Note: Descriptions are shown in the official language in which they were submitted.
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
ACCURATE, PRECISE MICROLITER DOSING SYRINGE
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Provisional
Application Serial No.
62/323,341, filed April 15, 2016, titled "Accurate, Precise Microliter Dosing
Syringe," which is
hereby incorporated by reference in its entirety.
FIELD OF THE INVENTION
[0002] This invention relates to syringes and, more specifically, to
syringes that can
deliver microliter-sized doses.
BACKGROUND OF THE INVENTION
[0003] Studies have shown that a number of factors contribute towards
inability of
standard syringes to deliver accurate and precise microliter sized doses. Most
conventional
syringes, which include components such as a cylindrical barrel, a plunger
rod, and a plunger
seal, are designed to deliver milliliter doses and are unable to deliver an
accurate and precise
dose. Variation in delivering microliter-sized doses using a conventional
syringe are often
caused by the inability of the user to precisely control the distance of
travel by the plunger rod.
Travel distance is controlled by setting the start of the dose and the end of
the dose. Studies have
shown that providing a better defined start and end of the dose can improve
accuracy of
delivering a microliter dose. However, this is insufficient to ensure
precision. Imprecision can
be due to variability from one user to another and to inherent human
limitations in establishing
limits and resolution of the travel distance. Studies have shown that this
variation could be as
much as 20% of the intended dose. Further, manufacturing issues may cause
variations when
establishing visual references and markings that define plunger travel. These
variations are
negligible when delivering milliliter sized doses, but are significant source
of variation when
delivering microliter sized doses.
[0004] Many clinical and non-clinical applications require that a
microliter sized dose be
delivered. Applications for microliter delivery include injectable drug
delivery into or onto the
eye, intracellular delivery, delivery of radioactive agents, chemotherapy,
etc. Both accuracy and
precision are important with drugs that have a small therapeutic window, and
where inadequate
accuracy and precision would put the amount of injected drug outside of its
therapeutic window.
1
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
In some cases, inaccuracy and imprecision can cause alterations in biophysical
properties at the
injected site, such as increase in pressure, rupture of cell walls, etc. In
the case of microliter
delivery of drugs, systems that are not compatible with either standard
pharmaceutical fill-finish
systems or standard prefillable syringe components may be unsuitable. Studies
have shown that
used of microliter delivery systems for intravitreous administration with only
conventional
prefillable syringe components results in a suboptimal outcome.
[0005] Irrespective of whether an injectable substance or drug is
prefilled or user-filled, a
microliter dosing syringe should be able to prime the needle or any other
delivery conduit to
ensure that any air is expelled before delivery. Priming is important to
ensure that once the dose
is set, an accurate dose is dispensed. In instances where the drug is
prefilled (i.e. not filled by the
end user), the ability to prime is critical to ensuring that the accuracy of
the administered dose is
independent of the accuracy of the drug fill.
[0006] There is a need for accurate, precise microliter dose setting
and delivery
mechanisms adaptable to conventional, commercially available syringes ¨
thereby enabling a
conventional syringe to deliver accurate, precise microliter volumes.
SUMMARY OF THE INVENTION
[0007] According to some embodiments, accurate and precise dosing
mechanisms can be
configured with a number of syringe configurations, including: prefilled (with
pre-attached
needle, with user-attached needle, with a retractable needle, etc.) and user
filled. According to
some embodiments, a system includes a dosing mechanism that is manufactured
and partly
assembled independent of the syringe and then coupled to the syringe to
provide the plunger rod
functionality. As such, the functioning of the accurate dosing mechanism may
include features
analogous to those of a plunger rod in a conventional syringe but with
improved resolution of
dose setting for accurate and precise microliter dose delivery.
[0008] According to some embodiments, the system may include a plunger rod
with lugs
and teeth. The lugs on the plunger rod may mechanically interact with internal
threads within
the thumb nut. The teeth of the plunger rod may mesh with a set of teeth of a
gear of a gear
train. Axial advancement of the plunger rod may generate a torsional force
that causes rotation
of the gear. The gear may be coupled with one or more additional gears. The
teeth of one of the
additional gears may mesh with the teeth of a drive rod. The drive rod may be
placed within a
prefilled syringe barrel and may abut a plunger adapter. The plunger adapter
may be screwed
2
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
into the back of a plunger seal. The thumb nut and gear train are all placed
within a housing. The
gear may be attached to the housing by way of a pin at its geometric center.
The flange of the
syringe barrel may be placed within the housing and a cover may mate with the
housing to
couple the dosing mechanism to the syringe.
[0009] According to some embodiments, a plunger rod assembly for a syringe
includes a
first plunger rod component comprising a first linear gear, a second plunger
rod component
comprising a second linear gear, a first rotational gear having a plurality of
gear teeth for
engaging the first linear gear, and a second rotational gear having a second
plurality of gear teeth
for engaging the second linear gear, wherein the first rotational gear is
coupled to the second
rotational gear such that translation of the first plunger rod component
causes translation of the
second plunger rod component.
[0010] In any of these embodiments, translation of the first plunger
rod component a first
amount may cause translation of the second plunger rod component a second
amount that is less
than the first amount. In any of these embodiments, the first rotational gear
and the second
rotational gear may be portions of a compound gear.
[0011] In any of these embodiments, the first rotational gear may be
spaced from the
second rotational gear. In any of these embodiments, the first and second
rotational gears may
be spaced by a third rotational gear. In any of these embodiments, the second
and third
rotational gears may be compound gears.
[0012] In any of these embodiments, the first set of gear teeth may have a
first pitch
diameter and the second set of gear teeth may have a second pitch diameter
that is different from
the first pitch diameter. In any of these embodiments, the first pitch
diameter may be greater
than the second pitch diameter.
[0013] In any of these embodiments, at least one of the first and
second sets of gear teeth
may include involute gear teeth. In any of these embodiments, the first and
second plunger rod
components may be configured for at least partial insertion into a barrel of a
syringe. In any of
these embodiments, at least a portion of the first plunger rod component may
include a
semicircular cross section and at least a portion of the second plunger rod
component may
include a complimentary semicircular cross section. In any of these
embodiments, the first
plunger rod component may include at least one protrusion and the assembly may
further include
a rotational component for engaging the at least one protrusion.
3
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
[0014] In any of these embodiments, the first plunger rod component
may be configured
to axially translate in response to rotation of the rotational component when
the rotational
component is engaged with at least one of the at least one protrusion. In any
of these
embodiments, the first plunger rod component may be configured to axially
translate in response
to a force having an axial component applied directly to the first plunger rod
component after the
rotational component disengages from the at least one protrusion.
[0015] In any of these embodiments, the rotational component may
include an internal
thread for engaging the at least one protrusion. In any of these embodiments,
the rotational
component may include at least one stop that engages one of the at least one
protrusion when the
plunger rod component reaches an axial position relative to the rotational
component. In any of
these embodiments, the assembly may include at least one ratchet component
that is engaged
with the rotational component such that the rotational component is prevented
from rotating in
one direction. In any of these embodiments, the assembly may include at least
one ratchet
component that is engaged with the rotational component such that the
rotational component
rotates more freely in a first direction than in a second direction.
[0016] In any of these embodiments, the assembly may include a
housing for mounting
on an end of a syringe barrel. In any of these embodiments, the assembly may
include a retainer
for engaging with the housing and the end of the syringe barrel for affixing
the plunger rod
assembly to the end of the syringe barrel. In any of these embodiments, the
retainer may affix to
the housing. In any of these embodiments, the retainer may engage with an
internal thread or
groove in the recess of the housing. In any of these embodiments, the first
plunger rod
component may extend through an aperture in the housing, and the perimeter of
the aperture may
engage with the first plunger rod component to prevent rotation of the first
plunger rod
component.
[0017] According to some embodiments, a syringe may include a barrel; a
delivery
conduit; an elastomeric or elastomer containing plunger seal disposed within
the barrel; and a
plunger rod assembly affixed to an end of the barrel, the plunger rod assembly
including a first
plunger rod component comprising a first linear gear, a second plunger rod
component disposed
at least partially in the barrel and engaged with the plunger seal, wherein
the second plunger rod
component comprises a second linear gear, a first rotational gear having a
plurality of gear teeth
for engaging the first linear gear, and a second rotational gear having a
second plurality of gear
teeth for engaging the second linear gear, wherein the first rotational gear
is coupled to the
4
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
second rotational gear such that translation of the first plunger rod
component causes translation
of the second plunger rod component.
[0018] In any of these embodiments, axial translation of the first
plunger rod component
a first amount may cause axial translation of the second plunger rod component
a second amount
that is less than the first amount. In any of these embodiments, the first
rotational gear and the
second rotational gear may be portions of a compound gear. In any of these
embodiments, the
first rotational gear may be spaced from the second rotational gear.
[0019] In any of these embodiments, the first and second rotational
gears may be spaced
by a third rotational gear. In any of these embodiments, the second and third
rotational gears
may be compound gears. In any of these embodiments, the first set of gear
teeth may have a first
pitch diameter and the second set of gear teeth may have a second pitch
diameter that is different
from the first pitch diameter. In any of these embodiments, the first pitch
diameter may be
greater than the second pitch diameter. In any of these embodiments, at least
one of the first and
second sets of gear teeth may include involute gear teeth. In any of these
embodiments, the first
and second plunger rod components may be configured for at least partial
insertion into a barrel
of a syringe.
[0020] In any of these embodiments, at least a portion of the first
plunger rod component
may include a semicircular cross section and at least a portion of the second
plunger rod
component may include a complimentary semicircular cross section. In any of
these
embodiments, the first plunger rod component may include at least one
protrusion and the
assembly may further include a rotational component for engaging the at least
one protrusion.
[0021] In any of these embodiments, the first plunger rod component
may be configured
to axially translate in response to rotation of the rotational component when
the second rotational
component is engaged with at least one of the at least one protrusion. In any
of these
embodiments, the first plunger rod component may be configured to axially
translate in response
to a force having an axial component applied directly to the first plunger rod
component after the
rotational component disengages from the at least one protrusion.
[0022] In any of these embodiments, the rotational component may
include an internal
thread for engaging the at least one protrusion. In any of these embodiments,
the rotational
component may include at least one stop that engages one of the at least one
protrusion when the
plunger rod component reaches an axial position relative to the rotational
component. In any of
these embodiments, the assembly may include at least one ratchet component
that is engaged
5
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
with the rotational component such that the rotational component is prevented
from rotating in
one direction. In any of these embodiments, the assembly may include at least
one ratchet
component that is engaged with the rotational component such that the
rotational component
rotates more freely in a first direction than in a second direction. In any of
these embodiments,
the assembly may include a housing mounted on an end of the barrel.
[0023] In any of these embodiments, the assembly may include a
retainer that is engaged
with the housing and the end of the barrel to affix the plunger rod assembly
to the end of the
syringe barrel. In any of these embodiments, the retainer may affix to the
housing. In any of
these embodiments, the retainer may engage with an internal thread or groove
in the recess of the
housing. In any of these embodiments, the first plunger rod component may
extend through an
aperture in the housing, and the perimeter of the aperture may engage with the
first plunger rod
component to prevent rotation of the first plunger rod component. In any of
these embodiments,
the syringe may be a prefilled or a prefillable syringe.
[0024] In any of these embodiments, the delivery conduit may include
an attached
needle, an attachable needle, an IV connector, an attachable tubing connector,
or an attachable
microneedle array. In any of these embodiments, the plunger seal may include
an adapter for
engagement with the second plunger rod component.
[0025] According to some embodiments, a plunger rod assembly for a
syringe includes a
first plunger rod component; and a second plunger rod component configured to
translate relative
to the first plunger rod component, wherein the second plunger rod component
engages with the
first plunger rod component such that the second plunger rod component
translates in response to
translation of the first plunger rod component.
[0026] In any of these embodiments, the second plunger rod component
may engage with
the first plunger rod component through at least one rotational gear. In any
of these
embodiments, the first plunger rod component may include a first linear gear;
the second plunger
rod component may include a second linear gear; and the at least one
rotational gear may include
a first gear having a first set of gear teeth for engaging the first linear
gear and a second gear
having a second set of gear teeth for engaging the second linear gear.
[0027] In any of these embodiments, the first gear and the second
gear may be portions
of a compound gear. In any of these embodiments, the first gear may be spaced
from the second
gear. In any of these embodiments, the assembly may further include a
rotational component
6
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
configured to engage with the first plunger rod component such that rotation
of the rotational
component causes axial translation of the first plunger rod component.
[0028] In any of these embodiments, the rotational component may
include an internal
thread for engaging one or more protrusions on the first plunger rod
component. In any of these
embodiments, the second plunger rod component engages with the first plunger
rod component
such that the second plunger rod component axially translates a first amount
in response to axial
translation of the first plunger rod component a second amount that is greater
than the first
amount.
[0029] In any of these embodiments, the assembly may include at least
one ratchet or
ratchet-like component that is engaged with the rotational component such that
the rotational
component is prevented from rotating in one direction. In any of these
embodiments, the
assembly may include at least one ratchet component that is engaged with the
rotational
component such that the rotational component rotates more freely in a first
direction than in a
second direction. In any of these embodiments, the assembly may include a
housing for
mounting on a non-patient end of a syringe barrel. In any of these
embodiments, the assembly
may include a retainer for engaging with the housing and the non-patient end
of the syringe
barrel for affixing the plunger rod assembly to the non¨patient end of the
syringe barrel.
[0030] In any of these embodiments, the first plunger rod component
may extend through
an aperture in the housing, and the perimeter of the aperture may be shaped to
prevent rotation of
the first plunger rod component.
[0031] According to some embodiments, a syringe includes a barrel; a
delivery conduit; a
plunger seal disposed within the barrel; and a plunger rod assembly affixed to
an end of the
barrel, the plunger rod assembly including a first plunger rod component; and
a second plunger
rod component disposed at least partially in the barrel and engaged with the
plunger seal,
wherein the second plunger rod component is configured to axially translate
relative to the first
plunger rod component and engages with the first plunger rod component such
that the second
plunger rod component axially translates in response to translation of the
first plunger rod
component.
[0032] In any of these embodiments, the second plunger rod component
may engage with
the first plunger rod component through at least one rotational gear. In any
of these
embodiments, the first plunger rod component may include a first linear gear;
the second plunger
rod component may include a second linear gear; and the at least one
rotational gear may include
7
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
a first gear having a first set of gear teeth for engaging the first linear
gear and a second gear
having a second set of gear teeth for engaging the second linear gear.
[0033] In any of these embodiments, the first gear and the second
gear may be portions
of a compound gear. In any of these embodiments, the first gear may be spaced
from the second
gear. In any of these embodiments, the syringe may further include a
rotational component
configured to engage with the first plunger rod component such that rotation
of the rotational
component causes axial translation of the first plunger rod component. In any
of these
embodiments, the rotational component may include an internal thread for
engaging one or more
protrusions on the first plunger rod component.
[0034] In any of these embodiments, the second plunger rod component may
engage with
the first plunger rod component such that the second plunger rod component
translates a first
amount in response to translation of the first plunger rod component a second
amount that is
greater than the first amount. In any of these embodiments, the assembly may
include at least
one ratchet component that is engaged with the rotational component such that
the rotational
component is prevented from rotating in one direction. In any of these
embodiments, the
assembly may include at least one ratchet component that is engaged with the
rotational
component such that the rotational component rotates more freely in a first
direction than in a
second direction.
[0035] In any of these embodiments, the assembly may include a
housing mounted on an
end of the barrel. In any of these embodiments, the assembly may include a
retainer that is
engaged with the housing and the end of the barrel to affix the plunger rod
assembly to the end
of the syringe barrel. In any of these embodiments, the first plunger rod
component may extend
through an aperture in the housing, and the perimeter of the aperture may be
shaped to prevent
rotation of the first plunger rod component. In any of these embodiments, the
syringe may be a
prefilled syringe.
[0036] In any of these embodiments, the delivery conduit may include
an attached
needle, an attachable needle, an attachable tubing connector, or an attachable
microneedle array.
In any of these embodiments, the plunger seal may include an adapter for
engagement with the
second plunger rod component.
[0037] According to some embodiments, a blister pack includes a pre-filled
syringe
according to any of the above embodiments, wherein the syringe has been
sterilized using EtO,
H202, NO2 or Vaporized Peracetic Acid.
8
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
[0038] In any of these embodiments, the outer surface of the syringe
may have at most 1
ppm EtO, H202, NO2 or Vaporized Peracetic Acid. In any of these embodiments,
the total EtO,
H202, NO2 or Vaporized Peracetic Acid residue on the outside of the syringe
and inside of the
blister pack may be at most 0.1 mg. In any of these embodiments, the syringe
may have been
sterilized with a Sterility Assurance Level of at least 10-6.
[0039] According to some embodiments, a method of delivering a dosage
using a syringe
according to any of the above embodiments includes while pointing the delivery
conduit of the
syringe upwards with respect to the barrel, advancing the plunger seal within
the barrel by
rotating the rotational component; setting a dosage by continuing to rotate
the rotational
component until the rotational component disengages from the first plunger
rod; and after the
rotational component disengages from the first plunger rod, delivering the
dosage by applying a
user force directly to an end of the first plunger rod to advance the plunger
seal.
[0040] In any of these embodiments, the method may include attaching
a needle to the
syringe prior to advancing the plunger seal within the barrel by rotating the
second rotational
component. In any of these embodiments, the syringe may be a prefilled
syringe.
[0041] In any of these embodiments, the prefilled syringe may be
filled with a drug used
for ophthalmic applications. In any of these embodiments, the number of 10
micrometer or
larger sized sub-visible particulates may be less than 50 per milliliter of
drug solution. In any of
these embodiments, the number of 25 micrometer or larger sized sub-visible
particulates may be
less than 5 per milliliter of the drug solution. In any of these embodiments,
the number of 50
micrometer or larger sized sub-visible particulates may be less than 2 per
milliliter of the drug
solution.
BRIEF DESCRIPTION OF THE DRAWINGS
[0042] The invention will now be described, by way of example only,
with reference to
the accompanying drawings, in which:
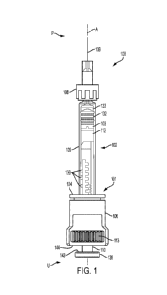
[0043] FIG. 1 is a side view of a dosing syringe system, according to
a first embodiment;
[0044] FIG. 2 is a cross-sectional view of the system of FIG. 1;
[0045] FIG. 3 is an exploded view of the system of FIG. 1;
[0046] FIG. 4 is a perspective view of a dosing mechanism, according
to an embodiment;
9
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
[0047] FIG. 5 is a side view of a plunger rod, according to an
embodiment;
[0048] FIG. 6 is a perspective view of a compound gear, according to
an embodiment;
[0049] FIG. 7A and 7B are perspective and cross-sectional views,
respectively, of a
thumb nut, according to an embodiment;
[0050] FIGS. 8A and 8B are perspective and side views, respectively, of a
drive rod,
according to an embodiment;
[0051] FIGS. 9A-9D are perspective, perspective cross-sectional,
cross-sectional, and
bottom views of a housing, according to one embodiment;
[0052] FIGS. 10A and 10B are cross-sectional views illustrating the
dose set
configuration of a system, according to an embodiment;
[0053] FIGS. 11A and 11B are cross-sectional views illustrating the
dose delivered
configuration of the system of FIGS. 10A and 10B;
[0054] FIG. 12 is a perspective view of a dosing syringe system
according to a second
embodiment;
[0055] FIG. 13 is a side view of the system of FIG. 12;
[0056] FIG. 14A and 14B are side views of the dosing system of FIG.
12 in a dose set
configuration and a dose delivered configuration, respectively;
[0057] FIG. 15 is a perspective view illustrating the coupling of a
dosing mechanism to a
syringe barrel, according to an embodiment;
[0058] FIGS. 16A and 16B are perspective and side views, respectively,
illustrating a
gear train, according to an embodiment;
[0059] FIGS. 17A-17C are cross-sectional views illustrating a dose
setting and dose
delivery process;
[0060] FIGS. 18A-18C illustrate the engagement of a thumb nut and
plunger rod,
according to an embodiment;
[0061] FIG. 19 illustrates a ratchet, according to an embodiment;
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
[0062] FIG. 20A illustrates a plunger rod, according to an
embodiment;
[0063] FIGS. 20B and 20C illustrate a drive rod, according to an
embodiment; and
[0064] FIGS. 21A and 21B are exploded views illustrating aspects of
an assembly
process of a plunger rod subassembly, according to some embodiments.
DETAILED DESCRIPTION OF EXEMPLARY EMBODIMENTS
[0065] Described herein are accurate and precise dosing mechanisms
and systems
incorporating the mechanisms with conventional (or custom) syringe bodies to
provide an
accurate and precise dosing syringe. According to some embodiments, the dosing
mechanism
translates user action into precisely controlled movements of a multi-
component plunger rod. A
gear train may couple plunger rod components such that the axial distance
travelled by the
plunger rod component that pushes on a syringe plunger seal is reduced
relative to the axial
distance travelled by the plunger rod component with which the user directly
engages. A thumb
nut engages with one of the plunger rod components to precisely control the
travel distances of
the plunger rod components. The dosing mechanism can include one or more
features for
coupling the mechanism to the plunger seal end of a conventional syringe.
[0066] The below description is provided to assist in an
understanding of exemplary
embodiments of the present disclosure with reference to the accompanying
figures.
Accordingly, those of ordinary skill in the art will recognize that various
changes to and
modifications of the exemplary embodiments described herein can be made
without departing
from the scope of the claimed invention. Also, descriptions of generally well-
known functions
and constructions are omitted for conciseness.
[0067] As used herein to describe the mechanism to deliver an
accurate, precise dose,
drug delivery syringes, or any of the relative positions of the components of
the present
invention, the terms "axial" or "axially" refer generally to a longitudinal
axis "A" around which
the mechanisms and syringes may be positioned, although not necessarily
symmetrically. The
term "radial" refers generally to all directions orthogonal to axis "A". The
term user end refers
generally to the end marked "U", and the patient end refers generally to the
end marked "P." As
used herein, the term "glass" should be understood to include other similarly
chemically inert
materials suitable for use in a pharmaceutical grade application that would
normally require
.. type I borosilicate glass, quartz, including but not limited to certain non-
reactive polymers such
as cyclic olefin copolymers (COC), cyclic olefin polymers (COP), and the like
used in
11
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
pharmaceutical prefillable syringes. These syringes may involve additional
treatments such
removal of subvisible particulates to make them appropriate for ophthalmic
drugs. Plastic also
refers to polymers such as polypropylene, polycarbonate and the like used in
hypodermic
syringes. The term "elastomer," "elastomeric" or "elastomeric materials
commonly used in the
manufacture of plunger seals in syringes. This also includes plunger seals
that may be coated to
afford chemical inertness for certain pharmaceutical applications. "Fluid"
refers primarily to
water, but can also refer to solutions such as polyethylene glycol, solids
suspended in solution,
immiscible substances in solution and refers to Newtonian as well as non-
Newtonian liquids; all
of these are injectable using a syringe. A system with needle safety can refer
to safety
implemented either with a retractable needle mechanism or an external
sheath/cover for the
needle. When a needle used is for administration, the needle is typically made
of stainless steel;
needles also include microneedles and microneedle arrays. Needle size used
could range from
21G through 40G in diameter and up to 1" in length. Administration could be
subcutaneous,
intravenous, intradermal, intravitreal, intraocular, suprachoroidal, sub-
conjunctival, intra-
tumoral, intracellular, topical, etc.
[0068] Embodiments of a mechanism to set and deliver an accurate,
precise injectable
dose and embodiments of drug delivery syringe(s) that could incorporate such
mechanisms are
described below. Such devices can be safe and easy to use, aesthetically
appealing, and designed
per ergonomic considerations of their users, which may include researchers,
veterinary health
practitioners, and other clinical practitioners. Ergonomic features may be
included that enable
activation, operation, and disposal of devices with minimal or no training.
Embodiments of
dose control mechanisms, fluid delivery syringes, and respective components
are described
further herein with reference to the accompanying figures.
[0069] FIGs. 1-3 illustrate a syringe-based system 100 incorporating
an accurate, precise
dose delivery mechanism, according to a first embodiment. System 100 includes
plunger rod
subassembly 101, prefillable syringe 102, plunger seal adapter 103, and cover
104. Cover 104 is
affixed to plunger rod subassembly 101 by the engagement of external threads
105 on cover 104
with internal threads 107 on the barrel opening end of housing 106. In other
embodiments, cover
104 may be affixed to housing 106 using other means, including press fitting,
snap-on features,
fasteners, etc. According to some embodiments, the prefillable syringe may
include a luer lock
adapter 108 or a staked/pre-attached needle. In some embodiments, a cartridge
may be used
instead of a syringe. In some embodiments, a delivery adapter may be used that
enables topical
delivery or intranasal delivery. The prefillable syringe 102 may be sprayed on
the inside of the
syringe barrel 109 with silicone oil such as Dow Corning 360 to provide
lubrication.
12
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
Alternatively, a siliconization emulsion such as Dow CORNING 365 may be
sprayed. The
syringes may be baked at a high temperature to bake the silicone onto the
inside of the syringe
barrel 109.
[0070] According to some embodiments, the plunger rod subassembly
101, an
embodiment of which is shown separately in FIG. 4, includes a multi-component
plunger rod
that includes plunger rod 110 and drive rod 112. Plunger rod subassembly 101
also includes
housing 106, gear 111, drive rod 112, thumb nut 113, and a spring 114.
[0071] Plunger rod 110 includes a set of linear gear teeth 128 along
a portion of its length
(FIG. 5). Teeth 128 mesh with a first set of larger pitch gear teeth 118 on
gear 111. Gear 111 is
pinned to the housing such that it can rotate but it cannot translate. Thus,
linear movement of
plunger rod 110 causes gear 111 to rotate. Gear 111 is a compound gear (FIG.
6) with a second
set of smaller pitch gear teeth 120 that engage with a set of linear gear
teeth 124 on drive rod
112. Thus, rotation of gear 111 causes axial advancement of drive rod 112 in
the patient "P"
direction, pushing the non-patient surface of the plunger adapter 136. This,
in turn, pushes the
filled fluid 133, dispensing it through the tip of the needle 139 (or other
component). Due to the
difference in pitch between the two sets of teeth of the compound gear, the
drive rod 112
advances a smaller amount than the plunger rod 110 and a mechanical advantage
is provided
such that less user force is required than would be required to directly push
plunger seal 132. In
other embodiments, the configuration of gear 111 may be reversed such that the
smaller pitch
gear teeth interface with the plunger rod and the larger pitch gear teeth
interface with the drive
rod.
[0072] Plunger rod 110 also includes a plurality of dosing pegs 129
that engage with an
internal thread 131 of thumb nut 113 (FIGs. 7A-7B). At least a portion of
plunger rod 110 may
include a non-circular cross-section that fits within a complimentary aperture
in the housing,
which prevents rotation of plunger rod 110. With plunger rod 110 constrained
against rotation,
the rotation of thumb nut 113 causes axial movement of plunger rod 110. The
engagement of
dosing pegs 129 with internal thread 131 also prevents a user from pushing
plunger rod 110 to
axially advance plunger rod 110. Continued axial advancement of the plunger
rod 110 via
turning of thumb nut 113 will result in a rearmost dosing peg 142 (the dosing
peg nearest the
user end of the plunger rod 110) escaping the internal thread 131 of thumb nut
113. Once it has
escaped, the thumb nut 113 no longer prevents a user from directly advancing
plunger rod 110
by pushing on plunger rod 110. According to some embodiments, lugs 130 are
provided on the
plunger rod 110 to help maintain orientation of the plunger rod 110 inside the
syringe barrel 109.
13
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
[0073] Assembly of the plunger rod subassembly 101 may include
placing the spring 114
in seat 115, which is cavity in thumb nut 113. The gear 111 may be attached to
the housing 106
using a cylindrical pin 116 such that the back surface 117 of the larger pitch
teeth 118 abut
housing 106. There may be interference between the pin 116 and two holes 122,
123 in the
housing 106 ensuring that there is no rotational slip. The gear 111 may rotate
freely about the
axis of the pin 116.
[0074] Spring 114 inside the thumb nut 113 may then be slid inside
housing 106 as an
assembly through opening 126 such that the open side of the spring 114 is
facing the patient end
"P" of the system. Spring 114 may preload the thumb nut 113 against the
housing to reduce any
axial play that may arise from component tolerances. The drive rod 112 may be
inserted from
the patient end P such that teeth 124 on drive rod 112 interact with smaller
pitch teeth 120. The
drive rod 112 may be pushed all the way until a hard stop when the shoulder
125 on the drive rod
(FIG. 8A and 8B) touches the surface of the housing 106. The plunger rod 110
may then be
inserted through the dorsal cavity 127 (see FIG. 9A-9D) in the housing 106
such that teeth 128
are pointed away from the user end "U" and in a manner that they interact with
the larger pitch
teeth 118. The pegs or lugs 129 on the plunger rod 110 interfere with the
internal thread 131 on
the thumb nut 113 such that rotation of the thumb nut 113 in one direction
advances the plunger
rod 110 in the direction of the patient end "P". The shape of dorsal cavity
127 of the housing 106
is matched with the cross-section of the plunger rod 110, and is designed such
that it would
prevent any rotation of the plunger rod 110. The plunger rod subassembly 101
is now assembled.
[0075] To assemble a prefilled syringe, according to some
embodiments, the fluid 133
may be filled from the non-patient end of the syringe and then a substantially
elastomeric
plunger seal 132 is inserted from the non-patient end towards the patient end.
This is now a
filled syringe. A plunger adapter 136 may be screwed into the back of the
plunger seal 132 with
matching, complementary threads 137. In order to attach the plunger rod
subassembly 101 to
this prefilled syringe, the patient end "P" of the plunger rod subassembly 101
may be inserted
from the user end "U" of prefilled syringe inside the syringe barrel 109. The
cover 104 with its
threads 105 oriented towards the user end "U" may then be slid across the
length of the syringe
barrel 109 until it mates with the threads 107 on the housing. The cover may
be turned until
tightened. The accurate, precise dosing syringe-based system 100 is now
completely assembled.
This assembled system is ready for use by the user, ready for secondary
packaging, and/or ready
for terminal sterilization as required for certain applications.
14
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
[0076] In this embodiment, when ready to use, the user can attach a
needle 139 by
twisting and turning on the luer lock adapter 108 of the syringe. By pointing
the patient end "P"
of the syringe upwards, the user rotates thumb nut 113 to set the dose.
Striated features 140 may
be provided on the outer curved surface of the thumb nut 113 to allow for
better grip and tactile
feel. Markings 141 on the dorsal surface of the thumb nut 113 may provide a
visual cue to the
user of the direction of rotation of the thumb nut 113 for dose setting. As
the thumb nut 113 is
rotated, the internal threads 131 act as a guide for pegs or lugs 129 on the
plunger rod 110,
thereby advancing the plunger rod 110.
[0077] As the user continues to rotate the thumb nut 113, the plunger
rod 110 is axially
advanced in direction "P" until the dose set peg 142 (the last peg) exits from
the thread 131
inside the thumb nut 113. After this point, subsequent rotation of the thumb
nut 113 does not
cause any axial translation of the plunger rod 110, and thus, does not cause
any more fluid to be
dispensed. The dose is now "set." This configuration is illustrated in FIG.
10A and 10B. The
user then inserts the needle into the target site for delivery and pushes the
thumb rest 138 on the
plunger rod 110 until the underside 143 of thumb rest 138 contacts the surface
144 of the
housing 106. This configuration is illustrated in FIG. 11A and 11B. An
accurate, precise dose is
now delivered and system 100 can be safely discarded.
[0078] FIGs. 12-15 illustrate a syringe-based system 1000
incorporating an accurate,
precise dose delivery mechanism, according to a second embodiment. System 1000
includes
plunger rod subassembly 1001 coupled to a prefillable syringe 1002.
Prefillable syringe 1002
may include luer lock adapter 1008 and a plunger seal 1032. Prefillable
syringe 1002 is shown
with a cap in FIG. 12 and with a needle in FIG. 13, for illustration purposes.
A plunger seal
adapter 1003 may be provided in the prefillable syringe 1002 to serve as the
interface between
the plunger seal 1032 and the plunger rod subassembly 1001.
[0079] The plunger rod subassembly 1001 is affixed to the user end of the
prefillable
syringe. The plunger rod subassembly 1001 includes drive rod 1012, which
drives the plunger
seal 1032 (e.g., via plunger seal adapter 1003) during dose delivery, and
plunger rod 1010, which
is driven by a user press during dose delivery. Plunger rod subassembly 1001
also includes
housing 1006, housing clip 1004 and thumb nut 1013. The operation of system
1000 is similar
to that of system 100 in that a user rotates thumb nut 1013 to set the dose
and then presses on
plunger rod 1010 to deliver the dose. Housing 1006 may include an indicator
1040 for indicating
the direction of rotation of thumb nut 1013 for dose setting. Rotation of
thumb nut 1013 causes
plunger rod 1010 to advance via the engagement of an internal thread on thumb
nut 1013 with
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
dosing lugs 1029 on the plunger rod 1010. Advancement of plunger rod 1010
causes
advancement of drive rod 1012 via a gear train that couples the movements of
the plunger rod
and the drive rod. Once the dosing lugs 1029 have cleared the internal thread
on the thumb nut
1013, the dose is set, and a user press on the user end of plunger rod 1010
causes plunger rod
1010 to fully advance, which in turn, causes drive rod 1012 to push plunger
seal 1032 to expel
the set dose.
[0080] FIG. 14A shows system 1000 in the dose set configuration. The
plunger rod 1010
has advanced within housing 1006 to the point that the dosing lugs 1029 have
cleared the
internal thread on the thumb nut 1013. In the illustrated embodiment, a dose
indication 1042
("20") can be seen through a window 1044 on the side of housing 1006 to
indicate that the dose
has been set and/or the amount of the dose that has been set. FIG. 14B shows
system 1000 in the
dose delivered configuration. A user press on the thumb rest 1038 of plunger
rod 1010 has
advanced plunger rod 1010 to its fully depressed position, against housing
1006. The patient end
of plunger rod 1010 has advanced within syringe barrel 1009. The drive rod
1012, plunger seal
adapter 1003, and plunger seal 1032 have also advanced within syringe barrel
1009 to a lesser
degree than plunger rod 1010 due to the gear reduction of the gear train, as
will be discussed in
more detail below. Thus, the plunger rod travel "X" is greater than the drive
rod travel "Y". In
the embodiment shown, the dose indication 1042 shown through window 1044 is
"0" to indicate
that the set dose has been fully dispensed.
[0081] FIG. 15 illustrates the assembly of plunger rod subassembly 1001
onto syringe
barrel 1009, according to some embodiments. The patient end of the plunger rod
subassembly
1001 may be inserted from the user end of syringe barrel 1009. Clip 1004 is
positioned around
syringe barrel 1009. Clip 1004 may be formed of two pieces that may register
to one another
with one or more pegs or other registering features. In some embodiments, the
pieces snap
together, for example, via an interference fit of registering features. The
clip pieces may be
screwed together, glued together, or otherwise affixed to one another using
any suitable method.
In some embodiments, clip 1004 is a unitary piece, such as cover 104 of system
100. Once clip
1004 is positioned around syringe barrel 1009, clip 1004 is slid toward the
user end of syringe
barrel 1009 to interface with the patent end of housing 1006. Clip 1004 may
include one or more
tabs 1046 that engage with an inner-facing groove 1048 in the patient end of
housing 1006 such
that turning clip 1004 once the tabs 1046 are engaged in groove 1048 (for
example, a quarter
turn) locks the housing 1006, syringe barrel 1009, and clip 1004 together. In
some
embodiments, clip 1004 includes an external thread that mates with an internal
thread on the
housing (for example, similar to system 100 described above). In some
embodiments, clip 1004
16
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
includes one or more recesses for insertion of a tool, such as a spanner
wrench, to turn clip 1004
in housing 1006. In some embodiments, compliance in one or more of clip 1004
and housing
1006 (for example, compliance of tabs 1046) results in preloading of the
housing, cover, and
syringe barrel once clip 1004 is tightened into housing 1006, which eliminates
free play between
.. the components. In some embodiments, a compliance component (such as a wave
spring, coil
spring, gasket, etc.) is included to preload the syringe barrel-to-housing
engagement
[0082] FIGs. 16A and 16B show a gear train 1011 that couples plunger
rod 1010 and
drive rod 1012, according to some embodiments. The gear train 1011 includes
rotor gear 1060,
lower force gear 1062, and higher force gear 1064. Lower force gear 1062 and
higher force gear
1064 are both compound gears, each having two sets of gear teeth. Rotor gear
1060 is pinned to
the housing through its axis of rotation and includes teeth that engage a set
of teeth on plunger
rod 1010 such that linear movement of plunger rod 1010 causes rotational
movement of rotor
gear 1060. The teeth on rotor gear 1060 also engage a first set of teeth 1062A
on lower force
gear 1062, which is also pinned to the housing, such that rotation of rotor
gear 1060 causes
rotation of lower force gear 1062. Lower force gear 1062 includes a second set
of gear teeth
1062B on the other side (see FIG. 17A). The second set of gear teeth 1062B
engage a first set of
gear teeth 1064A on higher force gear 1064, which is pinned to housing 1006,
such that rotation
of lower force gear 1062 causes rotation of higher force gear 1064. Higher
force gear 1064
includes a second set of gear teeth 1064B on the other side (see FIG. 17A).
The second set of
gear teeth 1064B engage a set of linear gear teeth 1024 on drive rod 1012 such
that rotation of
higher force gear 1064 causes linear movement of drive rod 1012. Thus, gear
train 1011
converts linear motion of plunger rod 1010 to linear motion of drive rod 1012.
[0083] The gear train may be configured to provide a stroke
reduction of the drive rod
relative to the plunger rod and a mechanical advantage through the
configuration of the gears.
As illustrated, the pitch of the first set of teeth 1062A on lower force gear
1062 may be greater
than the pitch of the teeth on rotor gear 1060 such that lower force gear 1062
rotates less than
rotor gear 1060. The pitch of the second set of gear teeth 1062B may be less
than the pitch of
the first set of teeth 1062A. The pitch of the first set of gear teeth 1064A
on higher force gear
1064 may be greater than the pitch of the second set of teeth 1062A and the
pitch of the second
set of gear teeth 1064B on higher force gear 1064 may be less than that of the
first set of gear
teeth 1064A. This configuration results in the drive rod 1012 moving a
fraction of the amount
that plunger rod 1010 moves and results in a mechanical advantage such that
the force required
to depress the plunger rod 1010 is less than it would be if no gear train were
provided.
17
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
[0084] One of skill in the art will readily understand that a gear
train may be configured
with any suitable combination of gears to achieve design requirements, such as
gear reduction,
mechanical advantage, compactness, etc. For example, according to some
embodiments, the
gear train may include just a single compound gear, such as gear 101 of system
100 described
above. A gear train may include two gears or four or more gears. Any of the
gears may include
just a single tooth pitch or may include two or more tooth pitches. A gear
train may include idler
gears, epicyclic gears, or any other suitable gears or gear arrangements.
Further, embodiments
may be configured to reverse the gear reduction described above such that
axial movement of the
drive rod is greater than axial movement of the plunger rod.
[0085] FIG. 17A-C are cross-sectional views illustrating the dose setting
and dose
delivery configurations of system 1000, according to some embodiments. FIG.
17A shows
system 1000 in an "as-delivered" state, prior to the dose being set. In this
state, the plunger rod
1010 cannot be pushed in by a user press because of the engagement of at least
one dosing lug
1029 on plunger rod 1010 with the internal thread of thumb nut 1013. To set
the dose, the user
turns thumb nut 1013 in the dose setting direction (for example, as indicated
by indicator 1040),
causing dosing lugs 1029 (and, thus, plunger rod 1010) to be advanced by the
internal thread of
thumb nut 1013.
[0086] System 1000 is shown in the dose set state in FIG. 17B. As
shown, plunger rod
1010 has moved toward the patient end but the thumb rest 1038 is still spaced
from the housing.
Through action of the gear train 1011, the drive rod 1012 has advanced within
the syringe barrel
1009, though to a lesser degree due to the gear reduction of the gear train
1011 described above.
Although not shown in FIG. 17B, all of the dosing lugs 1029 have escaped the
internal thread of
thumb nut 1013 such that further turning of thumb nut 1013 in the dose setting
direction causes
no further advancement of plunger rod 1010. In some embodiments, thumb nut
1013 includes
one or more stops that engage with dosing lugs 1029 to prevent further turning
of thumb nut
1013 in the dose setting direction, as will be described in more detail below,
which can provide
an indication to the user that the dose is set and can provide precise
positioning of the plunger
rod 1010. The system 1000 is now ready for dispensing the set dose. FIG. 17C
illustrates
system 1000 in the dispensed configuration. Plunger rod 1010 has been advance
fully to the
point that its thumb rest 1038 abuts the user end of housing 1006. As
illustrated, the drive rod
1012, plunger seal adapter 1003, and plunger seal 1032 have advanced within
syringe barrel
1009, expelling the set dose. As is readily apparent, the dosage delivered is
proportional to the
stroke of the drive rod 1010 during the dose delivery process. The stroke of
the drive rod 1012
during dose delivery is controlled by the gear train configuration and by the
distance between the
18
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
rearmost dosing lugs 1029 and the underside of the thumb rest 1038, which
determines the dose
delivery stroke of the plunger rod. Thus, the drive rod stroke can be tailored
for specific
applications by the dosing lug configuration and the gear train configuration.
[0087] FIG. 18A-C illustrate the engagement between plunger rod 1010
and thumb nut
1013, according to some embodiments. FIG. 18A shows plunger rod 1010 and thumb
nut 1013
in their relative positions when the system is in the dose set position.
Plunger rod 1010 includes
dosing lugs 1029 on two sides, which provides balanced loading on plunger rod
1010. In this
embodiment, the second side includes fewer lugs than the first side. The
rearmost lugs 1050A
and 1050B have both emerged from the groove 1031 within thumb nut 1013 (FIG.
18B) and are
abutting stops 1054A and 1054B at ends of groove 1031. These stops prevent
thumb nut from
being turned in the dose setting direction (the direction indicated by the
arrow of FIG. 18A). By
providing stops, the relative position of the lugs with respect to the thumb
nut can be precisely
controlled, allowing for precise control of the axial position of plunger rod
1010.
[0088] Plunger rod 1010, according to the illustrated embodiment,
includes dosage
indications 1042 on the side, which may show through window 1044 on the side
of housing 1006
to indicate to the user conformation that the dose is set and that the dose
delivery is complete.
For example, once the dose is set (FIG. 17B), the number "20" (in this
embodiment) may show
through window 1044 to indicate to the user that the dose has been properly
set. After the dose
has been set and the plunger rod 1010 advances further within the housing 1006
during dosage
delivery, the "20" marker moves axially out of window 1044 and the "0" marker
moves axially
into window 1044. Alignment of the "0" marker within window 1044 may indicate
to the user
that the dose has been completely delivered.
[0089] FIG. 19 illustrates a ratchet engagement between a pawl 1056
on the housing
1006 and grooves 1058 on the side of thumb nut 1013. This ratchet engagement
serves dual
purposes of preventing the thumb nut 1013 from being rotated in the direction
opposite to the
dose setting direction and providing an aural and/or tactile indication of the
dose setting process.
The ratchet engagement may also serve to urge thumb nut 1013 against the
housing to control
the axial positioning of the thumb nut. The pawl 1056 is configured such that
it is urged into the
grooves 1058 of the thumb nut 1013. One face of the pawl is configured such
that the pawl rides
out of a respective groove 1058 as the thumb nut 1013 is rotated in the dose
setting direction.
The pawl then snaps back into the next groove 1058, providing an aural and/or
tactile indication
of the movement of thumb nut 1013. In some embodiments, the opposite face of
pawl 1056 is
configured such that the pawl remains in the groove 1058 and prevents the
thumb nut 1013 from
19
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
being rotated in the counter direction such that the plunger rod 1010 can only
be advanced in the
dose-setting direction. In some embodiments, the opposite face of pawl 1056 is
configured such
that the pawl resists rotation of the thumb nut 1013 in the counter direction
but can rise out of the
groove 1058 if enough rotational force is applied to the thumb nut. In this
way, the thumb nut
can be counter rotated but the effort required to counter rotate the thumb nut
is greater than the
effort required to rotate the thumb nut in the dose-setting direction. In some
embodiments, the
opposite face of pawl 1056 is configured such that the pawl does not resist
rotation of the thumb
nut 1013 or, at most, resists counter rotation to the same degree as it
resists rotation in the dose-
setting direction. In this way, the ratchet provides aural and/or tactile
feedback but does not
generally favor rotation in one direction or the other.
[0090] FIG. 20A is a perspective view of a plunger rod 1010,
according to some
embodiments. Plunger rod 1010 includes spacing pegs 1030 for maintaining the
lateral position
of plunger rod 1010 within syringe barrel 1009, as discussed above. Plunger
rod 1010 includes a
longitudinal groove 1070 along a portion of the shaft nearest the thumb rest
1038. Portions of
the low and high force gears are located within this groove when the plunger
rod subassembly is
assembled. Plunger rod 1010 includes a cutout 1072 such that a cross section
perpendicular to
the longitudinal axis of plunger rod 1010 is semicircular. Cutout 1072
provides a bearing
surface for a portion of drive rod 1012, which has a complimentary
semicircular cross section, to
slide along. Drive rod 1012, according to some embodiments, is shown in FIGs.
20B-C. Drive
rod 1012 includes a set of linear gear teeth 1024, which engage the second set
of teeth 1064B of
higher force gear 1064. The cross-sectional profile of a portion of drive rod
1012 is configured
to fit with and slide along the cutout 1072 of plunger rod 1010, providing a
large bearing surface
interface that can ensure smooth relative axial motion between the plunger rod
and drive rod and
reduced lateral play. Drive rod 1012 can include a cylindrical end portion to
help concentrically
locate drive rod 1012 in syringe barrel 1009.
[0091] FIGs. 21A-B are exploded views illustrating aspects of the
assembly process of
plunger rod subassembly 1001, according to some embodiments. In the
illustrated embodiment,
housing 1006 includes four components¨main body 1080A, back cover 1080B, front
cover
1080C, and top cover 1080D. Pins 1082 in front cover 1080C fit into
corresponding bores 1083
in back cover 1080B to assemble the front and back covers to the main body.
Main body 1080A
includes a plurality of holes into which gear pins 1084 are inserted. Gear
pins 1084 provide
shafts for the gears of the gear train 1011. Main body 1080A also includes
grooves into which
window 1044 slides. Top cover 1080D includes pins that fit into corresponding
bores in the user
end of main body 1080A. Top cover 1080D includes a cavity 1027 shaped to match
the cros 5-
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
sectional profile of plunger rod 1010, which can prevent rotation of plunger
rod 1010, such that
plunger rod 1010 can translate but not rotate. In some embodiments, a
preloading ring 1086 may
be provided to preload the engagement of plunger rod subassembly 1001 with the
syringe barrel
1009. Preloading ring 1086 may be a coil spring, a wave spring, a ring of
compliant material,
.. such as plastic, foam, or rubber, or any other suitable component for
preloading the engagement.
[0092] Following is a description of an assembly process for plunger
rod subassembly
1001, according to some embodiments. The following process is intended to be
exemplary only.
Steps may be conducted in a different order, one or more of the steps may be
omitted, and/or one
or more additional steps may be included depending on the configuration of the
various
components of the particular embodiment. In a first step, top cover 1080D is
seated and press-fit
into main body 1080A, for example, using an arbor press. Next, the three gear
pins 1084 are
press-fit into the main body 1080A. Then, the thumb nut 1013 is inserted into
the corresponding
portion of main body 1080A, the back cover 1080B is seated onto the back side
of the housing,
and the preloading ring 1086 is inserted into the barrel opening of the
housing. Next, the higher
force gear 1064 is installed on the corresponding gear pin 1084¨the gear pin
farthest from the
thumb nut 1013. The lower force gear 1062 is then installed onto the center
gear pin 1084 and
aligned such that its second set of gear teeth 1062B engage with the first set
of gear teeth 1064A
on higher force gear 1064. The drive rod 1012 is then inserted through the
barrel opening in the
housing 1006, aligned with alignment features of the housing, and pushed such
that its teeth
1024 engage the second set of teeth 1064B on higher force gear 1064. The drive
rod 1012 is
inserted into the housing to a specified depth, which may depend on the
particular application
and which may be controlled using tooling.
[0093] In the next step, the plunger rod 1010 is inserted through the
top cover 1080D and
through the thumb nut 1013 until the lowest-most dosing lug 1029 prevents
further insertion.
The rotor gear 1060 is then installed onto the remaining gear pin 1084 and
aligned for
engagement with both the first set of teeth 1062A of the lower force gear 1062
and the teeth
1028 on plunger rod 1010. The thumb nut 1013 is rotated in the dose setting
direction to engage
the dosing lugs 1029 on plunger rod 1010 with the internal thread of thumb nut
1013. Plunger
rod 1010 is translated further into the housing 1006 through continued
rotation of thumb nut
1013 in the dose setting direction until plunger rod 1010 reaches a
predetermined depth.
[0094] Next, window 1044 may be inserted into the corresponding
groove in main body
1080A. The front cover 1080C is then aligned with features on the main body
1080A and/or
features on back cover 1080B and press fit into place. This completes assembly
of plunger rod
21
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
subassembly 1001, according to some embodiments. The assembled plunger rod
subassembly
1001 may then be assembled to a prefilled syringe or packaged, for example,
for storage and/or
shipment to the syringe filler for final assembly of a prefilled accurate and
precise dosing syringe
system.
[0095] Embodiments of the present invention may provide configurations
which allow
the use of standard, commercially-available components, thereby reducing
overall manufacturing
costs, streamlining assembly processes, and avoiding regulatory concerns often
associated with
non-standard materials and components. For example, syringe barrels may be
made of plastic,
glass, or any other material commonly used for medical grade products. One or
more
components may be made of any suitable plastic, such as polycarbonate
(including those sold
under the trade name "LEXAN" by SABIC Innovative Plastics of Pittsfield, Mass)
and the like.
Any suitable elastomeric polymers or rubbers may be utilized (such as the
rubber products sold
under the trade name "HELVOET" by Datwyler Pharma Packaging USA Inc. of
Pennsauken,
N.J.) for components such as the plunger seal. Various medical grade metals,
such as stainless
steel, may be utilized for one or more components, such as the plunger rod,
drive rod, gear pins,
gears, thumb nut, etc., as will be appreciated by an ordinarily skilled
artisan. Any of the
components described herein may be shaped or sized in any configuration to
meet desired
parameters. Any of the components described herein may be formed as singular
components or
may comprise multiple sub-components. Components may be built and/or assembled
by any
suitable process, including using glues or welding methods such as ultrasonic
welding.
[0096] A person of skill in the art will appreciate that dosing
mechanisms, syringes,
syringe systems, etc., according to the principles and features described
herein, can generally be
configured for any application including, injectable drug delivery into or
onto the eye,
intracellular delivery, delivery of radioactive agents, delivery of
chemotherapy, etc.
[0097] The foregoing description, for the purpose of explanation, has been
described
with reference to specific embodiments. However, the illustrative discussions
above are not
intended to be exhaustive or to limit the invention to the precise forms
disclosed. Many
modifications and variations are possible in view of the above teachings. The
embodiments were
chosen and described in order to best explain the principles of the techniques
and their practical
applications. Others skilled in the art are thereby enabled to best utilize
the techniques and
various embodiments with various modifications as are suited to the particular
use contemplated.
[0098] Although the disclosure and examples have been fully described
with reference to
the accompanying figures, it is to be noted that various changes and
modifications will become
22
CA 03018880 2018-09-24
WO 2017/180480
PCT/US2017/026684
apparent to those skilled in the art. Such changes and modifications are to be
understood as
being included within the scope of the disclosure and examples as defined by
the claims.
Finally, the entire disclosure of the patents and publications referred to in
this application are
hereby incorporated herein by reference.
23