Note: Descriptions are shown in the official language in which they were submitted.
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
Description
Title of Invention: METHOD FOR PRODUCING CELLULOSE FINE FIBER
Technical Field
[0001] The present invention relates to a method of producing cellulose fine
fibers.
Background Art
[0002] A cellulose fiber (cell wall unit) is an assembly of cellulose fine
fiber (microfibrils). The
microfibrils have been socially attracting close attention as a reinforcing
material because the
microfibrils have mechanical characteristics comparable to those of steel and
have
nanostructures each having a diameter of about 20 nm. However, the fine fibers
are bound
together through hydrogen bonds therebetween. Accordingly, in order that the
fine fibers may
be drawn out, the hydrogen bonds need to be cleaved to separate the
microfibrils (referred to as
"fibrillation"). Accordingly, a mechanical fibrillation method involving
applying an intense
physical force has been developed.
[0003] A method involving performing production by an underwater mechanical
fibrillation
method has been known as a method of producing cellulose nanofibers. In the
method,
cellulose is swollen with water to be brought into a soft state, and is
fibrillated into nanofibers by
.. strong mechanical shearing with, for example, a high-pressure homogenizer.
Natural cellulose
microfibrils each include a crystalline zone and a noncrystalline zone, and
when the
noncrystalline zone absorbs a swelling solvent, such as water, to be brought
into a swollen
state, the zone is deformed by strong shearing. Accordingly, damage is present
in the
resultant cellulose fine fibers, and hence the fine fibers are of such shapes
as to be liable to be
23519505.1
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
entangled and caught with each other.
[0004] In addition, a strong mechanical pulverization method involving using,
for example, a
ball mill causes a mechanochemical reaction intrinsic to a solid state. The
action makes the
breakage or dissolution of the crystal structure of the cellulose inevitable.
As a result, a yield
reduces and a crystallinity degree may reduce.
[0005] Another problem of the underwater fibrillation is that in order that
the resultant cellulose
fine fibers and a resin may be composited with each other, after the
fibrillation, the cellulose
nnicrofibrils need to be dehydrated and subjected to surface hydrophobic
modification. The
dehydration step requires high energy.
[0006] In addition, a method involving swelling and/or partially dissolving a
cellulose-based
substance with a mixed solvent containing an ionic liquid and an organic
solvent, and then
esterifying the resultant has been known as a method of producing cellulose
fine fibers having
esterified surfaces (Patent Literature 1). However, when the mixed solvent
containing the ionic
liquid and the organic solvent of Patent Literature 1 is used, there occurs a
problem in that cost
concerning the recovery and reuse of the ionic liquid is high.
[0007] In addition, with regard to the method of producing cellulose fine
fibers having esterified
surfaces, in Patent Literature 2, there is a disclosure of a method involving
mixing cellulose and
an organic solvent, adding an esterifying agent to the mixture, and then
performing an
esterification reaction together with strong mechanical crushing to esterify
and dissociate the
surface of the cellulose. However, in the method of Patent Literature 2, a
solution for fibrillation
23519505.1 2
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
that contains the esterifying agent and the organic solvent to be used as
described in Examples
thereof has low impregnability into the cellulose, and hence the organic
solvent and the
esterifying agent can be hardly impregnated into the cellulose during
mechanical pulverization
treatment. Accordingly, the production method is not chemical fibrillation but
a mechanical
fibrillation method requiring a strong mechanical force. Strong mechanical
crushing involves
the same problems as those described above because the crushing may damage
cellulose
nanofibers. In addition, the organic solvent and the esterifying agent more
hardly enter a
deeper portion of a pulp fiber from its surface, and hence the inside of the
pulp fiber is hardly
subjected to esterification modification. Accordingly, it can be assumed that
fine fibers in the
pulp fiber are fibrillated by mechanical fibrillation, but their surfaces can
be hardly modified. In
addition, in Patent Literature 3, there is a disclosure of a method of
producing cellulose fine
fibers modified with a surface aromatic substituent. However, cellulose cannot
be fibrillated by
the chemical modification step alone, and hence a strong mechanical
fibrillation step is required.
Citation List
Patent Literature
[0008] [PTL 1] JP 2010-104768 A
[PTL 2] JP 2015-500354 A
[PTL 3] JP 2011-16995 A
Summary of Invention
Technical Problem
[0009] The present invention provides a method of producing cellulose fine
fibers that are
nanosized, that have a high crystallinity degree, and that are less vulnerable
to fiber shape
23519505.1 3
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
damage, the method being an energy-saving method that does not require any
strong physical
pulverization, and a method of producing modified cellulose fine fibers formed
of such fine
fibers.
Solution to Problem
[0010] The inventors of the present invention have made extensive
investigations for
achieving the object, and as a result, have found a method of producing
cellulose fine fibers that
are nanosized, that have a high crystallinity degree, and that are less
vulnerable to fiber shape
damage, the method including impregnating cellulose with formic acid, a high-
concentration
formic acid aqueous solution, or a fibrillation solution containing, in an
aprotic solvent having a
donor number of 26 or more, formic acid or a high-concentration formic acid
aqueous solution to
fibrillate the cellulose without strong fibrillation with, for example, a high-
pressure homogenizer
or a water jet.
[0011] That is, the present invention has a feature of including the following
constructions, and
solves the above-mentioned problems.
[1] A method of producing cellulose fine fibers, including impregnating
cellulose with formic acid
or a high-concentration formic acid aqueous solution to fibrillate the
cellulose.
[2] A method of producing cellulose fine fibers, including impregnating
cellulose with a fibrillation
solution containing an aprotic solvent having a donor number of 26 or more and
formic acid or a
high-concentration formic acid aqueous solution to fibrillate the cellulose.
23519505.1 4
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
[3] A method of producing surface-modified cellulose fine fibers, including
impregnating
cellulose with a modification-reactive fibrillation solution, which is
obtained by adding a cellulose
modification reaction agent to formic acid, a high-concentration formic acid
aqueous solution, or
the fibrillation solution of the above-mentioned item [2], to fibrillate the
cellulose.
[4] The production method according to the above-mentioned item [3], wherein
the cellulose
modification reaction agent includes at least one kind selected from a
carboxylic acid halide, a
carboxylic acid anhydride, a carboxylic acid, an isocyanate, and an epoxy.
[5] The production method according to any one of the above-mentioned items
[1] to [4],
wherein a weight ratio of the cellulose to the formic acid or the high-
concentration formic acid
aqueous solution, of the cellulose to the fibrillation solution of the above-
mentioned item [2], or
of the cellulose to the modification-reactive fibrillation solution of the
above-mentioned item [3] is
from 0.5/99.5 to 25/75.
Advantageous Effects of Invention
[0012] In the present invention, the cellulose is fibrillated by impregnating
the cellulose with
the fibrillation solution containing the formic acid or the high-concentration
formic acid aqueous
solution without strong fibrillation with, for example, a high-pressure
homogenizer or a water jet.
Accordingly, damage to cellulose microfibrils is reduced, and hence cellulose
fine fibers having
a large aspect ratio can be produced.
[0013] Further, surface-modified cellulose fine fibers can be produced by
adding the
modification reaction agent to the fibrillation solution to subject hydroxy
groups on the surfaces
23519505.1 5
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
of the microfibrils to a modification reaction. The fibrillation solution is
impregnated into the
cellulose to modify the surfaces of the microfibrils while cleaving hydrogen
bonds between
fibers, between lamellae, and between the microfibrils, and hence the
cellulose is fibrillated
without the breakage of the crystal structure of naturally-derived cellulose
and microfibril
structures, and the surfaces of the microfibrils can be efficiently modified.
Accordingly,
cellulose fine fibers that are nanosized, that have a high crystallinity
degree, that are less
vulnerable to fiber shape damage, that have a large aspect ratio, and that are
each excellent in
redispersibility in a solvent or a resin after its drying can be simply and
efficiently produced by
an energy-saving method. In addition, the fibrillation solution of the present
invention can not
only fibrillate the cellulose but also cause the hydroxy groups on the
surfaces of the cellulose
fine fibers to react with various modification reaction agents. Accordingly,
various modification
functional groups can be introduced in accordance with applications. For
example, the
introduction of a hydrophobic functional group can further improve an affinity
between the
cellulose fine fibers and an organic medium, such as a resin. In addition,
when a terminal of a
modification functional group is modified with a modification reaction agent
having a reactive
group, such as an acrylic group, an epoxy group, an isocyanate group, or a
vinyl group, the
surfaces of the cellulose fine fibers to be obtained have reactive groups.
Accordingly, the
functionality and applications of the fine fibers can be further extended. For
example, an
improvement in reinforcing effect can be expected from an improvement in
interfacial adhesive
property by the occurrence of a chemical reaction between the cellulose fine
fibers and a resin
at the time of their com positing.
[0014] Further, in the method of producing cellulose fine fibers of the
present invention, a
cellulose substance can be fibrillated without the use of mechanical
fibrillation means based on
23519505.1 6
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
the action of a strong shear force. Accordingly, the resultant cellulose fine
fibers have
structures close to those of natural microfibrils and are less vulnerable to
damage, and hence
each have high strength.
.. [0015] Further, the formic acid brings together properties as a swelling
solvent, a fibrillation
aid, and a modification reaction catalyst, and hence the cellulose can be
fibrillated with the
single component. Further, the addition of the modification reaction agent
enables
simultaneous performance of the surface modification and the fibrillation.
Further, the formic
acid has a low boiling point, and hence processes for the purification and
recovery of the
.. cellulose fine fibers are simple and energy-saving.
Brief Description of Drawings
[0016] FIG. 1 is a SEM image of fine fibers obtained in Example 1.
FIG. 2 is a SEM image of fine fibers obtained in Example 2.
FIG. 3 is a SEM image of fine fibers obtained in Example 3.
FIG. 4 is a SEM image of fine fibers obtained in Example 4.
FIG. 5 is a SEM image of fine fibers obtained in Example 5.
FIG. 6 is a SEM image of fine fibers obtained in Example 6.
23519505.1 7
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
Description of Embodiments
[0017] A method of producing cellulose fine fibers of the present invention
has a feature of
including impregnating cellulose with formic acid, a high-concentration formic
acid aqueous
solution, or a fibrillation solution containing formic acid or a high-
concentration formic acid
aqueous solution (the foregoing are hereinafter sometimes collectively
referred to as "fibrillation
solution") to fibrillate the cellulose without mechanical crushing so that the
cellulose may be
fibrillated so as to be nanosized, to have a high crystallinity degree, and to
be less vulnerable to
fiber shape damage.
[0018] The cellulose serving as a raw material may be in the form of cellulose
alone, or may
be in a mixed form containing a non-cellulose component, such as lignin or
hemicellulose. A
preferred cellulose substance is a cellulose substance containing a type I
crystal cellulose
structure, and examples thereof include substances each containing wood-
derived pulp, wood,
bamboo, linter pulp, cotton, or cellulose powder.
[0019] The method of producing cellulose fine fibers of the present invention
has a feature in
that the formic acid, the high-concentration formic acid aqueous solution, or
the fibrillation
solution containing the formic acid or the high-concentration formic acid
aqueous solution is
used as the fibrillation solution for fibrillating the cellulose.
[0020] Formic acid having a purity close to 100 wt% may be used as the formic
acid or the
high-concentration formic acid aqueous solution. In consideration of the ease
of availability of
a raw material and the ease of handling, it is realistic to use the high-
concentration formic acid
aqueous solution.
23519505.1 8
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
[0021] The high-concentration formic acid aqueous solution is, for example, a
formic acid
aqueous solution having a concentration of 40 wt% or more. It is preferred
that a formic acid
aqueous solution having a concentration of 50 wt% or more, more preferably a
formic acid
aqueous solution having a concentration of 70 wt% or more, still more
preferably a formic acid
aqueous solution having a concentration of 85 wt% or more be used because the
impregnation
of the solution into the cellulose and the fibrillation become more rapid as
the formic acid
concentration increases. When the formic acid concentration becomes less than
40 wt%, a
side reaction between a modification reaction agent and water may occur.
[0022] In addition, the formic acid or the high-concentration formic acid
aqueous solution may
be used as a fibrillation solution by being mixed with an aprotic solvent
having a donor number
of 26 or more. In this embodiment, a fibrillation solution excellent in
modification reactivity can
be obtained.
[0023] When the cellulose is impregnated with the fibrillation solution, the
formic acid cleaves
hydrogen bonds between microfibrils while the impregnated fibrillation
solution swells the
cellulose, and hence the microfibrils can disentangle by themselves to provide
cellulose fine
fibers.
[0024] The ratio of the formic acid in the fibrillation solution is preferably
20 wt% or more with
respect to the entirety of the fibrillation solution. A case in which the
ratio is less than 20 wt%
is not preferred because the fibrillation may be insufficient owing to a
reduction in impregnability
of the fibrillation solution into the cellulose. Further, the formic acid can
also function as a
23519505.1 9
CA 03026213 2018-11-30
CA Application
Blokes Ref: 15803/00002
weakly acidic catalyst. Accordingly, the case in which the ratio is less than
20 wt% is not
preferred because there is a risk in that the rate of the surface modification
reaction of fine
cellulose fibers is slow and hence their modification ratio reduces.
When the
high-concentration formic acid aqueous solution is used, the high-
concentration formic acid
aqueous solution is used so that the ratio of the formic acid in the
fibrillation solution may fall
within the range.
The ratio of the formic acid in the fibrillation solution is more preferably
30 wt% or more,
still more preferably 50 wt% or more.
[0025] In addition, when the high-concentration formic acid aqueous solution
and the aprotic
solvent having a donor number of 26 or more are mixed, the ratio of the formic
acid in the
fibrillation solution is as described above. A formic acid aqueous solution
having as high a
concentration as possible (e.g., 40 wt% or more) is preferably used as the
high-concentration
formic acid aqueous solution to be used because a water concentration in the
fibrillation solution
is preferably set to 30 wt% or less.
[0026] Any appropriate solvent may be used as the aprotic solvent having a
donor number of
26 or more to be mixed with the formic acid or the high-concentration formic
acid aqueous
solution. When described in detail, out of the aprotic solvents each having a
donor number of
26 or more, a solvent having a donor number of from 26 to 35 is preferred, a
solvent having a
donor number of from 26.5 to 33 is more preferred, and a solvent having a
donor number of
from 27 to 32 is still more preferred. When the donor number is excessively
low, an improving
effect on the impregnability of the fibrillation solution containing the
formic acid into a space
23519505.1 10
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
between the microfibrils may not be expressed. The donor number is based on
the description
of the literature "Netsu Sokutei 28(3), 135-143."
[0027] Examples of the aprotic solvent include a sulfoxide, a pyridine, a
pyrrolidone, and an
amide. Those solvents may be used alone or in combination thereof.
[0028] Further, of the aprotic solvents each having a donor number of 26 or
more, at least one
kind selected from the group consisting of dimethyl sulfoxide (donor number:
29.8), pyridine
(donor number: 33.1), N,N-dimethylacetamide (donor number: 27.8), N,N-
dinnethylformamide
(donor number: 26.6), and N-methyl-2-pyrrolidone (donor number: 27.3) is more
preferred
because the impregnability of the formic acid into a space between the
microfibrils can be
promoted to a high extent.
[0029] The aprotic solvent is not particularly limited as long as the solvent
is the
above-mentioned solvent that does not impair the impregnation and the
fibrillation. Dimethyl
sulfoxide, N,N-dimethylacetamide, N,N-dimethylformamide, or N-methyl-2-
pyrrolidone is
preferred because the impregnability of the formic acid into a space between
the microfibrils can
be promoted.
[0030] The fibrillation solution may contain any appropriate aprotic solvent
having a donor
number of less than 26, such as acetonitrile, dioxane, acetone, or
tetrahydrofuran, as another
solvent. When the content of the solvent having a donor number of less than 26
is excessively
large, a reduction in impregnability of the fibrillation solution into a space
between the cellulose
microfibrils and a reduction in fibrillating effect on the cellulose may
occur. Accordingly, the
23519505.1 11
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
content of the solvent having a donor number of less than 26 is preferably 30
wt% or less, more
preferably 25 wt% or less.
[0031] The formic acid, the high-concentration formic acid aqueous solution,
or the fibrillation
solution containing the formic acid or the high-concentration formic acid
aqueous solution to be
used in the present invention may further contain a cellulose modification
reaction agent.
When the modification reaction agent is added, the fibrillation solution can
be impregnated as a
modification-reactive fibrillation solution into the cellulose to chemically
modify the surfaces of
the cellulose microfibrils while fibrillating the cellulose.
[0032] The ratio of the formic acid in the modification-reactive fibrillation
solution may be the
same as the concentration of the formic acid in the fibrillation solution. The
concentration of
the formic acid is preferably 30 wt% or more, more preferably 50 wt% or more.
In addition,
from the viewpoint of effective utilization of the modification reaction
agent, a formic acid
aqueous solution having as high a concentration as possible, or specifically a
concentration of
40 wt% or more is preferably used as the high-concentration formic acid
aqueous solution to be
added to the modification-reactive fibrillation solution. The concentration of
the formic acid is
more preferably 60 wt% or more, still more preferably 80 wt% or more.
[0033] In addition, the ratio of the modification reaction agent in the
modification-reactive
fibrillation solution is not particularly limited as long as the
impregnability of the fibrillation
solution into the cellulose is not reduced. The ratio of the modification
reaction agent is, for
example, 30 parts by weight or less, preferably from 0.1 part by weight to 30
parts by weight,
more preferably from 0.1 part by weight to 25 parts by weight, still more
preferably from 0.5 part
23519505.1 12
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
by weight to 20 parts by weight with respect to 100 parts by weight of the
modification-reactive
fibrillation solution. When the ratio of the modification reaction agent is
excessively large, the
fibrillation degree of the cellulose may reduce owing to a reduction in
impregnability of the
fibrillation solution.
[0034] The modification-reactive fibrillation solution only needs to contain
the
above-mentioned ratios of the formic acid and the modification reaction agent.
However, it is
not easy to use 100% formic acid, and hence the solution typically contains
water or water and
the aprotic solvent having a donor number of 26 or more in addition to the
foregoing. The ratio
of the water or the water and the aprotic solvent having a donor number of 26
or more in the
modification-reactive fibrillation solution only needs to be such that the
formic acid and the
modification reaction agent satisfy the above-mentioned contents, and the
ratio may be freely
selected in accordance with the purposes of the fibrillation and the
modification.
[0035] When the modification reaction agent is added to the fibrillation
solution, the cellulose
may be impregnated with the modification-reactive fibrillation solution
prepared by mixing the
fibrillation solution with the modification reaction agent, or the following
may be adopted: the
cellulose is impregnated with the fibrillation solution to which the
modification reaction agent is
not added, and after the fibrillation has advanced to some extent, the
modification reaction
agent is added to the solution, and the solution is impregnated as the
modification-reactive
fibrillation solution into the cellulose.
[0036] Any appropriate compound may be used as the cellulose modification
reaction agent.
The modification reaction agent is preferably at least one kind selected from
a carboxylic acid
23519505.1 13
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
halide, a carboxylic acid anhydride, a carboxylic acid, an isocyanate, and an
epoxy.
[0037] The cellulose modification reaction agents may be used alone or in
combination
thereof. Of those modification reaction agents, in terms of the fibrillation
property of the
cellulose and the reactivity of the solution, a modification reaction agent
having 2 to 7 carbon
atoms is preferred, and a modification reaction agent having 2 to 5 carbon
atoms is more
preferably used. Meanwhile, when the number of carbon atoms of the
modification reaction
agent becomes 8 or more, the impregnability of the solution into a space
between the
microfibrils and the reactivity thereof with a hydroxy group of the cellulose
may reduce.
Accordingly, the modification reaction agent is preferably added during the
fibrillation or after the
completion of the fibrillation. Further, a modification reaction agent having
a large number of
carbon atoms is preferably used in combination with a modification reaction
agent having a
small number of carbon atoms.
[0038] The carboxylic acid halide serving as the modification reaction agent
may be at least
one kind selected from the group consisting of carboxylic acid halides each
represented by the
following formula (1):
(1)
where R1 represents any one of an alkyl group having 1 to 24 carbon atoms, an
alkylene group,
.. a cycloalkyl group, and an aryl group, and X represents Cl, Br, or I.
[0039] As the carboxylic acid halide, any appropriate carboxylic acid halide
may be used.
Examples thereof include a carboxylic acid chloride, a carboxylic acid
bromide, and a carboxylic
acid iodide. Specific examples of the carboxylic acid halide include, but not
limited to, acetyl
23519505.1 14
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
chloride, acetyl bromide, acetyl iodide, propionyl chloride, propionyl
bromide, propionyl iodide,
butyryl chloride, butyryl bromide, butyryl iodide, benzoyl chloride, benzoyl
bromide, and benzoyl
iodide. Of those, a carboxylic acid chloride may be suitably adopted in terms
of reactivity and
handleability.
[0040] Any appropriate carboxylic acid anhydride may be used as the carboxylic
acid
anhydride serving as the modification reaction agent. Examples of the
carboxylic acid
anhydride include: monobasic carboxylic acid anhydrides [such as: an anhydride
of a saturated
aliphatic monocarboxylic acid, such as acetic acid, propionic acid,
(iso)butyric acid, or valeric
acid; an anhydride of an unsaturated aliphatic monocarboxylic acid, such as
(meth)acrylic acid
or oleic acid; an anhydride of an alicyclic monocarboxylic acid, such as
cyclohexanecarboxylic
acid or tetrahydrobenzoic acid; and an anhydride of an aromatic monocarboxylic
acid, such as
benzoic acid or 4-methylbenzoic acid]; dibasic carboxylic acid anhydrides
[such as: a saturated
aliphatic dicarboxylic acid anhydride, such as succinic anhydride or adipic
anhydride; an
unsaturated aliphatic dicarboxylic acid anhydride, such as maleic anhydride or
itaconic
anhydride; an alicyclic dicarboxylic acid anhydride, such as 1-cyclohexene-1,2-
dicarboxylic
anhydride, hexahydrophthalic anhydride, or methyltetrahydrophthalic anhydride;
and an
aromatic dicarboxylic acid anhydride, such as phthalic anhydride or naphthalic
anhydride]; and
polybasic carboxylic acid anhydrides (such as a polycarboxylic acid
(anhydride), such as
trimellitic anhydride or pyromellitic anhydride). Acetic anhydride, propionic
anhydride, or
butyric anhydride is preferred because the fibrillation can be satisfactorily
performed.
[0041] Any appropriate compound may be used as the isocyanate. The isocyanate
is, for
example, an isocyanate represented by the following formula (2) or (3):
23519505.1 15
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
R2-N=C=O (2)
0=C=1\1-R3-N=C--.0 (3)
where R2 or R3 represents an alkyl group having 1 to 24 carbon atoms, an
alkylene group, a
cycloalkyl group, or an aryl group.
[0042] Specific examples of the isocyanate include isocyanates, such as methyl
isocyanate
(MIC), diphenylmethane diisocyanate (MDI), hexamethylene diisocyanate (HDI),
toluene
diisocyanate (TDI), isophorone diisocyanate (IPDI), 2-isocyanatoethyl
methacrylate (M01), and
2-isocyanatoethy1 acrylate (A01). MO1 and A01 are preferred in terms of
compositing with an
acrylic resin. In addition, MIC, MDI, HDI, TDI, or IPDI is preferred in terms
of compositing with
a urethane resin.
[0043] Any appropriate compound may be used as the epoxy. The epoxy may be,
for
example, at least one kind selected from the group consisting of epoxies each
represented by
the following formula (4) or (5):
R4
\ (4)
0
/ R5
( 5)
0
where R4 or R5 represents an alkyl group having 1 to 24 carbon atoms, an
alkylene group, a
substituent derived from ethylene glycol, a substituent derived from bisphenol
A, a substituent
derived from bisphenol F, a cycloalkyl group, or an aryl group.
23519505.1 16
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
[0044] Specific examples of the epoxy include: a monofunctional epoxy
modification reaction
agent, such as allyl glycidyl ether, 2-ethylhexyl glycidyl ether, glycidyl
phenyl ether,
4-tert-butylphenyl glycidyl ether, or lauryl alcohol(E0)15 glycidyl ether; and
a bifunctional epoxy
modification reaction agent, such as bisphenol A epoxy, bisphenol F epoxy,
diglycidyl
terephthalate, or diglycidyl o-phthalate. A bifunctional epoxy modification
reaction agent is
preferred in terms of compositing with an epoxy resin.
[0045] Any appropriate compound may be used as the carboxylic acid. The
carboxylic acid
is, for example, an aliphatic carboxylic acid or a carboxylic acid having an
aryl group. The
carboxylic acid is specifically, for example, a carboxylic acid represented by
the following
formula (6):
R6-000H (6)
where R6 represents an alkyl group having 1 to 24 carbon atoms, an alkylene
group, a
cycloalkyl group, or an aryl group.
[0046] An acid catalyst may be further added to the fibrillation solution or
the
modification-reactive fibrillation solution. Any appropriate compound may be
used as the acid
catalyst. The catalyst is, for example, sulfuric acid, p-toluenesulfonic acid,
hydrochloric acid,
phosphoric acid, or nitric acid. The amount of the acid catalyst to be added
to the fibrillation
solution may be set to any appropriate amount. The addition amount of the acid
catalyst is
preferably from 0.01 wt% to 15 wt%, more preferably from 0.05 wt% to 10 wt%
with respect to
the fibrillation solution.
23519505.1 17
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
[0047] The weight ratio of the cellulose to the fibrillation solution or the
modification-reactive
fibrillation solution "former/latter" is, for example, from 0.5/99.5 to 25/75,
preferably from
1.0/99.0 to 20/80, more preferably from 2.0/98 to 15/85.
[0048] Next, the reason why the cellulose is fibrillated with the fibrillation
solution to be used in
the present invention may be assumed to be as described below. That is, the
formic acid has
a large solubility parameter (SP value, 13.5), a large donor number (donor
number: 19), a large
acceptor number (acceptor number: 83.6), and a large dielectric constant
(58.5). Accordingly,
the formic acid may cleave hydrogen bonds between cellulose fibers, between
lamellae, and
between the microfibrils, while being impregnated into the cellulose, to cause
the fibrillation.
The acceptor number is a value obtained by the Gutmann method.
Further, the formic acid contains an aldehyde functional group. Accordingly,
it is
assumed that the aldehyde group forms a henniacetal or an acetal with a
hydroxy group on the
surfaces of the microfibrils to cleave a hydrogen bond between the
microfibrils, and hence the
microfibrils are easily separated so that the cellulose may be fibrillated.
[0049] In addition, the fibrillation solution to be used in the present
invention is not
impregnated into the crystalline zones (domains) of the microfibrils, and
hence the resultant
cellulose fine fibers are less vulnerable to damage and have structures close
to those of natural
microfibrils. At the same time, in the process, the cellulose can be
fibrillated without the use of
mechanical fibrillation means based on the action of a shear force, and hence
damage by
physical action is reduced. Accordingly, each of the resultant modified
cellulose fine fibers can
be assumed to hold high strength. Further, the modified cellulose fine fibers
each have low
23519505.1 18
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
surface roughness, and hence can be easily redispersed in a solvent or a resin
even when dried
once.
[0050] Methods of producing cellulose fine fibers and surface-modified
cellulose fine fibers of
the present invention are each performed by impregnating the cellulose with
the fibrillation
solution containing the formic acid to cleave the hydrogen bonds between the
cellulose fibers,
between the lamellae, and between the microfibrils. In order to impregnate the
cellulose with
the fibrillation solution containing the formic acid, the fibrillation
solution and the cellulose only
need to be mixed, and a method involving adding the cellulose to the
fibrillation solution to mix
the solution and the cellulose or adding the fibrillation solution to the
cellulose to mix the solution
and the cellulose may be typically utilized.
In addition, the cellulose is preferably brought into a state of being torn
into a certain
size before its mixing with the fibrillation solution. The certain size is a
size smaller than a size
to be loaded into a fibrillation container.
[0051] Also in the case of the fibrillation and the modification with the
modification-reactive
fibrillation solution obtained by causing the modification reaction agent to
coexist in the
fibrillation solution, the same method as that in the case of the fibrillation
solution free of the
modification reaction agent is used. That is, when the modification-reactive
fibrillation solution
and the cellulose are mixed, the modification-reactive fibrillation solution
is impregnated into a
space between the microfibrils to cleave a hydrogen bond between the
microfibrils, and hence
the cellulose fine fibers obtained by modifying the surfaces of the fine
fibers can be obtained.
23519505.1 19
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
[0052] The modification-reactive fibrillation solution simultaneously performs
the modification
and the fibrillation by: uniformly mixing the fibrillation solution and the
modification reaction
agent through stirring or the like; and impregnating the cellulose with the
mixture. The order in
which the fibrillation solution and the modification reaction agent are mixed
is not particularly
limited, and the fibrillation solution and the modification reaction agent may
be mixed in any
appropriate order. A method involving adding the modification reaction agent
to the formic
acid, the formic acid aqueous solution, or the aprotic solvent containing the
formic acid or the
formic acid aqueous solution is typically used.
In addition, when the polarity of the modification reaction agent is low, in
order that the
impregnation rate of the fibrillation solution, and the swelling rate and
fibrillation rate of the
cellulose may not be reduced, the following is preferably adopted: first, the
cellulose is
impregnated with the fibrillation solution to which the modification reaction
agent is not added,
and after the fibrillation has advanced to some extent, the modification
reaction agent is added
to the fibrillation solution. In this case, the entirety of the modification
reaction agent may be
added to the fibrillation solution in one stroke, or the modification reaction
agent may be added
in several portions.
Further, a catalyst may be added after the cellulose has been impregnated with
the
modification-reactive fibrillation solution.
[0053] A fibrillation method is described in detail. In a fibrillation method
based on the
fibrillation solution to be used in the present invention, after the
fibrillation solution or the
modification-reactive fibrillation solution has been mixed with the cellulose,
the mixture may be
23519505.1 20
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
left to stand for from 0.5 hour to 1 hour or more, or after the mixing,
stirring may be further
performed to such an extent that the cellulose can maintain a uniform state in
the solution.
That is, although the fibrillation is advanced merely by mixing the
fibrillation solution with the
cellulose and leaving the mixture to stand, stirring may be performed with
stirring means for
.. promoting the impregnation of the solution or uniformity of the mixture.
The stirring may be
performed with any appropriate means, and in ordinary cases, the stirring only
needs to be
stirring comparable in intensity to stirring with a stirring machine or a
magnetic stirrer generally
used in organic synthesis. The stirring machine is not particularly limited,
and in ordinary
cases, the machine only needs to be an apparatus capable of stirring,
blending, or kneading.
A kneading machine, such as a kneader or an extruder, is also permitted. In
particular, when
the concentration of the cellulose is high, a kneader or an extruder that can
deal with a high
viscosity is preferred. In addition, the stirring may be performed
continuously or may be
performed intermittently.
[0054] With regard to a temperature in the fibrillation in the present
invention, there is no need
to heat the mixture of the solution and the cellulose, and the fibrillation or
the modification
reaction may be performed at room temperature. When the mixture of the
solution and the
cellulose is stirred for 2 hours or more, the cellulose can be chemically
fibrillated as described
above without the use of mechanical fibrillation means based on the action of
a shear force. In
.. the present invention, the cellulose can be fibrillated without the use of
excess energy. Heating
may be performed in the fibrillation treatment because when the fibrillation
temperature is made
higher than normal temperature, a treatment time required for the fibrillation
can be shortened.
In consideration of energy efficiency, a satisfactory result is obtained even
when no heating is
performed. The present invention has a feature in that the cellulose can be
fibrillated at normal
23519505.1 21
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
temperature without the use of excess energy.
Meanwhile, at the time of the preparation of the surface-modified cellulose
fine fibers
with the modification-reactive fibrillation solution, heating is preferably
performed for promoting
the modification reaction. When the solution and the cellulose are caused to
react with each
other at normal pressure, a heating temperature is, for example, 90 C or less,
preferably from
40 C to 90 C, more preferably 80 C or less, still more preferably 70 C or
less, particularly
preferably 50 C or less. When the solution and the cellulose are caused to
react with each
other while being pressurized, the heating temperature is preferably 180 C or
less, more
preferably 150 C or less, still more preferably 135 C or less. A case in which
the solution and
the cellulose are heated to a temperature of more than 180 C is not preferred
because the
cellulose may decompose. The case in which the solution and the cellulose are
heated to a
temperature of more than 180 C is not preferred also because of the following
reason: at any
such temperature, the modification reaction rate of the cellulose fine fibers
exceeds the
fibrillation rate of the cellulose in some cases, and in such cases, the
fibrillation degree of the
cellulose may deteriorate, or the yield of the surface-modified cellulose fine
fibers may reduce.
[0055] The fibrillation treatment time required for the fibrillation of the
cellulose in the present
invention varies depending on, for example, the concentration of the formic
acid, when an
aprotic polar solvent is used, the donor number of the solvent to be added, a
mixing ratio
between the fibrillation solution and the cellulose, a treatment temperature
in the fibrillation, and
the degree of the stirring of the solution and the cellulose. When the
fibrillation is performed in
the above-mentioned condition ranges, the fibrillation can be typically
performed in a time period
in the range of from 0.5 hour to 50 hours.
23519505.1 22
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
When the treatment time is excessively short, the impregnation of the
fibrillation
solution into a space between the microfibrils becomes insufficient, and hence
the fibrillation
becomes insufficient in some cases. In addition, when the treatment time is
excessively long,
.. the efficiency of the treatment deteriorates to reduce the yield in some
cases. The treatment
time is preferably from about 1 hour to about 36 hours, more preferably from
about 1.5 hours to
about 24 hours.
A treatment time in the case of the fibrillation and modification of the
cellulose with the
modification-reactive fibrillation solution also varies depending on, for
example, the
concentration of the formic acid, when an aprotic polar solvent is used, the
donor number of the
solvent to be added, the kind of the modification reaction agent, the donor
number of the solvent
to be added, a mixing ratio between the modification-reactive fibrillation
solution and the
cellulose, a treatment temperature in the fibrillation and the modification,
and the degree of the
stirring of the solution and the cellulose. When the fibrillation is
performed in the
above-mentioned condition ranges, the fibrillation and the modification can be
typically
performed in a time period in the range of from 0.5 hour to 50 hours.
The treatment time can be shortened by increasing the treatment temperature
(reaction
temperature) or increasing the rate of the stirring. However, when the
reaction time is
excessively short, there is a risk in that the impregnation of the
fibrillation solution into a space
between the nnicrofibrils becomes insufficient, and hence the fibrillation and
the modification
reaction become insufficient.
23519505.1 23
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
Further, when the modification reaction agent is added in the middle of the
reaction, the
reaction is preferably further advanced for from 0.5 hour to 5 hours or more
after the addition of
the modification reaction agent.
In addition, when the fibrillation is performed by using a carboxylic acid
anhydride
having a large number of carbon atoms or a carboxylic acid halide having a
large number of
carbon atoms as the modification reaction agent, the fibrillation treatment
time is preferably 2
hours or more, more preferably 3 hours or more, still more preferably 4 hours
or more.
[0056] In the case of the fibrillation with the fibrillation solution, the
cellulose may be treated in
any one of an open container and a closed container. When the cellulose is
fibrillated and
modified with the modification-reactive fibrillation solution, the
fibrillation and the modification
are preferably performed in the closed container in order that the
volatilization of the
modification reaction agent or a side reaction of the modification reaction
agent due to its water
absorption may be suppressed. Further, when the modification reaction agent is
liable to
undergo a side reaction with moisture, the fibrillation is preferably
performed under a nitrogen or
argon atmosphere. The treatment may be performed while the open container or
the closed
container is mounted with a stirring apparatus and a heating reflux apparatus.
[0057] The cellulose fine fibers obtained by the fibrillation may be separated
and purified by
any appropriate method (e.g., centrifugation, filtration, concentration, or
precipitation). The
cellulose fine fibers and the fibrillation solution may be separated from each
other by, for
example, centrifuging or filtering a fibrillation mixture containing the fine
fibers and the fibrillation
solution. Alternatively, the following may be performed: a solvent that can
dissolve the
23519505.1 24
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
components of the fibrillation solution (e.g., water, an alcohol, or a ketone)
is added to the
fibrillation mixture, and the whole is separated and purified (washed) by the
separation method
(any appropriate method), such as the centrifugation, the filtration, or the
precipitation. A
separation operation may be performed a plurality of times (e.g., about twice
to about five
times). When the modification reaction agent is added, the modification
reaction agent may be
deactivated with water, methanol, or the like after the completion of the
reaction, but is
preferably recovered by distillation and reused without being deactivated from
the viewpoint of
reuse.
[0058] The resultant cellulose fine fibers include cellulose fibrillated to
sizes of from
nanometers to several hundred nanometers, and their average fiber diameter is,
for example,
from 2 nm to 800 nm, preferably from 3 nm to 600 nm, more preferably from 5 nm
to 500 nm,
still more preferably from 10 nm to 300 nm. When the average fiber diameter is
excessively
large, an effect of the fine fibers as a reinforcing material may reduce. When
the average fiber
diameter is excessively small, the handleability and heat resistance of each
of the fine fibers
may reduce.
[0059] A strong mechanical force is not applied to the resultant cellulose
fine fibers, and hence
the fine fibers have fiber lengths longer than those of fine fibers obtained
by a conventional
mechanical fibrillation method, and their average fiber length is, for
example, 1 pm or more. In
addition, although the average fiber length of the cellulose fine fibers to be
obtained falls within
the range of from about 1 pm to about 200 pm, cellulose fine fibers having an
appropriate
average fiber length may be obtained by controlling reaction conditions in
accordance with their
applications. In general, the average fiber length is, for example, from 1 pm
to 100 pm,
23519505.1 25
,
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
preferably from 2 pm to 60 pm, more preferably from 3 pm to 50 pm. When the
average fiber
length is excessively short, the reinforcing effect and film-forming function
of the fine fibers may
reduce. In addition, when the average fiber length is excessively long, there
is a risk in that the
fine fibers are liable to be entangled with each other, and hence their
dispersibility in a solvent
or a resin reduces.
[0060] The aspect ratio of the fine fibers may be easily controlled by the
composition and
impregnation time of the fibrillation solution. In general, the aspect ratio
is preferably from 40
to 1,000. From the viewpoints of the dispersibility and the reinforcing
effect, the aspect ratio is
more preferably from about 50 to about 800, still more preferably from about
80 to about 600.
A case in which the aspect ratio is less than 40 is not preferred because the
reinforcing effect
and the strength of a free-standing film formed of the fine fibers are low,
though the fine fibers
can be easily dispersed. Meanwhile, when the aspect ratio is more than 1,000,
the
dispersibility may reduce owing to the entanglement of the fine fibers.
[0061] The cellulose fine fibers and the modified cellulose microfibrils
obtained in the present
invention, the cellulose fine fibers and the modified cellulose fine fibers
each having an average
fiber diameter of from 2 nm to 800 nm and an aspect ratio of from 40 to 1,000,
can be
redispersed as described below. The cellulose fine fibers that are not
subjected to any
modification treatment can be redispersed in water, an organic solvent, such
as cellosolve
containing ethylene glycol, and a monomer and a resin each having a SP value
of more than 10
after their drying. The cellulose fine fibers modified with the modification
reaction agent can be
redispersed in an organic solvent, a monomer, or a resin having a SP value of
10 or less after
their drying. Of the modified cellulose fine fibers, acetylated-modified
cellulose fine fibers can
23519505.1 26
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
be redispersed in organic solvents, such as cellosolve containing ethylene
glycol, ethanol,
acetone, 1,4-dioxane, and dimethylacetamide, an organic solvent having a SP
value of more
than 10, and an organic solvent, a monomer, and a resin each having a SP value
of 10 or less.
Cellulose fine fibers modified with a carboxylic acid anhydride whose alkyl
group has 3 or more
.. carbon atoms can be dispersed in a solvent, a monomer, or a resin having a
SP value of more
than 10.
[0062] Examples of the solvent having a SP value of 10 or less in which the
fine fibers can be
dispersed include acetone (9.9), 1,4-dioxane (10), 1-dodecanol (9.8),
tetrahydrofuran (9.4),
methyl ethyl ketone (MEK) (9.3), ethyl acetate (9.1), toluene (8.8), butyl
acetate (8.7), and
methyl isobutyl ketone (MIBK) (8.6). Examples of the resin having a SP value
of 10 or less
include polyurethane (10.0), an epoxy resin (9 to 10), polyvinyl chloride (9.5
to 9.7),
polycarbonate (9.7), polyvinyl acetate (9.4), a polymethyl methacrylate resin
(9.2), polystyrene
(8.6 to 9.7), NBR rubber (8.8 to 9.5), polypropylene (8.0), and polyethylene
(7.9).
[0063] The surfaces of the modified fine fibers obtained by the present
invention are uniformly
modified, and hence the fine fibers can be satisfactorily dispersed in an
organic solvent, a
monomer, or a resin. In particular, the dispersion of the fine fibers in a
solvent or a resin
having a SP value of 10 or less that cannot be achieved by the related art can
be performed. A
possible reason for the foregoing is as follows: the fine fibers obtained in
the present invention
are modified in elongated states in the fibrillation solution, and hence
hydroxy groups on their
surfaces are modified without unevenness; accordingly, the fine fibers can
maintain the
elongated states even after drying. Meanwhile, in the related art, in order
that surface-modified
cellulose fine fibers may be prepared, first, cellulose is fibrillated by
strong mechanical
23519505.1 27
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
pulverization or a strong shear force in water, and then a modification
reaction is performed by
replacing the water with an aprotic polar solvent, such as acetone or toluene.
At the time of the
solvent replacement, unmodified cellulose fine fibers are bonded to each
other, gather, or are
entangled with each other by themselves, and hence an aggregated state in
which the fine
fibers form a mass is established. Even when the fine fibers are loaded in the
state into a
reaction solvent, the fine fibers are present as an aggregate, and hence only
a hydroxy group
on the surface of the aggregate is modified. Accordingly, modified fine fibers
to be obtained
cannot be satisfactorily dispersed in a solvent or a resin.
[0064] The cellulose fine fibers obtained in the present invention can be
assumed to find
applications in the fields of, for example, a paint, an adhesive, and a
composited material. In
addition, in the case of dispersion in a resin, the modified cellulose fine
fibers obtained in the
present invention, the fine fibers having a dispersion effect higher than that
of related-art
modified cellulose fine fibers, exhibit a stronger reinforcing effect when
dispersed in the resin.
[0065] The ratio (aspect ratio) of the average fiber length of the cellulose
fine fibers to the
average fiber diameter thereof may be changed in accordance with their
applications. For
example, when the fine fibers are connposited with a resin, the aspect ratio
may be 30 or more,
and is preferably from 40 to 1,000, more preferably from 50 to 500, still more
preferably from 60
to 200, particularly preferably from 80 to 150.
[0066] With regard to a method of determining the average fiber diameter,
average fiber
length, and aspect ratio of the modified cellulose fine fibers, in the present
invention, these
values are each determined by a method involving randomly selecting 50 fibers
from an image
23519505.1 28
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
of a scanning electron microscope photograph, and adding and averaging
measured values to
calculate a target value.
[0067] In addition, modified cellulose fine fibers obtained by treatment with
a fibrillation
solution containing a modification reaction agent can be satisfactorily
dispersed in an organic
medium, such as an organic solvent or a resin, because each fiber or all
fibers are modified
without unevenness.
In order to cause the resin to effectively express the characteristics of
modified
cellulose fine fibers (e.g., low linear expansion characteristics, strength,
and heat resistance),
modified cellulose fine fibers each having high crystallinity are preferred.
[0068] The modified cellulose fine fibers obtained in the present invention
can maintain the
crystallinity of the raw material cellulose to a high extent because the
cellulose is chemically
fibrillated. The crystallinity degree of the modified cellulose fine fibers is
preferably as high as
possible because the physical properties of the modified cellulose fine fibers
are improved.
The crystallinity degree of the modified cellulose fine fibers is, for
example, 50% or more,
preferably 55% or more, more preferably 60% or more, still more preferably 65%
or more.
When the crystallinity degree is excessively small, the characteristics of the
fine fibers, such as
linear expansion characteristics and strength, may be reduced. The
crystallinity degree of the
modified cellulose fine fibers obtained in the present invention is affected
by the crystallinity
degree of the cellulose serving as a raw material, and hence the crystallinity
degree of the
modified cellulose fine fibers may be equal to or less than the crystallinity
degree of the
cellulose serving as a raw material. Therefore, the raw material cellulose
only needs to be
23519505.1 29
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
selected in accordance with the use purposes of the modified cellulose fine
fibers. The
crystallinity degree may be measured by a method described in Examples to be
described later.
[0069] The average degree of substitution of the modified cellulose fine
fibers (the average
number of substituted hydroxy groups per glucose serving as a basic
constituent unit for the
cellulose) may be determined by the diameters of the fine fibers and the kind
of the modification
reaction agent. The average sensitivity is, for example, 1.5 or less,
preferably from 0.02 to 1.2,
more preferably from 0.05 to 1.2, still more preferably from 0.1 to 1.2, still
more preferably from
0.15 to 1.0, particularly preferably from 0.25 to 0.9, more particularly
preferably from 0.3 to 0.9.
When the average degree of substitution is excessively large, the
crystallinity degree of the fine
fibers or the yield thereof may reduce. The average degree of substitution
(DS) is the average
number of substituted hydroxy groups per glucose serving as a basic
constituent unit for the
cellulose, and is described in, for example, Biomacromolecules 2007, 8, 1973-
1978, WO
2012/124652 Al, or WO 2014/142166 Al, the descriptions of which are
incorporated herein by
reference.
Examples
[0070] The present invention is described in more detail below on the basis of
Examples.
However, the present invention is not limited to these Examples. Details about
used raw
materials are as described below, and the characteristics of the resultant
modified cellulose fine
fibers were measured as described below. In Example or Comparative Example in
which the
temperature at which fibrillation was performed is not specified, the
fibrillation is performed at
room temperature.
23519505.1 30
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
[0071] (Used Raw Materials, Catalysts, and Solvents)
Cellulose pulp: Pulp obtained by tearing commercial wood pulp (manufactured by
Georgia-Pacific LLC, product name: FLUFF PULP ARC48000GP) into sizes that can
be loaded
into a sample bottle.
Formic acid, organic solvents, and carboxylic acid anhydrides: Products
manufactured
by Nacalai Tesque, Inc. An 88 wt% formic acid aqueous solution was used as
formic acid.
[0072] (Evaluation of Fibrillation Degree)
The fibrillation degree of cellulose in the resultant cellulose fine fibers
was observed
with an optical microscope ("OPTIPHOT-POL" manufactured by Nikon Corporation),
and was
evaluated by the following criteria.
C): Fibrillation advances, and hence substantially no fibers each having a
fiber
diameter of more than 500 nnn are present.
o: Most fiber diameters are 500 nm or less, but fibers each having a fiber
diameter of 1
pm or more are slightly present.
x: The fibers of the raw material cellulose remain as they are.
(Average Degree of Substitution of Cellulose Fine Fibers)
The surface modification ratio of modified cellulose fine fibers was
represented by an
average degree of substitution, and was measured by the following titration
method. The
average degree of substitution is the average of the number of modified
hydroxy groups
(number of substituents) per repeating unit of cellulose.
23519505.1 31
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
That is, 6 ml of methanol and 2 ml of distilled water were added to surface-
modified
cellulose fine fibers (solid content: 0.05 g) that had been washed with water
or an organic
solvent, and dried, and the mixture was stirred at from 60 C to 70 C for 30
minutes. After that,
10 ml of a 0.05 N sodium hydroxide aqueous solution was added to the mixture,
and the whole
was stirred at from 60 C to 70 C for 15 minutes, and was further stirred at
room temperature for
1 day. The resultant mixed liquid was titrated with a 0.02 N hydrochloric acid
aqueous solution
through the use of phenolphthalein, and the chemical modification ratio of the
fine fibers was
calculated from the following equation.
Here, the number of moles Q of a substituent introduced by the chemical
modification
is determined from the amount Z (ml) of the 0.02 N hydrochloric acid aqueous
solution required
for the titration by using the following equation.
Q (mol)=0.05 (N)x10 (m1)/1,000-0.02 (N)xZ (mI)/1,000
A relationship between the number of moles Q of the substituent and the
average
degree of substitution D of the fine fibers is calculated from the following
equation
[cellulose=(C605H10)n.(162.14)n, number of hydroxy groups per repeating
unit=3, molecular
weight of OH=17]:
D=162.14x0/[sample amount-(T-18)xo]
where T represents the molecular weight of a carboxylic acid serving as a
precursor of an
esterification substituent. In the case of, for example, acetylation
modification, T represents
the molecular weight of acetic acid.
23519505.1 32
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
(Shape Observation of Cellulose Fine Fibers)
The shapes of cellulose fine fibers were observed with a FE-SEM ("JSM-6700F"
manufactured by JEOL Ltd., measurement conditions: 20 mA and 60 seconds). The
average
fiber diameter and average fiber length of the fine fibers were each
calculated by randomly
selecting 50 fibers from an image of a SEM photograph, and adding and
averaging measured
values.
(Crystallinity Degree)
The crystallinity degree of the resultant modified cellulose fine fibers was
measured by
an XRD analysis method (Segal method) on the basis of a method described in
the reference:
Textile Res. J. 29: 786-794 (1959), and was calculated from the following
equation:
Crystallinity degree (%)=[(1200-IAM)/1200] x 100%
where 1200 represents the diffraction intensity of a lattice plane (002 plane)
(diffraction angle
28=22.6 ) in X-ray diffraction, and IAM represents the diffraction intensity
of an amorphous
portion (the lowest portion between the 002 plane and a 110 plane, diffraction
angle 28=18.5').
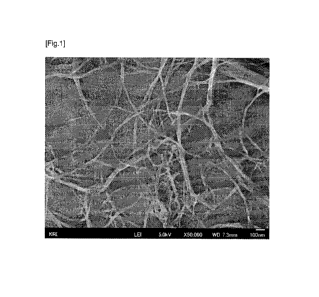
[0073] [Example 1]
10 g of the formic acid aqueous solution and 0.3 g of the cellulose pulp were
loaded
into a 20-milliliter sample bottle, and the mixture was stirred with a
magnetic stirrer for 3 hours.
After that, the mixture was transferred to a 200-milliliter centrifuge tube,
and 100 ml of distilled
water was added to the mixture, followed by washing through centrifugation.
Formic acid was
removed by performing centrifugation three times according to the same
procedure. Thus,
cellulose fine fibers were obtained. The modification ratio of the resultant
cellulose fine fibers
was determined by solid-state NMR. In addition, the fibrillation degree of the
cellulose pulp
23519505.1 33
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
was observed with an optical microscope. The shapes of the fine fibers were
observed with a
FE-SEM. The results are shown in Table 1. As shown in Table 1, it was found
that the
surfaces of the cellulose fine fibers were not modified. A SEM photograph of
the fine fibers is
shown in FIG. 1. The cellulose fine fibers had an average fiber diameter of
100 nm or less,
.. and an average fiber length of 13.9 pm. The resultant fine fibers were able
to be redispersed
in water or ethylene glycol after their drying at 105 C. The result of the
measurement of the
crystallinity degree of the fine fibers is shown in Table 1.
[0074] [Example 2]
Cellulose fine fibers were obtained in the same manner as in Example 1 except
that: 9
g of the formic acid aqueous solution and 1 g of propionic anhydride were used
instead of 10 g
of the formic acid aqueous solution; and the fibrillation time was changed
from 3 hours to 5
hours. The resultant cellulose fine fibers were evaluated in the same manner
as in Example 1.
The cellulose fine fibers had an average degree of ester substitution of 0.5,
an average fiber
diameter of 100 nm or less, which was substantially equal to that of Example
1, and an average
fiber length of 15.0 pm.
The resultant fine fibers were able to be redispersed in
dimethylacetamide, acetone, or methyl ethyl ketone after their drying with a
fan dryer at 105 C.
A SEM image of the fine fibers is shown in FIG. 2. The result of the
measurement of the
crystallinity degree of the fine fibers is shown in Table 1.
[0075] [Example 3]
Cellulose fine fibers were obtained in the same manner as in Example 2 except
that
acetic anhydride was used instead of propionic anhydride. The resultant
cellulose fine fibers
were evaluated in the same manner as in Example 2. The cellulose fine fibers
had an average
23519505.1 34
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
degree of ester substitution of 0.6, an average fiber diameter of 100 nm or
less, and an average
fiber length of 12.2 pm.
The resultant fine fibers were able to be redispersed in
dimethylacetamide after their drying with a fan dryer at 105 C. A SEM image of
the fine fibers
is shown in FIG. 3. The result of the measurement of the crystallinity degree
of the fine fibers
is shown in Table 1.
[0076] [Example 4]
Cellulose fine fibers were obtained in the same manner as in Example 2 except
that
butyric anhydride was used instead of propionic anhydride. The resultant
cellulose fine fibers
were evaluated in the same manner as in Example 2. The fine fibers had an
average degree
of ester substitution of 0.32, an average fiber diameter of 100 nm or less,
and an average fiber
length of 12.6 pm. The resultant fine fibers were able to be redispersed in
dimethylacetamide,
acetone, or methyl ethyl ketone after their drying with a fan dryer at 105 C.
A SEM image of
the fine fibers is shown in FIG. 4. The result of the measurement of the
crystallinity degree of
the fine fibers is shown in Table 1.
[0077] [Example 5]
Cellulose fine fibers were obtained in the same manner as in Example 1 except
that 5 g
of the formic acid aqueous solution and 5 g of DMSO were used instead of 10 g
of the formic
acid aqueous solution. The resultant cellulose fine fibers were evaluated in
the same manner
as in Example 1. The surfaces of the resultant cellulose fine fibers were not
modified, and the
fine fibers had an average fiber diameter of 100 nm or less, and an average
fiber length of 13.3
pm.
A SEM image of the fine fibers is shown in FIG. 5. The result of the
measurement of the
crystallinity degree of the fine fibers is shown in Table 1.
23519505.1 35
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
[0078] [Example 6]
Cellulose fine fibers were obtained in the same manner as in Example 1 except
that
instead of the fibrillation treatment at room temperature, the fibrillation
solution was stirred for 2
hours while being heated at 60 C. The resultant cellulose fine fibers were
evaluated in the
same manner as in Example 1. The average fiber diameter and average fiber
length of the fine
fibers were substantially equal to those of Example 1.
[0079] (Comparative Example 1)
Fibrillation was performed in the same manner as in Example 1 except that the
formic
acid aqueous solution was changed to acetic acid. The cellulose pulp
substantially remained
in the state of a sheet, and was not dispersed.
[0080] (Comparative Example 2)
Fibrillation was performed in the same manner as in Example 1 except that the
formic
acid aqueous solution was changed to propionic acid. The cellulose pulp
substantially
remained in the state of a sheet, and was not dispersed.
[0081] (Comparative Example 3)
Fibrillation was performed in the same manner as in Example 1 except that the
formic
acid aqueous solution was changed to a 35 wt% hydrochloric acid aqueous
solution. The
cellulose pulp was able to be dispersed to a fiber level, but as a result of
observation with an
optical microscope, its fibers were not fibrillated at all.
23519505.1 36
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
[0082] The results of the evaluations of the modified cellulose fine fibers
obtained in Examples
and Comparative Examples are shown in Table 1.
Table 1
Average
Composition Fibrillation Fibrillation
degree of Crystallinity
ester degree
(weight ratio) temperature/time degree
substitution (%)
(DS)
Formic acid
aqueous Room
Example 1 0 0 86
solution/pulp temperature/3 hr
(10/0.3)
Formic acid
aqueous
Room
Example 2 solution/propionic 0 0.38 83
temperature/5 hr
anhydride/pulp
(9/1/0.3)
Formic acid
aqueous
Room
Example 3 solution/acetic 0 0.50 85
temperature/5 hr
anhydride/pulp
(9/1/0.3)
Formic acid
aqueous
Room
Example 4 solution/butyric 0.31 84
temperature/5 hr
anhydride/pulp
(9/1/0.3)
Formic acid
aqueous Room
Example 5 0 0 86
solution/DMSO/pulp temperature/3 hr
(5/5/0.3)
Formic acid
Example 6 aqueous 60 C/3 hr 0 0 82
solution/pulp
(10/0.3)
Comparative Acetic acid/pulp Room
Example 1 (10/0.3) temperature/3 hr
Comparative Propionic acid/pulp Room
Example 2 (10/0.3) temperature/3 hr
35 wt%
Hydrochloric acid
Comparative Room
Example 3 aqueous temperature/3 hr
solution/pulp
(10/0.3)
23519505.1 37
CA 03026213 2018-11-30
CA Application
Blakes Ref: 15803/00002
As is apparent from the results of Table 1, while the fibrillation advanced in
each of the
cellulose fine fibers obtained in Examples, the fibrillation hardly advanced
in each of the
modified cellulose fine fibers obtained in Comparative Examples.
Industrial Applicability
[0083] Cellulose fine fibers to be produced by the production method of the
present invention
can be utilized in various composite materials and coating agents, and can
also be utilized by
being formed into a sheet or a film.
23519505.1 38