Note: Descriptions are shown in the official language in which they were submitted.
CA 03026708 2018-12-05
WO 2017/204787 1 PCT/US2016/033950
TITLE
ACCURATE DOSE CONTROL MECHANISMS
AND DRUG DELIVERY SYRINGES
FIELD
THIS INVENTION relates to accurate dose drug delivery syringes. More
particularly, this invention relates to accurate dose control mechanisms, drug
delivery
syringes which incorporate such control mechanisms, the methods of operating
such
devices, and the methods of assembling such devices.
BACKGROUND
Various studies have shown that the accuracy of dose delivery is affected by a
number of factors, including: injection methodologies employed by medical
practitioners, an inability to accurately read and control plunger travel
during dosing,
and the loss of dosage associated with the prime step used to evacuate air
from the
syringe prior to the dosing step. These effects are particularly magnified by
the use of
drug delivery syringes that have a high dose volume to axial translation ratio
(i.e., a
significant quantity of drug is dispensed for even incrementally small
distances of
plunger depression, as may be the case for large diameter syringes); this
problem is
more acute when delivering microliter size doses. While these causes for error
are
common, the need for accurate dose syringes remains. Such syringes are of
particular
importance in sensitive operations, such as in intravitreal injections, and
are very
desirable for low dose treatments where inaccurate dosing can lead to
substantial error
and potential patient harm.
Studies have shown that the amount of treatment delivered may vary
significantly depending on whether the medical practitioner chooses to deliver
5 [IL (5
microliters) of the treatment by depressing the syringe plunger from 10 [IL to
5 [IL or
by depressing the syringe from 5 [IL to 0 [tL. Additionally, due to the
uncertainty of
plunger travel limits some practitioners may depress the syringe past the
natural travel
limit and deliver excess treatment to the patient because of mechanical
compliance
between the stopper and the syringe barrel. For example, given a particular
syringe
barrel diameter, a practitioner may depress the plunger past the natural stop
for 0 [IL
and erroneously deliver up to 20% more dosage than necessary. This error is
magnified
because of the small dose volume requirements for particular treatments.
Because the
dosage amount and associated plunger travel distance are small, it is very
difficult for a
CA 03026708 2018-12-05
WO 2017/204787 2 PCT/US2016/033950
practitioner to gauge the fill amount of the dosing chamber and to control the
injection
amount as the treatment is applied to the patient. This inaccuracy in dosing
can lead to
substantial safety risks including, among other side effects, increased
pressure in the
target region and altered (reduced) drug efficacy.
A primary cause of the dosing inaccuracy is the inability to reliably set the
limits
of plunger travel, and the inherent variability in the degree to which the
plunger seal (or
stopper) is depressed at end of delivery during dosing. Also contributing to
inaccuracy
is the potential variability, during syringe manufacturing, in the placement
of reference
markings on the syringe barrel. Endemic to these causes of inaccuracy is the
high
sensitivity of volume dispensed to the axial travel of the plunger, as
described above.
Mechanical travel limits, however, are difficult to employ in such
applications because
of the challenges associated with reading and controlling the plunger travel
by the user
over the small distance of dosing. Simply put, because the dosage amounts are
so small,
it is difficult for a practitioner to identify the dosage measurements on the
syringe barrel
and accurately control the plunger depression and dosage amount during
injection.
In addition to improving dosing accuracy, it is useful to incorporate the
functionality of a priming step into a syringe design to reduce or eliminate
air bubbles
within the dosing chamber. This step is very useful to minimize safety risks,
improve
operational hygiene, and reduce pressure in the target site. Minimizing the
likelihood of
air bubbles during filling helps streamline the drug delivery process for the
clinician.
Employing pre-filled syringes may assist in the minimization of air bubbles.
However,
even pre-filled syringes are not fully devoid of air captured during the
filling process.
Accordingly, there is a substantial need for syringes which allow the user to
readily identify and control the dosage amount, minimize the presence of air
bubbles
within the dosage chamber prior to drug delivery, and ensure accurate delivery
of the
required drug dose. It is preferred that such a syringe would enable pre-
filling to take
advantage of benefits associated with the use of such products.
Further, some medications require mixing two fluids or reconstitution of dry
or
lyophilized drug prior to an accurate dose injection. This allows, for
example, a diluent
to be added to a dehydrated, lyophilized, desiccated or powdered active
substance
immediately prior to injection, which is particularly useful for substances
that are
subject to degradation or loss of activity when stored in a hydrated form.
This also
allows for mixing of two liquids, which are mixed just before an injection.
CA 03026708 2018-12-05
WO 2017/204787 3 PCT/US2016/033950
While it is known to provide syringes that comprise a mixing device for mixing
deliverable substances prior to injection, the market has been unable to
provide such
mixing syringes that are capable of providing accurate dosage delivery
required for
some medications and as discussed above. Examples of such mixing syringes are
disclosed, for example, in U.S. Patent Application No. 13/566,079, which is
assigned to
the assignee of this disclosure and incorporated by reference. In addition to
the
complexities of the structures themselves, the designs may require complex
assemblies,
multiple operation steps by the user, or other particular nuances that make
them difficult
to manufacture, assemble, or operate. Further, some mixing syringes must also
address
factors such as maintenance of container sterility, interaction of components
for sealing,
venting requirements, and distribution of internal forces, among others. Each
of these
challenges is further complicated when extreme dose accuracy is required.
SUMMARY
The present invention provides dose control mechanisms, which allow for the
accurate dosing and delivery of drug treatments, and drug delivery syringes
which
incorporate such control mechanisms. Such novel devices permit the
identification and
control of the dosage amount, permit the syringe to be "primed" (i.e.,
evacuated of air
bubbles) prior to drug delivery, and ensure the accurate delivery of
microliter volume
dosages, all within a device size that is similar to commonly used
conventional syringes
available in the marketplace. Such novel devices are safe and easy to use, and
are
aesthetically and ergonomically appealing for clinical practitioners without
significantly
altering technique currently employed by clinicians to administer injectable
medications. The novel devices of the present invention provide these
desirable features
without any of the problems associated with known prior art devices.
In a first embodiment, the present invention provides a dose control mechanism
for a syringe. The control mechanism includes a plunger having a coarse pitch
screw on
its exterior surface, a housing having a corresponding coarse pitch guide
along the
interior surface of the housing, a screw having a fine pitch screw which
interfaces with
a fine pitch nut of an adapter, wherein the plunger has an internal annular
space within
which screw at least partially resides. The plunger having the coarse pitch is
rotatable
upon the corresponding coarse pitch guide, and wherein at least a portion of
the plunger
is rotationally keyed to interface with a corresponding rotationally keyed
portion of the
screw. A pitch ratio between the coarse pitch screw and the fine pitch screw
is from
approximately 1 : 1 to approximately 20 : 1, more specifically from
approximately 2 : 1
CA 03026708 2018-12-05
WO 2017/204787 4 PCT/US2016/033950
to approximately 10 : 1, and more preferably from approximately 4: 1 to
approximately
8 : 1. In a preferred embodiment, the pitch ratio of the coarse pitch screw
14B and the
fine pitch screw 30B is approximately 4 : 1. The screw may further include a
screw
connection aspect and, optionally, a ring which function to connect the screw
to the
plunger seal directly or to a plunger rod. In at least one embodiment, the
housing has a
housing cover at its proximal end and a window to permit the user to view the
location
of the plunger within the housing. The plunger may have one or more dose
markings on
the external surface of the plunger and the housing may have one or more guide
markings with which to align plunger dose markings. Upon use by the user, the
plunger
axially translates a first distance D1 causing the screw to axially translate
a second
distance D2, wherein D1 is always greater than D2 by a factor determined by
the pitch
ratio.
In a second embodiment, the present invention provides an accurate dose drug
delivery syringe having a dose control mechanism, a barrel, a plunger seal,
and a barrel
adapter assembly having a barrel tip and a needle. The syringe may further
include a
plunger rod connected at one end to the screw and at another end to the
plunger seal.
The syringe may be a fill-at-time-of-use syringe, a pre-filled syringe, or a
safety
syringe, or a combination thereof. The housing of the syringe may have a
housing cover
at its proximal end to protect the interior of the housing from the
environment and a
window to permit the user to view the location of the plunger within the
housing. The
plunger may have one or more dose markings on the external surface of the
plunger,
and the housing may have one or more guide markings at the window with which
to
align plunger dose markings. Upon use by the user, the plunger axially
translates a first
distance D3 causing the screw to axially translate a second distance D4.
The dose control mechanism may also be designed to provide a desired axial
movement of the screw relative to the axial movement of the plunger. In other
words,
the dose control mechanism may be tailored to provide a desired feel to the
user by way
of the plunger, while providing a desired axial movement of the screw and
associated
administration of medication. In an embodiment, the plunger and housing are
coupled
together by way of a variable pitch screw. The pitch may be varied over the
length of
the travel of the plunger relative to the housing, or the travel may include
two or more
distinct or transitional pitches. In any case, however, the variable pitch
will provide at
least two pitches.
CA 03026708 2018-12-05
WO 2017/204787 5 PCT/US2016/033950
In an embodiment, for example, the housing includes at least one variable
pitch
thread along its inner diameter, and the plunger includes at least one thread
segment
disposed to engage the variable pitch thread of the housing. According to a
preferred
embodiment, the variable pitch thread of the housing includes a fine pitch
thread toward
its proximal end and a coarse pitch thread towards it distal end. Thus, if the
user applies
a substantially constant speed movement of the plunger as it is depressed, as
the thread
segment of the plunger moves along the fine pitch, the plunger and the keyed
screw will
rotate at a first speed, while the plunger and keyed screw will rotate at
slower speed as
the thread segments of the plunger engage the coarse thread disposed toward
the distal
end of the housing. In this way, the rotations of the plunger, and associated
screw, may
be tailored to a wide range of rotational speeds, and, therefore, axial
movements of the
screw by utilizing a variable pitch screw engagement between the plunger and
the
housing.
The fine portion of the variable pitch thread may have a 1:1 pitch ratio with
the
thread of the adapter while the coarse portion of the variable pitch thread
may have a
ratio of up to approximately 20:1 with the thread of the adapter as described
above.
The variable pitch thread may provide numerous advantages. For example, the
syringe may be configured to be filled at time of use. The variable pitch may
allow the
syringe to be filled more quickly.
The housing may be provided in one or more components. By way of example
only, the housing may include two or more housing sections that include
threads of
respective pitches, potentially providing advantages regarding the fabrication
process.
By way of further example, the housing may include a lower housing section
having a
coarse pitch and an upper housing section having a fine pitch. The components
of the
housing may be assembled by any appropriate coupling arrangement, including,
but not
limited to, spin welding, adhesive, or coupling structure, such as threads or
engaging
latches or the like.
In another embodiment, the dose control mechanism further includes a housing
including first and second housing sections that are moveable relative to one
another.
For example, a second housing section may be positioned between the first
housing
section and the plunger. In such an embodiment, the second housing section
includes an
internal thread¨which can be either constant pitch or variable
pitch¨configured to
engage the external thread segments of the plunger. The second housing section
is
configured such that, in a first configuration, it is able to axially
translate with respect to
CA 03026708 2018-12-05
WO 2017/204787 6 PCT/US2016/033950
the first housing section. In a second configuration, the second housing
section is fixed
in relation to the first housing section. As will be explained below, this
allows a syringe
to be quickly filled and primed in a way that is familiar to the user, while
providing
accurate dose control during delivery.
According to one aspect of the invention, there is provided a dose control
mechanism for a syringe, and a syringe including such a dose control
mechanism. One
embodiment of such a dose control mechanism includes a housing, an adapter, a
plunger, and a screw. The housing includes a longitudinally extending channel
having
an interior surface. The adapter includes a channel having a fine pitch
thread. The
plunger has an exterior surface and an axially extending channel; the axially
extending
channel includes a first key aspect. The screw includes a screw exterior
surface that
includes a second key aspect along a proximal portion of the screw exterior
surface. A
proximal end of the screw is disposed at least partially within the axially
extending
channel of the plunger. At least a portion of the second key aspect is
disposed within the
axially extending channel of the plunger and engaging the first key aspect for
sliding
movement such that rotational movement of the plunger causes rotational
movement of
the screw. A distal portion of the screw exterior surface includes a fine
pitch screw
thread at least partially disposed within and interfacing with the fine pitch
thread of the
adapter. An engaging screw thread arrangement is provided between the plunger
and
the housing. The engaging screw thread arrangement includes at least one
thread
segment and a pitch guide including a variable pitch thread. At least a
portion of the
longitudinally extending channel of the housing includes one of the pitch
guide and the
at least one thread segment, and the plunger includes the other of the pitch
guide and the
at least one thread segment. The plunger resides at least partially within the
housing
with the at least one thread segment engaged with the pitch guide. In at least
one
embodiment of the dose control mechanism, the variable pitch thread includes
at least
two different thread pitches. In at least an additional embodiment, the
variable thread
pitch continually varies along at least a portion of the variable thread
pitch.
At least an additional embodiment of such a dose control mechanism includes a
housing, an adapter, a plunger, and a screw wherein the housing has a
longitudinally
extending channel having an interior surface, and includes at least a first
housing
section and a second housing section disposed for telescoping movement
relative to one
another between a retracted position and an extended position. The adapter
includes a
channel having a fine pitch thread. The plunger has an exterior surface and an
axially
CA 03026708 2018-12-05
WO 2017/204787 7 PCT/US2016/033950
extending channel, the axially extending channel including a first key aspect.
A
proximal end of the screw is disposed at least partially within the axially
extending
channel of the plunger. The screw has a screw exterior surface including a
second key
aspect along a proximal portion. At least a portion of the second key aspect
is disposed
within the axially extending channel of the plunger and engaging the first key
aspect for
sliding movement such that rotational movement of the plunger causes
rotational
movement of the screw. a distal portion of the screw exterior surface includes
a fine
pitch screw thread at least partially disposed within and interfacing with the
fine pitch
thread of the adapter. An engaging screw thread arrangement is provided
between the
exterior surface of the plunger and the housing. The engaging screw thread
arrangement includes at least one thread segment and a pitch guide including a
thread.
At least a portion of the longitudinally extending channel of the housing
includes one of
the pitch guide and the at least one thread segment; the plunger includes the
other of the
pitch guide and the at least one thread segment. The plunger resides at least
partially
within the housing with the at least one thread segment engaged with the pitch
guide.
According to at least one embodiment, the first housing section and the second
housing
section are disposed for movement between a retracted position and a primed
position.
According to at least one embodiment, rotational movement is permitted between
the
first and second housing sections as they telescope relative to one another
between the
retracted and extended positions.
Additionally, in at least one embodiment, the dose control mechanism includes
a
dose feedback mechanism. The feedback mechanism may provide tactile feedback
to
the user for, for example, identification of the desired delivery volume. When
the user
dials the plunger rod/screw to their desired dose volume (i.e., when they the
desired
microliter setting in the window), they will feel a tactile notch or stop-
point so they
know that they should check to see if they have reached the desired dose
volume. The
feedback mechanism may include multiple volume-based detents to indicate, for
example, when the syringe is at the 20 microliter, 10 microliter, and 5
microliter
delivery volumes. In one embodiment, one or more clips can be attached to the
housing
to engage with the plunger rod/screw at axial locations which correspond with
one or
more desired set-points/stop-points. The clip may have one or more radially
inward
extending prongs which pass-through corresponding apertures in the housing at,
for
example, the screw-threaded portion of the housing. The clip prongs are
configured to
removably engage or contact one or more corresponding recesses, divots,
apertures or
CA 03026708 2018-12-05
WO 2017/204787 8 PCT/US2016/033950
the like in the plunger rod/screw. As the user axially rotates the plunger
rod/screw to
dial their desired delivery volume, the clip prongs are caused to
contact/engage the
screw recess which corresponds to a defined set-point/stop-point. The set-
points/stop-
points recesses are dimensioned such that each corresponds with the amount of
drug
volume in the syringe for drug delivery. Accordingly, multiple clips
containing clip
prongs can be used to engage the housing through multiple apertures in order
to engage
the plunger rod/screw at various set-point recesses along the axial length of
the plunger
rod/screw to give one or more tactile feedbacks to the user regarding the
dialed dose
volume within the syringe. In another embodiment, the clip prongs may be pre-
established and molded as radially inward aspects on the housing.
In a further embodiment, a method of manufacturing a syringe having a control
mechanism includes the steps of: (i) mounting a barrel adapter assembly to a
distal end
of a syringe barrel; (ii) mounting a plunger seal through a proximal end of
the syringe
barrel; and (iii) mounting a control mechanism to the proximal end of the
syringe barrel,
wherein the control mechanism may rest in contact with the plunger seal. The
method
may further include, before the step of (ii) mounting a plunger seal through a
proximal
end of the syringe barrel, the step of: filling the barrel at least partially
with a fluid
substance. In at least one embodiment, the adapter is a two component adapter
having a
proximal adapter portion and a distal adapter portion. The proximal adapter
portion has
one or more connection prongs and the distal adapter portion has corresponding
connection ports which, when forced together, connection prongs and
corresponding
connection ports merge, mate, or otherwise connect to unite the two portions
of the
adapter. Steps (i) and (ii), and the optional step of filling the barrel at
least partially with
a fluid substance, may be performed in a sterile environment to maintain the
container
integrity and sterility of the syringe.
The present invention further provides methods of assembling dose control
mechanisms, methods of manufacturing syringes having dose control mechanisms,
and
methods of operation of such mechanisms and syringes. Such novel devices and
methods permit the identification and control of the dosage amount, permit the
syringe
to be "primed" (i.e., evacuated of air bubbles) prior to drug delivery, and
ensure the
accurate delivery of microliter volume dosages, all within a device size that
is similar to
commonly used conventional syringes available in the marketplace. Throughout
this
specification, unless otherwise indicated, "comprise," "comprises," and
"comprising," or
related terms such as "includes" or "consists of," are used inclusively rather
than
CA 03026708 2018-12-05
WO 2017/204787 9 PCT/US2016/033950
exclusively, so that a stated integer or group of integers may include one or
more other
non-stated integers or groups of integers. As will be described further below,
the
embodiments of the present invention may include one or more additional
components
which may be considered standard components in the industry of medical
devices. The
components, and the embodiments containing such components, are within the
contemplation of the present invention and are to be understood as falling
within the
breadth and scope of the present invention.
BRIEF DESCRIPTION OF THE DRAWINGS
The following non-limiting embodiments of the invention are described herein
with reference to the following drawings, wherein:
FIG. 1A shows a side elevational view of a dose control mechanism, according
to at
least one embodiment of the present invention;
FIG. 1B shows a cross-sectional view in a plane "B" which is perpendicular to
axis "A"
of the dose control mechanism of FIG. 1A;
FIG. 2A shows a cross-sectional view of the dose control mechanism shown in
FIG. 1A
as the components may appear in a ready-to-inject stage of operation;
FIG. 2B shows a cross-sectional view of the dose control mechanism shown in
FIG. 1A
as the components may appear in an end-of-dose stage of operation;
FIG. 3A shows an exploded view, exploded along an axis "A," of the dose
control
mechanism shown in FIG. 1A;
FIG. 3B shows a cross-sectional exploded view, exploded along an axis "A," of
the
dose control mechanism shown in FIG. 1A;
FIG. 4A shows an isometric view of a drug delivery syringe which incorporates
a dose
control mechanism, according to a second embodiment of the present invention;
FIG. 4B shows an enlarged isometric view of the distal portion of the drug
delivery
syringe shown in FIG. 4A;
FIG. 5A shows an isometric view of another drug delivery syringe which
incorporates a
dose control mechanism, according to another embodiment of the present
invention;
FIG. 5B shows an enlarged isometric view of the distal portion of the drug
delivery
syringe shown in FIG. 5A;
FIG. 6A shows an isometric view of yet another drug delivery syringe which
incorporates a dose control mechanism, according to another embodiment of the
present invention;
CA 03026708 2018-12-05
WO 2017/204787 10 PCT/US2016/033950
FIG. 6B shows an enlarged isometric view of the distal portion of the drug
delivery
syringe shown in FIG. 6A;
FIG. 7A shows an isometric view of an initial assembly stage of a pre-filled
drug
delivery syringe which incorporates a dose control mechanism, according to at
least one embodiment of the present invention;
FIG. 7B shows an isometric view of the pre-filled drug delivery syringe shown
in
FIG. 7A after it has been assembled;
FIG. 7C shows an isometric view of the pre-filled drug delivery syringe shown
in
FIG. 7A in a ready-to-inject stage of operation;
FIG. 7D shows an isometric view of the pre-filled drug delivery syringe shown
in
FIG. 7A in an end-of-dose stage of operation
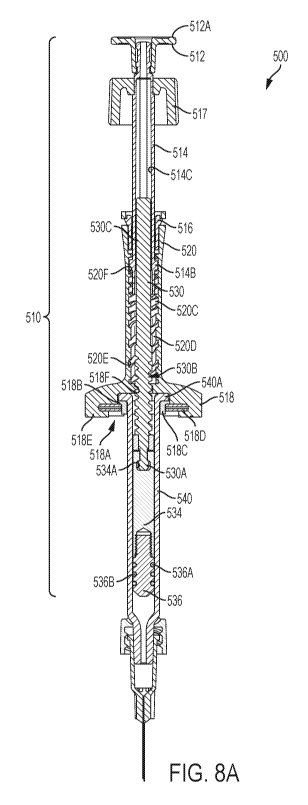
FIG. 8A shows a cross-sectional view of an alternate design of a dose control
mechanism as the components may appear in a ready-to-inject stage of
operation;
FIG. 8B shows a cross-sectional view of the dose control mechanism shown in
FIG. 8A
as the components may appear in a ready-to-inject stage of operation;
FIG. 8C shows a cross-sectional view of the dose control mechanism shown in
FIG. 8A
as the components may appear in an end-of-dose stage of operation;
FIG. 9A shows an exploded view, exploded along a longitudinal axis of the dose
control
mechanism shown in FIGS. 8A-8C;
FIG. 9B shows a cross-sectional exploded view, exploded along a longitudinal
axis of
the dose control mechanism shown in FIGS. 8A-8C;
FIG. 10A is a cross-sectional view of a syringe incorporating another
embodiment of a
dose control mechanism according to the present invention, the housing being
illustrated in an extended position;
FIG. 10B is a side elevational view of the syringe of FIG. 10A;
FIG. 10C is a cross-sectional view of the syringe of FIGS. 10A and 10B in a
primed
position;
FIG. 10D is a side elevational view of the syringe of FIGS. 10A and 10B in the
position
illustrated in FIG. 10C;
FIG. 10E is a cross-sectional view of the syringe of FIGS. 10A-10D at the
completion
of delivery;
FIG. 1OF is a side elevational view of the syringe of FIGS. 10A-10D in the
position
illustrated in FIG. 10E;
CA 03026708 2018-12-05
WO 2017/204787 11 PCT/US2016/033950
FIG. 11A is an exploded view of the dose control mechanism of FIGS. 10A-10F,
exploded along a longitudinal axis;
FIG. 11B is a cross-sectional exploded view of the dose control mechanism
shown in
FIGS. 10A-11A, exploded along a longitudinal axis;
FIG. 12A is a side elevational view of another embodiment of a dose control
mechanism according to the present invention, the housing being illustrated in
a
retracted position;
FIG. 12B is a side elevational view of the dose control mechanism of FIG. 12A,
the
housing being illustrated in an extended position;
FIG. 12C is a side elevational view of the dose control mechanism of FIGS. 12A
and
12B in a primed position;
FIG. 12D is a side elevational view of the dose control mechanism of FIGS. 12A-
12C
in a ready position for delivery;
FIG. 12E is a side elevational view of the dose control mechanism of FIGS. 12A-
12D
during delivery;
FIG. 13A is an enlarged fragmentary isometric view of the engaging aspects of
the first
and second housing sections of FIGS. 12A-12F in the retracted position
illustrated in FIG. 12A, the first housing section being shown partially
transparent to illustrate the internal structure;
FIG. 13B is an enlarged fragmentary isometric view of the engaging aspects of
the first
and second housing sections of FIGS. 12A-12F in the extended position
illustrated in FIG. 12B, the first housing section being shown partially
transparent to illustrate the internal structure;
FIG. 13C is an enlarged fragmentary isometric view of the engaging aspects of
the first
and second housing sections of FIGS. 12A-12F in the delivery position
illustrated in FIG. 12E, the first housing section being shown partially
transparent to illustrate the internal structure;
FIG. 14 is an enlarged isometric view of the second housing section of the
embodiment
of FIGS. 12A-13C.
FIG. 15 is an enlarged partial cross-sectional view of an embodiment of an
engaging
structure between first and second housing sections according to an embodiment
of the invention;
CA 03026708 2018-12-05
WO 2017/204787 12 PCT/US2016/033950
FIG. 16A is a side elevational view of a syringe incorporating a dose control
mechanism according to another embodiment of the invention incorporating a
tactile feedback feature;
FIG. 16B is a side elevational, partially cross-sectional view of the syringe
of FIG. 16B
partially broken away to illustrate the tactile feedback feature;
FIG. 16C is an enlarged view of the cross-sectioned portion of FIG. 16B.
DETAILED DESCRIPTION
As used herein to describe the dose control mechanisms, drug delivery
syringes,
or any of the relative positions of the components of the present invention,
the terms
"axial" or "axially" refer generally to a longitudinal axis "A" around which
the control
mechanisms and syringes are preferably positioned, although not necessarily
symmetrically there-around. The term "radial" refers generally to a direction
normal to
axis "A". The terms "proximal," "rear," "rearward," "back," or "backward"
refer
generally to an axial direction in the direction "P". The terms "distal,"
"front,"
"frontward," "depressed," or "forward" refer generally to an axial direction
in the
direction "D". As used herein, the term "glass" should be understood to
include other
similarly non-reactive materials suitable for use in a pharmaceutical grade
application
that would normally require glass, including but not limited to certain non-
reactive
polymers such as cyclic olefin copolymers (COC), cyclic olefin polymers (COP),
and
the like. The term "plastic" may include both thermoplastic and thermosetting
polymers. Thermoplastic polymers can be re-softened to their original
condition by
heat; thermosetting polymers cannot. As used herein, the term "plastic" refers
primarily
to moldable thermoplastic polymers such as, for example, polyethylene and
polypropylene, or an acrylic resin, that also typically contain other
ingredients such as
curatives, fillers, reinforcing agents, colorants, and/or plasticizers, etc.,
and that can be
formed or molded under heat and pressure. As used herein, the term "plastic"
is not
meant to include glass, non-reactive polymers, or elastomers that are approved
for use
in applications where they are in direct contact with therapeutic liquids that
can interact
with plastic or that can be degraded by substituents that could otherwise
enter the liquid
from plastic. The term "elastomer," "elastomeric" or "elastomeric material"
refers
primarily to cross-linked thermosetting rubbery polymers that are more easily
deformable than plastics but that are approved for use with pharmaceutical
grade fluids
and are not readily susceptible to leaching or gas migration under ambient
temperature
and pressure. "Fluid" refers primarily to liquids, but can also include
suspensions of
CA 03026708 2018-12-05
WO 2017/204787 13 PCT/US2016/033950
solids dispersed in liquids, and gasses dissolved in or otherwise present
together within
liquids inside the fluid-containing portions of syringes. According to various
aspects
and embodiments described herein, reference is made to a "biasing member",
such as in
the context of one or more biasing members for retraction of a needle or
needle
assembly. It will be appreciated that the biasing member may be any member
that is
capable of storing and releasing energy. Non-limiting examples include a
spring, such
as for example a coiled spring, a compression or extension spring, a torsional
spring,
and a leaf spring, a resiliently compressible or elastic band, or any other
member with
similar functions. In at least one embodiment of the present invention, the
biasing
member is a spring, preferably a compression spring.
The novel devices of the present invention provide dose control mechanism,
which allow for the accurate dosing and delivery of drug treatments, and drug
delivery
syringes which incorporate such control mechanisms. Such devices are safe and
easy to
use, and are aesthetically and ergonomically appealing for clinical
practitioners. The
devices described herein incorporate features which make activation,
operation, and
lock-out of the device simple for even untrained users. The novel devices of
the present
invention provide these desirable features without any of the problems
associated with
known prior art devices. Certain non-limiting embodiments of the novel dose
control
mechanisms, drug delivery syringes, and their respective components are
described
further herein with reference to the accompanying figures.
Various studies have shown that the accuracy of dose delivery using
conventional syringes is affected by a number of factors, including an
inability to
accurately read and control plunger travel during dosing. The use of
conventional drug
delivery syringes that have a high dose volume to axial translation ratio
(i.e., a
significant quantity of drug is dispensed for even incrementally small
distances of
plunger depression, as may be the case for large diameter syringes)
significantly
magnifies this inaccuracy. With the growth of high-cost, low-volume drug
treatments
entering the marketplace, it is increasingly important to accurately dose and
deliver
such low-volume treatments to the patient. The embodiments of the present
invention
overcome the challenges faced with the use of conventional syringes for the
dosing and
delivery of low-volume treatments by utilizing novel dose control mechanisms.
As will
be described further herein, the novel dose control mechanisms permit the user
to
accurately read and dose the desired volume of drug treatment for delivery to
the
patient. These devices permit the user to have a normal range of thumb travel,
as they
CA 03026708 2018-12-05
WO 2017/204787 14 PCT/US2016/033950
may otherwise expect with a conventional syringe, but transform that range of
thumb
travel to a very finite (e.g., smaller or incremental) range of plunger seal
travel. This
relationship allows the user to utilize the syringe without additional
training, but with
the significant benefit of incremental, low-volume dose control.
FIG. 1A shows an embodiment of a novel dose control mechanism for a syringe,
according to at least one embodiment of the present invention. The control
mechanism
includes a plunger 14, a housing 20, an adapter 18, and a screw 30. The
plunger 14
may include a button 12 as a unified or separate component. For example,
button 12
may be a preformed aspect at the proximal end of the plunger 14.
Alternatively, button
10 12 may be a separate component attached to the proximal end of plunger
14 by a snap-
fit. In a preferred embodiment, the button 12 may be attached to plunger 14
but allowed
to axially rotate freely from plunger 14, but rotationally fixed relative to
the
user's/clinician's finger. Regardless of the specific configuration and
relationship of
button 12 and plunger 14, button 12 is intended to have a user interface
surface 12A for
contact and control by a user (e.g., such as with the thumb or finger tip of
the user).
Housing 20 has a substantially cylindrical axial pass-through within which a
substantially cylindrical plunger 14 may at least partially reside. The distal
end of the
housing 20 is connected to, and/or resides partially within, a proximal
portion of adapter
18. The proximal and distal portions of adapter 18 may be separated by an
adapter
flange 18A which may additionally serve as a finger flange for use by the
user. The
internal aspects of these components will be described in further detail
herein with
reference to FIGS. 1B, 2A, 2B, and 3B. Screw 32 may reside at least partially
within
housing 20 and plunger 14, and extends distally beyond flange 18. Screw 30 may
have a
screw connection 30A aspect and, optionally, a ring 32, to facilitate
integration of the
control mechanism with a drug delivery syringe and to center the plunger rod.
Housing 20 may optionally include housing cover 16 at its proximal end, for
example, to close the interior of the housing 20 off from the environment
and/or to
axially align plunger 14 within housing 20, and to prevent removal of the
plunger rod
by functioning as a mechanical stop. Housing 20 may further include a window
20A,
which may be an opening (e.g., an aperture) in the housing or a transmissive
or
translucent component. Regardless of the particular configuration of window
20A, its
primary purpose is to permit the user to view the location of the plunger 14
within
housing 20. Plunger 14 may include one or more dose markings 14A on the
external
surface of the plunger 14. Housing 20 may have one or more reference or guide
CA 03026708 2018-12-05
WO 2017/204787 15 PCT/US2016/033950
markings 20B, such as at the window 20A, with which to align plunger dose
markings
14A. The plunger dose markings 14A may correspond to the relevant dose amounts
desired by the user. By employing the respective plunger and housing markings,
the
user can control the volumetric dose quantities desired for delivery to the
patient, as will
be explained further herein. In another embodiment, the window 20A may be
covered
by a lens, such as a clear lens, that provides visual magnification.
FIGS. 2A and 2B show cross-sectional views of the dose control mechanism,
according to at least one embodiment of the present invention, in a ready-to-
inject stage
and in an end-of-dose stage, respectively. The cross-sectional views show
certain other
aspects of the components which are internal to the mechanism. As shown,
plunger 14
has an internal annular space 14C within which screw 30 at least partially
resides.
Plunger 14 has a coarse pitch male thread 14B (visible in FIG. 3A) on its
exterior
surface which interfaces with the coarse pitch guide 20C along the interior
surface of
the housing 20 such that, in at least one embodiment, the pitch on guide 20C
is the same
as pitch on plunger thread 14B. Similarly, screw 30 has a fine pitch thread
30B which
interfaces with a fine pitch nut 18B of adapter 18 such that, in at least one
embodiment,
the pitch on screw thread 30B is the same as pitch on nut 18B. Also visible in
FIGS. 2A
and 2B are the proximal end 30C of screw 30 and ledge 18C of adapter 18. The
plunger
14 having the coarse pitch 14B is rotatable upon the corresponding (e.g.,
"female")
coarse pitch guide 20C, which is rotationally keyed to the screw 30 having the
fine pitch
thread 30B. The terms "male" and "female" are intended to describe
corresponding and
interfacing threads or surfaces, and can be used interchangeably to describe
corresponding aspects as would be readily appreciated in the art. The screw 30
having
the fine pitch screw 30B engages the female fine pitch nut 18B of the adapter
18.
Hence, rotation of plunger 14 results in axial translation of screw 30 and the
resolution
of axial travel is dictated by pitch 30B.
Because the plunger 14 and screw 30 are rotationally keyed, each having a
respective screw pitch, rotational translation of the plunger 14 rotates and
axially
translates the screw 30. The term "keyed" is used herein to mean any number of
internal
aspects which removably or slidably (in the axial sense) connect two or more
components. For example, the plunger 14 may be a hollow cylinder having a
coarse
pitch screw on at least some portion of the outer surface and a spline design
along at
least a portion of the inner surface. The spline design is configured to mate
with, and
transform or relay rotation to, a complimentary spline contained at a proximal
end of
CA 03026708 2018-12-05
WO 2017/204787 16 PCT/US2016/033950
the screw 30. This spline design element ensures that the plunger 14 and screw
30 are
rotationally keyed. The spline or rotationally keyed aspect is visible at the
proximal end
30C of screw 30 in FIG. 3A, and with its corresponding spline or rotationally
keyed
aspect in the annular space 14C of plunger 14 in FIG. 3B. Any number of
corresponding shapes may be utilized to impart a rotationally "keyed"
relationship
between these components such that the first component may removably or
slidably
engage the second component in a manner which enables the rotational keyed
relationship and permits axial slip. Such components may alternatively be
keyed to have
the shape of, for example, a cross or plus, a horizontal line or minus, a
star, or a semi-
circle shape, with the corresponding component having the inverse of the shape
on an
interior annular space. FIG. 1B shows a cross-sectional view in a plane "B"
which is
perpendicular to axis "A" of the dose control mechanism of FIG. 1A. As shown
in FIG.
1B, in at least one embodiment, screw 30 has a cross or plus shape in its
perpendicular
cross-section which is keyed to plunger 14. This arrangement or configuration
allows
the two components to be rotationally keyed while allowing them to axially
slip past
each other. Both screw 30 and plunger 14 reside, at least partially and/or at
some point
of operation, within housing 20.
Fine pitch nut 18B (or simply "nut"), having the same fine pitch of the screw
30,
may be used to brace the screw 30 and facilitate the transfer of the
rotational movement
of the plunger 14 into axial translation of the screw 30. The pitch ratio of
the coarse
pitch to the fine pitch dictates the degree or resolution of axial travel of
the screw 30,
i.e., the distance that the screw 30 axially translates for each rotation of
the plunger 14.
As a result, the medical practitioner is provided with an ease of operation
that enables
them to accurately read and set the dosage amount. The pitch ratio can be set
to enable
"fine tuning" of the dosage amount, which is of particular importance for low-
volume
dosage quantities where variance may be significantly affected by plunger
travel.
During operation of the dose control mechanism, the user may axially rotate
plunger 14 or depress the button 12 to control the desired dosage volume for
injection
into the patient. Axial rotation of the plunger 14 causes coarse pitch screw
14B (visible
in FIG. 3B) to travel within the corresponding coarse pitch guide 20C of
housing 20, as
shown in FIGS. 3A and 3B. This action causes the plunger 14 to axially
translate in the
distal direction thereby reducing the dosage volume within the drug chamber,
as is
explained in more detail herein. Because of the rotationally keyed interaction
between
plunger 14 and screw 30 within the annular space 14C, rotation of the plunger
14 causes
CA 03026708 2018-12-05
WO 2017/204787 17 PCT/US2016/033950
screw 30 to axially rotate and translate. However, because of the pitch ratio
between the
plunger 14 and screw 30, each unit measure of translation in the distal
direction of the
plunger 14 results in fractional (e.g., smaller, more resolved) translation of
the screw 30
in the distal direction. This has a number of benefits for accurate control
during delivery
of low-volume doses. Primarily, the pitch ratio relationship permits the user
to
accurately control the desired dose and delivery of a drug treatment.
Additionally, this
pitch ratio relationship allows the user to operate a syringe in a
conventional manner,
such as by depressing the plunger 14 a noticeable distance, while only
resulting in
fractional or small translation of the screw.
The novel dose control mechanisms of the present invention also utilize
features
which provide integrated and adjustable range-of-travel limits to ensure
accurate
delivery of low-volume drug treatments. This may be enabled, for example, by
incorporating features that prevent variable depression of the plunger seal
(or stopper)
(e.g., preventing the plunger from "bottoming out" during drug delivery)
within a
syringe. Specifically, the dose control mechanisms of the present invention
utilize
adjustable set mechanical end-points for the range of plunger axial travel
during drug
delivery. Such limits may be predefined, i.e., integrated and fixed into the
syringe
configuration in advance of use by the medical practitioner, or adjustable,
i.e., variably
controlled by a compounding pharmacist, a medical practitioner, or by a self-
administering patient using an integrated dosage setting mechanism. Such
mechanical
set-points permit a range of axial plunger travel that are, for example,
related to the
priming and dosing quantities, but also prevent the user from variably
depressing the
plunger and plunger seal as part of the dosing stroke or from bottoming out
these
components within the dosing chamber of a syringe. This novel control
mechanism
greatly increases the accuracy of the dose delivered to the patient.
Additionally,
embodiments of the present invention allow the user to prime the syringe to
evacuate
the dosing chamber of any residual air prior to delivering the dose to the
patient. The
prime step may be a fixed amount or a variable amount, depending on the
configuration
of the low dose syringe and variation in amount of drug or liquid
contained/filled in the
dosing chamber. The configuration of the novel syringe allows the user to
complete the
prime step while maintaining, or enabling, the ability of the syringe to
deliver an
accurate and precise dose to the patient.
As stated above, the mechanical set-point limits effectively function to
prevent
the user from variably depressing the plunger and plunger seal or from
bottoming out
CA 03026708 2018-12-05
WO 2017/204787 18 PCT/US2016/033950
these components within the dosing chamber of a syringe. This functionality
increases
the accuracy of the dose delivered to the patient because it reduces the
variability of the
delivered dose from the amount prescribed and intended to be delivered to the
patient.
The mechanical end-points may be readily identified and easily set by
employing the
pitch ratio between the plunger 14 having a coarse pitch screw 14B and the
screw 30
having a fine pitch screw 30B. For example, in one such embodiment a pitch
ratio
between the coarse pitch and a fine pitch may be 4 : 1, such that rotationally
"screwing"
or turning plunger 14 axially translates the plunger component four times as
far as the
axial translation of the screw component. Accordingly, the practitioner is
provided with
a significant ease of operation since they may more accurately set the
required dosage
amount. Such a pitch ratio may be, for example, anywhere from the range of 1 :
1 to
: 1, as may be necessary to obtain the required accuracy of the low-volume
dosage
amount. The "dialing-in" or "setting" may be facilitated by the dose markings
on the
plunger and guide markings on the housing described above.
15 As the user depresses the button 12, which rotates the plunger 14 to
set the
desired low-volume dosage for injection, they can perform what is known in the
art as a
"priming step." This priming step evacuates the dosing chamber of any residual
air
bubble captured in the dosing chamber during pre-filling, if any, and primes
the
attached needle (or catheter or an extension set) before delivery. After
priming and
20 setting of the dose by depression of the button 12 has been
completed, the button 12
may be depressed further to bottom out and, hence, inject the desired dose
amount to
the patient. Upon drug dose delivery, the plunger 14 is caused to "bottom out"
on ledge
18C of adapter 18 (as shown in FIG. 2B). Because of the pitch ratio between
the
plunger 14 and the screw 30, as plunger 14 is depressed or axially translated
in the
distal direction (i.e., in the direction of solid arrow in FIGS. 2A and 2B),
screw 30 is
caused to axially translate in the distal direction only a fraction of the
distance translated
by the plunger 14. This difference in axial translation distance between
plunger 14 and
screw 30 is visible by comparing distances D1 and D2 in FIGS. 2A and 2B. D1 is
the
distance that plunger 14 axially translates while D2 is the incremental
distance that
screw 30 axially translates. The difference in dimensions D1 and D2 is also
clear by the
reduction in the annular space 14C of plunger 14 (compare FIGS. 2A and 2B),
when
identifying the relative position of the proximal end 30C of the screw 30.
Accordingly,
the variable annular space 14C of plunger 14 is related to the mechanical set-
point
CA 03026708 2018-12-05
WO 2017/204787 19 PCT/US2016/033950
desired by the practitioner and provides space for translation of the screw 30
during the
dosage stroke.
Notably, the novel embodiments contemplated by the present invention
effectively prevent the plunger seal from "bottoming-out" within the dosing
chamber.
This pre-empts one aspect of user variability in either excess dosing by over-
depression
of the plunger or under dosing by under-depression of the plunger, ensuring
that the
quantity dosed to the patient is accurate and minimizes user error. This is of
particular
importance in low dosage treatments, where user-related errors can cause
significant
and undesirable variation and inaccuracy in the delivery of medication to the
patient.
The embodiments according to the present invention prevent such occurrences
and
work to effectively eliminate the dosing errors associated with prior syringe
configurations and delivery methodologies. Furthermore, depression of the
plunger in
this embodiment does not back-drive the screw.
The novel dose control mechanisms of the present invention can be integrated
into a number of drug delivery syringe configurations to provide accurate dose
delivery
capability to the user. For example, the control mechanisms may be utilized
with fill-at-
time-of-use syringes, pre-filled syringes, or safety syringes having
integrated needle
retraction or needle sheathing safety features, or a combination thereof
Further, dose
control mechanisms according to the teachings of this disclosure may be
utilized with
conventional syringes, as well as so-called mixing syringes. For example, the
dose
control mechanisms may be incorporated into syringes such as those disclosed
in U.S.
Patent Application No. 13/566,079, which is incorporated herein by reference
for all
disclosed therein.
Examples of such syringes which incorporate the novel dose control
mechanisms are provided below. By employing the respective plunger 14 and,
optionally, the dose markings 14A and guide markings 20B, the user can control
the
volumetric dose quantities within the syringe that is desired for delivery to
the patient.
The plunger dose markings 14A may correspond to the relevant dose amounts
desired
by the user. The user may initially utilize the plunger, such as by axially
depressing the
button or rotating the plunger, to identify and select the desired dose amount
by aligning
the desired dose marking 14A with the guide marking 20B. Axial rotation of the
plunger 14 causes the plunger 14 to axially translate in the distal direction,
which
motion is transferred by the above described mechanism to the screw 30. Axial
translation of the screw 30 in the distal direction causes drug fluid
contained within the
CA 03026708 2018-12-05
WO 2017/204787 20 PCT/US2016/033950
drug chamber of the syringe to be dispensed through the needle of the barrel
adapter
assembly. Once the desired dose has been identified and selected by the user,
the
remaining amount of drug fluid within the drug chamber is substantially the
exact
amount desired to be injected. Syringe may then be injected into the patient
for drug
delivery. After injection of the needle into the patient, the user may further
depress the
plunger 14 (and/or the button 12) axially in the distal direction to deliver
the drug dose.
Because of the novel aspects of the present invention, including the pitch
ratio and
mechanical stop mechanisms described above, the accuracy of the dose is finely
controlled and variability is reduced. In the embodiments of the present
invention
intended for fill-at-time-of-use syringes, the plunger 14 and screw 30 may
initially
function in reverse (e.g., axially translate in the proximal direction) to
draw-in drug
fluid from a vial or container to fill the drug chamber of the syringe. In the
embodiments of the present invention intended for retractable or safety
syringes, the
plunger 14 and screw 30 may function, substantially after the drug dose has
been
delivered, to initiate or engage a needle retraction or safety mechanism.
These
embodiments of the present invention are discussed in further detail below
with
reference to the accompanying figures.
FIG. 4A shows an embodiment of the dose control mechanism 10 as a
component of an exemplary fill-at-time-of-use drug delivery syringe 100, i.e.,
syringes
which can be drawn back and filled with a drug treatment by the user. As
shown, the
control mechanism 10 includes a plunger 14, a housing 20, an adapter 18, and a
screw
30. The plunger 14 may include a button 12 as a unified or separate component,
as
described above. Housing 20 may optionally include housing cover 16 at its
proximal
end, for example, to close the interior of the housing 20 off from the
environment
and/or to axially align plunger 14 within housing 20. Housing 20 may further
include a
window 20A, which may be an opening (e.g., an aperture) in the housing or a
transmissive, translucent, and/or optically magnifying component. Plunger 14
may
include one or more dose markings 14A on the external surface of the plunger
14.
Housing 20 may have one or more reference or guide markings 20B, such as at
the
window 20A, with which to align plunger dose markings 14A. The control
mechanism
10 may be attached, mounted, affixed, or otherwise connected at the proximal
end of
barrel 140 such that at least a portion of the screw 30 resides inside barrel
140.
FIG. 4B shows an enlarged isometric view of the distal portion of the drug
delivery syringe shown in FIG. 4A. Screw 30 may be connected to plunger seal
136
CA 03026708 2018-12-05
WO 2017/204787 21 PCT/US2016/033950
either directly or indirectly to drive the axial translation of the plunger
seal 136. In the
latter configuration, a plunger rod 134 may be utilized between screw 30 and
plunger
seal 136 to connect those components. The plunger rod 134 may be connected to
the
screw 30 at, for example, the screw connection 30A aspect. Optionally, a ring
32 near
the distal end of the screw 30 may be utilized to facilitate the connection of
the screw
30, the plunger rod 134 and the plunger seal 136. The screw connection 30A
aspect and
the ring are visible in FIGS. 2A, 2B, and 3B. In at least one embodiment, the
screw
connection 30A aspect is connected to the plunger rod 134 through a radial
opening in
the plunger rod. Additionally or alternatively, this connection may be a snap-
fit
connection, an interference-fit connection, or a number of other connection
methods
known in the industry. In at least one other embodiment, the screw connection
aspect is
connected to the plunger rod through a proximal opening in the plunger rod
such that
the screw connection aspect sits within a proximal pocket in the plunger rod.
Preferably,
the connection between the screw 30 and the plunger seal 136, or screw 30 and
plunger
rod 134 when a plunger rod is employed, is such that the screw is permitted to
axially
rotate while the plunger rod and/or the plunger seal remain rotationally
fixed.
Accordingly, as the plunger 14 and screw 30 of the control mechanism 10 are
axially
rotated and translated, the motion is relayed to the plunger seal 136 which is
also axially
translated.
When utilized within a fill-at-time-of-use syringe, the plunger 14 and screw
30
may initially function in reverse (e.g., axially translate in the proximal
direction) to
draw-in drug fluid from a vial or container to fill the drug chamber 138 of
the syringe
100. As described above, the control mechanism 10 may then be utilized by the
user to
identify and select drug dose for delivery. The user may then inject the
needle into the
patient for drug delivery. Subsequently, the button 12 and/or plunger 14 may
be
depressed by the user to cause the plunger 14 and screw 30 to axially
translate. Because
of the function of the control mechanism and the pitch ratio, any measure of
distal
translation of the plunger 14 causes only an incremental measure of distal
translation of
the screw 30, permitting accurate dose delivery control by the user. Axial
translation of
the screw 30 causes axial translation of the plunger seal 136. This axial
motion in the
distal direction of the plunger seal 136 forces drug fluid out of drug chamber
138 of
barrel 140, through the needle 154 of the barrel adapter assembly 150, for
injection and
delivery to the patient.
CA 03026708 2018-12-05
WO 2017/204787 22 PCT/US2016/033950
Similarly, the novel control mechanisms of the present invention may be
utilized
with pre-filled syringes, i.e., syringes which are filled with a drug
treatment by the
manufacturer and ready for injection by the user. FIG. 5A shows an embodiment
of the
dose control mechanism 10 as a component of an exemplary pre-filled drug
delivery
syringe 200. As shown, the control mechanism 10 includes a plunger 14, a
housing 20,
an adapter 18, and a screw 30. The plunger 14 may include a button 12 as a
unified or
separate component, as described above. Housing 20 may optionally include
housing
cover 16 at its proximal end, for example, to close the interior of the
housing 20 off
from the environment, to axially align plunger 14 within housing 20, and/or to
prevent
the plunger 14 being accidently removed by the user/clinician. Housing 20 may
further
include a window 20A, which may be an opening (e.g., an aperture) in the
housing or a
transmissive or translucent component. Plunger 14 may include one or more dose
markings 14A on the external surface of the plunger 14. Housing 20 may have
one or
more reference or guide markings 20B, such as at the window 20A, with which to
align
or view plunger dose markings 14A. The control mechanism 10 may be attached,
mounted, affixed, or otherwise connected at the proximal end of barrel 140
such that at
least a portion of the screw 30 resides inside barrel 140.
FIG. 5B shows an enlarged isometric view of the distal portion of the drug
delivery syringe shown in FIG. 5A. Screw 30 may be connected to plunger seal
236
either directly or indirectly to drive the axial translation of the plunger
seal 236. In the
latter configuration, a plunger rod 234 may be utilized between screw 30 and
plunger
seal 236 to connect those components. The plunger rod 234 may be connected to
the
screw 30 at, for example, the screw connection 30A aspect. In at least one
embodiment,
the screw connection aspect is connected to the plunger rod through a proximal
opening
in the plunger rod such that the screw connection aspect sits within a
proximal pocket in
the plunger rod. Additionally or alternatively, this connection may be a snap-
fit
connection, an interference-fit connection, or a number of other connection
methods
known in the industry. In at least one embodiment, as is described further
below with
reference to FIGS. 7A-7D, the screw, screw connection aspect, and plunger rod
are
configured to be readily connectable after the drug chamber has been filled
with a drug
fluid and the plunger seal and plunger rod have been inserted into the
proximal end of
the barrel. Preferably, the connection between the screw 30 and the plunger
seal 236, or
screw 30 and plunger rod 234 when a plunger rod is employed, is such that the
screw is
permitted to axially rotate while the plunger rod and/or the plunger seal
remain
CA 03026708 2018-12-05
WO 2017/204787 23 PCT/US2016/033950
rotationally fixed. Accordingly, as the plunger 14 and screw 30 of the control
mechanism 10 are axially rotated and translated, the motion is relayed to the
plunger
seal 236 which is also axially translated. When utilized within a pre-filled
syringe, the
control mechanism 10 is generally attached to the barrel 240 after the drug
chamber 238
of barrel 240 has been filled with a drug fluid. This is often desired so that
the syringe
200 may be filled and assembled in standard pharmaceutical fill-finish process
lines.
Once the syringe 200 has been filled and assembled, the control mechanism 10
may be
utilized by the user to identify and set the selected drug dose for delivery.
The user may
then inject the needle into the patient for drug delivery. Subsequently, the
button 12
and/or plunger 14 may be depressed by the user to cause the plunger 14 and
screw 30 to
axially translate. Because of the function of the control mechanism and the
pitch ratio,
any measure of distal translation of the plunger 14 causes only an incremental
measure
of distal translation of the screw 30, permitting accurate dose delivery
control by the
user. Axial translation of the screw 30 causes axial translation of the
plunger seal 236.
This axial motion in the distal direction of the plunger seal 236 forces drug
fluid out of
drug chamber 238 of barrel 240, through the needle 254 of the barrel adapter
assembly
250, for injection and delivery to the patient.
Furthermore, the novel control mechanisms of the present invention may be
utilized with safety syringes, such as retractable needle safety syringes
(i.e., syringes
which incorporate needle safety mechanisms). FIG. 6A shows an embodiment of
the
dose control mechanism 10 as a component of an exemplary retractable drug
delivery
syringe 300. As shown, the control mechanism 10 includes a plunger 14, a
housing 20,
an adapter 18, and a screw 30. The plunger 14 may include a button 12 as a
unified or
separate component, as described above. Housing 20 may optionally include
housing
cover 16 at its proximal end, for example, to close the interior of the
housing 20 off
from the environment, to axially align plunger 14 within housing 20, and/or to
prevent
accidental removal of plunger 14. Housing 20 may further include a window 20A,
which may be an opening (e.g., an aperture) in the housing or a transmissive,
translucent, and/or a component providing optical magnification. Plunger 14
may
include one or more dose markings 14A on the external surface of the plunger
14.
Housing 20 may have one or more reference or guide markings 20B, such as at
the
window 20A, with which to align or view plunger dose markings 14A. The control
mechanism 10 may be attached, mounted, affixed, or otherwise connected at the
CA 03026708 2018-12-05
WO 2017/204787 24 PCT/US2016/033950
proximal end of barrel 140 such that at least a portion of the screw 30
resides inside
barrel 140.
FIG. 6B shows an enlarged isometric view of the distal portion of the drug
delivery syringe shown in FIG. 6A. Screw 30 may be connected to plunger seal
336
either directly or indirectly to drive the axial translation of the plunger
seal 336. In the
latter configuration, a plunger rod 334 may be utilized between screw 30 and
plunger
seal 336 to connect those components. The plunger rod 334 may be connected to
the
screw 30 at, for example, the screw connection 30A aspect. The screw
connection
aspect may be connected to the plunger rod in the configuration described
above with
reference to FIGS. 4A and 4B, in the configuration described above with
reference to
FIGS. 5A and 5B, or any number of other connection methods known in the
industry.
Preferably, the connection between the screw 30 and the plunger seal 336, or
screw 30
and plunger rod 334 when a plunger rod is employed, is such that the screw is
permitted
to axially rotate while the plunger rod and/or the plunger seal remain
rotationally fixed.
Accordingly, as the plunger 14 and screw 30 of the control mechanism 10 are
axially
rotated and translated, the motion is relayed to the plunger seal 336 which is
also axially
translated. The plunger 14 and screw 30 may function, substantially after the
drug dose
has been delivered, to initiate or engage a needle retraction or safety
mechanism.
When utilized within a safety syringe, such as a retractable needle safety
syringe, the plunger 14 of the control mechanism 10 is capable of engaging or
initiating
a needle safety mechanism. Suitably, the needle safety mechanism is
facilitated by a
biasing member such as a spring, elastic or other member capable of storing
and
releasing energy to facilitate needle retraction, needle sheathing, or any
other method of
protecting the user from accidental needle stick injuries. It will be
appreciated that the
safety syringe may comprise any needle safety mechanism, such as a needle
retraction
safety mechanism or needle sheathing safety mechanism, which is operable with
the
control mechanisms and syringes disclosed herein. By way of example, the
needle
safety mechanism may be a needle retraction safety mechanism as described in
International Publication W02006/119570, International Publication
W02006/108243,
International Publication W02009/003234, International Publication
W02011/075760,
and/or U.S. Patent 8,702,653, although without limitation thereto. In at least
one
embodiment of the present invention, syringe 300 is a needle retraction safety
syringe
and incorporates the needle retraction safety mechanism 356 as disclosed in
U.S. Patent
8,702,653.
CA 03026708 2018-12-05
WO 2017/204787 25 PCT/US2016/033950
Such a needle retraction safety mechanism 356 may be assembled to the syringe
barrel 340, for example as part of the barrel adapter assembly 350, through
the distal
end of the barrel 340. The control mechanism 10 is generally attached to the
barrel 340
after the drug chamber 338 of barrel 340 has been filled with a drug fluid.
This is often
desired so that the syringe 300 may be filled and assembled in standard
pharmaceutical
fill-finish process lines. Once the syringe 300 has been filled and assembled,
the control
mechanism 10 may be utilized by the user to identify and set drug dose for
delivery.
The user may then inject the needle into the patient for drug delivery.
Subsequently, the
button 12 and/or plunger 14 may be depressed by the user to cause the plunger
14 and
screw 30 to axially translate. Because of the function of the control
mechanism and the
pitch ratio, any measure of distal translation of the plunger 14 causes only
an
incremental measure of distal translation of the screw 30, permitting accurate
dose
delivery control by the user. Axial translation of the screw 30 causes axial
translation of
the plunger seal 336. This axial motion in the distal direction of the plunger
seal 336
forces drug fluid out of drug chamber 338 of barrel 340, through the needle
354 of the
barrel adapter assembly 350, for injection and delivery to the patient. At the
end of drug
delivery, the plunger seal 336 is caused to contact a component of the needle
retraction
safety mechanism 356 to initiate the retraction mechanism thereby causing
retraction of
the needle 354 into the barrel 340 of syringe 300. The screw 30 and other
components
or the control mechanism 10 may be configured or adjusted to permit this
additional
range of axial translation in the distal direction after the desired drug dose
has been
delivered. As the needle 354 is then retracted into the barrel 340 of syringe
300,
components of the needle retraction safety mechanism 356 bear and push against
plunger seal 356 in the proximal direction. As that retraction force is
continued, the user
may control the rate of needle retraction by controllably reducing the force
they apply
on the button 12 and/or plunger 14 as the screw 30 and plunger 14 move in the
proximal
direction. The needle retraction safety mechanism 356 therefore provides a
number of
additionally desirable features to the novel syringes of the present
invention.
As would readily be appreciated by one having ordinary skill in the art, the
barrel adapter assembly may be attached, mounted, affixed, or otherwise
connected to
the distal end of the barrel by a number of known methods. For example, a luer
connection may be utilized to connect the barrel adapter assembly to the
syringe barrel.
Luer connection systems are a standard way of attaching syringes, catheters,
hubbed
needles, IV tubes, and the like to each other. Luer connections consist of
conical/tubular
CA 03026708 2018-12-05
WO 2017/204787 26 PCT/US2016/033950
male and female interlocking components slightly tapered to hold together
better. Luer
connections can either be a "luer slip", as shown in FIGS. 4A and 4B, which
are luer
connections with a simple pressure or twist fit; or luer connections be a
"luer lock", as
shown in FIGS. 5A and 5B, which can have an additional outer rim of threading
allowing them to be more secure. Alternatively, the connection may be
facilitated by a
barrel adapter connection. By way of example, the barrel adapter connection
may be as
described in International Publication W02011/137488 and/or U.S. Patent
8,702,653,
although without limitation thereto. Luer connections, interference fit
connections,
barrel adapter connections, or any number of other known connections may be
utilized
to attach the barrel adapter assembly to the barrel while remaining within the
breadth
and scope of the present invention. Regardless of the type of barrel adapter
assembly
utilized, the barrel adapter assembly generally comprises of a barrel tip 152,
252, 352
and a needle 154, 254, 354, respectively. In some configurations, the barrel
tip 152,
252, 352 may be a pre-formed aspect at the distal end of the barrel.
Alternatively, the
barrel tip 152, 252, 352 may be a separate component that is attached at the
distal end of
the barrel. The needle 154, 254, 354 may be any type of fluid conduit
including, for
example, a flexible cannula or a rigid needle, and may be made of any number
of
materials, including stainless steel. The type of connections described herein
can be
utilized regardless of the type of syringe with which they are shown. For
clarity, the luer
slip connection shown with the fill-at-time-of-use syringe in FIGS. 4A and 4B
may be
utilized with the pre-filled syringe in FIGS. 5A and 5B, or any other type of
connection
may be used with any other type of syringe described herein.
It will be appreciated from the foregoing that the novel dose control
mechanisms
and syringes disclosed herein provide an efficient and easily operated system
for the
accurate dose setting and delivery of drug treatments. Such devices are safe
and easy to
use, and are aesthetically and ergonomically appealing for clinical
practitioners. The
embodiments of the present invention overcome the challenges faced with the
use of
conventional syringes for the dosing and delivery of low-volume treatments by
utilizing
novel dose control mechanisms. The novel dose control mechanisms permit the
user to
accurately read and dose the desired volume of drug treatment for delivery to
the
patient. These devices permit the user to have a normal range of thumb travel,
as they
may otherwise expect with a conventional syringe, but transform that range of
thumb
travel to a very finite (e.g., smaller or incremental) range of plunger seal
travel. This
CA 03026708 2018-12-05
WO 2017/204787 27 PCT/US2016/033950
relationship allows the user to utilize the syringe without additional
training, but with
the significant benefit of incremental, low-volume dose control.
Assembly and/or manufacturing of control mechanism 10, syringe 100, syringe
200, or syringe 300, or any of the individual components may utilize a number
of
known materials and methodologies in the art. For example, a number of known
cleaning fluids such as isopropyl alcohol and hexane may be used to clean the
components and/or the devices. A number of known adhesives or glues may
similarly
be employed in the manufacturing process. For example, a glue or adhesive may
be
utilized to connect the distal end of the housing 20 to the proximal end of
adapter 18.
Similarly, a glue or adhesive may be utilized to connect the distal end of
adapter 18 to
the proximal end of the barrel. Additionally, known siliconization fluids and
processes
may be employed during the manufacture of the novel components and devices.
Furthermore, known sterilization processes may be employed at one or more of
the
manufacturing or assembly stages to ensure the sterility of the final product.
In one embodiment, a method of assembling the control mechanism includes the
steps of:
(i) threading a fine pitch screw at least partially through a fine pitch
nut of an
adapter;
(ii) threading a plunger, the plunger having a coarse pitch screw on its
outer surface
and an annular space within its inner surface, at least partially through an
interior axial pass-through of housing, wherein the housing interior has a
corresponding coarse pitch guide;
(iii) inserting at least a proximal portion of the screw into the annular
space of the
plunger through a distal portion of the plunger; and
(iv) attaching
the outer distal portion of the housing to a proximal aspect of the
adapter.
Additionally, the plunger may include a button at its proximal end. The button
may be a pre-formed aspect of the plunger or may be a separate component from
the
plunger. Preferably, the button is a separate component attached to plunger
by, for
example, snap-fit. Similarly, the housing may include a housing cover at its
proximal
end. The housing cover may be a pre-formed aspect of the housing or may be a
separate
component from the housing. As discussed above, a glue or adhesive may be
utilized to
affix one or more components of the control mechanism to each other.
Alternatively,
one or more components of the control mechanism may be a unified component.
For
CA 03026708 2018-12-05
WO 2017/204787 28 PCT/US2016/033950
example, the housing may be a separate component affixed by a glue to adapter,
or the
adapter may be a preformed aspect at the distal end of the housing which is
glued to the
barrel. Similarly, the housing cover may be affixed by a glue to the housing.
These
components may be sterilized individually or together, and may be assembled in
a
sterile environment or sterilized after assembly. The barrel may be
siliconized prior to
or after assembly.
The control mechanism may be utilized as a component of a syringe. In one
embodiment, the method of manufacturing a syringe comprising a control
mechanism
includes the steps of:
(i) mounting a barrel adapter assembly to a distal end of a syringe barrel;
(ii) mounting a plunger seal through a proximal end of the syringe barrel;
and
(iii) mounting a control mechanism to the proximal end of the syringe
barrel,
wherein the control mechanism may rest in contact with the plunger seal.
The method of manufacturing a syringe may further comprise, before the step of
(ii) mounting a plunger seal through a proximal end of the syringe barrel, the
step of:
filling the barrel at least partially with a fluid substance. Step (iii) may
further require
the step of connecting a screw connection aspect of a screw of the control
mechanism
directly to the plunger or indirectly through a plunger rod which is connected
at the
proximal end of the plunger seal. The connection between the plunger rod and
the
plunger seal may be any number of connections including, but not limited to,
screw-
type connection, snap-fit connections, interference connections, capture
connections,
and the like. In at least one embodiment, the screw connection aspect is
connected to
the plunger rod through a radial opening or a proximal opening in the plunger
rod such
that the screw connection aspect sits within a proximal pocket in the plunger
rod.
Additionally or alternatively, this connection may be a snap-fit connection,
an
interference-fit connection, or a number of other connection methods known in
the
industry. Preferably, the connection between the screw and the plunger seal,
or between
the screw and plunger rod when a plunger rod is employed, is such that the
screw is
permitted to axially rotate while the plunger rod and/or the plunger seal
remain
rotationally fixed.
One preferred method of manufacturing a syringe having a dose control
mechanism, according to one embodiment of the present invention, is described
herein
with reference to FIGS. 7A-7D. FIG. 7A shows a pre-filled syringe, such as
that
described with reference to FIGS. 5A-5B above, wherein the adapter is a
CA 03026708 2018-12-05
WO 2017/204787 29 PCT/US2016/033950
two-component adapter having a proximal adapter portion 418P and a distal
adapter
portion 418D. Proximal adapter portion 418P has one or more connection prongs
418E
and distal adapter portion 418D has corresponding connection ports 418F. When
forced
together, connection prongs 418E and corresponding connection ports 418F
merge,
mate, or otherwise connect to unite the two portions of the adapter 418P,
418D.
Initially, a cap 460 may be connected to the distal end of barrel 440 of
syringe 400. The
distal adapter portion 418D may be slidably mounted to the exterior of the
barrel. The
interior of the barrel 440, i.e. the drug chamber 438, may be filled with a
drug fluid or
substance through the open proximal end of the barrel. The plunger seal 436
may be
mounted into the barrel through the proximal end such that is in contact with
the fluid.
The optional plunger rod 434 may be connected to the plunger seal 436 prior
to, or
after, insertion of the plunger seal 436 into the barrel 440. These steps may
be
performed in a sterile environment to maintain the container integrity and
sterility of the
drug treatment.
The remainder of the syringe may then be assembled in a non-sterile or sterile
environment. The screw, as a component of the control mechanism, may then be
connected to the plunger seal or to the plunger rod when a plunger rod is
employed. The
distal adapter portion 418D may then be slid in the proximal direction along
the exterior
of the barrel to connect to the proximal adapter portion 418P as described
above. The
connection between the distal adapter portion 418D and the proximal adapter
portion
418P may capture a barrel flange 440A aspect of the barrel 440 in order to
retain the
control mechanism 10 at the proximal end of the barrel 440. Various glues or
adhesives
may be utilized to ensure that such components and connections are retained in
position
during assembly, filling, manufacturing, transportation, storage, and
operation of the
novel devices of the present invention. The final assembly of the syringe,
such as in the
pre-filled syringe 400, may appear as shown in FIG. 7B. This type of pre-
filled syringe
may be utilized when, for example, a syringe is to be filled with a standard
amount of
drug fluid by a pharmaceutical company or contract drug filler, when the drug
dose is
variably selectable by the user, when the needle length is variably selectable
by the user,
or in a number of other situations. FIG. 7C shows the pre-filled syringe with
a
selectable needle that is attached via a luer lock connection, as described
above. In such
a scenario, the syringe may be held such that the distal end of the syringe is
pointed
upwards. The cap 460 (shown in FIG. 7B) may be removed and replaced by a
barrel
adapter assembly 450. The barrel adapter assembly 450 includes a barrel tip
452 and
CA 03026708 2018-12-05
WO 2017/204787 30 PCT/US2016/033950
needle 454 which may be selected by the user and attached to the pre-filled
syringe just
prior to use. The drug dose may be identified and selected by the user, as
described
above. Comparison of the pre-filled syringe 400 in FIGS. 7C and 7D clarifies
the
differences in the pre-filled syringe just prior to, and after, injection and
delivery of the
drug dose to the patient. Because of the pitch ratio between the plunger 14
and the
screw 30, screw 30 is caused to axially translated in the distal direction
only
incrementally or to a lesser distance when plunger 14 is depressed or axially
translated
in the distal direction (i.e., in the direction of solid arrow in FIGS. 7C and
7D). This
difference in axial translation distance between plunger 14 and screw 30 is
visible by
comparing distances D3 and D4 in FIGS. 7C and 7D. D3 is the distance that
plunger 14
axially translates while D4 is the fractional distance that screw 30 axially
translates.
Yet another embodiment of a syringe 500 incorporating a dose control
mechanism 510 is illustrated in FIGS. 8A-8C. The embodiment of FIGS. 8A-8C
offers
advantages of both the dose control mechanisms of the earlier-described
embodiments,
and the advantages of conventional syringes, as will be explained below. FIGS.
8A,
8B, and 8C show cross-sectional views of the dose control mechanism in a ready-
to-
inject stage, partially injected stage, and in an end-of-dose stage,
respectively, while
FIGS. 9A and 9B illustrate partially exploded views of the dose control
mechanism 510
of this embodiment.
As with the earlier-disclosed embodiments, the dose control mechanism 510 for
a syringe 500 includes a plunger 514, a housing 520, an adapter 518, and a
screw 530.
The housing 520 has a substantially cylindrical axial pass-through within
which the
substantially cylindrical plunger 514 may at least partially reside. The
distal end of the
housing 520 includes the adapter 518. The housing 520 and the adapter 518 of
this
embodiment are formed as a unitary structure, the adapter 518 presenting a
finger flange
for engagement by a user during operation. It will be noted, however, that the
housing
520 and adapter 518 may be separately formed, as illustrated with regard to
other
embodiments.
The adapter 518 may couple the dose control mechanism 510 to the barrel 540
of a syringe 500 by any appropriate structure. In the illustrated embodiment,
adapter
518 is coupled to the barrel 540 by way of an insert 518A, which is received
in a
laterally extending opening 518B in the adapter 518. While the insert may be
of any
appropriate design, the illustrated insert 518A includes a gasket 518C and a
positioning
insert 518D. The barrel 540 of the syringe 500 is received within an opening
in the
CA 03026708 2018-12-05
WO 2017/204787 31 PCT/US2016/033950
gasket 518C, with the barrel flange 540A disposed along an upper surface of
the gasket
518C. The gasket 518C and the barrel 540 are inserted through an opening in
the
positioning insert 518D that may be slidably received within the laterally
extending
opening 518B of the adapter 518; laterally extending flanges 518E may serve to
maintain the insert 518A and the associated barrel 540 in position. Thus, in
assembly,
the barrel 540 may be inserted into openings in the gasket 518C and
positioning insert
518D, and then slide into position within the laterally extending opening 518B
in the
adapter 518.
As with the above embodiments, the screw 530 may be coupled to a plunger rod
534 in any appropriate manner, either directly or indirectly. For example, as
with the
embodiments of FIGS. 1A-7D, a connection aspect 530A of the screw 530 may be
received within a recess 534A at the proximal end of the plunger rod 534. In
the
illustrated embodiment, the distal end of the plunger rod 534 is coupled to a
plunger
seal 536 by a screw connection, although alternate connections known in the
art may be
provided. Likewise, the plunger seal 536 may be of any appropriate design. For
example, in the illustrated embodiment a plurality of ring seals 536A are
disposed
within a corresponding plurality of peripheral recesses 536B of the plunger
seal 536.
Housing 520 may optionally include housing cover 516 at its proximal end, for
example, to close the interior of the housing 520 off from the environment
and/or to
axially align plunger 514 within housing 520, and to prevent removal of the
plunger rod
by functioning as a mechanical stop.
Housing 520 may further include a dosage reference arrangement. For example,
as discussed in greater detail above, the housing 520 may be provided with a
window
520A (see FIG. 9B) to permit the user to view the location of the plunger 514
within
housing 520 by viewing the location of one or more dose markings on the
external
surface of the plunger 514. While not illustrated in detail in this
embodiment, those of
skill in the art will appreciate that the same or a similar arrangement may be
provided in
this embodiment as in the earlier embodiments.
The plunger 514 may include a button 512 presenting a user interface surface
512A for engagement by a user to translate the plunger 514 axially within the
housing
520. The button 512 and plunger 514 may be a unitary component, or separate
components. For example, button 512 may be a preformed aspect at the proximal
end of
the plunger 514. Alternatively, button 512 may be a separate component
attached to the
proximal end of plunger 514 by a snap-fit. In at least one embodiment, the
button 512
CA 03026708 2018-12-05
WO 2017/204787 32 PCT/US2016/033950
may be attached to plunger 514, but allowed to rotate freely about the
proximal end of
plunger 514. In this way, the button 512 may be rotationally fixed relative to
the
user's/clinician's finger while permitting the plunger 514 to rotate as the
plunger
translates axially.
The plunger 514 may additionally include a plunger dial 517 that may provide
an alternative or additional structure by which to manipulate the plunger 514.
In the
illustrated embodiment, for example, the plunger dial 517 is secured with the
plunger
514. As a result, by rotating the plunger dial 517, a user may directly rotate
the plunger
514 as desired. In this way, the plunger dial 517 may be rotated to either
draw in
medication or administer medication, depending upon which direction the
plunger is
rotated.
As with the embodiments of FIGS. 1A-7B, the screw 530 is disposed at least
partially within an axially extending channel 514C within the plunger 514. As
illustrated in FIG. 1B with regard to screw 30 and plunger 14, a proximal
length 530C
of the screw 530 is axially keyed with the plunger 514 for sliding relative
movement in
an axial direction. In this way, an axial rotation of the plunger 514 results
in an axial
rotation of the screw 530. As with the embodiments described above, those of
skill in
the art will appreciate that the axial keying may be other than as
specifically illustrated
in FIG. 1B.
As with the earlier-discussed embodiments, a distal length 530B of the screw
530 is externally threaded for complimentary engagement with an internally
threaded
portion 518F of the adapter 518. As a result, rotation of screw 530, as may
result from
the rotation of the axially keyed plunger 514, will result in rotation of the
screw 530
within the adapter 518. As the axial direction in which the screw 530
translates will be
dependent upon the rotational direction of the screw 530, the translation of
the
associated plunger rod 534 and plunger seal 536 likewise will be dependent
upon the
rotational direction of the screw 530.
The plunger 514 is received within a longitudinally extending channel 520D
within the housing 520. In order to provide axial and rotational movement of
the
plunger 514 relative to and within the housing 520, the longitudinally
extending channel
520D and plunger 514 are coupled by an engaging screw thread arrangement. To
this
end, one of the longitudinally extending channel 520D and plunger 514 includes
a
length of thread, while the other of the longitudinally extending channel 520D
and the
plunger 514 includes at least one thread segment disposed to engage the coarse
thread.
CA 03026708 2018-12-05
WO 2017/204787 33 PCT/US2016/033950
In the illustrated embodiment, the longitudinally extending channel 520D
includes a
thread 520C, while the plunger 514 includes a thread segment 514B; in this
case, as the
thread 520C of the longitudinally extending channel 520D is double threaded,
the
plunger 514 includes a pair of thread segments 514B.
Those of skill in the art will appreciate, however, that in at least one
embodiment, the configuration of engaging screw pitch relative to the plunger
514 and
housing 520 may be reversed. In other words, the outer surface of the plunger
514 may
include a length of coarse thread, while the longitudinally extending channel
520D
includes at least one thread segment disposed to engage the thread of the
plunger 514.
Those of skill in the art will further appreciate that the thread segment
along the inner
surface of the longitudinally extending channel 520D may include a segment of
a screw
thread recess within which the external thread of the plunger 514 may ride as
it rotates
and translates axially. For the purposes of this disclosure, the term "thread
segment"
will include both a thread recess that may receive a thread, and a thread that
may be
received within a thread recess.
According to an aspect of the embodiment of FIGS. 8A-9B, the engagement
between the plunger 514 and the housing 520 is provided by way of a variable
pitch
thread 520C, rather than a uniform coarse pitch thread, as illustrated with
regard to the
embodiment of FIGS. 1A-3B. In this way, it is possible to more precisely
tailor the
rotation of the plunger 514 relative to the axial translation of the plunger
514 within the
housing 520, and, as a result, the rotation and axial translation of the screw
530 relative
to the housing 520. Additionally, this arrangement may allow the user to fill
the syringe
more easily and quickly.
For the purposes of this disclosure, the term "variable pitch thread" 520C
means
that the thread includes at least two thread pitches for engagement by the
thread
segment(s) 514B (see FIG. 9B). A first thread pitch 520E is disposed toward
the distal
end of the housing 520, while a second thread pitch 520F is disposed
proximally within
the housing 520 from the first thread pitch 520E.
In this embodiment, the first thread pitch 520E is a coarse pitch thread, as
illustrated with regard to the embodiment of FIGS. 1A-3B, while the second
thread
pitch 520F is a relatively finer pitch than that of the first thread pitch
520E. As a result,
as the plunger 514 is rotated and the thread segment 514B engages the first
pitch thread
520E, the plunger 514 will move in an axial direction as explained above with
regard to
the embodiment of FIGS. 1A-3B. In other words, as the user advances the
plunger 520
CA 03026708 2018-12-05
WO 2017/204787 34 PCT/US2016/033950
with the thread segments 514B engaging the first thread pitch 520E, the
plunger 520
will move axially a greater distance than the keyed, relatively smaller pitch
screw 530.
Conversely, when the user advances the plunger 520 with the thread segments
514B
engaging the second thread pitch 520F, the thread pitches of the plunger 520
and the
screw 530 are more closely aligned. As a result, the axial distances traveled
by the
plunger 520 and the screw 530 will be more closely matched. In this way, when
the
user moves the plunger 520 in a proximal direction at a relatively constant
speed to
draw a medication into the barrel 540, medication will initially be drawn
slowly into the
barrel 540 as the thread segments 514B are disposed in the first thread pitch
520E, and
more rapidly when the thread segments 514B are disposed in the second thread
pitch
520F. Thus, the variable pitch thread 520C facilitates the more rapid draw of
larger
volumes of medication than the embodiment of FIGS. 1A-3B. Likewise, when the
plunger 520 is depressed to prime the syringe or administer medication, the
rate at
which the plunger seal 536 moves within the barrel 40 will be dependent upon
the
location of the thread segments 514B within the variable pitch thread 520C,
allowing an
initially more rapid prime, with a slower, final delivery of medication. In at
least one
embodiment, upon completion of the priming step of operation, thread segments
514B
are disposed in the first thread pitch 520E of the variable pitch thread 520C.
This allows
the medicament to be accurately delivered to the target using the mechanical
advantages
described above with reference to FIGS. 1A-3B.
Turning to FIG. 8A, the syringe 500 is illustrated with the plunger 514 in a
full
draw position. It will be noted that the thread segment 514B is disposed in
the second
thread pitch 520F portion of the variable pitch thread 520C. As the plunger
514 is
depressed, for example, to prime the syringe 500, the thread segment(s) 514B
traverses
the second thread pitch 520F of the variable pitch thread 520C, generally
moving into
the first thread pitch 520E, as illustrated in FIGS. 8B. During priming, air
is typically
expelled from the barrel 540, possibly along with a small amount of
medication. It will
be appreciated that, as the thread segment 514B enters the first thread pitch
520E, the
rotational speed of the screw 530 will decrease for a uniform axial movement
of the
plunger 514. In this way, the user may accurately prime the syringe 500 to a
desired
dosage. As the user then depresses the plunger 512 or rotates the plunger dial
517, the
primed dosage may be delivered, the thread segment 514B rotating relative to
the first
thread pitch 520E of the variable pitch thread 520C to provide a rotating
movement to
the screw 530 for delivery of the medication (see FIG. 8C). In this way, the
syringe may
CA 03026708 2018-12-05
WO 2017/204787 35 PCT/US2016/033950
be quickly filled and primed. For example, the pitch ratio of the first thread
pitch 520E
to the adapter nut may be 1:1. Thus, when thread segment 514B is disposed in
first
thread pitch 520E, axial translation of the plunger seal 536 will be equal to
the axial
translation of the plunger 512. This allows the syringe to perform as a
standard syringe
during the filling and priming steps, providing a familiar experience for
healthcare
professionals. After priming, with thread segments 514B disposed in second
thread
pitch 520F, the syringe provides for fine control of the volume of medicament
administered as described above with reference to FIGS. 1-7B.
It will thus be appreciated by those of skill in the art that the variable
pitch
thread 520C may thus be tailored to provide a desired rotational, and,
therefore, axial
movement of the screw 530 for a relatively uniform axial movement of the
plunger 512.
Further, it will be appreciated that variable pitch thread 520C may include
greater than
two pitches. For example, the variable pitch thread 520C may include three or
more
different pitches. The pitch may transition along its entire length, gradually
going from
one pitch to another, such as gradually transitioning from a coarse pitch to a
fine pitch.
Further, the variable pitch thread 520C may include a transitional pitch
between
different pitches, such as a transition pitch between a coarse pitch and a
fine pitch.
While FIGS. 8A-9B illustrate a unitarily formed housing 520, it will be
appreciated that housing 520 may be constructed of a plurality of components.
Those
of skill in the art will further appreciate that a housing that includes a
plurality of
components may not only facilitate manufacturing and assembly, but also
enhance the
customization options and functionality of the device.
For example, the housing 520 may include a lower housing and an upper
housing. FIG. 15, for example, shows an exemplary embodiment of the present
invention having a housing 520 including a lower housing 522 and an upper
housing
524. The lower housing 522 and the upper housing 524 may be assembled together
by,
for example, including threaded engagement, snap fit, interference fit,
hook/prong and
window engagement (as shown in cross-sectional FIG. 15) or in a broad range of
known
methodologies. As shown in FIG. 15, upper housing 524 may include one or more
hooks 524A configured to engage one or more windows 522A of lower housing 522.
In
yet another embodiment the adapter 518 may be formed separately from the lower
housing, and assembled to the lower housing 522.
In addition to aiding the manufacture and assembly of these components,
bifurcating or even trifurcating the housing 520 into multiple components may
have
CA 03026708 2018-12-05
WO 2017/204787 36 PCT/US2016/033950
additional functional benefits. For example, the pitch ratios of the
individual upper and
lower housings 524, 522 may be varied to provide customization options,
enabling
different accuracy or tuning of dose delivery. Thus, the remaining portions of
the
device may be uniform structures, regardless of the dose accuracy parameters,
but
specifically desired dose accuracy of each device may be altered simply by
changing or
selecting the correct lower and upper housing, along with the interfacing
screw-portion
of the plunger rod. Accordingly, the device may have substantial
customizability while
minimizing the components that need to change to meet the exact desired
delivery
parameters. Further customization may be provided by varying, for example, the
pitches of the screw 530 and the internally threaded portion 518F of the
adapter 518. In
such an arrangement, the adapter 518 may be formed with the lower housing 522
as
illustrated, or separately formed from and assembled to the lower housing 522.
In this
way, the device may be further customized by changing the adapter 518 and the
screw
530. Those of skill will appreciate that such an arrangement may provide a
wide array
of options in customizing the device through the utilization of a number of
standardized
components that may be mixed and matched to provide the desired delivery
parameters.
Varying these components can also permit the manufacturer, pharmaceutical
company,
or user to alter other parameters, such as drug delivery metering, applied
forces, and fill
volume, since all are dependent at least in part in the selected pitch ratio
of these sub-
components.
Similarly, the functions of one or more components may further be separated
into separate subcomponents. For example, the housing may be further sub-
divided
such that the upper housing has an inner upper housing and an outer upper
housing.
The inner upper housing in such an instance could include the screw-threaded
portion
and interface with the outer upper housing. This may further aid the
manufacturing and
assembly of the device, and/or improve the range of customization of the
devices by
replacement of just one sub-component. In this example, the inner upper
housing could
be readily replaced to alter the screw threading and, accordingly, the
accuracy or tuning
of drug delivery. Additionally, or alternatively, one or more components could
be
modified to serve the function of, a function similar to, or supplement the
function of,
another component described herein. For example, in at least one embodiment
the
cover 516 may be modified to incorporate a screw-threaded portion that
supplements
the screw-threaded portion of the upper housing. This may be utilized to
provide
further axial translation of the plunger and/or may be utilized to provide
another portion
CA 03026708 2018-12-05
WO 2017/204787 37 PCT/US2016/033950
of the plunger having a varied pitch ratio. In yet another embodiment of the
present
invention, the threaded cover may be elongated and combined with the inner
upper
housing such that the cover has a threaded portion that extends substantially
the length
of the housing or upper housing. Accordingly, the threaded portion of the
housing
could be a separate component from the housing outer. As a result, the
threaded portion
may be easily replaced.
Additionally, the threaded portions of the device and/or its sub-components
may
have any range of thread profiles or cross-sectional configurations. For
example, FIGS.
8A-9B illustrate configurations utilizing a rectangular thread profile. The
embodiments
shown in FIGS. 10A-11B illustrate configurations utilizing a triangular thread
profile.
The term profile in this sense is meant to refer to the cross-sectional shape
of each
thread of the screw-threaded portions of the device.
The thread profile or shape may be selected to meet the desired parameters of
the functioning device. For example, a triangular thread profile may reduce
the glide
forces felt by the user and provide a less sticky engagement between the
corresponding
threaded components. This may be because the engagement surfaces of the
corresponding threaded components are altered or occur at a different plane
that are
perceived by the user as more easily tactile or operated. Additionally, a
triangular thread
profile may enable more rotations in a smaller axial length. This may provide
finer
accuracy, tuning, or volume control to the device. Accordingly, while the
embodiments
of the present invention show a rectangular/squared thread profile or a
triangular square
profile, a number of thread profiles may be utilized by the present device
while
remaining within the scope of the presently claimed invention. Similarly, the
thread
direction may be altered while remaining within the scope of the presently
claimed
invention.
In the embodiments shown in FIGS. 10A-14, dose control mechanism 610
further includes a housing 620 having at least first and second housings 668,
670 which
are adapted for selective telescoping movement relative to one another between
a
retracted position and an extended position. In this way, the dose control
mechanism
610 may be provided in a retracted position, and then extended to draw a
medicament
into the barrel. In at least one embodiment, the first and second housings
668, 670 may
then be translated relative to one another to a primed position from which the
medicament may be administered. A first such embodiment is illustrated in
FIGS. 10A-
11B, and a second such embodiment is illustrated in FIGS. 12A-14. A difference
CA 03026708 2018-12-05
WO 2017/204787 38 PCT/US2016/033950
between the first and second such embodiments is the mechanisms by which the
relative
motion between the first and second housing sections 668, 670 are governed.
Accordingly, the same reference numbers are utilized for like components
between the
two embodiments. Those of skill in the art will appreciate that the
illustrated dose
control mechanism 600 of FIGS. 12A-12E may be coupled to the barrel of a
syringe in
a manner similar to the embodiment of FIGS. 10A-10F.
More specifically, in the illustrated embodiments, the second housing section
670 is positioned between the first housing section 668 and the plunger 614.
In such an
embodiment, the second housing section 670 includes an internal thread
670D¨which
can be either constant pitch or variable pitch¨configured to engage the
external thread
segments 614B of the plunger 614. The second housing section 670 is configured
such
that, in a first configuration, it is able to axially translate with respect
to the first housing
section 668. In a second configuration, the sleeve 670 is fixed in relation to
the first
housing section 668. As will be explained below, this allows a syringe to be
quickly
filled and primed in a way that is familiar to the user when the first and
second housing
sections 668, 670 are moved from the retracted position to the extended
position and
then to the primed position, while providing accurate dose control during
delivery.
To fill the syringe, the user pulls the button 612 in the proximal direction.
This
causes the plunger 614, second housing section 670, screw 630, plunger rod
634, and
plunger seal 636 to translate in the proximal direction relative to the first
housing
section 668 from the retracted position illustrated in FIGS. 12A, and 13A, to
the
extended position illustrated in FIGS. 10A, 10B, 12B, 13B, thereby drawing
fluid
contents into the barrel of the syringe. After filling the syringe, the user
may prime the
syringe by depressing the button 612 in the distal direction. This causes the
plunger 614,
second housing section 670, screw 630, plunger rod 634, and plunger seal 636
to move
as a unit and expel a portion of the fluid contained in the barrel (this
position is shown
in FIGS. 10C, 10D, and 12C). At the completion of this priming movement, the
second
housing section 670 engages the first housing section 668 such that the second
housing
section 670 cannot rotate or translate with respect to the first housing
section 668. In
this configuration, the dose control mechanism functions in like manner to the
embodiment described above with reference to FIGS. 1A-7B.
FIGS. 10A-11B illustrate one embodiment of a locking mechanism to restrict
relative movement of the second housing section 670 with respect to the first
housing
section 668. Second housing section 670 may include guide boss 670A and
locking tab
CA 03026708 2018-12-05
WO 2017/204787 39 PCT/US2016/033950
670B. First housing section 668 may include longitudinal slot 668H. As can be
seen in
FIG. 10B, as second housing section 670 is translated proximally to fill the
syringe,
both guide boss 670A and locking tab 670B are disposed within longitudinal
slot 668H.
This restricts rotation of the second housing section 670 with respect to the
first housing
section 668. As can be seen in FIG. 10D, as second housing section 670 is
translated in
the distal direction, for example to prime the syringe, locking tab 670B has
engaged
first housing section 668 to restrict subsequent translation of the second
housing section
670 with respect to first housing section 668. Additionally, or alternatively,
interaction
of guide boss 670A with longitudinal slot 668H may restrict distal translation
of second
housing section 670 with respect to first housing section 668.
In at least one embodiment, illustrated in FIGS. 12A-14, second housing
section
670 has a track 672 which engages a guide aspect of the first housing section
668.
Initially, the guide aspect 668G is disposed in first portion 672A of track
672, as seen in
FIG. 13A. As the plunger 614, second housing section 670, screw 630, plunger
rod 634,
and plunger seal 636 are translated in the proximal direction from the
retracted position
of FIGS. 12A and 13A, the interaction of the guide aspect 668G and track 672
causes
the second housing section 670 to rotate with respect to the first housing
section 668 to
the position shown in FIGS. 12B and 13B. That is, in addition to translating
axially, the
second housing section 670 rotates relative to the first housing section 668.
Subsequently, distal translation of the second housing section 670 relative to
the first
housing section 668 results in the guide aspect 668G traversing the second
portion 672B
of the track 672 as the second housing section translates from the extended
position
illustrated in FIGS. 12B and 13B to the primed position shown in FIGS. 12C and
13C.
The track 672 may include a locking aspect 672C which engages the guide aspect
to
restrict further translation of the second housing section 670 with respect to
the first
housing section 668.
During the steps of filling and priming, rotation of second housing section
670
and plunger 614 may be coupled to prevent relative rotation therebetween. For
example,
as shown in FIGS. 12A-14, dose control mechanism 610 may include coupler 680,
which is in a keyed relationship with plunger 614. During the steps of filling
and
priming, tab 680A of coupler 680 may be disposed within notch 670F of second
housing section 670, thereby restricting rotation of the second housing
section 670 with
respect to the coupler 680. Because of the keyed relationship of the coupler
680 and
plunger 614, this engagement also prevents rotation of the second housing
section 670
CA 03026708 2018-12-05
WO 2017/204787 40 PCT/US2016/033950
with respect to the plunger 614. The user may disengage the tab 680A of the
coupler
680 from the notch 670F of the second housing section 670 by translating
coupler 680
in the proximal direction (compare FIG. 12C and FIG. 12D). With the coupler
680 in
this position, the dose control mechanism may be operated as described
previously, the
plunger 614 rotating relative to the housing 620 the tab 680A riding along an
upper
ramped surface of the housing 620, to rotate the screw 630 to expel the
contents of the
syringe barrel. The remaining elements of the dose control mechanism 610 of
FIGS.
12A-14 are substantially as illustrated in FIGS. 10A-11B.
In any of the described embodiments, the dose control mechanism may include
one or more additional threaded components. This may provide additional
mechanical
advantage to the user. For example, the dose control mechanism may include an
inner
plunger and an outer plunger. The outer plunger has an external thread
engaging an
internal thread of the housing and an internal thread engaging an external
thread of the
inner plunger. The inner plunger also has an internal thread engaging the
screw. In this
way, the ratio of displacement between the knob and the plunger seal may be
increased.
According to yet another feature, some embodiments may provide tactile
feedback to the user, for example, in connection with the identification of
the desired
delivery volume. In this way, when the user dials the plunger rod/screw to
their desired
dose volume (e.g., when the plunger 514 is rotated until a particular
microliter setting is
visible in the window 520A), the user will feel a tactile notch or stop-point
to signal the
positioning for a preset dose volume. The dose control mechanism 510 may be
provided with multiple volume-based detents to indicate various dose volumes.
By way
of example only, the dose control mechanism 510 may include such feedback for
syringe delivery volumes of 20 microliter, 10 microliter, and 5 microliter.
While the tactile feedback may be provided by any appropriate arrangement, one
such embodiment is illustrated in FIGS. 16A-16C. For example, the housing 520
and
plunger 514 may include structures that, when aligned provide a tactile
feedback. As
most clearly shown in FIG. 16C, the housing 520 may include a protrusion 526
and the
plunger 514 may include at least one recess 514B, which, when aligned, offer
the user a
variation in the normal rotation of the plunger 514.
The recess 514B in the plunger 514 may be formed by any appropriate method.
For example, the recess 514B may be formed by adivot, or a bore extending
through
the wall of the plunger 514.
CA 03026708 2018-12-05
WO 2017/204787 41 PCT/US2016/033950
Similarly, the protrusion 526 may be provided by any appropriate structure,
such
as, a molded formation on the inner wall of the housing 520. In the
illustrated
embodiment, however, the housing 520 includes at least one radially extending
aperture
520G through which a prong extends radially inward to provide the protrusion
526. In
this embodiment, a pair of apertures 520G and a pair of protrusions 526 are
provided.
The protrusions 526 may extend from a separate clip 528 that may be attached
to the
outer surface of the housing 520, as illustrated in FIGS. 16A-C.
It will be appreciated that any number of such clips 528 may be provided, at
locations along the length of housing 520, to identify a corresponding number
of desired
set-points/stop-points identifying preset dose volumes. Alternatively or
additionally,
the plunger 514 may be provided with any number of recesses 514B that
correspond to
preset dose volumes. As the user axially rotates the plunger rod/screw to dial
their
desired delivery volume, the protrusions 526 extending radially from the clip
528 are
caused to contact/engage the recess 514B which corresponds to a defined set-
point/stop-
point. The recesses 514B and protrusions 526 are dimensioned such that each
corresponds with a preset dose volume in the syringe for drug delivery.
Any of the dose control mechanisms described above can be used in conjunction
with such a mixing syringe. Because the dose control mechanisms described
herein
allow for proximal translation of the plunger rod with respect to the drug
container, they
are particularly well-suited for such a mixing syringe.
Accordingly, the novel embodiments of the present invention provide dose
control mechanisms, which allow for the accurate dosing and delivery of drug
treatments, and drug delivery syringes which incorporate such control
mechanisms.
Such novel devices permit the identification and control of the dosage amount,
permit
the syringe to be "primed" (i.e., evacuated of air bubbles) prior to drug
delivery, and
ensure the accurate delivery of microliter volume dosages, all within a device
size that
is similar to commonly used conventional syringes available in the
marketplace. Such
novel devices are safe and easy to use, and are aesthetically and
ergonomically
appealing for clinical practitioners. The novel devices of the present
invention provide
these desirable features without any of the problems associated with known
prior art
devices.
A number of known filling processes and equipment may be utilized to achieve
the filling steps of the syringe manufacturing process. The barrel assembly,
needle,
plunger seal, plunger rod, and other components described in these
manufacturing and
CA 03026708 2018-12-05
WO 2017/204787 42 PCT/US2016/033950
assembly processes may be as described above or may be a number of similar
components which achieve the same functionality as these components.
Throughout the
specification, the aim has been to describe the preferred embodiments of the
invention
without limiting the invention to any one embodiment or specific collection of
features.
Various changes and modifications may be made to the embodiments described and
illustrated without departing from the present invention. The disclosure of
each patent
and scientific document, computer program and algorithm referred to in this
specification is incorporated by reference in its entirety.