Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 229
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 229
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
CA 03021185 2018-10-16
A00039
4
DESCRIPTION
FUSED HETEROCYCLIC COMPOUND
TECHNICAL FIELD
[0001]
The present invention relates to a novel compound having an inhibitory action
against
cdc2-like kinase (cdc2-like kinase; hereinafter sometimes abbreviated as CLK),
or a salt thereof.
The present invention also relates to a medicament which contains the compound
or salt thereof
and which is used for preventing or treating a CLK-related disease such as
cancer.
BACKGROUND ART
[0002]
Abnormal control of an alternative splicing mechanism has been reported in
various
diseases such as neurodegenerative disease, amyotrophic lateral sclerosis and
cancer.
Particularly in cancer, cancer-specific splicing variants produced by abnormal
alternative
splicing have been shown to play an important role in cancer survival and
invasion. In recent
years, it has been shown that spliceosome constituent factors such as SF3B1,
SRSF2 and U2AF1
are mutated with high frequency in the osteomyelodysplasia syndrome. These
findings indicate
that control of an alternative splicing mechanism plays an important role in
cancer.
CLK family kinase is a type of bispecific protein kinase retaining both
serine/threonine
kinase activity and tyrosine kinase activity, and include four types of
kinase: CLK1 to CLK4.
CLK phosphorylates SR proteins such as SRSF I to control localization of the
proteins, so that a
splicing regulation mechanism by SR proteins is controlled. It has been shown
that by
regulating alternative splicing by inhibition of CLK kinase activity, signals
essential for survival
of cancer can be hindered to inhibit growth of cancer cells. In addition, CLK2
has been shown
to act as an oncogene in breast cancer. Therefore, inhibition of kinase
activity of CLK may be a
promising treatment for cancer.
[0003]
Patent Literatures 1 and 2 disclose a fused heterocyclic compound as a
compound
having a tyrosine kinase inhibitory action. Patent Literatures 3 and 4
disclose a fused
heterocyclic compound having other pharmacological activities.
CITATION LIST
PATENT LITERATURE
[0004]
PATENT LITERATURE 1: WO 00/71129
1
CA 03021185 2018-10-16
A00039
PATENT LITERATURE 2: WO 2004/009784
PATENT LITERATURE 3: WO 2014/143610
PATENT LITERATURE 4: WO 2012/003576
SUMMARY OF INVENTION
TECHNICAL PROBLEM
[0005]
An object of the present invention is to provide a compound which has an
excellent
CLK inhibitory action, and is satisfactory as a pharmaceutical product.
SOLUTION TO PROBLEM
[0006]
The present inventors have found that a compound represented by the following
formula or a salt thereof has excellent CLK inhibitory activity, and is useful
for prevention and
treatment of cancer and the like. On the basis of this finding, the present
inventors have
conducted intensive studies, leading to completion of the present invention.
That is, the present
invention is as follows.
[0007]
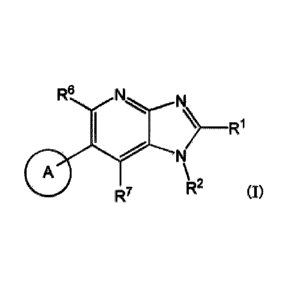
[1] A compound represented by formula (I), or
a salt thereof (herein sometimes abbreviated as "compound (I)"):
[Chemical Formula 1]
R6
) _________________________ R1
N
(I)
R7
R2
[wherein
Rl represents a substituent or a hydrogen atom;
R2 represents an optionally substituted hydrocarbon group or an optionally
substituted
heterocyclic group;
R6 and R7 each independently represent a hydrogen atom, a halogen atom or an
optionally substituted hydrocarbon group; and
ring A represents a bicyclic aromatic heterocyclic ring selected from the
following
formulas (1), (2), (3), (4) and (5):
[Chemical formula 2]
2
CA 03021185 2018-10-16
A00039
4=
4-
N)11"
N---N
R4 (
R4 R4
R4
NyX NyX N NN
R5 R5
R3 R3 R3 R3 R3
(1) (2) (3) (4) (5)
wherein Xs each independently represent N or C(R5); and
R3, R4 and R5 each independently represent a hydrogen atom or a substituent.].
[2] The compound or salt thereof according to [1], wherein
R2 i s
(I) a C1_6 alkyl group optionally having Ito 3 five- or six-membered
monocyclic
aromatic heterocyclic groups optionally having 1 to 3 optionally halogenated
C1_6 alkyl groups;
Or
(II) an optionally halogenated C7_16 aralkyl group.
[3] The compound or salt thereof according to [1], wherein ring A is a
bicyclic aromatic
heterocyclic ring represented by the following formula (1).
I
NyX
R3
(1)
[4] The compound or salt thereof according to [1], wherein R3 is a hydrogen
atom;
R4 is (a) an amino group optionally monosubstituted or disubstituted with a
C1_6 alkyl
group, or
(b) a Ci_6 alkoxy group; and
R5 is (a) a C1-6 alkoxy group, or
(b) a Ch6 alkyl group optionally having 1 to 3 hydroxy groups.
[5] The compound or salt thereof according to [1], wherein R1 is
(I) a Ci.6 alkyl group optionally having 1 to 5 substituents each selected
from
(a) a halogen atom,
3
CA 03021185 2018-10-16
A00039
4
(b) a hydroxy group,
(c) a cyano group,
(d) a C1_6 alkoxy group optionally having 1 to 3 substituents each selected
from
(i) a halogen atom, and
(ii) a hydroxy group, and
(e) a three- to eight-membered nonaromatic heterocyclic group having 1 to 3
halogen
atoms;
(II) a three- to eight-membered nonaromatic heterocyclic group optionally
having 1 to 5
substituents each selected from
(a) a C1_6 alkoxy group,
(b) a halogen atom, and
(c) a hydroxy group;
(III) a five- to fourteen-membered aromatic heterocyclic group optionally
having 1 to 3
optionally halogenated Ci_6 alkyl groups;
(IV) a C1_6 alkoxy group optionally having 1 to 5 substituents each selected
from
(a) a hydroxy group,
(b) a three- to eight-membered nonaromatic heterocyclic group, and
(c) a five- or six-membered monocyclic aromatic heterocyclic group optionally
having 1
to 3 optionally halogenated C1_6 alkyl groups;
(V) an optionally halogenated C3-10 cycloalkyl group; or
(VI) an amino group optionally monosubstituted or disubstituted with a CI-6
alkyl group
optionally having 1 to 5 substituents each selected from
(a) (i) halogen atom,
(ii) a hydroxy group, and
(iii) C1-6 alkoxy group;
R2 is
(I) a C1_6 alkyl group optionally having 1 to 3 five- to fourteen-membered
aromatic
heterocyclic groups optionally having 1 to 3 substituents each selected from
(a) a halogen atom,
(b) a C1_6 alkyl group optionally having 1 to 7 substituents each selected
from
(i) a halogen atom,
(ii) a hydroxy group, and
(iii) a C1-6 alkoxy group,
(c) an optionally halogenated C3-10 cycloalkyl group,
4
CA 03021185 2018-10-16
A00039
(d) a C1-6 alkoxy group,
(e) a C1-6 alkyl group-C3_10 cycloalkyl group,
(f) a Ci_6 alkyl group-three- to eight-membered monocyclic nonaromatic
heterocyclic
group, and
(g) an oxo group; or
(II) a C7_16 aralkyl group optionally having 1 to 3 substituents each selected
from
(a) a C1_6 alkyl group optionally having 1 to 3 substituents each selected
from
(i) a halogen atom, and
(ii) a hydroxyl group,
(b) a C1_6 alkoxy-carbonyl group,
(c) a halogen atom,
(d) a C1_6 alkoxy group optionally having 1 to 3 substituents each selected
from
(i) a halogen atom, and
(ii) a hydroxyl group
(e) a mono-or di-C1_6 alkyl-carbamoyl group,
(f) a cyano group,
(g) an amino group optionally monosubstituted or disubstituted with a
substituent
selected from
(i) a Ci_6 alkyl group, and
(ii) a C1_6 alkoxy-carbonyl group,
(h) a nitro group, and
(i) a carboxy group;
each of R6 and R7 is a hydrogen atom;
ring A is a bicyclic aromatic heterocyclic ring selected from the formulas
(1), (2), (3)
and (4);
Xs each independently represents C(R5) or N;
R3 is a hydrogen atom;
R4 is
(I) a hydrogen atom,
(II) a halogen atom,
(III) an optionally halogenated C1_6 alkoxy group, or
(IV) an amino group optionally monosubstituted or disubstituted with a C1-6
alkyl
group; and
R5 is
5
CA 03021185 2018-10-16
A00039
(I) a hydrogen atom,
(II) a halogen atom,
(III) a cyano group,
(IV) a Ci_6 alkyl group optionally having 1 to 5 substituents each selected
from
(a) a halogen atom,
(b) a C1-6 alkoxy group,
(c) a C1-6 alkoxy-C1_6 alkoxy group,
(d) a hydroxy group, and
(e) a C1_6 alkyl-carbonyloxy group,
(V) a C1.6 alkoxy group,
(VI) a C2-6 alkenyl group optionally having 1 to 3 Ci_6 alkoxy groups,
(VII) a C2..6 alkynyl group,
(VIII) a cyano-C6.14 aryl group,
(IX) a five- to fourteen-membered aromatic heterocyclic group optionally
having 1 or 2
C1_6 alkyl groups optionally having 1 to 3 substituents each selected from
(a) a halogen atom,
(b) a five- or six-membered monocyclic aromatic heterocyclic group, and
(c) a three- to eight-membered monocyclic nonaromatic heterocyclic group,
(X) a C1_6 alkyl-carbonyl group,
(XI) a hydroxy group,
(XII) a C1-6 alkyl-five- to fourteen-membered aromatic heterocyclic oxy group,
(XIII) a three- to eight-membered monocyclic nonaromatic heterocyclic group
optionally having 1 to 3 substituents each selected from
(a) a halogen atom,
(b) a C1_6 alkoxy group, and
(c) an oxo group, or
(XIV) an amino group optionally monosubstituted or disubstituted with an
optionally
halogenated C1-6 alkyl.
[6] 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methy1-1-((3-methy1-1,2,4-
thiadiazol-5-yl)methyl)-1H-imidazo[4,5-b]pyridine, or a salt thereof.
[7] 1-((5-(difluoromethyl)-1,3,4-oxadiazol-2-yOmethyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine, or a salt thereof.
[8] 1-((5-(1,1-difluoroethyl)-1,3,4-oxadiazol-2-y1)methyl)-6-(4-
methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine, or a salt thereof.
6
CA 03021185 2018-10-16
A00039
[9] 14(54(1R)-1-fluoroethy0-1,3,4-oxadiazol-2-yOmethyl)-6-(4-
methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine, or a salt thereof.
[10] A medicament including the compound or salt thereof according to [1].
[11] The medicament according to [10] which is a CLK inhibitor.
[12] The medicament according to [10] which is a prophylactic or therapeutic
agent for
cancer.
[13] A method for inhibiting CLK in a mammal, including administering to the
mammal
an effective amount of the compound or salt thereof according to [1].
[14] A method for preventing or treating cancer in a mammal, including
administering
to the mammal an effective amount of the compound or salt thereof according to
[1].
[15] The compound or salt thereof according to [1] for use in prevention and
treatment
of cancer.
[16] Use of the compound or salt thereof according to [1] for producing a
prophylactic
or therapeutic agent for cancer.
ADVANTAGEOUS EFFECTS OF INVENTION
[0008]
The compound (I) has an excellent CLK inhibitory action, and is useful for
prevention
and treatment of cancer and the like.
DESCRIPTION OF EMBODIMENTS
[0009]
Hereinafter, compounds of the present invention, methods for production
thereof, and
uses of thereof will be described in detail.
[0010]
The definition of each substituent used in the present specification is
described in detail
in the following. Unless otherwise specified, each substituent has the
following definition.
In the present specification, examples of the "halogen atom" include fluorine,
chlorine,
bromine and iodine.
In the present specification, examples of the "C1_6 alkyl group" include
methyl,
ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl,
isopentyl, neopentyl, 1-
ethylpropyl, hexyl, isohexyl, 1,1-dimethylbutyl, 2,2-dimethylbutyl, 3,3-
dimethylbutyl and 2-
ethylbutyl.
In the present specification, examples of the "optionally halogenated C1_6
alkyl
group" include a C 1 .6 alkyl group optionally having 1 to 7, preferably 1 to
5, halogen atoms.
Specific examples thereof include methyl, chloromethyl, difluoromethyl,
trichloromethyl,
7
CA 03021185 2018-10-16
A00039
trifluoromethyl, ethyl, 2-bromoethyl, 2,2,2-trifluoroethyl, tetrafluoroethyl,
pentafluoroethyl,
propyl, 2,2-di fluoropropyl, 3,3,3-trifluoropropyl, isopropyl, butyl, 4,4,4-
trifluorobutyl, isobutyl,
sec-butyl, tert-butyl, pentyl, isopentyl, neopentyl, 5,5,5-trifluoropentyl,
hexyl and 6,6,6-
trifluorohexyl.
In the present specification, examples of the "C2_6 alkenyl group" include
ethenyl, 1-propenyl, 2-
propenyl, 2-methyl-1-propenyl, 1-butenyl, 2-butenyl, 3-butenyl, 3-methyl-2-
butenyl, 1-pentenyl,
2-pentenyl, 3-pentenyl, 4-pentenyl, 4-methyl-3-pentenyl, 1-hexenyl, 3-hexenyl
and 5-hexenyl.
In the present specification, examples of the "C2.6 alkynyl group" include
ethynyl, 1-propynyl, 2-
propynyl, 1-butynyl, 2-butynyl, 3-butynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl,
4-pentynyl, 1-
hexynyl, 2-hexynyl, 3-hexynyl, 4-hexynyl, 5-hexynyl and 4-methyl-2-pentynyl.
In the present specification, examples of the "C3_10 cycloalkyl group" include
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl,
bicyclo[2.2.1]heptyl,
bicyclo[2.2.2]octyl, bicyclo[3.2.1]octyl and adamantyl.
In the present specification, examples of the "optionally halogenated C3_10
cycloalkyl
group" include a C3-10 cycloalkyl group optionally having 1 to 7, preferably 1
to 5, halogen
atoms. Specific examples thereof include cyclopropyl, 2,2-difluorocyclopropyl,
2,3-
difluorocyclopropyl, cyclobutyl, difluorocyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl and
cyclooctyl.
In the present specification, examples of the "C3_10 cycloalkenyl group"
include
cyclopropenyl, cyclobutenyl, cyclopentenyl, cyclohexenyl, cycloheptenyl and
cyclooctenyl.
In the present specification, examples of the "C6.14 aryl group" include
phenyl, 1-
naphthyl, 2-naphthyl, 1-anthryl, 2-anthryl and 9-anthryl.
In the present specification, examples of the "C7_16 arallcyl group" include
benzyl,
phenethyl, naphthylmethyl and phenylpropyl.
[0011]
In the present specification, examples of the "C1_6 alkoxy group" include
methoxy,
ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, sec-butoxy, tert-butoxy,
pentyloxy and
hexyloxy.
In the present specification, examples of the "optionally halogenated C1_6
alkoxy
group" include a C1.6 alkoxy group optionally having 1 to 7, preferably 1 to
5, halogen atoms.
Specific examples thereof include methoxy, difluoromethoxy, trifluoromethoxy,
ethoxy, 2,2,2-
trifluoroethoxy, propoxy, isopropoxy, butoxy, 4,4,4-trifluorobutoxy,
isobutoxy, sec-butoxy,
pentyloxy and hexyloxy.
In the present specification, examples of the "C3.10 cycloalkyloxy group"
include
8
CA 03021185 2018-10-16
A00039
cyclopropyloxy, cyclobutyloxy, cyclopentyloxy, cyclohexyloxy, cycloheptyloxy
and
cyclooctyloxy.
In the present specification, examples of the "C1_6 alkylthio group" include
methylthio,
ethylthio, propylthio, isopropylthio, butylthio, sec-butylthio, tert-
butylthio, pentylthio and
hexylthio.
In the present specification, examples of the "optionally halogenated C1_6
alkylthio
group" include a C1_6 alkylthio group optionally having 1 to 7, preferably 1
to 5, halogen atoms.
Specific examples thereof include methylthio, difluoromethylthio,
trifluoromethylthio, ethylthio,
propylthio, isopropylthio, butylthio, 4,4,4-trifluorobutylthio, pentylthio and
hexylthio.
In the present specification, examples of the "C1_6 alkyl-carbonyl group"
include acetyl,
propanoyl, butanoyl, 2-methylpropanoyl, pentanoyl, 3-methylbutanoyl, 2-
methylbutanoyl, 2,2-
dimethylpropanoyl, hexanoyl and heptanoyl.
In the present specification, examples of the "optionally halogenated C1_6
alkyl-carbonyl
group" include a C1_6 alkyl-carbonyl group optionally having 1 to 7,
preferably 1 to 5, halogen
atoms. Specific examples thereof include acetyl, chloroacetyl,
trifluoroacetyl, trichloroacetyl,
propanoyl, butanoyl, pentanoyl and hexanoyl.
In the present specification, examples of the "C1_6 alkoxy-carbonyl group"
include
methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl,
butoxycarbonyl,
isobutoxycarbonyl, sec-butoxycarbonyl, tert-butoxycarbonyl, pentyloxycarbonyl
and
hexyloxycarbonyl.
In the present specification, examples of the "C6_14 aryl-carbonyl group"
include
benzoyl, 1-naphthoyl and 2-naphthoyl.
In the present specification, examples of the "C7_16 aralkyl-carbonyl group"
include
phenylacetyl and phenylpropionyl.
In the present specification, examples of the "5- to 14-membered aromatic
heterocyclylcarbonyl group" include nicotinoyl, isonicotinoyl, thenoyl and
furoyl.
In the present specification, examples of the "3- to 14-membered non-aromatic
heterocyclylcarbonyl group" include morpholinylcarbonyl, piperidinylcarbonyl
and
pyrrolidinylcarbonyl.
[0012]
In the present specification, examples of the "mono- or di-C6 alkyl-carbamoyl
group"
include methylcarbamoyl, ethylcarbamoyl, dimethylcarbamoyl, diethylcarbamoyl
and N-ethyl-
N-methylcarbamoyl.
In the present specification, examples of the "mono- or di-C7.16 aralkyl-
carbamoyl
9
CA 03021185 2018-10-16
A00039
group" include benzylcarbamoyl and phenethylcarbamoyl.
In the present specification, examples of the "C1_6 alkylsulfonyl group"
include
methylsulfonyl, ethylsulfonyl, propylsulfonyl, isopropylsulfonyl,
butylsulfonyl, sec-
butylsulfonyl and tert-butylsulfonyl.
In the present specification, examples of the "optionally halogenated C1_6
alkylsulfonyl
group" include a C1_6 alkylsulfonyl group optionally having 1 to 7, preferably
Ito 5, halogen
atoms. Specific examples thereof include methylsulfonyl,
difluoromethylsulfonyl,
trifluoromethylsulfonyl, ethylsulfonyl, propylsulfonyl, isopropylsulfonyl,
butylsulfonyl, 4,4,4-
trifluorobutylsulfonyl, pentylsulfonyl and hexylsulfonyl.
In the present specification, examples of the "C6_14 arylsulfonyl group"
include
phenylsulfonyl, 1-naphthylsulfonyl and 2-naphthylsulfonyl.
[0013]
In the present specification, examples of the "substituent" include a halogen
atom, a
cyano group, a nitro group, an optionally substituted hydrocarbon group, an
optionally
substituted heterocyclic group, an acyl group, an optionally substituted amino
group, an
optionally substituted carbamoyl group, an optionally substituted
thiocarbamoyl group, an
optionally substituted sulfamoyl group, an optionally substituted hydroxy
group, an optionally
substituted sulfanyl (SH) group and an optionally substituted silyl group.
In the present specification, examples of the "hydrocarbon group" (including
"hydrocarbon group" of "optionally substituted hydrocarbon group") include a
C1_6 alkyl group, a
C2_6 alkenyl group, a C2-6 alkynyl group, a C3_10 cycloalkyl group, a C3_10
cycloalkenyl group, a
C6_14 aryl group and a C7-16 aralkyl group.
[0014]
In the present specification, examples of the "optionally substituted
hydrocarbon group"
include a hydrocarbon group optionally having substituent(s) selected from the
following
substituent group A.
[substituent group A]
(1) a halogen atom,
(2) a nitro group,
(3) a cyano group,
(4) an oxo group,
(5) a hydroxy group,
(6) an optionally halogenated C1_6 alkoxy group,
(7) a C6-14 aryloxy group (e.g., phenoxy, naphthoxy),
CA 03021185 2018-10-16
A00039
(8) a C7-16 aralkyloxy group (e.g., benzyloxy),
(9) a 5- to 14-membered aromatic heterocyclyloxy group (e.g., pyridyloxy),
(10) a 3- to 14-membered non-aromatic heterocyclyloxy group (e.g.,
morpholinyloxy,
piperidinyloxy),
(11) a C1-6 alkyl-carbonyloxy group (e.g., acetoxy, propanoyloxy),
(12) a C6-14 aryl-carbonyloxy group (e.g., benzoyloxy, 1-naphthoyloxy, 2-
naphthoyloxy),
(13) a C1_6 alkoxy-carbonyloxy group (e.g., methoxycarbonyloxy,
ethoxycarbonyloxy,
propoxycarbonyloxy, butoxycarbonyloxy),
(14) a mono- or di-C1_6 alkyl-carbamoyloxy group (e.g., methylcarbamoyloxy,
ethylcarbamoyloxy, dimethylcarbamoyloxy, diethylcarbamoyloxy),
(15) a C6_14 aryl-carbamoyloxy group (e.g., phenylcarbamoyloxy,
naphthylcarbamoyloxy),
(16) a 5- to 14-membered aromatic heterocyclylcarbonyloxy group (e.g.,
nicotinoyloxy),
(17) a 3-to 14-membered non-aromatic heterocyclylcarbonyloxy group (e.g.,
morpholinylcarbonyloxy, piperidinylcarbonyloxy),
(18) an optionally halogenated Ci_6 alkylsulfonyloxy group (e.g.,
methylsulfonyloxy,
trifluoromethylsulfonyloxy),
(19) a C6-14 arylsulfonyloxy group optionally substituted by a Ci_6 alkyl
group (e.g.,
phenylsulfonyloxy, toluenesulfonyloxy),
(20) an optionally halogenated C1_6 alkylthio group,
(21) a 5- to 14-membered aromatic heterocyclic group,
(22) a 3- to 14-membered non-aromatic heterocyclic group,
(23) a formyl group,
(24) a carboxy group,
(25) an optionally halogenated CI-6 alkyl-carbonyl group,
(26) a C6-14 aryl-carbonyl group,
(27) a 5- to 14-membered aromatic heterocyclylcarbonyl group,
(28) a 3- to 14-membered non-aromatic heterocyclylcarbonyl group,
(29) a C1_6 alkoxy-carbonyl group,
(30) a C6.14 aryloxy-carbonyl group (e.g., phenyloxycarbonyl, 1-
naphthyloxycarbonyl, 2-
naphthyloxycarbonyl),
(31) a C7-16 aralkyloxy-carbonyl group (e.g., benzyloxycarbonyl,
phenethyloxycarbonyl),
(32) a carbamoyl group,
(33) a thiocarbamoyl group,
(34) a mono- or di-C1.6 alkyl-carbamoyl group,
11
CA 03021185 2018-10-16
A00039
(35) a C6-14 aryl-carbamoyl group (e.g., phenylcarbamoyl),
(36) a 5- to 14-membered aromatic heterocyclylcarbamoyl group (e.g.,
pyridylcarbamoyl,
thienylcarbamoyl),
(37) a 3- to 14-membered non-aromatic heterocyclylcarbamoyl group (e.g.,
morpholinylcarbamoyl, piperidinylcarbamoyl),
(38) an optionally halogenated C1-6 alkylsulfonyl group,
(39) a C6-14 arylsulfonyl group,
(40) a 5- to 14-membered aromatic heterocyclylsulfonyl group (e.g.,
pyridylsulfonyl,
thienylsulfonyl),
(41) an optionally halogenated C1_6 alkylsulfinyl group,
(42) a C6_14 arylsulfinyl group (e.g., phenylsulfinyl, 1-naphthylsulfinyl, 2-
naphthylsulfinyl),
(43) a 5- to 14-membered aromatic heterocyclylsulfinyl group (e.g.,
pyridylsulfinyl,
thienylsulfinyl),
(44) an amino group,
(45) a mono- or di-C6 alkylamino group (e.g., methylamino, ethylamino,
propylamino,
isopropylamino, butylamino, dimethylamino, diethylamino, dipropylamino,
dibutylamino, N-
ethyl-N-methylamino),
(46) a mono- or di-C6_14 arylamino group (e.g., phenylarnino),
(47) a 5- to 14-membered aromatic heterocyclylamino group (e.g.,
pyridylamino),
(48) a C7-16 aralkylamino group (e.g., benzylamino),
(49) a formylamino group,
(50) a C1_6 alkyl-carbonylamino group (e.g., acetylamino, propanoylamino,
butanoylamino),
(51) a (C1_6 a1ky1)(Ci_6 alkyl-carbonypamino group (e.g., N-acetyl-N-
methylamino),
(52) a C6-14 aryl-carbonylamino group (e.g., phenylcarbonylamino,
naphthylcarbonylamino),
(53) a C1-6 alkoxy-carbonylamino group (e.g., methoxycarbonylamino,
ethoxycarbonylamino,
propoxycarbonylamino, butoxycarbonylamino, tert-butoxycarbonylamino),
(54) a C7-16 aralkyloxy-carbonylamino group (e.g., benzyloxycarbonylamino),
(55) a C1_6 alkylsulfonylamino group (e.g., methylsulfonylamino,
ethylsulfonylamino),
(56) a C6-14 arylsulfonylamino group optionally substituted by a C1-6 alkyl
group (e.g.,
phenylsulfonylamino, toluenesulfonylamino),
(57) an optionally halogenated C1_6 alkyl group,
(58) a C2-6 alkenyl group,
(59) a C2_6 alkynyl group,
(60) a C3-10 cycloalkyl group,
12
CA 03021185 2018-10-16
A00039
(61) a C3_10 cycloalkenyl group and
(62) a C6-14 aryl group.
[0015]
The number of the above-mentioned substituents in the "optionally substituted
hydrocarbon group" is, for example, 1 to 5, preferably 1 to 3. When the number
of the
substituents is two or more, the respective substituents may be the same or
different.
In the present specification, examples of the "heterocyclic group" (including
"heterocyclic group" of "optionally substituted heterocyclic group") include
(i) an aromatic
heterocyclic group, (ii) a non-aromatic heterocyclic group and (iii) a 7-to 10-
membered bridged
heterocyclic group, each containing, as a ring-constituting atom besides
carbon atom, 1 to 4
hetero atoms selected from a nitrogen atom, a sulfur atom and an oxygen atom.
[0016]
In the present specification, examples of the "aromatic heterocyclic group"
(including
"5- to 14-membered aromatic heterocyclic group") include a 5- to 14-membered
(preferably 5- to
10-membered) aromatic heterocyclic group containing, as a ring-constituting
atom besides
carbon atom, 1 to 4 hetero atoms selected from a nitrogen atom, a sulfur atom
and an oxygen
atom.
Preferable examples of the "aromatic heterocyclic group" include 5- or 6-
membered
monocyclic aromatic heterocyclic groups such as thienyl, fury', pyrrolyl,
imidazolyl, pyrazolyl,
thiazolyl, isothiazolyl, oxazolyl, isoxazolyl, pyridyl, pyrazinyl,
pyrimidinyl, pyridazinyl, 1,2,4-
oxadiazolyl, 1,3,4-oxadiazolyl, 1,2,4-thiadiazolyl, 1,3,4-thiadiazolyl,
triazolyl, tetrazolyl,
triazinyl and the like; and
8- to 14-membered fused polycyclic (preferably bi or tricyclic) aromatic
heterocyclic groups
such as benzothiophenyl, benzofuranyl, benzimidazolyl, benzoxazolyl,
benzisoxazolyl,
benzothiazolyl, benzisothiazolyl, benzotriazolyl, imidazopyridinyl,
thienopyridinyl,
furopyridinyl, pyrrolopyridinyl, pyrazolopyridinyl, oxazolopyridinyl,
thiazolopyridinyl,
imidazopyrazinyl, imidazopyrimidinyl, thienopyrimidinyl, furopyrimidinyl,
pyrrolopyrimidinyl,
pyrazolopyrimidinyl, oxazolopyrimidinyl, thiazolopyrimidinyl,
pyrazolotriazinyl, naphtho[2,3-
b]thienyl, phenoxathiinyl, indolyl, isoindolyl, 1H-indazolyl, purinyl,
isoquinolyl, quinolyl,
phthalazinyl, naphthyridinyl, quinoxalinyl, quinazolinyl, cinnolinyl,
carbazolyl, P-carbolinyl,
phenanthridinyl, acridinyl, phenazinyl, phenothiazinyl, phenoxazinyl and the
like.
[0017]
In the present specification, examples of the "non-aromatic heterocyclic
group"
(including "3- to 14-membered non-aromatic heterocyclic group") include a 3-
to 14-membered
13
CA 03021185 2018-10-16
A00039
(preferably 4- to 10-membered) non-aromatic heterocyclic group containing, as
a ring-
constituting atom besides carbon atom, 1 to 4 hetero atoms selected from a
nitrogen atom, a
sulfur atom and an oxygen atom.
Preferable examples of the "non-aromatic heterocyclic group" include 3- to 8-
membered
monocyclic non-aromatic heterocyclic groups such as aziridinyl, oxiranyl,
thiiranyl, azetidinyl,
oxetanyl, thietanyl, tetrahydrothienyl, tetrahydrofuranyl, pyrrolinyl,
pyrrolidinyl, imidazolinyl,
imidazolidinyl, oxazolinyl, oxazolidinyl, pyrazolinyl, pyrazolidinyl,
thiazolinyl, thiazolidinyl,
tetrahydroisothiazolyl, tetrahydrooxazolyl, tetrahydroisooxazolyl,
piperidinyl, piperazinyl,
tetrahydropyridinyl, dihydropyridinyl, dihydrothiopyranyl,
tetrahydropyrimidinyl,
tetrahydropyridazinyl, dihydropyranyl, tetrahydropyranyl,
tetrahydrothiopyranyl, morpholinyl,
thiomorpholinyl, azepanyl, diazepanyl, azepinyl, oxepanyl, azocanyl,
diazocanyl and the like;
and
9- to 14-membered fused polycyclic (preferably bi or tricyclic) non-aromatic
heterocyclic groups
such as dihydrobenzofuranyl, dihydrobenzimidazolyl, dihydrobenzoxazolyl,
dihydrobenzothiazolyl, dihydrobenzisothiazolyl, dihydronaphtho[2,3-b]thienyl,
tetrahydroisoquinolyl, tetrahydroquinolyl, 4H-quinolizinyl, indolinyl,
isoindolinyl,
tetrahydrothieno[2,3-c]pyridinyl, tetrahydrobenzazepinyl,
tetrahydroquinoxalinyl,
tetrahydrophenanthridinyl, hexahydrophenothiazinyl, hexahydrophenoxazinyl,
tetrahydrophthalazinyl, tetrahydronaphthyridinyl, tetrahydroquinazolinyl,
tetrahydrocinnolinyl,
tetrahydrocarbazolyl, tetrahydro-p-carbolinyl, tetrahydroacrydinyl,
tetrahydrophenazinyl,
tetrahydrothioxanthenyl, octahydroisoquinolyl and the like.
[0018]
In the present specification, preferable examples of the "7- to 10-membered
bridged
heterocyclic group" include quinuclidinyl and 7-azabicyclo[2.2.1]heptanyl.
In the present specification, examples of the "nitrogen-containing
heterocyclic group"
include a "heterocyclic group" containing at least one nitrogen atom as a ring-
constituting atom.
In the present specification, examples of the "optionally substituted
heterocyclic group"
include a heterocyclic group optionally having substituent(s) selected from
the aforementioned
substituent group A.
The number of the substituents in the "optionally substituted heterocyclic
group" is, for
example, 1 to 3. When the number of the substituents is two or more, the
respective
substituents may be the same or different.
[0019]
In the present specification, examples of the "acyl group" include a formyl
group, a
14
CA 03021185 2018-10-16
A00039
carboxy group, a carbamoyl group, a thiocarbamoyl group, a sulfino group, a
sulfo group, a
sulfamoyl group and a phosphono group, each optionally having "1 or 2
substituents selected
from a C1-6 alkyl group, a C2-6 alkenyl group, a C3-10 cycloalkyl group, a C3-
10 cycloalkenyl
group, a C6-I4 aryl group, a C7-16 aralkyl group, a 5- to 14-membered aromatic
heterocyclic group
and a 3-to 14-membered non-aromatic heterocyclic group, each of which
optionally has 1 to 3
substituents selected from a halogen atom, an optionally halogenated Ci_6
alkoxy group, a
hydroxy group, a nitro group, a cyano group, an amino group and a carbamoyl
group".
Examples of the "acyl group" also include a hydrocarbon-sulfonyl group, a
heterocyclylsulfonyl group, a hydrocarbon-sulfinyl group and a
heterocyclylsulfinyl group.
Here, the hydrocarbon-sulfonyl group means a hydrocarbon group-bonded sulfonyl
group, the heterocyclylsulfonyl group means a heterocyclic group-bonded
sulfonyl group, the
hydrocarbon-sulfinyl group means a hydrocarbon group-bonded sulfinyl group and
the
heterocyclylsulfinyl group means a heterocyclic group-bonded sulfinyl group.
Preferable examples of the "acyl group" include a formyl group, a carboxy
group, a C1_6
alkyl-carbonyl group, a C2_6 alkenyl-carbonyl group (e.g., crotonoyl), a C3_10
cycloalkyl-carbonyl
group (e.g., cyclobutanecarbonyl, cyclopentanecarbonyl, cyclohexanecarbonyl,
cycloheptanecarbonyl), a C3_10 cycloalkenyl-carbonyl group (e.g., 2-
cyclohexenecarbonyl), a C6_
14 aryl-carbonyl group, a C7_16 aralkyl-carbonyl group, a 5- to 14-membered
aromatic
heterocyclylcarbonyl group, a 3- to 14-membered non-aromatic
heterocyclylcarbonyl group, a
C1_6 alkoxy-carbonyl group, a C6_14 aryloxy-carbonyl group (e.g.,
phenyloxycarbonyl,
naphthyloxycarbonyl), a C7_16 aralkyloxy-carbonyl group (e.g.,
benzyloxycarbonyl,
phenethyloxycarbonyl), a carbamoyl group, a mono- or di-C1_6 alkyl-carbamoyl
group, a mono-
or di-C2_6 alkenyl-carbamoyl group (e.g., diallylcarbamoyl), a mono- or di-
C3_10 cycloalkyl-
carbamoyl group (e.g., cyclopropylcarbamoyl), a mono- or di-C6_14 aryl-
carbamoyl group (e.g.,
phenylcarbamoyl), a mono- or di-C7_16 aralkyl-carbamoyl group, a 5- to 14-
membered aromatic
heterocyclylcarbamoyl group (e.g., pyridylcarbamoyl), a thiocarbamoyl group, a
mono- or di-C1_
6 alkyl-thiocarbamoyl group (e.g., methylthiocarbamoyl, N-ethyl-N-
methylthiocarbamoyl), a
mono- or di-C2_6 alkenyl-thiocarbamoyl group (e.g., diallylthiocarbamoyl), a
mono- or di-C3_10
cycloalkyl-thiocarbamoyl group (e.g., cyclopropylthiocarbamoyl,
cyclohexylthiocarbamoyl), a
mono- or di-C6_14 aryl-thiocarbamoyl group (e.g., phenylthiocarbamoyl), a mono-
or di-C7_16
aralkyl-thiocarbamoyl group (e.g., benzylthiocarbamoyl,
phenethylthiocarbamoyl), a 5- to 14-
membered aromatic heterocyclylthiocarbamoyl group (e.g.,
pyridylthiocarbamoyl), a sulfino
group, a C1_6 alkylsulfinyl group (e.g., methylsulfinyl, ethylsulfinyl), a
sulfo group, a C1-6
alkylsulfonyl group, a C6_14 arylsulfonyl group, a phosphono group and a mono-
or di-C1_6
CA 03021185 2018-10-16
A00039
=
alkylphosphono group (e.g., dimethylphosphono, diethylphosphono,
diisopropylphosphono,
dibutylphosphono).
[0020]
In the present specification, examples of the "optionally substituted amino
group"
include an amino group optionally having "1 or 2 substituents selected from a
C1_6 alkyl group, a
C2_6 alkenyl group, a C3_10 cycloalkyl group, a C6_14 aryl group, a C7_16
arallcyl group, a C1_6 alkyl-
carbonyl group, a C6-14 aryl-carbonyl group, a C7-16 aralkyl-carbonyl group, a
5- to 14-membered
aromatic heterocyclylcarbonyl group, a 3- to 14-membered non-aromatic
heterocyclylcarbonyl
group, a C1_6 alkoxy-carbonyl group, a 5- to 14-membered aromatic heterocyclic
group, a
carbamoyl group, a mono- or di-C1_6 alkyl-carbamoyl group, a mono- or di-C7_16
aralkyl-
carbamoyl group, a Ci_6 alkylsulfonyl group and a C6.14 arylsulfonyl group,
each of which
optionally has 1 to 3 substituents selected from substituent group A".
Preferable examples of the optionally substituted amino group include an amino
group,
a mono- or di-(optionally halogenated Ci_6 alkyl)amino group (e.g.,
methylamino,
trifluoromethylamino, dimethylamino, ethylamino, diethylamino, propylamino,
dibutylamino), a
mono- or di-C2_6 alkenylamino group (e.g., diallylamino), a mono- or di-C3.10
cycloalkylamino
group (e.g., cyclopropylamino, cyclohexylamino), a mono- or di-C6_14 arylamino
group (e.g.,
phenylamino), a mono- or di-C7_16 aralkylamino group (e.g., benzylamino,
dibenzylamino), a
mono- or di-(optionally halogenated C1_6 alkyl)-carbonylamino group (e.g.,
acetylamino,
propionylamino), a mono- or di-C6_14 aryl-carbonylamino group (e.g.,
benzoylamino), a mono-
or di-C7_16 aralkyl-carbonylamino group (e.g., benzylcarbonylamino), a mono-
or di-5- to 14-
membered aromatic heterocyclylcarbonylamino group (e.g., nicotinoylamino,
isonicotinoylamino), a mono- or di-3- to 14-membered non-aromatic
heterocyclylcarbonylamino
group (e.g., piperidinylcarbonylamino), a mono- or di-C1_6 alkoxy-
carbonylamino group (e.g.,
tert-butoxycarbonylamino), a 5- to 14-membered aromatic heterocyclylamino
group (e.g.,
pyridylamino), a carbamoylamino group, a (mono- or di-C1_6 alkyl-
carbamoyl)amino group (e.g.,
methylcarbamoylamino), a (mono- or di-C7_16 aralkyl-carbamoyl)amino group
(e.g.,
benzylcarbamoylamino), a CI-6 alkylsulfonylamino group (e.g.,
methylsulfonylamino,
ethylsulfonylamino), a C6-14 arylsulfonylamino group (e.g.,
phenylsulfonylamino), a (C1-6
alkyl)(C1-6 alkyl-carbonyl)amino group (e.g., N-acetyl-N-methylamino) and a
(C1.6 alkyl)(C6_14
aryl-carbonyl)amino group (e.g., N-benzoyl-N-methylamino).
[0021]
In the present specification, examples of the "optionally substituted
carbamoyl group"
include a carbamoyl group optionally having "1 or 2 substituents selected from
a CI-6 alkyl
16
CA 03021185 2018-10-16
A00039
group, a C2_6 alkenyl group, a C3_10 cycloalkyl group, a C6_14 aryl group, a
C7_16 aralkyl group, a
C1-6 alkyl-carbonyl group, a C6-14 aryl-carbonyl group, a C7-16 aralkyl-
carbonyl group, a 5- to 14-
membered aromatic heterocyclylcarbonyl group, a 3- to 14-membered non-aromatic
heterocyclylcarbonyl group, a C1_6 alkoxy-carbonyl group, a 5- to 14-membered
aromatic
heterocyclic group, a carbamoyl group, a mono- or di-C1_6 alkyl-carbamoyl
group and a mono-
or di-C7_16 aralkyl-carbamoyl group, each of which optionally has 1 to 3
substituents selected
from substituent group A".
Preferable examples of the optionally substituted carbamoyl group include a
carbamoyl
group, a mono- or di-C1_6 alkyl-carbamoyl group, a mono- or di-C2_6 alkenyl-
carbamoyl group
(e.g., diallylcarbamoyl), a mono- or di-C3.10 cycloalkyl-carbamoyl group
(e.g.,
cyclopropylcarbamoyl, cyclohexylcarbamoyD, a mono- or di-C6_14 aryl-carbamoyl
group (e.g.,
phenylcarbamoyl), a mono- or di-C7_16 aralkyl-carbamoyl group, a mono- or di-
C1_6 alkyl-
carbonyl-carbamoyl group (e.g., acetylcarbamoyl, propionylcarbamoyl), a mono-
or di-C6_14
aryl-carbonyl-carbamoyl group (e.g., benzoylcarbamoyl) and a 5- to 14-membered
aromatic
heterocyclylcarbamoyl group (e.g., pyridylcarbamoyl).
[0022]
In the present specification, examples of the "optionally substituted
thiocarbamoyl
group" include a thiocarbamoyl group optionally having "1 or 2 substituents
selected from a CI-6
alkyl group, a C2_6 alkenyl group, a C3_10 cycloalkyl group, a C6.14 aryl
group, a C7_16 aralkyl
group, a C1_6 alkyl-carbonyl group, a C6-14 aryl-carbonyl group, a C7.16
aralkyl-carbonyl group, a
5- to 14-membered aromatic heterocyclylcarbonyl group, a 3- to 14-membered non-
aromatic
heterocyclylcarbonyl group, a C1_6 alkoxy-carbonyl group, a 5- to 14-membered
aromatic
heterocyclic group, a carbamoyl group, a mono- or di-C1.6 alkyl-carbamoyl
group and a mono-
or di-C7_16 aralkyl-carbamoyl group, each of which optionally has 1 to 3
substituents selected
from substituent group A".
Preferable examples of the optionally substituted thiocarbamoyl group include
a
thiocarbamoyl group, a mono- or di-C1_6 alkyl-thiocarbamoyl group (e.g.,
methylthiocarbamoyl,
ethylthiocarbamoyl, dimethylthiocarbamoyl, diethylthiocarbamoyl, N-ethyl-N-
methylthiocarbamoy1), a mono- or di-C2_6 alkenyl-thiocarbamoyl group (e.g.,
diallylthiocarbamoy1), a mono- or di-C3_10 cycloalkyl-thiocarbamoyl group
(e.g.,
cyclopropylthiocarbamoyl, cyclohexylthiocarbamoyl), a mono- or di-C6_14 aryl-
thiocarbamoyl
group (e.g., phenylthiocarbamoyl), a mono- or di-C7_16 aralkyl-thiocarbamoyl
group (e.g.,
benzylthiocarbamoyl, phenethylthiocarbamoyl), a mono- or di-C1.6 alkyl-
carbonyl-
thiocarbamoyl group (e.g., acetylthiocarbamoyl, propionylthiocarbamoyl), a
mono- or di-C6-14
17
CA 03021185 2018-10-16
A00039
aryl-carbonyl-thiocarbamoyl group (e.g., benzoylthiocarbamoyl) and a 5- to 14-
membered
aromatic heterocyclylthiocarbamoyl group (e.g., pyridylthiocarbamoyl).
[0023]
In the present specification, examples of the "optionally substituted
sulfamoyl group"
include a sulfamoyl group optionally having "1 or 2 substituents selected from
a C1_6 alkyl group,
a C2-6 alkenyl group, a C3-10 cycloalkyl group, a C6.14 aryl group, a C7-16
aralkyl group, a C1-6
alkyl-carbonyl group, a C6-14 aryl-carbonyl group, a C7-16 aralkyl-carbonyl
group, a 5- to 14-
membered aromatic heterocyclylcarbonyl group, a 3- to 14-membered non-aromatic
heterocyclylcarbonyl group, a Ci_6 alkoxy-carbonyl group, a 5- to 14-membered
aromatic
heterocyclic group, a carbamoyl group, a mono- or di-C1_6 alkyl-carbamoyl
group and a mono-
or di-C7_16 aralkyl-carbamoyl group, each of which optionally has 1 to 3
substituents selected
from substituent group A".
Preferable examples of the optionally substituted sulfamoyl group include a
sulfamoyl
group, a mono- or di-C1_6 alkyl-sulfamoyl group (e.g., methylsulfamoyl,
ethylsulfamoyl,
dimethylsulfamoyl, diethylsulfamoyl, N-ethyl-N-methylsulfamoyl), a mono- or di-
C2.6 alkenyl-
sulfamoyl group (e.g., diallylsulfamoy1), a mono- or di-C3_10 cycloalkyl-
sulfamoyl group (e.g.,
cyclopropylsulfamoyl, cyclohexylsulfamoyl), a mono- or di-C6_14 aryl-sulfamoyl
group (e.g.,
phenylsulfamoyl), a mono- or di-C7_16 aralkyl-sulfamoyl group (e.g.,
benzylsulfamoyl,
phenethylsulfamoyl), a mono- or di-C1_6 alkyl-carbonyl-sulfamoyl group (e.g.,
acetylsulfamoyl,
propionylsulfamoyl), a mono- or di-C6.14 aryl-carbonyl-sulfamoyl group (e.g.,
benzoylsulfamoyl)
and a 5- to 14-membered aromatic heterocyclylsulfamoyl group (e.g.,
pyridylsulfamoyl).
[0024]
In the present specification, examples of the "optionally substituted hydroxy
group"
include a hydroxyl group optionally having "a substituent selected from a C1-6
alkyl group, a C2-6
alkenyl group, a C3_10 cycloalkyl group, a C6i4 aryl group, a C7_16 aralkyl
group, a C1.6 alkyl-
carbonyl group, a C6-14 aryl-carbonyl group, a C7-16 aralkyl-carbonyl group, a
5- to 14-membered
aromatic heterocyclylcarbonyl group, a 3- to 14-membered non-aromatic
heterocyclylcarbonyl
group, a C1-6 alkoxy-carbonyl group, a 5- to 14-membered aromatic heterocyclic
group, a
carbamoyl group, a mono- or di-C1_6 alkyl-carbamoyl group, a mono- or di-C7_16
aralkyl-
carbamoyl group, a C1_6 alkylsulfonyl group and a C6-14 arylsulfonyl group,
each of which
optionally has 1 to 3 substituents selected from substituent group A".
Preferable examples of the optionally substituted hydroxy group include a
hydroxy
group, a C1.6 alkoxy group, a C2_6 alkenyloxy group (e.g., allyloxy, 2-
butenyloxy, 2-pentenyloxy,
3-hexenyloxy), a C3_10 cycloalkyloxy group (e.g., cyclohexyloxy), a C6-14
aryloxy group (e.g.,
18
CA 03021185 2018-10-16
A00039
phenoxy, naphthyloxy), a C7-16 aralkyloxy group (e.g., benzyloxy,
phenethyloxy), a C1..6 alkyl-
carbonyloxy group (e.g., acetyloxy, propionyloxy, butyryloxy, isobutyryloxy,
pivaloyloxy), a C6-
14 aryl-carbonyloxy group (e.g., benzoyloxy), a C7.16 aralkyl-carbonyloxy
group (e.g.,
benzylcarbonyloxy), a 5- to 14-membered aromatic heterocyclylcarbonyloxy group
(e.g.,
nicotinoyloxy), a 3- to 14-membered non-aromatic heterocyclylcarbonyloxy group
(e.g.,
piperidinylcarbonyloxy), a C1-6 alkoxy-carbonyloxy group (e.g., tert-
butoxycarbonyloxy), a 5- to
14-membered aromatic heterocyclyloxy group (e.g., pyridyloxy), a carbamoyloxy
group, a C1-6
alkyl-carbamoyloxy group (e.g., methylcarbamoyloxy), a C7_16 aralkyl-
carbamoyloxy group
(e.g., benzylcarbamoyloxy), a C1-6 alkylsulfonyloxy group (e.g.,
methylsulfonyloxy,
ethylsulfonyloxy) and a C6_14 arylsulfonyloxy group (e.g., phenylsulfonyloxy).
[0025]
In the present specification, examples of the "optionally substituted sulfanyl
group"
include a sulfanyl group optionally having "a substituent selected from a Ci_6
alkyl group, a C2_6
alkenyl group, a C3_10 cycloalkyl group, a C6_14 aryl group, a C7_16 aralkyl
group, a C1..6 alkyl-
carbonyl group, a C6-14 aryl-carbonyl group and a 5- to 14-membered aromatic
heterocyclic
group, each of which optionally has 1 to 3 substituents selected from
substituent group A" and a
halogenated sulfanyl group.
Preferable examples of the optionally substituted sulfanyl group include a
sulfanyl (-
SH) group, a C1.6 alkylthio group, a C2-6 alkenylthio group (e.g., allylthio,
2-butenylthio, 2-
pentenylthio, 3-hexenylthio), a C3_10 cycloalkylthio group (e.g.,
cyclohexylthio), a C6_14 arylthio
group (e.g., phenylthio, naphthylthio), a C7_16 arallcylthio group (e.g.,
benzylthio, phenethylthio),
a C1_6 alkyl-carbonylthio group (e.g., acetylthio, propionylthio, butyrylthio,
isobutyrylthio,
pivaloylthio), a C6-14 aryl-carbonylthio group (e.g., benzoylthio), a 5- to 14-
membered aromatic
heterocyclylthio group (e.g., pyridylthio) and a halogenated thio group (e.g.,
pentafluorothio).
[0026]
In the present specification, examples of the "optionally substituted silyl
group" include
a silyl group optionally having "1 to 3 substituents selected from a C1_6
alkyl group, a C2-6
alkenyl group, a C3-10 cycloallcyl group, a C6-14 aryl group and a C7-16
aralkyl group, each of
which optionally has 1 to 3 substituents selected from substituent group A".
Preferable examples of the optionally substituted silyl group include a tri-
C1_6 alkylsilyl
group (e.g., trimethylsilyl, tert-butyl(dimethyl)sily1).
In the present specification, examples of the "C1_6 alkylene group" include -
CH2-, -
(CH2)2-, -(CH2)3-, -(CH2)4-, -(CH2)5-, -(CH2)6-, -CH(CH3)-, -C(CH3)2-, -
CH(C2H5)-, -CH(C3H7)-,
-CH(CH(CH3)2)-, -(CH(CH3))2-, -CH2-CH(CH3)-, -CH(CH3)-CH2-, -CH2-CH2-C(CH3)2-,
-
19
CA 03021185 2018-10-16
A00039
C(CH3)2-C142-CH2-, -CH2-CH2-CH2-C(CH3)2- and -C(CH3)2-CH2-CH2-CH2-.
In the present specification, examples of the "C2_6 alkenylene group" include -
CH=CH-,
-CH2-CH=CH-, -CH=CH-CH2-, -C(CH3)2-CH=CH-, -CH=CH-C(CH3)2-, -CH2-CH=CH-CH2-, -
CH2-CH2-CH=CH-, -CH=CH-CH2-CH2-, -CH=CH-CH=CH-, -CH=CH-CH2-CH2-CH2- and -
CH2-CH2-CH2-CH=CH-.
In the present specification, examples of the "C2_6 alkynylene group" include
-
CH2-CC-, -C--=C-C(CH3)2-, -
-C---C-CH2-CH2-CH2- and -CH2-CH2-CH2-C-C-.
[0027]
In the present specification, examples of the "hydrocarbon ring" include a C6-
14 aromatic
hydrocarbon ring, C3-10 cycloalkane and C3_10 cycloalkene.
In the present specification, examples of the "C6_14 aromatic hydrocarbon
ring" include
benzene and naphthalene.
In the present specification, examples of the "C3_10 cycloalkane" include
cyclopropane,
cyclobutane, cyclopentane, cyclohexane, cycloheptane and cyclooctane.
In the present specification, examples of the "C3_10 cycloalkene" include
cyclopropene,
cyclobutene, cyclopentene, cyclohexene, cycloheptene and cyclooctene.
In the present specification, examples of the "heterocycle" include an
aromatic
heterocycle and a non-aromatic heterocycle, each containing, as a ring-
constituting atom besides
carbon atom, 1 to 4 hetero atoms selected from a nitrogen atom, a sulfur atom
and an oxygen
atom.
[0028]
In the present specification, examples of the "aromatic heterocycle" include a
5- to 14-
membered (preferably 5- to 10-membered) aromatic heterocycle containing, as a
ring-
constituting atom besides carbon atom, 1 to 4 hetero atoms selected from a
nitrogen atom, a
sulfur atom and an oxygen atom. Preferable examples of the "aromatic
heterocycle" include 5-
or 6-membered monocyclic aromatic heterocycles such as thiophene, furan,
pyrrole, imidazole,
pyrazole, thiazole, isothiazole, oxazole, isoxazole, pyridine, pyrazine,
pyrimidine, pyridazine,
1,2,4-oxadiazole, 1,3,4-oxadiazole, 1,2,4-thiadiazole, 1,3,4-thiadiazole,
triazole, tetrazole,
triazine and the like; and
8- to 14-membered fused polycyclic (preferably bi or tricyclic) aromatic
heterocycles such as
benzothiophene, benzofuran, benzimidazole, benzoxazole, benzisoxazole,
benzothiazole,
benzisothiazole, benzotriazole, imidazopyridine, thienopyridine, furopyridine,
pyrrolopyridine,
pyrazolopyridine, oxazolopyridine, thiazolopyridine, imidazopyrazine,
imidazopyrimidine,
CA 03021185 2018-10-16
A00039
thienopyrimidine, furopyrimidine, pyrrolopyrirnidine, pyrazolopyrimidine,
oxazolopyrimidine,
thiazolopyrimidine, pyrazolopyrimidine, pyrazolotriazine, naphtho[2,3-
b]thiophene,
phenoxathiin, indole, isoindole, 1H-indazole, purine, isoquinoline, quinoline,
phthalazine,
naphthyridine, quinoxaline, quinazoline, cinnoline, carbazole, f3-carboline,
phenanthridine,
acridine, phenazine, phenothiazine, phenoxazine and the like.
[0029]
In the present specification, examples of the "non-aromatic heterocycle"
include a 3- to
14-membered (preferably 4- to 10-membered) non-aromatic heterocycle
containing, as a ring-
constituting atom besides carbon atom, 1 to 4 hetero atoms selected from a
nitrogen atom, a
sulfur atom and an oxygen atom. Preferable examples of the "non-aromatic
heterocycle"
include 3- to 8-membered monocyclic non-aromatic heterocycles such as
aziridine, oxirane,
thiirane, azetidine, oxetane, thietane, tetrahydrothiophene, tetrahydrofuran,
pyrroline,
pyrrolidine, imidazoline, imidazolidine, oxazoline, oxazolidine, pyrazoline,
pyrazolidine,
thiazoline, thiazolidine, tetrahydroisothiazole, tetrahydrooxazole,
tetrahydroisoxazole,
piperidine, piperazine, tetrahydropyridine, dihydropyridine, dihydrothiopyran,
tetrahydropyrimidine, tetrahydropyridazine, dihydropyran, tetrahydropyran,
tetrahydrothiopyran,
morpholine, thiomorpholine, azepanine, diazepane, azepine, azocane, diazocane,
oxepane and
the like; and
9- to 14-membered fused polycyclic (preferably bi or tricyclic) non-aromatic
heterocycles such
as dihydrobenzofuran, dihydrobenzimidazole, dihydrobenzoxazole,
dihydrobenzothiazole,
dihydrobenzisothiazole, dihydronaphtho[2,3-b]thiophene,
tetrahydroisoquinoline,
tetrahydroquinoline, 4H-quinolizine, indoline, isoindoline,
tetrahydrothieno[2,3-c]pyridine,
tetrahydrobenzazepine, tetrahydroquinoxaline, tetrahydrophenanthridine,
hexahydrophenothiazine, hexahydrophenoxazine, tetrahydrophthalazine,
tetrahydronaphthyridine, tetrahydroquinazoline, tetrahydrocinnoline,
tetrahydrocarbazole,
tetrahydro-P-carboline, tetrahydroacridine, tetrahydrophenazine,
tetrahydrothioxanthene,
octahydroisoquinoline and the like.
In the present specification, examples of the "nitrogen-containing
heterocycle" include a
"heterocycle" containing at least one nitrogen atom as a ring-constituting
atom.
[0030]
In the present specification, "a group via a carbon atom" means a group having
an
atomic bonding on the carbon atom; examples include a cyano group, a
hydrocarbon group
optionally having substituent(s), an acyl group, an optionally esterified
carboxyl group, an
imidoyl group optionally having substituent(s), an amidino group optionally
having
21
CA 03021185 2018-10-16
A00039
substituent(s), a carbamoyl group optionally having substituent(s), a
thiocarbamoyl group
optionally having substituent(s), a heterocyclic group via a carbon atom,
optionally having
substituent(s), etc.
Examples of "a heterocyclic group via a carbon atom" in the "a heterocyclic
group via a
carbon atom, optionally having substituent(s)" include a heterocyclic group
having an atomic
bonding on the carbon atom in the "heterocyclic group".
Examples of "an acyl group via a carbon atom" include an acyl group having an
atomic
bonding on the carbon atom in the "acyl group"; examples include a formyl
group, a carboxyl
group, a carbamoyl group, a thiocarbamoyl group, etc, each optionally having
"1 or 2
substituents selected from a C1_6 alkyl group, a C2_6 alkenyl group, a C3_10
cycloalkyl group, a C3_
10 cycloalkenyl group, a C614 aryl group, a C7-16 aralkyl group, a 5- to 14-
membered aromatic
heterocyclic group and a 3- to 14-membered non-aromatic heterocyclic group,
each of which
optionally has 1 to 3 substituents selected from a halogen atom, an optionally
halogenated C1-6
alkoxy group, a hydroxy group, a nitro group, a cyano group, an amino group
and a carbamoyl
group".
[0031]
In the present specification, "a group via a nitrogen atom" means a group
having an
atomic bonding on the nitrogen atom; examples include heterocyclic groups via
a nitrogen atom
(1)a nitro group, (2)an amino group optionally having 1 or 2 of the above
described "a group via
a carbon atom", (3)an amino group optionally having 1 or 2 "a group via a
nitrogen atom", (4) a
heterocyclic group via a nitrogen atom, optionally having a substituent.
[0032]
In the present specification, "a group via an oxygen atom" means a group
having an
atomic bonding on the oxygen atom; examples include a hydroxy group optionally
having one
"group via a carbon atom" described above.
[0033]
In the present specification, "a group via a sulfur atom" means a group having
an
atomic bonding on the sulfur atom; examples include a sulfanyl group
optionally having one
"group via a carbon atom" or "group via a nitrogen atom" described above,
optionally being
oxidized.
[0034]
Hereinafter, the definition of each symbol in the formula (I) will be
described in detail.
[0035]
R' is preferably a substituent selected from
22
CA 03021185 2018-10-16
A00039
(I) a C1,6 alkyl group (particularly methyl or ethyl)optionally having 1 to 5
substituents
each selected from
(a) a halogen atom (particularly fluorine atom),
(b) a hydroxy group,
(c) a cyano group,
(d) a C1-6 alkoxy group (particularly methoxy or ethoxy) optionally having 1
to 3
substituents each selected from
(i) a halogen atom (particularly fluorine atom), and
(ii) a hydroxy group, and
(e) a three- to eight-membered nonaromatic heterocyclic group (particularly
azetidinyl)
having 1 to 3 halogen atoms (particularly fluorine atoms);
(II) a three- to eight-membered nonaromatic heterocyclic group (particularly
azetidinyl
or pyrrolidinyl) optionally having 1 to 5 (particularly 1 or 2) substituents
each selected from
(a) a C1-6 alkoxy group (particularly methoxy)
(b) a halogen atom (particularly fluorine), and
(c) a hydroxy group;
(III) a five- to fourteen-membered aromatic heterocyclic group (particularly
five- or six-
membered monocyclic aromatic heterocyclic group (preferably pyrazolyl or
oxazolyI))
optionally having 1 to 3 (particularly 1) optionally halogenated C1_6 alkyl
groups (particularly
methyl or difluoromethyl);
(IV) a C1_6 alkoxy group (particularly methoxy or ethoxy) optionally having 1
to 5
(particularly 1) substituents each selected from
(a) a hydroxy group,
(b) a three- to eight-membered nonaromatic heterocyclic group (particularly
tetrahydrofuranyl), and
(c) a five- or six-membered monocyclic aromatic heterocyclic group optionally
having 1
to 3 (particularly 1) optionally halogenated CI-6 alkyl groups (particularly
methyl or
difluoromethyl);
(V) an optionally halogenated C3_10 cycloalkyl group (particularly cyclopropyl
or
difluorocyclobutyl); and
(VI) an amino group optionally monosubstituted or disubstituted with a Ci_6
alkyl group
(particularly methyl, ethyl or isobutyl) optionally having 1 to 5
(particularly 1 to 3) substituents
each selected from
(a) (i) a halogen atom (particularly fluorine atom),
23
CA 03021185 2018-10-16
A00039
(ii) a hydroxy group, and
(iii) a C1,6 alkoxy group (particularly methoxy).
[0036]
RI is more preferably a substituent selected from
(I) a C1-6 alkyl group (particularly methyl) optionally having 1 to 3
substituents each
selected from
(a) a C1_6 alkoxy group (particularly methoxy) and
(b) a hydroxy group;
(II) a three- to fourteen-membered nonaromatic heterocyclic group
(particularly
azetidinyl);
(III) a C1-6 alkoxy group (particularly methoxy or ethoxy); and
(IV) an amino group.
[0037]
R1 is a C1-6 alkyl group (particularly methyl) optionally having 1 to 3
(preferably 1)
hydroxy groups.
[0038]
R2 is preferably
(I) a Ci_6 alkyl group (particularly methyl) optionally having 1 to 3
(particularly 1) five-
to fourteen-membered aromatic heterocyclic groups (particularly five- or six-
membered
monocyclic aromatic heterocyclic groups (preferably oxadiazolyl (preferably
1,2,4-oxadiazoly1
or 1,3,4-oxadiazoly1), thiadiazolyl (preferably 1,2,4-thiadiazolyl, 1,3,4-
thiadiazoly1 or 1,2,3-
thiadiazolyl), isoxazolyl, isothiazolyl, thiazolyl, triazolyl (preferably
1,2,3-triazoly1 or 1,2,4-
triazolyl), pyrazinyl, pyrazolyl, pyridyl, pyrimidinyl, oxazolyl or furyI))
optionally having 1 to 3
(particularly 1 or 2) substituents each selected from
(a) a halogen atom (particularly fluorine or chlorine),
(b) a C1-6 alkyl group (particularly methyl, ethyl, isobutyl, isopropyl or
propyl)
optionally having 1 to 7 (particularly 1 to 5) substituents each selected from
(i) a halogen atom (particularly fluorine atom),
(ii) a hydroxy group, and
(iii) a C1-6 alkoxy group (particularly methoxy),
(c) an optionally halogenated C3-10 cycloalkyl group (particularly, 2,2-
difluorocyclopropyl, cyclobutyl, cyclopropyl or difluorocyclobutyl
(particularly 3,3-
difluorocyclobutyl), fluorocyclobutyl (particularly 1-fluorocyclobutyl),
fluorocyclopropyl),
(d) a C1.6 alkoxy group (particularly methoxy),
24
CA 03021185 2018-10-16
A00039
(e) a C1-6 alkyl group-C3.10 cycloalkyl group (particularly methyl-
cyclopropyl),
(f) a C1_6 alkyl group-three- to eight-membered monocyclic nonaromatic
heterocyclic
group (particularly methyl-oxetanyl), and
(g) oxo group; or
(II) a C7-16 aralkyl group (particularly benzyl) optionally having 1 to 3
substituents each
selected from
(a) a C1_6 alkyl group (methyl or isopropyl) optionally having 1 to 3
substituents each
selected from
(i) a halogen atom (particularly fluorine atom), and
(ii) a hydroxyl group,
(b) a C1-6 alkoxy-carbonyl group (particularly methoxycarbonyl),
(c) a halogen atom (fluorine or chlorine)
(d) a Ci_6 alkoxy group (particularly methoxy or ethoxy) optionally having 1
to 3
substituents each selected from
(i) a halogen atom (particularly fluorine atom), and
(ii) a hydroxyl group
(e) a mono-or di-C1_6 alkyl-carbamoyl group (particularly methylcarbamoyl),
(f) a cyano group,
(g) an amino group optionally monosubstituted or disubstituted (particularly
monosubstituted) with a substituent selected from
(i) a Ci_6 alkyl group, and
(ii) a C1_6 alkoxy-carbonyl group (particularly tert-butoxy-carbonyl),
(h) a nitro group, and
(i) a carboxy group.
[0039]
R2 is more preferably
(I) a C1-6 alkyl group (particularly methyl) optionally having 1 to 3
(preferably 1) five-
or six-membered monocyclic aromatic heterocyclic groups (particularly
oxadiazolyl (preferably
1,2,4-oxadiazoly1 or 1,3,4-oxadiazoly1), thiadiazolyl (preferably 1,2,4-
thiadiazolyl, 1,3,4-
thiadiazolyl or 1,2,3-thiadiazolyl), isoxazolyl, thiazolyl, triazolyl
(preferably 1,2,3-triazoly1 or
1,2,4-triazoly1), pyrazinyl, pyrazolyl, pyridyl, pyrimidinyl or oxazoly1)
optionally having 1 to 3
(preferably 1) substituents each selected from
(a) a halogen atom (particularly fluorine),
(b) an optionally halogenated C1_6 alkyl group (particularly methyl,
difluoromethyl,
CA 03021185 2018-10-16
A00039
trifluoromethyl, ethyl, difluoroethyl (particularly 1,1-difluoroethyl), 2,2,2-
trifluoroethyl,
isopropyl, 1-fluoroisopropyl or 1-fluoroethyl), and
(c) an optionally halogenated C3-10 cycloalkyl group (particularly, 2,2-
difluorocyclopropyl, cyclobutyl, cyclopropyl or difluorocyclobutyl
(particularly 3,3-
difluorocyclobutyl), fluorocyclobutyl (particularly 1-fluorocyclobutyl),
fluorocyclopropyl); or
(II) a C7-16 aralkyl group (particularly benzyl) optionally having 1 to 3
(preferably 1 or
2) substituents each selected from
(a) a halogen atom (particularly fluorine or chlorine)
(b) an optionally halogenated C1_6 alkyl group (particularly trifluoromethyl),
and
(c) an optionally halogenated Ci_6 alkoxy group (particularly difluoromethoxy
or
trifluoromethoxy).
[0040]
R2 is still more preferably
(I) a Ci_6 alkyl group (particularly methyl) optionally having 1 to 3
(preferably 1) five-
or six-membered monocyclic aromatic heterocyclic groups (particularly
oxadiazolyl (preferably
1,2,4-oxadiazolyl or 1,3,4-oxadiazolyl), thiadiazolyl (preferably 1,2,4-
thiadiazoly1), isoxazoly1)
optionally having 1 to 3 (preferably 1) optionally halogenated C1_6 alkyl
groups (particularly
methyl, difluoromethyl, difluoroethyl, fluoroisopropyl or fluoroethyl); or
(II) an optionally halogenated C7_16 aralkyl (particularly difluorobenzyl
(preferably 3,5-
difluorobenzyl)).
R2 is even more preferably
a C1_6 alkyl group (particularly methyl) having 1 to 3 (preferably 1) five- or
six-
membered monocyclic aromatic heterocyclic groups (particularly oxadiazolyl
(preferably 1,2,4-
oxadiazolyl or 1,3,4-oxadiazoly1), thiadiazolyl (preferably 1,2,4-
thiadiazoly1), isoxazoly1) having
1 to 3 (preferably 1) halogenated Ci_6 alkyl groups (particularly methyl,
difluoromethyl,
difluoroethyl, fluoroisopropyl or fluoroethyl).
[0041]
R6 is preferably a hydrogen atom.
[0042]
R7 is preferably a hydrogen atom.
[0043]
R6 and R7 are preferably each a hydrogen atom.
[0044]
Ring A is preferably a bicyclic aromatic heterocyclic ring selected from the
formulas
26
CA 03021185 2018-10-16
A00039
(1), (2), (3) and (4).
[0045]
Ring A is more preferably a bicyclic aromatic heterocyclic ring represented by
the
formula (1).
[0046]
As preferable examples of R3, R4 and R5, in particular,
R3 is a hydrogen atom;
R4 is (I) a hydrogen atom,
(II) a halogen atom (particularly chlorine atom),
(III) an optionally halogenated C1..6 alkoxy group (particularly methoxy,
fluoromethoxy,
difluoromethoxy or ethoxy), or
(IV) an amino group optionally monosubstituted or disubstituted with a C1_6
alkyl group
(particularly methyl);
R5 is (I) a hydrogen atom,
(II) a halogen atom (particularly chlorine),
(III) a cyano group,
(IV) a Ci_6 alkyl group (particularly methyl, ethyl or isopropyl) optionally
having l to 5
(particularly I or 2) substituents each selected from
(a) a halogen atom (particularly fluorine),
(b) a CI-6 alkoxy group (particularly methoxy),
(c) a C1_6 alkoxy-C1_6 alkoxy group, and
(e) a C1_6 alkyl-carbonyloxy group (particularly, 2,2-dimethylpropanoyloxy),
(V) a C1-6 alkoxy group (particularly methoxy),
(VI) a C2_6 alkenyl group (particular 1-propenyl or ethenyl) optionally having
1 to 3
(particularly 1) C1-6 alkoxy groups (particularly methoxy),
(VII) a C2_6 alkynyl group (particularly ethynyl),
(VIII) a cyano-C6_14 aryl group (particularly cyano-phenyl),
(IX) a five- to fourteen-membered aromatic heterocyclic group (particularly
five- or six-
membered monocyclic aromatic heterocyclic group (preferably pyrazolyl,
isoxazolyl or pyridyl))
optionally having I or 2 C1-6 alkyl groups (particularly methyl or ethyl)
optionally having 1 to 3
substituents each selected from
(a) a halogen atom (particularly fluorine atom),
(b) a five- to six-membered monocyclic aromatic heterocyclic group
(particularly
pyridyl), and
27
CA 03021185 2018-10-16
A00039
(c) a three- to eight-membered monocyclic nonaromatic heterocyclic group
(particularly
morpholinyl),
(X) a Ci.6 alkyl-carbonyl group (particularly acetyl),
(XI) a hydroxy group,
(XII) a Ci_6 alkyl-five- to fourteen-membered aromatic heterocyclic oxy group
(particularly methylpyrazolyloxy),
(XIII) a three- to eight-membered monocyclic nonaromatic heterocyclic group
(particularly pyrrolidinyl, dihydropyranyl (preferably 3,6-dihydropyranyl),
azetidinyl, oxetanyl
or tetrahydropyranyl) optionally having 1 to 3 substituents each selected from
(a) a halogen atom (particularly fluorine),
(b) a C1_6 alkoxy group (particularly methoxy), and
(c) an oxo group, or
(XIV) an amino group (particularly 2,2-difluoroethylamino) optionally
monosubstituted
or disubstituted with an optionally halogenated C1_6 alkyl.
[0047]
As preferable examples of R3, R4 and R5,
1) ring A is a bicyclic aromatic heterocyclic ring of the formula (1),
R3 is a hydrogen atom,
R4 is a hydrogen atom or a C1-6 alkoxy group (particularly methoxy or ethoxy),
and
R5 is a hydrogen atom; or
2) ring A is a bicyclic aromatic heterocyclic ring of the formula (2),
R3 is a hydrogen atom, and
R5 is
(I) a hydrogen atom,
(II) a halogen atom (particularly chlorine),
(III) a cyano group,
(IV) a C1_6 alkyl group (particularly methyl, ethyl or isopropyl) optionally
having 1 to 5
(particularly 1 or 2) substituents each selected from
(a) a halogen atom (particularly fluorine),
(b) a C1_6 alkoxy group (particularly methoxy),
(c) a C1_6 alkoxy-C1_6 alkoxy group (particularly methoxy-ethoxy),
(d) a hydroxy group, and
(e) a C1-6 alkyl-carbonyloxy group (particularly, 2,2-dimethylpropanoyloxY),
(V) a C1-6 alkoxy group (particularly methoxy),
28
CA 03021185 2018-10-16
A00039
(VI) a C2-6 alkenyl group (particular 1-propenyl or ethenyl) optionally having
1 to 3
(particularly 1) C1.6 alkoxy groups (particularly methoxy),
(VII) a C2-6 alkynyl group (particularly ethynyl),
(VIII) a cyano-C6_14 aryl group (particularly cyano-phenyl),
(IX) a five- to fourteen-membered aromatic heterocyclic group (particularly
five- or six-
membered monocyclic aromatic heterocyclic group (preferably pyrazolyl,
isoxazolyl or pyridyl))
optionally having 1 or 2 C1..6 alkyl groups (particularly methyl or ethyl)
optionally having 1 to 3
substituents each selected from
(a) a halogen atom (particularly fluorine atom),
(b) a five- to six-membered monocyclic aromatic heterocyclic group
(particularly
pyridyl), and
(c) a three- to eight-membered monocyclic nonaromatic heterocyclic group
(particularly
morpholinyl),
(X) a C1-6 alkyl-carbonyl group (particularly acetyl),
(XI) a hydroxy group,
(XII) a C1..6 alkyl-five- to fourteen-membered aromatic heterocyclic oxy group
(particularly methylpyrazolyloxy),
(XIII) a three- to eight-membered monocyclic nonaromatic heterocyclic group
(particularly pyrrolidinyl, dihydropyranyl (preferably 3,6-dihydropyranyl),
azetidinyl, oxetanyl
or tetrahydropyranyl) optionally having 1 to 3 substituents each selected from
(a) a halogen atom (particularly fluorine),
(b) a C1.6 alkoxy group (particularly methoxy), and
(c) an oxo group, or
(XIV) an amino group (particularly 2,2-difluoroethylamino) optionally
monosubstituted
.. or disubstituted with an optionally halogenated C1.6 alkyl; or
3) ring A is a bicyclic aromatic heterocyclic ring of the formula (3),
R3 is a hydrogen atom,
R4 is
(I) a hydrogen atom,
(II) a halogen atom (particularly chlorine atom),
(III) an optionally halogenated C1.6 alkoxy group (particularly methoxy,
fluoromethoxy
or difluoromethoxy), or
(IV) an amino group optionally monosubstituted or disubstituted with a C1_6
alkyl group
(particularly methyl); and
29
CA 03021185 2018-10-16
A00039
R5 is a hydrogen atom; or
4) ring A is a bicyclic aromatic heterocyclic ring of the formula (4),
R3 is a hydrogen atom,
R4 is a hydrogen atom or a CI-6 alkoxy group (particularly methoxy), and
R5 is a hydrogen atom.
[0048]
As more preferable examples of R3, R4 and R5, in particular,
R3 is a hydrogen atom;
R4 is (a) an amino group optionally monosubstituted or disubstituted with a CI-
6 alkyl
group (particularly methyl), or
(b) a Ch6 alkoxy group (particularly methoxy); and
R5 is (a) a C1_6 alkoxy group (particularly methoxy), or
(b) a C1_6 alkyl group (particularly ethyl) optionally having 1 to 3 hydroxy
groups.
[0049]
As more preferable examples of R3, R4 and R5,
1) ring A is a bicyclic aromatic heterocyclic ring of the formula (1),
R3 is a hydrogen atom,
R4 is a Ci_6 alkoxy group (particularly methoxy), and
R5 is a hydrogen atom; or
2) ring A is a bicyclic aromatic heterocyclic ring of the formula (2),
R3 is a hydrogen atom, and
R5 is (a) a C1.6 alkoxy group (particularly methoxy), or
(b) a C1_6 alkyl group (particularly ethyl) optionally having 1 to 3
(preferably 1)
hydroxy groups; or
3) ring A is a bicyclic aromatic heterocyclic ring of the formula (3),
R3 is a hydrogen atom,
R4 is (a) an amino group optionally monosubstituted or disubstituted with a
Ci_6 alkyl
group (preferably methyl), or
(b) a C1_6 alkoxy group (particularly methoxy), and
R5 is a hydrogen atom; or
4) ring A is a bicyclic aromatic heterocyclic ring of the formula (4),
R3 is a hydrogen atom,
R4 is a hydrogen atom or a C1_6 alkoxy group (particularly methoxy), and
R5 is a hydrogen atom.
CA 03021185 2018-10-16
A00039
[0050]
More preferably, ring A is a bicyclic aromatic heterocyclic ring represented
by the
following formula (1)
[Chemical formula 3]
R4
N y X
R3
(1)
X is N,
R3 is a hydrogen atom, and
R4 is a CI-6 alkoxy group (particularly methoxy).
[0051]
Preferred specific examples of the compound (I) include the following.
Compound (A) which is a compound of the formula (I) or a salt thereof, wherein
R1 is
(I) a Ci_6 alkyl group (particularly methyl or ethyl)optionally having 1 to 5
substituents
each selected from
(a) a halogen atom (particularly fluorine atom),
(b) a hydroxy group,
(c) a cyano group,
(d) a C1_6 alkoxy group (particularly methoxy or ethoxy) optionally having 1
to 3
substituents each selected from
(i) a halogen atom (particularly fluorine atom), and
(ii) a hydroxy group, and
(e) a three- to eight-membered nonaromatic heterocyclic group (particularly
azetidinyl)
having 1 to 3 halogen atoms (particularly fluorine atoms);
(II) a three- to eight-membered nonaromatic heterocyclic group (particularly
azetidinyl
or pyrrolidinyl) optionally having 1 to 5 (particularly 1 or 2) substituents
each selected from
(a) a Ci_6 alkoxy group (particularly methoxy)
(b) a halogen atom (particularly fluorine), and
31
CA 03021185 2018-10-16
A00039
(c) a hydroxy group;
(III) a five- to fourteen-membered aromatic heterocyclic group (particularly
five- or six-
membered monocyclic aromatic heterocyclic group (preferably pyrazolyl or
oxazoly1))
optionally having 1 to 3 (particularly 1) optionally halogenated C 1_6 alkyl
groups (particularly
methyl or difluoromethyl);
(IV) a C1.6 alkoxy group (particularly methoxy or ethoxy) optionally having 1
to 5
(particularly 1) substituents each selected from
(a) a hydroxy group,
(b) a three- to eight-membered nonaromatic heterocyclic group (particularly
tetrahydrofuranyl), and
(c) a five- or six-membered monocyclic aromatic heterocyclic group optionally
having 1
to 3 (particularly 1) optionally halogenated C1_6 alkyl groups (particularly
methyl or
difluoromethyl);
(V) an optionally halogenated C3_10 cycloalkyl group (particularly cyclopropyl
or
difluorocyclobutyl); or
(VI) an amino group optionally monosubstituted or disubstituted with a C1_6
alkyl group
(particularly methyl, ethyl or isobutyl) optionally having 1 to 5
(particularly 1 to 3) substituents
each selected from
(a) (i) a halogen atom (particularly fluorine atom),
(ii) a hydroxy group, and
(iii) a C1.6 alkoxy group (particularly methoxy);
R2 is
(I) a C1_6 alkyl group (particularly methyl) optionally having Ito 3
(particularly 1) five-
to fourteen-membered aromatic heterocyclic groups (particularly five- or six-
membered
monocyclic aromatic heterocyclic groups (preferably oxadiazolyl (preferably
1,2,4-oxadiazoly1
or 1,3,4-oxadiazoly1), thiadiazolyl (preferably 1,2,4-thiadiazolyl, 1,3,4-
thiadiazoly1 or 1,2,3-
thiadiazolyl), isoxazolyl, isothiazolyl, thiazolyl, triazolyl (preferably
1,2,3-triazoly1 or 1,2,4-
triazolyl), pyrazinyl, pyrazolyl, pyridyl, pyrimidinyl, oxazolyl or furyl))
optionally having 1 to 3
(particularly 1 or 2) substituents each selected from
(a) a halogen atom (particularly fluorine or chlorine),
(b) a C1.6 alkyl group (particularly methyl, ethyl, isobutyl, isopropyl or
propyl)
optionally having 1 to 7 (particularly 1 to 5) substituents each selected from
(i) a halogen atom (particularly fluorine atom),
(ii) a hydroxy group, and
32
CA 03021185 2018-10-16
A00039
(iii) a Ci_6 alkoxy group (particularly methoxy),
(c) an optionally halogenated C3-10 cycloalkyl group (particularly, 2,2-
difluorocyclopropyl, cyclobutyl, cyclopropyl or difluorocyclobutyl
(particularly 3,3-
difluorocyclobutyl), fluorocyclobutyl (particularly 1-fluorocyclobutyl),
fluorocyclopropyl),
(d) a C1_6 alkoxy group (particularly methoxy),
(e) a C1_6 alkyl group-C3_10 cycloalkyl group (particularly methyl-
cyclopropyl),
(f) a C1_6 alkyl group-three- to eight-membered monocyclic nonaromatic
heterocyclic
group (particularly methyl-oxetanyl), and
(g) oxo group; or
(II) a C7-16 aralkyl group (particularly benzyl) optionally having 1 to 3
substituents each
selected from
(a) a Ci_6 alkyl group (methyl or isopropyl) optionally having 1 to 3
substituents each
selected from
(i) a halogen atom (particularly fluorine atom), and
(ii) a hydroxyl group,
(b) a C1_6 alkoxy-carbonyl group (particularly methoxycarbonyl),
(c) a halogen atom (fluorine or chlorine)
(d) a CI-6 alkoxy group (particularly methoxy or ethoxy) optionally having 1
to 3
substituents each selected from
(i) a halogen atom (particularly fluorine atom), and
(ii) a hydroxyl group
(e) a mono-or di-C1_6 alkyl-carbamoyl group (particularly methylcarbamoyl),
(f) a cyano group,
(g) an amino group optionally monosubstituted or disubstituted (particularly
.. monosubstituted) with a substituent selected from
(i) a C1-6 alkyl group (particularly methyl), and
(ii) a Ci_6 alkoxy-carbonyl group (particularly tert-butoxy-carbonyl),
(h) a nitro group, and
(i) a carboxy group;
each of R6 and R7 is a hydrogen atom;
ring A is a bicyclic aromatic heterocyclic ring selected from the formulas
(1), (2), (3)
and (4);
Xs each independently represents N or
R3 is a hydrogen atom;
33
CA 03021185 2018-10-16
A00039
R4 is (I) a hydrogen atom,
(II) a halogen atom (particularly chlorine atom),
(III) an optionally halogenated C1.6 alkoxy group (particularly methoxy,
fluoromethoxy,
difluoromethoxy or ethoxy), or
(IV) an amino group optionally monosubstituted or disubstituted with a C1_6
alkyl group
(particularly methyl);
R5 is (I) a hydrogen atom,
(II) a halogen atom (particularly chlorine),
(III) a cyano group,
(IV) a C1_6 alkyl group (particularly methyl, ethyl or isopropyl) optionally
having 1 to 5
(particularly 1 or 2) substituents each selected from
(a) a halogen atom (particularly fluorine),
(b) a CI-6 alkoxy group (particularly methoxy),
(c) a C1_6 alkoxy-Ci_6 alkoxy group (particularly methoxy-ethoxy),
(d) a hydroxy group, and
(e) a C1.6 alkyl-carbonyloxy group (particularly, 2,2-dimethylpropanoyloxy),
(V) a C1.6 alkoxy group (particularly methoxy),
(VI) a C2_6 alkenyl group (particular 1-propenyl or ethynyl) optionally having
1 to 3
(particularly 1) C1_6 alkoxy groups (particularly methoxy),
(VII) a C2_6 alkynyl group (particularly ethynyl),
(VIII) a cyano-C6_14 aryl group (particularly cyano-phenyl),
(IX) a five- to fourteen-membered aromatic heterocyclic group (particularly
five- or six-
membered monocyclic aromatic heterocyclic group (preferably pyrazolyl,
isoxazolyl or pyridyl))
optionally having 1 or 2 C1.6 alkyl groups (particularly methyl or ethyl)
optionally having 1 to 3
substituents each selected from
(a) a halogen atom (particularly fluorine atom),
(b) a five- to six-membered monocyclic aromatic heterocyclic group
(particularly
pyridyl), and
(c) a three- to eight-membered monocyclic nonaromatic heterocyclic group
(particularly
morpholinyl),
(X) a Ci_6 alkyl-carbonyl group (particularly acetyl),
(XI) a hydroxy group,
(XII) a C1-6 alkyl-five- to fourteen-membered aromatic heterocyclic oxy group
(particularly methylpyrazolyloxy),
34
CA 03021185 2018-10-16
A00039
(XIII) a three- to eight-membered monocyclic nonaromatic heterocyclic group
(particularly pyrrolidinyl, dihydropyranyl (preferably 3,6-dihydropyranyl),
azetidinyl, oxetanyl
or tetrahydropyranyl) optionally having 1 to 3 substituents each selected from
(a) a halogen atom (particularly fluorine),
(b) a C1_6 alkoxy group (particularly methoxy), and
(c) an oxo group, or
(XIV) an amino group (particularly 2,2-difluoroethylamino) optionally
monosubstituted
or disubstituted with an optionally halogenated C1_6 alkyl.
[0052]
Compound (A') which is the compound (A), wherein
1) ring A is a bicyclic aromatic heterocyclic ring of the formula (1),
R3 is a hydrogen atom,
R4 is a hydrogen atom or a Ci_6 alkoxy group (particularly methoxy or ethoxy),
R5 is a hydrogen atom; or
2) ring A is a bicyclic aromatic heterocyclic ring of the formula (2),
R3 is a hydrogen atom,
Rs is
(I) a hydrogen atom,
(II) a halogen atom (particularly chlorine),
(III) a cyano group,
(IV) a C1-6 alkyl group (particularly methyl, ethyl or isopropyl) optionally
having 1 to 5
(particularly 1 or 2) substituents each selected from
(a) a halogen atom (particularly fluorine)
(b) a C1_6 alkoxy group (particularly methoxy)
(c) a C1_6 alkoxy-C1..6 alkoxy group (particularly methoxy-ethoxy)
(d) a hydroxy group, and
(e) a C1-6 alkyl-carbonyloxy group (particularly, 2,2-dimethylpropanoyloxY),
(V) a C1,6 alkoxy group (particularly methoxy),
(VI) a C2-6 alkenyl group (particular 1-propenyl or ethynyl) optionally having
1 to 3
(particularly 1) C1õ6 alkoxy groups (particularly methoxy),
(VII) a C2-6 allcynyl group (particularly ethynyl),
(VIII) a cyano-C6,14 aryl group (particularly cyano-phenyl),
(a) a five- to fourteen-membered aromatic heterocyclic group (particularly
five- or six-
membered monocyclic aromatic heterocyclic group (preferably pyrazolyl,
isoxazolyl or pyridyl))
CA 03021185 2018-10-16
A00039
optionally having 1 or 2 C1_6 alkyl groups (particularly methyl or ethyl)
optionally having 1 to 3
substituents each selected from
(a) a halogen atom (particularly fluorine atom),
(b) a five- to six-membered monocyclic aromatic heterocyclic group
(particularly
pyridyl), and
(c) a three- to eight-membered monocyclic nonaromatic heterocyclic group
(particularly
morpholinyl),
(X) a C1-6 alkyl-carbonyl group (particularly acetyl),
(XI) a hydroxy group,
(XII) a Ci_6 alkyl-five- to fourteen-membered aromatic heterocyclic oxy group
(particularly methylpyrazolyloxy),
(XIII) a three- to eight-membered monocyclic nonaromatic heterocyclic group
(particularly pyrrolidinyl, dihydropyranyl (preferably 3,6-dihydropyranyl),
azetidinyl, oxetanyl
or tetrahydropyranyl) optionally having 1 to 3 substituents each selected from
(a) a halogen atom (particularly fluorine),
(b) a C1_6 alkoxy group (particularly methoxy), and
(c) an oxo group, or
(XIV) an amino group (particularly 2,2-difluoroethylamino) optionally
monosubstituted
or disubstituted with an optionally halogenated Ci_6 alkyl; or
3) ring A is a bicyclic aromatic heterocyclic ring of the formula (3),
R3 is a hydrogen atom,
R4 iS
(I) a hydrogen atom,
(II) a halogen atom (particularly chlorine atom),
(III) an optionally halogenated Ci_6 alkoxy group (particularly methoxy,
fluoromethoxy
or difluoromethoxy), or
(IV) an amino group optionally monosubstituted or disubstituted with a C1_6
alkyl group
(particularly methyl); and
R5 is a hydrogen atom; or
4) ring A is a bicyclic aromatic heterocyclic ring of the formula (4),
R3 is a hydrogen atom,
R4 is a hydrogen atom or a C1_6 alkoxy group (particularly methoxy), and
R5 is a hydrogen atom.
[0053]
36
CA 03021185 2018-10-16
A00039
Compound (B) which is the compound (A), wherein
R1 is
(I) a C1_6 alkyl group (particularly methyl) optionally having 1 to 3
substituents each
selected from
(a) a Ci_6 alkoxy group (particularly methoxy), and
(b) a hydroxy group;
(II) a three- to fourteen-membered nonaromatic heterocyclic group
(particularly
azetidinyl);
(III) a Ci_6 alkoxy group (particularly methoxy or ethoxy); or
(IV) an amino group;
R2 is
(I) a C1_6 alkyl group (particularly methyl) optionally having 1 to 3
(preferably 1) five-
or six-membered monocyclic aromatic heterocyclic groups (particularly
oxadiazolyl (preferably
1,2,4-oxadiazoly1 or 1,3,4-oxadiazoly1), thiadiazolyl (preferably 1,2,4-
thiadiazolyl, 1,3,4-
thiadiazolyl or 1,2,3-thiadiazolyl), isoxazolyl, thiazolyl, triazolyl
(preferably 1,2,3-triazoly1 or
1,2,4-triazoly1), pyrazinyl, pyrazolyl, pyridyl, pyrimidinyl or oxazoly1)
optionally having 1 to 3
(preferably 1) substituents each selected from
(a) a halogen atom (particularly fluorine),
(b) an optionally halogenated C1_6 alkyl group (particularly methyl,
difluoromethyl,
trifluoromethyl, ethyl, difluoroethyl (particularly 1,1-difluoroethyl), 2,2,2-
trifluoroethyl,
isopropyl, 1-fluoroisopropyl or 1-fluoroethyl), and
(c) an optionally halogenated C3-10 cycloalkyl group (particularly, 2,2-
difluorocyclopropyl, cyclobutyl, cyclopropyl or difluorocyclobutyl
(particularly 3,3-
difluorocyclobutyl), fluorocyclobutyl (particularly 1-fluorocyclobutyl),
fluorocyclopropyl); or
(II) a C7.16 aralkyl group (particularly benzyl) optionally having 1 to 3
(preferably 1 or
2) substituents each selected from
(a) a halogen atom (particularly fluorine or chlorine)
(b) an optionally halogenated C1_6 alkyl group (particularly trifluoromethyl),
and
(c) an optionally halogenated CI-6 alkoxy group (particularly difluoromethoxy
or
trifluoromethoxy);
R3 is a hydrogen atom;
R4 is (a) an amino group optionally monosubstituted or disubstituted with a
C1_6 alkyl
group (particularly methyl), or
(b) a C1_6 alkoxy group (particularly methoxy); and
37
CA 03021185 2018-10-16
A00039
R5 is (a) a C1-6 alkoxy group (particularly methoxy), or
(b) a C1_6 alkyl group (particularly ethyl) optionally having 1 to 3 hydroxy
groups.
[0054]
Compound (W) which is the compound (B), wherein
1) ring A is a bicyclic aromatic heterocyclic ring of the formula (1),
R3 is a hydrogen atom,
R4 is a C1_6 alkoxy group (particularly methoxy), and
R5 is a hydrogen atom; or
2) ring A is a bicyclic aromatic heterocyclic ring of the formula (2),
R3 is a hydrogen atom, and
R5 is (a) a C1_6 alkoxy group (particularly methoxy), or
(b) a C1_6 alkyl group (particularly ethyl) optionally having 1 to 3
(preferably 1)
hydroxy groups; or
3) ring A is a bicyclic aromatic heterocyclic ring of the formula (3),
R3 is a hydrogen atom,
R4 is (a) an amino group optionally monosubstituted or disubstituted with a
Ci_6 alkyl
group (preferably methyl), or
(b) a Ci_6 alkoxy group (particularly methoxy), and
R5 is a hydrogen atom; or
4) ring A is a bicyclic aromatic heterocyclic ring of the formula (4),
R3 is a hydrogen atom,
R4 is a hydrogen atom or a Ci_6 alkoxy group (particularly methoxy), and
R5 is a hydrogen atom.
[0055]
Compound (C) which is the compound (B), wherein
RI is a C1_6 alkyl group (particularly methyl) optionally having 1 to 3
(preferably 1)
hydroxy groups;
R2 is
(I) a C1_6 alkyl group (particularly methyl) optionally having 1 to 3
(preferably 1) five-
or six-membered monocyclic aromatic heterocyclic groups (particularly
oxadiazolyl (preferably
1,2,4-oxadiazoly1 or 1,3,4-oxadiazoly1), thiadiazolyl (preferably 1,2,4-
thiadiazoly1), isoxazoly1)
optionally having 1 to 3 (preferably 1) optionally halogenated C1_6 alkyl
groups (particularly
methyl, difluoromethyl, difluoroethyl, fluoroisopropyl or fluoroethyl); or
(II) an optionally halogenated C7-16 aralkyl group (particularly
difluorobenzyl
38
=
CA 03021185 2018-10-16 A00039
(preferably 3,5-difluorobenzyl));
ring A is a bicyclic aromatic heterocyclic ring represented by the following
formula (1)
[Chemical Formula 4]
R4
N.y.X
R3
(1)
X is N;
R3 is a hydrogen atom;
R4 is a Ci..6 alkoxy group (particularly methoxy).
In the compound (C), it is more preferable that R2 is a C1_6 alkyl group
(particularly
methyl) having 1 to 3 (preferably 1) five- or six-membered monocyclic aromatic
heterocyclic
groups (particularly oxadiazolyl (preferably 1,2,4-oxadiazoly1 or 1,3,4-
oxadiazoly1), thiadiazolyl
(preferably 1,2,4-thiadiazoly1), isoxazoly1) having 1 to 3 (preferably 1)
halogenated C1 _6 alkyl
groups (particularly methyl, difluoromethyl, difluoroethyl, fluoroisopropyl or
fluoroethyl).
[0056]
The salt of the compound (I) is preferably a pharmacologically acceptable
salt.
Examples thereof include a salt with an inorganic base, a salt with an organic
base, a salt with an
inorganic acid, a salt with an organic acid and a salt with a basic or acidic
amino acid.
Preferable examples of the salt with an inorganic base include: an alkali
metal salt such
as sodium salt, potassium salt and the like; an alkaline earth metal salt such
as calcium salt,
magnesium salt and the like; and an aluminum salt and an ammonium salt.
Preferable examples of the salt with an organic base include a salt with
trimethylamine,
triethylamine, pyridine, picoline, ethanolamine, diethanolamine,
triethanolamine, tromethamine
[tris(hydroxymethyl)methylamine], tert-butylamine, cyclohexylamine,
benzylamine,
dicyclohexylamine or N,N-dibenzylethylenediamine.
Preferable examples of the salt with an inorganic acid include a salt with
hydrochloric
acid, hydrobromic acid, nitric acid, sulfuric acid or phosphoric acid.
Preferable examples of the salt with an organic acid include a salt with
formic acid,
39
CA 03021185 2018-10-16
A00039
acetic acid, trifluoroacetic acid, phthalic acid, fumaric acid, oxalic acid,
tartaric acid, maleic acid,
citric acid, succinic acid, malic acid, methanesulfonic acid, benzenesulfonic
acid or p-
toluenesulfonic acid.
Preferable examples of the salt with a basic amino acid include a salt with
arginine,
lysine or ornithine.
Preferable examples of the salt with an acidic amino acid include a salt with
aspartic
acid or glutamic acid.
[0057]
The method for producing the compound of the present invention will be
described
below.
[0058]
A starting material or a reagent used in each step in the production method
given below
and the obtained compound may each form a salt. Examples of such a salt
include the same as
the aforementioned salt of the compound of the present invention, and the
like.
[0059]
When the compound obtained in each step is a free compound, this compound can
be
converted to a salt of interest by a method known per se in the art. On the
contrary, when the
compound obtained in each step is a salt, this salt can be converted to a free
form or another type
of salt of interest by a method known per se in the art.
[0060]
The compound obtained in each step may be used in the next reaction in the
form of its
reaction solution or after being obtained as a crude product. Alternatively,
the compound
obtained in each step can be isolated and/or purified from the reaction
mixture by a separation
approach such as concentration, crystallization, recrystallization,
distillation, solvent extraction,
fractionation, chromatography or the like according to a routine method.
[0061]
If a starting material or a reagent compound for each step is commercially
available, the
commercially available product can be used directly.
[0062]
In the reaction of each step, the reaction time may differ depending on the
reagent or the
solvent used and is usually 1 minute to 48 hours, preferably 10 minutes to 8
hours, unless
otherwise specified.
[0063]
In the reaction of each step, the reaction temperature may differ depending on
the
CA 03021185 2018-10-16
A00039
reagent or the solvent used and is usually -78 C to 300 C, preferably -78 C to
150 C, unless
otherwise specified.
[0064]
In the reaction of each step, the pressure may differ depending on the reagent
or the
solvent used and is usually 1 atm to 20 atm, preferably 1 atm to 3 atm, unless
otherwise
specified.
[0065]
In the reaction of each step, a microwave synthesis apparatus, for example,
Initiator
manufactured by Biotage Japan Ltd., may be used. The reaction temperature may
differ
depending on the reagent or the solvent used and is usually room temperature
to 300 C,
preferably 50 C to 250 C, unless otherwise specified. The reaction time may
differ depending
on the reagent or the solvent used and is usually 1 minute to 48 hours,
preferably 1 minute to 8
hours, unless otherwise specified.
[0066]
In the reaction of each step, the reagent is used at 0.5 equivalents to 20
equivalents,
preferably 0.8 equivalents to 5 equivalents, with respect to the substrate,
unless otherwise
specified. In the case of using the reagent as a catalyst, the reagent is used
at 0.001 equivalents
to 1 equivalent, preferably 0.01 equivalents to 0.2 equivalents, with respect
to the substrate.
When the reagent also serves as a reaction solvent, the reagent is used in the
amount of the
solvent.
[0067]
In the reaction of each step, this reaction is carried out without a solvent
or by
dissolution or suspension in an appropriate solvent, unless otherwise
specified. Specific
examples of the solvent include a solvent described in Examples and the
following:
alcohols: methanol, ethanol, tert-butyl alcohol, 2-methoxyethanol and the
like;
ethers: diethyl ether, diphenyl ether, tetrahydrofuran, 1,2-dimethoxyethane
and the like;
aromatic hydrocarbons: chlorobenzene, toluene, xylene and the like;
saturated hydrocarbons: cyclohexane, hexane and the like;
amides: N,N-dimethylformamide, N-methylpyrrolidone and the like;
halogenated hydrocarbons: dichloromethane, carbon tetrachloride and the like;
nitriles: acetonitrile and the like;
sulfoxides: dimethyl sulfoxide and the like;
aromatic organic bases: pyridine and the like;
acid anhydrides: acetic anhydride and the like;
41
CA 03021185 2018-10-16
A00039
organic acids: formic acid, acetic acid, trifluoroacetic acid and the like;
inorganic acids: hydrochloric acid, sulfuric acid and the like;
esters: ethyl acetate and the like;
ketones: acetone, methyl ethyl ketone and the like; and
water.
Two or more of these solvents may be used as a mixture at an appropriate
ratio.
[0068]
In the case of using a base in the reaction of each step, for example, the
following base
or a base described in Examples is used:
inorganic bases: sodium hydroxide, magnesium hydroxide,sodium carbonate,
calcium carbonate,
sodium hydrogen carbonate and the like;
organic bases: triethylamine, diethylamine, pyridine, 4-dimethylaminopyridine,
N,N-
dimethylaniline, 1,4-diazabicyclo[2.2.2loctane, 1,8-diazabicyclo[5.4.0]-7-
undecene, imidazole,
piperidine and the like;
metal alkoxides: sodium ethoxide, potassium tert-butoxide and the like;
alkali metal hydrides: sodium hydride and the like;
metal amides: sodium amide, lithium diisopropylamide, lithium
hexamethyldisilazide and the
like; and
organic lithiums: n-butyllithium and the like.
[0069]
In the case of using an acid or an acidic catalyst in the reaction of each
step, for
example, the following acid or acidic catalyst or an acid or an acidic
catalyst described in
Examples is used:
inorganic acids: hydrochloric acid, sulfuric acid, nitric acid, hydrobromic
acid, phosphoric acid
and the like;
organic acids: acetic acid, trifluoroacetic acid, citric acid, p-
toluenesulfonic acid, 10-
camphorsulfonic acid and the like; and
Lewis acids: boron trifluoride-diethyl ether complex, zinc iodide, anhydrous
aluminum chloride,
anhydrous zinc chloride, anhydrous iron chloride and the like.
[0070]
The reaction of each step is carried out according to a method known per se in
the art,
for example, a method described in The Fifth Series of Experimental Chemistry,
Vol. 13 to Vol.
19 (edited by The Chemical Society of Japan); Shin Jikken Kagaku Koza (New
Experimental
Chemistry in English), Vol. 14 to Vol. 15 (edited by The Chemical Society of
Japan); Syntheses
42
CA 03021185 2018-10-16
A00039
in the Organic Chemistry Laboratory, Revised, 2nd Ed. (L. F. Tietze, Th.
Eicher, Nankodo Co.,
Ltd.); Organic Name Reactions; The Reaction Mechanism and Essence, Revised
(Hideo Tougo,
Kodansha Ltd.); Organic Syntheses Collective Volume Ito VII (John Wiley &
Sons, Inc.);
Modem Organic Synthesis in the Laboratory: A Collection of Standard
Experimental Procedures
(Jie Jack Li, Oxford University Press); Comprehensive Heterocyclic Chemistry
III, Vol. 1 to Vol.
14 (Elsevier Japan KK); Strategic Applications of Named Reactions in Organic
Synthesis
(translated by Kiyoshi Tomioka, published by Kagaku-Dojin Publishing Company,
Inc.);
Comprehensive Organic Transformations (VCH Publishers, Inc.) (1989), etc., or
a method
described in Examples, unless otherwise specified.
[0071]
In each step, the protection or deprotection reaction of a functional group is
carried out
according to a method known per se in the art, for example, a method described
in "Protective
Groups in Organic Synthesis, 4th Ed." (Theodora W. Greene, Peter G. M. Wuts),
Wiley-
Interscience (2007); "Protecting Groups, 3rd Ed." (P.J. Kocienski) Thieme
Medical Publishers
(2004), etc., or a method described in Examples.
Examples of a protective group for a hydroxy group in an alcohol or a phenolic
hydroxy
group or the like include: an ether-type protective group such as
methoxymethyl ether, benzyl
ether, tert-butyldimethylsilyl ether, tetrahydropyranyl ether and the like; a
carboxylic acid ester-
type protective group such as acetic acid ester and the like; a sulfonic acid
ester-type protective
group such as methanesulfonic acid ester and the like; a carbonic acid ester-
type protective group
such as tert-butyl carbonate and the like; and the like.
Examples of a protective group for a carbonyl group in an aldehyde include: an
acetal-
type protective group such as dimethylacetal and the like; a cyclic acetal-
type protective group
such as 1,3-dioxane and the like; and the like.
Examples of a protective group for a carbonyl group in a ketone include: a
ketal-type
protective group such as dimethylketal and the like; a cyclic ketal-type
protective group such as
1,3-dioxane and the like; an oxime-type protective group such as 0-methyloxime
and the like; a
hydrazone-type protective group such as N,N-dimethylhydrazone and the like;
and the like.
Examples of a protective group for a carboxyl group include: an ester-type
protective
group such as methyl ester and the like; an amide-type protective group such
as N,N-
dimethylamide and the like; and the like
Examples of a protective group for a thiol include: an ether-type protective
group such
as benzyl thioether and the like; an ester-type protective group such as
thioacetic acid ester,
thiocarbonate, thiocarbamate and the like; and the like.
43
CA 03021185 2018-10-16
A00039
Examples of a protective group for an amino group or an aromatic heterocycle
such as
imidazole, pyrrole, indole or the like include: a carbamate-type protective
group such as benzyl
carbamate and the like; an amide-type protective group such as acetamide and
the like; an
alkylamine-type protective group such as N-triphenylmethylamine and the like;
a sulfonamide-
type protective group such as methanesulfonamide and the like; and the like.
These protective groups can be removed by use of a method known per se in the
art, for
example, a method using an acid, a base, ultraviolet light, hydrazine,
phenylhydrazine, sodium
N-methyldithiocarbamate, tetrabutylammonium fluoride, palladium acetate or
trialkylsilyl halide
(e.g., trimethylsilyl iodide, trimethylsilyl bromide) or a reduction method.
[0072]
In the case of carrying out reduction reaction in each step, examples of the
reducing
agent used include: metal hydrides such as lithium aluminum hydride, sodium
triacetoxyborohydride, sodium cyanoborohydride, diisobutyl aluminum hydride
(DIBAL-H),
sodium borohydride, tetramethylammonium triacetoxyborohydride and the like;
boranes such as
a borane-tetrahydrofuran complex and the like; Raney nickel; Raney cobalt;
hydrogen; formic
acid; triethylsilane and the like. In the case of reducing a carbon-carbon
double bond or triple
bond, a method using a catalyst such as palladium-carbon, a Lindlar's catalyst
or the like can be
used.
[0073]
In the case of carrying out oxidation reaction in each step, examples of the
oxidizing
agent used include: peracids such as m-chloroperbenzoic acid (mCPBA), hydrogen
peroxide,
tert-butyl hydroperoxide and the like; perchlorates such as tetrabutylammonium
perchlorate and
the like; chlorates such as sodium chlorate and the like; chlorites such as
sodium chlorite and the
like; periodates such as sodium periodate and the like; a high-valent iodine
reagent such as
iodosylbenzene and the like; a reagent having manganese, such as manganese
dioxide, potassium
permanganate and the like; leads such as lead tetraacetate and the like; a
reagent having
chromium, such as pyridinium chlorochromate (PCC), pyridinium dichromate
(PDC), Jones
reagents and the like; halogen compounds such as N-bromosuccinimide (NBS) and
the like;
oxygen; ozone; a sulfur trioxide-pyridine complex; osmium tetroxide; selenium
dioxide; 2,3-
dichloro-5,6-dicyano-1,4-benzoquinone (DDQ); and the like.
[0074]
In the case of carrying out radical cyclization reaction in each step,
examples of the
radical initiator used include: an azo compound such as azobisisobutyronitrile
(AIBN) and the
like; a water-soluble radical initiator such as 4-4'-azobis-4-cyanopentanoic
acid (ACPA) and the
44
CA 03021185 2018-10-16
A00039
like; triethylboron in the presence of air or oxygen; benzoyl peroxide; and
the like. Examples
of the radical reaction agent used include tributylstannane,
tris(trimethylsilyl)silane, 1,1,2,2-
tetraphenyldisilane, diphenylsilane, samarium iodide and the like.
[0075]
In the case of carrying out Wittig reaction in each step, examples of the
Wittig reagent
used include alkylidenephosphoranes and the like. The alkylidenephosphoranes
can be
prepared by a method known per se in the art, for example, the reaction
between a phosphonium
salt and a strong base.
[0076]
In the case of carrying out Horner-Emmons reaction in each step, examples of
the
reagent used include: phosphonoacetic acid esters such as methyl
dimethylphosphonoacetate,
ethyl diethylphosphonoacetate and the like; and a base such as alkali metal
hydrides, organic
lithiums and the like.
[0077]
In the case of carrying out Friedel-Crafts reaction in each step, examples of
the reagent
used include a combination of a Lewis acid and an acid chloride and a
combination of a Lewis
acid and an alkylating agent (e.g., alkyl halides, alcohols, olefins, etc.).
Alternatively, an
organic acid or an inorganic acid may be used instead of the Lewis acid, and
an acid anhydride
such as acetic anhydride or the like may be used instead of the acid chloride.
[0078]
In the case of carrying out aromatic nucleophilic substitution reaction in
each step, a
nucleophile (e.g., amines, imidazole, etc.) and a base (e.g., organic bases,
etc.) are used as
reagents.
[0079]
In the case of carrying out nucleophilic addition reaction using a carbanion,
nucleophilic
1,4-addition reaction (Michael addition reaction) using a carbanion or
nucleophilic substitution
reaction using a carbanion in each step, examples of the base used for
generating the carbanion
include organic lithiums, metal alkoxides, inorganic bases, organic bases and
the like.
[0080]
In the case of carrying out Grignard reaction in each step, examples of the
Grignard
reagent include: aryl magnesium halides such as phenyl magnesium bromide and
the like; and
alkyl magnesium halides such as methyl magnesium bromide and the like. The
Grignard
reagent can be prepared by a method known per se in the art, for example, the
reaction between
alkyl halide or aryl halide and metal magnesium with ether or tetrahydrofuran
as a solvent.
CA 03021185 2018-10-16
A00039
[0081]
In the case of carrying out Knoevenagel condensation reaction in each step, an
active
methylene compound sandwiched by two electron-attracting groups (e.g., malonic
acid, diethyl
malonate, malononitrile, etc.) and a base (e.g., organic bases, metal
alkoxides, inorganic bases)
are used as reagents.
[0082]
In the case of carrying out Vilsmeier-Haack reaction in each step, phosphoryl
chloride
and an amide derivative (e.g., N,N-dimethylformamide, etc.) are used as
reagents.
[0083]
In the case of carrying out azidation reaction of alcohols, alkyl halides or
sulfonic acid
esters in each step, examples of the azidating agent used include
diphenylphosphorylazide
(DPPA), trimethylsilylazide, sodium azide and the like. In the case of
azidating, for example,
alcohols, a method using diphenylphosphorylazide and 1,8-
diazabicyclo[5.4.0]undec-7-ene
(DBU), a method using trimethylsilylazide and a Lewis acid, or the like can be
used.
[0084]
In the case of carrying out reductive amination reaction in each step,
examples of the
reducing agent used include sodium triacetoxyborohydride, sodium
cyanoborohydride,
hydrogen, formic acid and the like. When the substrate is an amine compound,
examples of the
carbonyl compound used include paraformaldehyde as well as aldehydes such as
acetaldehyde
and the like, and ketones such as cyclohexanone and the like. When the
substrate is a carbonyl
compound, examples of the amines used include: ammonia; primary amine such as
methylamine
and the like; secondary amine such as dimethylamine and the like; and the
like.
[0085]
In the case of carrying out Mitsunobu reaction in each step, azodicarboxylic
acid esters
(e.g., diethyl azodicarboxylate (DEAD), diisopropyl azodicarboxylate (DIAD),
etc.) and
triphenylphosphine are used as reagents.
[0086]
In the case of carrying out esterification reaction, amidation reaction or
ureation
reaction in each step, examples of the reagent used include: an acyl halide
form such as acid
chloride, acid bromide and the like; and activated carboxylic acids such as an
acid anhydride, an
active ester form, a sulfuric acid ester form and the like. Examples of the
activating reagent for
carboxylic acid include: a carbodiimide condensing agent such as 1-ethy1-3-(3-
dimethylaminopropyl)carbodiimide hydrochloride (WSCD) and the like; a triazine
condensing
agent such as 4-(4,6-dimethoxy-1,3,5-triazin-2-y1)-4-methylmorpholinium
chloride-n-hydrate
46
=
CA 03021185 2018-10-16 A00039
(DMT-MM) and the like; a carbonic acid ester condensing agent such as 1,1-
carbonyldiimidazole (CDI) and the like; diphenylphosphorylazide (DPPA);
benzotriazol-1-
yloxy-trisdimethylaminophosphonium salt (BOP reagent); 2-chloro-1-methyl-
pyridinium iodide
(Mukaiyama reagent); thionyl chloride; lower alkyl haloformate such as ethyl
chloroformate and
the like; 0-(7-azabenzotriazol-1-y1)-N,N,N',N1-tetramethyluronium
hexafluorophosphate
(HATU); sulfuric acid; and combinations thereof; and the like. In the case of
using a
carbodiimide condensing agent, an additive such as 1-hydroxybenzotriazole
(HOBO, N-
hydroxysuccinimide (HOSu), dimethylaminopyridine (DMAP) or the like may be
further added
for the reaction.
[0087]
In the case of carrying out coupling reaction in each step, examples of the
metal catalyst
used include: a palladium compound such as palladium(II) acetate,
tetrakis(triphenylphosphine)palladium(0),
dichlorobis(triphenylphosphine)palladium(II),
dichlorobis(triethylphosphine)palladium(II),
tris(dibenzylideneacetone)dipalladium(0), 1,1'-
bis(diphenylphosphino)ferrocene palladium(II) chloride, palladium(II) acetate
and the like; a
nickel compound such as tetrakis(triphenylphosphine)nickel(0) and the like; a
rhodium
compound such as tris(triphenylphosphine)rhodium(III) chloride and the like; a
cobalt
compound; a copper compound such as copper oxide, copper(I) iodide and the
like; a platinum
compound; and the like. A base may be further added for the reaction. Examples
of such a
base include inorganic base and the like.
[0088]
In the case of carrying out thiocarbonylation reaction in each step,
diphosphorus
pentasulfide is typically used as a thiocarbonylating agent. A reagent having
a 1,3,2,4-
dithiadiphosphetane-2,4-disulfide structure such as 2,4-bis(4-methoxypheny1-
1,3,2,4-
dithiadiphosphetane-2,4-disulfide (Lawesson's reagent) or the like may be used
instead of
diphosphorus pentasulfide.
[0089]
In the case of carrying out Wohl-Ziegler reaction in each step, examples of
the
halogenating agent used include N-iodosuccinimide, N-bromosuccinimide (NBS), N-
chlorosuccinimide (NCS), bromine, sulfuryl chloride and the like. The reaction
can be
accelerated by the further addition of a radical initiator such as heat,
light, benzoyl peroxide,
azobisisobutyronitrile or the like for the reaction.
[0090]
In the case of carrying out halogenation reaction of a hydroxy group in each
step,
47
CA 03021185 2018-10-16
A00039
examples of the halogenating agent used include a hydrohalic acid and an acid
halide of an
inorganic acid, specifically, hydrochloric acid, thionyl chloride, phosphorus
oxychloride and the
like for chlorination, and 48% hydrobromic acid and the like for bromination.
Also, a method
for obtaining an alkyl halide foim from an alcohol by the action of
triphenylphosphine and
carbon tetrachloride or carbon tetrabromide or the like may be used.
Alternatively, a method
for synthesizing an alkyl halide form through 2-step reactions like involving
the conversion of an
alcohol to sulfonic acid ester and the subsequent reaction with lithium
bromide, lithium chloride
or sodium iodide may be used.
[0091]
In the case of carrying out Arbuzov reaction in each step, examples of the
reagent used
include: alkyl halides such as ethyl bromoacetate and the like; and phosphites
such as triethyl
phosphite, tri(isopropyl)phosphite and the like.
[0092]
In the case of carrying out sulfonic acid esterification reaction in each
step, examples of
the sulfonylating agent used include methanesulfonyl chloride, p-
toluenesulfonyl chloride,
methanesulfonic anhydride, p-toluenesulfonic anhydride and the like.
[0093]
In the case of carrying out hydrolysis reaction in each step, an acid or a
base is used as a
reagent. In the case of carrying out acid hydrolysis reaction of tert-butyl
ester, formic acid,
triethylsilane or the like may be added for reductively trapping a by-product
tert-butyl cation.
[0094]
In the case of carrying out dehydration reaction in each step, examples of the
dehydrating agent used include sulfuric acid, diphosphorus pentoxide,
phosphorus oxychloride,
N,N'-dicyclohexylcarbodiimide, alumina, polyphosphoric acid and the like.
[0095]
In the case of carrying out alkylation reaction in each step, examples of the
alkylating
agent include optionally substituted alkyl halide (e.g., iodomethane),
optionally substituted alkyl
having an optionally substituted C1_6 alkylsulfonyloxy group as a leaving
group, optionally
substituted alkyl having a C6_14 arylsulfonyloxy group optionally substituted
by a C1_6 alkyl
group. Examples of the base used include organic lithiums, metal alkoxides,
inorganic bases,
organic bases and the like. In order to activate the reaction, an additive
such as sodium iodide
or a quartemary ammonium salt like tetrabutylammonium iodide (1...BAD may
further be added
in the reasction.
[0096]
48
CA 03021185 2018-10-16
A00039
s.
Examples of the reagent to be used when an acylation reaction is carried out
in each
step include activated carboxylic acids such as acyl halides such as acid
chloride and acid
bromide, active esters, esters and sulfuric acid esters. Examples of the
carboxylic acid
activating agent include carbodiimide-based condensing agents such as acid
anhydrides (e.g.
acetic anhydride) and 1-ethy1-3-(3-dimethylaminopropyl) carbodiimide
hydrochloride (WSCD);
triazine-based condensing agents such as 4-(4,6-dimethoxy-1,3,5-triazin-2-y1)-
4-
methylmorpholinium chloride-n-hydrate (DMT-MM); carbonic acid ester-based
condensing
agents such as 1,1-carbonyldiimidazole (CDI); diphenylphosphoryl azide (DPPA);
benzotriazol-l-yloxy-trisdimethylaminophosphonium salts (BOP reagents); 2-
chloro-1-methyl-
pyridinium iodide (Mukaiyama Reagent); thionyl chloride; haloformic acid lower
alkyls such as
ethyl chloroformate; 0-(7-azabenzotriazol-1-y1)-N,N,N ',N'-tetramethyluronium
hexafluorophosphate (HATU); sulfuric acid; and combinations thereof. When a
carbodiimide-
based condensing agent is used, additives such as 1-hydroxybenzotriazole
(HOBt), N-
hydroxysuccinimide (HOSu), dimethylaminopyridine (DMAP) and the like may be
further added
to the reaction.
[0097]
Examples of the halogenating agent to be used when an aromatic compound
halogenation reaction is carried out in each step include N-bromosuccinimide
(NBS), N-
iodosuccinimide (NIS), N-chlorosuccinimide (NCS), 1,3-diiodo-5,5'-
dimethylhydantoin (DIH),
dibromoisocyanuric acid (DBI), N-bromophthalimide, N-iodophthalimide, N-
chlorophthalimide,
N-bromosaccharin, N-iodosaccharin, trimethylphenylammonium tribromide,
bromine, iodine
and chlorine. Further, by adding a radical initiator such as heat, light,
benzoyl peroxide or
azobisisobutyronitrile to the reaction, the reaction can be accelerated.
[0098]
As a reducing agent to be used when a nitro group reduction reaction is
carried out in
each step, a metal powder of reduced iron, zinc, tin or the like is used in
the Bechamp reduction
method, and a catalyst such as palladium-carbon or platinum-carbon nickel is
used in the
catalytic reduction method.
[0099]
A ligand may be added in a reaction system when a coupling reaction is carried
out in
each step, and examples of the ligand include phosphine ligands [e.g.
triphenylphosphine, 2,2'-
bis(diphenylphosphino)-1,1'-binaphthyl, 2-dicyclohexylphosphino-2',6'-
diisopropoxy-1,1'-
biphenyl, 2-(dicyclohexylphosphino)-3,6-dimethoxy-2',4',6'-triisopropy1-1,1'-
biphenyl, 2-di-tert-
butylphosphino-3,4,5,6-tetramethy1-2',4',6'-triisopropy1-1,1'-biphenyl, 2-(di-
tert-
49
.0 CA 03021185 2018-10-16
A00039
butylphosphino)biphenyl, 2-(dicyclohexylphosphino)biphenyl, 2-
dicyclohexylphosphino-2 ',6'-
dimethoxy-1,1'-biphenyl, 2-dicyclohexylphosphino-2',4',6'-triisopropy1-1,1'-
biphenyl, 2-
(dicyclohexylphosphino)-2'-(N,N-dimethy lamino)biphenyl, 1,1'-
bis(diphenylphosphino)
ferrocene, tri-tert-butylphosphine, tricyclohexylphosphine, 4,5-
bis(diphenylphosphino)-9,9-
dimethylxanthene], amine ligands (N,N'-dimethylethylenediamine, trans-1,2-
diaminocyclohexane, trans-N,N'-dimethy1-1,2-cyclohexanediamine, 1,10-
phenanthroline, 4,7-
dimethoxy-1,10-phenanthroline, 3,4,7,8-tetramethy1-1,10-phenanthroline and the
like), diketone
ligands (2-acetylcyclohexanone, 2-isobutyrylhexanone, 2,2,6,6-tetrarnethy1-3,5-
heptanedione
and the like), salicylaldoxime, and proline.
[0100]
Examples of the reagent to be used when an imidazole ring formation reaction
is carried
out in each step include activated carboxylic acids such as orthocarboxylic
acid esters (e.g.
trimethyl orthoacetate), N, N-dimethylcarboxylic acid amide dialkyl acetals
(e.g. N,N-
dimethylacetamide dimethyl acetal), imidate esters (e.g. methyl acetoimidate),
acyl halides such
as acid chloride and acid bromide, active esters, esters and sulfuric acid
esters. Examples of the
activating agent when a carboxylic acid is used include carbodiimide-based
condensing agents
such as propylphosphonic anhydride (cyclic trimer), acid anhydrides (e.g.
acetic anhydride) and
1-ethy1-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (WSCD); triazine-
based
condensing agents such as 4-(4,6-dimethoxy-1,3,5-triazin-2-y1)-4-
methylmorpholinium chloride-
n-hydrate (DMT-MM); carbonic acid ester-based condensing agents such as 1,1-
carbonyldiimidazole (CDI); diphenylphosphoryl azide (DPPA); benzotriazol-1-
yloxy-
trisdimethylaminophosphonium salts (BOP reagents); 2-chloro-l-methyl-
pyridinium iodide
(Mukaiyama Reagent); thionyl chloride; haloformic acid lower alkyls such as
ethyl
chloroformate; 0-(7-azabenzotriazol-1-y1)-N,N,N ',N'-tetramethyluronium
hexafluorophosphate
(HATU); sulfuric acid; and combinations thereof. Additives such as 1-
hydroxybenzotriazole
(HOBt), N-hydroxysuccinimide (HOSu), dimethylaminopyridine (DMAP), an acid and
a base
may be further added to the reaction. Alternatively, when an activated
carboxylic acid or a
carboxylic acid is used, a method may be used in which an imidazole is
synthesized through a
two-step reaction such that a reaction with an acid or base is carried out
after isolation with a
carboxylic acid amide.
[0101]
Examples of the reagent to be used when an alkyne synthesis reaction is
carried out in
each step include a-diazophosphonate compounds (Seyferth-Gilbert Reagent and
Ohira-
Bestmann Reagent (e.g. dimethyl (1-diazo-2-oxopropyl)phosphonate acid)), and
examples of the
CA 03021185 2018-10-16
A00039
base to be used include organic lithiums, metal alkoxides, inorganic bases and
organic bases.
[0102]
Examples of the halogenating agent to be used when a carbonyl group
halogenation
reaction is carried out in each step include thionyl chloride, phosphorus
oxychloride and
phosphorus oxybromide. N, N-dimethylformamide may be added to the reaction for
the
purpose of activating the reaction.
[0103]
When an aromatic nucleophilic substitution reaction is carried out in each
step,
alcohols, thiols and salts thereof can be used as nucleophilic agents.
[0104]
Hereinafter, a method for producing the compound (I), including the reaction
formulas,
will be described in more detail.
Each symbol in the following reaction schemes has the same meaning as
described
above unless otherwise specified.
In addition, unless otherwise specified, raw material compounds to be used in
the
following various production methods can be produced by a method known per se.
[0105]
The compound (I) has the same meaning as that of the compound represented by
the
following formula (Id) or a salt thereof.
[Chemical Formula 5]
11R
R6¨OgN
'112
Xl- X2
H R7 (Id)
R3
[wherein XI, X2, X3, X4 and X5 represent one of the following combinations:
(X1, )(2, x.3, ¨4,
A X5) = (CH, N, C, CR4, N) (a compound represented by this combination
is sometimes referred to as compound Al),
(CH, N, C, N, CR5) (a compound represented by this combination is sometimes
referred
to as compound A2),
(CH, N, C, CR4, CR5) (a compound represented by this combination is sometimes
referred to as compound A3),
(N, N, C, CR4, CR5) (a compound represented by this combination is sometimes
51
CA 03021185 2018-10-16
A00039
referred to as compound A4),
(CH, C, N, CR4, N) (a compound represented by this combination is sometimes
referred
to as compound AS),
(N, C, N, CR4, N) (a compound represented by this combination is sometimes
referred
to as compound A6), and
(CH, C, N, CR4, CR5) (a compound represented by this combination is sometimes
referred to as compound A7); and
each symbol has the same meaning as described above.]
[0106]
Hereinafter, a method for producing a compound represented by the formula (Id)
or a
salt thereof will be described.
[Production Method A-1]
A compound represented by each of the formulae A5 to A7 (compound represented
by
Ia in the following formula) or a salt thereof, and a compound represented by
each of formulae
Al to A4 (compound represented by lb in the following formula) can be produced
from the
compound (ha) by the following method.
[0107]
[Chemical Formula 6]
R4
R4
CNI'Y'l R4 (1).-1R4
Ny X NyX N N.y.N
R5 R5
R3 R3 R3 R3 R3
(1) (2) (3) (4) (5)
[0108]
[wherein VI and V2 are the same or different, and each represent a leaving
group (e.g.
hydrogen, an alkali metal (e.g. lithium or sodium sodium), a halogen atom
(e.g. fluorine,
chlorine, bromine or iodine), a C1_6 alkoxy group (e.g. methoxy), a C6_14
aryloxy group (e.g.
phenoxy), an optionally substituted acyl-oxy group (e.g. acetyloxy or
benzoyloxy), an optionally
substituted C1-6 alkoxysulfonyloxy group (e.g. methoxysulfonyloxy), an
optionally halogenated
C1_6 alkylsulfonyl-oxy group (e.g. methanesulfonyloxy, ethanesulfonyloxy,
trichloromethanesulfonyloxy, trifluoromethanesulfonyloxy (triflate)), or an
optionally substituted
C6_14 arylsulfonyl-oxy group (e.g. a C6-14 arylsulfonyloxy group (e.g.
benzenesulfonyloxy, m-
52
CA 03021185 2018-10-16
A00039
=
nitrobenzenesulfonyloxy, p-toluenesulfonyloxy or naphthylsulfonyloxy)
optionally having 1 to 3
substituents each selected from a C1_6 alkyl group (e.g. methyl, ethyl,
propyl, isopropyl, butyl,
isobutyl, sec-butyl, tert-butyl, pentyl or hexyl), a C1_6 alkoxy group (e.g.
methoxy, ethoxy,
propoxy, isopropoxy, butoxy, isobutoxy, sec-butoxy, pentyloxy or hexyloxy) and
a nitro group;
U represents a boryl group (e.g.
[Chemical Formula 7]
RIII I
N y X
(1)
etc.), an optionally substituted C1_6 alkylstannyl group (e.g. tributylstannyl
or the like),
an optionally substituted C2_6 alkenylstannyl group, or an optionally
substituted C1_6
alkynylstannyl group; and
each of other symbols has the same meaning as described above.]
[0109]
The compound (IV) in which U is a boryl group can be produced by a borylation
reaction of the compound (Ha). The borylation reaction includes a method using
a Miyaura-
Ishiyama-Hartwig borylation reaction and a transmetalation reaction as
described below, and by
using each reaction, a compound (IV) in which U is a boryl group can be
produced.
[0110]
i) Method using Miyaura-Ishiyama-Hartwig borylation reaction
The compound (IV) in which U is a boryl group can be produced by reacting the
compound (Ha) and a borylating agent under a metal catalyst and a base
(specifically Miyaura-
Ishiyama-Hartwig borylation reaction). Preferably, this reaction is carried
out under an inert
gas atmosphere. This reaction may be carried out in the presence of a ligand
and even under
microwave irradiation.
Specific examples of the borylating agent include bis(pinacolato)diboron and
pinacol
borane. Specific examples of the metal catalyst include palladium compounds
such as
palladium (II) acetate, tetrakis(triphenylphosphine)palladium (0),
dichlorobis(triphenylphosphine)palladium (II),
dichlorobis(triethylphosphine)palladium (II),
53
CA 03021185 2018-10-16
A00039
tris(dibenzylideneacetone)dipalladium (0), 1,11-
bis(diphenylphosphino)ferrocenepalladium (II)
chloride and palladium (II) acetate; nickel compounds such as
tetrakis(triphenylphosphine)nickel
(0); rhodium compounds such as tris(triphenylphosphine)rhodium (III) chloride;
cobalt
compounds; copper compounds such as copper oxide and copper (I) iodide;
platinum
compounds; and iridium compounds. Specific examples of the ligand include
phosphine
ligands [e.g. triphenylphosphine, 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl,
2-
dicyclohexylphosphino-2',6'-diisopropoxy-1,1'-biphenyl, 2-
(dicyclohexylphosphino)-3,6-
dimethoxy-2',4',6'-triisopropy1-1,1'-biphenyl, 2-di-tert-butylphosphino-
3,4,5,6-tetramethy1-
2',4',6'-triisopropy1-1,1'-biphenyl, 2-(di-tert-butylphosphino)biphenyl, 2-
(dicyclohexylphosphino)biphenyl, 2-dicyclohexylphosphino-2 ',6'-dimethoxy-1,1'-
biphenyl, 2-
dicyclohexylphosphino-2',4',6'-triisopropy1-1,1'-biphenyl, 2-
(dicyclohexylphosphino)-2'-(N,N-
dimethylamino)biphenyl, 1,11-bis(diphenylphosphino) ferrocene, tri-tert-
butylphosphine,
tricyclohexylphosphine, 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene],
amine ligands
(N,N'-dimethylethylenediamine, trans-1,2-diaminocyclohexane, trans-N,N'-
dimethy1-1,2-
cyclohexanediamine, 1,10-phenanthroline, 4,7-dimethoxy-1,10-phenanthroline,
3,4,7,8-
tetramethyl-1 ,10-phenanthroline and the like), diketone ligands (2-
acetylcyclohexanone, 2-
isobutyrylhexanone, 2,2,6,6-tetramethy1-3,5-heptanedione and the like),
salicylaldoxime, and
proline.
[0111]
ii) Method using transmetalation reaction
The compound (IV) in which U is a boryl group can be produced by reacting the
compound (Ha) with a borylating agent in the presence of an organometallic
reagent (e.g.
organolithium, metal alkoxide, alkali metal hydride, metal amide or isopropyl
magnesium
chloride) (specifically transmetalation reaction). Specific examples of the
borylating agent
include trialkoxyboranes (e.g. trimethoxyborane, triethoxyborane and
triisopropoxyborane).
[0112]
The compound (IV) in which U is an optionally substituted C1_6 alkylstannyl
group (e.g.
tributylstannyl or the like), an optionally substituted C2-6 alkenylstannyl
group, or an optionally
substituted C1_6 alkynylstannyl group can be produced by a stannylation
reaction of the
compound (ha). This reaction is carried out by reacting the compound (Ha) with
a stannylating
agent in the presence of an organometallic reagent (e.g. organolithium, metal
alkoxide, alkali
metal hydride or metal amide). Specific examples of the stannylating agent
include trimethyl
tin chloride, triphenyl tin chloride, trimethyl tin acetate, dimethyl tin
dichloride, dibutyl tin
dichloride, dimethyl tin diacetate and dibutyl tin diacetate.
54
CA 03021185 2018-10-16
A00039
[0113]
[Production Method A-2]
The compound (Id) can also be produced from a compound (Ic) contained in the
compound (Id) by the following method.
[0114]
[Chemical Formula 8]
R4
N y X
R3
(1)
[wherein V3 represents a halogen atom (e.g. fluorine, chlorine, bromine or
iodine); and
each of other symbols has the same meaning as described above.]
[0115]
[Production Method A-3]
The compound (Id) can also be produced from a compound (le) contained in the
compound (Id) by the following method.
[0116]
[Chemical Formula 9]
N 1N R
X1-X2
R1VCr'r
R2
R7
(Id)
R3
[0117]
[Production Method A-4]
The compound represented by the formula Al or each of the formulae A3 to A7
(compound represented by Ii in the following formula) or a salt thereof can be
produced from a
compound (Ig) contained in the compound (Ii) by the following method.
[Chemical Formula 10]
CA 03021185 2018-10-16
A00039
,.
,
Borylotion reaction Rorylotion reaction
Or or
stannylation reaction stannylation reaction
Ftk."(:i.rw _______________ je...qctIrR, Fr_111.47-Fc ____ R.
41.C_,1 orR'
R2 'R2 'IR3
R- If le
Ole) (1',0 V3 14 (1,4 /1
p).; ,,....
...._(t- g 3
R. (..)
0110 R
Coupling reaction 1.1.-00q..
-3
Coupling reaction
Y 1,),.... Rs (uk.) ,
Rs ono Fe_pelriv Coupllin reacr.on
ar atic !awhile
R 58 ,R.:'
Coupling reaction .R3
. H.,..g) R7 elnatuttee ( eaclan re
)431)Ce: et',
R3 fil
Os) (lb)
[0118]
[wherein V4 represents a halogen atom (e.g. fluorine, chlorine, bromine or
iodine); and
each of other symbols has the same meaning as described above.]
[0119]
A compound (Ih) can be produced by a halogenation reaction of a methoxy group
of the
compound (Ig). This reaction can be carried out similarly to the halogenation
reaction of a
carbonyl group.
[0120]
[Production Method A-5]
The compound represented by each of the formulae A2 to A4 or formula A7
(compound
represented by Id' in the following formula) or a salt thereof can be produced
from a compound
(Ij) contained in the compound (Id') by the following method.
[Chemical Formula 11]
¨ Q _
H3c, H3c
= -o e_B#H 0
FgH)2 = -BF3K
. 0 . b , = - 8 1- -NO-- : N
b-r '
cH3
cH3
o PDCH: ._BP-i-cH, P
=-u = =-e, ilo
._.: .3 --B, so¨vc.H3 .
o_< .3
.3 .
'
¨
._-BecD._k_e. ,,, a ,.. LIP, Na, Kt)
0
¨
[0121]
[wherein V5 represents a halogen atom (e.g. fluorine, chlorine, bromine or
iodine); and
each of other symbols has the same meaning as described above.]
[0122]
56
CA 03021185 2018-10-16 A00039
[Production Method A-6]
A compound (I1) contained in the compound (Id') can be produced from a
compound
(Ik) contained in the compound (Id') by the following method.
[Chemical Formula 12]
(!--qtrV3 Coupling reaction
or
aromatic nucleophilic N w
)12 substitution reaction .R2
117 xl,x21"--(
R
3105
tyl;
R3
R3
(Ic) (Id)
[wherein each symbol has the same meaning as described above.]
[0123]
[Production Method A-7]
Compounds (Jo), (Iq) and (Ir) contained in the compound (Id') can be produced
from a
compound (Im) contained in the compound (Id') by the following method.
[Chemical Formula 13]
N ng Alkyl
Re-pgr Coupling reaction
Or
=Ra x2 Oxidation
reaction R2 aromatic nucleophilic 171.1_94NrR
sEt2
X'= Ri substi tution
reaction Xlx2
Fir
______________________________________________________________________ . H1-
6,x4
t,eR7 , ;es
Y n = 1-2
R3 R3 Ft3
014 00
[wherein P1 represents a protecting group for a hydroxyl group;
R5a represents a hydrogen atom, a group bonded via a carbon atom, a group
bonded via
a nitrogen atom or a group bonded via an oxygen atom;
R51' represents a group bonded via a carbon atom; and
each of other symbols has the same meaning as described above.]
[0124]
Compound (r) can be produced by a fluorination reaction of a compound (Ip).
This
reaction can be carried out in accordance with a method known per se [e.g.
Journal of Medicinal
Chemistry, 33(1), 142-6 (1990), Journal of Medicinal Chemistry, 55(21), 9346-
9361 (2012),
Journal of Medicinal Chemistry, 50(15), 3427-3430 (2007), Bioorganic &
Medicinal Chemistry
Letters, 20 (16), 4753-4756 (2010), Journal of Medicinal Chemistry, 50(20),
5024-5033 (2007),
57
CA 03021185 2018-10-16
A00039
Tetrahedron, 65(33), 6611-6625 (2009), Synlett, (14), 2111-2114 (2008),
Organic Process
Research & Development, 14(2), 393-404 (2010), and Bioorganic & Medicinal
Chemistry, 23(2),
297-313 (2015)], or a similar method.
[0125]
[Production Method B-1]
The compound (Ha) to be used in [Production method A-1] can be produced by the
following method.
[Chemical Formula 14]
N oi
REL-M's
H¨T R61Coupling reaction N
, Halogenation reaction or
XIC2f-1.7 *R- of a methoxy group ,n2 aromaticpucleophilic
O.
Ar"
Me _____________________________________ R
7 IN substitution reaction )6x F24
y X5
R3 R3 R3
(h)
[0126]
[wherein R2a represents an optionally substituted aromatic ring, an optionally
substituted
aromatic heterocyclic ring, or an optionally substituted nonaromatic
heterocyclic ring; and
each of other symbols has the same meaning as described above.]
[0127]
The compound (Ha) can be produced by a dehydration cyclization reaction of a
compound (IX). This reaction can also be carried out by heating. Further, this
reaction can
also be carried out by heating in the presence of an acid (e.g. inorganic
acid, organic acid, Lewis
acid or the like) or a base (e.g. organolithium, metal alkoxide, inorganic
base, organic base or the
like).
[0128]
A compound (V) is available as a commercially available product, or is
produced by a
method known per se.
[0129]
[Production Method B-2]
The compound (Ha) to be used in [Production method A-1] can be produced from a
compound (VI) by the following method.
[Chemical Formula 15]
58
CA 03021185 2018-10-16
A00039
Re
r R1
Coupling reaction R
Or
*R2 aromatic nucleophilic
X1-)(2 Rz
substitution reaction Xl-x2
R7
R7
X'.
vs
R5
R3 R3
Ocn
[0130]
[wherein V6 represents a halogen atom (e.g. fluorine, chlorine, bromine or
iodine); and
each of other symbols has the same meaning as described above.]
[0131]
A compound (X) can be produced by a cyclization reaction of the compound (VI)
using
a carbonylating reagent. Examples of the carbonylating reagent include 1,1'-
carbonylbis-1H-
imidazole, diphosgene, triphosgene and phenyl chloroformate. The reaction can
also be carried
out in the presence of a base. Examples of the base include organic bases,
inorganic bases and
basic salts.
[0132]
The compound (XI) can be produced by a cyclization reaction of the compound
(VI)
using a thiocarbonylating reagent. Examples of the thiocarbonylating reagent
include carbon
disulfide, thiourea, ethyl xanthate, thiophosgene and 1,1'-
thiocarbonyldiimidazole. This
reaction can also be carried out in the presence of a base. Examples of the
base include organic
bases, inorganic bases and basic salts.
[0133]
A compound (IIc) can be produced by a halogenation reaction of a thiocarbonyl
group
of the compound (XI). This reaction can be carried out similarly to the
halogenation reaction of
a carbonyl group.
[0134]
[Production Method B-3]
A compound (He), a compound (Ilh), a compound (Ilk) and a compound (III)
contained
in the compound (Ha) to be used in [Production Method A-1] can be produced by
the following
method.
[Chemical Formula 16]
59
CA 03021185 2018-10-16
A00039
,.
,
N,rFti 14,,,Ri
XRa¨Mi
4
Ralqql.
,R2 Reduction reaction
HR7l- r-I--.
, 117:(0)
:::)
_____________________________________ , H x4R7
1 ffs ,...c.14t2
OW 00
[0135]
[wherein R2b and R2d each independently represent a hydrogen atom, a group
bonded
via a halogen atom or a carbon atom, a group bonded via a nitrogen atom, a
group bonded via an
oxygen atom, or a group bonded via a sulfur atom;
R2C represents a group bonded via a carbon atom; and
each of other symbols has the same meaning as described above.]
[0136]
A compound (lid) can be produced by an amidination reaction of a cyano group
of a
compound (HO. This reaction can be carried out in accordance with a method
known per se
[e.g. Bioorganic & Medicinal Chemistry Letters, 20(19), 5735-5738 (2010),
Tetrahedron, 45(20),
6511-18 (1989), Journal of Organic Chemistry, 53(5), 1085-7 (1988), European
Journal of
Medicinal Chemistry, 16(2), 175-9 (1981), Journal of Medicinal Chemistry,
33(4), 1230-41
(1990), European Journal of Medicinal Chemistry, 60, 395-409(2013), Journal of
Organic
Chemistry, 69(20), 6572-6589 (2004), Journal of Labelled Compounds and
Radiopharmaceuticals, 56(14), 722-725 (2013), European Journal of Medicinal
Chemistry, 103,
29-43 (2015), European Journal of Organic Chemistry, 2014(17), 3614-
3621(2014), and Journal
of the American Chemical Society, 107(9), 2743-8 (1985)], or a similar method.
[0137]
A compound (Iii) can be produced by subjecting a cyano group of the compound
(llf) to
a hydroxyamidation reaction in the presence of a hydroxyamidinating reagent.
Examples of the
hydroxyamidinating reagent include hydroxyamine or a salt thereof. This
reaction can be
carried out in the presence of an acid or a base. Examples of the acid include
inorganic acids,
organic acids and Lewis acids. Examples of the base include organic lithiums,
metal alkoxides,
inorganic bases and organic bases.
[0138]
A compound (IIg) can be produced by subjecting a cyano group of the compound
(Ili)
to a thioamidation reaction in the presence of a thioamidating reagent.
Examples of the
thioamidating reagent include phosphorus pentasulfide, hydrogen sulfide, 2,4-
bis(4-
CA 03021185 2018-10-16
A00039
methoxypheny1-1,3,2,4-dithiadiphosphetane-2,4-disulfide (Lowesson reagent),
dialkyl
dithiophosphates (e.g. 0,0'-diethyl dithiophosphate).
[0139]
The compound (lie) can be produced by subjecting the compound (lid) to a
pinner
pyrimidine synthesis reaction in the presence of a reagent. Examples of the
reagent include a
1,3-dicarbonyl compounds and a,-unsaturated carbonyl compounds. This reaction
can be
carried out in the presence of a base. Examples of the base include organic
lithiums, metal
alkoxides, inorganic bases and organic bases.
[0140]
The compound (Ilh) can be produced by subjecting the compound (hg) to a 1,2,4-
thiadiazole ring formation reaction in the presence of a reagent. Examples of
the reagent
include N, N-dialkylcarboxylic acid amide dialkyl acetals (e.g. N,N-
dimethylacetamide dimethyl
acetal) and hydroxyamine derivatives (e.g. hydroxylamine-O-sulfonic acid).
This reaction can
be carried out through a two-step reaction such that by the a N,N-
dialkylcarboxylic acid amide
dialkyl acetal is reacted with the compound (Hg) to isolate a N-
(dialkylaminomethylene)thiocarbonyl, and the isolated N-
(dialkylaminomethylene)thiocarbonyl
is reacted with a hydroxyamine derivative.
[0141]
The compound (II1) can be produced by subjecting the compound (Ili) to a 1,2,4-
__________ oxadiazole ring foi illation reaction in the presence of a
reagent. Examples of the reagent
include activated carboxylic acids such as acyl halides such as acid chloride
and acid bromide,
active esters, esters and sulfuric acid esters. Examples of the activating
agent in preparation of
the activated carboxylic acid include carbodiimide-based condensing agents
such as
propylphosphonic anhydride (cyclic trimer), acid anhydrides (e.g. acetic
anhydride) and 1-ethyl-
3-(3-dimethylaminopropyl) carbodiimide hydrochloride (WSCD); triazine-based
condensing
agents such as 4-(4,6-dimethoxy-1,3,5-triazin-2-y1)-4-methylmorpholinium
chloride-n-hydrate
(DMT-MM); carbonic acid ester-based condensing agents such as 1,1-
carbonyldiimidazole
(CDI); diphenylphosphoryl azide (DPPA); benzotriazol-1-yloxy-
trisdimethylaminophosphonium salts (BOP reagents); 2-chloro-l-methyl-
pyridinium iodide
(Mukaiyama Reagent); thionyl chloride; haloformic acid lower alkyls such as
ethyl
chloroformate; 0-(7-azabenzotriazol-1-y1)-N,N,N ',N'-tetramethyluronium
hexafluorophosphate
(HATU); sulfuric acid; and combinations thereof. When the reaction is carried
out, additives
such as 1-hydroxybenzotriazole (HOBt), N-hydroxysuccinimide (HOSu),
dimethylaminopyridine (DMAP), an acid and a base can be added to the
activating agent.
61
CA 03021185 2018-10-16
A00039
[0142]
[Production Method B-4]
A compound (Ho) and a compound (llq) contained in the compound (Ha) to be used
in
[Production Method A-1] can be produced from a compound (urn) contained in the
compound
(ha) by the following method.
[Chemical Formula 17]
Re-pFVµ
=Rt Deprotection Alkyiation reaction b
'Fe
_____________________________ = 141),e __
YAY%"
Ri ie. R" fe le
0.0 Ora 00)
Oxidation reaction resAgir N
alkyne synthesis
Fr'4(44:R7 reaction
PYLe
te
H=tf_QTR1
Fluorination reaction
______________________________________________________________ -
tWF
[0143]
[wherein P2 represents a protecting group for a carboxyl group, and
each of other symbols has the same meaning as described above.]
[0144]
The compound (llo) can be produced by subjecting a carboxyl group of the
compound
(In) to a 1,2,4-oxadiazole cyclization reaction in the presence of a reagent.
Examples of the
reagent include hydroxyamidines (e.g. N-hydroxyacetamidine). Examples of the
carboxylic
acid activating agent to be used in the reaction include carbodiimide-based
condensing agents
such as propylphosphonic anhydride (cyclic trimer), acid anhydrides (e.g.
acetic anhydride) and
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (WSCD); triazine-
based
condensing agents such as 4-(4,6-dimethoxy-1,3,5-triazin-2-y1)-4-
methylmorpholinium chloride-
n-hydrate (DMT-MM); carbonic acid ester-based condensing agents such as 1,1-
carbonyldiimidazole (CDI); diphenylphosphoryl azide (DPPA); benzotriazol-1-
yloxy-
trisdimethylaminophosphonium salts (BOP reagents); 2-chloro-1-methyl-
pyridinium iodide
(Mukaiyama Reagent); thionyl chloride; haloformic acid lower alkyls such as
ethyl
62
CA 03021185 2018-10-16
A00039
chloroformate; 0-(7-azabenzotriazol-1-y1)-N,N,N ',N'-tetramethyluronium
hexafluorophosphate
(HATU); sulfuric acid; and combinations thereof. When the reaction is carried
out, additives
such as 1-hydroxybenzotriazole (HOBt), N-hydroxysuccinimide (HOSu),
dimethylaminopyridine (DMAP), an acid and a base can be added to the
activating agent.
[0145]
The compound (IN) can be produced by subjecting a carboxyl group of the
compound
(In) to a 1,3,4-oxadiazole cyclization reaction in the presence of a reagent.
Examples of the
reagent include hydrazides (e.g. acetohydrazide). Examples of the carboxylic
acid activating
agent to be used in the reaction include carbodiimide-based condensing agents
such as thionyl
chloride, phosphorus oxychloride, phosphorus oxybromide, propylphosphonic
anhydride (cyclic
trimer), acid anhydrides (e.g. acetic anhydride) and 1-ethyl-3-(3-
dimethylaminopropyl)
carbodiimide hydrochloride (WSCD); triazine-based condensing agents such as 4-
(4,6-
dimethoxy-1,3,5-triazin-2-y1)-4-methylmorpholinium chloride-n-hydrate (DMT-
MM); carbonic
acid ester-based condensing agents such as 1,1-carbonyldiimidazole (CDI);
diphenylphosphoryl azide (DPPA); benzotriazol-l-yloxy-
trisdimethylaminophosphonium salts
(BOP reagents); 2-chloro-1-methyl-pyridinium iodide (Mukaiyama Reagent);
thionyl chloride;
haloformic acid lower alkyls such as ethyl chloroformate; 0-(7-azabenzotriazol-
1-y1)-N,N,N
',N'-tetramethyluronium hexafluorophosphate (HATU); sulfuric acid; and
combinations thereof.
When the reaction is carried out, additives such as 1-hydroxybenzotriazole
(HOBt), N-
hydroxysuccinimide (HOSu), dimethylaminopyridine (DMAP), an acid and a base
can be added
to the activating agent.
This reaction can be carried out through a two-step reaction such that N,N'-
diacylhydrazine is isolated, and the isolated N,N'-diacylhydrazine is
subjected to a dehydration
reaction in the presence of a sulfonating agent (e.g. methanesulfonyl
chloride, p-toluenesulfonyl
chloride, methanesulfonic anhydride or p-toluenesulfonic anhydride) or a
dehydrating agent (e.g.
sulfuric acid, diphosphorus pentoxide, phosphorus oxychloride, N,N1-
dicyclohexylcarbodiimide,
alumina or polyphosphoric acid).
[0146]
The compound (Hq) can be produced by subjecting a hydrazide group of a
compound
(IIp) to a 1,3,4-oxadiazole cyclization reaction in the presence of a reagent.
Examples of the
reagent include activated carboxylic acids such as acyl halides such as acid
chloride and acid
bromide, active esters, esters and sulfuric acid esters. Examples of the
carboxylic acid
activating agent when a carboxylic acid is used include carbodiimide-based
condensing agents
such as thionyl chloride, phosphorus oxychloride, phosphorus oxybromide,
propylphosphonic
63
CA 03021185 2018-10-16
A00039
anhydride (cyclic trimer), acid anhydrides (e.g. acetic anhydride) and 1-ethy1-
3-(3-
dimethylaminopropyl) carbodiimide hydrochloride (WSCD); triazine-based
condensing agents
such as 4-(4,6-dimethoxy-1,3,5-triazin-2-y1)-4-methylmorpholinium chloride-n-
hydrate (DMT-
MM); carbonic acid ester-based condensing agents such as 1,1-
carbonyldiimidazole (CDI);
diphenylphosphoryl azide (DPPA); benzotriazol-l-yloxy-
trisdimethylaminophosphonium salts
(BOP reagents); 2-chloro-1-methyl-pyridinium iodide (Mukaiyama Reagent);
thionyl chloride;
haloformic acid lower alkyls such as ethyl chloroformate; 0-(7-azabenzotriazol-
1-y1)-N,N,N
',N'-tetramethyluronium hexafluorophosphate (HATU); sulfuric acid; and
combinations thereof.
When the reaction is carried out, additives such as 1-hydroxybenzotriazole
(HOBt), N-
hydroxysuccinimide (HOSu), dimethylaminopyridine (DMAP), an acid and a base
can be further
added to the activating agent.
This reaction can be carried out through a two-step reaction such that N,N'-
diacylhydrazine is isolated, and the isolated N,N'-diacylhydrazine is
subjected to a dehydration
reaction in the presence of a sulfonating agent (e.g. methanesulfonyl
chloride, p-toluenesulfonyl
chloride, methanesulfonic anhydride or p-toluenesulfonic anhydride) or a
dehydrating agent (e.g.
sulfuric acid, diphosphorus pentoxide, phosphorus oxychloride, N,N'-
dicyclohexylcarbodiimide,
alumina or polyphosphoric acid).
[0147]
A compound (IIr) can be produced by subjecting a hydrazide group of a compound
(lip)
to a 1,3,4-thiadiazole cyclization reaction in the presence of a reagent.
Examples of the reagent
include thiocarbonylating reagents (e.g. diphosphorus pentasulfide, 2,4-bis(4-
methoxyphenyl-
1,3,2,4-dithiadiphosphetane-2,4-disulfide (Lowesson reagent), and activated
carboxylic acids
such as acyl halides such as acid chloride and acid bromide, active esters,
esters and sulfuric acid
esters. Examples of the activating agent when a carboxylic acid is used
include activating
agents similar to those mentioned as carboxylic acid activating agents usable
in production of the
compound (IIci) from the compound (lip).
[0148]
[Production Method C-1]
A compound (111a) to be used in [Production Method A-1] can be produced by the
following method.
[Chemical Formula 18]
64
CA 03021185 2018-10-16
A00039
..
,
NH, Imidazole ring N RI
1 P4Ht formation reaction RI b Fe Alkylation reaction Ri_pqr-
RI
or.L.
*1-1 VI 'Fe
VI Fe Fe
M (I1b) OW
Reductive NI42
amination reaction N Fri Imidazole ring
- I formation reaction
R?
VI
001)
Reduction reaction
1
Dehydration cyclization reaction
Ntit
Acylation reaction 0 =Nelitie
nt. 6
R,
w
MI)
NIM2 , /1142 Fe
Acylation reaction neri41yR1 Alkylation reaction ppetRyiti
Fe RT45
vi VI
NM (u9
[wherein each symbol has the same meaning as described above.]
[0149]
The compound (IIIa) can be produced by a borylation reaction or a stannylation
reaction
of the compound (Mb). The method for the reaction is similar to the method
described in
[Production Method A-1].
[0150]
A compound (Mb) is available as a commercially available product, or is
produced by a
method known per se.
[0151]
When a substituent of the compound (I) thus obtained is converted by applying
means
known per se (i.e. introduction of a substituent or conversion of a functional
group), another
compound included in the compound (I), or a salt thereof can be produced.
As a method for introduction of a substituent or conversion of a functional
group, a
general known method is used, and examples thereof include conversion of a
halogen atom (e.g.
fluorine, chlorine, bromine or iodine) or an optionally halogenated CI-6
allcylsulfonyl-oxy group
[e.g. methanesulfonyloxy, ethanesulfonyloxy, trichloromethanesulfonyloxy or
trifluoromethanesulfonyloxy (triflate)] into a methyl group, a cyclopropyl
group, a vinyl group, a
CA 03021185 2018-10-16
A00039
cyano group, a formyl group, a carbonyl group, a carboxyl group, a hydroxyl
group, an amino
group, a boryl group or the like; conversion of a formyl group into an ethynyl
group by Seyferth-
Gilbert homologation; conversion of an ester into a carboxy group by
hydrolysis; conversion of a
carboxy group into a carbamoyl group by arnidation; conversion of a carboxy
group into a
hydroxymethyl group by reduction; conversion of a carbonyl group into an
alcohol by reduction
or alkylation; reductive amination of a carbonyl group; oximation of a
carbonyl group; acylation
of an amino group; urea formation of an amino group; sulfonylation of an amino
group;
alkylation of an amino group; substitution or amination of an active halogen
by an amine;
alkylation of a hydroxy group; and substitution or amination of a hydroxy
group.
When a reactive part in which an unintended reaction takes place in the
introduction of
a substituent or conversion of a functional group is present, a compound
falling within the scope
of the present invention can be produced by introducing a protecting group
into the reactive part
beforehand by means known per se as necessary, carrying out an intended
reaction, and then
removing the protecting group by means known per se.
For example, when a raw material compound or an intermediate has an amino
group, a
carboxyl group or a hydroxyl group as a substituent, the group may be
protected with a
protecting group that is generally used peptide chemistry etc. In this case, a
target compound
can be obtained by removing the protective group as necessary after the
reaction.
[0152]
When a substituent of the compound (I) thus obtained is converted by applying
means
known per se (i.e. introduction of a substituent or conversion of a functional
group), another
compound included in the compound (1), or a salt thereof can be produced.
As a method for introduction of a substituent or conversion of a functional
group, a
general known method is used, and examples thereof include conversion of a
halogen atom (e.g.
fluorine, chlorine, bromine or iodine) or an optionally halogenated Ci_6
alkylsulfonyl-oxy group
[e.g. methanesulfonyloxy, ethanesulfonyloxy, trichloromethanesulfonyloxy or
trifluoromethanesulfonyloxy (triflate)] into a methyl group, a cyclopropyl
group, a vinyl group, a
cyano group, a formyl group, a carbonyl group, a carboxyl group, a hydroxyl
group, an amino
group, a boryl group or the like; conversion of a formyl group into an ethynyl
group by Seyferth-
Gilbert homologation; conversion of an ester into a carboxy group by
hydrolysis; conversion of a
carboxy group into a carbamoyl group by amidation; conversion of a carboxy
group into a
hydroxymethyl group by reduction; conversion of a carbonyl group into an
alcohol by reduction
or alkylation; reductive amination of a carbonyl group; oximation of a
carbonyl group; acylation
of an amino group; urea formation of an amino group; sulfonylation of an amino
group;
66
CA 03021185 2018-10-16
A00039
alkylation of an amino group; substitution or amination of an active halogen
by an amine;
alkylation of a hydroxy group; and substitution or amination of a hydroxy
group.
When a reactive part in which an unintended reaction takes place in the
introduction of
a substituent or conversion of a functional group is present, a compound
falling within the scope
of the present invention can be produced by introducing a protecting group
into the reactive part
beforehand by means known per se as necessary, carrying out an intended
reaction, and then
removing the protecting group by means known per se.
For example, when a raw material compound or an intermediate has an amino
group, a
carboxyl group or a hydroxyl group as a substituent, the group may be
protected with a
protecting group that is generally used peptide chemistry etc. In this case, a
target compound
can be obtained by removing the protective group as necessary after the
reaction.
[0153]
When the compound (I) has isomers such as optical isomers, stereoisomers,
regioisomers and rotational isomers, the compound (I) encompasses any one of
the isomers and
mixtures of the isomers. For example, when the compound (I) has optical
isomers, the
compound (I) also encompasses optical isomers divided from racemates. Each of
these isomers
can be obtained as a single product by a synthesis method or separation method
known per se
(e.g. concentration, solvent extraction, column chromatography or
recrystallization).
[0154]
The compound (I) may be in the form of a crystal, and the compound (I)
encompasses
either a single crystal form or a crystal form mixture. The crystal can be
produced by
performing crystallization using a crystallization method known per se.
In addition, the compound (I) may be in the form of a pharmaceutically
acceptable
cocrystal or cocrystal salt. Here, the cocrystal or cocrystal salt means a
crystalline substance
composed of two or more unique solids at room temperature, which have
different physical
properties (e.g. structure, melting point, heat of fusion, hygroscopic
property and stability). The
cocrystals or cocrystal salt can be produced in accordance with a
cocrystallization method known
per se.
The compound (I) may be any of a hydrate, a non-hydrate, a solvate and a non-
solvate,
.. all of which are encompassed in the compound (I).
The compound (I) also encompasses compounds each labeled with an isotope (e.g.
2H,
3H, llc, 14C, 18,-r, 15
or 1251) or the like. The compound (I) labeled or substituted with an
isotope can be used as, for example, a tracer (PET tracer) that is used in
positron emission
tomography (PET), and may be useful in the field of medical diagnosis etc.
67
CA 03021185 2018-10-16
A00039
[0155]
The compound (I) may be a prodrug.
The prodrug of the compound (I) is a compound that is converted into the
compound (I)
by a reaction with an enzyme, gastric acid or the like under physiological
conditions in a living
body, i.e. a compound that is enzymatically oxidized, reduced or hydrolyzed to
change into the
compound (I), or a compound that is hydrolyzed by gastric acid etc. to change
into the
compound (I).
[0156]
Examples of the prodrug of the compound (I) include:
(1) compounds in which the amino of the compound (I) is acylated, alkylated or
phosphorylated (e.g. compounds in which the amino of the compound (I) is
eicosanoylated,
alanylated, pentylaminocarbonylated, (5-methy1-2-oxo-1,3-dioxolen-4-
y1)methoxycarbonylated,
tetrahydrofuranylated, pyrrolidylmethylated, pivaloyloxymethylated, tert-
butylated,
ethoxycarbonylated, tert-butoxycarbonylated, acetylated or
cyclopropylcarbonylated);
(2) compounds in which the hydroxy of the compound (I) is acylated, alkylated,
phosphorylated or borated (e.g. compounds in which the hydroxy of the compound
(I) is
acetylated, palmitoylated, propanoylated, pivaloylated, succinylated ,
fumarylated, alanylated or
dimethylaminomethylcarbonylated); and
(3) compounds in which the carboxy of the compound (I) is esterified or
amidated (e.g.
carboxy of the compound (I) is ethyl-esterified, phenyl-esterified,
carboxymethyl-esterified,
dimethylaminomethyl-esterified, pivalolyloxymethyl-esterified,
ethoxycarbonyloxyethyl-
esterified, phthalidyl-esterified, (5-methy1-2-oxo-1,3-dioxolen-4-yl)methyl-
esterified,
cyclohexyloxycarbonylethyl-esterified or methylamidated). These compounds can
be produced
from the compound (I) by a method known per se.
[0157]
In addition, the prodrug of the compound (I) is a compound which is changed
into the
compound (I) under physiological conditions as described in "Development of
Pharmaceuticals"
published by Hirokawa Shoten Co., 1990, Vol. 7, Molecular Design, pages 163 to
198.
In this specification, the prodrug may form a salt, and examples of the salt
include salts
exemplified as the salt of the compound represented by the formula (I).
[0158]
The compound (I) or prodrug thereof (herein sometimes abbreviated as a
"present
invention compound" collectively) has CLK inhibitory activity, and may be
useful as a
prophylactic or therapeutic agent for cancer, a cancer growth inhibitor, or
cancer metastasis
68
CA 03021185 2018-10-16
A00039
inhibitor.
The present invention compound may be useful as a medicament because the
present
invention compound exhibits selective inhibitory activity against CLK, and is
excellent in
development of pharmacological effects, pharmacokinetics (e.g. absorbability,
distribution,
metabolism and excretion), solubility (e.g. water solubility), interaction
with other
pharmaceutical products (e.g. drug metabolizing enzyme inhibitory action),
safety (in terms of,
for example, acute toxicity, chronic toxicity, genotoxicity, reproductive
toxicity, cardiotoxicity,
carcinogenicity and central toxicity) and stability (e.g. chemical stability
and stability against
enzymes).
Since the present invention compound has low inhibitory activity against SCD
family
subtypes other than SCD1, the present invention compound may be useful as a
prophylactic/therapeutic agent for cancer, which has reduced toxicity to
normal cells.
Therefore, the compound of the present invention can be used for inhibiting
excess
(abnormal) CLK action on mammals (e.g. mouse, rat, hamster, rabbit, cat, dog,
bovine, ovine,
monkey and human).
The present invention compound can be used as a medicament such as a
prophylactic or
therapeutic agent for diseases which may be influenced by CLK (herein,
sometimes abbreviated
as "CLK related diseases"), for example cancers [e.g. colorectal cancer (e.g.
colon cancer, rectal
cancer, anal cancer, familial colorectal cancer, hereditary nonpolyposis
colorectal cancer and
gastrointestinal stromal tumor), lung cancer (e.g. non-small-cell lung cancer,
small cell lung
cancer and malignant mesothelioma), mesothelioma, pancreatic cancer (e.g.
pancreatic ductal
carcinoma and pancreatic endocrine tumor), pharyngeal cancer, laryngeal
cancer, esophageal
cancer, stomach cancer (e.g. papillary adenocarcinoma, mucous adenocarcinoma
and
adenosquamous carcinoma), duodenal carcinoma, small intestinal cancer, breast
cancer (e.g.
infiltrating duct carcinoma, noninfiltrating intraductal carcinoma and
inflammatory breast
cancer), ovarian cancer (e.g. epithelial ovarian cancer, extragonadal germ
cell tumor, ovarian
germ cell tumor and ovarian low malignant potential tumor), testicular tumor,
prostatic cancer
(e.g. hormone-dependent prostatic cancer, hormone-independent prostatic cancer
and castration-
resistant prostatic cancer), liver cancer (e.g. hepatic cell carcinoma,
primary hepatic cancer and
cancer of extrahepatic bile duct), thyroid cancer (e.g. thyroid medullary
carcinoma), kidney
cancer (e.g. renal cell carcinoma (e.g. clear cell type renal cell carcinoma)
and transitional cell
carcinoma of renal pelvis and ureter), uterine cancer (e.g. cervical cancer,
corpus uteri cancer and
uterine sarcoma), gestational choriocarcinoma, brain tumor (e.g.
medulloblastoma, glioma,
pineal astrocytoma, pilocytic astrocytoma, diffuse astrocytoma, anaplastic
astrocytoma and
69
CA 03021185 2018-10-16
A00039
pituitary adenoma), retinoblastoma, skin cancer (e.g. basal cell carcinoma and
malignant
melanoma), sarcoma (e.g. rhabdomyosarcoma, leiomyosarcoma, soft tissue sarcoma
and spindle
cell sarcoma), malignant bone tumor, bladder cancer, blood cancer (e.g.
multiple myeloma,
leukemia (e.g. acute myeloid leukemia), malignant lymphoma, Hodgkin's disease
and chronic
myeloproliferative disease), and cancer of unknown primary], a cancer growth
inhibitor, a cancer
metastasis inhibitor, an apoptosis promoting agent, or a therapeutic agent for
precancerous lesion
(e.g. osteomyelodysplasia syndrome).
In particular, the present invention compound can be used as a medicament for
osteomyelodysplasia syndrome, acute myeloid leukemia, multiple myeloma or
breast cancer.
From a different point of view, the present invention compound may be useful
as a
prophylactic or therapeutic agent, a growth inhibitor or a metastasis
inhibitor for
(i) cancer in which splicing is abnormal (e.g. osteomyelodysplasia syndrome,
acute
myeloid leukemia, lymphoma, lung cancer, pancreatic cancer, breast cancer,
melanoma, bladder
cancer and head and neck cancer)
(ii) cancer in which Myc (specifically c-Myc, N-Myc or L-Myc) is activated
(e.g.
lymphoma, multiple myeloma, neuroblastoma, breast cancer and lung cancer), and
(iii) high-CLK expressing cancer (e.g. breast cancer and multiple myeloma).
[0159]
The compound of the present invention can be orally or parenterally
administered
as a medicament containing the compound of the present invention alone or as a
mixture with a
pharmacologically acceptable carrier to a mammal (preferably a human).
Hereinafter, the medicament comprising the compound of the present invention
(also
referred to as the "medicament of the present invention") will be described in
detail. Examples
of the dosage form of the medicament of the present invention include an oral
preparation such
as tablets (e.g., sugar-coated tablets, film-coated tablets, sublingual
tablets, buccal tablets and
rapidly orally disintegrating tablets), pills, granules, powders, capsules
(e.g., soft capsules and
microcapsules), syrups, emulsions, suspensions, films (e.g., orally
disintegrating films and patch
films for application to the oral mucosa) and the like. Other examples of the
dosage form of the
medicament of the present invention include a parenteral preparation such as
injections,
transfusions, transdermal preparations (e.g., iontophoresis dermal
preparations), suppositories,
ointments, transnasal preparations, transpulmonary preparations, eye drops and
the like.
Alternatively, the medicament of the present invention may be a controlled-
release preparation
such as a rapid-release preparation, a sustained-release preparation (e.g., a
sustained-release
microcapsule) or the like.
CA 03021185 2018-10-16
A00039
[0160]
The medicament of the present invention can be produced by a production method
known in the art (e.g., a method described in Japanese Pharmacopoeia)
generally used in the
field of pharmaceutical technology. If necessary, the medicament of the
present invention can
appropriately contain an appropriate amount of an additive usually used in the
pharmaceutical
field, such as an excipient, a binder, a disintegrant, a lubricant, a
sweetener, a surfactant, a
suspending agent, an emulsifier, a colorant, a preservative, a fragrance, a
corrigent, a stabilizer, a
viscosity modifier and the like.
Examples of the pharmacologically acceptable carrier described above include
these
additives.
[0161]
For example, the tablets can be produced using an excipient, a binder, a
disintegrant, a
lubricant and the like. The pills and the granules can be produced using an
excipient, a binder
and a disintegrant. The powders and the capsules can be produced using an
excipient and the
like. The syrups can be produced using a sweetener and the like. The emulsions
or the
suspensions can be produced using a suspending agent, a surfactant, an
emulsifier and the like.
[0162]
Examples of the excipient include lactose, saccharose, glucose, starch,
sucrose,
microcrystalline cellulose, licorice powder, mannitol, sodium hydrogen
carbonate, calcium
phosphate and calcium sulfate.
Examples of the binder include a solution containing 5 to 10% by weight of
starch
paste, a solution containing 10 to 20% by weight of gum arabic or gelatin, a
solution containing
1 to 5% by weight of tragacanth, a carboxymethylcellulose solution, a sodium
alginate solution
and glycerin.
Examples of the disintegrant include starch and calcium carbonate.
Examples of the lubricant include magnesium stearate, stearic acid, calcium
stearate and
purified talc.
Examples of the sweetener include glucose, fructose, invert sugar, sorbitol,
xylitol,
glycerin and simple syrup.
Examples of the surfactant include sodium lauryl sulfate, polysorbate 80,
sorbitan
monofatty acid ester and polyoxyl 40 stearate.
Examples of the suspending agent include gum arabic, sodium alginate,
carboxymethylcellulose sodium, methylcellulose and bentonite.
Examples of the emulsifier include gum arabic, tragacanth, gelatin and
polysorbate 80.
71
CA 03021185 2018-10-16
A00039
[0163]
When the medicament of the present invention is, for example, tablets, the
tablets can
be produced according to a method known per se in the art by adding, for
example, an excipient
(e.g., lactose, saccharose, starch), a disintegrant (e.g., starch, calcium
carbonate), a binder (e.g.,
starch, gum arabic, carboxymethylcellulose, polyvinylpyrrolidone,
hydroxypropylcellulose) or a
lubricant (e.g., talc, magnesium stearate, polyethylene glycol 6000) to the
compound of the
present invention and molding the mixture by compression, followed by coating,
if necessary, by
a method known per se in the art for the purpose of taste masking, enteric
properties or
durability. For example, hydroxypropylmethylcellulose, ethylcellulose,
hydroxymethylcellulose, hydroxypropylcellulose, polyoxyethylene glycol, Tween
80, Pluronic
F68, cellulose acetate phthalate, hydroxypropylmethylcellulose phthalate,
hydroxymethylcellulose acetate succinate, Eudragit (manufactured by Rohm GmbH,
Germany,
methacrylic acid-acrylic acid copolymer) and a dye (e.g., iron red, titanium
dioxide) can be used
as coating agents for the coating.
[0164]
The injections include intravenous injections as well as subcutaneous
injections,
intracutaneous injections, intramuscular injections, intraperitoneal
injections, drip injections and
the like.
[0165]
Such injections are prepared by a method known per se in the art, i.e., by
dissolving,
suspending or emulsifying the compound of the present invention in a sterile
aqueous solution or
oily solution. Examples of the aqueous solution include saline, an isotonic
solution containing
glucose or an additional adjuvant (e.g., D-sorbitol, D-mannitol, sodium
chloride) and the like.
The aqueous solution may contain an appropriate solubilizing agent, for
example, an alcohol
(e.g., ethanol), a polyalcohol (e.g., propylene glycol, polyethylene glycol)
or a nonionic
surfactant (e.g., polysorbate 80, HCO-50). Examples of the oily solution
include sesame oil,
soybean oil and the like. The oily solution may contain an appropriate
solubilizing agent.
Examples of the solubilizing agent include benzyl benzoate, benzyl alcohol and
the like. The
injections may be further supplemented with a buffer (e.g., a phosphate buffer
solution, a sodium
acetate buffer solution), a soothing agent (e.g., benzalkonium chloride,
procaine hydrochloride),
a stabilizer (e.g., human serum albumin, polyethylene glycol), a preservative
(e.g., benzyl
alcohol, phenol) or the like. Ampules can usually be filled with the prepared
injection
solutions.
[0166]
72
CA 03021185 2018-10-16
A00039
The content of the compound of the present invention in the medicament of the
present
invention differs depending on the form of the preparation and is usually
approximately 0.01 to
approximately 100% by weight, preferably approximately 2 to approximately 85%
by weight,
more preferably approximately 5 to approximately 70% by weight, with respect
to the whole
preparation.
[0167]
The content of the additive in the medicament of the present invention differs
depending
on the form of the preparation and is usually approximately 1 to approximately
99.9% by weight,
preferably approximately 10 to approximately 90% by weight, with respect to
the whole
preparation.
[0168]
The compound of the present invention can be used stably, low toxically and
safely.
The daily dose of the compound of the present invention differs depending on
the status and
body weight of a patient, the type of the compound, an administration route,
etc. In the case of,
for example, oral administration to a patient for the purpose of treating
cancer, the daily dose in
adult (body weight: approximately 60 kg) can be approximately 1 to
approximately 1000 mg,
preferably approximately 3 to approximately 300 mg, more preferably
approximately 10 to
approximately 200 mg, of the compound of the present invention, which can be
administered in
one portion or in two or three portions.
[0169]
In the case of parenterally administering the compound of the present
invention, the
compound of the present invention is usually administered in the form of a
solution (e.g., an
injection). The single dose of the compound of the present invention also
differs depending on
a recipient, a target organ, symptoms, an administration method, etc. For
example, usually
approximately 0.01 to approximately 100 mg, preferably approximately 0.01 to
approximately
50 mg, more preferably approximately 0.01 to approximately 20 mg, of the
compound of the
present invention per kg of body weight can be administered by intravenous
injection.
[0170]
The compound of the present invention can be used in combination with an
additional
drug. Specifically, the compound of the present invention can be used in
combination with a
drug such as a hormone therapeutic, a chemotherapeutic, an immunotherapeutic,
an agent
inhibiting the effects of a cell growth factor and its receptor, or the like.
Hereinafter, the drug
that may be used in combination with the compound of the present invention is
referred to as a
concomitant drug.
73
CA 03021185 2018-10-16
A00039
[0171]
Examples of the "hormone therapeutic" that may be used include fosfestrol,
diethylstilbestrol, chlorotrianisene, medroxyprogesterone acetate, megestrol
acetate,
chlormadinone acetate, cyproterone acetate, danazol, allylestrenol,
gestrinone, mepartricin,
raloxifene, ormeloxifene, levormeloxifene, anti-estrogen (e.g., tamoxifen
citrate, toremifene
citrate), contraceptive pills, mepitiostane, testololactone,
aminoglutethimide, LH-RI-1 agonists
(e.g., goserelin acetate, buserelin, leuprorelin acetate), droloxifene,
epitiostanol, ethinyl estradiol
sulfonate, aromatase inhibitors (e.g., fadrozole hydrochloride, anastrozole,
letrozole, exemestane,
vorozole, formestane), anti-androgen (e.g., flutamide, bicalutamide,
nilutamide, enzalutamide),
5u-reductase inhibitors (e.g., finasteride, epristeride, dutasteride), adrenal
corticosteroid agents
(e.g., dexamethasone, prednisolone, betamethasone, triamcinolone), androgen
synthesis
inhibitors (e.g., abiraterone), retinoid and agents delaying retinoid
metabolism (e.g., liarozole),
thyroid hormones and DDS (drug delivery system) preparations thereof.
[0172]
Examples of the "chemotherapeutic" that may be used include an alkylating
agent, an
antimetabolite, an anticancer antibiotic and a plant-derived anticancer agent.
[0173]
Examples of the "alkylating agent" that may be used include nitrogen mustard,
nitrogen
mustard-N-oxide hydrochloride, chlorarnbucil, cyclophosphamide, ifosfamide,
thiotepa,
carboquone, improsulfan tosilate, busulfan, nimustine hydrochloride,
mitobronitol, melphalan,
dacarbazine, ranimustine, estramustine sodium phosphate, triethylenemelamine,
carmustine,
lomustine, streptozocin, pipobroman, etoglucid, carboplatin, cisplatin,
miboplatin, nedaplatin,
oxaliplatin, altretamine, ambamustine, dibrospidium hydrochloride,
fotemustine, prednimustine,
pumitepa, Ribomustin, temozolomide, treosulfan, trofosfamide, zinostatin
stimalamer,
adozelesin, cystemustine, bizelesin and DDS preparations thereof.
[0174]
Examples of the "antimetabolite" that may be used include mercaptopurine, 6-
mercaptopurine riboside, thioinosine, methotrexate, pemetrexed, enocitabine,
cytarabine,
cytarabine ocfosfate, ancitabine hydrochloride, 5-FU drugs (e.g.,
fluorouracil, tegafur, UFT,
doxifluridine, carmofur, galocitabine, emitefur, capecitabine), aminopterin,
nelarabine,
leucovorin calcium, tabloid, butocine, calcium folinate, calcium levofolinate,
cladribine,
emitefur, fludarabine, gemcitabine, hydroxycarbamide, pentostatin, piritrexim,
idoxuridine,
mitoguazone, tiazofurin, ambamustine, bendamustine and DDS preparations
thereof.
[0175]
74
CA 03021185 2018-10-16
A00039
Examples of the "anticancer antibiotic" that may be used include actinomycin
D,
actinomycin C, mitomycin C, chromomycin A3, bleomycin hydrochloride, bleomycin
sulfate,
peplomycin sulfate, daunorubicin hydrochloride, doxorubicin hydrochloride,
aclarubicin
hydrochloride, pirarubicin hydrochloride, epirubicin hydrochloride,
neocarzinostatin,
mithramycin, sarkomycin, carzinophilin, mitotane, zorubicin hydrochloride,
mitoxantrone
hydrochloride, idarubicin hydrochloride and DDS preparations (e.g., PEG
liposomal
doxorubicin) thereof.
[0176]
Examples of the "plant-derived anticancer agent" that may be used include
etoposide,
etoposide phosphate, vinblastine sulfate, vincristine sulfate, vindesine
sulfate, teniposide,
paclitaxel, docetaxel, cabazitaxel, vinorelbine and DDS preparations thereof.
[0177]
Examples of the "immunotherapeutic" that may be used include picibanil,
Krestin,
schizophyllan, lentinan, ubenimex, interferon, interleukin, macrophage colony-
stimulating
factor, granulocyte colony-stimulating factor, erythropoietin, lymphotoxin,
BCG vaccines,
Corynebacterium parvum, levamisole, polysaccharide K, procodazol, anti-CTLA4
antibodies
(e.g., ipilimumab, tremelimumab), anti-PD-1 antibodies (e.g., nivolumab,
pembrolizumab) and
anti-PD-L I antibodies.
[0178]
The "cell growth factor" in the "agent inhibiting the effects of a cell growth
factor and
its receptor" can be any substance that promotes the growth of cells. Typical
examples thereof
include a factor that is a peptide having a molecular weight of 20,000 or
smaller and exerts its
effects at a low concentration through binding to its receptor. Specific
examples of the cell
growth factor that may be used include (1) EGF (epidermal growth factor) or a
substance having
activity substantially identical thereto [e.g., TGFa], (2) insulin or a
substance having activity
substantially identical thereto [e.g., insulin, IGF (insulin-like growth
factor)-1, IGF-2], (3) FGF
(fibroblast growth factor) or a substance having activity substantially
identical thereto [e.g.,
acidic FGF, basic FGF, KGF (keratinocyte growth factor), FGF-10], and (4)
other cell growth
factors [e.g., CSF (colony stimulating factor), EPO (erythropoietin), IL-2
(interleukin-2), NGF
(nerve growth factor), PDGF (platelet-derived growth factor), TGFP
(transforming growth factor
[3), HGF (hepatocyte growth factor), VEGF (vascular endothelial growth
factor), heregulin,
angiopoietin].
[0179]
The "receptor of the cell growth factor" can be any receptor having the
ability to bind to
CA 03021185 2018-10-16
A00039
any of the cell growth factor described above. Specific examples of the
receptor that may be
used include EGF receptor, heregulin receptor (e.g., HER3), insulin receptor,
IGF receptor-1,
IGF receptor-2, FGF receptor-1 or FGF receptor-2, VEGF receptor, angiopoietin
receptor (e.g.,
Tie2), PDGF receptor and the like.
[0180]
Examples of the "agent inhibiting the effects of a cell growth factor and its
receptor"
that may be used include EGF inhibitors, TGFa inhibitors, heregulin
inhibitors, insulin
inhibitors, IGF inhibitors, FGF inhibitors, KGF inhibitors, CSF inhibitors,
EPO inhibitors, IL-2
inhibitors, NGF inhibitors, PDGF inhibitors, TGFp inhibitors, HGF inhibitors,
VEGF inhibitors,
angiopoietin inhibitors, EGF receptor inhibitors, HER2 inhibitors, HER4
inhibitors, insulin
receptor inhibitors, IGF-1 receptor inhibitors, IGF-2 receptor inhibitors, FGF
receptor-1
inhibitors, FGF receptor-2 inhibitors, FGF receptor-3 inhibitors, FGF receptor-
4 inhibitors,
VEGF receptor inhibitors, Tie-2 inhibitors, PDGF receptor inhibitors, Abl
inhibitors, Raf
inhibitors, FLT3 inhibitors, c-Kit inhibitors, Src inhibitors, PKC inhibitors,
Smo inhibitors, ALK
inhibitors, ROR1 inhibitors, Trk inhibitors, Ret inhibitors, mTOR inhibitors,
Aurora inhibitors,
PLK inhibitors, MEK (MEK1/2) inhibitors, MET inhibitors, CDK inhibitors, Akt
inhibitors,
ERK inhibitors, PI3K inhibitors and the like. More specific examples of the
agent that may be
used include anti-VEGF antibodies (e.g., bevacizumab, ramucirumab), anti-HER2
antibodies
(e.g., trastuzumab, pertuzumab), anti-EGFR antibodies (e.g., cetuximab,
panitumumab,
matuzumab, nimotuzumab), anti-HGF antibodies, imatinib, erlotinib, gefitinib,
sorafenib,
sunitinib, dasatinib, lapatinib, vatalanib, ibrutinib, bosutinib,
cabozantinib, crizotinib, alectinib,
vismodegib, axitinib, motesanib, nilotinib, 644-(4-ethylpiperazin-1-
ylmethyl)phenyl]-N-[1(R)-
phenylethy1]-7H-pyrrolo[2,3-d]pyrimidin-4-amine (AEE-788), vandetanib,
temsirolimus,
everolimus, enzastaurin, tozasertib, phosphoric acid 24N-[3-[4454N-(3-
fluorophenyl)carbamoylmethyl]-1H-pyrazol-3-ylamino]quinazolin-7-yloxy]propyl]-
N-
ethylamino]ethyl ester (AZD-1152), 4-[9-chloro-7-(2,6-difluoropheny1)-5H-
pyrimido[5,4-
d][2]benzazapin-2-ylamino]benzoic acid, N-[2-methoxy-5-[(E)-2-(2,4,6-
trimethoxyphenyl)vinylsulfonylmethyl]phenyl]glycine sodium salt (ON-1910Na),
volasertib,
selumetinib, trametinib, N-[2(R),3-dihydroxypropoxy]-3,4-difluoro-2-(2-fluoro-
4-
iodophenylamino)benzamide (PD-032590I), bosutinib, regorafenib, afatinib,
idelalisib, ceritinib,
dabrafenib and the like.
[018]]
In addition to the drugs described above, examples that may be used as the
concomitant
drug also include L-asparaginase, L-arginase, arginine deiminase, aceglatone,
procarbazine
76
CA 03021185 2018-10-16
A00039
hydrochloride, protoporphyrin-cobalt complex salt, mercury hematoporphyrin-
sodium,
topoisomerase I inhibitors (e.g., irinotecan, topotecan, indotecan,
indimitecan), topoisomerase II
inhibitors (e.g., sobuzoxane), differentiation inducers (e.g., retinoid,
vitamins D), other
angiogenesis inhibitors (e.g., fumagillin, shark extracts, COX-2 inhibitors),
a-blockers (e.g.,
tamsulosin hydrochloride), bisphosphonic acids (e.g., pamidronate,
zoledronate), thalidomide,
lenalidomide, pomalidomide, 5-azacytidine, decitabine, proteasome inhibitors
(e.g., bortezomib,
carfilzomib, ixazomib), NEDD8 inhibitors (e.g., pevonedistat), UAE inhibitors,
PARP inhibitors
(e.g., olaparib, niraparib, veliparib), antitumor antibodies such as anti-CD20
antibodies (e.g.,
rituximab, obinutuzumab), anti-CCR4 antibodies (e.g., mogamulizumab) and the
like, antibody-
drug conjugates (e.g., trastuzumab emtansine, brentuximab vedotin), MDM2
inhibitors or the
like.
[0182]
The combination of the compound of the present invention and the concomitant
drug
can produce excellent effects such as: (1) the dose of the compound of the
present invention or
the concomitant drug can be reduced as compared with the administration of the
compound of
the present invention or the concomitant drug alone; (2) the concomitant drug
can be selected for
combined use with the compound of the present invention according to the
symptoms (mild,
serious, etc.) of a patient; (3) the period of treatment can be set longer;
(4) a sustained therapeutic
effect can be achieved; (5) a synergistic effect can be obtained by the
combined use of the
compound of the present invention and the concomitant drug; and the like.
[0183]
Hereinafter, the combined use of the compound of the present invention and the
concomitant drug is referred to as the "combination drug of the present
invention".
For use of the combination drug of the present invention, the time of
administration of
the compound of the present invention and the time of administration of the
concomitant drug
are not limited, and the compound of the present invention and the concomitant
drug may be
administered simultaneously or in a staggered manner to a recipient. In the
case of
administration in a staggered manner, the staggered manner differs depending
on active
ingredients to be administered, a dosage form and an administration method. In
the case of first
administering, for example, the concomitant drug, the compound of the present
invention can be
administered within 1 minute to 3 days, preferably within 10 minutes to 1 day,
more preferably
within 15 minutes to 1 hour, after the administration of the concomitant drug.
In the case of
first administering the compound of the present invention, the concomitant
drug can be
administered within 1 minute to 1 day, preferably within 10 minutes to 6
hours, more preferably
77
CA 03021185 2018-10-16
A00039
within 15 minutes to 1 hour, after the administration of the compound of the
present invention.
The dose of the concomitant drug can abide by a dose clinically used and can
be appropriately
selected according to a recipient, an administration route, a disease, a
combination, etc.
[0184]
Examples of the administration mode of the compound of the present invention
and the
concomitant drug used in combination include (1) the administration of a
single preparation
obtained by simultaneously formulating the compound of the present invention
and the
concomitant drug, (2) the simultaneous administration through the same
administration route of
two preparations obtained by separately formulating the compound of the
present invention and
the concomitant drug, (3) the administration through the same administration
route in a staggered
manner of two preparations obtained by separately formulating the compound of
the present
invention and the concomitant drug, (4) the simultaneous administration
through different
administration routes of two preparations obtained by separately formulating
the compound of
the present invention and the concomitant drug, and (5) the administration
through different
administration routes in a staggered manner of two preparations obtained by
separately
formulating the compound of the present invention and the concomitant drug
(e.g.,
administration in the order of the compound of the present invention and then
the concomitant
drug, or in the reverse order).
The dose of the concomitant drug can be appropriately selected on the basis of
a dose
clinically used. The mixing ratio between the compound of the present
invention and the
concomitant drug can be appropriately selected according to a recipient, an
administration route,
a target disease, symptoms, a combination, etc. When the recipient is, for
example, a human,
0.01 to 100 parts by weight of the concomitant drug can be used with respect
to 1 part by weight
of the compound of the present invention.
[0185]
The compound of the present invention or the combination drug of the present
invention
can be further used in combination with a non-drug therapy. Specifically, the
compound of the
present invention or the combination drug of the present invention may be
combined with a non-
drug therapy, for example, (1) surgery, (2) induced hypertension chemotherapy
using angiotensin
II or the like, (3) gene therapy, (4) therrnotherapy, (5) cryotherapy, (6)
laser cauterization or (7)
radiotherapy.
[0186]
The compound of the present invention or the combination drug of the present
invention
is used, for example, before or after the surgery or the like or before or
after treatment involving
78
CA 03021185 2018-10-16
A00039
two or three of these therapies in combination to produce effects such as
prevention of
development of resistance, prolonged disease-free survival, inhibition of
cancer metastasis or
recurrence, life prolongation and the like.
[0187]
Also, the treatment with the compound of the present invention or the
combination drug
of the present invention may be combined with supportive care [(i) the
administration of an
antibiotic (e.g., a P-lactam antibiotic such as Pansporin and the like, a
macrolide antibiotic such
as clarithromycin and the like) against various intercurrent infections, (ii)
the administration of a
high-calorie infusion, an amino acid preparation or multivitamin for the
improvement of
malnutrition, (iii) the administration of morphine for pain relief, (iv) the
administration of a drug
improving adverse reactions such as nausea, vomiting, anorexia, diarrhea,
leukopenia,
thrombocytopenia, decreased hemoglobin concentration, alopecia, liver damage,
kidney damage,
DIC, fever and the like and (v) the administration of a drug for inhibiting
multidrug resistance of
cancer, etc.].
[0188]
The present invention will be described further specifically with reference to
Examples,
Formulation Examples and Test Examples given below. However, the present
invention is not
intended to be limited by them, and various changes or modifications may be
made therein
without departing from the scope of the present invention.
Examples
[0189]
In Examples below, the term "room temperature" usually means approximately 10
C to
approximately 35 C. A ratio used for a mixed solvent represents a volume ratio
unless
otherwise specified. % represents % by weight unless otherwise specified.
[0190]
In silica gel column chromatography, the term "NH" represents that an
aminopropylsilane-bound silica gel was used, the term "Diol" represents that a
3-(2,3-
dihydroxypropoxy)propylsilane-bound silica gel, was used, and the term "DiNH"
represents that
an N-(2-aminoethyl)-3-aminopropylsilane-bound silica gel. In HPLC (High Speed
Liquid
Chromatography) , the term "C18" represents that an octadecyl-bound silica gel
was used. A
ratio used for elution solvents represents a volume ratio unless otherwise
specified.
[0191]
In Examples below, the following abbreviations are used:
mp: melting point
79
CA 03021185 2018-10-16
A00039
MS: mass spectrum
[M+Hr, molecular ion peakM: molar concentration
N : normality
CDC13: deuterated chloroform
DMS0-4: deuterated dimethyl sulfoxide
1H NMER: proton nuclear magnetic resonance
LC/MS: liquid chromatograph-mass spectrometer
ES!: electrospray ionization
APCI: atmospheric pressure chemical ionization
THF : tetrahydrofuran
DME: 1,2-dimethoxyethane
DMF: N,N-dimethylformamide
DMA: N,N-dimethylacetamide
DMSO: dimethyl sulfoxide
HATU: 2-(7-azabenzotriazol-1-y1)-1,1,3,3-tetramethyluronium
hexafluorophosphate
HOBt: 1-hydroxybenzotriazole
CDI: 1,11-carbonyldiimidazole
Ty: pyridine
IPE: diisopropyl ether
DIPEA: N,N'-diisopropylethylamine
DMAP: N,N-dimethyl-4-aminopyridine
IPA: isopropanol
IPE :diisopropyl ether
KFIT/IDS: potassium bis(trimethylsilyl)amide
DIPA: diisopropylamine
NBS: N-bromosuccinimide
NMO: N-methylmorpholine N-oxide
TBAF: tetra-n-butylammonium fluoride
pTsCI: p-toluenesulfonyl chloride
TFA: trifluoroacetic acid
DIAD: diisopropyl azodicarboxylate
CPME: cyclopentyl methyl ether
EDC: 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
TLC: thin layer chromatography
CA 03021185 2018-10-16
A00039
IBX: 2-iodoxybenzoic acid
TBAI: tetrabutylammonium iodide
DIBAL: diisobutylaluminium hydride
AIBN: 2,2'-(E)-diazene-1,2-diyIbis(2-methylpropanenitrile)
[0192]
1H NMR was measured by Fourier transform NMR. ACD/SpecManager (trade name)
or the like was used in analysis. No mention was made about the very broad
peaks of protons
of a hydroxy group, an amino group and the like.
MS was measured by LC/MS. ESI or APCI was used as an ionization method. Data
was indicated by actually measured values (found). In general, molecular ion
peaks ([M+H]+,
and the like) are observed. For example, in the case of a compound having a
tert-
butoxycarbonyl group, a fragment ion peak derived from the elimination of the
tert-
butoxycarbonyl group or the tert-butyl group is observed. In the case of a
compound having a
hydroxy group, a fragment ion peak derived from the elimination of H20 may be
observed. In
the case of a salt, a molecular ion peak or fragment ion peak of a free form
is usually observed.
The unit of the sample concentration (c) in optical rotation (([a]D) is g/100
mL.
For the value of element analysis (Anal.), the calculated values (Calcd) and
measured values
(Found) are described.
[0193]
Example 1
2-(azetidin-l-y1)-1-(3,5-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-
d]pyrimidin-5-
y1)-1H-imidazo[4,5-b]pyridine
a) 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine
4-chloro-5H-pyrrolo[3,2-dlpyrimidine (10 g) was suspended in methanol (50 mL),
and
sodium methoxide (28% methanol solution) (30 mL) was added at room
temperature. Under a
dry atmosphere, the mixture was stirred at 60 C overnight. The reaction
mixture was
concentrated under reduced pressure. The residue was sequentially washed with
water and IPA,
and then dried under reduced pressure to obtain the title compound (6.44 g).
'H NMR (300 MHz, DMSO-d6) 5 4.07 (3H, s), 6.54 (1H, d, J = 3.0 Hz), 7.65 (1H,
d, J =
3.0 Hz), 8.40 (1H, s), 12.01 (1H, brs).
[0194]
b) 5-bromo-N3-(3,5-difluorobenzyppyridine-2,3-diamine
Acetic acid (5 mL) was added to a solution of 3,5-difluorobenzaldehyde (26.5
g) and 5-
bromopyridine-2,3-diamine (25 g) in THF (400 mL) at room temperature. Under a
dry
81
CA 03021185 2018-10-16
A00039
atmosphere, the mixture was stirred at room temperature for 10 hours. The
mixture was
neutralized with saturated aqueous sodium hydrogen carbonate solution at 0 C,
and extracted
with ethyl acetate (250 mL)/toluene (250 mL). The organic layer was washed
with water and a
saturated brine, dried over magnesium sulfate, and then concentrated to obtain
a residue.
Sodium borohydride (10.06 g) was suspended in THF (200 mL), methanol (200 mL)
was added
at 0 C, and the mixture was stirred for 10 minutes. The obtained suspension of
the residue in
THF (300 mL) was added to the reaction mixture at 0 C. Under a dry atmosphere,
the mixture
was stirred at room temperature for 2 hours. The reaction mixture was poured
into ice water at
0 C, and extracted with ethyl acetate. The organic layer was sequentially
washed with water
and a saturated brine, then dried over magnesium sulfate, and concentrated
under reduced
pressure. The residue was purified by silica gel column chromatography (ethyl
acetate/hexane)
to obtain the title compound (10 g).
1H NMR (300 MHz, DMSO-d6) M.35 (2H, d, J = 5.8 Hz), 5.71-5.86 (3H, m), 6.56
(1H,
d, J = 2.1 Hz), 7.03-7.16 (3H, m), 7.32 (1H, d, J = 2.1 Hz).
[0195]
c) 6-bromo-1-(3,5-difluorobenzy1)-1H-irnidazo[4,5-b]pyridin-2(3H)-one
Under a dry atmosphere, a mixture of 5-bromo-N3-(3,5-difluorobenzyl)pyridine-
2,3-
diamine (6 g), CDI (6.19 g) and THF (60 mL) was stirred at 60 C for 16 hours.
The reaction
mixture was diluted with water at room temperature, and extracted with ethyl
acetate. The
organic layer was sequentially washed with a saturated brine, then dried over
magnesium sulfate,
and concentrated under reduced pressure. The residue was washed with ethyl
acetate/lPE to
obtain the title compound (6.4 g).
1H NMR (300 MHz, DMSO-d6) 8 5.04 (2H, s), 7.03-7.23 (3H, m), 7.79 (1H, d, J =
2.1
Hz), 8.03 (1H, d, J = 2.0 Hz), 11.97 (1H, brs).
[0196]
d) 6-bromo-2-chloro-1-(3,5-difluorobenzy1)-1H-imidazo[4,5-b]pyridine
A mixture of 6-bromo-1-(3,5-difluorobenzy1)-1H-imidazo[4,5-b]pyridin-2(3H)-one
(6.4
g) and phosphorus oxychloride (45 mL) was stirred under a nitrogen atmosphere
at 100 C for 22
hours. The reaction mixture was concentrated under reduced pressure. The
residue was
diluted with THF, neutralized with a saturated aqueous sodium hydrogen
carbonate solution at
0 C, and extracted with ethyl acetate. The organic layer was washed with a
saturated brine,
dried over magnesium sulfate, and then concentrated. The residue was washed
with ethyl
acetate/WE to obtain the title compound (4.59 g).
1H NMR (300 MHz, DMSO-d6) 8 5.59 (2H, s), 7.00 (2H, dd, J = 8.3, 2.2 Hz), 7.22
(1H,
82
CA 03021185 2018-10-16
A00039
tt, J = 9.4, 2.2 Hz), 8.55 (2H, q, J 2.1 Hz).
[0197]
e) 2-(azetidin-1-y1)-6-bromo-1-(3,5-difluorobenzy1)-1H-imidazo[4,5-b]pyridine
A mixture of 6-bromo-2-chloro-1-(3,5-difluorobenzy1)-1H-imidazo[4,5-b]pyridine
(1.00
g), azetidine (0.8 mL) and THF (8 mL) was stirred under a dry atmosphere at
room temperature
for 16 hours. The reaction mixture was diluted with water at room temperature,
and extracted
with ethyl acetate. The organic layer was washed with a saturated brine, dried
over magnesium
sulfate, and then concentrated. The residue was washed with ethyl acetate to
obtain the title
compound (0.946 g).
1H NMR (300 MHz, DMSO-d6) 8 2.33 (2H, quin, J = 7.6 Hz), 4.19 (4H, t, J = 7.6
Hz),
5.31 (2H, s), 6.79-6.90 (2H, m), 7.18 (1H, tt, J = 9.4, 2.3 Hz), 7.86 (1H, d,
J ----- 2.2 Hz), 8.16 (1H,
d, J = 2.2 Hz).
[0198]
0 2-(azetidin-1-y1)-1-(3,5-difluorobenzyl)-6-(4-methoxy-5H-pyrrolo[3,2-
d]pyrimidin-5-
y1)-1H-imidazo[4,5-b]pyridine
A mixture of 2-(azetidin-1-y1)-6-bromo-1-(3,5-difluorobenzy1)-1H-imidazo[4,5-
b]pyridine (200 mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (157 mg), sodium t-
butoxide (101
mg), tris(dibenzylideneacetone)dipalladium (0) (33.8 mg), 2-di-tert-
butylphosphino-2',4',6'-
triisopropylbiphenyl (62.7 mg) and toluene (5 mL) was stirred under microwave
irradiation at
130 C for 2 hours. The mixture was diluted with water/methanol at room
temperature, and
extracted with ethyl acetate/THF. The organic layer was washed with a
saturated brine, dried
over magnesium sulfate, and then concentrated. The residue was purified by NH
silica gel
column chromatography (methanol/ethyl acetate), and the residue was washed
with ethyl acetate
to obtain the title compound (16.3 mg).
1H NMR (300 MHz, DMSO-d6) ö 2.36 (2H, quin, J = 7.6 Hz), 3.74 (3H, s), 4.22
(4H, t,
J = 7.6 Hz), 5.32 (2H, s), 6.77 (1H, d, J = 3.2 Hz), 6.86-6.94 (2H, m), 7.20
(1H, tt, J = 9.4, 2.3
Hz), 7.87 (2H, dd, J 2.8, 1.7 Hz), 8.27 (1H, d, J = 2.4 Hz), 8.48 (1H, s).
[0199]
Example 7
1-(3,5-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-
1H-
imidazo[4,5-b]pyridine
a) N3-(3,5-difluorobenzy1)-5-iodopyridine-2,3-diamine
A mixture of 3,5-difluorobenzaldehyde (14.4 g), 5-iodopyridine-2,3-diamine
(15.4 g),
acetic acid (10 mL) and THF (200 mL) was stirred under nitrogen atmosphere at
room
83
CA 03021185 2018-10-16
A00039
temperature for 13 hours. The reaction mixture was concentrated under reduced
pressure.
The residue was neutralized with a saturated aqueous sodium hydrogen carbonate
solution at
0 C, and extracted with ethyl acetate/THF. The organic layer was washed with a
saturated
aqueous sodium hydrogen carbonate solution and a saturated brine, dried over
magnesium
sulfate, and then concentrated to obtain a residue. Methanol (100 mL) was
added to a mixture
of sodium borohydride (6.6 g) and THF (200 mL) at 0 C. The obtained solution
of the residue
in THF (300 mL) was added to the reaction mixture at 0 C. Under a nitrogen
atmosphere, the
mixture was stirred at room temperature for 2 hours. Water and a saturated
aqueous ammonium
chloride solution were added to the reaction mixture at 0 C to stop the
reaction, and the mixture
was extracted with ethyl acetate. The organic layer was washed with a
saturated brine, then
dried over magnesium sulfate, and concentrated under reduced pressure. The
residue was
purified by silica gel column chromatography (ethyl acetate/hexane) to obtain
the title compound
(18.1 g).
1HNMR (300 MHz, DMSO-d6) 5 4.33 (2H, d, J = 5.9 Hz), 5.69 (1H, t, J = 5.8 Hz),
5.79
(2H, s), 6.65 (I H, d, J = 1.8 Hz), 7.01-7.18 (3H, m), 7.42 (1H, d, J = 1.8
Hz).
[0200]
b) 1-(3,5-difluorobenzy1)-6-iodo-2-methy1-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (1 mL) was added to a mixture of N3-(3,5-
_
difluorobenzy1)-5-iodopyridine-2,3-diamine (5 g) and trimethyl orthoacetate
(50 mL) at room
temperature. The mixture was stirred under a dry atmosphere at room
temperature for 2 hours.
The mixture was neutralized with a saturated aqueous sodium hydrogen carbonate
solution at
room temperature, and extracted with ethyl acetate/THF. The organic layer was
washed with a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was washed with ethyl acetate to obtain the title compound (3.46
g).
1HNMR (300 MHz, DMSO-d6) 5 2.55 (3H, s), 5.54 (2H, s), 6.80-6.92 (2H, m), 7.20
(1H, tt, J = 9.4,2.3 Hz), 8.39 (1H, d, J = 1.9 Hz), 8.53 (11-I, d, J = 2.0
Hz).
[0201]
c) 1-(3,5-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-
methyl-
IH-imidazo[4,5-b]pyridine
A mixture of 1-(3,5-difluorobenzyI)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine
(1.1
g), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (0.554 g), potassium phosphate
(1.819 g), trans-
N,N'-dimethylcyclohexane-1,2-diamine (0.406 g), copper (I) iodide (0.272 g)
and THF (26 mL)
was stirred under microwave irradiation at 110 C for 2 hours. The mixture was
diluted with
water at room temperature, and extracted with ethyl acetate. The organic layer
was washed
84
CA 03021185 2018-10-16
A00039
with a saturated brine, dried over magnesium sulfate, and then concentrated.
The residue was
purified by silica gel column chromatography (methanol/ethyl acetate), and the
residue was
crystallized from ethanol/WE to obtain the title compound (0.916 g).
1HNMR (300 MHz, DMSO-d6) 8 2.63 (3H, s), 3.73 (3H, s), 5.57 (2H, s), 6.83 (1H,
d,
= 3.2 Hz), 6.88-6.97 (2H, m), 7.22 (1H, tt, J = 9.4, 2.3 Hz), 7.97 (1H, d, J =
3.2 Hz), 8.29 (1H, d,
J = 2.4 Hz), 8.51 (1H, s), 8.55 (1H, d, J = 2.4 Hz).
[0202]
Example 10
2-ethoxy-1-(3-fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-1H-
imidazo[4,5-b]pyridine
a) N3-(3-fluorobenzy1)-5-iodopyridine-2,3-diamine
A mixture of 3-fluorobenzaldehyde (4.75 g), 5-iodopyridine-2,3-diamine (6 g),
acetic
acid (3.7 mL) and THF (80 mL) was stirred under a nitrogen atmosphere at room
temperature for
3 days. The reaction mixture was concentrated under reduced pressure. The
residue was
neutralized with a saturated aqueous sodium hydrogen carbonate solution at 0
C, and extracted
with ethyl acetate/THF. The organic layer was washed with a saturated aqueous
sodium
hydrogen carbonate solution and a saturated brine, dried over magnesium
sulfate, and then
concentrated to obtain a residue. Methanol (30 mL) was added to a mixture of
sodium
borohydride (2.83 g) and THF (80 mL) at 0 C. The obtained solution of the
residue in THF
(120 mL) was added to the reaction mixture at 0 C. Under a nitrogen
atmosphere, the mixture
was stirred at room temperature for 2 hours. Water and a saturated aqueous
ammonium
chloride solution were added to the reaction mixture at 0 C to stop the
reaction, and the mixture
was extracted with ethyl acetate. The organic layer was washed with a
saturated brine, then
dried over magnesium sulfate, and concentrated under reduced pressure. The
residue was
purified by silica gel column chromatography (ethyl acetate/hexane) to obtain
the title compound
(4.61 g).
1H NMR (300 MHz, DMSO-d6) S 4.31 (2H, d, J = 5.7 Hz), 5.67 (1H, t, J = 5.7
Hz),
5.79 (2H, s), 6.65 (1H, d, J = 1.8 Hz), 7.03-7.12 (1H, m), 7.13-7.23 (2H, m),
7.34-7.44 (2H, m).
[0203]
b) 1-(3-fluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridine-2(3H)-thione
Under a dry atmosphere, a mixture of N3-(3-fluorobenzy1)-5-iodopyridine-2,3-
diamine
(1.15 g), 1,1'-thiocarbonyldiimidazole (1.31 g) and THF (15 mL) was stirred at
60 C for 16
hours. The reaction mixture was quenched with water at room temperature, and
extracted with
ethyl acetate. The organic layer was sequentially washed with a saturated
aqueous ammonium
A00039
CA 03021185 2018-10-16
chloride solution, water and a saturated brine, then dried over magnesium
sulfate, and
concentrated under reduced pressure. The residue was washed with ethyl acetate
to obtain the
title compound (1.26 g).
11-1NMR (300 MI-1z, DMSO-d6) 8 5.49 (2H, s), 7.08-7.27 (3H, m), 7.39 (1H, td,
J = 7.9,
.. 6.2 Hz), 8.09 (1H, d, J = 1.7 Hz), 8.36 (1H, d, J = 1.7 Hz), 13.69 (1H,
brs).
[0204]
c) 2-ethoxy-1-(3-fluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridine
To a mixture of 1-(3-fluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridine-2(3H)-
thione
(1.25 g) and DMF (2 mL) was added thionyl chloride (5 mL). Under a dry
atmosphere, the
mixture was stirred at 700 C for 30 minutes. The reaction mixture was
concentrated under
reduced pressure. The residue was neutralized with a saturated aqueous sodium
hydrogen
carbonate solution at 0 C, and extracted with ethyl acetate/TI-IF. The organic
layer was washed
with a saturated brine, then dried over magnesium sulfate, and concentrated
under reduced
pressure. The residue was suspended in ethanol (5 mL)/THF (5 mL), and sodium
ethoxide
.. (21% ethanol solution) (7 mL) was added at room temperature. Under a dry
atmosphere, the
mixture was stirred at room temperature for 1 hour. The mixture was diluted
with water at
room temperature, and extracted with ethyl acetate. The organic layer was
washed with a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was purified by silica gel column chromatography (ethyl
acetate/hexane), and
washed with IPE to obtain the title compound (0.498 g).
IHNMR (300 MHz, DMSO-d6) 5 1.39 (3H, t, J = 7.0 Hz), 4.61 (2H, q, J = 7.1 Hz),
5.27
(2H, s), 7.06 (1H, d, J = 7.8 Hz), 7.10-7.18 (2H, m), 7.34-7.46 (1H, m), 8.22
(1H, d, J = 1.9 Hz),
8.38 (1H, d, J = 1.9 Hz).
[0205]
d) 2-ethoxy-1-(3-fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-
1H-
imidazo[4,5-b]pyridine
A mixture of 2-ethoxy-1-(3-fluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridine (300
mg),
4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (146 mg), potassium phosphate (481 mg),
trans-N,N'-
dimethylcyclohexane-1,2-diamine (107 mg), copper (1) iodide (71.9 mg) and THF
(4 mL) was
stirred under microwave irradiation at 110 C for 2 hours. The mixture was
diluted with water
at room temperature, and extracted with ethyl acetate. The organic layer was
washed with a
saturated brine, dried over magnesium sulfate, and then concentrated. The
residue was purified
by NH silica gel column chromatography (methanol/ethyl acetate), and the
residue was washed
with ethyl acetate to obtain the title compound (37.2 mg).
86
CA 03021185 2018-10-16
A00039
IHNNIR (300 MHz, DMSO-d6) 6 1.44 (3H, t, J = 7.0 Hz), 3.73 (3H, s), 4.67 (2H,
q, J =
7.1 Hz), 5.29 (2H, s), 6.81 (1H, d, J = 3.2 Hz), 7.10-7.24 (3H, m), 7.37-7.49
(1H, m), 7.92 (1H,
d, J = 3.2 Hz), 8.16 (1H, d, J = 2.4 Hz), 8.38 (1H, d, J = 2.3 Hz), 8.50 (1H,
s).
[0206]
Example 12
1-(3-fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-1H-
imidazo[4,5-b]pyridine
a) 1-(3-fluorobenzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (0.5 mL) was added to a mixture of N3-(3-
fluorobenzy1)-5-iodopyridine-2,3-diamine (1.5 g) and trimethyl orthoacetate
(20 mL) at room
temperature. The mixture was stirred under a dry atmosphere at room
temperature for 2 hours.
The mixture was neutralized with a saturated aqueous sodium hydrogen carbonate
solution at
room temperature, and extracted with ethyl acetate/THF. The organic layer was
washed with a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was washed with ethyl acetate to obtain the title compound (1.31
g).
IFINMR (300 MHz, DMSO-d6) 6 2.54 (3H, s), 5.54 (2H, s), 6.91 (1H, d, J = 7.6
Hz),
7.02 (1H, dt, J = 9.9, 2.0 Hz), 7.14 (1H, td, J = 8.5, 2.2 Hz), 7.39 (1H, td,
J = 8.0, 6.1 Hz), 8.39
(1H, d, J = 1.9 Hz), 8.52 (1H, d, J = 1.9 Hz).
[0207]
b) 1-(3-fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-
1H-
imidazo[4,5-b]pyridine
A mixture of 1-(3-fluorobenzy1)-6-iodo-2-methy1-1H-imidazo[4,5-b]pyridine (320
mg),
4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (169 mg), potassium phosphate (555 mg),
trans-N,N'-
dimethylcyclohexane-1,2-diamine (124 mg), copper (I) iodide (83 mg) and TI-IF
(5 mL) was
stirred under microwave irradiation at 110 C for 2 hours. The mixture was
diluted with water
and a 28% aqueous ammonia solution at room temperature, and extracted with
ethyl acetate.
The organic layer was washed with a saturated brine, dried over magnesium
sulfate, and then
concentrated. The residue was purified by NH silica gel column chromatography
(methanol/ethyl acetate), and the residue was washed with ethyl acetate to
obtain the title
compound (176 mg).
1HNMR (300 MHz, DMSO-d6) 6 2.62 (3H, s), 3.71 (311, s), 5.57 (211, s), 6.83
(1H, d, J
= 3.2 Hz), 6.99 (1H, d, J = 7.7 Hz), 7.07 (1H, dt, J = 9.9, 2.0 Hz), 7.16 (1H,
td, J = 8.5, 2.2 Hz),
7.42 (1H, td, J = 7.9, 6.1 Hz), 7.96 (1H, d, J = 3.2 Hz), 8.28 (1H, d, J = 2.4
Hz), 8.51 (1H, s),
8.54 (1H, d, J = 2.4 Hz).
87
CA 03021185 2018-10-16
A00039
[0208]
Example 13
(1-(3-fluorobenzyl)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-1H-
imidazo[4,5-
b]pyridin-2-yl)methanol
a) (1-(3-fluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridin-2-yl)methanol
A mixture of N3-(3-fluorobenzy1)-5-iodopyridine-2,3-diamine (1.5 g), 2-
hydroxyacetic
acid (8.31 g) and TI-IF (3 mL) was heated under microwave irradiation at 150 C
for 2 hours.
The reaction mixture was poured into a saturated aqueous sodium hydrogen
carbonate solution,
and extracted with ethyl acetate. The organic layer was washed with a
saturated brine, then
dried over magnesium sulfate, and concentrated under reduced pressure. The
residue was
purified by silica gel column chromatography (methanol/ethyl acetate) to
obtain the title
compound (1.2 g).
1H NIVIR (300 MHz, DMSO-d6) 8 4.76 (2H, d, J = 5.9 Hz), 5.61 (2H, s), 5.84-
5.92 (1H,
m), 7.00-7.18 (3H, m), 7.34-7.43 (1H, m), 8.31 (1H, d, J = 2.0 Hz), 8.56 (1H,
d, J = 2.0 Hz).
[0209]
b) (1-(3-fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-1H-
imidazo[4,5-b]pyridin-2-yl)methanol
A mixture of (1-(3-fluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridin-2-y1)methanol
(959
mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (485 mg), potassium phosphate (1594
mg), trans-
N,N'-dimethylcyclohexane-1,2-diamine (356 mg), copper (I) iodide (238 mg) and
THF (13 mL)
was stirred under microwave irradiation at 110 C for 2 hours. The mixture was
diluted with
water and a 28% aqueous ammonia solution at room temperature, and extracted
with ethyl
acetate. The organic layer was washed with a saturated brine, dried over
magnesium sulfate,
and then concentrated. The residue was purified by NH silica gel column
chromatography
(methanol/ethyl acetate), and the residue was washed with ethyl
acetate/ethanol to obtain the title
compound (153 mg).
1H NMR (300 MHz, DMSO-d6) 83.65 (3H, s), 4.83 (2H, d, J = 5.6 Hz), 5.64 (2H,
s),
5.93 (1H, t, J = 5.6 Hz), 6.83 (1H, d, J = 3.2 Hz), 7.07-7.21 (3H, m), 7.36-
7.47 (1H, m), 7.96
(1H, d, J = 3.2 Hz), 8.22 (1H, d, J = 2.2 Hz), 8.50 (1H, s), 8.59 (1H, d, J =
2.2 Hz).
[0210]
Example 17
1-(3,4-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-
111-
imidazo[4,5-b]pyridine
a) N3-(3,4-difluorobenzy1)-5-iodopyridine-2,3-diamine
88
CA 03021185 2018-10-16
A00039
A mixture of 3,4-difluorobenzaldehyde (7 g), 5-iodopyridine-2,3-diamine (7.3
g), acetic
acid (5 mL) and THF (100 mL) was stirred under nitrogen atmosphere at room
temperature for
13 hours. The reaction mixture was concentrated under reduced pressure. The
residue was
neutralized with a saturated aqueous sodium hydrogen carbonate solution at 0
C, and extracted
with ethyl acetate/THF. The organic layer was washed with a saturated aqueous
sodium
hydrogen carbonate solution and a saturated brine, dried over magnesium
sulfate, and then
concentrated to obtain a residue. Methanol (50 mL) was added to a mixture of
sodium
borohydride (2.9 g) and THF (100 mL) at 0 C. The obtained solution of the
residue in THF (50
mL) was added to the reaction mixture at 0 C. Under a nitrogen atmosphere, the
mixture was
stirred at room temperature for 2 hours. A saturated aqueous ammonium chloride
solution was
added to the reaction mixture at 0 C to stop the reaction, and the mixture was
extracted with
ethyl acetate. The organic layer was washed with a saturated brine, then dried
over magnesium
sulfate, and concentrated under reduced pressure. The residue was purified by
silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound (8
g).
1H islivLR (300 MHz, DMSO-d6) 5 4.28 (2H, d, J = 5.7 Hz), 5.64 (1H, t, J = 5.7
Hz), 5.78
(2H, s), 6.66 (1H, d, J = 1.8 Hz), 7.15-7.25 (1H, m), 7.33-7.48 (3H, m).
[0211]
b) 1-(3,4-difluorobenzy1)-6-iodo-2-methy1-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (300 L) was added to a mixture of N3-(3,4-
difluorobenzy1)-5-iodopyridine-2,3-diamine (1.1 g) and trimethyl orthoacetate
(5 mL) at room
temperature. The mixture was stirred at room temperature for 1 hour. The
reaction mixture
was concentrated under reduced pressure. The residue was washed with IPE to
obtain the title
compound (790 mg).
1H NMR (300 MHz, DMSO-d6) 2.55 (3H, s), 5.50 (2H, s), 6.89-7.02 (1H, m), 7.25-
7.50 (2H, m), 8.40 (1H, d, J = 1.9 Hz), 8.52 (1H, d, J = 2.0 Hz).
[0212]
c) 1-(3,4-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-
methyl-
1H-imidazo[4,5-b]pyridine
A mixture of 1-(3,4-difluorobenzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine
(202.7
mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (78 mg), potassium phosphate (335
mg), trans-
N,N'-dimethylcyclohexane-1,2-diamine (90 mg), copper (I) iodide (60.1 mg) and
THF (2 mL)
was stirred under microwave irradiation at 150 C for 3 hours. The mixture was
cooled to room
temperature, and insolubles were removed. The mixture was concentrated under
reduced
pressure. The residue was purified by NH silica gel column chromatography
(methanol/ethyl
89
CA 03021185 2018-10-16
A00039
acetate), and crystallized from ethanol to obtain the title compound (66 mg).
1H NMR (300 MHz, DMSO-d6) 6 2.63 (3H, s), 3.74 (3H, s), 5.53 (2H, s), 6.83
(1H, d, J
= 3.2 Hz), 6.96-7.09 (1H, m), 7.31-7.51 (2H, m), 7.96 (1H, d, J = 3.2 Hz),
8.29 (1H, d, J = 2.4
Hz), 8.46-8.58 (2H, m).
[0213]
Example 20
1-(2,3-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methy1-
1H-
imidazo[4,5-b]pyridine
a) N3-(2,3-difluorobenzy1)-5-iodopyridine-2,3-diamine
A mixture of 2,3-difluorobenzaldehyde (13.6 g), 5-iodopyridine-2,3-diamine (15
g),
acetic acid (7.31 mL) and TI-IF (100 mL) was stirred under nitrogen atmosphere
at room
temperature for 13 hours. The reaction mixture was concentrated under reduced
pressure.
The residue was neutralized with a saturated aqueous sodium hydrogen carbonate
solution at
0 C, and extracted with ethyl acetate/THF. The organic layer was washed with a
saturated
aqueous sodium hydrogen carbonate solution and a saturated brine, dried over
magnesium
sulfate, and then concentrated to obtain a residue. Methanol (50 mL) was added
to a mixture of
sodium borohydride (6.04 g) and THF (100 mL) at 0 C. The obtained solution of
the residue in
TI-IF (300 mL) was added to the reaction mixture at 0 C. Under a nitrogen
atmosphere, the
mixture was stirred at room temperature for 2 hours. Water and a saturated
aqueous ammonium
chloride solution were added to the reaction mixture at 0 C to stop the
reaction, and the mixture
was extracted with ethyl acetate. The organic layer was washed with a
saturated brine, then
dried over magnesium sulfate, and concentrated under reduced pressure. The
residue was
purified by silica gel column chromatography (ethyl acetate/hexane) to obtain
the title compound
(14.1 g).
1H NMR (300 MHz, DMSO-d6) 6 4.36 (2H, d, J 5.6 Hz), 5.54-5.66 (1H, m), 5.80
(2H,
s), 6.72 (1H, d, J = 1.6 Hz), 7.11-7.56 (4H, m).
[0214]
b) 1-(2,3-difluorobenzyl)-6-iodo-2-methy1-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (300 IlL) was added to a mixture of N3-(2,3-
difluorobenzyI)-5-iodopyridine-2,3-diamine (144.9 mg) and trimethyl
orthoacetate (3 mL) at
room temperature. The mixture was stirred at room temperature for 1 hour. The
reaction
mixture was concentrated under reduced pressure. The residue was purified by
NH silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound (105
mg).
1H NMR (400 MHz, DMSO-d6) 6 2.53 (3H, s), 5.64 (211, s), 6.76 (111, t, J = 7.3
Hz),
CA 03021185 2018-10-16
A00039
=
7.13-7.22 (1H, m), 7.36-7.46 (1H, m), 8.40 (1H, d, J = 2.0 Hz), 8.52 (1H, d, J
= 2.0 Hz).
[0215]
c) 1-(2,3-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-
methyl-
1H-imidazo[4,5-b]pyridine
A mixture of 1-(2,3-difluorobenzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine
(105.1
mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (44.8 mg), potassium phosphate (174
mg), trans-
N,N'-dimethylcyclohexane-1,2-diamine (46.6 mg), copper (I) iodide (31.2 mg)
and THF (2 mL)
was stirred under microwave irradiation at 150 C for 3 hours. The mixture was
cooled to room
temperature, poured into water, and extracted with ethyl acetate. The organic
layer was washed
with a saturated brine, then dried over magnesium sulfate, and concentrated
under reduced
pressure. The residue was purified by NH silica gel column chromatography
(methanol/ethyl
acetate), and crystallized from ethanol/IPE to obtain the title compound (44.6
mg).
1HNMR (300 MHz, DMSO-d6) 5 2.61 (3H, s), 3.72 (3H, s), 5.67 (2H, s), 6.77-6.85
(2H, m), 7.15-7.25 (1H, m), 7.36-7.48 (1H, m), 7.94 (1H, d, J = 3.2 Hz), 8.26
(1H, d, J = 2.4 Hz),
8.51 (1H, s), 8.55 (1H, d, J = 2.4 Hz).
[0216]
Example 21
1-benzy1-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-1H-imidazo[4,5-
b]pyridine
a) N3-Benzy1-5-iodopyridine-2,3-diamine
Acetic acid (200 L) was added to a solution of benzaldehyde (600 L) and 5-
iodopyridine-2,3-diamine (1.12 g) in THF (50 mL) at room temperature. The
mixture was
stirred overnight at room temperature. The reaction mixture was poured into a
saturated
aqueous sodium hydrogen carbonate solution, and extracted with ethyl acetate.
The organic
layer was washed with water and a saturated brine, dried over magnesium
sulfate, and then
concentrated. Sodium borohydride (0.27 g) was added to a solution of the
residue in methanol
(50 mL) at 0 C. The mixture was stirred at 0 C for 1 hour. The reaction
mixture was poured
into a saturated aqueous ammonium chloride solution, and extracted with ethyl
acetate. The
organic layer was washed with water and a saturated brine, then dried over
magnesium sulfate,
and concentrated under reduced pressure. The residue was purified by NH silica
gel column
chromatography (ethyl acetate/hexane) to obtain the title compound (1.13 g).
1H NMR (300 MHz, DMSO-d6) 5 4.28 (2H, d, J = 5.6 Hz), 5.64 (1H, t, J = 5.6
Hz), 5.78
(2H, s), 6.65 (1H, d, J = 1.8 Hz), 7.20-7.43 (6H, m).
[0217]
91
CA 03021185 2018-10-16
A00039
b) 1-benzy1-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (0.1 mL) was added to a mixture of N3-benzy1-5-
iodopyridine-2,3-diamine (300 mg) and trimethyl orthoacetate (4 mL) at room
temperature.
The mixture was stirred under a dry atmosphere at room temperature for 1 hour.
The mixture
was neutralized with a saturated aqueous sodium hydrogen carbonate solution at
room
temperature, and extracted with ethyl acetate/THF. The organic layer was
washed with a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was washed with ethyl acetate/hexane to obtain the title compound
(230 mg).
IHNMR (300 MHz, DMSO-d6) 6 2.54 (3H, s), 5.51 (2H, s), 7.05-7.17 (2H, m), 7.23-
7.44 (31-1, m), 8.38 (1H, d, J = 1.9 Hz), 8.51 (1H, d, J = 1.9 Hz).
[0218]
c) 1-benzy1-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-1H-
imidazo[4,5-
b]pyridine
A mixture of 1-benzy1-6-iodo-2-methyl-11-1-imidazo[4,5-b]pyridine (200 mg), 4-
methoxy-5H-pyrrolo[3,2-d]pyrimidine (111 mg), potassium phosphate (365 mg),
trans-N,N'-
dimethykyclohexane-1,2-diamine (0.09 mL), copper (1) iodide (54.5 mg) and TI-
IF (5 mL) was
stirred under microwave irradiation at 110 C for 2 hours. The mixture was
diluted with water
and a 28% aqueous ammonia solution at room temperature, and extracted with
ethyl acetate.
The organic layer was washed with a saturated brine, dried over magnesium
sulfate, and then
concentrated. The residue was purified by NH silica gel column chromatography
(methanol/ethyl acetate), and the residue was crystallized from water/ethanol
to obtain the title
compound (120 mg).
IFINMR (300 MHz, DMSO-d6) 5 2.61 (3H, s), 3.70 (3H, s), 5.55 (2H, s), 6.83
(1H, d, J
= 3.2 Hz), 7.13-7.24 (2H, m), 7.25-7.44 (3H, m), 7.96 (1H, d, J = 3.2 Hz),
8.27 (1H, d, J = 2.4
Hz), 8.50 (1H, s), 8.53 (1H, d, J = 2.4 Hz).
[0219]
Example 22
1-(4-fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-1H-
imidazo[4,5-b]pyridine
a) N3-(4-fluorobenzy1)-5-iodopyridine-2,3-diamine
A mixture of 4-fluorobenzaldehyde (0.507 mL), 5-iodopyridine-2,3-diamine (1.03
g),
TI-IF (10 mL) and acetic acid (0.632 mL) was stirred overnight at room
temperature. The
reaction mixture was poured into a saturated aqueous sodium hydrogen carbonate
solution, and
extracted with ethyl acetate. The organic layer was washed with a saturated
brine, dried over
92
CA 03021185 2018-10-16
A00039
magnesium sulfate, and then concentrated. Sodium borohydride (0.415 g) was
added to a
solution of the residue in methanol (10 mL) at room temperature. The mixture
was stirred
overnight at room temperature. The reaction mixture was poured into water, and
extracted with
ethyl acetate. The organic layer was washed with a saturated brine, then dried
over magnesium
sulfate, and concentrated under reduced pressure. The residue was purified by
silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound
(1.21 g).
11-1 NIVIR (300 MHz, DMSO-d6) 6 4.26 (2H, d, J = 5.5 Hz), 5.63 (1H, t, J = 5.6
Hz), 5.78
(2H, s), 6.65 (1H, d, J = 1.8 Hz), 7.12-7.23 (2H, m), 7.34-7.43 (3H, m).
[0220]
b) 1-(4-fluorobenzy1)-6-iodo-2-methy1-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (0.1 mL) was added to a mixture of N3-(4-
fluorobenzy1)-5-iodopyridine-2,3-diamine (300 mg) and trimethyl orthoacetate
(4 mL) at room
temperature. The mixture was stirred under a dry atmosphere at room
temperature for 1 hour.
The mixture was neutralized with a saturated aqueous sodium hydrogen carbonate
solution at
room temperature, and extracted with ethyl acetate/THF. The organic layer was
washed with a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was washed with ethyl acetate to obtain the title compound (244
mg).
IfINMR (300 MHz, DMSO-d6) l 2.54 (3H, s), 5.50 (2H, s), 7.13-7.25 (4H, m),
8.40
(1H, d, J -= 2.0 Hz), 8.51 (1H, d, J = 2.0 Hz).
[0221]
1-(4-fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-1H-
imidazo[4,5-b]pyridine
A mixture of 1-(4-fluorobenzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine (200
mg),
4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (106 mg), potassium phosphate (347 mg),
trans-N,N-
dimethylcyclohexane-1,2-diamine (0.086 mL), copper (I) iodide (51.9 mg) and
THF (5 mL) was
stirred under microwave irradiation at 110 C for 2 hours. The mixture was
diluted with water
and a 28% aqueous ammonia solution at room temperature, and extracted with
ethyl acetate.
The organic layer was washed with a saturated brine, dried over magnesium
sulfate, and then
concentrated. The residue was purified by NH silica gel column chromatography
(methanol/ethyl acetate), and the residue was crystallized from water/ethanol
to obtain the title
compound (88 mg).
1HNMR (300 MHz, DMSO-d6) 6 2.62 (3H, s), 3.73 (3H, s), 5.53 (2H, s), 6.83 (11-
1, d, J
= 3.2 Hz), 7.10-7.38 (4H, m), 7.96 (1H, d, J = 3.2 Hz), 8.29 (1H, d, J = 2.4
Hz), 8.51 (1H, s),
8.53 (1H, d, J = 2.4 Hz).
93
CA 03021185 2018-10-16
A00039
[0222]
Example 23
1-(2,5-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-
1H-
imidazo[4,5-b]pyridine
a) N3-(2,5-difluorobenzy1)-5-iodopyridine-2,3-diamine
Acetic acid (200 pi.) was added to a solution of 2,5-difluorobenzaldehyde (400
L) and
5-iodopyridine-2,3-diamine (0.72 g) in THF (50 mL) at room temperature. The
mixture was
stirred overnight at room temperature. The reaction mixture was poured into a
saturated
aqueous sodium hydrogen carbonate solution, and extracted with ethyl acetate.
The organic
layer was washed with water and a saturated brine, dried over magnesium
sulfate, and then
concentrated. Sodium borohydride (0.174 g) was added to a solution of the
residue in methanol
(50 mL) at 0 C. The mixture was stirred at 0 C for 1 hour. The reaction
mixture was poured
into a saturated aqueous ammonium chloride solution, and extracted with ethyl
acetate. The
organic layer was washed with water and a saturated brine, then dried over
magnesium sulfate,
and concentrated under reduced pressure. The residue was purified by NH silica
gel column
chromatography (ethyl acetate/hexane) to obtain the title compound (0.82 g).
1H NMR (300 MHz, DMSO-d6) 64.31 (2H, d, J = 5.6 Hz), 5.56 (1H, t, J = 5.6 Hz),
5.80
(2H, s), 6.72 (1H, d, J = 1.8 Hz), 7.07-7.35 (31-1, m), 7.44 (1H, d, J = 1.8
Hz).
[0223]
b) 1-(2,5-difluorobenzy1)-6-iodo-2-methy1-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (0.1 mL) was added to a mixture of N3-(2,5-
difluorobenzy1)-5-iodopyridine-2,3-diamine (300 mg) and trimethyl orthoacetate
(4 mL) at room
temperature. The mixture was stirred under a dry atmosphere at room
temperature for 1 hour.
The mixture was neutralized with a saturated aqueous sodium hydrogen carbonate
solution at
room temperature, and extracted with ethyl acetate/THF. The organic layer was
washed with a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was washed with ethyl acetate to obtain the title compound (249
mg).
1H NMR (300 MHz, DMSO-d6) 6 2.54 (3H, s), 5.56 (2H, s), 6.85-7.03 (1H, m),
7.16-
7.42 (2H, m), 8.30-8.58 (2H, m).
[0224]
c) 1-(2,5-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-
methyl-
1H-imidazo[4,5-b]pyridine
A mixture of 1-(2,5-difluorobenzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine
(200
mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (101 mg), potassium phosphate (331
mg), trans-
94
CA 03021185 2018-10-16
A00039
N,N'-dimethylcyclohexane-1,2-diamine (0.082 mL), copper (I) iodide (49.4 mg)
and THF (5
mL) was stirred under microwave irradiation at 110 C for 2 hours. The mixture
was diluted
with water and a 28% aqueous ammonia solution at room temperature, and
extracted with ethyl
acetate. The organic layer was washed with a saturated brine, dried over
magnesium sulfate,
and then concentrated. The residue was purified by NH silica gel column
chromatography
(methanol/ethyl acetate), and the residue was washed with ethyl acetate to
obtain the title
compound (131 mg).
1H NMR (300 MI-lz, DMSO-d6) 8 2.62 (3H, s), 3.72 (3H, s), 5.59 (2H, s), 6.83
(1H, d, J
= 3.3 Hz), 6.91-7.02 (1H, m), 7.17-7.42 (2H, m), 7.94 (1H, d, J = 3.3 Hz),
8.25 (1H, d, J = 2.4
Hz), 8.51 (1H, s), 8.54 (1H, d, J = 2.4 Hz).
[0225]
Example 24
1-(3-fluorobenzy1)-2-(methoxymethyl)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-
y1)-1H-imidazo[4,5-b]pyridine
a) 1-(3-fluorobenzy1)-6-iodo-2-(methoxymethyl)-1H-imidazo[4,5-b]pyridine
A mixture of N3-(3-fluorobenzy1)-5-iodopyridine-2,3-diamine (251 mg), 2-
methoxyacetic acid (86 mg), THE' (4 mL), propylphosphonic anhydride (50% in
ethyl acetate
solution) (0.58 mL) and DIPEA (0.35 mL) was heated under microwave irradiation
at 200 C for
2 hours. The mixture was diluted with a saturated aqueous sodium hydrogen
carbonate solution
at room temperature, and extracted with ethyl acetate. The organic layer was
washed with a
saturated brine, dried over magnesium sulfate, and then concentrated. The
residue was purified
by NH silica gel column chromatography (ethyl acetate/hexane) to obtain the
title compound
(232 mg).
1H NMR (300 MHz, DMSO-d6) 6 3.31 (3H, s), 4.72 (2H, s), 5.58 (2H, s), 6.98
(1H, d, J
= 7.7 Hz), 7.03-7.18 (2H, m), 7.39 (1H, td, J = 8.0, 6.1 Hz), 8.37 (1H, d, J =
2.0 Hz), 8.60 (1H, d,
J = 1.9 Hz).
[0226]
b) 1-(3-fluorobenzy1)-2-(methoxymethyl)-6-(4-methoxy-5H-pyrrolo[3,2-
d]pyrimidin-5-
y1)-1H-imidazo[4,5-b]pyridine
A mixture of 1-(3-fluorobenzy1)-6-iodo-2-(methoxymethyl)-1H-imidazo[4,5-
b]pyridine
(226 mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (110 mg), potassium phosphate
(362 mg),
trans-N,N'-dimethylcyclohexane-1,2-diamine (81 mg), copper (I) iodide (54.2
mg) and THF (4
mL) was stirred under microwave irradiation at 110 C for 2 hours. The mixture
was diluted
with water and a 28% aqueous ammonia solution at room temperature, and
extracted with ethyl
CA 03021185 2018-10-16
A00039
acetate. The organic layer was washed with a saturated brine, dried over
magnesium sulfate,
and then concentrated. The residue was purified by NI-1 silica gel column
chromatography
(methanol/ethyl acetate), and the residue was washed with ethyl acetate/WE to
obtain the title
compound (73.8 mg).
11-1 NIvIR (300 MHz, DMSO-d6) 6 3.35 (3H, s), 3.68 (3H, s), 4.79 (2H, s), 5.61
(2H, s),
6.84 (1H, d, J = 3.1 Hz), 7.01-7.22 (3H, m), 7.35-7.47 (1H, m), 7.96 (1H, d, J
= 3.1 Hz), 8.28
(1H, d, J = 2.1 Hz), 8.51 (1H, s), 8.63 (1H, d, J = 2.0 Hz).
[0227]
Example 27
5-(1-(3-fluorobenzy1)-2-methy1-1H-imidazo[4,5-b]pyridin-6-y1)-N-methy1-5H-
pyrrolo[3,2-d]pyrimidine-4-amine
a) 6-(4-chloro-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-1-(3-fluorobenzy1)-2-methy1-1H-
imidazo[4,5-b]pyridine
A mixture of 1-(3-fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-
2-
methyl-1H-imidazo[4,5-b]pyrimidine (155 mg) and phosphorus oxychloride (4.5
mL) was stirred
under a nitrogen atmosphere at 100 C for 2 hours. The reaction mixture was
concentrated
under reduced pressure. The residue was neutralized with a saturated aqueous
sodium
hydrogen carbonate solution at room temperature, and extracted with ethyl
acetate. The organic
layer was washed with a saturated brine, dried over magnesium sulfate, and
then concentrated.
The residue was purified by NH silica gel column chromatography (ethyl
acetate), and washed
with ethyl acetate to obtain the title compound (112 mg).
NMR (300 MHz, DMSO-d6) 6 2.64 (3H, s), 5.57 (2H, s), 6.96-7.07 (31-1, m), 7.14
(1H, td, J = 8.5, 2.2 Hz), 7.39 (1H, td, J = 8.0, 6.1 Hz), 8.24 (1H, d, J =
3.3 Hz), 8.37 (1H, d, J =
2.3 Hz), 8.57 (1H, d, J = 2.3 Hz), 8.75 (1H, s).
[0228]
b) 5-(1-(3-fluorobenzy1)-2-methyl-1H-imidazo[4,5-b]pyridin-6-y1)-N-methyl-5H-
pyrrolo[3,2-d]pyrimidin-4-amine
A mixture of 6-(4-chloro-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-1-(3-fluorobenzy1)-2-
methyl-1H-imidazo[4,5-b]pyridine (95 mg) and methylamine (2M in THF solution)
(4 mL) was
stirred under microwave irradiation at 120 C for 3 hours. The mixture was
purified by NH
silica gel column chromatography (ethyl acetate), and washed with ethyl
acetate to obtain the
title compound (42.7 mg).
11-1NMR (300 MHz, DMSO-d6) 6 2.57 (3H, s), 2.71 (3H, d, J = 4.5 Hz), 5.47 (1H,
d, J =
4.5 Hz), 5.57 (2H, s), 6.62 (1H, d, J = 3.2 Hz), 7.04-7.19 (3H, m), 7.36-7.46
(1H, m), 7.68 (1H,
96
CA 03021185 2018-10-16
A00039
d, J = 3.2 Hz), 8.08 (1H, d, J = 2.4 Hz), 8.29 (1H, s), 8.54 (1H, d, J = 2.4
Hz).
[0229]
Example 28
1-(2-fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-1H-
imidazo[4,5-b]pyridine
a) N3-(2-fluorobenzy1)-5-iodopyridine-2,3-diamine
Under a nitrogen atmosphere, a mixture of 2-fluorobenzaldehyde (0.792 g), 5-
iodopyridine-2,3-diamine (1 g), THF (100 mL) and acetic acid (0.487 mL) was
stirred at room
temperature for 13 hours. The reaction mixture was concentrated under reduced
pressure.
The residue was neutralized with a saturated aqueous sodium hydrogen carbonate
solution at
0 C, and extracted with ethyl acetate/THF. The organic layer was washed with a
saturated
aqueous sodium hydrogen carbonate solution and a saturated brine, dried over
magnesium
sulfate, and then concentrated to obtain a residue. Methanol (100 mL) was
added to a mixture
of sodium borohydride (0.402 g) and THF (100 mL) at 0 C. The obtained solution
of the
residue in THF (300 mL) was added to the reaction mixture at 0 C. Under a
nitrogen
atmosphere, the mixture was stirred at room temperature for 2 hours. Water and
a saturated
aqueous ammonium chloride solution were added to the reaction mixture at 0 C
to stop the
reaction, and the mixture was extracted with ethyl acetate. The organic layer
was washed with
a saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was purified by silica gel column chromatography (ethyl
acetate/hexane) to obtain
the title compound (1.12 g).
IFI NMR (300 MHz, DMSO-d6) 64.30 (2H, d, J = 5.5 Hz), 5.46-5.60 (1H, m), 5.79
(2H,
s), 6.70 (1H, d, J = 1.8 Hz), 7.12-7.52 (5H, m).
[0230]
b) 1-(2-fluorobenzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (300 ilL) was added to a mixture of N3-(2-
fluorobenzy1)-5-iodopyridine-2,3-diamine (500 mg) and trimethyl orthoacetate
(5 mL) at room
temperature. The mixture was stirred at room temperature for 1 hour. The
mixture was
concentrated under reduced pressure. The residue was washed with IPE to obtain
the title
compound (400 mg).
IHNIvIR (300 MHz, DMSO-d6) 62.53 (3H, s), 5.57 (2H, s), 6.94-7.48 (4H, m),
8.37
(1H, d, J = 1.6 Hz), 8.51 (1H, d, J = 1.9 Hz).
[0231]
c) 1-(2-fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-
1H-
97
CA 03021185 2018-10-16
A00039
imidazo[4,5-b]pyridine
A mixture of 1-(2-fluorobenzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine (200
mg),
4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (81 mg), potassium phosphate (347 mg),
trans-N,N'-
dimethylcyclohexane-1,2-diamine (93 mg), copper (I) iodide (62.2 mg) and TI-IF
(5 mL) was
stirred under microwave irradiation at 110 C for 2 hours. The mixture was
cooled to room
temperature, and insolubles were removed. The mixture was concentrated under
reduced
pressure. The residue was purified by NH silica gel column chromatography
(methanol/ethyl
acetate), and washed with ethanol to obtain the title compound (80 mg).
II-INMR (300 MHz, DMSO-d6) 82.61 (3H, s), 3.71 (3H, s), 5.61 (2H, s), 6.83
(1H, d, J
= 3.2 Hz), 7.00-7.47 (4H, m), 7.94 (1H, d, J = 3.2 Hz), 8.23 (1H, d, J = 2.4
Hz), 8.46-8.58 (2H,
m).
[0232]
Example 31
1-(2,4-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-
1H-
imidazo[4,5-b]pyridine
a) N3-(2,4-difluorobenzy1)-5-iodopyridine-2,3-diamine
A mixture of 2,4-difluorobenzaldehyde (0.237 mL), 5-iodopyridine-2,3-diamine
(392.4
mg), TI-IF (8 mL) and acetic acid (0.482 mL) was stirred overnight at room
temperature. The
reaction mixture was poured into a saturated aqueous sodium hydrogen carbonate
solution, and
extracted with ethyl acetate. The organic layer was washed with a saturated
brine, dried over
magnesium sulfate, and then concentrated. Sodium borohydride (158 mg) was
added to a
solution of the residue in methanol (8 mL) at room temperature. The mixture
was stirred at
room temperature for 3 hours. The reaction mixture was poured into water, and
extracted with
ethyl acetate. The organic layer was washed with a saturated brine, then dried
over magnesium
sulfate, and concentrated under reduced pressure. The residue was purified by
silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound (493
mg).
11-1 NMR (300 MHz, DMSO-d6) E. 4.27 (2H, d, J = 5.5 Hz), 5.52 (1H, t, J = 5.6
Hz), 5.78
(2H, s), 6.71 (1H, d, J = 1.8 Hz), 7.08 (1H, tdd, J = 8.5, 2.6, 0.9 Hz), 7.26
(1H, ddd, J = 10.5, 9.4,
2.6 Hz), 7.36-7.47 (2H, m).
[0233]
b) 1-(2,4-difluorobenzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (500 1.11-) was added to a mixture of N3-(2,4-
difluorobenzy1)-5-iodopyridine-2,3-diamine (492.8 mg) and trimethyl
orthoacetate (5 mL) at
room temperature. The mixture was stirred at 60 C for 3 hours. The mixture was
poured into
98
CA 03021185 2018-10-16
A00039
4.
a saturated aqueous sodium hydrogen carbonate solution, and extracted with
ethyl acetate. The
organic layer was washed with a saturated brine, then dried over magnesium
sulfate, and
concentrated under reduced pressure. The residue was purified by NH silica gel
column
chromatography (ethyl acetate/hexane) to obtain the title compound (350 mg).
1H NMR (300 MIlz, DMSO-d6) 8 2.54 (3H, s), 5.54 (2H, s), 7.02-7.21 (2H, m),
7.26-
7.36 (1H, m), 8.38 (1H, d, J = 1.9 Hz), 8.51 (1H, d, J = 2.0 Hz).
[0234]
c) 1-(2,4-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-
methyl-
1H-imidazo[4,5-b]pyridine
A mixture of 1-(2,4-difluorobenzy1)-6-iodo-2-methy1-1H-imidazo[4,5-b]pyridine
(156.9
mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (66.8 mg), potassium phosphate (259
mg), trans-
N,N'-dimethylcyclohexane-1,2-diamine (69.5 mg), copper (I) iodide (46.6 mg)
and THF (2.5
mL) was stirred under microwave irradiation at 150 C for 3 hours. The mixture
was cooled to
room temperature, then poured into a saturated aqueous ammonium chloride
solution, and
extracted with ethyl acetate. The organic layer was washed with a saturated
brine, then dried
over magnesium sulfate, and concentrated under reduced pressure. The residue
was purified by
silica gel column chromatography (methanol/ethyl acetate), and crystallized
from ethanol and
IPE to obtain the title compound (82 mg).
1H NMR (300 MHz, DMSO-d6) 8 2.61 (3H, s), 3.74 (3H, s), 5.58 (2H, s), 6.83
(1H, d, J
= 3.2 Hz), 7.04-7.24 (2H, m), 7.29-7.39 (1H, m), 7.94 (1H, d, J = 3.2 Hz),
8.23 (1H, d, J = 2.4
Hz), 8.51 (1H, s), 8.53 (1H, d, J = 2.4 Hz).
[0235]
Example 32
1-(3-chlorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-dipyrimidin-5-y1)-2-methyl-11-1-
imidazo[4,5-b]pyridine
a) N3-(3-chlorobenzy1)-5-iodopyridine-2,3-diamine
A mixture of 3-chlorobenzaldehyde (0.234 mL), 5-iodopyridine-2,3-diamine
(374.1
mg), TI-IF (8 mL) and acetic acid (0.23 mL) was stirred overnight at room
temperature. The
reaction mixture was poured into a saturated aqueous sodium hydrogen carbonate
solution, and
extracted with ethyl acetate. The organic layer was washed with a saturated
brine, dried over
magnesium sulfate, and then concentrated. Sodium borohydride (151 mg) was
added to a
solution of the residue in methanol (5 mL) at room temperature. The mixture
was stirred
overnight at room temperature. The reaction mixture was poured into water, and
extracted with
ethyl acetate. The organic layer was washed with a saturated brine, then dried
over magnesium
99
CA 03021185 2018-10-16
A00039
sulfate, and concentrated under reduced pressure. The residue was purified by
silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound (394
mg).
114 NMR (300 MHz, DMSO-d6) 6 4.30 (2H, d, J = 5.7 Hz), 5.67 (1H, t, J = 5.8
Hz), 5.78
(2H, s), 6.65 (1H, d, J = 1.8 Hz), 7.29-7.34 (2H, m), 7.35-7.44 (3H, m).
[0236]
b) 1-(3-chlorobenzy1)-6-iodo-2-methyl-IH-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (500 gL) was added to a mixture of N3-(3-
chlorobenzy1)-5-iodopyridine-2,3-diamine (393.8 mg) and trimethyl orthoacetate
(5 mL) at room
temperature. The mixture was stirred at 60 C for 3 hours. The mixture was
cooled to room
temperature, poured into a saturated aqueous sodium hydrogen carbonate
solution, and extracted
with ethyl acetate. The organic layer was washed with a saturated brine, then
dried over
magnesium sulfate, and concentrated under reduced pressure. The residue was
purified by NH
silica gel column chromatography (ethyl acetate/hexane) to obtain the title
compound (298 mg).
IFI NIVIR (300 MHz, DMSO-d6) 6 2.54 (311, s), 5.53 (2H, s), 6.97-7.04 (114,
m), 7.24-
7.27 (1H, m), 7.35-7.40 (2H, m), 8.40 (1H, d, J = 2.0 Hz), 8.52 (1H, d, J =
1.9 Hz).
[0237]
c) 1-(3-chlorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-
1H-
imidazo[4,5-b]pyridine
A mixture of 1-(3-chlorobenzy1)-6-iodo-2-methy1-1H-imidazo[4,5-b]pyridine
(129.3
mg), 4-methoxy-511-pyrrolo[3,2-d]pyrimidine (52.8 mg), potassium phosphate
(215 mg), trans-
N,N'-dimethylcyclohexane-1,2-diamine (57.5 mg), copper (I) iodide (38.5 mg)
and THF (2 mL)
was stirred under microwave irradiation at 150 C for 3 hours. The mixture was
cooled to room
temperature, then poured into a saturated aqueous ammonium chloride solution,
and extracted
with ethyl acetate. The organic layer was washed with a saturated brine, then
dried over
magnesium sulfate, and concentrated under reduced pressure. The residue was
purified by NH
silica gel column chromatography (methanol/ethyl acetate), and crystallized
from ethanol/IF'E to
obtain the title compound (51.3 mg).
1HNMR (300 MHz, DMSO-d6) 6 2.61 (3H, s), 3.70 (3H, s), 5.57 (2H, s), 6.83 (1H,
d, J
= 3.2 Hz), 7.05-7.12 (1H, m), 7.30 (1H, s), 7.35-7.45 (2H, m), 7.97 (111, d, J
= 3.2 Hz), 8.28 (1H,
d, J = 2.3 Hz), 8.51 (1H, s), 8.55 (1H, d, J = 2.3 Hz).
[0238]
Example 33
6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methy1-1-(3-
trifluoromethoxy)benzy1)-1H-imidazo[4,5-b]pyridine
100
CA 03021185 2018-10-16
A00039
a) 5-iodo-N3-(3-(trifluoromethoxy)benzyl)pyridine-2,3-diamine
A mixture of 3-(trifluoromethoxy)benzaldehyde (0.336 mL), 5-iodopyridine-2,3-
diamine (425 mg), THF (10 mL) and acetic acid (0.261 mL) was stirred overnight
at room
temperature. The reaction mixture was poured into a saturated aqueous sodium
hydrogen
carbonate solution, and extracted with ethyl acetate. The organic layer was
washed with a
saturated brine, dried over magnesium sulfate, and then concentrated. Sodium
borohydride
(171 mg) was added to a solution of the residue in methanol (5 mL) at room
temperature. The
mixture was stirred overnight at room temperature. The reaction mixture was
poured into
water, and extracted with ethyl acetate. The organic layer was washed with a
saturated brine,
then dried over magnesium sulfate, and concentrated under reduced pressure.
The residue was
purified by silica gel column chromatography (ethyl acetate/hexane) to obtain
the title compound
(673 mg).
NMR (300 MHz, DMSO-d6) 6 4.35 (2H, d, J = 5.7 Hz), 5.71 (1H, t, J = 5.8 Hz),
5.78
(211, s), 6.66 (1H, d, J = 1.9 Hz), 7.22-7.28 (1H, m), 7.34 (1H, s), 7.36-7.43
(2H, m), 7.45-7.53
(1H, m).
[0239]
b) 6-iodo-2-methyl-1-(3-(trifluoromethoxy)benzy1)-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (500 pt) was added to a mixture of 5-iodo-N3-(3-
(trifluoromethoxy)benzyl)pyridine-2,3-diamine (672.9 mg) and trimethyl
orthoacetate (5 mL) at
room temperature. The mixture was stirred at room temperature for 1 hour. The
mixture was
concentrated under reduced pressure. The residue was purified by NH silica gel
column
chromatography (ethyl acetate/hexane) to obtain the title compound (458 mg).
1HNMR (300 MHz, DMSO-d6) 6 2.54 (3H, s), 5.58 (2H, s), 7.06 (1H, d, J = 7.8
Hz),
7.25 (1H, s), 7.28-7.35 (1H, m), 7.44-7.52 (1H, m), 8.39 (11-1, d, J = 1.9
Hz), 8.52 (1H, d, J = 1.9
Hz).
[0240]
c) 6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methy1-1-(3-
trifluoromethoxy)benzy1)-1H-imidazo[4,5-b]pyridine
A mixture of 6-iodo-2-methy1-1-(3-(trifuoromethoxy)benzy1)-1H-imidazo[4,5-
.. b]pyridine (137.5 mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (49.7 mg),
potassium phosphate
(202 mg), trans-N,N'-dimethylcyclohexane-1,2-diamine (54.2 mg), copper (I)
iodide (36.3 mg)
and THF (2 mL) was stirred under microwave irradiation at 150 C for 3 hours.
The mixture
was cooled to room temperature, then poured into a saturated aqueous ammonium
chloride
solution, and extracted with ethyl acetate. The organic layer was washed with
a saturated brine,
101
CA 03021185 2018-10-16
A00039
then dried over magnesium sulfate, and concentrated under reduced pressure.
The residue was
purified by NH silica gel column chromatography (methanol/ethyl acetate) and
crystallized from
ethanol/IPE to obtain the title compound (58.5 mg).
NMR (300 MHz, DMSO-d6) 5 2.61 (3H, s), 3.68 (3H, s), 5.62 (2H, s), 6.83 (1H,
d, J
= 3.2 Hz), 7.11 (1H, d, J = 7.9 Hz), 7.26-7.37 (2H, m), 7.46-7.55 (1H, m),
7.95 (1H, d, J = 3.4
Hz), 8.28 (1H, d, J = 2.3 Hz), 8.50 (1H, s), 8.55 (1H, d, J = 2.4 Hz).
[0241]
Example 34
6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methy1-1-(3-
trifluoromethypbenzy1)-
1H-imidazo[4,5-b]pyridine
a) 5-iodo-N3-(3-(trifluoromethyl)benzyl)pyridine-2,3-diamine
A mixture of 3-(trifluoromethyl)benzaldehyde (0.319 mL), 5-iodopyridine-2,3-
diamine
(431 mg), THF (8 mL) and acetic acid (0.264 mL) was stirred overnight at room
temperature.
The reaction mixture was poured into a saturated aqueous sodium hydrogen
carbonate solution,
and extracted with ethyl acetate. The organic layer was washed with a
saturated brine, dried
over magnesium sulfate, and then concentrated. Sodium borohydride (173 mg) was
added to a
solution of the residue in methanol (8 mL) at room temperature. The mixture
was stirred
overnight at room temperature. The reaction mixture was poured into water, and
extracted with
ethyl acetate. The organic layer was washed with a saturated brine, then dried
over magnesium
sulfate, and concentrated under reduced pressure. The residue was purified by
silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound (539
mg).
IHNMR (300 MHz, DMSO-d6) 5 4.39 (2H, d, J = 5.7 Hz), 5.71 (1H, t, J = 5.8 Hz),
5.79
(2H, s), 6.69 (1H, d, J = 1.9 Hz), 7.41 (1H, d, J 1.9 Hz), 7.55-7.69 (3H, m),
7.72 (1H, s).
[0242]
b) 6-iodo-2-methyl-1-(3-(trifluoromethypbenzyl)-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (500 p.L) was added to a mixture of 5-iodo-N3-
(3-
(trifluoromethyl)benzyl)pyridine-2,3-diamine (538.5 mg) and trimethyl
orthoacetate (5 mL) at
room temperature. The mixture was stirred at 60 C for 3 hours. The reaction
mixture was
poured into a saturated aqueous sodium hydrogen carbonate solution, and
extracted with ethyl
acetate. The organic layer was washed with a saturated brine, dried over
magnesium sulfate,
and then concentrated. The residue was purified by NH silica gel column
chromatography
(ethyl acetate/hexane) to obtain the title compound (397 mg).
IHNIVIR (300 MHz, DMSO-d6) 5 2.54 (3H, s), 5.63 (2H, s), 7.28 (1H, d, J = 7.7
Hz),
7.53-7.61 (1H, m), 7.63 (1H, s), 7.65-7.71 (1H, m), 8.41 (1H, d, J = 1.9 Hz),
8.52 (1H, d, J = 2.0
102
CA 03021185 2018-10-16
A00039
Hz).
[0243]
c) 6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methy1-1-(3-
trifluoromethypbenzy1)-1H-imidazo[4,5-b]pyridine
A mixture of 6-iodo-2-methyl-1-(3-(trifuoromethypbenzy1)-1H-imidazo[4,5-
b]pyridine
(140.5 mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (52.7 mg), potassium
phosphate (214 mg),
trans-N,N'-dimethylcyclohexane-1,2-diamine (57.5 mg), copper (I) iodide (38.5
mg) and THF (2
mL) was stirred under microwave irradiation at 150 C for 2.5 hours. The
mixture was cooled
to room temperature, then poured into a saturated aqueous ammonium chloride
solution, and
extracted with ethyl acetate. The organic layer was washed with a saturated
brine, then dried
over magnesium sulfate, and concentrated under reduced pressure. The residue
was purified by
NH silica gel column chromatography (methanol/ethyl acetate) and crystallized
from
ethanol/WE to obtain the title compound (67 mg).
NMR (300 MHz, DMSO-d6) 6 2.62 (3H, s), 3.64 (3H, s), 5.67 (2H, s), 6.83 (1H,
d, J
= 3.2 Hz), 7.34 (1H, d, J = 7.7 Hz), 7.56-7.73 (3H, m), 7.95 (1H, d, J = 3.2
Hz), 8.29 (1H, d, J =
2.4 Hz), 8.50 (1H, s), 8.55 (1H, d, J = 2.3 Hz).
[0244]
Example 35
1-(2,6-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-methyl-
1H-
imidazo[4,5-b]pyridine
a) N3-(2,6-difluorobenzyl)-5-iodopyridine-2,3-diamine
Acetic acid (300 )1L) was added to a solution of 2,6-difluorobenzaldehyde (500
L) and
5-iodopyridine-2,3-diamine (0.7 g) in THF (50 mL) at room temperature. The
mixture was
stirred overnight at room temperature. The reaction mixture was poured into a
saturated
aqueous sodium hydrogen carbonate solution, and extracted with ethyl acetate.
The organic
layer was washed with water and a saturated brine, dried over magnesium
sulfate, and then
concentrated. Sodium borohydride (0.169 g) was added to a solution of the
residue in methanol
(50 mL) at 0 C. The mixture was stirred at 0 C for 1 hour. The reaction
mixture was poured
into an aqueous ammonium chloride solution, and extracted with ethyl acetate.
The organic
layer was washed with water and a saturated brine, then dried over magnesium
sulfate, and
concentrated under reduced pressure. The residue was purified by NH silica gel
column
chromatography (ethyl acetate/hexane) to obtain the title compound (0.263 g).
111 NMR (300 MHz, DMSO-d6) 6 4.24 (2H, d, J = 5.1 Hz), 5.33 (1H, t, J = 5.1
Hz), 5.78
(2H, s), 6.89 (1H, d, J = 1.8 Hz), 7.05-7.26 (2H, m), 7.35-7.55 (2H, m).
103
CA 03021185 2018-10-16
A00039
[0245]
b) 1-(2,6-difluorobenzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (0.1 mL) was added to a mixture of N3-(2,6-
difluorobenzy1)-5-iodopyridine-2,3-diamine (262.8 mg) and trimethyl
orthoacetate (4 mL) at
room temperature. Under a dry atmosphere, the mixture was stirred at room
temperature for 1
hour. The reaction mixture was neutralized with a saturated aqueous sodium
hydrogen
carbonate solution at room temperature, and extracted with ethyl acetate/THF.
The organic
layer was washed with a saturated brine, dried over magnesium sulfate, and
then concentrated.
The residue was washed with ethyl acetate to obtain the title compound (200
mg).
1H NMR (300 MHz, DMSO-d6) 8 2.54 (3H, s), 5.58 (2H, s), 7.09-7.26 (2H, m),
7.39-
7.58 (1H, m), 8.26-8.34 (1H, m), 8.50 (1H, d, J = 2.0 Hz).
[0246]
c) 1-(2,6-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-
methyl-
1H-imidazo[4,5-blpyridine
A mixture of 1-(2,6-difluorobenzy1)-6-iodo-2-methy1-1H-imidazo[4,5-b]pyridine
(200
mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (101 mg), potassium phosphate (331
mg), trans-
N,N-dimethylcyclohexane-1,2-diamine (0.082 mL), copper (I) iodide (49.4 mg)
and THF (5
mL) was stirred under microwave irradiation at 110 C for 2 hours. The mixture
was diluted
with an aqueous ammonia solution at room temperature, and extracted with ethyl
acetate. The
.. organic layer was washed with a saturated brine, then dried over magnesium
sulfate, and
concentrated under reduced pressure. The residue was purified by NH silica gel
column
chromatography (methanol/ethyl acetate), and washed with ethyl acetate to
obtain the title
compound (68.5 mg).
NMR (300 MHz, DMSO-d6) 8 2.61 (3H, s), 3.79 (3H, s), 5.62 (211, s), 6.84 (1H,
d, J
= 3.2 Hz), 7.12-7.26 (2H, m), 7.43-7.58 (1H, m), 7.93 (1H, d, J = 3.2 Hz),
8.10 (1H, d, J = 2.4
Hz), 8.44-8.60 (2H, m).
[0247]
Example 38
1-(3,5-difluorobenzy1)-2-methoxy-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-
1H-
imidazo[4,5-b]pyridine
a) 1-(3,5-difluorobenzy1)-6-iodo-2-(isopropylthio)-1H-imidazo[4,5-b]pyridine
Under a dry atmosphere, a mixture of N3-(3,5-difluorobenzy1)-5-iodopyridine-
2,3-
diamine (5 g), CDI (4.5 g) and THF (50 mL) was stirred at 60 C for 16 hours.
The reaction
mixture was quenched with water at room temperature, and extracted with ethyl
acetate. The
104
CA 03021185 2018-10-16
A00039
organic layer was sequentially washed with a saturated aqueous ammonium
chloride solution,
water and a saturated brine, then dried over magnesium sulfate, and
concentrated under reduced
pressure to obtain a white solid as 1-(3,5-difluorobenzy1)-6-iodo-1H-
imidazo[4,5-b]pyridin-
2(3H)-one. A mixture of the obtained solid and phosphorus oxychloride (50 mL)
was stirred at
100 C for 2 days. The reaction mixture was concentrated under reduced
pressure. The
residue was diluted with THF, poured into a saturated aqueous sodium hydrogen
carbonate
solution, and extracted with ethyl acetate. The organic layer was washed with
water and a
saturated brine, dried over sodium sulfate, and then concentrated. The residue
was diluted with
THF (100 mL). To the mixture was added sodium 2-propanethiolate (1.963 g) at 0
C. Under
a nitrogen atmosphere, the mixture was stirred at room temperature for 1 hour.
The reaction
mixture was poured into water, and extracted with ethyl acetate. The organic
layer was washed
with water and a saturated brine, dried over sodium sulfate, and then
concentrated. The residue
was purified by silica gel column chromatography (ethyl acetate/hexane) to
obtain the title
compound (5.73 g).
IFI NMR (300 MI-[z, DMSO-d6) 61.45 (6H, d, J = 6.8 Hz), 4.05-4.17(111, m),
5.43 (2H,
s), 6.87 (2H, dd, J = 8.4, 2.2 Hz), 7.14-7.28 (1H, m), 8.42 (1H, d, J = 2.0
Hz), 8.52 (1H, d, J =
1.9 Hz).
[0248]
b) 1-(3,5-difluorobenzy1)-2-(isopropylthio)-6-(4-methoxy-5H-pyrrolo[3,2-
d]pyrimidin-
5-yI)-1H-imidazo[4,5-b]pyridine
A mixture of 1-(3,5-difluorobenzy1)-6-iodo-2-(isopropylthio)-1H-imidazo[4,5-
b]pyridine (203 mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (88 mg), potassium
phosphate
(290 mg), trans-N,N'-dimethylcyclohexane-1,2-diamine (0.144 mL), copper (I)
iodide (87 mg)
and THF (5 mL) was stirred under microwave irradiation at 100 C for 2 hours.
The mixture
was diluted with water and an aqueous ammonia solution at room temperature,
and extracted
with ethyl acetate. The organic layer was washed with a saturated brine, then
dried over
magnesium sulfate, and concentrated under reduced pressure. The residue was
purified by
silica gel column chromatography (methanol/ethyl acetate), and washed with
ethyl acetate to
obtain the title compound (170 mg).
IFINMR (300 Wiz, DMSO-d6) 61.49 (6H, d, J = 6.8 Hz), 3.75 (3H, s), 4.10-4.25
(1H,
m), 5.46 (2H, s), 6.84 (1H, d, J = 3.3 Hz), 6.90-7.02 (2H, m), 7.18-7.30 (1H,
m), 7.97 (1H, d, J --
3.2 Hz), 8.33 (1H, d, J = 2.4 Hz), 8.52 (1H, s), 8.55 (1H, d, J = 2.4 Hz).
[0249]
c) 1-(3,5-difluorobenzy1)-2-methoxy-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-
y1)-
105
CA 03021185 2018-10-16
A00039
1H-imidazo[4,5-b]pyridine
m-Chloroperbenzoic acid (55.5 mg) was added to a solution of 1-(3,5-
difluorobenzy1)-
2-(isopropylthio)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-1H-imidazo[4,5-
b]pyridine
(60 mg) in acetonitrile (5 mL) at 0 C. The mixture was stirred for 10 minutes,
and then added
to a solution of sodium methoxide (20 mg) in acetonitrile (5 mL) at 0 C. The
mixture was
stirred under a nitrogen atmosphere at room temperature for 5 hours. The
reaction mixture was
poured into a saturated aqueous ammonium chloride solution at room
temperature, and extracted
with ethyl acetate. The organic layer was washed with water and a saturated
brine, dried over
magnesium sulfate, and then concentrated. The residue was purified by silica
gel column
chromatography (ethyl acetate/hexane) to obtain the title compound (47 mg).
1H NMR (300 MHz, DMSO-d6) 63.74 (3H, s), 4.24 (3H, s), 5.30 (2H, s), 6.82 (1H,
d, J
= 3.2 Hz), 7.02-7.09 (2H, m), 7.15-7.29 (1H, m), 7.93 (1H, d, J = 3.2 Hz),
8.18 (1H, d, J = 2.4
Hz), 8.41 (1H, d, J = 2.4 Hz), 8.51 (1H, s).
[0250]
Example 39
1-(3-fluorobenzy1)-2-methoxy-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-1H-
imidazo[4,5-b]pyridine
a) 1-(3-fluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridin-2(3H)-one
Under a dry atmosphere, a mixture of N3-(3-fluorobenzy1)-5-iodopyridine-2,3-
diamine
(2.56 g), CDI (2.5 g) and THF (30 mL) was stirred at 60 C for 16 hours. The
mixture was
quenched with water at room temperature, and diluted with ethyl acetate. The
organic layer
was sequentially washed with a saturated aqueous ammonium chloride solution,
water and a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was washed with ethyl acetate to obtain the title compound (2.4
g).
1H NMR (300 MHz, DMSO-d6) 65.02 (2H, s), 7.08-7.22 (3H, m), 7.39 (1H, td, J =
7.9,
6.1 Hz), 7.80 (1H, d, J = 1.8 Hz), 8.12 (1H, d, J = 1.8 Hz), 11.87 (1H, s).
[0251]
b) 2-ehloro-1-(3-fluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridine
A mixture of 1-(3-fluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridin-2(3H)-one
(2.39 g)
and phosphorus oxychloride (20 mL) was stirred under a nitrogen atmosphere at
100 C for 18
hours. The reaction mixture was concentrated under reduced pressure. The
residue was
diluted with THF, neutralized with a saturated aqueous sodium hydrogen
carbonate solution at
room temperature, and extracted with ethyl acetate. The organic layer was
washed with a
saturated brine, dried over magnesium sulfate, and then concentrated. The
residue was washed
106
CA 03021185 2018-10-16
A00039
with ethyl acetate/IPE to obtain the title compound (1.78 g).
MS: [M+H] 388.1.
[0252]
c) 2-chloro-1-(3-fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-
1H-
imidazo[4,5-b]pyridine
A mixture of 2-chloro-1-(3-fluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridine (300
mg),
4-methoxy-5H-pyn-olo[3,2-d]pyrimidine (150 mg), potassium phosphate (493 mg),
trans-N,N1-
dimethylcyclohexane-1,2-diamine (0.122 mL), copper (I) iodide (73.7 mg) and
THF (5 mL) was
stirred under microwave irradiation at 90 C for 2 hours. The mixture was
diluted with water,
and extracted with ethyl acetate. The organic layer was washed with a
saturated brine, then
dried over magnesium sulfate, and concentrated under reduced pressure. The
residue was
purified by silica gel column chromatography (methanol/ethyl acetate) to
obtain the title
compound (99 mg).
IHNIVIR (300 MHz, DMSO-d6) 63.74 (3H, s), 5.61 (2H, s), 6.86 (1H, d, J = 3.3
Hz),
7.04-7.12 (IH, m), 7.14-7.26 (2H, m), 7.37-7.50 (1H, m), 8.00 (1H, d, J = 3.3
Hz), 8.50 (1H, d, J
= 2.4 Hz), 8.53 (1H, s), 8.68 (1H, d, J = 2.4 Hz).
[0253]
d) 1-(3-fluorobenzy1)-2-methoxy-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-
1H-
imidazo[4,5-b]pyridine
Sodium methoxide (28% in methanol solution) (90 mg) was added to 2-chloro-1-(3-
fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-1H-imidazo[4,5-
b]pyridine (90
mg) in THF (5 mL) at room temperature. The mixture was stirred at room
temperature for 30
minutes. The reaction mixture was diluted with a saturated aqueous ammonium
chloride
solution at room temperature, and extracted with ethyl acetate. The organic
layer was washed
with water and a saturated brine, dried over magnesium sulfate, and then
concentrated. The
residue was purified by silica gel column chromatography (methanol/ethyl
acetate), and
crystallized from ethanol/water to obtain the title compound (56.8 mg).
1HNMR (300 MHz, DMSO-d6) 5 3.72 (3H, s), 4.24 (3H, s), 5.30 (2H, s), 6.81 (1H,
d, J
= 3.2 Hz), 7.08-7.23 (3H, m), 7.36-7.48 (1H, m), 7.92 (1H, d, J = 3.2 Hz),
8.16 (1H, d, J = 2.4
Hz), 8.39 (1H, d, J = 2.4 Hz), 8.50 (1H, s).
[0254]
Example 44
1-(3-(difluoromethoxy)benzy1)-6-(4-methoxy-5H-pyrrolo [3,2-d]pyrimidin-5-y1)-2-
methy1-1H-imidazo [4,5-b]pyridine
107
CA 03021185 2018-10-16
A00039
a) N3-(3-(difluoromethoxy)benzy1)-5-iodopyridine-2,3-diamine
A mixture of 3-(difluoromethoxy)benzaldehyde (429 mg), 5-iodopyridine-2,3-
diamine
(451 mg), THF (8 mL) and acetic acid (0.277 mL) was stirred overnight at room
temperature.
The reaction mixture was poured into a saturated aqueous sodium hydrogen
carbonate solution,
and extracted with ethyl acetate. The organic layer was washed with a
saturated brine, dried
over magnesium sulfate, and then concentrated. Sodium borohydride (181 mg) was
added to a
solution of the residue in methanol (8 mL) at room temperature. The mixture
was stirred
overnight at room temperature. The reaction mixture was poured into water, and
extracted with
ethyl acetate. The organic layer was washed with a saturated brine, then dried
over magnesium
sulfate, and concentrated under reduced pressure. The residue was purified by
silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound (660
mg).
IFINMR (300 MHz, DMSO-d6) 6 4.30 (2H, d, J = 5.8 Hz), 5.66 (111, t, J = 5.9
Hz), 5.78
(2H, s), 6.66 (1H, d, J = 1.9 Hz), 6.95-7.48 (2H, m), 7.06 (1H, dd, J = 8.0,
2.2 Hz), 7.16 (1H, s),
7.37-7.44 (2H, m).
[0255]
b) 1-(3-(difluoromethoxy)benzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (500 L) was added to a mixture of N3-(3-
(difluoromethoxy)benzy1)-5-iodopyridine-2,3-diamine (0.66 g) and trimethyl
orthoacetate (5
mL) at room temperature. The mixture was stirred at 60 C for 3 hours. The
reaction mixture
was poured into a saturated aqueous sodium hydrogen carbonate solution, and
extracted with
ethyl acetate. The organic layer was washed with a saturated brine, dried over
magnesium
sulfate, and then concentrated. The residue was purified by NH silica gel
column
chromatography (ethyl acetate/hexane) to obtain the title compound (0.492 g).
1H NMR (300 MI-Iz, DMSO-d6) 6 2.54 (3H, s), 5.53 (2H, s), 6.92 (1H, d, J = 7.6
Hz),
6.96-7.49 (1H, m), 7.01 (1H, s), 7.11 (1H, dd, J = 8.3, 2.2 Hz), 7.35-7.44
(1H, m), 8.39 (1H, d, J
= 1.9 Hz), 8.52 (1H, d, J = 1.9 Hz).
[0256]
c) 1-(3-(difluoromethoxy)benzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-
2-
methy1-1H-imidazo[4,5-b]pyridine
1-(3-(difuoromethoxy)benzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine (157.9
mg),
4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (59.6 mg), potassium phosphate (242 mg),
trans-N,N1-
dimethylcyclohexane-1,2-diamine (64.9 mg), copper (I) iodide (43.5 mg) and TI-
11F (2 mL) was
stirred under microwave irradiation at 150 C for 2.5 hours. The mixture was
cooled to room
temperature, then poured into a saturated aqueous ammonium chloride solution,
and extracted
108
CA 03021185 2018-10-16
A00039
with ethyl acetate. The organic layer was washed with a saturated brine, then
dried over
magnesium sulfate, and concentrated under reduced pressure. The residue was
fractionated by
NH silica gel column chromatography (methanol/ethyl acetate) and HPLC (C18,
mobile phase:
water/acetonitrile (0.1% TFA-containing system)) to obtain the title compound
(93 mg).
NMR (300 MHz, DMSO-d6) 82.62 (3H, s), 3.70 (3H, s), 5.57 (2H, s), 6.83 (1H, d,
J
= 3.2 Hz), 6.91-7.50 (5H, m), 7.96 (1H, d, J = 3.2 Hz), 8.29 (1H, d, J = 2.4
Hz), 8.51 (1H, s),
8.54 (1H, d, J = 2.4 Hz).
[0257]
Example 48
1-(3,5-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-1H-
imidazo[4,5-b]pyridin-2-amine
a) 1-(3,5-difluorobenzy1)-6-iodo-IH-imidazo[4,5-b]pyridin-2(3H)-one
Under a dry atmosphere, a mixture of N3-(3,5-difluorobenzy1)-5-iodopyridine-
2,3-
diamine (2.2 g), CDI (1.976 g) and THF (150 mL) was stirred at 60 C for 16
hours. The
mixture was quenched with water at room temperature, and diluted with ethyl
acetate. The
organic layer was sequentially washed with a saturated aqueous ammonium
chloride solution,
water and a saturated brine, then dried over magnesium sulfate, and
concentrated under reduced
pressure. The residue was washed with ethyl acetate to obtain the title
compound (2 g).
'H NMR (300 MHz, DMSO-d6) 85.02 (2H, s), 6.99-7.25 (3H, m), 7.84 (1H, d, J =
1.8
Hz), 8.13 (1H, d, J 1.8 Hz), 11.88 (1H, brs).
[0258]
b) 2-chloro-1-(3,5-difluorobenzy1)-6-iodo-IH-imidazo[4,5-b]pyridine
A mixture of I -(3,5-difluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridin-2(3H)-one
(2 g)
and phosphorus oxychloride (24.13 mL) was stirred under a nitrogen atmosphere
at 100 C for 17
hours. The reaction mixture was concentrated under reduced pressure. The
residue was
diluted with THF, neutralized with a saturated aqueous sodium hydrogen
carbonate solution at
0 C, and extracted with ethyl acetate. The organic layer was washed with a
saturated brine,
dried over magnesium sulfate, and then concentrated. The residue was washed
with LPE to
obtain the title compound (1.97 g).
NMR (300 MHz, DMSO-d6) 85.58 (2H, s), 6.98 (3H, d, J = 6.0 Hz), 8.62 (2H, dd,
J
= 11.5, 1.9 Hz).
[0259]
c) 2-chloro-1-(3,5-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-
yI)-1H-
imidazo[4,5-b]pyridine
109
CA 03021185 2018-10-16
A00039
A mixture of 2-chloro-1-(3,5-difluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridine
(980
mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (469 mg), potassium phosphate (1539
mg), trans-
N,N'-dimethylcyclohexane-1,2-diamine (0.381 mL), copper (1) iodide (230 mg)
and THF (5 mL)
was stirred under microwave irradiation at 90 C for 3 hours. The mixture was
diluted with
water and an aqueous ammonia solution at room temperature, and extracted with
ethyl acetate.
The organic layer was washed with a saturated brine, then dried over magnesium
sulfate, and
concentrated under reduced pressure. The residue was purified by NH silica gel
column
chromatography (methanol/ethyl acetate) to obtain the title compound (440 mg).
1H NMR (300 MI-Iz, DMSO-d6) 83.77 (3H, s), 5.61 (2H, s), 6.87 (1H, d, J = 3.2
Hz),
7.04-7.26 (3H, m), 8.00 (1H, d, J = 3.2 Hz), 8.47-8.59 (2H, m), 8.64-8.78 (1H,
m).
[0260]
d) 1-(3,5-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-1H-
imidazo[4,5-b]pyridin-2-amine
A mixture of 2-chloro-1-(3,5-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-
d]pyrimidin-5-y1)-1H-imidazo[4,5-b]pyridine (120 mg), ammonia (2 M in IPA
solution) (15 mL)
and THF (5 mL) was stirred under microwave irradiation at 100 C for 5 hours.
The mixture
was diluted with water at room temperature, and extracted with ethyl acetate.
The organic layer
was washed with a saturated brine, then dried over magnesium sulfate, and
concentrated under
reduced pressure. The residue was purified by NH silica gel column
chromatography
(methanol/ethyl acetate) to obtain the title compound (41 mg).
1H NMR (300 MHz, DMSO-d6) 83.70 (3H, s), 5.32 (2H, s), 6.76 (1H, d, J = 3.2
Hz),
6.91-7.00 (2H, m), 7.14-7.24 (1H, m), 7.30 (2H, s), 7.78 (1H, d, J = 2.4 Hz),
7.85 (1H, d, J = 3.2
Hz), 8.14 (1H, d, J = 2.4 Hz), 8.47 (1H, s).
[0261]
Example 68
1-(3-fluoro-5-(trifluoromethypbenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-
5-y1)-
2-methyl-1H-imidazo[4,5-b]pyridine
a) N3-(3-fluoro-5-(trifluoromethyl)benzy1)-5-iodopyridine-2,3-diamine
A mixture of 3-fluoro-5-(trifluoromethyDbenzaldehyde (0.72 mL), 5-iodopyridine-
2,3-
diamine (1.01 g), THF (10 mL) and acetic acid (0.62 mL) was stirred overnight
at room
temperature. The reaction mixture was poured into a saturated aqueous sodium
hydrogen
carbonate solution, and extracted with ethyl acetate. The organic layer was
washed with a
saturated brine, dried over magnesium sulfate, and then concentrated. Sodium
borohydride
(0.406 g) was added to a solution of the residue in methanol (5 mL) at room
temperature. The
110
CA 03021185 2018-10-16
A00039
mixture was stirred overnight at room temperature. The reaction mixture was
poured into
water, and extracted with ethyl acetate. The organic layer was washed with a
saturated brine,
then dried over magnesium sulfate, and concentrated under reduced pressure.
The residue was
purified by silica gel column chromatography (ethyl acetate/hexane) to obtain
the title compound
(1.51 g).
II-INNER (300 MHz, DMSO-d6) 8 4.41 (2H, d, J = 5.8 Hz), 5.71 (1H, t, J = 5.7
Hz), 5.80
(2H, s), 6.71 (1H, d, J 1.7 Hz), 7.43 (1H, d, J 1.9 Hz), 7.50-7.64 (3H, m).
[0262]
b) 1-(3-fluoro-5-(trifluoromethyl)benzyl)-6-iodo-2-methyl-1H-imidazo[4,5-
b]pyridine
Concentrated hydrochloric acid (500 p.L) was added to a mixture of N3-(3-
fluoro-5-
(trifuoromethyl)benzy1)-5-iodopyridine-2,3-diamine (1.51 g) and trimethyl
orthoacetate (5 mL)
at room temperature. Under a dry atmosphere, the mixture was stirred at 60 C
for 3 hours.
The reaction mixture was poured into a saturated aqueous sodium hydrogen
carbonate solution,
and extracted with ethyl acetate. The organic layer was washed with a
saturated brine, dried
over magnesium sulfate, and then concentrated. The residue was purified by NH
silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound
(1.03 g).
11-1NMR (300 MHz, DMSO-d6) 6 2.55 (3H, s), 5.63 (2H, s), 7.19-7.26 (1H, m),
7.45
(1H, s), 7.62-7.70 (11-1, m), 8.41 (1H, d, J = 2.0 Hz), 8.53 (1H, d, J = 1.9
Hz).
[0263]
c) 1-(3-fluoro-5-(trifluoromethyObenzyl)-6-(4-methoxy-5H-pyrrolo[3,2-
d]pyrimidin-5-
y1)-2-methyl-1H-imidazo[4,5-b]pyridine
A mixture of 1-(3-fluoro-5-(trifuoromethyl)benzy1)-6-iodo-2-methyl- 1H-
imidazo[4,5-
b]pyridine (139 mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (50 mg), potassium
phosphate
(203 mg), trans-N,N'-dimethylcyclohexane-1,2-diamine (0.06 mL), copper (1)
iodide (36.5 mg)
and THF (2 mL) was stirred under microwave irradiation at 100 C for 2 hours.
The mixture
was cooled to room temperature, then diluted with a saturated aqueous ammonium
chloride
solution, and extracted with ethyl acetate. The organic layer was washed with
a saturated brine,
then dried over magnesium sulfate, and concentrated under reduced pressure.
The residue was
purified by NH silica gel column chromatography (methanol/ethyl acetate) to
obtain the title
compound (113 mg).
'FIN-MR (300 MHz, DMSO-d6) 8 2.63 (3H, s), 3.68 (3H, s), 5.67 (2H, s), 6.83
(1H, d, J
= 3.3 Hz), 7.26 (1H, d, J = 9.3 Hz), 7.50 (11-1, s), 7.68 (1H, d, J = 8.7 Hz),
7.96 (1H, d, J = 3.2
Hz), 8.29 (1H, d, J = 2.4 Hz), 8.51 (1H, s), 8.56 (1H, d, J = 2.4 Hz).
[0264]
111
CA 03021185 2018-10-16
A00039
Example 79
1-(3-chloro-5-fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-
methyl-
1H-imidazo[4,5-b]pyridine
a) N3-(3-chloro-5-fluorobenzy1)-5-iodopyridine-2,3-diamine
A mixture of 3-chloro-5-fluorobenzaldehyde (0.623 g), 5-iodopyridine-2,3-
diamine
(0.77 g), THF (10 mL) and acetic acid (0.472 mL) was stirred overnight at room
temperature.
The reaction mixture was poured into a saturated aqueous sodium hydrogen
carbonate solution,
and extracted with ethyl acetate. The organic layer was washed with a
saturated brine, dried
over magnesium sulfate, and then concentrated. To a solution of the residue in
methanol (5
mL) was added sodium borohydride (0.31 g) at room temperature. The mixture was
stirred
overnight at room temperature. The reaction mixture was poured into water, and
extracted with
ethyl acetate. The organic layer was washed with a saturated brine, then dried
over magnesium
sulfate, and concentrated under reduced pressure. The residue was purified by
silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound (1
g).
1HNMR (300 MHz, DMSO-d6) 8 4.32 (2H, d, J = 5.8 Hz), 5.69 (1H, t, J ---- 5.9
Hz), 5.79
(2H, s), 6.67 (1H, d, J = 1.8 Hz), 7.16-7.23 (1H, m), 7.27-7.35 (2H, m), 7.43
(1H, d, J = 1.9 Hz).
[0265]
b) 1-(3-chloro-5-fluorobenzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine
Concentrated hydrochloric acid (500 p.L) was added to a mixture of N3-(3-
chloro-5-
fluorobenzy1)-5-iodopyridine-2,3-diamine (1 g) and trimethyl orthoacetate (5
mL) at room
temperature. The mixture was stirred at 60 C for 3 hours. The reaction mixture
was poured
into a saturated aqueous sodium hydrogen carbonate solution, and extracted
with ethyl acetate.
The organic layer was washed with a saturated brine, dried over magnesium
sulfate, and then
concentrated. The residue was purified by NH silica gel column chromatography
(ethyl
acetate/hexane) to obtain the title compound (0.77 g).
NMR (300 MHz, DMSO-d6) Et 2.54 (3H, s), 5.54 (2H, s), 6.92-7.00 (1H, m), 7.06
(1H, s), 7.40 (1H, dt, J = 8.7, 2.2 Hz), 8.40 (1H, d, J = 1.9 Hz), 8.53 (1H,
d, J = 1.9 Hz).
[0266]
c) 1-(3-chloro-5-fluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-2-
methyl-1H-imidazo[4,5-b]pyridine
A mixture of 1-(3-chloro-5-fluorobenzy1)-6-iodo-2-methy1-1H-imidazo[4,5-
b]pyridine
(110.7 mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (43.2 mg), potassium
phosphate (176 mg),
trans-N,N'-dimethylcyclohexane-1,2-diamine (0.052 mL), copper (I) iodide (31.5
mg) and THF
(2 mL) was stirred under microwave irradiation at 100 C for 2 hours. The
mixture was cooled
112
CA 03021185 2018-10-16
A00039
to room temperature, then poured into a saturated aqueous ammonium chloride
solution, and
extracted with ethyl acetate. The organic layer was washed with a saturated
brine, then dried
over magnesium sulfate, and concentrated under reduced pressure. The residue
was purified by
NH silica gel column chromatography (methanol/ethyl acetate) and crystallized
from
ethanol/TPE to obtain the title compound (54.7 mg).
1H NMR (300 MHz, DMSO-d6) 2.62 (3H, s), 3.72 (3H, s), 5.57 (2H, s), 6.83 (1H,
d, J
= 3.2 Hz), 6.98-7.05 (1H, m), 7.10-7.14 (1H, m), 7.42 (111, dt, J = 8.7, 2.1
Hz), 7.97 (1H, d, J =
3.2 Hz), 8.28 (1H, d, J = 2.4 Hz), 8.51 (1H, s), 8.55 (1H, d, J = 2.4 Hz).
[0267]
Example 82
(1-(3,5-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-1H-
imidazo[4,5-b]pyridin-2-yl)methanol
a) (1-(3,5-difluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridin-2-yl)methanol
A mixture of N3-(3,5-difluorobenzy1)-5-iodopyridine-2,3-diamine (9 g),
glycolic acid
(48 g) and THF (9 mL) was stirred under microwave irradiation at 150 C for 1.5
hours. The
mixture was neutralized with a saturated aqueous sodium hydrogen carbonate
solution at room
temperature, and extracted with ethyl acetate. The organic layer was washed
with a saturated
brine, dried over magnesium sulfate, and then concentrated. The residue was
purified by silica
gel column chromatography (ethyl acetate/hexame and methanol/ethyl acetate) to
obtain the title
compound (4.9 g).
1H NMR (300 MHz, DMSO-d6) ö 4.77 (2H, s), 5.61 (2H, s), 5.79-6.07 (1H, m),
6.81-
7.08 (2H, m), 7.12-7.30 (1H, m), 8.33 (1H, d, J = 1.9 Hz), 8.57 (1H, d, J =
2.0 Hz).
[0268]
b) 2-(((tert-butyldiphenylsilypoxy)methyl)-1-(3,5-difluorobenzyl)-6-iodo-lH-
imidazo[4,5-b]pyridine
1H-imidazole (105 mg) and tert-butylchlrodiphenylsilane (0.39 mL) were added
to a
solution of (1-(3,5-difluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridin-2-
yOmethanol (200 mg) in
DMF (1.5 mL). The mixture was stirred at room temperature for 10 minutes. The
reaction
mixture was quenched with a saturated aqueous sodium hydrogen carbonate
solution at 0 C, and
extracted with ethyl acetate. The organic layer was washed with water and a
saturated brine,
dried over magnesium sulfate, and then concentrated. The residue was purified
by silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound (256
mg).
1H NMR (300 MHz, DMSO-d6) so.90 (9H, s), 4.81-5.11 (2H, m), 5.54-5.83 (2H, m),
6.74-6.91 (2H, m), 7.12-7.23 (1H, m), 7.37-7.53 (6H, m), 7.57-7.65 (4H, m),
8.19-8.47 (1H, m),
113
CA 03021185 2018-10-16
A00039
8.55-8.71 (1H, m).
[0269]
c) 2-(((tert-butyldiphenylsilyl)oxy)methyl)-1-(3,5-difluorobenzyl)-6-(4-
methoxy-5H-
pyrrolo[3,2-d]pyrimidin-5-y1)-1H-imidazo[4,5-b]pyridine
A mixture of 2-(((tert-butyldiphenylsilyl)oxy)methyl)-1-(3,5-difluorobenzyl)-6-
iodo-
1H-imidazo[4,5-b]pyridine (4.92 g), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (1.2
g), potassium
phosphate (4.9 g), trans-N,N'-dimethylcyclohexane-1,2-diamine (2.76 mL),
copper (I) iodide
(1.46 g) and THF (72 mL) was stirred under microwave irradiation at 100 C for
1.5 hours. The
mixture was diluted with a saturated aqueous sodium hydrogen carbonate
solution and an
aqueous ammonia solution at room temperature, and extracted with ethyl
acetate. The organic
layer was concentrated under reduced pressure. The residue was purified by NH
silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound
(3.23 g).
MS: [M+Hr 661.2
[0270]
d) (1-(3,5-difluorobenzy1)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-1H-
imidazo[4,5-b]pyridin-2-y1)methanol
TBAF (1M in THF solution) (0.239 mL) was added to a solution of 2-(((tert-
butyldiphenylsilypoxy)methyl)-1-(3,5-difluorobenzy1)-6-(4-methoxy-5H-
pyrrolo[3,2-
d]pyrimidin-5-y1)-1H-imidazo[4,5-b]pyridine (105 mg) in THF (2 mL) at room
temperature.
The mixture was stirred at room temperature for 30 minutes. The residue was
purified by NH
silica gel column chromatography (methanol/ethyl acetate) to obtain the title
compound (44.3
mg).
1HNMR (300 MHz, DMSO-d6) 63.67 (3H, s), 4.66-4.96 (2H, m), 5.46-5.74 (2H, m),
5.82-6.09 (1H, m), 6.64-6.89 (1H, m), 6.93-7.09 (2H, m), 7.13-7.33 (1H, m),
7.96 (1H, d, J = 3.3
Hz), 8.16-8.32 (1H, m), 8.51 (2H, s).
[0271]
Example 96
146-fluoropyridin-2-yOmethyl)-6-(4-methoxy-51-I-pyrrolo[3,2-d]pyrimidin-5-y1)-
2-
methyl-1H-imidazo[4,5-b]pyridine
a) N3((6-fluoropyridin-2-yOmethyl)-5-iodopyridine-2,3-diamine
A mixture of 6-fluoropicolinealdehyde (2.97 g), 5-iodopyridine-2,3-diamine
(5.08 g),
THF (50 mL) and acetic acid (3.12 mL) was stirred overnight at room
temperature. The
reaction mixture was poured into a saturated aqueous sodium hydrogen carbonate
solution, and
extracted with ethyl acetate. The organic layer was washed with a saturated
brine, dried over
114
CA 03021185 2018-10-16
A00039
magnesium sulfate, and then concentrated. To a solution of the residue in
methanol (5 mL) was
added sodium borohydride (2.044 g) at room temperature. The mixture was
stirred overnight at
room temperature. The reaction mixture was poured into water, and extracted
with ethyl
acetate. The organic layer was washed with a saturated brine, then dried over
magnesium
sulfate, and concentrated under reduced pressure. The residue was purified by
silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound
(5.72 g).
NMR (300 MHz, DMSO-d6) 8 4.34 (2H, d, J 5.9 Hz), 5.77-5.89 (3H, m), 6.63 (1H,
d, J 1.8 Hz), 7.06 (1H, dd, J = 8.1, 2.4 Hz), 7.30 (111, dd, J 7.4, 2.4 Hz),
7.41 (1H, d, J ¨ 1.9
Hz), 7.89-8.04 (1H, m).
[0272]
b) 1-((6-fluoropyridin-2-yl)methyl)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine
A mixture of N3((6-fluoropyridin-2-yl)methyl)-5-iodopyridine-2,3-diamine (1.09
g),
acetic acid (0.218 mL), DMAP (0.019 g), propylphosphonic anhydride (50% in
ethyl acetate
solution) (2.79 mL), DIPEA (1.106 mL) and THF (12 mL) was stirred under
microwave
irradiation at 180 C for 2 hours. The reaction mixture was poured into a
saturated aqueous
sodium hydrogen carbonate solution, and extracted with ethyl acetate. The
organic layer was
washed with a saturated brine, dried over magnesium sulfate, and then
concentrated. The
residue was purified by NH silica gel column chromatography (ethyl
acetate/hexane) to obtain
the title compound (0.71 g).
1H NMR (300 MHz, DMSO-d6) 6 2.57 (3H, s), 5.59 (2H, s), 7.12 (1H, dd, J 8.2,
2.5
Hz), 7.28 (1H, dd, J 7.3, 2.4 Hz), 8.01 (1H, q, J = 8.2 Hz), 8.40 (1H, d, J .=
1.9 Hz), 8.50 (1H,
d, J = 1.9 Hz).
[0273]
c) 1-((6-fluoropyridin-2-yl)methyl)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-
y1)-2-
methyl-1H-imidazo[4,5-b]pyridine
A mixture of 1-((6-fluoropyridin-2-yl)methyl)-6-iodo-2-methyl-1H-imidazo[4,5-
b]pyridine (149.5 mg), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (63.6 mg),
potassium phosphate
(259 mg), trans-N,N'-dimethylcyclohexane-1,2-diamine (0.128 mL), copper (I)
iodide (77 mg)
and THF (1 mL) was stirred under microwave irradiation at 120 C for 2 hours.
The mixture
was poured into a saturated aqueous ammonium chloride solution, and extracted
with ethyl
acetate. The residue was purified by NH silica gel column chromatography
(methanol/ethyl
acetate) to obtain the title compound (75 mg).
1H NMR (300 MHz, DMSO-d6) 6 2.66 (3H, s), 3.70 (3H, s), 5.62 (2H, s), 6.83
(1H, d, J
= 3.2 Hz), 7.13 (1H, dd, J 8.0, 2.4 Hz), 7.31 (1H, dd, J 7.2, 2.4 Hz), 7.95
(1H, d, J = 3.2 Hz),
115
CA 03021185 2018-10-16
A00039
7.98-8.08 (1H, m), 8.26 (1H, d, J = 2.4 Hz), 8.50 (1H, s), 8.53 (1H, d, J =
2.4 Hz).
[0274]
Example 98
14(6-fluoropyridin-2-yOmethyl)-2-methoxy-6-(4-methoxy-5H-pyrrolo[3,2-
d]pyrimidin-
5-y1)-1H-imidazo [4,5-b]pyridine
a) 14(6-fluoropyridin-2-yl)methyl)-6-1H-imidazo[4,5-b]pyridin-2(3H)-one
Under a nitrogen atmosphere, a mixture of N3-((6-fluoropyridin-2-yOmethyl)-5-
iodopyridine-2,3-diamine (4.62 g), CDI (4.35 g) and THF (50 mL) was stirred
overnight at 60 C.
The mixture was diluted with THF, sequentially washed with a saturated aqueous
ammonium
chloride solution and a saturated brine, then dried over magnesium sulfate,
and concentrated
under reduced pressure. The residue was washed with ethyl acetate to obtain
the title
compound (3.89 g).
1HNMR (300 MI-Iz, DMSO-d6) 6 5.09 (2H, s), 7.09 (1H, dd, J = 8.1, 2.3 Hz),
7.25 (1H,
dd, J = 7.4, 2.3 Hz), 7.78 (1H, d, J = 1.7 Hz), 7.92-8.03 (1H, m), 8.13 (1H,
d, J = 1.8 Hz), 11.86
(1H, s).
[0275]
b) 2-chloro-14(6-fluoropyridin-2-yl)methyl)-6-iodo-1H-imidazo[4,5-b]pyridine
A mixture of 146-fluoropyridin-2-yOmethyl)-6-iodo-1H-imidazo[4,5-b]pyridin-
2(3H)-
one (3.88 g) and phosphorus oxychloride (50 mL) was stirred under a nitrogen
atmosphere at
100 C for 21 hours. The reaction mixture was concentrated under reduced
pressure. The
residue was neutralized with a saturated aqueous sodium hydrogen carbonate
solution at 0 C,
and extracted with ethyl acetate/THF. The organic layer was washed with a
saturated brine,
dried over magnesium sulfate, and then concentrated. The residue was purified
by NH silica
gel column chromatography (ethyl acetate/hexane), and washed with ethyl
acetate to obtain the
title compound (1.36 g).
MS: [M+H]1- 388.9
[0276]
c) 2-chloro-14(6-fluoropyridin-2-yl)methyl)-6-(4-methoxy-5H-pyrrolo[3,2-
d]pyrimidin-5-y1)-1H-imidazo[4,5-b]pyridine
A mixture of 2-chloro-14(6-fluoropyridin-2-yOmethyl)-6-iodo-1H-imidazo[4,5-
b]pyridine (1.18 g), 4-methoxy-5H-pyrrolo[3,2-d]pyrimidine (0.589 g),
potassium phosphate
(1.934 g), trans-N,N'-dimethylcyclohexane-1,2-diamine (0.864 g), copper (I)
iodide (0.578 g)
and THF (15 mL) was stirred under microwave irradiation at 95 C for 90
minutes. The mixture
was diluted with water at room temperature, and extracted with ethyl acetate.
The organic layer
116
CA 03021185 2018-10-16
A00039
was washed with a saturated brine, then dried over magnesium sulfate, and
concentrated under
reduced pressure. The residue was purified by silica gel column chromatography
(methanol/ethyl acetate) to obtain the title compound (770 mg).
MS: [M+H]-1- 410.2
[0277]
d) 1-((6-fluoropyridin-2-yl)methyl)-2-methoxy-6-(4-methoxy-5H-pyrrolo[3,2-
d]pyrimidin-5-y1)-1H-imidazo[4,5-b]pyridine
Sodium methoxide (28% in methanol solution) (0.2 mL) was added to 2-chloro-1-
((6-
fluoropyridin-2-yl)methyl)-6-(4-methoxy-5H-pyrrolo[3,2-d]pyrimidin-5-y1)-1H-
imidazo[4,5-
b]pyridine (380 mg) in THF (5 mL) at room temperature. Under a dry atmosphere,
the mixture
was stirred at room temperature for 30 minutes. The reaction mixture was
diluted with water at
room temperature, and extracted with ethyl acetate. The organic layer was
washed with water
and a saturated brine, dried over magnesium sulfate, and then concentrated.
The residue was
purified by NH silica gel column chromatography (ethyl acetate/hexane) and
HPLC (C18,
mobile phase: water/acetonitrile (0.1% TFA-containing system)), and washed
with ethyl
acetate/WE to obtain the title compound (56.8 mg).
NMR (300 MHz, DMSO-d6) 6 3.73 (3H, s), 4.19 (3H, s), 5.39 (2H, s), 6.80 (1H,
d, J
= 3.2 Hz), 7.12 (1H, dd, J = 8.1, 2.4 Hz), 7.25 (1H, dd, J = 7.4, 2.2 Hz),
7.91 (1H, d, J = 3.1 Hz),
7.96-8.09 (2H, m), 8.40 (1H, d, J = 2.4 Hz), 8.49 (1H, s).
[0278]
Example 102
1-(3-fluorobenzy1)-6-(7-methoxy-1H-pyrrolo[3,2-b]pyridin-l-y1)-2-methyl-lH-
imidazo[4,5-b]pyridine
a) 7-methoxy-1H-pyrrolo[3,2-b]pyridine
A mixture of 7-chloro-1H-pyrrolo[3,2-b]pyridine (1 g), methanol (20 mL) and
sodium
methoxide (28% in methanol solution) (1.817 mL) was stirred under microwave
irradiation at
150 C for 10 hours. The reaction mixture was poured into a saturated aqueous
ammonium
chloride solution at room temperature, and extracted with ethyl acetate. The
organic layer was
dried over magnesium sulfate, and concentrated under reduced pressure. The
residue was
purified by silica gel column chromatography (ethyl acetate/hexane) and NH
silica gel column
chromatography (ethyl acetate/hexane) to obtain the title compound (0.113 g).
NMR (300 MHz, DMSO-d6) 53.97 (3H, s), 6.48 (1H, dd, J = 3.1, 2.1 Hz), 6.73
(1H,
d, J = 5.4 Hz), 7.42-7.49 (1H, m), 8.18 (1H, d, J = 5.4 Hz), 11.45 (1H, brs).
[0279]
117
CA 03021185 2018-10-16
A00039
b) 1-(3-fluorobenzy1)-6-(7-methoxy-1H-pyrrolo[3,2-b]pyridin-l-y1)-2-methyl-1H-
, imidazo[4,5-b]pyridine
A mixture of 1-(3-fluorobenzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine (400
mg),
7-methoxy-1H-pyrrolo[3,2-b]pyridine (194 mg), potassium phosphate (730 mg),
trans-N,N'-
dimethylcyclohexane-1,2-diamine (190 mg), copper (I) iodide (125 mg) and THF
(3 mL) was
stirred under microwave irradiation at 130 C for 3 hours. The mixture was
poured into water at
room temperature, and extracted with ethyl acetate. The organic layer was
washed with a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was purified by NH silica gel column chromatography
(methanol/ethyl acetate) and
silica gel column chromatography (methanol/ethyl acetate) to obtain the title
compound (154
mg).
iff NIvIR (300 MHz, DMSO-d6) V.61 (3H, s), 3.55 (3H, s), 5.56 (2H, s), 6.76
(1H, d, J
= 3.3 Hz), 6.80 (1H, d, J = 5.5 Hz), 6.98 (1H, d, J = 7.7 Hz), 7.02-7.10 (1H,
m), 7.11-7.21 (1H,
m), 7.36-7.48 (1H, m), 7.72 (1H, d, J = 3.3 Hz), 8.16 (1H, d, J = 2.4 Hz),
8.29 (1H, d, J = 5.5
Hz), 8.48 (1H, d, J = 2.4 Hz).
[0280]
Example 105
1 -(1 -(3-fluorobenzy1)-2 -m ethy1-1H-im idazo[4,5-b]pyridin-6-y1)-7-m ethoxy-
1H-
pyrazolo [4,3-b]pyridine
a) 7-methoxy-1H-pyrazolo[4,3-b]pyridine
A mixture of 7-chloro-1H-pyrazolo[4,3-b]pyridine (300 mg), methanol (5 mL) and
sodium methoxide (555 mg) was stirred under microwave irradiation at 100 C for
3 hours and at
150 C for 8 hours. The reaction mixture was poured into a saturated aqueous
ammonium
chloride solution at room temperature, and extracted with ethyl acetate. The
organic layer was
dried over magnesium sulfate, and concentrated under reduced pressure. The
residue was
purified by silica gel column chromatography (ethyl acetate), and crystallized
from ethyl
acetate/hexane to obtain the title compound (93 mg).
1HNMR (300 MHz, DMSO-d6) 84.03 (3H, s), 6.91 (1H, d, J = 5.1 Hz), 8.19 (1H,
s),
8.36 (1H, d, J = 5.1 Hz), 13.55 (1H, brs).
[0281]
b) 1-(1-(3-fluorobenzy1)-2-methy1-1H-imidazo[4,5-b]pyridin-6-y1)-7-methoxy-1H-
pyrazolo[4,3-b]pyridine
A mixture of 1-(3-fluorobenzy1)-6-iodo-2-methy1-1H-imidazo[4,5-b]pyridine (224
mg),
7-methoxy-1H-pyrrolo[4,3-b]pyridine (100 mg), potassium phosphate (409 mg),
trans-N,N'-
118
CA 03021185 2018-10-16
A00039
dimethylcyclohexane-1,2-diamine (106 mg), copper (I) iodide (69.7 mg) and TI-
IF (3 mL) was
stirred under microwave irradiation at 150 C for 4 hours. The mixture was
poured into water at
room temperature, and extracted with ethyl acetate. The organic layer was
washed with a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was purified by NI-I silica gel column chromatography
(methanol/ethyl acetate) and
silica gel column chromatography (methanol/ethyl acetate), and crystallized
from ethyl
acetate/heptane to obtain the title compound (41.2 mg).
IFI NMR (300 MHz, DMSO-d6) 82.63 (3H, s), 3.69 (3H, s), 5.59 (2H, s), 6.97
(1H, d, J
= 7.9 Hz), 7.03 (1H, d, J= 5.2 Hz), 7.05-7.11 (1H, m), 7.12-7.21 (1H, m), 7.37-
7.48 (1H, m),
8.25 (1H, d, J = 2.4 Hz), 8.48 (1H, d, J = 5.2 Hz), 8.53 (1H, s), 8.59 (1H, d,
J = 2.4 Hz).
[0282]
Example 110
1-(3-fluorobenzy1)-6-(3-methoxy-5H-pyrrolo[2,3-b]pyrazin-5-y1)-2-methyl-1H-
imidazo[4,5-b]pyridine
a) 3-chloro-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazine
Sodium hydride (60%, oil) (0.846 g) was added to a mixture of 3-chloro-5H-
pyrrolo[2,3-b]pyrazine (2.5 g) and DMF (25 mL) at 0 C. The mixture was stirred
for 5
minutes, and 2-(trimethylsilyl)ethoxymethyl chloride (3.75 mL) was then added
to the mixture at
0 C. The mixture was stirred under an argon atmosphere at room temperature for
2 hours.
The reaction mixture was poured into a saturated aqueous ammonium chloride
solution at 0 C,
and extracted with ethyl acetate. The organic layer was washed with water and
a saturated
brine, dried over sodium sulfate, and concentrated under reduced pressure. The
residue was
purified by silica gel column chromatography (ethyl acetate/hexane) to obtain
the title compound
(4.42 g).
II-1 NMR (300 MHz, DMSO-d6) 8 -0.14--0.07 (9H, m), 0.76-0.88 (2H, m), 3.52
(2H, dd,
J = 8.5, 7.6 Hz), 5.60 (2H, s), 6.81 (1H, d, J = 3.7 Hz), 8.10 (1H, d, J = 3.8
Hz), 8.56 (1H, s).
[0283]
b) 3-methoxy-54(2-(trimethylsilypethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazine
A mixture of 3-chloro-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-
b]pyrazine
(1 g), N-methyl-2-pyrrolidone (10 mL) and sodium methoxide (28% methanol
solution) (0.68 g)
was stirred at 70 C for 2 hours. The reaction mixture was poured into water,
and extracted with
ethyl acetate. The organic layer was washed with water and a saturated brine,
dried over
sodium sulfate, and concentrated under reduced pressure. The residue was
purified by silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound
(0.693 g).
119
CA 03021185 2018-10-16
A00039
1HNMR (300 MHz, DMSO-do) -0.17--0.07 (9H, m), 0.76-0.90 (2H, m), 3.46-3.61
(2H, m), 3.96 (3H, s), 5.56 (2H, s), 6.64 (1H, d, J = 3.8 Hz), 7.70 (1H, d, J
= 3.7 Hz), 8.13 (1H,
s).
[0284]
c) 3-methoxy-5H-pyrrolo[2,3-b]pyrazine
TFA (3 mL) was added to 3-methoxy-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-
pyrrolo[2,3-b]pyrazine (1.02 g) at room temperature. Under a dry atmosphere,
the mixture was
stirred at room temperature for 1 hour. The reaction mixture was concentrated
under reduced
pressure. To the residue were added ethanol and ammonia (28% in aqueous
solution) at 0 C.
Under a dry atmosphere, the mixture was stirred at room temperature for 4
hours. The reaction
mixture was concentrated under reduced pressure. The residue was washed with
water and
IPE, and dried under reduced pressure to obtain the title compound (0.461 g).
1HNMR (300 MHz, DMSO-d6) 8 3.92 (3H, s), 6.54 (1H, dd, J = 3.5, 0.9 Hz), 7.49-
7.54
(1H, m), 8.06 (1H, s), 11.79 (1H, brs).
[0285]
d) 1-(3-fluorobenzyl)-6-(3-methoxy-5H-pyrrolo[2,3-b]pyrazin-5-y1)-2-methyl-1H-
imidazo[4,5-b]pyridine
A mixture of 1-(3-fluorobenzy1)-6-iodo-2-methyl-1H-imidazo[4,5-b]pyridine (222
mg),
3-methoxy-5H-pyrrolo[2,3-b]pyrazine (90 mg), potassium phosphate (384 mg),
trans-N,N'-
dinnethylcyclohexane-1,2-diamine (0.104 mL), copper (I) iodide (63.2 mg) and
THF (5 mL) was
stirred under microwave irradiation at 100 C for 3 hours. The mixture was
diluted with water
at room temperature, and extracted with ethyl acetate. The organic layer was
washed with a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was purified by silica gel column chromatography (methanoUethyl
acetate), and
pulverized with ethyl acetate to obtain the title compound (71.9 mg).
IHNMR (300 MHz, DMSO-d6) 6 2.62 (3H, s), 3.74 (3H, s), 5.62 (2H, s), 6.87 (1H,
d, J
= 3.8 Hz), 6.94-7.20 (3H, m), 7.33-7.45 (1H, m), 8.07 (1H, d, J = 3.8 Hz),
8.19 (1H, s), 8.54 (1H,
d, J = 2.4 Hz), 8.81 (1H, d, J ¨ 2.4 Hz).
[0286]
Example 134
1-(5-(1-(3,5-difluorobenzy1)-2-methoxy-1H-imidazo[4,5-b]pyridin-6-y1)-5H-
pyrrolo[2,3-b]pyrazin-3-yl)ethanol
a) 1-(5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazin-3-
yDethanone
A mixture of chloro(2-dicyclohexylphosphino-2',4',6'-triisopropy1-1,1'-
bipheny1)[2-
120
CA 03021185 2018-10-16
A00039
(2'-amino-1,1'-biphenyl)]palladium (II) (0.31 g), cesium fluoride (4.33 g), 3-
chloro-5-42-
, (trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazine (3.68 g),
tributy1(1-
ethoxyvinyl)stannane (5.8 mL) and DME (40 mL) was stirred under an argon
atmosphere at
80 C for 3 hours. The mixture was poured into an aqueous potassium fluoride
solution,
insolubles were removed, and the mixture was extracted with ethyl acetate. The
organic layer
was washed with an aqueous potassium fluoride solution and a saturated brine,
then dried over
sodium sulfate, and concentrated under reduced pressure. The residue was
purified by silica gel
column chromatography (ethyl acetate/hexane). The obtained residue (3.86 g)
was diluted with
methanol (100 mL)/hydrochloric acid (2 N in aqueous solution) (25 mL) at room
temperature.
The mixture was stirred at room temperature for 30 minutes. The mixture was
poured into
water, and extracted with ethyl acetate. The organic layer was washed with
water and a
saturated brine, then dried over sodium sulfate, and concentrated under
reduced pressure. The
residue was purified by silica gel column chromatography (ethyl
acetate/hexane) to obtain the
title compound (3.05 g).
11-1 NMR (300 MHz, DMSO-d6) 6 -0.16--0.09 (9H, m), 0.80-0.91 (2H, m), 2.71
(3H, s),
3.53-3.64 (2H, m), 5.72 (2H, s), 6.88 (11-1, d, J = 3.7 Hz), 8.38 (1H, d, J =
3.7 Hz), 9.05 (1H, s).
[0287]
b) 1-(5H-pyrrolo[2,3-b]pyrazin-3-yl)ethanol mono-TFA salt
Sodium borohydride (0.398 g) was added to a mixture of 1454(2-
(trimethylsilypethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazin-3-ypethanone (3.05 g)
and methanol
(60 mL) at 0 C. The mixture was stirred at room temperature for 15 minutes.
The mixture
was poured into hydrochloric acid (0.1 N in aqueous solution), and extracted
with ethyl acetate.
The organic layer was washed with water and a saturated brine, then dried over
sodium sulfate,
and concentrated under reduced pressure. A mixture of the residue and TFA (20
mL) was
stirred at room temperature for 1 hour, and concentrated under reduced
pressure. The residue
was diluted with ethanol/ammonia (28% in aqueous solution). The mixture was
stirred at 60 C
for 15 minutes, and then concentrated under reduced pressure. The residue was
purified by
silica gel column chromatography (ethyl acetate/hexane) to obtain the title
compound (2.22 g).
11-1NMR (300 MHz, DMSO-d6) 61.45 (3H, d, J = 6.5 Hz), 4.87 (1H, q, J = 6.5
Hz), 5.37
(1H, brs), 6.58 (1H, dd, J = 3.6, 1.8 Hz), 6.86-7.34 (1H, m), 7.79 (1H, dd, J
3.5, 2.8 Hz), 8.53
(1H, s), 11.89 (1H, brs).
[0288]
c) 1-(5-(1-(3,5-difluorobenzy1)-2-(isopropylthio)-1H-imidazo[4,5-b]pyridin-6-
y1)-5H-
pyrrolo[2,3-b]pyrazin-3-yl)ethanol
121
CA 03021185 2018-10-16
A00039
A mixture of 1-(3,5-difluorobenzyI)-6-iodo-2-(isopropylthio)-1H-imidazo[4,5-
' b]pyridine (707 mg), a 1-(5H-pyrrolo[2,3-b]pyrazin-3-yl)ethanol mono-
TFA salt (400 mg),
potassium phosphate (919 mg), trans-N,N'-dimethylcyclohexane-1,2-diamine
(0.249 mL),
copper (I) iodide (151 mg) and THF (8 mL) was stirred under microwave
irradiation at 100 C
for 3 hours. The mixture was diluted with water at room temperature, and
extracted with ethyl
acetate. The organic layer was washed with an aqueous ammonium chloride
solution and a
saturated brine, then dried over sodium sulfate, and concentrated under
reduced pressure. The
residue was purified by silica gel column chromatography (methanol/ethyl
acetate) to obtain the
title compound (610 mg).
1HNMR (300 MHz, DMSO-d6) M.39 (3H, d, J = 6.5 Hz), 1.49 (6H, d, J = 6.8 Hz),
4.11-4.25 (1H, m), 4.84 (1H, dd, J = 6.5, 4.8 Hz), 5.44-5.60 (3H, m), 6.89-
7.02 (3H, m), 7.17-
7.29 (1H, m), 8.33 (1H, d, J = 3.9 Hz), 8.52 (1H, d, J = 2.4 Hz), 8.69 (1H,
s), 8.85 (1H, d, J = 2.4
Hz).
[0289]
d) 1-(5-(1-(3,5-difluorobenzy1)-2-methoxy-1H-imidazo[4,5-blpyridin-6-y1)-5H-
pyrrolo[2,3-b]pyrazin-3-yl)ethanol
Methachloroperbenzoic acid (0.73 g) was added to a mixture of 1454143,5-
difluorobenzy1)-2-(isopropylthio)-1H-imidazo[4,5-b]pyridin-6-y1)-5H-
pyrrolo[2,3-b]pyrazin-3-
yl)ethanol (1.18 g) and acetonitrile (30 mL) at 0 C. The mixture was stirred
at 0 C for 2 hours,
and sodium methoxide (28% in methanol solution) (1.5 mL) was added at 0 C. The
mixture
was stirred at room temperature for 15 minutes. The reaction mixture was
poured into water at
room temperature, and extracted with ethyl acetate. The organic layer was
washed with water
and a saturated brine, dried over magnesium sulfate, and then concentrated.
The residue was
purified by silica gel column chromatography (methanol/ethyl acetate) to
obtain the title
compound (0.53 g).
NMR (300 MHz, DMSO-d6) 81.39 (3H, d, J = 6.5 Hz), 4.24 (3H, s), 4.77-4.89 (1H,
m), 5.35 (2H, s), 5.50 (1H, d, J = 4.8 Hz), 6.91 (1H, d, J = 3.8 Hz), 7.02-
7.12 (2H, m), 7.16-7.27
(1H, m), 8.29 (1H, d, J = 3.8 Hz), 8.36 (1H, d, J = 2.4 Hz), 8.64-8.71 (2H,
m).
[0290]
Example 158
(R)-1-(5-(1-(3,5-difluorobenzy1)-2-methoxy-1H-imidazo [4,5 -b]pyridin-6-y1)-5H-
pyrrolo[2,3-b]pyrazin-3-ypethanol (optical isomer)
A racemate of 1-(5-(1-(3,5-difluorobenzy1)-2-methoxy-1H-imidazo[4,5-b]pyridin-
6-y1)-
5H-pyrrolo[2,3-b]pyrazin-3-yl)ethanol (553.5 mg) was fractionated by SFC
(column:
122
CA 03021185 2018-10-16
A00039
CHIRALPAK ASH (LA005), 20 mm ID x 250 mm L, manufactured by Daicel Chemical
Industries Ltd, mobile phase: CO2/ethanol (containing 0.3% diethylamine) =
700/300), and
crystallized from ethanol/water to obtain the title compound (0.219 g) with a
smaller retention
time.
1H NMR (300 MHz, DMSO-d6) 81.39 (3H, d, J = 6.5 Hz), 4.24 (3H, s), 4.75-4.93
(1H,
m), 5.35 (2H, s), 5.50 (1H, d, J = 4.8 Hz), 6.91 (1H, d, J = 3.8 Hz), 7.01-
7.11 (2H, m), 7.16-7.27
(1H, m), 8.29 (1H, d, J = 3.8 Hz), 8.36 (1H, d, J = 2.4 Hz), 8.64-8.70 (2H,
m).
[0291]
Example 159
(S)-1-(5-(1-(3,5-difluorobenzy1)-2-methoxy-1H-imida7o[4,5-b]pyridin-6-y1)-5H-
pyrrolo[2,3-b]pyrazin-3-y1)ethanol (optical isomer)
A racemate of 1-(5-(1-(3,5-difluorobenzy1)-2-methoxy-1H-imidazo[4,5-b]pyridin-
6-y1)-
5H-pyrrolo[2,3-b]pyrazin-3-yl)ethanol (553.5 mg) was fractionated by SFC
(column:
CI-IIRALPAK ASH (LA 005), 20 mm ID x 250 mm L, manufactured by Daicel Chemical
Industries Ltd, mobile phase: CO2/ethanol (containing 0.3% diethylamine) =
700/300), and
crystallized from ethanol/water to obtain the title compound (0.197 g) with a
larger retention
time.
1H NMR (300 MHz, DMSO-d6) 81.39 (3H, d, J = 6.5 Hz), 4.24 (3H, s), 4.78-4.90
(1H,
m), 5.35 (2H, s), 5.50 (1H, d, J = 4.7 Hz), 6.91 (1H, d, J = 3.9 Hz), 7.01-
7.11 (2H, m), 7.16-7.27
(1H, m), 8.29 (1H, d, J = 3.9 Hz), 8.36 (1H, d, J = 2.4 Hz), 8.66-8.69 (2H,
m).
[0292]
Example 160
1-(5-(1-(3-fluorobenzy1)-2-methoxy-1H-imidazo[4,5-b]pyridin-6-y1)-5H-
pyrrolo[2,3-
b]pyrazin-3-yl)ethanol (optical isomer)
a) 1-(3-fluorobenzy1)-6-iodo-2-(isopropylthio)-1H-imidazo[4,5-b]pyridine
Under a dry atmosphere, a mixture of N3-(3-fluorobenzyl)-5-iodopyridine-2,3-
diamine
(5 g), CDI (4.75 g) and THF (50 mL) was stirred at 60 C for 16 hours. The
reaction mixture
was quenched with water at room temperature, and extracted with ethyl acetate.
The organic
layer was sequentially washed with a saturated aqueous ammonium chloride
solution, water and
a saturated brine, then dried over sodium sulfate, and concentrated under
reduced pressure to
obtain a brown solid as 1-(3-fluorobenzy1)-6-iodo-1H-irnidazo[4,5-b]pyridin-
2(3H)-one. A
mixture of the obtained solid and phosphorus oxychloride (50 mL) was stirred
at 100 C for 2
days. The reaction mixture was concentrated under reduced pressure. The
residue was diluted
with THF, poured into a saturated aqueous sodium hydrogen carbonate solution,
and extracted
123
CA 03021185 2018-10-16
A00039
with ethyl acetate. The organic layer was washed with water and a saturated
brine, dried over
sodium sulfate, and then concentrated. The residue was diluted with THF (100
mL). To the
mixture was added sodium 2-propanethiolate (2.066 g) at 0 C. Under a
nitrogen atmosphere,
the mixture was stirred overnight at room temperature. The reaction mixture
was diluted with
ethyl acetate. Insolubles were removed, and the mixture was concentrated under
reduced
pressure. The residue was purified by silica gel column chromatography (ethyl
acetate/hexane)
to obtain the title compound (5.87 g).
1H NMR (300 MHz, DMSO-d6) M.45 (61-1, d, J = 6.8 Hz), 4.05-4.16 (1H, m), 5.42
(2H,
s), 6.93 (1H, d, J = 7.7 Hz), 7.00-7.19 (2H, m), 7.34-7.44 (1H, m), 8.41 (1H,
d, J = 1.9 Hz), 8.51
(1H, d, J = 1.9 Hz).
[0293]
b) 1-(5-(1-(3-fluorobenzy1)-2-(isopropyl thio)-1H-imidazo[4,5-b]pyridin-6-y1)-
5H-
pyrrolo[2,3-b]pyrazin-3-ypethanol
A mixture of 1-(3-fluorobenzy1)-6-iodo-2-(isopropylthio)-1H-imidazo[4,5-
b]pyridine
(1017 mg), a 1-(5H-pyrrolo[2,3-b]pyrazin-3-yl)ethanol mono-TFA salt (600 mg),
potassium
phosphate (1378 mg), trans-N,N'-dimethylcyclohexane-1,2-diamine (0.374 mL),
copper (I)
iodide (227 mg) and THF (10 mL) was stirred under microwave irradiation at 100
C for 3 hours.
The mixture was diluted with water at room temperature, and extracted with
ethyl acetate. The
organic layer was washed with a saturated brine, then dried over sodium
sulfate, and
concentrated under reduced pressure. The residue was purified by silica gel
column
chromatography (methanol/ethyl acetate) to obtain the title compound (600 mg).
1H NMR (300 MHz, DMSO-d6) M .40 (3H, d, J = 6.4 Hz), 1.49 (6H, d, J = 6.8 Hz),
4.10-4.26 (1H, m), 4.72-4.91 (1H, m), 5.41-5.56 (3H, m), 6.93 (1H, d, J = 3.8
Hz), 7.02-7.24
(3H, m), 7.35-7.48 (111, m), 8.33 (11-1, d, J = 3.8 Hz), 8.53 (1H, d, J = 2.4
Hz), 8.68 (1H, s), 8.83
(1H, d, J = 2.3 Hz).
[0294]
c) 1-(5-(1-(3-fluorobenzy1)-2-methoxy-1H-imidazo[4,5-b]pyridin-6-y1)-5H-
pyrrolo[2,3-
b]pyrazin-3-ypethanol (optical isomer)
Methachloroperbenzoic acid (0.79 g) was added to a mixture of 1454143-
fluorobenzy1)-2-(isopropylthio)-111-imidazo[4,5-b]pyridin-6-y1)-5H-pyrrolo[2,3-
b]pyrazin-3-
yl)ethanol (1.24 g), DMF (5 mL) and ethyl acetate (30 mL) at 0 C. The mixture
was stirred at
room temperature for 3 hours, and then concentrated under reduced pressure.
The residue was
diluted with THF (20 mL), and sodium methoxide (28% in methanol solution) (2.8
mL) was
added at 0 C. The mixture was stirred at room temperature for 10 minutes. The
reaction
124
CA 03021185 2018-10-16
A00039
mixture was poured into water at room temperature, and extracted with ethyl
acetate. The
organic layer was washed with water and a saturated brine, dried over sodium
sulfate, and then
concentrated. The residue was purified by silica gel column chromatography
(methanol/ethyl
acetate) to obtain 1-(5-(1-(3-fluorobenzy1)-2-methoxy-1H-imidazo[4,5-b]pyridin-
6-y1)-5H-
pyrrolo[2,3-b]pyrazin-3-yl)ethanol. A racemate of 1-(5-(1-(3-fluorobenzy1)-2-
methoxy-1H-
imidazo[4,5-b]pyridin-6-y1)-5H-pyrrolo[2,3-b]pyrazin-3-yl)ethanol (688 mg) was
fractionated by
SFC (column: CHIRALPAK ASH (S90ASHSCZ-RA001), 30 mm ID x 250 mm L,
manufactured by Daicel Chemical Industries Ltd, mobile phase: CO2/ethanol =
800/200), and a
compound with a smaller retention time was crystallized from ethanol/water to
obtain the title
compound (335 mg).
NMR (300 MHz, DMSO-d6) M.40 (3H, d, J = 6.5 Hz), 4.24 (3H, s), 4.84 (1H, dd, J
= 6.5, 4.8 Hz), 5.34 (2H, s), 5.50 (1H, d, J = 4.8 Hz), 6.91 (11-1, d, J = 3.8
Hz), 7.10-7.25 (3H, m),
7.36-7.46 (1H, m), 8.28 (1H, d, J = 3.8 Hz), 8.37 (1H, d, J = 2.4 Hz), 8.64-
8.70 (2H, m).
[0295]
Example 161
1-(5-(1-(3-fluorobenzy1)-2-methoxy-1H-imidazo[4,5-b]pyridin-6-yI)-5H-
pyrrolo[2,3-
b]pyrazin-3-yl)ethanol (optical isomer)
A racemate of 1-(5-(1-(3-fluorobenzy1)-2-methoxy-1H-imidazo[4,5-b]pyridin-6-
y1)-5H-
pyrrolo[2,3-b]pyrazin-3-yl)ethanol (688 mg) was fractionated by SFC (column:
CHIRALPAK
ASH (S90ASHSCZ-RA001), 30 mm ID x 250 mm L, manufactured by Daicel Chemical
Industries Ltd, mobile phase: CO2/ethanol = 800/200), and a compound with a
larger retention
time was crystallized from ethanol/water to obtain the title compound (309
mg).
1H NMR (300 MHz, DMSO-d6) M.40 (3H, d, J = 6.4 Hz), 4.24 (3H, s), 4.79-4.90
(1H,
m), 5.34 (2H, s), 5.50 (1H, d, J = 4.7 Hz), 6.91 (1H, d, J = 3.8 Hz), 7.08-
7.25 (3H, m), 7.40 (1H,
d, J = 6.0 Hz), 8.28 (1H, d, J = 4.0 Hz), 8.37 (1H, d, J = 2.4 Hz), 8.64-8.70
(2H, m).
[0296]
Example 162
1-(3,5-difluorobenzy1)-6-(4-methoxypyrrolo[1,2-b]pyridin-5-y1)-2-methyl-1H-
imidazo[4,5-b]pyridine
a) 6-bromo-2-methyl-1H-imidazo[4,5-b]pyridine
A solution of 5-bromopyridine-2,3-diamine (83.5 g) in acetic acid (500 mL) was
heated
and refluxed for 2 days. The reaction mixture was concentrated under reduced
pressure. The
residue was pulverized with WE, and dried under reduced pressure to obtain the
title compound
(93 g).
125
84777231
1H NMR (300 MHz, DMSO-d6) 6 2.51-2.53 (3H, m), 8.12 (111, d, J = 2.2 Hz), 8.31
(1H,
d, J =-- 2.2 Hz), 12.96 (1H, brs).
[0297]
b) 6-bromo-1-(3,5-difluorobenzy1)-2-methy1-1H-imidazo[4,5-b]pyridine
Potassium hydroxide (2.381 g) was added to a mixture of 6-bromo-2-methy1-1H-
imidazo[4,5-b]pyridine (3 g), 1-(bromomethyl)-3,5-difluorobenzene (2.014 mL)
and THF (40
mL) at 60 C. The reaction mixture was stirred overnight at 60 C. The reaction
mixture was
cooled to room temperature, then poured into water, and extracted with ethyl
acetate. The
organic layer was washed with a saturated brine, then dried over magnesium
sulfate, and
concentrated under reduced pressure. The residue was purified by silica gel
column
chromatography (ethyl acetate/hexane), and crystallized from ethanol/IPE. The
obtained
precipitate was collected by filtration, and washed with WE to obtain the
title compound (2.000
8).
NMR (300 MHz, DMSO-d6) 6 2.55 (3H, s), 5.55 (2H, s), 6.82-6.93 (2H, m), 7.20
.. (1H, tt, J = 9.4, 2.2 Hz), 8.31 (1H, d, J = 2.2 Hz), 8.43 (1H, d, J = 2.2
Hz).
[0298]
c) 1-(3,5-difluorobenzy1)-2-methy1-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-
y1)-1H-
imidazo[4,5-b]pyridine
A mixture of 6-bromo-1-(3,5-difluorobenzy1)-2-methy1-1H-imidazo[4,5-b]pyridine
(400
mg), bis (pinacolato)diboron (468 mg), a [1,1'-
bis(diphenylphosphino)ferrocene]palladium (II)
dichloride dichloromethane complex (108 mg), potassium acetate (452 mg), THF
(9 mL) and
DMSO (0.9 mL) was stirred under microwave irradiation at 110 C for 1 hour. The
reaction
mixture was diluted with ethyl acetate, and insolubles were removed by
filtration through Celite TM.
The filtrate was poured into water, and extracted with ethyl acetate. The
organic layer was
washed with a saturated brine, then dried over magnesium sulfate, and
concentrated under
reduced pressure. The residue was collected by filtration, and washed with WE
to obtain the
title compound (387 mg).
NMR (300 MHz, DMSO-d6) 6 1.31 (12H, s), 2.57 (3H, s), 5.65 (2H, s), 6.71-6.85
(2H, m), 7.19 (1H, U, J ¨ 9.3, 2.2 Hz), 8.10 (1H, d, J= 1.5 Hz), 8.60 (111, d,
1= 1.5 Hz).
[0299]
d) Ethyl 4-oxo-1,4-dihydropyrrolo[1,2-b]pyridazine-3-carboxylate
A mixture of 1H-pyrrol-1 -amine (9.62 mL) and diethyl
(ethoxymethylene)malonate (30
mL) was stirred at 125 C for 2 hours. The reaction mixture was diluted with
diphenyl ether (40
mL), and the resulting mixture was stirred at 200 C for 3 hours. The reaction
mixture was
126
Date recue/Date received 2023-04-05
CA 03021185 2018-10-16
A00039
purified by silica gel column chromatography (ethyl acetate/hexane) to obtain
the title compound
(15.97g).
11-1. NMR (300 MHz, DMSO-d6) 6 1.35 (3H, t, J = 7.1 Hz), 4.37 (2H, q, J = 7.1
Hz), 6.85
(1H, dd, J = 4.4, 2.5 Hz), 6.99 (1H, dd, J = 4.4, 1.6 Hz), 7.97 (1H, dd, J =
2.6, 1.6 Hz), 8.32 (1H,
s), 12.11 (1H, brs).
[0300]
e) 4-oxo-1,4-dihydropyrrolo[1,2-b]pyridazine-3-carboxylic acid
A mixture of ethyl 4-oxo-1,4-dihydropyrrolo[1,2-b]pyridazine-3-carboxylate
(15.9 g), a
8 N aqueous sodium hydroxide solution (40 mL) and ethanol (150 mL) was heated
and refluxed
for 6 hours. The reaction mixture was concentrated under reduced pressure. To
the residue
were added 6 N hydrochloric acid (65 mL) and water. The precipitate was
collected by
filtration, and washed with water to obtain the title compound (13.40 g).
IFINMR (300 MHz, DMSO-d6) 6 6.80 (1H, dd, J = 4.4, 2.6 Hz), 6.89 (1H, dd, J =
4.4,
1.6 Hz), 7.89 (1H, dd, J = 2.6, 1.6 Hz), 8.26 (1H, s).
[0301]
I) Pyrrolo[1,2-b]pyridazin-4(1H)-one
A mixture of 4-oxo-1,4-dihydropyrrolo[1,2-b]pyridazine-3-carboxylic acid (13.0
g) and
DMSO (50 mL) was stirred under a nitrogen atmosphere at 150 C for 1 hour. The
reaction
mixture was concentrated under reduced pressure. The residue was purified by
silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound
(8.27 g).
NMR (300 MHz, DMSO-d6) 6 5.99 (1H, d, J = 5.3 Hz), 6.56 (1H, dd, J = 4.3, 1.7
Hz), 6.69 (1H, dd, J = 4.2, 2.6 Hz), 7.71 (1H, dd, J = 2.6, 1.7 Hz), 7.89 (1H,
d, J = 5.3 Hz), 11.37
(1H, s).
[0302]
g) 4-methoxypyrrolo[1,2-b]pyridazine
Cesium carbonate (5.90 g) was added to a solution of pyrrolo[1,2-b]pyridazin-
4(1H)-
one (2.00 g) and iodomethane (1.1 mL) in THF (15 mL) and DMF (5 mL) at 0 C.
The mixture
was stirred at room temperature for 3 hours. The reaction mixture was poured
into water, and
extracted with ethyl acetate. The organic layer was washed with a saturated
brine, then dried
over magnesium sulfate, and concentrated under reduced pressure. The residue
was purified by
silica gel column chromatography (ethyl acetate/hexane) to obtain the title
compound (1.846 g).
IHNMR (300 MHz, DMSO-d6) 6 3.98 (3H, s), 6.21 (1H, d, J = 5.5 Hz), 6.53 (1H,
dd, J
= 4.3, 1.7 Hz), 6.72 (1H, dd, J = 4.2, 2.7 Hz), 7.76 (1H, dd, J = 2.6, 1.7
Hz), 8.05 (1H, d, J = 5.4
Hz).
127
A00039
CA 03021185 2018-10-16
[0303]
h) 5,7-dibromo-4-methoxypyrrolo[1,2-b]pyridazine
1,3-dibromo-5,5-dimethylhydantoin (3.54 g) was added to a solution of 4-
methoxypyrrolo[1,2-b]pyridazine (1.83 g) in THF (50 mL) at 0 C. The mixture
was stirred at
0 C for 0.5 hours. The reaction mixture was concentrated under reduced
pressure. The
residue was purified by silica gel column chromatography (ethyl
acetate/hexane) to obtain the
title compound (3.54 g).
1H NMR (300 MHz, DMSO-d6) 3.99 (3H, s), 6.37 (1H, d, J = 5.6 Hz), 7.07 (1H,
s),
8.23 (1H, d, J = 5.6 Hz).
[0304]
i) 5-bromo-4-methoxypyrrolo[1,2-b]pyridazine
n-butyllithium (1.6 M in hexane solution) (7.1 mL) was added dropwise to a
solution of
5,7-dibromo-4-methoxypyrrolo[1,2-b]pyridazine (3.52 g) in THF (70 mL) at -78
C. The
mixture was stirred under a nitrogen atmosphere at -78 C for 0.5 hours. Water
was added to the
reaction mixture at -78 C, and the temperature was elevated to room
temperature, and the
mixture was extracted with ethyl acetate. The organic layer was washed with a
saturated brine,
then dried over magnesium sulfate, and concentrated under reduced pressure.
The residue was
purified by silica gel column chromatography (ethyl acetate/hexane) to obtain
the title compound
(2.501 g).
1H NMR (300 MHz, DMSO-d6) 5 3.96 (3H, s), 6.25 (1H, d, J = 5.6 Hz), 6.81 (1H,
dd, J
= 2.9, 0.3 Hz), 7.79 (1H, d, J = 2.9 Hz), 8.07 (1H, d, J = 5.5 Hz).
[0305]
j) 1-(3,5-difluorobenzy1)-6-(4-methoxypyrrolo[1,2-b]pyridin-5-y1)-2-methyl-lH-
imidazo[4,5-b]pyridine
A mixture of 5-bromo-4-methoxypyrrolo[1,2-b]pyridazine (80 mg), 1-(3,5-
difluorobenzy1)-2-methy1-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-y1)-1H-
imidazo[4,5-
b]pyridine (107 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II)
(20 mg), cesium carbonate (2 M in aqueous solution) (0.5 mL) and DME (10 mL)
was stirred
under microwave irradiation at 110 C for 1 hour. The reaction mixture was
diluted with THF.
Insolubles were removed by filtration through Celite, and washed with THE The
filtrate was
concentrated under reduced pressure. The residue was purified by NTI silica
gel column
chromatography (methanol/ethyl acetate). The obtained product was collected by
filtration, and
washed with ethyl acetate/IPE to obtain the title compound (54 mg).
1H NMR (300 MI-lz, DMSO-d6) 5 2.59 (3H, s), 3.67 (3H, s), 5.57 (2H, s), 6.24
(1H, d, J
128
CA 03021185 2018-10-16
A00039
= 5.6 Hz), 6.81-6.96 (3H, m), 7.21 (1H, tt, J = 9.4, 2.3 Hz), 7.88 (1H, d, J =
2.8 Hz), 7.97 (1H, d,
J = 2.1 Hz), 8.08 (1H, d, J = 5.4 Hz), 8.49 (1H, d, J = 2.1 Hz).
[0306]
Example 175
1-(3-fluorobenzy1)-6-(4-methoxypyrrolo[2,14][1,2,4]triazin-5-y1)-2-methyl-1H-
imidazo[4,5-b]pyridine
a) 4-methoxypyrrolo[2,1-f][1,2,4]triazine
A mixture of pyrrolo[2,1-f][1,2,4]triazin-4(3H)-one (14 g), DIPEA (18.1 mL),
N,N-
dimethylbenzeneamine (12.56 g), phosphoryl chloride (42.6 mL) and toluene (300
mL) was
stirred at 100 C for 12 hours. The reaction mixture was concentrated under
reduced pressure.
Sodium methoxide (28% in methanol solution) (140 g) was added to a suspension
of the residue
and THF (100 mL) at room temperature. The mixture was washed with a saturated
aqueous
sodium hydrogen carbonate solution at room temperature, and extracted with
ethyl acetate. The
organic layer was washed with a saturated brine, then dried over magnesium
sulfate, and
concentrated under reduced pressure. The residue was purified by silica gel
column
chromatography (ethyl acetate/hexane) to obtain the title compound (12.1 g).
1H NMR (300 MHz, DMSO-d6) 4.09 (3H, s), 6.73-6.91 (2H, m), 7.94 (1H, dd, J =
2.5,
1.6 Hz), 8.19 (1H, s).
[0307]
b) 5,7-dibromo-4-methoxypyrrolo[2,14][1,2,4]triazine
NBS (28.6 g) was added to a solution of 4-methoxypyrrolo[2,1-f][1,2,4]triazine
(12 g)
in THF (200 mL) at room temperature. The mixture was stirred under a nitrogen
atmosphere at
room temperature for 1 hour and at 50 C for 1 hour. The mixture was
neutralized with a
saturated aqueous sodium hydrogen carbonate solution at 0 C, and extracted
with ethyl acetate.
The organic layer was washed with water and a saturated brine, then dried over
magnesium
sulfate, and concentrated under reduced pressure. The residue was washed with
IPE to obtain
the title compound (22.5 g).
1H NMR (300 MHz, DMSO-d6) 54.10 (3H, s), 7.22 (1H, s), 8.33 (1 H, s).
[0308]
c) 5-bromo-4-methoxypyrrolo[2,1-f][1,2,4]triazine
n-butyllithium (1.6 M in hexane solution) (64.1 mL) was added to a solution of
5,7-
dibromo-4-methoxypyrrolo[2,1-f][1,2,4]triazine (22.5 g) in THF (450 mL) at -78
C. The
mixture was stirred under a nitrogen atmosphere at -78 C for 30 minutes. Water
was added to
the reaction mixture at -78 C, and extracted with ethyl acetate. The organic
layer was
129
CA 03021185 2018-10-16
A00039
sequentially washed with water and a saturated brine, then dried over
magnesium sulfate, and
= concentrated under reduced pressure. The residue was washed with hexane
to obtain the title
compound (14.5 g).
1H NMR (300 MHz, DMSO-d6) 84.09 (3H, s), 6.96 (1H, d, J = 3.0 Hz), 7.97 (1H,
d, J
2.8 Hz), 8.19 (1H, s).
[0309]
d) 6-bromo-2-methyl-1H-imidazo[4,5-b]pyridine
A solution of 5-bromopyridine-2,3-diamine (83.5 g) in acetic acid (500 mL) was
heated
and refluxed for 2 days. The reaction mixture was concentrated under reduced
pressure. The
residue was pulverized with IPE, and dried under reduced pressure to obtain
the title compound
(93 g).
1H NMR (300 MHz, DMSO-d6) 82.51-2.53 (3H, m), 8.12 (1H, d, J = 2.2 Hz), 8.31
(1H,
d, J = 2.2 Hz), 12.96 (1H, brs).
[0310]
e) 6-bromo-1-(3-fluorobenzy1)-2-methyl-1H-imidazo[4,5-b]pyridine
Potassium hydroxide (1.984 g) was added to a mixture of 6-bromo-2-methy1-1H-
imidazo[4,5-b]pyridine (2.5 g), 3-fluorobenzyl bromide (1.735 mL) and TI-IF
(30 mL) at 60 C.
The mixture was stirred overnight at 60 C. The reaction solution was cooled to
room
temperature, then poured into water, and extracted with ethyl acetate. The
organic layer was
washed with a saturated brine, then dried over magnesium sulfate, and
concentrated under
reduced pressure. The residue was purified by NH silica gel column
chromatography (ethyl
acetate/hexane) to obtain the title compound (1.23 g).
1H NIVIR (300 MHz, DMSO-d6) 52.54 (3H, s), 5.51 (2H, s), 6.93-7.19 (3H, m),
7.31-
7.44 (1H, m), 8.27 (1H, d, J = 2.0 Hz), 8.40 (1H, d, J = 2.0 Hz).
[0311]
0 1-(3-fluorobenzy1)-2-methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-y1)-
1H-
imidazo[4,5-b]pyridine
A mixture of 6-bromo-1-(3-fluorobenzy1)-2-methy1-1H-imidazo[4,5-b]pyridine
(412
mg), a [1,1'-bis(diphenylphosphino)ferrocene]palladium (II) dichloride
dichloromethane
complex (107 mg), bis(pinacolato)diboron (667 mg), potassium acetate (391 mg),
THF (5 mL)
and DMSO (0.5 mL) was stirred under microwave irradiation at 120 C for 40
minutes. The
mixture was diluted with a saturated aqueous sodium hydrogen carbonate
solution at room
temperature, and extracted with ethyl acetate. The organic layer was washed
with a saturated
brine, dried over magnesium sulfate, and then concentrated. The residue was
washed with WE
130
CA 03021185 2018-10-16
A00039
to obtain the title compound (108 mg).
MS, found:286.1.
[0312]
g) 1 -(3-fluorobenzy1)-6-(4-methoxypyrrolo[2,1 -f] [1,2,4]triazin-5-y1)-2-
methyl-1H-
imidazo[4,5-b]pyridine
A mixture of 5-bromo-4-methoxypyrrolo[2,1-f][1,2,4]triazine (52 mg), 1-(3-
fluorobenzy1)-2-methy1-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-y1)-1H-
imidazo[4,5-
b]pyridine (108 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II)
(14 mg), cesium carbonate (2 M in aqueous solution) (0.215 mL) and DME (1 mL)
was stirred
under microwave irradiation at 100 C for 40 minutes. The reaction mixture was
concentrated
under reduced pressure. The residue was purified by NH silica gel column
chromatography
(ethyl acetate/hexane), and the residue was washed with ethyl acetate to
obtain the title
compound (12 mg).
1H NMR (300 MHz, DMSO-d6) ö 2.60 (3H, s), 3.80 (3H, s), 5.58 (2H, s), 6.96
(1H, d, J
7.6 Hz), 7.01-7.09 (2H, m), 7.15 (1H, td, J = 8.8, 3.0 Hz), 7.42 (1H, td, J =
8.0, 6.0 Hz), 8.05
(1H, d, J = 2.7 Hz), 8.12 (1H, d, J = 2.0 Hz), 8.21 (1H, s), 8.57 (1H, d, J =
2.1 Hz).
[0313]
Example 176
(1-(3,5-difluorobenzy1)-6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-1H-
imidazo[4,5-
b]pyridin-2-yl)methanol
a) 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yl)pyrrolo[2,1-
f][1,2,4]triazine
A mixture of 5-bromo-4-methoxypyrrolo[2,1-f][1,2,4]triazine (10 g), a [1,1'-
bis(diphenylphosphino)ferrocene]palladium (II) dichloride dichloromethane
complex (3.58 g),
bis(pinacolato)diboron (17.82 g), potassium acetate (17.21 g), THF (70 mL) and
DMSO (10 mL)
was stirred under microwave irradiation at 120 C for 30 minutes. The mixture
was quenched
with a saturated aqueous sodium hydrogen carbonate solution, and extracted
with ethyl acetate.
The organic layer was washed with a saturated aqueous sodium hydrogen
carbonate solution,
dried over magnesium sulfate, and then concentrated. The residue was purified
by Diol silica
gel column chromatography (ethyl acetate/hexane) to obtain the title compound
(3.96 g).
1H NMR (300 MI-1z, DMSO-d6) M.30 (12H, s), 4.06(3H, s), 6.90-7.01 (1H, m),
7.86-
7.96 (1H, m), 8.24 (1H, s).
[0314]
b) (1-(3,5-difluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridin-2-yl)methanol
131
CA 03021185 2018-10-16
A00039
A mixture of N3-(3,5-difluorobenzy1)-5-iodopyridine-2,3-diamine (1 g),
glycolic acid (5
= g) and TI-[F (1 mL) was stirred under microwave irradiation at 150 C for
2.5 hours. The
mixture was diluted with ethyl acetate and water, neutralized with a 8 N
aqueous sodium
hydroxide solution, basified with a saturated aqueous sodium hydrogen
carbonate solution, and
extracted with ethyl acetate. The organic layer was washed with a saturated
brine, dried over
magnesium sulfate, and then concentrated. The residue was purified by silica
gel column
chromatography (ethyl acetate/hexane and methanol/ethyl acetate) and NH silica
gel column
chromatography (ethyl acetate/hexane and methanol/ethyl acetate) to obtain the
title compound
(0.65 g).
IH NMR (300 MHz, DMSO-d6) 4.77 (2H, d, J = 5.9 Hz), 5.61 (2H, s), 5.84-5.95
(1H,
m), 6.91-7.04 (2H, m), 7.19 (1H, U, J = 9.3, 2.4 Hz), 8.33 (1H, d, J = 2.0
Hz), 8.58 (1H, d, J = 2.0
Hz).
[0315]
c) (1-(3,5-difluorobenzy1)-6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-1H-
imidazo[4,5-b]pyridin-2-yl)methanol
A mixture of (1-(3,5-difluorobenzy1)-6-iodo-1H-imidazo[4,5-b]pyridin-2-
y1)methanol
(0.915 g), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yl)pyrrolo
[2,1-
f][1,2,4]triazine (2 g), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II)
(0.137 g), cesium carbonate (2 M in aqueous solution) (3.05 mL) and DME (14
mL) was stirred
under microwave irradiation at 100 C for 40 minutes. The reaction mixture was
concentrated
under reduced pressure. The residue was purified by NH silica gel column
chromatography
(methanol/ethyl acetate), and the residue was crystallized from ethanol/water
to obtain the title
compound (0.35 g).
IH NMR (300 MHz, DMSO-d6) 63.79 (3H, s), 4.83 (2H, d, J = 3.0 Hz), 5.65 (2H,
s),
5.91 (1H, brs), 6.95-7.09 (3H, m), 7.15-7.29 (1H, m), 8.02-8.10 (2H, m), 8.22
(1H, s), 8.62 (1H,
d, J = 1.9 Hz).
[0316]
Example 179
6-(4-methoxypyrrolo[2,14] [1,2,4]triazin-5-y1)-2-methyl-1-((1-methy1-1H-
pyrazol-5-
yOmethyl)-1H-imidazo[4,5-b]pyridine
a) 6-bromo-2-methyl-14(1-methyl-1H-pyrazol-5-yl)methyl)-1H-imidazo[4,5-
b]pyridine
Acetic acid (0.4 mL) was added to a solution of 2-methyl-2H-pyrazole-3-
carbaldehyde
(0.8 g) and 5-bromopyridine-2,3-diamine (1 g) in THF (20 mL) at room
temperature. The
mixture was stirred overnight at room temperature. The mixture was poured into
a saturated
132
CA 03021185 2018-10-16
A00039
aqueous sodium hydrogen carbonate solution, and extracted with ethyl acetate.
The organic
layer was washed with water and a saturated brine, dried over magnesium
sulfate, and then
concentrated to obtain a residue. Sodium borohydride (300 mg) was added to a
solution of the
residue in THF (20 mL)/methanol (50 mL) at 0 C, and the mixture was stirred
for 1 hour. The
reaction mixture was poured into a saturated aqueous ammonium chloride
solution, and extracted
with ethyl acetate. The organic layer was sequentially washed with water and a
saturated brine,
then dried over magnesium sulfate, and concentrated under reduced pressure.
The residue was
purified by NH silica gel column chromatography (ethyl acetate/hexane) to
obtain a pale yellow
solid as 5-bromo-N3-((1-methy1-1H-pyrazol-5-y1)methyl)pyridine-2,3-diamine
(0.38 g). A
mixture of the obtained pale yellow solid (300 mg), acetic acid (0.1 mL), DMAP
(10 mg),
propylphosphonic anhydride (50% in ethyl acetate solution) (0.938 mL), DIPEA
(0.464 mL) and
THY (10 mL) was stirred under microwave irradiation at 200 C for 2 hours. The
mixture was
purified by NH silica gel column chromatography (methanol/ethyl acetate) to
obtain the title
compound (188 mg).
1FINMR (300 MHz, DMSO-d6) 5 2.55 (3H, s), 3.81 (3H, s), 5.63 (2H, s), 5.70
(IH, d, J
= 1.9 Hz), 7.30 (1H, d, J = 1.9 Hz), 8.24 (1H, d, J = 2.2 Hz), 8.42 (1H, d, J
= 2.2 Hz).
[0317]
b) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triaz1n-5-y1)-2-methy1-1-((1-methyl-1H-
pyrazol-5-
yl)methyl)-1H-imidazo[4,5-b]pyridine
A mixture of 6-bromo-2-methy1-1-((1-methyl-1H-pyrazol-5-yl)methyl)-1H-
imidazo[4,5-b]pyridine (61 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
yl)pyrrolo[2,1-f][1,2,4]triazine (151 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (11 mg), cesium carbonate
(2 M in
aqueous solution) (0.2 mL) and DME (3 mL) was stirred under microwave
irradiation at 100 C
for 40 minutes. The mixture was purified by NH silica gel column
chromatography
(methanol/ethyl acetate), and the residue was crystallized from ethanol/ethyl
acetate/heptane to
obtain the title compound (33 mg).
1H NMR (300 MHz, DMSO-d6) 2.60 (3H, s), 3.83 (3H, s), 3.86 (3H, s), 5.65 (2H,
s),
5.72 (1H, d, J = 2.0 Hz), 7.03 (1H, d, J = 2.7 Hz), 7.31 (1H, d, J = 2.0 Hz),
8.02-8.08 (2H, m),
8.21 (1H, s), 8.56 (1H, d, J = 2.0 Hz).
[0318]
Example 186
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-14(3-methyl-1,2,4-
oxadiazol-
5-yl)methyl)-1H-imidazo[4,5-b]pyridine
133
CA 03021185 2018-10-16
A00039
a) tert-butyl 2-(6-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-l-y1)acetate
= Sodium hydride (60%, oil) (15.09 g) was added to a solution of 6-bromo-2-
methyl-1H-
imidazo[4,5-b]pyridine (40 g) in DMF (300 mL) at 0 C. The mixture was stirred
at 0 C for 30
minutes, and tert-butyl chloroacetate (40.6 mL) was then added to the mixture.
The mixture
was stirred at 0 C for 1 hour. The reaction was stopped with acetic acid (20
mL) and water at
0 C, and the mixture was extracted with ethyl acetate. The organic layer was
washed with a
saturated aqueous sodium hydrogen carbonate solution and a saturated brine,
dried over
magnesium sulfate, and then concentrated under reduced pressure to reduce the
amount by half.
The precipitate was collected by filtration, and washed with IPE to obtain the
title compound
(21.11 g).
114 NMR (300 MHz, DMSO-d6) l 1.43 (9H, s), 2.49 (3H, s), 5.15 (2H, s), 8.33
(1H, d, J
= 2.2 Hz), 8.41 (1H, d, J = 2.2 Hz).
[0319]
b) 5-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-3-methyl-1,2,4-
oxadiazole
A solution of tert-butyl 2-(6-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-1-
yl)acetate
(3.00 g) in TFA (10 mL) was stirred at room temperature for 1 hour. The
mixture was
concentrated under reduced pressure to obtain a brown oil. A mixture of the
obtained brown
oil, propylphosphonic anhydride (50% in ethyl acetate solution) (14.40 mL),
(Z)-N'-
hydroxyacetimidamide (1.00 g), DIPEA (8 mL) and ethyl acetate (10 mL) was
stirred at 80 C for
4 hours. The residue was purified by NH silica gel column chromatography
(methanol/ethyl
acetate) to obtain the title compound (1.980 g).
1HNMR (300 MHz, DMSO-d6) 8 2.28 (3H, s), 2.58 (3H, s), 5.97 (2H, s), 8.39 (1H,
d, J
= 2.2 Hz), 8.45 (1H, d, J = 2.2 Hz).
[0320]
c) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methy1-14(3-methyl-1,2,4-
oxadiazol-5-yl)methyl)-1H-imidazo[4,5-b]pyridin e
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yl)pyrrolo
[2,1-
f][1,2,4]triazine (680 mg), 5-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
yOmethyl)-3-
methyl-1,2,4-oxadiazole (576 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (105 mg), cesium
carbonate (2 M in
aqueous solution) (2.336 mL) and DME (14 mL) was stirred under microwave
irradiation at
100 C for 40 minutes. The mixture was concentrated under reduced pressure. The
residue
was purified by NH silica gel column chromatography (methanol/ethyl acetate),
and the obtained
134
CA 03021185 2018-10-16
A00039
solid was crystallized from THF/ethyl acetate/heptane to obtain the title
compound (310 mg).
1HNMR (300 MI-lz, DMSO-d6) 52.29 (31I, s), 2.63 (3H, s), 3.94 (3H, s), 6.00
(211, s),
7.06 (1H, d, J = 2.7 Hz), 8.06 (1H, d, J = 2.7 Hz), 8.14-8.30 (2H, m), 8.59
(1H, d, J = 2.1 Hz).
[0321]
Example 191
6-(4-methoxypyrrolo [2,14] [1,2,4]triazin-5-y1)-2-methy1-1-(1,3-thiazol-2-
ylmethyl)-1H-
imidazo [4,5-b]pyridine
a) 5-bromo-N3-(thiazol-2-ylmethyl)pyridine-2,3-diamine
Acetic acid (0.4 mL) was added to a solution of 2-thiazolecarboxyaldehyde
(1.553 mL)
and 5-bromopyridine-2,3-diamine (3 g) in THF (50 mL) at room temperature. The
mixture was
stirred overnight at room temperature. The mixture was poured into a saturated
aqueous
sodium hydrogen carbonate solution, and extracted with ethyl acetate. The
organic layer was
washed with water and a saturated brine, dried over magnesium sulfate, and
then concentrated.
Sodium borohydride (4 g) was added to a solution of the residue in THF (20
mL)/methanol (50
mL) at 0 C, and the mixture was stirred for 10 minutes. The reaction mixture
was poured into a
saturated aqueous ammonium chloride solution, and extracted with ethyl
acetate. The organic
layer was sequentially washed with water and a saturated brine, then dried
over magnesium
sulfate, and concentrated under reduced pressure. The residue was purified by
silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound
(2.32 g).
1HNMR (300 MHz, DMSO-d6) 6 4.65 (2H, d, J = 5.9 Hz), 5.81 (2H, s), 6.13 (1H,
t, J =
5.9 Hz), 6.66 (1H, d, J = 2.1 Hz), 7.34 (1H, d, J = 2.1 Hz), 7.63 (1H, d, J =
3.3 Hz), 7.77 (1H, d, J
= 3.3 Hz).
[0322]
b) 2((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-l-y1)methyl)thiazole
A mixture of 5-bromo-N3-(thiazol-2-ylmethyppyridine-2,3-diamine (620 mg),
acetic
acid (0.162 mL), DMAP (20 mg), propylphosphonic anhydride (50% in ethyl
acetate solution)
(1.918 mL), DIPEA (843 mg) and THF (10 mL) was stirred under microwave
irradiation at
200 C for 2 hours. The mixture was purified by NH silica gel column
chromatography (ethyl
acetate/hexane) to obtain the title compound (540 mg).
1HNMR (300 MHz, DMSO-d6) 6 2.63 (3H, s), 5.92 (2H, s), 7.70-7.81 (2H, m), 8.37-
8.45 (2H, m).
[0323]
c) 6-(4-methoxypyrrolo[2,1-1][1,2,4]triazin-5-y1)-2-methy1-1-(1,3-thiazol-2-
ylmethyl)-
1H-imidazo[4,5-b]pyridine
135
CA 03021185 2018-10-16
A00039
A mixture of 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
y1)methyl)thiazole
= (100 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (125 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (20 mg), cesium carbonate
(2 M in
aqueous solution) (0.3 mL) and DME (4 mL) was stirred under microwave
irradiation at 100 C
for 1 hour. The mixture was purified by NH silica gel column chromatography
(methanol/ethyl
acetate), and the obtained solid was crystallized from ethanol/ethyl
acetate/heptane to obtain the
title compound (47 mg).
114 NMR (300 MHz, DMSO-d6) 6 2.66 (3H, s), 3.88 (3H, s), 5.93 (2H, s), 7.05
(111, d, J
= 2.7 Hz), 7.73 (1H, d, J = 3.3 Hz), 7.80 (1H, d, J = 3.3 Hz), 8.06 (1H, d, J
= 2.7 Hz), 8.20 (1H,
d, J = 2.1 Hz), 8.22 (1H, s), 8.57 (1H, d, J = 2.1 Hz).
[0324]
Example 193
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1-((5-methyl-1,3,4-
oxadiazol-
2-yOmethyl)-1H-imidazo[4,5-b]pyridine
a) 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-5-methyl-1,3,4-
oxadiazole
Potassium hydroxide (2.00 g) was added to a solution of 6-bromo-2-methy1-1H-
imidazo[4,5-b]pyridine (5.80 g), 2-(chloromethyl)-5-methyl-1,3,4-oxadiazole
(3.70 g) and TBAI
(1.01 g) in THF (80 mL) at room temperature. The mixture was stirred at 60 C
for 2 hours.
The mixture was poured into water at room temperature, and extracted with
ethyl acetate. The
organic layer was washed with water and a saturated brine, dried over
magnesium sulfate, and
then concentrated under reduced pressure. The residue was purified by NH
silica gel column
chromatography (methanol/ethyl acetate) to obtain the title compound (1.550
g).
NMR (300 MHz, DMSO-d6) 6 2.47 (3H, s), 2.61 (3H, s), 5.86 (2H, s), 8.36 (1H,
d, J
= 2.2 Hz), 8.44 (1H, d, J = 2.2 Hz).
[0325]
b) 2-((6-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-l-y1)methyl)-5-methyl-1,3,4-
oxadiazole
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yppyrrolo[2,1-
f][1,2,4]triazine (550 mg), 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
yOmethyl)-5-
methyl-1,3,4-oxadiazole (450 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (50 mg), cesium carbonate
(2 M in
aqueous solution) (1.3 mL) and DME (10 mL) was stirred under microwave
irradiation at 100 C
136
CA 03021185 2018-10-16
A00039
for 1 hour. The residue was purified by NH silica gel column chromatography
(methanol/ethyl
acetate) and silica gel column chromatography (methanol/ethyl acetate). The
obtained solid
was crystallized from ethyl acetate/ethanol/heptane to obtain the title
compound (255 mg).
II-INMR (300 MHz, DMSO-d6) 6 2.47 (3H, s), 2.66 (3H, s), 3.97 (3H, s), 5.88
(2H, s),
7.07 (1H, d, J = 2.7 Hz), 8.07 (1H, d, J = 2.7 Hz), 8.20 (1H, d, J = 2.1 Hz),
8.23 (1H, s), 8.58
(1H, d, J = 2.1 Hz).
[0326]
Example 199
6-(4-methoxypyrrolo[2,14][1,2,4]triazin-5-y1)-2-methyl-1-((2-methyl-1,3-
oxadiazol-4-
yOmethyl)-1H-imidazo[4,5-b]pyridine
a) 4-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yOmethyl)-2-methyloxazole
Potassium hydroxide (0.287 g) was added to a mixture of 6-bromo-2-methy1-1H-
imidazo[4,5-b]pyridine (0.361 g), 4-(bromomethyl)-2-methyloxazole (0.30 g) and
THF (10 mL)
at 60 C. The mixture was stirred under a nitrogen atmosphere at 60 C for 4
hours. The
reaction mixture was poured into water, and extracted with ethyl acetate. The
organic layer was
washed with water and a saturated brine, then dried over sodium sulfate, and
concentrated under
reduced pressure. The residue was purified by NH silica gel column
chromatography (ethyl
acetate/hexane) to obtain the title compound (0.124 g).
1HNMR (300 MHz, DMSO-d6) 6 2.32 (3H, s), 2.67 (3H, s), 5.33 (2H, s), 8.09 (1H,
s),
8.34-8.37 (1H, m), 8.37-8.40 (1H, m).
[0327]
b) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methy1-142-methyl-1,3-
oxadiazol-
4-yl)methyl)-1H-imidazo[4,5-b]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (120 mg), 44(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
yOmethyl)-2-
methyloxazole (79.5 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium
(II) (13 mg), cesium carbonate (2 M in aqueous solution) (0.200 mL) and DME
(3.0 mL) was
stirred under microwave irradiation at 100 C for 15 minutes. The reaction
mixture was purified
by silica gel column chromatography (ethyl acetate/hexane and methanol/ethyl
acetate), and the
residue was crystallized from ethanol/ethyl acetate/heptane to obtain the
title compound (57.9
mg).
1HNMR (300 MHz, DMSO-d6) 5 2.33 (3H, s), 2.71 (3H, s), 3.96 (3H, s), 5.34 (2H,
s),
7.05 (1H, d, J = 2.7 Hz), 8.07 (1H, d, J = 2.7 Hz), 8.12 (1H, s), 8.17 (1H, d,
J = 2.1 Hz), 8.23
(1H, s), 8.52 (1H, d, J = 2.1 Hz).
137
CA 03021185 2018-10-16
A00039
[0328]
= Example 203
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1-((1-methyl-1H-
pyrazol-3-
yl)methyl)-1H-imidazo[4,5-b]pyridine
a) 5-iodo-N3-((1-methy1-1H-pyrazol-3-y1)methyl)pyridine-2,3-diamine
A mixture of 1-methyl-1H-pyrazole-3-carbaldehyde (0.516 g) and 5-iodopyridine-
2,3-
diamine (1 g), acetic acid (0.256 mL) and THF (20 mL) was stirred at room
temperature for 21
hours. The mixture was neutralized with a saturated aqueous sodium hydrogen
carbonate
solution, and extracted with THF and ethyl acetate. The organic layer was
washed with a
saturated aqueous sodium hydrogen carbonate solution and a saturated brine,
dried over
magnesium sulfate, and then concentrated to obtain a residue. Sodium
borohydride (0.485 g)
was added to a solution of the residue in THF (40 mL)/methanol (10 mL) at 0 C,
and the mixture
was stirred at room temperature for 1 hour. The reaction was stopped with
water and a
saturated aqueous ammonium chloride solution at room temperature, and the
mixture was
basified, and extracted with ethyl acetate. The organic layer was washed with
a saturated brine,
then dried over magnesium sulfate, and concentrated under reduced pressure.
The residue was
purified by silica gel column chromatography (ethyl acetate/methanol) to
obtain the title
compound (0.325 g).
MS: [M+H]+ 330.1.
[0329]
b) 6-iodo-2-methyl-1-((l-methyl-1H-pyrazol-3-yl)methyl)-1H-imidazo[4,5-
b]pyridine
A mixture of 5-iodo-N3-((1-methy1-1H-pyrazol-3-y1)methyl)pyridine-2,3-diamine
(325.1 mg), acetic acid (0.075 mL), DMAP (18 mg), propylphosphonic anhydride
(1.7 M in
ethyl acetate solution) (0.99 mL), DIPEA (0.346 mL) and THF (3 mL) was stirred
under
microwave irradiation at 160 C for I hour. The residue was purified by NH
silica gel column
chromatography (hexane/ethyl acetate), and the obtained solid was washed with
IPE to obtain
the title compound (240 mg).
MS: [M+H]+ 354Ø
[0330]
c) 6-(4-methoxypyrrolo [2,1-f] [1,2,4]triazin-5-y1)-2-methy1-1 -((l-methyl -1H-
pyrazol -3-
yl)methyl)-1H-imidazo[4,5-b]pyridine
A mixture of 6-iodo-2-methy1-1-((1-methyl-1H-pyrazol-3-yl)methyl)-1H-
imidazo[4,5-
b]pyridine (81 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (175 mg), bis(di-tert-buty1(4-
138
CA 03021185 2018-10-16
A00039
dimethylaminophenyl)phosphine)dichloropalladium (II) (12 mg), cesium carbonate
(2 M in
= aqueous solution) (0.195 mL) and DME (1 mL) was stirred under microwave
irradiation at
100 C for 1 hour. The solvent was distilled off, the residue was then purified
by NH silica gel
column chromatography (methanol/ethyl acetate), and the obtained solid was
washed with IPE to
obtain the title compound (31.3 mg).
IHNIVIR (300 MHz, DMSO-d6) 8 2.67 (3H, s), 3.76 (3H, s), 3.95 (3H, s), 5.41
(2H, s),
6.21 (1H, d, J = 2.3 Hz), 7.05 (1H, d, J =- 2.7 Hz), 7.62 (1H, d, J = 2.2 Hz),
8.06 (1H, d, J = 2.7
Hz), 8.15 (1H, d, J = 2.1 Hz), 8.22 (1H, s), 8.52 (1H, d, J = 2.1 Hz).
[0331]
Example 204
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-14(2-methy1-1,3-
thiazol-4-
yl)methyl)-1H-imidazo[4,5-b]pyridine
a) 5-iodo-N3-((2-methylthiazol-4-yl)methyl)pyridine-2,3-diamine
A mixture of 2-methylthiazole-4-carbaldehyde (390 mg) and 5-iodopyridine-2,3-
diamine (770 mg), acetic acid (0.198 mL) and THF (15 mL) was stirred under a
nitrogen
atmosphere at room temperature for 21 hours. The mixture was concentrated
under reduced
pressure. The residue was neutralized with a saturated aqueous sodium hydrogen
carbonate
solution at 0 C, and extracted with THF and ethyl acetate. The organic layer
was washed with
a saturated aqueous sodium hydrogen carbonate solution and a saturated brine,
dried over
magnesium sulfate, and then concentrated to obtain a residue. Sodium
borohydride (311 mg)
was added to a solution of the residue in THF (40 mL)/methanol (10 mL) at 0 C,
and the mixture
was stirred at room temperature for 30 min. The reaction was stopped with
water and a
saturated aqueous ammonium chloride solution at room temperature, and the
mixture was
basified, and extracted with ethyl acetate. The organic layer was washed with
a saturated brine,
then dried over magnesium sulfate, and concentrated under reduced pressure.
The residue was
purified by silica gel column chromatography (ethyl acetate/methanol) to
obtain the title
compound (708 mg).
MS: [M+H]+ 347.1.
[0332]
b) 4-((6-iodo-2-methyl-1H-imidazo[4,5-b]pyridin-l-y1)methyl)-2-methylthiazole
A mixture of 5-iodo-N3-((2-methylthiazol-4-yOmethyl)pyridine-2,3-diamine (300
mg),
acetic acid (0.075 mL), DMAP (16 mg), propylphosphonic anhydride (1.7 M in
ethyl acetate
solution) (0.868 mL), DIPEA (0.304 mL) and THF (2.5 mL) was stirred under
microwave
irradiation at 160 C for 1 hour. The residue was purified by NH silica gel
column
139
CA 03021185 2018-10-16
A00039
chromatography (hexane/ethyl acetate), and the obtained solid was washed with
1PE to obtain
the title compound (265 mg).
MS: [M+H]+ 370.9.
[0333]
c) 6-(4-methoxypyrrolo[2,1-f][1,2,41triazin-5-y1)-2-methy1-1-((2-methy1-1,3-
thiazol-4-
yl)methyl)-1H-imidazo[4,5-b]pyridine
A mixture of 4-06-iodo-2-methy1-1H-imidazo[4,5-b]pyridin-l-y1)methyl)-2-
methylthiazole (94 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (175 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (13 mg), cesium carbonate
(2 M in
aqueous solution) (0.218 mL) and DME (1 mL) was stirred under microwave
irradiation at
100 C for 1 hour. The solvent was distilled off, the residue was then purified
by NH silica gel
column chromatography (methanol/ethyl acetate), and the obtained solid was
washed with IPE to
obtain the title compound (57.3 mg).
IFI NMR (300 MHz, DMSO-d6) 8 2.58 (3H, s), 2.71 (3H, s), 3.93 (3H, s), 5.51
(2H, s),
7.04 (1H, d, J = 2.7 Hz), 7.52 (1H, s), 8.06 (1H, d, J = 2.7 Hz), 8.18 (1H, d,
J = 2.1 Hz), 8.22
(1H, s), 8.53 (1H, d, J = 2.1 Hz).
[0334]
Example 205
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methy1-1-(1,3-oxazol-2-
ylmethyl)-1H-
imidazo[4,5-b]pyridine
a) N-(2-amino-5-iodopyridin-3-yl)oxazole-2-carboxamide
A mixture of 5-iodopyridine-2,3-diamine (1 g), oxazole-2-carboxylic acid
(0.482 g),
HATU (2.27 g), Et3N (1.78 mL) and DMF (10 mL) was stirred at 0 C for 1 hour,
and then
stirred overnight at room temperature. The mixture was diluted with a
saturated aqueous
sodium hydrogen carbonate solution, and extracted with ethyl acetate. The
organic layer was
washed with a saturated brine, then dried over magnesium sulfate, and
concentrated under
reduced pressure. The residue was purified by silica gel column chromatography
(ethyl
acetate/hexane) to obtain the title compound (0.781 g). The title compound was
used for the
subsequent reaction without being further purified.
MS: [M+H]+ 330.9.
[0335]
b) 5-iodo-N3-(oxazol-2-ylmethyl)pyridine-2,3-diamine
A BH3-THF complex (1M in THF solution) (9 mL) was added dropwise to a solution
of
140
CA 03021185 2018-10-16
A00039
N-(2-amino-5-iodopyridin-3-yl)oxazole-2-carboxamide (750 mg) in THF (6 mL) at
room
temperature. The mixture was stirred under a nitrogen atmosphere at 60 C for 2
hours. The
reaction was stopped with water at room temperature, and the mixture was then
acidified with
HC1 (1 N in aqueous solution). The mixture was stirred at 60 C for 30 minutes.
The mixture
was neutralized with a saturated aqueous sodium hydrogen carbonate solution at
room
temperature, and then extracted with ethyl acetate. The organic layer was
washed with a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was purified by silica gel column chromatography (ethyl
acetate/hexane) to obtain
the title compound (153 mg).
MS: [M+H]+ 316.9.
[0336]
c) 2-((6-iodo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyDoxazole
A mixture of 5-iodo-N3-(oxazol-2-y1methyl)pyridine-2,3-diamine (152.8 mg),
acetic
acid (0.045 mL), DMAP (9 mg), propylphosphonic anhydride (1.7 M in ethyl
acetate solution)
(0.484 mL), DIPEA (0.195 mL) and THE (1.5 mL) was stirred under microwave
irradiation at
160 C for 1 hour. The residue was purified by NH silica gel column
chromatography
(hexane/ethyl acetate), and the obtained solid was washed with IPE to obtain
the title compound
(99 mg).
MS: [M+H]+ 341Ø
[0337]
d) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methy1-1-(1,3-oxazol-2-
ylmethyl)-
1H-imidazo[4,5-b]pyridine
A mixture of 2((6-iodo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yOmethyl)oxazole
(98
mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yppyrrolo[2,1-
f][1,2,4]triazine
(210 mg), bis(di-tert-buty1(4-dimethylaminophenyl)phosphine)dichloropalladium
(II) (16 mg),
cesium carbonate (2 M in aqueous solution) (0.261 mL) and DME (1.2 mL) was
stirred under
microwave irradiation at 100 C for 1 hour. The solvent was distilled off, the
residue was then
purified by NH silica gel column chromatography (methanol/ethyl acetate), and
the obtained
solid was washed with IPE to obtain the title compound (44.1 mg).
1H NMR (300 MHz, DMSO-d6) 8 2.64 (3H, s), 3.95 (3H, s), 5.77 (2H, s), 7.06
(1H, d, J
= 2.8 Hz), 7.22 (1H, d, J = 0.8 Hz), 8.06 (1H, d, J = 2.7 Hz), 8.14 (1H, d, J
= 0.8 Hz), 8.18 (1H,
d, J = 2.1 Hz), 8.23 (1H, s), 8.57 (1H, d, J = 2.0 Hz).
[0338]
Example 206
141
CA 03021185 2018-10-16
A00039
=
6-(4-methoxypyrrolo [2,1-f] [1,2,4]triazin-5-y1)-2-methy1-1-((1-methyl-1H-
1,2,3-triazol-
= 4-yl)methyl)-1H-imidazo[4,5-b]pyridine
a) N-(2-amino-5-iodopyridin-3-y1)-1-methy1-1H-1,2,3-triazole-4-carboxamide
A mixture of 5-iodopyridine-2,3-diamine (1 g), 1-methyl-1H-1,2,3-triazole-4-
carboxylic
acid (0.54 g), HATU (2.27 g), Et3N (1.78 mL) and DMF (10 mL) was stirred at 0
C for 1 hour,
and then stirred overnight at room temperature. The mixture was diluted with a
saturated
aqueous sodium hydrogen carbonate solution, and extracted with ethyl acetate.
The organic
layer was washed with a saturated brine, then dried over magnesium sulfate,
and concentrated
under reduced pressure. The residue was purified by silica gel column
chromatography (ethyl
acetate/hexane) to obtain the title compound (0.987 g). The title compound was
used for the
subsequent reaction without being further purified.
MS: [M-FH]+ 344.9.
[0339]
b) 5-iodo-N3-((1-methy1-1H-1,2,3-triazol-4-y1)methyl)pyridine-2,3-diamine
A BH3-THF complex (1M in THF solution) (11 mL) was added dropwise to a
solution
of N-(2-amino-5-iodopyridin-3-y1)-1-methy1-1H-1,2,3-triazole-4-carboxamide
(985 mg) in THF
(8 mL) at room temperature. The mixture was stirred under a nitrogen
atmosphere at 60 C for
2 hours. The reaction was stopped with water at room temperature, and the
mixture was then
acidified with HC1 (1 N in aqueous solution). The mixture was stirred at 60 C
for 30 minutes.
The mixture was neutralized with a saturated aqueous sodium hydrogen carbonate
solution at
room temperature, and then extracted with ethyl acetate. The organic layer was
washed with a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was purified by silica gel column chromatography (ethyl
acetate/methanol) to obtain
the title compound (682 mg).
MS: [M+1-1]+ 331Ø
[0340]
c) 6-iodo-2-methy1-1-((1-methyl-1H-1,2,3-triazol-4-yl)methyl)-1H-imidazo[4,5-
b]pyridine
A mixture of 5-iodo-N3-((1-methy1-1H-1,2,3-triazol-4-yl)methyppyridine-2,3-
diamine
(300 mg), acetic acid (0.084 mL), DMAP (16 mg), propylphosphonic anhydride
(1.7 M in ethyl
acetate solution) (0.910 mL), DIPEA (0.350 mL) and THF (2.5 mL) was stirred
under
microwave irradiation at 160 C for 1 hour. The residue was purified by NH
silica gel column
chromatography (methanol/ethyl acetate), and the obtained solid was washed
with IPE to obtain
the title compound (167 mg).
142
CA 03021185 2018-10-16
A00039
MS: [M+H]-1- 355Ø
= [0341]
d) 6-(4-methoxypyrrolo [2,1-f] [1,2,4]triazin-5-y1)-2-methy1-1-((1-methyl-IH-
1,2,3-
triazol-4-yl)methyl)-1H-imidazo[4,5-b]pyridine
A mixture of 6-iodo-2-methy1-1-((1-methyl-1H-1,2,3-triazol-4-yl)methyl)-1H-
imidazo[4,5-13]pyridine (97 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
yl)pyrrolo[2,1-f][1,2,4]triazine (200 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (15 mg), cesium carbonate
(2 M in
aqueous solution) (0.249 mL) and DME (1 mL) was stirred under microwave
irradiation at
100 C for 1 hour, and then stirred at 120 C for 20 minutes. The solvent was
distilled off, the
residue was then purified by NH silica gel column chromatography
(methanol/ethyl acetate), and
the obtained solid was washed with IPE to obtain the title compound (59.0 mg).
1HNMR (300 MHz, DMSO-d6) 2.72 (3H, s), 3.97 (3H, s), 3.99 (314, s), 5.56 (2H,
s),
7.07 (1H, d, J = 2.7 Hz), 8.07 (1H, d, J = 2.8 Hz), 8.14 (1H, s), 8.21 (1H, d,
J = 2.0 Hz), 8.23
(1H, s), 8.53 (1H, d, J = 2.1 Hz).
[0342]
Example 207
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1-((2-methyl-1,3-
oxadiazol-5-
y1)methyl)-1H-imidazo[4,5-b]pyridine
a) 5-46-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-l-y1)methyl)-2-methyloxazole
Potassium hydroxide (3.60 g) was added to a mixture of 6-bromo-2-methy1-1H-
imidazo[4,5-b]pyridine (4.53 g), 5-(bromomethyl)-2-methyloxazole (3.76 g) and
THF (100 mL)
at room temperature. The mixture was stirred under a nitrogen atmosphere
overnight at 60 C.
Insolubles were removed by filtration through a filter, and the filtrate was
concentrated under
reduced pressure. The residue was purified by NH silica gel column
chromatography (ethyl
acetate/hexane) to obtain the title compound (2.030 g).
114 NIVIR (300 MHz, DMSO-d6) 8 2.32 (3H, s), 2.66 (3H, s), 5.57 (2H, s), 7.19
(1H, s),
8.41 (2H, d, J = 0.7 Hz).
[0343]
b) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1-((2-methy1-1,3-
oxadiazol-
5-yl)methyl)-1H-imidazo[4,5-13]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (203 mg), 5-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
y1)methyl)-2-
methyloxazole (140 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium
143
CA 03021185 2018-10-16
A00039
(II) (18 mg), cesium carbonate (2 M in aqueous solution) (0.30 mL) and DME
(3.0 mL) was
= stirred under microwave irradiation at 100 C for 20 minutes. The reaction
mixture was purified
by silica gel column chromatography (ethyl acetate/hexane and methanol/ethyl
acetate), and the
residue was crystallized from ethanol/ethyl acetate/heptane to obtain the
title compound (78 mg).
111 NMR (300 MHz, DMSO-d6) 6 2.34 (3H, s), 2.70 (3H, s), 3.97 (3H, s), 5.58
(2H, s),
7.06 (1H, d, J = 2.7 Hz), 7.21 (1H, s), 8.07 (1H, d, J = 2.7 Hz), 8.20-8.26
(2H, m), 8.54 (1H, d, J
= 2.1 Hz).
[0344]
Example 208
6-(4-methoxypyrrolo[2,14][1,2,4]triazin-5-y1)-2-methyl-1-(pyrazin-2-ylmethyl)-
1H-
imidazo[4,5-b]pyridine
a) 6-bromo-2-methyl-1-(pyrazin-2-ylmethyl)-1H-imidazo[4,5-b]pyridine
6-bromo-2-methyl-1H-imidazo[4,5-b]pyridine (600 mg) was dissolved in THF (20
mL)
at 60 C, and potassium hydroxide (476 mg) and 2-(bromomethyl)pyrazine (490 mg)
were added.
The mixture was stirred at 60 C for 16 hours. The reaction solution was cooled
to room
temperature, then diluted with water, and extracted with ethyl acetate. The
organic layer was
washed with a saturated brine, then dried over magnesium sulfate, and
concentrated under
reduced pressure. The residue was purified by NH silica gel column
chromatography (ethyl
acetate/hexane), and the obtained solid was washed with ethyl acetate/IPE to
obtain the title
compound (88.3 mg).
11-INMR (300 MHz, DMSO-d6) 6 2.59 (3H, s), 5.71 (2H, s), 8.34 (1H, d, J = 2.2
Hz),
8.40 (1H, d, J = 2.2 Hz), 8.53 (1H, dd, J = 2.5, 1.6 Hz), 8.59 (1H, d, J 2.5
Hz), 8.82 (1H, d, J =
1.5 Hz).
[0345]
b) 6-(4-methoxypyrrolo[2,14][1,2,4]triazin-5-y1)-2-methyl-1-(pyrazin-2-
ylmethyl)-1H-
imidazo[4,5-b]pyridine
A mixture of 6-bromo-2-methyl-1-(pyrazin-2-ylmethyl)-1H-imidazo[4,5-b]pyridine
(82
mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,14][1,2,4]triazine
(100 mg), bis(di-tert-buty1(4-dimethylaminophenyl)phosphine)dichloropalladium
(II) (15.16
mg), cesium carbonate (2 M in aqueous solution) (0.3 mL) and DME (3 mL) was
stirred under
microwave irradiation at 100 C for 1 hour. The mixture was diluted with water,
and extracted
with ethyl acetate. The organic layer was washed with a saturated brine, dried
over magnesium
sulfate, and then concentrated. The residue was purified by NH silica gel
column
chromatography (methanol/ethyl acetate), and the obtained solid was washed
with ethyl
144
CA 03021185 2018-10-16
A00039
acetate/WE to obtain the title compound (45.9 mg).
= IFI NMR (300 MHz, DMSO-d6) 8 2.66 (3H, s), 3.83 (3H, s), 5.73 (2H, s),
7.03 (1H, d, J
= 2.7 Hz), 8.05 (1H, d, J = 2.7 Hz), 8.15 (1H, d, J = 2.0 Hz), 8.21 (1H, s),
8.54 (1H, d, J = 2.1
Hz), 8.57-8.62 (2H, m), 8.83 (1H, d, J = 1.3 Hz).
[0346]
Example 209
6-(4-methoxypyrrolo [2,1-f] [1,2,4]triazin-5-y1)-2-methyl-1-(pyrimidin-2-
ylmethyl)-1H-
imidazo[4,5-b]pyridine
a) 6-bromo-2-methyl-1-(pyrimidin-2-ylmethyl)-1H-imidazo[4,5-b]pyridine
6-bromo-2-methyl-1H-imidazo[4,5-b]pyridine (700 mg) was dissolved in THF (20
mL)
at 60 C, and potassium hydroxide (741 mg), tetrabutylarnmonium iodide (1829
mg) and 2-
(chloromethyl)pyrimidine hydrochloride (599 mg) were added. The mixture was
stirred at
60 C for 3 hours. The reaction solution was cooled to room temperature, then
diluted with
water, and extracted with ethyl acetate. The organic layer was washed with a
saturated brine,
then dried over magnesium sulfate, and concentrated under reduced pressure.
The residue was
purified by NH silica gel column chromatography (ethyl acetate/hexane), and
the obtained solid
was washed with ethyl acetate/WE to obtain the title compound (199 mg).
IHNMR (300 MHz, DMSO-d6) 8 2.50 (3H, s), 5.76 (2H, s), 7.45 (1H, t, J ---- 4.9
Hz),
8.26 (1H, d, J = 2.2 Hz), 8.40 (1H, d, J = 2.2 Hz), 8.76 (1H, s), 8.77 (1H,
s).
[0347]
b) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1-(pyrimidin-2-
ylmethyl)-
1H-imidazo[4,5-b]pyridine
A mixture of 6-bromo-2-methyl-1-(pyrimidin-2-ylmethyl)-1H-imidazo[4,5-
b]pyridine
(82 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yppyrrolo[2,141[1,2,4]triazine
(100 mg), bis(di-tert-buty1(4-dimethylaminophenyl)phosphine)dichloropalladium
(II) (15.16
mg), cesium carbonate (2 M in aqueous solution) (0.3 mL) and DME (3 mL) was
stirred under
microwave irradiation at 100 C for 1 hour. The mixture was diluted with water,
and extracted
with ethyl acetate. The organic layer was washed with a saturated brine, dried
over magnesium
sulfate, and then concentrated. The residue was purified by NH silica gel
column
chromatography (methanol/ethyl acetate), and the obtained solid was washed
with ethyl
acetate/WE to obtain the title compound (33.8 mg).
11-INMR (300 MHz, DMSO-d6) 8 2.57 (311, s), 3.82 (3H, s), 5.77 (2H, s), 7.04
(1H, d, J
2.6 Hz), 7.45 (1H, t, J = 4.9 Hz), 8.01-8.11 (2H, m), 8.20 (1H, s), 8.54 (1H,
s), 8.79 (2H, d, J =
5.0 Hz).
145
CA 03021185 2018-10-16
A00039
[0348]
= Example 210
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1-(pyridin-4-ylmethyl)-
1H-
imidazo[4,5-b]pyridine
a) 5-iodo-N3-(pyridin-4-ylmethyl)pyridine-2,3-diamine
Acetic acid (0.6 mL) was added to a solution of isonicotinaldehyde (1.34 g)
and 5-
iodopyridine-2,3-diamine (2 g) in THF (20 mL) at room temperature. The mixture
was stirred
at room temperature for 21 hours. The mixture was concentrated, a saturated
aqueous sodium
hydrogen carbonate solution was added to the residue at 0 C, and the mixture
was extracted with
THF/ethyl acetate. The organic layer was washed with a saturated aqueous
sodium hydrogen
carbonate solution and a saturated brine, dried over magnesium sulfate, and
then concentrated.
Sodium borohydride (0.644 g) was added to a solution of the residue in TI-IF
(30 mL)/methanol
(3 mL) at 0 C, and the mixture was stirred at room temperature for 1 hour. An
aqueous
ammonium chloride solution was added to the reaction mixture at 0 C, and the
mixture was
extracted with ethyl acetate. The organic layer was sequentially washed with
water and a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was purified by silica gel column chromatography (methanol/ethyl
acetate) to obtain
the title compound (933 mg).
1H NMR (300 MHz, DMSO-d6) 8 4.35 (2H, d, J = 5.8 Hz), 5.76 (1H, t, J = 5.9
Hz), 5.81
(2H, s), 6.59 (1H, d, J = 1.8 Hz), 7.32-7.35 (2H, m), 7.41 (1H, d, J = 1.8
Hz), 8.49-8.54 (2H, m).
[0349]
b) 6-bromo-2-methyl-1-(pyridin-4-ylmethyl)-1H-imidazo[4,5-b]pyridine
A mixture of 5-iodo-N3-(pyridin-4-ylmethyl)pyridine-2,3-diamine (917 mg),
acetic acid
(0.209 mL), DMAP (51.5 mg), propylphosphonic anhydride (50% in ethyl acetate
solution) (2.98
mL), D1PEA (0.982 mL) and THF (15 mL) was stirred under microwave irradiation
at I60 C for
1 hour. The mixture was diluted with a saturated aqueous sodium hydrogen
carbonate solution,
and extracted with ethyl acetate. The organic layer was washed with a
saturated brine, dried
over magnesium sulfate, and then concentrated. The residue was purified by N1-
1 silica gel
column chromatography (ethyl acetate/hexane), and the obtained solid was
washed with IPE to
obtain the title compound (606 mg).
11-1NMR (300 MHz, DMSO-d6) 8 2.51 (3H, s), 5.59 (2H, s), 7.05 (2H, d, J = 6.0
Hz),
8.38 (1H, d, J = 1.9 Hz), 8.49-8.57 (3H, m).
[0350]
c) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-yI)-2-methyl-1-(pyridin-4-
ylmethyl)-1H-
146
CA 03021185 2018-10-16
A00039
imidazo[4,5-b]pyridine
A mixture of 6-iodo-2-methyl-1-(pyridin-4-ylmethyl)-1H-imidazo[4,5-blpyridine
(95
mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yppyrrolo[2,1-
f][1,2,4]triazine
(100 mg), bis(di-tert-buty1(4-dimethylaminophenyl)phosphine)diehloropalladium
(II) (15.16
mg), cesium carbonate (2 M in aqueous solution) (0.3 mL) and DME (3 mL) was
stirred under
microwave irradiation at 100 C for 1 hour. The mixture was diluted with water,
and extracted
with ethyl acetate. The organic layer was washed with a saturated brine, dried
over magnesium
sulfate, and then concentrated. The residue was purified by NH silica gel
column
chromatography (methanol/ethyl acetate), and the obtained solid was washed
with ethyl
acetate/WE to obtain the title compound (46.1 mg).
1H NMR (300 MHz, DMSO-d6) 6 2.58 (3H, s), 3.74 (3H, s), 5.63 (2H, s), 7.05
(1H, d, J
= 2.6 Hz), 7.10 (2H, d, J = 5.7 Hz), 8.04 (1H, d, J = 2.6 Hz), 8.09 (1H, d, J
= 1.8 Hz), 8.20 (1H,
s), 8.53-8.60 (3H, m).
[0351]
Example 211
6-(4-methoxypyrrolo[2,141[1,2,4]triazin-5-y1)-2-methyl-1-(pyridin-3-ylmethyl)-
1H-
imidazo[4,5-b]pyridine
a) 6-bromo-2-methyl-1-(pyridin-3-ylmethyl)-1H-imidazo[4,5-b]pyridine
6-bromo-2-methyl-1H-imidazo[4,5-b]pyridine (600 mg) was dissolved in THE' (20
mL)
at 60 C, and potassium hydroxide (635 mg) and 3-(bromomethyl)pyridine (787 mg)
were added.
The mixture was stirred at 60 C for 3 hours. The reaction solution was cooled
to room
temperature, then diluted with water, and extracted with ethyl acetate. The
organic layer was
washed with a saturated brine, then dried over magnesium sulfate, and
concentrated under
reduced pressure. The residue was purified by NH silica gel column
chromatography (ethyl
acetate/hexane), and the obtained solid was washed with ethyl acetate/IPE to
obtain the title
compound (382 mg).
1H NMR (300 MHz, DMSO-d6) 6 2.58 (3H, s), 5.58 (2H, s), 7.36 (1H, ddd, J =
7.9, 4.8,
0.8 Hz), 7.52 (1H, dt, J = 8.2, 1.9 Hz), 8.36 (1H, d, J = 2.2 Hz), 8.42 (1H,
d, J = 2.2 Hz), 8.49-
8.55 (2H, m).
[0352]
b) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methy1-1-(pyridin-3-
ylmethyl)-1H-
imidazo[4,5-b]pyridine
A mixture of 6-bromo-2-methyl-1-(pyridin-3-ylmethyl)-1H-imidazo[4,5-b]pyridine
(82
mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yl)pyrrolo [2,1-f]
[1,2,4]triazine
147
CA 03021185 2018-10-16
A00039
(100 mg), bis(di-tert-buty1(4-dimethylaminophenyl)phosphine)dichloropalladium
(II) (15.16
= mg), cesium carbonate (2 M in aqueous solution) (0.3 mL) and DME (3 mL)
was stirred under
microwave irradiation at 100 C for 1 hour. The mixture was diluted with water,
and extracted
with ethyl acetate. The organic layer was washed with a saturated brine, dried
over magnesium
sulfate, and then concentrated. The residue was purified by NH silica gel
column
chromatography (methanol/ethyl acetate), and the obtained solid was washed
with ethyl
acetate/IPE to obtain the title compound (49.6 mg).
NMR (300 MHz, DMSO-d6) 2.63 (3H, s), 3.82 (3H, s), 5.61 (2H, s), 7.05 (1H, d,
J
= 2.8 Hz), 7.39 (1H, dd, J = 7.6, 4.5 Hz), 7.53-7.61 (1H, m), 8.05 (1H, d, J =
2.7 Hz), 8.17 (1H,
d, J = 2.0 Hz), 8.21 (1H, s), 8.52 (1H, dd, J = 4.7, 1.5 Hz), 8.56 (2H, d, J =
1.9 Hz).
[0353]
Example 212
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-14(5-methy1-1,2-
oxadiazol-3-
yl)methyl)-1H-imidazo[4,5-b]pyridine
a) 3-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-5-
methylisoxazole
Potassium hydroxide (1.081 g) was added to a mixture of 6-bromo-2-methy1-1H-
imidazo[4,5-b]pyridine (1.361 g), 3-(bromomethyl)-5-methylisoxazole (1.13 g)
and THF (30
mL) at room temperature. The mixture was stirred under a nitrogen atmosphere
at 60 C for 4
hours. The reaction mixture was poured into water at room temperature, and
extracted with
ethyl acetate. The organic layer was washed with water and a saturated brine,
then dried over
sodium sulfate, and concentrated under reduced pressure. The residue was
purified by NH
silica gel column chromatography (ethyl acetate/hexane), and the residue was
washed with IPE
to obtain the title compound (0.819 g).
11-1 NMR (300 MHz, DMSO-d6) 62.35 (3H, d, J = 0.8 Hz), 2.59 (3H, s), 5.58 (2H,
s),
6.21 (1H, d, J = 0.8 Hz), 8.34 (1H, d, J = 2.2 Hz), 8.42 (1H, d, J = 2.2 Hz).
[0354]
b) 6-(4-methoxypyrrolo [2,14] [1,2,4]triazin-5-y1)-2-methyl-1-((5-methy1-1,2-
oxadiazol-
3-yl)methyl)-1H-imidazo[4,5-b]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (144 mg), 3-46-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
yl)methyl)-5-
methylisoxazole (100 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (22 mg), cesium carbonate
(2 M in
aqueous solution) (0.330 mL) and DME (2.0 mL) was stirred under microwave
irradiation at
100 C for 40 minutes. The reaction mixture was purified by silica gel column
chromatography
148
CA 03021185 2018-10-16
A00039
=
(methanol/ethyl acetate), and the residue was washed with ethyl
acetate/heptane to obtain the
title compound (63.8 mg).
1H NMR (300 MHz, DMSO-d6) 82.35 (3H, s), 2.64 (3H, s), 3.94 (3H, s), 5.59 (2H,
s),
6.21 (1H, s), 7.07 (1H, d, J = 2.8 Hz), 8.07 (1H, d, J = 2.6 Hz), 8.16 (1H, d,
J = 1.9 Hz), 8.23
(11-1, s), 8.56 (1H, d, J = 1.7 Hz).
[0355]
Example 213
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1-((2-methy1-1,3-
thiazol-5-
yl)methyl)-1H-imidazo[4,5-b]pyridine
a) 5-((6-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-1-yl)methyl)-2-methylthiazole
Potassium hydroxide (0.709 g) was added to a mixture of 6-bromo-2-methy1-1H-
imidazo[4,5-b]pyridine (0.893 g), 5-(bromomethyl)-2-methylthiazole (0.809 g)
and THF (20
mL) at room temperature. The mixture was stirred under a nitrogen atmosphere
at 60 C for 4
hours. The reaction mixture was poured into water, and extracted with ethyl
acetate. The
organic layer was washed with water and a saturated brine, then dried over
sodium sulfate, and
concentrated under reduced pressure. The residue was purified by NH silica gel
column
chromatography (ethyl acetate/hexane) to obtain the title compound (0.0953 g).
1H NMR (300 MHz, CDC13) 8 2.67 (3H, s), 2.69-2.72 (3H, m), 5.40 (2H, s), 7.52
(1H,
s), 7.73 (1H, d, J = 2.1 Hz), 8.55 (1H, d, J = 2.1 Hz).
[0356]
b) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methy1-142-methyl-1,3-
thiazol-5-
yOmethyl)-1H-imidazo[4,5-b]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yppyrrolo[2,1-
f] [1 ,2 ,4] triazine (131 mg), 5-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-
1-yl)methyl)-2-
methylthiazole (95.3 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (20.0 mg), cesium
carbonate (2 M in
aqueous solution) (0.300 mL) and DME (2.0 mL) was stirred under microwave
irradiation at
100 C for 40 minutes. The residue was purified by silica gel column
chromatography
(methanol/ethyl acetate), and the residue was washed with ethyl
acetate/heptane to obtain the
title compound (51.5 mg).
1H NMR (300 MHz, DMSO-d6) 5 2.57 (3H, s), 2.66 (3H, s), 3.95 (3H, s), 5.73
(2H, s),
7.05 (1H, d, J = 2.7 Hz), 7.76 (1H, s), 8.07 (1H, d, J = 2.7 Hz), 8.21-8.27
(2H, m), 8.55 (1H, d, J
= 2.0 Hz).
[0357]
149
CA 03021185 2018-10-16
A00039
Example 214
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-14(5-methy1-1,2,4-
oxadiazol-
3-yl)methyl)-1H-imidazo[4,5-b]pyridine
a) 3-46-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-l-y1)methyl)-5-methyl-1,2,4-
oxadiazole
Potassium hydroxide (1.270 g) was added to a mixture of 6-bromo-2-methy1-1H-
imidazo[4,5-b]pyridine (1.600 g), 3-(chloromethyl)-5-methy1-1,2,4-oxadiazole
(1 g), TBAI (2.79
g) and THF (10 mL) at 60 C. The mixture was stirred under a nitrogen
atmosphere at 60 C for
2 hours. The mixture was poured into water at room temperature, and extracted
with ethyl
acetate. The organic layer was washed with water and a saturated brine, dried
over magnesium
sulfate, and concentrated under reduced pressure. The residue was purified by
NH silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound
(0.430 g).
NMR (300 MHz, DMSO-d6) 62.54 (3H, s), 2.60 (3H, s), 5.73 (2H, s), 8.33 (111,
d, J
= 2.2 Hz), 8.43 (1H, d, J = 2.2 Hz).
[0358]
b) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methy1-14(5-methy1-1,2,4-
oxadiazol-3-yOmethyl)-1H-imidazo[4,5-b]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (595 mg), 3-((6-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-l-
yOmethyl)-5-
methyl-1,2,4-oxadiazole (420 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (45.8 mg), cesium
carbonate (2 M in
aqueous solution) (2.045 mL) and DME (12 mL) was stirred under microwave
irradiation at
100 C for 40 minutes. The mixture was poured into ice water at room
temperature, and
extracted with ethyl acetate. The organic layer was washed with water and a
saturated brine,
dried over magnesium sulfate, and concentrated under reduced pressure. The
residue was
purified by NH silica gel column chromatography (methanol/ethyl acetate) to
obtain a solid.
The obtained solid was washed with ethyl acetate/IPE to obtain the title
compound (171 mg).
1H NMR (300 MHz, DMSO-d6) 62.55 (3H, s), 2.66 (311, s), 3.97 (311, s), 5.73
(2H, s),
7.06 (1H, d, J = 2.8 Hz), 8.06 (1H, d, J = 2.7 Hz), 8.17 (1H, d, J = 2.0 Hz),
8.23 (1H, s), 8.57
(1H, d, J 2.1 Hz).
[0359]
Example 215
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1-((3-methy1-1,2-
oxadiazol-5-
yl)methyl)-1H-imidazo[4,5-b]pyridine
150
CA 03021185 2018-10-16
A00039
a) 5((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yl)methyl)-3-methylisoxazole
Potassium hydroxide (1.588 g) was added to a solution of 6-bromo-2-methy1-1H-
imidazo[4,5-b]pyridine (2 g), 5-(bromomethyl)-3-methylisoxazole (1.8 g) and
TBAI (3.48 g) in
TI-IF at 60 C. The mixture was stirred under a nitrogen atmosphere at 60 C for
2 hours. The
reaction mixture was poured into water, and extracted with ethyl acetate. The
organic layer was
sequentially washed with water and a saturated brine, then dried over sodium
sulfate, and
concentrated under reduced pressure. The residue was purified by NH silica gel
column
chromatography (hexane/ethyl acetate) to obtain the title compound (0.89 g).
IHNMR (300 MHz, DMSO-d6) 8 2.18 (3H, s), 2.63 (3H, s), 5.71 (2H, s), 6.38 (1H,
s),
8.40-8.42 (1H, m), 8.42-8.46 (1H, m).
[0360]
b) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1-((3-methy1-1,2-
oxadiazol-
5-yOmethyl)-1H-imidazo[4,5-b]pyridine
A mixture of 5-46-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-l-yl)methyl)-3-
methylisoxazole (644.5 mg), 4-methyl-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-
2-
yl)pyrrolo[2,1-f][1,2,4]triazine (502 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (129 mg), cesium
carbonate (2 M in
aqueous solution) (1.825 mL) and DME (12 mL) was stirred under microwave
irradiation at
100 C for 1 hour. The mixture was purified by NH silica gel column
chromatography
(methanol/ethyl acetate), and the residue was crystallized from ethanol/water
to obtain the title
compound (138 mg).
IHNMR (300 MHz, DMSO-d6) 8 2.19 (3H, s), 2.66 (3H, s), 3.96 (311, s), 5.72
(2H, s),
6.40 (1H, s), 7.07 (1H, d, J = 2.8 Hz), 8.07 (1H, d, J = 2.7 Hz), 8.21-8.25
(2H, m), 8.57 (1H, d, J
=2.1 Hz).
[0361]
Example 220
b) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-14(5-methyl-1,3,4-
thiadiazol-2-yOmethyl)-1H-imidazo[4,5-b]pyridine
a) 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-l-y1)methyl)-5-methyl-1,3,4-
thiadiazole
Potassium hydroxide (600 mg) was added to a mixture of 6-bromo-2-methy1-1H-
imidazo[4,5-b]pyridine (720 mg), 2-(chloromethyl)-5-methy1-1,3,4-thiadiazole
(550 mg), TBAI
(1254 mg) and THF (10 mL) at room temperature. The mixture was stirred at 60 C
for 2 hours.
The mixture was poured into water at room temperature, and extracted with
ethyl acetate. The
151
CA 03021185 2018-10-16
A00039
organic layer was washed with water and a saturated brine, dried over
magnesium sulfate, and
concentrated under reduced pressure. The residue was purified by NH silica gel
column
chromatography (methanol/ethyl acetate) to obtain the title compound (182 mg).
11-1NIVIR (300 MHz, DMSO-d6) 5 2.62 (3H, s), 2.68 (3H, s), 6.00 (2H, s), 8.39-
8.46
(2H, m).
[0362]
b) 6-(4-methoxypyrrolo[2,141[1,2,4]triazin-5-y1)-2-methyl-1-(5-methy1-1,3,4-
thiadiazol-2-yl)methyl)-1H-imidazo[4,5-b]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yppyrrolo[2,1-
fl[1,2,4]triazine (100 mg), 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
y1)methyl)-5-
methyl-1,3,4-thiadiazole (80 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (11 mg), cesium carbonate
(2 M in
aqueous solution) (0.250 mL) and DME (3 mL) was stirred under microwave
irradiation at
100 C for 40 minutes. The residue was purified by NH silica gel column
chromatography
(methanol/ethyl acetate) to obtain a solid. The obtained solid was
crystallized from
ethanol/THF/heptane to obtain the title compound (33.5 mg).
1H NIvIR (300 MHz, DMSO-d6) 5 2.66 (3H, s), 2.67 (3H, s), 3.94 (3H, s), 6.02
(2H, s),
7.05 (1H, d, J = 2.8 Hz), 8.07 (1H, d, J = 2.8 Hz), 8.20-8.25 (2H, m), 8.58
(1H, d, J = 2.0 Hz).
[0363]
Example 229
6-(4-methoxypyrrolo [2,1-f] [1,2,4]triazin-5-y1)-2-methy1-1-((3-methyl-1,2,4-
thiadiazol-
5-yOmethyl)-1H-imidazo[4,5-b]pyridine
a) 2-(6-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-l-ypacetonitrile
A mixture of potassium tert-butoxide (11 g), 6-bromo-2-methyl-1H-imidazo[4,5-
b]pyridine (20 g) and THF (200 mL) was stirred at 50 C for 30 minutes. To the
mixture was
added dropwise a solution of bromoacetonitrile (7.00 mL) in THF (20 mL) at 50
C. The
mixture was stirred at 50 C for 2 hours, and then cooled to 5 C. The mixture
was diluted with
water (100 mL), and stirred at 5 C for 30 minutes. The precipitate was
collected by filtration,
and washed with water (100 mL) and ethyl acetate (50 mL) to obtain a solid
(13.3 g). A
suspension of the obtained solid (13.3 g) in ethyl acetate (260 mL) was
stirred at 70 C for 60
minutes. The suspension was cooled to 40 C, and the precipitate was then
collected by
filtration, and washed with ethyl acetate (100 mL) to obtain the title
compound (7.52 g).
MAR (300 MHz, DMSO-d6) 6 2.66 (3H, s), 5.63 (2H, s), 8.45-8.47 (1H, m), 8.47-
8.50 (1H, m).
152
CA 03021185 2018-10-16
A00039
[0364]
b) 2-(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)ethanethioamide
Diethyl phosphorodithioic acid (3.00 mL) was added to a solution of 2-(6-bromo-
2-
methy1-1H-imidazo[4,5-b]pyridin-1-yDacetonitrile (2.40 g) in THF (40 mL)/water
(4 mL) at
room temperature. The mixture was stirred at 70 C for 3 hours. The mixture was
cooled, and
the residue was purified by NH silica gel column chromatography
(methanol/ethyl acetate) to
obtain the title compound (2.150 g).
1H NMR (300 MHz, DMSO-d6) 5 2.50 (3H, s), 5.16 (2H, s), 8.20 (1H, d, J = 2.2
Hz),
8.38 (1H, d, J = 2.2 Hz), 9.51 (1H, brs), 9.94 (1H, brs).
[0365]
c) 5-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-l-yOmethyl)-3-methyl-1,2,4-
thiadiazole
N,N-dimethylacetamide dimethylacetal (900 ilL) was added to a suspension of 2-
(6-
bromo-2-methy1-11-1-imidazo[4,5-b]pyridin-1-ypethanethioamide (1.40 g) in
acetonitrile (50 mL)
at room temperature. The mixture was stirred at 40 C for 3 hours, and then
concentrated under
reduced pressure to obtain a brown oil. Pyridine (1.00 mL) was added to a
solution of the
obtained oil in methanol (10 mL) at 0 C. The mixture was stirred at 0 C for 10
minutes, and a
solution of hydroxylamine-O-sulfonic acid (600 mg) in methanol (15 mL) was
then added
dropwise to the mixture. The mixture was stirred overnight at room
temperature. The
precipitate was collected by filtration, and washed with IPA and water to
obtain the title
compound (644 mg). The filtrate was poured into a saturated aqueous sodium
hydrogen
carbonate solution, and extracted twice with ethyl acetate. The organic layer
was washed with
water and a saturated brine, dried over magnesium sulfate, and then
concentrated. The residue
was purified by NH silica gel column chromatography (ethyl acetate/hexane) to
obtain the title
compound (660 mg).
1H NMR (300 MHz, DMSO-d6) 5 2.51 (3H, s), 162 (3H, s), 6.08 (2H, s), 8.40-8.42
(1H, m), 8.43-8.45 (1H, m).
[0366]
d) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1-(3-methy1-1,2,4-
thiadiazol-5-yl)methyl)-1H-imidazo[4,5-b]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetrarnethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (1867 mg), 5-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
y1)methyl)-3-
methyl-1,2,4-thiadiazole (550 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (57.1 mg), cesium
carbonate (2 M in
153
CA 03021185 2018-10-16
A00039
aqueous solution) (2.54 mL) and DME (12 mL) was stirred under microwave
irradiation at
100 C for 40 minutes. The mixture was poured into ice water at room
temperature, and
extracted with ethyl acetate. The organic layer was washed with water and a
saturated brine,
dried over magnesium sulfate, and concentrated under reduced pressure. The
residue was
purified by NH silica gel column chromatography (methanol/ethyl acetate) to
obtain a solid.
The obtained solid was washed with ethyl acetate/IPE to obtain the title
compound (210 mg).
1H NMR (300 MHz, DMSO-d6) 62.54 (3H, s), 2.66 (3H, s), 3.86 (3H, s), 6.11 (2H,
s),
7.05 (1H, d, J = 2.8 Hz), 8.06 (1H, d, J = 2.8 Hz), 8.15-8.28 (21-1, m), 8.58
(1H, d, J = 2.1 Hz).
[0367]
Example 230
6-(4-methoxypyrrolo[2,1-f] [1,2,4]triazin-5-y1)-2-methy1-1-((1-methyl-1H-
pyrazol-4-
yl)methyl)-1H-imidazo[4,5-b]pyridine
a) 6-bromo-2-methy1-1-((1-methyl-1H-pyrazol-4-yl)methyl)-1H-imidazo[4,5-
b]pyridine
di-TFA salt
6-bromo-2-methyl-1H-imidazo[4,5-b]pyridine (700 mg) was dissolved in THF (20
mL)
at 60 C, and KOH (742 mg), TBAI (123 mg) and 4-(chloromethyl)-1-methyl-1H-
pyrazole
hydrochloride (663 mg) were added at 60 C. The mixture was stirred at 60 C for
2 hours.
The mixture was diluted with water at room temperature, and extracted with
ethyl acetate. The
organic layer was washed with a saturated brine, then dried over magnesium
sulfate, and
concentrated under reduced pressure. The residue was purified by NH silica gel
column
chromatography (ethyl acetate/methanol), the obtained solid was further
fractionated by HPLC
(C18, mobile phase: water/acetonitrile (0.1% TFA-containing system)), and the
fraction was
concentrated to obtain the title compound (305 mg).
MS: [M+H] 306Ø
[0368]
b) 6-(4-methoxypyrrolo[2,1-f] [1,2,4]triazin-5-y1)-2-methy1-1-(( I -methy1-1H-
pyrazol-4-
y1)methyl)-1H-imidazo[4,5-b]pyridine
A mixture of 6-bromo-2-methy1-1-((l-methyl-1H-pyrazol-4-yl)methyl)-1H-
imidazo[4,5-b]pyridine di-TFA salt (200 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-
1,3,2-
dioxaborolan-2-yl)pyrrolo[2,1-1][1,2,4]triazine (140 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (21 mg), cesium carbonate
(2 M in
aqueous solution) (0.330 mL) and DME (1.5 mL) was stirred under microwave
irradiation at
100 C for 1 hour, and then stirred at 120 C for 20 minutes. The mixture was
purified by NH
silica gel column chromatography (methanol/ethyl acetate), and the obtained
solid was washed
154
CA 03021185 2018-10-16
A00039
with WE to obtain the title compound (90 mg).
1HNMR (300 MHz, DMSO-d6) ö 2.68 (3H, s), 3.75 (3H, s), 3.93 (3H, s), 5.32 (2H,
s),
7.07 (1H, d, J = 2.8 Hz), 7.49 (111, s), 7.74 (1H, s), 8.07 (1H, d, J = 2.8
Hz), 8.18 (1H, d, J = 2.0
Hz), 8.23 (1H, s), 8.52 (111, d, J = 2.0 Hz).
[0369]
Example 231
6-(4-methoxypyrrolo[2,1-1] [1,2,4]triazin-5-y1)-2-methy1-141-methyl- 1 H-1,2,4-
triazol-
3-yl)methyl)-1H-imidazo[4,5-b]pyridine
a) N-(2-amino-5-bromopyridin-3-y1)-1-methy1-1H-1,2,4-triazole-3-carboxamide
A mixture of 5-bromopyridine-2,3-diamine (1.5 g), 1-methyl-1H-1,2,4-triazole-3-
carboxylic acid (1.02 g), HATU (4.26 g), Et3N (3.4 mL) and DMF (16 mL) was
stirred overnight
at room temperature. The mixture was diluted with a saturated aqueous sodium
hydrogen
carbonate solution, and extracted with ethyl acetate. The organic layer was
washed with a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was purified by silica gel column chromatography (ethyl
acetate/methanol) to obtain
the title compound (0.663 g). The title compound was used for the subsequent
reaction without
being further purified.
MS: [M+H]F 297Ø
[0370]
b) 5-bromo-N3-((l-methy1-1H-1,2,4-triazol-3-yOmethyppyridine-2,3-diamine
A BH3-THF complex (1M in THF solution) (7.7 mL) was added dropwise to a
solution
of N-(2-amino-5-bromopyridin-3-y1)-1-methy1-1H-1,2,4-triazole-3-carboxamide
(662.7 mg) in
THF (6 mL) at room temperature. The mixture was stirred under a nitrogen
atmosphere at
60 C for 2 hours. The/ reaction was stopped with water at room temperature,
and the mixture
was then acidified with HC1 (1 N in aqueous solution). The mixture was stirred
at 60 C for 30
minutes. The mixture was neutralized with a saturated aqueous sodium hydrogen
carbonate
solution at room temperature, and then extracted with ethyl acetate. The
organic layer was
washed with a saturated brine, then dried over magnesium sulfate, and
concentrated under
reduced pressure. The residue was purified by silica gel column chromatography
(ethyl
acetate/methanol) to obtain the title compound (348 mg).
MS: [M+Hr 283.1.
[0371]
c) 6-bromo-2-methy1-1-((1-methyl-1H-1,2,4-triazol-3-yOmethyl)-1H-imidazo[4,5-
b]pyridine
155
CA 03021185 2018-10-16
A00039
A mixture of 5-bromo-N3-((1-methy1-1H-1,2,4-triazol-3-yOmethyl)pyridine-2,3-
diamine (348 mg), acetic acid (0.09 mL), DMAP (22 mg), propylphosphonic
anhydride (1.7 M
in ethyl acetate solution) (1.1 mL), DIPEA (0.43 mL) and THF (2.5 mL) was
stirred under
microwave irradiation at 160 C for 50 minutes. The residue was purified by NH
silica gel
column chromatography (methanol/ethyl acetate) to obtain the title compound
(301 mg).
MS: [M+1-1]+ 307Ø
[0372]
d) 6-(4-methoxypyrrolo [2,1-f] [1,2,4]triazin-5-y1)-2-methy1-1-((l-methyl-1H-
1,2,4-
triazol-3-yl)methyl)-1H-imidazo[4,5-b]pyridine
A mixture of 6-bromo-2-methyl-1-((1-methyl-1H-1,2,4-triazol-3-yl)methyl)-1H-
imidazo[4,5-b]pyridine (90 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
yl)pyrrolo[2,14][1,2,4]triazine (120 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (18 mg), cesium carbonate
(2 M in
aqueous solution) (0.285 mL) and DME (1.5 mL) was stirred under microwave
irradiation at
120 C for 1 hour. The mixture was purified by NH silica gel column
chromatography
(methanol/ethyl acetate), and the obtained solid was washed with WE to obtain
the title
compound (45.0 mg).
IHNIVIR (300 MHz, DMSO-d6) 8 2.68 (3H, s), 3.80 (3H, s), 3.97 (3H, s), 5.53
(211, s),
7.06 (1H, d, J = 2.7 Hz), 8.06 (1H, d, J = 2.7 Hz), 8.14 (1H, d, J = 2.1 Hz),
8.22 (1H, s), 8.42
(1H, s), 8.54 (1H, d, J = 2.0 Hz).
[0373]
Example 237
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1-(pyridin-2-ylmethyl)-
1H-
imidazo[4,5-b]pyridine
a) 5-iodo-N3-(pyridin-2-ylmethyl)pyridine-2,3-diamine
Acetic acid (0.657 mL) was added to a solution of picolinaldehyde (0.476 mL)
and 5-
bromopyridine-2,3-diamine (1.07 g) in THF (10 mL) at room temperature. The
mixture was
stirred overnight at room temperature. The mixture was poured into a saturated
aqueous
sodium hydrogen carbonate solution, and extracted twice with ethyl acetate.
The organic layers
were combined, washed with a saturated brine, dried over magnesium sulfate,
and then
concentrated to obtain a residue. To a solution of the residue in methanol (10
mL) was added
sodium borohydride (0.431 g) at room temperature, and the mixture was stirred
overnight. The
reaction mixture was poured into water, and extracted twice with ethyl
acetate. The organic
layers were combined, then washed with a saturated brine, dried over sodium
sulfate, and
156
CA 03021185 2018-10-16
A00039
concentrated under reduced pressure. The residue was purified by silica gel
column
chromatography (ethyl acetate/hexane/methanol) to obtain the title compound
(0.26 g).
IFINMR (300 MHz, DMSO-d6) 6 4.37 (2H, d, J = 5.8 Hz), 5.72-5.90 (3H, m), 6.63
(1H,
d, J = 1.9 Hz), 7.28 (1H, ddd, J = 7.5, 4.8, 1.1 Hz), 7.35 (11-1, d, J = 7.8
Hz), 7.40 (1H, d, J = 1.9
Hz), 7.77 (1H, td, J = 7.7, 1.8 Hz), 8.51-8.58 (1H, m).
[0374]
b) 6-bromo-2-methyl-1-(pyridin-2-ylmethyl)-1H-imidazo[4,5-b]pyridine
A mixture of 5-iodo-N3-(pyridin-2-ylmethyl)pyridine-2,3-diamine (0.26 g),
acetic acid
(0.055 mL), DMAP (4.87 mg), propylphosphonic anhydride (50% in ethyl acetate
solution)
(0.703 mL), DIPEA (0.278 mL) and THF (3 mL) was stirred under microwave
irradiation at
180 C for 4 hours. The mixture was purified by NH silica gel column
chromatography
(hexane/ethyl acetate) to obtain the title compound (0.154 g).
IFI NMR (300 MHz, DMSO-d6) 6 2.56 (3H, s), 5.60 (2H, s), 7.27-7.39 (2H, m),
7.81
(1H, td, J = 7.7, 1.8 Hz), 8.38 (1H, d, J = 1.9 Hz), 8.45-8.50 (2H, m).
[0375]
c) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methy1-1-(pyridin-2-
ylmethyl)-1H-
imidazo[4,5-b]pyridine
A mixture of 6-iodo-2-methyl-1-(pyridin-2-ylmethyl)-1H-imidazo[4,5-b]pyridine
(153.6
mg), 4-methyl-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yppyrrolo[2,1-
f][1,2,4]triazine (127
mg), bis(di-tert-buty1(4-dimethylaminophenyl)phosphine)dichloropalladium (II)
(31.1 mg),
cesium carbonate (2 M in aqueous solution) (0.439 mL) and DME (2 mL) was
stirred under
microwave irradiation at 100 C for 1 hour. The mixture was purified by NH
silica gel column
chromatography (methanol/ethyl acetate), and the residue was crystallized from
ethanol/water to
obtain the title compound (74.2 mg).
NMR (300 MHz, DMSO-d6) 6 2.63 (3H, s), 3.80 (3H, s), 5.63 (2H, s), 7.04 (1H,
d, J
= 2.8 Hz), 7.29-7.37 (2H, m), 7.82 (1H, td, J = 7.7, 1.8 Hz), 8.04 (1H, d, J =
2.8 Hz), 8.11 (1H, d,
J = 2.1 Hz), 8.20 (1H, s), 8.49-8.53 (1H, m), 8.54 (11-1, d, J = 2.1 Hz).
[0376]
Example 242
1-((5-(difluoromethyl)-1,3,4-oxadiazol-2-yOmethyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
a) methyl 2-(6-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-1-yl)acetate
A mixture of potassium tert-butoxide (11.1 g), 6-bromo-2-methy1-1H-imidazo[4,5-
b]pyridine (20.0 g) and THF (200 mL) was stirred at 50 C for 20 minutes.
Methyl 2-
157
CA 03021185 2018-10-16
A00039
bromoacetate (17.4 g) was added to the mixture. The mixture was stirred at 50
C for 1 hour.
The reaction was stopped with acetic acid/water (1/10) at 0 C, and the mixture
was diluted with
ethyl acetate (100 mL) and a saturated aqueous sodium hydrogen carbonate
solution (100 mL).
The precipitate was collected by filtration, and the obtained solid was washed
with water (100
mL) and ethyl acetate (50 mL) to obtain the title compound (13.10 g).
NMR (300 MHz, DMSO-d6) 6 2.52 (3H, s), 3.73 (3H, s), 5.27 (2H, s), 8.33 (1H,
d, J
= 2.2 Hz), 8.41 (1H, d, J = 2.2 Hz).
[0377]
b) 2-(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)acetohydrazide
A suspension of methyl 2-(6-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-1 -
yl)acetate
(6.43 g) in ethanol (130 mL)/water (13 mL) was stirred at 70 C for 10 minutes.
Hydrazine
monohydrate (10 mL) was added to the mixture, and the mixture was stirred at
70 C for 1 hour,
and then stirred at 0 C for 2 hours. The precipitate was collected by
filtration, and washed with
ethanol (30 mL) to obtain the title compound (5.17 g).
IHNIVIR (300 MHz, DMSO-d6) 6 2.53 (3H, s), 4.36 (2H, brs), 4.87 (2H, s), 8.22
(1H, d,
J = 2.2 Hz), 8.39 (1H, d, J = 2.2 Hz), 9.49 (1H, brs).
[0378]
c) 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yl)methyl)-5-
(difluoromethyl)-
1,3,4-oxadiazole
Propylphosphonic anhydride (50% in ethyl acetate solution) (8.0 mL) was added
to a
suspension of triethylamine (3.00 mL), 2-(6-bromo-2-methy1-1H-imidazo[4,5-
b]pyridin-1-
y1)acetohydrazide (1.70 g) and difluoroacetic acid (500 p.L) in ethyl acetate
(55 mL) at room
temperature. The mixture was stirred at 50 C for 20 minutes, and then stirred
under microwave
irradiation at 140 C for 60 minutes. Insolubles were removed by filtration,
and the filtrate was
concentrated under reduced pressure. The residue was purified by NH silica gel
column
chromatography (ethyl acetate/hexane) to obtain a solid. The obtained solid
was washed with
ethyl acetate/hexane to obtain the title compound (0.730 g).
1HNMR (300 MHz, DMSO-d6) 6 2.63 (3H, s), 5.99 (2H, s), 7.19-7.73 (1H, m), 8.39
(1H, d, J = 2.2 Hz), 8.45 (1H, d, J 2.2 Hz).
[0379]
d) 1-((5-(difluoromethyl)-1,3,4-oxadiazol-2-y1)methyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetsamethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (900 mg), 24(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
yOmethyl)-5-
158
CA 03021185 2018-10-16
A00039
(difluoromethyl)-1,3,4-oxadiazole (305 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (30 mg), cesium carbonate
(2 M in
aqueous solution) (850 pt) and DME (8 mL) was stirred under microwave
irradiation at 100 C
for 1 hour. The residue was purified by silica gel column chromatography
(methanol/ethyl
acetate) to obtain a solid. The obtained solid was crystallized from
ethanol/water to obtain the
title compound (88 mg).
11-1 MAR (300 MHz, DMSO-d6) ö 2.67 (314, s), 3.96 (3H, s), 6.02 (211, s), 7.05
(1H, d, J
= 2.7 Hz), 7.25-7.69 (1H, m), 8.07 (1H, d, J = 2.7 Hz), 8.21-8.25 (211, m),
8.59 (1H, d, J ¨ 2.1
Hz).
[0380]
Example 243
145-(1,1-difluoroethyl)-1,2,4-oxadiazol-3-yOmethyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
a) 34(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yOmethyl)-5-(1,1-
difluoroethyl)-
1,2,4-oxadiazole
Sodium carbonate (1.266 g) was added to a mixture of hydroxylamine
monohydrochloride (0.850 g) and water (5.0 ml) at room temperature. The
mixture was stirred
for 10 minutes. Thereafter, to the mixture was added a mixture of 2-(6-bromo-2-
methy1-1H-
imidazo[4,5-b]pyridin-1-ypacetonitrile (2.00 g) and ethanol (30 ml), and the
resulting mixture
.. was stirred under a nitrogen atmosphere at 70 C for 1 hour. The reaction
mixture was
concentrated under reduced pressure, and diluted with water. Insolubles were
collected by
filtration, and washed with WE to obtain a brown solid (1.589 g) as 2-(6-bromo-
2-methy1-1H-
imidazo[4,5-b]pyridin-l-y1)-N'-hydroxyacetimidamide. The obtained solid (500
mg) was
added to a mixture of DIEA (0.867 ml), 2,2-difluoropropanoic acid (140 mg),
propylphosphonic
anhydride (50% in ethyl acetate solution) (1.477 ml) and ethyl acetate (4 ml)
at room
temperature, and the resulting mixture was stirred at room temperature for 2
days. The reaction
mixture was concentrated under reduced pressure, and the residue was purified
by NH silica gel
column chromatography (ethyl acetate/hexane) to obtain the title compound (77
mg).
IHNMR (300 MHz, DMSO-d6) 2.14 (3H, t, J = 19.8 Hz), 2.62 (311, s), 5.88 (2H,
s),
8.37 (1H, d, J = 2.1 Hz), 8.44 (1H, d, J = 2.1 I-1z).
[0381]
b) 1-((5-(1,1-difluoroethyl)-1,2,4-oxadiazol-3-y1)methyl)-6-(4-
methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-11-1-imidazo[4,5-b]pyridine
A mixture of 3-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yOmethyl)-5-(1,1-
159
CA 03021185 2018-10-16
A00039
difluoroethyl)-1,2,4-oxadiazole (77.0 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-
1,3,2-
dioxaborolan-2-yppyrrolo[2,1-f][1,2,4]triazine (99 mg) bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (15 mg), cesium carbonate
(2 M in
aqueous solution) (0.20 mL) and DME (2.0 mL) was stirred under microwave
irradiation at
100 C for 30 minutes. The reaction mixture was purified by silica gel column
chromatography
(ethyl acetate/hexane and methanol/ethyl acetate), and the residue was
crystallized from
ethanol/ethyl acetate/heptane to obtain the title compound (19.00 mg).
IFINMR (300 MHz, DMSO-d6) 6 2.14 (3H, t, J = 19.8 Hz), 2.67(311, s), 3.96 (3H,
s),
5.90 (2H, s), 7.06 (1H, d, J = 2.7 Hz), 8.07 (11-1, d, J = 2.7 Hz), 8.20-8.25
(2H, m), 8.58 (1H, d, J
= 2.0 Hz).
[0382]
Example 250
6-(4-methoxypyrrolo[2,14][1,2,4]triazin-5-y1)-2-methyl-1-((5-(trifluoromethyl)-
1,3,4-
oxadiazol-2-y1)methyl)-1H-imidazo[4,5-b]pyridine
a) 24(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-5-
(trifluoromethyl)-
1,3,4-oxadiazole
A mixture of potassium tert-butoxide (2.80 g), 6-bromo-2-methy1-1H-imidazo[4,5-
b]pyridine (5.00 g) and THF (60 mL) was stirred at 45 C for 30 minutes. To the
mixture were
added TBAI (8.71 g) and 2-(chloromethyl)-5-(trifluoromethyl)-1,3,4-oxadiazole
(5.00 g), and the
resulting mixture was stirred at 45 C for 1 hour. The mixture was neutralized
with aqueous
acetic acid, and extracted with ethyl acetate. The organic layer was washed
with a saturated
aqueous sodium hydrogen carbonate solution and a saturated brine, dried over
magnesium
sulfate, and then concentrated under reduced pressure. The residue was
purified by NH silica
gel column chromatography (ethyl acetate/hexane) to obtain the title compound
(0.094 g).
NMR (300 MHz, DMSO-d6) 6 2.64 (3H, s), 6.00 (2H, s), 8.37 (I H, d, J = 2.2
Hz),
8.45 (1H, d, J = 2.2 Hz).
[0383]
b) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methy1-14(5-
(trifluoromethyl)-
1,3,4-oxadiazol-2-y1)methyl)-1H-imidazo[4,5-b]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yl)pyrrolo
[2,1-
f][1,2,4]triazine (100 mg), 2-46-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
yOmethyl)-5-
(trifluoromethyl)-1,3,4-oxadiazole (94 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (10 mg), cesium carbonate
(2 M in
aqueous solution) (250 L) and DME (4 mL) was stirred under microwave
irradiation at 100 C
160
CA 03021185 2018-10-16
A00039
for 40 minutes. The residue was purified by NH silica gel column
chromatography
(methanol/ethyl acetate) to obtain a solid. The obtained solid was
crystallized from ethyl
acetate/ethanol/heptane to obtain the title compound (18.50 mg).
11-INIVIR (300 MHz, DMSO-d6) 8 2.68 (3H, s), 3.97 (3H, s), 6.02 (2H, s), 7.05
(1H, d, J
= 2.7 Hz), 8.07 (1H, d, J = 2.7 Hz), 8.19-8.27 (21-1, m), 8.59 (1H, d, J = 2.1
Hz).
[0384]
Example 252
1-((5-ethy1-1,3,4-oxadiazol-2-yOmethyl)-6-(4-methoxypyrrolo[2,14] [ I
,2,4]triazin-5-
y1)-2-methy1-1H-imidazo[4,5-b]pyridine
a) 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-5-ethyl-1,3,4-
oxadiazole
A solution of tert-butyl 2-(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
y1)acetate
(580 mg) in TFA (5 mL) was stirred at room temperature for 1 hour, and
concentrated under
reduced pressure. To a solution of the residue in ethyl acetate (6 ml) were
added TEA (1.239
.. ml), propionohydrazide (220 mg) and propylphosphonic anhydride (50% in
ethyl acetate
solution) (3.70 ml) at room temperature, and the mixture was stirred overnight
at 80 C, and
stirred under microwave irradiation at 150 C for 2 hours. The residue was
purified by NH
silica gel column chromatography (methanol/ethyl acetate) to obtain the title
compound (325
mg).
11-1 NMR (300 MHz, DMSO-d6) 6 1.23 (3H, t, J = 7.6 Hz), 2.61 (3H, s), 2.83
(2H, q, J
7.6 Hz), 5.86 (2H, s), 8.36 (1H, d, J = 2.2 Hz), 8.44 (1H, d, J = 2.2 Hz).
[0385]
b) 1-((5-ethyl-1,3,4-oxadiazol-2-yOmethyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-
y1)-2-methyl-1H-imidazo[4,5-b]pyridine
A mixture of 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-5-
ethyl-
1,3,4-oxadiazole (100 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-
2-
yl)pyrrolo[2,14][1,2,4]triazine (149 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (22 mg), cesium carbonate
(2 M in
aqueous solution) (0.24 mL) and DME (2.0 mL) was stirred under microwave
irradiation at
100 C for 20 minutes. The reaction mixture was purified by silica gel column
chromatography
(ethyl acetate/hexane acetate and methanol/ethyl acetate), and the residue was
crystallized from
ethanol/ethyl acetate/heptane to obtain the title compound (66.8 mg).
11-1NMR (300 MHz, DMSO-d6) 8 1.22 (3H, t, J = 7.5 Hz), 2.66 (3H, s), 2.78-2.89
(2H,
m), 3.97 (3H, s), 5.88 (2H, s), 7.06 (1H, d, J = 2.8 Hz), 8.07 (1H, d, J = 2.6
Hz), 8.20 (1H, d, J =
161
CA 03021185 2018-10-16
A00039
2.1 Hz), 8.23 (1H, s), 8.58 (1H, d, J = 1.9 Hz).
[0386]
Example 253
1-((5-cyclopropy1-1,3,4-oxadiazol-2-yOmethyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methy1-1H-imidazo[4,5-b]pyridine
a) 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yOmethyl)-5-cyclopropyl-
1,3,4-
oxadiazole
A solution of tert-butyl 2-(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
yl)acetate
(500 mg) in TFA (5.0 mL) was stirred at room temperature for 1 hour, and
concentrated under
reduced pressure. To a solution of the residue in ethyl acetate (2 ml) were
added TEA (1.068
ml), cyclopropanecarbohydrazide (220 mg) and propylphosphonic anhydride (50%
in ethyl
acetate solution) (3.19 ml) at room temperature, and the mixture was stirred
at room temperature
for 1 hour, and stirred under microwave irradiation at 150 C for 2 hours. The
residue was
purified by NH silica gel column chromatography (methanol/ethyl acetate) to
obtain the title
compound (61.2 mg).
NMR (300 MHz, DMSO-d6) 6 0.92-0.99 (2H, m), 1.07-1.15 (2H, m), 2.15-2.25 (1H,
m), 2.60 (3H, s), 5.80 (2H, s), 8.35 (1H, d, J = 2.2 Hz), 8.44 (1H, d, J = 2.2
Hz).
[0387]
b) 14(5-cyclopropy1-1,3,4-oxadiazol-2-yl)methyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methy1-1H-imidazo[4,5-b]pyridine
A mixture of 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yOmethyl)-5-
cyclopropyl-1,3,4-oxadiazole (61.2 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-
2-yppyrrolo[2,1-f][1,2,4]triazine (88 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (13 mg), cesium carbonate
(2 M in
aqueous solution) (0.15 mL) and DME (2.0 mL) was stirred under microwave
irradiation at
100 C for 20 minutes. The reaction mixture was purified by silica gel column
chromatography
(ethyl acetate/hexane acetate and methanol/ethyl acetate), and the residue was
crystallized from
ethanol/ethyl acetate/heptane to obtain the title compound (28.0 mg).
11-1 NMR (300 MHz, DMSO-d6) 6 0.91-1.00 (2H, m), 1.04-1.16 (2H, m), 2.15-2.25
(1H,
m), 2.65 (31-1, s), 3.97 (3H, s), 5.83 (2H, s), 7.06 (1H, d, J = 2.7 Hz), 8.07
(1H, d, J = 2.7 Hz),
8.19 (1H, d, J = 2.1 Hz), 8.23 (1H, s), 8.58 (1H, d, J = 2.1 Hz).
[0388]
Example 267
1-((5-isopropy1-1,3,4-oxadiazol-2-yl)methyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-
162
CA 03021185 2018-10-16
A00039
5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
a) 24(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yOmethyl)-5-isopropyl-1,3,4-
oxadiazole
A mixture of isobutyric acid (180 p L), TEA (1.10 mL), 2-(6-bromo-2-methy1-1H-
imidazo[4,5-b]pyridin-1-yl)acetohydrazide (450 mg), propylphosphonic anhydride
(50% in ethyl
acetate solution) (3.30 ml) and ethyl acetate (2.0 ml) was stirred under
microwave irradiation at
150 C for 30 minutes. The reaction mixture was purified by NH silica gel
column
chromatography (methanol/ethyl acetate) to obtain the title compound (337 mg).
1H NMR (300 MHz, DMSO-d6) 6 1.27 (6H, d, J = 7.0 Hz), 2.61 (3H, s), 3.10-3.24
(1H,
m), 5.86 (2H, s), 8.36 (1H, d, J = 2.2 Hz), 8.44 (1H, d, J = 2.2 Hz).
[0389]
b) 1-((5-isopropy1-1,3,4-oxadiazol-2-y1)methyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
A mixture of 2-((6-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-1-yl)methyl)-5-
isopropyl-1,3,4-oxadiazole (100 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
yl)pyrrolo[2,1-f][1,2,4]triazine (143 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (20 mg), cesium carbonate
(2 M in
aqueous solution) (0.24 mL) and DME (2.0 mL) was stirred under microwave
irradiation at
100 C for 20 minutes. The reaction mixture was purified by silica gel column
chromatography
.. (ethyl acetate/hexane and methanol/ethyl acetate), and the residue was
crystallized from
ethanol/ethyl acetate/heptane to obtain the title compound (61.1 mg).
1f1NMR (300 MHz, DMSO-d6) 6 L26 (6H, d, J = 6.9 Hz), 2.66 (3H, s), 3.12-3.23
(1H,
m), 3.96 (3H, s), 5.88 (2H, s), 7.06 (1H, d, J = 2.7 Hz), 8.07 (1H, d, J = 2.7
Hz), 8.20 (1H, d, J =
2.0 Hz), 8.23 (1H, s), 8.58 (1H, d, J = 2.0 Hz).
[0390]
Example 268
1-((5-cyclobuty1-1,3,4-oxadiazol-2-yOmethyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-
5-y1)-2-methyl-IH-imidazo[4,5-b]pyridine
a) 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-5-cyclobutyl-
1,3,4-
oxadiazole
A mixture of cyclobutanecarboxylic acid (130 [IL), TEA (0.73 ml), 2-(6-bromo-2-
methy1-1H-imidazo[4,5-b]pyridin-1-yl)acetohydrazide (300 mg), propylphosphonic
anhydride
(50% in ethyl acetate solution) (2.10 ml) and ethyl acetate (2.0 ml) was
stirred under microwave
irradiation at 150 C for 30 minutes. The reaction mixture was purified by NH
silica gel column
163
CA 03021185 2018-10-16
A00039
chromatography (methanol/ethyl acetate), and the residue was washed with ethyl
acetate/heptane
to obtain the title compound (210 mg).
1H NMR (300 MI-[z, DMSO-d6) 6 2.02 (2H, s), 2.20-2.41 (4H, m), 2.61 (3H, s),
3.67-
3.82 (1H, m), 5.85 (2H, s), 8.37 (1H, d, J -= 2.2 Hz), 8.44 (1H, d, J = 2.2
Hz).
[0391]
b) 1-((5-cyclobuty1-1,3,4-oxadiazol-2-yl)methyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
A mixture of 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-5-
cyclobutyl-1,3,4-oxadiazole (100 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
yl)pyrrolo[2,1-f][1,2,4]triazine (138 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (20 mg), cesium carbonate
(2 M in
aqueous solution) (0.24 mL) and DME (2.0 mL) was stirred under microwave
irradiation at
100 C for 20 minutes. The reaction mixture was purified by silica gel column
chromatography
(ethyl acetate/hexane and methanol/ethyl acetate), and the residue was
crystallized from
ethanol/ethyl acetate/heptane to obtain the title compound (67.1 mg).
1H NMR (300 MIHz, DMSO-d6) 6 1.84-2.11 (2H, m), 2.21-2.39 (4H, m), 2.66 (3H,
s),
3.66-3.82 (1H, m), 3.96 (3H, s), 5.88 (2H, s), 7.06 (1H, d, J = 2.7 Hz), 8.07
(1H, d, J = 2.7 Hz),
8.20 (1H, d, J 2.1 Hz), 8.23 (1H, s), 8.58 (1H, d, J = 2.1 Hz).
[0392]
Example 269
1-((5-(3,3-difluorocyclobuty1)-1,3,4-oxadiazol-2-y1)methyl)-6-(4-
methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
a) 24(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-5-(3,3-
difluorocyclobuty1)-1,3,4-oxadiazole
A mixture of 3,3-difluorocyclobutanecarboxylic acid (180 mg), TEA (0.73 ml), 2-
(6-
bromo-2-methyl-1H-imidazo[4,5-b]pyridin-l-yl)acetohydrazide (300 mg),
propylphosphonic
anhydride (50% in ethyl acetate solution) (2.10 ml) and ethyl acetate (2.0 ml)
was stirred under
microwave irradiation at 150 C for 20 minutes. The reaction mixture was
purified by NH silica
gel column chromatography (methanol/ethyl acetate) to obtain the title
compound (209 mg).
NMR (300 MHz, DMSO-d6) 6 2.62 (3H, s), 2.83-3.21 (4H, m), 3.62-3.80 (1H, m),
5.86 (2H, s), 8.36 (1H, d, .1 = 2.2 Hz), 8.44 (1H, d, J = 2.2 Hz).
[0393]
b) 14(5-(3,3-difluorocyclobuty1)-1,3,4-oxadiazol-2-yl)methyl)-6-(4-
methoxypyrrolo[2,14][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
164
CA 03021185 2018-10-16
A00039
A mixture of 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-5-(3,3-
difluorocyclobuty1)-1,3,4-oxadiazole (100 mg), 4-methoxy-5-(4,4,5,5-
tetramethy1-1,3,2-
dioxaborolan-2-yl)pyrrolo[2,14][1,2,4]triazine (315 mg) bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (11) (20 mg), cesium carbonate
(2 M in
aqueous solution) (0.20 mL) and DME (2.0 mL) was stirred under microwave
irradiation at
100 C for 20 minutes. The reaction mixture was purified by silica gel column
chromatography
(ethyl acetate/hexane and methanol/ethyl acetate), and the residue was
crystallized from
ethanol/ethyl acetate/heptane to obtain the title compound (61.1 mg).
NMR (300 MHz, DMSO-d6) 2.67 (3H, s), 2.85-3.17 (4H, m), 3.72 (1H, d, J = 7.9
Hz), 3.97 (3H, s), 5.89 (2H, s), 7.06 (1H, d, J = 2.8 Hz), 8.07 (1H, d, J =
2.8 Hz), 8.21 (1H, d, J =-
2.1 Hz), 8.23 (1H, s), 8.58 (1H, d, J =2.1 Hz).
[0394]
Example 273
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-1,2-dimethyl-1H-imidazo[4,5-
b]pyridine
a) 6-bromo-1,2-dimethy1-1H-imidazo[4,5-b]pyridine
Potassium hydroxide (15.88 g) was added to a solution of 6-bromo-2-methy1-1H-
imidazo[4,5-b]pyridine (15 g) and iodomethane (5.31 mL) in THF (150 mL) at
room
temperature. Under a nitrogen atmosphere, the mixture was stirred at 60 C for
3 hours. The
reaction mixture was poured into hydrochloric acid (1N in aqueous solution) at
room
.. temperature, and extracted with ethyl acetate. The organic layer was washed
with water and a
saturated brine, dried over magnesium sulfate, and then concentrated. The
residue was purified
by NH silica gel column chromatography (ethyl acetate/hexane) to obtain the
title compound
(8.3 g).
1H NMR (300 Wiz, DMSO-d6) ö 2.57 (3H, s), 3.75 (3H, s), 8.29 (1H, d, J = 2.26
Hz),
8.37 (1H, d, J = 2.26 Hz).
[0395]
b) 6-(4-methoxypyrrolo[2,1-1][1,2,4]triazin-5-y1)-1,2-dimethyl-1H-imidazo[4,5-
b]pyridine
A mixture of 6-bromo-1,2-dimethy1-1H-imidazo[4,5-b]pyridine (70 mg), 4-methoxy-
5-
(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yl)pyrrolo[2,1-1][1,2,4]triazine
(135 mg), bis(di-tert-
buty1(4-dimethylaminophenyl)phosphine)dichloropalladium (II) (10.41 mg),
cesium carbonate (2
M in aqueous solution) (0.464 mL) and DME (12 mL) was stirred under microwave
irradiation
at 100 C for 40 minutes. The reaction mixture was poured into ice water at
room temperature,
and extracted with ethyl acetate. The organic layer was sequentially washed
with water and a
165
CA 03021185 2018-10-16
A00039
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The mixture was purified by NH silica gel column chromatography (methanoUethyl
acetate), and
the residue was washed with ethyl acetate/lPE to obtain the title compound (27
mg).
1H NMR (300 MHz, DMSO-d6) 62.60 (3H, s), 3.80 (3H, s), 4.02 (3H, s), 7.08 (1H,
d, J
= 2.7 Hz), 7.99-8.12 (2H, m), 8.23 (1H, s), 8.53 (1H, d, J = 2.1 Hz).
[0396]
Example 274
1-((5-(1,1-(difluoroethyl)-1,3,4-oxadiazol-2-y1)methyl)-6-(4-
methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
a) 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yOmethyl)-5-(1,1-
difluoroethyl)-
1,3,4-oxadiazole
Propylphosphonic anhydride (50% in ethyl acetate solution) (2.57 mL) was added
to a
suspension of triethylamine (0.859 mL), 2-(6-bromo-2-methy1-1H-imidazo[4,5-
b]pyridin-1-
yl)acetohydrazide (350 mg) and 2,2-difluoropropanoic acid (200 mg) in ethyl
acetate (2 mL) at
room temperature. The mixture was stirred under microwave irradiation at 150 C
for 30
minutes. The residue was purified by NH silica gel column chromatography
(ethyl
acetate/hexane) to obtain the title compound (135 mg).
11-1 NMR (300 MHz, DMSO-d6) 6 2.15 (3H, t, J = 19.5 Hz), 2.63 (31-1, s), 5.97
(2H, s),
8.39 (1H, d, J = 2.2 Hz), 8.45 (1H, d, J = 2.2 Hz).
[0397]
b) 145-( 1 ,1-difluoroethyl)-1,3,4-oxadiazol-2-yOmethyl)-6-(4-
methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (135 mg), 24(6-bromo-2-methy1-1 H-imidazo[4,5-b]pyridin-1-
ypmethyl)-5-(1,1-
difluoroethyl)-1,3,4-oxadiazole (130 mg), bis (di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (15 mg), cesium carbonate
(2 M in
aqueous solution) (300 ttL) and DME (4 mL) was stirred under microwave
irradiation at 100 C
for 40 minutes. The residue was purified by NH silica gel column
chromatography
(methanol/ethyl acetate) and silica gel column chromatography (methanol/ethyl
acetate) to
obtain a solid. The solid was purified by preparative frpLc (C18, mobile
phase:
water/acetonitrile (10 mM ammonium hydrogen carbonate-containing system)), and
the target
fraction was concentrated under reduced pressure, and then crystallized from
ethanoUwater to
obtain the title compound (38.8 mg).
11-1NMR (300 MHz, DMSO-d6) 6 2.15 (3H, t, J = 19.5 Hz), 2.67 (3H, s), 3.96
(3H, s),
166
CA 03021185 2018-10-16
A00039
6.01 (2H, s), 7.05 (1H, d, J = 2.7 Hz), 8.07 (1H, d, J = 2.7 Hz), 8.23 (1H,
s), 8.24 (1H, d, J = 2.1
Hz), 8.59 (1H, d, J = 2.1 Hz).
[0398]
Example 276
1-((5-(2,2-difluorocyclopropy1)-1,3,4-oxadiazol-2-yl)methyl)-6-(4-
methoxypyrrolo [2,1-
f][1,2,4]triazin-5-y1)-2-methy1-1H-imidazo[4,5-b]pyridine
a) 24(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yOmethyl)-5-(2,2-
difluorocyclopropy1)-1,3,4-oxadiazole
Propylphosphonic anhydride (50% in ethyl acetate solution) (2.200 mL) was
added to a
suspension of triethylamine (0.736 mL), 2-(6-bromo-2-methyl-1H-imidazo[4,5-
b]pyridin-1 -
yl)acetohydrazide (300 mg) and 2,2-difluorocyclopropanecarboxylic acid (150
mg) in ethyl
acetate (6 mL) at room temperature. The mixture was stirred under microwave
irradiation at
150 C for 30 minutes. The residue was purified by NH silica gel column
chromatography
(methanol/ethyl acetate) to obtain the title compound (230 mg).
IFINMR (300 MHz, DMSO-d6) 8 2.04-2.23 (1H, m), 2.25-2.44 (1H, m), 2.61 (3H,
s),
3.41-3.60 (1H, m), 5.89 (2H, s), 8.37 (1H, d, J =- 2.1 Hz), 8.44 (1H, d, J =
2.1 Hz).
[0399]
b) 14(5-(2,2-difluorocyclopropy1)-1,3,4-oxadiazol-2-yl)methyl)-6-(4-
methoxypyrrolo[2,14][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (550 mg), 24(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
yOmethyl)-5-(2,2-
difluorocyclopropyl)-1,3,4-oxadiazole (220 mg), bis (di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (20 mg), cesium carbonate
(2 M in
aqueous solution) (300 1.1L) and DME (4 mL) was stirred under microwave
irradiation at 100 C
.. for 40 minutes. The residue was purified by silica gel column
chromatography (ethyl
acetate/hexame and methanol/ethyl acetate) to obtain the title compound (98
mg).
IFINMR (300 Ml-[z, DMSO-d6) 8 2.09-2.23 (1H, m), 2.28-2.39 (1H, m), 2.66 (3H,
s),
3.42-3.57 (1H, m), 3.96 (3H, s), 5.92 (2H, s), 7.06 (1H, d, J = 2.7 Hz), 8.07
(1H, d, J = 2.7 Hz),
8.21 (1H, d, J = 2.1 Hz), 8.23 (1H, s), 8.58 (1H, d, J = 2.1 Hz).
[0400]
Example 277
1-45-(2-fluoropropan-2-y1)-1,3,4-oxadiazol-2-yOmethyl)-6-(4-methoxypyrrolo
[2,1-
f][1,2,4]triazin-5-y1)-2-methy1-1H-imidazo[4,5-b]pyridine
a) 246-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-l-y1)methyl)-5-(2-fluoropropan-
2-
167
CA 03021185 2018-10-16
A00039
y1)-1,3,4-oxadiazole
Propylphosphonic anhydride (50% in ethyl acetate solution) (2.200 mL) was
added to a
suspension of triethylamine (0.736 mL), 2-(6-bromo-2-methy1-1H-imidazo[4,5-
b]pyridin-1-
y1)acetohydrazide (300 mg) and 2-fluoro-2-methylpropanoic acid (127 mg) in
ethyl acetate (6
mL) at room temperature. The mixture was stirred under microwave irradiation
at 130 C for 30
minutes. The residue was purified by NH silica gel column chromatography
(methanol/ethyl
acetate) to obtain the title compound (224 mg).
1H NMR (300 MHz, DMSO-d6) 6 1.79 (6H, d, J = 21.9 Hz), 2.62 (3H, s), 5.93 (2H,
s),
8.39 (1H, d, J = 2.2 Hz), 8.45 (1H, d, J = 2.2 Hz).
[0401]
b) 1-((5-(2-fluoropropan-2-y1)-1,3,4-oxadiazol-2-yl)methyl)-6-(4-
methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (300 mg), 2-46-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
yOmethyl)-5-(2-
fluoropropan-2-y1)-1,3,4-oxadiazole (110 mg), bis (di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (10 mg), cesium carbonate
(2 M in
aqueous solution) (300 L) and DME (4 mL) was stirred under microwave
irradiation at 100 C
for 40 minutes. The residue was purified by silica gel column chromatography
(ethyl
acetate/hexane and methanol/ethyl acetate) to obtain a solid. The obtained
solid was
crystallized from ethyl acetate/ethanol/heptane to obtain the title compound
(47.0 mg).
1H NMR (300 MHz, DMSO-d6) 61.78 (611, d, J = 21.9 Hz), 2.67(311, s), 3.95 (3H,
s),
5.97 (2H, s), 7.05 (1H, d, J = 2.7 Hz), 8.07 (1H, d, J = 2.7 Hz), 8.18-8.27
(2H, m), 8.59 (1H, d, J
= 2.0 Hz).
[0402]
Example 279
1-((5-(1-fluoroethyl)-1,3,4-oxadiazol-2-yOmethyl)-6-(4-methoxypyrrolo [2,1-
fl[1,2,4]triazin-5-y1)-2-methy1-1H-imidazo[4,5-b]pyridine
a) 24(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-5-(1-fluoroethyl)-
1,3,4-oxadiazole
A mixture of 2-fluoropropanoic acid (137 mg), Et3N (0.86 mL), propylphosphonic
anhydride (50% in ethyl acetate solution) (2.2 mL), 2-(6-bromo-2-methy1-1H-
imidazo[4,5-
b]pyridin-l-ypacetohydrazide (350 mg) and ethyl acetate (2.0 mL) was stirred
under microwave
irradiation at 135 C for 30 minutes. The mixture was purified by NH silica gel
column
chromatography (methanol/ethyl acetate) to obtain the title compound (188 mg).
168
CA 03021185 2018-10-16
A00039
MS: [M+H] 340Ø
[0403]
b) 1-((5-(1-fluoroethyl)-1,3,4-oxadiazol-2-y1)methyl)-6-(4-methoxypyrrolo[2,1-
fl[1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
A mixture of 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-5-(1-
fluoroethyl)-1,3,4-oxadiazole (186 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
yl)pyrrolo[2,1-f][1,2,4]triazine (500 mg) bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (30 mg), cesium carbonate
(2 M in
aqueous solution) (0.478 mL) and DME (2.5 mL) was stirred under microwave
irradiation at
100 C for 1 hour. The mixture was purified by NH silica gel column
chromatography
(hexane/ethyl acetate), and the obtained solid was washed with IPE to obtain
the title compound
(80 mg).
1HNMR (300 MHz, DMSO-d6) 8 1.60-1.81 (3H, m), 2.67 (3H, s), 3.96 (3H, s), 5.85-
6.13 (3H, m), 7.06 (1H, d, J = 2.7 Hz), 8.07 (1H, d, J = 2.8 Hz), 8.18-8.27
(2H, m), 8.59 (1H, d, J
= 2.1 Hz).
[0404]
Example 280
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methy1-1-(1,2,3-thiazol-4-
ylmethyl)-
1H-imidazo[4,5-b]pyridine
a) N-(2-amino-5-bromopyridin-3-y1)-1,2,3-thiadiazole-4-carboxamide
A mixture of 5-bromopyridine-2,3-diamine (723 mg), 1,2,3-thiadiazole-4-
carboxylic
acid (500 mg), HATU (2050 mg), Et3N (1.6 mL) and DMF (8 mL) was stirred
overnight at room
temperature. The mixture was diluted with a saturated aqueous sodium hydrogen
carbonate
solution, and extracted with ethyl acetate. The organic layer was washed with
a saturated brine,
then dried over magnesium sulfate, and concentrated under reduced pressure.
The residue was
purified by silica gel column chromatography (ethyl acetate/hexane) to obtain
the title compound
(754 mg). The title compound was used for the subsequent reaction without
being further
purified.
MS: [M+11] 299.9.
[0405]
b) N3-((1,2,3-thiadiazol-4-yl)methyl)-5-bromopyridine-2,3-diamine
A BH3-THF complex (1M in THF solution) (10 mL) was added dropwise to a
solution
of N-(2-amino-5-bromopyridin-3-y1)-1,2,3-thiadiazole-4-carboxamide (754 mg) in
THF (10 mL)
at room temperature. The mixture was stirred under a nitrogen atmosphere at 60
C for 2 hours.
169
CA 03021185 2018-10-16
A00039
The reaction was stopped with water at room temperature, and the mixture was
then acidified
with HCI (1 N in aqueous solution). The mixture was stirred at 60 C for 30
minutes. The
mixture was neutralized with a saturated aqueous sodium hydrogen carbonate
solution at room
temperature, and then extracted with ethyl acetate. The organic layer was
washed with a
saturated brine, then dried over magnesium sulfate, and concentrated under
reduced pressure.
The residue was purified by silica gel column chromatography (ethyl
acetate/methanol) to obtain
the title compound (135 mg).
MS: [M+Hr 286.2.
[0406]
c) 44(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yOmethyl)-1,2,3-thiadiazole
A mixture of N3-((1,2,3-thiadiazol-4-yl)methyl)-5-bromopyridine-2,3-diamine
(135
mg), acetic acid (0.045 mL), DMAP (8 mg), propylphosphonic anhydride (1.7 M in
ethyl acetate
solution) (0.505 mL), DIPEA (0.164 mL) and TI-IF (1.5 mL) was stirred under
microwave
irradiation at 140 C for 50 minutes. The residue was purified by NH silica gel
column
chromatography (methanol/ethyl acetate) to obtain the title compound (90 mg).
MS: [M+Hr 309.9.
[0407]
d) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1-(1,2,3-thiazol-4-
ylmethyl)-1H-imidazo[4,5-b]pyridine
A mixture of 4-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yl)methyl)-1,2,3-
thiadiazole (89 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (317 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (H) (19 mg), cesium carbonate
(2 M in
aqueous solution) (0.30 mL) and DME (1.5 mL) was stirred under microwave
irradiation at
100 C for 1 hour. The mixture was purified by NH silica gel column
chromatography
(hexane/ethyl acetate), and the obtained solid was washed with IPE to obtain
the title compound
(32.0 mg).
1H NMR (300 MHz, DMSO-d6) 6 2.75 (3H, s), 3.92 (3H, s), 6.01 (2H, s), 7.05
(1H, d, J
= 2.8 Hz), 8.06 (1H, d, J = 2.7 Hz), 8.22 (1H, s), 8.27 (1H, d, J = 2.1 Hz),
8.55 (1H, d, J = 2.0
Hz), 9.29 (1H, s).
[0408]
Example 281
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methy1-1-05-(2,2,2-
trifluoroethyl)-
1,3,4-oxadiazol-2-yl)methyl)-1H-imidazo[4,5-b]pyridine
170
CA 03021185 2018-10-16
A00039
a) N'-(2-(6-bromo-2-methyl-1H-imidazo[4,5 -b]pyridin-l-yl)acety1)-3,3,3 -
trifluoropropanehydrazide
3,3,3-trifluoropropanoyl chloride (185 mg) was added to a solution of 2-(6-
bromo-2-
methy1-1H-imidazo[4,5-b]pyridin-l-ypacetohydrazide (300 mg) in DMA (3 mL) at
room
temperature, and the mixture was stirred for 1 hour. The reaction mixture was
purified by NH
silica gel column chromatography (methanol/ethyl acetate) to obtain the title
compound (370
mg).
MS: [M-1-Hr 393.9.
[0409]
b) 2-((6-bromo-2-methyl-1H-imidazo[4,5-b]pyridin- 1 -yOmethyl)-5-(2,2,2-
trifluoroethyl)-1,3,4-oxadiazole
pTsC1 (470 mg) was added to a mixture of TEA (600 p.1), trimethylamine
monohydrochloride (198 mg), N'-(2-(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
ypacety1)-
3,3,3-trifluoropropanehydrazide (369.9 mg) and acetonitrile (10 ml) at room
temperature, and the
mixture was stirred overnight at room temperature. The reaction mixture was
purified by silica
gel column chromatography (ethyl acetate/hexame and methanol/ethyl acetate) to
obtain the title
compound (210 mg).
11-INNIR (300 Wiz, DMSO-d6) S 2.61 (3H, s), 4.32 (2H, d, J = 10.6 Hz), 5.95
(2H, s),
8.37 (1H, d, J = 2.2 Hz), 8.45 (1H, d, J = 2.2 Hz).
[0410]
c) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-145-(2,2,2-
trifluoroethyl)-
1,3,4-oxadiazol-2-yl)methyl)-1H-imidazo[4,5-b]pyridine
A mixture of 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-l-y1)methyl)-5-
(2,2,2-
trifluoroethyl)-1,3,4-oxadiazole (210 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-
1,3,2-dioxaborolan-
2-yl)pyrrolo[2,1-f][1,2,4]triazine (676 mg) bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (20 mg), cesium carbonate
(2 M in
aqueous solution) (0.40 mL) and DME (4.0 mL) was stirred under microwave
irradiation at
100 C for 20 minutes. The reaction mixture was purified by silica gel column
chromatography
(ethyl acetate/hexane and methanol/ethyl acetate), and the residue was
crystallized from
ethanol/ethyl acetate/heptane to obtain the title compound (49.6 mg).
1H NMR (300 MHz, DMSO-d6) 8 2.66 (3H, s), 3.95 (3H, s), 4.27-4.41 (2H, m),
5.98
(2H, s), 7.06 (1H, d, J = 2.7 Hz), 8.07 (1H, d, J = 2.7 Hz), 8.21 (1H, d, J =
2.1 Hz), 8.23 (1H, s),
8.59 (1H, d, J = 2.1 Hz).
[0411]
171
CA 03021185 2018-10-16
A00039
Example 282
6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methy1-1-(1,2,3-thiazol-5-
ylmethyl)-
1H-imidazo[4,5-b]pyridine
a) N-(2-amino-5-bromopyridin-3-y1)-1,2,3-thiadiazole-5-carboxamide
TEA (2.4 ml) was added to a mixture of 5-bromopyridine-2,3-diamine (1.08 g),
1,2,3-
thiadiazole-5-carboxylic acid (760 mg), HATU (2.93 g) and DMF (12 ml) at room
temperature,
and the mixture was stirred overnight. The reaction mixture was diluted with a
saturated
aqueous sodium hydrogen carbonate solution, and extracted with ethyl acetate.
The organic
layer was washed with a saturated brine, dried over sodium sulfate, and
concentrated under
.. reduced pressure. The residue was purified by silica gel column
chromatography (ethyl
acetate/hexane) to obtain the title compound (0.900 g).
IHNMR (300 MHz, DMSO-d6) 6 6.36 (2H, s), 7.74 (1H, d, J = 2.3 Hz), 7.98 (1H,
d, J =
2.3 Hz), 9.49 (1H, s), 10.34 (1H, s).
[0412]
b) N34(1,2,3-thiadiazol-5-yl)methyl)-5-bromopyridine-2,3-diamine
A borane-THF complex (1.0 M) (10 ml) was added to a mixture of N-(2-amino-5-
bromopyridin-3-y1)-1,2,3-thiadiazole-5-carboxamide (0.90 g) and THF (10 ml) at
0 C, and the
mixture was stirred overnight under a nitrogen atmosphere at room temperature.
To the
reaction mixture were added methanol and hydrochloric acid (1N) at 0 C, and
the mixture was
stirred at room temperature for 3 hours, and concentrated under reduced
pressure. The residue
was poured into an aqueous potassium carbonate solution, and extracted with
ethyl acetate.
The organic layer was washed with water and a saturated brine, dried over
sodium sulfate, and
concentrated under reduced pressure. The residue was purified by silica gel
column
chromatography (ethyl acetate/hexane) to obtain the title compound (0.330 g).
NMR (300 MHz, DMSO-d6) 6 4.83 (2H, d, J = 5.9 Hz), 5.77 (2H, s), 5.96 (1H, t,
J =
5.9 Hz), 6.76 (1H, d, J =2.1 Hz), 7.37 (1H, d, J = 2.1 Hz), 8.92 (1H, s).
[0413]
c) 5-((6-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-1-yl)methyl)-1,2,3-
thiadiazole
A mixture of acetic acid (130 1), DMAP (5.0 mg), DMA (0.403 ml), N3-((1,2,3-
thiadiazol-5-yl)methyl)-5-bromopyridine-2,3-diamine (330 mg), propylphosphonic
anhydride
(50% in ethyl acetate solution) (1.2 ml) and THF (6.0 ml) was stirred under
microwave
irradiation at 150 C for 1 hour. The residue was purified by silica gel column
chromatography
(ethyl acetate/hexame and methanol/ethyl acetate) to obtain the title compound
(220 mg).
IHNMR (300 MHz, DMSO-d6) 6 2.61 (3H, s), 6.04 (2H, d, J = 0.7 Hz), 8.44 (2H,
d, J =
172
CA 03021185 2018-10-16
A00039
1.3 Hz), 8.90 (1H, s).
[0414]
d) 6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1-(1,2,3-thiazol-5-
ylmethyl)-1H-imidazo[4,5-b]pyridine
A mixture of 5-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-1,2,3-
thiadiazole (210 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (820 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (40 mg), cesium carbonate
(2 M in
aqueous solution) (0.45 mL) and DME (4.0 mL) was stirred under microwave
irradiation at
.. 100 C for 50 minutes. The reaction mixture was purified by silica gel
column chromatography
(ethyl acetate/hexane and methanol/ethyl acetate) and NH silica gel column
chromatography
(methanol/ethyl acetate), and the residue was crystallized from ethanol/ethyl
acetate/heptane to
obtain the title compound (49.6 mg).
1HNMR (300 MHz, DMSO-d6) 5 2.66 (3H, s), 3.85 (3H, s), 6.08 (2H, s), 7.06 (1H,
d, J
= 2.8 Hz), 8.07 (1H, d, J = 2.7 Hz), 8.20-8.23 (2H, m), 8.58 (1H, d, J = 2.0
Hz), 8.92 (1H, s).
[0415]
Example 283
1-((5-(1-fluorocyclopropy1)-1,3,4-oxadiazol-2-y1)methyl)-6-(4-
methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
a) 2-46-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yl)methyl)-5-(1-
fluorocyclopropy1)-1,3,4-oxadiazole
Propylphosphonic anhydride (50% in ethyl acetate solution) (0.917 mL) was
added to a
suspension of triethylamine (0.307 mL), 2-(6-bromo-2-methy1-1H-imidazo[4,5-
b]pyridin-1-
y1)acetohydrazide (125 mg) and 1-fluorocyclopropanecarboxylic acid (50 mg) in
ethyl acetate (2
mL) at room temperature. The mixture was stirred under microwave irradiation
at 150 C for 30
minutes. The residue was purified by NH silica gel column chromatography
(ethyl
acetate/hexane) to obtain the title compound (84 mg).
IHNIVIR (300 MHz, DMSO-d6) ö 1.32-1.44 (2H, m), 1.61-1.77 (2H, m), 2.62 (3H,
s),
5.90 (2H, s), 8.38 (1H, d, J = 2.2 Hz), 8.44 (11-1, d, J = 2.2 Hz).
[0416]
b) 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yOmethyl)-5-(1-
fluorocyclopropy1)-1,3,4-oxadiazole
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (245 mg), 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
yl)methyl)-5-(1-
173
CA 03021185 2018-10-16
A00039
fluorocyclopropy1)-1,3,4-oxadiazole (84 mg), bis (di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (20 mg), cesium carbonate
(2 M in
aqueous solution) (220 L) and DME (4 mL) was stirred under microwave
irradiation at 100 C
for 40 minutes. The residue was purified by NH silica gel column
chromatography (ethyl
acetate/hexane and methanol/ethyl acetate) to obtain a solid. The obtained
solid was washed
with ethyl acetate/hexane to obtain the title compound (32.0 mg).
1H NMR (300 MHz, DMSO-d6) 1.31-1.45 (2H, m), 1.61-1.76 (2H, m), 2.66 (3H, s),
3.97 (31-1, s), 5.93 (2H, s), 7.06 (1H, d, J = 2.7 Hz), 8.07 (1H, d, J = 2.7
Hz), 8.20-8.27 (2H, m),
8.59 (1H, d, J = 2.1 Hz).
[0417]
Example 284
1-((5-(1-fluorocyclobuty1)-1,3,4-oxadiazol-2-yl)methyl)-6-(4-
methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
a) 246-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-5-(1-
fluorocyclobuty1)-1,3,4-oxadiazole
Propylphosphonic anhydride (50% in ethyl acetate solution) (0.917 mL) was
added to a
suspension of triethylamine (0.307 mL), 2-(6-bromo-2-methy1-1H-imidazo[4,5-
b]pyridin-1-
y1)acetohydrazide (125 mg) and 1-fluorocyclobutanecarboxylic acid (50 mg) in
ethyl acetate (2
mL) at room temperature. The mixture was stirred under microwave irradiation
at 150 C for 30
.. minutes. The residue was purified by NH silica gel column chromatography
(ethyl
acetate/hexane) to obtain the title compound (101 mg).
11-INMR (300 MHz, DMSO-d6) Ei 1.57-1.76 (1H, m), 1.86-2.04 (1H, m), 2.57-
2.77(7H,
m), 5.93 (2H, s), 8.39 (1H, d, J = 2.2 Hz), 8.45 (1H, d, J = 2.2 Hz).
[0418]
b) 1-((5-(1-fluorocyclobuty1)-1,3,4-oxadiazol-2-yl)methyl)-6-(4-
methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
1][1,2,4]triazine (283 mg), 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
yOmethyl)-5-(1-
fluorocyclobutyl)-1,3,4-oxadiazole (101 mg), bis (di-tert-buty1(4-
.. dimethylaminophenyl)phosphine)dichloropalladium (II) (10 mg), cesium
carbonate (2 M in
aqueous solution) (250 L) and DME (4 mL) was stirred under microwave
irradiation at 100 C
for 40 minutes. The residue was purified by NH silica gel column
chromatography (ethyl
acetate/hexane and methanol/ethyl acetate) to obtain a solid. The obtained
solid was washed
with ethyl acetate/hexane to obtain the title compound (42.0 mg).
174
CA 03021185 2018-10-16
A00039
1H NMR (300 MHz, DMSO-d6) 5 1.55-1.76 (1H, m), 1.84-2.05 (1H, m), 2.58-2.76
(7H,
m), 3.95 (3H, s), 5.96 (2H, s), 7.05 (1H, d, J = 2.7 I-1z), 8.07 (1H, d, J =
2.7 Hz), 8.19-8.27 (2H,
m), 8.59 (1H, d, J = 2.0 Hz).
[0419]
Example 286
1-((5-((1R)-1-fluoroethyl)-1,3,4-oxadiazol-2-y1)methyl)-6-(4-
methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-IH-imidazo[4,5-b]pyridine (optical isomer)
a) 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yOmethyl)-5-(1-fluoroethyl)-
1,3,4-oxadiazole
Propylphosphonic anhydride (50% in ethyl acetate solution) (6.0 mL) was added
to a
mixture of 2-fluoropropionic acid (0.58 mL), DIEA (3.0 mL), 2-(6-bromo-2-
methy1-1H-
imidazo[4,5-b]pyridin-l-yl)acetohydrazide (1.33 g) and ethyl acetate (40 ml)
at room
temperature, and the resulting mixture was stirred at 50 C for 1 hour. The
mixture was stirred
under microwave irradiation at 140 C for 2.5 hours. Insolubles were removed by
filtration
through a filter, and the filtrate was concentrated. The residue was purified
by NH silica gel
column chromatography (methanol/ethyl acetate), and washed with IPE to obtain
the title
compound (0.644 g).
NMR (300 MHz, DMSO-d6) S 1.64-1.77 (3H, m), 2.62 (3H, s), 5.86-6.12 (3H, m),
8.38 (1H, d, J = 2.2 Hz), 8.45 (1H, d, J = 2.2 Hz).
[0420]
b) 14(5-(1-fluoroethyl)-1,3,4-oxadiazol-2-yOmethyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
A mixture of 2-((6-bromo-2-methy1-1}1-imidazo[4,5-b]pyridin-1-y1)methyl)-5-(1-
fluoroethyl)-1,3,4-oxadiazole (186 mg), 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
yl)pyrrolo[2,1-f][1,2,4]triazine (500 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (30 mg), cesium carbonate
(2 M in
aqueous solution) (0.478 ml) and DME (2.5 ml) was stirred under microwave
irradiation at
100 C for 1 hour. The reaction mixture was purified by NH silica gel column
chromatography
(ethyl acetate/hexane), and the residue was washed with WE to obtain the title
compound (80
mg).
NMR (300 MHz, DMSO-d6) 5 1.56-1.86 (3H, m), 2.67 (3H, s), 3.96 (3H, s), 5.98
(3H, s), 7.06 (1H, d, J = 2.7 Hz), 8.07 (1H, d, J = 2.8 Hz), 8.19-8.35 (2H,
m), 8.59 (1H, d, J = 2A
Hz).
[0421]
175
CA 03021185 2018-10-16
A00039
c) 1-((5-((1R)-1-fluoroethyl)-1,3,4-oxadiazol-2-yOmethyl)-6-(4-
methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine (optical isomer)
A racemate of 1-((5-(1-fluoroethyl)-1,3,4-oxadiazol-2-yOmethyl)-6-(4-
methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
(66.5 mg) was
fractionated by SFC (column: CH1RALPAK ASH (LA005) 20 x 250 mm, 5 gm, mobile
phase:
CO2/methano1=800/200), and a compound with a smaller retention time was
crystallized with
ethanol/ethyl acetate/heptane to obtain the title compound (12.8 mg).
1H NMR (300 MHz, CDC13) 6 1.72-1.89 (3H, m), 2.84 (3H, s), 4.06 (3H, s), 5.57
(2H,
s), 5.61-5.87 (1H, m), 6.87 (1H, d, J = 2.8 Hz), 7.77 (1H, d, J = 2.7 Hz),
7.95 (111, d, J = 2.0 Hz),
8.07 (1H, s), 8.75 (1H, d, J = 2.0 Hz).
[0422]
Another method for producing 1-((5-((1R)-1-fluoroethyl)-1,3,4-oxadiazol-2-
y1)methyl)-
6-(4-methoxypyrrolo [2,1-f] [1,2,4]triazin-5-y1)-2-methy1-1H-imidazo [4,5-
b]pyridine
monohydrate (identical to the title compound in Example 286) is shown below.
a) (4-methoxypyrrolo[2,14][1,2,4]triazin-5-yl)boronic acid
To a solution of 5-bromo-4-methoxypyrrolo[2,1-f][1,2,4]triazine (60 g) and 2-
isopropoxy-4,4,5,5-tetramethy1-1,3,2-dioxaborolane (243 mL) in THF (3000 mL)
was added
dropwise n-butyllithium (1.6 M in hexane solution) (411 mL) under an argon
atmosphere at -
78 C to -60 C over 45 minutes. The mixture was stirred at -70 C to -60 C for
30 minutes
under an argon atmosphere. To the reaction mixture were added water (140 mL)
and
hydrochloric acid (2 N in aqueous solution) (600 mL), and the mixture was
warmed to room
temperature. The aqueous layer was extracted with isopropyl acetate. The
organic layer was
inversely extracted with a 2N aqueous sodium hydroxide solution (700 mL) and
water (300 mL).
The aqueous layer was acidified with hydrochloric acid (2N in aqueous
solution) (7100 mL) at
10 C, and the suspension was filtered. The obtained residue was washed with
water to obtain
the title compound (40.5 g).
IHNMR (300 MHz, DMSO-d6) 6 4.13 (311, s), 7.07 (1H, d, J = 2.3 Hz), 7.68 (2H,
s),
7.94 (1H, d, J = 2.6 Hz), 8.24 (1H, s).
[0423]
b) (R)-2-fluoropropanoic acid
(R)-Ethyl 2-fluoropropanoate (95 g) was suspended in 10% sulfuric acid (950
mL), and
heated and refluxed for 3 hours. After cooled, sodium chloride was added to
saturate the
aqueous layer, and the aqueous layer was extracted with t-butyl methyl ether
(900 mL x 4). The
obtained organic layer was dried over magnesium sulfate, and concentrated
under reduced
176
CA 03021185 2018-10-16
A00039
pressure to obtain the title compound (124 g, containing t-butyl methyl
ether).
1H NMR (300 MHz, DMSO-d6) 6 1.35-1.56 (311, m), 4.91-5.21 (1H, m), 13.19 (1H,
brs).
[0424]
c) (S)-2-amino-3-phenylpropane-1-01 (R)-2-fluoropropanoate
To a solution of (S)-2-amino-3-phenylpropan-l-ol (119 g) in ethanol (360 mL)
and
acetonitrile (1090 mL) was added dropwise a solution of (R)-2-fluoropropanoic
acid (72.7 g) in
acetonitrile (1090 mL) at 65 C to 70 C. The mixture was stirred at 60 C for 1
hour, and further
stirred at room temperature for 1 hour. Precipitated crystals were collected
by filtration, and
washed with acetonitrile (500 mL) to obtain white crystals (170 g). The
obtained crystals (140
g) were dissolved in ethanol (700 mL) at 60 C, and to the solution was added
acetonitrile (4200
mL) at 58 C to 65 C. The mixture was stirred at 60 C for 1 hour. The mixture
was cooled to
room temperature, and then stirred overnight at room temperature. The obtained
solid was
collected by filtration, and washed with acetonitrile to obtain the title
compound (109 g).
[0425]
d) (R)-2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-y1)methyl)-5-(1-
fluoroethyl)-
1,3,4-oxadiazole
(S)-2-Amino-3-phenylpropan-1-ol (R)-2-fluoropropanoate (109 g) was dissolved
in
hydrochloric acid ON in aqueous solution) (1500 mL) and a saturated brine
(1500 mL), and the
solution was extracted with t-butyl methyl ether (1000 mL x 4). The organic
layer was dried
over magnesium sulfate, and concentrated under reduced pressure to obtain a
colorless oil.
Propylphosphonic anhydride (50% in ethyl acetate solution) (419 mL) was added
to a suspension
of the obtained oil, 2-(6-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-1-
yl)acetohydrazide (100 g),
D1PEA (246 mL) and butyl acetate (3000 mL) at room temperature. The mixture
was stirred at
50 C for 30 minutes, propylphosphonic anhydride (50% in ethyl acetate
solution) (210 mL) was
added to the mixture, and the resulting mixture was heated and refluxed for 3
hours. The
mixture was cooled, a saturated aqueous sodium hydrogen carbonate solution
(3000 mL) was
then added to the mixture, and insolubles were removed. The liquid layer was
extracted twice
with ethyl acetate (1500 mL x 2), and the organic layer was washed with water
and a saturated
brine. The organic layer was purified with NH silica gel (ethyl acetate). The
residue was
concentrated under reduced pressure, and the obtained solid was washed with
IPE (3000 mL) to
obtain the title compound (57.8 g).
114 NMR (300 MHz, DMSO-d6) 6 1.62-1.79 (3H, m), 2.62 (3H, s), 5.83-6.14 (3H,
m),
8.38 (1H, d, J = 1.9 Hz), 8.45 (1H, d, J = 1.9 Hz).
177
CA 03021185 2018-10-16
A00039
[0426]
e) 1-((5-((lR)-1-fluoroethyl)-1,3,4-oxadiazol-2-yOmethyl)-6-(4-
methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine monohydrate
A mixture of (4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-yl)boronic acid (79 g),
(R)-2-((6-
bromo-2-methyl-1H-imidazo[4,5-b]pyridin-l-yOmethyl)-5-(1-fluoroethyl)-1,3,4-
oxadiazole (100
g), bis(di-tert-buty1(4-dimethylaminophenyl)phosphine)dichloropalladium (II)
(2.00 g), cesium
carbonate (2 M in aqueous solution) (295 mL) and DME (2000 mL) was stirred at
80 C for 1
hour. The mixture was cooled to 50 C, and then diluted with THF (1000 mL). The
mixture
was poured into an aqueous sodium hydrogen carbonate solution (1600 mL), and
extracted with
ethyl acetate (1000 mL x 3). The organic layer was washed with a 5% aqueous
ammonia
solution (1600 mL x 2) and a saturated brine (1600 mL), dried over magnesium
sulfate, and
concentrated under reduced pressure to obtain a yellow solid. NH silica gel
(2400 g) was added
to a solution of the obtained solid in THF (8000 mL) and water (200 mL), and
the mixture was
stirred at room temperature for 3.5 hours. Insolubles were removed, and washed
with THE (15
L). The obtained solution was concentrated under reduced pressure to obtain a
yellow solid.
The obtained solid was washed with t-butyl methyl ether to obtain pale yellow
crystals (98 g).
A mixture of the obtained crystals (115 g), activated carbon (Ecosorb) (33 g),
ethanol/water = 9/1 (2200 mL) and water (1100 mL) was stirred at 55 C for 1
hour. Insolubles
were removed, and washed with ethanol (550 mL). The obtained solution was
diluted with
water (1600 mL) at 55 C, and stirred overnight at room temperature. The
mixture was cooled
to 5 C, and then stirred for 3 hours. The obtained solid was collected by
filtration, and washed
with ethanol/water = 1/1 (1000 mL) to obtain the title compound (88 g).
1HNMR (300 MHz, DMSO-d6) 6 1.60-1.83 (3H, m), 2.67 (3H, s), 3.96 (3H, s), 5.83-
6.19 (3H, m), 7.06 (1H, d, J = 2.5 Hz), 8.06 (1H, d, J = 2.5 Hz), 8.17-8.30
(2H, m), 8.59 (1H, d, J
= 2.0 Hz).
[0427]
Example 287
1-((5-((1S)-1-fluoroethyl)-1,3,4-oxadiazol-2-yOmethyl)-6-(4-methoxypyrrolo[2,1-
1][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine (optical isomer)
A racemate of 1-((5-(1-fluoroethyl)-1,3,4-oxadiazol-2-yOmethyl)-6-(4-
methoxypyrrolo[2,1-f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
(66.5 mg) was
fractionated by SFC (column: CHLRALPAK ASH (LA005) 20 x 50 mm, 5 pm, mobile
phase:
CO2/methano1=800/200), and a compound with a larger retention time was
crystallized with
ethanol/ethyl acetate/heptane to obtain the title compound (15.7 mg).
178
CA 03021185 2018-10-16
A00039
NMR (300 MHz, CDC13) 6 L72-1.87 (3H, m), 2.84 (3H, s), 4.06 (3H, s), 5.57 (2H,
s), 5.62-5.87 (1H, m), 6.87 (1H, d, J = 2.8 Hz), 7.77 (1H, d, Jr= 2.7 Hz),
7.94 (1H, d, J = 1.9 Hz),
8.07 (1H, s), 8.75 (1H, d, J = 1.9 Hz).
[0428]
Example 288
1-((5-(difluoromethyl)-1,3,4-oxadiazol-2-yOmethyl)-6-(4-methoxypyrrolo[1,2-
b]pyridazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
a) 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yl)pyrrolo[1,2-
b]pyridazine
n-butyllithium (1.6 M in hexane solution) (4.5 mL) was added dropwise to a
solution of
5-bromo-4-methoxypyrrolo[1,2-b]pyridazine (1.20 g) in TI-if (30 mL) under a
nitrogen
atmosphere at -78 C. The mixture was stirred at -78 C for 20 minutes, and 2-
isopropoxy-
4,4,5,5-tetramethy1-1,3,2-dioxaborolane (1.8 mL) was then added. The mixture
was warmed to
room temperature, and stirred for 30 minutes. The reaction mixture was poured
into water, and
extracted with ethyl acetate. The organic layer was washed with a saturated
brine, then dried
over magnesium sulfate, and concentrated under reduced pressure. The residue
was purified by
silica gel column chromatography (ethyl acetate/hexane) to obtain the title
compound (0.707 g).
1HNMR (300 MHz, DMSO-d6) 6 1.28 (1211, s), 3.94 (3H, s), 6.30 (1H, d, J = 5.5
Hz),
6.87 (1H, d, J = 2.5 Hz), 7.75 (1H, d, J = 2.6 Hz), 8.11 (1H, d, J = 5.5 Hz).
[0429]
b) 1-45-(difluoromethyl)-1,3,4-oxadiazol-2-yOmethyl)-6-(4-methoxypyrrolo[1,2-
b]pyridazin-5-y1)-2-methyl-1H-imidazo[4,5-blpyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
b]pyridazine (91 mg), 24(6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-yOmethyl)-
5-
(difluoromethyl)-1,3,4-oxadiazole (120 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (11 mg), cesium carbonate
(2 M in
aqueous solution) (0.350 mL) and DME (3 mL) was stirred under microwave
irradiation at
100 C for 40 minutes. The residue was purified by silica gel column
chromatography (ethyl
acetate/hexane and methanol/ethyl acetate) to obtain a solid. The obtained
solid was
crystallized from ethanol/ethyl acetate/heptane to obtain the title compound
(57 mg).
IHNMR (300 MHz, DMSO-d6) 6 2.65 (3H, s), 3.81 (3H, s), 6.00 (2H, s), 6.27 (1H,
d, J
= 5.6 Hz), 6.91 (111, d, J = 2.8 Hz), 7.21-7.70 (1H, m), 7.90 (1H, d, J = 2.8
Hz), 8.04-8.13 (211,
m), 8.50 (1H, d, J = 2.0 Hz).
[0430]
Example 289
179
CA 03021185 2018-10-16
A00039
1-((5-(difluoromethyl)-1,3,4-thiadiazol-2-y1)methyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1H-imidazo[4,5-b]pyridine
a) 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-l-y1)methyl)-5-
(difluoromethyl)-
1,3,4-thiadiazole
A mixture of triethylamine (4.00 mL), difluoroacetic acid (0.50 mL),
propylphosphonic
anhydride (50% in ethyl acetate solution) (10.00 mL), 2-(6-bromo-2-methy1-1H-
imidazo[4,5-
b ]pyridin-l-yl)acetohydrazide (1.72 g), diphosphorus pentasulfide (3.33 g)
and ethyl acetate (50
mL) was heated overnight at 80 C. The mixture was purified by NH silica gel
column
chromatography (ethyl acetate). The obtained residue was purified by NH silica
gel column
chromatography (ethyl acetate/hexane) to obtain the title compound (0.711 g).
IH NMR (300 MHz, DMSO-d6) 6 2.63 (3H, s), 6.15 (2H, s), 7.33-7.78 (1H, m),
8.41-
8.50 (2H, m).
[0431]
b) 1-((5-(difluoromethyl)-1,3,4-thiadiazol-2-yOmethyl)-6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methy1-1H-imidazo[4,5-b]pyridine
A mixture of 4-methoxy-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-
yl)pyrrolo[2,1-
f][1,2,4]triazine (430 mg), 2-((6-bromo-2-methy1-1H-imidazo[4,5-b]pyridin-1-
y1)methyl)-5-
(difluoromethyl)-1,3,4-thiadiazole (150 mg), bis(di-tert-buty1(4-
dimethylaminophenyl)phosphine)dichloropalladium (II) (25 mg), cesium carbonate
(2 M in
aqueous solution) (500 4) and DME (10 mL) was stirred under microwave
irradiation at 100 C
for 2 hours. The residue was purified by silica gel column chromatography
(ethyl
acetate/hexane and methanol/ethyl acetate) to obtain a solid. The obtained
solid was washed
with ethanol/ethyl acetate/heptane to obtain the title compound (48.0 mg).
IH NMR (300 MHz, DMSO-d6) 6 2.68 (3H, s), 3.90 (3H, s), 6.18 (2H, s), 7.05
(1H, d, J
= 2.7 Hz), 7.34-7.78 (1H, m), 8.06 (1H, d, J = 2.7 Hz), 8.22 (1H, s), 8.24 (I
H, d, J = 2.0 Hz),
8.58 (1H, d, J = 2.0 Hz).
[0432]
The compounds of Examples 2 to 6, 8 and 9, 11, 14 to 16, 18 and 19, 25 and 26,
29 and
30, 36 and 37, 40 to 43, 45 to 47, 49 to 67, 69 to 78, 80 and 81, 83 to 95,
97, 99 to 101, 103 and
104, 106 to 109, 111 to 133, 135 to 155, 157, 163 to 174, 177 and 178, 180 to
185, 187 to 190,
192, 194 to 198, 200 to 202, 216 to 219, 221 to 228, 232 to 236, 238 to 241,
244 to 249, 251, 254
to 266, 270 to 272, 275, 278, 285 and 290 in the table below were produced in
accordance with
the methods shown in the above-described examples, or similar methods. The
example
compounds are shown in the table below. MS in the table indicates a measured
value.
180
CA 03021185 2018-10-16 A00039
..
[Table 1-1]
Example IUPAC NAME CHEMICAL
STRUCTURE Salt MS
N N.,- NI F
2-(azetidin-1-y1)-1-(3,5- r4
difluorobenzy1)-6-(4-methoxy-5H-
1 448.2
pyrrolo[3,2-d)pyrimidin-5-y)-1H- / N
imidazo[4.5-b]pyridine 0 F
--- `=-=
i
N --. N
--...--
N N /
¶
1-(3,4-difluorobenzy1)-6-(4-
\>-- NH
c_c i N
2
methoxy-5H-pyrrolo[32-
dipyrimidin-5-y1)-N-methy1-1H-
imidazo[4.5-b]pyridin-2-amine --- 0 F 422.2
N \ 1 \
F
1-(3,4-difluorobenzyI)- th 2-eoxy- I \>-0
6-(4-methoxy-5H-pyrrolo[3,2- / N '''' N
3
d)pyrimidin-5-y1)-1H-imidazo[4,5-
--- 0 ei F 437.2
b]pyridine -
\_- N
F
N F
2-(1,1-difluoroethyl)-1-(3-
s/1.1.Nr-lc--F
fluorobenzy1)-6-(4-methoxy-5 H-
4
\----Q¨ 439.2
PYrrolo[3,2-d]pyrimidin-5-yI)-1H- / N
imidazo[4,5-b]pyridine 0 F
i
N .... N
---.--
. ,
N N /
1-(3,4-difluorobenzy1)-N-methyl-
..L.,,..õ1,1 \>--- NH
6-(5H-pyrrolo[3,2-dipyrimidin-5- / N '' N y1)-1H-imidazo[4,5-
blpyridin-2- -- F 392.2
amine
N , /
\...- N
F
N
,10:-.7._ Ni.) F
2-(azetidin-1-0-1-(2,5-
difluorobenzy1)-6-(4-methoxy-5H- ,
6 \ N e
448.2
pyrrolo[3,2-d]pyrimidin-5-yI)-1H- / N
imidazo[4,5-b]pyridine 0 F
---- ---.
1
N-. N
---..--
N N, F
r---
1-(3,5-difluorobenzy0-6-(4- 0- N
methoxy-5H-pyrrolo[3,2-
1 c),.= ...,r 407.1
7
djpyrimidin-5-0-2-methy1-1H-
0 F
imidazo[4,5-b]pyridine --, ---
i
N , N
181
CA 03021185 2018-10-16 A00039
[Table 1-2]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt MS
,
F
1-(3,5-difluorobenzy1)-N-(2,2- N N
--- ----- F
, ---
difluoroethyl)-6-(4-methoxy-5H- 1 \,)-= NH
8 F 472.1
PYrrolo[3,2-d]Pyrimidin-5-y1)-1H- /
imidazo[4,5-b]pyridin-2-amine ¨ 0
N \ / =
\,..._ N
F
N pki
.....- .......... = . F
2-(3,3-difluoroazetidin-l-y1)-1- N< F
(3,5-difluorobenzy1)-6-(4-
c____c=-''--- N
9 methoxy-5H-pyrrolo[3,2- F 484.2
d]pyrimidin-5-y1)-1H-imidazo[4,5- _ 0
b]pyridine N .õ / \
\..- N
F
. -
.....N
....-j
2-ethoxy-1-(3-fluorobenzyl)-6-
(4-methoxy-5H-pyrrolo[3.2- / N N
N
d]pyrimidin-5-y1)-1H-imidazo[4,5-
419.1
b]pyridine ¨ 0
\ / \
\,..- N
F
N m
,õ,...... " 7--
2-ethoxy-1-((5-fluoropyridin-3-
c rNI ------"-------- N
11 yl)methyl)-6-(4-methoxy-5H- _ N 420.2
pyrrolo[3,2-d]pyrimidin-5-y1)-1H-
imidazo[4,5-b]pyridine N \ / \
\...- N
F
, .
N N
r- "IN--- \ )
1-(3-fluorobenzy1)-6-(4-methoxy-
N ---L-',1:.------- N
5H-pyrrolo[3,2-d]pyrimidin-5-y1)- / -
12 389.2
2-methyl-1H-imidazo[4,5-
¨' 0
b]pyridine
N / \
__._. N
F
N N OH
,
,r__,,,l,õ_ \> /
(1-(3-fluorobenzy1)-6-(4- c, c."' N
th
13
meoxy-5H-pyrrolo[3,2-
d]pyrimidin-5-y1)-1H-imidazo[4,5-
IP
¨ a 405.2
b]pyridin-2-yl)methanol
N 1 \
F
N N ¨ N _
3-(1-(3-fluorobenzy1)-6-(4-
, N
14 ,...U......
q,_,I ---`
methoxy-5H-pyrrolo[3,2-
d]pyrimidin-5-y1)-1H-imidazo[4,5-
le
¨ 0 428,3
b]pyridin-2-yl)propanenitrile N /r \
\-- N
F
182
CA 03021185 2018-10-16 A00039
_
[Table 1-3]
EXAMPLE (UPAC NAME CHEMICAL
STRUCTURE Salt MS
N 1-(4-(difluoromethoxy)benzy1)-6- N"- 4 F \r F
(4-methoxy-5H-pyrrolo [3,2- e
437.2
dlpyrimidin-5-y1)-2-methyl-1H- / o
imidazo[4,5-b]pyridine -... ----
1
N . N
.....
N NI
N
1-(3-fluorobenzy1)-6-(4-methoxy- --....--
1-i"--- N\1) C
- N
16
5H-pyrrolo[3,2-d]pyrimidin-5-yI)- / N 2-(1 -methyl-1 H-pyrazo1-4-y1)-
455.2
1H-imidazo[4,5-b]pyridine - 0
N / i \
..= _ N
F
N N
'13 1-(3,4-difluorobenzy1)-6-(4- -N
17 F
methoxy-5H-pyrrolo[3,2-
407.1
d]pyrimidin-5-y1)-2-methy1-1H- / N
0 F
imidazo[4.5-131pyridine ---. `-=
1
N .-N
--...---
,
N N
1-(1-(3-fluorobenzy1)-6-(4-
N>,.... \>- OH
NN
methoxy-5H-pyrrolo[3,2-
18 446.3
d]pyrimidin-5-y1)-1H-imidazo[4,5-
- .
b]pyridin-2-yl)azetidin-3-ol
\.- N
F
N N
2-(3-fluoroazetidin-l-y1)-1-(3- õ.,..1 \>-- N>
F
19 fluorobenzy1)-6-(4-methoxy-5H- / N N
PYrrolo[32-diPyrimidin-5-y1)-1 448.2
H-
imidazo[4,5-b]pyridine - 0
N \ / \
\.- N
F
. .
N N
1-(2.3-difluorobenzy1)-6-(4- 0- N
20 methoxy-5H-pyrrolo[32-
c,µ1,,.,.T..õ 407.1
d]pyrimidin-5-y1)-2-methy1-1H-
0
imidazo[4,5-b]pyridine ---.. ".-
F F
1
N . N
--,,..-
N N--- --..---
1-benzy1-6-(4-methoxy-5H-
c I I
:c -----'=:-."---- N
21 pyrrolo[3,2-d]pyrimidin-5-y1)-2- 371.2
methyl-1H-imidazo[4,5-b]pyridine , 0
\- N
183
CA 03021185 2018-10-16
A00039
[Table 1-4]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt MS
N N
1-(4-fluorobenzy1)-6-(4-methoxy-
5H-pyrrolo[3,2-d]pyrimidin-5-yI)- / N ---C-------- N
22 389.1
2-methyl-111-imidazo[4,5-
b]pyridine ¨ 0 F
N / \
\'_.- N
N N
1-(2,5-difluorobenzy1)-6-(4-
,....0
'' N F
23
methoxy-5H-pyrrolo[32-
c., s1 i_L)___
d]pyrimidin-5-y1)-2-methy1-1H-
407.1
imidazo[4,5-b]pyridine .--- 0
N / \
N F
N N 0 ¨
1-(3-fluorobenzyI)-2-
24 Xj \> /
(methoxymethyl)-6-(4-methoxy- / N N 5H-pyrrolo[3,2-d]pyrimidin-5-
y1)-
419.2
1H-imidazo[4,5-b]pyridine -- 0
F
N N
6-(4-chloro-5H-pyrrolo[3,2- N1
25 )
d]pyrimidin-5-y1)-1-(3- cyl _
fluorobenzy1)-2-methyl-1H-
imidazo[4,5-b]pyridine -- 'Cl 393.1
N /
.._ N
F
OH
2-((1-(3-fluorobenzy1)-6-(4- o N 0 I\
methoxy-5H-pyrrolo[3,2- f. z _
26 435.2
d]pyrimidin-5-yI)-1H-imidazo[4,5-
\--QF
b]pyridin-2-y1)oxy)ethanol /ill
0
Nri ---- '-
N ..- N
-....-
N N
5-(1-(3-fluorobenzy1)-2-methyl- r--.. 1 \>
--kz...-,------
1H-imidazo[4,5-b]pyridin-6-y1)-N- / N
N
27 methyl-5H-pyrrolo[3,2-
d]pyrimidin-4-amine -- NH 388.2
N / \
...... N F
N
N l,
28
142-fluorobenzyI)-6-(4-methoxy-
5H-pyrrolo[3,2-d]pyrimidin-5-yI)-
r1).,'s1 ..1_, 389.2
2-methyl-1H-imidazo[4,5-
0
b]pyridine ----.. --. F
i
N N
<,....-
184
CA 03021185 2018-10-16 A00039
,
[Table 1-5]
EXAMPLE IUPAC NAME CHEMICAL
STRUCTURE Salt MS
_
N N OH
1-(1-(3-fluorobenzyI)-6-(4-
29
(,\
N
methoxy-5H-pyrrolo[3,2- / N
419.3
dipyrimidin-5-y1)-1H-imidazo[4,5-
b]pyridin-2-ypethanol --- 0
IP
\-- N F
-
" N
6-(4- m eth o xy -5H - py r ro I o [3,2- 0- N
111P
d]pyrimidin-5-y1)-2-methyl-1-(3-
30 1,...1.....y1
=416.2
nitrobenzyI)-1H-imidazo[4,5- 0 N+ CY
b]pyridine 6'
N -.. N
NN,:),.---- ,
1-(2,4-difluorobenzyI)-6-(4- 5/5. N
F
methoxy-5H-pyrrolo[3,2-
31 N 407.1
d]pyrimidin-5-y1)-2-methy1-1H-
imidazo[4,5-b]pyridine '
---. ===== F
1 .
N N
.õ,,....,
N
N
1-(3-chlorobenzy1)-6-(4-methoxy-
0- N
5H-pyrrolo[3,2-d]pyrimidin-5-yI)-
32 cl
4051
2-methyl-1H-imidazo[4,5- 0 CI
bipyridine -,.. ---
1
N -N
-----
N
N..,--r---
6-(4-methoxy-5H-pyrrolo[32- (7
33 _____,1_ N AM
d]pyrimidin-5-y1)-2-methyl-1-(3- NW
,r1,...µ,....,,..r1
455.2
(trifluoromethoxy)benzyI)-1H-
0 0
imidazo[4,5-b]pyridine --, --
I X-- F
N ..- N F F
-----
- .
N
N...-.7.--
6-(4-methoxy-5H-pyrrolo[3,2-
51_ \- N
d]pyrimidin-5-yI)-2-methyl-1-(3-
34 cll,sy F 439.1
(trifluoromethyObenzyI)-1H-
imidazo[4,5-b]pyridine -...,. --,. F F
1
N -- N
-----
N N
1-(2,6-difluorobenzyI)-6-(4-
/qi F
. N
N
methoxy-5H-pyrrolo[3,2-
d]pyrimidin-5-y1)-2-methy1-1H-
407.1
imidazo[4,5-b]pyridine ---- 0
\ i \
N F
185
CA 03021185 2018-10-16 A00039
[Table 1-6]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt MS
N
6-(4-methoxy-5H-pyrrolo[32-
d]pyrimidin-5-y1)-2-methyl-1- N N
36 (2,3,4-trifluorobenzy1)-1H-
425.1
imidazo[4,5-b]pyridine 0
N
N
".õ1=1 N z
1-(2,3-difluorobenzyI)-2-ethoxy- -0
37
6-(4-methoxy-5H-pyrrolo[3,2- N N d3pyrimidin-5-yI)-1H-
imidazo[4,5-
437.2
b]pyridine 0
N
\-- N
N N
1-(3.5-difluorobenzyI)-2-methoxy- I
6-(4-methoxy-5H-pyrrolo[3,2- / N N
38
d]pyrimidin-5-yI)-1H-imidazo[4,5-
b]pyridine 0 423.1
N \
N
N N
\ ¨ 0
1-(3-fluorobenzyI)-2-methoxy-6-
N
39
(4-methoxy-5H-pyrrolo[3,2-
d]pyrimidin-5-yI)-1H-imidazo[4,5-
405.1
b]pyridine 0
N \
N
OH
N N
2-((1-(3,5-difluorobenzy1)-6-(4-
\>-- NH
methoxy-5H-pyrrolo[3,2- N d]pyrimidin-5-yI)-1H-
imidazo[4.5-
b]pyridin-2-yl)amino)ethanol -- 0 452.1
N
N
N N
/
1-(3,4-difluorobenzy1)-2-methoxy-
6-(4-methoxy-5H-pyrrolo[3.2- N
41 d]pyrimidin-5-yI)-1H-imidazo[4,5-
423.1
-- 0
b]pyridine
N ,
- N
0
N
1-(3.4-difluorobenzy1)-N-(2-
th -.
methoxyey0-6-(4-(4 meoxy-5H- , N N
42 \> NH 466.2
pyrrolo[3,2-d]pyrimidin-5-y1)-1H-
imidazo[4,5-b]pyridin-2-amine ¨ 0 F
N
N
186
CA 03021185 2018-10-16 A00039
[Table 1-7]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE
Salt MS
N N OH
------e-
1-(1-(3,4-difluorobenzyI)-6-(4-
\ ¨ N7
43 c N \----
methoxy-5H-pyrrolo[3,2-
d]pyrimidin-5-y1)-1H-imidazo[4,5- c
b]pyridin-2-yOpyrrolidin-3-ol -- 0 F 478.2
\¨ N F
N N 0-N
1-(3-(difluoromethoxy)benzyl)-6-
(4-methoxy-5H-pyrrolo[3,2-
44 N ft F 437.2
capyrimidin-5-y1)-2-methyl-1H- / o 0 ---(
imidazo[4,5-b]pyridine --.. ---- F
I
Nõ.....,-,N
14
tert-butyl (3-((6-(4-methoxy-5H-
pyrrolo[3,2-4211Pyrimidin-5-y1)-2- 4. 0
45 486.1
methyl-1H-imidazo[4,5-b]pyridin- r!',.,k 1,1 0 HN --1< /
1-yOmethyl)phenyOcarbamate 1 0 - \-----
N . N
----
NN.,-,i---
3- ( (6- (4- m e th o x y -5H - p y rro I o [3,2- 0___ N
capyrimidin-5-y1)-2-methy1-1H-
46 cti\lõ,, 386.1
imidazo[4,5-b]pyridin-1-
0
yOmethyDaniline --... NH2
i
N... N
N N
-""-- '----
6-(4-methoxy-5H-pyrrolo[3,2-
cl]pyrimidin-5-y1)-2-methyl-1-methyl
c c.1"-=------- N
47 372.2
(pyridin-2-yirnethyl)-1H-
imidazo[4,5-13]pyridine --- 0
N i \
___-N
N N
1-(3,5-difluorobenzy0-6-(4-
c...1--------- N F
methoxy-5H-pyrrolo[3,2-
48 408.2
capyrimidin-5-y1)-1H-imidazo[4,5-
b]pyridin-2-amine --- 0
N \ / \
\¨ N
F
OH
1 -((1-(3,5-difluorobenzyl)-6-(4- ,,,,N N / <
methoxy-5H-pyrrolo[3,2- ,.,..J =-- NH \
F
49 capyrimidin-5-y1)-1H-imidazo[4,5- / N N'
480.2
blpyridin-2-yDamino)-2- ---- 0
methylpropan-2-ol N N ii \
\¨ N
F
187
CA 03021185 2018-10-16 A00039
[Table 1-8]
EXAMPLE 1UPAC NAME CHEMICAL STRUCTURE
Salt MS
......;.N N /
1-(3,5-difluorobenzy0-6-(4- õ..õ.
ni '''' N F
(difluoromethoxy)-5H-pyrrolo[32- / = ' d]pyrimidin-5-yI)-N-methyl-1H-
458.1
imidazo[4,5-b]pyridin-2-amine --- 0
N. / )_ F
F F
N N
--- ....._.-- -
..
1-((5-fluoropyridin-3-yl)methyl)- .õ I \ ¨
6-(4-methoxy-5H-pyrrolo[3,2- / N -----'''''..----' N
51 - N 390.2
N\dipyrmidin-5-y1)-2-methyl-1H-
imidazo[4,5-b]pyridine --- 0 \ /
\ /
\_- N
F
,
N N..-- -----
---
1-((2-fluoropyridin-4-yOrnethyl)- 1 \>
6-(4-methoxy-5H-pyrrolo[3,2- / Nr---'-------'''- N
52 -__ 390.2
d]pyrimidin-5-y1)-2-methyl-1H-
imidazo[4,5-13]pyridine N
N \ / \
N.._ N
F
-----.
1-(3-chloro-4-fluorobenzyI)-6-(4-
53 N
metho xy-5H-pyrrolo[3,2-
423.1
d]pyrimidin-5-yI)-2-methyl-1H- / N
0- F
0 CI
imidazo[4.5-b]pyridine ---.. ---
. I
N ,-- N
-....--
1-(4-chloro-3-fluorobenzyI)-6-(4- CI
methoxy-5H-pyrrolo[3,2-
54 N 423.1
djpyrimidin-5-yI)-2-methyl-1H- /
imidazo[4,5-blpyridine 0
i
N .-N.
--....--
N N
methyl 3-((6-(4-methoxy-5H-
pyrrolo[3,2-d]pyrimidin-5-yI)-2-
429.2
methyl-1H-imidazo[4,5-b]pyridin- / N
0 0
1-yl)methyl)benzoate -- ---- \
1 0
N.. N
....--
N
N .zr--
3-((6-(4-methoxy-5H-pyrrolo[3,2- 0- N
d]pyrimidin-5-yI)-2-methyl-1H-
56 N 415.2
imidazo[4,5-b]pyridin-1- 0H
0
yl)methyl)benzoic acid / .õ, 1
0/
N , N
-....--
188
CA 03021185 2018-10-16 A00039
[Table 1-9]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt MS
3-((6-(4-methoxy-5H-pyrrolo[3,2-
c .
d]pyrimidin-5-y1)-2-methyl-1H-
1 400.2
57 imidazo[4,5-b]pyridin-1-
,1,..1 0 0--ni
yl)methyl)-N-methylaniline HN - .-- .,.
1
N . N
-...-
3-((6-(4-methoxy-5H-pyrrolo[3,2-
58
d]pyrimidin-5-y1)-2-methy1-1H-
/7-- N0__
, 428.2
imidazo[4,5-b]pyridin-1- 0 NH
yl)methyl)-N-methylbenzamide -Y-"--r- - --- 0 / \
1
N-.. N
N N
.-y-
2-(3-((6-(4-methoxy-5H- 0- N .
pyrrolo[3,2-d]pyrimidin-5-yI)-2-
59 N 429.2
methyl-1H-imidazo[4,5-b]pyridin- / 0 OH
1-yl)methyl)phenyl)propan-2-ol ..-- --.,
1
N . N
-...--
.,....N N /
2-methoxy-6-(4-methoxy-5H-
_...,____II \ - 0
pyrrolo[3,2-d]pyrimidin-5-yI)-1- / N '''. N
F
60 441.1
(2,4,5-trifluorobenzyI)-1H-
imidazo[4,5-b]pyridine ---- 0 F
N / \
N;..... N F
N N
--- /
1 \
1-benzy1-2-methoxy-6-(4-
61 >--0
methoxy-5H-pyrrolo[3,2-
___./ ----'''---"'L N
d]pyrimidin-5-yI)-1H-imidazo[4,5-
b]pyridine -- . 387.2
ID
N / \
\,..- N
/
N 0 F
62
51/.11...._,,,r
1-(2,5-difluorobenzy1)-2-methoxy-
6-(4-methoxy-5H-pyrrolo[3,2-
\ N e
423.1
dlpyrimidin-5-yI)-1H-imidazo[4,5- N
b]pyridine ,r1..y0 F
..--- '-
i
N... N
-,..-
N N
/
=,..._ \--
1-(2,3-difluorobenzy1)-2-methoxy-
LI > 0
...."- N
63
6-(4-methoxy-5H- mpyrrolo[3.2- / '" d]pyrimidin-5-yI)-
1H-imidazo[4,5-
423.1
b]pyridine --- 0
N / \
F
F
189
CA 03021185 2018-10-16
A00039
[Table 1-10]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt MS
2-methoxy-6-(4-methoxy-5H- 0
64 pyrrolo[3,2-d]pyrimidin-5-y0-1- 7 N ---- N
(2,3,4-trifluorobenzy0-1H-
imidazo[4,5-b1pyridine --- 0 F 441.1
N \ i \
\....- N F
F
=
N N /,....;õ -.......-
2-methoxy-6-(4-methoxy-5H-
pyrrolo[3,2-d]pyrimidin-5-y0-1- / N -.-----.--------- I Nµ>¨ F
(2,3,6-trifluorobenzy0-1H-
441.1
N\imidazo[4,5-b]pyridine -- 0
/
`..... N F
F
NN -,,----
(3-((6-(4-methoxy-5H- 513--N
PYrrolo[3.2-4pyrimidin-5-y1)-2-
66 401.2
methyl-1H-imidazo[4,5-blpyridin- / N 0 OH
1-y0methy0phenyOrnethanol --- -...
1
N. N
N N -,
1-(3,5-difluorobenzy0-6-(4- ----- '11-- \\..... N
methoxy-5H-pyrrolo[3,2- ..!_c-----1-...---"L N/ \--- N67
d]pyrimidin-5-y0-2-(1 -methyl- F 473.2
1H-pyrazol-4-y1)-1H-imidazo[4,5- ¨ 0
b]pyridine N \ i \
F
N N,,,.7....-- F
1-(3-fluoro-5-
(trifluoromethyl)benzy1)-6-(4- S13¨ N .
68 methoxy-511-pyrrolo[3,2- N F 457.1
cOpyrimidin-5-y1)-2-methyl-1H- / 0
imidazo[4,5-b]pyridine F F
N ..- N
-....--
5.1,111_N 0
,N 1-(3,5-dichlorobenzy0-6-(4-
methoxy-5H-pyrrolo[3,2-
439.1
69
d]pyrimidin-5-0-2-methy1-1H- / N o CI
imidazo[4,5-b]pyridine ,-.. *---
1
N -N.
------
1/ss_l_l_N,N
F
1-(3-fluoro-5-nitrobenzy0-6-(4-
N
methoxy-5H-pyrrolo[3,2-
434.1
d]pyrimidin-5-yI)-2-methyl-1H-i 0-
midazo[4,5-b]pyridine --- -..
1 6"
N- N
-.....-
190
CA 03021185 2018-10-16 A00039
.,
[Table 1-11]
EXAMPLE 1UPAC NAME CHEMICAL STRUCTURE Salt MS
IN
si_3_., NN
F
3-fluoro-5-((6-(4-methoxy-5H-
pyrrolo[3,2-d]pyrimidin-5-y1)-2-
71 N 404.2
methyl-1H-imidazo[4,5-bipyridin-
1-yOrnethyl)aniline NH2 ..- ' -...
1
N. N
-....--
, .
F
1-(3,5-difluorobenzy1)-2-(1- N N .1----
> \ C", F
(difluoromethyl)-1H-pyrazol-4-y1)- -
72 6-(4-methoxy-5H-pyrrolo[32- / N -- '''/ -- N N
507.2
clipyrimidin-5-0-1H-imidazo[4.5- ei F
b]pyridine ¨ 0
N \ / =
F
N ...,_._ õI:N ,
2-methoxy-6-(4-methoxy-5H-
pyrrolo[3,2-cOpyrimidin-5-y1)-1- / N -
N F
73 (2.3,5-trifluorobenzy1)-1H-
441.1
imidazo[4,5-b]Pyridine --- 0
ID
N i =
,.... N F
F
N
F
2-fluoro-4-((6-(4-methoxy-5H- 0- N ¨ N
pyrrolo[3,2-capyrimidin-5-y1)-2- ¨
74 N 414.1
methyl-1H-imidazo[4,5-bipyridin- / o
1-yOmethyObenzonitrile --.. ---
1
N . N
--.....-
. .
N
N
1-(5-fluoro-2-methoxybenzy1)-6- ¨ N
c
(4-methoxy-5H-pyrrolo[3,2-
75 N,y.
419.1
clipyrimid 0 in-5--2-methyl-1H-
0
imidazo[4,5-b]pyridine ---.. `-
0
i =
N . N
-,...-
-
1-(3,5-difluorobenzy1)-6-(4- .. N N
methoxy-5H-pyrrolo[3,2-
,, .ci L N F
76 c1pyrimidin-5-y1)-2-
493.2
(tetrahydrofuran-3-ylmethoxy)-
--- 0
1H-imidazo[4,5-b]pyridine N / =
=..- N
F
N ..,
1-(3,5-difluorobenzyI)-6-(4- N N /
, C
methoxy-5H-pyrrolo[3,2-
77 d]pyrimidin-5-y1)-2-((1 -methyl- / N F
'''-(-::=== N 503.1
1H-pyrazo1-4-yOrnethoxy)-1H-
¨ 0
imidazo[4,5-b]pyridine N i \
_... N
F
191
CA 03021185 2018-10-16 A00039
[Table 1-12]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt MS
N N F
1-(3,5-difluorobenzyI)-2- I \> (
(difluoromethy0-6-(4-methoxy- / N -' III N F F
78 5H-pyrrolo[3,2-d]pyrimidin-5-Y0 441.1
- --
1H-imidazo[4,5-b]pyridine --- 0 \ /
N / \
...- N
F
,
N
F
1-(3-chloro-5-fluorobenzyI)-6-(4- 0¨ N
methoxy-5H-pyrrolo[3.2-
79 N 423.1
d]pyrimidin-5-0-2-methyl-1H- /
imidazo[4,5-b]pyridine 0
---. ---- CI
I
N -- N
-----
N.7....- F
N
1-(3-fluoro-4-
0--'N F
(trifluoromethyl)benzy1)-6-(4- F
BO methoxy-5H-pyrrolo[3,2-
cN F 457.1
d]pyrimidin-5-y0-2-methyl-1H- 0
--.. "-=
imidazo[4,5-b]pyridine I
N , N
------
F
rcl_t_ F
2-((3,3-difluoroazetidin-1-
yOmethy0-1-(3.5-difluorobenzy!)- N N
1,j -UI N F 498.1
81 6-(4-methoxy-5H-pyrrolo[32- \>---/
d]pyrimidin-5-y0-1H-imidazo[4,5- /
b]pyridine ¨ 0
11)
N F ,
N N OH
...-- --...--
--
(1-(3,5-difluorobenzyI)-6-(4- 1 \> /
methoxy-5H-pyrrolo[3,2- / N ''''''''-. N F
82 d]pyrimidin-5-y1)-1H-imidazo[4,5-
423.1
b]pyridin-2-yOmethanol -- 0
N / \
".._.. N
F
N N
r.... i \>
1-((6-methoxypyridin-2-
83 /c. iscLI:.----- N
yOmethy0-6-(4-methoxy-5H-
PYrrolo[3,2-d]pyrirnidirr-5-yI)-2- -- 0 \-----(\i
methyl-1H-imidazo[4,5-b]pyridine N / \ 402.2
0
/
-
N N,=7---
1-((6-chloropyridin-2-y0methy0- 0¨ N(>
84 84 N \---Q71 / 406.1
d]pyrimidin-5-0-2-methyl-1H- /
imidazo[4,5-b]pyridine 0
--.. "-- Cl
1
N ..- N
192
CA 03021185 2018-10-16 A00039
,
[Table 1-13]
EXAMPLE ILIPAC NAME CHEMICAL STRUCTURE Salt
MS
OH
N
(1-(2.3-difluorobenzy1)-6-(4- (' - N
methoxy-5H-pyrrolo[3,2-
85
423.1
d]pyrimidin-5-yI)-1H-imidazo[4,5- N
b]pyridin-2-yOmethanol ci.r0-. F F
N N
--.."-
N N
---- --,---
-- ,
1 \>
1-(3,5-difluorobenzy1)-6-(4- _...._ ......"-. ,,. F
86
(fiuoromethoxy)-5H-pyrrolo[3,2- / N" ----- " d]pyrimidin-5-y1)-2-
methy1-1H-
425.1
imidazo[4,5-b]pyridine -- 0
N \ 1
\-- N
F F
..,õ N N 0 ---N\
.--
, 1 \>---- N
1-(3,5-difluorobenzy1)-6-(4-
methoxy-5H-pyrrolo[3,2-
cy.c
460.2
1" N F
87
d]pyrimidin-5-0-2-(1,3-oxazol-5-
yI)-1H-imidazo[4,5-b]pyridine --- 0
N / \
,...- N
F
f-- OH
N N 0 __/-
2-¶1-(3.5-difluorobenzy1)-6-(4- .
1 \> /
88
methoxy-5H-pyrrolo[3,2- _...N_c_ -' N F
d]pyrimidin-5-yI)-1H-imidazo[4,5-
b]pyridin-2-yOmethoxy)ethanol ' --. 0
467.1
\....- NF
F F
1-(3,5-difluorobenzyI)-6-(4- N N coil- F
E
methoxy-5H-pyrrolo[3,2-
N /
89 capyrimidin-5-y1)-2-((2,2,2- F
505.1
Cc
trifluoroethoxy)rnethy0-1H-
imidazo[4,5-b]pyridine , - 0
IP
N / \
\;...s_ N
F
F
J- F
1-(3,5-difluorobenzy1)-2-((2,2- ,N N 0
difluoroethoxy)methyl)-6-(4-
,,,L\> /
90 methoxy-5H-pyrrolo[3,2- / N --- N F
487.1
d]pyrimidin-5-0-1H-imidazo[4,5- '
b]pyridine - 0
N i \
F
.5:11.__N,
/-----
1-(2-furylmethy1)-6-(4-methoxy-
N 0
5H-pyrrolo[3,2-d]pyrimidin-5-y1)- "...---(0
91 N
361.1
2-methy1-1H-imidazo[4,5-
b]pyridine-"-
I
N , N
193
CA 03021185 2018-10-16 A00039
[Table 1-14]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE
Salt MS
..... N N
1-(3-fluorobenzyI)-2-(3-
\>-- -
methoxyazetidin-1-yI)-6-(4- , N__.,11 N N 0,,,
92 methoxy-5H-pyrrolo[3,2- N 0 460.2
d]pyrimidin-5-yI)-1H-imidazo[4,5- ¨ IP
b]pyridine \-- N \ F
,
N N ---
1-(3-furylmethy0-6-(4-methoxy- ____i_O
5H-pyrrolo[3,2-d]pyrimidin-5-y)-
93 361.1
2-methy1-114-imidazo[4,5-
cI)õ.= ,I 0
b]pyridine ---. '-
1
N ,- N
=-,...--
F
N \___;3
6-(4-methoxy-5H-pyrrolo[3,2-
5.1): ( ....2r-r-- N _ F
d]pyrimidin-5-y1)-2-methyl-1-((6-
(
94 (trifluoromethyl)pyridin-2- \ / 440.1 -/- INI\
yl)methyl)-1H-imidazo[4,5- 0
b]pyridine
N N
-...%
N N ..-- OH
2-(2-fluoro-4-((6-(4-methoxy-
r 449.1
---1
21µ1 lia
5H-pyrrolo[3,2-d]pyrimidin-5-yI)-
95 2-methyl-1H-imidazo[4,5- cN0
):., NW
b]pyridin-1- y 0 F
yOrnethyl)phenoxy)ethanol i
N N
--..="-
N N,
)'-----
1-((6-fluoropyridin-2-yl)methyI)- 0- N \Q-
6-(4-rnethoxy-5H-pyrrolo[3,2-
96 pl.,y N 390.1
cl]pyrimidin-5-y9-2-methyl-1H-
. . 0 F
Imidazo[4.5-1Apyridine '.... ""`-
i
N N
.....,-.
_N N.
1-(2-chloro-6-methylpyridin-4- r¨
N
97 yl)methyl)-6-(4-methoxy-5H- \ /
N
pyrrolo[3,2-d]pyrimidin-5-yI)-2- Cl 418.0 / 0
methyl-1H-imidazo[4,5-b]pyridine .,- --...
i
N. N
/
N N 0
1-((6-fluoropyridin-2-yl)methyl)- f\iiI -N ¨
2-meoxy-6-(4-methoxy-5H-
\---q
th 406.2
98
pyrrolo[3,2-d]pyrimidin-5-yI)-1H- N
imidazo[4,5-b]pyridine cr,.0--.. F
N sz_. , N
194
CA 03021185 2018-10-16 A00039
[Table 1-15]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE
Salt MS
'
N N /
.¶ >--
1-(3,4-difluorobenzyI)-N-methyl- / N \ NH --.- N
99 6-(1H-pyrrolo[3,2-b]pyridin-1 -yI)- 391.2
1H-imidazo[4,5-b]pyridin-2-amine -- F
N \ /
F
N N /
X1-(3,5-difluorobenzyI)-6-(7-
methoxy-1H-pyrrolo[3,2- / NN F
100 b]pyridin-1-y1)-N-methy1-1H-
421.2
imidazo[4,5-b]pyridin-2-amine --- 0
N \ / \
F
N N
,--..... ----- /
1-(3,5-difluorobenzyI)-2-ethoxy-
/ N --"/"--- Ni F
101
6-(7-methoxy-1H-pyrrolo[3,2- b]pyridin-1-y1)-1H-imidazo[4,5-
436.2
b]pyridine --- 0
N \ / N.
F
,
. .
'
N N,-- -.........-
I \>
1-(3-fluorobenzyl)-6-(7-methoxy- , N - N
102 1H-pyrrolo[3,2-b]pyridin-1-yI)-2- '
388.2
methyl-1H-imidazo[4,5-b]pyridine -- 0
F
.........N N /
1-(3,4-difluorobenzy1)-N-methyl- N
103 N
6-(1H-pyrazolo[4,3-b]pyridin-1- ,, ' N
yI)-1H-imidazo[4,5-b]pyridin-2- ¨ F 392.2
amine
N \ /
F
N N N /
1-(3,4-difluorobenzy1)-6-(7-
104 N)-- NH
======
methoxy-1H-pyrazolo[4,3- ,, ' N
¶ N
blpyridin-l-y1)-N-methyl-1H-
imidazo[4,5-b]pyridin-2-amine --- 0 F 422.2
N \ / \
F
N N....- -....--
1-(1-(3-fluorobenzyl)-2-methyl- N 1 \>
' 105 -------'---
1H-imidazo[4,5-b]pyridin-6-yi)7- /
N N
methoxy-1H-pyrazolo[4,3-
b]pyridine ---"" 389.2
0
N \ / \
F _
195
CA 03021185 2018-10-16
A00039
[Table 1-16]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt MS
N N ,
r- --r-
-
õ...Lz,...õ: ,>-- NH
1-(3,5-difluorobenzy0-6-(3-
N
,q F
methoxy-5H-pyrrolo[2,3-
106 ¨ 422.1
b]pyrazin-5-y0-N-methyl-1H- N
N.....4.
imidazo[4,5-b]pyridin-2-amine
F
0
/
N N
1-(3,5-difluorobenzy1)-N-methyl-
,..,C,-1,......1 \>-- N4
107
6-(5H-pyrrolo[2,3-b]pyrazin-5- / N
N F 392.1
y1)-1H-imidazo[4,5-1D]pyridin-2-
amine ..¨
N
F
r4 ,T..._ N OH
(1-(3-fluorobenzy1)-6-(3- cz----- N
methoxy-5H-py0rrol o[2.3-
108
b]pyrazin-5--1H-imidazo[4,5- ID
N 405.1
b]pyridin-2-yOmethanol
F
/
...-N-..,..... N OH
..,_
(1-0,5-difluorobenzy1)-6--(3- ...._, 1. j's\J"-:-.' N F
methoxy-5H-pyrrolo[2,3-
109 423.1
b]pyrazin-5-y1)-1H-imidazo[4,5- N ¨ / N
b]pyridin-2-yOmethanol
.v.i...iõ F
0
/
N N
-....
1-(3-fluorobenzy1)-6-(3-methoxy- 7 N '-i'-'21----- 1:'1>
110 5H-pyrrolo[2,3-b]pyrazin-5-y1)-2- 389.2
methyl-1H-imidazo[4,5-b]pyridine --- N
F
µ------\0 ¨
N N
XL.:::1
2-(5-(1-(3-fluorobenzy1)-2- (ANii --' N
methyl-1H-imidazo[4,5-b)pyridin-
111 417.1
6-0-5H-pyrrolo[2,3-b]pyrazin-3- N\ i N
yl)propan-2-ol
\--)__ OH F
NSi ",,,_-
i_3-ni op
1-(3-fluorobenzy1)-2-methy1-6-
112 (5H-pyrrolo[2,3-13]pyrazin-5-y1)- 1 N 359.1
1H-imidazo[4,5-b]pyridine
ZI'N F
1
N,L...õ....1--
196
CA 03021185 2018-10-16 A00039
[Table 1-17]
_
- ___________________________________________________________________
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE
Salt MS
N N
._.._z r --1-, - ..>
1-(5-(1-(3-fluorobenzy1)-2- ---<;>-:--L N
113
methyl-1H-imidazo[4,5-b]pyridin-
6-y0-5H-pyrrolo[2,3-b]pyrazin-3- ¨ N 401.2
yl)ethanone N ,... ji\sf. 0 F
N N
,
6-(3-chloro-5H-pyrrolo[2,3-
.....¶ \>
N
114
b]pyrazin-5-yI)-1-(3- ..,1__L\I fluorobenzy1}-2-1-1 H- _-
imidazo[4,5-b]pyridine N
N 393.1N....2_(
Ci F
N
N
A;[ --
1-(3-fluorobenzy1)-2-methyl-6-
N
2L.,(2?
115
(3-(1-methyl-1H-pyrazol-4-y1)- -
439.2
5H-pyrrolo[2,3-b]pyrazin-5-yI)- N \ /N
Z
1H-imidazo[4,5-b]pyridine
i7\N F
N'
I
,...õN N
5-(1-(3-fluorobenzyI)-2-methyl- N
116 1H-imidazo[4,5-b]Pyridin-6-yI)- N
375.2
5H-pyrrolo[2,3-b]pyrazin-3-ol -- N
OH F
N N
-,- ,,---
1-(3-fluorobenzy1)-2-methyl-6- i ...õ.. N>
/c.._ 1. j\s.1"-.."--------- N
117
(3-methyl-5H-pyrrolo[2,3- b]pyrazin-5-y1)-1H-imiclazo[4,5- --
b]pyrid 373.2ine N
N ,.....Lc F
" N,N,>___
..,11)...
N-(2,2-difluoroethyl)-5-(1-(3-
q - N
fluorobenzyl)-2-methyl-1H- =
118 N - N 438.2
imidazo[4.5-b]pyridin-6-yI)-5H- .\_.,(
pyrrolo[2,3-b]pyrazin-3-amine NH F
is\r_ F
F
N N
2-(5-(1-(3,5-difluorobenzy1)-2- õ...,...
cz- -----:---1N F
119
methyl-1H-imidazo[4,5-b]pyrdin-
6-yI)-5H-pyolo[23]pyrazin-3- ., ¨IN 435.2
''',_____cit._
yl)propan-2-ol F
HO
197
CA 03021185 2018-10-16 A00039
dr
=
[Table 1-18]
_
_____________________________________________________________________________
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
N N
L. __I__ = >___
1-(5-(1 -(3,5-difluorobenzy0-2- qi '"-- N F
120
methyl-1H-imidazo[4,5-b]pyridin-
6-y0-5H-pyolo[2,3-b]pyrazin-3- na ¨ i N
421.1
"
yl)ethanol ...._._(\r___ F
HO
......N N
5-(1 -(3-fluorobenzy0-2-methyl- - N
121
1H-imidazo[4,5-b]pyridin-6-0-
5H-pyrrolo[2,3-b]pyrazine-3- .'-- Z.¨ N1.'---1
384.1
carbonitrile
-\\ F
N N /
U\ ¨ (3 2-(5-(1-(3,5-difluorobenzy0-2- N F
e ri
methoxy-1H-imidazo[4,5-
122
451.2
b]pyridin-6-y9-5H-pyrrolo[2,3- -:-------\ N
b]pyrazin-3-y0propan-2-ol NL5c...._ F
OH
F
1\1.-- N / (
1-(3,5-difluorobenzy0-N-(2,2-
:C-J____ \>-- NH F
difluoroethyl)-6-(5H-pyrrolo[2,3- N --' N
123 /
442.1
b]pyrazin-5-y0-1H-imidazo[4,5- F
b]pyridin-2-amine --
N
NI =\1___9
F
'
N N /
\>¨ 0
1-(3,5-difluorobenzy0-2-methoxy- (-, - IN ''U: N F
124 6-(5H-pyrrolo[2,3-blpyrazin-5-
yI)-1H-imidazo[4,5-b]pyridine >7------\ N
393.1
N ..\\...jj
F
. .
1-(3,5-difluorobenzy0-6-(5H- ---...
N F
125 pyrrolo[2,3-b]pyrazin-5-y9-1H- /N'
378.2
imidazo[4,5-b]pyridin-2-amine --
N
N.<:=,..._ _I/
F
N N
...r.....:....il
6-(3-(1-(difluoromethy0-1H- ("71 N
pyrazol-4-0-5H-pyrrolo[2,3- ¨t-----N 1111
126 b]pyrazin-5-y9 NI
-1-(3- F
475.2
fluorobenzy1)-2-methyl-1H- sri-"\N
4,
imidazo[4,5-b]pyridine N.
F)-- F
198
CA 03021185 2018-10-16 A00039
,
:
I.
[Table 1-19]
_ -
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
J \
1-(3,5-difluorobenzy0-2-methyl- - N)
F
127 6-(5H-pyrrolo[2,3-b]pyrazin-5- <7- IN
377.2
y1)-1H-imidazo[4,5-b]pyridine
F
N N
1-(3,5-difluorobenzyI)-2-methyl- ,,.. ji \>---
N F
6-(3-((1-methy1-1H-pyrazol-4- / N
128 473.1
yl)oxy)-5H-pyrrolo[2,3-b]pyrazin-
5-y1)-1H-imidazo[4,5-b]pyridine N, i\--- / N _ N
`----\0.......,),,,.... F
õ..N N
1-(3,5-difluorobenzy1)-6-(3-(3- <-,- N ---J N1>-----
fluoroazetidin-1-0-5H- iiiii,,,,
129 rN
F
450.1
Ur
pyrrolo[2,3-b]pyrazin-5-y1)-2- N=--:
.\\____(/
methyl-1H-imidazo[4,5-b]pyridine
N ¨
F
I \
F
N N
.,u
,H \>---
1-(5-(1-(3,5-difluorobenzy1)-2- czAi -- N
130
methyl-1H-imidazo[4,5-b]pyridin- F 6-y1)-5H-py
460.3
rrolo(2,3-b]pyrazin-3- ,,, ¨ N
yl)pyrrolidin-2-one i'4,....4. 9
INJ,..., F
"
,N ,.._ N
)
1-(3,5-difluorobenzy1)-6-(3-(3,6-
IV F
/..--- ti
131
dihydro-2H-pyran-4-yI)-5H-
---"------j\
pyrrolo[2,3-b]pyrazin-5-yI)-2- N ., /N
459.2
methyl-1H-imidazo[4,5-b]pyridine F
A-,
.
N N
¶ ..>_
1-(3,5-difluorobenzy1)-2-methyl- .c.....Nci --- N F
1
6-(3-(1 H-pyrazol-1-y1)-5H-
132 1)
443.2
pyrrolo[2,3-b]pyrazin-5-y1)-1H- ¨ N
N___L(
imidazo[4,5-b]pyridine F
N - N
1.z.,
, N N
c--1-(3,5-difluorobenzy1)-2-methyl- ' -1---X1 N F
6-(3-(tetrahydro-2H-pyran-4-yI)- N \ /N
133
461.2
5H-pyrrolo[2.3-bipyrazin-5-0- F
1H-imidazo[4,5-b]pyridine
\---b)
199
CA 03021185 2018-10-16 A00039
0,
#
,
F
[Table 1-20]
,
_____________________________________________________________________________
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
-
N N ,
XII ,,,I77-
'. \
1-(5--(1-(3,5-difluorobenzy0-2- iql N F
methoxy-114-imidazo[4,5- ¨
134 N
437,2
Opyridin-6-y0-5H-pyrrolo[2,3- N ...:_o\rõ._
lajpyrazin-3-y0ethanol F
HO
- N
1
crA\44
1-(3,5-difluorobenzy0-2-methyl-
N F10
135
6-(3-(pyridin-2-y0-5H- ¨
N
\rne.c73õ,
pyrrolo[2,3-b]pyraz 0 N /
454.2
in-5--1H- F
imidazo[4,5-b]pyridine
N
-- ,
N N
1-(3,5-difluorobenzy0-2-methyl- /1- ri - N F
6-(3-(pyridin-4-0-511-
136
454.2
pyrrolo[2,3-Opyrazin-5-0-1H- N.,,.._..c_ ,),, i
imidazo[4,5-13]pyridine F
1
N
-
N
. ,
1-(3,5-difluorobenzy0-2-methyl-
6-(3-(pyridin-3-y0-5H- -C''r:C:¨ N--ji F N \>
137
454,2
pyrrolo[2,3-tdpyrazin-5-y0-1H- N , /
imidazo[4,5-Opyridine F
\----4\C
N-
, , _ ,I S I N
<71:1
;i L. 1 .. fi...> _....F
4-(5-(1-(3,5-difluorobenzy0-2-
methyl-1 H-imidazo[4,5-b]pyridin- N \ ,
138 F 478.2
6-y0-5H-pyrrolo[2,3-b]pyrazin-3-
y0benzonitrile fit
= N
N N
F
q
3-(5-(1-(3,5-difluorobenzy0-2-
139
methyl-1H-imidazo[4,5-bjpyridin- _.
N
\
6-0-5H-pyrrolo[2,3-b]pyrazin-3-
N /
yObenzonitrile -- N
F
478.2
--
200
CA 03021185 2018-10-16 A00039
t-
k
[Table 1-21]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt MS
c- -1
1-(3,5-difluorobenzy1)-2-methyl- rj
6-(3-(1-(2-(morpholin-4-yOethY1)- N - N
U-S..
140 1H-pyrazol-4-y0-5H-pyrrolo[2.3-
556,3
.. N
b]pyrazin-5-y1)-1H-imidazo[4,5- il ./..ti .."'= i't
F
b]pyridine
ir
,t4,.., FN
f
LI,sh Isil
1-(3,5-difluorobenzy1)-2-methy1-
6-(3-(1-(pyridin-2-ylmethyl)-1H-
N
141 pyrazol-4-y1)-5H-pyrrolo[2,3- F
534.2
b]pyrazin-5-yI)-1H-imidazo[4,5- Z-7- µN
PC
b]pyridine \ -:
N N
F
1-(3,5-difluorobenzy1)-2-methyl-
110
6-(3-(1-(pyridin-3-ylmethyl)-1H-
N
142 pyrazol-4-y1)-5H-pyrrolo[2,3- F
534.2
b]pyrazin-5-yI)-1H-imidazo[4,5- --A----/ N ..
b]pyridine
(17,N
N N
<j
.¶
'..." N "- ji
1-(3,5-difluorobenzy1)-2-methy1-
N F
6-(3-(1-methyl-1H-pyrazol-3-y1)- :-----IN IP
143 N,,,,..c.c..),:i./ 457,2
5H-pyrrolo[2,3-b]pyrazin-5-y1)-
F
1H-imidazo[4,5-b]pyridine
, .
N N
.C:3µ>---
-.."
1 -(3,5-difluorObenZy0-6-(3 J
-(1,3- <7 r ... N F
i
dimethy1-1H-pyrazol-4-y1)-5H-
111
144 -'----::\ N
471.2
pyrrolo[2,3-b]pyrazin-5-0-2- N
methyl-1H-imidazo[4,5-b]pyridine F
--f-----\N ..__
N.
N
1-(3,5-difluorobenzy1)-2-methyl-
zcz::/"." N F
6-(3-(1-methy1-3-
IP
145 (trifluoromethyl)-1H-pyrazol-5- N - N
525.2
y1)--5H-pyrrolo[2,3-b]pyrazin-5- F
yI)-1H-imidazo[4,5-b]pyridine - F
201
CA 03021185 2018-10-16 A00039
it=,
[Table 1-22]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt MS
N N
.....Xj \>......_
1-(3,5-difluorobenzy1)-6-(3-(3,5- (*-- IN N F
146
dimethy1-1,2-oxazol-4-y0-5H-
IP
:-..----.\N 472.2
pyrrolo[2,3-b]pyrazin-5-yI)-2-
methy1-1H-imidazo[4,5-b]pyridine F
\-------4-- 0
,N N
µ
1-(3,5-difluorobenzy1)-2-methyl- - N
F
<7.- IN,,.*..___
6-(3-vinyl-5H-pyrrolo[2,3-
147 403.1
b]pyrazin-5-yI)-1H-imidazo[4,5- -7---"--- \ N
b]pyridine N\.\ /
F
ii
,
N N
1-(3,5-difluorobenzyI)-6-(3-((1Z)- I \>
m ''''' N F
3-methoxyprop-1-en-l-y1)-5H- /
148 447.2
bYrrolo[2,3-b]pyrazin-5-yI)-2- /
methyl-1H-imidazo[4,5-b]pyridine N , N 0
......N N
,, s? F
(5-(1-(3,5-difluorobenzyI)-2-
N
N
methyl-1H-imidazo[4,5-b]pyridin-
149 ¨ N 491.2
6-yI)-5H-pyrrolor2,3-b]pyrazin-3- N...2_1
yOrnethyl pivalate \._ 0)..rk F
0
N N
1-(3,5-difluorobenzyI)-6-(3-(1- c_fsci '------j N F
150
methoxyethyl)-5H-pyrrolo[2,3-
¨
b]pyrazin-5-y1)-2-methyl-1 H¨ N 435.1
imidazo[4,5-b]pyridine N____9..... 0 F
N N
(5-(1-(3,5-difluorobenzyI)-2-
N N F
151
-"-
methyl-1H-imidazo[4,5-b]pyridin- / - 6-y1)-5H-pyrrolo[2 407.1
OH y1)methanol --- N
N...._ic.., OH F
.õ,..
1-(3,5-difluorobenzyI)- 11 6-(3- - N> çççF
(methoxymethyl)-5H-pyrrolo[2.3-
152 421.2
b]pyrazin-5-y1)-2-methyl-1H- >7-------N
imidazo[4,5-b]pyridine N ,.....Lcs___ 0 F
\
202
CA 03021185 2018-10-16
A00039
[Table 1-23]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt MS
N N
_._ \>
1-(3,5-difluorobenzy1)-6-(3- </: -, ', = t;sl -"..- N F ,
(difluoromethyl)-5H-pyrrolo[2,3-
153 b]pyrazin-5-y1)-2-methyl-1H- .7.-- N 427.1
imidazo[4,5-b]pyridine N /
----__. F F
F
N N
. J,.._.. \
1 -(3,5-difluorobenzy1)- 4.
6-(3- N N> F
ethyny1-5H-pyrrolo [2,3-b]pyrazin-
154 401.1
5-Y0-2-methyl-1H-imidazo[4,5- 11- N
b]pyridine N.. j...c
F
k\N\
N N
1 -(3,5-difluorobenzy1)-6-(3-((2- N
- N F
th methoxyeoxy)methyl)-5H-
55 C---CN..--C:.------ 465.1 1
pyrrolo12,3-blpyrazin-5-0-2- N i
methyl-1H-imidazo[4,5-b]pyridine ---c... 0 F
\ )
0
N N
1 -(3,5-difluorobenZY0- ,, r N>-- F6-(3-(3- ,, ,,1
methoxyoxetan-3-0-5H-
157 463.1
pyrrolo[2,3-b]pyrazin-5-y0-2- N \ /
methyl-1H-imidazo[4,5-b]pyridine \----s 0 F
\
0
N N ./
\X-C)
(
(R)-1-(5-(1-(3,5-difluorobenzy0- N F c-1.;=1
2-methoxy-1H-imidazo[4,5-
158
ID b]pyridin-6-y1)-5H-pyrrolo[2,3- 437.2 .:-...":-.\-- N
b]pyrazin-3-yOethanol N \ f
\--Kr OH F
N N /
1 .1,.,- ___,
(SH -(5-(1-(3,5-difluorobenzyI)- clsi\I N F
2-methoxy-1H-imidazo[4,5-
159 ¨
b]pyridin-6-y1)-5H-pyrrolo[2,3-
N\ 437.2
iN
b]pyrazin-3-yl)ethanol
\--1._ OH F
2,1õ,..._ N ,
..- 1oi
1-(5-(1-(3-fluorobenzy1)-2- q1
"-<-----------N
IP
methoxy-1H-imidazo[4,5-
160 ¨ 419.2
b]pyridin-6-y1)-5H-pyrrolo[2,3-
\--
N \ /N
b]pyrazin-3-yl)ethanol 4)..... OH F
203
CA 03021185 2018-10-16
A00039
..-
,
[Table 1-24]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
N,..,..._ N i
\>-O
1-(5-(1-(3-fluorobenzy0-2- q ''-- N
methoxy-1H-imidazo[4,5-
161 --
419.1
b]pyridin-6-y0-5H-pyrrolo[2,3- N 1110
,\..
b]pyrazin-3-yl)ethanol Nj.ci..._
OH F
,
F
1-(3,5-difluorobenzy0-6-(4-
methoxypyrrolo[l 2-b]pyridazin-5-
N
162
406.1
y0-2-methyl-1H-imidazo[4,5-
b]pyridine
(;4 1
N
N,-,,r--
/ \ N
1-((2-fluoropyridin-4-yOrnethyl)-
6-(4-methoxypyrrolo[1,2- \ /
163 389.2
b]pyridazin-5-y0-2-methyl-1H-
imidazo[4,5-b]pyridine N -.
N j
......N
6-(4-methoxypyrrolo[1,2- N
..-
b]pyridazin-5-0-2-methyl-1-((3- I \)
N
376.1
164 methyl-1,2,4-oxadiazol-5- ,"
/
yOmethyl)-1H-irnidazo[4,5- N \----<\0; N
b]pyridine 14 \ / 0\ N --c
/
N
N r\/..r..4....,NF
1-(3,5-difluorobenzy0-6-(4- .
methoxypyrrolo[1,2-b]pyridazin-5-
165 472.2
y0-2-(1-methy1-1H-pyrazol-4-0-
1H-imidazo[4,5-blpyridine / \ F
N
N , I
N
N.,-..1---
/ \
th N
6-(4-meoxypyrrolo[l ,2-
b]pyridazin-5-0-2-methyl-1-((1- \ N ' N
166
374.2
methyl-1H-pyrazol-5-y1)methyl)- / \ 0 /
1H-imidazo[4,5-b]pyridine
N . 1
OH
N
N.::r. ....j F
(1-(3,5-difluorobenzyl)-6-(4- f_ \ N 40
methoxypyrrolo[l ,2-b]pyridazin-5-
167
422.1
y0-1H-imidazo[4,5-b]pyridin-2- F
yl)methanol N -...
N . I
204
CA 03021185 2018-10-16
A00039
[Table 1-25]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt MS
OH
N N,>/_--c F
(1 s)-1-(1-(3,5-difluorobenzy0-6-
(4-methoxypyrrolo[l .2-
168 436.2
b]pyridazin-5-y0-1H-imidazo[4,5- / \
b]pyridin-2-y0ethanol N
N ..j
,
OH
N N,,,,T.... N,,,/ F
1-(1-(3,5-difluorobenzy0-6-(4-
methoxypyrrolo [1 ,2-b]pyridazin-5-
169 463.2
yi)-1H-imidazo[4,5-1:]pyridin-2- / µ
yl)azetidin-3-ol N 0 -... F
. N j
OH
Nz'
N'.s1.2.--,N' F
(1R)-1-(1-(3,5-difluorobenzyI)-6- / \\ 170 N
436.1
(4-methoxypyrrolo[1,2-
b]pyridazin-5-y0-1H-imidazo[4,5- / \ F
b]pyridin-2-ypethanol N 0..
0--
N N
_ :.=/---/
1-((2-fluoropyridin-4-y0methy0-
171
2-(methoxymethy0-6-(4- \ / N_< 417.1
methoxypyrrolo[12-b]pyridazin-5-
/ \
y0-1H-imidazo[4,5-b]pyridine 0 F
N
N N
_ ----4
2-cyolopropy1-1-((2-fluoropyridin- \ / N - N
413.1
4-y0methyl)-6-(4-
\ /
172
methoxypyrrolo[1,2-b]pyridazin-5- i \
y!)-1H-imidazo[4,5-b]pyridine N 0,., F
N
N _ Nõr N')
2-(azetidin-1-y0-1-((2-
173 \ / N ¨ ti
fluoropyridin-4-y0methy0-6-(4-
\ /
methoxypyrrolo[1,2-b]py 430.2ridazin-5- / \
yi)-1H-imidazo[4,5-13]pyridine N
1
N
N_ ..,..7...-- F
\ / N
1-(3,5-difluorobenzy0-2-methyl-
174 6-(pyrrolo[1,2-b]pyridazin-5-0)- 376.1
/ \
1H-imidazo[4,5-b]pyridine F
N
14 I ,
205
CA 03021185 2018-10-16 A00039
...
,
[Table 1-26]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
N N
- i \ > _
1-(3-fluorobenzy0-6-(4-
th -- " N
/ .-
meoxypyrrolo[2,1-
175 NN" 389.2
f][1,2,4]triazin-5-0-2-methyl-1H- il . ,
imidazo[4,5-13)pyridine ' N '
\
F
N N OH
/
(1-(3,5-difluorobenzy0-6-(4- </jIJIIIi
s)
---,,
methoxypyrrolo[2.1- / N F
176 10 ,2,4]triazin-5-0-1H-
N
imidazo[4,5-b]pyridin-2- 14 1 0
yl)methanol \
\N
F
_
N N
..---
i \>
1 -(3,5-difluorobenzyI)-2-methyl- ".=-= N F
/
177 6-(pyrrolo[2,1-f][1,2,41triazin-5-
/ 377.2
y0-1H-imidazo[4,5-b]pyridine N
rsi \ /
\:.- N
F
N m
,-- iN
I \>
1-((2-fluoropyridin-4-yOmethyl)- N
/
6-(4-methoxypyrrolo[2,1- /
178 --
390.2
\
1[1,2,4)triazin-5-0-2-methy1-1H- N / N
imidazo[4,5-bipyridine N. /
.--- N \
F
N- N
6-(4-methoxypyrrolo[2,1- -".:- ,-----
1[1,2,4]triazin-5-y1)-2-methyl-1- N
/
179 ((1-methyl-1H-pyrazol-5- /
375.2
yOmethy0-1H-imidazo[4,5- N
\------(7j) .
blpyridine N i N - N
/
N
..--
.
1-((2-fluoropyridin-4-yl)rnethy0- .,,_ lN 0 ¨
'') /
2-(methoxymethy0-6-(4- Z - N
180 methoxypyrrolo [2.1-
420.2
N
1[1,2,4]triazin-5-0-1H- \ / N
Ni / 0
imidazo[4,5-bipyridine \
\--\ N
F
N N OH
.- --.......-
I \> c
1-
1--(1-(3,5-difluorobenzy1)-6-(4- ''- N F
/
methoxypyrrolo[2, /
181
437.2
f][1,2,4]triazin-5-0-1H- N
imidazo[4,5-OPYridin-2-y0ethanol Ni /
F
206
CA 03021185 2018-10-16 A00039
4.
1
[Table 1-27]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
N N
2-(azetidin-1-yI)-1-((2-
fluoropyridin-4-yOrnethyl)-6-(4-
182 methoxypyrrolo[2,1- /
431.2
f][1,2,4]triazin-5-y1)-1H- N ¨
\ / N
imidazo[4,5-b]pyridine Ni 1 0
\
\--\ N
F
N N
6-(4-methoxypyrrolo[2,1- 1 \>
N
1[1,2,41triazin-5-y1)-2-methyl-1- / i
183 ((2-(trifluoromethyl)pyridin-4-
N 440.1
µ---Sr:----
/
yOmethyl)-1H-imidazo[4,5- Ni i O\
Il]pyridine ..,___ N
F
F F
N N
1-((2-methoxypyridin-4- . 1
yOmethyl)-6-(4- `= N
184 methoxypyrrolo[2,1- / I ----
402.1
f][1,2,41triaz1n-5-y1)-2-methyl-1H- N
imidazo[4,5-b]pyridine INI .\ i) \
\._.- N 0..
N N..-
1 \
1-((2-chloropyridin-4-yl)methyl)- N>
6-(4-methoxypyrrolo[2,1- / 1
185 --
406.1
1[1,2,4]triazin-5-y1)-2-methyl-1H- N
0
imidazo[4,5-b]pyridine NI \---)--- \
\-- N
Cl
N N.. -----
6-(4-methoxypyrrolo[2,1- .
1 \>
f][1,2,4]triazn-5-y1)-2-methyl-1- N
/
186 ((3-methyl-1,2,4-oxadiazol-5- /
N\ <Q ., 377.2
yOmethyl)-1H-imidazo[4,5- N "-----(\ ;"
b]pyridine Ni , 0\ N 4\
..- N
N N---
2-cyclopropy1-1-((2-fluoropyridin-
4-yl)methyl)-6-(4- / N
187 methoxypyrrolo[2,1- / --
416.1
N
1[1,2,4]triazin-5-yI)-1H-
------cN
imidazo[4,5-b]pyridine Ni 1 0\
F
N N
2-(3,3-difluorocyclobuty1)-1-((2- --;,- 1--- \)_<>< F
fluoropyridin-4-y1)methy0-6-(4- / N F
188 methoxypyrrolo [2,1- / \ N
466.2
f][1,2,4]triazin-5-y0-1H- N
-----c.,
imidazo[4,5-b]pyridine K , 0
---- N \
F
207
CA 03021185 2018-10-16
A00039
6.
1
[Table 1-28]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
N N OH
../...... -----
1-(1-(3,5-difluorobenzy1)-6-(4- , I \>
- N F
/
methoxypyrrolo[2,1- /
189 437.1
f][1,2,41triazin-5-y1)-1H- N
imidazo[4,5-b]pyridin-2-yDethanol Isi /
--- N \ F
,... N N OH
1-(1-(3,5-difluorobenzy1)-6-(4- .õ,_ I \>
- N F
/
methoxypyrrolo[2,1- /
190
437.1
f][1,2,4]triazin-5-yI)-1H- N
IP
imidazo[4,5-b]pyridin-2-yl)ethanol 14 /
\\-. N \
F
,
N -.., k 1
..., , IN
I \)
6-(4-methoxypyrrolo[2,1- 0 ''' N
/
191 f][1,2,4]triazin-5-y0-2-methy1-1- / N
(1,3-thiazol-2-ylmethyl)-1H- N V---</ .I)
378.2
imidazo[4,5-b]pyridine Ni , / S
\I- N \
.. , - N
_
3-(1-(3,5-difluorobenzy1)-6-(4-
N /
......_
methoxypyrrolo[2,1- - N F
192 / f][1,2,4]triazin-5-y1)-1H- /
446.1
imidazo[4,5-b]pyridin-2- N
yl)propanenitr Nile / O\"
F
N 6-(4-methoxypyrrolo[2.1 - ---.... --.... N--
f][1,2,4]triazin-5-0-2-methyl-1- I \>
N
193 ((5-methyl-1,3,4-oxadiazol-2-
/ N
377.2
yOrnethyl)-1H-imidazo[4,5- N N
0
A
b]pyridine N/
O\ \
\-\ N
N N
--
1 \>
1-((6-fluoropyridin-2-yOrnethyl)-
6-(4-methoxypyrrolo[2,1- /
194 / 390.1
f][1,2,4]triazin-5-y0-2-methyl-1H- N
imidazo[4,5-b]pyridine Ni /
INV--ci /-----
N \
F
= . N
1-((6-chloropyridin-2-yl)methyl)- / \ N
6-(4-methoxypyrrolo[2,1-
\----q
195 N
406.1
f][1,2,4]triazin-5-y1)-2-methyl-1H- / \
0 CI
imidazo[4,5-b)pyridine --.
N 1
N --. N
208
CA 03021185 2018-10-16
A00039
..
t
[Table 1-29]
-
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Set
MS ,
_
N N '
1-(1-(3.5-difluorobenzyI)-6-(4- I \>-. N>- OH
methoxypyrrolo[2,1- / '' N F
196 f][1,2,4]triazin-5-0-1H- / /
464.2
N
imidazo[4,5-b]pyridin-2- 0
N \
yl)azetidin-3-ol \\- N
F
N N /-- "----
2-ethozy-1-((2-fluoropyridin-4- I \ - 0
yl)methyl)-6-(4- / N
1
197 methoxypyrrolo[2,1- N --
420.2
f][1 2 0 ,4]triazin-5-yI)-1H- \
/ N
Ni \ /
\
imidazo[4,5-b]pyridine \- N
F
N N OH
I \> /
(1-(3,5-difluorobenzy0-6-(4- ''' N F
/
198
ethoxypyrrolo[2.1-1[1,2,4]triazin- /
5-y1)-1H-imidazo[4
437.1
,5-b]pyridin-2- N
yl)methanol 1,4 I 0
\-- N
F
N N
6-(4-methoxypyrrolo[2.1-
f][1,2,41triazin-5-0-2-methyl-1-
N
199 ((2-methy1-1,3-oxazol-4- /
/
376.2
yl)methyl)-1H-imidazo[4,5-
b]pyridine ni / , N =c
'
.
,
N N
..-
6-(4-metho xypyrrolo [2.1 -
N
f][1,2,4]triazin-5-y1)-2-methyl-1- /
200 i -...
386.1
((2-methylpyridin-4-yl)methyl)- N
1H-imidazo[4,5-b]pyridine 0
N \ / \
\.- N
N N
1-((2-chloro-6-methylpyridin-4-
yOmethyl)-6-(4- ''''= N
201 methoxypyrrolo[2,1-
420.1
f][1,2,4]triazin-5-y1)-2-methyl-1H- N 0 \ / N
imidazo[4,5-b]pyridine Ni , / \
\.- N
Cl
,
OH
N
(1-(3-fluorobenzy0-6-(4- N :7----j
methoxypyrrolo[2,1-
202 f][1.2,4]triazin-5-0-1H-
405.1
imidazo[4,5-b]pyridin-2- / 1 0 F
yOmethanol N -..
. 1
N-. N
-..--
209
CA 03021185 2018-10-16 A00039
,
t
[Table 1-30]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Sett
MS
N N
6-(4-methoxypyrrolo[2,1- .
1 \>
f][1,2,4)triazin-5-0-2-methyl-1- -.. N
1
203 ((1-methyl-1H-pyrazol-3- / N
375.2
N
\-------ciN ---
yl)methyl)-1 H-imidazo [4,5- 0
ni /
b]pyridine \
\--` N
N N
6-(4-methoxypyrrolo[2,1 - 1 \>
f3[1,2,4]triazin-5-0-2-methyl-l-
z
204 ((2-methyl-1 ,3-thiazol-4-
392.1
N
yl)methy1)-1 H-imidazo [4,5- Nj / 0 \ S
b]pyridine \
\ - N \
N N
--:- 1-----
\>6-(4-methoxypyrrolo[2,1-
N
1[1 ,2,4]triazin-5-y1)-2-methyl-1 - / i \ ,N
205
362.2
(1 ,3-oxazol-2-ylmethyl)-1 H- N
imidazo[4,5-1Apyridine 1,4 / o
o
\\-- N \
N N
6-(4-methoxypyrrolo[2,1-
1 `>---
I][i ,2,41triazin-5-y1)-2-methy1-1-
206 ((l-methyl-1H-1,2,3-triazol-4- 1
376.2
yOmethyl)-1H-imidazo[4,5- N = N
b]pyridine ni i
\.- N
N N
6-(4-methoxypyrrolo[2,1 - .
'"
1 \> -
1[1 ,2,4]triazin-5-y0-2-methy1-1-
N
207 ((2-methyl-1,3-oxazol-5- / ."
/ 0 ,.......õ
376.2
yl)methyl)-1H-imidazo[4,5- N
\¨ i /
b]pyridine Ni , --(e_. o N
--- N \
N N
6-(4-methoxypyrrolo[2,1 -
/UC I\/>----
fill ,2,43triazin-5-0-2-methy1-1- / 1 ___ N
208
373.2
th (pyrazin-2-ylmeyl)-1H- N
imidazo[4,5-b]pyridine Ni \---)¨ O\ ---(-
\11./2
N_- N
N N
...--
,
I \>
6-(4-methoxypyrrolo[2,1- N1µ ,N
0 373.2
209
al ,2,41triazin-5-0)-2-methyl-l- /
I
(pyrimidin-2-ylmethyl)-1H- N ------c N D- - -
i m i d a z o [ 4 , 5 - b ] p y r i d i n e Nj
N
, -----¨ \
\.;.¨ N
_
210
CA 03021185 2018-10-16 A00039
...
[Table 1-31]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt MS
N N.--
6-(4-methoxypyrrolo[2,1- ..'` N
372.2
f][1,2,41triazin-5-y1)-2-methy1-1-
,
210
(pyridin-4-ylmethyl)-1H- N
imidazo[4,5-bipYridine
ni
N N
, 1 \>
6-(4-methoxypyrrolo[2,1- - N
372.2
f][1,2,4]triazin-5-y1)-2-methy1-1- / 1
-- 211
(pyridin-3-ylmethyl)-1H- N
imidazo[4,5-b]pyridine 0 \ /
N i \ N
_ N
,
N N
..-
6-(4-methoxypyrrolo[2,1- i \>
f][1 ,2,4]triazin-5-0-2-methyl-1-
212 ((5-methyl-1,2-oxazol-3- / N 376.2
N
\----q)
yl)methyl)-1H-imidazo[4,5- ,,,; / 0 ¨
b]pyridine " \_-\ N \
, .
N 6-(4-methoxypyrrolo[2,1-
N
f][1,2,4]triazin-5-y1)-2-methy1-1- 1 N>
213 ((2-methyl-1,3-thiazol-5- / / S 392.1
yl)methyl)-1H-imidazo[4,5- N \---(L --r--"I
b]pyridine N i \ N
\¨\ N
N N
6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1- - N
/
214 ((5-methyl-1,2,4-oxadiazol-3- / N 377.2
yl)methyl)-1H-imidazo[4,5- N ----(' .
b]pyridine Ni , , 0\ 147=-
\-- N
N N
..-
6-(4-methoxypyrrolo[2,1- i `>--
f][1,2,4]triazin-5-y1)-2-methyl-1- 0
215 ((3-methyl-1,2-oxazol-5- / 376.2
N \ ; N
yOmethyl)-1H-imidazo [4,5- K \ i 0\
b]pyridine \-- N
N N
1-((3-cyclopropy1-1,2,4-oxadiazol-
---, I N\>---
5-yl)methyl)-6-(4- /
/ 0
216 methoxypyrrolo[2,1- N \-----(µ ; N 401.1
f][1.2,4]triazin-5-y1)-2-methyl-1H- i = 0
N \ \
imidazo[4,5-b]pyridine \-- N
-
211
CA 03021185 2018-10-16 A00039
...
,
[Table 1-32]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
N N
1-((3-(difluoromethyl)-1.2,4- I \>
-'" N
oxadiazol-5-yl)methyl)-6-(4- /
/
217 methoxypyrrolo[2,1- No ; N 411.1
f][1,2,4]triazin-5-y1)-2-methy1-1H- N' \ N ¨____
imidazotz1,5-b]pyridine \-- N \ F
F
N N
1-((1,3-dimethy1-1H-pyrazol-5- I I \>¨
yl)methyl)-6-(4- '- N I
/ N
218 methoxypyrrolo[2,1- 389.2
f][1,2,4)triazin-5-y1)-2-methy1-1H- N 0 \----c\ ;111
imidazo[4.5-b]pyridine
`N
N N..--.
--
I \>¨
1-((2--fluoropyridin-3-yOmethy0-- N
13-(4-methoxypyrrolo[2.1- / 1
219 390.2
f][1,2,41triazin-5-y1)-2-methyl-1H- N
imidazo[4,5-b]pyridine o
Ni , i \ \---1---;1N
\.¨ N F
,
N N
6-(4-methoxypyrrolo[2,1-
fill ,2,4]triazin-5-y1)-2-methy1-1-
220 ((5-methyl-1,3,4-thiadiazol-2- / N
393.2
yl)methyl)-1H-imidazo[4,5- 2.4\
b]pyridine
\_- N
N N
/
1-((4-chloropyridin-2-yOmethyl)-
I
6-(4-methoxypyrrolo[2,1- \ /
221 406.1
f][1,2,4]triazin-5-y1)-2-methy1-1H- / \ 0 CI
imidazo[4,5-b1pyridine N --.
,
N-. N
N N
6-(4-methoxypyrrolo[2.1- I
f][1,2,4]triazin-5-y1)-2-methyl-1-
222 ((3-methyl-1,2-thiazol-5-
b]pyridine 0 /
/ '''' 392.1
yOrnethyl)-1H-imidazo[4,5- N
--\\ t c
Ni i
\
\ - - -N N
, _
...,14 N r- OH
--
2-(1-(3,5-difluorobenzy1)-6-(4-
223 1 \> i
methoxypyrrolo[2,1- /
/ 437.2
f][1,2,4]triazin-5-y0-1H- N
N F
imidazo[4,5-b]pyridin-2-ypethanol Ni / 0
..-- N \
F
212
CA 03021185 2018-10-16 A00039
I.
,
[Table 1-33]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
,
N --__It,N
14 thy1-1,2-oxazol-4- / \ N(3,5-dime - =
.., 0
yl)methyl)-6-(4- ¨
224 methoxypyrrolo[2,1-
390.2
f][1,2,4]triazin-5-y1)-2-methy1-1H- 4/, \ 0 ,
imidazo[4,5-b]pyridine N i
N , N
N N
6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methyl-1- - N
/
225 ((4-methyl-1,3-thiazol-2- / v ,S
391.9
N
yl)methyl)-1H-imidazo[4,5- ----CN
\ \
b]pyridine Ni 1 0
\¨ N
N
, 1-((3-ethy1-1,2,4-oxadiazol-5- N
I \>---
yOmethy1)-6-(4- y N
/ N
226 methoxypyrrolo[2,1- N Võ.,1i0,N
389.0
f][1,2,4]triazin-5-y1)-2-methyl-1H- N, , 0
imidazo[4,5-blpyridine \s--- N \ N
N
,J ,,,-.7---
_
1-((5-fluoropyridin-3-yl)methyl)- N
_____. \ N
6-(4-methoxypyrrolo[2,1- \ /
227
390.2
f][1,2,4]triazin-5-0-2-methyl-1H- / \ F
imidazo[4,5-b]pyridine 0
N 1 ---
N-.. N
--....--
OH
(1-(3,4-difluorobenzy1)-6-(4- N N,,,r_j
F
methoxypyrrolo[2,1- ' \ N
228 f][1,2,4]triazin-5-y1)-1H-
423.1
imidazo[4.5-b]pyridin-2- / µ 0 F
yl)methanol N
. 1
N-.. N
--...-
N
6-(4-methoxypyrrolo[2,1- S - N
f][1,2,4]triazin-5-y1)-2-methyl-1-
229 ((3-methyl-1,2,4-thiadiazol-5- N
393.2
/ \
yOmethyl)-1H-imidazo[4,5- 0
N
b]pyridine . 1
N -.. N
N N
6-(4-methoxypyrrolo[2,1- .
1 \>
f][1,2,4]triazin-5-y1)-2-methy1-1- .'" N
230 ((1-methyl-1H-pyrazol-4- /
/ 3752
N
yOmethyl)-1H-imidazo[4,5- N \----__C--
b]pyridine Ni , T> 0\ N
\_- N
N.
213
CA 03021185 2018-10-16
A00039
s
[Table I-34]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
6-(4-methoxypyrrolo[2,1- . N N
I \>
f][1,2,4]triazin-5-0-2-methy1-1- t4\ õ
N
231 ((1-methy1-1H-1,2,4-triazol-3-
/ 376.2
yOmethyl)-1H-imidazo[4,5- 1(.1---(---------
0
b]pyridine N / N - N
---N \ N
N 6-(4-methoxypyrrolo[2,1- . N,
f][1,2,4]triazn-5-y1)-2-methy1-1- 1 `J
232 ((5-methyl-1,3-thiazol-2- /
/ --- N\._ ,s
392.1
yOmethyl)-1H-imidazo[4.5- N
b]pyridine N. N
\\_- N \
r
/N r--
3-((6-(4-methoxypyrrolo[2.1- \ N N - 0
f][12,4]triazin-5-y1)-2-methy1-1H- / \---</ )....,
233 imidazo[4,5-b]pyridin-1-
393.2
\
yOmethyl)-4-methyl-1,2,4- 0 /
N -...
oxadiazol-5(4H)-one i
N N
-
N
N -Z-7----
1 \ N p- N
6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methy1-1- N
234 ((3-(trifluoromethyl)-1,2,4- / 1
F F 429.0
0
oxadiazol-5-yl)methyl)-1H- N --.
imidazo[4,5-b)pyridine . 1
N --.. N
---...--
,
N
N,-...õ---=
6-(4-methoxypyrrolo[2,1-
235 ((2-methylpyr N
f][1,2,4]triazin-5-0-2-methy1-1-
387.2
imidin-4-yl)methyl)- / k r,
1 H-imidazo[4,5-b]pyridine N ' -...
, 1
N N
,...õ.õ
N
I -(2-furylmethyl)-6-(4-
methoxypyrrolo[21-
,
236 361.2
f][1,2,41triazin-5-0)-2-methyl-1H-
imidazo[4,5-b]pyridine N 1 ---.
N-.. N
N Nõ,,r---
/ \ N, IN ----\
6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-methy1-1- \-----2
237
372.2
(pyridin-2-ylmethyl)-1H- /N\ 1 0
imidazo[4,5-b]pyridine --..
N-.. N
---....
214
CA 03021185 2018-10-16
A00039
.
,
[Table 1-35]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
N N..-
1-((1,5-dimethy1-1H-pyrazol-3- ..--
1 \.>
yOmethyl)-6-(4- ..' N
238 methoxypyrrolo[2,1- /
389.2
f][1,2,4]triazin-5-y0-2-methyl-1H- N 0 \----_.(\'-'-:-('
Ni / N - N
imidazo[4,5-b]pyridine \ "..
\--\ N
0 --
2-(methoxymethyl)-6-(4- N N,y
methoxypyrrolo[2,1- - N
239 f3[1,2,4]triazin-5-y1)-1-((3-methyl- -
\----K\ * 405.0
N - --.
1,2,4-oxadiazol-5-yOmethyl)-1H- / \
0
imidazo[4,5-b]pyridine N
N N
1-q3-methoxy-1-methy1-1H- , N N
..-
pyrazol-5-yOmethy0-6-(4- , I \>--
N
240 methoxypyrrolo[2,1- /
/ 0
405.2
f][1,2,4]triazin-5-0-2-methyl-1 H- N \---{<7/r-
imidazo[4,5-b]pyridine N / (:), N - N
..- N ' /
F F
F
2,2,2-trifluoro-1-(1-((2-
N NI
, N'rl-OH
fluoropyridin-4-yOrnethyl)-6-(4- 1._ ' N
241 methoxypyrrolo[2,1- \ /
474.1
n[1.2,41triazin-5-y1)-1 H- / \
0 F
imidazo[4,5-b]pyridin-2-yl)ethanol N -...
i
N=-õ,..õ.N
N
N
1-((5-(difluoromethyl)-1,3,4- / \
' N N. N
oxadiazol-2-yl)methyl)-6-(4-
242 methoxypyrrolo[2,1- 0
413.2
/ \
OD ,2,4]triazin-5-y1)-2-methyl-1H- 0
imidazo[4,5-b]pyridine . = 1
N . N
N
N :7------
1-((5-(1,1-difluoroethyl)-1,2,4- / \ Ni
oxadiazol-3-yOmethyl)-6-(4-
243 methoxypyrrolo[2,1-
425.0
/ \
f][1,2,4]triazin-5-y1)-2-methyl-1H- 0
imidazo[4,5-b]pyridine . 1
N-. N
...--
N
N ,:r
1-((4,6-dimethylpyrimidin-2-
yOmethyl)-6-(4-
244 methoxypyrrolo[2,1- N -
401.2
f][1,2,4]triazin-5-y0-2-methyl-1H-
imidazo[4,5-b]pyridine
KI ,z.N
215
CA 03021185 2018-10-16 A00039
,
[Table 1-36]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
N F
1-((1-(difluoromethyl)-1H- / \ N N-N)--F
pyrazol-3-yl)methy1)-6-(4- --- /
--
411.2
245 methoxypyrrolo[2,1-
/ 1
f][1,2,4]triazin-5-0-2-methyl-1H- N 0
--...
imidazo[4.5-b]pyridine . 1
N --.. N
N N
6-(4-rnethoxypyrrolo[2,1-1-
f][1,2,41triazin-5-y1)- th 2-mey1-1- ,, / F ''' N F
246 ((1-methyl-3-(trifluoromethyl)-
443.2
N / i F
1H-pyrazol-5-yOmethyl)-1H- tj / N - N
imidazo[4,5-b]pyridine \-..- N \ /
N N
.--,... --....--
1-((1-(difluoromethyl)-1H- i \>
Kj.V...-------' N
pyrazol-5-yOmethyl)-6-(4-
/
247 methoxypyrrolo[2,1- N \------(27'7
411.2
f][1,2,4]triazin-5-0-2-methyl-1H- NI' , 0 N _ N
imidazo[4,5-b]pyridine \`µ-'- N \ F ----(F
,
N
N,-..."---
1-((6-chloropyrazin-2-yl)methyl)- N
_4
-
6-(4-methoxypyrrolo[2,1-
248 N
405.0
f3[1,2,4]triazin-5-0-2-methy1-1H- / µ
0 Cl
imidazo[4,5-b]pyridine -...
N 1
N-. N
--....-
N
N,-.1---
1-((2-methoxypyridin-3- / \ N ¨
yl)methyl)-6-(4- \ /
249 methoxypyrrolo[2,1-
402.2
f][1,2,4)triazin-5-y1)-2-methyl-1H-
imidazo[4,5-b]pyridine _. I \
N ... N
N
õN
6-(4-methoxypyrrolo[2.1- \ N N - N
f][1,2,4]triazin-5-yI)-2-methyl-1- --...
250 ((5-(trifluoromethyl)-1,3,4- \ ---(10 .L<
431.1
/ \ F
oxadiazol-2-yl)methyl)-1H- 0
N -... F
imidazo[4,5-b]pyridine , 1
N-. N
,
N
2-(3-((6-(4-methoxypyrrolo[2,1- N -:7----
' \ N "
f][1,2,4]triazin-5-0-2-methy1-1H-
251 imidazo[4,5-b]pyridin-1- N
421.2
yl)methyl)-1,2.4-oxadiazol-5- OH
--..
yl)proparr-2-ol N1
N-.. N
216
CA 03021185 2018-10-16 A00039
p
,
[Table 1-37]
EXAMPLE IUPAC NAME , CHEMICAL STRUCTURE
Salt , MS
N
1-((5-ethy1-1,3,4-oxadiazol-2- N ..--r-
/ \ N N-N
yOmethyl)-6-(4- __
252 methoxypyrrolo[2,1- ¨ 0
391.2
f][1,2,4]triazin-5-y1}-2-methy1-111- / \ o
imidazo[4,5-b]pyridine N '-
, I
N... N
-,--
N
N -:-/-----
1-((5-cyclopropy1-1,3,4-oxadiazol- / \ N ,N - N
2-yOmethyl)-6-(4-
0 -).----,7
253 methoxypyrrolo[241-
403.2
/ \
f][1,2,41triazin-5-y1}-2-methy1-1H- 0
N --...
imidazo[4,5-b]pyridine i
N N
..,....,....
NN.Z.)----
1-((5-(methoxymethyl)-1,3,4- // \ N ,N ' N
oxadiazol-2-yl)methyl)-6-(4-
0 -----
254 methoxypyrrolo[2,1-
407.2
f][1.2,4]triazin-5-y1)-2-methyl-1H-
imidazo[4,5-b]pyridine ... I
n/ , N
,..--
OH
(1-((2-chloro-6-methylpyridin-4-
yOmethyl)-6-(4- / \ N ¨
methoxypyrrolo[2,1- N
255 \ i 433.9
f][1,2,4]triazin-5-yI)-1H-
imidazo[4,5-b]pyridin-2-
yl)methanol N , N
OH
(1-(4-fluorobenzy1)-6-(4- N
/ \
methoxypyrrolo[2,1- F
256 f][1,2,4]triazin-5-yI)-1H- ____ N op
405.1
imidazo[4,5-b]pyridin-2- / \ 0
yOmethanol N
. 1 -..
N-.. N
OH
N
N
(1-(4-chlorobenzyI)-6-(4- ..--r---j
/
methoxypyrrolo[2,1-
257 f][1,2,4]triazin-5-yI)-1 \ N CI H-
421.2
imidazo[4,5-b]pyridin-2- / \
0
N-.
yl)methanol 1
N N
--:..--
N N
..---
.--
1-((1,4-dimethy1-1H-pyrazol-5- I \>
yOmethyl)-6-(4-
th Z -..." N
258 meoxypyrrolo[2,1-
389.3
. N \-------
f][1.2,4]triazin-5-y1)-2-methyl-1H- , 0 N - N
imidazo[4,5-b]pyridine N /
\
\---\ N /
217
CA 03021185 2018-10-16 A00039
s
[Table 1-38]
EXAMPLE IUPAC NAME CHEMICAL
STRUCTURE Salt MS
N N
.--
1-((1-(difluoromethyl)-1H- 1 =>_._
pyrazol-4-yl)methyl)-6-(4-- /
259 methoxypyrrolo[2,1- N \-----e N
411 2
.11[1 ,2,4]triazin-5-yI)-2-methyl-1H- Ni i 0 Nsr F
imidazo[4,5-b]pyridine \._-\ N \
F
N N
1-((2,5-dimethy1-1,3-oxazol-4- ..-
yl)methyl)-6-(4- -".= N
/
260 methoxypyrrolo[2,1- /
390.2
\------(20
f][1,2,4]triazin5-y1)-2-methy1-1H- N - Ni 1 0 IV ---:-
imidazo[4,5-b]pyridine N \
N ,õ.._ N
1-((2,4-dimethy1-1,3-oxazol-5-
yl)methyl)-6-(4- ''." N
/
261 methoxypyrrolo[2,1- N /
390.2
\--_(. -"==
f][1,2,4]triazin-5-y1)-2-methy1-1H- N 0\ 0
imidazo[4,5-b]pyridine N i
...-N N \.
N N
...=
1-(0 ,4-dimethy1-1H-pyrazol-3- I \>
yOmethyl)-6-(4-(4 / ' N
/
262 methoxypyrrolo[2,1- 389.2 N \---_(:::1
f][1,2,4]triazin-5-0-2-methy1-1H- = 0 N - N
imidazo[4,5-b]pyridine N \ i
`..- N \ \.
N
iNI ,:-7
.---
' \ -.--
6-(4-methoxypyrrolo[2,1-
N N
263
1][1,2,4]triazin-5-y1)-2-methyl-1-
N ((4-methylpy 387,2rimidin-2-yl)methyl)- / l 0
1 H-imidazo[4,5-b]pyridine N --..
. i
N -... N
N
N..õ.../..--=
6-(4-methoxypyrrolo[2,1- z \ N N ---c._
f][1.2,4]triazin-5-y1)-2-methyl-1-
264 ((4-(trifluoromethyl)pyrimidin-2- / \
N 441.1
O F
yOrnethyl)-1H-irnidazo[4,5- N --.
b]pyridine . 1
N -- N FE
---...,
N
N::,T----
1-((5-isobuty1-1,3,4-oxadiazol-2- ' \ N N - N,L
yOmethy0-6-(4- ___ \____<! ,
0
265 methoxypyrrolo[2,1-
419.2
f][1,2,4]triazin-5-y1)-2-methyl-1H-
imidazo[4,5-b]pyridine . 1
N , N
- -
218
CA 03021185 2018-10-16
A00039
[Table 1-39]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
N
6-(4-methoxypyrrolo[2,1- N ..,-.T...--
f][1,2,4]triazin-5-y1)-2-methy1-1- / \ N N -
((4-methyl-6-
(trifluoromethyl)pyr 266 455.2 imidin-2- / \ N
YOrnethyl)-1H-imidazo[4,5- N 0
--, F
b]PYridine . i F F
N-... N
N
;:r--
1-((5-isopropyl-1,3,4- N oxadiazol-2- / \ N N-N
y Dm ethyl)-6-(4- ¨ \----%
267 meth oxypy rrolo[2,1- 405.2
f][1,2,4]triazin-5-y1)-2-methyl-1H- / 1 0
imidazo[4,5-b]uyridine N
i -..
N-.. N
-,...--
.. .
N
N---7-----
1-((5-cyclobuty1-1,3,4-oxadiazol-
' \ N N - N
2-yOmethyl)-6-(4-
268 methoxypyrrolo[2,1- 417.2
f][12,4]triazin-5-y1)-2-methyl-1H- / 1 0
imidazo[4,5-b]pyridine N
N --, N
N N===r---
1-((5--(3,3-difluorocyclobuty1)- / \ N 1\1 - N
1,3,4-oxadiazol-2-yOmethyl)-6-(4-
269 methoxypyrrolo[2,1- F 453.2
/ 1
1f ,[1,2,4]triazin-5-y1)-2-methyl-1H-
F
imidazo[4,5-b]pyridine " 1
N .z...., N
. .
. N
N'-7--
6-(4-methoxypyrrolo[2,1- ' \ N N - N
f][1,2,4]triazin-5-y1)-2-m ethyl-1- ¨ \--%1K
270 ((5-(1-methylcyclopropy1)-1,3,4- 417.2
oxadiazol-2-yOmethyl)-1H-
imidazo[4,5-b]pyridine I
N -..z......,N
,
N
N:-T-----
1-((5-(1.1-difluoropropy1)-1,3,4- / \ N, j -N F
oxadiazol-2-yOmethyl)-6-(4-
¨ ¨- .(3-1-1---t:
271 meth oxypyrrolo[2,1- 441.2
/ 1 0 õõ.
f][1,2,43triazin-5-0 th -2-meyl-1H- N
imidazo[4,5-b]pyridine I
N ... N
N
N--,"-i----
6-(4-m ethoxypyrrolo [2,1- N - N F
f][1,2,4]triazin-5-y1)-2-m ethyl-1- ¨
F
272 ((5-(1,1 ,2,2-tetrafluoroethyl)- 461.0
/ 1
1,3,4-oxadiazol-2-yl)methyl)-1H-
F
0 F
N --,
imidazo[4,5-b]pyridine
N -, N
219
CA 03021185 2018-10-16
A00039
,
[Table 1-40]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE
Salt MS
N
N -:)-----
/ \ N
6-(4-methoxypyrrolo[2,1- ¨ \
273 fl[1,2,4]triazin-5-0-1,2-dimethyl- 295.1
1H-imidazo[4,5-b]pyridine
N. N
--....--
NN-,---
1-((5-(1,1-difluoroethyl)-1,3,4- / \ N N-N
oxadiazol-2-yDrnethyl)-6-(4- __
274 methoxypyrrolo[2,1- 0 427,1
f][1,2,41triazin-5-0 F F-2-methy1-1H- / \ 0
N --...
imidazo[4,5-b]pyridine , t
N-.. N
-.....--
N
6-(4-methoxypyrrolo[2,1- / \ N N."
f][1,2,4]triazin-5-0-2¨methy1-1¨ ¨ \----0 k-
275 ((5-(3-methyloxetan-3-y1)-1,3,4- 433.2
\
oxadiazol-2-yOmethyl)-1H- / 0 0
-..
imidazo[4,5-bipyridine N. I
N-.. N
--...--
,
N N=:!-----
1-((5-(2,2-difluorocyclopropyl)- / \ N 1'1 - N
1,3,4-oxadiazol-2-yOmethyl)-6-(4-
276 methoxypyrrolo[2,1- 439.2
f][1,2,4]triazin-5-y1)-2-methyl-1H-
imidazo[4,5-b]pyridine N
F F
N.. N
------
N Nr,._
1-((5-(2-fluoropropan-2-y1)-1,3,4- / \ N N- N
oxadiazol-2-yl)methyl)-6-(4- ¨ ----% )cF
277 methoxypyrrolo[2,1- 423.2
f][1,2,4]triazin-5-0-2-methy1-1H-
imidazo[4,5-b]pyridine N
N ,.. N
INJ ,:-7.----
1-((6-methoxypyrazin-2- / \ N, F--- N
yl)methyl)-6-(4- N---, 4
278 methoxypyrrolo[2,1- N 403.2
/ \
f][1,2,4]triazin-5-0-2-methyl-1H- 0 0 ¨
N ---
imidazo[4,5-b]pyridine
N.... .. .. , .. , õ N
N N
1-((5-(1-fluoroethyl)-1,3,4-
oxadiazol-2-yOmethyl)-6-(4- / N
i N
279 methoxypyrrolo[2,1- N \------(, ' N
409.2
f][1,2,4]triazin-5-y1)-2-methyl-1H- r4 , 0 0 -L5_
imidazo[4,5-b]pyridine \---\ N \
F
220
CA 03021185 2018-10-16 A00039
[Table 1-41]
EXAMPLE WRAC NAME CHEMICAL STRUCTURE Salt MS
N N
--'."-
6-(4-methoxypyrrolo[2,1- N
1 \)
-a [1,2,4]triazin-5-y1)-2-methyl-1-
280
(1,2,3-thiadiazol-4-ylmethyl)- 1 H- N \----- ,(:." S 379.2
imidazo[4,5-b]pyridine N : KI
_.- N \
N,
N -Zr
6-(4-methoxypyrrolo [2,1- / \ N " F F
f][1,2,4]triazin-5-y1)-2-methy1-1-1.....L__). F
281 ((5-(2,2,2-trifluoroethyl)-1,3,4-
0 445.2
/ \
oxadiazol-2-yOmethyl)-1H- 0
imidazo[4,5-b]pyridine 1
N . N
N
N -:27---- th N
/ \ F
6-(4-meoxypyrrolo[2,1-
282 ¨
f][1,2,4]triazin-5-0-2-methy1-1- S'IN
(1,2,3 N 379.2-thiadiazol-5-ylmethyl)-1H-
/ 1
0
imidazo[4,5-b]pyridine N
N . N
NN------
1-((5-(1-fluorocyclopropyI)-1,3,4- i \ INI 7 - N
oxadiazol-2-yOmethyl)-6-(4- ¨
.------7H
283 methoxypyrrol 0--- F
o[2,1- 421.2
/ \
f][1,2,4]tr1azin-5-0-2-methyl-1H- 0
N ,..
imidazo[4,5-b]pyridine I
N-. N
N
N :7----
1-((5-(1-fluorocyclobuty0-1,3,4- 1 \ N N- N
oxadiazol-2-0methyl)-6-(4- ¨ ----(10
284 methoxypyrrolo[2,1- 435.2
f][1,2,4]triazin-5-y1)-2-methy1-1H- / \ 0
imidazo[4,5-Opyridine N 1 ----.
1:1 , N
N N,
6-(4-methoxypyrrolo[2,1-
f][1.2,41triazin-5-y1)-2-methyl-1- ¨
S - N
285 ((4-methy1-1,2,3-thiadiazol-5- 393.2
/ \
yOmethyl)-1H-imidazo[4.5- 1II0
N ,..
b]pyridine I
1'.1 . N
-....--
N N
1-<(5-((1R)-1 -fluoroethyl)-1,3.4- / \ µ.--.- m
, N .. - N
oxadiazol-2-yOmethy0-6--(4-
/
----</
286 methoxypyrrolo[2,1- 0
imidazo[4,5-b]pyridine 409.3
1
f][1,2,4]triazin-5-y0-2-methy1-1H- 0
F
N
t`l , 1µ1
-,..---
221
CA 03021185 2018-10-16
A00039
[Table 1-42]
EXAMPLE IUPAC NAME CHEMICAL STRUCTURE Salt
MS
N
1-((5-(( 1 S)-1-fluoroethyl)-1,3,4- N N - N
oxadiazol-2-yl)methy0-6-(4- F
287 methoxypyrrolo[2,1- /
409.3
f][1,2,4]triazin-5-yI)-2-methyl-1H- N
imidazo[4,5-bipyridine
N
1-((5-(difluoromethyl)-1,3,4- / N ,N"N
oxadiazol-2-y1)methyl)-6-(4- --4\10 !õ\ F
288 methoxypyrrolo[1,2-b]pyridazin-5-
410.0
yI)-2-methyl-1H-imidazo[4,5- 0
b]pyridine
N
1-((5-(difluoromethyl)-1,3,4- / N N-N
thiadiazol-2-yl)methyl)-6-(4-
F
289 methoxypyrrolo[2,1-
429.2
/
fi [1,2,4]triazin-5-yI)-2-methyl-1H- 0
imidazo[4,5-b]pyridine
N N
p, N
I N
/N
6-(4-methoxypyrrolo[2,1-
f][1,2,4]triazin-5-y1)-2-rnethy1-1-
290
387.3
((6-methylpyrazin-2-yl)methyl)- \ 0
1H-imidazo[4,5-b]pyridine N
N. N
[0433]
Preparation Example 1
A medicament containing the present invention compound as an active ingredient
can
be produced in accordance with, for example, the following founulation.
[Equation 1]
1. capsule
(1) compound obtained in Example 1 40 mg
(2)lactose 70 mg
(3) microcrystalline cellulose 9 mg
(4) magnesium stearate 1 mg
222
CA 03021185 2018-10-16
A00039
one capsule 120 mg
Components (1), (2) and (3), and one-half of component (4) are mixed, and then
granulated. The remainder of component (4) is added thereto, and the whole of
the mixture is
encapsulated in a gelatin capsule.
[0434]
[Equation 2]
2. tablet
(1) compound obtained in Example 1 40 mg
(2) lactose 58 mg
(3) corn starch 18 mg
(4) microcrystalline cellulose 3.5 mg
(5) magnesium stearate 0.5 mg
one tablet 120 mg
Components (1), (2) and (3), two-thirds of component (4), and one-half of
component
(5) are mixed, and then granulated. The remainders of components (4) and (5)
are added to the
resulting granule, and the mixture is press-molded into a tablet.
[0435]
Preparation Example 2
50 mg of the compound obtained in each of examples is dissolved in 50 mL of
injection
distilled water in Japanese Pharmacopoeia, and injection distilled water in
Japanese
Pharmacopoeia Japanese is added so that the volume of the solution is 100 mL.
The solution is
filtered under sterile conditions, and 1 mL of the solution is then taken,
filled into an injection
vial under sterile conditions, freeze-dried, and hermetically sealed.
[0436]
Test example 1 (test method: measurement of CLK2 inhibitory activity)
A test compound dissolved in DMSO was added to a reaction solution (50 mM
HEPES
(pH: 7.5), 10 mM MgCl2, 1 mM EGTA, 2 mM DTT, 0.01% Tween 20, 0.01% BSA)
containing a
CLK2 enzyme (Thermo Fisher Scientific) and an ULight-labeled MBP peptide
(PerkinElmer),
and the mixture was then reacted at room temperature for 10 minutes. An ATP
solution was
added to a final concentration of 1 mM to initiate an enzyme reaction, and the
reaction was
carried out at room temperature for 60 minutes. A europium-labeled anti-
phosphorylated MBP
antibody (PerkinElmer) and a LANCE Detection buffer (PerkinElmer) containing
EDTA with a
final concentration of 20 mM were added to stop the reaction, the mixture was
left standing for
60 minutes, and the time-resolved fluorescence value (excitation 320 nm, and
emission 615 nm
223
CA 03021185 2018-10-16
A00039
and 665 rim) was measured by Envision (PerkinElmer). The inhibition ratio (%)
of CLK2 by
the test compound was calculated in accordance with the following expression.
Inhibition ratio (%) = (1-(count of test compound - blank) (control -
blank)) x 100
The count of the CLK2 enzyme reaction solution without addition of the
compound was
described as a control, and the count without addition of the compound and
addition of the CLK2
enzyme was described as a blank. The concentration at which the inhibition
ratio was 50% was
defined as an IC50 value.
[0437]
The test results are shown in Table 2.
[Table 2]
224
CA 03021185 2018-10-16
A00039
Table 2
CLK2 activity inhibition ratio at
Example No. compound concentration of 1 .M (ATP 1mM)
7 100%
102 100%
105 100%
110 IGO%
160 100%
161 100%
176 100%
=
176 100%
186 99%
193 100%
214 99%
215 100%
229 100%
242 100%
274 100%
277 100%
283 100%
286 100%
[0438]
These results showed that the present invention compound had CLK2 inhibitory
activity.
[0439]
Test Example 2 (measurement of growth inhibitory activity using HCT116 cells)
HCT 116 cells (ATCC) were seeded in a 384-well plate at a density of 6 x 102
225
CA 03021185 2018-10-16
A00039
cells/well, and cultured overnight in a cell culture medium McCoy's 5A (Thermo
Fisher
Scientific) containing 10% FBS (fetal bovine serum) and 1%
penicillin/streptomycin. The
dissolved test compound was added in the cell medium, and the cell medium was
left standing in
a CO2 incubator (37 C) for 3 days. A Cell Titer-Glo solution (Promega
Corporation) was
added, the mixture was stirred at room temperature for 10 minutes, and the
emission value was
measured by Envision (PerkinElmer). The inhibition ratio (%) of HCT116 cell
growth by the
test compound was calculated in accordance with the following expression.
Inhibition ratio (%) = (1-(count of test compound - blank) (control -
blank)) x 100
The count of the HCT116 cell suspension without addition of the compound was
described as a control, and the count without addition of the compound and
addition of HCT116
cells was described as a blank. The concentration at which the inhibition
ratio was 50% was
defined as an IC50 value.
The test results are shown in Table 3.
[Table 3]
226
CA 03021185 2018-10-16
A00039
Table 3
Cell growth inhibition ratio at
Example No. compound concentration of I RM
7 92%
102 96%
105 96%
110 92%
160 98%
161 97%
175 91%
176 85%
186 96%
193 98%
214 96%
215 96%
229 97%
242 94%
274 93%
277 95%
283 96%
286 95%
[0440]
These results showed that the present invention compound inhibited growth of
colon
adenocarcinoma cells.
[0441]
In vitro CLK2 phosphorylation inhibitory action and p53 activation action in
human
colon adenocarcinoma cells HCT 116
227
CA 03021185 2018-10-16
A00039
1000 p.1 (8,0000 cells/well) of a cell suspension of human colon
adenocarcinoma cells
HCT116 (purchased from ATCC) was seeded in a 12-well plate, and cultured at 37
C for 2 days
in a 5% CO2 gas incubator. The test compound solution was added to a final
concentration of
300 nM, and the mixture was cultured for 16 hours. The plate was washed with
PBS, a protein
extract (10% glycerol, 1% sodium dodecyl sulfate, 62.5 mM Tris-HC1 (pH: 7.5))
was then added
to dissolve the cells, and the solution was treated at 95 C for 5 minutes.
Thereafter, the amount
of protein was determined using BCA Protein Assay Kit (Thermo Fisher
Scientific).
The amount of the protein in each sample was adjusted, SDS-PAGE was then
performed, and the protein was transferred to a PVDF membrane using an iBlot
(registered
trademark) gel transfer system (Invitrogen). Blocking was performed using
StartingBlock T20
(PBS) Blocking Buffer (Thermo Fisher Scientific), and the sample was reacted
at 4 C overnight
with anti-phosphorylation CLK2 (Ser98), an anti-MDM4 antibody (Bethyl
Laboratories, Inc.,
A300-287A), an anti-p53 antibody (Santa Cruz, Inc., sc-126) and anti-p21
antibody (Santa Cruz,
Inc., sc-6246) each diluted 1000 times with Can Get Signal Immunoreaction
Enhancer Solution 1
(TOYOBO CO., LTD.). The membrane was washed with Tris-buffered saline (Bio-Rad
Laboratories, Inc.) containing 0.05% of Tween 20 (Bio-Rad Laboratories, Inc.),
and the sample
was then reacted at room temperature for 1 hour with a HRP-labeled rabbit IgG
polyclonal
antibody (NA 9340 from GE Healthcare) diluted 5000 times with Can Get Signal
Immunoreaction Enhancer Solution 2 (TOYOBO CO., LTD.). The membrane was washed
in
the same procedure as described above, a phosphorylated CLK2 protein, a MDM4
protein, a p53
protein and a p21 protein each labeled with an antibody were then made
chemiluminescent using
ImmunoStar ZETA (Wako Pure Chemical Industries, Ltd.), and luminescence was
detected with
LAS-3000 Image Analyzer (Fujifilm Corporation). The luminescence intensity was
determined
using Multi Gauge Ver. 3.1 (Fujifilm Corporation), and quantified.
[0442]
The CLK2 phosphorylation inhibitory activity of the test compound is
calculated as a
phosphorylated CLK2 residual ratio (%) in accordance with the following
expression, and shown
in Table 4.
Residual ratio (%) = (phosphorylated CLK2 signal of test compound
phosphorylated
CLK2 signal of control group) x 100
[Table 4]
Table 4
Test compound (Example No.) Concentration (nM) Residual ratio (%)
286 300 71.4
228
CA 03021185 2018-10-16
A00039
[0443]
The MDM4 inhibitory activity of the test compound is calculated as a residual
ratio ( /0)
in accordance with the following expression, and shown in the table.
Residual ratio (%)=(MDM4 signal of test compound MDM4 signal of control
group)
x
[Table 5]
Table 5
Test compound (Example No.) Concentration (nM) Residual ratio (%)
286 300 43.6
[0444]
The p53 induction activity of the test compound is calculated as an increase
ratio (%) in
accordance with the following expression, and shown in Table 5.
Increase ratio (%) = (p53 signal of test compound p53 signal of control
group)) x 100
[Table 6]
Table 6
Test compound (Example No.) Concentration (nM) Increase ratio (%)
286 300 620.7
[0445]
The p21 induction activity of the test compound is calculated as an increase
ratio (%) in
accordance with the following expression, and shown in Table 6.
Increase ratio (%) (121 signal of test compound p21 signal of control group))
x 100
[Table 7]
Table 7
Test compound (Example No.) Concentration (nM) Increase ratio (%)
286 300 556.4
[0446]
These results showed that the present invention compound inhibited
phosphorylation of
CLI(2, decreased the amount of the MDM4 protein, and increased the amounts of
the p53 and
p21 proteins in colon adenocarcinoma cells.
[0447]
In vitro MDM4 alternative splicing ratio in human colon adenocarcinoma cell
HCT 116
1000 I (20,0000 cells/well) of a cell suspension of human colon
adenocarcinoma cells
HCT116 (purchased from ATCC) was seeded in a 12-well plate, and cultured at 37
C for 1 day
in a 5% CO2 gas incubator. The test compound solution was added to a final
concentration of
300 nM, and the mixture was cultured for 6 hours. The plate was washed with
PBS, the cells
229
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 229
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 229
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: