Note: Descriptions are shown in the official language in which they were submitted.
=
Methods And Apparatus For Automatically Filling Dispensing Containers
DESCRIPTION
Field of the Invention
The invention relates to methods and apparatus for automatically filling
dispensing containers
with medication such as pills, tablets and capsules, and to the dispensing
containers for use with
such apparatus. The dispensing containers are typically used for organising
and storing mixed
medication for subsequent dispensation according to a pre-defined dosage
regimen. The
principle behind such multi-dosage dispensing containers is that a dosage
regimen of mixed
medication can be organised in advance for a period of a week or more, and a
patient or care-
giver can then remove from the container, at pre-defined times over the said
period, the one or
more pills, tablets and/or capsules to be administered on each occasion
according to the dosage
regimen.
Background Art
Dispensing containers for the storage and dispensing of pills, tablets and
capsules are known. A
typical dispensing container includes a tray into which is formed a plurality
of discrete cavities
and which is closed by a seal. The contents of each cavity may be removed by
pushing each pill,
tablet or capsule through a rupturable film or foil covering the cavities, or
in the case where the
seal has a removable portion per cavity, by removing a removable portion to
create an opening in
the seal through which the contents of the cavity can be dispensed. The
dispensing containers
can carry one unit dose of the same medication in each cavity or can be filled
with a mixture of
medications. The tray can have ap x q array of cavities corresponding to p pre-
defined
medication times per day over a q-day period, for example. In some cases not
all of the cavities
will be filled with medication.
The cavities of the dispensing container can be filled manually. However,
apparatus are now
available that can automatically fill the appropriate cavities with the
correct medication in
accordance with the patient's prescription. In one arrangement, a range of
different medication is
1
CA 3020810 2018-10-15
. .
stored in cartridges or cassettes within the apparatus and can be selectively
dispensed into the
cavities before the apparatus automatically applies a seal to the tray. The
patient's prescription
information can be entered into the apparatus manually or electronically ¨ for
example, by
importing or downloading information contained in an e-prescription where a
prescriber (e.g., a
doctor or other medical professional) transmits electronically a new or repeat
prescription to a
dispensing location such as a pharmacy.
Summary of the Invention
The present invention provides a dispensing container comprising:
a tray with one or more discrete cavities for receiving medication; and
a seal;
wherein the tray includes a machine-readable marker that encodes a unique
identifier for
the tray; and
the seal includes a pre-defined area for thermal printing.
Two different types of thermal printing can be used, namely direct thermal
printing and thermal
transfer printing. An apparatus specifically adapted for use with the
dispensing container can
include a thermal print head adapted to thermally print human-readable
information (i.e., data or
information that is in a format that can be naturally read by humans such as
text constructed from
alphanumeric characters or a suitable visual image such as a photograph, for
example) on to the
pre-defined area for thermal printing before, during or after the seal has
been applied to the tray.
The present invention further provides an automatic filling system comprising:
a dispensing container comprising:
a tray with one or more discrete cavities for receiving medication; and
a seal;
wherein the tray includes a machine-readable marker that encodes a unique
identifier for the tray; and
the seal includes a pre-defined area for thermal printing; and
2
CA 3020810 2018-10-15
an apparatus specifically adapted for use with the dispensing container, the
apparatus
comprising a reader, a controller, a medication dispenser, a sealing unit, and
a thermal print
head;
wherein, when a tray is positioned in the apparatus, the system carries out
the following
steps:
the reader reads the marker on the tray and obtains, from the marker, the
unique identifier
for the tray;
the controller receives patient-specific information and associates the
patient-specific
information with the unique identifier for the tray;
the medication dispenser automatically fills one or more cavities of the tray
with
medication with reference to the patient-specific information associated with
the unique
identifier;
the sealing unit automatically applies the seal to the tray; and
the thermal print head thermally prints human-readable information on to the
pre-defined
area for thermal printing ink before, during or after the seal has been
applied to the tray, the
human-readable information being directly derived from at least some of the
patient-specific
information associated with the unique identifier.
The medication dispenser preferably further comprises a plurality of cassettes
for storing
medication.
In the case of direct thermal printing, a patch of thermoprint ink can be pre-
applied to the pre-
defined area of the seal using a suitable process, e.g., flexographic
printing. It will be readily
appreciated that the seals for use with the direct thermal printing process
will be supplied with
the patch of thermoprint ink already applied so that they are ready for
thermal printing by the
apparatus. A plurality of seals can be provided as a continuous reel or
stacked in a seal feeder
unit of the apparatus until they are required. The thermoprint ink is heat-
sensitive and will
change colour, e.g., from light to dark, to give a permanent image when heated
by the thermal
print head of the apparatus. The thermoprint ink can also change opacity,
e.g., from substantially
3
CA 3020810 2018-10-15
=
transparent or translucent to opaque when heated. The activation temperature
of the thermoprint
ink can be selected to be compatible with the thermal print head of the
apparatus. The permanent
image will be determined by the thermal print head and will result in the
human-readable
information (e.g., text or image) being thermally printed on the seal. In
particular, the thermal
print head will normally include a plurality of heating elements, typically
arranged as a closely
spaced dot matrix. When the thermal print head is in close proximity to the
thermoprint ink
patch, the individual heating elements can be selectively controlled to apply
heat to activate the
thermoprint ink in such a way as to thermally print the human-readable
information as the
permanent image on the seal. The parts of the thermoprint ink patch to which
heat is not applied
by the thermal print head (i.e., which are not activated) will not change
colour or opacity.
Suitable thermoprint inks are supplied under the brand name Thermosil by
Siltech Limited of
Church Street, Lenton, Nottingham, NG7 2FH, United Kingdom.
In the case of thermal transfer printing, a transfer ribbon (or printer foil)
is interposed between
the thermal print head and the pre-defined area of the seal. The transfer
ribbon typically has a
wax-based ink on a backing layer. When the thermal print head is in close
proximity to the
transfer ribbon, the individual heating elements can be selectively
controlled, in a similar way to
direct thermal printing described above, to melt the ink onto the seal in such
a way as to
thermally print the human-readable information as a permanent image. When
cooled, the ink is
permanently adhered to the seal surface. Any wax-based ink which is not melted
by the thermal
print head remains adhered to the backing layer. Optionally, a patch of ink
and/or a suitable
primer can be pre-applied to the pre-defined area of the seal using a suitable
process. The ink is
then melted on to the pre-applied ink patch and/or primer during the thermal
transfer printing.
It will be readily appreciated that different heating elements of the thermal
print head can be
selected to create specific human-readable information (e.g., specific text or
image) for each
individual seal. In particular, a thermal print head controller can process
the patient-specific
information associated with the unique identifier, extract the part of the
patient-specific
4
CA 3020810 2018-10-15
=
information that is to be thermally printed (along with any other information
¨ see below), and
control the thermal print head accordingly. The apparatus controller can also
extract the part of
the patient-specific information that is to be thermally printed and send this
to the thermal print
head controller. In one arrangement, only the heating elements in the dot
matrix that correspond
to the specific text or image are controlled to apply heat to the thermoprint
ink or the transfer
ribbon.
In an alternative arrangement, at least part of the seal can be adapted for
direct thermal printing
without the need for a thermoprint ink patch. In other words, the seal will
change colour or
.. opacity to give a permanent image when heated by the above-described
thermal print head of the
apparatus. The seal can be coated with a thin layer of heat-sensitive material
or heat-sensitive
material can be integrated or dispersed within the seal itself, i.e., within
the seal material such as
a plastics material. Although only the pre-defined area of the seal needs to
be directly thermally-
printable, it will often be the case that the whole of the seal (or, in the
case of a multi-layer
construction, the whole of the top layer) is directly thermally-printable,
which provides
flexibility when selecting the location for the printed human-readable
information. The seal can
comprise a flexible film or sheet of plastics material (e.g., polypropylene)
and will typically be
substantially transparent or translucent. The thin layer of heat-sensitive
material can be coated
on all or part of the top surface of the flexible film or sheet, i.e., on the
seal surface that is
adjacent the thermal print head in use, or the heat-sensitive material can be
integrated or
dispersed within all or part of the of the seal material (or, in the case of a
multi-layered
construction, the whole or part of the top layer). The heat-sensitive material
will also typically
be substantially transparent or translucent before being heat activated. When
the thermal print
head is in close proximity to the seal, and in particular to the pre-defined
area for thermal
printing, the individual heating elements can be selectively controlled to
apply heat to activate
the heat-sensitive material in such a way as to thermally print the human-
readable information as
the permanent image on the seal.
5
CA 3020810 2018-10-15
= .
In one arrangement, the seal comprises a flexible sheet of plastics material
that is substantially
transparent or translucent, and the seal is directly thermally-printable with
human-readable
information in at least the pre-defined area for direct thermal printing.
The dispensing container can be a multi-dosage dispensing container.
The tray of the dispensing container can have any suitable number of discrete
cavities arranged
in any suitable p x q array, e.g., a 2 x 7, 3 x 7, 4 x 7 or 5 x 7 array of
cavities. The tray typically
includes a generally planar top surface into which the cavities are formed and
to which the seal is
adhered after one or more cavities have been automatically filled by the
apparatus.
The machine-readable marker can be pre-applied to the tray using any suitable
process, e.g., a
printing process. It will be readily appreciated that the trays for use with
the apparatus will be
supplied with the machine-readable marker already applied. The trays can be
manually inserted
into the apparatus or stacked in a tray feeder unit of the apparatus until
they are required.
The marker can be located on a suitable part of the tray, and in particular
where it is accessible to
the reader when correctly positioned in the apparatus. The marker can be any
suitable visual,
machine-readable, pattern that encodes a unique identifier for the tray, e.g.,
a one-dimensional
.. (or linear) barcode or a two-dimensional (or matrix) barcode. The marker
will be compatible
with the reader of the apparatus. The unique identifier can be a unique data
or information string
that allows the apparatus to register (and optionally record) the unfilled
tray for compliance
purposes and to associate or connect the unique data or information string
with the patient-
specific information received by the apparatus so that the tray can be
automatically filled with
the appropriate medication for the particular patient.
The seal can have any suitable single- or multi-layer construction and can be
rupturable or non-
rupturable, i.e., with a removable portion per cavity. In the case of a non-
rupturable seal, the seal
can include pre-formed lines of separation that define a removable portion per
cavity to retain the
6
CA 3020810 2018-10-15
medication in that cavity until it is removed along its line of separation.
Each removable portion
can be attached to the remainder of the seal by a frangible bridge region
defined by a gap in the
associated pre-formed line of separation. Each removable portion can include a
lug portion that
can be grasped by a user preparatory to removing the removable portion.
The seal is preferably a flexible plastics film or sheet (e.g., polypropylene)
of single- or multiple-
layer construction. The seal is preferably substantially transparent or
translucent so that the
contents of the cavities are visible through the seal. But in other
arrangements the seal can be
opaque and can be a metal foil, such as aluminium foil, or a metallized
polymeric film or paper
sheet, for example.
The seal is adapted to be adhered to the tray to seal the one or more discrete
cavities and the
sealing unit of the apparatus can use any suitable sealing process. In
particular, the seals can be
adhered to the tray using a heat seal or cold seal process and an appropriate
heat seal or cold seal
adhesive or lacquer can be pre-applied to the bottom surface of the seal
(i.e., the surface that is in
contact with the tray in use).
The pre-defined area for thermal printing will typically be positioned so that
it does not interfere
with or obscure any other printing on the seal, e.g., any visual markings or
text that indicate
when the patient should take the medication in the underlying tray cavity, or
any removable
portions if the seal is non-rupturable. The pre-defined area should also
preferably be in a part of
the seal that is readily visible to the patient or pharmacist. In the case of
direct thermal printing,
the thermoprint ink patch can be pre-applied to any suitable part of the seal,
and in particular
where it is accessible to the thermal print head for the thermal printing
process before, during, or
after the seal has been applied to the filled tray. The thermoprint ink patch
can have any suitable
size, shape and thickness (or coating weight).
The present invention further provides a method for automatically filling a
dispensing container
comprising a tray with one or more discrete cavities for receiving medication,
and a seal,
7
CA 3020810 2018-10-15
. .
wherein the tray includes a machine-readable marker that encodes a unique
identifier for the tray
and the seal includes a pre-defined area for thermal printing (i.e., direct
thermal printing or
thermal transfer printing), the method comprising the steps of:
reading the marker on the tray and obtaining, from the marker, the unique
identifier for
the tray;
receiving patient-specific information and associating the patient-specific
information
with the unique identifier for the tray;
automatically filling one or more cavities of the tray with medication with
reference to
the patient-specific information associated with the unique identifier;
automatically applying the seal to the tray; and
thermally printing human-readable information on to the pre-defined area for
thermal
printing before, during or after the seal has been applied to the tray, the
human-readable
information being directly derived from at least some of the patient-specific
information
associated with the unique identifier.
The patient-specific information can be provided manually or electronically,
e.g., from an e-
prescription that is transmitted electronically to a remote dispensing
location such as a pharmacy
where the apparatus is located. Electronic transmission can take place over
any wired or wireless
network and the apparatus mentioned above can include any appropriate
communication unit or
use any appropriate communication protocol to receive the patient-specific
information.
The patient-specific information can include at least patient identification
information and
patient prescription information. The patient identification information can
be any information
or data that can be used to identify the patient and includes inter alia the
patient's name, home
address, date of birth, NHS number, an image or photograph of the patient, and
personal medical
details. The patient prescription information can include inter alia details
about the prescribed
medication (which can be a mixture of medication), dosage instructions,
including if any of the
medication to be taken by the patient is particularly important, and whether
the prescription is a
new prescription or a repeat prescription. It will readily appreciated that
these lists are not
8
CA 3020810 2018-10-15
. .
intended to be exhaustive and that other patient identification information
and patient
prescription information can be utilised in the present invention as
appropriate.
The one or more cavities of the tray are automatically filled with medication
(e.g., by the
medication dispenser of the apparatus mentioned above) with reference to the
patient
prescription information. The prescribed medication (which can be a mixture of
medication) is
dispensed into the appropriate cavities of the tray depending on the dosage
instructions. For
example, if the tray includes a 4 x 7 array of cavities corresponding to four
pre-defined
medication times per day over a seven-day period, and the patient is
prescribed a first medication
type to be taken first thing every morning and a second medication type to be
taken every
evening, the seven cavities in the first array of the tray are automatically
filled with the required
dose of the first medication type and the seven cavities in the fourth array
are automatically filled
with the required dose of the second medication type. The cavities in the
second and third arrays
of the tray are left empty.
The human-readable information that is thermally printed on the seal can
include at least some of
the patient identification information, e.g., the patient's name. Typically
the human-readable
information will be sufficient to ensure that the filled dispensing container
is given to the correct
patient, and sensitive or confidential information about the patient or the
patient's prescription
will normally be avoided so that the empty dispensing container can be safely
disposed of
without compromising the patient's privacy.
Additional information, including information that is not patient-specific,
can also be thermally
printed on the seal. The additional information might be generated within the
apparatus, or be
apparatus- or pharmacist-specific and can include inter alia human-readable
information about
the pharmacy or the identity of the pharmacist or other healthcare
professional operating the
apparatus, warning notices, filling date or expiry date, for example. In some
arrangements,
machine-readable information (i.e., data, metadata or information that is in a
format that can be
understood by a computer) or a machine-readable marker can also be thermally
printed on the
9
CA 3020810 2018-10-15
. .
pre-defined area of the seal. The machine-readable information can be directly
derived from at
least some of the patient-specific information associated with the unique
identifier or can be not
patient-specific. A machine-readable marker thermally printed on the seal can
be any suitable
visual, machine-readable, pattern that encodes a unique identifier for the
seal, e.g., a one-
dimensional (or linear) barcode or a two-dimensional (or matrix) barcode. In
one arrangement,
the pre-applied machine-readable marker on the tray can be replicated on the
seal during the
filling process. In other words, the unique identifiers for the tray and seal
encoded in the
respective machine-readable markers can be the same. The machine-readable
marker thermally
printed on the pre-defined area of the seal can be read by the same or a
different reader to check
to see if the respective markers on the tray and seal match.
In practice, any combination of human-readable information and machine-
readable information
(both patient-specific and non-patient-specific) can be thermally printed on
the seal by the
thermal print head depending on the particular requirements.
Information might also be conventionally printed separately on a card, sheet
or label that is
adapted to be adhered to, or supplied with, the dispensing container. The
conventionally printed
information might include at least some of the patient-specific information
associated with the
unique identifier. It might, in part, replicate the information printed on the
seal. The printing
unit (e.g., a conventional printer) can be integrated with the apparatus for
automatically filling
the dispensing container, or connected to the apparatus by means of a wired or
wireless network
so that patient-specific information that is received by the controller can be
transmitted to the
printing unit. The conventionally printed information can include dosage
instructions for
explaining to the patient when the medication needs to be dispensed, although
this can also be
indicated by appropriate visual markings or text on the seal itself. The
conventionally printed
information can also include an image or photograph of the patient, details
about the filled
medication, location information (e.g., the patient's bed or room number in a
hospital or care-
home environment), the patient's address, details about the patient's doctor,
pharmacist or carer
etc.
CA 3020810 2018-10-15
=
Drawings
Figure 1 is a top view of a tray of a dispensing container according to the
present invention;
Figure 2 is a top view of a first seal of a dispensing container according to
the present invention
before thermal printing;
Figure 3 is a top view of a second seal of a dispensing container according to
the present
invention before thermal printing;
Figure 4 is a cross section view of a filled dispensing container according to
the present
invention;
Figure 5 is a schematic view of an apparatus according to the present
invention for automatically
filling a dispensing container with medication; and
Figure 6 is a top view of the seal of Figure 3 after thermal printing.
With reference to Figures 1 to 4 a dispensing container 1 includes a tray 2
made of a
substantially rigid plastics material into which is formed a 4 x 7 array of
discrete cavities 4. The
cavities 4 are closed by a seal which is adhered or secured to a generally
planar top surface 8 of
the tray 2.
The underside of the tray 2 is pre-printed with a machine-readable marker 10
in the form of a
two-dimensional (or matrix) barcode. The marker 10 encodes a unique
identifier, which can be a
unique data or information string.
The seal is a flexible sheet of substantially transparent or translucent
plastics material (e.g.,
polypropylene) which is formed with a 4 x 7 array of removable portions 12 in
a known manner.
The seal 6a shown in Figure 2 is specifically adapted for direct thermal
printing and heat-
sensitive material (not shown) is integrated or dispersed within the plastics
material. If the seal
6a has a multi-layer construction, i.e., with a top layer and a bottom layer,
the heat-sensitive
material need only be integrated or dispersed within one of the layers,
typically the top layer.
The heat-sensitive material can be integrated or dispersed within the entirety
of the seal (or
11
CA 3020810 2018-10-15
layer), or only within part of the seal (or layer) that is a pre-defined area
for direct thermal
printing.
The seal 6b shown in Figure 3 is also specifically adapted for direct thermal
printing and a
thermoprint ink patch 14 is pre-applied to the top surface of the seal by
flexographic printing.
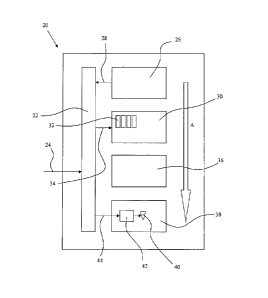
Figure 5 shows an apparatus 20 for automatically filling a dispensing
container with medication.
The apparatus 20 includes a controller 22 which receives electronic patient-
specific information
24 in the form of an e-prescription. For the purposes of the present
description, the patient-
specific information 24 includes the patient's name (so-called "patient
identification
information") and the patient's prescription and dosage instructions (so-
called "patient
prescription information"). In an alternative arrangement, the patient-
specific information can be
manually entered into the apparatus 20 by the pharmacist on receipt of the
patient's paper
prescription.
The apparatus 20 includes a barcode reader 26 which is adapted to read the
marker 10 on the
underside of the tray 2 when the tray is positioned in the apparatus. The
barcode reader 26
obtains, from the marker 10, the unique identifier for the tray 2 and sends
the unique data or
information string 28 to the controller 22. The controller 22 associates or
electronically matches
the patient-specific information 24 with the unique data or information string
28 obtained from
the marker 10.
The apparatus 20 includes a medication dispenser 30 with a plurality of
cassettes 32 for storing
different medication. The medication dispenser 30 receives patient
prescription information 34
from the controller 22.
The apparatus 20 includes a sealing unit 36 (e.g., a conventional heat seal
unit) that will
automatically apply the seal 6a or 6b to the tray 2 after it has been filled
with medication.
12
CA 3020810 2018-10-15
The apparatus 20 includes a direct thermal print unit 38 with a thermal print
head 40 and a
thermal print head controller 42 which receives patent identification
information 44 from the
controller 22.
The arrow A indicates the processing direction for the apparatus 20. The tray
4 can be moved
through a series of separate process stations, e.g., reading, filling, sealing
and printing, within the
apparatus 20.
When a tray 2 is positioned within the apparatus, its marker 10 is scanned by
the barcode reader
26 and the unique data or information string 28 is sent to the controller 22
which associates or
electronically matches it with the patient-specific information 24 that it has
already received
from the e-prescription. In particular, the controller 22 associates both the
patient identification
information 44 and the patient prescription information 34 with the unique
data or information
string 28.
The medication dispenser 30 uses the patient prescription information 34 to
fill the appropriate
cavities 4 of the tray 2 with the relevant medication. The medication can be
dispensed from the
cassettes 32.
Once the tray 2 has been filled, the sealing unit 36 will automatically adhere
the seal 6a or 6b to
the generally planar top surface 8 of the tray 2. A plurality of seals can be
stored in a seal feeder
tray (not shown) that forms part of the sealing unit 36.
The thermal print head 38 is then controlled to thermally print human-readable
information 46 on
to the seal 6a or 6b after it has been adhered to the tray 2. In an
alternative arrangement, the seal
can be thermally printed before it is adhered to the tray 2 by the sealing
unit 36, or during the
sealing process.
13
CA 3020810 2018-10-15
In the case of the seal 6a shown in Figure 2, the human-readable information
is directly
thermally printed on to the pre-defined area of the seal. In the case of the
seal 6b shown in
Figure 3, the human-readable information 46 is directly thermally printed on
to the thermoprint
ink patch 14 of the seal.
The human-readable information 46 that is thermally printed on the seal can be
data or
information that is in a format that can be naturally read by humans such as
text constructed from
alphanumeric characters or a visual image, for example. The human-readable
information 46 is
derived directly from the patient-specific information 24, and in particular
from the patient
identification information 44 that is determined by the controller 22. In this
specific example,
the patient identification information 44 that is sent to the thermal print
head controller 42 is the
patient's name, namely Joe Bloggs. The thermal print head 40 includes a
plurality of heating
elements arranged as a closely spaced dot matrix. The thermal print head
controller 42 selects
specific heating elements to create the text "JOE BLOGGS" for the particular
seal to be printed.
The heating elements apply heat to the seal 6a to activate the heat-sensitive
material that is
integrated or dispersed within the plastics material of the seal, or apply
heat to the patch 14 of the
seal 6b to activate the thermoprint ink and create a permanent image with the
text "JOE
BLOGGS". In the case of the seal 6a shown in Figure 2, the application of the
heat to the seal
will change the colour or opacity of the actual plastics material to create
the permanent image.
Figure 6 shows the seal 6b after it has been thermally printed ¨ it should be
noted that the tray
has been omitted both for clarity and to emphasise that the seal can be
thermally printed before it
is adhered to the tray in some arrangements.
The seal 6b shown in Figure 6 has also been thermally printed with additional
human-readable
information, namely "01/01/2017", that is not patient-specific. This
additional human-readable
information indicates the date on which the dispensing container was filled by
the apparatus 20.
In an alternative arrangement, the apparatus can include a thermal transfer
print unit instead of
the direct thermal print unit. Such a thermal transfer print unit would still
include a thermal print
14
CA 3020810 2018-10-15
head, but would also have means for interposing the transfer ribbon (or
printer foil) between the
thermal print head and the pre-defined area of the seal for thermal printing.
CA 3020810 2018-10-15