Note: Descriptions are shown in the official language in which they were submitted.
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
HIGH CONDUCTIVITY MAGNESIUM ALLOY
[0001] This invention claims priority on United States Provisional Patent
Application Serial
No. 62/340,074 filed May, 23, 2016, which is incorporated herein.
[0002] This invention was made with partial support from the US government
under Contract
No. NNX14CM36P awarded by National Aeronautics and Space Administration
(NASA). The
U.S. government has certain rights in the invention.
[0003] The present invention relates to composites and methods for
manufacture of a high
conductivity magnesium composite, particularly to a magnesium-based composite
and methods
for manufacture of such composite wherein the composite has improved thermal
and mechanical
properties, more particularly to a magnesium-based composite and method for
manufacture of
such composite wherein the composite has improved thermal and mechanical
properties by the
modification of grain boundary thermal resistance through the addition of
insoluble nanoparticles
that are generally high conductivity metal materials to the high strength
magnesium-based
composite, and still more particularly to a magnesium-based composite and
method for
manufacture of such composite wherein the composite has improved thermal and
mechanical
properties by the modification of grain boundary thermal resistance through
the addition of
insoluble nanoparticles that are generally high conductivity metal materials
to the magnesium-
based composite wherein the magnesium-based composite has a thermal
conductivity greater than
180 W/m-K. The magnesium-based composite can be used as a dissolvable
structure in oil drilling.
Specifically, the magnesium-based composite can be formed into a ball or other
structure in a well
drilling or completion operation, such as a structure that is seated in a
hydraulic operation, that can
be dissolved away after use so that that no drilling or removal of the
structure is necessary.
Primarily, dissolution is measured as the time it takes for the ball to remove
itself from the seat or
can become free floating in the system. Secondarily, dissolution is measured
as time it takes the
ball to fully dissolv into submicron particles. Furthermore, the magnesium-
based composite of
the present invention can be used in other well structures that also desire
the function of dissolving
after a period of time. The material is machinable and can be used in place of
existing metallic or
plastic structures in oil and gas drilling rigs including, but not limited to,
water injection and
hydraulic fracturing.
1
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
BACKGROUND OF INVENTION
[0004] The ability to control the dissolution of a down hole well structure
in a variety of
solutions is very important to the utilization of non-drillable completion
tools, such as sleeves frac
balls, hydraulic actuating tooling, and the like. Reactive materials for this
application, which
dissolve or corrode when exposed to acid, salt, and/or other wellbore
conditions, have been
proposed for some time. Generally, these consist of materials that are
engineered to dissolve or
corrode. Dissolving polymers and some powder metallurgy metals have been
disclosed and are
also used extensively for controlled release of drugs in the pharmaceutical
industry.
[0005] While these systems have enjoyed modest success in reducing well
completion costs,
their consistency and ability to specifically control dissolution rates in
specific solutions, as well
as other drawbacks such as limited strength and poor reliability, have
impacted their widespread
adoption. Ideally, these structures would be manufactured by a process that is
low cost, scalable,
and produces a controlled corrosion rate having similar or increased strength
as compared to
traditional engineering alloys such as aluminum, magnesium, and iron. Ideally,
traditional heat
treatments, deformation processing, and machining techniques would be used
without impacting
the dissolution rate and reliability of such structures.
[0006] Magnesium alloys can be strengthened through grain refinement and
second phase
additions. While pure magnesium has a relatively good thermal conductivity of
156 W/m-K (the
thermal conductivity is at about % of aluminum (237 W/m-K)), additions of
second phases and the
refining of the grain size in magnesium alloys have resulted in dramatic
reduction in the thermal
conductivity of the magnesium alloys. High strength casting alloys, such as
the AM series alloys
(Mg-Al-Mn alloy) and AZ series alloys (Mg-Al-Zn alloy), have conductivities
significantly below
100 W/m-K, less than half that of comparable strength aluminum alloys.
[0007] The addition of high thermal conductivity phases to some alloys,
such as carbon fibers
and diamond particles, has been known as a method of increasing thermal
conductivity of metals,
generally following the rule of mixtures enhancement of thermal conductivity.
While the addition
of a large volume percent of high thermal conductivity phases, such as SiC and
graphite or carbon
materials, to certain alloys has been shown to increase thermal conductivity
in select alloys, such
additions have typically resulted in increased brittleness of the alloy and
the inability to cast or
otherwise form the alloy. As such, these types of alloys have been
economically unattractive to
use.
2
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
[0008] Attempts to improve mechanical properties of magnesium alloys while
also enhancing
the thermal performance of such alloys by using smaller particles have been
generally
unsuccessful.
[0009] The addition of nanoparticle dispersions has been shown as a way of
increasing
mechanical properties in light metal alloys, including magnesium alloys.
However, the addition
of high surface area materials to the magnesium and other light metals is also
known to decrease
the thermal conductivity of magnesium and other light metal alloys due to high
interfacial
resistance.
[0010] Research published by Hasselman et al. (D.P. Hasselman and L.F.
Johnson; "Effective
Thermal Conductivity of Composites with Interfacial Thermal Barrier
Resistance", J. Composites
21: pp. 508-515, (1987)) determined that interfacial resistance dominates with
particles below 60-
100 microns, and that larger particles are needed to enhance thermal
conductivity of composites.
In aluminum composites, significant work was carried out (by the author and
others) using
controlled interfaces (such as tungsten) to enable finer particles to be
utilized, with some success.
However, additions of nanoscale high conductivity phases have generally not
been successful at
enhancing thermal properties, and most often degrade the properties of
aluminum alloys.
[0011] Li et al. (US 8,734,602) has demonstrated that specific fabrication
routes of adding
nanoparticles can lead to an enhancement of damping (acoustic/thermal loss)
internal resistances
using semi-solid mixing. Much work has been reported about methods for
incorporating
nanoparticles into metals, including adding nanoparticles in the semi-solid
state (which can lead
to high damping, per Li et al.), using high shear and ultrasonic mixing to
disperse particle
agglomerates, and using powder metallurgy to prepare predispersed materials
for melt addition.
Each of these methods, and their associated thermal and mechanical processing
history, lead to
different microstructures and degrees of dispersion of the nanoparticles in
the molten alloy.
[0012] Jin et al. (US 7,959,830) describes the concentrating of high
electrical conductivity
particles near the surface of copper alloys to create a structure with
improved wear resistance
without a significant degradation in electrical properties.
[0013] Angelie et al. (US 6,251,159) discloses a dispersion strengthening
method for metallic
melts that is used to form large particles. The method comprises adding
nanophase particles into
a molten metallic melt and dispersing the nanophase particles in the metallic
melt. The nanophase
3
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
particles comprise particles with diameters in the range of about 5 nanometers
to about 100
nanometers.
[0014] Lim et al. (US 5,614,684) discloses a method for the production of a
superplastic
magnesium-based composite by preparing a composite consisting of ceramic
particles formed of
at least one compound selected from among TiC, AIN, Si3N4, and TiB2 and a
matrix formed of a
magnesium alloy.
[0015] In view of the prior art, there remains a need to form a magnesium-
based composite
that has improved thermal conductivity and which magnesium-based composite
does not have
increased brittleness, and which alloy can be cast or otherwise formed.
SUMMARY OF THE INVENTION
[0016] The present invention is directed to a cast or wrought magnesium-
based composite
incorporating insoluble nanoparticles and optional insoluble micron-sized
particles, and method
for manufacture of such magnesium-based composite. The magnesium-based
composite has
improved thermal, physical, and mechanical properties as compared to prior art
magnesium alloys.
The nanoparticles and optional micron-sized particles can be selected and used
in quantities so that
the grain boundaries of the magnesium-based composite contain a desired
composition and
morphology to achieve the desired physical and chemical properties of the
composite and to
optionally obtain a specific galvanic corrosion rate in the entire composite
or along the grain
boundaries of the composite. The addition of insoluble particles to the metal
or metal alloy can be
used to enhance mechanical properties of the magnesium-based composite, such
as ductility and/or
tensile strength. The final casting (when used) can optionally be enhanced by
heat treatment as
well as deformation processing (such as extrusion, forging, or rolling) to
further improve the
strength of the final magnesium-based composite over the as-cast material. The
deformation
processing achieves strengthening by reducing the grain size of the magnesium-
based composite.
Further enhancements, such as traditional alloy heat treatments (such as
solutionizing, aging and
cold working) can optionally be used without dissolution impact if further
improvements are
desired.
[0017] In one non-limiting aspect of the invention, a cast or wrought
structure of the
magnesium-based composite can be made into almost any shape.
4
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
[0018] In another and/or alternative non-limiting aspect of the invention,
ultrasonic dispersion
and/or electro-wetting of insoluble nanoparticles can be used for further
enhancement of strength
and/or ductility the magnesium-based composite.
[0019] In still another and/or alternative non-limiting aspect of the
invention, a magnesium-
based composite is formed of by casting with at least one insoluble phase in
discrete particle form
in the metal or metal alloy. The discrete insoluble particles generally have a
different galvanic
potential from the base metal or metal alloy. The discrete insoluble particles
are generally
uniformly dispersed through the base metal or base metal alloy using
techniques such as
thixomolding, stir casting, mechanical agitation, electrowetting, ultrasonic
dispersion, and/or
combinations of these methods; however, this is not required. Due to the
insolubility and
difference in atomic structure in the melt material and the insoluble
particles, the insoluble particles
will be pushed to the grain boundary during casting solidification. Because
the insoluble particles
will generally be pushed to the grain boundary, such feature makes engineering
grain boundaries
to control the dissolution rate of the casting possible, if so desired. This
feature also allows for
further grain refinement of the final alloy through traditional deformation
processing to increase
tensile strength, elongation to failure, and/or other properties in the alloy
system that are not
achievable without the use of insoluble particle additions. The ratio of
insoluble particles in the
grain boundary is generally constant and the grain boundary to grain surface
area is typically
consistent even after deformation processing and heat treatment of the
magnesium-based
composite.
[0020] In yet another and/or alternative non-limiting aspect of the
invention, the magnesium-
based composite can be designed to corrode at the grains, the grain
boundaries, and/or the insoluble
particle additions.
[0021] In another and/or alternative non-limiting aspect of the invention,
when a slower
corrosion rate is desired, two or more different insoluble particle
compositions can be added to the
base metal or base metal alloy to be deposited at the grain boundary. When the
exposed surface
area of the second insoluble particle composition is removed from the system,
the system reverts
to the two previous embodiments described above until more particles of the
second insoluble
particle composition are exposed. This arrangement creates a mechanism to
retard the corrosion
rate with minor additions of the second insoluble particle composition.
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
[0022] In still another and/or alternative non-limiting aspect of the
invention, the rate of
corrosion in the entire casting system can be controlled by the surface area
and, thus, the particle
size and morphology of the insoluble particle additions.
[0023] In yet another and/or alternative non-limiting aspect of the
invention, there is provided
a magnesium-based composite wherein the grain boundary composition and the
size and/or shape
of the insoluble phase additions can be used to control the dissolution rate
of such composite. The
composition of the grain boundary layer can optionally include two added
insoluble particles
having a different composition with different galvanic potentials as compared
to the base metal or
base metal alloy. As defined herein, insoluble particles (e.g., insoluble
nanoparticles, insoluble
micron-sized particles) have a solubility in the base metal or base metal
alloy (e.g., magnesium or
magnesium alloy) of less than about 5% (e.g., 0%-4.9999% and all values and
ranges
therebetween) when forming the magnesium-based composite, typically less than
about 1%, and
more typically less than about 0.5%. The strength of the magnesium-based
composite can
optionally be increased using deformation processing and a change dissolution
rate of less than
about 20% (e.g., 0.0001-19.999% and all values and ranges therebetween),
typically less than
about 10%, and more typically less than about 5%. The ductility of the
magnesium-based
composite can optionally be increased using insoluble nanoparticle additions.
The magnesium-
based composite can optionally include chopped fibers.
[0024] The insoluble particle additions (e.g., insoluble nanoparticles,
insoluble micron-sized
particles) to the magnesium-based composite can be used to improved toughness
of the
magnesium-based composite. The magnesium-based composite can have improved
tensile
strength and/or elongation due to heat treatment without significantly
affecting the dissolution rate
of the magnesium-based composite. The magnesium-based composite can have
improved tensile
strength and/or elongation by extrusion and/or another deformation process for
grain refinement
without significantly affecting the dissolution rate of the magnesium-based
composite. In such a
process, the dissolution rate change can be less than about 10% (e.g., 0-10%
and all values and
ranges therebetween), typically less than about 5%, and more typically less
than about 1%. The
magnesium-based composite can optionally have controlled or engineered
morphology (particle
shape and size of the insoluble particle additions) to control the dissolution
rate of the magnesium-
based composite. The insoluble particles in the magnesium-based composite can
optionally have
a surface area of 0.001m2/g-200m2/g (and all values and ranges therebetween).
The insoluble
6
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
particles in the magnesium-based composite optionally are or include non-
spherical particles. The
insoluble nanoparticles in the magnesium-based composite optionally are or
include nanotubes
and/or nanowires. The non-spherical insoluble particles can be added to
control corrosion rates
without changing composition. The insoluble particles in the magnesium-based
composite
optionally are or include spherical particles. The spherical particles (when
used) can have the
same or varying diameters. Such particles are optionally used at the same
volume and/or weight
fraction to increase cathode particle surface area to control corrosion rates
without changing
composition. Particle reinforcement in the magnesium-based composite can
optionally be used to
improve the mechanical properties of the magnesium-based composite and/or to
act as part of the
galvanic couple. The insoluble particles in the magnesium-based composite can
optionally be used
as a grain refiner, as a stiffening phase to the base metal or base metal
alloy, and/or to increase the
strength of the magnesium-based composite. The insoluble particles in the
magnesium-based
composite can optionally be less than about 1 tm in size (e.g., 0.001-0.999 pm
and all values and
ranges therebetween), typically less than about 0.51.1m, more typically less
than about 0.11.im, and
more typically less than about 0.05 tm. The insoluble particles can optionally
be dispersed
throughout the magnesium-based composite using ultrasonic means, by
electrowetting of the
insoluble particles, and/or by mechanical agitation. The magnesium-based
composite can
optionally be used to form all or part of a device for use in hydraulic
fracturing systems and zones
for oil and gas drilling, wherein the device has a designed dissolving rate.
The magnesium-based
composite can optionally be used to form all or part of a device for
structural support or component
isolation in oil and gas drilling and completion systems, wherein the device
has a designed
dissolving rate.
[0025] In still yet another and/or alternative non-limiting aspect of the
invention, there is
provided a magnesium-based composite that includes a base metal or base metal
alloy and a
plurality of insoluble particles disbursed in said magnesium-based composite,
wherein the
insoluble particles have a melting point that is greater than a melting point
of the base metal or
base metal alloy, and at least 50% of the insoluble particles are located in
grain boundary layers of
the magnesium-based composite. The insoluble particles can optionally have a
selected size and
shape to control a dissolution rate of the magnesium-based composite. The
insoluble particles can
optionally have a different galvanic potential than a galvanic potential of
the base metal or base
metal alloy. The major component of the grain boundary layer optionally has a
different
7
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
composition than the base metal or base metal alloy. The insoluble particles
optionally: a) increase
ductility of said magnesium-based composite; b) improve toughness of said
magnesium-based
composite; c) improve elongation of said magnesium-based composite; d)
function as a grain
refiner in said magnesium-based composite; e) function as a stiffening phase
to said base metal or
base metal alloy; f) increase strength of said magnesium-based composite; or
g) combinations
thereof.
[0026] There is provided a method for forming a magnesium-based composite that
includes:
a) providing one or more metals used to form a base metal or base metal alloy;
b) providing a
plurality of insoluble nanoparticles having a melting point that is greater
than a melting point of
the base metal or base metal alloy; c) heating the one or more metals until
molten; d) mixing the
one or more molten metals and the plurality of insoluble particles to form a
mixture and to cause
the plurality of insoluble particles to disperse in the mixture; and e)
cooling the mixture to form
the magnesium-based composite; and, wherein the plurality of insoluble
particles are disbursed in
the magnesium-based composite. Generally, at least 50% of the plurality of the
insoluble
nanoparticles are located in the grain boundary layers of the magnesium-based
composite;
however, this is not required. The step of mixing optionally includes mixing
using one or more
processes selected from the group consisting of thixomolding, stir casting,
mechanical agitation,
electrowetting and ultrasonic dispersion. The method optionally includes the
step of heat treating
the magnesium-based composite to improve the tensile strength, elongation, or
combinations
thereof the magnesium-based composite without significantly affecting a
dissolution rate of the
magnesium-based composite. The method optionally includes the step of
extruding or deforming
the magnesium-based composite to improve the tensile strength, elongation, or
combinations
thereof of said magnesium-based composite without significantly affecting a
dissolution rate of
the magnesium-based composite. The method optionally includes the step of
forming the
magnesium-based composite into a device for: a) separating hydraulic
fracturing systems and
zones for oil and gas drilling; b) structural support or component isolation
in oil and gas drilling
and completion systems; or c) combinations thereof. There is provided a method
for forming a
magnesium-based composite that includes mixing a base metal or a base metal
alloy in molten
form with insoluble particles to form a mixture, and cooling the mixture to
form a magnesium-
based composite.
8
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
[0027] The magnesium-based composite includes a modification of grain boundary
thermal or
sliding resistance through the addition of insoluble nanoparticles to high
strength magnesium
alloys. The magnesium-based composite has a 10% or greater improvement in
thermal
conductivity, strength, or strain to failure compared to the base material.
For thermally-conductive
applications, the magnesium-based composite thermal conductivity is greater
than about 140 W/m-
K, typically greater than about 160 W/m-K, and more typically greater than
about 180 W/m-K.
The thermal conductivity of a typical AZ91 base material is about 90, pure Mg
is about 140.
[0028] It has been found that the addition of insoluble high thermal
conductivity nanoparticles
in a molten magnesium or magnesium alloy to cause such nanoparticles to
concentrate in the
interface/second phase regions at the junction of primary grains and at the
interfaces of secondary
particles and phases leads to a significant enhancement of both thermal
conductivity, as well as
strain tolerance and processability of the magnesium alloy. In one non-
limiting aspect of the
invention, the increase in thermal conductivity of the magnesium-based
composite (as compared
to magnesium or a magnesium alloy that is absent the insoluble nanoparticles)
is at least about
10%, typically at least about 15%, more typically at least about 20%, and
still more typically at
least about 30%. For some magnesium-based composite, the increase in thermal
conductivity of
the magnesium-based composite (as compared to magnesium or a magnesium alloy
that is absent
the insoluble nanoparticles) is at least 50%, and in some instances greater
than 100%.
[0029] By enhancing the strength of these boundary and interfacial regions,
while reducing
their thermal resistance, a high thermal conductivity magnesium alloy with
excellent processability
and mechanical properties (particularly toughness or ductility) can be
obtained. It was discovered
that these phases could be ex situ (e.g., added), such as carbon nanotubes or
nanodiamonds, or
could be internally formed during cooling, such as Mg-Cu intermetallics.
[0030] When the magnesium-based composite includes at least 0.1% by volume of
insoluble
nanoparticles in a magnesium alloy such as AZ91, the thermal conductivity of
engineering the
magnesium-based composite was found to be increased by 10-100% (and all values
and ranges
therebetween) or more, while strain to failure was found to increase by 10-
100% (and all values
and ranges therebetween) or more as compared to a AX91 alloy that was absent
the insoluble
nanoparticles. As an added benefit, the magnesium-based composite appeared to
inhibit
macrosegregation, thus increasing hot tear strength and melt fluidity in the
near-liquidus region as
compared magnesium or a magnesium alloy that was absent the insoluble
nanoparticles. In one
9
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
non-limiting embodiment of the invention, the magnesium-based composite
includes at least about
0.1 vol.% insoluble nanoparticles. In another non-limiting embodiment, the
magnesium-based
composite includes about 0.1-20 vol.% (and all values and ranges therebetween)
insoluble
nanoparticles, and typically the magnesium-based composite includes about 0.1-
10 vol.%
insoluble nanoparticles, more typically the magnesium-based composite includes
about 0.2-5
vol.% insoluble nanoparticles, still more typically the magnesium-based
composite includes about
0.5-4 vol.% insoluble nanoparticles, and even still more typically the
magnesium-based composite
includes about 0.5-3 vol.% insoluble nanoparticles. The insoluble
nanoparticles generally have an
average particle size and/or have at least one dimension of at least 10 nm,
and typically at least 60
nm, and typically no more than about 400 nm, typically the insoluble
nanoparticles have an average
particle size and/or have at least one dimension of no more than about 300 nm,
more typically the
insoluble nanoparticles have an average particle size and/or have at least one
dimension of no more
than about 250 nm, and still more typically the insoluble nanoparticles have
an average particle
size and/or have at least one dimension of no more than about 200 nm. In one
non-limiting
embodiment of the invention, at least about 5% of the insoluble nanoparticles
have an average
particle size and/or have at least one dimension of no more than about 200 nm,
typically at least
about 10% of the insoluble nanoparticles have an average particle size and/or
have at least one
dimension of no more than about 200 nm, more typically at least about 20% of
the insoluble
nanoparticles have an average particle size and/or have at least one dimension
of no more than
about 200 nm, and still more typically at least about 30% of the insoluble
nanoparticles have an
average particle size of no more than about 200 nm. Larger particles (e.g.,
insoluble nanoparticles
greater than 400 nm, and insoluble micron-sized particles) can be added to the
magnesium-based
composite to increase the hardness and the stiffness of the magnesium-based
composite. These
larger particles can generally be added in larger volumes than the 400 nm or
smaller insoluble
nanoparticles can be added to the magnesium-based composite without
percolation; however, this
is not required. These larger particles have been found to have less of
tendency to concentrate at
the grain boundaries or dislocations in the magnesium-based composite of the
magnesium-based
composite.
[0031] In accordance with another and/or alternative non-limiting aspect of
the invention, the
insoluble nanoparticles that are included in the magnesium-based composite are
caused to be at
least partially segregated so as to be located within about 200 nm of the
grain boundaries or
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
dislocations in the magnesium-based composite (e.g., 0-200 nm and all values
and ranges
therebetween), typically located within about 100 nm of the grain boundaries
or dislocations in the
magnesium-based composite, and more typically located within about 50 nm of
the grain
boundaries or dislocations in the magnesium-based composite.
[0032] In accordance with another and/or alternative non-limiting aspect of
the invention, the
insoluble nanoparticles can include one or more types of materials. In one non-
limiting
embodiment, the insoluble nanoparticles can include fullerenes (including
multi-walled and
single-walled carbon nanotubes, graphene, nanodiamonds, buckeyballs); inert
ceramics, including
submicron and nanoparticles (including nanotubes, platelets, and flakes) of W,
SiC, AIN, Be0,
BN, and TiB2, as well as high thermal conductivity MAX phase materials (e.g.,
MAX phases -
AB-X compounds with laminate structures); and/or intermetallic particles
containing high thermal
conductivity Cu, Ag, Al, Be, and/or Au compounds.
[0033] In accordance with another and/or alternative non-limiting aspect of
the invention,
there is provided a magnesium-based composite that optionally includes
insoluble micron-sized
particles. The addition of micron-sized particles to the magnesium-based
composite can be used
to increase the hardness and stiffness of the magnesium-based composition;
however, this is not
required. In another and/or alternative non-limiting embodiment of the
invention, the insoluble
micron-sized particles (when used) have an average particle size of about 1-
800 microns (and all
values and ranges therebetween), typically have an average particle size of
about 2-500 microns,
more typically have an average particle size of about 10-300 microns. In
accordance with another
and/or alternative non-limiting aspect of the invention, the insoluble micron-
sized particles
constitute at least 1% by volume of the magnesium-based composite, typically
about 0.1-49.5
vol.% (and all values and ranges therebetween) of the magnesium-based
composite, more typically
about 5-45 vol.% of the magnesium-based composite, and more typically 5-30
vol.% of the
magnesium-based composite. The maximum vol.% of insoluble micron-sized
particles that can
be included in the magnesium-based composition is generally greater than the
maximum vol.% of
insoluble nanoparticles having a size of no greater than 400 nm; however, this
is not required.
[0034] In accordance with another and/or alternative non-limiting aspect of
the invention,
when the magnesium-sized composite includes insoluble micron-sized particles,
the micron-sized
particles have an average high thermal conductivity of greater than about 140
W/m-K, typically
11
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
greater than about 160 W/m-K, more typically greater than about 180 W/m-K,
still more typically
greater than about 200 W/m-K, and yet still more typically greater than about
250 W/m-K.
[0035] In accordance with another and/or alternative non-limiting aspect of
the invention, the
insoluble micron-sized particles (when used), can include one or more types of
materials. In one
non-limiting embodiment, the micron-sized particles can include carbon fiber;
SiC particles, fibers
or whiskers; heat-treated graphite; AIN; BN; and/or other high thermal
conductivity, thermally-
stable materials.
[0036] In accordance with another and/or alternative non-limiting aspect of
the invention,
there is provided a magnesium-based composite that is formed from magnesium or
a magnesium
alloy and the addition of one or more types of insoluble nanoparticles. When a
magnesium alloy
is used as the base metal to form the magnesium-based composite, the magnesium
alloy can be
selected from: AE series alloys (e.g., Mg-Al-Re alloys such as AE42, etc.), AJ
series alloys (e.g.,
Mg-Al-Sr alloys), AM series alloys (e.g., Mg-Al-Mn alloys such as AM20, AM50,
AM60, etc.),
AS series alloys (AS21, AS 41, etc.), AX series alloys (Mg-Al-Ca), AXJ series
alloys (e.g., Mg-
Al-Ca-Sr alloys), AZ series alloys (e.g., Mg-Al-Zn alloys such as AZ31, AZ61,
AZ80, AZ91, etc.),
Elektron 21 series alloys (e.g., Mg-Gd-Nd-Zr alloys such as Elektron ZRE1,
etc.), LPSO alloys
(e.g., Mg-Zn-Re alloys, Mg-Zn-Y alloys, Mg-Zn-Dy alloys, Mg-Zn-Ho alloys, Mg-
Zn-Er alloys,
Mg-Zn-Tm alloys, Mg-Zn-Gd alloys, Mg-Zn-Tb alloys, etc.), QE series alloys
(e.g., Mg-Ag-Nd-
Zr alloys), WE series alloys (Mg-Y-RE), ZE series alloys (e.g., Mg-Zn-RE-Zr
alloys such as ZI-41,
ZE 63, etc.), ZK series alloys (Mg-Zn-Zr), Z1V15 series alloys (e.g., Mg¨Al¨Zn
alloy), ZMS series
alloys (Mg-Zn-Mn-Sn), or ZW series alloys (Mg-Zn-Y).
[0037] In accordance with another and/or alternative non-limiting aspect of
the invention,
there is provided a magnesium-based composite that has an improved hot tear
strength as
compared to a magnesium or a magnesium alloy that is absent insoluble
nanoparticle additions,
and which magnesium-based composite can be die-cast and formed into complex
thin-walled
shapes. In one non-limiting embodiment of the invention, the hot tear strength
of the magnesium-
based composite is increased by at least about 15% as compared to magnesium or
a magnesium
alloy that is absent insoluble nanoparticle additions, typically the hot tear
strength of the
magnesium-based composite is increased by more than 20% as compared to
magnesium or a
magnesium alloy that is absent insoluble nanoparticle additions, and more
typically the hot tear
12
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
strength of the magnesium-based composite is increased by more than 30% as
compared to
magnesium or a magnesium alloy that is absent insoluble nanoparticle
additions.
[0038] In accordance with another and/or alternative non-limiting aspect of
the invention, the
magnesium-based composite can be cast (e.g., sand casting, investment casting,
and die
casting/other permanent mold casting) to have a wall thickness of less than
about 2 mm, typically
the magnesium-based composite can be cast to have a wall thickness of less
than about 1.5 mm,
and more typically the magnesium-based composite can be cast to have a wall
thickness of less
than about 1 mm.
[0039] In accordance with another and/or alternative non-limiting aspect of
the invention, the
insoluble nanoparticles can be formed ex situ and added to the magnesium or
magnesium-based
alloy while the magnesium or magnesium-based alloy is molten, using various
methods to control
wetting and dispersion of the insoluble nanoparticles in the molten magnesium
or magnesium-
based alloy.
[0040] In accordance with another and/or alternative non-limiting aspect of
the invention, the
insoluble nanoparticles can be formed in situ, through addition of reactive
species or alloying
elements while the magnesium or magnesium-based alloy is in a molten state.
[0041] In summary, the invention pertains to a magnesium-based composite
that is selected
from magnesium or a magnesium alloy having good strength such as magnesium
alloys of AE
series alloys, AJ series alloys, AM series alloys, AS series alloys, AX series
alloys, AXJ series
alloys, AZ series alloys, Elektron 21 series alloys, LPSO alloys, QE series
alloys, WE series alloys,
"tli series alloys, ZK series alloys, ZM5 series alloys, ZMS series alloys, or
ZW series alloys, to
which about 0.1-20 vol.% insoluble nanoparticles are included in the magnesium
or magnesium
alloy (e.g., base magnesium metal or base magnesium alloy). The insoluble
nanoparticles can be
formed of one or more materials, and have the same or different size and
shape. Generally, a
majority of the insoluble nanoparticles have an average particle size and/or
have at least one
dimension that is no more than about 400 nm. The insoluble nanoparticles have
a thermal
conductivity that is greater than about 140 W/m-K. The insoluble nanoparticles
are caused to be
at least partially segregated so as to be located within about 200 nm of grain
boundaries or
dislocations in the magnesium or base magnesium alloy. The insoluble
nanoparticle additions
result in at least about a 10% increase in thermal conductivity, strength,
modulus, ductility, and/or
13
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
other selected property of the magnesium-based composite as compared to
magnesium or a
magnesium alloy that is absent the insoluble nanoparticles.
[0042] The magnesium-based composite can be subjected to deformation
processing to have
an tensile yield strength that is greater than about 35 ksi, and typically
greater than about 45 ksi,
while retaining significant ductility of more than 5%, and preferably more
than 15% strain to
failure.
[0043] The magnesium-based composite can be subjected to semi-solid
processing such as
thixomolding, thixocasting, continuous reheocasting, SIMA processing, and/or
other processing
techniques to further improve ductility of the material.
[0044] The magnesium-based composite can have a strain to failure exceeding
about 15%, and
preferably exceeds about 20%, compared to 3-14% for magnesium and magnesium
alloy.
[0045] The magnesium-based composite can be annealed to create a desired
microstructure
(e.g., stable LPSO phases, alpha-beta microstructures, homogenous solid
solutions, or uniform fine
precipitates) prior to deformation processing.
[0046] The magnesium-based composite can retain excellent mechanical
properties as-cast,
with or without heat treatment, to include an elongation to failure above
about 5%, and generally
above about 10%. Magnesium and magnesium alloys generally have an elongation
to failure of
no more than about 3%.
[0047] The magnesium-based composite can be die-cast and formed into complex
thin-walled
shapes. The magnesium-based composite can have improved hot tear strength of
about 10-15%
as compared to magnesium or magnesium alloys that are absent insoluble
nanoparticles.
[0048] The magnesium-based composite can have a wall thickness of the
casting (e.g., sand
casting, investment casting, and die casting/other permanent mold casting) of
less than about 2
mm, and as thin as about 0.3-0.5 mm. Such thickness can be useful in
applications such as
automotive and aerospace applications.
[0049] The magnesium-based composite can include one or more nanoparticles
that can
include fullerenes (including multi-walled and single-walled carbon nanotubes,
graphene,
nanodiamonds, buckeyballs); inert ceramics, including submicron and
nanoparticles (including
nanotubes, platelets, and flakes) of W, SiC, MN, Be0, BN, and TiB2, as well as
high thermal
conductivity MAX phase materials (e.g., MAX phases - AB-X compounds with
laminate
14
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
structures); and/or intermetallic particles containing high thermal
conductivity Cu, Ag, Al, Be,
and/or Au compounds.
[0050] The magnesium-based composite can include nanoparticles, and wherein
larger
nanoparticles can optionally be present. The magnesium-based composite can
optionally include
insoluble micron-sized particles. The average particle size of the insoluble
micron-sized particles
is about 1-800 microns (and all values and ranges therebetween). The insoluble
micron-sized
particles generally have a high thermal conductivity that is greater than
about 140 W/m-K. The
insoluble micron-sized particles can constitute 0.1-49.5 vol.% (and all values
and ranges
therebetween) of the magnesium-based composite. When insoluble micron-sized
particles are
included in the magnesium-based composite, such micron-sized particles can
include one or more
materials such as diamond; heat-treated carbon fiber; SiC particles, fibers or
whiskers; heat- treated
graphite; AIN; BN; and/or other high thermal conductivity, thermally-stable
materials.
[0051] When forming the magnesium-based composite, the insoluble nanoparticles
can be
formed ex situ and can be added to the magnesium or magnesium alloy while the
magnesium or
magnesium alloy is molten, and by using various methods to control wetting and
dispersion of the
insoluble nanoparticles in the molten metal. Wetting of the insoluble
nanoparticles can be
controlled by pre-dispersion or pre-alloying, by temperature, by surface
treatments, by high shear
forces, by mechanical (ultrasound or vibrational) or electromagnetic methods
to break metal
surface tension and force intimate contact and wetting.
[0052] When forming the magnesium-based composite, the insoluble nanoparticles
can be
formed in situ and can be added through addition of reactive species or
alloying elements. For
example, the addition of B to the molten magnesium or magnesium alloy can be
used to create
insoluble MgB2 nanoparticles. Also, the addition of polyacrylonitrile, phenol,
preceramic
polymer, or other prolyzable precursor can be used to create insoluble
nanoparticles while the
magnesium or magnesium alloy is in a molten state or during the process of
solidifying from the
molten state. Also, nitrogen, BC13, or other reactive gas can be added to the
molten magnesium or
magnesium alloy make corresponding insoluble nanoparticles, such as MgN or
MgB2.
[0053] The magnesium-based composite can also include galvanically-active
phases that
produce a controlled dissolution rate of the composite in the presence of tap
water or brine or
fracking liquid of 10-200 g/cm2/hr. (and all values and ranges therebetween)
at a temperature of at
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
least 55 C, typically at a temperature of at least 75 C, more typically at a
temperature of at least
about 90 C, and even more typically at a temperature of at least about 135 C.
[0054] The magnesium-based composite can optionally be solution treated
(e.g., to
homogenize the material by solutionizing precipitation species) at
temperatures from about 250-
550 C (and all values and ranges therebetween).
[0055] The magnesium-based composite can optionally be age hardened or
tempered at about
150-350 C (and all values and ranges therebetween). The time and temperature
generally are
selected to create a uniform dispersion of very fine precipitates in the
magnesium-based composite.
Longer times are used at lower temperatures, but with less sensitivity. Higher
temperatures for
shorter times can be used to achieve peak aging, overaging, or underaging to
tailor properties, but
with much more control over time and temperature than lower temperature aging
processes.
[0056] The magnesium-based composite can optionally be subjected to
thermomechanical
processes (e.g., solutionizing, deforming, and then annealing/aging).
[0057] The magnesium-based composite can optionally be extruded, rolled,
forged, drawn or
stamped at temperatures from 250-500 C (and all values and ranges
therebetween) to refine the
grain structure in the composite so as to improve the mechanical, thermal
and/or electrical
properties of the composite. Generally, improvements of greater than 15-20%,
and typically
greater than 30% are achieved in the magnesium-based composite by such further
processing as
compared to magnesium or magnesium alloys that are absent insoluble
nanoparticles.
[0058] The magnesium-based composite generally includes at least 80 wt.%
magnesium, and
typically about 80-97 wt.% (and all values and ranges therebetween).
[0059] The magnesium-based composite can be formed from a magnesium alloy
wherein the
magnesium content of the alloy is at least about 80 wt.% and the alloy
includes one or more of the
following alloying agents a) 0.1-10 wt.% aluminum, b) 0.1-9 wt.% calcium, c)
0.1-3 wt.%
strontium, d) 0.1-6 wt.% zinc, e) 0.1-1 wt.% zirconium, f) 0.1-5 wt.% niobium,
g) 0.1-10 wt.%
lithium, h) 0.1-8 wt.% tin, i) 0.1-10 wt.% lanthanide elements, and j) 0.1-10
wt.% yttrium. When
lanthanide elements or yttrium are included in the magnesium alloy, such
alloying agents can form
a long period stacking order, or LPSO phase in the magnesium alloy.
[0060] The magnesium-based composite generally retains at least 70% of its
room temperature
(e.g., 25 C) tensile strength properties at 150 C, and typically retains at
least 85% of its room
temperature tensile strength properties at 150 C. The magnesium-based
composite generally
16
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
retains at least 70% of its room temperature mechanical properties at 185 C,
and typically retains
at least 70% of its room temperature mechanical properties at 200 C.
[0061] The magnesium-based composite generally retains an elongation to
failure of at least
8% at 25 C.
[0062] The magnesium-based composite can optionally include 1-4 wt.% Ca to
increase the
ignition temperature of the magnesium-based alloy to above 700 C; however,
this is not require.
[0063] One non-limiting objective of the present invention is the provision
of a castable,
moldable, or extrudable magnesium-based composite using a metal or metallic
primary alloy that
includes insoluble particles dispersed in the metal or metallic primary alloy.
[0064] Another and/or alternative non-limiting objective of the present
invention is the
provision of selecting the type and quantity of insoluble particles so that
the grain boundaries of
the magnesium-based composite have a desired composition and/or morphology to
achieve a
specific galvanic corrosion rate in the entire composite and/or along the
grain boundaries of the
magnesium-based composite.
[0065] Still another and/or alternative non-limiting objective of the
present invention is the
provision of a magnesium-based composite that has insoluble particles located
at the grain
boundary during the solidification of the magnesium-based composite.
[0066] Yet another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite wherein the insoluble particles can
be controllably
located in the magnesium-based composite in the final casting, as well as the
surface area ratio,
which enables the use of lower cathode particle loadings compared to a powder
metallurgical or
alloyed composite to achieve the same dissolution rates.
[0067] Still yet another and/or alternative non-limiting objective of the
present invention is the
provision of a magnesium-based composite wherein the insoluble particles can
be used to enhance
mechanical properties of the composite, such as ductility and/or tensile
strength.
[0068] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that can be enhanced by heat
treatment as well as
deformation processing (such as extrusion, forging, or rolling) to further
improve the strength of
the final composite.
[0069] Still another and/or alternative non-limiting objective of the
present invention is the
provision of a magnesium-based composite that can be designed such that the
rate of corrosion can
17
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
be controlled through use of certain insoluble particle sizes (while not
increasing or decreasing the
volume or weight fraction of the insoluble particles) and/or by changing the
volume/weight
fraction (without changing the insoluble particle size).
[0070] Yet another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that can be can be made into almost
any shape.
[0071] Still yet another and/or alternative non-limiting objective of the
present invention is the
provision of a magnesium-based composite that, during solidification, the
insoluble particles are
pushed to the grain boundaries and the grain boundary composition is modified
to achieve the
desired dissolution rate.
[0072] Still yet another and/or alternative non-limiting objective of the
present invention is the
provision of a magnesium-based composite that can be designed such that
galvanic corrosion only
affects the grain boundaries and/or affects the grains based on the
composition of the nanoparticles.
[0073] Another and/or alternative non-limiting objective of the present
invention is the
provision of dispersing the insoluble particles in the magnesium-based
composite by
thixomolding, stir casting, mechanical agitation, electrowetting, ultrasonic
dispersion and/or
combinations of these processes.
[0074] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite with at least one insoluble phase in
discrete particle
form in the metal or metal alloy, and wherein the discrete insoluble particles
have a different
galvanic potential from the base metal or metal alloy.
[0075] Still another and/or alternative non-limiting objective of the
present invention is the
provision of a magnesium-based composite wherein the ratio of insoluble
particles in the grain
boundary is generally constant and the grain boundary to grain surface area is
typically consistent
even after deformation processing and/or heat treatment of the composite.
[0076] Yet another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite designed to corrode at the grains,
the grain boundaries,
and/or the insoluble particle additions depending on selecting where the
particle additions fall on
the galvanic chart.
[0077] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite wherein galvanic corrosion in the
grains can be
promoted by selecting a base metal or base metal alloy that sits at one
galvanic potential in the
18
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
operating solution of choice where its major grain boundary alloy composition
will have a different
galvanic potential as compared to the matrix grains (i.e., grains that form in
the casted base metal
or base metal alloy), and an insoluble particle addition can be selected that
also has a different
galvanic potential.
[0078] Still another and/or alternative non-limiting objective of the
present invention is the
provision of a magnesium-based composite having a slower corrosion rate by
adding two or more
different insoluble components to the base metal or base metal alloy to be
deposited at the grain
boundary, wherein the second insoluble component is the most anodic in the
entire system.
[0079] Still yet another and/or alternative non-limiting objective of the
present invention is the
provision of a magnesium-based composite wherein the rate of corrosion in the
entire system can
be controlled by the surface area and, thus, the insoluble particle size and
morphology of the
insoluble particle additions.
[0080] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite wherein the grain boundary
composition and the size
and/or shape of the insoluble particles can be used to control the dissolution
rate of such
magnesium-based composite.
[0081] Still another and/or alternative non-limiting objective of the
present invention is the
provision of a magnesium-based composite that includes two added insoluble
components with
different galvanic potentials, which insoluble components either are more
anodic or more cathodic
as compared to the base metal or base metal alloy.
[0082] Yet another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that includes insoluble particles
that have a solubility
in the base metal or base metal alloy of no more than about 5%.
[0083] Another and/or alternative non-limiting objective of the present
invention is the
provision of a method for producing a magnesium-based composite that includes
the steps: of a)
providing one or more metals used to form a base metal or base metal alloy; b)
providing a plurality
of insoluble nanoparticles have a melting point that is greater than a melting
point of the base metal
or base metal alloy; c) heating the one or more metals until molten; d) mixing
the one or more
molten metals and the plurality of insoluble particles to form a mixture and
to cause the plurality
of insoluble particles to disperse in the mixture; e) cooling the mixture to
form the magnesium-
19
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
based composite; and, wherein the plurality of insoluble particles are
disbursed in the magnesium-
based composite.
[0084] Yet another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite wherein at least 50% of the plurality
of the insoluble
nanoparticles is located in the grain boundary layers of the magnesium-based
composite.
[0085] Another and/or alternative non-limiting objective of the present
invention is the
provision of a method for producing a magnesium-based composite that includes
the optional step
of mixing using one or more processes selected from the group consisting of
thixomolding, stir
casting, mechanical agitation, electrowetting and ultrasonic dispersion.
[0086] Another and/or alternative non-limiting objective of the present
invention is the
provision of a method for producing a magnesium-based composite that includes
the optional step
of heat treating the magnesium-based composite to improve the tensile
strength, elongation, or
combinations thereof the magnesium-based composite without significantly
affecting a dissolution
rate of the magnesium-based composite.
[0087] Another and/or alternative non-limiting objective of the present
invention is the
provision of a method for producing a magnesium-based composite that includes
the optional step
of extruding or deforming the magnesium-based composite to improve the tensile
strength,
elongation, or combinations thereof of said magnesium-based composite without
significantly
affecting a dissolution rate of the magnesium-based composite.
[0088] Another and/or alternative non-limiting objective of the present
invention is the
provision of a method for producing a magnesium-based composite that includes
the optional step
of forming the magnesium-based composite into a device for: a) separating
hydraulic fracturing
systems and zones for oil and gas drilling; b) structural support or component
isolation in oil and
gas drilling and completion systems; or c) combinations thereof.
[0089] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that includes a modification of grain
boundary thermal
or sliding resistance through the addition of insoluble nanoparticles to high
strength magnesium
alloys.
[0090] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that has a 10% or greater improvement
in thermal
conductivity, strength, or strain to failure compared to the base material.
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
[0091] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite wherein the thermal conductivity is
greater than about
140 W/m-K, typically greater than about 160 W/m-K, and more typically greater
than about 180
W/m-K.
[0092] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite wherein the insoluble nanoparticles
concentrate in the
interface/second phase regions at the junction of primary grains and at the
interfaces of secondary
particles and phases leads.
[0093] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite wherein an increase in thermal
conductivity of the
magnesium-based composite as compared to magnesium or a magnesium alloy that
is absent the
insoluble nanoparticles is at least about 10%, typically at least about 15%,
more typically at least
about 20%, and still more typically at least about 30%.
[0094] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that has enhanced strength at the
boundary and
interfacial regions, while reducing its thermal resistance.
[0095] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that has enhanced strength at the
boundary and
interfacial regions, while reducing its thermal resistance with excellent
processability and
mechanical properties (particularly toughness or ductility).
[0096] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that includes at least 0.1% by volume
of insoluble
nanop articles.
[0097] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite wherein the thermal conductivity is
increased by at
least 10%, the strain to failure is increased by at least 10%.
[0098] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that inhibits macrosegregation, thus
increasing hot tear
strength and melt fluidity in the near-liquidus region as compared magnesium
or a magnesium
alloy that was absent the insoluble nanoparticles.
21
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
[0099] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that has insoluble nanoparticles
located within about
200 nm of the grain boundaries or dislocations in the magnesium-based
composite.
[00100] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite wherein the insoluble nanoparticles
can include one
or more types of materials.
[00101] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite wherein the insoluble nanoparticles
can include
fullerenes (including multi-walled and single-walled carbon nanotubes,
graphene, nanodiamonds,
buckeyballs); inert ceramics, including submicron and nanoparticles (including
nanotubes,
platelets, and flakes) of W, SiC, MN, Be0, BN, and TiB2, as well as high
thermal conductivity
MAX phase materials; and/or intermetallic particles containing high thermal
conductivity Cu, Ag,
Al, Be, and/or Au compounds. MAX phase materials are layered, hexagonal
carbides and nitrides
have the general formula: M
- -n-FiAXn, (MAX) where n = 1 to 3, M is an early transition metal, A is
an A-group (mostly IIIA and WA, or groups 13 and 14) element and X is either
carbon and/or
nitrogen. The layered structure consists of edge-sharing, distorted XIVI6
octahedra interleaved by
single planar layers of the A-group element.
[00102] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that optionally includes insoluble
micron-sized
particles.
[00103] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that includes insoluble micron-sized
particles
[00104] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that includes insoluble micron-sized
particles having
an average high thermal conductivity of greater than about 140 W/m-K,
typically greater than
about 160 W/m-K, more typically greater than about 180 W/m-K, still more
typically greater than
about 200 W/m-K, and yet still more typically greater than about 250 W/m-K.
[00105] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that includes insoluble micron-sized
particles such as
carbon fiber; SiC particles, fibers or whiskers; heat-treated graphite; AIN;
BN; and/or other high
thermal conductivity, thermally-stable materials.
22
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
[00106] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that is formed from magnesium or a
magnesium alloy
and the addition of one or more types of insoluble nanoparticles.
[00107] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that is formed from a magnesium alloy
and the
addition of one or more types of insoluble nanoparticles, and wherein the
magnesium alloy can be
selected from AE series alloys (e.g., Mg-Al-Re alloys such as AE42, etc.), AJ
series alloys (e.g.,
Mg-Al-Sr alloys), AM series alloys (e.g., Mg-Al-Mn alloys such as AM20, AM50,
AM60, etc.),
AS series alloys (AS21, AS 41, etc.), AX series alloys (Mg-Al-Ca), AXJ series
alloys (e.g., Mg-
Al-Ca-Sr alloys), AZ series alloys (e.g., Mg-Al-Zn alloys such as AZ31, AZ61,
AZ80, AZ91, etc.),
Elektron 21 series alloys (e.g., Mg-Gd-Nd-Zr alloys such as Elektron ZRE1,
etc.), LPSO alloys
(e.g., Mg-Zn-Re alloys, Mg-Zn-Y alloys, Mg-Zn-Dy alloys, Mg-Zn-Ho alloys, Mg-
Zn-Er alloys,
Mg-Zn-Tm alloys, Mg-Zn-Gd alloys, Mg-Zn-Tb alloys, etc.), QE series alloys
(e.g., Mg-Ag-Nd-
Zr alloys), WE series alloys (Mg-Y-RE), ZE series alloys (e.g., Mg-Zn-RE-Zr
alloys such as ZI-,41,
Lk, 63, etc.), ZK series alloys (Mg-Zn-Zr), ZM5 series alloys (e.g., Mg¨Al¨Zn
alloy), ZMS series
alloys (Mg-Zn-Mn-Sn), or ZW series alloys (Mg-Zn-Y).
[00108] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that has an improved hot tear
strength as compared to
magnesium or a magnesium alloy that is absent insoluble nanoparticle
additions, and which
magnesium-based composite can be die-cast and formed into complex thin-walled
shapes.
[00109] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite wherein the insoluble nanoparticles
can be formed ex
situ and added to the magnesium or magnesium-based alloy while the magnesium
or magnesium-
based alloy is molten, using various methods to control wetting and dispersion
of the insoluble
nanoparticles in the molten magnesium or magnesium-based alloy.
[00110] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite wherein the insoluble nanoparticles
can be formed in
situ through addition of reactive species or alloying elements while the
magnesium or magnesium-
based alloy is in a molten state.
[00111] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that can be subjected to deformation
processing to
23
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
have an tensile yield strength that is greater than about 35 ksi, and
typically greater than about 45
ksi, while retaining significant ductility of more than 5%, and preferably
more than 15% strain to
failure.
[00112] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that can be subjected to semi-solid
processing such as
thixomolding, thixocasting, continuous reheocasting, SIMA processing, and/or
other processing
techniques to further improve ductility of the material.
[00113] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that can have a strain to failure
exceeding about 15%,
and preferably exceeds about 20%, compared to 3-14% for magnesium and
magnesium alloy.
[00114] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that can be annealed to create a
desired microstructure
(e.g., stable LPSO phases, alpha-beta microstructures, homogenous solid
solutions, or uniform fine
precipitates) prior to deformation processing.
[00115] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that can retain excellent mechanical
properties as-cast,
with or without heat treatment, to include an elongation to failure above
about 5%, and generally
above about 10%.
[00116] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that can have improved hot tear
strength of about 10-
15% as compared to magnesium or magnesium alloys that are absent insoluble
nanoparticles.
[00117] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that can also include galvanically-
active phases that
produce a controlled dissolution rate of the composite in the presence of tap
water or brine or
fracking liquid of 10-200 Mg/cm2/hr. at a temperature of at least 55 C,
typically at a temperature
of at least 75 C, more typically at a temperature of at least about 90 C, and
even more typically at
a temperature of at least about 135 C.
[00118] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that retains at least 70% of its room
temperature (e.g.,
25 C) tensile strength properties at 150 C, and typically retains at least 85%
of its room
temperature tensile strength properties at 150 C.
24
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
[00119] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that retains at least 70% of its room
temperature
mechanical properties at 185 C, and typically retains at least 70% of its room
temperature
mechanical properties at 200 C.
[00120] Another and/or alternative non-limiting objective of the present
invention is the
provision of a magnesium-based composite that retains an elongation to failure
of at least 8% at
25 C.
[00121] Still yet another and/or alternative non-limiting objective of the
present invention, there
is provided a magnesium-based composite that can be used as a dissolvable,
degradable and/or
reactive structure in oil drilling. For example, the magnesium-based composite
of the present
invention can be used to form a frac ball or other structure in a well
drilling or completion
operation, such as a structure that is seated in a hydraulic operation, that
can be dissolved away
after use so that that no drilling or removal of the structure is necessary.
Other types of structures
can include, but are not limited to, sleeves, valves, hydraulic actuating
tooling and the like. Such
non-limiting structures or additional non-limiting structure are illustrated
in US Patent Nos.
8,905,147; 8,717,268; 8,663,401; 8,631,876; 8,573,295; 8,528,633; 8,485,265;
8,403,037;
8,413,727; 8,211,331; 7,647,964; US Publication Nos. 2013/0199800;
2013/0032357;
2013/0029886; 2007/0181224; and WO 2013/122712, all of which are incorporated
herein by
reference.
[00122] Other objects, advantages, and novel features of the present invention
will become
apparent from the following detailed description of the invention when
considered in conjunction
with the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
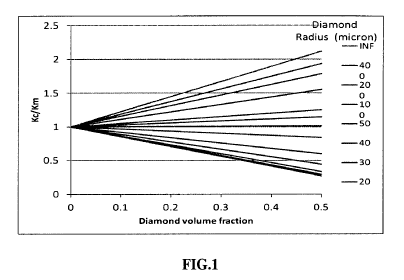
[00123] FIG. 1 is a Plot of Maxwell Model calculation for ratio of magnesium-
based composite
thermal conductivity versus magnesium thermal conductivity for various
particle sizes and
loadings of diamonds (1500 W/m-K) from 20 to 400 micron in size.
[00124] FIG. 2 is a photograph of a tabletop ultrasonic casting unit for
dispersing nanoparticles
into magnesium.
[00125] FIG. 3 is a photomicrograph of a typical AZ91 cast structure showing
the Mg-Al
primary (alpha) and the alpha plus gamma eutectic phases.
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
[00126] FIG. 4 is a photomicrograph of a rapidly quenched AZ91 showing the
uniform
distribution of the eutectic along primary alpha grain boundaries.
[00127] FIG. 5 is a graph illustrating thermal conductivity of the magnesium-
based composite
verses the particle size of the nanodiamond particles in the magnesium-based
composite.
[00128] FIG. 6 is an illustration of the microstructure of a Mg-CNT composite.
[00129] FIG. 7 illustrates an ultrasonication process to disperse
nanoparticles in molten metal
magnesium material.
[00130] FIG. 8 is a picture of the addition of nanoparticles to a molten
magnesium alloy.
[00131] FIG. 9 is graph illustrating time-temperature curves generated for
diffusivity
measurement of a magnesium component containing 3 wt.% CNT.
[00132] FIG. 10 is a graph illustrating tensile yield strengths for magnesium,
magnesium alloys
and magnesium-based composites that include nanoparticles.
DESCRIPTION OF THE INVENTION
[00133] The present invention is directed to a cast or wrought magnesium-based
composite
incorporating nanoparticle modifiers, and method for manufacture of such
magnesium-based
composite. The magnesium-based composite has improved thermal, physical, and
mechanical
properties as compared to prior art magnesium alloys. The magnesium-based
composite includes
a modification of grain boundary thermal or sliding resistance through the
addition of nanoscale
fillers to the high strength magnesium alloys. The magnesium-based composite
has a 15% or
greater improvement in thermal conductivity, strength, or strain to failure
compared to the base
material.
[00134] The magnesium-based composite includes magnesium or a magnesium alloy
having at
least one insoluble phase in discrete form that is disbursed in the base metal
or base metal alloy.
The magnesium-based composite is generally produced by casting. The discrete
insoluble
particles include nanoparticles that have a different galvanic potential from
the magnesium or a
magnesium alloy. The discrete insoluble particles are generally uniformly
dispersed through the
magnesium or a magnesium alloy using techniques such as, but not limited to,
thixomolding, stir
casting, mechanical agitation, electrowetting, ultrasonic dispersion and/or
combinations of these
methods; however, this is not required. In one non-limiting process, the
insoluble particles are
uniformly dispersed through the magnesium or a magnesium alloy using
ultrasonic dispersion.
26
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
Due to the insolubility and difference in atomic structure in the melted
magnesium or a magnesium
alloy and the insoluble particles, the insoluble particles will be pushed to
the grain boundary of the
mixture of insoluble particles and the melted magnesium or a magnesium alloy
as the mixture
cools and hardens during casting solidification. Because the insoluble
particles will generally be
pushed to the grain boundary, such feature makes it possible to
engineer/customize grain
boundaries in the magnesium-based composite to control the dissolution rate of
the magnesium-
based composite. This feature can be also used to engineer/customize grain
boundaries in the
magnesium-based composite through traditional deformation processing (e.g.,
extrusion,
tempering, heat treatment, etc.) to increase tensile strength, elongation to
failure, and other
properties in the magnesium-based composite that were not achievable in cast
metal structures that
were absent insoluble particle additions. Because the amount or content of
insoluble particles in
the grain boundary is generally constant in the magnesium-based composite, and
the grain
boundary to grain surface area is also generally constant in the magnesium-
based composite even
after and optional deformation processing and/or heat treatment of the
magnesium-based
composite, the corrosion rate of the magnesium-based composite remains very
similar or constant
throughout the corrosion of the complete magnesium-based composite.
[00135] The magnesium-based composite can be designed to corrode at the grains
in the
magnesium-based composite, at the grain boundaries of the magnesium-based
composite, and/or
the location of the insoluble particle additions in the magnesium-based
composite depending on
selecting where the insoluble particle additions fall on the galvanic chart.
For example, if it is
desired to promote galvanic corrosion only along the grain boundaries, a
magnesium-based
composite can be selected such that one galvanic potential exists in the base
metal or base metal
alloy where its major grain boundary alloy composition will be more anodic as
compared to the
matrix grains (i.e., grains that form in the casted base metal or base metal
alloy) located in the
major grain boundary, and then an insoluble particle addition will be selected
which is more
cathodic as compared to the major grain boundary alloy composition. This
combination will cause
corrosion of the material along the grain boundaries, thereby removing the
more anodic major
grain boundary alloy at a rate proportional to the exposed surface area of the
cathodic particle
additions to the anodic major grain boundary alloy. The current flowing in the
grain boundary can
be calculated by testing zero resistance current of the cathode to the anode
in a solution at a desired
solution temperature and pressure that includes the magnesium-based composite.
Corrosion of the
27
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
magnesium-based composite will be generally proportional to current
density/unit area of the most
anodic component in the grain boundary and/or grains until that component is
removed. If
electrical conductivity remains between the remaining components in the grain
boundary, the next
most anodic component in the grain boundary and/or grains will next be removed
at a desired
temperature and pressure.
[00136] Galvanic corrosion in the grains can be promoted in the magnesium-
based composite
by selecting a base metal or base metal alloy that has at least one galvanic
potential in the operating
solution of choice (e.g., fracking solution, brine solution, etc.) where its
major grain boundary
alloy composition is more cathodic as compared to the matrix grains (i.e.,
grains that form in the
casted base metal or base metal alloy), and an insoluble particle addition is
selected that is more
cathodic as compared to the major grain boundary alloy composition and the
base metal or base
metal alloy. This combination will result in the corrosion of the magnesium-
based composite
through the grains by removing the more anodic grain composition at a rate
proportional to the
exposed surface area of the cathodic non-soluble particle additions to the
anodic major grain
boundary alloy. The current flowing in the magnesium-based composite can be
calculated by
testing zero resistance current of the cathode to the anode in a solution at a
desired solution
temperature and pressure that includes the magnesium-based composite.
Corrosion of the
magnesium-based composite will be generally proportional to current
density/unit area of the most
anodic component in the grain boundary and/or grains until that component is
removed. If
electrical conductivity remains between the remaining components in the grain
boundary, the next
most anodic component in the grain boundary and/or grains will next be removed
at a desired
temperature and pressure.
[00137] If a slower corrosion rate of the magnesium-based composite is
desired, two or more
insoluble particle additions can be added to the magnesium-based composite to
be deposited at the
grain boundary. If the second insoluble particle is selected to be the most
anodic in the magnesium-
based composite, the second insoluble particle will first be corroded, thereby
generally protecting
the remaining components of the magnesium-based composite based on the exposed
surface area
and galvanic potential difference between second insoluble particle and the
surface area and
galvanic potential of the most cathodic system component. When the exposed
surface area of the
second insoluble particle is removed from the system, the system reverts to
the two previous
embodiments described above until more particles of second insoluble particle
are exposed. This
28
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
arrangement creates a mechanism to retard corrosion rate with minor additions
of the second
insoluble particle component.
[00138] The rate of corrosion in the magnesium-based composite can also be
controlled by the
surface area of the insoluble particle. As such, the particle size, particle
morphology, and particle
porosity of the insoluble particles can be used to affect the rate of
corrosion of the magnesium-
based composite. The insoluble particles in the magnesium-based composite can
optionally have
a surface area of 0.001m2/g-200m2/g (and all values and ranges therebetween).
The insoluble
particles in the magnesium-based composite optionally are or include non-
spherical particles. The
insoluble particles in the magnesium-based composite optionally are or include
nanotubes and/or
nanowires. The non-spherical insoluble particles can optionally be used at the
same volume and/or
weight fraction to increase cathode particle surface area to control corrosion
rates without changing
composition. The insoluble particles in the magnesium-based composite
optionally are or include
spherical particles. The spherical particles (when used) can have the same or
varying diameters.
Such particles are optionally used at the same volume and/or weight fraction
to increase cathode
particle surface area to control corrosion rates without changing composition.
[00139] The strength of the magnesium-based composite can optionally be
increased using
deformation processing and a change dissolution rate of the magnesium-based
composite of less
than about 20% (e.g., 0.01-19.99% and all values and ranges therebetween),
typically less than
about 10%, and more typically less than about 5%.
[00140] The ductility of the magnesium-based composite can optionally be
increased using
insoluble nanoparticle cathodic additions.
[00141] The magnesium-based composite can optionally include chopped fibers.
These
additions to the magnesium-based composite can be used to improve toughness of
the magnesium-
based composite.
[00142] The magnesium-based composite can have improved tensile strength
and/or elongation
due to heat treatment without significantly affecting the dissolution rate of
the magnesium-based
composite.
[00143] The magnesium-based composite can have improved tensile strength
and/or elongation
by extrusion and/or another deformation process for grain refinement without
significantly
affecting the dissolution rate of the magnesium-based composite. In such a
process, the dissolution
29
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
rate change can be less than about 10% (e.g., 0-10% and all values and ranges
therebetween),
typically less than about 5%, and more typically less than about 1%.
[00144] Particle reinforcement in the magnesium-based composite can optionally
be used to
improve the mechanical properties of the magnesium-based composite and/or to
act as part of the
galvanic couple.
[00145] The insoluble particles in the magnesium-based composite can
optionally be used as a
grain refiner, as a stiffening phase to the base metal or metal alloy (e.g.,
matrix material), and/or
to increase the strength of the magnesium-based composite.
[00146] The insoluble particles can optionally be dispersed throughout the
magnesium-based
composite using ultrasonic means, by electrowetting of the insoluble
particles, and/or by
mechanical agitation.
[00147] The magnesium-based composite can optionally be used to form all or
part of a device
for use in hydraulic fracturing systems and zones for oil and gas drilling,
wherein the device has a
designed dissolving rate. The magnesium-based composite can optionally be used
to form all or
part of a device for structural support or component isolation in oil and gas
drilling and completion
systems, wherein the device has a designed dissolving rate.
[00148] High conductivity nanoparticles (e.g., carbon (carbon nanotubes and
nano-diamond
particles), copper etc.) can be added to the magnesium-based composite to
increase thermal
conductivity of the magnesium-based composite by 100% or more via segregation
and
concentration in the eutectic alpha plus gamma phase, as well as segregation
to subgrain
boundaries and other lattice defects.
[00149] By adding high conductivity nanoparticles (e.g., 0.507 vol.%), either
ex situ (blending),
or formed in situ (e.g., from Cu additions, Ag additions, etc.), a significant
increase of the thermal
conductivity of the magnesium-based composite can be achieved as compared to
an alloy absent
such additions. This same phenomenon is not observed in single-phase magnesium
(e.g., pure
magnesium), but only in multiphase alloys where segregation to the eutectic
region and to the
phase interfaces is observed.
[00150] The nanoparticles are selected from the group consisting of fullerenes
(including multi-
walled and single-walled carbon nanotubes, graphene, nanodiamonds,
buckeyballs); inert
ceramics, including submicron and nanoparticles (including nanotubes,
platelets, and flakes) of
W, SiC, AIN, Be0, BN; and/or TiB2, high thermal conductivity MAX phase
materials; and/or Cu,
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
Ag, Al, Be, and/or Au compounds. At least about 30% of the nanoparticles
generally have
dimensions of less than about 200 nm. The nanoparticles generally constitute
about 0.1-15 vol.%
of the magnesium-based composite. The nanoparticles generally have at least
one dimension
below about 400 nm, and at least about 30% of the nanoparticles generally have
dimensions of
less than about 200 nm. The nanoparticles generally have a thermal
conductivity of greater than
about 140 W/m-K.
[00151] The micron-sized particles (when used) can include one or more
materials selected
from the group consisting of diamond; heat-treated carbon fiber; SiC
particles, fibers or whiskers;
heat- treated graphite; AIN; BN; and/or other high thermal conductivity,
thermally-stable material.
The size of the micron-sized particles (when used), is about 10-300 microns,
and the micron-sized
particles generally have a high thermal conductivity that is greater than
about 180 W/m-K. The
micron-sized particles (when used), constitute about 1-45 vol.% of the
magnesium-based
[00152] EXAMPLE 1
[00153] An AZ91D magnesium alloy having 9 wt.% aluminum, 1 wt.% zinc and 90
wt.%
magnesium was melted to above 700 C. About 2 vol.% nano iron particles and
about 2 vol.%
nano graphite particles were added to the AZ91D magnesium alloy using
ultrasonic mixing. The
melt was cast into steel molds. The iron particles and graphite particles did
not fully melt during
the mixing and casting processes. The material dissolved at a rate of 2 mg/cm2-
min in a 3% KC1
solution at 20 C. The material dissolved at a rate of 20 mg/cm2-hr in a 3% KC1
solution at 65 C.
The material dissolved at a rate of 100 mg/cm2-hr in a 3% KCl solution at 90
C. The dissolving
rate of magnesium-based composite for each these test was generally constant.
[00154] EXAMPLE 2
[00155] Carbon nanotubes and/or finely divided copper nanoparticle powder were
added to pure
magnesium and an AZ91 magnesium alloy (having 9 wt.% aluminum, 1 wt.% zinc and
90 wt.%
magnesium) when in molten form. The AZ91 magnesium alloy was melted to above
700 C.
Insoluble nanoparticles in the form of carbon nanotubes (multiwall, high
thermal conductivity)
were added to the molten AZ91 magnesium alloy. The insoluble carbon nanotubes
were added by
consolidating the carbon nanotubes into a magnesium rod by mechanically
blending the carbon
nanotubes with magnesium powder and then cold pressing the mixture of carbon
nanotubes and
magnesium powder into a rod. The rod containing the carbon nanotubes was
fed/inserted into the
molten AZ91 magnesium alloy. The insoluble carbon nanotubes were dispersed in
the molten
31
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
AZ91 magnesium alloy by ultrasonic mixing wherein the rod was directed into
the ultrasonic sweet
spot to melt the rod at a melt temperature of 700 C. The carbon nanotubes
constituted about 3
vol.% of the formed magnesium-base composite. The average particle size of the
carbon
nanotubes was less than 300 nm.
[00156] The copper nanoparticle powder was added to the molten AZ91 magnesium
alloy by
consolidating the copper nanoparticle powder with magnesium powder and then
cold pressing the
mixture of copper powder and magnesium powder into a rod. The rod containing
the copper
nanoparticle powder was fed/inserted into the molten AZ91 magnesium alloy. The
insoluble
copper nanoparticle powder was dispersed in the molten AZ91 magnesium alloy by
ultrasonic
mixing wherein the rod was directed into the ultrasonic sweet spot to melt the
rod at a melt
temperature of 700 C. The copper nanoparticles constituted about 3 vol.% of
the formed
magnesium-base composite. The average particle size of the copper
nanoparticles was less than
300 nm. When both carbon nanotubes and copper nanoparticles were added, the
carbon nanotubes
constituted about 2 vol.% of the formed magnesium-base composite and the
copper nanoparticles
constituted about 2 vol.% of the formed magnesium-base composite.
[00157] A 10 lb. casting of the magnesium-based composite in accordance with
Example 2 was
prepared in a steel permanent mold having a 3" diameter. After casting, the
cast materials were
extruded into 1/2" rods for mechanical and thermal testing at an extrusion
temperature of about
340 C. Table I illustrates the results of the Mg-CNT, AZ91-CNT and AZ91-Cu-CNT
composites
formed in accordance with the present invention as compared with casting
formed of pure
magnesium and AZ91 magnesium alloy.
[00158] Table 1: Comparative Results of Magnesium-Based Composite
Material Thermal Conductivity Ultimate Tensile Tensile Yield
(ksi)
Strength (ksi)
Mg 156 27.1 14.7
AZ91 108 49.5 41.7
Mg-Cu 179 38.4 35.5
Mg-CNT 89 35.5 26.8
AZ91-CNT 204 49.8 41.9
32
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
AZ91-Cu-CNT 271 52.3 44.6
[00159] The results in Table 1 illustrate that the AZ91-CNT and AZ91-Cu-CNT
composites
had a greater thermal conductivity, tensile strength, and yield strength than
the pure magnesium
and AZ91 magnesium alloy that was absent the nanoparticle additions. The Mg-
CNT composite
had a thermal conductivity that was less than the thermal conductivity of pure
magnesium, but had
a greater tensile strength and yield strength than pure magnesium.
[00160] Alternative alloy systems developed that demonstrate the mechanical
property
improvements and propensity for high thermally and electrically conductive
magnesium-based
composites were researched. These alloys were formed in a similar manner as
the alloy of
Example 2. The AZ91 magnesium alloy was substituted for magnesium alloys of
AXM4304,
AX50, ZMS616, WEK430, ZWK120, ZWE111 and ZWEK1450.
[00161] These alloys were also developed as 10 lb. castings, but cast into 1"
and 2" ingot sizes.
After casting, the parts were extruded into < " rods for mechanical
characterization using an 8:1
extrusion ratio and varying extrusion processing parameters. The ZMS alloys
were also put
through a double-aging process post extrusion and the LPSO phase alloys were
solution treated
prior to extrusion.
[00162] Table 2: Mechanical Properties of Alternative Alloy Systems
Material Tensile Ultimate (ksi) Tensile Yield (ksi) Elongation to
failure (%)
AXM4304 50.5 43.6 3.5
AX50 38.1 23.6 18
ZMS616 50.5 47.1 11
WEK430 33.3 21.3 15
ZWK120 34.5 22.0 20
ZWE111 32.3 18.7 25
ZWEK1450 38.5 22.1 18
[00163] FIG. 1 is a Plot of Maxwell Model calculation for ratio of magnesium-
based composite
thermal conductivity versus magnesium thermal conductivity for various
particle sizes and
loadings of diamonds (1500 W/m-K) from 20 to 400 micron in size. FIG. 1
illustrates that at least
33
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
60 microns are needed to have significant effect due to interface resistance
between electron
conductor (Mg, metal), and phonon conductor (carbon, ceramic, intermetallic,
diamond). FIG. 1
also illustrates that the addition of fine particles as a well-mixed composite
into the magnesium or
other light alloys (aluminum, etc.) leads to a reduction or no change in
thermal performance of the
magnesium-based composite.
[00164] FIG. 2 is a photograph of a tabletop ultrasonic casting unit for
dispersing nanoparticles
into magnesium. This unit can be used to obtain an initially uniform
dispersion in a single-phase
molten metal mixture of nanoparticles and dispersoids. Typically, this is done
under various
degrees of superheat, from about 675-725 C for magnesium, or from 25-150 C
degrees of
superheat, and typically from about 50-100 C degrees of superheat for light
metals.
[00165] FIG. 3 is a photomicrograph of a typical AZ91 cast structure showing
the Mg-Al
primary (alpha) and the alpha plus gamma eutectic phases. Segregation of ex
situ- and in situ-
added high conductivity particulates to the subgrain boundaries and eutectic
liquid resulted in
elimination of microstructural defects and an enhancement of strength and
conductivity of the
interfacial and inteiphasic regions.
[00166] FIG. 4 is a photomicrograph of a rapidly quenched AZ91 showing the
uniform
distribution of the eutectic liquid along primary alpha grain boundaries. By
changing solidification
and nucleating grains more homogenously, or through the use of other
techniques, a more uniform
distribution of the eutectic liquid can be obtained; thus, alloying lower
concentrations of additives
can be used to achieve the desired results in accordance with the present
invention.
[00167] It is surmised that the next generation of missile airframes and
spacecraft thermal
management systems could be designed using a magnesium-based composite with
the combination
of the lowest density, highest thermal conductivity, and highest strength. The
cast or wrought
magnesium-based composite incorporating nanoparticle modifiers can be formed
by a highly
scalable, low cost process that advances the state-of-the-art of metal matrix
thermal conductors to
reach a theoretical goal of 578 W/mK (up to 270W/mK achieved), a density less
than aluminum
(1.7g.cc achieved), and a yield strengths over 30 ksi (207 MPa, 42KSI achieved
at 8:1 extrusion
ratio). The addition of high conductivity reinforcements is limited due to
interfacial resistance,
requiring large particles to achieve significant improvements. Thermal
conductivity of the
magnesium-based composite versus diamond particle size (Type I, k=1500 w/m-K)
is illustrated
in Fig. 5. The system is a magnesium-diamond system that includes 20 vol.%
diamonds. By
34
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
adding high conductivity nanofibers in accordance with the present invention,
interfacial
resistances can be reduced or eliminated. This concept is illustrated in Fig.
6, illustrating the high
conductivity phases connected with "short circuit" nanotubes. Fig. 6 is an
illustration of the
microstructure of a Mg-CNT composite. In the past, the incorporation of high
conductivity carbon
nanofibers into metals and the production of highly grain-refined metals
(nanostructured metals)
has been found to improve mechanical and thermal properties of materials, with
limited technical
and commercial success. The majority of these efforts have focused on powder
metallurgy solid
state approaches, which are expensive and have poor scalability, not to
mention significant safety
concerns in magnesium alloys. The present invention focuses on enabling the
production of metal
matrix nanocomposites through advanced dispersion casting techniques,
combining the production
of engineered nanocomposite feedstocks with the addition of acoustic and
mechanical energy to
control nanophase dispersion and chemistry.
[00168] The problem of incorporating high-aspect-ratio, high-surface-area
particles (including
fiber and flake) with controlled and repeatable concentration and distribution
into molten metals
is a large undertaking, and must factor in the molten metal temperature,
composition, and surface
tension as well as particle surface area, reactivity, clustering, segregation,
and temperature and
time-dependent wetting phenomena. Direct feeding of the low-density high-
surface-area particles
into the melt does not work, as particles burn, float, react with the molten
metal, or do not stay in
the metal. Other feeding mechanisms attempted in the past (such as auger
feeding into the metal,
in situ formation, and stir casting) are cost prohibitive and not always
scalable. To solve these
problems, high-aspect-ratio nanoparticles (carbon nanofibers) in accordance
with the present
invention are incorporated into a pre-dispersed master-composite that enables
safe and reliable
feeding into molten magnesium to create a high-strength, high thermal-
conductivity magnesium-
based composite. As illustrated in Fig. 7, the process can be based on
ultrasonication technologies
with nanoparticle surface chemistry control to disperse nanoparticles into a
metal-compatible
binder that can be formed into controlled density pellets or rods similar to
grain refiners used
commercially. These pellets or rods can be directly inserted into a pool of
molten metal. Without
fabrication of these magnesium-based composites, the nanoparticles agglomerate
at the surface of
the molten metal, being burned or removed from the casting with slag, instead
of being
incorporated into the nanocomposite. The method has resulted in both strength
increase (>50%)
and improvement in thermal conductivity (>150%) in magnesium using multi-
walled carbon
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
nanotubes incorporated at low volume fractions into high strength
castable/wrought magnesium
alloys. These low-cost methods of achieving high-strength, high-conductivity
magnesium-based
composites using casting techniques have now been demonstrated, leading to an
exceptional
balance of properties and cost not achievable in currently available alloys or
materials.
[00169] After preparation of the master alloy, the high concentration
nanocomposites were
added to magnesium alloy melts using stircasting in 101b melts and a flux
cover as illustrated in
Fig. 8. The master alloy was fabricated into rods, which were thrust below the
flux cover manually,
and mixed using a steel stirring rod. The degree of superheat and mixing
procedures were
optimized through iterative development to obtain a good dispersion. After
casting, ingots were
extruded at ratios of 4-12:1, as well as roll-reduced to improve mechanical
properties and to
improve ductilities from the cast structures. A 175-ton press was used, and
extrusion temperatures
of 300-400 C were used to further process the ingots.
[00170] Thermal and mechanical testing were completed as a function of
nanotube loading,
alloy composition, and high conductivity filler loading. A steady state vacuum
thermal chamber
was used for thermal diffusivity measurement, calibrated to an aluminum
baseline. Fig. 9
illustrates time-temperature curves generated for diffusivity measurement.
Measured
conductivities, depending on alloy composition, diamond and CNT compositions
ranged from 145
W/m-K (Mg is 156), to 204-270 W/m-K (depending on alloy). 204 W/m-k was
achieved at low
CNT loadings in a low cost AZ91 alloy matrix, a nearly 100% increase in
conductivity. Strengths
of wrought alloys were maintained or slightly improved in the magnesium-based
composites, as
illustrated in Fig. 10. Fig. 10 illustrates tensile yield strengths for high
conductivity magnesium-
based composite in accordance with the present invention. Interestingly, the
addition of CNT's
significantly increased the elongation to failure in magnesium alloys over
equivalently processed
pure alloy.
[00171] The production of wrought magnesium-based composite is highly
scalable. The
magnesium-based composite can be cast into cast billets and extruded to form a
rod product which
can be used for the production of magnesium frac balls in widespread use in
the oil and gas
industry.
[00172] It will thus be seen that the objects set forth above, among those
made apparent from
the preceding description, are efficiently attained, and since certain changes
may be made in the
constructions set forth without departing from the spirit and scope of the
invention, it is intended
36
CA 03017752 2018-09-13
WO 2017/205281 PCT/US2017/033819
that all matter contained in the above description and shown in the
accompanying drawings shall
be interpreted as illustrative and not in a limiting sense. The invention has
been described with
reference to preferred and alternate embodiments. Modifications and
alterations will become
apparent to those skilled in the art upon reading and understanding the
detailed discussion of the
invention provided herein. This invention is intended to include all such
modifications and
alterations insofar as they come within the scope of the present invention. It
is also to be
understood that the following claims are intended to cover all of the generic
and specific features
of the invention herein described and all statements of the scope of the
invention, which, as a
matter of language, might be said to fall there between. The invention has
been described with
reference to the preferred embodiments. These and other modifications of the
preferred
embodiments as well as other embodiments of the invention will be obvious from
the disclosure
herein, whereby the foregoing descriptive matter is to be interpreted merely
as illustrative of the
invention and not as a limitation. It is intended to include all such
modifications and alterations
insofar as they come within the scope of the appended claims.
37