Note: Descriptions are shown in the official language in which they were submitted.
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
METHODS OF PROVIDING HIGHER QUALITY
LIQUID KEROSENE BASED-PROPULSION FUELS
The present application claims the benefit of pending U. S. Provisional
Application
Serial No. 62/270,176, filed 21 December 2015.
Field of the Invention
This invention relates to methods of providing higher quality kerosene-based
propulsion fuels.
Background of the Invention
Typical jet fuels and liquid kerosene rocket fuels are prepared in a refinery
from a
crude mineral oil source. Typically the crude mineral oil is separated by
means of
distillation into a distillate kerosene fraction boiling in the aviation fuel
range or a more
purified liquid kerosene rocket fuel. If required, these fractions are
subjected to
hydroprocessing to reduce sulfur, oxygen, and nitrogen levels.
Increasing demand for jet fuel and the environmental impact of aviation
related
emissions places the aviation industry at the forefront of today's global
energy challenge.
The increased demand for petroleum-based fuels has resulted in a higher
production of
greenhouse gases. In particular, the aviation industry accounts for about 2%
of global CO2
emissions. The aviation transport sector is growing 3-5% year on year, and due
to the
projected increasing demand for fuel and increasing production of CO2
emissions, there is
a need to explore methods to increase environmentally-friendly fuel sources
while meeting
jet fuel specifications.
Perhaps more tangible than the global impact of greenhouse gases is the impact
of
local emissions from aircraft. Emissions near and around airports have a
direct impact on
the air composition and therefore have been linked with poor local air
quality, which can
be further linked to impacts on human health. Sooty particulates and oxides of
sulfur and
nitrogen are considered to be contributors to poor local air quality. Thus,
local air quality
is seen as an integral element in the pursuit of environment-friendly fuels.
Petroleum-derived jet fuels inherently contain both paraffinic and aromatic
hydrocarbons. In general, paraffinic hydrocarbons offer the most desirable
combustion
cleanliness characteristics for jet fuels. Aromatics generally have the least
desirable
combustion characteristics for aircraft turbine fuel. In aircraft turbines,
certain aromatics,
such as naphthalenes, tend to burn with a smokier flames and release a greater
proportion
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
of their chemical energy as undesirable thermal radiation than other more
saturated
hydrocarbons.
The closest current option for reducing aviation emissions is blending
synthesized
paraffinic kerosene ("SPK") from Fischer-Tropsch or hydrogenated vegetable oil
with
conventional jet fuel. Up to 50% by volume of SPK is permitted by the
alternative jet fuel
specification ASTM D7566. If the resulting blend meets the specification, it
can be certified
and considered equivalent to conventional, petroleum-derived jet fuel.
Typically, these
synthesized paraffinic kerosenes contain a mixture of normal and branched
paraffin according
to ASTM D7566.
It is important that novel fuels meet their respective jet fuel specifications
without
having a detrimental impact on safety or aircraft performance. Because SPK is
purely
paraffinic and absent of both aromatics and sulfur, it does not exhibit all of
the desired
properties expected from a jet fuel. For example, a gas to liquids Fischer-
Tropsch-derived
fuel is not considered an on-spec fuel in its pure state due to its lower
density. Further,
SPK fuels tend to have low volumetric energy density, which may require more
fuel than can
be accommodated in aircraft fuel tanks for long distance flights.
Kerosene fuels can also be used as liquid rocket fuels. MIL-DTL-25576 defines
two grades of kerosene fuels, rocket propellant (RP) fuels known as RP-1 and
RP-2, for
use in rocket engines. These fuels, while still kerosene-type fuels, have some
different
property requirements from jet fuels. RP fuels have a higher minimum flash
point at 60 C,
a lower maximum freezing point at -51 C, higher temperature thermal stability
requirement at 355 C, lower maximum total aromatics content of 5% volume, and
reduced
density range of 799 ¨ 815 kg/m' at 15 C, and reduced distillation range, with
T10
between 185 C and 210 C and maximum distillation end point of 274 C.
Summary of the Invention
In accordance with certain of its aspects, provided is a method for producing
a
liquid rocket fuel useful as RP-1 or RP-2 grade rocket fuels comprising;
a. providing a quantity of kerosene range hydrocarbon component having a
boiling point in the range of 145 C to 300 C, at atmospheric pressure, flash
point of at
least 60 C or above measured by ASTM D56, a density at 15 C of at most 815
kg/m3;
b. providing a quantity of synthetic cyclo-paraffinic kerosene fuel
blending
component comprising component comprising at least 99.5 mass% of carbon and
hydrogen
content and at least 50 mass% of cyclo-paraffin, said cyclo-paraffinic
kerosene fuel
2
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
blending component having a boiling point of at most 300 C, at atmospheric
pressure,
flash point of at least 38 C, a density at 15 C of at least 799 kg/m3, and
freezing point of
-60 C or lower; and
c.
blending a quantity of the synthetic cyclo-paraffinic kerosene fuel blending
component and the kerosene range hydrocarbon component in amount sufficient to
meet a
flash point of at least 60 C and final boiling point of 274 C or lower to
produce the
blended liquid rocket fuel.
The features and advantages of the invention will be apparent to those skilled
in the
art. While numerous changes may be made by those skilled in the art, such
changes are
within the spirit of the invention.
Brief Description of the Drawings
The drawings illustrate certain aspects of some of the embodiments of the
invention, and should not be used to limit or define the invention.
Fig. 1 shows the volumetric energy density (MJ/m3) of the jet fuel blends
based on
paraffinic kerosene content (vol.%) in Jet A of various fuels from Examples
described
herein.
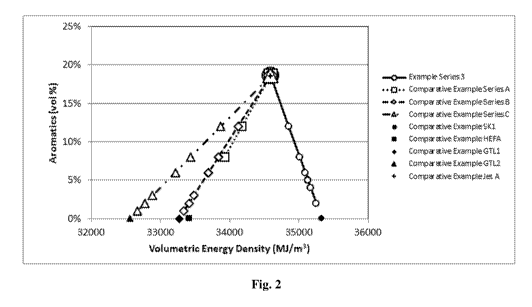
Fig. 2 shows a plot of the aromatics content (vol.%) versus volumetric energy
density (MJ/m3) of the various jet fuel blends from Examples described herein.
Fig 3 shows the smoke point increase of jet fuel with volumetric energy
density
(MJ/m3) of the various jet fuel blends from Examples described herein.
Fig 4 shows the freezing point ( C) of various jet fuel blends from Examples
described herein versus volumetric energy density (MJ/m3).
Fig. 5 shows the comparison of sub-zero viscosities of commercial RP rocket
fuels
versus the rocket fuel of the invention from Example 6.
Detailed Description of the Invention
It has been found that by blending a quantity of certain synthetic cyclo-
paraffinic
kerosene fuel blending components comprising at least 99.5 mass% of carbon and
hydrogen content and at least 50 mass% of cyclo-paraffin into a kerosene base
fuel, the
fuel can be upgraded or blended to meet certain specifications and/or increase
its
volumetric energy content for jet and rocket fuel applications. More
specifically, it has
been found that by blending a quantity of the certain synthetic cyclo-
paraffinic kerosene
fuel blending components into certain kerosene base fuel or a kerosene range
hydrocarbon
3
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
component, a fuel useful as a liquid rocket fuel (such as RP-1 or RP-2 grade
rocket fuels)
may be produced.
Such liquid rocket fuel may be produced by:
a. providing a quantity of kerosene range hydrocarbon component having a
boiling point in the range of 145 C to 300 C, at atmospheric pressure, flash
point of at
least 60 C or above measured by ASTM D56, a density at 15 C of at most 815
kg/m3;
b. providing a quantity of synthetic cyclo-paraffinic kerosene fuel
blending
component comprising component comprising at least 99.5 mass% of carbon and
hydrogen
content and at least 50 mass% of cyclo-paraffin, said cyclo-paraffinic
kerosene fuel
blending component having a boiling point of at most 300 C, at atmospheric
pressure,
flash point of at least 38 C, a density at 15 C of at least 799 kg/m3, and
freezing point of
-60 C or lower; and
c. blending a quantity of the synthetic cyclo-paraffinic kerosene fuel
blending
component and the kerosene range hydrocarbon component in amount sufficient to
meet a
flash point of at least 60 C and final boiling point of 274 C or lower to
produce the
blended liquid rocket fuel.
In one embodiment, it has also been found that the volumetric energy content
of a
fuel can be increased without increase in its aromatic content by:
a. providing a quantity of kerosene base fuel having a boiling point in the
range of 130 C to 300 C, at atmospheric pressure, flash point of 38 C or above
measured
by ASTM D56, and a density at 15 C of at least 760 kg/m3, preferably at least
770 kg/m3;
b. providing a quantity of a synthetic cyclo-paraffinic kerosene fuel
blending
component comprising at least 99.5 mass% of carbon and hydrogen content and at
least 50
mass% of cyclo-paraffin, said cyclo-paraffinic kerosene fuel blending
component having a
boiling point of at most 300 C, at atmospheric pressure, flash point of 38 C
or above, a
density at 15 C of at least 800 kg/m3, and freezing point of -60 C or lower;
and
c. blending a quantity of the synthetic cyclo-paraffinic kerosene fuel
blending
component to the kerosene base fuel in amount effective to increase the
volumetric energy
content, preferably at least 0.1% increase in the volumetric energy content.
Volumetric energy content can be calculated as energy per unit volume using
the
following equation:
Energy per unit volume (MJ/m3) = (energy per unit mass (MJ/kg)) * (density
(kg/m3))
4
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
Energy per unit mass can be obtained by one of several methods, including ASTM
D4529,
D3338, D4809, or IP12 by way of example. The increase in volumetric energy
content is
relative so any of these methods can be used as long as the same method is
used.
As used herein, "lower" in context of freezing points (e.g., the term "X C or
lower") means that the temperature is equal to or lower than the X
temperature. For
example, for a freezing point of "-60 C or lower", the temperature may be, for
example, -
60 C, -61 C, -65 C, -70 C, etc., as long as the temperature is not higher than
-60 C.
In certain embodiments, the kerosene-based fuel component may originate from
petroleum or be synthetically derived from biomass or other non-biomass
resources.
Aromatics content in a jet fuel can be determined by ASTM D1319. Aromatics
content for
synthetic blend components can be determined by ASTM D2425. The aromatic
content of
the blended jet fuel is typically determined by ASTM D1319. Equivalent total
aromatic
content between two fuels means the total aromatic content measured by these
methods
give an aromatic content within +/- 1.5 vol.%. Minimal increase of aromatic
content is
generally less than 3 vol.%, preferably less than 2 vol.%, more preferably
less than 1.5
vol.%, or more preferably without an increase that is within the precision of
measurement
for aromatic content, or even a decrease in aromatic content.
The method above may also produce a fuel having an improved smoke point as
compared with the kerosene base fuel component without the cyclo-paraffinic
kerosene
fuel blending component. In an embodiment, the smoke point is at least 1 mm
greater
than the kerosene base fuel as measured by ASTM D1322.
ASTM International ("ASTM") and the United Kingdom Ministry of Defence
("MOD") have taken the lead roles in setting and maintaining specification for
civilian
aviation turbine fuel and jet fuel. The respective specifications issued by
these two
organizations are very similar, but not identical. Many other countries issue
their own
national specifications for jet fuel, but are very nearly or completely
identical to either the
ASTM or MOD specification. ASTM D1655 is the Standard Specification for
Aviation
Turbine Fuels and includes specifications for Jet A and Jet A-1. Defense
Standard 91-91 is
the MOD specification for Jet A-1 and is the dominant fuel specification for
Jet A-1
outside of the United States.
Jet A-1 is the most common jet fuel and is produced to an internationally
standardized set of specifications. In the United States, Jet A is the primary
grade of jet
5
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
fuel. Another jet fuel that is used in civilian aviation is called Jet B. Jet
B is a wide-cut,
lighter fuel in the naphtha-kerosene region that is used for its enhanced cold-
weather
performance. Jet A and Jet A-1 are specified in ASTM D1655. Jet B is specified
in
ASTM D6615.
Alternatively, jet fuels are classified by militaries around the world with a
different
system of NATO or JP (Jet Propulsion) numbers. Some are almost identical to
their
civilian counterparts and differ only by the amounts of a few additives. For
example, Jet
A-1 is similar to JP-8. Both Jet A-1 and JP-8 specifications require a
freezing point of -
47 C or lower. Jet A specification requires a freezing point of -40 C or lower
as does the
military equivalent F-24. Jet B is similar to JP-4 that requires a freezing
point of ¨ 58 C
or lower. Other jet fuel specifications for militaries may include JP-5 that
requires a
freezing point of -46 C or lower and JP-7 that requires a freezing point of -
43.3 C or lower
and the RP grades that requires a freezing point of -51 C or lower.
Further, some jet fuel specifications have more stringent requirement for
flight in
more challenging environments. For cold climates, such as the Antarctic, AN-8
has a jet
fuel specification with a freezing point of -58 C or lower. AN-8 fuel is used
for turbine
engines and other power applications that require low freeze point for low
temperature
applications and storage.
Typically, jet fuel is a product boiling for more than 90 vol.% at from 130 C
to
300 C (ASTM D86), having a density in the range from 775 to 840 kg/m3,
preferably from
780 to 830 kg/m3, at 15 C (e.g. ASTM D4052), an initial boiling point in the
range 130 C
to 190 C and a final boiling point in the range 220 C to 300 C, at atmospheric
pressure, a
flash point of 38 C or above (ASTM D56), a kinematic viscosity at -20 C (ASTM
D445)
suitably from 1.2 to 8.0 mm2/s and a freeze point of -40 C or below for Jet A
specification,
preferably -47 C or below for Jet A-1 and JP-8 specifications, and preferably -
58 C or
below for AN-8 specification.
Jet fuel will typically meet one or more of the following civil standards. Jet
A-1
requirements are in ASTM D1655 or DEF STAN 91-91 (British Ministry of Defence
Standard DEF STAN 91-91/Issue 7 amendment 3 of 2 Feb. 2015 (or later issues)
for
Turbine Fuel, Aviation "Kerosene Type," Jet A-1, NATO code F-35, Joint Service
Designation AVTUR, or versions current at the time of testing), as well as
some airport
handling requirements of the IATA Guidance Material for Aviation Turbine Fuels
Specifications. Jet A requirements are in ASTM D1655. Military jet fuel
requirements are
6
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
similar to civil requirements but usually more stringent for select properties
and in the use
of additives; these requirements are published by respective governments. For
example,
these can include MIL-DTL-83133 which defines JP-8 as used by US federal
agencies.
Due to the differences in the specifications and depending on locations and
intended
use, it is desirable to upgrade the fuel to achieve the specification that the
fuel must meet in
order to fly in certain regions. For example, it may be desirable to upgrade a
jet fuel which
meets the Jet A specification to a fuel that has a lower freezing point
consistent with the Jet
A-1 specification requirement, particularly without an increase in its
aromatic content. In
another example, it may be desirable to upgrade a jet fuel to a cold climate
specification,
such as AN-8 jet fuel specification, which requires an even lower freezing
point.
It has been found that by blending a quantity of synthetic cyclo-paraffinic
kerosene
fuel blending component comprising at least 99.5 mass% of carbon and hydrogen
content
and at least 50 mass% of cyclo-paraffin, the cyclo-paraffinic kerosene fuel
blending
component having a boiling point of at most 300 C, at atmospheric pressure,
flash point of
38 C or above, and a density at 15 C of at least 800 kg/m3, and freezing point
of -60 C or
below, one can upgrade a kerosene base fuel to meet certain specifications.
As used herein, upgrading to meet a fuel specification means blending a fuel
that
does not meet the specification standard to meeting the standard for such fuel
specification.
For jet fuels, it is particularly desirable to upgrade the jet fuel without
increasing its
aromatic content. To meet a jet fuel specification property means that the jet
fuel meets the
requirements of at least one of the above mentioned specifications, as
determined by
standard test methods, such as from ASTM, IP, or other such industry-
recognized
standards bodies. Test methods for determining if a fuel meets a specification
may
include:
Table 1: Test for Jet Fuel Specification Properties
Test ASTM Method
Acidity (mgKOH/g) D3242
Density at 15 C (g/cm3) D4052
Hydrogen Content (mass%) D7171
Flash Point ( C) D56
Freeze Point ( C) D5972
Viscosity (mm2/s) D445
7
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
Total Sulfur (mass%) D4294
Mercaptan sulfur (mass%) D3227
Smoke Point (mm) D1322
Naphthalenes (vol.%) D1840
Aromatics (vol.%) D1319
Net Heat of Combustion (MJ/kg) D3338
Initial Boiling Point (IBP) ( C) D86
Final Boiling Point (FBP) ( C) D86
It is desirable to produce a quality liquid rocket fuel that meets the rocket
fuel
specifications. MIL-DTL-25576E specifies 2 grades of rocket fuel, RP-1 and RP-
2, which
are identical except for the maximum sulfur content. RP-1 has a maximum
allowable
sulfur content of 0.0030 mass%, while RP-2 has a maximum allowable sulfur
content of
0.00001 mass%. Both RP-1 and RP-2 have a maximum aromatics content of 5 vol.%,
a
10% distillation point between 185 C and 210 C, a distillation end point
maximum of
274 C, a minimum flash point of 60 C, a density range at 15 C of 799 ¨ 815
kg/m3, a
maximum freezing point of -51 C, a minimum hydrogen content of 13.8 mass %,
and a
thermal stability test temperature of 355 C.
Kerosene Base Fuel or Kerosene Range Hydrocarbon Component
A kerosene base fuel or kerosene range hydrocarbon component is any kerosene
that
may be useful as a jet or rocket fuel, or a jet or rocket fuel blending
component (other than
the synthetic cyclo-paraffinic kerosene fuel blending component described
herein) having a
boiling point in the range of 130 C to 300 C, at atmospheric pressure (as
measured by
ASTM D86), preferably in the range of 140 C to 300 C, and most preferably in
the range
of 145 C to 300 C. For a jet fuel blending component, the kerosene base fuel
(whether
single stream or a mixture) can have a flash point of 38 C or above (measured
by ASTM
D56), and a density at 15 C of at least 760 kg/m3 (as measured by D4052). For
liquid
rocket fuel, the kerosene range hydrocarbon component can have a boiling point
in the
range of 145 C to 300 C, preferably in the range of 145 C to 270 C; a flash
point of 60 C
or above, measured by ASTM D56; and a density at 15 C of at most 815 kg/m3.
The
kerosene base fuel or kerosene range hydrocarbon component may originate from
petroleum or be synthetically derived from biomass, or other non-biomass
resources. In
8
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
certain embodiments, the kerosene base fuel may be any petroleum-derived jet
fuel known
to skilled artisans, including kerosene fuels meeting at least one of Jet A,
Jet A-1, F-24,
JP-8, Jet B or AN-8 specification. Preferably the kerosene base fuel is a
kerosene that can
meet the jet fuel specification properties according to the invention.
For example, petroleum-derived kerosene fuels meeting Jet A or Jet A-1
requirements and a kerosene stream used in Jet A or Jet A-1 production are
listed in Table
2. It is also contemplated that petroleum-derived kerosene fuels which do not
meet Jet A
or Jet A-1 specifications may be used as kerosene base fuels that can be
upgraded to meet
such specifications according to the present invention.
Table 2
Jet Fuel Produced Using:
Straight run kerosene stream.
Caustic washing of straight run kerosene.
A sweetening process such as Merox , Merichem , or Bender process.
Hydroprocessed jet fuel.
As another example, the low boiling fraction as separated from a mineral gas
oil
may be used as such or in combination with petroleum-derived kerosene,
suitably made at
the same production location. As the low boiling fraction may already comply
with a jet
fuel specification, it is evident that the blending ratio between said
component and the
petroleum-derived kerosene may be freely chosen. The petroleum-derived
kerosene will
typically boil for more than 90 vol.% within the usual kerosene range of 145 C
to 300 C
(ASTM D86), depending on grade and use. It will typically have an initial
boiling point in
the range 130 C to 190 C, and a final boiling point in the range 220 C to 300
C. It will
typically have a density from 775 to 840 kg/m3 at 15 C (e.g., ASTM D4052 or IP
365).
Its kinematic viscosity at -20 C (ASTM D445) might suitably be from 1.2 to 8.0
mm2/s.
The kerosene base fuel or kerosene range hydrocarbon component may be a
straight
run kerosene fraction as isolated by distillation from a crude oil source or a
kerosene
fraction isolated from the effluent of typical refinery conversion processes,
preferably
hydrocracking. The kerosene fraction may also be the blend of straight run
kerosene and
kerosene as obtained in a hydrocracking process. Suitably the properties of
the mineral
derived kerosene are those of the desired jet fuel as defined above.
9
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
Aromatic content of the kerosene base fuel may vary in the range of 0 to 25
vol.%,
preferably 3 to 25 vol.%, more preferably 15 to 20 vol.% based on the fuel (as
measured by
ASTM 1319). Typical density of the petroleum-derived kerosene at 15 C is in
the range of
775 kg/m3 to 840 kg/m3 (as measured by D4052). The kerosene base fuel most
useful for
the inventive process may have a density of at least 760 kg/m3, more
preferably at least 775
kg/m3, to preferably at most 840 kg/m3, and more preferably at most 820 kg/m3.
The
aromatic content of the kerosene range hydrocarbon component for liquid rocket
fuel may
vary in the range of 0 to 10 vol.%, preferably 0 to 5 vol.%.
The kerosene base fuel may be a single stream from a refining stream
(petroleum-
derived kerosene), or a mixture of one or more refining streams, or a mixture
of refining
streams and one or more synthetic kerosene components, or one or more
synthetic
kerosene streams (other than the synthetic cyclo-paraffinic blending
component) approved
by ASTM D7566 or equivalent specifications.
For Example, kerosene range hydrocarbon component may be aliphatic mineral
spirits having flash points in the range of 60 C up to 120 C, preferably 63 C
up to 120 C.
Preferably, the aliphatic mineral spirits also have density at 15 C from 790
to 820 kg/m3.
These aliphatic mineral spirits are typically mixtures of normal-, iso- and
cyclo-paraffins.
Aliphatic mineral spirits are fractionated from selected feedstock. Their low
aromatics
content is obtained by deep hydrogenation. Commercially available kerosene
range
hydrocarbon component may include She11S01TM D (de-aromatised) grades
available from
Shell Chemical Co. such as for example, ShellSol D60, D70, D80, D90 and D100
or
suitably fractionated aliphatic mineral spirits having flash points in the
appropriate range.
Other aliphatic mineral spirits such as IsoparTM isoparaffinic fluids or
NORPARTM fluids
may be used. Kerosene range hydrocarbon component may also be kerosene base
fuel so
long as it can meet the kerosene range hydrocarbon component properties and
the final
blend can meet the rocket fuel specifications.
Synthetic Cyclo-paraffinic Kerosene Fuel Blending Component
The synthetic cyclo-paraffinic kerosene fuel blending component is generally
characterized as a liquid composed of individual hydrocarbons useable as a jet
fuel
blending component and having at least the following properties: comprising at
least 99.5
mass% of carbon and hydrogen content and at least 50 mass% of cyclo-paraffin.
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
For jet fuel applications, the cyclo-paraffinic kerosene fuel blending
component can
typically have a boiling point of at most 300 C, at atmospheric pressure;
flash point of
38 C, or above; a density at 15 C of at least 800 kg/m3, preferably at least
810 kg/m3,
preferably at most 845 kg/m3, more preferably at most 830 kg/m3, most
preferably in the
range of 810 to 818 kg/m3; and a freezing point of -60 C or below, preferably
of -65 C or
below, more preferably of -70 C or below.
For rocket fuel applications, preferably the synthetic cyclo-paraffinic
kerosene fuel
blending component is generally characterized as a liquid composed of
individual
hydrocarbons useable as a rocket fuel blending component and having at least
the
following properties: comprising at least 99.5 mass% of carbon and hydrogen
content and
at least 50 mass% of cyclo-paraffin. The cyclo-paraffinic kerosene fuel
blending
component can typically have a flash point of at least 38 C, preferably at
least 45 C,
preferably at least 50 C, more preferably at least 55 C, more preferably at
least 60 C; a
density at 15 C of at least 799 kg/m3; and a freezing point of -60 C or lower,
preferably of
-65 C or lower, more preferably of -70 C or lower. Further, the cyclo-
paraffinic kerosene
fuel blending component can have good thermal stability for use in rocket
fuel. The
cyclo-paraffinic kerosene fuel blending component typically has a final
boiling point below
300 C, more preferably below 290 C, more preferably below 280 C, most
preferably
below 274 C.
The synthetic cyclo-paraffinic kerosene fuel blending component preferably has
a
maximum iso-paraffin and n-paraffin content of less than 50 mass%, preferably
less than
40 mass%, less than 35 mass%, or less than 30 mass% (ASTM D2425 or optionally
can be
measured by GCxGC). The synthetic cyclo-paraffinic kerosene fuel blending
component
preferably has at least 60 mass%, at least 65 mass%, or at least 70 mass% of
cyclo-
paraffinic content (ASTM 1)2425 or optionally can be measured by GCxGC). The
aromatic content of the synthetic cyclo-paraffinic kerosene fuel blending
component is
preferably at most 1.5 mass%, at most 1 mass%, or at most 0.5 mass%. (ASTM
D2425 or
optionally can be measured by GCxGC).
In certain embodiments, the synthetic cyclo-paraffinic kerosene fuel blending
component is derived from biomass (bio-derived cyclo-paraffinic kerosene fuel
blending
component). As used herein, the term "biomass" refers to, without limitation,
organic
materials produced by plants (such as leaves, roots, seeds and stalks), and
microbial and
animal metabolic wastes. Common biomass sources include: (1) agricultural
residues,
11
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
including corn stover, straw, seed hulls, sugarcane leavings, bagasse,
nutshells, cotton gin
trash, and manure from cattle, poultry, and hogs; (2) wood materials,
including wood or
bark, sawdust, timber slash, and mill scrap; (3) municipal solid waste,
including recycled
paper, waste paper and yard clippings; and (4) energy crops, including
poplars, willows,
switch grass, miscanthus, sorghum, alfalfa, prairie bluestream, corn, soybean,
and the like.
The term also refers to the primary building blocks of the above, namely,
lignin, cellulose,
hemicellulose and carbohydrates, such as saccharides, sugars and starches,
among others.
Common biomass-derived feedstocks include lignin and lignocellulosic
derivatives,
cellulose and cellulosic derivatives, hemicellulose and hemicellulosic
derivatives,
carbohydrates, starches, monosaccharides, disaccharides, polysaccharides,
sugars, sugar
alcohols, alditols, polyols, and mixtures thereof. Preferably, the biomass-
derived feedstock
is derived from material of recent biological origin such that the age of the
compounds, or
fractions containing the compounds, is less than 100 years old, preferably
less than 40
years old, and more preferably less than 20 years old, as calculated from the
carbon 14
concentration of the feedstock.
The biomass-derived feedstocks may be derived from biomass using any known
method. Solvent-based applications are well known in the art. Organosolv
processes use
organic solvents such as ionic liquids, acetone, ethanol, 4-methyl-2-
pentanone, and solvent
mixtures, to fractionate lignocellulosic biomass into cellulose,
hemicellulose, and lignin
streams (Paszner 1984; Muurinen 2000; and Bozell 1998). Strong-acid processes
use
concentrated hydrochloric acid, phosphoric acid, sulfuric acid or other strong
organic acids
as the depolymerization agent, while weak acid processes involve the use of
dilute strong
acids, acetic acid, oxalic acid, hydrofluoric acid, or other weak acids as the
solvent.
Enzymatic processes have also recently gained prominence and include the use
of enzymes
as a biocatalyst to deconstruct the structure of the biomass and allow further
hydrolysis to
useable feedstocks. Other methods include fermentation technologies using
microorganisms, Fischer-Tropsch reactions and pyrolysis technologies, among
others.
In one embodiment, the synthetic cyclo-paraffinic kerosene fuel blending
component is derived from the conversion of a biomass-derived feedstock
containing one
or more carbohydrates, such as starch, monosaccharides, disaccharides,
polysaccharides,
sugars, and sugar alcohols, or derivatives from lignin, hemicellulose and
cellulose using a
bioreforming processes. As used herein, the term "bioreforming" refers to,
without
limitation, processes for catalytically converting biomass-derived oxygenated
12
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
hydrocarbons to lower molecular weight hydrocarbons and oxygenated compounds
using
aqueous phase reforming, hydrogenation, hydrogenolysis, hydrodeoxygenation
and/or
other conversion processes involving the use of heterogeneous catalysts.
Examples of
various bioreforming processes include those technologies described in U.S.
Patent Nos.
8053615, 8017818 and 7977517 (all to Cortright and Blommel, and entitled
"Synthesis of
Liquid Fuels and Chemicals from Oxygenated Hydrocarbons"); U.S. Patent No.
8642813
(to Qiao et al., and entitled "Reductive Biomass Liquefaction"); U.S. Patent
Application
Publication No. 2012/0198760 (to Blommel et al., and entitled Methods and
Systems for
Making Distillate Fuels from Biomass); and U.S. Patent Application Publication
No.
2013/0263498 (to Kania et al., and entitled Production of Distillate Fuels
from Biomass-
Derived Polyoxygenates); and U.S. Patent Application Pub. No. 2013/0036660 (to
Woods
et al. and entitled "Production of Chemicals and Fuels from Biomass"), all of
which are
incorporated herein by reference.
Alternatively, the synthetic cyclo-paraffinic kerosene fuel blending component
may
be produced using natural gas or syngas-derived feedstocks used in a
bioreforming process.
For example, certain alkanols and other mixed oxygenated hydrocarbons derived
from
natural gas or syngas using Fischer-Tropsch type reactions may have
application in the
above described bioreforming processes, and can be used as a feedstock to
provide the
synthetic cyclo-paraffinic kerosene fuel blending component of the present
invention.
In its application, a bioreforming process is used to convert oxygenated
hydrocarbons to an intermediate stream of mixed oxygenates, with the resulting
mixed
oxygenates subsequently converted to C8+ compounds containing the desired
synthetic
cyclo-paraffinic kerosene fuel blending component. Examples of various
oxygenated
hydrocarbons include any one or more sugars, such as glucose, fructose,
sucrose, maltose,
lactose, mannose or xylose, or sugar alcohols, such as arabitol, erythritol,
glycerol, isomalt,
lactitol, malitol, mannitol, sorbitol, xylitol, arabitol, glycol, and other
oxygenated
hydrocarbons. Additional non-limiting examples of oxygenated hydrocarbons
include
various alcohols, ketones, aldehydes, furans, hydroxy carboxylic acids,
carboxylic acids,
diols and triols.
The oxygenated hydrocarbons are reacted in an aqueous solution with hydrogen
over a deoxygenation catalyst to produce a stream of mixed oxygenates. The
oxygenates
will generally include, without limitation, oxygenated hydrocarbons having 1
to 4 oxygen
atoms (e.g., mono-, di-, tri- and tetra-oxygenated hydrocarbons). The mono-
oxygenated
13
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
hydrocarbons typically include alcohols, ketones, aldehydes, cyclic ethers,
furans, and
pyrans, while the di-oxygenated hydrocarbons typically include diols, hydroxy
ketones,
lactones, furfuryl alcohols, pyranyl alcohols, and carboxylic acids.
The deoxygenation catalyst is a heterogeneous catalyst having one or more
active
materials capable of catalyzing a reaction between hydrogen and the oxygenated
hydrocarbons to remove one or more of the oxygen atoms from the oxygenated
hydrocarbon to produce the oxygenates described above. The active materials
may
include, without limitation, Cu, Re, Fe, Ru, Ir, Co, Rh, Pt, Pd, Ni, W, Os,
Mo, Ag, Au,
alloys and combinations thereof, adhered to a support. The deoxygenation
catalyst may
include these elements alone or in combination with one or more Mn, Cr, Mo, W,
V, Nb,
Ta, Ti, Zr, Y, La, Sc, Zn, Cd, Ag, Au, Sn, Ge, P, Al, Ga, In, Tl, Ce and
combinations
thereof. The support may be any one of a number of supports, including a
support having
carbon, silica, alumina, zirconia, titania, tungsten, vanadia, chromia,
zeolites,
heteropolyacids, kieselguhr, hydroxyapatite, and mixtures thereof. The
deoxygenation
catalyst may also include an acidic support modified or constructed to provide
a desired
functionality. Heteropolyacids are a class of solid-phase acids exemplified by
such species
as H3+xPMo/2_xVxO4o, H4SiW12040, H3PW12040, and H6P2W18062, and have a well-
defined
local structure, the most common of which is the tungsten-based Keggin
structure.
To produce oxygenates, a stream of oxygenated hydrocarbons is combined with
water to provide an aqueous feedstock solution. The feedstock solution is then
reacted
with hydrogen in the presence of the deoxygenation catalyst at deoxygenation
temperature
and pressure conditions, and weight hourly space velocity, effective to
produce the desired
oxygenates. In condensed phase liquid reactions, the pressure within the
reactor must be
sufficient to maintain the reactants in the condensed liquid phase at the
reactor inlet. For
liquid phase reactions, the reaction temperature may be from about 80 C to 300
C, and the
reaction pressure from about 72 psig to 1300 psig. For vapor phase reactions,
the reaction
should be carried out at a temperature where the vapor pressure of the
oxygenated
hydrocarbon is at least about 0.1 atm (and preferably a good deal higher), and
the
thermodynamics of the reaction are favorable. This temperature will vary
depending upon
the specific oxygenated hydrocarbon compound used, but is generally in the
range of from
about 100 C to 600 C for vapor phase reactions.
The synthetic cyclo-paraffinic kerosene fuel blending component is
subsequently
produced using an acid condensation catalyst and a reactant stream that
includes the mixed
14
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
oxygenate stream above as a first reactant and a second reactant having an
average oxygen
to carbon ratio of 0.2 or less, in the presence of water. The first reactant
(i.e., the mixed
oxygenates produced above) can be generally described as having the formula
C,,HyOz,
with x representing 2 to 12 carbon atoms and z representing 1 to 12 oxygen
atoms, and an
average oxygen to carbon ratio of between 0.2 and 1Ø Collectively, the
average oxygen
to carbon ratio of the first reactant should be about 0.2 to 1.0, calculated
as the total
number of oxygen atoms (z) in the oxygenates of the first reactant divided by
the total
number of carbon atoms (x) in the oxygenates of the first reactant.
Alternatively, the first
reactant may have an average oxygen content per molecule of about 1 to 4,
calculated as
the total number of oxygen atoms (z) in the oxygenates of the first reactant
divided by the
total number of molecules of oxygenates in the first reactant. The total
number of carbon
atoms per molecule, oxygen atoms per molecule and total molecules in the first
reactant
may be measured using any number of commonly known methods, including (1)
speciation
by gas chromatography (GC), high performance liquid chromatography (HPLC), and
other
methods known to the art and (2) determination of total oxygen, carbon, and
water content
by elemental analysis. Oxygen present in water, carbon dioxide, or carbon
monoxide is
excluded from the determination of reactant oxygen to carbon ratio.
The second reactant includes one or more hydrocarbons and/or oxygenated
hydrocarbons having a general formula Cp1-1,0s, with E representing 2 to 7
carbon atoms
and s representing 0 to 1 oxygen atoms. When the second reactant is derived
from a
recycle stream as described below, the second reactant may also contain
residual
oxygenated hydrocarbons containing 2 oxygen atoms. Collectively, the average
oxygen to
carbon ratio of the second reactant should be less than 0.2, calculated as the
total number of
oxygen atoms (s) in the oxygenated hydrocarbons of the second reactant divided
by the
total number of carbon atoms (2) in the hydrocarbons and oxygenated
hydrocarbons of the
second reactant. Alternatively, the second reactant may have an average oxygen
per
molecule ratio of less than 1.5, calculated as the total number of oxygen
atoms (s) in the
oxygenated hydrocarbons of the second reactant divided by the total number of
molecules
of hydrocarbons and oxygenated hydrocarbons in the second reactant. The second
reactant
may also be characterized as having an average normal boiling point of less
than 210 C,
or less than 200 C, or less than 190 C.
The second reactant will generally include C7_ alkanes, C7_ alkenes, C7_
cycloalkanes, C7_ cycloalkenes, C7_ alcohols, C7_ ketones, C7_ aryls, and
mixtures thereof.
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
Examples of the second reactant compounds include, without limitation, C7_
alkanes and
C7_ alkenes having from 4 to 7 carbon atoms (C4_7 alkanes and C4_7 alkenes),
such as
butane, iso-butane, butene, isobutene, pentane, pentene, 2-methylbutane,
hexane, hexene,
2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, 2,3-dimethylbutane,
cyclohexane,
heptane, heptene, methyl-cyclohexane and isomers thereof. The C7- aryls will
generally
consist of an aromatic hydrocarbon having 6 or 7 carbon atoms, whether in
either an
unsubstituted (phenyl), mono-substituted or multi-substituted form. The C7_
cycloalkanes
and C7- cycloalkenes have 5, 6 or 7 carbon atoms and may be unsubstituted,
mono-
substituted or multi-substituted. In the case of mono-substituted and multi-
substituted
compounds, the substituted group may include straight chain C1_2 alkyls,
straight chain C2
alkylenes, straight chain C2 alkynes, or combinations thereof. Examples of
desirable C7_
cycloalkanes and C7_ cycloalkenes include, without limitation, cyclopentane,
cyclopentene,
cyclohexane, cyclohexene, methyl-cyclopentane, methyl-cyclopentene, ethyl-
cyclopentane,
ethyl-cyclopentene, and isomers thereof.
The second reactant may be provide from any source, but is preferably derived
from biomass or a biomass-derived feedstock. For example, although a biomass-
derived
feedstock is preferred, it is contemplated that all or a portion of the second
reactant may
originate from fossil fuel based compounds, such as natural gas or petroleum.
All or a
portion of the second reactant may also originate from any one or more
fermentation
technologies, gasification technologies, Fischer- Trop sch reactions, or
pyrolysis
technologies, among others. Preferably, at least a portion of the second
reactant is derived
from the product stream and recycled to be combined with the first reactant to
provide at
least a portion of the reactant stream.
When a portion of the second reactant is derived from the product stream
following
the condensation reaction, the product stream is separated into a first
portion containing
C8+ compounds and a second portion containing C7_ compounds to be recycled and
used as
a portion of the second reactant. Alternatively, the product stream may be
first separated to
a water fraction and an organic fraction, with the organic fraction then
separated into a first
portion containing the desired C8+ compounds and a second portion containing
the C7_
compounds to be recycled and used as a portion of the second reactant.
Processes for
separating liquid mixtures into their component parts or fractions are
commonly known in
the art, and often involve the use of a separator unit, such as one or more
distillation
columns, phase separators, extractors, purifiers, among others.
16
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
The condensation reaction is performed using catalytic materials that exhibit
acidic
activity. These materials may be augmented through the addition of a metal to
allow
activation of molecular hydrogen for hydrogenation/dehydrogenation reactions.
The acid
condensation catalyst may be either an acidic support or an acidic
heterogeneous catalyst
comprising a support and an active metal, such as Pd, Pt, Cu, Co, Ru, Cr, Ni,
Ag, alloys
thereof, or combinations thereof. The acid condensation catalyst may include,
without
limitation, aluminosilicates, tungstated aluminosilicates, silica-alumina
phosphates
(SAP05), aluminum phosphates (ALPO), amorphous silica alumina (ASA), acidic
alumina, phosphated alumina, tungstated alumina, zirconia, tungstated
zirconia, tungstated
silica, tungstated titania, tungstated phosphates, acid modified resins,
heteropolyacids,
tungstated heteropolyacids, silica, alumina, zirconia, titania, tungsten,
niobia, zeolites,
mixtures thereof, and combinations thereof. The acid condensation catalyst may
include
the above alone or in combination with a modifier or metal, such as Re, Cu,
Fe, Ru, Ir, Co,
Rh, Pt, Pd, Ni, W, Os, Mo, Ag, Au, alloys thereof, and combinations thereof.
Examples of applicable acidic condensation catalysts include bifunctional
pentasil
zeolites, such as ZSM-5, ZSM-8 or ZSM-11. The zeolite with ZSM-5 type
structure is a
particularly preferred catalyst. Other suitable zeolite catalysts include ZSM-
12, ZSM-22,
ZSM-23, ZSM-35 and ZSM-48. Zeolite ZSM-5, and the conventional preparation
thereof,
is described in U.S. Patent Nos. 3702886, Re. 29,948 (highly siliceous ZSM-5),
4100262
and 4139600, all incorporated herein by reference. Zeolite ZSM-11, and the
conventional
preparation thereof, is described in U.S. Patent No. 3709979, which is also
incorporated
herein by reference. Zeolite ZSM-12, and the conventional preparation thereof,
is
described in U.S. Patent No. 3832449, incorporated herein by reference.
Zeolite ZSM-23,
and the conventional preparation thereof, is described in U.S. Patent No.
4076842,
incorporated herein by reference. Zeolite ZSM-35, and the conventional
preparation
thereof, is described in U.S. Patent No. 4016245, incorporated herein by
reference.
Another preparation of ZSM-35 is described in U.S. Patent No. 4107195, the
disclosure of
which is incorporated herein by reference. ZSM-48, and the conventional
preparation
thereof, is taught by U.S. Patent No. 4375573, incorporated herein by
reference. Other
examples of zeolite catalysts are described in U.S. Patent No. 5019663 and
U.S. Patent
7022888, also incorporated herein by reference.
The specific C8+ compounds produced will depend on various factors, including,
without limitation, the make-up of the reactant stream, the type of oxygenates
in the first
17
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
reactant, the hydrocarbons and oxygenated hydrocarbons in the second reactant,
the
concentration of the water, condensation temperature, condensation pressure,
the reactivity
of the catalyst, and the flow rate of the reactant stream as it affects the
space velocity (the
mass/volume of reactant per unit of catalyst per unit of time), gas hourly
space velocity
(GHSV), and weight hourly space velocity (WHSV). The condensation temperature
and
pressure conditions may be selected to more favorably produce the desired
products in the
vapor-phase or in a mixed phase having both a liquid and vapor phase. In
general, the
condensation reaction should be conducted at a temperature and pressure where
the
thermodynamics of the reactions are favorable. In general, the condensation
temperature
should be between 100 C and 400 C and the reaction pressure between 72 psig
and 2000
psig.
The above condensation reactions result in the production of C8+ alkanes, C8+
alkenes, C8+ cycloalkanes, C8+ cycloalkenes, C8+ aryls, fused aryls, C8+
alcohols, C8+
ketones, oxygenated C8+ aryls, oxygenated fused aryls, and mixtures thereof.
The C8+
alkanes and C8+ alkenes have 8 or more carbon atoms, and may be branched or
straight
chained alkanes or alkenes. The C8+ alkanes and C8+ alkenes may also include
fractions
containing Cs, C9, Cm, C11, C12, C13, C14 compounds (C8_14 fraction), or C12,
C13, C14, C15,
C16, C17, C18, C19, C20, C21, C22, C23, C24 Compounds (C12-24 fraction), or
more than 25
carbon atoms (C25+ fraction), with the C8-14 fraction directed to the
synthetic cyclo-
paraffinic kerosene fuel blending component, the C12-24 fraction directed to
diesel fuel, and
the C25+ fraction directed to heavy oils and other industrial applications.
Examples of
various C8+ alkanes and C8+ alkenes include, without limitation, octane,
octene, 2,2,4,-
trimethylpentane, 2,3-dimethyl hexane, 2,3,4-trimethylpentane, 2,3-
dimethylpentane,
nonane, nonene, decane, decene, undecane, undecene, dodecane, dodecene,
tridecane,
tridecene, tetradecane, tetradecene, pentadecane, pentadecene, hexadecane,
hexadecane,
heptyldecane, heptyldecene, octyldecane, octyldecene, nonyldecane,
nonyldecene,
eicosane, eicosene, uneicosane, uneicosene, doeicosane, doeicosene,
trieicosane,
trieicosene, tetraeicosane, tetraeicosene, and isomers thereof.
The C8+ cycloalkanes and C8+ cycloalkenes have 8 or more carbon atoms and may
be unsubstituted, mono-substituted or multi-substituted. In the case of mono-
substituted
and multi-substituted compounds, the substituted group may include a branched
C3+ alkyl,
a straight chain Ci4 alkyl, a branched C3+ alkylene, a straight chain C2+
alkylene, a straight
chain C2+ alkyne, a phenyl or a combination thereof. In one embodiment, at
least one of
18
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
the substituted groups include a branched C3+ alkyl, a straight chain Ci+
alkyl, a branched
C3+ alkylene, a straight chain C2+ alkylene, a straight chain C2+ alkyne, a
phenyl or a
combination thereof. Examples of desirable Cg+ cycloalkanes and Cg+
cycloalkenes
include, without limitation, ethyl-cyclopentane, ethyl-cyclopentene, ethyl-
cyclohexane,
ethyl-cyclohexene, and isomers thereof.
The Cg+ product compounds may also contain high levels of alkenes, alcohols
and/or ketones, which may be undesirable in certain fuel applications or which
lead to
coking or deposits in combustion engines, or other undesirable combustion
products. In
such event, the Cg+ compounds may undergo a finishing step. The finishing step
will
generally involve a hydrotreating reaction that removes a portion of the
remaining carbon-
carbon double bonds, carbonyl, hydroxyl, acid, ester, and ether groups.
The moderate fractions above (Cs-Cis) may be separated for use as the
synthetic
cyclo-paraffinic kerosene fuel blending component, while the C12-C24 fraction
may be
separated for diesel fuel, and the heavier fraction (C25+) separated for use
as a heavy oil or
cracked to produce additional gasoline and/or diesel fractions. A C12-Cis
fraction can also
be separated for rocket fuel applications. Separation processes are well known
in the art
and generally involve one or more distillation columns designed to facilitate
the separation
of desired compounds from a product stream. The distillation will be generally
operated at
a temperature, pressure, reflux ratio, and with an appropriate equipment
design, to recover
the portion of the Cg+ compounds which conform to the boiling point
characteristics of the
synthetic cyclo-paraffinic kerosene fuel blending component as described
above.
Additional Propulsion Fuel Blending Component
The additional propulsion fuel blending component may be any fuel blending
component which can be considered a kerosene base fuel as described above. The
additional propulsion fuel blending component may also be naphtha generally
used for
blending to manufacture Jet B fuel.
Other Components
Optionally, the fuel composition may further comprise a fuel additive known to
a
person of ordinary skill in the art. In certain embodiments, the fuel additive
can be used
from about 0.00005% by weight to about 0.20 % by volume, based on the total
weight or
volume of the fuel composition. The fuel additive can be any fuel additive
approved for
use in jet fuel or rocket fuel known to those of skill in the art. In further
embodiments, the
fuel additive may be antioxidants, thermal stability improvers, lubricity
improvers, fuel
19
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
system icing inhibitors, metal deactivators, static dissipaters, other
aviation-approved
additives and combinations thereof.
The amount of a fuel additive in the fuel composition disclosed herein may be
from
about 0.00005% by weight to less than about 0.20% by volume, based on the
total amount
of the fuel composition. In some embodiments, the amount is in wt.% based on
the total
weight of the fuel composition. In other embodiments, the amount is in vol.%
based on the
total volume of the fuel composition. In yet other embodiments, the amount is
in mass per
volume of the fuel composition. The amount will normally be within limits
mandated or
recommended within the appropriate fuel specification.
Illustrative examples of fuel additives are described in greater detail below.
Lubricity improvers are one example. They were first used in aviation fuels as
corrosion
inhibitors to protect ferrous metals in fuel handling systems, such as
pipelines and fuel
storage tanks, from corrosion. It was discovered that they also provided
additional
lubricity performance, reducing the wear in components of the aircraft engine
fuel system,
such as gear pumps and splines, where thin fuel layers separate moving metal
components.
Nowadays, these additives are only used for lubricity improvement. The
lubricity
improver may be present in the fuel composition at a concentration up to about
23 mg/L,
based on the total volume of the fuel composition, and in accordance with jet
fuel
specification limits.
Antioxidants can also be used herein. Antioxidants prevent the formation of
gum
depositions on fuel system components caused by oxidation of fuels in storage
and/or
inhibit the formation of peroxide compounds in certain fuel compositions. The
antioxidant
may be present in the fuel composition at a concentration up to 24 mg/L, based
on the total
volume of the fuel composition.
Static dissipaters reduce the effects of static electricity generated by
movement of
fuel through high flow-rate fuel transfer systems. The static dissipater may
be present in
the fuel composition at a concentration up to about 5 mg/L, based on the total
volume of
the fuel composition.
Fuel system icing inhibitors (also referred to as anti-icing additives) reduce
the
freezing point of water precipitated from jet fuels due to cooling at high
altitudes and
prevent the formation of ice crystals which could restrict the flow of fuel to
the engine.
Certain fuel system icing inhibitors can also act as a biocide. The fuel
system icing
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
inhibitor may be present intentionally in the fuel composition at a
concentration from about
0.02 to about 0.2 volume %, based on the total volume of the fuel composition.
Metal deactivators suppress the catalytic effect that some metals,
particularly copper,
have on fuel oxidation. The metal deactivator may be present in the fuel
composition at a
concentration up to about 5.7 mg/L active matter, based on the total volume of
the fuel
composition.
Thermal stability improvers are used to inhibit deposit formation in the high
temperature areas of the aircraft fuel system. The thermal stability improver
may be
present in the fuel composition at a concentration up to about 256 mg/L, based
on the total
volume of the fuel composition.
Blending and Using
In certain embodiments, volumetric energy content of a jet fuel can be
increased with
minimal increase of the aromatic content of the fuel. By the term minimal
increase of
aromatic content, typically the increase in aromatic content is less than 2
vol%, preferably
less than 1.5 vol%, or preferably without an increase that is within the
precision of
measurement for aromatic content, or preferably even decreasing, based on the
jet fuel.
Higher volumetric energy content is usually associated with higher aromatics.
Thus, it is
unexpected to increase the volumetric energy content of a fuel without an
increase in its
aromatic content.
A quantity of kerosene base fuel as described above (which is different or
other than
cyclo-paraffinic kerosene fuel blending component) may be blended with a
quantity of the
synthetic cyclo-paraffinic kerosene fuel blending component in an amount
effective or
sufficient to increase the volumetric energy content of the final blended fuel
compared to
the kerosene base fuel, preferably at least 0.1% increase in the volumetric
energy content
as calculated from the Net Heat of Combustion estimated by ASTM D3338 and
multiplied
by density.
In some embodiments, the smoke point of the blended fuel may also increase
compared with the kerosene base fuel.
Optionally, the blended fuel may be blended with an additional propulsion fuel
blending component to produce the kerosene-based propulsion fuel.
The propulsion fuel may be blended at refineries or terminals, in tankers, or
at the
location of application, as well as at any other location that may have
blending capabilities.
Various methods and equipment required for such blending activities are
commonly known
21
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
in the art, and may be applied as needed depending on the particular
propulsion fuel
desired.
The amount of the synthetic cyclo-paraffinic kerosene fuel blending component
may
suitably be in an amount of 1 to 97 vol.%, preferably 3 to 97 vol.%,
preferably 5 to 97
vol.%, more preferably 10 to 97 vol.%, more preferably 15 to 97 vol.% provided
that the
amount is sufficient to increase volumetric energy content at least 0.1%. The
amount may
vary depending on the kerosene base fuel and/or the desired specification to
upgrade to
and/or amount of desired volumetric energy content increase desired. The
amount of the
synthetic cyclo-paraffinic kerosene fuel blending component of the blend is
preferably at
least 1 vol.%, preferably at least 3 vol.%, more preferably at least 5 vol.%,
more preferably
at least 10 vol.%, more preferably at least 15 vol.%, based on the blended
fuel. The
amount of the synthetic cyclo-paraffinic kerosene fuel blending component will
vary
depending on the kerosene base fuel used.
The kerosene base fuel may be upgraded to meet Jet A-1 specification or JP-8
specification (e.g., when the kerosene base fuel has a freezing point of above
-47 C) by
blending the synthetic cyclo-paraffinic kerosene fuel blending component in an
amount
effective or sufficient to lower the freezing point of the blended fuel to -47
C or lower.
For example, Jet A or F-24 jet fuel may be upgraded to meet Jet A-1 or JP-8
specification
in such manner.
In some embodiments, the kerosene base fuel may be upgraded to meet AN-8
specification (e.g., when the kerosene base fuel has a freezing point of above
-58 C) by
blending the synthetic cyclo-paraffinic kerosene fuel blending component in an
amount
effective or sufficient to lower the freezing point of the blended fuel to -58
C or lower.
For example, any one of Jet A, F-24, Jet A-1, JP-8, or JP-5 jet fuel may be
upgraded to
meet Jet AN-8 specification.
In some embodiments, the kerosene base fuel is upgraded to meet Jet A
specification
(e.g., when the kerosene base fuel have a freezing point of above -40 C) by
blending the
synthetic cyclo-paraffinic kerosene fuel in an amount effective or sufficient
to lower the
freezing point of the blended fuel to -40 C or lower. For example, refinery
streams,
synthetic fuel streams and mixtures thereof that have a freezing point of
above -40 C
and/or have a density of at least 760 kg/m3 may be upgraded to meet Jet A
specification.
In certain embodiments, a kerosene fuel can be upgraded to meet Jet A-1
specification or JP-8 specification by;
22
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
a.
providing a quantity of kerosene base fuel having a boiling point in the
range of 130 C to 300 C, at atmospheric pressure, flash point of 38 C or above
measured
by above measured by ASTM D56, a density at 15 C of at least 775 kg/m3 and
freezing
point of above -47 C;
b. providing a
quantity of synthetic cyclo-paraffinic kerosene fuel blending
component described above; and
c.
blending a quantity of the synthetic cyclo-paraffinic kerosene fuel blending
component to the kerosene base fuel in amount sufficient to lower the freezing
point of the
blended fuel to -47 C or lower.
In certain embodiments, a kerosene fuel can be upgraded to meet AN-8
specification
by;
a. providing a quantity of kerosene base fuel having a boiling point in the
range of 130 C to 300 C, at atmospheric pressure, flash point of 38 C or above
measured
by ASTM D56, and a density at 15 C of at least 775 kg/m3 and freezing point of
above -58 C;
b. providing a quantity of synthetic cyclo-paraffinic kerosene fuel
blending
component described above; and
c. blending a quantity of the synthetic cyclo-paraffinic kerosene fuel
blending
component to the kerosene base fuel in amount sufficient to lower the freezing
point of the
blended fuel to -58 C or lower.
In certain embodiments, a kerosene fuel can be upgraded to meet Jet A
specification
by;
a. providing a quantity of kerosene base fuel having a boiling point in the
range of 130 C to 300 C, at atmospheric pressure, flash point of 38 C or above
measured
by ASTM D56, a density at 15 C of at least 760 kg/m3 and freezing point of
above -40 C;
b. providing a quantity of synthetic cyclo-paraffinic kerosene fuel
blending
component described above; and
c. blending a quantity of the synthetic cyclo-paraffinic kerosene fuel
blending
component to the kerosene base fuel in amount sufficient to lower the freezing
point of the
blended fuel to -40 C or lower.
In some embodiments, the blended jet fuel may preferably have a density of
equal or
above 800 kg/m3. The blended jet fuel may preferably have an aromatic content
of less
than or equal to 25 vol%, more preferably less than or equal to 20 vol%.
23
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
In some embodiments, the inventive method may be used to meet any of the
standard
specifications for Aviation Turbine fuels described above.
The increase in volumetric energy content and/or smoke point increase can be
seen
by operating a jet engine comprising burning the jet fuel produced by the
method described
above in such jet engine.
In another aspect, a fuel system is provided comprising a fuel tank containing
the
fuel composition produced by the methods described above. Optionally, the fuel
system
may further comprise an engine cooling system having a recirculating engine
coolant, a
fuel line connecting the fuel tank with the internal combustion engine, and/or
a fuel filter
arranged on the fuel line. Some non-limiting examples of internal combustion
engines
include reciprocating engines (e.g., diesel engines), jet engines, some rocket
engines, and
gas turbine engines.
In some embodiments, the fuel tank is arranged with a cooling system so as to
allow heat transfer from the recirculating engine coolant to the fuel
composition contained
in the fuel tank. In other embodiments, the fuel system further comprises a
second fuel
tank containing a second fuel for a jet engine and a second fuel line
connecting the second
fuel tank with the engine. Optionally, the first and second fuel lines can be
provided with
electromagnetically operated valves that can be opened or closed independently
of each
other or simultaneously.
In another aspect, an engine arrangement is provided comprising an internal
combustion engine, a fuel tank containing the fuel composition disclosed
herein, a fuel line
connecting the fuel tank with the internal combustion engine. Optionally, the
engine
arrangement may further comprise a fuel filter and/or an engine cooling system
comprising
a recirculating engine coolant. In some embodiments, the internal combustion
engine is a
jet engine.
The smoke point increase can be seen by burning the jet fuel produced by the
methods described above by providing the jet fuel to the fuel system andlor
jet engine and
operating such fuel system and/or jet engine.
Rocket fuel can be used in a rocket engine system that includes a combustion
chamber, an oxidizer supply, a fuel delivery circuit connected to a fuel
supply, a faceplate
having a plurality of openings therethrough, and an injector assembly
positioned at the
combustion chamber. Such a system is described, for example, in U.S. Patent
No.
7685807 and U.S. Patent No. 7827781.
24
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
A liquid rocket fuel useful to meet RP-1 or RP-2 grade rocket fuels may be
produced by blending a quantity of the synthetic cyclo-paraffinic kerosene
fuel blending
component and a quantity of the kerosene range hydrocarbon component in amount
sufficient to meet a flash point of at least 60 C and a final boiling point of
274 C or lower.
The blended rocket fuel preferably have a freezing point of -51 C or below, a
flash point of
at least 60 C, a density in the range of 799 ¨ 815 kg/m3 at 15 C, and a
volumetric energy
density in the range of 34,380 - 35,070 MJ/m3. The blended rocket fuel can
also have a
hydrogen content of at least 13.8 mass%. In one embodiment, the net heat of
combustion
of the blended rocket fuel is at least 43.03 MJ/kg. The blended rocket fuel
can also have a
sulfur content of no more than 0.0030 mass%. The sulfur requirement for RP-4
is 0.0030
mass% or below and RP-2 0.00001 mass% or below by ASTM D-5623. The liquid
rocket
fuel may also be blended to meet a thermal stability requirement at a
temperature of at least
355 C. The preferable amount of synthetic cyclo-paraffinic kerosene fuel
blending
component in the final liquid rocket fuel is at least 1
preferably at least 3 vol.%,
preferably at least 5 vol.%, preferably at least 10 vol.%, preferably at least
15 vol.%,
preferably at least 20 vol.%, preferably at least 25 vol.%, or more preferably
at least 30
vol.%, based on the final rocket fuel blend. The preferable amount of
synthetic cyclo-
paraffinic kerosene fuel blending component in the final liquid rocket fuel is
at most 97
vol.%, preferably at most 95 vol.%, preferably at most 90 vol.%, preferably at
most 85
vol.%, preferably at most 80 vol.%, or more preferably at most 75 vol.%, based
on the final
rocket fuel blend.
By blending a quantity of the synthetic cyclo-paraffinic kerosene fuel
blending
component and a quantity of the kerosene range hydrocarbon component, it was
found that
a higher quality liquid fuel suitable for use as liquid rocket fuel may be
produced. The
blended liquid rocket fuel may be considered to be a biofuel containing rocket
fuel.
In an embodiment of the invention, it has been found that a rocket fuel blend
can be
produced that has a -34 C kinematic viscosity (measured according to ASTM D445
method) that is less than 10cSt, more preferably less than 9cSt, and most
preferably less
than 8cSt. Rocket fuels with lower viscosities at sub-zero temperatures can be
further
cooled, maximizing the benefit of improved fuel densities at lower active fuel
cooling
temperatures.
As used herein, a "low" or "lower" in the context of propulsion fuel
properties
embraces any degree of decrease or reduction compared to an average commercial
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
petroleum jet fuel property containing equivalent total aromatics content
under the same or
equivalent conditions.
As used herein, a "high" or "higher" in the context of propulsion fuel
properties
embraces any degree of increase compared to an average commercial petroleum
jet fuel
property containing equivalent total aromatics content under the same or
equivalent
conditions.
As used herein, an "increase" in the context of propulsion properties embraces
any
degree of increase compared to a previously measured jet fuel property under
the same or
equivalent conditions. Thus, the increase is suitably compared to the jet fuel
property of the
fuel composition prior to incorporation of the synthetic cyclo-paraffinic
kerosene fuel
blending component. Alternatively, the property increase may be measured in
comparison
to an otherwise analogous jet fuel composition (or batch or the same fuel
composition); for
example, which is intended (e.g., marketed) for use in a jet turbine engine,
without adding
the bio-based cyclo-paraffinic kerosene fuel blending component to it.
As used herein, a "decrease" or "reduction" in the context of propulsion fuel
properties embraces any degree of decrease or reduction compared to a
previously
measured jet fuel property under the same or equivalent conditions. Thus, the
decrease or
reduction is suitably compared to the property of the jet fuel composition
prior to
incorporation of the synthetic cyclo-paraffinic kerosene fuel blending
component.
Alternatively, the property decrease may be measured in comparison to an
otherwise
analogous jet fuel composition (or batch or the same fuel composition); for
example, which
is intended (e.g., marketed) for use in a jet turbine engine, without adding
the synthetic
cyclo-paraffinic kerosene fuel blending component to it.
In the context of the present invention, "use" of a synthetic cyclo-paraffinic
kerosene fuel blending component in a propulsion fuel composition means
incorporating
the component into the jet fuel, typically as a blend (i.e., a physical
mixture) with one or
more jet fuel components and optionally with one or more jet fuel additives.
Accordingly, in one embodiment of the invention, there is provided the use of
a
synthetic cyclo-paraffinic kerosene fuel blending component described above to
increase
the volumetric energy content of a jet fuel. Accordingly, in another
embodiment of the
invention, there is provided the use of a synthetic cyclo-paraffinic kerosene
fuel blending
component described above to upgrade a kerosene base fuel to meet a Jet A-1
specification. Accordingly, in another embodiment of the invention, there is
provided the
26
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
use of a synthetic cyclo-paraffinic kerosene fuel blending component described
above to
upgrade a kerosene base fuel to meet a Jet A specification. Accordingly, in
another
embodiment of the invention, there is provided the use of a synthetic cyclo-
paraffinic
kerosene fuel blending component described above to upgrade a kerosene base
fuel to meet
a Jet AN-8 specification.
Suitably, the synthetic cyclo-paraffinic kerosene fuel blending component
described
above is used in an amount to increase the smoke point, preferably to increase
the smoke
point at least 1 mm greater than the kerosene base fuel (e.g., petroleum based
jet fuel) as
measured by ASTM D1322 (automated method). When using a jet fuel composition
prepared by the method disclosed herein, a jet airplane equipped with a jet
turbine engine,
a fuel tank containing the jet fuel composition prepared according to methods
disclosed
herein, and a fuel line connecting the fuel tank with the jet turbine engine.
Thus, a jet
engine may be operated by burning in such jet engine a jet fuel described
herein.
Accordingly, in another embodiment of the invention, there is provided the use
of a
synthetic cyclo-paraffinic kerosene fuel blending component comprising at
least
99.5mass% of carbon and hydrogen content and at least 50 mass% of cyclo-
paraffin, said
cyclo-paraffinic kerosene fuel blending component having a boiling point of at
most
300 C, at atmospheric pressure, flash point of at least 38 C, a density at 15
C of at least
799 kg/m', and a freezing point of -60 C or lower, to produce a liquid rocket
fuel.
While the invention is susceptible to various modifications and alternative
forms,
specific embodiments thereof are shown by way of examples herein described in
detail. It
should be understood, that the detailed description is not intended to limit
the invention to
the particular form disclosed, but on the contrary, the intention is to cover
all
modifications, equivalents and alternatives falling within the spirit and
scope of the present
invention as defined by the appended claims. The person skilled in the art
will readily
understand that, while the invention is illustrated making reference to one or
more a
specific combinations of features and measures, many of those features and
measures are
functionally independent from other features and measures such that they can
be equally or
similarly applied independently in other embodiments or combinations.
The present invention will be illustrated by the following illustrative
embodiment,
which is provided for illustration only and is not to be construed as limiting
the claimed
invention in any way.
27
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
Illustrative Examples
Test Methods
Jet Fuel Specification Tests and Methods
A jet fuel can be verified to meet a given specification by testing the fuel's
properties specified by the governing specification.
Energy Per Unit Volume or Energy Per Unit Mass
The energy per unit mass (or gravimetric energy density) of a fuel is simply
its Net
Heat of Combustion as determined by ASTM D3338. The energy per unit volume (or
volumetric energy density) can be calculated by multiplying the fuel's Net
Heat of
Combustion (determined by ASTM D3338) by the fuel's density (determined by
ASTM
D4052).
Materials
Comparative Examples
A petroleum-derived jet fuel sourced from Convent Terminal in Convent,
Louisiana
is provided as a comparative example of Jet A or a kerosene base fuel
component. A
synthetic jet fuel component sourced from Shell Middle Distillate Synthesis
plant in
Bintulu, Malaysia having (99.9 wt.% paraffin content with iso-paraffin and n-
paraffin
content of 98.7 wt. %) is provided as a comparative example of GTL1. Another
synthetic
jet fuel component sourced from Pearl GTL plant in Qatar having (100.0 wt.%
paraffin
content with iso-paraffin and n-paraffin content of 96.3 wt.%) is provided as
a comparative
example of GTL2. A jet fuel component from hydroprocessed esters and fatty
acid sourced
from UOP having (98.1 mass% paraffins, 1.9 mass% cyclo-paraffins) is provided
as a
comparative example of HEFA. The specification properties for each comparative
example are summarized in Table 3 below.
Table 3: Specification Properties of Fuel Components
Test ASTM
Jet A GTL1 GTL2 HEFA
Method
Acidity (mgKOH/g) D3242 0.003 0.001 0.001 0.003
Density at 15 C (-kg/m3) D4052 798.4 735.9 753.8 756.7
Hydrogen Content (mass%) D5291 14.005 15.595 15.42 14.73
Flash Point ( C) D56 45 43 56.5 43
Freeze Point ( C) D5972 -43.2 -54.6 -49.3 -57.3
28
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
Viscosity (mm2/s) at -20 C D445 4.037 2.450 4.146 4.795
Total Sulfur (ppm) D5453 NA <1* 1 <1*
Total Sulfur (mass%) D4294 0.151 NA NA NA
Mercaptan sulfur (mass%) D3227 6 NA NA NA
Smoke Point D1322 24.3 >50.0* >50.0* >50.0*
(mm) (automated)
Naphthalenes (vol.%) D1840 1.26 NA 0.0 NA
Aromatics (vol.%) D1319 17.5 NA NA NA
D6379 NA 0.1 <0.1* 0.1
Net Heat of Combustion D3338 43.318 44.246 44.136 44.145
(MJ/kg)
Distillation Temperature at 10% D86 176.2 161.0 184.4 162.9
Boiling Point ( C)
Final Boiling Point ( C) D86 274.4 195.9 234.3 277.8
* Actual values were beyond the indicated detection limit
Example 1 - Production of Synthetic Cyclo-Paraffinic Kerosene from Corn Starch
A three step catalytic process as described above utilizing aqueous phase
reforming
(APR), dehydration/oligomerization (DHOG) and hydrotreating (HT), was used to
convert
corn syrup to cyclo-paraffin-rich organic product. Two distinct beds of APR
catalyst
developed by Virent, Inc. (Madison, WI) were used. The first APR catalyst
included
palladium, molybdenum, and tin metals on a tungsten modified zirconia support,
while the
second APR catalyst included palladium and silver metals on a tungsten
modified zirconia
support. The DHOG catalyst included palladium and silver metals on a tungsten
modified
zirconia support, also provided by Virent, Inc. The HT catalyst was prepared
by CRI with
a nickel metal loading on an alumina support.
The catalysts were loaded into separate fixed-bed, tubular reactors configured
in
series such that the liquid product from one step was fed to the next step. A
60% 43DE
corn syrup in water mixture by weight was fed across the system with the
process
conditions shown in Table 4.
Table 4. Start of Run Conditions for APR, DHOG, and HT
APR I APR II DHOG HT
WHSV Wtfeed/(Wtcatalyst hr) 0.8 0.8 0.8
1.6
Added Hydrogen molm/molfeed 1.4 0.8 0.5
Average Reactor Temperature C 210 250 280 370
Pressure Psig 1800 900 900 1300
29
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
A two-pass hydrotreating configuration was used. The hydrotreating process
included an intermediate distillation step in between each pass to remove the
components
heavier than the 300 C end point for jet fuel. The liquid from the HDO-DHOG-HT
train
was fractionated continuously within the same plant. The SK fraction was
collected,
combined all together, and re-fed across the HT catalyst and fractionation
portion of the
plant at the same conditions shown in Table 4 for the HT step.
The resulting product composition of the liquid organic product is shown in
Table
5, which includes a comparison of the composition and carbon number of the
product pre-
fractionation, after the first HT pass, and after the second HT pass.
For alternative applications, the fractionation can be tuned to produce a
diesel
fraction that is primarily C12-C24, or a rocket fuel application that is
primarily C12-Cis
(rocket fuel cut).
Table5. Liquid organic product composition by GCxGC - Full Range and SK
fraction
Speciation Full Range SK Fraction SK Fraction
Pre-fractionation Post-fractionation Post-
fractionation
1 Pass HT 2 Pass HT
Cyclo-paraffins wt. % 37.3 52.2 82.2
Paraffins wt. % 14.3 14.6 16.9
Aromatics wt. % 6.5 27.8 0.5
PNA wt. % 0.5 2.1 0.0
Unclassified* wt. % 41.4 3.4 0.6
Total wt. % 100.0 100.0 100.0
Carbon Number
C7- wt. % 33.2 1.7 0.8
C8-C18 wt. % 52.4 95.6 98.5
C19+ wt. % 13.6 2.4 0.4
Unclassified wt. % 0.8 0.4 0.3
Total wt. % 100.0 100.0 100.0
*Method set up to look for compounds between C7-C18 in jet fuel range, so C7-
and C19+ compounds are not classified
into a class. In case of "Full Range" material, majority of "Unclassified"
compounds were paraffins.
The process was run to produce greater than 420 liters (110 gallons) of
synthetic
cyclo-paraffinic kerosene for product testing. The product was stored in two
55 gallon
drums and one 16 gallon drum. 20 milligrams per liter of butylated
hydroxyltoluene
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
(BHT) anti-oxidant additive was added to each drum as is standard fuel
handling practice
for jet fuel. Examples 3 were tested fuel from this example.
Example 2 - Production of Synthetic Cyclo-Paraffinic Kerosene from
Lignocellulose
A woody biomass material was deconstructed by a 3rd party to produce a
hydrolysate. This hydrolysate was ion exchanged to remove inorganic impurities
and
diluted so the carbon containing fraction was 50% by weight, with the balance
being water.
A three step catalytic process as described in Example 1 utilizing aqueous
phase
reforming (APR), dehydration/oligomerization (DHOG) and hydrotreating (HT) was
used
to convert the hydrolysate to cyclo-paraffinic rich organic product under the
process
conditions shown in Table 6.
Table6. Process Conditions for APR and DHOG
APR I DHOG
WHSV Wtfeed/(Wtcatalyst 0.7 0.7
Added Hydrogen molm/molteed 6.4 1.1
Average Reactor Temperature C 210 270
Pressure psig 1050 600
An organic phase product from the APR-DHOG continuous system was collected
throughout the run, combined all together, and fed to a separate plant to
perform the
hydrotreating (HT) step. The HT step utilized a two-pass hydrotreating
configuration as
described in Example 1, which included an intermediate distillation step
between each pass
to remove the components heavier than the 300 C end point for jet fuel. The HT
catalyst
was prepared by CRI with a nickel metal loading on an alumina support and
loaded into a
fixed-bed, tubular reactor. A 1:1 co-loading of amorphous silica alumina was
used to
distribute the catalyst.
Table 7. Process Conditions for HT I and II
HT I HT II
WHSV Wtfeed(Wtcatalyst 3 1.7
hr)
Added Hydrogen molm/molteed 13.8 13.6
Average Reactor C 290 290
Temperature
Pressure psig 800 800
31
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
The product composition of the final SK liquid organic product is shown in
Table 8Error!
Reference source not found.. The 3rd column from Table 5 in Example 1 is
included to
show the similarity of the product from both feedstock sources. Since the
compositions of
the products are very similar, it follows that the physical properties are
very similar as well,
as shown in Table 9.
Table 8. Liquid organic product composition by ASTM D2425
Speciation Example 1 Example 2
Corn Syrup Woody Biomass
Cyclo-paraffins wt. % 83 74
Paraffins wt. % 17 25
Aromatics wt. % <0.3* <0.3*
Olefins wt. % <0.3* <0.3*
PNA wt. % <0.3* <0.3*
Other wt. % <0.3* <0.3*
Total wt. % 100 100
* Actual values were beyond the indicated detection limit
Table 9. Physical properties of SK produced from corn syrup and biomass-
derived feedstocks
Example Example SK 1 Example SK 2
Woody
Feedstock Corn Syrup
Biomass
ASTM
D1655
Test
Specification Test Jet MA-1
Method
Spec
Requirement
Aromatics, vol. % D1319 <25 0.0 0.2
Heat of Combustion
D4809 >42.8 43.3 43.3
(measured), MJ/kg
Distillation: D86/D7345**
IBP, C 149 146
10% recovered,
<205 178 172
C
50% recovered,
217 227
C
90% recovered,
266 280
C
EP, C <300 292 300
Residue, % vol. <1.5 1.2 2**
Loss, % vol. <1.5 0.7 0
Flash point, C D56 >38 44 45
Freezing Point, C D5972 <-47 <-78*
32
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
Density @ 15 C,
D4052 775 - 840 818 813
kg/m3
Thermal Stability
D3241 >260 >355 >325***
Breakpoint, C
Net Heat of
D3338 >42.8 43.3 43.3
combustion (MJ/kg)
* Actual values were beyond the indicated detection limit
**D7345 micro-distillation performed due to sample size, high residue likely
an artifact of test method. No HDO-SK
sample analyzed by D86 had a high residue.
*** Sample not tested higher than 325 C due to sample size, breakpoint at some
temperature higher.
Example 3 - Comparative Jet Fuel Blends
Several series of Example and Comparative Example jet fuel blends were
prepared
using Comparative Example Jet A, Example SK1 from Example 1, Comparative
Example
HEFA, Comparative Example GTL1, and Comparative Example GTL2. These jet fuel
blends and their indicated blend ratios are summarized in Table 10.
Table 10
Jet A content (Vol.%) SK1 content (Vol.%)
Example Series 3-1 64.5 35.5
3-2 43.0 57.0
3-3 32.3 67.7
3-4 26.9 73.1
3-5 21.5 78.5
3-6 10.8 89.2
Jet A content (Vol.%) HEFA content (Vol.%)
Comparative Example Series A-1 64.5 35.5
A-2 43.0 57.0
Jet A content (Vol.%) GTL1 content
(Vol.%)
Comparative Example Series B-1 64.5 35.5
B-2 43.0 57.0
B-3 32.3 67.7
B-4 16.1 83.9
B-5 10.8 89.2
B-6 5.4 94.6
Jet A content (Vol.%) GTL2 content
(Vol.%)
Comparative Example Series C-1 64.5 35.5
C-2 43.0 57.0
C-3 32.3 67.7
C-4 16.1 83.9
C-5 10.8 89.2
C-6 5.4 94.6
The Jet fuel Blends above were tested for jet fuel specification properties.
The results are
provided in Tables 11 below.
Aromatic contents of the blends were calculated by linear blending; that is,
multiplying the percentage of Kerosene base fuel (Jet A) in the comparative
example blend
by the aromatic content of the Kerosene base fuel (as determined by D1319).
Gravimetric
and volumetric energy densities of the two-component blends were calculated by
linear
33
CA 03009058 2018-06-18
WO 2017/112717 PCT/US2016/067902
blending. That is, for components a and (3 with respective volumetric contents
[a] and 1-
[a], respective gravimetric energy densities ya and yp, and respective
densities of pa and pp,
the gravimetric and volumetric energy densities of resulting blends can be
calculated as
follows:
Gravimetric energy density of blend of a and (3 = [arya + (14a])* yi3
Volumetric energy density of blend of a and (3 = [a]*ya*pa + (1-[a])* yp*pi3
Table 11-1: Key Specification Properties of Example Series 3
SK1 content in SK1/Jet A blend (vol.%)
0.0 35.5 57.0 67.7 73.1 78.5
89.2 100.0
Comparativ
Jet A 3-1 3-2 3-3 3-4 3-5 3-6
SK1
e Examples
ASTM
Test Method Property
Aromatic D1319 or
18.6 12.0 8.0 6.0 5.0 4.0 2.0
0
Content (vol.%) calculated
Density at 15 C
D4052 0.7984 0.8037 0.8071 0.8087 0.8096 0.8104 0.8121
0.8138
(g/cm3)
Freezing Point
D5972 -43.2 -48.8 NA -58.0 -61.1 -64.9 <-77.0* <-76.0*
( C)
Smoke Point
(mm) D1322 24.3 26.1 27.9 28.3 29.3 29.9
30.2 31.3
(automated)
Hydrogen
D5291 14.01 14.15 14.21 14.26 14.21
14.25 14.27 14.4
Content (mass%)
Net Heat of
Combustion or
Gravimetric D3338 or 43.3 43.3 43.3 43.3 43.3
43.3 43.3 43.4
calculated
Energy Density
(MJ/kg)
Volumetric
Energy Density Calculated 34,600 34,900 35,000 35,100 35,100 35,100
35,200 35,300
(MJ/m3)
* Actual values were beyond the indicated detection limit
Table 11-2: Key Specification Properties of Comparative Example Series A
HEFA content in HEFA/Jet A blend
(vol %)
0.0 43.0 64.5 100.0
Comparative Examples Jet A A-1 A-2 HEFA
Test ASTM Method Property
Aromatic Content (vol.%) D1319 or calculated 18.6 12 8 0.0
Density at 15 C (g/cm3) D4052 0.7984 0.7839 0.7753 0.7570
Freezing Point ( C) D5972 -43.2 -45.9 -49.3 -57.5
Smoke Point (mm)
D1322 24.3 30.65 36.35 >50.0*
(automated)
Hydrogen Content (mass%) D5291 14.01 14.45 14.73 15.60
Net Heat of Combustion or D3338 or calculated 43.3 43.6 43.8
44.1
34
CA 03009058 2018-06-18
WO 2017/112717 PCT/US2016/067902
Gravimetric Energy Density
(MJ/kg)
Volumetric Energy Density
Calculated 34,600 34,200 33,900 33,400
(MJ/m3)
* Actual values were beyond the indicated detection limit
Table 11-3: Key Specification Properties of Comparative Example Series B
GTL1 content in GTL1/Jet A blend (vol.%)
0.0 35.5 57.0 67.7 83.9 89.2
94.6 100.0
Comparative
Jet A B-1 B-2 B-3 B-4 B-5 B-6
GTL1
Examples
ASTM
Test Method Property
Aromatic D1319 or
18.6 12.0 8.0 6.0 3.0 2.0 1.0
0.0
Content (vol.%) calculated
Density at 15 C
D4052 0.7984 0.7761 0.7636 0.7568 0.7467 0.7433 0.7396
0.7359
(g/cm3)
Freezing Point
D5972 -43.2 -49.2 NA -58.1 NA NA -
55.8 -54.6
( C)
Smoke Point
(mm) D1322 24.3 32.5 39.05 43.7 >50.0* >50.0* >50.0* >50.0*
(automated)
Hydrogen
Content D5291 14.01 14.55 14.84 15.06 15.31 15.40
15.52 15.60
(mass%)
Net Heat of
Combustion or
Gravimetric D3338 or 43.3 43.6 43.8 43.9 44.1 44.1
44.2 44.2
calculated
Energy Density
(MJ/kg)
Volumetric
Energy Density Calculated 34,600 33,900 33,400 33,200 32,900
32,800 32,700 32,600
(MJ/m3)
* Actual values were beyond the indicated detection limit
CA 03009058 2018-06-18
WO 2017/112717 PCT/US2016/067902
Table 11-4: Key Specification Properties of Comparative Example Series C
GTL2 content in GTL2/Jet A blend (vol.%)
0.0 35.5 57.0 67.7 83.9 89.2
94.6 100.0
Comparative
Jet A C-1 C-2 C-3 C-4 C-5 C-6
GTL2
Examples
ASTM
Test Method Property
Aromatic D1319 or
18.6 12.0 8.0 6.0 3.0 2.0 1.0
0.0
Content (vol.%) calculated
Density at 15 C
D4052 0.7984 0.7823 0.7731 0.7682 0.7609 0.7586 0.7560
0.7538
(g/cm3)
Freezing Point
D5972 -43.2 -47.2 NA -50.1 NA NA -
49.5 -49.3
( C)
Smoke Point
(mm) D1322 24.3 31.4 37.6 42.3 49.9 >50.0* >50.0* >50.0*
(automated)
Hydrogen
D5291 14.01 14.47 14.78 14.93 15.18
15.21 15.32 15.42
Content (mass%)
Net Heat of
Combustion or
D3338 or
Gravimetric 43.3 43.6 43.8 43.9 44.0 44.0 44.1
44.1
calculated
Energy Density
(MJ/kg)
Volumetric
Energy Density Calculated 34,600 34,100 33,800 33,700 33,500 33,400
33,300 33,300
(MJ/m3)
* Actual values were beyond the indicated detection limit
As can be seen from Table 11-1, SK1 can be blended to Jet A to meet Jet A-1
specification as shown in Example 3-1 and can be blended to meet AN-8
specification as
shown in Example 3-3, particularly without loss, but increase in volumetric
energy density.
Figure 1 compares the volumetric energy density (MJ/m3) of the jet fuel blends
Example Series 3, Comparative Example Series A, Comparative Example Series B,
and
Comparative Example Series C based on paraffinic kerosene content in Jet A
(vol.%).
Also included for completeness are the volumetric energy densities of the neat
blend
components Comparative Example SK1, Comparative Example HEFA, Comparative
Example GTL1, Comparative Example GTL2, and Comparative Example Jet A. The
data
show a linear blending relationship for all blends. The slopes of all the
Comparative
Example Series data are negative, indicating increased paraffinic kerosene
content
(whether via HEFA, GTL1, or GTL2) typically results in an undesirable decrease
in
volumetric energy density. However, the slope of the Example 3 data is
positive, indicating
that increased SK1 cyclo-paraffinic kerosene content resulted in increased
volumetric
energy density. This demonstrates the unique ability to blend a cyclo-
paraffinic kerosene
product such as SK1 into a kerosene base fuel without decreasing, but rather
increase
volumetric energy density. This is desirable because higher volumetric energy
density
36
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
results in aircraft flying greater distances using the same volume of fuel, or
in other words,
with greater payload range.
Figure 2 compares the aromatics content (vol.%) versus volumetric energy
density
(MJ/m3) of the jet fuel blends Example Series 3, Comparative Example Series A,
Comparative Example Series B, and Comparative Example Series C. Also included
for
completeness are the aromatics contents of the neat blend components
Comparative
Example SK1, Comparative Example HEFA, Comparative Example GTL1, Comparative
Example GTL2, and Comparative Example Jet A. The data show a linear blending
relationship for all blends. The slopes of all the Comparative Example Series
data are
positive, indicating that increased volumetric energy density typically
requires an
undesirable increase in aromatics content. However, the slope of the Example
Series 3 data
is negative, indicating increased volumetric energy density with decreasing
aromatics
content. This demonstrates the unique ability to blend a cyclo-paraffinic
kerosene product
such as SK1 into a kerosene base fuel to decrease aromatics content without
decreasing,
but rather increase volumetric energy density. This is desirable because lower
aromatics
content improves engine operability and lifetime and reduces soot emissions;
and higher
volumetric energy density results in aircraft flying greater distances using
the same volume
of fuel, or in other words, with greater payload range.
Figure 3 compares the smoke point increase (mm) of jet fuel with volumetric
energy density (MJ/m3) of the jet fuel blends Example 3, Comparative Example
Series A,
Comparative Example Series B, and Comparative Example Series C. Also included
for
completeness are the smoke points of the neat blend components Comparative
Example
SK1, Comparative Example HEFA, Comparative Example GTL1, Comparative Example
GTL2, and Comparative Example Jet A. The data show a non-linear blending
relationship
for all blends. The slopes of all the Comparative Example Series data are
negative,
indicating increased volumetric energy density typically requires an
undesirable decrease
in smoke point. However, the slope of the Example 3 data is positive,
indicating increased
volumetric energy density with increasing smoke point. This demonstrates the
unique
ability to blend a cyclo-paraffinic kerosene product such as SK1 into a
kerosene base fuel
to increase volumetric energy density without decreasing, but rather increase
smoke point.
This is desirable because higher smoke point indicates a cleaner-burning fuel;
and higher
volumetric energy density results in aircraft flying greater distances using
the same volume
of fuel, or in other words, with greater payload range.
37
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
Figure 4 compares the freezing point increase ( C) of jet fuel with volumetric
energy density (MJ/m3) of the jet fuel blends Example Series 3, Comparative
Example
Series A, Comparative Example Series B, and Comparative Example Series C. Also
included for completeness are the freezing points of the neat blend components
Comparative Example SK1, Comparative Example HEFA, Comparative Example GTL1,
Comparative Example GTL2, and Comparative Example Jet A. The data show a non-
linear blending relationship for all blends. The Comparative Example Series
data indicate
increased volumetric energy density typically requires an undesirable increase
in freezing
point. However, the Example 3 data show increased volumetric energy density
with
decreasing freezing point. This demonstrates the unique ability to blend a
cyclo-paraffinic
kerosene product such as SK1 into a kerosene base fuel to increase volumetric
energy
density without increasing, but rather decrease the freezing point. This is
desirable because
a lower freezing point enables a fuel to meet more stringent specifications
(such as for AN-
8) or to fly more direct routes through colder areas, and have wider
applicability for cold
environments; and higher volumetric energy density results in aircraft flying
greater
distances using the same volume of fuel, or in other words, with greater
payload range.
Example 4 - Production of modified Synthesized Cyclo-Paraffinic Kerosene for
rocket
fuel applications
A fraction of cyclo-paraffinic kerosene can be produced in a similar manner to
Example 1. The last distillation step can be modified to meet a flash point of
greater than
60 C and a final boiling point less than 274 C. Estimated properties of the
product from
this modified fractionation are summarized in Table 12.
Table 12. Physical properties of SK produced with modified fractionation
Distillation
Initial BP (C) 180
Temp @ 10% Rec. (C) 189
Temp @ 20% Rec. (C) 197
Temp @ 50% Rec. (C) 215
Temp @ 90% Rec. (C) 251
Final BP (C) 270
Flash Point (C) 60
Density, 15C (kg/m3) 828
Example 5 ¨Rocket Fuel Blends
Liquid kerosene rocket fuel blends can be produced using cyclo-paraffinic
kerosene
(SK) from Example 1 and Example 4 and commercially available kerosene range
38
CA 03009058 2018-06-18
WO 2017/112717 PCT/US2016/067902
hydrocarbon component She11S01TM D60, D70, D9OS and DlOOS as indicated below.
These
liquid rocket fuel blends, their indicated blend ratios and their properties
are summarized in
Table 13. Remainder of vol.% is the respective ShellSol components.
Table 13-1: Key Specification Properties of Example Series 5 using Example 1
SK content from Example 1 in
respective ShellSol blend (vol. %)
62 37 72
ShC11S01TM Series D70 D60 D1005
Test ASTM Method Property
Initial Boiling Point ( C) Distillation 175 180 180
Final Boiling Point ( C) Distillation 265 234 274
Flash Point ( C) D56 >60 >60 >60
Density at 15 C (kg/m3) D4052 805 803 808
Freezing Point ( C) D5972 <-51 <-51 <-51
Viscosity@-34 C (cSt) D445 est. 9.5 est. 8.5 est.
11
Hydrogen Content (mass%) D5291 est. 14.3 est. 14.3 est.
14.3
Net Heat of Combustion or
Gravimetric Energy Density D3338 or calculated est. 43.9 est. 44.4 est.
43.8
(MJ/kg)
Table 13-2: Key Specification Properties of Example Series 5 using Example 4
SK content from Example 4 in
respective ShellSol blend (vol. %)
79 74 62 37
ShellSolTM Series D1005 D905 D70 D60
Test ASTM Method Property
Flash Point ( C) D56 >60 >60 >60 >60
Density at 15 C (kg/m3) D4052 821 819 815 809
Freezing Point ( C) D5972 <-51 <-51 <-51 <-51
Viscosity@-34 C (cSt) D445 est. 14 est. 13 est. 12.3
est. 10.5
Hydrogen Content (mass%) D5291 >13.8 >13.8 >13.8
>13.8
Net Heat of Combustion or
Gravimetric Energy Density D3338 or calculated >43.03 >43.03 >43.03
>43.03
(MJ/kg)
It is expected that the above blends will meet the RP rocket fuel
specifications.
39
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
Example 6 ¨ Proven Rocket Fuel Blend
Using the SK with modified fractionation as per Example 4 and the ShellSol D60
hydrocarbon component, a proven rocket fuel blend was produced and assessed
for
applicability in relation to the RP-1/RP-2 rocket fuel (MIL-DTL-25576)
specification. The
proven rocket fuel blend was tuned to meet the required limits of the MIL-DTL-
25576
specification as shown in results Table 14 below. The proven rocket fuel blend
disclosed
here relates to a 90% ShellSol D60 and 10% SK with modified fractionation
(Example 4)
blend, by volume.
Table 14: Specification data from the proven rocket fuel blend of 90% ShellSol
D60/10% SK
(Example 4) (by volume) as compared to the MIL-DTL-25576 (RP-1/RP-2)
specification
Proven
RP-1/RP-2 Rocket
ASTM Method Specification Fuel Blend
Initial Boiling Point (deg. C) Report 182
10% Boiling Point (deg. C) 185-210 191.9
50% Boiling Point (deg. C) D86 Report 197.3
90% Boiling Point (deg. C) Report 209.8
Final Boiling Point (deg. C) 274 max 233.9
Flash Point (deg. C) D56 60 min 60
Density @ 15C (g/cm3) D4052 0.799-0.815
0.8126
Freeze point (deg. C) D5972 -51.1 max <-80
Viscosity @ -34C (cSt) D445 16.5 max 7.759
Gravimetric Energy Density (MJ/kg) Calculated (D3338) 43.03 min 43.407
Table 14 therefore shows that this proven rocket fuel blend is viable in
relation to
the specification. Of additional interest is how this proven rocket fuel blend
compares
against typical values for an RP-1 and/or RP-2 rocket fuel. Typical values for
these rocket
fuels were determined through independent testing of commercially available
rocket fuel,
and are shown in Table 15.
Table 15: Typical RP-1/RP-2 Rocket Fuel (from commercially available sources)
specification
values
RP-1/RP-2 Typical Typical
ASTM Method Specification RP-1 RP-
2
Initial Boiling Point (deg. C) Report 185
182.1
10% Boiling Point (deg. C) 185-210 198.1
198.1
50% Boiling Point (deg. C) D86 Report 210.5
216.1
90% Boiling Point (deg. C) Report 230.8
244.3
Final Boiling Point (deg. C) 274 max 247.1
261.9
Flash Point (deg. C) D56 60 min 63 62
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
Density @ 15C (g/cm3) D4052 0.799-0.815 0.8096
0.8142
Freeze point (deg. C) D5972 -51.1 max <-80 <-
80
Viscosity @ -34C (cSt) D445 16.5 max 10.90
12.14
Gravimetric Energy Density (MJ/kg) Calculated (D3338) 43.03 min
43.483 43.449
Comparing and contrasting the results from the proven rocket fuel blend (Table
14)
and the typical values presented in Table 15 the values are all similar except
for the
viscosity at -34C (ASTM method D445). In particular, the gravimetric energy
density
differs by no more than 0.2% between RP-1/RP-2/proven rocket fuel blend,
indicating that
the specific impulse (the key parameter in rocketry which determines the
effective payload
that can be launched for a given quantity of propellant) should be consistent
between the
two base-case fuels (RP-1 and RP-2) and the proven rocket fuel blend. The
specific
impulse is a measure of the pounds of thrust produced by the consumption of
one pound of
propellant in the timeframe of one second, and is directly related to the
square of the
exhaust velocity of the exit gases from the rocket engine. It is common
knowledge in the
industry that the square of the exhaust velocity of the exit gases is
(approximately)
proportional to the temperature within the combustion chamber (in turn related
to the fuel
energy density) and inversely proportional to the molar mass of the exit
gases: since both
fuels are hydrocarbon-based with similar densities/hydrogen content the exit
gas molar
mass should also be similar. This logic points towards similar specific
impulse between
rocket fuels RP-1, RP-2 and the proven rocket fuel blend.
The proven rocket fuel blend does provide a distinct advantage over the RP-1
and
RP-2 fuels due to the lower viscosity profile at sub-zero temperatures. This
is particularly
beneficial on rockets that actively cool their fuel to get higher fuel
densities allowing more
fuel mass to be contained within a given fuel tank. Current practice for
active onboard
rocket fuel cooling is limited by the higher viscosities experienced as the
fuel is cooled,
negatively affecting the fuel's ability to flow and critically to disperse
effectively into the
oxidizer through the spray-forming hardware. Fuels with lower viscosities at
sub-zero
temperatures can therefore be further cooled, maximizing the benefit of
improved fuel
densities at lower active fuel cooling temperatures.
Table 16 shows the sub-zero viscosities for two independent temperatures for
RP-1,
RP-2 and the proven rocket fuel blend. Fig. 5 shows the same data graphically,
the benefit
of working with lower viscosity fuels on rockets with actively cooled fuel
tanks being that
they can be cooled to lower temperatures for a given limiting fuel viscosity.
41
CA 03009058 2018-06-18
WO 2017/112717
PCT/US2016/067902
Figure 5 is a graphical representation of data from Table 15; highlighting
that for a given
limiting viscosity a lower temperature viscosity profile is beneficial to
allow for additional
active fuel cooling to maximize fuel density.
Table 15: Sub-Zero temperature viscosity data for the base-case rocket fuels
RP-1, RP-2 and
the proven rocket fuel (RF) blend
Viscosity (cSt)
-20C -34C
RP-1 6.22 10.90
RP-2 6.97 12.14
Proven RF Blend 4.76 7.76
42