Note: Descriptions are shown in the official language in which they were submitted.
METHOD FOR PRODUCING HYDROCARBONS FROM SUBTERRANEAN
RESERVOIR WITH VARYING SOLVENT INJECTION TEMPERATURE
TECHNICAL FIELD
[1] This disclosure relates generally to solvent-based methods for in situ
hydrocarbon
production.
BACKGROUND
[2] Recovery of viscous hydrocarbons from subterranean reservoirs can be aided
by
injection of a selected solvent into the reservoir. The solvent can function
as a diluent
for viscous hydrocarbons. When the solvent is heated, it may also, to a
limited extent,
transfer heat to the hydrocarbons or the reservoir. Both effects can reduce
the viscosity
of viscous hydrocarbons and increase their mobility.
[3] A solvent may be used to aid a steam-assisted recovery process, in a so-
called
solvent-aided process (SAP). SAPs include both steam driven solvent processes,
where the amount of steam added is greater than the amount of solvent added,
and
solvent driven processes, where the amount of steam added is less than the
amount of
solvent added. To further reduce steam use, a solvent may be injected without
steam in
a production stage of a recovery process (processes including such a
production stage
are referred to herein as solvent-based recovery processes).
[4] In a solvent-based recovery process known as Vapor Extraction (VAPEX)
process,
a vaporized solvent is injected into the reservoir (formation) via an
injection well
situated above a production well. The injected solvent mobilizes viscous
hydrocarbons
in the formation, and the mobilized hydrocarbons drain downward and are
collected in
the production well and produced to surface. Drainage of the mobilized
hydrocarbons
leaves a hydrocarbon-depleted porous volume in the formation, through which
the
solvent vapor and other fluids can more easily travel, and this porous volume
can be
1
CA 3027052 2018-12-11
referred to as a "solvent chamber", similar to a "steam chamber" in a steam-
assisted
gravity drainage (SAGD) process. In fact, the well arrangement in a VAPEX
process
may be configured similarly to a SAGD well-pair arrangement, as can be
understood by
those skilled in the art.
[5] Compared with steam-assisted recovery processes such as SAGD processes, a
solvent-based recovery process may require less heating energy and less use of
water,
and reduce emission of greenhouse gases. However, existing solvent-based
recovery
processes face their own challenges. For example, the oil production rate is
typically
lower in a solvent-based recovery process than in a SAGD process or a SAP when
solvent injection is limited to keep the solvent to oil ratio (SolOR) within
practical limits.
[6] CA2299790, published 23 August 2001 proposed a method of enhanced oil
recovery, where a heated and vaporized solvent is injected under pressure into
the
formation and condensed in the formation to release heat of condensation to
the
formation. A liquid blend of the solvent and mobilized heavy oil is then
extracted from
the formation. However, this proposed technique requires injection of a
relatively high
volume of heated solvent, which increases production costs due to both
increased
heating cost and increased material cost.
[7] CA 2281276, published 28 January 2001 proposed a method of in situ
recovery of
viscous petroleum hydrocarbons from an underground formation, where vaporized
solvents are injected into the formation and the injected solvents are boiled
off by
indirect heating in the formation (termed as "reboil") to recycle the solvents
in the
reservoir.
[8] WO 2013/007297, published 17 January 2013 proposed a process for recovery
of
viscous hydrocarbons, where steam and/or one or more solvents are injected
into an
upper injection well, and the lower production well is electrically heated to
re-vaporize
(reflux) the steam and/or one or more solvents.
2
CA 3027052 2018-12-11
[9] However, commercial applications of solvent-based recovery processes have
been
limited to date. Challenges remain in providing solvent-based recovery
processes for
efficient and effective commercial application.
SUMMARY
[10] In one aspect, the present disclosure relates to a method of producing
hydrocarbons from a subterranean reservoir, comprising: injecting a solvent at
an
injection temperature (T) into the reservoir to mobilize viscous hydrocarbons
in the
reservoir, wherein T, is maintained above the boiling point temperature (Tbp)
of the
solvent at a reservoir pressure, whereby AT =1-1- Tbp is positive; producing
hydrocarbons mobilized by the solvent from the reservoir; and decreasing AT
over time
during production of the hydrocarbons.
[11] In another aspect, the present invention relates to a method of
producing
hydrocarbons from a subterranean reservoir, comprising: injecting a solvent at
an
injection temperature into the reservoir to mobilize viscous hydrocarbons in
the
reservoir, wherein the injection temperature is maintained above the boiling
point
temperature of the solvent at a reservoir pressure; producing from the
reservoir a fluid
comprising the solvent and hydrocarbons mobilized by the solvent; and in
response to
a change in a solvent to oil ratio in the produced fluid, adjusting the
injection
temperature during production.
[12] In an embodiment of a method described herein, the injection
temperature is decreased over time from a first temperature to a second
temperature,
the first temperature being at least 50 C above the boiling point temperature
of the
solvent at the reservoir pressure.
[13] In an embodiment of a method described herein, the injection
temperature is decreased over time from a first temperature to a second
temperature,
3
CA 3027052 2018-12-11
the first temperature being at least 100 C above the boiling point temperature
of the
solvent at the reservoir pressure.
[14] In an embodiment of a method described herein, the reservoir pressure
is
about 2.5 MPa to about 3.5 MPa.
[15] In an embodiment of a method described herein, the reservoir pressure
is
about 3 MPa.
[16] In an embodiment of a method described herein, the solvent is a C3-C7
alkane.
[17] In an embodiment of a method described herein, the solvent is propane.
[18] In an embodiment of a method described herein, the injection
temperature is about 200 C to about 300 C and the reservoir pressure is about
3.0
MPa.
[19] In an embodiment of a method described herein, the injection
temperature is about 200 C.
[20] In an embodiment of a method described herein, the injection
temperature is decreased from about 200 C to about 100 C over time.
[21] In an embodiment of a method described herein, the solvent is n-
butane,
iso-butane, or a mixture thereof.
[22] In an embodiment of a method described herein, the injection
temperature is about 300 C and the reservoir pressure is about 3.0 MPa.
[23] In an embodiment of a method described herein, the injection
temperature is decreased from about 300 C to about 140 C over time.
[24] In an embodiment of a method described herein, the injection
temperature is adjusted to maintain the solvent to oil ratio within a target
range.
4
CA 3027052 2018-12-11
[25] In an embodiment of a method described herein, the target range is
determined based on a predicted relationship between oil production rate and
produced
solvent to oil ratio.
[26] In an embodiment of a method described herein, the target range is
determined based on a predicted relationship between net injected solvent to
oil ratio
and produced solvent to oil ratio.
[27] In an embodiment of a method described herein, the solvent is propane
and the target range is about 1 to about 2 by liquid volume.
[28] In an embodiment of a method described herein, the solvent is butane
and the target range is about 2 to about 3 by liquid volume.
[29] In an embodiment of a method described herein, the solvent is injected
into the reservoir through an injection well and the hydrocarbons are produced
through
a production well.
[30] In an embodiment of a method described herein, one or both of the
injection well and the production well are heated with a downhole heater.
[31] In an embodiment of a method described herein, the solvent is heated
at
surface before injection into the reservoir.
[32] In an embodiment of a method described herein, injecting the solvent
comprises injecting into the reservoir a fluid consisting essentially of the
solvent.
[33] In an embodiment of a method described herein, a liquid mixture
comprising mobilized hydrocarbons and the solvent is produced through a
production
zone in the reservoir, the production zone having a temperature below the
bubble point
temperature of the solvent in the liquid mixture at the reservoir pressure.
[34] In an embodiment of a method described herein, the temperature in the
production zone is from about 50 C to about 105 C.
CA 3027052 2018-12-11
[35] Other aspects and features will become apparent to those of ordinary
skill
in the art upon review of the following description of specific embodiments of
the
invention in conjunction with the accompanying figures.
BRIEF DESCRIPTION OF THE DRAWINGS
[36] Selected illustrative embodiments are described in detail below, with
reference to the following drawings.
[37] FIG. 1 is a schematic side view of a well system for use in an
embodiment of the present disclosure.
[38] FIG. 2 is a schematic side section view of the injection well of FIG.
1.
[39] FIG. 3 is a schematic side section view of the production well of FIG.
1.
[40] FIG. 4 is a flow diagram for an exemplary solvent-based recovery
process, illustrative of an embodiment of the present disclosure.
[41] FIG. 5 is a schematic cross-sectional view of the reservoir and wells
of
FIG. 1 during operation.
[42] FIG. 6A is data graph showing representative experimental and
simulation results of viscosity as a function of temperature for different
sample
materials.
[43] FIGS. 6B and 6C are 3D data graphs showing representative simulation
results of mobility as a function of temperature and pressure for propane and
butane
aided recovery of bitumen, respectively.
[44] FIG. 7A is a line graph comparing calculated solvent to oil (SolOR)
ratios
in an embodiment of the present disclosure (with heating of the production
well during
6
CA 3027052 2018-12-11
production) and a comparison solvent-based recovery process (without heating
the
production well during production).
[45] FIG. 7B is a schematic diagram illustrating a reservoir model used for
simulation of production performances in different recovery processes and for
the
results shown in FIG. 7A.
[46] FIG. 7C is a line graph comparing simulation results of cumulative
heater
energy intensity (El) for different heating strategies.
[47] FIG. 8 is a line graph illustrating corresponding profiles of
electricity
usage in a power supply system and electricity usage for intermittent heating
of the
production zone in an embodiment of the present disclosure.
[48] FIG. 9 is a line graph of the oil production rate as a function of the
relative
density of the produced oil.
[49] FIG. 10 is a comparison of the energy intensity (El) at 70% recovery
factor (RE) between a SAGD process (cSOR=3.2) and a propane-based recovery
process (cSOR=0.2), with a reduction in El by 16 times (cSOR = cumulative
solvent to
oil ratio).
[50] FIG. 11 shows the relative changes of temperature, oil saturation in
the
formation (So %), and solvent saturation (propane concentration %) over time
in a
propane-based recovery process at temperatures below 100 'C.
[51] FIG. 12 shows comparison data with respect to FIG. 11 from a
comparison process with co-injection of steam and propane at temperatures up
to
about 240 C.
[52] FIG. 13 shows comparison data with respect to bitumen rates for a low
injection temperature process and a high injection temperature process.
7
CA 3027052 2018-12-11
[53] FIG. 14 shows comparison data with respect to net solvent rates for
the
low injection temperature process and the high injection temperature process
of FIG.
13.
[54] FIG. 15 shows comparison data with respect to cumulative net solvent
to
oil ratio (cSolOR) for the low injection temperature process and the high
injection
temperature process of FIGS. 13 and 14.
[55] FIG. 16 is a schematic showing bitumen rate and net solvent to oil
ratio
as a function of produced solvent to oil ratio for a low injection temperature
process, a
high injection temperature process and an injection temperature ranging
process.
[56] FIG. 17 illustrates a typical injection temperature profile for an
injection
temperature ranging process.
[57] FIG. 18 is a line graph showing oil rate as a function of produced
solvent
to oil ratio (by volume) for propane in a simulated injection temperature
ranging
process.
[58] FIG. 19 is a line graph showing oil rate as a function of produced
solvent
to oil ratio (by volume) for butane in a simulated injection temperature
ranging process.
[59] FIG. 20 is a line graph showing net injected solvent to oil ratio as a
function of produced solvent to oil ratio for butane in a simulated injection
temperature
ranging process.
[60] FIG. 21 is a graph showing the solvent chamber dynamics for the low
injection temperature process from the injector.
[61] FIG. 22 is a graph showing the solvent chamber dynamics for the low
injection temperature process from the producer.
[62] FIG. 23 is a graph showing the solvent chamber dynamics for the
injection temperature ranging process from the injector.
8
CA 3027052 2018-12-11
[63] FIG. 24 is a graph showing the solvent chamber dynamics for the
injection temperature ranging process from the producer.
[64] FIG. 25 is a cross sectional view of a well pair and portion of the
reservoir
showing propane concentration in the oil phase during the low injection
temperature
process.
[65] FIG. 26 is a cross sectional view of a well pair and a portion of the
reservoir showing propane concentration in the oil phase during the injection
temperature ranging process.
[66] FIG. 27 is a schematic depicting an idealized well system showing
solvent
chamber, bitumen and a dispersion zone.
[67] FIG. 28 is schematic depicting a magnified section of the dispersion
zone
of FIG. 27 including a typical solvent concentration profile and a linear
approximation
thereof.
DETAILED DESCRIPTION
[68] In brief overview, the present inventors have recognized that solvent-
based hydrocarbon recovery from a subterranean reservoir can be optimized by
separately or independently controlling the solvent injection temperature and
the
temperature in the production zone (referred to as the "production
temperature" herein).
For example, in different embodiments where the production temperature is
maintained
to be below the boiling point temperature of the solvent at the reservoir
pressure, the
solvent injection temperature may be controlled to be higher than the boiling
point
temperature and be:
= near the boiling point temperature of the solvent at reservoir pressure
(referred
to as the "low injection temperature process" herein), or
9
CA 3027052 2018-12-11
= substantially above the boiling point temperature of the solvent at
reservoir
pressure (referred to as the "high injection temperature process"), or
= varied, such as gradually decreased, over time during production of the
hydrocarbons (referred to as the "injection temperature ranging process").
[69] As can be appreciated by those skilled in the art, when the solvent is
injected above its boiling temperature at the reservoir pressure, the solvent
will be
substantially in the vapor phase when injected into the reservoir. Because the
temperature in the production zone is lower than the boiling temperature of
the solvent,
the solvent will be substantially in the liquid phase in the production zone.
[70] In the low injection temperature process, viscous hydrocarbons in the
reservoir are mobilized mainly by viscosity reduction due to solvent
dissolution in the
bitumen. Heat may be transferred from the injected solvent to the formation as
the
solvent vapor is cooled and eventually condenses in the reservoir. The heat
transfer
may improve mobility of the viscous hydrocarbons but viscosity reduction due
to
heating may be limited with solvent injection when the injection temperature
is relatively
low. At a lower solvent injection temperature, less energy will be required to
heat the
solvent before injection, and a low temperature injection process can result
in energy
savings over a conventional steam-assisted gravity drainage (SAGD) process.
However, at a lower solvent injection temperature, an increased amount of the
solvent
may need to be injected to achieve similar hydrocarbon recovery performance,
such as
when the reservoir has a lower permeability and/or is heterogeneous.
[71] When the solvent injection temperature is relatively high, viscous
hydrocarbons in the reservoir may be mobilized mainly by viscosity reduction
due to
heat transfer, which will be increased at elevated temperatures. In this case,
solvent
dissolution can still have an effect on mobilizing the hydrocarbons but such
effect may
be secondary depending on the injection temperature and other factors such as
solvent
type and injection pressure. At a higher injection temperature, the solvent
will have a
lower density at a given pressure and thus the same amount of solvent can
occupy
CA 3027052 2018-12-11
more space within the reservoir. Consequently, a lower amount of the solvent
may be
required to achieve similar hydrocarbon recovery at a higher solvent injection
temperature. However, to heat the solvent to these higher injection
temperatures, the
process may be more energy intensive.
[72] It has been recognized by the present inventors that, in some
embodiments, it may be beneficial to vary the solvent injection temperature
during
hydrocarbon production based on reservoir conditions and production progress,
so as
to balance and optimize energy efficiency, solvent usage, and hydrocarbon
recovery
performance. Varying the injection temperature may also provide other benefits
in
different embodiments. For example, the injection temperature ranging process
may be
optimized to realize the benefits of both the high injection temperature
process (e.g.
lower solvent condensation in the solvent chamber as well as less asphaltene
precipitation) and the low injection temperature process (e.g. higher solvent
solubility to
mobilize the hydrocarbons in the reservoir and increased bitumen upgrading).
Exemplary well system in a subterranean reservoir
[73] An illustrative embodiment of a well system in a subterranean
reservoir
will be described next with reference to the figures.
[74] FIG. 1 shows a reservoir 100 having a pay zone 102 under a cap layer
103. In the particular embodiment illustrated in FIG. 1, an injection well
(injector) 120
and a production well (producer) 140 are provided, which penetrate the pay
zone 102
of the reservoir 100.
[75] The reservoir 100 is a subterranean or underground reservoir
containing
recoverable viscous hydrocarbons. At least some of the viscous hydrocarbons
are
immobile under natural or original reservoir conditions (i.e. before the
reservoir 100 is
subjected to heating or before a treatment material has been injected into the
reservoir
to mobilize the hydrocarbons). Immobile materials include materials that are
not mobile
or not mobile enough to drain under gravity without further treatment. In the
reservoir
11
CA 3027052 2018-12-11
100, fluids such as gases and water may also have limited mobility due to a
relatively
high degree of viscous hydrocarbon saturation. In some typical bitumen
reservoirs
found in Alberta, Canada, the natural or original temperature in the reservoir
may be
between about 7 C and about 12 C, and the natural or original pressure in
the
reservoir may be between about 1 MPa and about 5 MPa. In different reservoirs,
the
original temperature and pressure may be different.
[76] Broadly, viscous hydrocarbons in the reservoir 100 may have a
viscosity
higher than about 1,000 centipoise (cP), 10,000 cP, 100,000 cP, or 1,000,000
cP. The
viscous hydrocarbons in the reservoir 100 may be a mixture of various
materials. A
variety of hydrocarbons in the reservoir 100 may exist, as viscous liquids, or
in semi-
solid or solid forms at native reservoir conditions. For example, the viscous
hydrocarbons in reservoir 100 may exist in the form of bitumen, heavy oil,
extra heavy
oil, bituminous sands (also referred to as oil sands), or combinations
thereof. In
bituminous sands, at least some viscous or immobile hydrocarbons are disposed
between, or attached to, sands. In the reservoir 100, hydrocarbons may exist
in
mixtures of varying compositions comprising hydrocarbons in the gaseous,
liquid or
solid states, which may also be in combination with other fluids (liquids and
gases) that
are not hydrocarbons. Bitumen is generally non-mobile under typical native
reservoir
conditions. The reservoir 100 also includes asphaltenes in the pay zone, which
may co-
exist or be mixed with the viscous hydrocarbons. As will be understood by
those skilled
in the art, asphaltenes are typical components of crude oil or petroleum which
are
insoluble in light paraffinic hydrocarbons, but at least partially soluble in
benzene,
chloroform, or carbon disulfide. Asphaltenes may include polycyclic aromatic
compounds, which may contain oxygen, sulfur, nitrogen, or a combination
thereof, in
addition to carbon and hydrogen. In some hydrocarbon reservoirs, the initial
asphaltene content in a pay zone may be from about 10 wt% to about 30 wt%, or
about
15 wt% to about 30 wt%, of the hydrocarbon content in the same pay zone.
[77] Each of the wells 120 and 140 has a horizontal section with a
perforated
section. The horizontal sections of the wells 120 and 140 are substantially
parallel to
12
CA 3027052 2018-12-11
one another and are vertically spaced by a distance, which may be about 5 to
about 8
m, with the production well 140 positioned below the injection well 120. The
horizontal
sections of the wells 120 and 140 may be about 800 m in length. The injection
well 120
is connected to an injection surface facility 220 (not shown in detail), and
the production
well 140 is connected to a production surface facility 240 (not shown in
detail). Further
details of the wells 120 and 140 are provided below with reference to FIGS. 2
and 3.
[78] The injection surface facility 220 is configured to supply an
injection fluid,
which includes a solvent, to the injection well 120 for injection into the pay
zone 102 of
the reservoir 100. The injection surface facility 220 may have a supply line
(not shown)
connected to an injection fluid source (not shown) for supplying the injection
fluid.
[79] The production surface facility 240 and the production well 140 are
configured to produce a fluid from the reservoir 100 to surface through
production well
140. The produced fluid may include a liquid mixture of the injected solvent,
mobilized
hydrocarbons, and asphaltenes. The production surface facility 240 may include
a fluid
transport pipeline (not shown) for conveying the produced fluid to a
downstream facility
(not shown) for processing or treatment.
[80] The injection surface facility 220 includes equipment for supplying
the
injection fluid to the injection well 120, and the production surface facility
240 includes
equipment for producing the produced fluid from the production well 140, as
can be
understood by those skilled in the art.
[81] The wells 120 and 140 may be configured and completed in a similar
manner as the horizontal wells used in a steam-assisted gravity drainage
(SAGD)
process, with suitable modifications to inject a solvent instead of, or in
addition to,
steam, and to heat the production zone as will be further explained below.
[82] For example, FIG. 2 schematically illustrates an embodiment of the
injection well 120. The injection well 120 is provided with a coiled tubing
122 for
injecting the solvent (and other possible injected fluids or materials), a
casing 124, a
13
CA 3027052 2018-12-11
liner assembly 126, and a liner hanger 128. The liner assembly 126 is slotted
to allow
injected fluids to pass through. The coiled tubing 122 may be connected to a
control
system (not shown) at the surface for controlling the injection operation, as
can be
understood by those skilled in the art. One or more downhole heaters 132 may
be
provided in the injection well 120, which may include a wire or rod coiled
around the
coiled tubing 122 along a length of the horizontal section of the injection
well 120. The
heater 132 may be an electric heater. An electric heater may be operated in
the direct-
current (DC) mode or in an alternating-current (AC) mode, and maybe operated
at an
operating frequency in the range of 1 Hz to 30 kHz. A temperature sensor 134
may be
provided in or on the coiled tubing 122. The temperature sensor 134 may
include a
distributed temperature sensing (DTS) device, and may include thermocouples.
Temperatures at multiple points along the well 120, such as 4 to 6 points or
more, may
be monitored during operation. Electrical signal and power lines (not
separately shown)
for the temperature sensors 134 and the heater 132 may be connected to the
surface
control system to provide temperature signals from the sensors 134 to the
control
system and to control operation of the heater 132. The power and signal lines
may be
attached to the coiled tubing 122 or a tubing string (not shown in FIG. 2).
Additional
necessary or optional components, tools, or equipment may be installed in the
injection
well 120, but they are not shown in FIG. 2 as they are not particularly
relevant for the
purpose of the present disclosure. For example, as is typical for steam- or
solvent-
aided or -assisted processes, sensors and devices (not shown) for measuring
downhole temperature (T) and pressure (P) may be provided in the well 120,
such as at
a heel portion of the well 120.
[83] In a specific embodiment, the injection well may have a true vertical
well
depth (TVD) of about 390 m, and a total depth (TD) of 1,500 mKB. This
particular well
is provided with a dual-heater string, four thermocouples (TC), and a DTS
fibre in the
coiled tubing.
[84] FIG. 3 illustrates an embodiment of the production well 140, which is
similarly constructed as injection well 120. In particular, the production
well 140 also
14
CA 3027052 2018-12-11
includes a coiled tubing 142, a casing 144, a slotted liner assembly 146, a
liner hanger
148, a heater 152, and a temperature sensor 154, which may be similarly
constructed
and configured as their counterparts in the injection well 120. The production
well 140
also additionally includes a pump 156 and a production tubing 158 for
producing fluids
entering the well 140 through the slotted liner assembly 146 to the surface.
As in the
injection well 120, signal and power lines (not shown) for the heater 152 and
temperature sensor 154 may be provided and connected to the surface control
system.
As in the injection well 120, additional necessary or optional components,
tools, or
equipment may be installed in the production well 140, but they are not shown
in FIG. 3
as they are not particularly relevant for the purpose of the present
disclosure.
However, it is noted that a pressure sensor may be optionally omitted in the
production
well 140 in some embodiments. In a specific embodiment, the production well
may
have a TVD of about 390 m, and a TD of 1,500 mKB. This well may also be
provided
with a dual-heater string, four or more TCs, and a DTS fibre in the coiled
tubing. The
production tubing may be landed at the heel of the well, with a TD of 595 mKB.
Low injection temperature process
[85] In an embodiment of the low injection temperature process, the process
comprises: injecting a solvent at an injection temperature (Ti) into the
reservoir to
mobilize viscous hydrocarbons in the reservoir, wherein T1 is maintained near
the
boiling point temperature (Tbp) of the solvent at a reservoir pressure,
whereby AT = T1 -
Tbp is relatively low; and producing hydrocarbons mobilized by the solvent
from the
reservoir.
[86] In the low injection temperature process AT remains more or less
stable
or is not varied substantially during production of the hydrocarbons. Further,
AT may
be a value from 0 C to about 50 C.
[87] An illustrative embodiment of the low injection temperature process
will
be described next with reference to the figures.
CA 3027052 2018-12-11
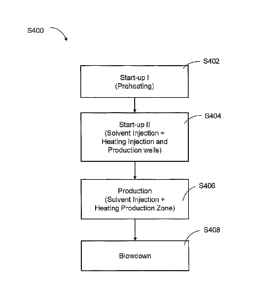
[88] During operation, an example recovery process S400 for producing
hydrocarbons from the reservoir 100 using the well pair of wells 120 and 140
may
include the stages illustrated in FIG. 4.
[89] As listed in FIG. 4, the process S400 includes a start-up stage, which
may
include a Start-up I sub-stage S402 and a Start-up ll sub-stage 3404, a
production
stage S406, and a blowdown stage 3408. The process S400 is discussed below
with
references to FIGS. 4 and 5.
[90] In the sub-stage S402 (which may be referred to as preheating), the
heaters 132 and 152 may be powered to heat an inter-well zone (or inter-well
region)
104 to soften the viscous hydrocarbons therein. The heating in the sub-stage
S402
may be provided at a selected power for a period of sufficient time to prepare
the
reservoir formation for the sub-stage S404, such as for about 1 month to about
7
months at a heating power/well length of up to 10,000 W/m, such as from about
500 to
5,000 Winn. As can be appreciated by those skilled in the art, heating the
materials in
the reservoir 100, particularly in the inter-well region 104, can soften, or
increase the
mobility of, viscous hydrocarbons within the inter-well zone 104, which can
facilitate
distribution and dispersion of the injected solvent in the inter-well region
104. At the end
of the sub-stage 3402, the temperature in the inter-well zone 104 is increased
as
compared to its native temperature, so that the viscous hydrocarbons in the
inter-well
region 104 are at least partially softened and mobilized. For example, the
average
temperature of the inter-well zone 104 may be about 95 C at the end of the
sub-stage
3402. The average temperature may vary from about 80 C to about 290 C for
propane at the operating pressure of about 3 MPa.
[91] In the sub-stage S404, an injection fluid 160 including a selected
solvent
is injected into the inter-well zone 104 from both the injection well 120 and
the
production well 140 at a selected pressure to establish fluid communication
between
the injection well 120 and the production well 140. The solvent may be
selected as
discussed below. The injection pressure and injection temperature may also be
16
CA 3027052 2018-12-11
selected as further discussed below. In a particular embodiment, the selected
solvent is
propane. When the solvent is propane, the injection temperature may be about
80 C to
about 100 C, and the injection pressure may be about 3 MPa to about 3.5 MPa.
The
propane is thus injected as a vapor at these selected temperatures and
pressures. The
injected solvent vapor will disperse into the pay zone 102 particularly the
inter-well
region 104, and will condense in the cooler regions as the solvent travels
away from
the wells 120 and 140. The latent heat transferred from the solvent to the pay
zone 102
further mobilizes the hydrocarbons therein. The condensed solvent liquid can
also
dilute the hydrocarbons it contacts, thus further softening or mobilizing the
hydrocarbons in the inter-well region 104. During the sub-stage S404, heaters
132 and
152 may both be activated to heat the inter-well region 104 to assist heating
of the
hydrocarbons therein.
[92] At some point in the sub-stage S404, a pressure differential between
the
injection well 120 and the production well 140 may be established to drive
fluid flow
from the injection well 120 towards the production well 140. For example,
injection of
solvent into the production well 140 may be terminated at a selected time, and
the
pump 156 may be operated to produce fluids in the well 140 to the surface,
while
injection of the solvent into the injection well 120 is maintained. As can be
appreciated,
a higher injection pressure or higher pressure differential between the wells
can drive
the solvent into the reservoir 100, or the fluid flow in the reservoir 100,
more quickly.
Eventually, a fluid path between the wells 120 and 140 will be formed and
fluid
communication between the wells is established. In some applications, it may
take
about 3 months or more to establish fluid communication between the wells in
the well
pair. The sub-stage S404 may continue after initial fluid communication
between the
wells in the well pair to provide improved communication there between. For
example,
it may be desirable to have generally uniform communication along the length
of the
horizontal sections of the wells 120 and 140.
[93] After fluid communication between the wells 120 and 140 is
established,
the production stage S406 may commence. At the beginning of the production
stage
17
CA 3027052 2018-12-11
S406, there may be a ramp-up phase (not shown in FIG. 4), in which the
production
rate is gradually increased, or increased in steps.
[94] At this point, some softened or mobilized hydrocarbons will have
drained
downward, leaving behind a porous volume, referred to as the solvent chamber
106, in
the pay zone 102. The solvent chamber 106 is analogous to the "steam chamber"
in
SAGD processes. The concept of a "steam chamber" is well known and understood
by
those skilled in the art. A solvent vapor can travel more easily and quickly
in the solvent
chamber 106 as compared to the original, much less porous, pay zone 102. In
the
ramp-up phase, the solvent chamber may grow and develop upwards above the
injection well 120, as the heated solvent vapor tends to rise in the solvent
chamber
106. The temperature in the central region of the solvent chamber 106 near the
injection well 120 is higher than the temperature at the edges (sometimes
referred to as
the "interface region" or the "chamber front") of the solvent chamber 106. The
interface
region is indicated in FIG. 5 by the dashed line. For example, the temperature
of the
central region of the solvent chamber 106 may be close to the injection
temperature, at
about 80 to about 100 C in the above discussed example. The temperature at
the
interface region may vary from about 70 C to about 20 C, for example, if the
temperature in regions outside the solvent chamber 106 is about 15 C.
[95] During the production stage S406, the selected solvent, propane in
this
particular example, is injected into the pay zone 102 of the reservoir 100
through the
injection well 120 only. The injection pressure may be about 3 MPa at this
stage. The
injection temperature may be about 75 C to about 100 C, such as about 80 C
to
about 90 C at this stage. The solvent, propane in this example, enters the
reservoir
100 mainly in the vapor form. The solvent may be vaporized at surface and
supplied to
the injection well 120 in the vapor phase, or provided as a liquid to the
injection well
120 and vaporized in the injection well 120 before entering the pay zone 102.
Alternatively, the solvent may be supplied to the injection well 120 as a
liquid-vapor
mixture. The ratio of liquid:vapor in the liquid-vapor mixture may be selected
such that
the liquid portion of the mixture at least mostly vaporizes at reservoir
conditions (e.g.,
18
CA 3027052 2018-12-11
due to the pressure differential between the injection well 120 and the
production well
140 and/or in the presence of the heater) to generate the solvent chamber.
[96] The solvent may be injected by injecting into the reservoir a fluid
consisting essentially of the solvent. The fluid may contain impurities or
small amounts
of other substances such as water, steam, methane or the like, but the total
weight or
molar concentration of such impurities and other substances are relatively
small, such
as below about 1-2 wt%.
[97] The heater 132 in the injection well 120 may be used to control the
injection temperature of the solvent, which may be at about 80 C to about 90
C for
propane, such that the propane is injected substantially in the vapor phase.
[98] The heated solvent vapor will initially travel generally upwards in
the
solvent chamber 106, as indicated by arrows 160 in FIG. 5. The solvent vapor
will
condense at the interface region due to the cooler temperature in the
interface region.
The solvent liquid will mix with the mobilized hydrocarbons to form a liquid
mixture 170
and drain generally downward.
[99] Eventually, the liquid mixture 170 drains into the production zone 108
around the production well 140, and is produced to the surface through the
production
well 140.
[100] It should be understood that a liquid mixture may contain some
limited
gaseous contents. For example, in the formation a solvent may be partially in
the liquid
phase and partially in the vapor phase, such as with up to 80 wt% of the
solvent in the
liquid phase. A liquid in the liquid mixture, such as a liquid solvent, may
also be
vaporized in the production well when being produced to surface. Some other
gases
such as methane, CO2, H2S, or a combination thereof may also be produced with
the
liquid mixture.
[101] During production, the heater 152 in the production well 140 is used
to
heat the production zone 108. The heating may be controlled by the surface
control
19
CA 3027052 2018-12-11
system (not shown) based on the temperature signal detected by the temperature
sensor 154, to maintain the temperature in the production zone 108 to be
within a
selected temperature range. The factors considered for selecting this range
will be
discussed in detail below. For injection of propane as the solvent in a
particular type of
reservoir formation, the lower threshold of the temperature range may be about
50 C,
and the upper threshold may be about 70 C, when the injection pressure is
about 3
MPa. The lower threshold temperature is selected in this case based on the
data
shown in FIG. 6A. Because the propane dew point temperature is about 77 C at
the
given operating pressure, the bubble point temperature of propane in the fluid
mixture
170 is also close to 77 C. Thus, the lower and upper thresholds are about 27
C and
about 7 C below the bubble point temperature of the liquid mixture at the
given
reservoir conditions, respectively. As the temperature in the production zone
108 is
maintained below the bubble point temperature of the liquid mixture 170, the
propane in
the liquid mixture 170 will not be significantly re-vaporized, or refluxed, in
the
production zone 108.
[102] Further, as the reservoir 100 contains asphaltenes, which may be
mixed
with the hydrocarbons, the liquid mixture 170 in the production zone 108 may
include
the condensed solvent, mobilized hydrocarbons, and asphaltenes. Depending on
the
amount of the asphaltenes and the sizes of the asphaltene particles in the
liquid
mixture 170, the liquid mixture 170 may contain the asphaltene-rich bitumen
(ARB)
phase. Conveniently, when the temperature in the production zone 108 is higher
than
about 50 C, the growth of the ARB phase may be limited or controlled. Thus,
the rate
of hydrocarbon production is expected to be high, as compared to a comparison
solvent based process in which the production zone or the production well is
not
separately heated with a heater. When the production zone is not heated with a
heater,
and the temperature in the production zone is relatively low, the hydrocarbon
production rate may be relatively low because the liquid mixture 170 may have
an
increased ARB phase.
CA 3027052 2018-12-11
[103] For example, with controlled heating of the production zone 108, the
asphaltene content in the produced fluid mixture may be limited to between
about 1
wt% and about 30 wt%, such as about 1 wt% to about 20 wt%.
[104] Hydrocarbon production may continue until the amount of the
hydrocarbons in the pay zone has been reduced to a level that is no longer
economical.
At S408, the blowdown stage may start as can be understood by those skilled in
the
art. During the blowdown stage S408, injection of the solvent is terminated.
The
residual hydrocarbons and solvent may still be produced for a period of time.
A non-
condensable gas (NCG) such as methane may be injected into the solvent chamber
106 to assist recovery of the residual solvent and the remaining hydrocarbons.
The
injected NCG may keep the pressure in the solvent chamber at a relatively high
level.
During the blowdown stage S408, the production zone 108 may be heated with the
heater 152 to keep the temperature in the production zone 108 between the
lower and
upper thresholds so that the hydrocarbon production is still efficient.
[105] In different embodiments solvents other than propane may be selected
and used, and the operating conditions may also vary depending on the selected
solvent and the native reservoir conditions. To improve the efficiency of
hydrocarbon
production, the solvent and the injection and heating conditions may be
selected or
determined based on a number of factors including those disclosed herein.
[106] In theory, a higher injection pressure is more desirable for
increasing the
hydrocarbon production rate. A higher injection pressure would drive the fluid
flow
faster. A higher pressure also allows the solvent to be injected at a higher
rate and to
condense at a higher temperature, both of which would increase the rate of
mobilizing
the viscous hydrocarbons. As can be appreciated, a hotter solvent liquid is
more
efficient for mobilizing hydrocarbons. Simulation tests have confirmed that
the
production rate increases as the injection pressure increases at the tested
conditions.
However, in practical applications, the injection pressure is typically
limited by
technical, safety, environmental, or other concerns and may be regulated by
local
21
CA 3027052 2018-12-11
authorities. Within the practical limitations, the injection pressure may be
selected to be
as high as is permitted.
[107] Given the possible injection pressure range, a suitable solvent
may be
selected so that the solvent can be injected as a vapor at the given injection
pressure
and at the possible temperature range and can condense at the expected
temperature
at the interface region of the solvent chamber. The selected solvent should
also be
effective for mobilizing the viscous hydrocarbons solvent at the reservoir
conditions.
Among the possible solvents, the solvents that would provide a similar
recovery rate at
relatively lower temperatures may be selected as heating a solvent and the pay
zone to
a lower temperature requires less energy and less cost. Other factors such as
chemical
compatibility, availability, pre- and post-injection treatment requirements,
costs, or the
like may also be considered when selecting the solvent. As can be appreciated,
a
solvent may be injected as a vapor at temperatures above the critical point of
the
solvent. In this regard, the critical point data are:
- Propane: 96 C, 4.26 MPa
- Butane: 152 C, 3.8 MPa
- Pentane: 197 C, 3.4 MPa
- Hexane: 235 C, 3.02 MPa
[108] In this regard, known data including simulation data may be
utilized for
selecting the solvent. For example, FIG. 6A shows experimental and simulation
results
of the viscosity of sample materials or mixtures at different temperatures, at
a selected
pressure of 2.89 MPa. It can be seen that the bitumen viscosities generally
decrease
as the temperature increases. For practical production, the viscosity of the
softened
bitumen should be lower than about 50 - 100 cP, such as from about 1 to about
20 cP,
although bitumen with even lower viscosity is generally easier to produce. The
data in
FIG. 6A indicates that propane, butane and pentane are all effective for
lowering the
bitumen viscosity to below about 10 cP, at temperatures from about 50 to about
22
CA 3027052 2018-12-11
250 C. In particular, propane is still effective for viscosity reduction in
the temperature
range of about 50 to about 100 C. Thus, on balance of consideration of other
factors,
propane may be selected as the solvent at the given pressure range.
[109] The heating temperature or the threshold temperatures for heating the
production zone 108 may then be selected as discussed earlier. For the given
solvent
and a selected solvent injection rate, the asphaltene content in the fluid
mixture
collected in the production zone 108, and how the ARB and solvent-rich bitumen
(SRB)
phases would change at different temperatures may be assessed, such as by
conducting experimental tests, or simulation tests, or both. The lower
temperature
threshold for heating the production zone 108 may be selected so that the ARB
phase
formation is controlled and limited to allow efficient hydrocarbon production.
For
example, based on simulation and laboratory tests, it is expected that for
propane and
the tested type of bitumen, the ARB phase formation is limited at temperatures
above
about 50 C at about 3 MPa when propane is used as the solvent. The
temperature 50
C can thus be selected as the lower threshold temperature in this instance.
The
bubble point temperature in the liquid mixture of propane and the tested
bitumen is
about 77 C at the pressure of about 3.5MPa. Thus, the upper threshold
temperature
may be selected to be a temperature below the bubble point temperature of 77
C to
prevent reflux of the solvent (re-vaporization) in the production zone 108.
For example,
the upper threshold temperature may be selected to be 70 C. The heating
temperature
may be at least 5 C below the bubble point temperature of the solvent in the
liquid
mixture in the production zone, or at least 15 C below the bubble point
temperature.
For propane, the heating temperature may be between 50 C and 70 C.
[110] For selecting solvents and injection and heating conditions, data
plots
such as shown in FIGS. 6B and 6C may be helpful. FIG. 6B shows mobility
dependence on pressure and temperature for propane, and FIG. 6C shows similar
data
for butane. For comparison purposes, the typical mobility of bitumen obtained
in a
conventional SAGD process at typical temperatures of 250 C or higher is
about 0.1 cP-1. For propane and butane, similar bitumen mobility may be
obtained at
23
CA 3027052 2018-12-11
much lower temperatures at pressures higher than 3 MPa. From FIG. 6C, it can
be
seen that the bitumen mobility can be higher than 0.1 cP-1 at temperatures
lower than
about 100 C and pressures of lower than 4 MPa. Thus, it can be expected that
butane
may also be a suitable solvent for a solvent-aided process as described
herein.
[111] The actual temperature control may be carried out by controlling the
heater 152 to maintain a set temperature point or range based on the detected
temperature from the temperature sensor 154.
[112] It is noted that in FIG. 6A, the viscosity data for different
materials or
mixtures are plotted. The materials include the following:
- "Saturated C3 Expt." = dead oil bitumen saturated with propane
liquid (experiment)
- "Liq-Liq C3-Bitumen Expt." = two liquid phase: a bitumen rich
phase and a propane (C3 alkane) rich phase, at equilibrium
(experiment)
- "Saturated C4 Expt." = dead oil bitumen saturated with butane
liquid (experiment)
- "Bitumen" (experiment)
- "Bitumen saturated with methane" (simulation)
- "Bitumen saturated with propane" (simulation)
- "Bitumen saturated with butane" (simulation)
- "Bitumen saturated with pentane" (simulation)
[113] It is worth noting that the data in FIGS. 6A, 6B and 6C indicate
that the
viscosity of certain alkane-saturated bitumen can decrease as the temperature
is
reduced. In other words, the mobility of such materials increases at lower
24
CA 3027052 2018-12-11
temperatures. In comparison, as can be seen in FIG. 6A, the viscosity of the
bitumen
material or a mixture of bitumen and methane increases as the temperature
decreases,
and their mobility correspondingly decreases. From the mobility data alone, it
might be
concluded that the lower the production temperature (i.e. the temperature in
the
production zone), the higher the production rate due to increased mobility
when
propane or butane is used to aid the production process. However, as noted
above,
when the production temperature is too low, formation or excess growth of the
ARB
phase in the production zone can negatively affect the production rate. Thus,
the lower
threshold for the production temperature should take this effect into account.
[114] Known analysis tools and methods may be used to aid the selection of
the solvent and operating conditions. For example, a known oil production
analysis
method is the SARA (Saturates, Aromatics, Resins, and Asphaltenes) analysis.
The
SARA analysis method is described in, for example, US 5,424,959, the entire
contents
of which are incorporated herein by reference.
[115] In some embodiments, propane may be selected as a suitable candidate
solvent for a number of reasons relating to thermo-physical characteristics of
propane
and propane-bitumen mixtures under the particular reservoir conditions. First,
propane
has a moderate dew point temperature (and the corresponding bubble point
temperature in a propane-bitumen mixture is also moderate), and thus it can be
readily
vaporized at a moderate temperature for injection through the injection well
120 and the
propane vapor can be readily condensed at the interface region of the solvent
chamber
106. Second, the viscosity of the propane-bitumen mixture decreases with
decreased
temperature at the temperature range of 50 to 70 C, which is just below the
propane
bubble point in the mixture at the given pressure of about 3MPa. The shaded
oval
region in FIG. 6A indicates a region for effective bitumen production. Based
on this
indicated region, a production temperature for propane may be indicated as
from about
50 C to about 105 C. For example, a preliminary lower temperature threshold
may be
selected based on this region, and selecting the lower temperature threshold
of about
50 C may provide a minimum acceptable viscosity level. From the preliminary
CA 3027052 2018-12-11
threshold, lab or field tests can be conducted to determine an optimal or
actual lower
threshold. The lower threshold may then be increased or decreased based on the
test
results or production performance during operation, such as to mitigate
against excess
formation of an undesirable ARB phase in the production zone 108.
[116] While the initial upper temperature threshold may be selected to
limit
solvent reflux or "reboil" in the production zone 108, the data such as shown
in FIGS.
6A, 6B or 6C may also be considered, and can be adjusted based on actual test
or
performance results. Limiting re-vaporization of the solvent in the production
zone 108
can reduce inefficient heating of the solvent and the production zone 108. For
example,
the upper temperature threshold may be set at 70 C, which is about 7 C below
the
propane dew point temperature under the stated reservoir conditions.
[117] For clarity, it is noted that an embodiment of a solvent-based
recovery
process may include injection of steam at different stages (such as the start-
up stage)
other than the oil production stage, where a solvent is injected in the
production stage
without steam. Embodiments of the present disclosure also include recovery
processes
in which a solvent is injected in an oil production stage to drive oil
production, but
steam is not co-injected with the solvent as a primary heating source to
maintain or
control the temperature in the production zone of the reservoir during the
production
stage.
[118] Conveniently, an embodiment of the solvent-based recovery process as
described herein may provide effective and efficient hydrocarbon production at
reduced
energy and solvent consumption and lower costs.
[119] For example, FIG. 7A compares the achievable solvent to oil ratios
(SolOR) in two different processes based on simulation results for propane.
The lower
lighter curve in FIG. 7A represents the SolOR for a process as described
herein where
the production zone is heated with a downhole heater in the production well at
the
heating temperature of 65 C (for subcool of about 10 C below the bubble
point
temperature of the produced fluid mixture containing propane). The upper
darker line in
26
CA 3027052 2018-12-11
FIG. 7A represents the SolOR for a comparison process, which is similar in
other
aspects but without using a downhole heater in the production well to heat the
production zone to control the temperature in the production zone. The solvent
injection
pressure and injection temperature are the same in both cases. The idealized
half
symmetry reservoir model used to generate the simulation results shown in FIG.
7A is
illustrated in FIG. 7B. The model was used to compare effects of production
using a
down hole heater in the production well to maintain the heating temperature in
the
production well at 65 C, against production without directly heating the
production well
and the production zone with a heater in the production well to control the
temperature
in the production zone. The simulated reservoir was assumed to be homogenous.
The
overall SolOR of the comparison process without separate heating of the
production
zone was higher as compared to the process with controlled heating of the
production
zone.
[120] As
illustrated in FIG. 7B, the simulated reservoir had a reservoir pay zone
700 and contained a shale barrier 702, which may alternatively be a baffle.
The pay
zone 700 was positioned below an overburden 704 and above an underburden 706.
In
the simulation model, the shale barrier 702 was positioned so that it would be
in the
region of the solvent chamber developed due to injection of the solvent.
Additional
reservoir properties of the simulation model are listed in Table I.
27
CA 3027052 2018-12-11
Table I. Simulated Reservoir Properties
PROPERTY VALUE
Formation Material McMurray Sand
Initial Reservoir Temperature 15 C
Initial Reservoir Pressure 3 MPa
Operating (Injection) Pressure 3.2 MPa
Injection Temperature 74 C
Initial Methane Fraction in Oil 20 mol%
Solvent Concentration in the Injection Fluid 100 wt%
Solvent C3H8
Electric Heater Temperature (Circulation) 260 C
Electric Heater Temperature (Normal Operation) 65 C
[121] It can be appreciated that fluid flow through or around the shale
barrier
702 is slower than fluid flow through the same region without the barrier, so
the fluid
path through the shale barrier is an inefficient fluid path. When an
inefficient fluid path
exists in the pay zone, more energy or heat is required to overcome the
barrier. One
possible way to meet such increased heat demand is to inject more solvent than
otherwise needed. However, because the latent heat of solvents is relatively
low
compared to steam, utilizing more solvent to provide the required heat energy
is not
efficient. Utilizing a heater in the production well to separately heat the
production zone
and control the temperature in the production zone may provide more efficient
heating.
[122] The representative simulation results shown in FIG. 7A indicate that
the
use of the heater to heat the production zone during oil production increased
production efficiency by about 7%. It is expected that a further increase of
production
efficiency may be obtained in actual reservoirs where the formation is
significantly more
heterogeneous and has more shale barriers/baffles than in the simulated
reservoir
shown in Fig. 7B.
28
CA 3027052 2018-12-11
[123] The present inventors have further recognized that it is less
efficient to
heat the solvent in the production zone to a temperature above the bubble
point of the
solvent in the fluid mixture to be produced, as compared to subcool heating
where the
heating temperature is maintained below the bubble point temperature. FIG. 7C
shows
the cumulative heater energy intensities for different heating strategies or
regimes,
including subcool heating to a subcool temperature of 65 C (i.e. 10 C below
the
bubble point of propane), and overheating to temperatures of 85 C (i.e. 10 C
above
the bubble point of propane) and 155 C (i.e. 80 C above the bubble point of
propane),
based on unit oil production. At the heating temperature of 85 C, propane in
the
production well and the production zone is partially re-vaporized. At the
heating
temperature of 155 C, propane in the production well and the production zone
is
completely or nearly completely re-vaporized. FIG. 7C shows that for the same
amount
of oil production, overheating requires more heat energy than subcool heating.
Even
when propane (the solvent) is only partially re-vaporized at 85 C, the
required heat
energy is about double (twice of) the heat energy required for subcool heating
at 65 C
where there is no or little re-vaporization. Heating to 155 C (complete re-
vaporization)
requires more than 3 times the heat energy, compared to subcool heating at 65
C to
produce the same amount of oil. These simulation results indicate that
overheating is
not as efficient as subcool heating the solvent in the production zone during
oil
production. This effect may be understandable in view of FIG. 6B, as in the
case of
partial re-vaporization of the solvent, the oil mobility may be increased due
to both
temperature increase and solvent diluting effects (some liquid solvent is
still dissolved
in the fluid mixture in the formation), but in the case of complete re-
vaporization, the oil
mobility increase comes mainly from temperature increase.
[124] The data shown in FIGS. 6A to 6C and FIGS. 7A and 7C were generated
using solvent partition coefficients (k values) in bitumen, solvent and
bitumen
viscosities as functions of temperature and pressure, and a log-linear mixing
rule for
bitumen saturated viscosities (with solvent). The simulation algorithm was
configured to
calculate expected bitumen viscosity at the given temperature and pressure.
29
CA 3027052 2018-12-11
[125] In a different embodiment, butane may be selected as the solvent, and
the operation parameters and conditions may be selected based on the approach
described above with regard to propane, and in view of the data shown in FIG.
6C.
[126] In view of the foregoing description of example embodiments, a
skilled
person will appreciate the working principles of the present disclosure, which
is in no
way bound to the example embodiments set out above or below. The foregoing
description will now be supplemented to elucidate other aspects and
embodiments of
the present disclosure.
[127] For instance, in different embodiments, different solvents may be
used as
a solvent in one or more selected stages of the recovery process. Example
candidates
for suitable solvents may include, for example, the following materials, and
may be
selected based on factors including the factors discussed below.
[128] Some factors to be considered for selecting the solvent include the
reservoir pressure, maximum operating pressure (may be dictated by local
regulatory
requirement), solvent solubility, solvent cost and availability, solvent-rock
interaction
properties, capital expenditure (capex) constraints, possible solvent losses,
and other
factors.
[129] Generally, an operator may not be able to change the reservoir
pressure
and the maximum permissible operating pressure, and may need to work within
these
constraints. For example, in a shallow reservoir with a regulatory constraint
that the
operating pressure should not be significantly above the initial reservoir
pressure,
lighter hydrocarbon solvents such as propane may be used.
[130] As an illustrative example, assume that the initial reservoir
pressure is 0.6
MPa and the upper limit on operating pressure is 1.0 MPa, from Fig. 6B, it can
be
expected that propane may not be a viable solvent due to low production
performance
at these conditions when propane is used as the solvent in a solvent-based
recovery
CA 3027052 2018-12-11
process. However, if butane is used as the solvent, reasonable production
rates can
still be expected at the pressure of 1 MPa or even less (see FIG. 6C).
[131] At a given operating pressure, the solvent injection temperature may
be
selected to match the highest mobility point in curves such as those shown in
Fig. 6B
and 6C, for propane and butane, respectively.
[132] Among solvents which can work within the same operating conditions
(pressure and temperature), the solvent that provides the highest oil mobility
within the
reservoir operating ranges may be selected and may be expected to provide
better
production performance than other solvents in the group. Alternatively, the
solvent
associated with the lowest operating temperature may be selected, such as when
it is
desirable to reduce energy consumption or to lower greenhouse gas (GHG)
emissions.
For example, at an operating pressure of 3 MPa and based on the data in FIGS.
6B
and 6C, selecting butane may provide better oil production rates than propane,
while
selecting propane may reduce energy requirements and GHG emissions compared to
butane.
[133] A person of skill in the art may also appreciate that objective
functions
(used in optimization) may be formulated by combining maximizing oil
production rates
and minimizing energy requirements and GHG emissions, with a selected weight
for
each objective.
[134] Solvent cost and availability are economic factors that can change
and
are mainly driven by demand and supply in the market. However, such economic
factors should also be considered along with other factors including technical
factors.
Economic considerations may be balanced against technical advantages or
disadvantages of selecting a particular solvent.
[135] Hydrocarbon solvents, as organic solvents, do not generally interact
with
the mineral rocks present in the reservoir, and may be used. However, non-
hydrocarbon solvents may also be used. When selecting a non-hydrocarbon
solvent for
31
CA 3027052 2018-12-11
use in a recovery process as described herein, one should consider the
possible
interaction between the particular solvent and the rock matrix in the
reservoir. If the
particular solvent would interact deleteriously with the rock matrix, it
should not be
used. For example, carbon dioxide (CO2) may not be a good solvent for
carbonate
reservoirs because CO2 can interact with the rock matrix to form calcium
carbonate
(CaCO3), which can precipitate and potentially block reservoir pores, thus
limiting or
preventing fluid flow in the reservoir and negatively affecting oil
production.
[136] The costs of obtaining and handling solvent should also be
considered.
On a balanced approach considering both economic and technical factors, in
some
cases a technically less optimal solvent (such as according to the type of
technical data
shown in FIGS. 6B and 6C) may be selected over the technically optimal
solvent.
[137] As another example, to reduce solvent residue (trapped solvent) in
the
reservoir formation (particularly before the blowdown phase or stage), heavier
solvents
may be selected as they are less likely to be trapped. However, heavier
solvents tend
to be more expensive. Thus, a detailed analysis may be required to determine
the
actual overall costs for selecting a heavier solvent over another lighter
solvent.
[138] In some embodiments, a mixture of solvents, such as propane and
butane, may be injected, which may provide some advantages over using a single
solvent. For example, the mixture may be selected to optimize a combined
objective
function of oil production rate and heater energy intensity. An example of
such a
combined objective function is the net present value (NPV) for a proposed
process,
which may take into account the amount of oil produced, the capital and
operating
costs required for the production, and carbon tax savings from possible GHG
emission
reductions.
[139] As a skilled person in the art will appreciate, in a liquid mixture
containing
multiple solvents, the bubble point condition of the liquid mixture is
different from the
bubble point condition of a mixture containing only one of the solvents.
32
CA 3027052 2018-12-11
[140] The candidate solvent should be suitable for dissolving at least one
of the
viscous hydrocarbons in the reservoir 100, such that it can function as a
diluent for the
hydrocarbons. Possible solvents may include non-polar solvents such as C3-C15
hydrocarbons, such as a C3, C4, C5, C6 or C7 alkane. In some embodiments, the
solvent
may be propane, iso-butane, n-butane, pentane, hexane, heptane, octane or a
combination thereof. Cyclohexane, 2,2-dimethylpentane, 2,2,4-trimethylpentane,
or
combinations thereof may also be suitable solvents alone or in combination
with other
non-polar solvents. Other possible solvents may include polar solvents. Polar
solvents
may include one or more of the following functional groups: an ether group, an
epoxide
group, a carboxylic acid group, an aldehyde group, a ketone group, an
anhydride
group, an ester group, an alcohol group, an amine group, and the like as
disclosed in
CA 1,887,405, which is incorporated by reference herein. Other possible
solvents may
be multi-component solvents such as natural gas liquids (NGLs), gas
condensates,
naphtha, diesel, other diluents, or combinations thereof.
[141] Not all solvents will work under all conditions, as would be
understood by
the skilled person. The solvent thus should be carefully selected for given
reservoir
conditions and for given overall production objectives. Some properties of the
solvent
may be readily recognized by a person skilled in the art. For example, the
skilled
person may be able to select a solvent that is vaporizable under given
injection
conditions (temperature and pressure) such that it can be injected into the
reservoir
100 in the gas (vapor) phase and so that it can substantially remain in the
vapor phase
until it reaches the interface region in the solvent chamber 106. In this
regard, heavier
solvents, such as C8-C15 hydrocarbons, may not be suitable under some
reservoir
conditions. If heavier solvents are desirable under such conditions, they may
be
combined with another lighter solvent to form a solvent mixture. The skilled
person may
also be able to recognize solvents that are condensable under given
temperature and
pressure conditions. In this regard, non-condensable solvent gases (under
reservoir
conditions), such as methane and ethane, are not suitable solvents for
embodiments
disclosed herein.
33
CA 3027052 2018-12-11
[142] In selecting a suitable solvent for use, the skilled person may be
guided,
by initially determining the pressure and temperature conditions of the
particular
reservoir. Typically, injection pressures and temperatures are also subject to
limitations
set by regulatory bodies. The skilled person may select an injection
pressure/temperature at a point which is at or near the upper
pressure/temperature
limit for the particular conditions in order to obtain maximum solvent
diffusivity and to
broaden the choice of solvents for use. Once the initial temperature and
pressure
conditions are set, the choice of potential solvents may be determined based
on the
guidance provided in this disclosure, and may be additionally based on routine
calculation, routine experimentation or routine simulation and analysis of
solvent
behaviour and properties in a given reservoir composition.
[143] In selecting a suitable solvent, the skilled person may also be
guided by
the solvent-crude hydrocarbon miscibility profiles for the solvents that meet
the
pressure/temperature requirements set out above. Solvent-crude hydrocarbon
miscibility profiles for a wide array of solvents are known, as discussed in
H.
Nouroozieh, M Kariznovi and J. Abedi, "Experimental and modeling studies of
phase
behavior for propane/Athabasca bitumen mixtures," Journal of Fluid Phase
Equilibria,
397 (2015) 37-43, the entire contents of which are incorporated by reference
herein. In
general, the skilled person may select a solvent which has a suitable solvent-
crude
hydrocarbon mixing coefficient, such that it will serve to mobilize
hydrocarbons within
the reservoir 100 during the development and expansion of the solvent chamber
106.
For this reason, highly polar solvents may not be appropriate under some
reservoir
conditions. Likewise, the skilled person may select a solvent which has a
suitable
solvent-asphaltene miscibility (or precipitation) coefficient. As different
solvents have
different solvent-asphaltene miscibility coefficients, the choice of the
solvent may affect
the selection of the lower temperature threshold for the production zone 108.
In order to
select an appropriate solvent for a particular set of reservoir conditions,
the skilled
person may also rely on the teachings in this disclosure, in combination with
routine
34
CA 3027052 2018-12-11
calculation, routine experimentation, or routine simulation related to solvent-
crude
hydrocarbon miscibility profiles, or solvent-asphaltene miscibility profiles.
[144] In selecting a suitable solvent, the skilled person may be further
guided
by the solvent bubble point in the fluid mixture in the production zone under
the
reservoir operating conditions. As noted, to avoid excess heating which is non-
productive or less efficient, substantial solvent re-vaporization within the
production
zone 108 should be prevented. Further, solvent re-vaporization may increase
the
viscosity of the liquid mixture in which the solvent acts as a diluent.
Solvents which
substantially evaporate or remain substantially in the vapor phase at a very
low
temperature, such as below about 50 to 60 C, may not be suitable, because if
such
solvents were used, the production zone would need to be maintained at even
lower
temperatures, and the oil mobility at these lower temperatures would be too
low to
allow efficient production. At such low temperatures, other potential problems
may arise
which may negatively affect the production process, such as hydrate formation
or the
like.
[145] In selecting a suitable solvent, the skilled person may be
additionally
guided by additional factors such as solvent cost, solvent recoverability,
solvent toxicity,
and solvent recyclability. A skilled person can weigh these exemplary
additional factors
when selecting an appropriate solvent without requiring undue experimentation
and
without requiring inventive ingenuity.
[146] As can be appreciated, the temperatures under original (natural)
conditions in different reservoirs may vary. For example, the original
temperature may
be from about 7 C to about 22 C, from about 9 C to about 15 C, or from about
10 C
to about 13 C, depending on the location of the reservoirs and the time. The
native
pressures may also vary in different reservoirs. For example, the native
pressure in a
reservoir may be from about 0.1 to about 4 MPa, from about 0.5 to about 3.5
MPa, or
from about 1 to about 3 MPa. The pressure and temperature profiles in a
reservoir may
also vary depending on the location and other characteristics of the
reservoir.
CA 3027052 2018-12-11
[147] The types of viscous hydrocarbons within different reservoirs may
also
vary. Depending on the in situ density and viscosity of the viscous
hydrocarbons, the
viscous hydrocarbons may comprise, for example, a combination of heavy oil,
extra
heavy oil and bitumen. Heavy oil, for example, may be defined as any liquid
petroleum
hydrocarbon having an American Petroleum Institute (API) Gravity of less than
about
200 and a viscosity greater than 1,000 mPa-s. Extra heavy oil, for example,
may be
defined as having a viscosity of over 10,000 mPa.s and about 10 API Gravity.
The API
Gravity of bitumen ranges from about 12 to about 70 and the viscosity is
greater than
about 100,000 mPa.s. For example, the bitumen in a reservoir may have an API
of 10
and a viscosity of about 110,000 mPa.s. API Gravity is also referred to as API
for
brevity.
[148] The recovery processes described herein are not limited to any
particular
type of reservoirs or hydrocarbon compositions in the reservoir.
[149] As noted earlier, in selected embodiments, the injection well 120 may
be
completed with, for example, a perforated or slotted liner along the
horizontal section of
the well. The production well 140 may also be completed with a slotted liner
along the
horizontal section of the well. In other embodiments, the wells may be
completed
differently as described above. For example, the injection or production well
may
include perforations, slotted liners, screens, outflow control devices
(injection well),
inflow control devices (production well), or a combination thereof as known to
one
skilled in the art.
[150] In selected embodiments, one or both of the wells 120 and 140 may be
provided with standard completion devices and equipment used in a typical
solvent
aided process, or used in wells that are suitable for use in a SAGD process
with
suitable modifications for solvent injection. Such devices and equipment may
include
flow control devices (FCDs), temperature measuring devices such as distributed
temperature sensing (DTS) devices or fibre optic measurement or control
components,
or the like.
36
CA 3027052 2018-12-11
[151] In selected embodiments, the injection well 120 may be vertically
spaced
from the production well 140 by a distance within a range of from 3m to 10 m,
or from
4 m to 6 m. These distances are exemplary and may be varied to optimize the
operation performance. A skilled person could select the well spacing by
considering
relevant processing parameters such as the temperature and pressure of the
reservoir
100 and the mobility of the viscous hydrocarbons present therein. In selected
embodiments, the length of the horizontal sections of the wells 120 and 140
may vary.
For example, in some embodiments, the horizontal sections of the wells 120 and
140
may have a length from 200 m to 1400 m, or from 600 m to 1000 m. The injection
well
120 and the production well 140 may be configured and completed in any
suitable
manner so long as the wells are suitable for injection of the selected solvent
and
production of a fluid from the reservoir as described herein. In some
embodiments, the
terminal sections of the wells 120 and 140 may be substantially parallel to
one another.
A person of skill in the art will appreciate that while there may be some
variation in the
vertical or lateral trajectory of the wells 120 and 140 (causing increased or
decreased
separation there between), such wells for the purpose of this application will
still be
considered substantially horizontal and substantially parallel to one another.
[152] In selected embodiments, the surface facility 220 may have a supply
line
(not shown) connected to an injection fluid source for supplying the solvent.
In selected
embodiments, one or more additional supply lines may be provided for supplying
other
fluids, additives or the like (not shown) for co-injection with the solvent.
Each supply
line may be connected to an appropriate source of supply, which may include,
for
example, a truck, a fluid tank, or the like. In some embodiments, co-injected
fluids or
materials may be pre-mixed before injection. In other embodiments, co-injected
fluids
may be separately supplied into the injection well 120.
[153] In selected embodiments, the surface facility 240 may include a fluid
transport pipeline (not shown) for conveying the produced fluids to a
downstream
facility (not shown) for processing or treatment. The surface facility 240 may
also
37
CA 3027052 2018-12-11
include additional optional equipment for producing a fluid from the
production well 140,
as can be understood by one skilled in the art.
[154] In selected embodiments, other necessary or optional surface
facilities
(not shown) may also be provided, as can be understood by one skilled in the
art. For
example, the surface facilities 220 and 240 may include one or more of a pre-
injection
treatment facility for treating a material to be injected into the formation,
a post-
production treatment facility for treating a produced material, a solvent
recycling facility,
and a control or data processing system for controlling production / operation
or for
processing collected operational data.
[155] Example heaters disclosed in CA 2,304,938 may be used as downhole
heaters in selected embodiments as described herein.
[156] The heaters 132 and 152 may include an electric heater. An electric
heater may include an insulated conductor. The conductor may be elongated,
such as
in the form of a wire or a rod, and may be coiled. The conductor may be
disposed and
enclosed in a conduit.
[157] A heater may also include a suitable heating system.
[158] For example, a heating system may generate heat by burning a fuel
external to or in a formation. The heating system may also include surface
burners,
downhole gas burners, flameless distributed combustors, and natural
distributed
combustors. In selected embodiments, heat provided to or generated in one or
more
heaters may be supplied by other sources of energy. The other sources of
energy may
directly heat a formation, or the energy may be applied to a transfer medium
that
directly or indirectly heats the formation. It is to be understood that one or
more heaters
that are applying heat to a formation may use different sources of energy.
Thus, for
example, for a given formation some heaters may supply heat from electric
heaters,
some heat sources may provide heat from combustion, and some heat sources may
provide heat from one or more other energy sources (for example, chemical
reactions,
38
CA 3027052 2018-12-11
solar energy, wind energy, biomass, or other sources of renewable energy). A
chemical
reaction may include an exothermic reaction (for example, an oxidation
reaction). A
heater may also include a heater that provides heat to a zone proximate to or
surrounding a heating location such as a heater well. The selection of an
appropriate
heater is within the purview of those skilled in the art. Such a selection is
typically made
having regard to, i.e., the output, efficiency, durability, control and
configuration of the
heater. The heater may be selected based on how far into the reservoir heat
from the
heater is expected to penetrate.
[159] In some selected embodiments, one or more of the heaters 132 and 152
may be powered to provide continuous desired or optimal heating. In other
selected
embodiments, one or more of the heaters 132 and 152 may be operated to provide
heating intermittently. Intermittent heating involves a first period of
heating at a lower
level, and a second period of heating at a higher level. The first and second
heating
levels may alternate and cyclically repeated. The first time period may
correspond to a
period of peak usage in the power grid that supplies heating power to the
heaters, and
the second time period may correspond to an off-peak period in the power grid.
Thus
the cycle of intermittent heating of the production zone may correspond to the
cycle of
peak and off-peak usage in the power grid.
[160] For example, heating may be reduced during a period of peak-demand
for electrical power as illustrated in FIG. 8, which shows a schematic
representation of
the power usage profiles of the heaters and the power grid. In general, the
heating
power applied in the heater 152 may have a profile that tracks or matches the
peak and
off-peak usage in the power source (a power grid in this example). Such
intermittent
heating may be more economical and, if scheduled appropriately, may not
negatively
affect the production performance significantly. The process may be, for
example
operated intermittently so as to reduce or minimize the operating expenses
(OPEX)
associated with electricity usage for heating the production zone. For
example, the
electricity cost to the operator of the recovery process may be substantially
reduced
during the off-peak period, as compared to the peak period. However,
cyclically
39
CA 3027052 2018-12-11
alternating between increased heating and reduced heating at 24 hour cycles
may not
significantly affect the average temperature in the production zone or the pay
zone in
general. That is, the temperature fluctuation in the reservoir, particularly
in the
production zone, may be limited and may not exceed or fall outside the
selected
temperature range (e.g. the selected lower and upper threshold temperatures).
Thus,
factors for selecting the varied heating powers may include the electricity
costs at
different time periods during a day or different days of the week. The
reservoir
pressure, reservoir temperature, injection rates, and production rates may
also
influence the optimizing of field operations, and may be included in the
consideration.
The heater(s) may be powered in any suitable manner to maintain the
temperature in
the production zone 108 between the upper threshold and the lower threshold.
[161] In various embodiments, the start-up sub-stage S402 may last for a
period of about 1 to about 12 months, or about 3 to about 9 months.
[162] In selected embodiments, other preheating measures may be employed
in the start-up sub-stage S402. Such measures may include, for example, the
application of geo-mechanical techniques or the use of one or more
microorganisms to
increase overall fluid mobility in a near-wellbore region. Another measure may
include
closed loop circulation (CLC) of a heating medium, for example steam. CLC
involves
running concentric pipe in the injection and production wells and circulating
the heating
medium through the concentric pipe, without injecting the heating medium into
the
reservoir formation. The heating mechanism in CLC is conduction. The heating
medium is never in contact with reservoir. Once the reservoir is pre-heated to
a target
temperature, the CLC concentric tubing may be removed from the injection and
production wells before production commences.
[163] In selected embodiments, the time period for the start-up sub-stage
S404
may vary. For example, the sub-stage S404 may last for a period of about 1 to
about 6
months, or about 2 to about 4 months.
CA 3027052 2018-12-11
[164] Instead of or in addition to solvents, other suitable injection
fluids such as
steam, diesel, natural gas liquids, gas condensate, C3-C15 hydrocarbons, non-
condensable gases (NCGs), or combinations thereof may be injected during the
start-
up stage S402 or S404, or both sub-stages. While not all of these fluids
solvents will
work under all conditions, a suitable fluid for use in the start-up stages
S402 or S404
may be selected by a person skilled in the art having regard to the particular
reservoir
conditions (e.g. temperature, pressure, composition), in view of the guidance
provided
in this disclosure. NCGs include, but are not limited to air, nitrogen, carbon
dioxide,
methane, natural gas, other light hydrocarbons, or a combination thereof. The
NCG
may facilitate maintaining at least a portion of the solvent in the vapor
phase due to a
partial pressure effect, allowing the solvent to travel further before
completely
condensing.
[165] The injection temperature and injection pressure for any given
injection
fluid in the start-up stages may also vary. Possible injection temperatures
may be, for
example, from the ambient temperature to about 250 C or about 290 C.
Possible
injection pressures may be from about 2 MPa to about 7 MPa.
[166] In selected embodiments, the time period of the production stage S406
may vary. For example, it may last for a period of about 1 year to about 10
years.
Likewise, the injection temperature and injection pressure during the
production stage
S406 may vary over time and may vary in different applications. The injection
temperatures may be, for example, from the ambient temperature to about 250 C
or
about 290 C, depending on the solvent selected and the reservoir conditions.
The
injection pressures may be from about 2 MPa to about 7 MPa.
[167] The wells 120 and 140 may be positioned towards the bottom of the pay
zone 102, which may be more efficient as the heated solvent vapor may tend to
rise up
in the solvent chamber 106. The heater 132 may be configured and operated to
provide
more heating power, as it may be used to heat a larger volume of the pay zone
102
41
CA 3027052 2018-12-11
than the heater 152. For example, the power ratio between the heaters 132 and
152
may be 60:40, 70:30, or 80:20.
[168] In select embodiments, the heater 152 may include a plurality of
heaters
positioned in various configurations throughout the horizontal section of the
production
well 140. For example, two or more heaters may be positioned at equal spacing
along
the horizontal sectional section of the production well 140, and the two or
more heaters
may be independently controlled. In such a configuration, heat may be applied
to a first
region of the production well 140, while a second region of the production
well 140 is
not heated. This location specific heating may be applied to account for, for
example,
heterogeneity in the production zone 108.
[169] In select embodiments, the upper temperature threshold and the lower
temperature threshold of the production zone 108 may vary during the
production stage
S406. The lower threshold may be selected to manage and control asphaltene
phase
equilibria such as precipitation, flocculation, and agglomeration, in order to
limit the
formation or growth of an asphaltene-rich bitumen (ARB) phase in the liquid
mixture to
be produced, and to limit the extent of asphaltene deposition in the
production zone.
The skilled person may select the lower threshold based on the teachings of
this
specification, routine calculations, routine experimentation and routine
simulation. The
upper threshold may be selected to prevent excessive re-vaporization of the
solvent in
the liquid mixture because solvent re-vaporization may reduce the mobility of
the liquid
mixture. The skilled person may select the upper threshold based on the
teachings of
this specification, routine calculations, routine experimentation and routine
simulation.
[170] In selected embodiments, the time period of the blowdown stage S408
may vary. For example, the blowdown stage S408 may last for a period of about
1
month to about 12 months. In selected embodiments, the injected fluid,
injection
temperature and pressure used during the blown-down stage S408 may vary.
Possible
fluids for blowdown may include methane, ethane, propane, N2, CO2 or the like.
Possible blowdown pressures may range from 2 MPa to 7 MPa, and possible
42
CA 3027052 2018-12-11
blowdown temperatures may range from ambient temperature to about 250 C or
about
290 C.
[171] As discussed earlier with respect to FIG. 5, the temperatures of the
various regions of the reservoir 100 generally decrease as the distance from
the
injection well 120 and the production well 140 becomes longer, towards the
interface
regions of the solvent chamber 106. In the interface region, the temperature
may
decrease quickly, and the temperature just outside the solvent chamber may be
close
to or at the reservoir native temperature. Thus, the temperature of the
injected solvent
may be the highest at the injection well 120, and may drop modestly as the
solvent
travels through the central region of the solvent chamber 106. In select
embodiments,
the injection temperature may be between 50 C and 150 C, and the injected
solvent
may cool down to a temperature between about 45 C and to about 145 C is it
passes
through the solvent chamber 106. As the solvent vapor contacts materials
within the
cooler interface region its temperature may decrease more quickly, and the
solvent
may condense and mix with hydrocarbons in the interface region to form a
liquid
mixture containing the solvent, mobilized hydrocarbons and asphaltenes. In
select
embodiments the temperature at the interface region may be between 25 C and
125 C.
[172] In different embodiments, the process parameters may be selected to
improve overall process efficiency, with an aim to recover the maximum amount
of oil
from the reservoir. The process may also be designed to reduce the amount of
the
solvent used, or to recapture injected solvent quickly. Convenient recycling
and re-use
of the solvent may be a factor, but reducing or avoiding solvent recycling may
be
beneficial in some embodiments; recycling a solvent may not be as efficient as
for
recycling steam because the gravity is not as efficient for driving solvent
drainage as
compared to driving steam drainage.
43
CA 3027052 2018-12-11
[173] Another factor to consider is overall reduced energy usage. Such
factor
may be assessed using a net energy intensity (El). The El for a given process
may be
assessed by a person skilled in the art based on known methods and tools.
[174] For example, some analysis has shown that substantial energy savings
can be obtained for a given recovery factor (RF) (such as at 70% RF) with an
embodiment of the present disclosure in a homogenous reservoir, as compared to
other processes. In particular, using the El of a typical SAGD process as the
base line,
using propane according to the present disclosure may reduce the El by as much
as
75%, and using butane may reduce the El by as much as 45%, at 3 MPa. In other
words, propane may reduce the El to about 1/14 of the SAGD value, butane may
reduce the [Ito about 1/8 of the SAGD value. Pentane may reduce the El to
about 1/4
of the SAGD value. The reduced effect of butane as compared to propane is
expected
to be largely due to the higher heating temperature permitted in the butane
process
(see FIGS. 6B and 6C).
[175] The process parameters may be selected to reduce or minimize the
amount or volume of injected solvent without sacrificing the production rate,
production
efficiency, or recovery factor.
[176] It is also noted that to upgrade bitumen in situ, some asphaltene
precipitation will occur. Generally, the more asphaltenes precipitate, the
more the
bitumen is upgraded. Asphaltenes can plug up reservoir pores and wellbore
liners, so it
may be desirable to control asphaltene production in a solvent driven process.
Controlled heating of the production zone as disclosed herein provides a
control
mechanism to control asphaltene precipitation and production. In an ideal
situation, the
optimal heating temperature set for a heater in the production well would
provide
maximum upgrading of the produced oil without, or with only minimal,
deleterious
asphaltene precipitation in the production zone.
[177] In practice, the oil production rate may be monitored in real time as
a
function of the asphaltene content in the produced oil, or as a function of
the API of the
44
CA 3027052 2018-12-11
produced oil (API value is indicative of the degree of oil upgrading), while
lowering the
temperature in the production well, and the heating temperature may be
selected as
the temperature at which the oil production rate is maximized or an overall
production
performance metric is optimized. Normally, it is desirable to increase both
the oil
production rate and the API of the produced oil, which may result from limited
and
controlled asphaltene precipitation. However, when the oil production rate
starts to
decrease while the API of the produced oil is still increasing, the asphaltene
precipitation in the production zone may be considered to have become
deleterious, as
the asphaltene precipitation may have possibly led to plugging of the pores or
wellbore
liners.
[178] FIG. 9 illustrates an example correlation between the oil production
rate
(Oil Rate) and the API of the produced oil (Oil API). The Oil API increases as
the
heating temperature in the production well is increased. As can be seen, the
oil
production rate initially increases as the Oil API increases and then starts
to fall at an
inflexion point. The corresponding heating temperature at the inflexion point
may
represent an optimal operating condition (temperature) for controlling the
heater(s) in
the production well. The asphaltene content in the produced oil may be
measured in a
laboratory using the SARA analysis. The Oil API may be determined by measuring
the
density of the produced oil, as can be understood by those skilled in the art.
[179] Tests were performed which indicated that using propane and butane as
the solvent, the produced oil could be upgraded by about 30%.
[180] An oil extraction lab test involving soaking an oil core (used as a
reservoir
model) with butane (no steam) at a temperature of 138 C and a pressure of
3.172
MPa over a period of about 30 h indicated that percent oil saturation (So) in
the core
was reduced from 88% to 16%. In comparison, soaking an oil core with de-
ionized (DI)
water at a temperature of 236 C and a pressure of 3.137 MPa over the same
time
period of about 30 h resulted in So in the core only being reduced from 88% to
67%.
Likewise, oil recovery of 83.63% of the original oil in place (00IP) was
significantly
CA 3027052 2018-12-11
higher with butane-only extraction compared to DI water, which yielded an oil
recovery
of just 24.03% of 00IP. Relevant data for this test is listed in Table II.
Table II. Solvent (Butane) Oil Extraction Results
Butane DI Water
Solvent:Steam Ratio 100:0 0:100
Core Weight (g) 121.4 120.36
Oil in Core (g, 18.22 18.05
Initial So (%) 88 88
Pressure of Operation (MPa) 3.172 3.137
Temperature of Operation ( C) 138 236
Mass Solvent Added (g) 115 0
Mass Water Added (g) 2 115
Oil Extracted (g) 15.23 4.34
Oil Recovery (% 00IP) 83.63 24.03
Final So (%) 16 67
Time (h) 30 30
[181] FIG. 10 compares the energy intensity (El) at 70% recovery factor
(RF)
between a SAGD process (cSOR=3.2) and a propane-based recovery process
(cSOR=0.2), with a reduction in El by 16 times (cSOR = cumulative solvent to
oil ratio).
The comparison is for a homogeneous reservoir.
[182] FIG. 11 shows the relative changes of temperature, oil saturation in
the
formation (So %), and solvent saturation (propane concentration A) over time
in a
propane-based recovery process at temperatures below 100 C. FIG. 12 shows
comparison data with respect to FIG. 11 from a comparison process with co-
injection of
steam and propane at temperatures up to about 240 C.
High injection temperature process
[183] The low injection temperature process can present challenges in some
cases. For example, the low injection temperature process may require high
gross and
net solvent in lower permeability and heterogeneous reservoirs to be
successful. This is
due to the solvent chamber containing two solvent phases - gas and liquid - at
low
injection temperatures. The gas phase is necessary to maintain a solvent
chamber and
46
CA 3027052 2018-12-11
also allow for solvent to diffuse/disperse into the pores of the reservoir so
that gas-
liquid partitioning of the solvent may occur beyond the solvent chamber walls.
However, the solvent in the liquid phase in the solvent chamber contributes
little to oil
recovery because limited mobile bitumen is present in the central portions of
the
solvent chamber, and the liquid solvent in the central portion of the solvent
chamber
increases solvent retention within the reservoir pore spaces.
[184] An alternative to the low injection temperature process described
above
involves injecting the solvent at an injection temperature substantially above
the boiling
point temperature of the solvent at reservoir pressure (the "high injection
temperature
process"). The well system and recovery process described above may be adapted
for
the high injection temperature process with modifications to inject the
solvent at higher
temperatures. At higher temperatures, viscosity reduction due to heat transfer
becomes more effective, but solvent dissolution can still contribute to
increasing the
mobility of the viscous hydrocarbons.
[185] In an embodiment of the high injection temperature process, the
process
comprises: injecting a solvent at an injection temperature (T,) into the
reservoir to
mobilize viscous hydrocarbons in the reservoir, wherein T1 is maintained
substantially
above the boiling point temperature (Tbp) of the solvent at a reservoir
pressure,
whereby AT = T1- Tbp is positive; and producing hydrocarbons mobilized by the
solvent
from the reservoir.
[186] In the high injection temperature process AT remains more or less
stable
or is not varied substantially during production of the hydrocarbons. Further,
AT may
be at least 50 C. In selected embodiments, AT may be at least 100 C.
[187] In selected embodiments, propane may be injected at an injection
temperature of about 200 C to about 300 C at a reservoir pressure of about 2.5
MPa to
about 3.5 MPa. For example, propane may be injected at an injection
temperature of
about 200 C at a reservoir pressure of about 3.0 MPa.
47
CA 3027052 2018-12-11
[188] In selected embodiments, butane may be injected at an injection
temperature of about 200 C to about 300 C at a reservoir pressure of about 2.5
MPa to
about 3.5 MPa. For example, butane may be injected at an injection temperature
of
about 300 C at a reservoir pressure of about 3.0 MPa.
[189] At the same reservoir pressure, solvent injected in the high
injection
temperature process has a decreased density compared to solvent injected in
the low
injection temperature process and will more readily occupy more space within
the
reservoir. At high injection temperatures, particularly when the injection
temperature is
substantially above the boiling point temperature, the solvent chamber is more
likely to
contain a single solvent phase, namely the gas phase. Consequently, the high
injection temperature process may require less solvent to operate, and may
result in a
lower solvent to oil ratio in the produced fluid from the reservoir.
[190] Despite using higher injection temperatures, the high injection
temperature process should still be effective at dispersing and condensing
solvent in
the porous reservoir. Indeed, calculations detailed below suggest that less
than 1% of
the solvent chamber need be at lower temperature conditions for the high
temperature
process to be successful.
[191] Because solvents have relatively low latent heats, this 1% condition
should always be met at any reasonable injection temperature; hence, injection
temperatures for the high injection temperature process should only be limited
by GHG
intensity targets and coking temperatures of bitumen and/or solvent, whichever
is less,
as economics will favor the highest possible injection temperatures giving the
lower
solvent requirement. For example, for butane solvent at a reservoir pressure
of 3MPa,
temperatures as high as 300 C may be needed (which gives limited flexibility
as the
coking temperature of bitumen is around 350 C), but for reservoir pressures
around
1MPa, injecting butane at 200 C could be relatively optimal (solvent
solubility
decreases with pressure) given other factors such as targeting reduced GHG
emissions intensity.
48
CA 3027052 2018-12-11
[192] FIGS. 13 and 14 show representative simulation results, using propane
as solvent, which illustrate why the high injection temperature process is
more
economical than the low injection temperature process; the high injection
temperature
process leads to higher bitumen rates and lower net solvent requirements for
the
simulated reservoir. FIG. 15 plots the pre-blowdown net cumulative solvent to
oil ratio
(cSolOR) for both the low and high temperature injection processes with an
average of
about 0.25 cSolOR for the high temperature injection process and 0.45 cSolOR
for the
low temperature injection process.
[193] The simulation model used to generate the data in FIGS. 13-15 is as
described above for the low injection temperature process with reservoir
properties
listed in Table I. In the case of the high injection temperature process, the
injection
temperature used was 200 C.
Injection temperature ranging process
[194] In some cases, the solvent-based recovery processes discussed herein
can be improved by varying the solvent injection temperatures over time during
oil
production, such as by decreasing the solvent injection temperature from a
higher
temperature at the beginning of the process to a lower temperature closer to
the end of
the process where the reservoir would have been heated over a period of time
to
relatively elevated temperatures and a porous solvent chamber has been formed
therein. When the solvent chamber is already hot, and has expanded to a
substantial
volume, the injected solvent may more easily travel through the solvent
chamber to
reach the chamber edges with limited condensation in the central portion of
the solvent
chamber. Thus, injecting the solvent at a reduced temperature, but still above
the
boiling point temperature, would still allow effective and efficient oil
production.
[195] As can be seen from FIGS. 13 and 14, in the early years of oil
production,
solvent injection at the higher temperature would provide relatively higher
bitumen
recovery (oil production) rate and lower net solvent usage; but in the later
years of oil
production, solvent injection at the lower temperature would provide
relatively higher
49
CA 3027052 2018-12-11
bitumen recovery (oil production) rate and lower net solvent usage. Thus, it
may be
expected that injecting the solvent at the higher temperature in the earlier
years and at
the lower temperature in the later years of oil production would provide
improved
overall oil recovery and reduced solvent usage. It may also be expected that
gradually
decreasing the injection temperature over time, based on the solvent chamber
development and other factors as indicated by the solvent to oil ratio in the
produced
fluid from the reservoir, may provide even better overall operation
performance and
efficiency.
[196] The well system and recovery processes described earlier may be
adapted for use in the improved process with modifications to allow control
and
variation of the solvent injection temperatures, as will be further described
below.
[197] In selected embodiments, the injection temperature ranging process
comprises: injecting a solvent at an injection temperature (T1) into the
reservoir to
mobilize viscous hydrocarbons in the reservoir, wherein T, is maintained above
the
boiling point temperature (Tbp) of the solvent at a reservoir pressure,
whereby AT = T, -
Tbp is positive; producing hydrocarbons mobilized by the solvent from the
reservoir; and
decreasing AT over time during production of the hydrocarbons.
[198] In selected embodiments, AT may be at least 50 C, or at least 100 C,
at
reservoir pressure before decreasing over time during production of
hydrocarbons.
[199] The injection temperature ranging process has an optimal operating
window for a solvent process which features both the high injection
temperature
benefits (lower solvent retention and less asphaltene precipitation) and the
low injection
temperature benefits (higher solvent solubility and increased bitumen
upgrading).
[200] The injection temperature ranging process may use electric heaters as
described herein but their purpose is dual in nature, i.e. preventing
significant
asphaltene precipitation and/or ensuring minimal solvent condensation within
the
solvent chamber. The injection well may also have an electric heater to help
maintain
CA 3027052 2018-12-11
solvent chamber temperature and minimize solvent condensation within the
solvent
chamber. Because injection temperatures are substantially above the boiling
point of
the solvent for the injection temperature ranging process, the production well
electric
heater can now be maintained at higher temperatures than the asphaltene
rejection
temperature limit of the low injection temperature process. In heterogeneous
reservoirs,
significantly higher solvent injection volumes may be required because
solvents have
relatively low latent heats. Use of the production well electric heater may
reduce such
process inefficiencies, just as in the low injection temperature process.
[201] Surface heating of solvent is limited to the coking temperature of
solvent
which is generally about 350 C for light alkanes. This means that the
theoretical
maximum solvent injection temperature for the injection temperature ranging
process
will be about 300 C if surface heating is used. Accounting for heat losses
within the
injector wellbore, the reservoir sandface might "see" temperatures that could
be lower
than optimal. One way to "insure" against this is to also use one or more
electric
heaters in the injection well. This will especially be the case as the carbon
number of
the solvent increases because injection temperatures increase with carbon
number and
maintaining a relatively high temperature is aided by the electric heater(s).
Note that
this isn't necessary for the low injection temperature process because
injected
temperatures are lower and more easily handled at surface.
[202] Because temperatures are significantly higher for the injection
temperature ranging process than for the low injection temperature process,
this opens
up the solvent choice to many other solvents such as pentane and hexane, the
dew/bubble points of which are significantly higher than, for example, propane
and
butane.
[203] In selected embodiments, the solvent is propane and
AT = 200 C ¨ Tbp (propane) and AT may be decreased to any value greater than
or
equal to zero during production of the hydrocarbons. In selected embodiments,
the
injection temperature is decreased to about 100 C during production of
hydrocarbons.
51
CA 3027052 2018-12-11
[204] In selected embodiments, the solvent is butane and
AT = 300 C ¨ Tbp(butane) and AT may be decreased to any value greater than or
equal
to zero during production of the hydrocarbons.
[205] Because the injection temperature is not expected to be constant
throughout the recovery process, a method to determine injection temperatures
is
required as a function of time. FIG. 16 conceptually illustrates one such
method that
involves monitoring the produced solvent to oil ratio and/or net injected
solvent to oil
ratio.
[206] The produced solvent to oil ratio may be calculated based on the
amount
of solvent present in the produced reservoir fluid and the amount of oil in
the produced
reservoir fluid, e.g., the volume ratio of the solvent to oil in the produced
reservoir fluid
on a liquid basis. This volume ratio may be referred to as the produced SolOR.
The net
injected solvent to oil ratio may be calculated based on the ratio of the
difference
between the total amount of injected solvent and the total amount of the
solvent
recovered from the reservoir, and the amount of oil produced. The net injected
solvent
to oil ratio indicates the solvent usage efficiency. When the net injected
solvent to oil
ratio is too high, the injected solvent is not efficiently utilized to assist
oil production.
[207] The left side of FIG. 16 represents a high injection temperature
process
where solvent retention is low but dilution of bitumen is not as efficient,
while the right
side of FIG. 16 represents a low injection temperature process where solvent
retention
is high but dilution of bitumen is more efficient. The central region of FIG.
16 represents
an injection temperature ranging process where bitumen rates and solvent
retention in
the reservoir are optimal due to the combined effect of temperature and
solvent
solubility for reducing bitumen viscosity.
[208] The objective is to maintain the produced solvent to oil ratio in a
target
range that results in the high bitumen rates shown in the central region of
FIG.16. The
target range is set to achieve optimal economic operating conditions, i.e.
high bitumen
recovery while balancing energy and solvent use. The injection temperature is
varied
52
CA 3027052 2018-12-11
to balance and optimize energy efficiency, solvent usage and bitumen recovery
performance.
[209] For example, if the injection temperature is too low, there may be
excessive solvent in the solvent chamber not aiding in hydrocarbon
mobilization.
Consequently, at low injection temperatures there may be energy savings at the
expense of solvent waste. If the injection temperature is too high, there may
be
excessive energy input for hydrocarbon mobilization. Consequently, at high
injection
temperatures there may be solvent savings at the expense of energy waste.
[210] In selected embodiments, the injection temperature ranging process
comprises: injecting a solvent at an injection temperature into the reservoir
to mobilize
viscous hydrocarbons in the reservoir, wherein the injection temperature is
maintained
above the boiling point temperature of the solvent at a reservoir pressure;
producing
from the reservoir a fluid comprising the solvent and hydrocarbons mobilized
by the
solvent; and in response to a change in a solvent to oil ratio in the produced
fluid,
adjusting the injection temperature during production.
[211] Simulation tests of the injection temperature ranging process were
conducted. The simulation model is similar to that described above for the low
injection
temperature process with reservoir properties listed in Table I. However, the
injection
temperatures used in the simulation for the injection temperature ranging
process are
outlined in FIG. 17.
[212] FIG. 17 shows the results of an optimization process in which the
solvent
injection temperature was allowed to vary with time so as to maximize an
economic
objective function. The results clearly show that the injected temperature
trend for the
injection temperature ranging process should be downward.
[213] As can be seen from FIG. 18, the optimal solvent to oil ratio by
volume, or
target range, for propane in the simulated injection temperature ranging
process is
53
CA 3027052 2018-12-11
about 1 to about 2. This means that the injection temperature should be ranged
to
maintain produced SolOR to a number between about 1 and about 2 for propane.
[214] As can be seen from FIG. 19, the optimal solvent to oil ratio by
volume, or
target range, for butane in the simulated injection temperature ranging
process is about
2 to about 3. This means that the injection temperature should be ranged to
maintain
produced SolOR to a number between about 2 and about 3 for butane.
[215] FIG. 20 plots the net injected solvent to oil ratio as a function of
the
produced SolOR for butane and shows that it mimics well the theoretical plot
of the
right vertical axis of FIG. 16. At low produced SolOR typical of high
injection
temperature, net SolOR does not change significantly (i.e. little benefit is
gained by
excessive high temperatures) but at high produced SolOR typical of low
injection
temperature, solvent losses within the reservoir significantly increase. It is
expected
that a plot for propane will be similar to FIG. 20 but shifted leftward.
[216] It is possible that the injection temperature ranging process may
have the
lowest energy intensity compared to the high injection temperature process
(high
temperatures but lower solvent volumes) and the low injection temperature
process
(low temperatures but higher solvent volumes), but this will depend on the
nature of the
reservoir and the solvent used.
[217] FIGS. 21 ¨ 24 compare the solvent chamber edge dynamics for the
injection temperature ranging process and the low injection temperature
process as a
function of distance from the injection and production wells. These FIGS. are
illustrative
of why the injection temperature ranging process requires less solvent than
the low
injection temperature process. In FIG. 21 for the low injection temperature
process, it
can be seen that almost 90% of the solvent chamber contains significant
solvent liquid,
while FIG. 23 shows this value is only about 25% for the injection temperature
ranging
process. This means that the injection temperature ranging process may be
significantly less wasteful of solvent compared to the low injection
temperature process
as solvent liquid within the chamber does very little for the process (except
of course to
54
CA 3027052 2018-12-11
ensure condensing conditions at the edge of the chamber). In other words, the
solvent
chamber only needs to be 25% condensing to achieve the low injection
temperature
process bitumen rates.
[218] This 25% is more than provided for by the low injection
temperature
process, but at the expense of wasting solvent that can be quite expensive,
because
solvent densities increase as condensing conditions improve. On the other
hand, the
injection temperature ranging process optimally allows for the 25% condensing
conditions by forcing a temperature gradient through the solvent chamber. In
the
chamber closer to the injection and production well pairs, there exists almost
no mobile
bitumen so condensing conditions are not necessary and the injection
temperature
ranging process minimizes solvent retention by primarily allowing solvent to
be in the
gaseous phase with a lower density. Moving away from the injection and
production
well pairs and closer to the cold bitumen wall, the injection temperature
ranging
process then allows for condensing conditions where they are most needed for
solvent
to partition into the bitumen. This is observed in FIGS. 25 and 26, which show
propane
concentration in the oil phase of the solvent chamber during the low injection
temperature process (FIG. 25) and the injection temperature ranging process
(FIG. 26),
respectively. In FIGS. 25 and 26, the darkness or brightness of a region
indicates the
relative propane concentration in the local region according to the relative
concentration scale shown at the right hand side of each figure. As can be
seen, in
FIG. 25, most of the central regions in the solvent chamber have relatively
high solvent
concentrations. In comparison, in FIG. 26, the regions near the injector have
much
lower solvent concentrations (as low as about 0.3-0.4 on the relative
concentration
scale), and the relative solvent concentration gradually increases towards the
edge of
the solvent chamber, to about 1 at the lateral edges and to about 0.6-0.8 at
the top
edge of the chamber. FIGS. 22 and 24 also show that the production zone has
relatively higher temperature during the injection temperature ranging process
than
during the low injection temperature process, which generally translates into
higher
bitumen recoveries as seen in FIG. 13.
CA 3027052 2018-12-11
Example ¨ Injection Temperature Ranging with Propane
[219] In a practical example of the injection temperature ranging process
with
propane as solvent, the injection pressure is set to be about 3.0 MPa. The
injection
pressure could be higher, as a higher pressure is more desirable for
increasing
hydrocarbon production rate, but the injection pressure may also be limited by
technical, safety and environmental concerns, as well as regulations by local
authorities.
[220] The solvent, propane in this example, is selected so that it can be
injected
as a vapor and condense at the interface region of the solvent chamber.
[221] The initial injection temperature of propane is set to be about 200
C,
which is substantially higher than the boiling point temperature (77 C) of
propane at
about 3.0 MPa. The high injection temperature is chosen to decrease the
density of
the injected propane causing every unit mass of injected propane to occupy
more
space in the solvent chamber and thus reducing the amount of propane required
compared to a low injection temperature process. The injection temperature
could be
higher, but is maintained below the coking temperature of propane, i.e. the
upper limit
for the injection temperature.
[222] Once the start-up stage of the injection and production wells is
completed, propane is injected into the reservoir via the injection well at
the above
mentioned temperature of 200 C and injection pressure of about 3.0 MPa for a
period
of time before any adjustment of the injection temperature.
[223] The propane enters the reservoir as a vapor and travels generally
upwards in the solvent chamber as the chamber develops. The propane will
condense
at the chamber edges due to the cooler temperatures. The temperature in a
solvent
chamber varies from the injection well towards the front of the solvent
chamber, and
the temperature at the chamber edges (also referred to as the "solvent front")
is still
relatively low, such as about 15 C to about 25 C. The liquid solvent will
mix with
56
CA 3027052 2018-12-11
hydrocarbons and drain generally downwards to the production zone around the
production well. A pump in the production well will produce fluids to the
above ground
facilities.
[224] The fluid produced from the production well is monitored in real time
to
determine the content of solvent and oil in the produced fluid, for example
using test
separators and meters.
[225] After start-up, it is expected that the initial produced solvent to
oil ratio will
be high given that the reservoir is relatively cold. As time progresses, there
should be
gradual convergence of the produced solvent to oil ratio by volume to about 1
to about
2. After convergence, the produced SolOR will generally start increasing thus
indicating the time to start adjusting injection temperature. It is expected
that the time
period before adjustment may be about 6 months.
[226] Once the injection temperature adjustment period begins, the
injection
temperature is adjusted to maintain the solvent to oil ratio in the target
range of about 1
to about 2. For example, if the solvent to oil ratio by volume measured in the
produced
fluid is 2 or more, the injection temperature is raised to effectively lower
the amount of
solvent used in the process. Likewise, if the solvent to oil ratio by volume
in the
produced fluid is 1 or lower, then the injection temperature is lowered to
effectively
raise the amount of solvent used in the process.
[227] While the injection temperature can be raised or lowered to maintain
the
target solvent to oil ratio, the injection temperature will generally trend
downward over,
for example, a 10-year production period to a final injection temperature of
about
100 C. It is expected that as the solvent chamber gets larger over time, the
reservoir
becomes depleted and requires less energy to raise its temperature.
Calculation of solvent chamber volume to be at lower temperature
[228] FIG. 27 illustrates schematically a solvent chamber in an idealized
high
injection temperature process during a growth phase of the solvent chamber.
The
57
CA 3027052 2018-12-11
region between the solid line (representing the edge of the solvent chamber at
the
interface between the solvent chamber and the reservoir) and the dashed line,
referred
to as a "dispersion zone", represents a volume in the reservoir into which the
solvent
may diffuse and disperse. FIG. 28 shows a magnified section of the dispersion
zone,
and indicates the distribution of the solvent concentration in the dispersion
zone.
[229] A diffusion/dispersion length scale may be computed using a
convection/dispersion equation, but as an approximation, a length value of up
to about
0.01m may be assumed (those skilled in the art will appreciate this as within
the order
of magnitude of a length scale for dispersion in porous media). As shown in
FIG. 28,
the injected solvent concentration may vary from 100% mole fraction at the
interface of
the solvent chamber (solid line) to 0% mole fraction at the end of the
dispersion zone
(dashed line). For a simplified analysis, a linear concentration profile that
gives an
average solvent concentration Cavg within the dispersion zone as 50% mole
fraction
may be assumed. Such a linear approximation may be conservative because the
convex non-linear concentration profile via the solution of the
convection/dispersion
equation guarantees that Cavg 5. 50%.
[230] Assuming that the region of the solvent chamber closest to the
dispersion
zone is the required lower temperature zone, then the minimum required lower
temperature zone volume must be equal to the dispersion zone volume in liquid
equivalent or mass basis. Hence, by computing the volume of liquid solvent in
the
dispersion zone at 50% solvent concentration, an upper limit of the minimum
required
gaseous solvent volume for the lower temperature zone may be computed. An
example
of this computation is given below.
[231] Considering a typical oil sands reservoir with a 15m thick rich
bitumen
zone and 100m well spacing between adjacent well pairs, it can be assumed that
the
well length is lm for simplicity of calculation without any loss of generality
because it
can also be assumed that the solvent has the idealized inverted triangular
prism shape
shown in FIG. 27. The dispersion zone can again be assumed to be up to 0.01m.
58
CA 3027052 2018-12-11
Further it is assumed that only solvent and bitumen exist in the dispersion
zone (i.e.
methane gas does not partition appreciably into bitumen in the presence of
higher
carbon number solvents such as propane and butane).
[232] Given that the solvent mole fraction in the dispersion zone is 0.5
and the
densities of bitumen and liquid solvent are known, the volume fraction of
solvent in the
dispersion zone can be computed as shown in equation (1).
1
vs =1+ xs Mb ps
[233] 1¨x5 Ms Pb 1
vs= Solvent volume fraction
x5 = Solvent mole fraction
Ms = Solvent molar mass
[234] where
Mb= Bitumen molar mass
ps= Solvent mass density
ph= Bitumen mass density
[235] Because xs = 0.5, equation 1 can be written as equation (2):
1
[236] VS__ 2.
1+ Mb ps
Ms Pb
[237] Substituting values for propane and typical bitumen gives the volume
fraction Its , 0.11, meaning that ¨ 11% of the liquid volume present in the
0.01m
dispersion zone is propane solvent for this hypothetical reservoir.
[238] Given the geometry of the idealized solvent chamber (see FIG. 27) and
solving for one-half of the symmetry element, the maximum volume of solvent in
the
dispersion zone will occur at the end of the growth phase with an interface
length of
59
CA 3027052 2018-12-11
, 152 + (1 oo 50.2m . This then equates to a solvent volume of -0.0574m3
per unit
k 2
well length in the dispersion zone. Converting this liquid volume to gaseous
volumes at
standard conditions gives 17.51 Sm3 per well length. For propane in the
gaseous
phase at 3MPa, the ideal lower temperature zone will be about 75 C, which then
gives
in situ gaseous volumes of -0.715m3 per unit well length associated with the
lower
temperature zone inside the solvent chamber.
[239] At the end of the growth phase, the total solvent chamber volume
will be
¨1x 50 x15 =375m3 per unit well length. Assuming only the lower temperature
zone
2
contributes the solvent in the dispersion zone (again another conservative
assumption),
then it means only (0.715/375) * 100 ==, 0.2% of the solvent chamber needs to
be at
lower temperature conditions. Using order of magnitude arguments and without
loss of
generality, a good rule of thumb for most bitumen reservoirs based on this
analysis is
that less than 1% of the solvent chamber needs to be at lower temperature
conditions
for the high injection temperature process to be successful.
CA 3027052 2018-12-11
CONCLUDING REMARKS
[240] It will be understood that any range of values herein is intended to
specifically include any intermediate value or sub-range within the given
range, and all
such intermediate values and sub-ranges are individually and specifically
disclosed.
[241] It will also be understood that the word "a" or "an" is intended to
mean
"one or more" or "at least one", and any singular form is intended to include
plurals
herein.
[242] It will be further understood that the term "comprise", including any
variation thereof, is intended to be open-ended and means "include, but not
limited to,"
unless otherwise specifically indicated to the contrary.
[243] When a list of items is given herein with an "or" before the last
item, any
one of the listed items or any suitable combination of two or more of the
listed items
may be selected and used.
[244] Of course, the above described embodiments are intended to be
illustrative only and in no way limiting. The described embodiments are
susceptible to
many modifications of form, arrangement of parts, details and order of
operation. The
invention is intended to encompass all such modification within its scope, as
defined by
the claims.
61
CA 3027052 2018-12-11