Note: Descriptions are shown in the official language in which they were submitted.
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
REGISTRATION OF LOT FIDUCIALS WITH CAMERA
CROSS REFERENCE TO RELATED APPLICATIONS
[1] This application is a continuation-in-part of and claims priority to
U.S. Patent
Application Serial No. 14/576,593, Attorney Docket No. 0M763U50, titled
"Confocal Laser
Eye Surgery Systems," filed December 19, 2014, which claims priority to U.S.
Provisional
Application Serial No. 61/970,854, filed March 26, 2014, and to U.S.
Provisional Application
Serial No. 62/043,749, filed August 29, 2014, the entire contents of all of
which applications are
incorporated herein as if fully set forth. Full Paris Convention priority is
hereby expressly
reserved.
BACKGROUND
[2] Cataract extraction is a frequently performed surgical procedure.
Cataracts are
formed when the crystalline lens of the eye opacifies. The cataract scatters
light passing through
the lens and may perceptibly degrade vision. A cataract can vary in degree
from slight to
complete opacity. Early in the development of an age-related cataract, the
power of the lens may
increase, causing near-sightedness (myopia). Gradual yellowing and
opacification of the lens
may reduce the perception of blue colors as those shorter wavelengths are more
strongly
absorbed and scattered within the cataractous lens. Over time, cataract
formation may progress
and gradually result in progressive vision loss.
[3] Cataract treatment often involves eye surgery to remove the opaque
crystalline
lens. The cataractous lens is then replaced with an artificial intraocular
lens (TOL). Each year,
an estimated 19 million cataract surgeries are performed worldwide.
[4] During cataract surgery, a technique termed phacoemulsification can be
used,
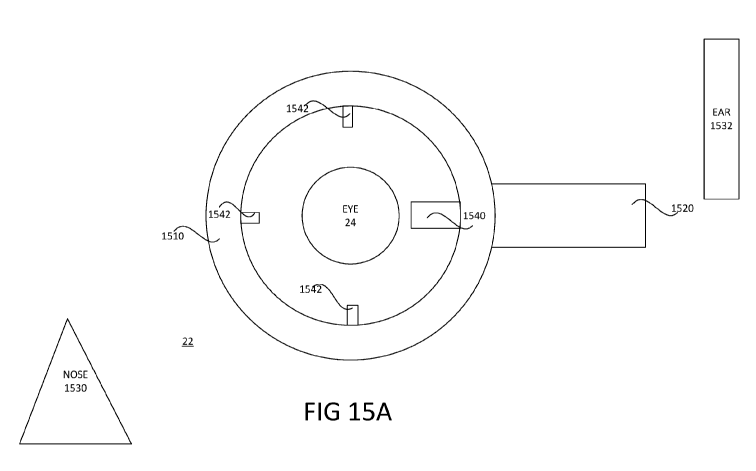
wherein an ultrasonic tip with associated irrigation and aspiration ports is
used to sculpt the
relatively hard nucleus of the lens to facilitate removal through an opening
made in the anterior
lens capsule. The nucleus of the lens is contained within an outer membrane of
the lens that is
referred to as the lens capsule. Access to the lens nucleus can be provided by
making an incision
in the shape of a small round hole in the anterior side of the lens capsule.
This procedure is
referred to as an anterior capsulorhexis when manual tools are used for making
the incisions, and
as an anterior capsulotomy when a surgical laser system is used instead.
1
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
[5] Previously, manual tools such as microkeratomes were used for making
incisions
such as those in the lens capsule to provide access to the lens nucleus. Over
the years, however,
surgical laser systems have become the tool of choice as they tend to lessen
the chance of
irregular, imprecise and inaccurate cuts and related complications. Laser eye
surgery systems
have been developed for various cataract procedures, including for instance:
(1) creating one or
more primary incisions or sideport incisions in the cornea to provide access
for a cataract
surgery instrument (such as a phacoemulsification tip) and/or to provide
access for implantation
of an intraocular lens, (2) incising the anterior lens capsule (anterior
capsulotomy) to provide
access for removing a cataractous lens, (3) segmenting and/or fragmenting a
cataractous lens, (4)
incising the posterior lens capsule (posterior capsulotomy) for various
cataract-related
procedures, and/or (5) creating one or more arcuate incisions in the cornea or
in the limbus to
reshape the cornea for treating refractive conditions.
[6] Accurate placement of a capsulotomy incision, a primary incision, a
sideport
incision, and an arcuate incision can be important for achieving a successful
outcome of cataract
surgery. In automated laser surgical procedures, physicians generally provide
the necessary
parameters for identifying the number, the placement and the size of incisions
based on pre-
treatment measurements. But, errors in data entry or lack of proper
calibration of the laser
surgical system can potentially lead to the placement of incisions at
locations other than at the
locations prescribed by the user. Moreover, some laser surgery systems do not
allow real time
confirmation of the location of the incision at the predetermined location, or
do not provide
warnings to the user if the actual placement of incisions during an automated
scan is different
from the intended location of those incisions.
[7] Thus, methods and systems that introduce additional safeguards, such as
verifying
the location of a laser scan or ocular incision, would be helpful for treating
patients with laser
surgical systems.
SUMMARY
[8] Hence, to obviate one or more problems due to limitations and
disadvantages of
the related art, many embodiments provide a method of verifying the placement
of a laser scan at
a predetermined location within an object comprises imaging at least a portion
of the object, the
2
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
resulting image comprising the predetermined location; identifying the
predetermined location in
the image, thereby establishing an expected scan location of the laser scan in
the image;
performing the laser scan on the object by scanning a focal point of a laser
beam in a scanned
area; detecting a luminescence from the scanned area and identifying an actual
scanned location
within the image based on the detected luminescence; and verifying whether the
laser scan was
at the predetermined location based on a difference between the actual scanned
location and
expected scan location. Preferably, the laser beam is a pulsed laser beam
having a wavelength of
320 nm to 370 nm. The luminescence preferably has a wavelength of 400 nm or
more. The step
of verifying the laser scan is at the predetermined location comprises
determining whether a
distance between the actual scanned location and the expected scan location is
within a
predetermined threshold.
[9] In many embodiments, the object is a human eye. In other embodiments,
the
object is a calibration apparatus.
[10] In many embodiments, the image comprises an array of pixels. The
expected
scan location preferably comprises one or more pixels selected from amongst
the array of pixels.
Also, the actual scanned location preferably comprises one or more pixels
selected from the
array of pixels. Preferably, verifying the laser scan at the predetermined
location comprises
determining whether a distance between the actual scanned location and the
expected scan
location is within a predetermined threshold.
[11] In many embodiments, the method further comprises periodically re-
imaging the
object, thereby obtaining one or more successive images of the object, and
identifying an actual
scanned location by comparing a detected luminescence of a same pixel in the
array between two
of the successive images. Preferably, the methods include identifying a
direction of the scan by
comparing an actual scanned location in between two or more of the successive
images.
[12] A method of verifying the placement of an ocular incision by a laser
surgical
system at a predetermined location within an eye comprises imaging at least a
portion of the eye,
the resulting image comprising the predetermined location for a laser scan
corresponding to the
ocular incision; identifying the predetermined location in the image, thereby
establishing an
expected scan location of the ocular incision in the image; performing a laser
scan on the object
by scanning a focal point of the laser beam in a scanned area, the laser scan
being configured in a
scan pattern for performing the ocular incision; detecting a luminescence from
the scanned area
3
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
and identifying an actual scanned location within the image based on the
detected luminescence;
and verifying the placement of an ocular incision based on the difference
between the actual
scanned location and expected scan location. The luminescence preferably has a
wavelength of
400 nm or more. The step of verifying the laser scan is at the predetermined
location comprises
determining whether a distance between the actual scanned location and the
expected scan
location is within a predetermined threshold.
[13] In many embodiments, the image comprises an array of pixels. The
expected
scan location preferably comprises one or more pixels selected from amongst
the array of pixels.
Also, the actual scanned location preferably comprises one or more pixels
selected from the
array of pixels. Preferably, verifying the laser scan at the predetermined
location comprises
determining whether a distance between the actual scanned location and the
expected scan
location is within a predetermined threshold.
[14] In many embodiments, the method further comprises periodically re-
imaging the
object, thereby obtaining one or more successive images of the eye, and
identifying an actual
scanned location by comparing a detected luminescence of a same pixel in the
array between two
of the successive images. Preferably, the methods include identifying a
direction of the scan by
comparing an actual scanned location in between two or more of the successive
images.
[15] In many embodiments, a method of verifying the calibration of a laser
eye
surgical system comprises imaging at least a portion of a calibration
apparatus having at least
one emissive surface, the resulting image comprising a predetermined location
for a laser scan;
identifying the predetermined location in the image, thereby establishing an
expected scan
location of the laser scan in the image; performing the laser scan of the
calibration apparatus by
scanning a focal point of the laser beam in a scanned area; detecting a
luminescence from the
scanned area and identifying an actual scanned location within the image based
on the detected
luminescence; and determining whether the laser surgical system is calibrated
based on a
difference between the actual scanned location and expected scan location. The
laser beam
preferably has a wavelength of 320 nm to 370 nm. The luminescence preferably
has a
wavelength of 400 nm or more. The step of verifying the laser scan is at the
predetermined
location preferably comprises determining whether a distance between the
actual scanned
location and the expected scan location is within a predetermined threshold.
[16] In many embodiments, the image comprises an array of pixels. The
expected
4
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
scan location preferably comprises one or more pixels selected from amongst
the array of pixels.
Also, the actual scanned location preferably comprises one or more pixels
selected from the
array of pixels. Preferably, verifying the laser scan at the predetermined
location comprises
determining whether a distance between the actual scanned location and the
expected scan
location is within a predetermined threshold.
[17] In many embodiments, the method further comprises periodically re-
imaging the
object, thereby obtaining one or more successive images of the calibration
apparatus, and
identifying an actual scanned location by comparing a detected luminescence of
a same pixel in
the array between two of the successive images. Preferably, the methods
include identifying a
direction of the scan by comparing an actual scanned location in between two
or more of the
successive images.
[18] In many embodiments, a laser eye surgical system, comprises a
laser source for
generating a pulsed laser beam; an imaging system comprising a detector;
shared optics
configured for directing the pulsed laser beam to an object to be sampled and
confocally
deflecting back-reflected light from the object to the detector; and a
controller operatively
coupled to the laser source, the imaging system and the shared optics. The
controller configured
to:
(a) receive one or more parameters defining one or more ocular incisions;
(b) image the eye with the imaging apparatus and identify an expected scan
location within the
image corresponding to the one or more ocular incisions based on the one or
more parameters;
(c) scan the focal point of a laser beam;
(d) detect luminescence from the region scanned;
(e) identify the actual scanned location within the image based on the
detected luminescence;
and
(f) provide a warning to the user if a difference between the actual scanned
location and the
expected is not within a predetermined threshold value.
[19] The controller may be configured to verify the laser scan is at
the predetermined
location when a distance between the actual scanned location and the expected
scan location is
within a predetermined threshold.
[20] The laser beam preferably has a wavelength of 320 nm to 370 nm,
and the
luminescence has a wavelength of 400 nm or more.
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
[21] In many embodiments, the image preferably comprises an array of
pixels. The
expected scan location preferably comprises one or more pixels selected from
amongst the array
of pixels, and the actual scanned location comprises one or more pixels
selected from the array
of pixels.
[22] The controller is preferably configured to periodically re-image the
eye, thereby
obtaining one or more successive images and identifying an actual scanned
location by
comparing a detected luminescence of a same pixel in the array between two of
the successive
images. The controller is also preferably configured to identify direction of
the scan by
comparing an actual scanned location in between two or more of the successive
images.
[23] This summary and the following description are merely exemplary,
illustrative,
and explanatory, and are not intended to limit, but to provide further
explanation of the invention
as claimed. Additional features, aspects, objects and advantages of
embodiments of this
invention are set forth in the descriptions, drawings, and the claims, and in
part, will be apparent
from the drawings and detailed description, or may be learned by practice. The
claims are
incorporated by reference.
BRIEF DESCRIPTION OF THE FIGURES
[24] The novel features of the invention are set forth with particularity
in the appended
claims. A better understanding of the features and advantages of the present
invention will be
obtained by referring to the following detailed description that sets forth
illustrative
embodiments using principles of the invention, as well as to the accompanying
drawings of
which:
[25] FIG. 1 is a schematic diagram of a laser surgery system, according to
many
embodiments, in which a patient interface device is coupled to a laser
assembly and a detection
assembly by way of a scanning assembly and shared optics that supports the
scanning assembly.
[26] FIG. 2 is a schematic diagram of an embodiment of the laser surgery
system of
FIG. 1.
[27] FIG. 3 is a schematic diagram of an embodiment of the laser surgery
system of
FIG. 1.
[28] FIG. 4 is a block diagram illustrating several acts of the methods and
acts for laser
6
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
scan verification in many embodiments.
[29] FIG. 5 illustrates an en face image of an eye.
[30] FIGS. 6A and 6B illustrate a calibration apparatus.
[31] FIG. 7A is a plan view illustrating a calibration plate, according to
many
embodiments, that can be used to calibrate the laser surgery system of FIG. 1.
[32] FIG. 7B is a schematic diagram illustrating using the calibration
plate of FIG.
10A to calibrate a camera of the laser surgery system of FIG. 1.
[33] FIG. 7C is a schematic diagram illustrating using the calibration
plate of FIG.
10A to calibrate the scanning assembly of the laser surgery system of FIG. 1.
[34] FIG. 8 shows a plan view of a capsulotomy incision locator and a cross-
sectional
view showing projection of the capsulotomy incision locator on the lens
anterior capsule,
according to many embodiments.
[35] FIGS. 9A, 9B and 9C illustrate aspects of arcuate incisions of a
cornea that can be
formed by the laser surgery system of FIG. 1, according to many embodiments.
[36] FIGS. 10A, 10B, 10C, 10D, 10E and 1OF illustrate aspects of primary
cataract
surgery access incisions of a cornea that can be formed by the laser surgery
system of FIG. 1,
according to many embodiments.
[37] FIGS. 11A, 11B, 11C, 11D and 11E illustrate aspects of sideport
cataract surgery
access incisions of a cornea that can be formed by the laser surgery system of
FIG. 1, according
to many embodiments.
[38] FIG. 12 is a schematic diagram illustrating the use of emission from
eye tissue to
verify the location scan with a camera of the laser surgery system of FIG. 1.
[39] FIG. 13 is a schematic diagram illustrating an en face image of the
eye projected
onto a monitor using a laser surgery system such as described in FIG. 1.
[40] FIG. 14 is a schematic diagram of aspects of a section scan and an
along-the-cut
scan for imaging areas of a cornea.
[41] FIG. 15A is a diagram showing an example alignment embodiment as
disclosed
herein.
[42] FIG. 15B is a diagram showing an example alignment embodiment as
disclosed
herein.
7
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
[43] FIG. 15C is a diagram showing an example alignment embodiment as
disclosed
herein.
[44] FIG. 15D is a diagram showing an example alignment embodiment as
disclosed
herein.
DETAILED DESCRIPTION
[45] The following description describes various embodiments of the present
invention. For purposes of explanation, specific configurations and details
are set forth so as to
provide a thorough understanding of the embodiments. It will also, however, be
apparent to one
skilled in the art that embodiments of the present invention can be practiced
without certain
specific details. Further, to avoid obscuring the embodiment being described,
various well-
known features may be omitted or simplified in the description.
[46] Systems for imaging and/or treating an eye of a patient are provided.
In many
embodiments, a shared optics provides a variable optical path by which a
portion of an
electromagnetic beam reflected from a focal point disposed within the eye is
directed to a path
length insensitive imaging assembly, such as a confocal detection assembly. In
many
embodiments, the shared optics is configured to accommodate movement of the
patient while
maintaining alignment between an electromagnetic radiation beam and the
patient. The
electromagnetic radiation beam can be configured for imaging the eye, can be
configured for
treating the eye, and can be configured for imaging and treating the eye.
[47] Referring now to the drawings in which like numbers reference similar
elements,
FIG. 1 schematically illustrates a laser surgery system 10, according to many
embodiments. The
laser surgery system 10 includes a laser assembly 12, a confocal detection
assembly 14, a shared
optics 16, a scanning assembly 18, an objective lens assembly 20, and a
patient interface device
22. The patient interface device 22 is configured to interface with a patient
24. The patient
interface device 22 is supported by the objective lens assembly 20. The
objective lens assembly
20 is supported by the scanning assembly 18. The scanning assembly 18 is
supported by the
shared optics 16. The shared optics 16 has a portion having a fixed position
and orientation
relative to the laser assembly 12 and the confocal detection assembly 14. In
many embodiments,
8
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
the patient interface device 22 is configured to interface with an eye of the
patient 24. For
example, the patient interface device 22 can be configured to be vacuum
coupled to an eye of the
patient 24 such as described in U.S. Publication No. 2014-0128821 Al (co-
pending U.S. Patent
Application serial number: 14/068994, entitled "Liquid Optical Interface for
Laser Eye Surgery
System," filed October 31, 2013). The laser surgery system 10 can further
optionally include a
base assembly 26 that can be fixed in place or repositionable. For example,
the base assembly
26 can be supported by a support linkage that is configured to allow selective
repositioning of the
base assembly 26 relative to a patient and secure the base assembly 26 in a
selected fixed
position relative to the patient. Such a support linkage can be supported in
any suitable manner
such as, for example, by a fixed support base or by a movable cart that can be
repositioned to a
suitable location adjacent to a patient. In many embodiments, the support
linkage includes setup
joints with each setup joint being configured to permit selective articulation
of the setup joint and
can be selectively locked to prevent inadvertent articulation of the setup
joint, thereby securing
the base assembly 26 in a selected fixed position relative to the patient when
the setup joints are
locked.
[48] In many embodiments, the laser assembly 12 is configured to emit an
electromagnetic radiation beam 28. The beam 28 can include a series of laser
pulses of any
suitable energy level, duration, and repetition rate.
[49] In certain embodiments, the laser assembly 12 incorporates femtosecond
(FS)
laser technology. By using femtosecond laser technology, a short duration
(e.g., approximately
10-13 seconds in duration) laser pulse (with energy level in the micro joule
range) can be
delivered to a tightly focused point to disrupt tissue, thereby substantially
lowering the energy
level required to image and/or modify an intraocular target as compared to
laser pulses having
longer durations. In other embodiments, the laser pulses have a pulse duration
generally between
1 ps and 100 ns.
[50] The laser assembly 12 can produce laser pulses having a wavelength
suitable to
treat and/or image tissue. For example, the laser assembly 12 can be
configured to emit an
electromagnetic radiation beam 28 such as emitted by any of the laser surgery
systems described
in U.S. Publication No. US 2014-0163534 A 1(co-pending U.S. Patent Application
serial number
14/069,042, entitled "Laser Eye Surgery System," filed October 31, 2013) and
US Publication
9
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
No. US 2011-0172649 Al (co-pending U.S. Patent Application serial number
12/987,069,
entitled "Method and System For Modifying Eye Tissue and Intraocular Lenses,"
filed January
7, 2011). For example, the laser assembly 12 can produce laser pulses having a
wavelength from
1020 nm to 1050 nm. For example, the laser assembly 12 can have a diode-pumped
solid-state
configuration with a 1030 (+/-5) nm center wavelength. As another example, the
laser assembly
12 can produce ultraviolet light pulses having a wavelength of between 320 nm
and 430 nm,
preferably between 320 and 400 nm, preferably between 320 to 370 nm, and more
preferably
between 340nm and 360 nm. In many embodiments, the laser pulses have a
wavelength of 355
nm. The 320 nm to 430 nm light source may be, for instance, a Nd:YAG laser
source operating
at the 3rd harmonic wavelength, 355nm.
[51] When an ultraviolet wavelength is used, the pulse energy of the laser
pulses is
generally between 0.010 and 5000. In many embodiments, the pulse energy will
be between
0.1 0 and 100 0, or more precisely, between 0.1 0 and 40 0, or between 0.1 0
and 10 0.
[52] When an ultraviolet wavelength is used, a pulse repetition rate of the
laser pulses
is generally between 500Hz and 500kHz. In many embodiments, the pulse
repetition rate is
between lkHz to 200 kHz, or between 1 KHz to 100 KHz.
[53] When an ultraviolet wavelength is used, spot sizes of the laser pulses
are
generally smaller than 10 p.m. In many embodiments, the spot size is
preferably smaller than 5
p.m, typically 0.5i.tm to 3i.t.m.
[54] When an ultraviolet wavelength is used, a pulse duration of the laser
pulses is
generally between 1ps and 100ns. In many embodiments, the pulse duration is
between 100 ps
to 10 ns, or between 100 ps and 1 ns. In a preferred embodiment, the pulse
duration is between
300 ps and 700 ps, preferably 400 ps to 700 ps.
[55] In some embodiments when an ultraviolet wavelength is used, the beam
quality,
also referred to as M2 factor, is between 1 and 1.3. The M2 factor is a common
measure of the
beam quality of a laser beam. In brief, the M2 factor is defined as the ratio
of a beam's actual
divergence to the divergence of an ideal, diffraction limited, Gaussian TEMOO
beam having the
same waist size and location as is described in ISO Standard 11146.
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
[56] In some embodiments when an ultraviolet wavelength is used, a peak
power
density, obtained by dividing the peak power of the laser pulse by the focal
spot size, is generally
expressed in units of GW/cm2. In general, the peak power density of the laser
pulses should be
sufficiently high to modify the ocular tissue to be treated. As would be
understood by those
ordinarily skilled, the peak power density depends upon a number of factors,
including the
wavelength of the selected laser pulses. In some embodiments, a peak power
density is generally
in the range of 100 GW/cm2 to 800 GW/cm2 will be used to cut ocular tissue
with 355 nm light.
[57] In some embodiments when an ultraviolet wavelength is used, the scan
range of
the laser surgical system is preferably in the range of 6 to 10 mm.
[58] In some embodiments when an ultraviolet wavelength is used, spot
spacing
between adjacent laser pulses is typically in the range of about 0.20 p.m to
10 p.m, preferably 0.2
inn to 6 p.m.
[59] In some embodiments when an ultraviolet wavelength is used, a
numerical
aperture should be selected that preferably provides for the focal spot of the
laser beam to be
scanned over a scan range of 6 mm to 10 mm in a direction lateral to a Z-axis
that is aligned with
the laser beam. The NA of the system should be less than 0.6, preferably less
than 0.5 and more
preferably in a range of 0.05 to 0.4, typically between 0.1 and 0.3. In some
specific
embodiments, the NA is 0.15. For each selected NA, there are suitable ranges
of pulse energy
and beam quality (measured as an M2 value) necessary to achieve a peak power
density in the
range required to cut the ocular tissue. Further considerations when choosing
the NA include
available laser power and pulse rate, and the time needed to make a cut.
Further, in selection of
an appropriate NA, it is preferable to ensure that there is a safe incidental
exposure of the iris,
and other ocular tissues, that are not targeted for cuts.
[60] When UV wavelengths are used, the tissue modification is carried out
using
chromophore absorption without plasma formation and/or without bubble
formation and an
associated cavitation event. Here, chromophore absorption refers to the
absorption of at least a
portion of the ultraviolet light by one or more chemical species in the target
area. The use of
ultraviolet light significantly reduces the threshold for plasma formation and
associated
formation of cavitation bubbles but also decreases the threshold energy
required for linear
absorption enhanced photodecomposition without the formation of cavitation
bubbles for a few
11
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
reasons. First, the focused spot diameter scales linearly with wavelength
which squares the peak
radiant exposure within the focal plane. Second, the linear absorption of the
material itself
allows an even lower threshold for plasma formation or low density
photodecomposition as
initially more laser energy is absorbed in the target structure. Third, the
use of UV laser pulses
in the nanosecond and sub-nanosecond regime enables linear absorption enhanced
photodecomposition and chromophore guided ionization.
[61] Furthermore, this chromophore guided ionization when using ultraviolet
wavelength strongly lowers the threshold for ionization in case of plasma
formation as well
lowers the threshold for low density photodecomposition for material
modification or alteration
without cavitation even under very weak absorption. The linear absorption also
allows for the
specific treatment of topical lens structures (e.g. the lens capsule) as the
optical penetration depth
of the laser beam is limited by the linear absorption of the lens. This is
especially true for aged
lenses which absorption in the UV-blue spectral region increases strongly
compared to young
lenses.
[62] The laser assembly 12 can include control and conditioning components.
For
example, such control components can include components such as a beam
attenuator to control
the energy of the laser pulse and the average power of the pulse train, a
fixed aperture to control
the cross-sectional spatial extent of the beam containing the laser pulses,
one or more power
monitors to monitor the flux and repetition rate of the beam train and
therefore the energy of the
laser pulses, and a shutter to allow/block transmission of the laser pulses.
Such conditioning
components can include an adjustable zoom assembly and a fixed optical relay
to transfer the
laser pulses over a distance while accommodating laser pulse beam positional
and/or directional
variability, thereby providing increased tolerance for component variation.
[63] In many embodiments, the laser assembly 12 and the confocal detection
assembly
14 have fixed positions relative to the base assembly 26. The beam 28 emitted
by the laser
assembly 12 propagates along a fixed optical path through the confocal
detection assembly 14 to
the shared optics 16. The beam 28 propagates through the shared optics 16
along a variable
optical path 30, which delivers the beam 28 to the scanning assembly 18. In
many embodiments,
the beam 28 emitted by the laser assembly 12 is collimated so that the beam 28
is not impacted
by patient movement induced changes in the length of the optical path between
the laser
12
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
assembly 12 and the scanner 16. The scanning assembly 18 is operable to scan
the beam 28
(e.g., via controlled variable deflection of the beam 28) in at least one
dimension. In many
embodiments, the scanning assembly 18 is operable to scan the beam 28 in two
dimensions
transverse to the direction of propagation of the beam 28 and is further
operable to scan the
location of a focal point of the beam 28 in the direction of propagation of
the beam 28. The
scanned beam is emitted from the scanning assembly 18 to propagate through the
objective lens
assembly 20, through the interface device 22, and to the patient 24.
[64] The shared optics 16 is configured to accommodate a range of movement
of the
patient 24 relative to the laser assembly 12 and the confocal detection
assembly 14 in one or
more directions while maintaining alignment of the beam 28 emitted by the
scanning assembly
18 with the patient 24. For example, in many embodiments, the shared optics 16
is configured to
accommodate a range movement of the patient 24 in any direction defined by any
combination
of unit orthogonal directions (X, Y, and Z).
[65] The shared optics 16 supports the scanning assembly 18 and provides
the variable
optical path 30, which changes in response to movement of the patient 24.
Because the patient
interface device 22 is interfaced with the patient 24, movement of the patient
24 results in
corresponding movement of the patient interface device 22, the objective lens
assembly 20, and
the scanning assembly 18. The shared optics 16 can include, for example, any
suitable
combination of a linkage that accommodates relative movement between the
scanning assembly
18 and, for example, the confocal detection assembly 24, and optical
components suitably tied to
the linkage so as to form the variable optical path 30.
[66] A portion of the electromagnetic radiation beam 28 that is reflected
by eye tissue
at the focal point propagates back to the confocal detection assembly 14.
Specifically, a
reflected portion of the electromagnetic radiation beam 28 travels back
through the patient
interface device 22, back through the objective lens assembly 20, back through
(and de-scanned
by) the scanning assembly 18, back through the shared optics 16 (along the
variable optical path
30), and to the confocal detection assembly 14. In many embodiments, the
reflected portion of
the electromagnetic radiation beam that travels back to the confocal detection
assembly 14 is
directed to be incident upon a sensor that generates an intensity signal
indicative of intensity of
the incident portion of the electromagnetic radiation beam. The intensity
signal, coupled with
13
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
associated scanning of the focal point within the eye, can be processed in
conjunction with the
parameters of the scanning to, for example, image/locate structures of the
eye, such as the
anterior surface of the cornea, the posterior surface of the cornea, the iris,
the anterior surface of
the lens capsule, and the posterior surface of the lens capsule. In many
embodiments, the
amount of the reflected electromagnetic radiation beam that travels to the
confocal detection
assembly 14 is substantially independent of expected variations in the length
of the variable
optical path 30 due to patient movement, thereby enabling the ability to
ignore patient
movements when processing the intensity signal to image/locate structures of
the eye.
[67] FIG. 2 schematically illustrates details of an embodiment of the laser
surgery
system 10. Specifically, example configurations are schematically illustrated
for the laser
assembly 12, the confocal detection assembly 14, and the scanning assembly 18.
As shown in
the illustrated embodiment, the laser assembly 12 can include an laser 32
(e.g., a femtosecond
laser), alignment mirrors 34, 36, a beam expander 38, a one-half wave plate
40, a polarizer and
beam dump device 42, output pickoffs and monitors 44, and a system-controlled
shutter 46. The
electromagnetic radiation beam 28 output by the laser 32 is deflected by the
alignment mirrors
34, 36. In many embodiments, the alignment mirrors 34, 36 are adjustable in
position and/or
orientation so as to provide the ability to align the beam 28 with the
downstream optical path
through the downstream optical components. Next, the beam 28 passes through
the beam
expander 38, which increases the diameter of the beam 28. Next, the expanded
beam 28 passes
through the one-half wave plate 40 before passing through the polarizer. The
beam exiting the
laser is linearly polarized. The one-half wave plate 40 can rotate this
polarization. The amount
of light passing through the polarizer depends on the angle of the rotation of
the linear
polarization. Therefore, the one-half wave plate 40 with the polarizer acts as
an attenuator of the
beam 28. The light rejected from this attenuation is directed into the beam
dump. Next, the
attenuated beam 28 passes through the output pickoffs and monitors 44 and then
through the
system-controlled shutter 46. By locating the system-controlled shutter 46
downstream of the
output pickoffs and monitors 44, the power of the beam 28 can be checked
before opening the
system-controlled shutter 46.
[68] As shown in the illustrated embodiment, the confocal detection
assembly 14 can
include a polarization-sensitive device such as a polarized or unpolarized
beam splitter 48, a
14
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
filter 50, a focusing lens 51, a pinhole aperture 52, and a detection sensor
54. A one-quarter
wave plate 56 is disposed downstream of the polarized beam splitter 48. The
beam 28 as
received from the laser assembly 12 is polarized so as to pass through the
polarized beam splitter
48. Next, the beam 28 passes through the one-quarter wave plate 56, thereby
rotating the
polarization axis of the beam 28. A quarter rotation is a presently preferred
rotation amount.
After reflecting from the focal point in the eye, the returning reflected
portion of the beam 28
passes back through the one-quarter wave plate 56, thereby further rotating
the polarization axis
of the returning reflected portion of the beam 28. Ideally, after passing back
through the one-
quarter wave plate 56, the returning reflected portion of the beam has
experienced a total
polarization rotation of 90 degrees so that the reflected light from the eye
is fully reflected by the
polarized beam splitter 48. The birefringence of the cornea can also be taken
into account if, for
example, the imaged structure is the lens. In such a case, the plate 56 can be
adjusted and/or
configured so that the double pass of the plate 56 as well as the double pass
of the cornea sum up
to a polarization rotation of 90 degrees. Because the birefringence of the
cornea may be different
from patient to patient, the configuration/adjustment of the plate 56 can be
done dynamically so
as to optimize the signal returning to the detection sensor 54. Accordingly,
the returning
reflected portion of the beam 28 is now polarized to be at least partially
reflected by the
polarized beam splitter 48 so as to be directed through the filter 50, through
the lens 51, and to
the pinhole aperture 52. The filter 50 can be configured to block wavelengths
other than the
wavelengths of interest. The pinhole aperture 52 is configured to block any
returning reflected
portion of the beam 28 reflected from locations other than the focal point
from reaching the
detection sensor 54. Because the amount of returning reflected portion of the
beam 28 that
reaches the detection sensor 54 depends upon the nature of the tissue at the
focal point of the
beam 28, the signal generated by the detection sensor 54 can be processed in
combination with
data regarding the associated locations of the focal point so as to generate
image/location data for
structures of the eye.
[69] In this embodiment, the same laser assembly may be used both for
treatment (i.e.
modification) and imaging of the target tissue. For instance, the target
tissue may be imaged by
raster scanning pulsed laser beam 28 along the target tissue to provide for a
plurality of data
points, each data point having a location and intensity associated with it for
imaging of the target
tissue. In some embodiments, the raster scan is selected to deliver a sparse
pattern in order to
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
limit the patient's exposure, while still discerning a reasonable map of the
intraocular targets. In
order to image the target tissue, the treatment laser beam (i.e. the laser
beam having the
parameters suitably chosen as described above for the modification of tissue)
is preferably
attenuated to the nanoJoule level for imaging of the structures to be treated.
When used for
imaging, the attenuated laser beam may be referred to as an imaging beam. In
many
embodiments, the treatment beam and the imaging beam may be the same except
that the pulse
energy of the laser source is lower than the treatment beam when the laser
beam is used for
imaging. In many embodiments, the pulse energy of the laser beam when used for
imaging is
preferably from about 0.1 nJ to 10 nJ, preferably less than 2 nJ and more
preferably less than 1.8
nJ. The use of the same laser beam for both treatment and imaging provides for
the most direct
correlation between the position of the focal locations for imaging and
treatment ¨ they are the
same beam. This attenuated probe beam can is preferably used directly in a
back reflectance
measuring configuration, but, alternatively, may be used indirectly in a
fluorescence detection
scheme. Since increases in both backscatter and fluorescence within tissue
structures will be
evident, both approaches have merit.
[70] In a preferred embodiment, imaging of a first target area to be
modified is
performed sequentially with the modification of the tissue in the first target
area before moving
on to a second, different, target area, i.e. imaging is performed sequentially
with treatment in a
predetermined target area. Thus, for instance imaging of the lens capsule is
preferably followed
by treatment of the lens capsule before imaging is carried out on other either
structures, such as
the cornea or iris. In another embodiment, imaging of a first target area
where a first incision to
be place is performed sequentially with the scanning the treatment beam to
perform the incision
in the first target area before moving on to a second target area for
performing a second incision,
i.e. imaging of the area to be incised is performed sequentially with scanning
the treatment beam
to perform in the predetermined target area.
[71] In another embodiment, a cataract procedure comprises a capsulotomy
incision,
and at least one of a cataract incision and a limbal relaxing incision. In one
embodiment,
imaging of the target tissue where the capsulotomy is to be performed is
followed by scanning of
the treatment to perform the capsulotomy, and then the treatment beam is
scanned to perform the
capsulotomy. Subsequently, imaging of the target tissue where the at least one
of the cataract
16
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
incisions (CI) and the limbal relaxing incision (LRI) is carried out and then
the treatment beam is
scanned to perform the at least one of the LRI and the CI. When an LRI is
selected, this
minimizes the chance for the patient to move between imaging and treatment for
the LRIs which
are the most critical / sensitive to eye movements between image and
treatment.
[72] As shown in the illustrated embodiment, the scanning assembly 18 can
include a
z-scan device 58 and a xy-scan device 60. The z-scan device 58 is operable to
vary a
convergence/divergence angle of the beam 28 and thereby change a location of
the focal point in
the direction of propagation of the beam 28. For example, the z-scan device 58
can include one
or more lenses that are controllably movable in the direction of propagation
of the beam 28 to
vary a convergence/divergence angle of the beam 28. The xy-scan device 60 is
operable to
deflect the beam 28 in two dimensions transverse to the direction of
propagation of the beam 28.
For example, the xy-scan device 60 can include one or more mirrors that are
controllably
deflectable to scan the beam 28 in two dimensions transverse to the direction
of propagation of
the beam 28. Accordingly, the combination of the z-scan device 58 and the xy-
scan device 60
can be operated to controllably scan the focal point in three dimensions, for
example, within the
eye of the patient.
[73] As shown in the illustrated embodiment, a camera 62 and associated
video
illumination 64 can be integrated with the scanning assembly 18. The camera 62
and the beam
28 share a common optical path through the objective lens assembly 20 to the
eye. A video
dichroic 66 is used to combine/separate the beam 28 with/from the illumination
wavelengths
used by the camera. For example, the beam 28 can have a wavelength of about
355 nm and the
video illumination 64 can be configured to emit illumination having
wavelengths greater than
450 nm. Accordingly, the video dichroic 66 can be configured to reflect the
355 nm wavelength
while transmitting wavelengths greater than 450 nm.
[74] FIG. 3 schematically illustrates a laser surgery system 300, according
to many
embodiments. The laser surgery system 300 includes the laser assembly 12, the
confocal
detection assembly 14, the shared optics 16, the scanning assembly 18, the
objective lens
assembly 20, the patient interface 22, communication paths 302, control
electronics 304, control
panel/graphical user interface (GUI) 306, and user interface devices 308. The
control
electronics 304 includes processor 310, which includes memory 312. The patient
interface 22 is
17
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
configured to interface with a patient 24. The control electronics 304 is
operatively coupled via
the communication paths 302 with the laser assembly 12, the confocal detection
assembly 14, the
shared optics 16, the scanning assembly 18, the control panel/GUI 306, and the
user interface
devices 308.
[75] The scanning assembly 18 can include a z-scan device and a xy-scan
device. The
laser surgery system 300 can be configured to focus the electromagnetic
radiation beam 28 to a
focal point that is scanned in three dimensions. The z-scan device can be
operable to vary the
location of the focal point in the direction of propagation of the beam 28.
The xy-scan device
can be operable to scan the location of the focal point in two dimensions
transverse to the
direction of propagation of the beam 28. Accordingly, the combination of the z-
scan device and
the xy-scan device can be operated to controllably scan the focal point of the
beam in three
dimensions, including within a tissue of the patient 24 such as within an eye
tissue of the patient
24. The scanning assembly 18 is supported by the shared optics 16, which may
be configured to
accommodate patient movement induced movement of the scanning assembly 18
relative to the
laser assembly 12 and the confocal detection assembly 14 in three dimensions.
[76] The patient interface 22 is coupled to the patient 24 such that the
patient interface
22, the objective lens assembly 20, and the scanning assembly 18 move in
conjunction with the
patient 24. For example, in many embodiments, the patient interface 22 employs
a suction ring
that is vacuum attached to an eye of the patient 24. The suction ring can be
coupled with the
patient interface 22, for example, using vacuum to secure the suction ring to
the patient interface
22.
[77] The control electronics 304 controls the operation of and/or can
receive input
from the laser assembly 12, the confocal detection assembly 14, the free-
floating assembly 16,
the scanning assembly 18, the patient interface 22, the control panel/GUI 306,
and the user
interface devices 308 via the communication paths 302. The communication paths
302 can be
implemented in any suitable configuration, including any suitable shared or
dedicated
communication paths between the control electronics 304 and the respective
system components.
[78] The control electronics 304 can include any suitable components, such
as one or
more processors, one or more field-programmable gate arrays (FPGA), and one or
more memory
storage devices. In many embodiments, the control electronics 304 controls the
control
18
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
panel/GUI 306 to provide for pre-procedure planning according to user
specified treatment
parameters as well as to provide user control over the laser eye surgery
procedure.
[79] The control electronics 304 can include a processor/controller 310
that is used to
perform calculations related to system operation and provide control signals
to the various
system elements. A computer readable medium 312 is coupled to the processor
310 in order to
store data used by the processor and other system elements. The processor 310
interacts with the
other components of the system as described more fully throughout the present
specification. In
an embodiment, the memory 312 can include a look up table that can be utilized
to control one or
more components of the laser system surgery system 300.
[80] The processor 310 can be a general purpose microprocessor configured
to execute
instructions and data, such as a Pentium processor manufactured by the Intel
Corporation of
Santa Clara, California. It can also be an Application Specific Integrated
Circuit (ASIC) that
embodies at least part of the instructions for performing the method according
to the
embodiments of the present disclosure in software, firmware and/or hardware.
As an example,
such processors include dedicated circuitry, ASICs, combinatorial logic, other
programmable
processors, combinations thereof, and the like.
[81] The memory 312 can be local or distributed as appropriate to the
particular
application. Memory 312 can include a number of memories including a main
random access
memory (RAM) for storage of instructions and data during program execution and
a read only
memory (ROM), in which fixed instructions are stored. Thus, the memory 312
provides
persistent (non- volatile) storage for program and data files, and may include
a hard disk drive,
flash memory, a floppy disk drive along with associated removable media, a
Compact Disk Read
Only Memory (CD-ROM) drive, an optical drive, removable media cartridges, and
other like
storage media.
[82] The user interface devices 308 can include any suitable user input
device suitable
to provide user input to the control electronics 304. For example, the user
interface devices 308
can include devices such as, for example, a touch-screen display/input device,
a keyboard, a
footswitch, a keypad, a patient interface radio frequency identification
(RFID) reader, an
emergency stop button, and a key switch.
19
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
[83] Certain acts or steps in connection with the methods and systems
of verifying the
location of a laser scan in an object, preferably an eye, are shown in Fig. 2.
In some
embodiments, the object is an eye and the methods and acts of verifying the
locations of the laser
scan is operable to verify the location of an incision in ocular surgical
procedures, including
cataract surgery. In other embodiments, the object is a calibration apparatus,
and the methods
and acts are operable to verify the calibration of a laser surgical system,
preferably a laser eye
surgical system.
84] The methods and/or acts of verifying the location of a laser scan
within an object
include, at Step 202 (Fig. 4), imaging the object, the resulting image
including a portion of the
object at a predetermined location to be scanned. The type or manner of
imaging is not
particularly limited, so long as the selected imaging method is capable of
imaging the portion of
the object in which the predetermined scan location is located. In many
embodiments, the
predetermined scan location includes the location of an incision that has been
prescribed or
identified by a health professional for placement in a tissue of the eye, such
as the lens capsule,
the lens, the cornea or the limbus. In this case, the selected imaging method
should be capable
of imaging the selected tissue. In one embodiment, the imaging method is
optical imaging by a
camera, and the image is presented as an en face image of the eye on monitor
as shown in Figure
5. The image may likewise be a video image in which successive images are
captured in real
time by a sensor and displayed on a monitor. The monitor may operate at, for
instance, 60 Hz,
120 Hz or 240 Hz.
[85] In another embodiment, the imaging method includes scanning the
location of a
focal point of a pulsed laser beam and confocally detecting light reflected
from the location of
the pulsed laser. Preferably, the pulsed laser beam is an ultraviolet pulsed
laser beam having a
wavelength of 320-370 nm. In many embodiments, the methods of verifying the
location of a
laser scan within an object include both video imaging and confocal imaging.
[86] The methods and/or acts of verifying the location of a laser scan
within an object
include, at Step 204, identifying an expected scan location within the image
corresponding to the
predetermined scan location. In many embodiments, a camera 62 in the imaging
system includes
a sensor having an orthogonal array of pixels (e.g., in x and y directions
where the corresponding
z direction is in the direction of propagation of the electromagnetic
radiation beam). Thus, in
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
many embodiments, the image is comprised of an array of pixels, preferably
color pixels. In
many embodiments, a calibration of the system according to the methods
described herein
provides a known relationship between the location of a pixel in the
orthogonal array of the
image and a location of the tissue in the treatment space. This known
correspondence between
the pixels in the image and a location in treatment space makes it possible to
identify an expected
scan location in the image corresponding to the predetermined scan location.
In many
embodiments, the expected scan location within the image is a set of pixels,
PEL illustrated
visually in FIG. 5 (not to scale), that is a subset of the array of pixels
comprising the image. The
set of pixels, PEL, may include a pixel denominated as an expected starting
point pixel of the
expected scan location, Pstart, a pixel may be identified as an expected
ending point pixel, Pend, of
the expected scan location or a pixel denominated as a midpoint pixel, Pd,
located at some
position between the starting point pixel and the ending point pixel.
[87] The methods and/or acts of verifying the location of a laser scan
within an object
include, at Step 206, conducting a laser scan of the object by scanning a
focal point of the laser
beam through at least a portion of the object. The location of the scan is not
particularly limited.
But, in many embodiments, it will preferably include the predetermined scan
location. The laser
beam is preferably a pulsed laser beam, and preferably a pulsed ultraviolet
laser beam. The laser
scan is preferably a raster scan of the pulsed laser beam. In some
embodiments, the laser beam
may be of sufficient energy to modify the eye tissue scanned, and such that a
succession of laser
pulses within the eye tissue is sufficient to incise the tissue scanned. In
other embodiments, the
energy of the laser beam will be insufficient to modify the tissue scanned.
The intensity of the
laser beam is also preferably insufficient to cause the formation of a plasma,
and also preferably
insufficient to generate one or more cavitation events, such as the formation
of a bubble.
[88] The methods and/or acts of verifying the location of a laser scan
within an object
include, at Step 208, detecting the luminescence region scanned by the laser
beam. As would be
understood by those ordinarily skilled, individual photons of the ultraviolet
laser beam, each
having an energy, hv, will be absorbed by various components in the tissue
scanned. This
absorbed light will then be re-emitted by the component as a photon of lower
energy (larger
wavelength) either by fluorescence or phosphorescence from the scanned tissue.
When
ultraviolet light is used for the laser scan, the emitted luminescence
generally includes light in
21
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
the blue, indigo and violet portions of the visible spectrum, having
wavelengths from about 400
nm to 475 nm. The emission of light from tissue, including by processes such
as fluorescence or
phosphorescence, is generally referred to herein as luminescence. In many
embodiments, the
luminescence, preferably in the range of 400 nm to 475 nm light is detected
using the same
camera 62 and same sensor having the orthogonal array of pixels which was used
to image an
object.
[89] The methods and/or acts of verifying the location of a laser scan
within an object
include, at Step 208, detecting the luminescence from the region scanned by
the laser beam. As
would be understood by those ordinarily skilled, each pixel has red (R), Green
(G) and Blue (B)
components ("R, G, B components"), each having an intensity, I, associated
with it that has a
value from Imin to Imax. In many embodiments, Inõõ=0 and Imax=255. According
to some
embodiments, the actual scanned location within the image may be determined
monitoring the
intensity, IB, of the B component of the pixels that make up the image. In
many embodiments,
the actual scanned location may be comprised of one or more Pixels, Pact in
the image. In many
embodiments, a pixel, Pact, is identified as being an actual scanned location
if the measured value
of lb for the pixel is greater than a predetermined threshold value, Ii,. More
than one Pact may be
identified in one image or frame. The predetermined threshold value may be
empirically
determined based on the object to be imaged. For instance, if the object to be
imaged contains
very few blue components, it may be possible to determine luminescence based
on a relatively
small lb. In contrast, if the object to be imaged contains a relatively large
amount of blue
components, it may be necessary to determine luminescence based on a
relatively large IB.
Those skilled in the art thus instructed can suitably determine the necessary
threshold for each
application. In some embodiments, the predetermined threshold value, Ii,, may
be 0.9Imax,
0.7Imax, 0.6lmax, 0.5Imax, 0.4lmax, 0.3Imax, 0.2Ima,, or 0.1Imax. This may be
termed a "pixel
thresholding" approach.
[90] In other embodiments, the actual scanned location within the image may
be
determined by comparing the intensity, IB, of the B component of a pixel in
successive images or
frames an image. In this embodiment, the actual scanned location is determined
by calculating a
difference between an Ib value of a pixel in a first frame, Ibi, and the Ib
value of the same pixel in
a second successive frame, Ib2. In many embodiments, a pixel is identified as
being an actual
22
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
scanned location if the measured value difference, Ib2- Ibi for a pixel is
greater than a
predetermined threshold value, Ip. The predetermined threshold value may be
empirically
determined based on the object to be imaged; however, since the identification
is based on a
difference in the same pixel in successive frames, the threshold may not be as
sensitive to the
amount of blue in the components of the image. In some embodiments, the
predetermined
threshold value, Ip, may be 0.9Imms, 0.8Imax, 0.71mws, 0.6Imax, 0.51mws,
0.4Imax, 0.3Imax, 0.2Imax, or
()Amax. This may be termed a "consecutive differential" approach.
[91] In other embodiments, the actual scanned location within the image may
be
determined by comparing an intensity, IB, of the B component of a pixel in a
first frame or image
and then calculating a difference in intensity value for the pixel in each
successive image or
frame compared to its intensity of the first frame. In this embodiment, the
actual scanned
location is determined by comparing an lb value of a pixel in a first frame,
Ibi, with the lb value
of the same pixel in each successive i=2, n frames, i.e. Ib2 Ib3, Ib4. -La
etc. In In many embodiments,
a pixel is identified as being an actual scanned location if the measured
value difference, Ibi-
for a pixel is greater than a predetermined threshold value, Ip. The
predetermined threshold
value may be empirically determined based on the object to be imaged; however,
since the
identification is based on a difference in the same pixel in successive
frames, the threshold may
not be as sensitive to the amount of blue in the components of the image. In
some embodiments,
the predetermined threshold value, Ip, may be 0.9Ima,, O.8lmax, 0.7Imax,
0.6Imax, 0.5Imax, 0.4Imax,
0.3Imax, 0.2Imax, or 0.1Imax. This may be termed an "absolute differential"
approach.
[92] In some embodiments, a statistical approach may be implemented for
determining
the actual scanned location within the image. In these probabilistic
approaches, the values for
the intensity, IB of the thresholding approach, the value of Ib2- Ibi in the
consecutive differential
approach and the value Lc Li in the absolute differential is assigned a
probability of being an
actual scanned location, and is determined to be an actual scanned location if
the value of the
probability is greater than a predetermined probability, for instance 50%
(i.e., 0.5), or 60%, 70%,
80% or 90%.
[93] Since a scan is conducted over a period of time, the pixels which are
identified as
being an actual scanned location, Pact, may change during the time course of
the scan. Analysis,
such as by overlaying successive frames or obtaining difference images between
frames, either
23
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
of individual pairs of frames or of all successive images/frames during the
scan permits the
determination of all the actual scanned locations and of the direction of the
scan during the scan.
In some embodiments, all actual scanned locations may be determined before a
comparison of
the actual scanned location with the expected scan location is completed.
[94] The methods and/or acts of verifying the location of a laser scan
within an object
include, at Step 214, providing a warning if a difference between the actual
location in the image
and an expected scan location in the image is greater than a threshold
distance, DT. The nature
of the warning is not particularly limited. For instance, a warning message
may be placed on the
image indicating a difference in the expected scan location and actual scan
location has been
detected. The warning may optionally include stopping the scan and alerting a
user. Where the
object is an eye, the warning may also optionally include reducing the
intensity of the laser beam
below a level necessary to incise the tissue.
[95] The manner of calculating the difference between the expected scan
location and
the actual scan location is not particularly limited. In many embodiments, the
calculated
difference may be a distance between the expected scan location and the actual
scanned location.
The distance may be between any of the one or more pixels, Pact, identified as
an actual scan
location and any pixel from the set of pixels, PEL, that comprises the
expected scan locations. In
some embodiments, one Pact from the actual scan locations is selected for the
distance
measurement and one pixel is selected from the set of PEL pixels for the
distance measurements.
In some embodiments, the selected expected scan location pixel may be either
Ps,tart Pend or a
Põ,d. The distance may be calculated as a number of pixels separating the
selected pixels.
Alternatively, the distance may be calculated as a physical distance in, for
instance, units of
microns. In another alternative, it may be suitable to calculate the distance
as an angular distance
between the pixels, for instance, by an angle theta, 0, around an axis
centered at the pupil center
in the direction of propagation of the laser light source. The threshold
difference, DT, may be
chosen based on the units selected. In the case of a distance measured in
microns, the threshold
difference DT, may be 5000 microns, or 1000 microns, or 500 microns or 200
microns or 100
microns, or 50 microns or 5 microns. In the case of angular distance, the
distance DT, may be
120 , or 90 , or 60 , or 45 , or 30 , or 15 .
24
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
[96] The methods and/or acts of verifying the location of a laser scan may
be used in
connection with laser eye surgery systems and methods to verify the placement
of one or more
ocular incisions, including in methods for cataract surgery using a laser eye
surgery system for
verifying the placement of incisions in a cataract surgery. The laser eye
surgery system may be
the one shown in FIGS. 1-3 and described herein. Thus, some embodiments are a
laser surgical
system configured to carry out the methods described herein. In some
embodiments, a user or
physician will define one or more incisions to be performed by the laser
surgical system during
cataract surgery selected from capsulotomy incisions, primary incisions,
sideport incisions and
arcuate incisions by entering the necessary parameters into system to define
the incision. The
laser surgical system is configured to receive those parameters, image the
eye, identify the
expected scan location within the image corresponding to the selected
incisions, conduct a laser
scan of the eye by scanning the focal point of a laser beam, detect
luminescence from the region
scanned, identify the actual scanned location within the image based on the
detected
luminescence, and provide a warning to the user if the difference between the
expect location in
the image and the actual location in the image is greater than a predetermined
threshold. In some
embodiments, the laser scan that is conducted is a confocal imaging scan of
the eye to verify that
the confocal imaging scan is imaging the actual location to be incised. In
some embodiment, the
laser scan conducted is a treatment scan of sufficient energy to incise the
tissue to be treated. In
other embodiments, the scan conducted is the same as the treatment scan but at
energies
insufficient to incise human tissue. This scan can be done in order to verify
the placement of the
incisions prior to conducting a treatment scan capable of incising tissue.
[97] The methods and/or acts of verifying the location of a laser scan may
be used in
connection with laser eye surgery systems and methods to verify the
calibration of an eye
surgical system prior to treatment. The methods or acts of verifying the
calibration may include
a calibration apparatus 300 shown in FIGS. 6A and 6B. The calibration
apparatus 300 includes
sidewall 320 and also comprises structures similar to structures of an eye.
For example, the
calibration apparatus 300 may include a container 350 having a viscous
substance or solid
substance that is similarly optically transmissive to the structures of the
eye. The material 350
may comprise of visco-elastic fluid, a gel or other optically transmissive
structure and material,
for example. The calibration apparatus 300 comprises an iris structure 310
and, optionally, a
lens structure 330, either of which can provide a suitable surface for
calibration. At least one of
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
the surfaces of lens structure 330 or iris structure 310 should emit blue
wavelength light when
irradiated by ultraviolet light. Here, a structure or property is "similar" if
it is within 10%,
preferably within 5% and more preferably within about 1% of a typical
measurement of that
structure or property in an adult human eye. The calibration structure 300 may
connect to the
patient interface as described herein and a fluid (note shown) can be provided
above the
calibration apparatus, for example.
[98] A method and/or acts of verifying the calibration of laser surgical
system,
including a laser eye surgical system, include imaging a calibration
apparatus, identifying an
expected scan location within the image corresponding to a predetermined scan
pattern within
the calibration apparatus, conducting a laser scan of the calibration
apparatus by scanning the
focal point of a laser beam, detecting luminescence from the region of the
calibration area
scanned, identifying the actual scanned location within the image based on the
detected
luminescence, and identifying the laser surgical system as not calibrated if a
difference between
the expected scan location in the image and the actual location in the image
is greater than a
predetermined threshold. The method can also include identifying the laser
surgical system as
calibrated if a difference between the expected scan location in the image and
the actual location
in the image is less than a predetermined threshold.
[99] System Calibration
[100] In many embodiments, a calibration of the system is carried out to
provide a
known relationship between the location of a pixel in the orthogonal array of
the image and a
location of the tissue in the treatment space. This known correspondence
between the pixels in
the image and a location in treatment space makes it possible to identify an
expected scan
location in the image corresponding to the predetermined scan location, an
actual scanned
location of a laser scan in the image and a difference between the actual
scanned location and the
expected scan location. The method for performing the calibration is not
particularly limited.
Examples of suitable calibration methods can be found, for instance, in U.S.
Publication No.
U.S. 2014-0128853 Al (U.S. Patent Application. No. 14/069,703, filed November
1, 2013,
entitled "Laser Surgery System Calibration") and U.S. Publication No. 2014-
0316389 Al (U.S.
Patent Application No. 14/191,095, filed February 26, 2014, entitled "Laser
Eye Surgery
System"), the entire contents of which are hereby incorporated by reference
herein in their
26
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
entirety.
[101] In brief, the laser surgery system 10 can be calibrated to relate
locations in a
treatment space with pixels in the camera 62 and with control parameters used
to control the
scanning assembly 18 such that the focal point of the electromagnetic
radiation beam can be
accurately positioned within the intraocular target. Such calibration can be
accomplished at any
suitable time, for example, prior to using the laser surgery system 10 to
treat a patient's eye.
[102] FIG. 7A is a top view diagram of a calibration plate 402 that can be
used to
calibrate the laser surgery system 10. In many embodiments, the calibration
plate 402 is a thin
plate having an array of target features, for example, through holes 404
therein. In alternate
embodiments, the calibration plate 402 is a thin plate having a field of small
dots as the target
features. While any suitable arrangement of the target features can be used,
the calibration plate
402 of FIG. 7A has an orthogonal array of through holes 404. Any suitable
number of the target
features can be included in the calibration plate 402. For example, the
illustrated embodiment
has 29 rows and 29 columns of the through holes 404, with three through holes
at each of the
four corners of the calibration plate 402 being omitted from the orthogonal
array of through
holes 404.
[103] In many embodiments, each of the through holes 404 is sized small
enough to
block a suitable portion of an electromagnetic radiation beam when the focal
point of the
electromagnetic radiation beam is not located at the through hole. For
example, each of the
through holes 404 can have a diameter slightly greater than the diameter of
the focal point of the
electromagnetic radiation beam so as to not block any of the electromagnetic
radiation beam
when the focal point is positioned at one of the through holes 404. In the
embodiment shown,
the through holes 404 have a diameter of 5 [tm, which is sized to be used in
conjunction with a
focal point diameter of 1 [tm.
[104] FIG. 7B schematically illustrates using the calibration plate 402 to
calibrate the
camera 62 of the laser surgery system 10. The calibration plate 402 is
supported at a known
fixed location relative to the objective lens assembly 20. In many
embodiments, the objective
lens assembly 20 is configured for telecentric scanning of the electromagnetic
radiation beam
and the calibration plate 402 is supported to be perpendicular to the
direction of propagation of
the electromagnetic radiation beam. The calibration plate 402 is disposed
between the objective
lens assembly 20 and a light source 406. The light source 406 is used to
illuminate the
27
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
calibration plate 402. A portion of the illumination light from the light
source 406 passes
through each of the through holes 404, thereby producing an illuminated
location within the field
of view of the camera 62 at each of the through holes 404. A light beam 408
from each of the
through holes 404 passes through the objective lens assembly 20, through the
video dichroic 66,
an into the camera 62. In many embodiments, the camera 62 includes a sensor
having an
orthogonal array of pixels (e.g., in x and y directions where the
corresponding z direction is in
the direction of propagation of the electromagnetic radiation beam). In many
embodiments, X
and Y pixel values for each of the light beams 408 is used in conjunction with
the known
locations of the through holes 404 relative to the objective lens assembly 20
to determine the
relationship between the camera X and Y pixel values and locations in the
treatment space for
dimensions transverse to the propagation direction of the electromagnetic
radiation beam.
[105] FIG. 7C schematically illustrates using the calibration plate 402 to
calibrate the
scanning assembly 18. The calibration plate 402 is supported at a known fixed
location relative
to the objective lens assembly 20. In many embodiments, the objective lens
assembly 20 is
configured for telecentric scanning of the electromagnetic radiation beam and
the calibration
plate 402 is supported to be perpendicular to the direction of propagation of
the electromagnetic
radiation beam. The calibration plate 402 is disposed between the objective
lens assembly 20
and a detector 410. The detector 410 is configured to generate a signal
indicative of how much
of the electromagnetic radiation beam is incident thereon, thereby being
indirectly indicative of
how much of the electromagnetic radiation beam is blocked by the calibration
plate 402. For
example, when the focal point of the electromagnetic radiation beam is
positioned at one of the
through holes 404 (as illustrated for the focal point disposed on the right
side of the detection
plate 402 in FIG. 7B), a maximum amount of the electromagnetic radiation beam
passes through
the through hole and is incident on the detector 410. In contrast, when the
focal point of the
electromagnetic radiation beam is not positioned at one of the through holes
404 (as illustrated
for the focal point disposed above the left side of the detection plate 402
in, a portion of the
electromagnetic radiation beam is blocked from reaching the detector 410.
[106] Control parameters for the z-scan device 58 and the xy-scan device 60
are varied
to locate the focal point of the electromagnetic radiation beam at each of a
suitable set of the
through holes, thereby providing data used to determine the relationship
between the control
parameters for the scanning assembly 18 and the resulting location of the
focal point of the
28
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
electromagnetic radiation beam. The z-scan device 58 is operable to vary a
convergence/divergence angle of the electromagnetic radiation beam, thereby
being operable to
control the distance of the focal point from the objective lens in the
direction of propagation of
the electromagnetic radiation beam. The xy-scan device 60 is operable to vary
a direction of the
electromagnetic radiation beam in two dimensions, thereby providing the
ability to move the
focal point in two dimensions transverse to the direction of propagation of
the electromagnetic
radiation beam.
[107] A suitable existing search algorithm can be employed to vary the
control
parameters for the z-scan device 58 and the xy-scan device 60 so as to
reposition the focal point
to be located at each of a suitable set of the through holes 404. In many
embodiments where the
objective lens assembly 20 is configured to telecentrically scan the
electromagnetic radiation
beam, the resulting control parameter data for the scanning assembly 18 can be
used to calibrate
the scanning assembly 18 relative to directions transverse to the direction of
propagation of the
electromagnetic radiation beam (e.g., x and y directions transverse to a z
direction of propagation
of the electromagnetic radiation beam).
[108] Application to Cataract Surgery
[109] In many embodiments, the methods and/or acts of verifying the
location of a laser
scan is used with laser eye surgery systems and methods to verify the
placement of one or more
ocular incisions. In many embodiments, the methods and/or acts are used in
cataract surgery
using a laser eye surgery system for verifying the placement of incisions in a
cataract surgery.
[110] In cataract surgery, a capsulotomy incision, often in the form of a
small round
hole is formed in the anterior side of the lens capsule to provide access to
the lens nucleus.
[111] In addition, cataract surgery may include three types of cornea
incisions: arcuate,
primary, and sideports. Parameters that may be used to define the capsulotomy
include shape
(i.e. circular, elliptical, rectangular or polygonal) and size. The systems
described herein are
designed to receive these parameters based on user or physician's input and
preferably, to
provide a prompt for their input where not received.
[112] Primary incisions and sideport incisions may have the same structure.
They are
generally multiplanar structures that create an opening that allow the
physician access into the
anterior chamber. The primaries are used for insertion of the aspiration tool
and the insertion of
the IOL. Sideport incisions may be used for inserting smaller instrumentation
into the anterior
29
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
chamber. The location and shape of both the primary incisions and the sideport
incisions are
determined by the user parameters and, optionally, by information from a
section scan as
described herein, where the cornea anterior and posterior surfaces may be
modeled by circles.
The anterior and posterior curvatures of the cornea as measured in the
circular fits of the section
scans may optionally be used to position the cuts. Parameters that may be used
to define the
primary cataract incision or the sideport incision are preferably selected
from the group
consisting of limbus offset, width, side cut angle, plane depth, and length.
The systems
described herein are designed to receive these parameters based on user or
physician's input and
preferably, to provide a prompt for their input where not received.
[113] Arcuate incisions may be used to correct a patient's astigmatism. For
instance,
they may adjust the curvature of the cornea to a more spherical shape by means
relaxing stresses
along the meridian on which they are placed. They are parts of a conical
surface that crosses
both the anterior and posterior surfaces of the cornea. In some embodiments,
the anterior
curvature and posterior curvature of the cornea, as measured in a circular fit
to a section scan, are
used to position an "along-the-cut" scan. The along-the-cut scan lays on the
surface of a cone
that transverses the cornea. The arcuate incision can be located within the
along-the-cut scan.
Parameter that may be used to define the arcuate incision may include the size
of the optical
zone, arc length, uncut anterior portion, uncut posterior portion and side cut
angle. The systems
described herein are designed to receive these parameters based on user or
physician's input and
preferably, to provide a prompt for their input where not received.
[114] Capsulotomy Incisions
[115] The laser surgery system 10 can be used to form any suitably shaped
capsulotomy. For example, while the anterior and posterior capsulotomies in
the illustrated
embodiments are circular, any other suitable shape, including but not limited
to, elliptical,
rectangular, and polygonal can be formed. And the anterior and/or posterior
capsulotomy can be
shaped to accommodate any correspondingly suitably shaped IOL.
[116] For example, referring now to FIG. 8, the laser surgery system 10 can
be used to
incise an anterior capsulotomy and/or a posterior capsulotomy in the anterior
portion of a lens
capsule 418. The focal point of the electromagnetic radiation beam can be
scanned to form an
anterior capsulotomy closed incision boundary surface 420 that transects the
anterior portion of
the lens capsule 418. Likewise, the focal point of the electromagnetic
radiation beam can be
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
scanned to form a posterior capsulotomy closed incision boundary surface 430
that transects the
posterior portion of the lens capsule 418.
[117] The anterior and/or posterior closed incision boundary surfaces 420,
430 can be
designated using any suitable approach. For example, a plan view of the
patient's eye can be
obtained using the camera 62. A capsulotomy incision designator 422 can be
located and shown
superimposed on the plan view of the patient's eye to illustrate the size,
location, and shape of a
planned capsulotomy relative to the patient's eye. The capsulotomy incision
designator 422 can
be manually defined by an operator of the laser surgery system 10 and/or the
laser surgery
system 10 can be configured to generate an initial capsulotomy incision
designator 422 for
operator verification and/or modification.
[118] The anterior capsulotomy closed incision boundary surface 420 can be
defined on
a projection of the capsulotomy incision designator 422 such that the anterior
capsulotomy
closed incision boundary surface 420 transects the anterior portion of the
lens capsule 418 at all
locations around the anterior capsulotomy incision boundary surface 420 for
all expected
variations in the location of the anterior portion of the lens capsule 418
relative to the projection
of the capsulotomy incision designator 422. For example, a curve corresponding
to the
capsulotomy incision designator 422 can be projected to define an intersection
with a minimum
depth mathematical surface model (e.g., a spherical surface) defining a
minimum expected depth
configuration for the anterior portion of the lens capsule 418 with the
resulting intersection being
an anterior capsulotomy upper closed curve 424 that defines an upper boundary
for the anterior
capsulotomy closed incision boundary surface 420. Likewise, the curve
corresponding to the
capsulotomy incision designator 422 can be projected to define an intersection
with a maximum
depth mathematical surface model (e.g., a spherical surface) defining a
maximum expected depth
configuration for the anterior portion of the lens capsule 418 with the
resulting intersection being
an anterior capsulotomy lower closed curve 426 that defines a lower boundary
for the anterior
capsulotomy closed incision boundary surface 420. Alternatively, the focal
point can be scanned
using a low imaging-only power level (e.g., a power level sufficient to
provide for imaging of the
intraocular target via processing of the signal generated by the detection
sensor 54 of the
confocal detection assembly 14 without modifying the intraocular target) along
the projection of
the capsulotomy incision designator 422 while varying the depth of the focal
point to determine
the depth of the anterior lens capsule at a sufficient number of locations
around the projection of
31
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
the capsulotomy incision designator 422. The measured depths of the anterior
lens capsule can
then be used to determine suitable anterior capsulotomy upper and lower
boundary curves 424,
426 of the anterior capsulotomy closed incision boundary surface 420.
[119] Corneal Incisions
[120] The laser surgery system 10 can be used to form any suitably shaped
arcuate,
primary or sideport incisions.
[121] FIGS. 9A through 9C illustrate aspects of arcuate incisions of a
cornea that can be
formed by the laser surgery system 10, according to many embodiments. FIG. 9A
shows an en
face view of arcuate incisions within the optical zone of the cornea that can
be formed using the
laser surgery system 10. The optical zone can be user-adjustable within, for
example, the range
of 2mm-l1mm. For asymmetric arcuate incisions, the optical zone can be
independently
adjustable for each incision. Arc length can be user-adjustable within, for
example, the range of
-120 .
[122] FIG. 9B shows a cross-sectional view of an arcuate incision in the
cornea that can
be formed using the laser surgery system 10 and that penetrates the cornea
anterior surface and
has an uncut posterior portion. FIG. 9C shows a cross-sectional view of an
arcuate intrastromal
incision in the cornea that can be formed using the laser surgery system 10.
The arcuate
intrastromal incision has an uncut anterior portion and an uncut posterior
portion. Side cut angle
can be user-adjustable within, for example, the range of 30 - 150 . Uncut
posterior and anterior
portions can be user-adjustable within, for example, the range of 100i.tm -
250iim or 20% - 50%
of the cornea thickness. Cornea thickness can be measured at the projected
intersection of the
incision with the cornea anterior/posterior measured at 90 to
anterior/posterior cornea surface
regardless of what side cut angle is chosen.
[123] FIG. 10A shows an en face view of a primary cataract incision in the
cornea that
can be formed using the laser surgery system 10. The primary cataract incision
provides access
to surgical tools used to, for example, remove a fragmented crystalline lens
nucleus and insert in
an IOL. FIG. 10B shows a cross-sectional view of a primary cataract incision
of the cornea that
can be formed using the laser surgery system 10. Limbus offset can be user-
adjustable within,
for example, the range of 0.0mm-5.0mm. Width can be user-adjustable within,
for example, the
range 0.2mm-6.5mm. Length can be user-adjustable within, for example, the
range of
0.5mm-3.0mm. Side Cut Angle can be user-adjustable within, for example, the
range of 30 -
32
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
150 . Plane depth can be user-adjustable within, for example, the range of
125i.tm-375iim or
25%-75% of the cornea thickness. Length can be defined as the en face view
distance between
the projected incision intersection with the cornea anterior and the cornea
posterior. FIG. 10C
shows a cross-sectional view of a primary cataract incision that includes an
uncut anterior
portion. FIG. 10D shows a cross-sectional view of a primary cataract incision
that includes an
uncut posterior portion. FIG. 10E shows a cross-sectional view of a primary
cataract incision
that includes an uncut central length. And FIG. 1OF shows a cross-sectional
view of a primary
cataract incision that includes no uncut portion. Side Cut Angle can be user-
adjustable within,
for example, the range of 30 -150 . Uncut central length can be user-
adjustable within, for
example, the range of 25i.tm-1000i.tm.
[124] FIG. 11A shows an en face view of a sideport cataract incision in the
cornea that
can be formed using the laser surgery system 10. The sideport cataract
incision provides access
for surgical tools used, for example, to assist in the removal of a fragmented
crystalline lens.
FIG. 11B shows a cross-sectional view of a sideport cataract incision of the
cornea that has an
uncut posterior portion and can be formed using the laser surgery system 10.
Limbus offset can
be user-adjustable within, for example, the range of 0.0mm-5.0mm. Width can be
user-
adjustable within, for example, the range 0.2mm-6.5mm. Length can be user-
adjustable within,
for example, the range of 0.5mm-3.0mm. FIG. 11C shows a cross-sectional view
of a sideport
cataract incision that includes an uncut anterior portion. FIG. 11D shows a
cross-sectional view
of a sideport cataract incision that includes an uncut central length. And
FIG. 11E shows a
cross-sectional view of a sideport cataract incision that includes no uncut
portion. Side Cut
Angle can be user-adjustable within, for example, the range of 30 -150 . Uncut
central length
can be user-adjustable within, for example, the range of 100m-250i.tm or 20%-
50% of the
cornea thickness. Cornea thickness can be measured at the projected
intersection location of the
incision with the cornea anterior/posterior measured at 90 to the
anterior/posterior cornea
surface regardless of what side cut angle is chosen.
[125] Video and Confocal Imaging of Incision Locations
[126] Although many different imaging techniques may be used in different
embodiments, a combination of video/camera imaging and confocal imaging based
on pulsed
laser raster scanning of the tissue to be treated is preferred.
33
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
[127] As illustrated in the embodiment of Fig. 1, video imaging of the
tissue to be
treated, preferably a human eye, can be achieved by a camera 62 and associated
video
illumination 64 integrated with the scanning assembly 18. The camera 62 and
the beam 28 share
a common optical path through the objective lens assembly 20 to the eye. A
video dichroic 66 is
used to combine/separate the beam 28 with/from the illumination wavelengths
used by the
camera. In one embodiment, the beam 28 can have a wavelength of between 320
and 370 nm,
preferably about 355 nm, and the video illumination 64 can be configured to
emit illumination
having wavelengths greater than 370 nm, or more than 400 or more than 450 nm.
Accordingly,
the video dichroic 66 can be configured to reflect the beam between 320 and
370 nm wavelength
while transmitting wavelengths greater than 370 nm, thus facilitating video
imaging of the eye
without interference from beam 28. The resulting video image is preferably an
en face image as
shown in Fig. 13. The location(s) of the capsulotomy incision and any corneal
incision specified
by the physician can be projected onto the video image prior to treatment as
expected scan
locations for each respective incision.
[128] In many embodiments, the imaging of the eye 24 further includes
confocally
imaging one or more portions of the tissue, preferably the eye, to be treated.
Any suitable
device, assembly, and/or system, such as described herein, can be used to
confocally image one
or more portions of the eye or other tissue to be imaged. The confocal imaging
methods used
herein generally include using a beam source, preferably a pulsed laser
source, to generate an
electromagnetic radiation beam; propagating the electromagnetic radiation beam
to a scanner
along an optical path to the eye; focusing the electromagnetic radiation beam
to a focal point at a
location within the eye; using the scanner to scan, preferably raster scan,
the focal point to
different locations within the eye; propagating a portion of the
electromagnetic radiation beam
reflected from the focal point location back along the shared optical path to
a sensor; and
generating an intensity signal indicative of the intensity of a portion of the
electromagnetic
radiation beam reflected from the focal point location and propagated to the
sensor. The method
can include modifying polarization of at least one of the electromagnetic
radiation beam and a
portion of the electromagnetic radiation beam reflected from the focal point
location. The
method can include using the polarization-sensitive device to reflect a
portion of the
electromagnetic radiation beam reflected from the focal point location so as
to be incident upon
the sensor.
34
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
[129] Based on the calibration of the system described herein, the focal
point location of
the confocally detected light can be related to the physical location of the
focal point within the
eye, and the location within the eye and the magnitude of the intensity at
each location can be
used to identify boundaries, edges and layers within the eye. Boundaries,
edges and layers may
be located in a confocal image by, for instance, Delaunay triangulation and
Dijkstra
segmentation. These confocal images, including the boundaries, edges and
layers can then be
displayed to a user as a graphical representation of the areas of the eye to
be treated.
[130] In many embodiments, the lens capsule, and optionally a portion or
all of the lens,
are imaged using confocal imaging, and preferably, these portions include the
area of the lens
capsule where the capsulotomy will be placed. In general, the parameters
necessary to define the
capsulotomy are input by a user or physician, and a raster scan with a pulsed
laser beam sweeps
through the relevant portion of the lens capsule for imaging the lens capsule.
Based on the
recorded location and magnitude of the confocally reflected intensity
measurements at each
location, the capsule is identified by image recognition, such as by Delaunay
triangulation and
Dijkstra segmentation, and the capsule shape is fit to the segmented image.
The resulting
confocal image of lens may then be shown to the physician for use in
visualizing the
capsulotomy incision.
[131] In many embodiments, the methods and systems may include confocally
imaging
a cornea by scanning one or more of portions of the cornea where a primary
incision, sideport
incision or arcuate incision is to be placed. In a preferred embodiment, one
sectional image of
the cornea is performed for each selected corneal incision. These images are
preferably in the
form of a section scan. As shown in FIG. 14, a section scan crosses cornea 500
along plane 510
and measures the confocal intensity at every location of a pulsed laser during
the scan.
Preferably, a section scan 510 comprises a raster scan of a pulsed laser beam
along the cornea
500, including the anterior surface 501 and posterior surface 502, on a
vertical plane 510
centered at the cornea incision center and oriented along an incision's
meridian. The trajectory
goes from deep to shallow, inside the eye, crossing the cornea. The posterior
and anterior
boundaries of the cornea may be identified in the image by, for instance,
Dijkstra segmentation
of the image, and the resulting image may be provided to the user.
[132] If the selected corneal incision is an arcuate incision, an "along-
the-cut" imaging
scan is also preferably performed. An along-the-cut imaging scan may assist a
physician in
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
choosing the correct location for the arcuate incision in order to maintain an
adequate depth and
avoid posterior penetration. The "along the cut" scan preferably has the same
conical shape as
the arcuate incision and is inclusive of the entire area to be covered arcuate
incision. The conical
sector in the "along the cut" scan is mapped into a rectangular domain 520
defined by the conical
coordinates. The resulting conical image is segmented and fit. Optionally, the
resulting fits to
the anterior and posterior surfaces of the cornea are used to construct the
arcs, which can then be
overlaid on their sections and "along the cut" scans.
[133] In many embodiments, the optical surface of the eye is fit with one
or more with
one or more of a Fourier transform, polynomials, a spherical harmonics, Taylor
polynomials, a
wavelet transform, or Zernike polynomials. The optical tissue surface may
comprise one or
more of the anterior surface of the cornea, the posterior surface of the
cornea, the anterior surface
of the lens capsule, the posterior surface of the lens capsule, an anterior
surface of the lens
cortex, a posterior surface of the lens cortex, an anterior surface of the
lens nucleus, a posterior
surface of the lens nucleus, one or more anterior surfaces of the lens having
a substantially
constant index of refraction, one or more posterior surfaces of the lens
having a substantially
constant index of refraction, the retinal surface, the foveal surface, a
target tissue surface to
correct vision such as a target corneal surface, an anterior surface of an
intraocular lens, or a
posterior surface of an intraocular lens, for example.
[134] Generating a Treatment Scan
[135] After the relevant portions of the lens, lens capsule and cornea have
been imaged,
the incisions defined by the physician parameters may be projected onto the
image, and a
treatment scan of the laser light beam is generated. The treatment scan
preferably consists of a
continuous set of x, y, z points arranged in space that are designed to carry
out the incisions
defined by the user. The location of the treatment scans are projected onto at
least one of the
video and confocal images in order to define the set of expected scan
locations of the incisions.
[136] Detecting an Actual Location of a Scan by Luminescence
[137] Certain components of eye tissue absorb light having wavelengths of
370 nm and
less and emit red-shifted light (due for instance, to either fluorescence or
phosphorescence) at
wavelengths greater than 370 nm. The emitted light from the eye tissue is also
passed by
dichroic 66 in FIG. 12. Thus, when the focal point of beam 28 is scanned
across the tissue to be
treated, the location of the focal point of beam 28 within the target tissue
can be tracked by
36
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
camera 62 based on the known relationship between the pixels of the camera 62
and the location
of the focal point in the treatment space as established by the calibration
described above.
[138] FIG. 12 schematically illustrates using a luminescence from an eye 24
to obtain a
video image of the actual location of a laser scan. Eye 24 includes one or
more components that
emit light in response to absorbing electromagnetic radiation at wavelengths
preferably less than
370 nm. The eye 24 is preferably connected to the objective 20 and scanning
assembly 18 via
patient interface 22. Light from light source 12 is directed to eye 24 via the
confocal assembly
14, the shared optics 16 the scanner 18 and the objective 20. With the focal
point of the
electromagnetic radiation beam from light source 12 disposed, preferably
sequentially, within the
eye, the camera 62 is used to detect the actual location of the resulting
emission from eye 24
based on the position of the focal point within eye 24. The luminescence is
generally detected as
one or more pixels, Pact. The observed location of the resulting fluorescent
emission can be used
in conjunction with calibration data for the camera 62 to determine x and y
coordinates of the
associated focal point in the treatment space and can be compared to the
expected scan location
of the incisions.
[139] The camera image of the eye is preferably presented to a user as an
en face image,
such as shown in Fig. 13 with the pixels, Pact, corresponding to the actual
location of the scan
illuminated on the image.
[140] The above described methods and systems permit a physician to verify
the actual
scan location of a contemplated incision in comparison to its expected
location and also to
confirm that the laser surgical system is adequately calibrated. For example,
if the physician
desired to make a cut at a predetermined position in an eye, the physical need
only enter the
necessary parameters to define the location and type of incision the physician
intends to make.
In one embodiment, the laser surgical system of the present invention is
configured to receive
these parameters and to project the defined incision onto a video image. In
some embodiments,
the video image then illustrates the expected position of the incision on the
image, by, for
instance, illuminating a set of Pixels, PEL, corresponding to the intended
location of the incision.
The laser system is also preferably configured to carry out a treatment scan
configured to make
the incision at the predetermined location. As the pulsed laser scans the
tissue, a resulting
luminescence from the location of the treatment scan is detected and
subsequently used to
identify the actual location in the eye where the treatment scan was
performed. Preferably, the
37
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
actual location is illustrated on the video image by illuminating a set of
pixels, Pact,
corresponding to the position of the actual scan. Thus, in some embodiments,
if the actual
location of the scan differs from the expected location, the physician can
visually make this
determination by inspection of the video image.
[141] In another embodiment, a warning is issued if a difference between
the actual
location and the expected location is greater than a predetermined threshold
amount. This makes
it possible to warn a physician or user, or stop the scan completely, even if
the physician is not
actively viewing the image.
[142] Preferably, the system and methods are used throughout the entirety
of the
treatment scan. Specifically, in some embodiments, the progression of the
treatment scan is
monitored by successive images/frames captured during the treatment. In a
preferred
embodiment, successive frames of the image capture the progression of the
treatment scan in real
time. In some embodiments, the difference in detected luminescence between
frames track the
actual location and actual direction of the treatment scan. For example, when
a confocal scan is
being taken, a video is taken at the same time. In this manner, the system and
methods can
ensure that the entirety of the incision is placed at its expected location.
[143] The methods described herein also provide a convenient method for
confirming
that a laser eye surgery system is adequately calibrated. With conventional
imaging, a number of
safeguards are generally in place to ensure proper calibration; however, a
physician may have
limited convenient procedures for determining whether the instrument is
calibrated. The present
invention allows the physician or other user to quickly assess the calibration
of the laser surgical
system.
[144] It is also noted that the present invention provides a safeguard
should the
physician inadvertently type in the wrong coordinates for his cuts. In that
scenario, the
calibration would not necessarily be wrong but the physician would notice that
the cutting was
not taking place in the correct locations. This event would presumably prompt
the physician to
double check to see if he typed in the correct geometric coordinates. Further,
this method would
provide a safeguard should the calibration be off before a procedure by the
inadvertent bumping
of the camera or things of that nature.
[145] In sum, many embodiments provide a method or system that detects an
actual
location of a laser scan within an object and verifies whether the laser scan
is at the expected
38
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
location. Other embodiments provide a method or system that detects an actual
placement of an
ocular incision within an eye and verifies whether the ocular incision is at
the intended location.
Other embodiments provide a method of verifying the calibration of a laser eye
surgical system.
[146] Alignment Examples
[147] In certain examples, when an operator is utilizing the systems above,
the operator
may only be able to view the patient eye through a camera image. In certain
examples, this may
be a view of just the eyeball and no other reference features. Without other
references, a user
may become disoriented as to the direction the image of the eye is relative to
the patient, and
even which eye is being viewed. In certain example embodiments, it may be
useful to include
fiducials in the patient interface, so that a user may be able to better
orient the image to the
patient.
[148] Referring now to FIG. 15A, various embodiments of the liquid optics
patient
interface 22 are discussed. The patient interface 22 allows for a liquid
optics docking to take
place between the eye 24 and the system 2 for conducting laser procedures on a
patient's eye as
described above.
[149] The patient interface 22 may be placed on a patient during procedure,
and can
hold the liquid used as the interface for the laser systems described above.
But during the
procedure, the surgeon user may view the patient interface 22 and the
patient's eye through a
camera arrangement. In doing so, the surgeon may become disoriented as to the
eye's orientation
in the patient's head. In order to help the surgeon to understand the
orientation of the patient's
eye, it may be useful to indicate orientation markers on the patient interface
itself, because the
eyeball, when viewed through the camera arrangement, may not indicate any type
of orientation.
[150] Thus, from the top down, a video camera 62 can capture an image of
the eye 24
and the patient interface 22. With the addition of various fiducials inside
the patient interface,
within the field of view of a camera looking down at the eye 24 and the
patient interface 22, a
surgeon or other operator could more easily understand the orientation of the
eye 24 to the
patient interface 22 and its relation to the ear 1532 and nose 1530 of the
patient.
[151] Any of various steps, dots, lines, arrows, notches, and/or other
fiducials could be
used in the patient interface 22 to show such orientation. Examples include
but are not limited to
raised steps, indented steps, colored indicators, lines, etched grooves,
raised bumps, notches,
arrows, boxes and/or any combination of these or other indicators.
39
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
[152] In certain example embodiments, the patient interface 22 may have a
rim 1510
and a cup for containing the liquid interface. The patient interface 22 may
include an optional
tool access portion 1520 which may extend laterally from the rim 1510 of the
patient interface
22. This tool access portion 1520 may be used in some embodiments and may be a
tunnel
through which the surgeon may insert tools to access the eye 24 during
procedure. In such
embodiments, this tool access portion 1520 requires the proper amount of space
for operation of
the various tools, it should be orientated on the patient so that the tool
access portion 1520 is
pointed temporally, toward the temple of the patient and not toward the bridge
of the nose of the
patient. This may allow for enough room for the surgical tools to access the
tool access portion
1520 from the side of the head area.
[153] In one example, as shown in FIG. 15A, a large step 1540 is shown. In
some
embodiments, this step 1540 is close to the tool access portion 1520 if one is
used. Certain
embodiments include steps at various intervals to help orientation. In one
example, at 90 degree
intervals, around the inside of the rim 1510 of the patient interface 22,
smaller steps 1542 are
shown. In this way, a surgeon looking down at the patient interface 22 would
quickly orient the
surgeon to the position of the interface 22 on the eye 24.
[154] In the example embodiment shown in of FIG. 15B, instead of four steps
at 90
degree intervals, only one large step 1540 is shown. In embodiments using a
tool access portion
1520, this large step is oriented near the tool access port 1520 so an
operator could orient the
patient interface 22 on the patient, with the nose 1530 and ear 1532 in the
correct orientation. It
is shown that the patient interface 22 is orientated with the tool access
portion 1520 orientated
toward the temple or ear 1532 of the patient and not toward the nose 1530 of
the patient. In
embodiments without such tool access portion, the steps can be used to orient
the interface 22 to
the patient.
[155] FIG. 15C shows another example where instead of a step, there is a
notch 1544
cut out of an interior rim 1546 of the patient interface. In some examples,
this notch is located
near the tool access portion 1520 just as the large step was located in FIG.
15A and FIG. 15B.
Again, certain embodiments may not use a tool access portion in which case the
notches 1544
may be used to merely indicate the patient orientation. In various
embodiments, any number of
notches could be arranged of various sizes and colors, in any combination.
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
[156] FIG. 15D shows an example side view of the patient interface 22
attached to a
patient eye 24 as for procedure. The nose 1530 and ear 1532 of the patient are
again shown for
orientation purposes. In this example side view, the tool access portion 1520
is properly oriented
toward the ear 1532 of the patient. In examples without such tool access
portions, the interface
may orient to the patient for the laser system only. The rim 1510 of the
patient interface 22 is
shown with the system 2 configured to dock with the patient interface 22 for
procedure.
[157] In the example, a large step 1540 is shown as a step of an interior
portion of the
patient interface 22. The large step 1540 is oriented to the patient interface
22 in the same
direction as the tool access portion 1520 if such a portion exists. Because
the large step 1540 is
positioned in line with the tool access portion 1520, the user looking down,
from the perspective
of the system 2 would be able to orient the image and know which direction the
patient interface
is oriented. In embodiments using a tool access portion 1520, such a portion
may be indicated by
the step 1540 as it may stick out of the patient interface 22.
[158] Other variations are within the spirit of the present invention.
Thus, while the
invention is susceptible to various modifications and alternative
constructions, certain illustrated
embodiments thereof are shown in the drawings and have been described above in
detail. It
should be understood, however, that there is no intention to limit the
invention to the specific
form or forms disclosed, but on the contrary, the intention is to cover all
modifications,
alternative constructions, and equivalents falling within the spirit and scope
of the invention, as
defined in the appended claims.
[159] The use of the terms "a" and "an" and "the" and similar referents in
the context of
describing the invention (especially in the context of the following claims)
are to be construed to
cover both the singular and the plural, unless otherwise indicated herein or
clearly contradicted
by context. The terms "comprising," "having," "including," and "containing"
are to be construed
as open-ended terms (i.e., meaning "including, but not limited to,") unless
otherwise noted. The
term "connected" is to be construed as partly or wholly contained within,
attached to, or joined
together, even if there is something intervening. Recitation of ranges of
values herein are merely
intended to serve as a shorthand method of referring individually to each
separate value falling
within the range, unless otherwise indicated herein, and each separate value
is incorporated into
the specification as if it were individually recited herein. All methods
described herein can be
performed in any suitable order unless otherwise indicated herein or otherwise
clearly
41
CA 03022457 2018-10-26
WO 2017/188936 PCT/US2016/029368
contradicted by context. The use of any and all examples, or exemplary
language (e.g., 'such
as") provided herein, is intended merely to better illuminate embodiments of
the invention and
does not pose a limitation on the scope of the invention unless otherwise
claimed. No language
in the specification should be construed as indicating any non-claimed element
as essential to the
practice of the invention.
[160] While certain illustrated embodiments of this disclosure have been
shown and
described in an exemplary form with a certain degree of particularity, those
skilled in the art will
understand that the embodiments are provided by way of example only, and that
various
variations can be made without departing from the spirit or scope of the
invention. Thus, it is
intended that this disclosure cover all modifications, alternative
constructions, changes,
substitutions, variations, as well as the combinations and arrangements of
parts, structures, and
steps that come within the spirit and scope of the invention as generally
expressed by the
following claims and their equivalents.
[161] It is intended that the following claims define the scope of the
invention and that
methods and structures within the scope of these claims and their equivalents
be covered thereby.
It is to be understood that the present invention is not limited to the
embodiment(s) described
above and illustrated herein, but encompasses any and all variations
explicitly and implicitly
derived therefrom. Although not shown in the figures, multiple imaging steps
can also be
employed in between treatment steps to account for any changes in position
and/or size due to
treatment and further insure the accurate disposition of laser energy in the
target tissue.
42