Note: Descriptions are shown in the official language in which they were submitted.
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
CYCLOPROPYL-AMIDE COMPOUNDS AS DUAL LSD1/HDAC INHIBITORS
TECHNICAL FIELD
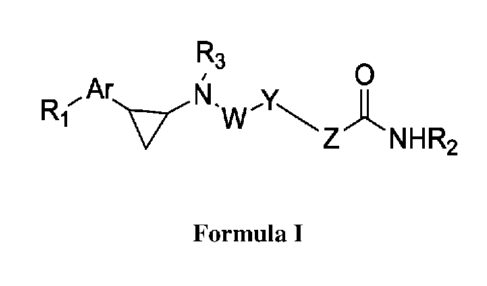
[0001] Described are novel derivatives of the Formula (I), their analogs,
tautomeric
forms, stereoisomers, geometrical isomers, polymorphs, hydrates, solvates,
pharmaceutically acceptable salts, pharmaceutical compositions, metabolites,
and
prodrugs thereof.
R3
0
Ar N. A
Ri' w
Z NHR2
(I)
Also, described herein is the process for the preparation of the above said
novel
derivatives of the Formula (I), their analogs, stereoisomers, diastereomers,
polymorphs,
hydrates, solvates, pharmaceutically acceptable salts, pharmaceutical
compositions,
metabolites, prodrugs, and intermediates useful in the preparation of such
compounds.
[0002] The compounds described herein are dual inhibitors of lysine specific
demethylase (LSD) and histone deacetylase (HDAC) and also arrest cell growth
in
neoplastic cells, thereby inhibiting proliferation. These compounds can be
used as
prophylactic or therapeutic agents for treating cancer, schizophrenia,
Alzheimer's
disease, Parkinson's disease, and the like.
BACKGROUND
[0003] Transcriptional regulation is a major event in cell differentiation,
proliferation
and apoptosis. Transcriptional activation of a set of genes determines
cellular function
and is tightly regulated by a variety of factors. One of the regulatory
mechanisms
involved in this process is an alteration in the tertiary structure of DNA,
which affects
transcription factors to their target DNA regiments. Nucleosomal integrity is
regulated
by the acetylation status of the core histone, with the result being
permissiveness to
transcription. The regulations of transcription factor are thought to involve
changes in
the structure of chromatin. Changing the affinity of histone proteins for
coiled DNA in
the nucleosome alters the structure of chromatin. Hypoacetylated histones are
believed
to have greater affinity to the DNA and form a tightly bound DNA-histone
complex and
render the DNA inaccessible to transcriptional regulation. The acetylating
status of the
histone is governed by the balanced activities of the histone acetyl
transferase (HAT)
and histone deacetylase (HDAC).
- 1 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
[0004] Human histone deacetylases (HDACs) are classified into two distinct
classes,
the HDACs and sirtuins. The HDACs are divided into two subclasses based on
their
similarity to yeast histone deacetylases, RPD 3 (class I includes HDAC 1, 2,
3, and 8)
and Hda 1 (class II includes HDAC 4, 6, 7, 9, and 10). All the HDACs have a
highly
conserved zinc dependent catalytic domain. There is growing evidence that the
acetylation state of proteins and thus the HDAC enzyme family plays a crucial
role in
the modulation of several biological processes, including transcription and
cell cycle.
Several structural classes of HDAC inhibitors have been identified and are
reviewed in
Marks et al., J. Natl. Cancer Inst., 2000, 92, 1210-1215; L. Zhang, eLaL,
Medicinal
Research Reviews, 2015, 35, 63-84; P.K. Agrawala, eLaL, HOAJ Biology 2013, 2,
1-
8. Other compounds that are able to inhibit HDAC activity are Trichostatin A
(TSA),
PXD101, Tropoxin (TPX), Sodium butyrate (NaB), Sodium valproate (VPA), Cyclic
hydroxamic acid containing peptides (CHAPs), Depsipeptide FK-228, MGCD0103
and MS-275. The above mentioned inhibitors can also de-repress tumor
suppressor
genes (e.g. p2lwafl/cf 1), resulting in antiproliferative effects in vitro and
anti tumor
effects in vivo. At present, there are four HDAC inhibitors that have been
approved by
FDA for the treatment of various cancers. Vorinostat, Isotdax and Belinostat
have been
approved for the treatment of Cutaneous T-Cell Lymphoma and panibinostat has
been
approved for the treatment of multiple myeloma.
[0005] Another group of enzymes known as lysine methyl transferases and lysine
demethylases are involved in the modulation of histone methylation. Lysine
demethylases (LSD1 and LSD2) are known to remove methyl group from mono and
dimethylated Lys4 of histone H3 (H3K4me1/2) through flavin adenine
dinucleotide
(FAD) dependent enzymatic oxidation and releasing formaldehyde as the
byproduct.
LSD1 mediated demethylation is not restricted to histones; other non-histone
substrates such as p53, STAT3, E2F1, and MYPT1 are also demethylated leading
to a
change in cellular functions. LSD1 is overexpressed in various cancer cells
and tissues,
neuroblastoma, prostate cancer, breast cancer, leukemia, lung cancer and
bladder
cancer cells. It is known that either inhibition of LSD1 with small molecule
or by
RNAi is associated with inhibition of cancer cell growth by modulating
prosurvival
gene expression and p53 transcriptional activity. Several novel irreversible
inhibitors
of LSD1 have been described in literature and two compounds ORY-1001 and GSK-
2879552 have entered phase 1 clinical trial, (N. Miyata, eLaL, J. Med.Chem,
54, 8236-
8250, 2011; R.P. Clausen, eLaL, Bioorg.Med.Chem., 19, 3625-3636, 2011; J. W.
- 2 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Hyjfeldt, et.al., Nature Drug Discovery, 12, 917-930, 2013, Manfred Jung and
et.al.,
Clinical Epigenetics (2016) 8:57).
[0006] Another recent report suggests that a cross talk between LSD1 and HDAC
is
associated with changes in gene expression that leads to growth inhibition and
apoptosis (Huanget.cd.Carcinogenesis, 34, 1196-1207, 2013). This and other
similar
studiessuggest that the inhibition of both LSD1 and HDAC can exhibit
synergyism in
modulating gene expression and in inducing growth inhibition. Singh, et al.,
(Neuro-
Oncology, 13, 894-903, 2011) have demonstrated that combined inhibition of
LSD1
and HDAC can lead to cooperative regulation of key pathways of cell death in
glioblastoma multiforme (GBM, a form of aggressive brain tumor). Fiskus, et
al.,
(Leukemia, 1-10, 2014) have shown that combined treatment of LSD1 inhibitor
SP2509 and HDAC inhibitor panobinostat was synergistically lethal against
cultured
and primary AML blasts. In mice engrafted with human AML cells, combined
treatment of both SP2509 and panobinostat significantly improved the survival
compared with either SP2509 or Panobinostat.
[0007] Cole, et al., have disclosed LSD1/HDAC dual inhibitors and their
utility in
treating various disease conditions or disorders (US2017/0029366).
[0008] Although, there are several chemotherapies and target therapies based
drugs
for cancer, an effective cure for cancer still remains elusive. Further,
development of
acquired resistance and disease relapse are major issues that still need to be
addressed.
Therefore, there is a need for novel mechanism-based approaches in the
treatment of
cancer, that would have a stronger effect on a signaling pathway and/or affect
multiple
pathways and mutually exclusive mechanisms in the cells. In this regard, novel
dual
inhibitors of LSD-1/HDAC will have better efficacy in treating multiple
cancers
compared to either treating with LSD-1 or HDAC inhibitors alone.
OBJECTIVE
[0009] One objective herein is to provide a compound of FFormula (I) their
analogs,
tautomeric forms, stereoisomers, polymorphs, solvates, intermediates,
pharmaceutically
acceptable salts, metabolites, and prodrugs thereof.
R3
0
Ar k .11
Z NHR2
(I)
- 3 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Another objective herein is to provide a pharmaceutical composition with the
novel
derivatives of the Formula (I).
[00010] Yet another objective herein is to provide a method of preventing or
treating
proliferative diseases by administering a therapeutic amount of novel compound
of the
Formula (I) or a pharmaceutically acceptable salt and/or prodrug.
SUMMARY
[00011] The present disclosure describes compound of Formula I
R3
0
Ar 11. .Y
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof,
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C 1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocycly1õC3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C 1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7-12 -alkylaryl, C7-12 -alkenylaryl, C7-15 -arylalkenyl, C2-12-
alkylheteroaryl,
-C 0-C 7_12 alkylaryl, -C 0-C 7_12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-
C1_8 alkyl-, -
NR6-C1_8 alkyl, -0-C 1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1-6
heteroaryl, C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C
7-12
- 4 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
alkenylaryl, -S02-05_6 aryl, -S02-C7_12 alkylaryl, -NR6S02-C7-12 alkylaryl,
C1_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, Ci_8 alkyl, Ci_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen, hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, Ci_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, Ci_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRc, -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, RE, and Rc
is
independently selected from the group consisting of hydrogen, Ci_8 alkyl, C3_8
cycloalkyl, C5-6 aryl, C7-15 arYlalkYl, C2-10 heterocyclyl, Ci_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkoxy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;R7 is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6
aryl, C2-10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6heteroaryl, C3_8 cycloalkyl, and C2-10heterocyclyl.
[00012] These and other features, aspects, and advantages of the present
subject matter
will become better understood with reference to the following description.
This
summary is provided to introduce a selection of concepts in a simplified form.
This
summary is not intended to identify key features or essential features of the
disclosure,
nor is it intended to be used to limit the scope of the subject matter.
- 5 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
BRIEF DESCRIPTION OF THE ACCOMPANYING DRAWINGS
[00013] The following drawings form part of the present specification and are
included to further illustrate aspects of the present disclosure. The
disclosure may be
better understood by reference to the drawings in combination with the
detailed
description of the specific embodiments presented herein.
[00014] Figure 1 depicts the modulation of Tubulin and Histone Acetylation in
MM. 1S cells, in accordance with an embodiment of the present disclosure.
[00015] Figure 2 depicts the efficacy study in multiple myeloma model, in
accordance
with an embodiment of the present disclosure.
[00016] Figure 3 depicts the modulation of CD86 and CD1 lb in MV411 cells, in
accordance with an embodiment of the present disclosure.
DETAILED DESCRIPTION
[00017] Those skilled in the art will be aware that the present disclosure is
subject to
variations and modifications other than those specifically described. It is to
be
understood that the present disclosure includes all such variations and
modifications.
The disclosure also includes all such steps, features, compositions and
compounds
referred to or indicated in this specification, individually or collectively,
and any and
all combinations of any or more of such steps or features.
Definitions
[00018] For convenience, before further description of the present disclosure,
certain
terms employed in the specification, and examples are collected here. These
definitions
should be read in the light of the remainder of the disclosure and understood
as by a
person of skill in the art. The terms used herein have the meanings recognized
and
known to those of skill in the art, however, for convenience and completeness,
particular terms and their meanings are set forth below.
[00019] The articles "a", "an" and "the" are used to refer to one or to more
than one
(i.e., to at least one) of the grammatical object of the article.
[00020] The terms "comprise" and "comprising" are used in the inclusive, open
sense,
meaning that additional elements may be included. Throughout this
specification,
unless the context requires otherwise the word "comprise", and variations,
such as
"comprises" and "comprising", will be understood to imply the inclusion of a
stated
element or step or group of element or steps but not the exclusion of any
other element
or step or group of element or steps.
- 6 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
[00021] The term "including" is used to mean "including but not limited to".
"Including" and "including but not limited to" are used interchangeably.
[00022] In the structural formulae given herein and throughout the present
disclosure,
the following terms have been indicated meaning, unless specifically stated
otherwise.
[00023] Furthermore, the compound of Formula (I) can be its derivatives,
analogs,
tautomeric forms, stereoi somer' s, diastereomers, geometrical isomers,
polymorphs,
solvates, intermediates, metabolites, prodrugs or pharmaceutically acceptable
salts and
compositions.
[00024] The compounds described herein may contain one or more chiral centers
and/or double bonds and therefore, may exist as stereoisomers, such as double-
bond
isomers (i.e., geometric isomers), regioisomers, enantiomers or diastereomers.
Accordingly, the chemical structures depicted herein encompass all possible
enantiomers and stereoisomers of the illustrated or identified compounds
including the
stereoisomerically pure form (e.g., geometrically pure, enantiomerically pure
or
diastereomerically pure) and enantiomeric and stereoisomeric mixtures.
Enantiomeric
and stereoisomeric mixtures can be resolved into their component enantiomers
or
stereoisomers using separation techniques or chiral synthesis techniques well
known to
the person skilled in the art. The compounds may also exist in several
tautomeric forms
including the enol form, the keto form and mixtures thereof. Accordingly, the
chemical
structures depicted herein encompass all possible tautomeric forms of the
illustrated or
identified compounds. It is also understood that some isomeric form such as
diastereomers, enantiomers and geometrical isomers can be separated by
physical
and/or chemical methods and by those skilled in the art. Pharmaceutically
acceptable
solvates may be hydrates or comprising of other solvents of crystallization
such as
alcohols, ether, and the like.
[00025] The term "solvate", as used herein, refers to a crystal lattice which
contains
solvent.
[00026] The term "hydrate" refers to a more specific form of solvate, wherein
the
solvent is water.
[00027] As used herein, the term "substituted" is contemplated to include all
permissible substituents of organic compounds. In a broad aspect, the
permissible
substituents include acyclic and cyclic, branched and unbranched, carbocyclic
and
heterocyclic, aromatic and nonaromatic substituents of organic compounds.
Illustrative
substituents, for example, include those described herein above. The
permissible
- 7 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
substituents can be one or more and the same or different for appropriate
organic
compounds. For purposes of this disclosure, the heteroatoms such as nitrogen
may
have hydrogen substituents and/or any permissible substituents of organic
compounds
described herein which satisfy the valences of the heteroatoms.
[00028] The term "polymorphs" refers to crystal forms of the same molecule,
and
different polymorphs may have different physical properties such as, for
example,
melting temperatures, heats of fusion, solubilities, dissolution rates and/or
vibrational
spectra as a result of the arrangement or conformation of the molecules in the
crystal
lattice.
[00029] The term "prodrugs" refers to the precursor of the compound of Formula
(I),
which on administration undergoes chemical conversion by metabolic processes
before
becoming active pharmacological substances. In general, such prodrugs will be
functional derivatives of a compound of the invention, which are readily
convertible in
vivo into a compound of the invention.
[00030] The term "alkyl" refers to straight or branched aliphatic hydrocarbon
groups
having the specified number of carbon atoms, which are attached to the rest of
the
molecule by a single atom, which may be optionally substituted by one or more
substituents. Preferred alkyl groups include, without limitation, methyl,
ethyl, n-
propyl, isopropyl, butyl, isobutyl, t-butyl, pentyl, hexyl, heptyl, octyl and
the like.
[00031] The term "aryl" refers to aromatic radicals having 6 to 14 carbon
atoms, which
may be optionally substituted by one or more substituents. Preferred aryl
groups
include, without limitation, phenyl, naphthyl, indanyl, biphenyl, and the
like.
[00032] The term "arylalkyl" refers to an aryl group directly bonded to an
alkyl group,
which may be optionally substituted by one or more substituents. Preferred
arylalkyl
groups include, without limitation, -CH2C6H5, -C2H4C6H5, and the like.
[00033] The term "heterocycly1" refers to a heterocyclic ring radical which
may be
optionally substituted by one or more substituents. The heterocyclyl ring
radical may
be attached to the main structure at any heteroatom or carbon atom that
results in the
creation of a stable structure.
[00034] Furthermore, the term "heterocycly1" refers to a stable 3 to 15
membered rings
radical, which consists of carbon atoms and from one to five heteroatoms
selected
from nitrogen, phosphorus, oxygen and sulfur. For purposes of this invention
the
heterocyclic ring radical may be monocyclic, bicyclic or tricyclic ring
systems, and the
nitrogen, phosphorus, carbon, or sulfur atoms in the heterocyclic ring radical
may be
- 8 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
optionally oxidized to various oxidation states. In addition, the nitrogen
atom may be
optionally quaternized; and the ring radical may be partially or fully
saturated.
Preferred heterocyclyl groups include, without limitation, azetidinyl,
acridinyl,
benzodioxolyl, benzodioxanyl, benzofuranyl, carbazolyl, cinnolinyl,
dioxolanyl,
indolizinyl, naphthyridinyl, perhydroazepinyl, phenazinyl, phenothiazinyl,
phenoxazinyl, phthalazinyl, pyridyl, pteridinyl, purinyl, quinazolinyl,
qunioxalinyl,
quinolinyl, isoquinolinyl, tetrazolyl, imidazolyl, tetrahydroisoquinolinyl,
piperidinyl,
piperazinyl, homopiperazinyl, 2-oxoazepinyl, azepinyl, pyrrolyl, 4-
piperidonyl,
pyrrolidinyl, pyrazinyl, pyrimidinyl, pyridazinyl, oxazolyl, oxazolinyl,
triazolyl,
indanyl, isoxazolyl, isoxazolidinyl, thiazolyl, thiazolinyl, thiazolidinyl,
isothiazolyl,
quinuclidinyl, isothiazolidinyl, indolyl, isoindolyl,
indolinyl, isoindolinyl,
octahydroindolyl, octahydroisoindolyl, quinolyl, isoquinolyl,
decahydroisoquinolyl,
benzimidazolyl, thiadiazolyl, benzopyranyl, benzothiazolyl, benzooxazolyl,
thienyl,
morpholinyl, thiomorpholinyl, thiamorpholinyl sulfoxide, furyl,
tetrahydrofuryl,
tetrahydropyranyl, chromanyl, and isochromanyl.
[00035] The term "heteroaryl" refers to an aromatic heterocyclic ring radical
as
defined above. The heteroaryl ring radical may be attached to the main
structure at any
heteroatom or carbon atom that results in the creation of stable structure.
[00036] The term "heteroarylalkyl" refers to a heteroaryl group directly
bonded to an
alkyl group, which may be optionally substituted by one or more substituents.
Preferred heteroarylalkyl groups include, without limitation, -CH2-pyridinyl, -
C2H4-
furyl and the like.
[00037] The term "fused heterocycly1" refers to monocyclic or polycyclic ring,
polycyclic ring system refers to a ring system containing 2 or more rings,
preferably
bicyclic or tricyclic rings, in which rings can be fused, bridged or spiro
rings or any
combinations thereof. A fused ring as used herein means that the two rings are
linked
to each other through two adjacent ring atoms common to both rings. The fused
ring
can contain 1-4 hetero atoms independently selected from N, 0, and S. The
rings can
be either fused by nitrogen or -CH- group.
[00038] The term"bridged ring" as used herein means that a ring comprises a
linker
group (C(Rq)2)p-linking together any two non-adjacent carbon or nitrogen atoms
of the
ring, where p is 1 or 2 and each independently is hydrogen or C1-4 alkyl.
[00039] The term "cycloalkyl" refers to non-aromatic mono or polycyclic ring
system
of about 3 to 12 carbon atoms, which may be optionally substituted by one or
more
- 9 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
substituents. The polycyclic ring denotes hydrocarbon systems containing two
or more
ring systems with one or more ring carbon atoms in common i.e. a spiro, fused
or
bridged structures. Preferred cycloalkyl groups include, without limitation,
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclooctanyl,
perhydronaphthyl,
adamantyl, noradamantyl and norbornyl groups, bridged cyclic groups or
spirobicyclic
groups e.g spiro [4.4] non-2-y1 and the like.
[00040] The term "alkoxy" refers to an alkyl group attached via an oxygen
linkage to
the rest of the molecule, which may be optionally substituted by one or more
substituents. Preferred alkoxy groups include, without limitation, ¨OCH3,
¨0C2H5 and
the like.
[00041] The term "alkylthio" refers to an alkyl group attached via a sulfur
linkage to
the rest of the molecule, which may be optionally substituted by one or more
substituents. Preferred alkylthio groups include, without limitation, ¨SCH3,
¨SC2H5
and the like.
[00042] The term "alkylamino" refers to an alkyl group as defined above
attached via
amino linkage to the rest of the molecule, which may be optionally substituted
by one
or more substituents. Preferred alkylamino groups include, without limitation -
NHCH3,
-N (CH3)2, and the like.
[00043] The term "alkenyl" refers to an aliphatic hydrocarbon group containing
a
carbon-carbon double bond and which may be straight or branched chain having
about
2 to 10 carbon atoms, which may be optionally substituted by one or more
substituents.
Preferred alkenyl groups include, without limitation, ethenyl, 1-propenyl, 2-
propenyl,
iso-propenyl, 2-methyl- 1 -propenyl, 1-butenyl, 2-butenyl and the like.
[00044] The term "alkynyl" refers to a straight or branched hydrocarbyl
radicals
having at least one carbon-carbon triple bond and having in the range of 2-12
carbon
atoms, which may be optionally substituted by one or more substituents.
Preferred
alkynyl groups include, without limitation, ethynyl, propynyl, butynyl and the
like.
[00045] The term "alkylaryl" refers to an alkyl group directly bonded to an
aryl group,
which may be optionally substituted by one or more substituents. Preferred
alkylaryl
groups include, without limitation, -CH2-phenyl, -C2H4-phenyl, C3H6-phenyl and
the
like.
[00046] The term "alkenylaryl" refers to an alkenyl group directly bonded to
an aryl
group, which may be optionally substituted by one or more substituents.
Preferred
- 10 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
alkenylaryl groups include, without limitation, -CH=CH-phenyl, -CH2-CH=CH¨
phenyl and the like.
[00047] The term "arylalkenyl" refers to an aryl group directly bonded to an
alkenyl
group, which may be optionally substituted by one or more substituents.
Preferred
arylalkenyl groups include, without limitation, -C6H5-CH=CH-, -C6H5-CH=CH-CH2
and the like.
[00048] The term "arylalkynyl" refers to an aryl group directly bonded to an
alkynyl
group, which may be optionally substituted by one or more substituents.
Preferred
arylalkenyl groups include, without limitation, -C6H5-ethynyl, -C6H5-propynyl,
and the
like
[00049] The term "-CO-alkylaryl" refers to a carbonyl group directly attached
to an
alkylaryl group which may be optionally substituted by one or more
substituents.
Preferred "-CO-alkylaryl groups include, without limitations, -CO-CH2-phenyl, -
CO-
C2H4-phenyl and the like
[00050] The term "-CO-alkenylaryl" refers to a carbonyl group directly
attached to an
alkenylaryl group which may be optionally substituted by one or more
substituents.
Preferred "-CO-alkenylaryl" groups include, without limitations, -CO-CH=CH-
phenyl,
-CO-CH2-CH=CH¨phenyl and the like.
[00051] The term "-CO-heterocycly1" refers to a carbonyl group directly
attached
through the heteratom or carbon atom of a heterocyclyl group which may be
optionally
substituted by one or more substitutents. Preferred "-CO-heterocycly1" groups
include,
without limitations, -CO-piperazinyl, -CO-N-piperdinyl (implies attachment is
through
the nitrogen of piperdinyl group), -CO-C-piperidinyl (implies the attachment
is
through the carbon of piperdinyl group)and the like
[00052] The term "alkyl-0-aryl-" refers to an alkyl group attached to aryl
through the
oxygen linker which may be optionally substituted by one or more subtitutents.
Preferred groups without limitations include-(CH2)2-0-phenyl- and the like.
[00053] The term- "-S02alkylaryl-"refers to a ¨SO2- group attached to
alkylaryl group
which may be optionally substituted by one or substitutents. Perferred ' -
S02alkylaryl-
'groups include ¨S02-CH2 ¨Aryl and the like.
[00054] It is understood that included in the family of compounds of Formula
(I) are
isomeric forms including diastereoisomers, enantiomers, tautomers, and
geometrical
isomers in "E" or "Z" configurational isomer or a mixture of `E' and 'Z'
isomers. It is
also understood that some isomeric form such as diastereomers, enantiomers and
- 11-
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
geometrical isomers can be separated by physical and/or chemical methods and
by
those skilled in the art.
[00055] Compounds disclosed herein may exist as single stereoisomers,
racemates and
or mixtures of enantiomers and/or diastereomers. All such single
stereoisomers,
racemates and mixtures thereof are intended to be within the scope of the
subject
matter described.
[00056] Compounds disclosed herein include isotopes of hydrogen, carbon,
oxygen,
fluorine, chlorine, iodine and sulfur which can be incorporated into the
compounds,
such as not limited to 2H (D), 3H (T), c 11C, 13C, 14C, 15N, 18F, 35s, 36C1
and 1251.
Compounds of this invention where in atoms were isotopically labeled for
example
, 14,,,
radioisotopes such as 3H, 13Cand the like can be used in metabolic studies,
kinetic studies and imaging techinques such as positron emission tomography
used in
understanding the tissue distribution of the drugs. Compounds of the invention
where
hydrogen is replaced with deuterium may improve the metabolic stability and
pharmacokinetics properties of the drug such as in vivo half life. Compounds
of the
invention where isotopically labeled 18F can be useful as PET imaging studies.
[00057] The phrase "pharmaceutically acceptable" refers to compounds or
compositions that are physiologically tolerable and do not typically produce
allergic or
similar untoward reaction, including but not limited to gastric upset or
dizziness when
administered to subjects.
[00058] Pharmaceutically acceptable salts forming part of this invention
include salts
derived from inorganic bases such as like Li, Na, K, Ca, Mg, Fe, Cu, Zn and Mn
and
ammonium, substituted ammonium salts, aluminum salts and the like.; salts of
organic
bases such as N, N' -diacetylethylenediamine, glucamine, triethylamine,
choline,
dicyclohexylamine, benzylamine, trialkylamine, thiamine, guanidine,
diethanolamine,
a-phenylethylamine, piperidine, morpholine, pyridine, hydroxyethylpyrrolidine,
hydroxyethylpiperidine and the like, salts also include amino acid salts such
as glycine,
alanine, cystine, cysteine, lysine, arginine, phenylalanine, guanidine etc.
Salts may
include acid addition salts where appropriate which are sulphates, nitrates,
phosphates,
perchlorates, borates, hydrohalides, acetates, tartrates, maleates, fumarates,
citrates,
succinates, palmoates, methanesulphonates, tosylates, benzoates, salicylates,
hydroxynaphtho ate s , benzenesulfonates , ascorb ate s , glycerophosphates ,
ketoglutarates
and the like.
- 12 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
[00059] Described herein are prodrugs of the compound of Formula (I), which on
administration undergo chemical conversion by metabolic processes before
becoming
active pharmacological substances. In general, such prodrugs will be
functional
derivatives of a compound of the invention, which are readily convertible in
vivo into a
compound of the invention.
[00060] The compounds described herein can also be prepared in any solid or
liquid
physical form, for example the compound can be in a crystalline form, in
amorphous
form and have any particle size. Furthermore, the compound particles may be
micronized or nanoized, or may be agglomerated, particulate granules, powders,
oils,
oily suspensions or any other form of solid or liquid physical forms.
[00061] The compounds described herein may also exhibit polymorphism. This
invention further includes different polymorphs of the compounds of the
present
invention. The term polymorph refers to a particular crystalline state of a
substance,
having particular physical properties such as X-ray diffraction, IR spectra,
melting
point and the like.
[00062] The terms "histone deacetylase" and "HDAC" are intended to refer to
any one
of a family of enzymes that remove acetyl groups from the c-amino groups of
lysine
residues at the N-terminus of a histone or tubulin. Unless otherwise indicated
by
context, the term "histone" is meant to refer to any histone protein,
including H1, H2A,
.. H2B, H3, H4 and H5, from any species. Human HDAC proteins or gene products
include but are not limited to, HDAC-1, HDAC-2, HDAC-3, HDAC-4, HDAC-5,
HDAC-6, HDAC-7, HDAC-8, HDAC-9, HDAC-10 and HDAC-11. The histone
deacetylase can also be derived from a protozoal or fungal source.
[00063] The term "histone deacetylase inhibitor" or "inhibitor of histone
deacetylase"
is used to identify a compound, which is capable of interacting with a histone
deacetylase and inhibiting its activity, more particularly its enzymatic
activity.
Inhibiting histone deacetylase enzymatic activity means reducing the ability
of a
histone deacetylase to remove an acetyl group from a histone or tubulin.
Preferably,
such inhibition is specific, i.e. the histone deacetylase inhibitor reduces
the ability of
histone deacetylase to remove an acetyl group from a histone or tubulin at a
concentration that is lower than the concentration of the inhibitor that is
required to
produce some other, unrelated biological effect.
[00064] The term "lysine demethylase inhibitor" or "inhibitor of lysine
demethylase" is
used to identify a compound, which is capable of interacting with a histone
- 13 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
demethylase and inhibiting its activity, more particularly its enzymatic
activity.
Inhibiting histone demethylase enzymatic activity means reducing the ability
of a
histone demethylase to remove a methyl group from a histone. Inhibitor of
histone
demethylase involves removal either mono methyl or dimethyl or trimethyl group
from
histones. Preferably, such inhibition is specific, i.e. the histone
demethylase inhibitor
reduces the ability of histone demethylase to remove a methyl group from a
histone at
a concentration that is lower than the concentration of the inhibitor that is
required to
produce some other, unrelated biological effect.
[00065] The term 'Dual inhibitor of LSD-1/HDAC' is capable of removing acetyl
group from histones or tublin and methyl group from histones. These inhibitors
are
capable of inhibiting more than one HDAC isozyme and all those isozymes are
covered in addition to inhibiting LSD-1 activity
[00066] The term dual inhibitor LSD1/HDAC6 is used to identify a compound
which
is capable of interacting selectively with HDAC6 enzymes in addition to having
enzymatic interactions for LSD-1. Dual inhibitor of LSD-1/HDAC6 is capable of
removing acetyl group from tublin and methyl group from histones.
[00067] The term dual inhibitor LSD1/HDAC1 is used to identify a compound
which
is capable of interacting selectively with HDAC1 enzymes in addition to having
enzymatic interactionsfor LSD-1. Dual inhibitor of LSD-1/HDAC 1 is capable of
removing acetyl group from histones and methyl group from histones.
[00068] The term dual inhibitor LSD1/HDAC8 is used to identify a compound
which
is capable of interacting selectively with HDAC8 enzymes in addition to having
enzymatic interactionsfor LSD-1. Dual inhibitor of LSD-1/HDAC8 is capable of
removing acetyl group from histones and methyl group from histones.
[00069] A term once described, the same meaning applies for it, throughout the
patent.
[00070] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar II. A
Ri' w
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
- 14 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -C 0-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6CO-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1-6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, O-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
.. alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2_10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbl2c, NRaC(S )NRbRc, -
N(Ra)S ORb , -N(Ra)S 02Rb, -NRaC (0 ) ORb , -NRaRb, - NRaC (0 )Rb- , -NRaC(S
)Rb- , -
S ONRaRb- , -S 02NRaRb-, -0Ra, - ORaC( 0 )0 Rb- , - OC (0 )NRaRb , OC( 0)Ra, -
OC( 0 )NRaRb- , -RaNRbRc, -RaORb- , -S Ra, -S ORa and -SO2Ra, wherein Ra, Rb
and Rc is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
- 15 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
wherein C7-12 arylalkoxy, C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, Ci_6 alkyl, C5_6 aryl, C 1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C 1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7 is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl,
C2-10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[00071] In another embodiment, the invention provides compound of Formula I
R3
0
Ar k .Y
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted C5_6
aryl, C1_6
heteroaryl, and C2_10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
and
substituted or unsubstituted C1_8 alkyl;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1_8 alkynyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocycicyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
- 16 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7-12 alkylaryl, C7-12 alkenylaryl, C7_15 arylalkenyl, C2-12
alkylheteroaryl, -
CO-C712 alkylaryl, -CO-C7-12 alkenylaryl, -C 0 NR6-C1_8 alkyl, -NR6C O-C 1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-C 0- 0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -SO2-056 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C 1_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen, hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
0C(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, Rb and 12c
is
.. independently selected from the group consisting of hydrogen, C1_8 alkyl,
C3_8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
hetroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocycicyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocycicyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, and substituted or
unsubstituted
C1_8 alkyl;
R2 is selected from the group consisting of ¨0127, aniline, amino C5_6 aryl,
and amino C1_
6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
- 17 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R7 is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl,
C2-10
heterocyclyl and¨COR8, wherein R8 is selected the group consisting of C1_8
alkyl, C5_6
aryl, Ci_6heteroaryl, C3_8cycloalkyl, and C210 heterocyclyl.
[00072] In yet another embodiment, the invention relates to compound of
Formula I
R3
0
Ar II. A
Ri' w
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of C5_6 aryl, and C2_10 heterocyclyl
with
heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
and
substituted or unsubstituted C1_8 alkyl;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8 cycloalkyl, -CO-,
and -CO-C2_10
heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocycicyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 alkylaryl, C7_12 alkenylaryl, C7_15 arylalkenyl, C2_12
alkylheteroaryl, -
CO-C7_12 alkylaryl, -CO-C712 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5_6 aryl, C1_6 heteroaryl,
C2-10
heterocyclycl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05-6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl or 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
- 18 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R1 is selected from the group consisting of hydrogen, halogen, hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, Ci_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, Ci_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S 02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, RE, and
12c is
independently selected from the group consisting of hydrogen, Ci_8 alkyl, C3_8
cycloalkyl, C5_6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, Ci_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocycicyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, Ci_6 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10 heter0CyClCyl, OX0
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0127, aniline, amino C5_6 aryl,
and amino Ci_
6heteroaryl;
wherein aniline, amino C5_6 aryl, and amino Ci_6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7 is selected from the group consisting of hydrogen, Ci_8 alkyl, C5-6 aryl,
C2-10
heterocyclyl and ¨COR8, wherein R8 is selected the group consisting of C1_8
alkyl, C5_6
aryl, C1_6 heteroaryl, C3_8 cycloalkyl, and C210 heterocyclyl.
[00073] In an embodiment of the present invention, there is provided compound
of
Formula I
R3
0
,Ar II. A
Ri w
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
- 19 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Ar is selected from the group consisting of substituted or unsubstituted C5_6
aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 is hydrogen;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1_8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7-12 alkylaryl, C7-12 alkenylaryl, C7-15 arylalkenyl, C2-12
alkylheteroaryl, -
CO-C712 alkylaryl, -CO-C712 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8 alkyl-
, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1-6 heteroaryl,
C2-10
heterocyclycl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -Ci_8alky1-0-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7-12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
C1_8 alkyl-
CONR6-05_6 aryl or 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, Ci_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen, C1_8 alkyl,
C1_8 haloalkyl,
C1_8 alkoxy, C1_8 haloalkoxy, C7-12 arylalkoxy, C3_8 cycloalkyl, C3_8
cycloalkyloxy, C5-6
aryl, C2_10 heterocyclyl, Ci_6 heteroaryl, -C(0)Rb, -C(0)NRaRb, wherein Ra,
and Rb is
independently selected from the group consisting of hydrogen, C1_8 alkyl, and
C5_6 aryl;
wherein C7-12 arylalkoxy, C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocycicyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5_6 aryl, oxo (=0), halogen, OH, amino, and cyano;
R3 is hydrogen;
R2 is selected from the group consisting of ¨0127, aniline, amino C5_6 aryl,
and amino C1_
6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
- 20 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R7 is selected from the group consisting of hydrogen, Ci_8alkyl, C5_6aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected the group consisting of C1_8
alkyl, C5_6
aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C210 heterocyclyl.
[00074] In another embodiment, the invention relates to compound of Formula I
R3
0
,Ar
Ri 11'W X Z N H R2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of C5_6 aryl, and C2_10 heterocyclyl
with
heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 is hydrogen;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocycicyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C7_12 alkylaryl, C7_15 arylalkenyl, C2_12 alkylheteroaryl, -CO-
C7_12alkylaryl, -CO- C7_
12alkenylaryl, -CONR6- Ci_8alkyl, C5_6aryl, Ci_6heteroaryl, -CO-C2_10
heterocyclyl, -
NR6- C5-6 aryl, -NR6- Ci_6heteroaryl, -0- C5_6 aryl, 0- Ci_6 heteroaryl, -
CONR6- C7-12
alkylaryl, -SO2- C5_6 aryl, -SO2- C7-12 alkylaryl, and -NR6S02-C712alkylaryl;
R6 is selected from the group consisting of hydrogen, and C1_8 alkyl;
R1 is selected from the group consisting of hydrogen, halogen, hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, Ci_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbl2c, NRaC(S )NRbRc, -
N(Ra)S ORb , -N(Ra)S 02Rb, -NRaC (0 ) ORb , -NRaRb, - NRaC (0 )Rb- , NRaC(S
)Rb-, -
S ONRaRb- , -S 02NRaRb-, -0Ra, - ORaC( 0 )0 Rb- , - OC (0 )NRaRb , OC( 0)Ra, -
- 21 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and
Rcis
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arYlalkYl, C2-10 heterocyclyl, Ci_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocycicyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, Ci_6 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0127, aniline, amino C5_6 aryl,
and amino Ci_
6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7 is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl,
C2-10
heterocyclyl and ¨COR8, wherein R8 is selected the group consisting of C1_8
alkyl, C5_6
aryl, C1_6 heteroaryl, C3_8 cycloalkyl, and C210 heterocyclyl.
[00075] In yet another embodiment, the invention relates to compound of
Formula I
R3
0
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of C5_6 aryl, and C2_10 heterocyclyl
with
heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 is hydrogen;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl,, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
- 22 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
.. Cu alkylaryl, C7_15 arylalkenyl, C2-12 alkylheteroaryl, -CO-C7-12
alkylaryl, -CO-C7-12
alkenylaryl, -CONR6-C1_8 alkyl, C5_6 aryl, C1-6 heteroaryl, -CO-C2_10
heterocyclyl, -NR6-
05_6 aryl, -NR6- C1_6 heteroaryl, -0-05_6 aryl, -0-Ci_6 heteroaryl, -CONR6-
C7_12 alkylaryl,
-SO2-056 aryl, -S 02-C7_12 alkylaryl, and -NR6S 02-C7-12 alkylaryl;
R6 is selected from the group consisting of hydrogen, and C1_8 alkyl;
.. R1 is selected from the group consisting of hydrogen, halogen, C1_8 alkyl,
C1_8 haloalkyl,
C1_8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3_8
cycloalkyloxy, C5_6
aryl, C2_10 heterocyclyl, C1_6 heteroaryl, -C(0)Rb, -C(0)NRaRb, wherein Ra,
and RE, is
independently selected from the group consisting of hydrogen, C1_8 alkyl, and
C5_6 aryl;
wherein C7-12 arylalkoxy, Ci_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5_6 aryl, oxo (=0), halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0127, aniline, amino C5_6 aryl,
and amino Ci_
6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7 is selected from the group consisting of hydrogen, C1_8 alkyl, C5_6 aryl,
C2-10
heterocyclyl and ¨COR8, wherein R8 is selected the group consisting of C1_8
alkyl, C5_6
aryl, C1_6 heteroaryl, C3_8 cycloalkyl, and C210 heterocyclyl.
[00076] In an embodiment, there is provided compound of Formula I
R3
0
Ar II. A
Ri' w
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
-23 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Ar is selected from the group consisting of C5_6 aryl, and C2_10 heterocyclyl
with
heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 is hydrogen;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C7_12 alkylaryl, C7-15 arYlalkenYl, C2-12 alkylhetero aryl, -C 0-C 7-12
alkylaryl, -CO-C712
alkenylaryl, -CONR6-C1_8 alkyl, C5_6 aryl, Ci_6heteroaryl, -CO-C2_10
heterocyclyl, -NR6-
05_6 aryl, -NR6-C1_6 heteroaryl, -0-05_6 aryl, -0-Ci_6heteroaryl, -CONR6-C7_12
alkylaryl,
-S02-056 aryl, - S 02-C7-12 alkylaryl, and -NR6S 02-C7-12 alkylaryl;
R6 is selected from the group consisting of hydrogen, and C1_8 alkyl;
R1 is selected from the group consisting of hydrogen, halogen, hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxY, C5-6 aryl, C2-10 heterocyclyl, Ci_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, -NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S 02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, Ci_6 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is hydrogen;
R2 is selected from the group consisting of ¨0R7, and aniline;
- 24 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
wherein aniline is optionally substituted with one or more of the groups
selected from
C1_8 alkyl, halogen, OH, amino, and cyano;
R7 is selected from the group consisting of hydrogen, and C1_8 alkyl.
[00077] In another embodiment, the invention relates to compound of Formula I
R3
0
,Ar II. A
Ri w
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of C5_6 aryl, and C2_10 heterocyclyl
with
heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 is hydrogen;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl,, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C7_12 alkylaryl, C7_15 arylalkenyl, C2_12 alkylheteroaryl, -CO-C7-12
alkylaryl, -CO-C7-12
alkenylaryl, -CONR6-C1_8 alkyl, C5_6 aryl, Ci_6heteroaryl, -CO-C2_10
heterocyclyl, -NR6-
05_6 aryl, -NR6-C1_6 heteroaryl, -0-05_6 aryl, -0-Ci_6heteroaryl, -CONR6-C7_12
alkylaryl,
-SO2-056 aryl, - S 02-C7-12 alkylaryl, and -NR6S 02-C7-12 alkylaryl;
R6 is selected from the group consisting of hydrogen, and C1_8 alkyl;
R1 is selected from the group consisting of hydrogen, halogen, hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, Ci_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRo NRaC(S )NRbRc, -
N(Ra)S ORb , -N(Ra)S 02Rb, -NRaC (0 ) ORb , -NRaRb, - NRaC (0 )Rb- , NRaC(S
)Rb- , -
S ONRaRb- , -S 02NRaRb-, -0Ra, - ORaC ( 0 )0 Rb- , - OC (0 )NRaRb , OC(0)Ra, -
- 25 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and 12,
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3_8
cycloalkyl, C5-6 aryl, C7-15 arYlalkYl, C2-10 heterocyclyl, Ci_6 heteroaryl,
and C2-12
hetroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, Ci_6 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is hydrogen;
R2 is selected from the group consisting of ¨0R7, and aniline;
wherein aniline is optionally substituted with one or more of the groups
selected from
C1_8 alkyl, halogen, OH, amino, and cyano.
[00078] In yet another embodiment, the invention relates to compound of
Formula I
R3
0
Ar N, x
Ri N\I w
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of C5_6 aryl, and C2_10 heterocyclyl
with
heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 is hydrogen;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1_8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C7-12 -alkylaryl, C7_15 -arylalkenYl, C2-12 - alkylhetero aryl, -C 0-C 7-12
alkylaryl, -CO-C712
alkenylaryl, -CONR6-C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, -CO-C210
heterocyclyl, -NR6-
- 26 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
C5_6 aryl, -NR6-C1_6 heteroaryl, -0-05_6 aryl, -0-C1_6 heteroaryl, -CONR6-
C7_12 alkylaryl,
-SO2-056 aryl, - S 02-C7-12 alkylaryl, and -NR6S 02-C7-12 alkylaryl;
R6 is selected from the group consisting of hydrogen, and C1_8 alkyl;
R1 is selected from the group consisting of hydrogen, halogen, hydroxy, nitro,
cyano,
.. azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-
8 alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, Ci_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRc, -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, Ci_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
.. wherein C7-12 arylalkoxy, C1_8 alkyl, C5-6 aryl, C1-6 heteroaryl, C2-10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, Ci_6 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10 heterocyclyl, OX0
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is hydrogen;
R2 is selected from the group consisting of ¨0R7, R7 is selected from the
group
consisting of hydrogen, and C1_8 alkyl.
[00079] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
,Ar N, y
R1 w-
ZNHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of C5_6aryl, and C240heterocycly1
with
heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 is hydrogen;
- 27 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_
8 alkyl, C 1_8 alkenyl, C 1_8 alkynyl, C5_6aryl, Ci_6heteroaryl, C2-
10heterocyclyl, C3
8cyc1oa1ky1, -CO-, and -CO-C2_10heterocycly1;
wherein C 1-8 alkyl, C5-6arY1, Ci_6heteroaryl, C2-10heterocyclyl,
C3_8cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of Ci_8alkyl,
Ci_8alkenyl,
C7_12-alkyl aryl, C7_15-arylalkenyl, C2_12-alkylhetero aryl, -C 0-C
7_12alkylaryl, -C O-C 7_
ualkenylaryi, _CONR6-C1_8a1ky1, C5_6aryl, Ci_6heteroaryl, -CO-
C2_10heterocyclyl, -NR6-
C5_6aryl, -NR6-C 1_6hetero aryl, -0-05_6aryl, -0-C 1_6hetero aryl, -CONR6-
C7_12alkylaryl, -
S 02-05_6aryl, -S 02-C742alkylaryl, and -NR6S 02-C742alkylaryl;
R6 is selected from the group consisting of hydrogen, and C18 alkyl;
R1 is selected from the group consisting of hydrogen, halogen, hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C 1_8
alkyl, C1_
8ha1oa1ky1, C 1_8a1koxy, C 1_8ha1oa1koxy, C7_12arylalkoxy,
C3_8cycloalkyl, C3_
8cyc10a1ky10xy, C5_6aryl, C240heterocyclyl, Ci_6heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRc, -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, Rb and
Rcis
independently selected from the group consisting of hydrogen, Ci_8alkyl,
C3_8cycloalkyl,
C5_6aryl, C7_15 arylalkyl, C2_10heterocyclyl, Ci_6heteroaryl, and
C2_12heteroarylalkyl with
heteroatoms selected from N, 0, S;
wherein C7_12arylalkoxy, C 1_8 alkyl, C5_6aryl, Ci_6heteroaryl,
C2_10heterocyclyl, C3_
8cyc10a1ky1, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5_6aryl, Ci_6heteroaryl, C240heterocyclyl, oxo (=0), C3-
8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is hydrogen;
R2 is selected from the group consisting of aniline, amino C5_6aryl, and amino
C1_
6heteroaryl, wherein aniline, amino C5_6aryl, and amino Ci_6heteroaryl, is
optionally
substituted with one or more of the groups selected from C18 alkyl, halogen,
OH,
amino, and cyano.
[00080] In an embodiment of the present invention, there is provided a
compound of
Formula I
- 28 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R3
0
Ar N, y
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, Ci_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl,, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -C 0-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1_6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-8
alkyl, C1-8
haloalkyl, C1_8 alkoxy, C1_8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyloxy,
C5_6 aryl,
C2_10 heterocyclyl, C1_6 heteroaryl, alkylamino, -COORa, -C(0)Rb, -C(S)Ra, -
- 29 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S )NRbRc, -N(Ra)S ORb, -
N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -S ONRaRb-, -
S 02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -0C(0)NRaRb-, -
RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, RE, and 12, is
independently
selected from the group consisting of hydrogen, C1_8 alkyl, C3_8 cycloalkyl,
C5_6 aryl, C7-
arylalkyl, C2-10 heterocyclyl, C1-6 heteroaryl, and C2-12 heteroarylalkyl with
heteroatoms selected from N, 0, S;
wherein C7-12 arylalkoxy, Ci_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
10 hydrogen, Ci_6 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl,
oxo (=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl,and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
15 Ci_6heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5_6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6heteroaryl, C3_8 cycloalkyl, and C2-10heterocyclyl.
[00081] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar N, x
Ri' w
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
- 30 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1_8 alkynyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocycly1õC3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-C 0-C7-12 alkylaryl, -C 0-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05_6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, Ci_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C2_10 heterocyclyl, C1_6 heteroaryl, alkylamino, -COORa, -
C(0)Rb, -
C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbR, NRaC(S)NRbRc, -N(Ra)SORb, -
N(Ra)S 02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -SONRaRb-, -
SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -0C(0)NRaRb-, -
RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and 12, is
independently
selected from the group consisting of hydrogen, C1_8 alkyl, C3_8 cycloalkyl,
C5_6 aryl, C7_
15 arylalkyl, C2-10 heterocyclyl, C1_6 heteroaryl, and C2_12 heteroarylalkyl
with
heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
-31 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
hydrogen, C1_6 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5_6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6 heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[00082] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar 11, y
Ri' vr
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted C5_6
aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocycly1õC3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
- 32-
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C712 alkylaryl, -CO-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C 1_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2_10 heterocyclyl, alkylamino, -COORa, -C(0)Rb, -
C(S)Ra, -
C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRc, -N(Ra)SORb, -
N(Ra)S 02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -SONRaRb-, -
S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -0C(0)NRaRb-, -
RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, RE, and 12, is
independently
selected from the group consisting of hydrogen, C1_8 alkyl, C3_8 cycloalkyl,
C5_6 aryl, C7_
15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl, and C2-12 heteroarylalkyl
with
heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
- 33 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6 heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[00083] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ri'
Ar kw -Y
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted C5_6
aryl, Ci_6
heteroaryl, and C2_10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C2_10 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl, C
1_8 alkenyl,
C1_8 alkynyl, C7-12 -alkylaryl, C7-12 -alkenylaryl, C7-15 -arylalkenyl, C2-12-
alkylheteroaryl,
-CO-C7_12 alkylaryl, -CO-C7_12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6CO-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5_6 aryl, C1_6 heteroaryl,
C2-10
heterocyclyl, -CO-C210 heterocyclyl, -NR6-C 0- 0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7-12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05-6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
C1_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
- 34 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
.. haloalkyl, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3_8
cycloalkyloxy, C5-6
aryl, C2-10 heterocyclyl, C1-6 heteroaryl, alkylamino, -COORa, -C(0)Rb, -C(S
)Ra, -
C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S )NRb12,, -N(Ra)S ORb, -
N(Ra)S 02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -S ONRaRb- , -
S 02NRaRb- , -0Ra, -0RaC(0)0Rb- , - 0C(0)NRaRb, 0C(0)Ra, - 0C(0)NRaRb- , -
.. RaNRbRc, -RaORb- , - S Ra, -S ORa and -S02Ra, wherein Ra, RE, and 12, is
independently
selected from the group consisting of hydrogen, C1_8 alkyl, C3_8 cycloalkyl,
C5_6 aryl, C7_
arylalkyl, C2-10 heterocyclyl, C1-6 heteroaryl, and C2-12 heteroarylalkyl with
heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, C 1_8 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
15 .. cycloalkyl, is optionally substituted with one or more of the groups
selected from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C 1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[00084] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar
R1 w
N, y
N\I -
Z NHR2
I
- 35 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, Ci_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1_8 alkynyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocycly1õC3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -CO-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6CO-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7_12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C3_8 cycloalkyl, C3_8 cycloalkyloxy,
C5_6 aryl, C2-
10 heterocyclyl, C1_6 heteroaryl, alkylamino, -COORa, -C(0)Rb, -C(S)Ra, -
C(0)NRaRb, -
C(S)NRaRb, -NRaC(0)NRbR, NRaC(S)NRbR, -N(Ra)SORb, -N(Ra)S02Rb, -
NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -SONRaRb-, -SO2NRaRb-, -0Ra, -
0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -0C(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -
SORa and -SO2Ra, wherein Ra, Rb and 12, is independently selected from the
group
- 36 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
consisting of hydrogen, C1_8 alkyl, C3_8 cycloalkyl, C5_6 aryl, C7-15
arylalkyl, C2-10
heterocyclyl, C1-6 heteroaryl, and C2-12 heteroarylalkyl with heteroatoms
selected from
N, 0, S;
wherein C7-12 arylalkoxy, Ci_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl,
C2_10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[00085] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
- 37 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1_8 alkynyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocycly1õC3-8
cycloalkyl, -CO-, and -CO-C2_10 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C712 alkylaryl, -CO-C7-12 alkenylaryl, -C ONR6-C 1_8 alkyl, -NR6C O-C 1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, hydroxy, nitro, cyano,
azido,
nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-8 alkyl,
C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbl2c, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with hetero atoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C1_8
alkyl, and C5_6 aryl;
- 38 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[00086] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar k X
Ri' w
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocycly1õC3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -CO-C712 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5_6 aryl, C1_6 heteroaryl,
C2_10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
- 39 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
NR6CO-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C
7-12
alkenylaryl, -S02-05_6 aryl, -S 02-C 7-12 alkylaryl, -NR6S 02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of halogen, hydroxy, nitro, cyano,
azido,
nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-8 alkyl,
C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxY, C5-6 aryl, C2-10 heterocyclyl, C 1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S 02Rb, -NRaC (0 ) ORb, -NRaRb, -NRaC (0 )Rb- , -NRaC(S )Rb-
, -
S ONRaRb- , -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C 1_6 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C 1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6 hetero aryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci-6heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[00087] In an embodiment of the present invention, there is provided a
compound of
Formula I
- 40 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R3
0
Ar N, y
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted C5_6
aryl, C1_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted Ci_8
alkyl, Ci_8 alkenyl, Ci_8 alkynyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocycly1õC3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
Ci_8alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -C 0-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1_6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen, hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
- 41 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbR, -N(Ra)SORb, -
N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -SONRaRb-, -
SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -0C(0)NRaRb-, -
RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, RE, and 12, is
independently
selected from the group consisting of hydrogen, C1_8 alkyl, C3_8 cycloalkyl,
C5_6 aryl, C7-
arYlalkYl, C2-10 heterocyclyl, C1-6 heteroaryl, and C2-12 heteroarylalkyl with
heteroatoms selected from N, 0, S;
wherein C7-12 arylalkoxy, Ci_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
10 hydrogen, Ci_6 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl,
oxo (=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
15 Ci_6heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5_6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6 heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[00088] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar N, x
Ri' w
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted C5_6
aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
- 42 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-C 0-C7-12 alkylaryl, -C 0-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05_6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, Ci_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen, hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2_10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRc, -N(Ra)SORb, -
N(Ra)S 02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -SONRaRb-, -
SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -0C(0)NRaRb-, -
RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and 12, is
independently
selected from the group consisting of hydrogen, C1_8 alkyl, C3_8 cycloalkyl,
C5_6 aryl, C7_
15 arylalkyl, C2-10 heterocyclyl, C1_6 heteroaryl, and C2_12 heteroarylalkyl
with
heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
-43 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
hydrogen, C1_6 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5_6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6 heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[00089] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar 11, y
Ri' vr
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted C5_6
aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1_8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
- 44 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7_12 alkylaryl, -CO-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7_12 alkylaryl,
C1_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C 1_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen, hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
haloalkyl,
C1_8 alkOXY, C1-8 halOalkOXY, C7-12 arylalkoxy, C3_8 cycloalkyl, C3_8
cycloalkyloxy, C5-6
aryl, C2_10 heterocyclyl, C1_6 heteroaryl, alkylamino, -COORa, -C(0)Rb, -
C(S)Ra, -
C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRc, -N(Ra)SORb, -
N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -SONRaRb-, -
S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -0C(0)NRaRb-, -
RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, RE, and 12, is
independently
selected from the group consisting of hydrogen, C1_8 alkyl, C3_8 cycloalkyl,
C5_6 aryl, C7_
15 arYlalkYl, C2-10 heterocyclyl, C1_6 heteroaryl, and C2-12 heteroarylalkyl
with
heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
- 45 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6 heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[00090] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ri'
Ar kw -Y
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted C1_6
heteroaryl,
and C2_10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C2_10 heterocyclyl;
.. wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl, C
1_8 alkenyl,
C1_8 alkynyl, C7-12 -alkylaryl, C7-12 -alkenylaryl, C7-15 -arylalkenyl, C2-12-
alkylheteroaryl,
-CO-C7_12 alkylaryl, -CO-C7_12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6CO-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5_6 aryl, C1_6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7-12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05-6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
C1_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
- 46 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, Ci_8
alkyl, C1-8
haloalkyl, C1_8 alkoxy, C1-8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1-6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRc, -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, RE, and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, Ci_8 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C1_6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[00091] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar
R1 w
N, y
N\I -
Z NHR2
I
- 47 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted C5_6
aryl, and C2_
10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1_8 alkynyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocycly1õC3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
.. alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-C 0-C7-12 alkylaryl, -C 0-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6CO-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen, hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5_6 aryl, C2_10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbl2c, NRaC(S )NRbRc, -
N(Ra)S ORb , -N(Ra)S 02Rb, -NRaC (0 ) ORb , -NRaRb, - NRaC (0 )Rb- , -NRaC(S
)Rb- , -
S ONRaRb- , -S 02NRaRb-, -0Ra, - ORaC ( 0 )0 Rb- , - OC (0 )NRaRb , OC ( 0)Ra,
-
OC ( 0 )NRaRb- , -RaNRbRc, -RaORb- , - S Ra, -S ORa and -SO2Ra, wherein Ra, Rb
and Rc is
- 48 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3_8
cycloalkyl, C5_6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkoxy, Ci_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[00092] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted C5_6
aryl, and C1_
6 heteroaryl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
- 49 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C2_10 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C712 alkylaryl, -CO-C7-12 alkenylaryl, -C ONR6-C 1_8 alkyl, -NR6C O-C 1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-C 0- 0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -SO2-056 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbl2c, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C1_8
alkyl, and C5_6 aryl;
- 50 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7 is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl,
C2-10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[00093] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar k X
Ri' w
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C210 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocycly1õC3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -CO-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5_6 aryl, C1_6 heteroaryl,
C2_10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
- 51 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
NR6CO-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S 02-C7-12 alkylaryl, -NR6S 02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxY, C5-6 aryl, C2-10 heterocyclyl, C 1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S 02Rb, -NRaC (0 ) ORb, -NRaRb, -NRaC (0 )Rb- , -NRaC(S )Rb-
, -
S ONRaRb- , -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, RE, and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, Ci_6 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of substituted or unsubstituted C1_8
alkyl, and
C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C1_6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[00094] In an embodiment of the present invention, there is provided a
compound of
Formula I
- 52 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R3
0
Ar N, y
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, Ci_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted Ci_8
alkyl, Ci_8 alkenyl, Ci_8 alkynyl, C5_6 aryl, Ci_6 heteroaryl, C2_10
heterocycly1õC3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
Ci_8alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -C 0-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1_6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
- 53 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, RE, and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkoxy, C 1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, Ci_6 alkyl, C5_6 aryl, C 1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7 is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl,
C2-10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[00095] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
iokr- N, x
Ri N\I w
Z NHR2
1
.. their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2_10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents CR4R5, wherein
- 54 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl,C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-C 0-C7-12 alkylaryl, -C 0-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
.. NR6CO-05_6 aryl-, -NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05_6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, Ci_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2_10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
0C(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5_6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
- 55 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
hydrogen, C1_6 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C 1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7 is selected from the group consisting of hydrogen, C1_8 alkyl, C5_6 aryl,
C2-10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6 heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[00096] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar 11, y
Ri' vr
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted C5_6
aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of substituted
or
unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from N,
0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1_8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
- 56 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C712 alkylaryl, -CO-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S 02-C7-12 alkylaryl, -NR6S 02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C 1_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
0C(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3_8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with hetero atoms selected from N, 0, S;
wherein C7_12 arylalkoxy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
- 57 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C 1_6 heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[00097] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ri'
Ar kw -Y
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C 1_6
heteroaryl, and C2_10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
C5_6 aryl,
and C 1-6 heteroaryl with heteroatoms selected from N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocycly1õC3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C 1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl, C
1_8 alkenyl,
Ci_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-C 0-C 7-12 alkylaryl, -C 0-C 7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-
C1_8 alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1-6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7-12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S 02-C 5-6 aryl, -S 02-C 7-12 alkylaryl, -NR6S 02-C7-12
alkylaryl, Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
- 58 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, Ci_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S 02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, RE, and Rc
is
independently selected from the group consisting of hydrogen, Ci_8 alkyl, C3_8
cycloalkyl, C5_6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, Ci_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, Ci_6 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10 heterocyclyl, OX0
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6heteroaryl,
wherein aniline, amino C5_6 aryl, and amino Ci_6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, Ci_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5-6 aryl, C1-6heteroaryl, C3_8 cycloalkyl, and C2-10heterocyclyl.
[00098] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
,Ar II. A
Ri w
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
- 59 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, Ci_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1_8 alkynyl, C 1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, -CO-
and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -C 0-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1-6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2_10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRo NRaC(S )NRbRc, -
N(Ra)S ORb , -N(Ra)S 02Rb, -NRaC (0 ) ORb , -NRaRb, - NRaC (0 )Rb- , -NRaC(S
)Rb- , -
S ONRaRb- , -S 02NRaRb-, -0Ra, - ORaC( 0 )0 Rb- , - OC (0 )NRaRb , OC( 0)Ra, -
OC( 0 )NRaRb- , -RaNRbRc, -RaORb- , -S Ra, -S ORa and -SO2Ra, wherein Ra, Rb
and Rc is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
- 60 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
wherein C7-12 arylalkoxy, C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, Ci_6 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[00099] In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ri w
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2_10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1_8 alkynyl, C5_6 aryl, C1_6 heteroaryl, C3_8
cycloalkyl, -CO-, and -
CO-C210 heterocyclyl;
- 61 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-C 0-C7-12 alkylaryl, -C 0-C7-12 alkenylaryl, -C ONR6-C 1_8 alkyl, -NR6C O-C
1_8 alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
C1_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, Ci_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRc, -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
S ONRaRb-, -S 02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3_8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C1_6 heteroaryl,
- 62 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5_6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6heteroaryl, C3_8 cycloalkyl, and C2-10heterocyclyl.
[000100]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar k .Y
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is selected from the group consisting of substituted or unsubstituted C1_8
alkyl, C1_8
alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, -CO-,
and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7-12 -alkylaryl, C7-12 -alkenylaryl, C7-15 -arylalkenyl, C2-12-
alkylheteroaryl,
-CO-C7_12 alkylaryl, -CO-C7_12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1_6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
- 63 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
C1_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, Ci_8 alkyl, Ci_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, Ci_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, Ci_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRc, -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, RE, and Rc
is
independently selected from the group consisting of hydrogen, Ci_8 alkyl, C3_8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, Ci_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, Ci_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6heteroaryl, C3_8 cycloalkyl, and C2-10heterocyclyl.
[000101]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
y
Ri'Ar\III=w- ).
Z NHR2
- 64 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, Ci_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, -CO-,
and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C712 alkylaryl, -CO-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1_8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbl2c, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
- 65 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and 12,
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3_8
cycloalkyl, C5-6 aryl, C7-15 arYlalkYl, C2-10 heterocyclyl, Ci_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3_8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, Ci_6 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6 heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[000102]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar ris A
Ri' w
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
- 66 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1_8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, -CO-, and -
CO-C2_10 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C712 alkylaryl, -CO-C7-12 alkenylaryl, -C ONR6-C 1_8 alkyl, -NR6C O-C 1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1_6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -SO2-056 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbl2c, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C1_8
alkyl, and C5_6 aryl;
- 67 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[000103]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ri' w
Z7NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1_8 alkynyl, C5_6 aryl, C2_10 heterocyclyl, C3_8
cycloalkyl, -CO-, and -
CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -CO-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5_6 aryl, C1_6 heteroaryl,
C2_10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8
alkyl, -
- 68 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
NR6CO-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxY, C5-6 aryl, C2-10 heterocyclyl, Ci_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
.. hydrogen, Ci_6 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6 hetero aryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci-6heteroaryl, C3_8 cycloalkyl, and C2-10heterocyclyl.
[000104]In an embodiment of the present invention, there is provided a
compound of
Formula I
- 69 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R3
0
Ar N, y
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, Ci_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted Ci_8
alkyl, Ci_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, and -CO-;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
Ci_8alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -C 0-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1_6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
- 70 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, RE, and Rc
is
.. independently selected from the group consisting of hydrogen, C1_8 alkyl,
C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkoxy, C 1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
.. hydrogen, C 1_6 alkyl, C5_6 aryl, C 1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C 1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
.. C1_6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C 1_8 alkyl, C5_6 aryl,
C2-10
.. heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C 1-6 heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[000105]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ri w
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
-71 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, and -CO-C2_10 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-C 0-C7-12 alkylaryl, -C 0-C7-12 alkenylaryl, -C ONR6-C 1_8 alkyl, -NR6C O-C
1_8 alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-C 0- 0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05_6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -SO2-056 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, Ci_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2_10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
0C(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5_6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
- 72 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
hydrogen, C1_6 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C 1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5_6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6 heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[000106]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar KI, y
Ri' vr
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C210 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1_8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
-73 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
Ci_8 alkynyl, C7-12 -alkylaryl, C7_15 -arylalkenyl, C2_12- alkylheteroaryl, -
CO-C12
alkylaryl, -C 0-C 7-12 alkenyl aryl, -CONR6-C1_8 alkyl, -NR6C O-C 1_8 alkyl-, -
NR6-C 1-8
alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, -
CO-C10 heterocyclyl, -NR6-C 0-0C 1_8 alkyl, 0-C 0-NR6-C 1_8 alkyl, -NR6C 0-
05_6 aryl-,
-NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -Ci_8 alkyl-O-05_6 aryl, -0-05_6 aryl, 0-
C1-6
heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12
alkenylaryl, -
S02-05_6 aryl, -S02-C7_12 alkylaryl, -NR6S02-C7_12 alkylaryl, C1_8 alkyl-CONR6-
05_6 aryl,
and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
0C(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3_8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
- 74 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C240heterocyclyl.
[000107]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar k .11
Ri' w
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, Ci_6
heteroaryl, and C240heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7-12 -alkylaryl, C7-12 -alkenylaryl, C7-15 -arylalkenyl, C2-12-
alkylheteroaryl,
-CO-C7_12 alkylaryl, -CO-C7_12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6CO-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5_6 aryl, C210
heterocyclyl, -CO-C2-
10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8 alkyl, -NR6CO-05_6 aryl-, -
NR6-
05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6 aryl, -0-05_6 aryl, 0-Ci_6
heteroaryl, -
NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12 alkenylaryl, -S02-05_6
aryl,
-502-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl, Ci_8 alkyl-CONR6-05_6 aryl, and
0-00-
NR6-05_6 aryl;
-75 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, Ci_8
alkyl, C1-8
haloalkyl, C1_8 alkoxy, C1-8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1-6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRc, -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, RE, and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, Ci_8 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from Ci_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10heterocyclyl.
[000108]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar N y
,
R1 N\I w-
Z NHR2
I
- 76 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, Ci_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted Ci_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted Ci_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
Ci_8alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -CO-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C1-6 heteroaryl, C2_10
heterocyclyl, -
CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8 alkyl, -NR6CO-05_6
aryl-,
-NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6 aryl, -0-05_6 aryl, 0-
C1-6
heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12
alkenylaryl, -
S02-05_6 aryl, -S02-C7_12 alkylaryl, -NR6S02-C712alkylaryl, Ci_8 alkyl-CONR6-
05_6 aryl,
and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5_6 aryl, C2_10 heterocyclyl, Ci_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRo NRaC(S )NRbRc, -
N(Ra)S ORb , -N(Ra)S 02Rb, -NRaC (0 ) ORb , -NRaRb, - NRaC (0 )Rb- , -NRaC(S
)Rb- , -
S ONRaRb- , -S 02NRaRb-, -0Ra, - ORaC ( 0 )0 Rb- , - OC (0 )NRaRb , OC ( 0)Ra,
-
OC ( 0 )NRaRb- , -RaNRbRc, -RaORb- , - S Ra, -S ORa and -SO2Ra, wherein Ra, Rb
and Rc is
- 77 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3_8
cycloalkyl, C5_6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkoxy, Ci_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[000109]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar k .sk
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
- 78 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C2_10 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C 1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z is selected from the group consisting of C1_8 alkyl, C1_8 alkenyl, C1_8
alkynyl, C7-12 -
alkylaryl, C7-12 -alkenylaryl, C7_15 -arylalkenyl, C2_12- alkylheteroaryl, -CO-
C712
alkylaryl, -CO-C712 alkenylaryl, -CONR6-C1_8 alkyl, -NR6CO-C 1_8 alkyl-, -NR6-
C 1-8
alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, -
CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8 alkyl, -NR6CO-05_6
aryl-,
-NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6 aryl, -0-05_6 aryl, 0-
C1-6
heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12
alkenylaryl, -
S02-05_6 aryl, -S02-C7_12 alkylaryl, -NR6S02-C7_12 alkylaryl, C1_8 alkyl-CONR6-
05_6 aryl,
and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C1_8
alkyl, and C5_6 aryl;
- 79 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[000110]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar k A
Ri' w
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkynyl,
C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylhetero aryl, -C O-C 7-12
alkylaryl, -C O-C 7-12 alkenyl aryl, -CONR6-C1_8 alkyl, -NR6C O-C 1_8 alkyl-, -
NR6-C1-8
alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, -
CO-C2_10 heterocyclyl, -NR6-00-0C 1_8 alkyl, 0-C 0-NR6-C1_8 alkyl, -NR6C 0-
05_6 aryl-,
- 80 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
-NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -Ci_8 alkyl-O-05_6 aryl, -0-05_6 aryl, 0-
C1-6
heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12
alkenylaryl, -
S02-05_6 aryl, -S02-C7_12 alkylaryl, -NR6S02-C7_12 alkylaryl, C1_8 alkyl-CONR6-
05_6 aryl,
and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxY, C5-6 aryl, C2-10 heterocyclyl, Ci_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
.. hydrogen, Ci_6 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C1_6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[000111]In an embodiment of the present invention, there is provided a
compound of
Formula I
- 81 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R3
0
Ri vv-
ZNHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7_12 alkylaryl, -CO-C7_12 alkenylaryl, -NR6CO-C1_8 alkyl-, -NR6-C1_8
alkyl, -0-C1-8
alkyl-, -CONR6-05-6 aryl-, C5-6 aryl, C1-6 heteroaryl, C2-10 heterocyclyl, -CO-
C2_10
heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8 alkyl, -NR6CO-05_6 aryl-, -
NR6-05_
6 arYl, -NR6-C16heteroaryl, -C1_8 alkyl-O-05_6 aryl, -0-05_6 aryl, 0-
Ci_6heteroaryl, -NR6-
00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12 alkenylaryl, -S02-05_6
aryl, -SO2-
C7_12 alkylaryl, -NR6S02-C7_12 alkylaryl, C1_8 alkyl-CONR6-05_6 aryl, and 0-CO-
NR6-05_
6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
- 82 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, RE, and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkoxy, C 1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C 1_6 alkyl, C5_6 aryl, C 1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C 1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C1_6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C 1_8 alkyl, C5_6 aryl,
C2-10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C 1-6 heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[000112]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ri w
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
- 83 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C2_10 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C 1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl, C
1_8 alkenyl,
C1_8 alkynyl, C7-12 -alkenylaryl, C7-15 -arylalkenyl, C2-12- alkylheteroaryl, -
CO-C7-12
alkylaryl, -CO-C712 alkenylaryl, -CONR6-C1_8 alkyl, -NR6CO-C 1_8 alkyl-, -NR6-
C 1-8
alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, -
CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8 alkyl, -NR6CO-05_6
aryl-,
-NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -Ci_8 alkyl-O-05_6 aryl, -0-05_6 aryl, 0-
C1-6
heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12
alkenylaryl, -
S02-05_6 aryl, -S02-C7_12 alkylaryl, -NR6S02-C7_12 alkylaryl, C1_8 alkyl-CONR6-
05_6 aryl,
and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2_10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5_6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
- 84 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
hydrogen, C1_6 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C 1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5_6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6 heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[000113]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar KI, y
Ri' vr
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C210 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1_8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
- 85 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, -CO-
C7_12 alkylaryl, -
CO-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8 alkyl-, -NR6-C1_8 alkyl,
-0-C1-8
alkyl-, -CONR6-05-6 aryl-, C5-6 aryl, C1-6 heteroaryl, C2_10 heterocyclyl, -CO-
C2-10
heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8 alkyl, -NR6CO-05-6 aryl-, -
NR6-05_
6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6 aryl, -0-05_6 aryl, 0-C1_6
heteroaryl, -NR6-
00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12 alkenylaryl, -S02-05_6
aryl, -S02-
C7_12 alkylaryl, -NR6S02-C7_12 alkylaryl, C1_8 alkyl-CONR6-05_6 aryl, and 0-CO-
NR6-05_
6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, Ci_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1-6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3_8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, Ci_8 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
- 86 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C240heterocyclyl.
[000114]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar k .11
Ri' w
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, Ci_6
heteroaryl, and C240heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7-12 -alkylaryl, C7-12 -alkenylaryl, C7-15 -arylalkenyl, C2-12-
alkylheteroaryl,
-CO-C7_12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6CO-C1_8 alkyl-, -NR6-C1_8 alkyl,
-0-C1-8
alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1-6 heteroaryl, C2-10 heterocyclyl, -CO-
C2_10
heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8 alkyl, -NR6CO-05_6 aryl-, -
NR6-05_
6 aryl, -NR6-C16heteroaryl, -C1_8 alkyl-O-05_6 aryl, -0-05_6 aryl, 0-
Ci_6heteroaryl, -NR6-
00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12 alkenylaryl, -S02-05_6
aryl, -S02-
C7_12 alkylaryl, -NR6S02-C7_12 alkylaryl, C1_8 alkyl-CONR6-05_6 aryl, and 0-CO-
NR6-05_
6 aryl;
- 87 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, Ci_8
alkyl, Ci_8
haloalkyl, C1_8 alkoxy, C1-8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1-6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRc, -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, Ci_8 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10heterocyclyl.
[000115]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar N y
,
R1 N\I w-
Z NHR2
I
- 88 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, Ci_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -CO-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl, Ci_8 alkyl-CONR6-
05_6 aryl,
and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5_6 aryl, C2_10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S )NRbRc, -
N(Ra)S ORb , -N(Ra)S 02Rb, -NRaC (0 ) ORb , -NRaRb, - NRaC (0 )Rb- , -NRaC(S
)Rb- , -
S ONRaRb- , -S 02NRaRb-, -0Ra, - ORaC ( 0 )0 Rb- , - OC (0 )NRaRb , OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and Rc
is
- 89 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3_8
cycloalkyl, C5_6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkoxy, Ci_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[000116]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar k .sk
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
- 90 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C2_10 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C712 alkylaryl, -CO-C7-12 alkenylaryl, -C ONR6-C 1_8 alkyl, -NR6C O-C 1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -SO2-056 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbl2c, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C1_8
alkyl, and C5_6 aryl;
- 91 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[000117]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ri' w
Z7NH R2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -CO-C712 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5_6 aryl, C1_6 heteroaryl,
C2_10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8
alkyl, -
- 92 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
NR6CO-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7-12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -NR6S02-C7_12 alkylaryl, C1_8 alkyl-CONR6-05_6
aryl, and 0-
CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1-8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxY, C5-6 aryl, C2-10 heterocyclyl, Ci_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, Ci_6 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C1_6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[000118]In an embodiment of the present invention, there is provided a
compound of
Formula I
- 93 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R3
0
Ri vv-
ZNHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted C5_6
aryl, C1_6
heteroaryl, and C2-10heterocycly1 with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
Ci_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -C 0-C7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, C1_6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C1-6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7-
12
alkenylaryl, -S02-05_6 aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl,
Ci_8 alkyl-
CONR6-05_6 aryl, and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1-8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
- 94 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, RE, and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkoxy, C 1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C 1_6 alkyl, C5_6 aryl, C 1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C 1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C1_6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C 1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C 1_8 alkyl, C5_6 aryl,
C2-10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C 1-6 heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[000119]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ri w
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
- 95 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C2_10 heterocyclyl;
wherein C1_8 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7_12 alkylaryl, -CONR6-C1_8 alkyl, -NR6CO-C1_8 alkyl-, -NR6-C1_8 alkyl, -
0-C1-8
alkyl-, -C ONR6-C 5-6 aryl-, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, -
CO-C2-10
heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8 alkyl, -NR6CO-05_6 aryl-, -
NR6-05_
6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6 aryl, -0-05_6 aryl, 0-C1-6
heteroaryl, -NR6-
00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12 alkenylaryl, -S02-05_6
aryl, -S02-
C7_12 alkylaryl, -NR6S02-C7_12 alkylaryl, C1_8 alkyl-CONR6-05_6 aryl, and 0-CO-
NR6-05_
6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, Ci_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2_10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5_6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
- 96 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
hydrogen, C1_6 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5_6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, C1-6 heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[000120]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar KI, y
Ri' vr
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1_8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
.. optionally substituted with one or more of the groups selected from
hydrogen, C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
- 97 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7_12 alkylaryl, -CO-C7-12 alkenylaryl, -C ONR6-C 1_8 alkyl, -NR6C O-C 1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -C 0-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
NR6C 0-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, 0-C1-6
heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12
alkenylaryl, -
S02-05_6 aryl, -S02-C7_12 alkylaryl, -NR6S02-C7_12 alkylaryl, C1_8 alkyl-CONR6-
05_6 aryl,
and 0-CO-NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C 1_6
haloalkyl, C3_8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
0C(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3_8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
Ci_6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
- 98 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[000121]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar k .11
Ri' w
Z NHR2
1
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C 1_6
heteroaryl, and C2_10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3_8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl, C
1_8 alkenyl,
C1_8 alkynyl, C7-12 -alkylaryl, C7-12 -alkenylaryl, C7-15 -arylalkenyl, C2-12-
alkylheteroaryl,
-CO-C7_12 alkylaryl, -CO-C7_12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5_6 aryl, C1_6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05_6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12 alkenylaryl, -
S02-05-6
aryl, -S02-C7-12 alkylaryl, -NR6S02-C7-12 alkylaryl, Ci_8 alkyl-CONR6-05_6
aryl, and 0-
CO-NR6-05_6 aryl;
- 99 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, Ci_8
alkyl, C1-8
haloalkyl, C1_8 alkoxy, C1-8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1-6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRc, -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, RE, and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7_12 arylalkoxy, Ci_8 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10heterocyclyl.
[000122]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar N y
,
R1 N\I w-
Z NHR2
I
- 100 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C 1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C 1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C 1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-C O-C 7-12 alkylaryl, -C O-C 7-12 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-
C1_8 alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8
alkyl, -
NR6CO-05_6 aryl-, -NR6-05-6 aryl, -C1_8 alkyl-O-05_6 aryl, -0-05_6 aryl, O-C 1-
6 heteroaryl,
-NR6-00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12 alkenylaryl, -S02-
05_6 aryl,
-S 02-C7_12 alkylaryl, -NR6S 02-C7-12 alkylaryl, C 1_8 alkyl-CONR6-05_6 aryl,
and 0-00-
NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7-12 arylalkoxy, C3-8 cycloalkyl, C3-
8
cycloalkyloxy, C5_6 aryl, C2_10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRo NRaC(S )NRbRc, -
N(Ra)S ORb , -N(Ra)S 02Rb, -NRaC (0 ) ORb , -NRaRb, - NRaC (0 )Rb- , -NRaC(S
)Rb- , -
S ONRaRb- , -S 02NRaRb-, -0Ra, - ORaC ( 0 )0 Rb- , - OC (0 )NRaRb , OC ( 0)Ra,
-
OC ( 0 )NRaRb- , -RaNRbRc, -RaORb- , - S Ra, -S ORa and -SO2Ra, wherein Ra, Rb
and Rc is
- 101 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3_8
cycloalkyl, C5_6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkoxy, Ci_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted Ci_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[000123]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ar k .sk
Z NHR2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
- 102 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1-8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C2_10 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, Ci_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
Ci_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7_12 alkylaryl, -CO-C7-12 alkenylaryl, -C ONR6-C 1_8 alkyl, -NR6C O-C 1_8
alkyl-, -
NR6-C1_8 alkyl, -0-Ci_8 alkyl-, -CONR6-05_6 aryl-, C5-6 aryl, Ci_6 heteroaryl,
C2-10
heterocyclyl, -NR6-00-0C1_8 alkyl, 0-CO-NR6-C1_8 alkyl, -NR6CO-05-6 aryl-, -
NR6-05_
6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6 aryl, -0-05_6 aryl, 0-C1_6
heteroaryl, -NR6-
00-0C5_6 aryl, -CONR6-C7_12 alkylaryl, -CONR6-C7_12 alkenylaryl, -S02-05_6
aryl, -S02-
C7_12 alkylaryl, -NR6S02-C7_12 alkylaryl, C1_8 alkyl-CONR6-05_6 aryl, and 0-CO-
NR6-05_
6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1-8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxy, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRo NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S02Rb, -NRaC(0)0Rb, -NRaRb, -NRaC(0)Rb-, -NRaC(S)Rb-, -
SONRaRb-, -SO2NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, OC(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -SO2Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, C1_6 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C1_8
alkyl, and C5_6 aryl;
- 103 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C16 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1_8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci_6heteroaryl, C3_8 cycloalkyl, and C2_10 heterocyclyl.
[000124]In an embodiment of the present invention, there is provided a
compound of
Formula I
R3
0
Ri' w
Z7NH R2
I
their analogs, tautomeric forms, stereoisomers, polymorphs, solvates,
intermediates,
pharmaceutically acceptable salts, metabolites, and prodrugs thereof;
wherein
Ar is selected from the group consisting of substituted or unsubstituted
C5_6aryl, C1_6
heteroaryl, and C2-10 heterocyclyl with heteroatoms selected from N, 0, S;
W represents a bond or CR4R5, wherein
R4 and R5 are independently selected from the group consisting of hydrogen,
substituted
or unsubstituted C1_8 alkyl, C5_6 aryl, and C1_6 heteroaryl with heteroatoms
selected from
N, 0, S;
Y is a bond or is selected from the group consisting of substituted or
unsubstituted C1_8
alkyl, C1_8 alkenyl, C1-8 alkynyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, -CO-, and -CO-C210 heterocyclyl;
wherein C1_8 alkyl, C5_6 aryl, C1_6 heteroaryl, C2_10 heterocyclyl, C3_8
cycloalkyl, is
optionally substituted with one or more of the groups selected from hydrogen,
C1_6
alkyl, oxo (=0), C3-8 cycloalkyl, halogen, OH, and cyano;
Z represents a bond or is selected from the group consisting of C1_8 alkyl,
C1_8 alkenyl,
C1_8 alkynyl, C7_12 -alkylaryl, C7_12 -alkenylaryl, C7_15 -arylalkenyl, C2_12-
alkylheteroaryl,
-CO-C7-12 alkylaryl, -CO-C712 alkenylaryl, -CONR6-C1_8 alkyl, -NR6C 0-C1_8
alkyl-, -
NR6-C1_8 alkyl, -0-C1_8 alkyl-, -CONR6-05_6 aryl-, C5_6 aryl, C1_6 heteroaryl,
C2_10
heterocyclyl, -CO-C2_10 heterocyclyl, -NR6-00-0C1_8 alkyl, 0 -C 0-NR6-C1_8
alkyl, -
- 104 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
NR6CO-05_6 aryl-, -NR6-05-6 aryl, -NR6-C1_6 heteroaryl, -C1_8 alkyl-O-05_6
aryl, -0-05-6
aryl, 0-C 1_6 heteroaryl, -NR6-00-0C5_6 aryl, -CONR6-C7_12 alkenylaryl, -S02-
05_6 aryl, -
SO2-C712 alkylaryl, -NR6S 02-C7-12 alkylaryl, Ci_8 alkyl-CONR6-05_6 aryl, and
0-00-
NR6-05_6 aryl;
R6 is selected from the group consisting of hydrogen, C1_8 alkyl, C1_6
haloalkyl, C3-8
cycloalkyl, C5_6 aryl, and C1_6 heteroaryl, with heteroatoms selected from N,
0, S;
R1 is selected from the group consisting of hydrogen, halogen hydroxy, nitro,
cyano,
azido, nitroso, oxo (=0), thioxo (=S), -SO2-, amino, hydrazino, formyl, C1_8
alkyl, C1_8
haloalkyl, C1-8 alkoxy, C1_8 haloalkoxy, C7_12 arylalkoxy, C3_8 cycloalkyl, C3-
8
cycloalkyloxY, C5-6 aryl, C2-10 heterocyclyl, C1_6 heteroaryl, alkylamino, -
COORa, -
C(0)Rb, -C(S)Ra, -C(0)NRaRb, -C(S)NRaRb, -NRaC(0)NRbRc, NRaC(S)NRbRo -
N(Ra)SORb, -N(Ra)S 02Rb, -NRaC (0 ) ORb , -NRaRb, -NRaC (0 )Rb- , -NRaC(S )Rb-
, -
S ONRaRb- , -S02NRaRb-, -0Ra, -0RaC(0)0Rb-, -0C(0)NRaRb, 0C(0)Ra, -
OC(0)NRaRb-, -RaNRbRc, -RaORb-, -SRa, -SORa and -S02Ra, wherein Ra, Rb and Rc
is
independently selected from the group consisting of hydrogen, C1_8 alkyl, C3-8
cycloalkyl, C5-6 aryl, C7-15 arylalkyl, C2_10 heterocyclyl, C1_6 heteroaryl,
and C2-12
heteroarylalkyl with heteroatoms selected from N, 0, S;
wherein C7-12 arylalkOXy, C1_8 alkyl, C5-6 aryl, C1_6 heteroaryl, C2_10
heterocyclyl, C3-8
cycloalkyl, is optionally substituted with one or more of the groups selected
from
hydrogen, Ci_6 alkyl, C5-6 aryl, C1-6 heteroaryl, C2_10 heterocyclyl, oxo
(=0), C3-8
cycloalkyl, halogen, OH, amino, and cyano;
R3 is selected from the group consisting of hydrogen, substituted or
unsubstituted C 1_8
alkyl, and C5_6 aryl;
R2 is selected from the group consisting of ¨0R7, aniline, amino C5_6 aryl,
and amino
C1_6 heteroaryl,
wherein aniline, amino C5_6 aryl, and amino C1-6 heteroaryl, is optionally
substituted
with one or more of the groups selected from C1_8 alkyl, halogen, OH, amino,
and
cyano;
R7is selected from the group consisting of hydrogen, C1-8 alkyl, C5-6 aryl, C2-
10
heterocyclyl and ¨COR8, wherein R8 is selected from the group consisting of
C1_8 alkyl,
C5_6 aryl, Ci-6heteroaryl, C3_8 cycloalkyl, and C2-10 heterocyclyl.
[000125] According to an embodiment, the present disclosure relates to a
compound of
Formula I or its stereoisomers, pharmaceutically acceptable salts, complexes,
hydrates,
- 105 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
solvates, tautomers, polymorphs, racemic mixtures, optically active forms and
pharmaceutically active derivative thereof, which is selected from a group
consisting of:
1) (E)3(4(((2(4-Cyclopropylphenyl)cyclopropyl)amino)methyl)pheny1)-N-
hydroxyacrylamide TFA salt
2) (E)-3-(4-112-(4-Fluoro-pheny1)-cyclopropylaminol -methyl} -pheny1)-N-
hydroxy-
acrylamide TFA salt
3) (E)-3-(4-(((2-(4-((4-
Fluorobenzyl)oxy)phenyl)cyclopropyl)amino)methyl)pheny1)-N-
hydroxyacrylamideTFA salt
4) (E)-N-hydroxy-3-(4-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-1-
yl)phenyl)
acrylamideTFA salt
5) (E)-3-(4-(((2-(4'-Chloro-11,1'-bipheny11-4-
yl)cyclopropyl)amino)methyl)pheny1)-N-
hydroxyacrylamideTFA salt
6) (E)-3-(4-(((2-(4-(3,5-Dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)pheny1)-N-hydroxyacrylamide. TFA salt
7) (E)-N-hydroxy-3-(4-(((2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino)methyl)phenyl) acrylamide TFA salt
8) 2-(4-(((2-(4-Fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-y1)-N-
hydroxy
pyrimidine-5-carboxamide TFA salt
9) 2-14-(2-Phenyl-cyclopropylamino)-piperidin-1-y11-pyrimidine-5-
carboxylicacid
hydroxyamide TFA salt
10) 2-14-12-(4-Fluoropheny1)-cyclopropylaminol -piperidin-l-y1} -p yrimidine-5-
carboxylic acid hydroxyamide TFA salt
11) 2-(4-(((2-(4-((4-
Fluorobenzyl)oxy)phenyl)cyclopropyl)amino)methyl)piperidin-l-
y1)-N-hydroxypyrimidine-5-carboxamideTFA salt
12) 2-(4-((2-(4-((4-Fluorobenzyl)oxy)phenyl)cyclopropyl)amino)piperidin-l-y1)-
N-
hydroxypyrimidine-5-carboxamideTFA salt
13) 2-(44(2-(4'-Chloro-11,1'-bipheny11-4-yl)cyclopropyl)amino)piperidin-1-y1)-
N-
hydroxypyrimidine-5-carboxamideTFA salt
14) 2-(4-(((2-(4'-Chloro-11,1'-bipheny11-4-
yl)cyclopropyl)amino)methyl)piperidin-1-
y1)-N-hydroxypyrimidine-5-carboxamide TFA salt
15) 2-(4-(((2-(4'-Fluoro-11,1'-bipheny11-4-
yl)cyclopropyl)amino)methyl)piperidin-1-
y1)-N-hydroxypyrimidine-5-carboxamide TFA salt
- 106 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
16) 2-(4-(((2-(4-(3,5-Dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-y1)-N-hydroxypyrimidine-5-
carboxamide. TFA salt
17) N-hydroxy-2-(4-(((2-(4-(pyrimidin-5-yl)phenyl)cyclopropyl)amino)methyl)
piperidin-l-yl)pyrimidine-5-carboxamide.TFA salt
18) N-hydroxy-2-(4-(((2-(4-methoxyphenyl)cyclopropyl)amino)methyl)piperidin-l-
yl)pyrimidine-5-carboxamideTFA salt
19) N-hydroxy-2-(4-((2-(4-methoxyphenyl)cyclopropyl)amino)piperidin-l-
yl)pyrimidine-5-carboxamide TFA salt
20) 2-(4-((((1R,2S)-2-(4-Fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-y1)-
N-
hydroxypyrimidine-5-carboxamide TFA salt
21) 2-(4-((((lS ,2R)-2-(4-Fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-
y1)-N-
hydroxypyrimidine-5-carboxamide TFA salt
22) 4-(4-(((2-(4-Fluorophenyl)cyclopropyl)amino)methyl)piperidin-l-y1)-N-
hydroxybenzamide TFA salt
23) N-hydroxy-2-(2-(((2-phenylcyclopropyl)amino)methyl)-5,6-dihydroimidazo[1,2-
a[pyrazin-7(8H)-yl)pyrimidine-5-carboxamide TFA salt
24) N-hydroxy-2-(2-(((2-(4-methoxyphenyl)cyclopropyl)amino)methyl)-5,6-
dihydroimidazo[1,2-a[pyrazin-7(8H)-yl)pyrimidine-5-carboxamide TFA salt
25) 2-(2-(((2-(4-Fluorophenyl)cyclopropyl)amino)methy1)-5,6-dihydroimidazo[1,2-
a[pyrazin-7(8H)-y1)-N-hydroxypyrimidine-5-carboxamide TFA salt
26) 3-(((2-(4-Bromophenyl)cyclopropyl)amino)methyl)-N-hydroxybenzamide TFA
salt
27) N-hydroxy-3-(((2-phenylcyclopropyl)amino)methyl)benzamide TFA salt
28) N-hydroxy-4-(((2-phenylcyclopropyl)amino)methyl)benzamide TFA salt
29) N-hydroxy-6-((2-phenylcyclopropyl)amino)hexanamide TFA salt
30) 4-(3-((2-(4-Fluorophenyl)cyclopropyl)amino)propy1)-N-hydroxybenzamide TFA
salt
31) N-(6-Hydroxycarbamoyl-hexyl)-4-[(2-phenyl-cyclopropylamino)-methyl[-
benzamide TFA salt
32) 4-(((2-(4-Fluorophenyl)cyclopropyl)amino)methyl)-N-(7-(hydroxyamino)-7-
oxoheptyl)benzamide TFA salt
33) 4-(2-Phenyl-cyclopropylamino)-cyclohexanecarboxylic acid hydroxyamide TFA
salt
- 107 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
34) (1S,4R)-N-hydroxy-44(1S)-1-((2-
phenylcyclopropyl)amino)ethyl)cyclohexanecarboxamide TFA salt
35) N-hydroxy-4-((4-(((2-(4-(1-methy1-6-oxo-1,6-dihydropyridin-3-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-yl)methyl)benzamide TFA Salt
36) N-Hydroxy-4-14- [(2-phenyl-cyclopropylamino)-methyl] -piperidin-l-ylmethyl
} -
benzamide TFA salt
37) 4-((4-(((2-(4-(3,5-Dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-y1) methyl)-N-hydroxybenzamide
TFA salt
38) N-hydroxy-4-((4-(((2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)methyl)benzamide TFA salt
39) 6-((4-(((2-(4-(3,5-Dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-yl)methyl)-N-
hydroxynicotinamide
TFA salt
40) N-hydroxy-4-((4-(((2-phenylcyclopropyl)amino)methyl)-1H-pyrazol-1-
y1)methyl)
benzamide TFA salt
41) N-hydroxy-4-((4-(((2-phenylcyclopropyl)amino)methyl)-1H-1,2,3-triazol-1-
y1)
methyl)benzamide TFA salt
42) N-hydroxy-4-(2-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-1-
yl)ethyl)benzamide TFA salt
43) N-hydroxy-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-1-
yl)propyl)benzamide TFA salt
44) N-hydroxy-4-(3-(4-((2-phenylcyclopropyl)amino)piperidin-1-
yl)propyl)benzamideTFA salt
45) N-hydroxy-4-(3-(4-((methyl (2-phenylcyclopropyl) amino) methyl) piperidin-
1-y1)
propyl)benzamide TFA salt
46) N-hydroxy-4-(3-(6-((2-phenylcyclopropyl)amino)-2-azaspiro[3.3]heptan-2-
1)propyl) benzamide TFA salt
47) 4- [3-(4-1 [2-(4-Fluoropheny1)-c yclopropylamino] -methyl} -piperidin-l-
y1)-propyll -
N-hydroxy-benzamide TFA salt
48) 4-(3-(3-(((2-(4-Fluorophenyl)cyclopropyl)amino)methyl)azetidin-1-
yl)propy1)-N-
hydroxy benzamide TFA salt
49) 4-(3-(4-(((2-(3-Fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-
yl)propy1)-N-
hydroxy benzamide TFA salt
- 108 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
50) 4-(3 -(4-(((2-(3 ,4-Difluorophenyl)c ycloprop yl) amino)methyl)piperidin-1-
yl)propy1)-N-hydroxybenzamide TFA salt
51) N-hydroxy-4- (3 -(4- (((2-(4-
methoxyphenyl)cyclopropyl)amino)methyl)piperidin- 1 -
yl)propyl)benzamide TFA salt
52) N-hydroxy-4- (3 -(4- (((2-(4-(morpholine-4-
carbonyl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA
salt
53) N-hydroxy-4- (3 -(4- (((2-(4-(morpholine-4-
carbonyl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA
salt
54) N-hydroxy-4- (3 -(4- (((2-(4-(piperidine- 1-
carbonyl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA
salt
55) N-(2-(Dimethylamino)ethyl)-4 -(2- (((1-(3 -(4-
(hydroxycarbamoyl)phenyl)propyl)piperidin-4-
yl)methyl)amino)cyclopropyl)benzamide TFA salt
56) 4-(3 -(4- (((2-(4'-Chloro- [1,1'-biphenyl[ -4-yl)c yclopropyl)
amino)methyl)piperidin- 1-
yl)propy1)-N-hydroxybenzamide TFA salt
57) 4-(3 -(4- (((2-(4'-Fluoro- [1, l'-biphenyl[ -4- yl)c yclopropyl)
amino)methyl)piperidin-1-
yl)propy1)-N-hydroxybenzamide TFA salt
58) 4-(3 -(3 - (((2-(4'-Fluoro- [1, l'-biphenyl[ -4- yl)c yclopropyl)
amino)methyl) azetidin-1-
yl) propy1)-N-hydroxybenzamide TFA salt
59) 4-(3 -(4- (((2-(4'-C yano- [1, l'-biphenyl[ -4- yl)c yclopropyl)
amino)methyl)piperidin-1-
yl)propy1)-N-hydroxybenzamideTFA salt
60) N-hydroxy-4- (3 -(4- (((2-(4-(1-methy1-2-oxo-1,2-dihydropyridin-4-
yl)phenyl)cyclopropyl) amino)methyl)piperidin-l-yl)propyl)benzamide. TFA salt
61) N-hydroxy-4- (3 -(4- (((2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
62) N-hydroxy-4- (3 -(4- (((2-(4-(1-methyl- 1H-p yrazol-4-yl)phenyl)c ycloprop
yl) amino)
methyl)piperidin-l-y1) propyl) benzamide TFA salt
63) N-hydroxy-4- (3 -(3 - (((2-(4-(1-methyl- 1H-p yrazol-4-yl)phenyl)c
ycloprop yl) amino)
methyl) azetidin-1- yl)prop yl)benz amide
64) 4-(3-(4-(((2-(4-(3,5-Dimethylisoxazol-4-
yl)phenyl)cycloprop yl)amino)methyl)piperidin- 1-yl)prop y1)-N-hydroxybenz
amide TFA
salt
- 109 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
65) 3-(3-(3-(((2-(4-(3,5-Dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)azetidin-l-yl)propy1)-N-hydroxybenzamideTFA
salt
66) N-hydroxy-4-(3-(4-(((2-(4-(6-(trifluoromethyl)pyridin-3-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamideTFA salt
67) N-hydroxy-4-(3-(4-(((2-(1-isopropy1-1H-pyrazol-4-
y1)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzamideTFA salt
68) N-hydroxy-4-(3-(4-(((2-(1-pheny1-1H-pyrazol-4-
yl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzamideTFA salt
69) N-hydroxy-4-(3-(4-(((2-(2-methylthiazol-5-
yl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzamideTFA salt
70) N-hydroxy-4-(3-(4-(((2-(pyridin-3-yl)cyclopropyl)amino)methyl)piperidin-1-
yl)propyl) benzamideTFA salt
71) N-hydroxy-4-(3-(2-(((2-(4-methoxyphenyl)cyclopropyl)amino)methyl)-5,6-
dihydroimidazo[1,2-a[pyrazin-7(8H)-yl)propyl)benzamide TFA salt
72) 4-(3-(2-(((2-(4-Fluorophenyl)cyclopropyl)amino)methyl)-5,6-
dihydroimidazo[1,2-
a] pyrazin-7(8H)-yl)propy1)-N-hydroxybenzamide TFA salt
73) 4-(3 -(4-(((2-(3 ,4-Difluorophenyl)c ycloprop yl)amino)methyl) - 1H-
imidazol- 1-y1)
propy1)-N-hydroxybenzamide TFA salt
74) N-hydroxy-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)-1H-imidazol-1-
y1)propyl) benzamide TFA salt
75) N-hydroxy-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)-1H-imidazol-1-
y1)propyl) benzamide
76) N-hydroxy-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)-1H-pyrazol-1-
yl)propyl) benzamide TFA salt
77) N-hydroxy-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)-1H-1,2,3-triazol-1-
y1)propyl)benzamide TFA salt
78) 4-(3-(6-(((2-(4-Fluorophenyl)cyclopropyl)amino)methyl)-3,4-
dihydroisoquinolin-
2-(1H)-yl)propy1)-N-hydroxybenzamide TFA salt
79) 4-((7-(((2-(4-Fluorophenyl)cyclopropyl)amino)methyl)-3,4-
dihydroisoquinolin-
2(1H)-yl)methyl)-N-hydroxybenzamide TFA salt
80) 4-((2-(((2-(4-Fluorophenyl)cyclopropyl)amino)methyl)-5,6-
dihydroimidazo[1,2-
a[pyrazin-7 (8H)-yl)methyl)-N-hydroxybenzamide TFA salt
- 110 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
81) N-hydroxy -4-(3-(4(((2-(1,3,3,-trimethyl -2-oxoindoline-5-
yl)cyclopropyl)amino)methyl) piperidine-1-yl)propyl)benzamide TFA salt
82) N-hydroxy-4-(3-oxo-3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-1-
yl)propyl)benzamide TFA salt
83) N-hydroxy-4-(3-oxo-3-(4-((2-phenylcyclopropyl)amino)piperidin-1-
yl)propyl)benzamide TFA salt
84) N-hydroxy-4-(2-oxo-2-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-l-
yl)ethyl) benzamide TFA Salt
84A. N-hydroxy-4-(2-oxo-2-(4-((((1R,25)-2-
phenylcyclopropyl)amino)methyl)piperidin-l-y1) ethyl)benzamide
84B. N-hydroxy-4-(2-oxo-2-(4-((((lS,2R)-2-
phenylcyclopropyl)amino)methyl)piperidin-l-y1) ethyl)benzamide
85) N-hydroxy-4-((4-(((2-phenylcyclopropyl)amino)methyl)piperidin-l-
yl)sulfonyl)
benzamide TFA salt
86) N-hydroxy-4-((N-(2-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-l-
yl)ethyl)
sulfamoyl)methyl)benzamideTFA salt
87) 4-(N-(2-(4-(((2-(4-fluorophenyl)cyclopropyl)amino)methyl)piperidin-l-
yl)ethyl)
sulfamoy1)-N-hydroxybenzamide
88) N-hydroxy-4-(2-((4-(((2-phenylcyclopropyl)amino)methyl)piperidin-1-
yl)sulfonyl)ethyl) benzamide TFA salt
89) N-hydroxy-N4-(2-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-l-
yl)ethyl)
terephthalamideTFA salt
90) N1-(2-(4-(((2-(3,4-difluorophenyl)cyclopropyl)amino)methyl)piperidin-l-
yl)ethyl)-
N4-hydroxyterephthalamide TFA salt
91) N-hydroxy-4-((4-(2-((2-phenylcyclopropyl)amino)acetyl)piperazin-l-
yl)methyl)benzamide TFA salt
92) N-hydroxy-4-(3-oxo-3-(4-(2-((2-phenylcyclopropyl)amino)acetyl)piperazin-1-
yl)propyl)benzamide TFA salt
93) N-hydroxy-4-(3-(1-(2-((2-phenylcyclopropyl)amino)acetyl)piperidin-4-
yl)propyl)benzamide TFA salt
94) N-hydroxy-4-(3-(2-oxo-4-(((2-phenylcyclopropyl)amino)methyl)piperidin-l-
y1)
propyl)benzamide TFA salt
95) N-hydroxy-4-(2-((2-phenylcyclopropyl)amino)ethoxy)benzamide TFA salt
- 111-
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
96) 6-(2-(4-(((2-(4-fluorophenyl)cyclopropyl)amino)methyl)piperidin-l-
yl)ethoxy)-N-
hydroxynicotinamide TFA salt
97) N-hydroxy-6-(2-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-l-
yl)ethoxy)nicotinamide TFA salt
98) 6-(2-(4-(((2-(4'-Fluoro-[1,1'-biphenyl[-4-
yl)cyclopropyl)amino)methyl)piperidin-1-
y1) ethoxy)-N-hydroxynicotinamide TFA salt
99) N-hydroxy-4-(2-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-l-
yl)ethoxy)benzamide TFA salt
100) N-hydroxy-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-1-
yl)propoxy)benzamide TFA salt
101) N-hydroxy-4-(3-((2-phenylcyclopropyl)amino)propoxy)benzamide TFA salt
102) 2-((2-(4-(((2-(4-Fluorophenyl)cyclopropyl)amino)methyl)piperidin-l-
yl)ethyl)amino)-N-hydroxypyrimidine-5-carboxamide TFA salt
103) 5-(2-((2-(4-Fluorophenyl)cyclopropyl)amino)acety1)-N-hydroxy-4,5,6,7-
tetrahydro thieno[3,2-c[pyridine-2-carboxamide TFA salt
103A) 5-(2-(((1R,2S)-2-(4-Fluorophenyl)cyclopropyl)amino)acety1)-N-hydroxy-
4,5,6,7-tetrahydrothieno[3,2-c[pyridine-2-carboxamide TFA salt
103B) 5-(2-(((lS ,2R)-2-(4-Fluorophenyl)c yclopropyl)amino)acetyl) -N-hydroxy-
4,5,6,7-tetrahydrothieno[3,2-c[pyridine-2-carboxamide TFA salt
104) 2-(2-((2-(4-Fluorophenyl)cyclopropyl)amino)acety1)-N-hydroxy-1,2,3,4-
tetrahydro isoquinoline-7-carboxamide TFA salt
104A) 2-(2-(((15,2R)-2-(4-Fluorophenyl)cyclopropyl)amino)acety1)-N-hydroxy-
1,2,3,4-tetrahydroisoquinoline-7-carboxamide TFA salt
104B) 2-(2-(((1R,25)-2-(4-Fluorophenyl)cyclopropyl)amino)acety1)-N-hydroxy-
1,2,3,4-tetrahydroisoquinoline-7-carboxamide TFA salt
105) 5-(4-((2-(4-Fluorophenyl)cyclopropyl)amino)butanoy1)-N-hydroxy-4,5,6,7-
tetrahydro thieno[3,2-c[pyridine-2-carboxamide TFA salt
106) 5-(4-(4-(((2-(4-Fluorophenyl)cyclopropyl)amino)methyl)piperidin-l-
yl)butanoy1)-N-hydroxy-4,5,6,7-tetrahydrothieno[3,2-c[pyridine-2-carboxamide
TFA
salt
107) 2-(4-((2-(4-Fluorophenyl)cyclopropyl)amino)butanoy1)-N-hydroxy-1,2,3,4-
tetrahydro isoquinoline-7-carboxamide TFA salt
108) 2-(4-((2-(4-Fluorophenyl)cyclopropyl)amino)butanoy1)-N-hydroxyisoindoline-
5-
carboxamide TFA salt
- 112 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
109) N-hydroxy-2-(4-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-l-
yl)butanoyl) isoindoline-5-carboxamide TFA salt
110) N-hydroxy-2-(3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-1-
yl)propyl)
thiazole-4-carboxamide TFA salt
111) 2-(3-(4-(((2-(4'-Fluoro- [1,1'-biphenyl[ -4-yl)c
yclopropyl)amino)methyl)piperidin-
1-yl)propy1)-N-hydroxythiazole-4-carboxamide TFA salt
112) N-hydroxy-2-(3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-l-
yl)propyl)
thiazole-5-c arboxamide TFA salt
113) N-hydroxy-2-(3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-l-
yl)propyl)
oxazole-4-carboxamide TFA salt
114) (E)-N-hydroxy-4-(3-oxo-3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-
1-
y1) prop-I-en-1- yl)benzamide TFA salt
114A) N-hydroxy-4-((E)-3-oxo-3-(4-((((1R,2S )-2-
phenylcyclopropyl)amino)methyl)
piperidin- 1-yl)prop- 1-en- 1-yl)benzamide TFA salt
114B) N-hydroxy-4-((E)-3-oxo-3-(4-((((lS ,2R)-2-
phenylcyclopropyl)amino)methyl)
piperidin- 1-yl)prop- 1-en- 1-yl)benzamide TFA salt
115) 4-((E)-3 -(4-((((lS ,2R)-2-(4-Fluorophenyl)c ycloprop yl)amino)methyl)
piperidin-
1-y1)-3 -oxoprop-l-en- 1-y1)-N-hydroxyb enzamide TFA salt
115A)4-((E)-3 -(4-((((lS ,2R)-2-(4-Fluorophenyl)cyclopropyl)amino)methyl)
piperidin-
1-y1)-3 -oxoprop-l-en- 1-y1)-N-hydroxyb enzamide TFA salt
116) (E)-4-(3-(4-(((2-(4-(3,5-Dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl) piperidin-1 -y1)-3 -oxoprop-l-en- 1-y1)-N-
hydroxybenzamide TFA salt
117) (E)-N-hydroxy-4-(3-oxo-3-(4-(((2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino)
methyl)piperidin-1- yl)prop- 1-en-1 -yl)benzamide TFA salt
118) (E)-4-(3 -(3 -(((2-(4-Fluorophenyl)c ycloprop yl)amino)methyl)azetidin- 1-
y1)-3 -
oxoprop-1-en-1- y1)-N-hydroxybenzamideTFA salt
119) (E)-N-hydroxy-4-(3-(3-(((2-(4-(1-methyl- 1H-pyrazol-4-
yl)phenyl)cycloprop yl)amino)methyl)azetidin- 1-y1)-3 -oxoprop- 1-en- 1-
yl)benz amide
TFA salt
120) (E)-N-(2-aminopheny1)-3-(4-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)phenyl)acrylamide TFA salt
121) N-(2- aminopheny1)-4-(3-(4-(((2-phenylc yclopropyl)amino)methyl)pip
eridin-1-
yl)propyl)benzamide TFA salt
- 113 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
122) N-(2-aminopheny1)-4-(3-(4-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
123) N-(2-aminopheny1)-4-(3-(4-(((2-(4-
methoxyphenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA
salt
124) N-(2-aminopheny1)-4-(3-(4-(((2-(3,4-
difluorophenyl)cyclopropyl)amino)methyl)
piperidin-l-yl)propyl)benzamide TFA salt
125) N-(2-aminopheny1)-4-(3-(4-(((2-(4-(piperidine-1-
carbonyl)phenyl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzamide TFA
salt
126) N-(2-aminopheny1)-4-(3-(3-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)azetidin-l-y1) propyl)benzamide TFA salt
127) N-(2-aminopheny1)-4-(3-(6-((2-phenylcyclopropyl)amino)-2-
azaspiro[3.3]heptan-
2-y1)propyl)benzamide TFA salt
128) N-(2-aminopheny1)-4-(3-(4-(((2-(1-isopropy1-1H-pyrazol-4-
y1)cyclopropyl)amino)methyl)piperidin-1-y1)propyl)benzamide TFA salt
129) N-(2-aminopheny1)-4-(3-(4-(((2-(1-pheny1-1H-pyrazol-4-
y1)cyclopropyl)amino)methyl)piperidin-1-y1)propyl)benzamide TFA salt
130) N-(2-aminopheny1)-4-(3-(4-(((2-(2-methylthiazol-5-
yl)cyclopropyl)amino)methyl) piperidin-l-yl)propyl)benzamide TFA salt
131) N-(2-aminopheny1)-4-(3-(4-(((2-(pyridin-3-
yl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
132) N-(2-amino-5-fluoropheny1)-4-(3-(4-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzamide TFA salt
133) N-(2-aminopheny1)-4-(3-oxo-3-(4-((2-phenylcyclopropyl)amino)piperidin-1-
yl)propyl)benzamide TFA salt
134) N-(2-aminopheny1)-4-(3-oxo-3-(4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
135) N-(2-aminopheny1)-4-(3-(4-(((2-(3,4-
difluorophenyl)cyclopropyl)amino)methyl)-
1H-imidazol-1-yl)propyl)benzamide TFA salt
136) N-(2-aminopheny1)-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)-1H-
imidazol-
1-yl)propyl)benzamide TFA salt
137) N-(2-aminopheny1)-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)-1H-1,2,3-
triazol-1-yl)propyl)benzamide TFA salt
138) N-(2-aminopheny1)-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)-1H-pyrazol-
1-
y1)propyl)benzamide TFA salt
- 114 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
139) N-(2-aminopheny1)-4-(2-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-l-
y1)
ethyl)benzamide TFA salt
140) N-(2-aminopheny1)-4-((4-((((lR,2S)-2-
phenylcyclopropyl)amino)methyl)piperidin-1-y1) methyl)benzamide TFA salt
141) N-(2-aminopheny1)-4-((4-(((2-(4-(1-methy1-6-oxo-1,6-dihydropyridin-3-
yl)phenyl) cyclopropyl)amino)methyl)piperidin-l-yl)methyl)benzamide TFA salt
142) N-(2-aminopheny1)-4-((4-(((2-(4-(1-methy1-1H-p yrazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)methyl)benzamide TFA salt
143) N-(2-aminopheny1)-4-((4-(((2-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino) methyl)piperidin-l-yl)methyl)benzamide TFA salt
144) N-(2-aminopheny1)-4-((4-(((2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)methyl)benzamide TFA salt
145) N-(2-aminopheny1)-4-((4-(((2-phenylcyclopropyl)amino)methyl)-1H-pyrazol-1-
y1)methyl)benzamideTFA salt
146) N-(2-aminopheny1)-4-((4-(((2-phenylcyclopropyl)amino)methyl)-1H-1,2,3-
triazol-1-yl)methyl)benzamideTFA salt
147) N-(2-aminopheny1)-4-(2-(4-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-l-y1) -2-oxoethyl)benzamide
TFA
salt
148) N-(2-aminopheny1)-4-(2-((2-(4-fluorophenyl) cyclopropyl) amino) ethoxy)
benzamide TFA salt
149) N-(2-aminopheny1)-6-(2-(4-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-l-yl)ethoxy)nicotinamide TFA
salt
150) N-(-2-aminopheny1)-2-((2-4(((2-(4-
flurophenyl)cyclopropyl)amino)methyl)piperdine-1-yl)ethyl)amino)pyrimidine-5-
carboxamide TFA salt
151) N-(2-aminopheny1)-5-((2-(4-fluorophenyl)cyclopropyl)glycy1)-4,5,6,7-
tetrahydrothieno[3,2-c]pyridine-2-carboxamide TFA salt
152) N-(2-aminopheny1)-2-(2-((2-(4-fluorophenyl)cyclopropyl)amino)acety1)-
1,2,3,4-
tetrahydroisoquinoline-7-carboxamide TFA salt
153) N-(2-aminopheny1)-2-(3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-1-
yl)propyl)oxazole-4-carboxamide TFA salt
154) N-(2-aminopheny1)-2-(3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-1-
yl)propyl)thiazole-5-carboxamide TFA salt
- 115 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
155) N-(2-aminopheny1)-4-((2-((2-(4-fluorophenyl)cyclopropyl)amino)acetamido)
methyl)benzamide TFA salt
156) (E)-N-(2-aminopheny1)-4-(3-(4-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-l-y1)-3-oxoprop-1-en-1-
yl)benzamide TFA salt
157) (E)-N-(2-aminopheny1)-4-(3-(3-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)azetidin-l-y1)-3-oxoprop-1-en-1-
y1)benzamide
TFA salt
158) N-(4-((2-aminophenyl)carbamoyl)benzy1)-4-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidine-l-carboxamide TFA salt
159) N-(2-aminopheny1)-4-(3-(2-oxo-4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
160) N-(2-aminopheny1)-4-((4-(((2-phenylcyclopropyl)amino)methyl)piperidin-1-
yl)sulfonyl) benzamide TFA salt
161) N-(2-aminopheny1)-4-(((4-(((2-phenylcyclopropyl)amino)methyl)piperidin-1-
yl)sulfonyl)methyl)benzamide TFA salt
162) N-(2-aminopheny1)-4-(2-((4-(((2-phenylcyclopropyl)amino)methyl)piperidin-
1-
yl)sulfonyl)ethyl)benzamide TFA salt
[000126] In an embodiment, the invention relates to a process of preparation
of
compounds of Formula (I) or its tautomers, polymorphs, stereoisomers,
prodrugs,
solvate, co-crystals or pharmaceutically acceptable salts thereof.
[000127] In another embodiment, the invention relates to a pharmaceutical
composition comprising a compound of Formula (I) or a pharmaceutically
acceptable
salt thereof of together with a pharmaceutically acceptable carrier,
optionally in
combination with one or more other pharmaceutical compositions.
[000128] In yet another embodiment, the invention relates to the
pharmaceutical
composition as described herein, wherein the composition is in the form
selected from
the group consisting of a tablet, capsule, powder, syrup, solution, aerosol,
and
suspension.
[000129] In an embodiment, the invention relates to the compound of Formula I
or a
pharmaceutically acceptable salt thereof for use in the manufacture of a
medicament
for inhibiting LSD1 enzymes in a cell.
- 116 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
[000130] In another embodiment, the invention relates to A method of
inhibiting
LSD1 in a cell, comprising treating said cell with an effective amount of the
compound of Formula I.
[000131] In yet another embodiment, the invention relates to amethod of
treating a
condition mediated by LSD1 comprising administering to a subject suffering
from a
condition mediated by LSD1, a therapeutically effective amount of the compound
of
Formula Ior the pharmaceutical composition described herein.
[000132] In an embodiment, the invention relates to a compound of Formula I or
a
pharmaceutically acceptable salt thereof for use in the manufacture of a
medicament
for inhibiting HDAC enzymes in a cell.
[000133] In another embodiment, the invention relates to amethod of inhibiting
HDAC in a cell comprising treating said cell with an effective amount of the
compound of Formula I.
[000134] In yet another embodiment, the invention relates to a method of
treating a
condition mediated by HDAC, comprising administering to a subject suffering
from a
condition mediated by HDAC, a therapeutically effective amount of the compound
of
Formula I or the pharmaceutical composition as described herein.
[000135] In an embodiment, the invention relates to a compound of Formula I or
a
pharmaceutically acceptable salt thereof for use in the manufacture of a
medicament
for inhibiting both LSD1 and HDAC enzymes in a cell.
[000136] In another embodiment, the invention relates to a method of
inhibiting both
LSD1 and HDAC in a cell comprising treating said cell with an effective amount
of
the compound of Formula I.
[000137] In yet another embodiment, the invention relates to a method of
treating a
condition mediated by both LSD1 and HDAC, comprising administering to a
subject
suffering from a condition mediated by both LSD1 and HDAC, a therapeutically
effective amount of the compound of Formula I or the pharmaceutical
composition.
[000138] In an embodiment, the invention relates to amethod for the treatment
and/or
prevention of a proliferative disorder or cancer, comprising administering to
a subject
suffering from the proliferative disorder or cancer a therapeutically
effective amount
of the compound of Formula I or the pharmaceutical composition. In another
embodiment, the invention relates to the method as described herein, wherein
said
compound or composition is administered in combination with at least one
compound
selected from cytotoxic agents and non-cytotoxic agents to a subject in need
thereof.
- 117 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
[000139] In yet another embodiment, the invention relates to use of the
compounds of
Formula I or the pharmaceutical composition for treatment of a condition
mediated by
LSD1; treatment and/or prevention of a proliferative disorder or cancer; or
treatment
of cancer together with other clinically relevant cytotoxic agents or non-
cytotoxic
agents.
[000140] In an embodiment, the invention relates to a method for the treatment
and/or
prevention of a condition mediated by LSD1 or a proliferative disorder or
cancer,
comprising administering to a subject suffering from the condition mediated by
LSD1
or the proliferative disorder or cancer, a therapeutically effective amount of
the
compound or the pharmaceutical composition.
[000141] In another embodiment, the invention relates to use of the compounds
of
Formula I or the pharmaceutical composition for: treatment of a condition
mediated
by HDAC; treatment and/or prevention of a proliferative disorder or cancer; or
treatment of cancer together with other clinically relevant cytotoxic agents
or non-
cytotoxic agents.
[000142] In yet another embodiment, the invention relates to a method for the
treatment and/or prevention of a condition mediated by HDAC or a proliferative
disorder or cancer, comprising administering to a subject suffering from the
condition
mediated by HDAC or the proliferative disorder or cancer, a therapeutically
effective
amount of the compound of Formula I or the pharmaceutical composition.
[000143] In an embodiment, the invention relates to use of the compounds of
Formula I or the pharmaceutical composition for: treatment of a condition
mediated
by both LSD1 and HDAC; treatment and/or prevention of a proliferative disorder
or
cancer; or treatment of cancer together with other clinically relevant
cytotoxic agents
or non-cytotoxic agents.
[000144] In another embodiment, the invention relates to a method for the
treatment
and/or prevention of a condition mediated by both LSD1 and HDAC or a
proliferative
disorder or cancer, comprising administering to a subject suffering from the
condition
mediated by both LSD1 and HDAC or the proliferative disorder or cancer, a
therapeutically effective amount of the compound of Formula I or the
pharmaceutical
composition.
[000145] In yet another embodiment, the invention relates to a method for the
treatment of cancer, said method comprising administering a combination of the
- 118 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
compounds of Formula I or the pharmaceutical composition, with other
clinically
relevant cytotoxic agents or non-cytotoxic agents to a subject in need
thereof.
[000146] In an embodiment, the invention relates to a method of treatment of
cancer,
said method comprising administering a combination of the compounds of Formula
I
or the pharmaceutical composition, with other clinically relevant immune
modulators
agents to a subject in need of thereof.
[000147] The invention also provides a method of treatment of cancer in
patients
including administration of a therapeutically effective amount of a compound
of
Formula (I).
[000148] The invention also provides a method for treatment of proliferative
conditions or cancer, comprising administering to a subject suffering from
proliferative conditions or cancer, a therapeutically effective amount of a
compound
of Formula (I), in the presence or absence of other clinically relevant
cytotoxic agents
or non-cytotoxic agents to a subject in need thereof.
[000149] The present invention provides a method of treatment of a disorder
caused
by, associated with or accompanied by disruptions of cell proliferation and/or
angiogenesis and the subsequent metastasis including administration of a
therapeutically effectiveamount of a compound of Formula (I).
[000150] The invention provides a method of treatment of cancer in patient
including
administration of effective amount of compounds of Formula (I). The cancer can
be
either a hematologic malignancy or solid tumor. Hematological malignancy is
selected
from the group consisting of B-cell lymphoma, T-cell lymphoma and leukemia. In
the
case of solid tumors, the tumors are selected from the group consisting of
breast
cancer, lung cancer, ovarian cancer, prostate cancer, head cancer, neck
cancer, renal
cancer, gastric cancer, colon cancer, pancreatic cancer and brain cancer.
[000151] As discussed above, the compounds of the present invention are useful
for
treating proliferative diseases. A proliferative disease includes, for
example, a tumor
disease and/or metastases. Compounds of the present invention are useful for
treating
a proliferative disease that is refractory to the treatment with other
chemotherapeutics;
or a tumor that is refractory to treatment with other therapeutics due to
multidrug
resistance.
[000152] Compounds of the present invention are able to slow tumor growth,
stop
tumor growth or bring about the regression of tumors and to prevent the
formation of
tumor metastasis (including micrometastasis) and the growth of metastasis
- 119 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
(includingmicrometastasis). In addition, they can be used in epidermal
hyperproliferation.
The compound of formula I of the present invention can be used as a
prophylactic or
therapeutic agent for cancer. Examples of the cancer include, but not
restricted
to,breast cancer, prostate cancer, pancreatic cancer, gastric cancer, lung
cancer, colon
cancer, rectal cancer, esophagus cancer, duodenal cancer, tongue cancer,
pharyngeal
cancer, brain tumor, neurinoma, non-small cell lung cancer, small cell lung
cancer,
liver cancer, kidney cancer, bile duct cancer, uterine body cancer, cervical
cancer,
ovarian cancer, urinary bladder, skin cancer, hemangioma, malignant lymphoma,
malignant melanoma, thyroid cancer, bone tumor, vascular fibroma,
retinoblastoma,
penile cancer, pediatric solid cancer, lymphoma, myeloma and leukemia
(including,
for example acute myelogenous leukemia (AML), chronic myelogenous leukemia
(CML), chronic neutrophilic leukemia, chronic eosinophilic leukemia, chronic
lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL) or hariy cell
leukemia) or cutaneous T-cell lymphoma (CTCL).
[000153] In one embodiment, the invention provides a method of inhibiting both
LSD-1 and HDACactivity comprising administering, to a patient in need of
treatment,
an amount of a composition comprising a compound of formula I and a
pharmaceutically acceptable carrier sufficient to inhibit both LSD-1 and HDAC
activity.
[000154] In one aspect of this embodiment, the invention provides a compound
of
formula I for use in inhibitingboth LSD-land HDAC. In a related aspect, the
invention
provides for the use of a compound of formula I for the manufacture of a
medicament
for inhibiting both LSD-1 and HDAC.
[000155] In one embodiment, the invention provides a method of treating and/or
preventing a neurodegenerative disease or disorder comprising administering,
to a
patient in need of treatment, a therapeutically effectively amount of a
composition
comprising a compound of formula I and a pharmaceutically acceptable carrier.
[000156] In one aspect of this embodiment, the invention provides a compound
of
formula I for use in treating and/or preventing a neurodegenerative disorder
or
condition. In a related aspect, the invention provides for the use of a
compound of
formula I for the manufacture of a medicament for treating and/or preventing a
neurodegenerative disorder or condition.
- 120 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
[000157] In another aspect, the compound may be administered in combination
therapy by combining the compound of Formula (I) with one or more separate
agents,
not limited to targets such as DNA methyltransferase, heat shock proteins
(e.g.
HSP90), kinase, epigenetic and other matrix metalloproteinases.
[000158] "Combination therapy" includes the administration of the subject
compounds in further combination with other biologically active ingredients
(such as,
but are not limited to, different antineoplastic agent) and non-drug therapies
(such as,
but are not limited to, surgery or radiation treatment). The compounds
described
herein can be used in combination with other pharmaceutically active
compounds,
preferably, whichwill enhance the effect of the compounds of the invention.
The
compounds can beadministered simultaneously or sequentially to the other drug
therapy.
[000159] In another aspect, the subject compounds may be combined with
theantineoplastic agents (e.g. small molecules, cytotoxic reagents, non-
cytotoxic
reagents,monoclonal antibodies, antisense RNA andfusion proteins) that inhibit
one or
more biological targets. Such combination mayenhance therapeutic efficacy over
the
efficacy achieved by any of the agents alone andmay prevent or delay the
appearance
of resistant variants.
[000160] In another aspect, the subject compounds may be combined with
immunoncology drugs not restricting to PDL-1, IDO, TDO, CTLA4 or any other
drugs which is involved in the immune modulation.
EXAMPLES
[000161] The following examples provide the details about the synthesis,
activities,
and applications of the compounds of the present disclosure. It should be
understood the
following is representative only, and that the invention is not limited by the
details set
forth in these examples.
[000162] There is also provided a process as shown in the following scheme-1,
for the
preparation of compounds of the Formula (I), wherein all the groups are as
defined
earlier.
- 121 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Aldehyde or ketone R3
R3
R( R3 N, R3 reducing agent Ar N. X, NH R2 Y
0
or Alkylation R( IN R1A r`\7" N w
Z NHR2
1 Step 1 2 Step 2 Formula I
Scheme 1
The said process for the preparation of the compounds of Formula (I) comprises
of the
following:
Step 1: Compound 1 werereacted with an aldehyde or ketone in protic solvents
such
as Me0H, etc., to give the intermediate imine which was reacted with sodium
borohydride (NaBH4) or its equivalent to give the compound 2 or compound of 1
were
alkylated with the corresponding substituted halo compound in the presence of
inorganic or organic base to give the compound 2.
Step 2: Hydrolyzing the intermediate compound 2 with an inorganic base gave
the
corresponding acid. Coupling the acid with activating agents such as EDCI.HC1
(1 (3-
dimethylaminopropy1)-3-ethylcarbodiimide hydrochloride) and HOBt (1-
hydroxybenzotriazole) or (1-propylphosphonic anhydride)T3P/triethylamine and
the
like in the presence of the respective amine NH2R2 to yield the compound of
the
general Formula (I) or alternatively reacting the intermediate compound 2 with
NH2R2
and an inorganic base gave the compound of Formula (I)
Synthesis of Intermediates
A-1-methyl 2-(4-formylpiperidin-1-yl)pyrimidine-5-carboxylate
HO
0
N
N y.LO
NO _______________________________________________ v.
I N N
CI N Step 1 HO.) Step 2
OHC
A-1
Step 1: methyl 2-(4-(hydroxymethyl) piperidin-1-y1) pyrimidine-5-carboxylate-
II
IsV 0
N N
HO II
)
To a stirred solution of methyl 2-chloropyrimidine-5-carboxylate (I, 2.5 g,
14.53
mmol) in DMF (25 mL) was added piperidin-4-ylmethanol (2 g, 17.44 mmol) and
potassium carbonate (4.01 g, 29.07 mmol) and stirred for 5 hours at room
temperature.
Progress of reaction followed TLC, after completion of reaction, the reaction
mixture
- 122 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
was concentrated and quenched with water (100 mL) extracted with ethyl acetate
(2 x
150 mL). The organic portion was washed with water, brine, dried over sodium
sulphate and concentrated under reduced pressure to afford the crude product
which
was purified by column chromatography using methanol-dichloromethane gradient
to
afford the titled product as off-white solid (II, 3.6 g, 83%). LC-MS m/z calcd
for
C12H17N303, 251.1, found 252.1[M+1-1] .
Step 2: methyl 2-(4-formylpiperidin-l-y1) pyrimidine-5-carboxylate-A-1
o
NlyLO
I
N
OHC
A-1
To a stirred solution of DMSO (5.6 g, 171.71 mmol) in dichloromethane (40 mL)
was
added oxalyl chloride (6.02 g, 47.81 mmol) at -78 C (drop-wise) and continue
stirred
for 30 C, methyl 2-(4-(hydroxymethyl)piperidin-1-yl)pyrimidine-5-carboxylate
(II, 3
g, 11.95 mmol) dissolved in dichloromethane (10 mL) was slowly added and
continue
stirred for 3 h at -78 C (drop-wise). To the reaction mixture triethylamine
(14.4 g,
143.42 mmol) was added and stirred for 12 h at room temperature. Progress of
reaction followed by TLC, reaction mixture quenched with ammonium chloride
(100
mL), extracted with ethylacetate (2 X 150 mL). The organic portion was washed
with
water and brine dried over sodium sulphate and concentrated under reduced
pressure
to afford the crude product which was purified by column chromatography using
methanol-dichloromethane gradient to afford the titled product as off-white
solid (A-1,
2 g, 67 %). LC-MS m/z calcd for C12H15N303, 249.1, found 250.1[M+H]t
A-2 methyl (E)-3-(4-(4-formylpiperidin-l-yl)phenyl)acrylate
OHC
N
0
/
0
The intermediate A-2 was synthesized using methyl-4-fluorocinnamic acid ester
and
piperidin-4-yl-methanol using the procedure for synthesizing A-1. LC-MS m/z
calcd
for C16H19NO3 273.1, found 274.1[M+1-1] .
A-3- ethyl 2-(4-oxopiperidin-l-yl)pyrimidine-5-carboxylate
- 123 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
NN
NrO
The intermediate A-3 was synthesized usingethyl 2-chloropyrimidine-5-
carboxylate
and 4-oxo-piperidine using the procedure for synthesizing A-1.
LC/MS m/z calcd for C12H15N303, 249.1, found 250.1 [M+H]
A-4 methyl 2-(2-formy1-5,6-dihydroimidazo[1,2-a]pyrazin-7(8H)-yl)pyrimidine-5-
carboxylate
p 10H Step2
Stepl
N OH
V
IV
N
I Step3 Step 4 N
N_
N
0 0
VI A-4
Step 1: 2-Hydroxymethy1-5,6-dihydro-8H-imidazo[1,2-a]pyrazine-7-carboxylic
acid tert-butyl ester (IV)
0
N OH
To a stirred solution of 7-tert-butyl 2-methyl 5,6-dihydroimidazo[1,2-
a]pyrazine-
2,7(8H)-dicarboxylate (III, 0.42 g, 1.42 mmol) in dry tetrahydrofuran (12 mL)
was
added diisobutylaluminium hydride (DIBAL-H) (4.97 mL, 4.98 mmol, 1M solution
of
THF) drop-wise at -35 C to -40 C. After completion of addition, the reaction
mixture was allowed to stir at room temperature for 3 h. The progress of the
reaction
was monitored by TLC. The reaction mixture was quenched with saturated
ammonium chloride solution at -30 C and was extracted with dichloromethane (3
x
50 mL). The combined organic extract was washed with water, brine dried over
sodium sulphate and concentrated under reduced pressure to get the titled
product as
an off-white solid (IV, 0.34 g, 94 %). LC-MS m/z calcd for C12H19N303, 253.1;
found
254.4 [M+1-1] .
- 124 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Step 2: (5,6,7,8-Tetrahydro-imidazo[1,2-a]pyrazin-2-y1)-methanol hydrochloride
(v)
CIH
N OH
To a solution of 2-hydroxymethy1-5,6-dihydro-8H-imidazo[1,2-a[pyrazine-7-
carboxylic acid tert-butyl ester (IV, 0.34 g, 1.34 mmol) in dry methanol (12
mL) was
added 20% HC1 in dioxane (18 mL) at 0 C and the resulting mixture was stirred
at
room temperature for 16 h. The solvent was evaporated under reduced pressure
to get
the crude product which was triturated with diethylether to afford the titled
product as
a pale-yellow solid (V, 0.25 g, 95 %). LC-MS m/z calcd for C7H11N30, 153.1;
found
154.2 [M+I-1] .
Step 3: 2-
(2-Hydroxymethy1-5,6-dihydro-8H-imidazo[1,2-a]pyrazin-7-y1)-
pyrimidine-5-carboxylic acid methyl ester (VI)
HON--(N\
I N
0
To a
suspension of (5,6,7 ,8-tetrahydro-imidazo [1,2- al pyrazin-2-y1)-methanol
hydrochloride (V, 0.35 g, 1.85 mmol) was added potassium carbonate (0.51 g,
3.71
mmol) at 0 C and stirred at that temperature for 5 min. Then, 2-chloro-
pyrimidine-5-
carboxylic acid methyl ester (0.38 g, 2.22 mmol) was added and the resulting
mixture
was stirred at room temperature for 15 h. The reaction mixture was quenched
with ice
and the solvent was evaporated to get the residue. Water was added and
precipitate
formed was filtered,ashed with water and n-hexane to afford the pure product
as an
off-white solid (VI, 0.36 g, 68%). LC-MS m/z calcd for C13H15N503, 289.1;
found
290.1 [M+H]t
Step 4: 2-(2-Formy1-5,6-dihydro-8H-imidazo[1,2-a]pyrazin-7-y1)-pyrimidine-5-
carboxylic acid methyl ester-Intermediate A-4
oNi
0
To a solution of 2-(2-Hydroxymethy1-5,6-dihydro-8H-imidazo[1,2-a[pyrazin-7-y1)-
pyrimidine-5-carboxylic acid methyl ester (VI, 0.36 g, 1.24 mmol) in dry
dichloromethane (15 mL) was added Dess-martin periodinane (1.32 g, 3.11 mmol)
at
0 Cand the resulting mixture was stirred at room temperature for 2h. The
progress of
- 125 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
the reaction was monitored by TLC. The reaction mixture was quenched with
saturated sodium bicarbonate solution. Aqueous solution of sodium thiosulphate
(10
mL, 10%)was added and stirred for 15 min. Then diluted with dichloromethane
and
the organic portion was washed with saturated sodium bicarbonate solution,
water and
brine solution, dried over sodium sulphate and concentrated under reduced
pressure to
afford the crude which was then triturated with n-pentane to afford the titled
product
as an off white solid (A-4, 0.35 g, 95%), LC-MS m/z calcd for C13H13N503,
287.1;
found 288.1 [M+H]t
A-5- methyl 7-(4-formylbenzamido)heptanoate-procedure
OHC 0 OHC 10 s
OH + H2N i
,OMe H
N ,OMe
0
0 VII 0 0
A-5
To a stirred solution of 4-formylbenzoic acid (1 g, 6.66 mmol) andmethyl 7-
aminoheptanoate (VII, 1.16 g, 7.33 mmol) in dichloromethane (30 mL) was added
triethylamine (2.3 mL, 16.6 mmol), the reaction mixture was stirred at room
temperature for 10min and then cooled reaction mixture to 0 C and added T3P
(6.35
mL, 10 mmol) and was stirred at room temperature for 3 h.Reaction was
monitored by
TLC.After completion of reaction, the mixture was quenched with ice.The
reaction
mixture was diluted with water and extracted with dichloromethane (3x25 mL).
The
organic portion was washed with water, brine, dried over sodium sulphate and
concentrated under reduced pressure to get the title compound as solid. (A-5,
1.8 g, 92
%). LC-MS m/z calcd for C16H21N04, 291.1; found 292.2 [M+H]t
A-6- 1-(2,2,2-trifluoroacetyl)piperidine-4-carbaldehyde
o o
NH Step 1 N)'LCF3 Step 2 N LCF3
HO) -I" HO)
VIII IX A-6
Step 1: 2,2,2-trifluoro-1-(4-(hydroxymethyppiperidin-1-ypethanone (IX)
0
N ACF3
HO)
To a stirred solution of piperidin-4-ylmethanol (VIII, 5.0 g, 4.3mmo1) in
dichloromethane (200 mL) was addedtriethylamine at 0 C and followed by
trifluoro
acetic anhydride, and the reaction mixture was stirred at room temperature
about 12
h.The progress of the reaction was monitored by TLC. The reaction mixture was
- 126 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
diluted with dichloromethane,the organic portion was washed with saturated
ammonium chloride, water, followed by brine solution, dried over sodium
sulphate
and concentrated under reduced pressure to get the product as sticky oil (IX,
8.5g, 92
%). LC-MS m/z calcd for C8H12F3N0, 211.1; found 212.1 [M+H]t
Step 2: 1-(2,2,2-trifluoroacetyl)piperidine-4-carbaldehyde (A-6)
0
tIiJN
).LCF3
C)
A solution of dimethyl sulfoxide (4 mL) and dichloromethane (60 mL) was cooled
to -
68 C.Oxaly1 chloride (3.2 mL) was slowly added drop-wise and the reaction
mixture
was stirred for 30 min at -68 C.Then a solution of 2,2,2-trifluoro-1-(4-
(hydroxymethyl)piperidin-l-yl)ethanone (IX, 2 g, 9.48 mmol) in 4 mL of
dichloromethane and was added dropwise at -68 C, after completion of addition,
the
reaction mixture was stirred for 1.5 h at -68 C and this was followed by drop-
wise
addition of triethylamine at -68 C. The reaction mixture was stirred at-68 C
for 4-6
h.The reaction mixture was then allowed to warm to room temperature and the
stirring
was continued for 16 h. The progress of the reaction was monitored by TLC. The
reaction mixture was diluted withethylacetate and the organic portion was
washed
with water, saturated ammonium chloride, brine, dried over sodium sulphate and
concentrated under reduced pressure to get theproduct as sticky oil (A-6, 1.9
g, 96 %),
LC-MS m/z calcd for C8I-110F3NO2, 209.1; found 210.1 [M+H]t
A-7: Ethyl 4-((4-formy1-1H-1,2,3-triazol-1-yl)methyl)benzoate
o o o
0r N 0 ...,----
,, ..,,
40 0 N 0
N3
-.:--N 4111
HO =--NI
4 - Step-1 Step-2 )--c
\----c N
H
X XI A-7
Step-1: Ethyl 4-44-(hydroxymethyl)-1H-1,2,3-triazol-1-y1)methyl)benzoate-XI
o
(:)
Ho, ii`lN
N 00
To a stirred solution of ethyl 4-(azidomethyl)benzoate (X, 2 g, 9.75 mmol) in
DMF
(80 mL) was added propargyl alcohol (0.6 mL, 10.7mmo1) andDIPEA (2.7 mL, 14.6
mmol) and then copper (I) iodide (0.9 g, 4.87 mmol) was added. The reaction
mixture
was stirred for 30 min at room temperature. Saturated ammonium chloride
solution
with few drop of ammonia (20 mL) was added and extracted with ethylacetate
(2x100
- 127 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
mL). The organic portion was washed with water, brine, dried over sodium
sulphate
and concentrated under reduced pressure to afford the crude compound which was
purified by column chromatography using ethylacetate-hexane gradient as eluent
to
afford the titled product as sticky oil (XI, 2.1 g, 87 %). LC-MS m/z calcd for
C13H15N303, 261.1; found 262.1 [M+1-1] .
Step-2: ethyl 4-((4-formy1-1H-1,2,3-triazol-1-yl)methyl)benzoate-Intermediate
A-
7
0
40 o-----
1---N
H
To a
stirred solution of ethyl 4-((4-(hydroxymethyl)-1H-1,2,3 -triazol-1-
yl)methyl)benzoate (XI, 1 g, 3.83 mmol) in ethylacetate (25 mL) was added IBX
(1.6
g, 5.74 mmol) and heated at 75 Cfor 16 h. Water (50 mL) was added and
extracted
with ethyl acetate (2x50 mL). The organic portion was washed with water,
brine,
dried over sodium sulphate and concentrated under reduced pressure to afford
the
crude compound which was purified by column chromatography using ethylacetate-
hexane gradient as eluentto afford the titled product as solid (A-7, 0.75 g,
76 %). LC-
MS m/z calcd for C13H13N303, 259.1; found 260.1 [M+H]t
A-8 and A-9: ethyl 4-(3-(4-formy1-1H-imidazol-1-yl)propyl)benzoate and ethyl 4-
(3-(5-formy1-1H-imidazol-1-yl)propyl)benzoate
H
0.....?,,
Br
0 N---'
+
N-- 0
A-8 A-9 0
xi!
To a stirred solution of sodium hydride (0.124 g, 5.20 mmol) in THF (3.5 mL)
was
added 1H-imidazole-4-carbaldehyde (0.5 g, 5.20 mmol) portion wise at 0 C.
After 1
h stirring, ethyl 4-(3-bromopropyl)benzoate (XII, 1.4 g, 5.20 mmol) and was 18-
crown ether (0.2 g) was added at 0 C and the temperature was allowed to warm
to
room temperature. The reaction mixture was then heated at 60 C for 18 h. The
reaction mixture was quenched with ice-water and extracted with ethylacetate
(2x50
mL). The organic portion was washed with water, brine, dried over sodium
sulphate
and concentrated under reduced pressure to afford the crude compound which was
purified by column chromatography using ethylacetate-hexane gradient as eluent
to
- 128 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
afford the titled product as sticky oil (A-8, 0.19 g, 13 %), itINMR (400 MHz,
DMSO-
d6): 6 9.68 (s, 1H), 8.09 (s, 1H), 7.88-7.84 (m, 3H), 7.33 (d, 2H, J=8 Hz),
4.28 (q, 2H,
J=6.8 Hz), 4.06 (t, 2H, J=6.8 Hz), 2.62 (t, 2H, J=7.2 Hz), 2.13-2.06 (m, 2H),
1.29 (t,
3H, J=7.2 Hz). LC-MS m/z calcd for C16H18N203, 286.1; found 287.1 [M+H] and
stick oil (A-9, 0.2 g, 15 %),1HNMR (400 MHz, DMSO-d6): 6 9.70 (s, 1H), 8.06
(s,
1H), 7.88 (s, 1H), 7.85 (d, 2H, J=8 Hz), 7.31 (d, 2H, J=7.6 Hz),4.32-4.25 (m,
4H),
2.62 (t, 2H, J=8 Hz), 2.04-1.95 (m, 2H), 1.29 (t, 3H,J=7.2 Hz). LC-MS m/z
calcd for
C16H18N203, 286.1; found 287.1 [M+H]t
A-10: ethyl 4-((4-formy1-1H-imidazol-1-yl)methyl)benzoate
iNz-_-1 ico
Intermediate A-10 was synthesized starting from ethyl 4-(bromomethyl)benzoate
and
1H-imidazole-4-carbaldehyde following protocol given for A-8. LC-MS m/z calcd
for
C14H14N203, 258.1, found 259.1 [M+H]t
A-11: ethyl 4- (3- (4-formyl- 1H-pyrazol- 1-yl)propyl)benzoate
0
Intermediate A-11 was synthesized starting from 1H-pyrazole-4-carbaldehyde and
methyl 4-(3-bromopropyl)benzoatepyrazole following protocol given for A-8. LC-
MS
m/z calcd for C16H18N203, 286.1; found 287.0 [M+H]t
A-12- methyl 4-((4-formy1-1H-pyrazol-1-yl)methyl)benzoate
OHC,,c
0,
0
Intermediate A-12 was synthesized starting from 1H-pyrazole-4-carbaldehyde and
ethyl 4-(bromomethyl)benzoate following protocol given for A-8. LC-MS m/z
calcd
for C14H14N203, 258.1; found 259.1 [M+H]t
A-13- methyl 4-(2-(4-formy1-1H-imidazol-1-ypethyl)benzoate
N=-\
OHC"-rN 0
0
- 129 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Intermediate A-13 was synthesized starting from 1H-pyrazole-4-carbaldehyde and
methyl 4-(bromoethyl)benzoate following protocol given for A-8. LC-MS m/z
calcd
for C14H14N203, 258.1; found 259.1 [M+H]t
A-14: Methyl 4-
42-formy1-5,6-dihydroimidazo[1,2-a]pyrazin-7(8H)-
yl)methyl)benzoate
0 0//
0
-
Step 1 HeN I-"N Step 2
I
I N
NH N
XIII
V A-14
Step 1: Methyl 4-42-(hydroxymethyl)-5,6-dihydroimidazo[1,2-a]pyrazin-7(8H)-
yl)methyl)benzoate (XIII)
0
0
HON
I
N
To a suspension of (5,6,7,8-Tetrahydro-imidazo[1,2-a[pyrazin-2-y1)-methanol
hydrochloride (V, 0.52 g, 2.75 mmol) was added potassium carbonate (1.14 g,
8.27
mmol) at 0 C and stirred at that temperature for 5 min. Then methyl 4-
(bromomethyl)benzoate (0.69 g, 3.03 mmol) was added and the resulting mixture
was
stirred at room temperature for 3 h. Reaction was monitored by TLC, after
completion
of the reaction, the reaction mixture was quenched with ice and extracted with
dichloromethane. The organic layer was washed with cold water, brine, dried
over
sodium sulphate and concentrated under reduced pressure to afford the required
product (XIII, 0.47 g, 52%). LC-MS m/z calcd for C16H19N303, 301.1; found
302.2
[M+I-1] .
Step 2: Methyl 4-42-formy1-5,6-dihydroimidazo[1,2-a]pyrazin-7(8H)-
yl)methyl)benzoate-Intermediate A-14
0
0
N
To a solution of methyl 4(2-hydroxymethyl)-5,6-dihydroimidazo[1,2-a[pyrazin-
7(8H)yl)methyl)benzoate (XIII, 0.43 g, 1.42 mmol) in dry dichloromethane (15
mL)
- 130 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
was added Dess-martin periodinane (1.51 g, 3.57 mmol) at 0 Cand the resulting
mixture was stirred at room temperature for 2 h. The progress of the reaction
was
monitored by TLC. The reaction mixture was quenched with saturated sodium
bicarbonate solution. A 10% aqueous solution of sodium thiosulphate (5 mL)was
added and stirred for 15 min. Then diluted with dichloromethane and the
organic
portion was washed with saturated sodium bicarbonate solution, water and brine
solution dried over sodium sulphate and concentrated under reduced pressure to
afford
the crude which was then triturated with n-pentane to afford the titled
product as an
off white solid (A-14, 0.41 g, 96%). LC-MS m/z calcd for C16H17N303, 299.1;
found
300.1 [M+H]t
A-15- methyl 4-(2-(4-formylpiperidin-1-yl)ethyl)benzoate
OHCO
0...,
0
Intermediate A-15 was synthesized starting from piperidin-4-yl-methanol and
methyl
4-(bromoethyl)benzoate following protocol given for A-14. LC-MS m/z calcd for
C16H21NO3, 275.1; found 276.1 [M+H]t
A-16- methyl 4-(3-(4-oxopiperidin-1-yl)propyl)benzoate
0
0
0
N
Intermediate A-16 was synthesized starting from 4-oxo-piperidine hydrochloride
salt
and methyl 4-(bromopropyl)benzoate following protocol given for A-14. LC-MS
m/z
calcd for C16H21NO3, 275.1; found 276.1 [M+H]t
A-17- ethyl 4-(3-(4-formy1-2-oxopiperidin-1-yl)propyl)benzoate
0
OHCO 0
N
Intermediate A-17 was synthesized starting from 2-oxo-piperidin-y1-4-methanol
and
methyl 4-(bromopropyl)benzoate following protocol given for A-14. LC-MS m/z
calcd for C18H23N04, 317.1; found 318.0 [M+H]t
A-18-
methyl 4-(3-(2-formy1-5,6-dihydroimidazo[1,2-a]pyrazin-7(8H)-
yl)propyl)benzoate
- 131 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
o o/¨
o
o
it
c)-- HO-0_, 11 step 2
V + H stepi N N
0 XIV
A-18
Stepl: 443-
(2-Hydroxymethy1-5,6-dihydro-8H-imidazo[1,2-a]pyrazin-7-y1)-
propy11-benzoic acid ethyl ester (XIV)
O/
HON.---- N
I ---\
'N N
To a stirred solution of (5,6,7,8-tetrahydro-imidazo[1,2-a[pyrazin-2-y1)-
methanol. HC1
salt (V, 0.1 g, 0.53 mmol) in methanol (8 mL) was added 4-(3-oxo-propy1)-
benzoic
acid ethylester (0.13 g, 0.63 mmol) and sodium bicarbonate (0.044 g, 0.53
mmol) and
molecular sieves (approx 1g) at room temperature and the resulting mixture was
heated to reflux for 30min. Cooled to room temperature and sodium
cyanoborohydride
(0.036 g, 0.58 mmol) was added and stirred at room temperature for 15 h. Ice
was
added and the reaction mixture was filtered. The solvent was evaporated to get
the
residue. Water was added and extracted with dichloromethane (2x30 mL). The
organic portion was washed with water and brine, dried over sodium sulphate
and
concentrated under reduced pressure to afford the crude compound which was
purified
by column chromatography to afford the titled product as a colourless oil.
(XIV, 0.09
g, 50 %). LC-MS m/z calcd for C19H25N303, 343.2; found 344.3 [M+H]t
Step 2: 4-[3-(2-Formy1-5,6-dihydro-8H-imidazo[1,2-a]pyrazin-7-y1)-propy11-
benzoic acid ethyl ester-Intermediate A-18
0
7-----
0
0---N
1 -----\
'N N
To a stirred solution of 4-[3-(2-hydroxymethy1-5,6-dihydro-8H-imidazo[1,2-
a[pyrazin-7-y1)-propyll -benzoic acid ethyl ester (XIV, 0.09 g, 0.26 mmol) in
dry
dichloromethane (10 mL) was added Dess-martin periodinane (0.28 g, 0.65 mmol)
at
0 Cand the resulting mixture was stirred at room temperature for 3 h. The
progress of
the reaction was monitored by TLC. The reaction mixture was quenched with
saturated sodium bicarbonate solution. A 10% aqueous solution of sodium
- 132 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
thiosulphate (1 mL)was added and stirred for 15 min, then diluted with
dichloromethane (20 mL) and the organic portion was washed with saturated
sodium
bicarbonate solution, water and brine solution dried over sodium sulphate and
concentrated under reduced pressure to afford the required product as yellow
solid (A-
18, 0.08 g, 90 %) which was carried to next step without further purification.
LC-MS
m/z calcd for C19H23N303, 341.1; found 342.2 [M+H]t
A-19- methyl 4-(2-(4-formylpiperidin-1-yl)propyl)benzoate
0
o
0
N
Intermediate A-19 was synthesized starting from piperidin-4-yl-methanol and 4-
(3-
Oxo-propy1)-benzoic acid ethylester following protocol given for A-18. LC-MS
m/z
calcd for C18H25NO3, 303.2; found 304.1 [M+H]t
A-20- methyl 4-(3-(7-formy1-3,4-dihydroisoquinolin-2(1H)-yl)propyl)benzoate
OHC
= N
0
\
0
Intermediate A-20 was synthesized starting from (1,2,3,4-tetrahydroisoquinolin-
6-
yl)methanol hydrochloride and 4-(3-oxo-propy1)-benzoic acid ethylester
following
protocol given for A-18. LC-MS m/z calcd for C21H23NO3, 337.2; found 338.1
[M+1-1] .
A-21- ethyl 4-(3-(6-formy1-3,4-dihydroisoquinolin-2(1H)-yl)propyl)benzoate
0
OHC 0
N
Intermediate A-21 was synthesized starting from (1,2,3,4-tetrahydroisoquinolin-
7-
yl)methanol hydrochloride and 4-(3-oxo-propy1)-benzoic acid ethylester
following
protocol given for A-18. LC-MS m/z calcd for C22H25NO3, 351.2; found 352.2
[M+1-1] .
A-22- methyl 4-47-formy1-3,4-dihydroisoquinolin-2(1H)-yl)methyl)benzoate
0
H N 00
0
- 133 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Intermediate A-22 was synthesized starting from (1,2,3,4-tetrahydroisoquinolin-
7-
yl)methanol hydrochloride and 4-formyl--benzoic acid methylester following
protocol
given for A-18. LC-MS m/z calcd for C19H19NO3, 309.1; found 310.1 [M+H]t
A-23: Methyl 4-(3-oxo-3-(4-oxopiperidin-1-yl)propyl)benzoate
0
0 0
0
+ HO
T3P/DCM 0
0
N N
H 0 0
A-23
To a stirred solution of 3-(4-(methoxycarbonyl)phenyl)propanoic acid (0.6 g,
2.88
mmol)and piperidine-4-onehydrochloride (0.57 g, 5.76 mmol), in
dichloromethane(15
mL) was added triethylamine (1.2 g, 8.64 mmol),the reaction was stirred at
room
temperature for 10min, then cooled reaction mixture to 0 C and added T3P (2.14
mL,7.20 mmol),and the resulting mixture was stirred at room temperature for 3
h.Reaction was monitored by TLC, after completion of reaction and the mixture
was
quenched with ice. The reaction mixture was diluted with water and extracted
with
dichloromethane (3x25 mL). The organic portion was washed with water, brine,
dried
over sodium sulphate and concentrated under reduced pressure to get the
required
product as pale-yellow oil. (A-23, 0.79 g, 94%). LC-MS m/z calcd for
C16H19N04,
289.1; found 290.2 [M+1-1] .
A-24- methyl 4-(2-(4-formylpiperidin-1-y1)-2-oxoethyl)benzoate-proceedure
HO Step 1 HO
HOD -).- N
+ 0
'H 0
0 (3.
0
XV 0
XVI
OHC,
Step 2
-).-
0 0
0
A-24
Step 1: methyl 4-(2-(4-(hydroxymethyl)piperidin-1-y1)-2-oxoethyl)benzoate-XVI
HOON
0 0
0
To a stirred solution of 2-(4-(methoxycarbonyl)phenyl)acetic acid (1 g,
5.15mmol)and
piperidin-4-ylmethanol (0.65 g, 5.67 mmol), in dichloromethane(25 mL) was
added
triethylamine (1..07 mL, 7.72mmo1),the reaction was stirred at room
temperature for
10min, then cooled reaction mixture to 0 C and added T3P (4.91 mL,7.72
mmol),and
- 134 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
the resulting mixture was stirred at room temperature for 3 h.Reaction was
monitored
by TLC, after completion of reaction,the mixture was quenched with ice. The
reaction
mixture was diluted with water and extracted with dichloromethane (3x25 mL).
The
organic portion was washed with water, brine, dried over sodium sulphate and
concentrated under reduced pressure to get the tiltle product as gummy solid.
(XVI,
1.2 g, 80%). LC-MS m/z calcd for C16H21N04, 291.1; found 292.1 [M+H]t
Step 2: methyl 4-(2-(4-formylpiperidin-l-y1)-2-oxoethyl)benzoate-A-24
OHCa
0 0
0
To a stirred solution of oxalyl chloride (0.23 mL, 2.69 mmol) in dry
dichloromethane
(5 mL) was added drieddimethylsulfoxide (0.28 mL, 4.06 mmol) dropwise at -78
C
and stirred for 15 min. A solution of methyl 4-(2-(4-(hydroxymethyl)piperidin-
1-y1)-
2-oxoethyl)benzoate (XVI,0.2 g, 0.68mmo1) in dry dichloromethane was added
drop-
wise followed by the slow addition of triethylamine (6.25 mL, 45.36 mmol) at -
78 C.
The resulting mixture was stirred at -78 C for 2 h. The reaction mixture was
diluted
with dichloromethane (100 mL). The organic portion was washed with water,
brine,
dried over sodium sulphate and concentrated under reduced pressure to afford
the
titled product as light yellow colour oil (A-24, 0.2 g, quantitative yield).
LC-MS m/z
calcd for C16H19N04, 289.1; found 290.1 [M+H]t
A-25- ethyl 5-
(4-oxobutanoy1)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine-2-
carboxylate
0
6 2
HO N 0 r......54-\ 0
ra=Sy.i0-\ I / I /
HN I / r ________________ -11-----B-N 0
0 step _2 H 0
Step-1 0 A45
XVII XVIII
Step-1 ethyl 5-(4-hydroxybutanoy1)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine-2-
carboxylate-XVIII
-i(3-\
HO----ThrN o
o
To a solution of ethyl 4,5,6,7-tetrahydrothieno[3,2-c]pyridine-2-carboxylate
TFA salt
(XVII, 0.5 g, 1.50 mmol) in triethylamine (1 mL) was added dihydrofuran-2(3H)-
one
(0.11 mL, 1.50 mmol) and heated at 1000 C for 16 h. The reaction mixture was
diluted
with dichloromethane (50 mL) and washed with 1N HC1 solution, water, brine
- 135 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
solution, dried over sodium sulphate and concentrated under vacuum to get
crude
product which was purified by column chromatography using methanol-
dichloromethane gradient to afford the titled product as sticky oil (XVIII,
0.2 g, 44
%). LC-MS m/z calcd for C14H19N04S, 297.1; found 298.2 [M+H]t
Step-2: ethyl 5-
(4-oxobutanoy1)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine-2-
carboxylate-A-25
H)HrN,
0
0
To a stirred solution of oxalyl chloride (0.23 mL, 2.69 mmol) in dry
dichloromethane
(5 mL) was added dry dimethylsulfoxide (0.28 mL, 4.06 mmol) drop-wise at -78
C
and stirred for 15 min. A solution of ethyl 5-(4-hydroxybutanoy1)-4,5,6,7-
tetrahydrothieno[3,2-c]pyridine-2-carboxylate (XVIII, 0.2 g, 0.67 mmol) in dry
dichloromethane was added drop-wise followed by the slow addition of
triethylamine
(6.25 mL, 45.36 mmol) at -78 C. The resulting mixture was stirred at -78 C
for 2 h.
The reaction mixture was diluted with dichloromethane (100 mL). The organic
portion
was washed with water and brine dried over sodium sulphate and concentrated
under
reduced pressure to afford the titled product as light yellow colour oil ( A-
25, 0.2 g,
quantitative yield). LC-MS m/z calcd for C14H17N04S, 295.1; found 296.2 [M+H]
.
A-26- ethyl 2-(4-oxobutanoy1)-1,2,3,4-tetrahydroisoquinoline-7-carboxylate
OHC
\--)r N
0 0
\----
0
Intermediate A-26 was synthesized starting from ethyl 1,2,3,4-
tetrahydroisoquinoline-
7-carboxylate and dihydrofuran-2(3H)-one following protocol given for A-25. LC-
MS
m/z calcd for C16H19N04, 289.1; found 290.1 [M+H]t
A-27- ethyl 2-(4-oxobutanoyl)isoindoline-5-carboxylate
OHC 0--./
0
0
Intermediate A-27 was synthesized starting from ethyl isoindoline-5-
carboxylate and
dihydrofuran-2(3H)-one following protocol given for A-25. LC-MS m/z calcd for
C15H17N04, 275.1; found 276.1 [M+H]t
A-28-ethyl 4-(3-(4-formy1-1H-1,2,3-triazol-1-yl)propyl)benzoate
- 136 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
NE-Ni
0
A-28 0
Intermediate A-28 was synthesized starting from ethyl 4-(3-
azidopropyl)benzoate
following protocol given for A-7. LC-MS m/z calcd for C15H17N303, 287.1; found
288.1 [M+H]t
A-29-methyl 4-(3-(4-formylpiperidin-l-y1)-3-oxopropyl)benzoate
OHCLO
0
A-29
Intermediate A-29 was synthesized starting from piperidin-4-ylmethanol and 3-
(4-
(methoxycarbonyl)phenyl)propanoic acid following the protocol given for A-24.
LC-
MS m/z calcd for C18H23N04, 317.1; found 318.2 [M+H]t
A-30: ethyl 2-(3-oxopropyl)thiazole-4-carboxylate
0 /-
0 /¨
\
step-, Step-2 0 HO Step-3
N
Br s
XIX HO XX )0(1 A-30
Step-1: ethyl-2(-3-hydroxyprop-1-yn-l-y1)thiazole-4-carboxylate-XX
0
HO
To a ethyl-2-bromothioazole-4-carboxylate (XIX, 4.0 g, 16.0mmo1) in a seal
tube was
added prop-2-yn-1-ol (1.07 g, 18 mmol), triethylamine (5.92 ml, 42.0 mmol),
CuI
(0.16 g, 0.8 mmol) and acetonitrile (60 mL) and then degassed with argon for 5
min.
PdC12(PPh3)2 (0.59 g, 8.0 mmol) was added and heated the seal tube at 80 C for
16 h.
After completion of reaction, the reaction mixture was cooled to room
temperature
and filtered through celite bed. The filtrate was concentratedunder vacuum to
afford
the crude product which was purified by flash column chromatography using
ethylacetate-hexane gradient to afford title product as brown color liquid
(XX, 1.3 g,
36%). LC-MS m/z calcd for C9H9N035, 211.0; found 212.0 [M+H]t
Step-2: ethyl 2-(3-hydroxypropyl)thiazole-4-carboxylate-XXI
- 137 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0 HO /-
Nz___?-0
S
To a stirred solution of ethyl 2(-3-hydroxyprop-1-yn-1-y1)thiazole-4-
carboxylate (XX,
1.3 g, 6.1 mmol) in ethanol (20 mL) was added Pt02(0.069 mg, 3.0 mmol),
triethylamine (0.6 mL, 4.3 mmol) and stirred under hydrogen gas at 30 psi for
3 h.
After completion of reaction, the reaction mixture was filtered through celite
bed. The
filtrate was concentrate under vacuum to get the crude product which was
purified by
flash column chromatography using ethylacetate-hexane gradient to afford the
titleproduct as yellow color liquid (XXI, 0.5 g,50%). LC-MS m/z calcd for
C9H13NO3S, 215.1; found 216.1 [M+H]t
Step-3:ethyl 2-(3-oxopropyl)thiazole-4-caroboxylate-A30
0 /¨
-
0
N
0-.-S?
To astirred solution of dimethylsulfoxide (0.85 mL, 20.0mmo1) in dry
dichloromethane (20 mL) was added oxalyl chloride (0.71 mL, 8.3 mmol) drop-
wise
at -70 C and stirred at same temperature for 30 min. Then solution of ethyl 2-
(3-
hydroxypropyl)thiazole-4-carboxylate (0.45 g, 2.0 mmol) in dichloromethane (4
mL)
was added drop-wise. After completion of addition, the reaction mixture was
stirred at
-70 C for additional 2 h. Triethylamine (3.3 mL, 24.0 mmol) was slowly added
and
stirred for 20 min. Then the reaction mixture was warmed to room temperature.
After
completion of reaction, the reaction was quenched with water, organic layer
was
separated. The organic layerwas washed with water, brine solution, dried over
sodium
sulphate and concentratedto get crude which was purified by flash column
chromatography using ethylacetate-hexane gradient to afford the title product
as
yellow liquid (A30, 0.4 g, 90%). LC-MS m/z calcd for C9th1NO3S, 213.0; found
214.1 [M+H]t
A-31: ethyl 2-(3-oxopropyl)thiazole-5-carboxylate
N /0
Hy---$ A ____________________________________ -\
S \\0
0
A-31
- 138 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using ethyl 2-bromothiazole-5-carboxylate
following
the procedure for synthesis of A-30. LC-MS m/z calcd for C9H11NO3S, 213.0;
found
214.1 [M+H]t
A-32: methyl 2-(3-oxopropyl)oxazole-4-carboxylate
o
TBDPSO OH Step-1
TBDPSO /10 Step-2
0
)001 OH
Mall
0.5_0
\ 0 0
N \ Step-3 ,--0
\
TBDPSOõii. .
N
HO 0-)
___________________________________ ,-,A3-0\ ____ Step-4 0
)0(IV
)0(V
A-32
Step-1: methyl (4-((tert-butyldiphenylsilyl)oxy)butanoyl)serinate-XXIII
w 0
7.iNij.L v
TBDPSO .., 0
0 OH
To a stirred solution of serine methyl ester (3.68 g, 23.7 mmol) in
acetonitrile (60 mL)
was added EDC.HC1 (4.55 g, 23.7 mmol),triethylamine (3.76 mL, 26.86 mmol) and
stirred for 5 min at room temperature and then 4-((tert-
butyldiphenylsilyl)oxy)butanoic acid (XXII, 0.54 g, 15.8 mmol) was added and
stirred
for 1 h at argon atmosphere. After completion of reaction, the reaction
mixture was
quenched with water (100 mL) and diluted with ethylacetate. The organic layer
was
separated and washed with 1N HC1 solution,water, brine, dried over sodium
sulphate
and concentrated to get crude product which was purified by flash column
chromatography using ethylacetate-hexane gradient to afford the title product
as
colorless liquid (XXIII, 2.46 g, 63%). LC-MS m/z calcd for C24H33NO5Si, 443.2;
found 444.2 [M+H]t
Step-2: methyl 2-(3-((tert-butyldiphenylsilyl)oxy)propyl)oxazole-4-carboxylate-
XXIV
0
0
\
N-i---
TBDPS00
- 139 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
To a stirred solution of methyl (4-
((tert-
butyldiphenylsilyl)oxy)butanoy1)(hydroxymethyl)carbamate (XXIII, 2.46 g, 5.50
mmol) in dichloromethane (25 mL) was added diethylaminosulfur trifluoride
(DAST,
0.8 mL, 6.10 mmol) at -78 C and then stirred for 2 h. Potassium carbonate
(2.27g,
16.50 mmol) was added and then stirred at -20 C for lh. After completion of
reaction,
the mixture was quenched with water (50 mL). The organic layer was separated,
dried
over sodium sulphate and concentrated under vacuum. The resultant crude
product
was dissolved in dichloromethane (35 mL) and then followed by addition of1,8-
Diazabicyclo(5.4.0)undec-7-ene (DBU, 2.46 mL, 16.5 mmol). The reaction mixture
was cooledto 0 C. The solution of CBrC13(1.14 mL, 11.5 mmol) in
dichloromethane
(4mL) was added drop-wise and stirred for 10 h at room temperature. After
completion of reaction, the mixture was diluted with ethylacetate (50mL) and
washed
with 1N HC1, water, saturated aqueous sodium bicarbonate solution,brine, dried
over
sodium sulphate and concentrated under vacuumto get crude product which was
purified by flash column chromatography using ethylacetate-hexane gradient to
afford
the title product colorless oil (XXIV, 0.86 g, 36%). LC-MS m/z calcd for
C24H29NO4Si, 423.3; found 424.3 [M+H]t
Step-3: methyl 2-(3-hydroxypropyl)oxazole-4-carboxylate-XXV
%___0
\
HO N-
0
To a stirred solution of methyl 2-(3-((tert-
butyldiphenylsilyl)oxy)propyl)oxazole-4-
carboxylate (XXIV, 0.86g, 2.0 mmol) in THF (5 mL) was added TBAF (4.1 mL, 4.10
mmol) and stirred for 1 h at room temperature under argon atmosphere. After
completion of reaction, the mixture was quenched with brine solution and
extracted
with ethylacetate(25 mL x 5). The organic layer was dried over sodium sulphate
and
concentrated under vacuum to get crude productwas purified by flash column
chromatography using ethylacetate-hexane gradient to afford the title product
brown
coloured liquid (XXV, 0.3 g, 81 %). LC-MS m/z calcd for C8H11N04, 185.1; found
186.1 [M+H] .
Step-4: methyl 2-(3-oxopropyl)oxazole-4-carboxylate-A32
- 140 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
0___
0
\
0
N-
A-32
To astirred solution of dimethylsulfoxide (0.57 mL, 8.1 mmol) in dry
dichloromethane
(10 mL) was added oxalyl chloride (0.46 mL, 5.0 mmol) drop-wise at -70 C and
stirred at same temperature for 30 min. Then solution of methyl 2-(3-
hydroxypropyl)oxazole-4-carboxylate (0.25 g, 1.35 mmol) in dichloromethane (1
mL)
was added drop-wise. After completion of addition, the reaction was stirred at
-70 C
for additional 2 h. Triethylamine (2.2 mL, 24.0 mmol) was slowly added and
stirred
for 20 min. Then the reaction mixture was warmed to room temperature. After
completion of reaction, the reaction was quenched with water, organic layer
was
separated. The organic layerwas washed with water, brine solution, dried over
sodium
sulphate and concentratedto get crude product which was purified by flash
column
chromatography using ethylacetate-hexane gradient to afford the title product
as
yellow liquid (A32, 0.2 g, 81%). LC-MS m/z calcd for C8H9N04, 183.1; found
184.1
[M+1-1] .
A-33- methyl (E)-4-(3-(4-formylpiperidin-1-y1)-3-oxoprop-1-en-1-yl)benzoate
0 0 H 0
Step-1 Step-2 (:).....ci
HO 0 HO---0 0 0
+
----'.0H HO ",... I
0 lOCVI 0 30CVII 0
A-33
Step-1: methyl (E)-4-(3-(4-(hydroxymethyl)piperidin-1-y1)-3-oxoprop-1-en-1-
yl)benzoate-XXVII
0
HON 0
I
0
To a stirred solution of (E)-3-(4-(methoxycarbonyl)phenyl)acrylic acid (XXVI,
3 g,
14.50 mmol) and piperidin-4-yl-methanol (2.2 g, 18.9 mmol) in DMF (50 mL) was
added EDC.HC1 (2.5 g, 16.0 mmol), HOBt (2.1 g, 16.0 mmol) and DIPEA (3.7 mL,
29.0 mmol) at0 C and the resulting mixture was stirred at room temperature
for 16 h.
The reaction mixture was concentrated under vacuum. The resultant crude was
diluted
with ethylacetate and washed with water, brine, dried over sodium sulphate and
concentrated under reduced pressure to get the crude product which was
purified by
column chromatography using methanol-dichloromethane gradient to afford the
titled
- 141 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
product as colorless solid (XXVII, 2.1 g, 48 %). LC-MS m/z calcd for
C17H21N04,
303.2; found 304.2 [M+H]t
Step-2: methyl (E)-4-(3-(4-formylpiperidin-l-y1)-3-oxoprop-1-en-1-y1)benzoate-
A33
H 0
0 0
1
0
A-33
To astirred solution of dimethylsulfoxide (2.8 mL, 39.6 mmol) in dry
dichloromethane
(40 mL) was added oxalyl chloride (2.2 mL, 26.0 mmol) drop-wise at -70 C and
stirred at same temperature for 30 min. Then a solution of methyl (E)-4-(3-(4-
(hydroxymethyl)piperidin-1-y1)-3-oxoprop-1-en-1-y1)benzoate (XXVI, 2 g, 6.60
mmol) in dichloromethane (10 mL) was added drop-wise. After completion of
addition, the reaction mixture was stirred at -70 C for additional 2 h.
Triethylamine
(11 mL, 79.2 mmol) was slowly added and stirred for 20 min. Then the reaction
mixture was warmed to room temperature. After completion of reaction, the
reaction
was quenched with water, organic layer was separated. The organic layerwas
washed
with water, brine, dried over sodium sulphate and concentratedto afford the
title
productA-33as yellow liquid (2 g, quantitative yield). LC-MS m/z calcd for
C17H19N04, 301.1; found 302.1 [M+I-1] .
Synthesis of Intermediates-Amines
B-1 and B-2- (1R,25)-2-(4-fluorophenyl)cyclopropanamine hydrochloride (I-8)
and (1S,2R)-2-(4-fluorophenyl)cyclopropanamine hydrochloride (I-9)
A
A . Stepl 0 " o
'N
F 0 ''N H2 -I.- F
o 41
Trans mixture- Trans mixture-
lOCVIII XXIX
A o
6 "'N N
Chiral spearation F + 0 F 0 410
_______________________ ..- .. 0
Isomer 1 Isomer 2
IDe-protection De-protection
A 0 ,, ,A... 'NH2 HCI IOC NH2
HCI
F F
B-1 B-2
- 142 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Step 1: 2-((lS ,2R)-2-(4-fluorophenyl)c ycloprop yl)is oindoline- 1,3 -dione
and 2-
((1 R,25 )-2-(4-fluorophenyl)c ycloprop yl)is oindoline- 1,3 -dione-XXIX
A 0
4." N 0 `"s=A*N
and
F Si 0 F 0
A mixture of 2-(4-fluoro-phenyl)-cyclopropylamine hydrochloride (XXVIII, 1.5
g,
7.99 mmol), isobenzofuran-1,3-dione (1.77 g, 11.99 mmol) and
diisopropylethylamine
(4.27 mL, 23.97 mmol) was heated in a sealed tube at 150 C for 12 h and cooled
to
room temperature. The reaction mixture was diluted with ethylacetate (3x50
mL). The
organic portion was washed with water and brine dried over sodium sulphate and
concentrated under reduced pressure to afford the crude compound which was
purified
by column chromatography to afford the racemic product (1.9 g). The racemic
product
was separated by chiral Prep. HPLC, Chiralpak ia (250 mm X 4.6mm X 51.tm)
using
0.1% TFA in ACN:Me0H(20:80%) solvent to get isomer 1 (0.73 g) and isomer 2
(0.77 g). LC-MS m/z calcd for C17I-112FN02, 281.1; found 282.2 [M+H]t
(1R,25)-2-(4-fluorophenyl)cyclopropanamine
-
40"' l\PNH2
F
To a
stirred solution of 2- [2-(4-fluoro-phenyl)-cyclopropyl] -is oindole-1,3 -
dione(isomer 2,0.77 g, 2.73 mmol) in dichloromethane and ethanol mixture (12
mL,
5:1) was added hydrazine hydrate (0.41 mL, 8.21 mmol) at room temperature and
the
resulting mixture was stirred at room temperature for 3 h. The progress of the
reaction
was monitored by TLC. A precipitate formed which was filtered and washed with
dichloromethane. The filtrate was evaporated to give the product as yellow oil
(0.47 g,
95%). The crude was carried to next step without further purification. LC-MS
m/z
calcd for C9I-110FN, 151.1; found 152.2 [M+H]t
B-2 (Isomer 2): (1R,25)-2-(4-fluorophenyl)cyclopropanamine hydrochloride
O=
N' A*N1H2 HCI
F
B-2
To a stirred solution of 2-(4-fluoro-phenyl)-cyclopropylamine (chirally pure,
0.47 g,
2.108 mmol) in dioxane (5 mL) was added HC1 in dioxane solution (2 mL) at 0 C
and
the resulting mixture was stirred at room temperature for 2 h. Cooled to room
- 143 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
temperature and the solvent was evaporated to get the residue which was
triturated
with diethyl ether to afford the product as an off-white solid (B-2, 0.42 g,
72 %). LC-
MS m/z calcd for C9I-110FN, 151.1; found 152.2 [M+H]t
B-1 (Isomer 1): (1S,2R)-2-(4-fluorophenyl)cyclopropanamine hydrochloride
The compound was synthesized from 24(1R,25 )-2-
(4-
fluorophenyl)cyclopropyl)isoindoline-1,3-dione (B-1, isomer 1) by following
the
same synthesis procedure of (1R,25)-2-(4-fluorophenyl)cyclopropanamine
hydrochloride, LC-MS m/z calcd for C9I-110FN, 151.1; found 152.2 [M+H] .
B-3- 2,2,2-trifluoro-N-(2-(4-fluorophenyl)cyclopropy1)-N-(piperidin-4-
ylmethyl)
acetamide hydrochloride.
A
A step 1
Step 2 A
NH2 HCI , 1401
OCF3 N,r0
F3C 0
XXVIII
)0(X Intermediate B-3
Step 1: tert-butyl 4-
((2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropypacetamido)methyppiperidine-1-carboxylate-XXX
A
fON 0
0 CF3
Cr:1
15 To a
stirred solution of 2-(4-fluorophenyl)cyclopropan- 1-amine hydrochloride
(XXVIII, 0.5 g, 2.66 mmol) in methanol (20 mL) was added tert-butyl 4-
formylpiperidine-1-carboxylate (0.57 g, 2.66 mmol) and sodium bicarbonate
(0.20 g,
2.30 mmol) and molecular sieves (approx. 1 g) at room temperature and the
resulting
mixture was heated to reflux for 2 h. Cooled to 0 C, then sodium borohydride
(0.1 g,
20 2.66
mmol) was added and stirred at room temperature for 1 h. Ice was added and the
reaction mixture was filtered. The solvent was evaporated to get the residue.
Water
was added and extracted with ethylacetate (2x100 mL). The organic portion was
washed with water, brine, dried over sodium sulphate and concentrated under
reduced
pressure to afford the crude compound. To a stirred solution of crude compound
in dry
25
dichloromethane (20 mL) was added TEA (0.92 mL, 6.65 mmol) and then cooled to
0
C.Then trifluoroacetic anhydride (0.56 mL, 3.99 mmol) was added drop-wise
cautiouslyand the resulting mixture was stirred for 2 h at that temperature.
The
progress of the reaction was monitored by TLC. The reaction mixture was
diluted with
dichloromethane and the organic portion was washed with water, brine solution,
dried
30 over
sodium sulphate and concentrated under reduced pressure to get the crude
- 144 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
product which was further purified by column chromatography using ethylacetate-
hexane gradient to afford the titled product as brown colour sticky oil (XXX,
1.1 g, 93
%). LC-MS m/z calcd for C22H28F4N203, 444.2; found 445.2 [M+H]t
Step 2:
2,2,2-trifluoro-N-(2-(4-fluorophenyl)cyclopropy1)-N-(piperidin-4-
ylmethyl)acetamide hydrochloride-Intermediate B-3
A
F F3e HCIL0 "
To a stirred solution of
tert-butyl 4-((2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropyl)acetamido)methyl)piperidine-l-carboxylate (XXX, 1.1
g,
2.40 mmol) in dioxane (3 mL) was added 20 % HC1 in dioxane (3 mL) at 0 C and
stirred for 3 h at room temperature. The reaction mixture was concentrated
under
vacuum and triturated with diethyl ether. The resultant solid was dried under
vacuum
to afford the title product as off-white solid (B-3, 0.8 g, 94 %). LC-MS m/z
calcd for
C17H20F4N20, 344.1; found 345.1 [M+H]t
B-4-N-(azetidin-3-ylmethyl)-2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropypacetamide
A
00 NH2 HCI
A A
NyOJ( ________________ Step-1 F 0 CF3 II
0 Step-2 401 0rc\0F3
0 . TFA
MOO Intermedlate-B-4
MOO
Step 1:tert-butyl 3-
42,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropypacetamido)methypazetidine-1-carboxylate-XXXII
A
40 ,,I7Ny(D,
0
)001
20 To a stirred solution of 2-(4-fluorophenyl)cyclopropan- 1-amine
hydrochloride (1.2 g,
6.41 mmol) in methanol (20 mL) was added tert-butyl 3-formylazetidine- 1-
carboxylate (XXXI, 1.2 g, 6.41 mmol) and sodium bicarbonate (0.48 g, 5.77
mmol)
and molecular sieves (approx. 1 g) at room temperature and the resulting
mixture was
heated to reflux for 2 h. Cooled to 0 C, then sodium borohydride (0.24 g,
6.41 mmol)
25 was added and stirred at room temperature for 1 h. Ice was added and the
reaction
mixture was filtered. The solvent was evaporated to get the residue. Water was
added
and extracted with ethylacetate (2x100 mL). The organic portion was washed
with
water, brine, dried over sodium sulphate and concentrated under reduced
pressure to
- 145 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
afford the crude compound. To a stirred solution of crude compound in dry
dichloromethane (10 mL) was added triethylamine (2.2 mL, 16.02 mmol) and then
cooled to 0 C.Then trifluoroacetic anhydride (0.98 mL, 7.05 mmol) was added
drop-
wise cautiously and the resulting mixture was stirred at that temperature for
2 h. The
progress of the reaction was monitored by TLC. The reaction mixture was
diluted with
dichloromethane and the organic portion was washed with water, brine solution,
dried
over sodium sulphate and concentrated under reduced pressure to get the crude
product which was purified by column chromatography using ethylacetate-hexane
gradient to afford the titled product as sticky oil (XXXII, 2.3 g, 86 %). LC-
MS m/z
calcd for C20I-124F4N203, 416.2; found 317.3 [M-Boc + H].
Step-2: N-(azetidin-3-ylmethyl)-2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropyl)
acetamide TFA salt-Intermediate B-4
A
TFA
0 CF3
To solution of compound (XXXII, 2.3 g, mmole) in dichloromethane (2.5 mL) was
added TFA (2.5 mL) and stirred at room temperature for 3 h. Then, the reaction
mixture was concentrated under vacuum. The resultant crude product was
triturated
with diethyl ether and then dried under vacuum to afford the titled product as
sticky
oil (B-4, 1.8 g, 78%). LC-MS m/z calcd for C151-116F4N20, 316.1; found 221.1
[M-
TFA].
B-5: N-(azetidin-3-
ylmethyl)-2,2,2-trifluoro-N-(2-(4-
iodophenyl)cyclopropyl)acetamide
A
.;C\N1 NH TFA
I 0 F
F F
Intermediate B-5 was synthesized following procedure for synthesizing B-4
LC-MS m/z calcd for C151-116F31N20, 424.0; found 425.0 [M+1] .
B-6- 2-(4((4-fluorobenzypoxy)phenyl)cyclopropan-1-amine
o A o
A OH
HO Ail
0- 40 Step 3 40
0, Step 1 Step 2 0 0 0 0
o 0 'w F F
F )00CIV
MOUll
A
A N,Boc 40 NH2 HCI
0
Ste 5
Step 4
0 P
F B-6
F 700CVI
Step-1: (E)-methy13-(4-((4-fluorobenzypoxy)phenypacrylate-XXXIII
- 146 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
\ 0
0 0
F
To a stirred solution of (E)-methyl 3-(4-hydroxyphenyl)acrylate (5.1 g, 28.6
mmol), in
dry DMF (50 mL) was added 1-(bromomethyl)-4-fluorobenzene (6.5 g, 34.3 mmol)
and potassium carbonate (11.86 g, 85.9 mmol) at room temperature and the
resulting
mixture was stirred at room temperature for 16h. Ice water was added to it and
then
extracted with ethylacetate (3x 50 mL). The combined organic extract was
washed
with water, brine, dried over sodium sulphate and concentrated under reduced
pressure
to afford the product as off-white solid (XXXIII, 8 g, 97 %). LC-MS m/z calcd
for
C17H15F03, 286.1; found 287.1 [M+H]t
Step-2: methyl 2-(4-((4-fluorobenzypoxy)phenyl)cyclopropanecarboxylate-
XXXIV
0
110 0 0
F
To a stirred solution of (E)-methyl 3-(4-((4-fluorobenzyl)oxy)phenyl)acrylate
(2 g, 6.9
mmol) in diethyl ether (50 mL) was added Pd(OAc)2 (0.31 g, 1.3 mmol)at 0 C
and
stirred for 20 min. A freshly prepared solution of diazomethane (30 eq) in
diethyl
ether was then added slowly and stirred at room temperature for16 h. The
reaction
mixture was filtered through celite bed and washed with dichloromethane. The
filtrate
was evaporated under reduced pressure to get crude product which was purified
by
column chromatography using ethylacetate-hexane gradient to afford the titled
product
as an off white solid (XXXIV, 1.96 g, 94 %). LC-MS m/z calcd for C18H17F03,
300.1;
found 301.1 [M+H]t
Step-3:2-(4-((4-fluorobenzypoxy)phenyl)cyclopropanecarboxylicacid-XXXV
JJA(OH
40 0 0
F
To a stirred solution of methyl 2-
(4-((4-
fluorobenzyl)oxy)phenyl)cyclopropanecarboxylate (1.86 g, 6.2 mmol) in
tetrahydrofuran (10 mL) and methanol (10 mL) was added lithium hydroxide (0.52
g,
- 147 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
12.4 mmol). The reaction mixture was heated at 50 C for 12 h. The reaction
was
concentrated under vacuum and then acidified to pH 2 with 2N aqueous HC1. The
resultant solid was filtered and dried under vacuum to get the title product
as a white
colour solid (XXXV, 1.65 g, 93%). LC-MS m/z calcd for C17H15F03, 286.1; found
285.1 [M-H].
Step-4: tert-butyl (2-(44(4-fluorobenzyl)oxy)phenyl)cyclopropyl)carbamate-
XXXVI
0
NA0
0
To a stirred solution of 2-(4-((4-
fluorobenzyl)oxy)phenyl)cyclopropanecarboxylic
acid (XXXV, 1.74 g, 6mmo1) in t-butanol (50 mL) was added triethylamine (1.26
mL,
9.10mmol) diphenylphosphorylazide (1.44 mL, 6.60mmo1) and then heated at 80 C
for 48 h. The reaction mixture was concentrated under vacuum. The resultant
crude
product was diluted with ethylacetate (100 mL) and washed with water, brine,
dried
over sodium sulphate and concentrated under reduced pressure which was
purified by
column chromatography using ethylacetate-hexane gradient to afford the titled
product
as yellow solid (XXXVI, 1.05 g, 48 %), LC-MS m/z calcd for C211-124FN03,
357.2;
found 358.2 [M+H]t
Step-5:2-(4-((4-fluorobenzyl)oxy)phenyl)cyclopropanaminehydrochloride-B-6
NH2.HCI
0
To a stirred solution of tert-butyl (2-(4-((4-
fluorobenzyl)oxy)phenyl)cyclopropyl)carbamate (XXXVI, 1.05 g, 2.9 mmol) in 1,4
-
dioxane (10 mL) was added 20 % HC1 in 4-dioxane (10 mL) at 0 C and then was
heated at 50 C for 3 h.The reaction mixture was concentrated under vacuum and
the
resultant solid was titurated with diethyl ether. The solid was filtered and
dried under
vacuum to get the titled product as white solid (B6, 0.76 g, 88%).
itINMR (400 MHz, DMSO-d6): 6 8.39 (bs, 3H), 7.47-7.44 (m, 2H),7.21-7.17 (m,
2H),
7.07 (d, 2H, J=8.8Hz), 6.91 (d, 2H, J=8.4Hz), 5.04(s, 2H), 2.71-2.67 (m, 1H),
2.28-
- 148 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
2.23 (m, 1H), 1.33-1.30 (m, 1H), 1.14-1.09 (m, 1H). LC-MS m/z calcdfor
C16H16FN0,
257.1, found 258.2.
B-7-2-(4-(1-methyl-1H-pyrazol-3-yl)phenyl)cyclopropan-1-amine hydrochloride
v.,,rB(OH)2
0
NA0 0
[
A A " X N qi Ns/
Step-1 Step-2
NH2HCI
;NI
)00CVII XXXIX Intermediate B-7
Step 1:tert-butyl (2-(4-(1-methyl-1H-pyrazol-3-yl)phenyl)cyclopropyl)carbamate-
XXXIX
0
NA0
N 11
H
To a stirred solution of tert-butyl (2-(4-iodophenyl)cyclopropyl)carbamate
(XXXVII,
1 g, 2.78 mmol) in mixture of dimethoxyethane (8 mL) and water (0.5 mL) was
added
(1-methyl-1H-pyrazol-4-y1)boronic acid (0.42 g, 3.34 mmol) andpotassium
carbonate
(0.76 g, 5.57 mmol) and then degassed for 5 min. 1,1'-
Bis(diphenylphosphino)ferrocene-palladium(II)dichloride dichloromethane
complex
(0.22 g, 0.27 mmol) was added and heated at 120 C in microwave for 2 h. Water
was
added and extracted with ethylacetate (2x100 mL). The organic portion was
washed
with water, brine, dried over sodium sulphate and concentrated under reduced
pressure
to afford the crude productwhich was purified by column chromatography using
methanol-dichloromethane gradient to afford the titled product as sticky oil
(XXXIX,
0.29 g, 40 %), LC-MS m/z calcd for C18H23N302, 313.2; found 214.2 [M-Boc+H]t
Step 2: 2-
(4-(1-methyl-1H-pyrazol-3-yl)phenyl)cyclopropan-1-amine
hydrochloride-Intermediate B-7
NH2 HCI
N
To a stirred solution of
tert-butyl (2-(4 -(1-methyl- 1H-pyrazol-3 -
yl)phenyl)cyclopropyl)carbamate (XXXIX, 0.29 g, 1.11 mmol) in dioxane (15 mL)
was added 20 % HC1 in dioxane ( 10 mL) at 0 C and stirred for 3 h at room
temperature. The reaction mixture was concentrated under vacuum to afford the
title
- 149 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
product as off-white solid (1-23, 0.18 g, 50 %), LC-MS m/z calcd for C13H15N3,
213.1;
found 214.1 [M+H]t
B-8- 2-(4-(3,5-dimethylisoxazol-4-yl)phenyl)cyclopropan-1-amine hydrochloride
NH2.HCI
N
The above intermediate B-8 was synthesized by following the experiment
procedure
of B-7. LC-MS m/z calcd for C14H16N20, 228.1; found 229.1 [M+H]t
B-9- 4-(4-(2-aminocyclopropyl)pheny1)-1-methylpyridin-2(1H)-one hydrochloride
NH2 HCI
The intermediate B-9 was synthesized by following the experiment procedure of
B-7.
LC-MS m/z calcd for C151-116N20, 240.1; found 241.1 [M+H]t
B-10: 2-(4'-chloro-[1,1'-bipheny1]-4-yl)cyclopropan-1-amine
NH2 HCI
CI
The intermediate B-10 was synthesized by following the experiment procedure of
B-7.
LC-MS m/z calcd for C15H14C1N, 243.1 found 244.1 [M+H]t
B-11- 2-(4-(pyrimidin-5-yl)phenyl)cyclopropan-1-amine
NH2 HCI
N
The intermediate B-11 was synthesized by following the experiment procedure of
B-7.
LC-MS m/z calcd for C13H13N3, 211.1, found 212.1 [M+H]t
B-12- 2-(4'-fluoro-[1,1'-bipheny1]-4-yl)cyclopropan-l-amine
NH2 HCI
The intermediate B-12 was synthesized by following the experiment procedure of
B-7.
LC-MS m/z calcd for C15H14FN, 227.1; found 228.1[M+H]t
- 150 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
B-13: 2-(4'-cyano-l1,1'-biphenyll-4-yl)cyclopropan-1-amine
NH2 HCI
NC
The intermediate B-13 was synthesized by following the experiment procedure of
B-7.
LC-MS m/z calcd for C16H14N2, 234.1; found 235.1 [M+H]t
B-14: 2-(4-(6-(trifluoromethyl)pyridin-3-yl)phenyl)cyclopropan-1-amine
NH2 HCI
I
F3C N
The intermediate B-14 was synthesized by following the experiment procedure of
B-7.
LC-MS m/z calcd for C151-113F3N2, 278.1; found 279.1[M+H]t
B-15- 2-(1-isopropyl-1H-pyrazol-4-yl)cyclopropan-1-amine
o o
\i7N
Step-1 Step-2 Step-3
XLI XLII
XL
0
Step-4 j< N Step-5 NH2 HCI
Ns u
N¨
XLIII XLIV
B-15
Step-1: ethyl (E)-3-(1-isopropyl-1H-pyrazol-4-ypacrylate-XLI
0
To a stirred solution of triethylphosphonoacetate (7.75mL, 39.13mmol) in
tetrahydrofuran (80 mL) was added 60% of sodium hydride (0.94 g,39.13mmol) at
0
C and then stirredfor 1 h. 1-Isopropyl-1H-pyrazole-4-carbaldehyde (XL, 4.5 g,
32.57
mmol) in tetrahydrofuran (20 mL) was added and stirred for 2h at room
temperature.
The reaction mixture was quenched with ice-water. Then the reaction mixture
was
concentrated under vacuum. The resultant crude was diluted with ethylacetate
(100
mL) and washed with water, brine, dried over sodium sulphate and concentrated
under
reduced pressure to result in crude product which was purified by column
chromatography using ethylacetate-hexane gradient to afford the titled product
as
- 151 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
colourless oil (XLI, 5.4 g, 80% yield). LC-MS m/z calcd for C11H16N202, 208.1;
found 209.1 [M+H]t
Step-2: ethyl 2-(1-isopropyl-1H-pyrazol-4-yl)cyclopropane-1-carboxylate-XLII
0
)¨Isel
'NJ--
To a stirred solution of ethyl (E)-3-(1-isopropyl-1H-pyrazol-4-y1)acrylate
(XLI, 0.5 g,
2.40mmo1) in diethyl ether (10 mL) was added Pd(OAc)2 (0.026 g, 0.12mmol) at 0
C
and stirred for 20 min. A freshly prepared solution of diazomethane (30 eq.)
in diethyl
ether was then added slowly and stirred at room temperature for 16 h. The
reaction
mixture was filtered through celite bed and washed with dichloromethane. The
filtrate
was evaporated under reduced pressure to get crude product which was purified
by
column chromatography using ethylacetate-hexane gradient to afford the titled
product
as an off-white solid (XLII, 0.37 g, 69 %). LC-MS m/z calcd for C12H18N202,
222.1;
found 223.1 [M+1-1] .
Step-3:2-(1-isopropyl-1H-pyrazol-4-yl)cyclopropane-1-carboxylic acid-XLIII
N
To a stirred solution of ethyl 2-(1-isopropy1-1H-pyrazol-4-y1)cyclopropane-1-
carboxylate (XLII, 0.37 g, 1.78mmo1) in water (8 mL) and methanol (2 mL) was
added sodium hydroxide (0.28 g, 7.11mmol). The reaction mixture was stirred at
room
temperature for 2 h. The reaction was concentrated under vacuum and then
acidified
to pH 5 with 2Naqueous HC1. The resultant stick solid was extracted with
dichloromethane (50 mL x 3). The combined organic layer was washed with brine
solution and concentrated under vacuum to get the title product as colourless
sticky oil
(XLIII, 0.3 g, 87 %). LC-MS m/z calcd for C10H14N202, 194.1; found 195.1
[M+H]t
Step-4: tert-butyl (2-(1-isopropyl-1H-pyrazol-4-yl)cyclopropyl)carbamate-XLIV
0
H
'NI¨
To a stirred solution of 2-(1-isopropy1-1H-pyrazol-4-y1)cyclopropane-1-
carboxylic
acid (XLIII, 0.3 g, 1.55mmo1) in t-butanol (10 mL) was added triethylamine
(0.65
mL, 4.64mmo1) diphenylphosphorylazide (0.5 mL, 2.32mmo1) and then heated at 80
- 152 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
C for 18 h. The reaction mixture was concentrated under vacuum. The resultant
crude
was diluted with ethylacetate (100 mL) and washed with water, brine solution,
dried
over sodium sulphate and concentrated under reduced pressure to result in
crude
product which was purified by column chromatography using ethylacetate-hexane
gradient to afford the titled product as yellow solid (XLIV, 0.13 g, 33 %). LC-
MS m/z
calcd for C14H23N302, 265.2; found 266.2 [M+H]t
Step-5: 2-(1-isopropy1-1H-pyrazol-4-yl)cyclopropan-1-amine hydrochloride-B15
To a stirred solution of
tert-butyl (2-(1-isoprop yl- 1H-pyrazol-4-
yl)cyclopropyl)carbamate (XLIV,0.13 g, 0.49mmo1) in 1,4 -dioxane (3 mL) was
added 20 % HC1 in 1,4-dioxane (3 mL) at 0 C and then was heated at room
temperaturefor 3 h. The reaction mixture was concentrated under vacuum and the
resultant solid was titurated with diethyl ether. The solid was filtered out
and dried
under vacuum to get the titled product as white solid (B-15, 0.06 g, 61%). LC-
MS m/z
calcd for C9Hi5N3, 165.1; found 166.1 [M+H]t
B-16- 2-(1-phenyl-1H-pyrazol-4-yl)cyclopropan-l-amine
Nrf&NH2 HCI
The intermediate B-16 was synthesized starting from 1-pheny1-1H-pyrazole-4-
carbaldehyde by following the experiment procedure of B-15. LC-MS m/z calcd
for
C12H13N3, 199.1; found 200.1 [M+H]t
B-17- 2-(2-methylthiazol-5-yl)cyclopropan-l-amine
HCI
The intermediate B-17 was synthesized starting from 2-methylthiazole-5-
carbaldehyde
by following the experiment procedure of B-15. LC-MS m/z calcd for C7H10N2S,
154.0; found 155.1[M+H]t
B-18- 2-(pyridin-3-yl)cyclopropan-l-amine
- 153 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
I
N.0
0
/ C OH
I
-.. .,.-
Step-1 L(:) Step-2 NI Step-3
I
N I 0
N N
XLV XLVI XLVII
0
Step-4 nA A Step-5
OH N 0
ff.ANHITFA
, I H
, I 0
N N N
XLVIII XLIX B-18
Step-1: (E)-N-methoxy-N-methyl-3-(pyridin-3-yl)acrylamide-XLVI
I
N-0
0
I
N
To a stirred solution of (E)-3-(pyridin-3-yl)acrylic acid (XLV, 10 g,
67.1mmol) and
N,0-dimethylhydroxylamine hydrochloride (13 g, 134 mmol) in DMF (300 mL) was
added EDC.HC1 (16.6 g, 87.1 mmol), HOBt (9 g, 67 mmol) and TEA (46 mL, 335
mmol) at 0 C and the resulting mixture was stirred at room temperature for 16
h. The
reaction mixture was concentrated under vacuum. The resultant crude was
diluted
with ethylacetate and washed with water, brine, dried over sodium sulphate and
concentrated under reduced pressure to get the crude product which was
purified by
column chromatography using methanol-dichloromethane gradient to afford the
titled
product as an sticky oil (XLVI, 8.2 g, 64 %). LC-MS m/z calcd for C10H12N202,
192.0; found 193.1 [M+H]t
Step-2: N-methoxy-N-
methy1-2-(pyridin-3-yl)cyclopropane-1-carboxamide-
XLVII
I
N,
0
1 0
N
To a stirred solution of trimethylsulfoxonium iodide (2.75g, 12.5mmo1) in dry
dimethyl sulfoxide (20 mL) was added 60% of sodium hydride (12.5 g,12.5mm01)
portion-wise atroom temperature and then stirredfor 3 h. (E)-N-methoxy-N-
methy1-3-
(pyridin-3-yl)acrylamide (1.2 g,6.25mm01) in dimethyl sulfoxide (10 mL)was
added
and stirred for 2h at room temperature. The reaction mixture was quenched with
ice-
water and then extracted with ethylacetate. The combined organic layer was
washed
- 154 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
with water, brine solution, dried over sodium sulphate and concentrated under
reduced
pressure which was purified by column chromatography using ethylacetate-hexane
gradient to afford the titled product as colourless oil (XLVII, 0.9 g, 70%),
LC-MS m/z
calcd for C11H14N202, 206.1; found 207.1 [M+H]t
Step-3: 2-(pyridin-3-yl)cyclopropane-1-carboxylic acid-XLVIII
OH
I 0
N
To a stirred solution of N-methoxy-N-methy1-2-(pyridin-3-yl)cyclopropane-1-
carboxamide (XLVII, 0.9 g, 4.3mmo1) in water (2 mL) andethanol (1 mL) was
addedpotassium hydroxide (0.731 g, 13mmol). The reaction mixture was stirred
at
room temperature for 16 h. The reaction was concentrated under vacuum and then
acidified to pH 5 with 2Naqueous HC1. The resultant crude was concentrated
under
vacuum and then methanol (5 mL) was added to it. The resultant solid was
filtrated
and the filtrate was concentrated under vacuum to get the title product as
colourless
sticky oil (XLVIII, 0.45 g, 64%). LC-MS m/z calcd for C9H9NO2, 163.1; found
164.1
[M+I-1] .
Step-4: tert-butyl (2-(pyridin-3-yl)cyclopropyl)carbamate-XLIX
0
r)AN A0
H
N
To a stirred solution of 2-(pyridin-3-yl)cyclopropane- 1-carboxylic acid
(XLVIII, 0.4
g, 2.43mmo1) in t-butanol (20 mL) was added triethylamine (0.845 mL,
6.07mmol),diphenylphosphorylazide (0.67 mL, 3.16mmol) and then heated at 80 C
for 18 h. The reaction mixture was concentrated under vacuum. The resultant
crude
was diluted with ethylacetate (100 mL) and washed with water, brine solution,
dried
over sodium sulphate and concentrated under reduced pressure which was
purified by
column chromatography using ethylacetate-hexane gradient to afford the titled
product
as colourlesssticky oil (XLIX, 0.11 g, 20%). LC-MS m/z calcd for C13H18N202,
234.1; found 235.1 [M+I-1] .
Step-5: 2-(pyridin-3-yl)cyclopropan-1-amine TFA salt-B-18
1 NH2.TFA
N
B-1 8
- 155 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
To a stirred solution of tert-butyl (2-(pyridin-3-yl)cyclopropyl)carbamate
(XLIX, 0.05
g,0.21 mmol) in dichloromethane (1 mL) was added trifluoroacetic acid (0.5 mL)
at 0
C and then was stirred at room temperaturefor 3 h. The reaction mixture was
concentrated under vacuum and the resultant solid was titurated with diethyl
ether.
The solid was filtered out and dried under vacuum to get the titled product as
cream
colour solid (B-18, 0.03 g, 62%). LC-MS m/z calcd for C8H10N2, 134.1; found
135.1
[M+1-1] .
B-19- 5-
(4-(2-aminocyclopropyl)pheny1)-1-methylpyridin-2(1H)-one
hydrochloride
NI
NH2 HCI
,O'B(OH)2
A
NIO _______________________________________________ H
N10 N
H jc" I
H x Step-2 0 N sh,p4 0 N
I Intermediate B49
SteP4 N Lõ
LI
Step 1:tert-butyl (2-(4-(6-methoxypyridin-3-yl)phenyl)cyclopropyl)carbamate-LI
0
N
H
1;) N
To a stirred solution of tert-butyl (2-(4-iodophenyl)cyclopropyl)carbamate (L,
1 g,
2.78 mmol) in mixture of dimethoxyethane (8 mL) and water (2 mL) was added (6-
methoxypyridin-3-yl)boronic acid (0.47 g, 3.06 mmol) andpotassium carbonate
(0.77
g, 5.56 mmol) and then degassed for 5 min.
Tetrakis(triphenylphosphine)palladium(0)
(0.16 g, 1.39) was added and heated at 60 C for 2 h. Water was added and
extracted
with ethylacetate (2x100 mL). The organic portion was washed with water,
brine,
dried over sodium sulphate and concentrated under reduced pressure to afford
the
crude product which was purified by column chromatography using methanol-
dichloromethaneto afford the titled product as sticky oil (LI, 0.84 g, 89 %).
LC-MS
m/z calcd for C201-124N203, 340.2; found 341.2 [M+H]t
Step 2: tert-butyl (2-
(4-(1-methy1-6-oxo-1,6-dihydropyridin-3-
yl)phenyl)cyclopropyl)carbamate-LII
0
NA0
H
0 N
To a stirred solution of
tert-butyl (2-(4-(6-methoxypyridin-3-
yl)phenyl)cyclopropyl)carbamate (LI, 0.85 g, 2.5 mmol) in acetonitrile (5 mL)
was
added methyl iodide(1.5 mL) and heated at 60 C for 16 h. The reaction mixture
- 156 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
concentrated under reduced pressure and the resultant crude product was
tritutrated
with diethyl ether to afford the titled product as light brown solid (LII,
0.80 g, 94 %).
LC-MS m/z calcd for C201424N203, 340.2; found 341.2 [M+H]t
Step 3: 5-
(4-(2-aminocyclopropyl)pheny1)-1-methylpyridin-2(1H)-one
hydrochloride- B-19
JJ'NH2 HCI
0 N
To a stirred solution of tert-butyl (2-(4-(1-methy1-6-oxo-1,6-dihydropyridin-3-
yl)phenyl) cyclopropyl) carbamate (LII, 0.85 g, 2.5 mmol) in dioxane (5 mL)
was
added 20 % HC1 in dioxane (3 mL) at 0 C and heated at 60 C for 16 h. The
reaction
mixture was concentrated under vacuum to afford the title product as off-white
solid
(B-19, 0.47 g, 68%). LC-MS m/z calcd for C15H16N20, 240.1; found 241.1 [M+H]t
B-20- 5-(2-aminocyclopropy1)-1,3,3-trimethylindolin-2-one hydrochloride
0 NH2.HCI
0
0
Step 1 Br Step 2 Br "====.
0 0 0
0
Step 3
LIV LV LVI
LIII
0
Step 4 Step 5~LA.YOH Step 6
N0
-).= 0 0 _),õ 0
0 0
LVII
LVIII LIX
Step 7
NH2 HCI
B-20
Step-1: 5-bromoindolin-2-one-LIV
=Br
0
To a stirred suspension of indolin-2-one (LIII, 4.0 g, 27.0 mmol) in
acetonitrile (160
mL), N-bromosuccinimide (6.24 g, 35.1 mmol) was added portion-wise at 0 C
under
nitrogen atmosphere and then stirredfor 3 h at 15-20 C. The reaction mixture
was
quenchedwith ice-water (100 mL) to afford solid. The resultant solid was
filteredthrough sintered funnel, washed with water and dried to afford the
title
- 157 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
compound as a solid (LIV, 6.0 g, 93%). LC-MS m/z calcd for C8H6BrNO, 210.9;
found 212Ø [M+H]t
Step 2: 5-Bromo -1,3,3-trimethylindolin-2-one-LV
Br
0
N
/
3
To a stirred solution of 5-bromoindolin-2-one (LIV, 7.25 g, 34.36 mmol) in
tetrahydrofuran (70 mL) under nitrogen atmosphere, was added sodium hydride
(5.9
g, 137.0 mmol) portion-wise at 0 C. After addition of sodium hydride, the
reaction
was stirred at room temperature for 30 min, then cooledto 0 C. Methyl iodide
(8.5
mL, 137.0 mmol) was added, and then allowed to stir at room temperature for
2h. The
reaction mass was cooled to 0 C and carefully quenched with ice-water. Then
the
reaction mixture was diluted with water (150 mL) and ethylacetate (150 mL).
The
organic layer was separated, washed with water, brine solution, dried over
sodium
sulphate and concentrated under reduced pressure to afford the titled product
as brown
colour solid (LV, 7.4 g, 85%). LC-MS m/z calcd for C11H12BrNO, 253.0; found
254.0
[M+1-1] .
Step-3: (E)-ethyl 3-(1,3,3-trimethy1-2-oxoindolin-5-yl)acrylate-LVI
o
o
N
/
4
To a stirred solution of 5-Bromo-1,3,3-trimethylindolin-2-one (LV, 9.0 g, 35.0
mmol)
in triethylamine (25 mL) wasadded Tetrakis(triphenylphosphine)palladium(0)
(1.92 g,
1.75 mmol) and ethyl acrylate (5.59 mL, 52.5 mmol). The reaction mixture was
heated
at 120 C for 12 h. The reaction mass was cooled to room temperature and then
diluted
with ethylacetate (50 mL). The reaction mixture was filteredout through with
celite
bed. The filtratewas washed with water (100 mL), 1.5N HC1 solution (100 mL),
water
(100 mL), dried over sodium sulphate and then concentrated under reduced
pressure to
afford the crude product which was purified by column chromatography using
ethylacetate-hexane gradient to afford the titled product as yellow solid
(LVI, 5.0 g,
70%). LC-MS m/z calcd for C16H19NO3, 273.1; found 274.1 [M+H]t
Step 4: ethyl 2-(1,3,3-trimethyl -2-oxoindolin-5-yl)cyclopropanecarboxylate-
LVII
- 158 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
o
N 0
/
To a stirred solution of (E)-ethyl, 3-(1,3,3-trimethy1-2-oxoindolin-5-
yl)acrylate (LVI,
2.0 g, 7.2 mmol) in diethyl ether (20 mL) was added Pd(OAc)2(0.32 g, 1.40
mmol), at
0 C and stirred for 20 min. A freshly prepared diazomethane (30 eq) in
diethyl ether
5 was then added slowly and stirred at room temperature for16 h. The
reaction mixture
was filtered through celite bed and washed with dichloromethane. The filtrate
was
evaporated under reduced pressure to get crude product which was purified by
column
chromatography using ethylacetate-hexane gradient to afford the titled product
as an
off white solid (LVII, 1.74 g, 82 %). LC-MS m/z calcd for C17H21NO3, 287.1;
found
288.1 [M+H]t
Step-5: 2-(1,3,3-trimethy1-2oxoindolin-5-yl)cyclpropanecarboxylicacid-LVIII
OH
0
N 0
/
6
To a stirred solution of
ethyl 2-(1,3,3-trimethy1-2-oxoindolin-5-
yl)cyclopropanecarboxylate (LVII, 1.7 g, 14.0 mmol) in tetrahydrofuran (5 mL),
methanol (5 mL) and water (2 mL)was added lithium hydroxide (0.62 g, 14.0
mmol).
The reaction mixture was heated at 50 C for 12 h. The reaction was
concentrated
under vacuum and then acidified to pH 2 with aqueous solution of 2N HC1. The
resultant solid was filteried and dried under vacuum to get the title product
as a white
colour solid (LVIII, 1.2 g, 79%). LC-MS m/z calcd for C15H17NO3, 259.1; found
260.1 [M+H]t
Step-6: tert-butyl (2-(1,3,3-trimethy1-2-oxoindolin-5-yl)cyclopropyl)carbamate-
LIX
0
NA0
0 H
N
/ 7
To a stirred solution of
2-(1,3,3 -trimethy1-2oxoindolin-5-
yl)cyclpropanecarboxylicacid (LVIII, 3.0 g,11.50 mmol) in t-butanol (200 mL)
was
added triethylamine (2.32 mL, 17.2 mmol) diphenylphosphoryl azide (2.86 mL,
12.6
mmol) and then heated at 80 C for 48 h. The reaction mixture was concentrated
under
vacuum to afford the crude product which was purified by column chromatography
- 159 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
using ethylacetate-hexane gradient to afford the titled product as yellow
solid (LIX,
2.5 g, 65%). LC-MS m/z calcd for C19H26N203, 330.2; found 331.2 [M+H]t
Step-7: 5-(2-aminocyclopropy1)-1,3,3-trimethylindolin-2-one hydrochloric acid-
B-20
0 NH2 HCI
To a stirred solution of tert-butyl (2-(1,3,3-trimethy1-2-oxoindolin-5-
yl)cyclopropyl)carbamate (LIX, 12 g, 51.0 mmol) in 1,4 -dioxane (50 mL) was
added
20 % HC1 in 4-dioxane (36 mL) at 0 C and then was heated at 50 C for 3 h.The
reaction mixture was concentrated under vacuum and the resultant solid was
titurated
with diethyl ether. The solid was filtered out and driedunder vacuum to get
the titled
product as white solid (B-20, 7.7g, 87%). LC-MS m/z calcd for C14H18N20,
230.1;
found 231.1 [M+1-1] .
B-21-
2,2,2-trifluoro-N-(2-phenylcyclopropy1)-N-(2-azaspiro[3.3]heptan-6-
ypacetamide hydrochloride
NH. HCI
_________________________________ A NH2.H01 .. y
Step-1 Fr 0J Step-2 A
_______________________________________________________ la (Li
F3c^0
XXVIII F3c-0
B-21
LX
Step-1: tert-butyl 6-
(2,2,2-trifluoro-N-(2-phenylcyclopropypacetamido)-2-
azaspiro[3.3]heptane-2-carboxylate-LX
":3C/No9
F3C0
To a stirred solution of 2-phenylcyclopropan-1-amine hydrochloride (XXVIII,
0.2 g,
1.17mmol) and tert-butyl 6-oxo-2-azaspiro[3.3]heptane-2-carboxylate (0.3 g,
1.41
mmol) in DCE (6 mL) was added sodium triacetoxyborohydride (0.89 g, 4.20mmo1)
and stirred at room temperature for 0.5 h. Methanol (1 mL) was added and then
followed by addition of ethylacetate (10 mL) and 1Mpotassium carbonate
solutionand
the stirring was continued for 30 min.. The organic layer was separated and
washed
with water, brine solution, dried over sodium sulphate and concentrated under
reduced
pressure to get the crude. The crude product was diluted with dry
dichloromethane (5
mL) and cooled to 0 C. Triethylamine (0.5 mL, 3.51 mmol)and trifluoroacetic
- 160 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
anhydride (0.25 mL, 1.70 mmol) were added to it. The reaction mixture was
stirred for
30 min. The reaction mixture was diluted with dichloromethane (50 mL) and
washed
with water, brine solution, dried over sodium sulphate and concentrated under
reduced
pressure to get crude which was purified by column chromatography using
ethylacetate-hexane gradient to afford the titled product as an brown colour
liquid
(LX, 0.2 g, 40 %). LC-MS m/z calcd for C22H27F3N203, 424.2; found 425.2 [M+H]t
Step-2: 2,2,2-trifluoro-N-(2-phenylcyclopropy1)-N-(2-azaspiro[3.3]heptan-6-
ypacetamide hydrochloride-Intermediate B-21
NI-1.HCI
A
01 N
F3C 0
B-21
To a stirred solution of tert-
butyl 6-(2,2,2-trifluoro-N-(2-
phenylcyclopropyl)acetamido)-2-azaspiro[3.3]heptane-2-carboxylate (LX, 0.2 g,
0.47mmo1) in 1,4 -dioxane (2 mL) was added 20 % HC1 in 1,4-dioxane (5 mL) and
then was refluxedfor 10 min. The reaction mixture was concentrated under
vacuum
and the resultant solid was titurated with diethyl ether. The solid was
filtered out and
dried under vacuum to get the titled product B-21as white solid (0.15 g, 98
%). LC-
MS m/z calcd for C17H19F3N20, 324.1; found 325.1 [M+H]t
B-22-N-(azetidin-3-ylmethyl)-2,2,2-trifluoro-N-(2-(4'-fluoro-[1,1'-biphenyl]-4-
yl)cyclopropyl)acetamide hydrochloride
H. HCI
F3C 0
F
B-22
The intermediate B-22 was synthesized starting from intermediate B-12
following
procedure given for the synthesis of B-4. LC-MS m/z calcd for C211-120F4N20,
392.1;
found 393.1 [M+H]t
B-23-N-(azetidin-3-ylmethyl)-2,2,2-trifluoro-N-(2-(4-(1-methyl-1H-pyrazol-4-
yl)phenyl)cyclopropyl)acetamide hydrochloride
- 161 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
..--..\NH.1-1C1
N F3C0
B-23
The intermediate B-23 was synthesized starting from intermediate B-7 following
procedure given for the synthesis of B-4. LC-MS m/z calcd for C19H21F3N40,
378.1;
found 379.1 [M+H]t
B-24-N-(azetidin-3-ylmethyl)-N-(2-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropy1)-2,2,2-trifluoroacetamide hydrochloride
H. HCI
N F3co
'
B-24
The intermediate B-24 was synthesized starting from intermediate B-8 following
procedure given for the synthesis of B-4. LC-MS m/z calcd for C201-122F3N302,
393.1;
found 394.2 [M+H]t
B-25-N-41-(2-aminoethyl)piperidin-4-yl)methyl)-2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropypacetamide hydrochloride
A A
FS
NH Step 1 0
F3C0
F F3C0 ='NNAe<
B-3 LXI
A
Step 2
F F3C0 NH2.HCI
B-25
Step 1: tert-butyl (2-
(4-((2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropypacetamido)methyl)piperidin-1-ypethyl)carbamate-LXI
A
0
101 F3C0
To a solution of 2,2,2-trifluoro-N-(2-(4-fluorophenyl)cyclopropy1)-N-
(piperidin-4-
ylmethyl)acetamide hydrochloride(B-3, 0.5 g, 1.40 mmol) in acetonitrile (5 mL)
was
added tert-butyl (2-bromoethyl)carbamate (0.35 g, 1.50 mmol) and N,N-
diisopropylethylamine (0.75 mL, 4.2 mmol). Then the reaction mixture was
heated at
50 C for 16 h. After completion of reaction, the reaction was diluted with
ethylacetate
- 162 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
(50 mL), washed with water, brine solution, dried over sodium sulfate and
concentrated under vacuum to afford the crude product which was further
purified by
flash chromatography using methanol-dichloromethane gradient to result in the
titled
product as a brown colour liquid (LXI, 0.6 g, 85 %). LC-MS m/z calcd for
C24H33F4N303, 487.2; found 488.2 [M+H]t
Step 2: N-41-(2-aminoethyl)piperidin-4-y1)methyl)-2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropypacetamide hydrochloride-B-25
A
N
F F3eL0 N H2 HCI
B-25
To a stirred solution of
tert-butyl (2-(4-((2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropyl)acetamido)methyl)piperidin-l-yl)ethyl)carbamate(LXI,
0.6
g, 1.20 mmol) in dioxane (5 mL) was added 20 % HC1 in dioxane (3 mL) at 0 C
and
stirred for 2 h at room temperature. The reaction mixture was concentrated
under
vacuum and triturated with diethyl ether. The resultant solid was dried under
vacuum
to afford the title product as off-white solid (B-25, 0.48 g, quantitative
yield). LC-MS
m/z calcd for C19H25F4N30, 387.2; found 388.2 [M+H]t
B-26- N-41-(2-aminoethyl)piperidin-4-y1)methyl)-2,2,2-trifluoro-N-(2-
phenylcyclopropypacetamide hydrochloride
A
F3eL0 H2. HCI
B-26
The intermediate B-26 was synthesized starting from 2,2,2-trifluoro-N-(2-
phenylcyclopropy1)-N-(piperidin-4-ylmethyl)acetamide and tert-
butyl (2-
bromoethyl)carbamate by following the experiment procedure of - B-25. LC-MS
m/z
calcd for C19H26F3N30, 369.2; found 370.1 [M+H]t
B-27- N-41-(2-aminoethyl)piperidin-4-yl)methyl)-N-(2-(3,4-difluorophenyl)
cyclopropy1)-2,2,2-trifluoroacetamide hydrochloride
FI\N
F3eL0 NNHHCI
B-27
The intermediate B-27 was synthesized starting from N-(2-(3,4-
difluorophenyl)cyclopropy1)-2,2,2-trifluoro-N-(piperidin-4-ylmethyl)acetamide
and
- 163 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
tert-butyl (2-bromoethyl)carbamate by following the experiment procedure of -B-
25.
LC-MS m/z calcd for C19H22F5N20, 389.2; found 390.1 [M+H]t
Synthesis of Intermediate Esters-I series
1-2 (E)-3-[4-
({tert-Butoxycarbonyl-[2-(4-fluoro-phenyl)-cyclopropy1]-aminol-
methyl)-phenyl]-acrylic acid methyl ester (LXII)
Step F
A
F A Step 2
__________________________________________________ ' 0 Nil
0 1 so NH Boc ..--- OMe
H2N F
V ---
OMe 1-2 0
LX11
)0CV111 0
Step 1: (E)-
3-(4-{ [2-(4-Fluoro-phenyl)-cyclopropylamino] -methyl}-phenyl)-
acrylic acid methyl ester 0
A
101 NH
F ---
OMe
0
To a stirred solution of 2-(4-fluoro-phenyl)-cyclopropylamine hydrochloride
(XXVIII, 0.2 g, 1.06 mmol) in methanol (20 mL) was added (E)-3-(4-formyl-
pheny1)-
acrylic acid methyl ester (0.24 g, 1.28 mmol) and sodium bicarbonate (0.08 g,
0.95
mmol) and molecular sieves (approx 1 g) at room temperature and the resulting
mixture was heated to reflux for 2.5 h. Cooled to 0 C and sodium borohydride
(0.036
g, 0.95 mmol) was added, stirred at room temperature for 1 h. Ice was added
and the
reaction mixture was filtered. The solvent was evaporated to get the residue.
Water
was added and extracted with dichloromethane (2x50 mL). The organic portion
was
washed with water and brine dried over sodium sulphate and concentrated under
reduced pressure to afford the crude productwhich was purified by column
chromatography using methanol-dichloromethane gradient to afford the titled
product
as yellow oil. (LXII, 0.3 g, 90 %). LC-MS m/z calcd for C20I-120FN02, 325.1;
found
326.3 [M+1-1] .
Step 2: (E)-3-[4-({tert-Butoxycarbonyl-[2-(4-fluoro-phenyl)-cyclopropy1]-
aminol-
methyl)-phenyl]-acrylic acid methyl ester (I-2)
N
F Bioc / OMe
0
To a stirred solution of (E)-3-(4-1}2-(4-fluoro-pheny1)-cyclopropylamino] -
methyl} -
phenyl)-acrylic acid methyl ester (XLVI, 0.25 g, 0.76 mmol) in tetrahydrofuran
and
- 164 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
water mixture (6 mL, 1:1) was added sodium bicarbonate (0.087 g, 2.3 mmol) and
Boc anhydride (0.22 mL, 0.92 mmol) at room temperature and the resulting
mixture
was stirred at that temperature for 2 h. The progress of the reaction was
monitored by
TLC. The reaction mixture was diluted with ethylacetate and the organic
portion was
washed with water and brine solution, dried over sodium sulphate and
concentrated
under reduced pressure to get the crude product which was purified by column
chromatography using ethylacetate-hexane gradient to afford the titled product
as
sticky oil (1-2, 0.19 g, 58 %). LC-MS m/z calcd for C25H28FN04, 425.2; found
326.3
[M-Boc+1] .
The following compounds were synthesized using procedure for the synthesize of
1-2
I-3methy1 (E)-
3-(4-(((tert-butoxycarbonyl)(2-(44(4-fluorobenzypoxy)phenyl)
cyclopropyl)amino)methyl)phenyl)acrylate
N
1
Boc
0 0 /
0
F
The compound was synthesized using amine B6 and (E)-3-(4-Formyl-phenyl)-
acrylic
acid methyl esterfollowing the procedure for the synthesis of 1-2. LC-MS m/z
calcd
for C32H34FN05, 531.2; found 532.2 [M+H]t
1-4 methyl (E)-
3-(4-(4-(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)phenyl)acrylate
A
0 rl
Boc N
0
/
0
The compound was synthesized using phenylcyclopropyl amine and aldehyde A2
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C30H38N204,
490.3; found 434.2 [M-56] .
1-5 methyl (E)-
3-(4-(((tert-butoxycarbonyl)(2-(4'-chloro-[1,1'-biphenyl]-4-
yl)cyclopropyl)amino)methyl)phenyl)acrylate
- 165 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
N
Bioc 0
/
0
CI
The compound was synthesized using amine B10 and methyl-4-formyl cinnamic acid
esterfollowing the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C311-132C1N04, 517.2; found 462.2 [M-56] .
1-6 methyl (E)-3-(4-
(((tert-butoxycarbonyl)(2-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)phenypacrylate
N
Bioc / 0
R
N¨ 0
The compound was synthesized using amine B8 and methyl-4-formyl cinnamic acid
esterfollowing the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C30H34N205, 502.2; found 503.2 [M+H] .
1-7 methyl (E)-
3-(4-(((tert-butoxycarbonyl)(2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl) amino)methyl)phenyl)acrylate
N
1
Boc / 0
N
N 0
The compound was synthesized using amine B11 and methyl-4-formyl cinnamic acid
esterfollowing the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C29H31N304, 485.2; found 486.2 [M+H]t
1-8 methyl 2-
(4-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-yl)pyrimidine-5-carboxylate
F Boc N N
II
N
0
- 166 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using 4-fluorophenyl cyclopropyl amine and
aldehyde
Al following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C26H33FN404, 484.2; found 485.2 [M+H]t
1-9 methyl 2-(4-((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)piperidin-1-
yl)pyrimidine-5-carboxylate
0
.)L
NV 1 0
NN
A
40 N
Bioc
The compound was synthesized using phenylcyclopropyl amine and ketone A3
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C25H32N404,
452.2; found
453.2[M+1-1] .
1-10 methyl 2-(4-((tert-
butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)piperidin-l-yl)pyrimidine-5-carboxylate
0
,)=Lo N ' 1
N)N
N)
Bioc
F
The compound was synthesized using 4-fluorophenylcyclopropyl amine and ketone
A3 following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C25H31FN404, 470.2; found 471.2 [M+H]t
1-11 methyl 2-
(4-(((tert-butoxycarbonyl)(2-(4-((4-
fluorobenzypoxy)phenyl)cyclopropyl) amino)methyl)piperidin-l-yl)pyrimidine-5-
carboxylate
A
11
Boc N N
0 0 =
N 0
F
0
The compound was synthesized using amine B-6 and aldehyde Al following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C33H39FN405, 590.3;
found
591.2 [M+1-1] .
- 167 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1-12 methyl 2-
(4-((tert-butoxycarbonyl)(2-(4-((4-
fluorobenzypoxy)phenyl)cyclopropyl)amino)piperidin-l-y1)pyrimidine-5-
carboxylate
o
..---..j... ...-
N ' 0
I I
N N
A
0 rs6loc
6 o
F
The compound was synthesized using amine B-6 and ketone A3 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C32H37FN405, 576.2;
found
577.3 [M+1-1] .
I-13methyl 2-
(4-((tert-butoxycarbonyl)(2-(4'-chloro-[1,1'-biphenyl[-4-
yl)cyclopropyl)amino)piperidin-1-yl)pyrimidine-5-carboxylate
0
N ).L0"---
I
isial N
Bioc
CI
The compound was synthesized using amine B-10 and ketone A3 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C31H35C1N404, 562.2;
found
563.2 [M+H]t
1-14 methyl 2-
(4-(((tert-butoxycarbonyl)(2-(4'-chloro-[1,1'-biphenyl[-4-
yl)cyclopropyl) amino)methyl)piperidin-1-yl)pyrimidine-5-carboxylate
1
Boc N N
0
The compound was synthesized using amine B-10 and aldehyde Al following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C32H37C1N404, 576.2;
found
577.2 [M+H] .
1-15 ethyl 2-(4-(((tert-
butoxycarbonyl)(2-(4'-fluoro-[1,1'-biphenyl]-4-
yl)cyclopropyl)amino)methyl)piperidin-l-yppyrimidine-5-carboxylate
- 168 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
r'
Boc N N
NO(¨/
F
0
The compound was synthesized using amine B-12 and aldehyde Al following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C33H39FN404, 574.3;
found
575.3 [M+1-1] .
1-16 methyl 2-(4-(((tert-
butoxycarbonyl)(2-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-y1)pyrimidine-5-carboxylate
ri
Boc N N
(3,
N¨ N 0
134
The compound was synthesized using amine B-8 and aldehyde Al following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C311-139N505, 561.2;
found
562.2 [M+1-1] .
1-17 methyl 2-
(4-(((tert-butoxycarbonyl)(2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino) methyl)piperidin-l-yl)pyrimidine-5-carboxylate
"
Boc N N N
N
-r -
N r0-
0
The compound was synthesized using amine B-11 and aldehyde Al following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C301436N604, 544.3,
found
545.2 [M+1-1] .
1-18 methyl 2-
(4-(((tert-butoxycarbonyl)(2-(4-
methoxyphenyl)cyclopropyl)amino)methyl)
piperidin-l-yl)pyrimidine-5-
carboxylate
A
o SI r(
Boc N N
-r -
N ((:)
0
- 169 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using 4-methoxyphenyl cyclopropyl amine and
aldehyde Al following the procedure for the synthesis of 1-2. LC-MS m/z calcd
for
C27H36N405, 496.2, found 497.3 [M+H]t
1-19 methyl 2-
(4-((tert-butoxycarbonyl)(2-(4-
methoxyphenyl)cyclopropyl)amino)piperidin-l-yl)pyrimidine-5-carboxylate
o
N
I
N N
A , j
lel
o N"
1
Boc
The compound was synthesized using 4-methoxyphenyl cyclopropyl amine and
ketone A3 following the procedure for the synthesis of 1-2. LC-MS m/z calcd
for
C26H34N405, 482.2, found 483.2 [M+H]t
1-20 methyl 2-(4-(((tert-
butoxycarbony1)41R,2S)-2-(4-
fluorophenyl)cyclopropyl)amino)
methyl)piperidin-l-yl)pyrimidine-5-
carboxylate
'WAY
F Boc N N
N.,(0
0
The compound was synthesized using 4-fluorophenyl cyclopropyl amine B1 and
aldehyde Al following the procedure for the synthesis of 1-2. LC-MS m/z calcd
for
C26H33FN404, 484.2, found 485.2 [M+H]t
1-21 methyl 2-
(4-(((tert-butoxycarbonyl)((lS,2R)-2-(4-
fluorophenyl)cyclopropyl)amino)
methyl)piperidin-l-yl)pyrimidine-5-
carboxylate
A ,
110 .'11
F
Boc N N
)r
N 0
0
The compound was synthesized using 4-fluorophenyl cyclopropyl amine B2 and
aldehyde Al following the procedure for the synthesis of 1-2. LC-MS m/z calcd
for
C26H33FN404, 484.2, found 485.2 [M+H]t
1-22 methyl 4-(4-(((tert-
butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl) piperidin-l-yl)benzoate
- 170 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
ri
F Boc N 0
spo
0
The compound was synthesized using 4-fluorophenyl cyclopropyl amine and
aldehyde
A2 following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C28H35FN204, 482.2, found 483.3 [M+H]t
1-23 methyl 2-(2-(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)-5,6-
dihydroimidazo[1,2-a]pyrazin-7(8H)-yl)pyrimidine-5-carboxylate
A
N Boo N\\
N
1 --\N--D N._ 0¨
_,µ
/ 0
The compound was synthesized using phenylcyclopropyl amine and aldehyde A4
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C27H32N604,
10 504.2, found 505.2 [M+H]t
1-24 methyl 2-
(2-(((tert-butoxycarbonyl)(2-(4-
methoxyphenyl)cyclopropyl)amino)methyl)-5,6-dihydroimidazo[1,2-a]pyrazin-
7(8H)-yl)pyrimidine-5-carboxylate
A
1.1 N N
B NN3 \
oc ¨ ,K(:)-
0 N¨ \0
I
The compound was synthesized using 4-methoxyphenylcyclopropyl amine and
aldehyde A4 following the procedure for the synthesis of 1-2. LC-MS m/z calcd
for
C28H34N605, 534.2, found 535.2 [M+H]t
1-25 methyl 2-
(2-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)-5,6-dihydroimidazo[1,2-a]pyrazin-
7(8H)-yl)pyrimidine-5-carboxylate
/ __________________________________ er<
N Isl---N,rN,
µ13oc Nr0
. 0
F
The compound was synthesized using 4-fluorophenylcyclopropyl amine and
aldehyde
A4 following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C27H31FN604, 522.2, found 523.2 [M+H]t
- 171 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1-26 methyl 3-
(((2-(4-bromophenyl)cyclopropyl)(tert-
butoxycarbonyl)amino)methyl)benzoate
0
40 0
Boc
Br
The compound was synthesized using 4-bromophenylcyclopropyl amine and methyl-
3-formyl benzoic acid ester following the procedure for the synthesis of 1-2.
LC-MS
m/z calcd for C23H26BrN04, 459.1, found 460.1 [M+H]t
1-27 methyl 3-
(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)benzoate
o
A
0 N 0 0
Bioc
The compound was synthesized using phenylcyclopropyl amine and methyl-3-formyl
benzoic acid ester following the procedure for the synthesis of 1-2. LC-MS m/z
calcd
for C23H27N04, 381.2, found 382.1 [M+H]t
1-28 methyl 4-
(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)benzoate
A
Boc iz)
0
The compound was synthesized using phenylcyclopropyl amine and methyl-4-formyl
benzoic acid ester following the procedure for the synthesis of intermediate 1-
2. LC-
MS m/z calcd for C23H27N04, 381.2, found 382.1 [M+H]t
1-29 ethyl 6-((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)hexanoate
A
BN1o,---....,....õ..-........r,c0
0 0
I
The compound was synthesized using phenylcyclopropyl amine and methyl 6-
oxohexanoatefollowing the procedure for the synthesis of 1-2. LC-MS m/z calcd
for
C22H33N04, 375.2, found 276.2 [M-BocH]t
1-30 ethyl 4-
(3-((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)propyl)benzoate
- 172 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
N
Bioc 0
F
0
The compound was synthesized using phenylcyclopropyl amine and methyl 4-(3-
oxopropyl)benzoate following the procedure for the synthesis of 1-2. LC-MS m/z
calcd for C26H32FN04, 441.2, found 386.2 [M-55]t
1-31 methyl 7-(4-(((tert-
butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)benzamido) heptanoate
A
Boc N .r0
0 0
The compound was synthesized using phenylcyclopropyl amine and aldehyde A5
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C301140N205,
508.3, found 509.3 [M+H]t
1-32 methyl 7-
(4-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl) benzamido)heptanoate
Y 40 H
F Boc N 0
0 0
The compound was synthesized using 4-fluorophenylcyclopropyl amine and
aldehyde
A5 following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C30H39FN205, 526.3, found 527.3 [M+H] .
1-33 methyl 4-
((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)cyclohexanecarboxylate
0
A
110 N
Boc
The compound was synthesized using phenylcyclopropyl amine and methyl 4-
oxocyclohexane- 1-carboxylate following the procedure for the synthesis of 1-
2. LC-
MS m/z calcd for C22H31N04, 373.2, found 374.2 [M+H]t
1-34 (1S,4R)-methyl 4-
((lS)-1-((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)ethyl)cyclohexane carboxylate
- 173 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
A j
el I3oc ONTiO
0
The compound was synthesized using phenylcyclopropyl amine and methyl (1R,4R)-
4-acetylcyclohexane-1-carboxylate following the procedure for the synthesize
of 1-2
LC-MS m/z calcd for C24H35N04, 401.2, found 402.2 [M+H]t
1-35 methyl 4-((4-
(((tert-butoxycarbonyl)(2-(4-(1-methy1-6-oxo-1,6-
dihydropyridin-3-yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-
yl)methyl)benzoate
0
N 0 e
N
I X
0 N
I
The compound was synthesized using amine B19 and methyl 4-((4-formylpiperidin-
1-
yl)methyl)benzoate following the procedure for the synthesis of 1-2. LC-MS m/z
calcd
for C35H43N305, 585.3, found 586.3 [M+H]t
1-36 methyl 4-
((4-(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)methyl)benzoate
0
A
0 N
giolON 40) 0
The compound was synthesized using Phenylcyclopropyl amine and methyl 4-((4-
formylpiperidin-1-yl)methyl)benzoate following the procedure for the synthesis
of 1-2.
LC-MS m/z calcd for C29H38N204, 478.3, found 479.3 [M+H]t
1-37 methyl 4-((4-(((tert-butoxycarbonyl)(2-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)
cyclopropyl)amino)methyl)piperidin-l-yl)methyl)benzoate
0
N
13:cON el ¨
q
N ¨
The compound was synthesized using amine B8 and methyl 4-((4-formylpiperidin-1-
yl)methyl)benzoate following the procedure for the synthesis of 1-2. LC-MS m/z
calcd
for C34H43N305, 573.3, found 574.3 [M+H]t
- 174 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1-38 methyl 4-
((4-(((tert-butoxycarbonyl)(2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino) methyl)piperidin-l-yl)methyl)benzoate
0
Boc N
N
N 1
The compound was synthesized using amine B11 and methyl 44(4-formy1piperidin-1-
yl)methyl)benzoate following the procedure for the synthesis of 1-2. LC-MS m/z
calcd
for C33H40N404,556.3, found 557.3 [M+H]t
1-39 methyl 6-((4-(((tert-butoxycarbonyl)(2-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)
cyclopropyl)amino)methyl)piperidin-l-yl)methyl)nicotinate
0
YO L ¨
Boc NN
R
N-
The compound was synthesized using amine B8 and methyl 64(4-formylpiperidin- 1-
yl)methyl)nicotinatefollowing the procedure for the synthesize of 1-2. LC-MS
m/z
calcd for C33H42N405, 574.3, found 575.3 [M+H]t
1-40 ethyl 4-((4-(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)-1H-
pyrazol-1-yl)methyl)benzoate
60c ¨N
.....-
0
The compound was synthesized using Phenylcyclopropyl amine and aldehyde Al2
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C28H33N304,
475.2, found 476.2 [M+H]t
1-41 ethyl 4-((4-(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)-1H-
1,2,3-triazol-1-yl)methyl)benzoate
110" N 0
µBoc
. 0
I
- 175 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using phenylcyclopropyl amine and aldehyde A7
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C27H32N404,
476.2, found 477.2 [M+H]t
1-42 methyl 4-
(2-(4-(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)ethyl)benzoate
A
0 1.11
(:)c) N SI
X (:)
0
The compound was synthesized using Phenylcyclopropyl amine and aldehyde A15
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C30H40N204,
492.66, found 393.6 [M+H-Boc]t
1-43 ethyl 4-(3-(4-
(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-1-y1) propyl)benzoate
0
A
0 ril
Boc N 0
The compound was synthesized using phenylcyclopropyl amine and aldehyde A19
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C32H44N204,
520.3, found 521.3 [M+H]t
1-44 ethyl 4-(3-(4-((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)piperidin-
1-
yl)propyl) benzoate
N
A ,)
el N
1
Boc 0 ()
The compound was synthesized using phenylcyclopropyl amine and aldehyde A16
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C31H42N204,
506.3, found 507.3 [M+H]t
1-46 ethyl 4-
(3-(6-(2,2,2-trifluoro-N-(2-phenylcyclopropypacetamido)-2-
azaspiro[3.3]heptan-2-y1)propyl)benzoate
LiCINH.HCI N
A = A. NOC/ ) N -111.-
F300 0
\-----
F3C0 0
1-46
B-21
- 176 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
To a solution of 2,2,2-trifluoro-N-(2-phenylcyclopropy1)-N-(2-
azaspiro[3.3]heptan-6-
y1)acetamide hydrochloride(B-21, 0.2 g, 0.5 mmol) in acetonitrile (2 mL) was
added
ethyl 4-(3-bromopropyl)benzoate (0.149 g, 0.5 mmol) and N,N-
diisopropylethylamine
(0.26 mL, 1.5 mmol). Then the reaction mixture was heated at 60 C for 16 h.
After
completion of reaction, the reaction was diluted with ethylacetate (50 mL),
washed
with water, brine solution, dried over sodium sulfate and concentrated under
vacuum
to get crude product which was purified by column chromatography using
ethylacetate-hexane gradient to afford the titled product as brown gummy solid
(1-46,
0.140 g, 49 %). LC-MS m/z calcd for C29H33F3N203, 514.2; found 515.3 [M+H]t
1-47 ethyl 4-(3-(4-
(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl) piperidin-l-yl)propyl)benzoate
0
A
F
Boo N
The compound was synthesized using 4-fluorophenylcyclopropyl amine and
aldehyde
A19 following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C32H43FN204, 538.3, found 539.3 [M+H]t
1-48 ethyl 4-
(3-(3-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl) azetidin-l-yl)propyl)benzoate-
A
F
o
0
A NC\ 0 0
01 ._ FN + Br F>rLO
N
F0 r..."--0 F
F
F
The intermediate I-48was synthesized usingB-4 and ethyl 4-(3-
bromopropyl)benzoate
following the procedure for the synthesis of 1-46. LC-MS m/z calcd for
C30H39FN204,
510.3, found 511.3 [M+1-1] .
1-49 ethyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(3-
fluorophenyl)cyclopropyl)amino)methyl) piperidin-l-yl)propyl)benzoate
0
A
110 rl
Boc N 0
F
The compound was synthesized using 3-fluorophenylcyclopropyl amine and
aldehyde
A19 following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C32H43FN204, 538.3, found 539.3 [M+H]t
- 177 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1-50 ethyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(3,4-
difluorophenyl)cyclopropyl)amino) methyl)piperidin-l-yl)propyl)benzoate
0
rij 0
Boc N
F
F
The compound was synthesized using 3,4-difluorophenylcyclopropyl amine and
aldehyde A19 following the procedure for the synthesis of 1-2. LC-MS m/z calcd
for
C32H42F2N204, 556.3, found 557.3 [M+H] .
1-51 ethyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(4-
methoxyphenyl)cyclopropyl)amino)methyl) piperidin-l-yl)propyl)benzoate
0
11 0
Boc N
0
The compound was synthesized using 4-methoxyphenylcyclopropyl amine and
aldehyde A19 following the procedure for the synthesis of 1-2. LC-MS m/z calcd
for
C33H46N205, 550.3, found 551.3 [M+H]t
1-52 ethyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(4-((4-
fluorobenzypoxy)phenyl)cyclopropyl)
amino)methyl)piperidin-1-
yl)propyl)benzoate
0
N 0
1
Boc N
F . CI
The compound was synthesized using amine B6 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C39H49FN205, 644.3,
found
645.4 [M+H] .
1-53: ethyl 4-(3-(4-
(((tert-butoxycarbonyl)(2-(4-
iodophenyl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzoate
- 178 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
o
09
A o
A 401 (:)
101 NH2 HCI 090
Step-1
LX111
LX1V
0
H2N
Isrn 09
Step-2 0
1-53
Step-I: To a stirred solution of 2-(4-iodophenyl)cyclopropan- 1-amine
hydrochloride
(LXIII, 1.0 g, 3.30 mmol) in methanol (50 mL) was added ethyl 4-(3-(4-
formylpiperidin-1-yl)propyl)benzoate (1-3, 1.13 g, 3.30 mmol) and sodium
bicarbonate (0.25 g, 2.90 mmol) and molecular sieves (approx 2 g) at room
temperature and the resulting mixture was heated to reflux for 2 h. Cooled to
0 C,
then sodium borohydride (0.12 g, 3.30 mmol) was added and stirred at room
temperature for 1 h. Ice was added and the reaction mixture was filtered. The
solvent
was evaporated to get the residue. Water was added and extracted with
ethylacetate
(2x200 mL). The organic portion was washed with water, brine, dried over
sodium
sulphate and concentrated under reduced pressure to afford the crude compound.
To a
stirred solution of crude compound in mixture of tetrahydrofuranand water (20
mL,
1:1) was added sodium bicarbonate (0.69 g, 8.25 mmol) and Boc anhydride (1.05
mL,
4.90 mmol) at room temperature and the resulting mixture was stirred at that
temperature for 2 h. The progress of the reaction was monitored by TLC. The
reaction
mixture was diluted with ethylacetate and the organic portion was washed with
water
and brine solution, dried over sodium sulphate and concentrated under reduced
pressure to get the crude product which was purified by column chromatography
using
ethylacetate-hexane gradient to afford the titled product as sticky oil (LXIV,
1 g, 54
%), LC-MS m/z calcd for C321L31N204, 646.2; found647.1 [M+H]t
Step-2: To a stirred solution of ethyl 4-(3-(4-(((tert-butoxycarbonyl)(2-(4-
iodophenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzoate (LXIV, 1 g,
1.5 mmol) in toluene (50 mL) was added N,N-dimethylethane-1,2-diamine (0.16 g,
1.80 mmol) and degassed with argon gas for 10 min. Then, palladium acetate
(0.008 g,
0.037 mmol), Bis[(2-diphenylphosphino)phenyl] ether (0.080 g, 0.15),
chloroform
(0.36 mL, 4.5 mmol) and cesium hydroxide hydrate (2.51 g, 15.0 mmol) was added
and heated at 100 C for 24 h. The reaction mixture was cooled to room
temperature.
Then, the reaction mixture was filtered through celite, washed with toluene
and
- 179 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
concentrated under vacuum. The crude product was purified by column
chromatography using methanol-dichloromethane gradient to afford the titled
product
as brown colour oil (1-53, 0.57 g, 58 %), LC-MS m/z calcd for C37H54N405,
634.4;
found 635.4 [M+H]t
1-54 ethyl 4-(3-(4-(((tert-butoxycarbonyl)(2-(4-(morpholine-4-carbonyl)phenyl)
cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzoate
0
N Boc N
0
The compound I-54was synthesized following the procedure for the synthesis of
1-53.
LC-MS m/z calcd for C37H51N306, 633.4, found 634.4 [M+H]t
1-55 ethyl 4-(3-(4-(((tert-butoxycarbonyl)(2-(4-(piperidine-1-carbonyl)phenyl)
cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzoate
0
N Boc N
0
The compound was synthesized following the procedure for the synthesis of 1-
53. LC-
MS m/z calcd for C38H53N305, 631.4, found 632.4 [M+H]t
1-56 methyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(4'-chloro-[1,1'-biphenyl[-4-
yl)cyclopropyl) amino)methyl)piperidin-l-yl)propyl)benzoate
0
ril 0
Boc N
CI
The compound was synthesized using amine B10 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C37H45C1N204, 616.3,
found
617.3 [M+H]t
1-57 ethyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(4'-fluoro-[1,1'-biphenyl[-4-
yl)cyclopropyl) amino)methyl)piperidin-l-yl)propyl)benzoate
- 180 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
r' c.
Boc N
F
The compound was synthesized using amine B12 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C38H47FN204, 614.3,
found
615.3 [M+H]t
1-58 methyl 4-(3-(3-
(((tert-butoxycarbonyl)(2-(4'-fluoro-[1,1'-biphenyl[-4-
yl)cyclopropyl)amino)methypazetidin-1-y1)propyl)benzoate
o
N\NI 0
F>rLO
F
F
F
The compound was synthesized using amine B22 and methyl 4-(3-
bromopropyl)benzoate following the procedure for the synthesis of 1-46. LC-MS
m/z
calcd for C32H32F4N203, 568.2, found 569.2 [M+H]t
1-59 ethyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(4'-cyano-[1,1'-biphenyl[-4-
yl)cyclopropyl)amino) methyl)piperidin-l-yl)propyl)benzoate
o
Boc N
NC
The compound was synthesized using amine B13 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C39H47N304, 621.3,
found
622.3 [M+1-1] .
1-60 ethyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(4-(1-methyl-2-oxo-1,2-
dihydropyridin-4-y1)
phenyl)cyclopropyl)amino)methyl)piperidin-l-
yl)propyl)benzoate
o
Boc N
I
N
o
The compound was synthesized using amine B9 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C38H49N305, 627.3,
found
628.3 [M+H] .
- 181 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1-61 ethyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzoate
0
N 0
1
Boc N
IsV
N 1
The compound was synthesized using amine B11 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C36H46N404, 598.3,
found
599.3 [M+1-1] .
1-62 ethyl 4-(3-(4-(((tert-butoxycarbonyl)(2-(4-(1-methyl-1H-pyrazol-4-
yl)phenyl)
cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzoate
0
ril 0
Boc N
---..
¨N
N-
The compound was synthesized using amine B7 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C35H46N404, 586.3,
found
587.3 [M+1-1] .
1-63 ethyl 4-
(3-(34(2,2,2-trifluoro-N-(2-(4-(1-methy1-1H-pyrazol-4-
yl)phenyl)cyclopropyl)
acetamido)methyl)azetidin-1-yl)propyl)benzoate-
Procedure
0
0
Nil C\N
N/ I F3C0
µN
/
The compound was synthesized using amine B23 and ethyl 4-(3-
bromopropyl)benzoatefollowing the procedure for the synthesis of 1-46. LC-MS
m/z
calcd for C311435F3N403, 568.2, found 569.2 [M+H]t
1-64 ethyl 4-(3-(4-
(((tert-butoxycarbonyl)(2-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-y1)propyl)benzoate
- 182 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
N 0
o
µIsl¨ X
The compound was synthesized using amine B8 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C37H49N305, 615.4,
found
616.4 [M+H]t
1-65 ethyl 4-(3-(3-4N-(2-(4-(3,5-dimethylisoxazol-4-yl)phenyl)cyclopropy1)-
2,2,2-
trifluoroacetamido)methypazetidin-1-y1)propyl)benzoate
0
0
N/ I F3C0
b
The compound was synthesized using amine B23 and ethyl 4-(3-
bromopropyl)benzoatefollowing the procedure for the synthesis of 1-46. LC-MS
m/z
calcd for C32H36F3N304, 583.2 found 584.3 [M+H]t
1-66 ethyl 4-(3-(4-(((tert-butoxycarbonyl)(2-(4-(6-(trifluoromethyppyridin-3-
y1)phenyl) cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzoate
0
Y sci'
Boc N
I
F3C N
The compound was synthesized using amine B14 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C38H46F3N304, 665.3;
found
666.3 [M+1-1] .
1-67 ethyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(1-isopropyl-1H-pyrazol-4-
yl)cyclopropyl) amino)methyl)piperidin-l-yl)propyl)benzoate
0
N 0
I --N
:1:-/BcONc
/ N
The compound was synthesized using amine B15 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C32H48N404, 552.4;
found
553.4 [M+H]t
- 183 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1-68 ethyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(1-phenyl-1H-pyrazol-4-
yl)cyclopropyl) amino)methyl)piperidin-l-yl)propyl)benzoate
0
N 0
I O. N
N
The compound was synthesized using amine B16 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C35H46N404, 586.4;
found
586.4 [M+1-1] .
1-69 ethyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(2-methylthiazol-5-
yl)cyclopropyl)amino)methyl) piperidin-l-yl)propyl)benzoate
0
Boc N
N
The compound was synthesized using amine B17 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C301-143N304S, 541.3;
found
542.3 [M+1-1] .
1-70 ethyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(pyridin-3-
yl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzoate
0
CAN 0
BoleN
N
The compound was synthesized using amine B18 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C31H43N304, 521.3;
found
522.3 [M+1-1] .
1-71 ethyl 4-
(3-(2-(((tert-butoxycarbonyl)(2-(4-
methoxyphenyl)cyclopropyl)amino)methyl)-5,6-dihydroimidazo[1,2-a]pyrazin-
7(8H)-y1)propyl)benzoate
0
0"--\
13oc
¨0
- 184 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using 4-methoxyphenylcyclopropylamine and
aldehyde A18 following the procedure for the synthesis of 1-2. LC-MS m/z calcd
for
C34H44N405, 588.3; found 589.3 [M+H]t
1-72 ethyl 4-
(3-(2-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)-5,6-dihydroimidazo[1,2-a]pyrazin-
7(8H)-yl)propyl)benzoate
0
ersi 0
N/ N-:--J,N
boc
F
The compound was synthesized using 4-fluorophenylcyclopropylamine and aldehyde
A18 following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C33H41FN404, 576.3; found 577.3 [M+H]t
1-73 ethyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-(3,4-
difluorophenyl)cyclopropyl)amino)methyl)-1H-imidazol-1-yl)propyl)benzoate
N/"-----eNN
F Boc
0
N/
F 0
The compound was synthesized using 3,4-difluorophenylcyclopropylamine and
aldehyde A8following the procedure for the synthesis of 1-2. LC-MS m/z calcd
for
C30H35F2N304, 539.3; found 540.3 [M+H]t
1-74 ethyl 4-(3-(5-(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)-
1H-imidazol-1-y1) propyl)benzoate
Boc
NI\___411
N
4110
0
/
- 185 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using phenylcyclopropylamine and aldehyde A9
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C30H37N304,
503.3; found 504.3 11\4+Hr.
1-75 ethyl 4-(3-(4-(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)-
1H-imidazol-1-yl)propyl)benzoate
N =_/
Boo N
0,
0
The compound was synthesized using phenylcyclopropylamine and aldehyde A8
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C30H37N304,
503.4; found 504.3 [M+H]t
1-76 ethyl 4-(3-(4-(((2-
phenylcyclopropyl)amino)methyl)-1H-pyrazol-1-
y1)propyl)benzoate
Boc 0_
0
The compound was synthesized using phenylcyclopropylamine and aldehyde All
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C30H37N304,
503.4 found 504.3 [M+H]t
1-77 ethyl 4-(3-(4-(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)-
1H-1,2,3-triazol-1-yl)propyl)benzoate
Nf---eNN
I N=N
Boc 0_
0
The compound was synthesized using phenylcyclopropylamine and aldehyde A28
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C29H36N404,
504.2; found 505.3 [M+H]t
1-78 ethyl 4-
(3-(6-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)-3,4-dihydroisoquinolin-2(1H)-
yl)propyl)benzoate
- 186 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
N 0
1
FBN
The compound was synthesized using 4-fluorophenylcyclopropylamine and aldehyde
A20 following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C36H43FN204, 586.3; found 587.3 [M+H]t
1-79 methyl 4-((7-
(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)-3,4-dihydroisoquinolin-2(1H)-
yl)methyl)benzoate
N N 0Bioc 0
F
0
The compound was synthesized using phenylcyclopropylamine and aldehyde A22
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C33H37FN204,
544.2; found 545.3 [M+H]t
1-80 methyl 4-
((2-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)-5,6-dihydroimidazo[1,2-a]pyrazin-
7(8H)-y1)methyl)benzoate
0
r. N 0 e
N/ \N.-----N
13oc
F
The compound was synthesized using 4-fluorophenylcyclopropylamine and aldehyde
A14 following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C30H35FN404, 534.2; found 535.3 [M+H]t
1-81 ethyl 4-(3-(4-(((tert-butoxycarbonyl)(2-(1,3,3-trimethy1-2-oxoindolin-5-
y1)
cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzoate
0
N 0
0 1
N Boc N
/
- 187 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using amine B20 and aldehyde A19 following the
procedure for the synthesis of 1-2. LC-MS m/z calcd for C37H51N305, 617.4;
found
618.4 [M+1-1] .
1-82 methyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-l-y1)-3-oxopropyl)benzoate
o
A
401
Boo N 0
o
The compound was synthesized using phenylcyclopropylamine and aldehyde A29
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for C311-
140N205,
520.3; found 465.2 [M-55] .
1-83 methyl 4-(3-(4-((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)piperidin-
l-y1)-3-oxopropyl)benzoate
0
A 0
0 N
Bioc 0
The compound was synthesized using phenylcyclopropylamine and ketone A23
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for C301-
138N205,
506.3; found 451.2 [M-55] .
1-84 methyl 4-
(2-(4-(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-l-y1)-2-oxoethyl)benzoate
A
0 l'
Boc N
0 0
o
The compound was synthesized using phenylcyclopropylamine and aldehyde A24
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for C301-
138N205,
506.3; found 451.2 [M-55] .
1-85 methyl 4-
((4-(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-1-y1) sulfonyl)benzoate-
- 188 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
A A o
S _. 40 N o
BO( 3H 0 00
0 0 N-g
X 8
1-85
1-85 4-44-42,2,2-trifluoro-N-(2-phenylcyclopropypacetamido)methyl)piperidin-
1-y1) sulfonyl)benzoic acid
To a stirred solution of tert-butyl (2-phenylcyclopropyl)(piperidin-4-
ylmethyl)carbamate (1 g, 3.03 mmol) in dichloromethane (20 mL) was added
triethylamine (0.63mL, 4.5 mmol) and methyl 4-(chlorosulfonyl)benzoate (0.78
g,
3.33 mmol) atO C and stirred at room temperature for 2 h. The reaction
mixture was
diluted with dichloromethane and washed with 10% aqueous NaHCO3 solution,
water,
brine solution, dried over sodium sulphate and concentrated under reduced
pressure to
afford the titled product as off-white solid (1-85, 1.5 g, 92%). LC-MS m/z
calcd for
C28H36N206S, 528.2; found 429.1 [M-Boc+H]t
1-86 ethyl 4-
4N-(2-(4-42,2,2-trifluoro-N-(2-
phenylcyclopropyl)acetamido)methyl)piperidin-1-
yl)ethyl)sulfamoyl)methyl)benzoate-
0
A
. N- 0 0
C)
F3C0 NN:S. lei
H
The compound was synthesized using amine B-26 and ethyl 4-
((chlorosulfonyl)methyl)benzoate following the procedure for the synthesize of
1-85.
LC-MS m/z calcd for C28H34F3N305S, 595.2; found 596.3 [M+H]t
1-87 methyl 4-(N-(2-(4-
42,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropypacetamido)
methyl)piperidin-l-
yl)ethyl)sulfamoyl)benzoate
N 0õ0
F F3CLO NNS' 110
H 0
0
- 189 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using amine B-25 and methyl 4-
(chlorosulfonyl)benzoate following the procedure for the synthesize of 1-85.
LC-MS
m/z calcd for C27H31F4N305S, 585.2; found 586.2 [M+H]t
1-88 methyl 4-
(24(44(2,2,2-trifluoro-N-(2-
phenylcyclopropypacetamido)methyl)piperidin-1-y1) sulfonyl)ethyl)benzoate
A
0 yN p
F30-0
0'
0 0,
0
The compound was synthesized using 2,2,2-trifluoro-N-(2-phenylcyclopropy1)-N-
(piperidin-4-ylmethyl)acetamide hydrochloride and
methyl 4-(2-
(chlorosulfonyl)ethyl)benzoate following the procedure for the synthesize of 1-
85. LC-
MS m/z calcd for C27H31F3N205S, 552.2; found 553.2 [M+H]t
1-89 methyl 4-
02-(44(2,2,2-trifluoro-N-(2-
phenylcyclopropyl)acetamido)methyl)piperidin-l-yl)ethyl)carbamoyl)benzoate
A
N 0
F3eL0 Nrs1 0
0
0
The compound was synthesized using amine B-26 and methyl 4-
(chlorocarbonyl)benzoate following the procedure for the synthesize of 1-85.
LC-MS
m/z calcd for C28H32F3N304, 531.2; found 532.2 [M+H]t
1-90 methyl
414(2-(44(N-(2-(3,4-difluorophenyl)cyclopropy1)-2,2,2-
trifluoroacetamido) methyl)piperidin-l-yl)ethyl)carbamoyl)benzoate
N7
F F3C'LO
H
F 0
0
The compound was synthesized using amine B-27 and methyl 4-
(chlorocarbonyl)benzoate following the procedure for the synthesize of 1-85.
LC-MS
m/z calcd for C28H30F5N304, 567.2; found 568.2 [M+H]t
1-91 methyl
414(4-(N-(tert-butoxycarbony1)-N-(2-
phenylcyclopropyl)glycyl)piperazin-1-y1) methyl)benzoate
- 190 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
NH Br 0 Step-1 r-N a Step-2 r-N
0,N) A
, ,. +
H-..õõoõN.õ) ilw
a, -I. C I H.HN........) MP 0
11
0 0 0 0
0
LXV LXVI LXVII
LXVIII
A rN
r' [11µ1) - 0
, Step-3 0 0,
Boc 0 0
1-91
Step-1: tert-butyl 4-(4-(methoxycarbonyl)benzyl)piperazine-l-carboxylate-
LXVII
.,0 Nrj 0
0
Y,
0 0
To a solution of tert-butyl piperazine- 1-carboxylate (LXV, 2 g, 10.8 mmol) in
acetonitrile (100 mL) was added potassium carbonate (1.7 g, 12.9 mmol) and
methyl
4-(bromomethyl)benzoate (LXVI, 2.4 g, 10.8 mmol) and stirred for 24 h at room
temperature. After completion of reaction, the reaction was concentrated under
vacuum and then diluted with ethylacetate (50 mL). The organic layer was
washed
with water, brine, dried over sodium sulfate and concentrated under vacuum to
get
crude product which was purified by flash column chromatography using
ethylacetate-
hexane gradient to afford the titled product as colourless liquid (LXVII, 2.7
g, 75 %).
LC-MS m/z calcd for C18H26N204, 334.2; found 335.2 [M+H]t
Step-2: methyl 4-(piperazin-l-ylmethyl)benzoate hydrochloride-LX VIII
rN a
CIH.HN) 0
0
To a
stirred solution of tert-butyl 4-(4-(methoxycarbonyl)benzyl)piperazine- 1-
carboxylate (LXVII, 2.7 g, 8.08 mmol) in 1,4-dioxane (50 mL) was added 20 %
HC1
in 1,4-dioxane (50 mL) and was stirred for 16 h. The reaction mixture was
concentrated under vacuum. The resultant solid was triturated with diethyl
ether. The
solid was filtered out and dried under vacuum to get the titled product as
white solid
(LXVIII, 1.8 g, 75 %). LC-MS m/z calcd for C13H18N202, 234.2; found 235.2
[M+1-1] .
Step-3: methyl 4-((4-((2-phenylcyclopropyl)glycyl)piperazin-l-
yl)methyl)benzoate-I-91
- 191 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
A rN 0
0 N(N)
1
Boc 0 0 C)
1-91
To a stirred solution of N-(tert-butoxycarbony1)-N-(2-
phenylcyclopropyl)glycine
(LXVIII, 0.10 g, 0.34 mmol) in dry dichloromethane (10 mL) was added methyl 4-
(piperazin-1-ylmethyl)benzoate hydrochloride (0.11 g, 0.37 mmol), then
triethylamine
(0.24 mL, 1.71mmol) and cooled to 0 C. T3P (0.6 mL, 0.86 mmol) was added and
stirred at room temperature for 16 h. After completion of the reaction, the
mixture was
quenched with ice-water and extracted with dichloromethane (10 mL x 3). The
organic layer was washed with water, brine, dried over sodium sulphate and
concentrated under reduced pressure to afford the crude product which was
purified
by column chromatography using methanol-dichloromethane to afford the titled
product as gummy solid (1-91, 0.1 g, 57 %). LC-MS m/z calcd for C29H37N305,
507.3;
found 508.3 [M+H]t
1-92 methyl 4-
((4-(2-((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)acetyl)piperazin-l-yl)methyl)benzoate
0
0 0
, r--N
Ho N+No 400 0, Stepl Hri 100 0, Step2 ,.. 010 NõIrNõ,)
400 0,
Elm 0 0
0 LMX 0 1.92
Step 1: Methyl 4-(3-oxo-3-(piperazin-1-yl)propyl)benzoate-LXIX
0
HN 0
N
0
To a stirred solution of 3-(4-(methoxycarbonyl)phenyl)propanoic acid (0.29 g,
1.41
mmol)and piperazine (0.36g, 4.25 mmol), in dichloromethane(15 mL) was added
triethylamine (0.60 g, 4.25 mmol),the reaction was stirred at room temperature
for
10min, then cooled to 0 C and added propylphosphonic anhydride (1.04 mL, 3.54
mmol), and the resulting mixture was stirred at room temperature for 3 h.The
reaction
was monitored by TLC, after completion of reaction,the mixture was quenched
with
ice. The reaction mixture was diluted with water and extracted with
dichloromethane
(3x25 mL). The organic portion was washed with water and brine, dried over
sodium
sulphate and concentrated under reduced pressure to get the crude light pale
yellow oil
(LXIX, 0.37 g, 93 %). LC-MS m/z calcd for C15H20N203, 276.2; found 278.3
[M+H]t
- 192 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Step 2: methyl 4-
(3-(4-(N-(tert-butoxycarbony1)-N-(2-
phenylcyclopropyl)glycyl)piperazin-l-y1)-3-oxopropyl)benzoate-I-92
0
N1) 0
0
0 0 0
To a solution of N-(tert-butoxycarbony1)-N-(2-phenylcyclopropyl)glycine (LXIX,
0.2
g, 0.69 mmol) in dichloromethane (15 mL) was added methyl 4-(3-oxo-3-
(piperazin-
1-yl)propyl)benzoate (0.23 g, 0.82 mmol), triethyl amine (0.29 mL, 2.05 mmol)
to 0
C and then T3P was added (0.50 mL, 1.72 mmol). The resulting mixture was
stirred at
room temperature for 3h. After completion of reaction, the mixture was diluted
with
dichloromethane (20 mL). The combined organic layer was washed with water,
brine
solution, dried over sodium sulphate and concentrated under reduced pressure
to
afford the title product as stick oil (0.37 g, quantitative yield). LC-MS m/z
calcd for
C311-139N306, 549.3; found 550.3 [M+H]t
1-93 methyl 4-
(3-(1-(2-((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)acetyl)piperidin-4-yl)propyl)benzoate
0
OH Step 1 21¨ND _________ /¨ E1 Step 2 _1=0 Step 3
--)¨( _________________________
0
0
0
0 0
Step 4 >cd=LN e Step 5
0
DOW
LX111
0
Step 6
HCIHN 0
0
00
LXXV /-\ 1-93
Step 1:tert-butyl 4-(2-hydroxyethyl)piperidine-1-carboxylate (LXXI)
0 OH
To a stirred solution of 2-(piperidin-4-yl)ethanol (LXX, lg, 7.72 mmol) in
tetrahydrofuran and water mixture (40 mL, 1:1) was added sodium bicarbonate
(1.62
g, 19.32 mmol) and Boc anhydride (2.6 mL, 11.6 mmol) at room temperature and
stirred for 3 h. The reaction mixture was diluted with ethyl acetate and the
organic
- 193 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
portion was washed with water and brine, dried over sodium sulphate and
concentrated under reduced pressure to get the crude product which was
purified by
flash column chromatography using ethylacetate-hexane gradient to afford the
titled
product as gummy solid (LXXI, 1.6 g, 88 %). LC-MS m/z calcd for C12H23NO3,
229.2; found 130.2 [M-Boc]t
Step 2: tert-butyl 4-(2-oxoethyl)piperidine-1-carboxylate (LXXII)
0 ¨0
----)-0)¨Nai¨
To a stirred solution of tert-butyl 4-(2-hydroxyethyl)piperidine-1-carboxylate
(LXXI,
1.5 g, 6.55 mmol) in dry dichloromethane (40 mL) was added Dess-Martin
periodinane (3.3 g, 7.86 mmol) at 0 Cand the resulting mixture was stirred at
room
temperature for 5 h. The reaction mixture was quenched with 10 % sodium
thiosulphate solution (20 mL) and saturated sodium bicarbonate solution (20
mL) and
then extracted with dichloromethane (2 x 50 mL).The organic portion was washed
with saturated sodium bicarbonate solution, water, brine, dried over sodium
sulphate
and concentrated under reduced pressure to afford the product as yellow semi-
solid
(LXXII, 0.9 g, 60 %).
Step 3: (E)-tert-butyl 4-(3-(4-(methoxycarbonyl)phenyl)allyl)piperidine-1-
carboxylate (LXXIII)
0
0
\
0 ¨
----),--N
¨0
To a stirred solution of methyl 4-((diethoxyphosphoryl)methyl)benzoate (0.9 g,
3.96
mmol) in dry THF (40 mL) was added 60 % sodium hydride at 0 Cand stirred
forlh.
Solution of tert-butyl 4-(2-oxoethyl)piperidine-1-carboxylate (LXXII, 1.1 g,
3.96
mmol) in dry THF was added and stirred further 2 h at room temperature. The
reaction
mixture was quenched with saturated ammonium chloride and then extracted with
ethyl acetate (100 mL). The organic portion was washed with water, brine,
dried over
sodium sulphate and concentrated under reduced pressure to get the crude
product
which was purified by column chromatographyu sing ethylacetate-hexane gradient
to
- 194 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
afford the titled product as colourless liquid (LXXIII, 0.7 g, 50 %). LC-MS
m/z calcd
for C21-129N04, 359.2; found 260.2 [M-Boc +H]t
Step 4: Tert-butyl 4-(3-(4-(methoxycarbonyl)phenyl)propyl)piperidine-1-
carboxylate (LXXIV)
0 0
>0A N ).L0
To a stirred solution of (E)-tert-butyl 4-(3-(4-
(methoxycarbonyl)phenyl)allyl)piperi-
dine- 1-carboxylate (LXXIII, 0.71 g, 1.97 mmol) in methanol (20 mL) was added
10
% Pd-Cand stirred for 0.5h in hydrogen balloon at room temperature. The
reaction
mixture was filtered out through celite and washed with methanol. The filtrate
was
concentrated under vacuum to afford the title product as colourless sticky
solid
(LXXIV, 0.71 g, 99%). LC-MS m/z calcd for C21t131N04, 361.2; found 262.2 [M-
Boc
Step 5: Methyl 4-(3-(piperidin-4-yl)propyl)benzoate hydrochloride-Intermediate
LXXV
o
HCI HN )L0
To a stirred solution of tert-butyl 4-(3-(4-
(methoxycarbonyl)phenyl)propyl)piperidine-
1-carboxylate (LXXIV, 0.7 g, 1.9 mmol) in dioxane (15 mL) was added 20 % HC1
in
dioxane at 0 C and stirred for 16h at room temperature. The reaction mixture
was
concentrated under vacuum to afford the title product as off-white solid
(LXXV, 0.41
g, 72%). LC-MS m/z calcd for C16H23NO2, 261.1; found 262.2 [M+H]t
Step 6: Methyl 4-
(3-(1-(N-(tert-butoxycarbony1)-N-(2-
phenylcyclopropyl)glycyl)piperidin-4-yl)propyl)benzoate-I-93
A I
o
0 ,,
y c N,
0
00
X
To a stirred solution of N-(tert-butoxycarbony1)-N-(2-
phenylcyclopropyl)glycine (0.1
g, 0.34 mmol) and methyl 4-(3-(piperidin-4-yl)propyl)benzoate hydrochloride
(LXXV, 0.11 g, 0.37 mmol) in DMF (2 mL) was added EDC.HC1 (0.058 g, 0.37
mmol), HOBt (0.05 g, 0.37 mmol) and DIPEA (0.13 mL, 1.03 mmol) at0 C and the
resulting mixture was stirred at room temperature for 16 h. The reaction
mixture was
diluted with water and extracted with ethyl acetate. Organic portion was
washed with
- 195 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
water and brine, dried over sodium sulphate and concentrated under reduced
pressure
to get the crude product which was purified by column chromatography using
ethylacetate-hexane gradient to afford the titled product as an sticky oil (1-
93, 0.12 g,
68 %). LC-MS m/z calcd for C32H42N205, 534; found 535 [M+H]t
1-94 ethyl 4-(3-(4-(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)-2-
oxopiperidin-1-yl)propyl)benzoate
0
A
S N,--.......õ..........f0
1
Boc N 0
The compound was synthesized using phenylcyclopropylamine and aldehyde A17
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C32H42N205,
534.3; found 535.2 [M+H]t
1-95 methyl 4-
(2-((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)ethoxy)benzoate
A NH2 +
1 Br 10/ Step 1 0 A
N() lei 0 101
IJOCV1
0 H
IJOCV11 0
A
IO
Step 2 N
SI 1
Boc 0 0
1-95 0
Step 1: Methyl 4-(2-((2-phenylcyclopropyl)amino)ethoxy)benzoate (LXXVII)
A
lel () N 0
H
0
0
To a stirred solution of methyl 4-(2-bromoethoxy)benzoate (LXXVI, 0.45g, 1.77
mmol) in dimethylformamide (15 mL) was added 2-phenylcyclopropanamine (0.5g,
2.95mmo1) and potassium carbonate (1.22 g, 8.84 mmol) and the resulting
mixture
was stirred at 60 C temperature for 12 h.Reaction was monitored by TLC, after
completion of reaction, reaction was quenched with ice and the solvent was
completely removed to get the residue. Water was added and the residue was
extracted
with dichloromethane (3x25 mL). The organic portion was washed with water and
brine, dried over sodium sulphate and concentrated under reduced pressure to
get the
crude product which was purified by combi-flash chromatography using
ethylacetate-
- 196 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
hexane gradient to afford the required product as white solid (LXXVII, 0.32 g,
35 %),
LC-MS m/z calcd for C19H21NO3, 311.1; found 312.2 [M+H]t
Step 2: Methyl 4-
(2-((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)ethoxy)benzoate (1-95)
A
101 N(3 0
0 0 0
.....--...... 0
To a stirred solution of methyl 4-
(2-((2-
phenylcyclopropyl)amino)ethoxy)benzoate(LXXVII, 0.2g, 0.64 mmol) in
tetrahydrofuran and water mixture (14mL, 1:1) was added sodium bicarbonate
(0.16 g,
1.92 mmol) and Boc anhydride (0.16 mL, 0.77 mmol) at room temperature and the
resulting mixture was stirred at that temperature for 1 h. The progress of the
reaction
was monitored by TLC. The reaction mixture was diluted with ethylacetate and
the
organic portion was washed with water and brine, dried over sodium sulphate
and
concentrated under reduced pressure to get the crude product which was
purified by
column chromatography using ethylacetate-hexane gradient to afford the titled
product
as pale-yellow oil (1-95, 0.16 g, 61 %). LC-MS m/z calcd for C24H29N05, 411.2;
found 312.1 [M-Boc +H]t
1-96 methyl 6-
(2-(44(2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropyl)acetamido)methyl) piperidin-l-yl)ethoxy)nicotinate
0
0 o A
r'j XIA nAci Step
1
BrO ,r)AV Step _________________________ 2 F '
0 N N
H 1-96
DOCV111
Step 1: methyl 6-(2-bromoethoxy)nicotinate
0
-)Li CY
I
BroN
To a solution of methyl 6-hydroxynicotinate (1.2 g, 7.84 mmol) in DMF (10 mL)
was
added 60 % of sodium hydride (0.75 g, 17.25 mmol) at 0 C. Then 1,2-
dibromoethane
(6.57 mL, 7.84 mmol) was added and then stirred for 16 h at room temperature.
After
completion of reaction, the reaction was quenched with ice and extracted with
ethylacetate (2 x 50 mL). The combined organic layer was washed with water,
brine
solution, dried over sodium sulfate and concentrated under vacuum to get crude
product which was purified by column chromatography using ethylacetate-hexane
- 197 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
gradient to afford the titled product as a white solid (LXXVIII, 0.73 g, 35%).
LC-MS
m/z calcd for C9H10BrNO3, 259.0; found 261.0 [M+H]t
Step 2: methyl 6-
(2-(4-42,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropypacetamido)methyl)piperidin-1-ypethoxy)nicotinate-I-
96
0
I
F3C0
To a solution of methyl 6-(2-bromoethoxy)nicotinate (LXXVIII, 0.54 g, 2 mmol)
in
acetonitrile (5 mL) was added 2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropy1)-N-
(piperidin-4-ylmethyl)acetamide hydrochloride (B-3, 0.80 g, 2 mmol) and N,N-
diisopropylethylamine (1.07 mL, 6 mmol). Then the reaction mixture was heated
at 40
C for 16 h. After completion of reaction, the reaction was diluted with ethyl
acetate
(50 mL), washed with water, brine, dried over sodium sulfate and concentrated
under
vacuum to get crude product which was purified by column chromatography
usingmethanol-dichloromethane gradient to afford the titled product as a brown
colour
liquid (1-96, 0.8 g, 74 %). LC-MS m/z calcd for C26H29F4N304, 523; found 524
[M+1-1] .
1-97 methyl 6-
(2-(4-((2,2,2-trifluoro-N-(2-
phenylcyclopropypacetamido)methyl)piperidin-1-y1) ethoxy)nicotinate
A
I
F3C0
The compound was synthesized using phenylcyclopropylamine following the
procedure for the synthesis of 1-96. LC-MS m/z calcd for C26H30F3N304, 505.2;
found
506.2 [M+1-1] .
1-98 methyl 6-
(2-(44(2,2,2-trifluoro-N-(2-(4'-fluoro-[1,1'-bipheny1]-4-
yl)cyclopropyl)acetamido)methyl)piperidin-1-ypethoxy)nicotinate
0
0
OCF3NJON
- 198 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using amine B-12 following the procedure for the
synthesis of 1-96. LC-MS m/z calcd for C32H33F4N304, 599.2; found 600.2 [M+H]t
1-99 methyl 4-
(2-(44(2,2,2-trifluoro-N-(2-
phenylcyclopropyl)acetamido)methyl)piperidin-l-yl)ethoxy)benzoate
o
A
N e
.I F3C0 N
.
The compound was synthesized using phenylcyclopropylamine following the
procedure for the synthesis of 1-95. LC-MS m/z calcd for C27H31F3N204, 504.2;
found
505.2 [M+1-1] .
1-100 methyl 4-
(3-(4-42,2,2-trifluoro-N-(2-
phenylcyclopropyl)acetamido)methyl)piperidin-l-yl)propoxy)benzoate
A
N
N 0
. F3C0
0
0
The compound was synthesized using phenylcyclopropylamine following the
procedure for the synthesis of 1-95. LC-MS m/z calcd for C28H33F3N204, 518.2;
found
519.2 [M+H]t
1-101 methyl 4-(3-
((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)propoxy)benzoate
o
0 N 0
Boc
The compound was synthesized using phenylcyclopropylamine following the
procedure for the synthesis of 1-95. LC-MS m/z calcd for C25H31N05, 425.2;
found
426.1 [M+H]t
1-102 methyl 2-
((2-(4-((2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropypacetamido)
methyl)piperidin-1-
yl)ethyl)amino)pyrimidine-5-carboxylate
- 199 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
A
,NL1' A
NJO
1 I
F 4111111)7. F3C 0 N NH2 HCI
F 4111111".P 0
CFNNN
1-102
To a solution of N-((1-(2-aminoethyl)piperidin-4-yl)methyl)-2,2,2-trifluoro-N-
(2-(4-
fluorophenyl)cyclopropyl)acetamide hydrochloride. (B-25, 0.48 g, 1.60 mmol) in
acetonitrile (5 mL) was added methyl 2-(ethylsulfonyl)pyrimidine-5-carboxylate
(0.4
g, 1.7 mmol) and N,N-diisopropylethylamine (0.86 mL, 4.8 mmol). Then the
reaction
mixture was heated at 50 C for 16 h. After completion of reaction, the
reaction was
diluted with ethylacetate (50 mL ), washed with water, brine solution, dried
over
sodium sulfate and concentrated under vacuum to get crude product which was
purified by column chromatography usingmethanol-dichloromethane gradient to
afford the titled product as brown colour sticky oil (1-102, 0.250 g, 41 %).
LC-MS m/z
calcd for C26H31F4N503, 537.2; found 538.2 [M+H]t
1-103 ethyl 5-
(2-((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)acety1)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine-
2-carboxylate
alaj
F H
Cr>
Boo, 2 s Boc,N FINa$4 ________________ 3-
S Step 2
Step 1 S 0
Step 3
IJOUX uooc
A
N N
OCS)4
10 6. 0 0_\
1-103
Stepl: 5-
(tert-butyl)-2-ethyl-6,7-dihydrothieno[3,2-c]pyridine-2,5(4H)-
dicarboxylate LXXIX
)
S 0
To a stirred solution of tert-butyl 6,7-dihydrothieno[3,2-c]pyridine-5(4H)-
carboxylate
(11.5 g, 48.09 mmol) in tetrahydrofuran (75 mL) was added 1.6M solution of n-
butyl
lithium in n-hexane(36 mL, 57.71 mmol) at -78 C and stirred for same
temperature
for 3 h, ethyl chloroformate (52.19 g, 480.9 mmol) was added drop wise at -78
C and
allowed to stirred for 12 h at room temperature. Progress of reaction followed
by TLC.
After completion of reaction,the mixture was quenched with ammonium chloride
(100
- 200 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
mL) and extracted with ethylacetate. The organic portion was washed with water
and
brine dried over sodium sulphate and concentrated under reduced pressure to
afford
the crude product which was purified by column chromatography using
ethylacetate-
hexane gradient to afford LXXIX as yellowish liquid (3.75 g, 23 %). LC-MS m/z
calcd for C15H21N04S, 311.1, found 212.1 [M-Boc+1-1] .
Step2: ethyl 4,5,6,7-tetrahydrothieno[3,2-c]pyridine-2-carboxylate TFA salt
LXXX
HN
L ,..._Th0
S 0
To a stirred solution of 5-(tert-buty1)-2-ethy1-6,7-dihydrothieno[3,2-
c[pyridine-
2,5(4H)-dicarboxylate (LXXIX, 0.5 g, 1.68 mmol) in dichloromethane (15 mL) was
added trifluoroacetic acid (1.5 g, 13.47 mmol) at 0 C and allowed to stirred
for 4 h at
room temperature. Progress of reaction was followed by TLC. After completion
of
reaction, the mixture was concentrated completely and washed with diethyl
ether to
afford product LXXX as a brown colour liquid (0.50 g, 91 %). LC-MS m/z calcd
for
C10H13NO2S, 211.0, found 212.1 [M+H]t
Step3: ethyl 5-
(N-(tert-butoxycarbony1)-N-(2-(4-
fluorophenyl)cyclopropyl)glycy1)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine-2-
carboxylate 1-103
s o
A I /
rryN
o¨\
10 F Boo 0
To a stirred solution of ethyl 4,5,6,7-tetrahydrothieno[3, 2-c[pyridine-2-
carboxylate
TFA salt 4 (0.2 g, 0.65 mmol), N-(tert-butoxycarbony1)-N-(2-(4-
fluorophenyl)cyclopropyl)glycine 5 (0.16 g, 0.78 mmol), triethylamine (0.261
g, 2.59
mmol) in dichloromethane (10 mL) was added propylphosphonic anhydride (T3P,
0.514 g, 1.62 mmol) and stirred for 12 h at room temperature. Progress of
reaction
followed by TLC. After completion, the reaction was quenched with water (20
mL)
and extracted with dichloromethane (2 x 30 mL). The organic portion was washed
with water,brine, dried over sodium sulphate and concentrated under reduced
pressure
to afford the crude product which was purified by column chromatography
chromatographyusing ethylacetate-hexane gradient to afford the titled product
1-103
- 201 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
as colourless liquid (0.15 g, 57%). LC-MS m/z calcd forC26H31FN205S, 502.2,
found
503.2 [M+H]t
1-104 methyl 2-
(2-((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)acety1)-1,2,3,4-tetrahydroisoquinoline-7-
carboxylate
A N -.rN 0
,.L 0 0
F 0 0
X
To a stirred solution of methyl 1,2,3,4-tetrahydroisoquinoline-7-carboxylate
hydrochloride (0.25 g, 1.1 mmol) and 2-((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)acetic acid (0.34 g, 1.1 mmol) in N,N-
dimethylformamide (5 mL), was added EDC.HC1 (0.42 g, 2.2 mmol), HOB t (0.18 g,
1.32mmo1) and triethylamine (0.61 mL, 4.4 mmol) at room temperature. The
resulting
mixture was stirred at that temperature for 16 h. The progress of the reaction
was
monitored by TLC. After completion, the reaction mixture was diluted with
water and
extracted with ethylacetate. Organic portion was washed with water and brine,
dried
over sodium sulphate and concentrated under reduced pressure to get the crude
product which was purified by column chromatography using ethylacetate-hexane
gradient to afford the titled product as an off-white solid (1-104, 0.25 g,
47%). LC-MS
m/z calcd for C27H31FN205, 482.2; found 483.1 [M+H]t
1-105 ethyl 5-
(4-((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)butanoy1)-4,5,6,7-tetrahydrothieno[3,2-
c]pyridine-2-carboxylate
S 0¨\
NO E) µ \
N . = ' 0
0
F 0 0
X
The compound was synthesized using 2-(4-fluorophenyl)cyclopropanamine and A-25
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C28H35FN205S,
530.2; found 531.2 [M+H]t
1-106 ethyl 5-
(4-(44(2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropyl)acetamido)methyl)
piperidin-1-yl)butanoy1)-4,5,6,7-
tetrahydrothieno[3,2-c]pyridine-2-carboxylate
- 202 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
N 0
F F3C0 =''''N-----\
µ
S 0
The compound was synthesized using amine B-3 and A25 following the procedure
for
the synthesis of 1-2. LC-MS m/z calcd for C311-137F4N304S, 623.2; found 624.3
[M+1-1] .
1-107 ethyl 2-(4-((tert-
butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)butanoy1)-1,2,3,4-tetrahydroisoquinoline-7-
carboxylate
O¨
N N
F Bioc 0 0
The compound was synthesized using 2-(4-fluorophenyl)cyclopropanamine and A-26
following the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C29H35FN205,
510.2; found 511.3 [M+1-1] .
1-108 methyl 2-
(4-((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)butanoyl) isoindoline-5-carboxylate
O¨
N 0
Bioc 0
F 1 A Nr
The compound was synthesized using 2-(4-fluorophenyl)cyclopropanamine and A-
27fo11owing the procedure for the synthesis of 1-2. LC-MS m/z calcd for
C28H33FN205, 496.2; found 497.2 [M+H]t
1-109 methyl 2-
(4-(4-42,2,2-trifluoro-N-(2-
phenylcyclopropyl)acetamido)methyl)piperidin-l-yl)butanoyl)isoindoline-5-
carboxylate
N 0-
N
410. F3C 0
The compound was synthesized using 2,2,2-trifluoro-N-(2-phenylcyclopropy1)-N-
(piperidin-4-ylmethyl)acetamide and ketone A27 following the procedure for the
synthesis of 1-2. LC-MS m/z calcd for C31H36F3N304, 571.2; found 572.3 [M+H]t
- 203 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
I-110 methyl 2-
(3-(4-02,2,2-trifluoro-N-(2-
phenylcyclopropyl)acetamido)methyl)piperidin-l-yl)propyl)thiazole-4-
carboxylate
0)\¨o
\
N
)t1
F3CLO N S
The compound was synthesized using 2,2,2-trifluoro-N-(2-phenylcyclopropy1)-N-
(piperidin-4-ylmethyl)acetamide and aldehyde A30 following the procedure for
the
synthesis of 1-2. LC-MS m/z calcd for C25H30F3N303S, 509.2; found 510.2 [M+H]t
I-111 methyl 2-
(3-(44(2,2,2-trifluoro-N-(2-(4'-fluoro-[1,1'-bipheny1]-4-
yl)cyclopropyl) acetamido)methyl)piperidin-l-yl)propyl)thiazole-4-carboxylate
0
--0
\
N
F3C 0 S
F
The compound was synthesized using 2,2,2-trifluoro-N-(2-phenylcyclopropy1)-N-
(piperidin-4-ylmethyl)acetamide and aldehyde A30 following the procedure for
the
synthesis of 1-2. LC-MS m/z calcd for C311-133F4N303S, 603.2;found 604.2
[M+H]t
1-112 ethyl 2-
(3-(4-((2,2,2-trifluoro-N-(2-
phenylcyclopropyl)acetamido)methyl)piperidin-l-yl)propyl)thiazole-5-
carboxylate
A
N N I e \ - _/
SI F3L0 N 11-1-S n
0
The compound was synthesized using amine 2,2,2-trifluoro-N-(2-
phenylcyclopropy1)-
N-(piperidin-4-ylmethyl)acetamide and aldehyde A31 following the procedure for
the
synthesis of 1-2. LC-MS m/z calcd for C26H32F3N303S, 523.2; found 524.2 [M+H]t
1-113 methyl 2-
(3-(4-02,2,2-trifluoro-N-(2-
phenylcyclopropypacetamido)methyl)piperidin-1-y1)
propyl)oxazole-4-
carboxylate
o
A o
\
N
1.1 F3eL0 N rtO\
- 204 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using amine 2,2,2-trifluoro-N-(2-
phenylcyclopropy1)-
N-(piperidin-4-ylmethyl)acetamide and aldehyde A32 following the procedure for
the
synthesis of 1-2. LC-MS m/z calcd for C25H30F3N304, 493.2; found 494.2 [M+H]t
1-114 (E)-methyl 4-
(3-(4-(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-1-y1)-3-oxoprop-1-en-1-yl)benzoate
o
A
0 N e
N
0 0 \
X 0
1-114
The compound was synthesized using 2-phenylcyclopropanamine hydrochloride and
aldehyde A33 following the procedure for the synthesis of 1-2. LC-MS m/z calcd
for
C31H38N205, 518.2; found 519.3 [M+H]t
1-115 Methyl 4-((E)-3-(4-
(((tert-butoxycarbonyl)((18,2R)-2-(4-
fluorophenyl)cyclopropyl)
amino)methyl)piperidin-1-y1)-3-oxoprop-1-en-1-
yl)benzoate
0
A .
10 9"ril 0
F Boc N \
0
The compound was synthesized using B1 and aldehyde A33 following the procedure
for the synthesis of 1-2. LC-MS m/z calcd for C31H37FN205, 536.2; found 537.2
[M+1-1] .
1-116 (E)-
methy14-(3-(4-(((tert-butoxycarbonyl)(2-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)
cyclopropyl)amino)methyl)piperidin-1-y1)-3-oxoprop-1-en-1-
yl)benzoate
0
r( 0
Boc N
R
N¨ 0
The compound was synthesized using B8 and aldehyde A33 following the procedure
for the synthesis of 1-2. LC-MS m/z calcd for C36H43N306, 613.3; found 614.2
[M+H] .
- 205 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1-117 (E)-
methy14-(3-(4-(((tert-butoxycarbonyl)(2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)
amino)methyl)piperidin-1-y1)-3-oxoprop-1-en-1-
yl)benzoate
0
Boc
0
N
I
The compound was synthesized using B11 and aldehyde A33 following the
procedure
for the synthesis of 1-2. LC-MS m/z calcd for C35H40N405, 596.3; found 597.3
[M+1-1] .
1-118 (E)-methy14-(3-oxo-3-(34(2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropyl)
acetamido)methyl)azetidin-1-yl)prop-1-en-1-yl)benzoate
NH.TFA
HO \ F 0
A
A y
F3C0
F3C 0 0
B-4 B-118
To a stirred solution of methyl (E)-4-(3-oxo-3-(3-((2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cycloprop yl)acetamido)methyl)azetidin- 1-yl)prop- 1-en-1-
yl)benzo ate
TFA salt (B-4, 0.50 g, 1.51 mmol) and(E)-3-(4-(methoxycarbonyl)phenyl)acrylic
acid
(0.40 g, 1.97 mmol) in dichloromethane (20 mL), was added HOBt (0.05 g, 0.30
mmol) and triethylamine (0.46 mL, 4.55 mmol) at room temperature and cooled to
0
C. Then, EDC.HC1 (0.43 g, 2.27 mmol) was added and stirred for 16 h at room
temperature. The reaction mixture was diluted with water and extracted with
dichloromethane. The combined organic portion was washed with water, brine,
dried
over sodium sulphate and concentrated under reduced pressure to get the crude
product which was purified by column chromatography using methanol-
dichloromethane gradient to afford the titled product as a off-white solid (B-
118, 0.49
g, 65 %). LC-MS m/z calcd for C26H24F4N204, 504.2; found 505.2 [M+H] .
1-119 (E)-methy14-(3-oxo-3-(34(2,2,2-trifluoro-N-(2-(4-(1-methyl-1H-pyrazol-4-
yl)phenyl) cyclopropyl)acetamido)methyl)azetidin-1-yl)prop-1-en-1-yl)benzoate
- 206 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
o o
A A
o'
I o'
0 niic-NN .... 0 nil '-c-AN
step-1 1 o'o
F3co
o X o
1J00(11
LJOOC1
0
e
_,....
Step-2 N / i 00
iq X 0
/
1-119
Step-1: methyl (E)-
4-(3-(3-(((tert-butoxycarbonyl)(2-(4-
iodophenyl)cyclopropyl)amino)methypazetidin-l-y1)-3-oxoprop-1-en-1-
y1)benzoate-LXXXII
0
0
y C\N
I 00
X 0
To a stirred solution of methyl (E)-4-(3-oxo-3-(3-((2,2,2-trifluoro-N-(2-(4-
iodophenyl)cycloprop yl)acetamido)methyl)azetidin-1- yl)prop-1 -en- 1-
yl)benzoate(LXXXI, 0.2 g, 0.33 mmol) in methanol (5 mL) was added potassium
carbonate (0.1 g, 2.40 mmol) at room temperature and the resulting mixture was
stirred at that temperature for 3 h. After completion of the reaction, solvent
was
evaporated under vacuum.The residue was mixed with tetrahydrofuran-water
mixture
(6 mL, 1:1). This was followed by addition of Boc anhydride (0.08 mL, 0.39
mmol)
and sodium bicarbonate (0.08 g, 0.98 mmol). The reaction mixture was stirred
for 2 h
at room temperature. The solvent was evaporated and then diluted with
dichloromethane. The combined portion was washed with water and brine
solution,
dried over sodium sulphate and concentrated under reduced pressure to get the
crude
to afford the titled product as stick oil (LXXXII, 0.25 g, 63 %). LC-MS m/z
calcd for
C29H33IN205, 616.1; found 617.1 [M+1-1] .
Step-2: Methyl (E)-4-(3-(3-(((tert-butoxycarbonyl)(2-(4-(1-methyl-1H-pyrazol-4-
yl)phenyl)cyclopropyl)amino)methypazetidin-l-y1)-3-oxoprop-1-en-l-
y1)benzoate-I-119
- 207 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
N
N/ I 0 0
IV X 0
/
1-119
To a stirred solution of methyl (E)-4-(3-(3-(((tert-butoxycarbonyl)(2-(4-
iodophenyl)cyclopropyl)amino)methyl)azetidin-l-y1)-3-oxoprop-1-en-1-
y1)benzoate
(LXXXII, 0.25 g, 0.41 mmol) in DMF (3 mL) was added (1-methy1-1H-pyrazol-4-
yl)boronic acid (0.06 g, 0.49 mmol) andpotassium carbonate (0.11 g, 0.82 mmol)
and
then degassed for 5 min.
1,1'-Bis(diphenylphosphino)ferrocene-
palladium(II)dichloride dichloromethane complex (0.016 g, 0.02 mmol) was added
and heated at 120 Cin microwave for 2 h. Water was added andthe residue was
extracted with ethylacetate (2x100 mL). The organic portion was washed with
water,
brine, dried over sodium sulphate and concentrated under reduced pressure to
afford
the crude product which was purified by column chromatography using methanol-
dichloromethane gradient to afford the titled product as sticky oil (1-119,
0.2 g, 86 %).
LC-MS m/z calcd for C33H38N405, 570.3; found 571.2 [M+H]t
Synthesis of Acid intermediates
1-120 (E)-
3-(4-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)phenyl)acrylic
acid
A
N
/ OMe Step 1 N
F $1 0 0 / F OH
- 0 0
/\ 0
1-2 /\ 1-120
To a stirred solution of (E)-methyl 3-(4-(((tetra-butoxycarbonyl)(2-(4-
florophenyl)cyclopropl)amino)methyl)phenyl)acrylate(I-2, 0.38 g, 0.89 mmol) in
methanol and water mixture (20 mL, 4:1) was added sodium hydroxide (0.11 g,
2.68
mmol) at room temperature and the resulting mixture was stirred at that
temperature
for 1 h. The progress of the reaction was monitored by TLC. After completion
of
reaction, solvent was evaporated and washed with ethylacetate. The reaction
mixture
was acidified to pH 5 with 2N HC1 and extracted with dichloromethaneand the
organic
portion was washed withwater,brine solution, dried over sodium sulphate and
- 208 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
concentrated under reduced pressure to afford the product as off-white solid
(1-120,
0.31 g, 86 %). LC-MS m/z calcd for C24H26FN04, 411.2; found 312.2 [M-Boc +H]t
1-121 4-
(3-(4-(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzoic acid
0
0
A
101 ,:=I =OH
X
The compound was synthesized using 1-43 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C301-140N204, 492.3; found 493.3 [M+H]t
1-122 4-
(3-(4-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzoic acid
0
r( OH
N
F Boc
The compound was synthesized using 1-47 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C301439FN204, 510.3; found 511.3 [M+H]t
1-123 4-
(3-(4-(((tert-butoxycarbonyl)(2-(4-
methoxyphenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzoic acid
0
ril OH
Boc N
0
The compound was synthesized using 1-51 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C311442N205, 522.3; found 523.3 [M+H]t
1-124 4-(3-(4-
(((tert-butoxycarbonyl)(2-(3,4-
difluorophenyl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzoic acid
0
r' OH
F Boc N
F
The compound was synthesized using 1-50 following the procedure for the
synthesis
of intermediate 1-120. LC-MS m/z calcd for C30H38F2N204, 528.3; found 529.3
[M+1-1] .
- 209 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1-125 4-
(3-(4-(((tert-butoxycarbonyl)(2-(4-(piperidine-1-
carbonyl)phenyl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzoic acid
0
........--,.., N OH
1
Boc N
0
The compound was synthesized using 1-54 following the procedure for the
synthesis
of intermediate 1-120. LC-MS m/z calcd for C36H49N305, 603.4; found 604.4
[M+H]t
1-127 4-
(3-(6-((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)-2-
azaspiro[3.3]heptan-2-yl)propyl)benzoic acid
j:FIN
AO =
NI:FIN
OH
is A 0 A
gtoc 0
F30-0
I-46 1-127
Step: 4-(3-(6-
((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)-2-
azaspiro[3.3]heptan-2-yl)propyl)benzoic acid
To a stirred solution of
ethyl 4-(3-(6-(2,2,2-trifluoro-N-(2-
phenylcyclopropyl)acetamido)-2-azaspiro[3.3]heptan-2-yl)propyl)benzoate (0.4
g,
0.77 mmol) in tetrahydrofuran and water mixture (10 mL, 1:1) was added lithium
hydroxide (0.097 g, 2.30 mmol) at room temperature and the resulting mixture
was
stirred at that temperature for 3 h. After disappearance of starting material
1-46, Boc
anhydride (0.33 mL, 1.50 mmol) was added and stirred for 2 h at room
temperature.
The reaction solvent was evaporated and then acidified with 2N HClsolution.
The
aqueous layer was extracted with dichloromethane (50 mL x 2). The combined
organic layer was washed with water, brine solution, dried over sodium
sulphate and
concentrated under reduced pressure to afford the titled product as brown
solid (0.31
g, 81 %). LC-MS m/z calcd for C301438N204, 490.3; found 489.3 [M-H]t
1-128 4-
(3-(4-(((tert-butoxycarbonyl)(2-(1-isopropy1-1H-pyrazol-4-
yl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzoic acid
0
)--
OH 111
N
Boc
N
The compound was synthesized using 1-67 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C301-144N404, 524.3; found 525.4 [M+H]t
- 210 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1-129 JBI-
XXX-4-(3-(4-(((tert-butoxycarbonyl)(2-(1-phenyl-1H-pyrazol-4-
yl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzoic acid
0
SN1-Ay OH
Boc N
N
The compound was synthesized using 1-68 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C33H42N404, 558.3; found 559.3 [M+H]t
1-130 4-
(3-(4-(((tert-butoxycarbonyl)(2-(2-methylthiazol-5-
yl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzoic acid
0
Boc N
N
The compound was synthesized using 1-69 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C28H39N304S, 513.3; found 514.3 [M+H]t
1-131 4-
(3-(4-(((tert-butoxycarbonyl)(2-(pyridin-3-
yl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzoic acid
0
C)/A OH
1 , Boc N
N
The compound was synthesized using 1-70 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C29H39N304, 493.3; found 494.3 [M+H]t
1-133 4-(3-(4-((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)piperidin-l-y1)-
3-oxopropyl)benzoic acid
o
N
A OH
0 N
o
...õ---...,
The compound was synthesized using 1-83 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C29H36N205, 492.2; found 491.2 [M-H]t
1-134 4-
(3-(4-(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-l-y1)-3-oxopropyl)benzoic acid
-211 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
A
0 niinN
(:)o OH
/\ 0
The compound was synthesized using 1-82 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C301438N205, 506.2; found 506.3 [M]t
1-135 4-
(3-(4-(((tert-butoxycarbonyl)(2-(3,4-
difluorophenyl)cyclopropyl)amino)methyl)-1H-imidazol-1-yl)propyl)benzoic acid
/-----rN
N
F 13oc --
OH
F 0
The compound was synthesized using 1-73 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C28H31F2N304, 511.2; found 512.2 [M+H]t
1-136 4-
(3-(4-(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)-1H-
imidazol-1-yl)propyl)benzoic acid
N1/--ell
10 Boc N¨
OH
0
The compound was synthesized using 1-75 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C28H33N304, 475.2; found 476.2 [M+H]t
1-137 4-
(3-(4-(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)-1H-
1,2,3-triazol-1-yl)propyl)benzoic acid
N-----e-N
* i3oc N=N OH
0
The compound was synthesized using 1-77 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C27H32N404, 476.2; found 477.2 [M+H]t
1-138 4-
(3-(4-(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)-1H-
pyrazol-1-yl)propyl)benzoic acid
'116" N/(NNTh
* 130c " OH
0
The compound was synthesized using 1-76 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C28H33N304, 475.2; found 476.3 [M+H]t
- 212 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1-139 4-
(2-(4-(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-1-yl)ethyl)benzoic acid
la A N.
IW 13oc N
0 OH
0
The compound was synthesized using 1-42 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C29H38N204, 478.2; found 479.3 [M+H]t
1-140
44(4- (((tert-butoxycarb onyl)((lR,2S)-2-
phenylcyclopropyl)amino)methyl)piperidin-1-yl)methyl)benzoic acid
0
10/µ'''y 0 OH
Boc N
The compound was synthesized using 1-36 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C28H36N204, 464.3; found 465.3 [M+H]t
1-141 4-
((4-(((tert-butoxycarbonyl)(2-(4-(1-methy1-6-oxo-1,6-dihydropyridin-3-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)methyl)benzoic acid
0
, N 0 OH
0 0 N
I X
0 N
I
The compound was synthesized using 1-35 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C34H41N305, 571.3; found 572.3 [M+H]t
1-142 4-
44-(((tert-butoxycarbonyl)(2-(4-(1-methyl-1H-pyrazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-y1)methyl)benzoic acid
0
rl 0 OH
Boc N
/
N I
'NI
/
The compound was synthesized following the procedure for the synthesis of
intermediate 1-120 using the corresponding ester (ester was synthesized using
B-7 and
methyl 4-((4-formylpiperidin- 1-yl)methyl)benzoate using the procedure
outlined for
synthesis of 1-2). LC-MS m/z calcd for C32H40N404, 544.3; found 545.3 [M+H]t
- 213 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1-143 4-
44-(((tert-butoxycarbonyl)(2-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-y1)methyl)benzoic acid
0
N ei OH
Boc N
0,
N¨
The compound was synthesized using 1-37 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C33H41N305, 559.3; found 560.3 [M+H]t
1-144 4-
((4-(((tert-butoxycarbonyl)(2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-yl)methyl)benzoic acid
0
N,..--.....s.,..---..õ
OH
1
Boc N
N '
N 1
The compound was synthesized using 1-38 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C32H38N404, 542.2; found 543.3 [M+H]t
1-145 4-
((4-(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)-1H-
pyrazol-1-yl)methyl)benzoic acid
A.{CN
* 130c -NI O OH
0
The compound was synthesized using 1-40 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C26H29N304, 447.2; found 448.2 [M+H]t
1-146 4-44-(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)-1H-1,2,3-
triazol-1-y1)methyl)benzoic acid
itt- mr-----e'N õ..1
* loc "=" IOH
0
The compound was synthesized using 1-41 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C25H28N404, 448.2; found 449.2 [M+H]t
1-147 4-
(2-(4-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-y1)-2-oxoethyl)benzoic acid
F. AO:c N 0
- 214 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized following the procedure for the synthesis of
intermediate 1-120 using the corresponding ester (ester was synthesized using
4-
fluorocyclopropylamine and aldehyde A-24 using the procedure outlined for
synthesis
of 1-2). LC-MS m/z calcd for C29H35FN205, 510.2; found 455.2 [M-55] .
1-148 4-(2-((tert-
butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)ethoxy)benzoic acid
A
N
60c w OH
0
The compound was synthesized following the procedure for the synthesis of
intermediate 1-120 using the corresponding ester (ester was synthesized using
4-
fluorocyclopropylamine and LXXVI, using the procedure outlined for synthesis
of I-
95). LC-MS m/z calcd for C23H26FN05, 415.2; found 416.2 [M+H]t
1-149 6-
(2-(4-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-ypethoxy)nicotinic acid
A
101 111--oN X--)LOH
F 00 Nr
The compound was synthesized using 1-95 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C28H36FN305, 513.2; found 514.3[M+H]t
1-150 2-
((2-(4-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-ypethypamino)pyrimidine-
5-carboxylic acid
A
101 1(
OH
Boc
The compound was synthesized using I-101 following the procedure for the
synthesize of 1-120. LC-MS m/z calcd for C27H36FN504, 513.3; found 512.3 [M-
H]t
1-151 5-
(N-(tert-butoxycarbony1)-N-(2-(4-fluorophenyl)cyclopropyl)glycy1)-
4,5,6,7-tetrahydrothieno[3,2-c[pyridine-2-carboxylic acid
Boo 0
- 215 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using 1-102 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C24H27FN205S, 474.2; found 475.2 [M+H]t
1-152 2-
(N-(tert-butoxycarbony1)-N-(2-(4-fluorophenyl)cyclopropyl)glycy1)-
1,2,3,4-tetrahydroisoquinoline-7-carboxylic acid
OH
NrN
FQBOCOO
The compound was synthesized using 1-103 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C26H29FN205, 468.2; found 469.2 [M+H]t
1-153 2-
(3-(4-(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-1-yl)propyl)oxazole-4-carboxylic acid
A OH
N
The compound was synthesized using 1-112 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C27H37N305, 483.2; found 484.2 [M+H]t
1-154 2-
(3-(4-(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-1-yl)propyl)thiazole-5-carboxylic
acid
A NThNDH
Boc S 0
The compound was synthesized using I-111 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C27H37N304S, 499.2; found 500.3 [M+H]t
1-155 4-
((2-((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)acetamido)methyl)benzoic acid
A H1OH
F Boc 0
This compound 1-155 was synthesized following the procedure for the synthesis
of I-
120 using the corresponding ester (ester was synthesized using 2-((tert-
butoxycarbonyl)(2-(4-fluorophenyl)cyclopropyl)amino)acetic acid and methyl 4-
(aminomethyl)benzoate using the procedure outlined for synthesis of 1-103). LC-
MS
m/z calcd for C24H27FN205, 442.2; found 443.2 [M+H]t
- 216 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1-156 (E)-
4-(3-(4-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-y1)-3-oxoprop-1-en-1-
yl)benzoic acid
o
A
0 Ill'ON OH
F 00
) 0
The compound was synthesized using 1-114 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C301-135FN205, 522.2; found 523.4 [M+H]t
1-157 (E)-4-(3-(3-(((2-(4-fluorophenyl)cyclopropyl)amino)methyl)azetidin-1-y1)-
3-
oxoprop-1-en-1-yl)benzoic acid
0
NH 'C \N OH
F
0
The compound was synthesized using 1-117 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C23H23FN203, 394.1; found 395.2 [M+H]t
1-158 4-
((4-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidine-1-
carboxamido)methyl)benzoic acid
0
0 0-
H2N 0
0
--- Step 2 0-7'Ci 0 e
Step 1 HOCN 40 0 H
NY N
HOONH
0
1JOOCIV
1.300C111
0
0 A
A Step 4 iii, OH
Step 3 io H 140
,L ,N N F CYLO --- NyNEI Si
-'- F 0 0 -.- y
X 0 X o
MOW 1-158
Step 1: methyl 4-
((4-(hydroxymethyl)piperidine-l-
carboxamido)methyl)benzoate-LXXXIII
0
HO
NI 0 e
Y
0
To a stirred solution of methyl 4-(aminomethyl)benzoate (1.0 g, 6.06 mmol) in
water
was added carbonyldiimidazole (1.18 g, 7.26 mmol) 0 C and stirred for 1 h and
then
warmed to room temperature. Then piperidine-4-ylmethanol (0.84 g, 7.26 mmol)
was
added and stirring continued for 12 h. The resultant white precipitate was
filtrated
- 217 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
through sintered funnel. The filtrate was extracted with dichloromethane (2 x
100 mL)
andthe combined organic portion was washed with water, brine, dried over
sodium
sulphate and concentrated under reduced pressure to get the product as off-
white solid
(LXXXIII, 0.26 g, 49%). LC-MS m/z calcd for C16H22N204, 306.1; found 307.2
[M+1-1] .
Step 2: methyl 4-((4-formylpiperidine-1-carboxamido)methyl)benzoate-LXXXIV
o
o e
.,NlyENII 00
0
To a stirred solution of dimethyl sulphoxide (0.55 ml, 7.84 mmol) in
dichloromethane
oxalyl chloride (0.45 mL, 5.22 mmol) was slowly added at -78 C. After 30 min
stirring, a solution of methyl
4-((4-(hydroxymethyl)piperidine- 1-
carboxamido)methyl)benzoate (LXXXIII, 0.4 g, 1.30 mmol) was added dropwise.
Then the reaction mixture was stirred for 3h at -78 C. Triethylamine (2.1 mL,
15.68
mmol) was added and stirred for 0.5 h. The reaction mixture was allowed to
warm to
room temperature. The reaction mixture was diluted with dichloromethane,
washed
with water, brine, dried over sodium sulphate and concentrated under reduced
pressure
to get the product as yellow brown oil (LXXXIV, 0.35g, 89%). LC-MS m/z calcd
for
C16H20N204, 304.1; found 305.1 [M+H]t
Step 3: methyl 4-
44-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)
methyppiperidine-1-
carboxamido)methyl)benzoate-LXXXV
0
A
SI H N
Boc N11.rN 40 e
F
0
To a stirred solution of 2-(4-flurophenyl)cyclopropylamine hydrochloride (0.2
g, 1.06
mmol) in methanol (15 mL) was added methyl 4-((4-formylpiperidine-1-
carboxamido)methyl)benzoate (LXXXIV, 0.39 g, 1.28 mmol), sodium bicarbonate
(0.08 g, 0.95 mmol), and molecular sieves (approx 1 g) at room temperature and
the
resulting mixture was heated to reflux for 2 h. The reaction mixture was
cooled to 0
C and sodium borohydride (0.35 g, 0.95 mmol) was added. Stirring was continued
at
room temperature for 1 h. Ice-water was added and the reaction mixture was
filtered.
The solvent was evaporated to get the residue. Water was added to the residue
and
extracted with dichloromethane (2 x 50 mL). The combined organic portion were
- 218 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
washed with water, brine, dried over sodium sulphate and concentrated under
reduced
pressure to afford the crude product as brown oil (0.44 g). The crude product
was
dissolved intetrahydrofuran-water mixture (20 mL, 1:1). Sodium bicarbonate
(0.26 g,
3.07 mmol) and Boc anhydride (0.26 mL, 1.25 mmol) were added at room
temperature. The resulting mixture was stirred at that temperature for 1 h.
The reaction
mixture was diluted with ethylacetate and was washed with water, brine
solution,
dried over sodium sulphate and concentrated under reduced pressure to get the
crude
product which was purified by column chromatography using methanol-
dichloromethane gradient to afford the titled product as brown thick oil
(LXXXV,
0.22 g, 40 %). LC-MS m/z calcd for C301438FN305, 539.3; found 540.3 [M+H]t
Step 4: 4-
((4-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)
piperidine-l-
carboxamido)methyl)benzoic acid (Intermediate 1-158)
o
A
N H OH
F 1.1 00 N y" 40
o
1-158
To a stirred solution of methyl 4-((4-(((tert-butoxycarbonyl)(2-(4-
fluorophenyl)cycloprop yl)amino)methyl)pip eridine-l-c arboxamido)methyl)benzo
ate
(LXXXV, 0.22g, 0.40 mmol), in mixture of tetrahydrofuran-water (6 mL, 1:1),and
lithium hydroxide (0.029 g, 1.22 mmol) was added and stirred for lh at room
temperature. After completion of the reaction, the mixture was evaporated, the
residue
was diluted with ice-water,and acidified to pH 5 with 2N HC1. The aqueous
layer was
extracted with dichloromethane (50 mL X 2). The combined organic layer was
washed
with water,brine, dried over sodium sulphate and concentrated under reduced
pressure
to get the product as off-white solid (1-158, 0.22g, quantitative yield). LC-
MS m/z
calcd for C29H36FN305, 525.2; found 526.2 [M+H]t
1-159 4-(3-(4-
(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)-2-
oxopiperidin-1-yl)propyl)benzoic acid
0
A
40 1
N OH
...--...õ...--y0
Boc .,N
The compound was synthesized using 1-93 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C301438N205, 506.2; found 507.2 [M+H]t
- 219 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
1-160 4-((4-(((tert-butoxycarbonyl)(2-phenylcyclopropyl)amino)methyl)piperidin-
1-yl)sulfonyl)benzoic acid
A A o
0 F3C0 .. H y-
_____________________________________ - 401 Ni, 0 ao OH
N 0^0 N,ii
X
0
1-160
1-160 4-44-42,2,2-trifluoro-N-(2-phenylcyclopropypacetamido)methyl)piperidin-
1-y1) sulfonyl)benzoic acid
To a stirred solution of 2,2,2-trifluoro-N-(2-phenylcyclopropy1)-N-(piperidin-
4-
ylmethyl)acetamide (1 g, 2.7 mmol) in dichloromethane (20 mL) was added
triethylamine (1.1 mL, 8.10 mmol) and 4-(chlorosulfonyl)benzoic acid (0.66 g,
2.7
mmol) atO C and stirred at room temperature for 2 h. The reaction mixture was
concentrated under vacuum. Then the resultant residue was mixed with
tetrahydrofuran-water (20 mL, 1:1) and lithium hydroxide (0.28 g, 6.7 mmol)
was
added at room temperature. After stirring for 3 h, Boc anhydride (0.88 mL, 4
mmol)
was added and stirring continued for 2 h at room temperature. The solvent was
evaporated and the residue was acidified with 2N HC1 and extracted with
dichloromethane. The combined organic portion was washed with water, brine,
dried
over sodium sulphate and concentrated under reduced pressure to afford the
titled
product as off-white solid (1-160, 1.2 g, 84 %). LC-MS m/z calcd for
C27H34N206S,
514.2; found 415.1 [M-Boc+H] .
1-161 4-(((4-(((tert-
butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)sulfonyl)methyl)benzoic acid-
ester procedure
A o'
A 0
0 N 0
F>rL o' step-10 NH.HCI + CI, /9
F 1 F
F ci
F
B-3 1.300(V1
A OH
Ni'
Step-2 0
BoIN, /9 0o
s
6
1-161
Step-1: The compound was synthesized using amine B-3 and methyl 4-
((chlorosulfonyl)methyl)benzoatefollowing the procedure for the synthesis of 1-
85.
- 220 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Step-2: Hydrolysis of ester LXXX VI, followed by protection with (Boc)20
resulted in
1-161 as white solid. LC-MS m/z calcd for C28H36N206S, 528.2; found 529.2
[M+H]t
1-162 4-
(2-((4-(((tert-butoxycarbonyl)(2-
phenylcyclopropyl)amino)methyl)piperidin-1-yl)sulfonyl)ethyl)benzoic acid
A
40 N
Boc
e SOH
o
The compound was synthesized using 1-88 following the procedure for the
synthesis
of 1-120. LC-MS m/z calcd for C29H38N206S, 542.2; found 543.2 [M+H]t
Examplel
Synthesis of (E)-3(4(((2(4cyclopropylphenyl)cyclopropyl)amino)methyl)pheny1)-
N-hydroxyacrylamide (XLIV)
N
H H
/ N,
OH
0
Step 1 NH Step 2
/ OMe
U000/11 DOOCV111 0
NH
/ NHOH
Example 1 0
Step-1:(E)-3-(44[2-(4-Cyclopropyl-pheny1)-cyclopropylamino]-methyll-pheny1)-
acrylic acid methyl ester (LXXXVIII)
NH
/ OMe
0
2-(4-Cyclopropyl-phenyl)-cyclopropylamine.HC1 (LXXXVII, 0.3 g, 1.43 mmol);
which was prepared through cycloproponation of alkene(as described in Bioorg.
Med.
Chem. Lett., 2008, 18, 3047-3051) was dissolved in dichloroethaneand
triethylamine
- 221 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
(approx 1 mL) was added and stirred for 5 min. The solvent was concentrated
under
reduced pressure to get the free amine.To a stirred solution of (E)-3-(4-
formyl-
pheny1)-acrylicacid methyl ester (0.22 g, 1.19mmol) which was synthesized
using
reported procedure (J. Org. Chem., 2011, 76(19), 8036-8041) in 1,2-
dichloroethane
(20 mL) was added to the free cyclopropylamine and the resulting mixture was
stirred
at 60 C for 1 h. Cooled to 0 C, sodiumtriacetoxyborohydride (0.5 g, 2.39
mmol) was
added and the resulting mixture was stirred at room temperature for 12 h. The
reaction
mixture was filtered and the filtrate was diluted with dichloromethane (50
mL). The
organic portion was washed with water and brine dried over sodium sulphate and
concentrated under reduced pressure to afford the crude. The crude product was
purified by column chromatography using ethylacetate-hexane gradient to obtain
titled
compound as gummy oil (LXXXVIII, 0.22 g, 55 %), LC-MS m/z calcd for
C23H25NO2, 347.1; found 348.2 [M+H]t
Step-2: (E)-3-(44[2-(4-Cyclopropyl-pheny1)-cyclopropylamino]-methyll-pheny1)-
N-hydroxy-acrylamide-Example 1
NH
NHOH
Example 1 0
To a solution of hydroxylamine hydrochloride (0.79 g, 11.41 mmol) in methanol
(5
mL) was added a solution of potassium hydroxide (0.64 g, 11.41 mmol) in
methanol
(5 mL) at 5-10 C and stirred at that temperature for 15 min. The formed
precipitate
was filtered through cotton plug and the filtrate was added to a solution of
(E)-3-(4-
[2-(4-cyclopropyl-pheny1)-cyclopropylamino] -methyl } -phenyl)-acrylic acid
methyl
ester (LXXXVIII, 0.22 g, 0.63 mmol) in methanol at room temperature. The
resulting
mixture was stirred at room temperature for 3h. The reaction mixture was
diluted with
water and extracted with ethylacetate (3x50 mL). The combined organic extract
was
dried over sodium sulphate and concentrated under reduced pressure to afford
the
crude product. The crude product was purified through trituration with
acetonitrile
solvent to afford the titled compound as an off-white solid (Example 1, 0.28
g, 13 %).
itINMR (400 MHz, DMSO-d6): 6 10.7 (bs, 1H), 8.98 (bs, 1H), 7.46-7.36 (m, 3H),
7.32 (d, 2H, J=8 Hz), 6.88 (d, 2H, J=7.6 Hz), 6.83 (d, 2H, J=8 Hz), 6.41 (d,
1H, J=16
Hz), 3.75 (s, 2H), 2.91-2.75 (m, 1H), 2.17-2.10 (m, 1H), 1.85-1.72 (m, 2H),
0.96-
0.91(m, 1H), 0.88-0.82 (m, 3H), 0.58-0.53 (m, 2H). LC-MS m/z calcd for
C22H24N202,
348.1; found 349.2 [M+H]t HPLC purity 98.6 %.
- 222 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Example2
(E)-3-(4-{ [2-(4-Fluoro-phenyl)-cyclopropylamino] -methyll-phenyl)-N-hydroxy-
acrylamide TFA salt
A
B 40 S step 1 F I N
1
Boc __________________________ / NHOH 1 N oc OMe .
F A LXXX1X 0
1-2 0 0
1 NHOH
Step 2 F
110 NH
___________ ...
V
TFA Salt
Example 2
Step 1: [2-(4-Fluoro-phenyl)-cyclopropyl[44-((E)-2-hydroxycarbamoyl-vinyl)-
benzyll-carbamic acid tert-butyl ester (LXXXIX)
N
1
Boc / F NHOH
0
To a solution of hydroxylamine hydrochloride (0.147 g, 2.11 mmol) in methanol
was
added a solution of potassium hydroxide (0.12 g, 2.11 mmol) in methanol at 5-
10 C
and stirred at that temperature for 15 min. The formed precipitate was
filtered through
cotton plug and the filtrate was added to a solution of (E)-344-(1 tert-
butoxycarbonyl-
}2-(4-fluoro-pheny1)-cyclopropyl]-amino }-methyl)-phenyl} -acrylic acid methyl
ester
(1-2, 0.05 g, 0.12 mmol) in methanol (4 mL) at room temperature. Potassium
hydroxide (0.12 g, 2.11 mmol) was added and the resulting mixture was stirred
at
room temperature for 1 h. The solvent was removed and water was added to the
resulting residue. The pH of the aqueous portion was adjusted to 7.0 with 10%
acetic
acid solution and then extracted with ethylacetate (3 x 30 mL). The combined
organic
extract was washed with brine, dried over sodium sulphate and concentrated
under
reduced pressure to afford the crude product which was triturated with water
and dried
to afford the title compound as a white solid (LXXXIX, 0.035 g, 73 %). LC-MS
m/z
calcd for C24H27FN204, 426.2; found 427.2 [M+H]t
Step 2: (E)-3-(4-112-(4-Fluoro-phenyl)-cyclopropylaminol-methyll-phenyl)-N-
hydroxy-acrylamide. TFA salt-Example 2
- 223 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
NHOH
F
V
TFA Salt
To a stirred solution of [2-
(4-fluoro-pheny1)-cyclopropyl]-[44(E)-2-
hydroxycarbamoyl-viny1)-benzyll-carbamic acid tert-butyl ester (LXXXIX, 0.15
g,
0.36 mmol) in dry dichloromethane (2 mL) was added trifluoroacetic acid (2 mL)
at 0
C and the resulting mixture was stirred at that temperature for 1 h. The
progress of
the reaction was monitored by TLC. The solvent was concentrated under reduced
pressure to get the crude product which was purified by reverse-phase HPLC
using
Chemsil C18(250mm X 4.6mm X 5mic) column with 0.1% TFA in water:ACN to
afford the pure product as off-white solid (Example 2, 0.04 g, 26 %).
itINMR (400 MHz, DMSO-d6): 6 10.76 (bs, 1H), 9.18 (bs, 1H), 9.04 (bs, 1H),
7.59
(d, 2H, J=7.6 Hz), 7.52-7.42 (m, 3H), 7.18-7.07 (m, 4H), 6.48 (d, 1H, J=16
Hz), 4.30
(s, 2H), 2.89 (bs, 1H), 2.41-2.32 (m, 1H), 1.45-1.37 (m, 1H), 1.32-1.25 (m,
1H). LC-
MS m/z calcd for C19H19FN202, 326.1; found 327.3 [M+H]t HPLC purity 97.1 %.
The following compounds were synthezied using the procedure exemplified in
Example 2
Example3 (E)-
3-(4-(((2-(4-((4-
fluorobenzypoxy)phenyl)cyclopropyl)amino)methyl)pheny1)-N-
hydroxyacrylamide TFA salt
A
40 ENI
'OH
0
F
The compound was synthesized using the 1-3 following the procedure for Example
2.
itINMR (400 MHz, DMSO-d6): 6 10.75 (bs, 1H), 9.02 (bs, 1H), 7.57-7.55 (m, 2H),
7.49-7.41 (m, 5H), 7.20 (t, 2H, J=9 Hz), 7.00 (d, 2H, J=8.4 Hz), 6.89 (d, 2H,
J=8.4
Hz), 6.47 (d, 1H, J=15.6 Hz), 5.04 (s, 2H), 4.19-4.14 (m, 2H), 2.76-2.45 (m,
1H),
2.24-2.15 (m, 1H), 1.31-1.22 (m, 1H), 1.14-1.10 (m, 1H). LC-MS m/z calcd for
C26H25FN203, 432.1, found 433.2 [M+H]t HPLC purity 96.3 %.
Example4 (E)-
N-hydroxy-3-(4-(4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)phenyl)acrylamide TFA salt
- 224 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
A N
No
H
0
The compound was synthesized using the 1-4 following the procedure for Example
2.
itINMR (400 MHz, DMSO-d6): 6 10.53 (bs, 1H), 8.78 (bs, 1H), 7.42-7.26 (m, 5H),
7.24-7.15 (m, 3H), 6.94 (d, 2H, J=8.8 Hz), 6.22 (d, 1H, J=15.6 Hz), 5.32 (bs,
1H),
3.86-3.80 (m, 2H), 3.65-3.59 (m, 1H), 3.15-3.11 (m, 1H), 3.08-2.96 (m, 3H),
2.79-
2.70 (m, 2H), 2.02-1.96 (m, 1H), 1.90-1.76 (m, 3H), 1.49-1.41 (m, 1H), 0.86-
0.80 (m,
1H). LC-MS m/z calcd for C24H29N302, 391.2; found 392.3 [M+H]t HPLC purity
99.4 %.
Example5 (E)-
3-(4-4(2-(4'-chloro-[1,1'-bipheny1]-4-
yl)cyclopropyl)amino)methyl)pheny1)-N-hydroxyacrylamide TFA salt
0
The compound was synthesized using the 1-5 following the procedure for Example
2.
itINMR (400 MHz, DMSO-d6): 6 10.75 (s, 1H), 9.20 (bs, 1H), 9.03 (s, 1H), 7.66
(d,
2H, J=8.4 Hz), 7.61-7.56 (m, 4H), 7.50-7.42 (m, 5H), 7.21-7.19 (m, 2H), 6.48
(d, 1H,
J=16 Hz), 4.32-4.25 (m, 2H), 1.48-1.32 (m, 2H), 1.25-1.15 (m, 2H). LC-MS m/z
calcd
for C25H23C1N202, 418.1; found 419.2 [M+H]t HPLC purity 92.8 %.
Example6 (E)-
3-(4-(((2-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)pheny1)-N-hydroxyacrylamide TFA Salt
N
N,OH---
N¨ TFA Salt 0
The compound was synthesized using the 1-6 following the procedure for Example
2.
itINMR (400 MHz, DMSO-d6): 6 10.74 (s,1H), 9.25 (bs, 2H), 9.02 (bs, 1H), 7.61
(d, J
= 7.6 Hz, 2H),7.50 (d, J = 8Hz, 2H), 7.44 (d, J = 15.6 Hz, 1H), 7.28 (d, J = 8
Hz, 2H),
7.21 (d, J= 8 Hz, 2H), 6.47 (d, J = 16Hz, 1H), 4.32 (s, 2H), 2.96 (m, 1H),
2.55 (m,
1H), 2.36 (s, 3H), 2.18 (s, 3H), 1.50-1.40 (m,1H), 1.40-1.30 (m,1H). LC-MS m/z
calcd for C24H25N303, 403.2; found 404.2 [M+H]t HPLC purity 98.8%.
Example7 (E)-
N-hydroxy-3-(4-(((2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino)methyl)phenyl)acrylamide TFA salt
- 225 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
N,OH N
Nr TFA salt 0
The compound was synthesized using the 1-7 following the procedure for Example
2.
11-1NMR (400 MHz, DMSO-d6): 6 10.76 (s,1H), 9.36 (bs, 2H), 9.25 (bs, 1H), 9.16
(s,
1H), 9.12 (s, 2H), 7.74 (d, J= 8.4Hz, 2H), 7.60 (d, J= 8.0Hz, 2H), 7.50 (d, J=
8.0Hz,
2H), 7.45 (d, J = 15.6 Hz, 1H), 7.29 (d, J = 8.0 Hz, 2H), 6.48 (d, J = 15.6Hz,
1H), 4.34
(s, 2H), 3.02 (m, 1H), 2.55 (m, 1H), 1.52-1.42 (m,1H), 1.42-1.34 (m,1H). LC-MS
m/z
calcd for C23H22N402, 386.2; found 387.2 [M+Hr.HPLC purity98.3%.
Example 8 2-(4-(((2-(4-fluorophenyl)cyclopropyl)amino)methyl)piperidin-l-y1)-
N-hydroxypyrimidine-5-carboxamide TFA salt
F NN
II H
N N,OH
0
The compound was synthesized using the 1-8 following the procedure for Example
2.
11-1NMR (400 MHz, DMSO-d6): 6 11.02 (s, 1H), 8.75 (bs, 2H), 8.64 (s, 2H), 7.23
¨
7.20 (dd, J = 5.6, 5.6 Hz, 2H), 7.14 ¨ 7.10 (dd, J = 9.2, 8.8 Hz, 2H), 4.70 ¨
4.67 (d, J
=13.6 Hz, 2H), 3.01 ¨ 2.92 (m, 5H), 2.44 ¨ 2.41 (m, 1H), 2.05-1.95 (m, 1H),
1.82 ¨
1.79 (m, 2H), 1.47 ¨ 1.42 (m, 1H), 1.30 ¨ 1.25 (q, 1H), 1.20 ¨ 1.12 (m, 2H).
LC-MS
m/z calcd [M+H] 385.2, found 386.2. HPLC purity 99.8%.
Example 9 244-(2-Phenyl-cyclopropylamino)-piperidin-l-y11-pyrimidine-5-
carboxylic acid hydroxyamide TFA salt
NHOH
A
asl
The compound was synthesized using the 1-9 following the procedure for Example
2.
11-1NMR (400 MHz, DMSO-d6): 6 10.96 (bs, 1H), 8.94 (bs, 1H), 7.24-7.18 (m,
2H),
7.12-7.08 (m, 1H), 7.04-7.00 (m, 2H), 4.50-4.4 (m, 2H), 3.20-3.10 (m, 2H),
2.90-2.80
(m, 1H), 2.28-2.20 (m, 2H), 1.88-1.75 (m, 3H), 1.28-1.16 (m, 2H), 0.98-0.92
(m, 2H),
0.86-0.81 (m, 1H). LC-MS m/z calcd for C19H23N502, 353.2; found 354.2 [M+H]t
HPLC purity 99.8 %.
- 226 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Example 10 2-
{4-[2-(4-Fluoro-pheny1)-cyclopropylamino]-piperidin-1-yll-
pyrimidine-5-carboxylic acid hydroxyamide TFA salt
0
NN1,01-1
I H
N1
A
The compound was synthesized using the 1-10 following the procedure for
Example 2.
5 iHNMR (400 MHz, DMSO-d6): 6 10.95 (bs, 1H), 8.93 (bs, 1H), 8.63 (s, 2H),
7.09-
7.01 (m, 4H), 4.50-4.41 (m, 2H), 3.18-3.08 (m, 2H), 2.88-2.80 (m, 1H), 2.25-
2.18 (m,
1H), 1.86-1.75 (m, 3H), 1.28-1.15 (m, 3H), 0.99-0.89 (m, 2H). LC-MS m/z calcd
for
C19H22FN502, 371.1; found 372.1 [M+H]t HPLC purity 97.7 %.
Example 11 2-
(4-(((2-(4-((4-
10 fluorobenzypoxy)phenyl)cyclopropyl)amino)methyl)piperidin-1-y1)-N-
hydroxypyrimidine-5-carboxamide TFA salt
0
NrN,OH
0
The compound was synthesized using the I-11 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.03 (bs, 1H), 8.97 (bs, 1H), 8.72 (bs, 2H),
8.66 (s,
2H), 7.48-7.45 (m, 2H), 7.23-7.18 (m, 2H), 7.11 (d, 2H, J=8.4 Hz), 6.94 (d,
2H, J=8
Hz), 5.06 (s, 2H), 4.71 (d, 2H, J=12.4 Hz), 3.08-2.85 (m, 6H), 2.40-2.34 (m,
2H),
2.05-1.94 (m, 1H), 1.84-1.77 (m, 2H), 1.42-1.36 (m, 1H), 1.24-1.11 (m, 1H). LC-
MS
m/z calcd for C27H30FN503, 491.2; found492.4 [M+H]t HPLC purity 96.7 %.
Example12 2-(4-42-(44(4-fluorobenzypoxy)phenyl)cyclopropyl)amino)piperidin-
1-y1)-N-hydroxypyrimidine-5-carboxamide TFA salt
0
N)LN
OH
H
=
o 1N-11
The compound was synthesized using the 1-12 following the procedure for
Example 2.
- 227 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
itINMR (400 MHz, DMSO-d6): 6 11.07 (bs, 1H), 9.0 (bs, 1H), 8.88 (bs, 1H), 8.84
(bs,
1H), 8.69 (s, 2H), 7.49-7.35 (m, 2H), 7.20 (t, 2H, J=8.8 Hz), 7.11 (d, 2H,
J=8.4 Hz),
6.94 (d, 2H, J=8.4 Hz), 5.06 (s, 2H), 4.77 (d, 2H, J=12.8 Hz), 3.63-3.54 (m,
1H), 3.05-
2.90 (m, 4H), 2.14-2.08 (m, 2H), 1.52-1.44 (m, 2H), 1.39-1.32 (m, 1H), 1.30-
1.23 (m,
1H). LC-MS m/z calcd for C26H28FN503, 477.2; found 476.2 [M-H] . HPLC purity
99.7 %.
Example13 2-(4-02-(4'-chloro-[1,1'-bipheny1]-4-yl)cyclopropyl)amino)piperidin-
1-y1)-N-hydroxypyrimidine-5-carboxamide TFA salt
0
NAN,OH
I H
CI
The compound was synthesized using the 1-13 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.07 (s, 1H), 9.01-8.96 (m, 3H), 8.68 (s, 2H),
7.67
(d, 2H, J= 8.4 Hz), 7.61 (d, 2H, J= 8.0 Hz), 7.50 (d, 2H, J= 8.4 Hz), 7.29 (d,
2H, J=
7.6 Hz), 4.78 (d, 2H, J= 12.8 Hz), 3.60 (bs, 1H), 3.02 (t, 4H, J= 12.4 Hz),
2.13 (d, 2H,
J= 10.8 Hz), 1.50-1.47 (m, 3H), 1.40-1.38 (m, 1H). LC-MS m/z calcd
forC25H26C1N502, 464.1; found 464.2 [M+H]t HPLC purity 98.8 %.
Example 14 2-
(4-0(2-(4'-chloro-[1,1'-bipheny1]-4-
yl)cyclopropyl)amino)methyl)piperidin-1-y1)-N-hydroxypyrimidine-5-
carboxamide TFA salt
N
N
TFA H
N N
CI
0
The compound was synthesized using the 1-14 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.03 (bs, 1H), 8.97 (bs, 1H), 8.89 (bs, 2H),
8.66 (s,
2H), 7.67 (d, 2H, J=11.2 Hz), 7.61 (d, 2H, J=8 Hz), 7.49 (d, 2H, J=8 Hz), 7.28
(d, 2H,
J=7.6 Hz), 4.71 (d, 2H, J=13.2 Hz), 3.09-2.91 (m, 5H), 2.09-1.96 (m, 1H), 1.86-
1.78
(m, 2H), 1.52-1.48 (m, 1H), 1.41-1.31 (m, 1H), 1.27-1.12 (m, 3H). LC-MS m/z
calcd
for C26H28C1N502, 477.1; found 476.4 [M-H]t HPLC purity 98.8 %.
- 228 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Example15 2-
(4-(42-(4'-fluoro-[1,1'-bipheny1]-4-
yl)cyclopropyl)amino)methyl)piperidin-1-y1)-N-hydroxypyrimidine-5-
carboxamide TFA salt
H
N
TFA
The compound was synthesized using the 1-15 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.02 (bs, 1H), 8.96 (bs, 1H), 8.80 (bs, 1H),
8.73
(bs, 1H), 8.65 (s, 2H), 7.69-7.64 (m, 2H), 7.59-7.55 (m, 2H), 7.28-7.23 (m,
4H), 4.74-
4.55 (m, 2H),3.08-2.93 (m, 6H),2.05-1.96 (m, 1H),1.84-1.80 (m, 2H), 1.51-1.44
(m,
1H), 1.36-1.32 (m, 1H), 1.24-1.15 (m, 2H). LC-MS m/z calcd for C26H38FN502,
461.2; found 462.2 [M+H]t HPLC purity 99.5 %.
Example16 2-
(4-(42-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-y1)-N-hydroxypyrimidine-5-
carboxamide TFA Salt
Nr--0õyrN
R
NI¨
TFA Salt NO
HN,OH
The compound was synthesized using the 1-16 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.02 (bs,1H), 8.95 (bs,1H), 8.85 (bs,1H), 8.73
(bs,1H), 8.65 (s, 2H), 7.31-7.26 (m, 4H), 4.69 (d, J=12.8 Hz, 2H), 3.08-2.92
(m, 5H),
2.55 (m, 1H), 2.35 (s,3H), 2.18 (s, 3H), 2.06-1.95 (m, 1H), 1.85-1.78 (m, 2H),
1.53-
1.45 (m,1H), 1.40-1.32 (m,1H), 1.25-1.10 (m, 2H). LC-MS m/z calcd for
C25H30N603,
462.2; found 463.2 [M+H]t HPLC purity 99.1%.
Example17 N-
hydroxy-2-(4-(((2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)pyrimidine-5-carboxamide
TFA salt
N
N H
kN N N-OH
TFA 0
The compound was synthesized using the 1-17 following the procedure for
Example 2.
- 229 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
itINMR (400 MHz, DMSO-d6): 6 11.02 (bs, 1H), 9.16 (s, 1H), 9.12 (s, 2H), 8.90
(bs,
2H), 8.83 (bs, 1H), 8.65 (s, 2H), 7.76 (d, J = 8.4Hz, 2H), 7.35 (d, J = 8Hz,
2H), 4.73-
4.67 (m, 2H), 3.08-2.93 (m, 5H), 2.08-1.92 (m, 2H), 1.84-1.78 (m, 2H), 1.54-
1.50 (m,
1H), 1.42-1.35 (m, 1H), 1.25-1.13 (m, 2H). LC-MS m/z calcd for C24H27N702,
445.2,
found 446.2 [M+H]t HPLC purity 99.8 %.
Example18 N-
hydroxy-2-(4-(((2-(4-
methoxyphenyl)cyclopropyl)amino)methyl)piperidin-l-yl)pyrimidine-5-
carboxamide TFA salt
H N
NrN,
OH
0
The compound was synthesized using the 1-18 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.03 (bs, 1H), 8.96 (bs, 1H), 8.79 (bs, 2H),
8.68 (s,
2H), 7.10 (d, 2H, J=8.4 Hz), 6.86 (d, 2H, J=8.8 Hz), 4.77 (d, 2H, J=13.2 Hz),
3.71 (s,
3H), 3.05-2.85 (m, 5H), 2.42-2.32 (m, 1H), 2.05-1.97 (m, 1H), 1.83-1.80 (m,
2H),
1.42-1.37 (m, 1H), 1.24-1.13 (m, 3H). LC-MS m/z calcd for C21H27N503, 397.2;
found 398.2 [M+H]t HPLC purity 96.2 %.
Example 19 N-hydroxy-2-(4-((2-(4-methoxyphenyl)cyclopropyl)amino)piperidin-
l-yl)pyrimidine-5-carboxamide TFA salt
0
N-LN,OH
ii H
N)
The compound was synthesized using the 1-19 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.06 (bs, 1H), 9.04 (bs, 2H), 8.67 (s, 2H), 7.19
(d,
2H, J=8.4 Hz), 6.85 (d, 2H, J=9.2 Hz), 4.78-4.73 (m, 2H), 3.70 (s, 3H), 3.61-
3.53 (m,
1H), 3.03-2.96 (m, 2H), 2.92-2.84 (m, 1H), 2.35-2.30 (m, 1H), 2.11-2.04 (m,
2H),
1.51-1.45 (m, 2H), 1.40-1.34 (m, 1H), 1.27-1.21 (m, 1H). LC-MS m/z calcd for
C20H25N503, 383.2; found 384.2 [M+H]t HPLC purity 97.1 %.
- 230 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Example 20 2-
(44(41R,2S)-2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-y1)-N-hydroxypyrimidine-5-
carboxamide TFA salt
F NN
ii H
NrN,
OH
0
The compound was synthesized using the 1-20 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.02 (bs, 1H), 8.95 (bs, 1H), 8.64(s, 2H), 7.22-
7.15 (m, 2H), 7.13-7.06 (m, 2H),4.72-4.62 (m, 2H), 3.01-2.92 (m, 4H), 2.89-
2.81 (m,
1H), 2.35-2.28 (m, 1H), 2.00-1.91 (m, 1H), 1.84-1.76 (m, 2H), 1.40-01.38 (m,
1H),
1.35-1.09 (m, 3H). LC-MS m/z calcd for C201-124FN502[M+H] 385.1, found 386.2.
HPLC purity 98.2 %.
Example 21 2-
(4-((((lS,2R)-2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-y1)-N-hydroxypyrimidine-5-
carboxamide TFA salt
ii H
NOH
0
The compound was synthesized using the 1-21 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.02 (bs, 1H), 8.95 (bs, 1H), 8.64 (s, 2H), 7.22-
7.15 (m, 2H), 7.13-7.06 (m, 2H), 4.72-4.62 (m, 2H), 3.01-2.92 (m, 4H), 2.89-
2.81 (m,
1H), 2.35-2.28 (m, 1H), 2.00-1.91 (m, 1H), 1.84-1.76 (m, 2H), 1.40-01.38 (m,
1H),
1.35-1.09 (m, 3H). LC-MS m/z calcd for C201-124FN502[M+H] 385.1, found 386.1.
HPLC purity 98.1 %.
Example 22 4-(4-(((2-(4-fluorophenyl)cyclopropyl)amino)methyl)piperidin-l-y1)-
N-hydroxybenzamide TFA salt
gH
N
'OH
0
The compound was synthesized using the 1-22 following the procedure for
Example 2.
- 231 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
itINMR (400 MHz, DMSO-d6): 6 10.98 (bs, 1H), 8.82 (bs, 2H), 7.61 (d, 2H, J=8.4
Hz), 7.24-7.20 (m, 2H), 7.16-7.08 (m, 2H), 6.92 (d, 2H, J=8.4 Hz), 3.95-3.80
(m, 2H),
3.04-2.90 (m, 3H), 2.79-2.70 (m, 2H), 2.42-2.35 (1H, m), 1.87-1.77 (m, 3H),
1.46-
1.41 (m, 1H), 1.34-1.20 (m, 3H). LC-MS m/z calcd for C22H26FN302, 383.2; found
384.2 [M+H]t HPLC purity 98.0 %.
Example 23 N-
hydroxy-2-(2-0(2-phenylcyclopropyl)amino)methyl)-5,6-
dihydroimidazo[1,2-a]pyrazin-7(8H)-yppyrimidine-5-carboxamide TFA salt
A
1.1 N N
H N- HN-OH
N 0
The compound was synthesized using the 1-23 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 8.71 (s, 2H), 7.22-7.15 (m, 2H), 7.09-7.05 (m,
1H),
7.01-6.94 (m, 2H), 6.86 (s, 1H), 4.86 (s, 2H), 4.45-4.38 (m, 2H), 4.02-3.93
(m, 2H),
3.61 (s, 2H), 2.30-2.25 (m, 1H), 1.82-1.75 (m, 1H), 1.02-0.88 (m, 2H). LC-MS
m/z
calcd for C21H23N702, 405.1; found 406.2 [M+H]t HPLC purity 98.2 %. (3
exchangeable proton merged with solvent)
Example24 N-hydroxy-2-(2-0(2-(4-methoxyphenyl)cyclopropyl)amino)methyl)-
5,6-dihydroimidazo[1,2-a]pyrazin-7(8H)-yppyrimidine-5-carboxamide TFA salt
A
N N
NHOH
N 3
0 N- \()
The compound was synthesized using the 1-24 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.14 (bs, 1H), 9.22 (bs, 2H), 8.76 (s, 2H), 7.23
(s,
1H), 6.96 (d, 2H, J=8.4Hz), 6.77 (d, 2H, J=8.4Hz), 5.03-4.91 (m, 2H), 4.29-
4.13 (m,
4H), 4.09-3.98 (m, 2H), 3.67 (s, 3H), 2.83-2.78 (m, 1H), 2.25-2.18 (m, 1H),
1.36-1.29
(m, 1H), 1.23-1.13 (m, 1H). LC-MS m/z calcd for C22H25N703, 435.2, found
436.1[M+H]t HPLC purity 99.6 %.
Example 25 2-(2-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)-5,6-
dihydroimidazo[1,2-a]pyrazin-7(8H)-y1)-N-hydroxypyrimidine-5-carboxamide
TFA salt
- 232 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
rfsl
II H
N/H N
NrN,OH
0
The compound was synthesized using the 1-25 following the procedure for
Example 2.
11-1NMR (400 MHz, DMSO-d6): 6 11.18 (bs, 1H), 9.28 (bs, 2H), 8.75 (s, 2H),
7.24 (s,
1H), 7.12-7.02 (m, 4H), 4.96 (q, 2H, J=17.2 Hz), 4.25-4.22 (m, 2H), 4.18-4.14
(m,
2H), 4.11-4.00 (m, 2H), 2.87 (t, 1H, J=3.2 Hz), 2.29 (s, 1H), 1.40-1.35 (m,
1H), 1.23
(t, 1H, J= 6.8 Hz). LC-MS m/z calcd for C211-122FN702, 423.2; found 424.4
[M+H]t
HPLC purity 99.5 %.
Example26 3-
(((2-(4-bromophenyl)cyclopropyl)amino)methyl)-N-
hydroxybenzamide TFA salt
0
,
El
Br 01-1
The compound was synthesized using the 1-26 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.09 (bs, 1H), 8.95 (bs, 1H), 7.71 (s, 1H), 7.57
(d,
1H, J=7.6 Hz), 7.41 (d, 1H, J=7.6 Hz), 7.35-7.31 (m, 3H), 6.92 (d, 2H, J=8.4
Hz),
3.77 (s, 2H), 2.92 (bs, 1H), 2.21-2.17 (m, 1H), 1.82-1.78 (m, 1H), 1.04-0.99
(m, 1H),
0.95-0.90 (m, 1H). LC-MS m/z calcd for C17H17BrN202, 360.0; found 361.0 [M+H]t
HPLC purity 98.0 %.
Example 27 N-hydroxy-3-(((2-phenylcyclopropyl)amino)methyl)benzamide TFA
salt
A _OH
N
The compound was synthesized using the 1-27 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.09 (bs, 1H), 8.95 (bs, 1H), 7.72 (s, 1H), 7.57
(d,
1H, J=7.2 Hz), 7.42 (d, 1H, J=7.6 Hz), 7.33 (t, 1H, J=7.6Hz), 7.18 (t, 2H,
J=7.6Hz),
7.08 (t, 1H, J=7.6 Hz), 7.0 (d, 2H, J=7.2 Hz), 3.79 (s, 2H), 2.92-2.81 (m,
1H), 2.22-
2.20 (m, 1H), 1.86-1.78 (m, 1H), 1.02-0.97 (m, 1H), 0.93-0.84 (m, 1H). LC-MS
m/z
calcd for C17H18N202 282.1; found 283.2 [M+H]t HPLC purity 99.8 %.
- 233 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Example 28 N-hydroxy-4-(((2-phenylcyclopropyl)amino)methyl)benzamide TFA
salt
A
SN
H
NOH
0
The compound was synthesized using the 1-28 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 9.20 (bs, 2H), 7.65 (d, 2H, J=7.6 Hz), 7.25 (d,
2H,
J=7.2 Hz), 7.18 (t, 2H, J=7.6 Hz), 7.07 (t, 2H, J=7.2 Hz), 6.80 (d, 2H, J=7.6
Hz), 3.75
(s, 2H), 2.88-2.75 (m, 1H), 2.23-2.15 (m, 1H), 1.85-1.75 (m, 1H), 0.98-0.85
(m, 1H).
LC-MS m/z calcd for C17H18N202 282.1; found 283.2 [M+H]t HPLC purity 99.5 %.
Example 29 N-hydroxy-6-((2-phenylcyclopropyl)amino)hexanamide TFA salt
A
NHOH
The compound was synthesized using the 1-29 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 10.31 (bs, 1H), 8.77 (bs, 2H), 7.32-7.25 (m, 2H),
7.23-7.14 (m, 3H), 3.09-3.00 (m, 2H), 2.98-2.91 (m, 1H), 2.42-2.33 (m, 1H),
1.98-
1.91 (m, 2H), 1.62-1.38 (m, 5H), 1.32-1.25 (m, 3H). LC-MS m/z calcd for
C15H22N202, 263.3; found 263.2 [M+H]t HPLC purity 96.4 %.
Example 30 4-
(3-42-(4-fluorophenyl)cyclopropyl)amino)propy1)-N-
hydroxybenzamide TFA salt
'OH
0
The compound was synthesized using the 1-30 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.12 (s, 1H), 8.94 (bs, 2H), 7.68 (d, 2H, J=8.0
Hz),
7.27 (d, 2H, J=8.0 Hz), 7.20-7.08 (m, 4H), 3.09-3.01 (m, 2H), 2.98-2.91 (m,
1H),
2.72-2.63 (m, 2H), 2.47-2.37 (m, 1H), 1.94-1.86 (m, 2H), 1.43-1.38 (m, 1H),
1.28-
1.21 (m, 1H). LC-MS m/z calcd for C19H21FN202, 328.1; found 329.4 [M+H]t HPLC
purity 96.6 %.
Example 31 N-(6-Hydroxycarbamoyl-hexyl)-4-[(2-phenyl-cyclopropylamino)-
methyl]-benzamide TFA salt
- 234 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
A
1101 110 IR II/\/\/.r NHOH
0 0
The compound was synthesized using the 1-31 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 10.29 (bs, 1H), 8.63 (bs, 1H), 8.32 (t, 1H, J=5.2
Hz), 7.74 (d, 2H, J=8Hz), 7.35 (d, 2H, J=8 Hz), 7.20-7.16 (m, 2H), 7.09-7.06
(m, 2H),
6.85 (d, 2H, J=7.6Hz), 3.79 (s, 2H), 3.34-3.19 (m, 2H), 2.91 (bs, 1H), 2.25-
2.17 (m,
1H), 1.92 (t, 1H, J=7.2 Hz), 1.84-1.80 (m, 1H), 1.55-1.42 (m, 4H), 1.32-1.20
(m, 4H),
1.02-0.97 (m, 1H), 0.94-0.89 (m, 1H). LC-MS m/z calcd for C24H31N303, 409.2;
found 410.3 [M+H]t HPLC purity 97.0 %.
Example 32 4-
(42-(4-fluorophenyl)cyclopropyl)amino)methyl)-N-(7-
(hydroxyamino)-7-oxoheptyl)benzamide TFA salt
H
NyNOH
0 0
The compound was synthesized using the 1-32 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 10.30 (bs, 1H), 9.39 (bs, 2H), 8.46-8.44 (m, 1H),
7.86 (d, 2H, J=8.4 Hz), 7.55 (d, 2H, J=8 Hz), 7.19-7.09 (m, 4H), 4.36 (s, 2H),
3.26-
3.21 (m, 2H), 2.93-1.88 (m, 1H), 2.44-2.43 (m, 1H), 1.95-1.91 (m, 2H), 1.52-
1.41 (m,
5H), 1.31-1.24 (m, 5H). LC-MS m/z calcd for C24H30FN303, 427.2; found428.5
[M+H]t HPLC purity 98.6 %.
Example 33 4-(2-Phenyl-cyclopropylamino)-cyclohexanecarboxylic acid
hydroxyamide TFA salt
0
cIAN,OH
The compound was synthesized using the 1-33 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 10.26 (bs, 1H), 8.54 (bs, 1H), 7.21 (t, 2H, J=7.2
Hz), 7.09 (t, 1H, J=7.2 Hz), 7.00 (d, 2H, J=7.6 Hz), 2.82 (s, 1H), 2.09 (s,
1H), 2.01-
1.97 (m, 1H), 1.84-1.59 (m, 5H), 1.50-1.26 (m, 5H), 1.01-0.92 (m, 2H). LC-MS
m/z
calcd C16H22N202[M+H]+275.1, found275.1. HPLC purity 95 %.
- 235 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Example 34
(1S,4R)-N-hydroxy-4- ((lS)-1 -((2-
phenylcyclopropyl)amino)ethyl)cyclohexanecarboxamide TFA salt
N H
0
The compound was synthesized using the 1-34 following the procedure for
Example 2.
iHNMR (400 MHz, DMSO-d6): 6 10.34 (bs, 1H), 8.76 (bs, 1H), 8.59 (bs, 1H), 7.33-
7.27 (m, 2H), 7.24-7.22 (m, 1H), 7.20-7.17 (m, 2H), 3.32-3.23 (m, 1H), 3.00-
2.91 (m,
1H), 2.44-2.31 (m, 2H), 1.97-1.89 (m, 1H), 1.72-1.65 (m, 5H), 1.43-1.29 (m,
5H),
1.19-1.00 (m, 3H). LC-MS m/z calcd for C18H26N202, 302.2; found 303.2 [M+H]t
HPLC purity 99.1 %.
Example35 N-hydroxy-4-
((4-(((2-(4-(1-methy1-6-oxo-1,6-dihydropyridin-3-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)methyl)benzamide TFA Salt
0
N N,OH
ON
,
0 N
TFA Salt
The compound was synthesized using the 1-35 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.29 (bs, 1H), 9.57 (bs, 1H), 9.00 (bs, 2H),
8.08 (s,
1H), 7.83 (d, J = 8.0 Hz, 2H), 7.79-7.76 (m, 1H), 7.55 (d, J = 8.0Hz, 2H),
7.52-7.46
(m, 2H), 7.21 (d, J= 8.0 Hz, 2H), 6.46 (d, J= 9.2 Hz, 1H), 4.31 (s, 2H), 3.49
(s, 3H),
3.44-3.34 (m, 2H), 3.25-3.10 (m, 1H), 3.05-2.85 (m, 5H), 2.02-1.80 (m, 3H),
1.51-
1.27 (m, 4H). LC-MS m/z calcd for C29H34FN403, 486.3; found 487.6 [M+H]t HPLC
purity 99.7%.
Example 36 N-Hydroxy-444-[(2-phenyl-cyclopropylamino)-methy1]-piperidin-1-
ylmethyll-benzamide TFA salt
0
A OH
1.1 NON 40:1 HN
The compound was synthesized using the 1-36 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 10.80 (bs, 1H), 8.95 (bs, 1H), 7.68 (d, 2H, J=8
Hz),
7.33 (d, 2H, J=8 Hz), 7.24-7.17 (m, 2H), 7.12-7.06 (m, 1H), 7.03-6.98 (m, 2H),
3.45
(s, 2H), 2.78-2.72 (m, 2H), 2.46-2.42 (m, 3H), 2.19-2.14 (m, 1H), 1.92-1.84
(m, 2H),
1.78-1.71 (m, 1H), 1.68-1.59 (m, 2H), 1.39-1.30 (m, 1H), 1.16-1.04 (m, 2H),
0.81-
- 236 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0.62 (m, 2H). LC-MS m/z calcd for C23H29N302, 379.2; found 380.2 [M+H]t HPLC
purity 99.8 %.
Example 37 4-
44-(42-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-yl)methyl)-N-
hydroxybenzamide TFA salt
0
N-OH
H
q
N- TFA
The compound was synthesized using the 1-37 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.28 (bs, 1H), 9.53 (bs, 1H), 9.04-8.84 (bs,
2H),
7.83 (d, J= 7.2Hz, 2H), 7.55 (d, J =7.2Hz, 2H), 7.32-7.24 (m, 4H), 4.32 (s,
2H), 3.50-
3.37 (m, 2H),3.06-2.92 (m, 5H),2.35 (s, 3H), 2.18 (s, 3H), 1.98-1.83 (m, 4H),
1.50-
1.32 (m, 4H). LC-MS m/z calcd for C28H34N403,474.3; found 475.3 [M+H]t HPLC
purity 99.9 %.
Example38 N-
hydroxy-4-((4-(((2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)methyl)benzamide TFA salt
0
rEl N-OH
N
I
TFA
The compound was synthesized using the 1-38 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.27 (bs, 1H), 9.70 (bs, 1H), 9.16 (s, 1H), 9.11
(s,
2H), 9.06 (bs, 1H), 7.82(d, 2H, J =8.4Hz), 7.75(d, 2H, J =8Hz), 7.55(d,2H, J
=8Hz),7.33(d, 2H, J =8Hz), 4.32 (s, 2H), 3.41-3.37 (m, 2H), 3.07-2.92 (m,
5H),2.00-
1.92 (m, 4H), 1.55-1.49 (m, 1H), 1.47-1.33 (m, 3H). LC-MS m/z calcd for
C27H31N502, 457.2; found 458.6[M+H]t HPLC purity 99.0 %.
Example 39 6-
44-(42-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-yl)methyl)-N-
hydroxynicotinamide TFA salt
0
LrEsii-oH
-
N TFA
- 237 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using the 1-39 following the procedure for
Example 2.
11-1NMR (400 MHz, DMSO-d6): 6 11.45 (bs, 1H), 9.97 (bs, 1H), 9.05 (bs, 2H),
9.00-
8.93 (m, 1H), 8.22 (d, 1H, J =7.2Hz), 7.60 (d, 1H, J =8.4Hzõ 7.33-7.24 (m,
4H), 4.52-
4.48 (m, 3H),3.46-3.40 (m, 2H),3.11-3.01 (m, 5H), 2.36 (s, 3H),2.18 (s,
3H),1.98-1.91
(m, 3H), 1.58-1.48 (m, 3H),1.38-1.31 (m,1H). LC-MS m/z calcdfor C27t133N503,
475.3, found 476.3 [M+Hr.HPLC purity 99.8 %.
Example 40 N-hydroxy-444-4(2-phenylcyclopropyl)amino)methyl)-1H-pyrazol-
1-yl)methyl)benzamide TFA salt
n(--CN
H N
'OH
0
The compound was synthesized using the 1-40 following the procedure for
Example 2.
11-1NMR (400 MHz, DMSO-d6): 6 11.16 (bs, 1H), 9.01 (bs, 3H), 7.87 (s, 1H),
7.69 (d,
2H, J=8 Hz), 7.54 (s, 1H), 7.32-7.24 (m, 4H), 7.23-7.19 (m, 1H), 7.11 (d, 2H,
J=7.2
Hz), 5.36 (s, 2H), 4.18 (s, 2H), 2.92-2.85 (m, 1H), 2.40-2.31 (m, 1H), 1.42-
1.36 (m,
1H), 1.32-1.23 (m, 1H). LC-MS m/z calcd for C211-122N402, 362.4; found 363.4
[M+H]t HPLC purity 99.1 %.
Example 41 N-hydroxy-4-44-4(2-phenylcyclopropyl)amino)methyl)-1H-1,2,3-
triazol-1-yl)methyl)benzamide TFA salt
NH NN 410 0
= HN
'OH
The compound was synthesized using the 1-41 following the procedure for
Example 2.
11-1NMR (400 MHz, DMSO-d6): 6 11.19 (bs, 1H), 9.39 (bs, 2H), 9.03 (bs, 1H),
8.21 (s,
1H), 7.73 (d, 2H, J=7.6 Hz), 7.35 (d, 2H, J=8 Hz), 7.32-7.24 (m, 2H), 7.22-
7.16 (m,
1H), 7.12-7.08 (m, 2H), 5.68 (s, 2H), 4.41 (s, 2H), 3.01-2.95 (m, 1H), 2.41-
2.34 (m,
1H), 1.43-1.36 (m, 1H), 1.32-1.22 (m, 1H). LC-MS m/z calcd for C201-121N502,
363.2;
found 364.2 [M+H]t HPLC purity 99.8 %.
Example 42 N-hydroxy-4-(2-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-
l-yl)ethyl)benzamide TFA salt
A
= so
'OH
0
- 238 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using the 1-42 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.17 (bs, 1H), 9.69 (bs, 1H), 9.11 (bs, 2H),
7.72
(d, 2H, J=7.6 Hz), 7.36-7.26 (m, 4H), 7.24-7.20 (m, 1H), 7.15 (d, 2H, J=7.2
Hz),
3.70-3.55 (m, 2H), 3.40-3.15 (m, 3H), 3.10-2.90 (m, 7H), 2.05-1.92 (m, 2H),
1.90-
1.78 (m, 1H), 1.55-1.42 (m, 3H), 1.35-1.25 (m, 1H). LC-MS m/z calcd for
C24H31N302, 393.5; found 394.5 [M+H]t HPLC purity 97 %
Example 43 N-hydroxy-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-
l-yl)propyl)benzamide TFA salt
0
NON
N,OH
The compound was synthesized using the 1-43 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 7.66 (d, 2H, J=8.4 Hz), 7.30-7.26 (t, 4H, J=7.6
Hz),
7.22-7.20 (t, 1H, J=6.8 Hz), 7.15 (d, 2H, J=7.6 Hz), 3.53 (m, 2H), 3.15-2.97
(m, 4H),
2.97-2.92 (m, 1H), 2.92-2.80 (m, 2H), 2.69-2.60 (m, 2H), 2.45-2.38 (m, 1H),
2.00-
1.88 (m, 5H), 1.46-1.35 (m, 3H), 1.30-1.22 (m, 1H). LC-MS m/z calcd for
C25H33N302, 407.2; found 408.3 [M+H]t HPLC purity 99 %.
Example 44 N-
hydroxy-4-(3-(4-((2-phenylcyclopropyl)amino)piperidin-l-
yl)propyl)benzamide TFA salt
A
N,
OH
0
The compound was synthesized using the 1-44 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.13 (bs, 1H), 9.45 (bs, 1H), 9.26 (bs, 2H),
7.69
(d, 2H, J= 7.6 Hz), 7.33-7.26 (m, 4H), 7.23-7.21 (m, 1H), 7.19-7.15 (m, 2H),
3.62-
3.54 (m, 3H), 3.50-3.41 (m, 2H), 3.35-3.28 (m, 1H), 3.06-2.91 (m, 5H), 2.70-
2.61 (m,
1H), 2.25-2.18 (m, 2H), 1.96-1.91 (m, 2H), 1.83-1.72 (m, 1H), 1.48-1.41 (m,
1H),
1.34-1.30 (m, 1H). LC-MS m/z calcd for C24H31N302, 393.2; found 394.2 [M+H]t
HPLC purity 99.6 %.
Example 45 N-hydroxy-4-(3-(4-((methyl (2-phenylcyclopropyl) amino) methyl)
piperidin-1-y1) propyl) benzamide TFA salt
- 239 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
A
NON
N,OH
To a stirred solution of example 43(0.05 g, 0.12 mmol) in methanol (5 mL) was
added
paraformaldehyde (0.007 g, 0.24 mmol) andTEA (0.037 g, 0.36 mmol) and continue
stirred for 1 h at room temperature, sodium borohydride (0.09 g, 0.245 mmol)
were
added and continue stirred for 30 minute. Reaction mixture was quenched with
water
(20 mL) and extracted with dichloromethane (2 x 20 mL). The organic portion
was
washed with water,brine, dried over sodium sulphate and concentrated under
reduced
pressure to afford the crude product compound which was purified by reverse-
phase
HPLCusing Chemsil C18 (250mm X 4.6mm X 5mic) column with 0.1% TFA in
water:ACN to afford(0.02 g, 20%).
itINMR (400 MHz, DMSO-d6): 6 11.16 (s, 1H), 9.35 (bs, 1H), 7.69 (d, J=8.4 Hz,
2H),
7.30-7.10 (m, 7H), 3.53-3.50 (m, 3H), 3.28-2.76 (m, 10H), 2.67-2.63 (m, 3H),
2.04-
1.83 (m, 4H), 1.48-1.28 (m, 4H). LC-MS m/z calcd for C26H35N302, 421.3; found
422.5 [M+H]t HPLC purity 99.5%.
Example 46 N-hydroxy-4-
(3-(64(2-phenylcyclopropyl)amino)-2-
azaspiro[3.3]heptan-2-1)propyl)benzamide TFA salt
0
,OH
1107
* ENL 'ON
The compound was synthesized using the 1-46 following the procedure for
Example
48. itINMR (400 MHz, DMSO-d6): 6 11.12 (bs, 1H), 9.97 (bs, 1H), 9.35 (bs, 1H),
9.26 (bs, 1H), 7.68 (d, 2H, J=8 Hz), 7.32-7.24 (m, 4H), 7.23-7.19 (m, 1H),
7.14 (d,
2H, J=7.2 Hz), 4.25-4.19 (m, 2H), 4.14-3.98 (m, 3H), 3.82-3.73 (m, 2H), 3.14-
3.02
(m, 2H), 2.89-2.78 (m, 1H), 2.69-2.60 (m, 3H), 2.94-2.84 (m, 2H), 1.79-1.70
(m, 2H),
1.43-1.35 (m, 1H), 1.30-1.26 (m, 1H). LC-MS m/z calcd for C25H31N302, 405.5;
found 406.5 [M+H]t HPLC purity 99.5 %.
Example 47 4-[3-(44[2-(4-Fluoro-phenyl)-cyclopropylamino]-methyll-piperidin-
1-y1)-propyli-N-hydroxy-benzamide TFA salt
0
_OH
- 240 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using the 1-47 following the procedure for
Example 2.
itINTMR (400 MHz, DMSO-d6): 6 11.14 (bs, 1H), 9.27 (bs, 1H), 8.96 (bs, 3H),
7.70
(d, 2H, J=8 Hz), 7.30 (d, 2H, J=8 Hz), 7.26-7.19 (m, 2H), 7.16-7.10 (m, 2H),
3.58-
3.46 (m, 2H), 3.08-2.98 (m, 4H), 2.96-2.82 (m, 3H), 2.69-2.63 (m, 2H), 2.00-
1.88 (m,
6H), 1.49-1.35 (m, 3H), 1.31-1.24 (m, 1H). LC-MS m/z calcd for C25H32FN302,
425.3; found 426.5 [M+H]t HPLC purity 97.1 %
Example 48 4-(3-(3-4(2-(4-fluorophenyl)cyclopropyl)amino)methypazetidin-1-
yl)propy1)-N-hydroxy benzamide TFA salt
0
A
N,OH
r\INI
TFA Salt
To a solution of hydroxylamine hydrochloride (0.38 g, 5.33 mmol) in methanol
was
added a solution of potassium hydroxide (0.3 g, 5.33 mmol) in methanol at 5-10
C
and stirred at that temperature for 15 min. The formed precipitate was
filtered through
cotton plug and the filtrate was added to a solution of ethyl 4-(3-(3-((2,2,2-
trifluoro-N-
(2-(4-fluorophenyl)cyclopropyl)acetamido)methyl)azetidin-l-yl)prop yl)benzo
ate (I-
48, 0.15 g, 0.3 mmol) in methanol (4 mL) at room temperature. Potassium
hydroxide
(0.3 g, 5.33 mmol) was added and the resulting mixture was stirred at room
temperature for 1 h. The solvent was removed and ice-water was added to the
resulting residue. The pH of the aqueous portion was adjusted to 7.0 with 10 %
acetic
acid solution. The crude product was extracted with dichloromethane (30 mL x
3).
The combined organic layer was washed with water, brine, dried over sodium
sulphate
and concentrated under reduced pressure to get the crude product which was
mixed
with dichloromethane (5 mL) and trifluoroacetic acid (0.5 mL) was added at 0
C. The
reaction mixture was stirred for 10 min at same temperature. The reaction
mixture was
concentrated under vacuum to get crude TFA salt of product which was purified
by
reverse-phase HPLC using Chemsil C18 (250mm X 4.6mm X 5mic) column with 0.1%
TFA in water:ACN to afford the pure product as colourless solid (0.04 g, 34 %
yield).
LC-MS m/z calcd for C23H28FN302, 397.5; found 398.5 [M+H]t
Example 49 4-(3-(4-4(2-(3-fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-
yl)propy1)-N-hydroxybenzamide TFA salt
N-
OH
ri'ON
A
- 241 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using the 1-49 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.14 (bs, 1H), 9.38 (bs, 1H), 9.07 (bs, 3H),
7.69
(d, 2H, J=8 Hz), 7.36-7.28 (m, 3H), 7.04-7.00 (m, 3H), 3.54-3.50 (m, 2H), 3.28-
3.17
(m, 1H), 3.10-2.98 (m, 5H), 2.93-2.82 (m, 2H), 2.70-2.61 (m, 2H), 2.00-1.85
(m, 5H),
1.53-1.30 (m, 3H). LC-MS m/z calcd for C25H32FN302, 425.3; found 426.5
[M+H] .HPLC purity 99.4 %.
Example 50 4-(3-(4-(42-(3,4-difluorophenyl)cyclopropyl)amino)methyl)piperidin-
1-y1)propyl)-N-hydroxybenzamide TFA salt
0
N-OH
The compound was synthesized using the 1-50 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.14 (bs, 1H), 9.06 (bs, 1H), 8.94 (bs, 1H),
7.70(d,
2H, J = 8Hz), 7.40-7.24 (m, 4H), 7.09-7.03 (m, 1H), 3.58-3.48 (m, 3H),3.30-
3.12 (m,
1H), 3.08-2.97 (m, 5H), 2.93-2.82 (m, 2H), 2.69-2.64 (m, 2H), 1.98-1.86 (m,
5H),
1.48-1.30 (m, 3H). LC-MS m/z calcdforC25H31F2N302, 443.2, found 444.5 [M+H]t
HPLC purity 99.6 %.
Example 51 N-
hydroxy-4-(3-(4-(((2-(4-
methoxyphenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA
salt
0
N_OH
0
The compound was synthesized using the 1-51 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.13 (bs, 1H), 9.35 (bs, 1H), 8.98 (bs, 1H),
7.69
(d, 2H, J=7.6 Hz), 7.29 (d, 2H, J=8.4 Hz), 7.08 (d, 2H, J=8.4 Hz), 6.84 (d,
2H, J=8.4
Hz), 3.70 (s, 3H), 3.53-3.50 (m, 2H), 3.07-3.01(m, 4H), 2.91-2.86 (m, 3H),
2.67-2.53
(m, 3H), 2.00-1.92 (m, 5H), 1.40-1.38 (m, 3H), 1.22-1.17 (m, 1H). LC-MS m/z
calcd
for C26H35N303 [M+Hr438.2, found438.3. HPLC purity 99.5 %.
Example 52 4-
(3-(4-(((2-(4-((4-
fluorobenzypoxy)phenyl)cyclopropyl)amino)methyl)piperidin-1-y1)propy1)-N-
hydroxybenzamide TFA salt
- 242 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
F
N-
OH
0
la
The compound was synthesized using the 1-52 following the procedure for
Example 2.
ifINMR (400 MHz, DMSO-d6): 6 11.14 (bs, 1H), 9.10 (bs, 1H), 8.96 (bs, 1H),
8.82
(bs, 2H), 7.71 (d, 2H, J=7.6 Hz), 7.48-7.42 (m, 2H), 7.31 (d, 2H, J=8 Hz),
7.20 (t, 2H,
J=8.8 Hz), 7.09 (d, 2H, J=8.4 Hz), 6.93 (d, 2H, J=8.4 Hz), 5.01 (s, 2H), 3.58-
3.50 (m,
2H), 3.06-2.97 (m, 4H), 2.92-2.85 (m, 4H), 2.70-2.62 (m, 2H), 2.40-2.35 (m,
1H),
2.00-1.88 (m, 5H), 1.45-1.34 (m, 3H). LC-MS m/z calcd for C32H38FN303, 531.3;
found 532.4 [M+H]t HPLC purity 99.9 %.
Example53 N-
hydroxy-4-(3-(4-(((2-(4-(morpholine-4-
carbonyl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide
TFA salt
0
0"1 N_OH
0
TFA salt
The compound was synthesized using the 1-54 following the procedure for
Example 2.
ifINMR (400 MHz, DMSO-d6): 6 11.07 (bs, 1H), 9.41 (bs, 1H), 9.27 (bs, 2H),
7.69
(d, 2H, J=8Hz), 7.36-7.29 (m, 3H), 7.27-7.22 (m, 3H), 3.65-3.45 (m, 7H), 3.41-
3.25
(m, 3H), 3.10-2.98 (m, 5H), 2.96-2.85 (m, 2H), 2.70-2.61 (m, 3H), 2.01-1.85
(m, 5H),
1.78-1.70 (m, 1H), 1.52-1.48 (m, 1H), 1.45-1.31 (m, 2H).LC-MS m/z calcd for
C30H40N404, 520.3; found 521.3 [M+H]; HPLC purity 99.6%.
Example54 N-
hydroxy-4-(3-(4-(((2-(4-(piperidine-1-
carbonyl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide
TFA salt
0
NON N¨OH
0
TFA
The compound was synthesized using the 1-55 following the procedure for
Example 2.
- 243 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
11-1N1MR (400 MHz, DMSO-d6): 6 11.14 (bs, 1H), 9.31 (bs, 1H), 9.15 (bs, 3H),
7.70(d,
2H, J = 8.4Hz), 7.32-7.26 (m, 4H), 7.24-7.20 (m, 2H), 3.56-3.50 (m, 6H), 3.27-
3.26
(m, 2H), 3.05-2.85 (m, 5H),2.93-2.83 (m, 2H), 2.68-2.63 (m, 2H), 2.00-1.90 (m,
5H),
1.62-1.57 (m, 2H), 1.53-1.32 (m, 7H). LC-MS m/z calcdfor C31H42N403, 518.3,
found
519.3 [M+H]t HPLC purity 99.4 %.
Example 55 N-
(2-(dimethylamino)ethyl)-4-(2-0(1-(3-(4-
(hydroxycarbamoyl)phenyl)propyl)piperidin-4-
yl)methypamino)cyclopropyl)benzamide TFA Salt
0
N_OH
0
The compound was synthesized using the 1-53 following the procedure for
Example 2.
11-1N1MR (400 MHz, DMSO-d6): 6 11.15 (bs, 1H), 9.51 (bs, 1H), 9.43 (bs, 1H),
9.16
(bs, 2H), 8.65 (bs, 1H), 7.79 (d, 2H, J= 8.4 Hz), 7.69 (d, 1H, J= 8.0 Hz),
7.62 (d, 1H,
J= 8.0 Hz), 7.30-7.23 (m, 4H),3.50-3.48 (m, 4H),3.28-3.21 (m, 2H),3.08-2.98
(m,
5H),2.90-2.81 (m, 8H),2.69-2.60 (m, 3H),2.00-1.82 (m, 5H),1.57-1.50 (m,
1H),1.46-
1.30 (m, 3H). LC-MS m/z calcd for C30H43N503, 521.3; found 522.2 [M+H]t HPLC
purity 99.97 %.
Example56 4-
(3-(4-0(2-(4'-chloro-[1,1'-bipheny1]-4-
yl)cyclopropyl)amino)methyl)piperidin-1-yl)propy1)-N-hydroxybenzamide TFA
salt
0
N_OH
CI
The compound was synthesized using the 1-56 following the procedure for
Example 2.
11-1N1MR (400 MHz, DMSO-d6): 6 11.13 (bs, 1H), 9.39 (bs, 1H), 9.08 (bs, 2H),
7.70-
7.64 (m, 4H), 7.59 (d, 2H, J=8.4 Hz), 7.48 (d, 2H, J=8.4 Hz), 7.30-7.25 (m,
4H), 3.53-
3.43 (m, 3H), 3.08-2.97 (m, 5H), 2.89-2.86 (m, 2H), 2.68-2.65 (m, 2H), 2.01-
1.90 (m,
5H), 1.52-1.32 (m, 4H). LC-MS m/z calcd for C31H36C1N302, 518.2; found 518.2
[M+H]t HPLC purity 99.8 %.
- 244 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Example 57 4-
(3-(4-(42-(4'-fluoro-[1,1'-biphenyl[-4-
yl)cyclopropyl)amino)methyl)piperidin-1-y1)propyl)-N-hydroxybenzamide TFA
salt
0
N N_OH
CN
TFA salt
The compound was synthesized using the 1-57 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.13 (bs, 1H), 9.14 (bs, 1H), 8.92 (bs, 2H),
7.75-
7.64 (m, 4H), 7.57 (d, J =8.1 Hz, 2H), 7.35- 7.20 (m, 6H), 3.6-3.5 (m, 2H),
3.10-2.96
(m, 5H), 2.94-2.82 (m, 2H), 2.72-2.62 (m, 3H), 2.02-1.86 (m, 5H), 1.54-1.44
(m, 1H),
1.44-1.28 (m, 3H). LC-MS m/z calcd for C31H36FN302, 501.3; found 502.3 [M+H]t
HPLC purity 99.7 %.
Example 58 4-
(3-(3-(42-(4'-fluoro-[1,1'-biphenyl[-4-
yl)cyclopropyl)amino)methypazetidin-l-y1) propy1)-N-hydroxybenzamide TFA
salt
0
N-OH
NCN
TFA salt
The compound was synthesized using the 1-58 following the procedure for
Example
48. itINMR (400 MHz, DMSO-d6): 6 11.13 (bs, 1H), 10.05 (bs, 1H), 9.17 (bs,
2H),
9.00 (bs, 1H), 7.70-7.64 (m, 4H), 7.57 (d, 2H, J=8.0 Hz), 7.28-7.24 (m, 6H),
4.25-4.15
(m, 1H), 4.14-4.10 (m, 2H), 3.92-3.81 (m, 2H), 3.48-3.31 (m, 2H), 3.11-3.07
(m, 3H),
2.65-2.61 (m, 2H), 2.48-2.43 (m, 1H), 1.75-1.73 (m, 2H), 1.49-1.41 (m, 1H),
1.39-
1.31 (m, 1H). LC-MS m/z calcd for C29H32FN302, 473.5; found 474.5 [M+H]t HPLC
purity 99.8 %.
Example 59 4-
(3-(4-(42-(4'-cyano-[1,1'-biphenyl[-4-
yl)cyclopropyl)amino)methyl)piperidin-1-yl)propy1)-N-hydroxybenzamide TFA
salt
0
N NHOH
NC
TFA Salt
The compound was synthesized using the 1-59 following the procedure for
Example 2.
- 245 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
itINMR (400 MHz, DMSO-d6): 6 11.13 (bs, 1H), 9.12 (bs, 1H), 8.92 (bs, 2H),
7.97-
7.93 (m, 3H), 7.72-7.66 (m, 5H), 7.35-7.24 (m, 4H), 3.70-3.45 (m, 4H), 3.18-
2.98 (m,
4H), 3.30-3.16 (bs, 2H), 3.08-2.98 (bs, 4H), 2.95-2.82 (m, 2H), 2.70-2.62 (m,
2H),
2.02-1.90 (m, 4H), 1.55-1.30 (m, 3H). LC-MS m/z calcd for C32H36N402, 508.3;
found 527.3[M+H+17] . HPLC purity 99.8 %.
Example 60 N-hydroxy-4-(3-(4-(((2-(4-(1-methy1-2-oxo-1,2-dihydropyridin-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
0
NO N-OH
N1/
TFA
The compound was synthesized using the 1-60 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.14 (bs, 1H), 9.21 (bs, 1H), 8.95 (bs, 3H),
8.07 (s,
1H), 7.81-7.76 (m, 1H), 7.69 (d, J =8.0Hz, 2H), 7.50 (d, J =8.0Hz, 2H),7.30
(d, J
=7.6Hz, 2H), 7.21 (d, J =7.6Hz, 2H), 6.49 (d, J =9.6Hz, 1H), 3.56-3.48 (m,
5H), 3.26-
3.18 (m, 1H), 3.07-2.97 (m, 5H), 2.92-2.82 (m, 2H), 2.69-2.63 (m, 3H), 2.02-
1.94 (m,
5H), 1.48-1.27 (m, 3H). LC-MS m/z calcd for C31H38N403, 514.3, found 513.3[M-
Hr. HPLC purity 99.3 %.
Example 61 N-
hydroxy-4-(3-(4-(((2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
0
N NHOH
N
I
TFA salt
The compound was synthesized using the 1-61 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.14 (s, 1H), 9.24 (bs, 1H), 9.16 (s, 1H), 9.11
(s,
2H), 8.98 (bs, 2H), 7. 75 (d, J=8.4 Hz, 2H), 7.69 (d, J=8.0 Hz, 2H), 7.34 (d,
J=8.4Hz,
2H), 7.29 (d, J=8.4 Hz, 1H), 3.52 (d, J=11.6 Hz, 2H), 3.30-3.18 (m, 1H), 3.10-
2.98
(m, 5H), 2.95-2.80 (m, 2H), 2.70-2.62 (m, 1H), 2.55 (m, 1H), 2.20-1.70 (m,
5H), 1.58-
1.48 (m, 1H), 1.46-1.32 (m, 2H). LC-MS m/z calcd for C29H35N502, 485.2; found
486.2 [M+Hr.HPLC purity 99.7%.
Example 62 N-
hydroxy-4-(3-(4-(42-(4-(1-methyl-1H-pyrazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-y1) propyl) benzamide TFA salt
- 246 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
Nr0H
¨N
TFA
The compound was synthesized using the 1-62 following the procedure for
Example 2.
11-1NMR (400 MHz, DMSO-d6): 11.13 (s, 1H), 9.17 (s, 1H), 8.91 (s, 2H), 8.07
(s, 1H),
7.80 (s, 1H), 7.70 -7.62 (d, J=8 Hz, 2H), 7.48 -7.40 (d, J=8.4 Hz, 2H), 7.35-
7.20 (d,
J=8 Hz, 2H), 7.15-7.10 (d, J=8.4 Hz, 2H), 3.83 (s, 3H), 3.54-3.51 (m, 2H),
3.24-3.19
(m, 1H), 3.02-2.95 (m, 4H), 2.89-2.86 (m, 3H), 2.66 (m, 2H), 2.48-2.30 (m,
1H), 2.05-
1.92 (m, 5H), 1.44-1.33 (m, 2H), 1.27-1.22 (m, 1H). LC-MS m/z calcd for
C29H37N502, 487.3; found 488.3[M+H]tHPLC purity 99.8%.
Example 63 N-
hydroxy-4-(3-(3-(42-(4-(1-methyl-1H-pyrazol-4-
yl)phenyl)cyclopropyl)amino)methyl)azetidin-l-yl)propyl)benzamide TFA salt
0
N,OH
TFA salt
The compound was synthesized using the 1-63 following the procedure for
Example
48. 11-1NMR (400 MHz, DMSO-d6): 6 11.13 (bs, 1H), 9.79 (bs, 1H), 8.99 (bs,
2H),
8.08 (s, 1H), 7.81 (s, 1H), 7.69 (d, 2H, J=8.0 Hz), 7.47 (d, 2H, J=8.4 Hz),
7.28-7.23
(m, 2H), 7.14 (d, 2H, J=8 Hz), 4.22-4.14 (m, 1H), 4.11-3.95 (m, 2H), 3.89-3.83
(m,
1H), 3.83 (s, 3H), 3.29-3.01 (m, 5H), 2.92-2.87 (m, 1H), 2.68-2.60 (m, 1H),
2.41-2.30
(m, 2H), 1.70-1.62 (m, 2H), 1.42-1.35 (m, 1H), 1.34-1.26 (m, 1H). LC-MS m/z
calcd
for C27H33N502, 459.6; found 460.6 [M+H]t HPLC purity 99 %.
Example64 4-
(3-(4-(((2-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propy1)-N-
hydroxybenzamide TFA salt
0
NJJ)L
N-
OH
N¨
TFA Salt
The compound was synthesized using the 1-64 following the procedure for
Example 2.
11-1NMR (400 MHz, DMSO-d6): M1.13 (bs, 1H), 9.14 (bs, 1H), 8.92 (bs, 2H),
7.69(d,
2H, J=7.6Hz), 7.32-7.28 (m, 6H), 3.54-3.51(m, 2H), 3.26-3.20 (m, 1H), 3.08-
2.98 (m,
5H), 2.92-2.85 (m,2H), 2.68-2.64 (m, 2H), 2.35 (s, 3H), 2.18 (s, 3H), 2.01-
1.90 (m,
- 247 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
4H), 1.78-1.64 (m, 1H), 1.51-1.47(m,1H), 1.40-1.32 (m, 3H). LC-MS m/z calcd
for
C30H38N403,502.3; found 503.3[M+H]t HPLC purity 99.5%.
Example 65 3-
(3-(3-(42-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)azetidin-l-yl)propy1)-N-hydroxybenzamide
TFA salt
Iraq
1µ1,/ 0
0
The compound was synthesized using the 1-65 following the procedure for
Example
48. itINMR (400 MHz, DMSO-d6): 6 11.13 (bs, 1H), 9.81 (bs, 1H), 8.97 (bs, 3H),
7.69 (d, 2H, J=8.0 Hz), 7.33-7.26 (m, 6H), 4.21-4.18 (m, 1H), 4.12-4.00 (m,
2H),
3.89-3.80 (m, 2H), 3.40-3.32 (m, 2H), 3.29-2.98 (m, 5H), 2.64-2.60 (m, 1H),
2.36 (s,
3H), 2.18 (s, 3H), 1.80-1.73 (m, 2H), 1.44-1.41 (m, 1H), 1.39-1.31 (m, 1H). LC-
MS
m/z calcd for C28H34N403, 474.6; found 475.6 [M+Hr.HPLC purity 99 %.
Example66 N-
hydroxy-4-(3-(4-(((2-(4-(6-(trifluoromethyl)pyridin-3-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
N_OH
HON
F3C N
The compound was synthesized using the 1-66 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.14 (bs, 1H), 9.18 (bs, 1H), 9.07 (s, 1H), 8.99
(bs,
2H), 8.33 (d, 1H, J=8 Hz), 7.96 (d, 1H, J=8.4 Hz), 7.77 (d, 2H, J=8 Hz),7.70
(d, 1H,
J=8 Hz), 7.62 (d, 1H, J=7.6 Hz), 7.35 (d, 2H, J=8 Hz), 7.30 (d, 1H, J=8 Hz),
7.25 (d,
1H, J=8 Hz), 3.55-3.48 (m, 2H), 3.30-3.16 (m, 1H), 3.10-2.84 (m, 2H), 2.69-
2.61 (m,
2H), 2.01-1.86 (m,5H), 1.56-1.50 (m,1H), 1.45-1.32 (m, 3H). LC-MS m/z calcd
for
C31H35F3N402, 552.3; found 553.3 [M+H]t HPLC purity 99.1 %.
Example 67 N-
hydroxy-4-(3-(4-(42-(1-isopropy1-1H-pyrazol-4-
yl)cyclopropyl)amino)methyl)piperidin-1-y1)propyl)benzamide TFA salt
N_OH
The compound was synthesized using the 1-67 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.14 (bs, 1H), 9.10 (bs, 1H), 8.78 (bs, 2H),
7.70
(d, 2H, J=8 Hz), 7.61 (s, 1H), 7.31-7.28 (m, 3H), 4.40-4.36 (m, 1H), 3.54-3.50
(m,
- 248 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
2H), 3.30-3.11 (m, 1H), 3.07-2.98 (m, 4H), 2.92-2.76 (m, 4H), 2.24-1.98 (m,
2H),
1.97-1.90 (m, 5H) 1.41-1.30 (m, 8H), 1.10-1.05 (m, 1H). LC-MS m/z calcd for
C25H37N502, 439.6; found 440.6 [M+H]t HPLC purity 99.2 %.
Example 68 N-
hydroxy-4-(3-(4-(42-(1-phenyl-1H-pyrazol-4-
yl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
N,oH
N
The compound was synthesized using the 1-68 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.14 (bs, 1H), 8.88 (bs, 3H), 8.36 (s, 1H), 7.75-
7.65 (m, 5H), 7.49-7.45 (m, 2H), 7.31-7.26 (m, 3H), 3.55-3.51 (m, 2H), 3.08-
2.98 (m,
4H), 2.95-2.84 (m, 3H), 2.71-2.63 (m, 2H), 2.00-1.89 (m, 6H), 1.45-1.34 (m,
3H),
1.25-1.19 (m, 1H). LC-MS m/z calcd for C28H35N502, 473.3; found 474.3
[M+H] .HPLC purity 99.5 %.
Example 69 N-
hydroxy-4-(3-(4-(((2-(2-methylthiazol-5-
yl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
41
N'OH/All.õõ,11,1
The compound was synthesized using the 1-69 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.10 (bs, 1H), 9.36 (bs, 1H), 9.10 (bs, 2H),
7.69
(d, 2H, J=8.4 Hz), 7.42 (s, 1H), 7.29 (d, 2H, J=7.6 Hz), 3.56-3.48 (m, 2H),
3.30-3.16
(m, 1H), 3.06-2.96 (m, 4H), 2.94-2.82 (m, 2H), 2.70-2.64 (m, 2H), 2.62-2.58
(m, 1H),
2.58 (s, 3H), 2.00-1.86 (m, 5H) 1.56-1.46 (m, 1H), 1.42-1.32 (m, 2H), 1.32-
1.26 (m,
1H). LC-MS m/z calcd for C23H32N402S, 428.5; found 429.5 [M+H]t
Example 70 N-
hydroxy-4-(3-(4-(((2-(pyridin-3-
yl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
N
NOH
(75A
The compound was synthesized using the 1-70 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.13 (bs, 1H), 9.35 (bs, 1H), 9.11 (bs, 2H),
8.57 (s,
1H), 8.51 (d, 1H, J=4.8 Hz), 7.75-7.67 (m, 3H), 7.49-7.45 (m, 1H), 7.29 (d,
2H, J=8.4
Hz), 3.59-3.53 (m, 2H), 3.08-2.98 (m, 5H), 2.94-2.82 (m, 2H), 2.73-2.67 (m,
2H), 2.02-
- 249 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1.91 (m, 5H), 1.78-1.71 (m, 1H), 1.55-1.51 (m, 1H), 1.45-1.38 (m, 3H). LC-MS
m/z
calcd for C24H32N402, 408.3; found 409.3 [M+H]t
Example 71 N-
hydroxy-4-(3-(2-(((2-(4-
methoxyphenyl)cyclopropyl)amino)methyl)-5,6-dihydroimidazo [1,2-a] pyrazin-
7(8H)-yl)propyl)benzamide TFA salt
N_OH
N/H
-0
The compound was synthesized using the 1-71 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.18 (bs, 1H), 9.31 (bs, 2H), 7.69 (d, 2H, J=7.6
Hz), 7.31-7.27 (m, 3H), 7.02 (d, 2H, J=9.2 Hz), 6.83 (d, 2H, J=8.4 Hz), 4.40-
4.29 (m,
2H), 4.23-4.11 (m, 4H), 3.69 (s, 3H), 3.60-3.50 (m, 2H) 3.16-3.08 (m, 2H),
2.88-2.76
(m, 1H) 2.71-2.65 (m, 2H), 2.32-2.24 (m, 1H), 2.08-1.98 (m, 2H), 1.36-1.31 (m,
1H),
1.22-1.14 (m, 1H). LC-MS m/z calcd for C27H33N503, 475.3; found 474.5 [M-
H] .HPLC purity 99.2 %.
Example 72 4-(3-(2-(42-
(4-fluorophenyl)cyclopropyl)amino)methyl)-5,6-
dihydroimidazo[1,2-a]pyrazin-7(8H)-y1)propyl)-N-hydroxybenzamide TFA salt
0
OH
NH N
The compound was synthesized using the 1-72 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.12 (s, 1H), 9.27 (bs, 2H), 7.68 (d, 2H, J=8.0
Hz),
7.29 (d, 2H, J=7.6 Hz), 7.24 (s, 1H), 7.13-7.07 (m, 4H), 4.16-4.12 (m, 2H),
3.89 (bs,
4H), 3.38-3.31 (m, 2H), 2.85 (s, 1H), 2.67 (t, 2H, J=7.2 Hz), 2.31 (d, 1H,
J=6.0 Hz),
1.96 (bs, 2H), 1.37 (t, 1H, J=4.4 Hz), 1.24 (s, 1H), 1.21 (s, 2H). LC-MS m/z
calcd for
C26H30FN502, 463.2; found 464.3 [M+H]t HPLC purity 99.6 %.
Example 73 4-(3-(4-(42-(3,4-difluorophenyl)cyclopropyl)amino)methyl)-1H-
imidazol-1-yl)propy1)-N-hydroxybenzamide TFA salt
- 250 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
FHNH
N-OH
0
The compound was synthesized using the 1-73 following the procedure for
Example 2.
11-1NMR (400 MHz, DMSO-d6): 6 11.12 (bs, 1H), 9.20 (bs, 2H), 8.05 (s, 1H),
7.67 (d,
1H, J=8.0 Hz), 7.60 (d, 1H, J=7.6 Hz), 7.38-7.17 (m, 5H),7.00-6.96 (m, 1H),
4.19 (s,
2H),4.05-3.92 (m, 2H),2.93-2.89 (m, 1H),2.58-2.50 (m, 2H),2.35-2.30 (m,
1H),2.07-
1.98 (m, 2H), 1.43-1.34 (m, 1H), 1.32-1.24 (m, 1H). LC-MS m/z calcd for
C23H24F2N402, 426.2; found 427.2 [M+H]t HPLC purity 99.4 %.
Example 74 N-
hydroxy-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)-1H-
imidazol-1-yl)propyl)benzamide TFA salt
NI-L(3
TFA
0
HN
\OH
The compound was synthesized using the 1-74 following the procedure for
Example 2.
11-1NMR (400 MHz, DMSO-d6): 6 11.13 (bs, 1H), 8.99 (s, 1H), 7.68 (d, 2H, J=8.0
Hz),7.57 (s, 1H), 7.31-7.24 (m, 4H), 7.22-7.18 (m, 1H), 7.08 (d, 2H, J=7.6
Hz),4.37
(s, 2H),4.20-4.16 (m, 2H),2.92-2.85 (m, 1H), 2.68-2.60 (m, 2H),2.28-2.21 (m,
1H),
2.12-2.04 (m, 2H), 1.38-1.30 (m, 1H), 1.25-1.18 (m, 1H). LC-MS m/z calcd for
C23H26N402, 390.2; found 391.2 [M+H]t HPLC purity 99.0 %.
Example 75 N-hydroxy-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)-1H-
imidazol-1-yl)propyl) benzamide TFA salt
TFA OH
0
The compound was synthesized using the 1-75 following the procedure for
Example 2.
11-1NMR (400 MHz, DMSO-d6): 6 11.14 (bs, 1H), 9.20 (bs, 2H), 8.31 (s, 1H),
7.67 (d,
2H, J=7.6 Hz), 7.46 (s, 1H), 7.27-7.23 (m, 4H), 7.19-7.15 (m, 1H), 7.08 (d,
2H, J=7.2
Hz), 4.30-4.23 (m, 2H), 4.08-4.00 (m, 2H), 2.94-2.90 (m, 1H), 2.59-2.55 (m,
2H),
-251 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
2.36-2.31 (m, 1H), 2.05-1.98 (m, 2H), 1.42-1.36 (m, 1H) 1.27-1.22 (m, 1H). LC-
MS
m/z calcd for C23H26N402, 390.2; found 391.2 [M+Hr.HPLC purity 99.7 %.
Example 76 N-
hydroxy-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)-1H-
pyrazol-1-yl)propyl)benzamide TFA salt
rv/CN
* H ¨N
0
The compound was synthesized using the 1-76 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.11 (bs, 1H), 9.03 (bs, 3H), 7.77 (s, 1H), 7.67
(d,
2H, J=8.4 Hz), 7.52 (s, 1H), 7.29-7.16 (m, 5H), 7.12 (d, 2H, J=7.6 Hz), 4.20-
4.17 (m,
2H), 4.07 (t, 2H, J=6.8 Hz), 2.93-2.87 (m, 1H), 2.65-2.53 (m, 2H), 2.37-2.32
(m, 1H),
2.06-1.99 (m, 2H), 1.43-1.37 (m, 1H) 1.31-1.26 (m, 1H). LC-MS m/z calcd for
C23H26N402, 390.2; found 391.2 [M+Hr.HPLC purity 99.7 %.
Example 77 N-hydroxy-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)-1H-1,2,3-
triazol-1-yl)propyl)benzamide TFA salt
1.1'eN
H N=N
OH
The compound was synthesized using the 1-77 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.12 (bs, 1H), 9.44 (bs, 2H), 8.99 (bs, 1H),
8.16 (s,
1H), 7.68 (d, 2H, J=8.4 Hz), 7.30-7.24 (m, 4H), 7.20-7.16 (m, 1H), 7.11-7.09
(m, 2H),
4.45 (s, 2H), 4.42-4.36 (m, 2H), 2.98-2.95 (m, 1H), 2.60-2.53 (m, 2H), 2.38-
2.33 (m,
1H), 2.13-2.05 (m, 2H), 1.43-1.38 (m, 1H) 1.29-1.24 (m, 1H). LC-MS m/z calcd
for
C22H25N502, 391.4; found 392.4 [M+Hr.HPLC purity 99.6 %.
Example78 4-
(3-(6-(42-(4-fluorophenyl)cyclopropyl)amino)methyl)-3,4-
dihydroisoquinolin-2(1H)-yl)propy1)-N-hydroxybenzamide TFA salt
0
NI-OH
The compound was synthesized using the 1-78 following the procedure for
Example 2.
- 252 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
itINMR (400 MHz, DMSO-d6): 6 11.14 (bs, 1H), 9.91 (bs, 1H), 9.37 (bs, 1H),
9.21
(bs, 1H), 8.95 (bs, 1H), 7.73-7.68 (m, 2H), 7.39-7.28 (m, 4H), 7.24-7.18 (m,
1H),
7.16-7.08 (m, 4H), 4.96-4.77 (m, 1H), 4.32-4.22 (m, 1H), 4.05-3.94 (m, 1H),
3.92-
3.84 (m, 1H), 3.78-3.66 (m, 1H), 3.35-3.28 (m, 2H), 3.26-3.27 (m, 2H), 3.07-
2.93 (m,
1H), 2.90-2.81 (m, 1H), 2.78-2.68 (m, 2H), 2.28-2.21 (m, 1H), 2.10-2.03 (m,
1H),
1.45-1.36 (m, 1H), 1.32-1.26 (m, 2H). LC-MS rniz calcd for C29H32FN302, 473.2;
found 474.2 [M+H]t HPLC purity 96.9 %.
Example79 4-
07-(02-(4-fluorophenyl)cyclopropyl)amino)methyl)-3,4-
dihydroisoquinolin-2(1H)-yl)methyl)-N-hydroxybenzamide TFA salt
N
NH
'OH
0
The compound was synthesized using the 1-79 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.28 (bs, 1H), 10.4 (bs, 1H), 9.30 (bs, 1H),
9.19
(bs, 1H), 7.86-7.77 (m, 2H), 7.63-7.56 (m, 2H), 7.37-7.32 (m, 1H), 7.27-7.20
(m, 2H),
7.11-7.09 (m, 4H), 4.56-4.46 (m, 2H), 4.28-4.20 (m, 4H), 3.75-3.66 (m, 2H),
3.13-
3.04 (m, 2H), 2.84-2.80 (m, 1H), 2.34-2.30 (m, 1H), 1.41-1.34 (m, 1H), 1.31-
1.23 (m,
1H). LC-MS rniz calcd for C27H28FN302, 445.2; found 446.1 [M+H]t HPLC purity
97.0 %.
Example 80 4-
02-(02-(4-fluorophenyl)cyclopropyl)amino)methyl)-5,6-
dihydroimidazo[1,2-a]pyrazin-7(8H)-y1)methyl)-N-hydroxybenzamide TFA salt
0
e N_OH N
NH
The compound was synthesized using the 1-80 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.17 (s, 1H), 9.14 (bs, 1H), 7.75-7.67 (m, 2H),
7.44-7.39 (m, 2H), 7.22-7.18 (m, 1H), 7.13-7.05 (m, 4H), 4.21-3.71 (m, 8H),
3.09-
2.97 (m, 1H), 2.96-2.89 (m, 1H), 2.86-2.81 (m, 1H), 2.32-2.26 (m, 1H), 1.41-
1.39 (m,
1H), 1.26-1.19 (m, 2H). LC-MS rniz calcd for C24H26FN502, 436.1; found 436.2
[M+H]t HPLC purity 99.8 %.
- 253 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Example 81 N-
hydroxy-4-(3-(4(((2-(1,3,3,-trimethy1-2-oxoindoline-5-
yl)cyclopropyl)amino)methyl)piperidine-1-yl)propyl)benzamide TFA salt
0
N_OH
0 NN
The compound was synthesized using the 1-81 following the procedure for
Example 2.
iHNMR (400 MHz, DMSO-d6): 6 11.16 (bs, 1H), 9.30 (bs, 1H), 8.98 (bs, 3H), 7.69
(d, 2H, J=8Hz), 7.29 (d, 2H, J=7.6 Hz), 7.16 (s, 1H), 7.06 (d, 1H, J=8Hz),
6.92 (d,
1H, J=8Hz), 3.54-3.50 (m, 2H), 3.09 (s, 3H), 3.07-2.98 (m, 3H), 2.95-2.82 (m,
3H),
2.68-2.63 (m, 2H), 2.46-2.38 (m, 2H), 1.98-1.91 (m, 5H), 1.46-1.36 (m. 3H),
1.29-
1.20 (m. 7H). LC-MS m/z calcd for C301L0N403, 504.3; found 505.5 [M+H]t HPLC
purity 99.8 %.
Example 82 N-
hydroxy-4-(3-oxo-3-(4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
0
A
N N_OH
1101
0
The compound was synthesized using the 1-82 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.09 (s, 1H), 8.90 (bs, 1H), 8.72 (bs, 2H), 7.64
(d,
2H, J=8.4Hz), 7.32-7.25 (m, 4H), 7.22-7.19 (m, 1H), 7.19-7.14 (m, 2H), 4.38-
4.32 (m,
1H), 3.89-3.83 (m, 1H), 3.00-2.81 (m, 4H), 2.83-2.79 (m, 2H), 2.64-2.61 (m,
3H),
2.59-2.40 (m, 2H), 1.90-1.80 (m, 1H), 1.73-1.66 (m, 2H), 1.46-1.40 (m, 1H),
1.30-
1.25 (m, 1H), 1.08-0.95 (m, 1H). LC-MS m/z calcd for C25H31N303, 421.2; found
422.3 [M+H]t HPLC purity 99.5 %.
Example 83 N-hydroxy-4-(3-oxo-3-(4-((2-phenylcyclopropyl)amino)piperidin-l-
yl)propyl)benzamide TFA salt
0
jal
0
The compound was synthesized using the 1-83 following the procedure for
Example 2.
- 254 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
11-1NMR (400 MHz, DMSO-d6): 6 11.1 (bs, 1H), 8.95 (bs, 2H), 7.64 (d, 2H, J=
8.4Hz),
7.32-7.26 (m, 4H), 7.23-7.20 (m, 1H), 7.19-7.15 (m, 2H), 4.46-4.39 (m, 1H),
4.01-
3.92 (m, 2H), 3.55-3.40 (m, 1H), 3.04-2.90 (m, 2H), 2.88-2.78 (m, 2H), 2.69-
2.61 (m,
2H), 2.59-2.52 (m, 1H), 2.41-2.31 (m, 1H) 2.08-1.95 (m, 2H), 1.45-1.31 (m,
4H). LC-
MS m/z calcd for C24H29N303, 407.2; found 408.2 [M+H]t HPLC purity 99.7 %.
Example84 N-
hydroxy-4-(2-oxo-2-(4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)ethyl)benzamide TFA salt
II H
0 N,
OH
0
The compound was synthesized using the 1-84 following the procedure for
Example 2.
11-1NMR (400 MHz, DMSO-d6): 6 11.13 (bs, 1H), 8.77 (bs, 2H), 7.66 (d, 2H,
J=8.4Hz), 7.34-7.23 (m, 4H), 7.22-7.13 (m, 3H), 4.36-4.33 (m, 1H), 3.97-3.91
(m,
1H), 3.74 (s,2H), 3.04-2.92 (m, 4H), 2.61-2.52 (m, 1H), 2.41-2.40 (m, 1H),
1.92-1.83
(m, 1H), 1.75-1.63 (m, 2H), 1.46-1.40 (m, 1H), 1.30-1.24 (m, 1H), 1.10-0.92
(m, 2H).
LC-MS m/z calcd for C24H29N303, 407.2; found 408.2 [M+1] . HPLC purity 99.8 %.
Example 84 A N-hydroxy-4-
(2-oxo-2-(4-(4(1R,2S)-2-
phenylcyclopropyl)amino)methyl)piperidin-l-y1) ethyl)benzamide TFA salt
"AN
101 N
0 Lço
TFA
NHOH
LC-MS m/z calcd for C24H29N303, 407.2; found 408.2 [M+1] .
Example 84 B N-
hydroxy-4-(2-oxo-2- (4- ((((lS,2R)-2-
phenylcyclopropyl)amino)methyl)piperidin-l-y1) ethyl)benzamide TFA salt
A
NN
0 0
TFA
NHOH
LC-MS m/z calcd for C24H29N303, 407.2; found 408.2 [M+1] .
Example 85 N-hydroxy-4-((4-(((2-phenylcyclopropyl)amino)methyl)piperidin-l-
yl)sulfonyl)benzamide TFA salt
- 255 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
=,9 NHOH
TFA salt 8
The compound was synthesized using the 1-85 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.43 (bs, 1H), 9.21 (bs, 1H), 8.71 (bs, 2H),
7.96
(d, 2H, J=8 Hz),7.80 (d, 2H, J=8 Hz), 7.30-7.24 (m, 2H), 7.21-7.16 (m, 1H),
7.14-7.10
(m, 2H), 3.71-3.62 (m, 2H), 3.02-2.87 (m, 3H), 2.41-2.32 (m, 1H), 2.31-2.20
(m, 2H),
1.83-1.73 (m, 2H), 1.71-1.59 (m, 1H), 1.42-1.36 (m, 1H), 1.31-1.19 (m, 3H). LC-
MS
m/z calcd for C22H27N304S, 429.5; found 430.5 [M+1] . HPLC purity 98.5 %.
Example 86 N-
hydroxy-4-((N-(2-(4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-
yl)ethyl)sulfamoyl)methyl)benzamide TFA salt
N_OH
lel 1)1 Ov0
The compound was synthesized using the 1-86 following the procedure for
Example
48. itINMR (400 MHz, DMSO-d6): 6 11.22 (bs, 1H), 9.33 (bs, 1H), 9.02 (bs, 3H),
7.75 (d, 2H, J=8 Hz), 7.43 (d, 2H, J=8 Hz), 7.32-7.27 (m, 2H), 7.24-7.21 (m,
1H),
7.19-7.14 (m, 2H), 4.47 (s, 2H), 3.56-3.49 (m, 2H), 3.46-3.25 (m, 2H), 3.21-
3.09 (m,
3H), 3.06-2.88 (m, 4H), 2.45-2.40 (m, 1H), 2.00-1.85 (m, 3H), 1.51-1.35 (m,
3H),
1.32-1.25 (m, 1H). LC-MS m/z calcd for C25H34N404S, 486.6; found 487.6 [M+H]t
HPLC purity 99.8 %.
Example 87 4-(N-(2-(4-4(2-(4-fluorophenyl)cyclopropyl)amino)methyl)piperidin-
1-ypethyl)sulfamoy1)-N-hydroxybenzamide TFA salt
NTh
F NOxp
H
0
The compound was synthesized using the 1-87 following the procedure for
Example
48. iHNMR (400 MHz, DMSO-d6): 6 11.42 (bs, 1H), 9.25 (bs, 2H), 8.89 (bs, 2H),
8.08 (bs, 1H), 7.89-7.83 (m, 2H), 7.87 (d, 2H, J=8.4 Hz), 7.27-7.19 (m, 2H),
7.16-7.08
(m, 2H), 3.56-3.48 (m, 3H), 3.22-3.08 (m, 5H), 3.06-2.89 (m, 5H), 1.96-1.81
(m, 3H),
1.48-1.36 (m, 2H), 1.32-1.27 (m, 1H). LC-MS m/z calcd for C24H31FN404S, 490.2;
found 491.5 [M+H]t HPLC purity 99.7 %.
- 256 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Example 88 N-hydroxy-4-(2-((4-(((2-phenylcyclopropyl)amino)methyl)piperidin-
l-yl)sulfonyl)ethyl)benzamide TFA salt
A
11%-(ON,s,P
'OH
0
The compound was synthesized using the 1-88 following the procedure for
Example 2.
5 iHNMR
(400 MHz, DMSO-d6): 6 11.14 (bs, 1H), 8.95 (bs, 1H), 8.75 (bs, 2H),7.66 (d,
2H, J=8Hz), 7.36 (d, 2H, J=8Hz), 7.32-7.26 (m, 2H), 7.23-7.15 (m, 3H), 3.64-
3.58 (m,
2H), 3.37-3.30 (m, 3H), 3.06-2.94 (m, 5H), 2.82-2.74 (m, 2H), 2.46-2.38 (m,
2H),
1.82-1.76 (m, 3H), 1.48-1.40 (m, 1H), 1.32-1.18 (m, 3H). LC-MS m/z calcd for
C24H31N304S, 457.2; found 458.3 [M+Hr.HPLC purity 99.1 %.
Example 89 N-hydroxy-N4-(2-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-
l-yl)ethyl)terephthalamide TFA salt
A
40 0
0
The compound was synthesized using the 1-89 following the procedure for
Example
48. itINMR (400 MHz, DMSO-d6): 6 11.32 (bs, 1H), 9.06 (bs, 2H), 8.86 (bs, 1H),
8.81 (bs, 2H), 7.90 (d, 2H, J=8.4 Hz), 7.84 (d, 2H, J=8 Hz),7.32-7.28 (m, 2H),
7.23-
7.21 (m, 1H), 7.18-7.16 (m, 2H), 3.70-3.58 (m, 3H), 3.36-3.30 (m, 1H), 3.28-
3.20 (m,
2H), 3.08-2.93 (m, 5H), 2.02-1.84 (m, 4H), 1.49-1.38 (m, 3H), 1.34-1.28 (m,
1H). LC-
MS m/z calcd for C25H32N403, 436.3; found 437.5 [M+H]t HPLC purity 99.7 %.
Example 90 N1-(2-(4-(((2-
(3,4-
difluorophenyl)cyclopropyl)amino)methyl)piperidin-1-ypethyl)-N4-
hydroxyterephthalamide TFA salt
A
40 0
\NN io NOH
TFA salt 0
The compound was synthesized using the 1-90 following the procedure for
Example
48. itINMR (400 MHz, DMSO-d6): 6 11.32 (bs, 1H), 9.10 (bs, 2H), 8.95 (bs, 1H),
8.81 (bs, 2H), 7.90 (d, 2H, J=8.4 Hz), 7.84 (d, 2H, J=8.4 Hz),7.40-7.32 (m,
1H), 7.30-
7.25 (m, 1H), 7.29-7.45 (m, 1H), 3.67-3.51 (m, 4H), 3.27-3.20 (m, 2H), 3.07-
2.94 (m,
- 257 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
5H), 2.00-1.82 (m, 4H), 1.48-1.32 (m, 4H). LC-MS m/z calcd for C25H30F2N403,
472.2; found 473.5 [M+H]t HPLC purity 99.4 %.
Example 91 N-hydroxy-4-((4-(2-((2-phenylcyclopropyl)amino)acetyl)piperazin-l-
yl)methyl)benzamide TFA salt
A
401 NN) N'OH
0
The compound was synthesized using the 1-91 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.27 (bs, 1H), 9.29 (bs, 2H), 7.82-7.77 (m, 2H),
7.54-7.47 (m, 2H), 7.31-7.24 (m, 2H), 7.22-7.18 (m, 1H), 7.14 (d, 2H,
J=6.8Hz,),
4.30-4.20 (m, 5H), 3.81-3.72 (m, 4H), 3.50-3.32 (m, 1H), 2.90-2.81 (m, 2H),
2.01-
1.92 (m, 1H), 1.47-1.28 (m, 1H), 1.74-1.51 (m, 1H), 1.27-1.21 (m, 1H). LC-MS
m/z
calcd for C23H28N403, 408.2; found 409.3 [M+H]t HPLC purity 98.7 %.
Example 92 N-
hydroxy-4-(3-oxo-3-(4-(2-((2-
phenylcyclopropyl)amino)acetyl)piperazin-l-yl)propyl)benzamide TFA salt
0
rN
A
N N
0 0
The compound was synthesized using the 1-92 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.10 (s, 1H), 9.21 (bs, 2H), 8.96 (bs, 1H), 7.64
(d,
2H, J= 8.4Hz), 7.35-7.26 (m, 4H), 7.23-7.18 (m, 1H), 7.18-7.14 (m, 2H), 4.28-
4.20
(m, 2H), 3.54-3.35 (m, 8H), 2.88-2.81 (m, 3H), 2.70-2.62 (m, 2H), 1.50-1.44
(m, 1H),
1.30-1.21 (m, 2H). LC-MS m/z calcd for C2511301\1404, 450.1; found 451.2
[M+H]t
HPLC purity 92.7 %.
Example93 N-hydroxy-4-(3-(1-(2-((2-phenylcyclopropyl)amino)acetyl)piperidin-
4-yl)propyl)benzamide TFA salt.
A
0 0 'OH
The compound was synthesized using the 1-93 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.09 (s, 1H), 9.11 (bs, 2H), 8.91 (bs, 1H), 7.65
(d,
2H, J=8.2Hz), 7.29-7.13 (m, 7H), 4.33-4.13 (m, 3H), 3.65-3.62 (m, 1H), 3.31-
2.84 (m,
2H), 2.87-2.82 (m, 1H), 2.65-2.57 (m, 3H), 1.73-1.65 (m, 2H), 1.58-1.44 (m,
4H),
- 258 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1.26-1.16 (m, 3H), 1.06-0.98 (m, 1H), 0.90-0.82 (m, 1H). LC-MS m/z calcd for
C26H33N303, 435.2; found 436.2 [M+H]t HPLC purity 99.9 %.
Example 94 N-
hydroxy-4-(3-(2-oxo-4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-y1) propyl)benzamide TFA salt
A
N-OH
H
N
The compound was synthesized using the 1-94 following the procedure for
Example 2.
LC-MS m/z calcd for C25H31N303, 421.5; found 422.5 [M+H]t
Example 95 N-hydroxy-4-(2-((2-phenylcyclopropyl)amino)ethoxy)benzamide
TFA salt
A
N
'OH
0
The compound was synthesized using the 1-95 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.06 (bs, 1H), 9.20 (bs, 2H), 7.73 (d, 2H,
J=8.8Hz), 7.29-7.22 (m, 2H), 7.26-7.18 (m, 1H), 7.12 (d, 2H, J=9.2Hz), 6.99
(d, 2H,
J=8.8Hz), 4.35-4.25 (m, 2H), 3.55-3.48 (m, 2H), 3.08-3.01 (m, 1H), 2.46-2.39
(m,
1H), 1.49-1.43 (m, 1H), 1.34-1.26 (m, 1H). LC-MS m/z calcd for C18H20N203,
312.1;
found 313.1 [M+H]t HPLC purity 99.6 %.
Example 96 6-(2-(4-4(2-(4-fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-
ypethoxy)-N-hydroxynicotinamide TFA salt
A
itrn NHOH
0 N
TFA Salt
The compound was synthesized using the 1-96 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.01 (bs, 1H), 9.17 (bs, 1H), 8.94 (bs, 2H),
8.29 (s,
1H), 7.77 (dd, J=9.2, 2.8Hz, 1H), 7.28-7.18 (m, 2H), 7.13 (t, J= 8.8 Hz, 2H),
6.46 (d,
J= 9.6Hz, 1H), 4.25-4.35 (m, 2H), 3.72-3.59 (m, 2H), 3.45-3.30 (m, 2H), 3.28-
3.14
(m, 1H), 3.08-2.90 (m, 5H), 2.04-1.85 (m, 3H), 1.48-1.24 (m, 4H). LC-MS m/z
calcd
for C23H29FN403, 428.2; found 429.2 [M+H]t HPLC purity 99.6%.
Example 97 N-hydroxy-6-(2-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-
l-yl)ethoxy)nicotinamide TFA salt
- 259 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
A )L1,1,0F1
110 I H
TFA Salt
The compound was synthesized using the 1-97 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.02 (bs, 1H),9.26(bs,1H), 9.03(bs,2H), 8.30 (d,
J=1.6 Hz, 1H), 7.77 (dd, J=9.6, 2.4Hz, 1H), 7.30(t,J=7.2Hz, 2H), 7.24-
7.16(m,3H),
6.46 (d,J=10Hz, 1H), 4.35-4.28 (m,2H), 3.80-3.62 (m,3H), 3.43-3.37(m,3H), 3.24-
3.12 (m, 1H), 3.08-2.91(m, 4H), 2.04-1.88 (m, 2H), 1.52-1.35 (m, 3H), 1.33-
1.25 (m,
1H). LC-MS m/z calcd for C23H30N403, 410.2; found 411.2 [M+H]t HPLC
purity=98.2%.
Example98 6-
(2-(4-(42-(4'-fluoro-[1,1'-biphenyl[-4-
yl)cyclopropyl)amino)methyl)piperidin-l-yl)ethoxy)-N-hydroxynicotinamide
TFA salt
n)ct N_OH
H I H
TFA Salt
The compound was synthesized using the 1-98 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.02(bs, 1H), 9.14 (bs, 1H), 8.93(bs, 3H),
8.30(s,
1H), 7.82-7.75 (m,1H),7.70-7.64(m, 2H), 7.58(d,J=8.4Hz, 2H), 7.32-7.24(m, 4H),
6.46 (d, J=9.2Hz, 1H), 4.34-4.28(m,2H), 3.71-3.65(m,3H), 3.26-3.24(m,1H), 3.08-
2.96 (m, 3H), 2.57-2.54(m,3H), 2.02-1.93(m, 3H), 1.52-1.39 (m, 4H). LC-MS m/z
calcd for C29H33FN403, 504.2; found 505.2[M+H]t HPLC purity 98.2%.
Example 99 N-hydroxy-4-(2-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-
1-yl)ethoxy)benzamide TFA salt
A
WO"
1$1 N
1101 H
The compound was synthesized using the 1-99 following the procedure for
Example 2.
itINMR (400 MHz, DMSO-d6): 6 11.0 (bs, 1H), 9.5 (bs, 1H), 8.9 (bs, 2H), 7.75
(d,
2H, J=8 Hz), 7.32-7.26 (m, 2H), 7.23-7.15 (m,3H), 7.03 (d, 2H, J= 8 Hz), 4.41-
4.35
(m, 2H), 3.52-3.45 (m, 2H), 3.35-3.28 (m, 2H), 3.10-2.95 (m, 5H), 2.00-1.91
(m,3H),
1.51-1.41 (m,3H), 1.32-1.25 (m, 1H). LC-MS m/z calcd for C24H31N303, 409.2;
found
410.2 [M+H]t HPLC purity 99.1 %.
- 260 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Example 100 N-hydroxy-4-(3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-
l-yl)propoxy)benzamide TFA salt
PH
101 N
N
'OH
The compound was synthesized using the 1-100 following the procedure for
Example
2.
itINMR (400 MHz, DMSO-d6): 6 11.04 (s, 1H), 9.12 (bs, 1H), 8.87 (bs, 1H), 7.72
(d,
2H,J=8.4Hz), 7.34-7.26 (m, 2H), 7.24-7.20 (m, 1H), 7.21-7.15 (m, 2H), 6.96 (d,
2H,
J=8.8Hz), 4.11-4.08 (m, 2H), 3.60-3.51 (m, 2H), 3.23-3.18 (m, 2H), 3.05-3.01
(m,
2H), 2.98-2.92 (m, 2H), 2.45-2.40 (m, 3H), 2.14-2.09 (m. 2H), 1.98-1.93 (m,
3H),
1.48-1.32 (m, 3H), 1.32-1.28 (m, 1H). LC-MS m/z calcd for C25H33N303, 423.2;
found 424.2 [M+H] . HPLC purity 99.2 %.
Example 101 N-hydroxy-4-(3-((2-phenylcyclopropyl)amino)propoxy)benzamide
TFA salt
0
,01-1
A
1101 N
The compound was synthesized using the 1-101 following the procedure for
Example
2.
itINMR (400 MHz, DMSO-d6): 6 11.03 (s, 1H), 8.92 (bs, 3H), 7.72 (d, 2H,
J=8.9Hz),
7.31-7.26 (m, 2H), 7.35-7.20 (m, 1H), 7.18-7.14 (m, 2H), 6.96 (m, 2H), 4.13-
4.07 (m,
2H), 3.28-3.21 (bs, 2H), 3.06-2.98 (m, 1H), 2.43-2.38 (m, 1H), 2.11-2.03 (m,
2H),
1.49-1.41 (m, 1H), 1.33-1.26 (m, 1H). LC-MS m/z calcd for C19H22N203, 326.3;
found 327.3 [M+H]t HPLC purity 99.7%.
Example 102 2-42-(4-4(2-(4-fluorophenyl)cyclopropyl)amino)methyl)piperidin-
1-ypethypamino)-N-hydroxypyrimidine-5-carboxamide TFA salt
A j)l, õOH
11 I0 I IN-11
NN
TFA Salt
- 261 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using the 1-102 following the procedure for
Example
2.
itINMR (400 MHz, DMSO-d6): 611.07(bs,1H),9.36(bs,1H), 9.14(bs, 2H), 8.66(s,
2H),
7.87(bs,1H), 7.26-7.19(m,2H), 7.15-7.08(m,2H), 3.72-3.58(m,4H),3.38-3.12(m,
3H),
3.08-2.88(m, 5H), 2.00-1.82 (m, 3H) 1.52-1.38(m,3H), 1.33-1.20 (m,1H). LC-MS
rniz
calcd for C22H29N602,428.2; found 429.3 [M+H]t HPLC purity99.8%.
Example 103 5-(24(2-(4-fluorophenyl)cyclopropyl)amino)acety1)-N-hydroxy-
4,5,6,7-tetrahydrothieno [3,2-c]pyridine-2-carboxamide TFA salt
ir-OH
A
1.1 0
The compound was synthesized using the 1-103 following the procedure for
Example
2.
itINMR (400 MHz, DMSO-d6): 6 11.16 (bs, 1H), 9.22 (bs, 2H), 9.06 (bs, 1H),
7.41-
7.35 (m, 1H), 7.25-7.16 (m, 2H), 7.13-7.07 (m, 2H) 4.59-4.48 (m, 2H), 4.43-
4.29 (m,
2H), 3.85-3.78 (m, 1H), 3.70-3.65 (m, 1H), 2.96-2.90 (m, 1H), 2.88-2.78 (m,
2H),
1.51-1.48 (m, 1H), 1.31-1.20 (m, 1H); LC-MS rniz calcd forC19H20FN303S, 389.1;
found 390.1 [M+H]t HPLC purity 99.3 %.
Example 103A 5-(2-4(1R,2S)-2-(4-fluorophenyl)cyclopropyl)amino)acety1)-N-
hydroxy-4,5,6,7-tetrahydrothieno[3,2-c]pyridine-2-carboxamide TFA salt
aS) (0NHOH
TFA
LC-MS rniz calcd forC19H20FN303S, 389.1; found 390.1 [M+H]
Example 103B 5-(2-(((lS,2R)-2-(4-fluorophenyl)cyclopropyl)amino)acety1)-N-
hydroxy-4,5,6,7-tetrahydrothieno[3,2-c]pyridine-2-carboxamide TFA salt
S 11N-OH
I /
Imor" 0
LC-MS rniz calcd for C19H20FN303S, 389.1; found 390.1 [M+H]
Example 104 2-(24(2-(4-fluorophenyl)cyclopropyl)amino)acety1)-N-hydroxy-
1,2,3,4-tetrahydroisoquinoline-7-carboxamide TFA salt
F
A
'OH
0 0
- 262 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using the 1-104 following the procedure for
Example
2.
itINMR (400 MHz, DMSO-d6): 6 11.16 (bs, 1H), 9.22 (bs, 2H), 8.96 (bs, 1H),
7.62-
7.51 (m, 2H), 7.28-7.15 (m, 3H), 7.13-7.08 (m, 2H), 4.66-4.64 (m, 2H), 4.35-
4.31 (m,
2H), 3.75-3.70 (m, 1H), 3.64-3.61 (m, 2H), 2.96-2.91(m, 1H), 2.89-2.80 (m,
2H),
1.49-1.46 (m, 1H), 1.29-1.25 (m, 1H). LC-MS m/z calcd for C21H22FN303, 383.2;
found 384.1 [M+H]t HPLC purity 99.5 %.
Example 104A 2-(2-(((lS,2R)-2-(4-fluorophenyl)cyclopropyl)amino)acety1)-N-
hydroxy-1,2,3,4-tetrahydroisoquinoline-7-carboxamide TFA salt
AN1cIcJLNHOH
TFA
LC-MS m/z calcd for C21H22FN303, 383.2; found 384.1 [M+H]t
Example 104B 2-(2-4(1R,2S)-2-(4-fluorophenyl)cyclopropyl)amino)acety1)-N-
hydroxy-1,2,3,4-tetrahydroisoquinoline-7-carboxamide TFA salt
AN.
N¨OH givo.
0 0
F
TFA
LC-MS m/z calcd for C21H22FN303, 383.2; found 384.1 [M+H]t
Example 105 5-(44(2-(4-fluorophenyl)cyclopropyl)amino)butanoy1)-N-hydroxy-
4,5,6,7-tetrahydrothieno[3,2-c]pyridine-2-carboxamide TFA salt
OH
A -
101
TFA salt
The compound was synthesized using the 1-105 following the procedure for
Example
2.
itINMR (400 MHz, DMSO-d6): 6 11.08 (bs, 1H), 9.04 (bs, 1H), 8.84 (bs, 2H),
7.35 (s,
1H), 7.28-7.16 (m, 2H), 7.11 (t, J=8.4 Hz, 2H), 4.50 (s, 2H),3.80-3.66 (m,
2H), 3.15-
3.05 (m, 2H), 3.00-2.90 (m, 1H), 2.92-2.80 (m, 1H), 2.82-2.70 (m, 1H), 2.60-
2.51(m,
2H), 2.42-2.34 (m,1H), 1.92-1.75 (m, 2H), 1.40 (q, 1H), 1.25 (q, 1H). LC-MS
m/z
calcd for C211-124FN303S, 417.2; found 418.4 [M+H]; HPLC purity 99.5 %.
- 263 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Example 106 5-(4-(4-4(2-(4-fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-
yl) butanoyl) -N- hydroxy -4,5,6,7- tetrahydrothieno [3,2-c] pyridine -2-
carboxamide
TFA salt
A H
F NHOH
µo
TFA salt
The compound was synthesized using the 1-106 following the procedure for
Example
48. iHNMR (400 MHz, DMSO-d6): 6 11.04 (bs, 1H), 9.05 (bs, 2H), 8.84 (bs, 2H),
7.34 (s, 1H), 7.22-7.20 (m, 2H), 7.15-7.11 (m, 2H), 4.51 (s, 2H), 3.77-3.70
(m, 2H),
3.55-3.50 (m, 2H), 3.06-3.00 (m, 4H), 2.97-2.86 (m, 4H), 2.77-2.75 (m, 1H),
2.58-
2.56 (m, 2H), 2.00-1.86 (m, 6H), 1.48-1.28 (m, 4H). LC-MS m/z calcd for
C27H35FN403S, 514.2; found 515.3 [M+H]t HPLC purity 99.2 %.
Example 107 2-(4-((2-(4-fluorophenyl)cyclopropyl)amino)butanoy1)-N-hydroxy-
1,2,3,4-tetrahydroisoquinoline-7-carboxamide TFA salt
A 11CCN NH-OH
0
TFA Salt
The compound was synthesized using the 1-107 following the procedure for
Example
2.
itINMR (400 MHz, DMSO-d6): 6 11.13 (bs, 1H), 8.86 (bs, 2H), 8.92 (bs, 2H),
7.59-
7.48 (m, 2H), 7.24-7.19 (m, 3H), 7.14-7.06 (t, J=8.4 Hz, 2H), 4.63 (d, J=10Hz,
2H),
3.69-3.53 (m, 2H), 3.14- 3.05 (m, 2H), 2.98-2.95 (m, 2H), 2.82-2.76 (m, 1H),
2.58-
2.56 (m, 2H), 2.45-2.38 (m, 1H), 1.90-1.82 (m, 2H), 1.45-1.38 (m, 1H), 1.32-
1.24 (m,
1H). LC-MS m/z calcd for C23H26FN303, 411.2; found 412.2[M+H] . HPLC purity
99.7 %.
Example 108 2-
(4-((2-(4-fluorophenyl)cyclopropyl)amino)butanoy1)-N-
hydroxyisoindoline-5-carboxamide TFA salt
HN¨OH
0
N
0
- 264 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using the 1-108 following the procedure for
Example
2.
itINMR (400 MHz, DMSO-d6): 6 11.19 (bs, 1H), 8.79 (bs, 2H), 7.75-7.62 (m, 2H),
7.43-7.35 (m, 1H), 7.25-7.21 (m, 2H), 7.15-7.10 (m, 2H),4.81 (s, 2H), 4.66 (s,
2H),
3.16-3.10 (m, 2H), 3.02-2.96 (m, 1H), 2.43-2.36 (m,3H), 1.94-1.88 (m,2H), 1.44-
1.40
(m, 1H), 1.33-1.27 (m, 1H). LC-MS m/z calcd for C22H24FN303, 397.2; found
398.2
[M+H]t HPLC purity 99.2 %.
Example 109 N-hydroxy-2-(4-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-
l-yl)butanoyl)isoindoline-5-carboxamide TFA salt
( NH /\N¨\
N¨OH
TFA Salt C¨N1
The compound was synthesized using the 1-109 following the procedure for
Example
48. itINMR (400 MHz, DMSO-d6): 6 11.20 (bs, 1H), 9.14 (bs, 2H), 8.88 (bs, 3H),
7.68 (d, 1H, J=8 Hz), 7.43-7.38 (m, 1H),7.32-7.28 (m, 2H), 7.24-7.16 (m, 3H),
4.84
(s, 2H), 4.66 (s, 2H), 3.60-3.54 (m, 2H), 3.34-3.15 (m, 1H), 3.10-2.88 (m,
8H), 2.01-
1.71 (m, 6H), 1.50-1.27 (m, 4H). LC-MS m/z calcd for C28H36N403, 476.3; found
477.6 [M+Hr.HPLC purity 99.6 %.
Example 110 N-hydroxy-2-(3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-
l-yl)propyl)thiazole-4-carboxamide TFA salt
A NH
.0H
TFA salt
The compound was synthesized using the 1-110 following the procedure for
Example
48.1HNMR (400 MHz, DMSO-d6): 6 11.32 (bs, 1H), 9.46 (bs, 1H), 9.09 (bs, 2H),
8.10
(s, 1H), 7.32-7.26 (m, 2H),7.23-7.13 (m, 3H), 3.56-3.51 (m, 2H), 3.30-2.83 (m,
9H),
2.43-2.40 (m, 1H), 2.17-2.08 (m, 2H), 2.06-1.71 (m, 3H), 1.50-1.35 (m, 3H),
1.30-
1.25 (m, 1H). LC-MS m/z calcd for C22H301\1402S, 414.2; found 415.5 [M+H]t
HPLC
purity 99.5 %.
Example 111 2-
(3-(4-(42-(4'-fluoro-[1,1'-biphenyl]-4-
yl)cyclopropyl)amino)methyl)piperidin-1-y1)propy1)-N-hydroxythiazole-4-
carboxamide TFA salt
- 265 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
NH H
TFA salt
The compound was synthesized using the I-111 following the procedure for
Example
48. iHNMR (400 MHz, DMSO-d6): 6 10.98 (bs, 1H), 9.13 (bs, 2H), 8.24 (bs, 2H),
8.13 (s, 1H), 7.69-7.64 (m, 2H), 7.58 (d, 2H, J=8.6 Hz), 7.30-7.25 (m, 4H),
3.60-3.55
(m, 2H), 3.19-3.12 (m, 3H), 3.10-3.06 (m, 6H), 2.98-2.86 (m, 3H), 2.18-2.09
(m, 2H),
2.00-1.94 (m, 2H), 1.50-1.44 (m, 1H), 1.42-1.36 (m, 2H). LC-MS m/z calcd for
C28H33FN402S, 508.2; found 509.6 [M+H]t HPLC purity 99.6 %.
Example 112 N-hydroxy-2-(3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-
1-yl)propyl)thiazole-5-carboxamide TFA salt
A HO
H
The compound was synthesized using the 1-112 following the procedure for
Example
48. itINMR (400 MHz, DMSO-d6): 6 11.32 (bs, 1H), 9.45 (bs, 1H), 9.09 (bs, 2H),
8.10 (s, 1H), 7.31-7.25 (m, 2H), 7.21-7.15 (m, 3H), 3.56-3.50 (m, 2H), 3.31-
3.10 (m,
3H), 3.09-3.02 (m, 3H), 2.97-2.86 (m, 2H), 2.47-2.42 (m, 2H), 2.15-2.07 (m,
2H),
2.00-1.89 (m, 2H), 1.80-1.70 (m, 1H), 1.50-1.35 (m, 3H), 1.32-1.27 (m, 1H). LC-
MS
m/z calcd for C22H301\1402S, 415.2; found 416.5 [M+H]t HPLC purity 99.3 %.
Example 113 N-hydroxy-2-(3-(4-(((2-phenylcyclopropyl)amino)methyl)piperidin-
l-yl)propyl)oxazole-4-carboxamide TFA salt
NH
bH
TFA Salt
The compound was synthesized using the 1-113 following the procedure for
Example
48. itINMR (400 MHz, DMSO-d6): 6 10.92 (bs, 1H), 9.49 (bs, 1H), 9.09 (bs, 2H),
8.47 (s, 1H), 7.32-7.14 (m, 5H), 3.60-3.51 (m, 2H), 3.32-3.12 (m, 3H), 3.08-
2.81 (m,
6H), 2.15-1.73 (m, 6H), 1.50-1.37 (m, 3H), 1.30-1.23 (m, 1H). LC-MS m/z calcd
for
C22H301\1403, 398.2; found 399.5 [M+H]t HPLC purity 99.6 %.
Example 114 (E)-
N-hydroxy-4-(3-oxo-3-(4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-1-yl)prop-1-en-1-yl)benzamide TFA
salt
- 266 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
A N _OH
101 N
N
0
The compound was synthesized using the 1-114 following the procedure for
Example
2.
11-1NMR (400 MHz, DMSO-d6): 6 11.24 (bs, 1H), 9.02 (bs, 1H), 8.76 (bs, 2H),
7.79-
7.72 (m, 4H), 7.47 (d, 1H, J=15Hz), 7.33 (d, 1H, J=15Hz), 7.33-7.27 (m, 2H),
7.22-
7.15 (m, 3H), 4.47-4.42 (m, 1H), 4.33-4.27 (m, 1H), 3.11-2.97 (m, 4H), 2.72-
2.64 (m,
1H), 2.48-2.38 (m, 1H), 2.00-1.91 (m, 1H), 1.84-1.75 (m, 2H), 1.48-1.41 (m,
1H),
1.33-1.27 (m, 1H), 1.19-1.09 (m, 2H). LC-MS m/z calcd for C25H29N303, 419.2;
found 420.2 [M+1] . HPLC purity 99.8 %.
Example 114A N-hydroxy-
44(E)-3-oxo-3-(44(((1R,2S)-2-
phenylcyclopropyl)amino)methyl)piperidin-1-yl)prop-1-en-1-yl)benzamide TFA
salt
0
NHOH
H
0
TFA
LC-MS m/z calcd for C25H29N303, 419.2; found 420.2 [M+1] .
Example 114B N-hydroxy-4-
((E)-3-oxo-3-(4-((((lS,2R)-2-
phenylcyclopropyl)amino)methyl)piperidin-1-yl)prop-1-en-1-yl)benzamide TFA
salt
NHOH
'ON
0
TFA Salt
LC-MS m/z calcd for C25H29N303, 419.2; found 420.2 [M+1] .
Example 115 44(E)-3-(4-
(4(1S,2R)-2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-y1)-3-oxoprop-1-en-1-y1)-N-
hydroxybenzamide TFA salt
NHOH
0
TFA Salt
- 267 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using the 1-115 following the procedure for
Example
2.
LC-MS m/z calcd for C25H28FN303, 437.5; found 438.5 [M+1] .
Example 115A
44(E)-3-(4-(4(1R,2S)-2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-y1)-3-oxoprop-1-en-1-y1)-N-
hydroxybenzamide TFA salt
N_OH
F
0
TFA Salt
LC-MS m/z calcd for C25H28FN303, 437.5; found 438.5 [M+1] .
Example 116 (E)-4-(3-(4-(((2-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-y1)-3-oxoprop-1-en-1-y1)-N-
hydroxybenzamide TFA salt
0
N,OH
N
0
N¨ 0
TFA Salt
The compound was synthesized using the 1-116 following the procedure for
Example
2.
itINMR (400 MHz, DMSO-d6): 6 11.24 (s, 1H), 9.02 (s, 1H), 8.84-8.72 (bs, 2H),
7.76
(m, 4H), 7.48 (d, J =16.0 Hz, 1H), 7.34 (d, J =16.0Hz, 1H), 7.30-7.26 (m, 4H),
4.4-4.3
(dd, 2H), 3.15-2.98 (m, 4H), 2.75-2.65 (m, 1H), 2.45-2.40 (m, 1H), 2.35 (s,
3H), 2.18
(s, 3H), 2.04-1.9 (m, 1H), 1.88-1.75 (m, 1H), 1.52-1.44 (m, 1H), 1.40-1.30 (q,
J
=6.8Hz,1H), 1.25-1.08 (m, 2H). LC-MS m/z calcd for C301434N404, 514.2; found
515.2 [M+H]t HPLC purity 99.8 %.
Example 117 (E)-
N-hydroxy-4-(3-oxo-3-(4-(((2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-yl)prop-1-en-1-yl)benzamide
TFA salt
N,OH
N
N
I 0
TFA salt
- 268 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using the 1-117 following the procedure for
Example
2.
itINMR (400 MHz, DMSO-d6): 6 11.24 (bs, 1H), 9.16 (s, 1H), 9.11 (s, 2H), 8.92-
8.72
(bs, 2H), 7.83-7.73 (m, 4H), 7.69 (d, 2H, J=8.4 Hz), 7.52-7.43 (m, 1H), 7.40-
7.33 (m,
J=8.4 Hz, 2H), 7.30 (d, J=16Hz, 1H), 4.55-4.26 (m, 2H), 3.18-3.00 (m, 4H),
2.75-2.60
(m, 2H), 2.05-1.90 (m, 1H), 1.88-1.72 (m, 2H), 1.58-1.48 (m, 1H),1.42-1.34 (m,
1H),
1.25-1.08 (m, 2H). LC-MS rniz calcd for C29H31N503, 497.2; found 498.2 [M+H]t
HPLC purity 99.6%.
Example 118 (E)-
4-(3-(3-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)azetidin-1-y1)-3-oxoprop-1-en-1-y1)-N-
hydroxybenzamide TFA salt
FrYX
N_OH
0
TFA
The compound was synthesized using the 1-118 following the procedure for
Example
2.
ltINMR (400 MHz, DMSO-d6): 6 11.25 (bs, 1H), 9.03 (bs, 1H), 8.91 (bs, 2H),
7.81-
7.61 (m, 4H), 7.45 (d, J =16Hz, 1H), 7.26-7.21 (m, 2H), 7.16-7.11 (m, 2H),6.74
(d, J
=15Hz, 1H), 4.47-4.40 (m, 1H), 4.10-4.02 (m, 2H), 3.81-3.76 (m, 1H), 3.48-3.43
(m,
3H), 3.10-2.93 (m, 2H), 1.44-1.40 (m,1H), 1.33-1.25 (m, 1H). LC-MS rniz calcd
for
C23H24FN303, 409.2; found 410.1[M+H]t HPLC purity 98.5 %.
Example 119 (E)-N-hydroxy-
4-(3-(3-(42-(4-(1-methy1-1H-pyrazol-4-
yl)phenyl)cyclopropyl)amino)methypazetidin-1-y1)-3-oxoprop-1-en-l-
y1)benzamide TFA salt
0
N_OH
0
N-
The compound was synthesized using the 1-119 following the procedure for
Example
2.
itINMR (400 MHz, DMSO-d6): 6 11.24 (bs, 1H), 9.01 (bs, 1H), 8.91 (bs, 2H),
8.08 (s,
1H), 7.81 (s, 1H), 7.56-7.70 (m, 4H), 7.50-7.43 (m, 3H), 7.15 (d, 2H, J=8 Hz),
6.74 (d,
1H, J=14 Hz), 4.46-4.38 (m, 1H), 4.08-4.02 (m, 2H), 3.83 (m, 3H), 3.80-3.77
(m, 1H),
- 269 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
3.01-2.94 (m,2H), 2.47-2.35 (m,3H), 1.46-1.40 (m, 1H), 1.35-1.28 (m, 1H). LC-
MS
m/z calcd for C27H29N503, 471.23; found 472.2 [M+H]t HPLC purity 99.8 %.
Example 120 (E)-
N-(2-aminopheny1)-3-(4-(42-(4-
fluorophenyl)cyclopropyl)amino)methyl)phenyl)acrylamide TFA salt
F
A
N NH2
H H
0 el
A A
6 y
/ OMe F Step 1 00 40 Nil
/ OH ____ Step 2 .
' F 00
/\ 0
1-2 /\ XC 0
A 6
F NH, F s A
H - Step 3 N
H
H 2NH
00 ...--- N 40 _,...
/ N 0
/.\ 0 0
XCI TFA
Example 120
Step 1: (E)-
3- (4- (((tert-butoxycarb onyl)(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)phenyl)acrylic acid (XC)
A
/ OH
F $1 Orli 0
/\ 0
To a stirred solution of (E)-methyl 3-(4-(((tetra-butoxycarbonyl)(2-(4-
florophenyl)cyclopropl)amino)methyl)phenyl)acrylate(I-2, 0.38 g, 0.89 mmol) in
methanol and water mixture (20mL, 4:1) was added sodium hydroxide (0.11 g,
2.68
mmol) at room temperature and the resulting mixture was stirred at that
temperature
for 1 h. The progress of the reaction was monitored by TLC. After completion
of
reaction, solvent was evaporated and washed with ethylacetate. The reaction
mixture
was acidified with 2N HC1 and extracted with dichloromethane and the organic
portion was washed withwater and brine, dried over sodium sulphate and
concentrated
under reduced pressure to get the crude to afford the titled product as off
white solid
(XC, 0.31 g, 86.2 %), LC-MS m/z calcd for C24H26FN04, 411.2; found 311.2 [M-
Boa'.
Step 2: (E)-tert-butyl 4-(342-aminophenyl)amino)-3-oxoprop-1-en-1-yl)benzyl(2-
(4-fluorophenyl)cyclopropyl)carbamate (XCI)
- 270 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
H NH2
F 011 0 N
0
To a stirred solution of
(E)-3-(4-(((tert-Butoxycarbony1)-(2-(4-
fluorophenyl)cyclopropyl)amino)methyl)phenyl)acrylic acid(XC, 0.28 g, 0.68
mmol)
in dry dichloromethane (8 mL), was added benzene-1,2-diamine(0.22g, 2.04
mmol),
thentriethylamine (0.28 mL, 2.04mmo1), cooled to 0 C and T3P (0.50mL, 1.70
mmol)
was added and the resulting mixture was stirred at room temperature for 3 h.
After
completion of the reaction, the mixture was quenched with ice-water and
extracted
with dichloromethane. The organic layer was washed with water, brine, dried
over
sodium sulphate and concentrated under reduced pressure to afford the crude
compound which was purified by flash column chromatography using ethylacetate-
hexane gradient to afford the titled product as yellow semi solid. (XCI, 0.26
g, 68%).
LC-MS m/z calcd for C30H32FN303, 501.2; found 502.3 [M+H]t
Step 3: (E)-
N-(2-aminopheny1)-3-(4-(02-(4fluorophenyl)
cyclopropyl)amino)methyl)phenyl)acrylamide-Example 120
NH2
N
TFA 0
To a stirred solution of(E)-tetra-butyl 4(3-((2-aminophenyl)amino)-3-oxoprop-1-
en-l-
y1)benzyl(2-(4-flurophenyl)cyclopropyl)carbamate(XCI,0.26g, 0.52 mmol) in dry
dichloromethane (10 mL) was added trifluoro acetic acid (0.63 mL, 8.30 mmol)
at 0
C and the resulting mixture was stirred at room temperature for 1 h. The
progress of
the reaction was monitored by TLC. The solvent was concentrated under reduced
pressure to get the crude product which was purified by reverse-phase HPLC
using
Chemsil C18 (250mm X 4.6mm X 5mic) column with 0.1% TFA in water:ACN to
afford the pure product as pale-yellow solid (Example 120: 0.204 g, 96 %).
itINMR
(400 MHz, DMSO-d6): 6 9.43 (bs, 1H), 9.34 (bs, 2H), 7.65-7.62 (m, 2H), 7.57-
7.49
(m, 3H), 7.34-7.30 (m, 1H), 7.19-7.06 (m, 4H), 6.95-6.89 (m, 2H), 6.78-6.74
(m, 1H),
6.62-6.56 (m, 1H), 4.36-4.32 (m, 2H), 2.93-2.89 (m, 1H), 2.46-2.35 (m, 1H),
1.49-
1.39 (m, 1H), 1.35-1.25 (m, 1H). LC-MS calcd for C25H24FN30, 401.2; found
402.4
[M+H] . HPLC purity 94.8 %.
-271 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Example 121 N-
(2-aminopheny1)-4-(3-(4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
A o
101 HNOH N
NH2
The compound was synthesized using 1-121 following the procedure for example
120.
iHNMR (400 MHz, DMSO-d6): 6 9.79 (bs, 1H), 9.59 (bs, 1H), 9.13 (bs, 2H), 7.94
(d,
2H, J=7.6 Hz), 7.36 (d, 2H, J=7.6 Hz), 7.31-7.27 (m, 2H), 7.25-7.13 (m, 4H),
7.09-
7.01 (m, 1H), 6.93-6.89 (m, 1H), 6.79-6.73 (m, 1H), 3.59-3.48 (m, 2H), 3.08-
2.82 (m,
7H), 2.74-2.67 (m, 2H), 2.06-1.87 (m, 6H), 1.50-1.38 (m, 3H), 1.30-1.20 (m,
1H). LC-
MS m/z calcd for C31H38N40, 482.3; found 483.3 [M+H]t HPLC purity 92.6 %.
Example 122 N-(2-
aminopheny1)-4-(3-(4-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzamide TFA
salt
F
A 0
N
NH2
TFA
The compound was synthesized using 1-122 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.73 (bs, 1H), 9.60 (bs, 1H), 9.14 (bs, 2H), 7.94
(d,
2H, J=7.6 Hz), 7.36 (d, 2H, J=7.6 Hz), 7.24-7.17 (m, 3H), 7.16-7.08 (m, 2H),
7.05-
7.00 (m, 1H), 6.90-6.85 (m, 1H), 6.75-6.68 (m, 1H), 3.56-3.48 (m, 2H), 3.30-
3.21 (m,
1H), 3.08-2.98 (m, 4H), 2.95-2.82 (m, 3H), 2.72-2.65 (m, 2H), 2.06-1.87 (m,
5H),
1.50-1.38 (m, 3H), 1.30-1.20 (m, 1H). LC-MS m/z calcd for C31H37FN40, 500.6;
found 501.2 [M+H]t HPLC purity 92.6 %.
Example 123 N-
(2-aminopheny1)-4-(3-(4-(((2-
(4methoxyphenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide
TFA salt
0
NH2
The compound was synthesized using 1-123 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.64 (s, 1H), 9.30 (bs, 1H), 8.92 (bs, 1H), 7.93
(d,
2H J=8 Hz), 7.36 (d, 2H,J=8 Hz), 7.15 (d, 2H, J=7.6 Hz), 7.08 (d, 2H,J= 8 Hz),
6.99-
6.96 (m, 1H), 6.86-6.81 (m, 2H), 6.65-6.60 (m, 1H), 3.70 (s, 3H), 3.54-3.50
(m, 3H),
- 272 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
3.08-3.00 (m, 4H), 2.94-2.82 (m, 3H), 2.75-2.68 (m, 2H), 2.42-2.34 (m, 1H),
2.04-
1.92 (m, 5H), 1.42-1.36 (m, 3H). LC-MS rniz calcd for C32H401\1402, 512.3;
found
513.3 [M+H]t HPLC purity 97.2 %.
Example 124 N-
(2-aminopheny1)-4-(3-(4-(((2-(3,4-
difluorophenyl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzamide TFA
salt
0
NH2
TFA
The compound was synthesized using 1-124 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.71 (bs, 1H), 9.35 (bs, 1H), 9.01 (bs, 2H),
7.94(d,
2H, J=7.6Hz), 7.37(d, 2H, J =7.6Hz), 7.32-7.26 (m, 1H), 7.18(d, 1H, J =7.6Hz),
7.08-
6.99 (m, 2H), 6.86(d, 2H, J =7.6Hz), 6.72-6.67 (m, 1H),3.56-3.51 (m, 2H), 3.29-
2.82
(m, 8H), 2.74-2.68 (m, 2H), 2.14-1.72 (m, 6H), 1.49-1.31 (m, 3H). LC-MS rniz
calcdfor C31H36F2N40, 518.3, found 519.6 [M+H].HPLC purity 99.6 %.
Example 125 N-
(2-aminopheny1)-4-(3-(4-(((2-(4-(piperidine-1-
carbonyl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide
TFA salt
0
N
H2N
0
The compound was synthesized using 1-125 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.70 (bs, 1H), 8.96 (bs, 1H), 8.90 (bs, 1H), 7.95
(bs,
1H), 7.94(d, 2H, J =8.4Hz), 7.37(d, 2H, J =7.6Hz), 7.29 (d, 2H, J =8Hz),
7.23(d, 2H, J
=8Hz), 7.17 (d, 1H, J =7.6Hz), 7.01 (t, 1H, J =7.6Hz), 6.85 (d, 1H, J =8Hz),
6.73-6.65
(m, 1H), 3.59-3.50 (m, 5H), 3.30-3.17 (m, 3H), 3.09-3.00 (m, 5H), 2.95-2.84
(m,
2H),2.77-2.67 (m, 2H), 2.05-1.90 (m, 5H), 1.55-1.32 (m, 9H). LC-MS rniz
calcdfor
C37H47N502, 593.4, found 594.4 [M+H]t HPLC purity 99.9 %.
Example 126 N-(2-
aminopheny1)-4-(3-(3-(((2-(4-
fluorophenyl)cyclopropyl)amino)methypazetidin-1-yl)propyl)benzamide TFA
salt
- 273 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0 Spi
H
H NH2
F
0 a
Ill HO 0 H
0
0 N
OH 40
HN -''''
Step-1 1101 HN Step-2 0 Step-3
HO HO NH2
XCIV Cy__
XCII XCIII
0 40
0 HN
0 40
N
A
40 ON
Step-5 F F2C 0
0
H
YBr 0 -a Br 0 e-
-
H NH Step-4 HN -
)(on 0,.._..-- HN
0
Y
0,-
min
xcv
Step-6 F A 0 40
_,... 0 N
i'VN 0 iti
NH2
Example 126
5 Step-1: N-(2-
aminopheny1)-4-(3-hydroxypropyl)benzamide-XCIII
0 .
N
HO HNH2
To a stirred solution of 4-(3-hydroxypropyl)benzoic acid (XCII, 0.7 g, 3.89
mmol) in
dry dichloromethane (15 mL) was added benzene-1,2-diamine (1.26 g, 11.66
mmol),
triethylamine (1.64 mL, 11.66mmo1) and cooled to 0 C. Then T3P (1.48 mL, 4.66
10 mmol)
was added and the resulting mixture was stirred at room temperature for 3 h.
After completion of the reaction, the mixture was quenched with ice water and
extracted with dichloromethane. The organic layer was washed with water,
brine,
dried over sodium sulphate and concentrated under reduced pressure to afford
the
crude compound which was purified by column chromatography using ethylacetate-
hexane gradient to afford the titled product as sticky oil. (XCIII, 0.6 g, 57
%). LC-MS
m/z calcd for C16H18N202, 270.1; found 271.0 [M+H]t
Step-2: tert-butyl (2-(4-(3-hydroxypropyl)benzamido)phenyl)carbamate-XCIV
0 0
N
HO HHN 0
0<
To a stirred solution of N-(2-aminopheny1)-4-(3-hydroxypropyl)benzamide
(XCIII,
0.3 g, 0.90 mmol) in tetrahydrofuran-water mixture (1:1, 10 mL) was added
sodium
- 274 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
bicarbonate (0.227 g, 2.71 mmol) and Boc anhydride (0.23 mL, 1.08 mmol) at
room
temperature. After 1 h, the reaction mixture was diluted with ethyl acetate
and was
washed with water, brine, dried over sodium sulphate and concentrated under
reduced
pressure to get the crude product which was purified by column chromatography
using
ethylacetate-hexane gradient to afford the titled product as thick oil (XCIV,
0.25 g,
61%). LC-MS m/z calcd for C21I-126N204, 370.1; found 371.0 [M+H]t
Step-3: N-(2-aminopheny1)-4-(3-bromopropyl)benzamide-XCV
0 el
N
Br HNH2
To a stirred solution of tert-butyl (2-
(4-(3-
hydroxypropyl)benzamido)phenyl)carbamate (XCIV, 0.3 g, 0.90 mmol) in
dichloromethane (5 mL) was added triphenylphosphine (0.31 g, 0.95 mmol) and
tetrabromomethane (0.41 g, 1.09 mmol) at 0 C. After 16 h, the reaction
mixture was
diluted with dichloromethane and was washed with water, brine solution, dried
over
sodium sulphate and concentrated under reduced pressure to get the crude
product
which was purified by column chromatography using ethylacetate-hexane gradient
to
afford the titled product as thick oil (XCV, 0.17 g, 70%). LC-MS m/z calcd for
C16H17BrN20, 332.0; found 333.1 [M+H]t
Step-4: tert-butyl (2-(4-(3-bromopropyl)benzamido)phenyl)carbamate-XCVI
0 0
N
Br H HN 0
0
To a stirred solution of N-(2-aminopheny1)-4-(3-bromopropyl)benzamide (XCV,
0.22
g, 0.66 mmol) in tetrahydrofuran-water mixture (1:1, 10 mL) was added sodium
bicarbonate (0.16 g, 1.98 mmol) and Boc anhydride (0.17 mL, 0.79 mmol) at room
temperature. After 1 h, the reaction mixture was diluted with ethylacetate and
was
washed with water, brine, dried over sodium sulphate and concentrated under
reduced
pressure to get the crude product which was purified by column chromatography
using
ethylacetate-hexane gradient to afford the titled product as thick oil (XCVI,
0.23 g,
82%). LC-MS m/z calcd for C21I-125BrN203, 432.1; found 433.0 [M+H]t
- 275 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Step-5:
tert-buty1(2-(4-(3-(34(2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropypacetamido)methypazetidin-l-
y1)propyl)benzamido)phenyl)carbamate-XCVII
A o 0
0 Nil
H
HN ,0
F F3C0 1
(:),.
To a solution of N-(azetidin-
3 -ylmethyl) -2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropyl)acetamide trifluoroacetate salt (XCVI, 0.18 g, 0.54
mmol)
in acetonitrile (5 mL) was added tert-butyl (2-
(4-(3-
bromopropyl)benzamido)phenyl)carbamate (0.28 g, 0.65 mmol) and N,N-
diisopropylethylamine (0.29 mL, 1.61 mmol). Then the reaction mixture was
heated at
60 C for 16 h. After completion of reaction, the reaction was diluted with
ethylacetate
(50 mL), washed with water, brine solution, dried over sodium sulfate and
concentrated under vacuum to get crude product which was purified by column
chromatography using methanol-dichloromethane gradient to afford the titled
product
as brown colour sticky oil (XCVII, 0.13 g, 36 %). LC-MS m/z calcd for
C36H40F4N404, 668.3; found 669.1 [M+H]t
Step-6: N-
(2-aminopheny1)-4-(3-(3-(42-(4-
fluorophenyl)cyclopropyl)amino)methypazetidin-1-yl)propyl)benzamide TFA
salt-Example 126
0 SI
N C\N N
H
H NH2
FCAO
To a solution of tert-butyl
(2-(4-(3-(3-((2,2,2-trifluoro-N-(2-(4-
fluorophenyl)cyclopropyl)
acetamido)methyl)azetidin-l-
yl)propyl)benzamido)phenyl)carbamate (XCVII, 0.17 g, 0.25 mmol) in methanol (5
mL) was added potassium carbonate (0.10 g, 0.76 mmol) at room temperature for
16
h. After completion of reaction, the reaction was concentrated under vacuum.
The
residue was diluted with dichloromethane and cooled to 0 C. TFA (0.46 mL) was
added to it and stirred for 1 h at same temperature. The solvent was
concentrated
under reduced pressure to get the crude product which was purified by reverse-
phase
HPLC using Chemsil C18 (250mm X 4.6mm X 5mic) column with 0.1% TFA in
water:ACN to afford the pure product as a colourless solid (Example 126, 0.04
g, 37
- 276 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
%).1HNMR (400 MHz, DMSO-d6): 6 9.87 (bs, 1H), 9.65 (s, 1H), 9.01 (bs, 2H),
7.93
(d, 2H, J=8 Hz), 7.34 (s, 2H, J=7.6 Hz), 7.25-7.19 (m, 2H), 7.16-7.10 (m, 3H),
6.99 (t,
1H,J=7.6 Hz), 6.81 (d, 1H, J=7.6 Hz), 6.65 (t, 1H, J=7.6 Hz),4.18 (m, 1H),
4.12-3.95
(m, 2H), 3.90-3.78 (m, 2H), 3.42-3.28 (m, 2H), 3.20-3.02 (m, 4H), 2.95-2.80
(m, 1H),
2.72-2.62 (m, 1H), 1.80-1.68 (m, 2H), 1.42-1.35 (m, 1H), 1.32-1.24 (m, 1H). LC-
MS
m/z calcd for C29H33FN40, 472.3; found 473.3 [M+H]t HPLC purity 99.8 %.
Example 127 N-(2-aminopheny1)-4-(3-(6-((2-phenylcyclopropyl)amino)-2-
azaspiro[3.3]heptan-2-yl)propyl)benzamide TFA salt
H NH2
H
0
The compound was synthesized using 1-127 following the procedure for example
120.
1HNMR (400 MHz, DMSO-d6): 6 10.48 (bs, 1H), 9.69 (s, 1H), 8.55 (bs, 2H), 7.93
(d,
2H,J=7.6 Hz), 7.40-7.28 (m, 4H), 7.25-7.14 (m, 4H), 7.00 (t, 1H,J=7.6 Hz),
6.85 (d,
1H, J=7.6 Hz), 6.68 (t, 1H, J=7.2 Hz), 4.38-4.4.26 (m, 1H), 3.75-3.62 (m, 1H),
3.38-
3.15 (m, 4H), 3.00-2.96 (m, 2H), 2.78-2.70 (m, 2H), 2.68-2.60 (m, 1H), 2.24-
2.04 (m,
3H), 2.00-1.88 (m, 2H), 1.70-1.55 (m, 2H), 1.45-1.32 (m, 1H). LC-MS m/z calcd
for
C31H36N40, 480.3; found 481.3 [M+H]t HPLC purity 99.6 %.
Example 128 N-
(2-aminopheny1)-4-(3-(4-(42-(1-isopropy1-1H-pyrazol-4-
yl)cyclopropyl)amino)methyl)piperidin-1-y1)propyl)benzamide TFA salt
)¨N
0 al
N
NH2
The compound was synthesized using 1-128 following the procedure for example
120.
1HNMR (400 MHz, DMSO-d6): 6 9.73 (bs, 1H), 9.22 (bs, 1H), 8.34 (bs, 2H), 7.94
(d,
2H, J=7.6 Hz), 7.61 (s, 1H), 7.37 (d, 2H, J=8 Hz),7.29 (s, 1H), 7.19 (d, 1H,
J=7.6
Hz),7.03 (t, 1H, J=7.6 Hz), 6.88 (d, 1H, J=8 Hz), 6.73 (t, 1H, J=7.2 Hz), 4.42-
4.35 (m,
1H), 3.55-3.52 (m, 2H), 3.08-2.98 (m, 4H), 2.96-2.87 (m, 2H), 2.83-2.78 (m,
1H),
2.75-2.68 (m, 2H), 2.23-2.19 (m, 1H), 2.06-1.90 (m, 5H), 1.46-1.40 (m, 9H),
1.21-
1.06 (m, 1H). LC-MS m/z calcd for C31H42N60, 514.3; found 515.3 [M+H]t HPLC
purity 98.8 %.
Example 129 N-
(2-aminopheny1)-4-(3-(4-(42-(1-pheny1-1H-pyrazol-4-
yl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
- 277 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
0
N
NH2
The compound was synthesized using 1-129 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.70 (bs, 1H), 9.54 (bs, 1H), 9.09 (bs, 2H), 8.37
(s,
1H), 7.94 (d, 2H, J=8 Hz), 7.73 (d, 2H, J=8 Hz),7.65 (s, 1H), 7.47 (t, 2H, J=8
Hz),7.36 (d, 2H, J=8 Hz),7.28 (t, 1H, J=7.6 Hz), 7.18 (d, 1H, J=8 Hz), 7.03-
6.98 (m,
1H), 6.85 (d, 1H, J=8 Hz), 6.71-6.66 (m, 1H), 3.58-3.51 (m, 2H), 3.10-3.02 (m,
4H),
2.95-2.84 (m, 3H), 2.74-2.68 (m, 2H), 2.38-2.32 (m, 1H), 2.03-1.94 (m, 5H),
1.50-
1.41 (m, 3H), 1.25-1.19 (m, 1H). LC-MS m/z calcd for C34H401\160, 548.3; found
549.3 [M+H]t HPLC purity 99 %.
Example 130 N-
(2-aminopheny1)-4-(3-(4-(42-(2-methylthiazol-5-
yl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
o
--e3/& N
NH2
The compound was synthesized using 1-130 following the procedure for example
120.
LC-MS m/z calcd for C29H37N50S, 503.7; found 504.7 [M+H]t
Example 131 N-
(2-aminopheny1)-4-(3-(4-(((2-(pyridin-3-
yl)cyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
o
N
H
NH2
The compound was synthesized using 1-131 following the procedure for example
120.
LC-MS m/z calcd for C30H37N50, 483.6; found 484.6 [M+H]t
Example 132 N-
(2-amino-5-fluoropheny1)-4-(3-(4-(42-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-yl)propyl)benzamide TFA
salt
o
1101 11 N
NH2
The compound was synthesized using 1-122 following the procedure for example
120.
- 278 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
itINMR (400 MHz, DMSO-d6): 6 9.53 (s, 1H), 9.42 (bs, 1H), 9.04 (bs, 2H), 7.95
(d,
2H, J=8.0 Hz), 7.34 (d, 2H, J=8.0 Hz), 7.23-7.19 (m, 2H), 7.14-7.06 (m, 3H),
6.55-
6.51 (m, 1H), 6.37-6.32 (m, 1H), 3.58-3.50 (m, 2H), 3.30-3.24 (m, 1H), 3.22-
3.14 (m,
1H), 3.08-2.98 (m, 3H), 2.95-2.81 (m, 3H), 2.74-2.64 (m, 2H), 2.06-1.85 (m,
5H),
1.46-1.36 (m, 3H), 1.29-1.24 (m, 1H). LC-MS rniz calcd for C31H36F2N40, 518.3;
found 519.2 [M+H]t HPLC purity 98.6 %.
Example 133 N-
(2-aminopheny1)-4-(3-oxo-3-(4-((2-
phenylcyclopropyl)amino)piperidin-l-yl)propyl)benzamide TFA salt
0
H NH2
A N
lel HN
0 VI
The compound was synthesized using 1-133 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.69 (s, 1H), 8.99 (bs, 2H), 7.88 (d, 2H, J=8
Hz),
7.36 (d, 2H, J=8 Hz), 7.31-7.27 (m, 2H), 7.26-7.16 (m, 4H), 7.05-7.00 (m, 1H),
6.88-
6.86 (m, 1H), 6.75-6.72 (m, 1H), 4.48-4.41 (m, 1H), 4.03-3.96 (m, 1H), 3.52-
3.42 (m,
1H), 3.04-2.97 (m, 2H), 2.90-2.82 (m, 2H), 2.72-2.65 (m, 2H), 2.60-2.52 (m,
1H),
2.42-2.34 (m, 1H), 2.06-2.01 (m, 2H), 1.42-1.30 (m, 4H). LC-MS rniz calcd for
C30H34N402 482.3, found 483.2 [M+H]t HPLC purity 94.3 %.
Example 134 N-
(2-aminopheny1)-4-(3-oxo-3-(4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)propyl)benzamide TFA salt
A 0 al
N N
NH2
0
The compound was synthesized using 1-134 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.70 (bs, 1H), 8.80 (bs, 2H), 7.88 (d, 2H,
J=8Hz),
7.36 (d, 2H,J=8Hz), 7.32-7.24 (m, 2H), 7.22-7.12 (m, 4H), 7.05-6.98 (m, 1H),
6.85-
6.83 (m, 1H), 6.75-6.70 (m, 1H), 4.37-4.34 (m, 1H), 3.89-3.84 (m, 1H), 3.02-
2.82 (m,
3H), 2.80-2.75 (m, 2H), 2.68-2.62 (m, 2H), 2.46-2.37 (m, 2H), 1.92-1.82 (m,
1H),
1.74-1.64 (m, 2H), 1.45-1.42 (m, 1H), 1.33-1.20 (m, 2H), 1.07-0.98 (m, 2H). LC-
MS
rniz calcd for C31H36N402, 496.3; found 497.4 [M+H]t HPLC purity 96.2 %.
Example 135 N-
(2-aminopheny1)-4-(3-(4-(((2-(3,4-
difluorophenyl)cyclopropyl)amino)methyl)-1H-imidazol-1-yl)propyl)benzamide
TFA salt
- 279 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
N
N
H N
H NH2
0 40
The compound was synthesized using 1-135 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.75 (bs, 1H), 8.16 (s, 1H), 7.92 (d, 2H, J=7.6
Hz),7.42 (s, 1H), 7.37-7.28 (m, 3H), 7.22-7.16 (m, 2H), 7.04 (t, 1H, J=7.6
Hz), 7.02-
6.96 (m, 1H), 6.90 (t, 1H,J=8.0 Hz), 6.76 (t, 1H, J=7.6 Hz),4.22 (s, 2H), 4.06-
4.00 (m,
2H), 2.93-2.90 (m, 1H), 2.65-2.60 (m, 2H), 2.37-2.30 (m, 1H), 2.08-2.00 (m,
2H),
1.42-1.38 (m, 1H), 1.33-1.28 (m, 1H). LC-MS m/z calcd for C29H29F2N50, 501.2;
found 502.2 [M-Ft1] . HPLC purity 99.5 %.
Example136 N-(2-aminopheny1)-4-(3-(4-0(2-phenylcyclopropyl)amino)methyl)-
1H-imidazol-1-y1) propyl)benzamide TFA salt
at. N/-----eNN
H N
H NH2
TFA Salt 0
The compound was synthesized using 1-136 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.73 (bs, 1H), 9.00 (bs, 1H), 8.09 (s, 1H), 7.92
(d,
2H, J=7.6 Hz), 7.39 (s, 1H),7.31 (d, 2H, J=7.6 Hz), 7.28-7.24 (m, 2H), 7.20-
7.16 (m,
2H), 7.09 (d, 2H, J=7.6 Hz),7.03 (t, 1H, J=7.6 Hz), 6.88 (d, 1H, J=8 Hz),6.74
(t, 1H,
J=7.2 Hz), 4.23-4.18 (m, 2H), 4.04-4.00 (m, 2H), 2.94-2.88 (m, 1H), 2.64-2.57
(m,
2H), 2.38-2.30 (m, 1H), 2.09-1.99 (m, 2H), 1.43-1.37 (m, 1H), 1.30-1.21 (m,
1H). LC-
MS m/z calcd for C29H31N50, 465.3; found 466.3 [M+Hr.HPLC purity 99.8 %.
Example 137 N-(2-aminopheny1)-4-(3-(4-0(2-phenylcyclopropyl)amino)methyl)-
1H-1,2,3-triazol-1-yl)propyl)benzamide TFA salt
Nr¨nq
H N=N H NH2
N
0 IV
The compound was synthesized using 1-137 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.71 (bs, 1H), 9.47 (bs, 2H), 8.18 (s, 1H), 7.92
(d,
2H, J=7.6 Hz), 7.32 (d, 2H, J=7.6 Hz), 7.29-7.24 (m, 2H), 7.20-7.16 (m, 2H),
7.11 (d,
2H, J=7.6 Hz),7.02 (t, 1H, J=7.6 Hz), 6.86 (d, 1H, J=8 Hz),6.71 (t, 1H, J=7.2
Hz),
4.23-4.18 (m, 2H), 4.04-4.00 (m, 2H), 2.94-2.88 (m, 1H), 2.64-2.57 (m, 2H),
2.38-
- 280 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
2.30 (m, 1H), 2.09-1.99 (m, 2H), 1.43-1.37 (m, 1H), 1.30-1.21 (m, 1H). LC-MS
m/z
calcd for C28H301\160, 466.2; found 467.3 [M+H]t HPLC purity 98.7 %.
Example 138 N-(2-aminopheny1)-4-(3-(4-4(2-phenylcyclopropyl)amino)methyl)-
1H-pyrazol-1-yl)propyl)benzamide TFA salt
H N H NH2
N
0 II
The compound was synthesized using 1-138 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.69 (bs, 1H), 9.09 (bs, 1H), 9.05 (bs, 1H), 7.91
(d,
2H, J=7.6 Hz), 7.78 (s, 1H), 7.53 (s, 1H), 7.32-7.25 (m, 4H), 7.20 (t, 2H,
J=7.6
Hz),7.12 (d, 2H, J=7.6 Hz),7.01 (t, 1H, J=7.6 Hz), 6.86 (d, 1H, J=8 Hz), 6.70
(t, 1H,
J=7.2 Hz), 4.42-4.17 (m, 2H), 4.10 (t, 2H, J=6.8 Hz), 2.94-2.86 (m, 1H), 2.65-
2.57
(m, 2H), 2.40-2.33 (m, 1H), 2.11-2.01 (m, 2H), 1.44-1.37 (m, 1H), 1.32-1.25
(m, 1H).
LC-MS m/z calcd for C29H31N50, 465.3; found 466.3 [M+H]t HPLC purity 99 %.
Example 139 N-(2-aminop
heny1)-4-(2- (4- (((2-
phenylcyclopropyl)amino)methyl)piperidin-1-yl)ethyl)benzamide TFA salt
A
N'ON Ed NH2
0
The compound was synthesized using 1-139 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.64 (bs, 1H), 9.36 (bs, 1H), 8.89 (bs, 2H), 7.96
(d,
2H, J=8.0 Hz), 7.40 (d, 2H, J=8.0 Hz), 7.34-7.28 (m, 2H), 7.23 (d, 1H, J=7.6
Hz),
7.21-7.12 (m, 3H), 6.98 (t, 1H, J=7.6 Hz), 6.80 (d, 1H, J=8.0 Hz), 6.62 (d,
1H, J=8.0
Hz), 3.68-3.60 (m, 2H), 3.36-3.28 (m, 3H), 3.10-3.02 (m, 4H), 3.01-2.92 (m,
3H),
2.04-1.96 (m, 2H), 1.95-1.88 (m, 1H), 1.50-1.40 (m, 3H), 1.34-1.28 (m, 1H). LC-
MS
m/z calcd for C30H36N40, 468.6; found 469.6 [M+H]t HPLC purity 99%.
Example 140 N-(2-
aminopheny1)-44(4-((((lR,2S)-2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)methyl)benzamide TFA salt
0
A-N
go -
NH2
TFA salt
-281 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
The compound was synthesized using 1-140 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.75 (s, 1H), 9.64 (bs, 1H), 8.94 (bs, 2H), 8.25
(d,
2H, J=8.0 Hz), 7.62 (d, 2H, J=8 Hz), 7.29 (t, 2H, J=7.2 Hz), 7.25-7.10 (m,
3H), 6.97
(t, 1H, J=7.6 Hz), 6.81 (d, 1H, J=8.0 Hz), 6.72 (t, 1H, J=6.8 Hz), 4.29 (s,
2H), 3.46-
3.34 (m, 2H), 3.26-3.10 (m, 1H), 3.06-2.86 (m, 5H), 2.02-1.78 (m, 3H), 1.52-
1.34 (m,
3H), 1.32-1.24 (m, 1H). LC-MS rniz calcd for C29H34N40, 454.2; found 455.2
[M+H]t HPLC purity 96.5%.
Example 141 N-
(2-aminopheny1)-4-04-0(2-(4-(1-methyl-6-oxo-1,6-
dihydropyridin-3-yl)phenyl)cyclopropyl)amino)methyl)piperidin-1-
yl)methyl)benzamide TFA salt
0
N
HN
NH2
0 N TFA Salt
The compound was synthesized using 1-141 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.86 (bs, 2H), 9.09 (bs, 2H), 8.08-8.60 (m, 3H),
7.79 (dd, J=9.2, 2Hz, 1H), 7.62 (d, J =8.0Hz, 2H), 7.49 (d, J =8.0Hz, 2H),
7.22-7.19
(m, 3H), 7.04 (t, J =7.6Hz, 1H), 6.89 (d, J =8.0Hz, 1H), 6.74 (d, J =7.2Hz,
1H), 6.46
(d, J =9.2Hz, 1H), 4.37 (s, 2H), 3.49 (s, 3H), 3.42-3.39 (m, 2H), 3.28-3.10
(m, 1H),
3.05-2.94 (m, 5H), 2.02-1.82 (m, 3H), 1.51-1.37 (m, 3H), 1.34-1.27 (m, 1H). LC-
MS
rniz calcd for C35H39N502, 561.3; found 560.6[M-H]t HPLC purity 99.9 %.
Example 142 N-
(2-aminopheny1)-44(4-(((2-(4-(1-methyl-1H-pyrazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)methyl)benzamide TFA salt
N 0 el
N H2
N I
T FA
The compound was synthesized using 1-142 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.77 (bs, 1H), 8.89 (bs, 1H), 8.08 (bs, 2H), 8.08
(bs,
3H), 7.81 (s, 1H), 7.61 (d, J =7.6Hz, 2H), 7.47 (d, J =8Hz, 2H), 7.17-7.13 (m,
3H),
7.04-6.98 (m, 1H), 6.85-6.80 (m, 1H), 6.68-6.62 (m, 1H), 4.46-4.32 (m, 4H),
3.83 (s,
3H), 3.43-3.38 (m, 2H), 3.25-3.12 (m, 2H), 3.06-2.96 (m, 5H), 1.98-1.84 (m,
3H),
- 282 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
1.46-1.36 (m, 3H), 1.30-1.26 (m, 1H). LC-MS m/z calcd for C35H39N502,534.3;
found
535.2 [M+H]t HPLC purity 99.8 %.
Example 143 N-
(2-aminopheny1)-4-44-4(2-(4-(3,5-dimethylisoxazol-4-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)methyl)benzamide TFA salt
o
N
NH2
TFA
The compound was synthesized using 1-143 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.76 (bs, 1H), 9.69 (bs, 1H), 9.03 (bs, 1H), 8.97
(bs,
1H), 8.07 (d, J =7.2Hz, 2H), 7.61 (d, J =7.2Hz, 2H), 7.34-7.25 (m, 4H), 7.16
(d, J
=7.6Hz, 1H), 7.00 (t, J =7.6Hz, 1H), 6.82 (d, J =8Hz, 1H), 6.65 (t, J =7.2Hz,
1H),
4.46-4.32 (m, 2H), 3.45-3.37 (m, 2H),3.26-3.13 (m, 1H), 3.08-2.91 (m, 5H),
2.36 (s,
3H), 2.18 (s, 3H), 2.00-1.82 (m, 3H), 1.51-1.31 (m, 4H). LC-MS m/z calcd for
C35H39N502,549.3; found 550.3 [M+H]t HPLC purity 99.6 %.
Example 144 N-
(2-aminopheny1)-4-((4-(((2-(4-(pyrimidin-5-
yl)phenyl)cyclopropyl)amino)methyl)piperidin-l-yl)methyl)benzamide TFA salt
o
N
NH2
I
TFA
The compound was synthesized using 1-145 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.77 (bs, 1H), 9.66 (bs, 1H), 9.16 (s, 1H), 9.12
(s,
2H), 9.02 (bs, 2H), 8.07 (d, J = 7.2Hz, 2H), 7.76(d, J = 8Hz, 2H), 7.62 (d, J
= 8Hz,
2H), 7.34 (d, J= 8Hz, 2H), 7.16 (d, J= 7.6Hz, 1H), 7.00 (t, J= 7.6Hz, 1H),
6.82 (d, J
= 7.6Hz, 1H), 6.65 (t, J = 7.6Hz, 1H), 4.37 (s, 2H),3.46-3.37 (m, 2H),3.27-
3.12 (m,
1H), 3.10-2.90 (m, 5H), 2.03-1.82 (m, 3H), 1.56-1.37 (m, 4H). LC-MS m/z calcd
for
C33H36N60,532.3; found 533.6 [M+H]t HPLC purity 99.9 %.
Example 145 N-(2-aminopheny1)-4-44-4(2-phenylcyclopropyl)amino)methyl)-
1H-pyrazol-1-yl)methyl)benzamide TFA salt
= Abe" N"0
H N kij NH2
o =
The compound was synthesized using 1-145 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.65 (bs, 1H), 9.02 (bs, 2H), 7.93 (d, 2H, J=7.6
Hz),
7.89 (s, 1H), 7.56 (s, 1H), 7.34-7.25 (m, 4H), 7.23-7.19 (m, 1H),7.18-7.10 (m,
- 283 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
3H),6.98 (t, 1H, J=7.6 Hz), 6.81 (d, 1H, J=8 Hz), 6.63 (t, 1H, J=7.2 Hz), 5.41
(s, 2H),
4.19 (s, 2H), 2.94-2.86 (m, 1H), 2.40-2.31 (m, 1H), 1.43-1.37 (m, 1H), 1.32-
1.22 (m,
1H). LC-MS m/z calcd for C27H27N50, 437.2; found 438.3 [M+H]t HPLC purity 98.7
%.
Example 146 N-(2-aminopheny1)-4-44-4(2-phenylcyclopropyl)amino)methyl)-
1H-1,2,3-triazol-1-yl)methyl)benzamide TFA salt
A416' Nr%i
H NN H NH2
0
The compound was synthesized using 1-146 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.72 (bs, 1H), 9.44 (bs, 2H), 8.24 (s, 1H),7.97
(d,
2H, J=7.6 Hz), 7.42 (d, 2H, J=8 Hz), 7.31-7.26 (m, 2H),7.23-7.15 (m, 2H),7.11
(d,
2H, J=7.2 Hz), 7.00 (t, 1H, J=7.6 Hz), 6.84 (d, 1H, J=8 Hz), 6.67 (t, 1H,
J=7.2 Hz),
5.73 (s, 2H), 4.43 (s, 2H), 2.99-2.98 (m, 1H), 2.40-2.37 (m, 1H), 1.44-1.37
(m, 1H),
1.30-1.24 (m, 1H). LC-MS m/z calcd for C26H26N60, 438.2; found 439.5 [M+H]t
HPLC purity 98.9 %.
Example 147 N-
(2-aminopheny1)-4-(2-(4-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-y1)-2-oxoethyl)benzamide
TFA salt
A
H NH2
0 N
0 VI
20 The compound was synthesized using 1-147 following the procedure for
example 120.
itINMR (400 MHz, DMSO-d6): 6 9.73 (s, 1H), 8.793 (bs, 2H), 7.91 (d, 2H,
J=8.0Hz),
7.35 (d, 2H, J=8.4Hz), 7.23-7.19 (m, 3H), 7.14-7.10 (m, 2H), 7.04-7.01 (m,
1H), 6.89-
6.87 (m, 1H), 6.76-6.70 (m, 1H), 4.38-4.35 (m, 1H), 3.99-3.96 (m, 1H), 3.79
(s, 2H),
3.05-2.92 (m, 4H), 2.65-2.55 (m, 1H), 2.48-2.42 (m, 1H), 1.92-1.85 (m, 1H),
1.76-
25 1.67 (m, 2H), 1.47-1.39 (m, 1H), 1.32-1.26 (m, 1H), 1.05-0.97 (m, 2H),
LC-MS m/z
calcd for C30H33FN402,500.1; found501.2 [M+H]t HPLC purity 99.9%.
Example 148 N-
(2-aminopheny1)-4-(2-((2-(4-
fluorophenyl)cyclopropyl)amino)ethoxy) benzamide TFA salt
- 284 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
A
NH2
FiNc)
F =
0
TFA
The compound was synthesized using 1-148 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.69 (s, 1H), 9.18 (bs, 2H), 7.98 (d, 2H, J= 8.8
Hz),
7.21-7.19 (m, 3H), 7.14-7.01 (m, 5H), 6.92-6.88 (m, 1H), 6.78-6.74 (m, 1H),
4.34-
4.29 (m, 3H), 3.59-3.51 (m, 2H), 3.09-3.02 (m, 1H), 1.48-1.43 (m, 1H), 1.34-
1.29 (m,
1H). LC-MS rniz calcd for C24H24FN302, 405.4; found 406.2 [M+H]t HPLC purity
99.7%.
Example 149 N-
(2-aminopheny1)-6-(2-(4-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-ypethoxy)nicotinamide TFA
salt
.).%
HN H NH2
TFA Salt
The compound was synthesized using 1-149 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.48(s,1H), 9.29(bs,1H), 8.98(bs,2H), 8.49(s,1H),
8.06(d, J=7.6Hz, 1H), 7.25-7.20(m,2H), 7.16-7.07 (m, 3H), 6.98(t, J=7.2Hz,
1H),
6.80(d,J=7.6 Hz, 1H), 6.61 (t,J=7.2Hz, 1H), 6.51(d,J=9.6Hz, 1H), 4.40-
4.30(m,2H),
3.70-3.65(m, 2H), 3.46-3.39(m,2H),3.30-3.25(m,1H),3.05-2.95(m,5H), 2.03-
1.95(m,3H), 1.49-1.39(m,3H),1.31-1.26 (m, 1H). LC-MS rniz calcd for
C29H34FN502,
503.2; found 504.3[M+H]t HPLC purity99.6%.
Example 150 N-(-
2-aminopheny1)-2-((2-4(((2-(4-
flurophenyl)cyclopropyl)amino)methyl)piperdine-1-ypethypamino)pyrimidine-5-
carboxamide TFA salt
A )
401 N N NH NH2
TFA Salt
The compound was synthesized using 1-150 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.68 (bs, 1H), 9.35 (bs, 1H), 9.08 (bs, 2H),8.88
(s,
2H), 7.98-7.93 (m, 1H), 7.24-7.10 (m, 5H), 7.03 (t, 1H, J=7.2Hz), 6.88 (d, 1H,
J=8Hz), 6.73 (t, 1H, J=7.6Hz), 3.76-3.60 (m, 4H), 3.34-3.21 (m, 3H), 3.05-2.92
(m,
- 285 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
4H), 2.46-2.41 (m, 1H), 2.01-1.92 (m,3H), 1.49-1.40 (m,3H), 1.30-1.24 (m,1H).
LC-
MS m/z calcd for C28H34FN70, 503.2; found 504.3[M+H]t HPLC purity 99.8%.
Example 151 N-(2-aminopheny1)-54(2-(4-fluorophenyl)cyclopropyl)glycyl)-
4,5,6,7-tetrahydrothieno[3,2-c]pyridine-2-carboxamide TFA salt
0:>
HN *H II
0
H2N
TFA
The compound was synthesized using 1-151 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 9.65 (s, 1H), 9.23 (s, 2H), 7.73 ¨ 7.71 (m, 1H),
7.22 ¨
7.20 (m, 2H), 7.13 ¨ 7.09 (m, 3H), 6.98 (bs, 1H), 6.80 (bs, 1H), 6.63 (bs,
1H), 4.62 (s,
1H), 4.55 (s, 1H), 4.37 ¨ 4.35 (m, 2H), 3.70 (s, 2H), 2.97 (s, 2H), 2.85 (s,
2H), 1.48
(bs, 1H), 1.28 ¨ 1.26 (m, 1H). LC-MS m/z calcd [M+H] 464.1, found 465Ø HPLC
purity 99.0 % .
Example 152 N-
(2-aminopheny1)-2-(2-((2-(4-
fluorophenyl)cyclopropyl)amino)acety1)-1,2,3,4-tetrahydroisoquinoline-7-
carboxamide TFA salt
F0&0
H2
N
H II
N
0
The compound was synthesized using 1-152 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.66 (bs, 1H), 9.26 (bs, 2H), 7.86-7.79 (m, 2H),
7.34-7.32 (d, 1H, J= 8.0Hz), 7.25-7.15 (m, 3H), 7.14-7.07 (m, 2H), 7.02-6.98
(m, 1H),
6.85-6.81 (m, 1H), 6.71-6.64 (m, 1H), 4.74-4.70 (m, 2H), 4.35 (s, 2H), 3.76-
3.74 (m,
3H), 3.02-2.96 (m, 1H), 2.90-2.82 (m, 2H), 1.52-1.48 (m, 1H), 1.32-1.26 (m,
1H). LC-
MS m/z calcd for C27H27FN402, 458.2; found 459.2 [M+H]t HPLC purity 99.2 %.
Example 153 N-
(2-aminopheny1)-2-(3-(4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)propyl)oxazole-4-carboxamide
TFA salt
EricN
3-
A NH NH2
The compound was synthesized using 1-153 following the procedure for example
120.
- 286 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
itINMR (400 MHz, DMSO-d6): 6 9.36 (bs, 2H), 8.94 (bs, 2H), 8.67 (s, 1H), 7.32-
7.26
(m, 3H), 7.23-7.15 (m, 3H),6.98 (t, J=7.6 Hz, 1H,), 6.83 (d, J=8 Hz, 1H,),
6.66 (t,
J=7.2 Hz, 1H), 3.60-3.54 (m, 2H), 3.23-3.14 (m, 3H), 3.07-2.90 (m, 7H), 2.15-
2.11
(m, 2H), 2.00-1.94 (m, 3H), 1.49-1.40 (m, 3H), 1.32-1.26 (m, 1H). LC-MS m/z
calcd
for C28H35N502, 473.3; found 474.3 [M+Hr.HPLC purity 99.8 %.
Example 154 N-
(2-aminopheny1)-2-(3-(4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)propyl)thiazole-5-carboxamide
TFA salt
H2N
A N HN
The compound was synthesized using 1-154 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.87 (bs, 1H), 9.35 (bs, 1H), 9.00 (bs, 2H), 8.46
(s,
1H), 7.32-7.26 (m, 2H), 7.23-7.09 (m, 4H),7.02 (t, 1H, J=7.6 Hz), 6.83 (d, 1H,
J=8
Hz), 6.66 (t, 1H, J=7.2 Hz), 3.59-3.52 (m, 2H), 3.28-2.88 (m, 9H), 2.46-
2.42(m, 1H),
2.18-2.11 (m, 2H), 2.00-1.94 (m, 3H), 1.49-1.39 (m, 3H), 1.32-1.22 (m, 1H). LC-
MS
m/z calcd for C28H35N50S, 489.3; found 490.3 [M+H]t HPLC purity 99.8 %.
Example 155 N-
(2-aminopheny1)-4-((2-((2-(4-
fluorophenyl)cyclopropyl)amino)acetamido)methyl)benzamide TFA salt
o
A
40 m
[10 NH
The compound was synthesized using 1-155 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.70 (s, 1H), 9.30 (bs, 2H), 8.97 (s, 1H), 7.94
(d,
2H, J=8 Hz), 7.39 (d, 2H, J=8 Hz), 7.22-7.18 (m, 3H), 7.13-7.09 (m, 2H), 7.03-
6.99
(m, 1H), 6.85 (d, 1H, J=7.2 Hz), 4.44 (d, 2H, J=6 Hz), 3.98-3.74 (m, 5H), 2.92
(m,
1H), 1.47-1.45 (m, 1H), 1.30-1.22 (m, 1H). LC-MS m/z calcd for C25H25FN402,
432.2; found 433.0 [M+H]t HPLC purity 99.8%.
Example 156 E)-
N-(2-aminopheny1)-4-(3-(4-(((2-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidin-1-y1)-3-oxoprop-1-en-1-
yl)benzamide TFA salt
- 287 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
A fg2N Ain
N
rEµilON
0
The compound was synthesized using 1-156 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.81 (s, 1H), 8.85 (bs, 2H), 8.00 (d, 2H, J=7.6
Hz),
7.85 (d, 2H, J=8 Hz), 7.53 (d, 1H, J=15.6 Hz), 7.40 (d, 1H, J=15.6 Hz), 7.25-
7.19 (m,
3H), 7.16-7.11 (m, 2H), 7.05-7.02 (m, 1H), 6.89-6.87 (m, 1H), 6.75-6.69 (m,
1H),
4.52-4.28 (m, 2H), 3.17-2.92 (m, 4H), 2.76-2.63 (m, 1H), 2.48-2.45 (m, 1H),
2.03-
1.92 (m, 1H), 1.87-1.76 (m, 2H), 1.47-1.43 (m, 1H), 1.32-1.16 (m, 3H). LC-MS
rniz
calcd for C31H33FN402, 512.6; found 513.2 [M+H]t HPLC purity 99.7%.
Example 157 (E)-
N-(2-aminopheny1)-4-(3-(3-(((2-(4-
fluorophenyl)cyclopropyl)amino)methypazetidin-1-y1)-3-oxoprop-1-en-l-
yl)benzamide TFA salt
o
N
40 11C`NI (13 NH2
0
TFA
The compound was synthesized using 1-157 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.80 (bs, 1H), 8.95 (bs, 2H), 8.00 (d, J =8Hz,
2H),
7.79 (d, J =8Hz, 2H), 7.50 (d, J =15.6Hz, 1H), 7.26-7.21 (m, 5H), 7.05-7.00
(m, 1H),
6.90-6.85 (m, 1H), 6.79 (d, J = 16Hz, 1H), 6.75-6.67 (m, 1H), 4.47-4.42 (m,
1H),
4.10-4.03 (m, 2H), 3.82-3.77 (m, 1H), 3.47-3.38 (m, 2H), 3.03-2.92 (m, 2H),
1.47-
1.40 (m, 1H), 1.33-1.27 (m, 1H). LC-MS rniz calcd for C29H29FN402, 484.2;
found
485.2 [M+H]t HPLC purity 99.6 %.
Example 158 N-(44(2-
aminophenyl)carbamoyl)benzy1)-4-(42-(4-
fluorophenyl)cyclopropyl)amino)methyl)piperidine-1-carboxamide TFA salt
A o
N N
F NH2
The compound was synthesized using 1-158 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.75 (s, 1H), 8.82 (bs, 2H), 7.91 (d, 2H, J=
7.6Hz),
7.35 (d, 1H, J= 8.0Hz), 7.26-7.18 (m, 3H), 7.16-7.08 (m, 3H), 7.08-7.02 (m,
1H),
6.94-6.88 (m, 1H), 6.79-6.75 (m, 1H), 4.32-4.28 (m, 2H), 4.05-3.94 (m, 2H),
3.07-
2.91 (m, 3H), 2.76-2.62 (m, 2H), 2.48-2.43 (bs, 1H), 1.90-1.81 (m, 1H), 1.74-
1.63 (m,
- 288 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
2H), 1.46-1.41 (m, 1H), 1.30-1.25 (m, 1H), 1.15-1.00 (m, 2H). LC-MS m/z calcd
for
C30H34FN502, 515.2; found 516.3 [M+H]t HPLC purity 99.9 %.
Example 159 N-
(2-aminopheny1)-4-(3-(2-oxo-4-(((2-
phenylcyclopropyl)amino)methyl) piperidin-l-yl)propyl)benzamide TFA salt
o
rN
NH2
The compound was synthesized using 1-159 following the procedure for example
120.
LC-MS m/z calcd for C31H36N402, 496.6; found 497.6 [M+H]t
Example 160 N-
(2-aminopheny1)-4-((4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)sulfonyl)benzamide TFA Salt
A
ir?!
H
NH,
-
N
0 IW
The compound was synthesized using 1-160 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.88 (bs, 1H), 8.73 (bs, 2H), 8.19 (d, 2H, J=8.0
Hz),
7.85 (d, 2H, J=7.6 Hz), 7.31-7.24 (m, 2H), 7.22-7.11 (m, 4H),7.02-6.96 (m,
1H), 6.82-
6.77 (m, 1H), 6.63-6.59 (m, 1H),3.72-3.67 (m, 2H), 3.02-2.92 (m, 4H), 2.38-
2.32 (m,
1H), 2.28-2.24 (m, 1H),1.84-1.77 (m, 2H), 1.68-1.61 (m, 1H), 1.43-1.38 (m,
1H),
1.34-1.21 (m, 3H). LC-MS m/z calcd for C28H32N403S, 504.2; found 505.3 [M+H]t
HPLC purity 99.4%.
Example 161 N-
(2-aminopheny1)-4-(((4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)sulfonyl)
methyl)benzamide
TFA salt
H2N
A HN
110N,,s 40 0
The compound was synthesized using 1-161 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.83 (bs, 1H), 8.88 (bs, 2H), 7.98 (d, 2H, J=8.0
Hz),
7.52 (d, 2H, J=7.6 Hz), 7.32-7.26 (m, 2H), 7.23-7.14 (m, 4H), 7.07-7.03 (m,
1H),
6.92-6.88 (m, 1H), 6.78-6.74 (m, 1H), 3.62-3.56 (m, 2H), 3.05-2.94 (m, 3H),
2.79-
2.68 (m, 2H), 2.43-2.39 (m, 1H), 1.80-1.70 (m, 3H), 1.46-1.42 (m, 1H), 1.31-
1.20 (m,
5H). LC-MS m/z calcd for C29H34N403S, 518.2; found 519.2 [M+H]t
- 289 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Example 162 N-
(2-aminopheny1)-4-(2-((4-(((2-
phenylcyclopropyl)amino)methyl)piperidin-l-yl)sulfonyl) ethyl)benzamide TFA
salt
A
0' 1j1
IN-1
H NH2
N
o
The compound was synthesized using 1-162 following the procedure for example
120.
itINMR (400 MHz, DMSO-d6): 6 9.73 (bs, 1H), 8.85 (bs, 2H), 7.92 (d, 2H, J=8.0
Hz),
7.43 (d, 2H, J=8 Hz), 7.31-7.26 (m, 2H), 7.22-7.14 (m, 4H), 7.04-7.00 (m, 1H),
6.89-
6.85 (m, 1H), 6.74-6.68 (m, 1H), 3.65-3.60 (m, 2H), 3.40-2.35 (m, 2H), 3.07-
3.00 (m,
4H), 2.98-2.93 (m, 1H), 2.84-2.76 (m, 2H), 2.43-2.40 (m, 1H), 1.82-1.78 (m,
3H),
1.48-1.42 (m, 1H), 1.31-1.20 (m, 3H). LC-MS m/z calcd for C30H36N403S, 532.3;
found 533.3 [M+H]t HPLC purity 99.4%.
BIOLOGY METHODS
TR-FRET assay for LSD1 (Perkin Elmer)
15[000163] LSD1 enzyme was produced in house. Tranylcypromine (TCP), LSD1
inhibitor
was procured from Selleckchem. LSD1 enzyme, TCP and Biotinylated peptide
substrate were diluted in assay buffer just before use. 2X inhibitor (10 [El,
diluted in
assay buffer) or Assay Buffer, and 5 nMenzyme were added to a 96 well plate
and
incubated at room temperature for 30 min. 5 [EL of biotinylated Histone
H3K4me1
peptide (4X) was added to each well and incubated at room temperature (RT) for
1
hour. Stop Solution containing 300 [EM tranylcypromine in 1X LANCE Detection
Buffer was added to the wells and incubated for 5 min at RT. Then, Detection
mix
containing 2 nM Eu-Ab and 50 nM ULight-Streptavidin in 1X LANCE Detection
Buffer was prepared and added to the reaction mix. This mixture was incubated
for 1
hour at room temperature. Readings were taken with the Pherastar Reader in TR-
FRET
mode (excitation at 337 nm & emission at A-665 nm, B-620nM).
Histone Deacetylase assay (BPS Biosciences)
[000164] Histone deacetylase assay was done as per manufacturer's
instructions.
Briefly, assay buffer, 200uM HDAC substrate (fluorogenic HDAC acetylated
peptide
substrate for class I HDACs (HDACs 1, 2, and 3) and class 2b HDACs (HDACs 6
and
- 290 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
10) and 1%BSA are taken as a master mix and aliquoted as 40u1 per well.
Compounds(10X) were diluted in assay buffer and were added to respective wells
of a
black 96 well plate. HDAC6 human recombinant enzyme was thawed on ice and 5 1
(7ng/u1) enzyme was added per well. The plate was incubated at 37 C for 1
hour.
Developer solution was then added (50 1 per well) and incubated at room
temperature
for 10 minutes. Fluorescence was measured at an excitation wave length of 350-
380 nm
and emission wavelength of 440-480 nm.
As described above, compounds were tested for LSD1, HDAC1, HDAC2 and HDAC6
enzyme inhibitory activities.
.. Anticancer activity: Alamar Blue Assay
[000165] Cells were seeded at 5000 cells/per well in 96-well tissue culture
plate and
incubated at 37 C/ 5% CO2. After 16-24 hours, fresh media was added to the
wells.
Compounds were then (1% DMS 0 conc.) added to the cells at 10 concentrations
ranging from 10-0.0005 uM prepared in 3-fold serial dilutions. Cells were
incubated for
68-72h at 37 C/ 5% CO2. Alamar BlueTM reagent was added and incubated for 1-3
hours at 37 C/ 5% CO2. Plates were read on fluorescence reader at 540 nm
excitation,
590 nm emission wave lengths.
As described above, compounds were tested for anticancer activities in
different tumor
cell lines and G150 were determined.
.. Metabolic Stability
[000166] The microsomal suspension was prepared by adding liver microsomes to
100
mM potassium phosphate buffer (pH7.4) to give a final protein concentration of
0.5
mg/mL. The stock solution of NCE (10 mM in DMSO) was added to the microsomes
to
provide a final concentration of 1 M. Incubations were undertaken with NADPH
(1
mM final concentration) for 0,5,15 and 30 min at 37 C, after which reactions
were
quenched with acetonitrile (quench ratio 1:1). Samples were vortexed and
centrifuged at
5,000 rpm for 10 min to remove proteins. Supernatant were analyzed on LC-
MS/MS.
Table 1: Selected list compounds with Enzymatic, cellular activity and
metabolic
stability
Compound LSD1 HDAC6 MM1S Metabolic
ICso ICso ECso stability
uM uM uM HLM/MLM
%remaining
in 30 min
- 291 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
1 0.083 ND ND 21/5
2 0.057 ND 0.023 41/19
3 0.020 ND 0.104 65/<5
4 0.684 ND ND ND
0.049 0.373 0.088 86/59
6 0.049 ND 0.034 44/<5
7 0.027 0.174 0.048 70/<5
8 0.040 0.088 0.007 88/52
9 0.101 ND 0.004 65/50
0.221 ND 0.009 81/55
11 0.019 ND 0.018 44/14
12 0.778 0.136 0.011 95/85
13 0.044 ND 0.024 57/23
14 0.367 ND 0.008 88/82
0.095 0.134 0.012 ND
16 0.089 ND 0.017 47/25
17 0.128 0.398 0.017 69/57
18 0.150 ND 0.003 41/28
19 0.309 ND 0.007 83/71
0.100 0.072 0.003 91/69
21 0.115 0.091 0.009 86/51
22 6.4 0.171 0.044 60/38
23 0.778 0.136 0.011 95/85
24 1.160 ND 0.009 71/74
0.840 ND 0.011 75/85
26 0.020 ND ND 30/13
27 0.016 >1.00 ND 75/51
28 0.023 0.264 ND 73/35
29 0.178 0.819 ND >95/90
0.119 0.202 0.045 16/51
31 0.111 ND ND 39/18
32 0.070 ND 0.017 41/22
33 2.250 > 1.00 ND ND
- 292 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
34 0.031 >1.00 0.094 90/79
35 0.034 0.199 1.429 ND
36 0.011 0.235 ND 63/53
37 0.043 0.141 1.731 86/55
38 0.053 0.169 3.642 87/>95
39 0.034 0.732 2.969 87/>95
40 0.178 0.037 ND ND
41 0.319 0.074 ND ND
42 0.015 0.49 0.19 ND
43 0.005 0.048 0.002 40/56
44 0.013 0.065 0.045 71/55
45 0.465 ND 0.048 ND
46 0.026 0477 0.071 54/47
47 0.006 0.051 0.033 54/66
48 0.029 0.184 0.025 85/<5
49 0.018 ND 0.062 48/34
50 0.029 0.212 0.035 86/80
51 0.006 0.038 0.002 74/91
52 0.004 0.012 0.003 79/56
53 0.022 0.291 0.024 89/82
54 0.023 0.214 0.038 65/72
55 0.022 0.130 0.189 40/36
56 0.002 0.019 0.001 87/75
57 0.021 0.059 0.006 92/75
58 0.025 0.043 0.015 85/91
59 0.032 0.204 0.018 76/75
60 0.021 0.046 0.024 73/72
61 0.012 0.121 0.049 77/66
62 0.043 0.066 0.007 77/66
63 0.031 1.315 0.191 86/69
64 0.059 0.058 0.011 82/77
65 0.038 0.383 0.026 ND
66 0.019 0.100 0.021 79/85
- 293 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
67 0.484 0.272 0.070 ND
68 ND 0.104 ND ND
71 1.1 0.028 ND 73/<5
72 0.730 0.018 0.343 67/<5
73 0.109 0.243 0.540 52/53
74 0.028 0.112 0.253 47/27
75 0.286 0.365 0.246 ND
76 0.084 0.022 0.042 ND
77 0.065 0.071 0.070 ND
78 0.103 0.183 0.060 72/32
79 0.079 0.024 0.039 18/6
80 1.5 0.068 0.331 82/87
81 0.394 0.125 0.022 >95/>95
82 0.094 0.035 0.619 77/72
83 0.090 0.179 0.153 69/82
84 0.057 0.045 0.112 78/40
84A 0.107 0.022 0.026 78/41
84B 0.230 0.025 0.259 75/45
85 0.062 0.575 >10 ND
86 0.018 0.554 0.676 71/58
87 0.025 1.061 9.107 ND
88 ND 0.077 ND ND
89 0.020 0.720 0.311 ND
90 0.021 0.170 0.339 80/93
91 0.332 0.138 0.468 85/79
92 0.273 0.115 1.4 95/91
93 0.072 0.187 1.04 1.040
95 0.033 0.350 0.144 75/55
96 0.022 6.608 >10 93/89
97 0.033 >10 >10 ND
98 0.037 5.96 >10 ND
99 0.004 0.051 0.027 63/76
100 0.023 ND 0.017 63/57
- 294 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
101 0.197 0.152 0.029 59/29
102 0.022 0.075 0.005 >99/>99
103 0.124 0.041 0.045 85/53
103A 0.667 0.022 0.052 87/68
103B 0.154 0.036 0.015 76/73
104 0.206 0.032 0.269 80/77
104A 0.455 0.053 0.183 74/68
104B 1.002 0.045 0.374 74/70
105 0.138 0.018 0.013 77/72
106 0.026 0.035 0.014 74/92
107 0.399 0.085 0.078 83/67
108 0.134 0.195 0.584 >95/85
109 0.024 0.107 1.1 ND
110 0.026 >10 >10 88/95
111 0.022 2.98 3.065 95/91
112 0.022 0.517 0.063 ND
113 0.026 >10 >10 78/76
114 0.051 0.068 0.044 83/56
114A 0.063 0.038 0.014 67/62
114B 0.285 0.033 0.097 73/43
115 0.065 0.053 0.010 75/45
115A 0.225 0.050 0.077 59/90
116 0.069 0.135 0.157 63/50
117 0.046 0.074 0.460 80/82
118 0.037 0.058 0.021 77/76
119 0.019 0.049 0.772 72/49
120 0.596 ND 0.361 27/16
121 0.006 ND 0.003 36/42
122 0.006 ND 0.015 77/72
123 0.007 ND 0.009 82/72
124 0.019 ND 0.014 73/68
125 0.021 ND 34/49
126 0.032 ND 0.012 79/76
- 295 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
127 0.764 >10 0.126 17/19
128 0.128 > 10 0.070 ND
129 ND >10 ND ND
132 0.005 >10 0.021 81/80
133 0.040 >10 ND 19/9
134 0.042 >10 0.174 10/10
135 0.145 >10 0.247 2/8
136 0.570 >10 0.174 4/3
137 0.139 >10 0.126 ND
138 0.099 >10 0.065 ND
139 ND >10 ND ND
140 0.002 >10 0.002 85/83
141 0.030 >10 0.194 88/75
142 0.012 >10 0.120 72/52
143 0.037 >10 0.102 37/50
144 0.041 >10 0.152 68/52
145 0.061 >10 0.123 ND
146 0.069 >10 0.106 ND
147 0.032 >10 0.029 48/<5
148 0.024 >10 0.040 46/34
149 0.021 >10 >10 ND
150 0.016 >10 0.411 >95/>95
151 0.216 >10 0.345 30/18
152 0.306 >10 0.566 36/31
153 0.024 >10 0.571 ND
154 ND >10 ND ND
155 0.271 >10 0.042 46/34
156 0.186 >10 0.097 58/49
157 0.058 >10 0.167 53/63
158 0.171 >10 0.058 50/<5
160 0.043 >10 0.178 ND
ND: Not determined
Table 3: Anticancer profileration (EC50 in pM)in different cell lines at 144hr-
- 296 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
Compound HEL- OCI- MV- CCRF- MDAMB231 A375
92.1.7 AML3 4-11 CEM
2 0.057 0.159 0.014 0.652 0.478 1.600
0.026 0.146 0.054 0.533 1.600 2.300
8 0.042 0.046 0.048 0.119 0.193 0.698
0.089 0.089 0.026 0.157 0.403 0.381
12 0.004 0.041 0.009 0.375 1.300 2.800
13 0.026 0.056 0.010 0.378 1.100 1.300
43 0.001 0.115 0.002 0.493 0.747 1.030
47 0.005 0.146 0.005 1.4 0.875 3.6
51 0.003 0.014 0.031 ND ND ND
52 0.005 0.022 0.001 0.371 0.891 1.7
56 0.01 0.01 0.007 ND ND ND
71 6.05 0.732 0.990 ND ND ND
72 4.8 0.573 0.978 ND ND ND
121 0.009 0.0007 0.004 ND ND ND
In vivo PK studies in Mice
[000167] All the animal experiments were approved by Institutional Animal
Ethical
5 Committee (IAEC/JDC/2015/72). Male Balb/C mice (n=24)were procured from
Vivo
Biotech, Hyderabad, India. The animals were housed in Jubilant Biosys animal
house
facility in a temperature (22 2 C) and humidity (30-70%) controlled room (15
air
changes/hour) with a 12:12 h light:dark cycles, had free access to rodent feed
(Altromin
Spezialfutter GmbH & Co. KG., Im Seelenkamp 20, D-32791, Lage, Germany) and
10 water for one week before using for experimental purpose. Following -4 h
fast (during
the fasting period animals had free access to water) animals were divided into
two
groups (n=12/group).Group I animals (27-29 g) received NCE orlaly at 10 mg/Kg
(strength: 1.0 mg/mL; dose volume: 10 mL/Kg), whereas Group II animals (29-31
g)
received NCE intravenously (strength: 0.1 mg/mL; dose volume: 10 mL/Kg) at 2.0
mg/Kg dose. Post-dosing serial blood samples (100 L, sparse sampling was done
and
at each time point three mice were used for blood sampling) were collected
using
Micropipettes (Microcaps ; catalogue number: 1-000-0500) through tail vein
into
polypropylene tubes containing K2.EDTA solution as an anti-coagulant at 0.25,
0.5, 1,
- 297 -
CA 03022561 2018-10-29
WO 2017/195216 PCT/IN2017/050167
2, 4, 8, 10 and 24 (for oral study) and 0.12, 0.25, 0.5, 1, 2, 4, 8 and 24
(for intravenous
study). Plasma was harvested by centrifuging the blood using Biofuge (Hereaus,
Germany) at 1760 g for 5 min and stored frozen at-80 10 C until
analysis.Animals
were allowed to access feed 2 h post-dosing.
[000168] The criteria for acceptance of the analytical runs encompassed the
following: (i) 67% of the QC samples accuracy must be within 85-115% of the
nominal concentration (ii) not less than 50% at each QC concentration level
must meet
the acceptance criteria (US DHHS, FDA, CDER, 2001).Plasma concentration-time
data of the compound was analyzed by non-compartmental method using Phoenix
WinNonlin Version 6.3 (Pharsight Corporation, Mountain View, CA).
Table 2: In vivo PK data
Compound Dose CO- CMax- AUC t112-hr Cl Vd F%
mg/kg ng/mL ng/mL ng/mL/hr IV/PO mL/min/Kg L/Kg
IV/PO IV IV/PO IV/PO IV IV
57 2/10 260 254/217 169/677 1.71/1.83 190
28 80
115A 2/10 452 452/354 301/638 0.87/2.78 108
8 43
142 2/50 198 150/555 357/3217 12.6/6.7
88 25 36
61 10/50 5340 2758/771
1287/1920 2.80/1.48 125 30 30
Expression of biomarkers assessed by western blotting
Cell lysates were prepared in RIPA buffer (150mM Tris-HC1, 150mM NaCl, 1%NP-
40, 0.5% sodium deoxycholate, 0.1%SDS, 0.5mM PMSF, 1X protease inhibitor
cocktail) and 5-1Oug of protein was loaded for SDS-PAGE. Proteins were then
transferred to a nitrocellulose membrane and then probed with respective
antibodies.
The bands of interest were visualized by chemiluminescence. Antibodies used
were
H3K4 mono, di and tri methyl from Abcam, Acetyl alpha tubulin and acetyl
histone
(K9) from Cell signaling technologies.
Expression of biomarkers assessed by PCR
RNA was extracted from cells or tumor samples using the TRI reagent
(manufacturer's protocol). Generally l[tg RNA per sample is used with 10mM
dNTPs
and 50[tM Random primers (Thermo). The samples are kept at 65 C for 5 minutes,
then 1 min on ice and then the master mix(5X strand buffer, 0.1M DTT, RNase
out
inhibitor, Superscript)is added to each sample anth then the RT reaction is
completed
in a PCR machine (25 C-5min, 50 C-60min, 70 C-15min). The 25-30ng of cDNA
- 298 -
CA 03022561 2018-10-29
WO 2017/195216
PCT/IN2017/050167
thus prepared is used for the QPCR using respective primers for CD86, CD11b,
GFi 1B and f3 actin. The SYBR Green qPCR plate is set up according to the
manufacturer's protocols.
Xenograft Studies
Tumor CellImplantation and Randomization of Animals
[000169] Five million (5x106) cells in 100 pl of serum free medium were mixed
with
equal amount of matrigel and the entire the mixture was injected
subcutaneously at the
right flank region. The tumors were measured with Vernier calipers
periodically after
first week of injection. When the tumor volume reached 120-150 mm3(3-4 weeks
after
injection) the animals were randomized into different groups so that their
tumor
volume is approximately similar in all groups.
Determination of in vivo efficacy and Tumor Growth Inhibition
[000170] For PO dosing, the compounds were prepared in the formulation
containing
0.5% Methyl cellulose and 0.01% Tween 80.Animals were dosed with compounds
prepared in specific formulations at the required doses. Tumors size and body
weights
were measured twice or thrice a week. Tumors were harvested at the end of the
study
after euthanizing the animals according to approved protocols. From the
harvested
tumor one part was snap frozen and given for PK studies and the other half was
homogenized and the lysates were tested for target inhibition using western
blotting.
Before the tumor was harvested, blood (¨ 200 L) was collected by ocular
bleeding for
PK studies.Changes in tumor volume (A volumes) for each treated (T) and
control (C)
group were calculated by subtracting the mean tumor volume on the first day of
treatment (starting day) from the mean tumor volume on the specified
observation
day. These values were used to calculate a percentage growth (% T/C) using the
formula:
% T/C = (AT/AC) X 100
where AT > 0, or
% T/C = (AT/ATi) X 100
Where AT <0 and Ti is the mean tumor volume at the start of the experiment.
Percentage tumor growth inhibition was calculated as [100 - % T/C].
- 299 -