Note: Descriptions are shown in the official language in which they were submitted.
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
TITLE: METHODS OF
USING CARBON QUANTUM DOTS TO
ENHANCE PRODUCTIVITY OF FLUIDS FROM WELLS
SPECIFICATION
Technical Field
[0001] Embodiments
of the disclosure relate generally to methods of forming carbon
quantum dots, methods and systems of using the carbon quantum dots to
determine at least
one property within subterranean formations and methods of using the carbon
quantum dots
to enhance the productivity of hydrocarbon-containing fluids from the
subterranean
formations.
Back!round
[0002] During
formation and operation of a wellbore, it may be desirable to measure at
least one property within a subterranean formation through which the wellbore
extends.
For example, a high pH may be a precursor of scale build-up and a low pH may
be a
precursor to corrosion of wellbore equipment. Thus, the pH of a foimation
fluid is
conventionally monitored to aid in reducing scale build-up and potential
corrosion of the
wellbore equipment.
[0003]
Conventionally, the pH of the formation fluid is determined by obtaining a
sample of the formation fluid and analyzing the sample in a laboratory.
However, as the
formation fluid is brought from formation conditions (e.g., high temperature
high pressure
conditions), acid gases and salts may come out of solution, irreversibly
changing the pH of
the sample. Thus the analyzed sample may not be an accurate representation of
the pH of
the formation fluid at formation conditions.
[0004] Other
methods of determining a pH of formation fluids include introducing a
dye (e.g., phenol red, methylene blue, and/or cresol red) into the formation
and correlating
the pH of the formation fluid to the color of the dye. However, such dyes may
not be
1
=
formulated to determine the pH of the formation fluid with a desired level of
accuracy. For
example, some dyes may only be sensitive within a narrow pH range, such as a
pH range of
about 3.0 pH units. In addition, the dyes may be chemically unstable under
formation
= conditions. Further, a continuous pH measurement may not be obtained
unless the dye is
continuously injected into the subterranean formation.
= 100051 Other properties of the subterranean formation (e.g.,
salinity, wettability of
formation surfaces, flow paths through the subterranean formation, etc.) may
be determined
using one or more tracer compounds, For example, water tracers may he
introduced into the
subterranean formation to estimate flow patterns between well during enhanced
oil recovery
processes, such as, for example, water flooding.
10006] In addition to measuring at least one property within
the formation through
which the wellbore extends, tracers have been used in reservoir monitoring.
Reservoir
monitoring refers to the gathering and analysis of information from reservoirs
during
production. Such monitoring is used to assess the productivity of producing
formations
or zones within the formations from which fluids are being produced.
Monitoring of
produced fluids is important in order to increase efficiency of a hydraulic
fracturing
oPeration. Reservoir monitoring is further used to determine water saturation
levels in
the well.
[0007] In the past, produced fluids have been monitored by
the use of tracers
placed in packs in strategic areas within the well. See, for instance, U.S.
Patent Nos.
3,991,827; 4,008,763; 5,892,147, and 7,560,690.
[0008] Tracers may include a fluorophore (i.e., a compound
that can re-emit light upon
light excitation) and a presence of the tracer may be determined by optical
spectroscopy
(absorbance, fluorescence and phosphorescence). However, the fluorophore may
include
= organic molecules and rare-earth complexes that are toxic and/or
radioactive and may
contaminate the subterranean formation (e.g., aquifers located in the
subterranean
= formation). Further, fluorophores may decompose at downhole conditions
and may be
subject to photobleaching (i.e., the photochemical alteration of the
fluorophore such that it
becomes permanently unable to fluoresce) and photo blinking (i.e.,
fluorescence
intermittency).
Brief Summary
10009] Embodiments disclosed herein include systems and
methods for determining at
least one property of a subterranean formation. Additional embodiments
disclosed herein
include methods of enhancing the productivity of hydrocarbon containing fluids
from a
subterranean formation penetrated by a well.
2
CA 3024488 2020-03-17
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
[00010] For example, in accordance with one embodiment, a system for
determining at
least one property of at least one fluid in at least one subterranean
formation comprises a
fluid delivery system configured and positioned to deliver a fluid into at
least one of at least
one subterranean formation and a wellbore extending through the at least one
subterranean
formation, a radiation source within the wellbore, the radiation source
configured to
generate excitation radiation, carbon quantum dots disposed in the fluid, and
a detector
within the wellbore, the detector configured to measure at least one optical
property of the
carbon quantum dots.
[00011] In additional embodiments, a system for determining at least one
property of at
least one subterranean formation comprises at least one fiber optic cable
within a wellbore
extending through at least one subterranean formation, the at least one fiber
optic cable
including at least one optical fiber comprising carbon quantum dots, a
radiation source
coupled to the at least one optical fiber, the radiation source configured to
generate
excitation radiation for transmission through the at least one optical fiber,
and a detector
coupled to the at least one fiber optic cable, the detector configured to
measure at least one
optical property of the carbon quantum dots.
[00012] In further embodiments, a method of forming carbon quantum dots
comprises
providing an electrolyte comprising a carbon source and a source of ions to an
electrochemical cell, introducing the electrolyte between platinum electrodes
of the
electrochemical cell, and applying electrical current between the platinum
electrodes to
form carbon quantum dots including carbon from the carbon source.
[00013] In further embodiments, a method of determining at least one
property within at
least one subterranean formation comprises introducing at least one fiber
optic cable into at
least one of at least one subterranean formation and a wellbore extending into
the at least
one subterranean formation, transmitting excitation radiation through the at
least one fiber
optic cable from a radiation source coupled to the at least one fiber optic
cable, exposing
carbon quantum dots disposed in a fluid in the wellbore or on the at least one
fiber optic
cable to the excitation radiation, receiving, at an optical sensor coupled to
the at least one
fiber optic cable, an emitted radiation from the carbon quantum dots
responsive to exposure
of the carbon quantum dots to the excitation radiation, and measuring at least
one of an
emission spectrum and a fluorescence intensity of the emitted radiation at a
detector
coupled to the at least one fiber optic cable.
[00014] In additional embodiments, a method of fracturing multiple zones of
a
subterranean formation penetrated by a well comprises: (a) pumping into each
zone of
the formation to be fractured a fracturing fluid, wherein the fracturing fluid
pumped
3
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
into each zone comprises a qualitatively distinguishable tracer comprising
carbon
quantum dots which are either hydrocarbon soluble, water soluble or both
hydrocarbon soluble and water soluble; (b) enlarging or creating a fracture in
the
formation; (c) recovering fluid from at least one of the multiple zones; and
(d)
identifying the zone within the subterranean formation from which the
recovered fluid
was produced by identifying the carbon quantum dots in the recovered fluid.
[00015] In other embodiments, a method of monitoring the production of
fluids
produced in multiple productive zones of a subterranean formation penetrated
by a
well comprises. (a) pumping fracturing fluid into the multiple productive
zones at a
pressure sufficient to enlarge or create fractures in each of the multiple
productive
zones, wherein the fracturing fluid comprises optically active carbon quantum
dots
which are either hydrocarbon soluble, water soluble or both hydrocarbon
soluble and
water soluble and further wherein the fluorescent carbon quantum dots pumped
into
each of the multiple productive zones is qualitatively and/or quantitatively
distinguishable; and (b) monitoring the amount of fluids produced from at
least one of
the multiple productive zones from the carbon quantum dots in the produced
fluid.
[00016] In other embodiments, a method for enhancing the production of
hydrocarbons from a production well penetrating a hydrocarbon-bearing
formation,
wherein one or more injector wells are associated with the production well,
comprises. (a) introducing into one or more of the injector wells an aqueous
fluid
comprising fluorescent carbon quantum dots; (b) flowing at least a portion of
the
aqueous fluid comprising the optically active carbon quantum dots from the one
or
more injector wells into the production well; and (c) recovering hydrocarbons
from
the production well.
[00017] In yet other embodiments, a method for determining water
breakthrough in
a production well associated with one or more injector wells, comprises: (a)
injecting
an aqueous fluid comprising optically active carbon quantum dots as tracer
into an
injector well; (b) flowing the aqueous fluid from the injector well into the
production
well; (c) producing fluid from the production well; and (d) determining water
breakthrough in the production well by qualitatively determining the presence
or
quantitatively measuring the amount of the fluorescent carbon quantum dots in
the
produced fluid.
[00018] In still other embodiments, a method of increasing hydrocarbon
production
from a production well penetrating a hydrocarbon-bearing reservoir, wherein
more
4
than one injection well is associated with the production well, comprises: (a)
injecting
an aqueous fluid having a water soluble tracer comprising carbon quantum dots
into
the more than one injection well and maintaining pressure in the hydrocarbon-
bearing
reservoir above the bubble point of the hydrocarbons in the reservoir, wherein
the
aqueous fluid pumped into each of the injection wells contains qualitatively
distinguishable carbon quantum dots; (b) identifying from hydrocarbons
recovered
from the production well, upon water breakthrough in the production well, the
injection well into which the breakthrough water was injected by qualitatively
determining the presence of the carbon quantum dots in the recovered
hydrocarbons;
and (c) shutting off the injector well identified in step (b).
[00018a] In yet other embodiments, a method of enhancing the productivity of
hydrocarbon containing fluids from a subterranean formation penetrated by a
well
comprises: pumping into the well a fluid comprising a tracer which is either
hydrocarbon soluble, water soluble or both hydrocarbon soluble and water
soluble and
further wherein the tracer comprises carbon quantum dots; and identifying the
tracer in
fluids produced from the well.
[00018b] In yet other embodiments, a method of fracturing multiple zones of a
subterranean formation penetrated by a well which comprises: pumping into each
zone of
the formation to be fractured a fracturing fluid, wherein the fracturing fluid
pumped
into each zone comprises a qualitatively distinguishable tracer comprising
carbon
quantum dots which are either hydrocarbon soluble, water soluble or both
hydrocarbon
soluble and water soluble; enlarging or creating a fracture in the formation;
recovering
fluid from at least one of the multiple zones; and identifying the zone within
the
subterranean formation from which the recovered fluid was produced by
identifying
the carbon quantum dots in the recovered fluid.
[00018c] In yet other embodiments, a method of monitoring the production of
fluids
produced in multiple productive zones of a subterranean formation penetrated
by a well
comprises: pumping fracturing fluid into the multiple productive zones at a
pressure
sufficient to enlarge or create fractures in each of the multiple productive
zones, wherein
Date Recue/Date Received 2020-08-07
the fracturing fluid comprises fluorescent carbon quantum dots which are
either
hydrocarbon soluble, water soluble or both hydrocarbon soluble and water
soluble and
further wherein the fluorescent carbon quantum dots pumped into each of the
multiple
productive zones is qualitatively and/or quantitatively distinguishable; and
monitoring
the amount of fluids produced from at least one of the multiple productive
zones from the
carbon quantum dots in the produced fluid.
[00018d] In yet other embodiments, a method for determining water breakthrough
in a
production well associated with one or more injector wells comprises: (a)
injecting an
aqueous fluid comprising fluorescent carbon quantum dots as tracer into an
injector well; (b)
flowing the aqueous fluid from the injector well into the production well; (c)
producing fluid
from the production well; (d) determining water breakthrough in the production
well by
qualitatively determining the presence or quantitatively measuring the amount
of the
fluorescent carbon quantum dots in the produced fluid.
[00018e] In yet other embodiments, a method of increasing hydrocarbon
production from a
production well penetrating a hydrocarbon-bearing reservoir, wherein more than
one
injection well is associated with the production well comprising: injecting an
aqueous fluid
having a water soluble tracer comprising carbon quantum dots into the more
than one
injection well and maintaining pressure in the hydrocarbon-bearing reservoir
above the
bubble point of the hydrocarbons in the reservoir, wherein the aqueous fluid
pumped into
each of the injection wells contains qualitatively distinguishable carbon
quantum dots;
identifying from hydrocarbons recovered from the production well, upon water
breakthrough
in the production well, the injection well into which the breakthrough water
was injected by
qualitatively determining the presence of the carbon quantum dots in the
recovered
hydrocarbons; and (c) shutting off the injector well identified in step (b).
5a
Date Recue/Date Received 2020-08-07
Brief Description of the Drawings
[00019]
FIG. I is a simplified schematic illustrating a system including a wellbore
within a subterranean formation, in accordance with embodiments of the
disclosure;
[00020]
FIG. 2A is a simplified cross-sectional view illustrating a fiber optic
cable, in
accordance with embodiments of the disclosure;
[00021]
FIG. 2B is a simplified cross-sectional view of the fiber optic cable taken
along
hue B-B of FIG. 2A;
[00022]
FIG. 2C is a simplified cross-sectional view of another fiber optic cable,
in
accordance with embodiments of the disclosure;
[00023]
FIG. 2D is a simplified cross-sectional view illustrating a fiber optic
cable, in
accordance with other embodiments of the disclosure;
[000241
FIG. 2E is a simplified schematic illustrating a measuring system including
a
fluid delivery system, in accordance with yet other embodiments of the
disclosure;
[00025]
FIG. 3A is a graph illustrating an absorption spectrum, an excitation
spectrum,
= and an emission spectrum of carbon quantum dots, in accordance with
embodiments of the
disclosure;
[00026]
FIG. 3B is a graph illustrating a change in intensity and a change in
wavelength
as a function of pH for carbon quantum dots exposed to an excitation radiation
having a
substantially monochromatic wavelength, in accordance with embodiments of the
disclosure;
and
[00027]
FIG. 4 is a simplified cross-sectional view of an electrochemical cell for
forming carbon quantum dots, in accordance with embodiments of the disclosure.
5b
CA 3024488 2020-03-17
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
Detailed Description
[00028] The following description provides specific details, such as
material types,
compositions, and processing conditions in order to provide a thorough
description of
embodiments of the disclosure. However, a person of ordinary skill in the art
will
understand that the embodiments of the disclosure may be practiced without
employing
these specific details. Indeed, the embodiments of the disclosure may be
practiced in
conjunction with conventional techniques employed in the industry. In
addition, the
description provided below does not form a complete process flow for measuring
properties
within a subterranean formation or for forming carbon quantum dots. Only those
process
acts and structures necessary to understand the embodiments of the disclosure
are described
in detail below.
[00029] As used herein, the term "optical property" means and includes any
qualitative
or quantitative property relating to optically active carbon quantum dots
(CQDs) which
may be determined by optical spectroscopy (such as absorbance, fluorescence
and
phosphorescence). As non-limiting examples, optical properties include a
wavelength at
which a material exhibits a peak absorption intensity, a wavelength at which a
material
exhibits a peak fluorescence intensity (e.g., a color of light emitted during
fluorescence,
such as when the fluorescence is in the visible spectrum), an excitation
spectrum, an
emission spectrum, an intensity of absorbed electromagnetic radiation, and an
intensity of
emitted electromagnetic radiation. The electromagnetic radiation may be
anywhere within
the electromagnetic spectrum, including, for example, the UV spectrum, the
visible
spectrum, and the infrared (IR) spectrum.
[00030] According to embodiments disclosed herein, a method of forming
carbon
quantum dots (CQDs) includes providing an electrochemical cell including an
electrolyte
comprising a carbon source, water, and at least another material. A current is
applied
across electrodes of the electrochemical cell to form carbon quantum dots
comprising
carbon from the carbon source. The carbon source may include at least one of
(i.e., one or
more of) nitrogen, boron, silicon, and phosphorus to form at least one of
nitrogen-doped,
boron-doped, silicon-doped, and phosphorus-doped carbon quantum dots,
respectively.
The carbon quantum dots may be water soluble, exhibit unique optical
properties
depending on a size and chemical composition (e.g., doping) of the carbon
quantum dots,
may be stable at wide pII ranges and temperatures (e.g., up to about 400 C),
and may be
resistant to photobleaching and photo blinking. Surfaces of the carbon quantum
dots may
6
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
be functionalized to form exposed hydrophilic surfaces, exposed hydrophobic
surfaces, or
exposed amphiphilic surfaces on the carbon quantum dots.
[00031] The optical properties of carbon quantum dots may be used to
determine at
least one property of at least one subterranean formation penetrated by a well
(e.g., a pH
of the formation fluid, a wettability of formation surfaces, a production zone
within the at
least one subterranean formation, an injection well contributing to the flow
of
breakthrough water, etc.). For example, the carbon quantum dots may exhibit an
optical
property that is related to a pH to which the carbon quantum dots are exposed.
[00032] Carbon quantum dots thus may be introduced into the subterranean
formation
penetrated by a well and exposed to excitation radiation (e.g., an excitation
wavelength),
such as fluorescence.
[00033] The well may be an oil well, gas well, water well or a geothermal
well.
[00034] The radiation source may be located within the wellbore and may be
configured
to provide the excitation radiation to the carbon quantum dots disposed within
the fluid and
be configured to measure at least one optical property of the carbon quantum
dots. For
example, a radiation source (e.g., a light source) may be coupled to a fiber
optic cable,
which may transmit the excitation radiation to the carbon quantum dots. The
carbon
quantum dots may be disposed within at least one optical fiber of the fiber
optic cable or
may be coated onto at least a portion of the at least one optical fiber.
Responsive to
exposure to the excitation radiation, the carbon quantum dots may fluoresce
(e.g., re-emit
radiation at a different wavelength than the excitation wavelength).
[00035] The emitted radiation may be transmitted through the at least one
optical fiber
to a detector that may be configured to measure at least one optical property
of the carbon
quantum dots.
[00036] The detection source may further be located above the wellbore. For
instance,
the source may be on the fly or at an external location distant from the
wellbore.
[00037] In some embodiments, the carbon quantum dots are disposed in a
fluid within
the wellbore. In one embodiment, the carbon quantum dots may be introduced or
pumped into a well as neutrally buoyant particles in the carrier fluid.
[00038] A fluid delivery system may be configured to provide (e.g.,
deliver) the carbon
quantum dots to the wellbore. The carbon quantum dots are compatible with
fluids
naturally present in the reservoir and within the rock itself. In addition,
the carbon
quantum dots are compatible with the fluids injected into the reservoir as
part of the
formation treatment. Further, the carbon quantum dots must be susceptible to
being
7
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
readily detected qualitatively and analyzed quantitatively in the presence of
the
materials' in the formation fluids.
[00039] The carbon quantum dots may be used to identify fluids produced
from the
well. Since the carbon quantum dots may be detected in recovered produced
fluids,
the methods described herein thus do not require downhole equipment for
detection.
Typically, fluids transported out of the well are evaluated and the carbon
quantum
dots are identified on the fly or at a location distant from the wellbore.
[00040] In addition, the carbon quantum dots may be used as tracers to
monitor fluid
flow through the subterranean formation. For example, carbon quantum dots
exhibiting
different optical properties may be introduced into different zones (e.g.,
producing zones,
aquifer zones, etc.) of the subterranean formation. A produced fluid
exhibiting an optical
property, such as fluorescence, corresponding to a property of carbon quantum
dots
introduced into a zone of the subterranean zone may be an indication that the
produced
fluid originated from the zone in which the carbon quantum dots were
introduced.
[00041] The carbon quantum dots may also be used to sweep a production well
in
an enhanced oil recovery (EOR) operation, such as flooding. Carbon quantum
dots
may be introduced into injection fluid and the injection fluid introduced into
the
formation. The injection fluid may be introduced by being pumped into one or
more
injection wells. Typically, the carbon quantum dots are soluble in the
injection fluid.
[00042] Injection fluids transporting carbon quantum dots into the
formation are
typically aqueous based, such as a brine, like a saturated potassium chloride
or
sodium chloride brine, salt water such as seawater, fresh water, a liquid
hydrocarbon,
a surfactant or a gas such as nitrogen or carbon dioxide. The carbon quantum
dots
may further be injected into the formation in liquefied gas, such as liquefied
natural
gas or liquefied petroleum gas as well as in foams, such as carbon dioxide,
nitrogen
and carbon dioxide/nitrogen. The injection fluid is preferably aqueous, steam
or gas
(water flooding, steam flooding or gas flooding).
[00043] The detection of the carbon quantum dots in fluids produced from
the
production well is indicative that the sweep, i.e., removal of the oil from
pore spaces
within the formation, has been completed.
[00044] Injection fluid pumped into the production well at different
locations may
contain qualitatively and/or quantitatively different carbon quantum dots. For
instance, different carbon quantum dots, distinguishable from each other, may
be
introduced in an aqueous fluid into different injection wells. Fluids produced
from
8
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
the well may be analyzed to determine if water breakthrough has occurred in
the
production well. By using different carbon quantum dots in different fluids,
the
injection well from which the water in the breakthrough water was pumped may
be
determined by optical spectroscopy. The injection well, into which the water
in the
breakthrough water has been determined to have been initially introduced, can
be shut
off Thus, the carbon quantum dots can be used to optimize enhancement of
hydrocarbons during secondary recovery operations by shutting down the
injection
well feeding into the formation into which sweep efficiency has been
maximized.
Thus, the flow of water from the injection well into that portion of the
formation
having been completely swept may be terminated.
[00045] Generally, fluids pumped into the production well do not require
excessive
amounts of the carbon quantum dots. Typically, the minimum amount of carbon
quantum dots in the fluid introduced into the formation, the production well
or
injection well is that amount sufficient to permit detection within a produced
fluid.
Typically, the amount of carbon quantum dots present in the introduced fluid
is
between from about 1 ppm to about 500,000 ppm.
[00046] In some embodiments, a mixture of hydrophilic and hydrophobic
carbon
quantum dots exhibiting different optical properties may be introduced into
the
subterranean formation, into the production well or into one or more injection
wells.
[00047] A ratio of hydrophilic carbon quantum dots to hydrophobic carbon
quantum
dots in a produced fluid may be determined by an optical property of the
produced fluid.
The ratio may be employed as an indication of a wettability of surfaces of the
subterranean
formation (e.g., a ratio of water wet surfaces to oil wet surfaces in the
subterranean
formation). It may also be indicative of the productivity of particular zones
within the
formation.
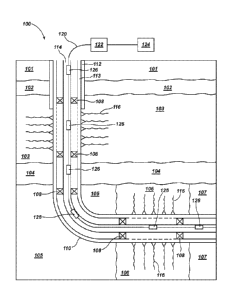
[00048] FIG. 1 is a simplified schematic illustration of a wellbore system
100 extending
through one or more subterranean formations. The subterranean formations may
include a
plurality of zones, including a first zone 101 proximate a surface of the
earth, an aquifer
zone 102 below the first zone, a second zone 103 below the aquifer zone 102, a
third zone
104 below the second zone 103, a fourth zone 105 below the third zone 104, and
a fifth
zone 106 horizontally adjacent to the fourth zone 105. The subterranean
formation may
include one or more additional zones, such as a sixth zone 107 horizontally
adjacent to the
fifth zone 106. At least some of the zones may be hydrocarbon-bearing zones.
For
example, the second zone 103 and the fifth zone 106 may be hydrocarbon-bearing
zones
9
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
and may include fractures 116 through which hydrocarbons to be produced may
travel
during production. The other zones (e.g., the third zone 104, the fourth zone
105, and the
sixth zone 107) may also contain hydrocarbons. Each of the zones may be
isolated from
other zones by at least one packer 108.
[00049] A wellbore 110 may extend through each of the different zones of
the
subterranean formation. Cement 112 may line the wellbore 110 at least through
the first
zone 101, the aquifer zone 102, and at least a portion of the second zone 103.
A liner string
113 may line at least a portion of the wellbore 110. A production string 114
may extend
through the subterranean formation and to a portion of the formation bearing
hydrocarbons
to be produced.
[00050] During formation and operation of the wellbore 110 (e.g., during
drilling,
completion, stimulation, production, etc.), it may be desirable to measure or
estimate
properties of fluids (e.g., drilling fluids, stimulation fluids, completion
fluids, formation
fluids, injection fluids, produced fluids etc.) located within the wellbore
110 and/or
subterranean formation through which the wellbore 110 extends. Further, it may
desirable
to determine the productivity of the zones within the formation they have been
subjected
to fracturing in a multi-zone fracturing operation, water breakthrough in
fluids produced
from the formation, sweep efficiency, etc. Such properties may be measured in
real
time.
[00051] Thus, as will be described in more detail below, at least one
optical property,
such as at least one of (i.e., one or more of) an absorption spectrum, an
absorption intensity,
a peak absorption wavelength, an emission spectrum, a peak emission
wavelength, and a
fluorescence intensity of carbon quantum dots, may be related to the
determination of at least
one property of at least one subterranean formation as referenced herein. In
addition, the at least
one optical property may identify one or more injection wells from which water
has been
introduced into a production well, etc..
[00052] The carbon quantum dots may be formulated to exhibit unique optical
properties associated with the size and the molecular composition of the
carbon quantum
dots. Carbon quantum dots which are qualitatively distinguishable by detection
means
and/or quantitatively distinguishable by detection means may be used. For
instance, in
the production of produced fluids from a formation, a first group of carbon
quantum
dots and at least a second group of carbon quantum dots may be used; the first
group of
carbon quantum dots formulated to exhibit a different detection property than
the at
least a second group of carbon quantum dots. At least one group of carbon
quantum
dots may be soluble in water and at least one other group of carbon quantum
dots may
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
be soluble in hydrocarbon. Alternatively, both groups may be soluble in
hydrocarbon
or water.
[00053] The carbon quantum dots may be formulated to fluoresce at
wavelengths
corresponding to a color of the visible spectrum (e.g., violet, blue, cyan,
green, yellow,
orange, and red). The color of fluorescence may depend at least in part upon
at least one
of a size and a chemical composition of the carbon quantum dots. In some
embodiments, the carbon quantum dots may be formulated to exhibit upconversion
properties. For example, in some embodiments, the carbon quantum dots may be
formulated to emit radiation at a shorter wavelength (and a corresponding
higher energy)
than radiation absorbed by the carbon quantum dots.
[00054] Accordingly, carbon quantum dots may be introduced into the
subterranean
formation at a zone where it is desired to determine the pH of a fluid within
the wellbore
(e.g., formation fluid). In some embodiments, the carbon quantum dots comprise
a part
of at least one optical fiber (e.g., the carbon quantum dots may comprise a
coating on an
optical fiber or the carbon quantum dots may be disposed within the optical
fiber). The
optical fiber including the carbon quantum dots may be exposed to fluid in
communication with the subterranean formation.
[00055] In other embodiments, the carbon quantum dots are introduced into
the
subterranean formation with a fluid delivery system configured to deliver a
fluid having
the carbon quantum dots suspended therein to the subterranean formation.
[00056] In other embodiments, the carbon quantum dots are introduced into
one or
more injection wells in a fluid delivery system configured to deliver a fluid
into the
injection well and to maintain pressure within the wellbore above the bubble
point of
fluids being extracted from the formation.
[00057] The pH of a fluid within the wellbore 110 may be determined by
exposing the
carbon quantum dots disposed within the wellbore 110 to an excitation
radiation and
measuring at least one of (i.e., one or more of) the absorption spectrum, the
absorption
intensity, the peak absorption wavelength (i.e., the peak of the absorption
spectrum), the
emission spectrum, the peak emission wavelength (i.e., the peak of the
emission spectrum),
and the fluorescence intensity of the carbon quantum dots responsive to
exposure to the
excitation radiation. The excitation radiation may be at a substantially
monochromatic
wavelength or may be at a plurality of wavelengths (i.e., polychromatic
wavelengths).
[00058] With continued reference to FIG. 1, the wellbore system 100 may
include a
fiber optic cable 120 extending from a surface location of the subterranean
formation to
locations adjacent to one or more zones within the subterranean formation. The
fiber optic
11
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
cable 120 may extend along an interior of the production string 114, similar
to a wireline,
as is known to those of ordinary skill in the art, and may be run into the
production string
114 as desired, or permanently deployed within the production string 114.
Although the
fiber optic cable 120 is illustrated as extending along an interior of the
production string
114, the fiber optic cable 120 may be located at any suitable location within
the wellbore
system 100 relative to the production string 114. For example, the fiber optic
cable 120
may be run along an exterior of the production string 114, or comprise part of
a self-
contained sensor package configured for wireless communication, as noted
below.
[00059] The fiber
optic cable 120 may be coupled to a radiation source 122 and to a
detector 124. In some embodiments, the radiation source 122 and the detector
124 may be
located at the surface above the subterranean formation, such as on or
adjacent to the rig
floor. As will be described herein, in other embodiments, one or more of the
radiation
source 122 and the detector 124 may be located within the wellbore 110. The
radiation
source 122 may be configured to emit electromagnetic radiation at one or more
wavelengths (i.e., the excitation radiation) which may be transmitted through
the fiber optic
cable 120 to one or more locations within the subterranean formation. In some
embodiments, the radiation source 122 comprises a laser configured to transmit
the
excitation radiation at a substantially monochromatic (e.g., a substantially
fixed and
uniform) wavelength. The
substantially monochromatic wavelength may be any
wavelength in the electromagnetic spectrum. In some embodiments, the
substantially
monochromatic wavelength may be within the ultraviolet spectrum, such as, for
example,
between about 100 nm and about 400 nm. In other embodiments, the radiation
source 122
includes a broadband radiation source configured to provide the excitation
radiation at more
than one wavelength (e.g., polychromatic wavelengths). By way of non-limiting
example,
the radiation source 122 may include a light-emitting diode (T FD) (e.g., a
collimated LED,
an uncollimated LED), a xenon lamp, a mercuty lamp, or other suitable
electromagnetic
radiation source. In some embodiments, the excitation radiation is transmitted
in pulses.
[00060] The fiber
optic cable 120 may include one or more optical sensors 126
configured to detect one or more fluorescence properties of the carbon quantum
dots in
the wellbore system 100. FIG. 2A is a simplified schematic representation of a
fiber optic
cable 120 including an optical sensor 126. The fiber optic cable 120 may
include at least
one optical fiber 128 within a sheath 132 configured to transmit the
excitation radiation
to the carbon quantum dots within the wellbore 110 and at least one optical
fiber 130
within the sheath 132 configured to receive the radiation emitted from the
carbon
quantum dots. Each of the optical fibers 128 may be coupled to the radiation
source 122
12
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
(FIG. 1) and each of the optical fibers 130 may be coupled to the detector 124
(FIG. 1).
The optical sensor126 may include at least one exposed portion of the optical
fiber 128 and
at least one exposed portion of the optical fiber 130.
[00061] Each of the optical fibers 128 may be configured to receive the
excitation
radiation independently of other optical fibers 128 and at differing
wavelengths, intensities
and, if applicable, pulse rates, radiation pulses from different optical
fibers 128 being sent
simultaneously or at offset time intervals. In other embodiments, each of the
optical fibers
128 may be configured to receive excitation radiation of substantially the
same wavelength,
intensity and, if applicable, pulse rates and intervals as the other optical
fibers 128. In yet
other embodiments, the radiation source 122 may be configured to provide the
excitation
radiation at a substantially monochromatic wavelength and intensity to one of
the optical
fibers 128 and excitation radiation of another substantially monochromatic
wavelength and
intensity to another of the optical fibers 128.
[00062] A distal end of the optical fiber 128 may include what is known in
the art as a
"mirror finished" or a "polished" end 134. The minor finished ends 134 of the
optical
fibers 128 may be angled with respect to a longitudinal axis of the optical
fiber 128 and
may be configured to reduce undesired reflection and/or scattering of the
excitation
radiation. For example, the mirror finished end 134 may be configured to
reduce
attenuation of the excitation radiation to be received through the optical
fibers 130. The
mirror finished ends 134 may be configured to substantially reflect light
emitted by the
carbon quantum dots to the detector 124.
[00063] At least a portion of at least one optical fiber 128 may include
carbon
quantum dots 129 disposed therein. The carbon quantum dots 129 may be disposed
within one or more optical fibers. FIG. 2B is a simplified cross-sectional
view of the
fiber optic cable 120 of FIG. 2A. The carbon quantum dots 129 may be disposed
within
and integral with the optical fibers 128. By way of non-limiting example,
carbon
quantum dots 129 may be dispersed in a composition (e.g., mixed in a molten
solution)
from which the optical fibers 128 are formed (e.g., extruded, drawn, cast,
etc.). It is
contemplated that, in some embodiments, the optical fibers 128 may include
materials
formulated to enhance optical properties of the optical fibers 128, such as,
for example,
titanium dioxide.
[00064] Portions of the optical fibers 128 may be exposed to a wellbore
fluid 140
(e.g., drilling fluids, stimulation fluids, completion fluids, formation
fluids, etc.). For
example, at least a distal end of the optical fibers 128 may be exposed to the
wellbore
fluid 140. The portions of the optical fibers 128 that are exposed to the
wellbore fluid
13
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
140 may include the carbon quantum dots 129 disposed therein. The excitation
radiation
from the radiation source 122 may be transmitted to the carbon quantum dots
129 of the
optical fibers 128. Responsive to exposure to the excitation radiation, the
carbon
quantum dots 129 may emit radiation exhibiting at least one fluorescence
property
related to the pH of the formation fluid 140 surrounding the exposed portions
of the
optical fibers 128. The optical fiber 130 may receive the radiation emitted by
the carbon
quantum dots 129 and transmit the emitted radiation to the detector 124.
[00065] In other embodiments, the carbon quantum dots 129 may be disposed
on at
least one surface of at least one optical fiber. For example, a surface of at
least one optical
fiber may have a coating of the carbon quantum dots. FIG. 2C is a simplified
cross-
sectional view of a fiber optic cable 120' substantially similar to the fiber
optic cable 120 of
FIG. 2B, except that the fiber optic cable 120 includes optical fibers 128'
having a coating
127 of carbon quantum dots thereon. In some embodiments, the coating 127
comprises a
monolayer of carbon quantum dots. The coating 127 may substantially surround
each of
the optical fibers 128'. The coating 127 may be a substantially continuous
layer around an
entire circumference of each of the optical fibers 128'. The coating 127 may
be in contact
with the wellbore fluid 140, which may affect at least one fluorescence
property of the
carbon quantum dots of the coating 127.
[00066] The coating 127 may be located at, for example, the distal end of
the optical
fiber 128'. The excitation radiation from the radiation source 122 may be
transmitted to
the carbon quantum dots on the coating 127. Responsive to exposure to the
excitation
radiation, the carbon quantum dots may emit radiation exhibiting at least one
fluorescence property related to the pH of the wellbore fluid 140 surrounding
the coating
127.
[00067] The optical fibers 130 may be configured to receive the radiation
emitted by the
carbon quantum dots (e.g., radiation emitted from the coating 127) and
transmit the emitted
radiation to the detector 124, which may be located at a surface location.
Each of the
optical fibers 130 may be coupled to the detector 124.
[00068] Accordingly, in some embodiments, the carbon quantum dots may be
introduced into the subterranean formation with the fiber optic cable 120,
120'. Radiation
emitted by the carbon quantum dots on or within the optical fibers 128, 128'
may be
received by the optical fiber 130 and transmitted to the detector 124. Thus,
the carbon
quantum dots may be configured to continuously measure the pH of the fluid in
the
wellbore 110 without continuously introducing new carbon quantum dots into the
subterranean formation.
14
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
[00069] In other embodiments, the carbon quantum dots may not be coated on the
optical fibers 128, 128', but may be disposed in the wellbore fluid 140. FIG.
2D illustrates
an embodiment of another fiber optic cable 120" according to another
embodiment of the
disclosure. The fiber optic cable 120" may include an optical sensor 126'
comprising
optical fibers 128" configured to transmit excitation radiation to carbon
quantum dots
disposed within the wellbore 110 and at least one optical fiber 130 within the
sheath 132
configured to receive the radiation emitted from the carbon quantum dots. The
carbon
quantum dots may be disposed in the wellbore fluid 140 proximate the optical
fibers 128",
130. The concentration of the carbon quantum dots in the wellbore fluid 140
may be
between about 50 parts per trillion (ppt) and about 10,000 parts per million
(ppm), such as
between about 50 ppt and about 500 ppt, between about 500 ppt and about 5,000
ppt (5
ppm), between about 5 ppm and about 500 ppm, or between about 500 ppm and
about
10,000 ppm.
[00070] Excitation radiation may be transmitted through the optical fibers
128" to the
carbon quantum dots in the wellbore fluid 140. Responsive to exposure to the
excitation
radiation, the carbon quantum dots may emit radiation that may be received by
the optical
fibers 130. The optical fiber 130 may transmit the emitted radiation to the
detector 124.
Thus, a pH of the fluid 140 proximate the optical fibers 128", 130 may be
determined by
disposing the carbon quantum dots in the wellbore fluid 140 and detecting at
least one
fluorescence property of the carbon quantum dots.
[00071] Accordingly, with reference again to FIG. 1, the radiation source
122 may be
configured to pulse the excitation radiation to the carbon quantum dots within
the wellbore
110. Carbon quantum dots proximate one or more of the optical sensors 126 may
absorb
the excitation radiation. Responsive to absorbing the excitation radiation,
the carbon
quantum dots may fluoresce at an emission wavelength (e.g., that may
correspond to, for
example, red light, yellow light, blue light, etc.). During fluorescence, the
carbon
quantum dots may re-emit radiation at a wavelength (i.e., an emission
wavelength) that is
different from the wavelength of the excitation radiation (i.e., the
excitation wavelength).
[00072] The detector 124 may be configured to continuously measure at least
one
fluorescence property (e.g., one or more of the absorption spectrum, the peak
absorption
wavelength, the absorption intensity, the emission spectrum, the peak emission
wavelength,
and the fluorescence intensity) of the carbon quantum dots. The measured
fluorescence
property may be correlated to a pH of the formation fluid. Accordingly, the pH
of the
formation fluid may be measured in situ and in real time. The detector 124 may
include
or be coupled to a processor configured to estimate the pH of the formation
fluid based on
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
one or more of the absorption spectrum, the peak absorption wavelength, the
absorption
intensity, the emission spectrum, the peak emission wavelength, and the
fluorescence
intensity of the carbon quantum dots. In some embodiments, the detector is a
spectrometer,
such as a fluorescence spectrometer.
[00073] Although FIG. 2A through FIG. 2D illustrate optical fibers 128,
128, 128"
configured to transmit the excitation radiation to the carbon quantum dots and
optical
fibers 130 configured to transmit the emitted radiation to the detector 124,
it is
contemplated that in some embodiments, the fiber optic cable 120 may include a
single
optical fiber. Excitation radiation may be transmitted through the optical
fiber in pulses,
such as every millisecond, every 10 milliseconds, or every 100 milliseconds.
The
fluorescence emitted by the carbon quantum dots may be transmitted back
through the
single optical fiber between excitation pulses and received by the detector
124. In other
words, the excitation pulses may be separated in time such that the carbon
quantum dots
may fluoresce and the emitted fluorescent radiation may be measured at the
detector 124
in between consecutive pulses of excitation radiation. Although FIG. 1
illustrates only
one fiber optic cable 120 extending into the wellbore 110, the wellbore system
100 may
include a plurality of fiber optic cables 120 extending into the wellbore 110.
For
example, in some embodiments, at least one fiber optic cable 120 may be
configured to
transmit the excitation radiation to the carbon quantum dots and at least one
fiber optic
cable 120 may be configured to receive and transmit the emitted radiation from
the
carbon quantum dots to the detector 124.
[00074] Although the radiation source 122 and the detector 124 are
illustrated as
being located at a surface location of the subterranean formation, at least
one of the
radiation source 122 and the detector 124 may be located within the wellbore
110. FIG.
2E is a simplified schematic illustrating a measuring system 150 according to
another
embodiment of the disclosure. The measuring system 150 includes a fluid
deliver
system 152 configured and positioned to deliver a carbon quantum dot-
containing fluid
154 into the wellbore 110, such as into the production string 114. The
radiation source
122 and the detector 124 may be located at a location downstream of the fluid
deliver
system 152. The wellbore fluid 140 may flow in the production string 114 in
the
direction indicated by arrow 156. The wellbore fluid 140 may carry the carbon
quantum
dot-containing fluid 154 to a location proximate the radiation source 122 and
the detector
124. In some embodiments, the fluid delivery system 152 is located proximate
the
radiation source 122 and the detector 124, such as, for example, within about
one meter
of the radiation source 122 and the detector 124. The carbon quantum dot-
containing
16
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
fluid 154 may substantially mix with the wellbore fluid 140 prior to being
exposed to
excitation radiation 160 from the radiation source 122.
[00075] As the carbon quantum dots in the carbon quantum dot-containing
fluid 154 are
exposed to the excitation radiation 160 from the radiation source 122, the
carbon quantum
dots may fluoresce. Responsive to exposure to the excitation radiation 160,
the carbon
quantum dots may emit radiation that may be received by the detector 124,
which, in some
embodiments, may be located directly across from the radiation source 122. In
other
embodiments, the detector 124 may be located adjacent the radiation source
122, such that
the carbon quantum dots pass the detector 124 directly after exposure to the
excitation
radiation 160. Accordingly, a pH of the wellbore fluid 140 may be detenuined
by
disposing the carbon quantum dots into the wellbore fluid 140 (e.g., via the
carbon-
quantum dot-containing fluid 154) and detecting at least one fluorescence
property of the
carbon quantum dots.
[00076] The detector 124 may be configured to transmit information about
the
detected fluorescence properties to the surface, such as by, for example, a
wire 158
coupled to the detector 124 and configured to transmit the data to the
surface, wireless
communications, mud pulse telemetry, or other method suitable to transmit the
data from
the detector 124 located within the wellbore 110 to the surface of the
subterranean
formation.
[00077] FIG. 3A illustrates an example of an absorption spectrum 202, an
excitation
spectrum 204, and an emission spectrum 206 of fluorescence of carbon quantum
dots in a
solution. The absorption spectrum 202 (y-axis of the absorption spectrum 202
illustrated on
the left side of FIG. 3A) graphs the absorption intensity of the carbon
quantum dots as a
function of an excitation wavelength to which the carbon quantum dots are
exposed. The
peak absorption intensity occurs at a wavelength of approximately 275 nm. The
excitation
spectrum 204 (y-axis of the excitation spectrum 204 illustrated on the right
side of FIG. 3A)
graphs a radiation intensity of the excitation radiation as a function of the
wavelength. The
peak excitation intensity occurs at an excitation wavelength of approximately
355 nm.
Although the excitation spectrum 204 is illustrated as shifted from the
absorption spectrum
202, in some embodiments, the excitation spectrum 204 may be more closely
aligned with
the absorption spectrum 202. The emission spectrum 206 (y-axis of the emission
spectrum
206 illustrated on the right side of FIG. 3B) graphs an intensity of the
emitted fluorescence
radiation emitted by the carbon quantum dots as a function of wavelength. The
emission
spectrum 206 illustrates that the peak fluorescence intensity occurs at an
emission
wavelength of approximately 480 nm (i.e., an emission of blue-colored light).
The intensity
17
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
of the peak emission wavelength of the emission spectrum 206 may increase or
decrease,
depending upon the pH of the solution in which the carbon quantum dots are
disposed.
Thus, the carbon quantum dots are exposed to excitation radiation at a
substantially
monochromatic wavelength and the wavelength of the peak emitted radiation
(i.e., the
wavelength of the peak fluorescence intensity) is shifted from the peak
wavelength of the
excitation radiation.
[00078] As described above, the absorption spectrum and the emission
spectrum
emitted by the carbon quantum dots may depend on a pH of the solution in which
the
carbon quantum dots are disposed. Thus, for the same excitation wavelength, a
change in
the pH of the formation fluid in which the carbon quantum dots are disposed
may
correspond to a change in one or more of the absorption spectrum, the
corresponding peak
absorption wavelength of excitation radiation absorbed by the carbon quantum
dot, the
emission spectrum, and the corresponding peak emission wavelength emitted by
the carbon
quantum dots.
[00079] FIG. 3B illustrates a change in intensity (e.g., one or more of an
absorption
intensity and an emission intensity) and a change in wavelength (e.g., a
change in one or
more of an excitation wavelength and an emission wavelength) as a function of
pH for
carbon quantum dots exposed to an excitation radiation at a substantially
monochromatic
wavelength. As illustrated in FIG. 3B, at a substantially monochromatic
wavelength, the
intensity of the carbon quantum dots (e.g., at the wavelength at which the
carbon quantum
dots exhibit the most fluorescence) may depend upon a pH of the solution in
which the
carbon quantum dots are disposed. Similarly, a wavelength at which carbon
quantum dots
absorb excitation radiation and an emission wavelength of the carbon quantum
dots may
change based on the pH to which the carbon quantum dots are exposed.
Accordingly, in
some embodiments, for a substantially monochromatic excitation wavelength, a
pH of a
formation fluid may be estimated based at least in part on one or more
fluorescence or
absorption properties of the carbon quantum dots, such as, for example, the
absorption
intensity (e.g., a change in the absorption intensity), the fluorescence
intensity (e.g., a
change in the fluorescence intensity), a change in the absorption wavelength
(e.g., a change
in the wavelength at which a highest intensity of excitation radiation is
absorbed), a change
in the emission radiation wavelength (e.g., a change in the wavelength at
which a highest
fluorescence intensity occurs), and combinations thereof For example, at a
substantially
monochromatic wavelength, the emission radiation wavelength of the carbon
quantum dots
may shift depending on the pH of the solution in which the carbon quantum dots
are
disposed.
18
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
[00080]
Hydrocarbons within the subterranean formation may include materials that
fluoresce responsive to exposure to the excitation radiation. Fluorescence of
such
materials may undesirably increase noise in at least one of the fluorescence
properties
measured by the detector 124. However, such materials may have a shorter
fluorescence
lifetime than a fluorescence lifetime of the carbon quantum dots. In some
embodiments,
the detector 124 may be configured to measure the at least one fluorescence
property of
the carbon quantum dots after a time delay, such as in time-resolved
fluorometric
detection. Measuring the fluorescence of the carbon quantum dots after a time
delay
may reduce background noise caused by fluorescence of the materials in the
hydrocarbons and increase the signal-to-noise ratio of the detector 124. The
time delay
may be between about 1 picosecond (ps) and about 100 microseconds (0), such as
between about 1 picosecond and about 1 nanosecond, between about 1 nanosecond
and
about 100 nanoseconds, between about 100 nanoseconds and about 1 microsecond,
between about 1 microsecond and about 10 microseconds, or between about 10
microseconds and about 100 microseconds.
[00081]
Accordingly, a fluid introduced into the subterranean formation may include
the carbon quantum dots, or the carbon quantum dots may comprise at least a
portion of
a fiber optic cable 120, 120', such as within the at least one optical fiber
128 or as a
coating on the at least one optical fiber 128. The carbon quantum dots may be
exposed
to the excitation radiation. As described above, responsive to exposure to the
excitation
radiation, the carbon quantum dots may exhibit a fluorescence property that
is, at least
partially, dependent upon the pH of the fluid surrounding the carbon quantum
dots. The
emitted radiation may be transmitted from the carbon quantum dots to the
detector 124,
where at least one fluorescence property of the carbon quantum dots may be
measured.
The pH of fluid in which the carbon quantum dots are disposed may be
determined based
on a fluorescence property of the carbon quantum dots. The carbon quantum
dots, either
within the wellbore fluid 140 or within the at least one optical fiber 128,
128', may be
substantially chemically inert (e.g., may not be subject to photobleaching)
and may
remain within the fluid or within the at least one optical fiber 128, 128 when
exposed to
formation conditions.
[00082] FIG. 4
illustrates a simplified cross-sectional view of a configuration that
may be used in a method of forming the carbon quantum dots described herein.
The
method includes providing an electrolyte 304 and electrodes 306 in a container
302 to
form an electrochemical cell 300. Electrical
current may be applied to the
19
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
electrochemical cell 300 to form carbon quantum dots from a carbon source
located in
the electrolyte 304.
[00083] The container 302 may be any vessel or container suitable for
holding the
electrolyte 304 before, during, or after the electrochemical process of the
disclosure, as
described in further detail below. By way of non-limiting example, the
container 302
may comprise a glass beaker configured to receive and hold the electrolyte 304
and the
electrodes 306.
[00084] The electrodes 306 may include at least one anode and at least one
cathode.
In some embodiments, each of the electrodes 306 comprises platinum. The
electrodes
306 may be coupled to a power supply configured to provide an electrical
current to the
electrochemical cell 300. A current may be applied to the electrochemical cell
300 for a
sufficient period of time to form carbon quantum dots from the electrolyte
304. By way
of non-limiting example, the applied current density may be within a range
extending
from about 100 milliamperes per square centimeter (mA/cm2) to about 1,100
mA/cm2
(e.g., from about 100 mA/cm2 to about 500 mA/cm2, from about 500 mA/cm2 to
about
1,000 mA/cm2, or from about 1,000 mAlcm2 to about 1,100 mA/cm2). In some
embodiments, the applied current density is approximately 1,100 mA/cm2. A
voltage
may be applied between the electrodes 306 during the electrochemical reaction
process.
In some embodiments, a voltage of approximately 10 volts may be applied
between the
electrodes 306. Accordingly, the carbon quantum dots may be formed without
using a
carbon-containing electrode, such as a graphite electrode. Even when using an
electrode
that includes carbon, such as a graphite electrode, the carbon in the
resulting carbon
quantum dots may not include any significant amount of carbon that originated
from the
electrode.
[00085] Although FIG. 4 illustrates two electrodes 306, the electrochemical
cell 300
may include any number of electrodes 306 (e.g., three, four, five, etc.).
[00086] After a suitable period of time, carbon quantum dots may form in
the
electrochemical cell 300. The electrolyte 304 may be evaporated and any solids
may be
collected. The solids may include amorphous carbon quantum dots. Accordingly,
carbon quantum dots exhibiting different fluorescence properties (e.g., peak
emission
wavelengths) may be formed in the electrochemical cell 300.
[00087] The electrolyte 304 may include at least one carbon source
formulated for
providing carbon for forming the carbon quantum dots during the
electrochemical
process. The electrolyte 304 may further include a source of ions, such as an
acid, a
base, or a buffer. In some embodiments, the source of ions includes a
hydroxide, such
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
as, for example, sodium hydroxide (NaOH), potassium hydroxide (KOH), cesium
hydroxide (Cs0H), magnesium hydroxide (Mg(OH)2), calcium hydroxide (Ca(OH)2),
and barium hydroxide (Ba(OH)2). In some embodiments, the at least another
material
has a concentration of about 1 molar and may be formulated such that the
electrolyte 304
has a pH between about 13 and about 14.
[00088] The carbon source may constitute between about 1 volume percent and
about
100 volume percent of the electrolyte 304, such as between about 1 volume
percent and
about 10 volume percent, between about 10 volume percent and about 25 volume
percent, between about 25 volume percent and about 50 volume percent, and
between
about 50 volume percent and about 100 volume percent. The carbon source may be
dispersed in water. In some embodiments, a ratio of the carbon source to water
is
approximately one to two (1:2).
[00089] The carbon source may include any water soluble carbon-containing
material. In some embodiments, the carbon source is an alcohol, such as
methanol,
ethanol, propanol, butanol, combinations thereof, etc. In some embodiments,
the carbon
source is ethanol. Carbon quantum dots formed from such carbon sources may
comprise
carbon, hydrogen, and oxygen (e.g., may be undoped).
[00090] The carbon-quantum dots may be formulated to include at least one
of
nitrogen, boron, silicon, and phosphorus. The electrolyte 304 may be
formulated to
include at least one of a nitrogen source, a boron source, a silicon source,
and a
phosphorus source. The nitrogen source, the boron source, the silicon source,
and the
phosphorus source may also include carbon. Suitable nitrogen-containing carbon
sources
may include amino alcohols, such as, for example, ethanolamine (C4-17N0),
diethanolamine (C4H11NO2), and triethanolamine (C6H15NO3). The nitrogen-
containing
carbon source may include a 2-aminoalcohol, such as, for example, 2-amino-1-
propanol
(alaninol) (C3H9N0), 2-amino-1,3-propanediol (serinol) (C3H9NO2), tryptophanol
(C11HI4N20), a 1-amino-2-propanol (C31-19N0), and a propanolamine, such as
metoprolol
(C151125NO3), nadolol (C17H27N04), and phenylpropanolamine (C9}113N0), or any
other
water soluble carbon source including nitrogen.
[00091] Suitable boron-containing carbon sources may include water soluble
organoboranes, such as, for example, a trialkylborane ORIR2R3B), wherein Ri,
R2, and
R3 are alkyl groups. Suitable trialkylboranes may include, for example,
trimethylborane
((CH3)3B), triethylborane ((C2H5)3B), and tripropylborane ((C3H7)3B). Other
boron-
containing sources may include diborane (H6B2), a carborane, decaborane
(B101114), a
boronic acid, such as, for example, phenylboronic acid (C6H7B02),
methylboroinic acid
21
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
(CH3B(OH)2), and propenylboronic acid (C3H5B(OH)2). Other boron-containing
sources
may include a boratobenzene (a borabenzene), such as, for example, 1-
bo ratanaphthal en e, 9-borataanthracene,
boracyclooctantetraene, and 2,2' -
diboratabiphenyl.
[00092] In some
embodiments, the electrolyte 304 includes a compound including a
nitrogen source and a boron source. For example, the electrolyte 304 may
include a
borane-amine complex, such as borane trimethylamine ((CH3)3NBH3) and borane
tert-
butylamine complex ((CH3)3CNH2BH3).
[00093] Suitable
silicon-containing carbon sources may include hydroxyalkylsilanes,
(e. g. , hydroxymethyltrimethylsilane (HOCH? Si
(CH3)3), hydroxyethoxysilatrane
(C81117NO5Si)), and other water soluble organosilicon compounds.
[00094] Suitable
phosphorus-containing compounds may include phosphate esters
such as, for example, a phosphatidylcholine, triphenylphosphate (OP(0C6H5)3),
cyclophosphamide (C7H15C12N20213), and parathion (CioRANO5PS), phosphonic
acids
and their esters, such as, for example, glyphosate (C3H8NO5P), phosphoranes,
such as,
for example, pentaphenylphosphorane (P(C6H5).5), and organophosphorus
compounds,
such as, for example, triphenylphosphine (P(C6H5)3), phosphites, phosphonites,
and
phosphinites.
[00095]
Accordingly, the carbon quantum dots may be doped with at least one of
nitrogen, boron, silicon, and phosphorus. The fluorescence properties of the
carbon
quantum dots may depend on the composition of the electrolyte 304 (e.g., the
carbon
source) from which the carbon quantum dots are formed.
[00096] At least
one of the nitrogen-containing carbon source, the boron-containing
carbon source, the silicon-containing carbon source, and the phosphorus-
containing
carbon source may constitute between about 0 volume percent and about 100
volume
percent of the carbon source, such as between about 1 volume percent and about
10
volume percent, between about 10 volume percent and about 25 volume percent,
between about 25 volume percent and about 50 volume percent, or between about
50
volume percent and about 100 volume percent of the carbon source.
[00097] The carbon quantum dots may further include C=C bonds and C-0
functional
groups. The carbon quantum dots may be undoped, nitrogen-doped, boron-doped,
silicon-doped, phosphorus-doped, and combinations thereof For example, at
least some
of the carbon quantum dots may include one of nitrogen, boron, silicon, and
phosphorus
and at least some of the carbon quantum dots may include at least another of
nitrogen,
boron, silicon, and phosphorus.
22
N00981 Any of the carbon quantum dots referenced herein may
be suitable for the
methods disclosed herein, especially those relating to the use of the carbon
quantum dots
in hydraulic fracturing including multi-zone fracturing, enhanced oil
recovery, flooding,
etc.
[00099] Typically, the carbon quantum dot's referenced herein
may be generally
õspherical in shape having diameters ranging from between about 1 um to about
10 nrn.
The carbon quantum dots may be separated into narrower size ranges by suitable
methods, which may include dialysis. For example, the carbon quantum dots may
be
passed through at least one membrane having a'pore size corresponding to a
desired size
of the carbon quantum dots. The separated carbon quantum dots may have a
diameter
ranging from between about 1 urn and about 3 nm, between about 3 nm and about
5 nm,
or between about 5 nm and about 10 nm. Carbon quantum dots having different
sizes
may exhibit different optical properties.
[000100] The carbon quantum dots may be soluble in aqueous-based solutions.
The
carbon quantum dots may include exposed hydroxyl groups, exposed carboxyl
groups,
exposed ether groups and combinations thereof. In some embodiments, exposed
surfaces
of the carbon quantum dots may be functionalized with at least one of
additional
hydrophilic functional groups or hydrophobic functional groups. Non-limiting
examples
of hydrophilic groups include, for example, a hy.droxyl group, a carboxyl
group, an amine
= group, a thiol group, an ether group and a phosphate group. Non-limiting
examples of
hydrophobic groups include, for example, an alkyl group, an alkenyl group, an
alkynyl
group, and an aryl group.
[000101] In some embodiments, a hydrophilic group or a hydrophobic group may
be
attached to the carbon quantum dots in a condensation reaction or a hydrolysis
reaction, =
such as described in U.S. Patent No. 9,708,525, filed Sanuary 31, 2014, and
titled
"NANO-SURFACTANTS FOR ENHANCED OIL RECOVERY, AND METHODS OF
.FORMING AND USING SUCH NANO-SURFACTANTS," or a reaction mechanism
described in U.S. Patent No. 9,873,827, filed October 21, 2014, and titled
"SUSPENSIONS FOR ENHANCED HYDROCARBON RECOVERY, AND METHODS
OF RECOVERING HYDROCARBONS USING THE SUSPENSIONS", For example, a =
hydrophilic precursor or a hydrOphobic precursor may include a hydrolyzable
group and
may be attached to a surface of the carbon quantum dots by hydrolyzing the
hydrolyzable
group. In other embodiments, a hydrophilic or hydrophobic group may be
attached to the
carbon
23
CA 3024488 2020-03-17
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
quantum dots by a condensation reaction between the carbon quantum dots and
one of a
hydrophilic precursor and a hydrophobic precursor.
[000102] The carbon quantum dots may be stable at elevated temperatures (e.g.,
up to
about 400 C) and a wide range of pH (e.g., a pH between about 0 and about
14.0).
Emission spectra of the carbon quantum dots may be dependent upon the size and
composition of the carbon quantum dots.
[000103] The carbon quantum dots may be formulated to interact with surfaces
of the
subterranean formation. For example, exposed surfaces of the carbon quantum
dots may
be functionalized with at least one functional group, such as with at least
one hydrophilic
group, at least one hydrophobic (e.g., oleophilic) group, and combinations
thereof (e.g.,
to form amphiphilic surfaces). Hydrophilic groups on surfaces of the carbon
quantum
dots may interact with water wet surfaces of the subterranean formation and
hydrophobic
groups may interact with oil wet surfaces of the subterranean formation.
[000104] In some embodiments, the hydrophilic carbon quantum dots may be
formulated
to exhibit a different optical property than the hydrophilic carbon quantum
dots. For
example, the hydrophilic carbon quantum dots may have a different size than
the
hydrophobic carbon quantum dots. In other embodiments, the hydrophilic carbon
quantum
dots are doped with at least one of nitrogen, boron, silicon, phosphorus,
etc., and the
hydrophobic carbon quantum dots are undoped or doped with at least another of
nitrogen,
boron, silicon, and phosphorus.
[000105] A mixture of hydrophilic and hydrophobic carbon quantum dots may be
introduced into the subterranean formation by pumping the mixture of carbon
quantum
dots into the well penetrating the formation. A produced fluid may include at
least one of
the hydrophilic carbon quantum dots and the hydrophobic carbon quantum dots.
[000106] The proportion of hydrophilic carbon quantum dots to hydrophobic
carbon
quantum dots may be determined by, for example, comparing the fluorescence
intensity at
the peak emission wavelength of the hydrophilic carbon quantum dots to the
fluorescence
intensity at the peak emission wavelength of the hydrophobic carbon quantum
dots.
[000107] A ratio of formation surfaces that are water wet relative to
formation surfaces
that are oil wet may correspond to a proportion of hydrophilic carbon quantum
dots to
hydrophobic carbon quantum dots in the produced fluid.
[000108] Information about the wettability of the formation surfaces may be
particularly useful where stimulation methods include expensive fluids, such
as those
including surfactants, micellar fluids, or polymers. Where the formation
includes more
water wet surfaces than oil wet surfaces, an aqueous-based stimulation fluid
may be used
24
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
during further stimulation procedures. Where the formation includes more oil
wet
surfaces than water wet surfaces, a non-polar stimulation fluid may be used
during
further stimulation procedures.
[000109] In some embodiments, the carbon quantum dots may be introduced into
the
subterranean formation during stimulation processes. Stimulation processes
such as, for
example, hydraulic fracturing (i.e., `Tracking") may be used to enhance
hydrocarbon
recovery from a hydrocarbon-bearing subterranean formation. In hydraulic
fracturing
operations, hydraulic fractures may be created or enlarged by injecting a
fluid containing
additives and including a suspended proppant material (e.g., sand, ceramics,
etc.) into a
targeted subterranean formation under elevated pressure conditions sufficient
to cause the
hydrocarbon-bearing formation material to fracture. The carbon quantum dots
may be
included in the fracturing fluid.
[000110] In addition to determining a chemical or physical parameter of the
formation
fluid (such as pH), it may be desirable to determine a location (e.g., a zone)
from which
produced fluids (e.g., hydrocarbons, water, etc.) originate. It is
contemplated that carbon
quantum dots exhibiting different optical properties may be introduced into
various
zones of the subterranean formation (as well as on an optical fiber). In some
embodiments, between about one and about twenty different types of carbon
quantum
dots, each exhibiting one or more different optical properties than the other
types of
carbon quantum dots, may be introduced into one or more different zones of the
subterranean formation.
[000111] As another example, carbon quantum dots may be introduced proximate
the
aquifer zone. Produced fluids may be analyzed to determine if the produced
fluids include
an optical property of the carbon quantum dots introduced into the aquifer
zone.
Identification of the corresponding optical property may be an indication that
the produced
fluid includes water from the aquifer zone.
[000112] In some embodiments, the carbon quantum dots may be used to identify
a
source of fluids produced from a production well. Carbon quantum dots
introduced
into each zone of the subterranean formation may exhibit a different optical
property than
carbon quantum dots introduced into other zones of the subterranean formation.
In
particular, by way of non-limiting example, the carbon quantum dots may be
dispersed
in produced fluids to indicate the source of the hydrocarbons. The optical
property,
such as fluorescence, of the carbon quantum dots in the hydrocarbons may be an
indication of the source of the hydrocarbons.
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
[000113] Thus, in some embodiments, carbon quantum dots exhibiting different
optical
properties may be introduced into multiple zones of the subterranean
formation. (The term
"zone" as used herein may refer to separate formations within a well or
separate areas
within a single formation within the well.) The carbon quantum dots introduced
in
one zone may be different from the carbon quantum dots introduced into another
zone
being treated. The carbon quantum dots introduced into different zones are
preferably
qualitatively (and preferably also quantitatively) distinguishable in order to
identify
the zone or area within the formation from which a produced fluid originates.
As
such, the carbon quantum dots introduced into each of the zones being treated
preferably exhibit unique absorption and optical properties such that the
properties of
carbon quantum dots introduced into one zone is unable to mask the properties
of carbon
quantum dots introduced into another zone.
[000114] For instance, carbon quantum dots haying a first chemical composition
(e.g.,
undoped, nitrogen-doped, boron-doped, silicon-doped, phosphorus-doped, and
combinations thereof) may be introduced into a first zone and carbon quantum
dots having
a different composition may be introduced into a second zone. Detection of an
optical
property in a produced fluid corresponding to an optical property of carbon
quantum dots
disposed in a zone of the subterranean formation may be an indication that the
produced
fluid originated from the corresponding zone. Detection of optical properties
in the
produced fluid that correspond to carbon quantum dots introduced into
different zones may
be an indication that the produced fluid comprises formation fluid originating
from each of
the corresponding zones.
[000115] Thus, for instance, a first fluid having fluorescent carbon quantum
dots
may be introduced into a first zone of a formation. A second fluid having
qualitatively distinguishable carbon quantum dots from the fluid introduced
into the
first zone) may be introduced into a second zone of a formation. A proportion
of
formation fluid originating from each zone may be determined by, for example,
the relative
value or intensity of the corresponding measured optical property in the
formation fluid.
(It is understood that the terms "first" and "second" need not be sequential
and only
denote the order of addition of the fluids into the formation or the order of
addition of
zones treated in a formation. In other words, the first zone is merely
penultimate to
the second zone. Thus, for example, the "first zone" may refer to a third zone
of a
multi-zone formation and the "second zone" may refer to a sixth zone of a
multi-zone
26
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
formation; the "first treatment fluid" may be a fourth treatment fluid
introduced while
the "second treatment fluid" may be the eighth treatment fluid introduced.)
[000116] As one non-limiting example, referring to FIG. 1, a first group of
carbon
quantum dots exhibiting a first optical property may be introduced into at
least one of the
first zone 101, the aquifer zone 102, the second zone 103, the third zone 104,
the fourth
zone 105, the fifth zone 106, and the sixth zone 107 and at least a second
group of carbon
quantum dots exhibiting a second optical property may be introduced into
another of the
first zone 101, the aquifer zone 102, the second zone 103, the third zone 104,
the fourth
zone 105, the fifth zone 106, and the sixth zone 107. An absorption spectrum,
an emission
spectrum or other optical property of produced fluids may be measured to
determine if any
of the first group of carbon quantum dots or the second group of carbon
quantum dots are
present in the produced fluid. For example, an emission spectrum of the
produced fluid
may be used to determine a proportion of the produced fluid that originated
from each zone
based on the fluorescence intensity of the carbon quantum dots introduced into
each zone.
By way of non-limiting example, carbon quantum dots introduced into a first
zone with a
first fracturing fluid may be formulated to fluoresce at wavelengths that
correspond to blue
light (e.g., at wavelengths of about 450 nm) and carbon quantum dots
introduced into a
second zone with a second fracturing fluid may be formulated to fluoresce at
wavelengths
that correspond to red light (e.g., at wavelengths of about 700 nm). An
emission spectrum
(e.g., a fluorescence color) of produced fluid may indicate whether the
produced fluid
originated from the first zone or the second zone.
[000117] Referring to the carbon quantum dots here, in some embodiments, the
first
group of carbon quantum dots may include undoped carbon quantum dots and the
at least a
second group of carbon quantum dots may be doped with one or more of nitrogen,
boron,
silicon, and phosphorus. In other embodiments, the first group of carbon
quantum dots
may be undoped or may be doped with nitrogen, boron, silicon, or phosphorus
and the at
least a second group of carbon quantum dots may be another of undoped or doped
with
nitrogen, boron, silicon, or phosphorus.
[000118] In addition to monitoring different zones in hydrocarbon production
wells
and determining the zone in which hydrocarbons have been produced from the
formation, the carbon quantum dots may also be used to monitor oil and gas for
flow
assurance and for maintaining regulatory compliance. The ability to analyze
the
fluids on-site, quickly and frequently, further assists operators to detect
flow
assurance, asset integrity and process problems early enabling them to take
27
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
preventative action to minimize the risks of production loss and to adapt the
treatment
operation.
[000119] Further, the carbon quantum dots may also be used to determine sites
of
flowback water and produced water as well as for detection or early warning of
phenomena such as water breakthrough.
[000120] In addition to their use in hydraulic fracturing, the carbon quantum
dots
may be included in fluids used in well treating applications near wellbore and
may be
directed toward improving wellbore productivity and/or controlling the
production of
formation sand. Particular examples include gravel packing and "frac-packs."
Typical gravel packing and frac packing methods.
[000121] In gravel packing, sand is used to pre-pack a screen to prevent the
passage
of formation particles or unconsolidated materials from the formation into the
wellbore during production of fluids from the formation. Gravel packing is
essentially a technique for building a two-stage filter downhole. The filter
consists of
gravel pack sand and a screen or liner. The gravel pack sand is sized
according to the
particle size distribution of the unconsolidated materials. The screen or
liner has
openings that are sized to retain the gravel pack sand. Thus the gravel pack
particulates retain the unconsolidated formation materials and the screen or
liner
retains the gravel pack particulates. The gravel pack particulates and the
screen or
liner act together to reduce or eliminate the production of the unconsolidated
formation materials with the oil or gas production. A slurry of sand
introduced into
the well further may contain the carbon quantum dots. The slurry is then
pumped into
the workstring within the well until the slurry is within about 150 to about
300 feet of
the primary port. Positioning of a crossover service tool allows the slurry to
travel
into the screen/casing annulus. Particulates are retained by the screen or
liner and the
remaining fluid leaks off into the formation allowing a tightly packed sand
filter to
remain in place. Monitoring the carbon quantum dots provides information of
the
type and amount of the produced fluid from the formation.
[000122] The carbon quantum dots may further be used in a frac pack operation
where the unconsolidated formation is hydraulically fractured while a two-
stage filter
of gravel pack is simultaneously built. In frac packing, the processes of
hydraulic
fracturing and gravel packing are combined into a single treatment to provide
stimulated production and an annular gravel pack to reduce formation sand
production.
28
CA 03024488 2018-11-16
WO 2016/205026 PCT/US2016/036305
[000123] Further, carbon quantum dots may be used in combination with an acid
in
an acid fracturing operation. Carbon quantum dots are stable in very low pH
also.
CQDs can be mixed with acids for acid fracturing operations. The acid is a
corrosive,
very low pH acid which reacts with the surrounding formation. The method is
particularly effective with sandstone and carbonate formations. Acids such as
hydrochloric acid, formic acid, and acetic acid are injected at high rates and
pressures
into the formation with the fluid to intentionally cause the formation to fail
by
inducing a fracture in the subterranean rock. In another embodiment, the fluid
of the
invention may contain the acid. Fractures, originating adjacent to the
wellbore,
initiate as two wings growing away from the wellbore in opposite directions.
The
acid is used to dissolve or etch channels or grooves along the fracture face
so that
after pressure is relieved and the fracture heals, there continues to exist
non-uniform
highly conductive channels, allowing unrestrained hydrocarbon flow from the
reservoir to the wellbore. In contrast, with propped fracturing, fracture
conductivity is
maintained by propping open the created fracture with a solid material, such
as sand,
bauxite, ceramic, and certain lighter weight materials. Conductivity in acid
fracturing
is obtained by etching of the fracture faces with an etching acid instead of
by using
proppants to prevent the fracture from closing. Monitoring of the carbon
quantum
dots provides information of the type and amount of the produced fluid from
the
formation and the success of the acid fracturing operation.
[000124] Carbon quantum dots may further be used, in addition to acid
fracturing, in
matrix acidizing. In matrix acidizing, a fluid containing an organic or
inorganic acid
or acid-forming material is injected into the formation below fracture
pressure such
that the acid or acid-forming material reacts with minerals in the formation.
A
channel or wormholes is created within the formation. As subsequent fluid is
pumped
into the formation, it tends to flow along the channel, leaving the rest of
the formation
untreated. Matrix acidizing is often used to enhance near-wellbore
permeability. In
addition to enhancing the production of hydrocarbons, blockages caused by
natural or
man-made conditions may further be removed during matrix acidizing. For
instance,
formation damage caused by drilling mud invasion and clay migration may also
be
removed during the process The use of matrix acidizing is often preferred in
the
treatment of carbonate formations since the reaction products are soluble in
the spent
acid. Monitoring of the carbon quantum dots during matrix acidizing infoinis
the
29
=
operator of the amount of fluids being produced during the operation and
further provides a
measurement on the value of the matrix acidizing operation.
[000125] In yet other embodiments, the carbon quantum dots may be used as a
tracer to
determine fluid flow paths through the subterranean formation and into
produced fluids. For
instance, the carbon quantum dots may be introduced into an injection fluids
during at least
one of water flooding, steam assisted gravity drainage, steam flooding, cyclic
steam
stimulation, or other enhanced oil recovery stimulation processes.
[000126] In other embodiments, 'different carbon quantum dots are preferably
introduced
into the aqueous fluid introduced into the different injection wells. Fluids
produced from
one or more production wells may be analyzed for the presence of the carbon
quantum dots
in the produced fluid. The presence of carbon quantum dots in produced fluids
from a
production well may indicate water breakthrough. Thus, not only can water
breakthrough in
the production well be determined but the injection well from which the water
has flowed in
into the production well can be identified. The injection well, into which the
water in the
breakthrough water has been determined to have been initially introduCed, can
be shut off.
Thus, the carbon quantum dots can be used to optimize enhancement of
hydrocarbons
'during secondary recovery operations by shutting down the injection well and
thus
terminating the flow of water from the injection well directly into the
production well.
[000127] The carbon quantum dots used in this embodiment are typically water
soluble.
Carbon quantum dots are introduced into the aqueous fluid which is then
introduced into
the injection well. The aqueous fluid introduced into each of the injection
wells contains
.qualitatively distinguishable carbon quantum dots. The aqueous fluid serves
to maintain
pressure in the hydrocarbon-bearing reservoir. The pressure is maintained
above the bubble
point. Should carbon quantum dots be detected in produced fluid from the
production well, the
operator would know to take remedial action and shut down the injection well
from which the
carbon quantum dots had originally been introduced. The injection well, once
shut down, may
be repaired to prevent further flow of aqueous fluid into the production well.
CA 3024488 2020-03-17
EXAMPLES
[000128] The methods described above can be performed in any desired suitable
order and are not necessarily limited to any sequence described herein.
Further, the
methods of the present disclosure do not necessarily require use of the
particular
embodiments shown and described herein, but are equally applicable with any
other
suitable structure, form and configuration of components.
[000129] While exemplary embodiments of the. disclosure have been shown and
described, many variations, modifications and/or changes of the system,
apparatus and
methods of the present disclosure, such as in the components, details of
construction
and operation, arrangement of parts and/or methods of use, are possible,
contemplated
by the patent applicant(s), and may be made and used by one of ordinary skill
in the
art without departing from the spirit or teachings of the disclosure. Thus,
all matter
herein set forth or shown in the accompanying drawings should be interpreted
as
illustrative, and the scope of the disclosure should not be limited to the
embodiments
= described and shown herein, .
[000130] Although the foregoing description contains many specifics, these are
not to be
= 'construed as limiting the scope of the disclosure, but merely as
providing certain embodiments.
Similarly, other embodiments may be devised that do not depart from the scope
of the
disclosure. For example, features described herein with reference to one
embodiment also may
be provided in others of the embodiments described herein. =
=
31
CA 3024488 2020-03-17