Note: Descriptions are shown in the official language in which they were submitted.
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
1
USE OF EMULSIFIER IN COLLECTOR COMPOSITION
Field of Invention
The present invention relates to the use of emulsifiers in branched alcohol
and/or alkoxylate-containing secondary collector compositions and the use of
such compositions for the froth flotation of non-sulfidic ores, especially
phosphate ores, in combination with a primary collector which is an anionic or
an amphoteric surface active-compound.
Background of the invention
Phosphate rocks contain calcium phosphate minerals largely in the form of
apatite, usually together with other minerals, e.g. silicate minerals and
carbonate minerals, such as calcite. Apatite is a generic name for a group of
calcium phosphate minerals also containing other elements or radicals, such as
fluorapatite, chlorapatite, hydroxylapatite, carbonate-rich fluorapatite and
carbonate-rich hydroxylapatite.
It is well-known to separate the valuable phosphate minerals from the gangue
by using a froth flotation process where the phosphate minerals are enriched
in
the float.
Good performance in a froth flotation process is achieved by a combination of,
on the one hand, a good separation of the valuable mineral from the gangue by
using a selective collector and, on the other hand, the froth characteristics.
The
froth characteristics include both the amount and the stability of the froth.
It is
important in the flotation process that the froth collapses as soon as
possible
after it leaves the flotation cell for the next step in the beneficiation
process. A
too stable froth will cause both entrainment of particles and froth product
pumping problems. Entrainment, especially on a large scale, will result in
decreased selectivity (grade, recovery). Problems with froth product pumping
will make a process of flotation technically impossible.
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
2
Collector performance may be improved by using collector combinations of a
primary (main) collector and a secondary collector (co-collector). In this
document the term "collector composition" shall be used to describe
compositions containing both a primary and a secondary collector.
For many decades secondary collectors have been used together with primary
ionic collectors in salt-type mineral flotation to improve the performance of
the
primary collector. Nonylphenol ethoxylates have been the dominating nonionic
surfactant used as a co-collector in a combination with sarcosine-type primary
collectors in selective flotation of apatite from calcite-containing ores.
US 4,814,070 discloses the flotation of non-sulfidic ores wherein alkyl
sulfosuccinates based on propoxylated and ethoxylated C8-C22 fatty alcohols
are employed as a collector. However, nowhere in this document are fatty
alcohols having a high degree of branching mentioned nor is it disclosed or
suggested that they can be used without the sulfosuccinate function.
US 4,789,466 discloses the flotation of apatite ores with a collector
composition
that contains two components, wherein one is an ethoxylated and propoxylated
fatty alcohol and the other is a cationic, anionic or ampholytic surfactant,
preferably a sulfosuccinamate surfactant. The surfactant is the primary
collector
and the alkoxylated fatty alcohol the secondary collector in the apatite ore
flotation process. The degree of branching of the used fatty alcohol is either
not
disclosed or fatty alcohols are applied which are known to have a degree of
branching that is 1 or less. US '466 moreover does not disclose mixtures of
two
alkoxylated alcohols wherein one is relatively highly branched and ethoxylated
with 1 to 4 ethylene oxide groups and the other has a higher amount of EO
groups than the first alkoxylated alcohol. Nor does this document disclose the
use of such mixtures as secondary collector in the flotation of apatite ores
or
suggest the benefits thereof.
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
3
SE 409291 discloses a method for foam flotation of calcium phosphate-
containing minerals, using an amphoteric surface-active compound as the
primary collector. The primary collector's flotating ability may further be
strengthened by the presence of a secondary collector, which is described as a
polar, water-insoluble, hydrophobic substance having affinity to the mineral
particles that have been coated by the primary collector. Examples of the
polar
components are e.g. water-insoluble soaps, such as calcium soaps, water-
insoluble surface-active alkylene oxide adducts, organic phosphate compounds,
such as tributyl phosphate, and esters of carbonic acids, such as tributyl
ester
of nitrilotriacetic acid. In the working examples nonylphenol that has been
reacted with two moles of ethylene oxide was used as the secondary collector.
The secondary collector disclosed in SE'291 is still considered a good choice
in
treating ores, as it provides for an excellent mineral recovery at a P205
grade of
higher than 30%. However, due to environmental concerns, an intense search
for a replacement of nonylphenol ethoxylates has been ongoing for a long time.
EP 0 270 933 A2 discloses mixtures as collectors for flotation of non-sulfidic
ores that contain an alkyl or alkenyl polyethylene glycol ether that is end-
capped
with a hydrophobic group and an anionic tenside. The end-capped alkyl or
alkenyl polyethylene glycol ether in some embodiments is based on a fatty
alcohol, preferably a 012 to 018 fatty alcohol. In comparative Examples in EP
0
270 933 also non-end-capped fatty alcohols are used together with anionic
tensides. In EP 0 270 933 no disclosure is made of using fatty alcohols having
a
degree of branching of 1 to 3, and the molecules exemplified in the EP'933
document, though more environmentally friendly than nonylphenol ethoxylates,
do not perform as well as these nonylphenol ethoxylates as collectors for
flotation of non-sulfidic ores in terms of mineral recovery at the desired
high
grades.
Thus, there is still a need for secondary collectors having a better
environmental
profile than nonylphenol ethoxylates that perform equally well.
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
4
Co-pending patent application PCT appl # EP 2015/071003 discloses the use of
a secondary collector, suitable for use with a primary collector of the
amphoteric
or anionic type, for the froth flotation of non-sulfidic ores to recover
oxides,
carbonates, phosphates and other salt-type minerals, especially calcium
.. phosphate-containing minerals, in which the secondary collector is a
branched
fatty alcohol-based compound selected from the group of fatty alcohols with 12-
16 carbon atoms having a degree of branching of 1-3, and their alkoxylates
with
a degree of ethoxylation of up to 3.
Summary of the invention
It has been found in the present invention that a secondary collector which
can
be used in combination with a primary collector of the amphoteric or anionic
type for the froth flotation of non-sulfidic ores to recover oxides,
carbonates,
phosphates and other salt-type minerals, especially calcium phosphate-
containing minerals, wherein the secondary collector is a mixture containing
at
least one compound (i) selected from the group of branched fatty alcohols with
12-16 carbon atoms having a degree of branching of 1-3.5 and their alkoxylates
with a degree of ethoxylation (DE) of up to 4, and at least one compound (ii)
selected from the group of alkoxylates of nonionic hydrocarbon compounds,
such as fatty amines, fatty alcohols, fatty (di)ethanolamides, fatty acids,
triglycerides, with a degree of ethoxylation (DE) of higher than 3, and
carbohydrate-based surfactants, leads to similar good efficiency in recovering
apatite in the presence of silicate and/or carbonate minerals as when using a
secondary collector only containing compound (i), and to the same good
environmental profile especially when compared to nonylphenol ethoxylates, but
that additionally compound (ii) has as a benefit that it helps to emulsify
compound (i) in the collector composition, enabling compound (i) to be used
more efficiently, like in a smaller amount.
Description of the drawings
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
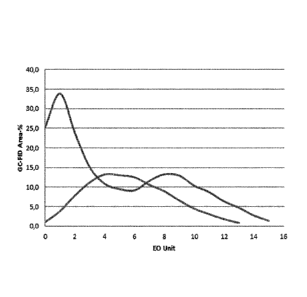
Figure 1 is the distribution of the degree of ethoxylation in a mixture
according
to the invention
Figure 2 is a schematic flow chart of a flotation procedure
Detailed description of the invention
5 In one aspect, the invention relates to (a secondary collector) mixture
of at least
one compound (i) selected from the group of branched fatty alcohols with 12-16
carbon atoms having a degree of branching of 1-3.5 and their alkoxylates with
a
degree of ethoxylation of up to 4, and at least one compound (ii) selected
from
carbohydrate-based surfactants and alkoxylates of nonionic hydrocarbon
compounds with a degree of ethoxylation of more than 3, such as alkoxylates of
hydrocarbon compounds of the group of fatty alcohols, fatty amines, fatty
ethanolamides, fatty diethanolamides, fatty acids, triglycerides with a degree
of
ethoxylation of more than 3, wherein when both compounds (i) and (ii) are
ethoxylated alcohols, the mixture has a bimodal degree of ethoxylation
distribution.
It should be noted that the mixture of compounds (i) and (ii) - in the
embodiments wherein both compounds are ethoxylated alcohol compounds -
has a bimodal DE distribution (bimodal meaning a statistical distribution with
two
maxima). Or in other words, the mixture is not an inherent mixture of more
than
.. one molecule that is obtained when a single ethoxylation reaction is
performed
with a hydrocarbon compound and wherein always some lower and higher
ethoxylated molecules are formed and wherein the DE distribution would be
unimodal (i.e. have a single maximum). Instead in the present invention the
mixture is a mixture obtained by mixing two separately ethoxylated alcohol
compounds (i) and (ii).
If both compounds (i) and (ii) are ethoxylated, preferably the degree of
ethoxylation of compound (ii) is higher than that of compound (i).
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
6
Additionally, the invention relates to the use of the mixture of the above
compounds (i) and (ii) as secondary collectors for the froth flotation of non-
sulfidic ores, especially to recover calcium phosphate-containing minerals,
such
as apatite, in combination with a primary collector which is an amphoteric or
anionic surfactant and to collector compositions containing such primary and
secondary collectors. Examples of other valuable minerals that may be
recovered using this combination of primary and secondary collectors include
scheelite, fluorspar, calcite and dolomite.
Furthermore, the invention relates to the use of compound (ii) as an
emulsifier
for compound (i) in a liquid, most prominently a carrier liquid in which a
collector
composition is present, or alternatively, it relates to a process to emulsify
compound (i) in a liquid. This process to emulsify a compound (i) in a liquid
contains a step of adding compound (i) to the liquid and a step of adding a
compound (ii) to the liquid, wherein the steps may be done one after the other
or simultaneously, optionally by premixing compounds (i) and (ii). In a
preferred
process compounds (i) and (ii) are first mixed and the so obtained mixture is
added to the carrier liquid. The carrier liquid for the collector composition
is
preferably an aqueous liquid.
In preferred mixtures the ratio of compound (i) to compound (ii) on a weight
basis is from 30:70 to 99:1, more preferably from 40:60 to 98:2, even more
preferably from 50:50 to 90:10.
By "the degree of branching" (DB) as used herein is meant the total number of
methyl groups present on the alkyl or alkenyl chain of the alcohol or
alkoxylate
thereof, minus one. The mean number of methyl groups in the molecules of a
sample can easily be determined by NMR spectroscopy. It should be
understood that the degree of branching (DB) in the 012-016 branched fatty
alcohol that delivers the branched alkyl or alkenyl chain for compound (i) is
an
average degree of branching for the fatty alcohol used. Fatty alcohols are
oftentimes available or applied as a mixture of several components and
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
7
therefore DB does not have to be an integer. Consequently, the degree of
branching for compound (i) is an average degree of branching, wherein the
average degree of branching is the statistical mean of the degree of branching
of the molecules of a sample.
.. In a preferred embodiment the DB in compound (i) is higher than 1, even
more
preferably it is higher than 1.5, most preferably higher than 2. In another
more
preferred embodiment the DB is lower than 3.2, most preferred it is 3 or
lower.The molecular formula of the secondary collector compounds (i) and (ii)
in
a preferred embodiment is
R-A (I),
wherein for compound (i) R is an alkyl or alkenyl group having 12-16,
preferably
12-15, carbon atoms and where said alkyl or alkenyl group has a degree of
branching of 1-3, and wherein for compound (ii) R is any alkyl, aryl or
alkenyl
group branched or linear having 8-24 carbon atoms; A is selected from the
groups
0-(P0)(E0)y(P0),1-1, for compounds (i) and (ii)
(CO)N(CH2CH20(P0)(E0)y(P0),1-1)2, for compound (ii)
(CO)NH(CH2CH20(P0)(E0)y(P0),1-1), for compound (ii)
(C0)0((P0)(E0)y(P0),1-1), for compound (ii)
N(P0)x(E0)y(P0),1-1)2, for compound (ii)
(C0)0((P0),;(E0)y(P0),OCH2CH(O(P0),;(E0)y(P0),O(CO)R)CH20((P0),;(E0)y(
P0),O(CO)R), for compound (ii), and
0(C6F11105)-(0-(C6H1105))m, for compound (ii)
wherein for compound (i) PO is a propyleneoxy unit and EO is an ethyleneoxy
unit; x is a number 0-2, preferably 0, y is a number 0-4, preferably 0-3, more
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
8
preferably 0-2.5, even more preferably 0-2.3 and most preferably 0-2, and z is
a
number 0-2, preferably 0, and wherein for compound (ii) PO is a propyleneoxy
unit and EO is an ethyleneoxy unit; x is a number 0-20, preferably 0, y is a
number higher than 3 up to 30, preferably 4-20, more preferably 5-15, even
more preferably 7-12, z is a number 0-20, preferably 0, and m is an integer of
0
to 5.
The mixture may in addition contain further components such as a liquid. In
preferred embodiments such liquid is an aqueous liquid, even more preferably
the liquid contains more than 95% of water. In such mixtures compound (ii)
acts
as an emulsifier for compound (i).
As is evident from formula (I) for compound (i), the alcohols as such, as well
as
their alkoxylates, may be used in the secondary collector mixtures.
The alkoxylated products according to formula (I) may be produced by
procedures well-known in the art by reacting the appropriate starting alcohol,
acid, amide, amine or triglyceride with ethylene oxide, or propylene oxide and
ethylene oxide, in the presence of a suitable catalyst, e.g. a conventional
basic
catalyst, such as KOH, or a so-called narrow range catalyst (see e.g. Nonionic
Surfactants: Organic Chemistry in Surfactant Science Series volume 72, 1998,
pp 1-37 and 87-107, edited by Nico M. van Os; Marcel Dekker, Inc). If both
propylene oxide and ethylene oxide are used, the alkoxides may be added as
blocks in either order, or may be added randomly. The products obtained from
reaction with only ethylene oxide are the most preferred.
Preferred compounds (i) are alcohol alkoxylates with a degree of ethoxylation
of
up to 3. Preferred compounds (ii) are alcohol alkoxylates with a degree of
ethoxylation of higher than 3, even more preferred higher than 4. In an
embodiment their degree of ethoxylation is up to 30, preferably up to 20. For
both compounds (i) and (ii) it is preferred that they have a degree of
propoxylation of less than 2, even more preferably less than 1, most
preferably
of about 0.
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
9
Carbohydrate-based surfactants, also referred to above as being of the formula
R-0-(C6F11105) in a preferred embodiment, are surfactants that are generally
nonionic and that in a preferred embodiment contain at least one unit chosen
from the group of carbohydrates, such as sorbitol (sorbitans), glucose
.. (glycosides), sucrose and/or their esters, amides.
The primary collectors used in the froth flotation according to the present
invention may be either amphoteric or anionic surface-active compounds. Below
some examples of formulae for the primary collectors are given, but these
should only be considered as suitable for the invention, and are not to be
regarded as limiting.
In one embodiment the primary collector for the above-mentioned froth
flotation
procedure has the formula (II)
R C)411 fl\I ¨
1 A ig Y (Mr+)1/r (II)
- - n
OH
wherein R1 is a hydrocarbyl group with 8-22, preferably 12-18, carbon atoms; A
is an alkyleneoxy group having 2-4, preferably 2, carbon atoms; p is a number
0
or 1; q is a number from 0 to 5, preferably 0; R2 is a hydrocarbyl group
having 1-
4 carbon atoms, preferably 1, or R2 is the group
cH2
R1-'1. O4 Arci
OH
,
wherein R1, A, p and q have the same meaning as above; r is selected from
the group consisting of C00- and S03-, preferably C00-; n is a number 1 or 2,
preferably 1; M is a cation, which may be monovalent or divalent, and
inorganic
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
or organic, and r is a number 1 or 2. The primary collector may also be used
in
its acid form, where the nitrogen is protonated and no external cation is
needed.
The compounds according to formula (II) can easily be produced in high yield
from commercially available starting materials using known procedures. US
5 4,358,368 discloses some ways to produce the compounds where R1 is a
hydrocarbyl group with 8-22 carbon atoms (col 6, line 9 ¨ col 7, line 52), and
in
US 4,828,687 (col 2, line 2¨ col 2, line 31) compounds where R2 is
CH2
R11. 44..-1Arq
OH
attached to the compound of formula (II) via the methylene group, are
described.
10 In another embodiment the primary collector has the formula (III)
COOM COOM
D/ D/
N.-.N COOM (III)
R2 D
- k
wherein R2 is a hydrocarbyl group with 8-22, preferably 12-18, carbon atoms, D
is ¨CH2- or -CH2CH2- , k is 0-4, preferably 0-3, and most preferably 0-2, and
M
is hydrogen or a cation, such as sodium or potassium.
These products are well known and are produced commercially by methods
well known in the art. The products where D is ¨CH2- are prepared by the
reaction between a fatty amine and chloroacetic acid or its salts, and the
products where D is -CH2CH2- are prepared by the reaction between a fatty
amine and acrylic acid or esters thereof, in the latter case the reaction is
followed by hydrolysis.
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
11
In a further embodiment the primary collector is selected from anionic surface-
active compounds such as fatty acids (with a 08 to 024-acyl group),
sulfonates,
alkyl phosphates, alkyl sulfates and compounds of formula (IV)
0 R2 0
R 0
X
/ \
\ 1 P n
R1 0 R3
_ ¨ m (IV)
where R is a hydrocarbyl group having from 7-23, preferably 11-21, carbon
atoms, optionally substituted; R1 is H or CH3, preferably H; R2 is H or a 01-
04
alkyl group, preferably H; R3 is H or CH3, preferably CH3; n is a number 1-20;
p
is a number 1-3, preferably 1; X is H+ or a cation which is organic or
inorganic,
and m represents the valency of the cation and is a number 1-2, preferably 1.
The cation is preferably selected from the group consisting of an alkali metal
cation, an alkaline earth metal cation, ammonium, and a substituted ammonium
group having one or more Ci to 03 alkyl and/or hydroxyalkyl groups.
For the production of compounds of formula (IV) see the description in WO
2015/000931 (corresponding to PCT/EP2014/064014).
Also it is possible to have mixtures of the above compounds as a primary
collector. In the case of mixing amphoteric and anionic surface-active
compounds as the primary collector, it is preferred to have up to 20 weight %
of
anionic surfactant on the amount of amphoteric surfactant.
In another aspect, the invention relates to a method for froth flotation of
non-
sulfidic ores, especially phosphate ores, to recover apatite minerals, in
which
method the collector mixture described above is used.
Such froth flotation method for phosphate ores may typically comprise the
steps
of:
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
12
a) conditioning a pulped ore, wherein the ore comprises a phosphate-containing
mineral, and gangue minerals in an aqueous medium, optionally, conditioning
the mixture with a flotation aid (in some embodiments a depressant); and
optionally, adjusting the pH
b) adding an aqueous composition (in some embodiments an aqueous
emulsion) containing the primary and the secondary collector described herein,
and, optionally, adjusting the pH
c) optionally, adding a frother; and
d) performing a froth flotation process to recover the phosphate-containing
mineral(s).
In yet another aspect the invention pertains to a collector composition
comprising a primary collector as defined herein and a secondary collector
mixture as defined herein.
The weight ratio between the primary collector and the secondary collector in
both the collector compositions and the flotation processes is preferably from
15:85, more preferably 20:80, most preferably 25:75 to 99:1, preferably 98:2,
most preferably 97:3. All weight ratios herein refer to the ratio of active
materials,
unless stated otherwise.
The amount of collector composition added to the ore will in general be in the
range of from 10 to 1000 g/ton dry ore, preferably in the range of from 20 to
500,
more preferably from 100 to 400 g/ton dry ore.
Further flotation aids that may be present in the flotation process are
depressants, such as a polysaccharide, alkalized starch or dextrin, extender
oils,
frothers/froth regulators, such as pine oil, MIBC (methylisobutyl carbinol)
and
alcohols such as hexanol and alcohol ethoxylates/propoxylates, inorganic
dispersants, such as silicate of sodium (water glass) and soda ash, and pH-
regulators.
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
13
The pH during the flotation process will preferably be in the range of 8-11.
The present invention is further illustrated by the following examples.
EXAMPLES
Example 1
General flotation procedure
A phosphate ore containing 20-25% of apatite, 30-40% of silicates and c. 20%
of iron oxides was crushed and ground to a desirable flotation size
(K80=110pm).
Three alkoxylates were prepared by reacting the alcohol Exxal 13 (ex Exxon),
which has a DB of 3, with ethylene oxide in an amount of 1.5, 5 and 8.5
equivalents, respectively, on the molar amount of the alcohol.
500 g of the ore were placed in a 1.4L Denver flotation cell, 500 ml of
process
water (25mg/I Ca2+, 40mg/I Mg2+) were added and the mixing was started. Then
5 minutes conditioning with 1000g/t of a 1`)/0(w/w) aqueous starch solution
was
performed, the collector (600g/t (or a mixture of primary acylglycide
collector
and secondary collectors)) was added to the flotation cell as a 1`)/0 aqueous
solution and conditioning was continued for 2.5 minutes. After the
conditioning
steps tap water was added so that a total volume of 1.4L was obtained, the pH
of the flotation mixture was adjusted to 9.5 with a 10% NaOH aqueous solution
and the flotation was started. The experiment was performed at RT (20 1 C).
The rougher flotation, followed by three cleaning steps, was performed. All
fractions (tailings, middlings and concentrate) were collected and analysed.
Reference is made to Figure 2 for the general procedure that was followed.
The flotation results and the composition of the collector formulation used
are
displayed in Table 1.
Table 1. Flotation results presented as P205 recovery and grade.
Amount of Average degree Amount of 2nd cleaner
concentrate 3rd cleaner concentrate
alcohol+XEO, g/t of ethoxylation acylglycide,
1.5E0 5E0 8.5E0 g/t Recovery, % Grade, %
Recovery, % Grade, %
Comparison 0 0 0 0 600 82.8 32.7
78.6 34.1
1
Invention 150 0 150 5 300 81.6 33.2
79.5 34.2
Comparison 0 300 0 5 300 76.6 33.4
72.4 35.0
2
4=,
oo
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
As one can see from Table 1, the flotation results can be improved only if use
is
made of the mixture of two nonionic surfactants as a secondary collector . In
Figure 1 the distribution of the degree of ethoxylation for the formulation
according to the invention and the comparison 2 formulation are graphically
5 represented. The results clearly show that the double peak distribution
plays a
crucial role in the flotation. In a combination with the primary collector the
mixture of two ethoxylated alcohols with a degree of ethoxylation equal to 1.5
and 8.5, respectively, provides much better recovery than the single
ethoxylated
alcohol with a degree of ethoxylation equal to 5.
Example 2
The emulsion formation and stability were tested by preparing aqueous 5 w%
solutions as follows: five (5) g of the surfactant or surfactant mixture were
added
to 150 ml beaker, diluted with 95 g of water and vigorously mixed. After 5 min
the mixing was stopped. Visual observation of the prepared solutions was done
after 1, 2, 3, 4, 5, 10 and 60 min.
The results are given in Table 2 below.
Table 2 Emulsion formation and stability results of mixture of the two alcohol
ethoxylates
Weight Ratio Under 1 min 2 min 3 min 4 min 5 min 10 60
1.5E0:8.5E0 mixing min min
100:0 2p 2p 2p 2p 2p 2p 2p 2 p
90:10 Em Em 2p 2p 2p 2p 2p 2 p
80:20 Em Em Em Em 2 p 2 p 2 p 2 p
50:50 Em Em Em Em Em Em Em 2p
Em means emulsion, 2p means 2 phases
From Table 2 it is clear that adding a compound having a degree of
ethoxylation
of higher than 3 to an alcohol ethoxylate that only has a degree of
ethoxylation
CA 03016794 2018-09-05
WO 2017/162563 PCT/EP2017/056516
16
of 1.5 helps considerably to form an emulsion. Adding more of the higher
ethoxylated product such as up to 50 wt% makes the emulsion much more
stable.