Note: Descriptions are shown in the official language in which they were submitted.
SURGICAL C-SECTION DRAPE WITH TUNNEL
TECHNICAL FIELD
[001] The present application relates to a drape for covering a patient
during a
surgical procedure.
BACKGROUND
[002] Currently, a traditional Cesarean section procedure, also known as "C-
section," is most commonly performed with the patient covered by a solid
surgical drape.
The drape is typically constructed of a multi-layer combination of spunbond
and
meltblown materials, as well as impervious films, commonly referred to as SMS
nonwoven fabric, or bilaminated and trilaminated impervious and absorbent
materials.
[003] Because the mother is typically awake and alert during the C-section,
it is
desirable to provide a barrier or screen to occlude the mother's view of the
surgical area
during the procedure. Many traditional drapes are in a "T" shape, with the top
portion of
the "T" acting as the anesthesia screen that obscures the patient's view of
the surgical
area. The drape is placed over the patient to isolate a sterile field near the
patient's
abdomen. The anesthesia screen is propped up on vertical standards at each
side of the
operating table near the patient's head or on a crossbar proximal the head.
10041 In this arrangement, the mother does not have an opportunity to
see her
newborn immediately after delivery. It is desirable in the first moments after
birth for the
mother and child to establish an immediate connection. It is especially
desirable for the
infant and mother to maintain skin-to-skin contact immediately after birth.
Such
maintenance of skin-to-skin contact is believed to provide a number of benefit
for both
the mother and the newborn infant. Because traditional surgical drapes obscure
the
mother's view of the newborn in the first moments after delivery, the mother
and child
do not have an opportunity to establish an immediate physical connection.
1
CA 3020589 2018-10-11
10051 To address this concern, it is known to provide surgical drapes
that
incorporates a coverable window and an opaque flap that can be attached and
detached
to alternately obscure and expose the window. It has now been realized that
many such
known drapes are undesirable in that they are configured in ways that might
allow for
contamination of the surgical field once the flap is removed, particularly if
the flap is
folded into the surgical field.
[006] A new surgical drape has now been devised. Generally, the drape
includes
a mainsheet having a mainsheet fenestration through which the Caesarean
section
procedure may be performed, the mainsheet having various edges and portions
including a head-oriented edge. The drape further includes a screen attached
to the
mainsheet at the head-oriented edge, the screen including a screen
fenestration and at
least one flap that covers the screen fenestration during the surgical
procedure.
Preferably, the screen includes at least two flaps, one disposed on the
patient-side of the
screen and the other disposed on the surgeon-side of the screen. Each flap is
substantially
opaque to thereby inhibit the patient's visual access through the screen
fenestration. The
screen is further equipped with a flexible tunnel material that is configured
to form a
tunnel extending from the surgeon-side to the patient-side, the tunnel
generally
extending in the direction of the patient. In use, the surgeon-side flap may
originally be
supplied in an occluding position while the patient-side flap may be disposed
in an open
position. Upon performance of the surgery and extraction of the newborn
infant, the
surgeon-side flap may be lifted and secured into an open position, whereupon
the infant
is passed through the tunnel to greet the mother. At this point the patient-
side flap is
closed and secured, and optionally the surgeon-side flap likewise may be re-
closed. In
this manner, the mother may view the birth of the infant, or may view the
infant
immediately after birth, and may establish skin-to-skin contact as quickly as
possible.
2
CA 3020589 2018-10-11
BRIEF DESCRIPTION OF THE DRAWINGS
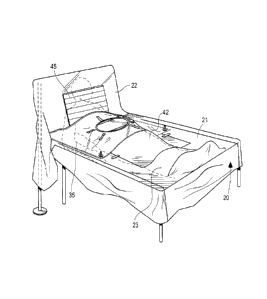
[007] Fig. 1 is a perspective view of a surgical drape in accordance with
one
embodiment of the invention, shown in use positioned over a patient in a
surgical bed.
[008] Fig. 2 is a top plan view of the surgical drape shown in Fig. 1.
[009] Fig. 3 is a top plan view of the surgical drape shown in Fig. 1 with
the
surgeon-side flap and optional absorbent pad removed.
[0010] Fig. 4 is a front perspective view of the surgical drape shown in
Fig. 1.
100111 Fig. 5 is a perspective view of the mainsheet fenestration region
of the
mainsheet, illustrating the fluid-collecting pouch.
[0012] Fig. 6 is a rear perspective view, partially cut away, of the
surgical drape
and bed shown in Fig. 1.
[0013] Fig. 7 is a perspective view of the tunnel-forming material that
is secured on
the patient-side of the screen to form an infant-transporting tunnel.
[0014] Fig. 8 is a perspective view illustrating the surgical drape in an
operating
position, showing the surgeon-side flap in a closed position.
[0015] Fig. 9 is a view similar to Fig. 8 but showing the surgeon-side
flap in an
open, secured position.
100161 Figs. 10A-E illustrate sequentially the passage of a newborn
infant through
the tunnel and screen fenestration to greet the mother; Figs 10A-D
illustrating the
surgeon-facing side of the screen and Fig. 10E illustrating the patient-facing
side of the
screen.
[0017] Fig. 11 is a perspective view of the drape taken from the patient-
facing side
of the screen after passage of the infant through the tunnel and after the
patient-side flap
has been secured in a closed position.
3
CA 3020589 2018-10-11
DETAILED DESCRIPTION
[0018] Referring to Figs. 1-3, the drape 20 includes generally a
mainsheet 21 and a
screen 22. With particular reference to Fig, 2, the mainsheet 21 includes a
central portion
23, side edges 25, 26, a foot-oriented edge 27, and a head-oriented edge 28.
As illustrated,
the screen 22 is disposed at the head-oriented edge 28, and is preferably
formed as a
separate piece of material that is secured to the mainsheet 21 via adhesive.
The mainsheet
21 includes a mainsheet fenestration 30 through which a surgery accessing a
patient's body
may be performed. The mainsheet fenestration 30 generally comprises an opening
formed
in the material of mainsheet 21, and is sized to allow sufficient access to a
patient's
abdominal region to perform a C-section procedure. The mainsheet fenestration
30 is
covered with a flexible adhesive film known in the industry as "incise film,"
which may be
formed from polyurethane or another suitable material, and a liner 31. In some
embodiments, the mainsheet fenestration 30 may be completely covered with
incise film to
form a "full incise" fenestration. It is contemplated that a "fenestrated
incise" structure (not
shown) alternatively may be employed, wherein there is adhesive disposed
around the
perimeter of the mainsheet fenestration leaving an opening in a center portion
of the
flexible adhesive film through which the patient's skin is exposed to permit
incisions to be
made directly though the exposed skin.
[0019] When the surgical drape 20 is laid over a patient, the "full
incise" film is first
covered with a removable backing (not shown), as is conventional. Before the
procedure is
performed and after the surgical drape 20 is laid over the patient, the
removable backing is
removed, exposing the adhesive bottom surface of the incise film and causing
the film to
adhere to the skin of the patient. When the procedure is performed, incisions
may be made
directly through the flexible adhesive film.
[0020] The illustrated mainsheet fenestration 30 is substantially
surrounded by an
optional fluid collection pouch 35. The fluid collection pouch 35 is composed
of a plastic
material that is impervious to fluid. The fluid collection pouch 35 surrounds
the mainsheet
4
CA 3020589 2018-10-11
fenestration 30 in a sealing fashion such that any fluids released from the
surgical site
during the procedure will run off the sheet into fluid collection pouch 35.
This prevents
fluids from running off the mainsheet 21 and onto the floor or other areas
where fluids are
not desired. The fluid collection pouch 35 may include one or more suction
ports 36 for
connection to suction equipment for aspirating the fluids from the fluid
collection pouch
35. The fluid collection pouch 35 may be secured to the mainsheet 31 via any
suitable
fashion, such as via double-sided adhesive tape.
100211 The fluid collection pouch 35 includes an opening 37 through which
the
surgeon may access the mainsheet fenestration 30. The edges of the opening 37
may be
bound by a formable material 39, such as a malleable wire encased in plastic.
Such formable
material 39 allows the surgeon to shape the opening of fluid collection pouch
35 to allow
for easier access to fenestration 30 or to reconfigure the shape of fluid
collection pouch 35
in a manner that is most effective for the particular procedure.
[0022] The illustrated mainsheet 21 includes malleable bars 40. The
malleable bars
40 are composed of a malleable metal material and of a plastic material, and
are present to
enable the surgeon to adjust the shape of the fenestration to improve fluids
collection. By
bending upwardly, the surgeon can adjust the shape and elevation of the open
fenestration.
[0023] The mainsheet 21 further may include one or more line retainers
(not shown)
for securing wires or lines to the surgical drape 20. Such line retainers
include may be
formed in any suitable fashion and may include two plies of hook and loop
material that
may be separated at one end, but attached at another end. When the plies of
the hook and
loop material are separated, a line may be inserted between the two plies, and
when the
plies are joined again, the hook and loop material captures the line between
the plies. The
tabs alternatively may be formed from tape or from other suitable material.
Additionally,
cord holding tabs (not shown) may be positioned near the head-oriented edge of
the
mainsheet.
CA 3020589 2018-10-11
[0024] The mainsheet 21 also may include an optional absorbent pad 42,
which, as
shown, is configured as a separate pad disposed on and secured to the
mainsheet 21. The
absorbent pad 42 is located near fenestration 30 and is composed of a material
that is
suitable for absorbing fluids that are generated during the surgical
procedure. This
absorbent pad 42 provides another measure of fluid retention in the instance
where fluids
are not collected by the fluid collection pouch 35. As shown in Figs. 1 and 2,
the drape 20
is further equipped with a surgeon-side flap 45, the purpose of which will be
discussed
below.
[0025] With particular reference to Fig. 3, the drape 20 may include
optional
armboard portions 43 (shown in hidden lines). The armboard portions are
intended to
cover armboard portions of a surgical bed or cot (not shown), and are composed
of
generally rectangular sheets of material that are secured to the mainsheet 21
and that are
folded behind the mainsheet 21 when not in use. Fig. 3 further illustrates
double-sided tape
48 that is useful in securing the surgeon-side flap 45 (not seen in Fig. 3) in
a closed position
with respect to the screen 22. As further illustrated, the screen 22 includes
a screen
fenestration 49, this screen fenestration 49 permitting passage of a newborn
infant
therethrough.
10026] With reference now to Fig. 7, the tunnel material 50 is in the
form of a
transparent, flexible structure having generally triangular first side 51,
second side 52, and
front face 53, and bottom 54. The first and second sides 51, 52 and front face
53 are
preferably secured to the bottom 54 via heat sealing or another suitable
method, and
perforations 55 optionally may be formed in the region of the sealed surfaces
to form tear
lines. This tunnel structure is secured to the patient-side of the screen 22
via adhesive or
other suitable form of connection. When the surgical drape 20 is laid over a
patient, or
shortly thereafter, the perforations 20 may be torn to open the tunnel
structure to form a
tunnel connecting the surgeon-facing side and the patient-facing side of the
screen 22.
6
CA 3020589 2018-10-11
100271 With reference now to Fig. 8, the surgeon-side flap 45 is provided
to cover
the screen fenestration 49. The flap 45 is secured to the screen 22 via tape
48 (Fig. 3) at the
bottom and sides of the surgeon-side flap 45, and via a permanent adhesive
connection
near the top of the surgeon-side flap 45. Upon birth of the infant, or when
birth is imminent,
a caregiver may lift the surgeon-side flap 45 and fold the flap in an
accordion-like manner
via pre-folded accordion folds 56. The tape 48 at the bottom of the surgeon-
side flap 45 may
be used to secure this flap in the open position, as shown in Fig. 9.
Thereafter, as shown in
Figs. 10A-E the infant 60 may be passed through the screen fenestration 49,
and through
the tunnel 62 that was formed by tearing open the tunnel structure, to then
greet the
mother. A sterile pad or blanket 64 may be placed over the patient to further
enhance
sterility.
100281 As shown in Fig. 11, the screen 22 is further provided with a
patient-side flap
66. This patient-side flap 66 may be similarly connected to the screen via
adhesive and via
double-sided tape. The patient-side flap 66 generally will be secured in an
open position
until the infant is passed through the tunnel 62. At that point, the patient-
side flap 66 may
be moved and secured via tape into a closed position to cover the screen
fenestration 49 to
prevent the patient from viewing subsequent stages of the operation. The
remainder of the
C-section procedure may then be performed with the patient's vision in the
direction
towards the surgical site occluded by at least the patient-side flap 66. For
purposes of
antisepsis, the surgeon-side flap 45 (not shown in Fig. 11) also may be
returned to the closed
position to inhibit contamination of the surgical field. Generally, because
the Cesarean
section procedure is an invasive operation, it is highly desirable that the
surgical side of the
drape remain as sterile as possible to protect the surgical field. The patient
side invariably
cannot remain as sterile. The tunnel is believed to assist in protecting the
sterility of the
surgical field when passing an infant through the screen fenestration 49.
100291 As supplied, the surgical drape 20 is initially provided in a
sterile folded state
wrapped in a sterile fabric wrapper (not shown) and an outer package (also not
shown), as
is conventional. The sterile fabric wrapper surrounds the surgical drape 20 to
protect the
7
CA 3020589 2018-10-11
surgical drape 20 and to maintain its sterile state. The wrapped surgical
drape then is
enclosed in a plastic pouch, which further protects the surgical drape and
maintains its
sterile condition.
100301 The mainsheet and screen and armboard covers may be constructed of
any
suitable material, and as shown are constructed of a multi-layer combination
of spunbond
and meltblown materials as well as impervious films, commonly referred to as
SMS
nonwoven fabric, or bilaminated and trilaminated impervious and absorbent
materials.
The tunnel material is preferably composed of a clear or translucent plastic
film material,
such as a polyethylene film. Other parts of the drape may be composed of
conventional
materials.
[0031] It is thus seen that a drape that allows the surgeon to
selectively permit the
patient to view the area near the surgical field is provided. Via the above-
described
configuration, the mother is immediately able to bond with the infant and to
greet the
infant immediately after birth, while minimizing the risk of contamination to
the surgical
field. The drape enables the mother and the newborn infant to establish
immediate skin-
to-skin contact.
100321 Uses of singular terms such as "a," "an," are intended to cover
both the
singular and the plural, unless otherwise indicated herein or clearly
contradicted by
context. The terms "comprising," "having," "including," and "containing" are
to be
construed as open-ended terms. Any description of certain embodiments as
"preferred"
embodiments, and other recitation of embodiments, features, or ranges as being
preferred,
or suggestion that such are preferred, is not deemed to be limiting. The
invention is
deemed to encompass embodiments that are presently deemed to be less preferred
and
that may be described herein as such. All methods described herein can be
performed in
any suitable order unless otherwise indicated herein or otherwise clearly
contradicted by
context. The use of any and all examples, or exemplary language (e.g., "such
as") provided
herein, is intended to illuminate the invention and does not pose a limitation
on the scope
8
CA 3020589 2018-10-11
of the invention. Any statement herein as to the nature or benefits of the
invention or of
the preferred embodiments is not intended to be limiting. This invention
includes all
modifications and equivalents of the subject matter recited herein as
permitted by
applicable law. Moreover, any combination of the above-described elements in
all possible
variations thereof is encompassed by the invention unless otherwise indicated
herein or
otherwise clearly contradicted by context. The description herein of any
reference or
patent, even if identified as "prior," is not intended to constitute a
concession that such
reference or patent is available as prior art against the present invention.
No unclaimed
language should be deemed to limit the invention in scope. Any statements or
suggestions
herein that certain features constitute a component of the claimed invention
are not
intended to be limiting unless reflected in the appended claims. Neither the
marking of
the patent number on any product nor the identification of the patent number
in connection
with any service should be deemed a representation that all embodiments
described herein
are incorporated into such product or service.
9
CA 3020589 2018-10-11