Note: Descriptions are shown in the official language in which they were submitted.
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
COMBINED BIOMARKER MEASUREMENT OF FIBROSIS
Technical Field of the Invention
The present invention relates to a sandwich immunoassay for detecting in a
biological sample cross-linked PIIINP, and its use in evaluating the efficacy
of drugs
targeting lysyl oxidases (LOXs). The invention also relates to a kit for
performing the
sandwich immunoassay.
Description of the Related Art
Fibrotic diseases (including those listed in Table 1) are a leading cause of
morbidity and mortality, e.g. cirrhosis with 800,000 deaths per year worldwide
(1).
Table 1. Different fibrotic diseases (2).
Tissue Examples of Causes
Liver Viral hepatitis
Schistosomiasis
Steatohepatitis (Alcoholic or non-alcoholic)
Lung Idiopathic pulmonary fibrosis (IPF)
Systemic sclerosis (Scleroderma)
Kidney Nephrogenic systemic fibrosis (NSF)
Diabetes
Untreated hypertension
Heart Heart attack
Hypertension
Atherosclerosis
Restenosis
Eye Macular degeneration, retinal and vitreal retinopathy
Skin Systemic sclerosis and scleroderma, keloids, hypertrophic
1
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
scars, burns, genetic factors
N FS
Pancreas Autoimmune/hereditary causes
Intestine Crohn's disease/inflammatory bowel disease
Brain Alzheimer's disease, AIDS
Bone Cancer, ageing
marrow
Multi-organ Surgical complications, chemotherapeutic drug-induced
fibrosis fibrosis, radiation-induced fibrosis, mechanical injuries
A 'fibrotic disease' is any disease giving rise to fibrosis, whether as a main
or a
secondary symptom. Fibrosis is the end result of chronic inflammatory
reactions
induced by a variety of stimuli including persistent infections, autoimmune
reactions,
allergic responses, chemical insults, radiation, and tissue injury.
Fibrosis is
characterized by the accumulation and reorganization of the extracellular
matrix (ECM).
Despite having obvious etiological and clinical distinctions, most chronic
fibrotic
disorders have in common a persistent irritant that sustains the production of
growth
factors, proteolytic enzymes, angiogenic factors, and fibrogenic cytokines,
which
together stimulate the deposition of connective tissue elements, especially
collagens
and proteoglycans, which progressively remodel and destroy normal tissue
architecture
(3,4). Despite its enormous impact on human health, there are currently no
approved
treatments that directly target the mechanisms of fibrosis (5).
Extracellular Matrix (ECM)
The ECM is a supramolecular structure with the ability to form aggregates of
proteins, thus forming a dynamic scaffold linking cells together in a three
dimensional
2
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
network. This scaffold controls cell-matrix interactions and cell fate through
up and
down regulation of proteases (6). The ECM consists of collagens, laminins,
proteoglycans, and other glycoproteins in various amounts and combinations,
thereby
providing a variety of biological components which can be modified by
proteases to
produce scaffolds with specific functions to meet the needs of the individual
tissue (7).
Collagen types I and III are the major structural proteins in the human body.
Collagen type III is essential for collagen type I fibrillogenesis in the
cardiovascular
system and other organs (8,9). During fibrillar assembly the N-terminal
propeptide of
type III procollagen (which consists of three identical a-chains with a total
molecular
weight of 42 kDa) is cleaved off by specific N-proteases prior to
incorporation of the
mature collagen in the ECM. The cleaved propeptides may either be retained in
the
ECM or released into the circulation. However, the cleavage of the propeptide
is
sometimes incomplete, leaving the propeptide attached to the molecule. This
results in
the formation of thin fibrils with abnormal cross-links, which in turn causes
the abnormal
molecule to be prone to rapid metabolic turnover (10,11). Thus, the level of
the N-
terminal propeptide of type III collagen (PIIINP) in a suitable sample can be
a marker of
formation and/or degradation of collagen type III.
Remodeling of the ECM plays an important role in the pathogenesis of various
diseases as altered components and non-coded modifications of the ECM leads to
tissue stiffness and changes in the signaling potential of the intact ECM and
fragments
thereof. ECM remodeling is an important prerequisite for tissue function and
repair, and
3
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
is tightly controlled by the enzymes responsible for the synthesis and
degradation of the
ECM.
During pathological events, such as fibrotic diseases, the balance between the
formation and the degradation of the ECM is disturbed, leading to an altered
composition of the ECM. Such an alteration results in altered tissue function
(12,13). It
has been suggested that PIIINP could be used as a biomarker for several
fibrotic
diseases, such as lung injury (14), viral and non-viral hepatitis (15),
systemic sclerosis
(16), vascular remodeling (17), and kidney diseases (18).
Limited attention has been given to the ECM remodeling in skeletal muscle
tissue. In rat models increased collagen gene expression and biosynthesis have
been
demonstrated in quadriceps femoris and tibialis anterior muscles after
exercise (19,20).
Additionally, increased serum levels of PIIINP have been demonstrated in
clinical
studies after exercise (21). Therefore, remodeling of the skeletal muscle
proteins
increases the quantity of PIIINP in the circulation and may serve as a
biomarker for
detecting early muscle anabolism. Serum levels of PIIINP have previously been
suggested as a biomarker of muscular tissue response to testosterone (22),
recombinant human growth hormone (23) or the combination thereof (24,25).
In liver fibrosis the fibrillar collagens type I and III are highly up-
regulated (26,27).
Type III collagen is dominant in the early stages of fibrosis, while up-
regulation of type I
collagen is related to the later stages of fibrosis. Fibrosis occuring in the
liver results in
the deposition of collagen and release of propeptides, predominantly PIIINP.
Consequently, PIIINP is one of the best studied markers for fibrogenesis
(28,29,30).
4
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
Through the years, several radioimmunoassays have been developed for the
quantification of PIIINP, with a sensitivity of up to 94% and specificity of
up to 81% for
the detection of cirrhosis (31,32); however none of the previous assays are
neo-epitope
specific. Additionally, the current commercially available assays for
quantification of
PIIINP utilise polyclonal antibodies or monoclonal antibodies targeting
internal
sequences of the procollagen or the propeptide and do not specifically
differentiate
between the formation and/or degradation of collagen type III (31,32).
Thus, to differentiate between formation and degradation of collagen type III
we
consider that it is necessary to determine and detect a neo-epitopic fragment
which is
solely produced in the formation process (i.e. a fragment which is produced in
the
formation of collagen type III but not produced in the degradation of collagen
type III).
WO 2014/170312disc1oses a monoclonal antibody which is specific for the C-
terminal PIIINP neo-epitope comprised in the terminal amino acids of the C-
terminal
amino acid sequence CPTGXQNYSP-COOH (SEQ ID NO:4), wherein X can be Gly or
Pro.
Brocks (31) discloses a polyclonal antibody directed to the modified Bovine C-
terminal PIIINP sequence IC*QSCPTGGENYSP-COOH (SEQ ID NO: 1) (C* =
acetamido protected Cys; Gin replaced with Glu (E)), however said antibodies
are non-
specific towards the terminal amino acids of the bovine PIIINP C-terminal
sequence
ICQSCPTGGQNYSP-COOH (SEQ ID NO: 2) and additionally said antibodies do not
recognise human PIIINP.
5
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
Bayer (33) discloses a sandwich ELISA which utilises a detector monoclonal
antibody directed to the sequence H2N-GSPGPPGICQSCPTGPQNYSP-COOH (SEQ
ID NO: 3), however the binding epitope is not defined.
The applicant has now found that a specific sandwich immunoassay which
utilises the neo-epitope specific antibody directed towards the C-terminal neo-
epitope of
PIIINP as disclosed in WO 2014/170312 may be useful in evaluating the efficacy
of
drugs that target lysyl oxidases (LOXs), particularly LOX antagonist drugs.
Enzymatic
collagen crosslinking by LOXs and processing of pro-collagens is key for
tissue
maturation and stability. In patients with organ fibrosis collagens become
highly cross-
linked, and thus are less prone to fibrosis resolution. LOXL2, a specific LOX,
is a main
driver in pathophysiological collagen crosslinking in fibrotic tissue, and
novel LOXL2
antagonists are currently undergoing clinical trials. Thus, an assay that
could be used
to evaluate the efficacy of drugs targeting LOXs, such as LOX antagonists,
would
clearly be a useful tool for the pharmaceutical industry.
Summary
The present invention is directed to a sandwich immunoassay for detecting in a
biological sample cross-linked PIIINP where the cross-linked PIIINP comprises
at least
two strands of PIIINP joined together by inter-strand cross-linking. The
method
comprises contacting the biological sample comprising the cross-linked PIIINP
with a
first monoclonal antibody bound to a surface, where each strand of PIIINP
comprised in
6
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
the cross-linked PIIINP has a C-terminal neo-epitope of PIIINP generated by N-
protease
cleavage of intact type Ill procollagen, and adding a second monoclonal
antibody. Both
monoclonal antibodies are specifically reactive with the C-terminal neo-
epitope of
PIIINP, and said neo-epitope is comprised in a C-terminal amino acid sequence
CPTGXQNYSP-COOH, where X is Gly or Pro. The method further comprises
determining the amount of binding of the second monoclonal antibody.
The present invention also is directed to a method for evaluating the efficacy
of
an antagonist drug targeting lysyl oxidases (LOXs). The method comprises using
the
sandwich immunoassay as described herein to quantify the amount of cross-
linked
PIIINP in at least two biological samples obtained from a subject at a first
time point and
at least one subsequent time point during a period of administration of the
antagonist
drug to the subject. A reduction in the quantity of cross-linked PIIINP from
the first time
point to the at least one subsequent time point during the period of
administration of the
antagonist drug is indicative of an efficacious antagonist drug targeting
LOXs.
The present invention is directed further to a kit for use in the sandwich
immunoassay as described herein. The kit comprises a solid support to which is
bound
the first monoclonal antibody as described above and a labelled second
monoclonal
antibody as described herein.
Figures
FIG. 1: Alignment of the targeted PIIINP al chain sequence in human (SEQ ID
NO: 14) and rat (SEQ ID NO: 15) species (highlighted by the box). Position of
the
7
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
corresponding human (¨) and rat (
_______________________________________________ ) sequences within the alpha 1
chain of the N-
terminal pro-peptide of type III collagen. The alignment was performed using
the NLP
CLUSTALW software.
FIG. 2: Western Blot showing the specific bands of N-terminal propeptide of
type
III collagen in Amniotic fluid from a) rat and b) human recognized by the
monoclonal
antibody NB61N62 (lane 1 and 3) and NB61N62 + selection peptide (lane 2+4).
Two
bands around 52-60 kDA was observed for the rat, whereas one band was observed
for
human. Addition of selection peptide resulted in weakness of band intensity
for both rat
and human.
FIGS. 3A-3D: PRO-C3 ELISA runs showing typical calibration curves and native
reactivity against human, rodent, and mouse material. FIG. 3A: Calibration
curve and
inhibition of the competitive PRO-C3 ELISA using healthy human serum, plasma,
and
amniotic fluid (AF). The calibrator curve was diluted in 2-fold from 76.31
ng/mL,
whereas native material was run diluted 1:2 to 1:16 as indicated (--). FIG.
3B:
Calibration curve and inhibition of the competitive PRO-C3 ELISA using healthy
rat
serum, plasma, and AF. The calibrator curve was diluted in 2-fold from 200
ng/mL,
whereas native material was run undiluted to 1:8 as indicated (--). FIG. 3C:
Calibration
curve and inhibition of the competitive PRO-C3 ELISA using healthy mouse serum
and
plasma. The calibrator curve was diluted in 2-fold from 200 ng/mL, whereas
native
material was run undiluted to 1:4 as indicated (--). FIG. 3D: Neo-epitope
specificity of
the PIIINP neo-epitope specific antibody using elongated peptide, i.e. peptide
sequence
of calibration peptide with one additional amino acid in the C-terminal end.
The
8
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
calibration curve, elongated peptide, and non-sense peptide were diluted in 2-
fold from
76.31 ng/mL. The signal is seen as the optical density at 450 nm, subtracting
the
background at 650nm, as a function of peptide concentration.
FIG. 4: Results of an in vitro model of lung fibroblasts ("scar-in-a-jar").
FIG. 5: A comparison of Pro-C3X levels in extractions from keloids and
extractions from normal skin.
FIGS. 6A-6B: Results of a study of patients with liver fibrosis.
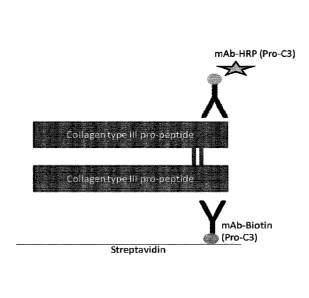
FIG. 7: Pictorial representation of the Pro-C3X assay.
FIG. 8: Results of a study of patients with Alcoholic steatohepatitis.
FIG. 9: Pro-C3X levels in supernatant collected at Day 10 from the Scar-in-a-
Jar
model. Significance was assessed by one-way ANOVA with Dunnett's multiple
comparisons test comparing each condition with TGF-8 alone. Data are shown as
mean
with SD. *"*p<0.0001. BAPN, (3-aminopropionitrile; TGF-8, transforming growth
factor
P.
Description of the Invention
As used herein the term "neo-epitope" refers to an N- or C-terminal peptide
sequence at the extremity of a polypeptide, i.e. at the N- or C- terminal end
of the of the
polypeptide, and is not to be construed as meaning in the general direction
thereof.
As used herein the term, the term "competitive ELISA" refers to a competitive
enzyme-linked immunosorbent assay and is a technique known to the person
skilled in
the art.
9
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
As used herein the term "sandwich immunoassay" refers to the use of at least
two antibodies for the detection of an antigen in a sample, and is a technique
known to
the person skilled in the art.
As used herein the term, the monoclonal antibody NB61N-62 refers to a neo-
epitope specific antibody directed towards the C-terminal neo-epitope of
PIIINP, said
neo-epitope comprising the C-terminal sequence CPTGXQNYSP-COOH (SEQ ID NO:
4), wherein X is Gly or Pro.
As used herein the term, the term "PRO-C3" is used to distinguish the herein
described PIIINP assay from the PIIINP assays known in the art which are not
based on
the specific binding of neo-epitopes originating from PIIINP.
As used herein the term "PRO-C3X" assay refers to the herein described
sandwich immunoassay for detecting and quantifying cross-linked PIIINP.
A monoclonal antibody suitable for use in the method of the invention was
disclosed in WO 2014/170312 and is specifically reactive with a C-terminal neo-
epitope
of PIIINP, said neo-epitope being comprised in a C-terminal amino acid
sequence
CPTGXQNYSP-COOH (SEQ ID NO:4), wherein X is Gly or Pro, and wherein said
monoclonal antibody does not substantially recognise or bind an elongated
version of
said C-terminal amino acid sequence which is CPTGXQNYSPQZ-COOH (SEQ ID NO:
5), wherein Z is absent or is one or more amino acids of the sequence of
collagen type
III.
Preferably, the monoclonal antibody is specifically reactive with the neo-
epitope
C-terminal sequence CPTGPQNYSP-COOH (SEQ ID NO: 6) in human PIIINP, which is
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
formed by the N-protease cleavage of PIIINP from intact procollagen type III
at the Pro-
Gin bond between amino acids P153-Q154 in human PIIINP.
Alternatively, the monoclonal antibody may be specifically reactive with the
neo-
epitope C-terminal sequence CPTGGQNYSP-COOH (SEQ ID NO: 7) in rodent PIIINP,
which said neo-epitope is formed by the N-protease cleavage of PIIINP from
intact
procollagen type III at the Pro-Gin bond between amino acids P154-Q155 in
rodent
PIIINP.
Preferably, the ratio of the affinity of the monoclonal antibody for amino
acid
sequence CPTGXQNYSP-COOH (SEQ ID NO: 4) to the affinity of said monoclonal
antibody for elongated amino acid sequence CPTGXQNYSPQZ-COOH (SEQ ID NO: 5)
is at least 10 to 1, preferably at least 100 to 1, more preferably at least
1,000 to 1, more
preferably at least 10,000 to 1, more preferably at least 100,000 to 1, and
most
preferably at least 1,000,000 to 1.
Preferably, the monoclonal antibody does not recognise or bind a shortened
version of a C-terminal neo-epitope of PIIINP, said shortened neo-epitope
having the
amino acid sequence CPTGXQNYS (SEQ ID NO: 8).
Preferably, the ratio of the affinity of the monoclonal antibody for amino
acid
sequence CPTGXQNYSP-COOH (SEQ ID NO: 4) to the affinity of said monoclonal
antibody for shortened amino acid sequence CPTGXQNYS-COOH (SEQ ID NO: 8) is at
least 10 to 1, preferably at least 100 to 1, more preferably at least 1,000 to
1, more
preferably at least 10,000 to 1, more preferably at least 100,000 to 1, and
most
preferably at least 1,000,000 to 1.
11
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
The present invention relates to a sandwich immunoassay for detecting in a
biological sample cross-linked PIIINP, said cross-linked PIIINP comprising at
least two
strands of PIIINP joined together by inter-strand cross-linking, said method
comprising:
contacting said biological sample comprising said cross-linked PIIINP with a
first
monoclonal antibody bound to a surface, wherein each strand of PIIINP
comprised in
the cross-linked PIIINP comprises a C-terminal neo-epitope of PIIINP generated
by N-
protease cleavage of intact type III procollagen;
adding a second monoclonal antibody; and
determining the amount of binding of said second monoclonal antibody;
wherein both said first monoclonal antibody and said second monoclonal
antibody are specifically reactive with said C-terminal neo-epitope of PIIINP,
said neo-
epitope being comprised in a C-terminal amino acid sequence CPTGXQNYSP-COOH,
wherein X is Gly or Pro.
Preferably, the monoclonal antibody does not substantially recognise or bind
an
elongated version of said C-terminal amino acid sequence which is CPTGXQNYSPQZ-
COOH, wherein Z is absent or is one or more amino acids of the sequence of
collagen
type III.
The herein described sandwich immunoassay uses the same antibody as both
catcher and detector antibody, therefore a double strand peptide (i.e. cross-
linked) can
be recognized by the assay.
12
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
Preferably, the sandwich immunoassay is used to quantify the amount of cross-
linked PIIINP in a biofluid, wherein said biofluid may be, but is not limited
to, serum,
plasma, urine, amniotic fluid, tissue supernatant or cell supernatant.
The sandwich immunoassay may be, but is not limited to, a radioimmunoassay,
fluorescence immunoassay, or an enzyme-linked immunosorbent assay.
In a preferred embodiment, the second monoclonal antibody may be labeled in
order to determine the amount of binding of said second monoclonal antibody.
Preferably, the second monoclonal antibody may be an enzyme-linked antibody.
The enzyme may be, but is not limited to, horseradish peroxidase (HRP).
Preferably, the second monoclonal antibody may be radiolabeled or linked to a
fluorophore.
Although these are preferred labels to be used with the invention, it is
envisaged
that any suitable labeling system may be employed, such as, but not limited
to, DNA
reporters or electrochemiluminescent tags.
Alternatively, a further labeled antibody which recognises the second
monoclonal
antibody may be used to determine the amount of binding of said second
monoclonal
antibody. The further labeled antibody may be labeled using a label as
described above.
In a preferred embodiment of the invention, the sandwich immunoassay may
further comprise correlating the quantity of cross-linked PIIINP determined by
said
method with standard fibrotic disease samples of known disease severity to
evaluate
the severity of a fibrotic disease. Such a fibrotic disease may be, but is not
limited to,
liver disease.
13
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
In a further aspect, the sandwich immunoassay described herein may be used in
a method for evaluating the efficacy of a drug targeting lysyl oxidases
(LOXs), such as
an antagonist drug targeting LOXs.
Accordingly, the present invention also relates to a method for evaluating the
.. efficacy of an antagonist drug targeting lysyl oxidases (LOXs), wherein
said method
comprises using the sandwich immunoassay described herein to quantify the
amount of
cross-linked PIIINP in at least two biological samples, said biological
samples having
been obtained from a subject at a first time point and at least one subsequent
time point
during a period of administration of the antagonist drug to said subject, and
wherein a
.. reduction in the quantity of cross-linked PIIINP from said first time point
to said at least
one subsequent time point during the period of administration of the
antagonist drug is
indicative of an efficacious antagonist drug targeting LOXs.
Preferably, the method quantifies the efficaciousness of the antagonist drug.
Preferably, the method evaluates the efficacy of an antagonist drug targeting
LOXL2.
In another aspect, the present invention relates to a kit for use in the
sandwich
immunoassay as described herein, the kit comprising a solid support to which
is bound
a first monoclonal antibody as described above; and a labelled second
monoclonal
antibody as described above.
14
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
Examples
Materials and general considerations
All reagents used in the experiments were high-standard chemicals from
companies such as Merck (Whitehouse Station, NJ, USA) and Sigma Aldrich (St.
Louis,
MO, USA). The synthetic peptides used for monoclonal antibody production and
validation were 1) Immunogenic peptide: Ovalbumine (OVA)-CGG-CPTGPQNYSP
(SEQ ID NO: 10), 2) Screening peptide: Biotin-CGG-CPTGPQNYSP (SEQ ID NO: 11),
and 3) Selection peptide: CPTGPQNYSP (SEQ ID NO 6). All synthetic peptides
were
purchased from the Chinese Peptide Company, Beijing, China.
Example 1 - Monoclonal antibody NB61-N62
Monoclonal antibody generation
The sequence for the N-terminal propeptide of type III collagen was aligned
between human, rat and mouse species and selected from homology between the
species and uniqueness among other ECM proteins by protein blasting. The amino
acid
sequence 145'-CPTGPQNYSP-'153 (SEQ ID NO: 6) in the al chain PIIINP is 100%
homologues between human and rat (FIG. 1). Generation of monoclonal antibodies
was
initiated by subcutaneous immunization of 4-5 week old Balb/C mice with 200 pl
emulsified antigen and 50 pg PIIINP neo-epitope C-terminal sequence (OVA-CGG-
CPTGPQNYSP (SEQ ID NO: 10)) using Freund's incomplete adjuvant. The
immunizations were repeated every 2 weeks until stable serum titer levels were
reached. The mouse with the highest serum titer was selected for fusion. The
mouse
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
was rested for a month and then boosted intravenously with 50 pg PIIINP neo-
epitope
C-terminal sequence in 100 pl 0.9% NaCI solution three days before isolation
of the
spleen. The spleen cells were fused with SP2/0 myeloma cells to produce
hybridoma as
described by (34), and cloned in culture dishes using the semi-medium method.
The
clones were plated into 96-well microtiter plates for further growth employing
the limited
dilution method to secure monoclonal growth. The supernatants were screened
for
reactivity against calibrator peptide and native material in an indirect ELISA
using
streptavidin-coated plates. Biotin-CGG-CPTGPQNYSP (SEQ ID NO: 11) was used as
screening peptide, while the free peptide CPTGPQNYSP (SEQ ID NO: 6) was used
as
calibrator to test for further specificity of clones.
Clone characterization
Native reactivity and affinity of the peptide were assessed using different
biological materials such as urine, serum, and amniotic fluid (AF) from both
humans and
rats in a preliminary ELISA using 2 ng/ml biotinylated peptide on streptavidin-
coated
microtiter plates and the supernatants from growing monoclonal hybridoma
cells.
Human AF was obtained from 30 women undergoing elective lower segment
Caesarean sections at the Beijing Obstetrics Gynecology Hospital over a 2
month
period. 100-200 ml AF was collected directly after incision and the fluid was
stored at -
C until use. The local ethical board had approved the study and all women
provided
20 written consent prior to collection. Rat AF was drawn from the uterus of
pregnant Wistar
rats two days prior to expected birth. Antibody specificity was tested in a
preliminary
assay using deselection and elongated peptides (i.e. calibrator peptide with
ten amino
16
acid substitutions and calibrator peptide with one additional amino acid at
the cleavage
site, respectively). The isotype of the monoclonal antibodies was determined
using the
Clonotyping System-HRP kit, cat. 5300-05 (Southern Biotech, Birmingham, AL,
USA).
Antibody characterization
Prior to Western Blotting, the total protein concentration of human and rat AF
was measured using Bicinchoninic acid (BCA) Protein Assay according to
manufacturer's instruction. Briefly, BCA was diluted 2-fold in PBS from 2
mg/ml to
produce a standard row for calculation of the samples. Samples were diluted
1:4 in lx
phosphate-buffered saline (PBS) and 25 pl sample was added to a microtiter
plate
along with 200 pl working reagent (Reagent A and B mixed in the ratio 50:1).
The
content was mixed on a plate shaker for 30 seconds followed by incubation for
30
minutes at 37 C. After ended incubation the plate was cooled to room
temperature and
the absorbance was measured in the ELISA reader at 562 nm (Molecular Devices,
SpectraMax M, CA, USA). Hereafter, rat or human AF was mixed with sample
buffer
(x2) and reducing agent (x10), heated at 70 C for 10 minutes, loaded on a 4-
20% tris-
glycein sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-page),
and
run for 1 hour at 180V. Protein bands were blotted onto a nitrocellulose
membrane
using the Invitrogen i-Blot gel transfer system according to manufacturer's
instruction.
The membrane was blocked in blocking buffer (5% skimmed milk in Tris-buffered
saline
with TweenTm (TBST) overnight at 4 C and incubated with 1 pg/ml horseradish
peroxidase (HRP)-conjugated PIIINP neo-epitope specific monoclonal antibody
NB61N-
62 for 2 hours. Specificity of the PIIINP neo-epitope specific monoclonal
antibody was
17
Date recue/Date received 2023-04-19
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
investigated by addition of excess PIIINP neo-epitope calibrator peptide and
antibody in
the ratio 10:1 and allowed to pre-incubate for 1 hour before it was added to
the
membrane for overnight incubation. After incubation the membranes was washed
4x10
minutes in TBST, incubated with 4 ml chemiluminescence detection kit (ECL),
and
developed using Amersham Hyperfilm.
Clone selection and characterization
The subtype was determined to be an IgG1 subtype. From the Western Blot
analysis it was seen that the PIIINP neo-epitope specific monoclonal antibody
NB61N-
62 recognized two bands with molecular sizes around 52-60 kDa in rat amniotic
fluid,
while only one band around 52 kDa was detected in human amniotic fluid. In
addition,
the signal could be partly inhibited by the selection peptide in the rat, and
inhibited in
human (FIG. 2). Native reactivity was observed using the NB61N-62 antibody in
the
ELISA. Native reactivity was seen towards human serum, plasma, and AF as well
as
against rodent serum, plasma, and AF (FIGS. 3A-3C). The signal was slightly
less
inhibited against mouse serum and plasma. The signal of the competitive ELISA
was
inhibited using from 1:2 to 1:16, undiluted to 1:8, or undiluted to 1:4 in
human, rodent,
and mouse native material, respectively. Dilution of the native material
approximately
followed the same dilution pattern as the calibrator curve for all three
species. Human
AF inhibited the signal up to 100%; 80% for rat AF; 70% for human serum and
plasma
and rat serum; 44% for rat plasma, and 35% for mouse serum and plasma. Zero
inhibition was observed using the elongated peptide (CPTGPQNYSPQ (SEQ ID NO:
6))
and non-sense peptide (GSPGKDGVRG (SEQ ID NO: 12)) (FIG. 3D).
18
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
Example 2¨ PRO-C3 ELISA using NB61N-62
Supernatant from the antibody producing hybridoma was collected and the
monoclonal antibody was purified using HiTrap affinity columns (GE Healthcare
Life
Science, Little Chalfont, Buckinghamshire, UK) and labeled with HRP using
Lightning-
LinkTM HRP Conjugation Kit (Innova Biosciences, Babraham, Cambridge, UK),
according to the manufacturer's instructions.
The PRO-C3 competitive ELISA procedure was as follows: A 96-well
streptavidin-coated ELISA plate from Roche, cat.11940279, was coated with the
biotinylated peptide Biotin-CGG-CPTGPQNYSP (SEQ ID NO: 11) dissolved in coater
buffer (50mM PBS-BTE + 10% sorbitol, pH 7.4), incubated for 30 min at 20 C in
the
dark and subsequently washed in washing buffer (20 mM Tris, 50 mM NaCI, pH
7.2).
Thereafter 20 pl of peptide calibrator or sample were added to appropriate
wells,
followed by 100 pl of HRP-conjugated monoclonal antibody NB61N-62 dissolved in
incubation buffer (50 mM PBS-BTB + 10% LiquidII (Roche), pH 7.4) and the plate
was
incubated for 20 hours at 4 C and washed. Finally, 100 pl
tetramethylbenzinidine (TMB)
(Kem-En-Tec cat.: 4380H) was added, the plate was incubated for 15 min at 20 C
in
the dark and in order to stop the reaction, 100 pl of stopping solution (1%
H2504) was
added and the plate was analyzed in the ELISA reader at 450 nm with 650 nm as
the
reference (Molecular Devices, SpectraMax M, CA, USA). A calibration curve was
plotted using a 4-parametric mathematical fit model.
19
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
Technical Evaluation
A 2-fold dilution of healthy serum and plasma samples from human and rats were
used to determine linearity and calculated as percentage of recovery of the
100%
sample. Antibody specificity was calculated as percentage of recovery of the
100%
calibrator peptide (CPTGPQNYSP (SEQ ID NO: 6)), elongated peptide
(CPTGPQNYSPQ (SEQ ID NO: 13)), and non-sense peptide (GSPGKDGVRG (SEQ ID
NO: 12)). Lower limit of detection (LLOD) was calculated as the mean +
3xStandard
Deviation (SD) of the blank from 21 determinations of standard K (i.e.
buffer). Upper
limit of detection (ULOD) was determined as the mean ¨ 3xSD of 10 measurements
of
Standard A. Lower limit of quantification (LLOQ) was determined as the lowest
concentration reproducibly measured with a precision lower than 30%. The intra-
and
inter-assay variation was determined by 10 independent runs of 8 QC samples,
with
each run consisting of double determination of the samples. Accuracy of the
samples
was measured in healthy human serum samples spiked with standard curve or
human
amniotic fluid at significant concentrations and calculated as the percentage
recovery of
the theoretical amount of serum. Interference was measured in healthy human
serum
spiked with hemoglobin, lipemia, and biotin at significant concentrations and
calculated
as the percentage recovery of the theoretical amount of serum.
Results
The measurement range of the human PRO-C3 ELISA was determined by
calculating ULOD and LLOQ providing a range from 0.867-60.1 ng/ml with a LLOD
of
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
0.606 ng/ml. The technical performance of the PRO-C3 ELISA showed acceptable
inter-
and intra assay variation of mean 11.03% and 4.11% (Table 1), with acceptance
range
below 15% and 10%, respectively.
Table 1: Inter- and intra-assay variation for the PRO-C3 assay using human
serum
quality control samples # 1-8 (HS1- HS8). The variation was calculated as the
mean
variation between 10 individual determinations of each sample.
Sample Value Intra-assay Inter-assay
(ng/mL) variability % variability %
HS1 24.24 2.28 5.94
HS2 11.62 2.90 6.45
HS3 8.40 5.31 11.99
HS4 6.54 4.46 11.31
HS5 6.36 3.88 13.09
HS6 5.23 3.98 12.31
HS7 4.29 3.53 12.94
HS8 2.98 4.66 18.56
Dilution recovery was performed using healthy serum and plasma samples from
humans, rat and mouse. The dilution recovery was within the acceptable 100 20%
recovery (Table 2). Further dilution resulted in measurements below LLOQ.
Table 2: Percentage dilution recovery for the PRO-C3 assay using human-, rat-,
and
mouse samples. Human serum (HS), Human plasma (HP), Rat serum (RS), Mouse
serum (MS), Mouse plasma (MP).
PIIINP HS HP RS MS MP
ng/ml (n=2) (n=3) (n=10) (n=2) (n=2)
21
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
Undiluted 100% - 100% 100% -
Dilution 1:2 98 100% 116 96 100%
Dilution 1:4 103 91 110 118 114
Dilution 1:8 114 87 - - -
Dilution 1:16 - 92 - - -
Mean 105 90 113 107 114
Spiking of calibrator peptide in serum or plasma resulted in a mean recovery
of
56% and 55%, respectively (Table 3).
Table 3: Spiking recovery of calibrator peptide in human serum or plasma, and
human
AF in human serum or plasma. The recovery was calculated as percent recovery
of
calculated peptide/AF in serum/plasma compared to pure serum/plasma.
Concentration
of calibrator peptide were 38.16 ng/ml (StdB), 19.08 ng/ml (StdC), 9.54 ng/ml
(StdD),
4.77 ng/ml (StdE), 2.39 ng/ml (StdF) and 1.19 ng/ml (StdG). AF was added in 2-
fold
dilution starting from 1:2.
Serum (n=3) Plasma Serum (n=3) Plasma
Added Std sRE% (n=3) Added AF sRE% (n=3)
sRE% sRE%
StdB 16 15 2x 101 103
StdC 29 25 4x 103 108
StdD 42 38 8x 106 113
StdE 58 54 16x 103 112
StdF 70 69 32x 104 115
StdG 82 83 64x 103 110
Buffer 92 100 Buffer 99 104
Mean sRE% 56 100 Mean sRE% 55 111
22
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
However, spiking of human AF in 2-fold dilution starting from 1:2 into healthy
human serum or plasma resulted in mean recovery of 100% and 111%,
respectively. No
interference was observed in serum spiked with different concentrations of
hemoglobin,
biotin, and lipemia (Table 4).
Table 4: Interference of hemoglobin, lipemia and biotin in human serum added
in
various concentrations. All data are shown as percent recovery compared to
pure
serum.
Hemoglobin Lipemia Biotin
mmol/L RE% mmol/L RE% ng/L RE%
0.5 68 0.56 101 160,000 134
_ -
0.25 74 0.28 103 80,000 113
0.13 81 0.14 99 40,000 96
0.063 81 0.07 104 20,000 97
0.031 82 0.04 101 10,000 94
0.016 86 0.00 100 5,000 87
_
0.008 95 2,500 100
0.000 100 0 100
,
Mean 83 101 103
The stability of the analyte was acceptable up to four freeze/thaw cycles with
100 20% recovery compared to 1 freeze/thaw cycle (Table 5).
Table 5: Analyte stability in three human serum and plasma samples in four
freeze/thaw
cycles. All data are shown as mean percent recovery compared to 1 freeze/thaw
cycle.
Freeze/thaw Serum EDTA plasma Heparin plasma Citrate plasma
cycle Mean recovery Mean recovery Mean recovery Mean recovery
% %
23
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
1 100% 100% 100% 100%
2 103 102 103 109
3 99 99 98 103
4 102 100 98 100
Example 3¨ Determining the ratio of binding affinity
To determine the ratio of the binding affinity of the monoclonal antibody for
the
target sequence to the binding affinity of the monoclonal antibody for the
elongated or
shortened sequence, each of the sequences are synthesized and used as
calibrator
peptides in the PRO-C3 ELISA as described in example 2. The resultant
calibration
curves are used to determine the IC50 values of each sequence/antibody
combination.
The ratio of IC5o[target] / IC50[elongated or shortened] defines the ratio of
binding
affinity.
Example 4 - PRO-C3X Assay
As noted above, enzymatic collagen crosslinking by lysyl oxidases (LOXs) and
processing of pro-collagens is key for tissue maturation and stability. Thus,
monitoring
inter-strand cross-linking of pro-collagen type III prior to enzymatic
processing may
prove useful for monitoring in-vivo activity of LOXs. This can be achieved by
detecting
and quantifying cross-linked PIIINP (i.e. two or more strands of PIIINP bound
together
by inter-strand links formed by LOXs in pro-collagen type III prior to
enzymatic
processing of the pro-collagen). A higher level of cross-linked PIIINP
detected in the
circulation would be indicative of greater LOX activity. Accordingly,
monitoring the level
24
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
of cross-linked PIIINP during drug trials for drugs targeting LOX, such as LOX
antagonists, could provide useful efficacy data for said drugs.
ELISA
Streptavidin-coated plates were coated with 100 p1/well of 1 pg/ml
biotinylated
catcher antibody (biotin-linked NB61-N62) and incubated at 20 C, 300 rpm
shaking for
30 minutes. Plates were washed five times in washing buffer (20 nM IRIS, 50 mM
NaCI, pH 7.2). Sample, standard or control (20 pl) was added and followed
immediately
by addition of 100 pl assay buffer and incubated at 4 C, 300 rpm shaking for
20 hours.
After incubation, plates were washed five times in washing buffer. 100 p1/well
of 1 pg/ml
HRP-labelled detector antibody (HRP-linked NB61-N62) was added and incubated
at
C, 300 rpm, shaking for 1 hour. After incubation, plates were washed five
times in
washing buffer. A volume of 100 pl 3,3',5,5' -Tetramethylbenzidine (TMB) was
added
and incubated for 15 min at 20 C in the dark. To stop the enzyme reaction of
TMB, 100
15 pl 0.1% sulphuric acid was added. The enzyme reaction was then read on an
ELISA
reader, using a quadratic curve fit. Each ELISA plate included both kit
control and in-
house quality control samples to monitor inter-assay variation. All samples
were
measured within the range of the specific assay. All samples below the level
of lower
limited of quantification (LLOQ) were assigned the value of LLOQ.
20 The technical characteristics of the assay are:
Parameter Results
Biological matrix Serum, plasma, supernatants, extraction
fluid.
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
Intra-assay variation 2% (accepted if <10%)
Inter-assay variation 6% (accepted if <15%)
Measurement range 0.965-17.586 ng/ml
Lower limit of detection 0.251 ng/ml
Normal range in healthy serum 4.022 ( 2.24) ng/ml
Required volume 30 pl serum/plasma; 60 pl
supernatant/extraction
Spiking recovery Peptide in serum: 94%
Serum in serum: 86%
Results
Scar Tissue
Preliminary results using an in vitro model of lung fibroblasts ("scar-in-a-
jar")
strongly suggest that an enzyme of the LOX family is the responsible for the
cross-link
in PIIINP (TGF-8 is known in the art to increase lysyl oxidase (LOX) enzyme
activity).
Briefly, Pro-C3X (i.e. cross-linked PIIINP) was generated by culturing lung
fibroblasts for
5 days in crowded conditions and under TGF48 stimulation. Pro-C3X was
significantly
elevated after 12 days of culturing with TGF-13, with negligible quantities of
Pro-C3X
being observed in the absence of TGF-8 (FIG. 4). Similarly, Pro-C3X was
elevated in
extractions from keloids when compared to extractions from normal skin (FIG.
5).
Liver Fibrosis
A study of patients with liver fibrosis was conducted using the "Pro-C3X"
assay
and compared to the herein described "Pro-C3" competitive ELISA. It was found
that
26
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
Pro-C3X was significantly elevated in later stages of disease, when fibrosis
is more
severe, with levels similar to healthy controls in early stages of disease
(FIG. 6A). In
comparison, Pro-C3 levels differed at all stages of the disease (FIG. 6B).
The difference in selectivity between the Pro-C3X assay and Pro-C3 assay is
attributed to the Pro-C3X assay only recognizing cross-linked PIIINP, whereas
the Pro-
C3 assay recognises both cross-linked and non-cross-linked PIIINP. FIG. 7
shows the
Pro-C3X assay and provides a pictorial explanation for the reasoning behind
this
conclusion:
Pro-C3X assay: if cross linked PIIINP is present then the first antibody will
bind to the free epitope on a first strand of PIIINP and subsequently the
second
antibody will bind to the free epitope on a second strand of PIIINP. However,
if non-
cross-linked PIIINP is present then the surface-bound antibody will bind to
the free
epitope of the non-cross linked collagen type III, but the second antibody
will fail to bind
as the binding epitope is already occupied, therefore addition of the second
antibody will
fail to produce a signal. Thus, the signal from the Pro-C3X assay is
exclusively due to
the detection of cross linked PIIINP.
Conversely, substantially all of the antibodies in the Pro-C3 assay will bind
to strands of PIIINP comprising a free binding epitope, irrespective of
whether the
PIIINP is or is not cross-linked. Thus, the signal obtained from the Pro-C3
assay is an
aggregate signal of cross-linked and non-cross-linked PIIINP.
Accordingly, it is this selectivity that prompts the use of the Pro-C3X assay
for
evaluating the efficacy of drugs targeting LOX; monitoring the level of PIIINP
cross-
27
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
linking which, as noted above, is suggested to be a result of LOX activity
during a period
of drug administration to a subject could be used to monitor drug activity and
thus
efficacy of said drug.
Alcoholic steatohepatitis
A study of patients with alcoholic steatohepatitis was conducted using the
"Pro-
C3X" assay and compared to the herein described "Pro-C3" assay. In alcoholic
steatohepatitis, both Pro-C3 and Pro-C3X were elevated in later stages of the
disease
(Metavir 2-4). However, for patients with cirrhosis Pro-C3 did not correlate
with MELD
(Model For End-Stage Liver Disease) score (P=0.527), whereas Pro-C3X strongly
correlated (corr coefficient 3.34, P<0.001). Moreover, ProC3 only correlated
negatively
with albumine, while ProC3X correlated with albumine, bilirubine and Gamma-
Glutamyl
Transpeptidase (GGT) (FIG. 8). The late stage increase in Pro-C3X suggests
increase
in cross-linking of PIIINP, which would be in accordance with increased
scarring of the
liver (i.e. increased LOX activity).
Example 5 Assessment of Pro-C3X in the Scar-in-a-Jar model
Background: Fibrosis is the accumulation of extracellular matrix (ECM) within
affected
tissues, which can lead to organ failure and ultimately death. Following
stimulation by
e.g. transforming growth factor (TGF)-13, fibroblasts are the main cell type
responsible
for the excessive accumulation of ECM proteins, especially collagens. Here we
describe
the use of the in vitro model "Scar-in-a-Jar", known to generate ECM and cross-
links, in
28
CA 03009445 2018-06-21
WO 2017/134172
PCT/EP2017/052271
combination with the Pro-C3X ELISA to investigate collagen formation and cross-
linking
during fibrogenesis. This tool can be used in the investigation of novel anti-
fibrotic
compounds by assessing the modulation of these fibrotic processes.
Methods: Healthy human lung fibroblasts (L248) were grown to confluence, after
which
they were seeded at a density of 30,000 cells/well. The cells were grown in
DMEM
media containing 0.4% FCS, 225 mg/mL ficoll 70, 150 mg/mL ficoll 400, and 1%
ascorbic acid for 18 days. The cells were stimulated with 1 ng/mL TGF-6 with
or without
the lysyl oxidase (LOX) inhibitor 6-aminopropionitrile (BAPN; 0.02 or 0.2 mM)
to inhibit
formation of cross-links. Unstimulated cells or cells grown in media not
containing ficoll
were used as controls. Media was changed on day 3, 6, 10, and 14. Fibroblast
viability
was assessed using the AlamarBlue assay. Pro-C3X levels were assessed in the
collected supernatant, using the Pro-C3X sandwich ELISA described above.
.. Results: TGF-6 stimulation induced the release of Pro-C3X from day 3 with a
peak in
Pro-C3X levels at day 10 showing a 14-fold increase as compared with
unstimulated
cells (p<0.0001; Figure 9). Treatment with 0.02 mM BAPN had no significant
effect, but
0.2 mM BAPN induced a significant decrease in Pro-C3X levels as compared with
TGF-
6 stimulation only (0.59-fold change, p<0.0001; Figure 9).
Conclusions: The pan LOX inhibitor BAPN significantly reduced Pro-C3X levels
at a
concentration of 0.2 mM, indicating that the Pro-C3X ELISA assesses a cross-
linked
29
epitope. Thus. the Pro-C3X ELISA can be used to evaluate fibroblast activity
and thus
be used to screen potential anti-fibrotic compounds. TGF-13 stimulation
induced the
release of Pro-C3X, a marker of the cross-linked collagen type III pro-
peptide.
In conclusion, the Pro-C3X assay is a second generation assay combining the
cleavage neo-epitope of the collagen type III pro-peptide and the presence of
cross-
linking in the molecule. This assay is therefore providing additional
information to the
measurement of Pro-C3, because it describes a different process in the
fibrosis
timeline; that is, the cross-linking of collagen molecules in the scar. The
Pro-C3X assay
can therefore be used to test the efficacy of drugs targeting LOX,
particularly LOX
antagonists/inhibitors, since the use of a LOX inhibitor was shown to reduce
the
presence of the cross-linked PIIINP biomarker.
In this specification, unless expressly otherwise indicated, the word 'or' is
used in
the sense of an operator that returns a true value when either or both of the
stated
conditions is met, as opposed to the operator 'exclusive or' which requires
that only one
of the conditions is met. The word 'comprising' is used in the sense of
'including' rather
than in to mean 'consisting of. No acknowledgement of any prior published
document
herein should be taken to be an admission or representation that the teaching
thereof
was common general knowledge in Australia or elsewhere at the date hereof.
***
In some aspects, embodiments of the present disclosure as described herein
include the following items:
Date recue/Date received 2023-04-19
Item 1. A sandwich immunoassay method for detecting in a biological sample
cross-linked PIIINP, said cross-linked PIIINP comprising at least two strands
of PIIINP
joined together by inter-strand cross-linking, said method comprising:
contacting said biological sample comprising said cross-linked PIIINP with a
first
monoclonal antibody bound to a surface, wherein each strand of PIIINP
comprised in
the cross-linked PIIINP comprises a C-terminal neo-epitope of PIIINP generated
by N-
protease cleavage of intact type III procollagen;
adding a second monoclonal antibody; and
determining the amount of binding of said second monoclonal antibody;
wherein both said first monoclonal antibody and said second monoclonal
antibody are specifically reactive with said C-terminal neo-epitope of PIIINP,
said neo-
epitope being comprised in a C-terminal amino acid sequence CPTGXQNYSP-COOH
of SEQ ID NO: 4, wherein X is Gly or Pro, wherein said first monoclonal
antibody and
said second monoclonal antibody bind to different strands of the crosslinked
PNIIIP.
Item 2. The sandwich immunoassay method of item 1, wherein the first
monoclonal antibody and/or the second monoclonal antibody does not recognise
or bind
an elongated version of said C-terminal amino acid sequence which is
CPTGXQNYSPQZ-COOH of SEQ ID NO: 5, wherein Z is absent or is one or more
amino acids of the sequence of collagen type III.
31
Date recue/Date received 2023-04-19
Item 3. The sandwich immunoassay method of item 1 01 2, wherein the sandwich
immunoassay method is used to quantify the amount of cross-linked PIIINP in
the
biological sample.
Item 4. The sandwich immunoassay method of item 3, further comprising
correlating the quantity of cross-linked PIIINP determined by said method with
standard
fibrotic disease samples of known disease severity to evaluate the severity of
a fibrotic
disease.
Item 5. The sandwich immunoassay method of item 4, wherein the fibrotic
disease is liver disease.
Item 6. The sandwich immunoassay method of any one of items 3 to 5, wherein
the biological sample is a biofluid.
Item 7. The sandwich immunoassay method of item 6, wherein said biofluid is
serum, plasma, urine, amniotic fluid, tissue supernatant or cell supernatant.
Item 8. The sandwich immunoassay method of any one of items 1 to 7, wherein
the sandwich immunoassay is a radioimmunoassay, fluorescence immunoassay, or
an
enzyme-linked immunosorbent assay.
32
Date recue/Date received 2023-04-19
Item 9. The sandwich immunoassay method of any one of items 1 to 8, wherein
the second monoclonal antibody is labeled.
Item 10. The sandwich immunoassay method of item 9, wherein the second
monoclonal antibody is an enzyme-linked antibody.
Item 11. The sandwich immunoassay method of item 10, wherein the enzyme is
horseradish peroxidase (HRP).
Item 12. The sandwich immunoassay method of item 9, wherein the second
monoclonal antibody is radiolabeled or linked to a fluorophore.
Item 13. The sandwich immunoassay method of any one of items 1 to 8, wherein
a further labeled antibody which recognises the second monoclonal antibody is
used to
determine the amount of binding of said second monoclonal antibody.
Item 14. A method for evaluating the efficacy of an antagonist drug targeting
lysyl
oxidases (LOXs), wherein said method comprises using the sandwich immunoassay
method of item 1 to quantify the amount of cross-linked PIIINP in at least two
biological
samples, said biological samples having been obtained from a subject at a
first time
point and at at least one subsequent time point during a period of
administration of the
antagonist drug to said subject, and wherein a reduction in the quantity of
cross-linked
33
Date recue/Date received 2023-04-19
PIIINP from said first time point to said at least one subsequent time point
during the
period of administration of the antagonist drug is indicative of an
efficacious antagonist
drug targeting LOXs.
Item 15. The method of item 14, wherein the method evaluates the efficacy of
an
antagonist drug targeting LOXL2.
The following references are cited herein:
.. 1. World Health Organization. Reducing Risks, Promoting Healthy Life.
Peducing
Risks, Promoting Healthy Life, Geneva: WHO, 2002:1-230.
2. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol
2008;214:199-
210.
3. Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and
therapeutic implications. Nat Clin Pract Gastroenterol Hepatol 2004;1:98-105.
4. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and
mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol
2002;3:349-
363.
5. Wynn TA. Common and unique mechanisms regulate fibrosis in various
fibroproliferative diseases. J Clin Invest 2007;117:524-529.
6. Bosman,F.T., and Stamenkovic,I. 2003. Functional structure and composition
of the
extracellular matrix. J. Pathol. 200:423-428.
7. Bruckner,P. 2010. Suprastructures of extracellular matrices: paradigms of
functions
controlled by aggregates rather than molecules. Cell Tissue Res. 339:7-18.
34
Date recue/Date received 2023-04-19
8. Bao X, Zeng Y, Wei S, Wang G, Liu C, Sun Y, Chen Q, and Li H. Developmental
changes of Col3a1 m RNA expression in muscle and their association with
intramuscular
collagen in pigs. J Genet Genomics 2007; 34(3): 223-228.
9. Jensen LT and Host NB. Collagen: scaffold for repair or execution.
Cardiovasc Res
1997; 33(3): 535-539.
10. Niemela 0, Risteli L, Parkkinen J, and Risteli J. Purification and
characterization of
the N-terminal propeptide of human type III procollagen. Biochem J 1985;
232(1): 145-
150.
11. Wang WM, Ge G, Lim NH, Nagase H, and Greenspan DS. TIMP-3 inhibits the
procollagen N-proteinase ADAMTS-2. Biochem J 2006; 398(3): 515-519.
12. Van den Steen PE, Opdenakker G, Wormald MR, Dwek RA, and Rudd PM. Matrix
remodelling enzymes, the protease cascade and glycosylation. Biochim Biophys
Acta
2001; 1528(2-3): 61-73.
13. Cuzner ML and Opdenakker G. Plasminogen activators and matrix
metalloproteases, mediators of extracellular proteolysis in inflammatory
demyelination
of the central nervous system. J Neuroimmunol 1999; 94(1-2): 1-14.
14. Meduri GU, Tolley EA, Chinn A, Stentz F, and Postlethwaite A. Procollagen
types I
and III aminoterrninal propeptide levels during acute respiratory distress
syndrome and
in response to methylprednisolone treatment. Am J Respir Crit Care Med 1998;
158(5
Pt 1): 1432-1441.
15. Teare JP, Sherman D, Greenfield SM, Simpson J, Bray G, Catterall AP,
Murray-
Lyon IM, Peters TJ, Williams R, and Thompson RP. Comparison of serum
procollagen
III peptide concentrations and PGA index for assessment of hepatic fibrosis.
Lancet
1993; 342(8876): 895-898.
16. Scheja A, Akesson A, and Horslev-Petersen K. Serum levels of aminoterminal
type
Date recue/Date received 2023-04-19
III procollagen peptide and hyaluronan predict mortality in systemic
sclerosis. Scand J
Rheumatol 1992; 21(1): 5-9.
17. Lin YH, Ho YL, Wang TD, Liu CP, Kao HL, Chao CL, Chien KL, Hung CS, Wu VC,
Tsai IJ, Yen RF, Shiau YC, and Chen WJ. The relation of amino-terminal
propeptide of
type III procollagen and severity of coronary artery disease in patients
without
myocardial infarction or hibernation. Clin Biochem 2006; 39(9): 861-866.
18. Teppo AM, Tornroth T, Honkanen E, and Gronhagen-Riska C. Urinary amino-
terminal propeptide of type Ill procollagen (PIIINP) as a marker of
interstitial fibrosis in
renal transplant recipients. Transplantation 2003; 75(12): 2113-2119.
19. Han XY, Wang W, Komulainen J, Koskinen SO, Kovanen V, Vihko V, Trackman
PC, and Takala TE. Increased mRNAs for procollagens and key regulating enzymes
in
rat skeletal muscle following downhill running. Pflugers Arch 1999; 437(6):
857-864.
20. Koskinen SO, Ahtikoski AM, Komulainen J, Hesselink MK, Drost MR, and
Takala
TE. Short-term effects of forced eccentric contractions on collagen synthesis
and
degradation in rat skeletal muscle. Pflugers Arch 2002; 444(1-2): 59-72.
21. Crameri RM, Langberg H, Teisner B, Magnusson P, Schroder HD, Olesen JL,
Jensen CH, Koskinen S, Suetta C, and Kjaer M. Enhanced procollagen processing
in
skeletal muscle after a single bout of eccentric loading in humans. Matrix
Biol 2004;
23(4): 259-264.
22. Chen F, Lam R, Shaywitz D, Hendrickson RC, Opiteck GJ, Wishengrad D, Liaw
A,
Song Q, Stewart AJ, Cummings CE, Beals C, Yarasheski KE, Reicin A, Ruddy M, Hu
X,
Yates NA, Menetski J, and Herman GA. Evaluation of early biomarkers of muscle
anabolic response to testosterone. J Cachexia Sarcopenia Muscle 2011; 2(1): 45-
56.
23. Longobardi S, Keay N, Ehrnborg C, Cittadini A, Rosen T, DaII R, Boroujerdi
MA,
Bassett EE, Healy ML, Pentecost C, Wallace JD, Powrie J, Jorgensen JO, and
Sacca L.
Growth hormone (GH) effects on bone and collagen turnover in healthy adults
and its
36
Date recue/Date received 2023-04-19
potential as a marker of GH abuse in sports: a double blind, placebo-
controlled study.
The GH-2000 Study Group. J Clin Endocrinol Metab 2000; 85(4): 1505-1512.
24. Bhasin S, He EJ, Kawakubo M, Schroeder ET, Yarasheski K, Opiteck GJ,
Reicin A,
Chen F, Lam R, Tsou JA, Castaneda-Sceppa C, Binder EF, Azen SP, and Sattler
FR.
N-terminal propeptide of type III procollagen as a biomarker of anabolic
response to
recombinant human GH and testosterone. J Clin Endocrinol Metab 2009; 94(11):
4224-
4233.
25. Nelson AE, Meinhardt U, Hansen JL, Walker IH, Stone G, Howe CJ, Leung KC,
Seibel MJ, Baxter RC, Handelsman DJ, Kazlauskas R, and Ho KK. Pharmacodynamics
of growth hormone abuse biomarkers and the influence of gender and
testosterone: a
randomized double-blind placebo-controlled study in young recreational
athletes. J Clin
Endocrinol Metab 2008; 93(6): 2213-2222.
26. Zachariae H, Heickendorff L, and Sogaard H. The value of amino-terminal
propeptide of type III procollagen in routine screening for methotrexate-
induced liver
fibrosis: a 10-year follow-up. Br J Dermatol 2001; 144(1): 100-103.
27. Gressner AM and Weiskirchen R. Modern pathogenetic concepts of liver
fibrosis
suggest stellate cells and TGF-beta as major players and therapeutic targets.
J Cell Mol
Med 2006; 10(1): 76-99.
28. Jarcuska P, Janicko M, Veseliny E, Jarcuska P, and Skladany L. Circulating
markers of liver fibrosis progression. Clin Chim Acta 2010; 411(15-16): 1009-
1017.
29. Frei A, Zimmermann A, and Weigand K. The N-terminal propeptide of collagen
type
III in serum reflects activity and degree of fibrosis in patients with chronic
liver disease.
Hepatology 1984; 4(5): 830-834.
30. Fabris P, Marranconi F, Bozzola L, Biasin MR, De Lazzari F, Plebani M,
Benedetti
P, Tositti G, Pellizzer G, Stecca C, and de LF. Fibrogenesis serum markers in
patients
with chronic hepatitis C treated with alpha-IFN. J Gastroenterol 1999; 34(3):
345-350.
37
Date recue/Date received 2023-04-19
31. Brocks DG, Steinert C, Gerl M, Knolle J, Neubauer HP, and Gunzler V. A
radioimmunoassay for the N-terminal propeptide of rat procollagen type III.
Application
to the study of the uptake of the N-terminal propeptide of procollagen type
III in isolated
perfused rat liver. Matrix 1993; 13(5): 381-387.
32. Rohde H, Vargas L, Hahn E, Kalbfleisch H, Bruguera M, and Timpl R.
Radioimmunoassay for type III procollagen peptide and its application to human
liver
disease. Eur J Clin Invest 1979; 9(6): 451-459.
33. Bayer Aktiengesellschaft. (1999) Monoclonal antibody and assay for
detecting
PIIINP. Patent Cooperation Treaty Appn. WO 99/61477.
34. Warming L, Hassager C, and Christiansen C. Changes in bone mineral density
with
age in men and women: a longitudinal study. Osteoporos Int 2002; 13(2): 105-
112.
38
Date recue/Date received 2023-04-19