas stream comprising SO3 and/or SO2;
and
v) subjecting the second calcine to a leaching process to obtain a leachate
solution of
sulphated REEs that is substantially free of gangue element sulphates, and a
solid
residue comprising the insoluble gangue element compounds.
[00140] The system implementation of the processes and methods of the
disclosure
are primarily dictated by the need to provide for:
33
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
1) Acid/concentrate mixing (as exemplified in Figure 16) and/or calcine
recycling
acid/concentrate mixing (as exemplified in Figure 17);
2) The controlled and continuous operation of thermal sulphation and
decomposition thermal vessels, including the feeding of material in and out of
the vessels, temperature control and material residence time in thermal
vessels;
3) The implementation of low acid use, and high strength acid recovery
strategies;
and
4) Heat recovery strategies to minimize energy consumption during heating and
overall operations.
[00141] Based on pilot testing conducted, in one embodiment, an exemplary
industrial
system and implementation protocol may comprise:
a. 3 Stages of acid/concentrate mixing @ 889 kg/t total acid addition
across the stages to provide the ratios of acid/concentrate indicated in 'b'
for each stage
b. 436 kg/T in stage 1; 665 kg/T stage 2; 889 kg/T stage 3
c. Heating at 273-291 C (sulphation) following each acid addition stage
in sequentially configured thermal vessels for a total of about one hour
in each vessel
d. Promoting agglomeration in a rotary kiln
c. Heating to 673 C in a rotary kiln (selective sulphate decomposition) for
about one hour
1001421 Based on other pilot tests, in another embodiment, the implementation
of an
industrial calcine recycle protocol for acid/mineral concentrate mixing may
comprise:
34
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
a. 809-840 kg/t Acid @ 47% calcine recycle ratio; calcine is obtained from
a previous cycle of thermal sulphation and the remaining calcine product
(53%) is subjected to further processing (desulphation)
b. Heating to 296 C
c. A "one kiln" configuration for acid/concentrate mixing with recycled
calcine
d. Dry crushing of calcine for recycling
e. Approximate calcine hot zone retention time of 99 min
f. Low temperature calcine product charged as is for heating to 663-669 C
in a second kiln
g. Approximate hot zone retention time of 71min
[00143] From pilot testing conducted according the above described protocols,
the
following results have been achieved:
Overall Extractions
a. 81% TREE w/ 22% Zr and 17% Fe
b. Both methods had similar extractions
Low Temperature Heating Off-Gas (sulphation stage)
c. Sulphur: 50% S03/SO4, 35% SO2, 15% particulate sulphur
d. Fluoride: All as HE
High Temperature Off-Gas (selective sulphate decomposition stage)
e. SO2 detected
Phosphor Addition (to enrich REF content in mineral concentrate)
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
f. No physical or chemical issues observed versus without Phosphor
powder
[00144] In one embodiment, a system for implementing the above protocols
comprises,
a feed (concentrate and recycled material) delivery system; heated acid /feed
mixing
equipment; an acid delivery system; one or more thermal vessels (e.g. kilns or
furnaces)
for carrying out sulphation and sulphate decomposition reactions on feed
materials; an
acid recovery and regeneration system; one or more off-gas treatment units;
calcine
handling equipment; waste material handling systems and storage facilities,
and an
instrumentation, control and data acquisition system, all operatively
associated in such
a way so as to carry out the processes and methods of the disclosure.
[00145] In another embodiment the system for carrying out the processes and
methods
of the disclosure is configured to process about 3,550,000 Mt of mined ore
material per
year. In an alternative embodiment, mine output is controlled to achieve
constant
flotation concentrate volume or grade.
[00146] In yet another embodiment, the system for carrying out the processes
and
methods of the disclosure is configured to process about 1,850,000 Mt/yr of
mill feed.
[00147] hi a further embodiment, the system for carrying out the processes and
methods of the disclosure is configured to process about 300,000 Mt/yr of
flotation
(mineral) concentrate.
[00148] In still a further embodiment, the system for carrying out the
processes and
methods of the disclosure is configured to input about 10,000 Mt/yr of lamp
(phosphor)
material.
[00149] In one embodiment, the system for carrying out the processes and
methods of
the disclosure is configured to produce about 11,270 Mt/yr of mixed REE
concentrate.
[00150] In related embodiment, the system for carrying out the processes and
methods
of the disclosure is configured to produce about 11,050 Mt/yr of pure RE0s.
36
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
[00151] It is understood that reference to various embodiments of the
processes of the
present disclosure and related applications thereof, including those described
in the
Examples and in the Figures are illustrative of certain embodiments of the
processes
disclosed herein and are not intended to limit the scope of the invention in
any way.
EXAMPLES
[00152] Embodiments of the invention are further illustrated in the following
examples.
Example 1
[00153] 500 g of flotation concentrate (grade provided below in Table 1) was
added to
a glass reactor fitted with a polytetrafluoroethylene (PTFE) impeller to
provide mixing.
In four steps, with high intensity mixing in-between each step, a total of
236.7 g of
concentrated (96%) sulphuric acid was added (909 kg acid/T concentrate) in a
total
time of one hour. During mixing (high intensity) the reactor was heated to 235-
259
degrees C. At the completion of mixing, agglomerated solids had been formed
and
were removed from the glass reactor and placed in a tray, which was placed in
pre-
heated muffle furnace heated to 600 degrees C. The furnace temperature was
allowed to
return/stabilize to 600 degrees C, and the sample was kept in the furnace for
an
additional 30 min. During the baking in the furnace, gas flow of 3L/min was
applied to
promote the desulphation reactions. After baking the sample weighed 563 g and
187 g
of this was added to 1470 mL of ambient temperature (25 degrees C) de-ionized
(DI)
water for leaching. Leaching was conducted in a glass reactor equipped with a
PTFE
impeller for 1 hr. The pulp was weighed and filtered, and the cake washed with
271 mL
of DI water. 1398 mL of final of leach filtrate and 260 mL of wash filtrate
were
collected. 159 g of dry residue was collected. Filtrate, washed and dry
leached residue
were analyzed. The results of the metallurgical balance are presented below.
Dy and Nd
extraction to solution were 86% and 91%, respectively, while Fe and Al
extraction
were both only 10%. The pH of the leach filtrate was only mildly acidic with
about 1.3
g/L of free acid. The solution was later further processed for the recovery of
high purity
mixed rare earth oxide.
37
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
[00154] Table 1 ¨ Feed Composition for Example 1
Feed: Concentrate #1 Unit
Mass: 166
Element: Assay
La 2020 g/t
Ce 4400 g/t
Pr 474 g/t
Nd 1630 g/t
Sm 390 g/t
Eu 23 g/t
Gd 414 g/t
Tb 89 g/t
Dy 629 g/t
Ho 141 g/t
3680 g/t
Er 446 g/t
Tm 69 g/t
Yb 426 g/t
Lu 59 g/t
Sc <25 g/t
Th 638 g/t
102 g/t
Zr 2.55
Nb 0.29
Ta 0.01
Si 29.4
Al 3.19
Fe 7.48
Mg 0.37
Ca 2.82
Na 2.62
2.29
Ti 0.45 %
0.03
Mn 0.21
1.36
Be 480 g/t
[00155] Table 2 - Metallurgical balance for Example 1 products:
Component: Final Unit Wash Unit Final Unit Extraction Unit
PLS residue to Solution:
Quantity: 1389 mL 260 mL 159
Element: Assay: Assay: Assay:
La 186 mg/L 83.5 mg/L 266 g/t 87
38
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
Ce 406 mg/L 177 mg/L 480 g/t 89 %
Pr 48.1 mg/L 20.0 mg/L 47 g/t 91 %
Nd 166 mg/L 69.1 mg/L _ 153 g/t 91
%
Sm 38.0 mg/L 15.3 mg/L 38 g/t 90 %
Eu 2.25 mg/L 0.92 mg/L 3 g/t 89 %
Gd 41.5 mg/L 16.3 , mg/L 44 g/t 90 %
Tb 8.44 mg/L 3.25 mg/L 11 g/t 88 %
Dy 59.9 mg/L 22.3 mg/L 89 , g/t 86 %
Ho 12.8 mg/L 4.64 mg/L 22 g/t 84 %
Y 355 mg/L 127 mg/L 541 g/t 86 %
Er 40.9 mg/L 14.5 mg/L 81 g/t 83 %
Tm 6.04 mg/L 2.05 mg/L 13 g/t 81 %
Yb 37.6 mg/L 12.6 mg/L , 88 g/t 80 %
Lu 4.99 mg/L 1.63 mg/L 12 g/t 79 %
Sc <0.2 mg/L(_)2 mg/L <25 g/t 8 %
Th 55.4 mg/L 17.0 mg/L 127 g/t , 80 %
U 8.78 mg/L 2.35 mg/L 23 g/t 78 %
Zr 509 mg/L 119 mg/L 2.12 % 18 %
Nb 27.0 mg/L 5.1 mg/L 0.26 % 9 %
Ta <0.9 mg/L <0.9 , mg/L 0.01 % 9 %
Si , 2.8 mg/L 3.2 mg/L 30.1 % 0
%
Al 326 mg/L 163 mg/L 2.91 % 10 %
Fe 845 mg/L 278 mg/L 7.06 % 10 %
Mg 372 mg/L 108 mg/L 0.01 % 97 %
Ca 667 mg/L 633 mg/L 2.14 , % 24 %
Na 525 mg/L 167 mg/L 2.17 % 18 %
K 264 mg/L 114 mg/L 2.12 % 11 %
Ti 54.1 mg/L 11.8 mg/L 0.41 % 11 %
P 10.0 mg/L <8 mg/L 0.03 % 28 %
Mn 116 mg/L 34.2 mg/L 0.12 % 48 %
F 31.0 mg/L 11.0 mg/L 0.27 % 10 %
Be 36.4 mg/L 10.7 mg/L 202 g/t 62 %
Example 2
[00156] A bench scale test was conducted to test the effect of multi-stage
acid addition
and selective thermal sulphation. 35 g of concentrated (96%) sulfuric acid was
added to
50 g of dry flotation concentrate. The acid and concentrate were mixed by hand
with a
plastic stick in a glass crucible to achieve as homogeneous a mixture as
possible. The
crucible was placed in a muffle furnace where it was heated until the sample
reached
approximately 280 C, and it was maintained at this temperature for 30 minutes.
The
sample was removed from the furnace and allowed to cool slightly. An
additional 25 g
39
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
of concentrated sulphuric acid was added and again mixed with the solids by
hand.
After mixing, the crucible was charged again into the muffle furnace. The
sample was
heated to 300 C and maintained at this temperature for an additional 30 mm.
The
sample was then heated further to 650 C, and maintained at close to this
temperature
for 30 minutes, before being removed and allowed to cool. At all stages of the
test, the
mixture was either in dry powder or granular agglomerate form. Paste formation
or
adhesion was avoided by adding the acid in multiple stages with heating in
between.
After baking the sample weighed 60 g and 55 g of this was added to 406 mL of
ambient
temperature (25 C) de-ionized (D1) water for leaching. Leaching was conducted
in a
glass reactor equipped with a PTFE impeller for lhr. The pulp was weighed and
filtered, and the cake washed with 75 mL of DI water. 464mL of combined final
leach
and wash filtrate were collected. 39 g of dry residue was collected. Filtrate,
and dry
leached residue were analyzed. The results of the metallurgical balance are
presented
below. Dy and Nd extraction to solution were 82% and 92%, respectively, while
Fe and
Al extraction were 26 and 34%, respectively. The pH of the leach filtrate was
only
mildly acidic with about 1.3 g/L of free acid.
[00157] Table 3 - Feed Composition for Example 2
Feed: 20% MP Concentrate #4 Unit
Mass: 46
Element: Assay
La 5380 g/t
Ce 11400 g/t
Pr 1280 g/t
Nd 4000 g/t
Sm 1000 g/t
Eu 56 g/t
Gd 889 g/t
Tb 205 g/t
Dy 1390 g/t
Ho 306 g/t
7540 g/t
Er 958 g/t
Tm 142 g/t
Yb 857 g/t
Lu 115 g/t
Sc <25 git
Th 1520 g/t
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
U 222 g/t
Zr 4.22 %
Si 21.0 %
Al 1.79 'A
Fe 13.15 %
_ Mg 0.36 %
Ca 6.09 %
Na 3.34 %
K 0.88 %
Ti 0.92 %
P 0.10 %
Mn 0.33 A)
1001581 Table 4 - Metallurgical balance for Example 2 products:
Component: PLS+ Unit Final Unit Extraction Unit
Wash residue to solution .
Quantity: 464 mL 39 g
Metal:
La 437 mg/L 403 g/t 93 %
Ce 912 mg/L _ 897 g/t 92
Pr 102 mg/L 99 g/t 93 %
Nd 353 mg/L 370 g/t 92
Sm 74.9 mg/L 108 g/t 89 %
Eu 4.37 mg/L 7 g/t 89 %
Gd 80.9 mg/L 135 g/t 88 %
Tb 15.4 mg/L 33 g/t 85 % _
Dy 102 mg/L 276 g/t 82
Ho 21.9 mg/L 67 g/t 80 % _
Y 596 mg/L 1520 g/t 83
Er 66.6 mg/L 229 g/t , 78 %
Tm 9.68 mg/L 36 g/t 76
Yb 59.2 mg/L 225 g/t , 76 %
Lu 7.39 mg/L 30 g/t 75 % ,
Sc <0.2 mg/L <25 g/t 9 %
Th 105 mg/L 342 g/t 79 %
U 14.3 mg/L 46 g/t 79 %
Zr 1310 mg/L 2.92 % 35 % _
Si mg/L 21.9 % 0 % ,
Al 559 mg/L 1.31 % 34 %
Fe 3100 mg/L 10.77 % 26 %
Mg 314 mg/L 0.04 % 90 %
Ca 823 mg/L 5.62 % 15 %
Na 688 mg/L 2.92 % 22 %
K 98 mg/L 0.84 % 12 %
41
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
Ti 237 mg/L 0.71 29
40.0 mg/L 0.06 44
Mn 147 mg/L 0.19 48
Example 3
[00159] Based on reasonable flotation performance targets set having regard to
experimentations conducted and available mineral concentrate samples, an
exemplary
estimate of maximum uranium and thorium content and activity in projected
flotation
concentrate was calculated as set out below. Given the putative targets and
available
resources, no representation is made with respect to the estimated
concentrations or
applicability of the calculations below with respect to any other ore samples,
or with
respect to as of yet unknown mine plans or installations:
[00160] Table 5 - Estimated maximum U+Th concentrations for Strange Lake
Mineral concentrate
Radionuclide Max Concentration (ppm)
Uranium (U) 600
Thorium (Th) 4300
[001611 Table 6 -Specific Activity Constants for U and Th
Radionuclide Specific Activity Constant (bq/g)
Uranium 238 12460
Thorium 232 4066
1001621 Based on the projected radionuclide levels in concentrate in Table 5
and the
activity constants in Table 6, the following calculation can be made:
Max Calculated activity (U+Th only) = 600/10^6*12460 4300/10^6*4066 = 7.5
bq/g (U) + 15.5 bq/g (Th) = 23 bq/g (U+Th)
1001631 In the entire decay chain there are 10 Th232 daughter products and 14
U238
daughter products, allowing the following calculation to be made:
42
SUBSTITUTE SHEET (RULE 26)
CA 03008040 2018-06-11
WO 2017/100933
PCT/CA2016/051489
Total estimated maximum activity = 600/10^6*12460*14 +4300/10^6*4066*10 =
75 bq/g (U series) + 155 bq/g (Th series) = 230 bq/g (Total U+Th series)
1001641 Radiation Dosage:
A previous sample with a calculated activity (U + Th only) of 13.2 bq/g
measured
approximately 2.5 .i.Sv/h near the surface of the sample. Assuming a linear
relationship
between dosage and U+Th activity, a sufficient quantity of this mineral
concentrate
could generate an activity of 4.4 Ov/h. An exposure of 227 hours directly
adjacent to
a material stockpile could possibly give rise to an annual external dose of 1
millisievert
(the annual dose limit for a member of public). There is also a minor
potential for
internal dose from inhaled dust.
[00165] Although the invention has been described with reference to certain
specific
embodiments, various modifications thereof will be apparent to those skilled
in the art
without departing from the spirit and scope of the invention. All such
modifications as
would be apparent to one skilled in the art are intended to be included within
the scope
of the following claims.
43
SUBSTITUTE SHEET (RULE 26)