Note: Descriptions are shown in the official language in which they were submitted.
ADDITIVE INFUSED BIOCHAR
RELATED APPLICATIONS
[0001] This application claims priority to U.S. Patent Application Serial No.
62/271,486
filed on December 28, 2015 titled ADDITIVE INFUSED BIOCHARS, U.S. Patent
Application Serial No. 62/290,285 tiled on February 2, 2016 titled ADDITIVE
INFUSED
BIOCHARS, U.S. Patent Application Serial No. 62/344,865 filed on June 2, 2016
titled
MINERAL SOLUBILIZING MICROORGANISMS INFUSED BIOCHARS and U.S.
Patent Application Serial No. 62/432,253 filed on December 9, 2016 titled
ADDITIVE
INFUSED BIOCHARS; this application is also claims priority to U.S. Patent
Application
Serial No. 15/350,920 filed on November 14, 2016 titled METHOD FOR ENHANCING
SOIL GROWTH USING 810-CHAR; this application also claims priority to U.S.
Patent
Application No. 15/156,256, filed on May 16, 2016, titled ENHANCED BIOCHAR.
Date Regue/Date Received 2023-06-16
INTENTIONALLY BLANK
2
Date Regue/Date Received 2023-06-16
FIELD OF INVENTION
[0002] The invention relates to porous carbonaceous structures having
increased
capabilities to retain additives for use in applications, including, but not
limited to,
agicultural applications. These additives include but arc not limited to
beneficial
nutrients, substances, microbes, or enzymes. The additives can be incorporated
with the
biochar in various ways, including but not limited to infusing the biochar
with the
additives to provide for the gradual delivery of the additive to the
surrounding
environment, such as soil.
BACKGROUND
[0003] During the last decades, soil degradation has increased due to
deforestation,
agricultural activities, industrial activities, vegetation overexploitation
and excessive
grazing. To avoid and potentially reverse soil degradation, many different
types of soil
enhancers have been developed and are already being used today. Among the
most.
common soil enhancers are fertilizers. Fertilizers improve the supply of
nutrients in the
soil, directly affecting plant growth. However, despite the wide spread use of
fertilizers,
conventional fertilizers are inefficient, particularly in soils with low
cation exchange
capacities and humid climate conditions. The demand for frequent application,
susceptibility to being washed out / leaching, and the need for nutrients to
be constantly
replenished are a few of the many problems associated with conventional
fertilizers.
Therefore, controlled released fertilizers (CRF) have become the preferred
type of
fertilizers to improve nutrient yield while minimizing losses. CRF's have the
ability to
supply nutrients gradually to soil and plants over a longer period of time. By
coinciding
with the nutrient requirements of the plant, CRF's ensure improved
effectiveness through
minimizing the losses between application and absorption, thus avoiding losses
by
leaching, runoff, and nutrient volatilization.
[0004] A second known type of soil enhancer consists of microbes, such as
beneficial
fungi or plant growth promoting bacteria ("PGPB"). PGPB are rhizosphere-
associateci
organisms that colonize the rhizosphere and rhizoplane and improve plant
growth when
artificially inoculated into soil. Kil-113 can both promote plant growth and
fight pathogenic
fungi. Current methods for deploying microbes into the environment often lead
to the
microbes being compromised and even dying before they can be fully
incorporated into
the environment. Thus a system or
3
Date Regue/Date Received 2023-06-16
method to deploy them which will better maintain their viability and
effectiveness is
needed for the industry to fully realize the benefits of microbial use.
[0005] A third known type of soil enhancer is biochar. Biochar has been known
for many
ycars as a soil enhancer. It contains highly porous, high carbon content
material similar
to the type of very dark, fertile anthropogenic soil found in the Amazon Basin
known as
Terra Preta, which has very high carbon content and historically has been made
from a
mixture of charcoal, bone, and manure. Biochar is created by the pyrolysis of
biomass,
which generally involves heating and/or burning of organic matter, in a
reduced oxygen
environment, at a predetermined rate. Such heating and/or burning is stopped
when the
matter reaches a charcoal like stage. The highly porous material of biochar is
suited to
host beneficial microbes, retain nutrients, hold water, and act as a delivery
system for a
range of beneficial compounds and additives suited to specific applications.
[0006] Raw biochar, while known for its soil enhancing characteristics, does
not always
benefit soil and, depending upon the biomass from which the biochar is
produced and the
method of production, can potentially be harmful to the soil, making it
unsuitable for
various types of crops or other productive uses. In particular, biochar can he
detrimental,
or even toxic, to 1) soil microbes involved in nutrient transport to the
plant; 2) plants and
3) humans. Biochars derived from different biomass or produced with differing
parameters, such as higher or lower pyrolysis temperature or variations in
residence time,
will have different physical and chemical properties and can behave (Trite
differently
when used in agriculture. For example, biochar having pH levels too high,
containing too
much ash, inorganics, or containing toxins or heavy metal content too high can
be harmful
and/or have minimal benefit to the soil and the plant life it supix)rts.
Biochar can also
contain unacceptable levels of residual organic compounds such as acids,
esters, ethers,
ketones, alcohols, sugars, phenyls, alkanes, alkenes, phenols, polychlorinated
biphenyls
or poly or mono aromatic hydrocarbons which arc either toxic or not beneficial
to plant
or animal life.
[0007] Due to the unpredictable performance of biochar and its potential to be
detrimental
to plant life and growth, it has mostly been a scientific curiosity, not round
wide spread
use, not found large scale commercial application, and has been relegated to
small niche
applications. It is, however, known, as noted above, that biochar, having
certain
characteristics can host
4
Date Regue/Date Received 2023-06-16
beneficial microbes, retain nutrients, hold water, and act as a delivery
system for a range
of beneficial compounds suited to specific applications. Thus, it has been a
continued
desire to capture the beneficial soil enhancing characteristics of biochar in
a more
consistent, predictable way. Biochar research has continued in an attempt to
harness
biochar having predictable, controllable, and beneficial results as a soil
amendment for
large scale applications.
WIN Additionally, attempts have been made to narrowly combine the benefits of
fertilizer with biochar by mixing it with, coating it with or submersing it in
the fertilizer.
The results of these attempts, however, have failed to adequately allow soil
nutrient
exposure and plant nutrient uptake to occur over a longer period of time
throughout a
growing season from the same application.
[00091 There are currently around 7 billion people in the world and this is
expected to
increase to approximately 8 billion around the year 2020. In light of both the
expected
worldwide population increase and the increasing environmental damage caused
by ever
greater levels of industrialization, it will become more and more of a
challenge to feed all
of the world's people, a problem that will only increase with time, Thus, a
need exists, in
order to feed this growing population, for a method of combining the benefits
of fertilizer,
beneficial fungi, POPB or other additives with biochar in a manner that
reduces the cost
and impact of the frequent application of nutrients to the soil and increases
agricultural
productivity in a sustainable and environmentally friendly manner.
Date Regue/Date Received 2023-06-16
SUMMARY
[00101 The present invention relates to biochar having increased capabilities
to retain and
then deploy additives more effectively. The biochar may be infused with
beneficial
additives to allow for a more gradual, prolonged release of thc compounds to
thc soil.
This time release effect in agricultural applications can dramatically reduce
the need for
high frequency application in the period immediately following planting and
can also
increase plant growth and sustain plant life. The present invention can be
used in
connection with any type of beneficial additive - including, but not limited
to, plant
nutrients, beneficial fungi, PGPB, hormones, enzymes, bio pesticides,
herbicides,
fungicides, nematicides, bacteriacides, fumigants among others additives, as
will be
described more fully below. In addition, the biochar can make a superior
microbial carrier
for various applications, including but not limited to agriculture, as the
properties of the
biochar can improve the viability of the microbes and their effectiveness
after deployment.
with the biochar.
[00111 The method includes producing an additive infused biochar that may
contain
biochar. plant nutrients, beneficial fungi, PGPB and/or other additives. The
method
includes impregnating at least some of the pores of the biochar with liquid
additives or
additives in liquid solution through an infusion process. The resulting
infused biochar
provides for gradual and/or steady delivery of the additives to the soil and
plants. The
utilization of additive infused biochar allows the delivery of inure nutrients
or additives
per unit of biochar and also provides for a more gradual release of the
additives to the
surrounding soil. In turn, this enables different soils to prov ide an
environment well suited
to the long term success of the desired plant. The use of additive infused
biochar results
in visibly fuller plants, increases plant yield with improved vitality and
longevity that can
be maintained with less frequent additive application and reduced additive
effectiveness
from leaching or runoff.
[0012] The present invention teaches treating the biochar in a manner that
forces,
accelerates or assists the infusion of additives into the pores of the
biochar. Treatment in
this manner allows for the impregnation or inoculation of the pores a the
biochar with
additives, which can be beneficial for the intended use of the biochar.
[0013] In one example of an implementation of the present invention, the
method for
treating the biochar includes placing porous carbonaceous materials in a tank
or chamber;
adding an additive
6
Date Regue/Date Received 2023-06-16
solution to the tank; and changing the pressure in the tank by, for example,
placing the
contents of the tank under a partial vacuum. In this example, the additive
solution may be
added to the tank either before the pressure change is applied or while the
pressure change
is being applied. In addition to subjecting thc contents to a partial vacuum,
the pores of
the biochar may be impregnated with the additive solution using a surfactant
solution
(e.g., a liquid solution containing 0.1 - 20% surfactant) or ultrasonic
treatment, as will be
further described below. Through the above treatment methods, at least 10% or
more of
the pore volume of the pores of the biochar material may be filled with the
additive
solution within a time period where it would not otherwise be possible to
achieve the
same results by simply contact or immersion of the biochar with the additive
solution
alone.
[0014] Other devices, apparatus, systems, methods, features and advantages of
the
invention are or will become apparent to one with skill in the art upon
examination of the
following figures and detailed description. It is intended that all such
additional systems,
methods, features and advantages be included within this description, be
within the scope
of the invention, and he protected by the accompanying claims.
7
Date Regue/Date Received 2023-06-16
BRIEF DESCRIPTION OF THE FIGURES
[0015] The invention may be better understood by referring to the following
figures. The
components in the figures are not necessarily to scale, emphasis instead being
placed upon
illustrating the principles of the invention.
[00161 Figure I illustrates a cross-section of one example of a raw biochar
particle.
[0017! Figure 2a is a SEM OKV x 3.00K 10.0n in) of pore morphology of treated
Noel] ar
made from pine.
[0018] Figure 2b is a SEM ( IOKV x 3.00K 10.0u.ni) of pore morphology of
treated
biochar made from birch.
[0019] Figure 2e is a SEM (10K V x 3.00K 10.0 m) of pore morphology of treated
biochar
made from coconut shells.
[00201 Figure 3 is a chart showing porosity distribution of various biochars.
[0021] Figure 4 is a flow chart process diagram of one implementation of a
process for
treating the raw biochar in accordance with the invention.
[0022] Figure 4a illustrates a schematic of one example of an implementation
of a biochar
treat processes that that includes washing, pH adjustment and moisture
adjustment.
[0023] Figure 4b illustrates yet another example of an implementation of a
biochar
treatment processing that includes inoculation.
[0024] Figure 5 is a schematic flow diagram of one example of a treatment
system for
use in accordance with the present invention.
[0025] Figure 6 is a chart showing the water holding capacities of treated
biochar as
compared to raw biochar and sandy clay loam soil and as compared to raw
biochar and
sunshine potting soil.
[0026] Figure 7 illustrates the different water retention capacities of raw
biochar versus
treated biochar measured gravimetrieally.
[0027] Figure 8 is a chart showing the retained water in vacuum impregnated
biochar
over other bioehars after a seven week period.
[0028] Figure 9 is a chart showing the weight loss of treated biochars versus
raw biochar
samples when heated at varying temperatures using a TGA testing method.
[0029] Figure 10 illustrates the plant available water in raw biochar, versus
treated
biochar and treated dried biochar.
8
Date Regue/Date Received 2023-06-16
[00301 Figure Ills a graph showing the pH of various starting biochars that
were made
from different starting materials and pyrolysis process temperatures.
[0031] Figure '12 is a chart showing various pH ranges and germination for
treated
biochars.
[0032] Figure 13 is a Thermogravimetric Analysis (TGA) plot showing the
ineasurement
of water content, heavy organics and light organics in a sample.
[0033] Figure 14 is a chart showing the impact of treatment on pores sizes of
biochar
derived from coconut.
[0034] Figure 15 is a chart showing the impact of treatment on pores sizes of
biochar
derived from pine.
[0035] Figure 16 is a chart showing the measured hydrophobicity index raw
biochar,
vacuum treated biochar and surfactant treated biochar.
[0036] Figure 17 is a flow diagram showing one example of a method for
infusing
hi ochar.
[0037] Figure 18 illustrates the improved liquid content of biochar using
vacuum
impregnation as against soaking the biochar in liquid_
[00381 Figure 19a is a chart comparing total retained water of treated biochar
after
soaking and after vacuum impregnation.
[0039] Figure 19b is a chart comparing water on the surface, interstitially
and in the pores
of biochar after soaking and after vacuum impregnation.
[0040] Figure 20 illustrates how the amount of water or other liquid in the
pores of
vacuum processed biochars can be increased varied based upon the applied
pressure.
[0041] Figure 21 illustrates the effects of NPK impregnation of biochar on
lettuce yield.
[0042] Figure 22 is a chart showing nitrate release curves of treated biochars
infused with
nitrate fertilizer.
[0043] Figures 23 and 24 arc images that show how different sized bacteria
will fit in
different biochar pore size structures.
[00441 Figure 25 illustrates release rate data verse total pore volume data
for both coconut
shell and pine based treated biochars inoculated with a releasable bacteria.
[0045] Figure 26 is a chart comparing examples of biochars.
[0046] Figures 27a, 27b, 27c are charts comparing different examples of
biochars.
9
Date Recue/Date Received 2023-06-16
[00471 Figure 28 is a chart comparing shoot biomass when the biochar added to
a soilless
mix containing soybean seeds is treated with microbial product containing
bradyrhizobium japonicum. and when it is untreated.
[0048] Figure 29 shows the comparison of root biomass in a treated verses an
untreated
en vironment.
[00491 Figure 30 is a chart comparing the nitrogen levels when the biochar is
inoculated
with the rhizobial inoculant verses when it is not inoculated.
[00501 Figure 31 illustrates the three day release rates of water infused
biochar compared
to other types of biochar.
[0051] Figure 32a is a SEM (10KV x 3.00K 10.0 m) of pore morphology of raw
biochar.
100521 Figure 32b is a SEM (10KV x 3.00K 10.0p.m) of pore morphology of raw
biochar
of Fig= 32a after it has been infused with microbial species.
100531 Figure 32c is a SEM (10KV x 3.00K 10.0um) of a pore morphology of
another
example of raw biochar of Figure 17a after it has been infused with microbial
species.
[00541 Figure 33 contains charts illustrating improved results obtained
through the use of
biochar s_
[00551 Figure 34 is an example of carbon dioxide production captured as a
continuous
gas bubble in BGB (left two tubes) and LTB (right two tubes) growth medium.
[0056] Figures 35 and 36 illustrate improved growth rates of colonies of
Streptomyces
lydicus using bioehars.
1.0
Date Regue/Date Received 2023-06-16
DESCRIPTION OF THE INVENTION
[0057] As illustrated in the attached figures, the present invention relates
to biochiu-
having increased capabilities to retain and then deploy additives more
effectively. In
addition, it includes additive infused biochars and methods for infusing
biochars with
additives that allow for the release of the additives into the environment
gradually. When
used in agricultural applications, the gradual release of the additive into
the soil, can result
in an increase of plant growth, vigor, or survivability, while minimizing the
loss of
beneficial compounds in the root zone. In particular, the biochar can make a
superior
microbial carrier for various applications, including but not limited to
agriculture, as the
properties of the biochar can improve the viability of the microbes and their
effectiveness
after deployment with the biochar. This present invention of the biochar as a
microbial
carrier can either be or not be in conjunction with the invention of the
additive infusion,
in which the additive infused is either the microbe itself or something else.
As described
below, through treatment, the properties of the raw biochar can be modified to
significantly increase the biochar' s ability to retain water, nutrients and
additives useful
for an end application while also, in many cases, creating an environment
beneficial to
microorganisms. Generally, for agricultural applications, such enhanced
abilities could
include holding water, and nutrients, e.g. fertilizer, or removing compounds,
such as
volatile organic compounds (VOCs), that may react with or negatively impact
either the
additive itself or microbial or plant life in general.
[0058] For example, through treatment, in addition to nutrients, other
material additives,
e.g., beneficial fungi. PGPB, herbicides and pesticides, can be utilized and
benefit from
the increased holding and retention capacities of the treated biochar. For
certain biochar,
the processing can also ensure that properties of the biochar, including its
pH, used in the
present application is suitable for creating conditions beneficial for plant
or microbial
growth, which has been a known challenge for raw biochars.
[0059] Generally, treated biochar of the present invention eau be used
throughout the
world, in numerous soil types. agricultural applications, horticultural, large
and small
scale farming, organic farming, and in a variety of soil management
applications and
systems, and combinations and variations of these. Examples of these
applications
include for example, use in acidic and highly weathered tropical field soils,
use in
temperate soils of higher fertility,
1.11
Date Regue/Date Received 2023-06-16
use in large commercial applications, use for the production of large scale
crops such as,
soybean, corn, sugarcane and rice, in forestry applications, for golf courses
(e.g. , greens,
fairways), for general purpose turf grasses, wine grapes, table grapes, raisin
grapes, fruit
and nut trees, ground fruits (e.g. , strawberries, blueberries, blackberries),
row crops (e.g.
, tomatoes, celery, lettuce, leafy greens), root crops (e.g. , tubers,
potatoes, beets, carrots),
mushrooms, and combinations and variations of these and other agricultural
applications.
As discussed in more detail below, biochar treated in this way may also be
used in other
applications, such as animal feed, composting, water treatment, and heavy
metal
remediation, to name a few.
[0060] For purposes of this application, the term "biochar" shall be given its
broadest
possible meaning and shall include any solid carbonaceous materials obtained
from the
pyrolysis, torrefaction, gasification or any other thermal and/or chemical
conversion of a
biomass. For puiposes of this application, the solid carbonaceous material may
include,
but not be limited to, BMF char disclosed and taught by 'U.S . Patent
8,317,891. Pyrolysis
is generally defined as a thennochemical decomposition of organic material at
elevated
temperatures in the absence of, or with reduced levels of oxygen. When the
biochar is
referred to as "treated' or undergoes "treatment," it shall mean raw,
pyrolyzed biochar
that has undergone additional physical, biological, and/or chemical
processing.
[0061] As used herein, unless specified otherwise, the terms "carbonaceous",
"carbon
based", "carbon containing", and similar such lerMS are to be given their
broadest possible
meaning, and would include materials containing carbon in various states,
crystallinities,
forms and compounds.
[0(162] As used herein, unless stated otherwise, room temperature is 25 C.
and, standard
temperature and pressure is 25 C and I atmosphere. Unless stated otherwise,
generally,
the term "about" is meant to encompass a variance or range of +10%, the
experimental or
instrument error associated with obtaining the stated value, and preferably
the larger of
these.
[0063] A. Biochars
[00641 Typically, biochars include porous carbonaceous materials, such as
charcoal, that
are used as soil amendments or other suitable applications. Biochar most
commonly is
created by pyrolysis of a biomass. In addition to the benefits to plant
growth, yield and
quality, etc.;
1.7
Date Regue/Date Received 2023-06-16
biochar provides the benefit of reducing carbon dioxide (C07) in the
atmosphere by
serving as a method of carbon sequestration. Thus, biochar has the potential
to help
mitigate climate change, via carbon sequestration. However, to accomplish this
important, yet ancillary benefit, to any meaningful cxtcnt, thc use of biochar
in
agricultural applications must become widely accepted, e.g., ubiquitous.
Unfortunately,
because of the prior failings in the biochar arts, this has not occurred. It
is believed that
with the solutions of the present invention may this level of use of biochar
be achieved;
and more importantly, yet heretofore unobtainable, realize the benefit of
significant
carbon dioxide sequestration.
I00651 In general, one advantage of putting biochar in soil includes long term
carbon
sequestration. It is theorized that as worldwide carbon dioxide emissions
continue to
mount, benefits may be obtained by, controlling, mitigating and reducing the
amount of
carbon dioxide in the atmosphere and the oceans. It is further theorized that
increased
carbon dioxide emissions are associated with the increasing industrial
development of
developing nations, and are also associated with the increase in the world's
population. In
addition to requiring more energy, the increasing world population will
require more
food. Thus, rising carbon dioxide emissions can be viewed as linked to the
increasing use
of natural resources by an ever increasing global population. As some suggest,
this larger
population brings with it further demands on food production requirements.
Biochar
uniquely addresses both of these issues by providing an effective carbon sink,
e.g., carbon
sequestration agent, as well as, an agent for improving and increasing
agricultural output.
In particular, biochar is unique in its ability to increase agricultural
production, without
increasing carbon dioxide emission, and preferably reducing the amount of
carbon
dioxide in the atmosphere. However, as discussed above, this unique ability of
biochar
has not been realized, or seen, because of the inherent problems and failings
of prior
biochars including, for example, high pH, phytotoxicity due to high metals
content and/or
residual organics, and dramatic product inconsistencies.
[0066] Biochar can be made from basically any source of carbon, for example,
from
hydrocarbons (e.g., petroleum based materials, coal, lignite, peat) and from a
biomass
(e.g., woods, hardwoods, softwoods, waste paper, coconut shell, manure, chaff,
food
waste, etc.). Combinations and variations of these starting materials, and
various and
different members of
1.3
Date Regue/Date Received 2023-06-16
each group of starting materials can be, and are, used. Thus, the large number
of vastly
different starting materials leads to biochars having different properties_
[0067] Many different pyrolysis or carbonization processes can be, and are
used, to create
biochars. In general, these processes involve heating thc starting material
under positive
pressure, reduced pressure, vacuum, inert atmosphere, or flowing inert
atmosphere,
through one or more heating cycles where the temperature of the material is
generally
brought above about 400C, and can range from about 300 C to about 900 C. The
percentage of residual carbon formed and several other initial properties are
strong
functions of the temperature and time history of the heating cycles. In
general, the faster
the heating rate and the higher the final temperature the lower the char
yield. Conversely,
in general, the slower the heating rate or the lower the final temperature the
greater the
char yield. The higher final temperatures also lead to modifying the char
properties by
changing the inorganic mineral matter compositions, which in turn, modify the
char
properties. Ramp, or heating rates, hold times, cooling profiles, pressures,
flow rates, and
type of atmosphere can all be controlled, and typically are different from one
biochar
supplier to the next_ These differences potentially lead to a biochar having
different
properties, further framing the substantial nature of one of the problems that
the present
inventions address and solve. Generally, in carbonization most of the non-
carbon
elements, hydrogen and oxygen arc first removed in gaseous form by the
pyrolytic
decomposition of the starting materials, e.g., the biomass. The free carbon
atoms group
or an-ange into crystallographic formations known as elementary graphite
crystallites.
Typically, at this point the mutual arrangement of the crystallite is
irregular, so that free
interstices exist between them. Thus, pyrolysis involves thermal decomposition
of
carbonaceous material, e.g., the biomass, eliminating non-carbon species, and
producing
a fixed carbon structure.
[0068] As noted above, raw or untreated biochar is generally produced by
subjecting
biomass to either a uniform or varying pyrolysis temperature 300 C
to 550' C to
750' C or more) for a prescribed period of time in a reduced oxygen
environment. This
process may either occur quickly, with high reactor temperature and short
residence
times, slowly with lower reactor temperatures and longer residence times, or
anywhere in
between. To achieve better results, the biomass from which the char is
obtained may be
first stripped of debris, such as bark, leaves and small branches, although
this is not
necessary. The biomass may further include feedstock
1.4
Date Regue/Date Received 2023-06-16
to help adjust the pH and particle size distribution in the resulting raw
biochar. In some
applications, it is desirous to have biomass that is fresh, less than six
months old, and with
an ash content of less than 3%. Further, by using biochar derived from
different biomass,
e.g., pine, oak, hickory, birch and coconut shells from different regions, and
understanding the stalling properties of the raw biochar, the treatment
methods can be
tailored to ultimately yield a treated biochar with predetermined, predictable
physical and
chemical properties.
10069j In general. biochar particles can have a very wide variety of particle
sizes and
distributions, usually reflecting the sizes occurring in the input biomass.
Additionally,
biochar can be ground or crushed after pyrolysis to further modify the
particle sizes.
Typically, for agricultural uses, biochars with consistent, predictable
particle sizes are
more desirable. By way of example, the biochar particles can have particle
sizes as shown
or measured in Table 1 below. When refening to a batch having 'A inch
particles, the
batch would have particles that will pass through a 3 mesh sieve, but will not
pass through
(i.e., are caught by or sit atop) a 4 mesh sieve.
Table 1
U.S. Mesh Inches Microns w= Milli meter.:
(i.e., mesh) (14m) (mm)
3. 0.2650 6730 , 6.370
4 0.1870 4760 4.760
0.1570 ,t0Q) 4(X)0
,
6 0:1320 3360 , 3.360
7 0:1110 2830 ;1..830
8. 0.0937 2380 ' 2.380
0_0787 2000 2.000
12 ¨ : 0M661 1680 . 1.6RO
i
14 0.0535 1410 ; 1.410
16 0.0469 1190 1 1.190
18 0.0394 1000 ' 1.000
0.0331 843 0.841
. .
,:..,
. 0,0280 71)7 030/
,
' 0.0232 , 59.5 0.595
:
= . 0.097 500 .: 0:500
,
0.1.)165 400 0-400
,
:
. 0:0138 354 .
. 0:354
0..0117 297 0:297
:
. .
.
0.0(..19 ' 250 0:250
, ..
is
Date Recue/Date Received 2023-06-16
70 0.0083 ' 210 0.210
0.0070 .1 177 0.177
õ r =
100 0.0054 149 0.149
120 0.0049 125 0,125
140 0.0041 105 0.105
170 0.0035 38 0.083
0-0029 74 0.074
230 0-0024 6,3. 0-063
270 0.0021 53 0.053
311 0.0017 44 0.044
400 0.015 I 3-7 0.037
1.00701 For most applications, it is desirable to use biochar particles having
particle sizes
from about 3/4 mesh to about 60/70 mesh, about 4/5 mesh to about 20/25 mesh,
or about
4/5 mesh to about 30/35 mesh. It being understood that the desired mesh size,
and mesh
size distribution can vary depending upon a particular application for which
the biochar
is intended.
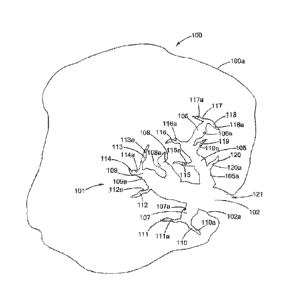
[00711 Figure 1 illustrates a cross-section of one example of a raw biochar
particle. As
illustrated in Fig= 1, a biochar particle 100 is a porous structure that has
an outer surface
100a and a pore structure 101 formed within the biochar particle 100. As used
herein,
unless specified otherwise, the terms "porosity", "porous", "porous
structure", and
"porous morphology" and similar such terms are to he given their broadest
possible
meaning, and would include materials having open pores, closed pores, and
combinations
of open and closed pores, and would also include macropores, mesopores, and
micropores
and combinations, variations and continuums of these morphologies. Unless
specified
otherwise, the term "pore volume" is the total volume occupied by the pores in
a particle
or collection of particles; the term "inter- particle void volume" is the
volume that exists
between a collection of particle; the term "solid volume or volume of solid
means" is the
volume occupied by the solid material and does not include any free volume
that may be
associated with the pore or inter-particle void volumes; and the term "bulk
volume" is the
apparent volume of the material including the particle volume, the inter-
particle void
volume, and the internal pore volume.
[00721 The pore structure 101 rot ms an opening 121 in the outer surface 100a
of the
biochar particle 100. The pore structure 101 has a macropore 102, which has a
macropore
surface 102a, and which surface 102a has an area, i.e., the macropore surface
area. (In
this diagram only a
16
Date Recue/Date Received 2023-06-16
single micropore is shown. If multiple micropores are present than the sum of
their surface
areas would equal the total macropore surface area for the biochar particle.)
From the
macropore 102, several mesopores 105, 106, 107, 108 and 109 are present, each
having
its respective surfaces 105a, 106a, 107a, 108a and 109a. Thus, each mesopore
has its
respective surface area; and the sum of all inesopore surface areas would be
the total
mesopore surface area for the particle. From the mesopores, e.g., 107, there
are several
m ieropores 110, 111, 112, 113, 114, 115, 116, 117, 118, 119 and 120, each
having its
respective surfaces 110a, I Ila, 112a, 113a, 114a, 115a, 116a, 117a, 118a, 1.
19a and 120a.
Thus, each micropore has its respective surface area and the sum of all
micropore surface
areas would be the total micropore surface area for the particle. The sum of
the macropore
surface area, the mesopore surface area and the micropore surface area would
be the total
pore surface area for the particle.
100731 Macropores are typically defined as pores having a diameter greater
than 300 nm,
mesopores are typically defined as diameter from about 1-300 nm, and micmpores
are
typically defined as diameter of less than about 1 nm, and combinations,
variations and
continuums of these morphologies. The macropores each have a macropore volume,
and
the sum of these volumes would be the total macropore volume. The mesopores
each
have a mesopore volume, and the sum of these volumes would be the total
mesopore
volume. The mieropores each have a micropore volume, and the sum of these
volumes
would be the total mieropore volume. The sum of the inaeropore volume, the
inesopure
volume and the micropore volume would be the total pore volume for the
particle.
[0074] Additionally, the total pore surface area, volume, mesopore volume,
etc., for a
batch of biochar would be the actual, estimated, and preferably calculated sum
of all of
the individual properties for each biochar particle in the batch.
[00751 It should be understood that the pore morphology in a biochar particle
may have
several of the porc structures shown, it may have mesoporcs opening to the
particle
surface, it may have micropores opening to particle surface, it may have
micropores
opening to macropore surfaces, or other combinations or variations of
interrelationship
and structure between the pores. It should further be understood that the pore
morphology
may be a continuum, where moving inwardly along the pore from the surface of
the
particle, the pore transitions, e.g., its diameter
1.7
Date Regue/Date Received 2023-06-16
becomes smaller, from a macropore, to a mesopore, to a micropore, e.g.,
macropore 102
to mesopore 109 to inicroixife 114.
[0076] In general, the biochars have porosities that can range from 0.2
cm3/cm3 to about
0.8 em3/cm3 and more preferably about 0.2 cm3/c m3 to about 0.5 cm3/cm3.
Unless stated
otherwise, porosity is provided as the ratio of the total pore volumes (the
sum of the micro
+ meso + macro pore volumes) to the solid volume of the biochar. Porosity of
the biochar
particles can be determined, or measured, by measuring the micro-, nrieso-,
and macro
pore volumes, the bulk volume, and the inter particle volumes to determine the
solid
volume by difference. The porosity is then calculated from the total pore
volume and the
solid volume.
100771 As noted above, the use of different biomass potentially leads to
biochars having
different properties, including, but not limited to different pore structures.
By way of
example, Figures 2A, 2B and 2C illustrate Scanning Electron Microscope ("SEM")
images of various types of treated biochars showing the different nature of
their pore
morphology. Figure 2A is biochar derived from pine. Figure 2B is biochar
derived from
birch. Figure 2C is biochar derived from coconut shells.
[0078] The surface area and pore volume for each type of pore, e.g., macro-,
meso- and
micro- can be determined by direct measurement using CO2 adsorption for micro-
, N2
adsorption for memo- and macro pores and standard analytical surface area
analyzers and
methods, for example, particle analyzers such as Micrometrics instruments for
meso- and
micro pores and impregnation capacity for macro pore volume. Mercury
porosirnetry,
which measures the macroporosity by applying pressure to a sample immersed in
mercury
at a pressure calibrated for the minimum pore diameter to be measured, may
also be used
to measure pore volume.
[00791 The total micropore volume can be from about 2% to about 25% of the
total pore
volume. The total mesopore volume can be from about 4% to about 35% of the
total pore
volume. The total maeropore volume can be from about 40% to about 95% of the
total
pore volume. By way of example. Figure 3 shows a bar chart setting out
examples of the
pore volumes for sample biochars made from peach pits 201, juniper wood 202, a
first
hard wood 203, a second hard wood 204, fir and pine waste wood 205, a first
pine 206, a
second pine 207, birch 208 and coconut shells 209.
1.8
Date Regue/Date Received 2023-06-16
[0080] As explained further below, treatment can increase usable pore volumes
and,
among other things, remove obstructions in the pores, which leads to increased
retention
properties and promotes further performancc characteristics of thc biochar.
Knowing thc
properties of the starting raw biochar, one can treat the biochar to produce
controlled,
predictable and optimal resulting physical and chemical properties.
[0081] B. Treatment
[0082] The rationale for treating the biochar after pyrolysis is that given
the large pore
volume and large surface are of the biochars, it is most efficient to make
significant
changes in the physical and chemical properties of the biochar by treating
both the internal
and external surfaces and internal pore volume of the char. Testing has
demonstrated that
if the biochar is treated, at least partially, in a manner that causes the
forced infusion
and/or diffusion of liquids into and/or out of the biochar pores (through
mechanical,
physical, or chemical means), Certain properties of the biochar can be altered
or improved
over and above simply contacting these liquids with the biochar. By knowing
the
properties of the raw biochar and the optimal desired properties of the
treated biochar, the
raw biochar can then be treated in a manner that results in the treated
biochar having
controlled optimized properties.
[0083] For purposes of this application, treating and/or washing the biochar
in accordance
with the present invention involves inure than a simple wash or soak, which
generally
only impacts the exterior surfaces and a small percentage of the interior
surface area.
"Washing" or "treating" in accordance with the present invention, and as used
below,
involves treatment of the biochar in a manner that causes the forced,
accelerated or
assisted infusion and/or diffusion of liquids and/or additivities into and/or
out of the
biochar pores (through mechanical, physical, or chemical means) such that
certain
properties of the biochar can be altered or improved over and above simply
contacting
these liquids with the Wm:bar or so that treatment becomes more efficient or
rapid front
a time standpoint over simple contact or immersion.
[00841 in particular, effective treatment processes can mitigate deleterious
pore surface
properties, remove undesirable substances from pore surfaces or volume, and
impact
anywhere from between 10% to 99% or more of pore surface area of a biochar
particle.
By modifying the usable pore surfaces through treatment and removing
deleterious
substances from the pore volume, the treated biochars can exhibit a greater
capacity to
retain water and/or other nutrients
1.9
Date Regue/Date Received 2023-06-16
as well as being more suitable habitats for some forms of microbial life.
Through the use
of treated biochars, agricultural applications can realize increased moisture
control,
increased nutrient retention, reduced water usage, reduced water requirements,
reduced
runoff or leaching, increased nutrient efficiency, reduced nutrient usage,
increased yields,
increased yields with lower water requirements and/or nutrient requirements,
increases in
beneficial microbial life, improved peribrmance and/or shelf life for
inoculated bacteria,
and any combination and variation of these and other benefits.
1100851 Treatment further allows the biochar to be modified to possess certain
known
properties that enhance the benefits received from the use of biochar. While
the selection
of feedstock, raw biochar and/or pyrolysis conditions under which the biochar
was
manufactured can make treatment processes less cumbersome, more efficient and
further
controlled, treatment processes can be utilized that provide for the biochar
to have desired
and generally sustainable resulting properties regardless of the biochar
source or pyrolysis
conditions. As explained further below, treatment can (1) repurpose
problematic biochars,
(ii) handle changing biochar material sources, e.g. , seasonal and regional
changes in the
source of biomass, (iii) provide for custom features and functions of biochar
for particular
soils, regions or agricultural purposes; (iv) increase the retention
properties of biochar,
(v) provide for large volumes of biochar having desired and predictable
properties, (vi)
provide for biochar having custom properties, (vii) handle differences in
biochar caused
by variations in pyrolysis conditions or manufacturing of the "raw" biochar;
and (viii)
address the majority, if not all, of the problems that have, prior to the
present invention,
stifled the large scale adoption and use of biochars.
[00861 Treatment can wash both the interior and exterior pore surfaces, remove
harmful
chemicals, introduce beneficial substances, and alter certain properties of
the biochar and
the pore surfaces and volumes. This is in stark contrast to simple washing
which generally
only impacts the exterior suifaces and a small percentage of the interior
surface area.
Treatment can further be used to coat substantially all of the biochar pore
surfaces with a
surface modifying agent or impregnate the pore volume with additives or
treatment to
provide a predetermined feature to the biochar, e.g., surface charge and
charge density,
surface species and distribution, targeted nutrient addition, magnetic
modifications, root
growth facilitator, and water absorptivity and water retention properties.
'Just as
importantly, treatment can also be used to
Date Regue/Date Received 2023-06-16
remove undesirable substances from the biochar, such as dioxins or other
toxins either
through physical removal or through chemical reactions causing neutralization.
[0087] Figure 4 is a schematic flow diagram of one example treatment process
400 for
use in accordance with the present invention. As illustrated, thc treatment
process 400
starts with raw biochar 402 that may be subjected to one or more reactors or
treatment
processes prior to bagging 420 the treated biochar for resale. For example,
404 represents
reactor 1, which may be used to treat the biochar. The treatment may be a
simple water
wash or may be an acid wash used for the purpose of altering the pH of the raw
biochar
particles 402. The treatment may also contain a surfactant or detergent to aid
the
penetration of the treatment solution into the pores of the biochar. The
treatment may
optionally be heated, cooled, or may be used at ambient temperature or any
combination
of the three. For some applications, depending upon the properties of the raw
biochar, a
water and/or acid/alkaline wash 404 (the latter for pH adjustment) may he the
only
necessary treatment prior to bailing the biochar 420. If, however, the
moisture content
of the biochar needs to be adjusted, the treated biochar may then be put into
a second
reactor 406 for purposes of reducing the moisture content in the washed
biochar. From
there, the treated and moisture adjusted biochar may be bagged 420.
[00881 Again, depending upon the starting characteristics of the raw biochar
and the
intended application for the resale product, further processing may still be
needed or
desired. In this case, the treated moisture adjusted biochar may then be
passed to a third
reactor 408 for inoculation, which may include the impregnation of biochar
with
beneficial additives, such as nutrients, bacteria, microbes, fertilizers or
other additives.
Thereafter, the inoculated biochar may be bagged 420, or may be yet further
processed,
for example, in a fourth reactor 410 to have further moisture removed from or
added to
the biochar. Further moisture adjustment may be accomplished by placing the
inoculated
biochar in a fourth moisture adjustment reactor 410 or circulating the biochar
back to a
previous moisture adjustment reactor (e.g. reactor 406). Those skilled in the
art will
recognize that the ordering in which the raw biochar is processed and certain
processes
may he left out, depending on the properties of the starting raw biochar and
the desired
application for the biochar. For example, the treatment and inoculation
processes may be
performed without the moisture adjustment step, inoculation processes may
21
Date Regue/Date Received 2023-06-16
also be performed with or without any treatment, pH adjustment or any moisture
adjustment. All the processes may be completed alone or in the conjunction
with one or
more of the others.
[0089J For example, Figure 4a illustrates a schematic of one example of an
implementation of biochar processing that includes washing the pores and both
pH and
moisture adjustment. Figure 4b illustrates yet another example of an
implementation of
biochar processing that includes inoculation.
[0090] As illustrated in Figure 4a, raw biochar 402 is placed into a reactor
or tank 404. A
washing or treatment liquid 403 is then added to a tank and a partial vacuum,
using a
vacuum pump, 405 is pulled on the tank. The treating or washing liquid 403 may
be used
to clean or wash the pores of the biochar 402 or adjust the chemical or
physical properties
of the surface area or pore volume, such as p11 level, usable pore volume, or
VOC content,
among other things. The vacuum can be applied after the treatment liquid 403
is added or
while the treatment liquid 403 is added. Thereafter, the washed/ adjusted
biochar 410 may
be moisture adjusted by vacuum exfiltration 406 to pull the extra liquid from
the
washed/moisture adjusted hiochar 410 or may he placed in a centrifuge 407,
heated or
subjected to pressure gradient changes (e.g., blowing air) for moisture
adjustment. The
moisture adjusted biochar 412 may then be bagged or subject to further
treatment. Any
excess liquids 415 collected from the moisture adjustment step may be disposed
of or
recycled, as desired. Optionally, biochar fines may be collected frum the
excess liquids
415 for further processing, for example, to create a slurry, cakes, or biochar
extrudates.
[0091] Optionally, rather than using a vacuum pump 405, a positive pressure
pump may
be used to apply positive pressure to the tank 404. In some situations,
applying positive
pressure to the tank may also function to force or accelerate the washing or
treating liquid
403 into the pores of the biochar 402. Any change in pressure in the tank 404
or across
the surface of the biochar could facilitate the exchange of gas and/or
moisture into and
out of the pores of the biochar with the washing or treating liquid 403 in the
tank.
Accordingly, changing the pressure in the tank and across the surface of the
biochar,
whether positive or negative, is within the scope of this invention.
[0092] As illustrated Figure 4b, the washed/ adjusted biochar 410 or the
washed/ adjusted
and moisture adjusted biochar 412 may be further treated by inoculating or
impregnating
the pores
27
Date Regue/Date Received 2023-06-16
of the biochar with an additive 425. The biochar 410, 412 placed back in a
reactor 401,
an additive solution 425 is placed in the. reactor 401 and a vacuum, using a
vacuum pump,
405 is applied on the tank. Again, the vacuum can be applied after the
additive solution
425 is added to thc tank or while the additive solution 425 is being added to
the tank.
Thereafter, the washed, adjusted arid inoculated bioehar 428 can be bagged.
Alternatively,
if further moisture adjustment is requited, the biochar can be further
moisture adjusted by
vacuum filtration 406 to pull the extra liquid from the washed/moisture
adjusted biochar
410 or may be placed in a centrifuge 407 for moisture adjustment. The
resulting biochar
430 can then be bagged. Any excess liquids 415 collected from the moisture
adjustment
step may be disposed of or recycled, as desired. Optionally, biochar
particulates or "fines"
which easily are suspended in liquid may be collected from the excess liquids
415 for
further processing, for example, to create a slurry, biocharextrudates, or
merely a biochar
product of a consistently smaller particle size. As described above, both
processes of the
Figure 4a and 4b can be performed with a surfactant solution in place of, or
in conjunction
with, the vacuum 405.
[00931 While known processes exist for the above described processes, research
associated with the present invention has shown improvement and the. ability
to better
control the properties and characteristics of the biochar if the processes are
performed
through the infusion and diffusion of liquids into and out of the biochar
pores. One such
treatment process that can be used is vacuum impregnation and vacuum and/or
centrifuge
extraction. Another such treatment process that can be used is the addition of
a surfactant
to infused liquid, which infused liquid may be optionally heated, cooled, or
used at
ambient temperature or any combination of the three.
[00941 Since research associated with the present invention has identified
what physical
and chemical properties have the highest impact on plant growth and/or soil
health, the
treatment process can be geared to treat different forms of raw biochar to
achieve treated
biochar properties known to enhance these characteristics. For example, if die
pH of the
biochar needs to be adjusted to enhance the raw biochar performance
properties, the
treatment may be the infusion of an acid solution into the pores of the
biochar using
vacuum, surfactant, or other treatment means. This treatment of pore infusion
through,
for example, the rapid, forced infusion of liquid into and out the pores of
the biochar, has
further been proven to sustain the
23
Date Regue/Date Received 2023-06-16
adjusted H levels of the treated biochar for much longer periods than biochar
that is
simply immersed in an acid solution for the same period of time. By way of
another
example, if the moisture content needs to be adjusted, then excess liquid and
other
selected substances (e.g. chlorides, dioxins, and other chemicals, to include
those
previously deposited by treatment to catalyze or otherwise react with
substances on the
interior or exterior surfaces of the biochar) can be extracted from the pores
using vacuum
and/or centrifuge extraction or by using various heating techniques. The above
describes
a few examples of treatment that result in treated biochar having desired
performance
properties identified to enhance soil health and plant life.
[0095] Figure 5 illustrates one example of a system 500 that utilizes vacuum
impregnation
to treat raw biochar. Generally, raw biochar particles, and preferably a batch
of biochar
particles, are placed in a reactor, which is connected to a vacuum pump, and a
source of
treating liquid (i.e. water or acidic/basis solution). When the valve to the
reactor is closed,
the pressure in the reactor is reduced to values ranging from 750 Ton to 400
Torr to 10
Torr or less. The biochar is maintained under vacuum ("vacuum hold time") for
anywhere
from seconds to 1 minute to 10 minutes, to 100 minutes, or possibly longer. By
way of
example, for about a 500 pound batch of untreated biochar, a vacuum hold time
of from
about 1 to about 5 minutes can be used if the reactor is of sufficient size
and sufficient
infiltrate is available to adjust the necessary properties. While under the
vacuum the
treating liquid may then be introduced into the vacuum chamber containing the
biochar.
Alternatively, the treating liquid may be introduced into the vacuum chamber
before the
biochar is placed under a vacuum. Optionally, treatment may also include
subjecting the
biochar to elevated temperatures from ambient to about 250 C or reduced
temperatures
to about -25 C or below, with the limiting factor being the temperature and
time at which
the infiltrate can remain flowable as a liquid or semi-liquid.
[0096] The infiltrate or treating liquid is drawn into the biochar poreõ and
preferably
drawn into the macropures and mesopores. Depending upon the specific doses
applied
and pore structure of the biochar, the infiltrate can coat anywhere from 10%
to 50% to
100% of the total macropore and mesopore surface area and can fill or coat
anywhere
from a portion to nearly all (10% - 100%) of the total macropore and mesopore
volume.
[00971 As described above, the treating liquid can be left in the biochar,
with the hatch
being a treated biochar batch ready for packaging, shipment and use in an
agricultural or
other
24
Date Regue/Date Received 2023-06-16
application. The treating liquid may also be removed through drying,
subsequent vacuum
processing, centrifugal force (e.g., cyclone drying machines or centrifuges),
with the
batch being a treated biochar batch ready for packaging, shipment and use in
an
agricultural application. A second, third or more infiltration, removal,
infiltration and
removal, and combinations and variations of these may also be performed on the
biochar
with optional drying steps between infiltrations to remove residual liquid
from and
reintroduce gasses to the pore structure if needed. in any of these stages the
liquid may
contain organic or inorganic surfactants to assist with the penetration of the
treating
liquid.
[0098] As illustrated in Figure 5, a system 500 for providing a biochar,
preferably having
predetermined and uniform properties. The system 500 has a vacuum infiltration
tank
501. The vacuum infiltration tank 501 has an inlet line 503 that has a valve
504 that seals
the inlet line 503. In operation, the starting biochar is added to vacuum
infiltration tank
501 as shown by arrow 540. Once the tank is filled with the starting biochar,
a vacuum is
pulled on the tank, by a vacuum pump connected to vacuum line 506, which also
has
valve 507_ The starting biochar is held in the vacuum for a vacuum hold time.
Infiltrate,
as shown by arrow 548 is added to the tank 501 by line 508 having valve 509.
The
infiltrate is mixed with the biochar in the tank 501 by agitator 502. The
mixing process is
done under vacuum for a period of time sufficient to have the infiltrate fill
the desired
amount of pore volume, e.g., up to 100% of the macropores and inesopores.
[0099] Alternatively, the infiltrate may be added to the vacuum infiltration
tank 501
before vacuum is pulled on the tank. In this manner, infiltrate is added in
the tank in an
amount that can be impregnated into the biochar. As the vacuum is pulled, the
biochar is
circulated in the tank to cause the infiltrate to fill the pore volume. To one
skilled in the
art, it should be clear that the agitation of the biochar during this process
can be performed
through various means, such as a rotating tank, rotating agitator, pressure
variation in the
tank itself, or other means. Additionally, the biochar may be dried using
conventional
means before even the first treatment. This optional pre-drying can remove
liquid from
the pores and in some situations may increase the efficiency of impregnation
due to
pressure changes in the tank.
[0100] Pressure is then restored in the tank 501 and the infiltrated biochar
is removed, as
shown by arrow 541, from the tank 501 to bin 512, by way of a sealing gate 511
and
removal line 510.
Date Regue/Date Received 2023-06-16
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
The infiltrated biochar is collected in bin 512, where it can be further
processed in several
different ways. The infiltrated biochar can be shipped for use as a treated
biochar as shown by
arrow 543. The infiltrated biochar can be returned to the tank 501 (or a
second infiltration tank).
If returned to the tank 501 the biochar can be processed with a second
infiltration step, a vacuum
drying step, a washing step, or combinations and variations of these. The
infiltrated biochar
can be moved by conveyor 514, as shown by arrow 542, to a drying apparatus
516, e.g., a
centrifugal dryer or heater, where water, infiltrate or other liquid is
removed by way of line 517,
and the dried biochar leaves the dryer through discharge line 518 as shown by
arrow 545, and
is collected in bin 519. The biochar is removed from the bin by discharge 520.
The biochar
may be shipped as a treated biochar for use in an agriculture application, as
shown by arrow
547. The biochar may also be further processed, as shown by 546. Thus, the
biochar could be
returned to tank 501 (or a second vacuum infiltration tank) for a further
infiltration step. The
drying step may be repeated either by returning the dry biochar to the drying
apparatus 516, or
by running the biochar through a series of drying apparatus, until the
predetermined dryness of
the biochar is obtained, e.g., between 50% to less than 1% moisture.
[0101] The system 500 is illustrative of the system, equipment and processes
that can be used
for, and to carry out the present inventions. Various other implementations
and types of
equipment can be used. The vacuum infiltration tank can be a sealable off-axis
rotating vessel,
chamber or tank. It can have an internal agitator that also when reversed can
move material
out, empty it, (e.g., a vessel along the lines of a large cement truck, or
ready mix truck, that can
mix and move material out of the tank, without requiring the tank's
orientation to be changed).
Washing equipment may be added or utilized at various points in the process,
or may be carried
out in the vacuum tank, or drier, (e.g., wash fluid added to biochar as it is
placed into the drier
for removal). Other steps, such as bagging, weighing, the mixing of the
biochar with other
materials, e.g., fertilized, peat, soil, etc, can be carried out. In all areas
of the system referring
to vacuum infiltration, optionally positive pressure can be applied, if
needed, to enhance the
penetration of the infiltrate or to assist with re-infusion of gaseous vapors
into the treated char.
Additionally, where feasible, especially in positive pressure environments,
the infiltrate may
have soluble gasses added which then can assist with removal of liquid from
the pores, or
gaseous treatment of the pores upon equalization of pressure.
26
l01021 As noted above, the biochar may also be treated using a surfactant. The
same or
similar equipment used in the vacuum infiltration process can be used in the
surfactant
treatment process. Although it is not necessary to apply a vacuum in the
surfactant
treatment process, the vacuum infiltration tank or any other rotating vessel,
chamber or
tank can be used. In the surfactant treatment process, a surfactant, such as
yucca extract,
is added to the infiltrate, e.g., acid wash or water. The quantity of the
surfactant added to
the infiltrate may vary depending upon the surfactant used. For example,
organic yucca
extract can be added at a rate of between 0.1 - 20%, but more preferably 1-5%
by volume
of the infiltrate. The infiltrate with surfactant is then mixed with the
biochar in a tumbler
for several minutes, e.g., 3-5 minutes, without applied vacuum. Optionally, a
vacuum or
positive pressure may be applied with the surfactant to improve efficiency,
but is not
necessary. Additionally, infiltrate to which the surfactant or detergent is
added may be
heated or may be ambient temperature or less. Similarly, the mixture of the
surfactant or
detergent, as well as the char being treated may be heated, or may be ambient
temperature,
or less. After tumbling, excess free liquid can be removed in the same manner
as described
above in connection with the vacuum infiltration process. Drying, also as
described above
in connection with the vacuum infiltration process, is an optional additional
step. Besides
yucca extract, a number of other surfactants may be used for surfactant
treatment, which
include, but are not limited to, the following: nonionic types, such as,
ethoxylated
alcohols, phenols- lauryl alcohol ethoxylates, Fatty acid esters- surbitan,
twecnTM 20,
amines, amides- imidazoles; anionic types, such as sulfonates- arylalkyl
sulfonates and
sulfate- sodium dodecyl sulfate; cationic types, such as alkyl- amines or
ammoniums-
quaternary ammoniums: and amphoteric types, such as betaines- coca midopropyl
betaine.
[0103) Optionally, the biochar may also be treated by applying ultrasonics. In
this
treatment process, the biochar may be contacted with a treating liquid that is
agitated by
ultrasonic waves. By agitating the treating liquid, contaminants may be
dislodged or
removed from the biuchar due to bulk motion of the lluid in and around the
biocarbon,
pressure changes, including cavitation in and around contaminants on the
surface, as well
as pressure changes in or near pore openings (cavitation bubbles) and internal
pore
cavitation.
[01041 In this manner, agitation will cause contaminants of many forms to be
released
from the internal and external structure of the biochar. The agitation also
encourages the
exchange of
27
Date Regue/Date Received 2023-06-16
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
water, gas, and other liquids with the internal biochar structure.
Contaminants are transported
from the internal structure to the bulk liquid (treating fluid) resulting in
biochar with improved
physical and chemical properties. The effectiveness of ultrasonic cleaning is
tunable as bubble
size and number is a function of frequency and power delivered by the
transducer to the treating
fluid
[0105] In one example, applying ultrasonic treatment, raw wood based biochar
between 10
microns to 10 mm with moisture content from 0% to 90% may be mixed with a
dilute mixture
of acetic acid and water (together the treating liquid) in a processing vessel
that also translates
the slurry (the biochar/treating liquid mixture). During translation, the
slurry passes near an
ultrasonic transducer to enhance the interaction between the fluid and
biochar. The biochar
may experience one or multiple washes of dilute acetic acid, water, or other
treating fluids. The
biochar may also make multiple passes by ultrasonic transducers to enhance
physical and
chemical properties of the biochar. For example, once a large volume of slurry
is made, it can
continuously pass an ultrasonic device and be degas sed and wetted to its
maximum, at a rapid
processing rate. The slurry can also undergo a separation process in which the
fluid and solid
biochar are separated at 60% effectiveness or greater.
[0106] Through ultrasonic treatment, the pH of the biochar, or other physical
and chemical
properties may be adjusted and the mesopore and macropore surfaces of the
biochar may be
cleaned and enhanced. Further, ultrasonic treatment can be used in combination
with bulk
mixing with water, solvents, additives (fertilizers, etc.), and other liquid
based chemicals to
enhance the properties of the biochar. After treatment, the biochar may be
subject to moisture
adjustment, further treatment and/or inoculation using any of the methods set
forth above.
[0107] C. Benefits of Treatment
[0108] As illustrated above, the treatment process, whether using vacuum,
surfactant or
ultrasonic treatment, or a combination thereof, may include two steps, which
in certain
applications, may be combined: (i) washing and (ii) inoculation of the pores
with an additive.
When the desired additive is the same and that being inoculated into the
pores, e.g., water, the
step of washing the pores and inoculating the pores with an additive may be
combined.
10109] While not exclusive, washing is generally done for one of three
purposes: (i) to modify
the surface of the pore structure of the biochar (i.e., to allow for increased
retention of liquids);
28
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
(ii) to modify the pH of the biochar; and/or (iii) to remove undesired and
potentially harmful
compounds or gases.
[0110] 1. Increases Water Holdinta Canadtv/VVater Retention CaDacitv
101111 As demonstrated below, the treatment processes of the invention modify
the surfaces of
the pore structure to provide enhanced functionality and to control the
properties of the biochar
to achieve consistent and predicable performance. Using the above treatment
processes,
anywhere from at least 10% of the total pore surface area up to 90% or more of
the total pore
surface area may be modified. In some implementations, it may be possible to
achieve
modification of up to 99% or more of the total pore surface area of the
biochar particle. Using
the processes set forth above, such modification may be substantially and
uniformly achieved for
an entire batch of treated biochar.
[0112] For example, it is believed that by treating the biochar as set forth
above, the
hydrophilicity of the surface of the pores of the biochar is modified,
allowing for a greater water
retention capacity. Further, by treating the biochars as set forth above,
gases and other substances
are also removed from the pores of the biochar particles, also contributing to
the biochar particles'
increased water holding capacity. Thus, the ability of the biochar to retain
liquids, whether water
or additives in solution, is increased, which also increases the ability to
load the biochar particles
with large volumes of inoculant, infiltrates and/or additives.
[0113] A batch of biochar has a bulk density, which is defined as weight in
grams (g) per cm3 of
loosely poured material that has or retains some free space between the
particles. The biochar
particles in this batch will also have a solid density, which is the weight in
grams (g) per cm3 of
just particles, i.e., with the free space between the particles removed. The
solid density includes
the air space or free space that is contained within the pores, but not the
free space between
particles. The actual density of the particles is the density of the material
in grams (g) per cm3 of
material, which makes up the biochar particles, i.e., the solid material with
pore volume removed.
[0114] In general, as bulk density increases the pore volume is expected to
decrease. When
the pore volume is macro or mesoporous, the ability of the material to hold
infiltrate, e.g.,
inoculant is directly proportional to the pore volume, thus it is also
expected to decrease as bulk
density increases. With the infiltration processes, the treated biochars can
have impregnation
capacities that are larger than could be obtained without infiltration, e.g.,
the treated biochars
29
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
can readily have 30%, 40%, 50%, or most preferably, 60%400% of their total
pore volume
filled with an infiltrate, e.g., an inoculant. The impregnation capacity is
the amount of a liquid
that a biochar particle, or batch of particles, can absorb. The ability to
make the pores surface
hydrophilic, and to infuse liquid deep into the pore structure through the
application of positive
or negative pressure and/or a surfactant, alone or in combination, provides
the ability to obtain
these high impregnation capabilities. The treated biochars can have
impregnation capacities,
i.e., the amount of infiltrate that a particle can hold on a volume held/total
volume of a particle
basis, that is greater than 0.2 cm3/cm3 to 0.8 cm3/cm3. Resulting bulk
densities of treated biochar
can range from 0.1- 0.6 g/cm3 and sometimes preferably between 0.3 - 0.6 g/cm3
and can have
solid densities ranging from 0.2 ¨ 1.2 g/cm3.
10115]
[0116] Accordingly, by using the treatment above, the water retention capacity
of biochar can be
greatly increased over the water retention capacities of various soil types
and even raw biochar,
thereby holding water and/or nutrients in the plant's root zone longer and
ultimately reducing the
amount of applied water (through irrigation, rainfall, or other means) needed
by up to 50% or
more. Figure 6 is a chart showing the water retention capacities of soils
versus raw and treated
biochar. The soils sampled are loam and sandy clay soil and a common
commercial horticultural
mix. The charts show the retained water as a function of time.
[0117] In chart A, the bottom line represents the retained water in the sandy
claim loam soil over
time. The middle line represents the retained water in the sandy clay soil
with 20% by volume
percent of unprocessed raw biochar. The top line represents the retained water
in the sandy clay
loam soil with 20% by volume percent of treated biochar (adjusted and
inoculated biochar). Chart
B represents the same using a soilless potting mix rather than sandy clay loam
soil.
[0118] As illustrated in Figure 7, testing showed a treated biochar had an
increased water
retention capacity of approximately 1.5 times that of the raw biochar from the
same feedstock.
Similar results have been seen with biochars derived from various feedstocks.
With certain
biochar types, the water retention capacity of treated biochar could be as
great as three times that
of raw biochar.
10119] "Water holding capacity," which may also be referred to as "Water
Retention Capacity,"
is the amount of water that can be held both internally within the porous
structure and in the
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
interparticle void spaces in a given batch of particles. While a summary of
the method of measure
is provided above, a more specific method of measuring water holding
capacity/water retention
capacity is measured by the following procedure; (i) drying a sample of
material under
temperatures of 105 C for a period of 24 hours or using another
scientifically acceptable
technique to reduce the moisture content of the material to less than 2%, less
than 1%; and
preferably less than .5% (ii) placing a measured amount of dry material in a
container; (iii) filling
the container having the measured amount of material with water such that the
material is
completely immersed in the water; (iv) letting the water remain in the
container having the
measured amount of material for at least ten minutes or treating the material
in accordance with
the invention by infusing with water when the material is a treated biochar,
(v) draining the water
from the container until the water ceases to drain; (vi) weighing the material
in the container (i.e.,
wet weight); (vii) again drying the material by heating it under temperatures
of 105 C for a period
of 24 hours or using another scientifically acceptable technique to reduce the
moisture content of
the material to less than 2% and preferably less than 1%; and (viii) weighing
the dry material
again (i.e., dry weight) and, for purposes of a volumetric measure,
determining the volume of the
material.
[0120] Measured gravimetrically, the water bolding/water retention capacity is
determined by
measuring the difference in weight of the material from step (vi) to step
(viii) over the weight of
the material from step (viii) (i.e., wet weight-dry weight/dry weight). Figure
8 illustrates the
different water retention capacities of raw biochar versus treated biochar
measured
gravimetrically. As illustrated, water retention capacity of raw biochar can
be between 100-
200%, whereas treated biochar can have water retention capacities measured
gravimetrically
between 200-400%.
[0121] Water holding capacity can also be measured volumetrically and
represented as a percent
of the volume of water retained in the biochar after gravitationally draining
the excess
water/volume of biochar The volume of water retained in the biochar after
draining the water can
be determined from the difference between the water added to the container and
water drained
off the container or from the difference in the weight of the wet biochar from
the weight of the
dry biochar converted to a volumetric measurement. This percentage water
holding capacity for
treated biochar may be 50-55 percent and above by volume.
31
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
[0122] Given biochar's increased water retention capacity, the application of
the treated biochar
and even the raw biochar can greatly assist with the reduction of water and/or
nutrient application.
It has been discovered that these same benefits can be imparted to
agricultural growth.
[0123] Treated biochar of the present invention has also demonstrated the
ability to retain more
water than raw biochar after exposure to the environment for defmed periods of
time. For
purposes of this application "remaining water content" can be defined as the
total amount of water
that remains held by the biochar after exposure to the environment for certain
amount of time.
Exposure to environment is exposure at ambient temperature and pressures.
Under this definition,
remaining water content can be measured by (i) creating a sample of biochar
that has reached its
maximum water holding capacity; (ii) determining the total water content by
thermogravimetric
analysis (H20 (TGA)), as described above on a sample removed from the output
of step (i) above,
(iii) exposing the biochar in the remaining sample to the environment for a
period of 2 weeks (15
days, 360 hrs.); (iv) determining the remaining water content by
thermogravimetric analysis (H20
(TGA)); and (v) normalizing the remaining (retained) water in mL to 1 kg or 1
L biochar. The
percentage of water remaining after exposure for this two-week period can be
calculated by the
remaining water content of the biochar after the predetermined period over the
water content of
the biochar at the commencement of the two-week period. Using this test,
treated biochar has
shown to retain water at rates over 4x that of raw biochar. Testing has
further demonstrated that
the following amount of water can remain in treated biochar after two weeks of
exposure to the
environment: 100-650 mL/kg; 45-150 ml IL; 12-30 gal/ton; 3-10 gal/yd3 after
360 hours (15 days)
of exposure to the environment. In this manner, and as illustrated in Figure
8, biochar treated with
a process consistent with those described in this disclosure can increase the
amount of retained
water in biochar about 3 times compared to other methods even after seven
weeks. In general,
the more porous and the higher the surface area of a given material, the
higher the water retention
capacity. Further, it is theorized that by modifying the
hydrophilicity/hydrophobicity of the pore
surfaces, greater water holding capacity and controlled release may be
obtained. Thus, viewed
as a weight percent, e.g., the weight of retained water to weight of biochar,
examples of the present
biochars can retain more than 5% of their weight, more than 10% of their
weight, and more than
15% of their weight, and even more than 50% of their weight compared to an
average soil which
may retain 2% or less, or between 100-600 ml/kg by weight of biochar.
32
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
[0124] Tests have also shown that treated biochars that show weight loss of >
1% in the interval
between 43-60 C when analyzed by the Thermal Gravimetric Analysis (TGA) (as
described
below) demonstrate greater water holding and content capacities over raw
biochars. Weight loss
of > 5%-15% in the interval between 38-68 C when analyzed by the Thermal
Gravimetric
Analysis (TGA) using sequences of time and temperature disclosed in the
following paragraphs
or others may also be realized. Weight percentage ranges may vary from between
> 1% - 15% in
temperature ranges between 38-68 C, or subsets thereof, to distinguish
between treated biochar
and raw biochar.
[0125] Figure 9 is a chart 900 showing the weight loss of treated biochars 902
versus raw biochar
samples 904 when heated at varying temperatures using the TGA testing
described below. As
illustrated, the treated biochars 902 continue to exhibit weight loss when
heated between 40-60
C when analyzed by the Thermal Gravimetric Analysis (TGA) (described below),
whereas the
weight loss in raw biochar 804 between the same temperature ranges levels off.
Thus, testing
demonstrates the presence of additional moisture content in treated biochars
902 versus raw
biochars 904.
[0126] In particular, the treated biochars 902 exhibit substantial water loss
when heated in inert
gas such as nitrogen following treatment, More particularly, when heated for
25 minutes at each
of the following temperatures 20, 30, 40, 50 and 60 C the treated samples lose
about 5-% to 15%
in the interval 43 - 60 C and upward of 20-30% in the interval between 38- 68
C. The samples
to determine the water content of the raw biochar were obtained by mixing a
measured amount
of biochar and water, stirring the biochar and water for 2 minutes, draining
off the water,
measuring moisture content and then subjecting the sample to TGA. The samples
for the treated
biochar were obtained by using the same measured amount of biochar as used in
the raw biochar
sample and using treatment process consistent with those described in this
disclosure. .The
moisture content is then measured and the sample is subjected to TGA described
above.
[0127] The sequences of time and temperature conditions for evaluating the
effect of biochars
heating in inert atmosphere is defined in this application as the "Bontchev-
Cheyne Test" ("BCT").
The BCT is run using samples obtained, as described above, and applying
Thermal Gravimetric
Analysis (TGA) carried out using a Hitachi STA 7200 analyzer under nitrogen
flow at the rate of
110 mL/min. The biochar samples are heated for 25 minutes at each of the
following
33
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
temperatures: 20, 30, 40, 50 and 60 C. The sample weights are measured at the
end of each dwell
step, at the beginning and at the end of the experiment. The analyzer also
continually measures
and records weight over time. Biochars treated with a process consistent with
those described in
this disclosure to enhance water holding or retention capacities typically
exhibit weight loss of >
5% in the interval between 38-68 C,> 1% in the interval between 43-60 'C.
Biochars with
greater water holding or retention capacities can exhibit > 5% weight loss in
the interval between
43-60 C measured using the above described BCT.
[0128] Lastly, as illustrated in Figure 10, plant available water is greatly
increased in treated
biochar over that of raw biochar. Figure 10 illustrates the plant available
water in raw biochar,
versus treated biochar and treated dried biochar and illustrates that treated
biochar can have a
plant available water percent of greater than 35% by volume.
[0129] "Plant Available Water" is the amount of unbound water in a material
available for plants
to uptake. This is calculated by subtracting the volumetric water content at
the permanent wilting
point from the volumetric water content at field capacity, which is the point
when no water is
available for the plants. Field capacity is generally expressed as the bulk
water content retained
in at ¨33 J/kg (or ¨0.33 bar) of hydraulic head or suction pressure. Permanent
wilting point is
generally expressed as the bulk water content retained at -1500J/kg (or -15
bar) of hydraulic head
or suction pressure. Methods for measuring plant available water are well-
known in the industry
and use pressure plate extractors, which are commercially available or can be
built using well-
known principles of operation.
[0130] 2. Adiusts pH
[0131] With regard to treatment for pH adjustment, the above described vacuum
infiltration
processes and/or surfactant treatment processes have the ability to take raw
biochars having
detrimental or deleterious pHs and transform those biochars into a treated
biochar having pH that
is in an optimal range for most plant growth, and soil health. Turning to
Figure 11, a graph 1100
is provided that shows the pH of various starting raw biochars that were made
from different
starting materials and pyrolysis process temperatures, including coconut
shells 1104, pistachio
shells 1101, corn at 500 C 1105, corn at 900 C 1102, bamboo 1103, mesquite
1106, wood and
coffee 1108, wood (Australia) 1109, various soft woods 1110, 1111, 1112, 1113,
1114, 1115,
1116, 1117, red fir at 900 C 1107, various grasses at 500 C 1118, 1119, 1120,
grass 1121, and
34
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
grass at 900 C 1123. The treatment processes described in this disclosure, can
be used to alter
the pH from the various undesirable pH levels and bring the pH into the
pefeired, optimal range
1124 for most plant growth, soil health and combinations of these. Figure 12
is a chart 1200
showing percentage of germination for lettuce plants for particular pHs, and a
desired germination
range 1201. A control 1204 is compared with an optimal pH range 1202, and a
distribution 1203
of growth rates across pHs is shown.
[0132] If treated for pH adjustment, the treated biochar takes a few days
after treatment for the
pH to normalize. Once normalized, tests have proven that pH altered biochar
remains at a stable
pH, typically the treatment is used to lower the stable pH to below that of
the raw biochar, for up
to 12 months or more after treatment. Although in certain situations, the pH
could be altered to
be higher than the raw biochar when needed.
[0133] For example, the treatment process of the present invention can remove
and/or neutralize
inorganic compounds, such as the calcium hydroxide ((CaOH)2), potassium oxide
(K20K20K20),
magnesium oxide (MgO), magnesium hydroxide (Mg(OH)2), and many others that are
formed
during pyrolysis, and are fixed to the biochar pore surfaces. These
inorganics, in particular
calcium hydroxide, adversely affect the biochar's pH, making the pH in some
instances as high
as 8.5, 9.5, 10.5 and 11.2. These high pH ranges are deleterious, detrimental
to crops, and may
kill or adversely affect the plants, sometimes rendering an entire field a
loss.
[0134] The calcium hydroxide, and other inorganics, cannot readily and quickly
be removed by
simple washing of the biochar, even in an acid bath. It cannot be removed by
drying the biochar,
such as by heating or centrifugal force. It is theorized that these techniques
and methodologies
cannot reach or otherwise affect the various pore surfaces, e.g., macro-, meso-
and micro- in any
viable or efficacious manner; and thus cannot remove or otherwise neutralize
the calcium
hydroxide.
[0135] Upon modification of the pore surface area by removal and/or
neutralization of deleterious
substances, such as calcium hydroxide, the pH of the biochar can be reduced to
the range of about
pH 5 to about pH 8, and more preferably from about pH 6.4 to about 7.2, and
still more preferably
around 6.5 to 6.8, recognizing that other ranges and pHs are contemplated and
may prove useful,
under specific environmental or agricultural situations or for other
applications. Thus, the present
treated biochars, particles, batches and both, have most, essentially all, and
more preferably all,
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
of their pore surfaces modified by the removal, neutralization and both, of
the calcium hydroxide
that is present in the starting biochar material. These treated biochars have
pHs in the range of
about 5 to about 8, about 6.5 to about 7.5, about 6.4 to about 7, and about
6.8. Prior to and before
testing, biochar is passed through a 2mm sieve before pH is measured. All
measurements are
taken according to Rajkovich et. al, Corn growth and nitrogen nutrition after
additions of
biochars with varying properties to a temperate soil, Biol. Fertil. Soils
(2011), from which the
International Biochar Initiative (BI) method is based.
[0136] There are a wide variety of tests, apparatus and equipment for making
pH measurements.
For example, and preferably when addressing the pH of biochar, batches,
particles and pore
surfaces of those particles, two appropriates for measuring pH are the Test
Method for the US
Composting Council ("TMCC") 4.11-A and the pH Test Method promulgated by the
International
Biochar Initiative. The test method for the TMCC comprises mixing biochar with
distilled water
in 1 :5 [mass volume] ratio, e.g., 50 grams of biochar is added to 250 ml of
pH 7.0 0.02 water
and is stirred for 10 minutes; the pH is then the measured pH of the slurry.
The pH Test Method
promulgated by the International Biochar Initiative comprises 5 grams of
biochar is added to 100
ml of water pH= 7.0 0.02 and the mixture is tumbled for 90 minutes; the pH
of the slurry is
measured at the end of the 90 minutes of tumbling.
[0137] 3. Rem:wino/Neutralizing Deleterious Materials
[0138] Further, the treatment processes are capable of modifying the pore
surfaces to remove or
neutralize deleterious materials that are otherwise difficult, if not for all
practical purpose,
impossible to mitigate. For example, heavy metals, transition metals, sodium
and phytotoxic
organics, polycyclic aromatic hydrocarbons, volatile organic compounds (VOCs),
and perhaps
other phytotoxins. Thus, by treating the biochar in accordance with the
treatment processes set
forth and described above, the resulting treated biochar has essentially all,
and more preferably
all, of their pore surfaces modified by the removal, neutralization and both,
of one or more
deleterious, harmful, o r potentially harmful material that is present in the
starting biochar
material.
[0139] For example, treatment can reduce the total percentage of residual
organic compounds
(ROC), including both the percentage of heavy ROCs and percentage of VOCs.
Through
treatment, the total ROC can be reduced to 0-25% wt.%, percentage heavy ROC
content can
36
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
be reduced to 0-20% wt.% and VOC content can be reduced to less than 5% wt.%.
For
purposes of this application, "Residual organic compounds" (ROCs) are defined
as compounds
that burn off during thermogravimetric analysis, as defined above, between 150
degrees C and
950 degrees C. Residual organic compounds include, but are not limited to,
phenols,
polyaromatic hydrocarbons, monoaromatic hydrocarbons, acids, alcohols, esters,
ethers, ketones,
sugars, alkanes and alkenes. Of the ROCs, those that burn off using
thermogravimetric analysis
between 150 degrees C and 550 degrees are considered light organic compounds
(volatiles or
VOCs), and those that burn off between 550 degrees C and 950 degrees C are
heavy residual
organic compounds. It should be noted that there may be some inorganic
compounds which also
are burned off during TGA analysis in these temperature ranges, but these are
generally a very
low percentage of the total emission and can be disregarded in the vast
majority of cases as slight
variations. In any of these measurements, a gas chromatograph mass
spectrometer may be used
if needed for higher degrees of precision.
[0140] The percent water, total organic compounds, total light organic
compounds (volatiles or
VOC) and total heavy organic compounds, as referenced in this application as
contained in a
biochar particle or particles in a sample may all be measured by
thermogravimetric analysis.
Thermogravimetric analysis is performed by a Hitachi STA 7200 analyzer or
similar piece of
equipment under nitrogen flow at the rate of 110 mUmin. The biochar samples
are heated for
predetermined periods of time, e.g., 20 minutes, at a variety of temperatures
between 100 and 950
'C. The sample weights are measured at the end of each dwell step and at the
beginning and at
the end of the experiment. Thermogravimetric analysis of a given sample
indicating percentage
water in a sample is determined by % mass loss measured between standard
temperature and 150
degrees C. Thermogravimetric analysis of a given sample indicating percentage
of residual
organic compounds is measured by percentage mass loss sustained between 150
degrees C and
950 degrees C. Thermogravimetric analysis of a given sample indicating
percentage of light
organic compounds (volatiles) is measured by percentage mass loss sustained
between 150
degrees C and 550 degrees C. Thermogravimetric analysis of a given sample
indicating
percentage of heavy organic compounds is measured by percentage mass loss
sustained between
550 degrees C and 950 degrees C. Figure 13 is an example of a
Thermogravimetric Analysis
37
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
(TGA) plot outlining the above explanation and the measure of water, light
organics and heavy
organic s.
[0141] As noted above, treatment can remove or neutralize heavy metals,
transition
metals, sodium and phytotoxic organics, polycyclic aromatic hydrocarbons,
volatile organic
compounds (VOCs), other phytotoxins, and even dioxins. Thus, by treating the
biochar in
accordance with the treatment processes set forth and described above, the
resulting treated
biochar has essentially all, and more preferably all, of their pore surfaces
modified by the
removal, neutralization or both, of one or more deleterious, harmful, or
potentially harmful
material that is present in the starting biochar material.
[0142] Dioxins may also be removed through the treatment processes of the
present
invention. Dioxins are released from combustion processes and thus are often
found in raw
biochar. Dioxins include polychlorinated dibenzo-p-dioxins (PCDDs) (i.e., 75
congeners (10
are specifically toxic)); polychlorinated dibenzofurans (PCDFs) (i.e., 135
congeners (7 are
specifically toxic)) and polychlorinated biphenyls (PCBs) (Considered dioxin-
like compounds
(DLCs)).
[0143] Since some dioxins may be carcinogenic even at low levels of
exposure over
extended periods of time, the FDA views dioxins as a contaminant and has no
tolerances or
administrative levels in place for dioxins in animal feed. Dioxins in animal
feed can cause
health problems in the animals themselves. Additionally, the dioxins may
accumulate in the fat
of food-producing animals and thus consumption of animal derived foods (e.g.
meat, eggs, milk)
could be a major route of human exposure to dioxins. Thus, if biochar is used
in animal
applications, where the animals ingest the biochar, the ability to remove
dioxins from the raw
biochar prior to use is of particular significance.
[0144] Results have proven the removal of dioxins from raw biochar by
applying the
treatment process of the present invention, To demonstrate the removal of
dioxins, samples of
both raw biochar and biochar, treated within the parameters set forth above,
were sent out for
testing. The results revealed that the dioxins in the raw biochar were removed
through treatment
as the dioxins detected in the raw biochar sample were not detected in the
treated biochar
sample. Below is a chart comparing the test results of measured dioxins in the
raw verses the
treated biochar.
38
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
Dioxins Amount Detected in Raw Biochar Amount Detected in Treated
Biochar
Sample Sample
Tetra dioxins 26.4 ng/Kg-dry Not detectable
Pent a clioxi n s 5_86 ng/Kg-dry Not detectable
Hexadloxins 8.41 ng/Kg-dry Not detectable
[0145] A number of different dioxins exist, several of which are known to
be toxic or
undesirable for human consumption. Despite the test results above, it is
possible that any
number of dioxins could be present in raw biochar depending on the biomass or
where the
biomass is grown. It is shown, however, in the above testing, that the
treatment process of the
present invention can be used to eliminate dioxins present in raw biochar.
[0146] Seventeen tetra-octo dioxins and furan congeners are the basis for
regulatory
compliance. Other dioxins are much less toxic. Dioxins are generally regulated
on toxic
equivalents (TEQ) and are represented by the sum of values weighted by Toxic
Equivalency
TEQ = 1[CiI x TEF,
Factor (TEF)
10147] 2,3,7,8-TCDD has a TEF of 1 (most toxic). ThQ is measured as ng/kg
WHO-
PCDD/F-TEQ//kg NDs are also evaluated. Two testing methods are generally used
to determine
TEQ values: EPA Method 8290 (for research and understanding at low levels (ppt-
ppq); and
EPA Method 1613B (for regulatory compliance). Both are based on high
resolution gas
chromatography (HRGC)/high resolution mass spectrometry (HRMS).
[0148] The required EU Feed Value is equal to or less than 0.75 ng/kg WHO-
PCDD/F-
TEQ//kg. Treated biochar, in accordance with the present invention, has shown
to have TEQ
dioxins less than 0.5 ng/kg WHO-PCDD/F-TEQ//kg, well below the requirement for
EU Feed
limits of 0.75 ng/kg WHO-PCDD/F-TEQ//kg. As further set forth above, treatment
can reduce
the amount of detectable dioxins from raw biochar such that the dioxins are
not detectible in
treated biochar. Two methods are used: EPA Method 8290 (for research and
understanding
at low levels (ppt-ppq); and EPA Method 1613B (for regulatory compliance).
Both are based
on high resolution gas chromatography (HRGC)/high resolution mass spectrometry
(HRMS).
39
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
10149] 4, Pore Volume
[0150] Generally, a treated biochar sample has greater than 50% by volume
of its porosity
in macropores (pores greater than 300 nanometers). Further, results indicate
that greater than
75% of pores in treated biochar are below 50,000 nanometers. Also, results
indicate that greater
than 50% by volume of treated biochar porosity are pores in the range of 500
nanometers and
100,000 nanometers. Bacterial sizes are typically 500 nanometers to several
thousand
nanometers. Bacteria and other microbes have been observed to fit and colonize
in the pores of
treated biochar, thus supporting the pore size test results.
[0151] Macropore volume is determined by mercury porosimetry, which
measures the
meso and/or macro porosity by applying pressure to a sample immersed in
mercury at a pressure
calibrated for the minimum pore diameter to be measured (for macroporosity
this is 300
nanometers). This method can be used to measure pores in the range of 3 nm to
360,000 nm.
Total volume of pores per volumetric unit of substance is measured using gas
expansion method.
[0152] Depending upon the biomass from which the biochar is derived,
mercury
porosimetry testing has shown that washing under differential pressure, using
the processes
described above, can increase the number of both the smallest and larger pores
in certain biochar
(e.g., pine) and can increase the number of usable smaller pores. Treatment of
biochar using
either vacuum or surfactant does alter the percentage of total usable pores
between 500 to
100,000 nanometers and further has varying impact on pores less than 50,000
nanometers and
less than 10,000 nanometers.
[0153] Figure 14 is a chart 1400 showing the impact of treatment on pores
sizes of biochar
derived from coconut. The majority of the coconut based biochar pores are less
than 10 microns.
Many are less than 1 micron. Vacuum processing of the biochar results in small
reduction of
to 50 micron pores, with increase of smaller pores on vacuum processing. The
mercury
porosimetry results of the raw biochar are represented by 1402 (first column
in the group of
three). The vacuum treated biochar is represented by 1404 (second column in
the group of
three) and the surfactant treated biochar is 1406 (third column in the group
of three).
[0154] Figure 15 is a chart 1500 showing the impact of treatment on pores
sizes of biochar
derived from pine. The majority of the pine based biochar pores are 1 to 50
microns, which is
a good range for micro-biologicals. Vacuum processing results in significant
reduction of the
to 50 micron pores, with an increase of smallest and largest pores. The
mercury
porosimetry results of the raw biochar are represented by 1502 (first column
in the group
of three). The vacuum treated biochar is represented by 1504 (second column in
the group
of three) and thc surfactant treated biochar is 1506 (third column in the
group of three).
[0155] 5. Electrical Conductivity
[01561 The electrical conductivity (EC) of a solid material-water mixture
indicates the
amount of salts present in the solid material. Salts are essential for plant
growth. The EC
measurement detects the amount of cations or anions in solution; the greater
the amount
of ions, the greater the EC. The ions generally associated with salinity are
Ca2+, Mg2+,
K+, Nat, HNOV. S0.42, Cl HCO3", OH-. Electrical conductivity testing of
biochar
was done following the method outlined in the USDA's Soil Quality Test Kit
Guide and
using a conventional EC meter, The biochar sample is mixed with DI water in a
I:1
biochar to water ratio on a volume basis. After thorough mixing, the EC (dS/m)
is
measured while the biochar particles are still suspended in solution.
Treatment, as
outlined in this disclosure can be used to adjust the ions in the char.
Testing of treated
biochar shows its EC is generally greater than 0.2 dS/m and sometimes greater
than 0.5
dS/m.
[0157] 6. Cation Exchange Capacity
[0158] One method tor cation exchange capacity ("CEC") determination is the
use of
ammonium acetate buffered at pH 7.0 (see Schollenberger, C.J. and Dreibelbis,
ER. 1930,
Analytical methods in base-exchange investigations on soils, Soil Science, 30,
161-173).
The material is saturated with 1M ammonium acetate, (NH40Ac), followed by the
release
of the NH4 + ions and its measurement in meq/100 g (mil I iequivalents of
charge per 100 g
of dry soil) or cmolc/kg (centimoles of charge per kilogram of dry soil).
Instead of
ammonium acetate another method uses barium chloride according to Mehlich,
1938, Use
of triethanolamine acetate -barium hydroxide buffer for the determination of
some base
exchange properties and lime requirement of soil, Soil Sci. Sue, Am. Proc.
29:374-378,
M BaC12 is used to saturate the exchange sites followed by replacement with
either
MgSO4 or MgCl2.
[01591 Indirect methods for CEC calculation involves the estimation of
extracted Ca2+,
Mg2+, IC, and Na* in a standard soil test using IViehlich 3 and accounting for
the
exchangeable acidity (sum of H-1-, A13+, Mn21-, and 1e2+) if the pH is below
6.0 (see
Mehlich, A. 1984, Mehlich-
41
Date Regue/Date Received 2023-06-16
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
3 soil test extractant: a modification of Mehlich-2 extractant, Commun. Soil
Sci. Plant Anal.
15(12): 1409-1416). When treated using the above methods, including but not
limited by
washing under a vacuum, treated biochars generally have a CEC greater than 5
millieq/1 and
some even have a CEC greater than 25 (millieq/l). To some extent, treatment
can be used to
adjust the CEC of a char.
101601 7. Anion Exchanze Capacity
[0161] Similar to CEC measurements, anion exchange capacity ("AEC") may be
calculated
directly or indirectly- saturated paste extraction of exchangeable anions, Cr,
NO3, S042", and
P043- to calculate anion sum or the use of potassium bromide to saturate
anions sites at different
pHs and repeated washings with calcium chloride and final measurement of
bromide (see
Rhoades, J.D. 1982, Soluble salts, p. 167-179. In: A. L. Page et al. (ed.)
Methods of soil
analysis: Part 2: Chemical and microbiological properties; and Michael
Lawrinenkoa and David
A. Laird, 2015, Anion exchange capacity of biochar, Green Chem., 2015, 17,
4628-4636).
When treated using the above methods, including but not limited by washing
under a vacuum,
treated biochars generally have an AEC greater than 5 millieq/1 and some even
have an AEC
greater than 20 (millieq/l). To some extent, treatment can be used to adjust
the CEC of a char.
[0162] 8. Hydrophilicity/Hydrophobicity
[0163] The ability to control the hydrophilicity of the pores provides the
ability to load the
biochar particles with larger volumes of inoculant. The more hydrophilic the
more the biochars
can accept inoculant or infiltrate. Tests show that biochar treated in
accordance with the above
processes, using either vacuum or surfactant treatment processes increase the
hydrophilicity of
raw biochar. Two tests may be used to test the hydrophobicity/hydrophilicity
of biochar: (i)
the Molarity of Ethanol Drop ("MED") Test; and (ii) the Infiltrometer Test.
[0164] The MED test was originally developed by Doerr in 1998 and later
modified by
other researchers for various materials, The MED test is a timed penetration
test that is noted
to work well with biochar soil mixtures. For 100% biochar, penetration time of
different
mixtures of ethanol/water are noted to work better. Ethanol/Water mixtures
verses surface
tension dynes were correlated to determine whether treated biochar has
increased hydrophilicity
over raw biochar. Seven mixtures of ethanol and deionized water were used with
a sorption
time of 3 seconds on the biochar.
42
CIL 03009977 2019-06-27
WO 2017/117302 PCT/US2016/069037
101651 Seven solutions of deionized ("Dr') water with the following
respective
percentages of ethanol: 3,5, 11, 13, 18,24 and 36, were made for testing. The
test starts with a
mixture having no DL If the solution is soaked into the biochar in 3 seconds
for the respective
solution, it receives the corresponding Hydrophobicity Index value below.
Ett/anol.?.';:. Ilytitophobicit y Index .
= .= . õ. .
11,1111*11;)11 n111111111.0)11%.
..:1;. ...0:
IRV ..11:i1-11111..:111"
1,111111111(.3E111,41111 IlL,1111M?,j1lv,>11L1`;õ,p1111111114111j1
01,71011
[0166] To start the test the biochar ("material/substrate") is placed in
convenient open
container prepared for testing. Typically, materials to be tested are dried
1100C overnight and
cooled to room temperature. The test starts with a deionized water solution
having no ethanol.
Multiple drips of the solution are then laid onto the substrate surface from
low height. If drops
soak in less than 3 seconds, test records substrate as "0". If drops take
longer than 3 seconds or
don't soak in, go to test solution 1. Then, using test solution 1, multiple
drops from dropper are
laid onto the surface from low height. If drops soak into the substrate in
less than 3 seconds,
test records material as "1". If drops take longer than 3 seconds, or don't
soak in, go to test
solution 2. Then, using test solution 2, multiple drops from dropper laid onto
the surface from
low height_ If drops soak into the substrate in less than 3 seconds, test
records material as "2".
If drops take longer than 3 seconds, or don't soak in, go to test solution 3.
Then, using test
solution 3, multiple drops from dropper laid onto the surface from low height.
If drops soak
into the substrate in less than 3 seconds, test records material as "3". If
drops take longer than
3 seconds, or don't soak in, go to solution 4.
10167] The process above is repeated, testing progressively higher numbered
MED
solutions until the tester finds the solution that soaks into the substrate in
3 seconds or less. The
43
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
substrate is recorded as having that hydrophobicity index number that
correlates to the solution
number assigned to it (as set forth in the chart above).
[0168] Example test results using the MED test method is illustrated below.
MATERIAL HYDROPHOBICITY INDEX
Raw Biochars 3 to 5
Treated Biochars 1 to 3
[0169] Another way to measure and confirm that treatment decreases
hydrophobicity and
increases hydrophilicity is by using a mini disk infiltrometer. For this test
procedure, the bubble
chamber of the infiltrometer is filled three quarters full with tap water for
both water and ethanol
sorptivity tests. Deionized or distilled water is not used. Once the upper
chamber is full, the
infiltrometer is inverted and the water reservoir on the reserve is filled
with 80 mL. The
infiltrometer is carefully set on the position of the end of the mariotte tube
with respect to the
porous disk to ensure a zero suction offset while the tube bubbles. If this
dimension is changed
accidentally, the end of the mariotte tube should be reset to 6 mm from the
end of the plastic
water reservoir tube. The bottom elastomer is then replaced, making sure the
porous disk is
firmly in place. If the infiltrometer is held vertically using a stand and
clamp, no water should
leak out.
[0170] The suction rate of 1 cm is set for all samples. If the surface of
the sample is not
smooth, a thin layer of fine biochar can be applied to the area directly
underneath the
infiltrometer stainless steel disk. This ensures good contact between the
samples and the
infiltrometer. Readings are then taken at 1 min intervals for both water and
ethanol sorptivity
test. To be accurate, 20 mL water or 95% ethanol needs to be infiltrated into
the samples.
Record time and water/ethanol volumes at the times are recorded.
[0171] The data is then processed to determine the results. The data is
processed by the
input of the volume levels and time to the corresponding volume column. The
following
equation is used to calculate the hydrophobicity index of R
/ = at +
a: Infiltration Rate, cm/s
44
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
b: Sot ptivity, cm/s1/2
bethanot
R = 1.95 *
uwater
101721 Figure 16 illustrates one example of the results of a hydrophobicity
test performed
on raw biochar, vacuum treated biochar and surfactant treated biochar. As
illustrated, both the
vacuum treated and surfactant treated biochar are more hydrophilic than the
raw biochar based
upon the lower Index rating. In accordance with the test data in Figure 16,
the hydrophobicity
of raw biochar was reduced 23% by vacuum processing and 46% by surfactant
addition.
[0173] As an example, raw biochar and treated biochar were tested with
ethanol and water,
five times for each. The results below show that the hydrophobicity index of
the treated biochar
is lower than the raw biochar. Thus, tests demonstrate that treating the
biochar, using the
methods set forth above, make the biochar less hydrophobic and more
hydrophilic.
MATERIAL HYDROPHOBICITY INDEX -
Dried Raw Biochar 12.9
Dried Vacuum Treated Biochar 10.4
Dried Surfactant Treated Biochar 7.0
As Is Raw Biochar 5.8
As Is Vacuum Treated Biochar 2.9
10174] Further, through the treatment processes of the present invention,
the biochar can
also be infused with soil enhancing agents. By infusing liquid into the pore
structure through
the application of positive or negative pressure and/or a surfactant, alone or
in combination,
provides the ability to impregnate the macropores of the biochar with soil
enhancing solutions
and solids. The soil enhancing agent may include, but not be limited to, any
of the following:
water, water solutions of salts, inorganic and organic liquids of different
polarities, liquid
organic compounds or combinations of organic compounds and solvents, mineral
and organic
oils, slurries and suspensions, supercritical liquids, fertilizers, plant
growth promoting
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
rhizobacteria, free-living and nodule-forming nitrogen fixing bacteria,
organic decomposers,
nitrifying bacteria, phosphate solubilizing bacteria, biocontrol agents,
bioremediation agents,
saprotrophic fungi, ectomycorrhizae and endomycorrhizae, among others.
[0175] 9. Impregnation and/or Inoculation with Infiltrates or Additives
[0176] In addition to mitigating or removing deleterious pore surface
properties, by treating
the pores of the biochar through a forced, assisted, accelerate or rapid
infiltration process, such as
those described above, the pore surface poperties of the biochar can be
enhanced. Such
treatment processes may also permit subsequent processing, may modify the pore
surface to
provide predetermined properties to the biochar, and/or provide combinations
and variations
of these effects. For example, it may be desirable or otherwise advantageous
to coat
substantially all, or all of the biochar macropore and mesopore surfaces with
a surface
modifying agent or treatment to provide a predetermined feature to the
biochar, e.g., surface
charge and charge density, surface species and distribution, targeted nutrient
addition,
magnetic modifications, root growth facilitator, and water absorptivity and
water retention
properties.
[0177] By infusing liquids into the pores of biochar, it has been discovered
that additives infused
within the pores of the biochar provide a time release effect or steady flow
of some beneficial
substances to the environment, e.g. root zones of the plants, and also can
improve and provide a
more beneficial environment for microbes which may reside or take up residence
within the pores
of the biochar. In particular, additive infused biochars placed in the soil
prior to or after planting
can dramatically reduce the need for high frequency application of additives,
minimize losses
caused by leaching and runoff and/or reduce or eliminate the need for
controlled release
fertilizers. They can also be exceptionally beneficial in animal feed
applications by providing an
effective delivery mechanism for beneficial nutrients, pharmaceuticals,
enzymes, microbes, or
other substances.
[0178] For purposes of this application, "infusion" of a liquid or liquid
solution into the pores
of the biochar means the introduction of the liquid or liquid solution into
the pores of the biochar
by a means other than solely contacting the liquid or solution with the
biochar, e.g., submersion.
The infusion process, as described in this application in connection with the
piesent invention,
includes a mechanical, chemical or physical process that facilitates or assist
with the penetration
46
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
of liquid or solution into the pores of the biochar, which process may
include, but not be limited
to, positive and negative pressure changes, such as vacuum infusion,
surfactant infusion, or
infusion by movement of the liquid and/or biochar (e.g., centrifugal force
and/or ultrasonic
waves) or other method that facilitates, assists, forces or accelerates the
liquid or solution into
the pores of the biochar.
101791 Prior to infusing the biochar, the biochar, as described in detail
above, may be washed
and/or moisture adjusted. Figure 17 is a flow diagram 1700 of one example of a
method for
infusing biochar with an additive. Optionally, the biochar may first be washed
or treated at step
1702, the wash may adjust the pH of the biochar, as described in more detail
above, or may be
used to remove elemental ash and other harmful organics that may be unsuitable
for the desired
infused additive. Optionally, the moisture content of the biochar may then be
adjusted by drying
the biochar at step 1704, also as described in further detail above, prior to
infusion of the additive
or inoculant at step 1706.
[0180] In summary, the infusion process may be performed with or without any
washing, prior
pH adjustment or moisture content adjustment. Optionally, the infusion process
may be
performed with the wash and/or the moisture adjustment step. All the processes
may be
completed alone or in the conjunction with one or more of the others.
10181] Through the above process of infusing the additive into the pores of
the biochar, the
pores of the biochar may be filled by 25%, up to 100%, with an additive
solution, as compared
to 1-20% when the biochar is only submerged in the solution or washed with the
solution for a
period of less than twelve hours. Higher percentages may be achieved by
washing and/or drying
the pores of the biochar prior to infusion.
[0182] Data have been gathered from research conducted comparing the results
of soaking or
immersion of biochar in liquid versus vacuum impregnation of liquid into
biochar. These
data support the conclusion that vacuum impregnation provides greater benefits
than simple
soaking and results in a higher percentage volume of moisture on the surface,
interstitially and
in the pores of the biochar.
[0183] In one experiment, equal quantities of pine biochar were mixed with
equal quantities
of water, the first in a beaker, the second in a vacuum flask. The mixture in
the beaker was
continuously stirred for up to 24 hours, then samples of the suspended solid
were taken,
47
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
drained and analyzed for moisture content. The mixture in the vacuum flask was
connected
to a vacuum pump and negative pressure of 15" was applied. Samples of the
treated solid
were taken, drained and analyzed for moisture content. Figure 18 is a chart
illustrating the
results of the experiment. The lower graph 1802 of the chart, which shows the
results of
soaking over time, shows a Wt.% of water of approximately 52%. The upper graph
1804 of
the chart, which shows the results of vacuum impregnation over time, shows a
Wt.% of water
of approximately 72%.
[0184] Figures 19a and 19b show two charts that further illustrate that the
total water and/or
any other liquid content in processed biochar can be significantly increased
using vacuum
impregnation instead of soaking. Figure 19a compares the mL of total water or
other liquid
by retained by 1 mL of treated biochar. The graph 1902 shows that
approximately .17 mL of
water or other liquid are retained through soaking, while the graph 1904 shows
that
approximately .42 mL of water or other liquid are retained as a result of
vacuum impregnation.
Figure 19b shows that the retained water of a biochar subjected to soaking
consists entirely of
surface and interstitial water 1906, while the retained water of a biochar
subjected to vacuum
impregnation consists not only of surface and interstitial water 1908a, but
also water
impregnated in the pores of the biochar 1908b.
[0185] In addition, as illustrated by Figure 20, the amount of moisture
content impregnated
into the pores of vacuum processed biochars by varying the applied (negative)
pressure during
the treatment process. The graphs of four different biochars all show how the
liquid content
of the pours of each of them increase to 100% as the vacuum is increased.
[0186] The pores may be substantially filled or completely filled with
additives to provide
enhanced performance features to the biochar, such as increased plant growth,
nutrient
delivery, water retention, nutrient retention, disadvantageous species
control, e.g., weeds,
disease causing bacteria, insects, volunteer crops, etc. By infusing liquid
deep into the pore
structure through the application of positive or negative pressure, surfactant
and/or ultrasonic
waves, alone or in combination, provides the ability to impregnate the
mesopores and
macropores of the biochar with additives, that include, but are not limited
to, soil enhancing
solutions and solids. It should be noted that using these infusion techniques
allows for
impregnating the pores with additives that are more fragile. For example,
since heating is not a
48
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
requirement for these infusion techniques, microbes, chemicals, or compounds
can be infused
without risk of destroying the microbes or changing chemicals or compounds due
to high
temperatures. Also the process can be done at low temperatures to infuse
chemicals that have
low boiling points to keep them a liquid.
[0187] The additive may be a soil enhancing agent that includes, but is not be
limited to, any of
the following: water, water solutions of salts, inorganic and organic liquids
of different polarities,
liquid organic compounds or combinations of organic compounds and solvents,
mineral and
organic oils, slurries and suspensions, supercritical liquids, fertilizers,
PGPB (including plant
growth promoting rhizobacteria, free-living and nodule-forming nitrogen fixing
bacteria, organic
decomposers, nitrifying bacteria, and phosphate solubilizing bacteria),
enzymes, biocontrol
agents, bioremediation agents, saprotrophic fungi, ectomycorrhizae and
endomycorrhizae, among
others.
[0188] Fertilizers that may be infused into the biochar include, but are not
limited to, the
following sources of nitrogen, phosphorous, and potassium: urea, ammonium
nitrate, calcium
nitrate, sulfur, ammonium sulfate, monoammonium phosphate, ammonium
polyphosphate,
potassium sulfate, or potassium chloride.
[0189] Similar beneficial results are expected from other additives, such as:
bio pesticides;
herbicides; insecticides; nematicides; plant hormones; plant pheromones;
organic or inorganic
fungicides; algicides; antifouling agents; antimicrobials; attractants;
biocides, disinfectants and
sanitizers; miticides; microbial pesticides; molluscicides; bacteriacides;
fumigants; ovicides;
repellents; rodenticides, defoliants, desiccants; insect growth regulators;
plant growth
regulators; beneficial microbes; and, microbial nutrients or secondary signal
activators, that may
also be added to the biochar in a similar manner as a fertilizer.
Additionally, beneficial macro-
and micro- nutrients such as, calcium, magnesium, sulfur, boron, zinc, iron,
manganese,
molybdenum, copper and chloride may also be infused into the biochar in the
form of a water
solution or other solvent solution.
[0190] Examples of compounds, in addition to fertilizer, that may be infused
into the pores of
the biochar include, but are not limited to; phytohomiones, such as, abscisic
acid (ABA), auxins,
cytokinins, gibberellins, brassinosteroies, salicylic acid, jasmonates, planet
peptide hormones,
polyamines, karrikins, strigolactones; 2,1,3-Benzothiadiazole (BTH), an
inducer of systemic
49
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
acquired resistance that confers broad spectrum disease resistance (including
soil borne
pathogens); signaling agents similar to BTH in mechanism or structure that
protects against a
broad range or specific plant pathogens; EPSPS inhibitors; synthetic auxins;
photosystem I
inhibitors photosystem II inhibitors; and HPPD inhibitors.
[0191] In one example, a 1000 ppm NO3- N fertilizer solution is infused into
the pores of the
biochar. As discussed above, the method to infuse biochar with the fertilizer
solution may be
accomplished generally by placing the biochar in a vacuum infiltration tank or
other sealable
mixing vessel, chamber or tank. When using vacuum infiltration, a vacuum may
be applied to
the biochar and then the solution may be introduced into the tank.
Alternatively, the solution
and biochar may both be introduced into the tank and, once introduced, a
vacuum is applied.
Based upon the determined total pore volume of the biochar or the incipient
wetness, the amount
of solution to introduce into the tank necessary to fill the pore of the
biochar can be determined.
When infused in this manner, significantly more nutrients can be held in a
given quantity of
biochar versus direct contact of the biochar with the nutrients alone.
[0192] When using a surfactant, the biochar and additive solution may be added
to a tank along
with 0.01 - 20% of surfactant, but more preferably 1-5% of surfactant by
volume of fertilizer
solution. The surfactant or detergent aids in the penetration of the wash
solution into the pores
of the biochar. The same or similar equipment used in the vacuum infiltration
process can be
used in the surfactant treatment process. Although it is not necessary to
apply a vacuum in the
surfactant treatment process, the vacuum infiltration tank or any other mixing
vessel, chamber
or tank can be used. Again, while it is not necessary to apply a vacuum, a
vacuum may be
applied or the pressure in the vessel may be changed. Further, the surfactant
can be added with
or without heat or cooling either of the infiltrate, the biochar, the vessel
itself, or any
combination of the three.
[0193] The utility of infusing the biochar with an additive is that the pores
in biochar create a
protective "medium" for carrying said additive to the environmeni As an
example when the
additive is a fertilizer the nutrient infused biochar provides a more constant
supply of available
nutrients to the soil and plants and continues to act beneficially,
potentially sorbing more
nutrients or nutrients in solution even after introduction to the soil. By
infusing the nutrients
in the pores of the biochar, immediate oversaturation of the soil with the
nutrients is prevented
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
and a time released effect is provided. This effect is illustrated in
connection with Figures 18
and 19 below. As demonstrated in connection with Figures 18 & 19 below,
biochars having
pores infused with additives, using the infusion methods described above, have
been shown to
increase nutrient retention, increase c op yields and provide a steadier
flow of fertilizer to the
root zones of the plants.
101941 Figure 21 is a chart showing improved mass yield in lettuce with
fertilizer infused
biochar using vacuum impregnation. Figure 21 compares the mass yield results
of lettuce grown
in different environments. One set of data measurements represents lettuce
grown in soil over
a certain set time period with certain, predetermined amounts of fertilizer
infused into the
biochar. A second set of data represents lettuce grown in soil over a certain
set period of time
with the same amount of unimpregnated biochar added at the beginning of the
trial and certain
predetermined amounts of NPK solution added to the soil over time. Growth
comparisons were
made between the same amount of fertilizer solution infused into the biochar
as added directly
to the soil, using the same watering schedule. As illustrated, the test
results demonstrated a 15%
yield increase in growth when infusing approximately 750 mg/pot of NPK into
the biochar than
when applying it directly to the soil. Similarly, the same mass yield of
lettuce is achieved at
400 mg NPK/pot with infused biochar than at 750 mg/pot when adding the
fertilizer solution
directly to the soil.
[0195] Figure 22 is a chart illustrating the concentration of nitrate (N)
found in distilled water
after washing differentially treated biochar. In the illustrated example, two
biochar samples
(500 ml each) mixed with 1000 ppm NO3- N fertilizer solution were washed with
distilled
water. The resulting wash was then tested for the presence of nitrate (N),
measured in ppm.
In one sample, the biochar was submerged in and mixed with the nutrient
solution. In the
other example, the biochar was mixed or washed with a nutrient solution
augmented with 1%
surfactant by volume (i.e., 1 ml of surfactant per 100 ml of fertilizer
solution) in a tumbler. In
both examples, the biochar was not dried completely before infusion with the
NO3- N fertili7er
solution, but used as received with a moisture content of approximately 10-
15%. In both
examples, the biochar was mixed with solution and/or surfactant (in the case
of a second
sample) with a bench scale tumbler, rotating the drum for four (4) minutes
without vacuum.
The results demonstrate that the biochar treated with the 1% surfactant
increases the efficiency
51
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
of infiltrating nitrate fertilizer into biochar and then demonstrates the
release of the nutrient
over time. To yield the above data, the test was repeated six times for each
treatment sample,
with 10 washes for each sample per repeat test.
101961 The above are only a few examples of how additive infused biochar may
be produced
for different uses. Those skilled in the art will recognize that there may be
other mechanisms
for infusing fertilizer or other soil additives into the pores of the biochar
without departing
from the scope of the invention. Those skilled in the art will further
recognize that the present
invention can be used on any type of soil application, including, but not
limited to, the
following: crops, turf grasses, potted plants, flowering plants, annuals,
perennials, evergreens
and seedlings, as will be further described below.
10197] For example, in another implementation, additive infused biochar may be
produced for
use for consumption by animals and/or humans. Biochar may be infused in the
same manner
as described above with nutrients (such as carbohydrates, minerals, proteins,
lipids), vitamins,
drugs and/or other supplements (such as enzymes or hormones, to name a few),
or a
combination of any of the foregoing, for consumption by either humans and/or
animals.
Coloring, flavor agents and/or coating may also be infused into the pores of
the biochar or
applied to the surface. The foregoing may be included to enhance the
performance of the
substance in the digestive tract or to ease or facilitate the ingestion of the
biochar.
[0198] D. Biochar as a Habitat for Microorganisms
[0199] Biotechnology, specifically the use of biological organisms, usually
microorganisms, to
address chemical, industrial, medical, or agricultural problems is a growing
field with new
applications being discovered daily. To date, much research has focused on
identifying,
developing, producing and deploying microbes for various uses. However,
despite much work
on the microbes themselves, relatively little work has been performed on how
to carry, deliver,
and encourage the successful establishment of these microbes in their targeted
environment. Most
current technology for microbial carriers in agriculture is based on
technologies or products that
are highly variable and, in many cases, lead to highly unpredictable
performance of microbes in
the field. For example, many commercial microbes in agricultural settings are
delivered on peat,
clay, or other carriers derived from natural sources, accompanied by limited
engineering or
process control.
52
102001 Biochar have a proclivity to interact positively with many microbes
relevant to
plant health, animal health, and human public health applications. In fact,
there has been
a level of initial research focused on inoculating biochar with microbes
and/or using
biochar in conjunction with microbes or materials with microbes, e.g. compost.
See co-
owned US Patent 8,317,891 Method lin- Enhancing Soil Growth Using Bio-char and
Fischer et al., Synergisms between compost and biochar Jar sustainable soil
amelioration
2012, Management of organic waste, 1, 167-198.
102011 However, biochars, especially in raw form, often suffer from many
characteristics
which make their interaction with microbial organisms extremely unpredictable.
Key
among these undesirable characteristics is a high deLn-ee of variability.
Because of this
and other factors, biochar has been, to date, unused in large scale commercial
biotechnology applications. There are several methods by which this
variability can be
ameliorated. At a high level, the methods to overcome these challenges fall
into two
categories: (i) making the biochar a more favorable habitat for the microbes -
either by
modifying its properties, adding materials beneficial to microbes, or removing
materials
deleterious to microbes, or (ii) inoculating, applying, or immobilizing the
microbes on
the biochar in ways that mitigate the underlying variability in the material.
Both of these
high-level methods can be used independently or in conjunction and have been
shown to
have a significant impact on the suitability of biochar in many biotechnology
applications.
[0202] Before delving into the varying treatment methods that will turn the
biuchar into
a microbial carrier or co-deploying with microbes, it is important to be able
to view
biochar as a habitat for microbes. Biochar, especially treated biochar, has
many physical
properties that make it interesting as a microbial habitat. The most obvious
of these is its
porosity (most biochars have a surface area of over 100 m2/g and total
porosity of 0.10
cm3/ern3 or above). Furthermore, many biochars have significant water holding
and
nutrient retention characteristics which may be beneficial to microbes.
Previous
disclosure has outlined how these characteristics can be further improved with
treatment,
e.g., US Patent Application Serial No. 15/156,256, filed on May 16, 2016, and
titled
Enhanced Biochar.
53
Date Regue/Date Received 2023-06-16
I02031 However, recent data indicates that the Earth may be home to more than
one
trillion independent species of microbes (See Kenneth J. Locey and Jay T.
Lemion,
Scaling laws predict global microbial diversity, Proceedings of the National
Academy of
Science, vol. 113 no. 21. Clearly, each of these microbial species does not
require an
identical habitat. In fact, many have evolved in different conditions and
thrive in different
environments. Biochar, due to its organic origins, porosity, and amenability
to treatment
seems to be an extremely desirable base product to be used in the construction
of
microbial carriers or co-deployment of microbes. If the properties of the
biochar can be
made to match the properties expected by particular microbes, or groups of
microbes,
empirical data has shown that a much greater impact can be delivered in many
applications - whether the targeted biochar is used as a carrier, substrate,
co-deployed
product, or merely is introduced into the same environment at a separate time.
It stands
to reason, as many real- world environments are composed of very complex
microbial
ecosystems, that giving certain microbes in these ecosystems a more favorable
habitat,
can ultimately help those microbes to become more successfully established,
and
potentially shift the entire ecosystem based on their improved ability to
compete for
resources. Clearly this is a very desirable characteristic when the successful
deployment
and establishment of a targeted microbe into a new environment is a desired
outcome.
[02041 There are many properties of a habitat which may be important to
certain
microbes, but sonic of the must important are: pH, hydrophobicity or
hydruphilicity,
ability to hold moisture, ability to retain and exchange certain types of
nutrients, ion
exchange capacity (cationic and anionic), physical protection from predatory
or
competitive microbes or protozoa (usable and inhabitable porosity), presence
or absence
of nutrients, micronutrients, or sources of metabolic carbon, ability to host
other
symbiotic microbes or plant systems (such as plant root tissue), or others
which may be
important to various types or species of microbes. Ability to either enhance
or suppress
the availability of certain enzymes can also be an extremely important factor
in building
a viable habitat. This invention focuses on methods and systems that can be
used to
consistently produce biochar which has these targeted characteristics, methods
that can
be used to effectively create a particular formulation of biochar targeted to
match a
particular microbe or
54
Date Regue/Date Received 2023-06-16
CA 03001977 2018..06-27
WO 2017/117302 1PCT/US2016/069037
group of microbes. and techniques for deploying the desired microbes along
with this targeted
biochar, through inoculation, co-deployment, integrated growth / fermentation,
or other methods.
[0205] By using treatment properties disclosed previously, proper feedstock
selection, and
control of the pyrolysis process, the following are some, but not all, of the
properties that can be
consistently targeted and controlled at production scales to improve the
bi.ochar for use with
microbes or as a microbial carrier. Examples of those properties include (1)
pH, (2)
hydrophobicity, (3) sodium levels, (4) usable pore size distribution and
usable pore volume, (5)
particle size and distribution, (6) exterior and interior surface geometry,
(7) nutrient exchange,
(8) exterior and interior surface geometry, (9) useable carbon or energy
source, (10) toxic
materials or compounds, (11) surface structure/crystals/tortuosity, (12)
compatibility with biofilm
formations, and (13) enzyme activity.
[0206] 1. pit
[0207] It is well known that various microbes prefer varying levels of acidity
or alkalinity. For
example. acidophiles have evolved to inhabit extremely acidic environments.
Likewise,
alkaliphiles prefer more basic (alkali) environments. It has been clearly
shown that the methods
outlined for treating biochars can product targeted pH values that can be
sustained over long
periods of time.
[0208] 2. Hydrophobicity
10209] There are several common sources of hydrophobicity in porous
carbonaceous materials.
One of them is the occurrence of hydrophobic organic compounds on the surface
of the char ¨
typically residual from the pyrolysis process. Targeted removal of these
compounds is a method
to improve the hydrophobicity of porous carbonaceous substances. These
compounds can be
removed in a non-selective way by increasing the pyrolysis temperature of the
biomass to a level
at which the compounds will disassociate with the material and become gaseous.
This method,
while useful, is very broad, and can also remove other desirable compounds as
well as changing
the surface chemistry of the residual carbon, increasing ash percentages, or
reducing carbon yield
by reacting and removing more carbon than. is necessary. These compound.s can
also be
selectively removed by the application of a targeted solvent using the
mechanisms previously
disclosed to infiltrate liquids into the pore volume of the material. This
method is also effective,
and has shown to be much more predictable in the removal, of certain
compounds. Since the vast
CA 03001977 2018..06-27
WO 2017/117302 PCT/US2016/069037
majority of microbes rely heavily on water for both transport and life, the
easy association of
water with a material has a large bearing on its ability to sustain microbial
life.
[0210] 3. sodium Levels
[0211] Differing types of microbes have varying proclivities for the presence
of sodium. Some
microbes Halobacterium spp., Salinibucter ruber, Wallentia ichihyophaga prefer
high levels of
salinity, while others prefer moderate or limited levels of sodium. Sodium can
be removed from
biochar by either simple washing, or more preferably and effectively,
treatment methods which
infuse a solvent (most commonly water, although others may be used) into the
pores of the
material. Sodium can be added, by using the same methods except instead of
using a solvent, the
liquid being washed with or infused is a solution high in sodium content.
Additionally, since
sodium usuall.y manifests itself as a cation in solution, temporary or
permanent adjustment of the
cationic exchange capacity (CEC) of the material through treatment which
impacts the ability of
the material to exchange cations. Lowering the CEC of the material will in
many cases reduce
its ability to exchange sodium cations, while raising the CEC wil.1 typically
enhance the ability of
the material to exchange sodium cations, with exceptions occurring if other
cations are present in
quantities that cause them to preferentially exchange instead of the sodium
cations present.
Finally, differing biomass feedstock contains differing levels of sodium ¨
selecting an appropriate
feedstock prior to pyrolysis will result in a raw or untreated biochar with
reasonably controlled
levels of sodium. For example, pine wood, when pyrolyzed, results in a raw
char with lower
levels of sodium, while coconut shells result in char with higher levels of
sodium after pyrolysis.
ASH Composition Untreated Coconut Untreated Pine Untreated Pine
Shell Biochar Biochar #1 Biochar #2
Ultimate Analysis ¨
Moisture free results
4
Ash 6.7% 9.2% 3.6%
Ash Composition
Sodium Oxide, as 5.7 % 1.2% 0.8%
Na2O
Regardless, it should be clear that there are various methods available to
produce final product
with a targeted sodium level., making it suitable for various microbes
depending on their
preference for an environment with a certain sodium level.
[0212] 4. Usable Pore Size Diptribution and Usable Pore Volume
CA 03001977 2018..06-27
WO 2017/117302 PCT/US2016/069037
10213] One very important quality of a microbial habitat is the availability
of shelter from
environmental or biological hazards. A few examples of environmental hazards
are high
temperature, UV radiation, or low moisture, while an example of a biological
hazard is the
existing of predatory multicellular microbes such as protozoa, including both
flagellates and
cil.iates. In order for a particle or material to provide shelter for
microbes, at least two conditions
must be present: (i) The material must consist of pores or openings of a size
which can be
inhabited by the microbe in question (ii) but prevent the hazard from entering
(e.g. pore size
smaller than the size of predators, such as protozoa, or deep enough to be
shaded from UV rays)
and, (iii) the pores mentioned previously must be usable ¨ namely, they should
not be occupied
by solid matter (clogged) and/or they should not contain substances that are
toxic or undesirable
for the microbe in question. In. some cases, the pore size distribution of a
biochar can be adjusted
by the selection of the biomass feedstock to be pyrolyzed and the conditions
of the pyrolysis
process itself. For example, pine wood has a relatively narrow pore size
distribution, with most
pores falling in the range from 10-70 pm. Coconut shells, on the other hand,
have a much wider
size distribution, with many pores below 1pm, and also a high percentage of
porosity above
I 00pm. It is theorized that materials with pores of a single size or where
most pores are of similar
size can potentially be good carriers or habitats for certain, targeted
microbes, while materials
consisting of broader ranges of pore sizes may be better habitats for
communities, consortia or
groups of microbes, where each microbe may prefer a slightly different pore
size. Furthermore,
the pore size of a material may also be controlled during the pyrolysis
process by increasing
temperature or performing "activation" or other steps common in activated
carbon production to
react or remove carbon, leaving larger pores, or exposing availability of
pores that were once
inaccessible from the exterior surface of the material. Adjusting the particle
size of the material
may also change the pore size distribution in at least two ways: (i) exposing
pores that were not
available or accessible previously, or (ii) destroying larger pores by
fracturing, splitting, or
dividing them. In many cases, raw biochar may contain a proper pore size
distribution, but for
one reason or another, the pores are not usable by the microbes in question.
In other cases, the
pore size distribution provided by the natural feedstock may be undesirable.
Both properties may
also be impacted through treatment of the raw biochar itself. Larger pores can
be created using
strong acids or other caustic substances either by simple washing or through
forced or rapid
57
CIL 03009977 2010-04-27
WO 2017/117302 PCT/US2016/069037
infusion into the pores. Conversely, a material with fewer usable pores may be
created by
intentionally "clogging" or filling the larger pores with either solids, gums,
or liquids designed to
stay resident in the pores themselves. This treatment may be done in a
controlled way to only
partially fill the pores. For example, one could infuse a limited amount of
heated liquid, such as
a resin, that will become solid at normal atmospheric temperatures. If the
volume of liquid used
is less than the available pore volume of the material being infused, some of
the porosity of the
material will be left untreated and available for use. Most importantly, and
most commonly,
usable pore volume may be increased through the act of simply removing
contaminants (physical
or chemical) from the pores. Rapid infusion and extraction of liquids may be
used to accomplish
this. As discussed previously, appropriate solvents may be infused or
extracted to remove
chemical contaminants. Additionally, gasses or liquids may be driven into or
out of the pores to
force the removal of many solid obstructions, such as smaller particles of ash
or simply smaller
particles of raw biochar which may have become lodged in the pore in question.
Regardless of
the mechanism used, it has been shown that the available, uncontaminated,
usable pore volume
and pore size has a major role in determining the efficacy of biochars in
microbial roles.
[0214] Figures 23 and 24 are images that show how different sized bacteria
will fit in different
biochar pore size structures. Figure 23 is rod-shaped gram-positive bacteria,
Bacillus
thuringiensis israelensis, in a treated pine biochar, with pore openings of -
10-20 pm and bacteria
of -2-5 gm. Figure 24 is rod-shaped gram-negative bacteria, Serratia
liquefaciens, in a treated
coconut shell biochar, with pore openings of -2-10 pm and bacteria of - 1-2
gm.
[0215] In addition, total pore volume in the size of 5-50 gm has been shown to
correlate with
microbial release rate after inoculation on treated biochar. Figure 25
illustrates release rate data
verse total pore volume data for both coconut shell and pine based treated
biochars inoculated
with a releasable bacteria. As illustrated in Figure 25, the data was plotted
in a graph, and clearly
shows that as pore volume increases so does the release rate.
[0216] 5. Exterior and Interior Surface Geometry
[0217] Two important properties of microbial carriers are: (i) their ability
to release microbes
from their surfaces and (ii) their ability to immobilize or stabilize microbes
on their surfaces.
Depending on the final application or use of the carrier, one or both of these
properties may be
desired. For example, for carriers designed to quickly release a microbe into
a targeted domain
58
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
such as a lake, river, or other waterway, the release characteristics of the
material are paramount.
For other applications, such as applications of certain symbiotic microbes in
agriculture, rapid
release may be undesirable, rather it may be important to sustain the microbes
within the
porosity of the material until plant tissue, such as root biomass, is nearby
to piovide nutrition
for the microbes in question. The surface and pore geometry of the material
used as a carrier
can be critical to determine this behavior. For example, material with
generally smooth, uniform
surfaces will typically release many microbes much more effectively, while
material with more
rugged, varied, tortuous pore surfaces and geometry will typically retain and
immobilize
microbes more effectively. The biomass used in the production of the fmal
material is one of
the most important factors in surface geometry. However, even this quality can
be altered
through treatment. Specifically, smooth surfaces may be etched by implementing
the treatment
and infusion processes previously disclosed with strong acids, rendering them
rougher.
Conversely, rough surfaces may be treated with either organic or inorganic
compounds to coat
and remove contour. Mechanical means may also be used to affect changes in
particle geometry.
Many forms of charred material have relatively low crush strength and are
relatively brittle. The
method used to grind, or size particles can have a large impact on the
geometry of the final
particles. For example, particles milled using a ball mill or other type of
grinding technology
will typically have a smoother exterior geometry after the milling is complete
and may lose a
good amount of their porosity through the simple mechanical crushing of pores.
However,
particles sized using ultrasonic vibrations or even simple physical vibrations
to shatter, rather
than crush larger particles into smaller ones, will typically retain their
geometry, or sometimes
result in smaller particles with more rugged geometries than the particles at
the beginning. It
should be apparent to one skilled in the art that there are various mechanical
mechanisms
available to effect these changes, but the resulting particles can be tailored
to meet a particular
microbial release or immobilization outcome.
[0218] 6. Particle Size and Distribution
[0219] It is well known that the particle size and particle size distribution
of a material has a
key impact on its formulation as a microbial carrier. In many cases, these
factors are very
different for porous carbonaceous materials than they are for other common
microbial carriers.
In standard carriers, typically the reduction of particle size is a method
used to increase surface
59
CA 03001977 2018..06-27
WO 2017/117302 PCT/US2016/069037
area, and thus the area available to support, immobilize, and carry microbes.
However, in porous
materials, specifically materials with a large volume of usable interior
porosity, sometimes a
reduction in particle size does not cause a large increase in the usable
surface area =-== specifically
because the interior surfaces of the material were already exposed, and
reducing the size of the
particle does not change that fact. This leads to a somewhat counterintuitive
behavior in some
cases in which the reduction of the particle size of a porous material
actually degrades its
performance as a microbial carrier, due to the phenomena that surfaces that
were once sheltered
inside the material are exposed as exterior surfaces when the material is
split or crushed, making
the material less desirable as a habitat for microbes that require shelter
from the surrounding
environment. Additionally, at times the actual distribution of particle sizes
can be a key factor
in perforrrtance. As a simple example, imagine an aggregated material, which
consists of only
two particle sizes: 1mm arid 1 IA in. Furthermore, imagine that 50% of the
mass of the aggregate
resided in the lnarn particles with the remainder in the 1prn particles.
Lastly, imagine that the
lmm particles were porous carbonaceous particles with an average pore size of
approximately
50gm. It should be clear that if this aggregate was placed in a container and
agitated, that a
good portion of the 1. gm particles would end up inhabiting the pore volume of
the lmm particles,
impacting their usability. In fact, this is the behavior that we see in
practice. Therefore, for
certain microbial applications, it is desirable to remove extremely small
particles, often referred
to as fines, from the aggregate. This has the additional benefit of reducing
dust during
application, which is particularly important in aerial applications, and
reducing the level of
surface runoff for applications in water, which also is important in certain
microbial
applications. The small particles may be removed through several methods such
as sieving,
blowing or aerodynamic removal, separation with either stationary or moving
liquids
(hydrostatic or hydrodynamic separation) of various viscosities, temperatures,
flow rates, etc.
However, at times, having a mixture of smaller and larger particles can be
desirable. The most
common cases are when communities of microbes are to be deployed, or the
aggregate is to
remain generally intact for a period of time (fermentation applications, long
term storage
applications, or preparation for other formulation uses such as
palletization), in which case, the
interparticle void space is also an important factor and can be. optimized for
a particular microbe
or set of microbes by providing a range of particle sizes and geometries.
CM 030011977 201111:0627
WO 2017/117302 PCT/US2016/069037
102201 7. Nutrient Exchanze
[0221] The ability of a material to hold or exchange nutrients is an
incredibly important
characteristic, not only for microbial, but also for general agricultural
applications. There are
two primary mechanisms that porous carbonaceous materials can exchange
nutrients: (i)
sorption or retention of the nutrients on the interior and exterior surfaces
of the material, and (ii)
retention of the nutrients either in suspension or solution in liquid or
gasses residing in the pore
volume of the material. Both mechanisms are very useful, but also very
different in function.
Surface sorption or retention is driven by two main properties, among others:
(1) ion exchange
capacity of the material and. (ii) reactivity or electrical charge of
compounds present on or
coating the surfaces of the material. Retention of nutrients in solution or
suspension are
impacted by other, different characteristics of the material, such as
hydrophilicity, oil sorption
capacity, usable pore volume and pore size distribution, and interior pore
geometry and
tortuosity. The surface retention of nutrients can be targeted by selecting
the feedstock biomass
(some materials render a char after pyrolysis with vastly differing ionic
exchange capacities
(CEC and AEC) than others). It can also be impacted by adjusting pyrolysis
conditions. Higher
pyrolysis temperatures tend to reduce CEC and nutrient adsorption capability.
See Gal, Xiapu
et al. "Effects of Feedstock and Pyrolysis Temperature on Biochar Adsorption
of Ammonium
and Nitrate." Ed. Jonathan A. Coles. PLoS ONE 9.12 (2014): el13888. PMC. Web.
19 Nov.
2016. In addition, the surface retention of nutrients can be impacted by
treating the surfaces of
the material with substances targeted towards adjusting the ionic exchange
characteristics. For
example, using the previously disclosed treatment methods to infuse FI202 into
the pores of the
carbonaceous material and then evaporating the liquid can increase the
cationic exchange
properties of the material.
102221 Furthermore, another way to exchange nutrients more efficiently is to
use the pore
volume rather than, or in addition to, the pore surfaces ¨ namely keeping the
nutrients in solution
or liquid or gaseous form and placing them in the volume of the pores rather
than attempting to
sorb them on the surfaces of the material. This can be an incredibly useful
technique not only
for plant life and soil health, but also for microbes. The food sources can
vary from simple to
complex such as glucose, molasses, yeast extract, kelp meal, or bacteria media
(e.g.
MacConkey, Tryptic Soy, Luria-Bertani). When using the pore volume to exchange
nutrients
61
CM 030011977 201111:0627
WO 2017/117302 PCT/US2016/069037
in this way, it should be clear that a wide variety of nutrients may be used,
and targeted
combinations of pore volume, size, and nutrition can be produced to assist in
the delivery,
establishment, or successful colonization of targeted microorganisms or groups
of
microorganisms. It should be clear by this point that merely immersing the
biochar or porous
carbonaceous material in a liquid nutrient broth may be partially effective in
filling the pore
volume or coating the pore surfaces with these nutrients and should be
considered within the
scope of this invention, however using the treatment techniques outlined in
this and related
disclosures is much more effective at both coating the surfaces and infusing
nutrition into the
pore volume of the material itself. Since many microbes rely on liquid for
mobility, placing
liquid into the pore volume of the material is in many cases a prerequisite
for successfully
infusing, carrying, or delivering microbes.
10223] 8. Usable Carbon or Enerev Sources
[0224] Related to the ability to improve nutrient exchange is the ability to
treat the pore volume,
pore surfaces, exterior surfaces, or any combination of these with not only
custom broths or
growth media, but also other forms of carbon known to be beneficial to
microbes and plant life.
Some examples of this are carbohydrates (simple and complex), humic
substances, plant macro
and micronutrients such as nitrogen (in many forms, such as ammonium and
nitrates),
phosphorous, potassium, iron, magnesium, calcium, and sulfur and trace
elements such as
manganese, cobalt, zinc, copper, molybdenum. These nutrients may either be
infused in liquid
or gaseous form, or even as a suspended solid in liquid. The liquid ma.y be
left in the pores, or
may be removed. If removed through evaporation, nutrients in solution or
suspended solids
may be left behind, while if removed by mechanical or physical means, a
portion of the liquid
may be left behind as well as some solids. It should be noted that the various
forms of removal
have differing advantages and disadvantages and that many energy sources may
be added either
at the same time or in sequence, with one, or many, removal steps i.n between
treatment or
infusion steps.
[0225] 9. Toxic Materials or Compounds
[0226] The selective addition or removal of materials or substances known to
be toxic to a
certain microbe or lifeform is a key step in preparation of biochar for use as
a microbial habitat
or carrier. It has been shown, that through treatment, potentially toxic
compounds can be
62
CM 030011977 201111:0627
WO 2017/117302 PCT/U82016/069037
removed with much greater effectiveness than through simple pyrolysis alone.
Some examples
of the potentially deleterious compounds that may be removed are: volatile
organic compounds
(VOCs), monoaromatics, polycyclic aromatic hydrocarbons (PAHs), heavy metals,
and
chlorinated compounds (e.g. dioxins and furans). A proven approach to remove
these
substances is to wash the exterior surfaces with and/or rapidly infuse a
solvent into the pore
volume of the material targeted to remove these substances. Following the
infusion with either
mechanical extraction, drying, or other methods to remove the solvent, laden
with the substances
in question, from the pores and interparticle spaces is a desirable, but not
strictly necessary step
to further reduce the levels of toxicity. For example, the following data
shows removal of
dioxins using the treatment process of the present invention.
Raw coconut Treated coconut Raw pine Treated pine
shell biochar shell biochar biochar biochar
'rEQ ng/kg 0.7 0.4 9.6 OA
(method
8290A)
102271 Another approach for some toxic compounds (benzene as one example) is,
rather than
removing the compounds in question, to react them in place with other
compounds to neutralize
the toxicant. This approach can be used either with washing, or forced /
assisted infusion, and
in these cases a removal step is less necessary ¨ although it still can be
used to prepare the
material for another, subsequent phase of treatment.
10228] Much attention is given to the removal of toxic compounds, but it
should be also be
noted that at times, it can be extremely beneficial to actually add or treat
the material with toxic
compounds. A primary example of this is sterilization, or preparation for
selective infusion.
Even after pyrolysis, residual biological life has been found to potentially
establish itself in
biochars given the right conditions. Treating, washing, or infusing the
material with antiseptics
such as methanol, ethanol, or other antibacterial or antiviral substances can
be a key step in
removing contamination and preparing the material for use in microbial
applications. A
variation on this approach is to infuse, treat, or wash the material with a
selectively toxic
compound, such as a targeted antibiotic or pharmaceutical targeted towards
interrupting the
lifecycle of a. specific set of microorganisms or organisms, thereby giving
other microbes, either
through infusion or merely contact in situ the opportunity to establish. Some
examples of this
63
treatment would be the use of antifungals such as cycioheximide to suppress
fungal
growth and provide an environment more well suited toward the establishment of
bacteria. As has been stated previously, the methods may be used alone, or in
combination
with one another. Specifically, a toxic compound such as ethanol, may be
infused,
removed, and then steps may be taken to remove other toxic compounds, followed
by
steps to add carbon sources or growth media.
[02291 10. Surface Structures / Crystals / Tortuosity
[02301 The physical surface and pore structure of the material is critically
important to its
suitability as a microbial habitat. There are many factors that contribute to
the surface
structure of the material. The most notable of these factors is the biomass
used to produce
the carbonaceous material - the cellular structure of the biomass dictates the
basic shape
of many of the pores. For example, pyrolyzed coconut shell s typically have
less surface
area, and a more diverse distribution of pore sizes than pyrolyzed pine wood,
which, when
pyrolyzed at the same temperature, has greater surface area, but a more
uniform (less
diverse) pore size distribution. Tortuosity, or the amount of curvature in a
given path
through a selected pore volume is also an extremely important characteristic
of
engineered porous carbonaceous materials.
[02311 Figure 26 shows the total fungi/bacteria ratio for two biochars derived
from
different biochar stalling materials, e.g., feedstocks. Each biochar was
loaded with
different levels of moisture, and the total fungi/bacteria ratio was monitored
during the
first week. Biochar A 2601 showed a constant total fungi/bacteria ratio of
0.08 across
moisture levels rang5000ng from 15% to 40%, while Biochar B 2602 showed a
constant
total fungi/bacteria ratio of 0.50 for moisture levels ranging from 30% to
40%. It is
theorized that, a fungi/bacteria ratio between 0.05 and 0.60 is an effective
prescription for
a stable biochar composition. This composition allows a commercially viable
product,
which has sufficient shelf life that it can be delivered to storage houses
waiting for the
proper planting window.
[02321 it is theorized that the difference in the observed total fungi/total
bacteria ratios of
may also be explainable by the structures of the biochars. Biochar' s having
an open pore
structure, e.g., more interconnected pores, promotes more bacteria formation;
while
closed pores, e.g., relatively non-connected nature of the pores, tends to
promote fungi
formation. Biochars with differing microbial communities may be beneficial for
specific
applications in
64
Date Regue/Date Received 2023-06-16
commercial agriculture, Thus, custom or tailored loading of the microbial
population may
be a desired implementation of the present invention.
[0233] Intentionally Blank
[0234] Intentionally Blank
[0235] Intentionally Blank
[02361 For example, as shown in Figures 27a, 27b and 27c, Biochar A 2701 shows
that it
has a greater population of, i.e., is inhabited by, more gram negative, gram
positive and
actinomycetes than Biochar B 2702. Thus, for example, Biochar A would be more
applicable for use with certain agricultural crops in which Plant Growth
Promoting
Bacteria (PGPB) species in the actinomycetes, gram (-) pseudomonas, and
bacillus groups
are used for nutrient utilization and uptake.
[0237] It should be noted that both pyrolysis and post-treatment can be used
to further
modify the shape of these pores and structures. Pyrolyzing at higher
temperatures, injecting
select gasses or liquids during pyrolysis, or both typically will increase the
pore volume
and surface area of the material in question. Steam is the most readily
available gas to
cause this effect, but hydrogen sulfide, carbon dioxide, carbon monoxide, as
well as other
reactive gasses can be used. Prior art has clearly shown that the surface area
of a biochar
changes based on feedstock and pyrolysis temperature. Post treatment focused
on a forced
infusion of a strong acid, or other reactive substance into the pore space of
the
carbonaceous material can also be used to modify the pore size and pore volume
of material
by removing or breaking down the carbon matrix which forms the structure of
the biochar,
or other porous carbonaceous material. Acid etching or infusion can also be
used to make
smoother surfaces rougher. Rough surfaces can be very useful in the attachment
and
immobilization of microbes. Smooth surfaces can be useful for the easy release
of carried
microbes. Coating the surface area with materials such as starches is a
technique to make
rough surfaces smoother. Ultrasound, with or without a transmission media
(gel, liquid,
oil, or other) can also be used to rupture interpore divisions and create more
porn space.
Flash gasification, either at atmospheric pressure, or under negative or
positive pressure,
of liquid infused into the pores by the methods previously disclosed can also
be used to
crack, disrupt, or fracture solid material separating adjacent pores.
[0238] While much attention is given to modifying the pore structure by
removing
carbonaceous material, it should be noted that the pore structure can also be
modified by
the coating, forced infusion, and/or addition of materials which will bond to
the carbon and
consume pore volume, smooth surfaces, add tortuosity, change the exterior
surfaces, or all
of these. In the
Date Regue/Date Received 2023-06-16
CA 0300,977 2018.436-27
WO 2017/117302 1PCT/US2016/069037
most simple form, it should be clear that materials may be added to coat
surfaces or fill pore
volume either through forced infusion, simple contact, or other means.
However, if the material
is infused or even simply contacted with a super saturated solution of a
substance that will
crystalize, such as sucrose, sodium chloride, or other common or uncommon
substances known
to form crystals. It should be noted that the crystals or substances used to
create them do not need
to be water soluble, and in fact in many cases it is desirable if they are
not. The crystals may also
be composed of nutrients or substances which may be beneficial to microbial or
plant life_
Examples of this are sucrose and monoammonium phosphate, both known for their
ability to
easily crystalize and be beneficial for microbial and plant life respectively.
By adding material
or even growing crystals on the carbon, a hybrid material is formed which can
have many
properties that are exceptionally useful for the delivery and establishment of
microbial systems.
Crystallization is also way to add tortuosity to a carbonaceous material and
typically is much
more effective in this aspect than coating with solids alone.
[0239] 11. Compatibility with IliofiIm Formation
[0240] Biofilms can be an important factor in tlic survival of a microbe in cx
irerne or challenging
conditions. Bacterial communities can shift their morphology to increase
nutritional access and
decrease predation. One such modification is that the bacteria may attach to
surfaces, such as
those found in biochar, in a densely compacted community. In this compacted
form, they may
form an e.xtracellular polymeric substance (EPS) matrix called a biofilm.
These communities
can contain hundreds of different species which find shelter under the
protective EPS coating
from predatory protozoa, pathogens, contaminants, and other environmental
stressors. In some
cases, usually related to public health or healthcare, biofilms are
undesirable as they typically
allow pathogenic microbes to survive exposure to antiseptics, antibiotics,
predatory microbes
such as protozoa, or other agents which may eliminate, them or negatively
impact their prospects
for survival. But in agricultural settings, encouraging target biofilm
establishment could lead to
improved microbe survival and thus improved agricultural or crop benefits.
[0241] As outlined in the article titled The Effect of Environmental
Conditions on Biolilm
Formation af Burkholderia psudomallei Clinical Isolates, it can be seen that
certain bacteria
require certain environmental factors, among them surface pH, for the creation
of biofilms. See
Ramli, et al., The Effect of Environmental Conditions on Biofilm Formation of
Burkholderia
66
psudomallei Clinical Isolates (September 6, 2012), e4-4104, It is believed
that other
surface characteristics (ragged vs. smooth surfaces, surface charge, and
more), along with
moisture levels and relative humidity also play a large role in biofilm
formation.
[0242] But for certain microbes requiring deployment into environments known
to
present survival challenges, optimizing a delivery material to encourage the
formation of
these protective biofilms can provide the targeted microbes with a significant
advantage.
Also, many vegetable and short cycle row crops such as tomatoes, lettuce, and
celery
form mutualistic relationships with bacteria that lead to the formation of
biofilms on root
hairs that function not only in nutrient uptake but also in plant pathogen
resistance.
10243l As outlined in previous disclosure, treatment of raw biochar can be
used to adjust
the surface pH to a level suitable for biofilm formation. Similarly, adjusting
the humidity
by selectively leaving a measured or controlled amount of water resident in
the pore
volume of the material can also provide benefit. Lastly, the techniques
outlines for
modifying the physical surface properties of the material either by smoothing
or
roughening, can be key factors also.
[0244] It should be clear that these factors can also be reversed to create an
environment
that is unsuitable for biofilm formation in applications where the formation
of biofilms
On the carrier is not desirable - e.g. delivery or applications where quick
release of
microbes from the carrier is important.
[0245] 12. Surface Charge
[0246] The surface charge of a porous carbonaceous material can be crucially
important
in t e association and establishment of targeted microbes with or on the
material. For
example, most bacteria have a net negative surface charge and in certain
conditions a
specific bacterium may favor attachment to positively charged stufaces. In
some
biological applications, this attachment may be preferred, in others,
attachment may not
be preferred. However, modifying the surface charge of the material is clearly
a way to
impact the suitability for attachment of certain microbes. There are many ways
in which
the surface charge of a carbonaceous material may be changed or modified. One
way to
accomplish this is by treating the surface area of the material with a
solution containing
a metal, such as Mn, Zn, Fe, or Ca. This can be performed either by doping the
material
with these metals prior to or during pyrolysis, or more preferably, by using a
forced
67
Date Regue/Date Received 2023-06-16
CA 03001977 2018..06-27
WO 2017/117302 1PCT/US2016/069037
infusion or treatment technique after pyrolysis to deposit these substances on
the interior and/or
exterior surfaces of the carbonaceous material. By controlling the amount and
or types of
substances infused, the surface charge of the material can be modified by
encouraging loading of
02.- or other anions. or conversely, N, NH), or other cations. This
modification of surface charge
can have a profound impact on the ability of certain microorganism to be
immobilized on the
interior and exterior surfaces of the material.
10247] Another application of surface charge can be found by temporarily
charging the
carbonaceous material during inoculation with microbes. Carbon is used as a
cathode or anode
in many industrial applications. Because of its unique electrical properties,
carbon, or more
specifically porous carbonaceous materials, may be given a temporary surface
charge by the
application of a difference in electrical potential. One application of this
mechanism is to create
a temporarily positively charged surface to encourage microbial attachment.
Then, while the
charge is maintained, allowing the microbes to attach themselves to and
colonize the carrier.
Once the colonization is complete, the charge can be released and the carrier,
laden with microbes
can either be deployed as is, or can undergo further treatment to stabilize
the microbes such as
lyophilization, or freeze drying.
[0248] 13. Enzyme Activity
[0249] For some types of microbes, enzyme activity, or the presence of certain
enzymes is every
bit as important as the availability of energy or nutrition. Enzymes can be
critical in the ability
of microbes to metabolize nutrition, which in turn can be a key element of
reproduction, survival,
and effective deployment. There are six main types of enzymes: hydrolases,
isom.erases, ligases,
lyases, oxidoreductases, and transferases. These enzymes can be important in
microbial
applications. Through treatment or even simple contact, enzymes, like
nutrients and energy
sources, can be deposited on the surfaces or within the pore volume of porous
carbonaceous
materials, either as solids, or i.n solution/suspension, ensuring the enzymes
are not degraded
through the process. However, forced infusion of enzymes through the treatment
processes
previously outlined allows for much greater storage capacity and much greater
levels of contact
with the interior surfaces of the biochar, and as such, is preferable to
simple contact. In some
cases, the carbonaceous material can be used to deliver enzymes alone into an
environment where
68
CM 030011977 201111:0627
WO 2017/117302 PCT/US2016/069037
both a habitat and enzymes are needed to promote or encourage the growth of
certain indigenous
microbes.
10250] Another important aspect of enzyme activity is that some bacteria
make extra-cellular
enzymes which could be bound by the biochar or either reduce or even stop
biochemical reactions.
Thus, i.n certain situations when application is appropriate the carbonaceous
material can be used
to inhibit or make certain enzymes ineffective. For example, if the biochar is
being used as a
carrier for food or certain chemicals that are vulnerable to breakdown by
enzymatic degradation
and these specific enzymes would be bound by the biochar, then using the
carbonaceous material
as the carrier would provide for greater shelf-life and viability of the
product versus traditional
carriers.
102511 14. &erilizatioit
102521 In many cases, it is desirable to remove potential unwanted microbes
from the surfaces
and pore volume of the material through sterilization. At outlined above,
infusion with antiseptics
or antibiotics are a way to accomplish this. Boiling, or more preferably,
forced infusion of steam
is also a technique that can be used to remove resident microbial life.
Heating to a temperature
above 100 degrees C, and preferably between 100 and 150 degrees C is also
effective for
removing some microbial life. Heating may be required for ideally 30 minutes
or more,
depending on volume, method, and extent (temperature, radiation). Autoclaving
can also be used
30 minutes, 121 degrees C, 20 psig. For applications requiring a high level of
sterility, gamma
irradiation can be used, with dosages adjusted for the level of sterility
needed in ranges of 5 to 10
kGy or even 50 to 100 kGy or even higher dosage levels. For all sterilization
methods, the extent
of treatment required will depend on the volume of material and the required
level of sterilization.
In general, sterilization, using heat, should be done for at least 30 minutes,
but should be adjusted
as needed.
10253] At this point, it should be clear that all or these properties can
be controlled and
modified to create a treated, controlled biochar that is suitable for use as a
microbial carrier,
delivery system, habitat, fermentation substrate, or environmental (soil,
water or other)
enhancement. By controlling these properties and producing a material matched
to the application
and the microbe(s) in question, effectiveness can be dramatically improved
over both traditional
biological carriers, and many forms of raw, untreated, uncontrolled biochar.
Furthermore,
69
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
varying materials, with varying properties, may be aggregated to provide
delivery systems or
habitats targeted towards consortia, communities, or groups of microbes.
[0254] E. Inoculating, Applying, or Immobilizing the Microbes on the
Biochar
102551 Typically, the prior art teaches either placing biochar on soils alone
or combining the
biochar with compost and using this mixture as a soil amendment. The nature of
the microbial
population in this compost mixture is poorly disclosed by the prior art. Thus
using more targeted
methods to get the desired microbes into the suitable habitat created by the
raw biochar, or more
preferably treated or controlled biochar is desired. The following are some
but not all, methods
and systems that can be used to inoculaw., deploy, or otherwise associate
microbial life with a
treated or untreated biochar:
[0256] 1. Co-Oenloymeni
[0257] This method focuses on deploying the microbes at the same time as the
biochar. This
can be done either by deploying the biochar into the environment first,
followed by microbes or
by reversing the order, or even deploying the two components simultaneously.
An example of
this would be the deployment of a commercial brady rhizobium inoculant
simultaneously with
the introduction of a treated biochar into the soil media. The system here is
the combination use
of a biochar and microbes in the environment, and more preferably a char
treated to have suitable
properties for a target microbe or group of microbes which it is used with in
a targeted
application for a specified purpose, for example a symbiotic crop of said
microbe(s).
[0258] In one experiment, various biochar feedstocks with various post-
treatments were added
to a soilless mix containing soybean seeds that had been treated with a
commercial microbial
product containing bradyrhizobium japonicum. and compared to both a control
with microbe
inoculant and one without. Some of the treated biochars co-deployed with the
inoculant
increased seed germination rates, one by 29%. Others increased nodulation
measured at 10
weeks, one more than doubled the number of nodules. The use of the microbial
inoculant
increased shoot biomass in all treatments. Figure 28 is a chart comparing
shoot biomass when
the biochar added to a soilless mix containing soybean seeds is treated with
microbial product
containing bradyrhizobium japonicurn. and when it is untreated. As illustrated
in Figure 28,
shoot biomass increased with the biochar was treated.
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
[0259] Figure 29 shows the comparison of root biomass in a microbial
inoculated environment
versus one without inoculation. As illustrated in Figure 29, when inoculated,
root biomass
decreased with the inoculant alone yet increased with the use of all the
treated biochars with or
without inoculant.
[0260] In addition leaf tissue analysis was done which showed some of the
treated biochars co-
deployed with the rhizobial inoculant showed a significant increase in
nitrogen uptake. Figure
30 is a chart comparing the nitrogen levels when the biochar is inoculated
with the rhizobial
inoculant verses when it is not inoculated. Statistical significance in the
chart in Figure 30 is
marked with a star. In all cases, nitrogen levels increase with inoculation.
[0261] As outlined in these results, the addition of a treated biochar
suitable for co-
deployment with this particular microbe increased nodulation, increased
nitrogen
fixation/availability, and resulted in substantially increased root mass. It
should be noted that
to demonstrate the differing performance of varying formulations, two
formulations were tested,
each showing different interactions with the microbe in question, along with
significant
variations in performance. This is just one example to demonstrate the
invention of how the
specific combination of biochar feedstock, biochar treatment, co-deployed
microbe, and
application (this case plant species) can lead to improved microbial
effectiveness and thus
improved results (this case plant vigor), versus no treatment, applying the
microbe alone, or
applying the biochar alone. Another example of co-deployment benefit could be
using a biochar
that has strong absorption properties in combination with fertilizer (or
infused with fertilizer)
and microbes in an agricultural setting. The biochar properties that help
retain and then slowly
release nutrients and ions will also help the targeted microbe population to
establish and grow
without being impacted by the high levels of fertilizer salts or nutrients
which can often impede
and sometimes kill the microbes being deployed.
[0262] 2. Basic Inoculation
[0263] A more advanced method of inoculation centers on mixing the microbe or
microbes in
question with the treated or untreated biochar before deployment. In some
cases, the biochar in
question can be treated, produced, or controlled to assist with this
deployment, making this case
slightly different than merely inoculating a microbe on untreated biochar. In
one form, microbes
suspended in liquid (either water, growth media, or other liquids) are
deposited on the biochar
71
and mixed together until both materials are well integrated and then the
material is
deployed as a granular solid. It has been shown that materials that have been
treated to be
more hydrophilic typically accept this inoculation more readily than
hydrophobic
materials - demonstrating yet another way in which the treatment of biochar
can enhance
performance. In another form of basic inoculation, the biochar is delivered in
suspension
in the liquid also carrying the microbes. This biochar/liquid/microbe slurry
is then
deployed as a liquid. In this form, sizing the biochar particles in such a way
that their
sulface properties and porosity is maintained is a key element of
effectiveness.
Additionally, ensuring that the pores are treated to allow easy association of
both liquid
and microbes with the surfaces of the biochar is important. An example of a
basic
inoculation method of biochar for a bacteria in lab scale is as follows:
I) Isolate Pseudomonas protege= on a plate with 1.5% wiv Tryptic Soy Broth
solidified with 1.5% w/v agar and incubate at 30"C for 12h
2) Take an isolated colony of Pseudomonas protege= and grow up in a 1.5% w/v
TSB solution (90 ml) along with 10 g sterile biochar (sterilized at 110C in
small
hatches for 15-20 min) and combine both in a sterile 250 ml Erlenmeyer flask
3) Shake contents of flask at I 50 rpm at 30 C for 12h, or greater
4) Transfer contents of flask into a sterilized ultracentrifuge tube (250 ml)
and spin
at 10,000 x g for 10 min
5) Carefully remove supernatant liquid fraction by filtering throug,h a
WhannanTm
No 4 filter with a vacuum filtration system to separate out the bulk liquid
from
biochar.
After basic inoculation, the material and the microbes may be deployed
immediately,
stored for future use, or stabilized using technology such as lyophilization,
[02641 3. Assisted Inoculation
[0265j Another form of inoculation, which appears to have greater efficacy
with some
microbial systems, is assisted inoculation. Assisted inoculation involves
providing
mechanical, chemical, or biological assistance to move the targeted microbe
either into
the pore volume of the carrier or onto interior surfaces of the material that
normally may
not be accessible. Realizing that many microbes require liquid, and preferably
water, for
mobility, the most straightforward method of assisted inoculation requires
infiltrating the
pore volume of the material with water prior to contact with the targeted
microbes. This
water infusion can be done using the treatment
77
Date Regue/Date Received 2023-06-16
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
methods described previously in this disclosure. It has been shown that, with
certain microbes,
making this change alone will have a positive impact on the ability of
microbes to associate with
and infiltrate the material. In one experiment, it was shown that water
infusion improved release
rate on both a treated pine biochar with granular particles and with a coconut
biochar powder.
Figure 31 illustrates the three-day release rates of water infused biochar
compared to other types
of biochar. As illustrated, results vary depending upon the biomass.
[0266] Changes can also be made in the media to reduce surface tension and
increase fiowability
through the addition of a surfactant to the water, either into the liquid used
to carry the microbes,
or into the pores of the material itself, through simple contact, or
preferably forced infusion.
[0267] Additionally, the microbes themselves may be assisted into the pores
using the treatment
techniques previously outlined. Care needs to be taken to match the microbe to
the technique
used, but many microbes are capable of surviving vacuum infiltration if
performed at relatively
gentle, lower pressure differentials (+/- 10% of standard temperature and
pressure). Some
microbes, and many spores however are capable of surviving vacuum infiltration
even at
relatively large pressure differentials (+/- 50, 75, or even 90 or 95% or more
variation from
standard temperature and pressure). When this technique is used, a liquid
mixture is constructed
containing both liquid to be infused and the microbe or microbes in question.
The liquid is then
used as the "infiltrant" outlined in previous disclosure related to placing
liquid into the pore
volume of the material. The final material, infiltrated with microbes, may
then be heated to
incubate the microbes, cooled to slow development of the microbes or stabilize
the microbes, or
have other techniques applied such as lyophilization. The material may then be
delivered in
solid granular form, powdered, further sized downward by grinding or milling,
upward by
agglomerating, aggregating, or bonding, or suspended in a liquid carrier. A
clear advantage to
this assisted infusion approach is that the material can be processed or
handled after inoculation
with more microbial stability because the targeted microbes are inhabiting the
interior pore
volume of the material and are less prone to degradation due to contact with
exterior surfaces,
or other direct physical or environmental contact. This method may be applied
repeatedly, with
one or more microbes, and one to many moisture removal steps. It may also be
combined with
the other inoculation methods disclosed here either in whole or in part.
73
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
[0268] Figures 32a, 32b and 32c show scanning electron microscopy (SEM) images
of raw
biochar compared to ones that have been processed by being infused under
vacuum with bio-
extract containing different microbial species.
102691 Figure 32a is a SEM (10KV x 3.00K 10.0 m) of pore morphology of raw
biochar.
Figure 32b is a SEM (10KV x 3.00K 10.0um) of pore morphology of raw biochar of
Figure 32a
after it has been infused with microbial species. Figure 32c is a SEM (10KV x
3.00K 10.0 m)
of a pore morphology of another example of raw biochar of Figure 32a after it
has been infused
with microbial species. The images confirm the ability to incorporate
different microbes into
the pores of biochar by treatment. In turn, these beneficial microbes can
interact with and
enhance the performance of the environment they are deployed into, for example
the plants'
root systems when the inoculated biochar is mixed with the soil in the root
zone.
[0270] Compared to a biochar that has immersed in a compost tea, which may
have a
relatively short, e.g., a few days for the life of the microbes, the
impregnated populations of
examples of the present treated biochars, are stable over substantially longer
periods of time,
e.g., at least an 8 week period and in some cases 1 year or more as measured
by
PLFA(Phospholipid-derived fatty acids) analysis. PLFA analysis extracts the
fatty acid side
chains of phospholipid bilayers and measures the quantity of these biomarkers
using GC-
MS. An estimate of the microbial community population can thus be determined
through
PLFA analysis. The microbial activity may also be inferred through PLFA
analysis by
monitoring the transformation of specific fatty acids. Thus, the impregnation
of the biochar
with a microbial population provides for extended life of the microbes by at
least 5x, 10x,
or more over simple contact or immersion. In fact, some microbes may be better
suited to
surfactant infiltration versus vacuum infiltration and vice versa and this may
impact the shelf
life, penetration, viability, or other characteristics of the microbes.
[0271] As used herein, unless stated otherwise, the stable shelf life of an
example of a biochar
product having a microbial population is the period of time over which the
product can be
stored in a warehouse, e.g., dry environment, temperature between 40 F- 90 F,
with a less
than 50% decrease in microbial population.
10272] 4. Integrated Growth / Deployable Substrate
74
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
[0273] With many microbes, especially fungi, it can be helpful to develop or
"grow" the microbes
on the material itself. With porous materials, rather than mechanically or
chemically assisting
the infiltration of the microbes, it can be beneficial to allow the microbes
themselves to inhabit
the pore volume of the material prior to deployment. In fact, with materials
constructed to
effectively immobilize microbes, this can be the most efficient technique to
stabilize, store, and
ultimately deploy the microbes in question.
[0274]
An example of this method involves preparing the biochar material for the
microbes, sometimes
through thorough cleansing, other times through addition of either enzymes or
energy sources
needed by the microbe in question, preferably using the treatment techniques
described
previously in this disclosure. Once the material is prepared, the microbes are
placed onto the
material, or infused into the material and then incubated for a period of
time. In the case of
many microbial systems, the microbes themselves will inhabit the material and
form close
affiliations with available surfaces and pore volume. At this point, the
material can be deployed
with the microbes actively attached and affiliated. With many microbes,
especially fungi, this
is a preferred method of deployment and shows many advantages over co-
deployment, or basic
inoculation because of the tight integration of biological life with the
material itself.
10275] An example of an integrated growth inoculation method of biochar for a
fungus in lab
scale is as follows:
1) Make petri dishes containing corn meal agar (17 g/L), glucose (10 g,/L),
and yeast extract
(1 g/L)
2) Inoculate plates with Sordaria fimicola and incubate between 22-30C for at
least 1 day
to produce hyphae
3) Sterilize an inoculating loop and slice "plugs" of the hyphae and agar
generating cubes
that are agar and hyphal mass
4) Inoculate a sterile plate with a "plug" in the center of the plate, around
perimeter have
sterile biochar
5) Incubate plate for at least a day and remove biochar (that are now covered
with grown
over hyphae)]
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
[0276] It should be noted that because of this effect, biochars, and
specifically treated biochars
can also be extremely effective substrates for solid state fermentation ¨
particularly when growth
media or energy sources are added to the pore volume of the material. So, once
incubation is
ongoing, the material can either be removed, with the integrated microbes, and
deployed, or it can
be stabilized for long term storage, or it can be left in situ and used as a
fermentation or growth
substrate to develop or grow more microbes ¨ especially those that require a
solid to propagate
and develop.
[0277] 5. Media and/or Enzyme Infiltration
[0278] As mentioned previously, growth media, energy sources, enzymes, or
other beneficial /
necessary components for microbial growth may be infused into the pore volume
or coated onto
the surfaces of the material in question. This method can be combined with any
of the other
inoculation techniques disclosed here. It has been shown that with certain
microbes and certain
types of material, inoculation with growth media or enzymes can significant
impact the
effectiveness of the biochar material as a carrier.
10279] 6. Habitat Pre-Establishment (Enhanced Rhizosphere)
[0280] There are certain microbes which, because of symbiotic associations
with host organisms,
such as plants, prefer to develop in the vicinity of the organism, such as the
active root or other
plant tissue. An effective method for deploying these organisms can be to
develop and deploy
the plant/microbe/habitat (biochar) system together as a unit.
[0281] An example of this is germinating seed or transplanting a seedling or
developing juvenile
plant in the presence of treated or untreated biochar, and the targeted
microbes. Biochar that has
been treated to encourage hydrophilicity and neutral pH typically allows for
easier affiliation of
plant root tissue with the material. As this affiliation occurs, a habitat for
symbiotic organisms is
developed within the material itself due to the proximity of active plant
tissue to microbes reliant
on the tissue for energy. As this symbiosis continues, the number, activity,
and colony size of the
targeted microbes will continue to grow. At this point, the plant and biochar
can be deployed
together into the target environment, acting as a pre-established habitat and
carrying the microbes
along with them.
[0282] Another option is to develop and then remove the biochar from the
"incubation" system
either by stripping the biochar material from the symbiotic organism, such as
the root mass, or by
76
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
sieving or sifting the media used to grow the plant. At this point, the
microbes can either be
deployed directly or stabilized for storage.
[0283] Thus, through more controlled inoculation of the biochar particles, one
can achieve a
predetermined and controllable amount of a microbial community, e.g.,
population, into the
soil. This integration of a microbial community with a biochar particle, and
biochar batches
provides the ability to have controlled addition, use and release of the
microbes in the
community. In agricultural applications, this integration can further enhance,
promote and
facilitate the growth of roots, e.g., micro- roots, in the biochar pores,
e.g., pore morphology,
pore volume.
[0284] Other methods than those listed above exist for integrating a microbial
community with
an untreated or previously infused biochar particle. Different manners and
methods would be
preferred depending on needs to minimize contamination, encourage biochar pore
colonization/infiltration, minimize labor and cost and producing a uniform, or
mostly uniform,
product.
[0285] Other methods for integrating a microbial community with a biochar
particle may
include, but are not be limited to the following : while under vacuum, pulling
the microbial
solution through a treated biochar bed that is resting on a membrane filter;
spraying a microbial
solution on top of a treated biochar bed; lyophilizing a microbial solution
and then blending said
freeze dried solution with the treated biochar; again infusing, as defined
previously, the treated
biochar with a microbial solution; adding treated biochar to a growth medium,
inoculating with
the microbe, and incubating to allow the microbe to grow in said biochar
containing medium;
infusing, as defined previously, the biochar with a food source and then
introducing the substrate
infused biochar to a microbe and incubating to allow the microbes to grow;
blending
commercially available strains in dry form with treated biochar; adding the
treated biochar to a
microbial solution and then centrifuging at a high speed, potentially with a
density gradient in
order to promote the biochar to spin down with the microbes; densely packing a
column with
treated biochar and then gravity flowing a microbial solution through the
column and possibly
repeating this multiple times; or adding the microbe to a solution based
binder that is well known
to enter the treated biochar pores and then adding said solution to the
treated biochar. In order
to insure the proper microbial community the treated biochar may need to be
sterilized prior to
77
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
these methods for integrating a microbial community. All or parts of the above
manners and
methods may be combined to create greater efficacy. In addition, those skilled
in the art will
recognize that there may be additional manners or methods of infusing biochars
with
microbiaLs, including those created by the combination of one or more of the
manners and
methods listed above, without departing from the scope of the present
invention.
[0286] F. Using Microbial Inoculated Biochars
[0287] Thus, treated biochar can have a microbial community in its pores
(macro-, meso-, and
combinations and variations of these), on its pore surfaces, embedded in it,
located on its
surface, and combinations and variations of these. The microbial community can
have several
different types, e.g., species, of biologics, such as different types of
bacteria or fungi, or it
may have only a single type. For example, a preferred functional biochar, can
have a preferred
range for bacterial population of from about 50-5000000 micrograms/g biochar;
and for fungi,
from about 5 to 500000 micrograms/g biochar. A primary purpose in agricultural
settings,
among many purposes, in selecting the microbial population is looking toward a
population
that will initiate a healthy soil, e.g., one that is beneficial for, enhances
or otherwise advance
the desired growth of plants under particular environmental conditions. Two
types of
microbial infused biochars will be discussed further for agricultural
settings: bacteria and
fungi. However, the microbes may also be used in other applications, including
but not limited
to animal health, either directly or through interactions with other microbes
in the animals'
digestive tract and public health applications, such as microbial larvicides
(e.g. Bacillus
thuringiensis var. israeletzsis (Bti)) and Bacillus sphaericus used to fight
Malaria).
[0288] G. Bacteria Inoculated Biochars
[0289] PGPB include, for example, plant growth promoting rhizobacteria, free-
living and
nodule-forming nitrogen fixing bacteria, organic decomposers, nitrifying
bacteria, phosphate
solubilizing bacteria, biocontrol agents, bioremediation agents, archea,
actinomycetes,
thermophilic bacteria, purple sulfur bacteria, cyanobacteria, and combinations
and variations
of these.
[0290] PGPB may promote plant growth either by direct stimulation such as iron
chelation,
phosphate solulbilization, nitrogen fixation and phytohormone production or by
indirect
stimulation, such as suppression of plant pathogens and induction of
resistance in host plants
78
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
against pathogens. In addition, some beneficial bacteria produce enzymes
(including chitinases,
cellulases, -1,3 glucanases, proteases, and lipases) that can lyse a portion
of the cell walls of
many pathogenic fungi. PGPB that synthesize one or more of these enzymes have
been found
to have biocontrol activity against a range of pathogenic fungi including
Botrytis cinerea,
Sclerotium rolfsii, Fusarium axysporum, Phytophthora spp., Rhizoctonia solani,
Pythium
ultimum.
[0291] Currently the most economic conventional solid carrier used to deliver
microbes is peat.
A solid carrier allows for a relatively long shelf life and a more direct
application to a plant's
root system compared to a microbial liquid solution, which would be sprayed
directly.
[0292] Research has shown a substantial increase in PGPB growth and
distribution resulting
from being infused in biochar. For example, data resulting from research
conducted to compare
the effects upon CO2 production (an indicator of bacterial growth) using peat
and biochars show
the beneficial effects of using various biochars in promoting PGPB growth. As
illustrated in
the left-hand chart in Figure 33, peat results in CO2 production of between
approximately 10%
and 30% (depending upon the grown medium), whereas biochars result in CO2
production of
approximately 48% and 80%. Replicated experimental results using different
biochars confirm
CO2 production of approximately 30% to 70% (depending on the grown medium), as
compared
to approximately 10% to 20% for the peat control.
[0293] The method developed for determining this CO2 production as an
indicator of bacterial
growth consists of the following. The substrate, here biochar or peat, is
sterilized by heating at
110C for 15 hours. A bacterial stock solution is then created, here Tryptic
Soy Broth was
solidified with agar at 1.5% w/v in petri plates to isolate the gram negative
non-pathogenic
organism Escherichia coli ATCC 51813 (15h growth at 37 C). Then an isolated
colony is
captured with an inoculating loop and suspend in 10 ml sterile buffer
(phosphate buffer saline
or equivalent) to create the bacterial stock solution. Lactose containing
assays are then used,
hem, test tubes that contain 13 ml of either Lauryl Tryptose Broth (Lill) or
Brilliant Green
Broth (BGB) that also contain a Durham tube. A negative control is generated
by adding 10 'IL
of sterile buffer to triplicate sets of LTB and BOB tubes. A positive control
is generated by
adding 10 L of bacterial stock solution to triplicate sets of LTB and BOB
tubes. A negative
substrate is generated by adding 1.25 ml (-1% v/v) of sterile substrate to
triplicate sets of LTB
79
CA 03009977 2010-06-27
WO 2017/117302 PCT/US2016/069037
and BGB tubes. A positive substrate is generated by adding 1.25 ml (-1% v/v)
of sterile
substrate and 10 L of bacterial stock solution to triplicate sets of LTB and
BGB tubes. The
tubes of the four treatments are then incubated statically in a test tube rack
at 37 C for at least
15h. The tubes are then carefully observed and any gas bubbles captured by the
Durham tube
within respective LTB or BGB tubes are closely measured with a ruler. Small
bubbles <0.2 mm
should not be considered. A continuous bubble as shown in individual tubes in
Figure 34 are
what are observed and quantified. Figure 34 is an example of carbon dioxide
production
captured as a continuous gas bubble in BGB (left two tubes) and LTB (right two
tubes) growth
medium. The percent carbon dioxide production is then calculated by dividing
the recorded
bubble length by the total Durham tube length and multiplying by 100.
[0294] Further tests were conducted using the Streptomyces lidicus WYEC 108
bacterium found
in one of the commercially available products sold under the Actinovate brand.
Actinovate
products are biofungicides that protect against many common foliar and soil-
borne diseases
found in outdoor crops, greenhouses and nurseries. The formulations are water-
soluble.
[0295] Figure 35 illustrates the effects upon the growth of Streptomyces
lidicus using
conventional peat versus biochars. In the test illustrated by the photograph
on the left of Figure
35, an Actinovate powder was blended with peat, placed in an inoculated media
and incubated
at 25 C. The photograph shows the distribution and density of white colonies
after 3 days. In
the test illustrated by the photograph on the right of Figure 35, an
Actinovate powder was
blended with the treated biochar, placed in an inoculated media and incubated
at 25 C. The
photograph also shows the distribution and density of white colonies after 3
days, the
distribution and density of which are significantly greater than those
achieved with peat.
[0296] Figure 36 further illustrates the improved growth of the Actinovate
bacterium using
biochar versus peat. The left photograph shows only limited and restricted
growth away from
the peat carrier. The right photograph shows abundant growth of the bacterium
spread much
farther out from the biochar carrier.
[0297] Another application of using biochar inoculated with bacteria would be
in the biofuel
industry, where methanotroph inoculated biochar could be used to create
methanol.
Methanotrophic bacteria are proteobacteria with diverse respiration
capabilities, enzyme types,
and carbon assimilation pathways. However, Methylosinus trichosporium OB3b is
one of the
few methanotrophs that can be manipulated by environmental conditioning to
exclusively
produce methanol from methane. M. trichosporium OB3b is one of the most well
studied
aerobic Ci degraders and can be grown in either batch or continuous systems.
As
mentioned earlier, the large pore volume and surface area of biochar is
suitable for
bacterial colonization and should subsequently increase substrate access to
enzyme
activation sites. To improve the conversion rate, copper, nitrate, and
phosphate should be
included in the system. The use of biochar as a support material for the
aerobic
bioconversion of methane to methanol provides a pore distribution suitable for
both
adsorptions of methane and impregnation with bacteria. By providing biological
and
adsorptive functionality the biochar can intensify the bacteria in the biochar
and increases
the conversion rate.
[02981 In general, bacteria communicate via the distribution of signaling
molecules which
trigger a variety of behaviors like swarming (rapid surface colonization),
nodulation
(nitrogen fixation), and virulence. Biochars can bind signaling molecules and
in particular
it is believed can bind a major signaling molecule to their surface. This
binding ability
can be dependent upon many factors including on the pyrolysis temperature.
This
dependency on pyrolysis temperature and other factors can be overcome,
mitigated, by
the use of examples of the present vacuum infiltration techniques. For
example, a
signaling molecule that is involved in quorum sensing- multicellular-like
cross-talk found
in prokaryotes can be bound to the surface of bioc bars. Concentration of
biochars required
to bind the signaling molecule decreased as the surface area of biochars
increased. These
signaling molecules may be added to the surface of a biochar and may be used
to
manipulate the behavior of the bacteria. An example of such a use would be to
bind the
molecules which inhibit cell-to-cell communication and could be useful in
hindering plant
pathogens; using techniques in the present invention signaling molecules may
be added
to the surface of a biochar to engineer specific responses from various
naturally occurring
bacteria.
[02991 H. Fungi Inoculated Biochars
[03001 Beneficial fungi include, for example, saprotrophic fungi, biocontml
fungi,
ectomycorrhizae, endomycorrhi zae, ericoid mycon-hr zae, and combinations and
variations of
Date Regue/Date Received 2023-06-16
these, It is further theorized that, in general, biochars with greater .fungal
development
may be better suited for perennial crops such as gapes, almonds, blueberries,
and
strawberries in which symbiotic relationships with arbuscular mycorrhizal
fungi (AMF)
arc favored over PGPBs. The presence of high concentrations of AMF spores in
biochars
can therefore rapidly promote fungal colonization of plant root hairs leading
to extensive
mycelia] development. Increased plant root associations with mycelial
filaments would
consequently increase nutrient and water uptake.
[03011 Mycorrhizal fungi, including but not limited to Endomycorrhizae and
Ectornycorrhizae, are known to be an important component of soil life. The
mutualistic
association between the fungi and the plant can be particularly helpful in
improving plant
survivability in nutrient-poor soils, plant resistance to diseases, e.g.
microbial soil-borne
pathogens, and plant resistance to contaminated soils, e.g. soils with high
metal
concentrations. Since mycorrhizal root systems significantly increase the
absorbing area
of plant roots, introducing mycorrhizal fungi may also reduce water and
fertilizer
requirements for plants.
[03021 Typically mycorrhizae are introduced into soil as a liquid formulation
or as a solid
in powder or granular form and contain dormant mycorrh iz al spores and/or
colonized root
fragments. Often the most economic and efficient method is to treat the seeds
themselves,
but dealing with traditional liquid and powder inoculums to coat the seed can
be difficult.
In accordance with the present invention, inoculated biochar may be used to
coat the seeds
by, for example, using a starch binder on the seeds and then subjecting the
seeds to
inoculated biochar fines or powder. Another method is by placing the
mycorrhizae
inoculum in the soil near the seeding or established plant but is more costly
and has
delayed response as the plants initial roots form without a mycorrhizal
system. This is
because the dormant mycorrhizae are only activated when they come close enough
to
living roots which exude a signaling chemical. In addition if the phosphorus
levels arc
high in the soil, e.g. greater than 70 ppm, the dormant myrorrhizae will not
be activated
until the phosphorus levels are reduced. Thus applying inoeulant with or near
fertilizers
with readily available phosphorus levels can impede the desired mycorrhizal
fungi
growth. A third option is to dip plant roots into an inoculant solution prior
to replanting,
but this is costly as it is both labor and time intensive and only applicable
to transplanting.
82.
Date Regue/Date Received 2023-06-16
[03031 If the colonization of mycorrhizae can be quickened and the density of
the
mycorrhizae' s hyphal network can be increased then the beneficial results of
mycorrhizal
root systems, e.g. increased growth, increased survivability, reduced water,
and reduced
fertilizer needs, can bc realized sooner. Prior art shows that compost,
compost teas,
humates, and fish fertilizers can improve microbial activities and in more
recent studies
have shown physically combining arbuscular mycorrhizal fungi (ANIF) inoculant
with
raw biochar has resulted in additional plant yield compared to each alone. See
Hammer,
et. al. Biochar Increases Arbuscular Mycon-hizal Plant Growth Enhancement and
Ameliorates Salinity Stress, Applied Soil Ecology Vol 96, Nov 2015 (pg. 114-
121).
[03041 An ideal carrier for the mycorrhizae would have moisture, air, a
neutral pH, a
surface for fungi to attach, and a space for the roots and fungi to meet. Thus
a previously
infused biochar created by the method disclosed above would be a better
carrier than raw
biochar alone. The infused biochar could be physically mixed with a solid
mycorrhizal
fungi inoculant or sprayed with a liquid mycorrhizal inoculant prior to or
during
application at seeding or to established plants. Additionally, the infused
biochar and
mycorrhizal fungi inoculant could be combined to form starter cubes, similar
to Organo-
Cubes, rockwool, oasis cubes, and peat pots.
[03051 The infused biochar could be further improved upon by initially
infusing or further
infusing the biochar with mieronutricnts for mycorrhizal fungi, for example
but not
limited to 'Runic acid, molasses, or sugar. The growth nutrient infused
biochar would
further expedite the colonization of the mycorrhizal fungi when physically
combined with
the inoculant and applied to seeds or plants.
[03061 Another improvement to the infused biochar would be to initially infuse
or further
infuse the biochar with the signaling molecules of mycorrhizal fungi. The
signaling
molecule infused biochar would further expedite the colonization of the
mycorrhizal
fungi when physically combined with the inoculant and applied to seeds or
plants, as it
would bring the unycorrhizac out of dormancy quicker and thus establish the
mycorrhizal
root system quicker.
[03071 Another method for establishing and improving mycorrhizal fungi
colonies would
be by growing mycorrhizae into the infused biochar and then applying the
mycorrhizal
fungi inoculated biochar to seeds or plants. Similar to a sand culture (Ojala
and Jarrell
1980; Donald Lester, 2009, Buying and Applying Mycorrhizat Fungi, Maximum
Yield
USA)
83
Date Regue/Date Received 2023-06-16
a bed of infused biochar is treated with a recycled inoculated nutrient
solution by passing
it through the bed multiple times.
[0308] 1. Batch Treatment/Bulk Production
[0309] As demonstrated above, thc treatment processes described above arc
particularly
well suited For large scale production of biochar. The processes and bioehars
of the
present invention provides a unique capability to select starting materials
and pyrolysis
techniques solely on the basis of obtaining a particular structure, e.g. pore
size, density,
pore volume, amount of open pores, interconnectivity, tortuosity, etc. Thus,
these starting
materials and processes can be selected without regard to adverse, harmful,
phytotoxic
side effects that may come from the materials and processes. This is possible,
because the
infiltration steps have the capability of mitigating, removing or otherwise
address those
adverse side effects. In this manner, a truly custom biochar can be made, with
any adverse
side effects of the material selection and pyrolysis process being mitigated
in later
processing steps.
[0310] Further, the processes are capable of treating a large, potentially
variable, batch of
biochar to provide the same, generally uniform, predetermined customized
characteristics
for which treatment was designed to achieve, e.g., pH adjustment. Treatment
can result
in treated biochar batches in which 50% to 70 % to 80% to 99% of the biochar
particles
in the batch have same modified or customized characteristic, e.g.,
deleterious pore
surface materials mitigated, pure surface modified w provide beneficial
surface, pure
volume containing beneficial additives.
[03111 Accordingly, the ability to produce large quantities of biochar having
a high level
of consistency, predictability and uniformity, provides numerous advantages in
both large
and small agricultural applications, among other things. For example, the
ability to
provide large quantities of biochar having predetermined and generally uniform
properties will find applications in large scale agriculture applications.
Thus, treated
biochar batches from about 100Ibs up to 50,000+lbs and between may have
treated biochar
particles with predetermined, uniform properties.
[0312] As the treated biochar batches are made up of individual biochar
particles, when
referring to uniformity of such batches it is understood that these batches
are made up of
tens and hundreds of thousands of particles. Uniformity is thus based upon a
sampling
and testing
84
Date Regue/Date Received 2023-06-16
method that statistically establishes a level of certainty that the particles
in the batch have
the desired uniformity.
[0313] Thus, when referring to a treated batch of biochar as being "completely
uniform"
or having "complete uniformity" it means that at least about 99% of all
particles in the
batch have at least one or more property or feature that is the same. Same
being within
appropriately set tolerances for said property. When a treated batch of
biochar is referred
to as "substantially uniform" or having "substantial uniformity" it means that
at least
about 95% of all particles in the batch have at least one or more property or
feature that
is the same. When a treated batch of biochar is referred to as "essentially
uniform" or
having "essential uniformity" it means that at least about 80% of all
particles in the batch
have at least one or more property or feature that is the same. The batches
can have less
than 25%, 20% to 80%, and 80% or more particles in the batch that have at
least one or
more property or feature that is the same. Further, the batches can have less
than 25%,
20% to 80%, and 80% or more particles in the batch that have at one, two,
three, four, or
all properties or features that are the same.
[0314] J. Applications
[0315] Generally, treated biochar of the present inventions can be used
throughout the
world, in numerous soil types, agricultural applications, horticultural, large
and small
scale farming, organic farming, and in a variety of soil manaizement
applications and
systems, and combinations and variations of these. ln fact, this particular
solution
provides the capability to custom-manufacture biochar for a particular
climate,
environment, geographical area, soil type, plant type, microbe type, or
application by
more precisely controlling key characteristics.
[0316] Examples of these applications include for example, use in acidic and
highly
weathered tropical field soils, use in temperate soils of higher fertility,
use in large
commercial applications, use for the production of large scale crops such as,
soybean,
corn, sugarcane and rice, in forestry applications, for golf courses (e.g. ,
greens, fairways),
for general purpose turf grasses, wine grapes, table grapes, raisin grapes,
fruit and nut
trees, ground fruits (e.g. , strawberries, blueberries, blackberries), row
crops (e.g. ,
tomatoes, celery, lettuce, leafy greens), root crops (e.g. , tubers, potatoes,
beets, carrots),
mushrooms, and combinations and variations of these.
Date Regue/Date Received 2023-06-16
[0317] Treated biochars and agriculture practices and methods, provide for
improved soil
structure, increased water retention capability, increased water holding
ability of the soil
over time, reduced runoff or leaching, increased holding ability for
nutrients, increase
holding of nutrients over time, and combinations and variations of these, and
other
features that relate to the increased holding and retention features and soil
aggregation of
the present biochars and processes. It further being understood that in
addition to
nutrients, other material additives, (e.g., herbicide, pesticide), can be
utilized and benefit
from the increased holding and retention capacities of the present biochars,
systems, and
methods.
[0318] Treated biochar may also be used in other applications, for example,
such mixing
with manure in holding ponds to potentially reduce gaseous nitrogen losses,
soil remedial
(for example absorption and capture of pesticide, contaminates, heavy metals,
or other
undesirable, disadvantageous soil components), ground water remediation, other
bioremediations, storm water runoff remediation, mine remediation, mercury
remediation
and as a cattle or poultry feed additive.
[0319] Further, the present invention could be used to clean and/or infiltrate
the pores of
biochar with a variety of substances, for a number of purposes, including but
not limited
to, infiltrating the pores of biochar with nutrients, vitamins, microbes,
drugs and/or other
supplements, or a combination of any of the foregoing, for consumption by
either humans
and/or animals. The treated biochar may also be applied to animal pens,
bedding, and/or
other areas where animal waste is present to reduce odor and emission of
unpleasant or
undesirable vapors. Furthermore it may be applied to compost piles to reduce
odor,
emissions, and temperature to enable the use of the food waste and animal feed
in
composting. Biochar can also be applied to areas where fertilizer or pesticide
runoff is
occurring to slow or inhibit leaching and runoff. Additionally, it can be used
in the public
health sector to help control disease, for example carrying or deploying
larvicides to
reduce numbers of certain disease carrying or transmitting insects. The
biochar may also
be treated with additives which make it easier to dispense or apply, such as
non-toxic oils,
anti-clumping/binding additives, surface drying agents, or other materials.
[0320] In general, in the agricultural application of biochar to soil, the
biochar should be
located near the soil's surface in the root zone, or in or adjacent to the
rhizosphere, where
86
Date Regue/Date Received 2023-06-16
the bulk of nutrient cycling and uptake by plants takes place. Although
benefits may be
obtained from the application of biochar in layers above, below, in and
combinations and
variation of these, the root zone, for example during landscaping for carbon
sequestration,
or if using biochar for moisture management. Layering of biochar at various
depths above,
below, in and combinations and variation of these, the root zone, the surface,
and
combinations and variations of these, may also be employed. The biochar layers
may have
different predetermined properties for each layer, based upon, for example,
the depth of
the layer, soil type, geography, crop. climate and other factors.
[0321] The above are only a few examples of how additive infused or microbial
carrying
biochar may be produced for different uses. Those skilled in the art will
recognize that
there may be other mechanisms for infusing additives into the pores of the
biochar without
departing from the scope of the invention. Those skilled in the art will
further recognize
that the present invention can be used on any type of soil application,
including, but not.
limited to, the following: crops, turf grasses, potted plants, flowering
plants, annuals,
perennials, evergeens and seedlings. By way of example, treated biochar may be
incorporated into or around the root zone of a plant_ As most trees, rows, and
specialty
crops extract large percentage of their water from the first twenty-four
inches below the
soil surface, the above applications will generally be effective incorporating
the biochar
around the root zone from the top surface of the soil and up to a depth of 24"
below the
top surface of the soil, depending on the plant type and species, or
alternatively, within a
24" radius surrounding the roots regardless of root depth or proximity from
the top surface
of the soil. When the plant roots are closer to the surface, the incorporation
of the biochar
within the top 2-6" inches of the soil surface may also be effective. Greater
depths are
more beneficial for plants having larger root zones, such as trees.
[0322] In certain examples of biochar applications, the treated biochar can be
applied in
amounts (e.g., rates of addition as measured by weight of treated biochar per
area of field)
of from about 0.001 ton of treated biochar per acre to about 150 tons of
treated bitx:har
per acre, from about 2.5 tons of treated biochar per acre to about 100 tons of
treated
biochar per acre, and from about 5 tons of treated biochar per acre to about
70 tons of
treated biochar per acre, although larger and smaller amounts inay be used.
Additional
rates of from about 1/2 tons of treated biochar to about 10 tons of treated
biochar may be
used. For example,
87
Date Regue/Date Received 2023-06-16
application rates of 1 ton of treated biochar was added per acre to a soil for
a lettuce crop
where the soil had a pH of about 7. In another example, about 3 tons per acre
of treated
biochar was added to soil for a strawberry crop. In these examples, the plants
showed
enhanced growth rates and yields.
[0323] Generally, for conventional field cropping systems, biochar can be
preferably
added using existing farm equipment and incorporated into existing farming
operations.
For example, treated biochar can be applied and incorporated together with
lime, since
lime is often applied as a fine solid, which must be well incorporated into
soil. However,
it is also contemplated that the examples of the present inventions may give
rise to new
equipment and utilizations based upon the features, performance and
capabilities of the
present inventions. Generally, treated biochar may be applied to fields, by
way of example
through the use of manure or compost spreaders, lime spreaders, plowing method
(e.g.,
from hand hoes, animal draft plows, disc harrows, chisels, rotary hoes, etc.),
large scale
tillage equipment, including rotary tillers, mulch finishers, draw offset
discs, and disc
harrows (such as for example JOHN DEERE Thl DH5 I, DH52F, PC 10, RT22, and
RC22).
Treated hiochar may also he applied by modified large scale nutrient
applicators (such as,
for example, JOHN DEERErm 24 10C, 2510H, and 2510S Strip-Till Medium Residue
Applicator), large scale draw dry spreaders (such as JOHN DEEREIm DN345),
large
scale no-till planters, large scale dry fertilizer sub- surface applicators,
and liquid slurry
surface or subsurface applicators. Similar, and various other types of large
farming, and
earth moving and manipulation equipment may be used to apply the treated
biochar to the
field, such as for example, drop spreaders or drills.
[0324] For example, treated biochar may he applied using banding techniques,
which is
an operation involving applying the biochar in a narrow band, using equipment
that cuts
the soil open, without disturbing the entire soil surface. Using this
technique the biochar
can be placed inside the soil while minimizing soil disturbance, making it
possible to
apply biochar after crop establishment, among other applications.
103251 In other examples, treated biochar may be mixed with other soil
amendments, or
other materials, such as for example manure, sand, topsoil, compost, turf
grass substrate,
peat, peat moss, or lime before soil application, which are already scheduled
or part of
the
88
Date Regue/Date Received 2023-06-16
existing operations, and in this manner by combining these steps (e.gõ biochar
application
with existing application step) can improve efficiency by reducing the number
of field
operations required. In other examples, treated biochar can also be mixed with
liquid,
(e.g., liquid manures) and applied as a slurry. Finer biochar particles may be
preferably
used with this type of slurry application using existing application
equipment, and dust
problems associated with these finer particles may be mitigated, managed or
eliminated.
[0326] In further examples, treated biochar can be top dressed on perennial
pastures or
other perennial vegetation, such as spaces between fruit trees in orchards.
Treated biochar
may also be applied with individual plants while transplanting or mixed with
topsoil and
other amendments while preparing raised beds. In forestry or similar
operations where
replanting of seedlings takes place, treated biochar can be applied by
broadcasting (e.g.,
surface application) or incorporation over the entire planting area, it can be
added in the
plantino, holes, and combinations and variations of these. Before or after
tree
establishment, biochar could also be applied by traditional and subsurface
banding or top-
dressed over perennial vegetation in orchards, but care should be taken to
minimize root
damage and soil compaction.
[0327] In other examples of applications, treated biochar can be applied in
trenches
radiating out from the base of established trees ("radial trenching") or in
holes dug at
some distance from the base of the tree ("vertical mulching"); biochar could
also
potentially be applied to soil using "air excavation tools". These tools use
pressurized air
to deliver material, e.g., compost, under the soil surface and reduce
compaction.
Alternatively, the soil around tree roots can be excavated and treated biochar
applied
before covering with soil.
[0328] While, in some examples, particle size distribution of treated biochar
materials
may vary widely depending on the feedstock and the pyrolysis technique used to
produce
the biochar, uniformity if required or preferred, can be achieved by various
milling and
grinding techniques that may be employed during processing or during the
distribution
and application to soil. When smaller particles are utilized, and in
particular for surface
applications, care should be taken to apply the treated biochar in ways that
minimize loss
due to wind or water erosion.
89
Date Regue/Date Received 2023-06-16
l032911 As set forth above, the treated biochar of the present invention may
be used in
various agriculture activities, and the. fields of edaphology and pedology, as
well as other
activities and in other fields. Additionally, the treated biochar may be used,
for example,
with: farming systems and technologies, operations or activities that may be
developed
in the future; and with such existing systems, operations or activities which
may be
modified, in-part, based on the teachings of this specification. Further, the
various treated
biochar and treatment processes set forth in this specification may he used
with each other
in different and various combinations. Thus, for example, the processes and
resulting
biochar compositions provided in the various examples provided in this
specification may
be used with each other; and the scope of protection afforded the present
inventions
should not be limited to any particular example, process, configuration,
application or
arrangement that is set forth in a particular example or figure.
[0330] Although this specification focuses on agriculture, soil modification
and plant.
growth, it should be understood that the materials, compositions, structures,
apparatus,
methods, and systems, taught and disclosed herein, may have applications and
uses for
many other activities in addition to agriculture for example, as filters,
additives, and in
remed iation activities, among other things.
[0331] It being understood that one or more of these may be preferred for one
application,
and another of these may be preferred for a different application. Thus, these
are only a
general list of preferred features and are not required, necessary and may not
be preferred
in all applications and uses.
[0332] It is noted that there is no requirement to provide or address the
theory underlying
the novel and groundbreaking functionality, performance or other beneficial
features and
properties that are the subject of, or associated with, embodiments of the
present
inventions. Nevertheless, to the extent that various theories are provided in
this
specification to further advance the art in this important area. These
theories put forth in
this specification, and unless expressly stated otherwise, in no way limit,
restrict or
narrow the scope of protection to be afforded the claimed inventions. These
theories many
not be required or practiced to utilize the present inventions. It is further
understood that
the present inventions may lead to new, and heretofore unknown theories to
explain the
functionality, performance or other
Date Regue/Date Received 2023-06-16
beneficial features and properties that are the subject of, or associated
with, embodiments
of the methods, articles, materials, and devices of the present inventions;
and such later
developed theories shall not limit the scope of protection afforded the
present inventions.
[0333] Those skilled in the art will recognize that there arc other methods
that may be
used to treat bioehar in a manner that forces the infusion of liquids into the
pores of the
biochar without departing from the scope of the invention. The foregoing
description of
implementations has been presented for purposes of illustration and
description. It is not
exhaustive and does not limit the claimed inventions to the precise form
disclosed.
Modifications and variations are possible in light of the above description or
may be
acquired from practicing the invention. The claims and their equivalents
define the scope
of the invention.
91
Date Regue/Date Received 2023-06-16