Note: Descriptions are shown in the official language in which they were submitted.
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
WELL TREATMENT METHODS AND COMPOSITIONS
Field of the Invention
[0001] The present invention relates to methods and compositions for use
in subterranean
operations to restore the permeability of a wellbore or an underground
formation near a wellbore
that has become impeded by sludge or tar deposits.
Background of the Invention
[0002] Hydraulic fracturing, also known as hydro-fracking or fracking, is
used by the
petroleum industry to stimulate production of oil or gas from a well.
Hydrocarbons such as oil
and natural gas can be obtained from subterranean hydrocarbon bearing
geological formations by
pumping a pressurized fluid into a well to create a fracture in the formation
or to enlarge a pre-
existing fracture in the formation. In a procedure known as slick water
fracturing,
polyacrylamide polymers are commonly used to reduce pumping pressure by
reducing the
frictional drag of the water against the well inner tubular walls, the
interface of the well tube and
geological formation at the puncture points of the well tube, and the
penetrated crevices formed
by fracturing. Such fracturing fluids have a high viscosity.
[0003] The polymers used in slick water fracturing often persist within
the producing
formation. The polymer residue can plug the permeability of the rock and
hinder recovery of the
water used in the fracturing treatment. The polymer residue also combines with
heavy oil tar by-
products. The resulting polymer/tar residue sludge deposits accumulate in
production tubing and
equipment. The sludge deposits can form a filter cake at the injector near
wellbore, resulting in
damage to the injectors, loss of injectivity, and a loss of capacity once the
well is put into
production. The polymer/tar residue can plug the permeability of the rock and
hinder recovery of
the water used in the fracturing treatment. In addition, the residue can foul
surface equipment
such as solids screening equipment. The frequent cleaning needed to remove the
accumulated
deposits is time-consuming and reduces the efficient recovery of petroleum
products.
[0004] Strategies to mitigate polymer persistence include the use of
oxidizers producing
free radicals to break down the viscosity of polyacrylamide in the slick
water. However, these
1
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
approaches were generally developed for the use under conditions of high
temperatures and/or
acidic environments. Many wells are located in cold climates. Slick water
typically has a
neutral to alkaline pH. Strategies for removal of wellbore tar incursion have
relied generally on
mechanical methods, such as increased hydrostatic pressure in the wellbore, or
chemical
methods, based on solubilizers or dispersants. These approaches typically do
not mitigate
polymer persistence. There is a continuing need for methods of efficient
methods of well
cleaning that are effective under field conditions.
Summary of the Invention
[0005] Provided herein are methods and compositions for treating a
wellbore or
underground formation in order to restore the permeability of a wellbore or a
formation that has
become impeded by sludge or tar deposits. The method can include the steps of
introducing an
aqueous composition comprising an oxidizer, a chelated metal activator, and a
surfactant into the
wellbore, wherein the wellbore comprises one or more sludge deposits on the
wellbore, and
contacting the sludge deposits with the aqueous composition. In some
embodiments, the
oxidizer can be hydrogen peroxide, carbamide peroxide, peracetic acid, sodium
persulfate or
potassium persulfate. In some embodiments, the chelated metal activator can be
FeEDTA,
CuEDTA, FeDTPA, ferric citrate, ferrous citrate, ferrous isocitrate, ferrous
aconitate, ferrous
salicylate, zinc gluconate, copper citrate, ferrous lactate, or ferrous
gluconate. In some
embodiments the surfactant can be ethoxylated castor oil, ethoxylated fatty
acids, D-limonene,
alcohol sulfates, alcohol ethoxylates, amine N-oxides or a combination
thereof. The sludge
deposit comprises a synthetic polymer and an oil tar or oil tar byproduct.
Brief Description of the Drawings
[0006] Figure lA is a graph depicting the results of an experiment
analyzing the effect of
500 ppm of sodium hypochlorite (NaC10) on the viscosity of cross-linked
partially hydrolyzed
polyacrylamide gel. Figure 1B is a graph depicting the results of an
experiment analyzing the
effect of 5000 ppm of sodium hypochlorite (NaC10) on the viscosity of cross-
linked partially
hydrolyzed polyacrylamide gel.
[0007] Figure 2A is a graph depicting the results of an experiment
analyzing the effect of
580 ppm of hydrogen peroxide activated by FeSO4 on the viscosity of cross-
linked partially
2
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
hydrolyzed polyacrylamide gel. Figure 2B is a graph depicting the results of
an experiment
analyzing the effect of 5015 ppm of hydrogen peroxide activated by FeSO4on the
viscosity of
cross-linked partially hydrolyzed polyacrylamide gel.
[0008] Figure 3 is a graph depicting the results of an experiment
analyzing the effect of
pH reduction to 5.0 on the effect of 5015 ppm of hydrogen peroxide activated
by FeSO4on the
viscosity of cross-linked partially hydrolyzed polyacrylamide gel.
[0009] Figure 4 is a graph depicting the results of an experiment
analyzing the effect of
1000 ppm of hydrogen peroxide activated by 340 ppm FeEDTA on the viscosity of
cross-linked
partially hydrolyzed polyacrylamide gel.
[0010] Figure 5 is a graph depicting the results of an experiment
analyzing the effect of
2000 ppm of urea peroxide activated by 1315 ppm FeEDTA on the viscosity of
cross-linked
partially hydrolyzed polyacrylamide gel.
[0011] Figure 6 is a graph depicting the results of an experiment
analyzing the effect of
2000 ppm of urea peroxide activated by 130 ppm FeEDTA on the viscosity of
cross-linked
partially hydrolyzed polyacrylamide gel.
[0012] Figure 7 is a graph depicting the results of an experiment
analyzing the effect of
1000 PPM of hydrogen peroxide activated by 50 PPM FeEDTA on the viscosity of
slick water.
Detailed Description
[0013] This description of preferred embodiments is intended to be read
in connection
with the accompanying drawings, which are to be considered part of the entire
written
description of this invention. The drawing figures are not necessarily to
scale and certain
features of the invention may be shown exaggerated in scale or in somewhat
schematic form in
the interest of clarity and conciseness. In the description, relative terms
such as "horizontal,"
"vertical," "up," "down," "top" and "bottom" as well as derivatives thereof
(e.g., "horizontally,"
"downwardly," "upwardly," etc.) should be construed to refer to the
orientation as then described
or as shown in the drawing figure under discussion. These relative terms are
for convenience of
description and normally are not intended to require a particular orientation.
Terms including
3
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
"inwardly" versus "outwardly," "longitudinal" versus "lateral" and the like
are to be interpreted
relative to one another or relative to an axis of elongation, or an axis or
center of rotation, as
appropriate. Terms concerning attachments, coupling and the like, such as
"connected" and
"interconnected," refer to a relationship wherein structures are secured or
attached to one another
either directly or indirectly through intervening structures, as well as both
movable or rigid
attachments or relationships, unless expressly described otherwise. The term
"operatively
connected" is such an attachment, coupling or connection that allows the
pertinent structures to
operate as intended by virtue of that relationship. When only a single machine
is illustrated, the
term "machine" shall also be taken to include any collection of machines that
individually or
jointly execute a set (or multiple sets) of instructions to perform any one or
more of the
methodologies discussed herein. In the claims, means-plus-function clauses, if
used, are intended
to cover the structures described, suggested, or rendered obvious by the
written description or
drawings for performing the recited function, including not only structural
equivalents but also
equivalent structures.
[0014] The present invention is directed to methods and compositions for
treating a
wellbore or an underground formation in order to restore the permeability of
the wellbore or
formation that has become impeded by sludge or tar deposits. Agents for
reducing viscosity in
polymer-containing well treatment fluids are typically referred to as
breakers. The inventors
have found that treatment of cross-linked partially hydrolyzed polyacrylamide
gel with peroxide
that had been activated by a chelated metal compound resulted in a rapid
decrease in viscosity of
the gel. Moreover, the treatment was effective at low temperatures (<120 F)
and the relatively
high pH commonly found in slick water. The inventors have also found that the
combination of
viscosity reducing agents with a surfactant effectively solubilized HPAM-
containing sludge.
[0015] The methods disclosed herein are generally useful for treatment of
a wellbore or
formation in order to restore the permeability of the wellbore that has become
impeded by
synthetic polymer -containing sludge or tar deposits. The methods generally
include reducing
the viscosity of a polymer-containing aqueous well treatment fluid in a
subterranean
environment, oxidizing or decomposing the gelled polymer sludge, and
solubilizing the water
insoluble tar deposits. The methods can include introducing an oxidizer, a
chelated metal
activator, and a surfactant into the polymer-containing aqueous well treatment
fluid to form a
4
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
well treatment composition. The concentration of the oxidizer, the chelated
metal activator, and
the surfactant can vary. Regardless of the specific concentrations of
oxidizer, chelated metal
activator, and surfactant in the well treatment composition, the well
treatment composition is
directed into the subterranean environment in an amount and for a time
sufficient to reduce the
viscosity of the polymer-containing aqueous well treatment fluid and
solubilize the tar deposits.
The methods can be used in a hydraulic fracturing operation to break down or
defragment
polyacrylamide and thus to decompose a polyacrylamide containing sludge or
filter cake formed
during operation of a well. In some embodiments, the subterranean environment
can be a
geological formation that has been penetrated by a wellbore.
[0016] Even though the polymers used in slick water fracturing are water
soluble, they
can become deposited in the sand or rock formation adjacent the wellbore,
reducing permeability
of the formation. The polymer molecules are typically too large to penetrate
the permeable
matrix of the formation. The polymer residue can combine with heavy oil tar by-
products,
forming a tacky substance that can readily adhere to any surface that it
contacts, including the
surfaces of the well bore and/or any equipment utilized during the drilling
operation. This
polymer residue/tar sludge can also incorporate soil and rock solids
(including, but not limited to
sand) into a persistent amalgam. The compositions of the invention can be
injected into a
wellbore to contact sludge deposits on surfaces of the wellbore, and drilling
equipment as well as
formations near the wellbore. Exposure of the sludge deposits to the
compositions of the
invention can provide dissolution and dispersal of the sludge deposits in
situ.
[0017] The oil tar that combines with synthetic polymers used in drilling
operations is
typically a mixture of hydrocarbons, for example, nonpolar hydrocarbons,
asphaltenes and
parrafins. We may refer to the resulting mixture as a sludge, a deposit, and
amalgam or a
residue. Filter cake generally refers to deposits of such insoluble material
left on filters or other
drilling equipment and which can substantially impede the flow of liquid.
Slick water generally
refers to an aqueous solution containing a friction reducing polymer, for
example, acrylamide. In
some embodiments, slick water can include a breaker.
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
Well Treatment Fluids and Polymers
[0018] Hydraulic fracturing typically relies on solid particulate
material, for example
inorganic solids like sand, silica, quartz, diatomaceous earth, in coated or
uncoated form, or
gravel, to keep fractures open and provide improved fluid conductivity through
the matrix. In
general, the viscosity modifying polymers increase the ability of the well
treatment fluid to retain
particulates in suspension. Viscosity-modifying polymers or copolymers used in
well treatment
fluids function can increase the fluid viscosity or promote a thickened or
viscous or semi-gelled
or gelled state in the aqueous well treatment fluid.
[0019] The methods and compositions disclosed herein can be used with
polymer- or
copolymer-containing aqueous well treatment fluids that are typically used in
subterranean oil-
and gas-field well operations, for example, well drilling, formation
fracturing, productivity
enhancement, and secondary recovery. Such aqueous well treatment fluids
typically contain one
or more viscosity-modifying polymers or copolymers.
[0020] Viscosity modifying polymers and copolymers generally function as
viscosity
enhancers (as a thickener or gelling agent). Some polymers and copolymers may
alternatively
function as flowing friction reducers. This functionality, viscosity
enhancement or flowing
friction reduction, is often concentration dependent. As a general rule,
higher concentrations of a
(dual function) viscosity-modifying polymer/copolymer provide viscosity-
enhancement in an
aqueous well treatment fluid, but low concentrations of the same
polymer/copolymer provide
flowing friction-reduction functionality.
[0021] Viscosity-enhancing polymers are typically used in amounts of
about 0.01 to
about 10 wt %, or about 0.1 to about 5 wt %, based on the weight of the
aqueous fluid. A
viscosity-enhanced well fluid can include an amount of viscosity-enhancing
polymer sufficient
to provide a fluid viscosity in excess of at least about 20 cP, at least about
25 cP, at least about
30 cP, at least about 35 cP, at least about 40 cP, at least about 45 cP, at
least about 50 cP, at least
about 55 cP, at least about 60 cP, at least about 65 cP, at least about 70 cP,
at least about 75 cP,
at least about 80 cP, at least about 85 cP, at least about 90 cP, at least
about 95 cP, at least about
100 cP or more.
6
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
[0022] The viscosity-modifying polymer can be a natural polymers,
including modified
forms of natural polymers, or a synthetic polymer, including synthetic
polymers and copolymers
and their derivatives. The viscosity-modifying polymers can be water-soluble
at the
concentrations employed in the aqueous well treatment fluids.
[0023] Viscosity-modifying polymers that can increase the fluid viscosity
include
synthetic polymers such as acrylamide-derived polymers and copolymers and
acrylate-derived
polymers and copolymers, often in crosslinked form. Synthetic polymers used in
slick water
fracturing include polyacrylamide, polymers containing acrylamide monomer, and
adducts
thereof such as partially hydrolyzed acrylamide or alkoxylated acrylamide.
These polymers
range in molecular weight from about 500,000 to about 10,000,000 or more.
[0024] Acrylamide-derived polymers can include, for example,
polyacrylamide,
acrylamide-acrylate (acrylic acid) copolymers, acrylic acid-methacrylamide
copolymers,
partially hydrolyzed polyacrylamide copolymers (PHPA), partially hydrolyzed
polymethacrylamides, and acrylamide-methyl-propane sulfonate copolymers
(AMPS). Cross-
linked acrylamide-based polymers that exhibit viscosity-enhancing
functionality have been
described in U.S. Pat. No. 4,995,461 of Sydansk (Marathon Oil) and in U.S.
Pat. No. 5,268,112
of Hutchins et al. (Union Oil of California).
[0025] Other viscosity-enhancing polymers include natural and synthetic
water-soluble
polysaccharides, including guar and guar derivatives such as hydroxypropyl
guar and
carboxymethyl hydroxypropyl guar; xanthan and xanthan derivatives; alginates
and alginate
derivatives; carrageenan; cellulosic polymers and cellulosic derivatives such
as
hydroxyethylcellulo se, hydroxypropylcellulose and
carboxymethylhydroxyethylcellulose; and
other biopolymers or synthetic polymers or copolymers that exhibit gelling or
viscosity-
enhancing functionality, and any combination thereof.
[0026] The polymers may either be linear (non-crosslinked) or
crosslinked, e.g., using
cross-linking agents such as borate or zirconate or titanate in the case of
polysaccharides like
guar, or other known crosslinkers in the case of synthetic polymers and
copolymers like
acrylamide-derived polymers and copolymers.
7
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
[0027] Crosslinked polymers, such as crosslinked hydroxypropyl guar
(HPG), provide
the advantage of high fluid viscosities at lower usage rates, as compared with
non-crosslinked or
linear polymers/copolymers. Such crosslinked polymers can provide viscous
fluid
characteristics even at high fluid temperatures and are generally superior to
linear polymers or
copolymers in their proppant suspension capabilities.
[0028] Polymers and copolymers may also function to reduce the flowing
friction, reduce
flow turbulence, or to improve flow characteristics of the aqueous well
treatment fluid. Such
polymers are used in amounts that provide flowing friction-reducing
functionality in the aqueous
fluid. Flowing polymers are typically used in relatively small amounts of
about 0.01 to about 1
wt %, or about 0.05 to about 0.5 wt %, based on the weight of the aqueous
fluid. A friction-
reduced well fluid typically contains an amount of flowing friction-reducing
polymer sufficient
to provide a fluid viscosity of about 10 cP, about 9 cP, about 8 cP, about 7
cP, about 6 cP, about
cP, about 4 cP, about 3 cP, about 2 cP or less. (Pure water has a viscosity of
about 1 cP).
[0029] Viscosity-reducing polymers that can serve as friction reducers
include
acrylamide-derived polymers and copolymers, such as polyacrylamide (sometime
abbreviated as
PAM), acrylamide-acrylate (acrylic acid) copolymers, acrylic acid-
methacrylamide copolymers,
partially hydrolyzed polyacrylamide copolymers (PHPA), partially hydrolyzed
polymethacrylamides, and acrylamide-methyl-propane sulfonate copolymers
(AMPS). Various
derivatives of such polymers and copolymers, e.g., quaternary amine salts,
hydrolyzed versions,
are also within the scope of the polymers and copolymers disclosed herein.
[0030] Commercial acrylamide-based polymer products that have friction-
reducing
functionality include, for example, NewDrillTM products (Baker Hughes,
Houston, Tex.), FRW-
friction reducer (BJ Services, Houston, Tex.), and FR56TM friction reducer
(Halliburton,
Houston, Tex.). Acrylamide-based polymers and copolymers have also been
described in the
patent literature for use as friction reducers in oil-field applications such
as well fracturing, e.g.,
U.S. Pat. No. 3,254,719 of Root (Dow Chemical) and U.S. Pat. No. 4,152,274 of
Phillips et al.
(Nalco Chemical).
[0031] Other viscosity-reducing polymers (besides acrylamide-derived
polymers and
copolymers) that can serve as friction reducers include guar and guar
derivatives, acrylate-
8
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
derived polymers and copolymers like polymethylmethacrylate, ethylene oxide-
derived polymers
such as polyethyleneoxide, alkoxylated alkanolamides, and other biopolymers or
synthetic
polymers or copolymers that exhibit viscosity-reducing or friction-reducing
functionality.
[0032] Regardless of the specific polymer that is used and regardless of
whether the
objective is to increase fluid viscosity or reduce friction, the well drilling
fluid can include any
combination of two or more of the polymers listed above, for example, three,
four, five, six, or
more polymers.
[0033] In some embodiments, the well treatment fluids can also contain
additional
additives. Additional well treatment additives can include, for example,
surfactants, scale
preventers, biocides, bacteriocides, stabilizers, corrosion inhibitors, fluid
loss control additives,
permeability modifiers, nanoparticles, or any combination thereof.
Oxidizers
[0034] The methods and compositions disclosed herein include an oxidizer.
The
oxidizer or oxidizing agent can reduce the molecular weight of the friction-
reducing polymer.
The fragmented components of the polymer can then be readily removed from the
wellbore, thus
minimizing damage to the formation. The oxidizer can be, for example, hydrogen
peroxide,
carbamide peroxide, peracetic acid, sodium persulfate or potassium persulfate.
[0035] We may refer to a peroxide as a compound containing an oxygen-
oxygen single
bond or the peroxide anion (022). In some embodiments, a peroxide can be
hydrogen peroxide,
e.g., H202. In some embodiments, a peroxide can be a bonded hydrogen peroxide,
for example,
urea-peroxide [(NH2)2C0 = H202] Urea peroxide, also known as carbamide
peroxide, is solid
adduct of one mole of hydrogen peroxide with one mole of urea. Urea peroxide
is a water-
soluble crystalline compound that acts as a source of peroxide. Other
peroxides within the scope
of the invention include bonded or stabilized hydrogen peroxide, such as
sodium perborate or
sodium percarbonate to generate peroxide in situ; organic peroxides, for
example,
peroxycarboxylic acid; peroxyacids, such as peracetic acid or peroxyoctanoic
acid and mixtures
thereof (C2 to Cs) in combination and Caro's acid (H2S05); aliphatic
carboxylic acids, e.g.
9
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
benzoyl peroxide, salicylic acids and phenolic carboxy acids; and salts of
peroxyacids, such as
sodium persulfate, potassium persulfate and potassium caroate.
[0036] Peroxides can be obtained as aqueous stock solutions and diluted
for use.
Aqueous hydrogen peroxide stock solutions can contain at least about 8 wt %
H202, at least
about 15 wt % H202, at least about 20 wt % H202., at least about 27% H202, at
least about 35 wt
% H202. Aqueous hydrogen peroxide stock solutions with these concentrations,
suitable for use
in the invention, are readily available from commercial suppliers as
stabilized H202 solutions.
[0037] Highly concentrated aqueous hydrogen peroxide stock solutions
(significantly
above 50 wt % H202) can also be used. Aqueous H202 stock solutions above about
50 wt %
H202 generally require stringent handling and safety measures. Thus, the
aqueous hydrogen
peroxide stock solutions can have a concentration in the range of about 8 wt %
H202 to about 70
wt % H202, about 15 wt % H202 to about 50 wt % H202, about 25 wt % H202 to
about 40 wt %
H202 Useful stock solutions can have a concentration in the range about 30 wt
% H202 to about
40 wt % H202
[0038] Regardless of the concentration of the aqueous hydrogen peroxide
stock solution,
a sufficient amount of the stock solution is added to the aqueous well
treatment fluid to provide
the desired concentration. In some embodiments, useful concentrations of the
peroxide, for
example hydrogen peroxide or urea peroxide, in the well treatment composition
can be within a
range of about 400 ppm to about 25,000 ppm. Thus, the peroxide concentration
can be about
400 ppm, about 500 ppm, about 550 ppm, about 580 ppm, about 600 ppm, about 650
ppm, about
700 ppm, about 750 ppm, about 800 ppm, about 850 ppm, about 900 ppm, about 950
ppm, about
1000 ppm, about 1500 ppm, about 2000 ppm, about 2500 ppm, about 3000 ppm,
about 3500,
ppm, about 4000 ppm, about 4500 ppm, about 5000 ppm, about 5500 ppm, about
6000 ppm,
about 8000 ppm, about 10,000 ppm, about 15,000 ppm, about 20,000 ppm, or about
25,000 ppm.
In some embodiments, the peroxide concentration can be from about 500 ppm to
about 5000
ppm, from about 500 ppm to about 4000 ppm, from about 500 ppm to about 3500
ppm, from
about 500 ppm to about 3000 ppm, from about 500 ppm to about 2500 ppm, from
about 500 ppm
to about 2000 ppm, from about 500 ppm to about 1000 ppm, from about 1000 ppm
to about 2000
ppm,
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
[0039] In some embodiments, the oxidizer can be a peracetic acid.
Peracetic acid
solutions exist as equilibrium solutions containing peracetic acid, hydrogen
peroxide, acetic acid
and water. Solutions are often identified by the concentration of peracetic
acid and hydrogen
peroxide. For example, a 15/23 formulation contains 15% by weight of peracetic
acid and 23%
by weight hydrogen peroxide. Commercially available peracetic acid solutions
have typical
formulations containing 2-35% peracetic acid and 5-30% hydrogen peroxide, with
the remainder
being acetic acid and water. In some embodiments, useful peracetic acid
concentrations in the
well treatment compositions can be about 500 ppm, about 1000 ppm, about 1500
ppm, about
2000 ppm, about 2500 ppm, about 3000 ppm, about 3500 ppm, about 4000 ppm,
about 4500
ppm, about 5000 ppm, about 7500 ppm, or about 10,000 ppm.
[0040] In some embodiments, the oxidizer can be a persulfate. The
persulfate can be a
mono-or dipersulfate, or a mixture of a mono or dipersulfate. The dipersulfate
can be, for
example sodium persulfate or potassium persulfate. In some embodiments, useful
sodium
persulfate concentrations in the well treatment compositions can be about 500
ppm, about 1000
ppm, about 1500 ppm, about 2000 ppm, about 2500 ppm, about 3000 ppm, about
3500 ppm,
about 4000 ppm, about 4500 ppm, about 5000 ppm, about 7500 ppm, about 10,000
ppm, about
15,000 ppm, about 20,000 ppm or about 25,000 ppm.
Activators
[0041] Peroxides are typically strong oxidizing agents. However, peroxide-
mediated
oxidation of polymers such as polyacrylamide polymers generally takes place
relatively slowly.
Activators, also known as catalytic activators, can be used to convert the
peroxide, for example
hydrogen peroxide, into free radicals, for more efficient oxidation. The
catalytic activation of
hydrogen peroxide involves its dissociation or ionization into free radicals,
which include
hydroxyl (OH-) and hydroperoxyl (also called perhydroxyl) (00H-) radicals.
Exemplary
activation reactions are believed to occur by the cleavage of either an O¨H
bond or an 0-0
bond in the hydrogen peroxide molecule. Decomposition of hydrogen peroxide
also results in
the decomposition products of oxygen gas and water.
[0042] Useful activators should persist in the environment for a
relatively long time
period, efficiently destroy the polymer, and be compatible with the oxidant.
The activator-
11
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
oxidizer combinations should be cost-effective and be efficient in a large
variety of specific site
conditions.
[0043] The inventors have found that chelated metal activators
effectively activated
peroxide to degrade polyacrylamide in polyacrylamide gels. This observation
was unexpected
because the classic Fenton reaction, which involves oxidation of iron by
hydrogen peroxide to
form free radicals, tends to be limited by pH. Fenton's reagent is an aqueous
solution containing
Fe2+ and hydrogen peroxide. Fenton chemistry typically is most effective at
acidic pH of 3 or
below. Slick water often has a pH in the neutral to near neutral basic range,
that is, greater than
6.
[0044] A catalyst can include a divalent and trivalent transition metal
such as Fe (II), FE
(III), Cu (II), Mn (II) and Zn (II). Chelating agents can include
aminopolycarboxylic acids and
salts such as ethylenediamine tetraacetic acid (EDTA), diethylenetriamine
pentaacetic acid
(DTPA), nitrilotriacetic acid (NTA), Hydroxyethylethylenediaminetriacetic acid
(HEDTA) as
well as citric acid, isocitric acid, aconitic acid, ascorbic acid, lactic
acid, gluconic acidõ
phosphonates, and glucoheptonates. Useful chelated metal activators include
ferric
ethylenediamine tetraacetic acid (FeEDTA), ferric diethylenetriamine
pentaacetic acid
(FeDTPA), ferric citrate, ferrous citrate, ferrous isocitrate, ferrous
aconitate, ferrous lactate,
ferrous salicylate, zinc gluconate, copper citrate and
ethylenediaminetetraacetic acid copper(II)
disodium salt (CuEDTA).
[0045] The chelated metal activator is added to the peroxide in a
concentration effective
to activate the peroxide. In some embodiments, useful concentrations of the
chelated metal
activator, for example FeEDTA, in the well treatment composition can be within
a range of
about 50 ppm to about 1000 ppm (as Fe concentration). Thus, the chelated metal
activator
concentration can be about 50 ppm, about 52 ppm, about 55 ppm, about 60 ppm,
about 65 ppm,
about 70 ppm, about 75 ppm, about 80 ppm, about 85 ppm, about 90 ppm, about 95
ppm, about
100 ppm, about 120 ppm, about 150 ppm, about 180 ppm, about 200 ppm, about 250
ppm, about
300 ppm, about 350 ppm, about 400 ppm, about 450 ppm, about 500 ppm, about 550
ppm, about
600 ppm, about 650 ppm, about 700 ppm, about 750 ppm, about 800 ppm, about 850
ppm, about
900 ppm, about 950 ppm, about 1000 ppm. In some embodiments, the chelated
metal activator
12
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
concentration in the well treatment composition can be from about 50 ppm to
about 1000 ppm,
about 50 ppm to about 800 ppm, about 50 ppm to about 750 ppm, about 50 ppm to
about 600
ppm, about 100 ppm to about 1000 ppm, about 200 ppm to about 1000 ppm, about
100 ppm to
about 500 ppm.
[0046] In some embodiments, useful concentrations of the chelated metal
activator, for
example FeEDTA, in the well treatment composition can be within a range of
about 50 ppm to
about 2000 ppm (expressed as total solid amount). Thus, the chelated metal
activator
concentration can be about 50 ppm, about 52 ppm, about 55 ppm, about 60 ppm,
about 65 ppm,
about 70 ppm, about 75 ppm, about 80 ppm, about 85 ppm, about 90 ppm, about 95
ppm, about
100 ppm, about 120 ppm, about 150 ppm, about 180 ppm, about 200 ppm, about 250
ppm, about
300 ppm, about 350 ppm, about 400 ppm, about 450 ppm, about 500 ppm, about 550
ppm, about
600 ppm, about 650 ppm, about 700 ppm, about 750 ppm, about 800 ppm, about 850
ppm, about
900 ppm, about 950 ppm, about 1000 ppm, about 1200 ppm, about 1300 ppm, about
1400 ppm,
about 1500 ppm, about 1600 ppm, about 1800 ppm, or about 2000 ppm. In some
embodiments,
the chelated metal activator concentration in the well treatment composition
can be from about
50 ppm to about 2000 ppm, about 50 ppm to about 800 ppm, about 50 ppm to about
750 ppm,
about 50 ppm to about 600 ppm, about 100 ppm to about 1000 ppm, about 200 ppm
to about
1000 ppm, about 100 ppm to about 1500 ppm, about 100 ppm to about 500 ppm.
Surfactants
[0047] The compositions and methods disclosed herein also contain one or
more
surfactants. The surfactant can be an ionic or a non-ionic surfactant. The non-
ionic surfactant
can be an ethoxylate. Exemplary surfactants include ethoxylated castor oil,
ethoxylated fatty
acids, D-limonene, alcohol sulfates, alcohol ethoxylates, amine N-oxides and
mixtures thereof.
The concentration of surfactant can vary depending upon the specific
surfactant or combination
of surfactants used. Generally the surfactant concentration in the well
treatment compositions
can be from between about 0.05% to about 5.0% wt/vol. The surfactant can be
obtained as a
concentrated stock solution and diluted for use. In some embodiments, useful
surfactant
concentrations in the well treatment compositions can be about 0.05%, about
0.1%, about 0.2%,
about 0.3%, about 0.4%, about 0.5%, about 0.6%, about 0.75%, about 0.8%, about
1.0%, about
13
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
1.2%, about 1.25%, about 1.5%, about 1.75%, about 2.0%, about 2.25%, about
2.5%, about
3.0%, about 3.5%, about 4%, about 4.5%, or about 5%.
Co-solvents
[0048] In some embodiments, the aqueous well treatment fluids can include
or exclude a
co-solvent. Co-solvents are typically added to the wellbore fluid to prevent
its freezing in cold
climates. Exemplary co-solvents include methanol or isopropanol or a mixture
thereof. Co-
solvent concentrations can range from about 1% to about 15%. In some
embodiments, useful co-
solvent concentrations can be about 1%, about 2%, about 3%, about 4%, about
5%, about 6%,
about 7%, about 8%, about 9%, about 10%, about 12%, or about 15%.
pH
[0049] The pH of the polymer-containing aqueous well treatment fluids can
range from
about neutral to alkaline. The pH can be at least about pH 6.0, at least about
pH 6.5, at least
about pH 6.8, at least about pH 7.0, at least about pH 7.1, at least about pH
7.2, at least about pH
7.3, at least about pH 7.4, at least about pH 7.5, at least about pH 7.6, at
least about pH 7.7, at
least about pH 7.8, at least about pH 7.9, at least about pH 8.0, at least
about pH 8.1, at least
about pH 8.2, at least about pH 8.3, at least about pH 8.4, at least about pH
8.5, at least about pH
8.6, at least about pH 8.7, at least about pH 8.8, at least about pH 8.9, at
least about pH 9.0, at
least about pH 9.1, at least about pH 9.2, at least about pH 9.3, at least
about pH 9.4, at least
about pH 9.5, at least about pH 9.6, at least about pH 9.7, at least about pH
9.8, at least about pH
9.9, at least about pH 10.0, at least about pH 10.1, at least about pH 10.2,
at least about pH 10.3,
at least about pH 10.4, at least about pH 10.5, at least about pH 10.6, at
least about pH 10.7, at
least about pH 10.8, at least about pH 10.9, or at least about pH 11Ø In
general, the pH of the
polymer -containing aqueous well treatment fluids will be about pH 12 or less,
although certain
high temperature applications may range up to pH 14.
[0050] The pH of the well treatment compositions of the invention can
range from about
neutral to alkaline. The pH can be at least about pH 6.0, at least about pH
6.5, at least about pH
6.8, at least about pH 7.0, at least about pH 7.1, at least about pH 7.2, at
least about pH 7.3, at
least about pH 7.4, at least about pH 7.5, at least about pH 7.6, at least
about pH 7.7, at least
about pH 7.8, at least about pH 7.9, at least about pH 8.0, at least about pH
8.1, at least about pH
14
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
8.2, at least about pH 8.3, at least about pH 8.4, at least about pH 8.5, at
least about pH 8.6, at
least about pH 8.7, at least about pH 8.8, at least about pH 8.9, at least
about pH 9.0, at least
about pH 9.1, at least about pH 9.2, at least about pH 9.3, at least about pH
9.4, at least about pH
9.5, at least about pH 9.6, at least about pH 9.7, at least about pH 9.8, at
least about pH 9.9, at
least about pH 10.0, at least about pH 10.1, at least about pH 10.2, at least
about pH 10.3, at least
about pH 10.4, at least about pH 10.5, at least about pH 10.6, at least about
pH 10.7, at least
about pH 10.8, at least about pH 10.9, or at least about pH 11Ø
[0051] Adjustment of the fluid pH may or may not be needed with the
aqueous well
treatment fluid containing a viscosity-modifying polymer. The typical pH found
in slick water
fluids is around 6.0 or greater. If an adjustment of pH to the alkaline range
is needed, the pH can
be increased by the addition of an alkaline or basic compound or base.
Exemplary alkaline
compounds include, sodium hydroxide, calcium hydroxide, potassium hydroxide,
sodium
bicarbonate, sodium carbonate, any of the sodium phosphates, inorganic or
organic alkaline
compounds, and mixtures thereof. If an adjustment of the pH to an acidic range
is needed, the
pH can be decreased by the addition of an acid or acidic compound. Exemplary
acidic
compounds include sulfuric acid, hydrochloric acid, nitric acid, phosphoric
acid, citric acid,
acetic acid, tartaric acid, succinic acid, inorganic or organic acids, or
acidic compounds or a
combination thereof.
Temperature
[0052] The well treatment fluid compositions disclosed herein are useful
for treatment of
wells located in environments in which lower temperatures prevail. For
example, environmental
temperature can range from at least about -10 C to at least about 95 C. The
temperature of the
subterranean environment may vary depending upon the depth of the well, the
nature of the
geological formation, the location, and the surface environmental temperature.
Thus the
temperature can be at least about -10 C, at least about -5 C, at least about
0 C, at least about 5
C, at least about 10 C, at least about 15 C, at least about 20 C, at least
about 25 C, at least
about 30 C, at least about 35 C, at least about 40 C, at least about 45 C,
at least about 50 C,
at least about 55 C, at least about 60 C, at least about 65 C, at least
about 70 C, at least about
75 C, at least about 80 C, at least about 85 C, at least about 90 C, or at
least about 95 C. In
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
some embodiments, the environmental temperature can range from at least about -
10 C to at
least about 95 C, at least about -10 C to at least about 50 C, at least
about 0 C to at least about
30 C, at least about 10 C to at least about 80 C, at least about 15 C to
at least about 75 C, at
least about 20 C to at least about 60 C, at least about 25 C to at least
about 50 C, or at least
about 30 C to at least about 45 C. Other exemplary environmental
temperatures include
temperatures from about -5 C to at least about 160 C.
[0053] Since large volumes of water are employed in the aqueous well
treatment fluids
that are introduced into subterranean environments, the actual well treatment
fluid temperature is
tends to be the ambient temperature of the subterranean formation. Heating of
the aqueous well
treatment fluid at the surface is normally impractical or uneconomic because
of the large
volumes of fluid involved. The temperature at which the aqueous well treatment
fluid of the
invention is subjected or maintained is typically the formation temperature,
i.e., the temperature
of the subterranean formation where the well treatment fluid is employed,
i.e., the bottom hole
temperature. These temperatures may vary depending upon the depth of the well,
the nature of
the formation, and the environment.
Duration of treatment
[0054] The methods disclosed herein provide effective viscosity reduction
of
polyacrylamide-based slick water in relatively short time periods. The actual
time periods may
vary depending upon environmental conditions, fracture length, and the
decrease in viscosity
required to recover the hydrocarbons from the treated subterranean formation.
Typically
polymer or copolymer-containing well treatment fluids are used to provide the
viscosity-
enhancing functionality over only a few hours after their initial introduction
into the subterranean
environment, e.g., between 1-12 hours or typically 2-5 hours. In the case of
thickened or
partially gelled aqueous well treatment fluids, e.g., fracturing fluids
containing crosslinked or
partially crosslinked polymers or copolymers or other viscosity-enhancing
polymers or
copolymers or circumstances in which there has been substantial deposition of
sludge on the
surfaces of the well bore, filters or equipment, a substantial or significant
viscosity reduction is
desirable for efficient removal or recovery of the gas or oil product from the
treated subterranean
formation. For efficient removal of an acrylamide containing filter cake or
sludge having high
16
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
molecular weight petroleum hydrocarbons and minerals in its matrix, treatment
times may
extend to 24 hrs.
[0055] The fluid viscosity of the polymer-containing aqueous well
treatment fluid,
especially fluids containing guars and other cross-linked polymers, can be
reduced to a viscosity
of less than about 100 cP. In some embodiments the reduction can be less than
about 50 cP, less
than about 40 cP, less than about 30 cP, less than about 20 cP, less than
about 15 cP, less than
about 10 cP, less than about 5 cP, less than about 1 cP, less than about 0 cP,
In slickwater
applications, the initial viscosities can be substantially lower than other
applications and
viscosity breaking is achieved with reductions from <100 cP to as low as about
5 cP. These
viscosity reductions are based on the presumption that the initial aqueous
well treatment fluid
viscosity is well in excess of these stated reduced viscosity target values.
In the case of aqueous
fluid compositions that contain polymers or copolymers used to achieve flowing
friction
reduction, e.g., slick water fluids, the initial viscosity of such fluids is
relatively low (in
comparison to thickened fracturing fluids). In some embodiments, viscosity
reductions that
approach the viscosity of water containing no additives, about 1 cP, can be
desirable.
[0056] In some embodiments, the oxidizer, the activator, and the
surfactant are
introduced into to the wellbore separately. In some embodiments, the oxidizer,
the activator and
the surfactant can be combined and then the combined oxidizer, activator, and
surfactant can be
introduced into the wellbore. Ratios of peroxide to activator can range from
about 50:1 to about
1:2 by weight. In some embodiments, a useful ratio can be 10:1 by weight.
[0057] Also provided is a method of reducing the viscosity of a polymer-
containing
aqueous well treatment fluid in a subterranean environment, the method
comprising: a)
introducing a peroxide and a chelated metal activator into the polymer-
containing aqueous well
treatment fluid to form a well treatment composition; and b) directing the
well treatment
composition into the subterranean environment in an amount and for a time
sufficient to reduce
the viscosity of the polymer-containing aqueous well treatment fluid. The
subterranean
environment can be a geological formation that has been penetrated by a
wellbore. The
temperature of the subterranean geological formation can be from about -10 C
to about 50 C.
The temperature of the subterranean geological formation can be from about 0
C to about 30 C.
17
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
The polymer-containing well treatment fluid can have a pH of about 6.0 to
about 10Ø The
polymer-containing well treatment fluid can include an acrylamide-derived
polymer. The
acrylamide-derived polymer is selected from the group consisting of
polyacrylamide,
acrylamide-acrylate (acrylic acid) copolymers, acrylic acid-methacrylamide
copolymers,
partially hydrolyzed polyacrylamide copolymers, partially hydrolyzed
polymethacrylamides, and
acrylamide-methyl-propane sulfonate copolymers. The acrylamide-derived polymer
can be a
cross-linked polymer. The peroxide is hydrogen peroxide or urea peroxide. The
peroxide
concentration in the well treatment composition can be from about 400 ppm to
about 3000 ppm.
The peroxide concentration in the well treatment composition can be about 1000
ppm. The
chelated metal activator is selected from the group consisting of FeEDTA,
CuEDTA, FeDTPA,
ferric citrate, ferrous citrate, ferrous isocitrate, ferrous aconitate,
ferrous salicylate, zinc
gluconate, copper citrate, ferrous lactate, and ferrous gluconate. The
chelated metal activator
can be FeEDTA. The concentration of the chelated metal activator can be from
about 100 ppm
to about 2000 ppm. The amount of the well treatment composition can be
sufficient to reduce
the viscosity of the aqueous well treatment fluid to less than about 1 cp.
Examples
Example 1: Materials and Methods
[0058] Hydrogen peroxide solutions were prepared by dilution of a 27%
stock solution
(PeroxyChem, LLC). Sodium hypochlorite (NaC10) solutions were prepared from a
14.5%
stock solution (Sigma Aldrich).
[0059] Test samples: a sludge sample containing cross-linked partially
hydrolyzed
polyacrylamide gel was obtained from Canadian oil fields. The liquid was
separated from the
oily black tar layer. The initial viscosity was 23 cps. The initial pH was
8.6.
[0060] Viscosity of the gel was measured using Viscometer Grace M3500. 70
grams of
gel were placed into a beaker and initial viscosity was measured before
treatment at room
temperature. Then oxidizer and activator were added and viscosity was
monitored during 1-2
hours or until the viscosity approached that of water. Viscosity was measured
at 300 rpm using a
standard R1 bob. All measurements were done at room temperature.
18
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
Example 2
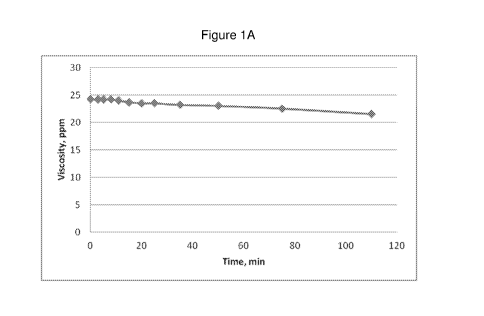
[0061] As shown in Figure 1A, the viscosity of the gel was only slightly
diminished in
the presence of 500 ppm of sodium hypochlorite (NaC10) after two hours. As
shown in Figure
1B, at a higher dosage of 5,000 ppm, viscosity of the gel decreased by about
60% in two hours.
Example 3
[0062] Treatment of the gel with a low concentration of hydrogen peroxide
(580 ppm)
combined with FeSO4 (75 ppm) as an activator resulted in a minimal effect on
gel viscosity as
shown in Figure 2A. Increasing the concentrations of the hydrogen peroxide
(5015 ppm) and the
FeSO4 (475 ppm) also had no effect on gel viscosity after two hours as shown
in Figure 2B. The
following day the viscosity remained relatively high, at 15.6 cP.
[0063] Adjusting the pH to 5.0 after 20 minutes of incubation by the
addition of H2SO4
resulted in a substantial decrease in viscosity, as shown in Figure 3. The
data in Figure 3 also
showed that a reduced pH also resulted in increased efficiency at a lower
dosage of peroxide
(1000 ppm) and FeSO4 (270 ppm) However, a fine heavy precipitate was formed in
the samples
shown in Figure 3. Taken together, the data in Figure 2 and Figure 3 indicated
that FeSO4
without pH adjustment was not effective for cross-linked partially hydrolyzed
polyacrylamide
gel oxidation because of high pH of the samples. Reduction in the pH by the
addition of acid
resulted in effective oxidation, but also led to the formation of a heavy
precipitate, which is
undesirable in field conditions.
Example 4
[0064] We then tested alternative catalytic activators which would not
require a pH
adjustment. We found surprisingly that the combination of 1000 ppm hydrogen
peroxide with
FeEDTA (Akzo Nobel) as a catalyst resulted in a drop in viscosity without the
formation of a
precipitate. As noted in this example on the examples below, the amount of
FeEDTA use was
expressed as ppm of the total solid amount. Figure 4 shows the results of an
experiment using an
even lower concentration of FeEDTA(340 ppm) in the presence of 1000 ppm of
hydrogen
peroxide. As shown in Figure 4, viscosity was reduced to less than 5 cP in
only 20 minutes
without the formation of a precipitate.
19
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
Example 5
[0065] We also tested hydrogen peroxide in a bonded form in order to
provide a slower
steady release of the oxidizer. Urea-peroxide (Sigma-Aldrich) was used at 2000
ppm, which
corresponds to only 723 ppm H202. FeEDTA was added at 1315 ppm (as Fe). As
shown in
Figure 5, we observed a rapid decrease in viscosity with the reduction to less
than 5 cP within 12
minutes. The liquid following the treatment had a viscosity similar to that of
water and a pH of
7.35. The liquid was homogeneous, light in color and no precipitate wa.s
formed. Residual 1-1202
was 30 ppm.
[0066] The results of an experiment in which the FeEDTA concentration was
reduced to
130 ppm are shown in Figure 6. The viscosity of the gel decreased to less than
5 eP in about 40
minutes. The liquid following the treatment had a viscosity similar to that of
water and a pH of
7.71. The liquid was homogeneous, light in color and no precipitate was
formed. Residual H202
was 50 ppm.
Example 6
[0067] For the studies described in Examples 7-11, the following materials
and methods
were used. Hydrogen peroxide solutions were prepared by dilution of a 27%
stock solution
(PeroxyChem, LLC). Sodium hypochlorite (NaC10) solutions were prepared from a
14.5%
stock solution (Sigma Aldrich). Peracetic acid was an equilibrated aqueous
containing about
15% peracetic acid, 23% hydrogen peroxide and 16% acetic acid (PeroxyChem,
LLC).
[0068] Test samples were obtained from Canadian oil fields. The slick
water sample
contained partially hydrolyzed polyacrylamide polymer (HPAM). The liquid was
separated from
the oily black tar layer and used in viscosity tests described in Examples
below. The sludge
sample was a viscous sticky black substance composed mostly of black tar and
gelled HPAM;
this sludge was insoluble and not dispersible in water.
Example 7
[0069] This experiment was performed as described in Example 1, using
hydrogen
peroxide as the oxidizer. Treatment of the slick water sample with hydrogen
peroxide at 1,000
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
ppm resulted only in a minimal effect on gel viscosity in 2 hours. Treatment
of the slick water
sample with the simultaneous addition of hydrogen peroxide at 1,000 ppm plus
50 ppm of
ethylenediaminetetraacetic acid iron (III) sodium salt (FeEDTA, Akzo Nobel) at
70 F resulted in
a relatively rapid decrease in viscosity over the course of 1 hour. A. The
results are shown in
Figure 7. No precipitate or gas evolution were observed during either during
the first hour or 24
hours later. These data show that the combination of hydrogen peroxide plus
the activator,
FeEDTA, resulted in a time-dependent reduction in slick water sample
viscosity.
Example 8
[0070] The slick water sample was treated as described in Example 1 using
hydrogen
peroxide, peracetic acid or sodium persulfate as oxidizers. Ferrous sulfate,
ferrous lactate,
FeEDTA and NaOH were used as activators. Treatments were carried out at either
70 F or 40 F.
Viscosity was assayed after 1 and 12 hours of treatment. The results are shown
in Table 1.
Table 1
Oxidizer Oxidizer Activator Activator Temp. ( F) Viscosity
Conc., Conc., Reduction, %
ppm ppm
1 hr 12 hr
None - None - 70 0 0
H202 1,000 None - 70 3 8
H202 5,000 FeS 04 500 70 13 31
H202 1,000 Fe EDTA 250 70 92 93
H202 500 Fe EDTA 50 70 74 93
PAA 2,500 None - 70 16 48
PAA 5,000 None - 70 24 84
PAA 2,000 Fe EDTA 500 70 24 88
Sodium 10,000 Fe Lactate 500 70 33 63
persulfate
21
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
Sodium 5,000 NaOH 100 70 0 0
persulfate
Sodium 5,000 Fe EDTA 500 70 25 32
persulfate
H202 2,500 Fe EDTA 250 40 52 91
H202 5,000 Fe EDTA 500 40 70 92
[0071] As shown in Table 1, the combination of hydrogen peroxide and Fe
EDTA
resulted in a substantial reduction in the slick water sample viscosity after
a 1 hour treatment
even at a temperature of 40 C. A reduction more than 90% was observed after
12 hours at both
70 C and 40 . The combination of hydrogen peroxide and ferrous sulfate
resulted in the
formation of a brown precipitate, which is undesirable in field conditions.
Peracetic acid
treatment in the absence of activator produced a decrease in slick water
sample viscosity, but at
relatively higher concentrations compared to hydrogen peroxide. Peracetic acid
in the absence of
activator also produced a decrease in pH of the treated solution. Sodium
persulfate also
produced a drop in slick water sample viscosity but at higher concentrations
compared to
hydrogen peroxide and at higher concentrations of activator. Of the activators
tested, sodium
hydroxide had no effect on slick water sample viscosity.
Example 10
[0072] We analyzed the effect of surfactant on solubilization of the HPAM
sludge
component. Test solutions included hydrogen peroxide or urea/hydrogen peroxide
as the
oxidizer and 250 ppm FeEDTA as the activator. VeruSOL (VeruTEK Technologies),
a
biodegradable, plant-based surfactant was used as the surfactant. For
analysis, about 2.0 g of
sludge was placed into a pre-weighed test tube. Test solutions were prepared
in tap water and 50
mL of the solution was added to each tube. Oxidizers and surfactants were
added in the amounts
shown in Table 2. In some tests, an alcohol ("co-solvent") was added to
simulate the slickwater
operations at low temperatures, in which an alcohol is added to prevent
freezing of the liquid.
Then the test tubes were shaken using a lab shaker for 2 hours at 40 F or 70
F. The liquid was
then removed from the tubes. The tubes were dried in an oven for one hour at
100 F. The dried
tubes were reweighed and the weight of remaining tar/sludge was calculated as
percent of the
22
CA 03012476 2018-07-24
WO 2017/132253 PCT/US2017/014930
control test where sludge was dried in oven without any treatment. The results
are shown in the
Table 2.
Table 2
Oxidizer Oxidizer Temp. Surfactant, Co-
solvent, % Sludge
Conc., ( F) % removed,
PPm %
None - 70 - 6
H202 2,500 70 - 34
H202 2,500 70 0.50 69
H202 2,500 70 0.75 84
H202 2,500 70 0.75 Methanol, 5.0 90
H202 2,500 70 0.75 Isopropanol, 7.0 92
Urea/H202 5,000 70 0.75 88
H202 2,500 40 - 19
H202 3,500 40 0.50 58
Urea/H202 5,000 40 0.75 Methanol, 5.0 76
[0073] As shown in Table 2, the addition of surfactant increased the
percentage of sludge
removed from the tubes. This increase was noted both in samples containing
hydrogen peroxide
and in samples containing bonded hydrogen peroxide (urea/hydrogen peroxide).
Addition of co-
solvent also increased the percentage of sludge removed from the tubes.
Example 11
[0074] We analyzed the stability of the hydrogen peroxide/ Fe EDTA oxidizer-
activator
system. Solutions of H202 were prepared without stabilizer, or with the
addition of either urea or
citric acid. Then the activator (Fe EDTA) was added to the oxidizer solutions.
The combined
solutions were incubated at room temperature, 70 F. Periodically a sample of
liquid was taken
23
CA 03012476 2018-07-24
WO 2017/132253
PCT/US2017/014930
and the % of the remaining H202 was measured by titration with 0.1N KMn04. The
results are
shown in the Table 3.
Table 3
H202, FeEDTA, Stabilizer Stabilizer Surfactant, Co-solvent, H202, %
% ppm Conc., % % remaining
PPm
241 90
hrs hrs 4 1.25 500 - - - - 2
1.25 500 Urea 2.20 - - 83 74
1.25 500 Citric 0.125 - - 70 50
Acid
5.00 1,000 Urea 8.90 0.50
Isopropanol, 61 37
7.0
1.25 500 Citric 0.125 0.75
Isopropanol, 88 74
Acid 7.0
1.25 500 Urea 2.20 0.37 Methanol, 92 81
5.0
[0075] As
shown in Table 3, the percentage of hydrogen peroxide remaining after 24
hours was relatively higher in those samples that were stabilized with either
urea or citric acid.
By 90 hours, the hydrogen peroxide was nearly completely decomposed in those
samples that
did not include a stabilizer. The addition of citric acid or urea resulted in
retention of 50% and
nearly 75%, respectively, of hydrogen peroxide after 90 hours. The addition of
surfactant and
co-solvent also increased the stability of hydrogen peroxide after 90 hours.
24