Note: Descriptions are shown in the official language in which they were submitted.
STABLE PEMETREXED FORMULATIONS COMPRISING PROPYLENE GLYCOL
BACKGROUND OF THE INVENTION
Compounds exhibiting anti-folate activity have a well known role as
chemotherapeutic
agents. One such compound is pemetrexed, which has the chemical name N-V142-(2-
amino-4,7-
dihydro-4-oxo-1H-pyffolo [2,3-d]pyrimidin-5-yl)ethylibenzoy1R-glutamic acid
and the
structure of formula (1):
0
0 OH
/
0
HN
HN
Pemetrexed is used in the treatment of pleural mesothelioma and non-small cell
lung
cancer. AL1MTATm, Eli Lilly's pemetrexed product is presently supplied in 100
mg and 500 mg
vials of lyophilized pemetrexed disodium for injection. According to the
prescribing
information, in order to prepare AL1MTATm for infusion the vials are
reconstituted in sufficient
0.9% Sodium Chloride Injection (preservative free) to give a solution
containing 25 mg/mL of
ALIMTATm. This concentrated solution is then further diluted into a solution
of 0.9% Sodium
Chloride Injection (preservative free). The prescribing information cautions
that reconstitution
and further dilution is only recommended with 0.9% Sodium Chloride Injection
(preservative
free), and that "ALIMTATm is physically incompatible with diluents containing
calcium, including
Lactated Ringer's Injection, USP and Ringer's Injection, USP and therefore
these should not be
used."
Calcium containing diluents, such as Lactated Ringer's Injection and Ringer's
Injection,
are common solutions used in medical settings for the reconstitution and/or
dilution of drug
products prior to intravenous administration. There is a need for pemetrexed
dosage forms that
are chemically stable after reconstitution and/or dilution with diluents
containing calcium. In
addition to being useful with a wider range of available diluents, the use of
such a dosage form
would minimize both the loss of dosage forms due to improper reconstitution or
dilution and the
1
Date Regue/Date Received 2022-07-11
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
risk that a patient would be administered pemetrexed reconstituted or diluted
in an incompatible
diluent.
In solution, pemetrexed undergoes hydrolysis and degrades rapidly. Due to this
rapid
degradation, pemetrexed formulations must either be lyophilized for long term
stability or
comprise stabilizers. However, reconstitution of a lyophilized formulation
requires multiple
steps, each of which increases the risk of user error. In addition,
reconstitution of a lyophilized
formulation is clinically inconvenient and can take up to 30 minutes.
While stable, ready to use formulations of pemetrexed are known, they require
stabilizers, such as anti-oxidants or amino acids as described in US6,686,365;
CN 101081305;
and WO 2012015810, or high levels of non-aqueous solvents, as described in
W02013144814. It
would be advantageous to minimize patient exposure to these additional
ingredients.
As such, there is a need for a stable, non-lyophilized pemetrexed composition
with a
minimal amount of additional ingredients. To this end, we have developed a
stable pemetrexed
formulation.
SUMMARY OF THE INVENTION
In certain embodiments, the invention is directed to a pharmaceutical
composition
comprising pemetrexed and a non-aqueous solvent present at a concentration
less than
0.30m1/mL, wherein upon dilution with a pharmaceutically acceptable diluent to
an initial dosage
concentration of pemetrexed, the composition comprises at least 90% of the
initial dosage
concentration of pemetrexed after storage at a temperature of 2 C to 8 C for
at least 24 hours.
In further embodiments, the invention is directed to a pharmaceutical
composition
comprising 25 mg/mL pemetrexed, 250 IlL/mL propylene glycol, and water,
wherein upon
dilution with a pharmaceutically acceptable diluent to an initial dosage
concentration of
pemetrexed, the composition comprises at least 90% of the initial dosage
concentration of
pemetrexed after storage at a temperature of 2 C to 8 C for at least 24 hours.
In still further embodiments, the invention is directed to a pharmaceutical
composition
comprising pemetrexed at an initial concentration of 10 to 50 mg/mL and a non-
aqueous solvent
2
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
present at a concentration less than 0.30m1/inL, wherein the composition
comprises at least 90%
of the initial pemetrexed concentration after storage at a temperature of 2 C
to 8 C for at least 12
months.
In further embodiments, the invention is directed to a pharmaceutical
composition
comprising 25 mg/mL pemetrexed, 250 1AL/mL propylene glycol, and water,
wherein the
composition comprises at least 900/ of the initial pemetrexed concentration
after storage at a
temperature of 2 C to 8 C for at least 12 months.
In certain embodiments, the invention is directed to a pharmaceutical
composition
comprising pemetrexed and a non-aqueous solvent present at a concentration
less than
0.30m1/mL, wherein upon dilution with a pharmaceutically acceptable diluent to
an initial dosage
concentration of pemetrexed, the composition comprises no more than 8% w/w
total impurities
after storage at a temperature of 2 C to 8 C for at least 24 hours.
In further embodiments, the invention is directed to a pharmaceutical
composition
comprising 25 mg/mL pemetrexed, 250 IAL/mL propylene glycol, and water,
wherein upon
dilution with a pharmaceutically acceptable diluent to an initial dosage
concentration of
pemetrexed, the composition comprises no more than 8% w/w total impurities
after storage at a
temperature of 2 C to 8 C for at least 24 hours.
In still further embodiments, the invention is directed to a pharmaceutical
composition
comprising pemetrexed at an initial concentration of 10 to 50 mg/mL and a non-
aqueous solvent
present at a concentration less than 0.30m1/mL, the composition comprises no
more than 8% w/w
total impurities after storage at a temperature of 2 C to 8 C for at least 24
hours.
In further embodiments, the invention is directed to a pharmaceutical
composition
comprising 25 mg/mL pemetrexed, 250 itiL/mL propylene glycol, and water, the
composition
comprises no more than 8% w/w total impurities after storage at a temperature
of 2 C to 8 C for
at least 24 hours.
Additional embodiments of the invention include:
3
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
1. A pharmaceutical composition comprising:
pemetrexed and
b) a non-aqueous solvent present at less than 0.30 mUrnL;
wherein upon dilution with a pharmaceutically acceptable diluent to an initial
dosage
concentration of pemetrexed, the composition comprises at least 90% of the
initial dosage
concentration of pemetrexed after storage at a temperature of 2 C to 8 C for
at least 12
hours.
2. The pharmaceutical composition of embodiment 1 wherein the composition
retains at
least 90% of the initial dosage concentration of pemetrexed upon dilution and
storage for
a period selected from:
a) at least 24 hours, and
b) at least 48 hours.
3. The pharmaceutical composition of embodiment 1 wherein the composition
retains at
least 95% of the initial dosage concentration of pemetrexed upon dilution and
storage for
a period selected from:
a) at least 12 hours,
b) at least 24 hours, and
c) at least 48 hours.
4. The pharmaceutical composition of embodiment 1 wherein the composition
retains at
least 98% of the initial dosage concentration of pemetrexed upon dilution and
storage for
a period selected from:
a) at least 12 hours,
b) at least 24 hours, and
c) at least 48 hours.
5. The pharmaceutical composition of embodiment 1 wherein the
pharmaceutically
acceptable diluent is selected from the group consisting of normal saline,
water for
injection, 5% dextrose in water, Ringer's Injection, and Lactated Ringer's
Injection.
4
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
6. The pharmaceutical composition of embodiment 1 comprising 10 to 50 mg/mL
pemetrexed.
7. The pharmaceutical composition of embodiment 6 comprising 25 mg/mL
pemetrexed.
8. The pharmaceutical formulation of embodiment 1 wherein the non-aqueous
solvent is
selected from the group consisting of propylene glycol, alcohol, polyethylene
glycol, or
combinations thereof.
9. The pharmaceutical formulation of embodiment 8 wherein the non-aqueous
solvent is
propylene glycol.
10. The pharmaceutical formulation of embodiment 9 wherein propylene glycol
is present at
250 pL/mL.
11. The pharmaceutical composition of embodiment 1 comprising at least
0.50mL/mL water.
12. The pharmaceutical composition of embodiment 1 wherein the pemetrexed
is in the form
of pemetrexed diacid.
13. The pharmaceutical composition of embodiment 1 wherein the pemetrexed
is in the form
of pemetrexed disodium.
14. The pharmaceutical composition of embodiment 1 substantially free of an
anti-oxidant.
15. A pharmaceutical composition comprising
a) 25 mg/mL pemetrexed, and
b) propylene glycol at 250 pL/mL
c) water
wherein upon dilution with a pharmaceutically acceptable diluent to an initial
dosage
concentration of pemetrexed, the composition comprises at least 90% of the
initial dosage
concentration of pemetrexed after storage at a temperature of 2 C to 8 C for
at least 24
hours.
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
16. The pharmaceutical composition of embodiment 15 wherein upon dilution
with a
pharmaceutically acceptable diluent to an initial dosage concentration of
pemetrexed, the
composition comprises at least 90% of the initial dosage concentration of
pemetrexed
after storage at a temperature of 2 C to 8 C for at least 48 hours.
17. A pharmaceutical composition comprising:
a) pemetrexed at an initial concentration of 10 to 50 mg/mL and
b) a non-aqueous solvent present at less than 0.30 mL/mL,
wherein the composition comprises at least 90% of the initial pemetrexed
concentration
after storage at a temperature of 2 C to 8 C for at least 12 months.
18. The pharmaceutical composition of embodiment 17 wherein the composition
comprises
at least 90% of the initial pemetrexed concentration after storage for a
period selected
from:
a) at least 18 months, and
b) at least 24 months.
19. The pharmaceutical composition of embodiment 17 wherein the composition
comprises
at least 95% of the initial pemetrexed concentration after storage for a
period selected
from:
a) at least 12 months,
b) at least 18 months, and
c) at least 24 months.
20. The pharmaceutical composition of embodiment 17 wherein the composition
comprises
at least 98% of the initial pemetrexed concentration after storage for a
period selected
from:
a) at least 12 months,
b) at least 18 months, and
c) at least 24 months.
6
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
21. The pharmaceutical composition of embodiment 17 having an initial
pemetrexed
concentration of 25 mg/mL.
22. The pharmaceutical formulation of embodiment 17 wherein the non-aqueous
solvent is
selected from the group consisting of propylene glycol, alcohol, polyethylene
glycol, or
combinations thereof.
23. The pharmaceutical formulation of embodiment 22 wherein the non-aqueous
solvent is
propylene glycol.
24. The pharmaceutical formulation of embodiment 23 wherein propylene
glycol is present at
250 pL/mL.
25. The pharmaceutical composition of embodiment 17 comprising at least
0.50mL/mL
water.
26. The pharmaceutical composition of embodiment 17 wherein the pemetrexed
is in the
form of pemetrexed diacid.
27. The pharmaceutical composition of embodiment 17 wherein the pemetrexed
is in the
form of pemetrexed disodium.
28. The pharmaceutical composition of embodiment 17 substantially free of
an anti-oxidant.
29. A pharmaceutical composition comprising
a) an initial pemetrexed concentration of 25 mg/rnL,
b) propylene glycol at 250 ItL/rnL, and
c) water;
wherein the composition comprises at least 90% of the initial pemetrexed
concentration
after storage at a temperature of 2 C to 8 C for at least 12 months.
30. The pharmaceutical composition of embodiment 29 wherein the composition
comprises
at least 90% of the initial pemetrexed concentration after storage at a
temperature of 2 C
to 8 C for at least 24 months.
7
CA 03014755 2018-08-15
WO 2017/142556
PCT/US2016/018703
31. A pharmaceutical composition comprising:
a) pemetrexed and
b) a non-aqueous solvent present at less than 0.30 mL/mL,
wherein upon dilution with a pharmaceutically acceptable diluent to an initial
dosage
concentration of pemetrexed the composition comprises no more than 8% w/w
total
impurities after storage at a temperature of 2 C to 8 C for at least 12 hours.
32. The pharmaceutical composition of embodiment 31 wherein the composition
comprises
no more than 8% w/w total impurities after dilution and storage for a period
selected
from:
a) at least 24 hours, and
b) at least 48 hours.
33. The pharmaceutical composition of embodiment 31 wherein the composition
comprises
no more than 5% w/w total impurities after dilution and storage for a period
selected
from:
a) at least 12 hours,
b) at least 24 hours, and
c) at least 48 hours.
34. The pharmaceutical composition of embodiment 31 wherein the composition
comprises
no more than 2% w/w total impurities after dilution and storage for a period
selected
from:
a) at least 12 hours,
b) at least 24 hours, and
c) at least 48 hours.
8
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
35. The pharmaceutical composition of embodiment 31 wherein the
pharmaceutically
acceptable diluent is selected from the group consisting of normal saline,
water for
injection, 5% dextrose in water, Ringer's Injection, and Lactated Ringer's
Injection.
36. The pharmaceutical composition of embodiment 31 comprising 10 to 50
mg/mL
pemetrexed.
37. The pharmaceutical composition of embodiment 36 comprising 25 ing/mL
pemetrexed.
38. The pharmaceutical formulation of embodiment 31 wherein the non-aqueous
solvent is
selected from the group consisting of propylene glycol, alcohol, polyethylene
glycol, or
combinations thereof.
39. The pharmaceutical formulation of embodiment 38 wherein the non-aqueous
solvent is
propylene glycol.
40. The pharmaceutical formulation of embodiment 39 wherein propylene
glycol is present at
250 RL/mL.
41. The pharmaceutical composition of embodiment 31 comprising at least
0.50mL/mL
water.
42. The pharmaceutical composition of embodiment 31 wherein the pemetrexed
is in the
form of pemetrexed diacid.
43. The pharmaceutical composition of embodiment 31 wherein the pemetrexed
is in the
form of pemetrexed disodium.
44. The pharmaceutical composition of embodiment 31 substantially free of
an anti-oxidant.
45. A pharmaceutical composition comprising
a) 25 mg/mL pemetrexed,
b) propylene glycol at 250 gL/mL, and
c) water;
9
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
wherein upon dilution with a pharmaceutically acceptable diluent to an initial
dosage
concentration of pemetrexed the composition comprises no more than 8% w/w
total
impurities after storage at a temperature of 2 C to 8 C for at least 24 hours.
46. The pharmaceutical composition of embodiment 45 wherein upon dilution
with a
pharmaceutically acceptable diluent to an initial dosage concentration of
pemetrexed the
composition comprises no more than 8% w/w total impurities after storage at a
temperature of 2 C to 8 C for at least 48 hours.
47. A pharmaceutical composition comprising:
a) pemetrexed at an initial concentration of 10 to 50 mg/mL and
b) a non-aqueous solvent present at less than 0.30 mL/mL,
wherein the composition comprises no more than 8% w/w total impurities after
storage at
a temperature of 2 C to 8 C for at least 12 months.
48. The pharmaceutical composition of embodiment 47 wherein the composition
comprises
no more than 8% w/w total impurities after storage for a period selected from:
a) at least 18 months, and
b) at least 24 months.
49. The pharmaceutical composition of embodiment 47 wherein the composition
comprises
no more than 5% w/w total impurities after storage for a period selected from:
a) at least 12 months,
b) at least 18 months, and
c) at least 24 months.
50. The pharmaceutical composition of embodiment 47 wherein the composition
comprises
no more than 2% w/w total impurities after storage for a period selected from:
a) at least 12 months,
b) at least 18 months, and
c) at least 24 months.
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
51. The pharmaceutical composition of embodiment 47 comprising 25 mg/mL
pemetrexed.
52. The pharmaceutical formulation of embodiment 47 wherein the non-aqueous
solvent is
selected from the group consisting of propylene glycol, alcohol, polyethylene
glycol, or
combinations thereof.
53. The pharmaceutical formulation of embodiment 52 wherein the non-aqueous
solvent is
propylene glycol.
54. The pharmaceutical formulation of embodiment 53 wherein propylene
glycol is present
at 250 tiL/mL.
55. The pharmaceutical composition of embodiment 47 comprising at least
0.50mUrnI.,
water.
56. The pharmaceutical composition of embodiment 47 wherein the pemetrexed
is in the
form of pemetrexed diacid.
57. The pharmaceutical composition of embodiment 47 wherein the pemetrexed
is in the
form of pemetrexed disodium.
58. The pharmaceutical composition of embodiment 47 substantially free of
an anti-oxidant.
59. A pharmaceutical composition comprising
a) an initial pemetrexed concentration of 25 mg/mL,
b) propylene glycol at 250 L/mL, and
c) water;
wherein the composition comprises no more than 8% w/w total impurities after
storage at
a temperature of 2 C to 8 C for at least 12 months.
60. The pharmaceutical composition of embodiment 59 wherein the composition
comprises
no more than 8% w/w total impurities after storage at a temperature of 2 C to
8 C for at
least 24 months.
11
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
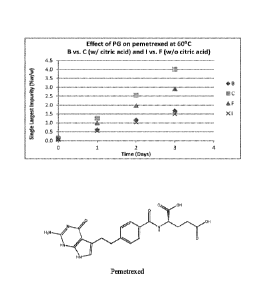
BRIEF DESCRIPTION OF THE FIGURES
FIGS 1A-1D: Depicts the effect of propylene glycol (PG) on the stability of
certain
pemetrexed formulations.
DETAILED DESCRIPTION
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as is commonly understood by one of ordinary skill in the art to which
this invention
belongs. In the event that there is a plurality of definitions for a term used
herein, those
definitions in this section prevail unless stated otherwise.
As used herein "single largest impurity" refers to the impurity with the
largest HPLC
peak by percentage.
As used herein "initial dosage concentration of pemetrexed" refers the
concentration of
pemetrexed at the time of dilution, prior to storage.
As used herein "initial pemetrexed concentration" refers to the concentration
of
pemetrexed at the time of formulation, prior to dilution and/or storage.
As used herein "room temperature" is about 20 C to about 25 C.
Pemetrexed or a pharmaceutically acceptable salt thereof is present in the
compositions
of the present invention at concentrations of between about 10 mg/mL to about
50 mg/mL when
calculated as anhydrous pemetrexed diacid. In certain embodiments of the
invention,
pemetrexed is present at about 10 mg/mL to about 40 mg/mL, at about 10 mg/mL
to about 30
mg/mL, at about 10 mg/mL to about 20 mg/mL, at about 20 mg/mL to about 50
mg/mL, at about
20 mg/mL to about 40 mg/mL, at about 20 mg/mL to about 30 mg/mL, at about 30
mg/mL to
about 50 mg/mL, at about 30 mg/mL to about 40 mg/mL, or at about 40 mg/mL to
about 50
mg/mL. In further embodiments of the invention, pemetrexed is available at
about 10 mg/mL,
about 15 mg/mL, 20 mg/mL, about 25 mg/mL, about 30 mg/mL, about 35 mWm.L,
about 40
mg/mL, about 45 mg/mL, or at about 50 mg/mL.
Pemetrexed is present in the composition as the diacid, monoacid, a
pharmaceutically
12
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
acceptable salt, or as combinations thereof. In certain embodiments of the
invention, pemetrexed
is present as pemetrexed disodium, in further embodiments of the invention,
pemetrexed is
present as pemetrexed dipotassium. In yet further embodiments of the
invention, pemetrexed is
present as pemetrexed meglumine. In still further embodiments of the
invention, pemetrexed is
present as pemetrexed tromethamine.
Non-Aqueous Solvents
Suitable non-aqueous solvents include, but are not limited to alcohols,
ketones, esters,
ethers, aromatic hydrocarbons, nitriles, aprotic polar solvents, acidic
solvents, and mixtures of
any two or more thereof. Useful alcohols include, for example, methanol,
ethanol, denatured
spirits, n-propanol, isopropanol, n-butanol, isobutanol, t-butanol,
polyhydroxy alcohols example
glycerin, propylene glycol, polyethylene glycol, diethylene glycol,
diglycerin, triethylene glycol,
tetraethylene glycol, trimethylolpropane and the like. Useful ketones include
propanone, 2-
butanone, and the like. Useful esters include, for example, ethyl acetate, n-
propyl acetate,
isopropyl acetate, n-butyl acetate, t-butyl acetate, and the like. Useful
ethers include, for
example, dimethyl ether, diethyl ether, methyl t-butyl ether, ethyl methyl
ether, diisopropyl ether,
and the like. Useful aromatic hydrocarbons include, for example, and the like.
Useful nitriles
include acetonitrile, propionitrile, and the like. Useful aprotic polar
solvents include N,N-
dimethylformide (DMF), dimethylsulfoxide (DMSO), N,N-dimethylacetamide (DMA),
and the
like.
In certain embodiments of the invention, the non-aqueous solvent is an
alcohol. In
further embodiments of the invention, the non-aqueous solvent is a polyhydroxy
alcohol. In still
further embodiments of the invention, the non-aqueous solvent is propylene
glycol. In yet further
embodiments of the invention, the non-aqueous solvent is polyethylene glycol.
In particular
embodiments of the invention, the non-aqueous solvent is low molecular weight
polyethylene
glycol. In other embodiments of the invention, the non-aqueous solvent is
selected from the
group consisting of polyethylene glycol 200, polyethylene glycol 300,
polyethylene glycol 400,
or combinations thereof. In certain embodiments of the invention, more than
one non-aqueous
solvent is present, such as, but not limited to polyethylene glycol and
propylene glycol.
13
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
In certain embodiments of the invention, the non-aqueous solvent is present at
a
concentration of about 50 mg/mL to 300 mg/mL prior to dilution. In further
embodiments of the
invention, the non-aqueous solvent is present at about 50 mg/mL to 100 mg/mL,
50 mg/mL to
200 mg/mL, 50 mg/mL to 250 mg/mL, 50 mg/mL to 300 mg/mL, 100 mg/mL to 200
mg/mL,
100 mg/mL to 250 mg/mL, 100 mg/mL to 300 mg/mL, 200 mg/mL to 250 mg/mL, 200
mg/mL
to 300 mg/mL, or 250 mg/mL to 300 mg/mL prior to dilution.
In particular embodiments, the non-aqueous solvent is present at a
concentration of no
more than 30 weight percent (wt%) of the formulation prior to dilution. In
further embodiments,
the non-aqueous solvent is present at a concentration of at least 5 weight
percent (wt.%) of the
formulation prior to dilution. In certain embodiments of the invention the non-
aqueous solvent is
present at 5-30 weight percent (wt%) of the formulation prior to dilution. In
particular
embodiments of the invention the non-aqueous solvent is present at about 10-30
wt.%, 15-30
wt.%, 20-30 wt.%, 25-30 wt.%, 5-28%, 10-28 wt.%, 15-28 wt.%, 20-28 wt%, 25-28
wt.%, 5-
25%, 10-25 wt.%, 15-25 wt.%, 20-25 wt.%, 25-28 wt.%, 5-20%, 10-20 wt.%, 15-20
wt.%, 5-
15%, 10-15 wt.%, or 5-10 wt% prior to dilution.
In particular embodiments, the non-aqueous solvent is present at a
concentration of no
more than 300 pL/mL prior to dilution. In further embodiments, the non-aqueous
solvent is
present at a concentration of at least 50 pL/mL prior to dilution. In certain
embodiments of the
invention, the non-aqueous solvent is present at a concentration of about 50
pL/mL to 300
LtL/mL. In further embodiments of the invention, the non-aqueous solvent is
present at about 50
pL/mL to 100 L/mL, 50 pL/mL to 200 pL/mL, 50 p.L/mL to 250 pL/mL, 50 pL/mL to
275
pL/mL, 50 pL/mL to 300 pL/mL, 100 pL/mL to 200 pL/mL, 100 pL/mL to 250 pL/mL,
100
pLitnL to 275 pL/mL, 100 L/mL to 300 pL/mL, 200 pL/mL to 250 ILL/mL, 200
pL/mL to 300
pL/mL, or 250 pL/mL to 300 pL/mL prior to dilution.
In certain embodiments, water is present at a concentration of at least about
500 L/mL
prior to dilution. In other embodiments of the invention, water is present at
a concentration of at
least about 600 pL/mL, 750 plJrnL, or 950 pL/mL prior to dilution. In further
embodiments of
the invention, water is present at a concentration of about 500 pL/mL to 950
pL/mL prior to
dilution. In still further embodiments of the invention, water is present at
about 500 pL/mL to
14
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
800 liL/mL, 500 pL/mL to 700 pL/mL, 500 pL/mL to 600 1.1L/mL, 600 pL/mL to 750
pL/mL,
600 pL/mL to 800 pL/mL, 600 pL/mL to 950 pL/mL, 700 pL/mL to 800 pL/mL, 700
pL/mL to
950 pL/mL, or 750 p.L/mL to 950 L/mL prior to dilution.
In particular embodiments, water is present at a concentration of at least
about 50 wt% of
the formulation prior to dilution. In further embodiments, water is present at
a concentration of at
least about 60 wt.%, 75 w wo, or 95 wt.% prior to dilution. In certain
embodiments of the
invention water is present at a concentration of at least about 50-95 wt.%
prior to dilution, of the
invention water is present at a concentration of at least about 50-60 wt.%, 50-
70 wt.%, 50-80
wt.%, 60-70 wt.%, 60-80 wt.%, 60-95 wt.%, 70-80 wt.%, or 70-95 wt.% prior to
dilution
In certain embodiments of the invention the formulation is substantially free
of anti-
oxidants and/or amino acids. In particular embodiments of the invention, the
formulation is
substantially free of anti-oxidants. In further embodiments of the invention,
the formulation is
substantially free of chelating agents. As used herein, substantially free of
anti-oxidants, amino
acids, and/or chelating agents means the formulation does not comprise one or
more anti-
oxidants, amino acids, and/or chelating agents, at a concentration sufficient
to have a stabilizing
effect.
In particular embodiments, the formulation is substantially free of additives
selected from
the group consisting of ascorbic acid and derivatives, tocopherols and
derivatives, propyl gallate,
thioglycerol, lactobionic acid, methionine, tertiary butylhydroquinone (TBHQ),
butylated
hydroxyanisole (BHA), butylated hydroxytoluene (BHT), sodium formaldehyde
sulfoxylate,
sodium hydrogen sulfite, Eethyl en ed iami netetraacetic acid (EDTA) and
derivatives,
monoethanolamine gentisate, glutathione, propionic acid, acetone sodium
bisulfite, sodium
dithionite, citric acid and derivatives, tribasic (txi sodium citrate
dihydrate), or suitable mixtures
thereof. As used herein, substantially free means the formulation does not
comprise one or more
additives listed above at a concentration sufficient to have a stabilizing
effect.
In particular embodiments of the invention, the formulation comprises
tromethamine. In
certain embodiments of the invention, the formulation comprises about 12 to 24
mg/mL
tromethamine. In further embodiments of the invention, the formulation
comprises about 12 to
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
14 mg/mL tromethamine, about 12 to 16 mg/mL tromethamine, about 14 to 16 mg/mL
tromethamine, about 14 to 18 mg/mL tromethamine, about 16 to 18 mg/mL
tromethamine, about
16 to 20 mg/mL tromethamine, about 17 to 19 mg/mL tromethamine, about 17 to 21
mg/mL
tromethamine, about 18 to 20 mg/mL tromethamine, about 18 to 22 mg/mL
tromethamine,
about 20 to 22 mg/mL tromethamine, about 20 to 24 mg/mL tromethamine, or about
22 to 24
mg/mL tromethamine. In yet further embodiments of the invention, the
formulation comprises
about 18 mg/mL tromethamine.
In certain embodiments of the invention, the formulation is not substantially
degraded
after storage at room temperature for at least about 6 months. In particular
embodiments of the
invention, the formulation is not substantially degraded after storage at room
temperature for at
least about 1 year. In further embodiments of the invention, the formulation
is not substantially
degraded after storage at room temperature for at least about 18 months. In
still further
embodiments of the invention, the formulation is not substantially degraded
after storage at room
temperature for at least about 2 years.
In further embodiments of the invention, the formulation is not substantially
degraded
after storage at 2-8 C for at least about 6 months. In certain embodiments of
the invention, the
formulation is not substantially degraded after storage at 2-8 C for at least
about 1 year. In
further embodiments of the invention, the formulation is not substantially
degraded after storage
at 2-8 C for at least about 18 months. In still further embodiments of the
invention, the
formulation is not substantially degraded after storage at 2-8 C for at least
about 2 years.
In other embodiments of the invention, the formulation comprises no more than
about
10% w/w impurities, no more than about 8% w/w impurities, no more than about
6% w/w
impurities, no more than about 5% w/w impurities, no more than about 4% w/w
impurities, no
more than about 3.5% w/w impurities, no more than about 3% w/w impurities, no
more than
about 2.5% w/w impurities, no more than about 2% w/w impurities, no more than
about 1.5%
w/w impurities, no more than about 1% w/w impurities, no more than about 0.5%
w/w
impurities, no more than about 0.2% w/w impurities, no more than about 0.1%
w/w impurities,
after storage at room temperature for at least about 6 months, about 12
months, about 18,
months, or about 24 months.
16
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
In further embodiments of the invention, the formulation comprises no more
than about
10% w/w impurities, no more than about 8% w/w impurities, no more than about
6% w/w
impurities, no more than about 5% w/w impurities, no more than about 4% w/w
impurities, no
more than about 3.5% w/w impurities, no more than about 3% w/w impurities, no
more than
about 2.5% w/w impurities, no more than about 2% w/w impurities, no more than
about 1.5%
w/w impurities, no more than about 1% w/w impurities, no more than about 0.5%
w/w
impurities, no more than about 0.2% w/w impurities, no more than about 0.1%
w/w impurities,
after storage at 2-8 C for at least about 6 months, about 12 months, about 18,
months, or about
24 months.
In still further embodiments of the invention, the formulation comprises no
more than
about 10% w/w of the single largest impurity, no more than about 8% w/w of the
single largest
impurity, no more than about 6% w/w of the single largest impurity, no more
than about 5% w/w
of the single largest impurity, no more than about 4% w/w of the single
largest impurity, no more
than about 3.5% w/w of the single largest impurity, no more than about 3% w/w
of the single
largest impurity, no more than about 2.5% w/w of the single largest impurity,
no more than about
2% w/w of the single largest impurity, no more than about 1.5% w/w of the
single largest
impurity, no more than about 1% w/w of the single largest impurity, no more
than about 0.5%
w/w of the single largest impurity, no more than about 0.2% w/w of the single
largest impurity,
no more than about 0.1% w/w of the single largest impurity, after storage at
room temperature
for at least about 6 months, about 12 months, about 18, months, or about 24
months.
In yet further embodiments of the invention, the formulation comprises no more
than
about 10% w/w of the single largest impurity, no more than about 8% w/w of the
single largest
impurity, no more than about 6% w/w of the single largest impurity, no more
than about 5% w/w
of the single largest impurity, no more than about 4% w/w of the single
largest impurity, no more
than about 3.5% w/w of the single largest impurity, no more than about 3% w/w
of the single
largest impurity, no more than about 2.5% w/w of the single largest impurity,
no more than about
2% w/w of the single largest impurity, no more than about 1.5% w/w of the
single largest
impurity, no more than about 1% w/w of the single largest impurity, no more
than about 0.5%
w/w of the single largest impurity, no more than about 0.2% w/w of the single
largest impurity,
no more than about 0.1% w/w of the single largest impurity, after storage at 2-
8 C for at least
17
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
about 6 months, about 12 months, about 18, months, or about 24 months.
In particular embodiments of the invention, the formulation retains at least
about 85% of
its initial pemetrexed concentration, at least about 90% of its initial
pemetrexed concentration,
the formulation retains at least about 92% of its initial pemetrexed
concentration, at least about
95% of its initial pemetrexed concentration, the formulation retains at least
about 97% of its
initial pemetrexed concentration, at least about 98% of its initial pemetrexed
concentration, at
least about 99% of its initial pemetrexed concentration, at least about 99.5%
of its initial
pemetrexed concentration, after storage at room temperature for at least about
6 months, about
12 months, about 18, months, or about 24 months.
In further embodiments of the invention, the formulation retains at least
about 85% of its
initial pemetrexed concentration, at least about 90% of its initial pemetrexed
concentration, the
formulation retains at least about 92% of its initial pemetrexed
concentration, at least about
95% of its initial pemetrexed concentration, the formulation retains at least
about 97% of its
initial pemetrexed concentration, at least about 98% of its initial pemetrexed
concentration, at
least about 99% of its initial pemetrexed concentration, at least about 99.5%
of its initial
pemetrexed concentration, after storage at 2-8 C for at least about 6 months,
about 12 months,
about 18, months, or about 24 months.
In certain embodiments of the invention, the formulation is further diluted in
a
pharmaceutically acceptable diluent Suitable diluents include, but are not
limited to saline,
dextrose, water, Ringer's Injection, and Lactated Ringer's Injection. In
certain embodiments of
the invention, the formulation can be diluted in a calcium containing diluent,
such as Ringer's
Injection or Lactated Ringer's Injection.
In particular embodiments of the invention, the formulation is diluted in a
pharmaceutically acceptable diluent to a suitable initial dosage concentration
of pemetrexed. In
certain embodiments of the invention the initial dosage concentration of
pemetrexed is based on
various factors, such as, but not limited to the patient's weight, age, and
condition as well as the
volume of diluent and can be determined by a practitioner or one of skill in
the art. In further
embodiments, pemetrexed is diluted to an initial dosage concentration and can
be further diluted
18
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
prior to administration.
In other embodiments of the invention, the formulation retains at least about
85% of its
initial dosage concentration of pemetrexed, at least about 90% of its initial
dosage concentration
of pemetrexed, at least about 92% of its initial dosage concentration of
pemetrexed, at least about
95% of its initial dosage concentration of pemetrexed, at least about 97% of
its initial dosage
concentration of pemetrexed, at least about 98% of its initial dosage
concentration of
pemetrexed, at least about 99% of its initial dosage concentration of
pemetrexed, at least about
99.5% of its initial dosage concentration of pemetrexed, after dilution and
storage at about 2 C to
about 8 C for at least about 12 hours.
In still other embodiments of the invention, the formulation retains at least
about 85% of
its initial dosage concentration of pemetrexed, at least about 90% of its
initial dosage
concentration of pemetrexed, at least about 92% of its initial dosage
concentration of
pemetrexed, at least about 95% of its initial dosage concentration of
pemetrexed, at least about
97% of its initial dosage concentration of pemetrexed, at least about 98% of
its initial dosage
concentration of pemetrexed, at least about 99% of its initial dosage
concentration of
pemetrexed, at least about 99.5% of its initial dosage concentration of
pemetrexed, after dilution
and storage at about 2 C to about 8 C for at least about24 hours.
In yet other embodiments of the invention, the formulation retains at least
about 85% of
its initial dosage concentration of pemetrexed, at least about 90% of its
initial dosage
concentration of pemetrexed, at least about 92% of its initial dosage
concentration of
pemetrexed, at least about 95% of its initial dosage concentration of
pemetrexed, at least about
97% of its initial dosage concentration of pemetrexed, at least about 98% of
its initial dosage
concentration of pemetrexed, at least about 99% of its initial dosage
concentration of
pemetrexed, at least about 99.5% of its initial dosage concentration of
pemetrexed, after dilution
and storage at about 2 C to about 8 C for at least about 48 hours.
In further embodiments of the invention, the formulation comprises no more
than about
10% w/w impurities, no more than about 8% w/w impurities, no more than about
6% w/w
impurities, no more than about 5% w/w impurities, no more than about 4% w/w
impurities, no
19
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
more than about 3.5% w/w impurities, no more than about 3% w/w impurities, no
more than
about 2.5% w/w impurities, no more than about 2% w/w impurities, no more than
about 1.5%
w/w impurities, no more than about 1% w/w impurities, no more than about 0.5%
w/w
impurities, no more than about 0.2% w/w impurities, no more than about 0.1%
w/w impurities,
after dilution and storage at about 2 C to about 8 C for at least about 12
hours.
In other embodiments of the invention, the formulation comprises no more than
about
10% w/w impurities, no more than about 8% w/w impurities, no more than about
6% why
impurities, no more than about 5% w/w impurities, no more than about 4% w/w
impurities, no
more than about 3.5% w/w impurities, no more than about 3% w/w impurities, no
more than
about 2.5% w/w impurities, no more than about 2% w/w impurities, no more than
about 1.5%
w/w impurities, no more than about 1% w/w impurities, no more than about 0.5%
w/w
impurities, no more than about 0.2% w/w impurities, no more than about 0.1%
w/w impurities,
after dilution and storage at about 2 C to about 8 C for at least about 24
hours.
In yet other embodiments of the invention, the formulation comprises no more
than about
10% w/w impurities, no more than about 8% w/w impurities, no more than about
6% w/w
impurities, no more than about 5% w/w impurities, no more than about 4% w/w
impurities, no
more than about 3.5% w/w impurities, no more than about 3% w/w impurities, no
more than
about 2.5% w/w impurities, no more than about 2% w/w impurities, no more than
about 1.5%
w/w impurities, no more than about 1% w/w impurities, no more than about 0.5%
w/w
impurities, no more than about 0.2% w/w impurities, no more than about 0.1%
w/w impurities,
after dilution and storage at about 2 C to about 8 C for at least about 48
hours.
In still other embodiments of the invention, the formulation comprises no more
than
about 10% w/w of the single largest impurity, no more than about 8% w/w of the
single largest
impurity, no more than about 6% w/w of the single largest impurity, no more
than about 5% w/w
of the single largest impurity, no more than about 4% w/w of the single
largest impurity, no more
than about 3.5% w/w of the single largest impurity, no more than about 3% w/w
of the single
largest impurity, no more than about 2.5% w/w of the single largest impurity,
no more than about
2% w/w of the single largest impurity, no more than about 1.5% w/w of the
single largest
impurity, no more than about 1% w/w of the single largest impurity, no more
than about 0.5%
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
w/w of the single largest impurity, no more than about 0.2% w/w of the single
largest impurity,
no more than about 0.1% w/w of the single largest impurity, after dilution and
storage at about
2 C to about 8 C for at least about 12 hours.
In further embodiments of the invention, the formulation comprises no more
than about
10% w/w of the single largest impurity, no more than about 8% w/w of the
single largest
impurity, no more than about 6% w/w of the single largest impurity, no more
than about 5% w/w
of the single largest impurity, no more than about 4% w/w of the single
largest impurity, no more
than about 3.5% w/w of the single largest impurity, no more than about 3% w/w
of the single
largest impurity, no more than about 2.5% w/w of the single largest impurity,
no more than about
2% w/w of the single largest impurity, no more than about 1.5% w/w of the
single largest
impurity, no more than about 1% w/w of the single largest impurity, no more
than about 0.5%
w/w of the single largest impurity, no more than about 0.2% w/w of the single
largest impurity,
no more than about 0.1% w/w of the single largest impurity, after dilution and
storage at about
2 C to about 8 C for at least about 24 hours.
In still further embodiments of the invention, the formulation comprises no
more than
about 10% w/w of the single largest impurity, no more than about 8% w/w of the
single largest
impurity, no more than about 6% w/w of the single largest impurity, no more
than about 5% w/w
of the single largest impurity, no more than about 4% w/w of the single
largest impurity, no more
than about 3.5% w/w of the single largest impurity, no more than about 3% w/w
of the single
largest impurity, no more than about 2.5% w/w of the single largest impurity,
no more than about
2% w/w of the single largest impurity, no more than about 1.5% w/w of the
single largest
impurity, no more than about 1% w/w of the single largest impurity, no more
than about 0.5%
w/w of the single largest impurity, no more than about 0.2% w/w of the single
largest impurity,
no more than about 0.1% w/w of the single largest impurity, after dilution and
storage at about
2 C to about 8 C for at least about 48 hours.
In other embodiments of the invention, the formulation retains at least about
85% of its
initial dosage concentration of pemetrexed, at least about 90% of its initial
dosage concentration
of pemetrexed, at least about 92% of its initial dosage concentration of
pemetrexed, at least
about 95% of its dosage concentration of pemetrexed, at least about 97% of its
dosage
21
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
concentration of pemetrexed, at least about 98% of its dosage concentration of
pemetrexed, at
least about 99% of its dosage concentration of pemetrexed, at least about
99.5% of its dosage
concentration of pemetrexed, after dilution and storage at room temperature
for at least about 12
hours.
In still other embodiments of the invention, the formulation retains at least
about 85% of
its initial dosage concentration of pemetrexed, at least about 90% of its
initial dosage
concentration of pemetrexed, at least about 92% of its initial dosage
concentration of
pemetrexed, at least about 95% of its dosage concentration of pemetrexed, at
least about 97% of
its dosage concentration of pemetrexed, at least about 98% of its dosage
concentration of
pemetrexed, at least about 99% of its dosage concentration of pemetrexed, at
least about 99.5%
of its dosage concentration of pemetrexed, after dilution and storage at room
temperature for at
least about 24 hours.
In yet other embodiments of the invention, the formulation retains at least
about 85% of
its initial dosage concentration of pemetrexed, at least about 90% of its
initial dosage
concentration of pemetrexed, at least about 92% of its initial dosage
concentration of
pemetrexed, at least about 95% of its initial pemetrexed concentration, the
formulation retains
at least about 97% of its initial pemetrexed concentration, at least about 98%
of its initial
pemetrexed concentration, at least about 99% of its initial pemetrexed
concentration, at least
about 99.5% of its initial pemetrexed concentration, after dilution and
storage at about room
temperature for at least about 48 hours.
In further embodiments of the invention, the formulation comprises no more
than about
10% w/w impurities, no more than about 8% w/w impurities, no more than about
6% w/w
impurities, no more than about 5% w/w impurities, no more than about 4% w/w
impurities, no
more than about 3.5% w/w impurities, no more than about 3% w/w impurities, no
more than
about 2.5% w/w impurities, no more than about 2% w/w impurities, no more than
about 1.5%
w/w impurities, no more than about 1% w/w impurities, no more than about 0.5%
w/w
impurities, no more than about 0.2% w/w impurities, no more than about 0.1%
w/w impurities,
after dilution and storage at room temperature for at least about 12 hours.
22
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
In other embodiments of the invention, the formulation comprises no more than
about
10% w/w impurities, no more than about 8% w/w impurities, no more than about
6% w/w
impurities, no more than about 5% w/w impurities, no more than about 4% w/w
impurities, no
more than about 3.5% w/w impurities, no more than about 3% w/w impurities, no
more than
about 2.5% w/w impurities, no more than about 2% w/w impurities, no more than
about 1.5%
w/w impurities, no more than about 1% w/w impurities, no more than about 0.5%
w/w
impurities, no more than about 0.2% w/w impurities, no more than about 0.1%
w/w impurities,
after dilution and storage at room temperature for at least about 24 hours.
In yet other embodiments of the invention, the formulation comprises no more
than about
10% w/w impurities, no more than about 8% w/w impurities, no more than about
6% w/w
impurities, no more than about 5% w/w impurities, no more than about 4% w/w
impurities, no
more than about 3.5% w/w impurities, no more than about 3% w/w impurities, no
more than
about 2.5% w/w impurities, no more than about 2% w/w impurities, no more than
about 1.5%
w/w impurities, no more than about 1% w/w impurities, no more than about 0.5%
w/w
impurities, no more than about 0.2% w/w impurities, no more than about 0.1%
w/w impurities,
after dilution and storage at room temperature for at least about 48 hours.
In further embodiments of the invention, the formulation comprises no more
than about
10% w/w of the single largest impurity, no more than about 8% w/w of the
single largest
impurity, no more than about 6% w/w of the single largest impurity, no more
than about 5% w/w
of the single largest impurity, no more than about 4% w/w of the single
largest impurity, no more
than about 3.5% w/w of the single largest impurity, no more than about 3% w/w
of the single
largest impurity, no more than about 2.5% w/w of the single largest impurity,
no more than about
2% w/w of the single largest impurity, no more than about 1.5% w/w of the
single largest
impurity, no more than about 1% w/w of the single largest impurity, no more
than about 0.5%
w/w of the single largest impurity, no more than about 0.2% w/w of the single
largest impurity,
no more than about 0.1% w/w of the single largest impurity, after dilution and
storage at room
temperature for at least about 12 hours.
In yet further embodiments of the invention, the formulation comprises no more
than
about 10% w/w of the single largest impurity, no more than about 8% w/w of the
single largest
23
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
impurity, no more than about 6% w/w of the single largest impurity, no more
than about 5% w/w
of the single largest impurity, no more than about 4% w/w of the single
largest impurity, no more
than about 3.5% w/w of the single largest impurity, no more than about 3% w/w
of the single
largest impurity, no more than about 2.5% w/w of the single largest impurity,
no more than about
2% w/w of the single largest impurity, no more than about 1.5% w/w of the
single largest
impurity, no more than about 1% w/w of the single largest impurity, no more
than about 0.5%
w/w of the single largest impurity, no more than about 0.2% w/w of the single
largest impurity,
no more than about 0.1% w/w of the single largest impurity, after dilution and
storage at room
temperature for at least about 24 hours.
In still further embodiments of the invention, the formulation comprises no
more than
about 10% w/w of the single largest impurity, no more than about 8% w/w of the
single largest
impurity, no more than about 6% w/w of the single largest impurity, no more
than about 5% w/w
of the single largest impurity, no more than about 4% w/w of the single
largest impurity, no more
than about 3.5% w/w of the single largest impurity, no more than about 3% w/w
of the single
largest impurity, no more than about 2.5% w/w of the single largest impurity,
no more than about
2% w/w of the single largest impurity, no more than about 1.5% w/w of the
single largest
impurity, no more than about 1% w/w of the single largest impurity, no more
than about 0.5%
w/w of the single largest impurity, no more than about 0.2% w/w of the single
largest impurity,
no more than about 0.1% w/w of the single largest impurity, after dilution and
storage at a room
temperature for at least about 48 hours.
In particular embodiments of the invention the formulation is in a vial with a
headspace
oxygen concentration of less than about 20 v/v%, 18 v/v%, 16 v/v%, 14 v/v%, 12
v/v%, 10
v/v%, 8 v/v%, 6 v/v%, 5 v/v%, 4 v/v%, 3 v/v%, 2 v/v%, or 1 v/v% oxygen.
In certain embodiments of the invention, the formulation is in a single-dose
vial. In
further embodiments of the invention, the formulation is in a multi-dose vial.
In yet further
embodiments of the invention, the formulation is in a multi-dose vial intended
for use by the
same patient In still further embodiments of the invention, the formulation is
in a multi-dose
vial intended for use by different patients.
24
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
In another embodiment, the invention relates to a method of administering
pemetrexed to
a patient in need thereof comprising administering an effective amount of a
formulation as
described herein. In a further embodiment, the invention relates to a method
of administering
pemetrexed to a patient in need thereof comprising administering an effective
amount of a
formulation comprising pemetrexed and a non-aqueous solvent present at less
than 30 wt%. In a
further embodiment, the invention relates to a method of administering
pemetrexed to a patient
in need thereof comprising administering an effective amount of a formulation
comprising
pemetrexed and propylene glycol wherein the propylene glycol is present at 5-
30 wt%.
EXAMPLES
Example I: Elfrct of Non-Aqueous Solvents on Pemetrexed Stability
Formulations as described in Table 1 were prepared as follows:
Water for Injection, a suitable base solution (0.1 ¨ 1 N NaOH or KOH), and
citric acid, if
present, were combined and mixed to yield a visually uniform mixture.
Pemetrexed diacid was
incrementally added to the mixture under continuous agitation, and the
resulting homogenous
suspension was agitated until all solids were completely dissolved. The pH of
the solution was
adjusted to about 7.4-7.6. Propylene glycol, if present, was then added to the
pemetrexed
solution, and the mixture was agitated until a visually uniform mixture was
obtained. The pH of
the solution was adjusted to 7.4-7.6.
.. ,
.:;,0Rimimi: 25 2 25 25
Citric kcid 13.2 13.2
250 250
aOH qs to qs to qs to __ qs to
pH 7.5 pH 7.5 pH 7.5 pH 7.5
E**4441:0*.W4i0g qs to qs to qs to qs to
pH. 7.5 PH 7.5 PH 7.5 PH 7.5
WFJ qs qs qs qs
The stability of Formulations B, C, F, and I was tested under accelerated
conditions at
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
60 C and 40 C/75% RH over the course of several days. Samples were taken at
various time
points and diluted with HPLC diluents (60:40 (v/v, Water:ACN)) prior to
testing. Impurities
were measured by HPLC.
The results are shown in Tables 2A-2D and Figures 1A-1D.
Table 2A: Single Largest Impurity (% w/w) after storage at 60 C
B C F 1 ,
Initial 0.09 0.15 0.10 0.07
1 day 0.61 1.26 1.00 0.56 ,
2 day 1.16 2.56 1.97 I 04
3 day 1.68 4.01 2.91 I.:50
Table 2B: Total Impurities (% w/w) after storage at 60 C
Total
B C F I
Impurity
Initial 0 69 0.92 0.74 0.70
1 day 1.50 2.35 2.02 1.39
2 day 2.20 3.93 3.31 2.04
3 day 2.86 5.63 4.54 2.65
Table 2C: Single Largest impurity (% w/w) after storage at 40 C/75% RH
R11.T 0.87 B C F I
Initial 0.09 , 0.15 0.10 0.07
_
2 days 1.70 6.36 _ 3.36 1.33 ,
4 days , 3.29 13.44 9.24 2.35
Table 2D: Total Impurities after (% w/w) storage at 40 C/75% RH
Total
B C F I
Impurity
Initial 0.69 0.92 0.74 0.70
2 days 3.63 11.75 7.28 2.75
4 days 6.05 20.94 16.71 4.31
The long term stability, real-time, and projected, of formulations B, I, and K
(See Table
4, prepared as described above) were also measured. Results are shown in Table
3
26
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
Table 3: Long Term Stability of Pemetrexed Formulations B, I, and K
Storage Single Projected Projected
at 2-8C Largest Total Impurities
space
TI in SLI in 24 Assay
Head
(Month Impurity (% who 24 M M (% (%LC)
s) (% w/w) (% w/w) w/w)
oxygen
Formulation 13 15 0.57 4.70 7.52 0.91 93.6 7.59
18.1
Formulation 1 15 0.31 1.70 2.72 0.50 97.8 7.50
20.9
Formulation K 14 0.42 2.96 5.07 0.72 95.2 7.47
19.6
Table 4: Pemetrexed Formulation K
Pemetrexed diacid 25.0 mg
Propylene Glycol 250 !IL
Tromethamine qs to pH 7.4-7.6
Hydrochloric Acid qs to pH 7.4-7.6
Water for Injection qs to 1 mL
Example 2: Stability of Pemetrexed Formulation K after dilution.
The stability of pemetrexed for up to 48 hours after dilution of Formulation K
was
evaluated. . Normal saline, water for injection and 5% dextrose in water,
Ringer's Injection, and
Lactated Ringer's Injection were purchased directly and used as is. The pH of
each diluent was
tested and recorded in Table 6.
Table 6: pH of diluents
Normal Saline (NS) 6.8
Water for Injection (WFI) 7.01
Ringer's Injection (LR) 6.6
Lactated Ringer's Injection (LAS) 6.5
5% Dextrose in Water (D5W) 7.5
27
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
Formulation K (25 mg/mL) was diluted to 0.15, 1.5, and 15 mg/mL in the
diluents listed
above. The mixtures were stored at 2-8 C and tested at 12, 24, and 48 hours
for appearance,
assay, impurities, and pH. A sample was taken immediately after dilution for
the time zero
sample. The 0.15 mg/mL samples were analyzed as is without further dilution,
while the 1.5
mg/mL and 15 mg/mL samples were diluted with HiPLC diluents (60:40 (v/v,
Water:ACN)) prior
to testing. Results are in Tables 6 & 7 (each Table represents separate
studies).
Table 6: Stability of Formulation K after dilution in Normal Saline or Water
for Injection
Theoretical Assay
*Percentage of Total
Diluents Time (hrs) Pemetrexed conc Theoretical
Impurity
(mg/mL) ( mg/g)
Pemetrexed % (%
w/w)
Bulk
25.11 24.80 99.2 7.50
0.16
Solution
7
0.10
.ii 15
: __
.
0i.... ,õ
NS () 14.55 98.84 7.47
0.08
24 14.61 99.25 7.54
0.19
48 14.71 99.93 7.58 O.
r
177.77.7'777.777=7777.=Ti777==:7.7777..777;777.13)0 007===7777.77.7:77=7-
77t,T777.7=7-7'=
0 I 1) :1310.20 7 3n
014
: .24 I n:747 017
====
VVFI 0 14.60 99.18 7.521
11.14
24 14.40 97.82 7.58
0.16
48 14.70 99.86 7.59
0.11
Table 7: Stability of Formulation K after dilution in Ringer's Injection,
Lactated Ringer's
Injection, or 5% Dextrose
Theoretical *Percentage of
Time Assay (
Total
Diluents Pemetrexed conc Theoretical pH
(hrs) LC) Impurity
(mg/mL) Pemetrexed %
28
CA 03014755 2018-08-15
WO 2017/142556 PCT/US2016/018703
Bulk - 25.0 97.6 NA 7.37 0.35
.
.................,.....õ.õõõõõõõ
solution
......õ.õ,õõõõ..õ:õõõ,õõõ:õõõõ......õ,,,,,,,:,:::.....:*okIN...m=H i',Hi=:].:-
.:-,Ø.1.:,.=g].:-.:R::0::::I !,...:1===Mti.J.1.':::IE
t..M'ilY::.;.44:::M:::,',i
1 ---',""",'""""'",::
:,,],=,:::],,::::9,3,tz:..]]:]::;: ]]::.:i]=:]=-,i=-,51=8i]la=R::m: .::-..A
S)4
Z:M:::
'..-...ai'i...i.i.i..:0.-,:]]ii:i:..:
2. 9= ..':i.i.''...'..:ii:'111:i.:il'ii.
.l....':.]',J9:'.....i..1i.i.1..:T.',J!i!'..F: E'-
,11:::.0T,',$U)i'!')i'!.:.?4..iii..'iiiiiiiii-.'iiilig
".' -- = -"" = --- = :<-: -- ' -- -- --*:,:"::: v.,:imi,,,
i..,:,.,,..ii.]ii.::::',i.ii.ii.]-;Ii.lim];]-;ii.]:=:,:,
,::.=:':Ø11.::.,::i..-::.,...,.,,,...,...,n,...A4c..-.0,..,:i.i,:i
.6gIi',1:;:g:3.::,:;:';..;::;Z:Lg Ø5'.4,1.4:HH IHR'-.HE.-.93:..:.2.':E.-
.1-.Hg-.H 7.45 0.31
- - - - - - -
==== ===== === 'v.'''. = -=-=""L'=""': ""='='="""L'=""'='====9'
".=='''''"''''''='=''='='='. ='=' '7:==:=.'..5THA.He::Rs=0:=34.
-...R..b,tv:: ::::-.;-.2'.:,:::;,;.--...-
:.::::.:.,:::::-.4 -.:::::.::.,Øi.-...90.2.:=...,....,...]. ]]...]]:].
.H.,.......: .. ....,..:-:]*-*.- . ':-:.::, =:..,:... : ...:., ,:... :
.. ... .. ...i.
RS 1:
24-':,:g ''.::..-,:':':':'';'':g --:1.-
.::::....i.':,':::..95:,.1....--MS: :1:-=1E1H--
T.97:Wi',....i.ia;:...aii.i.,. =.,i'......=,=,.='::::::.7.'44...:-
....i.ff...=.. =-g....:...::::::,.....iØ...',10....i.a:.,.....
- ......= --- =-= '''..----
===========-========""'"""""""""""""""""?.i"===::':==::: -
:=:"=,?'...'...:::'='"...".=AP=>.:.n'i=======
:i]:::ig::i;',...=:=.::=01.:=====:::.....,....:::.=-=N======:::'.:. :=-
:::.....:J.:.:=:,.:=-=ft5.3:::::.i=iti::::.:=.:. :.':====.:=.E-:=.:.==:',A3C:-
.:=.':==b:-.:-.:=.':
.::::::=-.:=.i'..i'..4=0':::::.:=.:-.:=.:g:'::-.:=.',',:::::=.':===g:-.:=.:-
.:=.0:-.:.:::',.]:=.a:::=,:=.]:=....M:=...::.i.,:===:::=w::i] =-
===========:::?,?:'=====:.'==)-- ':*==='======'='''======':' '''"''''""'=-
=="''"-=':="''''===='='=:='-'='='=:===='=-=: ============== == ==== =
- - = =
0 97 c
.8 100.2 7.47 J.3 -
,s
12 98.3 100.7 7.55 0.35
._ 15
24 97.4 99.7 7.39 0.36
48 95.6 98.0 7.53 0.33
...............- ' .. - . ' ' ' ' " " ' """ " " ' " ''''......'''''''''
'='='"'='='''''94'....i.=='='.====='..=
'===:=='.==',==='...?]=',='..4:4)0S1':;'.';.',......'...i..i...'....i=.1=.!...,
.',.='=:':!...'..1=.']=,.':1=:17..5if....i.,'=::'=::';',...i.:i
=:..',Iti.:i.f.',Iil...i.:ii047:,.',1:ii...i:'iY:1:ii...-i:i
1-17.77117.73..?::=:: 1:1:::::',..1..i....i...1..i..,..:::ti..1..t
i.,..1..;t.i...;:;::::::i..........',:i......
i;];iii.....',:i.....:1,1;i:i.9ir9Mlig:iiiii',.'...Mligt91.!..41.':ii=Oiiii:::'
,W::Inii.,:i!T.41:::V.=: ]=:.=:iii].,..,.:=>=:-=::.i..=,i,i...,....ii
24.1iliiiiiiiiiii',:iiiiIiillilililiiiiiiiiii:liiiiiiii:':.!iiiilli-f-
;illiklilililili;',Iililigliilili 9K 009 ',.:.:R.,...,:.:;-
.?!,,:::::',,::.;!,,,::,:::,=!..:,<T,,,,:::!1::
......"--. "..õ::::, ,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,
:..]]]-:::..g:::4;...g..m7::::.:. ::::.:.:.:::B,:=::104:2--
:.::,:.t::,:,.i:, i::,:,4::..61..f,.:::::',]]:: N,::]]]],:::-9,.-
..1,....;.]:].:.:::
4.ff",iii.:';',:ii,...,,.:i.::.::,:i,..,:i.::.::iiii...,...f::ii,.i,.i..i.::.::
,:i,..,:i:ii:ii.i,...,....,..,.:,..:,...,.*:,.i,.iy.,.,M.:.::,:,:.-,-,.:....,-
,- . -,.....,,-,-..... --- .. .....,:,: ,õ,,,.,.:,,,,,,
.,.,,,,,,,,,,,....:.,.-.1,.,.,,,:,,,,,.,,
98.4. .:.-..:,: ]]:.-,]:.-..:,:j]]9..).E....-....:.....:....,]..,,-...,,.,:..
,.,......',',7,:.-..5...i.,...:... g4,...::...,:.
.........1..12:::E:::::: ':'...1.,';;I:*':=,':%:''n''1'::'::'''''' 'O''''''
98 ..,.1 .. 39.04-.:T.......liiii......iiii$:!1......iiii
iii......i......iiii,Iiii7ARiii......iiii,Iiii...... l':..-
1.,.:i......i,..i....iiii......9.:;1:::::...E:::
LRS :,!:::::-..-=:-;;-:::::::::::,-.....' i.,:.,.........@.!.:-.R:-
.,.0,i!.MH,,R,,,,,0 ,. , , 4,17,4 .;,--....::;:;',.','-
,1.,.i...4:ii.:iii'fiji....ig:::.4 .*:;!'.:;',4,..if:..'-4 7.33 0.34
'......-.....'''''''''........- ' 1 - .:i::-
..f.i.'.:]*.i.-i.-: . 11'..n.:.?...6.':i...ini...i...i.,: 7.58 0.35
:...M::::.:=......4.&::::in::::.::
.,:=::....;....i.,::::...:=...,i::::::..:=:...t...=:...i,:=:::::=....i,fi,::::-
...:=:....,=.],:,:i;]HM ,..: . 9:?.;...,,,
..:,i:...,..,,,,...,..,..1,,,,F..,..,..:::::::::::::::::::::::.: ,. . ,-
. ............. i... _,, ..... ..
0 97.8 100.2 7.41 J.3
12 98.3 100.7 7.58 0.32
15
_____
24 98.2 100.6 7.36 0.34
48 98.7 101.1 7.46 0.33
........-
....õ:õ..õõõ.....õõ,.õõõ:,õõ,..:::::õõ,..,i,i..õ.õ....õ,i,.õ.õ...,..,i..õ.õ...,
.....,,,,,..,,,õõ.õ..:õ.*.õ..,,,,,:,,,.õ4,nc.,,,,::,,.T,:::
.,,,..,:.õ:.::,,,:,,,::õ:::,,,:,,,i,::.100.z..m...m:::,,. .;::,,.gm
:,5.0:..,....,...,;,.:::= i,:i.,......::,.1:,-
.,,:.,,,:....:0:,.:.,..4Ø.:,,,,,,,,,,,,,,....7.1iiii
t.!iiiiiiiii:'..,',1',1111411111.E.:111=1.:1_':',..n9Iiq:::i'ii'::::V2:::',A,gi
;i':::$1P4gliii!!:.1:.1g.,'!':','',' 7:7 0.46
-,:''','"::=:::"""'""#-"t''-
'"'"""""'"""="""':,:i',.,*:*:..i*t*"'i:.:':',',""'," ''='''''1==-
kiW'fm".'=""'='='"''' ''.=''''''.7=1'==4=-'"=-':'
.=:'''.'=.::='""0.4'S.==';'n'''::="'
'''''''.'"1!1'=Iiii=:=1'1:=1"14112=ERIMEEML'i'i''''.'iiifiliN$i44.giiiiiiiikH
.'::H.',.iliiiiiiiiliii.,:iiiittltiiiiniig iiiii,:,,...;.,,i7$.7aio
,..:g:,:i.,,..,,.,:.i.,9.5...-..:',i.,i.::::.:.:,i,
. ' H'.':'
.":::97.....JW :H.,.......=-..:l:H:='=.?9%=41=r=RIM:E:
:H:1]:.'7J,:3=..:..9.',HIZ :1H:11().=:.=:. 1.:11:HIZ
() , = - == -... = =96.6= :
==''' =:''''''':"...'==:""'''.= 99.0 ':':''=====1'.62=I'''''...
'.'.'.=:.J=,.....'...=::',...........0310........Ai
D5W ii,,,:i?..-.:;...i..?;,:;=:=:::;;....!..".-A. i].;...;.:..;
;.:.:......,:,.,..1.,,,.;...::::.,,,,H,1:;-;;t, ...=,a'::::.,:t:-
..:`,,,:ivi4a::::::e:::::µ,..,, ',.,,...!.ig:-.1.:!.µ,,'.,4,..,..!.,,,,i6..
.tr.:.:.:,a,..,E.: 7.30 0.40
A41.::::.:....,..,,,.,..,.. ,-..,,,-.L.]=.j....,,.,...,i],.-.,,i,ii...
....::,...........!,..,.......,..,.......................,.....................
....,..............,.........................._:,,,.. .. ,...,...,
.........._....._.........õ..........õ,......õ,.._,...,..,,......,.:õ......:.,.
:
4t,..kiniii ='.....g=-j-A=:=-31-J-.0=-*A]::ii.:H:ii;::::i;;:
......,:::]::::µ,..:0:-.iiii97.:,26:=::::::::::::-.]:-.... .:].-
.....,....:::::].-....:....:::::::.:.-...::::99.:....Ø.......:.,.,.:,:.,..
,.........7.53...õ.:..õ:õ..................00....3.3 .T . ..............
0 97.7 100.1 7.47
12 98.0 100.3 7.61 0.34
15 '
24 97.9 100.3 7.40 0.32
48 98.0 100.4 7.59 0.34
The foregoing detailed description has been given for clearness of
understanding only
and no unnecessary limitations should be understood there from as
modifications will be obvious
to those skilled in the art.
While the invention has been described in connection with specific embodiments
thereof,
29
it will be understood that it is capable of further modifications and this
application is intended to
cover any variations, uses, or adaptations of the invention following, in
general, the principles of
the invention and including such departures from the present disclosure as
come within known or
customary practice within the art to which the invention pertains and as may
be applied to the
essential features hereinbefore set forth and as follows in the scope of the
appended claims.
Date Regue/Date Received 2022-07-11