Note: Descriptions are shown in the official language in which they were submitted.
MOTORCYCLE LUBRICANT
FIELD OF THE INVENTION
The present invention relates to a motorcycle having a four cycle engine and a
transmission including a clutch assembly, the engine crankcase and the clutch
assembly being
lubricating by a lubricating oil provided from a common sump, and lubricating
oil
compositions suitable for lubricating the engine crankcase and clutch assembly
of such
motorcycles.
BACKGROUND OF THE INVENTION
In a typical motorcycle, a common sump lubricates the engine, transmission and
wet
clutch. Such universal lubricating fluids as used in motorcycles, therefore,
must have a
balance of both desirable friction properties and lubricity properties. In
particular, whilst it
is desirable to reduce friction in the engine crankcase to improve fuel
economy it is important
to maintain sufficient friction in the clutch assembly to allow it to function
efficiently.
It is well known to use molybdenum-containing additives as friction modifiers
in
crankcase lubricants for passenger car engine oils. However, use of molybdenum-
containing
additives in motorcycle lubricants is problematic due to the need to maintain
sufficient
friction in the clutch assembly.
This problem was considered in International patent application number
WO 2015/195614, which discloses a method of operating a 4-stroke motorcycle
engine
comprising supplying to the engine and clutch a lubricant comprising (a) an
antimony
dialkyldithiocarbamate compound and (b) an ash-free friction modifier
comprising at least
one of long chain fatty acid derivatives of amines, long chain fatty esters,
derivatives of long
chain fatty epoxides, fatty imidazolines; amine salts of alkylphosphoric acids
or fatty esters
amides or imides of hydroxyl-carboxylic acids, wherein the lubricating
composition comprise
less than 50 weight percent of a synthetic ester having a kinematic viscosity
of 5.5 to 25
mm2/s when measured at 100 C. The lubricant of WO 2015/195614 may also
comprise a
N-containing molybdenum additive other than a molybdenum dithiocarbamate,
which latter
may result in undesirable friction properties.
1
CA 3012205 2018-07-24
However, it has been discovered that a lubricant comprising molybdenum
additives,
including, but not limited to molybdenum dithiocarbamate complexes, can be
successfully
used to lubricate the engine crankcase and the clutch assembly from a common
sump of a
motorcycle having a four cycle engine by including an ash-free friction
modifier in the
lubricant.
SUMMARY OF THE INVENTION
According to a first aspect the present invention provides a motorcycle having
a four
cycle engine and a transmission including a clutch assembly, the engine
crankcase and the
clutch assembly being lubricated by a lubricating oil composition provided
from a common
sump, wherein said lubricating oil composition comprises a major amount of oil
of
lubricating viscosity and minor amounts of (A) an oil soluble molybdenum
compound and
(B) an ashless organic friction modifier.
According to a second aspect the present invention further provides a method
of
operating a motorcycle having a four cycle engine and a transmission including
a clutch
assembly, the engine crankcase and the clutch assembly being lubricating by a
lubricating oil
composition provided from a common sump, said method comprising supplying to
the engine
crankcase and clutch assembly a lubricating oil composition comprising a major
amount of
oil of lubricating viscosity and minor amounts of (A) an oil soluble
molybdenum compound
and (B) an ashless organic friction modifier.
According to a third aspect the present invention also provides a lubricating
oil
composition comprising a major amount of oil of lubricating viscosity and
minor amounts of
(A) an oil soluble molybdenum compound and (B) an ashless organic friction
modifier, which
lubricating oil composition exhibits a JASO clutch friction of at least MA1
when measured
according to the JASO T 903:2016 clutch friction test. In a preferred
embodiment the
lubricating oil composition of the third aspect of the invention exhibits a
JASO clutch friction
of at MA2 when measured according to the JASO T 903:2016 clutch friction test.
According to a fourth aspect the present invention provides the use of a
combination
of (A) an oil soluble molybdenum compound and (B) an ashless organic friction
modifier in
2
CA 3012205 2018-07-24
a lubricating oil composition to achieve at least an MA1 in the JASO T
903:2016 clutch
friction test.
In this specification, the following words and expressions, if and when used,
have the
meanings given below:
"active ingredients" or "(a.i.)" refers to additive material that is not
diluent or solvent;
"comprising" or any cognate word specifies the presence of stated features,
steps, or
integers or components, but does not preclude the presence or addition of one
or more
other features, steps, integers, components or groups thereof The expressions
"consists of', or "consists essentially of' or cognates may be embraced within
"comprises" or cognates, wherein "consists essentially of' permits inclusion
of
substances not materially affecting the characteristics of the composition to
which it
applies;
"hydrocarbyl" means a chemical group of a compound that contains hydrogen and
carbon atoms and that is bonded to the remainder of the compound directly via
a
carbon atom. The group may contain one or more atoms other than carbon and
hydrogen provided they do not affect the essentially hydrocarbyl nature of the
group.
Those skilled in the art will be aware of suitable groups (e.g., halo,
especially chloro
and fluor , amino, alkoxyl, mercapto, alkylmercapto, nitro, nitroso, sulfoxy,
etc.).
Preferably, the group consists essentially of hydrogen and carbon atoms,
unless
specified otherwise. Preferably, the hydrocarbyl group comprises an aliphatic
hydrocarbyl group. The term "hydrocarbyl" includes "alkyl", "alkenyl", "ally1"
and
"aryl" as defined herein;
"alkyl" means a C1 to C30 alkyl group which is bonded to the remainder of the
compound directly via a single carbon atom. Unless otherwise specified, alkyl
groups
may, when there are a sufficient number of carbon atoms, be linear (i.e.
unbranched)
or branched, be cyclic, acyclic or part cyclic/acyclic. Preferably, the alkyl
group
comprises a linear or branched acyclic alkyl group. Representative examples of
alkyl
groups include, but are not limited to, methyl, ethyl, n-propyl, iso-propyl, n-
butyl,
sec-butyl, iso-butyl, tert-butyl, n-pentyl, iso-pentyl, neo-pentyl, hexyl,
heptyl, octyl,
3
CA 3012205 2018-07-24
dimethyl hexyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetradecyl,
pentadecyl,
hexadecyl, heptadecyl, octadecyl, nonadecyl, icosyl and triacontyl;
"aryl" means a C6 to C18, preferably C6 to CI 0, aromatic group, optionally
substituted
by one or more alkyl groups, halo, hydroxyl, alkoxy and amino groups, which is
bonded to the remainder of the compound directly via a single carbon atom.
Preferred
aryl groups include phenyl and naphthyl groups and substituted derivatives
thereof,
especially phenyl and alkyl substituted derivatives thereof;
"alkenyl" means a C2 to C30, preferably a C2 to C12, group which includes at
least one
carbon to carbon double bond and is bonded to the remainder of the compound
directly via a single carbon atom, and is otherwise defined as "alkyl";
"alkylene" means a C2 to C20, preferably a C2 to Cio, more preferably a C2 to
C6
bivalent saturated acyclic aliphatic radical which may be linear or branched.
Representative examples of alkylene include ethylene, propylene, butylene,
isobutylene, pentylene, hexylene, heptylene, octylene, nonylene, decylene, 1-
methyl
ethylene, 1-ethyl ethylene, 1-ethy1-2-methyl ethylene, 1,1-dimethyl ethylene
and
1-ethyl propylene;
"polyol" means an alcohol which includes two or more hydroxyl functional
groups
(i.e. a polyhydric alcohol) but excludes a "polyalkylene glycol" (component
B(ii))
which is used to form the oil-soluble or oil-dispersible polymeric friction
modifier.
More specifically, the term "polyol" embraces a diol, triol, tetrol, and/or
related
dimers or chain extended polymers of such compounds. Even more specifically,
the
term "polyol" embraces glycerol, neopentyl glycol, trimethylolethane,
trimethylolpropane, trimethylolbutane, pentaerythritol,
dipentaerythritol,
tripentaerythritol and sorbitol;
"polycarboxylic acid" means an organic acid, preferably a hydrocarbyl acid,
which
includes 2 or more carboxylic acid functional groups. The term "polycarboxylic
acid"
embraces di-, tri- and tetra- carboxylic acids;
"halo" or "halogen" includes fluoro, chloro, bromo and iodo;
"oil-soluble" or "oil-dispersible", or cognate terms, used herein do not
necessarily
indicate that the compounds or additives are soluble, dissolvable, miscible,
or are
4
CA 3012205 2018-07-24
capable of being suspended in the oil in all proportions. These do mean,
however,
that they are, for example, soluble or stably dispersible in oil to an extent
sufficient to
exert their intended effect in the environment in which the oil is employed.
Moreover,
the additional incorporation of other additives may also permit incorporation
of higher
levels of a particular additive, if desired;
"ashless" in relation to an additive means the additive does not include a
metal;
"ash-containing" in relation to an additive means the additive includes a
metal;
"major amount" means in excess of 50 mass % of a composition expressed in
respect
of the stated component and in respect of the total mass of the composition,
reckoned
as active ingredient of the component;
"minor amount" means less than 50 mass % of a composition, expressed in
respect of
the stated additive and in respect of the total mass of the composition,
reckoned as
active ingredient of the additive;
"effective minor amount" in respect of an additive means an amount of such an
additive in a lubricating oil composition so that the additive provides the
desired
technical effect;
"ppm" means parts per million by mass, based on the total mass of the
lubricating oil
composition;
"metal content" of the lubricating oil composition or of an additive
component, for
example detergent metal, molybdenum or boron content or total metal content of
the
lubricating oil composition (i.e. the sum of all individual metal contents),
is measured
by ASTM D5185;
"TBN" in relation to an additive component or of a lubricating oil composition
of the
present invention, means total base number (mg KOH/g) as measured by ASTM
D2896;
"KV 1 00" means kinematic viscosity at 100 C as measured by ASTM D445;
"phosphorus content" is measured by ASTM D5185;
"sulfur content" is measured by ASTM D2622; and,
"sulfated ash content" is measured by ASTM D874.
5
CA 3012205 2018-07-24
All percentages reported are mass % on an active ingredient basis, i.e.
without regard
to carrier or diluent oil, unless otherwise stated.
Also, it will be understood that various components used, essential as well as
optimal
and customary, may react under conditions of formulation, storage or use and
that the
invention also provides the product obtainable or obtained as a result of any
such reaction.
Further, it is understood that any upper and lower quantity, range and ratio
limits set
forth herein may be independently combined.
Also, it will be understood that the preferred features of each aspect of the
present
invention are regarded as preferred features of every other aspect of the
present invention.
BRIEF DESCRIPTION OF THE FIGURES
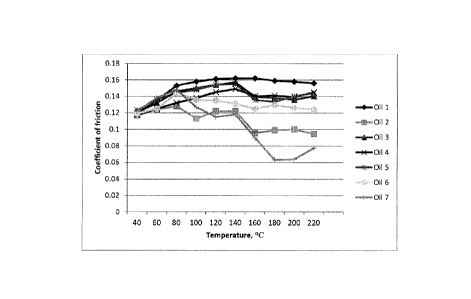
Fig. 1 represents graphically, the results shown in Table 2.
Fig. 2 represents graphically, the results shown in Table 3.
Figs. 3 - 6 represent graphically, the results of Example 2.
Fig. 7 represents graphically, the results shown in Table 5.
Fig. 8 represents graphically, the results shown in Table 6.
DETAILED DESCRIPTION OF THE INVENTION
OIL OF LUBRICATING VISCOSITY
The oil of lubricating viscosity (sometimes referred to as "base stock" or
"base oil")
is the primary liquid constituent of a lubricant, into which additives and
possibly other oils
are blended, for example to produce a final lubricant (or lubricant
composition). A base oil
is useful for making concentrates as well as for making lubricating oil
compositions
therefrom, and may be selected from natural (vegetable, animal or mineral) and
synthetic
lubricating oils and mixtures thereof
The base stock groups are defined in the American Petroleum Institute (API)
publication "Engine Oil Licensing and Certification System", Industry Services
Department,
Fourteenth Edition, December 1996, Addendum 1, December 1998. Typically, the
base stock
will have a viscosity preferably of 3-12, more preferably 4-10, most
preferably 4.5-8, mm2/s
(cSt) at 100 C.
6
CA 3012205 2018-07-24
Definitions for the base stocks and base oils in this invention are the same
as those
found in the American Petroleum Institute (API) publication "Engine Oil
Licensing and
Certification System", Industry Services Department, Fourteenth Edition,
December 1996,
Addendum 1, December 1998. Said publication categorizes base stocks as
follows:
a) Group I base stocks contain less than 90 percent saturates and/or greater
than 0.03
percent sulphur and have a viscosity index greater than or equal to 80 and
less than
120 using the test methods specified in Table E-1.
b) Group II base stocks contain greater than or equal to 90 percent saturates
and less
than or equal to 0.03 percent sulphur and have a viscosity index greater than
or equal
to 80 and less than 120 using the test methods specified in Table E-1.
c) Group III base stocks contain greater than or equal to 90 percent saturates
and less
than or equal to 0.03 percent sulphur and have a viscosity index greater than
or equal
to 120 using the test methods specified in Table E-1.
d) Group IV base stocks are polyalphaolefins (PAO).
e) Group V base stocks include all other base stocks not included in Group I,
II, III,
or IV.
Table E-1: Analytical Methods for Base Stock
Property Test Method
Saturates ASTM D 2007
Viscosity Index ASTM D 2270
Sulphur ASTM D 2622
ASTM D 4294
ASTM D 4927
ASTM D 3120
Other oils of lubricating viscosity which may be included in the lubricating
oil
composition are detailed as follows.
Natural oils include animal and vegetable oils (e.g. castor and lard oil),
liquid
petroleum oils and hydrorefined, solvent-treated mineral lubricating oils of
the paraffinic,
7
CA 3012205 2018-07-24
naphthenic and mixed paraffinic-naphthenic types. Oils of lubricating
viscosity derived from
coal or shale are also useful base oils.
Synthetic lubricating oils include hydrocarbon oils such as polymerized and
interpolymerized olefins (e.g. polybutylenes, polypropylenes, propylene-
isobutylene
.. copolymers, chlorinated polybutylenes, poly(1-hexenes), poly(1-octenes),
poly(1-decenes));
alkylbenzenes (e.g. dodecylbenzenes, tetradecylbenzenes, dinonylbenzenes, di(2-
ethylhexyl)benzenes); polyphenols (e.g. biphenyls, terphenyls, alkylated
polyphenols); and
alkylated diphenyl ethers and alkylated diphenyl sulfides and the derivatives,
analogues and
homologues thereof.
Another suitable class of synthetic lubricating oils comprises the esters of
dicarboxylic acids (e.g. phthalic acid, succinic acid, alkyl succinic acids
and alkenyl succinic
acids, maleic acid, azelaic acid, suberic acid, sebasic acid, fumaric acid,
adipic acid, linoleic
acid dimer, malonic acid, alkylmalonic acids, alkenyl malonic acids) with a
variety of
alcohols (e.g. butyl alcohol, hexyl alcohol, dodecyl alcohol, 2-ethylhexyl
alcohol, ethylene
glycol, diethylene glycol monoether, propylene glycol). Specific examples of
these esters
include dibutyl adipate, di(2-ethylhexyl) sebacate, di-n-hexyl fumarate,
dioctyl sebacate,
diisooctyl azelate, diisodecyl azelate, dioctyl phthalate, didecyl phthalate,
dieicosyl sebacate,
the 2-ethylhexyl diester of linoleic acid dimer, and the complex ester formed
by reacting one
mole of sebacic acid with two moles of tetraethylene glycol and two moles of 2-
ethylhexanoic
acid.
Esters useful as synthetic oils also include those made from C5 to C12
monocarboxylic
acids and polyols, and polyol ethers such as neopentyl glycol,
trimethylolpropane,
pentaerythritol, dipentaerythritol and tripentaerythritol.
Unrefined, refined and re-refined oils can be used in the compositions of the
present
invention. Unrefined oils are those obtained directly from a natural or
synthetic source
without further purification treatment. For example, a shale oil obtained
directly from
retorting operations, a petroleum oil obtained directly from distillation or
ester oil obtained
directly from an esterification process and used without further treatment
would be unrefined
oil. Refined oils are similar to the unrefined oils except they have been
further treated in one
.. or more purification steps to improve one or more properties. Many such
purification
8
CA 3012205 2018-07-24
techniques, such as distillation, solvent extraction, acid or base extraction,
filtration and
percolation are known to those skilled in the art. Re-refined oils are
obtained by processes
similar to those used to obtain refined oils applied to refined oils which
have been already
used in service. Such re-refined oils are also known as reclaimed or
reprocessed oils and
often are additionally processed by techniques for approval of spent additive
and oil
breakdown products.
Other examples of base oil are gas-to-liquid ("GTL") base oils, i.e. the base
oil may
be an oil derived from Fischer-Tropsch synthesised hydrocarbons made from
synthesis gas
containing H2 and CO using a Fischer-Tropsch catalyst. These hydrocarbons
typically
require further processing in order to be useful as a base oil. For example,
they may, by
methods known in the art, be hydroisomerized; hydrocracked and
hydroisomerized;
dewaxed; or hydroisomerized and dewaxed.
Whilst the composition of the base oil will depend upon the particular
application of
the lubricating oil composition and the oil formulator will chose the base oil
to achieve
desired performance characteristics at reasonable cost, the oil of a
lubricating viscosity of the
lubricating oil composition according to all aspects of the present invention
typically
comprises Group II or Group III base oil in the majority. The oil of a
lubricating viscosity of
the lubricating oil composition according to all aspects of the present
invention may comprise
at least 50 mass% Group III and/or Group II base oil, such as at least 70
mass% or even at
least 80 mass% Group III and/or Group II base oil, based on the mass of the
oil of lubricating
viscosity in the lubricating oil composition. The oil of lubricating viscosity
may comprise
100 mass% of Group III and/or Group II base oil, based on the mass of the oil
of lubricating
viscosity in the lubricating oil composition.
Preferably, the volatility of the oil of lubricating viscosity or oil blend,
as measured
by the NOACK test (ASTM D5800), is less than or equal to 20 %, preferably less
than or
equal to 16 %, preferably less than or equal to 12 %, more preferably less
than or equal to
10 %. Preferably, the viscosity index (VI) of the oil of lubricating viscosity
is at least 95,
preferably at least 110, more preferably up to 120, even more preferably at
least 120, even
more preferably at least 125, most preferably from about 130 to 140.
9
CA 3012205 2018-07-24
Preferably, the oil of lubricating viscosity is present in an amount of
greater than 55
mass %, more preferably greater than 60 mass %, even more preferably greater
than 70
mass %, based on the total mass of the lubricating oil composition.
Preferably, the oil of
lubricating viscosity is present in an amount of less than 98 mass %, more
preferably less
than 95 mass %, even more preferably less than 90 mass %, based on the total
mass of the
lubricating oil composition.
The lubricating oil composition of each aspect of the present invention may be
a
multigrade oil identified by the viscometric descriptor SAE 20WX, SAE 15WX,
SAE lOWX,
SAE 5WX or SAE OWX, where X represents any one of 20, 30, 40 and 50; the
characteristics
of the different viscometric grades can be found in the SAE J300
classification. In an
embodiment of each aspect of the invention, independently of the other
embodiments, the
lubricating oil composition is in the form of an SAE 1 OWX, SAE 5WX or SAE
OWX,
preferably in the form of a SAE lOWX or SAE 5WX viscosity grade, wherein X
represents
any one of 20, 30, 40 and 50. Preferably X is 30 or 40.
OIL-SOLUBLE MOLYBDENUM COMPOUND (A)
For the lubricating oil compositions of all aspects of the present invention,
any
suitable oil-soluble or oil-dispersible molybdenum compound having friction
modifying
properties in lubricating oil compositions may be employed.
Preferably, the oil-soluble or oil-dispersible molybdenum compound is an oil-
soluble
or oil-dispersible organo-molybdenum compound. As examples of such organo-
molybdenum compounds, there may be mentioned molybdenum dithiocarbamates,
molybdenum dithiophosphates, molybdenum dithiophosphinates, molybdenum
xanthates,
molybdenum thioxanthates, molybdenum sulfides, and the like, and mixtures
thereof.
Particularly preferred are molybdenum dithiocarbamates, molybdenum
dialkyldithiophosphates, molybdenum alkyl xanthates and molybdenum
alkylthioxanthates.
Especially preferred organo-molybdenum compounds are molybdenum
dithiocarbamates. In
an embodiment of the present invention the oil-soluble or oil-dispersible
molybdenum
compound consists of either a molybdenum dithiocarbamate or a molybdenum
dithiophosphate or a mixture thereof, as the sole source of molybdenum atoms
in the
CA 3012205 2018-07-24
lubricating oil composition. In an alternative embodiment of the present
invention the oil-
soluble or oil-dispersible molybdenum compound consists of a molybdenum
dithiocarbamate, as the sole source of molybdenum atoms in the lubricating oil
composition.
The molybdenum compound may be mono-, di-, tri- or tetra-nuclear. Di-nuclear
and
tri-nuclear molybdenum compounds are preferred.
Suitable dinuclear or dimeric molybdenum dialkyldithiocarbamate are
represented by
the following formula:
R1 S I X2 )(114 s
\II/ \II/ \ /R3
N ¨ C Mo Mo C ¨N
\ \ R2 R4 X3
wherein RI through R4 independently denote a straight chain, branched chain or
aromatic
hydrocarbyl group having 1 to 24 carbon atoms; and Xi through X4 independently
denote an
oxygen atom or a sulfur atom. The four hydrocarbyl groups, R1 through R4, may
be identical
or different from one another.
Other molybdenum compounds useful in the compositions of this invention are
organo-molybdenum compounds of the formulae Mo(ROCS2)4 and Mo(RSCS2)4, wherein
R
is an organo group selected from the group consisting of alkyl, aryl, aralkyl
and alkoxyalkyl,
generally of from 1 to 30 carbon atoms, and preferably 2 to 12 carbon atoms
and most
preferably alkyl of 2 to 12 carbon atoms. Especially preferred are the
dialkyldithiocarbamates
of molybdenum.
Suitable tri-nuclear organo-molybdenum compounds include those of the formula
Mo3SkL,Qz and mixtures thereof wherein L are independently selected ligands
having organo
groups with a sufficient number of carbon atoms to render the compound soluble
or
dispersible in the oil, n is from 1 to 4, k varies from 4 through 7, Q is
selected from the group
of neutral electron donating compounds such as water, amines, alcohols,
phosphines, and
ethers, and z ranges from 0 to 5 and includes non-stoichiometric values. At
least 21 total
carbon atoms should be present among all the ligands' organo groups, such as
at least 25, at
least 30, or at least 35 carbon atoms.
11
CA 3012205 2018-07-24
The ligands are independently selected from the group of:
¨X¨ R 1,
XI \
¨ /C¨ R 2,
X2
XI \ /R
3,
X2
and mixtures thereof, wherein X, Xi, X2, and Y are independently selected from
the group of
oxygen and sulfur, and wherein Ri, R2, and R are independently selected from
hydrogen and
organo groups that may be the same or different. Preferably, the organo groups
are
hydrocarbyl groups such as alkyl (e.g., in which the carbon atom attached to
the remainder
of the ligand is primary or secondary), aryl, substituted aryl and ether
groups. More
preferably, each ligand has the same hydrocarbyl group.
Importantly, the organo groups of the ligands have a sufficient number of
carbon
atoms to render the compound soluble or dispersible in the oil. For example,
the number of
carbon atoms in each group will generally range between about 1 to about 100,
preferably
from about 1 to about 30, and more preferably between about 4 to about 20.
Preferred ligands
include dialkyldithiophosphate, alkylxanthate, and dialkyldithiocarbamate, and
of these
dialkyldithiocarbamate is more preferred. Organic ligands containing two or
more of the
above functionalities are also capable of serving as ligands and binding to
one or more of the
cores. Those skilled in the art will realize that formation of the compounds
of the present
invention requires selection of ligands having the appropriate charge to
balance the core's
charge.
Compounds having the formula Mo3SkLnQz have cationic cores surrounded by
anionic ligands and are represented by structures such as
12
CA 3012205 2018-07-24
S
ml Mof
and
vmo.)141
\/
and have net charges of +4. Consequently, in order to solubilize these cores
the total charge
among all the ligands must be -4. Four mono-anionic ligands are preferred.
Without wishing
to be bound by any theory, it is believed that two or more tri-nuclear cores
may be bound or
interconnected by means of one or more ligands and the ligands may be
multidentate. This
includes the case of a multidentate ligand having multiple connections to a
single core. It is
believed that oxygen and/or selenium may be substituted for sulfur in the
core(s).
Oil-soluble or oil-dispersible tri-nuclear molybdenum compounds can be
prepared by
reacting in the appropriate liquid(s)/solvent(s) a molybdenum source such as
(N1-14)2Mo3S13.n(H20), where n varies between 0 and 2 and includes non-
stoichiometric
values, with a suitable ligand source such as a tetralkylthiuram disulfide.
Other oil-soluble
or dispersible tri-nuclear molybdenum compounds can be formed during a
reaction in the
appropriate solvent(s) of a molybdenum source such as of (NH4)2M03S13.n(H20),
a ligand
source such as tetralkylthiuram disulfide, dialkyldithiocarbamate, or
dialkyldithiophosphate,
and a sulfur abstracting agent such as cyanide ions, sulfite ions, or
substituted phosphines.
Alternatively, a tri-nuclear molybdenum-sulfur halide salt such as
[M'121Mo3S7A6], where M'
is a counter ion, and A is a halogen such as Cl, Br, or I, may be reacted with
a ligand source
13
CA 3012205 2018-07-24
such as a dialkyldithiocarbamate or dialkyldithiophosphate in the appropriate
liquid(s)/solvent(s) to form an oil-soluble or dispersible trinuclear
molybdenum compound.
The appropriate liquid/solvent may be, for example, aqueous or organic.
A compound's oil solubility or dispersibility may be influenced by the number
of
carbon atoms in the ligand's organo groups. Preferably, at least 21 total
carbon atoms should
be present among all the ligands' organo groups. Preferably, the ligand source
chosen has a
sufficient number of carbon atoms in its organo groups to render the compound
soluble or
dispersible in the lubricating composition.
The amount of oil-soluble molybdenum compound will depend upon the particular
performance requirements of the lubricating oil composition. Suitably, the
lubricating oil
composition of all aspects of the present invention contains the molybdenum
compound in
an amount providing the composition with at least 30ppm or at least 50ppm of
molybdenum
(ASTM D5185). The lubricating oil composition of all aspects of the present
invention may
contain the molybdenum compound in an amount providing the composition with up
to 1000
ppm, or up to 500ppm or up to 200ppm, or up to 150 ppm of molybdenum (ASTM
D5185).
ASHLESS ORGANIC FRICTION MODIFIER (B)
Ashless friction modifiers suitable for use in the lubricating oil composition
of all
aspects of the present invention include nitrogen-free organic friction
modifiers and include
esters formed by reacting carboxylic acids and anhydrides with alkanols. Other
suitable
friction modifiers include a polar terminal group (e.g. carboxyl or hydroxyl)
covalently
bonded to an oleophilic hydrocarbon chain. Esters of carboxylic acids and
anhydrides with
alkanols are described in US 4,702,850. Examples of other conventional organic
friction
modifiers are described by M. Belzer in the "Journal of Tribology" (1992),
Vol. 114, pp. 675-
682 and M. Belzer and S. Jahanmir in "Lubrication Science" (1988), Vol. 1, pp.
3-26.
Preferred organic ashless nitrogen-free friction modifiers are esters or ester-
based; a
particularly preferred organic ashless nitrogen-free friction modifier is
glycerol monooleate
(GMO).
Other preferred ashless organic friction modifiers include alkenyl substituted
anhydrides, such as octadecenyl succinic anhydride.
14
CA 3012205 2018-07-24
Ashless aminic or amine-based friction modifiers may also be used and include
oil-soluble alkoxylated mono- and di-amines, which improve boundary layer
lubrication.
One common class of such metal free, nitrogen-containing friction modifier
comprises
ethoxylated alkyl amines. They may be in the form of an adduct or reaction
product with a
boron compound such as a boric oxide, boron halide, metaborate, boric acid or
a mono-, di-
or tri-alkyl borate. Another metal free, nitrogen-containing friction modifier
is an ester
formed as the reaction product of (i) a tertiary amine of the formula RI R2R3N
wherein RI, R2
and R3 represent aliphatic hydrocarbyl, preferably alkyl, groups having 1 to 6
carbon atoms,
at least one of R1, R2 and R3 having a hydroxyl group, with (ii) a saturated
or unsaturated
fatty acid having 10 to 30 carbon atoms. Preferably, at least one of RI, R2
and R3 is an alkyl
group. Preferably, the tertiary amine will have at least one hydroxyalkyl
group having 2 to 4
carbon atoms. The ester may be a mono-, di- or tri-ester or a mixture thereof,
depending on
how many hydroxyl groups are available for esterification with the acyl group
of the fatty
acid. The ashless organic friction modifier of all aspects of the present
invention may
comprise a mixture of esters formed as the reaction product of (i) a tertiary
hydroxy amine of
the formula R1R2R3N wherein RI, R2 and R3 may be a C2-C4 hydroxy alkyl group
with (ii) a
saturated or unsaturated fatty acid having 10 to 30 carbon atoms, with a
mixture of esters so
formed comprising at least 30-60 mass%, preferably 45-55 mass% diester, such
as 50 mass%
diester, 10-40 mass%, preferably 20-30 mass% monoester, e.g. 25 mass%
monoester, and
10-40 mass%, preferably 20-30 mass% triester, such as 25 mass% triester.
Suitably, the ester
is a mono-, di- or tri-carboxylic acid ester of triethanolamine and mixtures
thereof.
Typically, the total amount of ashless organic friction modifier (B) in the
lubricating
oil composition of all aspects of the present invention does not exceed 5 mass
%, based on
the total mass of the lubricating oil composition and preferably does not
exceed 2 mass %
and more preferably does not exceed 0.5 mass %.
ASHLESS DISPERSANT (C)
The lubricating oil of all aspects of the present invention may also comprise
a
dispersant additive.
CA 3012205 2018-07-24
A dispersant is an additive whose primary function is to hold solid and liquid
contaminations in suspension, thereby passivating them and reducing engine
deposits at the
same time as reducing sludge depositions. For example, a dispersant maintains
in suspension
oil-insoluble substances that result from oxidation during use of the
lubricant, thus preventing
sludge flocculation and precipitation or deposition on metal parts of the
engine.
Dispersants are usually "ashless", as mentioned above, being non-metallic
organic
materials that form substantially no ash on combustion, in contrast to metal-
containing, and
hence ash-forming materials. They comprise a long hydrocarbon chain with a
polar head,
the polarity being derived from inclusion of e.g. an 0, P, or N atom. The
hydrocarbon is an
oleophilic group that confers oil-solubility, having, for example 40 to 500
carbon atoms.
Thus, ashless dispersants may comprise an oil-soluble polymeric backbone.
The ashless dispersant suitable for all aspects of the present invention is
preferably an
ashless, nitrogen-containing dispersant.
Suitable ashless dispersant may be made from polyalkenes that have been
functionalised exclusively by the thermal "ene" reaction, a known reaction.
Such
polyalkenes are mixtures having predominantly terminal vinylidene groups, such
at least 65,
e.g. 70, more preferably at least 85, %. As an example, there may be mentioned
a polyalkene
known as highly reactive polyisobutene (HR-PIB), which is commercially
available under
the tradename GlissopalTM (ex BASF). US-A-4 152 499 describes the preparations
of such
polymers.
Alternatively, the ashless dispersant may be made from polyalkenes that have
been
functionalised by the so-called chlorination method, which results in a
product where minor
percentage of its polymer chains (e.g. less than 20%) have terminal vinylidene
groups.
Preferred monounsaturated reactants that may be used to functionalize the
polyalkene
comprise mono- and dicarboxylic acid material, i.e., acid, anhydride, or acid
ester material,
including (i) monounsaturated C4 to CIO dicarboxylic acid wherein (a) the
carboxyl groups
are vicinyl, (i.e., located on adjacent carbon atoms) and (b) at least one,
preferably both, of
said adjacent carbon atoms are part of said mono unsaturation; (ii)
derivatives of (i) such as
anhydrides or CI to C5 alcohol derived mono- or diesters of (i); (iii)
monounsaturated C3 to
Cio monocarboxylic acid wherein the carbon-carbon double bond is conjugated
with the
16
CA 3012205 2018-07-24
carboxy group, i.e., of the structure -CC-CO-; and (iv) derivatives of (iii)
such as CI to C5
alcohol derived mono- or diesters of (iii). Mixtures of monounsaturated
carboxylic materials
(i) - (iv) also may be used. Upon reaction with the polyalkene, the
monounsaturation of the
monounsaturated carboxylic reactant becomes saturated. Thus, for example,
maleic
anhydride becomes polyalkene-substituted succinic anhydride, and acrylic acid
becomes
polyalkene-substituted propionic acid. Exemplary of such monounsaturated
carboxylic
reactants are fumaric acid, itaconic acid, maleic acid, maleic anhydride,
acrylic acid,
methacrylic acid, crotonic acid, cinnamic acid, and lower alkyl (e.g., CI to
C4 alkyl) acid
esters of the foregoing, e.g., methyl maleate, ethyl fumarate, and methyl
fumarate.
To provide the required functionality, monounsaturated carboxylic reactants,
preferably maleic anhydride, typically will be used in an amount ranging from
equimolar to
100, preferably 5 to 50, wt. % excess, based on the moles of polyalkene.
Unreacted excess
monounsaturated carboxylic reactant can be removed from the final dispersant
product by,
for example, stripping, usually under vacuum, if required.
The functionalised oil-soluble polyalkene is then derivatized with a
nucleophilic
reactant, such as an amine, amino-alcohol, alcohol, or mixture thereof, to
form a
corresponding derivative containing the dispersant. Useful amine compounds for
derivatizing functionalized polymers comprise at least one amine and can
comprise one or
more additional amine or other reactive or polar groups. These amines may be
hydrocarbyl
amines or may be predominantly hydrocarbyl amines in which the hydrocarbyl
group
includes other groups, e.g., hydroxy groups, alkoxy groups, amide groups,
nitriles and
imidazoline groups. Particularly useful amine compounds include mono- and
polyamines,
e.g., polyalkene and polyoxyalkylene polyamines of 2 to 60, such as 2 to 40
(e.g., 3 to 20),
total carbon atoms having 1 to 12, such as 3 to 12, preferably 3 to 9, most
preferably 6 to 7,
nitrogen atoms per molecule. Mixtures of amine compounds may advantageously be
used.
Preferred amines are aliphatic saturated amines, including, for example, 1,2-
diaminoethane;
1,3-diaminopropane; 1,4-diaminobutane; 1,6-diaminohexane; polyethylene amines
such as
diethylene triamine; triethylene tetramine; tetraethylene pentamine; and
polypropyleneamines such as 1,2-propylene diamine; and di-(1,2-
propylene)triamine. Such
polyamine mixtures, known as PAM, are commercially available. Particularly
preferred
17
CA 3012205 2018-07-24
polyamine mixtures are mixtures derived by distilling the light ends from PAM
products.
The resulting mixtures, known as "heavy" PAM, or HPAM, are also commercially
available.
The properties and attributes of both PAM and/or HPAM are described, for
example, in U.S.
Patent Nos. 4,938,881; 4,927,551; 5,230,714; 5,241,003; 5,565,128; 5,756,431;
5,792,730;
and 5,854,186.
Other useful amine compounds include: alicyclic diamines such as
1,4-di(aminomethyl) cyclohexane and heterocyclic nitrogen compounds such as
imidazolines. Another useful class of amines is the polyamido and related
amido-amines as
disclosed in U.S. Patent Nos. 4,857,217; 4,956,107; 4,963,275; and 5,229,022.
Also usable
is tris(hydroxymethypamino methane (TAM) as described in U.S. Patent Nos.
4,102,798;
4,113,639; 4,116,876; and UK 989,409. Dendrimers, star-like amines, and comb-
structured
amines may also be used. Similarly, condensed amines, as described in U.S.
Patent No.
5,053,152 may be used. The functionalized polymer is reacted with the amine
compound
using conventional techniques as described, for example, in U.S. Patent Nos.
4,234,435 and
5,229,022, as well as in EP-A-208,560.
A dispersant of the present invention preferably comprises at least one
dispersant that
is derived from polyalkenyl-substituted mono- or dicarboxylic acid, anhydride
or ester, which
has from greater than 1.3 to 1.7, preferably from greater than 1.3 to 1.6,
most preferably from
greater than 1.3 to 1.5, functional groups (mono- or dicarboxylic acid
producing moieties)
per polyalkenyl moiety (a medium functionality dispersant). Functionality (F)
can be
determined according to the following formula:
F = (SAP x M)/((112,200 x A.I.) - (SAP x MW)) (1)
wherein SAP is the saponification number (i.e., the number of milligrams of
KOH consumed
in the complete neutralization of the acid groups in one gram of the succinic-
containing
reaction product, as determined according to ASTM D94); M,, is the number
average
molecular weight of the starting olefin polymer; A.I. is the percent active
ingredient of the
succinic-containing reaction product (the remainder being unreacted olefin
polymer, succinic
anhydride and diluent); and MW is the molecular weight of the mono- or
dicarboxylic acid
producing moieties (e.g., 98 for maleic anhydride).
18
CA 3012205 2018-07-24
Generally, each mono- or dicarboxylic acid-producing moiety will react with a
nucleophilic group (amine, alcohol, amide or ester polar moieties) and the
number of
functional groups in the polyalkenyl-substituted carboxylic acylating agent
will determine
the number of nucleophilic groups in the finished dispersant.
The polyalkenyl moiety of the dispersant of the present invention may have a
number
average molecular weight of at least 900, suitably at least 1500, preferably
between 1800 and
3000, such as between 2000 and 2800, more preferably from about 2100 to 2500,
and most
preferably from about 2200 to about 2400. The molecular weight of a dispersant
is generally
expressed in terms of the molecular weight of the polyalkenyl moiety; this is
because the
precise molecular weight range of the dispersant depends on numerous
parameters including
the type of polymer used to derive the dispersant, the number of functional
groups, and the
type of nucleophilic group employed.
Polymer molecular weight, specifically MI, can be determined by various known
techniques. One convenient method is gel permeation chromatography (GPC),
which
additionally provides molecular weight distribution information (see W. W.
Yau, J. J.
Kirkland and D. D. Bly, "Modern Size Exclusion Liquid Chromatography", John
Wiley and
Sons, New York, 1979). Another useful method for determining molecular weight,
particularly for lower molecular weight polymers, is vapor pressure osmometry
(see, e.g.,
ASTM D3592).
The polyalkenyl moiety in a dispersant of the present invention preferably has
a
narrow molecular weight distribution (MWD), also referred to as
polydispersity, as
determined by the ratio of weight average molecular weight (Mw) to number
average
molecular weight (Me). Polymers having a Mw/Me of less than 2.2, preferably
less than 2.0,
are most desirable. Suitable polymers have a polydispersity of from about 1.5
to 2.1,
preferably from about 1.6 to about 1.8.
Suitable polyalkenes employed in the formation of the dispersants of the
present
invention include homopolymers, interpolymers or lower molecular weight
hydrocarbons.
One family of such polymers comprise polymers of ethylene and/or at least one
C3 to C28
alpha-olefin having the formula H2C¨CHR1 wherein RI is a straight or branched
chain alkyl
radical comprising 1 to 26 carbon atoms and wherein the polymer contains
carbon-to-carbon
19
CA 3012205 2018-07-24
unsaturation, and a high degree of terminal ethenylidene unsaturation.
Preferably, such
polymers comprise interpolymers of ethylene and at least one alpha-olefin of
the above
formula, wherein RI is alkyl of from 1 to 18 carbon atoms, and more preferably
is alkyl of
from 1 to 8 carbon atoms, and more preferably still of from 1 to 2 carbon
atoms
Another useful class of polymers is polymers prepared by cationic
polymerization of
monomers such as isobutene and styrene. Common polymers from this class
include
polyisobutenes obtained by polymerization of a C4 refinery stream having a
butene content
of 35 to 75% by wt., and an isobutene content of 30 to 60% by wt., by the
thermal "ene"
reaction. A preferred source of monomer for making poly-n-butenes is petroleum
feedstreams such as Raffinate II. These feedstocks are disclosed in the art
such as in U.S.
Patent No. 4,952,739. A preferred embodiment utilizes polyisobutylene prepared
from a pure
isobutylene stream or a Raffinate I stream to prepare reactive isobutylene
polymers with
terminal vinylidene olefins as described above.
The dispersant(s) of the invention are preferably mono- or bis-succinimides.
The dispersant(s) of the present invention can be borated by conventional
means, as
generally taught in U.S. Patent Nos. 3,087,936, 3,254,025 and 5,430,105.
Boration of the
dispersant is readily accomplished by treating an acyl nitrogen-containing
dispersant with a
boron compound such as boron oxide, boron halide boron acids, and esters of
boron acids, in
an amount sufficient to provide from 0.1 to 20 atomic proportions of boron for
each mole of
acylated nitrogen composition.
The boron, which appears in the product as dehydrated boric acid polymers
(primarily
(HB02)3), is believed to attach, for example, to dispersant imides and
diimides as amine salts,
e.g., the metaborate salt of the diimide. Boration can be carried out by
adding a sufficient
quantity of a boron compound, preferably boric acid, usually as a slurry, to
the acyl nitrogen
compound and heating with stirring at from 135C to 190, e.g., 140 to 170, C,
for from 1 to
5 hours, followed by nitrogen stripping. Alternatively, the boron treatment
can be conducted
by adding boric acid to a hot reaction mixture of the dicarboxylic acid
material and amine,
while removing water. Other post-reaction processes known in the art can also
be applied.
Typically, the lubricating oil composition may contain from 1 to 20, such as 3
to 15,
preferably 3 to 12, mass % dispersant.
CA 3012205 2018-07-24
The ashless dispersant (D) of all aspects of the present invention may
comprise a
mixture of ashless dispersant compounds. In a preferred embodiment of all
aspects of the
present invention, the lubricating oil composition comprises an ashless
dispersant made by
the thermal "ene" process. If the lubricating oil composition comprises a
mixture of ashless
dispersant additives, an ashless dispersant made by the thermal "ene" process
preferably
provides the majority of the ashless dispersant. For example, the ashless
dispersant (D) may
comprise at least 50 mass%, or at least 70% or at least 75% ashless dispersant
made by the
thermal process. In an embodiment of all aspects of the present invention the
ashless
dispersant (D) comprises only dispersant made by the thermal process.
The amount of nitrogen in a lubricating oil composition according to the
present
invention will depend upon the particular application of the oil. Typically, a
lubricating oil
composition according to the present invention contains at least 0.02, such as
at least 0.03 or
0.04 mass % nitrogen, based on the total mass of the composition and as
measured according
to ASTM method D5291. Suitably, the lubricating oil composition will contain
no more than
0.20, such as no more than 0.15 or no more than 0.12 mass % nitrogen based
upon the total
mass of the composition and as measured according to ASTM D5291.
METAL-CONTAINING DETERGENT (E)
Suitably the lubricating oil composition of all aspects of the present
invention further
comprises at least one metal-containing detergent additive.
Metal-containing detergents function both as detergents to reduce or remove
deposits
and as acid neutralizers or rust inhibitors, thereby reducing wear and
corrosion and extending
engine life. Detergents generally comprise a polar head with a long
hydrophobic tail, with
the polar head comprising a metal salt of an acidic organic compound. The
salts may contain
a substantially stoichiometric amount of the metal in which case they are
usually described
as normal or neutral salts, and would typically have a total base number or
TBN (as can be
measured by ASTM D2896) of from 0 to 80 mg KOH/g. A large amount of a metal
base
may be incorporated by reacting excess metal compound (e.g., an oxide or
hydroxide) with
an acidic gas (e.g., carbon dioxide). The resulting overbased detergent
comprises neutralized
detergent as the outer layer of a metal base (e.g. carbonate) micelle. Such
overbased
21
CA 3012205 2018-07-24
detergents may have a TBN of 150 mg KOH/g or greater, and typically will have
a TBN of
from 250 to 450 mg KOH/g or more. In the presence of the compounds of Formula
I, the
amount of overbased detergent can be reduced, or detergents having reduced
levels of
overbasing (e.g., detergents having a TBN of 100 to 200 mg KOH/g), or neutral
detergents
can be employed, resulting in a corresponding reduction in the SASH content of
the
lubricating oil composition without a reduction in the performance thereof
Detergents that may be used include oil-soluble neutral and overbased
sulfonates,
phenates, sulfurized phenates, thiophosphonates, salicylates, and naphthenates
and other oil-
soluble carboxylates of a metal, particularly the alkali or alkaline earth
metals, e.g., sodium,
potassium, lithium, calcium, and magnesium. The most commonly used metals are
calcium
and magnesium, which may both be present in detergents used in a lubricating
oil
composition according to any aspect of the present invention. Combinations of
detergents,
whether overbased or neutral or both, may be used.
Sulfonates may be prepared from sulfonic acids which are typically obtained by
the
sulfonation of alkyl substituted aromatic hydrocarbons such as those obtained
from the
fractionation of petroleum or by the alkylation of aromatic hydrocarbons.
Examples included
those obtained by alkylating benzene, toluene, xylene, naphthalene, diphenyl
or their halogen
derivatives such as chlorobenzene, chlorotoluene and chloronaphthalene. The
alkylation may
be carried out in the presence of a catalyst with alkylating agents having
from about 3 to more
than 70 carbon atoms. The alkaryl sulfonates usually contain from about 9 to
about 80 or
more carbon atoms, preferably from about 16 to about 60 carbon atoms per alkyl
substituted
aromatic moiety. The oil soluble sulfonates or alkaryl sulfonic acids may be
neutralized with
oxides, hydroxides, alkoxides, carbonates, carboxylate, sulfides,
hydrosulfides, nitrates,
borates and ethers of the metal. The amount of metal compound is chosen having
regard to
the desired TBN of the final product but typically ranges from about 100 to
220 mass %
(preferably at least 125 mass %) of that stoichiometrically required.
Metal salts of phenols and sulfurized phenols are prepared by reaction with an
appropriate metal compound such as an oxide or hydroxide and neutral or
overbased products
may be obtained by methods well known in the art. Sulfurized phenols may be
prepared by
reacting a phenol with sulfur or a sulfur containing compound such as hydrogen
sulfide,
22
CA 3012205 2018-07-24
sulfur monohalide or sulfur dihalide, to form products which are generally
mixtures of
compounds in which 2 or more phenols are bridged by sulfur containing bridges.
Carboxylate detergents, e.g., salicylates, can be prepared by reacting an
aromatic
carboxylic acid with an appropriate metal compound such as an oxide or
hydroxide and
.. neutral or overbased products may be obtained by methods well known in the
art. The
aromatic moiety of the aromatic carboxylic acid can contain heteroatoms, such
as nitrogen
and oxygen. Preferably, the moiety contains only carbon atoms; more preferably
the moiety
contains six or more carbon atoms; for example benzene is a preferred moiety.
The aromatic
carboxylic acid may contain one or more aromatic moieties, such as one or more
benzene
rings, either fused or connected via alkylene bridges.
Preferred substituents in oil-soluble salicylic acids are alkyl substituents.
In alkyl -
substituted salicylic acids, the alkyl groups advantageously contain 5 to 100,
preferably 9 to
30, especially 14 to 20, carbon atoms. Where there is more than one alkyl
group, the average
number of carbon atoms in all of the alkyl groups is preferably at least 9 to
ensure adequate
oil solubility.
The metal-containing detergent (E) may comprise one of more metal detergents
that
are neutral or overbased alkali or alkaline earth metal salicylates. Highly
preferred salicylate
detergents include alkaline earth metal salicylates, particularly magnesium
and calcium,
especially, calcium salicylates. The metal salicylate may be the sole metal-
containing
detergent present in the lubricating oil composition of all aspects of the
present invention.
Alternatively, other metal-containing detergents, such as metal sulfonates or
phenates, may
be present in the lubricating composition. Preferably, the salicylate
detergent provides the
majority of the detergent additive in the lubricating oil composition.
The total amount of metal-containing detergent additive present in the
lubricating oil
composition according to any aspect of the present invention is suitably in
the range of 0.1-10
mass%, preferably from 0.5 to 5 mass% on an active matter basis.
CO-ADDITIVES
Lubricating oil compositions according to each aspect of the invention may
additionally comprise one or more co-additives, which are different from
additive
23
CA 3012205 2018-07-24
components (B), (C), (D) and (E). Suitable co-additives and their common treat
rates are
discussed below. All the values listed are stated as mass percent active
ingredient in a fully
formulated lubricant.
Additive Mass % Mass %
(Broad) (Preferred)
Corrosion Inhibitor 0¨ 5 0¨ 1.5
Metal Dihydrocarbyl Dithiophosphate 0 ¨ 10 0 ¨ 4
Anti-Oxidants 0 ¨ 5 0.01 ¨ 3
Pour Point Depressant 0.01 ¨ 5 0.01 ¨ 1.5
Anti-Foaming Agent 0¨ 5 0.001 ¨0.15
Viscosity Modifier (1) 0 ¨ 10 0.01 ¨4
Mineral or Synthetic Base Oil Balance Balance
(1) Viscosity modifiers are used only in multi-graded oils.
The final lubricating oil composition, typically made by blending the or each
additive
into the base oil, may contain from 5 to 25, preferably 5 to 18, typically 7
to 15, mass % of
the additives; the remainder being oil of lubricating viscosity.
The above mentioned co-additives are discussed in further detail as follows;
as is
known in the art, some additives can provide a multiplicity of effects, for
example, a single
additive may act as a dispersant and as an oxidation inhibitor.
Anti-wear agents reduce friction and excessive wear and are usually based on
compounds containing sulfur or phosphorous or both, for example that are
capable of
depositing polysulfide films on the surfaces involved. Noteworthy are
dihydrocarbyl
dithiophosphate metal salts wherein the metal may be an alkali or alkaline
earth metal, or
aluminium, lead, tin, molybdenum, manganese, nickel, copper, or preferably,
zinc.
Dihydrocarbyl dithiophosphate metal salts may be prepared in accordance with
known techniques by first forming a dihydrocarbyl dithiophosphoric acid
(DDPA), usually
by reaction of one or more alcohols or a phenol with P2S5 and then
neutralizing the formed
DDPA with a metal compound. For example, a dithiophosphoric acid may be made
by
reacting mixtures of primary and secondary alcohols.
Alternatively, multiple
dithiophosphoric acids can be prepared where the hydrocarbyl groups on one are
entirely
secondary in character and the hydrocarbyl groups on the others are entirely
primary in
24
CA 3012205 2018-07-24
character. To make the metal salt, any basic or neutral metal compound could
be used but
the oxides, hydroxides and carbonates are most generally employed. Commercial
additives
frequently contain an excess of metal due to the use of an excess of the basic
metal compound
in the neutralization reaction.
The preferred zinc dihydrocarbyl dithiophosphates (ZDDP) are oil-soluble salts
of
dihydrocarbyl dithiophosphoric acids and may be represented by the following
formula:
¨ S ¨
RO
\ 11
P ¨ S Zn
/
R0
¨ ' ¨2
wherein R and R' may be the same or different hydrocarbyl radicals containing
from 1 to 18,
preferably 2 to 12, carbon atoms and including radicals such as alkyl,
alkenyl, aryl, arylalkyl,
alkaryl and cycloaliphatic radicals. Particularly preferred as R and R' groups
are alkyl groups
of 2 to 8 carbon atoms. Thus, the radicals may, for example, be ethyl, n-
propyl, i-propyl,
n-butyl, i-butyl, sec-butyl, amyl, n-hexyl, i-hexyl, n-octyl, decyl, dodecyl,
octadecyl,
2-ethylhexyl, phenyl, butylphenyl, cyclohexyl, methylcyclopentyl, propenyl,
butenyl. In
order to obtain oil solubility, the total number of carbon atoms (i.e. R and
R') in the
dithiophosphoric acid will generally be about 5 or greater. The zinc
dihydrocarbyl
dithiophosphate can therefore comprise zinc dialkyl dithiophosphates.
The ZDDP is added to the lubricating oil compositions in amounts sufficient to
provide at least 800 ppm such as at least 900ppm or at least 1000ppm by mass
of phosphorous
to the lubricating oil, based upon the total mass of the lubricating oil
composition, and as
measured in accordance with ASTM D5185.
The ZDDP is suitably added to the lubricating oil compositions in amounts
sufficient
to provide no more than 1200 ppm by mass of phosphorous to the lubricating
oil, based upon
the total mass of the lubricating oil composition, and as measured in
accordance with ASTM
D5185.
CA 3012205 2018-07-24
Viscosity modifiers (VM) function to impart high and low temperature
operability to
a lubricating oil. The VM used may have that sole function, or may be
multifunctional.
Multifunctional viscosity modifiers that also function as dispersants are also
known. Suitable
viscosity modifiers are polyisobutylene, copolymers of ethylene and propylene
and higher
alpha-olefins, polymethacrylates, polyalkylmethacrylates, methacrylate
copolymers,
copolymers of an unsaturated dicarboxylic acid and a vinyl compound, inter
polymers of
styrene and acrylic esters, and partially hydrogenated copolymers of styrene/
isoprene,
styrene/butadiene, and isoprene/butadiene, as well as the partially
hydrogenated
homopolymers of butadiene and isoprene and isoprene/divinylbenzene. Preferred
viscosity
modifiers for all aspects of the present invention are copolymers of an
unsaturated
dicarboxylic acid and a vinyl compound, inter polymers of styrene and acrylic
esters, and,
most preferably, partially hydrogenated copolymers of styrene/ isoprene,
styrene/butadiene,
and isoprene/butadiene, as well as the partially hydrogenated homopolymers of
butadiene
and isoprene and isoprene/divinylbenzene. The preferred partially hydrogenated
copolymers
of styrene/ isoprene, styrene/butadiene, and isoprene/butadiene, may be random
copolymers
but are preferably block copolymers. The preferred, partially hydrogenated
copolymers of
styrene/isoprene, styrene/butadiene, and isoprene/butadiene, and partially
hydrogenated
homopolymers of butadiene and isoprene and isoprene/divinylbenzene viscosity
modifiers
may be linear polymers or star (radial) polymers.
Linear block copolymers useful in the practice of the present invention may be
represented by the following general formula:
A,(B-A)y-Bx
wherein:
A is a polymeric block comprising predominantly monoalkenyl aromatic
hydrocarbon
monomer units;
B is a polymeric block comprising predominantly conjugated diolefin monomer
units;
x and z are, independently, a number equal to 0 or 1; and
y is a whole number ranging from 1 to about 15.
Useful tapered linear block copolymers may be represented by the following
general
formula:
26
CA 3012205 2018-07-24
A-A/B-B
wherein:
A is a polymeric block comprising predominantly monoalkenyl aromatic
hydrocarbon
monomer units;
B is a polymeric block comprising predominantly conjugated diolefin monomer
units; and
A/B is a tapered segment containing both monoalkenyl aromatic hydrocarbon and
conjugated
diolefin units.
Star or radial homopolymers or random copolymers of diene(s) (e.g., isoprene
and/or
butadiene) may be represented, generally, by the following general formula:
(B)n-C
wherein:
B and C are as previously defined; and
n is a number from 3 to 30;
C is the core of the radial polymer formed with a polyfunctional coupling
agent;
B' is a polymeric block comprising predominantly conjugated diolefin units,
which B' may
be the same or different from B; and
n' and n" are integers representing the number of each type of arm and the sum
of n' and n"
will be a number from 3 to 30.
Star or radial block copolymers may be represented, generally, by the
following
general formula:
(B,,-(A-B)y-Az)n-C; and
(B'õ-(A-B)rAz)n¨C(B')n-
wherein:
A, B, x, y and z are as previously defined;
n is a number from 3 to 30;
C is the core of the radial polymer formed with a polyfunctional coupling
agent;
B' is a polymeric block comprising predominantly conjugated diolefin units,
which B' may
be the same or different from B; and
n' and n" are integers representing the number of each type of arm and the sum
of n' and n"
will be a number from 3 to 30.
27
CA 3012205 2018-07-24
As used herein in connection with polymer block composition, the term
"predominantly" means that the specified monomer or monomer type which is the
principle
component in that polymer block is present in an amount of at least 85% by
weight of the
block.
Suitably, the lubricating oil composition according to all aspects of the
present
invention comprises one or more star polymer viscosity modifier. The
lubricating oil
composition according to all aspects of the present invention may comprise a
mixture of
linear and star polymer viscosity modifiers. In a preferred embodiment, the
lubricating oil
composition according to all aspects of the present invention comprises only
star polymer
.. viscosity modifier(s).
Oil-soluble viscosity modifying polymers generally have weight average
molecular
weights of from 10,000 to 1,000,000, preferably 20,000 to 500,000, which may
be determined
by gel permeation chromatography or by light scattering.
Anti-oxidants, sometimes referred to as oxidation inhibitors, increase the
resistance
of the composition to oxidation and may work by combining with and modifying
peroxides
to render them harmless, by decomposing peroxides, or by rendering oxidation
catalysts inert.
Oxidative deterioration can be evidenced by sludge in the lubricant, varnish-
like deposits on
the metal surfaces, and by viscosity growth.
Examples of suitable antioxidants are selected from copper-containing
antioxidants,
sulfur-containing antioxidants, aromatic amine-containing antioxidants,
hindered phenolic
antioxidants and dithiophosphates derivative. Preferred anti-oxidants ashless
antioxidants.
Preferred ashless antioxidants are ashless aromatic amine-containing
antioxidants, ashless
hindered phenolic antioxidants and mixtures thereof In a preferred embodiment,
one or more
antioxidant is present in a lubricating oil composition of all aspects of the
present invention.
Rust inhibitors selected from the group consisting of nonionic polyoxyalkylene
polyols and esters thereof, polyoxyalkylene phenols, and anionic alkyl
sulfonic acids may be
used.
Copper and lead bearing corrosion inhibitors may be used in some embodiments
of
the invention, and when these compounds are included in the lubricating
composition, they
are preferably present in an amount not exceeding 0.2 wt. % active ingredient.
However, in
28
CA 3012205 2018-07-24
a preferred embodiment of the present invention, no copper-containing
additives are present
in the lubricating oil composition. When present, suitable such compounds are
the thiadiazole
polysulfides containing from 5 to 50 carbon atoms, their derivatives and
polymers thereof
Derivatives of 1, 3, 4 thiadiazoles such as those described in U.S. Patent
Nos. 2,719,125;
2,719,126; and 3,087,932; are typical. Other similar materials are described
in U.S. Patent
Nos. 3,821,236; 3,904,537; 4,097,387; 4,107,059; 4,136,043; 4,188,299; and
4,193,882.
Other additives are the thio and polythio sulfenamides of thiadiazoles such as
those described
in UK Patent Specification No. 1,560,830. Benzotriazoles derivatives also fall
within this
class of additives.
A small amount of a demulsifying component may be used. A preferred
demulsifying
component is described in EP 330522. It is obtained by reacting an alkylene
oxide with an
adduct obtained by reacting a bis-epoxide with a polyhydric alcohol. The
demulsifier should
be used at a level not exceeding 0.1 mass % active ingredient. A treat rate of
0.001 to 0.05
mass % active ingredient is convenient.
Pour point depressants, otherwise known as lube oil flow improvers, lower the
minimum temperature at which the fluid will flow or can be poured. Such
additives are well
known. Typical of those additives which improve the low temperature fluidity
of the fluid
are C8 to Cis dialkyl fumarate/vinyl acetate copolymers,
polyalkylmethacrylates and the like.
Foam control can be provided by many compounds including an antifoamant of the
polysiloxane type, for example, silicone oil or polydimethyl siloxane.
The individual additives may be incorporated into a base stock in any
convenient way.
Thus, each of the components can be added directly to the base stock or base
oil blend by
dispersing or dissolving it in the base stock or base oil blend at the desired
level of
concentration. Such blending may occur at ambient or elevated temperatures.
Preferably, all the additives except for the viscosity modifier and the pour
point
depressant are blended into a concentrate or additive package described herein
as the additive
package that is subsequently blended into base stock to make the finished
lubricant. The
concentrate will typically be formulated to contain the additive(s) in proper
amounts to
provide the desired concentration in the final formulation when the
concentrate is combined
with a predetermined amount of a base lubricant.
29
CA 3012205 2018-07-24
The concentrate is preferably made in accordance with the method described in
US
4,938,880. That patent describes making a pre-mix of ashless dispersant and
metal detergents
that is pre-blended at a temperature of at least about 100 C. Thereafter, the
pre-mix is cooled
to at least 85 C and the additional components are added.
The final crankcase lubricating oil formulation may employ from 2 to 20,
preferably
4 to 18, and most preferably 5 to 17, mass % of the concentrate or additive
package with the
remainder being base stock.
The lubricating oil composition of the present invention may have a sulphated
ash
content of less than or equal to 1.2, preferably less than or equal to 1.1,
more preferably less
than or equal to 1.0, mass % (ASTM D874) based on the total mass of the
composition. The
lubricating oil composition of the present invention suitably has a sulphated
ash content of at
least 0.4, preferably at least 0.5, such as at least 0.6 mass % (ASTM D874)
based on the total
mass of the composition. Suitably the sulphated ash content of the lubricating
oil
composition is in the range of 0.04-1.2 mass%, preferably in the range of 0.06
to 1.0 mass%
(ASTM D874).
The amount of sulfur in the lubricating oil composition will depend upon the
particular application of the lubricating oil composition. The lubricating oil
composition may
contain sulphur in an amount of up to 0.4, such as, up to 0.35 mass % sulphur
(ASTM D2622)
based on the total mass of the composition. Generally the lubricating oil
composition will
contain at least 0.1, or even at least 0.2 mass% sulphur (ASTM D2622) based on
the total
mass of the composition.
Suitably, the lubricating oil composition of all aspects and embodiments of
the present
invention may have a total base number (TBN), as measured in accordance with
ASTM
D2896, of 4 to 15, preferably 4 to 10 mg KOH/g.
EXAMPLES
The invention will now be described in the following examples which are not
intended
to limit the scope of the claims hereof.
CA 3012205 2018-07-24
A series of 10W-30 oils as set out in Table 1 were blended. These oils were
subjected
to a variety of testing, as set out in Examples 1 and 2 below. The test
methods used are
described here.
The High Frequency Reciprocating Rig (HFRR¨ supplied by PCS Instruments) to
evaluate the boundary regime friction characteristics of the oils.
The rig was set up with a 6mm ball on a lOmm disc. The test protocol employed
was
as follows:
Test Duration (mins) 1 min hold and 5 min run at each temperature stage
Test Load (N) 4
Frequency (Hz) 40
Stroke Length 1,000
(microns)
Temperature ( C) 40, 60, 80, 100, 120, 140 (low temperature stage)
160, 180, 200, 220 (high temperature stage)
The test has 6 stages in the low temperature runs and 4 stages in the high
temperature
runs. The average friction at each temperature stage is measured and the
overall average
friction across all stages.
The JASO T 903:2016 clutch friction test measures clutch friction based on the
SAE
#2 test rig. The test runs at 3600rpm and duration of 1000 cycles of
engagement and
disengagement of the friction test plates. The friction coefficient of the
test oil is measured
in the test cycles. The test generates three different friction indices namely
the Static Friction
Index (SFI), Dynamic Friction Index (DFI) and Stop Time Index (SFI). These
indices are
calculated based on the friction coefficients of the test oil against two
standard reference oils.
These three indices will determine the classification of the JASO friction
performance to
MA2, MA1, MA or MB based on the limits specified in the JASO T 903:2016
specification.
The limits for each classification are set out below:
31
CA 3012205 2018-07-24
MB MA MA1 MA2
DFI 20.40 and <1.35 >1.35 and <2.5 21.35 and <1.50 >1.50 and
<2.5
SFI 20.40 and <1.45 21.45 and <2.5 21.45 and <1.60 21.60 and
<2.5
STI >0.40 and <1.40 >1.40 and <2.5 >1.40 and <1.60 >1.60 and
<2.5
The mini traction machine (MTM) supplied by PCS Instruments, is a computer
controlled traction and wear measurement instrument which provides controlled
traction
mapping of fluids. It measures the friction coefficient between a rotating
ball on a rotating
disk at variable entrainment speed. Contact was formed between 3/4 inch ball
mounted on a
pivoting shaft, which is automatically loaded against a rotating 46mm diameter
disc
horizontally mounted in the test fluid reservoir. Variation of the entrainment
speed simulates
variation in the thickness of the lubricating oil film between the surfaces.
As the MTM does
not incorporate reciprocating motion, it generally correlates with the mixed
and
hydrodynamic lubrication regimes that are typical in bearings, pump and piston
rings. The
test was run at four temperatures, 60 C, 80 C, 100 C and 145 C.
Test Duration (mins) 1 min hold and 5 min run at each temperature
stage
Test Load (N) 400
Frequency (Hz) 50
Stroke Length 3,000
(microns)
Temperature ( C) 60, 80, 100, 120, 140, 160
The Schwingung Reibung Verschleiss "SRV", supplied by Optimol, is used to
evaluate friction and wear properties of liquid lubricants across a broad
range of applications.
There are different specimens and configurations that can be used in SRV; in
these examples
the rig was set up with a 15x22mm cylinder on a 24x7.9mm disk. The test has 6
temperature
stages and you can record the average friction at each temperature stage and
the overall
average friction across all stages. The test protocol employed was as follows:
32
CA 3012205 2018-07-24
The test oil forms a film in between the cylinder and disk, the cylinder is
engaged in
a sliding or reciprocating stroke across the disk and friction between the
metal-metal contact
is measured. This is used to evaluate the boundary regime friction
characteristics of the oils.
33
CA 3012205 2018-07-24
n Table 1
w
0 Additive Oil 1 Oil 2 Oil 3 Oil
4 Oil 5 Oil 6 Oil 7 Oil 8 Oil 9
I-
I'.)
._
r.)
o Additive Package ' 7.09
7.09 7.09 7.09 7.09 7.09 7.09 7.09 7.096
co
_
Molybdenum 0.23
0.11 0.11 0.045 0.045
r.)
o
Dithiocarbamate2 .
_
1-.
co Ashless Friction Modifier
0.25
' 13
o .
,i
1 Ashless Friction Modifier 0.5
0.25
r.) 24
al. . .
. Ashless Friction Modifier 0.5
Viscosity modifier' 4.5 4.5 4.5 4.5
4.5 4.5 4.5 4.5 . 4.5
_
Group 11 base oil Balance Balance Balance Balance
Balance Balance Balance Balance Balance
_ _
_
-
SASH, mass% 0.820 0.820 0.820 0.820
' 0.820 0.820 0.820 0.820 - 0.820
(ASTM D874)
.
P, mass% 0.093 0.093 0.093 0.093
0.093 0.093 0.093 0.093 0.093
t.,..) (ASTM D5185) .
-i.
B, ppm (ASTM D5185) 100 100 100 100
100 100 100 100 100
Mo, ppm (ASTM D5185) 0 275 0 0 0
137 , 137 55 55
TBN (ASTM D2896) 6.63 6.73 7.07 6.63
6.63 6.68 , 6.90 6.65 6.61
N, mass% (ASTM D5291) 0.064 0.069 , 0.075 0.064
0.064 0.066 , 0.072 0.065 0.065
Ca, mass % (ASTM 0.184 0.184 0.184 0.184
0.184 0.184 0.184 0.184 0.184
D5185)
.
5, mass% (ASTM D2622) 0.249 0.299 0.249 0.249
0.249 0.274 0.274 0.259 0.259
' The additive package had the same composition for all oils in Table 1 and
comprised a dispersant combination comprising non-borated and borated
polyisobutenyl succinimide dispersant, a
calcium sulphonate dertergent, a combination of hindered phenol and aromatic
amine antioxidants, zinc dialkyldithiophosphate, silicone antifoam, pour point
depressant and diluent oil.
2 The molybdenum dithiocarbamate was a trimeric molybdenum dithiocarbamate
additive available from Infineum UK Limited as Infineum C94558.
3 Ashless friction modifier 1 was glycerol monooleate.
-3 Ashless friction modifier 2 was a tallow ester of triethanol amine.
5 Ashless friction modifier 3 was octadecylsuccinic anhydride.
6 The "chloro" non-borated dispersant in the additive package of Oils 1-8 was
replaced in the additive package of Oil 9 by "thermal" non-borated dispersant,
at equivalent nitrogen content.
7 The viscosity modifier was a hydrogenated styrene-diene star polymer.
Example 1
Each of Oils 1-7 were tested in the HFRR and the average coefficient at each
different
temperature is set out in Table 2 below:
Table 2
Temperature, C Oil 1 Oil 2 Oil 3 Oil 4 Oil 5 Oil 6
Oil 7
40 0.122 0.117 0.120 0.117 0.123 0.119
0.122
60 0.135 0.124 0.132 0.125 0.136 0.125
0.138
80 0.153 0.128 0.146 0.132 0.144 0.144
0.148
100 0.158 0.113 0.150 0.138 0.148 0.136
0.127
120 0.161 0.122 0.154 0.145 0.154 0.135
0.115
140 0.162 0.122 0.157 0.149 0.155 0.131
0.118
160 0.162 0.096 0.140 0.140 0.136 0.125
0.090
180 0.159 0.099 0.138 0.141 0.134 0.130
0.063
200 0.158 0.100 0.136 0.139 0.140 0.126
0.064
220 0.156 0.095 0.141 0.145 0.144 0.124
0.078
This data is also graphically represented in Fig. 1.
Each of Oils 1-7 were also tested in the SRV and the average coefficient at
each
different temperature is set out in Table 3 below:
Table 3
Temperature, C Oil 1 Oil 2 Oil 3 Oil 4 Oil 5 Oil 6
Oil 7
60 0.170 - 0.082 0.161 0.162 0.160
0.134 0.135
80 0.165
0.090 0.161 0.163 0.160 0.147 0.152
100 0.167
0.125 0.162 0.165 0.161 0.149 0.154
120 0.171 0.135 0.161 0.167 0.164 0.151
0.153
140 0.173 0.127 0.161 0.166 0.167 0.151
0.150
160 0.171 0.111 0.167 0.164 0.169 0.147
0.135
This data is also graphically represented in Fig. 2
It can be seen from the data that as expected the oil with 0.23 mass%
molybdenum
dithiocarbamate has generally the lowest coefficient of friction and this
reduces as the
CA 3012205 2018-07-24
temperature increases. Halving the amount of molybdenum dithiocarbamate in Oil
6
increases the friction coefficient at higher temperature. Oils 3, 4 and 5 with
ashless friction
modifier and no molybdenum compound generally have a higher friction
coefficient than the
molybdenum-containing oils, though still perform better than the reference Oil
1, which
contains neither ashless friction modifier nor molybdenum compound. It is
noted that Oil 7,
which contains half the amount of molybdenum dithiocarbamate and half the
amount of
ashless friction modifier 2 also performs well compared with Oil 2 with the
higher
molybdenum content.
Oils 6, 7 and 8 were tested in the JASO T 903:2016. Oil 8, which comprises a
lower
content of molybdenum achieved an MA2 rating, which is the highest friction
level that can
be achieved in this test and is desirable for good clutch operation. Oil 6,
which has a higher
molybdenum content achieved only an MB rating, which is the lowest rating in
this test and
not desirable for good functioning of a clutch assembly. These results are to
be expected,
since it is known that molybdenum compounds, especially molybdenum
dithiocarbamates,
are effective friction modifiers and at higher treat rates provide too much
friction reduction
for acceptable functioning of a clutch assembly.
Oil 7 though, which comprises the same higher molybdenum content as Oil 6, but
additionally contains ashless friction modifier, achieves an MA1 rating. This
is a good
operational rating for a clutch assembly.
It can be seen from these results that use of the ashless friction modifier in
combination with the molybdenum additive allows the use of higher treat rates
of the
molybdenum additive, which is beneficial for the engine lubrication, whilst
still maintaining
a clutch friction that is acceptable in use.
Thus, it can be seen from both the HFRR, SRV data and the JASO T 903:2016
data,
that a combination of oil-soluble molybdenum compound and ashless organic
friction
modifier can be used in a lubricating oil composition provided to lubricate
the engine
crankcase and the clutch assembly from a common sump and provide a good
coefficient of
friction in the engine whilst maintaining acceptable friction in the clutch
assembly.
36
CA 3012205 2018-07-24
Example 2
Oils 8 and 9, which differed only in the type of non-borated dispersant, were
tested
in the MTM. Figs. 3-6 show that Oil 9 with the "thermal" dispersant in place
of the
"chloro" dispersant exhibits reduced friction coefficient, which reduction is
particularly
marked at higher temperature.
Example 3
Three further oils, Oils 9-11, were blended and tested in the HFRR, SRV and
JASO
T 903:2016. The oils were compared to a commercial 10W-30 oil, composition
unknown,
which oil was measured as having an MA2 qualification in the JASO T 903:2016.
The composition of Oils 9-11 is set out in Table 4 below, the amounts being
mass%
active matter.
Table 4
Additive Oil 9 Oil 10 Oil 11
Additive Package' 7.9 7.9 7.9
Ashless Friction Modifier 24 0.2 0.2 0.2
Molybdenum Dithiocarbamate2 0.045 0.045 0.045
Viscosity Modifier' 11 9.5 15.3
Group II base oil Balance
Group III base oil Balance Balance
SAE Viscosity Grade 5W-30 10W-30 10W-40
SASH, mass %, (ASTM D874) 0.688 0.688 0.688
P, ppm (ASTM D5185) 0.086 0.086 0.086
Mo, ppm (ASTM D5185) 55 55 55
S, mass% (ASTM D2622) 0.198 0.198 0.198
N, mass% (ASTM D5291) 0.08 0.08 0.08
B, ppm (ASTM D5185) 86 86 86
Ca, mass% (ASTM D5185) 0.148 0.148 0.148
TBN, (ASTM D2896) 6.59 6.59 6.59
8 The additive package was the same for each of Oils 9 to 11 and contained a
combination of borated and non-borated
polyisobutenylsuccinimide dispersants, calcium salicylate detergent, ZDDP,
aromatic amine and hindered phenol antioxidant, silicone
antifoam PPD and diluent oil.
37
CA 3012205 2018-07-24
2 The molybdenum dithiocarbamate was a trimeric molybdenum dithiocarbamate
additive available from Infineum UK Limited as Infineum
C9455B.
4 Ashless friction modifier 2 was a tallow ester of triethanol amine.
7 The viscosity modifier was a hydrogenated styrene-diene star polymer.
Oils 9 to 11 vary only in the amount of viscosity modifier and the base oil,
in order
to obtain the different SAE viscosity grades indicated.
The reference oil and each of Oils 9-11 where tested in the HFRR and the
average
coefficient at each different temperature is set out in Table 5 below:
Table 5
Temp ( C) Reference Oil 9 Oil 10 , Oil 11
40 0.126 0.115 , 0.112 0.116
60 0.1335 0.124 0.119 0.125
80 0.154 0.143 0.138 0.144
100 0.1635 0.143 0.1395 0.1465
120 0.165 0.129 0.1305 0.139
140 0.166 0.101 0.0975 0.1335
160 0.157 , 0.148 0.150 0.147
180 0.158 0.133 0.136 0.144
200 0.157 0.091 0.093 0.097
220 0.160 0.086 0.089 0.089
The results are also represented graphically below, in Fig. 7.
The reference oil and each of Oils 9-11 where tested in the SRV and the
average
coefficient at each different temperature is set out in Table 6 below:
Table 6
Temp ( C) Reference Oil 9 Oil 10 Oil 11
60 0.164 0.160 0.160 0.159
80 0.159 0.156 0.156 0.154
100 0.163 0.158 0.158 0.157
120 0.168 0.160 0.160 0.159
140 0.172 0.158 0.159 0.159
160 0.174 0.148 0.147 0.152
38
CA 3012205 2018-07-24
The results are also represented graphically below, in Fig. 8.
It can be seen from the SRV data, that all of Oil 9-11 exhibited improved
friction
performance in the SRV compared to the commercial reference oil.
Oil 10 was also tested in the JASO T 903:2016 clutch friction test and found
to have
a MA2 performance level. Thus, Oil 10 exhibits comparable clutch friction
performance to
the commercial reference oil, but improved engine friction performance in the
SRV.
39
CA 3012205 2018-07-24