Note: Descriptions are shown in the official language in which they were submitted.
CA 03026335 2018-12-03
DESCRIPTION
ELECTRODE CATALYST, METHOD FOR PRODUCING THE SAME, AND ELECTRODE
CATALYST LAYER USING ELECTRODE CATALYST
TECHNICAL FIELD
[0001]
The present invention relates to an electrode catalyst, a method
for producing the same, and an electrode catalyst layer that uses
the electrode catalyst.
BACKGROUND ART
[0002]
A solid polymer electrolyte fuel cells (PEFC) using a proton
conductive solid polymer membrane works at a lower temperature than
other types of fuel cells, for example, solid oxide fuel cells and
molten carbonate fuel cells. For this reason, the polymer
electrolyte fuel cell is expected as a stationary power source and
a power source for moving bodies such as motor vehicles, and it has
been started to put the polymer electrolyte fuel cells to practical
use as well.
[0003]
In such a solid polymer type fuel cell, generally, highly
expensive metal catalysts represented by platinum (Pt) or Pt alloys
are used, and the metal catalysts have caused the high prices of such
fuel cells. Therefore, there is a demand for the development of a
technology capable of reducing the amount of use of noble metal
catalysts and reducing the production cost of fuel cells.
[0004]
JP 2008-181696 A discloses a catalyst for fuel cells, the
catalyst having metal oxide microparticles and platinum-based metal
- 1 -
CA 03026335 2018-12-03
catalyst microparticles supported on a carbon support.
SUMMARY OF INVENTION
[0005]
However, the catalyst described in JP 2008-181696 A still cannot
exhibit sufficient catalytic activity.
[0006]
Therefore, the present invention was achieved in view of such
circumstances, and it is an object of the invention to provide an
electrode catalyst capable of enhancing the catalytic activity.
[0007]
The inventors of the present invention conducted a thorough
investigation in order to solve the problems described above. As a
result, the inventors found that the problems can be solved by an
electrode catalyst in which a catalyst metal particle(s) and a
spacer ( s ) having a particular average diameter ratio are co-supported
on a catalyst support. Thus, the inventors completed the present
invention.
BRIEF DESCRIPTION OF DRAWINGS
[0008]
Fig. 1 is a schematic cross-sectional view illustrating an
electrode catalyst according to an embodiment of the present
invention.
Fig. 2 is a diagram illustrating in detail the correlation
between a catalyst metal particle and a spacer existing in the nearest
vicinity of the catalyst metal particle in the electrode catalyst
of Fig. 1.
Fig. 3 is a schematic cross-sectional view illustrating the
state in which the electrode catalyst according to an embodiment of
- 2 -
the present invention is covered with an electrolyte.
Fig. 4 is a magnified view of the interface between the electrode
catalyst and the electrolyte illustrated in Fig. 3.
Fig. 5 is a schematic cross-sectional view illustrating the
basic configuration of a solid polymer type fuel cell containing the
electrode catalyst according to an embodiment of the present
invention.
Fig. 6 is a SEM image of the electrode catalyst according to
an embodiment of the present invention.
DESCRIPTION OF EMBODIMENTS
[0009]
An electrode catalyst according to the present invention has
a catalyst metal particle (s) and a spacer (s) supported on a catalyst
support, and a ratio (dspidcat) of an average diameter of the spacer (s)
(dsp) with respect to an average diameter of the catalyst metal
particle (s) (dcat) is from 3.5 to 10. When the electrode catalyst
according to the present invention and an electrolyte are mixed to
form a catalyst layer, the spacer suppresses or prevents the catalyst
metal particles from being completely covered by the electrolyte.
Therefore, the poisoning action which the catalyst metal particle
receives is attenuated, and the catalytic activity (particularly,
oxygen reduction reaction (ORR) activity and/or ORR specific
activity) is enhanced.
[0010]
It is described in JP 2008-181696 A that the disclosed catalyst
has high catalytic activity (paragraph "0014") . However, it still
could not be said that the above-mentioned catalyst had sufficient
catalytic activity. In JP 2008-181696 A, the catalyst is produced
by supporting metal oxide microparticles on a carbon support and then
- 3 ¨
CA 3026335 2019-01-28
CA 03026335 2018-12-03
supporting platinum-based metal catalyst microparticles (catalyst
metal particles) thereon. Since the catalyst metal particle has a
strong interaction with the metal oxide microparticle, in the case
of this production method, a significant quantity of the catalyst
metal particles are not directly supported on the carbon support but
are supported on the metal oxide microparticles that are supported
on the carbon support. Asa result, the catalyst metal particles are
localized on protrusions of the catalyst. Therefore, when a catalyst
layer is formed by mixing the catalyst with an electrolyte, most of
the catalyst metal particles are covered by the electrolyte.
Therefore, the chance for reactive gas (particularly, oxygen) to be
brought into contact with the catalyst metal particle surface is
reduced (the poisoning action which the catalyst metal particle
receives is increased), and the catalytic activity of the catalyst
metal particle(s) is impaired.
[0011]
In this regard, the electrode catalyst according to the present
invention can exhibit high catalytic activity (particularly, oxygen
reduction reaction (ORR) activity and/or ORR specific activity), even
when the electrode catalyst is mixed with an electrolyte to form a
catalyst layer. The inventors of the present invention found that
an electrode catalyst can be effectively utilized by forming a
three-phase interface (reaction site) by means of reactive gas
(particularly, oxygen), a catalyst metal particle, and water. From
this, the present inventors thought that when a catalyst layer is
formed by mixing the electrode catalyst with an electrolyte, the
electrolyte brings poisoning action to the catalyst metal particles
and lowers the catalytic activity. That is, the inventors thought
that as the coverage of the catalyst metal particles by the electrolyte
is lower, the apparent ORR activity (ORR specific activity) is
¨ 4 ¨
CA 03026335 2018-12-03
enhanced. Here, the "poisoning action" means that since the
interaction between an electrolyte and a catalyst metal particle is
strong, the chance for reactive gas (particularly oxygen) to be
brought into contact with the surface of the catalyst metal particle
is reduced. Based on the findings described above, the inventors of
the present invention conducted a thorough investigation on the design
of the electrode catalyst, for the purpose of reducing the coverage
of the catalyst metal particles) by the electrolyte, that is, for
the purpose of reducing the poisoning action which the catalyst metal
.. particle receives. As a result, the inventors found that the coverage
of the catalyst metal particles by the electrolyte (poisoning action)
can be reduced by controlling the catalyst metal particle (s) and the
spacer (s) that are supported on the catalyst support, to have a
particular average diameter ratio. More particularly, in the
electrode catalyst according to the present invention, a catalyst
metal particle ("22" in Fig. 1) and a spacer ("23" in Fig. 1) that
is larger than the catalyst metal particle co-exist on the surface
of a catalyst support. The spacer has an outer circumferential region
("24" in Fig. 2) that is located on the outer side of the particle
diameter than the catalyst metal particle. When an electrode
catalyst having such a configuration is mixed with an electrolyte,
since the electrolyte (particularly, polymer electrolyte) is viscous,
as illustrated in Fig. 4, a void ("29" in Fig. 4) is formed between
the electrolyte, the spacer, the catalyst metal particle, and the
catalyst support. A part of the catalyst metal particle surface
touching the void is not brought into contact with the electrolyte.
The catalyst metal particle that is not in contact with the electrolyte
is hardly subjected to, or is not subjected to, the poisoning action
caused by the electrolyte. As a result, the chance for reactive gas
(particularly oxygen) to be brought into contact with the catalyst
- 5 -
CA 03026335 2018-12-03
metal particle surface is increased, and the formation of a
three-phase interface between reactive gas (particularly oxygen) ,
the catalyst metal particle, and water is promoted. Thus, the
catalytic activity (particularly, the ORR specific activity) is
enhanced. The above-described mechanism is only a speculation, and
the present invention is not intended to be limited by the
above-described speculation.
[0012]
Therefore, the electrode catalyst of the present invention can
have enhanced catalytic activity (particularly, ORR specific
activity) .
[0013]
In the following description, embodiments of the electrode
catalyst according to the present invention and an electrode catalyst
layer using this will be explained. Meanwhile, the present invention
is not intended to be limited to the following embodiments only.
Furthermore, the dimensional ratios of the drawings are exaggerated
for the convenience of explanation and may be different from the actual
ratios.
[0014]
In the present specification, the expression "X to Y" represents
a range including X and Y, and the expression means "X or more and
Y or less". In the present specification, the "maximum diameter" of
a particle refers to the longest length among the distances between
any arbitrary two points on the contour line of the particle.
Furthermore, unless particularly stated otherwise, the operations
and the measurements of physical properties and the like are measured
under the conditions of room temperature (20 C to 25 C) /relative
humidity of 40% to 50%.
.. [0015]
- 6 -
<Electrode catalyst>
The electrode catalyst according to the present invention is
fm-ry.r1 by supporting A catalyst metal particle (s) and a spacer (s)
on a catalyst support, and the ratio (dspidcat.) of an average diameter
of the spacer (s) (dsp) with respect to an average diameter of the
catalyst metal particle (s) (dcat) is from 3.5 to 10. In a case in which
the ratio dsp/dcat is less than 3.5, the difference in the size between
the catalyst metal particle and the spacer is small. Therefore, the
effect provided by the spacer for suppressing and preventing the
coverage of the catalyst metal particles by an electrolyte is
insufficient, and sufficient catalytic activity cannot be exhibited.
On the other hand, in a case in which the ratio dap/dcat is more than
10, it is difficult for the spacer to be supported in unoccupied spaces
on the catalyst support, and therefore, the supported ratio of the
spacer is decreased. Accordingly, the effect by which the spacer
suppresses or prevents the coverage of the catalyst metal by an
electrolyte is insufficient, and sufficient catalytic activity cannot
be exhibited. The ratio dsp/dcat is preferably from 3.7 to 6.0, and
more preferably from 3.7 to 5.9. When the ratio is in such a range,
in a case in which an electrode catalyst layer is formed using the
electrode catalyst and an electrolyte, coverage of the catalyst metal
particle surface by the electrolyte is more satisfactorily suppressed
by the effect of the spacer. Therefore, an electrode catalyst formed
by supporting a catalyst metal particle (s) and a spacer (s) in such
a range can have further enhanced catalytic activity (particularly,
ORR specific activity) .
[0016]
In regard to the electrode catalyst of the present invention,
the catalyst metal particle (s) and the spacer (s) substantially exist
on the surface of the catalyst support. Here, the phrase "the catalyst
- 7 ¨
CA 3026335 2019-01-28
CA 03026335 2018-12-03
metal particle ( s ) and the spacer ( s ) substantially exist on the surface
of the catalyst support" implies that substantially all of the
catalyst metal particles and the spacers exist on the surface of the
catalyst support. Specifically, the phrase implies that the number
of the catalyst metal particle(s) supported on the catalyst support,
with the spacer(s) being interposed therebetween, is 30% or less,
and preferably less than 20%, with respect to the total number of
the catalyst metal particles. Meanwhile, the term "catalyst metal
particle(s) supported on the catalyst support, with the spacer(s)
being interposed therebetween" refers to a catalyst metal particle ( s )
supported on the surface of the spacer, in a state of being in contact
with the spacer(s) only, without contacting the catalyst support.
Specifically, such a catalyst metal particle refers to a catalyst
metal particle 22' in Fig. 1. That is, according to a preferred
embodiment of the present invention, the number of the catalyst metal
particle(s) supported on the catalyst support, with the spacer(s)
being interposed therebetween, is less than 20% with respect to the
total number of the catalyst metal particles. More preferably, the
number of the catalyst metal particle(s) supported on the catalyst
support, with the spacer(s) being interposed therebetween, is 15%
or less, 10% or less, 9% or less, 8% or less, 7% or less, 6% or less,
5% or less, 4% or less, 3% or less, 2% or less, or 1% or less, with
respect to the total number of the catalyst metal particles supported
on the catalyst support, and it is more preferable as this value is
smaller (lower limit: 0%). When the value is in such a range, almost
no catalyst metal particle exists on the protrusions of the electrode
catalyst. Therefore, when the electrode catalyst and the electrolyte
are mixed, the number of the catalyst metal particles existing in
a void formed between the electrolyte, the spacer, and the catalyst
support can be further increased. Therefore, the surface area of the
¨ 8 -
CA 03026335 2018-12-03
catalyst metal particles covered by the electrolyte can be reduced
more effectively. As a result, the chance for reactive gas
(particularly, oxygen) to be brought into contact with the catalyst
metal particle surface increases, and the formation of a three-phase
interface by the reactive gas (particularly, oxygen), the catalyst
metal particle, and water is accelerated. Thus, the catalytic
activity is enhanced. Here, regarding the proportion of the number
of the catalyst metal particle(s) supported on the catalyst support,
with the spacer(s) being interposed therebetween, with respect to
the total number of the catalyst metal particles supported on the
catalyst support, a value measured according to the following method
is employed.
[0017]
[Method for calculating proportion of number of catalyst metal
particles supported on catalyst support, with the spacer being
interposed therebetween, with respect to total number of catalyst
metal particles supported on catalyst support]
0.01 g of an electrode catalyst is observed under a scanning
electron microscope (SEM) (1,000,000 times) manufactured by JEOL,
Ltd. The number of catalyst metal particles supported on the catalyst
support in a visual field of 250 nm x 250 nm is measured, and this
is designated as the total number of catalyst metal particle(s) (X
particle(s)) supported on the catalyst support. Furthermore, in a
visual field such as described above, catalyst metal particle(s)
supported on the catalyst support, with a spacer(s) being interposed
therebetween, are measured, and this is designated as the number of
catalyst metal particles (Y particle(s)) supported on the catalyst
support, with the spacer(s) being interposed therebetween. Based on
these values, the proportion (%) of the number of catalyst metal
particle(s) supported on the catalyst support, with the spacer(s)
¨ 9 ¨
CA 03026335 2018-12-03
being interposed therebetween, with respect to the total number of
catalyst metal particle ( s ) supported on the catalyst support [= (Y/X)
x 100] is determined. The measurement conditions described above may
be modified as appropriate. The method described above is only an
example, and even in a case in which the proportion is calculated
using a method other than the method described above, substantially
an equivalent proportion is calculated.
[0016]
Furthermore, in a case in which the differences in the size of
the catalyst metal particles can be neglected (for example, the
standard deviation of the particle size is 30% or less), this value
can be handled to be equivalent to percentage by weight (wt%) or
percentage by volume (vol%). That is, in this case, the amount of
the catalyst metal particles supported on the catalyst support, with
the spacer(s) being interposed therebetween, is preferably less than
20% by weight, 15% by weight or less, 10% by weight or less, 9% by
weight or less, 8% by weight or less, 7% by weight or less, 6% by
weight or less, 5% by weight or less, 4% by weight or less, 3% by
weight or less, 2% by weight or less, or 1% by weight or less, with
respect to the total amount of the catalyst metal particles supported
on the catalyst support, and it is more preferable as the value is
smaller (lower limit: 0% by weight). Meanwhile, the term "catalyst
metal particle(s) supported on the catalyst support" includes not
only a catalyst metal particle supported on the outer surface of the
catalyst support but also a catalyst metal particle supported on the
pore surface inside the catalyst support.
[0019]
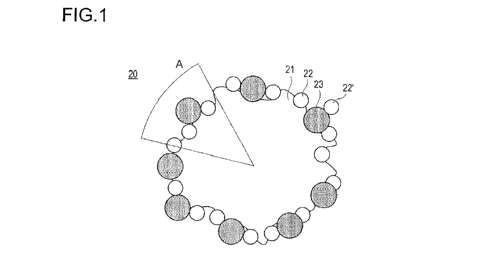
Fig. 1 is a schematic cross-sectional view illustrating an
electrode catalyst according to an embodiment of the present invention.
The electrode catalyst 20 according to the present embodiment has
- 10 -
CA 03026335 2018-12-03
a configuration in which catalyst metal particles 22 and 22' and a
spacer 23 larger than the catalyst metal particles are supported on
the surface of a catalyst support 21. The catalyst metal particle
22 is directly supported on the surface of the catalyst support 21
and is substantially not supported by means of the spacer 23 supported
on the catalyst support 21. That is, the catalyst metal particle 22
and the spacer 23 exist substantially on the surface of the catalyst
support 21. Furthermore, it is preferable that the spacer 23 exists
in the vicinity of the catalyst metal particle 22 on the surface of
the catalyst support 21. Here, the phrase "exists in the vicinity"
implies existing to be adjacent, or adjoining while being separated
by a gap. Thereby, the number of catalyst metal particles existing
in the void formed between the electrolyte, the spacer, and the
catalyst support is further increased, and the surface area of the
catalyst metal particles that is not covered by the electrolyte can
be increased more effectively. In the following description, the
term "catalyst metal particle(s) that is/are not covered by the
electrolyte" is also referred to as "non-covered catalyst metal
particle(s)".
[0020]
Fig. 2 is a diagram illustrating the correlation between a
catalyst metal particle and a spacer existing in the nearest vicinity
of the catalyst metal particle in the electrode catalyst of Fig. 1
(magnified view of the part surrounded by line Ain Fig. 1). In Fig.
2, a spacer 23 existing in the nearest vicinity of a catalyst metal
particle 22 has an outer circumferential region 24 that is located
on the outer side of the particle diameter of the catalyst metal
particle 22. In other words, when the radius of a circle 26
circumscribing the catalyst metal particle 22 is designated as dl,
and the radius of a circle 27 circumscribing the spacer 23 is
¨ 11 ¨
CA 03026335 2018-12-03
designated as d2, respectively with reference to the center 25 of the
catalyst support, the relation: di < d2 establishes. When an electrode
catalyst having such a configuration is mixed with an electrolyte,
the protruding spacer acts as a steric hindrance, and thus the viscous
electrolyte (for example, polymer electrolyte) cannot be brought into
contact, or can be only partially brought into contact, with the
catalyst metal particle existing in the vicinity of the spacer.
Therefore, voids as described below can be easily formed between the
catalyst metal particle (s) and the electrolyte, and the surface area
.. of the non-covered catalyst metal particle (s) can be increased. Here,
the "spacer existing in the nearest vicinity of the catalyst metal
particle" refers to a spacer for which, when a catalyst metal particle
is observed using an observation means such as a scanning electron
microscope (SEM) , the distance between the center of this catalyst
metal particle and the center of the spacer is the shortest.
[0021]
Fig. 3 is a schematic cross-sectional view illustrating the
state in which the electrode catalyst according to an embodiment of
the present invention is covered with an electrolyte. Furthermore,
Fig. 4 is a magnified view of the interface between the electrode
catalyst and the electrolyte in Fig. 3 (magnified view of the part
surrounded by line B in Fig. 3) . On the surface of the catalyst support
21 of the electrode catalyst, a spacer 23 that is larger than a catalyst
metal particle 22 exists in the vicinity of the catalyst metal particle
22. Therefore, even if the electrode catalyst 20 is covered with an
electrolyte 28, since the spacer acts as a steric hindrance, the
surface of the catalyst metal particle 22 is not completely covered
by the electrolyte 28. As a result, as illustrated in Fig. 4, a void
29 is formed between the catalyst support 21, the catalyst metal
particle 22, the spacer 23, and the electrolyte 28. That is, the
- 12 -
CA 03026335 2018-12-03
portion of the surface of the catalyst metal particle 22 that is in
contact with the void 29, is not brought into contact with the
electrolyte 28. A catalyst metal particle that is not in contact with
the electrolyte as such is not easily subjected to, or is not subjected
to, the poisoning action caused by the electrolyte. As a result, the
chance for oxygen to be brought into contact with the catalyst metal
particle surface is increased, the formation of a three-phase
interface between the reactive gas (particularly oxygen), the
catalyst metal particle, and water is promoted, and thus the catalytic
activity (ORR specific activity) is enhanced.
[0022]
In the following description, the various constituent
components of the electrode catalyst according to the present
invention will be explained.
[0023]
[Spacer]
The spacer according to the present invention plays the role
of suppressing or preventing the coverage of the catalyst metal
particle surface by an electrolyte when the electrode catalyst is
mixed with the electrolyte.
[0024]
Regarding the shape of the spacer, objects having a granular
shape, a fiber shape (fibrous shape), a scaly shape, a layered shape
or the like can be used; however, a preferred shape is a granular
shape or a fiber shape.
[0025]
According to the present specification, "an average diameter
(d2p) of the spacer(s)" represents an average height of the spacer(s)
supported on the catalyst support. For example, in regard to Fig.
2, when the radius of a circle 27 circumscribing the spacer 23 is
¨ 13 -
CA 03026335 2018-12-03
designated as d2, and the shortest distance between the center 25 of
the cata]yst support and the contact point between the spacer 23 and
the catalyst support 21 is designated as d3, dsp is a value obtained
by subtracting d3 from d2 (d2 - d3). For example, in a case in which
the spacer has a granular shape, cis', represents an average primary
particle size, and a value calculated as an average value of particle
size of the particles observed in several visual fields to several
ten visual fields using an observation means such as a scanning
electron microscope (SEM) or a transmission electron microscope (TEM)
will be employed. Furthermore, in a case in which the spacer has a
fiber shape (fibrous shape), dsp represents the diameter (diameter),
and similarly, a value calculated by SEM or TEN is employed. The
average diameter of the spacer(s) (dsp) is not particularly limited
as long as the ratio (dspielcat) between the average diameter of the
spacer(s) (dsp) and the average diameter of the catalyst metal
particle(s) (dear) satisfies in a range of 3.5 to 10. The average
diameter (dsp) of the spacer(s) is preferably from 5 nm to 40 nm, more
preferably from 10 nm to 30 nm, and even more preferably from 12 nm
to 20 nm. When the ratio is in such a range, the spacers are supported
on the catalyst support in a highly dispersed manner, and the spacers
can exhibit the function as a spacer. Furthermore, in a case in which
the spacer has a fiber shape (fibrous shape), the length of the fiber
is not particularly limited; however, when the dispersibility on the
catalyst support or the like is considered, the length of the fiber
is about 1 to 50 m, and preferably 5 to 20 m.
[0026]
The supported amount (supported ratio) of the spacer(s) is not
particularly limited; however, when the dispersibility on the
catalyst support or the like is considered, the supported amount is
preferably from 0.5% by weight to 30% by weight, more preferably from
- 14 -
CA 03026335 2018-12-03
1% by weight to 20% by weight, and even more preferably from 1.5%
by weight to 12% by weight, when the weight of the electrode catalyst
is designated as 100% by weight . The supported amount of the spacer (s)
can be investigated according to a conventionally known method such
as inductively coupled plasma emission analysis (ICE atomic emission
spectrometry), inductively coupled plasma mass analysis (ICP mass
spectrometry), or fluorescent X-ray analysis (XRF).
[0027]
The ratio (d3/d5) of the average diameter of the spacer(s)
(dsp) to the average diameter of the catalyst support(s) (dsup) is not
particularly limited; however, the ratio is preferably from 0.01 to
0.1, more preferably from 0.02 to 0.08, and even more preferably from
0.03 to 0.06. When the ratio is in such a range, the spacers are
supported on the catalyst support in a highly dispersed manner, and
the function as a spacer is satisfactorily exhibited.
[0028]
The material for the spacer may be any of an inorganic compound,
an organic compound, and an organic/inorganic hybrid compound;
however, from the viewpoint of stabi]ity (low reactivity), it is
preferable that the spacer is formed of an inorganic compound.
Examples of the inorganic compound include, but are not limited to,
oxides, nitrides, and the like of metals such as silicon, aluminum,
titanium, zirconium, cerium, and tin. Among these, it is more
preferable that the spacer is a metal oxide. It is particularly
preferable that the spacer includes at least one selected from the
group consisting of SiO2, Al2O3, and TiO2.
[0029]
Regarding the spacer, any of a synthesized product and a
commercially available product may be used.
Examples of the
commercially available product include SNOWTEX (registered
- 15 -
CA 03026335 2018-12-03
trademark) 20, 30, 40, OS, 0, OS, OXS, XS, 0-40, C, N, S, 20L, OL
(all manufactured by Nissan Chemical Industries, Ltd. ) , SiO2: Product
No. 637238, 637246, and 791334; A1203: Product No. 718475, 634131,
551643, 790915, and 790923; and TiO2: Product No. 718467, 798525,
798509, and 798495 (all manufactured by Sigma-AldrichCo. LLC. ) . The
spacers mentioned above may be used singly, or two or more kinds
thereof may be used in combination.
[0030]
[Catalyst support]
The catalyst support functions as a support for supporting the
catalyst metal particle (s) which will be described below and the
spacer (s) , and as an electron conduction path participating in the
donation and acceptance of electron between the catalyst particles
and other members. Regarding the catalyst support, any support
material may be used as long as it has a specific surface area for
supporting the catalyst metal particles in a desired dispersed state,
and the catalyst support may be any of a carbon support and a non-carbon
support. Here, the term "carbon support" refers to a support
containing carbon atoms as a main component. The phrase "contains
carbon atoms as a main component" is a concept including both "composed
only of carbon atoms" and "substantially composed of carbon atoms",
and elements other than carbon atoms may also be included. The phrase
"substantially composed of carbon atoms" means that incorporation
of impurities at a proportion of 2% to 3% by weight or less is acceptable.
A non-carbon support refers to a support that does not fall under
the definition of the carbon support as described above, and examples
include metal oxides.
[0031]
Specific examples of the carbon support include acetylene black,
Ketjen black, thermal black, oil furnace black, channel black, lamp
- 16 ¨
CA 03026335 2018-12-03
black, and graphitized carbon. More specific examples include VULCAN
(registered trademark) XC-72R, VULCAN (registered trademark) P, BLACK
PEARLS (registered trademark) 880, BLACK PEARLS (registered
trademark) 1100, BLACK PEARLS (registered trademark) 1300, BLACK
PEARLS (registered trademark) 2000, REGAL (registered trademark) 400
(all manufactured by Cabot Japan K.K. ) KETJENBLACK (Registered
trademark) EC300J, KETJENBLACK (registered trademark) EC600JD (all
manufactured by Lion Specialty Chemicals Co., Ltd. ) , #3150, #3250
(manufactured by Mitsubishi Chemical Corp. ) , and DENKA BLACK
(registered trademark) (manufactured by Denka Company Limited. ) .
[0032]
Regarding the shape of the catalyst support, the catalyst
support can have any arbitrary shape such as a granular shape, a plate
shape, a pillar shape, a tubular shape, or an irregular shape.
[0033]
The size of the catalyst support is not particularly limited.
From the viewpoint of controlling the ease of supporting, the catalyst
utilization factor, and the thickness of the electrode catalyst layer
to be in appropriate ranges, an average diameter of the catalyst
support (s) (dsup) is preferably 100 to 2,000 nm, more preferably 200
to 1,000 nm, and even more preferably 300 to 500 nm. Furthermore,
in a case in which primary particles are connected or aggregated to
form a catalyst support, an average primary particle size is
preferably 5 to 30 nm, and more preferably 10 to 20 nm. Regarding
the average primary particle size, a value measured by SEM or TEM
is employed. The ''average diameter of the catalyst support (s) (dsup) "
can be measured as the average value of the crystallite diameter that
is determined from the half-value width of a diffraction peak of the
catalyst support in X-ray diffraction (XRD) , or as the average value
of the particle size of the catalyst support that is detectable by
- 17 -
CA 03026335 2018-12-03
transmission electron microscopy (TEN) . In
the present
specification, the "average diameter of the catalyst support (s)
(dsup) " is the average value of the maximum diameter of the catalyst
support that is detectable from transmission electron microscopic
image for a statistically meaningful number (for example, at least
200, and preferably at least 300) samples
[0034]
The BET specific surface area of the catalyst support is
desirably a specific surface area sufficient for supporting the
catalyst metal particles and the spacers in a highly dispersed manner,
and the BET specific surface area is preferably 10 to 5,000 m2/g, more
preferably 50 to 2,000 m2/g, even more preferably 100 to 1,000 m2/g,
and particularly preferably 300 to 800 m2/g. With such a specific
surface area, sufficient catalyst metal particles can be supported
on the catalyst support, and high catalytic activity can be exhibited.
[0035]
The "BET specific surface area (m2/g of the support) " of the
support is measured by a nitrogen adsorption method. More
particularly, about 0.04 to 0.07 g of a catalyst powder is precisely
weighed and sealed in a sample tube. This sample tube is preliminarily
dried in a vacuum dryer at 90 C for several hours, and the content
is used as a sample for measurement. For the weighing, an electronic
balance (AW220) manufactured by SHIMADZU CORPORATION is used. In the
case of a coated sheet, a net mass of about 0.03 to 0.04 g of the
coating layer calculated by subtracting the mass of TEFLON (registered
trademark) (base material) having the same area from the total mass
of the coated sheet is used as the sample mass. Next, the BET specific
surface area is measured under the following measurement conditions.
A BET plot is produced over a relative pressure (P/Po) range of about
0.00 to 0.45 on the adsorption side of an adsorption/desorption
¨ 18 ¨
CA 03026335 2018-12-03
isotherm, and the BET specific surface area is calculated from the
gradient and the intercept.
[0036]
[Chem. 1]
<Measurement conditions>
Measuring apparatus: Fully automated high-precision gas adsorption
apparatus manufactured by EEL Japan, Inc., BELSORP36
Adsorption gas: N2
Dead volume measurement gas: He
Adsorption temperature: 77 K (liquid nitrogen temperature)
Treatment before measurement: Vacuum drying at 90 C for several hours
(after He purge, mounted on a measurement stage)
Measurement mode: Isothermal adsorption process and desorption
process
Relative measurement pressure P/Po: About 0 to 0.99
Balance set time: 180 sec for 1 relative pressure
[0037]
[Catalyst metal particle(s)]
The catalyst metal particle has a function of implementing
catalytic action in an electrochemical reaction. Regarding the
catalyst metal particle, a catalyst met al particle containing at least
platinum is preferably used in order to enhance the catalytic activity,
the resistance to poisoning against carbon monoxide or the like, heat
resistance, and the like. That is, the catalyst metal particle
contains platinum, or contains platinum and a metal component other
than platinum.
[0038]
Regarding the metal component other than platinum, any known
catalyst component can be similarly used without any particular
limitations, and specific examples include metals such as ruthenium,
iridium, rhodium, palladium, osmium, tungsten, lead, iron, copper,
silver, chrome, cobalt, nickel, manganese, vanadium, molybdenum,
gallium, aluminum, and zinc. The metal component other than platinum
may be one kind of metal or a mixture of two or more kinds. Among
- 19 -
CA 03026335 2018-12-03
them, from the viewpoint of the catalytic performance, the metal
component is preferably a transition metal. Here, a transition metal
atom refers to the atoms of elements ranging from an element of Group
3 to an element of Group 12, and the type of the transition metal
atom is not particularly limited. From the viewpoint of the catalytic
activity, it is preferable that the transition metal atom is selected
from the group consisting of vanadium, chromium, manganese, iron,
cobalt, copper, zinc, and zirconium.
[0039]
A composition of the alloy may be varied depending on the kind
of the metal to be alloyed, but for example, it is preferable that
a content of platinum is set to from 30% to 90% by atom and a content
of the metal to be alloyed with platinum is set to from 10% to 70%
by atom. Incidentally, an alloy is generally a generic term that it
comprises one or more kinds of metal elements or non-metal elements
added to a metal element and has metallic properties. As the
construction of the alloy, there are a eutectic alloy of a so-called
mixture in which constituent elements are separate crystals, one in
which constituent elements completely melt into each other to form
a solid solution, one in which constituent elements form an
intermetallic compound or a compound of a metal and a nonmetal, and
the like, and the construction of the alloy may be any of these in
the present embodiment.
[0040]
The shape of the catalyst metal particle is not particularly
limited, and the shape may be a spherical shape, a plate shape, a
needle shape, a pillar shape, a rectangular shape, a polyhedral shape,
or the like.
[0041]
An average diameter of the catalyst metal particle(s) (dcat) is
- 20 -
CA 03026335 2018-12-03
preferably from 1 nm to 30 nm, more preferably from 2 nm to 10 nm,
and even more preferably from 3 nm to 5 nm. When the average diameter
is in such a range, dissolution or aggregation of the catalyst metal
particles can be suppressed, while the activity per unit weight
(weight specific activity) of the catalyst metal particle(s) is
increased. In the present specification, the "average diameter of
the catalyst metal particle (s) (dcat) represents the maximum diameter
of the catalyst metal particle(s). In a case in which the catalyst
metal particle has a spherical shape, dcat represents an average
crystallite diameter. The "average crystallite diameter" is
calculated using Scherrer's equation from an XRD spectrum near 410,
which originates from Pt(111). In addition, there are occasions in
which the size of the particles is represented as the average particle
size; however, the average particle size is substantially equivalent
to the average crystallite diameter representing the size of the
catalyst metal particle(s). Therefore, it is also preferable that
the average particle size of the catalyst metal particle(s) is in
the range described above. Meanwhile, the "average particle size"
is the average value of the particle sizes of particles observed in
several visual fields to several ten visual fields using an
observation means such as scanning electron microscopy (SEM) or
transmission electron microscopy (TEM).
[0042]
The supported amount (supported ratio) of the catalyst metal
particles is not particularly limited; however, when the weight of
an electrode catalyst precursor (total weight of the catalyst support
and the catalyst metal particles) is designated as 100% by weight,
the supported amount is preferably from 2% by weight to 60% by weight.
When the supported amount is adjusted to such a range, aggregation
between catalyst metal particles is suppressed, and an increase in
¨ 21 ¨
CA 03026335 2018-12-03
the thickness of the electrode catalyst layer can be suppressed, which
is preferable. The supported amount is more preferably from 5% by
weight to 50% by weight, even more preferably from 10% by weight to
40% by weight, and particularly preferably from 20% by weight to 30%
by weight. When the supported amount is in such an amount, the balance
between the dispersibility of the catalyst metal particles on the
catalyst support and the catalytic activity can be appropriately
controlled. Meanwhile, the supported amount of the catalyst metal
particles can be examined according to any conventionally known method
such as inductively coupled plasma emission analysis (ICP atomic
emission spectrometry) , inductively coupled plasma mass analysis (ICP
mass spectrometry) , or fluorescent X-ray analysis (XRF) .
[0043]
In regard to the electrode catalyst according to the present
invention, it is preferable that the supported amount of the catalyst
metal particles is larger than the supporting amount of the spacer.
Specifically, the proportion of the supported amount of the catalyst
metal particles with respect to the supporting amount of the spacer
(=supported amount of catalyst metal particle (s) /supported amount
of spacer (s) ) is preferably from 1.1 times to 50 times, more preferably
from 1.5 times to 20 times, and even more preferably from 2.0 times
to 15 times. With such a mixing proportion between the catalyst metal
particles and the spacers, the catalyst metal particle (s) and the
spacer (s) exist sufficiently close to each other on the catalyst
support. Therefore, when the electrode catalyst is mixed with an
electrolyte, the spacer acts satisfactorily as a steno hindrance
and more effectively suppresses or prevents the electrolyte from being
brought into contact with the catalyst metal particle (s ) existing
in the vicinity of the spacer (s) . Therefore, voids are formed more
efficiently between the catalyst metal particl e (s) and the
¨ 22 ¨
CA 03026335 2018-12-03
electrolyte, and the surface area of non-covered catalyst metal
particles can be further increased.
[0044]
[Method for producing electrode catalyst]
The electrode catalyst according to the present invention may
be produced by any method as long as the electrode catalyst has the
configuration described above. According to a preferred embodiment
of the present invention, the electrode catalyst according to the
present invention is produced by a step of supporting the catalyst
metal particle(s) on the catalyst support to produce an electrode
catalyst precursor (Step 1); and a step of supporting the spacer(s)
on the electrode catalyst precursor (Step 2). Since small catalyst
metal particles are first supported on a catalyst support and then
large spacers are supported as such, most of the spacers are not
disposed on the smaller catalyst metal particles. Furthermore,
although a spacer exists on the catalyst metal particle, since the
spacer exists unstably on the catalyst metal particle due to the
difference in size, the spacer moves onto the catalyst support to
exist more stably. Therefore, when the electrode catalyst is
produced in this order, theoretically there is no catalyst metal
particle supported on the catalyst support, with the spacer being
interposed therebetween. That is, for example, as illustrated in Fig.
2, the spacer 23 is supported on the surface of the catalyst support
21 in the form of being protruded (having an outer circumferential
region 24), as compared to the catalyst metal particle 22 in the
vicinity. Thereby, when an electrode catalyst layer is formed by
mixing the electrode catalyst with an electrolyte, the amount (surface
area) of the catalyst metal particles that are in contact with the
electrolyte can be reduced. As a result, the chance for reactive gas
(particularly oxygen) to be brought into contact with the catalyst
¨ 23 ¨
CA 03026335 2018-12-03
metal particle surface is increased, the formation of a three-phase
interface between the reactive gas, the catalyst metal particle, and
water is promoted, and thus high catalytic activity can be exhibited.
That is, the method for producing an electrode catalyst according
to an embodiment of the present invention is a method for producing
an electrode catalyst ,which has supporting a catalyst metal
particle(s) on a catalyst support to produce an electrode catalyst
precursor, and mixing the electrode catalyst precursor with a
spacer(s) to produce the electrode catalyst.
[0045]
(Step 1: Production of electrode catalyst precursor)
The method for producing an electrode catalyst precursor
(method for supporting the catalyst metal particle (s) on the catalyst
support) is not particularly limited, and any conventionally known
method can be used. For example, methods such as a liquid phase
reduction method, an evaporation and drying method, a colloid
adsorption method, a spraying and thermal decomposition method, and
reverse micelles (microemulsion method) can be used.
[0046]
Examples of the liquid phase reduction method include a method
of depositing the catalyst metal particle(s) on the surface of the
catalyst support and then subjecting the catalyst metal particle(s)
to a heat treatment. Specific example, for example, a method of
immersing a catalyst support in a solution of a precursor of the
catalyst metal particle to reduce the precursor and then subjecting
the catalyst metal particle(s) to a heat treatment method may be
mentioned.
[0047]
The precursor of the catalyst metal particle is not particularly
limited and is appropriately selected depending on the kind of the
¨ 24 ¨
CA 03026335 2018-12-03
catalyst metal particle to be used. Specific examples thereof may
include a chloride, a nitrate, a sulfate, a chloride, an acetate,
and an amine compound of the catalyst metals such as platinum. More
specific examples thereof may preferably include chlorides such as
platinum chloride (hexachloroplatinic acid hexahydrate), palladium
chloride, rhodium chloride, ruthenium chloride, and cobalt chloride;
nitrates such as palladium nitrate, rhodium nitrate, and iridium
nitrate; sulfates such as palladium sulfate and rhodium sulfate;
acetates such as rhodium acetate; and ammine compounds such as
dinitrodiammine platinum nitrate and dinitrodiammine palladium. A
solvent to be used in the preparation of a precursor solution of
catalyst metal is not particularly limited as long as it can dissolve
a precursor of the catalyst metal, and it can be appropriately selected
depending on the kind of the precursor of the catalyst metal to be
used. Specifically, examples thereof may include water, an acid, an
alkali, and an organic solvent. A concentration of the precursor of
the catalyst metal in the precursor solution of the catalyst metal
is not particularly limited, but it is preferably from 0.1% to 50%
by weight and more preferably from 0.5% to 20% by weight in terms
of metal.
[0048]
Examples of the reductant may include hydrogen, hydrazine,
sodium borohydride, sodium thiosulfate, citric acid, sodium citrate,
L-ascorbic acid, sodium borohydride , formaldehyde, methanol, ethanol,
ethylene, and carbon monoxide. Incidentally, a substance, which is
gaseous at room temperature, such as hydrogen, can also be supplied
by bubbling. An amount of the reductant is not particularly limited
as long as the precursor of the catalyst metal can be reduced to the
catalyst metal, and a known amount can be applied in the same manner.
[0049]
¨ 25 ¨
CA 03026335 2018-12-03
The deposition conditions are not particularly limited as long
as conditions in which a catalyst metal particle(s) can be deposited
on the catalyst support are employed. For example, the deposition
temperature is preferably a temperature near the boiling point of
the solvent (solvent boiling point 10 C, more preferably solvent
boiling point 5 C) , and more preferably from room temperature to 100 C.
Furthermore, the deposition time is preferably 1 to 10 hours, and
more preferably 2 to 8 hours. Meanwhile, the deposition process may
be carried out with stirring and mixing, if necessary. Thereby, the
precursor of the catalyst metal is reduced, and the catalyst metal
particles are produced on the catalyst support.
[0050]
Regarding the heat treatment conditions, for example, the heat
treatment temperature is preferably 300 C to 1,200 C, more preferably
500 C to 1,150 C, even more preferably 700 C to 1,000 C, and
particularly preferably 900 C to 1,000 C. Furthermore, the heat
treatment time is preferably 0.02 to 3 hours, more preferably 0.1
to 2 hours, and even more preferably 0.2 to 1.5 hours. Meanwhile,
from the viewpoint of an effect of accelerating reduction of the
catalyst metal precursor, it is preferable that the heat treatment
process is carried out in an atmosphere containing hydrogen gas, and
more preferably in a hydrogen atmosphere.
[0051]
Alternatively, it is also acceptable to produce an electrode
catalyst precursor by producing the catalyst metal particle(s) in
advance and then supporting the catalyst metal particle(s) on the
catalyst support. In the case of this method, highly active catalyst
metal particles having a special form can be supported on the catalyst
support while maintaining their activity.
[0052]
- 26 -
CA 03026335 2018-12-03
(Step 2: Supporting of spacer(s) on electrode catalyst
precursor)
The method for supporting the spacer(s) on the electrode
catalyst precursor is not particularly limited, and any
conventionally known method can be used. For example, methods such
as an adsorption method, an impregnation method, a liquid phase
reduction supporting mcthod, an evaporation drying method, a spraying
thermal decomposition method, and a sputtering method can be used.
[0053]
Examples of the adsorption method include a method of dispersing
an electrode catalyst precursor and the spacer(s) in a liquid medium,
mixing the dispersion, and filtering and drying the resultant.
[0054]
Here, the means for dispersing the electrode catalyst precursor
and the spacer(s) is not particularly limited, and appropriate
dispersing means such as a homogenizer, an ultrasonic dispersing
apparatus, and a magnetic stirrer may be combined as appropriate.
Furthermore, the electrode catalyst precursor and the spacer(s) may
be dispersed in a liquid medium all at once, or may be dispersed in
two divided parts by, for example, first dispersing the spacer(s)
and then dispersing the electrode catalyst precursor.
[0055]
The liquid medium is not particularly limited as long as the
electrode catalyst precursor and the spacer(s) can be uniformly
dispersed therein, and examples include n-hexanol. These may be used
singly, or two or more kinds thereof may be used in combination.
[0056]
Regarding the mixing conditions, for example, the mixing
temperature is preferably 20 C to 50 C. The mixing time is preferably
0.5 to 24 hours.
¨ 27 ¨
CA 03026335 2018-12-03
[0057]
Regarding the drying conditions, for example, the drying
temperature is preferably 20 C to 80 C, and more preferably 40 C to
60 C. Furthermore, the drying time is preferably 0.5 to 24 hours.
.. [0058]
Meanwhile, in the method described above, an electrode catalyst
can be obtained by dispersing the electrode catalyst precursor and
the spacer (s) in a liquid medium, adsorbing and supporting and the
spacer (s) , and then filtering and drying the resultant.
.. [0059]
<Electrode catalyst layer>
The present invention also provides an electrode catalyst layer
containing the electrode catalyst described above.
[0060]
[Electrolyte]
ectrolyte ]
The electrode catalyst layer according to the present invention
preferably contains an electrolyte, an addition to the electrode
catalyst described above. The electrolyte used in the electrode
catalyst layer is not particularly limited; however, from the
viewpoint of the difficulties for covering the electrode catalyst,
it is preferable that the electrolyte is a polymer (polymer
electrolyte) .
[0061]
The polymer electrolyte is not particularly limited, and
conventionally known knowledge can be appropriately referred to. The
polymer electrolyte is roughly classified into a fluorine-based
polymer electrolyte and a hydrocarbon-based polymer electrolyte
depending on the kind of ion exchange resin as a constituent material.
[0062]
Examples of the ion exchange resin constituting the
- 28 -
CA 03026335 2018-12-03
fluorine-based polymer electrolyte may include perfluerocarbon
sulfonic acid-based polymers such as Nafion (registered trademark,
manufactured by DuPont) , Aciplex (registered trademark, manufactured
by Asahi Kasei Corp.), and FLEMION (registered trademark,
manufactured by Asahi Glass Co., Ltd.), a perfluorocarbonphosphonic
acid-based polymer, a trifluorostyrene sulfonic acid-based polymer,
an ethylene-tetrafluoroethylene-g-styrene sulfonic acid-based
polymer, an ethylene-tetrafluoroethylene copolymer, and a
polyvinylidene fluoride-perfluorocarbon sulfonic acid-based polymer.
From the viewpoint of excellent heat resistance, chemical stability,
durability, and mechanical strength, the fluorine-based polymer
electrolytes are preferably used and a fluorine-based polymer
electrolyte composed of a perfluorocarbon sulfonic acid-based polymer
is particularly preferably used.
[0063]
Specific examples of the hydrocarbon-based polymer electrolyte
may include sulfonated polyethersulfone (S-PES), sulfonated
polyarylether ketone, sulfonated polybenzimidazole alkyl,
phosphonated polybenzimidazole alkyl, sulfonated polystyrene,
sulfonated polyether ether ketone (S-PEEK), and sulfonated
polyphenyiene (S-PPP). The hydrocarbon-based polymer electrolytes
are preferably used from the viewpoint of production that the raw
material is inexpensive, the production process is simple, and the
selectivity for materials is high. Incidentally, only one kind of
the ion exchange resins described above may be used singly or two
or more kinds thereof may be used concurrently. In addition, the
polymer electrolyte is not limited to the materials described above,
and other materials may be used.
[0064]
Furthermore, the electrolyte included in the electrode catalyst
- 29 -
CA 03026335 2018-12-03
layer of the present invention may include a non-polymer compound
to the extent that the operating effects of the present invention
are not impaired. This non-polymer compound includes a low molecular
weight compound having a weight average molecular weight (Mw) of
10,000 or less, for example, a raw material (for example, a monomer)
or an intermediate product (for example, an oligomer) for a polymer
electrolyte such as NAFION (registered trademark); however, the
non-polymer compound is not limited to this.
[0065]
[Method for producing electrode catalyst layer]
The method for producing the electrode catalyst layer is not
particularly limited, and an electrode catalyst layer can be obtained
by, for example, mixing the electrode catalyst, the electrolyte, a
solvent, and other additives as necessary to produce a catalyst ink,
and applying and drying this catalyst ink.
[0066]
The amount of incorporation of the electrolyte in the catalyst
ink is not particularly limited; however, the amount of incorporation
is preferably from 0.1 parts by weight to 2 parts by weight, more
preferably from 0.2 parts by weight to 1 part by weight, and even
more preferably from 0.3 parts by weight to 0.5 parts by weight, with
respect to 1 part by weight of the electrode catalyst.
[0067]
The solvent used for the production of a catalyst ink is not
particularly limited as long as the solvent can uniformly disperse
or dissolve an electrode catalyst and an electrolyte and can be removed
after application. Specific examples include lower alcohols having
1 to 6 carbon atoms, such as n-hexanol, cyclohexanol, methanol,
ethanol, n-propanol (n-propyl alcohol), isopropanol, n-hutanol,
sec-butanol, isobutanol, and tert-butanol; propylene glycol, benzene,
- 30 -
CA 03026335 2018-12-03
toluene, and xylene. In addition to these, butyl alcohol acetate,
dimethyl ether, ethylene glyco1, and the like may be used. These may
be used singly or in the form of a mixed liquid of two or more kinds
thereof.
[0068]
The solid content concentration of the catalyst ink is not
particularly limited; however, the solid content concentration is
preferably 0.1 to 10 mg/mL, more preferably 0.2 to 5 mg/mL, even more
preferably 0 . 3 to 2 mg/mL, and particularly preferably 0 . 5 to 1 mg/mL.
[0069]
In the catalyst ink, if necessary, additives such as a water
repellant, a dispersant, a thickener, and a pore-forming agent may
be incorporated. In the case of using these additives, the amount
of addition for each of the additives is preferably 5% to 20% by weight
with respect to the total amount of the catalyst ink.
[0070]
When a catalyst ink such as described above is applied on an
object base material, an electrode catalyst layer is formed. At this
time, the conditions for forming the electrode catalyst layer are
not particularly limited, and known methods can be similarly used,
or can be used after appropriate modification is applied. For example,
the catalyst ink is applied on an intended substrate such that the
thickness after drying becomes a desired thickness, and drying is
carried out in a vacuum dryer or under reduced pressure. The drying
temperature is not particularly limited; however, the drying
temperature is 25 C to 150 C, more preferably 25 C to 100 C, and even
more preferably 25 C to 50 C. The drying time is not particularly
limited; however, the drying time is 1 to 24 hours, more preferably
5 to 24 hours, and even more preferably 12 to 24 hours.
[0071]
- 31 -
CA 03026335 2018-12-03
The film thickness (dried film thickness) of the electrode
catalyst layer is preferably 0.05 to 30 gm, more preferably 1 to 20
gm, even more preferably 1 to 10 gm, and particularly preferably 1
to 5 gm. The above-described conditions are applicable to both a
cathode catalyst layer and an anode catalyst layer. However, the
cathode catalyst layer and the anode catalyst layer may be identical
or different.
[0072]
The electrode catalyst according to the present invention has
excellent catalytic activity as well as excellent durability.
Therefore, the electrode catalyst according to the present invention
can be more suitably applied to fuel cell usage applications where
superior performance is required, such as power supplies for domestic
use or for driving mobile bodies. That is, a membrane electrode
assembly and a fuel cell, both of which have the electrode catalyst
according to the present invention in a catalyst layer, exhibit
excellent power generation performance. In the following
description, a membrane electrode assembly (MEA) and a fuel cell,
which include a catalyst layer containing the electrode catalyst
according to the present invention, will be explained.
[0073]
<Membrane electrode assembly (MEA)>
The electrode catalyst according to the present invention can
be suitably used in a membrane electrode catalyst (MEA). That is,
the present invention also provides a membrane electrode assembly
(MEA), particularly a membrane electrode assembly (MEA) for a fuel
cell, which includes the electrode catalyst of the present invention.
Such a membrane electrode assembly (MEA) can exhibit high power
generation performance (particularly weight specific activity) and
durability.
¨ 32 -
CA 03026335 2018-12-03
[0074]
For the membrane electrode assembly (MEA) including the
electrode catalyst of the present invention, a similar configuration
can be applied, except that the electrode catalyst (catalyst)
according to the present invention is used instead of a conventional
electrode catalyst. In the following description, preferred
embodiments of the MEA of the present invention will be explained;
however, the present invention is not intended to be limited to the
following embodiments.
[0075]
A MEA is configured to include an electrolyte membrane; and an
anode catalyst layer and an anode gas diffusion layer, and a cathode
catalyst layer and a cathode gas diffusion layer, which are
sequentially formed on both surfaces of the electrolyte membrane.
In regard to this membrane electrode assembly (MEA), the electrode
catalyst according to the present invention is used in at least one
of the cathode catalyst layer and the anode catalyst layer.
[0076]
[Electrolyte membrane]
The electrolyte membrane is constructed from, for example, a
solid polymer electrolyte membrane. This solid polymer electrolyte
membrane has a function of, for example, selectively permeating
protons produced in an anode catalyst layer to a cathode catalyst
layer along the film thickness direction at the time of operating
a fuel cell (PEFC or the like). Furthermore, the solid polymer
electrolyte membrane also has a function as a barrier for preventing
mixing of a fuel gas supplied to the anode side and an oxidizing gas
supplied to the cathode side.
[00771
The electrolyte material that constitutes the solid polymer
¨ 33 ¨
CA 03026335 2018-12-03
electrolyte membrane is not particularly limited, and conventionally
known knowledge can be referred to as appropriate. For example, the
fluorine-based polymer electrolyte or hydrocarbon-based polymer
electrolyte described above can be used. At this time, it is not
necessarily essential to use the same polymer electrolyte as that
used in the catalyst layer.
[0078]
The thickness of the electrolyte membrane may be determined as
appropriate while the characteristics of the fuel cell thus obtainable
are considered, and there are no particular limitations. The
thickness of the electrolyte membrane is usually about 5 to 300 m.
When the thickness of the electrolyte membrane has a value in such
a range, a balance between the strength at the time of membrane
formation, durability at the time of use, and output power
characteristics at the time of use can be appropriately controlled.
[0079]
[Catalyst layer]
The catalyst layer is a layer in which a cell reaction actually
proceeds. Specifically, an oxidation reaction of hydrogen proceeds
in the anode catalyst layer, and a reduction reaction of oxygen
proceeds in the cathode catalyst layer. Here, the electrode catalyst
of the present invention may exist in any of the cathode catalyst
layer or the anode catalyst layer. When the necessity for enhancement
of the oxygen reduction activity is considered, it is preferable that
the electrode catalyst of the present invention is used in at least
the cathode catalyst layer. However, the catalyst layer according
to the embodiment described above may be used as an anode catalyst
layer, or may be used as both a cathode catalyst layer and an anode
catalyst layer, without any particular limitations.
[00801
- 34 -
CA 03026335 2018-12-03
The catalyst layer contains the electrode catalyst according
to the present invention and an electrolyte. The electrolyte is
preferably an ion-conductive polymer electrolyte. Since the polymer
electrolyte accomplishes the role of transferring protons generated
around a catalytically active material on the fuel electrode side,
the polymer electrolyte is also referred to as proton-conductive
polymer. Regarding the polymer electrolyte, those compounds listed
in the section [Electrolyte] described above can be used.
[0081]
In the polymer electrolyte responsible for the transfer of
protons, conductance of protons is important. When EW of the polymer
electrolyte is too large, ion conductivity in the whole catalyst layer
decreases. Accordingly, the catalyst layer of the present embodiment
preferably contains a polymer electrolyte having a small EW.
Specifi ca] ly, the catalyst layer of the present embodiment preferably
contains a polymer electrolyte having an EW of 1500 g/eq. or less,
more preferably contains a polymer electrolyte having an EW of 1200
g/eq. or less, and particularly preferably contains a polymer
electrolyte having an EW of 1000 g/eq. or less. Meanwhile, when the
EW is too small, hydrophilicity becomes too high, and smooth movement
of water becomes difficult. From such a viewpoint, the EW of the
polymer electrolyte is preferably 600 g/eq. or higher. Meanwhile,
the equivalent weight (EW) represents an equivalent weight of an
exchange group having proton conductivity. The equivalent weight is
the dry weight of an ion exchange membrane per equivalent of an ion
exchange group and is represented by the unit "g/eq.".
[0082]
It is preferable that the catalyst layer contains two or more
kinds of polymer electrolytes having different EWs in a power
generation surface and a polymer electrolyte having the lowest EW
- 35 -
CA 03026335 2018-12-03
among the polymer electrolytes is used in a region in which relative
humidity of gas in a flow path is 90% or less. By adopting such a
material disposition, resistance decreases regardless of a current
density region and cell performance can be improved. It is desirable
that EW of a polymer electrolyte to be used in the region in which
the relative humidity of gas in the flew path is 90% or less, namely,
a polymer electrolyte having the lowest EW is 900 g/eq. or less. By
this, the effect described above can be more reliably and remarkably
exerted.
[0083]
Further, it is desirable to use a polymer electrolyte having
the lowest EW in a region in which a temperature is higher than an
average temperature of cooling water at an inlet and an outlet. By
this, resistance decreases regardless of a current density region
and cell performance can be improved.
[0084]
Furthermore, it is desirable to use a polymer electrolyte having
the lowest EW in a region in the range to be within 3/5 from a gas
supply port of at least either of a fuel gas or an oxidant gas with
respect to a flow path length from the viewpoint of decreasing
resistance of a fuel cell system.
[0085]
A thickness (dried film thickness) of the catalyst layer is
preferably from 0.05 to 30 gm, more preferably from 1 to 20 gm, and
still more preferably from 2 to 15 gm. Incidentally, the thickness
above can be applied to both the cathode catalyst layer and the anode
catalyst layer. However, the thicknesses of the cathode catalyst
layer and the anode catalyst layer may be the same as or different
from each other.
[0086]
¨ 36 ¨
CA 03026335 2018-12-03
(Gas Diffusion Layer)
Gas diffusion layers (anode gas diffusion layer and cathode gas
diffusion layer) serves to promote diffusion of gas (fuel gas or
oxidant gas) supplied through gas flow paths of a separator into the
catalyst layers and serves as an electron conduction path.
[0087]
A material constituting the substrate of the gas diffusion
layers is not particularly limited, and conventionally known
knowledge can be appropriately referred to. Examples thereof may
include a sheet-like material exhibiting conductivity and porosity
such as a carbon fabric, a paper-like papermaking body, a felt, or
a nonwoven fabric. A thickness of the substrate may be appropriately
determined in consideration of characteristics of the gas diffusion
layer to be obtained, but it may be about from 30 to 500 gm. When
the thickness of the substrate is within such a range, balance between
mechanical strength and diffusibility of gas, water and the like can
be properly controlled.
[0088]
The gas diffusion layer preferably contains a water repellent
for the purpose of further enhancing water repellency and preventing
a flooding phenomenon and the like. The water repellent is not
particularly limited, but examples thereof may include fluorine-based
polymer materials such as polytetrafluoroethylene (PTFE),
polyvinylidene fluoride (PVdF), polyhexafluoropropylene, and a
tetrafluoroethylene-hexafluoropropylene copolymer (FEP),
polypropylene, and polyethylene.
[0089]
In addition, the gas diffusion layer may have a carbon particle
layer (microporous layer; MPL, not illustrated) composed of carbon
particles containing a water repellent on the catalyst layer side
- 37 --
CA 03026335 2018-12-03
of the substrate in order to further improve water repellency.
[0090]
The carbon particles to be contained in the carbon particle
layer are not particularly limited, and conventionally known
materials such as carbon black, graphite, and expanded graphite can
be appropriately adopted. Among these, carbon black such as oil
furnace black, channel black, lampblack, thermal black, or acetylene
black can be preferably used since it exhibits excellent electron
conductivity and has a large specific surface area. An average
particle size of the carbon particles is preferably set to about from
10 to 100nm. This makes it possible to improve contact property with
the catalyst layer as well as to obtain high drainage property by
capillary force.
[0091]
Examples of the water repellent to be used in the carbon particle
layer may include the same water repellents as those described above.
Among these, a fluorine-based polymer material can be preferably used
since it exhibits excellent water repellency, corrosion resistance
at the time of electrode reaction, and the like.
[0092]
A mixing ratio of the carbon particles to the water repellent
in the carbon particle layer is preferably set to be about from 90 :
10 to 40 : 60 (carbon particles : water repellent) in terms of weight
ratio in consideration of better balance between water repellency
and electron conductivity. A thickness of the carbon particle layer
is also not particularly limited and may be appropriately determined
in consideration of water repellency of the gas diffusion layer to
be obtained.
[0093]
[Method for Producing Membrane Electrode Assembly]
¨ 38 ¨
CA 03026335 2018-12-03
A method for fabricating the membrane electrode assembly is not
particularly limited, and a conventionally known method can be used.
It is possible to use, for example, a method of transferring or
applying a catalyst layer on an electrolyte membrane by hot pressing,
drying this, and bonding a gas diffusion layer to the resultant; or
a method of applying a catalyst layer in advance on the microporous
layer side of a gas diffusion layer (in the case where the gas diffusion
layer does not include a microporous layer, one surface of the base
material layer), drying the catalyst layer, thereby producing two
sheets of a gas diffusion electrode (GDE), and bonding these gas
diffusion electrodes on both surfaces of a solid polymer electrolyte
membrane by hot pressing, can be used. The conditions for application
and bonding such as hot pressing may be adjusted as appropriate
depending on the type of the polymer electrolyte (perfluorosulfonic
acid-based or hydrocarbon-based polymer electrolyte) in the solid
polymer electrolyte membrane or the catalyst layer.
[0094]
<Fuel cell>
The membrane electrode assembly (MEA) described above can be
suitably used in a fuel cell. That is, the present invention also
provides a fuel cell that is formed using a membrane electrode assembly
(MEA) including the electrode catalyst according to the present
invention. Such a fuel cell can exhibit superior power generation
performance (particularly, weight specific activity) and durability.
[0095]
Here, the fuel cell has a membrane electrode assembly (MEA);
and a pair of separators composed of an anode-side separator having
fuel gas flow channels through which a fuel gas flows, and a
cathode-side separator having oxidizing gas flow channels through
which an oxidizing gas flows. The fuel cell of the present invention
- 39 -
CA 03026335 2018-12-03
has excellent durability and can exhibit superior power generation
performance.
[0096]
In the following description, an embodiment of a membrane
electrode assembly (MEA) and a fuel cell, both of which have a catalyst
layer using the electrode catalyst according to the present invention,
will be explained in detail with reference to the drawings as
appropriate. However, the present invention is not limited only to
the following embodiment. The respective drawings are described in
an exaggerated manner for convenience, and the dimensional ratios
of the various constituent elements in the respective drawings may
be different from the actual dimensional ratios. Furthermore, when
an embodiment of the present invention is explained with reference
to the drawings, an identical reference numeral is assigned to
identical elements in the explanation for the drawings, and any
overlapping description will not be repeated.
[0097]
Fig. 5 is a schematic diagram illustrating the basic
configuration of a solid polymer type fuel cell (PEFC) 1 according
to an embodiment of the present invention. PEFC 1 first has a solid
polymer electrolyte membrane 2; and a pair of catalyst layers (anode
catalyst layer 3a and cathode catalyst layer 3c) having the
electrolyte membrane interposed thcrebetween. Then, the laminate of
the solid polymer electrolyte membrane 2 and the catalyst layers (3a
and 3c) are interposed between a pair of gas diffusion layers (GDL)
(anode gas diffusion layer 4a and cathode gas diffusion layer 4c).
As such, the solid polymer electrolyte membrane 2, a pair of catalyst
layers (3a and 3c), and a pair of gas diffusion layers (4a and 4c)
constitute, in a laminated state, a membrane e]ectrode assembly (MEA)
10.
¨ 40 ¨
CA 03026335 2018-12-03
[0098]
In the PEFC 1, MEA 10 is further interposed between a pair of
separators (anode separator 5a and cathode separator 5c). In Fig.
5, the separators (5a and Sc) are depicted such that the separators
are positioned at both ends of the MEA 10 depicted therein. However,
in a fuel cell stack formed by a plurality of MEA' s laminated together,
it is general that a separator is also used as a separator for an
adjacent PEFC (not illustrated in the diagram). In other words, in
a fuel cell stack, MEA's constitute a stack by being sequentially
laminated, with separators being interposed therebetween. In an
actual fuel cell stack, gas seals are disposed between the separators
(5a and Sc) and the solid polymer electrolyte membrane 2, or between
a PEFC 1 and another PEFC adjacent thereto; however, in Fig. 5, such
a description will not be illustrated.
[0099]
The separators (5a and 5c) are obtained by, for example, molding
a thin plate having a thickness of 0.5 mm or less into a concavo-convex
shape as illustrated in Fig. 5, by subjecting the thin plate to a
pressing treatment. Convexities as viewed from the MEA side of the
separator (5a or Sc) are in contact with the MEA 10. Thereby,
electrical connection between the convexities and the MEA 10 is
secured. Furthermore, concavities (spaces between the separator and
the MEA produced due to the concavo-convex shape of the separator)
as viewed from the MEA side of the separator (5a or Sc) function as
gas flow channels for circulating a gas at the time of operating the
PEFC 1. Specifically, a fuel gas (for example, hydrogen) is
circulated in a gas flow channel 6a of the anode separator 5a, and
an oxidizing gas (for example, air) is circulated in the gas flow
channel 6c of the cathode separator Sc.
[01001
¨ 41 ¨
CA 03026335 2018-12-03
Meanwhile, the concavities as viewed from the opposite side of
the MEA side of the separator (5a or 5c) are regarded as coolant flow
channels 7 for circulating a coolant (for example, water) for cooling
the PEFC at the time of operating the PEFC 1. Furthermore, a separator
is usually provided with a manifold (not illustrated in the diagram) .
This manifold functions as a connection means for connecting various
cells when a stack is constructed. By adopting such a configuration,
the mechanical strength of the fuel cell stack can be secured.
[0101]
In the embodiment illustrated in Fig. 5, the separators (5a and
5c) are formed into a concavo-convex shape. However, the separators
are not limited only to such a concavo-convex shape, and the separators
may also have any arbitrary shape such as a flat plate shape or a
partially doncavo-convex shape, as long as the separators can exhibit
the functions as gas flow channels and coolant flow channels.
[0102]
[Separator]
The separator serves to electrically connect respective cells
in series when a plurality of single cells of a fuel cell such as
a polymer electrolyte fuel cell are connected in series to constitute
a fuel cell stack. In addition, the separator also has a function
as a partition wall, which separates a fuel gas, an oxidant gas, and
a refrigerant from each other. In order to secure flow paths therefor,
it is preferable that each of the separators is provided with gas
flow paths and a cooling flow path as described above. As a material
constituting the separator, conventionally known materials such as
carbon such as dense carbon graphite and a carbon plate, and a metal
such as stainless steel can be appropriately adopted without being
limited. A thickness and size of the separator, a shape and size of
each flow path to be provided, and the like are not particularly
- 42 -
CA 03026335 2018-12-03
limited and can be appropriately determined in consideration of
desired output characteristics and the like of a fuel cell to be
obtained.
[0103]
A method for producing a fuel cell is not particularly limited,
and knowledge conventionally known in the field of fuel cells can
be appropriately referred to.
[0104]
Further, a fuel cell stack having a structure in which a
plurality of membrane electrode assemblies (MEAs) are stacked via
a separator and connected in series may be formed so that the fuel
cell can exert a desired voltage. A shape and the like of the fuel
cell are not particularly limited and may be appropriately determined
so as to obtain cell characteristics such as a desired voltage.
[0105]
The PEFC and membrane electrode assembly (MEA) described above
use a catalyst layer exhibiting excellent power generation
performance and durability. Consequently, the PEFC and the membrane
electrode assembly (MEA) exhibit excellent power generation
performance and durability.
[0106]
The PEFC of the present embodiment and a fuel cell stack using
the same can be mounted on, for example, a vehicle as a power source
= for driving.
[0107]
A fuel cell such as described above exhibits excellent power
generation performance. Here, the type of the fuel cell is not
particularly limited, and in the explanation given above, a solid
polymer type fuel cell has been explained as an example. However,
in addition to these, examples of the fuel cell include an alkali
¨ 43 ¨
CA 03026335 2018-12-03
type fuel cell, a direct methanol type fuel cell, and a micro fuel
cell. Among them, a preferred example may be a solid polymer type
fuel cell (PEFC) that is small-sized and is capable of having high
density and high power output. Furthermore, the fuel cell is useful
as a stationary power supply or the like, in addition to a power supply
for a mobile body such as a vehicle having a limited mounting space.
Above all, it is particularly preferable that the fuel cell is used
as a power supply for a mobile body such as a vehicle where a high
output voltage is required after stoppage of driving for a relatively
long time.
[0108]
The fuel used when the fuel cell is driven is not particularly
limited. For example, hydrogen, methanol, ethanol, 1-propanol,
2-propanol, 1-butanol, secondary butanol, tertiarybutanol, dimethyl
ether, diethyl ether, ethylene glycol, and diethylene glycol can be
used. Among them, hydrogen or methanol is preferably used from the
viewpoint of being capable of obtaining high output power.
[0109]
Furthermore, the application usage of the fuel cell is not
particularly limited; however, it is preferable that the fuel cell
is applied to vehicles. A membrane electrode assembly including the
electrode catalyst of the present invention has excellent power
generation performance and durability, and size reduction can be
realized. Therefore, the fuel cell of the present invention is
particularly advantageous when the fuel cell is applied to a vehicle,
from the viewpoint of onboard mountability.
Examples
[0110]
The effects of the present invention will be described using
the following Examples and Comparative Examples. However, the
¨ 44 ¨
CA 03026335 2018-12-03
technical scope of the present invention is not intended to be limited
to the following Examples only. In the following Examples, unless
particularly stated otherwise, the operation was carried out at room
temperature (25 C). Furthermore, unless particularly stated
otherwise, the units "percent (%)" and "parts" mean "percent () by
weight" and "parts by weight", respectively.
[0111]
<Production of electrode catalyst>
(Synthesis Example 1)
46 g of acetylene black (OSAB, BET specific surface area: 800
m2/g, average secondary particle size: 300 to 400 nm) as a catalyst
support (manufactured by Denka Co., Ltd.), 1,000 g of a
dinitrodiammine(II)platinum nitrate solution having a platinum
concentration of 4.6% by weight (platinum content 46 g), and 100 mL
of ethanol as a reducing agent were added, and the mixture was mixed
for 7 hours at 80 C. Subsequently, platinum was chemically reduced.
This was filtered and dried for 12 hours at room temperature (25 C)
Subsequently, the resultant was subjected to a heat treatment for
1 hour at 900 C in a hydrogen atmosphere, and thus an electrode catalyst
precursor was obtained. In regard to this electrode catalyst
precursor, the physical property values of platinum were as follows:
average crystallite diameter: 3.4 nm, supported ratio (with respect
to the weight of the electrode catalyst precursor): 25.6% by weight,
and specific surface area: 83 m2/g.
[0112]
(Synthesis Example 2)
In regard to the electrode catalyst precursor obtained in
Synthesis Example 1, SiO2 particles as a spacer were supported, and
thus an electrode catalyst 1 was produced. Specifically, 3.8 mg of
Si02 particles (SNOWTEX (registered trademark) OXS manufactured by
¨ 45 -
CA 03026335 2018-12-03
Nissan Chemical Industries, Ltd.) having an average primary particle
size of 5 nm were dispersed in 25 mL of n-hexanol. 75 mg of the
electrode catalyst precursor obtained in Synthesis Example 1 was added
thereto, and the mixture was stirred for 4 hours at 25 C. This was
filtered and then dried for 4 hours at 60 C, and thus an electrode
catalyst 1 was obtained. In the electrode catalyst 1, the Si02
supported ratio was 5.4% by weight, and the ratio of the average
diameter of the Si02 particles (cl,p) with respect to the average
diameter of the platinum particles (dcat) was 1.5. Furthermore, the
results obtained by observing the surface of the electrode catalyst
1 by scanning electron microscopy (SEM) are shown in Fig. 6. In Fig.
6, reference numeral 31 represents a catalyst support; reference
numeral 32 represents a SiO2 particle; reference numeral 33 represents
a platinum particle that are directly supported on the catalyst
support; and reference numeral 34 represents a platinum particle
supported on the catalyst carrier, with SiO2 particles being
Interposed therebetween. As the result of SEM observation, the
number of platinum particles supported on the catalyst support, with
Si02 particles being interposed therebetween, was 2 with respect to
any arbitrary 100 platinum particles supported on the catalyst support.
That is, the number of platinum particles supported on the catalyst
support, with Si02 particles being interposed therebetween, was 2%
with respect to the total number of platinum particles supported on
the catalyst support.
[0113]
(Synthesis Example 3)
An electrode catalyst 2 was obtained in the same manner as in
Synthesis Example 2, except that the Si02 particles having an average
primary particle size of 5 nm used in Synthesis Example 2 were changed
to SiO2 particles having an average primary particle size of 12.5 nm
- 46 -
(manufactured by Sigma-Aldrich Company, Product No. 637238).
In the electrode catalyst 2, the SiO2 supported ratio was
11.3% by weight, and the ratio of the average diameter of the
SiO2 particles (dsp) with respect to the average diameter of
the platinum particles (dcat) was 3.7. Furthermore, according
to a SEM observation, the number of platinum particles
supported on the catalyst support, with SiO2 particles being
interposed therebetween, was 5% or less with respect to the
total number of platinum particles supported on the catalyst
support.
[0114]
(Synthesis Example 4)
In regard to the electrode catalyst precursor obtained in
Synthesis Example 1, A1203 particles were supported as a
spacer, and thus an electrode catalyst was produced.
Specifically, 1 mg of A1203 particles (manufactured by Sigma-
Aldrich Company, Product No. 718475) having an average primary
particle size of 13 nm were dispersed in 25 mL of n-hexanol,
15 mg of the electrode catalyst precursor obtained in
Synthesis Example 1 was added thereto, and the mixture was
stirred for 4 hours at 25 C. This
mixture was filtered and
dried, and thus an electrode catalyst 3 was obtained. In
regard to the electrode catalyst 3, the A1203 supported ratio
was 1.8% by weight, and the ratio of the average diameter of
the A1203 particles (dsp) with respect to the average diameter
of the platinum particles (dcat) was 3.8.
Furthermore,
according to a SEM observation, the number of platinum
particles supported on the catalyst support, with A1203
particles being interposed therebetween, was 5% or less with
respect to the total number of platinum particles supported on
the catalyst support.
[0115]
(Synthesis Example 5)
In regard to the electrode catalyst precursor obtained in
- 47 -
CA 3026335 2019-12-18
Synthesis Example 1, TiO2 particles were supported as a spacer,
and thus, an electrode catalyst was produced.
Specifically, 1
mg of TiO2 particles (manufactured by Sigma-Aldrich Company,
Product No. 718467) having an average primary particle size of
15 nm were dispersed in 25 mL of n-hexanol, 15 mg of the
electrode catalyst precursor obtained in Synthesis Example 1 was
added thereto, and the mixture was stirred for 4 hours at 25 C.
This mixture was filtered and dried, and thus an electrode
catalyst 4 was obtained. In regard to the electrode catalyst 4,
the TiO2 supported ratio was 7.9% by weight, and the ratio of the
average diameter of the TiO2 particles (dsp) with respect to the
average diameter of the platinum particles (dcat) was 4.4.
Furthermore, according to a SEM observation, the number of
platinum particles supported on the catalyst support, with TiO2
particles being interposed therebetween, was 5% or less with
respect to the total number of platinum particles supported on
the catalyst support.
[0116]
(Synthesis Example 6)
In regard to the electrode catalyst precursor obtained in
Synthesis Example 1, Al2O3 nanofibers were supported as a
spacer, and thus an electrode catalyst was produced.
Specifically, 1 mg of A1203 nanofibers having a diameter of 20 nm
and a length of 10 gm (manufactured by Sigma-Aldrich Company,
Product No.: 790915) were dispersed in 25 mL of n-hexanol, 15 mg
of the electrode catalyst precursor obtained in Synthesis
Example 1 was added thereto, and the mixture was stirred for 4
hours at 25 C. This mixture was filtered and dried, and thus an
electrode catalyst 5 was obtained. In regard to the electrode
catalyst 5, the Al2O3 supported ratio was 6.2% by weight, and the
ratio of the average diameter of the A1203 nanofibers (dsp) with
respect to the average diameter of the platinum particles (dcat)
was 5.9. Furthermore, according to a SEM observation, the
- 48 -
CA 3026335 2019-12-18
number of platinum particles supported on the catalyst
support, with A1203 nanofibers being interposed therebetween,
was 5% or less with respect to the total number of platinum
particles supported on the catalyst support.
[0117]
(Synthesis Example 7)
Acetylene black (OSAB, BET specific surface area: 800
m2/g, average secondary particle size: 300 to 400 nm)
(manufactured by Denka Co., Ltd.) as a catalyst support, and
SiO2 particles (manufactured by Sigma-Aldrich Company, Product
No. 637238) having an average primary particle size of 12.5 nm
were dispersed in hexanol, and the mixture was stirred for 4
hours at 25 C. This mixture was filtered and then dried for 4
hours at 60 C. The
powder (amount corresponding to 54 g of
OSAB) obtained here, 1,000 g of a dinitrodiammine(II)platinum
nitrate solution having a platinum concentration of 4.6% by
weight (platinum content: 46 g), and 100 mL of ethanol as a
reducing agent were added, and the mixture was mixed for 7
hours at 80 C. Subsequently, platinum was chemically reduced.
This was filtered and dried for 12 hours at room temperature
(25 C), and then the resultant was subjected to a heat treatment
for one hour at 900 C in a hydrogen atmosphere. Thus,
an
electrode catalyst 6 was obtained. In regard to the electrode
catalyst 6, the physical property values of platinum were as
follows: average crystallite diameter: 3.5 nm, supported ratio
(with respect to the weight of the electrode catalyst 6): 17.6%
by weight, specific surface area: 105 m.2/g, and the spacer
supported ratio with respect to the weight of the electrode
catalyst 6 was 5.8% by weight. Furthermore, according to a SEM
observation, the number of platinum particles supported on the
catalyst support, with SiO2 particles being interposed
therebeween, was more than 30% with respect to the total
- 49 -
CA 3026335 2019-12-18
CA 03026335 2018-12-03
number of platinum particles supported on the catalyst support.
[0118]
<Production of rotating disc electrode (RDE) apparatus>
(Comparative Example 1)
13.25 mg of the electrode catalyst precursor produced in
Synthesis Example 1, 0.1 ml of a 5 wt% electrolyte dispersion liquid
(NAFION (registered trademark) D520 manufactured by DuPont Company)
(specific gravity 1.0) , and 25 mL of n-hexanol were sufficiently mixed,
and a catalyst ink was produced. An aliquot of the catalyst ink
equivalent to 20 g of the amount of catalyst support was collected
using a micropipette . The catalyst ink was dropped on a rotating disc
electrode (RDE) apparatus (manufactured by HOKUTO DENKO CORPORATION)
made of glassy carbon and having a diameter of 6 mm, and then the
catalyst ink was dried for 21 hours at 25 C. Thus, an RDE apparatus
coated with an electrode catalyst layer having a film thickness of
1 m was produced.
[0119]
(Comparative Example 2)
An RDE apparatus was produced in the same manner as in
Comparative Example 1, except that the electrode catalyst precursor
used in Comparative Example 1 was changed to 13.97 mg (amount of
electrode catalyst precursor: 13.25 mg) of the electrode catalyst
1 produced in Synthesis Example 2.
[0120]
(Comparative Example 3)
An RDE apparatus was produced in the same manner as in
Comparative Example 1, except that the electrode catalyst precursor
used in Comparative Example 1 was changed to 14.07 mg (amount of
electrode catalyst precursor: 13.25 ma) of the electrode catalyst
6 produced in Synthesis Example 7.
¨ 50 ¨
CA 03026335 2018-12-03
[0121]
(Example 1)
An RDE apparatus was produced in the same manner as in
Comparative Example 1, except that the electrode catalyst precursor
used in Comparative Example 1 was changed to 14.75 mg (amount of
electrode catalyst precursor: 13.25 mg) of the electrode catalyst
2 produced in Synthesis Example 3.
[0122]
(Example 2)
An RDE apparatus was produced in the same manner as in
Comparative Example 1, except that the electrode catalyst precursor
used in Comparative Example 1 was changed to 13.49 mg (amount of
electrode catalyst precursor: 13.25 mg) of the electrode catalyst
3 produced in Synthesis Example 4.
[0123]
(Example 3)
An RDE apparatus was produced in the same manner as in
Comparative Example 1, except that the electrode catalyst precursor
used in Comparative Example 1 was changed to 14.30 mg (amount of
electrode catalyst precursor: 13.25 mg) of the electrode catalyst
4 produced in Synthesis Example 5.
[0124]
(Example 4)
An RDE apparatus was produced in the same manner as in
Comparative Example 1, except that the electrode catalyst precursor
used in Comparative Example 1 was changed to 14.07 mg (amount of
electrode catalyst precursor: 13.25 mg) of the electrode catalyst
5 produced in Synthesis Example 6.
[0125]
<Evaluation of performance of rotating disc electrode>
¨ 51 --
CA 03026335 2018-12-03
Using the rotating disc electrode (RDE) apparatuses produced
in Examples 1 to 4 and Comparative Examples 1 to 3, the oxygen reduction
reaction (ORR) activity and the effective electrochemical surface
area were measured by the following method, and thus the ORR specific
activity was calculated. For the evaluation conditions and the
evaluation protocol, reference was made to reference literature
(Proposal of Goals, Research and Development Issues, and Evaluation
Methods for Solid Polymer Type Fuel Cells, p. 17-22, Fuel Cell
Commercialization Conference of Japan (FCCJ), January 2011).
[0126]
Specifically, first, an RDE apparatus was sufficiently cleaned
by ultrasonic cleaning and boiling water washing, and a liquid
electrolyte (perchloric acid, HC104) adjusted to a concentration of
0.1 M was poured onto the RDE apparatus. Next, while the RDE apparatus
was controlled to be at 25 C, nitrogen bubbling was performed in the
RDE apparatus. In a state in which the speed of rotation of the
electrode was set to zero, cycljc voltammetry was performed in order
to perform cleaning of the electrode surface and measurement of the
surface area. The potential range was set to 0.05 to 1.20V vs. RHE,
and the scan rate was 50 mV/s. After a reproducible voltammogram was
obtained, the effective electrochemical surface area (m2/g Pt) of
platinum was calculated from the electric amount of hydrogen
adsorption and the platinum weight of the electrode according to the
reference literature described above. Subsequently, oxygen (purity
99.99995% or higher) was bubbled for about 30 minutes. After
completion of bubbling, convection voltammetry was performed under
the conditions of a scan rate of 10 mV/s in the direction of potential
scan of 0.05 V to 1.2 V, and the current value (A) at a potential
of 0.9 V was measured. The ORR activity (A/g_Pt) of the RDE apparatus
was calculated using this value, and the ORR specific activity
- 52 -
( A/cm2_Pt) was calculated by dividing the ORR activity by the
effective electrochemical surface area.
[0127]
The evaluation results for the RDE apparatuses according to
Examples 1 to 4 and Comparative Examples 1 to 3 are presented in Table
1.
[0128]
[Table 1]
Effective
ORR specific
d ORR activity electrochemical activity
sp/d cat
(A/g_Pt) surface area
( A/cm2 Pt)
(m2/g_Pt)
Comparative
127 51 250
Example 1
Comparative
1.5 130 66 196
Example 2
Comparative
3.7 120 66 182
Example 3
Example 1 3.7 160 44 364
Example 2 3.8 147 50 296
Example 3 4.4 191 62 309
Example 4 5.9 133 45 298
[0129]
From the results of Table 1, it was found that an RDE having
the electrode catalyst according to the present invention in the
electrode catalyst layer exhibits excellent catalytic activity (ORR
specific activity). From these results, it is speculated that the
electrode catalyst of the present invention has enhanced arrival
efficiency of reactive gas (02) to the catalyst metal particle surface
due to the effect of the spacer, and can exhibit high catalytic
activity.
[0130]
- 53 -
CA 3026335 2019-01-28
Reference Signs List
[0131]
1 Solid polymer type fuel cell (PEFC)
2 Solid polymer electrolyte membrane
3 Catalyst layer
3a Anode catalyst layer
3c Cathode catalyst layer
4a Anode gas diffusion layer
4c Cathode gas diffusion layer
5a Anode separator
5c Cathode separator
6a Anode gas flow channel
6c Cathode gas flow channel
7 Coolant flow channel
10 Membrane electrode assembly (MEA)
Electrode catalyst
21 Catalyst support
20 22, 22' Catalyst metal particle
23 Spacer
24 Outer circumferential region
Center of catalyst support
26 Circle circumscribing catalyst metal particle
25 27 Circle circumscribing spacer
28 Electrolyte
29 Void
31 Catalyst support
32 SiO2 particle
33 Platinum particle directly supported on catalyst support
¨ 54 ¨
CA 3026335 2019-01-28
CA 03026335 2018-12-03
34 Platinum
particle supported on catalyst support, with SiO2
particle being interposed therebetween
¨ 55 ¨