Note: Descriptions are shown in the official language in which they were submitted.
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
SYSTEMS AND METHODS FOR MONITORING PATIENT MEDICATION
ADHERENCE
CROSS REFERENCE TO RELATED APPLICATIONS
This application is a continuation in part of, and claims the priority benefit
of, US patent
application 15/060,514, "SYSTEMS AND METHODS FOR MONITORING PATIENT
MEDICATION ADHERENCE" filed March 3, 2016.
BACKGROUND OF THE INVENTION
FIELD OF THE INVENTION
This invention is in the field of patient operated medical diagnostic devices
that can be used
to determine if a patient is following health care provider medication
recommendations.
DESCRIPTION OF THE RELATED ART
Patient non-adherence to health care provider mediation recommendations is a
major medical
problem. Center for Disease Control (CDC) materials suggest that between 20-
30% of
medication prescriptions are never filled, and medication is not taken as
prescribed in up to
50% of all cases.
For example, studies have shown that only about 51% of patients being treated
for
hypertension are adherent to their medication therapy on a long term basis. In
this context,
"long term" should be viewed as being about six months, since other studies
have shown that
medication adherence rates drop off after the first six months of treatment.
This is a large
scale problem. At present over 133 million Americans have a long term chronic
condition
requiring medication.
It has also been estimated that medication non-adherence can result in up to
125,000 excess
deaths annually; also incurring economic costs (due to higher subsequent
patient expenses)
estimated at $100 billion to $300 billion dollars per year.
Thus methods to monitor and encourage patent adherence to prescribed
medications are of
high interest in the art. Patient adherence to hypertension medication is
particularly critical.
Patients, in particular elderly patients, are often put on multiple different
medications at the
same time. For example, to control hypertension, patients may be put on
various
combinations of diuretics, angiotensin converting enzyme (ACE) inhibitors,
angiotensin
receptor blockers (ARBs), beta-blockers, vasodilators, calcium channel
blockers, aldosterone
1
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
antagonists, refill inhibitors, alpha blockers, and the like. No one drug
alone may be totally
effective, but in combination, several drugs may produce the desired results.
Patients with other types of disorders, such as lung disease, chronic
obstructive pulmonary
disease, epilepsy, diabetes, and the like are of course not immune to
hypertension. Many of
these patients, sometimes in addition to anti-hypertension drugs, also take
additional types of
drugs for these disorders. It is not uncommon for these other drugs to also
have an impact on
cardiovascular system function as well.
In order to improve patient medication adherence, the patient should ideally
receive frequent
feedback that would promptly warn the patient whenever the patient is not
adhering to their
prescribed medication properly, or when this medication has otherwise become
less than fully
effective.
A few such patient operated medical diagnostic tests are presently on the
market, such as
home blood glucose tests, home blood pressure tests, home pulse oximeters, and
even home
ECG tests.
With the exception of home tests for blood glucose, there are presently few
home diagnostic
tests that can warn a patient when he or she is out of compliance for a
particular medication.
Here prior art home blood pressure tests illustrate the problem ¨ if a
patient's blood pressure
is non-ideal, is this because the patient skipped one of several anti-
hypertensive medications
that the patient has been taking, or is it simply because the patient is
having a bad day? If the
patient skipped a drug, which one was skipped?
Thus further improvements in the art of using patient operated medical
diagnostics to monitor
patient adherence to medication would be desirable.
BRIEF SUMMARY OF THE INVENTION
The invention is based, in part, on the insight that various types of patient
operated
instrumentation, such as blood pressure monitors, pulse oximeters, ECG readers
and the like
discard a huge amount of data in the course of obtaining their various
different types of pulse
wave measurements. This invention is also based, in part, on the insight that
with proper
analysis, this massive amount of blood pressure data, pulse oximeter data, ECG
reading data,
and other types of data could be usefully employed to help solve the major
problem of
monitoring patient medication adherence.
2
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
In some embodiments, the invention may be a method, device or system for
determining a
patient's adherence to a prescribed medication regime. The invention relies on
a plurality of
different types of measured (actual) patient pulse wave measurements, such as
some
combination of oscillometric blood pressure data, oscillometric pulse oximeter
data, and ECG
data, as well as other types of patient physiological measurements as
available.
The invention further relies on additional information, such as patient
reference (baseline)
information that reports on the various patient pulse wave measurements in the
absence of
various types of medication, medication schedule information (which informs
the invention
as to what types of drugs/medications that patient should be taking, and
when), and
medication impact parameters, which informs the invention as to how the
various individual
medications would be expected to impact (alter) various specific types of
patient pulse wave
measurements.
The invention will typically use at least one processor to obtain a various
different types of
actual patient pulse wave measurements. It will then use its various types of
additional
information to determine if the actual data is as expected based on the
patient baseline pulse
wave information, expected medication schedule, and expected impact of these
medications
on the patient baseline pulse wave information. If the results are
inconsistent, then the
invention will typically conclude that the patient is not properly adhering to
his medication
schedule, and will report this lack of medication adherence accordingly.
Other types of patient physiological readings, such as temperature, motion,
lung function
(e.g. stethoscope-like microphone pickups and automated sound analysis,
spirometers), brain
wave function (EEG) and the like may also be obtained, and these additional
types of
readings can be used to extend the range of different types of
drugs/medications that the
system can successfully monitor.
BRIEF DESCRIPTION OF THE DRAWINGS
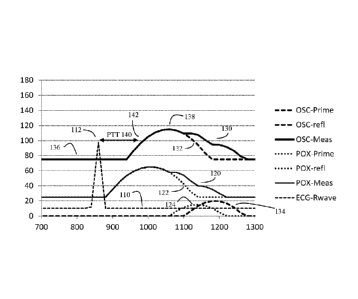
Figure 1 shows a simplified drawing of patient reference pulse wave
measurements for a
normal (healthy patient) in the absence of medication. Three different types
of patient pulse
wave measurements (oscillometric blood pressure measurements, pulse oximeter
type
measurements, and ECG measurements) are being shown simultaneously, along with
some of
the underlying patient physiological mechanisms that create some of these
various patient
pulse waves. Here the time elapsed from the last previous ECG "R" pulse (in
milliseconds)
is shown on the -X" (horizontal) axis. The -Y" vertical axis shows (for the
blood pressure
3
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
measurements) the blood pressure in millimeters of mercury (mm Hg), or
arbitrary units for
the other pulse wave measurements.
Figure 2 shows a simplified version the reference pulse wave measurements for
a different
(older patient suffering from hypertension) patient in the absence of
medication. This is this
patient's "baseline" pulse wave information. Note the overall higher blood
pressure, and
different timing of various components of the various pulse waves, relative to
Figure 1.
Figure 3 shows a how a specific type medication (here type "A" medication) can
impact the
pulse wave measurements for the hypertension patient from Figure 2 (above).
The changes
in the various and shapes of the curves can be considered to be the "impact
parameter- for
this type of medication. The impact parameters can be expressed either
analytically in terms
of the impact of the drug on the underlying patient physiology, and/or
empirically in terms of
the changes in the shapes of the curves (without needing to understand the
mechanism by
which the medication impacts the patient's physiology). Here drug "A" lowers
the patient's
blood pressure overall without otherwise causing much of a change in the
timing of the
various components of the various pulse waves.
Figure 4 shows how a different specific type of medication (here type "B"
medication)
impacts the pulse wave measurements for the hypertension patient from Figure 2
above.
Here drug "B" has altered the timing of the ECG "R" pulse, and has also
lowered the blood
pressure overall.
Figure 5 shows how yet another different specific type of medication (here
type "C"
medication) impacts the pulse wave measurements for the hypertension patient
from Figure 2
above. Here drug "C" has done several things. It has somewhat altered the
timing between
the ECG "R- pulse, and the onset in the rise in blood pressure. This drug has
also altered the
timing of some of the various underlying pulse waves (here direct wave and
reflected waves)
so that they don't superimpose (augment) with each other in an unfavorable
manner. This
helps reduce the peak (systolic) blood pressure.
Figure 6 shows the effect of all three mediations (type -A" and type "B" and
type "C") on the
pulse wave measurements for the hypertension patient from Figure 2 above. In
this case, the
effect of all three drugs is additive, and the hypertension patient's blood
pressure is brought
back to almost "normal" or acceptable values.
Figure 7 shows a flow chart showing of some of the various steps that may be
carried out by
the medication adherence device's processor in order to determine if the
various
4
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
physiological measurements taken by the device's various sensors are showing
that the
patient is likely following his or her assigned medication schedule, or not.
Figure 8 shows an example of one type of patient operable instrumentation
that, with
upgrades as described herein, may be used according to the present invention.
Figure 9 shows an example of a different type of patient operable
instrumentation that, with
upgrades as described herein, may be used according to the present invention.
In this
embodiment, the patient operable instrumentation is intended to be worn by the
patient over a
period of time.
DETAILED DESCRIPTION OF THE INVENTION
Hypertension is a very common and very serious disease that is frequently
treated by multiple
anti-hypertensive drugs simultaneously. Often these different types of anti-
hypertensive drugs
(medications) have different, and sometimes even well understood, mechanisms
of action on
the user's cardiovascular system.
In this discussion, we will first examine some of the various types of
cardiovascular system
related pulse wave data that may be obtained by patient operable
instrumentation, such as the
easy to use multiple sensor instrumentation discussed in more detail in US
patent applications
14/186,151 and 62/138,377, and shown in Figures 8 and 9. In these examples, we
will
examine some hypothetical automated oscillometric cuff type blood pressure
pulse wave
profiles, automated oscillometric pulse oximeter type pulse wave profiles, and
automated
electrocardiogram (ECG) pulse wave profiles, as well as some of the underlying
physiological changes brought about by hypertension and various drugs on these
pulse wave
profiles. These examples are intended to make the general principles behind
the invention
easier to understand, but are otherwise not intended to be limiting.
In this discussion, automated oscillometric cuff type blood pressure sensors
will be
commonly abbreviated as "oscillometric" or -OSC" sensors. The automated
oscillometric
pulse oximeter type sensors will be commonly abbreviated as "pulse oximeter-
or "PDX"
type sensors, and automated electrocardiogram sensors will be commonly
abbreviated as
"ECG" sensors. See applications 14/186,151 and 62/138,377 for further
discussion.
Figures 1-6 are based on a simplified model of the cardiovascular system.
These figures
show both the actual measurements that may be obtained by the various pulse
wave sensors,
as well as a few details of some of the underlying physiological mechanisms
that produce
these actual measurements.
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
In these simplified examples, assume that the major components of the
patient's systolic
blood pressure caused by a combination of the patient's primary pulse pressure
(caused by
contraction of the patient's left ventricle), augmented by a reflected wave
produced when the
primary pulse pressure wave effectively -bounces" off of the patient's major
arteries. In
younger or healthier patients, these arteries are more elastic, and this tends
to delay the timing
of the return "bounce" or "reflected- pressure wave so that it does not
significantly augment
the pressure of the primary pulse pressure wave. However in older or less
healthier patients,
the arteries are less elastic, and this tends to speed up the timing of the
return "bounce"
reflected pressure wave so that the reflected wave pressure and the primary
wave pressure
additively superimpose and augment each other, thus producing a higher (and
typically
unhealthy) diastolic pressure.
Figure 1 shows a simplified drawing of patient reference pulse wave
measurements for a
normal (healthy patient) in the absence of medication. Here the
instrumentation is providing
three types of "actual" patient "baseline" pulse wave measurements. These
types include
pulse waves from an electrocardiograph (ECG) type sensor (110), pulse waves
obtained from
an oscillometric pulse oximeter type sensor (PDX) (120), and pulse waves
obtained from an
oscillometric cuff type blood pressure monitor type sensor (OSC) (130).
The actually measured oscillometric type blood pressure waveforms are shown by
the solid
"OSC-Meas" line (130). These actually measured waveforms are produced by the
combination of a primary pulse pressure wave "OSC-Prime" shown in dashed lines
(132),
and a reflected pulse pressure wave "OSC-refl" shown by the dashed line (134).
For these
-0SC" waveforms, the vertical -Y" axis should be assumed to read in
millimeters of mercury
(mm Hg).
The actually measured pulse oximeter blood pressure waveforms are shown by the
solid
"PDX-Meas- line (120). These actually measured waveforms are also produced by
the
combination of a primary pressure wave "PDX-Prime" shown in dotted lines (122)
and a
reflected pulse pressure wave "PDX-refl" (124). Although these measurements
could also be
expressed in mm Hg, for better readability, the Y axis pulse oximeter readings
are shown as
being offset from the oscillometric blood pressure readings. Note that the
timing and the
shape of the pulse oximeter waveforms is not quite the same as the
oscillometric type
waveforms. This is because the pulse oximeter sensor will typically be located
in a different
part of the patient's body (e.g. ear lobe, fingertip) than the oscillometric
blood pressure
sensor (arm, wrist), and the pulse wave will thus take differing amounts of
time to reach the
6
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
two sensors, with the time being controlled by the pulse wave velocity (PWV).
Here assume
that in this case, the patient's pulse oximeter is mounted on an ear lobe,
while the
oscillometric blood pressure sensor is mounted on a wrist, and the signal
pulse wave reaches
the ear lobe first.
In this simplified model, assume that the horizontal "X" axis represents the
time (in
milliseconds) since the peak of the last ECG -R" wave, which occurs at the
same frequency
as the patient's pulse. Assume also that the pulse transit time "PTT" (140) is
the time delay
between the peak of the ECG "R" wave (112) and the rise in the measured pulse
(142).
Here also, assume that the baseline of the measured oscillometric waveform
(OSC-Meas)
(136) represents the patient's diastolic blood pressure, while the peak of the
measured
oscillometric waveform (138) represents the patient's systolic blood pressure.
These same
conventions and numbering scheme are used throughout Figures 1-6.
In Figure 1, this ideal normal patient has ideal cardiac parameters, such as a
pulse rate of 70
beats per minute, and a blood pressure of 115/75 mm/Hg. When the patient's
heart beats
(here shown triggered by the ECG "R" wave 112), the contraction of the
patient's ventricle
produces both a primary wave (OSC-Prime 132) and (due to rebound from the
patient's
arteries), a time-delayed reflected wave (OSC-refl 134) that, depending on the
timing of the
reflected wave, can augment or not augment the systolic blood pressure (138)
caused by the
primary wave.
In this example, assume that the patient has young and flexible arteries. As a
result, the
reflected wave (134) bounces more slowly, and is thus sufficiently delayed so
that the peak
pressure of the reflected wave (134) does not add to the peak pressure of the
primary wave
(132), thus helping to keep the peak systolic pressure (138) at a desired
level. Additionally,
due to the patient's more flexible arteries, the speed of the pulse transit
time (140) (e.g. time
between the peak ECG "R" wave (112) and the raising part of the pulse wave
142) also tends
to be somewhat longer, and the patient's diastolic blood pressure (136) is
also at a lower
level.
In this example, the processed pulse oximeter readings (120), (122), (124) are
generally
similar to the oscillometric cuff type blood pressure readings, but due to
differences in the
location of the two types of sensing device, differ somewhat in timing and
pulse shape. Thus
to summarize, in Figure 1, the patient has a normal pulse rate of 70 beats per
minute (this can
be seen by the fact that the ECG -R" wave (112) has a peak at 857
milliseconds), the patient
7
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
has a systolic blood pressure reading (138) of 115 mm Hg, a diastolic blood
pressure reading
(136) of 70 mm Hg, the reflected wave (134) arrives 130 milliseconds after the
primary wave
(138), and the patent has a pulse transition time (PPT, 140) of 200
milliseconds.
Figure 2 shows a simplified version of the same type of patient reference
information, but in
this case obtained from an older patient suffering from hypertension. In the
absence of any
drugs, this older hypertension patient has a somewhat higher pulse rate (75
beats per minute)
(note that the ECG "R" wave now is at 800 milliseconds), a higher diastolic
blood pressure
(136) of about 95 mm Hg because this patient has generally stiffer arteries.
Additionally, this
patient's coronary arteries are also stiffer, thus unfortunately producing a
faster acting
reflected wave (134) arriving only 60 milliseconds after the primary wave
(138). Due to this
faster arrival time, the peak of the reflected wave (134) acts to augment the
systolic blood
pressure produced by the peak of the patient's primary wave (132), producing a
high peak
systolic blood pressure of 160 over 95 (138). Additionally, the pulse transit
time (140) (e.g.
time between the peak ECG "R" wave (112) and the raising part of the pulse
wave (142))
tends to be somewhat shorter, and is here 150 milliseconds.
Drugs (medications) alter patient physiology. Sometimes the underlying
mechanisms are
known, and sometimes the underlying mechanisms are not known. When the
underlying
mechanisms are known (or at least well characterized from an analytical
perspective), then
the effects of the drugs can be analyzed by decomposing the various measured
signals into
the underlying physiological mechanisms or known analytical coefficients. When
the
underlying mechanisms are less well-known, or analytical methods less
adequate, alternative
and more empirical methods, such as curve fitting, can instead be used to
analyze patient
data. Here, these hypertension examples are useful, because both analytical
and empirical
methods may be used.
Figure 3 shows a simplified diagram showing, with respect to Figure 2, the
medication
impact parameter for older hypertension patent 2 for a "type A- specific type
of medicine.
Assume in this example that this medication lowers both the patient's systolic
and diastolic
pressure by a certain amount (here assume 10 mm Hg for both values), but
otherwise does
not alter other cardiovascular parameters. Thus Figure 3 generally resembles
Figure 2,
except that the systolic (138) and diastolic values (136) are both 10 mm Hg
lower.
Figure 4 shows a simplified diagram showing. with respect to Figure 2, the
medication
impact parameter for patent 2 for a type "13- specific type of medicine.
Assume that this
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
medication reduces the patient pulse rate from 75 beats per minute to 70 beats
per minute,
and the beneficial effects of this medication also reduce the systolic and
diastolic values by
about 10 mm Hg each (down to 150/85), but otherwise do not impact the other
cardiovascular
parameters. Here, drug -B" has not only lowered the systolic (138) and
diastolic (136) blood
pressure readings by about 10 mm Hg each, but it has also changed the timing
of the ECG
"R" pulse (112) from 800 milliseconds to 857 milliseconds. The timing of the
other pulse
waveforms has also been altered accordingly.
Figure 5 shows a simplified diagram showing, with respect to Figure 2, the
medication
impact parameter for patent 2 for a type "C" specific type of medicine. Assume
that this
medication makes the patient's arteries more flexible, and thus changes
(delays) the timing
for the reflected wave (s) so that the peak of the reflected wave (134, 124)
does not augment
the pressure effects of the peak of the primary wave (132, 122) in such an
unfavorable
manner. This change in reflected wave timing (say from an unhealthy 60
milliseconds to a
better 110 milliseconds, or a plus 50 millisecond increase) decreases the
magnitude of the
systolic portion of the blood pressure (138) from 160 mm Hg to 145 mm Hg.
Additionally
assume that this drug also makes the patient's other major arteries more
flexible, such that the
more flexible arteries have a longer pulse transit time (140) from 150
milliseconds to 190
milliseconds, for a net gain of 40 milliseconds (ms) over that patient's
original baseline
values (shown in Figure 2). Thus the time between the peak ECG "R" wave (112)
and the
raising part of the pulse wave (142) tends to be somewhat longer as well.
Figure 6 shows a simplified diagram showing, with respect to Figure 2, the
combined
medication impact parameters for patient 2 when the patient is taking all
three drugs at once
(e.g. drug "A", drug "B" and drug "C"). Here the three drugs all act
synergistically to bring
the patient's blood pressure from a formerly unacceptable level of 160/95
(systolic/diastolic)
to an acceptable target level of about 126/75 mm Hg.
Here, the fact that the different types of drugs have both different
underlying mechanisms of
actions (on an analytical level), as well as different effects on the shapes
of the patient's pulse
wave data (on an empirical level) can be used by the invention to help assess
if the patient is,
or is not, in compliance with his or her medication schedule for these three
types of drugs.
For example we know, (and this information can be encoded into the machine's
plurality of
medication impact parameters) that drug "B", if taken properly (e.g. according
to that
patient's medication schedule information) should both slow the pulse rate
(see the change in
9
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
the timing of the ECG "R- wave), and also lower both systolic and diastolic
blood pressure
by a certain expected amount (e.g. 10 mm Hg for each). Thus differences in
pulse rate timing
(e.g. the ECG "R" pulse 112) can be used to infer the presence of drug "B"
using either
analytical or empirical methods.
We also know that drug "C" changes the timing of the reflected wave(s) (124,
134) relative to
the primary waves (122, 132). This reduces the magnitude to which the peak of
the reflected
wave augments the peak of the primary wave during the systolic portion of the
pulse. This
drug also slows down the pulse transit time (here the distance from the "R"
wave to the rise
of the pulse) to a more normal level, and this shows up in other waveform
changes as well.
Thus differences in the timing of the reflected waves relative to the primary
waves can be
used to infer the presence or absence of drug "C".
Here the analytical or empirical methods differ however. Using an analytical
approach, the
shape of the measured waveform(s) such as OCS-Meas (130) and/or PDX-Meas (120)
could
be analyzed and decomposed (e.g. using Fourier analysis) into the underlying
OSC-prime
(132), OSC-refl (134) and/or PDX-prime (122), PDX-refl (124) waveforms, and
inferences as
to the drug effects on the timing of the primary and reflected waves can be
drawn directly.
In the analytical method, then with respect to the drug impact parameters for
drug "C", the
drug impact parameters could report that at this dosage, drug "C" changes the
timing
differences between the primary wave (132) and the reflected wave (134) by a
time value
such as from 60 milliseconds to 110 milliseconds, (e.g. + 50 milliseconds).
Thus this
particular medication impact parameter, for an analytical approach, might be a
simple number
such as +50 milliseconds. It might also be a different number, such as an
artery elasticity
parameter, that might in turn be converted to a different time delay
parameter. But in either
event, we are decomposing the observed or "actual" waveforms into some
underlying
equations, and the medication impact parameters can report on the effects that
the various
drugs each have on the coefficients of the underlying equations.
By contrast, in an empirical approach, which can be more useful when the
underlying
mechanisms are not so well known, the medication impact parameters might
instead store an
average pulse waveform shape for this patient when on the drug. The empirical
method
might instead look at the patient 2 reference waveform (e.g. Figure 2 OSC-Meas
130) and the
pulse waveform shape assumed to occur when this patent is taking drug C (e.g.
Figure 5) and
determine by curve fitting (e.g. model fitting, different types of statistical
regression analysis)
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
if the observed patient 2 data fit the drug C pulse waveform shapes above a
certain
significance threshold). In essence we attempt to see how much of the actual
or observed
data fits a drug "C" like pulse wave profile, and how much it resembles a
baseline profile
without drug, and attempt to infer the presence of drug "C" without otherwise
decomposing
the waveform profiles into underlying equation coefficients.
In terms of analyzing combinations of drugs, note that drug -A" acts to
further decrease both
the systolic and diastolic blood pressure to a greater extent than would be
expected by the
combination of drugs "B and "C" alone. Thus the system can also assume (or
infer or
deduce) that any further unexpected drop in systolic and diastolic blood
pressure, not already
assigned to drugs "B" and "C", can likely be attributed to drug "A".
Here, more channels of information (e.g. different types of actual patent
pulse wave
measurements, obtained from different sensors) are highly useful. This is
because combining
pulse wave measurements from at least two different types of sensors (e.g. a
plurality of
different types of actual patient pulse wave measurements) reduces noise,
helps confirm
positive signals, and can provide more insight than can any single type of
measurement by
itself.
It should be evident that in the examples above, any two of the various types
of pulse waves
will give better results than one type of pulse wave information. Thus here,
combinations
such as at least ECG and Oscillometric measurements, ECG and pulse oximeter
measurements, or oscillometric and pulse oximeter measurements may be used.
Combinations of three or more types of pulse wave data can be still more
useful and accurate.
Thus in some embodiments, the invention may be a method, device, or system for
determining a patient's adherence to a medication regime. In some embodiments,
the
invention may employ multi-sensor patient operable instrumentation, such as
that previously
described in US patent applications 14/186,151 and 62/138,377. See Figures 9
and 10 for
some examples.
This patient operable instrumentation will typically comprise at least one
computer processor
(microprocessor, microcontroller, etc.), memory (at least one of local or
remote memory) and
various different types of physiological monitoring devices, each configured
to obtain a
plurality of different types of actual patient pulse wave measurements. The
instrumentation
will typically be designed so that it is at least capable of operation by an
average patient in
the absence of a healthcare practitioner. Of course in some cases, this
instrumentation may
11
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
alternatively be operable by an average caregiver who will typically not need
to be a licensed
or professional healthcare practitioner. The idea in any event is to make the
invention's
instrumentation convenient and easy to use so that it is used very frequently.
In a preferred embodiment, this patient operable instrumentation and its
various physiological
monitoring devices will be configured to provide various different types of
actual patent
pulse wave measurements. Preferably at least two different types of pulse wave
measurements will be obtained. For example, these various types of patient
pulse wave
measurements can include oscillometric pulse oximeter data (e.g. pulse waves
that report on
varying blood oxygen saturation levels), electrocardiograph (ECG) readings,
and pulse waves
that report on blood pressure measurements (e.g. oscillometric measurements
from a cuff
type device).
In a preferred embodiment, the patient operable instrumentation will not
consist entirely of
independent stand-alone monitoring devices. Instead at least some and
preferably all of the
monitoring devices will form a unitized system where all devices are managed
by at least one
common processor, preferably at least one common processor local to the
patient operable
instrumentation. Here again, see the patient operable instrumentation shown in
Figures 9 and
as specific examples. This patient operable instrumentation will typically be
referred to as
"the device" for these discussions.
In order to determine if a patient is adhering to a particular medication, the
device, system, or
method will, in addition to patient baseline physiological data (e.g. data in
the absence of
drugs/medication) also need to know the patent's medication schedule
information (e.g. what
drugs is the patient supposed to take, and when) and the expected impact of
these drugs (or at
least the drugs where monitoring is desired) on the various patient
physiological parameters.
Thus the invention will typically be configured to store and retrieve a
variety of different
types of information. This information can include medication schedule
information that
pertains to at least one medication and medication dosing schedule for at
least one given
patient. For the patient shown in Figures 2-6, for example, this can be a list
or simple record
stored in computer memory showing that this patient takes drugs "A", "B", and
"C" on a
daily basis, usually around 9:00 pm. Thus the device, for example, can store
medication
schedule information pertaining to at least one medication and medication
dosing schedule
for that patient.
12
CA 03016315 2018-08-30
WO 2017/151164 PCT/US2016/033784
Table 1: Medication schedule information example
Drug Days Times
A Daily 9:00 pm
Daily 9:00 pm
Daily 9:00 pm
The invention will also typically store a plurality of medication impact
parameters. Here
each individual medication impact parameter provides information on how a
specific
medication alters a specific type of pulse wave measurements. Here various
types of data can
be stored and used depending on if the system processor is going to be using
either an
analytical approach, an empirical approach, or both approaches. An example of
the
medication impact parameters for drugs "A", "13", and "C" on a patent similar
to that shown
in Figures 2-6 is shown below:
Table 2: Medication impact parameters example
Drug A ECG-R A PTT A Reflected A systolic A diastolic
Waveform example(s)
A 0 0 0 -10 mm/Hg -10 mm/Hg Fig. 3 curves
+ 57 ms 0 0 -10 mmflig -10 mm/Hg Fig. 4 curves
0 +40 ins + 50 ms computed computed Fig. 5 curves
In this case, an "individual medication parameter" corresponds to a single row
in the above
list or record stored in memory, and the plurality of medication impact
parameters
corresponds to the above table as a whole.
The invention will also store a plurality of patient reference information,
where each
individual patient reference information provides information on a specific
type of patient
baseline pulse wave measurements in an absence of a specific type medication.
For the
patient in Figure 2, the patient baseline information in the absence of all
medication can
include, for example, all of the information shown or discussed in Figure 2.
According to the invention, at least one processor (preferably at least one
local device
processor, but alternatively also may be a remote processor) is configured so
that when the
patient operable instrumentation is used on the patient, this at least one
processor will analyze
the plurality of different types of actual patient pulse wave measurements.
Here the
13
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
processor will know what time this analysis is done relative to the medication
schedule
information. This time information can be used to adjust the analysis
accordingly. Thus the
processor may be configured so that if the readings are taken just before the
patient was
scheduled to take the medication, then any aberrant readings can be
discounted. However if
the readings are taken at several hours after the medication was expected, and
aberrant
readings are still obtained, then the aberrant results may be given a
correspondingly higher
weighting and be reported accordingly.
The invention will then use at least one processor to determine which of the
various actual
patient pulse wave measurements are inconsistent with calculated patient pulse
wave
measurements that the system would normally expect for that patient. This
calculation is
based on the medication schedule information, the time (here time means time
and the date)
the actual data was taken, the various patient reference information, and the
various
medication impact parameters.
The invention can then do various things with the results, in a preferred
embodiment varying
depending on what is found. The invention may just store the findings in
memory, or inform
the patient, or inform caregivers, or inform relevant healthcare
professionals, or keep human
readable records. At a minimum, however, the invention will least store a
record in the
invention's memory (either local and/or remote) of at least those medications
where
inconsistent findings were found (i.e. evidence that the patient was not
responding to the
medication as would be expected).
In a preferred embodiment, the invention's at least one processor may be
further configured
to provide patient alarm information. This patient alarm information could
report when
various patient physiological parameters, such as blood oxygen saturation,
blood pressure
measurements, or electrocardiograph readings fall outside of previously
established
boundaries. The invention may also be configured so that the device uses this
patient alarm
information, as well as those medications where inconsistent findings were
obtained, to
determine and report that the patient may be out of compliance with taking
these expected
medications.
Value of combining different types of pulse wave measurements:
In a preferred embodiment, the invention will use its at least one processor
to determine if
inconsistent findings were obtained over more than one different type of
actual patient pulse
wave measurements. For example, do the oscillometric cuff blood pressure and
pulse
14
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
oximeter finds agree? Are any of these results consistent with the ECG
results? Here the
invention can be configured so when at least two different types of actual
patient pulse wave
measurements both report inconsistent findings (i.e. both report a possible
medication
adherence problem, so both are consistent with each other), the device can
more definitively
determine and report that the patient may be out of compliance with taking
those particular
medications.
Methods of obtaining medication impact parameters:
In general, any method of obtaining the medication impact parameters may be
used. Here,
however, although the gold standard would almost certainly be experimenting on
the patient
by selectively withholding all medications, and then introducing one at a
time, and
monitoring results, this method will often not be practical. This is because
both doctors and
patients may (quite properly) object to deliberately withholding important
medication. Thus
various substitute methods will often be needed in order to obtain the
medication impact
parameters in a safer and more ethically responsible way.
Often methods that attempt to estimate the mediation impact parameters based
on the patients
data in the known presence of adequate medication, as corrected by the typical
known effects
of a given medication may be used. Here the probable changes in the various
patient
waveforms caused by the various drugs can be calculated, and these values used
for the
medication impact parameters. Not as good as performing the unethical
experiment, but
better than nothing.
In some embodiments, these various medication impact parameters can be
obtained by taking
averages over a plurality of similar type patients in the presence and absence
of a given
medication (here results from clinical studies may be used), and the
differences between the
various pulse waveforms in the presence and absence of a given medication can
be calculated
based on such clinical study data. Again, not as good as performing unethical
experiments on
the actual patient being monitored, but better than nothing.
Similarly, the various types of patient reference information (e.g. various
pulse wave
waveforms in the absence of drugs) may be obtained by averaging over a
plurality of similar
type patients in the absence of the various medications. Here again, data from
clinical studies
may be used. Alternatively, various mathematical models may be used. Again,
not as good
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
as performing unethical experiments on the actual patient being monitored, but
better than
nothing.
As will be described later on in this disclosure, the invention may also be
configured so as to
take advantage of "natural experiments" when the patient has forgotten to take
one or more
medications to ethically obtain more accurate patient reference information.
In some embodiments, the invention may be configured so that the device's at
least one
processor calculates the expected patient pulse wave measurements from the
previously
stored medication schedule information (and the time), plurality of patient
reference
information, and plurality of medication impact parameters. Here the invention
can use its
least one processor to transform the plurality of patient reference
information into the
expected patient pulse wave measurements by various mathematical operations.
For each
individual medication (as expected from the medication schedule information),
these
operations can include:
1: selecting corresponding individual medication impact parameters from list
or record of
various medication impact parameters, thereby retrieving the selected
individual medication
impact parameters.
2: Applying (using either analytical methods based on underlying equations
that attempt to
reproduce aspects of the patients physiology, or by empirical curve fitting
methods), these
selected individual medication impact parameters to the various patient
reference
information, thereby producing intermediate transformed patient reference
information. For
example, if the patient reference information is as shown in Figure 2, then
applying drug "C"
to this patient reference information would produce the results shown in
Figure 5, and so on.
3: Repeating the above steps until all of the individual medication (drugs) in
the medication
schedule information has been processed, thereby producing the expected
patient pulse wave
measurements. For example, going down through the list, and applying drug "A",
drug "B",
and drug "C" to the patient reference data from Figure 2, thus producing
Figure 6. If the
actual patient data resembles Figure 6, and the timing is appropriate, then
the patient is
probably taking all three drugs. If the actual patient data does not match,
the invention can
then alternatively try further by seeing if any partial combination of drugs
fits the observed
data. The system can then report on its findings (based on which combination
of expected
drugs best fits the observed data, and which deficiency in the combination of
expected drugs
best fits the observed data.)
16
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
Other types of medication and patient monitoring sensors:
Although for simplicity, much of the discussion so far has focused on pulse
wave
measurements and anti-hypertension drugs, it should be evident that the
concepts disclosed
herein may be used for a broader range of patient physiological measurements
and a broader
range of drugs as well,
In some embodiments, the invention may be further configured with additional
types of
sensors. These sensors could include one or more additional types of sensors
such as body
temperature sensors, patent motion sensors (e.g. accelerometers), lung
function sensors (e.g.
microphones, spirometers), ECG electroencephalographic (EEG) sensors, and the
like. In
these embodiments, the various categories of stored information would be
extended to
accommodate these additional types of physiological data, drug types, and
medication impact
data.
Thus, for example, the patient reference information data would be extended to
further
comprise baseline body information pertaining to these additional types of
sensors. This can
be, for example, at least one of baseline body temperature information,
baseline patient
motion data, baseline patient lung status data, or baseline patient EEG data.
Similarly the individual medication impact parameters can be extended to
further provide
information on how a specific medication alters at least one of these
additional types of
sensor data, such as baseline body temperature information, baseline patient
motion data,
baseline patient lung function, and patient baseline EEG data.
In these embodiments, the invention's at least one processor can be further
configured to use
this (suitably extended) medication schedule information, the known time of
data acquisition,
the various patient reference information and at least some of the individual
medication
impact parameters to further determine if at least some of these additional
(e.g. non-
hypertension) medications are also producing inconsistent findings (e.g.
provide evidence
that the patient is also not adhering to this additional medication as well).
To better visualize these various steps, processes, and methods, consider
Figure 7. Figure 7
shows a flow chart of some of the various steps that may be carried out by the
device's
processor. Here the patient's various medication impact parameters (702),
which report on
how each individual medication taken by the patent alters a specific type of
pulse wave
measurements, are stored in memory. Additionally, patient reference
information (704),
which provides information on an individual patents pulse wave measurements in
the absence
17
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
of either all medications, (exemplified by Figure 2), or in the absence of at
least some
medications, are also stored in memory. The patient's medication schedule
information
(reporting on which medications the patient is taking, and when (e.g. the
dosing schedule) is
also stored in memory (706).
When the patient operable instrumentation and its plurality of physiological
monitoring
devices (here exemplified by patient pulse wave data such as rapidly time
varying blood
oxygen saturation levels (e.g. using a pulse oximeter type sensor), blood
pressure readings
(e.g. using an oscillometric type blood pressure sensor), and
electrocardiograph readings (e.g.
using one or more ECG electrodes) is taken, this data is also stored in memory
as well (708)
as a series of different types of pulse wave measurements.
Once the patient pulse wave measurements (data) have been obtained, the
invention will then
use at least one processor to analyze this data. As previously discussed,
there are two general
approaches that can be used here, and the invention may use either approach or
both
approaches.
In a first, more analytical approach, the invention's processor(s) will
attempt to analyze the
various patient pulse wave measurements according to one or more analytical
models. Using
cardiac pulse waves as an example, examples of such analytical models can
include, but are
not limited, to models such as Moens-Korteweg equation, the Bramwell-Hill
equation, the
Waterhammer equation, Windkessel theory, and the like. The invention's
processor's may
attempt to obtain fundamental values such as the time differences between
cardiac forward
waves and reflected waves, systolic and diastolic blood pressure, pulse
transit times (PTT),
pulse wave velocities (PWV) and the like. This is shown in (710).
In a second, more empirical approach, the invention's processor(s) attempt to
analyze the
various patient pulse wave measurements according to a best fit to underlying
basis curves
approach (712). Here, the invention will draw upon various underlying pulse
wave curves
that describe the patient's pulse waves according to the effect of each
different type of drug
on that patient's baseline (e.g. in the absence of all drugs) profile. When
this option is used,
the underlying patient pulse waves may be stored in memory as well. Here, for
example, the
patient baseline curves may be stored as either part of the patient reference
information (704),
while the empirical impact of various drugs on that patient's various types of
pulse waves
may be stored along with the medication impact parameters (702). Other data
storage
schemes can also be used.
18
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
Although the invention may use either the model based (710) or the empirical
approach (712)
on a stand-alone basis, in a preferred embodiment, which may have the
advantages of being
more noise resistant in some cases, the invention may determine which approach
fits the
underlying data best, and then combine the results from both approaches to
produce a
weighted average of the two. Here, the approach that fits the underlying data
the best (best fit
may be determined by a least squares approach or other best fit determination
algorithm) may
be given a higher weight. This optional but preferred embodiment is shown as
(714).
Using either approach, the invention will then determine which likely
mediation mix best fits
the available pulse wave data (716).
In step (718), the invention's processors will then compare the observed
medication mix
information (716) with the medication mix expected from the patient's
medication schedule
information (706). The processor may employ various rules or rubrics to help
implement this
comparison process. For example, the processor may use rules such as, "the
patient is likely
to take all medications at once, or skip all mediations" in this analysis. The
medication
impact parameter information (706) may further contain information regarding
the half-lives
of the various drugs. Thus, for example, if a patient stopped taking all
medication, the effects
of the drug with the shortest half-life would diminish first, and the effects
of the drugs with
longer half-lives would diminish later.
If in step (718), the pulse wave results are clearly inconsistent with the
medication schedule
information (706), then the analysis can clearly determine already that there
is a problem, and
report it at step (720) (or at least store this in memory, and preferably also
notify a person or
machine).
In some embodiments, the invention may further double check its apparently OK
results,
and/or or alternatively report other types of potential mediation problems. In
these
embodiments, the invention may often monitor other types of physiological
parameters as
well. For example, the patient operable instrumentation may be further
configured to include
other types of sensors, such as accelerometers (particularly useful for
patient worn
instrumentation), patient temperature sensors, electroencephalographic (EEG)
reading
sensors, breathing status sensors (stethoscope-like microphones and sound
analysis
algorithms, spirometer sensors), and the like. This other data from the other
types of sensors
will be stored in memory as (722).
19
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
In this "extended other data- embodiment, the patient reference information
(704) may
further include information pertaining to this "other data", such as
information pertaining to
the patient's typical motion (e.g. does the patient tend to shake or move in
an atypical
manner), typical body temperatures, typical EEG waveforms (e.g. example
baseline
waveform for a patient with epilepsy), breathing parameters (e.g. respiratory
data pertaining
to conditions such as asthma) and the like. This can not only act as a double
check to help
insure that any cardiovascular drugs are being adequately monitored, but can
also be used to
extend the variety of different types of drugs and medical conditions that can
be successfully
monitored by the invention.
Here the medication impact parameters (702) and medication schedule
information will also
typically be extended to report on the impact of these other types of
medications on the
patient, as well as report on the schedule by which the patient takes such
other types of drugs.
For example, consider a patient with epilepsy or Parkinson's disease. Here,
improper
epilepsy or Parkinson's medication may not show up in the patient's pulse wave
measurements, but may show up as either abnormal motion data (e.g. patient
trembling,
patient abnormal motion) or as abnormal EEG readings, or even abnormal
temperature
readings.
At the same time, patients with other ailments, such as epilepsy or
Parkinson's disease, are
hardly immune from common high blood pressure and other cardiac difficulties.
Thus in
some embodiments, it is useful to have the same patient operable
instrumentation monitor
drugs directed towards completely different types of disease states.
In some other types of disorders, in particular breathing or lung disorders
(e.g. (asthma,
chronic obstructive pulmonary disease, other chronic lung disorders), the
underlying medical
condition and the various medications used to treat the lung disorder, can
interrelate with the
patient pulse wave measurements. Thus in these embodiments of the invention,
the invention
will also simultaneously evaluate these other factors as well.
Consider the case where the patient's other (non-pulse wave) data (722) is
also inconsistent
with the patient's typical reference information (704) for this other type of
(non-pulse wave
data). This consistency can be checked at step (724). The relationship between
findings
obtained from this other (non-pulse wave) data at step (724) and the pulse
wave data findings
(718), and the medication schedule information (706) can help with the
analysis of both types
of data.
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
For example, given that on a statistical basis, a patient that has forgotten
one type of
medication is also more likely to forget to take another type of medication;
findings of
abnormal pulse wave values tend to make other types of abnormal data more
significant.
Similarly findings of abnormal non-pulse wave data tend to make findings of
abnormal pulse
wave data more significant.
Due to measurement noise and general physiological variability, often the
invention's
methods may not produce results are entirely clear cut. Instead the invention
will typically
obtain compliance data that is best expressed in a more probabilistic numeric
score, in which
the system may judge, for example, that the probability that a patient is in
compliance with a
given drug, such as drug "C", at any time is a probability number, such as
70%.
In some embodiments, data obtained from the non-pulse wave "other" data may be
used to
change the weighting or significance level that the system uses to interpret
its findings and
report results. A patient with a borderline pulse and blood pressure data, as
obtained from the
pulse wave data, may be more likely to be scored as non-compliant with the
medication
schedule information if the patient is also exhibiting breathing problems, and
vice versa.
Thus in some embodiments, it may be useful to compare findings between the
pulse wave
and non-pulse wave wings of the analysis and use this comparison to alter
(728) the
significance threshold (726) by which the system reports problematic findings.
This option
(728) may be most useful when the significance of any abnormal findings is
somewhat
uncertain. If it is very clear that abnormal patient physiological parameters
are being
detected by the "other data" wing (722, 724), then these clearly abnormal
results may be
reported immediately (730). Here, the medication schedule information (706)
may be used to
suggest which medications may be at issue here.
Reporting findings, and interfacing with remote network connected devices:
In some embodiments, the invention may further be configured with means (e.g.
touch
screens, buttons, voice interface, network interface) to receive compliance
information from
the patient. Here the patient (or caregiver) can also report on periods when
it is known that
the patient was out of compliance with at least one known medication. This in
essence
constitutes an ethical "natural experiment" that can be used to obtain further
calibration
information for the invention. To take advantage of this ethical "natural
experiment', the
invention's at least one processor can be further configured to use this
compliance (or lack of
compliance) information to select at least some of the actual patient pulse
wave
21
WO 2017/151164
PCT/US2016/033784
measurements for use in establishing or refining at least some of the
patient's medication
impact parameters and/or the patient reference information.
Say that the patient has forgotten all medications for several days. Here the
system can at
least take advantage of this unfortunate fact and gather more accurate patient
reference
information.
In some embodiments, the invention may be further configured with network
interface means
(e.g. WiFi or Bluetooth(tm) or wired or other type of computer network
interface) to allow
the invention to connect with at least one remote computerized device over a
network
(preferably a computer network). Here the invention's at least one processor
can be further
configured to report at least some of those medications where inconsistent
findings were
obtained to a remote computerized device (e.g. a caretaker device, a patient
smartphone
tablet, or computer device, a healthcare professional computerized device, and
so on).
In these embodiments, the invention's at least one processor can be further
configured to
enable any of the various medication impact parameters, patient reference
information, and
medication schedule information to be uploaded or downloaded over a network
from a
remote computerized device. Here, for example, a healthcare professional, upon
prescribing
a new medication, might also contact the invention and upload the medication,
preferred use
schedule, and medication impact information to the invention so that patient
compliance can
then be monitored.
Examples of patient operable instrumentation:
Figure 8 shows an example of one type of patient operable instrumentation that
may be used
according to the present invention. This type of device is discussed in more
detail in
copending application 141186,151 and its provisional application 61;767,839 .
Figure 9 shows an example of a different type of patient operable
instrumentation that may be
used according to the present invention. In this embodiment, the patient
operable
instrumentation is intended to be worn by the patient over a period of time.
This type of
device is discussed in more detail in provisional patent application
62/138,377. This device
(900) can comprise an optional ear attachment device (902) that may have any
of a
temperature sensor and a pulse oximeter sensor. The device can also comprise a
neck
mounted device (904) that may contain an ECG sensor, batteries, and a computer
processor.
This neck mounted device may also comprise other types of sensors such as
accelerometers,
22
Date Recue/Date Received 2021-10-07
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
lung function sensors (e.g. microphones and audio processing circuitry to
provide stethoscope
like lung function assessment), and the like.
In some embodiments, other types of devices may also be attached to the neck
mounted
device. These other devices may include arm connectors (906) and armature
wires that
extend down to patient worn ECG electrodes (908), and the like.
User medication adherence methods, and Machine learning methods:
In some embodiments, the invention may be configured to receive input from the
user
pertaining to which medications the user believes he or she has taken. Here,
the invention
may query the user regarding these medications (usually via a graphical user
interface such as
a touch sensitive display, or other methods such as audio queries and machine
language
recognition of audio responses).
For example, the invention may ask the user questions or surveys such as: "Did
you take
your medication [here the system may also provide an optional list of
medications] on time
today?" and give appropriate statistical weight to the user's responses. Here,
for example, a
"no" answer or a "non-response" answer might make the system give higher
weight to the
possibility that the user (patient) is not properly adhering to his or her
medication schedule.
Similarly, the invention may also be configured to receive input relating to
other indicia of
user/patient cooperation with healthcare professional recommendations. For
example, input
pertaining if the patient is adhering to other parts of the user/patient's
health care plan, such
as taking vital sign readings, adhering to scheduled meetings/consultations,
providing on-time
answers to questionnaires, and the like can also provide further information
as to that
user/patient's likely adherence to their medication schedule.
Thus, for example, in situations where the physiological readings as to
medication adherence,
such as in Figure 7 steps (724 and 726) are unclear, evidence that the patient
has indicia of
not cooperating with other healthcare recommendations may be used to alter the
significance
levels at which medication non-adherence is reported as being a potential
problem.
Additionally, user surveys or behavioral data with regards to other aspects of
healthcare
compliance can also be used to extend the utility of the invention for a
broader range of
medications. This user survey or behavioral data may, for example, be
particularly useful for
patents (e.g. psychiatric patients) that may also be on medication that may
not produce
obvious changes in pulse wave data, for example.
23
CA 03016315 2018-08-30
WO 2017/151164
PCT/US2016/033784
Additionally, in some embodiments, various types of machine learning methods
may also be
used to enhance the utility of the invention. Examples of such machine
learning methods
include supervised learning approaches, such as the k-nearest neighbors
algorithm (KNN)
(see N. S. Altman, -An Introduction to Kernel and Nearest-Neighbor
Nonparametric
Regression", The American Statistician 46(3), 1992, pages 175-185). Other
machine
learning methods, such as artificial neural networks (ANNs) methods (Ganesan
et. al.,
"Application of Neural Networks in Diagnosing Cancer Disease Using Demographic
Data",
International Journal of Computer Applications (0975 - 8887) Volume 1 - No.
26, 2010); use
of support vector machines or support vector networks (SVMs), (Cortes and
Vapnick,
"Support-vector networks", Machine Learning September 1995, Volume 20, Issue
3, pp 273-
297) and the like may also be used.
These machine learning models may be pre-trained using data from
investigational studies.
Alternatively or additionally, the input for these supervised machine learning
techniques can
be obtained from various combinations of the patient or user input, including
healthcare
professional input, and/or from the patient caretaker input as well.
In some embodiments, these machine learning models will also employ features
that could
include any combination of the inputs gathered within the invention, such as
the various
physiological signals (or derived metrics), patient alarms, medication
scheduling, patient
demographic information, and the like.
The machine learning models (or indeed the invention in general) may in turn
provide various
types of output pertaining to patient medication adherence. In some
embodiments, the output
may be represented as a binary output (e.g. an automated "yes"/"no" assessment
as to
medication adherence).
In other embodiments, the learning models (or again, the invention in general)
may provide
multi-value outputs pertaining to patient medication adherence. For example,
the patient may
be scored on a non-binary scale (such as very compliant, moderately compliant,
and non-
compliant). In other embodiments, the learning models (or again, the invention
in general)
may provide numerical scores as to likely patient compliance (e.g. a 0.0-10.0
score, such as
9.8 out of 10.0). Other compliance output ratings, such as graphical or
calendar data that
show likely periods of compliance and non-compliance, and the like may also be
provided.
In general, any graphical, alphanumeric, sound or even tactile output
pertaining to patient
medication compliance may be provided by the invention.
24