Note: Descriptions are shown in the official language in which they were submitted.
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
COMPOUNDS AND METHODS FOR MODULATING SEROTONIN RECEPTORS IN THE PERIPHERY
FIELD OF THE INVENTION
[0001] This invention relates to, in part, compositions and methods that are
useful for the treatment of various
diseases, including those linked to serotonin receptor binding, such as, for
example, gastrointestinal and
cardiopulmonary disorders or conditions.
BACKGROUND
[0002] The gastrointestinal (GI) tract is the largest producer of serotonin (5-
hydroxytryptamine (5-HT)) in the
body, and as such it is intimately connected with GI function and physiology.
Serotonin produced by
enterochromaffin (EC) cells is an important enteric mucosal signaling molecule
and has been implicated in a
number of gastrointestinal disorders or conditions, including inflammatory
bowel disease and irritable bowel
syndrome.
[0003] Alosetron (LOTRONEX), a 5-HT3 receptor antagonist, became the first
agent approved by the US Food
and Drug Administration for the treatment of diarrhea-predominant IBS in 2000.
However, the drug has
demonstrated a difficult post-approval side effect profile that has resulted
in a temporary market withdrawal.
Meanwhile, activation of the serotonin 5-HT2B receptor is linked to cardiac
valvulopathy and pulmonary
hypertension (Fitzgerald et al. Mol Pharmacol. 2000; 57: 75-81; Setola et al.
Mol Pharmacol. 2005 68:20-33,
Launay J eta,'. Nat Med 2002 8: 1129-1135), and attenuation of 5-HT2B receptor
signaling may prevent and/or
reverse cardiopulmonary disorders (Janssen et al. Biomed Res Int.
2015;2015:438403; Rothman et al. Expert
Opin Drug Saf. 2009 May;8(3):317-29).
[0004] There remains a need for agents that can safely modulate serotonin
receptors (5-hydroxytryptamine
receptors or 5-HT receptors) for the treatment of gastrointestinal and
cardiopulmonary disorders or conditions.
SUMMARY OF THE INVENTION
[0005] Accordingly, the present invention provides for compositions and
methods that agonize or antagonize
one or more serotonin receptors and which find use in, for example, the
treatment of various gastrointestinal or
cardiopulmonary disorders or conditions.
[0006] In one aspect, the invention provides a compound, or a pharmaceutical
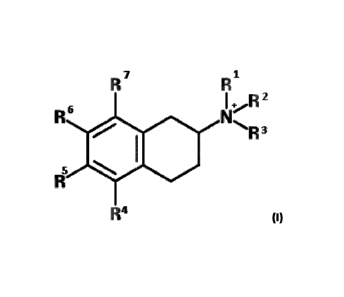
composition comprising a
compound, having the structure of Formula I or Formula II:
1
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
I
, '..
..,..., -..
1 +.,....,h 4.
.:,-..õ 6
..% Nt.,.....
leaX 1 '
x i
.1 (I) or
i
0 7
, 1.
1
n2
I A-Fe
s---,, A 6
\ k
NNõ,.,....
$1.
tint:
34
(II)
where R1 to R7 are hydrogen or independently selected substituents. Suitable
substituents are disclosed herein.
In various embodiments, none of R1, R2, and R3 is hydrogen. For example, in
some embodiments, R1, R2, and R3
are alkyl, e.g. methyl, or may come together to form a heterocyclic ring and
each of R4, R5, R6, and R7 is
independently hydrogen, hydroxy, sulfoxy, halo, acyl, acyloxy, alkyl,
heteroalkyl, alkenyl, heteroalkenyl, alkynyl,
heteroalkynyl, alkoxy, cycloalkyl, heterocycloalkyl, aryl, arylalkyl,
arylhalo, arylhydroxy, arylcyano,
aryltrifluoromethyl, aryltrifluoromethoxy, arylnitro, aryltrifluoromethoxy,
arylnitro, and arylether, arylester,
arylsulfonyl, arylsulfinyl, arylsulfonamidyl, arylsulfonate, arylsulfoxyl,
arylphosphate ester, arylcarbonyl,
arylcarboxylate, arylcarbamate, arylamine,
arylimide, heteroaryl, heteroarylalkyl, heteroarylhalo,
heteroarylhydroxy, heteroarylcyano,
heteroaryltrifluoromethyl, aryltrifluoromethoxy, arylnitro,
heteroaryltrifluoromethoxy, heteroaryl nitro, and
heteroarylether, heteroarylester, heteroarylsulfonyl,
heteroarylsulfinyl, heteroarylsulfonamidyl, heteroarylsulfonate,
heteroarylsulfoxyl, heteroarylphosphate ester,
heteroarylcarbonyl, heteroarylcarboxylate, heteroarylcarbamate,
heteroarylamine, heteroarylimide, quinidine,
morpholine, and any ring structure is optionally substituted with any of the
substituents described herein, with the
proviso that any two adjacent substituents can come together to form a
carbocyclic or heterocyclic ring system.
[0007] In various embodiments, any of R4, R5, R6, and R7 is a hydrocarbon or
heterocyclic ring system. In some
embodiments, the hydrocarbon or heterocyclic ring system is independently
selected from phenyl, thienyl,
furanyl, pyrimidinyl, oxazoyl, thiazolyl, pyridyl, naphthyl, quinolinyl,
indolyl, benzothiophenyl, benzofuranyl,
pyrrolyl, imidazolyl, pyrazole, triazolyl, isoxazolyl, pyridazinyl, pyzazinyl,
pyrimidinyl, oxadiazolyl, benzimidazolyl,
and triazinyl, each of which may contain substituents (i.e. is optionally
substituted). In some embodiments, the
2
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
heterocyclic ring system may contain one or more heteroatoms selected from the
group consisting of oxygen,
sulfur, nitrogen, and combinations thereof.
[0008] In a specific embodiment, only R4 is a hydrocarbon or heterocyclic ring
system which is optionally
substituted. In a specific embodiment, only R4 is a hydrocarbon or
heterocyclic ring system which is optionally
substituted, all of R1, R2, and R3 are alkyl, and each of R5, R6, and R7 is
independently hydrogen, hydroxy,
sulfoxy, halo, acyloxy, alkyl, heteroalkyl, alkenyl, heteroalkenyl, alkynyl,
heteroalkynyl, acyl, and alkoxy.
[0009] In various embodiments the compound or composition does not
substantially accumulate in the human
brain. In various embodiments the compound or composition is not rapidly
metabolized in the periphery. In
various embodiments the nitrogen bearing R1, R2, and R3 in the above formulae
is positively charged. For
example, in various embodiments, the compound or composition of the invention
has the structure of Formula I
or Formula ll and all of R1, R2, and R3 are alkyl groups, including methyl,
ethyl, propyl, isopropyl, n-butyl, iso-
butyl, sec-butyl isobutyl, tertiary butyl, pentyl, isopentyl, neopentyl, and
hexyl, which may be optionally
substituted. In various embodiments, all of R1, R2, and R3 are identical alkyl
groups. In various embodiments, two
of or three of R1, R2, and R3 are different alkyl groups. In some embodiments,
R1, R2, and R3 are each methyl.
[0010] In various embodiments, the compound or composition is a modulator of
one or more of 5-HT1
receptors (e.g. one or more of 5-HT1A, 5-HTic, 5HT1D, 5HT1E, 5-HT1B), 5-HT2
receptors (e.g. one or more
of 5-HT2A, 5-HT2B, 5-HT2c); 5-HT3 receptors; 5-HT4 receptors; 5-HT5 receptors
(e.g. 5-HT5A); 5-HT6 receptors; and
HT7 receptors. In some embodiments, the modulated 5-HT receptor is present in
the GI tract (e.g. one or more of
the stomach, small intestine, large intestine and rectum and includes all
subsections thereof (e.g. duodenum,
jejunum and ileum, colon transversum, colon descendens, colon ascendens, colon
sigmoidenum and cecum).
[0011] In various embodiments, the compound or composition is substantially
enantiomerically pure. In some
embodiments, the composition comprises at least about 70%, or at least about
80%, or at least about 85%, or at
least about 90%, or at least about 95%, or at least about 97%, or at least
about 99% of a single enantiomer. In
various embodiments, a least about 85%, or at least about 90%, or at least
about 95%, or at least about 97%, or
at least about 99% of a single enantiomer. In some embodiments, the single
enantiomer has functional
properties (e.g. serotonin receptor selectivity and/or receptor affinity) that
are not found in a corresponding
enantiomer.
[0012] In various embodiments, the pharmaceutical compositions of the
invention are formulated for long-
action or sustained-release. In some embodiments, the formulation is suitable
for oral delivery and/or
transmucosal delivery (e.g. a capsule, tablet, patch, or lozenge).
[0013] In some aspects, the present invention relates to method for treating
or preventing a gastrointestinal
disorder or condition, optionally selected from inflammatory bowel disease,
irritable bowel syndrome, celiac
disease, and an enteric infection, comprising administering the compound or
composition described herein to a
patient in need thereof.
3
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
[0014] In some aspects, the present invention relates to a method for treating
or preventing a functional GI
disorder comprising administering the composition described herein to a
patient in need thereof.
[0015] In some aspects, the present invention relates to a method for treating
or preventing a cardiopulmonary
disorder comprising administering the composition described herein to a
patient in need thereof.
DETAILED DESCRIPTION OF THE INVENTION
[0016] The present invention is based, in part, on the discovery of novel
serotonin receptor-modulating
compounds that do not substantially accumulate in the brain and therefore are
useful in treating diseases or
disorders of the periphery, including gastrointestinal or cardiopulmonary
disorders or conditions.
[0017] In some aspects, the invention provides a compound, or a pharmaceutical
composition, comprising a
compound, having the structure of Formula I or Formula II:
7
-1 2
, S.3
-"=i; 4
(I) or
7 1
2
6
(II)
where each of R1, R2, R3, R4, R5, R6, and R7 is hydrogen or a substituent, for
example, as defined above.
[0018] In various embodiments, any of R4, R5, R6, and R7 is a hydrocarbon or
heterocyclic ring system. In some
embodiments, the hydrocarbon or heterocyclic ring system is independently
selected from phenyl, thienyl,
furanyl, pyrimidinyl, oxazoyl, thiazolyl, pyridyl, naphthyl, quinolinyl,
indolyl, benzothiophenyl, benzofuranyl,
pyrrolyl, imidazolyl, pyrazole, triazolyl, isoxazolyl, pyridazinyl, pyzazinyl,
pyrimidinyl, oxadiazolyl, benzimidazolyl,
and triazinyl, each of which may contain substituents (i.e. is optionally
substituted). In some embodiments, the
4
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
heterocyclic ring system may contain one or more heteroatoms selected from the
group consisting of oxygen,
sulfur, nitrogen, and combinations thereof.
[0019] In a specific embodiment, only R4 is a hydrocarbon or heterocyclic ring
system which is optionally
substituted. In a specific embodiment, only R4 is a hydrocarbon or
heterocyclic ring system which is optionally
substituted, each of R1, R2, and R3 is alkyl, and each of R5, R6, and R7 is
independently hydrogen, hydroxy,
sulfoxy, halo, acyloxy, alkyl, heteroalkyl, alkenyl, heteroalkenyl, alkynyl,
heteroalkynyl, acyl, and alkoxy. In these
embodiments, the total number of atoms that comprise R4 may be at least about
5, and not more than about 20
atoms, not including hydrogen atoms (e.g. between about 5 to about 10 non-
hydrogen atoms, or between about
to about 15 non-hydrogen atoms, or between about 10 to about 15 non-hydrogen
atoms).
[0020] In various embodiments the nitrogen bearing R1, R2, and R3 in the above
formulae is positively charged
(e.g. a quaternary ammonium). For example, in various embodiments, the
compound or composition of the
invention has the structure of Formula I or Formula ll and all of R1, R2, and
R3 are alkyl groups, including methyl,
ethyl, propyl, isopropyl, n-butyl, iso-butyl, sec-butyl isobutyl, tertiary
butyl, pentyl, isopentyl, neopentyl, and hexyl,
which may be optionally substituted. In various embodiments, all of R1, R2,
and R3 are identical alkyl groups (e.g.,
methyl). In various embodiments, two of or three of R1, R2, and R3 are
different alkyl groups.
[0021] The compounds of Formula I may be referred to herein as "5-PAT" or "5-
APT" compounds. The
compounds of Formula ll may be referred to herein as "4-PAT" or "4-APT"
compounds. "PAT" is 1-Pheny1-3-
dimethylaminotetralin. Such nomenclature is derived from the position of the
attachment of R4to the bicyclic core
of Formula I or Formula II, i.e. the tetrahydronaphthyl (tetralin) moiety
bearing substituents R1-R3 and R5-R7.
[0022] In some embodiments, each of R1, R2, R3, R4, R5, Rs, and R7 is
independently hydrogen, alkyl, aryl,
halo, nitro, amino, heteroaryl, cycloalkyl, heterocyclic, or alkoxy. In
various embodiments, none of R1, R2, and R3
is hydrogen. In some embodiments, all of R1, R2, and R3are alkyl, such as
methyl.
[0023] In some embodiments, at least one of R4, R5, R6, and R7 is not
hydrogen. In some embodiments, at
least one of R4, R5, R6, and R7 is halo. In some embodiments, two of R3, R4,
R5, R6, and R7 are halo. In some
embodiments, one of R4, R5, R6, and R7 is halo. In some embodiments, at least
one of R4, R5, R6, and R7 is
fluoro. In some embodiments, only R4is a substituent other than H and it is a
hydrocarbon ring that is substituted
with a halo, including, for example fluoro or chloro.
[0024] In some embodiments, any one of R6 and R7 or R5 and R6 form a phenyl
ring, which is substituted or
unsubstituted. In various embodiments, the phenyl ring is substituted with a
halo, optionally selected from fluoro
and chloro and optionally at one or more of the ortho, meta, and para
positions. In various embodiments, the
phenyl ring is unsubstituted.
[0025] In various embodiments, there is an optionally substituted hydrocarbon
or heterocyclic ring system, e.g.
a phenyl ring or naphthyl ring connected to the 5 position of the bicyclic
core of Formula I (the core being the top
of the structure, i.e. the tetrahydronaphthyl moiety bearing R5,-R7 and the
amino group bearing R1, R2 and R3). In
5
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
some embodiments, these phenyl or naphthyl rings are substituted with a halo,
optionally selected from fluoro
and chloro. In some embodiments, the halo substitution is in the ortho, meta,
or para position of the phenyl or the
2, 3, 4, 5, 6, or 7 position of the naphthyl ring (where the attachment
position to the compounds's core is at
position 1 or 8 of the naphthyl ring). In some embodiments, the halo
substitution is in the ortho of the phenyl or
the 2 or 7 position of the naphthyl ring (where the attachment position to the
compounds's core is at position 1 or
8 of the naphthyl ring).
[0026] In various embodiments, the total number of atoms that comprise
substituents R4 are at least about 5,
and not more than about 20 atoms, not including hydrogen atoms (e.g. between
about 5 to about 10 non-
hydrogen atoms, or between about 5 to about 15 non-hydrogen atoms, or between
about 10 to about 15 non-
hydrogen atoms). In some embodiments, the composition is not a good substrate
for one or more forms of P450
at levels administered. For instance, without wishing to be bound by theory,
larger numbers of non-hydrogen
atoms that comprise substituents R4 may prevent metabolism associated with
P450 (such metabolism may be,
without wishing to be bound by theory, oxidation-mediated). For example, in
some embodiments, the
composition is not a substrate for one or more families of P450 (e.g. CYP1,
CYP2, CYP3, CYP4, CYP5, CYP7,
CYP8, CYP11, CYP17, CYP19, CYP20, CYP21, CYP24, CYP26, CYP27, CYP39, CYP46,
and CYP51). In some
embodiments, the composition is not a substrate for one or more of CYP1A2,
CYP2C19, CYP2C9, CYP2D6,
CYP2E1, and CYP3A4. In these embodiments, the compound is suited for avoiding
P450-mediated reduction in
a pharmacological effect.
[0027] In various embodiments, the compound or composition is a derivative any
one of the 4-PAT or 5-PAT
genera or species found in International Patent Publication Nos. WO
2008/156707, WO 2008/154044, WO
2009/061436, and WO 2010/129048, and International Patent Application No.
PCT/US2015/031523, the
contents of which are hereby incorporated by reference in their entireties. In
various embodiments, the
derivitization is at the nitrogen attached to position 2 of the compound or
composition's tetrahydronaphthyl
(tetralin) core. For clarity, the tetrahydronaphthyl core is shown below and
position 2 bears an amino group:
7
6 SS 2 3
4
In Formula I or Formula ll herein, this amino group at position 2 is denoted
as bearing R1, R2, and R3.
Accordingly, in various embodiments, any of the compounds of the above
incorporated documents bear an
amino group at position 2 and the amino group bears three of any of the
substituents described herein to provide
a net positive charge e.g. a quaternary ammonium. For example, any of the 4-
PAT or 5-PAT compounds in the
6
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
above incorporated documents are derivatized to comprise an amino group at
position 2 that bears three alkyl
substituents (a g. three methyl substituents).
[0028] For clarity, with reference to WO 2008/156707, the present compounds
and compositions include
derivatives at position R1 of formula (I) therein, which is a genus of 4-PAT
compounds. For instance, R1 of
formula (I) of WO 2008/156707, as well as compounds PAT # 1-31 (e.g. page 21
of WO 2008/156707, the
contents of which are hereby incorporated by reference in their entirety) is,
in the present invention, an amino
group bearing three substituents described herein (e.g. three methyl groups)
and therefore a positive charge. In
some embodiments, such compounds may agonize 5-HT2b. In some embodiments, such
compounds may
antagonize 5-HT2a and/or 5-HT2b.
[0029] Further, with reference to WO 2008/154044, the present compounds and
compositions include
derivatives at the amino group of position 2 of formula (I) therein, which is
a genus of 5-PAT compounds (see
pages 3-5 and FIG. 1 of WO 2008/154044, the contents of which are hereby
incorporated by reference in their
entirety). For instance, the amino group of position 2 of formula (I) of WO
2008/154044, as well as compounds
PAT # 32-40 (e.g. page 16 of 2008/154044, the contents of which are hereby
incorporated by reference in their
entirety) is, in the present invention, an amino group bearing three
substituents described herein (e.g. three
methyl groups) and therefore a positive charge. In some embodiments, such
compounds may agonize 5-HT2b. In
some embodiments, such compounds may antagonize 5-HT2a and/or 5-HT2b.
[0030] Further still, with reference to WO 2009/061436, the present compounds
and compositions include
derivatives at the amino group of position 2 (Ri) of the 4-PAT compounds
therein (see Table 1-3, pages 15-16,
compounds PAT #1-40 and 41(a)-(p), the contents of which are hereby
incorporated by reference in their
entirety). For instance, the amino group of the compounds of Table 1-3 (or Ri)
is, in the present invention, an
amino group bearing three substituents described herein (e.g. three methyl
groups) and therefore a positive
charge.
[0031] Further still, with reference to WO 2010/129048, the present compounds
and compositions include
derivatives at position R1 of any of formulae (I) - (VI) therein, which are
genera of 4-PAT compounds. For
instance, R1of any of formulae (I) - (VI) of WO 2010/129048, compounds of
Tables 1-3 therein (e.g. pages 31-35
of WO 2010/129048, the contents of which are hereby incorporated by reference
in their entirety), and
compounds formed by adding the groups of Table 7 e.g. at the 4- or 5- position
of the tetrahydronaphthyl core
(Table 7, pages 70-89 of WO 2010/129048 is hereby incorporated by reference in
its entirety), is, in the present
invention, an amino group bearing three substituents described herein (e.g.
three methyl groups) and therefore a
positive charge. In some embodiments, such compounds may agonize 5-HT2b. In
some embodiments, such
compounds may antagonize 5-HT2a and/or 5-HT2b.
[0032] Further still, with reference to WO 2010/129048, the present compounds
and compositions include
derivatives at position Ri of formula (V) therein, which is a genus of 5-PAT
compounds. For instance, Ri of
formula (V) of WO 2010/129048, the 5-PAT compounds of Table 3 (e.g. page 35 of
WO 2010/129048, the
7
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
contents of which are hereby incorporated by reference in their entirety) and
compounds formed by adding the
groups of Table 7 e.g. at the 4- or 5- position of the tetrahydronaphthyl core
(Table 7, pages 70-89 of WO
2010/129048 is hereby incorporated by reference in its entirety), is, in the
present invention, an amino group
bearing three substituents described herein (e.g. three methyl groups) and
therefore a positive charge. In some
embodiments, such compounds may agonize 5-HT2c. In some embodiments, such
compounds may antagonize
5-HT2a and/or 5-HT2b.
[0033] Further still, with reference to International Patent Application No.
PCT/US2015/031523, the present
compounds and compositions include, with reference to Formula (I') therein,
instances in which all of Ri to R3 are
identical e.g. bearing the substituents described herein (e.g. three methyl
groups) and therefore have a positive
charge or, with reference to Formula (I) therein, the amino group at position
2 bears a further substituent in
addition to R1 and R2, the additional substituent and R1 and R2 being
substituents described herein (e.g. three
methyl groups) and therefore having a positive charge.
[0034] In some embodiments, the compound or composition is
I +
N
silt
ei
[0035] In some embodiments, the compound or composition is the (+) enantiomer
of the above compound.
[0036] In some embodiments, the compound or composition is:
I +
N
41111 rk'''''
ill ci
[0037] In some embodiments the compound or composition is the (+) enantiomer
of the above compound.
[0038] In some embodiments, the compound or composition is
8
CA 03015750 2018-08-24
WO 2016/187377
PCT/US2016/033185
,,e"1
1
I
õ.-Nt,1/4
,N.õ,
1
[0039] In some embodiments, the compound or composition is the (-) enantiomer
of the above compound.
[0040] In various embodiments, the compound or composition may be any one of:
:
.....õ . ,,,, N 4-;,., ,,,--y,,,T
-,.=,,,,e 1 i
. ...."-=,,
i I
N....,'..,....õ--= õ:;:. N
,
\
1 0 I
0
,
!.
N 4-
,,,---
F 0
: -I
;and
9
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
:
=
:
N +
,,,-- ,,,--------'--õ--. i ---
'''' --"'-µ,..-----'
C---,
I
"7-N,
\i
_______________________________ 1
[0041] In some embodiments, the compound or composition is the (-) or (-F)
enantiomer of any of the above
compounds.
[0042] In various embodiments, the compositions of the present invention
comprise at least about 70%, or at
least about 80%, or at least about 85%, or at least about 90%, or at least
about 95%, or at least about 97%, or at
least about 99% of a single enantiomer. In various embodiments, a least about
85%, or at least about 90%, or at
least about 95%, or at least about 97%, or at least about 99% of a single
enantiomer.
[0043] In some embodiments, the compositions of the present invention
comprises less than about 30%, or
less than about 20%, or less than about 15%, or less than about 10%, or less
than about 5%, or less than about
3%, or less than about 1% of a single enantiomer.
[0044] In some embodiments, the present compounds and compositions are
substantially in the form of a
single enantiomer and essentially free of the corresponding enantiomer.
[0045] In some embodiments, a single enantiomer has functional properties
(e.g. serotonin receptor selectivity)
that are not found in a corresponding enantiomer. For example, in some
embodiments, the (-F) enantiomer has
serotonin-receptor modulating properties that are not found in the
corresponding (-) enantiomer (e.g. the (-)
enantiomer does not bind or modulate one of more serotonin receptors at
physiological levels). By way of further
example, in some embodiments, the (-) enantiomer has serotonin-receptor
modulating properties that are not
found in the corresponding (-F) enantiomer (e.g. the (-F) enantiomer does not
bind or modulate one of more
serotonin receptors at physiological levels). Such a stereoselectivity, in
some embodiments, also applies to (R)-
and (S)- and d- and I-. Accordingly, in some embodiments, the invention does
not provide racemates or racemic
mixtures. In some embodiments, the present compounds and compositions find use
as entiopure drugs.
[0046] In various embodiments, the present invention requires separation of a
racemate into its components,
the pure enantiomers, i.e. chiral resolution. Techniques to achieve
substantially enantiomerically pure
compounds are known and include, by way of non-limiting example,
crystallization, chromatography (e.g. HPLC),
and enzymatic resolution. Various techniques for chiral resolution are found
in Proter Pure App!. Chem. 63 (8):
1119-1122, the contents of which are hereby incorporated by reference in their
entirety. In various embodiments,
measurement of the Eudysmic ratio may assist in determining the final
properties of the composition. For
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
example, one enantiomer is the eutomer while the other enantiomer is the
distomer and comparison of two may
involve the quotient of activity or binding measurements (e.g. ECK or 1050).
[0047] In further embodiments, the present compounds and compositions are
entiopure drugs. However, in
instances in which corresponding enantiomers provide different but desirable
properties (e.g. physiologically
relevant modulation at different serotonin receptors), such enantiomers may be
combined as a combination
composition of known amounts.
[0048] In various embodiments, the present compounds or compositions modulate
one or more serotonin
receptors. For example, one or more of 5-H-f1 receptors (e.g. one or more of 5-
H-f1A, 5-H-fic, 5-HT up, 5-
5-HT2 receptors (e.g. one or more of 5-HT2A, 5-HT2B, 5-HT2c); 5-HT3 receptors;
5-HT4 receptors; 5-
HT5 receptors (e.g. 5-HT5A); 5-HT6 receptors; and 5-HT7 receptors may be
modulated. In various embodiments,
the present compounds or compositions bind one or more serotonin receptors
with a binding affinity (K,) of less
than about 100 nM, or less than about 50 nM, or less than about 25 nM, or less
than about 20 nM, or less than
about 10 nM, or less than about 5 nM, or less than about 2 nM, or less than
about 1 nM. In various
embodiments, the present compounds or compositions bind one or more serotonin
receptors with a binding
affinity (K,) of about 100 nM, or about 90 nM, or about 80 nM, or about 75 nM,
or about 70 nM, or about 60 nM, or
about 50 nM, or about 40 nM, or about 30 nM, or about 25 nM, or about 20 nM,
or about 10 nM, or about 5 nM,
or about 4 nM, or about 3 nM, or about 2 nM, or about 1 nM.
[0049] In various embodiments, the present compounds or compositions may be
full agonist, partial agonist,
antagonist, inverse agonist, etc. at one or more receptors described herein
(e.g. one or more serotonin
receptors). In some embodiments, the invention provides for partial agonists
at one or more receptors described
herein (e.g. one or more serotonin receptors) specifically. In some
embodiments, the present compounds or
compositions are not full agonists at one or more receptors described herein
(e.g. one or more serotonin
receptors). In some embodiments, such partial agonism may provide constant,
weak level of activity at the
receptor, as compared to a full agonist. Further, without wishing to be bound
by theory, such partial agonism may
prevent adaptive regulatory mechanisms that may develop after repeated
exposure to potent full agonists or
antagonists. Further, in embodiments relating to serotonin receptor
modulation, partial agonism of the present
compounds or compositions may reduce or eliminate the risk of serotonin
syndrome (e.g., fever, cardiac
arrhythmia, seizures, loss of consciousness). For example, some 5-H-f1
agonists (e.g. triptans) are known to
cause serotonin syndrome. Partial agonism is believed to avoid this
deleterious side effect.
[0050] In various embodiments, the present compound or composition binds to
one or more of the serotonin 5-
HT7 and 5-H-f1A receptor at physiologically relevant levels. In various
embodiments, the present compound or
composition is a dual agonist of the serotonin 5-HT7 and 5-H-f1A receptors. In
various embodiments, the present
compound or composition binds to the serotonin 5-HT7 receptor with a binding
affinity (K,) of less than about 100
nM, or less than about 50 nM, or less than about 25 nM, or less than about 20
nM, or less than about 10 nM, or
less than about 5 nM, or less than about 2 nM, or less than about 1 nM. In
various embodiments, the present
11
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
compound or composition binds to the serotonin 5-HT7 receptor with a binding
affinity (K) of about 100 nM, or
about 90 nM, or about 80 nM, or about 75 nM, or about 70 nM, or about 60 nM,
or about 50 nM, or about 40 nM,
or about 30 nM, or about 25 nM, or about 20 nM, or about 10 nM, or about 5 nM,
or about 4 nM, or about 3 nM,
or about 2 nM, or about 1 nM. In various embodiments, the present compound or
composition binds to the
serotonin 5-HT1A receptor with a binding affinity (K) of less than about 100
nM, or less than about 50 nM, or less
than about 25 nM, or less than about 20 nM, or less than about 10 nM, or less
than about 5 nM, or less than
about 2 nM, or less than about 1 nM. In various embodiments, the present
compound or composition binds to the
serotonin 5-HT1A receptor with a binding affinity (K) of about 100 nM, or
about 90 nM, or about 80 nM, or about
75 nM, or about 70 nM, or about 60 nM, or about 50 nM, or about 40 nM, or
about 30 nM, or about 25 nM, or
about 20 nM, or about 10 nM, or about 5 nM, or about 4 nM, or about 3 nM, or
about 2 nM, or about 1 nM.
[0051] In various embodiments, the present compound or composition does not
bind or modulate one or more
of the serotonin 5-HT2A and 5-HT2c receptors at physiologically-relevant
levels. In various embodiments, the
present compound or composition binds one or more of the serotonin 5-HT2A and
5-HT2c receptors with an
affinity of greater than about 300 nM, or greater than about 400 nM, or
greater than about 500 nM, or greater
than about 750 nM, or greater than about 1 pM. In some embodiments, the
present compound or composition
binds one or more of the serotonin 5-HT2A and 5-HT2c receptors with an
affinity of about 10 pM, or about 5 pM,
or about 1 pM, or about 900 nM, or about 800 nM, or about 750 nM, or about 700
nM, or about 600 nM, or about
500 nM, or about 400 nM, or about 250 nM.
[0052] Accordingly, in some embodiments, the present compound or composition
selectively binds to serotonin
5-HT7 and 5-HT1A receptors over other serotonin receptors. In various
embodiments, the present compound or
composition binds one or more of the serotonin 5-HT7 and 5-HT1A receptors with
at least about 10-fold, or at least
about 20-fold, or at least about 30-fold, or at least about 40-fold, or at
least about 50-fold, or at least about 75-
fold, or at least about 100-fold higher affinity than one or more of the
serotonin 5-HT2A and 5-HT2c receptors. In
various embodiments, the present compound or composition binds one or more of
the serotonin 5-HT7 and 5-
HT1A receptors with about a 10-fold, or 20-fold, or 25-fold, or 30-fold, or 40-
fold, or 50-fold, or 60-fold, or 70-fold,
or 75-fold, or 80-fold, or 90-fold, or 100-fold higher affinity than one or
more of the serotonin 5-HT2A and 5-HT2c
receptors.
[0053] In various embodiments, the present compound or composition binds to
one or more of the 5-HT2a, 5-
HT2b, and 5-HT2c receptors at physiologically relevant levels. In various
embodiments, the present compound or
composition binds to the one or more of the 5-HT2a, 5-HT2b, and 5-HT2c
receptor with a binding affinity (K) of less
than about 100 nM, or less than about 50 nM, or less than about 25 nM, or less
than about 20 nM, or less than
about 10 nM, or less than about 5 nM, or less than about 2 nM, or less than
about 1 nM. In various
embodiments, the present compound or composition binds to the one or more of
the 5-HT2a, 5-HT2b, and 5-HT2c
receptor with a binding affinity (K) of about 100 nM, or about 90 nM, or about
80 nM, or about 75 nM, or about 70
nM, or about 60 nM, or about 50 nM, or about 40 nM, or about 30 nM, or about
25 nM, or about 20 nM, or about
nM, or about 5 nM, or about 4 nM, or about 3 nM, or about 2 nM, or about 1 nM.
In various embodiments, the
12
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
present compound or composition selectively binds the serotonin 5-HT2c
receptor 5-HT2A relative to 5-HT2, or 5-
H T213.
[0054] In some embodiments, the selective binding to certain serotonin
receptors over other serotonin
receptors (e.g. a preference for 5-HT7 and 5-HT1A receptors) is enantiomer-
mediated. That is, in some
embodiments, one enantiomer of a compound displays the selectively binding
while the other does not. For
example, in some embodiments, the present compound or composition binds one or
more of the serotonin 5-HT7
and 5-HT1A receptors with an at least about 10-fold, or at least about 20-
fold, or at least about 30-fold, or at least
about 40-fold, or at least about 50-fold, or at least about 75-fold, or at
least about 100-fold higher affinity than a
corresponding enantiomer. In some embodiments, the present compound or
composition binds one or more of
the serotonin 5-HT7 and 5-HT1A receptors with about 10-fold, or about 20-fold,
or about 30-fold, or about 40-fold,
or about 50-fold, or about 75-fold, or about 100-fold higher affinity than a
corresponding enantiomer
[0055] In various embodiments, the present compound or composition does not
bind or modulate one or more
of the histamine H1 receptor, dopamine D2, and adrenergic am and alB receptors
at physiologically-relevant
levels.
[0056] In various embodiments, the present compound or composition binds one
or more of the histamine H1
receptor, dopamine D2, and adrenergic am and alB receptors with an affinity of
greater than greater than about
500 nM, or greater than about 750 nM, or greater than about 1 pM. In various
embodiments, the present
compound or composition binds one or more of the histamine H1 receptor,
dopamine D2, and adrenergic am and
alB receptors with an affinity of 10 pM, or about 5 pM, or about 1 pM, or
about 900 nM, or about 800 nM, or
about 750 nM, or about 700 nM, or about 600 nM, or about 500 nM, or about 400
nM, or about 250 nM.
[0057] Accordingly, in some embodiments, the present compound or composition
selectively binds one or more
serotonin receptor over one or more of the histamine H1 receptor, dopamine D2,
and adrenergic am and alB
receptors. In various embodiments, the present compound or composition binds
one or more serotonin receptor
with at least about 10-fold, or at least about 20-fold, or at least about 30-
fold, or at least about 40-fold, or at least
about 50-fold, or at least about 75-fold, or at least about 100-fold higher
affinity than one or more of the histamine
H1 receptor, dopamine D2, and adrenergic am and alB receptors. In various
embodiments, the present
compound or composition binds one or more serotonin receptor with about a 10-
fold, or 20-fold, or 25-fold, or 30-
fold, or 40-fold, or 50-fold, or 60-fold, or 70-fold, or 75-fold, or 80-fold,
or 90-fold, or 100-fold higher affinity than
one or more of the histamine H1 receptor, dopamine D2, and adrenergic am and
alB receptors.
[0058] In various aspects, the present invention relates to a compound or
pharmaceutical composition of the
structure represented by Formula (la):
13
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
N _____________________________________________
(la)
or pharmaceutically acceptable salt thereof, where each of R1 ,R2, and R3 are
defined as above for Formula I
and X is independently hydrogen, alkyl, aryl, halo, nitro, amino, heteroaryl,
cycloalkyl, heterocyclic, or alkoxy.
[0059] In some embodiments, the compound is the (-F) enantiomer. In some
embodiments, the compound is
the (-) enantiomer.
[0060] In some embodiments, each of R1 ,R2, and R3 is independently hydrogen,
alkyl, aryl, halo, nitro, amino,
heteroaryl, cycloalkyl, heterocyclic, or alkoxy. In some embodiments, R1 ,R2,
and R3 are identical. In some
embodiments, R1 ,R2, and R3 are methyl.
[0061] In some embodiments, X is a halo. In some embodiments, X is fluoro. In
some embodiments, X is
chloro. In some embodiments, X is bromo. In some embodiments, X is iodo. In
some embodiments, Xis in the
ortho position. In some embodiments, Xis in the meta position. In some
embodiments, Xis in the para position.
[0062] In some embodiments, the compound of Formula la is enantiomerically
pure. In some embodiments,
the compound of Formula la is substantially the (-F) enantiomer. In some
embodiments, the compound of
Formula la is substantially devoid of the (-F) enantiomer. In some
embodiments, the compound of Formula la is
substantially the (-) enantiomer. In some embodiments, the compound of Formula
la is substantially devoid of
the (-) enantiomer.
[0063] In some embodiments, the compounds and compositions of the present
invention may take the form of
a pharmaceutically acceptable salt. A pharmaceutically acceptable acid
addition salt is formed from a
pharmaceutically acceptable acid, as is well known in the art. Such salts
include the pharmaceutically acceptable
salts listed in, for example, Journal of Pharmaceutical Science, 66, 2-19
(1977) and The Handbook of
Pharmaceutical Salts; Properties, Selection, and Use. P. H. Stahl and C. G.
Wermuth (eds.), Verlag, Zurich
(Switzerland) 2002, which are hereby incorporated by reference in their
entirety.
[0064] In some embodiments, the compounds and compositions of the present
invention can be administered
to a subject as a component of a composition that comprises a pharmaceutically
acceptable carrier or vehicle.
14
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
Such compositions can optionally comprise a suitable amount of a
pharmaceutically acceptable excipient so as
to provide the form for proper administration.
[0065] Pharmaceutical excipients can be liquids, such as water and oils,
including those of petroleum, animal,
vegetable, or synthetic origin, such as peanut oil, soybean oil, mineral oil,
sesame oil and the like. The
pharmaceutical excipients can be, for example, saline, gum acacia, gelatin,
starch paste, talc, keratin, colloidal
silica, urea and the like. In addition, auxiliary, stabilizing, thickening,
lubricating, and coloring agents can be used.
In one embodiment, the pharmaceutically acceptable excipients are sterile when
administered to a subject.
Water, saline solutions and aqueous dextrose and glycerol solutions can also
be employed as liquid excipients.
Suitable pharmaceutical excipients also include starch, glucose, lactose,
sucrose, gelatin, malt, rice, flour, chalk,
silica gel, sodium stearate, glycerol monostearate, talc, sodium chloride,
dried skim milk, glycerol, propylene,
glycol, water, ethanol and the like. Any agent described herein, if desired,
can also comprise minor amounts of
wetting or emulsifying agents, or pH buffering agents.
[0066] The compounds and compositions of the present invention can be present
in various formulations. Any
compound and composition (and/or additional agents) described herein can take
the form of solutions,
suspensions, emulsion, drops, tablets, pills, pellets, capsules, capsules
containing liquids, powders, sustained-
release formulations, or any other form suitable for use. In one embodiment,
the composition is in the form of a
capsule (see, e.g., U.S. Patent No. 5,698,155). Other examples of suitable
pharmaceutical excipients are
described in Remington's Pharmaceutical Sciences 1447-1676 (Alfonso R. Gennaro
eds., 19th ed. 1995),
incorporated herein by reference.
[0067] Where necessary, the compounds and compositions can also include a
solubilizing agent. Also, the
compounds and compositions can be delivered with a suitable vehicle or
delivery device as known in the art.
Combination therapies outlined herein can be co-delivered in a single delivery
vehicle or delivery device.
[0068] The formulations comprising the compounds and compositions of the
present invention may
conveniently be presented in unit dosage forms and may be prepared by any of
the methods well known in the
art of pharmacy. Such methods generally include the step of bringing the
therapeutic agents into association with
a carrier, which constitutes one or more accessory ingredients. Typically, the
formulations are prepared by
uniformly and intimately bringing the therapeutic agent into association with
a liquid carrier, a finely divided solid
carrier, or both, and then, if necessary, shaping the product into dosage
forms of the desired formulation (e.g.,
wet or dry granulation, powder blends, etc., followed by tableting using
conventional methods known in the art)
[0069] Routes of administration include, for example: intradermal,
intramuscular, intraperitoneal, intravenous,
subcutaneous, intranasal, epidural, oral, sublingual, intranasal,
intracerebral, intravaginal, transdermal, rectally,
by inhalation, or topically, particularly to the ears, nose, eyes, or skin.
[0070] In some embodiments, the administering is effected orally. In some
embodiments, the administering is
by absorption through epithelial or mucocutaneous linings (e.g., oral mucosa).
Various delivery systems are
known, e.g., encapsulation in liposomes, microparticles, microcapsules,
capsules, etc., and can be used to
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
administer. In various embodiments, the compounds and compositions of the
present invention are formulated to
be suitable for oral delivery. In various embodiments, the compounds and
compositions of the present invention
are formulated to be suitable for transmucosal delivery (see, e.g. Msatheesh,
etal. Expert Opin Drug Deliv. 2012
Jun;9(6):629-47, the entire contents of which are hereby incorporated by
reference).
[0071] Compositions or compounds for oral delivery can be in the form of
tablets, lozenges, aqueous or oily
suspensions, granules, powders, emulsions, capsules, syrups, or elixirs, for
example. In some embodiments, the
compounds and compositions of the present invention are in the form of a
capsule, tablet, patch, or lozenge.
Orally administered compositions can comprise one or more agents, for example,
sweetening agents such as
fructose, aspartame or saccharin; flavoring agents such as peppermint, oil of
wintergreen, or cherry; coloring
agents; and preserving agents, to provide a pharmaceutically palatable
preparation. Moreover, where in tablet or
pill form, the compositions can be coated to delay disintegration and
absorption in the gastrointestinal tract
thereby providing a sustained action over an extended period of time.
Selectively permeable membranes
surrounding an osmotically capsule containing a driving compound capable of
active driving any compound or
composition described herein is also suitable for orally administered
compositions. In these latter platforms, fluid
from the environment surrounding the capsule is imbibed by the driving
compound, which swells to displace the
agent or agent composition through an aperture. These delivery platforms can
provide an essentially zero order
delivery profile as opposed to the spiked profiles of immediate release
formulations. A time-delay material such
as glycerol monostearate or glycerol stearate can also be useful. Oral
compositions can include standard
excipients such as mannitol, lactose, starch, magnesium stearate, sodium
saccharin, cellulose, and magnesium
carbonate. In one embodiment, the excipients are of pharmaceutical grade.
Suspensions, in addition to the
active compounds, may contain suspending agents such as, for example,
ethoxylated isostearyl alcohols,
polyoxyethylene sorbitol and sorbitan esters, microcrystalline cellulose,
aluminum metahydroxide, bentonite,
agar-agar, tragacanth, etc., and mixtures thereof.
[0072] In various embodiments, the compounds and compositions of the present
invention, or formulations
thereof, do not substantially accumulate in the brain. In various embodiments,
the compounds and compositions,
or formulations thereof, deliver a physiological amount of present compound or
composition to the periphery for
at least about 6 hours, or at least about 9 hours, or at least about 12 hours,
or at least about 15 hours, or at least
about 18 hours, or at least about 21 hours, or at least about 24 hours. In
various embodiments, the compounds
and compositions, or formulations thereof, deliver a physiological amount of
present compound or composition to
the periphery for about 6 hours, or about 9 hours, or about 12 hours, or about
15 hours, or about 18 hours, or
about 21 hours, or about 24 hours.
[0073] Further, the present compounds or compositions may find use as a
combination therapy or co-
formulation with one or more additional therapeutic agents. For example, such
additional therapeutic agents can
include an anti-bacterial agent, which includes, but is not limited to,
cephalosporin antibiotics (e.g. cephalexin,
cefuroxime, cefadroxil, cefazolin, cephalothin, cefaclor, cefamandole,
cefoxitin, cefprozil, and ceftobiprole);
fluoroquinolone antibiotics (e.g. cipro, Levaquin, floxin, tequin, avelox, and
norflox); tetracycline antibiotics (e.g.
16
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
tetracycline, minocycline, oxytetracycline, and doxycycline); penicillin
antibiotics (e.g. amoxicillin, ampicillin,
penicillin V, dicloxacillin, carbenicillin, vancomycin, and methicillin);
monobactam antibiotics (a g. aztreonam);
and carbapenem antibiotics (e.g. ertapenem, doripenem, imipenem/cilastatin,
and meropenem). In some
embodiments, the additional therapeutic agent is metronidazole (e.g. FLAGYL),
fidaxomicin (e.g. DIFICID), or
vancomycin (e.g. VANCOCIN), rifaximin, charcoal-based binders/adsorbents (e.g.
DAV132), fecal
bacteriotherapy, probiotic therapy (see, e.g., Intnarl J Inf Dis, 16 (11):
e786, the contents of which are hereby
incorporated by reference). In some embodiments, the additional therapeutic
agent is an antidiarrheal agent,
which include, but are not limited to, DPP-IV inhibitors, natural opioids,
such as tincture of opium, paregoric, and
codeine, synthetic opioids, such as diphenoxylate, difenoxin and loperamide,
bismuth subsalicylate, lanreotide,
vapreotide and octreotide, motiln antagonists, COX2 inhibitors like celecoxib,
glutamine, thalidomide and
traditional antidiarrheal remedies, such as kaolin, pectin, berberine and
muscarinic agents. In some
embodiments, the additional therapeutic agent is an anti-inflammatory agent
such as steroidal anti-inflammatory
agents or non-steroidal anti-inflammatory agents (NSAIDS). Steroids,
particularly the adrenal corticosteroids and
their synthetic analogues, are well known in the art.
[0074] Any compound or composition (and/or additional therapeutic agents)
described herein can be
administered by controlled-release or sustained-release means or by delivery
devices that are well known to
those of ordinary skill in the art. Examples include, but are not limited to,
those described in U.S. Patent Nos.
3,845,770; 3,916,899; 3,536,809; 3,598,123; 4,008,719; 5,674,533; 5,059,595;
5,591,767; 5,120,548; 5,073,543;
5,639,476; 5,354,556; and 5,733,556, each of which is incorporated herein by
reference in its entirety. Such
dosage forms can be useful for providing controlled- or sustained-release of
one or more active ingredients
using, for example, hydropropylmethyl cellulose, other polymer matrices, gels,
permeable membranes, osmotic
systems, multilayer coatings, microparticles, liposomes, microspheres, or a
combination thereof to provide the
desired release profile in varying proportions. Suitable controlled- or
sustained-release formulations known to
those skilled in the art, including those described herein, can be readily
selected for use with the active
ingredients of the agents described herein. The invention thus provides single
unit dosage forms suitable for oral
administration such as, but not limited to, tablets, capsules, gelcaps, and
caplets that are adapted for controlled-
or sustained-release.
[0075] Controlled- or sustained-release of an active ingredient can be
stimulated by various conditions,
including but not limited to, changes in pH, changes in temperature,
stimulation by an appropriate wavelength of
light, concentration or availability of enzymes, concentration or availability
of water, or other physiological
conditions or compounds.
[0076] In another embodiment, polymeric materials can be used (see Medical
Applications of Controlled
Release, Langer and Wise (eds.), CRC Pres., Boca Raton, Florida (1974);
Controlled Drug Bioavailability, Drug
Product Design and Performance, Smolen and Ball (eds.), Wiley, New York
(1984); Ranger and Peppas, 1983,
J. Macromol Sci. Rev. Macromol. Chem. 23:61; see also Levy et al., 1985,
Science 228:190; During et al., 1989,
Ann. Neurol 25:351; Howard et al., 1989, J. Neurosurg. 71:105).
17
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
[0077] Administration of any compounds or compositions (and/or additional
agents) described herein can,
independently, be one to four times daily. Administration can be for the
duration of one day or one month, two
months, three months, six months, one year, two years, three years, and may
even be for the life of the subject.
Chronic, long-term administration will be indicated in many cases. The dosage
may be administered as a single
dose or divided into multiple doses. In general, the desired dosage should be
administered at set intervals for a
prolonged period, usually at least over several weeks or months, although
longer periods of administration of
several months or years or more may be needed.
[0078] The present compounds and compositions also find use in various
therapeutic methods.
[0079] In some aspects, the present invention relates to method for treating
or preventing a gastrointestinal
disorder or condition, optionally selected from inflammatory bowel disease,
irritable bowel syndrome, celiac
disease, and an enteric infection, comprising administering the compound or
composition described herein to a
patient in need thereof.
[0080] In some aspects, the present invention relates to a method for treating
or preventing a functional GI
disorder comprising administering the composition described herein to a
patient in need thereof.
[0081] Illustrative gastrointestinal disorders or conditions include, but are
not limited to, hyperproliferative
diseases, for example, carcinomas (e.g., colorectal cancer); autoimmune and
inflammatory bowel diseases
(IBD), for example, Celiac's disease, Crohn's disease, and colitis (e.g.,
ulcerative colitis); irritable bowel
syndrome; infectious diseases of the intestine, for example, C. difficile
infection (ODD and/or a C. difficile-
associated disease, pseudomembranous colitis, amebiasis or intestinal
tuberculosis; parasitic disorders, colonic
polyps; cysts; diverticular disease (e.g., diverticulosis, diverticulitis, and
diverticular bleeding); constipation;
intestinal obstruction; malabsorption syndromes; rectal diseases; ulceration
of the mucosa; intestinal dysbiosis
(e.g., intestinal bacteria overgrowth); dyspepsia; gastroesophageal reflux
disease; gastroparesis; nausea;
vomiting; and diarrhea. Additional diseases, disorders and conditions which
are suitable for treatment with the
compositions and methods of the invention include those listed in Table 3 of
WO 2014/121298, the entire
contents of which are incorporated herein by reference.
[0082] In some embodiments, the present invention provides methods for
treating or preventing autoimmune
and inflammatory bowel diseases (IBD), for example, Celiac's disease, Crohn's
disease, and colitis (e.g.,
ulcerative colitis), comprising administering an effective amount of a
pharmaceutical composition and/or
formulation (and/or additional therapeutic agent) described herein to a
subject or a patient need thereof.
[0083] In some embodiments, the present invention provides methods for
treating or preventing irritable bowel
syndrome (IBS), comprising administering an effective amount of a
pharmaceutical composition and/or
formulation (and/or additional therapeutic agent) described herein to a
subject or a patient need thereof. In an
embodiment, the IBS is IBS with constipation (I BS-C). In an embodiment, the
IBS is IBS with diarrhea (IBS-D). In
an embodiment, the IBS is mixed IBS (I BS-M).
18
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
[0084] In some embodiments, methods of the invention are useful for treating
constipation, dyspepsia,
gastroesophageal reflux disease, gastroparesis, nausea, vomiting, diarrhea,
and a diverticular disease (e.g.,
diverticulosis, diverticulitis, and diverticular bleeding).
[0085] In some aspects, the present invention relates to method for modulating
a serotonin receptor in the GI
tract, comprising administering the compound or composition described herein
to a patient in need thereof.
[0086] In some aspects, the present invention relates to a method for treating
or preventing a cardiopulmonary
disorder comprising administering the composition described herein to a
patient in need thereof.
[0087] Illustrative cardiopulmonary disorders include cardiac valvulopathy and
pulmonary hypertension.
Cardiopulmonary disorders include right-sided and/or left-sided cardiac
fibrosis, inclusive of cardiomyopathy and
congestive heart failure. Also included are ventricular tachyarrhythmia, left
ventricular (LV) dysfunction, and heart
failure, as well as right ventricular failure (RVF) and left ventricular
failure (LVF). A further cardiopulmonary
disorder is heart valve disease, which includes left heart diseases (e.g.
diseases of the aortic valve (e.g. aortic
valve stenosis, aortic valve insufficiency, aortic valve incompetence, aortic
valve regurgitation) and/or the mitral
valve (e.g. mitral valve stenosis, mitral valve insufficiency, mitral valve
incompetence, mitral valve regurgitation)
and/or right heart diseases (e.g. diseases of the tricuspidvalve (e.g.
tricuspid valve stenosis, tricuspid valve
insufficiency, tricuspid valve incompetence, tricuspid valve regurgitation)
and/or the pulmonary valve (e.g.
pulmonary valve stenosis, pulmonary valve insufficiency, pulmonary valve
incompetence, pulmonary valve
regurgitation). Further, cardiopulmonary disorders are heart valve dysplasia;
tetralogy of Fallot, Ebstein's
anomaly, cardiornyoeatnies (including, for example, dilated carcliomyopathy,
restrictive cacilornyopathy and
hypertropnic cardiernyopathy), elevated heart rate. vasoconstrictor:, and
heart failure.
[0088] In various embodiments, e.g. those pertaining to treating or preventing
a cardiopulmonary disorder,
combination therapy or co-formulation with an additional therapeutic agent may
be provided. Illustrative
additional therapeutic agents include: anticoagulents (e.g. dalteparin
(FRAGMIN), danaparoid (ORGARAN),
enoxaparin (LOVENOX), heparin, tinzaparin (INNOHEP), and warfarin (COUMADIN));
Antiplatelet Agents (e.g.
aspirin, ticlopidine, clopidogrel, and dipyridamole); ACE Inhibitors (e.g.
benazepril (LOTENSIN), captopril
(CAPOTEN), enalapril (VASOTEC), fosinopril (MONOPRIL), lisinopril (PRINIVIL,
ZESTRIL), moexipril
(UNIVASC), perindopril (ACEON), quinapril (ACCUPRIL), ramipril (ALTACE) and,
trandolapril (MAVIK));
Angiotensin II Receptor Blockers (or Inhibitors) (e.g. candesartan (ATACAND),
eprosartan (TEVETEN),
irbesartan (AVAPRO), losartan (COZAAR), telmisartan (MICARDIS) and, valsartan
(DIOVAN)); beta-blockers
(e.g. acebutolol (SECTRAL), atenolol (TENORMIN), betaxolol (KERLONE),
bisoprolol/hydrochlorothiazide (ZIAC),
bisoprolol (ZEBETA), carteolol (CARTROL), metoprolol (LOPRESSOR, TOPROL XL),
nadolol (CORGARD),
propranolol (INDERAL), sotalol (BETAPACE) and, timolol (BLOCADREN)); calcium
channel blockers (e.g.
amlodipine (NORVASC, LOTREL), bepridil (VASCOR), diltiazem (CARDIZEM, TIAZAC),
felodipine (PLENDIL),
nifedipine (ADALAT, PROCARDIA), nimodipine (NIMOTOP), nisoldipine (SULAR), and
verapamil (CALAN,
ISOPTIN, VERELAN); diuretics (e.g. amiloride (MIDAMOR), bumetanide (BUMEX),
chlorothiazide (DIURIL),
19
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
chlorthalidone (HYGROTON), furosemide (LASIX), hydrochlorothiazide (ESIDRIX,
HYDRODIURIL), indapamide
(LOZOL), and Spironolactone (ALDACTONE); vasodilators (e.g. isosorbide
dinitrate (ISORDIL), nesiritide
(NATRECOR), hydralazine (APRESOLINE), nitrates and minoxidil); digitalis
preparations (e.g. lanoxin); and
statins (a g., atorvastatin, pravastatin, fluvastatin).
Definitions
[0089] The term "acyl" means both substituents of the formula Rx¨C(0)¨ , where
Rx is alkyl, heteroalkyl,
alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl,
and heteroarylalkyl.
[0090] The term "alkyl" means both "unsubstituted alkyls" and "substituted
alkyls," the latter of which refers to
alkyl moieties having substituents replacing a hydrogen on one or more carbons
of the hydrocarbon backbone.
Such substituents can include, for example, halogen, hydroxyl,
alkylcarbonyloxy, arylcarbonyloxy,
alkoxycarbonyloxy, aryloxycarbonyloxy, carboxyl ate,
alkylcarbonyl, alkoxycarbonyl, aminocarbonyl,
alkylthiocarbonyl, alkoxyl, phosphate, phosphonato, phosphinato, cyano, amino
(including alkyl amino,
dialkylamino, arylamino, diarylamino, and alkylarylamino), acylamino
(including alkylcarbonylamino,
arylcarbonylamino, carbamoyl and ureido), amidino, imino, sulfhydryl,
alkylthio, arylthio, thiocarboxylate, sulfates,
sulfonate, sulfamoyl, sulfonamido, nitro, trifluoromethyl, cyano, azido,
heterocyclyl, alkylaryl, or an aromatic or
heteroaromatic moiety. It will be understood by those skilled in the art that
the moieties substituted on the
hydrocarbon chain can themselves be substituted, if appropriate. Cycloalkyls
can be further substituted, e.g., with
the substituents described above. An "alkylaryl" moiety is an alkyl
substituted with an aryl (e.g., phenylmethyl
(benzyl)). The term "alkyl" also includes unsaturated aliphatic groups
analogous in length and possible
substitution to the alkyls described above, but that contain at least one
double or triple bond respectively. In
some embodiments, the alkyl group may have from 1 to 12 carbon atoms, e.g.
about 1 carbon atom, or about 2
carbon atoms, or about 3 carbon atoms, or about 4 carbon atoms, or about 5
carbon atoms, or about 6 carbon
atoms, or about 7 carbon atoms, or about 8 carbon atoms, or about 9 carbon
atoms, or about 10 carbon atoms,
or about 11 carbon atoms, or about 12 carbon atoms. Illustrative alkyl groups
include methyl, ethyl, propyl,
isopropyl, n-butyl, iso-butyl, sec-butyl isobutyl, tertiary butyl, pentyl,
isopentyl, neopentyl, hexyl, septyl, octyl,
nonyl and decyl.
[0091] The terms "alkoxyalkyl," "polyaminoalkyl" and "thioalkoxyalkyl" refer
to alkyl groups, as described above,
which further include oxygen, nitrogen or sulfur atoms replacing one or more
carbons of the hydrocarbon
backbone, e.g., oxygen, nitrogen or sulfur atoms. Illustrative alkoxy
substituents include, but are not limited to,
methoxy, ethoxy, propoxy, isopropoxy, cyclopropyloxy and cyclohexyloxy. In
some embodiments, the alkoxy is a
lower alkoxy (containing one to six carbon atoms). The alkoxy substituent is
optionally substituted.
[0092] The terms "alkenyl" and "alkynyl" refer to unsaturated aliphatic groups
analogous in length and possible
substitution to the alkyls described above, but that contain at least one
double or triple bond, respectively. In
some embodiments, the "alkenyl" or "alkynyl" group may have from 2 to 12
carbon atoms, e.g. about 2 carbon
atoms, or about 3 carbon atoms, or about 4 carbon atoms, or about 5 carbon
atoms, or about 6 carbon atoms, or
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
about 7 carbon atoms, or about 8 carbon atoms, or about 9 carbon atoms, or
about 10 carbon atoms, or about
11 carbon atoms, or about 12 carbon atoms.
[0093] Amino or "amine" substituents include those of the formula ¨N(Rb)2,
where Rb is hydrogen, alkyl,
(halo)alkyl, alkenyl, alkynyl, carbocyclyl, carbocyclylalkyl, aryl, aralkyl,
heterocycloalkyl, heterocycloalkylalkyl,
heteroaryl heteroarylalkyl, or other substituent described herein. When
¨N(Rb)2 has two Rb substituents other
than hydrogen, they can be combined with the nitrogen atom to form a 4-, 5-, 6-
or 7-membered ring. For
example, ¨N(Rb)2 is intended to include, for example, pyrrolidinyl and
morpholinyl.
[0094] Amide or "amido" substituents include those of the formula ¨G(0)N(R)2
or ¨NHC(0)Ry, where Ry is
selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl,
carbocyclyl, carbocyclylalkyl, cycloalkyl,
aryl, heteroaryl, or other substituent described herein. The RY of ¨N(R)2 of
the amide may optionally be taken
together with the nitrogen to which it is attached to form a 4-, 5-, 6- or 7-
membered ring.
[0095] The term "aryl" as used herein, refers to the radical of aryl groups,
including 5- and 6- membered single-
ring aromatic groups that may include from zero to four heteroatoms, for
example, benzene, pyrrole, furan,
thiophene, imidazole, benzoxazole, benzothiazole, triazole, tetrazole,
pyrazole, pyridine, pyrazine, pyridazine and
pyrimidine, and the like. Aryl groups also include polycyclic fused aromatic
groups such as naphthyl, quinolyl,
indolyl, and the like. Those aryl groups having heteroatoms in the ring
structure may also be referred to as "aryl
heterocycles," "heteroaryls" or "heteroaromatics." The aromatic ring can be
substituted at one or more ring
positions with such substituents as described above, as for example, halogen,
hydroxyl, alkoxy, alkylcarbonyloxy,
arylcarbonyloxy, alkoxycarbonyloxy, aryloxycarbonyloxy,
carboxyl ate, alkylcarbonyl, alkoxycarbonyl,
aminocarbonyl, alkylthiocarbonyl, phosphate, phosphonato, phosphinato, cyano,
amino (including alkyl amino,
dialkylamino, arylamino, diarylamino, and alkylarylamino), acylamino
(including alkylcarbonylamino,
arylcarbonylamino, carbamoyl and ureido), amidino, imino, sulfhydryl,
alkylthio, arylthio, thiocarboxylate, sulfates,
sulfonato, sulfamoyl, sulfonamido, nitro, trifluoromethyl, cyano, azido,
heterocyclyl, alkylaryl, or an aromatic or
heteroaromatic moiety. Aryl groups can also be fused or bridged with alicyclic
or heterocyclic rings which are not
aromatic so as to form a polycycle (e.g., tetralin).
[0096] The terms "halogen" and "halo" designate fluoro, chloro, bromo or iodo.
Thus, substituents include,
without limitation, haloalkyl, haloalkenyl, haloalkynyl and haloalkoxy.
[0097] The term "heteroaryl" means an aromatic heterocyclyl typically
containing from 5 to 18 ring atoms. A
heteroaryl may be a single ring, or two or more fused rings. Non-limiting
examples of five-membered heteroaryls
include imidazolyl; furanyl; thiophenyl (or thienyl or thiofuranyl);
pyrazolyl; oxazolyl; isoxazolyl; thiazolyl; 1,2,3-,
1,2,4-, 1,2,5-, and 1,3,4-oxadiazoly1; and isothiazolyl. Non-limiting examples
of six-membered heteroaryls include
pyridinyl; pyrazinyl; pyrimidinyl; pyridazinyl; and 1,3,5-, 1,2,4-, and 1,2,3-
triazinyl. Non- limiting examples of 6/5-
membered fused ring heteroaryls include benzothiofuranyl, isobenzothiofuranyl,
benzisoxazolyl, benzoxazolyl,
purinyl, and anthranilyl. Non-limiting examples of 6/6-membered fused ring
heteroaryls include quinolinyl;
isoquinolinyl; and benzoxazinyl (including cinnolinyl and quinazolinyl).
21
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
[0098] The terms "heterocyclic" or "heterocyclo" or "heterocyclyl" refer to a
saturated (e.g., "heterocycloalkyl"),
partially unsaturated (e.g., "heterocycloalkenyl" or "heterocycloalkynyl") or
completely unsaturated (e.g.,
"heteroaryl") ring system typically containing from 3 to 18 ring atoms, where
at least one of the ring atoms is a
heteroatom (i.e., nitrogen, oxygen or sulfur), with the remaining ring atoms
being independently selected from the
group consisting of carbon, nitrogen, oxygen and sulfur. A heterocyclyl group
can be linked to the parent
molecular moiety via any substitutable carbon or nitrogen atom in the group,
provided that a stable molecule
results. A heterocyclyl may be, without limitation, a single ring, which
typically contains from 3 to 14 ring atoms,
from 3 to 8 ring atoms, from 3 to 6 ring atoms, or from 5 to 6 ring atoms. Non-
limiting examples of single-ring
heterocyclyls include furanyl, dihydrofuranyl, pyrrolyl, isopyrrolyl,
pyrrolinyl, pyrrolidinyl, imidazolyl, isoimidazolyl,
imidazolinyl, imidazolidinyl, pyrazolyl, pyrazolinyl, pyrazolidinyl,
triazolyl, tetrazolyl, dithiolyl, oxathiolyl, oxazolyl,
isoxazolyl, thiazolyl, isothiazolyl, thiazolinyl, isothiazolinyl,
thiazolidinyl, isothiazolidinyl, thiodiazolyl, oxathiazolyl,
oxadiazoly, pyranyl, dihydropyranyl, pyridinyl, piperidinyl, pyridazinyl,
pyrimidinyl, pyrazinyl, piperazinyl, triazinyl,
isoxazinyl, oxazolidinyl, isoxazolidinyl, oxathiazinyl, oxadiazinyl,
morpholinyl, azepinyl, oxepinyl, thiepinyl, or
diazepinyl. A heterocyclyl may also include, without limitation, two or more
rings fused together, such as, for
example, anthracene, naphthyridinyl, thiazolpyrimidinyl, thienopyrimidinyl,
pyrimidopyrimidinyl, or
pyridopyrimidinyl. A heterocyclyl may comprise one or more sulfur atoms as
ring members; and in some cases,
the sulfur atom(s) is oxidized to SO or SO2. The nitrogen heteroatom(s) in a
heterocyclyl may or may not be
quaternized, and may or may not be oxidized to N-oxide. In addition, the
nitrogen heteroatom(s) may or may not
be N-protected.
[0099] The term "heteroatom" as used herein means an atom of any element other
than carbon or hydrogen.
Preferred heteroatoms are nitrogen, oxygen, sulfur and phosphorus.
[00100] The term "hydroxyl" means -OH.
[00101] The term "optionally substituted" is intended to encompass groups that
are unsubstituted or are
substituted by other than hydrogen at one or more available positions,
typically 1, 2, 3, 4 or 5 positions, by one or
more suitable groups (which may be the same or different). Such optional
substituents include, the group
substituents described above and for example, hydroxy, halogen, cyano, nitro,
Ci-Csalkyl, C2-C8 alkenyl, 02-
C8alkynyl, Ci-Csalkoxy, C2-C8alkyl ether, C1-C8alkanone, Ci-Csalkylthio,
amino, mono- or di- (CI-Csalkyl)amino,
haloCi-Csalkyl, haloCi-Csalkoxy, Ci-Csalkanoyl, C2-C8alkanoyloxy, Ci-
Csalkoxycarbonyl, -COOH, -CONH2,
mono- or di-(Ci-Csalkyl)aminocarbonyl, -502NH2, and/or mono or di(CI-
Csalkyl)sulfonamido, as well as
carbocyclic and heterocyclic groups. Optional substitution is also indicated
by the phrase "substituted with from 0
to X substituents," where X is the maximum number of possible substituents.
Certain optionally substituted
groups are substituted with from 0 to 2, 3 or 4 independently selected
substituents (i.e., are unsubstituted or
substituted with up to the recited maximum number of substituents).
[00102] "Substituted" means having substituents replacing an atom and includes
one or more of halo, acyl,
acyloxy, alkyl, heteroalkyl, alkenyl, heteroalkenyl, alkynyl, heteroalkynyl,
alkoxy, alkoxycarbonyl, cycloalkyl,
22
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, hydroxy,
cyano, trifluoromethyl, trifluoromethoxy, nitro,
and trimethylsilanyl, ether, ester, sulfide, disulfide, sulfonyl, sulfinyl,
sulfonamidyl, sulfonate, sulfoxyl, phosphate
ester, phosphine, borate ester, carbonyl, carboxylate, carbamate, amine,
imide, and quinidine. Such substituents
can also include, for example, halogen, hydroxyl, alkylcarbonyloxy,
arylcarbonyloxy, alkoxycarbonyloxy,
aryloxycarbonyloxy, carboxylate, alkylcarbonyl, alkoxycarbonyl, aminocarbonyl,
alkylthiocarbonyl, alkoxyl,
phosphate, phosphonato, phosphinato, cyano, amino (including alkyl amino,
dialkylamino, arylamino,
diarylamino, and alkylarylamino), acylamino (including alkylcarbonylamino,
arylcarbonylamino, carbamoyl and
ureido), amidino, imino, sulfhydryl, alkylthio, arylthio, thiocarboxylate,
sulfates, sulfonate, sulfamoyl, sulfonamido,
nitro, trifluoromethyl, cyano, azido, heterocyclyl, alkylaryl, or an aromatic
or heteroaromatic moiety.
[00103] The term "sulfhydryl" or "thiol" means -SH.
[00104] The term "treat" or "treatment" means any degree of reduction of
symptoms or causation of a disease or
medical condition. The term "prevent" or "prevention" means any degree of
avoiding the onset or acquisition of a
disease or medical condition.
[00105] As used herein, "consisting essentially of" allows the inclusion of
materials or steps that do not
materially affect the basic and novel characteristics of the claim. Any
recitation herein of the term "comprising"
can be exchanged with "consisting essentially of" or "consisting of".
[00106] This invention is further illustrated by the following non-limiting
examples.
EXAMPLES
Example 1: 5-PAT Synthesis
[00107] Synthesis of compounds described herein is shown in Scheme 1, which
involves 6-steps. Briefly, 5-Br-
tetralone (1), obtained by reacting 1-tetralone with bromine/A1013, was
reduced to give the corresponding alcohol
(2) that was treated with pTSA to obtain the olefin (3) to obtain the epoxide
(4), that was treated with pTSA to
obtain key intermediate 5-Br-2-tetralone (5). 5-Br-2-tetralone can be reacted
with a wide variety of commercially-
available boronic acid derivatives (6), these organoboron when used in the
Suzuki-Miyaura cross coupling
reaction allow synthesis of the compounds described herein. Thus, in Scheme 1,
5-bromo-2-tetralone (5) was
reacted with Tetrakis triphenylphosphine Pd [0], the mixture was degassed, and
the 2'-F- or 2'-Cl-phenylboronic
acid was added. The reaction mixture was stirred at 80 C for 3h and then
cooled to room temperature before
adding H202 to quench excess boronic acid to obtain the 5-(2'-F- or 2'-C1)-
phenyl-2-tetralones (7). Reductive
amination with dimethylamine gave 5-(2'[o]- F or C1)-phenyl-2-
dimethylaminotetralin racemates (8), resolved by
polysaccharide-based chiral stationary phase (CSP)-HPLC to obtain 25 mg each
(2R) and (25)-o-F-PAT and -0-
01-5-PAT. Other compounds synthesized herein involve this general process.
23
CA 03015750 2018-08-24
WO 2016/187377
PCT/US2016/033185
Scheme .1 OH 0
0 Nam-4, 0 C Alight pTSA, Benzene
mcpba, NaHCO3
It VW' IP *lb
01101 T600lun,effite0Ovil WHIP so oc, 15 min OCM, 2 h., 75%
Br 3
i: r 2 85% Br 4
1 pTSA, Benzene.
: r
N/ 80 C, 30 min, 80%õ.
N 0 1
fri2--F or CI Pd col K7CO3 3 h '
11611111P i4(1002, Nei 4040 .. .
,
R*2,6=1-1 al (o.F. or 116 st Fr2
NatitH3CN, PV HO..6 an R`2 13'OH R mf, CI 1111141111111. ,
,t
o-CI-5-PAT) THIF/Mo01-1, R6 at" R2 2 t,
,
t:
R"3 55 *C, 12 h R' litillF IT fir R3e-H Br 5
,
,
chiral+IPLC R*5 '*4 8 5 3 ,
t:
gives 2S er 2R 65% i ... 6
4 ...
4 R, R3
* 5-Br-
2-Tetralone
¨ Key
intermed.
[00108] In addition, the boronic acid derivatives in Chart 1 are available to
synthesize the corresponding 5-
substituted-PAT analogs, according to Scheme 1. Thus analogs are proposed with
multiple substitutions to the 5-
phenyl moiety, as listed under Hydrogen and Halogen Binding Moieties in Chart
1. Thus, racemic analogs will be
synthesized and separated to yield ( )- and (-)-5 (2R)-5-PAT analogs and/or
(2R) and (25)-5-PAT analogs.
Chart 1 Hydrogen and Halogen Binding Moieties
Hydrophobic
and Aromatic
(xeMi*
NM:::034. ,d,L, _.Esiotik,
e i
CrX,, N,
i."
Kkoics.k r:)-
"13tot4
: 1 CPA s.s. . kw**
tifOir&
r,:1V = '
roN,T.Sdlqqh, N.. PA4z PC
M3
`)10c:r04041.,
M..43.,
6r4:::r CF)-""ats'a
F..1 Pei )0'-osoy
OP*0
..õCiZEk.
e'-'s, ,Aarig 1
,Crealk 1 CiipPiaz,Z4leC, ,,..
&Vik C).0:).
- ......
Crisi4=3, ' '
0
0
CH.c' 1\Nreh -64 14}a L
Ega%
r.....6,
õs,õ,,.õ,j,..õ , ,Eig ..\''k ".'õ,== 0. '....4-rd
Rom ROM NC+% r
b ^,
Eskaisa
orrye,4.krw ROA=k=
e'-y-aPtli;.
24
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
Example 2: 4-PAT Synthesis
[00109] Synthesis of compounds of Formula ll is described in WO 2010/1290048,
for example "Synthetic
Schemes" of pages 53-55, and WO 2008/156707, for example 11 of pages 53-57,
the entire contents of which
are hereby incorporated by reference.
Example 3: Derivitization of the Amino Group at the 2 Position of the Tetralin
Core
[00110] Any of the compounds described herein may be derivatized at an amino
group at 2 position of the
tetralin core via the reaction shown below:
oR2
0 e.g., NH(Me)2. HCI, N(CH3)3 N
* CI $1 R3
NaBH3CN, R4
R4 THF/Me0H,
55 C, 12 h
oR2
0 e.g., NH(Me)2. HCI, N(CH3)3* CI 605 N.113
401$
NaBH3CN, R5
R5 THF/Me0H,
55 C, 12 h
Example 4: (-0-5-FPI is Orally Active And Readily Crosses the Blood-Brain
Barrier
[00111] The compound (-0-5-FPT (5-(2'-fluoropheny1)-N,N-dimethy1-1,2,3,4-
tetrahydronaphthalen-2-amine) falls
outside of Formula I as is lacks a positive charge on the nitrogen bearing R1,
R2, and R3:
40140 N
di is s F
1 I
[00112] Adult, male, C5781/6J mice, approximately six months old, and
treatment-naive for at least six weeks
prior to testing, were injected subcutaneously with (-0-5-FPT (3.0 mg/kg) and
returned to their home cages. At
30, 60, or 90 min later, mice were euthanized by rapid cervical dislocation
and decapitation. Trunk blood was
collected in pre-chilled, heparin-coated tubes. Brains were quickly excised
and frozen in liquid nitrogen. Plasma
was collected from blood after centrifugation for 5 min at 13,000 g. Whole
brain samples were wrapped in foil,
and brain and plasma samples were labeled and stored at -80 C until liquid
chromatography-mass
spectrometry/mass spectrometry (LC-MS/MS) assays were performed. Frozen brain
samples were weighed and
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
homogenized in phosphate buffered saline (PBS), pH 7.4. After the first
analysis, the extra brain homogenate
was stored at -80 C until they were thawed for a second, more dilute,
analysis. Plasma samples were used
directly upon arrival. The proteins from each plasma sample and a portion of
each brain homogenate were
immediately precipitated with 1:1 methanol:acetonitrile (4x starting volume)
and internal standard ((¨)-MBP68)
followed by centrifugation at 14,000 g for 5 minutes at 4 C. The resulting
supernatants from each sample were
dried under nitrogen. Each sample was reconstituted in methanol, vortexed,
sonicated briefly, and centrifuged
prior to LC-MS/MS analysis. Calibration curves were constructed from the
ratios of the peak areas of 5-FPT
versus (¨)-MBP in extracted standards made in mouse plasma or homogenized
mouse brain.
[00113] LC-MS/MS analysis was performed using an Agilent 1100 series HPLC and
a Thermo Finnigan
Quantum Ultra triple quad mass spectrometer. The mobile phases used were 0.1%
formic acid in water (A) and
0.1% formic acid in methanol (B) in a 5 minute gradient. Samples of 10 pL each
were injected onto a
Phenomenex Gemini C18 column (2 x 50 mm, 5 p) with a C18 guard column. 5-FPT
and its internal standard ((¨
)-MBP) were ionized in ESI+ and detected in SRM mode. Internal standards were
used for quantification of the
compound level per g tissue or per pL plasma. Four mice were included per
group, but plasma levels from one
mouse were not detectable due to low volume of blood collected.
[00114] Unlike compounds of the present invention, (+)-5-FPT readily crosses
the blood-brain barrier, as
evidenced by detection of pg levels 30, 60, and 90 min after systemic
administration (Table 4). Notably, levels of
(+)-5-FPT were substantially lower in plasma relative to brain tissue as soon
as 30 min post-administration,
indicating that (+)-5-FPT is rapidly cleared in the periphery. Meanwhile, the
attenuating effects of (+)-5-FPT (5.6
mg/kg) on the DOI HTR remained significant for up to 2 hrs. post-
administration; at 3 hrs. post-administration,
(+)-5-FPT did not block the DOI HTR.
Time after injection
30 min 60 min 90 min
Plasma (p g/mL) 0.114(0.03) 0.11 8(0.01) 0.0
70(0.01)
Brain (p g/g) 1.78(0.24) 2.1E0.17)
1.460109)
Tab1e4. Plasma and brain concentrations of (+)-5-FFT
after 3.0 mg/kg subcutaneous administration. Data are
expressed as mean (S EM).
Example 5: Compounds Bearing a Postivelv Charged Amino Group at the 2 Position
of the Tetralin Core Do Not
Readily Cross the Blood-Brain Barrier
[00115] Adult, male, C57 BI/6J mice, approximately six months old, and
treatment-naïve for at least six weeks
prior to testing, are injected sc with any of the compounds described above,
bearing a positively charged amino
group at the 2 position of the tetralin core (the "test compound") at a dose
of about 3.0 mg/kg and returned to
their home cages. At 30, 60, or 90 min later, mice are euthanized by rapid
cervical dislocation and decapitation.
Trunk blood is collected in pre-chilled, heparin-coated tubes. Brains are
quickly excised and frozen in liquid
26
CA 03015750 2018-08-24
W02016/187377
PCT/US2016/033185
nitrogen, Plasma is collected from blood after centrifugation for 5 min at
13,000 g. Whole brain samples are
'mapped in foil, and brain and plasma samples are labeled and stored at -80 C
until liquid chromatography-mass
spectrometry/mass spectrometry (LC-MBIMS) assays are performed.
[00116] Frozen brain samples are weighed and homogenized in phosphate buffered
saline (PBS), pH 7.4. After
the first analysis, the extra brain homogenate is stored at -80*C until they
are thawed for a second, more dilute,
analysis. Plasma samples are used directly upon arrival. The proteins from
each plasma sample and a portion of
each brain homogenate are immediately precipitated with 1:1
methanohanetonitrile (4x starting volume) and
internal standard (e,g, (¨)-MBP68) followed by centrifugation at 14,000 g for
5 Minutes at 4"C. The resulting
supernatants from each sample are dried under nitrogen. Each sample is
reconstituted in methanol, vonexed,
sonicated briefly, and centrifuged prior to 1..C-MSIMS analysis. Calibration
curves are constructed from the ratios
of the peak areas of test compound versus internal standard in extracted
standards made in mouse plasma or
homogenized mouse brain.
NO 1171 LC-MS/MS analysis is performed using an Agilent 1100 series HPLC and a
Thermo Finnigan Quantum
Ultra triple quad mass spectrometer. The mobile phases used are 0.1% formic
acid in water (A) and 0.1% forrnic
acid in methanol (B) in a 5 minute gradient. Samples of 10 pl. each are
injected onto a Phenornenex Gemini C18
column (2 x 50 mai. 5 p) with a C18 guard column. The test compound and its
internal standard ((¨)-MBP) were
ionized in ESI+ and detected in SRM mode. Internal standards are used for
quantification of the test compound
level per g tissue or per pi. plasma.
pm] The test compounds are not expected to accumulate in the brain and,
rather, are expected to be more
prevalent In the plasma.
Examole 6: 5-HT7 Receptor Binding Affinity of 5-PAT Compounds
1001191 Table 5 shows a group of 5-PAT compounds that were synthesized and
tested for their in vitro binding
affinity to serotonin 5-HT? receptors using human embryonic kidney 293
(HEK293) naffs as described in Canal et
ACS (Them. Neurosci. 2015. 6, 1259-1270. The compounds are tertiary amines and
available to bind 5-HT7
receptors both in the brain and in the periphery. Each of the compounds can be
dedvatized to a corresponding
positively-charged quaternary amine, for example, by the addition of a third
alkyl group, R3. to render it
impermeable to the blood-brain barrier and specific for peripheral 5--HT7
receptors.
Table 5. 5-Substituted-2-aminotetralins.
Ri
N
IC S
2 i 9
R5
27
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
R /R Substituent R Substituent Human
1 2 5 5-HT KJ (nM)
7
(S)-Methyl Phenyl 9 0.4
(R)-Methyl Phenyl 4400 930
(S)-Methyl Phenyl-2'-F 6 0.7
(R)-Methyl Phenyl-2'-F 260 53
(S)-Methyl Phenyl-2'-CI 6 0.6
(R)-Methyl Phenyl-2'-CI 58 12
(R/S)-Methyl Phenyl-2'-NH3 91 6.8
(R/S)-Methyl Phenyl-3'-F 34 6.2
(R/S)-Methyl Phenyl-3'-CI 18 2.4
(R/S)-Methyl Phenyl-3'-CF3 63 6.6
(R/S)-Methyl Phenyl-3'-0Me 37 9.0
(R/S)-Methyl Phenyl-3', 5'-F 180 32
(R/S)-Methyl Phenyl-3', 5,-CI 40 60
(S)-Methyl Phenyl-3', 5'-CF3 910 260
(R)-Methyl Phenyl-3', 5'-CF3 >10,000
(R/S)-Methyl Phenyl-4'-F 120 17
(S)-Methyl Pheny-4'-C1 72 5.8
(R/S)-Methyl Pheny1-2'-C1-4'-F 30 10
(S)-Methyl Napthalene 17 2.4
(R)-Methyl Napthalene 28 5.1
(S)-Methyl Furanyl 33 2.5
(R)-Methyl Furanyl >10,000
(R/S)-Methyl Cyclopentyl 240 35
(R/S)-Methyl Anthracene 440 110
(R/S)-Methyl Isoquinoline 93 10
(R/S)-Methyl O-Benzyl >10,000
(S)-Propyl 2'-F-Phenyl 35 5.2
(R)-Propyl 2'-F-Phenyl 63 2.7
(R)-Propyl Phenyl 180 31
(S)-Propyl Phenyl 83 5.8
(R/S)-Methyl 7,8-di0Me-5-Phenyl >10,000
EQUIVALENTS
[00120] While the invention has been described in connection with specific
embodiments thereof, it will be
understood that it is capable of further modifications and this application is
intended to cover any variations,
uses, or adaptations of the invention following, in general, the principles of
the invention and including such
departures from the present disclosure as come within known or customary
practice within the art to which the
invention pertains and as may be applied to the essential features
hereinbefore set forth and as follows in the
scope of the appended claims.
[00121] Those skilled in the art will recognize, or be able to ascertain,
using no more than routine
experimentation, numerous equivalents to the specific embodiments described
specifically herein. Such
equivalents are intended to be encompassed in the scope of the following
claims.
28
CA 03015750 2018-08-24
WO 2016/187377 PCT/US2016/033185
INCORPORATION BY REFERENCE
[00122] All patents and publications referenced herein are hereby incorporated
by reference in their entireties.
[00123] The publications discussed herein are provided solely for their
disclosure prior to the filing date of the
present application. Nothing herein is to be construed as an admission that
the present invention is not entitled to
antedate such publication by virtue of prior invention.
[00124] As used herein, all headings are simply for organization and are not
intended to limit the disclosure in
any manner. The content of any individual section may be equally applicable to
all sections.
[00125] This application claims the benefit of U.S. Provisional Application
No. 62/163,652, filed on 19 May 2015
and entitled "Compounds and Methods for Modulating Serotonin Receptors in the
Periphery", which is
incorporated herein by reference in its entirety.
29