Note: Descriptions are shown in the official language in which they were submitted.
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
COMPOSITIONS AND METHODS FOR ENHANCED GENE
EXPRESSION OF PKLR
CROSS REFERENCE TO RELATED APPLICATIONS
This application claims priority to U.S. Provisional Application No.
62/325,397, filed
on April 20, 2016, which is incorporated by reference herein in its entirety.
FIELD OF THE INVENTION
[0001] This invention pertains to gene therapy of Pyruvate Kinase Deficiency.
BACKGROUND OF THE INVENTION
[0002] Pyruvate Kinase Deficiency (PKD) is a monogenic metabolic disease
caused by
mutations in the PKLR gene that leads to hemolytic anemia of variable
symptomatology and
that can be fatal during the neonatal period. PKD recessive inheritance trait
and its curative
treatment by allogeneic bone marrow transplantation provide an ideal scenario
for developing
gene therapy approaches.
[0003] Among many other hereditary enzymatic defects affecting the
erythrocytes,
Pyruvate Kinase deficiency (PKD) is the most frequent one causing chronic
nonspherocytic
hemolytic anemia (CNSHA) (Zanella et al. 2007). Onset and severity of PKD are
very
variable and range from mild to severe neonatal anemia, becoming fatal during
the childhood
in the most severe cases (Pissard et al 2006). Growth retardation, hydrops
fetalis and death
during the neonatal period have also been reported with low frequency (Gilsanz
et al. 1993).
PKD prevalence has been estimated at 1:20,000 in the general Caucasian
population (Beutler
et al 2000) and, so far, more than 195 different mutations in the PKLR gene
have been
identified (http://www.lovd.nl/pk1r). Allogeneic bone marrow transplantation
(BMT) has
been successfully used to cure severe PKD patients (Tanphaichitr et al 2000),
but the low
availability of histocompatible donors and the serious complications
associated with the BMT
of these patients (i.e. graft versus host disease, opportunistic infections,
etc.) make periodic
blood transfusions and splenectomy the main therapeutic options for most of
the severe forms
of PKD (Zanella et al 2005), dramatically increasing patient morbidity and
mortality
(Hilgard et al 2005). The limited efficacy and side effects of the therapeutic
options for
severe PKD patients and its recessive inheritance trait make PKD a suitable
disease to be
treated by gene therapy.
1
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[0004] PKD is caused by defects in the Pyruvate Kinase (PK) enzyme (Zanella
2005) that
catalyses the last ATP-generating reaction of the glycolysis pathway in all
cells. In mature
erythrocytes PK becomes essential as RBCs only express the R-type specific
isoform (RPK)
(Kanno et al 1992) due to the regulation of the erythroid specific alternative
promoter of the
PKLR locus (Noguchi et al 1987). Thus, any loss of RPK activity impairs RBC
metabolism
and lifespan (Zanella 2005), leading to CNSHA.
[0005] A promising approach to treating and preventing genetic and other
diseases and
disorders is delivery of therapeutic agents with a gene therapy vector.
Currently, viral vectors
show the greatest efficiency in gene transfer, and for correction of genetic
diseases such that
persistent gene expression is required, herpesvirus, retrovirus, lentivirus,
adenovirus, or AAV
based vectors are desirable due to the integrating nature of the viral life
cycle.
[0006] Gene therapy for monogenic diseases, particularly those affecting the
hematopoietic
system, has provided convincing evidence that genetic correction of autologous
hematopoietic stem cells (HSCs) is an alternative therapeutic option to
allogeneic HSCT,
avoiding its major complications (Cartier et al 2009; Cavazzana-Calvo et al
2010; Cartier et
al 2012; Aiuti et al 2013; Biffi et al 2013). Genetic correction for diseases
affecting the
erythrocyte such as 0-thalassemia and sickle cell disease, have been addressed
in animal
models (Pestina et al 20091 Breda et al 2012) and also in humans (Cavazzana-
Calvo et al
2010). However, gene therapy approaches for inherited erythroid metabolic
deficiencies such
as PKD are still limited. The feasibility of HSC gene therapy for PKD has been
demonstrated
both in mouse (Tani et al 1994; Meza et al 2009) and in dog RPK deficient
experimental
models (Trobridge et al 2012) showing that donor chimerism and transduction
levels are key
points to reach an efficient correction of the hemolytic phenotype (Richard et
al 2004) given
the lack of selective advantage of donor gene-corrected HSCs. Previous work
with a PKD
mouse model demonstrated that retrovirally-derived human RPK expression was
capable of
fully correcting PKD phenotype when over 25% genetically corrected cells were
transplanted
(Meza et al 2009). A similar therapeutic threshold of corrected cells was
recently reported in
one PKD Basenji dog infused with in vivo expanded and foamy vector-corrected
HSCs
(Trobridge et al 2012).
[0007] A number of challenges remain with regard to designing polynucleotide
cassettes
and expression vectors for use in gene therapy. One significant challenge is
obtaining
sufficient expression of the transgene in target cells. A longstanding unmet
need in the art has
been sufficiently robust expression of transgenes following gene transfer. In
some cases,
more efficient expression is required for the efficacy of certain vectors, for
example plasmid
2
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
DNA vectors. In other cases, more efficient gene expression cassettes are
desirable to allow
for a lower therapeutic dose that has a more favorable safety profile or a
less invasive route of
administration.
[0008] High levels of transgene expression can be achieved when
gammaretroviral
(gamma-RV) vectors are used due to the fact that the therapeutic transgene
expression is
regulated by their LTR sequences. However, the first clinical trials based on
this type of
vector raised safety concerns, as several patients developed unexpected
leukemias (Hacein-
Bey-Abina et al 2008) The strong promoter activity of LTR sequences could
affect the
regulation of surrounding genes, either by activation of proto-oncogene
promoters or by
inhibition of tumour suppressor genes, leading to insertional mutagenesis (Ott
et al 2006;
Howe et al 2008; Stein et al 2010; Braun et al 2014). These findings
highlighted the need to
use safer and more efficient vectors than gamma retroviral vectors for the PKD
gene therapy.
SUMMARY OF THE INVENTION
[0009] In one embodiment, the present invention provides an expression
cassette
comprising a polynucleotide sequence comprising: a) a promoter sequence; b) a
sequence
encoding a gene product; and c) an ribonucleic acid (RNA) export signal,
wherein the
promoter sequence is operably linked to the sequence encoding the pyruvate
kinase
polypeptide, and optionally where a)-c) are present in the expression cassette
in 5' to 3'
order. In certain embodiments, the promoter is a phosphoglycerate kinase (PGK)
promoter. In
some embodiments, the gene product is a therapeutic gene product. In some
embodiments,
the therapeutic gene product is a pyruvate kinase (PK) polypeptide, optionally
a pyruvate
kinase, liver and red blood cell (PKLR) polypeptide. In certain embodiments,
the sequence
encoding the gene product is codon-optimized. In particular embodiments, the
RNA export
signal is a mutated post-transcriptional regulatory element of the woodchuck
hepatitis virus
(Wpre). In certain embodiments, the mutated Wpre is a chimeric Wpre comprising
a
sequence having at least 80% identity to SEQ ID NO: 1. In some embodiments,
the expression
cassette further comprising one or more enhancer sequences. In some
embodiments, the
expression cassette further comprises a polypurine tract (PPT) or
polyadenylation (polyA)
signal sequence. In some embodiments, the expression cassette further
comprises one or more
of the following sequences: i) a packing signal sequence; ii) a truncated Gag
sequence; iii) a
Rev responsive element (RRE); iv) a central polypurine tract (cPPT); v) a
central terminal
sequence (CTS); and vi) an upstream sequence element (USE), optionally from
simian virus
3
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
40 (SV40-USE). In some embodiments, the expression cassette further comprises
5' and 3'
long terminal repeat sequences.
[0010] In a related embodiment, the present invention provides a recombinant
gene delivery
vector comprising an expression cassette disclosed herein. In certain
embodiments, the
recombinant gene delivery vector is a virus or viral vector. In certain
embodiments, the virus
or viral vector is a lentivirus (LV).
[0011] In another related embodiment, the present invention provides a cell
comprising an
expression cassette or gene delivery vector disclosed herein. In some
embodiments, the cell is
a blood cell. In some embodiments, the cell is an erythroid cell. In some
embodiments, the
cell is a bone marrow cell, e.g., a lineage depleted bone marrow cell. In some
embodiments,
the cell is a hematopoietic stem cell. In some embodiments, the cell is a
CD34+
hematopoietic stem cell. In some embodiments, the cell is a committed
hematopoietic
erythroid progenitor cell.
[0012] In yet another related embodiment, the present invention provides a
pharmaceutical
composition comprising a pharmaceutically acceptable excipient and recombinant
gene
delivery vector or cell disclosed herein.
[0013] In another embodiment, the present invention provides a method of
treating or
preventing a disease or disorder in a subject in need thereof, comprising
providing to the
subject an expression cassette, gene delivery vector, or pharmaceutical
composition disclosed
herein. In one embodiment, the disease or disorder is a Pyruvate Kinase
Deficiency (PKD)
and the gene product is a pyruvate kinase (PK) polypeptide, optionally a
pyruvate kinase,
liver and red blood cell (PKLR) polypeptide. In certain embodiments, the
pharmaceutical
composition comprises the recombinant gene delivery vector. In other
embodiments, the
pharmaceutical composition comprises the cell. In one embodiment, the cell is
autologous to
the subject. In another embodiment, the cell is allogeneic to the subject.
[0014] In a related embodiment, the present invention provides a method for
expressing a
transgene in erythroid cells, comprising contacting one or more erythroid
cells with an
effective amount of a recombinant viral vector, wherein the vector comprises a
human
phosphoglycerate kinase promoter, a codon optimized version of a human
pyruvate kinase,
liver and red blood cell (PKLR) cDNA transgene, and a mutated post-
transcriptional
regulatory element of the woodchuck hepatitis virus, wherein following said
contacting,
PKLR is expressed at detectable levels in the one or more erythroid cells.
4
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
BRIEF DESCRIPTION OF THE DRAWINGS
[0015] The novel features of the disclosure are set forth with particularity
in the appended
claims. A better understanding of the features and advantages of the present
invention will be
obtained by reference to the following detailed description that sets forth
illustrative
embodiments, in which the principles of the invention are utilized, and the
accompanying
drawings of which:
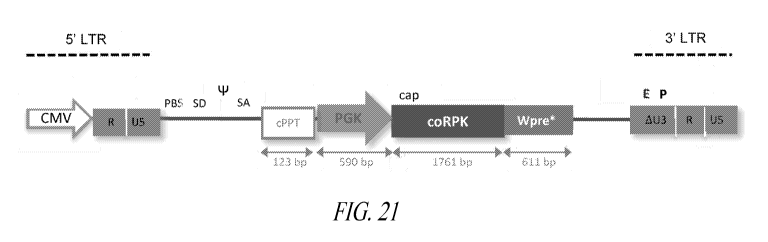
[0016] FIG. 1 depicts a scheme reflecting the position of the different
described elements
present in the backbone of the lentiviral vector.
[0017] FIG. 2A is a schematic representation of the self-inactivating
lentiviral vectors used
throughout gene therapy experiments harboring the human PGK promoter
regulating the
expression of the EGFP transgene in the control vector (upper diagram) or the
expression of a
codon-optimized sequence of the PKLR gene cDNA (coRPK) in the therapeutic
vector (lower
diagram).
[0018] FIG. 2b is a schematic gene therapy protocol performed to address the
functionality
of the developed PGK-coRPK lentiviral vector.
[0019] FIGS. 3a-d depict data showing correction of PKD phenotype in
peripheral blood of
primary recipients after genetic correction. FIG. 3a RBCs and FIG. 3b are
reticulocyte
levels in healthy (black bar, n=5) and PKD anemic mice (gray bar, n=6), and
PKD anemic
mice that were tranplanted with the EGFP (white bar, n=9) or coRPK transduced
cells
(scratched bar, n=17). Data are represented as the average SEM and were
analyzed by non-
parametric Kruskal-Wallis test. FIG. 3c shows the flow cytometry strategy used
to detect the
biotin labelled RBCs throughout the time and FIG. 3d RBC survival kinetics in
healthy
(black line, n=2), anemic (gray line, n=2) and genetically corrected mice
(discontinuous line,
n=4). Data are represented as the average SEM and were analyzed by two-way
ANOVA
test. Healthy, non-transplanted control mice; PKD, non-transplanted PKD mice;
coRPK, PKD
mice expressing the therapeutic transgene.
[0020] FIGS. 4a-c show multi-lineage hematopoietic reconstitution in secondary
transplanted mice. FIG. 4a is a diagram of the flow cytometry strategy used to
identify the
different hematopoietic lineages by labeling with CD3-PE, B220-PE, B220-PECy5,
Grl-
Biotin and Macl-Biotin antibodies plus SAV-PE-Cy5. FIG. 4b depicts
representative dot-
plots and FIG. 4c depicts percentages of each lineage in PB at 140 days after
transplant. Bars
represent the average percentage SEM of healthy (n=2, black bar) and PKD
mouse (n=2,
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
grey bar) controls and secondary transplanted mice expressing the coRPK
therapeutic
transgene (n=4, scratched bar).
[0021] FIGS. 5a-d depict PKD phenotype correction in secondary transplanted
mice. FIG.
5a shows Brilliant Cresyl blue staining of blood smears from non-transplanted
mice and
secondary recipients to identify reticulocyte population (in blue). FIG. 5b is
Flow cytometry
analysis of reticulocyte levels in peripheral blood. FIG. Sc represents RBC
percentage and
FIG. 5d reticulocyte percentage in secondary transplanted mice expressing the
coRPK
transgene (scratched bar, n=4), in healthy mice (black bar, n=3) and in anemic
control mice
(grey bar, n=3). Data are represented the average SEM and were analyzed by
non-
parametric two-tailed Mann-Whitney test.
[0022] FIGS. 6a-c show quantification of proviral integrations. FIG. 6a shows
vector copy
number per cell in BM CFUs from individual transplanted mice at 120 to 170
days after
transplant. Transduction and chimerism percentages are also shown. FIG. 6b
shows provirus
copy number in cells from different hematopoietic compartments. Columns
represent the
average SEM of the different groups of transplanted mice. FIG. 6c shows the
kinetics of
proviral integrations in BM cells from individual transplanted EGFP-expressing
mice (grey
lines) and mice carrying the coRPK transgene (black lines).
[0023] FIGS. 7a-c depict the normalization of the erythroid differentiation
pattern in
genetically corrected mice. FIG. 7a shows percentages of the different
erythroid
subpopulation in bone marrow and spleen at 140 days after transplant. FIG. 7b
depicts
representative dot plots of the flow cytometry strategy used. The expression
intensity of the
CD71 and Ter119 markers allows for identifying four erythroid subpopulations.
population I:
med high
early proerythroblasts (Ter119 CD71 ), population II: basophilic
erythroblasts
high high
(Ter119 CD71 ), population III: late basophilic and polychromatophilic
erythroblasts
high med
(Ter119 CD71 ) and population IV: orthochromatophilic erythroblasts,
reticulocytes and
high low
mature erythroid cells (Ter119 CD71 ). FIG. 7c shows plasma Epo levels
measured by
ELISA in non-transplanted and transplanted mice. Dots represent values of
individual mice.
Lines represent average SEM and were analyzed by non-parametric Kruskal-
Wallis test.
Healthy, non-transplanted control mice; PKD, non-transplanted PKD mice; EGFP,
PKD mice
expressing the EGFP transgene; coRPK, PKD mice expressing the therapeutic
transgene.
[0024] FIGS. 8a-b show hematopoietic progenitor assays in control mice and
transplanted
mice with transduced cells. The data demonstrate total CFUs from spleen (FIG.
8a) and bone
marrow (FIG. 8b) at 140 days after transplant. Dots represent number of
colonies per mouse
6
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
analyzed and lines represent average SEM in each group. Data were
statistically analyzed
by non-parametric Kruskal-Wallis test.
[0025] FIGS. 9a-c demonstrate reversion of splenomegaly and organ pathology in
genetically corrected mice at 140 days post-transplantation. FIG. 9a are
pictures of
representative spleens and FIG. 9b shows ratio of spleen weight to total body
weight from
primary and secondary transplanted PKD mice. Dots represent values of
individual mice.
Lines represent average SEM per group. Data were analyzed by non-parametric
Kruskal-
Wallis test. FIG. 9c shows the histological study of spleen and liver from
primary
transplanted PKD mice. First and second column show the representative
histology sections
of spleen and liver stained with hematoxylin-eosin and photographed using a 4x
and 10x
objective, respectively, in a light microscope. Arrows point to erythroid cell
clusters
indicative of extramedullary erythropoiesis. Third column shows Prussian blue
staining (Fe)
of liver sections to detect iron deposits indicated by arrowheads. Photographs
were taken
nd
using a 20x objective. Group legends as in Fig. 7. 2 coRPK, secondary
recipients.
[0026] FIGS. 10a-g depict metabolic profiling in RBC samples from mice
transplanted
with genetically modified cells. Analysis of significant metabolic profile
changes in healthy
and transplanted mice by comparison to PKD animals in two independent
experiments. FIG.
10a shows the complete RBC heat map obtained by untargeted profiling, where
higher and
lower metabolite levels are represented in red and blue respectively.
Metabolites listed have
at least one comparison that is significant using the following criteria:
absolute fold
change>1.5; minimal signal>2000; Adjusted p-value<0.01. Black boxes highlight
cluster of
metabolite changes with distinct profile among the groups. FIGS. 10b, 10c, and
10d depict
ATP, ADP and pyruvate levels in RBCs, respectively, measured by untargeted
profiling by
comparison to PKD mice at 140 days after transplant. Assay 1: Healthy mice
(black bars)
n=1, PKD (grey bars) n=1, hPGK-EGFP (white bars) n=2, hPGK-coRPK (scratched
bars)
n=3. Assay 2: Healthy mice n=2, PKD n=2, hPGK-EGFP n=6, hPGK-coRPK n=10. FIGS.
10e, 10f, and lOg depict RBC targeted metabolic profiling of a selected number
of
metabolites involved in the glycolytic pathway (PEP, 3-phosphoglyceric acid
and D-lactic
acid, respectively) at 280 days post-transplantation. Dots represent values of
individual mice.
Lines represent average SEM and were analyzed by non-parametric Kruskal-
Wallis test.
Assay 2: Healthy mice n=7, PKD n=5, hPGK-EGFP n=3, hPGK-coRPK n=5.
[0027] FIGS. ha-c depict pyruvate Kinase activity, FIG. lib hexokinase
activity and
FIG. 11c ratio of Pyruvate Kinase and Hexokinase enzymatic activities in RBCs
from control
7
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
mice and mice transplanted with transduced cells. RBCs were purified from
blood samples
through a cellulose column to avoid leukocyte PK activity contamination and
subjected to
enzyme activity evaluation. Black bars, healthy mice (n=2); white bars, mice
transplanted
with cells transduced with the EGFP expressing vector (n=3); scratched bars,
mice
transplanted with cells transduced with the coRPK expressing vector (n=3).
Checkered bars
represent values from a healthy volunteer (n=1). Data represent the average
SEM of each
group.
[0028] FIGS. 12a-d show untargeted metabolic profiling in WBC samples from
mice
transplanted with genetically modified cells. FIG. 12a represents the
principal component
analysis of untargeted metabolite profile in RBCs (red dots; left and center)
and WBCs (blue
dots; cluster on right) in control and transplanted mice. FIGS. 12b, 12c, and
12d depict ATP,
ADP and pyruvate levels, respectively, in WBCs by comparison to PKD mice.
Assay 1:
healthy mice (black bars) n=1, PKD (grey bars) n=1, hPGK-EGFP (white bars)
n=2, hPGK-
coRPK (scratched bars) n=3. Assay 2: healthy mice n=2, PKD n=2, hPGK-EGFP n=6,
hPGK-coRPK n=10. Data represent the average SEM per group and were analyzed
by non-
parametric Kruskal-Wallis test.
[0029] FIG. 13 shows a gel image of LAM-PCR products generated with Tsp509I
enzyme
for samples harvested from all mice at different time points and tissues.
Vector integration
sites were identified by LAM-PCR amplification of 3'vector LTR-genome
junctions. A
MultiNA automated system was used, generating a pattern characterized by
several bands.
Vector backbone derived Tsp5091 internal control band (IC) is indicated by an
arrow.
[0030] FIG. 14 shows a gel image of LAM-PCR products generated with HpyCH4IV5
enzyme for samples harvested from all mice at different time points and
tissues. Vector
integration sites were identified by LAM-PCR amplification of 3' vector LTR-
genome
junctions. A MultiNA automated system was used, generating a pattern
characterized by
several bands. Vector backbone derived HpyCH4IV5 internal control band (IC) is
indicated
by an arrow.
[0031] FIG. 15 depicts a general scheme of the analysis of integration site
mapping
performed in mice transplanted with genetically modified hematopoietic
progenitors. Bone
marrow and white blood cell samples from transplanted mice belonging to two
independent
experiments (Table 3) and harvested at different time-points after transplant
were analyzed as
described in supplementary methods following the showed pipeline.
[0032] FIGS. 16a-b show the distribution of LV integrations along the genome
of
transplanted mice. FIG. 16a depicts Integration site (IS) frequency
distribution around
8
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
Transcription Start Site (TSS) of the nearest RefSeq gene, spanning 500 Kb
upstream and
downstream the TSS. Numbers on the top are the number of IS detected for all
samples and
time-points. FIG. 16b depicts chromosomal distribution of LV integration sites
in
transplanted mice expressing the EGFP transgene (black bars) or the coRPK
therapeutic
transgene (grey bars), showing no skewing towards any particular chromosome.
[0033] FIGS. 17a-c demonstrates clonal abundance analysis of coRPK-LV
transduced
cells. Dots plot representation of clonal abundance of pooled integrations in
each mouse from
assays 1 (FIG. 17a) and 2 (FIGS. 17b and 17c). The relative percentage (y-
axis) for each
integration site is relative to the total number of sequences reads obtained
in each dataset. IS,
integration site; BM, bone marrow; PB, peripheral blood; coRPK1-14, mice
transplanted with
hematopoietic cells transduced with the therapeutic vector.
[0034] FIG. 18 presents the tracked shared integrations between primary and
secondary
recipient mice carrying the therapeutic PGK-coRPK LV vector. Integrations
detected in either
mouse in any organ and at any time are pooled. Secondary recipients received
the pooled BM
from transplanted mice coRPK11 to 14. The rest of the IS detected were
detected or in the
primary or in the secondary recipients. Numbers in the boxes show the
representativeness in
percentage of the corresponding integration in the referred mouse. In addition
to? 5% filter
applied on integration analysis, all integration with a sequence count < 3
were eliminated.
[0035] FIG. 19 demonstrates clonal abundance analysis of EGFP- LV transduced
cells.
Dots plot representation of clonal abundance of pooled integrations in each
mouse in bone
marrow. The relative percentage (y-axis) for each integration site is relative
to the total
number of sequences reads obtained in each dataset. Similarly to co-RPK
transduced cells
(Fig. 17), the graph indicates that the vast majority of transplanted mice
show a polyclonal
pattern of hematopoietic repopulation. IS, Integration site
[0036] FIG. 20 depicts the LV genomic integration profile. Gene Ontology (GO)
analysis
was performed using the GREAT software on samples from transplanted mouse. All
integrations retrieved from this study (N=2220) showed overrepresentations of
the gene
functions indicated on the left part of the figure. To address if the most
abundant integrations
were enriched on specific gene classes, all integration sites with a relative
sequence count
>5% of the entire dataset (shown in Fig. 17) were selected, showing no GO gene
classes
overrepresented.
[0037] FIG. 21 depicts a schematic diagram of the medicinal product (PGK-coRPK
LV).
[0038] FIGS. 22a-b depicts the mechanism of action of the medicinal product.
FIG. 22a
depicts the ectopic expression of the PGK-coRPK LV medicinal product will
rescue the wild-
9
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
type phenotype of PKD erythrocytes, otherwise unable to generate a functional
RPK protein
to produce sufficient energy to carry out their functions. FIG. 22b shows the
gene therapy
strategy for PKD patients based on the ex vivo transduction of RPK deficient
CD34+
hematopoietic progenitors with the medicinal product and subsequent
transplantation into the
patient. The developed medicinal product carrying the therapeutic human PKLR
gene cDNA
will be integrated in the patient CD34+ cell genome upon ex vivo transduction.
These
genetically corrected cells will be then infused back into the patient, where
they will produce
RBCs expressing the therapeutic transgene, and therefore, producing functional
RPK proteins
that will correct the PKD pathological phenotype. Figure modified from the
Boston
Children's Hospital blog.
DETAILED DESCRIPTION OF THE INVENTION
DEFINITIONS
[0039] A "vector" as used herein refers to a macromolecule or association of
macromolecules that comprises or associates with a polynucleotide and which
can be used to
mediate delivery of the polynucleotide to a cell. Illustrative vectors
include, for example,
plasmids, viral vectors, liposomes, and other gene delivery vehicles.
[0040] The term "LV" is an abbreviation for lentivirus, and may be used to
refer to the virus
itself or derivatives thereof The term covers all subtypes and both naturally
occurring and
recombinant forms, except where required otherwise.
[0041] As used herein, the term "gene" or "coding sequence" refers to a
nucleotide
sequence in vitro or in vivo that encodes a gene product. In some instances,
the gene consists
or consists essentially of coding sequence, that is, sequence that encodes the
gene product. In
other instances, the gene comprises additional, non-coding, sequence. For
example, the gene
may or may not include regions preceding and following the coding region, e.g.
5'
untranslated (5' UTR) or "leader" sequences and 3' UTR or "trailer" sequences,
as well as
intervening sequences (introns) between individual coding segments (exons).
[0042] As used herein, a "therapeutic gene" refers to a gene that, when
expressed, confers a
beneficial effect on the cell or tissue in which it is present, or on a mammal
in which the gene
is expressed. Examples of beneficial effects include amelioration of a sign or
symptom of a
condition or disease, prevention or inhibition of a condition or disease, or
conferral of a
desired characteristic. Therapeutic genes include genes that correct a genetic
deficiency in a
cell or mammal.
[0043] As used herein, a transgene is a gene that is delivered to a cell by a
vector.
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[0044] As used herein, the term "gene product" refers to the desired
expression product of a
polynucleotide sequence such as a polypeptide, peptide, protein or interfering
RNA including
short interfering RNA (siRNA), miRNA or small hairpin RNA (shRNA).
[0045] As used herein, the terms "polypeptide," "peptide," and "protein" refer
to polymers
of amino acids of any length. The terms also encompass an amino acid polymer
that has been
modified; for example, disulfide bond formation, glycosylation, lipidation,
phosphorylation,
or conjugation with a labeling component.
[0046] By "comprising" it is meant that the recited elements are required in,
for example,
the composition, method, kit, etc., but other elements may be included to form
the, for
example, composition, method, kit etc. within the scope of the claim. For
example, an
expression cassette "comprising" a gene encoding a therapeutic polypeptide
operably linked
to a promoter is an expression cassette that may include other elements in
addition to the gene
and promoter, e.g. poly-adenylation sequence, enhancer elements, other genes,
linker
domains, etc.
[0047] By "consisting essentially of", it is meant a limitation of the scope
of the, for
example, composition, method, kit, etc., described to the specified materials
or steps that do
not materially affect the basic and novel characteristic(s) of the, for
example, composition,
method, kit, etc. For example, an expression cassette "consisting essentially
of" a gene
encoding a therapeutic polypeptide operably linked to a promoter and a
polyadenylation
sequence may include additional sequences, e.g. linker sequences, so long as
they do not
materially affect the transcription or translation of the gene. As another
example, a variant, or
mutant, polypeptide fragment "consisting essentially of" a recited sequence
has the amino
acid sequence of the recited sequence plus or minus about 10 amino acid
residues at the
boundaries of the sequence based upon the full length naïve polypeptide from
which it was
derived, e.g. 10, 9, 8, 7, 6, 5, 4, 3, 2 or 1 residue less than the recited
bounding amino acid
residue, or 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 residues more than the recited
bounding amino acid
residue.
[0048] By "consisting of", it is meant the exclusion from the composition,
method, or kit of
any element, step, or ingredient not specified in the claim. For example, an
expression
cassette "consisting of" a gene encoding a therapeutic polypeptide operably
linked to a
promoter, and a post-transcriptional regulatory element consists only of the
promoter,
polynucleotide sequence encoding the therapeutic polypeptide, and post-
transcriptional
regulatory element. As another example, a polypeptide "consisting of" a
recited sequence
contains only the recited sequence.
11
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[0049] An "expression vector" as used herein encompasses a vector, e.g.
plasmid,
minicircle, viral vector, liposome, and the like as discussed above or as
known in the art,
comprising a polynucleotide which encodes a gene product of interest, and is
used for
effecting the expression of a gene product in an intended target cell. An
expression vector
also comprises control elements operatively linked to the encoding region to
facilitate
expression of the gene product in the target. The combination of control
elements, e.g.
promoters, enhancers, UTRs, miRNA targeting sequences, etc., and a gene or
genes to which
they are operably linked for expression is sometimes referred to as an
"expression cassette."
Many such control elements are known and available in the art or can be
readily constructed
from components that are available in the art.
[0050] A "promoter" as used herein encompasses a DNA sequence that directs the
binding
of RNA polymerase and thereby promotes RNA synthesis, i.e., a minimal sequence
sufficient
to direct transcription. Promoters and corresponding protein or polypeptide
expression may
be ubiquitous, meaning strongly active in a wide range of cells, tissues and
species or cell-
type specific, tissue-specific, or species specific. Promoters may be
"constitutive," meaning
continually active, or "inducible," meaning the promoter can be activated or
deactivated by
the presence or absence of biotic or abiotic factors. Also included in the
nucleic acid
constructs or vectors of the invention are enhancer sequences that may or may
not be
contiguous with the promoter sequence. Enhancer sequences influence promoter-
dependent
gene expression and may be located in the 5' or 3' regions of the native gene.
[0051] An "enhancer" as used herein encompasses a cis-acting element that
stimulates or
inhibits transcription of adjacent genes. An enhancer that inhibits
transcription also is termed
a "silencer". Enhancers can function (i.e., can be associated with a coding
sequence) in either
orientation, over distances of up to several kilobase pairs (kb) from the
coding sequence and
from a position downstream of a transcribed region.
[0052] A "termination signal sequence" as used herein encompasses any genetic
element
that causes RNA polymerase to terminate transcription, such as for example a
polyadenylation signal sequence.
[0053] As used herein, the terms "operatively linked" or "operably linked"
refers to a
juxtaposition of genetic elements, e.g. promoter, enhancer, termination signal
sequence,
polyadenylation sequence, etc., wherein the elements are in a relationship
permitting them to
operate in the expected manner. For instance, a promoter is operatively linked
to a coding
region if the promoter helps initiate transcription of the coding sequence.
There may be
12
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
intervening residues between the promoter and coding region so long as this
functional
relationship is maintained.
[0054] As used herein, the term "heterologous" means derived from a
genotypically distinct
entity from that of the rest of the entity to which it is being compared. For
example, a
polynucleotide introduced by genetic engineering techniques into a plasmid or
vector derived
from a different species is a heterologous polynucleotide. As another example,
a promoter
removed from its native coding sequence and operatively linked to a coding
sequence with
which it is not naturally found linked is a heterologous promoter. Thus, for
example, an LV
vector that includes a heterologous nucleic acid encoding a heterologous gene
product is an
LV vector that includes a nucleic acid not normally included in a naturally-
occurring, wild-
type LV, and the encoded heterologous gene product is a gene product not
normally encoded
by a naturally-occurring, wild-type LV.
[0055] The term "endogenous" as used herein with reference to a nucleotide
molecule or
gene product refers to a nucleic acid sequence, e.g. gene or genetic element,
or gene product,
e.g. RNA, protein, that is naturally occurring in or associated with a host
virus or cell.
[0056] The term "native" as used herein refers to a nucleotide sequence, e.g.
gene, or gene
product, e.g. RNA, protein, that is present in a wildtype virus or cell.
[0057] The term "variant" as used herein refers to a mutant of a reference
polynucleotide or
polypeptide sequence, for example a native polynucleotide or polypeptide
sequence, i.e.
having less than 100% sequence identity with the reference polynucleotide or
polypeptide
sequence. Put another way, a variant comprises at least one amino acid
difference (e.g.,
amino acid substitution, amino acid insertion, amino acid deletion) relative
to a reference
polynucleotide sequence, e.g. a native polynucleotide or polypeptide sequence.
For example,
a variant may be a polynucleotide having a sequence identity of 70% or more
with a full
length native polynucleotide sequence, e.g. an identity of 75% or 80% or more,
such as 85%,
90%, or 95% or more, for example, 98% or 99% identity with the full length
native
polynucleotide sequence. As another example, a variant may be a polypeptide
having a
sequence identity of 70% or more with a full length native polypeptide
sequence, e.g. an
identity of 75% or 80% or more, such as 85%, 90%, or 95% or more, for example,
98% or
99% identity with the full length native polypeptide sequence. Variants may
also include
variant fragments of a reference, e.g. native, sequence sharing a sequence
identity of 70% or
more with a fragment of the reference, e.g. native, sequence, e.g. an identity
of 75% or 80%
or more, such as 85%, 90%, or 95% or more, for example, 98% or 99% identity
with the
native sequence.
13
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[0058] As used herein, the terms "biological activity" and "biologically
active" refer to the
activity attributed to a particular biological element in a cell. For example,
the "biological
activity" of an "immunoglobulin", "antibody" or fragment or variant thereof
refers to the
ability to bind an antigenic determinant and thereby facilitate immunological
function. As
another example, the biological activity of a polypeptide or functional
fragment or variant
thereof refers to the ability of the polypeptide or functional fragment or
variant thereof to
carry out its native functions of, e.g., binding, enzymatic activity, etc. As
a third example, the
biological activity of a gene regulatory element, e.g. promoter, enhancer,
kozak sequence,
and the like, refers to the ability of the regulatory element or functional
fragment or variant
thereof to regulate, i.e. promote, enhance, or activate the translation of,
respectively, the
expression of the gene to which it is operably linked.
[0059] The terms "administering" or "introducing", as used herein, refer to
delivery of a
vector for recombinant protein expression to a cell, to cells and/or organs of
a subject, or to a
subject. Such administering or introducing may take place in vivo, in vitro or
ex vivo. A
vector for expression of a gene product may be introduced into a cell by
transfection, which
typically means insertion of heterologous DNA into a cell by physical means
(e.g., calcium
phosphate transfection, electroporation, microinjection or lipofection);
infection, which
typically refers to introduction by way of an infectious agent, i.e. a virus;
or transduction,
which typically means stable infection of a cell with a virus or the transfer
of genetic material
from one microorganism to another by way of a viral agent (e.g., a
bacteriophage).
[0060] "Transformation" is typically used to refer to bacteria comprising
heterologous DNA
or cells which express an oncogene and have therefore been converted into a
continuous
growth mode such as tumor cells. A vector used to "transform" a cell may be a
plasmid, virus
or other vehicle.
[0061] Typically, a cell is referred to as "transduced", "infected";
"transfected" or
"transformed" dependent on the means used for administration, introduction or
insertion of
heterologous DNA (i.e., the vector) into the cell. The terms "transduced",
"transfected" and
"transformed" may be used interchangeably herein regardless of the method of
introduction
of heterologous DNA.
[0062] The term "host cell", as used herein refers to a cell which has been
transduced,
infected, transfected or transformed with a vector. The vector may be a
plasmid, a viral
particle, a phage, etc. The culture conditions, such as temperature, pH and
the like, are those
previously used with the host cell selected for expression, and will be
apparent to those
14
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
skilled in the art. It will be appreciated that the term "host cell" refers to
the original
transduced, infected, transfected or transformed cell and progeny thereof
[0063] The terms "treatment", "treating" and the like are used herein to
generally mean
obtaining a desired pharmacologic and/or physiologic effect. The effect may be
prophylactic
in terms of completely or partially preventing a disease or symptom thereof,
e.g. reducing the
likelihood that the disease or symptom thereof occurs in the subject, and/or
may be
therapeutic in terms of a partial or complete cure for a disease and/or
adverse effect
attributable to the disease. "Treatment" as used herein covers any treatment
of a disease in a
mammal, and includes: (a) preventing the disease from occurring in a subject
which may be
predisposed to the disease but has not yet been diagnosed as having it; (b)
inhibiting the
disease, i.e., arresting its development; or (c) relieving the disease, i.e.,
causing regression of
the disease. The therapeutic agent may be administered before, during or after
the onset of
disease or injury. The treatment of ongoing disease, where the treatment
stabilizes or reduces
the undesirable clinical symptoms of the patient, is of particular interest.
Such treatment is
desirably performed prior to complete loss of function in the affected
tissues. The subject
therapy will desirably be administered during the symptomatic stage of the
disease, and in
some cases after the symptomatic stage of the disease.
[0064] The terms "individual," "host," "subject," and "patient" are used
interchangeably
herein, and refer to a mammal, including, but not limited to, human and non-
human primates,
including simians and humans; mammalian sport animals (e.g., horses);
mammalian farm
animals (e.g., sheep, goats, etc.); mammalian pets (dogs, cats, etc.); and
rodents (e.g., mice,
rats, etc.).
[0065] The terminology used herein is for the purpose of describing particular
embodiments
only and is not intended to be limiting of the invention. As used herein, the
singular forms
"a", "an" and "the" are intended to include the plural forms as well, unless
the context clearly
indicates otherwise. Furthermore, to the extent that the terms "including",
"includes",
"having", "has", "with", or variants thereof are used in either the detailed
description and/or
the claims, such terms are intended to be inclusive in a manner similar to the
term
"comprising".
[0066] The term "about" or "approximately" means within an acceptable error
range for the
particular value as determined by one of ordinary skill in the art, which will
depend in part on
how the value is measured or determined, i.e., the limitations of the
measurement system.
For example, "about" can mean within 1 or more than 1 standard deviation, per
the practice
in the art. Alternatively, "about" can mean a range of up to 20%, preferably
up to 10%, more
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
preferably up to 5%, and more preferably still up to 1% of a given value.
Alternatively,
particularly with respect to biological systems or processes, the term can
mean within an
order of magnitude, preferably within 5-fold, and more preferably within 2-
fold, of a value.
Where particular values are described in the application and claims, unless
otherwise stated
the term "about" meaning within an acceptable error range for the particular
value should be
assumed.
[0067] Unless otherwise indicated, all terms used herein have the same meaning
as they
would to one skilled in the art and the practice of the present invention will
employ
conventional techniques of microbiology and recombinant DNA technology, which
are
within the knowledge of those of skill of the art.
[0068] The practice of the present invention employs, unless otherwise
indicated,
conventional techniques of cell biology, molecular biology (including
recombinant
techniques), microbiology, biochemistry and immunology, which are within the
scope of
those of skill in the art. Such techniques are explained fully in the
literature, such as,
"Molecular Cloning: A Laboratory Manual", second edition (Sambrook et al.,
1989);
"Oligonucleotide Synthesis" (M. J. Gait, ed., 1984); "Animal Cell Culture" (R.
I. Freshney,
ed., 1987); "Methods in Enzymology" (Academic Press, Inc.); "Handbook of
Experimental
Immunology" (D. M. Weir & C. C. Blackwell, eds.); "Gene Transfer Vectors for
Mammalian
Cells" (J. M. Miller & M. P. Cabs, eds., 1987); "Current Protocols in
Molecular Biology" (F.
M. Ausubel et al., eds., 1987); "PCR: The Polymerase Chain Reaction", (Mullis
et al., eds.,
1994); and "Current Protocols in Immunology" (J. E. Coligan et al., eds.,
1991), each of
which is expressly incorporated by reference herein.
[0069] In certain embodiments, the present disclosure provides
polynucleotides,
polynucleotide cassettes and expression vectors for the expression of a gene
in cells. Also
provided are pharmaceutical compositions and methods for the use of any of the
compositions in promoting the expression of a gene in cells, for example, in
an individual,
e.g. for the treatment or prophylaxis of a disorder. These and other objects,
advantages, and
features of the invention will become apparent to those persons skilled in the
art upon reading
the details of the compositions and methods as more fully described below.
[0070] The present invention relates generally to the fields of molecular
biology and
virology, and in particular, to genetic expression cassettes, and vectors
comprising them
useful for the delivery of nucleic acid segments encoding selected therapeutic
constructs
(including for example, peptides, polypeptides, ribozymes, and catalytic RNA
molecules), to
selected cells and tissues of vertebrate animals. In particular, these genetic
constructs are
16
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
useful in the development of gene therapy vectors, including for example,
lentiviral vectors,
for the treatment of mammalian, and in particular, human, diseases, disorders,
and
dysfunctions.
[0071] The disclosed compositions may be utilized in a variety of
investigative, diagnostic
and therapeutic regimens, including the prevention and treatment of a variety
of human
diseases. The various compositions and methods of the invention are described
below.
[0072] Although particular compositions and methods are exemplified herein, it
is
understood that any of a number of alternative compositions and methods are
applicable and
suitable for use in practicing the invention. It will also be understood that
an evaluation of the
expression constructs and methods of the invention may be carried out using
procedures
standard in the art.
[0073] In certain embodiments, methods and compositions are provided for
preparation of
gene therapy vector compositions, e.g., viral vectors, comprising these
genetic expression
cassettes for use in the preparation of medicaments useful in central and
targeted gene
therapy of diseases, disorders, and dysfunctions in an animal, and in humans
in particular.
[0074] In some embodiments, the present invention provides for gene therapy
for PKD
based on a lentiviral vector harbouring the hPGK eukaryotic promoter that
drives the
expression of the PKLR cDNA. This therapeutic vector may be used to transduce
mouse PKD
hematopoietic stem cells (HSCs) and subsequently transplanted into
myeloablated PKD mice.
Ectopic RPK expression normalizes the erythroid compartment correcting the
hematological
phenotype and reverting organ pathology. Metabolomic studies demonstrate
functional
correction of the glycolytic pathway in RBCs derived from genetically
corrected PKD HSCs,
with no metabolic disturbances in leukocytes. The analysis of the lentiviral
insertion sites in
the genome of transplanted hematopoietic cells demonstrates no evidence of
genotoxicity in
any of the transplanted animals. Overall, the results underscore the
therapeutic potential of
the hPGK-coRPK lentiviral vector and provide high expectations towards the
gene therapy of
PKD and other erythroid metabolic genetic disorders.
[0075] In certain embodiments, the present invention provides an RPK
lentiviral vector
(LV) for the genetic correction of PKD. Genetic modification of murine PKD-
HSCs with this
vector can efficiently correct the hemolytic phenotype and the RBC metabolite
profile in
transplanted PKD mice. Remarkably, no evidence of metabolic disturbances in
leukocytes
and genotoxicity derived from the vector integration are observed, supporting
the therapeutic
potential of the PGK-coRPK LV vector. Overall, results provide encouraging
evidence of the
feasibility of gene therapy for PKD with a LV designed for clinical
application.
17
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[0076] Certain embodiments of the present invention comprise a self-
inactivating lentiviral
vector expressing a codon-optimized version of human PLKR gene. The expression
vector
comprises a promoter region, a coding sequence, and a post-transcriptional
regulatory
element.
[0077] Certain embodiments of polynucleotide cassettes of the present
invention comprise a
promoter region comprising a promoter sequence, or a functional fragment
thereof In one
embodiment, the promoter is a human phosphoglycerate kinase (PGK) promoter.
[0078] Some embodiments of the present invention comprise polynucleotide
cassettes for
the enhanced expression of pyruvate kinase. In some embodiments, the
polynucleotide
cassette comprises a codon-optimized version of the human PKLR cDNA (coRPK) to
increase mRNA stability upon transcription. For the optimization, GeneArt
software may be
used, increasing the GC content and removing cryptic splice sites in order to
avoid
transcriptional silencing and therefore increase transgene expression. The
coRPK optimized
sequence showed 80.4% homology with the human PKLR gene, with no changes in
the
amino acids sequence of the protein. Alternatively, any optimization method
known in the
art may be used.
[0079] In some embodiments, the polynucleotide cassette comprises an RNA
export signal
downstream of the second enhancer. The RNA export signal may comprise
woodchuck
hepatitis virus post-transcriptional element (WPRE) sequence. In some
embodiments, a
mutated post-transcriptional regulatory element of the woodchuck hepatitis
virus (Wpre),
lacking any residual open reading frame (Schambach, Bohne et al. 2006) is also
included to
improve the level of expression and stability of the therapeutic gene.
[0080] In some aspects of the invention, gene delivery vectors are provided
comprising a
polynucleotide cassette of the present invention. In some embodiments, the
gene delivery
vector is a lentivirus.
[0081] In some aspects of the invention, pharmaceutical compositions are
provided
comprising a polynucleotide cassette of the invention and a pharmaceutical
excipient. In
some embodiments, the pharmaceutical composition comprises a gene delivery
vector of the
invention and a pharmaceutical excipient.
[0082] In some aspects of the invention, methods are provided for expressing a
transgene in
mammalian cells. In some embodiments, the method comprises contacting one or
more
mammalian cells with an effective amount of a polynucleotide cassette of the
invention or a
gene delivery vector of the invention, wherein the transgene is expressed at
detectable levels
in the one or more mammalian cells. In some embodiments, the method comprises
18
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
contacting one or more mammalian cells with an effective amount of a
polynucleotide
cassette of the invention or a gene delivery vector of the invention, wherein
the transgene is
expressed at therapeutic levels in the one or more mammalian cells. In some
embodiments,
the method is in vitro. In other embodiments, the method is in vivo.
[0083] In some aspects of the invention, methods are provided for the
treatment or
prophylaxis of a disease or disorder in a mammal in need of treatment or
prophylaxis for a
disease or disorder. In some embodiments, the method comprises administering
to the
mammal an effective amount of a pharmaceutical composition of the invention,
wherein the
coding sequence encodes a therapeutic gene product.
COMPOSITIONS
[0084] In some aspects of the disclosure, compositions are provided for the
expression of a
transgene in a eukaryotic cell(s). In some aspects, the eukaryotic cell is a
mammalian cell. In
some aspects, the mammalian cell is a hematopoietic stem cell. In some
embodiments, the
cell is a bone marrow cell, e.g., a lineage depleted bone marrow cell. In some
aspects, the
mammalian cell is a committed hematopoietic erythroid progenitor.
[0085] In some embodiments of the disclosure, the composition is a
polynucleotide
cassette. By a "polynucleotide cassette" is meant a polynucleotide sequence
comprising two
or more functional polynucleotide sequences, e.g. regulatory elements,
translation initiation
sequences, coding sequences, and/or termination sequences, etc., typically in
operable
linkage to one another. Likewise, by a "polynucleotide cassette for the
expression of a
transgene in a mammalian cell," it is meant a combination of two or more
functional
polynucleotide sequences, e.g. promoter, enhancer, 5'UTR, translation
initiation sequence,
coding sequence, and/or termination sequences, etc. that promotes the
expression of the
transgene in a cell.
[0086] For example, in some embodiments, the polynucleotide cassette
comprises: human
phosphoglycerate kinase (PGK) promoter, a codon-optimized version of the human
PKLR
cDNA (coRPK), and a mutated post-transcriptional regulatory element of the
woodchuck
hepatitis virus (Wpre).
[0087] In particular embodiments of any of the expression cassettes and gene
delivery
vectors described herein, the human PKLR promoter comprises or consists of the
following
sequence, a functional fragment thereof, or a sequence having at least 80%, at
least 85%, at
least 90%, at least 95%, at least 98%, or at least 99% identity to the
following sequence:
19
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
ATTATGGTAAATC CAC TTAC TGTCTGC C CTC GTAGC CATC GAGATAAAC C CTAC C
GGGTAGGGGAGGCGCTTTTCCCAAGGCAGTCTGGAGCATGCGCTTTAGCAGCCC
CGCTGGGCACTTGGCGCTACACAAGTGGCCTCTGGCCTCGCACACATTCCACATC
CACCGGTAGGCGCCAACCGGCTCCGTTCTTTGGTGGCCCCTTCGCGCCACCTTCT
ACTC CTC CC CTAGTC AGGAAGTTCC CCC CCGCCC CGC AGCTCGC GTC GTGC AGGA
CGTGACAAATGGAAGTAGCACGTCTCACTAGTCTCGTGCAGATGGACAGCACCG
CTGAGCAATGGAAGCGGGTAGGCCTTTGGGGCAGCGGCCAATAGCAGCTTTGCT
CCTTCGCTTTCTGGGCTCAGAGGCTGGGAAGGGGTGGGTCCGGGGGCGGGCTCA
GGGGC GGGCTCAGGGGC GGGGC GGGC GC C C GAAGGTC CTC C GGAGGC C C GGCA
TTCTGCACGCTTCAAAAGCGCACGTCTGCCGCGCTGTTCTCCTCTTCCTCATCTCC
GGGCCTTTCGACCTGCAGCCC (SEQ ID NO:4).
[0088] In particular embodiments of any of the expression cassettes and gene
deliver)/
vectors described herein, the human PKLR promoter comprises or consists of the
following
sequence, a functional fragment thereof, or a sequence having at least 80%, at
least 85%, at
least 90%, at least 95%, at least 98%, or at least 99% identity to the
following sequence:
TC CAC GGGGTTGGGGTTGC GC C TTTTC CAAGGCAGC C C TGGGTTTGC GC AGGGAC
GC GGCTGCTCTGGGC GTGGTTC C GGGAAAC GCAGC GGC GC C GAC C CTGGGTC TC
GCACATTCTTCACGTCCGTTCGCAGCGTCACCCGGATCTTCGCCGCTACCCTTGTG
GGCCCCCCGGCGACGCTTCCTCGTCCGCCCCTAAGTCGGGAAGGTTCCTTGCGGT
TC GC GGC GTGC C GGAC GTGACAAAC GGAAGC C GCAC GTC TC ACTAGTAC C CTC G
CAGAC GGACAGC GC C AGGGAGC AATGGC AGC GC GC C GAC C GC GATGGGC TGTG
GC CAATAGC GGC TGC TC AGC AGGGGC GC C C GAGAGC AGC GGC C GGGAAGGGGC
GGTGC GGGAGGC GGGGTGTGGGGC GGTAGTGTGGGC C CTGTTC CTGC C C GC GC G
GTGTTCCGCATTCTGCAAGCCTCCGGAGCGCACGTCGGCAGTCGGCTCCCTCGTT
GACCGAATCACCGACCTCTCTCCCCAG (SEQ ID NO: 8).
[0089] In particular embodiments of any of the expression cassettes and gene
deliver)/
vectors described herein, the RPE sequence comprises or consists of the
following sequence,
or a sequence having at least 80%, at least 85%, at least 90%, at least 95%,
at least 98%, or at
least 99% identity to the following sequence:
TC CTTGGGTTCTTGGGAGCAGCAGGAAGCAC TATGGGC GCAGC GTCAATGAC GC
TGACGGTACAGGCCAGACAATTATTGTCTGGTATAGTGCAGCAGCAGAACAATT
TGCTGAGGGCTATTGAGGCGCAACAGCATCTGTTGCAACTCACAGTCTGGGGCAT
CAAGCAGCTCCAGGCAAGAATCCTGGCTGTGGAAAGATACCT (SEQ ID NO:3).
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[0090] In particular embodiments of any of the expression cassettes and gene
delivery
vectors described herein, the psi sequence is an HIV-1 psi sequence or the psi
sequence
comprises or consists of the following sequence, or a sequence having at least
80%, at least
85%, at least 90%, at least 95%, at least 98%, or at least 99% identity to the
following
sequence:
TCGACGCAGGACTCGGCTTGCTGAAGCGCGCACGGCAAGAGGCGAGGGGCGGC
GACTGGTGAGTACGCCAAAAATTTTGACTAGCGGAGGCTAGAAGGAGAGAGATG
GGTGCGAGAGCGTCAGTATTAAGCGGGGGAG (SEQ ID NO:5).
[0091] In particular embodiments of any of the expression cassettes and gene
delivery
vectors described herein, the 5' LTR comprises or consists of the following
sequence, or a
sequence having at least 80%, at least 85%, at least 90%, at least 95%, at
least 98%, or at
least 99% identity to the following sequence:
TGGAAGGGCTAATTCACTCCCAACGAAGACAAGATCTGCTTTTTGCTTGTACTGG
GTCTCTCTGGTTAGACCAGATCTGAGCCTGGGAGCTCTCTGGCTAACTAGGGAAC
CCACTGCTTAAGCCTCAATAAAGCTTGCCTTGAGTGCTTCAAGTAGTGTGTGCCC
GTCTGTTGTGTGACTCTGGTAACTAGAGATCCCTCAGACCCTTTTAGTCAGTGTG
GAAAATCTCTAGCAGT (SEQ ID NO:6).
[0092] In particular embodiments of any of the expression cassettes and gene
delivery
vectors described herein, the 3' LTR comprises or consists of the following
sequence, or a
sequence having at least 80%, at least 85%, at least 90%, at least 95%, at
least 98%, or at
least 99% identity to the following sequence:
TGGAAGGGCTAATTCACTCCCAACGAAGACAAGATCTGCTTTTTGCTTGTACTGG
GTCTCTCTGGTTAGACCAGATCTGAGCCTGGGAGCTCTCTGGCTAACTAGGGAAC
CCACTGCTTAAGCCTCAATAAAGCTTGCCTTGAGTGCTTCAAGTAGTGTGTGCCC
GTCTGTTGTGTGACTCTGGTAACTAGAGATCCCTCAGACCCTTTTAGTCAGTGTG
GAAAATCTCTAGCAG (SEQ ID NO:7).
[0093] In some embodiments, the polynucleotide cassettes of the present
disclosure provide
for enhanced expression of a transgene in mammalian cells. As demonstrated by
the working
examples of the present disclosure, the present inventors have discovered a
number of
polynucleotide elements, i.e. improved elements as compared to those known in
the art,
which individually and synergistically provide for the enhanced expression of
transgenes in
mammalian cells. In certain embodiments, the arrangement of the two or more
functional
polynucleotide sequences within the polynucleotide cassettes of the present
disclosure
provide for enhanced expression of a transgene in mammalian cells. By
"enhanced" it is
21
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
meant that expression of the transgene is increased, augmented, or stronger,
in cells carrying
the polynucleotide cassettes of the present disclosure relative to in cells
carrying the
transgene operably linked to comparable regulatory elements, e.g. as known in
the art. Put
another way, expression of the transgene is increased, augmented, or stronger,
from the
polynucleotide cassettes of the present disclosure relative to expression from
a polynucleotide
cassette not comprising the one or more optimized elements of the present
disclosure, i.e. a
reference control. In certain embodiment, the enhanced expression is specific
for or limited to
one or more desired cell types.
[0094] For example, expression of the transgene may be enhanced, or augmented,
or
stronger, in cells comprising a polynucleotide cassette comprising a promoter
disclosed
herein than in cells that carry the transgene operably linked to a different
promoter, e.g. as
known in the art. As another example, expression of the transgene may be
enhanced, or
increased, augmented, or stronger, in cells comprising a polynucleotide
cassette comprising
an enhancer sequence disclosed herein than in cells that carry the transgene
operably linked
to a different enhancer sequence.
[0095] Promoter and enhancer elements can be tissue specific or stage-
specific. For
example, a tissue ¨specific promoter or enhancer preferentially drives
expression (or a higher
level of expression) in one or more particular cell type. Examples of cell
types include but
are not limited to: hematopoietic stem cells, long term hematopoietic stem
cells, short term
hematopoietic stem cells, multipotent progenitors, hematopoietic CD34+ cells
and any cluster
differentiation subpopulation within the CD34+ population. A stage-specific
promoter or
enhancer preferentially drives expression (or higher level of expression)
during one or more
specific stages of the cell cycle or development. These include but are not
limited to beta-
globin locus control region, spectrin promoter, and an erythroid specific
promote.
[0096] Without wishing to be bound by theory, enhanced expression of a
transgene in cells
is believed to be due to a faster build-up of gene product in the cells or a
more stable gene
product in the cells. Thus, enhanced expression of a transgene by the
polynucleotide
cassettes of the subject disclosure may be observed in a number of ways. For
example,
enhanced expression may be observed by detecting the expression of the
transgene following
contact of the polynucleotide cassette to the cells sooner, e.g. 2 days
sooner, 7 days sooner, 2
weeks sooner, 3 weeks sooner, 4 weeks sooner, 8 weeks sooner, 12 weeks sooner
or more,
than expression would be detected if the transgene were operably linked to
comparable
regulatory elements, e.g. as known in the art. Enhanced expression may also be
observed as
an increase in the amount of gene product per cell. For example, there may be
a 2-fold
22
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
increase or more, e.g. a 3-fold increase or more, a 4-fold increase or more, a
5-fold increase
or more, or a 10-fold increase or more in the amount of gene product per
mammalian cell.
Enhanced expression may also be observed as an increase in the number of
mammalian cells
that express detectable levels of the transgene carried by the polynucleotide
cassette. For
example, there may be a 2-fold increase or more, e.g. a 3-fold increase or
more, a 4-fold
increase or more, a 5-fold increase or more, or a 10-fold increase or more in
the number of
mammalian cells that express detectable levels of the transgene. As another
example, the
polynucleotide of the present invention may promote detectable levels of the
transgene in a
greater percentage of cells as compared to a conventional polynucleotide
cassette; for
example, where a conventional cassette may promote detectable levels of
transgene
expression in, for example, less than 5% of the cells in a certain region, the
polynucleotide of
the present invention promotes detectable levels of expression in 5% or more
of the cells in
that region; e.g. 10% or more, 15% or more, 20% or more, 25% or more, 30% or
more, 35%
or more, 40% or more, or 45% or more, in some instances 50% or more, 55% or
more; 60%
or more, 65% or more, 70% or more, or 75% or more, for example 80% or more,
85% or
more, 90% or more, or 95% or more of the cells that are contacted, will
express detectable
levels of gene product. Enhanced expression may also be observed as an
alteration in the
viability and/or function of the cells.
[0097] The polynucleotide cassettes of the present disclosure typically
comprise a promoter
region. Any suitable promoter region or promoter sequence therein can be used
in the subject
polynucleotide cassettes, so long as the promoter region promotes expression
of a coding
sequence in eukaryotic cells. In certain embodiments, the promoter region
promoter
expression of a coding sequence in mammalian cells. In some instances, the
promoter is a
ubiquitous promoter, i.e., it is a promoter that is active in a wide range of
cells, tissues and
species. In other instances, the promoter is a human phosphoglycerate kinase
(PGK)
promoter.
[0098] In some embodiments, the polynucleotide comprises one or more
enhancers.
Enhancers are nucleic acid elements known in the art to enhance transcription,
and can be
located anywhere in association with the gene they regulate, e.g. upstream,
downstream,
within an intron, etc. Any enhancer element can be used in the polynucleotide
cassettes and
gene therapy vectors of the present disclosure, so long as it enhances
expression of the gene
when used in combination with the promoter.
[0099] The coding sequence to be expressed in the cells can be any
polynucleotide
sequence, e.g. gene or cDNA that encodes a gene product, e.g. a polypeptide or
RNA-based
23
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
therapeutic (siRNA, antisense, ribozyme, shRNA, etc.). The coding sequence may
be
heterologous to the promoter sequence to which it is operably linked, i.e. not
naturally
operably associated with it. Alternatively, the coding sequence may be
endogenous to the
promoter sequence to which it is operably linked, i.e. is associated in nature
with that
promoter. The gene product may act intrinsically in the mammalian cell, or it
may act
extrinsically, e.g. it may be secreted. For example, when the transgene is a
therapeutic gene,
the coding sequence may be any gene that encodes a desired gene product or
functional
fragment or variant thereof that can be used as a therapeutic for treating a
disease or disorder.
In various preferred embodiments, the transgene encodes human PKLR.
[00100] In one embodiment of the invention, the transgene coding sequence is
modified, or
"codon optimized" to enhance expression by replacing infrequently represented
codons with
more frequently represented codons. The coding sequence is the portion of the
mRNA
sequence that encodes the amino acids for translation. During translation,
each of 61
trinucleotide codons are translated to one of 20 amino acids, leading to a
degeneracy, or
redundancy, in the genetic code. However, different cell types, and different
animal species,
utilize tRNAs (each bearing an anticodon) coding for the same amino acids at
different
frequencies. When a gene sequence contains codons that are infrequently
represented by the
corresponding tRNA, the ribosome translation machinery may slow, impeding
efficient
translation. Expression can be improved via "codon optimization" for a
particular species,
where the coding sequence is altered to encode the same protein sequence, but
utilizing
codons that are highly represented, and/or utilized by highly expressed human
proteins (Cid-
Arregui et al., 2003; J. Virol. 77: 4928). In one aspect of the present
invention, the coding
sequence of the transgene is modified to replace codons infrequently expressed
in mammal or
in primates with codons frequently expressed in primates. For example, in some
embodiments, the coding sequence encoded by the transgene encodes a
polypeptide having at
least 85% sequence identity to a polypeptide encoded by a sequence disclosed
above or
herein, for example at least 90% sequence identity, e.g. at least 95% sequence
identity, at
least 98% identity, at least 99% identity, wherein at least one codon of the
coding sequence
has a higher tRNA frequency in humans than the corresponding codon in the
sequence
disclosed above or herein.
[00101] In an additional embodiment of the invention, the transgene coding
sequence is
modified to enhance expression by termination or removal of open reading
frames (ORFs)
that do not encode the desired transgene. An open reading frame (ORF) is the
nucleic acid
sequence that follows a start codon and does not contain a stop codon. ORFs
may be in the
24
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
forward or reverse orientation, and may be "in frame" or "out of frame"
compared with the
gene of interest. Such open reading frames have the potential to be expressed
in an expression
cassette alongside the gene of interest, and could lead to undesired adverse
effects. In one
aspect of the present invention, the coding sequence of the transgene has been
modified to
remove open reading frames by further altering codon usage. This was done by
eliminating
start codons (ATG) and introducing stop codons (TAG, TAA, or TGA) in reverse
orientation
or out-of-frame ORFs, while preserving the amino acid sequence and maintaining
highly
utilized codons in the gene of interest (i.e., avoiding codons with frequency
<20%). In the
present invention, the transgene coding sequence may be optimized by either of
codon
optimization and removal of non-transgene ORFs or using both techniques. As
will be
apparent to one of ordinary skill in the art, it is preferable to remove or
minimize non-
transgene ORFs after codon optimization in order to remove ORFs introduced
during codon
optimization.
[00102] In some embodiments, the polynucleotide cassette of the present
invention further
comprises an RNA export signal. Exemplary RNA export sequences include but are
not
limited to sequences from woodchuck hepatitis virus post-transcriptional
element (WPRE).
The woodchuck hepatitis virus (WHV) post-transcriptional regulatory element
(Wpre)
significantly increases transgene expression in target cells, by increasing
RNA stability in a
transgene, promoter and vector-independent manner (Zuffrey et al, 1999).
However, it can
express a truncated 60-amino acid protein derived from the WHV X gene involved
in liver
cancer (Kingsman et al, 2005). Therefore, most pre-clinical protocols and
clinical trials
include a mutated version of the Wpre element (Zanta-Boussif et al, 2009). On
the other
hand, the use of two 5V40-USE elements in SIN-LV vectors has been seen to be
more
efficient than WPRE sequence in supressing transcriptional read through
(Schambach et al,
2007). More precisely, the WPRE disclosed herein is a chimeric WPRE that
carries 589
nucleotides from the modified WPRE performed by Axel Schambach (nucleotides 1-
589)
(WO 2008136670 A2; [51) and 88 from a former WPRE (nucleotide 590-677)
(Zuffrey et al,
1999). Data disclosed herein shows this chimeric wpre works better than the
former WPRE.
The chimeric WPRE sequence comprises the sequence listed in the table below.
[00103] Table 1. Modified WPRE sequence
CGAGCATCTTACCGCCATTTATTCCCATATTTGTTCTG 1 1 1 1 1
CTTGATTTGGGTATACATTTAAATGTTAATAAA
ACAAAATGGTGGGGCAATCATTTACA 1 1 1 1 1 AG G GATATGTAATTACTAGTTCAG GTGTATTG
CCACAAGACA
AACATGTTAAGAAACTTTCCCGTTATTTACG CTCTGTTCCTGTTAATCAACCTCTG GATTACAAAATTTGTG AAA
GATTGACTGATATTCTTAACTATGTTGCTCCTTTTACGCTGTGTGGATATGCTGCTTTAATGCCTCTGTATCATG
CTATTGCTTCCCGTACGGCTTTCGTTTTCTCCTCCTTGTATAAATCCTGGTTGCTGTCTCTTTATGAGGAGTTGTG
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
GCCCGTTGTCCGTCAACGTGG CGTGGTGTGCTCTGTGTTTGCTGACG CAACCCCCACTGGCTG G G G CATTG
CC
ACCACCTGTCAACTCCTTTCTGG GACTTTCGCTTTCCCCCTCCCGATCG CCACG GCAGAACTCATCGCCG
CCTGC
CTTGCCCGCTGCTGGACAGGGGCTAGGTTGCTGGG CACTGATAATTCCGTGGTGTTGTCG GGGAAG GGCCTG
CTGCCGGCTCTGCGG CCTCTTCCGCGTCTTCGCCTTCGCCCTCAGACGAGTCG GATCTCCCTTTG GGCCGCCTC
CCCGCCTG (SEQ ID NO:1)
[00104] The present invention also include a nucleic acid, e.g., a
polynucleotide sequence,
comprising a sequence having at least 80%, at least 85%, at least 90%, at
least 95%, at least
98%, or at least 99% identity to the sequence set forth in SEQ ID NO:1 or SEQ
ID NO:9. In
particular embodiments, the polynucleotide sequence comprises the sequence set
forth in
SEQ ID NO:1 or SEQ ID NO:9.
[00105] In particular embodiments of any of the expression cassettes and gene
delivery
vectors described herein, the Wpre sequence comprises or consists of the
sequence of SEQ
ID NO:1, or a sequence having at least 80%, at least 85%, at least 90%, at
least 95%, at least
98%, or at least 99% identity to the sequence of SEQ ID NO:1.
[00106] In particular embodiments of any of the expression cassettes and gene
delivery
vectors described herein, the Wpre sequence comprises or consists of the
following sequence,
or a sequence having at least 80%, at least 85%, at least 90%, at least 95%,
at least 98%, or at
least 99% identity to the following sequence:
CGAGCATCTTACCGCCATTTATTCCCATATTTGTTCTGTTTTTCTTGATTTGGGTATACATT
TAAATGTTAATAAAACAAAATGGTGGGGCAATCATTTACATTTTTAGGGATATGTAATTA
CTAGTTCAGGTGTATTGCCACAAGACAAACATGTTAAGAAACTTTCCCGTTATTTACGCT
CTGTTCCTGTTAATCAACCTCTGGATTACAAAATTTGTGAAAGATTGACTGATATTCTTAA
CTATGTTGCTCCTTTTACGCTGTGTGGATATGCTGCTTTAATGCCTCTGTATCATGCTATT
GCTTCCCGTACGGCTTTCGTTTTCTCCTCCTTGTATAAATCCTGGTTGCTGTCTCTTTATGA
GGAGTTGTGGCCCGTTGTCCGTCAACGTGGCGTGGTGTGCTCTGTGTTTGCTGACGCAAC
CCCCACTGGCTGGGGCATTGCCACCACCTGTCAACTCCTTTCTGGGACTTTCGCTTTCCCC
CTCCCGATCGCCACGGCAGAACTCATCGCCGCCTGCCTTGCCCGCTGCTGGACAGGGGCT
AGGTTGCTGGGCACTGATAATTCCGTGGTGTTGTCGGGGAAGGGCCTGCTGCCGGCTCTG
CGGCCTCTTCCGCGTCTTCGCCTTCGCCCTCAGACGAGTCGGATCTCCCTTTGGGCCGCCT
CCCCGCCTG (SEQ ID NO:9).
[00107] In certain embodiments, an expression cassette or gene delivery
vector, e.g., a
lentivirus, comprises a polynucleotide sequence comprising the following
sequences in 5' to
3' order:
a) a PGK promoter sequence, optionally a human PGK promoter sequence;
26
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
b) a sequence encoding a pyruvate kinase polypeptide, optionally a codon
optimized
RPK coding or cDNA sequence; and
c) a mutant Wpre sequence, optionally comprising or consisting of the sequence
of
SEQ ID NO: 1.
[00108] In certain embodiments, an expression cassette or gene delivery
vector, e.g., a
lentivurus, comprises a polynucleotide sequence comprising the following
sequences in 5' to
3' order:
a) a cPPT sequence;
b) PGK promoter sequence, optionally a human PGK promoter sequence;
c) a sequence encoding a pyruvate kinase polypeptide, optionally a codon
optimized
RPK coding or cDNA sequence; and
d) a mutant Wpre sequence, optionally comprising or consisting of the sequence
of
SEQ ID NO: 1.
[00109] In certain embodiments, an expression cassette or gene delivery
vector, e.g., a
lentivurus, comprises a polynucleotide sequence comprising the following
sequences in 5' to
3' order:
a) a 5' LTR, optionally a modified 5' LTR;
b) a cPPT sequence;
c) PGK promoter sequence, optionally a human PGK promoter sequence;
d) a sequence encoding a pyruvate kinase polypeptide, optionally a codon
optimized
RPK coding or cDNA sequence;
e) a mutant Wpre sequence, optionally comprising or consisting of the sequence
of
SEQ ID NO:1; and
f) a 3' LTR, optionally a modified 3' LTR.
[00110] In certain embodiments, the gene delivery vector is PGK-coRPK LV, or
comprises
the elements depicted in FIG. 21.
[00111] In particular embodiments of any of the expression cassettes and gene
delivery
vectors described herein, the codon optimized RPK cDNA or coding sequence
encodes a
PKLR polypeptide that comprises or consists of the sequence disclosed in any
of GenBank
accession Nos. XP 016856982. I, XP011507942.1. XP006711449. 1. NF __.870986.
I, or
NP 000289.1 or a functional fragment of any of these sequences, or a sequence
having at
least 80%, at least 85%, at least 90%, at least 95%, at least 98%, or at least
99% identity to
any of these sequences.
27
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[00112] Other combinations of elements both as disclosed herein or as known in
the art will
be readily appreciated by the ordinarily skilled artisan.
[00113] Additionally, as will be recognized by one of ordinary skill in the
art, the
polynucleotide cassettes may optionally contain other elements including, but
not limited to
restriction sites to facilitate cloning and regulatory elements for a
particular gene expression
vector.
[00114] In some aspects of the present invention, the subject polynucleotide
cassettes are
used to deliver a gene to cells of an animal, e.g. to determine the effect
that the gene has on
cell viability and/or function, to treat a cell disorder, etc. Accordingly, in
some aspects of the
invention, the composition that provides for the expression of a transgene in
mammalian cells
is a gene delivery vector, wherein the gene delivery vector comprises the
polynucleotide
cassettes of the present disclosure.
[00115] Any convenient gene therapy vector that finds use delivering
polynucleotide
sequences to mammalian cells is encompassed by the gene delivery vectors of
the present
disclosure. For example, the vector may comprise single or double stranded
nucleic acid, e.g.
single stranded or double stranded DNA. For example, the gene delivery vector
may be
DNA, e.g., a naked DNA, e.g. a plasmid, a minicircle, etc. The vector may
comprise single-
stranded or double-stranded RNA, including modified forms of RNA. In another
example,
the gene delivery vector may be an RNA, e.g., an mRNA or modified mRNA.
[00116] As another example, the gene delivery vector may be a viral vector
derived from a
virus, e.g. an adenovirus, an adeno-associated virus, a lentivirus (LV), a
herpes virus, an
alpha virus or a retrovirus, e.g., Moloney murine leukemia virus (M-MuLV),
Moloney
murine sarcoma virus (MoMSV), Harvey murine sarcoma virus (HaMuSV), murine
mammary tumor virus (MuMTV), gibbon ape leukemia virus (GaLV), feline leukemia
virus
(FLV), spumavirus, Friend murine leukemia virus, Murine Stem Cell Virus (MSCV)
and
Rous Sarcoma Virus (RSV)) or lentivirus. While embodiments encompassing the
use of
lentivirus are described in greater detail below, it is expected that the
ordinarily skilled artisan
will appreciate that similar knowledge and skill in the art can be brought to
bear on non-LV
gene therapy vectors as well. In some embodiments, the gene delivery vector is
a self-
limiting lentivirus.
[00117] In such embodiments, the subject polynucleotide cassette is flanked on
the 5' and 3'
ends by functional long terminal repeat (LTR) sequences. In one embodiment,
the position of
different elements present in the backbone of the lentiviral vector is
depicted in Figure 1.
Both LTR sequences have been modified to generate self-inactivating (SIN) LV
vectors. SIN
28
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
vectors have a 400 bp deletion in the 3'-LTR, covering the promoter/enhancer
elements from
the U3 region. Expression of the transgene is thereby dependent on internal
promoters,
reducing the risk of RCLs and decreasing promoter interference (Ginn et al,
2003). This 3'-
LTR deletion removes the TATA box, preventing transcription initiation
(Miyoshi et al.
1998; Zuffrey et al 1998); and therefore inactivating the vector. The U3
region of the 5'-LTR
has been replaced by other heterologous promoting sequences (i.e. CMV or RSV)
to achieve
a Tat-independent transcription and to increase genomic RNA synthesis,
resulting in the
increase of the viral titer. Because 5'-U3 region drives the expression of
primary transcripts,
its modifications will not be present in transduced cells (Schambach et al.
2009). Exogenous
elements, such as 0-globin or SV40 polyadenylation signals (Iwakuma et al,
1999) or the
upstream sequence element (USE) from simian virus 40 (5V40-USE) (Schambach et
al.
2007), have also been included in the R region of the viral 3'LTR in order to
decrease the
transcriptional readthrough from the internal promoters (Zaiss et al, 2002) or
from remnants
of the deleted U3 region of SIN-LV vectors (Almarza et al. 2011) preventing
the potential
transcriptional activation of the downstream genes. The leader region contains
the packaging
signal (tP), and LV vectors were thought to require approximately 300 bp of
the Gag gene in
this region. Currently, this Gag sequence has been reduced to just 40 bp
(Figure 1). The Rev
responsive element (RRE) has also been included to improve the efficiency of
gene transfer,
although it contains surrounding Env remnants. The central polypurine tract
(cPPT), which
facilitates nuclear translocation of the pre-integration complexes, together
with the central
terminal sequence (CTS) involved in the separation of reverse transcriptase,
has been seen to
improve viral titer (Zennou, et al. 2000; Follenzi et al. 2000). In particular
embodiments, the
cPPT present in any of the expression cassettes or gene delivery vectors
described herein
comprises or consists of the following sequence:
TTTAAAAGAAAAGGGGGGATTGGGGGGT (SEQ ID NO:2), or a sequence having at
least 80%, at least 85%, at least 90%, at least 95%, at least 98%, or at least
99% identity to
SEQ ID NO:2.
[00118] The dNEF/PPT signal is essential for reverse transcription, and its
incorporation
significantly improves LV vector production.
[00119] Gene therapy vectors encapsulating the polynucleotide cassettes of the
present
disclosure may be produced using standard methodology. For example, in the
case of LV
virions, an LV expression vector according to the invention may be introduced
into a
producer cell, followed by introduction of an LV helper construct, where the
helper construct
includes LV coding regions capable of being expressed in the producer cell and
which
29
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
complement LV helper functions absent in the LV vector. This is followed by
introduction of
helper virus and/or additional vectors into the producer cell, wherein the
helper virus and/or
additional vectors provide accessory functions capable of supporting efficient
LV virus
production. The producer cells are then cultured to produce LV. These steps
are carried out
using standard methodology.
[00120] Any suitable method for producing viral particles for delivery of the
subject
polynucleotide cassettes can be used, including but not limited to those
described in the
examples that follow. Any concentration of viral particles suitable to
effectively transducer
mammalian cells can be prepared for contacting mammalian cells in vitro or in
vivo. For
example, the viral particles may be formulated at a concentration of 108
vector genomes per
ml or more, for example, 5x108 vector genomes per mL; i09 vector genomes per
mL; 5 x 109
vector genomes per mL, 1019 vector genomes per mL, 5x1019 vector genomes per
mL; 1011
vector genomes per mL; 5 x1011vector genomes per mL; 1012 vector genomes per
mL; 5x1012
vector genomes per mL; 1013 vector genomes per mL; 1.5 x1013 vector genomes
per mL;
3x1013 vector genomes per mL; 5x1013 vector genomes per mL; 7.5x10'3 vector
genomes per
mL; 9x1013 vector genomes per mL; 1 x 1014 vector genomes per mL, 5 x 1014
vector
genomes per mL or more, but typically not more than 1 x 1015 vector genomes
per mL.
[00121] In preparing the subject LV compositions, any host cells for producing
LV virions
may be employed, including, for example, mammalian cells (e.g. 293 cells),
insect cells (e.g.
SF9 cells), microorganisms and yeast. Host cells can also be packaging cells
in which the LV
rep and cap genes are stably maintained in the host cell or producer cells in
which the LV
vector genome is stably maintained and packaged. Exemplary packaging and
producer cells
are derived from SF-9, 293, A549 or HeLa cells. LV vectors are purified and
formulated
using standard techniques known in the art.
[00122] In certain embodiments, the present invention includes a cell
comprising an
expression cassette or gene delivery vector disclosed herein. In related
embodiments, the cell
is transduced with a viral vector comprising an expression cassette disclosed
herein or has an
expression cassette disclosed herein integrated into the cell's genome. In
certain
embodiments, the cell is a cell used to produce a viral gene delivery vector.
In other
embodiments, the cell is a cell to be delivered to a subject in order to
provide to the subject
the gene product encoded by the expression cassette. Thus, in certain
embodiments, the cell
is autologous to the subject to be treated or was obtained from the subject to
be treated. In
other embodiments, the cell is allogeneic to the subject to be treated or was
obtained from a
donor other than the subject to be treated. In particular embodiments, the
cell is a
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
mammalian cell, e.g., a human cell. In certain embodiments, the cell is a
blood cell, an
erythrocyte, a hematopoietic progenitor cell, a bone marrow cell, e.g., a
lineage depleted bone
marrow cell, a hematopoietic stem cell (e.g., CD34+) or a a committed
hematopoietic
erythroid progenitor cell
[00123] The present invention includes pharmaceutical compositions comprising
a
polynucleotide cassette, gene delivery vector, or cell described herein and a
pharmaceutically-acceptable carrier, diluent or excipient. The subject
polynucleotide cassette,
gene delivery vector, or cell can be combined with pharmaceutically-acceptable
carriers,
diluents and reagents useful in preparing a formulation that is generally
safe, non-toxic, and
desirable, and includes excipients that are acceptable for primate use. Such
excipients can be
solid, liquid, semisolid, or, in the case of an aerosol composition, gaseous.
Examples of such
excipients, carriers or diluents include, but are not limited to, water,
saline, Ringer's solutions,
dextrose solution, and 5% human serum albumin. Supplementary active compounds
can also
be incorporated into the formulations. Solutions or suspensions used for the
formulations can
include a sterile diluent such as water for injection, saline solution, fixed
oils, polyethylene
glycols, glycerine, propylene glycol or other synthetic solvents;
antibacterial compounds such
as benzyl alcohol or methyl parabens; antioxidants such as ascorbic acid or
sodium bisulfite;
chelating compounds such as ethylenediaminetetraacetic acid (EDTA); buffers
such as
acetates, citrates or phosphates; detergents such as Tween 20 to prevent
aggregation; and
compounds for the adjustment of tonicity such as sodium chloride or dextrose.
The pH can be
adjusted with acids or bases, such as hydrochloric acid or sodium hydroxide.
In particular
embodiments, the pharmaceutical compositions are sterile.
[00124] Pharmaceutical compositions suitable for use in the present invention
further include
sterile aqueous solutions or dispersions and sterile powders for the
extemporaneous
preparation of sterile injectable solutions or dispersion.
[00125] Sterile solutions can be prepared by incorporating the active compound
in the
required amount in an appropriate solvent with one or a combination of
ingredients
enumerated above, as required, followed by filtered sterilization. Generally,
dispersions are
prepared by incorporating the active compound into a sterile vehicle that
contains a basic
dispersion medium and the required other ingredients from those enumerated
above. In the
case of sterile powders for the preparation of sterile injectable solutions,
methods of
preparation are vacuum drying and freeze-drying that yields a powder of the
active ingredient
plus any additional desired ingredient from a previously sterile-filtered
solution thereof
31
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[00126] In one embodiment, the compositions are prepared with carriers that
will protect the
gene cassette or expression vector against rapid elimination from the body,
such as a
controlled release formulation, including implants and microencapsulated
delivery systems.
Biodegradable, biocompatible polymers can be used, such as ethylene vinyl
acetate,
polyanhydrides, polyglycolic acid, collagen, polyorthoesters, and polylactic
acid. Methods
for preparation of such formulations will be apparent to those skilled in the
art. The materials
can also be obtained commercially.
[00127] It is especially advantageous to formulate oral, ocular or parenteral
compositions in
dosage unit form for ease of administration and uniformity of dosage. Dosage
unit form as
used herein refers to physically discrete units suited as unitary dosages for
the subject to be
treated; each unit containing a predetermined quantity of active compound
calculated to
produce the desired therapeutic effect in association with the required
pharmaceutical carrier.
The specification for the dosage unit forms of the invention are dictated by
and directly
dependent on the unique characteristics of the active compound and the
particular therapeutic
effect to be achieved, and the limitations inherent in the art of compounding
such an active
compound for the treatment of individuals.
[00128] The pharmaceutical compositions can be included in a container, pack,
or dispenser,
e.g. syringe, e.g. a prefilled syringe, together with instructions for
administration.
[00129] The pharmaceutical compositions of the invention encompass any
pharmaceutically
acceptable salts, esters, or salts of such esters, or any other compound
which, upon
administration to an animal comprising a human, is capable of providing
(directly or
indirectly) the biologically active metabolite or residue thereof
[00130] The term "pharmaceutically acceptable salt" refers to physiologically
and
pharmaceutically acceptable salts of the compounds of the invention: i.e.,
salts that retain the
desired biological activity of the parent compound and do not impart undesired
toxicological
effects thereto. A variety of pharmaceutically acceptable salts are known in
the art and
described, e.g., in in "Remington's Pharmaceutical Sciences", 17th edition,
Alfonso R.
Gennaro (Ed.), Mark Publishing Company, Easton, PA, USA, 1985 (and more recent
editions
thereof), in the "Encyclopaedia of Pharmaceutical Technology", 3rd edition,
James
Swarbrick (Ed.), Informa Healthcare USA (Inc.), NY, USA, 2007, and in J.
Pharm. Sci. 66: 2
(1977). Also, for a review on suitable salts, see Handbook of Pharmaceutical
Salts:
Properties, Selection, and Use by Stahl and Wermuth (Wiley-VCH, 2002).
[00131] Pharmaceutically acceptable base addition salts are formed with metals
or amines,
such as alkali and alkaline earth metals or organic amines. Metals used as
cations comprise
32
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
sodium, potassium, magnesium, calcium, and the like. Amines comprise N-N'-
dibenzylethylenediamine, chloroprocaine, choline, diethanolamine,
dicyclohexylamine,
ethylenediamine, N-methylglucamine, and procaine (see, for example, Berge et
al.,
"Pharmaceutical Salts," J. Pharma Sci., 1977, 66, 119). The base addition
salts of said acidic
compounds are prepared by contacting the free acid form with a sufficient
amount of the
desired base to produce the salt in the conventional manner. The free acid
form may be
regenerated by contacting the salt form with an acid and isolating the free
acid in the
conventional manner. The free acid forms differ from their respective salt
forms somewhat
in certain physical properties such as solubility in polar solvents, but
otherwise the salts are
equivalent to their respective free acid for purposes of the present
invention.
[00132] The subject polynucleotide cassette, gene delivery vector, e.g.,
recombinant virus
(virions), or cell (e.g., transduced with a gene delivery vector disclosed
herein) can be
incorporated into pharmaceutical compositions for administration to mammalian
patients,
particularly primates and more particularly humans. The subject polynucleotide
cassette, gene
delivery vector, e.g. virions, or cell can be formulated in nontoxic, inert,
pharmaceutically
acceptable aqueous carriers, preferably at a pH ranging from 3 to 8, more
preferably ranging
from 6 to 8. Such sterile compositions will comprise the vector or virion
containing the
nucleic acid encoding the therapeutic molecule dissolved in an aqueous buffer
having an
acceptable pH upon reconstitution.
[00133] In some embodiments, the pharmaceutical composition provided herein
comprise a
therapeutically effective amount of a cell, vector or virion disclosed herein
in admixture with
a pharmaceutically acceptable carrier and/or excipient, for example saline,
phosphate
buffered saline, phosphate and amino acids, polymers, polyols, sugar, buffers,
preservatives
and other proteins. Exemplary amino acids, polymers and sugars and the like
are
octylphenoxy polyethoxy ethanol compounds, polyethylene glycol monostearate
compounds,
polyoxyethylene sorbitan fatty acid esters, sucrose, fructose, dextrose,
maltose, glucose,
mannitol, dextran, sorbitol, inositol, galactitol, xylitol, lactose,
trehalose, bovine or human
serum albumin, citrate, acetate, Ringer's and Hank's solutions, cysteine,
arginine, carnitine,
alanine, glycine, lysine, valine, leucine, polyvinylpyrrolidone, polyethylene
and glycol.
Preferably, this formulation is stable for at least six months at 4 C.
[00134] In some embodiments, the pharmaceutical composition provided herein
comprises a
buffer, such as phosphate buffered saline (PBS) or sodium phosphate/sodium
sulfate, tris
buffer, glycine buffer, sterile water and other buffers known to the
ordinarily skilled artisan
such as those described by Good et al. (1966) Biochemistry 5:467. The pH of
the buffer in
33
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
which the pharmaceutical composition comprising the tumor suppressor gene
contained in
the adenoviral vector delivery system, may be in the range of 6.5 to 7.75,
preferably 7 to 7.5,
and most preferably 7.2 to 7.4.
[00135] In certain embodiments, viral vectors may be formulated into any
suitable unit
dosage, including, without limitation, 1x108 vector genomes or more, for
example, 1x109,
lx101 , lx1011, lx1012, or lx1013 vector genomes or more, in certain
instances, lx1014 vector
genomes, but usually no more than 4x1015 vector genomes. In some cases, the
unit dosage is
at most about 5x1015 vector genomes, e.g. lx1014 vector genomes or less, for
example lx1013,
lx1012, lx1011, lx101 , or 1x109 vector genomes or less, in certain instances
lx108vector
genomes or less, and typically no less than 1x108 vector genomes. In some
cases, the unit
dosage is lx101 to lx1011 vector genomes. In some cases, the unit dosage is
lx101 to
3x1012 vector genomes. In some cases, the unit dosage is 1x109 to 3x1013
vector genomes.
In some cases, the unit dosage is 1x108 to 3x1014 vector genomes. In one
embodiment, the
range is from about 5x101 to about lx1011 vector genomes. In some
embodiments, the range
is from about 1x109 to about lx101 vector genomes.
[00136] In some cases, the unit dosage of a pharmaceutical composition may be
measured
using multiplicity of infection (MOI). By MOI it is meant the ratio, or
multiple, of vector or
viral genomes to the cells to which the nucleic acid may be delivered. In some
cases, the
MOI may be 1x106. In some cases, the MOI may be 1x105 -1x107. In some cases,
the MOI
may be 1x104 -1x108. In some cases, recombinant viruses of the disclosure are
at least about
lx101, lx102, lx103, lx104, lx105, lx106, lx107, lx108, lx109, lx101 , lx1011,
lx1012,
1X1013, 1X1014, 1X1015, 1X1016, lx1017, and lx1018MOI. In some cases,
recombinant viruses
of this disclosure are 1x108 to 3x10'4 MOI. In some cases, recombinant viruses
of the
disclosure are at most about lx101, 1x102, 1x103, 1x104, 1x105, 1x106, 1x107,
1x108, 1x109,
lx101 , lx1011, lx1012, lx1013, lx1014, lx1015, lx1016, lx1017, and lx1018MOI.
In some,
embodiments the range is from about 20 to about 400 MOI.
[00137] In some aspects, the amount of pharmaceutical composition comprises
about 1 x 108
to about 1 x 1015 recombinant viruses, about 1 x 109 to about 1 x 1014
recombinant viruses,
about 1 x 1010 to about 1 x 1013 recombinant viruses, or about 1 x 1011 to
about 3 x 1012
recombinant viruses.
METHODS
[00138] As disclosed herein, the subject polynucleotide cassettes and gene
delivery vectors,
referred to collectively herein as the "subject compositions", find use in
expressing a
34
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
transgene in cells of an animal. For example, the subject compositions may be
used in
research, e.g. to determine the effect that the gene has on cell viability
and/or function. As
another example, the subject compositions may be used in medicine, e.g. to
treat or prevent a
disease or disorder. Thus, in some aspects of the invention, methods are
provided for the
expression of a gene in cells, the method comprising contacting cells with a
composition of
the present disclosure. In some embodiments, contacting occurs in vitro or ex
vivo. In some
embodiments, contacting occurs in vivo, i.e., the subject composition is
administered to a
subject.
[00139] For instances in which mammalian cells are to be contacted in vitro or
ex vivo with
a subject polynucleotide cassette or gene delivery vector comprising a subject
polynucleotide
cassette, the cells may be from any mammalian species, e.g. rodent (e.g. mice,
rats, gerbils,
squirrels), rabbit, feline, canine, goat, ovine, pig, equine, bovine, primate,
human. Cells may
be from established cell lines or they may be primary cells, where "primary
cells", "primary
cell lines", and "primary cultures" are used interchangeably herein to refer
to cells and cells
cultures that have been derived from a subject and allowed to grow in vitro
for a limited
number of passages, i.e. splittings, of the culture. For example, primary
cultures are cultures
that may have been passaged 0 times, 1 time, 2 times, 4 times, 5 times, 10
times, or 15 times,
but not enough times go through the crisis stage. Typically, the primary cell
lines of the
present invention are maintained for fewer than 10 passages in vitro.
[00140] Embodiments of the present invention comprise mammalian cells (e.g.,
CD34+
cells) transduced with a viral delivery vector, e.g., a lentiviral vector
containing the human
liver and erythroid pyruvate kinase (PKLR) gene. Accordingly, the present
invention includes
a method of transducing a mammalian cell, e.g. a human hematopoietic stem cell
or other cell
described herein, comprising contacting the cell with a viral delivery vector,
e.g., a lentiviral
vector, comprising an expression cassette described herein. In certain
embodiments, the cell
was previously obtained from a subject to be treated, or from another donor.
In particular
embodiments, the subject was diagnosed with PKD, and the cell is transduced
with a LV
comprising an expression cassette encoding pyruvate kinase, e.g., a codon-
optimized RPK
coding region or cDNA. It is understood that the disclosed methods, e.g.,
those used to
deliver a pyruvate kinase gene product, e.g., using a coPRK cDNA sequence, to
a subject
may also be used to treat hemolytic anemia, and/or normalize erythroid
differentiation,
increase the number of functional mature erythrocytes, reduce extramedullar
erythropoiesis,
reduce splenomegaly and other secondary effects of hemolytic anemia or PKD.
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[00141] To promote expression of the transgene, the subject polynucleotide
cassette or gene
delivery vector comprising a subject polynucleotide cassette will be contacted
with the cells
for about 30 minutes to 24 hours or more, e.g., 1 hour, 1.5 hours, 2 hours,
2.5 hours, 3 hours,
3.5 hours 4 hours, 5 hours, 6 hours, 7 hours, 8 hours, 12 hours, 16 hours, 18
hours, 20 hours,
24 hours, etc.
[00142] The subject polynucleotide cassette or gene delivery vector comprising
a subject
polynucleotide cassette may be provided to the subject cells one or more
times, e.g. one time,
twice, three times, or more than three times, and the cells allowed to
incubate with the
agent(s) for some amount of time following each contacting event e.g. 16-24
hours, after
which time the media is replaced with fresh media and the cells are cultured
further.
Contacting the cells may occur in any culture media and under any culture
conditions that
promote the survival of the cells. The culture may contain growth factors to
which the cells
are responsive. Growth factors, as defined herein, are molecules capable of
promoting
survival, growth and/or differentiation of cells, either in culture or in the
intact tissue, through
specific effects on a transmembrane receptor. Growth factors include
polypeptides and non-
polypeptide factors.
[00143] Typically, an effective amount of subject polynucleotide cassette or
gene delivery
vector comprising a subject polynucleotide cassette is provided to produce the
expression of
the transgene in cells. As discussed elsewhere herein, the effective amount
may be readily
determined empirically, e.g. by detecting the presence or levels of transgene
gene product, by
detecting an effect on the viability or function of the cells, etc. Typically,
an effect amount of
subject polynucleotide cassette or gene delivery vector comprising a subject
polynucleotide
cassette will promote greater expression of the transgene in cells than the
same amount of a
polynucleotide cassette as known in the art. Typically, expression will be
enhanced 2-fold or
more relative to the expression from a reference, or control, polynucleotide
cassette e.g. as
known in the art, for example 3-fold, 4-fold, or 5-fold or more, in some
instances 10-fold, 20-
fold or 50-fold or more, e.g. 100-fold.
[00144] For instances in which cells are to be contacted in vivo with a
subject polynucleotide
cassette or gene delivery vector comprising a subject polynucleotide cassette,
the subject may
be any mammal, e.g. rodent (e.g. mice, rats, gerbils), rabbit, feline, canine,
goat, ovine, pig,
equine, bovine, or primate. In a further preferred embodiment, the primate is
a human. In a
further embodiment, the cells are CD34+ cells.
[00145] The methods and compositions of the present disclosure find use, e.g.,
in the
treatment of pyruvate kinase deficiency.
36
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[00146] In another embodiment, the present invention includes a method of
treating a disease
in a subject in need thereof comprising providing to the subject an effective
amount of cells
transduced with a gene delivery vector, e.g., a viral vector, that expresses a
therapeutic gene
product in the cells. In particular embodiments, the cells are autologous to
the subject. In
certain embodiments, the cells are erythroid cells, e.g., hematopoietic stem
cells or committed
hematopoietic erythroid progenitor cells. In some embodiments, the cell is a
bone marrow
cell, e.g., a lineage depleted bone marrow cell. In particular embodiments,
the method is used
to treat PKD, and the viral vector is a LV comprising an expression construct
disclosed herein
comprising a human PGK promoter operably linked to a codon optimized human
PKLR gene
cDNA or coding sequence, and a mutated Wpre disclosed herein. In particular
embodiments,
the cells are provided to the subject parenterally, e.g., via intravenous
injection.
[00147] In another embodiment, the present invention includes a method of
treating PKD in
a subject in need thereof, comprising providing to the subject an effective
amount of
autologous C34+ stem cells transduced with a lentiviral vector that expresses
a codon
optimized PKLR cDNA in the cells, wherein the lentiviral vector comprises a
human PGK
promoter operably linked to the codon optimized human PKLR cDNA or coding
sequence,
and a mutated Wpre sequence disclosed herein. In particular embodiments, the
cells are
hematopoietic stem cells or committed hematopoietic erythroid progenitor
cells, e.g., bone
marrow cells. In particular embodiments, the cells are provided to the subject
parenterally,
e.g., via intravenous injection.
[00148] In another embodiment, the present invention provides a of treating a
disease in a
subject in need thereof comprising providing to the subject an effective
amount of a gene
delivery vector, e.g., a viral vector, that expresses a therapeutic gene
product in the subject. In
particular embodiments, the method is used to treat PKD, and the viral vector
is a LV
comprising an expression construct disclosed herein comprising a human PGK
promoter
operably linked to a codon optimized human PKLR gene cDNA or coding sequence,
and a
mutated Wpre disclosed herein. In particular embodiments, the gene delivery
vector are
provided to the subject parenterally, e.g., via intravenous injection.
[00149] In particular embodiments, the cells or gene delivery vectors are
provided to the
subject in pharmaceutical compositions.
[00150] In some embodiments, the subject methods result in a therapeutic
benefit, e.g.
preventing the development of a disorder, halting the progression of a
disorder, reversing the
progression of a disorder, etc. In some embodiments, the subject method
comprises the step
of detecting that a therapeutic benefit has been achieved. The ordinarily
skilled artisan will
37
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
appreciate that such measures of therapeutic efficacy will be applicable to
the particular
disease being modified, and will recognize the appropriate detection methods
to use to
measure therapeutic efficacy.
[00151] Expression of the transgene using the subject transgene is expected to
be robust.
Accordingly, in some instances, the expression of the transgene, e.g. as
detected by
measuring levels of gene product, by measuring therapeutic efficacy, etc. may
be observed
two months or less after administration, e.g. 4, 3 or 2 weeks or less after
administration, for
example, 1 week after administration of the subject composition. Expression of
the transgene
is also expected to persist over time. Accordingly, in some instances, the
expression of the
transgene, e.g. as detected by measuring levels of gene product, by measuring
therapeutic
efficacy, etc., may be observed 2 months or more after administration of the
subject
composition, e.g., 4, 6, 8, or 10 months or more, in some instances 1 year or
more, for
example 2, 3, 4, or 5 years, in certain instances, more than 5 years.
[00152] In certain embodiments, the method comprises the step of detecting
expression of
the transgene in the cells or in the subject, wherein expression is enhanced
relative to
expression from a polynucleotide cassette not comprising the one or more
improved elements
of the present disclosure. Typically, expression will be enhanced 2-fold or
more relative to
the expression from a reference, i.e. a control polynucleotide cassette, e.g.
as known in the
art, for example 3-fold, 4-fold, or 5-fold or more, in some instances 10-fold,
20-fold or 50-
fold or more, e.g. 100-fold, as evidenced by, e.g. earlier detection, higher
levels of gene
product, a stronger functional impact on the cells, etc.
[00153] Typically, if the subject composition is an LV comprising the subject
a
polynucleotide cassette of the present disclosure, an effective amount to
achieve a change in
will be about 1x108 vector genomes or more, in some cases 1x109, lx101 ,
lx1011, lx1012, or
lx1013 vector genomes or more, in certain instances, lx1014 vector genomes or
more, and
usually no more than lx1015 vector genomes. In some cases, the amount of
vector genomes
that is delivered is at most about lx1015 vector genomes, e.g. lx1014 vector
genomes or less,
for example lx1013, lx1012, lx1011, lx101 , or 1x109 vector genomes or less,
in certain
instances 1x108 vector genomes, and typically no less than 1x108 vector
genomes. In some
cases, the amount of vector genomes that is delivered is lx101 to lx1011
vector genomes. In
some cases, the amount of vector genomes that is delivered is lx101 to 3x1012
vector
genomes. In some cases, the amount of vector genomes that is delivered is
1x109 to 3x1013
vector genomes. In some cases, the amount of vector genomes that is delivered
is 1x108 to
3x1014 vector genomes.
38
CA 03021572 2018-10-18
WO 2017/184903 PCT/US2017/028695
[00154] In some cases, the amount of pharmaceutical composition to be
administered may be
measured using multiplicity of infection (MOD. In some cases, MOI may refer to
the ratio,
or multiple of vector or viral genomes to the cells to which the nucleic may
be delivered. In
some cases, the MOI may be 1x106. In some cases, the MOI may be 1x105 -1x107.
In some
cases, the MOI may be lx iO4 -1x108. In some cases, recombinant viruses of the
disclosure
are at least about lx101, 1x102, 1x103, 1x104, 1x105, 1x106, 1x107, 1x108,
1X109, 1X101 ,
1X1011, 1X1012, 1X1013, 1X1014, 1X1015, 1X1016, lx1017, and lx1018 MOI. In
some cases,
recombinant viruses of this disclosure are 1x108 to 3x1014 MOI. In some cases,
recombinant
viruses of the disclosure are at most about lx101, 1x102, 1x103, 1x104, 1x105,
1x106, 1x107,
1x108, 1x109, lx101 , lx1011, lx1012, lx1013, lx1014, lx1015, lx1016, lx1017,
and lx1018
MOI.
[00155] In some aspects, the amount of pharmaceutical composition comprises
about 1 x 108
to about 1 x 1015 particles of recombinant viruses, about 1 x 109 to about 1 x
1014 particles of
recombinant viruses, about 1 x 1010 to about 1 x 1013 particles of recombinant
viruses, or
about 1 x 1011 to about 3 x 1012 particles of recombinant viruses.
[00156] Any total number of viral particles suitable to provide appropriate
transduction of
cells to confer the desired effect or treat the disease can be administered to
the mammal. In
various preferred embodiments, at least 108; 5x108; 109; 5 x 109, 1010;
5x101o; 1-11;
U 5 x1011;
1012; 5x1012; 1-13;
u 1.5 x1013; 3x1013; 5x1013; 7.5x1013; 9x1013, 1 x 1014 viral particles, or
5 x
1014 viral particles or more, but typically not more than 1 x 1015 viral
particles are injected..
Any suitable number of administrations of the vector to the mammal or the
primate eye can
be made. In one embodiment, the methods comprise a single administration; in
other
embodiments, multiple administrations are made over time as deemed appropriate
by an
attending clinician. In some embodiments at least 2 x 108 VG/ml of 5 x 105
cells/ml is
required in a single administration (24 hours transduction) to result in high
transduction
efficiencies.
[00157] Individual doses are typically not less than an amount required to
produce a
measurable effect on the subject, and may be determined based on the
pharmacokinetics and
pharmacology for absorption, distribution, metabolism, and excretion ("ADME")
of the
subject composition or its by-products, and thus based on the disposition of
the composition
within the subject. This includes consideration of the route of administration
as well as
dosage amount. Effective amounts of dose and/or dose regimen can readily be
determined
empirically from preclinical assays, from safety and escalation and dose range
trials,
39
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
individual clinician-patient relationships, as well as in vitro and in vivo
assays such as those
described herein and illustrated in the Examples.
[00158] Several aspects of the invention are described herein with reference
to example
applications for illustration. It should be understood that numerous specific
details,
relationships, and methods are set forth to provide a full understanding of
the invention. One
having ordinary skill in the relevant art, however, will readily recognize
that the invention
can be practiced without one or more of the specific details or with other
methods. The
present invention is not limited by the illustrated ordering of acts or
events, as some acts may
occur in different orders and/or concurrently with other acts or events.
Furthermore, not all
illustrated acts or events are required to implement a methodology in
accordance with the
present invention.
[00159] It is further noted that the claims may be drafted to exclude any
optional element. As
such, this statement is intended to serve as antecedent basis for use of such
exclusive
terminology as "solely", "only" and the like in connection with the recitation
of claim
elements, or the use of a "negative" limitation.
[00160] The publications discussed herein are provided solely for their
disclosure prior to the
filing date of the present application. Nothing-herein is to be construed as
an admission that
the present invention is not entitled to antedate such publication by virtue
of prior invention.
Further, the dates of publication provided may be different from the actual
publication dates
which may need to be independently confirmed.
[00161] All of the above U.S. patents, U.S. patent application publications,
U.S. patent
applications, foreign patents, foreign patent applications and non-patent
publications referred
to in this specification and/or listed in the Application Data Sheet, are
incorporated herein by
reference, in their entirety, e.g., to disclose and describe the methods
and/or materials in
connection with which the publications are cited. It is understood that the
present disclosure
supersedes any disclosure of an incorporated publication to the extent there
is a contradiction.
[00162] From the foregoing it will be appreciated that, although specific
embodiments of the
invention have been described herein for purposes of illustration, various
modifications may
be made without deviating from the spirit and scope of the invention.
Accordingly, the
invention is not limited except as by the appended claims.
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
EXAMPLES
[00163] The following examples are put forth so as to provide those of
ordinary skill in the
art with a complete disclosure and description of how to make and use the
present invention,
and are not intended to limit the scope of what the inventors regard as their
invention nor are
they intended to represent that the experiments below are all or the only
experiments
performed. Efforts have been made to ensure accuracy with respect to numbers
used (e.g.
amounts, temperature, etc.) but some experimental errors and deviations should
be accounted
for. Unless indicated otherwise, parts are parts by weight, molecular weight
is weight
average molecular weight, temperature is in degrees Centigrade, and pressure
is at or near
atmospheric.
Experimental Methods
[00164] Vectors and lentiviral supernatant production. LVs were generated as
described
herein. CoRPK sequence was designed using the GeneArt0 software to increase
the GC
content of the sequence and to prevent cryptic splice sites. Vectors were
developed using the
pCCL.sin.ppt.hPGK-EGFP-Wpre* construct as backbone, generously provided by Dr.
Naldini (HSR-TIGET, San Raffaele Telethon Institute, Milano, Italy). Vector
stocks of VSV-
G pseudotyped LVs were prepared by 3-plasmid calcium phosphate-mediated
transfection in
293T cells (ATCC: CRL-1573, Rockeville, MD, USA), as previously described
[Follenzi A,
et al. (2000). Nat Genet 25: 217-2221. Titers of infective LVs were determined
in HT1080
cells (ATCC: CCL-121) by qPCR as described elsewhere [Charrier S, et al.
(2005). Gene
Ther 12: 597-6061. Lentiviral stocks of 107-108 viral particles (vp)/mL titers
were routinely
obtained.
[00165] Purification and transduction of murine HSCs. BM from 8-14 week-old
male
PKD mice was harvested from the leg bones and lineage negative cells (Lin-)
were purified
using the Lin- Cell Depletion kit (Miltenyi Biotec, Gladbach, Germany),
obtaining 70-90%
purity. Lin- cells were pre-stimulated with 100 ng/mL of recombinant human IL-
11
(Peprotech EC Ltd., London, UK) and 100 ng/mL of recombinant murine SCF (R&D
Systems Inc., Minneapolis, MN) in IMDM-Glutamax medium supplemented with 20%
FBS
and 0.5% antibiotics (50 U/mL penicillin and 50 pg/mL streptomycin, (Thermo
Fisher
Scientific, Waltham, MA) for 24h, and then transduced with EGFP or coRPK
carrying LVs
in two cycles of transduction at MOIs of 1-10 vp/cell. Each transduction was
carried out for
24h in the presence of the aforementioned cytokines on plates previously
coated with CH-296
fibronectin fragment (2pg/cm2; Retronectin, TakaraShuzo, Otsu, Japan)
overnight at 4 C.
41
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[00166] In vivo RBC survival. Transplanted mice carrying the coRPK transgene
were
injected with three consecutive intravenous injections (12h apart) of Biotin 3-
sulfo-N-
hydroxysuccinimide ester sodium salt (50 mg/kg) (Sigma Aldrich, Saint Louis,
MO). Twelve
hours after the last injection, tail vein blood was harvested and labelled
with 2 pg/mL of anti-
mouse Ter119-PE (BD Bioscience, San Jose, CA) and streptavidin-FITC (50 pg/mL,
BD
Biosciences, San Jose, CA) for 30 min at 4 C. Samples were analyzed in an
EPICS XL flow
cytometer (Beckman Coulter, Brea, CA) every 2-4 days for 40 days after the
injection. RBC
survival kinetics was measured by the percentage of biotinylated cells within
the total RBC
population.
[00167] CFC Assay. CFC assay was performed in BM and spleen from control and
transplanted mice according to manufacturer's procedure from Methocult medium
GF M3434
(Stem Cell Technologies, Vancouver, Canada). BM cells were harvested at
different time-
points after transplant from all groups of mice, and CFUs (clusters of 30 or
more cells) were
scored 7 days after seeding in a Nikon Diaphot-TMD microscope.
[00168] Identification of hematopoietic lineages. PBMCs were obtained from the
tail vein
of transplanted animals and labelled with a panel of antibodies to detect
different
hematopoietic cells. Myeloid cells were detected with anti-GR-1 and anti-Mac-1
biotinylated
antibodies (BD Bioscience, San Jose, CA, 5 pg/mL), while lymphoid cells were
detected
using anti-CD3-PE antibody for T-cells, and anti-B220-PE and anti-B220-PECy5
antibodies
for B-cells (BD Bioscience, San Jose, CA, 10 pg/mL), together with SAV-TRC
secondary
antibody (Invitrogen, Thermo Fisher Scientific, Waltham, MA). Samples were
analysed in a
BD LSR Fortessa Cytometer (BD Bioscience, San Jose, CAõ USA) adding DAPI
(Boehringer, Ingelheim, Germany, 2 pg/mL) to exclude death cells.
[00169] Structural and histological studies. Spleens were collected,
photographed and
weighed on precision scales to determine the presence of splenomegaly.
Histological studies
were performed on spleen and liver sections obtained following conventional
histological
methods, and stained with hematoxylin (Gill-2 Haematoxylin, Thermo,
Pittsburgh, USA) and
eosin (Eosin Alcoholic, Thermo Fisher Scientific, Waltham, MA). Iron deposits
were also
studied in the spleen by Prussian Blue or Perls' staining (Sigma Aldrich,
Saint Louis, MO)
following manufacturer's instructions. All sections were examined using an
Olympus BX40
light microscope and photographed with an Olympus DP21 camera, with a final
magnification of 100x or 200x.
[00170] Erythroid differentiation. Flow cytometry analysis of Ter119 and CD71
marker
intensities in BM and spleen were used to identify the different erythroid
subpopulations as
42
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
described elsewhere [Socolovsky M, et al. (2001). Blood 98: 3261-3273] using 4
pg/mL of
anti-mouse Ten 19-PE antibody (BD Bioscience, San Jose, CA,), 10 pg/mL of
biotinylated
anti-CD71 antibody (BD Bioscience, San Jose, CA,) and streptavidin-tricolor
(Invitrogen,
Thermo Fisher Scientific, Waltham, MA). Cells were then analyzed in an EPICS
XL flow
cytometer (Beckman Coulter, Brea, CA) using propidium iodide (IP, 2 pg/mL) to
detect live
cells.
[00171] Provirus quantification. Detection and quantification of integrated
provirus per cell
was accomplished using complementary primers to the packaging proviral
sequence (tP) and
the mouse Titin housekeeping gene. Total BM and peripheral blood samples were
collected
periodically, and genomic DNA from nucleated cells was isolated using the
DNeasy Blood &
Tissue kit (Qiagen, Venlo, Limburg, The Netherlands). Twenty to 50 ng of
genomic DNA
(gDNA) were amplified by multiplex qPCR using the 7500 Fast Real-Time PCR
System
(Applied Biosystems, Thermo Fisher Scientific, Waltham, MA) and primers and
probes
previously described [Charrier S, et al. (2011). Gene Ther 18: 479-4871.
[00172] Chimerism. Presence of donor cells was quantified by qPCR detecting
the Y
chromosome SRY gene and the mouse 13-Actin housekeeping gene. Primers and
probes
previously described [Navarro S et al (2006). Mol Ther 14: 525-5351 were used
and genomic
DNA from PB of transplanted mice was amplified using the 7500 Fast Real-Time
PCR
System (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA). Standard
curves
were prepared using gDNA extracts from samples containing 0% to 100% of BM
cells from
male/female mouse mixtures and chimerism was calculated as: % of donor
engraftment = 100
x 2(CtPAct - CtSRY)
[00173] LAM-PCR procedure. In order to identify vector integration sites, 3'
vector LTR-
genome junctions were amplified by LAM-PCR following the method published by
Schmidt
et al. 2007 [Nat Methods 4: 1051-1057]. The starting linear amplification (100
cycles) was
performed using biotinylated LTR specific primers and up to 100 ng of gDNA as
template.
Linear amplification products were purified using streptavidin magnetic beads
and followed
by complementary strand synthesis, parallel digestion with 2 different
restriction enzymes
(Tsp509I and HpyCH4IV) and two ligation reactions using linker cassettes
complementary to
the ends left by the enzyme's cut. The fragments generated were amplified by
two additional
exponential PCR steps. LAM-PCR products were separated and quantified by gel
electrophoresis on a MultiNA automated system (Shimadzu).
[00174] Setup of LAM-PCR products for Illumina MiSeq sequencing. Following the
method published by Parazynski et al [Paruzynski A, et al. (2010). Nat Protoc
5: 1379-13951,
43
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
40 ng of the second exponential PCR products generated by Tsp5091 and HpyCH4IV
enzymes were re-amplified using fusion primers containing specific sequences
that allow
paired end sequencing on an Illumina MiSeq sequencer. LAM-PCR samples were
adapted for
454-pyrusequencing by fusion PCR to add the Roche 454 GS-FLX adaptors: adaptor
A plus
an 8-nucleotide barcode was added to the LTR end of the LAM-PCR amplicon;
adaptor B
was added to the linker cassette side. In 5'-3' orientation the final amplicon
was composed as
follow: adaptor A, barcode, LTR sequence, unknown genome sequence, linker
cassette
sequence and primer B. Purified fusion primer PCR products were run and
quantified on a
MultiNA automated electrophoresis system, and pooled together in order to
obtain a final
equimolar library of 10 nM. The final library was then re-quantified using a
KAPA Library
Quantification Kit for Illumina Sequencing Platform (Kapa Biosystems,
Wilmington, MA) on
a Viia7 real-time PCR system (Applied Biosystems, Thermo Fisher Scientific,
Waltham,
MA), obtaining an estimated concentration of 16.35 nM. Finally, libraries were
sequenced
using the Illumina MiSeq Reagent Kit.
[00175] Bioinformatics analysis. To extract vector integration sites (IS) from
a high-
throughput sequencing platform, both Roche 454 and Illumina MiSeq/HiSeq, a
pipeline
taking in input the row data (typically in FastQ file format) was designed,
providing the list
of reliable IS and the nearest gene. Superior level analyses for clonal
abundance
quantification and gene ontology enrichment were performed using Excel,
GraphPad Prism
(TM) and available online tools.
[00176] NGS data processing and pipeline usage. The step of NGS data
processing deals
with the management of high-throughput data from Illumina MiSeq sequencing
platforms
and aims at identify IS in which all valid sequence reads are aligned to the
reference genome.
Data processing comprises two main activities: 1. Data quality inspection and
analysis, in
which lentiviral vector sequences and other contaminants are trimmed. 2.
Integration site
identification, in which all valid sequence reads are aligned to the genome of
reference and
valid ISs are retrieved.
[00177] Data quality analysis. In order to identify IS from Illumina MiSeq raw
data a
bioinformatics pipeline was developed. Standard LAM-PCR products contain a LTR
sequence, a flanking human genomic sequence and a linker cassette (LC)
sequence. The 459
technology allowed retrieval of LAM-PCR sequences with length ranging from
10bp to 900
bp. Similar results were retrieved from Illumina MiSeq paired-ends reads.
These length
boundaries are important parameters to consider in the quality analysis
process since they
affect both, the subsequent alignment procedure and the algorithm of the
vector components
44
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
identification. Sequences too short to be correctly aligned to the reference
gene were
discarded, as well as those exceeding the maximum size reachable with NSG
technology to
avoid missing part or all of the LC sequence. Once the pipeline ends for each
pool, all
integration sites were collected both in files (archived in the TIGET network
attached file
storage -NAS-) and in the internal database, and maintained in a storage
server that keeps
track of the modified copies.
[00178] Integration site identification. To identify unique integration sites
and extract the
excel file with all IS in rows and each sample in columns (IS matrix) with the
closest gene
annotations, we run the following steps: 1. Creation of the IS matrix using
the program called
create matrix, enabling the collision detection inter projects. This program
will produce a
tab-separated file (TSV); 2.Annotation of the IS matrix file using the program
annotate bed,
that will be called as follows for each pool using the input TSV file: awk
'{print
"chr"$1"It"$2"It"$2}' TSV FILE tail -n +2> TSV FILE.bed; annotate bed ¨a
/opt/genome/mouse/mm9/annotation/mm9.refGene.TIGET.gtf -b TSV FILE. bed -o
TSV FILE.annotated.bed; 3. Import both annotation and matrix file into a new
Excel
worksheet, here on called XLS.
[00179] Collision detection. In order to obtain a reliable dataset of ISs from
each
transplanted mouse, we filtered data from potential contaminations/collisions
and from false
positives based on sequence counts. An additional step of data normalization
was required to
combine integration sites resulting from different experiments.
[00180] The term "collision" is used to identify the presence of identical IS
in independent
samples. In our experimental setting, the integration of vector in the very
same genomic
position in different cells is a very low probability event. Thus, the
detection of identical ISs
in independent samples likely derives from contamination, which may occur at
different
stages of wet laboratory procedures (sample purification, DNA extraction, LAM-
PCRs and
sequencing). Although our working pipeline is designed to minimize the
occurrence of inter-
samples contacts, the high-throughput analysis of ISs intrinsically carries a
certain degree of
background contamination. Identification of the extent of contamination
between samples is
crucial also because the retrieval of the same IS in different samples
obtained from the same
mouse is used in subsequent steps to make inference on biological properties
of the vector-
marked hematopoietic cells (i.e. multi-lineage potential and sustained
clonogenic activity).
Thus, we must be able to distinguish the actual occurrence of the same IS in
different samples
(from the same mouse) from a contamination/collision. To address these issues,
we assessed
the extent of shared IS among samples derived from different test items and
mice as a way to
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
measure the extent of collision in our analyses and then design rules to
discard from each
mouse's data set those IS that can be ascribed to collision and minimize the
likelihood of
scoring false positive when looking for shared IS between samples from the
same mouse. We
designed a collision detection process allowing the validation of each
integration locus. The
overall result should be that, given the set I of integration loci, in case of
classification of an
integration locus i in I as collision, i is discarded from I. We applied
collision detection
process between 3 independent transplantation groups: 1. coPKR170s: mice from
assay 1
euthanized at 170 days after transplant with Lin- cells transduced with the
coRPK expressing
LV vector (coRPK 1-3). 2. EGFP: mice from assay 2 transplanted with Lin- cells
carrying
the EGFP expressing LV vector (EGFP 1-6). 3.coPKR-TC: mice from assay 2
transplanted
with Lin- cells transduced with the coPKR expressing LV vector (coRPK 1-14),
whose blood
and BM was analysed at different time-points, including secondary recipients
transplanted
with a pooled BM from a sub-group of primary transplanted mice (coRPK 11-14).
[00181] Each identical IS has different sequence reads (sequence count) among
the different
mice. Sequence counts can be used to determine whether samples from one mouse
contaminated the other mice' samples based on the abundance criterion. In our
rationale, an
integration found in two mice will be assigned to the mouse that shows the
highest
abundance, while in the other mouse it will be considered as a contaminant.
Therefore, we
could identify a threshold of differential sequence count that allows
assigning a given
collision to a mouse and removing from the others. We retrieved the threshold
value from our
data obtaining a value of 10, meaning that for each IS, among all TI, if an IS
has got an
abundance value (percentage sequence count ratio) 10 times lower than the
highest
abundance value (percentage sequence count) of the other TIs, then it is
discarded from the
current TI. We applied these rules both among the TI and the selected groups,
and Excel files
were used to compute the collisions detection by applying the following rules
(here detailed
for TI filtering but that are extended to groups filtering as well): 1.
Isolating each TI, group
all samples of the same TI together by summing the sequence counts. 2. For the
three TIs
obtained, for each IS, compute the percentage ratio of the IS sequence count
versus the
overall sum of reads for the TI. 3.Then, applying the following rule to
compute the decision
step with the threshold of 10 that allowed to assigning each IS to a reliable
TI.
[00182] Once an IS was detected to remove, reads of that IS were removed from
the group
so that it will no longer assigned also to that group. The filter described
above was applied
between the mice transplanted with different ex-vivo transduced cell
populations (one cohort
of EGFP expressing mice from assay 2, and two cohorts of coPKR mice belonging
to two
46
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
independent transplantation experiments). Moreover for the coPKR-TC group
(assay 2), the
filter method mentioned above was modified in two ways: a) To better highlight
the sharing
of integrations between time-points in the context of the clonal abundance
analysis we
added the following rule: if one integration is shared between one or more
mice then the
integration will be kept for all time points even if their sequence count is
less than the 10% of
the maximum sequence count among mice; b) For lineage tracking relationships
we applied
a more stringent filter by eliminating the IS with a sequence count lower than
3 and the 10%
sequence count filter for sharing between time-points. Meaning that an
integration shared
between two time points will be kept or discarded only if is more or less than
the other
respectively.
[00183] Gene ontology analysis. All gene ontology analyses were made using the
GREAT
online software (http://bejerano.stanford.edu/great/public/html/). The web
page allow to
upload the genomic coordinates of the integrations of each dataset and
calculates the
enrichment levels in the tested dataset by correlating positional information
(based on the
binomial distribution analysis for p-value calculations) and annotated
function of the genes
nearest to the integration sites (based on the hypergeometric distribution
analysis for p-value
calculations) [Groeschel S, et al. (2011). J Inherit Metab Dis 34: 1095-11021.
Biological
processes and molecular functions of the Gene ontology database were chosen
for enrichment
analysis. Only the gene classes with a false discovery rate <0.05 for both
statistical analyses
were considered (Fig. 20).
[00184] Data storage. All data, both row data and results, are stored in TIGET
network
attached file storage (NAS) in the root folder, in which all alignments from
the pipeline are
available, as well as the abundance matrix and plots. NAS storage is secured
by
authentication and authorization policies, and was built on a reliable and
scalable
infrastructure using redundancy array of disks RAID 5, and it is under backup
on our
CrashPlan software registered in TIGET.
Example 1
PGK-coRPK therapeutic lentiviral vector leads to a stable and long-term
correction of the
anemic phenotype in genetically corrected PKD mice.
[00185] In vivo efficacy of the PGK-coRPK LV (Fig. 2a) was assayed by
transduction and
transplantation of lineage depleted BM cells (Lin- cells) from PKD mice (Fig.
2b). Fig. 2a is
a schematic representation of the self-inactivating lentiviral vectors used
throughout gene
47
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
therapy experiments harboring the human PGK promoter regulating the expression
of the
EGFP transgene in the control vector (upper diagram) or the expression of a
codon-optimized
sequence of the PKLR gene cDNA (coRPK) in the therapeutic vector (lower
diagram). The
coRPK sequence showed 80.4% homology with the human PKLR cDNA and 76.5%
homology with the mouse Pklr cDNA, with no changes in the amino acid sequence.
Figure
2b is a schematic of the gene therapy protocol performed to address the
functionality of the
developed PGK-coRPK lentiviral vector. Correction of the PKD phenotype was
studied for 4
to 9 months after transplant in PB and BM through hematological analysis and
metabolic
profiling. Integration analysis was performed in different tissues and time-
points from all
mice to address LV vector safety. At 280 days post-transplantation, total BM
from primary
transplanted mice carrying the coRPK transgene was transplanted again into
lethally-
irradiated female PKD mice (secondary recipients) to test the stability and
safety of the
engraftment. Lethally irradiated PKD mice transplanted with deficient cells
transduced with
the coRPK LV showed a significant improvement in all tested blood erythroid
parameters
when compared to non-transplanted PKD littermates or to mice transplanted with
cells
transduced with an EGFP LV (Fig. 3 and Table 2).
[00186] Table 2. Hematological variables recorded 140 days post-
transplantation in
peripheral blood.
Group HGB (g/dL) HTC (%) MCV MCH (pg)
PKD (n=9) 9.70 0.55 28.91 1.53
51.56 0.50 17.23 0.31
MGFP01#8)MniAgliai068MMa2132w141n49:43aitailmit5A6w0A2a=
tmwmOinsidimininimINNEiniaimmniAn]]]]]]]MiuNggeingiuimiNginiOiNkdia
coRPK (n=17) 10.67 0.53* 31.09 45.65 0.84
15.35 0.53
1.45*
ttgtioatrjgmmmgaZgnge67ittio543itgmmm4.6a5iwini.i5Niakgikig9Miog
g(iii*.4)mmEmEmEmEmEtA9PimmEmEmEmEmEmEmiiiiii
[00187] Data represent the mean SEM and were statistically analysed by
comparison to
EGFP-expressing mice using the Kruskal-Wallis non-parametric test. *p<0.05;
**p<0.01.
[00188] RBC counts increased as soon as 40 days post-transplantation (Fig. 3a)
and
constitutive reticulocytosis, which is one of the most common signs of PKD,
was
significantly reverted in mice carrying the PGK-coRPK transgene, reaching
levels close to
those observed in healthy controls for at least 9 months after transplant
(Fig. 3b). On the
48
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
contrary, PKD animals transplanted with EGFP LV-transduced cells showed anemia
and
remarkable reticulocytosis in parallel with PKD mice at all time-points
analyzed.
Hemoglobin levels (HGB), hematocrit index (HTC), mean corpuscular volume (MCV)
and
mean corpuscular hemoglobin (MCH) values were also corrected in mice
transplanted with
lentivirally corrected cells when compared to non-transplanted PKD littermates
(Table 2).
This hematological correction was achieved with 63.66 4.45 % of donor
chimerism and
transduction efficacies ranging from 60% to 90% (Table 3).
[00189] Table 3 Relevant molecular parameters in mice transplanted with
genetically
modified cells.
Donor
Vector copy number Transduction
chimerism
(VCN/cell)
Assay Groups
Provirus+
WBC rotal BM CFU SRY PB
CFUs
cells
1 0.42
EGFP (n=2) .83 0.05 .42 0.03 57.73 12.28 n.d
MO! 0.00
1-4 3.07+
coRPK (n=3) .76 0.28 .58 0.34 91.08 3.67 n.d
0.76
3.93 61.82
EGFP (n=6) .56 0.50 .19 1.29 92.32+ 7.68
0.98 3.61
2
coRPK 1.89+ 63.66+
MO! .65 0.08 .99 + 0.13 62.06+ 11.73
(n=14) 0.42 4.45
2nd coRPK 62.89
.44 0.08 n.d n.d 63.15 0.31a
(n=4) 5.61
49
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[00190] Data represent the mean SEM, n.d, not determined, a estimated
transduction
percentage obtained by interpolation in the linear regression built from
experiment 1 (X axis:
VCN/WBC, Y axis: 0/0 provirus+ CFUs).
[00191] Transduced cells showed on average 1.65 0.08 integrated vector
copies per cell,
indicating that PGK-coRPK LV-vector provided enough human RPK transgenic
expression
to revert the hemolytic anemia. Remarkably, the expression of the coRPK
transgene led to an
extension of erythroid cell half-life when compared to non-transplanted PKD
mice (Figs.
3c,d). On average, PKD mice showed a RBC half-life of 19 days, while in
genetically
corrected mice this was extended to 25 days (6 days' extension) reaching
values close to
wild¨type RBC half-life (Fig. 3d). Thus, RBCs of coRPK-expressing mice
displayed
intermediate survival kinetics between healthy and deficient control mice
(Fig.3c), most
likely because full chimerism was not achieved in these animals (Table 3).
[00192] Nine months after transplant hematopoietic progenitors from primary
recipients
were transplanted into secondary recipients that maintained engraftment levels
(62.89 5.61
%) and VCN (1.44 0.08 copies) (Table 3). Secondary transplanted recipients
showed a
multi-lineage hematopoietic reconstitution up to 5 months post-transplantation
(Fig. 4) and a
significant improvement in all PB erythroid parameters (Fig. 5 and Table 2).
Figure 4a is a
diagram of the flow cytometry strategy used to identify the different
hematopoietic lineages
by labeling with CD3-PE, B220-PE, B220-PECy5, Grl-Biotin and Macl-Biotin
antibodies
plus SAV-PE-Cy5. Figure 4b depicts representative dot-plots and percentages
(Figure 4c) of
each lineage in PB at 140 days after transplant. Bars represent the average
percentage SEM
of healthy (n=2, black bar) and PKD mouse (n=2, grey bar) controls and
secondary
transplanted mice expressing the coRPK therapeutic transgene (n=4, scratched
bar). In
addition, proviral integrations were detected in differently committed
hematopoietic
progenitors (Figs. 6a,b) and its number remained constant over time (Fig.6c)
demonstrating
the stability of the genetic correction and highlighting the safety of the PGK-
coRPK LV.
Figure 6a shows the vector copy number per cell in BM CFUs from individual
transplanted
mice at 120 to 170 days after transplant. Transduction and chimerism
percentages are also
shown. Figure 6b shows provirus copy number in cells from different
hematopoietic
compartments. Columns represent the average SEM of the different groups of
transplanted
mice. Figure 6c shows the kinetics of proviral integrations in BM cells from
individual
transplanted EGFP-expressing mice (grey lines) and mice carrying the coRPK
transgene
(black lines).
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
Example 2
Lentiviral-derived RPK expression normalizes erythroid differentiation and
allows the
production of functional mature erythrocytes.
PKD mice show a characteristic expansion of the erythroid compartment caused
by the
compensatory erythropoiesis mechanism (Min-oo et al 2004). The study of the
erythroid
differentiation pattern in transplanted mice indicated that the ectopic RPK
expression
reverted this mechanism (Figs. 7a,b). PKD and EGFP-expressing mice showed a
predominance of immature erythroid precursors (subpopulation I:
proerythroblasts and
subpopulation II: basophilic erythroblasts) in BM and spleen, and a remarkable
fall in late
erythroid cells (population IV: reticulocytes and mature erythrocytes), while
mice
transplanted with cells transduced with the coRPK LV showed a significant
reduction of
immature erythroid precursors (subpopulations I and II) in BM and spleen, and
a significant
increase of the latest erythroid compartment (subpopulation IV) equivalent to
healthy mice
(Figs. 7a,b). In addition, unlike PKD and EGFP-expressing mice, those carrying
the coRPK
transgene showed a significant reduction of erythropoietin (Epo) levels in
plasma (Fig. 7c).
Figure 8a shows the total CFUs from spleen and Figure 8b shows the bone marrow
at 140
days after transplant. Dots represent number of colonies per mouse analyzed
and lines
represent average SEM in each group. Data were statistically analyzed by non-
parametric
Kruskal-Wallis test. The normalization of the erythropoiesis in PKD mice
treated with the
therapeutic vector was accompanied by a reduction of the splenic number of
progenitors
content to normal levels (Fig. 8a), although no changes in the BM CFU content
were noted
(Fig. 8b).
Example 3
Transplantation of cells transduced with the coRPK lentiviral vector reverts
extramedullar
erythropoiesis and organ pathology.
[00193] Due to the active destruction of RPK deficient erythrocytes, PKD and
EGFP-
expressing mice displayed acute splenomegaly with an increase of spleen weight
and size of
over 200% when compared to healthy controls (Figs. 9a,b). A disorganized
structure of the
splenic tissue and the expansion of the spleen red pulp were also observed in
these animals,
indicating an intense extramedullar erythropoiesis also supported by the
presence of erythroid
cell clusters in PKD and EGFP-expressing liver sections (Fig. 9c). Remarkably,
the ectopic
expression of coRPK transgene completely reverted spleen and liver pathology
in genetically
corrected mice, reducing the RBC accumulations and normalizing spleen
histological
51
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
structure and size (Fig. 9). Additionally, histological studies revealed the
total absence of iron
deposits in the liver of genetically corrected mice, whereas PKD mice either
from the non-
transplanted group or the group transplanted with HSCs transduced with the
EGFP-carrying
vector displayed an intense iron overload due to the continuous hemolytic
process (Fig. 9c).
Overall, the transplant of genetically corrected HSCs in PKD mice restored the
normal status
of the erythropoiesis and all the secondary effects caused by the hemolytic
anemia.
Example 4
PGK-coRPK LV-derived expression restores the glycolysis pathway in RBCs
without
modifying the WBC metabolic balance.
Next, we performed an extensive metabolomic analysis of all transplanted and
control mice
to study the functional correction of RPK enzymatic activity. Following an
untargeted
profiling strategy, we observed significant changes of glycolytic
intermediates in RBCs
among the different groups, identifying three broad clusters of metabolite
patterns with
distinct trends (Fig.10a). RBCs from coRPK-expressing mice showed an increase
of
metabolites from cluster 1 similar to healthy controls but different from
transplanted mice
carrying the EGFP transgene. Likewise, cluster 3 reflected a reduced
metabolite trend in
genetically corrected mice similar to wild type mice and different from EGFP-
expressing
mice. Nevertheless, cluster 2 from assay 1 showed no differences of metabolite
profile
between transplanted mouse groups (EGFP and coRPK expressing mice) (Fig. 10a).
The
untargeted metabolic profiling also showed that the genetic modification was
capable of
modifying some important glycolytic intermediates, achieving an increase in
ATP (Fig. 10b),
ADP (Fig. 10c) and pyruvate (Fig. 10d) levels in erythrocytes isolated from
mice
transplanted with PGK-coRPK LV-transduced HSCs. Considering these metabolic
trends, we
then analysed other metabolites located closer to the PK-catalysed reaction
using a targeted
profiling approach. Levels of the direct PK substrate phosphoenolpyruvate
(PEP) (Fig. 10e)
and the 3-phosphoglycerate (3-PG) (Fig. 10f), located upstream of the PK-
catalysed reaction,
approached to that of healthy control mice. Deficient erythrocytes expressing
the coRPK
transgene also produced an increase of D-lactate (Fig. 10g), the final product
of the anaerobic
glycolysis, when compared to PKD and EGFP expressing mice. To test whether the
compensation in the glycolytic metabolites was a result of the normalization
in the PK
activity in mature erythrocytes, we measured the activity of this enzyme and
normalized it in
relation to the Hexokinase activity in order to avoid the influence of high
amounts of
reticulocytes in the deficient animals. RBCs were purified through a cellulose
column to
52
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
prevent leukocyte PK activity contamination. A complete compensation of PK
activity was
observed in the animals expressing the coRPK that reach ratios similar to
those obtained from
wild type healthy animals and from a normal healthy blood donor volunteer
(Fig. 11). Fig.
ha shows Pyruvate Kinase activity, Fig. lib shows Hexokinase activity and Fig.
lie shows
ratio of Pyruvate Kinase and Hexokinase enzymatic activities in RBCs from
control mice and
mice transplanted with transduced cells. RBCs were purified from blood samples
through a
cellulose column to avoid leukocyte PK activity contamination and subjected to
enzyme
activity evaluation. Black bars, healthy mice (n=2); white bars, mice
transplanted with cells
transduced with the EGFP expressing vector (n=3); scratched bars, mice
transplanted with
cells transduced with the coRPK expressing vector (n=3). Checkered bars
represent values
from a healthy volunteer (n=1). Data represent the average SEM of each
group.
[00194] Principal component analysis showed that metabolite pattern of RBCs
was different
depending on the group and markedly different to WBC profile (Fig. 12a). On
the contrary,
WBC sub-groups clustered together with very little difference among groups,
indicating no
changes in the metabolic balance of leukocytes when expressing the ectopic
coRPK (Fig.
12a). Additionally, specific metabolite changes observed in the RBC untargeted
profiling
were not present in WBCs (Figs. 12b-d).
Example 5
PGK-coRPK LV transduced cells render polyclonal hematopoietic reconstitution
without
evidence of vector genotoxicity.
[00195] Integration profile of LVs carrying either the coRPK or the EGFP
transgene was
analysed in transplanted mice. Resulting from the genome-wide integration
profile of LVs,
each insertion creates a unique genetic mark that can be used to track the
clonal behaviour in
individual transduced cells. Genomic DNA (gDNA) was obtained from WBCs and
from BM
cells and from primary and secondary transplanted mice, as well as from
transduced cell
pools before transplant (Lin- cells). Linear Amplification Mediated PCR (LAM-
PCR) (Fig.
13 and 14) was used to amplify vector/genome junctions, and to identify vector
insertion
sites (ISs). Figure 13 demonstrates vector integration sites were identified
by LAM-PCR
amplification of 3' vector LTR-genome junctions. A MultiNA automated system
was used,
generating a pattern characterized by several bands. Vector backbone derived
Tsp5091
internal control band (IC) is indicated by an arrow. Figure 14 demonstrates
vector
integration sites were identified by LAM-PCR amplification of 3' vector LTR-
genome
junctions. A MultiNA automated system was used, generating a pattern
characterized by
53
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
several bands. Vector backbone derived HpyCH4IV5 internal control band (IC) is
indicated
by an arrow.
[00196] PCR products were sequenced by MiSeq Illumina platform and the
obtained
sequences were mapped onto the mouse genome by bioinformatics pipeline and
filtered for
collisions as described in the Methods section above (Fig. 15). Figure 15 is
the general
scheme of the analysis of integration site mapping performed in mice
transplanted with
genetically modified hematopoietic progenitors. Bone marrow and white blood
cell samples
from transplanted mice belonging to two independent experiments (Table 3) and
harvested at
different time-points after transplant were analyzed as described in
supplementary methods
following the showed pipeline.
[00197] Overall, we mapped 5,173,892 sequencing reads on the transplanted
mouse genome,
resulting in 2,220 unique vector integration sites. The genomic distribution
of ISs from two
independent experiments matched the previously reported LV preference for
integration
within transcriptional units (particularly within the first 50 Kb downstream
of the
transcription start site -TSS-) (Fig. 16a), showing no skewing towards any
particular
chromosome in the mouse genome (Fig. 16b). Figure 16a shows Integration site
(IS)
frequency distribution around Transcription Start Site (TSS) of the nearest
RefSeq gene,
spanning 500 Kb upstream and downstream the TSS. Numbers on the top are the
number of
IS detected for all samples and time-points. Figure 16b shows chromosomal
distribution of
LV integration sites in transplanted mice expressing the EGFP transgene (black
bars) or the
coRPK therapeutic transgene (grey bars), showing no skewing towards any
particular
chromosome.
[00198] Safety of the PGK-coRPK LV-based gene therapy was studied by clonal
abundance
estimations, calculating the percentage of sequence count for each IS (a
clonal mark) with
respect to the total number of sequences of the dataset. Dot plots and heat
map
representations of the relative abundance of each IS retrieved for each mouse
(Figs.17, 18
and 19) showed strong fluctuations in clonal composition of the different mice
and of in vitro
cultured Lin- cells. Figure 17 is a chart of tracked shared integrations
between primary and
secondary recipient mice carrying the therapeutic PGK-coRPK LV vector.
Integrations
detected in either mouse in any organ and at any time are pooled. Secondary
recipients
received the pooled BM from transplanted mice coRPK11 to 14. The rest of the
IS detected
were detected or in the primary or in the secondary recipients. Numbers in the
boxes show
the representativeness in percentage of the corresponding integration in the
referred mouse.
In addition to > 5% filter applied on integration analysis, all integration
with a sequence
54
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
count < 3 were eliminated. Figure 19 presents dot plot representation of
clonal abundance of
pooled integrations in each mouse in bone marrow. The relative percentage (y-
axis) for each
integration site is relative to the total number of sequences reads obtained
in each dataset.
Similarly to co-RPK transduced cells (Fig. 17), the graph indicates that the
vast majority of
transplanted mice show a polyclonal pattern of hematopoietic repopulation.
[00199] Also, it was possible to appreciate that for several samples, a small
number of
integrations contributed to a large amount of sequence reads (Figs. 17 and
18), revealing a
polyclonal pattern of repopulation of transduced HSCs. In addition, tracked
shared
integration between primary mice carrying the therapeutic PGK-coRPK LV and
subsequently
transplanted secondary mice showed no strong sharing of integrations between
the groups,
confirming the absence of clonal dominance (Fig. 18).
[00200] To determine whether hallmarks of insertional mutagenesis were present
in
transplanted mice, we assessed the occurrence of Common Insertion Sites (CIS)
similar to
currently ongoing LV-mediated clinical trials. CIS are insertional hotspots
that may result
from integration bias at the time of transduction or in vivo selection of
clones harbouring
vector integrations that confer growth advantage. CIS were identified using an
algorithm
based on Abel and cols and the Grubbs test for outliers finding no CIS and
thus no alarming
signs of genotoxicity by this readout. Moreover, gene ontology (GO) analysis
revealed no
skewing towards gene classes involved in cancer, cell proliferation or
regulation of apoptosis
in any of the integration datasets sorted by tissue-distribution, time point
or abundance of
repopulating hematopoietic cell clones (Fig. 20). Figure 20 represents LV
genomic
integration profile. Gene Ontology (GO) analysis was performed using the GREAT
software
on samples from transplanted mouse. All integrations retrieved from this study
(N=2220)
showed overrepresentations of the gene functions indicated on the left part of
the figure. To
address if the most abundant integrations were enriched on specific gene
classes, all
integration sites with a relative sequence count >5% of the entire dataset
(shown in Fig. 17)
were selected, showing no GO gene classes overrepresented.
[00201] These results suggest neutrality of vector integration and demonstrate
the safety of
the PGK-coRPK LV in a preclinical setting.
Example 6
Human Clinical Trial
[00202] A clinical trial is conducted to evaluate safety and preliminary
efficacy of
autologous hematopoietic stem cell transplantation (HSCT) using the
EU/3/14/1130
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
medicinal product (autologous CD34+ hematopoietic stem cells transduced with
the lentiviral
vector containing the RPK gene) in patients with pyruvate kinase deficiency
with a history of
severe and transfusion dependent anemia refractory to splenectomy.
[00203] The ODD EU/3/14/1130 comprises a self-inactivating lentiviral vector
expressing
the codon-optimized version of the therapeutic human PKLR gene (Figure 21).
[00204] Self-inactivating lentiviral vectors (SIN-LV) provide a more robust
expression (Ellis
2005) and are less susceptible to transcriptional silencing than gamma-
retroviral vectors
(Pfeifer, Ikawa et al. 2002). They also show a much safer integration profile
(Schroder, Shinn
et al. 2002) (Mitchell, Beitzel et al. 2004) (Wu, Li et al. 2003), and because
of the 400 bp
deletion that they carry in the 3' LTR sequence (Miyoshi, Blomer et al. 1998)
(Zufferey, Dull
et al. 1998), transgene expression is regulated by internal promoters,
increasing the safety of
the LV-based genetic modification.
[00205] Vector sequence of the accepted lentiviral vector also includes
several modifications
to improve transgene expression and safety in target cells.
[00206] One modification is the use of the human phosphoglycerate kinase (PGK)
promoter,
already characterized by its stable in vivo activity and improved safety
properties compared
to other promoters used in gene therapy (Montini, Cesana et al. 2006, Modlich,
Navarro et al.
2009, Montini, Cesana et al. 2009, Biffi, Montini et al. 2013). PGK leads to a
more
physiological expression of the transgene and a lower susceptibility to
transcriptional
silencing (Gerolami, Uch et al. 2000, Zychlinski, Schambach et al. 2008).
[00207] Another modification is a codon-optimized version of the human PKLR
cDNA
(coRPK) to increase mRNA stability upon transcription. For the optimization
the GeneArt
software has been used, increasing the GC content and removing cryptic splice
sites in order
to avoid transcriptional silencing and therefore increase transgene
expression. The coRPK
optimized sequence showed 80.4% homology with the human PKLR gene, with no
changes
in the amino acids sequence of the protein.
[00208] Another modification is a mutated post-transcriptional regulatory
element of the
woodchuck hepatitis virus (Wpre), lacking any residual open reading frame
(Schambach,
Bohne et al. 2006) is also included to improve the level of expression and
stability of the
therapeutic gene. The backbone, promoter and Wpre* sequences of this
lentiviral vector
(PGK-coRPK LV) are the same as the one corresponding to the medicinal product
"Lentiviral
vector containing the Fanconi anemia A (FANCA) gene for the therapy of Fanconi
anemia
Type A patients" (Ref 141/2000), as well as vector backbone used in the
currently ongoing
clinical trial for the metachromatic leukodystrophy (MLD) (Biffi, Montini et
al. 2013).
56
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[00209] Mode of action
[00210] After harvesting the CD34+ progenitor cells from PKD patients, either
from bone
marrow (BM) or mobilized peripheral blood cells, they will be transduced ex
vivo with the
medicinal product and the therapeutic vector will be integrated in the genome
of the cells.
Once integrated, the therapeutic human gene (coRPK) will be transcribed and
translated
within deficient cells to produce the therapeutic RPK protein that is missing
or reduced in
PKD mature erythrocytes. Transduced PKD hematopoietic progenitors will be then
genetically corrected, and thus able to produce RBCs with sufficient amounts
of ATP to
accomplish their functions (Figure 22). These genetically corrected
hematopoietic
progenitors (which will constitute the medicinal product) will be then
transplanted back into
the patient, where once engrafted will generate normal erythrocytes, life-long
curing the
disease.
[00211] The active principle will consist in a cellular suspension of
corrected hematopoietic
stem cells (CD34+) cells with the therapeutic lentiviral vector PGK.coRPK.wpre
designed as
orphan drug by the European Commission (ODD EU/3/14/1130) for the treatment of
pyruvate kinase deficiency. Thus, this new medicament should be included in
the groups of
advanced therapy developments within the gene therapy sub-class.
[00212] The active principle will be composed by of a genetically modified
cellular
suspension of at least 2x106CD34+ cell/Kg of body weight with at least 0.1
copies of the
therapeutic vector per cell. The cells will be suspended in a saline buffer
with 2% HSA.
[00213] The final therapeutic product will be produced according to the GMP
rules, so the
product requirements for its liberation and its infusion in the patient will
be related with the
quality of the product. In relation to this these specifications will be
cellular viability >30%,
sterility (Gram test and sterility by Pharmacopeia), absence of mycoplasma,
absence of
replicative competitive lentiviral particles, and demonstration of the potency
of the
therapeutic potential by the detection of the presence of at least 0.1 vector
copies per cell by
quantitative PCR. Additionally, investigation and research studies of the
content of
hematopoietic progenitors and the vector copy number in these will be
performed. Three
independent validations will be performed with healthy control cells to assure
the procedure
reaches the above described requirements.
[00214] The final product will be finished packaged in a transfer bag sealed
by heat and
especially for freezing and storage until its infusion to the patient;
previously, samples will be
collected for the precise corresponding quality controls.
[00215] Mobilization
57
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[00216] Patients will be mobilized in their respective hospitals, although the
two first
patients will be mobilized in the Hospital del Nilio Jeans, Madrid (Spain).
Mobilization
process will consist in the administration of recombinant stimulating factor
granulocyte
colony (G-CSF, Neupogen, Amgen, Thousand Oaks, CA, USA) at doses of 12 mg/kg
twice a
day for up to eight days from birth, Plerixafor 4th day 240 mg/kg/d (Mozobil0,
Genzyme
Europe By, Naarden, Netherlands) to four subcutaneous doses for 4 consecutive
days. The
hematopoietic progenitor cells from peripheral blood will be collected by
leukapheresis large
volumes from day 5 of mobilization through a cell separator and according to
standard
protocols in Hospital Niiio Jeans in Madrid. All the instruments and solutions
are CE marked
and meet the specifications of the legislation for medical devices.
[00217] CD34+ cell purification
[00218] Consistent with the mobilization process, the apheresis will take
place in the hospital
where the patient is mobilized. Apheresis will be processed immediately to
select
hematopoietic progenitors (CD34+ cells) through MACS "magnetic cell sorting"
technology
(Miltenyi Biotec, Germany) which permits separation of cells by a high
magnetic field
gradient, through a powerful permanent magnet and a separation column with a
ferromagnetic matrix. The CliniMACS (Miltenyi Biotec, Bergisch Gladbach,
Germany)
system consists on a computer (CliniMACSOplus Instrument), a specific CD34+
selection
software, a set of sterile tubes (CliniMACS Tubing Sets), magnetic-controlled
reactive sterile
instrument (CliniMACS CD34 Reagent) and a sterile buffer (CliniMACS PBS/EDTA
Buffer). The instrument and reagents used will be CE marked and will meet the
specifications
of the legislation for medical devices. During this phase and in later washes
nonspecific
immunoglobulin (intravenous Flebogamma 5% 0.5 gr, Grifols) and human albumin
(human
albumin Grifols 20%, Grifols) are employed, which are later removed by
washing after
centrifugation. The CD34+ cells will be then quantified. Microbiological
controls on the
products obtained will be performed by taking standard fungal, aerobic
bacteria and
anaerobes samples for cultures following specific protocols.
[00219] CD34+ transduction
[00220] Transduction of the purified CD34+ cells with the ODD EU/3/14/1130
will be done
under GMP conditions in a time window within 48h since the extraction of the
cells from the
patient (apheresis). Ex-vivo culture of the cells will last less than 48
hours, and will be
cultured following stablished standards including the use of properly
formulated media X-
vivo-20 (Lonza), addition of hematopoietic growth factors (10Ong/m1hrSCF,
10Ong/m1 hrFlt-
3, 10Ong/m1 TPO and 20 ng/mL IL-3 (all from Prepotech), 1 g/mL Pulmozyme and
58
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
controlled 5% 02 concentration. Transduction will be performed with a GMP
lentiviral batch
of the ODD EU/3/14/1130 produced by VIVEbiotech (San Sebastian, Spain). After
transduction cells will be washed in X-vivo-20 (Lonza) to be finally packaged
in a transfer
bag suitable for cryopreservation. Specific samples will be collected to
determine if the final
product meets all the specifications already mentioned for its final
liberation. Three
independent validations will be performed to evaluate the stability of the
product. All
products and solutions, including the vector, will meet the specifications of
the legislation for
medical devices and clinical use. Before production, all raw materials
(including
consumables, biological reagents and chemical powders) will be inspected by
the Quality
Control (QC) Unit of CliniStem according to standard operating procedures
(SOPs).
[00221] Conditioning
[00222] Patients will be conditioned according to standardized and specific
protocols
considered for the trial. To consider a patient for conditioning an
alternative a back-up of
2x106 unmanipulated CD34+ cells/kg will be kept frozen to be used in case the
prepared
product do not completely reconstitute the haematopoiesis of the treated
patient.
[00223] Infusion
[00224] Previous to infusion, patient eligibility will be checked to ensure
they meet the study
requirements. The day of infusion, premedication and prophylactic medication
used will be
recorded.
Example 7
Non-clinical development
[00225] Previous work demonstrated the feasibility of HSC gene therapy for PKD
in mice
when above 25% genetically corrected cells were transplanted. These results
suggest that a
significant number of donor gene-corrected HSCs (Zaucha, Yu et al. 2001)and
high levels of
transgene expression are needed to achieve a therapeutic effect in PKD. We
have developed a
new therapeutic lentiviral vector proposed for this clinical trial, harbouring
the hPGK
eukaryotic promoter driving the expression of the PKLR cDNA that was
designated as
Orphan Drug on August 2014 (EU/3/14/1130). With this vector we conducted a
preclinical
gene therapy protocol for PKD in a mouse model of the disease. With lentiviral
dosages
based on clinical standards, ectopic RPK expression was able to normalize the
erythroid
compartment, correcting the haematological phenotype and reverting organ
pathology.
Metabolomic studies demonstrated the functional correction of the glycolytic
pathway in
genetically corrected RBCs, with no metabolic disturbances observed in
leukocytes.
59
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
Remarkably, WBCs analyzed in parallel showed no alterations of the metabolic
balance in
leukocytes when RPK is ectopically expressed under the activity of an
ubiquitous promoter
such as PGK, ruling out a leukocyte metabolic advantage as possible safety
concern and
reinforces the therapeutic potential of the EU/3/14/1130 vector.
[00226] The multi-lineage reconstitution and the absence of any leukemic event
or clonal
expansion in secondary recipients after the proliferative stress induced by BM
re-transplant
demonstrate the long-term stability and safety of the PGK-coRPK LV vector-
based protocol.
The use of the human PGK eukaryotic promoter that i) likely led to a more
physiological
expression of the RPK transgene, ii) has been proven to be a weak
transactivator and iii) is
being currently used in the clinical trial for metachromatic leukodystrophy
(MLD), could also
account for the safety of the whole procedure.
[00227] To assess the long-term safety of HSC gene therapy through the
analysis of vector
integration sites, next generation sequencing was used to predict the risk of
insertional
oncogenesis in HSC. More than 5,173,892 sequences reads were mapped on the
mouse
genome to a total of 2220 unique vector IS, finding no evidence of in vivo
expansion or
selection of clones harboring IS. Rather, our data show the clonal composition
and dynamics
of hematopoiesis after transplantation of transduced HSCs in mice, suggesting
a genuine and
stable genetic in vivo modification of HSC over time. Overall, the analysis of
the vector
integration pattern emphasizes the safety properties of the PGK-coRPK LV
vector that
provides PKD genetic correction with no evidence of genotoxicity.
Example 8
Clinical development
[00228] So far, no clinical studies have been conducted with the medicinal
product. This is
the first time protocol assistance is requested to a Regulatory Agency. Our
aim is to perform
a clinical trial sponsored by the European Commission. The ForGeTPKD
Consortium,
composed by different clinicians and basic researchers in Europe, has been
established to
focus in PKD research and development of new therapeutic strategies. ForGeTPKD
clinical
trial will be the first administration of this medicinal product in humans. It
is designed as an
International, Multicenter, Phase I/II Open Label Study to evaluate the Safety
and Efficacy of
Transplantation of Autologous CD34+ Cells Transduced Ex Vivo with a Lentiviral
vector
containing the red-cell type Pyruvate Kinase (RPK) gene (EU/3/14/1130) in
patients with
Severe Pyruvate Kinase Deficiency.
[00229] Regulatory status
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[00230] The medicinal product has no marketing authorization at present time.
The objective
of the PKD Consortium is to move forward to the clinical development of the
medicinal
product in order to eventually receive a marketing authorization.
[00231] The mentioned final product will be produced with a lentiviral vector
that has
received the Orphan Drug Designation related to:
[00232] Indication: Treatment of Pyruvate Kinase Deficiency
[00233] Criteria: The only curative treatment for PKD is allogeneic BMT, which
has been
used in patients with transfusion-dependent severe anemia refractory to other
measures.
However, allogeneic BMT is not a widely accepted treatment for PKD (only one
patient
reported in the literature (Tanphaichitr, Suvatte et al. 2000)) as it is
associated with severe
complications related to intensive pre-allo-BMT conditioning by chemotherapy
or chemo-
radiotherapy, as well as acute and chronic graft-versus-host disease (GVHD).
Our hypothesis
is that gene therapy using autologous hematopoietic stem cells transduced with
viral vectors
containing the wild type version of the gene, provided by the ODD
EU/3/14/1130, may
represent a potential curative opportunity for these patients, avoiding the
risks of GVHD, the
main cause of failure of a hematopoietic progenitors transplant.
[00234] Active substance: Autologous CD34+ hematopoietic stem cells,
transduced with
Lentiviral vector containing the red-cell type Pyruvate Kinase (RPK) gene (ODD
EU/3/14/1130), expressing the wild type version of the protein
[00235] Finished product: Frozen bag containing at least 2x106 active
substances/kg body
weight of the patient, suspended in a saline buffer with 2% HAS
Example 9
Pharmacology
[00236] Completed studies: The medicinal product developed includes several
modifications in its sequence that provides some advantages for the gene
therapy for PKD: 1)
The use of a SIN-LV vector design allowed a relatively easy and safe
production of viral
stocks, able to efficiently transduce HSCs; 2) the use of a weak and
eukaryotic promoter such
as hPGK, which is less susceptible to silencing by methylation (Gerolami, Uch
et al. 2000),
leads to a more physiological expression of the transgene, achieving
therapeutic levels with a
viral dosage (1.65 VCN) within the clinical standards (Matrai, Chuah et al.
2010); and 3) the
codon optimized transgene sequence and the presence of the mutated Wpre
sequence increase
transgene mRNA stability: no reporter gene was included in the therapeutic
vector sequence,
61
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
avoiding possible immunogenic problems (Morris, Conerly et al. 2004);
(Stripecke, Carmen
Villacres et al. 1999).
[00237] The developed hPGK-coRPK LV medicinal product efficiently reverted PKD
pathology in both primary and secondary deficient mice transplanted with
progenitors
transduced and corrected with the ODD EU/3/14/1130. The correction was
achieved with
cells carrying on average 1.65 copies per cell of the therapeutic transgene.
[00238] Human PGK promoter was potent enough to express clinically relevant
levels of
coRPK protein, restoring the hemolytic phenotype in transplanted mice.
[00239] The genetic correction was able to: Extend RBC half-life; Normalize
the
hematological variables and reticulocytes levels; Revert the compensatory
erythropoiesis
constitutively activated in PKD mice; Rescue the pathology in spleen and
liver, remarkably
reducing the iron overload, which is one of the life-threatening complications
of PKD. In
addition, the ectopic expression of human RPK corrected the energetic defect
in RBCs
without altering the metabolic balance in WBCs, emphasizing the efficacy and
safety of the
medicinal product.
Example 10
Ongoing studies
[00240] Transduction of human hematopoietic progenitors from healthy donors
and PKD
patients for the study of: 1) efficiency of transduction of the ODD
EU/3/14/1130 in human
cells; 2) definition of the optimal vector copy number/cell to get efficient
and therapeutic
expression of the RPK therapeutic protein; and 3) definition of the optimal
conditions to get
therapeutic transduction levels without losing hematopoietic stem cell
ability.
[00241] Planned studies include set up of the conditions for large scale
transduction at the
GMP facility and pre-validation and 3 validation studies to set up the optimal
conditions to
reach the required specifications defined for the final therapeutic product.
[00242] Toxicology
[00243] Completed studies include 1)The ectopic expression of human RPK
corrected the
energetic defect in RBCs without altering the metabolic balance in WBCs; 2)
Genome
integration analysis of the vector has demonstrated that (i)The analysis of
the relative
abundance of specific cell clones revealed an oligoclonal hematopoietic
reconstitution for
some mice, showing no clonal dominance for any primary and secondary
transplanted mice;
(ii) Common Integration Sites (CIS, dense clusters of vector integrations in
defined genomic
intervals), considered a hallmark of insertional mutagenesis did not show any
sign of
62
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
genotoxicity, neither an abnormal enrichment of CIS over time and detected CIS
from the
two independent gene therapy experiments performed in mice were not
represented by high
sequence counts and did not preferentially target oncogenes; (iii) Gene
ontology (GO)
analysis of the genes targeted by the lentivirus integration and study of the
position of the
vector integrations in specific regions of the genome demonstrated no skewing
towards gene
classes involved in cancer, cell proliferation or regulation of apoptosis; and
(iv)Overall,
medicinal product integration analysis did not show any evidence of
genotoxicity.
[00244] Planned studies include 1) Analysis of Recombinant Competent
Lentiviruses (RCL)
production: Human T lymphocytes from healthy donors and from PKD patients will
be
transduced with the ODD EU/3/14/1130 and culture in vitro for extended periods
of time.
Presence of viral p24 protein will be analyzed in the supernatants by ELISA to
evaluate the
potential generation or RCLs and 2) Biodistribution of the medicinal product:
Mouse
hematopoietic progenitors will be tranduced with the ODD EU/3/14/1130 and
transplanted
into lethally irradiated recipients. One month post-transplant animals will be
sacrificed and
different organs (gonads, liver, kidney, brain, bone marrow, spleen and
peripheral blood) will
be analyzed for the presence of vector DNA; and 3) Vector integrome in human
cells:
Hematopoietic progenitors from healthy donors and from PKD patients will be
transduced
with the ODD EU/3/14/1130 and transplanted into severe immunodeficient mice to
allow the
engraftment and proliferation of human hematopoietic cells. At different time
points (1, 2 and
3 months post-transplant) blood and BM transplants will be taken, sorted for
human cells and
subjected to vector integrome analysis as already performed with mouse cells.
Example 11
Human Clinical Trial
[00245] To test clinical efficacy the ForgetPKD trial will be conducted. The
proposed
clinical trial aims to evaluate safety and preliminary efficacy of autologous
hematopoietic
stem cell transplantation (HSCT) using the EU/3/14/1130 medicinal product
(autologous
CD34+ hematopoietic stem cells transduced with Lentiviral vector containing
the red-cell
type Pyruvate Kinase (RPK) gene) in patients with pyruvate kinase deficiency
with a history
of severe and transfusion dependent anemia refractory to splenectomy.
[00246] The primary objective is to evaluate treatment safety and
tolerability/feasibility. The
following endpoints will be measured in accordance: 1) incidence and
characterization of
Adverse Events (AE), including: AE related to the infusion of the transduced
cells, AE
derived from conditioning prior to cell infusion, and AE derived from clonal
evolution related
63
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
with the transduced cells; and 2) number of patients with stem cell
engraftment at 30 days
post-transplant.
[00247] Secondary objectives are to evaluate preliminary treatment efficacy.
The following
endpoints will be measured in accordance: Number of patients who become
"transfusion
independent" at the end of the study; In patients who still need transfusions
after treatment,
ratio between the mean numbers of transfusions needed within the study period
(1 year) with
respect to the mean number of transfusion in the last 1.5 years before
baseline evaluation;
Clinically significant reduction of anemia, defined as number of patients with
rise in
Hemoglobin levels in 2 gr/dL from baseline at the end of the study; Clinically
significant
reduction of reticulocytosis, defined as number of patients with a reduction
of 50% from
baseline evaluation at the end of the study; and Number of patients with stem
cell
engraftment where 1% transduced cells can be detected at 6 and 12 month post-
cell infusion
and at the end of the study.
[00248] An exploratory objective is to evaluate treatment impact on patient's
quality of life.
The following end-point will be measured in accordance: Improvement in quality
of life from
baseline at the end of the study, using a quality of life questionnaire (SF-36
for adults or
PEDSQL for children) and its translated validated versions in the language of
the participant
countries (Italian, Dutch and Spanish).
[00249] ForGetPKD Trial is a multicenter, international trial, which will be
carried out in 3
EU member states: Spain, Italy and The Netherlands. Participating centers
include Reference
National Investigators and Institutions for PKD diagnosis and treatment.
[00250] The trial will represent the first administration to humans of the
described product. It
is designed as a non-comparative, open label, single-dose, Phase I/II study.
[00251] Global Study duration will be 2 years from the first visit of the
first patient to the
last visit-last patient. This includes 1 year of recruitment period and 1 year
of treatment
period and early (immediate) follow-up. After the end of the trial, included
subjects will be
asked to participate in a subsequent follow up study that will monitor safety
and efficacy for
up to 5 years after transplant.
[00252] According to disease incidence and study design, we are planning to
include 6
patients in one year. This estimate has been decided considering that there
are 3 potential
participants already identified.
[00253] Study procedures include a Screening Period, a Treatment Period and a
Follow-up
Period. Details of visits on each phase and associated study procedures are
detailed below
and summarized in Table 4.
64
CA 03021572 2018-10-18
WO 2017/184903 PCT/US2017/028695
[00254] Table 4.
Treatment
Period Screening Period Follow up period
Period
-1 1 2
Visit 0 3 4 5
Pre- Treatment + 1
Baseline +3m +6m +12m
screening period m
Study procedures
Informed consent X
Inclusion/exclusion X X
Criteria
Medical History X
Physical X X X
Examination
CBCs X X X X X X
Biochemistry X X X X X X
Coagulation X X X X X X
Conditioning X
regimen
Transduced cells X
infusion
Discharge X
Bone Marrow X
sampling
Cell engraftment in X X X X
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
peripheral blood
Average vector copy X X X X
number in peripheral
blood
Vector integration X X X X
pattern in peripheral
blood
Cell engraftment in X X
bone marrow
Average vector copy X X
number in bone
marrow
Vector integration X X
pattern in bone
marrow
Cell extraction X
Availability of viable X
cells
Quality of life X X
questionnaire ( SF-
36 or PEDSQL )
Concomitant X X X X X X X
Medication
Adverse Events X X X X X X X
[00255] Screening Period
[00256] Visit -1: Pre-screening visit
[00257] Potential candidates will be informed of the aims and characteristics
of the trial, and
written informed consent will be taken, fulfilled and signed by the patient
(or legal
representative if underage), by duplicate. To be eligible for the study,
patients must fulfill all
inclusion criteria and none of the exclusion criteria, which will be checked.
This includes a
pre-treatment procedure to mobilize and obtain viable CD34+ cells, to A. Store
2x106 CD34+
cells/kg body weight to serve as a backup in case of non-engraftment, B.
transduce at least
66
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
6x106 CD34+ cells/kg body weight with the EU/3/14/1130 vector to generate the
medicinal
product and to perform all the quality control needed for the liberation of
the medicinal
product. Only patients with enough transduced cells available after liberation
of the medicinal
product (2x106 transduced CD34+ cells/kg body weight) will be included in the
study.
[00258] The following procedures will be also performed in this visit:
- Register of relevant medical o surgery history.
- Register of demographic data and clinically relevant physical examination
findings
- Register of relevant concomitant medications.
- Peripheral blood testing for routine cell blood counts (CBC),
Biochemistry,
Coagulation determination and serology
- Echocardiogram, Lung function test and thorax X-ray
- Quality of life questionnaire (SF-36 or PEDSQL)
- Genetic diagnosis of PKD
[00259] To be eligible for the study, patients have to fulfill all of the
following inclusion
criteria and none of the exclusion criteria.
[00260] Inclusion criteria are male or female patients, Age >2 year old at the
time of
recruitment, willing to give signed informed consent (which will be signed by
their parents or
legal representative in case of children under 18 years old), previous
diagnosis for PKD
confirmed by genetic testing, history of severe transfusion-dependent anemia,
not responsive
to splenectomy, Candidate to Autologous Hematopoietic Stem Cell Transplant, >
2x106
transduced CD34+ cells/kg body weight available, and treated and followed for
at least the
past 2 years in a specialized center that maintained detailed medical records,
including
transfusion history.
[00261] Exclusion criteria are positive for presence of human immunodeficiency
virus type 1
or 2 (HIV 1 and HIV 2), uncorrected bleeding disorder, presence of other
causes of
hemolysis, any prior or current malignancy or myeloproliferative or
immunodeficiency
disorder, immediate family member with a known or suspected Familial Cancer
Syndrome
(including but not limited to hereditary breast and ovarian cancer syndrome,
hereditary non-
polyposis colorectal cancer syndrome and familial adenomatous polyposis), in
patients with
previous allogeneic transplant, presence of residual cells of donor origin,
patients with severe
complications that after medical evaluation are considered to suffer grade
III/IV cardiac,
pulmonary, hepatic or renal function abnormalities, uncontrolled seizure
disorder, diffusion
capacity of carbon monoxide (DLco) <50% of predicted (corrected for
hemoglobin), any
other evidence of severe iron overload that, in the Investigator's opinion,
warrants exclusion,
participation in another clinical study with an investigational drug within 30
days of
67
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
Screening, availability of a HLA-identical family donor for allogeneic bone
marrow
transplant, pregnant or breast-feeding women, patients that, according to
investigator criteria,
will not be able to understand study purposes, benefits and risks and/or to
comply with study
procedures, an poor functional status, evidenced by a Karnofsky Index <80 in
adults or
Lansky <80 in children.
[00262] The statistical analysis of the study will be descriptive. Qualitative
endpoints will be
described by frequencies and percentages. Qualitative endpoints include the
adverse events,
the number of patients with stem cell engraftment, the number of patients who
become
"transfusion independent", the number of patients who have a clinically
significant reduction
of anemia, the number of patients who have a clinically significant reduction
of
reticulocytosis, the number of patients with stem cell engraftment where
presence of
transduced cells can be detected, and the improvement in quality of life from
baseline
measured by SF-36 or PEDSQL questionnaire. Quantitative endpoints will be
described by
mean and standard deviation or by median and quartiles. Quantitative endpoints
include the
reductions of anemia and reticulocytosis from baseline, the number of
transfusions needed
within the study period with respect to the number of transfusion in the last
1 year before
baseline evaluation, and the vector copy number in peripheral blood and bone
marrow. All
endpoints will be described at the end of the study.
[00263] Previous work demonstrated the feasibility of HSC gene therapy for PKD
in mice
when above 25% genetically corrected cell were transplanted. These results
suggest that a
significant number of donor gene-corrected HSCs (Zaucha, Yu et al. 2001) and
high levels of
transgene expression are needed to achieve a therapeutic effect in PKD. We
have developed a
new therapeutic lentiviral vector proposed for this clinical trial, harboring
the hPGK
eukaryotic promoter driving the expression of the PKLR cDNA that was
designated as
Orphan Drug on August 2014 (EU/3/14/1130). With this vector we conducted a
preclinical
gene therapy protocol for PKD in a mouse model of the disease. With lentiviral
dosages
based on clinical standards, ectopic RPK expression was able to normalize the
erythroid
compartment, correcting the hematological phenotype and reverting organ
pathology.
Metabolomic studies demonstrated the functional correction of the glycolytic
pathway in
genetically corrected RBCs, with no metabolic disturbances observed in
leukocytes.
Remarkably, WBCs analyzed in parallel showed no alterations of the metabolic
balance in
leukocytes when RPK is ectopically expressed under the activity of an
ubiquitous promoter
such as PGK, ruling out a leukocyte metabolic advantage as possible safety
concern and
reinforces the therapeutic potential of the EU/3/14/1130 vector.
68
CA 03021572 2018-10-18
WO 2017/184903
PCT/US2017/028695
[00264] The multi-lineage reconstitution and the absence of any leukemic event
or clonal
expansion in secondary recipients after the proliferative stress induced by BM
re-transplant
demonstrate the long-term stability and safety of the PGK-coRPK LV vector-
based protocol.
The use of the human PGK eukaryotic promoter that likely led to a more
physiological
expression of the RPK transgene, that has been proven to be a weak
transactivator and is
being currently used in the clinical trial for metachromatic leukodystrophy
(MLD) could also
account for the safety of the whole procedure.
[00265] To assess the long-term safety of HSC gene therapy through the
analysis of vector
integration sites, next generation sequencing was used to predict the risk of
insertional
oncogenesis in HSC. More than 5,173,892 sequences reads were mapped on the
mouse
genome to a total of 2220 unique vector IS, finding no evidence of in vivo
expansion or
selection of clones harboring IS. Rather, our data show the clonal composition
and dynamics
of hematopoiesis after transplantation of transduced HSCs in mice, suggesting
a genuine and
stable genetic in vivo modification of HSC over time. Overall, the analysis of
the vector
integration pattern emphasizes the safety properties of the PGK-coRPK LV
vector that
provides PKD genetic correction with no evidence of genotoxicity.
69