Note: Descriptions are shown in the official language in which they were submitted.
1 CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
TITLE OF THE INVENTION
METHOD FOR PRODUCING (METH)ACRYLIC ACID
Technical Field
[0001] The
present invention relates to a method for
producing (meth)acrylic acid, the method including extraction
of (meth)acrylic acid by an organic solvent. More
particularly, the present invention relates to a method for
stably producing high-quality (meth)acrylic acid, by
performing an energetically advantageous operation resulting
from a lowered heat load in a distillation step of a
production process of (meth)acrylic acid, and by preventing
the occurrence of an oil-water suspension state in an
extraction step and maintaining good separation of water in
the extraction step.
Background Art
[0002] Herein
(meth)acrylic acid [acrylic acid and/or
methacrylic acid] is obtained as an oxidation reaction gas
through vapor-phase catalytic oxidation of propylene or
propane, which is a hydrocarbon having three carbon atoms (C3),
or isobutylene, butenes or tertiary butanol, which is a
hydrocarbon having four carbon atoms (C4), together with air
as an oxygen source and steam or nitrogen, using two types of
solid catalyst. The obtained oxidation reaction gas is cooled,
1
1
* CA 03009033 2018-06-18
1 *
16A00512W00 (0P-16414-PCT)
and thereafter is brought into gas-liquid contact with
absorbing water that contains a polymerization inhibitor, in
an absorption column, as a result of which the oxidation
reaction gas is separated in the form of a (meth)acrylic acid
aqueous solution.
The gas not having been absorbed is
introduced into a waste gas treatment step, to be rendered
harmless and then disposed of, although part of the gas is
recycled to an oxidation reaction step.
[0003]
The (meth)acrylic acid aqueous solution obtained
from the absorption column is ordinarily purified through
extraction or azeotropic distillation, to
produce
(meth)acrylic acid thereby (Non-Patent document 1).
In the
extraction method, the (meth)acrylic acid aqueous solution is
subjected to an extraction treatment in an extraction column,
using an extraction solvent selected from among ketones,
alcohols, ethers, esters and hydrocarbons, to yield an extract
solution which is a mixed solution of (meth)acrylic acid and
the extraction solvent.
The extract solution is further
introduced into a (meth)acrylic acid purification system, and
is subjected to a purification treatment by distillation,
crystallization or the like, to yield (meth)acrylic acid as
the product.
[0004]
In the production process of (meth)acrylic acid,
contamination in the form of polymerization products and the
like accumulates in equipment and piping, and accordingly the
2
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
equipment must be opened and cleaned periodically.
Retained
solution within the various equipment items is once discharged
and held in a reservoir such as a tank.
Discharge solutions
are generated also during partial or complete process shutdown,
upon occurrence of operational problems. These
discharge
solutions include large amounts of valuable products such as
acrylic acid, the discarding of which is undesirable in terms
of production intensity. Accordingly, it is necessary to feed
the discharge solutions once more into the process, after
restart of process operations once cleaning is complete. In
some instances the discharge solutions may contain large
amounts of water. Herein
it would be ideal to feed the
discharge solutions to an extraction step in which water is
removed from acrylic acid. At the same time, however, these
discharge solutions include also components that adversely
affect oil-water separation, for causes that are not exactly
clear.
Accordingly, a concern has arisen in that in cases
where these discharge solutions are fed to the extraction step,
separation may be defective due to the formation of an oil-
water suspension state, and also operational problems may
occur, depending on the content of components that impair oil-
water separation and depending on operation conditions. In
conventional art, therefore, these discharge solutions have
been fed to a distillation process (see Patent document 1, for
instance).
3
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
Citation List
Patent Literature
[0005]
Patent document 1: Japanese Patent
Application
Publication No. 2003-292470
Patent document 2: Japanese Patent
Application
Publication No. 2013-151455
Non-Patent Literature
[0006]
Non-Patent document 1: Eizo OMORI, "Acrylic Acid and its
Polymers [I]", 3rd edition, Shokodo Co., Ltd., April 28, 1978,
pp. 10 to 13 (1.4 Purification Methods of Acrylic Acid)
Summary of Invention
Technical Problem
[0007] As
described above, components of large latent heat
of vaporization, such as water, are also present in the
discharge solutions that are discharged from the (meth)acrylic
acid production process. When
implementing the operations
involved in the above methods, it has been necessary to adopt
measures such as increasing the number of plates of
distillation columns and increasing the heat load during
operations, all of which is energetically disadvantageous. A
4
=
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
further concern is the increased frequency of equipment
opening and cleaning derived from contamination of reboilers
and draw-out piping of the distillation columns, arising from
the impact of components such as oligomers and polymers of
(meth)acrylic acid, maleic acid derivatives, phenolic resins
and the like, contained in the discharge solutions.
It is an object of the present invention to provide a
method for stably producing high-quality (meth)acrylic acid,
by performing an energetically advantageous operation
resulting from a lowered heat load in a distillation step of a
production process of (meth)acrylic acid, and by preventing
the occurrence of an oil-water suspension state in an
extraction step and maintaining good separation of water in
the extraction step.
Solution to Problem
[0008]
As a result of diligent research aimed at solving
the above problem, the inventors found that by feeding to the
extraction step the discharge solutions having been discharged
from the (meth)acrylic acid production process, the heat load
in the distillation step can be eased and an energetically
favorable operation can be carried out, while contamination
derived from the discharge solutions can be reduced.
As
described above, components that adversely affect oil-water
separation may in some instances be present in the discharge
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
solutions that are discharged from the (meth)acrylic acid
production process.
By being fed to the extraction step,
these components may impair oil-water separation in the
extraction step. If oil-water separation becomes poor in the
extraction step, water flows downstream contained in the
extraction solvent, which may translate into greater
downstream purification load and poorer product quality. Oil-
water separation may conceivably be carried out reliably by
keeping a liquid load no greater than a given level, in order
to prevent the above occurrence.
However, this requires
imposing limitations to processing capacity during the
operation, which may result in decreased production capacity.
[0009]
Further research by the inventors aimed at solving
the above problem revealed that impairment of oil-water
separation in the extraction step can be prevented, and high-
quality (meth)acrylic acid can be produced stably, by
performing the operations set forth below.
(a) A solution containing a discharge solution that is
discharged from a (meth)acrylic acid production process is
held as a collected solution, and the held collected solution
is fed to the above extraction step.
(b) The collected solution is fed to the same site as a
site to which the (meth)acrylic acid aqueous solution is
supplied in the extraction step.
6
=
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
(c) The concentration of Michael adducts in a mixed
composition of the (meth)acrylic acid aqueous solution and the
collected solution is set to lie in the range of 0.05 to 0.7
wt%.
(d) The collected solution is distilled, and the
resulting distillate is thereafter fed to the extraction step.
(e) The collected solution is subjected to oil-water
separation, after which the oil phase is fed to the extraction
step.
(f) The extraction temperature in the extraction step is
adjusted to 30 C to 90 C.
[0010]
The above problems are not found in conventional
art.
That is, the invention has discovered and solved
problems that were wholly unknown conventionally. A
person
skilled in the art unaware of the above problems would not
ordinarily incur a cost liability, for instance, in terms of
adding, to a production process, a new step such as holding,
distillation, oil-water separation and the like of the
collected solution, or adding equipment associated with these
steps. It
is moreover a basic feature of continuous
production to set constant-quantity charge amounts and charge
ratios in an extraction step; by contrast, feeding to the
extraction step a solution obtained as a result of operations
such as holding, distillation, oil-water separation and so
forth of the collected solution, may give rise to fluctuations
7
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
in the above charge amounts and charge ratios, and thus such
feeding is ordinarily not performed. In the
production of
(meth)acrylic acid, specifically, it is not usual to have a
discharge solution go through extra operations and thereafter
feed the discharge solution to an extraction step.
[0011] The present invention is as follows.
<1> A method for producing (meth)acrylic acid, the method
comprising:
an oxidation reaction step of obtaining a reaction gas
containing (meth)acrylic acid, through vapor-phase catalytic
oxidation; an absorption step of bringing the reaction gas
into gas-liquid contact with water, to thereby yield a
(meth)acrylic acid aqueous solution; an extraction step of
bringing the (meth)acrylic acid aqueous solution and an
extraction solvent into contact with each other, to thereby
extract crude (meth)acrylic acid; and a distillation step of
distilling (meth)acrylic acid from the crude (meth)acrylic
acid,
wherein a solution containing a discharge solution that
is discharged from at least one of the steps is used as a
collected solution, the collected solution is held for 1 day
to 60 days, and the held collected solution is fed to the
extraction step.
<2> A method for producing (meth)acrylic acid, the method
comprising:
8
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
an oxidation reaction step of obtaining a reaction gas
containing (meth)acrylic acid, through vapor-phase catalytic
oxidation; an absorption step of bringing the reaction gas
into gas-liquid contact with water, to thereby yield a
(meth)acrylic acid aqueous solution; an extraction step of
bringing the (meth)acrylic acid aqueous solution and an
extraction solvent into contact with each other, to thereby
extract crude (meth)acrylic acid; and a distillation step of
distilling (meth)acrylic acid from the crude (meth)acrylic
acid,
wherein a solution containing a discharge solution that
is discharged from at least one of the steps is used as a
collected solution, the collected solution is subjected to
oil-water separation, and an oil phase resulting from the oil-
water separation is fed to the extraction step.
<3> A method for producing (meth)acrylic acid, the method
comprising:
an oxidation reaction step of obtaining a reaction gas
containing (meth)acrylic acid, through vapor-phase catalytic
oxidation; an absorption step of bringing the reaction gas
into gas-liquid contact with water, to thereby yield a
(meth)acrylic acid aqueous solution; an extraction step of
bringing the (meth)acrylic acid aqueous solution and an
extraction solvent into contact with each other, to thereby
extract crude (meth)acrylic acid; and a distillation step of
9
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
distilling (meth)acrylic acid from the crude (meth)acrylic
acid,
wherein a solution containing a discharge solution that
is discharged from at least one of the steps is used as a
collected solution, the collected solution is distilled, and
the resulting distillate is fed to the extraction step.
<4> The method for producing (meth)acrylic acid according to
any one of <1> to <3>, wherein in at least one of the steps,
supply of a reaction product in the step and discharge of the
discharge solution in the step are carried out simultaneously.
<5> The method for producing (meth)acrylic acid according to
any one of <1> to <4>, wherein the collected solution contains
water.
<6> The method for producing (meth)acrylic acid according to
any one of <1> to <5>, wherein the collected solution that is
fed to the extraction step is supplied to the same site as a
site to which the (meth)acrylic acid aqueous solution is
supplied in the extraction step.
<7> The method for producing (meth)acrylic acid according to
any one of <1> to <6>, wherein the concentration of Michael
adducts in a mixed composition of the collected solution and
the (meth)acrylic acid aqueous solution is 0.05 to 0.7 wt%.
<8> The method for producing (meth)acrylic acid according to
any one of <1> to <7>, wherein the extraction temperature in
the extraction step is 30 C to 90 C.
=
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
<9> The method for producing (meth)acrylic acid according to
any one of <1> to <8>, wherein the extraction solvent is a
solvent having, as a main component, a water-insoluble
aromatic compound having a lower boiling point than the
boiling point of (meth)acrylic acid.
<10> The method for producing (meth)acrylic acid according to
<9>, wherein the water-insoluble aromatic compound is at least
one compound selected from the group consisting of benzene,
toluene and xylene.
Advantageous Effects of Invention
[0012]
The present invention allows reducing heat load in
a distillation step of a production process of (meth)acrylic
acid, performing an energetically favorable operation, and
reducing contamination derived from discharge solutions.
Further, the invention allows producing stably high-quality
(meth)acrylic acid, while maintaining the performance of
removal of water and other impurities at a high level, by
preventing impairment of oil-water separation in an extraction
step.
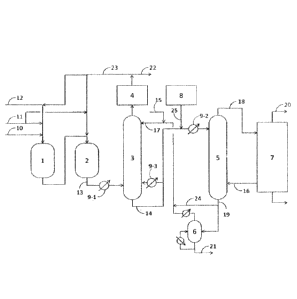
Brief Description of Drawing
[0013]
11
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
Fig. 1 is a schematic diagram illustrating an example of
a production facility of (meth)acrylic acid of the present
invention.
Description of Embodiments
[0014] Embodiment of the method for producing
(meth)acrylic acid of the present invention will be explained
next in detail.
The method for producing (meth)acrylic acid of the
present invention has: an oxidation reaction step of obtaining
a reaction gas containing (meth)acrylic acid, through vapor-
phase catalytic oxidation; an absorption step of bringing the
reaction gas into gas-liquid contact with water, to thereby
yield a (meth)acrylic acid aqueous solution; an extraction
step of bringing the (meth)acrylic acid aqueous solution and
an extraction solvent into contact with each other, to thereby
extract crude (meth)acrylic acid; and a distillation step of
distilling (meth)acrylic acid from the crude (meth)acrylic
acid, wherein operations are carried out in such a manner that,
necessarily, a solution containing a discharge solution having
been discharged from at least one of the above steps is held
as a collected solution, and the held collected solution is
fed to the extraction step (condition (a) below), and
preferably, in such a manner that the conditions below ((b) to
(f)) are satisfied.
12
CA 03009033 2018-06-18
=
=
16A00512W00 (0P-16414-PCT)
(a) A solution containing a discharge solution that is
discharged from a (meth)acrylic acid production process is
held as a collected solution, and the held collected solution
is fed to the above extraction step.
(b) The collected solution is fed to the same site as a
site to which the (meth)acrylic acid aqueous solution is
supplied in the extraction step.
(c) The concentration of Michael adducts in a mixed
composition of the (meth)acrylic acid aqueous solution and the
collected solution is set to lie in the range of 0.05 to 0.7
wt%.
(d) The collected solution is distilled, and the
resulting distillate is thereafter fed to the extraction step.
(e) The collected solution is subjected to oil-water
separation, after which the oil phase is fed to the extraction
step.
(f) The extraction temperature in the extraction step is
adjusted to 30 C to 90 C.
[0015] As set forth in (a), it is a characterizing feature
of the present invention to use, as a collected solution, a
solution containing discharge solutions having been discharged
from the (meth)acrylic acid production process, and holding
the collected solution, for instance, in a storage tank. The
collected solution may be collected during the production or
upon discontinuation of the production of (meth)acrylic acid.
13
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
Supply of the reaction product and discharge of the discharge
solutions in each step may be carried out simultaneously.
Through holding of the collected solution, the discharge
solutions having various compositions are mixed, whereby
components are homogenized, and the concentration of
components that impair oil-water separation is diluted.
Moreover, an effect of facilitating removal of impairing
components can be achieved through settling of solid matter
and through oil-water separation. In the
present invention,
the term discharge solutions that are discharged from the
(meth)acrylic acid production process may denote discharge
solutions that are discharged from the process during non-
steady operation, for instance, periodic repairs/cleaning or
plant problems, and denotes specifically solutions discharged,
for instance, from an absorption column, an extraction column,
an evaporator, or a distillation column. The term holding in
the present invention denotes the operation of keeping the
solutions to a storage tank such as a buffer tank, an off-spec
tank or a rundown tank, with the holding time being set to 1
day to 60 days, preferably 2 days to 40 days and more
preferably 2 days to 10 days. In the
present specification,
the term 1 day refers to 24 hours. If
this period is too
short, the effect of diluting the concentration of components
that impair oil-water separation, and the effects of solid
matter settling and oil-water separation fail to be achieved
14
* CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
sufficiently, whereas if the above period is too long, oil-
water separability is conversely impaired due to the
generation of new impurities such as Michael adducts and
polymerization products.
If the solutions discharged from the (meth)acrylic acid
production process are supplied quickly to the extraction step
without having undergone the holding operation, oil-water
separation may be impaired, and water may flow downstream
contained in the extraction solvent, which may translate into
greater downstream purification load and poorer product
quality.
[0016] As described above, components that impair oil-
water separation are contained in the discharge solutions that
are discharged from the (meth)acrylic acid production process,
with the content of substances that impair oil-water
separation fluctuating depending on the timing at which the
discharge solutions are discharged. Maintaining the
concentrations of these components within appropriate ranges,
while monitoring the concentrations, would conceivably be the
most efficient operation method herein, but such monitoring
has proved difficult, since there are multiple components that
can imaginably impair oil-water separation but have not been
identified clearly.
[0017] As a result of further research to address this
problem, it was found in the present invention that Michael
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
adducts can be used as an indicator substance of oil-water
separation, and that impairment of oil-water separation can be
prevented by managing the concentration of Michael adducts to
lie within a given range. As set forth in (c), a method for
managing oil-water separability involves preferably managing
oil-water separability in such a manner that the concentration
of Michael adducts in a mixed composition of the (meth)acrylic
acid aqueous solution and the collected solution is 0.05 to
0.7 wt%.
The concentration of Michael adducts is more
preferably 0.05 to 0.6 wt%, and yet more preferably 0.1 to 0.5
wt%. If the concentration of Michael adducts is too low, the
conditions in the oxidation reaction step and the absorption
step become constrained, whereas if the concentration is too
high, oil-water separation in the extraction step is impaired.
As used herein, the term mixed composition denotes the
composition of a mixed solution in a case where the collected
solution is mixed with the (meth)acrylic acid aqueous solution,
within piping, while in a case where the collected solution is
supplied to the extraction step through piping separate from
that of the (meth)acrylic acid aqueous solution, the term
mixed composition denotes an assumed composition of the
resulting mixture, calculated on the basis of respective
compositions and flow ratios.
The concentration of Michael
adducts can be measured in accordance with known methods. For
16
CA 03009033 2018-06-18
=
16A00512W00 (0P-16414-PCT)
instance, the concentration of Michael adducts can be measured
by gas chromatography.
[0018] A
conceivable method for maintaining the above
concentration ratio involves adjusting the flow rate of the
collected solution, or adjusting the concentration of Michael
adducts by separately supplying high-purity acrylic acid.
However, the treatment throughput is constrained in such a
case.
Accordingly, it is preferable to perform the
distillation operation before feeding of the collected
solution to the extraction step, as described in (d). By
performing the distillation operation and feeding then the
distillate to the extraction step it becomes possible to
prevent impairment of oil-water separation in the extraction
step. In
a case where the distillation operation is
accomplished using a distillation column, the term distillate
denotes herein a condensate of components obtained from the
top of a distillation column. There are conceivably multiple
components that impair oil-water separation in the extraction
step.
Although not clearly identified, these components are
deemed to include Michael adducts of (meth)acrylic acid,
polymerization products of (meth)acrylic acid, as well as
other high-boiling point compounds and solid matter.
Accordingly, it is estimated that removal of these components
by distillation should contribute to preventing impairment of
oil-water separation.
17
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
[0019] As
set forth in (e), impairment of oil-water
separation in the extraction step can be prevented also by
performing an oil-water separation operation on the collected
solution. Although the underlying reasons are unclear, it is
deemed that impairment of oil-water separation in the
extraction step can be prevented by feeding to the extraction
step an oil phase after oil-water separation, since components
having solubility in water are present among the components
that impair oil-water separation.
[0020] The
collected solution is preferably fed to the
extraction step during production of (meth)acrylic acid, and
,more preferably is fed to the extraction step during
continuous production, after having undergone at least one
step selected from the group consisting of holding,
distillation and oil-water separation. As
set forth in (b),
the site to which the collected solution is fed is preferably
identical to the site of supply of the (meth)acrylic acid
aqueous solution in the extraction step. Conceivable methods
to that end include mixing the collected solution into a pipe
through which the (meth)acrylic acid aqueous solution flows,
or a method of supplying the collected solution, through
separate piping, to the site to which the (meth)acrylic acid
aqueous solution is supplied. In a
case where an extraction
column is used as the extraction step and the (meth)acrylic
acid aqueous solution is supplied to the top of the column, a
18
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
conceivable example of the latter method involves supplying a
collected solution to the top of the column through separate
piping. The
extraction efficiency of (meth)acrylic acid is
lowered in a case where this method is not implemented and the
collected solution is instead supplied, for instance, to the
same site as that of the extraction solvent.
[0021] The
temperature during extraction is ordinarily a
low temperature, of about 20 C to 30 C, since the mutual
solubility of oil and water increases with rising temperature
at the time of oil-water separation (see Patent document 2,
for instance).
However, the extraction temperature is
preferably not excessively low, from the viewpoint of
preventing an oil-water suspension state, since the oil-water
separation rate is low at low temperatures. An
optimal
temperature in terms of the best balance between oil-water
mutual solubility and oil-water separation rate, and in terms
of suppressing, for instance, new generation of components
that impair oil-water separability, lies preferably in the
range of 30 C to 90 C, more preferably 40 C to 80 C and yet more
preferably 40 C to 60 C, as set forth in (f). As a method for
adjusting the temperature, it is most efficient herein to
adjust the temperature of the collected solution or the
(meth)acrylic acid aqueous solution supplied to the extraction
step, or the temperature of a mixed solution of the foregoing,
so as to lie in an appropriate range.
19
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
[0022] The
present invention will be explained next by
illustrating the production of acrylic acid using propylene as
a starting material, as a typical example of the production of
(meth)acrylic acid, with reference to Fig. 1 that depicts
schematically an example of a production facility of
(meth)acrylic acid.
However, the present invention is not
limited to the production of acrylic acid using propylene as a
starting material, and can be utilized in the production of
acrylic acid or methacrylic acid in general, using starting
materials in the form of hydrocarbons having three carbon
atoms or hydrocarbons having four carbon atoms.
[0023] 1) Oxidation reaction step
Air (11), steam and/or nitrogen as a diluent (12), and
further propylene (10) as a reaction starting material, are
mixed and supplied to an oxidation reactor (first-stage
reactor) (1). The
first-stage reactor (1) is packed with a
solid catalyst made up of a molybdenum (Mo)-bismuth (Bi)-based
composite metal oxide, the temperature of the reactor being
controlled through circulation of a heat medium. The
structure of the first-stage reactor (1) is ordinarily that of
a multi-tubular heat exchanger type or of a plate heat
exchanger type. The
reaction product gas resulting from
conversion of propylene to acrolein in the first-stage reactor
(1) is next supplied to an oxidation reactor (second-stage
reactor) (2). The
air (11) and so forth may be added to the
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
second-stage reactor (2). The second-stage reactor (2) is
packed with a molybdenum (Mo)-vanadium (V)-based composite
metal oxide catalyst, the temperature of the reactor being
controlled through circulation of a heat medium. A structure
similar to that of the first-stage reactor (1) is adopted as
the structure of the second-stage reactor (2). Acrolein is
converted to acrylic acid in the second-stage reactor (2), and
there is obtained an oxidation reaction gas (13).
[0024] 2) Absorption step
The oxidation reaction gas (13) is cooled to 150 C to
200 C using a heat exchanger (9-1), and thereafter is
introduced to an absorption column (3), to yield an acrylic
acid aqueous solution, with the temperature being controlled
by a heat exchanger (9-3).
Specifically, the oxidation
reaction gas having been cooled in the heat exchanger (9-1) is
introduced into the absorption column (3), and the oxidation
reaction gas and absorbing water (17) containing a
polymerization inhibitor (15), and supplied from the column
top of the absorption column (3), are brought into gas-liquid
contact, as a result of which acrylic acid and so forth in the
oxidation reaction gas is absorbed to yield an acrylic acid
aqueous solution (14). A packed column or plate column having
to 20 theoretical plates is ordinarily used as the
absorption column (3).
The temperature of the top of the
absorption column (3) is ordinarily 30 C to 70 C, while the
21
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
temperature of the bottom is ordinarily 35 C to 85 C, the
foregoing temperatures being controlled using the heat
exchanger (9-3).
[0025]
Waste gas from the top of the absorption column (3)
contains nitrogen as a main component, water, as well as
unreacted oxygen and propylene. The waste gas contains carbon
dioxide (CO2), acetic acid, formaldehyde and the like, as by-
products of the oxidation reaction, and also small amounts of
acrylic acid that failed to be absorbed.
Part of the waste
gas may be recycled as-is to the oxidation reaction step.
Ordinarily, however, the waste gas is rendered harmless in a
waste gas treatment step (4), after which the gas is recycled
(23) to the oxidation reactor (1) or (2), and the rest is
discarded as waste gas (22).
[0026] The
concentration of acrylic acid in the acrylic
acid aqueous solution (14) of the column bottoms is about 30
to 70 wt%. The
acrylic acid aqueous solution (14) contains,
for instance, by-products such as formaldehyde, acetic acid,
maleic acid and phthalic acid, as well as, for instance, the
polymerization inhibitor that is added to the top of the
absorption column (3).
[0027]
Various substances have been proposed for use as
the polymerization inhibitor in the production process of
acrylic acid. Examples thereof include phenol compounds such
as hydroquinone and hydroquinone monomethyl ether, and also
22
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
phenothiazine compounds, copper salt compounds, manganese salt
compounds, amine compounds, nitroso compounds, and N-oxyl
compounds.
[0028]
Examples of the foregoing compounds include
phenothiazine and bis-(a-methylbenzyl)phenothiazine as the
phenothiazine compound.
The copper salt compound, which is not particularly
limited, may be an inorganic salt, or an organic salt, and
numerous compounds can be used.
Examples thereof include
copper dialkyldithiocarbamate, copper acetate, copper acrylate,
copper naphthenate, copper sulfate, copper paratoluenate,
copper nitrate and copper carbonate. However, the solution in
the absorption column (3) is an aqueous solution, and hence
the copper salt compound is preferably a water-soluble
compound, for instance, copper acetate, copper acrylate,
copper carbonate, copper sulfate or copper paratoluenate.
Examples of suitable manganese salt compounds include
manganese acetate, manganese formate, manganese acrylate,
manganese naphthenate, manganese sulfate and manganese
carbonate.
Examples of nitroso compounds and amine compounds include
p-nitrosophenol, N-nitrosophenylhydroxylamine and ammonium
salts thereof, and N-nitrosodiphenylamine and ammonium salts
thereof.
23
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
Examples of N-oxyl compounds include tertiary butyl
nitrooxide, 2,2,6,6-tetramethylpiperidine-1-oxyl,
2,2,6,6-
tetramethy1-4-hydroxypiperidine-l-oxyl, and
4,4',4"-
tris(2,2,6,6-tetramethylpiperidinooxyl)phosphite.
[0029] 3) Waste gas treatment step
The waste gas discharged from the top of the absorption
column (3) ordinarily contains organic substances such as
acetic acid, formaldehyde and acrylic acid, together with
water, oxygen (02) and carbon dioxide (002). The treatment for
rendering the waste gas harmless is ordinarily carried out in
accordance with a catalytic combustion scheme.
Examples of
the catalyst include honeycomb-like catalysts.
[0030] 4) Extraction step
The acrylic acid aqueous solution (14) from the bottom of
the absorption column (3) undergoes heat exchange in a heat
exchanger (9-2), the temperature of the solution is adjusted
to about 20 C to 90 C, the solution is supplied to an
extraction column (5), and undergoes a liquid-liquid contact
treatment with an extraction solvent (16), to be separated
into an extract solution (18) of acrylic acid and into
raffinate water (19).
[0031] If the
extraction temperature in the extraction
column (5) is excessively high, the mutual solubility between
water and the extraction solvent increases, whereas if the
extraction temperature is too low, oil-water separation takes
24
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
some time. Accordingly, the extraction temperature is
preferably set to about 30 C to 90 C.
[0032] The
water-insoluble solvents described below are
preferably used as the extraction solvent (16). The
water
concentration in the extract solution (18) after extraction
decreases as a result of extraction using the water-insoluble
solvent, and accordingly it becomes possible to prevent, for
instance, clogging due to polymerization of acrylic acid in a
subsequent acrylic acid purification step (7); at the same
time, it becomes possible to reduce the heat load in the
purification step (7). By
using a water-insoluble solvent,
moreover, there can be reduced the concentration of impurities
such as acetic acid and maleic acid in the extract solution
(18) after extraction. It is
likewise preferable to use a
water-insoluble solvent, in order to treat discharge solutions
from the various equipment items, containing such impurities,
in the extraction step.
[0033] For
instance, a water-insoluble aromatic compound
solvent can be used as the water-insoluble solvent that is
utilized for extracting acrylic acid. A
compound the main
component of which has a lower boiling point than the boiling
point of acrylic acid is preferably used as the solvent, in
terms of separation efficiency of the solvent during acrylic
acid purification. The
above main component is herein a
water-insoluble aromatic compound having a boiling point lower
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
than the boiling point of acrylic acid and being 50 wt% or
more, preferably 60 wt% or more, and more preferably 80 wt% or
more of the extraction solvent.
Typical types of the water-insoluble aromatic compound
solvent include water-insoluble aromatic hydrocarbon solvents.
Water-insoluble aromatic hydrocarbon solvents are advantageous
in the extraction operation in that the ratio of the
distribution coefficients in a ratio of extraction agent /
water for acrylic acid and acetic acid is high, and acrylic
acid selectivity is thus high. A solvent having a solubility
in water at 20 C of 1.5 wt% or less is selected as the above
water-insoluble aromatic hydrocarbon solvent, but the
solubility in water is more preferably 0.5 wt% or less, yet
more preferably 0.1 wt% or less, and most preferably 0.06 wt%
or less.
Examples of water-insoluble aromatic hydrocarbon
solvents include benzene, toluene, xylene, ethyl benzene and
mesitylene, preferred among the foregoing are benzene, toluene
and xylene, since these boast high extraction efficiency and
low solubility in water. The
water-insoluble aromatic
hydrocarbon solvent may be used singly or in the form of a
mixed solvent of two or more types.
Although the water-insoluble aromatic hydrocarbon solvent
can be used in the form of a mixed solvent with other water-
insoluble solvents, in this case the water-insoluble aromatic
hydrocarbon solvent is preferably 70 wt% or more of the mixed
26
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
solvent.
Toluene is most preferred herein as the water-
insoluble aromatic hydrocarbon solvent, by virtue of having
high extraction efficiency and low solubility in water.
[0034] The
weight ratio (S/F ratio) of the extraction
solvent (16) with respect to the acrylic acid aqueous solution
(14) is ordinarily set to lie in the range of 1 to 5. When
the S/F ratio is lower than 1, the concentration of acrylic
acid in the extract solution (18) increases, but the
extraction rate of acrylic acid decreases while the water
concentration in the extract solution (18) increases, which is
undesirable. When
the S/F ratio excesses 5, the extraction
rate increases but the concentration of acrylic acid in the
extract solution (18) decreases, and thereby large amounts of
separation equipment and energy are required in the subsequent
purification step (7), which is undesirable.
Preferably, the
S/F ratio is set to lie in the range of 1 to 3.5, in order to
reduce the water concentration in the extract solution (18) as
much as possible.
[0035] The
theoretical plate number of the extraction
column (5) is ordinarily 4 or greater, preferably 6 or greater
and most preferably 7 or greater. The
extraction rate of
acrylic acid increases with a rising theoretical plate number.
The upper limit of the theoretical plate number of the
extraction column (5) is not particularly restricted, but is
ordinarily of 20 or smaller. The
extraction rate of acrylic
27
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
acid is ordinarily 95% or higher, preferably 98% or higher and
most preferably 99% or higher.
[0036] As the
extraction column (5) there is used a plate
extraction column, a rotating disc extraction column (RDC
column) or a reciprocating plate extraction column (for
instance, a Karr column). A
reciprocating plate extraction
column is preferred herein, for instance, in terms of
theoretical plate number and treatment throughput.
[0037] The
concentration of acrylic acid in the extract
solution (18) obtained in the extraction column (5) is
ordinarily 10 to 40 wt%, with small amounts of acetic acid and
water being provided in the extract solution (18). The
raffinate water (19) contains oxidation reaction by-products
such as acetic acid, formaldehyde and maleic acid, and also a
polymerization inhibitor and the like. The composition of the
extract solution (18) and the raffinate water (19) is
determined by, for instance, the liquid-liquid equilibrium
composition, the S/F ratio, and the theoretical plate number
of the extraction column (5).
[0038] 5) Acrylic Acid purification step
The present step includes a distillation step of
separating acrylic acid from crude acrylic acid by
distillation.
Impurities such as the extraction solvent and
acetic acid in the extract solution (18) obtained from the
column top of the extraction column (5) are separated by
28
=
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
purification means, such as the above distillation separation,
in the acrylic acid purification step (7). Acrylic acid (20)
is thus produced. The
extraction solvent (16) separated by
distillation is recycled to the extraction column (5). The
concentration of acrylic acid in the extraction solvent (16)
affects significantly the acrylic acid extraction rate in the
extraction column (5). The
concentration of acrylic acid in
the extraction solvent (16) is preferably 1 wt% or less, more
preferably 0.5 wt% or less, since the lower the concentration
the higher the extraction rate is. In
order to bring the
concentration of acrylic acid in the extraction solvent (16)
to 0.4 wt% or less it is necessary to increase the number of
plates in the distillation column, and to increase reflux, for
the purpose of separating the solvent and acrylic acid by
distillation. This
entails a significant energy demand, and
accordingly the foregoing conditions are controlled to
appropriate conditions based on a relationship between the
extraction rate of acrylic acid and the distillation load.
[0039] 6) Raffinate water treatment step
The raffinate water (19) from the extraction column (5)
is discharged as a waste solution that must be treated.
Combustion treatments and activated sludge treatments are
herein ordinary instances of waste solution treatment methods,
but a combustion treatment of large amounts of raffinate water
requires significant energy input, while an activated sludge
29
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
treatment is difficult, since the raffinate water (19)
contains formaldehyde. Accordingly, the raffinate water (19)
is preferably recycled to the absorption column (3) and is re-
used as absorbing water.
By-products (for instance, maleic
acid and phthalic acid) from the oxidation reaction step that
remain without having been extracted by the extraction solvent
in the extraction column (5) become concentrated in the
raffinate water (19).
Accordingly, it is necessary to
separate the by-products from the raffinate water (19) and to
discharge the separated by-products out of the system in the
form of a waste solution, in order to prevent accumulation of
such by-products in the system, and for the purpose of
recycling to the absorption column (3).
To treat the
raffinate water (19), part thereof is recycled (24) as-is, in
the form of absorbing water, to the absorption column (3), and
just the rest alone is subjected to a heating concentrating
treatment, to separate thereby the waste solution.
As a
result, it becomes possible to reduce the size of the
equipment for the heating concentrating treatment, while
optimizing energy.
[0040]
The equipment used for heat-concentrating the
raffinate water (19) is generally an evaporator (6) such as
the one illustrated in Fig. 1.
The evaporator (6) has an
evaporation tank, a reboiler for heating distillation and a
condenser for condensing evaporated vapor. A mist separator
=
. CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
for preventing entrainment may be provided in the tank, or
distillation parts such as trays may be provided at the top
section of the tank.
The heating concentrating equipment is
not limited to an evaporator, and, for instance, there can be
used a multiple effect evaporator, a stirring tank equipped
with a heating jacket or with a heat exchanger, a membrane
separation apparatus, a stripping column, a thin-film
evaporator or a centrifugal thin-film evaporator (for instance,
Kontro).
[0041]
7) Step of collecting discharge solutions from
various equipment items
The matter described above applies, for instance, to
preferred conditions of holding, distillation and oil-water
separation of the collected solution, and to feeding of the
collected solution, set out in the present section.
In anticipation of non-steady operation, for instance,
due to problems or during periodic inspection, the production
method of the present invention involves holding the discharge
solutions from the various equipment items once in a storage
tank (8) such as a buffer tank or an off-spec tank during non-
steady operation.
The discharge solutions having various
compositions are mixed through such holding, whereby
components are homogenized, and the concentration of
components that impair oil-water separation is diluted.
Through settling of solid matter and through oil-water
31
=
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
separation it becomes possible to achieve moreover the effect
of facilitating removal of impairing components. These
discharge solutions are preferably supplied, in the form of a
collected solution (25), to the same site as a site to which
the acrylic acid aqueous solution (14) is supplied in the
extraction step.
Conceivable methods to that end include
mixing the collected solution into a pipe through which the
acrylic acid aqueous solution (14) flows, or a method of
supplying collected solution through separate piping, to the
site to which the acrylic acid aqueous solution is supplied.
In a case where the extraction column (5) is used as the
extraction step and the acrylic acid aqueous solution is
supplied to the top of the column, a conceivable example of
the latter method involves supplying the collected solution to
the top of the extraction column (5) through separate piping.
The extraction efficiency of acrylic acid may drop in a case
where this method is not implemented and the collected
solution is instead supplied, for instance, to the same site
as that of the extraction solvent (16).
The collected solution may be fed as-is to the extraction
step, but is preferably distilled using distillation equipment,
with the resulting distillate after distillation being then
fed to the extraction step, in order to prevent the occurrence
of an oil-water suspension state in the extraction step. An
evaporator can be used as the distillation equipment. The
32
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
evaporator has an evaporation tank, a reboiler for heating
distillation and a condenser for condensing evaporated vapor.
A mist separator for preventing entrainment may be provided in
the tank, or a distillation part such as a tray may be
provided at the top section of the tank. The
distillation
equipment is however not limited to an evaporator, and, for
instance, there can be used a multiple effect evaporator, a
stirring tank equipped with a heating jacket or with a heat
exchanger, a stripping column or a thin-film evaporator.
Steam or a heat medium is used as heat source for heating
in the evaporator and so forth. A high
temperature process
fluid or the like can also be used alternatively.
In some instances an oil-water separation operation of
the collected solution may be performed, with feeding of the
oil phase alone, with a view to suppressing the occurrence of
an oil-water suspension state in the extraction step.
Preferably, the extraction temperature in the extraction step
is set to 30 C to 90 C. More preferably, the temperature is
set to 40 C to 80 C, and yet more preferably to 40 C to 60 C.
As a method for adjusting the temperature, it is most
efficient to adjust the temperature of the collected solution
or the acrylic acid aqueous solution that is supplied to the
extraction step, or the temperature of a mixed solution of the
foregoing, so as to lie in an appropriate range. An
33
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
appropriate range denotes herein a range of 20 C to 90 C, more
preferably 30 C to 90 C and yet more preferably 30 C to 70 C.
[0042]
Preferably, the operation of the extraction step,
including supply of the collected solution, is managed using
Michael adducts as an indicator substance. The concentration
of Michael adducts in a mixed composition of the acrylic acid
aqueous solution and the collected solution is preferably set
to 0.05 to 0.7 wt%, more preferably 0.05 to 0.6 wt% and yet
more preferably 0.1 to 0.5 wt%. If the
concentration of
Michael adducts is too low, the conditions in the oxidation
reaction step and the absorption step become constrained,
whereas if the concentration is too high, oil-water separation
in the extraction step may be impaired. Herein
it is
effective to perform the above-described distillation or oil-
water separation operation of the collected solution, as a
method for maintaining the above concentration ratio.
Conceivable alternative methods include, for instance,
adjusting the flow rate of the collected solution, and
adjusting the concentration of Michael adducts by separately
supplying high-purity acrylic acid.
[0043] 8) Waste solution treatment step
The waste solution (21) discharged from the evaporator
(6) contains, for instance, polymerization inhibitor; high-
boiling point components and formaldehyde that are generated
in the oxidation reaction step and that remain within the
34
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
raffinate water (19) in the extraction column (5). The waste
solution (21) is treated, for instance, by being incinerated.
Examples
[0044] The present invention will be explained next more
specifically by way of examples, but the invention is however
not meant, within the gist thereof, to be limited to the
examples.
[0045] [Analysis of a sampling solution]
The substances contained in a sampling solution were
identified, and the substances were quantified, through
analysis by gas chromatography. Gas
chromatography was
conducted using GC-14A, by Shimadzu Corporation. A capillary
column (HP-FFAP) by Agilent Technologies, Inc. was used as a
separation column, and FID and TCD were used as detectors.
[0046] (Reference example 1)
Acrylic acid was produced in the steps below using the
acrylic acid production facility described in Fig. 1.
Specifically, acrylic acid was produced as a result of: an
oxidation reaction step of obtaining an oxidation reaction gas
derived from a vapor-phase catalytic oxidation reaction of
propylene; an absorption step of bringing the oxidation
reaction gas and absorbing water, containing a polymerization
inhibitor, into gas-liquid contact, to yield thereby an
acrylic acid aqueous solution; an extraction step of obtaining
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
an extract solution from the acrylic acid aqueous solution,
using toluene as an extraction solvent; and an acrylic acid
purification step of obtaining acrylic acid through
purification of the extract solution by distillation.
The extraction test below was carried out in order to
monitor the above extraction step in a simple manner.
Colorimetric tubes were charged with 33 mL of an acrylic
acid aqueous solution (acrylic acid: 57.0 wt%, acrylic acid
dimer: 0.31 wt%, acrylic acid trimer: 0.0013 wt%, water: 38.3
wt%, acetic acid: 2.6 wt%, maleic acid: 0.44 wt%) obtained in
the absorption step. Next,
67 mL of toluene used in the
extraction step were charged into the colorimetric tubes. The
colorimetric tubes were set in a rotator (Taitec RT50), and
rotary mixing was carried out in a 21 C room-temperature
environment, at 30 rpm, for 1 minute. The tube was thereafter
allowed to stand, whereupon the oil-water separation state was
checked visually. It was
seen that the oil phase containing
acrylic acid, toluene and so forth and the aqueous phase
containing water, acetic acid, maleic acid and so forth
separated quickly, within 50 seconds.
[0047] (Example 1)
In Example 1, the extraction step in Reference example I
was modified to an extraction step that involved obtaining an
extract solution, using toluene, from a mixed solution
resulting from mixing of the acrylic acid aqueous solution
36
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
obtained in the absorption step and a collected solution
obtained by holding the discharge solution having been
discharged from the acrylic acid production process.
The extraction test below was carried out in order to
monitor the above absorption step in a simple manner.
Colorimetric tubes were charged with 33 mL of a mixed
solution resulting from mixing, at a ratio by weight of 10:1,
the acrylic acid aqueous solution (acrylic acid: 57.0 wt%,
acrylic acid dimer: 0.31 wt%, acrylic acid trimer: 0.0013 wt%,
water: 38.3 wt%, acetic acid: 2.6 wt%, maleic acid: 0.44 wt%)
obtained in the absorption step of Reference example 1 and a
collected solution (acrylic acid: 63.3 wt%, acrylic acid
dimer: 2.1 wt%, acrylic acid trimer: 0.022 wt%, water: 11.4
wt%, acetic acid: 0.030 wt%) after holding of the discharge
solution having been discharged from the acrylic acid
production process, for 4 days, in a storage tank.
Next, 67
mL of toluene used in the extraction step of Reference example
1 were charged into the colorimetric tubes. The same mixing
operation as that of Reference example 1 was carried out, and
the oil-water separation state was checked. It was seen that
the oil phase containing acrylic acid, toluene and so forth
and the aqueous phase containing water, acetic acid, maleic
acid and so forth separated quickly, within 50 seconds.
[0048] (Comparative example 1)
37
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
In Comparative example 1, the extraction step in
Reference example 1 was modified to an extraction step that
involved obtaining an extract solution, using toluene, from a
mixed solution resulting from mixing of the acrylic acid
aqueous solution obtained in the absorption step and the
discharge solution (without holding) discharged from the
acrylic acid production process.
The extraction test below was carried out in order to
monitor the above extraction step in a simple manner.
Colorimetric tubes were charged with 33 mL of a mixed
solution resulting from mixing, at a ratio by weight of 10:1,
the acrylic acid aqueous solution (acrylic acid: 57.0 wt%,
acrylic acid dimer: 0.31 wt%, acrylic acid trimer: 0.0013 wt%,
water: 38.3 wt%, acetic acid: 2.6 wt%, maleic acid: 0.44 wt%)
obtained in the absorption step of Reference example 1 and the
discharge solution (without holding) (acrylic acid: 73.3 wt%,
acrylic acid dimer: 5.9 wt%, acrylic acid trimer: 0.052 wt%,
water: 20.4 wt%, acetic acid: 0.16 wt%) discharged from the
acrylic acid production process. Next, 67 mL of toluene used
in the extraction step of Reference example 1 were charged
into the colorimetric tubes. The
same mixing operation as
that of Reference example 1 was carried out, and the oil-water
separation state was checked. The oil-water suspension phase
persisted even at the time of lapse of 90 seconds, and the oil
phase and the aqueous phase failed to separate.
38
*
= = CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
[0049] (Example 2)
In Example 2, the extraction step in Reference example 1
was modified to an extraction step that involved obtaining an
extract solution, using toluene, from a mixed solution
resulting from mixing of the acrylic acid aqueous solution
obtained in the absorption step and a distillate obtained
through simple distillation of a collected solution resulting
from holding of the discharge solution having been discharged
from the acrylic acid production process.
The extraction test below was carried out in order to
monitor the above extraction step in a simple manner.
The acrylic acid aqueous solution (acrylic acid: 57.0 wt%,
acrylic acid dimer: 0.31 wt%, acrylic acid trimer: 0.0013 wt%,
water: 38.3 wt%, acetic acid: 2.6 wt%, maleic acid: 0.44 wt%)
obtained in the absorption step of Reference example 1 was
sampled.
Then a 200 cc flask was charged with 100 g of a
collected solution resulting from holding, for 2 days in a
storage tank, the discharge solution having been discharged
from the acrylic acid production process, and a distillate
obtained through simple distillation at 80 C and 10 kPa was
sampled. Next, colorimetric tubes were charged with 33 mL of
a mixed solution resulting from mixing the acrylic acid
aqueous solution and the distillate at a ratio by weight of
10:1.
Further, 67 mL of toluene used in the extraction step
of Reference example 1 were charged into the colorimetric
39
= = CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
tubes. The same mixing operation as that of Reference example
1 was carried out, and the oil-water separation state was
checked. It was seen that the oil phase containing acrylic
acid, toluene and so forth and the aqueous phase containing
water, acetic acid, maleic acid and so forth separated quickly,
within 50 seconds.
[0050] (Example 3)
In Example 3, the extraction step in Reference example 1
was modified to an extraction step that involved obtaining an
extract solution, using toluene, from a mixed solution
resulting from mixing of the acrylic acid aqueous solution
obtained in the absorption step and an oil phase obtained
through oil-water separation of a collected solution obtained
by holding the discharge solution having been discharged from
the acrylic acid production process.
The extraction test below was carried out in order to
monitor the above extraction step in a simple manner.
The acrylic acid aqueous solution (acrylic acid: 57.0 wt%,
acrylic acid dimer: 0.31 wt%, acrylic acid trimer: 0.0013 wt%,
water: 38.3 wt%, acetic acid: 2.6 wt%, maleic acid: 0.44 wt%)
obtained in the absorption step of Reference example 1 was
sampled. Then a collected solution resulting from holding,
for 2 days in a storage tank, the discharge solution having
been discharged from the acrylic acid production process was
collected in calorimetric tubes, toluene was added in a ratio
= = CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
by weight of 1:1, the colorimetric tubes were set in a rotator
(Taitec RT50), and rotary mixing was carried out in a 21 C
room-temperature environment, at 30 rpm, for 1 minute.
The
tube was thereafter allowed to stand, to elicit oil-water
separation, and the oil phase was sampled. Next, colorimetric
tubes were charged with 33 mL of a mixed solution resulting
from mixing the acrylic acid aqueous solution and the oil
phase at a ratio by weight of 10:1. Further, 67 mL of toluene
used in the extraction step of Reference example 1 were
charged into the colorimetric tubes.
The same mixing
operation as that of Reference example 1 was carried out, and
the oil-water separation state was checked. It was seen that
the oil phase containing acrylic acid, toluene and so forth
and the aqueous phase containing water, acetic acid, maleic
acid and so forth separated quickly, within 50 seconds.
[0051] (Reference example 2)
Acrylic acid was produced in the steps below using the
acrylic acid production facility described in Fig. 1.
Specifically, acrylic acid was produced as a result of: an
oxidation reaction step of obtaining an oxidation reaction gas
derived from a vapor-phase catalytic oxidation reaction of
propylene; an absorption step of bringing the oxidation
reaction gas and absorbing water, containing a polymerization
inhibitor, into gas-liquid contact, to yield thereby an
acrylic acid aqueous solution; an extraction step of obtaining
41
= CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
an extract solution from the acrylic acid aqueous solution,
using toluene as an extraction solvent; and an acrylic acid
purification step of obtaining acrylic acid through
purification of the extract solution by distillation.
The extraction test below was carried out in order to
monitor the above extraction step in a simple manner.
Colorimetric tubes were charged with 33 mL of an acrylic
acid aqueous solution (acrylic acid: 57.5 wt%, acrylic acid
dimer: 0.25 wt%, acrylic acid trimer: 0.0014 wt%, water: 37.7
wt%, acetic acid: 2.6 wt%, maleic acid: 0.46 wt%) obtained in
the absorption step. Next, 67 mL of toluene used in the
extraction step were charged into the colorimetric tubes. The
colorimetric tubes were set in a rotator (Taitec RT50), and
rotary mixing was carried out in a 2100 room-temperature
environment, at 50 rpm, for 1 minute. The tube was thereafter
allowed to stand, whereupon the oil phase containing acrylic
acid, toluene and so forth and the aqueous phase containing
water, acetic acid, maleic acid and so forth separated quickly,
within 40 seconds.
[0052] (Comparative example 2)
In Comparative example 2, the extraction step in
Reference example 2 was modified to an extraction step that
involved obtaining an extract solution, using toluene, from a
mixed solution resulting from mixing of the acrylic acid
aqueous solution obtained in the absorption step and the
42
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
discharge solution (without holding) discharged from the
acrylic acid production process.
The extraction test below was carried out in order to
monitor the above extraction step in a simple manner.
Colorimetric tubes were charged with 33 mL of a mixed
solution resulting from mixing, at a ratio by weight of 10:1,
the acrylic acid aqueous solution (acrylic acid: 57.5 wt%,
acrylic acid dimer: 0.25 wt%, acrylic acid trimer: 0.0014 wt%,
water: 37.7 wt%, acetic acid: 2.6 wt%, maleic acid: 0.46 wt%)
obtained in the absorption step of Reference example 2 and the
discharge solution (without holding) (acrylic acid: 73.3 wt%,
acrylic acid dimer: 5.9 wt%, acrylic acid trimer: 0.052 wt%,
water: 20.4 wt%, acetic acid: 0.16 wt%) discharged from the
acrylic acid production process. Next, 67 mL of toluene used
in the extraction step of Reference example 2 were charged
into the colorimetric tubes. The
same mixing operation as
that of Reference example 2 was carried out, and the oil-water
separation state was checked. The oil-water suspension phase
persisted even at the time of lapse of 90 seconds, and the oil
phase and the aqueous phase failed to separate.
[0053] (Example 4)
In Example 4, the extraction step in Reference example 2
was modified to an extraction step that involved obtaining an
extract solution, using toluene, from a mixed solution
resulting from mixing of the acrylic acid aqueous solution
43
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
obtained in the absorption step and a distillate from simple
distillation of a collected solution resulting from holding of
the discharge solution having been discharged from the acrylic
acid production process.
The extraction test below was carried out in order to
monitor the above extraction step in a simple manner.
The acrylic acid aqueous solution (acrylic acid: 57.5 wt%,
acrylic acid dimer: 0.25 wt%, acrylic acid trimer: 0.0014 wt%,
water: 37.7 wt%, acetic acid: 2.6 wt%, maleic acid: 0.46 wt%)
obtained in the absorption step of Reference example 2 was
sampled. Then a
200 cc flask was charged with 100 g of a
collected solution used in Comparative example 2 and resulting
from holding, for 2 days in a storage tank, the discharge
solution having been discharged from the acrylic acid
production process, and a distillate obtained through simple
distillation at 80 C and 10 kPa was sampled. Next,
colorimetric tubes were charged with 33 mL of a mixed solution
resulting from mixing the acrylic acid aqueous solution and
the distillate at a ratio by weight of 10:1.
Further, 67 mL
of toluene used in the extraction step of Reference example 2
were charged into the colorimetric tubes. The
same mixing
operation as that of Reference example 2 was carried out, and
the oil-water separation state was checked. It was seen that
the oil phase containing acrylic acid, toluene and so forth
44
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
and the aqueous phase containing water, acetic acid, maleic
acid and so forth separated quickly, within 40 seconds.
[0054] (Example 5)
In Example 5, the extraction step in Reference example 2
was modified to an extraction step that involved obtaining an
extract solution, using toluene, from a mixed solution
resulting from mixing of the acrylic acid aqueous solution
obtained in the absorption step and an oil phase from oil-
water separation of a collected solution resulting from
holding the discharge solution discharged from the acrylic
acid production process.
The extraction test below was carried out in order to
monitor the above extraction step in a simple manner.
The acrylic acid aqueous solution (acrylic acid: 57.5 wt%,
acrylic acid dimer: 0.25 wt%, acrylic acid trimer: 0.0014 wt%,
water: 37.7 wt%, acetic acid: 2.6 wt%, maleic acid: 0.46 wt%)
obtained in the absorption step of Reference example 2 was
sampled. Then a
collected solution resulting from holding,
for 2 days in a storage tank, the discharge solution having
been discharged from the acrylic acid production process was
collected in colorimetric tubes, toluene was added in a ratio
by weight of 1:1, the colorimetric tubes were set in a rotator
(Taitec RT50), and rotary mixing was carried out in a 21 C
room-temperature environment, at 30 rpm, for 1 minute. The
tube was thereafter allowed to stand, to elicit oil-water
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
separation, and the oil phase was sampled. Next, colorimetric
tubes were charged with 33 mL of a mixed solution resulting
from mixing the acrylic acid aqueous solution and the oil
phase at a ratio by weight of 10:1. Further, 67 mL of toluene
used in the extraction step of Reference example 2 were
charged into the colorimetric tubes. The
same mixing
operation as that of Reference example 2 was carried out, and
the oil-water separation state was checked. It was seen that
the oil phase containing acrylic acid, toluene and so forth
and the aqueous phase containing water, acetic acid, maleic
acid and so forth separated quickly, within 40 seconds.
[0055] (Reference example 3)
Acrylic acid was produced in the steps below using the
acrylic acid production facility described in Fig. 1.
Specifically, acrylic acid was produced as a result of: an
oxidation reaction step of obtaining an oxidation reaction gas
derived from a vapor-phase catalytic oxidation reaction of
propylene; an absorption step of bringing the oxidation
reaction gas and absorbing water, containing a polymerization
inhibitor, into gas-liquid contact, to yield thereby an
acrylic acid aqueous solution; an extraction step of obtaining
an extract solution from the acrylic acid aqueous solution,
using toluene as an extraction solvent; and an acrylic acid
purification step of obtaining acrylic acid through
purification of the extract solution by distillation.
46
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
The extraction test below was carried out in order to
monitor the above extraction step in a simple manner.
Colorimetric tubes were charged with 33 mL of an acrylic
acid aqueous solution (acrylic acid: 57.5 wt%, acrylic acid
dimer: 0.25 wt%, acrylic acid trimer: 0.0014 wt%, water: 37.7
wt%, acetic acid: 2.6 wt%, maleic acid: 0.46 wt%) obtained in
the absorption step. Next,
67 mL of toluene used in the
extraction step were charged into the colorimetric tubes. The
colorimetric tubes were immersed for 10 minutes in a hot water
bath at 42 C, and thereafter were set in a rotator (Taitec
RT50), where rotary mixing was carried out at 50 rpm for 1
minute. The
tube was thereafter allowed to stand, whereupon
the oil phase containing acrylic acid, toluene and so forth
and the aqueous phase containing water, acetic acid, maleic
acid and so forth separated quickly, within 50 seconds.
[0056) (Example 6)
In Example 6, the extraction step in Reference example 2
was modified to an extraction step that involved obtaining an
extract solution at a temperature of 42 C, using toluene, from
a mixed solution resulting from mixing of the acrylic acid
aqueous solution obtained in the absorption step and a
collected solution obtained by holding the discharge solution
having been discharged from the acrylic acid production
process.
47
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
The extraction test below was carried out in order to
monitor the above extraction step in a simple manner.
The acrylic acid aqueous solution (acrylic acid: 57.5 wt%,
acrylic acid dimer: 0.25 wt%, acrylic acid trimer: 0.0014 wt%,
water: 37.7 wt%, acetic acid: 2.6 wt%, maleic acid: 0.46 wt%)
obtained in the absorption step of Reference example 3 was
sampled. The
temperature of the discharge solution (acrylic
acid: 73.3 wt%, acrylic acid dimer: 5.9 wt%, acrylic acid
trimer: 0.052 wt%, water: 20.4 wt%, acetic acid: 0.16 wt%)
discharged from the acrylic acid production process was
measured, whereupon a value of 20 C was obtained. A collected
solution resulting from holding the discharge solution for 2
days in a storage tank was sampled. Next, colorimetric tubes
were charged with 33 mL of a mixed solution resulting from
mixing the acrylic acid aqueous solution and the collected
solution at a ratio by weight of 100:6.
Further, 67 mL of
toluene used in the extraction step of Reference example 3
were charged into the colorimetric tubes. In the same way as
in Reference example 3, the colorimetric tubes were immersed
for 10 minutes in a hot water bath at 42 C, after which a
mixing operation was carried out, and the oil-water separation
state was checked. It was found that the oil phase containing
acrylic acid, toluene and so forth and the aqueous phase
containing water, acetic acid, maleic acid and so forth
separated quickly, within 50 seconds.
48
=
=
CA 03009033 2018-06-18
=
16A00512W00 (0P-16414-PCT)
[0057]
[Table 1]
49
-
,
Feeding of solution discharged from acrylic acid
Concentration (wt%)
Extraction
production process to extraction step Rotational speed Oil-water
separation
of Michael adducts
temperature
of rotator (rpm)
time .
Discharge Collected in mixed solution
CC)
No feeding
solution fed , solution fed
_
Reference
030 21 Within 50 seconds
example 1 0.31*1
_ Example 1 0 0.47 30
21 Within 50 seconds
Comparative
No separation
0 0.82
30 21
example 1
within 90 seconds
_
Example 2 0 0.33 30
21 Within 50 seconds
Example 3 0 0.43 30
21 Within 50 seconds
Reference
0 0.25*1
50 21 Within 40 seconds
example 2
P
.
,..
Comparative
No separation .
.
0 0.77
50 21 ,0
example 2
within 90 seconds ,..
u-i -
,..
o Example 4 0 0.28
50 21 Within 40 seconds 10
,
Example 5 0 0.38 50
21 Within 40 seconds 0
,
.
.
,
Reference,
0 0.25*/
50 42 Within 50 seconds 03
example 3
i--,
cs,
Example 6 0 0.77 50 42
Within 50 seconds >
CD
*1 The "concentration of Michael adducts" in reference examples denotes
concentration in the acrylic acid aqueous solution. o
c_n
1-µ
N)
0
D
¨
0
1-0
1
1--s
0-)
az,
1--,
,I.
1
1-0
0
H
*
* CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
[0058]
The results of Comparative example 1 revealed
impairment of oil-water separability in the case of addition
of the discharge solution having been discharged from the
acrylic acid production process. The concentration of Michael
adducts at this time, encompassing acrylic acid dimers and
acrylic acid trimers, exceeded 0.7 wt%.
When holding,
distillation and the oil-water separation operation were
carried out, in Examples 1, 2 and 3, there was by contrast no
impairment of oil-water separability, and the Michael adduct
concentration was 0.7 wt% or less. A comparison between the
results of Comparative example 2 and the results of Examples 4
and 5 reveals that the above tendency holds even when mixing
intensity is increased through an increase in rotational speed.
The results of Reference example 3 and Example 6,
meanwhile, revealed that oil-water separability was not
impaired in a case where the extraction temperature was set to
42 C, even when the concentration of Michael adducts exceeded
0.7 wt%, upon addition of discharge solution having been
discharged from the acrylic acid production process.
Reference Signs List
[0059]
1, 2 Oxidation reactor
3 Absorption column
4 Waste gas treatment step
51
CA 03009033 2018-06-18
16A00512W00 (0P-16414-PCT)
Extraction column
6 Evaporator
7 Acrylic acid purification step
8 Storage tank
9-1, 9-2, 9-3 Heat exchanger
Propylene
11 Air
12 Diluent (steam or nitrogen)
13 Oxidation reaction gas
14 Acrylic acid aqueous solution
Polymerization inhibitor
16 Extraction solvent
17 Absorbing water
18 Extract solution
19 Raffinate water
Acrylic acid
21 Waste solution
22 Waste gas
23 Recycled gas
24 Raffinate water recycling
Discharge solution / collected solution from acrylic acid
production process
52