Note: Descriptions are shown in the official language in which they were submitted.
,
,
UNSTENTED HEART VALVE WITH FORMED IN PLACE SUPPORT
STRUCTURE
Priority Information
[0001] This application claims the priority benefit of (1)
U.S. Provisional
Application 60/568,402, filed May 5, 2004, (2) U.S. Provisional Application
60/572,561,
filed May 19, 2004, (3) U.S. Provisional Application 60/581,664, filed June
21, 2004, (4)
U.S. Provisional Application 60/586,054, filed July 7, 2004, (5) U.S.
Provisional Application
60/586,110, filed July 7, 2004, (6) U.S. Provisional Application 60/586,005,
filed July 7,
2004, (7) U.S. Provisional Application 60/586,002, filed July 7, 2004, (8)
U.S. Provisional
Application 60/586,055, filed July 7, 2004, (9) U.S. Provisional Application
60/586,006,
filed July 7, 2004, (10) U.S. Provisional Application 60/588,106, filed July
15, 2004, (11)
U.S. Provisional Application 60/603,324, filed August 20, 2004, (12) U.S.
Provisional
Application 60/605,204, filed August 27, 2004 and (13) U.S. Provisional
Application
60/610,269 filed September 16, 2004, the entire contents of which are hereby
incorporated
by reference herein.
Background of the Invention
Field of the Invention
[0002] The present invention relates to medical methods and
devices, and, in
particular, to methods and devices for percutaneously implanting a stentless
valve having a
formed in place support structure.
Description of the Related Art
[0003] According to recent estimates, more than 79,000
patients are diagnosed
with aortic and mitral valve disease in U.S. hospitals each year. More than
49,000 mitral
valve or aortic valve replacement procedures are performed annually in the
U.S., along with
a significant number of heart valve repair procedures.
[0004] The circulatory system is a closed loop bed of
arterial and venous vessels
supplying oxygen and nutrients to the body extremities through capillary beds.
The driver of
the system is the heart providing correct pressures to the circulatory system
and regulating
flow volumes as the body demands. Deoxygenated blood enters heart first
through the right
- 1 -
CA 3014203 2018-08-14
atrium and is allowed to the right ventricle through the tricuspid valve. Once
in the right
ventricle, the heart delivers this blood through the pulmonary valve and to
the lungs for a
gaseous exchange of oxygen. The circulatory pressures carry this blood back to
the heart via
the pulmonary veins and into the left atrium. Filling of the left atrium
occurs as the mitral
valve opens allowing blood to be drawn into the left ventricle for expulsion
through the
aortic valve and on to the body extremities. When the heart fails to
continuously produce
normal flow and pressures, a disease commonly referred to as heart failure
occurs.
[0005] Heart failure simply defined is the inability for the heart to
produce output
sufficient to demand. Mechanical complications of heart failure include free-
wall rupture,
septal-rupture, papillary rupture or dysfunction aortic insufficiency and
tamponade. Mitral,
aortic or pulmonary valve disorders lead to a host of other conditions and
complications
exacerbating heart failure further. Other disorders include coronary disease,
hypertension,
and a diverse group of muscle diseases referred to as cardiomyopothies.
Because of this
syndrome establishes a number of cycles, heart failure begets more heart
failure.
[0006] Heart failure as defined by the New York Heart Association in
a
functional classification.
I. Patients with cardiac disease but without resulting limitations
of
physical activity. Ordinary physical activity does not cause undue
fatigue, palpitation, dyspnea, or anginal pain.
Patient with cardiac disease resulting in slight limitation of physical
activity. These patients are comfortable at rest. Ordinary physical
activity results in fatigue, palpitation, dyspnea, or anginal pain.
Patients with cardiac disease resulting in marked limitation of physical
activity. These patients are comfortable at rest. Less than ordinary
physical activity causes fatigue palpitation, dyspnea, or anginal pain.
IV. Patients with cardiac disease resulting in inability to carry on
any
physical activity without discomfort. Symptoms of cardiac
insuffiency or of the anginal syndrome may be present even at rest. If
any physical activity is undertaken, discomfort is increased.
- 2 -
CA 3014203 2018-08-14
[0007]
There are many styles of mechanical valves that utilize both polymer and
metallic materials. These include single leaflet, double leaflet, ball and
cage style, slit-type
and emulated polymer tricuspid valves. Though many forms of valves exist, the
function of
the valve is to control flow through a conduit or chamber. Each style will be
best suited to
the application or location in the body it was designed for.
[0008]
Bioprosthetic heart valves comprise valve leaflets formed of flexible
biological material. Bioprosthetic valves or components from human donors are
referred to
as homografts and xenografts are from non-human animal donors. These valves as
a group
are known as tissue valves. This tissue may include donor valve leaflets or
other biological
materials such as bovine pericardium. The leaflets are sewn into place and to
each other to
create a new valve structure. This structure may be attached to a second
structure such as a
stent or cage or other prosthesis for implantation to the body conduit.
[0009]
Implantation of valves into the body has been accomplished by a surgical
procedure and has been attempted via percutaneous method such as a
catheterization or
delivery mechanism utilizing the vasculature pathways. Surgical implantation
of valves to
replace or repair existing valves structures include the four major heart
valves (tricuspid,
pulmonary, mitral, aortic) and some venous valves in the lower extremities for
the treatment
of chronic venous insufficiency. Implantation includes the sewing of a new
valve to the
existing tissue structure for securement.
Access to these sites generally include a
thoracotomy or a sternotomy for the patient and include a great deal of
recovery time. An
open-heart procedure can include placing the patient on heart bypass to
continue blood flow
to vital organs such as the brain during the surgery. The bypass pump will
continue to
oxygenate and pump blood to the body's extremities while the heart is stopped
and the valve
is replaced. The valve may replace in whole or repair defects in the patient's
current native
valve. The device may be implanted in a conduit or other structure such as the
heart proper
or supporting tissue surrounding the heart. Attachments methods may include
suturing,
hooks or barbs, interference mechanical methods or an adhesion median between
the implant
and tissue.
[0010]
Although valve repair and replacement can successfully treat many
patients with valvular insufficiency, techniques currently in use are attended
by significant
- 3 -
CA 3014203 2018-08-14
morbidity and mortality. Most valve repair and replacement procedures require
a
thoracotomy, usually in the form of a median sternotomy, to gain access into
the patient's
thoracic cavity. A saw or other cutting instrument is used to cut the sternum
longitudinally,
allowing the two opposing halves of the anterior or ventral portion of the rib
cage to be
spread apart. A large opening into the thoracic cavity is thus created,
through which the
surgical team may directly visualize and operate upon the heart and other
thoracic contents.
Alternatively, a thoracotomy may be performed on a lateral side of the chest,
wherein a large
incision is made generally parallel to the ribs, and the ribs are spread apart
and/or removed in
the region of the incision to create a large enough opening to facilitate the
surgery.
100111 Surgical intervention within the heart generally requires
isolation of the
heart and coronary blood vessels from the remainder of the arterial system,
and arrest of
cardiac function. Usually, the heart is isolated from the arterial system by
introducing an
external aortic cross-clamp through a sternotomy and applying it to the aorta
to occlude the
aortic lumen between the brachiocephalic artery and the coronary ostia.
Cardioplegic fluid is
then injected into the coronary arteries, either directly into the coronary
ostia or through a
puncture in the ascending aorta, to arrest cardiac function. The patient is
placed on
extracorporeal cardiopulmonary bypass to maintain peripheral circulation of
oxygenated
blood.
[0012] Since surgical techniques are highly invasive and in the
instance of a heart
valve, the patient must be put on bypass during the operation, the need for a
less invasive
method of heart valve replacement has long been recognized. At least as early
as 1972, the
basic concept of suturing a tissue aortic valve to an expandable cylindrical
"fixation sleeve"
or stent was disclosed. See U.S. Patent No. 3,657,744 to Ersek. Other early
efforts were
disclosed in U.S. Patent No. 3,671,979 to Moulopoulos and 4,056,854 to
Boretos, relating to
prosthetic valves carried by an expandable valve support delivered via
catheter for remote
placement. More recent iterations of the same basic concept were disclosed,
for example, in
patents such as 5,411,552, 5,957,949, 6,168,614, and 6,582,462 to Anderson, et
al., which
relate generally to tissue valves carried by expandable metallic stent support
structures which
are crimped to a delivery balloon for later expansion at the implantation
site.
- 4 -
CA 3014203 2018-08-14
[0013] In each of the foregoing systems, the tissue or artificial
valve is first
attached to a preassembled, complete support structure (some form of a stent)
and then
translumenally advanced along with the support structure to an implantation
site. The
support structure is then forceably enlarged or allowed to self expand without
any change in
its rigidity or composition, thereby securing the valve at the site.
[0014] Despite the many years of effort, and enormous investment of
entrepreneurial talent and money, no stent based heart valve system has yet
received
regulatory approval, and a variety of difficulties remain. For example, stent
based systems
have a fixed rigidity even in the collapsed configuration, and have inherent
difficulties
relating to partial deployment, temporary deployment, removal and navigation.
[0015] Thus, a need remains for improvements over the basic concept
of a stent
based prosthetic valve. As disclosed herein a variety of significant
advantages may be
achieved by eliminating the stent and advancing the valve to the site without
a support
structure. Only later, the support structure is created in situ such as by
inflating one or more
inflatable chambers to impart rigidity to an otherwise highly flexible and
functionless
subcomponent.
Summary of the Invention
[0016] Accordingly, one embodiment of the present invention comprises
a
cardiovascular prosthetic valve that includes an inflatable cuff The cuff
comprises at least
one inflatable channel that forms, at least in part, a distal inflatable
toroidal structure and a
proximal inflatable toroidal structure. The inflatable cuff also comprises a
waist that extends
between the distal inflatable toroidal structure and the proximal inflatable
toroidal structure.
A valve is coupled to the inflatable cuff The valve is configured to permit
flow in a first
axial direction and to inhibit flow in a second axial direction opposite to
the first axial
direction.
[0017] Another embodiment of the present invention comprises a
prosthetic
valve for replacing an aortic valve positioned between the left ventricle and
the aorta of the
heart. The valve includes an inflatable structure that has a distal end and a
proximal end. A
valve member is coupled to the inflatable structure. The valve member is
positioned
generally between the distal and proximal ends of the inflatable structure.
The distal end of
- 5 -
CA 3014203 2018-08-14
,
the inflatable structure is configured to be positioned within the left
ventricle and the
proximal end of the inflatable structure is configured to be positioned within
the aorta.
[0018] Another embodiment of the present invention comprises a
cardiovascular
prosthetic valve that comprises an inflatable body. The inflatable body has at
least a first
inflatable chamber and a second inflatable chamber that is not in fluid
communication with
the first inflatable chamber. The inflatable body is to form, at least in
part, a generally
annular ring. A valve is coupled to the inflatable body. The valve is
configured to permit
flow in a first axial direction and to inhibit flow in a second axial
direction opposite to the
first axial direction. A first inflation port is in communication with the
first inflatable
chamber. A second inflation port in communication with the second inflatable
chamber.
[0019] Another embodiment of the present invention comprises a
cardiovascular
prosthetic valve that includes a cuff and an inflatable structure. The cuff
has a distal end and
a proximal end. The inflatable structure is coupled to the cuff and has at
least one inflatable
channel that forms a toroidal structure. A valve is coupled to the cuff. The
valve is
configured to permit flow in a first axial direction and to inhibit flow in a
second axial
direction opposite to the first axial direction. The distal end of the cuff
has a non-circular
cross-section with respect to the flow. The non-circular cross-section is
configured to affect
the performance of an adjacent valve.
[0020] Another embodiment of the present invention comprises a
cardiovascular
prosthetic valve that includes a flexible cuff having a distal end and a
proximal end. An
inflatable structure is coupled to the cuff and having at least one inflatable
channel that forms
a toroidal structure. A valve is mounted to the cuff. The valve is configured
to permit flow
in a first axial direction and to inhibit flow in a second axial direction
opposite to the first
axial direction. At least anchor is moveable between a first position in which
the anchor
extends in a radial direction to engage an adjacent anatomical structure and a
second position
in which the anchor has a reduced radial profile.
[0021] Another embodiment of the present invention comprises a
cardiovascular
prosthetic valve that includes an inflatable body. A valve is coupled to the
body. The valve
is configured to permit flow in a first axial direction and to inhibit flow in
a second axial
- 6 -
CA 3014203 2018-08-14
,
direction opposite to the first axial direction. At least two control wires
are detachably
coupled to the inflatable body.
[0022] Yet another embodiment of the present invention comprises a
cardiovascular prosthetic valve that includes an inflatable body comprising at
least one
inflation channel. A valve is coupled to the body. The valve is configured to
permit flow in a
first axial direction and to inhibit flow in a second axial direction opposite
to the first axial
direction. An inflation port is in communication with the at least one
inflatable channel. A
plug is positioned within the inflation port. An inflation tube extends
through the inflation tube
in communication with the at least one inflation channel. A balloon is coupled
to the inflation
tube. The balloon is configured to expand between a first, inflated position
in which the
balloon prevents the inflation tube from decoupling from the inflation port
and a second,
deflated position in which the inflation tube can be decoupled from the
inflation port.
[0023] Another embodiment of the present invention comprises a
method of
implanting a prosthetic valve within a heart. A prosthetic valve comprising an
inflatable
structure is translumenally advanced to a position proximate a native valve of
the heart.
Aportion of the inflatable structure that is distal to the native valve is
inflated. A portion of
the inflatable structure that is proximal to the native annular valve is
inflated.
[0024] Another embodiment of the invention involves a method of
implanting a
prosthetic valve within the heart that comprises translumenally advancing a
prosthetic valve
that has an inflatable structure to a position proximate a native valve of the
heart. A distal
portion of the inflatable structure is inflated. The valve is proximally
retracted to seat the
distal portion of the inflatable structure against a distally facing portion
of the native valve.
[0025] Another embodiment of the invention comprises a method of
implanting a
prosthetic valve within the heart. A prosthetic valve comprising an inflatable
structure is
advanced, translumenally, to a position proximate a native valve of the heart.
A first chamber
of the inflatable structure is inflated. A second chamber of the inflatable
structure is
independently inflated.
[0026] Another embodiment of the present invention relates to a
method of
implanting a prosthetic valve within the heart win which a prosthetic valve
comprising an
inflatable structure is advanced translumenally to a position proximate a
native valve of the
- 7 -
CA 3014203 2018-08-14
heart. The inflatable structure is inflated, to deploy the prosthetic valve.
The prosthetic
valve is stapled or sutured to an adjacent anatomical structure.
[0027] Another embodiment of the present invention is a method of
treating a
patient. The method comprises translumenally advancing a prosthetic valve a
position
proximate a native valve of the heart, fully deploying the prosthetic valve at
the
cardiovascular site, testing a performance characteristic of the prosthetic
valve, at least
partially reversing the deployment of the prosthetic valve, repositioning the
prosthetic valve;
and re-deploying the prosthetic valve.
[0028] Another embodiment of the present invention involves advancing
deployment catheter to a position proximate a native valve of the heart, the
deployment
catheter comprising an inflation tube and a prosthetic valve comprising an
inflatable
structure in communication with the inflation tube, inflating the inflatable
structure with the
inflation tube, removing the deployment catheter from the patient while the
inflation tube
remains coupled to the inflatable catheter, advancing a removal catheter over
the inflation
tube, deflating the inflatable structure, retracting the prosthetic valve into
the removal
catheter; and withdrawing the prosthetic valve and the removal catheter from
the patient.
[0029] Another embodiment of the invention comprise a method of
treating a
patient that includes advancing deployment catheter to a position proximate a
native valve of
the heart, the deployment catheter comprising a prosthetic valve and a linking
member
coupled to the prosthetic valve, deploying the prosthetic valve, removing the
deployment
catheter from the patient while linking member remains coupled to the
prosthetic valve,
advancing a removal catheter over the linking member, retracting the
prosthetic valve into
the removal catheter; and withdrawing the prosthetic valve and the removal
catheter from the
patient.
[0030] Another embodiment of the present invention comprises
identifying a
patient with a minimum cross-minimum flow area through an aortic valve of no
greater than
0.75 square cm, enlarging the minimum cross-minimum flow area through the
valve; and
deploying a prosthetic valve which provides a minimum cross-sectional flow
area of ate least
about 1.75 square cm.
- 8 -
CA 3014203 2018-08-14
,
,
[0031] Yet another embodiment of the preset invention
involves a method of
treating a patient. The methods comprises inflating an inflatable structure of
a temporary
valve at a cardiovascular site in fluid communication with a native valve,
translumenally
removing at least a portion of the native valve, deploying a prosthetic valve
to compliment or
replace a native valve, and removing the temporary valve.
[0032] Another embodiment of the present invention comprises
a method of
performing a procedure on a beating heart. In the method, a temporary valve is
positioned in
series fluid flow with a native valve. An inflatable prosthetic valve is
deployed upstream of
the temporary valve. The temporary valve is then removed.
[0033] Yet another embodiment of the present invention
comprises a temporary
heart valve catheter, for enabling minimally invasive procedures on a valve in
a beating heart.
The catheter includes an elongate, flexible catheter body, having a proximal
end and a distal
end, a valve on the distal end, the valve comprising an inflatable structure;
and at least one link
between the catheter and the valve to prevent detachment of the valve from the
catheter.
[0034] Another embodiment of the present invention comprises
a method of in
situ formation of a prosthetic valve support. A prosthetic valve is attached
to a flexible
support component which is incapable of retaining the valve at a functional
site in the arterial
vasculature. The support component extends both proximally and distally of the
base of the
valve. The valve is positioned at the site. The flexible support component is
supplemented
to increase the rigidity of the support component sufficiently to retain the
valve at the site.
[0035] Another embodiment of the present invention involves
an implantable
prosthetic valve that has an in situ formable support structure. The valve
comprises a
prosthetic valve, having a base and at least one flow occluder. A first
flexible component is
incapable of retaining the valve at a functional site in the arterial
vasculature. The first
component extends proximally of the base of the valve. A second flexible
component is
incapable of retaining the valve at a functional site in the arterial
vasculature. The second
component extends distally of the base of the valve. At least one rigidity
component
combines with at least one of the first and second flexible components to
impart sufficient
rigidity to the first or second components to retain the valve at the site.
- 9 -
CA 3014203 2018-08-14
[0036] There is provided in accordance with one embodiment of the
present
invention, a method of treating a patient. The method comprises deploying a
temporary
valve at a cardiovascular site in fluid communication with a native valve. At
least a portion
of the native valve is transluminally removed, and a prosthetic valve is
deployed to
complement or replace the native valve. The temporary valve is thereafter
removed.
[0037] In one embodiment, the deploying a temporary valve step may
comprise
transluminally advancing the temporary valve to the site while the valve is in
a first, reduced
cross sectional configuration, and transforming the valve to a second,
enlarged configuration
to enable the valve to function at the site. The removing the temporary valve
step may
comprise transforming the valve in the direction of the first configuration,
and transluminally
removing the temporary valve. In certain embodiments, the temporary valve is
permanently
affixed to a temporary valve deployment catheter, to facilitate valve removal.
The method
may be accomplished on a beating heart.
[0038] The deploying a temporary valve step may comprise deploying a
valve
with tissue leaflets. Alternatively, the deploying a temporary valve step may
comprise
deploying a valve with synthetic leaflets. The valve may be supported within a
self
expandable stent, a balloon expandable stent, or an inflatable cuff. The
removing the
temporary valve step may comprise retracting the valve into a tubular sheath.
[0039] The transluminally removing at least a portion of the native
valve step may
comprise mechanically cutting native valve tissue. Mechanical cutting may be
accomplished
with an axially reciprocating cutter, or a rotational cutter. Cutting or
decalcification may also
be accomplished using a thermal source, such as a laser, or ultrasound.
[0040] The method may additionally comprise the step of capturing
embolic
material dislodged into the blood stream from the valve procedure. This may be
achieved by
filtration or extraction of the material through an aspiration process.
[0041] In accordance with another embodiment of the present
invention, there is
provided a method of performing a procedure on a beating heart. The method
comprises the
steps of positioning a temporary valve in series fluid flow with a native
valve, and
performing a procedure on the native valve. The temporary valve is thereafter
removed. The
- 10 -
CA 3014203 2018-08-14
,
,
valve may be the aortic valve, the mitral valve, or other valves. The
procedure may be a
valve repair, or a valve replacement.
[0042] In accordance with another embodiment of the present
invention, there is
provided a temporary heart valve catheter, for enabling minimally invasive
procedures on a
valve in a beating heart. The catheter comprises an elongate flexible catheter
body, having a
proximal end and a distal end. A valve is carried by the distal end. At least
one link is
provided between the catheter and the valve to prevent detachment of the valve
from the
catheter. The valve may be supported by a support frame, which is connected to
a pull wire
or wires extending axially throughout the length of the catheter. Axial
tensioning of the pull
wire relative to the catheter body deploys the valve into its functional
configuration.
Proximal retraction of the pull wire causes the valve to reduce in cross
section and draw into
the distal end of the catheter, such as for placement or removal. The link may
comprise a
connection between the pull wire and a valve support.
[0042a] In accordance with another embodiment of the present invention, there
is
provided a prosthetic valve comprising a tubular elastic stent. The tubular
elastic stent has a
distal end and a proximal end with a passage extending from the distal end to
the proximal
end of the elastic stent. The prosthetic valve further comprises a valve
positioned within the
passage, and an annular sealing structure positioned on an exterior of the
elastic stent at the
distal end of the elastic stent.
[0042b] In accordance with another embodiment, there is provided prosthetic
valve comprising an inflatable cuff movable between a first position in which
the inflatable
cuff extends in a radial direction to engage an adjacent anatomical structure
and a second
position in which the inflatable cuff has a reduced radial profile. The
prosthetic valve further
comprises a valve positioned within an axial passage of the prosthetic valve.
The prosthetic
valve has a distal end diameter that is larger than a middle portion diameter
when in the first
position, which provides an anchoring mechanism for the prosthetic valve.
[0043] Further features and advantages of the present
invention will become
apparent from the detailed description of preferred embodiments which follows,
when
considered together with the attached drawings and claims.
- 1 1 -
CA 3014203 2018-08-14
,
=
Brief Description of the Drawings
[0044] Figure 1 is a cross-sectional schematic view of a
heart and its major blood
vessels.
[0045] Figure 2 is a partial cut-away view a left ventricle
and aortic with an
prosthetic aortic valve implant according to one embodiment of the present
invention
positioned therein.
[0046] Figure 2A is a side view of the implant of Figure 2
positioned across a
native aortic valve.
[0047] Figure 2B is a schematic top illustration of a
modified embodiment of an
implant positioned across the aortic valve.
[0048] Figure 2C is a schematic cross-sectional view of a
modified embodiment
of an implant.
[0049] Figure 2D is a side cross-sectional view of another
embodiment of an
implant positioned at the aortic valve.
[0050] Figures 2E and 2F are side and bottom views of another
embodiment of
an implant.
[0051] Figures 2G and 2H are side and bottom views of another
embodiment of
an implant.
[0052] Figure 3A is a front perspective view of the implant
of Figure 2.
[0053] Figure 3B is a cross-sectional side view of the
implant of Figure 3A.
[0054] Figure 3C is an enlarged cross-sectional view of a
lower portion of Figure
3B.
[0055] Figure 3D is a front perspective view of an inflatable
support structure of
the implant of Figure 3A.
[0056] Figure 4 is a front perspective view of a modified
embodiment of an
implant.
[0057] Figure 5A is a front perspective view of another
modified embodiment of
an implant.
[0058] Figure 5B is cross-sectional view taken through line
5B-5B of Figure 5A
[0059] Figure 6 is a front perspective view of another
embodiment of an implant.
- 12 -
CA 3014203 2018-08-14
,
,
[0060] Figure 7A is a front perspective view of another
embodiment of an
implant.
[0061] Figure 7B is cross-sectional view taken through line
7B-7B of Figure 7A.
[0062] Figure 8A is a front perspective view of another
embodiment of an
implant.
[0063] Figure 8B is cross-sectional view taken through line
8B-8B of Figure 8A
[0064] Figure 9A is a front perspective view of another
embodiment of an
implant.
[0065] Figure 9B is cross-sectional view taken through line
9B-9B of Figure 9A.
[0066] Figure 10 is an embodiment of a cross-section of an
inflation channel.
[0067] Figure 11 is a front perspective view of another
embodiment of an
implant.
[0068] Figure 12 is a cross-sectional side view of the
implant of Figure 11
positioned across an aortic valve.
[0069] Figures 13A-D are front perspective views of three
modified
embodiments of a valve implant.
[0070] Figure 14 is a side perspective view of a method of
forming a lumen in an
valve implant.
[0071] Figure 15 is a top perspective view of a method of
attaching a valve to a
valve implant.
[0072] Figure 16A-B are front perspective views of two
modified embodiments
of a valve implant.
[0073] Figure 17A-B are front perspective views of two
modified embodiments
of a non-inflatable valve implant.
[0074] Figures 18A-C are time sequence steps of deploying a
non-inflatable
valve implant.
[0075] Figure 19 is a side view of an un-deployed non-
inflatable valve implant.
[0076] Figure 19A is a cross-sectional view taken at line 19A-
19A of Figure 19.
[0077] Figure 19B is a side view of another embodiment of un-
deployed non-
inflatable valve implant.
- 13 -
CA 3014203 2018-08-14
,
,
[0078] Figure 19C is a top view of the valve implant of
Figure 19B in a deployed
state.
[0079] Figure 20 is side view of another embodiment of an un-
deployed non-
inflatable valve.
[0080] Figure 20A is a cross-sectional view taken at line 20A-
20A of Figure 20.
[0081] Figures 21A-B are time sequenced steps of deploying a
non-inflatable
valve implant
[0082] Figures 22A-B illustrate the deployment of a modified
embodiment of a
non-inflatable valve implant.
[0083] Figure 23 are top views of a modified embodiment of a
non-inflatable
valve implant in an expanded and compressed configuration.
[0084] Figures 24A-B are side perspective views of a modified
embodiment of a
non-inflatable valve implant in an expanded and compressed configuration.
[0085] Figures 25A-C are side perspective views of a modified
embodiment of a
non-inflatable valve implant in an expanded, compressed and assembled
configuration.
[0086] Figure 25D is a side perspective view of another
embodiment of a non-
inflatable valve implant.
[0087] Figures 25E-F are side perspective views of another
embodiment of a
non-inflatable valve implant.
[0088] Figure 26 is a side perspective view of an anchor for
an implant valve.
[0089] Figures 27A-C are time sequenced steps of securing an
implant to the
aorta with a staple or clip.
[0090] Figures 27D are side views of another embodiment of
securing an implant
to the aorta with a staple or clip.
[0091] Figure 28 is a side perspective view of another
embodiment of an anchor
for an implant valve.
[0092] Figure 28A is a side perspective view of another
embodiment of an
anchor for an implant valve.
[0093] Figure 29 is a side perspective view of another
embodiment of an anchor
for an implant valve.
- 14 -
CA 3014203 2018-08-14
[0094] Figure 30 is a side perspective view of another embodiment of
an anchor
for an implant valve.
[0095] Figure 30A is a side perspective view of another embodiment of
an
anchor for an implant valve in a deployed and un-deployed configuration.
[0096] Figure 31 is a side perspective view of another embodiment of
an anchor
for an implant valve in a deployed and un-deployed configuration.
[0097] Figures 32 is a top and side views of another embodiment of an
anchor for
an implant valve in a deployed and un-deployed configuration.
[0098] Figure 32A is a side perspective view of another embodiment of
an
anchor for an implant valve.
[0099] Figure 33 is a side perspective view of another embodiment of
an anchor
for an implant valve.
[0100] Figure 34 is a side view of a deployment catheter.
[0101] Figure 35 is a side view of the deployment catheter of Figure
34 with an
outer sheath partially withdrawn.
[0102] Figures 35A and 35B are side views of a modified embodiment of
the
distal end of the deployment catheter of Figure 35.
[0103] Figure 36 is a side view of the deployment catheter of Figure
35 with an
outer sheath partially withdrawn and the implant deployed.
[0104] Figure 36A is an enlarged view of the distal portion of the
deployment
catheter shown in Figure 36.
[0105] Figure 36B is a cross-sectional view taken through line 36B-
36B of
Figure 36A.
[0106] Figure 37 is a side view of the deployment catheter of Figure
35 with an
outer sheath partially withdrawn and the implant deployed and detached.
[0107] Figure 37A is a side view of another embodiment of a
deployment
catheter.
[0108] Figures 38A-C are schematic partial cross-sectional views of a
modified
embodiment of a deployment catheter with the implant in a stored, partially
deployed and
deployed position.
- 15 -
CA 3014203 2018-08-14
[0109] Figures 39A-D are cross-sectional side views of four
embodiments of a
sealing mechanism.
[0110] Figures 40A-B are cross-sectional side views of a sealing and
connection
mechanism in a connected and disconnected confirmation.
[0111] Figure 41 is a cross-sectional side view of a sealing and
connection
mechanism.
[0112] Figures 42 is cross-sectional side view of a sealing and
connection
mechanism in a connected and disconnected confirmation.
[0113] Figure 43 is a cross-sectional side view of a sealing and
connection
mechanism.
[0114] Figure 44 is a side perspective view of an embodiment of
connecting a
control wire to a prosthetic valve implant.
[0115] Figures 45A-C illustrates time sequence steps of partially
deploying and
positioning an artificial valve implant.
[0116] Figures 45A-C illustrates time sequence steps of deploying and
withdrawing an artificial valve implant.
[0117] Figures 47A-E illustrates time sequence steps of deploying,
testing and
repositioning an artificial valve implant.
[0118] Figure 48 is a side perspective view of an embodiment of
connecting a
control wire to a prosthetic valve implant.
[0119] Figure 49A is a side view of an embodiment of a control wire
with
controlled flexibility.
[0120] Figure 49B is a side view of another embodiment of a control
wire with
controlled flexibility.
[0121] Figure 49C is a cross-sectional front view of another
embodiment of a
control wire with controlled flexibility in a first position.
[0122] Figure 49D is a cross-sectional front view the control wire of
Figure 49C
in a second position.
[0123] Figure 50 is a side view of a distal end of a recapture
device.
- 16 -
CA 3014203 2018-08-14
[0124] Figure 51 is a side view of a distal end of another embodiment
of a
recapture device.
[0125] Figure 52A is a partial cross-sectional view of the heart and
the aorta with
a temporary valve positioned therein.
[0126] Figure 52B is a partial cross-sectional view of the heart and
the aorta with
protection device positioned therein
[0127] Figure 53A is a side view of an embodiment of an excise
device.
[0128] Figure 53B is a closer view of a portion of Figure 53A.
[0129] Figure 54A is a closer view of the distal end of the excise
device of Figure
53A.
[0130] Figure 54B is a cross-sectional view taken through line 54B-
54B of
Figure 53A.
[0131] Figure 54C is a cross-sectional view taken through line 54C-
54C of
Figure 53A.
[0132] Figure 55A is a cross-sectional view of a distal end of
another
embodiment of an excise device.
[0133] Figure 55B is a cross-sectional view taken through line 55B-
55B of
Figure 55A.
[0134] Figure 56A is a side view of a distal end of another
embodiment of an
excise device.
[0135] Figure 56B is a cross-sectional view taken through line 56B-
56B of
Figure 56A.
[0136] Figure 56C is a cross-sectional view taken through line 56C-
56C of
Figure 56A.
[0137] Figure 56D is a side view of another embodiment of a debulking
device.
[0138] Figures 57A-0 are time sequenced steps of an embodiment of a
method
for deploying a temporary valve, an excise device and a prosthetic valve
implant.
Detailed Description of the Preferred Embodiments
[0139] Figure 1 is a schematic cross-sectional illustration of the
anatomical
structure and major blood vessels of a heart 10. Deoxygenated blood is
delivered to the right
- 17 -
CA 3014203 2018-08-14
atrium 12 of the heart 10 by the superior and inferior vena cava 14, 16. Blood
in the right
atrium 12 is allowed into the right ventricle 18 through the tricuspid valve
20. Once in the
right ventricle 18, the heart 10 delivers this blood through the pulmonary
valve 22 to the
pulmonary arteries 24 and to the lungs for a gaseous exchange of oxygen. The
circulatory
pressures carry this blood back to the heart via the pulmonary veins 26 and
into the left
atrium 28. Filling of the left atrium 28 occurs as the mitral valve 30 opens
allowing blood to
be drawn into the left ventricle 32 for expulsion through the aortic valve 34
and on to the
body extremities through the aorta 36. When the heart 10 fails to continuously
produce
normal flow and pressures, a disease commonly referred to as heart failure
occurs.
[0140] One cause of heart failure is failure or malfunction of one or
more of the
valves of the heart 10. For example, the aortic valve 34 can malfunction for
several reasons.
For example, the aortic valve 34 may be abnormal from birth (e.g., bicuspid,
calcification,
congenital aortic valve disease), or it could become diseased with age (e.g.,
acquired aortic
valve disease). In such situations, it can be desirable to replace the
abnormal or diseased
valve 34.
[0141] Figure 2 is a schematic illustration of the left ventricle 32,
which delivers
blood to the aorta 36 through the aortic valve 34. The aorta 36 comprises (i)
the ascending
aorta 38, which arises from the left ventricle 32 of the heart 10, (ii) the
aortic arch 10, which
arches from the ascending aorta 38 and (iii) the descending aorta 42 which
descends from the
aortic arch 40 towards the abdominal aorta (not shown). Also shown are the
principal
branches of the aorta 14, which include the innomate artery 44 that
immediately divides into
the right carotid artery (not shown) and the right subclavian artery (not
shown), the left
carotid 46 and the subclavian artery 48.
[0142] Inflatable prosthetic aortic valve implant
[0143] With continued reference to Figure 2, a prosthetic aortic
valve implant
100 in accordance with an embodiment of the present invention is shown
spanning the native
abnormal or diseased aortic valve 34, which has been partially removed as will
be described
in more detail below. The implant 100 and various modified embodiments thereof
will be
described in detail below. As will be explained in more detail below, the
implant 100 is
- 18 -
CA 3014203 2018-08-14
preferably delivered minimally invasively using an intravascular delivery
catheter 200 or
trans apical approach with a trocar.
[0144] In the description below, the present invention will be
described primarily
in the context of replacing or repairing an abnormal or diseased aortic valve
34. However,
various features and aspects of methods and structures disclosed herein are
applicable to
replacing or repairing the mitral 30, pulmonary 22 and/or tricuspid 20 valves
of the heart 10
as those of skill in the art will appreciate in light of the disclosure
herein. In addition, those
of skill in the art will also recognize that various features and aspects of
the methods and
structures disclosed herein can be used in other parts of the body that
include valves or can
benefit from the addition of a valve, such as, for example, the esophagus,
stomach, ureter
and/or vesice, biliary ducts, the lymphatic system and in the intestines.
[0145] In addition, various components of the implant and its
delivery system
will be described with reference to coordinate system comprising "distal" and
"proximal"
directions. In this application, distal and proximal directions refer to the
deployment system
300, which is used to deliver the implant 100 and advanced through the aorta
36 in a
direction opposite to the normal direction of blood through the aorta 36.
Thus, in general,
distal means closer to the heart while proximal means further from the heart
with respect to
the circulatory system.
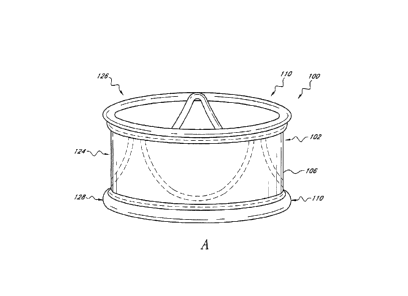
[0146] With reference now to Figures 3A-D, the implant 100 of the
illustrated
embodiment generally comprises an inflatable cuff or body 102, which is
configured to
support a valve 104 (see Figure 2) that is coupled to the cuff 102. As will be
explained in
more detail below, the valve 104 is configured to move in response to the
hemodynamic
movement of the blood pumped by the heart 10 between an "open" configuration
where
blood can throw the implant 100 in a first direction (labeled A in Figure 3B)
and a "closed"
configuration whereby blood is prevented from back flowing through the valve
104 in a
second direction B (labeled B in Figure 3B).
[0147] In the illustrated embodiment, the cuff 102 comprises a thin
flexible
tubular material 106 such as a flexible fabric or thin membrane with little
dimensional
integrity. As will be explained in more detail below, the cuff 102 can be
changed preferably,
in situ, to a support structure to which other components (e.g., the valve
104) of the implant
- 19 -
CA 3014203 2018-08-14
100 can be secured and where tissue ingrowth can occur. Uninflated, the cuff
102 is
preferably incapable of providing support. In one embodiment, the cuff 102
comprises
Dacron, PTFE, ePTFE, TFE or polyester fabric 106 as seen in conventional
devices such as
surgical stented or stent less valves and annuloplasty rings. The fabric 106
thickness may
range from about 0.002 inches to about 0.020 inches of an inch depending upon
material
selection and weave. Weave density may also be adjusted from a very tight
weave to prevent
blood from penetrating through the fabric 106 to a looser weave to allow
tissue to grow and
surround the fabric 106 completely. Additional compositions and configurations
of the cuff
102 will be described in more detail below.
[0148] With continued reference to Figures 3B-3D, in the illustrated
embodiment,
the implant 100 includes an inflatable structure 107 that forms one or more of
inflation
channels 120, which in illustrated embodiment are formed in part by a pair of
distinct balloon
rings or toroids 108a, 108b. The rings 108a, 108b in this embodiment are
positioned at the
proximal and distal ends 126, 128 of the cuff 102. As will be explained below,
the rings 108
can be secured to the body 102 in any of a variety of manners. With reference
to Figure 3C, in
the illustrated embodiment, the rings 108 are secured within folds 110 formed
at the proximal
and distal ends 126, 128 of the cuff 102. The folds 110, in turn, are secured
by sutures or
stitches 112. See Figure 3C.
[0149] The illustrated inflatable structure 107 also includes
inflatable struts 114,
which in the illustrated embodiment are formed from an annular zig-zag pattern
having three
proximal bends 116 and three distal bends 118. As best seen in Figure 3C, the
struts 114 can
be secured to the cuff 102 within pockets 115 of cuff material by sutures 112.
Of course, as
will be explained in more detail, other embodiments other configurations can
be can be used
to secure the struts 114 to the fabric 106.
[0150] As mentioned above, the inflatable rings 108 and struts 114
form the
inflatable structure 107, which, in turn, defines the inflation channels 120.
The inflation
channels 120 receive inflation media 122 to generally inflate the inflatable
structure 107.
When inflated, the inflatable rings and struts 108, 114 provide can provide
structural support
to the inflatable implant 100 and/or help to secure the implant 100 within the
heart 10.
Uninflated, the implant 100 is a generally thin, flexible shapeless assembly
that is preferably
- 20 -
CA 3014203 2018-08-14
uncapable of support and is advantageously able to take a small, reduced
profile form in
which it can be percutaneously inserted into the body. As will be explained in
more detail
below, in modified embodiments, the inflatable structure 107 may comprise any
of a variety
of configurations of inflation channels 120 that can be formed from other
inflatable members
in addition to or in the alternative to the inflatable rings 108 and struts
114 shown in Figures
3A and 3B. In addition, the inflatable media 122 and methods for inflating the
inflatable
structure 107 will be described in more detail below.
[0151] With particular reference to Figure 3D, in the illustrated
embodiment, the
proximal ring 108a and struts 114 are joined such that the inflation channel
120 of the proximal
ring 108a is in fluid communication with the inflation channel 120 of the
struts 114. In
contrast, the inflation channel 120 of the distal ring 108b is not in
communication with the
inflation channels 120 of the proximal ring 108a and struts 114. In this
manner, the inflation
channels of the (i) proximal ring 108a and struts 115 can be inflated
independently from the
(ii) distal ring 108b. As will be explained in more detail below, the two
groups of inflation
channels 120 are preferably connected to independent fluid delivery devices to
facilitate the
independent inflation. It should be appreciated that in modified embodiments
the inflatable
structure can include less (i.e., one common inflation channel) or more
independent inflation
channels. For example, in one embodiment, the inflation channels of the
proximal ring 108a,
struts 114 and distal ring 108b can all be in fluid communication with each
other such that they
can be inflated from a single inflation device. In another embodiment, the
inflation channels of
the proximal ring the proximal ring 108a, struts 114 and distal ring 108b can
all be separated
and therefore utilize three inflation devices.
[0152] With reference to Figure 3B, in the illustrated embodiment,
the proximal
ring 108a has a cross-sectional diameter of about 0.090 inches. The struts
have a cross-
sectional diameter of about 0.060 inches. The distal ring 108b has a cross-
sectional diameter
of about 0.090 inches diameter.
[0153] In prior art surgically implanted valves, the valve generally
includes a
rigid inner support structure that is formed from polycarbonate, silicone or
titanium wrapped
in silicone and Dacron. These surgical valves vary in diameter for different
patients due to
-21 -
CA 3014203 2018-08-14
the respective implantation site and orifice size. Generally the largest
diameter implantable
is the best choice for the patient. These diameters range from about 16 mm to
30 mm.
[0154] As mentioned above, the implant 100 allows the physician to
deliver a
valve via catheterization in a lower profile and a safer manner than currently
available.
When the implant 100 is delivered to the site via a delivery catheter 300, the
implant 100 is a
thin, generally shapeless assembly in need of structure and definition. At the
implantation
site, the inflation media 122 (e.g., a fluid or gas) may be added via a
catheter lumen to the
inflation channels 120 providing structure and definition to the implant 100.
The inflation
media 122 therefore comprises part of the support structure for implant 100
after it is
inflated. The inflation media 122 that is inserted into the inflation channels
120 can be
pressurized and/or can solidify in situ to provide structure to the implant
100. Additional
details and embodiments of the implant 100, can be found in U.S. Patent No.
5,554,185 to
Block, the disclosure of which is expressly incorporated in its entirety
herein by reference.
[0155] With reference to Figure 2A,in the illustrated embodiment, the
implant
100 has shape that can be viewed as a tubular member or hyperboloid shape
where a waist
124 excludes the native valve or vessel 34 and proximally the proximal end 126
forms a
hoop or ring to seal blood flow from re-entering the left ventricle 32
Distally, the distal end
128 also forms a hoop or ring to seal blood from forward flow through the
outflow track.
Between the two ends 126, 128, the valve 104 is mounted to the body 102 such
that when
inflated the implant 100 excludes the native valve 34 or extends over the
former location of
the native valve 34 and replaces its function. The distal end 128 should have
an appropriate
size and shape so that it does not interfere with the proper function of the
mitral valve, but
still secures the valve adequately. For example, there may be a notch, recess
or cut out in the
distal end 128 of the device to prevent mitral valve interference. The
proximal end 126 is
designed to sit in the aortic root. It is preferably shaped in such a way that
it maintains good
apposition with the wall of the aortic root. This prevents the device from
migrating back into
the ventricle 32. In some embodiments, the implant 100 is configured such that
it does not
extend so high that it interferes with the coronary arteries.
[0156] Any number of additional inflatable rings or struts may be
between the
proximal and distal end 126, 128. The distal end 126 of the implant 100 is
preferably
- 22 -
CA 3014203 2018-08-14
positioned within the left ventrical 34 and can utilize the aortic root for
axial stabilization as
it may have a larger diameter than the aortic lumen. This may lessen the need
for hooks,
barbs or an interference fit to the vessel wall. Since the implant 100 may be
placed without
the aid of a dilatation balloon for radial expansion, the aortic valve 34 and
vessel may not
have any duration of obstruction and would provide the patient with more
comfort and the
physician more time to properly place the device accurately. Since the implant
100 is not
utilizing a support member with a single placement option as a plastically
deformable or
shaped memory metal stent does, the implant 100 may be movable and or
removable if
desired. This could be performed multiple times until the implant 100 is
permanently
disconnected from the delivery catheter 300 as will be explained in more
detail below. In
addition, the implant 100 can include features, which allow the implant 100 to
be tested for
proper function, sealing and sizing, before the catheter 300 is disconnected.
When the
disconnection occurs, a seal at the device may be required to maintain the
fluid within the
inflation channels 120. Devices for providing such a seal will be described in
more detail
below.
[0157]
With reference to Figure 2B, in a modified embodiment, the shape of the
distal end 128 of the implant 100 can be configured so that the impact to the
shape of the
mitral valve annulus is minimized. This is particularly important in the
implant 100 extends
into or beyond the native annulus 35 and into the left ventrical 32 as shown
in Figure 2A. In
general, the distal end 128 can be shaped so that the chordae and leaflet
tissue from the
mitral valve are not impacted or abraded by the implant 100 during their
normal motion. In
this manner, the implant 100 does not apply or only applies minimal pressure
to the major
conduction pathways of the heart. Several different embodiment of the valve
100 address
these issues. In the embodiment shown in Figures 2B, 2E and 2F, the distal end
128 of the
implant has of a "D" shaped cross section where the flat side of the "D" is
positioned to
correspond with the mitral valve 22 location. In another embodiment shown in
Figure 2C,
the distal end 128 of the implant 100 has a generally elliptical cross
section, where the minor
axis of the ellipse extends generally from the mitral valve location to the
septal wall. In yet
another embodiment, the distal end 128 of the implant 100 contains feet or
enlarged pads,
designed to contact the native anatomy at the desired locations. For example,
the desired
- 23 -
CA 3014203 2018-08-14
locations are just below the annulus in the areas on either side of the mitral
valve. The feet
may be inflatable structures or separate mechanical structures such as
deployable anchors
may be made from materials such as stainless steel or nitinol. These anchors
can deployed
by the inflation media or a secondary system. Figures 2G and 2H illustrate an
embodiment
in which the distal end of the valve 100 has a pair of generally opposing flat
sides 128a.
[0158] In yet another embodiment of the implant 100, the implant 100
is
configured such that it does affect the mitral valve 22. In such an
embodiment, the distal end
128 of the implant 100 has a protrusion or feature that pushes on the annulus
of the mitral
valve 22 from the aortic root or aortic valve annulus. In this way, mitral
regurgitation is
treated by pushing the anterior leaflet closer 22a to the posterior leaflet
22b and improving
the coaptation of the valve. This feature can be a separate device from the
implant 100
and/or it may be actuated by a secondary mechanism, or it may simply be a
function of the
shape of the implant 100.
[0159] In yet another modified embodiment the implant 100 (see Figure
2D), for
an aortic valve replacement application, the implant 100 uses both the top and
bottom of the
aortic root for securement. In this case, the axial force pushing the implant
100 away from
the heart 10 is resisted by a normal force from the upper portion of the
aortic root. A implant
100 designed to be implanted in this configuration can have a different
configuration than an
implant designed to anchor around the annulus (e.g., the implant 100 shown in
Figure 2A).
For example, as shown in Figure 2D, the implant 100 can have a cylindrical or
partially
spherical shape, where the diameter in the mid portion 124 of the device is
larger than the
diameter at the proximal or distal portions 126, 128. The valve 104 can be
located in the
distal portion 128 of the implant 100 below the coronary arteries, preferably
in a supra-
annular position but an intra-annular position would also be possible. Anchors
(not shown)
can also be used with a device of this configuration. The anchors preferably
have a length of
1 to 4 mm and a diameter for .010 to .020 inches.
[0160] With reference back to Figures 3A and 3B, the body 102 may be
made
from many different materials such as Dacron, TFE, PTFE, ePTFE, woven metal
fabrics,
braided structures, or other generally accepted implantable materials. These
materials may
also be cast, extruded, or seamed together using heat, direct or indirect,
sintering techniques,
- 24 -
CA 3014203 2018-08-14
laser energy sources, ultrasound techniques, molding or thermoforming
technologies. Since
the body 102 generally surrounds the inflation lumens 120, which can be formed
by separate
members (e.g., rings 108), the attachment or encapsulation of these lumens 120
can be in
intimate contact with the body material 106 or a loosely restrained by the
surrounding
material 106. These inflation lumens 120 can also be formed also by sealing
the body
material 106 to create an integral lumen from the body 102 itself. For
example, by adding a
material such as a silicone layer to a porous material such as Dacron, the
fabric 106 can resist
fluid penetration or hold pressures if sealed. Materials may also be added to
the sheet or
cylinder material to create a fluid tight barrier. However, in the illustrated
embodiment of
Figures 3A and 3B, the inflation lumens 120 are formed by balloons 111 (see
Figure 4C),
which form the separate inflation components 108a, 108b, 122, which are, in
turn, secured to
the material 106.
[0161]
Various shapes of the body 102 may be manufactured to best fit
anatomical variations from person to person. As described above, these may
include a
simple cylinder, a hyperboloid, a device with a larger diameter in its mid
portion and a
smaller diameter at one or both ends, a funnel type configuration or other
conforming shape
to native anatomies. The shape of the implant 100 is preferably contoured to
engage a
feature of the native anatomy in such a way as to prevent the migration of the
device in a
proximal or distal direction. In one embodiment the feature that the device
engages is the
aortic root or aortic bulb 34 (see e.g., Figure 2A), or the sinuses of the
coronary arteries. In
another embodiment the feature that the device engages is the native valve
annulus, the
native valve or a portion of the native valve. In certain embodiments, the
feature that the
implant 100 engages to prevent migration has a diametral difference between 1%
and 10%.
In another embodiment the feature that the implant 100 engages to prevent
migration the
diameter difference is between 5% and 40%. In certain embodiments the diameter
difference
is defined by the free shape of the implant 100. In another embodiment the
diameter
difference prevents migration in only one direction. In another embodiment.
the diameter
difference prevents migration in two directions, for example proximal and
distal or
retrograde and antigrade. Similar to surgical valves, the implant 100 will
vary in diameter
ranging from about 14mm to about 30mm and have a height ranging from about 1
Omm to
- 25 -
CA 3014203 2018-08-14
about 30mm in the portion of the implant 100 where the leaflets of the valve
104 are
mounted. Portions of the implant 100 intended for placement in the aortic root
may have
larger diameters preferably ranging from about 20 to about 45mm
[0162] Different diameters of valves will be required to replace
native valves of
various sizes. For different locations in the anatomy, different lengths of
valves or anchoring
devices will also be required. For example a valve designed to replace the
native aortic
valve needs to have a relatively short length because of the location of the
coronary artery
ostium (left and right arteries). A valve designed to replace or supplement a
pulmonary
valve could have significantly greater length because the anatomy of the
pulmonary artery
allows for additional length.
[0163] Figure 4 illustrates a modified embodiment of the implant 100
in which
the implant 100 includes a distal inflation ring 130 with three commissural
inflatable
supports posts 132, which are arranged in a manner similar to that described
above. The
valve 104 is supported by the distal inflation ring 130 and support posts 132.
This shape is
similar to a commercially available valve sold by Edwards Life Science under
the trade name
of MagnaTM and many other commercially available surgical valves. However, the
illustrated embodiment is advantageous because of the inflation channels (not
shown) in the
distal inflation ring 130 and supports posts 132. As described above, the
inflation channels
of the inflation ring 130 and support posts 132 can be in fluid connection or
separated.
[0164] Other variations of inflatable valve shapes may include an
implant 100 in
which entire or substantially the entire cuff 102 forms an cylindrical pocket
that is filled with
fluid creating a cylinder shape with commissural supports defined by
sinusoidal patterns cut
from a cylindrical portion of the body 102. In such an embodiment, there may
be a desire to
seam or join the body 102 together at points or areas to provide passageways
for fluid to flow
or be restricted. This may also allow for wall definition of the body 102
defining a thickness
of the cylinder. It may be desired to maintain a thin body wall allowing the
largest area
where blood or other fluids may pass through the valve. The wall thickness of
the inflated
implant 100 may vary from 0.010 to 0.100 of an inch depending upon
construction, pressures
and materials. There also may be a desire to vary the thickness of the cuff
wall from distal to
proximal or radially. This would allow for other materials such as fixed
pericardial tissue or
- 26 -
CA 3014203 2018-08-14
polymer valve materials to be joined to the wall where support is greatest, or
allow the
maximum effective orifice area in the area of the implant 100 its self The
implant 100 may
be sealed fluid tight by glue, sewing, heat or other energy source sufficient
to bond or fuse
the body material together. There can be secondary materials added to the cuff
for stiffness,
support or definition. These may include metallic elements, polymer segments,
composite
materials.
[0165] Figures 5A and 5B illustrate an example of such the embodiment
described above. In the illustrated embodiment, the body 102 defines a
generally sleeve
shaped lumen 132. The top surface 134 of the body 102 is scalloped shaped. The
peaks or
commissars 136 of the top surface 134 are supported by elongated members 138
positioned
within or along the outer surface of the body 102. The leaflets 104 are
supported within the
body 102 with its edges corresponding to the supported commissars 136. The
members 138
can comprise metallic wire or laser-cut elements. These elements 138 may be
attached by
conventional techniques such as sewing, gluing or woven to the body 102. The
elements 138
can range in cross section from round, oval, square or rectangular.
Dimensionally they can
have a width and or thickness from 0.002 to 0.030 inches. Materials for these
elements 138
can be stainless steel, Nitinol, Cobalt-Chromium such as MP35N or other
implant grade
materials. These elements 138 can provide visualization under conventional
imaging
techniques such as fluoroscopy, echo, or ultrasound. Radiopaque markers may be
desired to
define the proximal and distal ends of the cuff and these markers may be
materials such as
gold, platinum iridium, or other materials that would provide an imaging
element on body
102
[0166] Figure 6 illustrates another embodiment of the valve 100,
which includes
a body 102, with distal and proximal ends 126, 128 supported by rings (not
shown) as
described above. As compared to the embodiment of Figures 3A and 3B, in this
embodiment, the inflatable struts 114 are replaced by elongated stiffening
members 140.
The stiffening members 140 can be positioned on the body 102 to generally
correspond to
the commissars 136 of a scalped to surface 134 as described above. The
stiffening members
140 can be coupled to the body 102 in any of a variety of manners. In the
illustrated
- 27 -
CA 3014203 2018-08-14
,
=
embodiment, the stiffening member 140 are coupled to the body 102 through a
combinations
of sutures 112 and loops 142 that extend through the body 102.
[0167] The stiffening members 140 can be metallic wire,
ribbon or tube. They
may vary in thickness from 0.005 to 0.050 inches and taper or vary in
thickness, width or
diameter. As mentioned embodiment, the members 140 can be used to support the
valve
commissars 136, and/or define the height of the cuff or be attachment points
for the
deployment catheter. These members 140 may be sewn to or woven into the cuff
material
106 through conventional techniques as described above and may be shaped with
hoops to
accept thread or wires. The members 140 may also be formed from a hypotube,
allowing
deployment control wires or a deployment control system as will be described
below to pass
through the stiffening wires or to attach to them. Other lengths of stiffening
wires are also
possible, in some instances a shorter wire may be preferred, either to allow a
smaller profile,
better conform to a calcified valve annulus, or to ensure positive engagement
of an anchor.
Short sections of stiffening wires may also be positioned in directions other
than the axial
direction. Positioning wires off axis may allow the valve to move more
naturally relative to
the native tissue, or prevent anchors from rotating and disengaging. The
stiffening members
140 may be substantially straight pieces of wire.
[0168] Figures 7A and 7B illustrate yet another embodiment of
the implant 100
in which substantially the entire body 102 is filled with fluid creating an
hour glass shape.
Between the proximal and distal ends 126, 128, the body 102 includes axially
extending
channels 46which form axially extending lumens 48 for extending over the
native valve or
valve stem.
[0169] In the embodiments described herein, the inflation
channels 120 may be
configured such that they are of round (see Figure 8A), oval, square (Figure
10), rectangular
(see Figure 98) or parabolic shape in cross section. Round cross sections may
vary from
.020 ¨ 0.100 inches in diameter with wall thicknesses ranging from 0.0005 ¨
0.010 inches.
Oval cross sections may have an aspect ratio of two or three to one depending
upon the
desired cuff thickness and strength desired. In embodiments in which the
lumens 120 are
formed by balloons 111, these lumens 120 can be constructed from conventional
balloon
materials such as nylon, polyethylene, PEEK, silicone or other generally
accepted medical
- 28 -
CA 3014203 2018-08-14
device material. They may be helically coiled into a cylinder shape creating a
tube (see
Figure 8A) or looped radially to create a series of toroids (see Figure 9A) or
undulate (see
Figure 3C) to create a sinusoidal pattern to provide support both radially and
axially. A
combination of these patterns may be desired to best suit the patient and
desired valve. For
example, a combination of single a single toroid proximal and distal may be
the preferred
pattern however any number of toroids may be located between proximal and
distal portions
of the device to provide additional tissue and or calcium support throughout
the height of the
device.
[0170] With reference now to Figures 11 and 12, the implant 100 can
include one
or more windows 150 cut or otherwise formed in the body 102 of the valve 120
to supply
blood to the coronary arteries 152. The number of windows 150 can range from
one to
twenty. In the illustrated embodiment, the windows 150 are generally located
radially
between the proximal and distal ends 126, 128. Depending upon the
configuration of the
implant 100, these windows 150 can be defined, at least in part, by inflation
lumens, support
structures such as metallic or polymer struts or be cut into the body material
as a step in the
manufacturing process. In one embodiment, the locations of the windows 150 is
denoted by
radio-opaque markers to ensure the proper orientation of the windows 150. In
another
embodiment, the rotational orientation of the implant 100 is controlled by the
orientation that
the implant 100 is loaded into the deployment catheter 300. In this
embodiment, the
deployment catheter 300 can have a preset curve or a preferred bending plane,
oriented such
that as the catheter 300 is delivered over the aortic arch or some other
native anatomy, the
implant 100 is oriented in the proper rotational position. The area of the
windows 150 is
preferably between about 1 square centimeter and about 6 square centimeters.
In one
embodiment, the area of the window 150 is between about 1.5 square centimeters
and about
3 square centimeters. A larger sized window advantageously can permit some
tolerance in
the placement of the window 150 relative to the coronary ostia. Windows 150
may also be
placed in a stent segment of a prosthetic valve.
[0171] In other embodiments configured for maintaining patent flow
through the
coronary arteries 152, the cuff 102 has an open mesh structure that allows
patent flow in any
orientation. The mesh structure is preferably sufficiently configured that not
more than one
- 29 -
CA 3014203 2018-08-14
or two of its threads or wires would cross an ostium at any position. It is
also possible to
access the coronary arteries with an angioplasty balloon and deform the mesh
structure away
from the ostium, provided that the mesh is manufactured from a plastically
deformable
material, such as stainless steel, or any of the biocompatable materials with
similarly
appropriate mechanical properties.
[0172] In order to visualize the position and orientation of the
implant 100,
portions of the body 102 would ideally be radio-opaque. Markers made from
platinum gold
or tantalum or other appropriate materials may be used. These may be used to
identify
critical areas of the valve that must be positioned appropriately, for example
the valve
commissures may need to be positioned appropriately relative to the coronary
arteries for an
aortic valve. Additionally during the procedure it may be advantageous to
catheterize the
coronary arteries using radio-opaque tipped guide catheters so that the ostia
can be
visualized. Special catheters could be developed with increased radio-opacity
or larger than
standard perfusion holes. The catheters could also have a reduced diameter in
their proximal
section allowing them to be introduced with the valve deployment catheter.
[0173] As mentioned above, during delivery, the body 102 is limp and
flexible
providing a compact shape to fit inside a delivery sheath. The body 102 is
therefore
preferably made form a thin, flexible material that is biocompatible and may
aid in tissue
growth at the interface with the native tissue. A few examples of material may
be Dacron,
ePTFE, PTFE, TFE, woven material such as stainless steel, platinum, MP35N,
polyester or
other implantable metal or polymer. As mentioned above with reference to
Figure 2, the
body 102 may have a tubular or hyperboloid shape to allow for the native valve
to be
excluded beneath the wall of the cuff. Within this body 102 the inflation
channels 120 can
be connected to a catheter lumen for the delivery of an inflation media to
define and add
structure to the implant 100. As described above, these channels 120 can have
any of a
variety of configurations. In such configurations, the channels 120 may number
from one to
fifty and may have a single lumen communicating to all channels or separate
lumens for
communication separate channels or groups of channels. In one embodiment, the
cuff or
sleeve 102 contains 2 to 12 lumens, in another the cuff 102 contains 10 to 20
lumens. As
described above, the channels 120 can be part of or formed by the sleeve 102
material 106
- 30 -
CA 3014203 2018-08-14
and/or be a separate component attached to the cuff such as balloon 111. The
valve 104,
which is configured such that a fluid, such as blood, may be allowed to flow
in a single
direction or limit flow in one or both directions, is positioned within the
sleeve 102. The
attachment method of the valve 104 to the sleeve 102 can be by conventional
sewing,
gluing, welding, interference or other means generally accepted by industry.
[0174] The cuff 102 would ideally have a diameter of between 15 and
30 mm and
a length of between 6 to 70 mm. The wall thickness would have an ideal range
from 0.01
mm to 2.00mm. As described above, the cuff 102 may gain longitudinal support
in situ from
members formed by fluid channels or formed by polymer or solid structural
elements
providing axial separation. The inner diameter of the cuff 102 may have a
fixed dimension
providing a constant size for valve attachment and a predictable valve open
and closure
function. Portions of the outer surface of the cuff 102 may optionally be
compliant and
allow the implant 100 to achieve interference fit with the native anatomy.
101751 Many embodiments of inflatable structure 107 shapes have been
described
above. In addition, as described above, the implant 100 can have various
overall shapes
(e.g., an hourglass shape to hold the device in position around the valve
annulus, or the
device may have a different shape to hold the device in position in another
portion of the
native anatomy, such as the aortic root). Regardless of the overall shape of
the device, the
inflatable channels 120 can be located near the proximal and distal ends 126,
128 of the
implant 100, preferably forming a configuration that approximates a ring or
toroid. These
channels 120 may be connected by intermediate channels designed to serve any
combination
of three functions: (i) provide support to the tissue excluded by the implant
100, (ii) provide
axial and radial strength and stiffness to the 100, and/or (iii) to provide
support for the valve
104. The specific design characteristics or orientation of the inflatable
structure 107 can be
optimized to better serve each function. For example if an inflatable channel
120 were
designed to add axial strength to the relevant section of the device, the
channels 120 would
ideally be oriented in a substantially axial direction. If an inflatable
channel 120 were
designed primarily to add radial strength to the relevant section of the
device the channel
would ideally be oriented generally circumferentially. In order to prevent
tissue from
-31 -
CA 3014203 2018-08-14
extending between the inflatable channels the channels 120 should be spaced
sufficiently
close together to provide sufficient scaffolding.
[0176] Additionally depending on the manufacturing process used
certain
configurations may be preferred. For example a single spiraling balloon (see
e.g., Figure 8A)
that forms the proximal, mid and distal inflation channels may be simplest to
manufacture if
a balloon is placed within a sewing cuff as described with referenced to
Figure 3C. Figure
3D illustrates an embodiment that utilizes rings 108 and struts 114 that are
positioned within
folds 110 of the cuff 102.
[0177] In other embodiments, the implant 100 is manufactured from
multiple
layers that are selectively fused together, then the inflation channels 120
are defined by the
unfused or unjoined areas between fused areas 152. In this case any of a
variety
configurations of inflation channels 120 can be used. For example, as shown in
Figure 13A,
the implant 100 can comprise distal and proximal rings 108 with undulating
channels 120
positioned therebetween. Figure 13B illustrates an embodiment in which the
inflation 120
generally formed a cylinder with axially extending fused portions forming
axially extending
ribs 156. Figure 13C is similar to the embodiment of Figure 13B, however, the
fused
portions 152 are larger to form narrow ribs 156. In these embodiments, the
inflation
channels 120 are preferably configured so that the inflation media can flow
into all of the
channels without forming pockets of trapped air or pre inflation fluid.
[0178] The cuff 102 and inflation channels 120 of the implant 100 can
be
manufactured in a variety of ways. In one embodiment the cuff 102 is
manufactured from a
fabric, similar to those fabrics typically used in endovascular grafts or for
the cuffs of
surgically implanted prosthetic heart valves. The fabric is preferably woven
into a tubular
shape for some portions of the cuff 102. The fabric may also be woven into
sheets. The yarn
used to manufacture the fabric is preferably a twisted yarn, but monofilament
or braided
yarns may also be used. The useful range of yarn diameters is from
approximately 0.0005 of
an inch in diameter to approximately 0.005 of an inch in diameter. Depending
on how tight
the weave is made. Preferably, the fabric is woven with between about 50 and
about 500
yarns per inch. In one embodiment, a fabric tube is woven with a 18mm diameter
with 200
yarns per inch or picks per inch. Each yarn is made of 20 filaments of a PET
material. The
- 32 -
CA 3014203 2018-08-14
,
final thickness of this woven fabric tube is 0.005 inches for the single wall
of the tube.
Depending on the desired profile of the implant 100 and the desired
permeability of the
fabric to blood or other fluids different weaves may be used. Any
biocompatible material
may be used to make the yarn, some embodiments include nylon and PET. Other
materials
or other combinations of materials are possible, including Teflon,
floropolymers, polyimide,
metals such as stainless steel, titanium, Nitinol, other shape memory alloys,
alloys comprised
primarily of a combinations of cobalt, chromium, nickel, and molybdenum.
Fibers may be
added to the yarn to increases strength or radiopacity, or to deliver a
pharmaceutical agent.
The fabric tube may also be manufactured by a braiding process.
[0179] The cut edges of the fabric are melted or covered with an
adhesive
material, or sutured over, in order to prevent the fabric from unraveling.
Preferably the edges
are melted during the cutting process, this can be accomplished using a hot-
knife. The blade
of the tool is heated and used to cut the material. By controlling temperature
and feed rate as
well as the geometry of the blade, the geometry of the cut edge is defined. In
one
embodiment the hot knife blade is 0.060 inches thick sharpened to a dull edge
with a radius
of approximately 0.010 inches. The blade is heated to approximately 400
degrees F and used
to cut through a Dacron fabric at a speed of about 20 inches per minute.
Preferably the
cutting parameters are adjusted so that the cut edge is sealed with a thin
layer of melted
fabric, where the melted area is small enough to remain flexible, and prevent
cracking, but
thick enough to prevent the fabric from unraveling. The diameter of the bead
of melted
fabric is preferably between 0.0007 and 0.0070 inches in diameter.
[0180] Two edges of a fabric may be sealed together by clamping
the edges
together to form a lap joint, and then melting the free edge. This may be
accomplished with
a flame, laser energy, a heated element that contacts the fabric, such as a
hot-knife or a
heating element that passes near the fabric, or a directed stream of a heated
gas such as air.
The bead of melted fabric joining the two edges is preferably between 0.0007
and 0.0070
inches in diameter.
[0181] The fabric is stitched, sutured, sealed, melted, glued or
bonded together to
form the desired shape of the implant 100. The preferred method for attaching
portions of the
fabric together is stitching. The preferred embodiment uses a polypropylene
monofilament
- 33 -
CA 3014203 2018-08-14
suture material, with a diameter of approximately 0.005 of an inch. The suture
material may
range from 0.001 to 0.010 inches in diameter. Larger suture materials may be
used at higher
stress locations such as where the valve commisures attach to the cuff The
suture material
may be of any acceptable implant grade material. Preferably a biocompatible
suture material is
used such as polypropylene. Nylon and polyethylene are also commonly used
suture materials.
Other materials or other combinations of materials are possible, including
Teflon,
flouropolymers, polyimides, metals such as stainless steel, titanium, Kevlar,
Nitinol, other
shape memory alloys, alloys comprised primarly of a combinations of cobalt,
chromium,
nickel, and molybdenum such as MP35N. Preferably the sutures are a
monofilament design.
Multi strand braided or twisted suture materials also may be used. Many suture
and stitching
patterns are possible and have been described in various texts. The preferred
stitching method
is using some type of lock stitch, of a design such that if the suture breaks
in a portion of its
length the entire running length of the suture will resist unraveling. And the
suture will still
generally perform its function of holding the layers of fabric together.
[0182] Figure 13D illustrates aonther embodiment of an implant 100 in
which an
outer portion 156 of the cuff 102, which is in contact with the calcified
annulus contains a
material selected for its abrasion resistance. In one embodiment, the abrasion
resistant
material is a synthetic fiber such a Kevlar or other Aramid fiber. In another
embodiment, the
abrasion resistant material is a metal such as MP35N or stainless steel. In
one embodiment,
the fabric is woven entirely from the abrasion resistant material. In another
embodiment, the
fabric is woven from a combination of materials including an abrasion
resistant material and
a second material, designed to optimize other properties, such as tissue in-
growth. The fibers
of different materials may be twisted together into a single yarn, or multiple
yarns of
different materials may be woven together as the fabric is manufactured.
Alternatively, an
abrasion resistant layer may be added to the outside of the finished device or
implanted first
as a barrer or lattice to protect the valve device.
[0183] As mentioned above, the cuff 102 may be manipulated in several
ways to
form inflation channels 120. In many embodiments, the implant 100 is not
provided with
separate balloons 111, instead the fabric 106 of the cuff 102 itself can form
the inflation
channels 100. For example, in one embodiment two fabric tubes of a diameter
similar to the
- 34 -
CA 3014203 2018-08-14
,
,
desired final diameter of the implant 100 are place coaxial to each other. The
two fabric
tubes are stitched, fused, glued or otherwise coupled together in a pattern of
channels 120
that is suitable for creating the geometry of the inflatable structure 107. In
one embodiment
the stitching pattern consists of a spiral connecting the two tubes. The
spiral channel formed
between the sutured areas becomes the inflation channel (see e.g., Figure 8A).
In another
embodiment the two coaxial fabric tubes are actually a single tube folded over
its self In
another embodiment, the tubes are sewn together in a pattern so that the
proximal and distal
ends of the fabric tubes form an annular ring or toroid. See e.g., Figure 13C.
In yet another
embodiment of the design the middle section of the device contains one or more
inflation
channels shaped in a sinusoidal pattern. See e.g., Figure 13A.
[0184] With reference to Figure 14, in another embodiment,
the implant 100 is
formed from a single fabric tube 160 similar to the final diameter of the
implant 100.
Smaller fabric tubes 162 of a diameter suitable for an inflation channel are
attached to the
larger tube 160. The smaller tubes 162 cab be attached to the inside or the
outside of the
larger tube 160 in any pattern desired to provide the inflatable structure 107
with the desired
properties. In one embodiment, the tubes 162 are attached in a spiral pattern,
in another
embodiment the tubes 162 are attached in a sinusoidal pattern simulating the
shape of the
connection of the leaflet to the cuff As shown in Figure 14, an optional
skived hypotube or
similar component 164 can be positioned within the smaller tubes 162. The
smaller tubes
162 can be sutured, glued, fused or otherwised coupled to the larger tube 160.
In the
illustrated embodiment, sutures 112 applied via a needle 166 and thread 168 to
secure the
smaller tube 162 to the larger tube 164.
[0185] In another embodiment, a single fabric tube similar to
the final diameter
of the prosthetic implant 100 is used. The ends or an end of the tube is
turned inside out
forming two layers of tube for a short length at one or both ends of the tube.
The layers of
tube are sewn or otherwise attached together to form a ring shaped inflation
channel at the
end of the tube in a manner similar to that shown in Figure 3C. Alternatively
the layers may
be sewn together in a different pattern to form an inflation channel with a
different shape
such as a spiral or a sinusoid.
- 35 -
CA 3014203 2018-08-14
,
,
101861 If a porous fabric is used for the cuff 102, it may be
desired to use a liner
(e.g., as shown in Figure 14) or coating to prevent the inflation media from
escaping from
the inflation lumens 120. This portion of the fabric may be coated, filled or
encapsulated in
a polymer or other dealing agent to better seal the fabric. The entire fabric
portion may be
treated or, a specific portion of the fabric may be treated. The fabric may be
treated before
the cuff 102 is manufactured, or after the cuff 102 is manufactured. In one
embodiment, the
treatment is a polymer suspended in a solvent. After the solvent evaporates or
is otherwise
removed the polymer is left behind sealing the fabric. In another embodiment,
the sealing
agent is applied as a liquid or paste, and then cured by moisture, heat
external energy, such as
UV light, light of another wave length or a chemical reaction caused by mixing
two or more
components together. In another embodiment, the sealing agent is a silicone.
101871 In the preferred embodiment, the fabric inflation
channels contain a liner
in a form of the balloons 111 as described with reference to Figures 3A-C. The
balloon 111
preferably is a thin wall tube made from a biocompatible material. In one
embodiment, the
balloon 111 is blown from nylon tubing the tubing diameter is about 0.030 of
an inch with a
0.005 inches wall thickness. The tubing is then necked to an outside diameter
of
approximately 0.020 inches the tubing is then placed inside a mold and
pressurized to about
200 PSI the mold is then heated in the area where the balloon should be
formed. The heating
step may be accomplished using a stream of heated air at approximately 300
degrees F. The
final diameter of the balloon in this embodiment is 0.060 inches at one
portion of the balloon
and 0.090 inches a second portion of the balloon. The total length of the
balloon 111 is
approximately 18cm. The balloon 111 may be blown in a shape that conforms to
the cuff, or
the balloon may be shaped to conform to the cuff in a secondary step.
Alternatively the liner
may be a different shape than the fabric cuff, where the liner is larger than
the fabric cuff,
allowing the assembly to inflate to a size determined by the fabric.
101881 Several embodiments of the inflatable prosthetic
implant 100 described
above utilize circular or ringed shaped balloon members 111. These balloons
111 can be
manufactured using a glass tube bent in a helix. The balloon 111 is then blown
inside the
tube using methods similar to those used to manufacture balloons for
angioplasty. For
example, the glass mold may be heated using air, water, steam infared elements
and pressure
- 36 -
CA 3014203 2018-08-14
=
,
and tension may be applied to blow the balloon to a specific diameter and
length. Secondary
processes may be added to "set" the balloon's shape by providing a second
heating process
to hold the balloon as it relaxes and ages. The balloons can be blown from
many different
materials; Nylon pebax and polyethylene are particularly suitable polymers.
The balloon
tubing is inserted through the mold, and sealed at one end. A knot tied in the
tubing is
sufficient for sealing. The other end of the tubing is connected to a pressure
source,
providing pressure in the range of 80 to 350 psi. The required pressure
depends on the
material and dimensions of the tubing. The balloon is then heated in a
localized area, while
tension is optionally applied to either end of the tubing. After the tubing
expands to match
the inside diameter of the glass mold, the heat source is advanced along the
length of the
mold, at a rate that allows the tubing to grow to match the inside diameter of
the mold. The
balloon and mold may then be cooled. One method for cooling is blowing
compressed air
over the mold. The balloon is then removed from the mold. Optionally a release
agent may
be used to facilitate this step. Acceptable mold release agents include
silicone, Polyvinyl
alcohol (PVA) and Polyethylene oxide (PEO) Additionally balloons may be
produced by
wrapping braiding or weaving a material such as EPTFE over a mandrel to
produce a shape
desired the material is then bonded to itself by a process such as sintering
or gluing.
[0189]
With reference back to Figures 3A-D, in a preferred embodiment, the
implant 100 is manufactured from a single layer of woven fabric tube 106 of a
diameter
similar to the desired diameter of the finished prosthetic valve. The diameter
of the tube 106
is approximately 1 inch. A length of tube 106 approximately 1.2 inches long is
used. The
ends of the tube 106 are cut using a hot knife to prevent the edges from
unraveling. A
second piece 115 of woven fabric tubing with a diameter of approximately 0.065
inches is
cut to length of approximately 7 inches long using a hot-knife so that the
edges of the tube
115 do not unravel. The smaller diameter tube 115 is then sewed to the middle
portion of
the inner diameter of the larger diameter fabric tube 106, in a shape
producing three cusps
near the top edge of the fabric tube 106. The cusps are located approximately
0.15 inches
from the top edge of the fabric tube 106. The portion of the smaller tube 115
between the
cusps is sewed to the middle section of the larger diameter tube, in
approximately a 0.5
inches in radius. The bottom portion of the radius is positioned about 0.27
inches from the
- 37 -
CA 3014203 2018-08-14
,
,
bottom edge of the larger diameter fabric tube 106. The bottom edge of the
larger diameter
fabric tube 106 is then folded inside out over its outside diameter. A suture
112 is placed
through the two layers of the larger diameter fabric tube 106, located about
0.1 inches from
the folded edge. This suture 112 is spaced approximately 0.05 inch from the
cut edge of the
fabric tube 106, and approximately .05in from the lower edge of the radii
formed from the
attachment of the smaller diameter fabric tube 115.
[0190] With reference to Figure 15, in the embodiment of
Figure 3A-D, a tubular
section of the valve 104 (preferably fixed pericardial tissue, of
approximately 1 inches in
diameter and 0.6 inches inlength) is inserted into the inside diameter of the
larger fabric tube
106. Small squares of fabric 166 approximately 0.08 inches by 0.18 inches are
placed at each
valve cusp inside the tubular section of pericardial tissue 104. Sutures 168
are passed through
the square of fabric and the pericardial tissue 104, and then between two
segments of the
smaller diameter fabric tube 115 that form the cusp 116, and through the
larger diameter fabric
tube 106. In this manner, the top edge of the pericardial tissue tube is
attached to the cuff 102
at the three locations that form the cusps 116 and valve commisures. The
bottom edge of the
pericardial tissue tube is then attached to the bottom edge of the cuff 102 by
suturing the tissue
in the location between the smaller fabric tube 115 and the suture that forms
the bottom ring
shaped inflation channel.
[0191] The balloon members 111 are then placed inside each
channel formed by
the cuff 102. See e.g. Figure 3C. In another embodiment, the cuff 102 is
manufactured from
a nonporous polymer sheet or tube, or from polymer sheet or tube with minimal
porosity,
where a secondary sealing member such as a balloon is not required.
[0192] Figures 16A and 16B illustrate a modified embodiment a
stented implant
170 that can be delivered percutaneously as described by Andersen in U.S.
Patent 6,168,614,
which is hereby incorporated by reference herein. The implant 170 generally
comprises a stent-
like structure 172 that comprises a one or more elongated members arranged in
an annular zig-
zag pattern comprising proximal and distal bends to form a self-expandable
stent. A valve 174
is coupled to the structure 172. The implant 170 can include one (Figure 16A)
or more (Figure
16B) inflatible cuffs 176 configured in a manner as described above. The
inflatable cuff 176 is
configured to minimize or eliminate peri-valvular leaks. For example, the
inflatable cuff 176
- 38 -
CA 3014203 2018-08-14
can be positioned on the implant 170 so that when it is in inflated it
prevents or restricts fluid
flow around the fixed edge of each leaflet of the valve 174. In the embodiment
of Figure 16A,
the valve 170 includes a single circular cuff 174 attached to the outer
surface of the stent 172 in
a location where the fixed edge of the leaflets of the valve 174 are attached
to the stent 172.
After the stent 172 is expanded, the inflatable cuff 176 is filled with
inflation media. The cuff
176 is inflated to a pressure adequate to seal the outer surface of the
implant 170 to the native
anatomy. A passive structure such as an 0-ring that has no inflatable passage
but does serve to
form a seal between the vessel wall and the valve 174 could also be provided.
In such an
embodiment, the sealing structure is preferably made from a low durometer
material or foam so
that it can easily conform to the anatomy. A silicone or silicone foam can
also be used to
produce an adequate sealing member.
[0193] Another problem with an expandable stent based valve
prosthesis is that if
the stent is over-expanded the valve leaflets may not coapt. This results in a
central leak, and
an incompetent valve. In one embodiment, the inflatable sealing cuff 176
described above is
designed so that if the operator detects a central leak the operator can
inflate the cuff to a high
pressure causing the stent 172 to decrease in diameter at the prosthetic
valves annulus. The
operator monitors any regurgant flow using an imaging technique such as
echocardiography.
Guided by this information the cuff 176 can be inflated to the minimum
pressure that
eliminates the leak. Using the minimum pressure insures that the maximum
possible area is
available for blood flow. This technique would allow for a reduction in the
initial deployed
diameter or a resizing of the structure to properly fit the implantation area.
[0194] Non-inflatable prosthetic aortic valve implants
[0195] Figures 17A-20A illustrate another embodiment of a implant
180, which
utilizes a different technique to secure a valve 182 at the implantation site.
In this
embodiment, the implant 180 comprises at least one member 184 that is attached
to the valve
182 and provides the valve 182 shape as it is deployed into the body. In
general, the member
184 forms a ring or annular shape when it is actuated and deployed. However,
during
delivery the member 184 is flexible and generally elongated with a reduced
profile, while the
leaflets 183 of the valve 182 are wrapped around the support member 184 (see
Figures 20
and 20A) so as to pass through a delivery catheter . During deployment
leaflets 183 of the
- 39 -
CA 3014203 2018-08-14
valve 182 unwrap and take a second shape to form a seal with the vessel and
function as a
single direction gate for blood flow. See also Figures 21A and 21B which show
the
deployment of the valve 180 within the heart 10.
[0196] A latch or lock mechanism 181maintains the tension in the wire
or locks
the distal end to a location near the proximal end. This tension mechanism may
be driven
from the handle through a tension wire, a hydraulic system, a rotational
member to drive a
screw. Furthermore the tensioning members may utilize a locking means to
maintain the
desired circular shape, such as a suture, an adhesive, or a mechanical snap
together type lock
actuated by the tension wire.
[0197] With initial reference to Figures 18A-C, in one embodiment the
structure
184 or a portion of the structure, is manufactured from a stainless steel tube
185 with slots
188 cut on one side (e.g., as seen in Published Application number US
2002/0151961 Al,
which is hereby incorporated by reference herein) to provide flexibility
during delivery. A
wire 186 located inside the tube is tensioned providing a bias to shape the
device as
determined by the patterning and width of the transverse slots 188 cut into
the member 184.
These slots 188 and tension wire 186 cause the device to form into a circular
shape as shown
in Figures 17A and 18C. In another embodiment, the slots 188 can be oriented
such that the
ring is three dimensional, possibly incorporating cusps or high points at the
valve
commissars as shown in Figure 17B. Additionally, the member may incorporate
integral
struts 190 to support the commissars of the valve 182 as shown in Figure 17A.
[0198] Figures 19B and 19C illustrate an embodiment in which the
slots 188
have a chevron type shape. The wire inside the device is tensioned providing a
bias to shape
the device as determined by the patterning and width of the transverse slots
188, causing the
device to form into a circular shape as shown in Figure 19C. In another
embodiment, the
slots 188 can be oriented such that the ring has a three dimensional shape
when tensioned.
[0199] Figures 22A and type shape 22B illustrate a modified
embodiment in
which the member 180 is formed from elements 191 that are configured to
provide the
member 180 with a preformed shape as the member 180 is rotated. For example,
as shown
in the figures, the elements 191 may have a trapezoidal shape.
- 40 -
CA 3014203 2018-08-14
[0200] Figure 23 illustrates an embodiment of an implant 194 in which
the
implant 194 comprises a ring 195. As shown, the device 194 can be constrained
in a catheter
by bending the ring 105 into an oval with a large aspect ratio. Once expelled
from the
catheter, the implant 194 would assume its free state of a circle or more
round shape. A
tissue valve 196 could be attached by conventional manners such as sewing or
seaming the
tissue together. Figures 24A and 24B illustrate a similar embodiment in which
the ring 195
has an undeformed configuration that includes elongated members 197.
[0201] Figures 25A-C illustrate another modified embodiment in which
the ring
195 needs to be assembled in situ. In this embodiment, the ring 195 comprises
a series of
distal and proximal bends 197a, 197b. As shown in Figure 25B, the ring 195 can
be
elongated and compressed for delivery via a catheter. Once expelled from the
catheter, the
ring 195 is assembled by coupling together connection points 199a, 199b
through the use of
sutures etc.
[0202] In the embodiments described above with reference to Figures
17A-17C,
the implant must be released or disconnected from a delivery catheter. Those
of skill in the
art will recognize in light of the disclosure herein that many different
release disconnect
methods are possible. For example if rotational motion is used to deploy the
device, then a
disconnect that can transmit torque is typically provided such as a threaded
connection. In
other embodiments, the device is pushed out of the catheter by a pusher
element. In still
other embodiments, a mechanical release mechanism such as a pin joint,
unscrewing the
device from the catheter delivery system, a tethered link such as a thread or
wire, a fusible
link as used in a GDC coil deployment, a cutting tool to sever a attachment of
the device
from the catheter, a threaded knot to tether the catheter to the device where
the as the knot
could be untied or cut, a hydraulic mechanism to deploy, expand or fracture a
link between
the catheter and the device.
[0203] Figure 25D illustrates another embodiment of a prosthesis 700.
In this
embodiment, the prosthesis 700 includes a flexible fabric cuff 702. The fabric
cuff 702
includes one or more channels 704 where a permanent support structure 706 can
later be
located. In one embodiment, the permanent support structure is woven through
the channels
704 that will later contain the support structure. In another embodiment, the
support structure
- 41 -
CA 3014203 2018-08-14
706 is preloaded into the cuff in a flexible configuration. In one embodiment
of use, a catheter
contains at least one lumen through which the support structure can be
advanced and the
assembly can be fitted inside a retractable delivery sheath. The cuff 702 is
delivered to the
desired valve annulus, and the support structure 706 is advanced into a
portion (e.g., a channel
704) of the device 700. This provides structure to the prosthesis 700 such
that it can support a
valve (not shown) that is coupled to the cuff 702, and allows it to be
positioned in the native
annulus and to function. In one embodiment, the support structure 706 is a
wire. If the
operator is satisfied with the size and position of the prosthesis 700
additional support structure
may be added to stiffen or secure the prosthsesis 700. After the prosthesis
700 is positioned the
delivery catheter may optionally be withdrawn or disconnected, leaving, the
valve cuff 702 and
support structure 706 in place. Alternatively, the delivery catheter may be
left in place for any
length of time to allow later adjustment or removal of the prosthesis 700.
[0204] In the illustrated embodiment, the cuff 702 contains a spiral
channel 704
allowing the delivery of a wire 706, which takes a helical shape after it is
inserted into the
cuff The helix extends from the proximal end of the device 700 to the distal
end of the
valve with the individual coils spaced close together as shown in Figure 25D.
[0205] The preferred wire material is Nitinol, although many other
metals and
polymers have suitable properties. Nitinol provides an advantage that its
chemistry and
thermal history can be used to tune the temperature at which it undergoes a
phase change.
By adjusting this transition temperature to fall at a temperature just below
body temperature
the support structure 706 can be delivered (e.g., within the cuff 702) with
one set of
mechanical properties and after delivery, and after the support structure 706
has equalized in
temperature with the body, the support structure 706 assumes a second set of
mechanical
properties (e.g., shape). Other materials that undergo a phase change near
body temperature,
such as other shape memory alloys may provide similar benefits.
[0206] In one embodiment, the catheter that is attached to the
channels 704 in the
cuff 702 is preferably in an orientation that allows the wire 706 to be
delivered with minimal
friction making a minimum number of excessively sharp bends. The catheter may
optionally
include an inflation portion to allow an inflation media to temporarily act as
a support
structure during the process of positioning the prosthesis 700.
- 42 -
CA 3014203 2018-08-14
,
[0207] Figures 25E and 25F illustrate another embodiment of a
prosthesis 750. In
this embodiment, the prosthesis 750 includes a flexible fabric cuff 752, which
can be coupled
to a valve 754. As shown in Figure 25E, the prosthesis 750 has a highly
flexible shape in this
configuration, which delivery within a catheter. Once the device 750 is
positioned near the
delivery site, the device 750 can be given structure through the use of one or
more stents 756.
The stents 756 can be self-expandable or balloon expandable. In the
illustrated embodiment,
the stents 756 are positioned generally at the proximal and distal ends of the
device 750. The
stents 756 provide structure to the prosthesis 750 such that it can support
the valve 754 that is
coupled to the cuff 752, and allows it to be positioned in the native annulus
and to function.
[0208] Leaflet subassembly
[0209] With reference back to the embodiments of Figures 1-16B,
the valve 104
preferably is a tissue-type heart valve that includes a dimensionally stable,
pre-aligned tissue
leaflet subassembly. Pursuant to this construction, an exemplary tissue valve
104 includes a
plurality of tissue leaflets that are templated and attached together at their
tips to form a
dimensionally stable and dimensionally consistent coapting leaflet
subassembly. Then, in
what can be a single process, each of the leaflets of the subassembly is
aligned with and
individually sewn the cuff 102, from the tip of one commissure uniformly,
around the leaflet
cusp perimeter, to the tip of an adjacent commissure. As a result, the sewed
sutures act like
similarly aligned staples, all of which equally take the loading force acting
along the entire
cusp of each of the pre-aligned, coapting leaflets. Once inflated, the cuff
102 supports the
comissures with the inflation media and its respective pressure which will
solidify and create
a system similar to a stent structure. The resulting implant 100 thereby
formed reduces stress
and potential fatigue at the leaflet suture interface by distributing stress
evenly over the entire
leaflet cusp from commissure to commissure. This improved, dimensionally
stable, reduced-
stress assembly is operatively attached to the top of a previously prepared
cloth-covered cuff
102 to clamp the tissue leaflet cusps on a load-distributing cloth seat formed
by the top of the
cloth-covered cuff without distorting the leaflets or disturbing their
relative alignment and
the resultant coaptation of their mating edges. Because the tissue leaflets
experience lower,
more evenly distributed stresses during operation, they are less likely to
experience distortion
- 43 -
CA 3014203 2018-08-14
in use. Thus, a more stable, long lived, functional closure or coaptation of
the leaflets is
provided by this even distribution of attachment forces.
[0210] A number of additional advantages result from the use of the
implant 100
and the cuff 102 construction utilized therein. For example, for each key area
of the cuff 102,
the flexibility can be optimized or customized. If desired, the coapting
tissue leaflet
commissures can be made more or less flexible to allow for more or less
deflection to relieve
stresses on the tissue at closing or to fine tune the operation of the valve.
Similarly, the base
radial stiffness of the overall valve 100 structure can be increased or
decreased by pressure or
inflation media to preserve the roundness and shape of the valve 100.
[0211] Attachment of the valve 104 to the cuff 102 can be completed
in any
number of conventional methods including sewing, ring or sleeve attachments,
gluing,
welding, interference fits, bonding through mechanical means such as pinching
between
members. An example of these methods are described in Published Application
from Huynh
et al 06102944 or Lafrance et al 2003/0027332 or Peredo U.S. Patent Number
6,409,759,
which are hereby incorporated by reference herein. These methods are generally
know and
accepted in the valve device industry. As mentioned above, the cuff 102 may
additionally
house an inflation mold where the structure is formed within the body or the
cuff made be
the mold where the fluid is injected to create the support structure. The
valve, whether it is
tissue, engineered tissue, mechanical or polymer, may be attached before
packaging or in the
hospital just before implantation. Some tissue valves are native valves such
as pig, horse,
cow or native human valves. Most of which are suspended in a fixing solution
such as
Glutaraldehyde.
[0212] Although mechanical heart valves with rigid pivoting occluders
or leaflets
have the advantage of proven durability through decades of use, they are
associated with
blood clotting on or around the prosthetic valve. Blood clotting can lead to
acute or subacute
closure of the valve or associated blood vessel. For this reason, patients
with implanted
mechanical heart valves remain on anticoagulants for as long as the valve
remains implanted.
Anticoagulants impart a 3-5% annual risk of significant bleeding and cannot be
taken safely
by certain individuals.
- 44 -
CA 3014203 2018-08-14
[0213] Besides mechanical heart valves, heart valve prostheses can be
constructed with flexible tissue leaflets or polymer leaflets. Prosthetic
tissue heart valves can
be derived from, for example, porcine heart valves or manufactured from other
biological
material, such as bovine or equine pericardium. Biological materials in
prosthetic heart
valves generally have profile and surface characteristics that provide
laminar, nonturbulent
blood flow. Therefore, intravascular clotting is less likely to occur than
with mechanical
heart valve prostheses.
[0214] Natural tissue valves can be derived from an animal species,
typically
mammalian, such as human, bovine, porcine canine, seal or kangaroo. These
tissues can be
obtained from, for example, heart valves, aortic roots, aortic walls, aortic
leaflets, pericardial
tissue such as pericardial patches, bypass grafts, blood vessels, human
umbilical tissue and
the like. These natural tissues are typically soft tissues, and generally
include collagen
containing material. The tissue can be living tissue, decellularized tissue or
recellularized
tissue.
[0215] Tissue can be fixed by crosslinking. Fixation provides
mechanical
stabilization, for example by preventing enzymatic degradation of the tissue.
Glutaraldehyde or
formaldehyde is typically used for fixation, but other fixatives can be used,
such as other
difunctional aldehydes, epoxides, genipin and derivatives thereof Tissue can
be used in either
crosslinked or uncrosslinked form, depending on the type of tissue, use and
other factors.
Generally, if xenograft tissue is used, the tissue is crosslinked and/or
decellularized.
[0216] The implants 100 can further include synthetic materials, such
as polymers
and ceramics. Appropriate ceramics include, for example, hydroxyapatite,
alumina, graphite
and pyrolytic carbon. Appropriate synthetic materials include hydrogels and
other synthetic
materials that cannot withstand severe dehydration. Heart valve prostheses can
include
synthetic polymers as well as purified biological polymers. These synthetic
polymers can be
woven or knitted into a mesh to form a matrix or similar structure.
Alternatively, the synthetic
polymer materials can be molded or cast into appropriate forms.
[0217] Appropriate synthetic polymers include without limitation
polyamides (e.g.,
nylon), polyesters, polystyrenes, polyacrylates, vinyl polymers (e.g.,
polyethylene,
polytetrafluoroethylene, polypropylene and polyvinyl chloride),
polycarbonates, polyurethanes,
- 45 -
CA 3014203 2018-08-14
poly dimethyl siloxanes, cellulose acetates, polymethyl methacrylates,
ethylene vinyl acetates,
polysulfones, nitrocelluloses and similar copolymers. Bioresorbable polymers
can also be used
such as dextran, hydroxyethyl starch, gelatin, derivatives of gelatin,
polyvinylpyrolidone,
polyvinyl alcohol, poly[N-(2-hydroxypropyl)methacrylamide], poly (hydroxy
acids),
poly(epsilon-caprolactone), polylactic acid, polyglycolic acid, poly(dimethyl
glycolic acid),
poly(hydroxy buterate), and similar copolymers. These synthetic polymeric
materials can be
woven or knitted into a mesh to form a matrix or substrate. Alternatively, the
synthetic polymer
materials can be molded or cast into appropriate forms.
[0218]
Biological polymers can be naturally occurring or produced in vitro by
fermentation and the like or by recombinant genetic engineering. Recombinant
DNA
technology can be used to engineer virtually any polypeptide sequence and then
amplify and
express the protein in either bacterial or mammalian cells. Purified
biological polymers can
be appropriately formed into a substrate by techniques such as weaving,
knitting, casting,
molding, extrusion, cellular alignment and magnetic alignment.
Suitable biological
polymers include, without limitation, collagen, elastin, silk, keratin,
gelatin, polyamino acids,
polysaccharides (e.g., cellulose and starch) and copolymers thereof.
[0219] A
tissue-based valve prosthesis can maintain structural elements, such as
leaflets, from its native form and/or structural elements can be incorporated
into the prosthesis
from the assembly of distinct pieces of tissue. For example, the valve
prosthesis can be
assembled from a porcine heart valve, from bovine pericardium or from a
combination thereof.
Porcine tissue valves, for example, the Toronto SPV® valve marketed by St.
Jude
Medical, Inc. St. Paul, Minn., can be implanted in the patient using the tools
described herein.
The Toronto SPV® valve is designed for implantation in an aortic heart
valve position.
See, for example, David et al., J. Heart Valve Dis. 1:244-248 (1992). It will
be appreciated by
those skilled in the art that the tools of the present invention are
applicable to any valve,
especially any tissue valve prosthesis, that is adapted for implanting in a
patient.
[0220] A
reinforcement may be placed along the inner surface of the valve
commissure supports and/or scallops. In alternative embodiments, the
reinforcement is
placed on the outer surface of the valve, such as at the valve commissure
supports. The
reinforcement preferably includes apertures through which the fasteners extend
or can be
- 46 -
CA 3014203 2018-08-14
inserted. The reinforcements are thin strips of relatively strong material.
The reinforcement
can prevent or reduce damage to the prosthesis when the fasteners are inserted
and after
implantation of the heart valve prosthesis in the patient. The reinforcement,
thus, can protect
and support the commissure supports from potential damage generated by the
presence of the
fasteners. In alternative embodiments, the reinforcement is placed on the
outside of the aorta
such that the fastener pierces the reinforcement after passing through the
prosthetic valve.
[0221] Tissue valves whether implanted surgically or percutaneously
have a risk
of calcification after implantation. To prevent or minimize the calcification
several
treatments have been employed before the tissue is fixed. Some strategies
include treating
the valves with ethanol, metallic salts, detergents, biophosphonates,
coimplants of polymeric
controlled release drug delivery systems, and covalent attachment of
anticalcifying agents.
In the preferred embodiment the valve tissue is treated in 40% to 80% ethanol
for 20 to 200
hours before fixation in a buffered glutaraldehyde solution. The ethanol
pretreatment may
prevent calcification in the valve after implantation and serves to remove
cholesterol and
phospholipids from the tissue before fixation. (ref Prevention of
Bioprosthetic Heart Valve
Calcification by Ethanol Preincubation, Vyavahare et al)
[0222] Inflation Media
[0223] The inflatable structure 107 can be inflated using any of a
variety of
inflation media 122, depending upon the desired performance. In general, the
inflation
media can include a liquid such water or an aqueous based solution, a gas such
as CO2, or a
hardenable media which may be introduced into the cuff 102 at a first,
relatively low
viscosity and converted to a second, relatively high viscosity. Viscosity
enhancement may
be accomplished through any of a variety of known UV initiated or catalyst
initiated
polymerization reactions, or other chemical systems known in the art. The end
point of the
viscosity enhancing process may result in a hardness anywhere from a gel to a
rigid structure,
depending upon the desired performance and durability.
[0224] Useful inflation media generally include those formed by the
mixing of
multiple components and that have a cure time ranging from a few minutes to
tens of
minutes, preferably from about three and about twenty minutes. Such a material
should be
biocompatible, exhibit long-term stability (preferably on the order of at
least ten years in
- 47 -
CA 3014203 2018-08-14
vivo), pose as little an embolic risk as possible, and exhibit adequate
mechanical properties,
both pre and post-cure, suitable for service in the cuff of the present
invention in vivo. For
instance, such a material should have a relatively low viscosity before
solidification or curing
to facilitate the cuff and channel fill process. A desirable post-cure elastic
modulus of such
an inflation medium is from about 50 to about 400 psi--balancing the need for
the filled body
to form an adequate seal in vivo while maintaining clinically relevant kink
resistance of the
cuff The inflation media ideally should be radiopaque, both acute and chronic,
although this
is not absolutely necessary.
[0225] Details of compositions suitable for use as an inflation
medium in the
present invention are described in greater detail in U.S. patent application
Ser. No.
09/496,231 to Hubbell et al., filed Feb. 1, 2000 and entitled "Biomaterials
Formed by
Nucleophilic Addition Reaction to Conjugated Unsaturated Groups" and U.S.
patent
application Ser. No. 09/586,937 to Hubbell et al., filed Jun. 2, 2000 and
entitled "Conjugate
Addition Reactions for the Controlled Delivery of Pharmaceutically Active
Compounds".
The entirety of each of these patent applications is hereby incorporated
herein by reference.
[0226] Below is listed one particular three-component medium.
[0227] This medium comprises:
(1) polyethylene glycol diacrylate (PEGDA), present in a proportion ranging
from
about 50 to about 55 weight percent; specifically in a proportion of about 52
weight percent,
(2) pentaerthyritol tetra 3(mercaptopropionate) (QT) present in a proportion
ranging
from about 22 to about 27 weight percent; specifically in a proportion of
about 24 weight
percent, and
(3) glycylglycine buffer present in a proportion ranging from about 22 to
about 27
weight percent; specifically in a proportion of about 24 weight percent.
[0228] Variations of these components and other formulations as
described in
copending U.S. patent application Ser. Nos. 09/496,231 and 09/586,937, both to
Hubbell et
al., may be used as appropriate. In addition, we have found PEGDA having a
molecular
weight ranging from about 350 to about 850 to be useful; PEGDA having a
molecular weight
ranging from about 440 to about 560 are particularly useful.
- 48 -
CA 3014203 2018-08-14
[0229] Radiopaque materials as previously discussed may be added to
this 3-
component system. We have found that adding radiopacifiers such as barium
sulfate,
tantalum powder, and soluble materials such as iodine compounds to the
glycylglycine buffer
is useful.
[0230] Applicants have found that triethanolamine in phosphate-
buffered saline
may be used as an alternative to glycylglycine buffer as the third component
described above
to form an alternative curable gel suitable for use in embodiments of the
present invention.
[0231] An alternative to these three-component systems is a gel made
via
polymer precipitation from biocompatible solvents. Examples of such suitable
polymers
include ethylene vinyl alcohol and cellulose acetate. Examples of such
suitable
biocompatible solvents include dimethylsulfoxide (DMSO), n-methyl pyrrolidone
(NMP)
and others. Such polymers and solvents may be used in various combinations as
appropriate.
[0232] Alternatively, various siloxanes may be used as inflation
gels. Examples
include hydrophilic siloxanes and polyvinyl siloxanes (such as STAR-VPS from
Danville
Materials of San Ramon, Calif. and various silicone products such as those
manufactured by
NuSil, Inc. of Santa Barbara, Calif.).
[0233] Other gel systems useful as an inflation medium or material
for the
present invention include phase change systems that gel upon heating or
cooling from their
initial liquid or thixotropic state. For example, materials such as n-
isopropyl-polyacrylimide
(NIPAM), BASF F-127 pluronic polyoxyamer, and polyethylene glycol (PEG)
chemistries
having molecular weights ranging between about 500 and about 1,200 are
suitable.
102341 Effective gels may also comprise thixotropic materials that
undergo
sufficient shear-thinning so that they may be readily injected through a
conduit such as a
delivery catheter but yet still are able to become substantially gel-like at
zero or low shear
rates when present in the various channels and cuffs of the present invention.
[0235] In the case of the three-component PEDGA-QT-glycylglycine
formulation
described above, a careful preparation and delivery protocol should be
followed to ensure
proper mixing, delivery, and ultimately clinical efficacy. Each of the three
components is
typically packaged separately in sterile containers such as syringes until the
appropriate time
for deploying the device. The QT and buffer (typically glycylglycine) are
first continuously
- 49 -
CA 3014203 2018-08-14
and thoroughly mixed, typically between their respective syringes for
approximately two
minutes. PEGDA is then mixed thoroughly with the resulting two-component
mixture for
approximately three minutes. This resulting three-component mixture is then
ready for
introduction into the cuff as it will cure into a gel having the desired
properties within the
next several minutes. Cure times may be tailored by adjusting the
formulations, mixing
protocol, and other variables according to the requirements of the clinical
setting. Details of
suitable delivery protocols for these materials are discussed in U.S. patent
application Ser.
No. 09/917,371 to Chobotov et al.
[0236] The post-cure mechanical properties of these gels may be
highly tailorable
without significant changes to the formulation. For instance, these gels may
exhibit moduli
of elasticity ranging from tens of psi to several hundred psi; the formulation
described above
exhibits moduli ranging from about 175 to about 250 psi with an elongation to
failure
ranging from about 30 to about 50 percent.
[0237] It may be helpful to add an inert biocompatible material to
the inflation
material. In particular, adding a fluid such as saline to the PEGDA-QT-
glycylglycine
formulation (typically after it has been mixed but before significant curing
takes place)
lowers the viscosity of the formulation and results in greater ease when
injecting the
formulation into cuffs and channels without sacrificing the desired physical,
chemical, and
mechanical properties of the formulation or its clinical efficacy. In the
appropriate volume
percentages, adding materials such as saline may also reduce the potential for
the inflation
material such as PEGDA-QT-glycylglycine to pose an embolic risk in case of
spillage or
leakage. Saline concentrations as a volume percentage of the final
saline/three-component
formulation combination may range from zero to as high as sixty percent or
more;
particularly suitable are saline concentrations ranging from about twenty to
about forty
percent. A saline volume concentration of about thirty percent to be most
suitable.
Alternatives to saline may include biocompatible liquids, including buffers
such as
glycylglycine.
[0238] In more general terms, it is desirable to use an inflation
medium in which
each of its components is biocompatible and soluble in blood. A biocompatible
inflation
medium is desirable so to manage any toxicity risk in the case the inflation
medium were
- 50 -
CA 3014203 2018-08-14
inadvertently released into the patient's vasculature. A soluble inflation
medium is desirable
so to manage any embolism risk if released into the vasculature. Such an
inflation medium
should not disperse nor gel or solidify if spilled into flowing blood before
curing. In the
event of a spill, the normal blood flow would then rapidly disperse the
components and their
concentration would fall below the level required for crosslinking and
formation of a solid.
These components would then be eliminated by the body through standard
pathways without
posing an embolic risk to the patient. Among the many possibilities of an
inflation medium
example in which all of the components are soluble in blood is the combination
polyethylene
glycol diacrylate, a thiolated polyethyleneamine, and a buffer.
[0239] As previously discussed, more than one type of inflation
medium, or more
than one variant of a single type of inflation medium may be used in a single
graft to
optimize the graft properties in the region in which it is disposed.
[0240] For example, in the cuffs 102 of the various embodiments of
the present
invention, the inflation material serves as a conformable sealing medium to
provide a seal
against the lumen wall. Desirable mechanical characteristics for the inflation
medium in the
proximal and distal cuffs would therefore include a low shear strength so to
enable the cuff
to deform around any luminal irregularities (such as calcified plaque
asperities) and to
conform to the luminal profile, as well as a high volumetric compressibility
to allow the fill
material to expand the cuffs as needed to accommodate any late lumen
dilatation and
maintain a seal.
[0241] Another inflation media that has proven especially useful is
an epoxy
based two part inflation media, where one part contains the reaction product
of
epichlorohydrin and bisphenol A, and Butaneddiol diglyceridyl ether. And where
one part
contains 2,2,4-trimethy1-1, 6-hexanediamine. Whereas the material may have a
viscosity of
about 100-200 cPs (@100rpm/23 C) but most preferably they may be readily
injected
through a small lumen to be introduced to the implant from outside the body.
The operating
temperature range may be from about ¨55 to about +125 C but would be most
advantageous
at the body temperature of +37 C. Other properties may include a hardness of
about 81 on
the Shore D scale and a lap shear strength of 1,700 PSI. An example of this
would be EPO-
TEK 301 supplied by 14 Fortune Drive Billerica, MA.
-51 -
CA 3014203 2018-08-14
=
[0242] The mixed uncured inflation media preferably has a
viscosity less than
2000cps In one embodiment the epxy based inflation media has a viscosity of
100-200cps.
In another embodiment the inflation media has a viscosity less than 1000cps.
[0243] In one embodiment the inflation media contains a foaming
agent. The
foaming inflation media is beneficial because the foaming action can generate
pressure
within the inflatable portion of the device. Therefore less inflation media
needs to be
injected. Additionally any pressure loss from the disconnection process is
compensated for
by the foaming action of the inflation media. Many appropriate foaming medias
are
possible; one example is a urethane foam.
[0244] In another embodiment the balloon or inflation channel may
be connected
to the catheter on both ends. This allows the balloon to be preinflated with a
nonsolidifying
material such as a gas or liquid. If a gas is chosen CO2 or helium are likely
choices, these
gasses are used to inflate intraortic balloon pumps. Preferably the
preinflation media is
radiopaque so that the balloon position can be determined by angiography.
Contrast media
typically used in interventional cardiology could be used to add sufficient
radiopacity to most
liquid preinflation medias. When it is desired to make the implant permanent
and exchange
the preinflation media for the permanent inflation media, the permanent
inflation media is
injected into the inflation channel through a first catheter connection. As
the permanent
inflation media is injected the preinflation media is expelled out a second
catheter
connection. The catheter connections are positioned in such a way that
substantially all of
the preinflation media is expelled as the permanent inflation media is
injected. In one
embodiment an intermediate inflation media is used to prevent entrapment of
preinflation
media in the permanent inflation media. In one embodiment the intermediate
inflation media
is a gas and the preinflation media is a liquid. In another embodiment the
intermediate
inflation media or preinflation media functions as a primer to aid the
permanent inflation
media to bond to the inner surface of the inflation channel. In another
embodiment the
preinflation media or the intermediate inflation media serves as a release
agent to prevent the
permanent inflation media from bonding to the inner surface of the inflation
channel.
[0245] The permanent inflation media may have a different
radiopacity than the
preinflation media. A device that is excessively radiopaque tends to obscure
other nearby
- 52 -
CA 3014203 2018-08-14
features under angiography. During the preinflation step it may be desirable
to visualize the
inflation channel clearly, so a very radiopaque inflation media may be chosen.
After the
device is inflated with the permanent inflation media a less radiopaque
inflation media may
be preferred. The feature of lesser radiopacity is beneficial for
visualization of proper valve
function as contrast media is injected into the ventricle or the aorta.
[0246] Anchoring mechanisms
[0247] In the embodiments described above, it may be necessary or
desirable to
incorporate an anchoring mechanism 220 into the cuff 102. The anchoring
mechanism 220
can comprise any of a variety of anchors or barbs such as those that have been
used
extensively on interventional devices, such as grafts for the treatment of
abdominal aortic
aneurysms, atrial appendage closure devices and filters. Most of the
traditional retention
mechanisms used for percutaneously implantable valves rely on an interference
fit between
the implant and the vessel to provide a significant portion of the retention
force, or to
activate the retention means. However, in the case of a replacement mitral or
aortic valve, it
can be desirable to minimize the radial force at the valve annulus, because
excessive dilation
of either annulus may have a detrimental effect on the function of another
other valve.
[0248] With reference to Figure 26, the anchoring mechanism 220
generally
comprises a radially extending flange 222 that protrudes radially outward from
the implant
100 to engage the tissue thus securing the implant 100 from migration. The
radially
extending flange 222 can include a sharpened tip 224 as shown in Figure 26.
With reference
to the particular embodiment shown in Figure 26, the anchor 220 can comprise a
looped base
226 that is coupled to the cuff 102 by sutures 228. The base 226 can be
sutured to a
reinforced area 230 of the cuff 102. Of course, those of skill in the art will
recognize in light
of the disclosure herein various other configurations of the anchor 220 and
the manner of
securing the anchor 220 to the implant 100.
[0249] In another embodiment, the valve 100 is sutured to the native
anatomy.
For example, the valve 100 can include a sewing ring configured allow sutures
to be easily
attached to the implant 100. A percutaneous or minimally invasive sewing
device can also
be incorporated or used as a secondary procedure. This device would contain at
least one
needle remotely actuated to attach the valve 100 to the tissue, or to a second
device
- 53 -
CA 3014203 2018-08-14
previously implanted at the desired valve location. Other methods may utilize
a balloon or
other force mechanism to push or pull the suture into position. These needles
can be made
from metallic or polymer elements or utilize sutures that may be inserted
through the
anatomy. They would range in diameters from 0.002 inches to about 0.040 inches
and may
protrude into the anatomy from 0.005 inches to about 0.090 inches depending
upon the
anatomy.
[0250] With reference to Figures 27A-C, in yet another embodiment,
the valve
100 is stapled or clipped into place with a single or multiple detachable
staples, clips, barbs
or hooks. As shown in Figure 27A, the valve 100 can be positioned over the
native aortic
valve 24. In this embodiment, the valve 100 is temporarily secured by control
wires 230 as
will be explained in more detail below. A surgically inserted or
percutaneously insert tool
232 is positioned near the valve 100 and is used to insert clips 234 or other
type of anchor
around the annulus and allows them to engage the tissue and/or portion of the
valve 100.
The staples, clips, hooks or barbs could also be delivered percutaneously with
a device that
positions the staples, clips, hooks or barbs near or below to the native
valve. These could be
attached through a balloon, pull wire or other force mechanism to push or pull
them into
position. The tool 232 used to stapled in place the valve 100 can be similar
to those used to
connect the mitral valve leaflets together by the company E-Valve and
described in US
patent publication 2004/0087975 Lucatero, Sylvester et al, which is hereby
incorporated by
reference herein. Figures 27D and 27E illustrate an embodiment in which the
tool 232
includes a tensioning wire 233, which has a distal end that is preferably
coupled to the distal
end of the device 232 and has a proximal end that extend through the device
232. By
applying tension to the wire 233, the top of the too 232 can be bent towards
the wall of the
aorta as shown in Figures 27D and 27E.
[0251] In one embodiment wires similar to, the control wires 230
described in
this application serve as guide wires over which the secondary anchoring
catheter is
delivered. This allows the precise placement of the anchors, staples, sutures
etc. relative to
the prosthesis, because the anchor catheter will follow the wire right to the
desired anchor
location. In one embodiment the anchor location is at the valve commisures. In
another
embodiment the anchor location is at the proximal end of the device. The
anchor delivery
- 54 -
CA 3014203 2018-08-14
,
catheter may consist of a multi lumen tube where one lumen serves to track
over the wire
and the second lumen or additional lumens deliver the anchor. In one
embodiment the
anchor is a screw which is actuated with a rotational motion and threaeded
through the
prosthesis and in to the aortic wall. Other anchor designs described in this
application may
also be adapted to the anchor delivery catheter.
[0252] In another embodiment, an adhesive is used to secure the
valve 100 to the
tissue. For example, adhesives such as a fibrin glue or cyanoacrylate could be
delivered
percutaneously or surgically to attach the valve 100 to the tissue. A method
for
percutaneously delivering an adhesive includes channeling it through a tubular
support
member, which has openings around its outer surface to allow the adhesive to
be released.
The adhesive could be used in conjunction with other anchoring methods to
ensure that no
blood leaks around the valve 100. Adhesion enhancing surfaces can be provided,
such as
ePTFE patches or jackets, to promote cellular in-growth for long term
anchoring.
[0253] With reference to Figure 28, in another embodiment, a barb,
anchor, hook
or pin 220 is located within a fold 110 of the cuff 102. When the inflation
channels 120 are
not inflated, the flange 222 of the anchor 220 does not extend in a radial
direction. As the
inflation channels 120 in the cuff 102 are inflated and deployed, the anchor
220 is configured
to unfold moving the flange 222 of the anchoring mechanism 220 into a radially
protruding
position. In such an embodiment, a section of the cuff 102 can be reinforced
to inhibit the
anchoring mechanism from puncturing the fabric or inflation passages 120 of
the cuff 102.
Preferably, the anchoring mechanism 220 is located so that the sharp end 224
of the anchor
mechanism is designed to engage the tissue is not located near an inflation
passage 120, and
is oriented so that it is unlikely that the anchoring mechanism 220 could
damage an inflation
passage during normal use of the device. The anchor mechanism 220 could be
attached to
the cuff 102 in many ways, for example the end of the anchor mechanism 220 not
intended
to engage tissue could be sutured, glued or crimped to the cuff In this case
the sutured end
of the anchor mechanism 220 can have a shape that prevents disengagement from
the
sutures. The anchor mechanism 220 may have holes through it, which the sutures
pass
through, or the anchor mechanism may be made from wire and shaped in a
configuration that
does not allow the disengagement of the sutures. One suitable pattern is a
generally circle, or
- 55 -
CA 3014203 2018-08-14
oval shape. Others would be apparent to one skilled in the art. Figure 29
illustrated a
modified embodiment in which the anchor mechanism is positioned on an
inflatable strut. In
yet another embodiment, the anchors 220 can be fixed to the device at or near
the attachment
point of the deployment control wires 230 to provide a solid engagement for
each anchor,
and to test the engagement of each anchor individually.
[0254] In the embodiment of Figures 28 and 29, the anchor 220 can
comprise a
laser cut tubular member attached to the inflation lumens such that they
deploy and expand
radially when inflated and provide an exposure to a point or hook 224. These
expansion
members could be cut from stainless steel and be plastically deformable or a
super-elastic
material such as Nitinol and recover as the inflation lumen is deflated thus
hiding the point
or hook from tissue exposure. It may be desirable to wrap these devices around
the inflation
lumen and attach them to the cuff for stability. A longer device may provide
better stability
since the forces would be spread out over a longer distance. A single device
or multiple
hooks may be required to anchor the cuff properly. The hooks 224 may be
pointed either
proximally or distally or in both directions if desired. The hooks 224 in
these embodiments
would preferably be bent from the axial direction between 40 and 95 degrees.
[0255] Figure 28A illustrates an other embodiment of an anchor 224.
In this
embodiment, the anchor is supported between a pair of annular stents 221 that
are formed
with proximal and distal bends in a generally sinusoidal pattern. The stents
221 can be
wrapped around an inflation lumen as shown. In one embodiment, the hook 224 is
moved
into a radially extending position as the stents 221 are expanded by the
inflation lumen.
[0256] In another embodiment, the distal and proximal ends 128, 126
of the
implant 100 can be sized to provide an anchor functions For example, as
described above
with reference to Figure 3A, the valve 100 can utilize a distal or proximal
ends 128, 126 of
larger diameter than the middle portion 124 of the valve 100. In a preferred
embodiment, the
implant 100 includes both an enlarged distal and an enlarged proximal ends
128, 126. This
produces a device with an hourglass shape as shown in Figure 2A. The enlarged
sections
128, 126 of the valve 100 inhibit the device from migrating proximally or
distally. It is also
possible to shape the transitions of the implant 100 so that the cone shape
produces a wedge
effect in a desired location, thereby increasing the radial force.
Alternatively it is possible to
- 56 -
CA 3014203 2018-08-14
,
shape the transitions with a shallow angle so that the implant is shaped like
a rivet and the
radial force caused by the application of axial force is minimized. The axial
force is applied
to the implant by the pressure of the blood acting on the area of the implant.
This axial force
must be reacted by a normal force on the surface of the implant. The implant
100 can be
designed so that the radial component of the normal force at any desired
location is any
desired ratio of the axial force.
[0257] For an implant 100 that utilizes an hourglass shape as
described above,
the orientation of the anchoring mechanisms 220 described above can be adapted
from
radially expandable applications can be reevaluated and reapplied. For example
barbs could
be placed on the most distal portion 128 of the hourglass shaped structure and
the barbs
would preferably be oriented approximately parallel to the axial direction.
See e.g., Figure
Figure 28. During the deployment procedure, the implant can be pulled back
into the
annulus after the distal portion 128 inflated. An axial force is then applied
by the inflation
lumens 120 to the anchoring mechanism 220.
[0258] Figure 30 illustrates an embodiment of an actuated
anchoring mechanism
240. In this embodiment, a rod member 242 is coaxially positioned within a
tube 244
positioned generally on the outer surface of the valve 100. A radially
extending hook or barb
246 is attached to the rod member 242 and extends through a slot 248 formed in
the tube
244. In a first position, the barb 246 extends generally against the outer
surface of the valve
100. When the rod 242 is rotated, the barb 246 rotates away from the valve 100
to expose
the barb 246 and form an anchor. When rotated back, the barb 246 would
unexposed such
that the valve 100 can be delivered or repositioned. In the illustrated
embodiment, the rod
member 242 is coupled to the control wire 230. The slot 248 forms a ramp or
guide that
promotes rotational movement and exposure of the barb 246 as the rod member
242 is
axially moved within the tub 246. The mechanism could also be driven
hydraulically by the
inflation of the device.
[0259] Figure 30A illustrates another embodiment of an actuated
anchor
mechanism 240. In this embodiment, the mechanism 240 comprises a proximal tube
portion
250 and a distal tube portion 252, which interface at corresponding tapered
faces 254a, 254b.
By applying a force to the two sections of tube, the distal portion 252 moves
both
- 57 -
CA 3014203 2018-08-14
longitudinally and horizontally exposing a sharp section 256 of the lower
portion 252 to the
tissue wall. Once exposed and engaged to the wall of the tissue, the device
could be locked
by maintaining a force on control wire 230 or by using an interference fit
such as a screw and
nut to hold the device in place.
[0260] Figure 31 illustrates another embodiment of an actuated
anchoring
mechanism 240. In this embodiment, the anchor 240 comprises a tubular member
260, with a
pattern 262 cut into the tubular member 260. The control wire 230 extends
through the tubular
member 260 and is attached to a distal stop 264. The tubular member 260 is
attached to the
cuff 102 by sutures, adhesives etc. By pulling on the control wire 230,
longitudinal
compression forces cause the tube to buckle exposes a hook or barb 266 to the
tissue wall. The
tube 260 may be made from a metallic material such as stainless steel or
Nitinol. If the tube
260 is super-elastic it can be possible to recover the hook 266 when the force
is released. If
made from a stainless steel or the like, the anchor 240 can be plastically
deformed and the
exposure of the hook 266 would be set. The actuation of this anchor 240
generally requires a
longitudinal force to buckle the tube 260 and may require a lock to hold the
tension in the pull
wire 230. This lock could be maintained by an interference fit such as a screw
and nut.
[0261] In this embodiment, the hook 266 can be cut from a hypotube
260 of
slightly larger inside diameter than the deployment control wire 230 outside
diameter.
Preferably these diameters are in the range of 0.01 to 0.03 inch. The hook 266
preferably
extends from the device at an angle of 10 to 80 degrees, more preferably at an
angle of 20 to
45 degrees.
[0262] Figure 32 illustrates another embodiment of an actuating
anchor
mechanism 240. In this embodiment, the anchor 240 comprises a pre-shaped
finger 270 that
was cut into a tube 272 and formed such that it would bend through the tubes
inner diameter
274 and expose a point on the opposite side of the tube. A window 276 cut
through both
walls of the tube 272 would allow for this exposure of the hook 270. A wire
230 can be
placed through the tube 272 to would interfere with the hook to hide it for
delivery and
recovery. This pivoting hook 272 could also be used on the same wall side if
the attachment
to the tube was in the center of the hook 272. Similar locking devices for the
wire could be
- 58 -
CA 3014203 2018-08-14
used if necessary described above. In the illustrated embodiment, the tube 272
includes slots
278 cut into the wall of the tube 272 to enhance the flexibility of the tube
272.
[0263] Figure 32A illustrates yet another embodiment of an actuating
anchor
mechanism 240a. In this embodiment, the anchor 240a also comprises a pre-
shaped finger
270a that was cut into a tube 272a and formed such that it would a first end
273a bend through
the tube's inner diameter 274a and expose a point 273b on the opposite side of
the tube 272a.
A window 276a cut through both walls of the tube 272a would allow for this
exposure of the
hook 270a. A wire 230 can be placed through the tube 272 to would interfere
with the side
272a of the hook 270 to deflect the point 273b for delivery and recovery.
[0264] Figure 33 illustrates another embodiment of an actuating
anchor 240. In
this embodiment, a tubular member 280 is attached to the cuff 102. A coaxial
member 282
(e.g., a distal end of the control wire 230) is positioned within the tubular
member 280 and
provided with a hook 284 that can be attached or integral to the coaxial
member 282. When
the coaxial member 282 is moved longitudinally within the tubular member 280
the hook
284 is exposed through a window or opening 286 in the tube 280. If pre-shaped
Nitinol is
used the hook 284 can be recoverable and hidden back into the tube 280 for
removal. The
hook 284 can face either proximal or distally or both directions for device
stability.
[0265] Delivery Catheter
[0266] Figures 34-37 illustrate an exemplary embodiment of a delivery
catheter
300 that can be used to deliver the valve 100 describe above. In general, the
delivery
catheter 300 can be constructed with extruded tubing using well known
techniques in the
industry. In some embodiments, the catheter 300 can incorporates braided or
coiled wires
and or ribbons into the tubing for providing stiffness and rotational
torqueability. Stiffening
wires may number between 1 and 64. More preferably, a braided configuration is
used that
comprises between 8 and 32 wires or ribbon. If wires are used the diameter can
range from
about 0.0005 inches to about 0.0070 inches. If a ribbon is used the thickness
is preferably
less than the width, and ribbon thicknesses may range from about 0.0005 inches
to about
0.0070 inches while the widths may range from about 0.0010 inches to about
0.0100 inches.
In another embodiment, a coil is used as a stiffening member. The coil can
comprise
between 1 and 8 wires or ribbons that are wrapped around the circumference of
the tube and
- 59 -
CA 3014203 2018-08-14
,
embedded into the tube. The wires may be wound so that they are parallel to
one another
and in the curved plane of the surface of the tube, or multiple wires may be
wrapped in
opposing directions in separate layers. The dimensions of the wires or ribbons
used for a
coil can be similar to the dimensions used for a braid.
[0267] With initial reference to Figure 34, the catheter 300
generally comprises
an outer tubular member 301 having a proximal end 302 and distal end 304 and
an inner
tubular member 305 also having a proximal end 303 and a distal end 307. The
inner tubular
member 305 extends generally through the outer tubular member 301, such that
the proximal
and distal ends 303, 307 of the inner tubular member 305 extend generally past
the proximal
end and distal ends 302, 304 of the outer tubular member 301. The proximal end
303 of the
inner tubular member 305 includes a connection hub or handle 306 to mate other
lab tools
and to grasp and move the inner member 305 with respect to the outer member. A
hemostasis valve 308 is preferably provided between the inner and outer
members 301, 305
at the proximal end 302 of the outer tubular member 301. A strain relief 313
is preferably
provided between the inner tubular member 305 and the handle 306 to limit
strain on the
inner member 305. The proximal end 302 of the outer tubular member 301 can
include a
grasping member or handle (not shown) for holding the outer tubular member 301
stationary
with respect to the inner tubular member 305.
[0268] In one embodiment, the outer diameter of the catheter
300 measures
generally about 0.030 inches to 0.200 inches with a wall thickness of the
outer tubular
member 301 being about 0.005 inches to about 0.060 inches. In another
embodiment, the
outer diameter ranges from about 0.15 inches to about 0.35 inches or from
about 12 French
to about 27 French. In this embodiment, the wall thickness of the outer tube
301 is between
about 0.005 inches and about 0.030 inches. The overall length of the catheter
300 ranges
from about 80 centimeters to about 320 centimeters.
[0269] As mentioned above, the catheter 300 includes a
connection hub or handle
306 that is configured to allow wires, devices and fluid to pass as will be
explained in more
detail below. The connection hub 306 is preferably compatible with normal cath-
lab
components and can utilize a threaded end and a taper fit to maintain seal
integrity. The
inner diameter of the inner member 305 of the catheter 300 is configured allow
for coaxial
- 60 -
CA 3014203 2018-08-14
,
use to pass items such as guidewires, devices, contrast and other catheters.
An inner lining
material such as Teflon may be used to reduce friction and improve performance
in tortuous
curves. Additionally, slippery coatings such as DOW 360, MDX silicone or a
hydrophilic
coating from BSI Corporation may be added to provide another form of friction
reducing
elements.
[0270] Multidurometer materials in the catheter 300 can help
to soften the
transition zones and add correct stiffness for pushability. Transition zones
may also be
achieved through an extrusion process know as bump tubing, where the material
inner and
outer diameter change during the extrusion process. The entire catheter shafts
301, 305 can
be produced in one piece. Another method for producing such a catheter shaft
is to bond
separate pieces of tubing together by melting or gluing the two components
together and
forming a single tube with multiple diameters and or stiffness. The
application of heat can
be applied by laser or heated air that flows over the shaft material or other
methods of heat
application sufficient to flow the materials together.
[0271] With continued reference to Figure 34, the distal end
304 of the outer
sheath 301 comprises an enlarged diameter section 309, which is configured to
cover the
implant 100. In one embodiment, the diameter of the enlarged diameter section
309 where
the implant 100 is contained is between about 0.20 inches and about 0.32
inches in diameter
with a length between about 0.5 in and about 5.0 inches. A second portion 310
of reduced
diameter and increased flexibility is located proximal to the section 309 that
covers the
implant 100. This section ranges from about 0.10 inches to about 0.25 inches
in diameter.
In the preferred embodiment, the distal section 309 is about 0.29 inches
diameter, and about
0.08 inches in length and the proximal section 310 has an outside diameter of
about 0.19
inches. The enlarged distal portion 309 can be made from a material with a
higher
durometer than the proximal portion 310 of the catheter 300. In one
embodiment, the
material of the enlarged distal portion 309 is a biocompatible material. In
another
embodiment, the material is a metallic material such as stainless steel. In
another
embodiment, the material is a polymer such as FEP, PEEK or a polyimide. In
another
embodiment, the enlarged distal portion 309 of the device which covers the
implant 100 is
capable of transmitting light in the visible spectrum. This allows the
orientation of the
- 61 -
CA 3014203 2018-08-14
,
,
implant 100 to be visualized within the catheter 300. The distal end 304 may
have a
radiopaque marker (not shown) to locate the catheter 300 under fluoroscopy.
102721 With continued reference to Figures 34-37 and in
particular Figures 36A
and 36B, multiple tubes extend through the inner member 305. Specifically, in
illustrated
embodiment, a guidewire tube 318, two inflation tubes 320 and three control
wire tubes 316
extend from the proximal end 303 to the distal end 307 of the inner member
307. Of course,
in modified embodiments, various other numbers and combinations of tubes 316,
318, 320
can be used depending upon the configuration of the implant 100 and the
deployment
procedure. These tubes may be extruded from materials such as polyethene,
polypropylene,
nylon, PEEK, polyimid or other accepted polymer materials. They may also
combine
metallic elements such as coils or braids for additional support or be made
from metallic
tubings such as Nitinol or stainless steel. As will be explained below, the
guidewire tube
318 is configured to receive a guidewire. The inflation tubes 320 are
configured to delivery
inflation media to the implant 100 and the control wire tubes 316 receive the
control wires
230, which are coupled to the implant 100. As will be explained in more detail
below, the
inflation tubes 320 can include inner and outer members 320a, 320b (see Figure
36B) for
providing an inflation disconnect mechanism as described below with reference
to Figures
40A and 40B.
[0273] The inner member 305 material may also consist of
stiffening members
for transition zones or bump extrusions to reduced diameter and maintain
correct pushability.
Conventional guidewire passage through the catheter such as "over-the-wire"
may be used or
technology such as "rapid-exchange" may aid in procedure ease and catheter
exchanges.
Since multiple devices may be placed in a single catheterization, rapid-
exchange may be
preferred but not essential. Other features that may aid in ease of use
include a slippery
coating on the outer and or inner diameter such as mineral oil, MDX (silicone)
or a
hydrophilic layer to allow easy access to tortuous anatomy, or easier more
controlled motion
of one portion of the catheter relative to another portion of the catheter. It
may be necessary
or desirable to utilize a balloon to initiate radial contact of the device to
its final position and
location. In one embodiment, an inflation lumen and balloon placed distal to
the hubis used.
This balloon is used to pre-dilate the native valve annulus, vessel or ostium
where the valve
- 62 -
CA 3014203 2018-08-14
may be implanted. Elements to transmit signals externally could be imbedded
into the
catheter 300 for pressure and flow readings or Doppler information. These may
include
electro-mechanical sensors, such as piezo-electric devices, electrical
sensors, wires, pressure
portal or lumens or optical fibers.
[0274] As mentioned above, delivery of the implant 100 via
catheterization of the
implantation site can include a mechanism to deploy or expel the implant 100
into the vessel.
This mechanism may include a push or pull member to transmit forces to the
distal portion
of the catheter 300. These forces may be applied externally to the body and
utilize a handle
at the proximal end of the catheter. Devices to transmit forces to the distal
end may also
include a rotational member to loosen or tighten, convert a torque into a
translational force
such as a threaded screw and nut or to add or subtract stiffness to the
catheter or device, or to
cause the device to assume a specific shape. The handle mechanism may also
include a port
for hydraulic pressures to be transmitted to the distal portion of the
catheter or have the
ability to generate hydraulic forces directly with the handle. These forces
may include a
pushing or pulling transmitted to the device or catheter, an exposure of the
device to allow
for implantation or to expel the device from the catheter. Further forces may
include a radial
or longitudinal expansion of the device or catheter to implant or size the
location of
implantation. The handle may also include connections to electrical signals to
monitor
information such as pressures, flow rates, temperature and Doppler
information.
[0275] With reference to Figures 34 and 36, in the illustrated
embodiment, the
implant 100 is loaded between the distal portion 309 of the outer sheath 301
and the inner
sheath 305. The distal portion 309 therefore forms a receptacle for the
implant 100. A distal
tip 312 can be coupled to the guidewire tube 318. The tip 312 can be used to
close the
receptacle when the catheter 300 is being advanced. The tip 312 can be
distanced from the
outer sheath 301 by proximally retracting the outer sheath 301, while holding
the guidewire
tube 318 stationary. Alternatively, the guidewire tube 318 can be advanced
while holding
the outer sheath 301 stationary. Control wires 230, which extend through the
control wire
tubes 316, can be coupled to implant 100 as described below and used to hold
the implant
100 stationary as the implant outer sheath 301 is retracted. Alternatively the
outer sheath
301 can be retracted with respect to the inner sheath 305, which acts as a
pusher to push the
- 63 -
CA 3014203 2018-08-14
,
implant 110 outer of the distal portion 309 of the outer sheath. The inflation
channels 120 of
the implant 100 are preferably connected to the inflation tubes 318 of the
catheter by an
inflation connection members 321 as will be described in more detail below.
[0276] With continued reference to Figure 36, the inflation
tubes 318, guidewire
tube 320 and control wire tube 316 preferably extend to the proximal end 303
of the inner
member 305. A connection hub 323 can be provided for connecting an inflation
fluid source
to the inflation tube 318. Various control mechanism (not shown) and sealing
devices can
also be provided for connecting to the control wires 230 and control wire
tuibes 316.
[0277] As will be described in more detail below, the control
wires 230 and/or
inflation lumen 318 can form part of a deployment mechanism for the implant
100. As the
implant is navigated to the site, attachment between the implant 100 and
catheter 300 is
important. Many detachment mechanisms have been used to deploy devices such as
stents and
embolic coils through balloon expansion and simple pushable coils expelled
from the distal end
of a catheter. The implant 100 can utilize many different methods to implant
100 at the
selected site such as an expulsion out the end of the catheter, a mechanical
release mechanism
such as a pin joint, unscrewing the device from the catheter delivery system,
a tethered link
such as a thread or wire, a fusible link as used in a GDC coil deployment, a
cutting tool to sever
a attachment of the device from the catheter, a threaded knot to tether the
catheter to the device
where the as the knot could be untied or cut, a hydraulic mechanism to deploy,
expand or
fracture a link between the catheter and the device. All above mentioned
concepts can be
enhanced by the utilization of the flexible tip 312 to allow acute
articulation of the device and
delivery catheter 300 to gain access to the implantation site.
[0278] As will be explained in more detail below, after the
implant 100 has been
temporarily deployed or positioned, it may be advantageous to recapture or
reposition the
implant for optimal results. This may include a rotation or translation of the
implant 100 or a
complete removal and exchange for a different diameter, length or style
device. Capture of
an implanted device may require a second catheter to reengage the device to
remove or
reposition to a proper location. This catheter may be constructed from polymer
tubing as
described above including coils, braids, etc. Additionally there may be a
braided section at
- 64 -
CA 3014203 2018-08-14
the distal most potion of the catheter to accept or capture the device for
retrieval from the
body.
[0279] As mentioned above, the guidewire tube 320 preferably extends
through
the inner sheath 305 and the tip 312. The guidewire tube 320 may have an
inside diameter of
.035 to .042 in so that the device is compatible with common .035 or .038
guide wires. A
modified embodiment includes a lumen .014 to .017 inches in diameter for
compatibility
with .014in diameter guide wires. In a third embodiment, the guidewire lumen
320 is .039 to
.080 in diameter, so that the device may be delivered over a larger than
standard guide wire,
or a diagnostic catheter, such as a pig tail catheter. This provides the
advantage of a stiffer
support to facilitate easier delivery through calcified valves. If a
diagnostic catheter is used
as a guidewire it may also serve as a port for contrast injection.
[0280] The guidewire tube 320 can be made from a lubricious material
such as
Teflon, polypropolene or a polymer impregnated with Teflon. It may also be
coated with a
lubricious or hydrophilic coating. The tube 320 can be constructed of multiple
layers of
material, including a lubricious inner layer and an outer layer to facilitate
bonding to other
catheter components.
[0281] The catheter 300 may be delivered over a guide wire to aid in
positioning.
The guide wire may pass coaxially through the entire length of the catheter or
in modified
embodiments may pass coaxially though only a portion of the catheter in a
configuration
known as rapid exchange. This allows shorter guide wires to be used if devices
are to be
exchanged out.
[0282] In the illustrated embodiment, the catheter 300 comprises the
outer
catheter shaft 301 and the inner catheter shaft 305 which move relative to one
another. In
order to minimize the risk of guidewire damage in a rapid exchange design
where the
catheter must pass through the wall of two sheaths which move relative to one
another, a slot
feature is desirable. Either the inner or outer elongate tube may contain a
longitudinal slot in
the area where the guide wire passes from the inner diameter to the outer
diameter of the
catheter assembly. The other elongate tube preferably contains a hollow pin to
engage the
slot and prevent the excessive movement of the two elongate members. The guide
wire
- 65 -
CA 3014203 2018-08-14
passes through the opening in the hollow pin. The inner diameter of the hollow
pin is
preferably oriented at an acute angle to the central axis of the catheter.
[0283] Another design to enable rapid exchange like performance is
for the guide
wire to enter the catheter tip through a side hole distal to the location of
the prosthetic valve.
The guidewire exits the tip of the system near the center of the catheter tip.
This design
enables the catheter to follow the guide wire across the native valve, while
still allowing
multiple devices to be exchanged easily on a short length guide wire.
[0284] As described above, the internal lumens of the catheter 300
can include
the deployment control wires lumens 316, the inflation lumens 320, and an
inner sheath 307
that encapsulates these lumens 316, 320. See e.g., FIG. 36B. With reference to
FIG. 37A, in
one embodiment of the delivery system 300, a portion of, or all, of the
internal lumens 316,
320 are located within the delivery catheter 300 at the distal portion 304 of
the catheter, and
pass through a hole 650 in for example a middle portion 652 of the delivery
catheter 300 so
that they are located generally parallel to the delivery catheter 300 at the
proximal end 306 of
the catheter 300. In one embodiment, the hole through 650 which the lumens
316, 320 pass
can be located between about 2 and about 20 cm from the distal end 304 of the
device 300.
The outside diameter of the delivery catheter 300 is substantially reduced
proximal to the
hole as shown in FIG. 37A, so that the entire device 300 may pass through most
common
introducers that are large enough to accept the distal portion 304 of the
device 300.
[0285] This catheter configuration advantageously allows the operator
to easily
switch between the delivery sheath 300 and a recovery sheath (described
herein) in the event
that the device 100 needs to be recovered, because the delivery sheath 300 can
be retracted
out of the body over relatively short internal lumens 316, 320, while still
maintaining a
portion of the lumens 316, 320 outside the catheter so that the operator can
manipulate them
as necessary.
[0286] Because of its shorter length the recovery sheath may not
require the
exchange hole 650, and it may be possible to locate the internal lumens
coaxially within the
recovery sheath. However in the preferred embodiment the recovery sheath also
includes a
hole in a similar location allowing the internal lumens to pass coaxially
through the distal
- 66 -
CA 3014203 2018-08-14
,
portion of the sheath, through the hole, and be located generally parallel to
the recovery
sheath in the proximal portion.
[0287] In one embodiment contrast media is passed through a lumen
(e.g., the
guidewire tube 320) of the device, and the lumen passes through the prosthetic
valve 100.
This allows visual evaluation of valve function by angiography, without
crossing the valve
with an additional device. In the preferred embodiment the lumen crosses the
valve while
the valve is in the catheter. In the preferred embodiment the lumen also
serves as the
guidewire tube 320, where the device is delivered over a guide wire. The wire
may be
removed from the lumen to allow more cross sectional area for contrast
injection. The
proximal end of the lumen near the handle of the device attaches to a fitting
to allow the
injection of contrast media with a power injector tool. The inner diameter of
the lumen may
range from 0.014 to 0.100 inch. The diameter of the lumen may vary along the
length of the
catheter, for example, Preferably the portion of the lumen which passes
through the
prosthetic valve is of a minimum possible diameter to allow both sufficient
flow and the use
of an adequate sized guidewire. This portion is preferably in the range of
diameters from
.014 to .080. The portion of the lumen extending along the length of the
catheter proximal to
the implant may be of larger diameter, the larger diameter allows flow of
contrast media at
lower pressure gradients, and the corresponding larger outside diameter does
not increase the
profile of the complete device. This portion of the lumen is preferably in the
inside diameter
range of 0.035 to 0.100 in. The distal portion of the lumen may contain a
diffuser or
transition to a larger diameter to minimize the pressure required to inject a
sufficient volume
of contrast media through the lumen. Multiple exit ports positioned around a
nose cone also
facilitate the flow of contrast media.
[0288] Access for the catheter 300 may be gained through a major
artery such as
the femoral artery. This access site is particularly appropriate for aortic
valve replacement.
Alternative access methods may be better suited for other valves. For example
the tricuspid
valve and possibly the pulmonary valve could best be accessed through the
venous system.
In this case, access would be gained through either a femoral vein or a
jugular vein. The
catheter would then be passed into the right atrium through the superior or
inferior vena
cava. Some embodiment of the current invention utilize a relatively large
diameter catheter,
- 67 -
CA 3014203 2018-08-14
,
,
which may not be compatable with the diameter of all patients femoral
arteries. In these
patients it may be desirable to access the common iliac artery or to use a
transeptal approach
and acess the heart through the venous system.
[0289] As mentioned above, the catheter 300 includes an
atraumatic tip 312 to
allow the device to be easily placed through the hemostasis valve of the
introducer, and to
easily cross the calcified aortic valve. The tip 312 may be cone shaped bullet
shaped or
hemispherical on the front end. The largest diameter of the tip 312 is
preferably
approximately the same as the distal portion 309 of the outer sheath 301. The
tip 312
preferably steps down to a diameter slightly smaller than the inside diameter
of the distal
portion 309 of the outer sheath 301, so that the tip can engage the outer
sheath 301 and
provide a smooth transition. In the illustrated embodiment, the tip 312 is
connected to the
guide wire tube 320, and the guide wire lumen passes through a portion of the
tip 312. The
proximal side of the tip 312 also has a cone, bullet or hemispherical shape,
so that the tip can
easily be retraced back across the deployed valve 100, and into the deployment
catheter 300.
The tip 312 can be manufactured from a rigid polymer such as polycarbonate, or
from a
lower durometer material that allows flexibility, such as silicone.
Alternatively, the tip 312
may be made from multiple materials with different durometers. For example,
the portion of
the tip 312 that engages the distal portion 309 of the outer sheath 301 can be
manufactured
from a rigid material, while the distal and or proximal ends of the tip are
manufactured from
a lower durmoter material.
[0290] With reference to Figures 35A and 35B, in a modified
embodiment, the
area where the tip 312 of the device is located to house a balloon 312a for
dilatation. This
balloon 312a could use the lumen where a guidewire passes through (as shown in
the
illustrated embodiment) or a separate lumen for inflation and deflation. Since
the distal
portion 309 is rather large (10-24 French) it can be advantageous place to
locate a large
diameter balloon that could be used to pre or post dilate the valve area.
There may also be a
stent or other structure mounted to this balloon 312a for device securement or
anchor
deployment. The balloon 312a could also be covered with a thin membrane
material similar
to the "SOX" device commercialized by Boston Scientific and seen in US patent
number
6,280,412 Pederson Jr. et al. This covering would allow the device to be
hidden during
- 68 -
CA 3014203 2018-08-14
delivery and could be exposed when inflated. In another embodiment, a tear-
away sheath
that covered the balloon 312a for protection can be used.
[0291] Figures 38A-38C illustrate one embodiment of a retractable
sheath 340
that may be used in combination with the deployment catheter 300 described
above. Many
implantable medical devices have been delivered using retractable sheaths. For
example,
some devices include self-expanding stents, and grafts to percutaneously treat
abdominal
aortic aneurisms. On problem with this design is that the catheter must slide
over the
implant, resulting in a scraping and shear forces. For a delicate implant such
as a tissue
valve or abdominal aortic aneurism graft, this scraping or shearing may result
in damage to
the implant. In less fragile devices such as self-expanding stents the sheath
material may be
scraped off and embolized. Several medical devices have solved this problem
using a
radially expandable shear barrier, as described by Chobotov. This shear
barrier in practice
typically consists of a thin walled piece of tubing, slit along its length in
several places. As
the outer sheath is retracted, it slides along the slit tubing. Once the outer
sheath has
retracted past the slit tubing, it can expand radially allowing the device to
be released.
[0292] The retractable sheath 340 of Figures 38A-C serves a similar
function to
the radially expandable shear barrier describe above, but provides several
advantages. For
example, as explained below, it does not have sharp edges and it can be made
from a softer
material, so it is less likely to cause trauma to the patient, or damage to
the implant. In
addition, it can be made from a thinner material allowing the device 340 to
have a lower
profile. And it does not protrude the full length of the implant 100 after the
outer sheath 340
has been retracted.
[0293] As shown in Figures 38A-C, in the illustrated embodiment, the
catheter
300 the outer sheath 301 is retracted to deploy the implant 100 and the inner
sheath 305,
which is stationary relative to the outer sheath 301, acts as a pusher and
prevents the implant
100 from moving back with the outer sheath 301 during deployment. A thin
flexible
membrane 340 connects to the outer surface 342 of the pusher 305 and passes
between the
implant 100 and the outer sheath 301 and acts as a shear barrier. The flexible
shear barrier
340 then attaches to the outer distal end 344 of the outer sheath 301.
Preferably the
membrane or shear barrier 340 extends out the tip of the outer sheath 301 and
then is pulled
- 69 -
CA 3014203 2018-08-14
inside out over the outer sheath 301 as shown in Figure 28A. The membrane or
shear barrier
340 is then bonded to the outer sheath 301 on its outer surface 342 near the
tip of the outer
sheath 301. In a modified embodiment, the flexible shear barrier 340 is bonded
to the inner
surface 346 of the outer sheath 301. The shear barrier 340 is preferably made
from a
polymer and has a thickness of about 0.0002 inches to about 0.0020 inches. In
one
embodiment, the polymer is nylon. The shear barrier 340 can be manufactured by
an
extrusion process or by a balloon blowing process where a polymer tubing is
inflated inside a
mold using heat and pressure.
[0294] As shown in Figures 38B and 38C, as the outer sheath 301 is
pulled back
the membrane 340 turns inside out and retracts from the implant 100, doubling
over on its
self. The sliding occurs between the flexible membrane 340 and the inner
surface 346 of the
outer, retractable sheath 301. Advantageously, little or no relative motion
occurs between
the implant 100 and the portion of the membrane 340 in contact with the
implant 100. This
minimizes any potential damage to the implant 100, and the risk of embolizing
particles from
the sheath 301. A lubricant can be applied between the outer sheath 301 and
the membrane
340 and between the outer sheath 301 and the pusher 305. The membrane 340
advantageously serves to isolate the implant 100 and the patient from the
lubricant. This
embodiment reduces the force necessary to deploy the implant 100, and allows
for a
smoother more controlled deployment.
[0295] With reference back to Figure 34, the hemostasis valve 308 is
preferably
is attached to the proximal end of the outer sheath 301 to prevent blood from
leaking past the
inner and outer sheaths 301, 305. In one embodiment, the valve 308 is a touhy-
borscht
design valve, or simiar valve where the radial compression is easily
adjustable. By adjusting
the valve it is possible to lock the outer sheath 301 to the inner sheath 305
of the catheter 300
to prevent their accidental relative motion during delivery of the implant. At
the proximal
end 304 of the catheter 300, an additional hemostasis valve (not shown) is
preferably
provided to provide a seal for the multiple inflation lumens, and deployment
control wires
that must pass through the inner sheath 305. An additional port (not shown)
can also be
provided to allow the catheter 300 to be flushed to remove any traped air
before the catheter
300 is inserted into the patient.
- 70 -
CA 3014203 2018-08-14
[0296] Connection between Implant and Inflation Lumens
[0297] As described above, in many embodiments, the implant 100
includes an
inflatable structure 107, which defines inflation channels 120. In these
embodiments, the
inflation channels 120 are inflated with inflation media 122 to provide
structure to the
implant 100. As shown in Figures 34-37, the deployment catheter 300 includes
at least one
inflation tube 318 and in the illustrated embodiment two inflation tubes 318
that extend
through from the proximal end 304 to the distal end of 302 of the catheter
300. The inflation
tubes are placed in communication with the inflation channels 120 such that
inflation media
122 can be supplied to the infltable structure 107. It will be appreciated
that after the
inflatable structure 107 is inflated the inflation tubes 318 will need to be
disconnected or
uncoupled from the implant 100. Various devices and methods for uncoupling the
implant
100 from the inflation tubes 318 will now be described.
[0298] In general, in embodiments in which the inflation media 122 is
not self
sealing the inflation channels 122 will need to be sealed as the inflation
lumen 318 is
disconnected from the implant 100. Sealing of these lumens could utilize many
different
techniques known to one skilled in the art. For example, as explained below,
the inflation
lumen can be placed through a valve, in such a way that it forces the valve
into the open
position. The valve could be one of a variety of normally closed or one way
(check) valves.
[0299] For example, Figure 39A illustrates an embodiment of a
connection
mechanism 350 that includes a check valve 352 comprising a spring 354 and a
ball member
356. The spring 354 and ball member 356 are positioned within a chamber 358
having a
first open end 360 that is in communication with the inflation channels 120
and a second
open end 362 that is in communication with the inflation tube 318. The spring
354 is
supported by a narrowed portion 364 of the first open end 360. The spring 354
biases the
ball 356 against a valve seat 366 formed by the second end 362 of the chamber
358. In the
biased closed position, the ball 356 prevents inflation media 122 from exiting
the inflation
channels 120. When inflation media 122 is applied under pressure to the
inflation channels
120, the pressure pushes the ball away from the valve seat 366 and into the
chamber 358
allowing inflation media 122 to flow into the inflation channels 120. When the
pressure is
removed, the spring 354 forces the ball 356 against the valve seat 366 to
prevent the inflation
- 71 -
CA 3014203 2018-08-14
,
media 122 from escaping. A pin 368 can extend through the inflation lumen 318
and can be
used to push against the ball 356, disabling the check valve 352 and allowing
deflation of the
inflation channels 120.
[0300] Figure 39B illustrates another embodiment of a check
valve 352. In this
embodiment, check valve 352 comprises a duck-bill valve that includes at least
two flanges
or bills 370a, 370b that are biased towards each other to close the inflation
channel 120. As
with the ball valve described above, a pin 368 can be used to open the valve
325 and allow
deflation of the inflation channels 120.
[0301] Figure 39C illustrates another embodiment of a sealing
mechanism 350.
In this embodiment, the inflation lumens 120 are inflated using a needle (not
shown) placed
through a soft polymer plug 372 positioned between the inflation lumen 318 and
the inflation
channels 120. The needle is withdrawn from the plug 372 and the plug closes
the hole
formed by the needle, preventing the loss of fluid or pressure. In the
preferred embodiment
the plug 372 is silicone inside a nylon, PE or PET tube 374. After the
silicone is cured and
bonded to the tube the tube may optionally be necked 376 to place a
compressive force on
the silicone plug 372. The proximal and distal sections of the tube
surrounding to the plug
can be necked to an even smaller diameter, to prevent the migration of the
plug. The
diameter of the needle may range from 0.010 to 0.050in with a diameter of
about 0.020in as
the currently preferred diameter. The plug 372 diameter may range from 0.020
to 0.120in.
In the illustrated embodiment, the plug 372 also includes an enlarged distal
section 376,
which abuts against a distally facing ledge 378 provided within the tube 374
to secure the
axial position of the plug 272. The proximal end 280 of the plug 372 can have
an outward
taper as shown to further secure the plug 372 within the tube 374.
[0302] Figure 39D illustrates another embodiment in which the
connection
mechanism comprises a rupture disk 375, which is secured within an inside
surface of a fluid
tight chamber 377. The disk 375 is configured to rupture and allow the
inflation of the
inflation channels 120 when sufficient pressure is applied.
[0303] In some embodiments, it is advantageous to configure
the deployment
catheter 300 and the implant 100 such that the inflation tube 318 cannot
disconnected
unintentionally. For example, in one embodiment, the inflation tube 318 is
connected to a
- 72 -
CA 3014203 2018-08-14
deployment control wire 230 so that the inflation lumen 218 can not be removed
from the
implant 100 unless the deployment control wire 230 is also disconnected from
the implant
100.
[0304] Figures 40A and 40B illustrates one embodiment of sealing and
connection mechanism 399. In this embodiment, the balloon 111 is connected to
a piece of
tubing 400. Within the tubing 400, is positioned a seal-sealing plug 402,
which can be
configured as described above with reference to Figure 39C. A tip 404 of the
inflation
lumen 318 is configured to be inserted through the plug 402 such that
inflation media can be
injected into the balloon 111. A connection balloon 406 is positioned
generally around the
tip 404 and proximally to the plug 402 within the tubing 400 A fluid channel
408 connects
the connection balloon 406 to an inflation port 410 on the proximal end of the
catheter 300.
In use, the balloon 11 is inflated with inflation media provided through the
tip 404. To
disconnect the inflation lumen 318 from the tubing 400, the connection balloon
406 is
deflated as shown in Figure 40B allowing the inflation lumen 318 to be
withdrawn with
respect to the plug 402 and tubing 400. A stop or narrowed region (not shown)
can be
provided within the tubing 400 to enhance the connection between the inflated
connection
balloon 406 and tubing 400.
[0305] Figure 41 illustrates another embodiment of sealing and
connection
mechanism 399. In this embodiment, the mechanism 399 comprises a ball and
spring type
check valve 412, which can be arranged as described above with the connection
portion 351
of the balloon 111. A connection mechanism 416 comprises an outer layer 418
and inner
layer 420 of coaxial tubes. The inner layer 418 includes an engagement feature
such as a
bump 422 that engages a corresponding engagement feature 424 on an outer
surface 426 of
the balloon 111 or other portion of the implant 100. As shown in Figure 41,
the outer layer
418 extends over the engagement features 424, 426. The outer layer 418 is
provided with a
diameter that it forces the engagement feature 422 on the inner layer 420 to
remain engaged
in the engagement feature 424 on the balloon 411. As the outer layer 418 is
retracted, the
inner layer 410 in the area of the feature 422 is free to disengage from the
engagement
feature 424 on the balloon 422. In the illustrated embodiment, the inner layer
420 defines in
-73 -
CA 3014203 2018-08-14
part the inflation lumen 318 . A push wire 368 can be provided as described
above for
deactivating the ball valve 414 and allowing deflation of the balloon 111.
[0306] Figure 42 illustrates another embodiment of a sealing and
connection
mechanism 399. In this embodiment, the mechanism 250 comprises a duck valve
430
positioned in a connection portion 351 of the balloon 111. When the catheter
300 is engaged,
the delivery tube 318 extends through the duckbill valve 420 allowing both
inflation and
deflation of the balloon 11. The tube 318 that extends through the valve 420
also extends
through a lock mechanism 432, which holds the inflation lumen attached to the
balloon. In the
illustrated embodiment, the lock mechanism 432 comprises of a lock tubing 434
that extends
approximately the length of the catheter 3000. The distal end of the lock
tubing 434 has an
enlarged ridge 436, and longitudinal slits 438 extending through the ridge
426. The distal end
of the lock tubing 424 fits in an orifice plug 440, which is inserted into the
connection portion
351 of the balloon 111 in line with the duckbill type valve 430. The orifice
has a groove recess
442 to receive the enlarged ridge 436 of the lock tubing 434. The longitudinal
slits 438 in the
lock tubing 434 allow it to collapse sufficiently to easily engage and
disengage from the groove
442 and the orifice 440. The inflation tube 318 extends through the lock
tubing 434 preventing
it from collapsing and releasing from the balloon 111.
[0307] After the balloon 111 has been inflated with the desired
inflation media
and the operator has chosen to disconnect the catheter 300 from the implant
100, the
inflation tube 318 is withdrawn past the duckbill valve 430. At this time
suction may be
applied to remove as much inflation material as possible from the area past
the valve 430. A
rinse procedure could also be used to remove additional fluid. The inflation
tube 318 is then
withdrawn past the enlarged ridge 436 and the slit portion of the lock tubing
434. The lock
tubing 434 can then be withdrawn from the orifice 440, and the implant 100 is
separated
from the catheter 300.
[0308] Figure 43 illustrates another embodiment of a sealing and
connection
mechanism 399. In this embodiment, connection portion 351 of the balloon 111
comprises a
threaded bore 448 and a valve seat 450 positioned generally proximally of the
threaded bore
448 within a fluid channel 452. A threaded portion 454 of a screw 456 is
positioned within
the bore 448. An enlarged, sealing portion 457 of the screw 456 is positioned
within the
- 74 -
CA 3014203 2018-08-14
fluid channel 452 proximal to the valve seat 450. As the screw 456 is threaded
into the bore
448, the head 457 engages the seat 450 to seal the fluid channel 452 formed in
the
connection portion 351. The delivery or connection tube 318 includes a distal
end 460 that
can be inserted into the connection portion 351 of the balloon 111 to place
the delivery
lumen 318 in communication with the fluid channel 352. The distal end 460 can
be provided
with releasable tangs 462 that engage a corresponding groove 464 formed on the
inner
surface of the connection portion 351. The screw 458 is activated by a driver
466 that
extends through the inflation tube 318 as shown in Figure 43.
[0309] Control wires
[0310] As discussed previously above, one advantage of many of the
embodiments described herein is that the deployment of the implant 100 can be
controlled.
In one embodiment, the deployment of the implant is controlled via the use of
control wires
230 that can be detachably coupled to the implant. Various mechanisms for
detachably
coupling the control wires 230 to the implant 10 will now be described.
[0311] With initial reference to Figure 44, of the control wires 230
are attached to
the cuff 102 of the implant 100 so that the implant 100 can be controlled and
positioned after
it is removed from the sheath or delivery catheter 300. The wires 230 are
preferably stiff
enough to prevent the implant 100 from rotating in a direction that would
reduce the
effectiveness or prevent the valve 104 from performing its function, of
allowing blood flow
only in a correct direction. Advantageously, the wires 230 would attach to the
implant 100
in a proximal location and in a distal location. This would limit the degrees
of freedom of
the implant 100 relative to the wire 230, and minimize the possibility of the
valve 104 or
implant 100 of being damaged by the distal end of the wire 230.
[0312] With continued reference to Figure 44, in the illustrated
embodiment, the
mechanism for coupling the wires 230 to the implant 100 incorporates a sheath
470 that
extends over most of the length of the wire 230. The sheath is skived in at
least one
preferably two, locations to form skive(s) 272. At the skive or skives 472, a
portion 474 of
the cuff 102 or a portion of a member attached to the cuff 102 passes between
the wire 230
and the sheath 470. With this method, the wire 230 may be released from the
cuff 102 by
withdrawing the wire 230 from the sheath 470 until the tip of the wire 230
extends past the
- 75 -
CA 3014203 2018-08-14
,
,
skive or skives 472. In a preferred embodiment, the sheath 470 can be formed
from part of
the control wire tubes 316 that extend through the deployment catheter 300.
[0313]
Preferably, three wires 230 are used, but any number between 1 and 10
can provide good results. The diameter of the wire 230 can range from about
0.002 inches to
0.020 inches. The wires 230 can be manufactured from a metal suitable for
blood contact
such as nitinol, stainless steel or one of many cobalt chrome nickel and/or
iron based alloys.
The wires 230 can also be made of a polymer that has the desired mechanical
properties such
as a polyimide. The sheath 472 can be manufactured from the many polymers
suitable for
blood contact including nylons Teflon PBX polyethylene polypropylene
polyimides etc. The
sheath 470 is preferably sufficiently rigid in the axial direction to prevent
the accidental
disconnection of the valve 100, so the dimensions of the sheath depend on the
axial stiffness
of the material. A polyimide sheath 470 with a 0.026 inches outside diameter
and a 0.005
inch thick single wall has proven adequate, while a grillamid nylon sheath
with a 0.030 inch
outside diameter and a 0.007 inch thick single wall has also proven adequate.
Preferably the
polymer sheath 470 ranges in outside diameter from about 0.018 inches to 0.040
inches and
in wall thickness from about 0.003 inches to about 0.010 inches. Additionally
a stainless
steel, nitinol or other metallic sheath cab be utilized. In this case, smaller
diameters and
thinner wall thicknesses are generally desirable. In one embodiment, the
stainless steel
sheath 470 has a 0.014 outer diameter with an inner diameter of about 0.011
inch and the
wire 230 with a 0.009 inchs outer diameter. With a metallic sheath 470, the
preferred wall is
about 0.0005 inch to about 0.0050 inch thick and the preferred outside
diameter is about
0.007 inches to about 0.025 inches. The inside diameter of the sheath 470
should provide
clearance to move freely over the wire 230. A clearance of 0.00 to about 0.007
inches
should provide adequately free motion. A lubricant or hydrophilic coating may
be applied to
the inside diameter of the sheath 470, or the outside diameter of the wire
230. Different
clearances may be required with less lubricious polymers. In addition,
extrusion parameters
may be adjusted to produce a surface finish on the inner diameter of the tube
470 that
optimizes the motion of the sheath 470 relative to the wire 230. With some
polymers a
rougher surface may result in reduced friction. As mentioned above, the ideal
wall thickness
- 76 -
CA 3014203 2018-08-14
,
of the sheath 470 depends on the strength and stiffness of the particular
material selected, but
likely ranges between .002 and .020 inches, single wall thickness.
[0314] The proximal end of the deployment control wires 230
preferably contains
a lock mechanism (not shown) to prevent the unintended relative motion of the
wire relative
to the sheath 470. The wires 230 may also be attached to a handle section that
allows the
relative movement of one wire individually or multiple wires together. In one
embodiment
the three wires 230 are attached to a ring, equally spaced around the edge of
the ring. As the
ring is moved proximal or distal relative to the main handle component the
implant 100
moves proximal or distal relative to the catheter tip. As the ring is tilted
off axis with the
axis of the catheter handle, the implant 100 is tilted in a similar direction.
[0315] The deployment control mechanism can performs several
functions. First
as described above, during the initial deployment of the implant 100, it
prevents the implant
100 from rotating off axis. Additionally the deployment control mechanism
allows the
implant 100 to be repositions after it has been removed from the sheath. The
wires described
above could be used to move the implant 100 proximally and distally.
[0316] With reference to Figures 45A-C, in one embodiment,
the implant 100 is
initially deployed partially in the ventricle 32(Figure 45A) and then later
pulled back into
position at or near the native valve 34 annulus (Figure 45B). Preferably, the
valve 100 itself
is placed just above the native valve annulus in the aortic root. The implant
100 can then be
fully deployed (e.g., inflated) such that extends across the native valve
annulus extending
slightly to either side. See Figure 45C. The deployment control wires 230
provide a
mechanism for force transmition between the handle of the deployment catheter
300 and the
implant 100. By moving all of the deployment control wires 230 together the
device can be
advanced or retracted in a proximal or distal direction. By advancing only a
portion of the
deployment control wires 230 relative to the other deployment control wires
230, the angle
or orientation of the wires can be adjusted relative to the native anatomy.
Radiopaque
markers on the implant 100 or on the deployment control wires 230 or the radio-
opacity of
the wires 230 themselves, help to indicate the orientation of the implant 100
as the operator
positions and orients the implant 100.
- 77 -
CA 3014203 2018-08-14
[0317] With reference to Figures 46A-C, the deployment control device
also
provides a method for retracting the implant 100 back into the deployment
catheter 300 if the
result is not satisfactory, or if the sizing of the implant could be
optimized. Thus, after the
implant 100 is fully or partially deployed (Figure 46A), in addition to
providing a mechanism
to transmit axial force to the implant 100, the wires 230 described above
provide a guide or
ramp to pull the implant 100 back into the deployment catheter 300 as it is
retracted as
shown in Figures 46B and 46C. The implant 100 could be recovered into the
deployment
catheter 300, or a larger recovery sheath (see. e.g., FIG 50 item 502) could
be introduced
over the deployment catheter 300 for recovery of the implant 100.
[0318] Figures 47A-E illustrate another advantage of the deployment
control
system. As shown in Figure 47A, the implant 100 can be partially deployed and
the wires
used to seat the implant 100 against the native aortic valve 34. The implant
100 can then be
fully deployed as in shown in Figure 47B and then tested as shown in Figure
47C. If
justified by the test, the implant 100 can be deflated and moved as shown in
Figure 47D to a
more optimum position. The implant 100 can then be fully deployed and released
from the
control wires as shown in Figure 48E.
[0319] The deployment control systems described herein could be used
with the
cast in place support structure described in this application, or on a self
expanding stent
structure, or on a inflatable structure as described by herein. The deployment
control device
may also be used on other non-vascular devices such as stent grafts for
aneurysm exclusion
or self-expanding stents for treating stenosis.
[0320] Figure 48 illustrates another embodiment of a deployment
control system.
In this embodiment, the control wires 230 include a small balloon 480 attached
to the distal
end of the wires 230. The balloons 480 are inserted through a small tube 482
provided on
the implant 100. In one embodiment, the tube 482 is formed of a fabric and can
be the same
fabric used to form the cuff 102. The deployment control wires 230 are
released by deflating
the balloon 480. The balloon 480is preferably about 0.02 to 0.12 inches
diameter and the
tube 482 preferably has a slightly smaller inner diameter than the outer
diameter of the
inflated balloon 480. The proximal and distal ends of the tube 482 may
additionally have
- 78 -
CA 3014203 2018-08-14
,
section(s) of reduced diameter, where the diameter is significantly smaller
than the diameter
of the inflated balloon 480.
[0321] As described above, the deployment control wires 230
can be used to
allow the repositioning of the implant 100 after it has been unsheathed. The
deployment
control wires 230 are preferably rigid enough to allow the operator to
reposition the implant
100 and to prevent the implant 100 from migrating due to the force of blood
flow and
pressure. Once the implant 100 is inflated, it is desirable for the wires 230
to be flexible and,
in one embodiment, as flexible as the tip of a conventional guidewire. This
flexibility allows
the implant 100 to take the same shape and position that it will take after
the wires 230 are
removed. This allows both the securement and function of the implant 100 to be
tested and
evaluated before the operator commits to permanently implanting the implant
100. The
increased flexibility is preferably provided in a plane tangent to the
generally cylindrical
shape defined by the vessel, where the valve 100 is implanted. Therefore, in a
preferred
embodiment, the control wires 230 will be particularly flexible at the tips
allowing the device
to be nearly free from forces exerted by the catheter 300, as it would be when
disconnected.
[0322] Many embodiments of a wire that fulfills the
requirements of flexibility
and stiffness are possible. In one embodiment, the wires are manufactured to
have a flexible
tip and a less flexible proximal section. Techniques for manufacturing wires
with these
properties are widely known to those skilled in the art of guide wire design
and manufacture.
Techniques include grinding a tapered control wire as shown in Figure 49A and
or stepped
shoulders to the diameter of the wires. In another embodiment, the wire is
wrapped with
coils of similar type or different materials.to provide a soft feel to the
distal section.
[0323] In another embodiment, which is illustrated in Figure
49B, the
deployment control wire 230 comprises of an inner wire 482 and an outer tube
484 over the
inner wir 482. When a stiff system is desired, the inner wire 482 and tube 484
are used
together. When a more flexible control wire 230 is desired, either the inner
wire 482 or the
tube 484 is used alone. In one embodiment, the inner wire 482 is preferably
manufactured
from a metal such as nitinol or stainless steel and the tube 484 can be
metallic or polymeric.
The tube 484 may be cut in a spiral pattern or have segments cut out of it or
a skive cut in it
to create the desired flexibility in the required areas. In another
embodiment, patterns can be
- 79 -
CA 3014203 2018-08-14
cut in the tube 484 as seen in U.S. Patent Publication 2002/0151961 Al to
Lashinski et al.,
which is hereby incorporated by reference herein. In this embodiment, there
are patterns cut
in the tube 484 to provide defined shape as the tube is deflected. In other
embodiments,
guidewires utilizing slots cut into a tube as seen by neurovascular products
from Boston
Scientific/Target Therapeutics can be used.
[0324] With reference to Figures 49C and Figure 49D, in another
embodiment,
deployment control wires with variable stiffness are created by utilizing a
wire 486 and a
sheath 488 as a system where each has a preferred bending plane. When the wire
486 and
the sheath 488 are rotated so that their preferred bending planes align (see
Figure 49D) they
have good flexibility in the plane where flexibility is required. When a
stiffer system is
desired, the wire 486 and sheath 484 are rotated so that their preferred
bending planes are out
of alignment (see Figure 49C), preferably approximately 90 degrees out of
alignment. In this
configuration a less flexible system is produced. The wires 486 and sheath
495cross
sectional profile may be round with single or multiple flats to create a "D"
shaped cross
section, for example, as shown in the illustrated embodiment of Figures 49C
and 49D. The
[0325] Recovery tools and techniques
[0326] Current valve systems are often deployed through a stent-based
mechanism where the valve is sewn to the support structure. In the inflated
embodiments
described herein, the structure is added to the implant secondarily via the
inflation fluid.
This allows the user to inflate or pressureize the implant with any number of
media including
one that will solidify. As such, if the operator desires, the implant 100 can
be moved before
the inflation media is solidified or depressurization can allow for movement
of the implant
within the body. Since catheter based devices tend to be small in diameter to
reduce trauma
to the vessel and allow for easer access to entry, it often difficult to
remove devices such as
stents once they have been exposed or introduced into the vasculature.
However, as will be
explained below, a device described herein enables a percutaneous aortic valve
to be
recovered from the body and reintroduced retrograde to the introducer.
[0327] Figure 50 illustrates one embodiment of a device 500 for
recapturing an
implant 100. As shown, the device 500 comprises an outer tubular sheath 502. A
tubular
recovery sheath 504 is inserted through the outer sheath 502. The recovery
sheath 504
- 80 -
CA 3014203 2018-08-14
,
,
includes a sox or braided structure 506, which is coupled to the distal end of
the sheath 504
and is configured to capture the implant into the device 500 without harm to
the patient.
Relative movement of the recovery sheath 504 with respect to the outer sheath
502 would
expose the braid 506 when introduced into the body. By pulling a implant 100
into the
braided section it may be safely reintroduced into a introducer or sheath. The
braid 506
allows the implant to be guided into an introducer without harm or worry of
the implant
being tethered or compiled to a larger diameter where it may not fit into the
inner diameter of
a sheath.
[0328]
A hemostasis valve (not shown) is preferably attached to the proximal end
of the device 500. Also at the proximal end, a flush port and stop-cock can be
provided for
fluid introduction. In one embodiment, the inner shaft 504 would have a length
of about 40
to 60 centimeters and a diameter of about 2 to 18 millimeters. In a modified
embodiment,
the distal end 508 of the braid section 506 could be attached to end of the
outer coaxial
sheath 502. This would allow relative motion between the two sheaths 502, 504
and allow
the braided section 506 to be inverted upon it self. The braided section 506
can be formed or
shaped into a funnel as shown in Figure 50 so that it is in contact with the
aortic wall when
introduced into the body. The braid 506 may be constructed with materials such
as
polymeric strands or Nitinol, stainless steel or MP35N wire and attached by
glue or thermal
bonding techniques know in the industry. This wire, strand or ribbon may have
a diameter or
dimension of about 0.002 to 0.020 of an inch. The set or expanded shape would
be about
1.00 to 1.50 inches and the length of the braid 506 would measure about 6 to 9
inches in
length. It is also possible to have the inner sheath 504 and outer sheath 504
connected
leaving the braid 506 fixed in length and diameter. The relative motion
between the two
sheaths 502, 504 would be limited or eliminated depending upon the
construction of the
device 500. Both configurations may require a capture sheath to collapse the
braided section
506 while it is being inserted into the introducer. The diameter of this
sheath would be about
24 F or similar in diameter to the introducer. Once inserted through the
sheath, the device
500 is expelled out the introducer and exposed to the descending aorta.
Hemostasis valves
will prevent blood from leaking proximally out the catheter shaft.
-81 -
CA 3014203 2018-08-14
[0329] In another embodiment the slit tubing is replaced by a fabric
cone, where
the fabric cone may contain a feature such as a preshaped wire or a balloon to
facilitate its
opening.
[0330] The braided cone 506 can be formed by heat setting or other
manners into
a cone shape with a free diameter slightly larger than the patients aorta. In
another
embodiment, the braided cone is manufactured from loops of wire so that the
cut ends of the
wire are all located at the proximal end of the cone. The wires used to
manufacture the cone
preferably have a diameter from 0.002in to 0.020 in. The wires may also be
replaced by
ribbons having a thickness between 0.002in and 0.020in and a width between
0.003in and
0.030in. The diameter of the small end of the cone is preferably between
0.007in and 0.3in
the cone is preferably be capable of collapsing to a diameter small enough to
pass through
the desired introducer size. The large end of the cone section preferably
expands to a
diameter similar to or slightly larger than the typical human aorta, or 0.75in
to 1.50in.
[0331] In one embodiment, the separate recovery device 500 is
supplied to
facilitate the recapture of the implant in the event that the prosthetic valve
did not produce
the desired result in the patient. To recapture an inflatable aortic implant
100 as describe
herein, the delivery catheter 300 for the device would be removed leaving
inflation tubes 318
and or deployment control tubes 316 tethered to the implant 100. By inserting
the retrieval
catheter 500 over these connections the implant 100 is now coaxial to the
retrieval system
500 and ready to be removed from the body. By advancing the retrieval catheter
500 over
the implant 100 or by pulling the control lines 230, the implant 100 can be
retracted into the
braided section 506. The implant 100 is now covered and may safely be pulled
into the
sheath 502 and removed from the body.
[0332] Figure 51 illustrated another embodiment of a retrieval
device/system.
500. In this embodiment, the distal end of the inner sheath 502 includes a
spilt section 510
that is flared to funnel the implant into the device 500. In one embodiment,
the distal end of
the inner sheath 504 would be slit longitudinally about 1 to 2 inches in
length and radially is
4 to 12 times. This would leave a series of narrow bands or strips 512 to be
preshaped open
or rolled back. In the illustrated embodiments, the strips512 are curved
outward away from
the center line of the tube. This would require the retrieval outer sheath 502
to be advanced
- 82 -
CA 3014203 2018-08-14
over the flairs 512 to capture the implant 100 to be removed. The implant 100
can include
control wires 230 spaced radially to gather the device into in a similar
manner as described
above. The wires may be stainless steel, Nitinol or other suitable materials
generally
accepted in medical devices. Formation of this wires would allow them to be
radially
expandable to contact the aortic wall allowing the device to be pulled into
the sheath.
[0333] Other applications for these recapturing systems may be
advantageous for
devices such as stents (coronary and peripheral), PFO and ASD closure devices,
micro coils
and other implantable devices that may need retrieval from the body. Currently
snares and
other tools are used to drag devices out of the body however, many devices
will be hung up
on catheters or introducers as they are removed. By creating a basket to
protect the device
from these events, removal becomes simpler and safer.
[0334] Another method for device recovery includes providing a string
woven
through the prosthetic valve 100. As tension is applied to the string the
prosthetic valve 100
collapses back down, to a size small enough to be recovered into the delivery
sheath, the
introducer or a recovery sheath.
[0335] Excision and debulking devices
[0336] The procedure of implanting a valve preferably begins with
enlarging the
valve annulus. This could be performed with a simple balloon valvuloplasty.
However, in
many instances this is not sufficiently. Thus, before a prosthetic valve is
replaced in a surgical
procedure, the surgeon often modifies or removes the native valve leaflets,
and especially any
calcification or vegetations in the area As will be explained in more detail
below, in order to
preserve outflow from the heart, between the time that the native aortic valve
is excised or
debulked and the time that a prosthetic valve is implanted, a temporary valve
520 (see Figure
52A) can be installed. The temporary valve 520 can be placed in the aorta 36
in the arch or in
the descending or ascending aorta. Examples of these types of valves are
described in U.S.
Patent No. 3,671,979 and U.S. Patent No. 4,056,854, which are hereby
incorporated by
reference herein. Many other temporary valve designs are possible; however,
flexible polymer
or tissue valves are the preferred valve type because they can be easily
delivered via catheter.
Several versions of flexible polymer valves are possible, for example, a "duck
bill", tricuspid or
bicuspid style valve can be used. Alternatively, an umbrella style valve or a
windsock type
- 83 -
CA 3014203 2018-08-14
,
valve could be used. The temporary valve 520 can be sealing and temporarily
engaged to the
wall of the aorta 36 by several methods including a self-expanding stent or an
inflatable balloon
like structure at the base of the valve. Additionally, the temporary 520 may
be entirely
inflatable or utilize combinations of polymers such as nylon, Teflon, Dacron
or polypropylene
with metallic elements including Nitinol, stainless steel, or other generally
acceptable materials
use in medical devices. There may be radiopaque markers attached to the
temporary valve for
proper placement and anchors deployable from the temporary valve or passively
attached may
aid the device in securment.
[0337] In one embodiment, the temporary valve 520 can be
configured in a
manner similar to the implant 100 described above. In such an embodiment, the
temporary
valve 520 would be delivered via catheterization technique by delivering a
collapsed
temporary valve and filling the valve body or cuff with fluid to provide
structure or by
compressing a valve assembly into a catheter for delivery and introducing the
valve by
removing a sheath to introduce the device to the targeted implantation site.
It is also possible
to unroll or unwrap a valve assembly from a catheter for delivery. Any method
of delivery
will suffice as long as the device can be safely removed once the removal and
introduction of
the new valve has been completed.
[0338] The temporary valve 520 should provide a manner for a
catheter to pass
across the temporary valve while still maintaining flow. The temporary valve
520 can be
delivered with a guidewire advanced through the valve to allow guide wire
compatible
devices to be easily advanced across the valve. If an umbrella type valve is
used blood flows
between the device and the wall of the aorta. In this case the guidewire or
catheter may pass
around the valve rather than through the valve.
[0339] A modified method to using a temporary valve is to use a
percutaneous
bypass procedure. When this procedure is performed it is no longer necessary
to maintain
the flow through the aortic outflow tract. The aorta may be occluded during
the excision
step and the debris and fluid from the excised area may be aspirated after or
during the
excision step. In a percutaneous bypass procedure blood is oxygenated
extracorporally and
reintroduced into the body. A cardiopelegia solution is used to stop the heart-
beat.
- 84 -
CA 3014203 2018-08-14
[0340]
With reference to Figure 52B, an embolic protection device 522 is
desirable, or necessary because as the calcified or diseased valve is removed
or dissected
embolic debris may likely be released. It may be desirable to locate the
embolic protection
device 522 downstream from the temporary valve 520 so that it will capture any
embolized
thrombosis and debris from the valve 34. It also may be desirable to locate
the embolic
protection device 522 below the ostia 521 of the coronary arteries. If this
location is selected
it may be difficult or impossible to locate the filter 522 downstream from the
temporary
valve 520. Filtration size can range from about 25 microns to 500 microns in
size. The filter
524 of the protection device 522 may be made from Nitinol, MP35N, stainless
steel or any
acceptable polymeric material used in medical devices.
[0341]
Many various tools are capable of removing portions of the aortic valve
34 or for removing calcification from the aortic valve 34. Examples of such
tools that are
known for surgical applications or for percutaneous applications include
ultrasonic energy
sources such as CUSA, hand tools such as cutters or knives and fluids that may
dissolve or
soften the tissue and or calcium to be removed. As shown in Figure 52B, in one
embodiment, the excise tool 530 is positioned generally within the filter 524.
[0342] In
one embodiment, an ultrasound transducer may be positioned near a
catheter tip and used as a tool to break up calcium and cause it to release
from the valve
tissue.
This method was used for the surgical repair of calcified aortic valves.
Unfortunately, the procedure can also damaged the healthy portions of the
leaflets causing
aortic insufficiency chronically. Typically, the aortic insufficiency would
develop in one to
two years. In some patients, the native valve was destroyed during the
procedure. As a
preparation for valve removal, a percutaneous adaptation of this technique may
be
appropriate. In addition to the ultrasound catheter, some method of collection
the calcified
tissue is often required. One method is the embolic protection filter
described in this
application. Alternatively, suction could be applied to the catheter tip, to
remove the small
particles. With either method, large nodules of calcium may be released from
the native
tissue. If the nodules are larger than the catheter they must be broken up
before they can be
safely removed percutaneously. Preferably, the ultrasound transducer can be
manipulated to
break up these large nodules into particles small enough that they can be
removed. This
- 85 -
CA 3014203 2018-08-14
technology is described in patent numbers 4,827,911, 4,931,04, 5,015,227,
4,750,488,
4,750,901 and 4,922,902, which are hereby incorporated by reference herein.
The frequency
range for these devices is often about 10-50 KHz but seems to be optimal at
about 35Khz.
[0343] Another tool that can be used to excise the native valve 34
may comprise
multiple external energy sources that are focused on the tissue to be removed
from different
directions. This technique can be used with several energy sources, for
example ultrasound
energy may be used in this way. Radiation energy may also be used in this way,
by a method
referred to as a gamma knife.
[0344] A heated wire system can also be used to cut the aortic valve
out from the
annulus. In such an embodiment, the wire may be mounted on a catheter and
heated by
means such as electric resistance or RF energy. The wire may be manipulated in
the area of
the valve to be removed, and located by balloons or wires. Wire sizes may
range from
0.005-0.100 inches in diameter and are typically made from a Ni-chrome
material.
[0345] In another embodiment, a laser can be used to cut the
calcified tissue
apart. The laser energy could be transmitted fiber optically through a
catheter and applied to
the calcified tissue at the catheter tip. The catheter tip may be manipulated
by the operator to
direct energy to the site-specific area causing ablation or cutting the tissue
and or diseased
material. It is important that the laser wavelength is correct and will couple
to the material to
be affected. There may be a need to adjust the wavelength, rep rate and energy
density to
customize the removal process.
[0346] In yet another embodiment, the calcified valve tissue may be
broken up
and removed using a cutting balloon, or an inflatable balloon with metal or
rigid plastic
blades along its length. An example of this is U.S. patent 5,616,149, which is
hereby
incorporated by reference herein. As the balloon is expanded the blades are
forced into the
tissue causing it to break apart. Mulitple inflations may be required to
create a sufficiently
large valve area. In one embodiment the balloon is mounted on a torquable
catheter allowing
a partially inflated balloon to be torqued scraping tissue away from the valve
annulus. This
balloon source may be used in the "hot-wire" application above to cut the
tissue in a pie
shaped pattern before removal or exclusion.
- 86 -
CA 3014203 2018-08-14
[0347] Several of the tools described for removing portions of the
aortic valve
may remove portions of valve or calcium that are larger than can pass through
a catheter. In
these cases a catheter with a provision to pulverize and extract the excised
material may be
needed. In one embodiment the catheter includes a rotating auger near its tip
to break up the
large particles and feed them back through the catheter shaft. Suction may
also be applied to
the catheter to prevent smaller particles from exiting the catheter tip.
Examples of this may
include the RotoBlader device produced by Boston Scientific but may be housed
in a
catheter to limit the escape of particles down stream.
[0348] Figures 53A-54C illustrate one embodiment of an excise device
530
which comprises a punch and die that can be used to punch out sections of
tissue. The
device 530 comprises a punch or cutter 532 with a sharp edge 534 that is
moveably
positioned within a channel or cavity 535 of a catheter body 540 to collect
the removed
sections of tissue. As will be explained below, the punch 532 can be actuated
by pushing or
pulling a wire 539 through the length of the catheter, by a hydraulic
actuation, or by a screw
device near the catheter tip that translates a rotational force transmitted
through a flexible
shaft into an axial or linear force that actuates the punch 532.
[0349] With continued reference to Figures 53A-54C, the cutting
action is
preferably from distal to proximal to move the material into a catheter's
inner diameter.
Although not shown, the device 530 can use a spring force to eject the
material and a trap or
door to retain the material once in the catheter shaft. As shown, a cutting
edge 542 is formed
in by a window 544 formed in the catheter body 540. The widow 544 forms a
cutting edge
that is generally perpendicular to the diameter of the catheter 540 or, in a
modified
embodiment, at an angle to provide a lower cutting force. The cutting edge 542
and or the
puncher 532 can have any of a variety of shapes such as hyperboloid,
triangular, diamond or
serrated that would aid in cutting the material to be removed. The cutting
edge 542 and/or
punch 532 can also use a vibrating or ultrasonic energy to lower the forces
required to cut the
material. These can be delivered through the catheter 540 and may include
transducers,
motors or RF energy. In one modified embodiment, the punch 532 is replaced
with a
rotating blade. The entire device 530 is preferably flexible and configured to
use normal
catheterization tools including contrast, introducers, saline, guidewires
etc/.
- 87 -
CA 3014203 2018-08-14
[0350] In the preferred embodiment, the cutting action is performed
by pulling
the punch 532 proximally into the cutting edge 542. The punch 532 is coupled
to the wire
539, which extends through the catheter and is actuated by a handle 546
provided at the
proximal end of the device 530. By cutting in this direction the excised
tissue is pulled into
the catheter 540, and the wire 539 which transmits the force is loaded in
tension. An
aspiration function is also incorporated into the lumen 535 into which the
excised tissue is
pulled. By maintaining a minimal fluid flow out through the catheter lumen 535
the risk of
embolic events may also be minimized. A spring (not shown) can be provided at
the distal
end 552 of the device to pull the punch 532 distally after the wire 539 is
released.
[0351] For a device such that described above or a DCA device, it is
advantageous for the cutting portion of the device to be movable to engage the
tissue. A
balloon or forced wires which forces the cutting portion against tissue, is
traditionally used
with a DCA device, however this prevents perfusion. In the illustrated
embodiment of
Figures 54A-54C, a strap or straps 550 extend the length of the catheter 540
and aid the
device 530 in engagement. The straps 550 are attached near the catheter tip
552 to the
catheter 540 and on the opposing side of the cutting edge 542. A section 551
of the strap or
straps 540 is free in the area near the cutting portion of the device 530. As
the straps 550 are
advanced relative to the catheter shaft 540, they are forced to bow out away
from the cutting
portion of the device 530. This forces the cutting portion of the device 530
into the tissue.
The operator may rotate the device 530 to engage the desired tissue.
[0352] In a modified embodiment, the straps 550 extend axially across
the
portion of the catheter where the cutting takes place, and attach to an
elongate member which
is free to move axially relative to the elongate member that is attached to
the cutting
mechanism. The two elongate members are preferably located coaxially. In one
embodiment both elongate members are polymer tubes.
[0353] Figures 55A and 55B illustrate another embodiment of the
excise device.
This embodiment is similar to the embodiment described above with reference to
Figures
53A-54C in that it includes a catheter body 540, a cutting edge 542 and a
tissue punch 532.
In this embodiment, the tissue punch 532 is coupled to a return spring 554 and
is actuated by
pressurized fluid that is supplied through an inflation lumen 556 to a chamber
558 at the
- 88 -
CA 3014203 2018-08-14
distal end 552 of the catheter body 540. A seal 560 is provided between the
punch 532 and
the catheter body 540 to seal the chamber 558. By increasing the pressure in
the pressurized
chamber 558, the punch 532 is moved proximally against the cutting edge 542.
When the
pressure is decreased, the punch 532 is moved distally by the spring 554.
Barbs 560 can be
provided in the catheter body 540 to retain tissue introduced through the
window 544. The
inflation lumen 556 can be attached to the catheter body 540 by an adhesive
564 as shown in
Figure 55B.
[0354] Figures 56A-C illustrate another modified embodiment of an
excise
device 530. In this embodiment, cutting wires 570 extend through lumens 572
provided in a
catheter body 574. The cutting wires 570 can be mounted at their distal end to
the distal
portion of the catheter body 574. Most of the length of the cutting wires is
encapsulated in
the lumens 572 of the catheter body 574. A skive 576 is provided at the distal
portion to
expose a short portion 578 of the wires 570 just proximal to the point where
the wires 570
are attached to the catheter body 574. In one embodiment, the skive 576 is
between about 5
and 100 mm in length preferably between about 10 and 30 mm in length. As the
proximal
ends of the wires 570 are advanced relative to the catheter body 574 the
distal portions 578
bow out away from the catheter body 574 through the skive 576. The wires 570
may have a
cross section that provides a preferential bending plane and prevents their
rotation within the
lumen 572 of the catheter body 574. This may help the wires 570 deploy in a
controlled
orientation. The exposed portions 578 of the wire 570 can include cutting
surfaces that are
exposed to the tissue when the wires 570 are advanced. In another embodiment,
this device
530 can be configured such that the wires 570 can be deployed, heated and then
advanced or
retracted through the valve annulus or it may be heated and then actuated
within the valve
annulus. The catheter body 574 can also include stiffening wires 580
positioned in lumens
582 as shown in Figure 56B.
[0355] Figure 56D illustrates another modified embodiment of an
excise device
530. In this embodiment, the device comprises an outer protective sheath 900,
an inner
sheath 902 that can track over a guidewire 904 and an intermediate member 906
positioned
between the outer and inner sheaths 900, 902. The intermediate member 906
includes an
cutting structure 908, which can expand as the outer sheath 900 is withdrawn
to expose the
- 89 -
CA 3014203 2018-08-14
cutting structure 908. In this embodiment, the cutting structure 906 comprises
a plurality of
elongated cutting members 910, which are supported by annular spring members
912. The
device can be positioned within the valve and then the outer sheath 900 is
withdrawn to
expose the cutting members 910. The device can be rotated to provide a cutting
action.
[0356] In yet another embodiment, an atherectomy catheter device (not
shown)
includes a housing at the distal end of a substantially round housing torque
cable. A cutter
torque cable is disposed within the housing and includes a rotatable and
translatable cutter at
its distal end. The housing includes a window into which an atheroma
protrudes. The cutter
severs the atheroma. A nose cone attached to the distal end of the housing
collects and stores
severed atheroma. A stabilizing member is attached to the exterior of the
housing opposite
the window. A stabilizing member can be provided and includes a balloon having
an
inflation lumen disposed within the housing. In a modified embodiment, a
mechanical
stabilizing member provided and includes a distal end attached to the distal
end of the
housing or to the nose cone, and a proximal end coupled to a stabilizing cable
disposed
within a cable lumen of the housing torque cable. The stabilizing cable can be
advanced
distally to bow the stabilizing member away from the housing and withdrawn
proximally to
flatten the stabilizing member against the housing, alternately urging the
window side of the
housing onto the atheroma and allowing it to retreat therefrom.
103571 Another method for removing calcification and vegetation from
the valve
area is with a pharmacological agent. For example, an agent that dissolves
calcium is
secreted by osteoblasts. An agent similar to this could be utilized prior to
the valve
replacement procedure. Alternatively an agent like this could be coated on the
valve leaflets
or on another portion of the prosthesis so that it slowly elutes over the life
of the valve. This
would prevent or minimize the calcification that contributes to the
deterioration of the valve.
The agent could be contained in a polymer coating, in a porous metallic
coating, or in the
tissue itself
[0358] To aid removal or debulking, the calcified tissue may be
visualized by
echocardiography and or fluoroscopy, ECHO, MRI, CT scan as is known in the
art.
[0359] With reference back to Figure 52B, an access sheath attached
to the
protection filter device 522 allows the excise device 530 or other tools to
access the work
- 90 -
CA 3014203 2018-08-14
area between the left ventricle 32 and the filter 530. The access catheter may
be made of a
flexible material which can be folded inside a delivery catheter. This allows
the delivery
catheter to be a low profile device while relatively large profile devices may
be introduced
through the access catheter. In one embedment, a delivery catheter containing
the temporary
valve and embolic protection device and access catheter is advanced through
the vasculature.
The devices are deployed and the delivery catheter is removed completely from
the patient.
The access catheter then expands to an inside diameter large enough for the
required devices
for valve removal and replacement to pass through.
[0360] Many of the devices described above for removing or cutting
the valve
commisures could benefit from the use of a centering balloon to locate the
catheter in the
center of the native annulus while the cutting occurs. The centering balloon
could be located
proximal or distal to the valve, or balloons could be located both proximal
and distal. The
balloons could optionally contain perfusion lumens.
[0361] In a modified embodiment, the method of enlarging the annulus
involves
a process of shrinking tissue instead of or in addition to removing tissue.
For example, it is
possible to shrink collagen type tissue by the application of heat. In such an
embodiment,
the tissue is preferably heated to a temperature of 50 to 65 C. More
preferably the tissue is
heated to 55 to 60C in one embodiment the tissue is heated to a temperature of
59C. The
heating may be accomplished from a variety of energy sources, one particularly
advantageous energy source for a percutaneous application is RF energy.
Accordingly, a
catheter with a heated element on the tip may be used to heat specific
portions of the valve.
[0362] In one embodiment, the catheter incorporates a needle near the
heated
portion. The portion of the catheter intended to transfer heat to the leaflet
tissue is
positioned below the surface of the leaflet. This minimizes the transmission
of heat into the
bloodstream, while maximizing the transmission of heat to the leaflet tissue.
[0363] In another embodiment the heating step is applied by a tool
that also
dilates the annulus. This tool may be a balloon inflated with a heated
solution or a dilation
device that contains heating elements, such as those described in this
application using
deflected straps.
- 91 -
CA 3014203 2018-08-14
[0364] In general, the application of heat is intended to affect the
portions of the
leaflets nearest to the center of the valve. Excessive shrinking of the outer
portion of the
valve annulus may cause the effective orifice area to be reduced. Shrinking an
area near the
tip or free edge of each leaflet will cause the effective orifice area to be
increased. It may
additionally release the calcium deposits within the valve tissue thus
providing a large
effective orifice area to implant a new valve.
[0365] Procedures for deploying the implant
[0366] Various procedures and methods for deploying an implant 100 in
the
aortic position will now be described. In one embodiment, the method generally
comprises
gaining access to the aorta, most often through the femoral artery. A balloon
valvuloplasty
may optionally be performed in the case of aortic stenosis, or another method
may be used to
remove or debulk the native valve as described above. A delivery sheath or
catheter is
advanced over the aortic arch and past the aortic valve. The outer sheath of
the catheter is
retracted exposing the valve and cuff Fluid is used to inflate the valve and a
second
inflation fluid may be used to partially form the implant. This allows the
distal portion of the
implant to open to its full diameter. The proximal portion of the implant may
be slightly
restricted by the deployment control mechanism. In general, the amount that
the
deployment-control mechanism restricts the diameter of the proximal end of the
device
depends on the length of the wires extend past the outer sheath, which an be
adjusted by the
operator. Alternatively, in some embodiments, the implant contains multiple
inflation ports
to allow the operator to inflate specific areas of the implant different
amounts. In another
embodiment, burst discs or flow restricters are used to control the inflation
of the proximal
portion of the implant 100. The implant is then pulled back into position. The
distal ring
seats on the ventricular side of the aortic annulus. A balloon may be used to
dilate or redilate
the device if necessary. At this time, the deployment control wires may act to
help separate
fused commisures by the same mechanism a cutting balloon can crack fibrous or
calcified
lesions. Additional casting material may be added to inflate the implant
fully. The inflation
lumen is then disconnected, and the deployment control wire(s) are then
disconnected, and
the catheter is withdrawn leaving the device behind. In modified embodiments,
these steps
may be reversed or their order modified if desired.
- 92 -
CA 3014203 2018-08-14
[0367] The above-describe method generally describes an embodiment
for the
replacement of the aortic valve. However, similar methods could be used to
replace the
pulmonary valve or the mitral or tricuspid valves. For example, the pulmonary
valve could
be accessed through the venous system, either through the femoral vein or the
jugular vein.
The mitral valve could be accessed through the venous system as described
above and then
trans-septaly accessing the left atrium from the right atrium. Alternatively,
the mitral valve
could be accessed through the arterial system as described for the aortic
valve, additionally
the catheter can be used to pass through the aortic valve and then back up to
the mitral valve.
[0368] For mitral valve replacement, the implant may require a
shorter body
length (e.g., 1-4cm) and would mount in the native mitral valve area. It may
be delivered
from the right side of the heart from the femoral vein up through the inferior
vena cava and
into the right atrium. From there, a transeptal-puncture may be made for entry
into the left
atrium and access to the mitral valve. Once in the left atrium, the implant
would be
delivered with the valve pointing down to allow flow from the left atrium to
the left
ventrical. A similar shape would allow the device to be deployed in the left
atrium and
advanced into the left ventrical. The proximal ring may require inflation to
hold the device
in the left ventical by creating a diameter difference between the mitral
orifice and the
proximal cuff diameter. Here as with the aortic replacement, the mitral valve
may require
partial removal or cutting of the valve or chorde to allow the native valve to
be excluded and
provide room for the replacement valve to be implanted. This may be achieved
by balloon
valvuloplasty, cutting techniques such as a cutting balloon or by utilizing a
hot-wire or knife
to cut slits in the native valve to allow for exclusion. Once the native valve
has been
prepared for the new valve, the mitral valve orifice may be crossed with the
distal portion of
the implant and the distal potion may be inflated for proper shape and
structure. At this time,
the native valve will have been excluded and the replacement valve will be
fully operational.
[0369] Other methods of mitral replacement would include a
transapical delivery
where the patent would receive a small puncture in the chest cavity where the
operator could
access the apex of the heart similar to a ventricular assist device
implantation. Once access
is gained to the left ventrical, the aortic and mitral valves are a direct
pathway for
implantation of the replacement valve. In this case, the aortic valve would be
delivered with
- 93 -
CA 3014203 2018-08-14
the flow path in the same direction as the catheter. For the mitral valve, the
flow path would
be against the direction of implantation. Both may still utilize the base of
the implant to
anchor the device using diameter differences to secure the device. It may be
desirable to also
use a hook or barb that could protrude from the cuff either passively or
actively as the cuff is
filled with fluid. The barb could be singular or a plurality of barbs or hooks
could be used
where the length could be between 1-5 millimeters in length depending upon the
tissue
composition. Where a longer barb may be required if the tissue is soft or
flexible. It may be
desired to have shorter lengths if the tissue is a stiffer more fibrous
structure where the barbs
could hold better.
[0370] For the pulmonary and tricuspid valve placement, the operator
could
access the femoral vein or internal jugular (LT) vein for insertion of the
delivery system. As
with the transeptal mitral valve approach the delivery system and device would
be introduced
either superiorly or inferiorly to the vena cava and to the right atrium and
right ventrical
where the pulmonary and tricuspid valves are accessible. The femoral approach
is preferable
due to the acute bends the delivery system would be required to make from a
superior or IJ
access. Once in the right ventrical the device could be delivered similarly to
the aortic
method where the cuff utilizes the base of the pulmonary valve for a positive
anchor with a
diameter difference holding it from migrating distally. It may be desirableto
also use a hook
or barb that could protrude from the cuff either passively or actively as the
cuff is filled with
fluid. The barb could be singular or a plurality of barbs or hooks could be
used where the
length could be between 1-5 millimeters in length depending upon the tissue
composition.
Where a longer barb may be required if the tissue is soft or flexible. It may
be desired to
have shorter lengths if the tissue is a stiffer more fibrous structure where
the barbs could
hold better.
[0371] In any placement, the proper valve configuration would be
chosen by the
performance of each required application. For instance the aortic valve may
require a two or
three-leaflet valve that will require a high degree of resistance to stress
and fatigue due to the
high velocities and movement. The pulmonary valve may require a lesser valve
due to the
more passive nature or the lower pressure that the valve is required to
support. Lengths may
vary and will be dependant upon the valve and structure surrounding them. A
shorter valve
- 94 -
CA 3014203 2018-08-14
(1-4 centimeters) may be required for the mitral but the aortic may allow for
a longer valve
(1-8 centimeter) where there is more room to work. In any application, the
maximum orifice
size is generally desired since the cross sectional area helps determine the
outflow volume.
The aortic cross sectional area may vary from nearly 0.00 square centimeters
in a heavily
calcified valve to about 5 square centimeters in a healthy valve. Most cases
the desire in
replacement is to increase a cross sectional area for additional flow.
[0372] During the procedure or during patient selection, or follow-
up, various
imaging techniques can be used. These include fluoroscopy, chest x-ray, CT
scan and MRI.
In addition, during the procedure or during patient selection, or follow-up,
various flows and
pressures may be monitored, for example echocardiography may be used to
monitor the flow
of blood through the relevant chambers and conduits of the heart. Pulmonary
wedge
pressure, left atrial pressure and left ventricular pressures may all be
recorded and monitored.
It may be desirable to use a measurement tool to determine the size of valve
required or to
determine if the anatomy provides enough room to allow implantation of a
valve. In the
past, marker-wires have been used to measure linear distance and a similar
technique could
be used in this application to measure a distance such as the distance from a
coronary artery
to the annulus of the aortic valve. To measure the diameter of a valve, a
balloon with a
controlled compliance could be used. Ideally, the balloon would be very
compliant and
inflated with volume control, but a semi compliant balloon could also be used
and inflated
with a normal interventional cardiology inflation device. The compliance curve
of the
balloon could then be used to relate the pressure to the diameter. The
diameter range of
valves in the heart may range from 10-50 mm in diameter and 2-40 mm in length.
A similar
sizing balloon has been used for sizing septal defects.
[0373] In one embodiment, implantation of a prosthetic valve includes
the step of
dilating the valve after it is positioned and functioning within the native
anatomy. If the
dilation step is used to replace a balloon valvuloplasty prior to the
inflation of the balloon the
cuff will minimize the embolization from the dilation. The dilation of the
functional implant
step may also be used in patients where a valvuloplasty is performed prior to
implantation of
the device, but where the outflow area is not as large as desired. Certain
embodiments of the
implantable prosthetic valve include deployment control wires or stiffening
wires. If these
- 95 -
CA 3014203 2018-08-14
features are present in the implant at the time of post dilation, then the
features may act to
concentrate the force from the deployment of the balloon in a mechanism
similar to the
function of a cutting balloon commonly known in interventional cardiology.
103741 To
gain access to the aortic valve the femoral arteries (radial, brachial,
carotid) can be used to introduce tools into the vascular system. Once in the
arterial
conduits, catheters may be advanced to the aortic arch and the native aortic
valve. As
discussed above, it may be necessary to install a temporary valve to allow
gating of the blood
flow while the work is being completed on the native valve. This will provide
time fro the
interventional cardiologist to prepare for removal and installation of a new
aortic valve. The
placement of the temporary valve could be between the native valve and the
coronary
arteries, or the valve could be placed in a location between the coronary
arteries and the
location where the great vessels branch off from the aorta or at any other
location within the
patients aorta. Placing a valve in these non native locations to treat aortic
insufficiency has
been proven effective in clinical experience by the use of the Huffnagel
valve. Placing a
temporary valve in these locations has been described by Moulolupos and
Boretos. A
guidewire or pig-tail catheter may be used to pass a stiffer catheter through
the stenotic hole
in the aortic valve. It may be necessary to install a filtration device to
protect any vessels
including the coronary tree from debris as the valve is loosened and removed.
This filter
may be placed in the region of the aortic valve just before the coronary ostia
or distal to the
sinus and just before the great vessels. Once through the valve opening a
balloon may be
passed into the aortic valve to predilate the region and loosen any calcium.
This may aid in
the removal of the tissue that may be calcified and or fibrosed. The use of a
catheter to
deliver energy such as ultrasonic, RF, heat or laser may additionally break or
loosen the
tissue including the calcification in and on the leaflets. There are chemical
treatments that
have shown some promise in dissolving the calcium such as Corazon Inc. of
California (see
US patent number 6,755,811). The ultrasonic energy device is described in
detail through
US patent number 4,827,911 and has a proven track record known as CUSA to
remove
calcium in a surgical suite from valve tissue. This has shown promise acutely
but will
denature the collagen tissue and result in a degeneration of the valve tissue
remaining in
about a year leaving a poorly functioning valve. After a filter has been
installed and the
- 96 -
CA 3014203 2018-08-14
valve tissue has been softened, a template may be used to define the area to
be removed.
This template will define the hole and prevent the removal of healthy tissue.
At this time the
valve is ready to be removed with adequate time since the temporary valve will
be
functioning when the native valve is removed. This will be important to not
allow the
patient to go from aortic stenosis to aortic insuffiency. The removal tool may
now be passed
through the stenotic valve and begin the removal process of the native valve.
As mentioned
above and in patents and US applications such as 20040116951 Rosengart there
are many
ways to remove tissue from this region.
[0375] The embodiments described above provide a technique that lends
itself
well to delivering a catheter based valve removal tool. Through a pushing and
pulling force
the pin and die set as seen in the drawings will allow the valve to be removed
in a controlled
manner while leaving the material in a catheter shaft for removal. It is
asserted that this is
the first that allows the aortic outflow track to be gated or valve
temporarily. Though an
aortic balloon pump may function as a temporary or supplementary valve in some
conditions, the balloon pump is ineffective and dangerous in patients with
aortic
insufficiency. A removed or partially removed aortic valve constitutes severe
aortic stenosis.
[0376] Figures 57A-570 will now be used to describe a embodiment of
procedure for installing an prostethetic aortic valve 100, which utilizes some
of the
procedures described above. In particular, the illustrate embodiment includes
the steps of
placing a temporary valve, optionally placing an embolic protection device,
removing or
debulking or destroying all or part of the stenotic valve, implanting a
permanent prosthetic
valve, and then removing the temporary valve and embolic protection device. Of
course
those of skill in the art will recognize that not all of these steps are
required and/or that the
order of certain steps can be changes. In addition, those of skill in the art
will recognize
various modified embodiments of the steps described herein.
[0377] As shown in Figure 57A, access to the aorta can be provided by
an access
sheath 600 through the femoral artery 602. A deployment catheter 604 is
advanced over
guidewires 606 through the access sheath and through the femoral artery toward
the aortic
arch 10 (Figure 57B) The deployment catheter 620 is used to implant a
temporary valve 520,
as described above with reference to Figure 52A. The temporary valve 520 is
implanted
- 97 -
CA 3014203 2018-08-14
preferably as a first step, although an embolic protection filter may also be
implanted as a
first step. For the treatment of a stenosed aortic valve 34, the temporary
valve 520 is placed
in the aorta 36. The valve 520 may be placed in the ascending or descending
aorta. A valve
520 in this position has been proven moderately effective by experience with
the Hufnagel
valve, and was described in similar designs disclosed by Moulolupos 3,671,979
and Boretos
4,056,854. Although a valve placed beyond the coronary arteries does not
provide ideal
performance as a long term implant, the function of the valve in this location
has been
proven sufficient for short-term use. In a healthy patient, the coronary
arteries fill during
diastole, however in a patient with severe aortic insufficiency the pressure
required to fill the
coronaries in diastole is not present. These patients are able to perfuse the
coronary arteries
sufficiently for survival.
103781
Alternatively, the temporary valve 520 may be placed so that it acts
between the native aortic valve and the coronary arteries although its
physical position would
likely extend well above the coronary arteries. In this embodiment the inlet
side of the
temporary valve would seal to the aortic wall just below the coronary
arteries. The outlet
side of the valve would extend up beyond the coronary arteries. The mid
portion of the valve
and the outlet side of the valve would have an outside diameter smaller than
the inside
diameter of the patients aorta. This would allow blood flow from the outlet of
the valve,
around the outside of the valve back towards the ostia of the coronary
arteries. In this
embodiment the valve would have a sealing portion on the inlet side of the
valve, the sealing
portion would have an outside diameter to match the patients aortic root
diameter. This
diameter would range from about 18mm to about 38mm. Multiple sized valves are
required
to accommodate differing patient anatomies. The sealing portion of the valve
may be
expandable or compliant to improve sealing and best conform to a wide range of
patient
anatomies. The length of the sealing portion is limited by the position of the
valve and the
position of the coronary arteries, the length of the sealing portion may range
from about lmm
to about 5mm, preferably about 3mm. The mid and outlet portions of the valve
are
preferably between 30% and 90% the diameter of the native aorta. This allows
sufficient
room for blood to flow back around the valve and perfuse the coronary
arteries. The valve
- 98 -
CA 3014203 2018-08-14
may also incorporate a secondary retaining mechanism, securing the outlet or
mid portion of
the valve beyond the coronary arteries
[0379] Alternatively, the valve can be replaced by a pump similar to
a device
designed by Medtronic known as a Hemo Pump, which is placed in the aorta. The
pump
moves blood out from the ventricle into the aorta, serving the function of
both the native
aortic valve and the contracting left ventricle. The pump may consist of a
screw type pump
actuated by a rotating shaft, where the motor is located outside the body. The
inlet of the
pump located on the distal end of the catheter may optionally be isolated from
the outlet of
the pump by a balloon. The balloon inflates between the outside diameter of
the pump and
the inner diameter of the aorta, in a location between the pump inlet and the
pump outlet.
Alternatively a pump using two occlusion balloons, both between the inlet and
the outlet of
the pump could isolate an area between the balloons for treatment. The valve
removal
procedure could take place in this area.
[0380] The temporary valve designs described by Moulolupos and
Boretos in US
patents 3,671,979 and 4,056,854 respectively, include umbrella valve designs
that allow the
blood to flow in one direction between the valve and the wall of the aorta.
The valves
prevent flow in an opposite direction as the valve seals against the wall of
the aorta. These
valves can be attached to a temporary valve catheter and adapted for use with
the present
invention.
[0381] Other valve designs are also possible for a temporary valve
including a
ball and cage valve, a tilting leaflet valve, bi-leaflet valve a reed type
valve, a windsock style
valve, a duckbill valve, or a tricuspid valve. In addition to these valves
made from synthetic
materials including polyurethane or tissue valve may also be utilized.
Commonly used in
permanent valve replacements valves constructed from bovine pericardium or
porcine aortic
valves, are adequate. To produce a low profile percutaneous device the
preferred
embodiment is a thin flexible polymer valve of either a duckbill design or
umbrella valve
design.
[0382] The temporary valve should be placed in such a way that it can
be easily
removed at the end of the procedure and also in such a way that the operator
has access
across the valve for performing the remaining steps. A guidewire or catheter
lumen placed
- 99 -
CA 3014203 2018-08-14
through the valve or around the valve before the valve is positioned in the
body allows the
required access for downstream procedures.
[0383] Alternatively an inflatable structure may be used. The
inflatable structure
provides the advantage of improved sealing characteristics with the vessel
wall, and the
inflatable structure may produce a lower profile device with some valve
designs. The
inflatable valve structure could be designed to be recoverable using wires as
described in
previous direct flow disclosures for permanent valve replacement devices, an
inflatable
prosthetic valve was first described by Block in 5,554,185 and is also
described herein. The
inflatable structure preferably inflates to an outside diameter between about
18mm and about
35mm.
[0384] In another embodiment, the temporary valve structure is a
recoverable
self-expanding stent. The stent could be a Z-stent formed from wires segments
shaped into
rings or a coil. Alternatively the Z-stent could be cut from a tube using a
process like laser
cutting. With a Z-type stent careful design of the stent shape is required to
make the stent
recoverable. It must be ensured that no crown hangs up on the recovery sheath.
One method
to accomplish this is to attach each crown to the crown of the next stent
segment by welding,
fusing or other joining techniques. Or the stent could be braided from wires
in a design
similar to a Wall Stent as produced by Boston Scientific. The material for the
stent is
preferably a superelastic material such as nitinol. Alternatively a material
with a relatively
high yield strength and/or a relatively low modulus of elasticity, such as a
cobalt chrome
alloy, or titanium, could be used. These non superelastic materials are most
appropriate for
use in a stent manufactured by a braiding process.
[0385] In another embodiment, the structure for the temporary valve
520 consists
of an unwrapable structure, similar to the structure described by Yang in US
patent
6,733,525 or as described herein. The structure is delivered in its wrapped
position. After
the structure is positioned the structure is unwrapped and expanded to its
final diameter.
[0386] In general, any of a wide variety of valve structures may be
utilized for the
temporary valve in accordance with the present invention. Since the temporary
valve is only
intended to remain functional at an intraluminal site for a relatively short
period of time (e.g.
less than a few hours), the temporary valve of the present invention is not
plagued by many
- 100 -
CA 3014203 2018-08-14
of the deficiencies of prior permanent implantable valves (thrombogenicity,
efficiency,
durability, etc.). Thus, valve design can be selected to minimize the initial
crossing profile
and optimize removal.
[0387] For example, in the example described previously in which a
valve is
supported by a Z-stent structure, each of the proximal apexes of the stent may
be attached to
a pull wire, which merge into a common axially moveable control wire which
runs the length
of the temporary valve deployment catheter. Following transluminal navigation
to the
desired temporary valve site, an outer sheath may be proximally retracted
relative to the
control wire, thereby enabling the stent and valve to be deployed from the
distal end of the
catheter. Following completion of the procedure, the temporary valve may be
removed by
applying proximal traction to the control wire and/or distal force on the
outer sheath. The
plurality of control filaments will cause the Z-stent to collapse, as it is
drawn back into the
tubular sheath.
[0388] Thus, the temporary valve of the present invention is
preferably
permanently attached to its deployment catheter. In this regard, the term
"deployment" refers
to the conversion of the temporary valve from a reduced cross sectional
profile such as for
transluminal navigation, to an enlarged cross sectional profile for
functioning as a valve in a
vascular environment. However, at no time does the valve become detached from
the
deployment catheter. This eliminates the complexity of snaring or otherwise
recapturing the
temporary valve, for retraction into a catheter. Alternatively, the present
invention may be
practiced by the use of a detachable temporary valve, which must be captured
prior to
removal.
[0389] The preferred temporary valve is therefore preferably carried
by an
elongate flexible catheter body, having a proximal control for advancing the
valve into a
functional configuration, and retracting the valve into a collapsed
configuration for
transluminal navigation into or away from the temporary valve site. Activation
of the control
to retract the valve back into the temporary valve catheter does not
necessarily need to
preserve the functionality of the valve. Thus, proximal retraction of the
valve into the
temporary valve catheter may involve a disassembly, stretching, unwinding, or
other
- 101 -
CA 3014203 2018-08-14
destruction of the valve if that is desirable to facilitate the step of
removing the temporary
valve.
[0390] Although tissue valves may be used for the temporary valve in
accordance
with the present invention, due to the short duration of the intended working
life of the valve,
any of a variety of polymeric valves may be adapted for use in the present
context.
Polymeric membranes may be configured to mimic the leaflets on a normal heart
valve, or
may be configured in any of a wide variety of alternative forms, as long as
they are moveable
between a first, open configuration and a second, closed configuration for
permitting blood
flow and essentially only a single direction. Thus, polymeric membranes may be
formed into
any of a wide variety of flapper valves, duck bill valves, or other
configurations.
[0391] Regardless of the valve leaflet construction, the temporary
valve may be
supported by an inflatable cuff as has been disclosed elsewhere herein. The
temporary valve
deployment catheter is provided with an inflation lumen extending between a
proximal
source of inflation media and a distal point of attachment to the inflatable
cuff. Once
positioned at the desired site, the temporary valve may be released such as by
proximal
retraction of an outer delivery sheath. Inflation media may thereafter be
expressed from the
source to inflate the cuff to enable the valve and provide a seal with the
vessel wall.
Following the procedure, the inflation media is aspirated out of the cuff by
way of the
inflation lumen 318 to deflate the cuff, and the temporary valve is withdrawn
from the
patient.
[0392] Alternatively, the temporary valve may take the form of an
inflatable
balloon, with an inflation cycle which is synchronized to the heart beat so
that it is deflated
to permit forward flow but inflated to inhibit reverse flow in the artery.
[0393] An embolic protection filter may be mounted to the temporary
valve or to
the temporary valve structure. The filter may be attached to the outlet
section of a duckbill
type valve. Alternatively the filter may be mounted on its own support
structure.
[0394] With the temporary valve deployed as shown in Figures 57D and
57E, a
filter or embolic protection device 522 may be used during the procedure of
implanting a
percutaneous valve. Several methods of embolic protection are possible as
described above.
In the illustrated embodiment of Figure 57F, a filtering basket 524 is placed
down stream of
- 102 -
CA 3014203 2018-08-14
the temporary valve 520 as shown , the basket 524 catches any debris that is
embolized or
cut from the native valve (see Figure 57G) and the basket 524 is then
recovered.
[0395] A trapping size of about 35 to 250 micron and may be treated
with an
anti-thrombogenic coating to prevent clotting. A basket of similar design
could be mounted
to the catheter shaft of a device designed for percutaneous treatment of a
coronary valve, in a
case where the valve is approached from a retrograde direction. In an
application where the
device is placed in an antegrade direction, a larger version of a conventional
wire based
embolic protection device could be used.
[0396] In an application for aortic valve treatment it may be
desirable to place the
embolic protection very close to the annulus of the valve because the ostia of
the coronary
arteries are very close to the area being treated. In a balloon, valvuloplasty
used as a
pretreatment for valve replacement or alone as an independent therapy, the
embolic
protection filter may be attached to the proximal end of the balloon or to the
catheter shaft
very near the proximal end of the balloon, specifically within lcm of the
proximal end of the
balloon. The filter could be positioned similarly on a catheter for the
delivery of a
percutaneous prosthetic valve, this configuration is especially beneficial for
a balloon
expandable prosthetic valve.
[0397] An alternative method of embolic protection applicable to a
balloon
valvuloplasty or implantation of a percutaneous prosthetic valve by means that
prevent flow
through the aortic valve is described as follows. Flow is occluded in a
position at the
treatment site or, preferably beyond the treatment site, in either a
retrograde or antegrade
direction. The treatment is performed. The treatment site is disengaged from
the device.
The treatment area is aspirated. Because the flow is prevented by the
occlusion the embolic
material does not travel. The occlusion is then removed. The preferred
embodiment for an
aortic application is a valvuloplasty balloon with dual balloons. A larger
distal balloon is
inflated within the ventricle. The balloon is pulled back so that the aortic
outflow is
obstructed, the balloon is sized so that it is significantly larger than the
aortic valve. The
second smaller diameter balloon located immediately proximal to the first
balloon is then
inflated to dilate the valve annulus. The second balloon is then deflated and
the entire area
aspirated with an aspiration catheter. The first balloon is then deflated to
restore aortic
- 103 -
CA 3014203 2018-08-14
outflow. Alternatively, there may be a tube central to these balloons
providing flow while
this operation in occurring. This would be a limited by-pass of oxygenated
blood around the
area being decalcified. During this by-pass, a cutting mechanism may be
introduced where
as the valve and calcium may be mechanically removed. Examples of cutting
mechanisms
would include a rotating burr, an oscillating pin and die to punch the
material out in
segments or ultrasound energy to fragment the material free for aspiration
removal. It may
be necessary to additionally canulate the coronary arteries to continue flow
to these critical
vessels.
[0398] The system could further contain a perfusion lumen to
reintroduce the left
ventricular outflow in a location that does not cause the movement of blood in
the area of the
aortic root. For example blood could be reintroduced in the coronary arteries
or in the aortic
arch or in the carotid arteries.
[0399] It may also be possible to have the filter device 522 mounted
to the
delivery catheter and actuated by the handle to open and close the filter to
the vessel wall.
This device would be placed between the aortic valve and the great vessels in
the arch. A
secondary catheter system could also be used to filter debris from the aorta
and delivered
from another vessel to the arch. This filter could also be attached to the
temporary valve
assembly providing filtration protection with valve support as the native
valve is removed or
decalcified. A filter could also be mounted to the excision tool protecting
the down stream
vessels from emboli. By protecting each individual vessel such as the
carotids, great vessels,
and the aorta separately, devices would be required in each of these vessels
to protect them
from emboli. These filters could be a simple windsox style as seen by EPI
(Boston
Scientific) and could recover the emboli through a catheter. Other systems for
filtration
include the Percusurge device sold by Medtronic where balloons protect the
area of interest
and aspiration withdrawals the emboli.
[0400] Filtration devices may be set directly on the calcified aortic
valve to
prevent any material from escaping. This filtration device may be made from a
woven or
braided wire such as Nitinol or stainless steel, MP35N, polymeric fibers or
other suitable
material commonly used in medical devices. The materials may be composed of
round, oval
or flat ribbon material. This may provide benefits when designing low profile
device. These
- 104 -
CA 3014203 2018-08-14
wire would be have cross sectional diameters ranging from 0.001-0.030 inches.
These wires
may be supported by larger extension wires to hold the filter material open as
seen in. The
filter may require a support structure such as a stent or series of struts to
provide dimensional
integrity. This stent structure could be a common Z-stent or an inflatable
structure to hold
the filter open and sealed to the valve base or vessel wall. The support
structure would be
expanded or deployed by exposing the device from a sheath or by actively
providing a force
to move the structure from a beginning shape to a final shape. Housed in a
catheter for
delivery, the device would be constrained to a small cross section and expand
to a larger
cross sectional diameter or area as allowed. The deployed device would have a
general
conical shape with the open large diameter facing down or toward the valve.
The opposite
end would come together at the catheter and be retrievable by the introduction
catheter or a
second retrieval catheter to remove any debris captured. These catheters may
have a
diameter of about 8-24 French. The filtration material could be located inside
or outside the
support structure depending upon what flow characteristics were required. For
instance, if
the filter material was located on the outside of the support structure the
filter may be in
contact with the coronary ostia. It may be more desirable to have the filter
material on the
inside of the support structure holding it away from the ostia of the coronary
arteries. The
filtration would trap particles from about 35-250 microns in size and allow
adequate flow
through the aorta. The distal portion of the filter may have a ring or
template at the distal
end to allow for a patterned removal of the native aortic valve. The distal
portion would fit
between the aortic wall of the sinus and the calcium deposits to be removed.
The template
would provide a pattern that may be traced by a removal tool as described in
paragraphs
above. By using a template the pattern would be close to the native healthy
orifice. An
acceptable cross sectional area would be about 2-3 cm2. This would provide
adequate room
to place a new valve and provide the patient good hemodynamic flow. This
template could
be as simple as a guide provided by a wire ring or a pattern with three arches
as seen in a
healthy valve similar to a clover. It may however be simpler to provide a
round hole than a
complex shape to begin. This template may be above or below the native valve
and may
require more than one shape and or size.
- 105 -
CA 3014203 2018-08-14
[0401] Another design for a filter is to utilize a braided Nitinol
stent that will
provide support to the filter material but is still recoverable inside the
catheter. In this
embodiment, the filter material would be inside the braided structure and the
braid would be in
contact with the aortic wall. This would provide a seal between the filter
device and the vessel
directing flow through the filter element and allowing the coronary arteries
to be patent
[0402] After the temporary valve 520 and embolic protection filter
524 are in
place, a debulking or valve removal step is performed as shown in Figures 57F-
H. If
possible, a guidewire may be advanced across the native valve. In some
severely calcified
native valves it may not be possible or practical to advance a wire across the
valve. In these
cases a new lumen may be formed through the valve. This can be done using a
sharp wire
needle or a heated wire cutting tool, or a rotating drill type cutting tool. A
centering device
may be used to ensure that the new lumen is created near the center of the
native valve.
Designs for centering devices known in interventional cardiology for the
treatment of
chronically occluded arteries may be used. These devices typically include a
centering
balloon. Alternatively an expandable wire basket may be used to center the
wire while
maintaining flow. An expandable basket could be cut from a superelastic
hypotube such as a
nitinol hypotube. One basket design includes a short uncut section of tube at
both the
proximal and distal ends this tube segment is about 1 to about 3 mm in length.
The proximal
and distal tube sections are connected by at least three struts. The struts
are from about 30 to
about 60 mm in length, and may be stabilized to each other by one ore more
connectors
along their length. The tube is then heat set or otherwise formed so that the
middle portion is
expanded to between 18mm and 35mm. The proximal and distal uncut hypotube
sections
support a central lumen for guidewire access, while the struts push out
against the vessel
walls to center the device.
[0403] After wire access has been gained it still may be difficult or
impossible to
pass some embodiments of the cutting device across the stenossed native valve.
If necessary
a preliminary cutting step may be performed to enlarge the valve opening
sufficiently that a
second cutting device may be inserted. In one embodiment the primary cutting
device
includes a rotating burr centered on a guidewire. The burr is mounted to a
small flexible
hypotube or solid shaft, which is spun by a motor outside the body. Preferably
the hypotube
- 106 -
CA 3014203 2018-08-14
has an inside diameter of .014 to .040 in and the shaft has a diameter of
about 0.010-0.030
inches. The rotating burr preferably has an outside diameter slightly larger
than the
secondary cutting tool this is preferably in a range of 2 to 6 mm diameter. A
similar rotating
burr device is marketed by Boston Scientific, under the trade name Rotoblader,
for the
treatment of stenotic arteries.
[0404] A cutting device of this design could also be used to open the
calcified
valve to the desired diameter. In this case a larger burr may be used ranging
in diameter
from about 3 to about 9mm in diameter. A steerable catheter may be required to
center the
newly enlarged opening in the native anatomy. A steerable catheter may consist
of a flexible
elongate tube with a pull wire located off center in at least a portion of the
elongate tube.
When tension is applied to the pullwire, it causes the catheter to bend in the
direction to
which the wire is offset. Multiple pullwires may be used to allow the catheter
to be steered
in multiple areas or directions. The catheter my also be manufactured with a
preferred
bending plane, allowing even a centered pullwire to steer the catheter, and
providing more
precise control of the catheter shape. The catheter is preferably of an
outside diameter
between 3mm and 9mm.
[0405] Several embodiments of cutting devices are possible some of
which are
describe above. In one embodiment, the cutting device 530 consists of a tool
that pushes or
pulls a sharpened punch into a die as described with reference to Figure 53A.
This cuts
segments of calcified tissue away from the valve annulus and pulls them back
into the
catheter shaft. From there suction may optionally be applied to extract the
calcified tissue
from the body through a catheter. This design has the advantage of producing a
minimum of
embolic debris, because most of the tissue is forced into the catheter shaft.
Preferably the
cutting die is manufactured from a hardened pin ground at an angle so that the
cutting forces
are primarily piercing the material first with a high force per square inch.
The cutter is
preferably ground at an angle between 20 and 80 degrees from the axial
direction of the pin.
Secondary angles may also be ground on the pin near the pint formed by the
primary
grinding angle. This minimizes the force required to start the cut. Preferably
the pin
diameter is between 3mm and lOmm.
- 107 -
CA 3014203 2018-08-14
[0406] Alternatively a similar cutting device could be used where the
cutting
portion consists of a rotating cutter. The cutter is pulled back through the
die portion forcing
the material into the catheter shaft in a similar manner to the device
described above. The
rotating edge of the cutter may be sharpened to an edge to minimize embolic
material as
much as possible or may be serrated to maximize the cutting ability of the
device. This
device is very similar in function to devices commonly used for DCA or
directional coronary
atherectomy. Typically DCA devices cut in a push mode, capturing the cut out
section in a
cavity near the distal tip of the device. The devices described above operate
in a pull mode,
which allows the cut out material to be evacuated out the catheter shaft or
fill a larger area
within the catheter shaft. However either cutting device described could be
manufactured to
operate in a push mode rather than a pull mode. It may be desired to have the
helix direction
pull the material back or proximally to the catheter handle. This would allow
for convenient
removal of the debris from the body.
[0407] The cutting device may include a device to engage the cutting
portion of
the device to the tissue. In one embodiment a balloon possibly a perfusion
balloon is
attached to the non-cutting side of the device. As the balloon is inflated the
cutter is moved
laterally to engaged into the tissue. To maintain flow out of the heart the
balloon inflation
and cutting may be accomplished during the time that the aortic valve would be
closed. This
could be synchronized to the patients heart rate by echocardiography or
similar sensing
techniques or the patient could be placed on a temporary pace maker and the
pacemaker
output could be used to time the inflation of the balloon. The inflation media
could be a
liquid or a gas. A gas such as helium or CO2 would allow the quickest
inflation time
through a small lumen. Helium would provide an even quicker inflation time
than CO2,
however CO2 may be better dissolved in the blood in the event of a balloon
burst.
[0408] Preferably the engagement method allows flow to pass around
the
engagement device as shown in Figure 53A. One way to accomplish this is with a
single or
plurality of metal straps as described above with reference to Figure 53A that
expand out
from the catheter against the native valve. The straps may be made to bow out
from the
catheter shaft by moving the mounting points of the straps towards each other,
or by sliding
the straps through the proximal section of the catheter. Alternatively the
straps may be made
- 108 -
CA 3014203 2018-08-14
self expanding and be constrained by a sheath or other means, during delivery.
A self
expanding sheathed device may consist of other geometries besides a simple
strap. For
example the expanding device could be formed from a braided mesh similar to a
recoverable
self expanding stent. These straps would be about 0.005-0.020 inches in cross
section and
have a length of about 40-80 mm.
[0409] Another engagement method that allows flow past the catheter
includes a
steerable catheter mechanism. The device can be bent in such a way that the
window of the
cutting device is pushed against the tissue, while this force is opposed by a
section of the
catheter bushed against tissue in an opposite direction. A DCA device marketed
by the
company Foxhollow uses this mechanism to engage tissue.
[0410] The engagement means may be adjustable to a predetermined
range of
sizes from the catheter handle. The cutting tool is advanced or retracted into
the annulus and
successive cuts are made by the operator. The catheter may be rotated slightly
between each
cut. Once the new annulus is cut out large enough for the engagement means to
pass through
the annulus the operator knows that the annulus has been enlarged to a size
that corresponds
with the adjustment, or size of the engagement means. If this is a
sufficiently large cross
sectional area for adequate flow after the permanent prosthetic valve is
implanted, then the
cutting device may be removed. If a larger annulus is desired the engagement
means may be
adjusted or replaced with a larger size, and the process repeated. If the
distal end of wire
straps are attached at the distal end of the cutting device and the proximal
end of the wire
straps are attached to the distal end of a sheath mounted over the cutting
tool shaft, then the
advancement of the sheath will cause the wire straps to bow out and engage the
tissue. The
distance that the sheath is advanced corresponds to the diameter that the
engagement
mechanism will pass through. Markings on the cutter shaft show the operator
what diameter
the engagement means is expanded to. Preferably the engagement means is
expandable to at
least about 2cm. This provides an effective orifice area over 3 cm2.
[0411] The cutting device 530 may include a lumen for contrast or
therapeutic
agent injection. The injection of contrast allows the operator to visualize
the size and
position of the cut out area relative to the aortic root and the ventricle,
under fluoroscopy,
MRI, NMR or other imaging techniques used in interventional cardiology. The
injection of a
- 109 -
CA 3014203 2018-08-14
therapeutic agent may be used to have any desired effect on the heart or
ventricle. Certain
therapeutic agents such as antibiotics may aid in reducing the risk of
endocarditis or in the
treatment of a valve damaged by endocarditis. Other therapeutic agents may
increase or
decrease the heart rate or hearts output as desired by the physician. The
diameter of the
inflation lumen is preferably between 0.010 and 0.060in. in diameter.
104121 As the valve 34 is being removed imaging the procedure is
important.
The operator must be able to visualize the position of the cutout relative to
the aortic wall
and the aortic root. Two-dimensional imaging techniques such as fluoroscopy
need to be
performed on multiple axis to allow the cutting procedure to be performed
safely. The
operator must be careful not to cut through the aortic wall or through the
ventricle. Electrical
conduction paths near the annulus such as the bundle of his may require
special attention and
care. The area between the anterior leaflet of the mitral valve and the aortic
valve must not
be damaged, and the mitral leaflets and chordae must be avoided. To visualize
these and
other obstacles during the procedure any number of common imaging techniques
may be
employed either during the procedure or prior to the procedure in a road-
mapping step.
Echocardiography may be employed in one of several forms to image the
necessary areas.
TEE or trans esophageal echocardiography may be particularly useful in imaging
the valve
area before the procedure begins and during the procedure as well. TTE may
also be used
with the benefit of being less invasive to the patient, but it is limited by a
reduced image
quality and the fact that the operator's hand must be near the patient's
chest. This makes the
simultaneous use of fluoroscopy and other imaging techniques unsafe for the
operator.
During the procedure fluoroscopy or MRI or NMR or similar imaging techniques
may be
used to visualize the size and shape of the newly cut out opening and the
position of the
opening relative to all the relevant structures of the native anatomy.
[0413] The cutting device 830 could be actuated by a simple lever
type handle
moving the cutter in either a proximal or a distal direction as the handle is
squeezed. In
addition the handle could contain a rotational swivel or union that allows the
catheter to be
rotated while the handle is held in a fixed position. Further, the function of
the catheter
rotation could be incorporated into the actuation of the handle. The handle
mechanism could
be designed or adjusted sot that the catheter rotates a predetermined amount
each time the
- 110 -
CA 3014203 2018-08-14
cutter is actuated. This could be accomplished with a simple cam and sprag
mechanism, or
using a stepper motor. The actuation of the cutting mechanism could also be
powered
electronically or pneumatically to minimize operator fatigue and to prevent
the overloading
of the device. In this case the operator would simply depress a button to
actuate the cutting
function. The handle may also contain an aspiration lumen to assist in the
removal of debris
from within the catheter shaft, and an injection lumen to inject contrast
media or a
therapeutic agent, or a fluid such as saline. Other means of providing energy
to the device
include an impact or momentum drive where a high velocity rate would contact
the area to
be removed providing a high degree of force to the calcific valve. Drive or
propultion
methods may include a gaseous discharge or chemical reaction to generate a
hydraulic force
to drive an object into or through the calcified valve. Other predictiable
forces may include
preloading a spring mechanism and releasing the energy stored to drive an
object into or
through the calcified valve.
[0414] The valve could also be cut out in sections using a laser or
heated wire.
Severely calcified areas could be broken up with Cavitation Ultrasound energy,
prior to
removal with a cutting tool or the calcified areas may be broken up with
ultrasound and the
debris captured in a filter. Similarly a chemical compound could be used to
dissolve or
break up the calcium.
[0415] With reference to Figures 57I-57L, the valve implantation step
includes
the installation of an inflatable valve 100 as described above or any stent
based valve such as
Edwards/PVT, CoreValve's self-expanding system. This step is described in
previous filings
by Lashinski from Direct Flow Medical and by Anderson from PVT/HeartPort both
of
California.
[0416] With reference now to Figures 57M- 570, as a final step in the
illustrated
embodiment, the two items to be removed after successful implantation of the
new valve are
the filtration device 522 which may hold emboli or debris and it's delivery
catheter. This
step may require aspiration and or suction to capture any items not trapped
within the filter.
Once the filter moved through the temporary valve 520, the filter 524 may need
to be
redeployed to capture any particles that the temporary valve 520 may hold or
dislodge during
removal. Once the temporary valve 520 is either deflated or drawn back into
the sheath, the
- 111 -
CA 3014203 2018-08-14
filter 524 and it's delivery system may be removed leaving the new valve
100functioning
properly.
[0417] The illustrated embodiment provides a method of implanting a
percutaneous prosthetic valve assembly, where the outflow tract is not blocked
at any time
during the implantation process. In heart failure patients, blocking the
aortic output can have
serious consequences, such as death. Another less significant problem with
blocking aortic
output is the contracting ventricle can exert significant pressure on the
device, making
positioning very difficult, and possibly forcing the device away from the
desired location
before it is completely deployed or anchored. To overcome this issue in some
cases patients
have been rapidly paced. By increasing the patients heart rate to such an
extent that the heart
does not effectively pump blood. This may not be required during the
implantation of this
inflatable device.
[0418] In contrast, devices such as those disclosed in Andersen
family of US
patents (5,411,552 6,168,614 6,582,462) result in the complete or nearly
complete
obstruction of the aortic valve during deployment. For example as a balloon
expandable
valve structure is expanded the balloon blocks the aortic output. In one
embodiment
Andersen describes the use of multiple balloons to deploy the valve, as was
common with a
balloon valvuloplasty. Using multiple balloons would provide a very small path
for fluid to
flow between the balloons when the balloons are fully inflated to a pressure
high enough that
they take on their natural generally round cross section. However when the
balloons are
partially inflated or during the inflation process the multiple balloons
conform to and occlude
the lumen, resulting in complete or nearly complete blockage of the outflow
tract.
[0419] The self-expanding valve support structures disclosed in
Andersen and
Leonhardt (6,582,462 and 5,957,949) also block aortic outflow as they are
deployed. As the
sheath is retracted from the distal portion of the device the device opens and
begins to
conform to the native vessel. The portion of the valve structure designed to
seal to the valve
annulus or other portion of the native anatomy comes in contact with the
native anatomy. At
the same time, the proximal portion of the device is still restrained within
the deployment
catheter, preventing the valve from opening. At this stage of deployment the
devices
effectively block all aortic output.
- 112 -
CA 3014203 2018-08-14
[0420] The simplest extension of existing technology to allow
implantation of a
prosthetic valve without blocking flow is the use of a perfusion balloon with
a balloon
expandable support structure. The perfusion balloon would have a lumen through
the
balloon large enough to allow significant perfusion through the balloon during
deployment.
Perfusion balloon technology is well developed, and known. Wasicek et al
describe a
perfusion balloon catheter is 6,117,106
[0421] Using a self-expanding valve support structure it would be
possible to
maintain flow past the valve using a tube section placed through the affected
valve, outside
the self expanding support structure. After the self-expanding support
structure is
completely deployed the tube section could be withdrawn. The tube section is
longer than at
least the sealing portion of the self-expanding valve support structure, and
preferably
attached to an elongate member to allow its withdrawal. Alternatively the tube
section could
be located inside the valve support structure. In this case the tube section
would allow fluid
(usually blood) to flow into the deployment catheter. Perfusion holes in the
deployment
catheter would allow blood to flow out into the native conduit.
[0422] Relating to the current inflatable prosthetic valve or cast in
place support
structure described herein, a different deployment procedure is used which
allows outflow to
be maintained. This deployment method could also be used with some self-
expanding
percutaneous valves. The deployment method is described as follows for an
aortic valve
replacement. The procedure could be easily adapted to any other coronary
valve. The
deployment catheter is advanced across the aortic valve. The prosthetic valve
and inflatable
cuff are unsheathed in the ventricle, but remain attached to the deployment
control wires.
The distal end of the inflatable cuff is inflated. The sheath is retracted far
enough that the
deployment control wires allow the prosthetic valve to function. The device is
then
withdrawn across the native valve annulus. The device is then fully inflated.
The valve
function may be tested using various diagnostic techniques. If the valve
function is sufficient
the inflation media may be exchanged for the permanent inflation media. The
deployment
control wires and the inflation lumens are then disconnected and the catheter
withdrawn. In
this procedure the key to maintaining the outflow tract is the use of
deployment control
wires. The deployment control wires allow the device to be moved an
appreciable distance
- 113 -
CA 3014203 2018-08-14
from the deployment sheath before the device is permanently positioned in the
desired
location. Other deployment control devices could be used to have similar
effect. For
example a sheath used as a shear barrier between the retractable sheath and
the implant
having longitudinal slots could be configured to produce a similar function.
It may be
desirable to predilate the native valve annulus with a balloon before device
implantation.
This may allow for a larger effective orifice area to implant the device and
precondition the
valve area. Secondarily, an additional dilatation may be desired after
implantation to ensure
the device is apposed to the wall of the annulus and seated properly.
[0423] The current percutaneous valve replacement devices do not
provide a
means for testing the function of the valve before committing to the position
of the valve.
[INSERT REFERENCE TO PREVIOUSLY DESCRIBED FIGURES] These devices are
deployed at a location and if the location was a wrong location or if the
valve does not have a
good effect, the valves can-not be removed. The present invention includes a
method of
valve implantation consisting of the steps of positioning the valve, enabling
the valve, testing
the function of the valve, and finally deploying the valve.
[0424] Relating to the current inflatable prosthetic valve or cast in
place cuff, a
unique deployment procedure is used, consisting of the steps of position,
enable, test, and
reposition or deploy. This deployment method could also be adapted to a valve
with a self-
expanding support structure or to other implantable devices. The deployment
method is
described as follows for an aortic valve replacement. The procedure could be
easily adapted
to any other coronary valve. The deployment catheter is advanced across the
aortic valve.
The prosthetic valve and inflatable cuff are unsheathed in the ventricle, but
remain attached
to the deployment control wires. The distal end of the inflatable cuff is
inflated. The sheath
is retracted far enough that the deployment control wires allow the prosthetic
valve to
function. The device is then withdrawn across the native valve annulus. The
device is then
fully inflated, enabling the valve to function. The valve function may be
tested using various
diagnostic techniques. If the valve function, sizing or securement is not
sufficient or ideal
the valve may be partially deflated, and advanced or retracted, and then
reinflated or the
valve may be fully deflated and retracted into the deployment catheter or
another slightly
larger catheter, and removed. Once a valve is positioned, sized and secured
acceptably or
- 114 -
CA 3014203 2018-08-14
ideally the inflation media may be exchanged for a permanent inflation media,
which may
jell, set or cure. The inflation catheters and deployment control wires are
then disconnected
and the catheter removed, fully deploying the valve.
[0425] If the technology from a known self-expanding recoverable
stent is
adapted to a valve support structure, the stent is only recoverable from a
partially deployed
state. A self-expanding support structure of a length sufficient only to
support and retain the
valve would not allow testing of the valve function, until the valve was fully
deployed. This
is because the proximal portion of the support structure contained within the
device would
prevent normal function of the valve. A proximal extension of the support
structure could be
added to act as a deployment control device allowing the valve function to be
tested in a
configuration where it is still possible to remove or reposition the valve.
The proximal
extension could be a continuation of the braided or laser cut stent structure,
provided that the
cell structure is open enough to allow blood flow through the stent. In an
aortic valve
application the required length of the proximal extension would most likely
extend beyond
the ostia of the coronary arteries. In this case the shape of the stent
structure may be
designed to permit unobstructed flow to the coronary arteries or to permit
adequate flow to
the coronary arteries. Another possibility is to design the proximal extension
so that it acts
as multiple individual wires. This could be done by laser cutting or by
changing a braid
pattern. This would also allow the proximal portion of the implant to act as a
deployment
control device.
[0426] A method for recapturing a self-expanding stent is described
by Johnson
et al in US Patent 5,817,102, as follows.
[0427] There is provided an apparatus for deploying a radially self-
expanding
stent within a body lumen. The apparatus includes a stent confining means for
elastically
compressing a radially self-expanding stent into a delivery configuration in
which the self-
expanding stent has a reduced radius along its entire axial length. The
apparatus includes an
elongate and flexible stent delivery device having a proximal end, a distal
end and a distal
region near the distal end. The distal region is used in delivering the
radially self-expanding
stent into a body lumen, and in positioning at a treatment site within the
body lumen with the
stent surrounding the delivery device along the distal region. The proximal
end of the
- 115 -
CA 3014203 2018-08-14
delivery device remains outside of the body. An axial restraining means is
disposed along the
distal region of the delivery device. A control means is operable associated
with the delivery
device and the confining means. The control means moves the confining means
axially
relative to the delivery device toward and away from a confinement position in
which the
confining means compresses the self-expanding stent into the delivery
configuration, and
urges the stent into a surface engagement with the axial restraining means.
The restraining
means, due to the surface engagement, tends to maintain the self-expanding
stent axially
aligned with the deployment device as the confining means is moved axially
away from the
confinement position to release the stent for radial self-expansion.
[0428] Preferably the stent delivery device is an elongate and
flexible length of
interior tubing, with a central lumen for accommodating a guidewire. The stent
confining
means can be an elongate and flexible length of tubing, having a lumen for
containing the
interior tubing. The second (or outer) tubing surrounds the stent to confine
it.
[0429] The preferred axial restraining means is a low durometer
sleeve
surrounding the interior tubing along the distal region. If desired, an
adhesive can be applied
to an exterior surface of the sleeves. Alternatively, the axial restraining
means can consist of
several elongate strips disposed along the distal region, with adhesive
applied to radially
outward surfaces of the strips, if desired.
[0430] In either event, so long as the exterior tubing surrounds the
stent to
radially compress the stent, it also maintains the stent in surface engagement
with the sleeve
or strips. As the exterior tubing is axially withdrawn to allow part of the
stent to radially self-
expand, the rest of the stent remains confined against the sleeve or the
strips. As a result, the
stent does not travel axially with the exterior tubing. Rather, the stent
remains substantially
fixed in the axial direction with respect to the interior tubing. This
structure affords several
advantages. First, the interior tubing can be used as a means to positively
maintain the
radially self-expanding stent in the desired axial position during deployment.
The interior
tubing can itself be employed as a reliable indicator of stent position, both
prior to and
during deployment. Further, should the need arise to retract the stent after a
partial
deployment, the outer tubing can be moved back into the confinement position,
without
tending to carry the stent along with it.
- 116 -
CA 3014203 2018-08-14
[0431] The current percutaneous valve replacement devices are not
removable or
repositionable. These devices are deployed at a location and if the location
was a wrong
location or if the valve does not have a good effect, the valves can not be
removed,
recaptured or repositioned percutaneously. The present invention includes a
method of
implantation facilitating percutaneously repositioning, recapturing and/or
removing, a
prosthetic valve
[0432] A balloon expandable support structure is more difficult to
make
recapturable, repositionable or removable. One method would be to use a shape
memory
alloy, such as Nitinol. In this case if Nitinol was used it would be in the
martensitic phase at
body temperature. Martensitic Nitinol is not superelastic, but soft and
conformable. It
would be somewhat suitable as a balloon expandable support structure material,
except the
yield strength is very low. This requires relatively thick cross sections to
be used. The
balloon expandable support structure is deployed in any way desired, such as
by the methods
described in Andersen. If the location or performance of the valve is not
acceptable the
support structure may be caused to contract by changing its temperature,
causing it to return
to its preset "remembered" shape, which in this case is a smaller, radially
collapsed shape.
The temperature controlling media could be a fluid such as saline, and could
be delivered
while a catheter or balloon is inserted through the support structure. This
would cause the
valve and valve support structure to collapse down on the balloon or catheter
allowing
removal or possibly redeployment. Other shape memory materials are available,
and may
have more desirable mechanical properties for use as a balloon expandable
support structure.
In some cases the biocompatibility of these alloys is not known.
[0433] It would be possible to construct a self-expanding valve that
would be
capable of being recaptured. This could be done using technology from
recapturable self-
expanding stents. Typically these devices are braided from a superelastic or
high strength
alloy and have relatively low radial strength. As they are pulled back into a
sheath they
collapse on their diameter and lengthen facilitating recapturability. Not all
braided self-
expanding structures are recapturable. To our knowledge this technology has
not yet been
applied to valve support structures.
- 117 -
CA 3014203 2018-08-14
[0434] Relating to the current inflatable prosthetic valve or cast in
place support
structure, a different deployment procedure is used which allows the device to
be
repositionable recapturable, and removable. . This deployment method could
also be used
with some self-expanding percutaneous valve support structures. The deployment
method is
described as follows for an aortic valve replacement. The procedure could be
easily adapted
to any other coronary valve. The deployment catheter is advanced across the
aortic valve.
The prosthetic valve and inflatable cuff are unsheathed in the ventricle, but
remain attached
to the deployment control wires. The distal end of the inflatable cuff is
inflated. The sheath
is retracted far enough that the deployment control wires allow the prosthetic
valve to
function. The device is then withdrawn across the native valve annulus. The
device is then
fully inflated. The valve function may be tested using various diagnostic
techniques. If the
valve function, sizing or securement is not sufficient or ideal the valve may
be partially
deflated, and advanced or retracted, and then reinflated or the valve may be
fully deflated and
retracted into the deployment catheter or another slightly larger catheter,
and removed. Once
a valve is positioned, sized and secured acceptably or ideally the inflation
media may be
exchanged for a permanent inflation media which may jell, set or cure. The
inflation
catheters and deployment control wires are then disconnected and the catheter
removed.
This deployment method provides many advantages including the ability to
reposition
recapture and remove the device.
[0435] In an alternative delivery method (surgical) transapical
access would
allow for the device to be placed in a less invasive surgical procedure. This
may still be a
beating-heart procedure but would limit the access incision area. Through the
apex of the
heart a tube may be inserted to introduce the device to the aortic valve from
a antigrade
approach. This would allow the device to be placed and or moved in the same
manner
previously described in a catheter delivery.
[0436] The prosthetic valve with inflatable cuff may also be
delivered surgically.
The inflatable cuff aids in sealing the valve to the native anatomy. A valve
of this design
may be placed in any coronary valve position as well as in a vein, lung,
ureter, or any area of
the body known to benefit from the implantation of a valve or flow control
device. In one
embodiment the native valve is sutured in place similar to known coronary
prosthetic valves.
- 118 -
CA 3014203 2018-08-14
The inflatable cuff is then expanded to form a tight seal with the native
anatomy. In another
embodiment the valve is placed in the desired location and the valve is
expanded. The valve
is held in place by physical interference with the native anatomy. The
geometry of the
implant may be similar to the percutaneous applications for the inflatable
prosthetic valve
described in previously.
[0437] The valve may be further secured by additional methods such as
sutures or
staples. The surgical procedure may also be performed in a less invasive
manner, for
example a smaller opening in the atrium or aorta could be used to implant the
valve, because
the valve attachment process is less critical. In another embodiment the valve
may be
implanted with a minimally invasive surgical device. A device of this design
for an aortic
valve application punctures the chest wall and the ventricular wall near the
apex of the heart.
The device is then advanced across the native valve annulus and implanted in a
manner
consistent with the percutaneous embodiments of the invention. This procedure
may be
guided by echocardiography, angiography, thorascopy or any other appropriate
visualization
method commonly known.
[0438] One Step Implantation
[0439] By deploying the device at the site in one step the native
valve may be
excluded while the new valve is being placed. It is conceived that the device
may have a
shape similar to a tubular hyperbola to exclude the old valve by trapping it
under the new
structure during deployment. This may aid in patient comfort and safety if the
vessel is not
occluded during implantation by a balloon deployed stent system. As the
sheathed device is
delivered via catheter through the vessel past the aortic valve, it may be
reveled or exposed
by removing the sheath partially or completely and allowing proper placement
at or beneath
the native valve. Once in the vessel, the device may be moved proximal or
distal and the
fluid may be introduced to the cuff providing shape and structural integrity.
It may be
necessary to add or retract the fluid for proper positioning or removal. Once
the cuff is
positioned properly and the fluid is added creating the structure and sealing
the device to the
vessel wall, the delivery catheter may be disconnected and removed leaving the
now
functioning valve device as a permanent implant. The disconnection method may
included
cutting the attachments, rotating screws, withdrawing or shearing pins,
mechanically
- 119 -
CA 3014203 2018-08-14
decoupling interlocked components, electrically separating a fuse joint,
removing a trapped
cylinder from a tube, fracturing a engineered zone, removing a colleting
mechanism to
expose a mechanical joint or many other techniques known in the industry.
[0440] Two Step Implantation
[0441] It may be desirable to implant the valve structure in two
steps. It is
desireable to attach the valve to the native tissue securely and without
leaks. Also it is
desireble to avoid blocking the flow of blood for a long period of time. For
these reasons it
may be desirable to first implant a retention-sealing device as a first step
and then as a
second step implant the cuff with the valve attached. The retention-sealing
device could be a
stent like structure expanded in place or a ring shaped support structure
where the valve is
secondarily attached. The ring shaped structure could utilize the fluid
inflation method as
mentioned above and could be a separate system and catheter. It could
incorporate barbs for
anchoring. It could also incorporate a sealing material to help prevent blood
from leaking
around the valve. The device could incorporate a mechanism to attach the
support structure
to. The retention mechanism could be a shoulder or a channel that the support
registers in.
Once in position, the deployment of the valve could take place as mentioned in
the One Step
Implantation description above
[0442] In an alternative embodiment a support structure, such as a
stent is
delivered in one step and the valve is delivered in a later step. The valve is
then attached to
the support structure. The support structure may be an expandable scaffold or
stent designed
to produce a physical interference with the native vessel. The support
structure could also
use the geometry of the native anatomy as described in other embodiments, for
[0443] Deflate balloons after anchoring
[0444] In another embodiment the balloon inflation step is used to
enable the
device and the support structure and anchoring device are delivered in a later
step. In one
embodiment the support structure is a balloon expandable stent. The stent is
placed inside
the inflated cuff The stent may also extend proximal or distal from the cuff
More than one
stent can be used. Preferably a stent is placed proximal to the valve portion
of the implant
and a stent is placed distal to the valve portion of the implant, or a portion
of the stent
extends across the valve. In one embodiment the balloons are left as part of
the implant in a
- 120 -
CA 3014203 2018-08-14
deflated state. The balloons are disconnected from the catheter by a mechanism
described in
this application with the exception that the sealing feature is not required.
Other detachment
mechanisms are also possible. In another embodiment the balloon is removed
from the
device after it is deflated. The balloon may be placed in a channel in the
cuff and simply
retracted after deflation. Alternatively the balloon may be attached to the
implant with
sutures designed to break as the balloon is inflated. After the balloon is
inflated and deflated
the balloon can be retracted.
[0445] Stent on device
[0446] A method of delivering a valve attached to a cuff as a first
step, and
delivering an expandable structure as a second step. The structure may be a
stent or an
unwrapable band, engaged coaxially inside the cuff The cuff may be positioned
using an
inflatable cuff, where the cuff remains inflated after the device is
disconnected from the
catheter. In this case the inflation serves the function of temporary
securement and of
permanent sealing. Alternatively the cuff may contain a removable balloon. In
this
embodiment the inflation provides a means of temporary support until the
permanent support
structure is deployed. Yet another alternative involves a valve and cuff
assembly that
contains no inflation provision. The cuff is held in place using deployment
control wires that
are shaped in a way to cause the expansion of the prosthesis. The stent or
expandable
support structure is then delivered to a position located coaxially within the
cuff The stent is
then deployed, securing the device.
[0447] Creating support structure in vivo
[0448] The present invention includes a method of creating a support
structure
inside the body of a patient. The preferred embodiment includes manufacturing
the support
structure by a casting method. In this method fluid is injected into a mold or
cuff that is
attached to the valve and delivered percutaneously. The fluid then jells
hardens or solidifies
forming the support structure.
[0449] There are other methods of manufacturing a support structure
in vivo. In
one embodiment the support structure can be assembled from many small solid
particles.
The particles can be attached to one another by various means, including a
thread woven
through the particles, in such a way that when the thread is tensioned the
thread and the
- 121 -
CA 3014203 2018-08-14
particles form a rigid structure. The particles could be attached to one
another by a sintering
process, with an adhesive or by another method. The support structure could
also be
manufactured in place from wire, which is woven and inserted into the shape of
a support
structure in vivo.
[0450] The support structure could also be manufactured in place
using a
biological reaction such as forming calcium deposits on the appropriate
portion of the valve.
The support structure could be assembled by nanomachines.
[0451] The support structure could also be manufactured from a fluid
that
solidifies jells or hardens that is not contained inside a mold. The fluid
could be applied to
an area on the outer surface of the valve or the inner surfaces oft the area
where the valve is
to be applied, in vivo. The support structure could be manufactured from a
material that
solidifies hardens or becomes more rigid by the addition of a catalyst, heat,
cold or other
energy source. The material could be applied to the outer surface of the
prosthetic valve
before the valve is installed and then activated in vivo. The support
structure could be
excited or activated by an electronic energy. This source could also be
activated by magnets
through a suspension fluid that solidifies in a magnetic field.
[0452] Attachment of valve to non-structural element
[0453] In the present invention the valve is attached only to a
nonstructural
element. In the preferred embodiment the nonstructural element is the sewing
cuff or mold.
The support structure is later manufactured within the mold. Other examples of
valves
permanently attached only to nonstructural elements are possible. A valve
could be attached
to an unsupported tubular section of fabric. After the fabric graft and valve
are positioned in
the patient a stent or other support structure could be deployed within the
graft anchoring the
graft in place. The stent could utilize barbs or fangs to puncture the graft
and anchor the
devices solidly to the native tissue. The stent could also be placed so that
it only partially
overlaps the graft. In this way barbs or fangs could be placed that do not
puncture the graft.
In another embodiment, rigid structural elements such as commissural support
posts or barbs
or anchors are attached to the cuff and delivered with the nonstructural
element.
[0454] The various methods and techniques described above provide a
number of
ways to carry out the invention. Of course, it is to be understood that not
necessarily all
- 122 -
CA 3014203 2018-08-14
objectives or advantages described may be achieved in accordance with any
particular
embodiment described herein. Thus, for example, those skilled in the art will
recognize that
the methods may be performed in a manner that achieves or optimizes one
advantage or
group of advantages as taught herein without necessarily achieving other
objectives or
advantages as may be taught or suggested herein.
[0455] Furthermore, the skilled artisan will recognize the
interchangeability of
various features from different embodiments disclosed herein. Similarly, the
various
features and steps discussed above, as well as other known equivalents for
each such feature
or step, can be mixed and matched by one of ordinary skill in this art to
perform methods in
accordance with principles described herein. Additionally, the methods which
is described
and illustrated herein is not limited to the exact sequence of acts described,
nor is it
necessarily limited to the practice of all of the acts set forth. Other
sequences of events or
acts, or less than all of the events, or simultaneous occurrence of the
events, may be utilized
in practicing the embodiments of the invention.
[0456] Although the invention has been disclosed in the context of
certain
embodiments and examples, it will be understood by those skilled in the art
that the
invention extends beyond the specifically disclosed embodiments to other
alternative
embodiments and/or uses and obvious modifications and equivalents thereof.
Accordingly,
the invention is not intended to be limited by the specific disclosures of
preferred
embodiments herein
- 123 -
CA 3014203 2018-08-14