Note: Descriptions are shown in the official language in which they were submitted.
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
1
SMOKELESS ORAL TOBACCO PRODUCT AND PREPARATION THEREOF
Field
The present invention relates to a smokeless oral tobacco product and methods
of making
the same.
Background
Smokeless oral tobacco products comprise smokeless materials, such as
smokeless
tobacco, that are designed to be placed in the oral cavity of a user for a
limited period of
time. Smokeless oral tobacco products include snuff, which can be provided in
dry or moist
form. Smokeless oral tobacco products can be portioned or non-portioned.
In some embodiments, the present invention seeks to provide an improved
smokeless oral
tobacco product and a method for the production thereof.
Summary
In accordance with some embodiments described herein, a smokeless oral tobacco
product
is provided comprising a tobacco material and a particulate material which is
other than
tobacco, the particulate material having the following properties:
i) a mass median particle size measured by sieve analysis of from about 0.3 mm
to about 3
mm;
ii) a bulk density of less than about 0.6 g/cm3; and
iii) a combined starch and sugar content of less than about 7% based on the
weight of the
particulate material.
In accordance with some embodiments described herein, a method is provided for
the
production of a smokeless oral tobacco product comprising a tobacco material
and a
particulate material which is other than tobacco, the particulate material
having the following
properties:
i) a mass median particle size measured by sieve analysis of from about 0.3 mm
to about 3
mm;
ii) a bulk density of less than about 0.6 g/cm3; and
iii) a combined starch and sugar content of less than about 7% based on the
weight of the
particulate material, the method comprising:
(a) providing a tobacco material;
(b) processing the tobacco material; and
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
2
(c) adding the particulate material to the tobacco material either prior to
the
processing step (b), during the processing step (b) or after the processing
step (b),
wherein the tobacco material comprises tobacco, or a tobacco replacement or
substitute.
In accordance with some embodiments described herein, a consumer package is
provided
comprising the smokeless oral tobacco product according to the first aspect or
produced by
a method according to the second aspect of the invention.
In accordance with some embodiments described herein, there is provided the
use of a
particulate material which is other than tobacco, the particulate material
having the following
properties:
i) a mass median particle size measured by sieve analysis of from about 0.3 mm
to about 3
mm;
ii) a bulk density of less than about 0.6 g/cm3; and
iii) a combined starch and sugar content of less than about 7% based on the
weight of the
particulate material
for improving mouthfeel of a smokeless oral tobacco product.
In accordance with some embodiments described herein, there is provided the
use of a
particulate material which is other than tobacco, the particulate material
having the following
properties:
i) a mass median particle size measured by sieve analysis of from about 0.3 mm
to about 3
mm;
ii) a bulk density of less than about 0.6 g/cm3; and
iii) a combined starch and sugar content of less than about 7% based on the
weight of the
particulate material
for reducing irritation at the back of the throat of a user during use of a
smokeless oral
tobacco product.
In accordance with some embodiments described herein, there is provided the
use of a
particulate material which is other than tobacco, the particulate material
having the following
properties:
i) a mass median particle size measured by sieve analysis of from about 0.3 mm
to about 3
mm;
ii) a bulk density of less than about 0.6 g/cm3; and
iii) a combined starch and sugar content of less than about 7% based on the
weight of the
particulate material
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
3
for maintaining in use structure of a smokeless oral tobacco product.
Brief Description of the Figures
Embodiments of the present invention are described, by way of example only,
with reference
to the accompanying drawings in which:
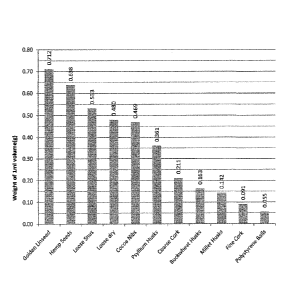
Figure 1 shows a chart that includes the densities measured for a number of
different
materials. The weight of the materials is plotted on the y-axis, where the
weight was
measured per cm3 of product.
Figure 2 shows an exemplary test sieving equipment for measuring mass median
particle
size of the particulate material.
Figure 3 shows exemplary settings for the test sieving equipment for measuring
mass
median particle size of the particulate material.
Figure 4 shows a graph of percentage cumulative mass of cork against mesh size
of the
sieves in an exemplary sieve analysis method of Example la for measuring mass
median
particle size of cork.
Figure 5 shows a graph of percentage cumulative mass of millet husks against
mesh size of
the sieves in an exemplary sieve analysis method of Example lb for measuring
mass
median particle size of millet husks.
Figure 6 shows a comparison of a smokeless oral tobacco product which is tin
comprising
20g standard white snus together with the pouched products shown above
(right), and a
sample comprising cork which is a tin comprising 10g cork snus together with
the pouches
products shown above (left).
Detailed Description
The present invention relates to a smokeless oral tobacco product.
The "particulate material" for use in the present invention is a particulate
material which is
other than tobacco. Thus, as will be understood by one skilled in the art, the
smokeless oral
tobacco product includes tobacco and particulate material as defined herein,
wherein the
particulate material is other than tobacco.
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
4
"Smokeless tobacco product" is used herein to denote any tobacco product which
is not
intended for combustion.
"Smokeless oral tobacco product" is used herein to denote any smokeless
tobacco product
designed to be placed in the oral cavity of a user for a limited period of
time, during which
there is contact between the user's saliva and the product. The term
"smokeless oral
tobacco product", as used herein, does not include tobacco heating products.
A smokeless oral tobacco product can be provided to the user in a portioned or
a non-
portioned format. Portioned smokeless oral tobacco products can reduce or
eliminate the
handling of the tobacco by the user, which can offer significant advantages in
terms of better
hygiene, convenience and/or ease of use.
In some embodiments, the smokeless oral tobacco product of the present
invention is a
portioned product.
The smokeless oral tobacco product comprises a tobacco material.
"Tobacco material" as used herein includes a material which comprises tobacco
and/or a
tobacco replacement or substitute. In some embodiments, the tobacco material
comprises
tobacco. In some embodiments, the tobacco material is tobacco. In some
embodiments,
the tobacco material comprises a tobacco replacement or substitute. In some
embodiments,
the tobacco material is a tobacco replacement or substitute.
"Tobacco" as used herein includes any part, such as the leaves, flowers, or
stems, of any
member of the genus Nicotiana and reconstituted materials thereof. In some
embodiments,
it includes treated tobacco. In some embodiments, it includes derivatives such
as specific
compounds found in natural tobacco, such as nicotine, whether extract or
synthesized, as
well as structural derivatives such as the fibrous portion of a tobacco leaf.
The term
"tobacco" as used herein includes tobacco extract.
The term "tobacco replacement or substitute" as used herein includes tobacco
substitutes
which comprise individual chemicals and/or complex chemical entities which,
when
appropriately prepared, physically resemble natural tobacco. Alternatively or
in addition, the
term "tobacco replacement or substitute" as used herein includes materials
which deliver
nicotine to the user and provide a similar mouthfeel to tobacco.
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
When the tobacco comprises plant material, defined amounts of the different
parts of the
plant may be used. For example, the amount of stem in the tobacco blend may be
up to
50%, up to 60%, or up to 70% by weight of the tobacco. In some embodiments,
the amount
of stem in the tobacco is from 5% to 70% by weight of the tobacco, such as
from 10% to
5 65% by weight of the tobacco, such as from 15% to 65% by weight of the
tobacco, such as
from 20% to 60% by weight of the tobacco, such as from 25% to 55% by weight of
the
tobacco, such as from 30% to 50% by weight of the tobacco.
Tobaccos used in the present invention may include types of tobaccos such as
dark air-
cured tobacco, flue-cured tobacco, Burley tobacco, Oriental tobacco, Maryland
tobacco, dark
tobacco, dark-fired tobacco and Rustica tobaccos, as well as other rare or
specialty
tobaccos.
In some embodiments, the tobacco is ground tobacco and/or is in particulate
form. In some
embodiments, the tobacco is not in the form of strands or cut lamina.
In some embodiments, the tobacco may be snuff in dry or moist form. "Snuff" is
used herein
to generally describe a class of smokeless tobacco product which typically
comprises cured
tobacco which has been dried and ground to have mass median particle size
measured by
sieve analysis of between 0.01 and 5mm, such as between 0.01 and 3mm, such as
between
0.01 and 1.0 mm.
In some embodiments, the tobacco may be dry snuff. In some embodiments, the
moisture
content of the tobacco is less than 16% by weight of the total smokeless oral
tobacco
product, such as less than 12% by weight of the total smokeless oral tobacco
product, such
as less than 10% by weight of the total smokeless oral tobacco product, such
as less than
5% by weight of the total smokeless oral tobacco product, such as less than 3%
by weight of
the total smokeless oral tobacco product, such as less than 2% by weight of
the total
smokeless oral tobacco product, such as less than 1% by weight of the total
smokeless oral
tobacco product.
In some embodiments, some or all of the tobacco is in moist form. The moist
tobacco may
be in any form that is suitable for incorporation into a smokeless oral
tobacco product. In
some embodiments, the moist tobacco comprises moist snuff.
In some embodiments, the moist snuff comprises Swedish-style snuff, which may
also be
referred to as snus-style tobacco or snus. In some embodiments, the moist
snuff is
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
6
Swedish-style snuff (snus). Snus is a moist powder tobacco product originating
from a
variant of dry snuff. As used herein, snus is an oral tobacco product which is
not fermented,
but is rather heat-treated, such as by pasteurisation. Snus is typically used
by placing it
under the upper lip for extended periods of time.
In some embodiments, the moisture content of the tobacco is at least 20% by
weight of the
total smokeless oral tobacco product, such as at least 25% by weight of the
smokeless oral
tobacco product, such as at least 30% by weight of the smokeless oral tobacco
product,
such as at least 35% by weight of the smokeless oral tobacco product, such as
at least 40%
by weight of the smokeless oral tobacco product, such as at least 45% by
weight of the
smokeless oral tobacco product, such as at least 50% by weight of the
smokeless oral
tobacco product, such as at least 60% by weight of the smokeless oral tobacco
product.
In embodiments in which the smokeless oral tobacco product comprises snus, the
snus may
comprise salt and/or other flavourants. Alternatively, or in addition, the
snus may be
pasteurised or may undergo a process similar to pasteurisation and may
optionally be
matured, to reach the desired pH and/or moisture content of the snus. Methods
and
apparatus suitable for pasteurisation and maturation are known to the person
skilled in the
art.
Alternatively or in addition, the moist snuff may be in the form of dipping
tobacco. In
embodiments in which the smokeless oral tobacco product comprises dipping
tobacco, the
dipping tobacco may be treated by fermentation or may undergo a process
similar to
fermentation and may optionally undergo one or more further processes such as
aging.
Methods and apparatus suitable for the treatment of dipping tobacco are known
to the
person skilled in the art.
Alternatively or in addition, the tobacco may be in the form of US moist snuff
and/or chewing
tobacco.
The amount of tobacco within the tobacco formulation may vary. In some
embodiments, the
amount of tobacco within the smokeless oral tobacco product is at least about
5% by weight
of the smokeless oral tobacco product, such as at least about 10% by weight of
the
smokeless oral tobacco product, such as at least about 15% by weight of the
smokeless oral
tobacco product, such as at least about 20% by weight of the smokeless oral
tobacco
product, such as at least about 25% by weight of the smokeless oral tobacco
product, such
as at least about 30% by weight of the smokeless oral tobacco product, such as
at least
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
7
about 40% by weight of the smokeless oral tobacco product, such as at least
about 50% by
weight of the smokeless oral tobacco product, such as at least about 55% by
weight of the
smokeless oral tobacco product, such as at least about 60% by weight of the
smokeless oral
tobacco product.
In some embodiments, the amount of tobacco within the smokeless oral tobacco
product is
no greater than about 90% by weight of the smokeless oral tobacco product,
such as no
greater than about 85% by weight of the smokeless oral tobacco product, such
as no greater
than about 80% by weight of the smokeless oral tobacco product, such as no
greater than
about 75% by weight of the smokeless oral tobacco product, such as no greater
than about
70% by weight of the smokeless oral tobacco product.
In some embodiments, the amount of tobacco within the smokeless oral tobacco
product is
between about 20% and 90% by weight of the smokeless oral tobacco product,
such as
between 20% and 85% by weight of the smokeless oral tobacco product, such as
between
about 20% and 80% by weight of the smokeless oral tobacco product, such as
between
about 20% and 75% by weight of the smokeless oral tobacco product, such as
between
about 20% and 70% by weight of the smokeless oral tobacco product, such as
between
about 20% and 65% by weight of the smokeless oral tobacco product, such as
between
about 20% and 60% by weight of the smokeless oral tobacco product, such as
between
about 25% and 60% by weight of the smokeless oral tobacco product, such as
between
about 30% and 60% by weight of the smokeless oral tobacco product, such as
between
about 35% and 60% by weight of the smokeless oral tobacco product.
In some embodiments, the smokeless oral tobacco product can be provided to the
user in a
portioned format. In some known portioned smokeless oral tobacco products, the
tobacco
material is surrounded by a pouch. For example, a common method of providing
moist snuff
is to seal the tobacco material in a permeable pouch.
A pouch holds the tobacco material in place, while at the same time allowing
substances
such as flavours and nicotine to diffuse through the pouch and into the mouth
of the user for
absorption through the user's mucous membranes.
In some embodiments, the smokeless oral tobacco product can be provided to the
user in a
non-portioned format. In one embodiment, the smokeless oral tobacco product is
packaged
in loose form in a container, such as a can, sachet or tin.
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
8
In some embodiments, the smokeless oral tobacco product can be provided to the
user in a
preformed format, such as that described in EP 2649889. Such a format
typically does not
require the use of pouches to contain the product, but may still be portioned.
In addition to a tobacco material, the smokeless oral tobacco product
comprises a particulate
material. The particulate material in accordance with the present invention
has the following
properties:
i) a mass median particle size measured by sieve analysis of from about 0.3 mm
to about 3
mm;
ii) a bulk density of less than about 0.6 g/cm3; and
iii) a combined starch and sugar content of less than about 7% based on the
weight of the
particulate material.
As used herein, the term "particulate" means that the average length to width
ratio of
particles of the material is less than 2:1, such as less than 1.5:1, such as
about 1:1. In some
embodiments the particles of the particulate material are substantially
spherical. In some
embodiments, the particulate material is granular.
In some embodiments, the particulate material has a mass median particle size
measured
by sieve analysis of from about 0.3 mm to about 2.5 mm, such as from about 0.3
mm to
about 2 mm, such as from about 0.3 mm to about 1.5 mm, such as from about 0.3
mm to
about 1 mm, such as from about 0.3 mm to about 0.7 mm.
In some embodiments, the particulate material has a mass median particle size
measured
by sieve analysis of from about 0.4 mm to about 2.5 mm, such as from about 0.4
mm to
about 2 mm, such as from about 0.4 mm to about 1.5 mm, such as from about 0.4
mm to
about 1 mm, such as from about 0.4 to about 0.7 mm.
As used herein, the term "mass median particle size" is defined as the size at
which one half
of the total mass of all particles in a sample is contributed by particles
with a size smaller
than the mass median particle size, and one half of the total mass of all
particles in a sample
is contributed by particles with a size larger than the mass median particle
size.
The mass median particle size of the particulate material may be measured by
sieve
analysis. As the skilled person will readily appreciate, sieve analysis
(otherwise known as a
gradation test) is a method used to measure the particle size distribution of
a particulate
material. Typically, sieve analysis involves a nested column of sieves which
comprise
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
9
screens, preferably in the form of wire mesh cloths. A pre-weighed sample may
be
introduced into the top or uppermost sieve in the column, which has the
largest screen
openings or mesh size (i.e. the largest pore diameter of the sieve). Each
lower sieve in the
column has progressively smaller screen openings or mesh sizes than the sieve
above.
Typically, at the base of the column of sieves is a receiver portion to
collect any particles
having a particle size smaller than the screen opening size or mesh size of
the bottom or
lowermost sieve in the column (which has the smallest screen opening or mesh
size).
In some embodiments, the column of sieves may be placed on or in a mechanical
agitator.
The agitator causes the vibration of each of the sieves in the column. The
mechanical
agitator may be activated for a pre-determined period of time in order to
ensure that all
particles are collected in the correct sieve. In some embodiments, the column
of sieves is
agitated for a period of time from 0.5 minutes to 10 minutes, such as from 1
minute to 10
minutes, such as from 1 minute to 5 minutes, such as for approximately 3
minutes.
Once the agitation of the sieves in the column is complete, the material
collected on each
sieve is weighed. The weight of each sample on each sieve may then be divided
by the total
weight in order to obtain a percentage of the mass retained on each sieve.
In some embodiments, the mass median particle size of the particulate material
may be
measured as follows:
= the cumulative mass of the sample of particulate material is measured
before sieving
of the sample;
= after sieving of the sample using the method described above, the
percentage of
aggregate particulate material retained in each sieve is calculated using the
following equation:
MSieve
%Retained = x100%
MTotal
where Msieve is the mass of the aggregate material in the sieve, and NATotal
is the
cumulative mass (or total mass) of the whole sample;
= the cumulative percentage of aggregate sample retained in each sieve is
then
calculated by adding up the total amount of aggregate material that is
retained in
each of the previous sieves (i.e. each of the sieves above the relevant
sieve). The
cumulative percentage of the aggregate sample retained in each sieve is found
by
subtracting the relative percentage retained in each of the previous sieves
from
100%, as follows:
%Cumulative = 100% ¨ %Retained on previous sieves
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
e a graph of percentage cumulative mass is then plotted on the y-axis
against sieve
size on the x-axis;
= the mass median particle size is then determined by calculating the value
of x when
y=50% (i.e. when percentage cumulative mass is 50%).
5
As the skilled person will readily appreciate, the screen opening sizes or
mesh sizes for each
sieve in the column used for sieve analysis may be selected based on the
granularity or
known maximum/minimum particle sizes of the sample to be analysed.
10 In some embodiments, a column of sieves may be used for sieve analysis,
wherein the
column comprises from 2 to 20 sieves, such as from 5 to 15 sieves. In some
embodiments,
a column of sieves may be used for sieve analysis, wherein the column
comprises 10
sieves. In some embodiments, the screen opening or mesh sizes of the 10 sieves
may be
as follows for determining the mass median particle size of the particulate
material:
Sieve Number Mesh Size (pm)
1 1000
2 900
3 800
4 710
5 600
6 500
7 400
8 180
9 90
10 <90
As used herein, the density of the particulate material is defined as the bulk
density of the
material. As used herein, bulk density is defined as the mass of a number of
particles of the
particulate material divided by the total volume they occupy. As used herein,
the term "bulk
density" refers to the untapped or freely settled bulk density. In other
words, bulk density is
measured as the untapped density (i.e. before any specified compaction
process, which may
involve vibration of the container).
In some embodiments, the particulate material has a bulk density of less than
about 0.55
g/cm3, such as less than about 0.5 g/cm3, such as less than about 0.45 g/cm3,
such as less
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
11
than about 0.4 g/cm3, such as less than about 0.35 g/cm3, such as less than
about 0.3
g/cm3, such as less than about 0.25 g/cm3, such as less than about 0.2 g/cm3,
such as less
than about 0.15 g/cm3.
As used herein, the term "starch" is defined as a carbohydrate comprising at
least 200
glucose units joined by glycosidic bonds.
As used herein, the term "sugar" is defined as compounds selected from the
group
consisting of monosaccharides (such as glucose and fructose), disaccharides
(such as
sucrose and maltose), and oligosaccharides.
In some embodiments, the combined amount of sucrose, fructose, maltose and
glucose in
the particulate material is less than about 7% based on the weight of the
particulate material.
Thus, in a preferred aspect, a smokeless oral tobacco product is provided
comprising a
tobacco material and a particulate material, the particulate material having
the following
properties:
i) a mass median particle size measured by sieve analysis of from about 0.3 mm
to
about 3 mm;
ii) a bulk density of less than about 0.6 g/cm3; and
iii) a combined content of sucrose, fructose, maltose and glucose of less than
about
7% based on the weight of the particulate material
In some embodiments, the particulate material has a combined starch and sugar
content of
less than about 7% based on the weight of the particulate material. In some
embodiments,
the particulate material has a combined starch and sugar content of less than
about 6%
based on the weight of the particulate material, such as less than about 5.5%
based on the
weight of the particulate material, such as less than about 5% based on the
weight of the
particulate material, such as less than about 4.5% based on the weight of the
particulate
material, such as less than about 4% based on the weight of the particulate
material, such
as less than about 3.5% based on the weight of the particulate material, such
as less than
about 3% based on the weight of the particulate material, such as less than
about 2.5%
based on the weight of the particulate material, such as less than about 2%
based on the
weight of the particulate material, such as less than about 1.5% based on the
weight of the
particulate material, such as less than about 1% based on the weight of the
particulate
material, such as less than about 0.5% based on the weight of the particulate
material.
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
12
In some embodiments, the particulate material has a total sugar content of
less than about
7% based on the weight of the particulate material. In some embodiments, the
particulate
material has a total sugar content of less than about 6% based on the weight
of the
particulate material, such as less than about 5.5% based on the weight of the
particulate
material, such as less than about 5% based on the weight of the particulate
material, such
as less than about 4.5% based on the weight of the particulate material, such
as less than
about 4% based on the weight of the particulate material, such as less than
about 3.5%
based on the weight of the particulate material, such as less than about 3%
based on the
weight of the particulate material, such as less than about 2.5% based on the
weight of the
particulate material, such as less than about 2% based on the weight of the
particulate
material, such as less than about 1.5% based on the weight of the particulate
material, such
as less than about 1% based on the weight of the particulate material, such as
less than
about 0.5% based on the weight of the particulate material.
In some embodiments, the particulate material has a total starch content of
less than about
7% based on the weight of the particulate material. In some embodiments, the
particulate
material has a total starch content of less than about 6% based on the weight
of the
particulate material, such as less than about 5.5% based on the weight of the
particulate
material, such as less than about 5% based on the weight of the particulate
material, such
as less than about 4.5% based on the weight of the particulate material, such
as less than
about 4% based on the weight of the particulate material, such as less than
about 3.5%
based on the weight of the particulate material, such as less than about 3%
based on the
weight of the particulate material, such as less than about 2.5% based on the
weight of the
particulate material, such as less than about 2% based on the weight of the
particulate
material, such as less than about 1.5% based on the weight of the particulate
material, such
as less than about 1% based on the weight of the particulate material, such as
less than
about 0.5% based on the weight of the particulate material.
In one embodiment, the particulate material has a mass median particle size
measured by
sieve analysis of from about 0.3 mm to about 1 mm, a bulk density of less than
about 0.4
gicrns, and a combined starch and sugar content of less than about 7% based on
the weight
of the particulate material.
In one embodiment, the particulate material has a mass median particle size
measured by
sieve analysis of from about 0.3 mm to about 0.7 mm, a bulk density of less
than about 0.25
g/cms, and a combined starch and sugar content of less than about 5% based on
the weight
of the particulate material.
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
13
In some embodiments, the particulate material is hydrophobic. In some
embodiments, the
particulate material is a hydrophobic particulate material selected from the
group consisting
of aerogels, hollow particles and semispheres, foams, textiles, materials of
plant origin,
materials of wood or tree origin, materials of animal origin, materials of
mineral origin, and
mixtures thereof.
In some embodiments, the particulate material is a hydrophobic material
selected from the
group consisting of husks or hulls of grains, materials of wood or tree
origin, animal feathers,
diatomaceous earth, perlite, foams, hollow particles and semispheres,
aerogels, and
mixtures thereof.
The husks or hulls of grains may encapsulate the nutritious seed of the grain
and are
typically a waste product of the food industry. In some embodiments, the husks
or hulls of
grains may be selected from buckwheat husk, millet husk, and mixtures thereof.
Such husks
or hulls of grains are typically hydrophilic in nature, but may be made
hydrophobic for the
purposes of the present invention by surface treatment.
In some embodiments, the animal feathers are selected from chicken feathers,
down and a
mixture thereof. Outer feathers of animals are typically hydrophobic in their
natural state.
Down, however, is naturally hydrophilic; however, several treatments are
available to make
down hydrophobic as its natural hydrophilicity was a major drawback for use in
outdoor
clothing. Various outdoor companies now offer articles with treated down,
which repels
moisture. Brands include Rab Hydrophobic Down, The North Fact ProDownTM,
Berghaus
HydroDownTM and Mountain Hardwear Q.Shield Down.
Diatomaceous earth (also known as diatomite or kieselgur/kieselguhr) is a
naturally
occurring soft, siliceous sedimentary rock. Diatomaceous earth typically
comprises ¨80%
silica, and ¨20% diatoms, which are a type of hard-shelled algae. Diatomaceous
earth is
naturally hydrophilic, but treatments are available to obtain hydrophobic
grades.
Perlite is a light material of volcanic origin, principally comprising
aluminium silicate. Perlite
is often found in expanded form after heat treatment. As with diatomaceous
earth, perlite is
naturally hydrophilic, but treatments are available to obtain hydrophobic
grades.
Foams are typically formed by trapping pockets of gas in a material. The foam
may include
open cell foam and/or closed cell foam. In some embodiments, the foam is
formed from raw
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
14
materials such as polymers, silicone, natural rubber, nanocellulose, alginates
and mixtures
thereof. In some embodiments, the foam is selected from sodium alginate,
polyethylene,
polystyrene, and low density chewing gums which are described in WO
2013/090653. The
low density chewing gum may comprise at least 50% polymer and less than 40% of
bulking
agent and filler combined.
In some embodiments, the particulate material is a foamed alginate, such as
that described
in WO 2014/096816. In some embodiments, the foamed alginate is foamed sodium
alginate.
The hollow particles and semispheres may be formed from polymers that are
filled with air to
ensure a lighter density than the bulk material. The hollow particles and
semispheres may
be selected from the group comprising Expancel (AkzoNobel), Dualitee (Henkel),
and
SunspheresTM (The DOW Chemical Company). Hydrophobic grades of each of these
hollow
particles and semispheres are available. The hollow particles and semispheres
may also be
formed from sodium alginate particles.
An aerogel may be a dry, low-density, porous solid framework of a gel isolated
intact from
the gel's liquid component. Aerogels may be open-porous and have pores in the
range of
<1 to 100 nm in diameter. In some embodiments, the aerogels may be made from a
wide
variety of substances, including silica, metal oxides, organic polymers,
biological polymers
(such as gelatine, pectin and agar).
In some embodiments, the particulate material is a hydrophobic particulate
material of wood
or tree origin. For example, in some embodiments, the particulate material is
sawdust.
Sawdust (otherwise referred to as wood dust) is a by-product of cutting,
grinding, drilling,
sanding or otherwise pulverising wood. It is typically composed of fine
particles of wood. In
some embodiments, the particulate material is cork. Cork is a prime subset of
bark tissue
harvested primarily from Quercus suber. Cork comprises suberin, a hydrophobic
substance.
In some embodiments, the particulate material is a hydrophobic particulate
material selected
from the group consisting of cork, millet husk, polystyrene, sodium alginate
microspheres,
foamed alginates and mixtures thereof.
In some embodiments, the particulate material is cork. Cork is a material
having a lower
density than tobacco. In some embodiments, the particulate material is cork,
wherein the
bulk density of cork is from 0.05 to 0.4 g/cm2, such as from 0.05 to 0.3
g/cm3, such as from
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
0.05 to 0.29 g/cm3, such as from 0.05 to 0.28 g/cm3, such as from 0.05 to 0.27
g/cm3, such
as from 0.05 to 0.26 g/cm3. In some embodiments, the density of the cork is
from 0.05 to 0.1
g/cm3. In some embodiments, the density of the cork is from 0.17 to 0.26
g/cm3.
5 Figure 1 shows a chart that includes the densities measured for a number
of different
materials. The weight of the materials is plotted on the y-axis, where the
weight was
measured per cm3 of product.
A suitable weight ratio between the tobacco material and the particulate
material in the
10 .. smokeless oral tobacco product depends on the desired properties of the
smokeless oral
tobacco product. For example, the weight ratio between the tobacco material
and the
particulate material will depend on the desired dryness and density of the
smokeless oral
tobacco product.
15 In some embodiments, the weight ratio of the particulate material to
tobacco material in the
smokeless oral tobacco product is from 20:1 to 1:20, such as from 15:1 to
1:15, such as from
10:1 to 1:10, such as from 9:1 to 1:9, such as from 8:1 to 1:8, such as from
7:1 to 1:7, such
as from 6:1 to 1:6, such as from 5:1 to 1:5, such as from 4:1 to 1:4, such as
from 3:1 to 1:3,
such as from 2:1 to 1:2.
In some embodiments, the particulate material is cork. In some embodiments,
the weight
ratio of the cork to tobacco material in the smokeless oral tobacco product is
from 20:1 to
1:20, such as from 15:1 to 1:15, such as from 10:1 to 1:10, such as from 9:1
to 1:9, such as
from 8:1 to 1:8, such as from 7:1 to 1:7, such as from 6:1 to 1:6, such as
from 5:1 to 1:5,
.. such as from 4:1 to 1:4, such as from 3:1 to 1:3, such as from 2:1 to 1:2.
In some embodiments, the weight ratio of the particulate material to tobacco
material in the
smokeless oral tobacco product is from 1:1.5 to 1:10, such as from 1:1.5 to
1:9, such as from
1:1.5 to 1:8, such as from 1:1.5 to 1:7, such as from 1:1.5t0 1:6, such as
from 1:1.5 to 1:5,
such as from 1:1.5 to 1:4, such as from 1:1.5 to 1:3, such as from 1:1.5 to
1:2.
In some embodiments, the particulate material is cork. In some embodiments,
the weight
ratio of the cork to tobacco material in the smokeless oral tobacco product is
from 1:1.5 to
1:10, such as from 1:1.5 to 1:9, such as from 1:1.5 to 1:8, such as from 1:1.5
to 1:7, such as
from 1:1.5 to 1:6, such as from 1:1.5 to 1:5, such as from 1:1.5 to 1:4, such
as from 1:1.5 to
1:3, such as from 1:1.5 to 1:2.
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
16
In some embodiments, the particulate material is included in an amount of from
1 to 80% by
weight of the smokeless oral tobacco product, such as in an amount of from 2
to 80% by
weight of the smokeless oral tobacco product, such as in an amount of from 3
to 80% by
weight of the smokeless oral tobacco product, such as in an amount of from 4
to 80% by
weight of the smokeless oral tobacco product, such as in an amount of from 5
to 80% by
weight of the smokeless oral tobacco product, such as in an amount of from 5
to 75% by
weight of the smokeless oral tobacco product, such as in an amount of from 5
to 70% by
weight of the smokeless oral tobacco product, such as in an amount of from 5
to 65% by
weight of the smokeless oral tobacco product, such as in an amount of from 5
to 60% by
weight of the smokeless oral tobacco product, such as in an amount of from 5
to 55% by
weight of the smokeless oral tobacco product, such as in an amount of from 5
to 50% by
weight of the smokeless oral tobacco product, such as in an amount of from 5
to 45% by
weight of the smokeless oral tobacco product, such as in an amount of from 5
to 40% by
weight of the smokeless oral tobacco product, such as in an amount of from 10
to 40% by
weight of the smokeless oral tobacco product.
In some embodiments, the particulate material is cork. In some embodiments,
the cork is
included in an amount of from 1 to 80% by weight of the smokeless oral tobacco
product,
such as in an amount of from 2 to 80% by weight of the smokeless oral tobacco
product,
such as in an amount of from 3 to 80% by weight of the smokeless oral tobacco
product,
such as in an amount of from 4 to 80% by weight of the smokeless oral tobacco
product,
such as in an amount of from 5 to 80% by weight of the smokeless oral tobacco
product,
such as in an amount of from 5 to 75% by weight of the smokeless oral tobacco
product,
such as in an amount of from 5 to 70% by weight of the smokeless oral tobacco
product,
such as in an amount of from 5 to 65% by weight of the smokeless oral tobacco
product,
such as in an amount of from 5 to 60% by weight of the smokeless oral tobacco
product,
such as in an amount of from 5 to 55% by weight of the smokeless oral tobacco
product,
such as in an amount of from 5 to 50% by weight of the smokeless oral tobacco
product,
such as in an amount of from 5 to 45% by weight of the smokeless oral tobacco
product,
such as in an amount of from 5 to 40% by weight of the smokeless oral tobacco
product,
such as in an amount of from 10 to 40% by weight of the smokeless oral tobacco
product.
In some embodiments, the particulate material does not interfere with the
aroma/smell of the
tobacco material. For example, cork is advantageously neutral in aroma, and
does not
interfere with the aroma/smell of the tobacco material, and therefore does not
adversely
affect the user experience.
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
17
An advantage of the present invention is that the amount of tobacco used in
the smokeless
oral tobacco products according to the present invention may be reduced whilst
maintaining
the organoleptic properties of the products.
Without wishing to be bound, the use of the particulate material having the
properties
described herein in the present invention may advantageously increase the
release of
components from the tobacco material, such as flavour and/or nicotine, in the
smokeless
oral tobacco product, which may enhance user experience. The inclusion of the
particulate
material having the properties described herein results in a less dense
smokeless oral
tobacco product, which has a more open structure. This may result in more free
movement
of fluid in the tobacco product and in the mouth, and subsequently may result
in improved
release of components from the smokeless oral tobacco product.
The present inventors have surprisingly found that modifying the weight ratio
of the
particulate material included in the smokeless oral tobacco product results in
a modification
of the density and compactness of the product, and thereby may enable
regulation of the
extraction rate of the flavour and nicotine.
Another advantage of the present invention is the ability to regulate the
amount of tobacco
provided to the user without adversely affecting the user experience. For
smokeless oral
tobacco products which are provided in portions (optionally in pouches), it is
undesirable to
decrease the size of the portion provided to the user because this can
adversely affect their
experience since they may be used to using a certain size of portion under
their upper lip.
For smokeless oral tobacco products in loose form, in some embodiments, it may
be
possible to vary the amount of tobacco provided to the user, whilst still
maintaining the
amount of product which the user may take from the container (i.e. a "pinch"
of product).
Furthermore, for smokeless oral tobacco products in loose form, it has
surprisingly been
found that the products are perceived by the user as having a softer and
springier feel when
a "pinch" of product is taken by the user from the container. This results in
an improved
overall user experience as the products may be more pleasant to the touch when
taken from
the container in loose form.
The use of the particulate material having the properties described herein in
the present
invention enables a decrease in the overall density of the smokeless oral
tobacco product
compared to conventional smokeless oral tobacco products which do not comprise
the
particulate material. Therefore, it has surprisingly been found that the
weight of a portion of
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
18
the product may be varied whilst maintaining the overall size of the portion
provided to the
user. For example, the weight of the product may be reduced without any
corresponding
reduction in the volume of the product.
A further advantage of the present invention is the ability to reduce the
amount of irritation at
the back of the throat perceived by the consumer during use of the product.
One problem
associated with smokeless oral tobacco products is the sensitisation of a
nerve at the back
of the throat of a user which occurs during use. This may result in an
irritation at the back of
the throat, commonly referred to by consumers as "drip". It has surprisingly
been found that
the inclusion of the particulate material in accordance with the present
invention results in a
product which is perceived by the consumer as being drier relative to standard
oral tobacco
products not comprising the particulate material, and also reduces the
perceived extent of
irritation at the back of the throat of a user of the smokeless oral tobacco
product.
Therefore, in one embodiment, there is provided use of the particulate
material having the
following properties:
i) a mass median particle size measured by sieve analysis of from about 0.3 mm
to about 3
mm;
ii) a bulk density of less than about 0.6 g/cm3; and
iii) a combined starch and sugar content of less than about 7% based on the
weight of the
particulate material
for reducing irritation at the back of the throat of a user during use of a
smokeless oral
tobacco product.
In one embodiment, there is provided use of the particulate material having
the following
properties:
i) a mass median particle size measured by sieve analysis of from about 0.3 mm
to about 3
mm;
ii) a bulk density of less than about 0.6 g/cm3; and
iii) a combined starch and sugar content of less than about 7% based on the
weight of the
particulate material
for reducing oral harshness during use of a smokeless oral tobacco product.
In addition, it has been found that the smokeless oral tobacco products of the
present
invention have an improved mouthfeel compared with smokeless oral tobacco
products
which do not comprise the particulate material having the properties described
herein.
Without wishing to be bound, it is considered that the improved mouthfeel is
due to the
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
19
improved flexibility and improved shape retention during use of the products
of the present
invention.
Therefore, the amount of tobacco in the smokeless oral tobacco products may be
varied
without any adverse effect on the total volume of product provided to the
user, and with an
improvement in the mouthfeel of the product.
In this case, the smokeless oral tobacco product may comprise a high nicotine
content
tobacco. Therefore, the overall nicotine content of the smokeless oral tobacco
product may
be maintained despite use of lower amounts of tobacco by weight.
In some embodiments, the nicotine content of the tobacco material is from
0.02% to 7.5% by
weight of the tobacco material. In some embodiments, the nicotine content of
the tobacco
material is from 0.03% to 7.5% by weight of the tobacco material, such as from
0.04% to
7.5% by weight of the tobacco material, such as from 0.05% to 7.5% by weight
of the
tobacco material, such as from 0.06% to 7.5% by weight of the tobacco
material, such as
from 0.07% to 7.5% by weight of the tobacco material, such as from 0.08% to
7.5% by
weight of the tobacco material, such as from 0.09% to 7.5% by weight of the
tobacco
material, such as from 0.1% to 7.5% by weight of the tobacco material, such as
from 0.1% to
7% by weight of the tobacco material.
The smokeless oral tobacco product may further comprise other components.
These
components may, for example, be included in order to alter the organoleptic
properties of the
formulation, contributing to the sensory perception by the consumer. The
particular
components and the amounts in which they are included in the smokeless oral
tobacco
product of the present invention will vary depending upon the desired flavour,
texture, and
other characteristics.
For example, flavouring agents, preservatives, binders, humectants, buffering
agents,
disintegration aids and/or colourants may be included in the smokeless oral
tobacco product.
As used herein, the terms "flavour" and "flavourant" refer to materials which,
where local
regulations permit, may be used to create a desired taste or aroma in a
product for adult
consumers. They may include extracts (e.g., licorice, hydrangea, Japanese
white bark
magnolia leaf, chamomile, fenugreek, clove, menthol, Japanese mint, aniseed,
cinnamon,
herb, wintergreen, cherry, berry, peach, apple, Drambuie, bourbon, scotch,
whiskey,
spearmint, peppermint, lavender, cardamom, celery, cascarilla, nutmeg,
sandalwood,
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
bergamot, geranium, honey essence, rose oil, vanilla, lemon oil, orange oil,
cassia, caraway,
cognac, jasmine, ylang-ylang, sage, fennel, piment, ginger, anise, coriander,
coffee, or a
mint oil from any species of the genus Mentha), flavour enhancers, bitterness
receptor site
blockers, sensorial receptor site activators or stimulators, sugars and/or
sugar substitutes
5 (e.g., sucralose, acesulfame potassium, aspartame, saccharine,
cyclamates, lactose,
sucrose, glucose, fructose, sorbitol or mannitol), and other additives such as
charcoal,
chlorophyll, minerals, botanicals, or breath freshening agents. They may be
imitation,
synthetic or natural ingredients or blends thereof. They may be in any
suitable form, for
example, oil, liquid or powder.
The present invention also provides a consumer package comprising the
smokeless oral
tobacco product as described herein. In some embodiments, the smokeless oral
tobacco
product is non-portioned, and the consumer package comprises a container, such
as a box
or a can. In some embodiments, the smokeless oral tobacco product is
portioned. The
portioned smokeless oral tobacco product may be provided in a pouch.
Therefore, in one embodiment, there is provided a pouch comprising the
smokeless oral
tobacco product as described herein.
The present invention also provides a method of manufacturing a smokeless oral
tobacco
product comprising a tobacco material and a particulate material, the
particulate material
having the following properties:
i) a mass median particle size measured by sieve analysis of from about 0.3 mm
to about 3
mm;
ii) a bulk density of less than about 0.6 g/cm3; and
iii) a combined starch and sugar content of less than about 7% based on the
weight of the
particulate material, wherein the tobacco material comprises tobacco, or a
tobacco
replacement or substitute comprising the steps of:
(a) providing a tobacco material;
(b) processing the tobacco material; and
(c) adding the particulate material to the tobacco material either prior to
the processing
step (b), during the processing step (b) or after the processing step (b).
In order to produce a tobacco formulation for snus, the blend of tobacco
particles may be
.. mixed with water and, typically, salt. Residual moisture from the tobacco
and the added
water combine to raise the moisture levels of the mixture to at least 20%, and
in some
embodiments to at least 25%, and in some embodiments to about 20 to 60%, and
in some
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
21
embodiments to about 25 to 60%. Salt is one form of flavourant; optionally it
may be
excluded and/or another flavourant may be added at this stage. The tobacco
blend may be
mixed with water and/or salt either prior to the processing step (b), during
the processing
step (b) or after the processing step (b). In one embodiment, the tobacco
blend is mixed
with water, and optionally salt, prior to the processing step (b).
In one embodiment, the tobacco formulation is particulate, comprising tobacco
particles
having a mass median particle size measured by sieve analysis of from about
0.01-1 mm,
for example, a mass median particle size of about 0.1-0.5 mm, or a mass median
particle
size of about 0.25-0.4 mm.
In one embodiment, the particulate tobacco comprises a mixture of tobacco stem
particles
and tobacco lamina particles. In some embodiments, the particulate tobacco
comprises
tobacco stem particles in an amount of from 5% to 70% by weight of the
combined amount
of tobacco stem particles and tobacco lamina particles.
In embodiments wherein the tobacco comprises particulate snus, the particulate
snus
tobacco may be humidified to 20 to 50% moisture, and/or may be salted. In
embodiments
wherein the tobacco comprises particulate snus, the particulate snus tobacco
may be
humidified to about 50% moisture, and/or may be salted.
The amount of tobacco within the tobacco formulation may vary. In some
embodiments, the
amount of tobacco within the tobacco formulation is at least about 25% on a
wet weight
basis, such as at least about 30% on a wet weight basis, such as at least
about 40% on a
wet weight basis.
In some embodiments, the tobacco blend (together with any water and salt) is
heat treated
for a period of time long enough and at a temperature high enough to meet the
demands for
pasteurisation. The tobacco blend is typically heat treated for one hour to
meet the
demands for pasteurisation. As tobacco is a natural product it can be expected
that there is
some degree of microbial presence in the blend. The heat treatment can take
any form
which is sufficient to produce a product having an acceptably low level of
spoilage
microorganisms. The heat treatment may meet the demands for pasteurisation,
and thus
may take a form that results in sterilisation of the product. Examples include
high or low
temperatures; e.g., heat treatment at about 80-140 C for about at least 30
minutes via hot
air, steam, microwaves, or other means, or cold pasteurisation. Other examples
include
irradiation and chemical treatment. One limitation on the heat treatment
method selected is
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
22
that it is appropriate for use with a product intended for human oral
consumption. Another is
that is should not present undue adverse effect on the taste, consistency or
other
organoleptic properties of the final smokeless oral tobacco product.
The heat treatment also gives texture and colour to the mixture.
It should be apparent to skilled workers that, where the present invention is
practised using
tobacco replacements or substitutes, the heat treatment step will comprise
ensuring the
tobacco replacement or substitute is not contaminated with microorganisms, and
that this
step may not require any other manipulation of the product.
After the heat treatment, it may be desirable to check and potentially adjust
the pH of the
blend. To achieve the desired characteristics of certain commercial blends of
smokeless
oral tobacco products, a pH of approximately 7 to 12 may be preferred at this
stage of the
process.
The heat treated blend may optionally be matured. This may be done by slowly
mixing the
blend while holding it at a constant temperature such as approximately 40-75
C, preferably
at about 50 C. The gentle stirring can continue for about 1 to 24 hours,
particularly for about
3 to 15 hours. This stage may be effective at reducing pH and possibly
reducing moisture to
about 20-55% based on the weight of the smokeless oral tobacco product,
depending on the
type of tobacco used and the particular snus product being manufactured.
A flavourant may be added during the maturation stage. Typically the
flavourant added at
this stage is in a liquid form and is added to about 0.1-5% by weight of the
smokeless oral
tobacco product. The liquid flavourant is often sprayed onto the tobacco
blend. Where
employed, humectants are traditionally added at this stage as well.
However, in some embodiments, flavourants and other additives (such as
preservatives,
binders, humectants, buffering agents, disintegration aids and colourants) may
be added to
the tobacco blend before or after pasteurisation and/or maturation. In some
embodiments,
flavourants and other additives (such as preservatives, binders, humectants,
buffering
agents, disintegration aids and colourants) may be added to the tobacco blend
before or
after the stage of pasteurisation and/or maturation.
According to the present invention, the particulate material may be added to
the tobacco
material prior to, during or after heat treatment. In some embodiments, the
particulate
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
23
material is added prior to the heat treatment step. In some embodiments, the
particulate
material is added during the heat treatment step. In some embodiments, the
particulate
material is added after the heat treatment step.
Preferably, the particulate material is mixed with the tobacco material prior
to heat treatment
so that the particulate material is heat treated in the same manner as the
tobacco material.
Examples
Example 1 ¨ Measurement of mass median particle size of sample
A sieve analysis method in accordance with the present invention is described
herein.
Example la ¨ Measurement of mass median particle size of cork
A sample of cork particles were sieved using a test sieving equipment (Haver
EML 200
digital plus N) using Haver & Boecker sieves, from Haver & Boecker OHG,
Partikelanalyse
presse, Ennigerloher St. 64, D-59302 OeIde, Germany. This equipment was used
to
mechanically separate fractions of the cork according to particle size.
The above-identified test sieving equipment is shown in Figure 2, and
comprises 10 sieves
having the following mesh sizes:
Sieve Number Mesh Size (pm)
1 1000
2 900
3 800
4 710
5 600
6 500
7 400
8 180
9 90
10 <90
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
24
The sieves were agitated using a mechanical agitator for 3 minutes using the
following
settings (which are shown in Figure 3):
= 2.4mm setting on amplitude
O Interval: 5 seconds on, and 1 second off
No sieve balls were used in the method according to this example.
Three samples of cork particles were tested using the method described above.
The
following results were obtained for the cork particles analysed:
Total Total Total
Mesh mass of mass of mass of Mass of Mass of Mass of
Mass of Mean
Size sieve + sieve + sieve + sample 1 sample 2 sample 3
sieve (g)
mass (g)
(Pm) sample 1 sample 2 sample 3 (g) (g) (9)
(9) (g) (9)
1000 454.16 454,49 454.66 455.43 0.33 0.50 1.27 0.70
900 441.46 447.98 447.71 448.28 6.52 6.25 6.82 6.53
800 427.19 432.76 432.23 433.30 5.57 5.04 6.11 5.57
710 449.34 458.30 458.10 457.77 8.96 8.76 8.43 8.72
600 434.00 442.39 442.27 442.17 8.39 8.27 8.17 8.28
500 424.94 433.91 434.05 433.52 8.97 9.11 8.58 8.89
400 405.06 411.87 412.35 411.52 6.81 7.29 6.46 6.85
180 386.45 390.63 390.44 390.30 4.18 3.99 3.85 4.01
90 373.71 373.82 373.79 373.73 0.11 0.08 0.02 0.07
<90 287.32 287.78 287.32 287.32 0.46 0.00 0.00 0.15
The mean cumulative mass of the samples was calculated to be 49.77g.
The cumulative percentage mass of aggregate collected on each sieve was thus
calculated
as follows:
Mass retained on Cumulative mass
Mesh Size (pm) Mean mass (g)
sieve (%) (%)
1000 0.70 1.41 100.00
900 6.53 13.12 98.59
800 5.57 11.20 85.47
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
710 8.72 17.52 74.27
600 8.28 16.63 56.76
500 8.89 17.86 40.13
500 6.85 13.77 22.27
180 4.01 8.05 8.50
90 0.07 0.14 0.45
<90 0.15 0.31 0.31
A graph plotting percentage cumulative mass (y-axis) against mesh size or each
sieve (x-
axis) is shown in Figure 4. A linear line of best-fit has been drawn through a
number of the
data points.
5
The equation for this plot was calculated as y=0.1526x - 36.534. Therefore,
when y=50%,
the mass median particle size of the cork is calculated as 667.06 pm
(equivalent to 0.567
mm).
10 Example lb - Measurement of mass median particle size of millet husks
The same method as described in Example la was used to determine the mass
median
particle size of a sample of millet husks.
The results were as follows:
Total Total Total
Mesh mass of
mass of mass of Mass of Mass of Mass of
Mass of Mean
Size sieve +
sieve + sieve + sample 1 sample 2 sample 3
sieve (g)
mass (g)
(1-1m) sample 1 sample 2 sample 3 (g) (0) (0)
(0) (0) (0)
1000 454.16 459.25 458.8 460.08 5.09 4.64 5.92 5.22
900 441.46 443.3 443.36 443.35 1.84 1.9 1.89 1.88
800 427.19 433.91 433.98 434.35 6.72 6.79 7.16 6.89
710 449.34 455.01 454.98 454.74 5.67 5.64 5.4 5.57
600 434 442.37 442.7 442.6 8.37 8.7 8.6 8.56
500 424.94 431.71 431.79 431.43 6.77 6.85 6.49 6.70
400 405.06 411.1 411.21 410.73 6.04 6.15 5.67 5.95
180 386.45 394.29 394.41 393.65 7.84 7.96 7.2 7.67
90 373.71 374.93 374.96 374.96 1.22 1.25 1.25 1.24
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
26
<90 287.32 287.82 287.78 287.85 0.5 0.46 0.53 0.50
The mean cumulative mass of the samples was calculated to be 50.17g.
The cumulative percentage mass of aggregate collected on each sieve was thus
calculated
as follows:
Mass retained on Cumulative mass
Mesh Size (pm) Mean mass (g)
sieve (`)/0) (%)
1000 5.22 10.40 100.00
900 1.88 3.74 89.60
800 6.89 13.73 85.86
710 5.57 11.10 72.13
600 8.56 17.06 61.03
500 6.70 13.36 43.97
500 5.95 11.87 30.61
180 7.67 15.28 18.74
90 1.24 2.47 3.46
<90 0.50 0.99 0.99
A graph plotting percentage cumulative mass (y-axis) against mesh size (x-
axis) is shown in
Figure 5. A linear line of best-fit has been drawn through a number of the
data points.
The equation for this plot was calculated as y=0.1371x - 23.841. Therefore,
when y=50%,
the mass median particle size of the cork is calculated as 538.59 pm
(equivalent to 0.539
mm).
Example 2
A snus batch was made using a 60:40 weight ratio (tobacco:cork) of pre-treated
materials by
adding 3,600g of tobacco and 2,400g of cork (cork granules at 0.055-0.060g/cm3
bulk
density; mass median particle size approximately 0.55-0.65 mm, supplied by
Cork Link,
Portugal), water, sodium chloride, and other ingredients to a horizontal,
cylindrical,
ploughshare mixer. The mix of ingredients was then stirred, and heat applied
for a long
enough duration to ensure safe microbiological status of snus. Following heat
treatment, the
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
27
product had a maturation period of several hours at elevated temperature with
continuous
intermittent stirring at low speed of mixer blades inside the mixer. The
processing was
finished by adding flavour, adjusting pH to about 8.5 by adding sodium
carbonate, and water
content measured to ensure that end moisture content was 45% (w/w).
Finished cork-containing snus was then transferred to plastic bags, and
allowed to mature in
a cold room for one week. Following this, snus was packed to pouches using a
Mertz snus
packer (Merz Verpackungsmaschinen GmbH, Bahnhofstrape 25, D-35423 Lich,
Deutschland), and bulk density was measured. Bulk density was measured using
an
Engelsmann Jolting Voltumeter STAV II. Bulk density was calculated pre-
jolting, for a given
volume of a certain mass of particulate material.
As a final step, sensory properties of the cork-containing snus were assessed.
Example 3
A snus batch containing an 80:20 tobacco:cork weight ratio was prepared and
analysed
using the method of Example 2, where 4,000g of tobacco was added to the
mixture, and
1,000g of cork added.
Example 4
A snus batch containing a 95:5 tobacco:cork weight ratio was prepared and
analysed using
the method of Example 2, where 4,750g of tobacco was added to the mixture, and
250g of
cork added.
Example 5 (Comparative)
A snus batch containing no cork (i.e. a tobacco:cork weight ratio of 100:0)
was prepared and
analysed using the method of Example 2, where 5,000g of tobacco was added to
the
mixture, and Og of cork added.
Results
The smokeless oral tobacco products in accordance with the present invention
(Examples 2
to 4) were analysed to determine total starch and sugar content, and the
following results
obtained:
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
28
O Glucose < 0.04g/100g
O Fructose <0.04g/100g
= Sucrose <0.04g/100g
0 Maltose <0.04g/100g
= Total starch & sugars < 1% w/w
The properties of the smokeless oral tobacco products prepared in Examples 2
to 5 are
shown in Table 1.
Table 1 ¨ Measured bulk densities and pouch weights of cork-containing snus of
a standard
White snus blend at 45% moisture, with varying levels of cork
Example Cork (% w/w)
Bulk Density (g/cm3) Mass of pouch (g)
2 40 0.207 0.4
3 20 0.260 0.5
4 5 0.350 0.6
5 0 0.425 0.8
It is noted that the stated level of cork does not refer to the ratio of cork
in the final snus
product as this depends on moisture level and other ingredients. In the case
of a white snus
mix containing 40% cork, at a finalmoisture of 45%, the final weight
percentage of cork is
around 18-20% w/w. However, in the Examples and Table 1 above, the amount of
cork is
specified as a ratio of total ground, pre-treated tobacco mix, prior to any
other ingredients
having been added.
As shown in Table 1, the inclusion of cork in the snus products results in a
considerable
decrease in bulk density and resulting pouch mass of the smokeless oral
tobacco product at
a similar fill value.
The products of Examples 2 and 5 are shown in Figure 6, where the product
comprising
40% w/w cork (Example 2) is shown on the left hand side, and the product
comprising 0%
w/w cork (Example 5) is shown on the right hand side.
Indeed, the product comprising 40% w/w cork (Example 2) had a bulk density
51.3% lower
than the product comprising 0% w/w of cork (Example 5) for the same weight of
product.
CA 03016766 2018-09-05
WO 2017/153718
PCT/GB2017/050559
29
A standard snus product contains 0.9g of tobacco. 0.9g of the snus product
according to
Example 5 having 0% w/w cork has a volume calculated as 1.8cm3. With a bulk
density of
0.207 g/cm3, the weight of the snus product according to Example 2 comprising
40% w/w
cork required to fill a volume of 1.8cm3 is 0.3726g. This results in a 58.6%
reduction in
material weight for the same volume.
In addition, the inventors surprisingly found that the samples comprising cork
are very soft
and flexible. The sample comprising 0% w/w cork (Example 5) started to
collapse and feel
flat when tested, whereas the samples comprising cork (Example 2) kept their
shape over an
extended period of time. In addition, the samples comprising cork (Example 2)
were
perceived as dry compared to the sample in Example 5.
It was also found that cork has a neutral aroma and taste, and thus did not
add any
noticeable aromas or tastes to the tobacco products.
The various embodiments described herein are presented only to assist in
understanding
and teaching the claimed features. These embodiments are provided as a
representative
sample of embodiments only, and are not exhaustive and/or exclusive. It is to
be
understood that advantages, embodiments, examples, functions, features,
structures and/or
other aspects described herein are not to be considered limitations on the
scope of the
invention as defined by the claims or limitations on equivalents to the
claims, and that other
embodiments may be utilised and modifications may be made without departing
from the
scope of the claimed invention. Various embodiments of the invention may
suitably
comprise, consist of, or consist essentially of appropriate combinations of
the disclosed
elements, components, features, parts, steps, means, etc., other than those
specifically
described herein. In addition, this disclosure may include other inventions
not presently
claimed, but which may be claimed in future.