Note: Descriptions are shown in the official language in which they were submitted.
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 1 -
FLUID CATALYTIC CRACKING OF TIGHT OIL RESID
FIELD
[0001] Systems and methods are provided for processing of tight oil
fractions.
BACKGROUND
[0002] Fluid catalytic cracking (FCC) processes are commonly used in
refineries as a method
for converting heavy oil feedstocks, without requiring additional hydrogen, to
produce lower
boiling fractions suitable for use as fuels. FCC processes can be valuable for
converting vacuum
gas oil boiling range feeds to lower boiling compounds suitable for use as
naphtha or distillate
fuels while avoiding excessive consumption of hydrogen. However, the catalysts
used for FCC
processes are typically susceptible to metal poisoning. To avoid exposing FCC
catalysts to
excessive metal contents, vacuum fractionation can be used to remove a vacuum
resid fraction
from a heavy oil feedstock prior to FCC processing.
[0003] U.S. Patent 8,007,662 describes methods for direct feed / effluent
heat exchange in fluid
catalytic cracking. The methods are described as being suitable for FCC
processing of feeds having
reduced quantities of coke precursors. Suitable feeds are described as having
a total metals content
of less than about 5 wppm, a Conradson Carbon residue of less than about 3
wt%, and a sulfur
content of less than about 500 wppm.
SUMMARY
[0004] In an aspect, a method is provided for processing a tight oil
fraction, the method
including separating a tight oil fraction at a pressure of at least 5 psig (35
kPa) to form at least a
higher boiling fraction having a T5 boiling point of at least about 650 F (343
C) and a second
fraction having a lower T5 boiling point than the higher boiling fraction, the
higher boiling fraction
comprising about 5 wppm to about 10 wppm of metals and at least about 20 wt%
of 1050 F+
compounds; and exposing the higher boiling fraction to a cracking catalyst
under effective fluid
catalytic cracking conditions to form a cracked effluent comprising at least
about 5 wt% of
1050 F+ compounds, or at least about 10 wt%.
[0005] In another aspect, a fluid catalytic cracking effluent is provided,
the fluid catalytic
cracking effluent comprising a light catalytic naphtha fraction, a heavy
catalytic naphtha fraction,
a light cycle oil, and a main column bottoms, the light catalytic naphtha
fraction having a research
octane number of at least 90 (or at least 93) and an olefin content of at
least 35 wt% (or at least 40
wt%), the heavy catalytic naphtha having a research octane number of at least
90 (or at least 92)
and optionally an olefin content of at least 6 wt% (or at least 8 wt% or at
least 9 wt%), the light
cycle oil having a cetane index of at least 20.0, and the main column bottoms
optionally having an
API gravity of at least -10, or at least -9.
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 2 -
BRIEF DESCRIPTION OF THE FIGURES
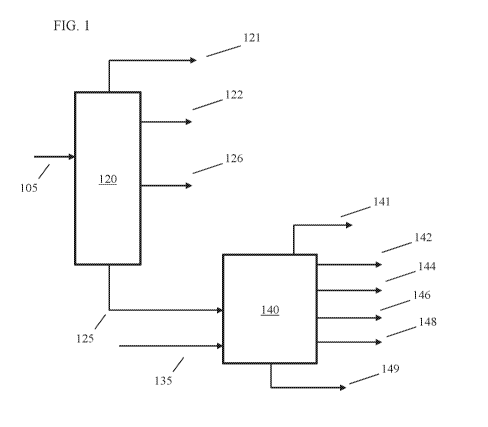
[0006] FIG. 1 shows a configuration suitable for performing a fluid
catalytic cracking process
on a tight oil fraction.
[0007] FIG. 2 shows examples of properties of fluid catalytic cracking
effluents.
DETAILED DESCRIPTION
[0008] Shale oil or "tight oil" represents an increasingly important source
of crude oil. It has
been determined that tight oils can have a somewhat different compositional
profile in comparison
with conventional mineral crude oils. In particular, the heavier (higher
boiling) portions of a tight
oil can tend to have lower sulfur contents and/or lower total metals contents
than other mineral
crude oils.
[0009] In various aspects, systems and methods are provided for FCC
processing of
atmospheric resid boiling range feedstocks derived from tight oils. Due to low
contents of metals,
sulfur, and/or coke-forming compounds, an atmospheric resid boiling range
fraction derived from
a tight oil feedstock that includes a substantial 1050 F+ (566 C+) portion can
be suitable for
processing in an FCC reactor. A substantial 1050 F+ (566 C) portion can
correspond to an
atmospheric resid boiling range fraction having at least 15 wt% of 1050 F+
compounds, or at least
about 20 wt%, or at least about 25 wt%, or at least about 30 wt%, such as up
to about 40 wt%.
This can allow the atmospheric resid boiling range portion of a tight oil
feedstock to be processed
in an FCC reactor without requiring a vacuum distillation. Instead, a tight
oil fraction can be
topped, such as by separation in an atmospheric tower, a flash tower, or a
flash drum, to form one
or more fuels boiling range fractions and a bottoms fraction. The one or more
fuels boiling range
fractions can include naphtha, kerosene, and/or diesel boiling range
fractions. By separating such
fuels boiling range fractions prior to FCC processing, further cracking of
these fuels boiling range
fractions to lower value products can be reduced or minimized. The bottoms
fraction remaining
after separation of fuels boiling range fractions, such as a 650 F+ (343 C+)
fraction, can be
processed in an FCC reactor.
[0010] One of the difficulties with processing feeds using FCC processing
is the accumulation
of metals on the FCC catalyst. Crude oil sources that include distillate
boiling range compounds
also typically include vacuum resid boiling range compounds. Unfortunately,
mineral sources of
vacuum resid fractions also typically contain metals such as nickel and
vanadium. Such metals
can accumulate on an FCC catalyst and can cause deactivation. In order to
reduce or minimize the
rate of deactivation under conventional methods, the vacuum resid portion of a
feed can be
separated from the distillate portion by vacuum distillation. The
fractionation cut point in such a
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 3 -
separation can be selected to achieve a desired target metals content in the
resulting distillate
portion. Typical target metals contents for FCC feeds are 2 ¨ 3 wppm.
[0011] Crude oils derived from shale oil or tight oil sources can have an
atmospheric resid
boiling range fraction that includes both a distillate and a 1050 F+ (566 C)
or vacuum resid
portion, similar to other mineral sources. However, it has been determined
that the 1050 F+
portion of atmospheric resids derived from a tight oil sources can have
unexpectedly low metal
contents. The unexpectedly low metal contents can correspond to metal contents
for an
atmospheric resid boiling range fraction of about 5 wppm to about 15 wppm, or
about 5 wppm to
about 10 wppm, or about 5 wppm to about 7 wppm. Additionally or alternately,
an atmospheric
resid boiling range fraction can have a combined content of Ni, Cr, and/or V
of about 5 wppm to
about 15 wppm, or about 5 wppm to about 10 wppm, or about 5 wppm to about 7
wppm. While
the metal content of a 1050 F+ portion of a feed derived from tight oil
sources is higher than the
feedstock metals content for conventional FCC processing, it has been
determined that 1050 F+
portion of a feed derived from tight oil sources can be suitable for FCC
processing.
[0012] Because the 1050 F+ portion of a feed derived from a tight oil can
be suitable for FCC
processing, a crude oil derived from tight oil can be prepared for FCC
processing using atmospheric
distillation, as opposed to using both an atmospheric distillation and a
vacuum distillation.
Performing an atmospheric distillation can allow fuels boiling range compounds
to be removed
from the feed prior to FCC processing. This can avoid cracking of diesel
boiling range compounds
to naphtha and/or cracking of naphtha to light ends.
[0013] In some aspects, an atmospheric distillation can be tightly
controlled in order to increase
the amount of diesel boiling range compounds that are separated out prior to
FCC processing.
Alternatively, another type of distillation or fractionation technique could
be used instead of an
atmospheric distillation, such as a flash fractionation. A flash fractionation
can tend to produce a
wider overlap in boiling range between the two resulting fractions as compared
with performing a
distillation in a distillation column or tower. Depending on the nature of the
fractionation, the
fractionation can be performed at a pressure of about 5 psig (35 kPa) to about
30 psig (207 kPa).
This is in contrast to a vacuum fraction, which can typically be performed at
a pressure of about 2
psig (about 15 kPa) or less. Depending on the nature of the fractionation, the
fractionation can be
used to form a higher boiling fraction having a T10 boiling point of about 700
F (371 C) or less,
or about 650 F (343 C) or less, or 600 F (316 C) or less; a T5 boiling point
of about 700 F (371 C)
or less, or about 650 F (343 C) or less, or 600 F (316 C) or less; and/or a T2
boiling point of about
700 F (371 C) or less, or about 650 F (343 C) or less, or 600 F (316 C) or
less. The higher boiling
fraction, or at least a portion of it, corresponds to a tight oil atmospheric
resid that can be processed
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 4 -
in an FCC reactor. The boiling points for the higher boiling fraction can be
determined by a
suitable ASTM method, such as ASTM D2887.
[0014] Another option for characterizing the separation of a tight oil into
(at least) a lower
boiling fraction and a higher boiling fraction can be based on the boiling
range of the lower boiling
fraction. In some aspects, it can be desirable to increase or maximize the
amount of diesel boiling
range compounds in the lower boiling fraction. This can be accomplished by
performing a
fractionation to form a lower boiling fraction having a T90 boiling point of
about 650 F (343 C)
or less, or about 690 F (365 C) or less, as determined by ASTM D86.
[0015] In this discussion, a tight oil or tight oil fraction is defined in
accordance with the U.S.
Energy Information Administration definition, which defines a tight oil as a
mineral crude oil or
crude oil fraction that is extracted from formations that must be
hydraulically fractured to produce
oil at a commercial rate. In this discussion, production of oil at a
commercial rate is defined as at
least 100 barrels per day, but in some aspects a lower threshold of 10 barrels
per day can be used.
A tight oil fraction is defined to include the limiting case of a fraction
that corresponds to a whole
tight oil crude.
[0016] In some aspects, reference is made to conversion of a feedstock
relative to a conversion
temperature T. Conversion relative to a temperature T is defined based on the
portion of the
feedstock that boils at a temperature greater than the conversion temperature
T. The amount of
conversion during a process (or optionally across multiple processes) is
defined as the weight
percentage of the feedstock that is converted from boiling at a temperature
above the conversion
temperature T to boiling at a temperature below the conversion temperature T.
As an illustrative
hypothetical example, consider a feedstock that includes 40 wt% of components
that boil at 700 F
(371 C) or greater. By definition, the remaining 60 wt% of the feedstock boils
at less than 700 F
(371 C). For such a feedstock, the amount of conversion relative to a
conversion temperature of
700 F (371 C) would be based only on the 40 wt% that initially boils at 700 F
(371 C) or greater.
If such a feedstock is exposed to a process with 30% conversion relative to a
700 F (371 C)
conversion temperature, the resulting product would include 72 wt% of
components boiling below
700 F (371 C) and 28 wt% of components boiling above 700 F (371 C).
[0017] In various aspects of the invention, reference may be made to one or
more types of
fractions generated during distillation of a petroleum feedstock. Such
fractions may include
naphtha fractions, kerosene fractions, diesel fractions, vacuum gas oil or
distillate fractions, and
atmospheric resid fractions. Each of these types of fractions can be defined
based on a boiling
range, such as a boiling range that includes at least 90 wt% of the fraction,
or at least 95 wt% of
the fraction. For example, for many types of naphtha fractions, at least 90
wt% of the fraction, and
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 5 -
preferably at least 95 wt%, can have a boiling point in the range of 85 F (29
C) to 350 F (177 C).
For some heavier naphtha fractions, at least 90 wt% of the fraction, and
preferably at least 95 wt%,
can have a boiling point in the range of 85 F (29 C) to 400 F (204 C). For a
kerosene fraction, at
least 90 wt% of the fraction, and preferably at least 95 wt%, can have a
boiling point in the range
of 300 F (149 C) to 600 F (288 C). Alternatively, for a kerosene fraction
targeted for some uses,
such as jet fuel production, at least 90 wt% of the fraction, and preferably
at least 95 wt%, can have
a boiling point in the range of 300 F (149 C) to 550 F (288 C). For a diesel
fraction, at least 90
wt% of the fraction, and preferably at least 95 wt%, can have a boiling point
in the range of 400 F
(204 C) to 750 F (399 C). For a (vacuum) gas oil fraction, at least 90 wt% of
the fraction, and
preferably at least 95 wt%, can have a boiling point in the range of 650 F
(343 C) to 1100 F
(593 C). Optionally, for some gas oil fractions, a narrower boiling range may
be desirable. For
such gas oil fractions, at least 90 wt% of the fraction, and preferably at
least 95 wt%, can have a
boiling point in the range of 650 F (343 C) to 1000 F (538 C), or 650 F (343
C) to about 900 F
(482 C). For an atmospheric resid boiling range fraction, the lower end of the
boiling range can
be similar to a vacuum gas oil. This can correspond to having a T5 boiling
point of at least about
600 F (316 C), or at least about 650 F (343 C), or at least about 700 F (371
C). Such a T5 boiling
point can be measured by a suitable method, such as ASTM D2887. An atmospheric
resid boiling
range fraction is different from a vacuum gas oil fraction due to the lack of
a well-defined T95
boiling point and/or final boiling point. Instead, an atmospheric resid
boiling range fraction can
also include higher boiling point compounds, such as 1050 F+ compounds.
Feedstock ¨ Tight Oil Atmospheric Resid
[0018] A tight oil atmospheric resid can correspond to a high boiling
fraction, such as a bottoms
fraction, from an atmospheric distillation or other type of fractionation as
described above. A
variety of properties of a tight oil fraction and/or a tight oil atmospheric
resid boiling range fraction
can be characterized to specify the nature of the fraction.
[0019] Crude oil produced from shale rock formations using hydraulic
fracturing (i.e., a tight
oil) can generally be light, such as greater than 35 API gravity. Such crude
oil can also generally
having a sulfur content of less than about 0.5 wt%.
[0020] After fractionation to remove diesel fuel boiling range and lower
boiling compounds,
an atmospheric resid fraction (or other similar boiling range fraction)
derived from a tight oil can
include a 1050 F+ (566 C) portion corresponding to at least about 15 wt% of
the atmospheric resid
fraction, or at least about 20 wt%, or at least about 25 wt%, or at least
about 30 wt%. Based in part
on the increased amount of 1050 F+ compounds, a tight oil atmospheric resid
fraction can also
have an increased content of Conradson Carbon Residue (CCR) relative to a
conventional feed for
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 6 -
FCC processing. In various aspects, a tight oil atmospheric resid boiling
range fraction can have
a CCR content of at least about 2 wt%, or at least about 5 wt%, or at least
about 8 wt%, or at least
about 10 wt%.
[0021] Density, or weight per volume, of an atmospheric resid boiling range
fraction derived
from a tight oil can also be characterized. In various aspects, the density of
the atmospheric resid
boiling range fraction at 60 F can be 0.88 g/cc to 0.98 g/cc, or 0.89 g/cc to
0.98 g/cc, or 0.88 g/cc
to 0.97 g/cc, or 0.89 g/cc to 0.97 g/cc.
[0022] Contaminants such as nitrogen and sulfur are typically found in an
atmospheric resid
boiling range fraction, often in organically-bound form. Nitrogen content can
range from about 50
wppm to about 3000 wppm elemental nitrogen, or about 75 wppm to about 2000
wppm, or about
100 wppm to about 1000 wppm elemental nitrogen, or about 250 wppm to about 500
wppm, based
on total weight of the atmospheric resid boiling range fraction. The nitrogen
containing
compounds can be present as basic or non-basic nitrogen species. Examples of
nitrogen species
can include quinolones, substituted quinolones, carbazoles, and substituted
carbazoles.
[0023] The sulfur content of an atmospheric resid boiling range fraction
can be at least about
200 wppm elemental sulfur, based on total weight of the atmospheric resid
boiling range fraction.
Generally, the sulfur content of a atmospheric resid boiling range fraction
can range from about
200 wppm to about 20,000 wppm elemental sulfur based on total weight of the
heavy component,
or from about 200 wppm to about 10,000 wppm, or from about 200 wppm to about
5,000 wppm,
or from about 200 wppm to about 4,000 wppm, or from about 200 wppm to about
3,000 wppm, or
from about 500 wppm to about 20,000 wppm, or from about 500 wppm to about
10,000 wppm, or
from about 500 wppm to about 5,000 wppm, or from about 500 wppm to about 4,000
wppm, or
from about 500 wppm to about 3,000 wppm, or from about 1,000 wppm to about
20,000 wppm,
or from about 1,000 wppm to about 10,000 wppm, or from about 1,000 wppm to
about 5,000
wppm, or from about 1,000 wppm to about 4,000 wppm, or from about 1,000 wppm
to about 3,000
wppm. Sulfur can usually be present as organically bound sulfur. Examples of
such sulfur
compounds include the class of heterocyclic sulfur compounds such as
thiophenes,
tetrahydrothiophenes, benzothiophenes and their higher homologs and analogs.
Other organically
bound sulfur compounds include aliphatic, naphthenic, and aromatic mercaptans,
sulfides, di- and
polysulfides.
FCC Processing
[0024] In various aspects, at least a portion of a tight oil atmospheric
resid can be used as a
feed for processing in a Fluid Catalytic Cracking ("FCC") unit. The tight oil
atmospheric resid can
be processed alone in the FCC process, or the hydrotreated effluent can be
combined with another
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 7 -
suitable feed for processing in an FCC process. Such other suitable
feedstreams can include feeds
(such as gas oils or distillates) boiling in the range of about 430 F to about
1050 F (221 to 566 C).
The FCC feed may also comprise recycled hydrocarbons, such as light or heavy
cycle oils.
[0025] An example of a suitable reactor for performing an FCC process can
be a riser reactor.
Within the reactor riser, the FCC feedstream can be contacted with a catalytic
cracking catalyst
under cracking conditions thereby resulting in spent catalyst particles
containing carbon deposited
thereon and a lower boiling product stream. The cracking conditions can
typically include:
temperatures from about 900 to about 1060 F (482 to 571 C.), or about 950 to
about 1040 F (510
to 560 C); hydrocarbon partial pressures from about 10 to 50 psia (70-345
kPa), or from about 20
to 40 psia (140-275 kPa); and a catalyst to feed (wt/wt) ratio from about 3 to
8, or about 5 to 6,
where the catalyst weight is total weight of the catalyst composite. Steam may
be concurrently
introduced with the feed into the reaction zone. The steam may comprise up to
about 5 wt% of the
feed. In some aspects, the FCC feed residence time in the reaction zone is
less than about 5
seconds, or from about 3 to 5 seconds, or from about 2 to 3 seconds.
[0026] Catalysts suitable for use within the FCC reactor herein are fluid
cracking catalysts
comprising either a large-pore molecular sieve or a mixture of at least one
large-pore molecular
sieve catalyst and at least one medium-pore molecular sieve catalyst. Large-
pore molecular sieves
suitable for use herein can be any molecular sieve catalyst having an average
pore diameter greater
than 0.7 nm which are typically used to catalytically "crack" hydrocarbon
feeds. In various aspects,
both the large-pore molecular sieves and the medium-pore molecular sieves used
herein may be
selected from those molecular sieves having a crystalline tetrahedral
framework oxide component.
For example, the crystalline tetrahedral framework oxide component can be
selected from the
group consisting of zeolites, tectosilicates, tetrahedral aluminophosphates
(ALP0s) and tetrahedral
silicoaluminophosphates (SAP0s). Preferably, the crystalline framework oxide
component of both
the large-pore and medium-pore catalyst is a zeolite. It should be noted that
when the cracking
catalyst comprises a mixture of at least one large-pore molecular sieve
catalyst and at least one
medium-pore molecular sieve, the large-pore component is typically used to
catalyze the
breakdown of primary products from the catalytic cracking reaction into clean
products such as
naphtha and distillates for fuels and olefins for chemical feedstocks.
[0027] Large pore molecular sieves that are typically used in commercial
FCC process units
are also suitable for use herein. FCC units used commercially generally employ
conventional
cracking catalysts which include large-pore zeolites such as USY or REY.
Additional large pore
molecular sieves that can be employed in accordance with the present invention
include both
natural and synthetic large pore zeolites. Non-limiting examples of natural
large-pore zeolites
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 8 -
include gmelinite, chabazite, dachiardite, clinoptilolite, faujasite,
heulandite, analcite, levynite,
erionite, sodalite, cancrinite, nepheline, lazurite, scolecite, natrolite,
offretite, mesolite, mordenite,
brewsterite, and ferrierite. Non-limiting examples of synthetic large pore
zeolites are zeolites X,
Y, A, L, ZK-4, ZK-5, B, E, F, H, J, M, Q, T, W, Z, alpha and beta, omega, REY
and USY zeolites.
In some aspects, the large pore molecular sieves used herein can be selected
from large pore
zeolites. In such aspects, suitable large-pore zeolites for use herein can be
the faujasites,
particularly zeolite Y, USY, and REY.
[0028] Medium-pore size molecular sieves that are suitable for use herein
include both medium
pore zeolites and silicoaluminophosphates (SAP0s). Medium pore zeolites
suitable for use in the
practice of the present invention are described in "Atlas of Zeolite Structure
Types", eds. W. H.
Meier and D. H. Olson, Butterworth-Heineman, Third Edition, 1992, which is
hereby incorporated
by reference. The medium-pore size zeolites generally have an average pore
diameter less than
about 0.7 nm, typically from about 0.5 to about 0.7 nm and includes for
example, MFI, MFS, MEL,
MTW, EUO, MTT, HEU, FER, and TON structure type zeolites (IUPAC Commission of
Zeolite
Nomenclature). Non-limiting examples of such medium-pore size zeolites,
include ZSM-5,
ZSM-12, ZSM-22, ZSM-23, ZSM-34, ZSM-35, ZSM-38, ZSM-48, ZSM-50, silicalite,
and
silicalite 2. An example of a suitable medium pore zeolite is ZSM-5, which is
described in U.S.
Pat. Nos. 3,702,886 and 3,770,614. Other suitable zeolites can include ZSM-11,
described in U.S.
Pat. No. 3,709,979; ZSM-12 in U.S. Pat. No. 3,832,449; ZSM-21 and ZSM-38 in
U.S. Pat. No.
3,948,758; ZSM-23 in U.S. Pat. No. 4,076,842; and ZSM-35 in U.S. Pat. No.
4,016,245. As
mentioned above SAPOs, such as SAPO-11, SAPO-34, SAPO-41, and SAPO-42, which
are
described in U.S. Pat. No. 4,440,871 can also be used herein. Non-limiting
examples of other
medium pore molecular sieves that can be used herein are chromosilicates;
gallium silicates; iron
silicates; aluminum phosphates (ALPO), such as ALP0-11 described in U.S. Pat.
No. 4,310,440;
titanium aluminosilicates (TASO), such as TASO-45 described in EP-A No.
229,295; boron
silicates, described in U.S. Pat. No. 4,254,297; titanium aluminophosphates
(TAPO), such as
TAPO-11 described in U.S. Pat. No. 4,500,651 and iron aluminosilicates. All of
the above patents
are incorporated herein by reference.
[0029] The medium-pore size zeolites used herein can also include
"crystalline admixtures"
which are thought to be the result of faults occurring within the crystal or
crystalline area during
the synthesis of the zeolites. Examples of crystalline admixtures of ZSM-5 and
ZSM-11 are
disclosed in U.S. Pat. No. 4,229,424 which is incorporated herein by
reference. The crystalline
admixtures are themselves medium-pore size zeolites and are not to be confused
with physical
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 9 -
admixtures of zeolites in which distinct crystals of crystallites of different
zeolites are physically
present in the same catalyst composite or hydrothermal reaction mixtures,
[0030] In some aspects, the large-pore zeolite catalysts and/or the medium-
pore zeolite
catalysts can be present as "self-bound" catalysts, where the catalyst does
not include a separate
binder. In other aspects, the large-pore and medium-pore catalysts can be
present in an inorganic
oxide matrix component that binds the catalyst components together so that the
catalyst product is
hard enough to survive inter-particle and reactor wall collisions. The
inorganic oxide matrix can
be made from an inorganic oxide sol or gel which is dried to "glue" the
catalyst components
together. Preferably, the inorganic oxide matrix can be comprised of oxides of
silicon and
aluminum. It is also preferred that separate alumina phases be incorporated
into the inorganic
oxide matrix. Species of aluminum oxyhydroxides-y-alumina, boehmite, diaspore,
and transitional
aluminas such as a-alumina, 13-alumina, y-alumina, 6-alumina, 6-alumina, K-
alumina, and
p-alumina can be employed. Preferably, the alumina species is an aluminum
trihydroxide such as
gibbsite, bayerite, nordstrandite, or doyelite. The matrix material may also
contain phosphorous
or aluminum phosphate. Optionally, the large-pore catalysts and medium-pore
catalysts be present
in the same or different catalyst particles, in the aforesaid inorganic oxide
matrix.
[0031] In the FCC reactor, the cracked FCC product is removed from the
fluidized catalyst
particles. Preferably this is done with mechanical separation devices, such as
an FCC cyclone.
The FCC product is removed from the reactor via an overhead line, cooled and
sent to a fractionator
tower for separation into various cracked hydrocarbon product streams. These
product streams
may include, but are not limited to, a light gas stream (generally comprising
C4 and lighter
hydrocarbon materials), a naphtha (gasoline) stream, a distillate (diesel
and/or jet fuel) steam, and
other various heavier gas oil product streams. The other heavier stream or
streams can include a
bottoms stream.
[0032] In the FCC reactor, after removing most of the cracked FCC product
through
mechanical means, the majority of, and preferably substantially all of, the
spent catalyst particles
are conducted to a stripping zone within the FCC reactor. The stripping zone
will typically contain
a dense bed (or "dense phase") of catalyst particles where stripping of
volatiles takes place by use
of a stripping agent such as steam. There will also be space above the
stripping zone wherein the
catalyst density is substantially lower and which space can be referred to as
a "dilute phase". This
dilute phase can be thought of as either a dilute phase of the reactor or
stripper in that it will
typically be at the bottom of the reactor leading to the stripper.
[0033] The majority of, and preferably substantially all of, the stripped
catalyst particles are
subsequently conducted to a regeneration zone wherein the spent catalyst
particles are regenerated
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 10 -
by burning coke from the spent catalyst particles in the presence of an oxygen
containing gas,
preferably air thus producing regenerated catalyst particles. This
regeneration step restores catalyst
activity and simultaneously heats the catalyst to a temperature from about
1200 F to about 1400 F
(649 to 760 C). The majority of, and preferably substantially all of the hot
regenerated catalyst
particles can then be recycled to the FCC reaction zone where they contact
injected FCC feed.
[0034] It is noted that the CCR content of an atmospheric resid boiling
range fraction can be
higher than a typical FCC feed due to the presence of a substantial portion of
1050 F+ compounds.
This can cause coke to form at an increased rate. At conventional catalyst
circulation rates, this
could lead to increased temperatures during regeneration due to the additional
amount of coke
present on the catalyst. In various aspects, the increased temperature in the
regeneration zone can
be mitigated at least in part by increasing the catalyst replacement rate.
Introduction of fresh
catalyst at a lower temperature can reduce the overall temperature of catalyst
introduced into the
FCC reactor.
Hydrotreatment of FCC Effluent Portions
[0035] After FCC processing, one or more resulting naphtha boiling range
fractions, diesel
boiling range fractions, and/or distillate boiling range fractions can be
separated from the FCC
effluent. These types of fractions can sometimes be referred to as catalytic
naphtha and various
cycle oils, such as light catalytic cycle oil or heavy catalytic cycle oil.
The one or more naphtha
and/or diesel boiling range fractions can be hydrotreated to meet desired
sulfur content targets.
[0036] In various aspects, a naphtha boiling range portion of an FCC
effluent can be
hydrotreated (sometimes referred to as hydrodesulfurized) to reduce the sulfur
content of the higher
boiling portion. Such a hydrodesulfurization process can correspond to a
selective or a non-
selective hydrodesulfurization process. A selective hydrodesulfurization
process can refer to a
process where the hydrotreatment catalyst and/or the hydrotreatment conditions
are selected based
on a desire to preserve the olefin content of the hydrotreated product. A non-
selective
hydrodesulfurization process can refer to a process where a substantial
portion of the olefins
present in an naphtha feed are saturated during hydrodesulfurization. While a
selective
hydrodesulfurization process can be used to treat the higher boiling portion,
it is not necessary.
[0037] A (optionally selective) hydrodesulfurization process can be
performed in any suitable
reaction system. The hydrodesulfurization can be performed in one or more
fixed bed reactors,
each of which can comprise one or more catalyst beds of the same, or
different,
hydrodesulfurization catalyst. Optionally, more than one type of catalyst can
be used in a single
bed. Although other types of catalyst beds can be used, fixed beds are
preferred. Non-limiting
examples of such other types of catalyst beds that may be used in the practice
of the present
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 11 -
invention include fluidized beds, ebullating beds, slurry beds, and moving
beds. Interstage cooling
between reactors, or between catalyst beds in the same reactor, can be
employed since some olefin
saturation can take place, and olefin saturation as well as the
desulfurization reaction are generally
exothermic. A portion of the heat generated during hydrodesulfurization can be
recovered by
conventional techniques. Where this heat recovery option is not available,
conventional cooling
may be performed through cooling utilities such as cooling water or air, or by
use of a hydrogen
quench stream. In this manner, optimum reaction temperatures can be more
easily maintained.
[0038] In various aspects, suitable (optionally selective)
hydrodesulfurization catalysts include
catalysts that are comprised of at least one Group VIII metal oxide,
preferably an oxide of a metal
selected from selected from Co and/or Ni, more preferably Co; and at least one
Group VI metal
oxide, preferably an oxide of a metal selected from Mo and W, more preferably
Mo, on a support
material, such as silica or alumina. Other suitable hydrotreating catalysts
include zeolitic catalysts,
as well as noble metal catalysts where the noble metal is selected from Pd and
Pt. It is within the
scope of the present invention that more than one type of hydrotreating
catalyst be used in the same
reaction vessel. The Group VIII metal oxide of a selective
hydrodesulfurization catalyst can be
present in an amount ranging from about 0.1 to about 20 wt. %, preferably from
about 1 to about
12%. The Group VI metal oxide can be present in an amount ranging from about 1
to about 50 wt.
%, preferably from about 2 to about 20 wt. %. All metal oxide weight percents
are on support. By
"on support" we mean that the percents are based on the weight of the support.
For example, if the
support were to weigh 100 g. then 20 wt. % Group VIII metal oxide would mean
that 20 g. of
Group VIII metal oxide is on the support.
[0039] The hydrodesulfurization catalysts can be supported catalysts. Any
suitable refractory
catalyst support material, such as inorganic oxide support materials, can be
used as supports for
the catalyst of the present invention. Non-limiting examples of suitable
support materials include:
zeolites, alumina, silica, titania, calcium oxide, strontium oxide, barium
oxide, carbons, zirconia,
magnesia, diatomaceous earth, lanthanide oxides including cerium oxide,
lanthanum oxide,
neodynium oxide, yttrium oxide, and praesodymium oxide; chromia, thorium
oxide, urania, niobia,
tantala, tin oxide, zinc oxide, and aluminum phosphate. Preferred are alumina,
silica, and silica-
alumina. It is to be understood that the support material can also contain
small amounts of
contaminants, such as Fe, sulfates, silica, and various metal oxides that can
be introduced during
the preparation of the support material. These contaminants are present in the
raw materials used
to prepare the support and will preferably be present in amounts less than
about 1 wt. %, based on
the total weight of the support. It is more preferred that the support
material be substantially free
of such contaminants. In another embodiment, about 0 to about 5 wt. %,
preferably from about
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 12 -
0.5 to about 4 wt. %, and more preferably from about 1 to about 3 wt. % of an
additive can be
present in the support, which additive is selected from the group consisting
of phosphorus and
metals or metal oxides from Group IA (alkali metals) of the Periodic Table of
the Elements.
[0040] Generally, (optionally selective) hydrodesulfurization conditions
can include
temperatures from about 425 F (218 C) to about 800 F (427 C), or about 500 F
(260 C) to about
675 F (357 C). Other (optionally selective) hydrodesulfurization conditions
can include a pressure
of from about 60 psig (414 kPa) to about 800 psig (5516 kPa), preferably from
about 200 psig
(1379 kPa) to about 500 psig (3447 kPa), more preferably from about 250 psig
(1724kPa) to about
400 psig (2758 kPa). The hydrogen feed rate can be from about 500 standard
cubic feet per barrel
(scf/b) (84.2 m3/m3) to about 6000 scf/b (1011 m3/m3), preferably from about
1000 scf/b (168.5
m3/m3) to about 3000 scf/b (505.5 m3/m3). The liquid hourly space velocity can
be from about of
about 0.5 hr-1 to about 15 hr-1, preferably from about 0.5 hr' toabout 10 hr-
1, more preferably from
about 1 hr' to about 5 hr'.
[0041] In various aspects, a goal of a (optionally selective)
hydrodesulfurization process can
be to produce a naphtha product having a desired level of sulfur. In an
aspect, the desired level of
sulfur can be at least about 1 wppm to about 50 wppm, or about 1 wppm to about
30 wppm, or
about 1 wppm to about 20 wppm, or about 1 wppm to about 10 wppm, or about 5
wppm to about
50 wppm, or about 5 wppm to about 30 wppm, or about 5 wppm to about 20 wppm,
or about 5
wppm to about 10 wppm, or about 10 wppm to about 50 wppm, or about 10 wppm to
about 30
wppm, or about 10 wppm to about 20 wppm. In other aspects, the desired level
of sulfur can be
about 10 wppm or less, or about 5 wppm or less.
[0042] In various aspects, one or more diesel and/or distillate boiling
range portions of an FCC
effluent can be hydrotreated. Catalysts used for hydrotreatment of a diesel
boiling range or
distillate boiling range portion can include conventional hydroprocessing
catalysts, such as those
that comprise at least one Group VIII non-noble metal (Columns 8 ¨ 10 of IUPAC
periodic table),
preferably Fe, Co, and/or Ni, such as Co and/or Ni; and at least one Group VI
metal (Column 6 of
IUPAC periodic table), preferably Mo and/or W. Such hydroprocessing catalysts
can optionally
include transition metal sulfides. These metals or mixtures of metals are
typically present as oxides
or sulfides on refractory metal oxide supports. Suitable metal oxide supports
include low acidic
oxides such as silica, alumina, titania, silica-titania, and titania-alumina.
Suitable aluminas are
porous aluminas such as gamma or eta having average pore sizes from 50 to 200
A, or 75 to 150
A; a surface area from 100 to 300 m2/g, or 150 to 250 m2/g; and a pore volume
of from 0.25 to 1.0
cm3/g, or 0.35 to 0.8 cm3/g. The supports are preferably not promoted with a
halogen such as
fluorine as this generally increases the acidity of the support.
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 13 -
[0043] The at least one Group VIII non-noble metal, in oxide form, can
typically be present in
an amount ranging from about 2 wt% to about 40 wt%, preferably from about 4
wt% to about 15
wt%. The at least one Group VI metal, in oxide form, can typically be present
in an amount ranging
from about 2 wt% to about 70 wt%, preferably for supported catalysts from
about 6 wt% to about
40 wt% or from about 10 wt% to about 30 wt%. These weight percents are based
on the total
weight of the catalyst. Suitable metal catalysts include cobalt/molybdenum (1-
10% Co as oxide,
10-40% Mo as oxide), nickel/molybdenum (1-10% Ni as oxide, 10-40% Co as
oxide), or
nickel/tungsten (1-10% Ni as oxide, 10-40% W as oxide) on alumina, silica,
silica-alumina, or
titania.
[0044] Alternatively, the hydrotreating catalyst for a diesel boiling range
or distillate boiling
range portion of an FCC effluent can be a bulk metal catalyst, or a
combination of stacked beds of
supported and bulk metal catalyst. By bulk metal, it is meant that the
catalysts are unsupported
wherein the bulk catalyst particles comprise 30-100 wt. % of at least one
Group VIII non-noble
metal and at least one Group VIB metal, based on the total weight of the bulk
catalyst particles,
calculated as metal oxides and wherein the bulk catalyst particles have a
surface area of at least 10
m2/g. It is furthermore preferred that the bulk metal hydrotreating catalysts
used herein comprise
about 50 to about 100 wt%, and even more preferably about 70 to about 100 wt%,
of at least one
Group VIII non-noble metal and at least one Group VIB metal, based on the
total weight of the
particles, calculated as metal oxides. The amount of Group VIB and Group VIII
non-noble metals
can easily be determined VIB TEM-EDX.
[0045] Bulk catalyst compositions comprising one Group VIII non-noble metal
and two Group
VIB metals are preferred. It has been found that in this case, the bulk
catalyst particles are
sintering-resistant. Thus the active surface area of the bulk catalyst
particles is maintained during
use. The molar ratio of Group VIB to Group VIII non-noble metals ranges
generally from 10:1-
1:10 and preferably from 3:1-1:3. In the case of a core-shell structured
particle, these ratios of
course apply to the metals contained in the shell. If more than one Group VIB
metal is contained
in the bulk catalyst particles, the ratio of the different Group VIB metals is
generally not critical.
The same holds when more than one Group VIII non-noble metal is applied. In
the case where
molybdenum and tungsten are present as Group VIB metals, the
molybdenum:tungsten ratio
preferably lies in the range of 9:1-1:9. Preferably the Group VIII non-noble
metal comprises nickel
and/or cobalt. It is further preferred that the Group VIB metal comprises a
combination of
molybdenum and tungsten. Preferably, combinations of
nickel/molybdenum/tungsten and
cobalt/molybdenum/tungsten and nickel/cobalt/molybdenum/tungsten are used.
These types of
precipitates appear to be sinter-resistant. Thus, the active surface area of
the precipitate is
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 14 -
maintained during use. The metals are preferably present as oxidic compounds
of the
corresponding metals, or if the catalyst composition has been sulfided,
sulfidic compounds of the
corresponding metals.
[0046] It is also preferred that the bulk metal hydrotreating catalysts
used herein have a surface
area of at least 50 m2/g and more preferably of at least 100 m2/g. It is also
desired that the pore
size distribution of the bulk metal hydrotreating catalysts be approximately
the same as the one of
conventional hydrotreating catalysts. Bulk metal hydrotreating catalysts have
a pore volume of
0.05-5 ml/g, or of 0.1-4 ml/g, or of 0.1-3 ml/g, or of 0.1-2 ml/g determined
by nitrogen adsorption.
Preferably, pores smaller than 1 nm are not present. The bulk metal
hydrotreating catalysts can
have a median diameter of at least 50 nm, or at least 100 nm. The bulk metal
hydrotreating catalysts
can have a median diameter of not more than 5000 pm, or not more than 3000 pm.
In an
embodiment, the median particle diameter lies in the range of 0.1-50 pm and
most preferably in
the range of 0.5-50 pm.
[0047] The hydrotreatment of a diesel boiling range portion or distillate
boiling range portion
can be carried out in the presence of hydrogen. A hydrogen stream is,
therefore, fed or injected
into a vessel or reaction zone or hydroprocessing zone in which the
hydroprocessing catalyst is
located. Hydrogen, which is contained in a hydrogen-containing "treat gas," is
provided to the
reaction zone. Treat gas, as referred to in this invention, can be either pure
hydrogen or a hydrogen-
containing gas, which is a gas stream containing hydrogen in an amount that is
sufficient for the
intended reaction(s), optionally including one or more other gasses (e.g.,
nitrogen and light
hydrocarbons such as methane), and which will not adversely interfere with or
affect either the
reactions or the products. Impurities, such as H2S and NH3 are undesirable and
would typically be
removed from the treat gas before it is conducted to the reactor. The treat
gas stream introduced
into a reaction stage will preferably contain at least about 50 vol. % and
more preferably at least
about 75 vol. % hydrogen.
[0048] Hydrotreating conditions for a diesel boiling range portion and/or
distillate boiling
range portion can include temperatures of about 200 C to about 450 C, or about
315 C to about
425 C; pressures of about 250 psig (1.8 MPag) to about 5000 psig (34.6 MPag)
or about 300 psig
(2.1 MPag) to about 3000 psig (20.8 MPag); liquid hourly space velocities
(LHSV) of about 0.1
hr-1 to about 10 hr-1; and hydrogen treat rates of about 200 scf/B (35.6
m3/m3) to about 10,000 scf/B
(1781 m3/m3), or about 500 (89 m3/m3) to about 10,000 scf/B (1781 m3/m3).
Product Properties ¨ Hydrotreated Effluent and FCC Products
[0049] The FCC effluent and/or hydrotreated FCC effluent from processing
the atmospheric
resid boiling range portion of a tight oil can be characterized in various
manners.
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 15 -
[0050] In
some aspects, the FCC effluent can include at least about 10 wt% of 1050 F+
(566 C) compounds, or at least about 15 wt%. Additionally or alternately, the
FCC effluent can
include about 1 wppm to about 3 wppm of metals, such as about 1 wppm to about
3 wppm of Ni,
Cr, and/or V. Additionally or alternately, can include about 1 wt% to about 5
wt% of Conradson
Carbon Residue (CCR).
[0051] In
some aspects, the FCC effluent can include one or more naphtha boiling range
fractions, one or more diesel boiling range fractions, and a catalytic slurry
oil or bottoms fraction.
Optionally, a combined yield of the one or more naphtha boiling range
fractions from the FCC
effluent and the naphtha boiling range fraction(s) separated from the tight
oil fraction can
correspond to 20 wt% to 50 wt% of the tight oil fraction. Optionally, a
combined yield of the one
or more diesel boiling range fractions from the FCC effluent and the diesel
boiling range fraction(s)
separated from the tight oil fraction can correspond to 20 wt% to 50 wt% of
the tight oil fraction.
Optionally, a combined yield of the various fuel boiling range fractions
(naphtha boiling range
fractions and diesel boiling range fractions from the FCC effluent and
separated from the tight oil
fraction) can correspond to at least 60 wt% of the tight oil fraction, or at
least 70 wt%, or at least
80 wt%.
[0052] In
various aspects, the FCC effluent derived from a tight oil feed can also
provide a
combination of unexpected properties. The unexpected combination of properties
can include
properties related to the fuel value of underlying fractions, the olefin
content of underlying
fractions, and/or the quality of the bottoms fraction (i.e., main column
bottoms) generated during
FCC processing.
[0053] FIG.
2 provides a comparison of selected properties (as generated by an emprical
compositional model) of FCC effluents from a conventional feed and a tight oil
feed. The
conventional feed corresponds to a synthetic crude derived from a tar sands
formation. The FCC
effluent was fractionated to form at least a light catalytic naphtha fraction,
a heavy catalytic naphtha
fraction, and a light cycle oil having the boiling ranges shown in FIG. 2. The
remaining heavier
portion of the effluent corresponded to the main column bottoms.
[0054] In
the modeled results shown in FIG. 2, the light catalytic naphtha and heavy
catalytic
naphtha from the tight oil feed have similar octane numbers but higher olefin
contents relative to
the fractions derived from the conventional feed. Additionally, the cetane
number of the light cycle
oil from the tight oil feed is higher than the corresponding fraction from the
conventional feed.
Also, the API gravity of the main column bottoms is lower for the tight oil
feed, which can be
beneficial for identifying processing options for making further use of the
bottoms fraction. This
combination of higher olefin contents in fuel fractions, improved cetane for
the light cycle oil while
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 16 -
maintaining similar octane for the naphtha fractions, and/or a higher API
gravity bottoms is
unexpected.
[0055] More generally, the FCC effluent derived from a tight oil feed can
have one or more of
the following properties: 1) a light catalytic naphtha fraction (T5 ¨ T95
boiling range of 28 C to
165 C) having a research octane number of at least 90, or at least 93, a motor
octane number of at
least 80, or at least 81, and/or an olefin content of at least 35 wt%, or at
least 40 wt%; 2) a heavy
catalytic naphtha fraction (T5 ¨ T95 boiling range of 150 C to 255 C) having a
research octane
number of at least 90, or at least 92, a motor octane number of at least 78,
or at least 79, and/or an
olefin content of at least 6 wt%, or at least 8 wt%, or at least 9 wt%; 3) a
light cycle oil (T5 ¨ T95
boiling range of 225 C to 400 C) having a cetane index of at least 20.0; and
4) a main column
bottoms having an API gravity of at least -10 or at least -9.
Configuration Example ¨ FCC Processing of Tight Oil Atmospheric Resid
[0056] FIG. 1 shows an example of a configuration for processing a tight
oil fraction (possibly
corresponding to a whole tight oil crude) using fluid catalytic cracking and
without exposing the
tight oil fraction to a fractionation process having a pressure of less than
about 15 kPa. In the
configuration shown in FIG. 1, a tight oil fraction 105 is passed into an
atmospheric distillation
unit 120. The tight oil fraction 105 is separated in atmospheric distillation
unit 120 to form a light
ends (C4-) fraction 121, one or more naphtha boiling range fractions 122, one
or more distillate
boiling range fractions 126, and a bottoms fraction 125. The bottoms fraction
125 can have a T5
boiling point of at least about 650 F (343 C), or at least about 700 F (371
C). Optionally, if prior
separations have been performed on tight oil fraction 105, the tight oil
fraction may not include
lower boiling range material. In this situation, the light ends fraction 121
and/or the one or more
naphtha boiling range fractions 122 may not be formed. As an alternative to
using an atmospheric
distillation unit 120, a flash fractionator or another type of separator may
be used. In such aspects,
a single lower boiling fraction may be formed along with bottoms fraction 125,
as opposed to the
configuration in FIG. 1 where distinct distillate boiling range fraction(s)
126 and naphtha boiling
range fraction(s) 122 are formed.
[0057] After separation, the one or more naphtha boiling range fractions
122 and/or the one or
more distillate boiling range fractions 126 can optionally be hydrotreated.
The bottoms fraction
125 can be passed into a fluid catalytic cracking unit 140 for fluid catalytic
cracking of the bottoms
fraction 125. Optionally, a co-feed 135 having a metals content of about 1 ¨ 3
wppm of metals
can also be introduced into fluid catalytic cracking unit 140. Examples of a
suitable co-feed 135
can include, but are not limited to, distillate boiling range feeds, vacuum
gas oil boiling range
feeds, heavy coker gas oils, and other feeds having a sufficiently low metals
content. The effluent
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 17 -
from the fluid catalytic cracking unit 140 can correspond to a variety of
fractions. In the
configuration shown in FIG. 1, the various fractions can include a light ends
fraction 141, a light
catalytic naphtha 142, a heavy catalytic naphtha 144, a light cycle oil 146, a
heavy cycle oil 148,
and a bottoms or catalytic slurry oil fraction 149. In other configurations,
fewer types of fractions
may be formed from the fluid catalytic cracking effluent. For example, a heavy
cycle oil may not
be formed, so that the effluent includes a light cycle oil fraction and a
catalytic slurry oil fraction.
One or more of these fractions can emerge from the fluid catalytic cracking
unit 140 as a single
effluent that is subsequently separated (not shown) using a distillation
column or another suitable
separator. The light catalytic naphtha 142, the heavy catalytic naphtha 144,
the light cycle oil 146,
and/or the heavy cycle oil 148 can optionally be hydrotreated to reduce the
sulfur content of the
fraction(s).
Additional Embodiments
[0058] Embodiment 1. A method for processing a tight oil fraction:
separating a tight oil
fraction at a pressure of at least 5 psig (35 kPa) to form at least a higher
boiling fraction having a
T5 boiling point of at least about 650 F (343 C) and a second fraction having
a lower T5 boiling
point than the higher boiling fraction, the higher boiling fraction comprising
about 5 wppm to about
wppm of metals and at least about 20 wt% of 1050 F+ compounds, or at least
about 30 wt%, or
at least about 40 wt%; and exposing the higher boiling fraction to a cracking
catalyst under
effective fluid catalytic cracking conditions to form a cracked effluent
comprising at least about 5
wt% of 1050 F+ compounds, or at least about 10 wt%.
[0059] Embodiment 2. The method of Embodiment 1, further comprising
separating the
cracked effluent to form a first naphtha boiling range fraction, a first
diesel boiling range fraction,
and a catalytic slurry oil fraction, the catalytic slurry oil fraction
comprising the at least about 10
wt% of 1050 F+ compounds of the cracked effluent.
[0060] Embodiment 3. The method of Embodiment 2, wherein the second
fraction comprises
at least a second naphtha boiling range portion and a second diesel boiling
range portion, a
combined yield of the first naphtha boiling range portion, the second naphtha
boiling range portion,
the first diesel boiling range portion, and the second diesel boiling range
portion being at least
about 60 wt% of the tight oil fraction, or at least about 70 wt%, or at least
about 80 wt%.
[0061] Embodiment 4. The method of Emobdiment 2 or 3, further comprising
hydrotreating
at least a portion of the cracked naphtha boiling range fraction, at least a
portion of the diesel
boiling range fraction, or a combination thereof
[0062] Embodiment 5. The method of any of the above embodiments, wherein
the higher
boiling fraction comprises at least about 25 wt% of 1050 F+ compounds, or at
least about 30 wt%.
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 18 -
[0063] Embodiment 6. The method of any of the above embodiments, wherein
the higher
boiling fraction comprises about 5 wppm to about 10 wppm of Ni, Cr, and V, or
wherein the higher
boiling fraction comprises about 5 wppm to about 7 wppm of metals, or a
combination thereof
[0064] Embodiment 7. The method of any of the above embodiments, wherein
the higher
boiling fraction comprises about 3 wt% to about 10 wt% of Conradson Carbon
Residue, the cracked
effluent comprising about 1 wt% to about 5 wt% of Conradson Carbon Residue.
[0065] Embodiment 8. The method of any of the above embodiments, wherein
the higher
boiling fraction is exposed to the cracking catalyst without being exposed to
a pressure of less than
15 kPag.
[0066] Embodiment 9. The method of any of the above embodiments, wherein
exposing the
higher boiling fraction to a cracking catalyst comprises exposing the higher
boiling fraction and a
co-feed to the cracking catalyst, the co-feed having a T95 boiling point of
about 1050 F or less and
a metal content of about 1 ¨ 3 wppm.
[0067] Embodiment 10. The method of any of the above embodiments, wherein
the higher
boiling fraction comprises an atmospheric resid fraction.
[0068] Embodiment 11. The method of any of the above embodiments, wherein
the higher
boiling fraction comprises about 500 wppm to about 20,000 wppm sulfur, or
about 500 wppm to
about 5000 wppm sulfur.
[0069] Embodiment 12. The method of any of the above embodiments, wherein
the tight oil
fraction is derived from a crude oil extracted from a formation having a
production rate of less than
100 barrels per day in the absence of hydraulic fracturing, or less than 10
barrels per day in the
absence of hydraulic fracturing.
[0070] Embodiment 13. A fluid catalytic cracking effluent formed according
to the method of
any of the above embodiments.
[0071] Embodiment 14. A fluid catalytic cracking effluent comprising alight
catalytic naphtha
fraction, a heavy catalytic naphtha fraction, a light cycle oil, and a main
column bottoms, the light
catalytic naphtha fraction having a research octane number of at least 90 (or
at least 93) and an
olefin content of at least 35 wt% (or at least 40 wt%), the heavy catalytic
naphtha having a research
octane number of at least 90 (or at least 92) and optionally an olefin content
of at least 6 wt% (or
at least 8 wt% or at least 9 wt%), the light cycle oil having a cetane index
of at least 20.0, and the
main column bottoms optionally having an API gravity of at least -10, or at
least -9.
[0072] Embodiment 15. The fluid catalytic cracking effluent of claim 15,
wherein the light
catalytic naphtha fraction has a motor octane number of at least 80, or at
least 81, or wherein the
CA 03008603 2018-06-14
WO 2017/105871 PCT/US2016/064525
- 19 -
heavy catalytic naphtha fraction has a motor octane number of at least 78, or
at least 79, or a
combination thereof
[0073] When numerical lower limits and numerical upper limits are listed
herein, ranges from
any lower limit to any upper limit are contemplated. While the illustrative
embodiments of the
invention have been described with particularity, it will be understood that
various other
modifications will be apparent to and can be readily made by those skilled in
the art without
departing from the spirit and scope of the invention. Accordingly, it is not
intended that the scope
of the claims appended hereto be limited to the examples and descriptions set
forth herein but rather
that the claims be construed as encompassing all the features of patentable
novelty which reside in
the present invention, including all features which would be treated as
equivalents thereof by those
skilled in the art to which the invention pertains.
[0074] The present invention has been described above with reference to
numerous
embodiments and specific examples. Many variations will suggest themselves to
those skilled in
this art in light of the above detailed description. All such obvious
variations are within the full
intended scope of the appended claims.