Note: Descriptions are shown in the official language in which they were submitted.
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
SEED-SPECIFIC AND EMBRYO-PREFERENTIAL PROMOTERS AND USES THEREOF
FIELD OF THE INVENTION
[1] The present invention relates to materials and methods for the
expression of a gene of interest specifically
in seeds of plants. In particular, the invention provides an expression
cassette for regulating seed-specific and
embryo-preferential expression in plants.
BACKGROUND
[2] Modification of plants to alter and/or improve phenotypic
characteristics (such as productivity or quality)
requires the overexpression or down-regulation of endogenous genes or the
expression of heterologous genes in
plant tissues. Such genetic modification relies on the availability of a means
to drive and to control gene expression
as required. Indeed, genetic modification relies on the availability and use
of suitable promoters which are effective
in plants and which regulate gene expression so as to give the desired
effect(s) in the transgenic plant.
[3] For numerous applications in plant biotechnology a tissue-specific or a
tissue-preferential expression
profile is advantageous, since beneficial effects of expression in one tissue
may have disadvantages in others.
[4] Seed-preferential or seed-specific promoters are useful for expressing
or down-regulating genes
.. specifically in the seeds to get the desired function or effect, such as
improving disease resistance, herbicide
resistance, modifying seed or grain composition or quality, such as modifying
starch quality or quantity, modifying
oil quality or quantity, modifying amino-acid or protein composition,
improving tolerance to biotic or abiotic stress,
increasing yield, or altering metabolic pathways in the seeds.
[5] Examples of seed-preferential or seed-specific promoters include the
Tonoplast Intrinsic Protein alpha
promoter from Arabidopsis thaliana (US patent application U52009/0241230), the
KNAT411 promoter from
Arabidopsis thaliana (US Pat. No. 6,342,657), an oleosin promoter, a 2S
storage protein promoter or a legumin-like
seed storage protein promoter from Linum usitatissimum (US Pat. No.
7,642,346), the acyl carrier protein promoter
from Brassica napus (US Pat. Application No. 1994/0129129), the 13-amylase
promoter of barley (US Pat.
Application No. 1997/0793599), and the Ha ds10 G1 promoter of sunflower (US
Pat. No. 6,759,570).
[6] There remains thus an interest in the isolation of novel late stage
seed-specific promoters having embryo-
preferential activity. It is thus an objective of the present invention to
provide Brassica promoters having late stage
seed-specific activity and embryo-preferential activity. This objective is
solved by the present invention as herein
further explained.
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 2 -
SUMMARY
[7] In one aspect, the invention provides an isolated nucleic acid
comprising late stage seed-specific and
embryo-preferential promoter activity selected from the group consisting of
(a) a nucleic acid comprising a
nucleotide sequence of any one of SEQ ID NOs: 2 to 10 or a functional fragment
thereof; and (b) a nucleic acid
comprising a nucleotide sequence having at least 80% sequence identity to any
one of SEQ ID NOs: 2 to 10 or a
functional fragment thereof.
[8] A further embodiment provides a recombinant gene comprising the nucleic
acid according to the invention
operably linked to a heterologous nucleic acid sequence encoding an expression
product of interest, and optionally
a transcription termination and polyadenylation sequence, preferably a
transcription termination and
polyadenylation region functional in plant cells. In a further embodiment,
said expression product of interest is an
RNA capable of modulating the expression of a gene or is a protein.
[9] Yet another embodiment provides a host cell, such as an E. coli cell,
an Agrobacterium cell, a yeast cell, or
a plant cell, comprising the isolated nucleic acid according to the invention,
or the recombinant gene according to
the invention.
[10] In a further embodiment, a plant is provided comprising the
recombinant gene according to the invention.
Yet a further embodiment provides seeds obtainable from the plant according to
the invention. In another
embodiment, the plants or plant parts according to the invention are seed crop
plants or seeds.
[11] Yet another embodiment provides a method of producing a transgenic
plant comprising the steps of (a)
introducing or providing the recombinant gene according to the invention to a
plant cell to create transgenic cells;
.. and (b) regenerating transgenic plants from said transgenic cell.
[12] Further provided is a method of effecting late stage seed-specific and
embryo-preferential expression of a
nucleic acid comprising introducing the recombinant gene according to the
invention into the genome of a plant, or
providing the plant according to the invention. Also provided is a method for
altering seed properties of a plant or to
produce a commercially relevant product in a plant, said method comprising
introducing the recombinant gene
according to the invention into the genome of a plant, or providing the plant
according to the invention. In another
embodiment, said plant is a seed crop plant.
[13] Also provided is the use of the isolated nucleic acid according to the
invention to regulate expression of an
operably linked nucleic acid in a plant, and the use of the isolated nucleic
acid according to the invention, or the
recombinant gene according to the invention to alter seed properties of a
plant or to produce a commercially
relevant product in a plant. In a further embodiment, said plant is a seed
crop plant.
[14] Yet another embodiment provides a method of producing food, feed, or
an industrial product comprising
(a) obtaining the plant or a part thereof, according to the invention; and (b)
preparing the food, feed or industrial
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 3 -
product from the plant or part thereof. In another embodiment, said food or
feed is oil, meal, grain, starch, flour or
protein, or said industrial product is biofuel, fiber, industrial chemicals, a
pharmaceutical or a nutraceutical.
BRIEF DESCRIPTION OF THE DRAWINGS
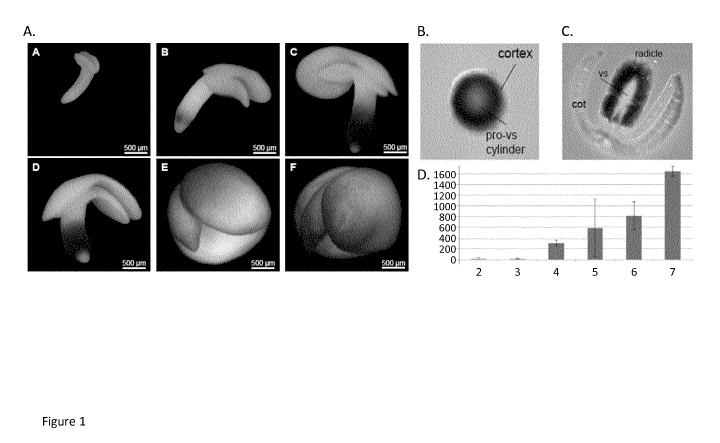
[15] Figure 1: GUS staining in the embryos carrying PcruP2 BnA2::GUS. A:
GUS labelling in whole embryos at
different developmental stages: sub-panel A: 10 to 12 DAF, sub-panel B: 15 to
18 DAF, sub-panel C: 20 to 23 DAF;
sub-panel D: 23 to 27 DAF; sub-panel E: 27 to 32 DAF; sub-panel F: 35 to 40
DAF. B: GUS labelling in sectioned
embryo radicle at early stage. C: GUS labelling in sectioned embryo at the
mature embryo stage. Cot: cotyledon,
vs: vasculature. D: Semi quantitative assessment of GUS labelling (pU*mg-1
fresh weightt) in transgenic lines
carrying a PcruP2 BnA2::GUS 1-DNA. Embryos stained at the following stages: 2:
13 to 15 DAF, 3: 16 to 18 DAF,
4: 19 to 23 DAF, 5: 25 to 29 DAF, 6: 31 to 35 DAF, 7: 37 to 40 DAF.
[16] Figure 2: Expression profile analysis in seeds carrying PcruP2
BnA2::GUS. A: GUS labelling in the
endosperm and seed coat at late stage. The arrow indicates the endosperm. B:
Semi quantitative assessment of
GUS labelling (p Wmg-1 fresh weightt) in seed coats of seeds from transgenic
lines carrying a PcruP2 BnA2::GUS
1-DNA. Seed coats stained at the following stages: 2: 13 to 15 DAF, 3: 16 to
18 DAF, 4: 19 to 23 DAF, 5: 25 to 29
DAF, 6: 31 to 35 DAF.
[17] Figure 3: Alignment of the amino acid sequence of different Brassica
CRUP2 proteins. Amino acid
residues conserved in all proteins are indicated by an asterisk, conserved
amino acid substitutions are indicated by
a colon. The lowest identity between any two CRUP2 proteins is about 83%.
[18] Figure 4: Relative expression levels of different CRUP2 transcripts in
different plant tissues. A: CRUP2
BnA2; B: CRUP2 BnA1; C: CRUP2 BnC1; D: CRUP2 Br2; E: CRUP2 Br1; F: CRUP2 Bo;
G: CRUP2 BjA2; H:
CRUP2 BjA1 and I: CRUP2 BjB1. Different tissues for A to F: AM33: Apical
meristem 33 days after sowing (DAS);
BFB42: Big flower buds 42 DAS; CTYL10: Cotyledons 10 DAS; 0F52: Open flowers
52 DAS; Pod2: Pods 14-20
DAS; Pod3: Pods 21-25 DAS; Ro2w: Roots 14 DAS; 5FB42: Small flower buds 42
DAS; 5eed2: Seeds 14-20 days
after flowering (DAF); 5eed3: Seeds 21-25 DAF; 5eed4: Seeds 26-30 DAF; Seed5:
Seeds 31-35 DAF; 5eed6:
Seeds 42 DAF; 5eed7: Seeds 49 DAF; 5t2w: Stem 14 DAS; 5t5w: Stem 33 DAS; YL33:
Young leaf 33 DAS.
Different tissues for G to I: AM22: Apical meristem 22 days after sowing
(DAS); BFB35: Big flower buds 35 DAS;
CTYL8: Cotyledons 8 DAS; 0F35: Open flowers 35 DAS; Pod2: Pods 14-20 DAS;
Pod3: Pods 21-25 DAS; Pod4:
Pods 26-30 DAS; Pod5: Pods 31-35 DAS; Ro2W: Roots 14 DAS; 5FB35: Small flower
buds 35 DAS; 5eed2: Seeds
14-20 days after flowering (DAF); 5eed3: Seeds 21-25 DAF; 5eed4: Seeds 26-30
DAF; Seed5: Seeds 31-35 DAF;
5eed6: Seeds 42 DAF; 5eed7: Seeds 49 DAF; 5t2w: Stem 14 DAS; 5t3w: Stem 22
DAS; YL22: Young leaf 22
DAS; 0L22: Old leaf 22 DAS.
[19] Figure 5: Relative expression levels of different CRUP2 transcripts in
different seed sub-tissues. A:
CRUP2 BnA2; B: CRUP2 BnA1; C: CRUP2 BnC1; D: CRUP2 Br2; E: CRUP2 Br1 and F:
CRUP2 Bo. Different seed
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 4 -
sub-tissues: a: Endosperm, 18 days after flowering (DAF); b: Endosperm, 24
DAF; c: embryonic hypocotyl, 18 DAF;
d: embryonic hypocotyl, 24 DAF; e: embryonic hypocotyl, 28 DAF; f: embryonic
hypocotyl, 32 DAF; g: embryonic
hypocotyl, 46 DAF; h: embryonic inner cotyledon, 18 DAF; i: embryonic inner
cotyledon, 24 DAF; j: embryonic inner
cotyledon, 28 DAF; k: embryonic inner cotyledon, 32 DAF; I: embryonic inner
cotyledon, 46 DAF; m: embryonic
outer cotyledon, 18 DAF; n: embryonic outer cotyledon(inner part), 24 DAF; o:
embryonic outer cotyledon(inner
part), 28 DAF; p: embryonic outer cotyledon(inner part), 32 DAF; q: embryonic
outer cotyledon(inner part), 46 DAF;
r: embryonic outer cotyledon(outer part), 24 DAF; s: embryonic outer
cotyledon(outer part), 28 DAF; t: embryonic
outer cotyledon(outer part), 32 DAF; u: embryonic outer cotyledon(outer part),
46 DAF.
[20]
Figure 6: Alignment of the 3' end of the nucleotide sequence of the Brassica
PcruP2 promoters from
Brassica napus, Brassica juncea, Brassica oleracea and Brassica rapa. The
predicted TATA box is indicated by a
frame. The transcription start is marked in bold. Conserved motifs are
underlined and numbered. Motif 1 has the
sequence of SEQ ID NO: 29, motif 2 has the sequence gtctaaya, motif 3 has the
sequence of SEQ ID NO: 30, motif
4 has the sequence tcatcttaa, motif 5 has the sequence gakcarttc, motif 6 has
the sequence of SEQ ID NO: 31,
motif 7 has the sequence of SEQ ID NO: 32, motif 8 has the sequence of SEQ ID
NO: 33, motif 9 has the sequence
of SEQ ID NO: 34, motif 10 has the sequence of SEQ ID NO: 35, motif 11 has the
sequence of SEQ ID NO: 36,
motif 12 has the sequence of SEQ ID NO: 37, motif 13 has the sequence of SEQ
ID NO: 38, motif 14 has the
sequence of SEQ ID NO: 39, motif 15 has the sequence of SEQ ID NO: 40 and
motif 16 has the sequence of SEQ
ID NO: 41.
DETAILED DESCRIPTION
[21] The present invention is based on the observation that SEQ ID NOs: 2
to 10 have late stage seed-specific
promoter activity and embryo-preferential promoter activity in Brassica.
[22]
SEQ ID NOs: 2 to 10 depict the region upstream (i.e. located 5' upstream of)
from the first ATG start codon
of the CRUP2 BnA2, CRUP2 BnA1, CRUP2 BnC1, CRUP2 Br2, CRUP2 Br1, CRUP2 Bo,
CRUP2 BjA2, CRUP2
BjA1, and CRUP2 BjB1 respectively.
[23] CRUP2 BnA2, CRUP2 BnA1 and CRUP2 BnC1 are the 3 copies present in
Brassica napus of the
orthologous gene to the Arabidopsis thaliana Cruciferin 2 gene At1g03880.
CRUP2 Bo is the one copy present in
Brassica oleracea of the orthologous gene to the Arabidopsis thaliana
Cruciferin 2 gene At1g03880. CRUP2 Br2
and CRUP2 Br1 are the 2 copies present in Brassica rapa of the orthologous
gene to the Arabidopsis thaliana
Cruciferin 2 gene At1g03880. CRUP2 BjA2, CRUP2 BjA1, CRUP2 BjB1 are the 3
copies present in Brassica juncea
of the orthologous gene to the Arabidopsis thaliana cruciferin gene At1g03880.
The cruciferin complex has an
octameric structure (Nietzel et al. 2013). Sjodahl et al. 1993 studied the
expression pattern of each cruciferin gene
family in Brassica napus seed development. They concluded that the transcripts
of the 3 families accumulate in the
embryo axis and in the cotyledons. They showed that the transcript of the
Cruciferin 2 gene family are absent from
the root cap and the provascular cells but did not disclose the corresponding
promoter sequence. Sjodahl et al.
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
-5-
1995 characterized the promoter region of the Cruciferin 1 gene of Brassica
napus and Bilodeau et al. 1994
characterized the promoter region of the Cruciferin 3 gene of Brassica napus.
[24] In one aspect, the invention provides an isolated nucleic acid
comprising late stage seed-specific and
embryo-preferential promoter activity selected from the group consisting of
(a) a nucleic acid comprising a
nucleotide sequence of any one of SEQ ID NOs: 2 to 10 or a functional fragment
thereof; and (b) a nucleic acid
comprising a nucleotide sequence having at least 80% sequence identity to
anyone of SEQ ID NOs: 2 to 10, or a
functional fragment thereof.
[25] The nucleic acid comprising the late stage seed-specific and embryo-
preferential promoter activity
according to the invention may also be comprised in a larger DNA molecule.
[26] "Seed-specific promoter activity" in the context of this invention
means the promoter activity is at least 10
times, or at least 20 times, or at least 50 times, or at least 100 times, or
at least 200 times, or at least 500 times, or
even at least 1000 times higher in seeds than in other tissues. In other
words, in seed-specific promoter activity,
transcription of the nucleic acid operably linked to the promoter of the
invention in the seeds is at least 10 times, or
at least 20 times, or at least 50 times, or at least 100 times, or at least
200 times, or at least 500 times or even at
least 1000 times higher than in other tissues. In other words, the seed-
specific promoter drives seed-specific
expression of the nucleic acid operably linked to the seed-specific promoter.
[27]
"Late stage seed" development in the context of this invention refers to seeds
in which the embryo
developmental stage ranges from green cotyledon stage to the mature embryo
stage. These developmental stages
are reached from 26 days after flowering.
[28] "Seed-specific promoter activity" encompasses "embryo-preferential
promoter activity".
[29] "Embryo-preferential promoter activity" in the context of this
invention means the promoter activity is at
least 2 times, or at least 5 times, or at least 10 times, or at least 20 times
or even at least 100 times higher in
embryonic tissues than in other seed tissues. In other words, in embryo-
preferential promoter activity, transcription
of the nucleic acid operably linked to the promoter of the invention in the
embryo is at least 2 times, or at least 5
times, or at least 10 times, or at least 20 times or even at least 100 times
higher than in other seed tissues. In other
words, the embryo-preferential promoter drives embryo-preferential expression
of the nucleic acid operably linked
to the embryo-preferential promoter.
[30] The phrase "operably linked" refers to the functional spatial
arrangement of two or more nucleic acid
regions or nucleic acid sequences. For example, a promoter region may be
positioned relative to a nucleic acid
sequence such that transcription of a nucleic acid sequence is directed by the
promoter region. Thus, a promoter
region is "operably linked" to the nucleic acid sequence. "Functionally
linked" is an equivalent term.
[31] The phrases "DNA", "DNA sequence," "nucleic acid sequence," "nucleic
acid molecule "nucleotide
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 6 -
sequence" and "nucleic acid" refer to a physical structure comprising an
orderly arrangement of nucleotides. The
DNA sequence or nucleotide sequence may be contained within a larger
nucleotide molecule, vector, or the like. In
addition, the orderly arrangement of nucleic acids in these sequences may be
depicted in the form of a sequence
listing, figure, table, electronic medium, or the like.
[32] As used herein, "promoter means a region of DNA sequence that is
essential for the initiation of
transcription of DNA, resulting in the generation of an RNA molecule that is
complementary to the transcribed DNA;
this region may also be referred to as a "5 regulatory region." Promoters are
usually located upstream of the coding
sequence to be transcribed and have regions that act as binding sites for RNA
polymerase II and other proteins
such as transcription factors (trans-acting protein factors that regulate
transcription) to initiate transcription of an
operably linked gene. Promoters may themselves contain sub-elements (i.e.
promoter motifs) such as cis-elements
or enhancer domains that regulate the transcription of operably linked genes.
The promoters of this invention may
be altered to contain "enhancer DNA to assist in elevating gene expression. As
is known in the art, certain DNA
elements can be used to enhance the transcription of DNA. These enhancers
often are found 5 to the start of
transcription in a promoter that functions in eukaryotic cells, but can often
be inserted upstream (5') or downstream
(3') to the coding sequence. In some instances, these 5 enhancer DNA elements
are introns. Among the introns
that are useful as enhancer DNA are the 5 introns from the rice actin 1 gene
(see US5641876), the rice actin 2
gene, the maize alcohol dehydrogenase gene, the maize heat shock protein 70
gene (see US5593874), the maize
shrunken 1 gene, the light sensitive 1 gene of Solanum tuberosum, the
Arabidopsis histon 4 intron and the heat
shock protein 70 gene of Petunia hybrida (see US5659122). Thus, as
contemplated herein, a promoter or promoter
region includes variations of promoters derived by inserting or deleting
regulatory regions, subjecting the promoter
to random or site-directed mutagenesis, etc. The activity or strength of a
promoter may be measured in terms of the
amounts of RNA it produces, or the amount of protein accumulation in a cell or
tissue, relative to a promoter whose
transcriptional activity has been previously assessed or relative to a
promoter driving the expression of a
housekeeping gene.
[33] A promoter as used herein may thus include sequences downstream of the
transcription start, such as
sequences coding the 5' untranslated region (5' UTR) of the RNA, introns
located downstream of the transcription
start, or even sequences encoding the protein. A functional promoter fragment
according to the invention may
comprise its own 5'UTR comprising the nucleotide sequence of SEQ ID NO: 2 from
nucleotide 1495 to nucleotide
1543, or comprising the nucleotide sequence of any one of SEQ ID NOs: 3 to 9
from nucleotide 1452 to nucleotide
1500, or comprising the nucleotide sequence of SEQ ID NO: 10 from nucleotide
1451 to nucleotide 1500.
Alternatively, 5'UTR fragments from other Brassica CRUP2 genes may be used.
For example, a promoter fragment
of SEQ ID NO: 2 may have the nucleotide sequence of said sequence from
position 1495 to 1543 replaced by the
nucleotide sequence of any one of SEQ ID NOs: 3 to 9 from nucleotide 1452 to
nucleotide 1500. A promoter
fragment of SEQ ID NO: 2 may have the nucleotide sequence of said sequence
from position 1495 to 1543
replaced by the nucleotide sequence of SEQ ID NO: 10 from nucleotide 1451 to
nucleotide 1500. A promoter
fragment of any one of SEQ ID NOs: 3 to 9 may have the nucleotide sequence of
said sequence from position 1452
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 7 -
to 1500 replaced by the nucleotide sequence of SEQ ID NO: 2 from nucleotide
1495 to nucleotide 1543. A promoter
fragment of any one of SEQ ID NOs: 3 to 9 may have the nucleotide sequence of
said sequence from position 1452
to 1500 replaced by the nucleotide sequence of any one of SEQ ID NOs: 3 to 9
from nucleotide 1452 to nucleotide
1500. As another example, a promoter fragment of any one of SEQ ID NOs: 3 to 9
may have the nucleotide
sequence of said sequence from position 1452 to position 1500 replaced by the
nucleotide sequence of SEQ ID
NO: 10 from nucleotide 1451 to nucleotide 1500. A promoter fragment of SEQ ID
NO: 10 may have the nucleotide
sequence of said sequence from position 1451 to position 1500 replaced by the
nucleotide sequence of SEQ ID
NO: 2 from nucleotide 1495 to nucleotide 1543. A promoter fragment of SEQ ID
NO: 10 may have the nucleotide
sequence of said sequence from position 1451 to position 1500 replaced by the
nucleotide sequence of any one of
SEQ ID NOs: 3 to 9 from nucleotide 1452 to nucleotide 1500.
[34] Such a promoter fragment may be at least about 500 bp, at least
about 550 bp, at least about 600 bp, at
least about 700 bp, at least about 800 bp, at least about 900 bp, at least
about 1000 bp, at least about 1100 bp, at
least about 1200 bp, at least about 1300 bp, at least about 1400 bp, at least
about 1500 bp, or at least about 1550
bp upstream of the first ATG start codon of the CRUP2 transcripts and have
late stage seed-specific and embryo-
preferential promoter activity. In combination with the above described
promoter fragments, a promoter fragment
according to the invention may thus comprise the nucleotide sequence of SEQ ID
NO: 2 from the nucleotide at
position 1043 to the nucleotide at position 1543, the nucleotide sequence of
SEQ ID NO: 2 from the nucleotide at
position 993 to the nucleotide at position 1543, the nucleotide sequence of
SEQ ID NO: 2 from the nucleotide at
position 893 to the nucleotide at position 1543, the nucleotide sequence of
SEQ ID NO: 2 from the nucleotide at
position 793 to the nucleotide at position 1543, the nucleotide sequence of
SEQ ID NO: 2 from the nucleotide at
position 693 to the nucleotide at position 1543, the nucleotide sequence of
SEQ ID NO: 2 from the nucleotide at
position 593 to the nucleotide at position 1543, the nucleotide sequence of
SEQ ID NO: 2 from the nucleotide at
position 493 to the nucleotide at position 1543, the nucleotide sequence of
SEQ ID NO: 2 from the nucleotide at
position 393 to the nucleotide at position 1543, the nucleotide sequence of
SEQ ID NO: 2 from the nucleotide at
position 293 to the nucleotide at position 1543, the nucleotide sequence of
SEQ ID NO: 2 from the nucleotide at
position 193 to the nucleotide at position 1543, or the nucleotide sequence of
SEQ ID NO: 2 from the nucleotide at
position 93 to the nucleotide at position 1543. A promoter fragment according
to the invention may also comprise
the nucleotide sequence of SEQ ID NO: 5 from the nucleotide position 1000 to
the nucleotide position 1500, the
nucleotide sequence of SEQ ID NO: 5 from the nucleotide position 950 to the
nucleotide position 1500, the
nucleotide sequence of SEQ ID NO: 5 from the nucleotide position 900 to the
nucleotide position 1500, the
nucleotide sequence of SEQ ID NO: 5 from the nucleotide position 800 to the
nucleotide position 1500, the
nucleotide sequence of SEQ ID NO: 5 from the nucleotide position 700 to the
nucleotide position 1500, the
nucleotide sequence of SEQ ID NO: 5 from the nucleotide position 600 to the
nucleotide position 1500, the
nucleotide sequence of SEQ ID NO: 5 from the nucleotide position 500 to the
nucleotide position 1500, the
nucleotide sequence of SEQ ID NO: 5 from the nucleotide position 400 to the
nucleotide position 1500, the
nucleotide sequence of SEQ ID NO: 5 from the nucleotide position 300 to the
nucleotide position 1500, the
nucleotide sequence of SEQ ID NO: 5 from the nucleotide position 200 to the
nucleotide position 1500, or the
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 8 -
nucleotide sequence of SEQ ID NO: 5 from the nucleotide position 100 to the
nucleotide position 1500. A promoter
fragment according to the invention may also comprise the nucleotide sequence
of any one of SEQ ID NOs: 3, 6
and 9 from the nucleotide position 950 to the nucleotide position 1500, the
nucleotide sequence of any one of SEQ
ID NOs: 3, 6 and 9 from the nucleotide position 900 to the nucleotide position
1500, the nucleotide sequence of any
one of SEQ ID NOs: 3, 6 and 9 from the nucleotide position 800 to the
nucleotide position 1500, the nucleotide
sequence of any one of SEQ ID NOs: 3, 6 and 9 from the nucleotide position 700
to the nucleotide position 1500,
the nucleotide sequence of any one of SEQ ID NOs: 3, 6 and 9 from the
nucleotide position 600 to the nucleotide
position 1500, the nucleotide sequence of any one of SEQ ID NOs: 3, 6 and 9
from the nucleotide position 500 to
the nucleotide position 1500, the nucleotide sequence of any one of SEQ ID
NOs: 3, 6 and 9 from the nucleotide
position 400 to the nucleotide position 1500, the nucleotide sequence of any
one of SEQ ID NOs: 3, 6 and 9 from
the nucleotide position 300 to the nucleotide position 1500, the nucleotide
sequence of any one of SEQ ID NOs: 3,
6 and 9 from the nucleotide position 200 to the nucleotide position 1500, or
the nucleotide sequence of any one of
SEQ ID NOs: 3, 6 and 9 from the nucleotide position 1000 to the nucleotide
position 1500. A promoter fragment
according to the invention may also comprise the nucleotide sequence of any
one of SEQ ID NOs: 4, 7, 8 and 10
from the nucleotide position 900 to the nucleotide position 1500, the
nucleotide sequence of any one of SEQ ID
NOs: 4, 7, 8 and 10 from the nucleotide position 800 to the nucleotide
position 1500, the nucleotide sequence of
any one of SEQ ID NOs: 4, 7, 8 and 10 from the nucleotide position 700 to the
nucleotide position 1500, the
nucleotide sequence of any one of SEQ ID NOs: 4, 7, 8 and 10 from the
nucleotide position 600 to the nucleotide
position 1500, the nucleotide sequence of any one of SEQ ID NOs: 4, 7, 8 and
10 from the nucleotide position 500
to the nucleotide position 1500, the nucleotide sequence of any one of SEQ ID
NOs: 4, 7, 8 and 10 from the
nucleotide position 400 to the nucleotide position 1500, the nucleotide
sequence of any one of SEQ ID NOs: 4, 7, 8
and 10 from the nucleotide position 300 to the nucleotide position 1500, the
nucleotide sequence of any one of SEQ
ID NOs: 4, 7, 8 and 10 from the nucleotide position 200 to the nucleotide
position 1500, or the nucleotide sequence
of any one of SEQ ID NOs: 4, 7, 8 and 10 from the nucleotide position 100 to
the nucleotide position 1500.
[35] Promoter activity for a functional promoter fragment in seeds may be
determined by those skilled in the art,
for example using analysis of RNA accumulation produced from the nucleic acid
which is operably linked to the
promoter as described herein, whereby the nucleic acid which is operably
linked to the promoter can be the nucleic
acid which is naturally linked to the promoter, i.e. the endogenous gene of
which expression is driven by the
promoter.
[36] The late stage seed-specific expression capacity and the embryo-
preferential expression capacity of the
identified or generated fragments of the promoters of the invention can be
conveniently tested by determining levels
of the transcript of which expression is naturally driven by the promoter of
the invention, i.e. endogenous transcript
levels, such as, for example, using the methods as described herein in the
Examples. Further, the late stage seed-
specific and embryo-preferential expression capacity of the identified or
generated fragments of the promoters of
the invention can be conveniently tested by operably linking such DNA
molecules to a nucleotide sequence
encoding an easy scorable marker, e.g. a beta-glucuronidase gene, introducing
such a chimeric gene into a plant
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 9 -
and analyzing the expression pattern of the marker in seeds at different
developmental stages as compared with
the expression pattern of the marker in other parts of the plant or in seeds
at other developmental stages. Other
candidates for a marker (or a reporter gene) are chloramphenicol acetyl
transferase (CAT) and proteins with
fluorescent properties, such as green fluorescent protein (GFP) from Aequora
victoria, or proteins with luminescent
properties such as the Renilla luciferase or the bacterial lux operon. To
define a minimal promoter region, a DNA
segment representing the promoter region is removed from the 5' region of the
gene of interest and operably linked
to the coding sequence of a marker (reporter) gene by recombinant DNA
techniques well known to the art. The
reporter gene is operably linked downstream of the promoter, so that
transcripts initiating at the promoter proceed
through the reporter gene. Reporter genes generally encode proteins, which are
easily measured, including, but not
limited to, chloramphenicol acetyl transferase (CAT), beta-glucuronidase
(GUS), green fluorescent protein (GFP),
beta-galactosidase (beta-GAL), and luciferase. The expression cassette
containing the reporter gene under the
control of the promoter can be introduced into an appropriate cell type by
transfection techniques well known to the
art. To assay for the reporter protein, cell lysates are prepared and
appropriate assays, which are well known in the
art, for the reporter protein are performed. For example, if CAT were the
reporter gene of choice, the lysates from
cells transfected with constructs containing CAT under the control of a
promoter under study are mixed with
isotopically labeled chloramphenicol and acetyl-coenzyme A (acetyl-CoA). The
CAT enzyme transfers the acetyl
group from acetyl-CoA to the 2- or 3-position of chloramphenicol. The reaction
is monitored by thin-layer
chromatography, which separates acetylated chloramphenicol from unreacted
material. The reaction products are
then visualized by autoradiography. The level of enzyme activity corresponds
to the amount of enzyme that was
made, which in turn reveals the level of expression and the late stage seed-
specific and embryo-preferential
functionality from the promoter or promoter fragment of interest. This level
of expression can also be compared to
other promoters to determine the relative strength of the promoter under
study. Once activity and functionality is
confirmed, additional mutational and/or deletion analyses may be employed to
determine the minimal region and/or
sequences required to initiate transcription. Thus, sequences can be deleted
at the 5' end of the promoter region
and/or at the 3' end of the promoter region, and nucleotide substitutions
introduced. These constructs are then
again introduced in cells and their activity and/or functionality determined.
[37] The activity or strength of a promoter may be measured in terms of the
amount of mRNA or protein
accumulation it specifically produces, relative to the total amount of mRNA or
protein. The promoter preferably
expresses an operably linked nucleic acid sequence at a level greater than
about 0.01%, about 0.02%, more
preferably greater than about 0.05% of the total mRNA. Alternatively, the
activity or strength of a promoter may be
expressed relative to a well-characterized promoter (for which transcriptional
activity was previously assessed).
[38] It will herein further be clear that equivalent late stage seed-
specific and embryo-preferential promoters
can be isolated from other plants. To this end, equivalent promoters can be
isolated using the coding sequences of
the genes driven by the promoters of any one of SEQ ID NOs: 2 to 10 to screen
a genomic library (e.g. by
hybridization or in silico) of a crop of interest. When sufficient identity
between the coding sequences is obtained
(for example, higher than 80% identity) then promoter regions can be isolated
upstream of the orthologous genes.
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 10 -
[39] Suitable to the invention are nucleic acids comprising late stage
seed-specific and embryo preferential
promoter activity which comprise a nucleotide sequence having at least 40%, at
least 50%, or at least 60%, or at
least 70%, or at least 80%, or at least 85%, or at least 90%, or at least 95%,
or at least 98% sequence identity to
the herein described promoters and promoter regions or functional fragments
thereof and are also referred to as
variants. The term "variant with respect to the transcription regulating
nucleotide sequences SEQ ID NOs: 2 to 10
of the invention is intended to mean substantially similar sequences.
Naturally occurring allelic variants such as
these can be identified with the use of well-known molecular biology
techniques, as, for example, with polymerase
chain reaction (PCR) as herein outlined before. Variant nucleotide sequences
also include synthetically derived
nucleotide sequences, such as those generated, for example, by using site-
directed mutagenesis of any one of
SEQ ID NOs: 2 to 10. Generally, nucleotide sequence variants of the invention
will have at least 40%, 50%, 60%, to
70%, e.g., preferably 71%, 72%, 73%, 74%, 75%, 76%, 77%, 78%, to 79%,
generally at least 80%, e.g., 81% to
84%, at least 85%, e.g., 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%,
96%, 97%, to 98% and 99%
nucleotide sequence identity to the native (wild type or endogenous)
nucleotide sequence or a functional fragment
thereof. Derivatives of the DNA molecules disclosed herein may include, but
are not limited to, deletions of
sequence, single or multiple point mutations, alterations at a particular
restriction enzyme site, addition of functional
elements, or other means of molecular modification which may enhance, or
otherwise alter promoter expression.
Techniques for obtaining such derivatives are well-known in the art (see, for
example, J. F. Sambrook, D. W.
Russell, and N. Irwin (2000) Molecular Cloning: A Laboratory Manual, 3rd
edition Volumes 1, 2, and 3. Cold Spring
Harbor Laboratory Press). For example, one of ordinary skill in the art may
delimit the functional elements within the
promoters disclosed herein and delete any non-essential elements. Functional
elements may be modified or
combined to increase the utility or expression of the sequences of the
invention for any particular application. Those
of skill in the art are familiar with the standard resource materials that
describe specific conditions and procedures
for the construction, manipulation, and isolation of macromolecules (e.g., DNA
molecules, plasmids, etc.), as well
as the generation of recombinant organisms and the screening and isolation of
DNA molecules. As used herein, the
term "percent sequence identity refers to the percentage of identical
nucleotides between two segments of a
window of optimally aligned DNA. Optimal alignment of sequences for aligning a
comparison window are well-
known to those skilled in the art and may be conducted by tools such as the
local homology algorithm of Smith and
Waterman (Waterman, M. S. Introduction to Computational Biology: Maps,
sequences and genomes. Chapman &
Hall. London (1995), the homology alignment algorithm of Needleman and Wunsch
(J. Mol. Biol., 48:443-453
(1970), the search for similarity method of Pearson and Lipman (Proc. Natl.
Acad. Sci., 85:2444 (1988), and
preferably by computerized implementations of these algorithms such as GAP,
BESTFIT, FASTA, and TFASTA
available as part of the GCG (Registered Trade Mark), Wisconsin Package
(Registered Trade Mark from Accelrys
Inc., San Diego, Calif.). An "identity fraction" for aligned segments of a
test sequence and a reference sequence is
the number of identical components that are shared by the two aligned
sequences divided by the total number of
components in the reference sequence segment, i.e., the entire reference
sequence or a smaller defined part of the
reference sequence. Percent sequence identity is represented as the identity
fraction times 100. The comparison of
one or more DNA sequences may be to a full-length DNA sequence or a portion
thereof, or to a longer DNA
sequence.
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 11 -
140] A
nucleic acid comprising a nucleotide sequence having at least 80% sequence
identity to any one of SEQ
ID NOs: 2 to 10 can thus be a nucleic acid comprising a nucleotide sequence
having at least at least 80%, or at
least 85%, or at least 90%, or at least 95%, or at least 98%, or 100% sequence
identity to anyone of SEQ ID NOs:
2 to 10.
[41] A "functional fragment" of a nucleic acid comprising late stage seed-
specific and embryo-preferential
promoter denotes a nucleic acid comprising a stretch of the nucleic acid
sequences of any one of SEQ ID NOs: 2 to
10, or of the nucleic acid having at least 80% sequence identity to any one of
SEQ ID NOs: 2 to 10 which still exerts
the desired function, i.e. which has late stage seed-specific and embryo-
preferential promoter activity. Assays for
determining late stage seed-specific and embryo-preferential promoter activity
are provided herein. Preferably, the
functional fragment of the late stage seed-specific and embryo-preferential
promoter contains the conserved
promoter motifs, such as, for example, conserved promoter motifs as described
in DoOP (doop.abc.hu, databases
of Orthologous Promoters, Barta E. et al (2005) Nucleic Acids Research Vol.
33, D86-D90). A functional fragment
may be a fragment of at least about 500 bp, at least about 550 bp, at least
about 600 bp, at least about 700 bp, at
least about 800 bp, at least about 900 bp, at least about 1000 bp, at least
about 1100 bp, at least about 1200 bp, at
least about 1300 bp, at least about 1400 bp, at least 1500 bp from the
translation start site.
[42] A nucleic acid comprising the nucleotide sequence of any one of SEQ ID
NOs: 2 to 10 which further
comprises insertion, deletion, substitution of at least 1 nucleotide up to 20
nucleotides, at least 1 nucleotide up to 15
nucleotides, at least 1 nucleotide up to 10 nucleotides, at least 1 nucleotide
up to 5 nucleotides, at least 1
nucleotide up to 4 nucleotides, at least 1 nucleotide up to 3 nucleotides, or
even at least 1 nucleotide up to 2
nucleotides may cover at least about 500 bp, at least about 550 bp, at least
about 600 bp, at least about 700 bp, at
least about 800 bp, at least about 900 bp, at least about 1000 bp, at least
about 1100 bp, at least about 1200 bp, at
least about 1300 bp, at least about 1400 bp, at least 1500 bp from the
translation start site.
[43] A number of consensus elements (sequence motifs) were identified on
the promoter sequence disclosed
herein.
[44] Variants of the promoter described herein include those which comprise
the identified motifs - motif 1
(SEQ ID NO: 29), motif 2 (gtctaaya), motif 3 (SEQ ID NO: 30), motif 4
(tcatcttaa), motif 5 (gakcarttc), motif 6 (SEQ
ID NO: 31), motif 7 (SEQ ID NO: 32), motif 8 (SEQ ID NO: 33), motif 9 (SEQ ID
NO: 34), motif 10 (SEQ ID NO: 35),
motif 11 (SEQ ID NO: 36), motif 12 (SEQ ID NO: 37), motif 13 (SEQ ID NO: 38),
motif 14 (SEQ ID NO: 39), motif 15
(SEQ ID NO: 40) and/or motif 16 (SEQ ID NO: 41) - but have otherwise been
modified to delete nucleotide
stretches within the sequence which are not needed for the promoter to be
functional in seed-specific and embryo-
preferential manner. For example, any nucleotide stretch located between the
motifs and /or between the
translational start and the first motif may be at least partially deleted to
result in a shorter nucleotide sequence than
the about 1500 bp sequence of anyone of SEQ ID NO: 2 to SEQ ID NO: 10.
[451 A
number of putative response elements were identified on the promoter sequence
disclosed herein. The
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 12 -
search was limited to seed-specific elements and two RY-repeat elements
(CATGCA) were identified in all the
promoters herein described. RY-repeat elements have been described as
necessary for seed-specific expression in
Brassica napus (Ezcurra et al. 1999).
[46] Variants of the promoters described herein include those which
comprise the identified RY- repeat
elements, but have otherwise been modified to delete nucleotide stretches
within the sequence which are not
needed for the promoter to be function in a late stage seed-specific and
embryo-preferential manner.
[47] "Isolated nucleic acid", used interchangeably with "isolated DNA" as
used herein refers to a nucleic acid
not occurring in its natural genomic context, irrespective of its length and
sequence. Isolated DNA can, for example,
refer to DNA which is physically separated from the genomic context, such as a
fragment of genomic DNA. Isolated
DNA can also be an artificially produced DNA, such as a chemically synthesized
DNA, or such as DNA produced
via amplification reactions, such as polymerase chain reaction (PCR) well-
known in the art. Isolated DNA can
further refer to DNA present in a context of DNA in which it does not occur
naturally. For example, isolated DNA can
refer to a piece of DNA present in a plasmid. Further, the isolated DNA can
refer to a piece of DNA present in
another chromosomal context than the context in which it occurs naturally,
such as for example at another position
in the genome than the natural position, in the genome of another species than
the species in which it occurs
naturally, or in an artificial chromosome.
[48] A further embodiment provides a recombinant gene comprising the
nucleic acid according to the invention
operably linked to a heterologous nucleic acid sequence encoding an expression
product of interest, and optionally
a transcription termination and polyadenylation sequence, preferably a
transcription termination and
polyadenylation region functional in plant cells. In a further embodiment,
said expression product of interest an RNA
capable of modulating the expression of a gene or is a protein.
[49] The term "expression product refers to a product of transcription.
Said expression product can be the
transcribed RNA. It is understood that the RNA which is produced is a
biologically active RNA. Said expression
product can also be a peptide, a polypeptide, or a protein, when said
biologically active RNA is an mRNA and said
protein is produced by translation of said mRNA.
[50] Alternatively, the heterologous nucleic acid, operably linked to the
promoters of the invention, may also
code for an RNA capable of modulating the expression of a gene. Said RNA
capable of modulating the expression
of a gene can be an RNA which reduces expression of a gene. Said RNA can
reduce the expression of a gene for
example through the mechanism of RNA-mediated gene silencing.
[51] Said RNA capable of modulating the expression of a gene can be a
silencing RNA down-regulating
expression of a target gene. As used herein, "silencing RNA" or "silencing RNA
molecule" refers to any RNA
molecule, which upon introduction into a plant cell, reduces the expression of
a target gene. Such silencing RNA
may e.g. be so-called "antisense RNA", whereby the RNA molecule comprises a
sequence of at least 20
consecutive nucleotides having 95% sequence identity to the complement of the
sequence of the target nucleic
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 13 -
acid, preferably the coding sequence of the target gene. However, antisense
RNA may also be directed to
regulatory sequences of target genes, including the promoter sequences and
transcription termination and
polyadenylation signals. Silencing RNA further includes so-called "sense RNA"
whereby the RNA molecule
comprises a sequence of at least 20 consecutive nucleotides having 95%
sequence identity to the sequence of the
target nucleic acid. Other silencing RNA may be "unpolyadenylated RNA"
comprising at least 20 consecutive
nucleotides having 95% sequence identity to the complement of the sequence of
the target nucleic acid, such as
described in W001/12824 or U56423885 (both documents herein incorporated by
reference). Yet another type of
silencing RNA is an RNA molecule as described in W003/076619 (herein
incorporated by reference) comprising at
least 20 consecutive nucleotides having 95% sequence identity to the sequence
of the target nucleic acid or the
complement thereof, and further comprising a largely-double stranded region as
described in W003/076619
(including largely double stranded regions comprising a nuclear localization
signal from a viroid of the Potato
spindle tuber viroid-type or comprising CUG trinucleotide repeats). Silencing
RNA may also be double stranded
RNA comprising a sense and antisense strand as herein defined, wherein the
sense and antisense strand are
capable of base-pairing with each other to form a double stranded RNA region
(preferably the said at least 20
consecutive nucleotides of the sense and antisense RNA are complementary to
each other). The sense and
antisense region may also be present within one RNA molecule such that a
hairpin RNA (hpRNA) can be formed
when the sense and antisense region form a double stranded RNA region. hpRNA
is well-known within the art (see
e.g W099/53050, herein incorporated by reference). The hpRNA may be classified
as long hpRNA, having long,
sense and antisense regions which can be largely complementary, but need not
be entirely complementary
(typically larger than about 200 bp, ranging between 200 and 1000 bp). hpRNA
can also be rather small ranging in
size from about 30 to about 42 bp, but not much longer than 94 bp (see
W004/073390, herein incorporated by
reference). Silencing RNA may also be artificial micro-RNA molecules as
described e.g. in W02005/052170,
W02005/047505 or US 2005/0144667, or ta-siRNAs as described in W02006/074400
(all documents incorporated
herein by reference). Said RNA capable of modulating the expression of a gene
can also be an RNA ribozyme.
[52] Said RNA capable of modulating the expression of a gene can modulate,
preferably down-regulate, the
expression of other genes (i.e. target genes) comprised within the seeds or
even of genes present within a
pathogen or pest that feeds upon the seeds of the transgenic plant such as a
virus, fungus, insect, bacteria.
[53]
The nucleic acid sequence heterologous to the promoters according to the
invention may generally be any
nucleic acid sequence effecting increased, altered (e.g. in a different organ)
or reduced level of transcription of a
gene for which such expression modulation is desired. The nucleic acid
sequence can for example encode a
protein of interest. Exemplary genes for which an increased or reduced level
of transcription may be desired in the
seeds are e.g. nucleic acids that can provide an agriculturally or
industrially important feature in seeds. Suitable
heterologous nucleic acid sequences of interest include nucleic acids
modulating expression of genes conferring
resistance to diseases, stress tolerance genes, genes involved at different
stages of fatty acid biosynthesis or
degradation, in acyl editing, in storage compound storage or breakdown, genes
encoding epoxidases,
hydroxylases, cytochrome P450 mono-oxygenases, desaturases, tocopherol
biosynthetic enzymes, carotenoid
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 14 -
biosynthesis enzymes, amino acid biosynthetic enzymes, steroid pathway
enzymes, starch branching enzymes,
genes encoding proteins involved in starch synthesis, glycolysis, carbon
metabolism, oxidative pentose phosphate
cycle, protein synthesis, organelle organization and biogenesis, DNA
metabolism, DNA replication, cell cycle, cell
organization and biogenesis, cell proliferation, chromosome organization and
biogenesis, microtubule-based
processes, microtubule-based movement, cytoskeleton-dependent intracellular
transport, cytoskeleton organization
and biogenesis, chromatin assembly or disassembly, DNA-dependent DNA
replication, chromosome organization
and biogenesis, DNA packaging, establishment and/or maintenance of chromatin
architecture, regulation of
progression through the cell cycle, regulation of the cell cycle, nucleobase,
nucleoside, nucleotide and nucleic acid
metabolism, chromatin assembly, macromolecule biosynthesis, intracellular
transport, establishment of cellular
localization, cellular localization, nucleosome assembly, macromolecule
metabolism, or M-phase; genes involved in
secondary metabolism or genes involved in seed and/or seed coat architecture.
[54] Genes involved in the fatty acid biosynthesis or degradation include
but are not limited to genes encoding
an acyl-CoA synthetase, a glycerol-phosphate acyltransferase, an 0-
acyltransferase, a lyso-phosphatidic acid
acyltransferase, a phosphatidic acid phosphatase, a diacylglycerol
acyltransferase, an oleate desaturases, a
linoleate desaturases, an acyl-CoA hydroxylase, an acyl-lipid hydroxylase, a
fatty acid epoxidase, a
phospholipid:sterol acyltransferase, a phospholipid:diacylglycerol
acyltransferase, a diacylglycerol transacylase, a
lysophosphatidylcholine acyltransferase, a phosphatidylcholine:diacylglycerol
cholinephosphotransferase, an acyl-
CoA elongase, an acyl-lipid elongase, a phosphatidylglycerol-phosphate
synthetase, a phosphatidylglycerol-
phosphate phosphatase, a CDP-diacylglycerol synthetase, a phosphatidylinositol
synthase, a phosphatidylserine
synthase, a choline kinase, an ethanolamine kinase, a CDP-choline synthetase,
a CDP-ethanolamine synthetase, a
phosphatidylserine decarboxylase, a lipoxygenase, a phospholipase, a lipase, a
carboxylesterase, a fatty alcohol
reductase, a wax ester synthase, a bifunctional acyltranferases/wax synthase,
a ketoacyl-CoA synthase, a ketoacyl-
CoA reductase, a hydroxylacyl-CoA dehydrase, an enoyl-CoA reductase, an
alcohol-forming fatty acyl-CoA
reductase, an aldehyde-forming fatty acyl-CoA reductase, an aldehyde
decarbonylase, a wax ester hydrolase, a
glycerol-3-P-dehydrogenase, a CDP-choline:1,2-diacylglycerol
cholinephosphotransferase, an oxidase, a
ketosphinganine reductase, a ceramide synthase, an
acylglycerophosphorylcholine acyltransferase, an
acylglycerol-phosphate acyltransferase, a phosphoethanolamine N-
methyltransferase, a ceramide sphingobase
desaturase, a glucosylceramide synthase, a acyl-ceramide synthase, a
triacylglycerol lipase, a monoacylglycerol
lipase, an acyl-CoA oxidase, an hydroxyacyl-CoA dehydrogenase, a dienoyl-CoA
reductase, a fatty acid omega-
alcohol oxidase, a monoacylglycerol lipase, an acyl-CoA oxidase, a hydroxyacyl-
CoA dehydrogenase, a dienoyl-
CoA reductase, a fatty acid omega-alcohol oxidase, a fatty acid/acyl-CoA
transporter, a acyl-CoA dehydrogenase, a
diacylglycerol-phosphate kinase, a lysophosphatidic acic phosphatase, a
peroxygenase; a A4-desaturase; a A5-
desaturase, a A6-desaturase; a A9-desaturase, a Al2-desaturase or a A15-
desaturase.
[55] Genes involved in cell proliferation include but are not limited to
genes encoding Dal (Li et al., 2008,
Genes Dev 22:1331, W02015/067943), Da2, E0D1 or E0D3 (W02015/022192,
PCT/GB2013/050072).
[56] A "transcription termination and polyadenylation region" as used
herein is a sequence that drives the
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 15 -
cleavage of the nascent RNA, whereafter a poly(A) tail is added at the
resulting RNA 3' end, functional in plant
cells. Transcription termination and polyadenylation signals functional in
plant cells include, but are not limited to,
3'nos, 3'35S, 3'his and 3'g7.
[57] The term "protein" interchangeably used with the term "polypeptide" as
used herein describes a group of
molecules consisting of more than 30 amino acids, whereas the term "peptide"
describes molecules consisting of up
to 30 amino acids. Proteins and peptides may further form dimers, trimers and
higher oligomers, i.e. consisting of
more than one (poly)peptide molecule. Protein or peptide molecules forming
such dimers, trimers etc. may be
identical or non-identical. The corresponding higher order structures are,
consequently, termed homo- or
heterodimers, homo- or heterotrimers etc. The terms "protein" and "peptide"
also refer to naturally modified proteins
.. or peptides wherein the modification is effected e.g. by glycosylation,
acetylation, phosphorylation and the like.
Such modifications are well known in the art.
[58] The term "heterologous" refers to the relationship between two or more
nucleic acid or protein sequences
that are derived from different sources. For example, a promoter is
heterologous with respect to an operably linked
DNA region, such as a coding sequence if such a combination is not normally
found in nature. In addition, a
.. particular sequence may be "heterologous" with respect to a cell or
organism into which it is inserted (i.e. does not
naturally occur in that particular cell or organism).
[59] The term "recombinant gene refers to any gene that contains: a) DNA
sequences, including regulatory
and coding sequences that are not found together in nature, or b) sequences
encoding parts of proteins not
naturally adjoined, or c) parts of promoters that are not naturally adjoined.
Accordingly, a recombinant gene may
.. comprise regulatory sequences and coding sequences that are derived from
different sources, or comprise
regulatory sequences, and coding sequences derived from the same source, but
arranged in a manner different
from that found in nature.
[60] Any of the promoters and heterologous nucleic acid sequences described
above may be provided in a
recombinant vector. A recombinant vector typically comprises, in a 5 to 3
orientation: a promoter to direct the
transcription of a nucleic acid sequence and a nucleic acid sequence. The
recombinant vector may further comprise
a 3 transcriptional terminator, a 3 polyadenylation signal, other untranslated
nucleic acid sequences, transit and
targeting nucleic acid sequences, selectable markers, enhancers, and
operators, as desired. The wording "5 UTR"
refers to the untranslated region of DNA upstream, or 5 of the coding region
of a gene and "3 UTR" refers to the
untranslated region of DNA downstream, or 3 of the coding region of a gene.
Means for preparing recombinant
vectors are well known in the art. Methods for making recombinant vectors
particularly suited to plant transformation
are described in U54971908, U54940835, U54769061 and U54757011. Typical
vectors useful for expression of
nucleic acids in higher plants are well known in the art and include vectors
derived from the tumor-inducing (Ti)
plasmid of Agrobacterium tumefaciens. One or more additional promoters may
also be provided in the recombinant
vector. These promoters may be operably linked, for example, without
limitation, to any of the nucleic acid
sequences described above. Alternatively, the promoters may be operably linked
to other nucleic acid sequences,
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 16 -
such as those encoding transit peptides, selectable marker proteins, or
antisense sequences. These additional
promoters may be selected on the basis of the cell type into which the vector
will be inserted. Also, promoters which
function in bacteria, yeast, and plants are all well taught in the art. The
additional promoters may also be selected
on the basis of their regulatory features. Examples of such features include
enhancement of transcriptional activity,
inducibility, tissue specificity, and developmental stage-specificity.
[61] The recombinant vector may also contain one or more additional nucleic
acid sequences. These additional
nucleic acid sequences may generally be any sequences suitable for use in a
recombinant vector. Such nucleic
acid sequences include, without limitation, any of the nucleic acid sequences,
and modified forms thereof,
described above. The additional structural nucleic acid sequences may also be
operably linked to any of the above
described promoters. The one or more structural nucleic acid sequences may
each be operably linked to separate
promoters. Alternatively, the structural nucleic acid sequences may be
operably linked to a single promoter (i.e. a
single operon).
[62] Yet another embodiment provides a host cell, such as an E. coli cell,
an Agrobacterium cell, a yeast cell, or
a plant cell, comprising the isolated nucleic acid according to the invention,
or the recombinant gene according to
the invention.
[63] Other nucleic acid sequences may also be introduced into the host cell
along with the promoter and
structural nucleic acid sequence, e. g. also in connection with the vector of
the invention. These other sequences
may include 3 transcriptional terminators, 3' polyadenylation signals, other
untranslated nucleic acid sequences,
transit or targeting sequences, selectable markers, enhancers, and operators.
Preferred nucleic acid sequences of
the present invention, including recombinant vectors, structural nucleic acid
sequences, promoters, and other
regulatory elements, are described above.
[64] In further embodiments, a plant and a plant cell are provided
comprising the recombinant gene according
to the invention. Yet a further embodiment provides seeds obtainable from the
plant according to the invention. In
another embodiment, the plants or seeds according to the invention are seed
crop plants or seeds.
[65] The plant cell or plant comprising the recombinant gene according to
the invention can be a plant cell or a
plant comprising a recombinant gene of which either the promoter, or the
heterologous nucleic acid sequence
operably linked to said promoter, are heterologous with respect to the plant
cell. Such plant cells or plants may be
transgenic plant in which the recombinant gene is introduced via
transformation. Alternatively, the plant cell or plant
may comprise the promoter according to the invention derived from the same
species operably linked to a nucleic
acid which is also derived from the same species, i.e. neither the promoter
nor the operably linked nucleic acid is
heterologous with respect to the plant cell, but the promoter is operably
linked to a nucleic acid to which it is not
linked in nature. A recombinant gene can be introduced in the plant or plant
cell via transformation, such that both
the promoter and the operably linked nucleotide are at a position in the
genome in which they do not occur
naturally. Alternatively, the promoter according to the invention can be
integrated in a targeted manner in the
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 17 -
genome of the plant or plant cell upstream of an endogenous nucleic acid
encoding an expression product of
interest, i.e. to modulate the expression pattern of an endogenous gene. The
promoter that is integrated in a
targeted manner upstream of an endogenous nucleic acid can be integrated in
cells of a plant species from which it
is originally derived, or in cells of a heterologous plant species.
Alternatively, a heterologous nucleic acid can be
integrated in a targeted manner in the genome of the plant or plant cell
downstream of the promoter according to
the invention, such that said heterologous nucleic acid is expressed seed-
specifically and embryo-preferentially.
Said heterologous nucleic acid is a nucleic acid which is heterologous with
respect to the promoter, i.e. the
combination of the promoter with said heterologous nucleic acid is not
normally found in nature. Said heterologous
nucleic acid may be a nucleic acid which is heterologous to said plant species
in which it is inserted, but it may also
naturally occur in said plant species at a different location in the plant
genome. Said promoter or said heterologous
nucleic acid can be integrated in a targeted manner in the plant genome via
targeted sequence insertion, using, for
example, the methods as described in W02005/049842.
[66] Plants comprising at least two recombinant genes according to the
invention wherein the nucleic acid
comprising seed-specific and embryo-preferential promoter activity is
different in each recombinant gene are, for
example, plants comprising a first recombinant gene comprising a nucleotide
sequence having at least 95%
sequence identity to SEQ ID NO: 2 or a functional fragment thereof, and a
second recombinant gene comprising a
nucleotide sequence having at least 95% sequence identity to any one of SEQ ID
NO: 3 to SEQ ID NO: 10 or a
functional fragment thereof. It will be clear that, when the first recombinant
gene comprises a nucleotide sequence
having at least 95% sequence identity to SEQ ID NO: x or a functional fragment
thereof, wherein SEQ ID NO: x is
selected from any one of SEQ ID NO: 2 to SEQ ID NO: 10, the second recombinant
gene may comprise a
nucleotide sequence having at least 95% sequence identity to any one of the
sequences according to the invention
or a functional fragment thereof, except to SEQ ID NO: x. Said plants are
suitable to express different genes with
the same tissue-specificity, however without the negative features associated
with the repeated use of one
promoter, such as gene silencing or recombination of a vector comprising the
recombinant genes. The at least two
recombinant genes according to the invention may be present at one locus in
the genome of said plant, and may be
derived from the same transforming DNA molecule.
[67] Plants according to the invention may comprise one or more recombinant
genes according to the
invention, but may in addition contain a recombinant gene comprising a nucleic
acid comprising promoter activity
which is preferential or specific to other plant tissues, such as apical
meristem, flower buds, cotyledons, flowers,
pods, roots, and leaves or other seed developmental stages, operably linked to
a nucleic acid sequence encoding
an expression product of interest. The recombinant gene according to the
invention and the recombinant gene
comprising a nucleic acid comprising another promoter activity may be present
at one locus and may be derived
from the same transforming DNA molecule.
[68] Yet another embodiment provides a method of producing a transgenic
plant comprising the steps of (a)
introducing or providing the recombinant gene according to the invention to a
plant cell to create transgenic cells;
and (b) regenerating transgenic plants from said transgenic cell.
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 18 -
[69] "Introducing" in connection with the present application relates to
the placing of genetic information in a
plant cell or plant by artificial means. This can be effected by any method
known in the art for introducing RNA or
DNA into plant cells, protoplasts, calli, roots, tubers, seeds, stems, leaves,
seedlings, embryos, pollen and
microspores, other plant tissues, or whole plants. "Introducing" also
comprises stably integrating into the plant's
genome. Introducing the recombinant gene can be performed by transformation.
[70] The term "transformation" herein refers to the introduction (or
transfer) of nucleic acid into a recipient host
such as a plant or any plant parts or tissues including plant cells,
protoplasts, calli, roots, tubers, seeds, stems,
leaves, seedlings, embryos and pollen. Plants containing the transformed
nucleic acid sequence are referred to as
"transgenic plants". Transformed, transgenic and recombinant refer to a host
organism such as a plant into which a
heterologous nucleic acid molecule (e.g. an expression cassette or a
recombinant vector) has been introduced. The
nucleic acid can be stably integrated into the genome of the plant.
[71] As used herein, the phrase "transgenic plant refers to a plant having
an introduced nucleic acid stably
introduced into a genome of the plant, for example, the nuclear or plastid
genomes. In other words, plants
containing transformed nucleic acid sequence are referred to as "transgenic
plants". Transgenic and recombinant
refer to a host organism such as a plant into which a heterologous nucleic
acid molecule (e.g. the promoter, the
chimeric gene or the vector as described herein) has been introduced. The
nucleic acid can be stably integrated
into the genome of the plant.
[72] Transformation methods are well known in the art and include
Agrobacterium-mediated transformation.
Agrobacterium-mediated transformation of cotton has been described e.g. in US
patent 5,004,863, in US patent
6,483,013 and W02000/71733. Plants may also be transformed by particle
bombardment: Particles of gold or
tungsten are coated with DNA and then shot into young plant cells or plant
embryos. This method also allows
transformation of plant plastids. Viral transformation (transduction) may be
used for transient or stable expression
of a gene, depending on the nature of the virus genome. The desired genetic
material is packaged into a suitable
plant virus and the modified virus is allowed to infect the plant. The progeny
of the infected plants is virus free and
also free of the inserted gene. Suitable methods for viral transformation are
described or further detailed e. g. in WO
90/12107, WO 03/052108 or WO 2005/098004. Further suitable methods well-known
in the art are microinjection,
electroporation of intact cells, polyethyleneglycol-mediated protoplast
transformation, electroporation of protoplasts,
liposome-mediated transformation, silicon-whiskers mediated transformation
etc. Said transgene may be stably
integrated into the genome of said plant cell, resulting in a transformed
plant cell. The transformed plant cells
obtained in this way may then be regenerated into mature fertile transformed
plants.
[73] Further provided is a method of effecting late stage seed-specific and
embryo-preferential expression of a
nucleic acid comprising introducing the recombinant gene according to the
invention into the genome of a plant, or
providing the plant according to the invention. Also provided is a method for
altering seed properties of a plant or to
produce a commercially relevant product in a plant, comprising introducing the
recombinant gene according to the
invention into the genome of a plant, or providing the plant according to the
invention. In another embodiment, said
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 19 -
plant is a seed crop plant.
[74] "Seed properties" as used herein are properties of the seed. Seed
properties can, for example, be seed
yield, seed storage compound production, seed compound accumulation, seed
nutrient accumulation; seed
micronutrient accumulation; seed storage compound quality, seed compound
composition, seed quality, biotic
stress tolerance such as disease tolerance, abiotic stress tolerance,
herbicide tolerance, seed dormancy, seed
imbibition, seed germination, seed vigor. Seed storage compounds can, for
example, be, seed oil, seed starch, or
seed protein.
[75] Seed properties may be modulated by modulating metabolic pathways,
such as starch metabolism, sugar
metabolism, inositol phosphate metabolism, glycolysis, amino acid
biosynthesis, carbon metabolism, nucleotide
metabolism, oxidative pentose phosphate cycle, fatty acid biosynthesis,
protein synthesis, or phytate metabolism,
and modulating secondary metabolism pathways. Another example is the methyl
recycling metabolic activity
impacting chromatin remodeling, phospholipid biosynthesis and cell wall
lignification. Such metabolic pathways can
be modulated by, for example, overexpressing or down-regulating a gene
involved in one or more of the metabolic
pathways using the early stage seed-specific and embryo-preferential promoter
according to the invention.
[76] Yield as used herein can comprise yield of the plant or plant part
which is harvested, such as seed,
including seed oil content, seed protein content, seed weight, seed number.
Increased yield can be increased yield
per plant, and increased yield per surface unit of cultivated land, such as
yield per hectare. Yield can be increased
by modulating, for example, by increasing seed size or oil content or
indirectly by increasing the tolerance to biotic
and abiotic stress conditions and decreasing seed abortion.
[77] Quality as used herein can comprise quality of the seed or grain such
as beneficial carbohydrate
composition or level, beneficial amino acid composition or level, beneficial
fatty acid composition or level, nutritional
value, seed and fiber content.
[78]
Abiotic stress tolerance as used herein can comprise resistance to
environmental stress factors such as
drought, extreme (high or low) temperatures.
[79] Biotic stress tolerance as used herein can comprise pest resistance,
such as resistance or fungal,
bacterial, bacterial or viral pathogens or insects.
[80] Also provided is the use of the isolated nucleic acid according to the
invention to regulate expression of an
operably linked nucleic acid in a plant, and the use of the isolated nucleic
acid according to the invention, or the
recombinant gene according to the invention to alter seed properties of a
plant or to produce a commercially
relevant product in a plant. In a further embodiment, said plant is a trait as
used herein refers to beneficial
properties of the plant, such as commercially beneficial properties of a
plant.
[81] Also provided is the use of the isolated nucleic acid according to the
invention to identify other nucleic
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 20 -
acids comprising late stage seed-specific and embryo preferential promoter
activity.
[82] The promoters according to the invention can further be used to create
hybrid promoters, i.e. promoters
containing (parts of) one or more of the promoters(s) of the current invention
and (parts of) other promoter which
can be newly identified or known in the art. Such hybrid promoters may have
optimized tissue specificity or
expression level.
[83] Yet another embodiment provides a method of producing food, feed, or
an industrial product comprising
(a) obtaining the plant or a part thereof, according to the invention; and (b)
preparing the food, feed or industrial
product from the plant or part thereof. In another embodiment, said food or
feed is oil, meal, grain, starch, flour or
protein, or said industrial product is biofuel, fiber, industrial chemicals, a
pharmaceutical or a nutraceutical.
[84] A "seed crop" or "seed crop plant" as used herein is a crop grown for
its seeds or material derived from the
seeds. Examples of seed crops are rice, maize, wheat, barley, millet, rye,
oats, camelina, crambe, Linum, castor
bean, calendula, safflower, sunflower, soybean, cotton, or Brassica species,
such as Brassica napus, Brassica
juncea, Brassica carinata, Brassica rapa, Brassica oleracea, and Brassica
nigra.
[85] "Brassicaceae" or "Brassicaceae plant" as used herein refers to plants
belonging to the family of
Brassicaceae plants, also called Cruciferae or mustard family. Examples of
Brassicaceae are, but are not limited to,
Brassica species, such as Brassica napus, Brassica oleracea, Brassica rapa,
Brassica carinata, Brassica nigra, and
Brassica juncea; Raphanus species, such as Raphanus caudatus, Raphanus
raphanistrum, and Raphanus sativus;
Matthiola species; Cheiranthus species; Camelina species, such as Camelina
sativa; Crambe species, such as
Crambe abyssinica and Crambe hispanica; Eruca species, such as Eruca
vesicaria; Sinapis species such as
Sinapis alba; Diplotaxis species; Lepidium species; Nasturtium species;
Olychophragmus species; Armoracia
species, Eutrema species; Lepidium species; and Arabidopsis species.
[86] Said Brassicaceae plant can be a Brassica plant. "Brassica plant"
refers to allotetraploid or amphidiploid
Brassica napus (AACC, 2n=38), Brassica juncea (AABB, 2n=36), Brassica carinata
(BBCC, 2n=34), or to diploid
Brassica rapa (syn. B. campestris) (AA, 2n=20), Brassica oleracea (CC, 2n=18)
or Brassica nigra (BB, 2n=16).
[87] Crop plants of the Brassica species are, for example, Brassica napus,
Brassica juncea, Brassica carinata,
Brassica rapa (syn. B. campestris), Brassica oleracea or Brassica nigra.
[88]
The plants according to the invention may additionally contain an endogenous
or a transgene, which
confers herbicide resistance, such as the bar or pat gene, which confer
resistance to glufosinate ammonium
(Liberty , Basta or Ignite ) [EP 0 242 236 and EP 0 242 246 incorporated by
reference]; or any modified EPSPS
gene, such as the 2mEPSPS gene from maize [EPO 508 909 and EP 0 507 698
incorporated by reference], or
glyphosate acetyltransferase, or glyphosate oxidoreductase, which confer
resistance to glyphosate
(RoundupReady0), or bromoxynitril nitrilase to confer bromoxynitril tolerance,
or any modified AHAS gene, which
confers tolerance to sulfonylureas, imidazolinones,
sulfonylaminocarbonyltriazolinones, triazolopyrimidines or
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 21 -
pyrimidyl(oxy/thio)benzoates, such as oilseed rape imidazolinone-tolerant
mutants PM1 and PM2, currently
marketed as Clearfield canola. Further, the plants according to the invention
may additionally contain an
endogenous or a transgene which confers increased oil content or improved oil
composition, such as a 12:0 ACP
thioesteraseincrease to obtain high laureate, which confers pollination
control, such as such as barnase under
control of an anther-specific promoter to obtain male sterility, or barstar
under control of an anther-specific promoter
to confer restoration of male sterility, or such as the Ogura cytoplasmic male
sterility and nuclear restorer of fertility.
[89] The plants or seeds of the plants according to the invention may be
further treated with a chemical
compound, such as a chemical compound selected
from the following lists:
Herbicides: Clethodim, Clopyralid, Diclofop, Ethametsulfuron, Fluazifop,
Glufosinate, Glyphosate, Metazachlor,
Quinmerac, Quizalofop, Tepraloxydim, Triflural
in.
Fungicides / PGRs: Azoxystrobin, N-[9-(dichloromethylene)-1,2,3,4-tetrahydro-
1,4-methanonaphthalen-5-y1]-3-
(difluoromethyl)-1-methy1-1H-pyrazole-4-carboxamide (Benzovindiflupyr,
Benzodiflupyr), Bixafen, Boscalid,
Carbendazim, Carboxin, Chlormequat-chloride, Coniothryrium minitans,
Cyproconazole, Cyprodinil,
Difenoconazole, Dimethomorph, Dimoxystrobin, Epoxiconazole, Famoxadone,
Fluazinam, Fludioxonil,
Fluopicolide, Fluopyram, Fluoxastrobin, Fluquinconazole, Flusilazole,
Fluthianil, Flutriafol, Fluxapyroxad, 1prodione,
lsopyrazam, Mefenoxam, Mepiquat-chloride, Metalaxyl, Metconazole,
Metominostrobin, Paclobutrazole, Penflufen,
Penthiopyrad, Picoxystrobin, Prochloraz, Prothioconazole, Pyraclostrobin,
Sedaxane, Tebuconazole,
Tetraconazole, Thiophanate-methyl, Thiram, Triadimenol, Trifloxystrobin,
Bacillus firmus, Bacillus firmus strain I-
1582, Bacillus subtilis, Bacillus subtilis strain GB03, Bacillus subtilis
strain QST 713, Bacillus pumulis, Bacillus.
pumulis strain GB34.
Insecticides: Acetamiprid, Aldicarb, Azadirachtin, Carbofuran,
Chlorantraniliprole (Rynaxypyr), Clothianidin,
Cyantraniliprole (Cyazypyr), (beta-)Cyfluthrin, gamma-Cyhalothrin, lambda-
Cyhalothrin, Cypermethrin,
Deltamethrin, Dimethoate, Dinetofuran, Ethiprole,
Flonicamid, Flubendiamide, Fluensulfone,
Fluopyram,Flupyradifurone, tau-Fluvalinate, lmicyafos, lmidacloprid,
Metaflumizone, Methiocarb, Pymetrozine,
Pyrifluquinazon, Spinetoram, Spinosad, Spirotetramate, Sulfoxaflor,
Thiacloprid, Thiamethoxam, 1-(3-chloropyridin-
211)-N-[4-cyano-2-methy1-6-(methylcarbamoyl)pheny1]-3-1[5-(trifluoromethyl)-2H-
tetrazol-2-yl]methy11-1H-pyrazole-
5-carboxamide, 1-(3-chloropyridin-2-y1)-N-[4-cyano-2-methy1-6-
(methylcarbamoyl)pheny1]-3-1[5-(trifluoromethyl)-1H-
tetrazol-1-yl]methy11-1H-pyrazole-5-carboxamide, 1-
12-fluoro-4-methy1-5-[(2,2,2-trifluorethyl)sulfinyl]phenyll-3-
(trifluoromethyl)-1H-1,2,4-triazol-5-amine,
(1E)-N-[(6-chloropyridin-3-yl)methyl]-N'-cyano-N-(2,2-
difluoroethyl)ethanimidamide, Bacillus firmus, Bacillus firmus strain 1-1582,
Bacillus subtilis, Bacillus subtilis strain
GB03, Bacillus subtilis strain QST 713, Metarhizium anisopliae F52.
[90] Whenever reference to a "plant" or "plants" according to the invention
is made, it is understood that also
plant parts (cells, tissues or organs, seed pods, seeds, severed parts such as
roots, leaves, flowers, pollen, etc.),
progeny of the plants which retain the distinguishing characteristics of the
parents, such as seed obtained by selfing
or crossing, e.g. hybrid seed (obtained by crossing two inbred parental
lines), hybrid plants and plant parts derived
there from are encompassed herein, unless otherwise indicated.
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 22 -
[91] In some embodiments, the plant cells of the invention as well as plant
cells generated according to the
methods of the invention, may be non-propagating cells.
[92] The obtained plants according to the invention can be used in a
conventional breeding scheme to produce
more plants with the same characteristics or to introduce the same
characteristic in other varieties of the same or
related plant species, or in hybrid plants. The obtained plants can further be
used for creating propagating material.
Plants according to the invention can further be used to produce gametes,
seeds (including crushed seeds and
seed cakes), seed oil, embryos, either zygotic or somatic, progeny or hybrids
of plants obtained by methods of the
invention. Seeds obtained from the plants according to the invention are also
encompassed by the invention.
[93] "Creating propagating material", as used herein, relates to any means
know in the art to produce further
plants, plant parts or seeds and includes inter alia vegetative reproduction
methods (e.g. air or ground layering,
division, (bud) grafting, micropropagation, stolons or runners, storage organs
such as bulbs, corms, tubers and
rhizomes, striking or cutting, twin-scaling), sexual reproduction (crossing
with another plant) and asexual
reproduction (e.g. apomixis, somatic hybridization).
[94] As used herein "comprising" is to be interpreted as specifying the
presence of the stated features, integers,
steps or components as referred to, but does not preclude the presence or
addition of one or more features,
integers, steps or components, or groups thereof. Thus, e.g., a nucleic acid
or protein comprising a sequence of
nucleotides or amino acids, may comprise more nucleotides or amino acids than
the actually cited ones, i.e., be
embedded in a larger nucleic acid or protein. A chimeric gene comprising a
nucleic acid which is functionally or
structurally defined, may comprise additional DNA regions etc.
[95] Furthermore, the disclosed invention is expected to yield similar
results in other seed crop plant species.
Particularly, it is expected to drive late stage seed-specific and embryo-
preferential expression in soybean. It is also
expected to drive late stage seed-specific and embryo-preferential expression
in wheat. The disclosed promoter
may lead to a late stage seed-specific and embryo-preferential expression in
cotton.
[96] The sequence listing contained in the file named õBCS16-
2004_5T25.txt", which is 84 kilobytes (size as
measured in Microsoft Windows ), contains 41 sequences SEQ ID NO: 1 through
SEQ ID NO: 41 is filed herewith
by electronic submission and is incorporated by reference herein.
[97] In the description and examples, reference is made to the following
sequences:
SEQUENCES
SEQ ID NO: 1: nucleotide sequence of the T-DNA PcruP2 BnA2::GUS.
SEQ ID NO: 2: nucleotide sequence of the promoter PcruP2-4 BnA2.
SEQ ID NO: 3: nucleotide sequence of the promoter PcruP2-4 BnA1.
SEQ ID NO: 4: nucleotide sequence of the promoter PcruP2-4 BnC1.
CA 03020397 2018-10-09
WO 2017/178368
PCT/EP2017/058390
- 23 -
SEQ ID NO: 5: nucleotide sequence of the promoter PcruP2-4 Br2.
SEQ ID NO: 6: nucleotide sequence of the promoter PcruP2-4 Br1.
SEQ ID NO: 7: nucleotide sequence of the promoter PcruP2-4 Bo.
SEQ ID NO: 8: nucleotide sequence of the promoter PcruP2-4 BjA2.
SEQ ID NO: 9: nucleotide sequence of the promoter PcruP2-4 BjA1.
SEQ ID NO: 10: nucleotide sequence of the promoter PcruP2-4 BjB1.
SEQ ID NO: 11: amino acid sequence of CRUP2 BnA2.
SEQ ID NO: 12: amino acid sequence of CRUP2 BnA1.
SEQ ID NO: 13: amino acid sequence of CRUP2 BnC1.
SEQ ID NO: 14: amino acid sequence of CRUP2 Br2.
SEQ ID NO: 15: amino acid sequence of CRUP2 Br1.
SEQ ID NO: 16: amino acid sequence of CRUP2 Bo.
SEQ ID NO: 17: amino acid sequence of CRUP2 BjA2.
SEQ ID NO: 18: amino acid sequence of CRUP2 BjA1.
SEQ ID NO: 19: amino acid sequence of CRUP2 BjB1.
SEQ ID NO: 20: nucleotide sequence of the coding sequence of CRUP2 BnA2.
SEQ ID NO: 21: nucleotide sequence of the coding sequence of CRUP2 BnA1.
SEQ ID NO: 22: nucleotide sequence of the coding sequence of CRUP2 BnC1.
SEQ ID NO: 23: nucleotide sequence of the coding sequence of CRUP2 Br2.
SEQ ID NO: 24: nucleotide sequence of the coding sequence of CRUP2 Br1.
SEQ ID NO: 25: nucleotide sequence of the coding sequence of CRUP2 Bo.
SEQ ID NO: 26: nucleotide sequence of the coding sequence of CRUP2 BjA2.
SEQ ID NO: 27: nucleotide sequence of the coding sequence of CRUP2 BjA1.
SEQ ID NO: 28: nucleotide sequence of the coding sequence of CRUP2 BjB1.
SEQ ID NO: 29: motif 1.
SEQ ID NO: 30: motif 3.
SEQ ID NO: 31: motif 6.
SEQ ID NO: 32: motif 7.
SEQ ID NO: 33: motif 8.
SEQ ID NO: 34: motif 9.
SEQ ID NO: 35: motif 10.
SEQ ID NO: 36: motif 11.
SEQ ID NO: 37: motif 12.
SEQ ID NO: 38: motif 13.
SEQ ID NO: 39: motif 14.
SEQ ID NO: 40: motif 15.
SEQ ID NO: 41: motif 16.
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 24 -
EXAMPLES
[98] Unless stated otherwise in the Examples, all recombinant DNA
techniques are carried out according to
standard protocols as described in Sambrook and Russell (2001) Molecular
Cloning: A Laboratory Manual, Third
Edition, Cold Spring Harbor Laboratory Press, NY, in Volumes 1 and 2 of
Ausubel et al. (1994) Current Protocols in
Molecular Biology, Current Protocols, USA and in Volumes I and II of Brown
(1998) Molecular Biology LabFax,
Second Edition, Academic Press (UK). Standard materials and methods for plant
molecular work are described in
Plant Molecular Biology Labfax (1993) by R.D.D. Croy, jointly published by
BIOS Scientific Publications Ltd (UK)
and Blackwell Scientific Publications, UK. Standard materials and methods for
polymerase chain reactions can be
found in Dieffenbach and Dveksler (1995) PCR Primer: A Laboratory Manual, Cold
Spring Harbor Laboratory Press,
and in McPherson at al. (2000) PCR - Basics: From Background to Bench, First
Edition, Springer Verlag, Germany.
Example 1 - Generation of expression constructs with the PcruP2 BnA2 promoter
of Brassica napus
operably linked to the GUS reporter gene (PcruP2 BnA2::GUS)
[99] The promoter sequence of the Brassica napus CRUP2 A2 promoter (SEQ ID
NO: 2 or 5' to 3' position 139
to 1681 of SEQ ID NO:1) isolated from an in house developed Brassica napus
line, the GUS gene (13-
glucuronidase) with intron (5' to 3' position 1682 to 3682 of SEQ ID NO: 1)
and a fragment of the 3' untranslated
region (UTR) of the gene 7 of Agrobacterium tumefaciens octopine Ti plasmid
(5' to 3' position 3739 to 3942 of
SEQ ID NO: 1) were assembled in a vector which contains the bar selectable
marker cassette (position 4023 to
6533 of SEQ ID NO: 1) to result in the T-DNA PcruP2 BnA2::GUS (SEQ ID NO: 1).
Example 2- Generation of transgenic plants comprising the PcruP2 BnA2::GUS
[100] In a next step the recombinant vectors comprising the expression
cassette of example 1, i. e. PcruP2
BnA2, were used to stably transform Brassica napus.
Example 3 - In planta expression pattern of PcruP2 BnA2::GUS in Brassica napus
[101] The in planta expression pattern of PcruP2 BnA2::GUS in the different
seed tissues and non-seed tissues
of Brassica napus seeds was monitored according to the method of Jasik et al.
2011.
[102] No GUS activity was detected in the assessed non-seed tissues, namely
young leaves, young stems,
flower buds, flowers and pods, thereby confirming the seed-specificity of the
selected promoters.
[103] Figure 1 shows the GUS labelling of the reporter gene in the embryo at
different developmental stages.
Staining is first detected in the embryos at the "walking stick" cotyledon
stage (panel A, sub-panel B). The
expression of the reporter gene (GUS) is then restricted to the radicle until
the end of the curled cotyledon stage
(panel A, sub-panel D). The radicle cap is then not stained. Transverse
section of the radicle at the curled cotyledon
stage indicates that the cortex is intensely stained while the pro-vascular
cylinder is less stained (panel B). From the
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 25 -
green cotyledon stage on (panel A, sub-panels E and F) the expression of the
reporter gene is detected in the
radicle, radicle cap included, as well as in the cotyledons. Longitudinal
section of a mature embryo (panel C) shows
that the vascular tissues of the radicle are not stained. Concerning the
staining in the cotyledons, the outer
cotyledon shows more intense staining than the inner cotyledon and the
parenchyma tissues are preferentially
stained over the vascular structures. The semi quantitative assessment of the
GUS labelling in the embryo of
transgenic lines carrying the PcruP2 BnA2::GUS T-DNA (panel D) shows that the
intensity of the embryo staining
increases many fold towards maturation.
[104] Figure 2 panel A shows the GUS labelling of the reporter gene in the
seed coat at late stage. The seed
coat is labelled in the inner integument only. The endosperm is also weakly
labelled. Panel B provides the semi
quantitative assessment of the GUS labelling in the seed coat of the
transgenic lines carrying the PcruP2
BnA2::GUS T-DNA. The GUS staining in the seed coat can be detected at very low
level.
[105] The strong staining in the late stage embryo and the weak staining in
the seed coat and endosperm
indicate that the PcruP2 BnA2 promoter has embryo-preferential promoter
activity.
Example 4 - Identification of the different Brassica napus copies of CRUP2
BnA2 and of the orthologues of
CRUP2 in Brassica rapa, Brassica oleracea and Brassica juncea
[106] The sequences of the different Brassica napus copies of CRUP2 as well as
their orthologues in Brassica
rapa, Brassica oleracea and Brassica juncea were obtained by blasting the
coding sequence of the CRUP2 BnA2
against an in-house database of Brassica napus, Brassica rapa, Brassica
oleracea and Brassica juncea sequences.
[107] The nucleotide sequences obtained in this way are given in SEQ ID NO: 20
to SEQ ID NO: 28. These
nucleotide sequences were translated into amino acid sequences, given in SEQ
ID NO: 11 to SEQ ID NO: 19.
[108] Figure 3 shows the alignment of the retrieved amino acid sequence. Any
two of these sequences share at
least 83% identity.
Example 5 - RNA isolation from different tissues of Brassica napus and
Brassica juncea
[109] The following tissues were isolated from Brassica napus:
a. Apical meristem 33 days after sowing (DAS) (including smallest leaves)
(AM33)
b. Big flower buds (>5 mm) 42 DAS (BFB42)
c. Cotyledons (with hypocotyl) 10 DAS (CTYL10)
d. Open flowers 52 DAS (0F52)
e. Pods 14-20 DAS (Pod2)
f. Pods 21-25 DAS (Pod3)
g. Roots 14 DAS (Ro2w)
h. Small flower buds 5 mm 42 DAS (5FB42)
CA 03020397 2018-10-09
WO 2017/178368
PCT/EP2017/058390
- 26 -
i. Seeds 14-20 days after flowering (DAF) (5eed2)
j. Seeds 21-25 DAF (5eed3)
k. Seeds 26-30 DAF (5eed4)
I. Seeds 31-35 DAF (5eed5)
m. Seeds 42 DAF (5eed6)
n. Seeds 49 DAF (5eed7)
o. Stem 14 DAS (5t2w)
p. Stem 33 DAS (5t5w)
q. Young leaf 33 DAS 3 cm leaf next to apical meristem) (YL33)
111101 The following tissues were isolated from Brassica juncea:
a. Apical meristem 22 days after sowing (DAS) (including smallest
leaves) (AM22)
b. Big flower buds (>5 mm) 35 DAS (BFB35)
c. Cotyledons (with hypocotyl) 8 DAS (CTYL8)
d. Open flowers 35 DAS (0F35)
e. Pods 14-20 DAS (Pod2)
f. Pods 21-25 DAS (Pod3)
g. Pods 26-30 DAS (Pod4)
h. Pods 31-35 DAS (Pod5)
i. Roots 14 DAS (Ro2w)
j. Small flower buds 5 mm 35 DAS (5FB35)
k. Seeds 14-20 days after flowering (DAF) (5eed2)
I. Seeds 21-25 DAF (5eed3)
m. Seeds 26-30 DAF (5eed4)
n. Seeds 31-35 DAF (Seed5)
o. Seeds 42 DAF (5eed6)
p. Seeds 49 DAF (5eed7)
q. Stem 14 DAS (5t2w)
r. Stem 22 DAS (5t3w)
s. Young leaf 22 DAS 3 cm leaf next to apical meristem) (YL22)
t. Old leaf 22 DAS (0L22)
[1 1 1] The following seed sub-tissues were isolated from Brassica napus:
a. Endosperm, 18 days after flowering (DAF)
b. Endosperm, 24 DAF
c. Embryonic hypocotyl, 18 DAF
d. Embryonic hypocotyl, 24 DAF
e. Embryonic hypocotyl, 28 DAF
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 27 -
f. Embryonic hypocotyl, 32 DAF
g. Embryonic hypocotyl, 46 DAF
h. Embryonic inner cotyledon, 18 DAF
i. Embryonic inner cotyledon, 24 DAF
j. Embryonic inner cotyledon, 28 DAF
k. Embryonic inner cotyledon, 32 DAF
I. Embryonic inner cotyledon, 46 DAF
m. Embryonic outer cotyledon, 18 DAF
n. Embryonic outer cotyledon(inner part), 24 DAF
o. Embryonic outer cotyledon(inner part), 28 DAF
p. Embryonic outer cotyledon(inner part), 32 DAF
q. Embryonic outer cotyledon(inner part), 46 DAF
r. Embryonic outer cotyledon(outer part), 24 DAF
s. Embryonic outer cotyledon(outer part), 28 DAF
t. Embryonic outer cotyledon(outer part), 32 DAF
u. Embryonic outer cotyledon(outer part), 46 DAF
111121 For the isolation of the seed sub-tissues, freshly harvested seeds were
frozen at -80 C and cut into 20pm
sections. Sections were placed on PET-membranes, lyophilized at -20 C, and
then used for laser-assisted
microdissection (PALM Laser-Microbeam instrument; Bernried/Germany) (for
details see Schiebold et al., 2011,
Plant Methods 7:19). Up to 5 distinct embryonic tissues plus endosperm were
targeted. Tissue dissection was
applied to seeds at 18, 24, 28, 32 and 46 DAF, covering the developmental
period from onset of storage activity
until late maturation. RNA was extracted (purification of total RNA by RNeasy
Micro kit; Qiagen) and amplified (C&E
version ExpressArt mRNA amplification Nano kit; Amp-tec) as detailed in
Schiebold et al. 2011 (supra).
111 1 3] Total RNA from the non-seed sub-tissues was isolated according to
standard methods.
111141 In our growth conditions, the correspondence between embryo
developmental stages and the selected
time points is as follows:
a. Between 10 and 13 DAF: torpedo stage
b. 5eed2 or between 14 and 20 DAF: "walking stick" cotyledon stage
c. 5eed3 or between 21 and 25 DAF: curled cotyledon stage
d. 5eed4 and Seed5 or between 26 and 35 DAF: green cotyledon stage
e. 5eed6 and 5eed7 or after 36 DAF: mature embryo
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 28 -
Example 6 - In silico expression analyses of the different copies of CRUP2 of
Brassica napus and their
orthologues
[115] Figures 4 shows the relative expression levels of the endogenous
transcripts of the different Brassica
napus (A-C), Brassica rapa (D-E) and Brassica oleracea (F) and Brassica juncea
(G-I) copies of CRUP2 in different
tissues, as isolated in Example 5.
[116] The CRUP2 BnA2 transcript (panel A) is abundantly detected in the Seed4,
Seed5, Seed6 and Seed7
tissues, and only barely detectable in the Seed3 tissues. This result
confirms, as determined in planta, that PcruP2
BnA2 has late stage seed-specific promoter activity.
[117] The CRUP2 BnA1 transcript (panel B) is abundantly detected in the Seed4,
Seed5, Seed6 and Seed7
tissues, and only barely detectable in the Seed3 tissues. This result
indicates that PcruP2 BnA1 has late stage
seed-specific promoter activity.
[118] The CRUP2 BnC1 transcript (panel C) is abundantly detected in the Seed4,
Seed5, Seed6 and Seed7
tissues, and only barely detectable in the Seed3 tissues. This result
indicates that PcruP2 BnC1 has late stage
seed-specific promoter activity.
[119] The CRUP2 Br2 transcript (panel D) is abundantly detected in the Seed4,
Seed5, Seed6 and Seed7
tissues, and only barely detectable in the Seed2, Seed3, Pod2, Pod3 and 5 week-
old stem tissues. This result
indicates that PcruP2 Br2 has late stage seed-specific promoter activity.
[120] The CRUP2 Br1 transcript (panel E) is abundantly detected in the Seed4,
Seed5, Seed6 and Seed7
tissues, and only barely detectable in the Seed3, Pod2, 2 week-old roots,
small flower buds and 5 week-old stem
tissues. This result indicates that PcruP2 Br1 has late stage seed-specific
promoter activity.
[121] The CRUP2 Bo transcript (panel F) is abundantly detected in the Seed4,
Seed5, Seed6 and Seed7
tissues, and only barely detectable in the Seed2, Seed3, Pod2, Pod3,
cotyledons, 2 week-old roots, 5 week-old leaf
and small flower buds tissues. This result indicates that PcruP2 Bo has late
stage seed-specific promoter activity.
[122] The CRUP2 BjA2 transcript (panel G) is clearly detected in the Seed4,
Seed5 and Seed6 tissues, mildly
detected in the Seed7 tissues and only barely detectable in the Seed2, Seed3
and Pod2 tissues. This result
indicates that PcruP2 BjA2 has late stage seed-specific promoter activity.
[123] The CRUP2 BjA1 transcript (panel H) is abundantly detected in the Seed4,
Seed5, Seed6 and Seed7
tissues, and only barely detectable in the Seed2, Seed3, Pod2, Pod3, Pod4,
Pod5, apical meristem, cotyledons,
open flowers and old leaves tissues. This result indicates that PcruP2 BjA1
has late stage seed-specific promoter
activity.
[124] The CRUP2 BjB1 transcript is abundantly detected in the Seed4, Seed5 and
Seed6 tissues, mildly
detected in the Seed3 and Seed7 tissues and only barely detectable in the
Seed2, Pod2, Pod3, Pod4, Pod5, apical
meristem, cotyledons, open flowers and old leaves tissues. This result
indicates that PcruP2 BjB1 has late stage
seed-specific promoter activity.
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 29 -
[125] Figure 5 shows the relative expression levels of the endogenous
transcripts of the different Brassica napus
(A-C), Brassica rapa (D-E) and Brassica oleracea (F) copies of CRUP2 in
different seed sub-tissues, as isolated in
Example 5.
[126] The CRUP2 BnA2 transcript (panel A) is abundantly detected in the embryo
tissues (except at 18 DAF),
but is absent from the endosperm tissues. This result confirms, as determined
in planta, that PcruP2 BnA2 has
embryo-preferential promoter activity.
[127] The CRUP2 BnA1 transcript (panel B) is abundantly detected in the embryo
tissues (except at 18 DAF),
but is absent from the endosperm tissues. This result indicates that PcruP2
BnA1 has embryo-preferential promoter
activity.
[128] The CRUP2 BnC1 transcript (panel C) is abundantly detected in the embryo
tissues (except at 18 DAF),
but is absent from the endosperm tissues. This result indicates that PcruP2
BnC1 has embryo-preferential promoter
activity.
[129] The CRUP2 Br2 transcript (panel D) is abundantly detected in the embryo
tissues (except at 18 DAF), but
is absent from the endosperm tissues. This result indicates that PcruP2 Br2
has embryo-preferential promoter
activity.
[130] The CRUP2 Br1 transcript (panel E) is abundantly detected in the embryo
tissues (except at 18 DAF), but
is absent from the endosperm tissues. This result indicates that PcruP2 Br1
has embryo-preferential promoter
activity.
[131] The CRUP2 Bo transcript (panel F) is abundantly detected in the embryo
tissues (except at 18 DAF), but is
absent from the endosperm tissues. This result indicates that PcruP2 Bo has
embryo-preferential promoter activity.
Example 7 - Sequence analysis of the promoters of the CRUP2 genes from
Brassica rapa, Brassica juncea,
Brassica oleracea and Brassica napus
[132] For each CRUP2 gene identified the about 1.5kb of genomic DNA sequence
upstream of the translation
start was retrieved from an in-house database of Brassica napus, Brassica
rapa, Brassica oleracea and Brassica
juncea sequences. The nucleotide sequences obtained in this way are given in
SEQ ID NO: 3 to SEQ ID NO: 10.
[133] A promoter analysis was carried out using publicly available databases
such as PLACE
(www.dna.affrc.go.jp/PLACE/), RegSite
(linuxtsoftberry.com/berry.phtml?topic=regsitelist), PlantCare (Lescot et
al., 2002; available at bioinformatics.psb.ugent.be/webtools/plantcare/html/)
and AtcisDB (Davuluri et al., 2003).
The search was limited to seed-specific elements and two RY-repeat elements
could be predicted in all promoters
disclosed herein. The exact sequence of the RY-repeat element is CATGCA and
was observed from position 1310
and from position 1327 on SEQ ID NO: 2, from position 1215 and from position
1232 on SEQ ID NO: 3, 4, 6, 9 and
CA 03020397 2018-10-09
WO 2017/178368 PCT/EP2017/058390
- 30 -
10, from position 1267 and from position 1284 on SEQ ID NO: 5, from position
1216 and from position 1233 on
SEQ ID NO: 7, from position 1214 and from position 1231 on SEQ ID NO: 8.
[134] Figure 6 shows the alignment of the 3' end sequence of the promoter
sequences (SEQ ID NO: 2 to SEQ
ID NO: 10). These promoters share a surprisingly high level of conservation in
this region. Sixteen consensus
-- sequences (motifs) were identified. The promoters comprise the following
motifs in their 3' about 500 bp sequence:
a. motif 1 is given in SEQ ID NO: 29;
b. motif 2 has the sequence gtctaaya;
c. motif 3 is given in SEQ ID NO: 30;
d. motif 4 has the sequence tcatcttaa;
e. motif 5 has the sequence gakcarttc;
f. motif 6 is given in SEQ ID NO: 31;
g. motif 7 is given in SEQ ID NO: 32;
h. motif 8 is given in SEQ ID NO: 33;
i. motif 9 is given in SEQ ID NO: 34;
j. motif 10 is given in SEQ ID NO: 35;
k. motif 11 is given in SEQ ID NO: 36;
I. motif 12 is given in SEQ ID NO: 37;
m. motif 13 is given in SEQ ID NO: 38;
n. motif 14 is given in SEQ ID NO: 39;
o. motif 15 is given in SEQ ID NO: 40;
p. motif 16 is given in SEQ ID NO: 41.
[135] The high degree of conservation of these motifs in all analyzed promoter
sequences described herein
indicate that these motifs may be required for the observed seed-specific and
embryo-preferential expression
pattern.
-- [136] Consequently, as PcruP2 BjA1 sequence comprises the motifs 1 to 16,
it can be concluded that it has
embryo-preferential promoter activity. As PcruP2 BjA2 sequence comprises the
motifs 1 to 16, it can be concluded
that it has embryo-preferential promoter activity. As PcruP2 BjB1 sequence
comprises the motifs 1 to 16, it can be
concluded that it has embryo-preferential promoter activity.
[137] More generally, these results indicate that a Brassica promoter
comprising the motifs 1 to 16 would have
late stage seed-specific and embryo-preferential promoter activity.