Note: Descriptions are shown in the official language in which they were submitted.
CA 03022230 2018-10-25
WO 2017/194074 PCT/EP2016/025046
Port Needle
Field
The present invention is directed to a port needle that is particularly
adapted to penetrate skin,
tissue and/or a port membrane of an implanted port to transfer fluid into a
port chamber of the
port.
Background
Port needles or punctuation needles into port catheters often need to remain
in a body of a
patient for an extended period of time. Up to seven days the needles remain in
the body.
Port needles are hollow needles designed to apply liquids into a prior
implanted port or port
catheter are commonly used and known. Various constructions can be easily or
safely used by
medical personal.
While often syringe-type devices are utilized for vascular access ports in
acute or short-term
situations, a special type of device is utilized for longer term infusion
therapy. Such an infusion
assembly device generally consists of a needle and wing assembly that lies
flat against the
skin in an insertion position, the needle having a proximal end attached to a
wing assembly
that is in angular relation to the needle shank, the angle being approximately
90 . As
mentioned, when this longer term needle device is inserted into the vascular
access port, it lies
flat against the patient's skin and is adhered thereto as described, for
example, in U.S. Pat. No.
4710176, which is herewith incorporated by reference.
With respect to the needle tip of the needle used in infusion assemblies for
vascular access
ports, a non-coring configuration is generally utilized due to repeated entry
of the needle into
the septum or membrane of the vascular access port. When the tip of a
hypodermic or other
traditional needle advances through the membrane or septum, coring occurs if
any portion of
the septum material is forced inside the shaft of the needle through the
opening in the tip
thereof. That portion of the septum material forced inside a needle in this
process is in effect
severed from the rest of the body of the septum material. Such septum coring
produces small,
detached particles of the septum that are likely to enter the fluid that is
infused by the implanted
vascular access system into the vascular system of the patient. These
particles can obstruct
fluid flow through the outlet stem of the vascular access port, or if escaping
through the outlet
stem of the vascular access port, can become trapped in the cardiovascular
system of the
1
CA 03022230 2018-10-25
WO 2017/194074 PCT/EP2016/025046
patient. In addition, septum coring produces small passageways through the
body of a septum.
On occasion these passageways extend entirely through the septum, from the
exterior thereof
to the fluid reservoir inside the vascular access port.
The inwardly directed forces imposed on the installed septum by the housing of
a vascular
access port should initially urge the material of the body of the septum
inwardly upon itself to
close such passageways after the shaft of the needle is withdrawn therefrom.
Nonetheless,
continued coring eventually leads to various forms of septum failure that
cannot be overcome
by such inwardly directed forces. The material continuity of the septum is
increasingly
compromised, resulting in crumbled areas of the septum matrix.
Eventually, leakage of fluid can be expected through the septum from the fluid
reservoir in the
vascular access port. Once such fluid escapes to the exterior of the vascular
access port,
necrosis will occur of the tissue surrounding the subcutaneous packet in which
the vascular
access port is implanted, causing many undesirable consequences. Therefore,
non-coring or
.. Huber needles are preferably used in conjunction with infusion assemblies
for vascular access
ports. These needles, in contradistinction to the standard or traditional
hypodermic needles
pierce the septum like a knife, facilitating the resealing thereof so that the
aforementioned
problems are largely averted.
As with any needle-type device, there exists the problem of inadvertent needle
sticks, which
generally occurs when the needle-type device is withdrawn from the patient
prior to appropriate
disposal thereof. Of course, inadvertent needle sticks introduce a variety of
concerns due to
unwanted transmission of blood from the patient to the medical practitioner.
Inadvertent needle
sticks can occur because of carelessness on the part of the medical
practitioner or due to
accidents in the handling of devices with exposed needle tips. With respect to
Huber needles
specifically, needle stick accidents can occur due to difficulty in removal as
well. This difficulty
results from the configuration of the Huber needle, which gets hooked into the
port making it
difficult to remove. Increased pulling force on behalf of the medical
practitioner to dislodge the
needle from the port results in less control over the tip of the needle when
freed from the port,
causing the inadvertent needle stick.
To address the inadvertent needle stick problem in winged infusion assemblies,
such as those
described herein, various safety devices have been designed to encase the
needle after it is
2
CA 03022230 2018-10-25
WO 2017/194074 PCT/EP2016/025046
withdrawn from the port. One such safety device is described in U.S. Pat. No.
5,755,694 that
discloses a needle base disposed over a segment of the needle at its proximal
end, comprising
two generally flat wings made of flexible material with one hinge connecting
each of the wings
to the needle base. Upon removal of the needle from the patient the wings flex
against a
moveable member that keeps them adjacent the needle until the needle is
completely removed
from the skin of a patient after which the wings surround the needle to
prevent the tip from
making contact outside of the needle base. This patent is herewith
incorporated by reference.
Another type of safety device is described in U.S. Pat. No. 5,951,522 in which
the configuration
of the Huber needle is described. Patent '522 discloses a safety enclosure
comprising a wing
assembly mounted to the aft end of an angled Huber needle, the wing assembly
having a
configuration consisting of either a single integral member having a plurality
of spaced apart
fold lines which divide the member into a plurality of interconnected panels,
or a pair of wing
members mounted in a scissors-type arrangement. In each embodiment, when the
medical
practitioner removes the needle from the patient, the wing assembly closes
around the needle
and locks together, encasing it therein. This patent is herewith incorporated
by reference.
A further invention has disclosed a safety needle in U.S. application
2013/0218085, which
describes a safety device for protecting a port needle or Huber needle against
shifting. It
permits particularly good fixing of the position of the port needle or Huber
needle since that part
of the port needle or Huber needle that lies on the surface of the skin of the
patient is received
in the inner opening of the protective device. The frame-like spacer element
surrounds this part
of the port needle or Huber needle lying on the surface of the skin of the
patient and extends
over a large part of the height of the needle. In this way, a kind of
embankment is created which
provides protection all the way round the port needle or Huber needle. This
application is
herewith incorporated by reference.
However, infections of unknown origin have been detected by the prior art
devices, particularly
at the location, where the port needle permeates the skin.
3
CA 03022230 2018-10-25
WO 2017/194074 PCT/EP2016/025046
Summary
Port catheters are by principle always intended to feed medical substances
into and/or retrieve
fluids from a human or animal body. This therapy is named "parenteral
therapy", i.e. the transfer
of the fluid in either direction circumvents the digestive system. A plurality
of applications is
commonly in practical use comprising parenteral vascular and/or arterial
medication with all
appropriate and approved infusion therapies, parenteral intrathecal medication
with all
appropriate and approved infusion therapies, parenteral peritoneal medication
with all
appropriate and approved infusion therapies, parenteral intraspinal or
epidural medication with
all appropriate and approved infusion therapies, constant negative aspiration
of ascites and/or
any combinations thereof.
The present invention is specified in the claims as well as in the below
description. Preferred
embodiments are particularly specified in the dependent claims and the
description of various
embodiments.
The present invention is directed to a port needle that is particularly
adapted to penetrate skin,
tissue and/or a port septum of a port to transfer fluid into a port chamber of
the port.
The port can optionally be provided to be operated outside a body of a human
or an animal.
The fluid is then transported just into the chamber, e.g. for simulation
purposes and/or training
purposes.
The port needle comprises a distal section adapted to receive the fluid and a
proximal section
adapted to deliver the fluid to the chamber of the port. The fluid can be
received from any
reservoir, container etc. containing any appropriate kind of fluid and/or
material for a patient.
The fluid will then be fed or conveyed to the proximal section. The proximal
section can be the
section directly communicating with a port or being adjacent the port. It also
comprises the
section penetrating the port's septum and/or the skin and/or tissue of a
patient.
At least one lumen can define or form at least one inner surface, the lumen
being adapted to
transfer the fluid from the distal section to the proximal section. More than
one lumen for
different purposes can also be provided. The purposes can be cooling, heating,
normalizing
temperatures, feeding other fluids and/or retrieving fluids. The port needle
can then be
designed to also provide the respective structures for all these functions or
just parts of them.
4
CA 03022230 2018-10-25
WO 2017/194074 PCT/EP2016/025046
At least one membrane defines at least one outer surface of the proximal
section. The
membrane can be a solid and/or rigid wall and/or any layer or coating on a
solid and/or rigid
substrate. A substrate can be a wall as well on which then the membrane is
arranged,
preferably in a fixed manner. In case of one lumen the wall forming the
membrane or the
substrate can have round cross-section or any other form, such as square or
polygonal or any
.. combination thereof. In case of more than one lumen, the outer wall can
also have such shapes
in accordance with the arrangement of the lumens.
The outer surface of the proximal section comprises a metal, forming a
compound or alloy
containing nickel (Ni) with a maximum content of 0.05% and/or being adapted to
release at
most 0.2 pg/cm2/week of Ni and/or of any Ni compound. The content of Ni and/or
any compound
and/or the capability to release Ni and/or any compound can also be 0 (zero)
or get practically
close to 0 (zero).
The membrane can be impermeable to liquids and/or gases. This depends on the
fluids used
and also the constitution of the wall or substrate. In case the latter are
impermeable the
membrane may be permeable to fluids but for sterility reasons an
impermeability is preferred
so that no fluid and unwanted materials, bacteria, germs etc. can easily rest.
The membrane can be arranged as at least one outer layer on a substrate and/or
is forming an
outer wall at least of the proximal section. The membrane can be made of metal
and/or metal
alloy, such as steel, preferably stainless steel. Alternatively, at least the
membrane of the
proximal portion of the port needle or the entire port needle comprises and/or
is made of a non-
ferric metal or a non-metallic material and preferably comprises carbon and/or
CFRP materials.
The section modulus of the port needle is at least 0.004 mm3, preferably 0.01
mm3, more
preferably 0.1 mm3, even more preferably 0.2 mm3 and/or at most 0.3 mm3,
preferably 0.2 mm3,
more preferably 0.1 mm3, even more 0.01 mm3. This is meaningful, because a
port needle
device, even if it is attached to a patient, and the needle itself is placed
in a port chamber,
carries the risk of breakage. Fracturing of the needle, when in use, could
lead to splinters that
could potentially carry lethal risk for the patient.
The membrane can be defined by a coating and/or a layer provided over at least
the proximal
section of the port needle, preferably adhered by a shrink fit and/or an
adhesive.
The inner surface of the port needle can comprise Ni or any Ni compound of at
most 0.05%
and/or being adapted to release at most 0.2 pg/cm2/week of Ni or of any Ni
compound. The
5
CA 03022230 2018-10-25
WO 2017/194074 PCT/EP2016/025046
content of Ni and/or any compound and/or the capability to release Ni and/or
any compound
can also be 0 (zero) or get practically close to 0 (zero).
The proximal section can have a maximum length of 50 mm, preferably of 40 mm,
more
preferably of 30 mm, even more preferably of 20 mm, and even more preferably
of 10 mm
and/or a minimum length of 2 mm, preferably of 5 mm, more preferably of 10 mm,
even more
preferably of 20 mm, even more preferably of 30 mm.
The solid membrane and/or the membrane arranged as a layer together with the
underlying
substrate has/have a maximum outer diameter of 1.5 mm, preferably of 1.2 mm,
more
preferably of 1.0 mm, even more preferably of 0.9 mm, even more preferably of
0.8 mm, even
more preferably of 0.7 mm and/or a minimum diameter of 0.3 mm, preferably of
0.4 mm, more
preferably of 0.5 mm, even more preferably of 0.6 mm, even more preferably of
0.7 mm, even
more preferably of 0.9 mm.
Theoretically port needles are available from 6 G till 30 G. Nowadays needles
are generally
used having the sizes 22G, 21G, 20G and 19G (G meaning the gauge identifying
specific inner
diameters). 19 G is commonly used for blood, albumin, thrombocyte concentrates
etc. Sizes of
20 to 22 are commonly used for common or uncommon infusion fluids, such as
chemotherapeutic compositions, total and/or partly parenteral feeding, also
named parenteral
nutrition, antibiosis, virustasis, antibody-therapies, etc.
The proximal section of the port needle can terminate in a blunt end, a
bevelled end and/or an
end with at least one lateral window.
The proximal section can comprise a dull end and the port needle can further
comprise a
piercing element with a sharp tip being invertible at least into the lumen so
that the sharp tip
extends out of the proximal section for penetrating the skin, tissue and/or
port lumen and the
piercing element being adapted to be taken out of the lumen. Such piercing
element is also
called a mandrain.
The invention is also directed to a needle for the medical use as a port
needle as specified
before and below.
The present invention also relates to a method of penetrating skin, tissue
and/or a port septum
of a port by a port needle to transfer fluid into a port chamber of the port.
The method can also
be practiced for the penetration of a port septum of a port outside the human
or animal body.
6
CA 03022230 2018-10-25
WO 2017/194074 PCT/EP2016/025046
The present invention can comprise the following steps: receiving the fluid by
a distal section;
delivering the fluid to the chamber of the port by a proximal section;
transferring the fluid from
the distal section to the proximal section by at least one lumen defining at
least one inner
surface; defining at least one outer surface of the proximal section by at
least one membrane;
and providing the outer surface of the proximal section with a Ni and/or any
Ni compound with
a content of at most 0.05% and/or the outer surface being adapted to release
at most 0.2
pg/cm2/week of Ni and/or of any Ni compound.
The word "coated" is intended to comprise a process to be described as plated
or metallized,
electroplated, sputtered, shrinked, glued, adhered etc.
The word "body" and/or "organism" is intended to comprise the human and/or any
warm-
blooded animal body.
The word "fluid" is intended to also comprise liquids, gases, emulsions and/or
any combination
thereof.
The word "thorn" comprises a punctuation thorn, penetration tip, rod,
mandrain, etc.
The word "membrane" comprises at least one sheath, film, lamina and/or
covering layer and/or
any combination thereof.
The word "compound" also comprises an alloy, mixture, combination, blend
and/or any
combination thereof.
The word "skin" also comprises cutaneous, subcutaneous tissue, subcutaneous
fat, muscle.
It has been surprisingly found that allergic reactions can be avoided to a
large extent by a
modification of the port needle giving patients great relief.
7
CA 03022230 2018-10-25
WO 2017/194074 PCT/EP2016/025046
Brief description of the drawings
The skilled person will understand that the drawings, described below, are for
illustration
purposes only. The drawings are not intended to limit the scope of the present
teachings in
any way.
FIG. 1 shows an example of a port needle arrangement 1 or port needle 1 from
the bottom. At
the side the distal section 17 can be seen receiving any fluids. A body 20 is
holding all
components or is arranged around at least part of them and may assist in
holding them in place.
At the body 20 an optional resting arrangement or resting layer 21 can be
seen. A resting layer
may be provided as an antibiotic means. Moreover, a proximal section 10 of the
port needle 1
is shown that extends perpendicular to the paper plane. A grip 18 can be
integrated for
convenience purposes.
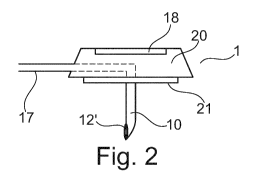
FIG. 2 exemplifies a side view of the port needle 1. The same or similar
elements bear the
same reference numerals. An optional resting portion 21 is arranged at the
bottom of the port
needle the resting portion 21 being provided to rest adjacent the port (not
shown) or the skin
and/or tissue in close proximity to the port. The proximal section 10 extends
down, preferably
in a perpendicular orientation or in a generally downward orientation.
FIG. 3 is further showing the port needle 1 with a septum 2. In addition to
the figure before a
punctuation thorn 25 is shown that can penetrate through the needle-septum 2,
the thorn 25
having a sharp end 19. The sharp end 19 is brought into a position extending
beyond the end
of the proximal section 10 optionally having a blunt end 11. The thorn 25
assists in penetrating
a port-septum 31 of a port 3, shown below, and can be retreated upon insertion
of the port
needle 1 in the port 3. The proximal end 10 can then rest in the port without
a sharp end and
may have a safe and more stable position therein. As the blunt end may bluntly
rest against
the bottom of a port chamber 30 a window 11' can be provided ensuring flow of
fluid out of the
proximal section 10 through the window 11'.
FIG. 4 shows an optional end 11 of proximal section 10. The before disclosed
membrane 13
can be solid and can define a lumen 12 with an interior surface 14 of the
membrane 13 and an
outer surface 15 of the membrane 13. In case the membrane is a layer or
coating on a
substrate, the membrane will define the outer surface 15 while the substrate
will define the
inner surface 14.
FIG. 5 shows two alternatives for designs of ends of the proximal sections 10
that also may be
combined with each other. A window 11', an inclined or truncated end or an
inclined end with
8
CA 03022230 2018-10-25
WO 2017/194074 PCT/EP2016/025046
a window 11 ' can be provided either alone or in combination. The embodiment
of the proximal
section 12' can be formed as a "Huber needle" and/or any other non-coring
needle-tip.
Description of various embodiments
In the following, exemplary embodiments of the invention will be described,
referring to the
figures. These examples are provided to provide further understanding of the
invention, without
limiting its scope.
In the following description, a series of features and/or steps are described.
The skilled person
will appreciate that unless required by the context, the order of features and
steps is not critical
for the resulting configuration and its effect. Further, it will be apparent
to the skilled person
that irrespective of the order of features and steps, the presence or absence
of time delay
between steps, can be present between some or all of the described steps.
As used herein, including in the claims, singular forms of terms are to be
construed as also
including the plural form and vice versa, unless the context indicates
otherwise. Thus, it should
be noted that as used herein, the singular forms "a", "an" and "the" include
plural references
unless the context clearly dictates otherwise.
Throughout the description and claims, the terms "comprise", "including",
"having", and
"contain" and their variations should be understood as meaning "including but
not limited to",
and are not intended to exclude other components.
The present invention also covers the exact terms, features, values and ranges
etc. in case
these terms, features, values and ranges etc. are used in conjunction with
terms such as about,
around, generally, substantially, essentially, at least etc. (i.e., "about 3"
shall also cover exactly
3 or "substantially constant" shall also cover exactly constant).
The term "at least one" should be understood as meaning "one or more", and
therefore includes
both embodiments that include one or multiple components. Furthermore,
dependent claims
that refer to independent claims that describe features with "at least one"
have the same
meaning, both when the feature is referred to as "the" and "the at least one".
It will be appreciated that variations to the foregoing embodiments of the
invention can be made
while still falling within the scope of the invention. Alternative features
serving the same,
equivalent or similar purpose can replace features disclosed in the
specification, unless stated
9
CA 03022230 2018-10-25
WO 2017/194074 PCT/EP2016/025046
otherwise. Thus, unless stated otherwise, each feature disclosed represents
one example of a
generic series of equivalent or similar features.
The abbreviation "Ni" stands for the element nickel and/or comprising all
isotope variations.
Use of exemplary language, such as "for instance", "such as", "for example"
and the like, is
merely intended to better illustrate the invention and does not indicate a
limitation on the scope
of the invention unless so claimed. Any steps described in the specification
may be performed
in any order or simultaneously, unless the context clearly indicates
otherwise.
All of the features and/or steps disclosed in the specification can be
combined in any
combination, except for combinations where at least some of the features
and/or steps are
mutually exclusive. In particular, preferred features of the invention are
applicable to all aspects
of the invention and may be used in any combination.
The same reference numerals used for different embodiments are intended to
identify parts or
features of different embodiments with the same or similar function. In case
the same reference
numerals are not identified in other embodiments, this is by no means intended
to mean that
the corresponding features designated by these reference numerals are not
present.
10